Abstract
Astaxanthin is a high-value carotenoid currently being produced by chemical synthesis and by extraction from the biomass of the microalga Haematococcus pluvialis. Other microalgae, such as Chlorella zofingiensis, have the potential for being used as sources of astaxanthin. The differences between the synthetic and the microalgae derived astaxanthin are notorious: not only their production and price but also their uses and bioactivity. Microalgae derived astaxanthin is being used as a pigment in food and feed or aquafeed production and also in cosmetic and pharmaceutical products. Several health-promoting properties have been attributed to astaxanthin, and these were summarized in the current review paper. Most of these properties are attributed to the high antioxidant capacity of this molecule, much higher than that of other known natural compounds. The aim of this review is to consider the main challenges and opportunities of microalgae derived products, such as astaxanthin as food. Moreover, the current study includes a bibliometric analysis that summarizes the current research trends related to astaxanthin. Moreover, the potential utilization of microalgae other than H. pluvialis as sources of astaxanthin as well as the health-promoting properties of this valuable compound will be discussed.
Keywords: Haematococcus pluvialis, Chlorella zofingiensis, carotenoids, xanthophylls, pigments, bibliometric analysis, functional foods, bioactive compounds, antioxidant capacity, biotechnology
1. Introduction
Consumer demands for sophisticated and innovative foods have increased. Moreover, consumers are now more aware of the water–energy–food nexus and the relationship between food and health. In this context, the food industry is making significant efforts to increase the sustainability of food production and to produce foods that are not only tasty but also nutritious, functional, and sustainable.
Microalgae are gaining importance in the food industry, and the number of foods containing microalgae that are launched into the market is increasing every year [1]. These microorganisms have several advantages in terms of the consumption of resources and energy when compared to other foods, and they are rich in bioactive compounds, including high value pigments and polyunsaturated fatty acids [2,3]. In the European Union, the use of microalgae as food must comply with Regulation (EU) 2015/2283 on novel foods. Because of their long history of use, some microalgal strains, such as Arthrospira (Spirulina), can be consumed in the EU without the need to comply with this regulation [4]. However, other valuable strains, such as Schizochytrium or Haematococcus, cannot yet be commercialized as food. However, some bioactive compounds derived from these sources have been approved as human foods. For example, oil that is rich in docosahexaenoic acid (DHA) derived from Schizochytrium sp. and astaxanthin derived from Haematococcus pluvialis can be consumed up to a certain degree in the EU [5,6]. The latter is the most relevant carotenoid pigment present in aquatic animals, being present in salmon, trout, and lobster, among others [7]. Astaxanthin is important for their pigmentation, and a valuable pigment has essential biological functions, such as protection against the oxidation of polyunsaturated fatty acids (PUFAs), immune response, reproductive behavior, and protection against negative ultraviolet light effects [7]. Because of the many positive health effects of astaxanthin, it was recently suggested by one of the world’s leading market intelligence agencies as one of the three main ingredients to watch and a niche ingredient with strong potential [1].
The interest in astaxanthin is supported by a large amount of evidence that suggests that this compound has a strong antioxidant capacity that leads to many positive health outcomes upon consumption [7,8,9,10]. However, the high production cost of natural astaxanthin as well as several technological problems (that are common to all microalga derived products) are currently limiting the mass production of this ingredient. For example, the cost of producing natural astaxanthin using the microalga H. pluvialis in the EU is approximately 1500 €·kg−1 in Greece and 6400 €·kg−1 in the Netherlands [11]. The cost of producing synthetic astaxanthin is around 880 €·kg−1; therefore, natural astaxanthin cannot compete in terms of cost (for food applications). However, synthetic astaxanthin cannot be used as food in the EU and in the US, and, therefore, natural astaxanthin produced in the EU and the US is the only permitted ingredient for food and the preferred option for high-end applications [12]. Conversely, in China, a recent study predicted the production cost of natural astaxanthin to be as low as 610 €·kg−1, which is encouraging as this value would allow the microalgal process to compete with the synthetic alternative [13]. Most of the microalgal astaxanthin being sold today is produced in Asia, although facilities in the US, Europe, and Israel are also producing this valuable compound. To reduce costs, efforts are being undertaken to increase biomass productivity and astaxanthin accumulation. Genetic engineering is a promising strategy that is being studied by many research groups around the globe [14,15,16]. The optimization of photobioreactors to maximize biomass productivity is also being widely investigated, as well as the utilization of alternative culture media produced using low-value nutrients [17].
The aim of the current review is to highlight the challenges and opportunities of astaxanthin use as a food ingredient and to summarize and discuss the most relevant research and industry findings and achievements on the topic. The health benefits of astaxanthin are also discussed and a bibliometric analysis summarized to identify current research trends related to astaxanthin use in the food industry.
2. Microalgae as Food: Current Challenges and Opportunities
Microalgae are gaining increased importance as added-value ingredients in foods. Indeed, several of the most important food companies have developed foods containing microalgal biomass, and the number of products being launched into the market is increasing every year [1]. For example, products containing astaxanthin are already commercially available, most of them sold as food supplements containing 1–8 mg of astaxanthin (Table 1). The most common products that contain microalgae that are being commercialized are snacks, baked goods, and beverages. However, the majority of the microalgal biomass currently commercialized is sold as a food supplement as capsules or as a dried powder.
Table 1.
Commercial food products containing natural astaxanthin.
| Brand | Company | Description | Astaxanthin Content |
|---|---|---|---|
| Better Foods Astaxanthin | Better Foods GmbH, Göttingen, Germany | Food supplement rich in astaxanthin extracted from H. pluvialis. Contains 80 capsules, and the recommended daily intake is two capsules. | 4 mg per capsule |
| Time Health Astaxanthin | Time Health Ltd., Uckfield, UK | Capsules containing 350 mg of powdered H. pluvialis. Commercialized as super antioxidant, GMO free, gluten free, and 100% vegan. The recommended daily intake is one capsule. | 7 mg per capsule |
| Natural Astaxanthin | Nutravita Ltd., Maidenhead, UK | Food supplement containing astaxanthin-rich oleoresin from H. pluvialis. Softgels commercialized as free from artificial colors and flavors, GMO free, and easy to swallow | 0.9 mg per softgel |
| Astaxanthin 10 mg | Solgar Inc., New Jersey, USA | Softgels containing natural astaxanthin labelled as non-GMO; glute, wheat, and dairy free. Product commercialized as an antioxidant support and a support for a healthy skin | 10 mg per softgel |
| Sila Astaxanthin | Sila, Hsinchu, Taiwan | Food supplement containing astaxanthin extracted from H. pluvialis plus vitamins and Omega-3. | 12 mg per capsule |
| Vivanaturals Astaxanthin from microalgae | Viva Naturals Inc., North York, Canada | Softgels sold as GMO free, dairy free, and gluten free that are good for inspiring firm and healthy skin, supporting immune health, and providing antioxidant protection. | 4 mg per softgel |
| Ox Nature Natural Hawaiian Astaxanthin | Ox Nature, Lisbon, Portugal | Softgels commercialized as 100% pure, vegan, GMO free, and wheat free. | 4 mg per softgel |
These microorganisms have emerged as one of the most promising foods to feed an increasing population. The main reasons include their high photosynthetic efficiency and growth rate, higher than those of terrestrial plants [18], and their very high nutritional value. The composition of microalgal biomass depends on many factors, including environmental conditions, the composition of the culture medium, and operational conditions, among others. The composition of the biomass also depends largely on the produced strain. For example, a hierarchical Bayesian analysis of data compiled from the scientific literature recently concluded that (in general) prokaryotic cyanobacteria have higher protein and carbohydrate and lower lipid content than eukaryotic microalgae. That same study reported that the Cryptophyta have higher protein content than, for example, the Ochrophyta, with a particularly high content of lipids [19]. What makes microalgae especially interesting in terms of human nutrition is not only their protein or lipid content but the quality of the proteins and lipids they produce and also their potential to produce and accumulate bioactive compounds with health-promoting properties. Microalgae have been reported to produce and accumulate polyunsaturated fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [20], and carotenoids, such as β-carotene, lutein, zeaxanthin, and astaxanthin [21].
Other advantages of microalgae include that they can be produced on non-arable land, and, therefore, they do not compete with conventional crops for land, and that some of them can be produced using seawater. Microalgae are well accepted by consumers as these microorganisms are normally considered as sustainable, nutritious, and safe [22]. However, in general, consumers are not aware of the health and environmental benefits of microalgae consumption and production [22]. For example, a recent study suggested a negative attitude towards microalgal biorefineries in Italy and Spain [23], and, therefore, further efforts are needed to increase consumers’ awareness of the environmental benefits of microalgae.
The incorporation of microalgae into foods faces some challenges that need to be overcome. The strong “marine” flavor and the intense (generally) green color of microalgae has been suggested as a drawback depending on the traditional diet of the target population [24]. The traditional consumption of algae is common in many countries in Asia but is limited to a few regions in Europe and America. However, the acceptability of foods containing microalgae is generally high [25,26,27,28,29,30,31], and strategies such as masking the color/flavor using ingredients such as spices or chocolate are common [1]. The encapsulation of microalgal biomass was investigated as an efficient method to hide the strong physical characteristics of microalgae [32]. This strategy has the added advantage of promoting the bioactivity and shelf-life of the products. Indeed, the encapsulation of astaxanthin using sodium alginate and pectin [33] or chitosan [34] led to increased shelf-life and improved photoprotection, respectively. One of the main challenges that the industry is currently facing is, although microalgae production is increasing every year, the current capacity is yet several orders of magnitude below the requirements of the food industry. When used as food, microalgae are not consumed alone but as an ingredient introduced into food matrices generally at concentrations ranging from 1–5% w/w [1]. Still, microalgae production capacity needs to increase to meet the requirements of the food industry. A second important challenge is that microalgae production costs are still high when compared to other food commodities. The cost of producing microalgal biomass depends largely on the location of the reactor and on the overall quality of the biomass. In Europe, the cost of producing microalgal biomass is around 10–50 €·kg−1, much higher than that of soya, wheat, rice, or other common food ingredients. For this reason, microalgae are generally produced using raceway reactors, such as those shown in Figure 1, which are cheaper to construct and easier to scale up and operate when compared to closed systems. These are open ponds divided into channels where the culture is recirculated, generally by means of a paddlewheel. The mixing section can include a sump, used to bubble air and carbon dioxide and simultaneously increase mass transfer and remove dissolved oxygen. Their surface-to-volume ratio generally varies between 5 and 10 m−1, and their areal biomass productivity is in the range of 20–25 g·m−2·day−1 when located in areas with sufficient light and adequate temperatures [35].
Figure 1.
Pilot-scale raceway reactors located inside a greenhouse in Almería, Spain.
Closed systems, such as tubular reactors, are easier to control and the quality of the produced biomass is higher, but these are more expensive and, therefore, used for a lower number of applications. These reactors consist of glass/plastic tubes that are used to recirculate the microalgal culture using pumps or air streams. Their surface-to-volume ratio is much higher than that of raceways, generally around 80 m−1, allowing much higher biomass productivities to be achieved [36]. As highlighted before, microalgae produce and accumulate valuable compounds, such as polyunsaturated fatty acids, proteins, and carotenoids, rendering the process economically viable. Indeed, the production of high value strains, such as H. pluvialis, is conducted using closed systems, as will be discussed below. In this sense, it is important to select not only a productive and robust strain but also one that can provide a benefit to the product.
The current legislation is also delaying the incorporation of microalgae into the food market. As highlighted before, microalgae must be commercialized in the EU under Regulation (EU) 2015/2283, known as the novel foods regulation. Because of their long history of use, Spirulina (Arthrospira platensis or Arthrospira maxima) and Chlorella can be commercialized in the EU without the need to comply with the novel foods regulation. For this reason, most of the products launched into the European market contain biomass of these two strains (mainly Spirulina). The dried biomass of Tetraselmis chuii was recently authorized by the European Food Safety Authority (EFSA) as a novel food [37], and products such as Plancton Marino Veta la Palma (Fitoplancton Marino S.L., Spain) are currently commercially available. The aim of the novel foods regulation is to ensure that businesses that bring novel and innovative foods to the EU market maintain a high level of food safety for European consumers. However, the commercialization of other strains with potential use as food, such as H. pluvialis, Nannochloropsis, Dunaliella, or Isochrysis, among others, is being delayed. Several strains are currently under evaluation, and more will be submitted for assessment in the coming years. This is also happening in the US, where the Food and Drug Administration (FDA) ensures the safety of novel ingredients, and in other countries.
3. Astaxanthin, More than Just a Red Pigment
As highlighted before, astaxanthin is mainly used in food and food applications as a pigment because of its intense red color. However, because of the many positive health outcomes attributed to this carotenoid, the research and industrial interest in astaxanthin has increased exponentially.
3.1. Astaxanthin Research 2010–2020
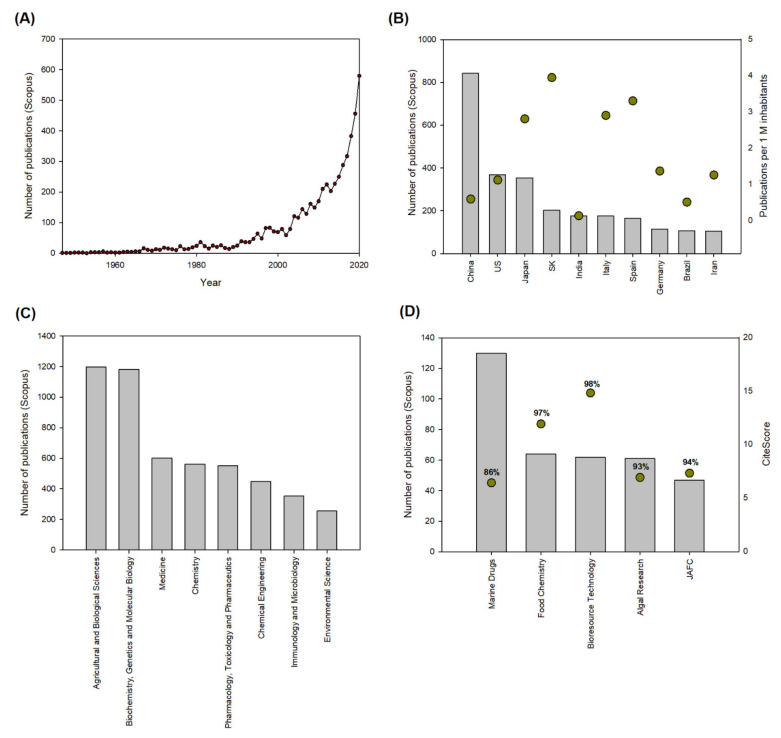
The current section will summarize a bibliometric analysis conducted following a previously described methodology [17]. Briefly, a complete search of the Elsevier Scopus database was carried out using the option “Article title, Abstract, Keywords” and the term “Astaxanthin”. The search resulted in 5715 documents published from 1947 to 2021, as shown in Figure 2A. Overall, the results revealed that the research interest in astaxanthin has increased exponentially during the last few years, with almost 600 publications in 2020 compared to only 69 publications in 2000. These research findings led to the construction of many facilities, and several products containing astaxanthin launched into the market, such as Astapure® (Igennus, Cambridge, UK), which are capsules containing 4 mg of astaxanthin derived from the microalga H. pluvialis. These capsules are recommended to be consumed once or twice a day. Recently, the EFSA concluded that an intake of 8 mg of astaxanthin per day from food supplements is safe for adults, even in combination with a high exposure from the background diet [5]. Figure 2B shows the top-10 countries with a higher number of publications including the term astaxanthin either in the title, abstract, or as a keyword. China ranks as the country with the largest number of publications. Indeed, the five institutions with the highest number of publications during the last decade (2010–2020) were: (i) the Chinese Academy of Sciences, 169; (ii) Ocean University of China, 65; (iii) Ministry of Education China, 47; Pilot National Laboratory of Marine Science and technology, 42; and Zheijian University, 38. The United States and Japan were the second- and third-placed countries in terms of publications, respectively. When considering the population of the different countries, South Korea and then Spain and Italy were the most productive countries (population accessed from https://www.worldometers.info/world-population/population-by-country/ accessed on 15 June 2021).
Figure 2.
Bibliometric analysis. (A) Distribution of publications per year and most relevant (B) countries, (C) subject areas, and (D) source titles of articles published 2010–2020. Abbreviations: US, United States; SK, South Korea; JAFC, Journal of Agricultural and Food Chemistry.
In addition, in terms of subject areas, most of the publications were included within the group of Agricultural and Biological Sciences and Biochemistry, Genetics, and Molecular Biology. One reason is that many efforts are being undertaken to increase the capacity of microalgae to produce and accumulate astaxanthin by the genetic improvement of strains through genetic engineering [14,15,16,38]. Because of the many health benefits of consuming natural astaxanthin, which will be discussed in the following section, a large number of the publications identified during the last decade were included in the subject areas of Medicine, Immunology, and Microbiology, and, finally, Pharmacology, Toxicology, and Pharmaceutics.
Most of the publications between 2010 and 2020 were published in high-impact journals, as seen in Figure 2D. Most of the studies published during the last decade were published in open access and high-quality journals. The most common journal was the open access journal Marine Drugs, followed by Food Chemistry, Bioresource Technology, Algal Research, and the Journal of Agricultural and Food Chemistry. All of these journals publish research on high-end applications, namely: food, cosmetics, and pharmaceutics. They rank within the top 75% of their respective areas, and four of them have their highest percentile over 90%, which demonstrates the importance and interest related to astaxanthin research.
3.2. Industrial Use of Natural Astaxanthin
The bright red color of some fish and crustaceans is one of the major attributes determining quality, purchase intention, and market price. Astaxanthin was first commercialized as an aquafeed ingredient used to increase the astaxanthin content in farmed salmonids and increase the characteristic orange/red color of their flesh [8]. Different microalgae rich in astaxanthin have been used to increase the red color of marine animals. For example, Monoraphidium sp. GK12 led to an improvement in the pigmentation of prawns, comparable to what was achieved when using synthetic astaxanthin [39]. Synthetic astaxanthin is more commonly used in aquafeeds when compared to natural astaxanthin or astaxanthin containing microalgae (Table 2).
Table 2.
Differences between synthetic and natural astaxanthin.
| Synthetic Astaxanthin | Natural Astaxanthin | Reference | |
|---|---|---|---|
| Stereochemistry | (3R,3′R), (3R,3′S), (3S,3′S) optical isomers-1:2:1 |
(3R,3′R), (3R,3′S), (3S,3′S) optical isomers-1:2:22 |
[40] |
| Structure | Non-esterified | More than 95% of the natural astaxanthin molecules are esterified | [40,41] |
| Industrial uses | Aquaculture feed | Food and dietary supplements, cosmetics, nutraceuticals, aquaculture feed | [41,42] |
| Market Price | USD 2000 per kg | USD 7000 per kg | [43] |
| Production |
|
|
[44] |
| Production Price * | 800–900 €·kg−1 | 1000–7000 €·kg−1 | [43] |
However, natural astaxanthin led to improved quality in several reports, and the main advantages and disadvantages of both natural and synthetic astaxanthin have been summarized previously [45]. The main advantages of synthetic astaxanthin include high availability, lower cost, and long lifespan, while the main disadvantages include the use of petrochemical reactants, negative environmental impact, and a complex biosynthesis route that is not sustainable and renewable. In turn, natural astaxanthin has a higher antioxidant capacity, is safer, and has a lower environmental impact. Moreover, natural astaxanthin demonstrated greater pigmentation efficiency than its synthetic counterpart in shrimps fed different diets (25, 50, 75, 100, and 150 mg of astaxanthin per kg) during an 8-week trial [46]. Not only the color of farmed marine animals but also their wellbeing and nutritional quality were improved after the consumption of astaxanthin containing microalgae. Indeed, diets containing microalgae achieved an elevated survival rate when used at low concentrations (5–10 ng of astaxanthin per gram of muscle) over a 4-week period [47]. The carotenoid content of juvenile lobsters fed natural astaxanthin showed an astaxanthin dose-response increase as well as the darkening pigmentation of their exoskeletons [48]. Moreover, a recent study demonstrated that natural astaxanthin promotes the disease resistance of Asian seabass against Vibrio alginolyticus infection [49].
Astaxanthin has also been assessed as a feed ingredient for land animals. Dietary supplementation with Phaffia rhodozyma, which is a yeast rich in astaxanthin, led to a higher content of total free amino acids and to improved texture and sensorial attributes on broiler chicken meat [50]. The consumption of astaxanthin at 0.25 mg per kg of body weight per day led to increased milk yield in buffaloes and, when combined with prill fat, a better health status was observed, higher than that of prill fat supplementation alone [51]. Several reports demonstrated the potential use of astaxanthin to promote the wellbeing of animals. Recent findings suggested that this compound can affect heat stress and inflammation in broiler chickens and laying hens under high ambient temperatures [52]. Similar results were reported for Sahiwal and Karan Fries heifers, where astaxanthin supplementation alleviated the adverse effect of heat stress and simultaneously improved weight gain and helped in the early attainment of puberty [53].
In terms of human consumption, natural astaxanthin derived from microalgae is generally commercialized as a nutritional supplement as capsules. Several products are currently commercially available, and these include Natural Astaxanthin 5 mg Softgels (Solgar, NJ, USA), Astapure® Astaxanthin Complex (Igennus Healthcare Nutrition, Cambridge, UK), and those listed in Table 1. Food products enriched in astaxanthin have also been formulated, and the results reported to date suggest great potential for further development. For example, raw and cooked lamb patties containing astaxanthin showed reduced lipid oxidation during storage, even improving the performance of metabisuplhite (450 ppm) and ascorbate (500 ppm) [54]. In that same study, not only the quality but also the consumer visual preference was increased after the addition of astaxanthin into the patties [54]. In a different study, the incorporation of 10 mg of astaxanthin per kg of chicken steaks led to minimal lipid oxidation during storage and the preservation of microbiological quality [55]. Similar results were obtained in a different study, where the use of 0.30 and 0.45 g of H. pluvialis per kg of minced pork meat delayed lipid oxidation and improved color stability as well as consumer acceptance on the last day of storage [56]. In a different study, astaxanthin encapsulated with zein and oligochitosan was used to increase the antioxidant activity of liquor, apple vinegar, and rice vinegar. The encapsulation of astaxanthin increased the products’ storage stability, especially in terms of stability against ultraviolet light [57]. Because of the health benefits of consuming astaxanthin, this pigment can be used to formulate functional foods that are sold at higher prices and have gained increased consumer interest, especially foods containing marine ingredients [58]. Moreover, the search for and utilization of natural pigments is a top trend in the food industry, especially if the pigment can also act as a radical scavenger [59,60].
Astaxanthin is also being increasingly used in the cosmetics industry. Masks with natural antioxidant are highly appreciated. In a recent study, a mask containing astaxanthin was compared against another mask formulated using vitamin E and demonstrated a superior antioxidant capacity [61]. In that study, the half-life of the mask was calculated as 70 weeks [61]. The same half-life value was reported for an astaxanthin essence stored at 4 °C and produced from Jerusalem artichoke using Phaffia rhodozyma [62]. Cosmetics containing microalgae are available in the market, such as the products Bloom Orchid Face Cream or Green Vitamin Concentrate Serum (Freshly Cosmetics, Barcelona, Spain) and Astaxanthin Collagen All-in-One Gel (DHC, Tokyo, Japan), which is a facial moisturizer commercialized in the US as “powdered by astaxanthin, a natural antioxidant proven to be 6000 times more effective than vitamin C” (https://www.dhccare.com/astaxanthin-collagen-all-in-one-gel.html accessed on 15 June 2021). Although the number of cosmetics containing astaxanthin is still limited, because of the extremely high radical scavenging capacity of this molecule, it is likely that further studies will be conducted and products launched into the market.
3.3. Health Benefits of Natural Astaxanthin
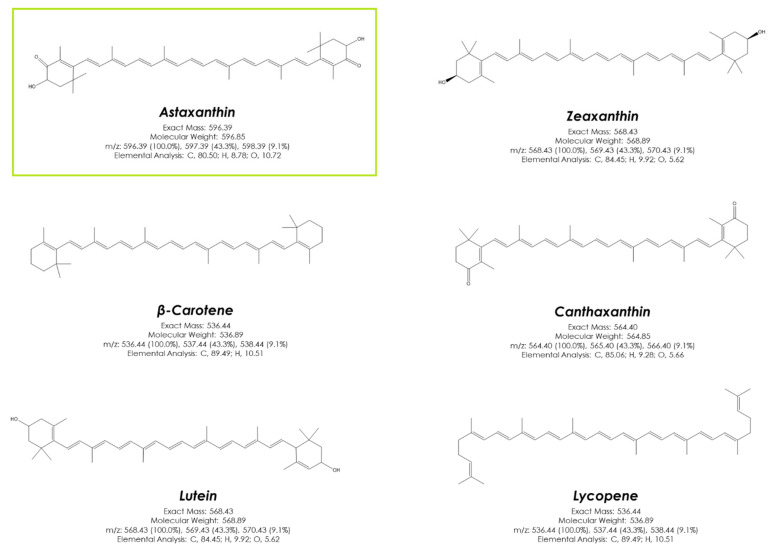
Free radicals and reactive oxygen species are naturally produced during normal aerobic metabolism. However, when oxidative molecules are accumulated in excess, they may react with cellular components, causing protein and lipid oxidation or DNA damage, which are associated with various diseases [63]. A correct balance between oxidants and antioxidants, which act as free radical scavengers, is necessary for proper physiological functioning. Antioxidants can be produced by the human body in situ, but most of them, such as carotenoids, are incorporated through diet [64]. Astaxanthin is related to other carotenoids, such as zeaxanthin, lutein, or β-carotene (Figure 3). However, the presence of a keto- and a hydroxyl group on each end of astaxanthin leads to a higher bioactivity of astaxanthin [8]. Indeed, astaxanthin has the highest antioxidant capacity reported for a natural compound, higher than that of other carotenoids [65]. For example, it is 100 times more antioxidant than vitamin E in neutralizing singlet oxygen [66].
Figure 3.
Chemical structure of industry-relevant carotenoids. Astaxanthin, the most potent natural antioxidant is highlighted in green.
Most of the health benefits of consuming astaxanthin are attributed to its strong antioxidant capacity. The recent findings are summarized in Table 3, and the main effects of astaxanthin are shown in Figure 4. An important challenge is that, to act as antioxidant in vivo, astaxanthin would have to be transported to the right place and in sufficient quantities to exert a beneficial effect. The bioavailability of bioactive compounds is generally overlooked in scientific literature. Because of the rigid cell wall of microalgae, cell wall disruption steps are generally necessary to liberate intracellular bioactives [60]. Polar carotenoids, such as astaxanthin, tend to have higher bioavailability than apolar compounds, such as lycopene, although the bioavailability of carotenoids is generally low [9]. The antioxidant capacity of astaxanthin was explored in vivo, demonstrating that astaxanthin consumption (2 mg·kg−1·day−1) exhibited higher antioxidant enzymes, such as catalase or superoxide dismutase, in the brains of mice [67]. Astaxanthin is able to promote the activity of these enzymes, therefore boosting the brain’s glutathione level [67]. Not only bioavailability but the resistance of bioactivity to food processing and storage should also be considered. Only a limited number of studies assessed the effect of storage on the stability of natural astaxanthin. In this regard, a previous report concluded that the optimal processing and storage conditions of H. pluvialis biomass were freeze-drying followed by vacuum-packed storage at −20 °C [68]. Further studies on the storage and processing stability of natural astaxanthin are needed.
Table 3.
Health benefits of natural astaxanthin when used as food.
| Health Benefit | Conditions Studied | Comments | Reference |
|---|---|---|---|
| Protecting against lipid peroxidation | Two 4-mg astaxanthin (Astaxin®) capsules daily for 3 months | Astaxanthin consumption decreased the plasma levels of 12- and 15-fatty acids of healthy non-smoking men | [69] |
| Decreasing TG levels and increasing HDL-cholesterol | 12 mg·day−1 astaxanthin for 12 weeks | Non-obese subjects with fasting serum triglyceride of 120–200 mg/dL and without diabetes and hypertension, aged 25–60 years. | [70] |
| Reducing oxidative stress and reversing age-related morphological changes of skin | 4 mg·day−1 astaxanthin for 4 weeks | The study included 31 volunteers (17 men and 14 women) over the age of 40. | [71] |
| Improving glucose metabolism and reducing blood pressure | 8 mg·day−1 astaxanthin for 8 weeks | The study enrolled 44 participants with type 2 diabetes. | [72] |
| Recovering from mental fatigue | 3 mg·day−1 and 5 mg·day−1 of sesamin for 4 weeks | The study included 24 healthy volunteers. | [73] |
| Protecting against UV-induced skin deterioration | 4 mg·day−1 astaxanthin for 10 weeks | The study included 23 healthy participants. | [74] |
| Maintaining the body’s antioxidant capacity after high-intensity exercise | 12 mg·day−1 astaxanthin for 4 weeks | The study enrolled 16 male students. | [75] |
| Decreasing a DNA damage biomarker and enhancing immune response | 2–8 mg·day−1 astaxanthin for 8 weeks | The study included 42 young healthy females. | [76] |
Abbreviations: TG, triglycerides; HDL, high-density lipoprotein; UV, ultraviolet.
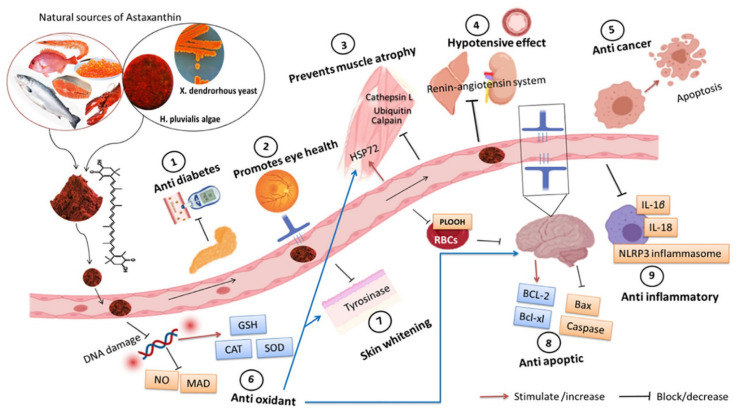
Figure 4.
Main effects of astaxanthin. Figure reprinted from [67] with permission from Elsevier.
Astaxanthin can be used to prevent or treat inflammation, a complex sequence of immune responses that take place as defense mechanisms or reactions to bodily injuries [77]. Uncontrolled inflammation reactions induce damage to host cells and tissues and play an important role in several neurodegenerative conditions. Astaxanthin can stop the onset of inflammation and, therefore, play an important role in the prevention of disorders of the central nervous system [78,79]. This valuable carotenoid also decreases gastric inflammation caused by bacterial infections and has an impact on intestinal microbiota, minimizing inflammation and oxidative stress at local and systemic levels in mice [80]. Astaxanthin consumption has been targeted as a potential strategy to mitigate Parkinson’s disease, Alzheimer’s disease, depression, and neuropathic pain, among other disorders [81]. It was reported that microalgae derived astaxanthin can inhibit the expression or production of cytokines and inflammatory mediators and inhibit the expression of inducible nitric oxide synthase and cyclooxygenase-2, with implications for such diseases as brain inflammatory disease, atherosclerosis, or inflammatory bowel disease, among others [9]. Moreover, in a different study, astaxanthin preserved redox-sensitive and key structures of lymphocytes, which could have been caused by its anti-inflammatory activity [82]. It has been suggested that astaxanthin can decrease oxidative stress and inflammation and also enhance the immune response in humans. The consumption of astaxanthin may also affect the immune system as the administration of astaxanthin at a dosage of 60 and 120 mg per kg of body weight prevented intestinal mucosa damage in cyclophosphamide-induced immunodeficient mice [83]. These results must be taken with caution as the doses tested are much higher than the acceptable daily intake of 0.2 mg per kg of body weight set by the EFSA in 2020 [5]. In a different study, an ELISA analysis revealed that astaxanthin could enhance interferon production in response to lipopolysaccharide or concanavalin stimulation, suggesting that astaxanthin can modulate lymphocytic immune responses [84].
Moreover, the antimicrobial potential of astaxanthin has direct health implications as this molecule was capable of reducing the bacterial load and gastric inflammation in mice infected with Helicobacter pylori [85,86]. In a different study, the antimicrobial capacity of astaxanthin was validated against different bacteria, including Escherichia coli, Proteus mirabilis, Aliivibrio fischeri, Enterobacter aerogenes, and L. monocytogenes, suggesting the potential of astaxanthin not only to produce safer foods but also to extend shelf-life [87]. Different antimicrobial polymers based on natural astaxanthin have been developed, with antimicrobial activities demonstrated in vitro with different pathogens minimizing bacterial growth and the formation of biofilms [88]. Extracts of H. pluvialis rich in astaxanthin also showed antiviral effects. Indeed, ethanolic extracts of this microalga at concentrations of 75 mg·L-1 inhibited the infection of herpes simplex virus type-1 (HSV-1) on Vero cells (African green monkey kidney cell line) by 85% and also affected HSV-1 intracellular replication [89]. Further studies assessing the antimicrobial and antiviral activity of astaxanthin are necessary as the amount of data currently available in the literature are limited. Other health-promoting properties of astaxanthin reported in the literature, assessed using animal models, include: the preservation of visual function and the protection of retinal ganglion cells apoptosis after ischemic results [90], the promotion of growth and liver function [91], benefits on skin health [92], and anti-apoptotic activity [67].
Overall, astaxanthin demonstrated different bioactivities both in vitro and in vivo in animal models. Further studies are needed to fully elucidate the potential of this valuable bioactive molecule. Assessments of bioavailability and resistance to storage and food processing are of key importance, and data reported to date are scarce. Moreover, in vivo studies with human subjects have been conducted with a limited number of participants, and this needs to be improved in order to achieve health claims for astaxanthin or H. pluvialis biomass.
4. Astaxanthin Producing Microalgae
Although different microorganisms can produce astaxanthin, natural astaxanthin is industrially obtained from the microalga H. pluvialis. Higher plants (with the exception of Adonis annua) cannot produce astaxanthin because they lack the enzyme β-carotene ketolasa, which is responsible for introducing keto-moieties to the 4,4′ position of β-ionon rings of carotene and zeaxanthin [93]. Production of H. pluvialis mainly occurs in Asia, although there are also production facilities in the US, New Zealand, Chile, Sweden, and Israel, among other countries [41]. This microalga is a freshwater strain that can accumulate astaxanthin up to 3.8–5.0% of dry weight depending on the strain used, the cultivation conditions, and the photobioreactor design [45]. The large-scale production of astaxanthin using H. pluvialis is already a reality but still remains a challenge, especially in terms of the high energy requirements and difficult downstream processing. The production of H. pluvialis is performed in two steps: a green motile stage and a red non-motile stage. The first stage is equal to that of other microalgal strains, where the microalga is produced in a nutrient-rich medium designed to maximize biomass productivity. At this stage, the most common reactors used are tubular reactors. These are more expensive and complex to operate than open systems. However, because of their capacity to achieve higher biomass concentrations and the high cost of H. pluvialis biomass, these systems are the most widely used. Once the maximum concentration is achieved, the biomass is generally (not always) transferred to other reactors where the stress induced by a lack of nutrients leads to the production and accumulation of astaxanthin inside the microalgal cells. Different strategies are being studied to increase the astaxanthin content of H. pluvialis, including the addition of exogenous compounds that enhance astaxanthin production [94] or the use of artificial illumination [95]. Artificial illumination is generally prohibited for microalgae production but can be viable for the production of high-end strains and products, such as astaxanthin. One of the strategies with more potential is the genetic modification of H. pluvialis strains. Several modified strains have recently been reviewed, achieving, in some cases, an increase in the astaxanthin content higher than 100% [41].
The microalga C. zofingiensis can also be used as a feedstock for astaxanthin production and has some advantages when compared to H. pluvialis. For example, the growth of C. zofingiensis is faster and higher biomass concentrations are able to be achieved. It can be produced phototrophically, heterotrophically, and mixotrophically [93]. The heterotrophic production of C. zofingiensis permits reaching higher biomass concentrations. However, the astaxanthin content reached when producing the biomass heterotrophically is generally around 1 mg·g−1 [96]. One advantage of C. zofingiensis is that this strain can be produced in one step. However, two-step processes have also been developed. In this case, the process consists of an initial fermentation step that allows very high cell densities to be achieved, close to 100 g·L−1, and a second step where the astaxanthin content of the cells is increased using outdoor reactors [97]. Xanthophyllomyces dendrorhous can accumulate a lower amount of astaxanthin when compared to the above-mentioned strains. Generally, the astaxanthin content of X. dendrorhous is below 0.1% of dry weight [93]. However, some X. dendrorhous mutants were able to achieve higher concentrations, and the use of phytohormones during biomass production allowed a 24% increase in the astaxanthin content of this microalga to be achieved [98]. Moreover, a recent study concluded that the consumption of X. dendrorhous could have the same effect as astaxanthin in preventing obesity caused by a high-fat diet [99]. This strain is being widely studied along with different processes to produce biomass of X. dendrorhous using low-cost nutrients, such as fruit and vegetable wastes [100,101], dairy waste [102], and oak leaf extract [103]. In this last study, the supplementation of oak leaf extract with inorganic phosphate (KH2PO4, 3 mM) resulted in a 1.4-fold increase in the astaxanthin content of the biomass, reaching approximately 2 mg·g−1 of dry biomass [103]. Overall, the astaxanthin content of X. dendrorphous is lower than that of H. pluvialis. However, the possibility of producing the biomass using a low-value nutrient source as well as the potential production of astaxanthin in one step makes the process interesting from a scientific and industrial point of view.
5. Conclusions
Natural astaxanthin has several health-promoting properties, most of them as a result of its very high antioxidant capacity. Astaxanthin consumption is thought to prevent and/or control different medical disorders. To date, most of the studies carried out were conducted in vitro and in vivo using animal models. Further in vivo human studies are needed to fully understand the potential of this valuable compound. The results reported so far are promising and suggest a bright future for producers of microalgal astaxanthin. Indeed, natural astaxanthin derived from microalgae is a top trend in food research and in the food industry. Different food products and nutritional supplements are currently available in the market, and the number is expected to increase in the coming years together with the number of publications and processes exploiting astaxanthin. Different microalgae can produce and accumulate astaxanthin, H. pluvialis being the most studied and the only one (to the best of the authors’ knowledge) being industrially produced as a source of astaxanthin. The mass production of H. pluvialis remains a challenge because of high production costs and the two-step requirement of the process. Other strains, such as C. zofingiensis and X. dendrorhous, show potential for being used as novel sources for astaxanthin, and several research groups worldwide are currently working on the optimization of their production and downstream processing steps.
Author Contributions
S.V., M.C., A.M.-E., A.S.-Z., G.A.-F., and T.L. conceived, designed, and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
S.V. thanks the University of Almería (BIM2021/026). A.S.-Z. thanks the Spanish Ministry of Education (FPU16/05996). T.L. thanks the Spanish Ministry of Science, Innovation, and Universities (IJC2018-035287-I). This work forms part of the ALGFOOD Project funded by the Andalucía European Regional Development Fund Operational Programme 2014–2020 (UAL2020-AGR-A1945). Work produced with the support of a 2020 Leonardo Grant for Researchers and Cultural Creators, BBVA Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lafarga T. Effect of microalgal biomass incorporation into foods: Nutritional and sensorial attributes of the end products. Algal Res. 2019;41:101566. doi: 10.1016/j.algal.2019.101566. [DOI] [Google Scholar]
- 2.Smetana S., Sandmann M., Rohn S., Pleissner D., Heinz V. Autotrophic and heterotrophic microalgae and cyanobacteria cultivation for food and feed: Life cycle assessment. Bioresour. Technol. 2017;245:162–170. doi: 10.1016/j.biortech.2017.08.113. [DOI] [PubMed] [Google Scholar]
- 3.Sudhakar M.P., Kumar B.R., Mathimani T., Arunkumar K. A review on bioenergy and bioactive compounds from microalgae and macroalgae-sustainable energy perspective. J. Clean. Prod. 2019;228:1320–1333. doi: 10.1016/j.jclepro.2019.04.287. [DOI] [Google Scholar]
- 4.Lafarga T., Fernández-Sevilla J.M., González-López C., Acién-Fernández F.G. Spirulina for the food and functional food industries. Food Res. Int. 2020;137:109356. doi: 10.1016/j.foodres.2020.109356. [DOI] [PubMed] [Google Scholar]
- 5.EFSA Safety of astaxanthin for its use as a novel food in food supplements. EFSA J. 2020;18:5993. doi: 10.2903/j.efsa.2020.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EFSA Safety of Schizochytrium sp. oil as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2020;18:6242. doi: 10.2903/j.efsa.2020.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerin M., Huntley M.E., Olaizola M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003;21:210–216. doi: 10.1016/S0167-7799(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 8.Davinelli S., Nielsen M.E., Scapagnini G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients. 2018;10:522. doi: 10.3390/nu10040522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan J.P., Peng J., Yin K., Wang J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011;55:150–165. doi: 10.1002/mnfr.201000414. [DOI] [PubMed] [Google Scholar]
- 10.Ambati R.R., Moi P.S., Ravi S., Aswathanarayana R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications-A review. Mar. Drugs. 2014;12:128–152. doi: 10.3390/md12010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panis G., Carreon J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016;18:175–190. doi: 10.1016/j.algal.2016.06.007. [DOI] [Google Scholar]
- 12.Stachowiak B., Szulc P. Astaxanthin for the Food Industry. Molecules. 2021;26:2666. doi: 10.3390/molecules26092666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J., Zhu D., Niu J., Shen S., Wang G. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol. Adv. 2011;29:568–574. doi: 10.1016/j.biotechadv.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Kathiresan S., Sarada R. Towards genetic improvement of commercially important microalga Haematococcus pluvialis for biotech applications. J. Appl. Phycol. 2009;21:553–558. doi: 10.1007/s10811-009-9414-0. [DOI] [Google Scholar]
- 15.Galarza J.I., Gimpel J.A., Rojas V., Arredondo-Vega B.O., Henríquez V. Over-accumulation of astaxanthin in Haematococcus pluvialis through chloroplast genetic engineering. Algal Res. 2018;31:291–297. doi: 10.1016/j.algal.2018.02.024. [DOI] [Google Scholar]
- 16.Kathiresan S., Chandrashekar A., Ravishankar G.A., Sarada R. Regulation of astaxanthin and its intermediates through cloning and genetic transformation of β-carotene ketolase in Haematococcus pluvialis. J. Biotechnol. 2015;196:33–41. doi: 10.1016/j.jbiotec.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Garrido-Cardenas J.A., Manzano-Agugliaro F., Acien-Fernandez F.G., Molina-Grima E. Microalgae research worldwide. Algal Res. 2018;35:50–60. doi: 10.1016/j.algal.2018.08.005. [DOI] [Google Scholar]
- 18.Kumar V., Sharma N., Jaiswal K.K., Vlaskin M.S., Nanda M., Tripathi M.K., Kumar S. Microalgae with a truncated light-harvesting antenna to maximize photosynthetic efficiency and biomass productivity: Recent advances and current challenges. Process Biochem. 2021;104:83–91. doi: 10.1016/j.procbio.2021.03.006. [DOI] [Google Scholar]
- 19.Finkel Z.V., Follows M.J., Liefer J.D., Brown C.M., Benner I., Irwin A.J. Phylogenetic diversity in the macromolecular composition of microalgae. PLoS ONE. 2016;11:e0155977. doi: 10.1371/journal.pone.0155977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Remize M., Brunel Y., Silva J.L., Berthon J.Y., Filaire E. Microalgae n-3 PUFAs Production and Use in Food and Feed Industries. Mar. Drugs. 2021;19:113. doi: 10.3390/md19020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lafarga T., Sanchez-Zurano A., Morillas-España A., Acien-Fernandez F.G. Extremophile microalgae as feedstock for high-value carotenoids: A review. Int. J. Food Sci. Technol. 2021 doi: 10.1111/ijfs.15069. [DOI] [Google Scholar]
- 22.Lafarga T., Rodríguez-Bermúdez R., Morillas-España A., Villaró S., García-Vaquero M., Morán L., Sánchez-Zurano A., González-López C.V., Acién-Fernández F.G. Consumer knowledge and attitudes towards microalgae as food: The case of Spain. Algal Res. 2021;54:102174. doi: 10.1016/j.algal.2020.102174. [DOI] [Google Scholar]
- 23.Lafarga T., Pieroni C., D’Imporzano G., Maggioni L., Adani F., Acién G. Consumer Attitudes towards Microalgae Production and Microalgae-Based Agricultural Products: The Cases of Almería (Spain) and Livorno (Italy) ChemEngineering. 2021;5:27. doi: 10.3390/chemengineering5020027. [DOI] [Google Scholar]
- 24.Chacón-Lee T.L., González-Mariño G.E. Microalgae for “Healthy” Foods-Possibilities and Challenges. Compr. Rev. Food Sci. Food Saf. 2010;9:655–675. doi: 10.1111/j.1541-4337.2010.00132.x. [DOI] [PubMed] [Google Scholar]
- 25.Hernández-López I., Benavente Valdés J.R., Castellari M., Aguiló-Aguayo I., Morillas-España A., Sánchez-Zurano A., Acién-Fernández F.G., Lafarga T. Utilisation of the marine microalgae Nannochloropsis sp. and Tetraselmis sp. as innovative ingredients in the formulation of wheat tortillas. Algal Res. 2021;58:102361. doi: 10.1016/j.algal.2021.102361. [DOI] [Google Scholar]
- 26.Lafarga T., Mayre E., Echeverria G., Viñas I., Villaró S., Acién-Fernández F.G., Castellari M., Aguiló-Aguayo I. Potential of the microalgae Nannochloropsis and Tetraselmis for being used as innovative ingredients in baked goods. LWT. 2019;115:108439. doi: 10.1016/j.lwt.2019.108439. [DOI] [Google Scholar]
- 27.Lafarga T., Acién-Fernández F.G., Castellari M., Villaró S., Bobo G., Aguiló-Aguayo I. Effect of microalgae incorporation on the physicochemical, nutritional, and sensorial properties of an innovative broccoli soup. LWT. 2019;111:167–174. doi: 10.1016/j.lwt.2019.05.037. [DOI] [Google Scholar]
- 28.García-Segovia P., García Alcaraz V., Tárrega A., Martínez-Monzó J. Consumer perception and acceptability of microalgae based breadstick. Food Sci. Technol. Int. 2020;26:493–502. doi: 10.1177/1082013220906235. [DOI] [PubMed] [Google Scholar]
- 29.Qazi W.M., Ballance S., Uhlen A.K., Kousoulaki K., Haugen J.E., Rieder A. Protein enrichment of wheat bread with the marine green microalgae Tetraselmis chuii–Impact on dough rheology and bread quality. LWT. 2021;143:111115. doi: 10.1016/j.lwt.2021.111115. [DOI] [Google Scholar]
- 30.García-Segovia P., Pagán-Moreno M.J., Lara I.F., Martínez-Monzó J. Effect of microalgae incorporation on physicochemical and textural properties in wheat bread formulation. Food Sci. Technol. Int. 2017;23:437–447. doi: 10.1177/1082013217700259. [DOI] [PubMed] [Google Scholar]
- 31.Fradique Ḿ., Batista A.P., Nunes M.C., Gouveia L., Bandarra N.M., Raymundo A. Incorporation of Chlorella vulgaris and Spirulina maxima biomass in pasta products. Part 1: Preparation and evaluation. J. Sci. Food Agric. 2010;90:1656–1664. doi: 10.1002/jsfa.3999. [DOI] [PubMed] [Google Scholar]
- 32.Vieira M.V., Pastrana L.M., Fuciños P. Microalgae Encapsulation Systems for Food, Pharmaceutical and Cosmetics Applications. Mar. Drugs. 2020;18:644. doi: 10.3390/md18120644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vakarelova M., Zanoni F., Lardo P., Rossin G., Mainente F., Chignola R., Menin A., Rizzi C., Zoccatelli G. Production of stable food-grade microencapsulated astaxanthin by vibrating nozzle technology. Food Chem. 2017;221:289–295. doi: 10.1016/j.foodchem.2016.10.085. [DOI] [PubMed] [Google Scholar]
- 34.Alarcón-Alarcón C., Inostroza-Riquelme M., Torres-Gallegos C., Araya C., Miranda M., Sánchez-Caamaño J.C., Moreno-Villoslada I., Oyarzun-Ampuero F.A. Protection of astaxanthin from photodegradation by its inclusion in hierarchically assembled nano and microstructures with potential as food. Food Hydrocoll. 2018;83:36–44. doi: 10.1016/j.foodhyd.2018.04.033. [DOI] [Google Scholar]
- 35.Lafarga T., Sánchez-Zurano A., Villaró S., Morillas-España A., Acién G. Industrial production of spirulina as a protein source for bioactive peptide generation. Trends Food Sci. Technol. 2021;116:176–185. doi: 10.1016/j.tifs.2021.07.018. [DOI] [Google Scholar]
- 36.Molina E., Fernández J., Acién F.G., Chisti Y. Tubular photobioreactor design for algal cultures. J. Biotechnol. 2001;92:113–131. doi: 10.1016/S0168-1656(01)00353-4. [DOI] [PubMed] [Google Scholar]
- 37.AECOSAN Report of the Scientific Committee of the Spanish Agency for Food Safety and Nutrition on a request for initial assessment for marketing of the marine microalgae Tetraselmis chuii under Regulation (EC) No 258/97 on novel foods and novel food ingredients. Rev. Com. Cient. 2017;25:1–10. [Google Scholar]
- 38.Sharon-Gojman R., Maimon E., Leu S., Zarka A., Boussiba S. Advanced methods for genetic engineering of Haematococcus pluvialis (Chlorophyceae, Volvocales) Algal Res. 2015;10:8–15. doi: 10.1016/j.algal.2015.03.022. [DOI] [Google Scholar]
- 39.Fujii K., Nakashima H., Hashidzume Y., Uchiyama T., Mishiro K., Kadota Y. Potential use of the astaxanthin-producing microalga, Monoraphidium sp. GK12, as a functional aquafeed for prawns. J. Appl. Phycol. 2010;22:363–369. doi: 10.1007/s10811-009-9468-z. [DOI] [Google Scholar]
- 40.Zhang C., Jin Y., Yu Y., Xiang J., Li F. Effects of natural astaxanthin from microalgae and chemically synthetic astaxanthin supplementation on two different varieties of the ridgetail white prawn (Exopalaemon carinicauda) Algal Res. 2021;57:102347. doi: 10.1016/j.algal.2021.102347. [DOI] [Google Scholar]
- 41.Li X., Wang X., Duan C., Yi S., Gao Z., Xiao C., Agathos S.N., Wang G., Li J. Biotechnological production of astaxanthin from the microalga Haematococcus pluvialis. Biotechnol. Adv. 2020;43:107602. doi: 10.1016/j.biotechadv.2020.107602. [DOI] [PubMed] [Google Scholar]
- 42.García J.L., de Vicente M., Galán B. Microalgae, old sustainable food and fashion nutraceuticals. Microb. Biotechnol. 2017;10:1017–1024. doi: 10.1111/1751-7915.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jannel S., Caro Y., Bermudes M., Petit T. Novel Insights into the Biotechnological Production of Haematococcus pluvialis-Derived Astaxanthin: Advances and Key Challenges to Allow Its Industrial Use as Novel Food Ingredient. J. Mar. Sci. Eng. 2020;8:789. doi: 10.3390/jmse8100789. [DOI] [Google Scholar]
- 44.Nguyen K.D. Astaxanthin: A Comparative Case of Synthetic vs. Natural Production. Faculty Publications and Other Works—Chemical and Biomolecular Engineering; The University of Tennessee; Knoxville, TN, USA: 2013. [(accessed on 15 June 2021)]. Available online: https://trace.tennessee.edu/cgi/viewcontent.cgi?article=1094&context=utk_chembiopubs. [Google Scholar]
- 45.Khoo K.S., Lee S.Y., Ooi C.W., Fu X., Miao X., Ling T.C., Show P.L. Recent advances in biorefinery of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2019;288:121606. doi: 10.1016/j.biortech.2019.121606. [DOI] [PubMed] [Google Scholar]
- 46.Ju Z.Y., Deng D.F., Dominy W.G., Forster I.P. Pigmentation of Pacific White Shrimp, Litopenaeus vannamei, by Dietary Astaxanthin Extracted from Haematococcus pluvialis. J. World Aquac. Soc. 2011;42:633–644. doi: 10.1111/j.1749-7345.2011.00511.x. [DOI] [Google Scholar]
- 47.Li Y., Xiao G., Mangott A., Kent M., Pirozzi I. Nutrient efficacy of microalgae as aquafeed additives for the adult black tiger prawn, Penaeus monodon. Aquac. Res. 2016;47:3625–3635. doi: 10.1111/are.12815. [DOI] [Google Scholar]
- 48.Barclay M.C., Irvin S.J., Williams K.C., Smith D.M. Comparison of diets for the tropical spiny lobster Panulirus ornatus: Astaxanthin-supplemented feeds and mussel flesh. Aquac. Nutr. 2006;12:117–125. doi: 10.1111/j.1365-2095.2006.00390.x. [DOI] [Google Scholar]
- 49.Lim K.C., Yusoff F.M., Shariff M., Kamarudin M.S. Dietary astaxanthin augments disease resistance of Asian seabass, Lates calcarifer (Bloch, 1790), against Vibrio alginolyticus infection. Fish Shellfish Immunol. 2021;114:90–101. doi: 10.1016/j.fsi.2021.03.025. [DOI] [PubMed] [Google Scholar]
- 50.Perenlei G., Tojo H., Okada T., Kubota M., Kadowaki M., Fujimura S. Effect of dietary astaxanthin rich yeast, Phaffia rhodozyma, on meat quality of broiler chickens. Anim. Sci. J. 2014;85:895–903. doi: 10.1111/asj.12221. [DOI] [PubMed] [Google Scholar]
- 51.Somagond Y.M., Singh S.V., Deshpande A. Effect of dietary supplementation of astaxanthin, prill fat and combination on stress indicators, milk yield and composition during heat stress in buffaloes. Biol. Rhythm Res. 2019 doi: 10.1080/09291016.2019.1658426. [DOI] [Google Scholar]
- 52.Tolba S.A., Magnuson A.D., Sun T., Lei X.G. Dietary supplemental microalgal astaxanthin modulates molecular profiles of stress, inflammation, and lipid metabolism in broiler chickens and laying hens under high ambient temperatures. Poult. Sci. 2020;99:4853–4860. doi: 10.1016/j.psj.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar S., Singh S.V. Influence of astaxanthin supplementation on attainment of puberty and lipid peroxidation in Sahiwal and Karan Fries (Holstein × Tharparkar) heifers during summer season. Biol. Rhythm Res. 2020;51:15–28. doi: 10.1080/09291016.2018.1512298. [DOI] [Google Scholar]
- 54.Carballo D.E., Caro I., Andrés S., Giráldez F.J., Mateo J. Assessment of the antioxidant effect of astaxanthin in fresh, frozen and cooked lamb patties. Food Res. Int. 2018;111:342–350. doi: 10.1016/j.foodres.2018.05.054. [DOI] [PubMed] [Google Scholar]
- 55.Abdelmalek B.E., Sila A., Ghlissi Z., Taktak M.A., Ayadi M.A., Bougatef A. The Influence of Natural Astaxanthin on the Formulation and Storage of Marinated Chicken Steaks. J. Food Biochem. 2016;40:393–403. doi: 10.1111/jfbc.12224. [DOI] [Google Scholar]
- 56.Pogorzelska E., Godziszewska J., Brodowska M., Wierzbicka A. Antioxidant potential of Haematococcus pluvialis extract rich in astaxanthin on colour and oxidative stability of raw ground pork meat during refrigerated storage. Meat Sci. 2018;135:54–61. doi: 10.1016/j.meatsci.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 57.Jiang G.L., Zhu M.J. Preparation of astaxanthin-encapsulated complex with zein and oligochitosan and its application in food processing. LWT. 2019;106:179–185. doi: 10.1016/j.lwt.2019.02.055. [DOI] [Google Scholar]
- 58.Lafarga T., Gallagher E., Walsh D., Valverde J., Hayes M. Chitosan-containing bread made using marine shellfishery byproducts: Functional, bioactive, and quality assessment of the end product. J. Agric. Food Chem. 2013;61:8790–8796. doi: 10.1021/jf402248a. [DOI] [PubMed] [Google Scholar]
- 59.Leong H.Y., Show P.L., Lim M.H., Ooi C.W., Ling T.C. Natural red pigments from plants and their health benefits: A review. Food Rev. Int. 2018;34:463–482. doi: 10.1080/87559129.2017.1326935. [DOI] [Google Scholar]
- 60.Lafarga T. Cultured Microalgae and Compounds Derived Thereof for Food Applications: Strain Selection and Cultivation, Drying, and Processing Strategies. Food Rev. Int. 2020;36:559–583. doi: 10.1080/87559129.2019.1655572. [DOI] [Google Scholar]
- 61.Cheng X.Y., Xiong Y.J., Yang M.M., Zhu M.J. Preparation of astaxanthin mask from Phaffia rhodozyma and its evaluation. Process Biochem. 2019;79:195–202. doi: 10.1016/j.procbio.2018.12.027. [DOI] [Google Scholar]
- 62.Jiang G.L., Zhou L.Y., Wang Y.T., Zhu M.J. Astaxanthin from Jerusalem artichoke: Production by fed-batch fermentation using Phaffia rhodozyma and application in cosmetics. Process Biochem. 2017;63:16–25. doi: 10.1016/j.procbio.2017.08.013. [DOI] [Google Scholar]
- 63.Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 64.Lafarga T., Hayes M. Bioactive peptides from meat muscle and by-products: Generation, functionality and application as functional ingredients. Meat Sci. 2014;98:227–239. doi: 10.1016/j.meatsci.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 65.Naguib Y.M.A. Antioxidant Activities of Astaxanthin and Related Carotenoids. J. Agric. Food Chem. 2000;48:1050–1064. doi: 10.1021/jf991106k. [DOI] [PubMed] [Google Scholar]
- 66.Fakhri S., Abbaszadeh F., Dargahi L., Jorjani M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018;136:1–20. doi: 10.1016/j.phrs.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 67.Raza S.H.A., Naqvi S.R.Z., Abdelnour S.A., Schreurs N., Mohammedsaleh Z.M., Khan I., Shater A.F., Abd El-Hack M.E., Khafaga A.F., Quan G., et al. Beneficial effects and health benefits of Astaxanthin molecules on animal production: A review. Res. Vet. Sci. 2021;138:69–78. doi: 10.1016/j.rvsc.2021.05.023. [DOI] [PubMed] [Google Scholar]
- 68.Ahmed F., Li Y., Fanning K., Netzel M., Schenk P.M. Effect of drying, storage temperature and air exposure on astaxanthin stability from Haematococcus pluvialis. Food Res. Int. 2015;74:231–236. doi: 10.1016/j.foodres.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 69.Karppi, Rissanen, Nyyssönen, Kaikkonen, Olsson, Voutilainen, Salonen Effects of Astaxanthin Supplementation on Lipid Peroxidation. Int. J. Vitam. Nutr. Res. 2013;77:3–11. doi: 10.1024/0300-9831.77.1.3. [DOI] [PubMed] [Google Scholar]
- 70.Yoshida H., Yanai H., Ito K., Tomono Y., Koikeda T., Tsukahara H., Tada N. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis. 2010;209:520–523. doi: 10.1016/j.atherosclerosis.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 71.Chalyk N.E., Klochkov V.A., Bandaletova T.Y., Kyle N.H., Petyaev I.M. Continuous astaxanthin intake reduces oxidative stress and reverses age-related morphological changes of residual skin surface components in middle-aged volunteers. Nutr. Res. 2017;48:40–48. doi: 10.1016/j.nutres.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 72.Sokri N., Phd M., Zakerkish M., Mohammadiasl Phd J., Phd M.Z., Mohammadshahi M., Hossein M., Msc H. Astaxanthin improves glucose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus. Asia Pac. J. Clin. Nutr. 2018;27:341–346. doi: 10.6133/apjcn.052017.11. [DOI] [PubMed] [Google Scholar]
- 73.Imai A., Oda Y., Ito N., Seki S., Nakagawa K., Miyazawa T., Ueda F. Effects of Dietary Supplementation of Astaxanthin and Sesamin on Daily Fatigue: A Randomized, Double-Blind, Placebo-Controlled, Two-Way Crossover Study. Nutrients. 2018;10:281. doi: 10.3390/nu10030281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ito N., Seki S., Ueda F. The Protective Role of Astaxanthin for UV-Induced Skin Deterioration in Healthy People—A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2018;10:817. doi: 10.3390/nu10070817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu L., Sun Z., Chen A., Guo X., Wang J. Effect of astaxanthin and exercise on antioxidant capacity of human body, blood lactic acid and blood uric acid metabolism. Sci. Sports. 2019;34:348–352. doi: 10.1016/j.scispo.2018.12.008. [DOI] [Google Scholar]
- 76.Park J.S., Chyun J.H., Kim Y.K., Line L.L., Chew B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010;7:1–10. doi: 10.1186/1743-7075-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turrin N.P., Rivest S. Molecular and cellular immune mediators of neuroprotection. Mol. Neurobiol. 2006;34:221–242. doi: 10.1385/MN:34:3:221. [DOI] [PubMed] [Google Scholar]
- 78.Galasso C., Orefice I., Pellone P., Cirino P., Miele R., Ianora A., Brunet C., Sansone C. On the neuroprotective role of astaxanthin: New perspectives? Mar. Drugs. 2018;16:247. doi: 10.3390/md16080247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grimmig B., Kim S.-H., Nash K., Bickford P.C., Douglas Shytle R. Neuroprotective mechanisms of astaxanthin: A potential therapeutic role in preserving cognitive function in age and neurodegeneration. GeroScience. 2017;39:19–32. doi: 10.1007/s11357-017-9958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bhuvaneswari S., Arunkumar E., Viswanathan P., Anuradha C.V. Astaxanthin restricts weight gain, promotes insulin sensitivity and curtails fatty liver disease in mice fed a obesity-promoting diet. Process Biochem. 2010;45:1406–1414. doi: 10.1016/j.procbio.2010.05.016. [DOI] [Google Scholar]
- 81.Fakhri S., Aneva I.Y., Farzaei M.H., Sobarzo-Sánchez E. The neuroprotective effects of astaxanthin: Therapeutic targets and clinical perspective. Molecules. 2019;24:2640. doi: 10.3390/molecules24142640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bolin A.P., Macedo R.C., Marin D.P., Barros M.P., Otton R. Astaxanthin prevents in vitro auto-oxidative injury in human lymphocytes. Cell Biol. Toxicol. 2010;26:457–467. doi: 10.1007/s10565-010-9156-4. [DOI] [PubMed] [Google Scholar]
- 83.Zhang L., Cao W., Gao Y., Yang R., Zhang X., Xu J., Tang Q. Astaxanthin (ATX) enhances the intestinal mucosal functions in immunodeficient mice. Food Funct. 2020;11:3371–3381. doi: 10.1039/C9FO02555C. [DOI] [PubMed] [Google Scholar]
- 84.Lin K.H., Lin K.C., Lu W.J., Thomas P.A., Jayakumar T., Sheu J.R. Astaxanthin, a carotenoid, stimulates immune responses by enhancing IFN-γ and Il-2 secretion in primary cultured lymphocytes in vitro and ex vivo. Int. J. Mol. Sci. 2015;17:44. doi: 10.3390/ijms17010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bennedsen M., Wang X., Willén R., Wadström T., Andersen L.P. Treatment of H. pylori infected mice with antioxidant astaxanthin reduces gastric inflammation, bacterial load and modulates cytokine release by splenocytes. Immunol. Lett. 2000;70:185–189. doi: 10.1016/S0165-2478(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 86.Wang X., Willen R., Wadstrom T. Astaxanthin-rich algal meal and vitamin C inhibit Helicobacter pylori infection in BALB/cA mice. Antimicrob. Agents Chemother. 2000;44:2452–2457. doi: 10.1128/AAC.44.9.2452-2457.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mageswari A., Subramanian P., Srinivasan R., Karthikeyan S., Gothandam K.M. Astaxanthin from psychrotrophic Sphingomonas faeni exhibits antagonism against food-spoilage bacteria at low temperatures. Microbiol. Res. 2015;179:38–44. doi: 10.1016/j.micres.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 88.Weintraub S., Shpigel T., Harris L.G., Schuster R., Lewis E.C., Lewitus D.Y. Astaxanthin-based polymers as new antimicrobial compounds. Polym. Chem. 2017;8:4182–4189. doi: 10.1039/C7PY00663B. [DOI] [Google Scholar]
- 89.Santoyo S., Jaime L., Plaza M., Herrero M., Rodriguez-Meizoso I., Ibañez E., Reglero G. Antiviral compounds obtained from microalgae commonly used as carotenoid sources. J. Appl. Phycol. 2011;24:731–741. doi: 10.1007/s10811-011-9692-1. [DOI] [Google Scholar]
- 90.Lin W.-N., Kapupara K., Wen Y.-T., Chen Y.-H., Pan I.-H., Tsai R.-K. Haematococcus pluvialis-Derived Astaxanthin Is a Potential Neuroprotective Agent against Optic Nerve Ischemia. Mar. Drugs. 2020;18:85. doi: 10.3390/md18020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao W., Fang H.-H., Liu Z.-Z., Huang M.-Q., Su M., Zhang C.-W., Gao B.-Y., Niu J. A newly isolated strain of Haematococcus pluvialis JNU35 improves the growth, antioxidation, immunity and liver function of golden pompano (Trachinotus ovatus) Aquac. Nutr. 2021;27:342–354. doi: 10.1111/anu.13188. [DOI] [Google Scholar]
- 92.Ng Q.X., de Deyn M.L.Z.Q., Loke W., Foo N.X., Chan H.W., Yeo W.S. Effects of Astaxanthin Supplementation on Skin Health: A Systematic Review of Clinical Studies. J. Diet. Suppl. 2020;18:169–182. doi: 10.1080/19390211.2020.1739187. [DOI] [PubMed] [Google Scholar]
- 93.Liu J., Sun Z., Gerken H., Liu Z., Jiang Y., Chen F. Chlorella zofingiensis as an Alternative Microalgal Producer of Astaxanthin: Biology and Industrial Potential. Mar. Drugs. 2014;12:3487–3515. doi: 10.3390/md12063487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang C., Zhang L., Liu J. Exogenous sodium acetate enhances astaxanthin accumulation and photoprotection in Haematococcus pluvialis at the non-motile stage. J. Appl. Phycol. 2018;31:1001–1008. doi: 10.1007/s10811-018-1622-z. [DOI] [Google Scholar]
- 95.Katsuda T., Shimahara K., Shiraishi H., Yamagami K., Ranjbar R., Katoh S. Effect of flashing light from blue light emitting diodes on cell growth and astaxanthin production of Haematococcus pluvialis. J. Biosci. Bioeng. 2006;102:442–446. doi: 10.1263/jbb.102.442. [DOI] [PubMed] [Google Scholar]
- 96.Wang Y., Peng J. Growth-associated biosynthesis of astaxanthin in heterotrophic Chlorella zofingiensis (Chlorophyta) World J. Microbiol. Biotechnol. 2008;24:1915–1922. doi: 10.1007/s11274-008-9692-8. [DOI] [Google Scholar]
- 97.Zhang Z., Huang J.J., Sun D., Lee Y., Chen F. Two-step cultivation for production of astaxanthin in Chlorella zofingiensis using a patented energy-free rotating floating photobioreactor (RFP) Bioresour. Technol. 2017;224:515–522. doi: 10.1016/j.biortech.2016.10.081. [DOI] [PubMed] [Google Scholar]
- 98.Pan X., Wang B., Duan R., Jia J., Li J., Xiong W., Ling X., Chen C., Huang X., Zhang G., et al. Enhancing astaxanthin accumulation in Xanthophyllomyces dendrorhous by a phytohormone: Metabolomic and gene expression profiles. Microb. Biotechnol. 2020;13:1446–1460. doi: 10.1111/1751-7915.13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang J., Liu S., Wang H., Xiao S., Li C., Li Y., Liu B. Xanthophyllomyces dendrorhous-Derived Astaxanthin Regulates Lipid Metabolism and Gut Microbiota in Obese Mice Induced by A High-Fat Diet. Mar. Drugs. 2019;17:337. doi: 10.3390/md17060337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gervasi T., Santini A., Daliu P., Salem A.Z.M., Gervasi C., Pellizzeri V., Barrega L., De Pasquale P., Dugo G., Cicero N. Astaxanthin production by Xanthophyllomyces dendrorhous growing on a low cost substrate. Agrofor. Syst. 2019;94:1229–1234. doi: 10.1007/s10457-018-00344-6. [DOI] [Google Scholar]
- 101.Hara K.Y., Kageyama Y., Tanzawa N., Hirono-Hara Y., Kikukawa H., Wakabayashi K. Development of astaxanthin production from citrus peel extract using Xanthophyllomyces dendrorhous. Environ. Sci. Pollut. Res. 2020;28:12640–12647. doi: 10.1007/s11356-020-11163-7. [DOI] [PubMed] [Google Scholar]
- 102.Gervasi T., Pellizzeri V., Benameur Q., Gervasi C., Santini A., Cicero N., Dugo G. Valorization of raw materials from agricultural industry for astaxanthin and β-carotene production by Xanthophyllomyces dendrorhous. Nat. Prod. Res. 2017;32:1554–1561. doi: 10.1080/14786419.2017.1385024. [DOI] [PubMed] [Google Scholar]
- 103.Kothari D., Lee J.-H., Chon J.-W., Seo K.-H., Kim S.-K. Improved astaxanthin production by Xanthophyllomyces dendrorhous SK984 with oak leaf extract and inorganic phosphate supplementation. Food Sci. Biotechnol. 2019;28:1171–1176. doi: 10.1007/s10068-019-00604-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.