Hospital-free days (HFDs) is increasingly selected as the primary or secondary outcome in clinical trials among critically and seriously ill patients. This outcome measure, alternatively known as days alive and outside the hospital (DAOH), was first used as a primary outcome in the 2005 ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) Trial assessing the effectiveness of pulmonary artery catheterization in the management of congestive heart failure (1). Since then, DAOH or HFDs have been used as a primary outcome in several studies across a broad range of medical (2–7) and surgical conditions (8). HFDs offers many advantages to traditional endpoints used in clinical trials but also presents potential challenges. In this perspective, we highlight important considerations relevant to HFDs using real-world examples from recent and upcoming clinical trials. In doing so, we identify opportunities for future scholarship to advance HFDs as a patient-centered clinical outcome measure.
Definition and Nomenclature
We define HFDs as all days alive that are spent outside of an acute-care hospital, long-term acute-care hospital (LTACH), or in an emergency department (ED), including days spent wholly or in part under “observation” status. All other days, including days spent in a long- or short-stay nursing facility, inpatient hospice facility, or rehabilitation facility count as hospital-free, as would all days at home, including those with home-based medical services (Table 1). This definition aligns with how others have operationalized DAOH (1–4). However, we advocate specifically for the term HFDs based on discussions with seriously ill patients and caregivers who joined a Patient and Family Advisory Committee guiding a recently proposed pragmatic randomized trial using HFDs as the primary outcome. These stakeholders preferred the term HFDs over DAOH for two reasons. First, patients and caregivers noted that the phrase “hospital-free” emphasizes the value they place on avoiding hospitalization and emergency room visits. Second, the phrase “days alive” was viewed as overemphasizing survival as the primary goal, which stakeholders identified as a common characteristic of traditional measures that (intentionally or not) fail to recognize that patients and families value multiple health outcomes as equal to or worse than death (5–7).
Table 1.
Example Calculations of HFDs for Patients with Different Acute Care Use Patterns during 6 mo of Follow-up
| HFD Value | Compatible Case Description(s) |
|---|---|
| 0 | 1. Patient dies during initial hospitalization |
| 2. Patient remains in acute care hospital or long-term acute care hospital for 6 mo without dying | |
| 1–178 | 1. Patient discharged 4 d after enrollment and lives 6 mo, during which she experiences 1 readmission of 7 d plus 2 emergency room visits of 1 d each (HFDs = 169) |
| 2. Patient discharged 4 d after enrollment to a long-term acute care hospital where she resides for 14 d. She is subsequently discharged home and experiences 1 readmission from Day 90–95 and dies on Day 100 (HFDs = 76) | |
| 175–180 | 1. Patient discharged to acute rehabilitation facility after initial hospitalization of 5 d or fewer who is subsequently discharged home and then survives 6 mo without additional hospital contact |
Definition of abbreviation: HFDs = hospital-free days.
Why Consider HFDs?
Interest in improving outcome measures for trials of patients with critical and serious illness is driven by the paucity of patient-centered outcomes that can be reliably measured in trials among patients at high risk of death. Mortality endpoints require large sample sizes (8, 9) and wrongly assume that death is the worst possible outcome for all critically and seriously ill patients (5–7). Longitudinal measures, such as quality of life or length of stay, are often informatively missing because of death and selective loss to follow-up, thereby introducing a high potential for biased results (10–13). Traditional “duration” endpoints (e.g., length of stay or duration of mechanical ventilation) can be misleading because an intervention that reduces mortality may paradoxically lengthen the average lengths of stay or duration of mechanical ventilation. Composite endpoints, including organ-failure–free days, suffer from similarly weighting dissimilar outcomes (14, 15), have not been shown to be patient-centered, and tend to be most applicable to narrowly defined populations. Finally, despite the prioritization of “goal-concordant care” as a top outcome metric in serious illness research (16), including designation as one of five recommended practices by the 2021 Critical Care Choosing Wisely group (17), methods to reliably and efficiently measure this construct remain elusive (18, 19).
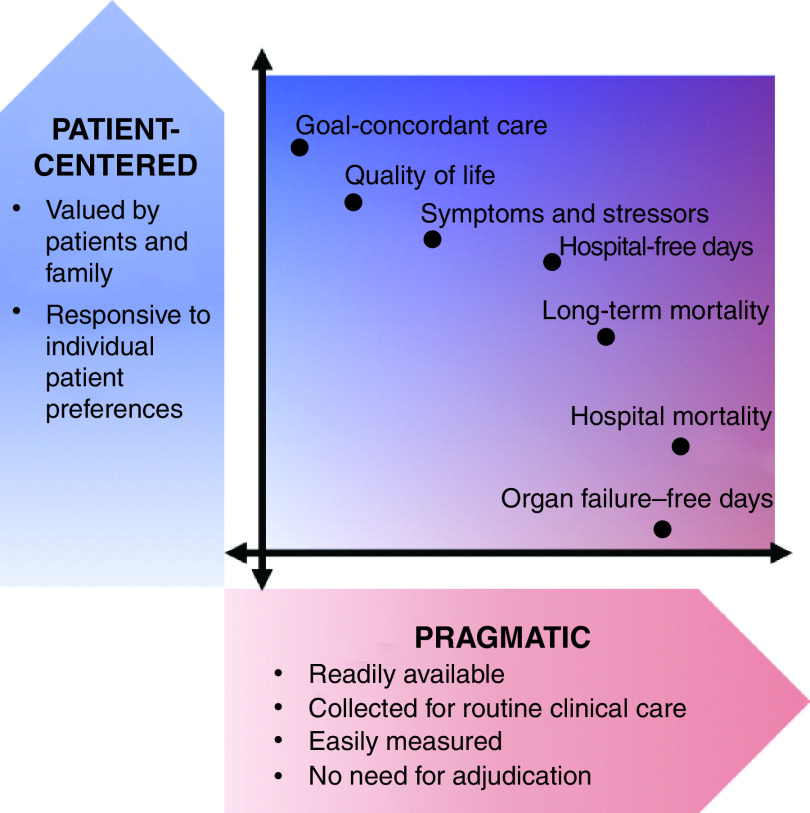
By contrast, HFDs is both highly patient centered and relatively pragmatic to measure (Figure 1). HFDs are patient centered because nearly all patients prefer longer lives to shorter ones and to have more of those days spent outside a hospital than within (6, 20, 21). Although short-term hospitalizations may be preferable to the experience of inadequately managed symptoms at home, HFDs will consistently be preferred when measured over a sufficient time horizon. HFDs can be reliably measured using pragmatic data sets such as payers’ claims data, states’ hospital utilization data, or health systems’ electronic health record (EHR) data—data that are typically available at relatively low cost without substantial missingness (22, 23).
Figure 1.
Conceptual model depicting the trade-offs between degree of patient centeredness and pragmatism of various outcome measures commonly used in or proposed for clinical trials. Relative rankings reflect a combination of published research on stakeholders’ values, the authors’ engagement of a patient and family advisory committee, and the authors’ experiences as clinicians and researchers.
An additional advantage of HFDs over traditional outcome measures is that it enables direct comparison of interventions with either restorative or palliative intents. Too often, interventions with restorative intents are evaluated in terms of survival, whereas interventions with palliative intents are evaluated in terms of quality of life or other patient- or caregiver-reported outcomes. However, neither choice enables comparisons between interventions that are primarily restorative or palliative in nature, which often represents the clinically relevant decision frame for seriously and critically ill patients. For example, older patients with advanced chronic obstructive pulmonary disease might face reasonable choices between high-risk lung transplantation or home-based palliative care. To inform this choice, a trial could compare HFDs between patients receiving each treatment. HFDs would capture a potential transplant-associated mortality benefit alongside a possibly lengthy postoperative hospital stay and perhaps subsequent readmissions for side effects, pain management, or debility. In summary, because HFDs can be increased by interventions that improve survival time, functional recovery, or avoidance of burdensome interventions, it provides a uniform metric by which to compare seemingly divergent interventions.
What Constitutes a Hospital Day?
We consider observation days, other ED days, and LTACH days as identical to full acute-care hospital admission days because all such days occur in an acute-care hospital setting and may considerably limit patients’ abilities to experience their optimal quality of life. We account for LTACH days as extensions of acute-care hospital stays given the intensive care LTACHs provide to a complex patient population. Although survivors of LTACH stays after prolonged mechanical ventilation and spinal cord injury ultimately view this time as valuable (24, 25), data are not available regarding valuations by all patients undergoing such care. Notably missing from these data are valuations by the one-third of LTACH-admitted patients who die in the LTACH (26). Furthermore, in prolonged mechanical ventilation, LTACH days and ICU days are often used interchangeably, as hospitals with higher LTACH discharge rates have shorter ICU lengths of stay with similar outcomes. Future work revealing how all patients rate time spent in LTACHs and other facilities would enable more nuanced HFD measurement approaches (27). Such weightings may be particularly important for patients with refractory symptoms at the end of life, such as pain or dyspnea, that may be best managed in the inpatient, or even ICU, setting (28).
Related Constructs
It is important to distinguish the construct of HFDs as presented here from the outcome of 28-day HFDs as reported in prior trials in the critical care literature. For example, in the SALT-ED (Saline against Lactated Ringer's or Plasma-Lyte in the Emergency Department) trial (29) comparing different crystalloid solutions administered in the ED among patients with sepsis, the primary outcome was DAOH from the index ED visit through 28 days later. Patients who died during the index hospitalization and those hospitalized for more than 28 days were classified as having zero HFDs. For patients discharged alive before Day 28, HFDs were censored at the time of hospital discharge such that HFDs equaled 28 minus the hospital length of stay. We do not recommend using this censored approach because of information loss and conflation of dissimilar outcomes. For example, a patient discharged alive on Day 8 who dies on Day 11 and a patient discharged alive on Day 8 who survives for the next year without any further admissions would both incur 20 HFDs using this approach. Similar to organ-failure-support–free days, such as ventilator-free days, this representation of HFDs as a composite measure of mortality and hospital length of stay is challenging to interpret, as it assigns similar weight to outcomes that would be rated dissimilarly by patients (15). Without a sufficiently long time horizon and the ability to capture potentially important outcomes such as return ED visits and hospital readmissions, such measures are at best surrogates for patient-centered outcomes.
The CESAR (Conventional Ventilatory Support versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure) trial (30) comparing conventional ventilatory support and extracorporeal membrane oxygenation used another endpoint that differs importantly from our conceptualization of HFDs. In this trial, the primary outcome was death or severe disability at 6 months. Despite the virtue of the longer time horizon than used in SALT-ED (Saline against Lactated Ringer's or Plasma-Lyte in the Emergency Department), this binary endpoint equates deaths on the day after randomization or 6 months later, fails to account for the functional status or location of care between initial hospital discharge and the 6-month time point, and assumes that the authors’ definition of severe disability would be rated as a state equal to death at 6 months.
Other related outcome measures also have important differences from HFDs. Measures of institution-free days, such as “home time” (31) or “days at home” (21, 32), assign zero value to time spent in an any institutional setting such as a rehabilitation facility, nursing home, or inpatient hospice. The Centers for Medicare and Medicaid are currently exploring a “healthy days at home” measure that additionally discounts days spent receiving home health or home hospice services (23, 33). However, several arguments suggest that such days should be counted as equivalent to days at home. First, for many underresourced populations, the home may not be sufficiently comfortable or equipped to be preferred over such facilities. Thus, measures that discount days spent in nonhome facilities might propagate or create disparities by disincentivizing care for persons with fewer opportunities for home-based care. Second, reliably measuring days spent in these facilities requires access to data that may significantly reduce the pragmatism of the measure and may introduce bias owing to differential missingness. Finally, and most importantly, days spent in rehabilitation and short-stay nursing facilities may be valued positively because they tend to hasten recovery, prevent rehospitalization, and extend survival (34–36). As different measures of HFDs and home time gain popularity, determining the patient-reported value of each type of healthcare day and evaluating their equity when used across heterogeneous demographics will be essential. When data are available, investigators should consider conducting sensitivity analyses to assess institution-free days (also termed “home time”) alongside HFDs to more fully appreciate the impact of interventions on post–acute care and well-being.
Data Collection and Measurement
An important virtue of HFDs is its facility of use in pragmatic randomized trials, in which outcome assessment using existing data sources is a substantive feature (37). Indeed, HFDs will often be calculated from algorithmic evaluation of administrative and EHR data. For example, in the PROSPER (Patient-centered Research into Outcomes Stroke patients Prefer and Effectiveness Research) study of statin prescription at hospital discharge among more than 75,000 patients admitted with an ischemic stroke (38), the primary outcome, termed DAOH in this study, was collected by linking registry data with Medicare claims data, including Medicare skilled nursing facility files given the investigators’ choice to consider days in such facilities as equivalent to hospital days. In another example, a randomized trial of default options in advance directives among 515 patients with serious illnesses (39), HFDs were calculated using a combination of EHR data, the Social Security Death Index, and state databases that capture all hospital admissions.
Comparisons of HFDs can also be obtained through secondary analyses of the adverse event data already collected for many clinical trials. This approach was demonstrated in a reanalysis of data from TRILOGY-ACS (Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes), a randomized, multinational clinical trial comparing antiplatelet agents among over 9,000 patients with non-ST elevation myocardial infarction (4). This study demonstrated the feasibility of assessing HFDs using site-submitted adverse event reporting data with a median follow-up of 17 months.
Medicare claims data can be especially helpful for identifying hospital days across institutions and capturing time spent in an LTACH (26, 40). Unfortunately, even in some integrated health systems, LTACH data are often not easily obtained through EHR data alone. Thus, although there are strong conceptual reasons to exclude LTACH days from counts of HFDs, accounting for these days may decrease pragmatism depending on the available data and population being studied. Thus, improved methods are needed to reliably assess LTACH admissions and lengths of stay from non-Medicare data sources, particularly for patients experiencing chronic critical illness (41).
Are All HFDs Equal?
Although our definition of HFDs is more patient centered than many traditional outcomes, it does not account for the functional, quality-of-life, and social effects prioritized by many patients and their families (5, 6, 42, 43). Thus, novel methods to integrate quality weighting of days into HFDs assessments could improve the patient centeredness of this outcome measure. Integrating quality weights of days may also facilitate comparisons of interventions with palliative versus restorative intents by more accurately defining “good” days, which may include inpatient stays that enable relief of acute symptoms, foster additional rehabilitation, or serve genuine interests of patients in feeling that all possible restorative approaches had been attempted (44). Still, research is needed to demonstrate the extents to which such virtues manifest with quality weighting as well as whether pragmatic approaches to obtaining quality weights, such as through mobile applications, can sufficiently preserve the measure’s utility in low-cost pragmatic trials.
When to Start and Stop Counting HFDs?
The time when HFDs begin to accrue and the appropriate duration of follow-up will vary based on the research question, the timing of the intervention (or exposure) of interest, and the expected effects of the intervention. Careful attention is needed when defining the start and stop times to mitigate bias and enable the observation of important treatment effects.
In general, outcome ascertainment should not begin before patients have had the opportunity to receive the trial intervention. For most trials enrolling hospitalized patients, HFDs have included the index hospitalization. However, depending on the population or intervention being studied, there may be justification for excluding all or part of the index hospitalization from outcome ascertainment. For example, studies of an intervention designed to occur near hospital discharge might exclude days earlier in the hospitalization to avoid immutable time bias (11).
The duration of follow-up used to measure HFDs is another important consideration. To date, studies have evaluated HFDs through 30 days and for up to 2 years, depending on the population under study and the expected impact of the intervention. HFDs through 30 and 90 days have been used frequently in trials of critically ill (45, 46) and surgical populations (47), with HFDs to Day 90 recently identified as a key, though not yet adequately validated, endpoint for phase II trials in critically ill patients by the Australian and New Zealand Intensive Care Society Clinical Trials Group (48).
Significantly longer durations of follow-up were used in the aforementioned PROSPER trial, which assessed DAOH at 2 years after index hospital discharge (38), and in the trial of default options in advance directives, which compared HFDs during a median follow-up of 18 months (39). This latter trial established a final date for follow-up, such that patients enrolled earlier were followed longer. This approach is statistically efficient as long as patients are randomly assigned to treatment arms in equal proportions across the enrollment period, as may be achieved through blocked randomization. Selected real-world trials with various durations of follow-up and underlying rationale are presented in Table 2.
Table 2.
Selected Real-World Clinical Studies Demonstrating the Variability in Start of Accrual and Follow-up Duration for HFDs
| Trial | Study Summary | Start of Accrual of HFDs | Follow-up Duration | Notes on Measurement and Interpretation |
|---|---|---|---|---|
| IMPACTS (45) | Pragmatic RCT comparing clinical outcomes between sepsis survivors who receive usual care versus care delivered through the Sepsis Transition and Recovery Program | Index hospital discharge | 30 and 90 d | Excluding the index hospital stay is appropriate to avoid immutable time bias because intervention begins close to discharge |
| SPRY-Metformin (47) | Adaptive clinical trial of perioperative metformin doses among high-risk surgical patients | Day of surgery | 90 d | Follow-up duration will capture most but not all prolonged postsurgical stays and complications and readmissions related to the surgery or study interventions |
| Selepressin for septic shock (46) | Blinded, adaptive RCT of selepressin for adults in septic shock | Not specified | 90 d | Accrual of HFDs should begin at randomization to fully capture the effects of the intervention |
| PROSPER (38) | Effect of statin prescription at hospital discharge among patients admitted with an ischemic stroke | Index hospital discharge | 2 yr | Longer follow-up times important when studying conditions with known persistent risk of rehospitalization or decompensation |
| Default options in ADs (39) | RCT comparing impact ADs with preselected comfort-focused care or life-extending care to standard (blank) ADs among patients with serious illness | Enrollment | 18 mo* | Longer follow-up times important when studying interventions that begin in and/or persist into the ambulatory setting |
| COVID-19 monitoring trial (60) | Trial evaluating the effect of an automated remote monitoring program for home-dwelling adults diagnosed with COVID-19 | Day of ED discharge | 30 d | Choice of 30-d facilitates complete data capture. However, if intervention saves lives by recommending earlier hospital evaluation for decompensating patients, it may have no effect on (or even worsen) HFDs during this short time horizon |
Definition of abbreviations: ADs = advance directives; COVID-19 = coronavirus disease; ED = emergency department; HFDs = hospital-free days; IMPACTS = Improving Morbidity during Post-Acute Care Transitions for Sepsis; PROSPER = Patient-centered Research into Outcomes Stroke Patients Prefer and Effectiveness Research; RCT = randomized clinical trial; SPRY = Strategies to Promote Resiliency.
Study established a final date for follow-up, such that patients enrolled earlier were followed longer. Median follow-up was 18 mo, with an interquartile range of 11–27 mo and total range of 6–35 mo.
Longer follow-up times may also be appropriate for mechanically ventilated patients with acute respiratory distress syndrome and other forms of respiratory failure, in whom somewhat lengthy initial hospitalizations may be required for long-term recovery. Longer follow-up would enable patient-centered benefits of interventions for these disorders to be realized while also distinguishing between patients who regain enough function to be discharged and those who remain alive but in states of chronic critical illness. It is particularly important to capture sufficient follow-up time such that most subjects have either died or been discharged to avoid recreating the problem of rating the disparate outcomes of death within the hospital and prolonged hospitalization equally. Longer durations of follow-up also have the added benefit of allowing sufficient time to capture repeat ED visits and hospitalizations, thereby minimizing the risk of observing small differences in HFDs owing only to changes in the index hospital length of stay, including those introduced by time spent awaiting post–acute care facility placement (11).
Given these considerations, and evidence that shorter endpoints might not predict longer ones (49), we agree with the Australian and New Zealand Intensive Care Society Clinical Trials Group recommendation to follow HFDs through at least Day 90 (48), particularly for trials of patients experiencing critical illness and acute lung injury. However, we also advocate for longer time horizons whenever feasible, pending future work into how different durations of follow-up affect the patient centeredness and statistical power of HFDs. In the meantime, there may be benefits to reporting HFDs at multiple, prespecified time points, chosen based on the interventions’ hypothesized effects.
Statistical and Analytic Considerations
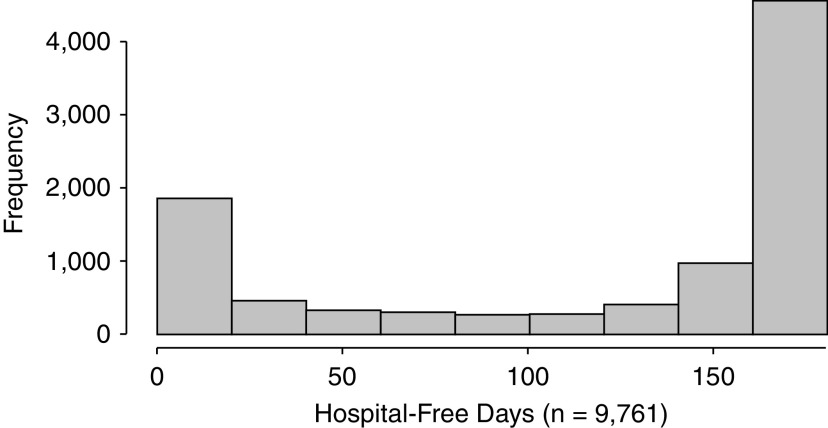
The statistical distributions of HFDs among seriously and critically ill patients present a final challenge that must be addressed for HFDs to be used broadly in clinical trials among such patients. In most populations, the distribution of HFDs will have a peak near the maximal value, restricting the count distribution. Several count-based models (e.g., negative binomial regression) can account for such peaks. However, among populations of patients with a high risk of mortality, including many critically ill or hospitalized populations, HFDs may have a second peak at or near zero, representing in-hospital deaths or inpatient stays that exceed the duration of follow-up. For example, to guide the calculation of statistical power in a recently proposed randomized clinical trial, we evaluated 9,761 patients admitted in 2019 to five hospitals in the University of Pennsylvania Health System who had a 6-month mortality risk ⩾30% based on an internally derived predictive model (50). As seen in Figure 2, 11.1% of these patients had 0 HFDs through 6 months.
Figure 2.
Hospital-free days through 6 months among 9,761 patients admitted to five hospitals in 2019 with ⩾30% risk of death within 6 months. Patients were identified using a validated electronic health record mortality prediction model based on age, sex, admission type, Elixhauser comorbidities, and laboratory values from the first 2 days of hospitalization.
There are three strategies for handling such distributions with overrepresentation of both zero and maximum values, and the choice of strategy is best determined by the clinical context and research question (51, 52). First, investigators may select a single model, such as a zero-inflated β-binomial model, that is applicable to restricted count data, accounts for the inflation in the proportion of zeros, and provides an overall intervention effect on the entire distribution of responses. Second, investigators could decompose the HFDs outcome into two parts—a binary outcome indicating whether each patient has any HFDs and a count outcome indicating the number of HFDs for patients with more than 0 HFDs—and then use an appropriate “two-part model.” (53–55) Third, investigators could again decompose the HFDs outcome into two parts but fit a “marginalized two-part model” to estimate the overall marginal mean intervention effect. In these latter two approaches (56), the value of the second part is subtracted from the maximum number of HFDs to estimate the number of days spent in the hospital (the inverse of the remaining HFDs, chosen to align with standard distributional assumptions), and an offset term is used to account for the number of days during follow-up. In addition to considering the distribution of HFDs in a particular context, investigators should ensure that the selected model’s estimands—the entity being measured—reflect the trial objectives and that their interpretation answers a clinically meaningful question.
These two-part models may be necessary for trials of interventions with both highly intensive and palliative intents. For example, consider a hypothetical trial comparing extracorporeal membrane oxygenation for patients with severe acute respiratory distress syndrome to standard of care. The intervention might decrease overall mortality but also increase hospital length of stay among survivors. Alternatively, a systems intervention in which specialist palliative care was integrated into the treatment of critically ill patients with advanced malignancies might increase hospital mortality by enabling earlier withdrawal of intensive care when its continuation is incompatible with patients’ goals. The same intervention might also increase HFDs among survivors by increasing uptake of home palliative care and reducing repeat hospitalizations at the end of life.
The unique distributional characteristics of HFDs also have implications for statistical power. Because HFDs is a continuous outcome that will often be measured reliably among all study participants, it should generally have greater statistical power than a dichotomous outcome or one with significant measurement error. However, particularly large peaks at either 0 or the maximal value of HFDs could reduce power, depending on the analytic model chosen, and simulation methods will generally be needed to estimate the required sample size before beginning a trial. An empiric assessment of different modeling strategies and their impact on statistical power and effect estimation could be undertaken using simulation studies (57, 58) and reanalyses of trial data to inform choices for future trials and provide guidance for the broader research community. Developing and testing strategies that add a quality weighting to days spent outside the hospital may also increase the variance of HFDs, thereby improving the chances of detecting treatment effects of a given size without increasing the sample size. Finally, future research will be needed to identify the minimal clinically important difference in HFDs at different time horizons, thereby informing these power simulations.
Conclusions
HFDs has been reported in numerous clinical studies, and multiple active trials of critically and seriously ill patients include HFDs as a primary or secondary outcome. Despite the advantages in patient centeredness and pragmatism offered by HFDs, important considerations remain about its definition, data collection, measurement, and analysis that warrant future investigation. Table 3 provides an overview of key considerations, with suggestions for current practice and future investigation. Of course, no single outcome measure can fully capture the diverse priorities of patients, clinicians, researchers, and policy makers (59). Thus, although HFDs may, with future development, prove to be a highly meritorious primary outcome in many trials of seriously and critically ill patients, it will remain important for trialists to also measure carefully selected secondary outcomes.
Table 3.
Barriers to Using HFDs as an Outcome Measure, Conceptual Arguments, and Proposed Solutions
| Key Considerations When Using HFDs | Conceptual Argument(s) | Proposed Solution(s) or Opportunity for Future Inquiry |
|---|---|---|
| HFDs might miss days spent under “observation status.” | From the patient’s point of view, days spent under observation compared to inpatient status are likely equivalent. | When using claims or EHR data, account for inpatient days spent under observation as hospital days. |
| HFDs consider all days spent in any acute care setting as equally bad. | Patients may not value time spent in each of these locations equivalently, particularly among populations with long hospitalizations in LTACHs. Some hospitalization days may also be of very high value, particularly if necessary for the relief of symptoms, such as refractory dyspnea. | Exploration of patient and family perspectives on how days spent in an acute care hospital compare to an ambulatory setting. |
| Development and validation of methods to quality-weight days spent in hospital settings. | ||
| Not all HFDs will be spent “at home,” and some of those days will involve the use of home health services. | For many underserved populations, the home may not be sufficiently resourced or comfortable to be preferred over an institutional setting. The use of home health services may be driven by patient preferences and differential ability to recover at home based on resources and access. | Empiric work to understand how the use HFDs, home time, or HDAH might propagate or create disparities. |
| Exploration of patient and family perspectives on the value of days spent in various post-acute care settings. | ||
| All HFDs are considered equally good. | Patients may not value all days spent outside of a hospital equivalently, particularly if there is variation in their level of comfort, function, or quality of life. | Development and validation of methods to quality-weight HFDs. |
| Optimal duration of follow-up is unknown, but we recommend at least 90 d and often longer. | If an intervention decreases mortality among the sickest patients, it may paradoxically increase hospital length of stay and thus HFDs if using shorter follow-up time. | Consideration of using multiple endpoints, all prespecified and determined purposefully based on anticipated impact of intervention. |
| Empiric work to determine how different durations of follow-up affect the patient centeredness and statistical power of HFDs. | ||
| Distribution of HFDs, with peaks at 0 or near maximum values, present statistical modeling and interpretive challenges. | Interventions may have differential impact on likelihood of survival, index hospitalization length of stay, and risk of recurrent hospitalization during follow-up. | Simulation studies to understand how different modeling strategies might impact results and statistical power. |
| Testing of strategies to quality-weight HFDs to improve statistical power. |
Definition of abbreviations: EHR = electronic health record; HDAH = healthy days at home; HFDs = hospital-free days; LTACH = long-term acute-care hospital.
Footnotes
Supported by an NIH/NHLBI training grant (5T32HL098054-09) and an NIH/NHLBI Loan Repayment Program award (C.L.A.); National Institute of Nursing Research (R01NR018434) and National Libraries of Medicine (R21LM013373) (S.P.T.); NIH/NHLBI grant R00HL141678 and a Patient-Centered Outcomes Research Institute Project Program Award (ME-2020C1-19220) (M.O.H.); NIH/NHLBI grant K23HL143181 (K.R.C.); and NIH/NHLBI grant K24HL143289 (S.D.H.). All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the NIH, Patient-Centered Outcomes Research Institute, or its Board of Governors or Methodology Committee.
Originally Published in Press as DOI: 10.1164/rccm.202104-1063PP on July 28, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Binanay C, Califf RM, Hasselblad V, O’Connor CM, Shah MR, Sopko G, et al. ESCAPE Investigators and ESCAPE Study Coordinators. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 2. Ariti CA, Cleland JGF, Pocock SJ, Pfeffer MA, Swedberg K, Granger CB, et al. Days alive and out of hospital and the patient journey in patients with heart failure: Insights from the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) program. Am Heart J. 2011;162:900–906. doi: 10.1016/j.ahj.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 3. Lopes RD, Macedo AVS, de Barros E Silva PGM, Moll-Bernardes RJ, Dos Santos TM, Mazza L, et al. BRACE CORONA Investigators. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. 2021;325:254–264. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fanaroff AC, Cyr D, Neely ML, Bakal J, White HD, Fox KAA, et al. Days alive and out of hospital: exploring a patient-centered, pragmatic outcome in a clinical trial of patients with acute coronary syndromes. Circ Cardiovasc Qual Outcomes. 2018;11:e004755. doi: 10.1161/CIRCOUTCOMES.118.004755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rubin EB, Buehler AE, Halpern SD. States worse than death among hospitalized patients with serious illnesses. JAMA Intern Med. 2016;176:1557–1559. doi: 10.1001/jamainternmed.2016.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Auriemma CL, Harhay MO, Haines KJ, Barg FK, Halpern SD, Lyon SM. What matters to patients and their families during and after critical illness: a qualitative study. Am J Crit Care. 2021;30:11–20. doi: 10.4037/ajcc2021398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rubin EB, Buehler A, Halpern SD. Seriously ill patients’ willingness to trade survival time to avoid high treatment intensity at the end of life. JAMA Intern Med. 2020;180:907–909. doi: 10.1001/jamainternmed.2020.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spragg RG, Bernard GR, Checkley W, Curtis JR, Gajic O, Guyatt G, et al. Beyond mortality: future clinical research in acute lung injury. Am J Respir Crit Care Med. 2010;181:1121–1127. doi: 10.1164/rccm.201001-0024WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Auriemma CL, Zhuo H, Delucchi K, Deiss T, Liu T, Jauregui A, et al. Acute respiratory distress syndrome-attributable mortality in critically ill patients with sepsis. Intensive Care Med. 2020;46:1222–1231. doi: 10.1007/s00134-020-06010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fielding S, Ogbuagu A, Sivasubramaniam S, MacLennan G, Ramsay CR. Reporting and dealing with missing quality of life data in RCTs: has the picture changed in the last decade? Qual Life Res. 2016;25:2977–2983. doi: 10.1007/s11136-016-1411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harhay MO, Ratcliffe SJ, Halpern SD. Measurement error due to patient flow in estimates of intensive care unit length of stay. Am J Epidemiol. 2017;186:1389–1395. doi: 10.1093/aje/kwx222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scharfstein D, Robins JM, Eddings W, Rotnitzky A. Inference in randomized studies with informative censoring and discrete time-to-event endpoints. Biometrics. 2001;57:404–413. doi: 10.1111/j.0006-341x.2001.00404.x. [DOI] [PubMed] [Google Scholar]
- 13. Wang C, Scharfstein DO, Colantuoni E, Girard TD, Yan Y. Inference in randomized trials with death and missingness. Biometrics. 2017;73:431–440. doi: 10.1111/biom.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tomlinson G, Detsky AS. Composite end points in randomized trials: there is no free lunch. JAMA. 2010;303:267–268. doi: 10.1001/jama.2009.2017. [DOI] [PubMed] [Google Scholar]
- 15. Yehya N, Harhay MO, Curley MAQ, Schoenfeld DA, Reeder RW. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med. 2019;200:828–836. doi: 10.1164/rccm.201810-2050CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sudore RL, Heyland DK, Lum HD, Rietjens JAC, Korfage IJ, Ritchie CS, et al. Outcomes that define successful advance care planning: a Delphi Panel consensus. J Pain Symptom Manage. 2018;55:245–255.e8. doi: 10.1016/j.jpainsymman.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Society of Critical Care Medicine. https://www.choosingwisely.org/societies/society-of-critical-care-medicine/
- 18. Halpern SD. Goal-concordant care—searching for the holy grail. N Engl J Med. 2019;381:1603–1606. doi: 10.1056/NEJMp1908153. [DOI] [PubMed] [Google Scholar]
- 19. Ernecoff NC, Wessell KL, Bennett AV, Hanson LC. Measuring goal-concordant care in palliative care research. J Pain Symptom Manage. 2021;62:e305–e314. doi: 10.1016/j.jpainsymman.2021.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sayer C.https://catalyst.nejm.org/doi/abs/10.1056/CAT.16.0854
- 21. Groff AC, Colla CH, Lee TH. Days spent at home—a patient-centered goal and outcome. N Engl J Med. 2016;375:1610–1612. doi: 10.1056/NEJMp1607206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fonarow GC, Liang L, Thomas L, Xian Y, Saver JL, Smith EE, et al. Assessment of home-time after acute ischemic stroke in medicare beneficiaries. Stroke. 2016;47:836–842. doi: 10.1161/STROKEAHA.115.011599. [DOI] [PubMed] [Google Scholar]
- 23. Burke LG, Orav EJ, Zheng J, Jha AK. Healthy days at home: a novel population-based outcome measure. Healthc (Amst) 2020;8:100378. doi: 10.1016/j.hjdsi.2019.100378. [DOI] [PubMed] [Google Scholar]
- 24. Jubran A, Grant BJB, Duffner LA, Collins EG, Lanuza DM, Hoffman LA, et al. Long-term outcome after prolonged mechanical ventilation. A long-term acute-care hospital study. Am J Respir Crit Care Med. 2019;199:1508–1516. doi: 10.1164/rccm.201806-1131OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Charlifue S, Apple D, Burns SP, Chen D, Cuthbert JP, Donovan WH, et al. Mechanical ventilation, health, and quality of life following spinal cord injury. Arch Phys Med Rehabil. 2011;92:457–463. doi: 10.1016/j.apmr.2010.07.237. [DOI] [PubMed] [Google Scholar]
- 26. Makam AN, Tran T, Miller ME, Xuan L, Nguyen OK, Halm EA. The clinical course after long-term acute care hospital admission among older Medicare beneficiaries. J Am Geriatr Soc. 2019;67:2282–2288. doi: 10.1111/jgs.16106. [DOI] [PubMed] [Google Scholar]
- 27. Hall WB, Willis LE, Medvedev S, Carson SS. The implications of long-term acute care hospital transfer practices for measures of in-hospital mortality and length of stay. Am J Respir Crit Care Med. 2012;185:53–57. doi: 10.1164/rccm.201106-1084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rolnick JA, Ersek M, Wachterman MW, Halpern SD. The quality of end-of-life care among ICU versus ward decedents. Am J Respir Crit Care Med. 2020;201:832–839. doi: 10.1164/rccm.201907-1423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Self WH, Semler MW, Wanderer JP, Wang L, Byrne DW, Collins SP, et al. SALT-ED Investigators. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378:819–828. doi: 10.1056/NEJMoa1711586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 31. Van Houtven CH, Smith VA, Sperber NR, Coffman CJ, Hastings SN. Advancing the science of population-based measures of home-time. Healthc (Amst) 2020;8:100463. doi: 10.1016/j.hjdsi.2020.100463. [DOI] [PubMed] [Google Scholar]
- 32. Maclagan LC, Wu F, Liu N, Tanuseputro P, Stukel TA, Guan J, et al. Association between palliative care, days at home, and health care use in patients with advanced COPD: a cohort study. Ann Am Thorac Soc. 2021 doi: 10.1513/AnnalsATS.202007-859OC. [DOI] [PubMed] [Google Scholar]
- 33.Martin LT, Berdahl C, Burns RM, Hoch E, Peet ED, Hussey PS. Measures and methodology for international comparisons of health care system performance. 2021. [Google Scholar]
- 34. Feltner C, Jones CD, Cené CW, Zheng ZJ, Sueta CA, Coker-Schwimmer EJL, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160:774–784. doi: 10.7326/M14-0083. [DOI] [PubMed] [Google Scholar]
- 35. Connor SR, Pyenson B, Fitch K, Spence C, Iwasaki K. Comparing hospice and nonhospice patient survival among patients who die within a three-year window. J Pain Symptom Manage. 2007;33:238–246. doi: 10.1016/j.jpainsymman.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 36. Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 37. Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. doi: 10.1136/bmj.h2147. [DOI] [PubMed] [Google Scholar]
- 38. O’Brien EC, Greiner MA, Xian Y, Fonarow GC, Olson DM, Schwamm LH, et al. Clinical effectiveness of statin therapy after ischemic stroke: primary results from the statin therapeutic area of the patient-centered research into outcomes stroke patients prefer and effectiveness research (PROSPER) study. Circulation. 2015;132:1404–1413. doi: 10.1161/CIRCULATIONAHA.115.016183. [DOI] [PubMed] [Google Scholar]
- 39. Halpern SD, Small DS, Troxel AB, Cooney E, Bayes B, Chowdhury M, et al. Effect of default options in advance directives on hospital-free days and care choices among seriously ill patients: a randomized clinical trial. JAMA Netw Open. 2020;3:e201742. doi: 10.1001/jamanetworkopen.2020.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Makam AN, Nguyen OK, Miller ME, Shah SJ, Kapinos KA, Halm EA. Comparative effectiveness of long-term acute care hospital versus skilled nursing facility transfer. BMC Health Serv Res. 2020;20:1032. doi: 10.1186/s12913-020-05847-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nelson JE, Cox CE, Hope AA, Carson SS. Chronic critical illness. Am J Respir Crit Care Med. 2010;182:446–454. doi: 10.1164/rccm.201002-0210CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Auriemma CL, O’Donnell H, Jones J, Barbati Z, Akpek E, Klaiman T, et al.
- 43. Turnbull AE, Sepulveda KA, Dinglas VD, Chessare CM, Bingham CO, III, Needham DM. Core domains for clinical research in acute respiratory failure survivors: an international modified Delphi consensus study. Crit Care Med. 2017;45:1001–1010. doi: 10.1097/CCM.0000000000002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wunsch H. When intensive care becomes good end-of-life care. Am J Respir Crit Care Med. 2020;201:762. doi: 10.1164/rccm.202001-0076ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kowalkowski M, Chou S-H, Mcwilliams A, Lashley C, Murphy S, Rossman W, et al. Structured, proactive care coordination versus usual care for Improving Morbidity during Post-Acute Care Transitions for Sepsis (IMPACTS): a pragmatic, randomized controlled trial. Trials. 2019;20:660. doi: 10.1186/s13063-019-3792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lewis RJ, Angus DC, Laterre PF, Kjølbye AL, van der Meulen E, Blemings A, et al. Rationale and design of an adaptive phase 2b/3 clinical trial of selepressin for adults in septic shock. Selepressin evaluation programme for sepsis-induced shock—adaptive clinical trial. Ann Am Thorac Soc. 2018;15:250–257. doi: 10.1513/AnnalsATS.201708-669SD. [DOI] [PubMed] [Google Scholar]
- 47. Reitz KM, Seymour CW, Vates J, Quintana M, Viele K, Detry M, et al. Strategies to Promote ResiliencY (SPRY): a randomised embedded multifactorial adaptative platform (REMAP) clinical trial protocol to study interventions to improve recovery after surgery in high-risk patients. BMJ Open. 2020;10:e037690. doi: 10.1136/bmjopen-2020-037690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Young P, Hodgson C, Dulhunty J, Saxena M, Bailey M, Bellomo R, et al. ANZICS Clinical Trials Group. End points for phase II trials in intensive care: recommendations from the Australian and New Zealand Clinical Trials Group consensus panel meeting. Crit Care Resusc. 2012;14:211–215. [PubMed] [Google Scholar]
- 49. Ritch CR, Cookson MS, Chang SS, Clark PE, Resnick MJ, Penson DF, et al. Impact of complications and hospital-free days on health related quality of life 1 year after radical cystectomy. J Urol. 2014;192:1360–1364. doi: 10.1016/j.juro.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 50. Courtright KR, Chivers C, Becker M, Regli SH, Pepper LC, Draugelis ME, et al. Electronic health record mortality prediction model for targeted palliative care among hospitalized medical patients: a pilot quasi-experimental study. J Gen Intern Med. 2019;34:1841–1847. doi: 10.1007/s11606-019-05169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Neelon B, O’Malley AJ, Smith VA. Modeling zero-modified count and semicontinuous data in health services research part 1: background and overview. Stat Med. 2016;35:5070–5093. doi: 10.1002/sim.7050. [DOI] [PubMed] [Google Scholar]
- 52.Cheng J, Small DS.Semiparametric models and inference for the effect of a treatment when the outcome is nonnegative with clumping at zero. Biometrics. 2020. [DOI] [PMC free article] [PubMed]
- 53. Dagne GA. Joint two-part Tobit models for longitudinal and time-to-event data. Stat Med. 2017;36:4214–4229. doi: 10.1002/sim.7429. [DOI] [PubMed] [Google Scholar]
- 54. Farewell VT, Long DL, Tom BDM, Yiu S, Su L. Two-part and related regression models for longitudinal data. Annu Rev Stat Appl. 2017;4:283–315. doi: 10.1146/annurev-statistics-060116-054131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhao J, Zhao Y, Xiang L, Khanal V, Binns CW, Lee AH. A two-part mixed-effects model for analyzing clustered time-to-event data with clumping at zero. Comput Methods Programs Biomed. 2020;187:105196. doi: 10.1016/j.cmpb.2019.105196. [DOI] [PubMed] [Google Scholar]
- 56. Preisser JS, Das K, Long DL, Divaris K. Marginalized zero-inflated negative binomial regression with application to dental caries. Stat Med. 2016;35:1722–1735. doi: 10.1002/sim.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Arnold BF, Hogan DR, Colford JM, Jr, Hubbard AE. Simulation methods to estimate design power: an overview for applied research. BMC Med Res Methodol. 2011;11:94. doi: 10.1186/1471-2288-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Burton A, Altman DG, Royston P, Holder RL. The design of simulation studies in medical statistics. Stat Med. 2006;25:4279–4292. doi: 10.1002/sim.2673. [DOI] [PubMed] [Google Scholar]
- 59. Halpern SD, Temel JS, Courtright KR. Dealing with death as an outcome in supportive care clinical trials. JAMA Intern Med. 2021;181:895–896. doi: 10.1001/jamainternmed.2021.1816. [DOI] [PubMed] [Google Scholar]
- 60.University of Pennsylvania. https://clinicaltrials.gov/ct2/show/NCT04581863?term=covid+pulse+ox&draw=2&rank=3