Abstract
It is well established that cell survival signals stimulated by growth factors, cytokines, and oncoproteins are initiated by phosphoinositide 3-kinase (PI3K)- and Akt-dependent signal transduction pathways. Oncogenic Ras, an upstream activator of Akt, requires NF-κB to initiate transformation, at least partially through the ability of NF-κB to suppress transformation-associated apoptosis. In this study, we show that oncogenic H-Ras requires PI3K and Akt to stimulate the transcriptional activity of NF-κB. Activated forms of H-Ras and MEKK stimulate signals that result in nuclear translocation and DNA binding of NF-κB as well as stimulation of the NF-κB transactivation potential. In contrast, activated PI3K or Akt stimulates NF-κB-dependent transcription by stimulating transactivation domain 1 of the p65 subunit rather than inducing NF-κB nuclear translocation via IκB degradation. Inhibition of IκB kinase (IKK), using an IKKβ dominant negative protein, demonstrated that activated Akt requires IKK to efficiently stimulate the transactivation domain of the p65 subunit of NF-κB. Inhibition of endogenous Akt activity sensitized cells to H-Ras(V12)-induced apoptosis, which was associated with a loss of NF-κB transcriptional activity. Finally, Akt-transformed cells were shown to require NF-κB to suppress the ability of etoposide to induce apoptosis. Our work demonstrates that, unlike activated Ras, which can stimulate parallel pathways to activate both DNA binding and the transcriptional activity of NF-κB, Akt stimulates NF-κB predominantly by upregulating of the transactivation potential of p65.
Akt, also known as PKB (protein kinase B) (3, 13), is a serine/threonine protein kinase that has been shown to regulate cell survival signals in response to growth factors, cytokines, and oncogenic Ras (19, 23, 40). Akt becomes activated via the phosphoinositide-3-OH kinase (PI3K) pathway (18, 24, 29) and by other upstream kinases, including the recently identified Ca2+- and calmodulin-dependent kinase protein kinase kinase (64). Akt inhibits cell death pathways by directly phosphorylating and inactivating proteins involved in apoptosis, including Bad, procaspase 9, and members of the Forkhead transcription factor family (7, 8, 15, 16, 36, 55). Phosphorylation of Bad by Akt at serine (Ser) residues 112 and 136 enables the 14-3-3 protein to interact with and sequester the inactivated Bad protein in the cytoplasm (15, 67). Akt also phosphorylates the procaspase 9 protease at Ser-196, which has been shown to contribute to the resistance of Ras-transformed cells to overcoming apoptotic agents (8). Finally, members of the Forkhead transcription factor family have been shown to be directly phosphorylated by Akt (7, 36, 55) and the inactivation of the Forkhead family member FKHRL1 promotes cell survival (7). In addition to directly phosphorylating and inactivating proapoptotic protein targets, Akt can stimulate signaling pathways that upregulate the activity of the transcription factor NF-κB (31, 44, 49, 52). Importantly, the antiapoptotic signals elicited by platelet-derived growth factor (PDGF) were shown to require Akt-induced NF-κB transcriptional activity (49).
Classical NF-κB, a heterodimer composed of p50 and p65 subunits, is a potent activator of gene expression from NF-κB sites due to the presence of transactivating domains located in the C-terminal 120 amino acids of the p65 (also called RelA) protein (1, 21). Thus, NF-κB is regulated through mechanisms that target the transcription function of NF-κB (22, 47, 58, 68, 69). Additionally, NF-κB activity is also regulated by the IκB family of proteins that interact with and sequester the transcription factor in the cytoplasm. Following cellular stimulation, IκB proteins become phosphorylated by the multisubunit IκB kinase (IKK) complex, which subsequently targets IκB for ubiquitination and degradation by the 26S proteasome (66). IKK-dependent degradation of IκB liberates NF-κB, allowing this transcription factor to translocate to the nucleus, where it upregulates transcription (1, 25). Thus, as is the case with several transcription factors, NF-κB is regulated through signaling mechanisms that control nuclear translocation (such as IKK) and through mechanisms that are responsible for upregulating the transactivation function of NF-κB.
We have previously demonstrated that oncogenic Ras stimulates NF-κB-dependent transcription (20) and that NF-κB is required for Ras-mediated transformation (22). Moreover, Ras activates NF-κB to suppress oncogene-induced apoptosis (41). NF-κB was originally found to be required to block apoptosis in response to tumor necrosis factor (TNF) (2, 37, 56, 57) and in response to genotoxic agents (57–59). Subsequently, it was shown that NF-κB blocks TNF-induced apoptosis through the transcriptional activation of genes encoding antiapoptotic proteins (12, 26, 57, 58, 59a, 70). Although we have demonstrated that oncogenic Ras upregulates NF-κB to suppress Ras-induced apoptosis, the signaling pathways utilized for NF-κB-dependent cell survival under these conditions have not been elucidated. Because Akt provides a strong cell survival signal in response to activated Ras signaling (33, 34), we asked whether H-Ras(V12) utilizes Akt to activate NF-κB and to provide an antiapoptotic signal. Thus, we demonstrate that H-Ras(V12) stimulates NF-κB-dependent transcription in a PI3K- and Akt-dependent manner. Recently, several groups have reported that PI3K and Akt are involved in the activation of NF-κB in response to TNF, interleukin 1β (IL-1β), phorbol myristate acetate (PMA), pervanadate, and PDGF signaling (4, 5, 31, 44, 46, 49, 52). Several of these papers indicated that IKK activity is involved in the ability of Akt to stimulate NF-κB transcriptional activity (30, 44, 49), presumably through direct mechanisms involving enhanced NF-κB nuclear translocation. However, we found that constitutively activated forms of either PI3K or Akt stimulated NF-κB transcriptional activity predominantly through signaling pathways that targeted the transactivation domain of the p65 subunit. Consistent with the importance of Akt in cell survival, we found that the inhibition of endogenous Akt activity resulted in a loss of NF-κB transcriptional activity and sensitization of cells to H-Ras(V12)-induced apoptosis. Moreover, Akt-induced resistance to etoposide is mediated, in part, by the ability of this serine/threonine kinase to upregulate the transcriptional activity of NF-κB. This study indicates that in addition to inhibiting preexisting proapoptotic proteins, like Bad, procaspase-9, and the Forkhead transcription factors, Akt provides long-term cell survival signals by activating pathways that target NF-κB-dependent transcription.
MATERIALS AND METHODS
Cell culture and reagents.
Murine NIH 3T3 fibroblasts were grown in Dulbecco modified Eagle medium (DMEM; Gibco/BRL) supplemented with 10% calf serum (Hyclone Laboratories, Logan, Utah) and penicillin-streptomycin unless otherwise indicated. Human 293T kidney and Rat-1 fibroblast cells were grown in DMEM supplemented with 10% fetal bovine serum (FBS; Hyclone Laboratories) and penicillin-streptomycin unless otherwise indicated in the figure legends. The H-Ras(V12)-inducible Rat1:iRas cell line has been described previously (42). Oncogenic Ras was induced by the addition of 5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) to complete medium. Cells expressing dominant negative forms of Akt were generated by transfecting Akt K179A mutants into Rat-1:iRas cells. Stable transfectants were selected and subcloned in medium containing 1 μg of puromycin (Sigma, St. Louis, Mo.) per ml. Clones were verified by Western blotting using an Akt-specific rabbit polyclonal antibody (New England Biolabs, Beverly, Mass.).
Plasmid constructs.
3x-κB luciferase (3x-κB-Luc) reporter constructs contain four κB DNA binding consensus sites from the major histocompatibility complex class I promoter fused upstream to firefly luciferase. Mutant 3x-κB-Luc reporter constructs contain two inactivating base pair mutations in each κB site. The Gal4 luciferase (Gal4-Luc) constructs contain four Gal4 DNA consensus binding sites derived from the Saccharomyces cerevisiae GAL4 gene upstream of luciferase, and Gal4-p65 constructs have the yeast Gal4 DNA binding domain fused to the transactivation domain of p65 (50). Activated PI3K and Akt as well as dominant negative constructs have been described previously (34, 61).
Transfection and luciferase reporter assays.
NIH 3T3 and 293T cells at 60 to 80% confluence were transiently transfected using the Superfect reagent (Qiagen, Valencia, Calif.) according to the manufacturer's instructions. Briefly, plasmid constructs (1 μg of total DNA) were diluted in serum-free medium and mixed with the Superfect reagent. Complexes were allowed to form for 10 min before serum-containing medium was added to the mixture. The cells were washed once with 1× phosphate-buffered saline (PBS) and Superfect-DNA complexes were added to the cells and placed in a humidified incubator at 37°C with 5% CO2. Three hours postaddition, cells were washed with 1× PBS and replenished with fresh serum-containing medium. Twenty-four hours posttransfection, cells were washed once with 1× PBS and lysed in Reporter Lysis Buffer (Promega, Madison, Wis.) for 10 min at room temperature. Extracts were collected and cleared by centrifugation at high speed. Rat-1:iRas cells expressing mutant Akt constructs were similarly transfected. However, cells were growth factor deprived in 0.5% serum for 12 to 18 h posttransfection and H-Ras(V12) was induced by the addition of IPTG for 12 h prior to cell harvest. Protein concentration was determined with the Bio-Rad (Hercules, Calif.) protein assay dye reagent. Luciferase assays were performed on equal amounts of protein (50 μg/sample). d-Luciferin was used as a substrate, and relative light units were measured using an AutoLumat LB953 luminometer (Berthold Analytical Instruments). Results were normalized to those with an internal β-galactosidase (β-Gal)-expressing plasmid (pCMV-LacZ) by a β-Gal colorimetric assay followed by spectrophotometric quantitation (Promega). In addition, cells transfected with Akt or PI3K mutant constructs were cotransfected with pCMV-LacZ and assayed for transfection efficiency and/or cell death by counting β-Gal-positive cells as previously described (41).
EMSAs and Western blot analysis.
Preparation of nuclear and cytoplasmic extracts and electrophoretic mobility shift assays (EMSAs) were performed as described previously (41). Briefly, nuclear extracts were prepared 48 h posttransfection and incubated with [α-32P]dCTP-labeled, double-stranded probe containing a κB consensus site from the class I major histocompatibility complex promoter. Labeled probe-nuclear extract complexes were incubated for 10 min at room temperature and separated on a 5% polyacrylamide gel. Subsequently, the gel was dried and exposed to X-ray film. Antibody supershift experiments were performed as previously described (41). Western blot analysis was performed by preparing whole-cell extracts in the presence of protease inhibitors. Total protein (50 μg) was separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes. The indicated primary antibodies were incubated for 30 min, washed, and visualized by incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies and enhanced chemiluminescence (ECL) reagents (Amersham, Piscataway, N.J.).
Apoptosis assays and adenovirus infections.
Cell viability assays were performed using Rat-1:iRas cells expressing dominant negative forms of Akt [Akt(K179A)] and/or a vector control. Cells were split to subconfluence in medium containing 2% FBS for 24 to 36 h. Subsequently, supernatants containing apoptotic cells were centrifuged at low speed in a clinical centrifuge and washed gently with serum-free medium. Pellets were resuspended in 150 μl of serum-free medium, and apoptotic cells were counted under light microscopy using a modified improved heamacytometer. Modified terminal deoxynucleotidyltransferase-mediated dUTP nicked-end labeling (TUNEL) assays were performed per the instructions of the manufacturer (Boehringer Mannheim, Indianapolis, Ind.). Adenovirus infections were performed as previously described (27). Briefly, Rat1-1 or Rat-1:iRas cells were plated at a concentration of 5 × 105 cells/well in 2% FBS. Twelve to 18 h later, the medium was aspirated and replaced with 2% medium plus FBS containing adenovirus encoding the super repressor (SR) IκBα (Ad-SRIκBα) or adenovirus encoding the cytomegalovirus (CMV) promoter but not IκBα (Ad-CMV) (control virus) at a concentration of 50 PFU/cell. Infection proceeded for 1 h, and then 2 ml of medium plus 2% FBS was added to the well with the addition of 5 mM IPTG, as indicated in the figures. Apoptotic cells were counted and assayed by TUNEL 48 h after the initial infection.
Kinase assays.
Akt activity was measured by a phosphorylation assay using the Crosstide peptide (14) specific to the Akt phosphorylation sequence from GSK3 per the instructions of the manufacturer (Upstate Biotechnology, Lake Placid, N.Y.). Briefly, Rat-1:iRas cells were grown to subconfluence and pretreated with LY 294002 (10 μM) for 1 h and 5 mM IPTG was added for 12 h prior to harvest. Immunoprecipitations were performed by combining 500 μg of protein with 4 μg of Akt monoclonal antibody (New England Biolabs) and incubating with protein A/E-agarose beads for 2 h at 4°C. Beads were washed and incubated with a PKA inhibitor peptide (17 μM) and 100 μCi of [γ-32P]dATP for 30 min at 30°C with constant agitation. Supernatants were spotted onto P81 phosphocellulose membranes, washed, and counted in a scintillation counter.
RESULTS
Activation of the PI3K pathway stimulates NF-κB-dependent transcription.
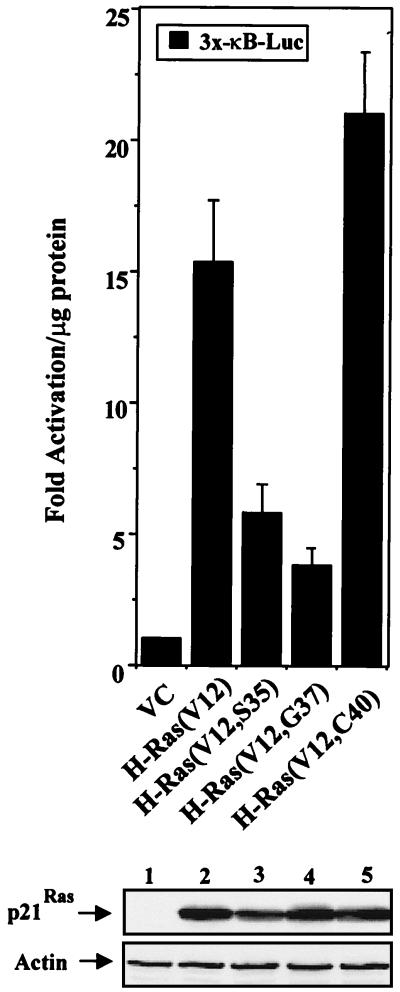
Ras directly interacts with effector targets, including the serine/threonine kinase Raf, PI3K, and the exchange factor Ral.GDS (63). Each of these targets is required for Ras-induced transformation (63). Since H-Ras(V12) is known to activate NF-κB (9, 20, 22), it was important to elucidate which Ras effector pathways were being utilized to stimulate NF-κB-dependent transcription. To address this question, NIH 3T3 cells were transiently cotransfected with an NF-κB-responsive reporter (3x-κB-Luc) and with either the empty vector control or plasmids bearing genes encoding various activated H-Ras effector mutants (63). As shown in Fig. 1, the H-Ras(V12, C40) effector mutant, which activates PI3K but not Raf or Ral.GDS, stimulated NF-κB-dependent transcription as efficiently as activated H-Ras(V12) in NIH 3T3 cells. However, expression of either H-Ras(V12, S35) or H-Ras(V12, G37), which activates either the Raf kinase or the Ral.GDS exchange factor, respectively, was less effective at stimulating the transcriptional activity of NF-κB (Fig. 1). Consistent with this data, our laboratory has recently demonstrated that the expression of the H-Ras(V12, C40) effector mutant effectively stimulates the transcriptional activity of NF-κB (43). These results suggested that H-Ras(V12) can activate NF-κB through signaling pathways involving PI3K.
FIG. 1.
Stimulation of NF-κB-dependent transcription by the H-Ras(V12, C40) effector mutant. NIH 3T3 cells were transiently cotransfected with an NF-κB-responsive reporter (3x-κB-Luc, 0.5 μg) and with expression plasmids bearing the gene encoding H-Ras(V12), H-Ras effector mutants, or an empty vector control (VC) (1 μg each). Cell lysates were harvested 24 h posttransfection, and luciferase activity was assayed as described in Materials and Methods. Data are presented as multiples of the level of activation obtained for the vector control group, which was normalized to 1. Results are the means ± standard deviations of results of three independent experiments. (Gel) Total protein was isolated from a representative transfection experiment, and immunoblot analysis was performed for transgene expression. Protein samples (50 μg per lane) were resolved on a 10% polyacrylamide gel, transferred to nitrocellulose, and probed for hemagglutinin-tagged p21Ras proteins with a hemagglutinin-specific antibody (BABCO, Berkeley, Calif.). Immunoblot assays for actin expression confirmed that relatively equal amounts of proteins were loaded in each lane.
H-Ras(V12) requires PI3K and Akt to upregulate the transcriptional activity of NF-κB.
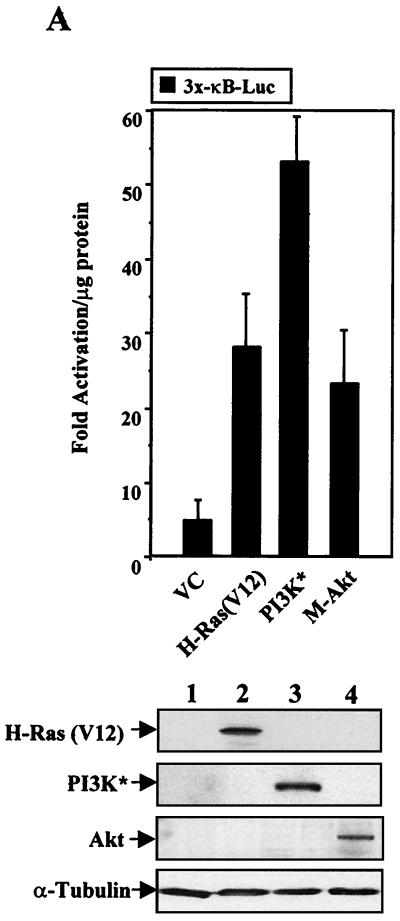
Activation of PI3K is known to stimulate at least two signal transduction pathways, one involving Rac and another involving Akt (28). Since we have recently demonstrated the requirement of Rac in H-Ras(V12)-induced activation of NF-κB (43) and since Akt signaling has been clearly implicated in Ras-mediated cell survival (33, 34), we were interested in determining whether an activated form of either PI3K or Akt could stimulate the transcriptional activity of NF-κB. NIH 3T3 cells were transiently transfected with an NF-κB-responsive reporter and with expression constructs bearing genes encoding activated forms of H-Ras(V12), PI3K, or Akt. As shown in Fig. 2A, cells transfected with activated PI3K or Akt constructs demonstrated an increase in NF-κB transcriptional activity. These results were similar to transcriptional levels observed in cells expressing H-Ras(V12) (Fig. 2A). The increases in 3x-κB-Luc reporter activities observed following PI3K and Akt expression were not due to differences in transfection efficiencies, since all luciferase values were normalized to that of an internal β-Gal control. Moreover, cells transfected with wild-type forms of H-Ras, PI3K, or Akt displayed a ≤2-fold increase in 3x-κB luciferase activity relative to the level in vector control cells, indicating that the increase in NF-κB transcriptional activity was associated with activated forms of these proteins (data not shown).
FIG. 2.
H-Ras(V12) stimulates NF-κB-dependent transcription through PI3K- and Akt-dependent pathways. (A) Activated forms of PI3K (PI3K*) and Akt stimulate the transcriptional activity of NF-κB as effectively as H-Ras(V12). NIH 3T3 cells were cotransfected with the 3x-κB-Luc reporter (0.5 μg), an internal control plasmid reporter (pCMV-LacZ, 1 μg), and various expression constructs (1 μg each). Luciferase and β-Gal activities were assayed 24 h posttransfection, and fold luciferase activity was determined by normalizing values to total protein levels and to β-Gal enzyme levels. Results are expressed as multiples of the level of activation obtained with the mutant 3x-κB-Luc reporter, which contains mutated cis elements that are no longer capable of binding NF-κB. Data are representative of results of at least three independent experiments, and the means ± standard deviations are shown. (Gel) Immunoblot analysis demonstrates that transfected cells effectively express the various transgenes. (B) H-Ras(V12)-induced NF-κB transcriptional activity requires PI3K- and Akt-dependent signaling pathways. NIH 3T3 cells were transiently transfected with the 3x-κB-Luc reporter (0.5 μg), in either the absence (−) or presence (+) of H-Ras(V12) (1 μg). Additionally, cells were transfected with the empty vector control (VC) or with expression constructs bearing genes encoding dominant negative PI3K (ΔPI3K) or Akt (Akt T308A and Akt K179A, 1 μg each). Luciferase levels were measured 24 h posttransfection in order to avoid potential pitfalls associated with cell death. Data are multiples of the level of activation obtained with the mutant 3x-κB-Luc control, as described above. Results are representative of at least three independent experiments and were normalized to an internal β-Gal-expressing plasmid. (Gel) Immunoblot analysis shows that transfected NIH 3T3 cells express the dominant negative P13K (ΔPI3K) and Akt (DN Akt) constructs. (C) PI3K requires Akt to stimulate NF-κB-dependent transcription. NIH 3T3 cells were transfected with the 3x-κB-Luc reporter (0.5 μg), a vector control (VC), activated PI3K (PI3K*), or dominant negative Akt (Akt K179A) (1 μg each). Forty-eight h posttransfection whole-cell extracts were harvested and assayed for luciferase activity. Results are plotted as multiples of the level of activation of the vector control and are representative of three independent experiments. The means ± standard deviations are shown.
Based on these results, it was important to establish whether oncogenic Ras required PI3K and/or Akt activities to stimulate the transcriptional activity of NF-κB. NIH 3T3 cells were cotransfected with the 3x-κB-Luc reporter and with either the empty vector control or the plasmid bearing the gene encoding H-Ras(V12). In addition, some groups were also transfected with expression constructs bearing genes encoding dominant negative forms of either PI3K or Akt [Akt(T308A) or Akt(K179A)]. H-Ras(V12)-induced NF-κB transcriptional activation was inhibited in NIH 3T3 cells coexpressing dominant negative forms of either PI3K or Akt (Fig. 2B). The ability of dominant negative PI3K and Akt constructs to block H-Ras(V12)-induced NF-κB activity was not due to cell death, since luciferase levels were normalized to that of an internal β-Gal-expressing reporter (pCMV-LacZ) (data not shown). Additionally, cell extracts were harvested 24 h posttransfection, a time frame which precedes the induction of Ras-induced cell death (41). This point is further supported by the observation that expression of either dominant negative PI3K or Akt constructs did not repress the basal transcriptional activity of NF-κB (Fig. 2B). Finally, the inability of H-Ras(V12) to stimulate NF-κB-mediated transcription in the presence of the dominant negative proteins was not due to a suppression of the H-Ras(V12) expression vector, since cell extracts displayed similar levels of protein expression (Fig. 2B). These results indicate that H-Ras(V12) requires PI3K- and Akt-dependent signal transduction pathways to stimulate the transcriptional activity of NF-κB.
To elucidate whether PI3K activates NF-κB through an Akt-dependent manner, additional transfection experiments were performed. Consistent with previous results (Fig. 2A), expression of activated PI3K (PI3K*) upregulated the transcriptional activity of NF-κB in NIH 3T3 cells (Fig. 2C). However, expression of the dominant negative Akt [Akt(K179A)] blocked PI3K*-induced activation of NF-κB (Fig. 2C). Equal levels of activated PI3K protein were expressed in transfected cells, indicating that the inability of PI3K* to stimulate NF-κB transcriptional activity was not due to suppression of the pCMV-PI3K* expression vector (data not shown). These results indicate that oncogenic H-Ras is capable of stimulating the transcriptional activity of NF-κB through signaling pathways that involve PI3K and Akt.
PI3K and Akt stimulate NF-κB by stimulating the transactivation domain 1 (TAD 1) of the p65 subunit.
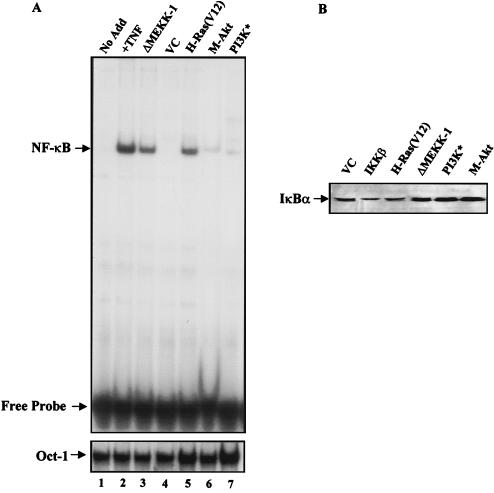
NF-κB is regulated, in part, through a cellular process involving phosphorylation and degradation of its inhibitory protein IκB, which allows NF-κB to translocate to the nucleus and activate transcription (1, 25). Upon cellular stimulation, signal transduction pathways are activated and subsequently stimulate the activation of the IKK signalsome complex to phosphorylate the IκB protein (66). To address whether PI3K or Akt could stimulate NF-κB nuclear translocation and subsequent DNA binding, 293T cells were transiently transfected with expression constructs bearing genes encoding activated forms of H-Ras, MEKK-1, PI3K, or Akt (M-Akt). Since 293T cells demonstrate ≥70% efficiency of transfection, we are able to transfect cells with plasmids bearing genes encoding the various transgenes, isolate nuclear proteins, and then analyze these extracts for nuclear NF-κB activity by performing EMSAs. Nuclear extracts isolated from TNF-stimulated cells were used as a positive control for NF-κB DNA binding activity. As shown in Fig. 3A, 293T cells expressing H-Ras(V12) or constitutively active MEKK-1 (ΔMEKK-1) demonstrated an increase in the DNA binding activity of NF-κB, compared to that of cells transfected with an empty vector control plasmid. This result is consistent with those of previous reports demonstrating that both H-Ras(V12) and ΔMEKK-1 can stimulate nuclear translocation and DNA binding of NF-κB (41, 65). Expression of H-Ras(V12) and ΔMEKK-1 in 293T cells induced DNA-protein complexes which contained both p65 and p50, as detected by supershift analysis (data not shown). Interestingly, nuclear extracts from 293T cells expressing an activated form of either PI3K or Akt failed to demonstrate significant increases in NF-κB DNA binding activity (Fig. 3A). Consistent with the inability of activated PI3K to stimulate nuclear translocation of NF-κB, expression of plasmids bearing genes coding for H-Ras(V12, C40) in 293T cells also failed to increase the DNA binding activity of NF-κB (data not shown). The inability of activated PI3K and Akt to stimulate NF-κB DNA binding in 293T cells was not due to lack of protein expression (data not shown). This effect was also not caused by the quality of the nuclear extracts or the amount of proteins analyzed during EMSAs, since reanalysis of nuclear extracts isolated from transfected cells demonstrated similar levels of Oct-1 DNA-protein complexes (Fig. 3A, bottom gel). These results demonstrate that unlike with activated H-Ras or ΔMEKK-1, the expression of an activated form of PI3K or Akt in 293T cells did not result in an increase in NF-κB nuclear translocation and DNA binding activity. In support of our results, Kane et al. failed to detect nuclear translocation of NF-κB following the expression of activated Akt in the absence of PMA induction (31).
FIG. 3.
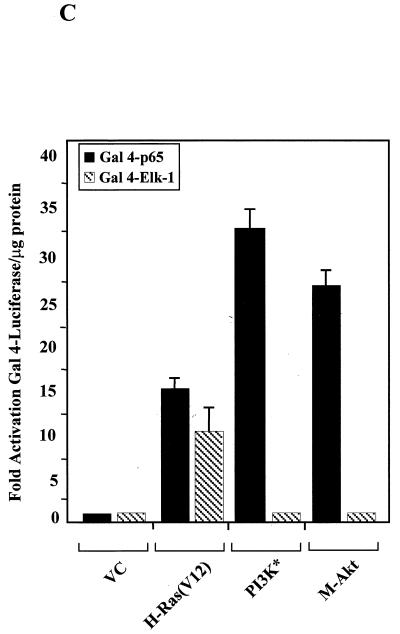
PI3K and Akt activate NF-κB by upregulating the transactivation potential of the p65 subunit. (A) Activated H-Ras(V12) and MEKK stimulate cellular pathways which result in nuclear translocation and increased DNA binding of NF-κB. Human 293T cells were transiently transfected with the vector control (VC) or activated forms of MEKK, H-Ras, Akt, or PI3K (PI3K*) (3 μg each). Nuclear extracts were prepared and EMSAs were performed to assess the presence of NF-κB DNA binding activity. Nuclear extracts isolated from TNF-stimulated 293T cells (15 ng/ml for 15 min) served as a positive control for NF-κB DNA binding activity. (Bottom) Nuclear extracts analyzed for NF-κB DNA binding activity were reanalyzed using a 32P-labeled Oct-1-specific double-stranded probe to confirm the quality of the nuclear proteins. Note that the Oct-1-specific DNA-protein complex but not the free probe is shown. No Add, no addition. (B) IKKβ or H-Ras(V12) expression stimulates IκBα degradation. NIH 3T3 cells were transfected with plasmids bearing the genes encoding the Flag-tagged IκBα and His-tagged LacZ (2 μg each). Additionally, cells were transfected with the vector control (VC) or with plasmids bearing genes encoding wild-type IKKβ or activated forms of H-Ras, MEKK, PI3K, or Akt (1 μg each). Forty-eight hours posttransfection, cells were lysed and proteins (80 μg per lane) were resolved by PAGE. Transfected IκBα protein was detected using a Flag-specific antibody (M2; Sigma). Protein loading and transfection efficiencies were controlled by performing immunoblot analysis for His-tagged LacZ expression within each experimental group (data not shown). (C) Activated PI3K and Akt stimulate the transactivation domain of NF-κB. NIH 3T3 cells were transiently transfected with the Gal4-luciferase reporter (100 ng) and with plasmids bearing the genes encoding either Gal4-p65 or Gal4–Elk-1 (100 ng each). Cells were also transfected with the empty vector control (VC) or activated H-Ras(V12), PI3K, or Akt. Luciferase levels were measured and expressed as multiples of the level of activation of the empty vector control. Data presented are the means ± standard deviations of results of three independent experiments.
Since the nuclear translocation of NF-κB is controlled, at least in part, through IKK-dependent phosphorylation of IκB, it was important to elucidate whether PI3K or Akt could stimulate signaling pathways that would phosphorylate and degrade IκBα. This was an important issue to address in our cell model system, since recent reports indicate that Akt controls NF-κB activity though mechanisms involving IKK-dependent phosphorylation and subsequent degradation of IκBα (31, 44, 49). To explore this possibility, NIH 3T3 cells were cotransfected with plasmids bearing genes encoding Flag-tagged wild-type IκBα and LacZ. Additionally, cells were cotransfected with either the empty vector control or plasmids bearing genes encoding H-Ras(V12), ΔMEKK-1, activated PI3K, or M-Akt. As shown in Fig. 3B, the expression of H-Ras(V12) in NIH 3T3 cells resulted in the degradation of the Flag-tagged IκBα protein. However, cells expressing either activated PI3K or Akt failed to stimulate signaling pathways that were associated with IκBα degradation (Fig. 3B). Expression of IKKβ served as positive control for inducing IκBα degradation. The differences in Flag-tagged IκBα protein levels observed in cells transfected with various expression constructs were not due to differences in plasmid transfection efficiencies, since cell extracts displayed similar levels of β-Gal protein (data not shown). These results are consistent with our observations that activated forms of PI3K or Akt alone are not capable of stimulating signaling pathways that lead to the phosphorylation and degradation of IκBα as well as subsequent nuclear translocation and the DNA binding activity of NF-κB (Fig. 3A and B). In support of our observations, we failed to detect significant activation of endogenous IKK activity in response to activated PI3K or AKT in 293T cells (data not shown). However, cells expressing H-Ras(V12) and ΔMEKK-1 proteins displayed increased IKK activity (data not shown). Thus, we found that activated forms of either PI3K or Akt when expressed alone do not induce signaling pathways that are capable of stimulating endogenous, IκBα-specific IKK activity, IκBα degradation, or nuclear translocation and DNA binding of NF-κB.
Various cellular stimuli can activate NF-κB-dependent transcription, at least in part, through mechanisms independent of signaling pathways which influence nuclear translocation. These signaling pathways stimulate the transactivation domain of the p65 subunit of NF-κB, presumably by targeting basal or induced levels of NF-κB in the nucleus (21, 47, 59a, 60, 68, 69). Since activated forms of PI3K and Akt can increase the transcriptional activity of NF-κB without stimulating nuclear translocation (Fig. 2A and 3A), we were interested in determining whether these signaling molecules could activate NF-κB by targeting TAD 1 of the p65 subunit. To address this question, we used a plasmid bearing DNA encoding the Gal4-p65 fusion protein, where sequences encoding the DNA binding domain of Gal4 have been joined with sequences encoding TAD 1 of p65 (50). This plasmid, when cotransfected with a Gal4-Luc reporter, allowed us to determine whether cellular signals upregulate gene expression by specifically targeting TAD 1 of the p65 subunit of NF-κB. NIH 3T3 cells were cotransfected with a 4x-Gal4-Luc reporter, a Gal4-p65 expression construct, and plasmids bearing genes encoding activated H-Ras(V12), PI3K, or Akt. For control purposes, cells were also transfected under the same conditions, except expression constructs bearing genes encoding the Gal4–Elk-1 fusion protein were used instead of Gal4-p65. As shown in Fig. 3C, activated PI3K and Akt, as well as H-Ras(V12), stimulated TAD 1 of p65. Importantly, the ability of PI3K and Akt to activate TAD 1 of p65 was specific, since Gal4–Elk-1-mediated activity was stimulated by H-Ras(V12) but not by activated PI3K or Akt (Fig. 3C). These results indicate that PI3K and Akt, like H-Ras(V12), stimulate NF-κB by targeting TAD 1 of the p65 subunit. However, unlike H-Ras(V12), which activates signal transduction pathways that increase NF-κB transcriptional activity through both nuclear translocation and an increased p65 transactivation potential, PI3K and Akt upregulate primarily the transactivation potential of the p65 subunit of NF-κB. Consistent with this hypothesis, we detected basal levels of nuclear NF-κB in proliferating cells used in our experiments (data not shown). These findings suggest that PI3K and Akt stimulate the transcriptional activity of NF-κB by targeting basal levels of nuclear NF-κB and that these molecules effectively upregulate the transactivation potential of this transcription factor.
Activated Akt requires IKK to upregulate the transactivation domain of the p65 subunit of NF-κB.
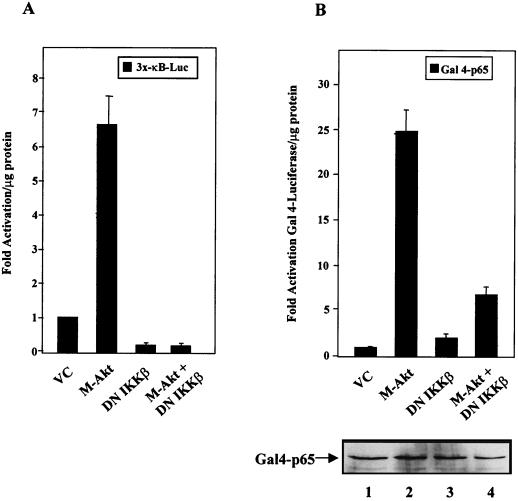
In order to further explore a possible role of the IKK signalsome in Akt-induced activation of NF-κB, we were interested in determining whether Akt required IKK to regulate NF-κB transcriptional activity. To answer this question, NIH 3T3 cells were either transiently transfected with the 3x-κB-Luc reporter or cotransfected with plasmids bearing genes encoding the Gal4-p65 and the Gal4-Luc reporters. In addition, cells were transfected with the vector control or with plasmids bearing the gene encoding M-Akt, dominant negative IKKβ, or M-Akt plus dominant negative IKKβ. Consistent with the ability of IKK to control NF-κB nuclear translocation, NIH 3T3 cells expressing the dominant negative IKKβ protein displayed reduced basal 3x-κB luciferase activity (Fig. 4A). These results suggest that dominant negative IKKβ blocked the accumulation of basal nuclear NF-κB activity. However, as shown in Fig. 4A, the expression of dominant negative IKKβ in NIH 3T3 cells blocked M-Akt-induced activation of NF-κB, suggesting that M-Akt required endogenous IKK activity to regulate NF-κB transcriptional activity. Since Akt did not induce nuclear localization of NF-κB and since IKK activity has been implicated in controlling Akt activity (31, 44, 49), we were interested in determining whether IKK activity was required for Akt to stimulate TAD 1 of the p65 subunit of NF-κB. As shown in Fig. 4B, expression of dominant negative IKKβ blocked the ability of M-Akt to stimulate the transactivation potential of the Gal4-p65 protein. Importantly, expression of the dominant negative IKKβ protein did not block expression of Gal4-p65 (Fig. 4B, gel). The ability of dominant negative IKKβ to block Gal4-p65 activity was somewhat unexpected, because Gal4-p65 transcriptional activity is not under the control of IκB-dependent phosphorylation and degradation events. The loss of Gal4-p65-induced luciferase activity in NIH 3T3 cells cotransfected with M-Akt and dominant negative IKKβ was not due to the ability of dominant negative IKKβ to inhibit pCMV-M-Akt expression, since relatively equal amounts of hemagglutinin-tagged Akt were observed (data not shown). Our results suggest that Akt activation alone does not induce NF-κB through mechanisms involving IκB degradation. Instead, our data indicate that Akt requires IKK to modulate TAD 1 of p65 through mechanisms independent of IκB phosphorylation and degradation (see Discussion). These results may explain, at least to some degree, the observed dependence of IKK activity on Akt-dependent induction of NF-κB activity (31, 49).
FIG. 4.
Activated PI3K and Akt stimulate the p65 transactivation domain of NF-κB in a manner dependent on IκB kinase. (A) Akt requires IKKβ to activate NF-κB-dependent transcription. NIH 3T3 cells were transfected with the 3x-κB-Luc reporter (0.5 μg) and with the empty vector control (VC), M-Akt, or dominant negative IKKβ (DN IKKβ) alone or with M-Akt plus DN IKKβ (1 μg each). Forty-eight hours posttransfection, whole-cell extracts were harvested and assayed for luciferase activity. Results are plotted as multiples of the level of activation obtained with the vector control and are averages ± standard deviations of results of three independent experiments. (B) Akt requires IKK to stimulate TAD 1 of the p65 subunit of NF-κB. NIH 3T3 cells were transfected with the Gal4-luciferase reporter, Gal4-p65 (100 ng each), and the indicated constructs described above (1 μg each). Results are expressed as multiples of the level of activation obtained with the vector control. The data are the means ± standard deviations of results of three independent experiments. (Gel) Western blot analysis of transfected Gal4-p65. Whole-cell extracts of the transfections described above (25 μg of protein each) were separated by SDS–10% PAGE, transferred to nitrocellulose, and assayed with an antibody specific for the Gal4 DNA binding domain (sc-510; Santa Cruz Biotech, Santa Cruz, Calif.). Primary antibodies were detected using an HRP-labeled secondary antibody and by performing ECL.
H-Ras(V12) requires Akt to stimulate the NF-κB transactivation potential and to suppress oncogene-induced apoptosis.
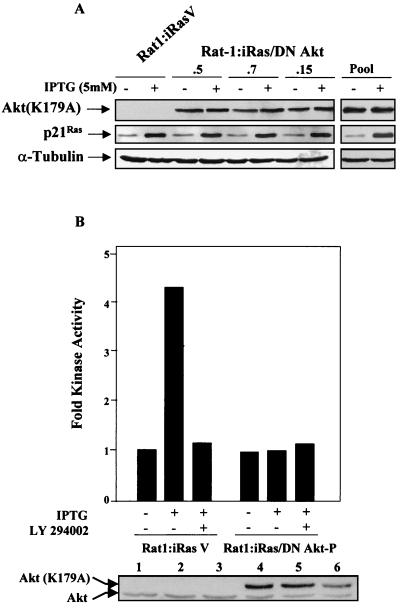
In order to investigate the physiological relevance of NF-κB activation by Akt, it was important to inhibit endogenous Akt activities under conditions in which H-Ras(V12) was expressed. To experimentally address this point, we used the inducible Rat-1:iRas cell line, which contains stably integrated H-Ras(V12) under the control of an IPTG-responsive promoter (42). This cell line has been used previously to demonstrate that NF-κB provides a cell survival role in H-Ras(V12) signaling (41). Rat-1:iRas cells were transfected with a plasmid bearing the gene encoding the dominant negative Akt(K179A) protein, and stable clones were generated. As shown in Fig. 5A, Rat-1:iRas–dominant negative Akt clones which expressed similar levels of the dominant negative Akt(K179A) protein were selected. Additionally, Rat-1:iRas–dominant negative Akt stable clones displayed levels of IPTG-induced H-Ras expression similar to that of the vector control cells (Rat-1:iRasV) (Fig. 4A). Rat-1:iRas–dominant negative Akt clones (namely, .5, .7, and .15) were combined to create a pooled cell line (Rat-1:iRas–dominant negative Akt-P) (Fig. 5A).
FIG. 5.
Characterization of Rat-1:iRas cells expressing a dominant negative Akt protein. (A) Characterization of the Rat-1:iRas–dominant negative Akt cells. Rat-1:iRas cells etopically expressing a plasmid bearing the gene encoding the dominant negative Akt(K179A) protein (DN Akt) or the vector control were generated, as described in the Materials and Methods. Total protein (50 μg) was isolated from Rat-1:iRasV cells (vector control cells), three Rat-1:iRas–dominant negative Akt clones (.5, .7, and .15), and Rat-1:iRas–dominant negative Akt-P cells (Pool) in the absence or presence of IPTG (5 mM). Protein samples were resolved on an SDS–10% polyacrylamide gel, transferred to membrane, and analyzed for the presence of Akt, Ras, and α-tubulin. Akt(K179A) protein was detected using a hemagglutinin-specific antibody (BABCO). IPTG-induced p21Ras expression was detected using a pan-Ras monoclonal antibody (Calbiochem, San Diego, Calif.). To ensure equal levels of protein loading, blots were reanalyzed with an α-tubulin-specific antibody (Sigma). Primary antibodies were detected using an HRP-labeled secondary antibody and by performing ECL. (B) Expression of the dominant negative Akt protein blocks H-Ras(V12)-induced endogenous Akt activity. Subconfluent Rat-1:iRasV and Rat-1:iRas–dominant negative Akt-P cells were grown overnight in medium containing 2% FBS. Eighteen hours later cells were washed and cultured for 4 h without serum and with or without IPTG (5 mM). Some groups received LY 294002 (10 μM) 3 h after serum deprivation. Immunocomplex kinase assays for Akt were performed as described in Materials and Methods. The fold Akt activity was determined by obtaining values for Rat-1:iRasV and Rat-1:iRas–dominant negative Akt-P cells (grown in the absence of IPTG) and normalizing these numbers to 1. Data presented are representative of results of at least three different assays, which generated similar results. (Gel) Immunoblot analysis demonstrating that relatively equal amounts of total Akt protein were immunoprecipitated during the course of the experiment. Both endogenous Akt and hemagglutinin-tagged dominant negative Akt(K179A) proteins are shown.
To determine whether cells expressing the dominant negative Akt(K179A) protein displayed a reduction in H-Ras(V12)-induced Akt activity, immune complex kinase assays were performed using Crosstide as an Akt-specific peptide substrate (14). As shown in Fig. 5B, Rat-1:iRasV control cells demonstrated a fourfold increase in Akt activity following IPTG-induced H-Ras(V12) expression. In contrast, Rat-1:iRas–dominant negative Akt-P cells, constitutively expressing Akt(K179A) protein, failed to display increases in Akt kinase activity following IPTG addition (Fig. 5B). Phosphorylation of the crosstide by endogenous Akt was specific to IPTG-induced H-Ras(V12) expression, and kinase activity was inhibited by the PI3K inhibitor LY 294002 (Fig. 5B). Differences in kinase activities between Rat-1:iRasV and Rat-1:iRas–dominant negative Akt-P cells were not due to unequal amounts of immunoprecipitated Akt, since analysis of input protein demonstrated that similar levels of protein were analyzed for the kinase activity (Fig. 5B, gel). These results indicate that the expression of the dominant negative Akt protein blocked the ability of H-Ras(V12) to stimulate endogenous Akt activity in Rat-1:iRas–dominant negative Akt-P cells.
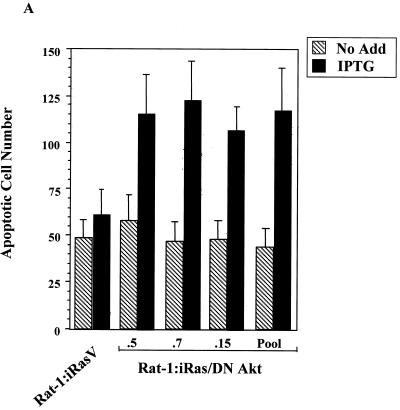
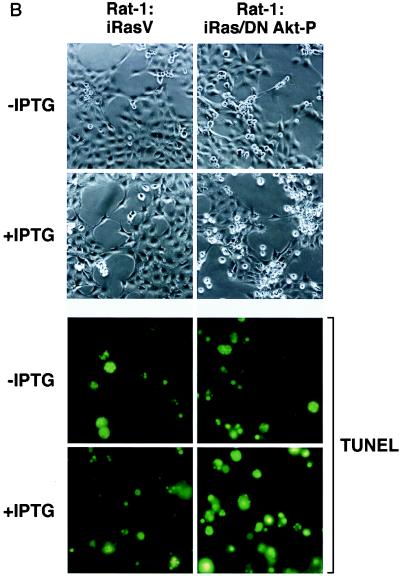
To determine whether the loss of endogenous Akt activity would sensitize cells to H-Ras(V12)-induced apoptosis in our model system, Rat-1:iRasV and Rat-1:iRas–dominant negative Akt clones were grown in complete medium containing a reduced concentration of serum (2% FBS) in either the absence or presence of IPTG to induce H-Ras(V12) expression. Forty-eight hours after IPTG addition, cellular supernatants containing detached cells were harvested, fixed, and stained with Hoechst dye and cells displaying fragmented nuclei were counted as described in Materials and Methods. As shown in Fig. 6A, both Rat-1:iRasV and Rat-1:iRas–dominant negative Akt clones displayed elevated basal apoptotic cell numbers (without IPTG) when cells were cultured with a reduced concentration of serum (2% FBS). However, this effect was not observed in cells cultured under normal conditions (10% FBS) (data not shown). Consistent with the antiapoptotic nature of Akt, we found that cells expressing the dominant negative Akt(K179A) protein were more susceptible to H-Ras(V12)-induced apoptosis than the control Rat-1:iRasV cells (Fig. 6A). Compared to vector control Rat-1:iRasV cells, IPTG-induced Rat-1:iRas–dominant negative Akt-P cells displayed enhanced morphological signs of apoptosis, including retraction of cellular processes, nuclear condensation, and loss of adherence (Fig. 6B, top). Moreover, IPTG-induced H-Ras(V12) expression stimulated apoptosis in Rat-1:iRas–dominant negative Akt-P cells, as detected by the appearance of TUNEL-positive cells (Fig. 6B, bottom). These results are consistent with reports indicating that Akt provides a cell survival signal downstream of activated H-Ras (33, 48) and indicate that Akt is one of the major antiapoptotic mediators of oncogenic Ras signaling.
FIG. 6.
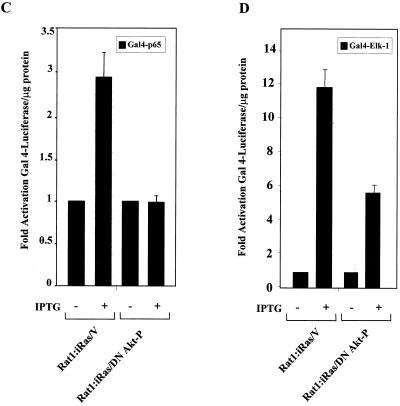
The inhibition of endogenous Akt activity sensitizes cells to H-Ras(V12)-induced apoptosis. (A) Expression of H-Ras(V12) is associated with an increase in apoptosis in cells expressing the dominant negative Akt protein (DN Akt). Rat-1:iRasV, Rat-1:iRas–dominant negative Akt clones (.5, .7, and .15), and Rat-1:iRas–dominant negative Akt-P cells (pooled clone) were grown overnight in medium containing a reduced concentration of serum (2% FBS). Eighteen hours later, cells were treated in either the absence or presence of IPTG (5 mM). Apoptotic cells were harvested from the supernatants 48 h after IPTG addition, fixed in paraformaldehyde, and stained with Hoechst dye, and cells displaying nuclear fragmentation and condensation were counted, as described in Materials and Methods. Results are expressed as numbers of apoptotic cells (means ± standard deviations). Assays were repeated at least three independent times. (B) Rat-1:iRasV and Rat-1:iRas–dominant negative Akt-P cells were cultured and stimulated with IPTG as described above. Cell morphologies were analyzed 48 h after IPTG addition. Paraformaldehyde-fixed cells were analyzed for apoptosis by performing TUNEL analysis (Boehringer Mannheim). The upper four images show phase-contrast microscopy (magnification, ×20). The bottom four images show fluorescence microscopy of TUNEL-positive cells (magnification, ×40). (C) H-Ras(V12) no longer stimulates the p65 transactivation domain in cells stably expressing the dominant negative Akt protein. Rat-1:iRasV and Rat-1:iRas–dominant negative Akt-P cells (pooled clone) were transfected with the Gal4-luciferase reporter (100 ng) and with constructs bearing the gene encoding the Gal4-p65 fusion protein (100 ng). Eighteen hours following transfections, cells were stimulated with IPTG (5 mM). Twenty-four hours following IPTG addition, cell extracts were isolated and luciferase activities were determined. Results are the averages ± standard deviations from three independent experiments performed in triplicate. (D) H-Ras(V12) stimulates Gal4–Elk-1 in the vector control and cells expressing dominant negative Akt [Akt(K179A)]. Cells were transfected with Gal4-luciferase and Gal4–Elk-1 (100 ng each). Twenty-four hours posttransfection, cells were incubated in either DMEM containing 10% serum and IPTG (5 mM) or DMEM plus 10% serum alone for an additional 24 h. Whole-cell extracts were isolated and assayed for luciferase levels. Results are expressed as multiples of the level of activation obtained with the vector control or dominant negative Akt-P without IPTG incubation and are the averages ± standard deviations of results of three independent experiments performed in triplicate.
To elucidate whether H-Ras(V12)-induced apoptosis, following the inhibition of Akt, was associated with a loss in NF-κB transcriptional activity, transient-cotransfection assays were performed. As shown in Fig. 6C, the addition of IPTG to the control Rat-1:iRasV cells led to a subsequent increase in H-Ras(V12)-induced Gal4-p65 transcriptional activity. However, Rat-1:iRas–dominant negative Akt-P cells failed to show an increase in Gal4-p65 transcriptional activity following IPTG-induced H-Ras(V12) expression (Fig. 6C). To show that Akt-mediated signaling pathways defective in Rat-1:iRas–dominant negative Akt-P cells were specific for Gal4-p65, similar experiments were performed using Gal4–Elk-1. Rat-1:iRas–dominant negative Akt-P and vector control cells were capable of stimulating Gal4–Elk-1 activity following IPTG-induced H-Ras(V12) expression (Fig. 6D). Thus, cells expressing the dominant negative Akt (K179A) still retain the ability to signal to other Ras effector pathways. Collectively, our results indicate that the inhibition of endogenous Akt activity is associated with a loss of H-Ras-induced NF-κB-dependent transcription and enhanced susceptibility of these cells to apoptosis.
Akt-transformed Rat-1 cells require NF-κB to suppress apoptosis induced by etoposide.
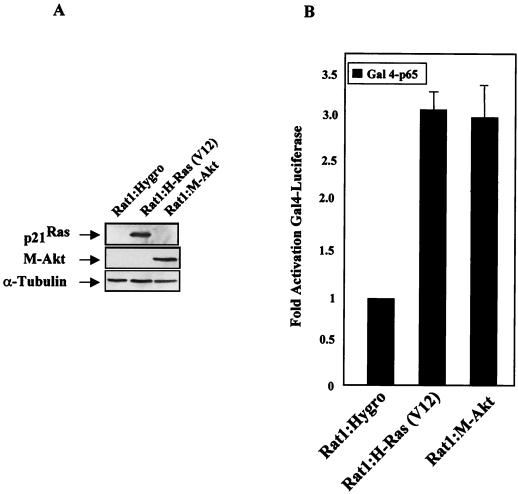
Although our data implicate NF-κB as a downstream mediator of the Akt cell survival response, we needed to determine whether the ability of Akt to transcriptionally upregulate NF-κB contributes to the antiapoptotic function of this kinase. To address this point, we made stable cell lines which expressed activated forms of Ras [Rat-1:H-Ras(V12)] or Akt (Rat-1:M-Akt) or which contained the vector control plasmid (Rat-1:hygro [Hygro]). As shown in Fig. 7A, Rat-1 clones which express relatively equal levels of either H-Ras(V12) or M-Akt were selected. Unlike the Rat-1:Hygro control cells, both Rat-1:H-Ras(V12) and Rat-1:M-Akt cells displayed characteristics of transformed cells, as indicated by their abilities to form foci and to grow in soft agar (data not shown). Consistent with transient-transfection assays (Fig. 3C), we found that both Rat-1:H-Ras(V12) and Rat-1:M-Akt cells displayed elevated Gal4-p65 transcriptional activity, compared to that of the Rat-1:Hygro control cells (Fig. 7B). These results are consistent with the ability of H-Ras(V12) and M-Akt to stimulate NF-κB by targeting TAD 1 of the p65 subunit.
FIG. 7.
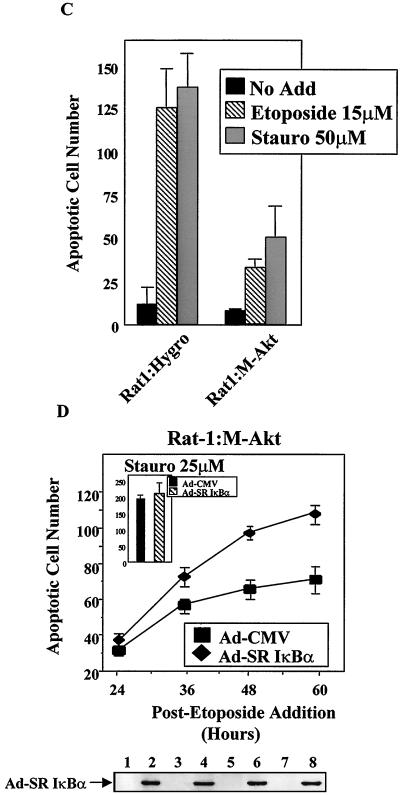
M-Akt-transformed cells require NF-κB to block etoposide-induced apoptosis. (A) Generation of H-Ras(V12)- and M-Akt-transformed Rat-1 cells. Rat-1 cells stably expressing activated H-Ras(V12) or M-Akt were generated as described in Materials and Methods. Total protein (50 μg) was isolated from Rat-1:Hygro, Rat-1:H-Ras(V12), and Rat-1:M-Akt cells, resolved by performing PAGE, and transferred to nitrocellulose membrane. Immunoblot analysis was performed to detect transgenic expression of p21ras and M-Akt using the pan-Ras antibody or the hemagglutinin antibody, respectively. (B) The p65 transactivation domain is activated in Rat-1 cells transformed with either H-Ras(V12) or M-Akt. Rat-1:Hygro, Rat-1:H-Ras(V12), and Rat-1:M-Akt cells were transiently transfected with a Gal4-luciferase reporter (100 ng) and with constructs bearing the genes encoding Gal4-p65 (100 ng). Forty-eight hours following the start of transfection, cell extracts were harvested and luciferase activities were determined. Data are the averages ± standard deviations of results of three experiments performed in triplicate. (C) Rat-1:M-Akt cells are resistant to apoptotic induction agents. Rat-1:Hygro and Rat-1:M-Akt cells were either left untreated (No Add) or given etoposide (15 μM) or staurosporine (50 μM). Apoptotic cell numbers were determined 18 h following the addition of either etoposide or staurosporine (Stauro). Results presented here are the means ± standard deviations of results of two independent experiments performed in duplicate. (D) M-Akt requires NF-κB to overcome etoposide-induced apoptosis. Rat1:M-Akt cells were cultured overnight in medium containing 2% FBS, after which cells were infected with either Ad-CMV or Ad-SRIκBα (50 PFU/cell). Six hours following adenovirus-mediated gene delivery, cells were either left untreated (No Add) or treated with either etoposide (5 μM) or staurosporine (25 μM). Apoptotic cell numbers were analyzed in etoposide-treated Rat-1:M-Akt cells over the time course indicated, while the apoptotic cell numbers detected for staurosporine were analyzed 60 h after the drug addition. Data presented are the averages ± standard deviations of results of two different experiments where the numbers of apoptotic cells were counted in triplicate. (Gel) Immunoblot analysis demonstrating that Ad-SRIκBα is effectively expressed in the Rat-1 cell lines. IκBα proteins were detected using a rabbit polyclonal antibody (C-21; Santa Cruz Biotech). Protein samples analyzed in lanes 1, 3, 5, and 7 are from Rat-1:M-Akt cells infected with Ad-CMV, while those in lanes 2, 4, 6, and 8 are from cells infected with Ad-SRIκBα.
To further confirm that NF-κB is a downstream target of Akt, we took advantage of the fact that it has been recently demonstrated that Akt provides a cell survival signal that blocks apoptosis initiated by the PKC inhibitor, staurosporine, or by the topoisomerase II inhibitor, etoposide (8). Importantly, unlike staurosporine, which does not activate NF-κB (58), etoposide is known to stimulate endogenous NF-κB-responsive gene transcription (32, 59a). To determine whether M-Akt expression provides a cell survival function in response to proapoptotic agents, Rat-1:Hygro and Rat-1:M-Akt cells were treated in either the absence or presence of etoposide (15 μM) or staurosporine (50 μM) and apoptotic cell numbers were determined 18 h after drug addition. As shown in Fig. 7C, Rat-1:M-Akt cells were more resistant to etoposide- and staurosporine-induced apoptosis than the Rat-1:Hygro control cells. These results are consistent with previous findings demonstrating that activated Akt is capable of suppressing apoptosis by phosphorylating and inactivating procaspase-9 (8). The addition of etoposide to Rat-1:M-Akt cells stimulated NF-κB-induced transcription in transient-transfection assays. However, staurosporine was unable to stimulate the transcriptional activity of NF-κB in these cells (data not shown). Therefore, despite the ability of M-Akt to overcome apoptosis induced by etoposide and staurosporine, only etoposide-induced stress signals are capable of activating NF-κB.
To determine whether M-Akt-transformed cells required NF-κB to suppress apoptosis in response to etoposide, we used the SR IκBα (6). This mutant IκBα protein acts as a transdominant negative protein because it binds to NF-κB and inhibits nuclear translocation and DNA binding (41, 47, 57). Importantly, expression of the SR IκBα protein blocks the activation of NF-κB-responsive genes (27, 58). Rat-1:M-Akt cells were infected with Ad-SRIκBα or with Ad-CMV, and cells were treated with either etoposide or staurosporine. Adenovirus-mediated delivery of transgenes is extremely effective in Rat-1:M-Akt cells, where ≥95% of cells effectively express transgenes 12 h following viral infection (Fig. 7D, gel, and data not shown). Rat-1:M-Akt cells were more susceptible to etoposide-induced apoptosis following the inhibition of NF-κB by Ad-SRIκBα than cells infected with Ad-CMV (control virus) (Fig. 7D). However, compared to Rat-1:Hygro control cells, Rat-1:M-Akt cells were more resistant to apoptotic signals induced by etoposide even after the inhibition of NF-κB transcriptional activity (data not shown). Although NF-κB functions as a cell survival factor, these results suggest that other NF-κB-independent pathways which provide strong antiapoptotic signals exist. Therefore, we presume that since Rat-1 cells express both Bad and caspase-9, these downstream targets are likely to be controlled by H-Ras(V12)-induced Akt activity in the absence of NF-κB transcriptional activity. Although Rat-1:M-Akt cells were more resistant to apoptosis induced by staurosporine, the expression of SR-IκBα did not sensitize staurosporine-treated cells to programmed cell death (Fig. 7D, inset). These results indicate that Rat-1:M-Akt cells require NF-κB to overcome proapoptotic death pathways induced by etoposide but not signals stimulated by staurosporine. Thus, Akt is an important antiapoptotic signaling molecule that contributes to cell survival not only by directly phosphorylating proapoptotic targets but also by stimulating the transcriptional activity of NF-κB.
DISCUSSION
The data presented here indicate that one mechanism whereby Akt-controlled signaling pathways inhibit apoptosis is through the activation of the transcription factor NF-κB. Loss of endogenous Akt is associated with a downregulation in the transcriptional activity of NF-κB and with H-Ras(V12)-induced apoptosis. Moreover, we find that activated Akt requires NF-κB to partially suppress etoposide-induced apoptosis. Consistent with previous findings which demonstrate that Akt can inhibit multiple proapoptotic molecules through direct phosphorylation events (7, 8, 15, 16, 36), we found that the loss of NF-κB transcriptional activity did not completely sensitize Akt-expressing cells to apoptosis in response to etoposide (Fig. 7D). Thus, our work and the work of others indicate that Akt mediates cell survival signals through immediate phosphorylation of proapoptotic proteins and through longer-term transcription-dependent mechanisms. These findings are consistent with extensive evidence for an antiapoptotic role for NF-κB in blocking certain apoptotic stimuli and indicate that NF-κB is a downstream target of Akt-induced cell survival signals.
We explored the potential involvement of Akt as a mediator in signaling between oncogenic Ras and NF-κB. Oncogenic Ras has been demonstrated to induce both proapoptotic and antiapoptotic signals (40, 51), and we have shown previously that oncogenic Ras stimulates the transcriptional activity of NF-κB to overcome H-Ras(V12)-induced apoptosis (41). Since both PI3K and Akt have been shown to provide an antiapoptotic signal in response to oncogenic Ras expression, we asked whether PI3K and Akt provide the link to NF-κB activation. In this study, we have found that H-Ras(V12) requires the antiapoptotic pathways involving PI3K and Akt to stimulate the transcriptional activity of NF-κB. Consistent with this, we demonstrate that inhibition of endogenous Akt kinase activity suppresses H-Ras(V12)-induced NF-κB transcription and sensitizes cells to apoptosis. Although Akt was required to suppress apoptosis induced by H-Ras(V12), Ras is able to utilize additional signaling pathways to activate NF-κB-dependent cell survival. This supposition is supported by our observations that inhibition of NF-κB with the SR IκBα further sensitized Rat-1:iRas–dominant negative Akt-P cells to Ras-mediated apoptosis (data not shown). Interestingly, unlike H-Ras(V12), M-Akt-transformed cells did not undergo apoptosis following the inactivation of NF-κB with the SR IκBα protein (data not shown). This observation suggests that, unlike H-Ras(V12), activated Akt does not stimulate signaling pathways which induce cell death. An alternative possibility, however, is that the overexpression of M-Akt is such a powerful antiapoptotic factor that it overcomes programmed cell death signals following the inactivation of NF-κB. Nevertheless, the evidence that Akt-induced activation of NF-κB provides a cell survival function is shown by the ability of NF-κB to protect Akt-expressing cells from etoposide-induced apoptosis (Fig. 7D).
The regulation of NF-κB by Akt is likely to be important in cytokine and growth factor signaling, since many of the physiological inducers of Akt also stimulate the transcriptional activity of NF-κB. Recently, physiological stimuli, including TNF-α, IL-1β and PDGF, have been shown to activate NF-κB in a PI3K- and Akt-dependent manner (31, 44, 49, 52). It is intriguing that the cell survival mechanisms and potentially the mitogenic pathways associated with growth factor signaling is controlled, at least partially, by NF-κB-dependent mechanisms. Consistent with this point, we and others (27, 30) have recently shown that NF-κB can promote cell growth by controlling the transcription of the cyclin D1 gene. It will be important to determine if Akt-derived mitogenic signals involve the transcription function of NF-κB, possibly through the controlled upregulation of cyclin D1 or other functionally related proteins.
Previous work has indicated that PI3K and Akt are involved in NF-κB activation. For example, it was shown (4) that activation of NF-κB in response to the tyrosine phosphatase inhibitor pervanadate was blocked by the PI3K inhibitor wortmannin. It was proposed that tyrosine-phosphorylated IκBα associated with the p85 subunit of PI3K, allowing nuclear translocation of NF-κB. In that study, wortmannin did not block the ability of TNF to induce nuclear translocation of NF-κB. Stark and colleagues have shown recently (52) that IL-1β stimulates the association of the IL-1 receptor accessory protein with the p85 subunit of PI3K and that, consistent with our results, this leads to the stimulation of the transactivation potential of the p65 subunit of NF-κB. Interestingly, it was shown that this response also leads to phosphorylation of the p65 subunit, but the site of this phosphorylation was not mapped. Additionally, Kane and colleagues (31) have shown that Akt expression augmented the ability of PMA to activate NF-κB through enhanced IκBα phosphorylation and degradation. The ability of Akt to synergize with PMA on the activation of an NF-κB promoter was blocked by a kinase-inactive IKK. Interestingly, Akt was unable to activate NF-κB nuclear translocation on its own, consistent with our data. More recently, two reports implicate Akt in NF-κB activation induced by TNF (44) and by PDGF (49). Several such reports implicate IKK activity as being required for the Akt-controlled response.
Our data indicate that Akt activation alone is insufficient to activate endogenous IKK activity and NF-κB nuclear translocation. However, our data demonstrate that Akt signals to a pathway that stimulates the transcriptional potential of the p65 subunit of NF-κB through activation of TAD 1. Even though Akt is a serine/threonine kinase, it is unlikely that this protein directly phosphorylates p65 since the TAD 1 region does not contain consensus Akt phosphorylation sites. Moreover, we have been unsuccessful at detecting protein-protein interactions between p65 and Akt. (L. V. Madrid, A. S. Baldwin, Jr., and M. W. Mayo, unpublished observations). However, mutation of serine 529 in TAD 1 of p65, which is known to be phosphorylated in response to TNF signaling (60), inhibits the ability of Akt to activate p65 transcription. (Madrid et al., unpublished observations). As mentioned earlier, and in apparent contrast with reports which indicate that the role of Akt in inducing NF-κB activity occurs through IKK-dependent degradation of IκBα (31, 44, 49), the overexpression of activated Akt is unable to stimulate endogenous IKK activity or IκBα degradation (Fig. 3B and data not shown). In contrast, H-Ras(V12) expression in cells is able to stimulate endogenous IKK activity, induce degradation of IκBα, and increase nuclear translocation and DNA binding of NF-κB (Fig. 3A and B and data not shown). These results suggest that H-Ras(V12) regulates NF-κB through two signals: one that leads to nuclear localization of NF-κB and one that activates the transactivation function of the p65 subunit. Although M-Akt does not stimulate endogenous IKK activity directed towards IκBα degradation, the IKK signalsome complex is still important in regulating the ability of Akt to stimulate NF-κB. This hypothesis is supported by the observation that a dominant negative IKKβ protein inhibited the ability of Akt to stimulate TAD 1 of p65 (Fig. 4B). These results suggest that IKK activity (possibly independent of IκBα phosphorylation) or some structural aspect of IKK is required for the ability of Akt to stimulate NF-κB transactivation function. The fact that Gal4-p65 is not regulated through an IκB-dependent mechanism suggests that IKK is required for Akt to activate TAD 1 of p65, independent of nuclear translocation signals. Collectively, these results suggest that the IKK signalosome complex is capable of regulating NF-κB through signaling events involved in nuclear translocation as well as Akt-induced transactivation pathways. Although our data indicate that Akt alone is unable to stimulate IκBα degradation on its own, they do not rule out the possibility that Akt is required (but not sufficient) for activation of IκBα phosphorylation and degradation in response to certain signal transduction cascades.
Our results demonstrating that H-Ras(V12) requires PI3K and Akt to stimulate NF-κB-dependent transcription and cell survival are very likely to be important in cancer biology. Human tumors displaying upregulated endogenous Akt (10, 11, 23) or lacking tumor suppressor gene products which modulate PI3K activities, such as the PTEN phosphotidylinoside phosphatase (39, 53), may utilize elevated transcriptional activities of antiapoptotic and proproliferative transcription factors, like NF-κB, to enhance oncogenic potential. Therefore, loss of PTEN, which occurs in a variety of tumors (17, 37, 45, 54, 62), is predicted to lead to the upregulation of NF-κB, providing signals potentially relevant to oncogenesis (27, 41).
The data provided here are consistent with those of other reports indicating that transforming events which upregulate Akt activity have profound effects not only on cell survival in terms of oncogenesis but also on chemoresistance. Along these lines, the activation of Akt in tumor cells may synergize with a chemotherapeutic response (such as that induced by etoposide) to provide an enhanced antiapoptotic function. Thus, the induction of NF-κB nuclear translocation by etoposide plus an Akt signal to stimulate transactivation function may lead to a potent antiapoptotic response through enhanced regulation of antiapoptotic genes. In support of this point, we demonstrate that M-Akt provides resistance to the apoptotic agent etoposide and that M-Akt-mediated resistance to etoposide requires the transcriptional activity of NF-κB. Since NF-κB functions to positively upregulate gene products which are known to overcome chemotherapy-induced apoptosis, such as c-IAP1, c-IAP2, and A1 (12, 26, 58, 59a, 70), future experiments will determine whether constitutively active Akt potentiates chemoresistance by regulating expression of NF-κB-controlled antiapoptotic gene products.
ACKNOWLEDGMENTS
We thank Channing Der (University of North Carolina) for kindly providing H-Ras(V12) and the activated Ras effector mutants, Phillip Hawkins (The Babraham Institute, Cambridge, United Kingdom) for providing dominant negative Akt(T308A) constructs, and Anke Klippel (Chiron Corporation, Emeryville, Calif.) for providing the other PI3K and Akt constructs used in this study. We also thank Michael J. Weber and Sandy Westerheide for critical readings of the manuscript.
This research was supported by NIH grants awarded to M.W.M. (K01 78595), A.S.B. (CA72771), A.S.B. and M.W.M. (CA75080), and C.-Y.W. (DE/CA13196-01A1).
L. V. Madrid and M. W. Mayo contributed equally to the scientific merit and preparation of this paper.
REFERENCES
- 1.Baldwin A S., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 2.Beg A A, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 3.Bellacosa A, Testa J R, Staal S P, Tsichlis P N. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 4.Beraud C, Henzel W J, Baeuerle P A. Involvement of regulatory and catalytic subunits of phosphoinositide 3-kinase in NF-kappaB activation. Proc Natl Acad Sci USA. 1999;96:429–434. doi: 10.1073/pnas.96.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bird T A, Schooley K, Dower S K, Hagen H, Virca G D. Activation of nuclear transcription factor NF-kappaB by interleukin-1 is accompanied by casein kinase II-mediated phosphorylation of the p65 subunit. J Biol Chem. 1997;272:32606–32612. doi: 10.1074/jbc.272.51.32606. [DOI] [PubMed] [Google Scholar]
- 6.Brockman J A, Scherer D C, McKinsey T A, Hall S M, Qi X, Lee W Y, Ballard D W. Coupling of a signal response domain in IκBα to multiple pathways for NF-κB activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 8.Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E, Frisch S, Reed J C. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 9.Chang H W, Aoki M, Fruman D, Auger K R, Bellacosa A, Tsichlis P N, Cantley L C, Roberts T M, Vogt P K. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science. 1997;276:1848–1850. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- 10.Cheng J Q, Altomare D A, Klein M A, Lee W C, Kruh G D, Lissy N A, Testa J R. Transforming activity and mitosis-related expression of the AKT2 oncogene: evidence suggesting a link between cell cycle regulation and oncogenesis. Oncogene. 1997;14:2793–2801. doi: 10.1038/sj.onc.1201121. [DOI] [PubMed] [Google Scholar]
- 11.Cheng J Q, Ruggeri B, Klein W M, Sonoda G, Altomare D A, Watson D K, Testa J R. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci USA. 1996;93:3636–3641. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu Z L, McKinsey T A, Liu L, Gentry J J, Malim M H, Ballard D W. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci USA. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coffer P J, Woodgett J R. Molecular cloning and characterisation of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur J Biochem. 1991;201:475–481. doi: 10.1111/j.1432-1033.1991.tb16305.x. [DOI] [PubMed] [Google Scholar]
- 14.Cross D A, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 15.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 16.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 17.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi P P. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 18.Downward J. Lipid-regulated kinases: some common themes at last. Science. 1998;279:673–674. doi: 10.1126/science.279.5351.673. [DOI] [PubMed] [Google Scholar]
- 19.Downward J. Ras signalling and apoptosis. Curr Opin Genet Dev. 1998;8:49–54. doi: 10.1016/s0959-437x(98)80061-0. [DOI] [PubMed] [Google Scholar]
- 20.Finco T S, Baldwin A S., Jr Kappa B site-dependent induction of gene expression by diverse inducers of nuclear factor kappa B requires Raf-1. J Biol Chem. 1993;268:17676–17679. [PubMed] [Google Scholar]
- 21.Finco T S, Baldwin A S. Mechanistic aspects of NF-kappa B regulation: the emerging role of phosphorylation and proteolysis. Immunity. 1995;3:263–272. doi: 10.1016/1074-7613(95)90112-4. [DOI] [PubMed] [Google Scholar]
- 22.Finco T S, Westwick J K, Norris J L, Beg A A, Der C J, Baldwin A S., Jr Oncogenic Ha-Ras-induced signaling activates NF-kappaB transcriptional activity, which is required for cellular transformation. J Biol Chem. 1997;272:24113–24116. doi: 10.1074/jbc.272.39.24113. [DOI] [PubMed] [Google Scholar]
- 23.Franke T F, Kaplan D R, Cantley L C. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 24.Fruman D A, Meyers R E, Cantley L C. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh S, May M J, Kopp E B. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 26.Grumont R J, Rourke I J, Gerondakis S. Rel-dependent induction of A1 transcription is required to protect B cells from antigen receptor ligation-induced apoptosis. Genes Dev. 1999;13:400–411. doi: 10.1101/gad.13.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guttridge D C, Albanese C, Reuther J Y, Pestell R G, Baldwin A S., Jr NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller R D, Krishna U M, Falck J R, White M A, Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- 29.Hawkins P T, Welch H, McGregor A, Eguinoa A, Gobert S, Krugmann S, Anderson K, Stokoe D, Stephens L. Signalling via phosphoinositide 3OH kinases. Biochem Soc Trans. 1997;25:1147–1151. doi: 10.1042/bst0251147. [DOI] [PubMed] [Google Scholar]
- 30.Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-κB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol Cell Biol. 1999;19:2690–2698. doi: 10.1128/mcb.19.4.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kane L P, Shapiro V S, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 32.Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green D R. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kappa B and AP-1. Mol Cell. 1998;1:543–551. doi: 10.1016/s1097-2765(00)80054-4. [DOI] [PubMed] [Google Scholar]
- 33.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 34.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne P H, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klippel A, Kavanaugh W M, Pot D, Williams L T. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol Cell Biol. 1997;17:338–344. doi: 10.1128/mcb.17.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kops G J, de Ruiter N D, De Vries-Smits A M, Powell D R, Bos J L, Burgering B M. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner S H, Giovanella B C, Ittmann M, Tycko B, Hibshoosh H, Wigler M H, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 38.Liu Z G, Hsu H, Goeddel D V, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 39.Maehama T, Dixon J E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 40.Marte B M, Downward J. PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem Sci. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 41.Mayo M W, Wang C-Y, Cogswell P C, Rogers-Graham K S, Lowe S W, Der C J, Baldwin A S., Jr Requirement of NF-kappaB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- 42.McCarthy S A, Samuels M L, Pritchard C A, Abraham J A, McMahon M. Rapid induction of heparin-binding epidermal growth factor/diphtheria toxin receptor expression by Raf and Ras oncogenes. Genes Dev. 1995;9:1953–1964. doi: 10.1101/gad.9.16.1953. [DOI] [PubMed] [Google Scholar]
- 43.Norris J L, Baldwin A S., Jr Oncogenic ras enhances NF-kappaB transcriptional activity through raf-dependent and raf-independent mitogen-activated protein kinase signaling pathways. J Biol Chem. 1999;274:13841–13846. doi: 10.1074/jbc.274.20.13841. [DOI] [PubMed] [Google Scholar]
- 44.Ozes O N, Mayo L D, Gustin J A, Pfeffer S R, Pfeffer L M, Donner D B. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 45.Podsypanina K, Ellenson L H, Nemes A, Gu J, Tamura M, Yamada K M, Cordon-Cardo C, Catoretti G, Fisher P E, Parsons R. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci USA. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reddy S A, Huang J H, Liao W S. Phosphatidylinositol 3-kinase in interleukin 1 signaling. Physical interaction with the interleukin 1 receptor and requirement in NFkappaB and AP-1 activation. J Biol Chem. 1997;272:29167–29173. doi: 10.1074/jbc.272.46.29167. [DOI] [PubMed] [Google Scholar]
- 47.Reuther J Y, Reuther G W, Cortez D, Pendergast A M, Baldwin A S., Jr A requirement for NF-kappaB activation in Bcr-Abl-mediated transformation. Genes Dev. 1998;12:968–981. doi: 10.1101/gad.12.7.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 49.Romashkova J A, Makarov S S. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 50.Schmitz M L, Baeuerle P A. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. EMBO J. 1991;10:3805–3817. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serrano M, Lin A W, McCurrach M E, Beach D, Lowe S W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 52.Sizemore N, Leung S, Stark G R. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-κB p65/RelA subunit. Mol Cell Biol. 1999;19:4798–4805. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stambolic V, Suzuki A, de la Pompa J L, Brothers G M, Mirtsos C, Sasaki T, Ruland J, Penninger J M, Siderovski D P, Mak T W. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 54.Steck P A, Pershouse M A, Jasser S A, Yung W K, Lin H, Ligon A H, Langford L A, Baumgard M L, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng D H, Tavtigian S V. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 55.Tang E D, Nuñez G, Barr F G, Guan K L. Negative regulation of the Forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 56.Van Antwerp D J, Martin S J, Verma I M, Green D R. Inhibition of TNF-induced apoptosis by NF-kappa B. Trends Cell Biol. 1998;8:107–111. doi: 10.1016/s0962-8924(97)01215-4. [DOI] [PubMed] [Google Scholar]
- 57.Wang C-Y, Mayo M W, Baldwin A S., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 58.Wang C-Y, Mayo M W, Korneluk R G, Goeddel D V, Baldwin A S., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 59.Wang C-Y, Cusack J C, Liu R, Baldwin A S., Jr Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med. 1999;5:412–417. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- 59a.Wang C-Y, Guttridge D C, Mayo M W, Baldwin A S., Jr NF-κB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol. 1999;19:5923–5929. doi: 10.1128/mcb.19.9.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang D, Baldwin A S., Jr Activation of nuclear factor-kappaB-dependent transcription by tumor necrosis factor-alpha is mediated through phosphorylation of RelA/p65 on serine 529. J Biol Chem. 1998;273:29411–29416. doi: 10.1074/jbc.273.45.29411. [DOI] [PubMed] [Google Scholar]
- 61.Welch H, Eguinoa A, Stephens L R, Hawkins P T. Protein kinase B and rac are activated in parallel within a phosphatidylinositide 3OH-kinase-controlled signaling pathway. J Biol Chem. 1998;273:11248–11256. doi: 10.1074/jbc.273.18.11248. [DOI] [PubMed] [Google Scholar]
- 62.Whang Y E, Wu X, Suzuki H, Reiter R E, Tran C, Vessella R L, Said J W, Isaacs W B, Sawyers C L. Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression. Proc Natl Acad Sci USA. 1998;95:5246–5250. doi: 10.1073/pnas.95.9.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.White M A, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler M H. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 64.Yano S, Tokumitsu H, Soderling T R. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature. 1998;396:584–587. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- 65.Yin M J, Christerson L B, Yamamoto Y, Kwak Y T, Xu S, Mercurio F, Barbosa M, Cobb M H, Gaynor R B. HTLV-I Tax protein binds to MEKK1 to stimulate IkappaB kinase activity and NF-kappaB activation. Cell. 1998;93:875–884. doi: 10.1016/s0092-8674(00)81447-6. [DOI] [PubMed] [Google Scholar]
- 66.Zandi E, Karin M. Bridging the gap: composition, regulation, and physiological function of the IκB kinase complex. Mol Cell Biol. 1999;19:4547–4551. doi: 10.1128/mcb.19.7.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zha J, Harada H, Yang E, Jockel J, Korsmeyer S J. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3, not Bcl-X1. Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 68.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. The transcriptional activity of NF-kappaB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell. 1997;89:413–424. doi: 10.1016/s0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 69.Zhong H, Voll R E, Ghosh S. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998;1:661–671. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- 70.Zong W X, Edelstein L C, Chen C, Bash J, Gelinas C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev. 1999;13:382–387. doi: 10.1101/gad.13.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]