Abstract
Rationale: Animal studies of cardiac arrest suggest that shorter epinephrine dosing intervals than currently recommended (every 3–5 min) may be beneficial in select circumstances.
Objectives: To evaluate the association between epinephrine dosing intervals and pediatric cardiac arrest outcomes.
Methods: Single-center retrospective cohort study of children (<18 years of age) who received ⩾1 minute of cardiopulmonary resuscitation and ⩾2 doses of epinephrine for an index in-hospital cardiac arrest. Exposure was epinephrine dosing interval ⩽2 minutes (frequent epinephrine) versus >2 minutes. The primary outcome was survival to hospital discharge with a favorable neurobehavioral outcome (Pediatric Cerebral Performance Category score 1–2 or unchanged). Logistic regression evaluated the association between dosing interval and outcomes; additional analyses explored duration of cardiopulmonary resuscitation (CPR) as a mediator. In a subgroup, the effect of dosing interval on diastolic blood pressure was investigated.
Measurements and Main Results: Between January 2011 and December 2018, 125 patients met inclusion/exclusion criteria; 33 (26%) received frequent epinephrine. Frequent epinephrine was associated with increased odds of survival with favorable neurobehavioral outcome (adjusted odds ratio, 2.56; 95% confidence interval, 1.07–6.14; P = 0.036), with 66% of the association mediated by CPR duration. Delta diastolic blood pressure was greater after the second dose of epinephrine among patients who received frequent epinephrine (median [interquartile range], 6.3 [4.1 to 16.9] vs. 0.13 [−2.3 to 1.9] mm Hg; P = 0.034).
Conclusions: In patients who received at least two doses of epinephrine, dosing intervals ⩽2 minutes were associated with improved neurobehavioral outcomes compared with dosing intervals >2 minutes. Mediation analysis suggests that improved outcomes are largely due to frequent epinephrine shortening duration of CPR.
Keywords: child, heart arrest, epinephrine
At a Glance Commentary
Scientific Knowledge on the Subject
Although the American Heart Association recommends bolus doses of epinephrine every 3–5 minutes in pediatric cardiac arrest, evidence for an ideal dosing interval is limited and conflicting. Previous studies have used estimations of epinephrine dosing intervals.
What This Study Adds to the Field
This study used documented epinephrine administration times to show that dosing epinephrine more frequently than currently recommended (at intervals of ⩽2 min, i.e., “frequent epinephrine”) is associated with higher intraarrest diastolic blood pressure, shorter cardiopulmonary resuscitation (CPR) duration, and better survival outcomes compared with dosing at intervals >2 minutes. The effect of frequent epinephrine on the probability of return of spontaneous circulation is time dependent and most notable in the first 10 minutes of CPR. In a mediation analysis, the effect of frequent epinephrine on neurologic survival is largely mediated by shortening the duration of CPR.
In-hospital cardiac arrest (IHCA) affects approximately 15,000 children in the United States each year, and most occur in ICUs (1, 2). Fewer than half of these children survive to hospital discharge, and neurobehavioral morbidity is common among survivors (3, 4). Moreover, survival rates have not improved in nearly 10 years (3, 5), highlighting the need for continued investigation into optimal resuscitation strategies.
Epinephrine has been the pharmacologic cornerstone of cardiopulmonary resuscitation (CPR) for decades. Its mechanism of action is to acutely increase coronary perfusion pressure, which is critical for achieving return of spontaneous circulation (ROSC) (6) and may lead to higher rates of survival (7). Current pediatric resuscitation algorithms for IHCA recommend the administration of bolus doses of epinephrine every 3–5 minutes, although evidence to support an optimal dosing frequency is limited (8). Pharmacologic studies suggest that epinephrine administered more frequently than the American Heart Association recommends may be beneficial (9–12); yet, retrospective observational studies are conflicting, supporting both less and more frequent epinephrine administration in pediatric and adult cardiac arrest (13–16). Previous observational registry studies all relied on estimations of epinephrine dosing intervals (i.e., actual administration timing of all doses was not available); thus, more rigorous investigation is warranted to support future recommendations.
To that end, the objective of this study was to evaluate the association between epinephrine dosing intervals and pediatric IHCA outcomes by leveraging a single-center database that overcomes limitations of previous work on this topic. We hypothesized that in patients who received at least two doses of epinephrine, 1) frequent epinephrine administration (intervals ⩽2 min) would be associated with improved patient outcomes and higher intraarrest diastolic blood pressure (DBP), 2) frequent epinephrine administration would improve outcomes by shortening the duration of CPR, and 3) the benefit of more frequent epinephrine would be most evident during the first ∼10 minutes of resuscitation (i.e., if frequent epinephrine did not facilitate ROSC early in the resuscitation, continued frequent administration would likely not provide additional benefit).
Methods
Setting and Design
This retrospective cohort study used a single quaternary care center cardiac arrest database of prospectively collected data to investigate the relationship between epinephrine dosing intervals and 1) patient outcomes and 2) intraarrest blood pressure. An intensive 24/7 screening process ensured identification and inclusion of consecutive cardiac arrests. The study was approved with a waiver of informed consent by the Institutional Review Board at the Children’s Hospital of Philadelphia.
Patient Population
Patients <18 years of age who experienced an index IHCA event in either the pediatric or cardiac ICU and who received at least 1 minute of CPR were included. Patients were excluded if 1) fewer than two doses of epinephrine were administered, 2) a bolus dose of a different vasopressor was given (e.g., vasopressin), 3) extracorporeal membrane oxygenation was required to obtain return of circulation, or 4) data related to the primary exposure or primary outcome were missing. Patients receiving extracorporeal CPR were specifically excluded to allow comparison to prior work (13) but also because our hypothesis was that more frequent epinephrine dosing would increase the likelihood of adequate DBP (7) and thus likelihood of prompt ROSC and subsequent improved neurobehavioral outcome. As such, shorter time to ROSC is in the causal pathway from epinephrine frequency to improved neurobehavioral outcome. This mechanism would not apply to patients who required extracorporeal support to achieve return of circulation.
Measurements
Epinephrine dosing times were abstracted from bedside resuscitation records and recorded through the end of CPR up to a maximum of 10 doses of epinephrine, regardless of the time over which they were administered or the availability of waveform data. Times were rounded to the nearest minute if documented in seconds. Individual intervals for each dose subsequent to the first dose were calculated and an event-level average of all intervals was calculated for each patient. Of note, paper code sheets are the standard at this institution, with trained bedside nurses performing documentation. The nurse documenter is trained to use the same clock, synchronized to the institutional monitors from which hemodynamic data are gathered, to record epinephrine administration times. The primary independent variable was the epinephrine dosing interval, classified as ⩽2 minutes (frequent epinephrine) or >2 minutes, an inflection point supported by pharmacodynamic data in translational models (9–12). Secondary analyses included 1) a trichotomous predictor that included a category consistent with the Pediatric Advanced Life Support (PALS)-recommended interval (<3, ⩾3 to ⩽5, and >5 min) and 2) this same predictor with estimated dosing intervals calculated by the following equation: (CPR duration after the first dose of epinephrine) / (total doses of epinephrine – 1) to allow direct comparison to prior work (13). An exploratory, more granular analysis of 1-minute intervals ± 2 minutes around the a priori 2-minute dosing cutoff (⩽1, 1 to ⩽2, 2 to ⩽3, 3 to ⩽4, and >4 min) was performed to ensure the cutoff was supported by the data. For all analyses, epinephrine doses that were <0.01 mg/kg (unless the patient received adult dosing of 1 mg) or those given before CPR initiation or after CPR cessation were not included.
To investigate the effect of epinephrine dosing interval on intraarrest arterial DBP, bedside monitor waveform data in printed or electronic format (BedmasterEX; Excel Medical) were collected and analyzed for patients with invasive arterial BP monitoring in place during CPR. Similar to previous work, only the first 10 minutes of waveform data were collected for each patient to limit the size of the data files. Printed waveforms were manually digitized by a trained research coordinator (K.G.) blinded to other clinical data (Plot Digitizer; https://sourceforge.net/projects/plotdigitizer). DBP was sampled during mid-diastole for each compression. Please see previous publications for more details on DBP data extraction (7, 17).
Outcomes
The primary outcome was survival to hospital discharge with favorable neurobehavioral outcome defined by a Pediatric Cerebral Performance Category score (18) of 1–2 or no change from baseline. Secondary outcomes were survival to hospital discharge, ROSC for >20 minutes, DBP, and CPR duration (beginning of chest compressions to end of chest compressions resulting in sustained ROSC >20 minutes or cessation of resuscitation efforts and death [19]).
Statistical Analysis
Categorical data are presented as frequencies and percentages. Normally distributed continuous data are presented using means and SDs. Non–normally distributed data are presented using medians and interquartile ranges. Univariate associations between exposures and outcomes were evaluated using chi-square or Fisher exact test for categorical data and Wilcoxon rank-sum test for continuous data.
Logistic and linear regression models were used to evaluate the association between epinephrine dosing intervals and outcomes, after adjusting for the effects of confounders. Potential confounders of the epinephrine interval–outcome relationship were location (20), age (21), initial CPR rhythm (22), time of day/week (23), and delayed time to first epinephrine dose (>5 min [24]) and were chosen a priori based on previously published associations with survival. As in previous work on this topic (13), regression analyses were stratified by presence of a vasoactive infusion at the time of arrest, a variable hypothesized to modify the relationship between the exposure (frequent epinephrine) and outcomes.
Cumulative probability distributions of ROSC were created, and the Kolmogorov-Smirnov test was used to investigate whether the association between frequent epinephrine and ROSC was time dependent.
Mediation analysis was planned to test whether the effect of frequent epinephrine on favorable neurobehavioral outcome is direct (independent) or indirect (i.e., mediated through CPR duration). The mediation analysis was conducted by incorporating mediation techniques into the outcome models using the Mediation package in R (www.R-project.org, version 3.6.1), which used a quasi-Bayesian Monte Carlo simulation–based approach (25). This method jointly modeled two separate regressions: the first regression examined the change in CPR duration as a function of frequent epinephrine and covariates, and the second regression examined the change in neurobehavioral outcome as a function of covariates, frequent epinephrine, and change in CPR duration. This package subsequently estimated average causal effects for the total effect (i.e., the overall relationship between frequent epinephrine and favorable neurobehavioral outcome), the indirect effect (i.e., the estimated [mediated] effect operating through CPR duration), and the direct effect (i.e., the estimated [unmediated] effect through unknown pathways). In addition, the software calculated the proportion of mediation, which represents the proportion of the total effect that was mediated by CPR duration. This analysis was completed for the overall cohort and after stratification by presence of a vasoactive infusion at the time of arrest.
To minimize potential bias from excluding subjects who achieved ROSC before a second dose of epinephrine (i.e., good outcomes among brief arrests that did not receive a second dose of epinephrine potentially because of a longer dosing interval), a sensitivity analysis including only patients who received at least 4 minutes of CPR was performed. The exploratory analysis of 1-minute intervals ± 2 minutes around the a priori 2-minute dosing cutoff (⩽1, 1 to ⩽2, 2 to ⩽3, 3 to ⩽4, and >4 min) used mean outcome within each interval with 95% confidence intervals (CIs) to show the proportion of patients achieving each outcome.
For the subset of patients with arterial lines in place at the time of the cardiac arrest, invasive blood pressure measurements after epinephrine boluses were analyzed in 30-second epochs in an exploratory analysis to evaluate whether there was hemodynamic evidence of improved DBP, a surrogate of coronary perfusion pressure, after frequent epinephrine (⩽2-min intervals). The primary hemodynamic analysis was to characterize the change in DBP from “baseline” (after the first dose of epinephrine) to after the second dose of epinephrine within groups using the Wilcoxon signed-rank test and across groups using the Wilcoxon rank-sum test. For all tests, a P value <0.05 was considered statistically significant.
Results
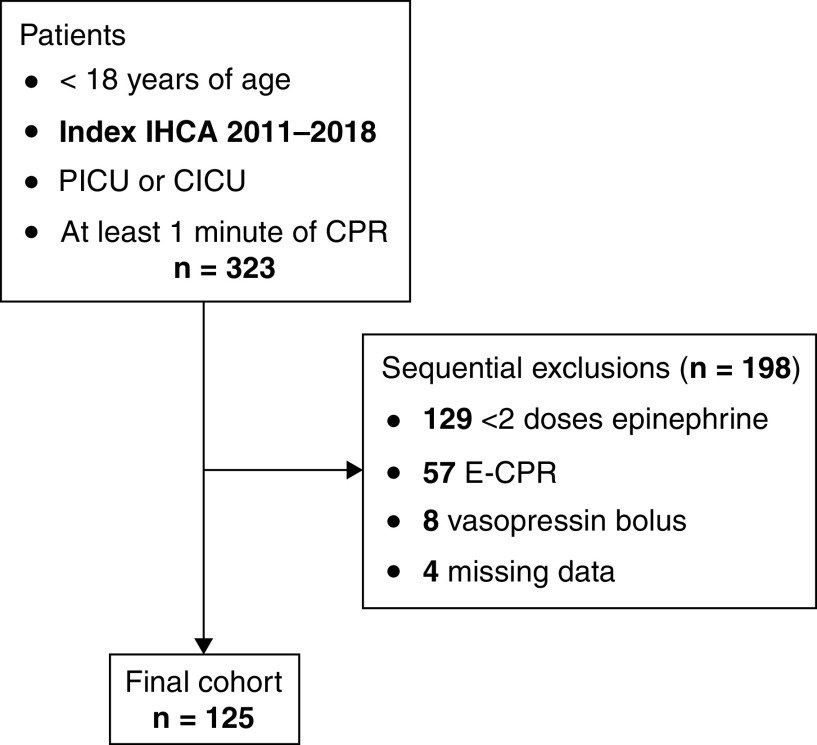
Between January 2011 and December 2018, 569 index IHCAs occurred, of which 323 (57%) met inclusion criteria; 125 were included in the final cohort (Figure 1). The median age was 1.6 years (interquartile range [IQR], 0.3–6.2) and 59 (47%) were male. Thirty-three (26%) patients received frequent epinephrine. Baseline patient characteristics of the cohort are listed in Table 1. The most common initial rhythm was asystole/pulseless electrical activity (95/125 [76%]). Patients who received frequent epinephrine had shorter durations of CPR and fewer doses of epinephrine overall. Cardiac arrest event characteristics are listed in Table 2.
Figure 1.
Consolidated Standards of Reporting Trials diagram. CICU = cardiac ICU; CPR = cardiopulmonary resuscitation; E-CPR = extracorporeal cardiopulmonary resuscitation; IHCA = in-hospital cardiac arrest; PICU = pediatric ICU.
Table 1.
Patient Characteristics
| Overall (N = 125) | Frequent Epinephrine |
|||
|---|---|---|---|---|
| Yes (N = 33) | No (N = 92) | P Value | ||
| Age, yr | 0.74 | |||
| <1 | 50 (40) | 14 (42) | 36 (39) | |
| ⩾1 | 75 (60) | 19 (58) | 56 (61) | |
| Sex | 0.56 | |||
| M | 59 (47) | 17 (52) | 42 (46) | |
| F | 66 (53) | 16 (48) | 50 (54) | |
| Race | 0.77 | |||
| White | 47 (38) | 13 (39.5) | 34 (37) | |
| Black | 29 (23) | 8 (24) | 21 (23) | |
| Asian | 3 (2) | 0 (0) | 3 (3) | |
| Other | 46 (37) | 12 (36.5) | 34 (37) | |
| Ethnicity | 0.33 | |||
| Non-Hispanic | 98 (78.5) | 24 (73) | 74 (80) | |
| Hispanic | 14 (11) | 6 (18) | 8 (9) | |
| Unknown | 13 (10.5) | 3 (9) | 10 (11) | |
| Preexisting conditions | ||||
| Respiratory failure | 76 (61) | 19 (58) | 57 (62) | 0.66 |
| Circulatory failure | 58 (46) | 15 (45) | 43 (47) | 0.90 |
| Pneumonia/sepsis | 20 (16) | 7 (21) | 13 (14) | 0.34 |
| Malignancy | 9 (7) | 1 (3) | 8 (9) | 0.28 |
| Congenital heart disease | 65 (52) | 18 (55) | 47 (51) | 0.73 |
| Baseline PCPC | 0.44 | |||
| 1: Normal | 63 (50.5) | 14 (42.5) | 49 (53) | |
| 2: Mild disability | 28 (22.5) | 11 (33.5) | 17 (18) | |
| 3: Moderate disability | 13 (10.5) | 4 (12) | 9 (10) | |
| 4: Severe disability | 13 (10.5) | 3 (9) | 10 (11) | |
| 5: Vegetative state | 8 (6) | 1 (3) | 7 (8) | |
Definition of abbreviation: PCPC = Pediatric Cerebral Performance Category.
Baseline patient characteristics by exposure to frequent epinephrine. Data are shown as n (%).
Table 2.
Event Characteristics
| Overall (N = 125) | Frequent Epinephrine |
P Value | ||
|---|---|---|---|---|
| Yes (N = 33) | No (N = 92) | |||
| Location of CPR event | 0.68 | |||
| PICU | 72 (58) | 18 (55) | 54 (59) | |
| CICU | 53 (42) | 15 (45) | 38 (41) | |
| Time of day | 0.27 | |||
| Nights/weekends | 67 (54) | 15 (45) | 52 (57) | |
| Weekdays | 58 (46) | 18 (55) | 40 (43) | |
| Immediate cause | ||||
| Hypotension/shock/sepsis | 77 (62) | 20 (61) | 57 (62) | 0.89 |
| Respiratory decompensation | 66 (53) | 19 (58) | 47 (51) | 0.52 |
| Arrhythmia | 7 (6) | 2 (6) | 5 (5) | 0.89 |
| Initial CPR rhythm | 0.19 | |||
| Bradycardia* | 15 (12) | 4 (12) | 11 (12) | |
| Asystole/PEA | 95 (76) | 24 (73) | 71 (77) | |
| VF/VT | 5 (4) | 0 (0) | 5 (5.5) | |
| Not documented | 10 (8) | 5 (15) | 5 (5.5) | |
| Duration of CPR, min | 13 (6–25) | 5 (4–10) | 16.5 (10–31) | <0.01 |
| Interventions in place | ||||
| Arterial catheter | 58 (46) | 18 (55) | 40 (43) | 0.27 |
| Vasoactive infusion | 63 (50) | 13 (39) | 50 (54) | 0.14 |
| Pharmacologic interventions | ||||
| Time to first epi dose, min | 2 (0–3) | 1 (0–2) | 2 (0–3) | 0.34 |
| Total epinephrine doses | 3 (2–5) | 2 (2–3) | 4 (2–5.5) | <0.01 |
| Calcium | 77 (62) | 16 (48) | 61 (66) | 0.07 |
| Sodium bicarbonate | 93 (74) | 21 (64) | 72 (78) | 0.10 |
Definition of abbreviations: CICU = cardiac ICU; CPR = cardiopulmonary resuscitation; epi = epinephrine; PEA = pulseless electrical activity; PICU = pediatric ICU; VF = ventricular fibrillation; VT = ventricular tachycardia.
Baseline event characteristics by exposure to frequent epinephrine. Data are shown as n (%) or median (interquartile range).
Bradycardia with poor perfusion.
Outcomes
Among the 125 patients included in the study, 91 (73%) achieved ROSC, 50 (40%) survived to hospital discharge, and 42 (34%) survived with favorable neurobehavioral outcome. After adjustment for a priori selected confounders, frequent epinephrine was associated with higher rates of survival with favorable neurobehavioral outcome (adjusted odds ratio [aOR], 2.56; 95% CI, 1.07–6.14; P = 0.036), survival to discharge (aOR, 2.69; 95% CI, 1.12–6.43; P = 0.027), and ROSC (aOR, 8.88; 95% CI, 1.91–41.3; P = 0.005). CPR duration was also significantly shorter in patients who received frequent epinephrine compared with those who did not (adjusted effect estimate, −15.7 min; 95% CI, −21.8 to −9.61; P < 0.01) (Table 3). After stratification by presence of a vasoactive infusion at the time of arrest, effect estimates were similar but not statistically significant (Table 4). In a sensitivity analysis of arrests ⩾4 minutes, effect estimates for the primary outcome were similar (aOR, 2.86; 95% CI, 1.11–7.4; P = 0.03). An exploratory analysis of 1-minute intervals ± 2 minutes around the a priori 2-minute dosing cutoff (⩽1, 1 to ⩽2, 2 to ⩽3, 3 to ⩽4, and >4 min) supported the 2-minute cutoff as an inflection point for evaluation in our analyses (see Figure E1 in the online supplement).
Table 3.
Association between Frequent Epinephrine and Outcomes: All Patients
| Unadjusted OR (95% CI) | P Value | Adjusted OR (95% CI)* | P Value | |
|---|---|---|---|---|
| Survival with favorable neurobehavioral outcome | 2.85 (1.25 to 6.48) | 0.013 | 2.56 (1.07 to 6.14) | 0.036 |
| Survival to discharge | 2.67 (1.18 to 6.03) | 0.018 | 2.69 (1.12 to 6.43) | 0.027 |
| ROSC | 8.27 (1.86 to 36.8) | 0.006 | 8.88 (1.91 to 41.3) | 0.005 |
| Duration of CPR† | −15.2 (−21.1 to −9.18) | <0.01 | −15.7 (−21.8 to −9.61) | <0.01 |
Definition of abbreviations: CI = confidence interval; CPR = cardiopulmonary resuscitation; OR = odds ratio; ROSC = return of spontaneous circulation.
All models adjusted for location, age category, initial rhythm, time of day, and delayed time to first epinephrine dose (>5 min).
Effect estimate (minutes) from linear regression models.
Table 4.
Association between Frequent Epinephrine and Outcomes: Stratified by Presence of Vasoactive Infusion
| Outcome | Infusion | Unadjusted OR (95% CI) | P Value | Adjusted OR (95% CI)* | P Value |
|---|---|---|---|---|---|
| Survival with favorable neurobehavioral outcome | No | 3.02 (0.99 to 9.16) | 0.051 | 3.33 (0.96 to 11.6) | 0.06 |
| Yes | 2.02 (0.51 to 8.06) | 0.32 | 2.58 (0.36 to 18.4) | 0.35 | |
| Survival to discharge | No | 2.57 (0.83 to 7.96) | 0.10 | 2.83 (0.79 to 10.1) | 0.11 |
| Yes | 2.22 (0.60 to 8.15) | 0.23 | 4.93 (0.78 to 31.3) | 0.09 | |
| ROSC | No | — | — | — | — |
| Yes | 3.98 (0.80 to 19.9) | 0.092 | 4.80 (0.76 to 30.4) | 0.10 | |
| Duration of CPR† | No | −14.0 (−20.5 to −4.32) | <0.01 | −13.4 (−19.9 to −6.9) | <0.01 |
| Yes | −16.0 (−26.9 to −5.1) | <0.01 | −16.7 (−29.4 to −3.9) | 0.012 |
Definition of abbreviations: CI = confidence interval; CPR = cardiopulmonary resuscitation; OR = odds ratio; ROSC = return of spontaneous circulation.
All models adjusted for location, age category, initial rhythm, time of day, and delayed time to first epinephrine dose (>5 min).
Effect estimate (minutes) from linear regression models. Note: Model for ROSC did not converge because 100% of those patients exposed to frequent epinephrine in this subgroup achieved ROSC.
DBP within and across Groups
Among 58 patients with arterial catheters in place at the time of arrest, 16 (28%) had evaluable DBP data. Comparisons within epinephrine interval groups (Figure 2A) demonstrated that DBP increased significantly after a second dose of epinephrine in the frequent epinephrine group (median, 35 [IQR, 30–43] to 46 [IQR, 39–52] mm Hg, P = 0.031) but not in the less frequent epinephrine group (median, 40 [IQR, 30–45] to 37 [IQR, 29–49] mm Hg; P = 0.99). When comparing the delta in diastolic blood pressure across groups (Figure 2B), the change in DBP after the second dose of epinephrine was greater among patients receiving frequent epinephrine versus those given less frequent epinephrine (median 6.3 [IQR, 4.1 to 16.9] vs. 0.13 [IQR, −2.3 to 1.9] mm Hg; P = 0.034).
Figure 2.
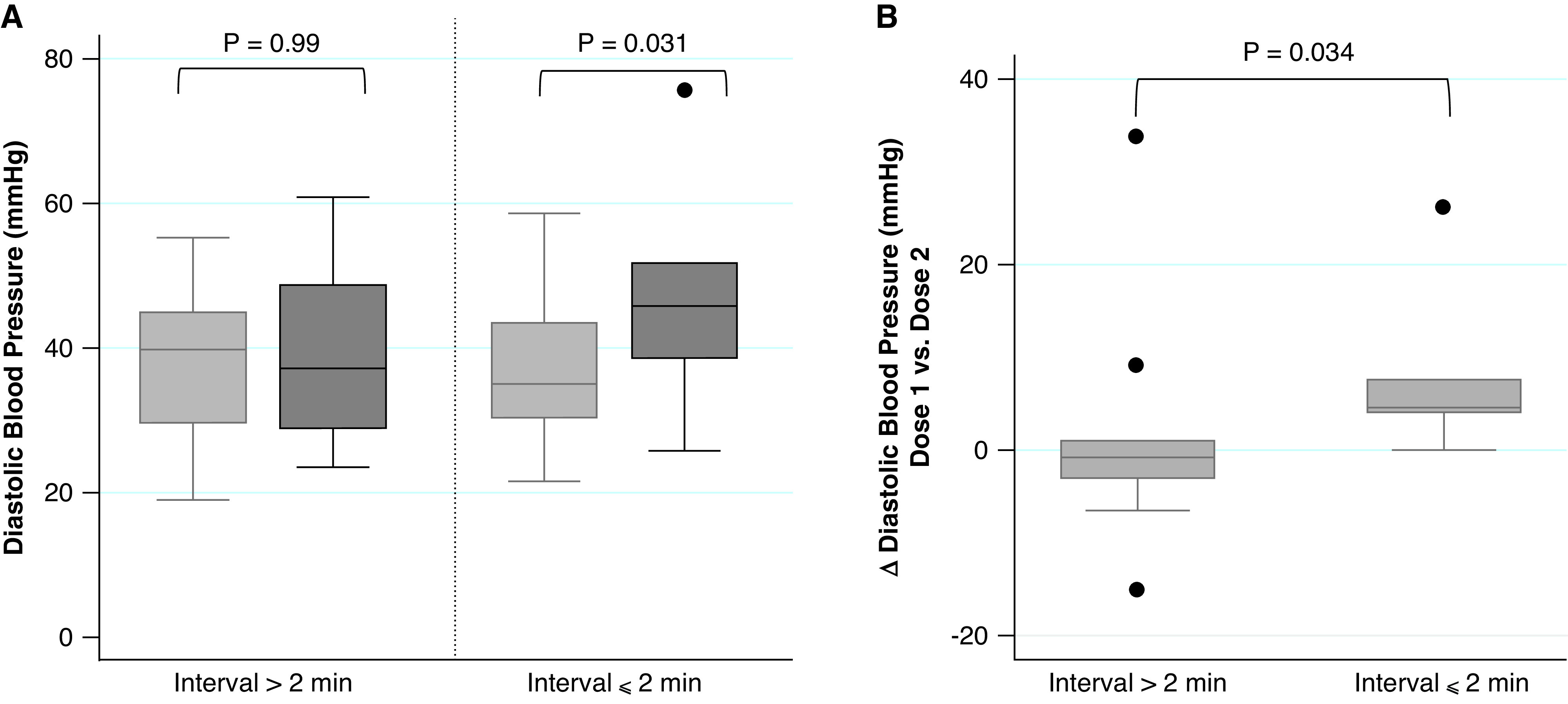
Diastolic blood pressure analyses. (A) Within-group response: baseline is average diastolic blood pressure after first epinephrine dose (light gray); response is average diastolic blood pressure after second epinephrine dose (dark gray). (B) Comparison of delta (dose 1 to dose 2) across groups. P values calculated within groups using the Wilcoxon signed-rank test (A) and across groups using the Wilcoxon rank-sum test (B).
PALS-recommended Dosing Interval and Outcomes
When the PALS-recommended dosing interval was used as part of a trichotomous predictor (<3, ⩾3 to ⩽5, and >5 min), odds of survival to discharge with favorable neurobehavioral outcome were not different between groups (Table E1). In the analysis using the same predictor with estimated dosing intervals, odds of survival with favorable neurobehavioral outcome were not different between groups (Table E2).
CPR Duration as an Effect Mediator
Using the mediation approach, the average causal effect (total effect) of frequent epinephrine on favorable neurobehavioral outcome was 0.20 (95% CI, 0.03–0.36). By incorporating causal mediation analysis into our logistic regression model, we could decompose the total effect into an indirect pathway through CPR duration (estimated indirect effect was 0.13 [95% CI, 0.04–0.23; P = 0.01]) and a direct effect through unknown pathways. These results indicate that the indirect effect (CPR duration as a mediator) explained 66% of the total effect of frequent epinephrine on favorable neurobehavioral outcome (i.e., frequent epinephrine at least partly improves outcomes by shortening the duration of CPR). The effect of CPR duration as a mediator was most evident among patients not on a vasoactive infusion at the time of arrest (Table E3).
Frequent Epinephrine and ROSC: Time-Dependent Analysis
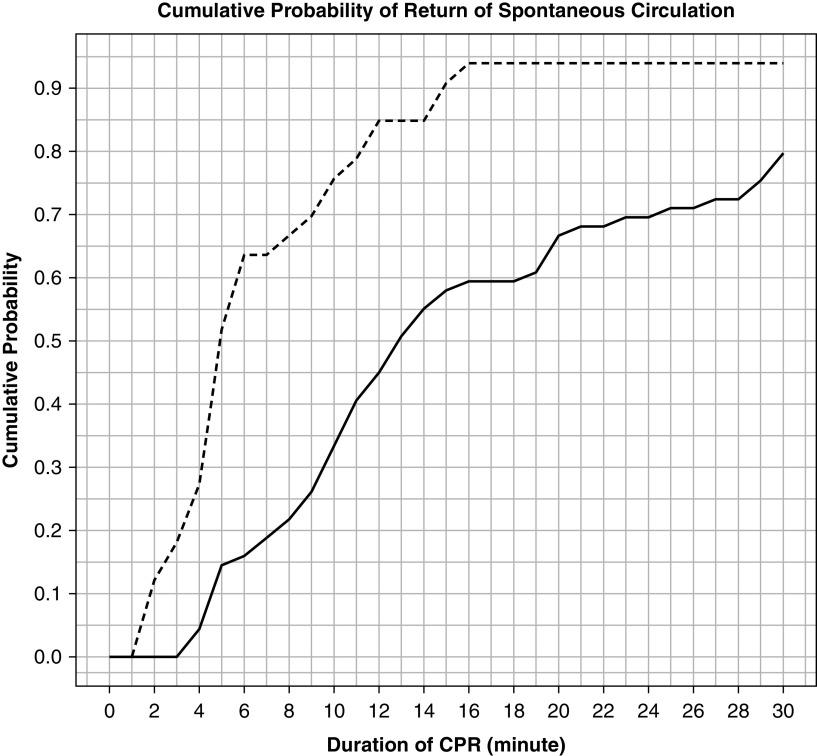
Cumulative probabilities of ROSC across all time points of the first 30 minutes of CPR were higher for those exposed to frequent epinephrine (P < 0.001), although the most notable divergence in the probability curves was evident during the first 10 minutes of CPR (Figure 3).
Figure 3.
Cumulative probabilities of return of spontaneous circulation. The figure depicts the analysis to evaluate whether the association between frequent epinephrine and return of spontaneous circulation was time dependent. The dashed line represents the frequent epinephrine group; the solid line represents the nonfrequent epinephrine group. CPR = cardiopulmonary resuscitation.
Discussion
In this cohort of pediatric ICU patients with IHCA who received at least two doses of epinephrine, epinephrine dosing intervals ⩽2 minutes were associated with significantly higher rates of survival with favorable neurobehavioral outcome, survival to hospital discharge, and ROSC compared with epinephrine dosing intervals >2 minutes. The association between frequent epinephrine administration and favorable neurobehavioral outcome was largely mediated by CPR duration. In the subset of patients with invasive blood pressure measurements during CPR, those exposed to frequent epinephrine experienced a more substantial rise in DBP after the second dose of epinephrine, providing a biologically plausible mechanism by which more frequent epinephrine may improve outcomes (i.e., increased coronary perfusion pressure that allows ROSC to be achieved more quickly). Taken together, these results suggest that a more frequent epinephrine dosing interval than recommended in current guidelines, at least during the initial minutes of resuscitation, may be a strategy to improve outcomes from pediatric cardiac arrest.
These findings must be considered in the context of the existing body of literature regarding epinephrine during CPR. First, although earlier administration of the first dose of epinephrine is associated with improved pediatric IHCA outcomes (24), there have been mixed observational data regarding the optimal dosing interval. Specifically, similar to our findings, an adult study of out-of-hospital cardiac arrest found an association between shorter epinephrine dosing intervals and improved outcomes (16). Conversely, a large registry study by Hoyme and colleagues observed an association between shorter epinephrine dosing intervals and worse pediatric cardiac arrest outcomes (13). Similar to other studies of cardiac arrest, the analytic plan undertaken by Hoyme and colleagues controlled for duration of CPR as an important confounder. We intentionally did not control for CPR duration in our multivariable models because we postulated that CPR duration was acting as a mediator in the relationship between frequent epinephrine and improved survival outcomes. Specifically, by improving hemodynamics and increasing the likelihood of ROSC (6), shorter epinephrine dosing intervals would shorten CPR duration and improve outcomes. This conceptual model and analysis is consistent with the recently published innovative approach to address the effects of early defibrillation on outcomes from ventricular fibrillation cardiac arrest (26). Our causal mediation analysis and the cumulative probability of ROSC at continuous time points during CPR support this hypothesis and our analytic plan.
Although our data suggest that more frequent epinephrine dosing may be a strategy to improve pediatric cardiac arrest outcomes, the potentially deleterious effects of epinephrine deserve comment. First, it has been known for decades that high-dose epinephrine (a 10-fold dose above that currently recommended) is associated with worse outcomes from pediatric cardiac arrest (27). At such doses, the potential risks of increased myocardial oxygen consumption (28) and decreased during CPR (29), and a toxic hyperadrenergic state after resuscitation (e.g., myocardial dysfunction and arrhythmias [30]), become more evident. Importantly, in the present study, patients who received more frequent epinephrine actually received fewer doses of epinephrine, and thus a lower cumulative dose, over the course of the resuscitation because of the aforementioned shorter cardiac arrest duration. Second, epinephrine may have adverse effects on the cerebral microvascular circulation (31, 32). Although epinephrine during CPR may improve systemic hemodynamics, global cerebral blood flow, and cerebral tissue oxygenation (33), attention to these other potentially problematic effects is necessary. Of note, experimental laboratory data demonstrate that the beneficial effects of epinephrine on systemic hemodynamics and cerebral blood flow diminish with each subsequent administration during CPR (12, 33–35). Our analysis highlighting a time dependence to the relationship between frequent epinephrine and ROSC in patients treated with frequent epinephrine is consistent with these translational data and suggests that if ROSC is not achieved within approximately 10 minutes of CPR onset with frequent epinephrine, continued administration is likely only to expose the patient to the aforementioned risks. In these cases, other strategies should be considered, including activation of extracorporeal CPR systems when available (36). Similarly, although effect estimates were similar between groups, our analyses suggest that patients on vasoactive infusions may see less benefit from increased epinephrine frequency. This may simply be a reflection of a more critically ill group of patients or may represent catecholamine nonresponsiveness in the setting of refractory shock as a precipitant of the arrest. In any case, a patient’s vasoactive requirement should be one additional consideration when deciding if more frequent epinephrine administration is warranted.
This study has limitations. First, it is retrospective in nature; thus, its results demonstrate association and not causation. Second, it is a single-center study, which potentially limits generalizability. Third, we limited our analysis to the first 10 doses of epinephrine given during an arrest and as such could have missed important dosing interval variations that occurred later in the resuscitation. However, 95% of the arrests within the final cohort included 10 or fewer doses of epinephrine. Fourth, we may have been underpowered to detect an association between longer dosing intervals and improved outcomes owing to small numbers of patients receiving epinephrine at longer intervals. Fifth, chart documentation of epinephrine dose does not necessarily equate to time of drug administration, and although its reliability and accuracy are unknown, it is presumably superior to previously used estimation techniques. Sixth, we did not rigorously collect data on postresuscitation myocardial dysfunction and as such cannot comment if more frequent epinephrine administration was associated with a toxic adrenergic state after resuscitation. Because patients receiving more frequent epinephrine received less cumulative epinephrine and had superior outcomes, even if present, this potential consequence of frequent epinephrine was not clinically significant. Finally, hemodynamic data were only available for 16/58 (28%) arrests with invasive arterial monitoring because of known limitations related to waveform capture (e.g., lack of arterial waveform due to line interruption for blood draw, truncation of BP waveform obscuring DBP [7]). As such, this convenience sample is subject to selection bias and, furthermore, resulted in an underpowered sample to consider important confounders (e.g., etiology of arrest) in our evaluation of the association between epinephrine intervals and DBP. In addition, CPR mechanics data (e.g., chest compression depth) were unavailable for the patients in the hemodynamic analysis, precluding evaluation of CPR quality as it related to outcomes.
Conclusions
In this single-center study of children receiving at least two doses of epinephrine during IHCA, frequent epinephrine dosing at intervals of ⩽2 minutes was associated with improved rates of survival with favorable neurobehavioral outcome, survival to discharge, and ROSC. The mechanisms by which this occurs may include earlier increases in coronary perfusion pressure necessary to achieve ROSC, shorter CPR duration, and, thereby, less neurologic injury. Despite these promising observational results, randomized controlled trials are warranted to better discover the optimal epinephrine dosing interval during pediatric IHCA.
Acknowledgments
Acknowledgment
The authors thank Dr. Ron Reeder (University of Utah) for providing statistical consultation.
Footnotes
Supported by the Department of Anesthesiology and Critical Care Medicine at The Children’s Hospital of Philadelphia. R.W.M. was supported by a career development award from the NHLBI (K23HL148541).
Author Contributions: M.F.K., K.G., W.P.L., and R.M.S. were responsible for acquisition of the data. J.A.F. and H.K. were responsible for the statistical analysis. All authors made substantial contributions to the conception/design of the work, interpretation of data, drafting/revising the submitted manuscript, and final approval of the submitted version and agree to be accountable for all aspects of the work.
This article has an online supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202012-4437OC on July 15, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Holmberg MJ, Ross CE, Fitzmaurice GM, Chan PS, Duval-Arnould J, Grossestreuer AV, et al. American Heart Association’s Get With The Guidelines–Resuscitation Investigators. Annual incidence of adult and pediatric in-hospital cardiac arrest in the United States. Circ Cardiovasc Qual Outcomes. 2019;12:e005580. [PMC free article] [PubMed] [Google Scholar]
- 2. Berg RA, Sutton RM, Holubkov R, Nicholson CE, Dean JM, Harrison R, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network and for the American Heart Association’s Get With the Guidelines-Resuscitation (formerly the National Registry of Cardiopulmonary Resuscitation) Investigators. Ratio of PICU versus ward cardiopulmonary resuscitation events is increasing. Crit Care Med. 2013;41:2292–2297. doi: 10.1097/CCM.0b013e31828cf0c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holmberg MJ, Wiberg S, Ross CE, Kleinman M, Hoeyer-Nielsen AK, Donnino MW, et al. Trends in survival after pediatric in-hospital cardiac arrest in the United States. Circulation. 2019;140:1398–1408. doi: 10.1161/CIRCULATIONAHA.119.041667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berg RA, Nadkarni VM, Clark AE, Moler F, Meert K, Harrison RE, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Incidence and outcomes of cardiopulmonary resuscitation in PICUs. Crit Care Med. 2016;44:798–808. doi: 10.1097/CCM.0000000000001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Girotra S, Nallamothu BK, Spertus JA, Li Y, Krumholz HM, Chan PS. American Heart Association Get with the Guidelines–Resuscitation Investigators. Trends in survival after in-hospital cardiac arrest. N Engl J Med. 2012;367:1912–1920. doi: 10.1056/NEJMoa1109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paradis NA, Martin GB, Rivers EP, Goetting MG, Appleton TJ, Feingold M, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263:1106–1113. [PubMed] [Google Scholar]
- 7. Berg RA, Sutton RM, Reeder RW, Berger JT, Newth CJ, Carcillo JA, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN) PICqCPR (Pediatric Intensive Care Quality of Cardio-Pulmonary Resuscitation) Investigators. Association between diastolic blood pressure during pediatric in-hospital cardiopulmonary resuscitation and survival. Circulation. 2018;137:1784–1795. doi: 10.1161/CIRCULATIONAHA.117.032270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Topjian AA, Raymond TT, Atkins D, Chan M, Duff JP, Joyner BL, Jr, et al. Pediatric Basic and Advanced Life Support Collaborators. Part 4: Pediatric basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020;142:S469–S523. doi: 10.1161/CIR.0000000000000901. [DOI] [PubMed] [Google Scholar]
- 9. Brown CG, Werman HA, Davis EA, Hobson J, Hamlin RL. The effects of graded doses of epinephrine on regional myocardial blood flow during cardiopulmonary resuscitation in swine. Circulation. 1987;75:491–497. doi: 10.1161/01.cir.75.2.491. [DOI] [PubMed] [Google Scholar]
- 10. Kosnik JW, Jackson RE, Keats S, Tworek RM, Freeman SB. Dose-related response of centrally administered epinephrine on the change in aortic diastolic pressure during closed-chest massage in dogs. Ann Emerg Med. 1985;14:204–208. doi: 10.1016/s0196-0644(85)80440-6. [DOI] [PubMed] [Google Scholar]
- 11. Hardig BM, Götberg M, Rundgren M, Götberg M, Zughaft D, Kopotic R, et al. Physiologic effect of repeated adrenaline (epinephrine) doses during cardiopulmonary resuscitation in the cath lab setting: A randomised porcine study. Resuscitation. 2016;101:77–83. doi: 10.1016/j.resuscitation.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 12. Putzer G, Martini J, Spraider P, Hornung R, Pinggera D, Abram J, et al. Effects of different adrenaline doses on cerebral oxygenation and cerebral metabolism during cardiopulmonary resuscitation in pigs. Resuscitation. 2020;156:223–229. doi: 10.1016/j.resuscitation.2020.06.024. [DOI] [PubMed] [Google Scholar]
- 13. Hoyme DB, Patel SS, Samson RA, Raymond TT, Nadkarni VM, Gaies MG, et al. American Heart Association Get With the Guidelines–Resuscitation Investigators. Epinephrine dosing interval and survival outcomes during pediatric in-hospital cardiac arrest. Resuscitation. 2017;117:18–23. doi: 10.1016/j.resuscitation.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 14. Warren SA, Huszti E, Bradley SM, Chan PS, Bryson CL, Fitzpatrick AL, et al. American Heart Association’s Get With the Guidelines-Resuscitation (National Registry of CPR) Investigators. Adrenaline (epinephrine) dosing period and survival after in-hospital cardiac arrest: a retrospective review of prospectively collected data. Resuscitation. 2014;85:350–358. doi: 10.1016/j.resuscitation.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang CH, Huang CH, Chang WT, Tsai MS, Yu PH, Wu YW, et al. The influences of adrenaline dosing frequency and dosage on outcomes of adult in-hospital cardiac arrest: a retrospective cohort study. Resuscitation. 2016;103:125–130. doi: 10.1016/j.resuscitation.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 16. Grunau B, Kawano T, Scheuermeyer FX, Drennan I, Fordyce CB, van Diepen S, et al. The association of the average epinephrine dosing interval and survival with favorable neurologic status at hospital discharge in out-of-hospital cardiac arrest. Ann Emerg Med. 2019;74:797–806. doi: 10.1016/j.annemergmed.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 17. Morgan RW, Landis WP, Marquez A, Graham K, Roberts AL, Lauridsen KG, et al. Hemodynamic effects of chest compression interruptions during pediatric in-hospital cardiopulmonary resuscitation. Resuscitation. 2019;139:1–8. doi: 10.1016/j.resuscitation.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 18. Becker LB, Aufderheide TP, Geocadin RG, Callaway CW, Lazar RM, Donnino MW, et al. American Heart Association Emergency Cardiovascular Care Committee; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Primary outcomes for resuscitation science studies: a consensus statement from the American Heart Association. Circulation. 2011;124:2158–2177. doi: 10.1161/CIR.0b013e3182340239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nolan JP, Berg RA, Andersen LW, Bhanji F, Chan PS, Donnino MW, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein Resuscitation Registry Template for In-Hospital Cardiac Arrest: A consensus report from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia) Circulation 2019140e746–e757.. [DOI] [PubMed] [Google Scholar]
- 20. Matos RI, Watson RS, Nadkarni VM, Huang HH, Berg RA, Meaney PA, et al. American Heart Association’s Get With The Guidelines–Resuscitation (Formerly the National Registry of Cardiopulmonary Resuscitation) Investigators. Duration of cardiopulmonary resuscitation and illness category impact survival and neurologic outcomes for in-hospital pediatric cardiac arrests. Circulation. 2013;127:442–451. doi: 10.1161/CIRCULATIONAHA.112.125625. [DOI] [PubMed] [Google Scholar]
- 21. Meaney PA, Nadkarni VM, Cook EF, Testa M, Helfaer M, Kaye W, et al. American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators. Higher survival rates among younger patients after pediatric intensive care unit cardiac arrests. Pediatrics. 2006;118:2424–2433. doi: 10.1542/peds.2006-1724. [DOI] [PubMed] [Google Scholar]
- 22. Nadkarni VM, Larkin GL, Peberdy MA, Carey SM, Kaye W, Mancini ME, et al. National Registry of Cardiopulmonary Resuscitation Investigators. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295:50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 23. Bhanji F, Topjian AA, Nadkarni VM, Praestgaard AH, Hunt EA, Cheng A, et al. American Heart Association’s Get With the Guidelines–Resuscitation Investigators. Survival rates following pediatric in-hospital cardiac arrests during nights and weekends. JAMA Pediatr. 2017;171:39–45. doi: 10.1001/jamapediatrics.2016.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Andersen LW, Berg KM, Saindon BZ, Massaro JM, Raymond TT, Berg RA, et al. American Heart Association Get With the Guidelines–Resuscitation Investigators. Time to epinephrine and survival after pediatric in-hospital cardiac arrest. JAMA. 2015;314:802–810. doi: 10.1001/jama.2015.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R package for causal mediation analysis J Stat Softw 2014. 59 1 38 26917999 [Google Scholar]
- 26. Patel KK, Spertus JA, Khariton Y, Tang Y, Curtis LH, Chan PS. American Heart Association’s Get With the Guidelines–Resuscitation Investigators. Association between prompt defibrillation and epinephrine treatment with long-term survival after in-hospital cardiac arrest. Circulation. 2018;137:2041–2051. doi: 10.1161/CIRCULATIONAHA.117.030488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perondi MB, Reis AG, Paiva EF, Nadkarni VM, Berg RA. A comparison of high-dose and standard-dose epinephrine in children with cardiac arrest. N Engl J Med. 2004;350:1722–1730. doi: 10.1056/NEJMoa032440. [DOI] [PubMed] [Google Scholar]
- 28. Lindner KH, Ahnefeld FW, Schuermann W, Bowdler IM. Epinephrine and norepinephrine in cardiopulmonary resuscitation. Effects on myocardial oxygen delivery and consumption. Chest. 1990;97:1458–1462. doi: 10.1378/chest.97.6.1458. [DOI] [PubMed] [Google Scholar]
- 29. Chase PB, Kern KB, Sanders AB, Otto CW, Ewy GA. Effects of graded doses of epinephrine on both noninvasive and invasive measures of myocardial perfusion and blood flow during cardiopulmonary resuscitation. Crit Care Med. 1993;21:413–419. doi: 10.1097/00003246-199303000-00020. [DOI] [PubMed] [Google Scholar]
- 30. Berg RA, Otto CW, Kern KB, Sanders AB, Hilwig RW, Hansen KK, et al. High-dose epinephrine results in greater early mortality after resuscitation from prolonged cardiac arrest in pigs: a prospective, randomized study. Crit Care Med. 1994;22:282–290. doi: 10.1097/00003246-199402000-00020. [DOI] [PubMed] [Google Scholar]
- 31. Ristagno G, Tang W, Sun S, Weil MH. Cerebral cortical microvascular flow during and following cardiopulmonary resuscitation after short duration of cardiac arrest. Resuscitation. 2008;77:229–234. doi: 10.1016/j.resuscitation.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 32. Ristagno G, Sun S, Tang W, Castillo C, Weil MH. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation. Crit Care Med. 2007;35:2145–2149. doi: 10.1097/01.ccm.0000280427.76175.d2. [DOI] [PubMed] [Google Scholar]
- 33. Mavroudis CD, Ko TS, Morgan RW, Volk LE, Landis WP, Smood B, et al. Epinephrine’s effects on cerebrovascular and systemic hemodynamics during cardiopulmonary resuscitation. Crit Care. 2020;24:583. doi: 10.1186/s13054-020-03297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wenzel V, Lindner KH, Krismer AC, Miller EA, Voelckel WG, Lingnau W. Repeated administration of vasopressin but not epinephrine maintains coronary perfusion pressure after early and late administration during prolonged cardiopulmonary resuscitation in pigs. Circulation. 1999;99:1379–1384. doi: 10.1161/01.cir.99.10.1379. [DOI] [PubMed] [Google Scholar]
- 35. Cairns CB, Niemann JT. Hemodynamic effects of repeated doses of epinephrine after prolonged cardiac arrest and CPR: preliminary observations in an animal model. Resuscitation. 1998;36:181–185. doi: 10.1016/s0300-9572(98)00018-5. [DOI] [PubMed] [Google Scholar]
- 36. Lasa JJ, Rogers RS, Localio R, Shults J, Raymond T, Gaies M, et al. Extracorporeal cardiopulmonary resuscitation (E-CPR) during pediatric in-hospital cardiopulmonary arrest is associated with improved survival to discharge: a report from the American Heart Association’s Get With The Guidelines-Resuscitation (GWTG-R) Registry. Circulation. 2016;133:165–176. doi: 10.1161/CIRCULATIONAHA.115.016082. [DOI] [PMC free article] [PubMed] [Google Scholar]