To the Editor:
Extracorporeal membrane oxygenation (ECMO) is an established treatment option for severe acute respiratory failure (1). In the context of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic with the occurrence of many severe acute respiratory distress syndrome cases, ECMO is increasingly being used worldwide depending on the available resources. Data from high-volume centers show that ECMO therapy may reduce the in-hospital mortality rate of ventilated patients who would otherwise reach more than 50–80% mortality (2, 3). When 10,021 hospitalized patients being treated in 920 different German hospitals during the first wave of the pandemic were analyzed, ECMO was reportedly used in 119 patients (1.2%) with a mortality rate of 71% (4). In contrast, a recent worldwide meta-analysis revealed a lower in-hospital mortality rate of 37% in 1,896 patients (5). The recent data of the European Extracorporeal Life Support Organization point in the same direction (6). The aim of the current research letter was to determine the in-hospital mortality rate during the first and second coronavirus disease (COVID-19) waves in Germany, a country that maintained quantitively sufficient healthcare resources during the pandemic without major restrictions.
We therefore report unbiased and unselected follow-up claims data of the largest German health insurance company, including a total of 768 patients with COVID-19 who underwent ECMO admitted to hospitals between February and December 2020. The largest German health insurance provider, Allgemeine Ortskrankenkasse, provides statutory health insurance for roughly 32% of the German population. All patients included in the study completed the hospital treatment and either died or were discharged from the hospital.
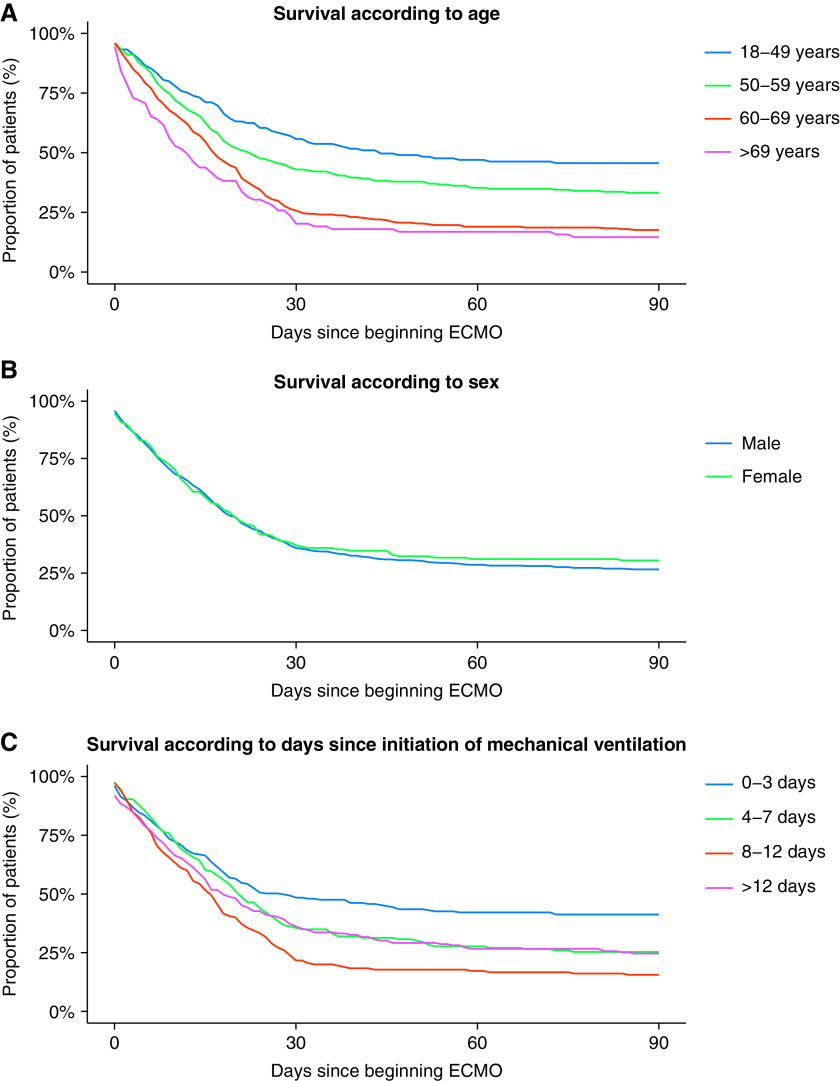
Baseline characteristics of the patients are depicted in Table 1. All patients included into the analysis had SARS-CoV-2 infection confirmed by PCR at a time when variants of concern were almost not present in Germany. The mean age of the patients reached 58 years (SD: 11 yr), 78% of whom were men with a median length of hospital stay of 44 days (SD: 39 d) and a mean length of ventilation time of 31 days (SD: 24 d). Sixty-one percent of patients were tracheotomized and 60% required dialysis. Unfortunately, in-hospital mortality reached 73%. In more detail, in-hospital mortality was 56% for patients 18–49 years of age, 67% for patients 50–59 years of age, 83% for patients 60–69, and 88% for patients >69 years of age (Figure 1A, P < 0.05 for all groups compared with the youngest age group). We found no significant survival difference between men and women (Figure 1B, P = 0.47). Regarding the time of initiation of ECMO therapy after onset of mechanical ventilation (either noninvasive or invasive, Figure 1C), we found the lowest mortality in those patients with early onset of ECMO therapy within the first 3 days after initiation of mechanical ventilation. However, no linear relation was observed, although all other groups demonstrated significantly higher mortality rates (Figure 1C, P < 0.05). We also had no information on the time from infection to intubation or ECMO therapy.
Table 1.
Baseline Characteristics of Patients Being Treated with ECMO according to Time of ECMO Initiation after Establishment of Invasive or Noninvasive Ventilation
| Initiation of ECMO Therapy according to Time Point after Initiation of Noninvasive or Invasive Mechanical Ventilation |
|||||
|---|---|---|---|---|---|
| Variable | All Patients | Days 0–3 | Days 4–7 | Days 8–12 | >12 Days |
| Patients, n | 768 | 223 | 166 | 180 | 199 |
| Age, mean (SD), yr | 57.7 (11.4) | 54.5 (13.4) | 57.7 (10.8) | 59.5 (10.5) | 59.8 (9.6) |
| Sex, M, n (%) | 601 (78.3) | 164 (73.5) | 123 (74.1) | 141 (78.3) | 173 (86.9) |
| Length of hospital stay, mean (SD), d | 44.0 (38.7) | 39.7 (36.1) | 37.0 (27.9) | 39.9 (32.9) | 58.4 (49.4) |
| Ventilation, mean (SD), d | 30.9 (23.7) | 23.1 (19.2) | 27.5 (19.6) | 29.1 (19.3) | 44.1 (29.2) |
| Tracheostomy, n (%) | 466 (60.7) | 117 (52.5) | 89 (53.6) | 115 (63.9) | 145 (72.9) |
| Dialysis, n (%) | 462 (60.2) | 123 (55.2) | 108 (65.1) | 103 (57.2) | 128 (64.3) |
| In-hospital mortality, n (%) | 564 (73.4) | 133 (59.6) | 126 (75.9) | 153 (85.0) | 152 (76.4) |
| Charlson comorbidity index, n (%) | |||||
| 0 | 148 (19.3) | 42 (18.8) | 30 (18.1) | 36 (20.0) | 40 (20.1) |
| 1 | 219 (28.5) | 68 (30.5) | 37 (22.3) | 53 (29.4) | 61 (30.7) |
| 2 | 162 (21.1) | 44 (19.7) | 40 (24.1) | 40 (22.2) | 38 (19.1) |
| 3–4 | 166 (21.6) | 48 (21.5) | 41 (24.7) | 37 (20.6) | 40 (20.1) |
| ⩾5 | 73 (9.5) | 21 (9.4) | 18 (10.8) | 14 (7.8) | 20 (10.1) |
Definition of abbreviation: ECMO = extracorporeal membrane oxygenation.
Figure 1.
(A–C) In-hospital survival according to age (A), sex (B), or days after onset of mechanical ventilation (C) (0–3, 4–7, 8–12, and >12 d). Regression analysis revealed that older age groups showed a significantly higher mortality rate than the age group of 18–49 years (P < 0.05; A); no significant difference between men and women (P = 0.47; B); and that all analyzed groups showed a significantly higher mortality rate than the group with the shortest time before initiation of mechanical ventilation (Days 0–3; P < 0.05; C). ECMO = extracorporeal membrane oxygenation.
What Might Be the Reasons for the High In-Hospital Mortality Rate in Patients with COVID-19 Who Underwent ECMO in Germany?
The mean age of 58 years with a strong predominance of men indicates the typical patient population of patients with COVID-19 in 2020 being infected with the wild type of the SARS-CoV-2 virus. As the average age of all patients with COVID-19 in German ICUs in 2020 was 68 years (4), the current analysis reveals that patients on ECMO were substantially younger. However, mean age was still 6 years higher than in the recent meta-analysis (5), and age is one of the most important, if not the most important, risk factors for a poor prognosis in COVID-19 (7). This might explain in part the worse outcome reported in this letter. Compared with the data of the European Extracorporeal Life Support Organization registry (8) and the French data (3), the higher observed mortality rate may not only be explained by the higher age (58 years vs. 52 and 49 years, respectively), prolonged median time from onset of mechanical ventilation to ECMO, increased proportion of patients with noninvasive or invasive mechanical ventilation for more than 10 days, increased severity of COVID-19 with a need for dialysis (60% vs. 43% and 44%, respectively), and heterogeneous experiences of ICUs deploying ECMO treatment. Importantly, the French group recently suggested that shorter time between intubation and ECMO, younger age, and treatment in centers managing at least 30 venovenous ECMO cases annually were independently associated with improved 90-day survival. However, all these factors do not fully explain the huge mortality difference even in patients aged below 49 years. It might partly be that more device-associated complications occurred, as COVID-19 severely affects coagulation. A critical future analysis should put a special emphasis on sooner recognizing coagulation-associated severe complications, which might reduce mortality.
Another reason for the high in-hospital mortality rate might be that indications for the insertion of ECMO are still influenced by individual attitudes, emotions, and the healthcare system’s strategies (resources, reimbursement, or expectations by the media). In a recent survey on ethical factors determining ECMO allocation during the COVID-19 pandemic compared with usual times (9), ECMO specialists from various countries reported giving more ethical weight to the benefit of ECMO to other patients not yet admitted, whereas before the pandemic they tended to focus more on the individual patient. Most of those taking part in the survey reported that when thinking about decisions about ECMO, it is most important to do the most good overall, even if that means not giving every patient an equal chance. Such an attitude was present under the circumstances of limited resources, which was not applicable in Germany.
We hypothesize that in Germany—on the basis of a well-prepared and fitted healthcare system—these attitudes on maximal care for the individual patient without fears of resource restrictions might have influenced a liberal indication for ECMO, whereas in other countries these indications were handled much more restrictively (possibly resulting in less “futile” applications). Second, in contrast to other healthcare systems, in Germany the concept of “clinical freedom of physicians” to “meet the health needs of the population” (10) is a highly ranked feature, and physicians mostly have a free decision—for example, to indicate ECMO—without any commercial or legal restrictions. Third, such a freedom of application of various highly sophisticated techniques like ECMO might result in a lack of a stringent quality control, such as for mandatory registries or certifications. Such a lack could lead to a certain variation in assessment and securing of quality criteria in connection with ECMO treatment regimens.
Our data confirm that ICU treatment in these critically ill patients with COVID-19 with a high mortality risk requires extended ICU resources, reflected by a mean length of ventilation of 31 days (SD: 24 d) beside ECMO therapy. Thus, ECMO indications for COVID-19 need to be considered cautiously, particularly in the pandemic context with at least regionally limited resources. Although mortality rate reasonably increases with higher age and prolonged time from initiation of ventilation to ECMO, an individual patient-centered decision has yet to be considered, as long-term survival after immediate hospital discharge seems to be preferential.
As a limitation of the analysis, the claims data do not include a high granularity of data, especially for use of prone positioning or neuromuscular blockade. However, the high mortality in general limits an adjustment for various confounders.
What Lessons Do We Learn from This High Mortality during the Pandemic?
An individual patient-centered decision for or against ECMO therapy during a pandemic with less resources and high pressure seems to be even more difficult. Dedicated inclusion and exclusion criteria and standardization of treatment regimens might be key for wise selection of patients to reduce the high in-hospital mortality observed now in Germany during the first and second wave of the pandemic. It also seems reasonable to harmonize and strengthen criteria for ECMO initiation, in particular under the pressure of decreased human resources caused by the pandemic. This might help to prevent a shortage of ICU resources, improve survival rates, and reduce the emotional stress caused by high mortality rates in patients with a high risk of death.
Footnotes
Supported by grants from the German Ministry of Research and Education (C.K.).
Originally Published in Press as DOI: 10.1164/rccm.202105-1145LE on July 20, 2021
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. EOLIA Trial Group, REVA, and ECMONet. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 2. Shekar K, Slutsky AS, Brodie D. ECMO for severe ARDS associated with COVID-19: now we know we can, but should we? Lancet Respir Med. 2020;8:1066–1068. doi: 10.1016/S2213-2600(20)30357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lebreton G, Schmidt M, Ponnaiah M, Folliguet T, Para M, Guihaire J, et al. Paris ECMO-COVID-19 investigators. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: a multicentre cohort study. Lancet Respir Med. doi: 10.1016/S2213-2600(21)00096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8:853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramanathan K, Shekar K, Ling RR, Barbaro RP, Wong SN, Tan CS, et al. Extracorporeal membrane oxygenation for COVID-19: a systematic review and meta-analysis. Crit Care. 2021;25:211. doi: 10.1186/s13054-021-03634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Broman LM, Eksborg S, Coco VL, De Piero ME, Belohlavek J, Lorusso R. EuroECMO COVID-19 Working Group; Euro-ELSO Steering Committee. Extracorporeal membrane oxygenation for COVID-19 during first and second waves. Lancet Respir Med. doi: 10.1016/S2213-2600(21)00262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, et al. Extracorporeal Life Support Organization. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dao B, Savulescu J, Suen JY, Fraser JF, Wilkinson DJC. Ethical factors determining ECMO allocation during the COVID-19 pandemic. BMC Med Ethics. 2021;22:70. doi: 10.1186/s12910-021-00638-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Busse R, Blümel M. Germany: Health system review. Health Syst Transit. 2014;16:1–296, xxi. [PubMed] [Google Scholar]