Abstract
Wild ungulate species provide a much-needed protein source to many communities in developed and developing countries. Frequently, these game meat animals are slaughtered, and the meat is unknowingly contaminated by microorganisms and released to the unsuspecting public. This review investigates the global usage of organic acids (lactic and acetic acids) as microbial decontamination strategies during slaughter. The results show that there is a more open-minded approach to adopting possible decontamination plans as a tool to improve meat safety during slaughter. Developed countries continue to adopt these strategies, while developing countries are lagging behind. While decontamination of carcasses can lead to a reduction of microbial load on these carcasses, this strategy must not be seen as a replacement of hygiene management during the animals’ slaughter.
Keywords: abattoir, illegal slaughter, wild ungulate
1. Introduction
Food microorganisms can be found throughout meat processing plants. This suggests and highlights the importance of monitoring, controlling and ensuring that these organisms, especially pathogenic organisms, do not contaminate carcasses during slaughter [1,2]. Generally, carcasses are free of microorganisms when slaughtered in a hygienic manner, and the meat derived from the animal slaughtered remains safe and healthy after slaughter [3]. However, the processing of game/wild animals slaughter could cause meat contamination during killing and dressing in the field or slaughter at the abattoir from various sources such as faecal material, paunch and hide, processing tools and equipment, the facility, human contact, the environment (air, water, etc.), and carcass-to-carcass where insufficient space is left between already dressed carcasses and undressed carcasses on the slaughter line [4,5]. Under normal circumstances, there are two game-meat animal-killing methods employed in the field: killing with a single projectile shot or killing with a shotgun (utilising numerous pellets). These in-field killing processes coupled with exsanguination (neck slitting and/or thoracic sticking) and evisceration utilising spear cuts have the potential to leave open cuts on the body, thus exposing the meat to microbial contamination [6]. As stated by [7], slaughter processes, if not well monitored, may result in dangerous microorganisms being transferred from one carcass to another. While it can be argued that this type of contamination can be avoided by proper training of slaughter operators, meat inspection and the general abattoir hygiene application by personnel, total elimination of microorganisms cannot be achieved. It is noted that while carcass trimming on observable contaminated surfaces is mandatory during meat inspection, this practice could be seen as meat wastage and throwing away of good protein that is usable [8]. In response to this challenge, many researchers expressed the possible effective use of decontamination strategies to remove organisms that could be present on carcasses before being released to consumers [9,10,11]. The use of organic acids on surfaces of meat products or meat during processing has been investigated in the past [12,13]. Investigations have been done on the efficiency of reducing microorganisms by introducing organic acids producing bacteria on surfaces of meat products [14,15]. Most of the studies conducted were on processed meat products and poultry carcasses and a few were on red meat carcasses, and the usage of organic acids on fresh carcasses during slaughter still needs to be further investigated [13,16,17,18,19]. In describing microbial decontamination, Han et al. [19] state that these processes expose food products or carcasses to a specific agent, or a combination thereof, such as steam, chlorination, trisodium phosphate, pulsatile light exposure, pulsed electric fields or ionizing radiation, and organic acid solutions with the aim of reducing the amount or concentration of the microorganisms. In other instances, hot steam is used as a form of decontamination [20,21]. While it can be confirmed that decontamination can be used to effectively reduce the number of microorganisms, it must be viewed as a meat safety strategy to be added to existing programs of hygiene, such as the use of a two-knifes system during animal slaughter and dressing, prevention of animal hide from coming into contact with already dressed sides and meat inspection/trimming to physically remove visible contamination already implemented during slaughter [22]. Reference [23] note that various processes of meat decontamination are not generally accepted across the globe. For example, the United States of America (USA) has effectively implemented a carcass decontamination plan, whilst some European countries do not fully endorse the use of decontaminates as a form of improving the safety of meat products, with an exception of lactic acid and potable (chlorinated) water. A few developing countries approve the use of decontamination agents; this is mainly caused by a lack of data or information on the implementation of the decontamination plan in these countries. This situation is no different in South Africa, where only chlorinated water can be used to rinse fresh carcasses after slaughter before chilling; no other methods of decontamination are approved yet. However, the obligation lies with industry to prove the efficiency and effectiveness of a decontamination system before it can be approved to be used in the meat industry [24]. Given the ever-changing environments and the ever-growing demand for meat and thus demand for slaughter, measures that can benefit the industry and at the same time improve the safety of a specific product must be developed and implemented [23]. Many researchers have identified citric, lactic and acetic acid as possible organic acids that can be used to reduce some types and numbers of microorganisms in wild ungulate species meat. As a perishable product, meat of animal origin also carries a significant number of microorganisms. These organisms include but are not limited to Salmonella, Campylobacter and Escherichia coli and some strains of Listeria monocytogenes [7]. These microorganisms and many others must be identified, monitored and controlled in a food processing plant such as an abattoir [25].
2. Decontamination Plans for Game Meat Animals during Slaughter
While there are many decontamination plans and systems adopted in food processing, the situation is different at slaughter plants or abattoirs, where fewer decontamination plans may be used [18]. These include a combination of water used to wash carcasses and chilling. The chilling effect that the residual water may have during evaporative chilling can help to reduce the number of microbes on the carcass surfaces. Other strategies include the use of chlorinated water, organic acids such as lactic, acetic and citric acids, and hot steam [10,12,19]. The main challenges of these interventions are as follows [9,20]:
Most decontamination strategies may change the appearance and texture of a product.
Specific concentrations must be maintained to ensure that they do not alter the texture of meat products.
There is a lack of sufficient data or information on the usage of different decontamination regiments on carcasses whilst still maintaining the quality of the product.
There can be a large cost of implementing a decontamination plan on top of the general hygiene prescripts that must be followed during animal slaughter at an abattoir.
According to [21], the usage of specific organic acids at lower concentrations can achieve the desired effect of reducing or killing microorganisms without influencing the quality, texture, smell and appearance of meat. The important factors to be considered are the time of application, the simplicity of the process, the availability of the decontaminant and the concentration of the acid. Reference [8] note that microbial decontamination strategies or plans should be used as a secondary measure of limiting micro-organisms on carcasses and must not replace the general hygiene application and good manufacturing practices employed by meat processors with respect to hygiene requirements.
This clearly implies that if slaughter is done correctly with proper hygiene management, there should not be a need to do any additional decontamination [22]. For the purpose of this review, the usage of acetic acid and lactic acid was examined to determine their usage as a microbial decontaminant during the slaughter of game meat animals. The selection of these acids was influenced by their availability and usage in food processing and the fact that they are also organic in nature and thus more acceptable to food processors and authorities [1,8,22]. It must be emphasized that in South Africa, no form of carcass decontamination/treatment is yet approved. Forthe benefit of meat safety and improving the principles of hazards control during slaughter, the potential use of organic acids as forms of decontamination plans should be investigated.
2.1. Organic Acid Usage
Treatment of carcass surfaces with organic acids can have a positive result in the inhibition of microbial growth [11,21]. This is mainly due to the fact that organic acids tend to promote the disruption of the proton motive force (PMF) created by microorganisms on the cell surface. This disruption subsequently leads to the creation of an unfavorable environment for microorganisms to thrive [11]. As confirmed by [10], organic acids tend to influence the microbial activity on the treated surfaces such as fresh meat; this then leads to the increase in the pH of the surface to a level intolerable by general microorganisms. It is through these processes that an organic acid is able to reduce or kill microorganisms on treated surfaces.
2.2. Lactic Acid Treatment
Lactic acid (LA) is a naturally occurring acid and is used effectively by the food industry during food processing. Another source of LAs is food waste, particularly of dairy products and especially sour milk [24]. The reason behind this widespread usage includes its ability to mix well with water and its anti-microbial capabilities [11,21]. It is also used as a preservative in food products and in cleaning and sanitation of food and food contact surfaces [25]; LA has been effectively and extensively used as a decontaminant in the food industry for general microorganisms, some of which are pathogenic, such as Salmonella and Escherichia coli [26].
2.3. Acetic Acid Treatment
Acetic acid (AA) is another organic acid extensively used in the food industry. In addition to its preservation capabilities, AA can be used to kill or reduce other microorganisms of interest in food or meat products [27,28]. Researchers have argued that while the use of AA in its concentrated form could be beneficial in reducing microorganisms, its strong pungent smell could be a deterrent to its usage on fresh carcasses [10,29]. This could be overcome by mixing it to less than 4% or lower in concentration and spraying this mixture onto areas prone to contamination such as the neck area around the bleeding cuts, bullet entry points in the case of body kill on game meat animals, first and second spear cuts’ areas, hind legs opening lines, evisceration points and the brisket areas [30]. Researchers [10,11,21] have identified citric, lactic and acetic acids as possible organic acids that can be used to reduce the number of microorganisms in red meat. It is the aim of this review to critically evaluate the use of organic acids in raw game meat and game meat products with the purpose of their use as microbial decontamination agents.
3. Materials and Methods
This review was compiled from English scholarly literature as sourced from Google Scholar; Science Direct; PubMed between 2011 and 2021. The procedure used to search included entering the following key terms: “Carcases OR Game meat OR Wild meat AND Decontamination OR Lactic Acid OR Acetic Acids OR Carcass wash AND Africa OR Europe OR South America OR North America OR Asia OR Australia OR Oceania OR Antarctica”. Grey material from web pages of the Codex Alimentarius (www.fao.org) (Accessed: 28 June 2020) was also searched for the latest update regarding the implementation of decontamination plans by food authorities for fresh carcasses at abattoir levels across the globe (Table 1). Figure 1 depicts the search and review methodology of the literature pursued on organic acid usage as a decontaminant of fresh meat across the world as per the PRISM diagram adapted from [26].
Table 1.
Summary of the use of acetic and lactic acid to decontaminate carcasses derived from research (2011–2021). The order of presentation in ascending order of publication date.
| Country | Aim | Product Investigated | Experimental Conditions | Study Findings and Recommendations | Reference * |
|---|---|---|---|---|---|
| United States of America | To compare spray washing at 55.4 °C of a 2% levulinic acid with lactic or acetic acid for decontamination of pathogenic bacteria inoculated onto meat surfaces and their residual protection against later growth of pathogenic bacteria | Red meat and poultry |
|
|
[33] |
| Switzerland | To examine antibacterial activity of LA, AA and steam as decontamination treatments for cattle hides and beef carcasses | Beef |
|
|
[34,35,36] |
| United States of America | To examine mechanisms of reducing contamination by C. jejuni in broiler carcasses that were vaccinated with Lactobacilli as chicks. | Poultry |
|
|
[37] |
| Serbia | To investigate possible interventions of controlling Salmonella contamination during poultry, beef and pig slaughter | Poultry, beef and pork |
|
|
[38,39,40] |
| United States of America | To determine the effectiveness of eight antimicrobial compounds including LA and AA in a laboratory. | Beef surfaces |
|
|
[41] |
| Turkey | To compare the inhibitory effects of various decontamination agents at different OA concentrations on Listeria monocytogenes contaminated raw beef samples. | Beef |
|
|
[42] |
| Mexico | To investigate microbial adaptation to OA as antimicrobials to control Salmonella in meat and poultry products. | Poultry |
|
|
[43,44] |
| Canada | To investigate microbial decontamination of raw and ready-to-eat meats using OA | Raw and ready to eat meat |
|
|
[44,45] |
| Singapore | To establish different intervention technologies ensuring microbial safety of meat | Raw meat |
|
|
[46,47,48] |
| Spain | To investigate effective control and treatment plans for Campylobacter in abattoirs. | Poultry |
|
|
[49,50] |
| United States of America | To establish the efficiency and effect of different concentrations of LA, AA, citric and propionic acid dipping solutions on bacterial contamination of raw chicken skin | Poultry |
|
|
[51] |
| United States of America | To investigate the survival and adaptation of Salmonella spp. when subjected to acidic conditions on carcass surfaces. | Beef and porcine |
|
|
[52] |
| Greece | To analyse carcass decontamination strategies employable in slaughterhouses: a review | Meat animal carcass |
|
|
[35,53] |
| Sri Lanka | To investigate the effect of natural compounds and acids on Salmonella typhimurium in broiler chicken meat | Poultry |
|
|
[54] |
| United states of America | To determine the bactericidal activity of lactic acid (LA), levulinic acid (LV) and sodium dodecyl sulfate (SDS) applied individually and in combination with Shiga toxin-producing Escherichia coli (STEC) under laboratory conditions | Beef cuts-experiments on trimmings |
|
|
[55] |
| France | To investigate lactic acid bacteria (LAB) and their controversial role in fresh meat spoilage | Raw meat |
|
|
[56,57] |
| United States of America | To investigate antimicrobial formulations and sanitation methods for meat and poultry product processing. | Poultry |
|
|
[58,59] |
| Pakistan | Postharvest intervention technologies for safety enhancement of meat and meat-based products; a critical review | Beef |
|
|
[4,60] |
| United States of America | To evaluate the ability of a bromine-based antimicrobial lactic acid (LA) and peroxyacetic acid (PAA) applied in a final carcass wash to reduce non-pathogenic Escherichia coli. | Bovine |
|
|
[61] |
| Spain | To test the efficiency of lactic acid concentrations on the reduction of microbial load yet minimally impact the colour and sensory characteristics of beef | Beef |
|
|
[62] |
| Canada | To investigate possible pathogens reduction strategies employable at the primary production level especially in relation to multi drug-resistant strains | Raw meat |
|
|
[63] |
| Romania | To assess the efficiency of organic acids LA, AA and citric acid in different concentrations on pathogens such as Salmonella, Listeria and Escherichia on beef. | Beef |
|
|
[27] |
| Japan | To evaluate the effect of LA with and without organic material at various post-treatment recovery times on the heat resistance of Listeria monocytogenes. | Bovine products |
|
|
[18] |
| Egypt | To test the antibacterial effect of lactic acid (LA) and acetic acid (AA) on sheep carcass surface after 20 min of spraying. | Sheep carcasses |
|
|
[64] |
| United States of America | To investigate the effectiveness of organic acids (LA) on Salmonella ssp. reduction on ground beef. | Beef |
|
|
[65] |
| United States of America | To establish the interactions of organic acids (LA and AA) with Campylobacter coli from swine | Red meat |
|
|
[66] |
| Australia | To investigate meat safety risks for the Australian red meat market | Red meat |
|
|
[67,68] |
| Egypt | To investigate the effect of LA, AA and trisodium phosphate (TSP) spray on the microbiological population. | Beef carcasses |
|
|
[69] |
* Some of the original source papers predate 2011. LA = Lactic acid. AA = Acetic acid. OA = Organic acid.
Figure 1.
Methodology of search followed during the review process.
Records with no specific reference to carcass decontamination by acetic acid, lactic acid or organic acid; studies in languages other than English; and postgraduate theses were excluded from this study as they did not relate to the objective of the study. An overview of studies conducted between 2011 and 2021 globally is presented in Table 1.
4. Results
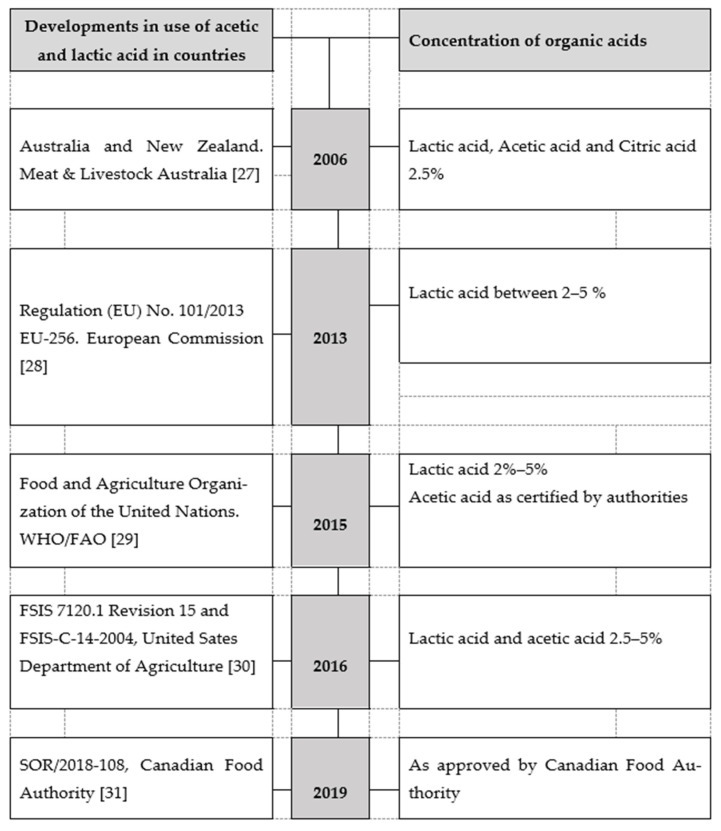
In an “any time” search on Google Scholar using the above-mentioned keywords, the first recommendation on the use of organic acids to decontaminate slaughter animal carcasses was as early as 1990 in Egypt [31]. It is evident that the concept of carcass decontamination is not new in the world. Strides have been made to improve and make these carcass decontamination plans applicable to wild ungulate species, especially in developed countries. Figure 2 provides a timeline of legislative advances and guidelines globally from 2006 (first year of detection during study) to the present. Evaluating country-to-country, Table 1 presents the frequency of acetic and lactic acids studies conducted between 2011 and 2021. The numbers of studies conducted were as follows in the United States of America (10), Canada (2), Spain (2), Serbia (1), Egypt (2), Switzerland (1), Australia (1) Japan (1), Greece (1), Sri Lanka (1), France (1), Pakistan (1), Romania (1), Turkey (1), Mexico (1), and Singapore (1). No information could be found on research done in any of the other countries of the world.
Figure 2.
Regulations and guidelines on the use of acetic and lactic acid to decontaminate carcasses globally. Note: The legislation and guidelines, or standards are from the inception of any relevant legislation. Existing and current regulations or guidelines are included from the date they became applicable or date of release. Some of the countries started developing these guidelines as early as 2006.
Figure 2 provides a summary of regulations and guidelines on the use of acetic and lactic acid to decontaminate carcasses globally.
While meat decontamination is continuously investigated globally, by the end of 2020, few countries had developed guidelines or regulations that dealt with carcass decontamination during slaughter [27,28,29,30,32]. As noted by [31], developing countries typically rely on the guidelines of the Food and Agriculture Organization (FAO) when applying carcass decontamination programs. However, the responsibility of approving such a plan belongs to the country where such a program is implemented.
5. Discussion
This review has shown that while extensive research has been done on meat animals, there is still more to be done in the application of organic acids (OA) in developing countries such as South Africa. Globally, South Africa is regarded as a diverse wild meat-producing country. A large proportion of these wild animals cohabitate with livestock, a situation that could lead to cross-contamination of microorganisms between these two groups of animals [22,70]. In fact, antibiotic-resistant microorganisms have been found between wildlife and farmed species in South Africa [71,72]. It is therefore surprising that no published works on adapted decontamination plans from livestock abattoirs for wildlife are available. While the demand for meat continues to grow, advances in improved slaughter, decontamination methods and legislation to produce safer meat products have been slow in developing countries and Africa as a continent [3,49]. This situation is no different from that of the consumption of (microbially safe) wild meat animals. In general, it can be accepted that the use of OA as a form of a microbial decontamination intervention during the slaughter of game meat animals can be adopted.
The decontamination of carcasses by organic acids such as LA and AA is reliant on the following factors: uniform application of the OA over the carcass at appropriate intervals, concentration of the OA and pH, temperature of the acid and of the carcass being treated, pressure of the application, contact time and a combination of decontamination strategies. Industries must decide on the best OA application plan to ensure an effective and efficient decontamination response. Reference [33] confirms that the majority of microorganisms do not survive in stronger acids; however, the concentration of these acids should be such that they are efficient whilst still retaining acceptable sensory qualities of the treated meat portion [21,73]. This is confirmed by [40], who noted that when higher concentrations of organic acids are used, secondary rinsing with potable water may be needed to remove the acids following their application. This is done to balance the sensory qualities of treated meat products. This could prove to be a challenge in wild animals as they are normally slaughtered in field-abattoirs where potable water is scarce [74].
The optimal pH values for an effective OA range between 2.5 and 3; while this could be seen as viable and possible to achieve, studies have also highlighted that the contact time between an OA and a surface is important in the reduction of microorganisms [55]. While the killing of microorganisms could occur within seconds, it is important to ensure that sufficient time between 2 and 10 min is observed to ensure sufficient treatment [65,75]. Additionally, the temperature of the acidic solution and the temperature of the surface or product could determine the efficiency of a decontamination plan. As noted by [31,76] an increase in the temperature of the OA solution and the application of OA while the carcass temperature was still warm led to the desired results of microbial reduction [38,73]; this could be enhanced by applying the OA solution in the form of steam between 50 and 55 °C while the carcass is still warm during the slaughter process [10,21]. This suggestion might be applicable in formal abattoirs where heating systems and potable water are readily available, but in field abattoirs where wild animals are frequently processed, heated water is limited; most decontamination systems make use of concentrated chlorine solutions [74].
Reference [77] explain that the influence of the steam solution temperature and carcass temperature can also be enhanced by increasing the pressure of the applicator; increasing the application pressure of the organic acid applicator achieved a log reduction of between 1 and 2 logs of aerobic microorganisms in raw meat [34]. This was confirmed by [78,79] where higher pressure yielded better microbial decontamination of Campylobacter on carcass surfaces. It can be accepted that there is no perfect system(s) that could guarantee the total elimination of microorganisms on meat surfaces during slaughter. It is important to ensure that the best possible microbial treatment interventions or a combination of interventions are adopted during slaughter. This is generally termed the hurdle technology approach. Hurdle technology can be described as using different microbial hurdles to achieve the basic condition of effective decontamination of carcasses during slaughter [80]. This technology could be applied effectively by using a combination of interventions such as improved hygiene application, improved meat inspection, trimming of suspected carcases/areas and introduction of organic acids in appropriate concentrations and pH on a carcass and swift chilling of dressed carcasses.
The OA could be applied as a single solution or in combination with other organic acids in the form of steam and/or water at varying pressures [66,81]. The development and use of OA in meat decontamination strategies must be done in a responsible and controlled manner to prevent the development of resistant strains of microorganisms.
References [71,72,82,83], as well as the study by [79], showed that prolonged usage of specific acids in a food facility may facilitate the development of resistant strains. As there are currently insufficient data available on microorganisms resistant to OAs, this situation should be monitored and controlled. Authors should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.
6. Conclusions
While decontamination is intended to remove or reduce the concentrations of microorganisms on carcass surfaces, the role of hygiene during slaughter should never be compromised. It is clear that given advancing research, more and more ideas for decontaminating game meat will be developed. Similarly, these ideas must be investigated for their effectiveness as well as usability, given that there are few factors that may determine the efficiency of these treatments, including: (1) temperature of the mixture, (2) temperature of the carcass, (3) time contact allowed settling on the carcass surfaces, (4) type of meat surface and (5) the pH of the carcass. These systems should include microbial monitoring at farm areas, minimisation of stress during the killing of animals, training of slaughter operators on hygiene application during slaughter and application of an accepted decontamination plan. It is important to note that in other instances, a combination regime of OA could be useful in the fight against microorganisms in game meat. In the South African context of the game meat industry, it is important that more investigations at abattoir or slaughter levels are conducted to determine the practical application of these treatments/methods (e.g., using heated/steam mixtures). It can be accepted that while there are many OAs used for carcass decontamination interventions, lactic acid (LA) and acetic acid (AA) have been adopted predominantly in meat products and carcasses in developed countries. Most of these interventions are product or species-based and cannot be used broadly for all meat animal species. There is clear evidence that LA and AA can reduce microbial colonies in beef, poultry, porcine and other meat products; what remains in question in general is their application in game meat and as part of an alternative process within a food safety management plan for the game meat industry.
Author Contributions
D.V.N. conducted the research as part of his doctorate degree in environmental health. This study was supervised by J.L.B. and L.C.H. All authors commented on early and final versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by the South African Research Chairs Initiative (SARChI) and partly funded by the South African Department of Science and Technology (UID number: 84633), as administered by the National Research Foundation (NRF) of South Africa, and partly by the Department of Trade and Industry’s THRIP program (THRIP/64/19/04/2017) with Wildlife Ranching South Africa as a partner and by Stellenbosch University. Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s), and the National Research Foundation does not accept any liability in this regard.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Matthews K.R., Kniel K.E., Montville T.J. Food Microbiology: An Introduction. John Wiley & Sons; Hoboken, NJ, USA: 2019. [Google Scholar]
- 2.Santos E.C.C.d., Castro V.S., Cunha-Neto A., Santos L.F.d., Vallim D.C., Lisbôa R.d.C., Carvalho R.C.T., Junior C.A.C., Figueiredo E.E.d.S. Escherichia coli O26 and O113: H21 on carcasses and beef from a slaughterhouse located in Mato Grosso, Brazil. Foodborne Pathog. Dis. 2018;15:653–659. doi: 10.1089/fpd.2018.2431. [DOI] [PubMed] [Google Scholar]
- 3.Mallhi I.Y., Sohaib M., Khan A.U., Nawaz M. Evaluating food safety knowledge, practices, and microbial profile of meat in abattoirs and butchery shops in Lahore, Pakistan. J. Food Saf. 2019;39:e12612. doi: 10.1111/jfs.12612. [DOI] [Google Scholar]
- 4.Sohaib M., Anjum F.M., Arshad M.S., Rahman U.U. Postharvest intervention technologies for safety enhancement of meat and meat based products; a critical review. J. Food Sci. Technol. 2016;53:19–30. doi: 10.1007/s13197-015-1985-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wardhana D.K. Risk Factors for Bacterial Contamination of Bovine Meat during Slaughter in Ten Indonesian Abattoirs. Vet. Med. Int. 2019;2019:2707064. doi: 10.1155/2019/2707064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hedman H.D., Varga C., Duquette J., Novakofski J., Mateus-Pinilla N.E. Food Safety Considerations Related to the Consumption and Handling of Game Meat in North America. Vet. Sci. 2020;7:188. doi: 10.3390/vetsci7040188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gouws P.A., Shange N., Hoffman L.C. Game Meat Hygiene: Food Safety and Security. Wageningen Academic Publishers; Wageningen, The Nearthelands: 2017. Microbial quality of springbok (Antidorcas marsupialis) meat in relation to harvesting and production process; pp. 67–92. [Google Scholar]
- 8.Kautto A.H., Vågsholm I., Niskanen R. Game Meat Hygiene–Food Safety and Security. Wageningen Academic Publishers; Wageningen, The Nearthelands: 2017. Reindeer–wild game ante and post mortem; pp. 141–152. [Google Scholar]
- 9.Yang X., Tran F., Wolters T. Microbial ecology of decontaminated and not decontaminated beef carcasses. J. Food Res. 2017;6:85–91. doi: 10.5539/jfr.v6n5p85. [DOI] [Google Scholar]
- 10.Hochreutener M., Zweifel C., Corti S., Stephan R. Effect of a commercial steam-vacuuming treatment implemented after slaughtering for the decontamination of cattle carcasses. Ital. J. Food Saf. 2017;16:6864. doi: 10.4081/ijfs.2017.6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Ba H., Seo H.-W., Pil-Nam S., Kim Y.-S., Park B.Y., Moon S.-S., Kang S.-J., Choi Y.-M., Kim J.-H. The effects of pre-and post-slaughter spray application with organic acids on microbial population reductions on beef carcasses. Meat Sci. 2018;137:16–23. doi: 10.1016/j.meatsci.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Barcenilla C., Ducic M., López M., Prieto M., Álvarez-Ordóñez A. Application of lactic acid bacteria for the biopreservation of meat products: A systematic review. Meat Sci. 2022;183:108661. doi: 10.1016/j.meatsci.2021.108661. [DOI] [PubMed] [Google Scholar]
- 13.Da Costa R.J., Voloski F.L., Mondadori R.G., Duval E.H., Fiorentini Â.M. Preservation of meat products with bacteriocins produced by lactic acid bacteria isolated from meat. J. Food Qual. 2019;2019:4726510. doi: 10.1155/2019/4726510. [DOI] [Google Scholar]
- 14.Hilbig J., Loeffler M., Herrmann K., Weiss J. Application of exopolysaccharide-forming lactic acid bacteria in cooked ham model systems. Food Res. Int. 2019;119:761–768. doi: 10.1016/j.foodres.2018.10.058. [DOI] [PubMed] [Google Scholar]
- 15.Doyle N., Mbandlwa P., Kelly W.J., Attwood G., Li Y., Ross R.P., Stanton C., Leahy S. Use of lactic acid bacteria to reduce methane production in ruminants, a critical review. Front. Microbiol. 2019;10:2207. doi: 10.3389/fmicb.2019.02207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casas D.E., Vargas D.A., Randazzo E., Lynn D., Echeverry A., Brashears M.M., Sanchez-Plata M.X., Miller M.F. In-Plant Validation of Novel On-Site Ozone Generation Technology (Bio-Safe) Compared to Lactic Acid Beef Carcasses and Trim Using Natural Microbiota and Salmonella and E. coli O157: H7 Surrogate Enumeration. Foods. 2021;10:1002. doi: 10.3390/foods10051002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aykın-Dinçer E., Ergin F., Küçükçetin A. Reduction of Salmonella enterica in Turkey breast slices kept under aerobic and vacuum conditions by application of lactic acid, a bacteriophage, and ultrasound. J. Food Saf. 2021:e12923. [Google Scholar]
- 18.Omori Y., Miake K., Nakamura H., Kage-Nakadai E., Nishikawa Y. Influence of lactic acid and post-treatment recovery time on the heat resistance of Listeria monocytogenes. Int. J. Food Microbiol. 2017;257:10–18. doi: 10.1016/j.ijfoodmicro.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Han J., Luo X., Zhang Y., Zhu L., Mao Y., Dong P., Yang X., Liang R., Hopkins D.L., Zhang Y. Effects of spraying lactic acid and peroxyacetic acid on the bacterial decontamination and bacterial composition of beef carcasses. Meat Sci. 2020;164:108104. doi: 10.1016/j.meatsci.2020.108104. [DOI] [PubMed] [Google Scholar]
- 20.Dan S.D., Mihaiu M., Reget O., Oltean D., Tabaran A. Lucrari Stiintifice-Medicina Veterinara. Volume 60. Universitatea de Stiinte Agricole si Medicina Veterinara “Ion. Ionescu de la Brad” Iasi; Iași, Romania: 2017. Influence on week organic acids on pathogens on swine carcasses; pp. 265–273. [Google Scholar]
- 21.Kure C.F., Axelsson L., Carlehög M., Måge I., Jensen M.R., Holck A. The effects of a pilot-scale steam decontamination system on the hygiene and sensory quality of chicken carcasses. Food Control. 2020;109:106948. doi: 10.1016/j.foodcont.2019.106948. [DOI] [Google Scholar]
- 22.Shange N., Gouws P., Hoffman L.C. Campylobacter and Arcobacter species in food-producing animals: Prevalence at primary production and during slaughter. World J. Microbiol. Biotechnol. 2019;35:1–16. doi: 10.1007/s11274-019-2722-x. [DOI] [PubMed] [Google Scholar]
- 23.Pohlman F., Dias-Morse P., Pinidiya D. Product safety and color characteristics of ground beef processed from beef trimmings treated with peroxyacetic acid alone or followed by novel organic acids. J. Microbiol. Biotechnol. Food Sci. 2019;2019:93–101. doi: 10.15414/jmbfs.2014.4.2.93-101. [DOI] [Google Scholar]
- 24.South Africa . In: Meat Safety Act. Act 40 of 2000. Agriculture D.O., editor. Goverment Gazzet; Pretoria, South Africa: 2000. No. 1106. [Google Scholar]
- 25.South Africa . Standard for Microbiological Monitoring of Meat Process Hygiene and Cleaning. Government Gazette; Pretoria, South Africa: 2010. VPN/15/2010/01. [Google Scholar]
- 26.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Dan S.D., Mihaiu M., Reget O., Oltean D., Tăbăran A. Pathogens Contamination Level Reduction on Beef Using Organic Acids Decontamination Methods. Bull. UASVM Vet. Med. 2017;74:2. doi: 10.15835/buasvmcn-vm:0052. [DOI] [Google Scholar]
- 28.European Commission Commission Regulation (EU) No 101/2013 of 4 February 2013 concerning the use of lactic acid to reduce microbiological surface contamination on bovine carcasses. Off. J. Eur. Union L. 2013;34:1–3. [Google Scholar]
- 29.WHO. FAO . Microbiological Risk Assessment Series 30. FAO; Rome, Italy: 2016. Interventions for the control of non-typhoidal Salmonella spp. in beef and pork. [Google Scholar]
- 30.United States Department of Agriculture (USDAS) In: Safe and Suitable Ingredients Used in the Production of Meat, Poultry, and Egg Products. Directive F., editor. USDA ERS; Washington, DC, USA: 2020. 7120.1 Rev. 55. [Google Scholar]
- 31.Signorini M., Costa M., Teitelbaum D., Restovich V., Brasesco H., García D., Superno V., Petroli S., Bruzzone M., Arduini V. Evaluation of decontamination efficacy of commonly used antimicrobial interventions for beef carcasses against Shiga toxin-producing Escherichia coli. Meat Sci. 2018;142:44–51. doi: 10.1016/j.meatsci.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Agency C.F.I. SOR/2018-108. Minister of Justice; Ottawa, ON, Canada: 2021. Safe Food for Canadians Regulations. [Google Scholar]
- 33.Carpenter C., Smith J., Broadbent J. Efficacy of washing meat surfaces with 2% levulinic, acetic, or lactic acid for pathogen decontamination and residual growth inhibition. Meat Sci. 2011;88:256–260. doi: 10.1016/j.meatsci.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 34.Loretz M., Stephan R., Zweifel C. Antibacterial activity of decontamination treatments for cattle hides and beef carcasses. Food Control. 2011;22:347–359. doi: 10.1016/j.foodcont.2010.09.004. [DOI] [Google Scholar]
- 35.Algino R., Ingham S., Zhu J. Survey of antimicrobial effects of beef carcass intervention treatments in very small state-inspected slaughter plants. J. Food Sci. 2007;72:M173–M179. doi: 10.1111/j.1750-3841.2007.00386.x. [DOI] [PubMed] [Google Scholar]
- 36.Bell K.Y., Cutter C.N., Sumner S.S. Reduction of foodborne micro-organisms on beef carcass tissue using acetic acid, sodium bicarbonate, and hydrogen peroxide spray washes. Food Microbiol. 1997;14:439–448. doi: 10.1006/fmic.1997.0108. [DOI] [Google Scholar]
- 37.Neal-McKinney J.M., Lu X., Duong T., Larson C.L., Call D.R., Shah D.H., Konkel M.E. Production of organic acids by probiotic lactobacilli can be used to reduce pathogen load in poultry. PLoS ONE. 2012;7:e43928. doi: 10.1371/journal.pone.0043928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buncic S., Sofos J. Interventions to control Salmonella contamination during poultry, cattle and pig slaughter. Food Res. Int. 2012;45:641–655. doi: 10.1016/j.foodres.2011.10.018. [DOI] [Google Scholar]
- 39.Carlson B.A., Geornaras I., Yoon Y., Scanga J.A., Sofos J.N., Smith G.C., Belk K.E. Studies to evaluate chemicals and conditions with low-pressure applications for reducing microbial counts on cattle hides. J. Food Prot. 2008;71:1343–1348. doi: 10.4315/0362-028X-71.7.1343. [DOI] [PubMed] [Google Scholar]
- 40.Castillo A., Lucia L., Goodson K., Savell J., Acuff G. Decontamination of beef carcass surface tissue by steam vacuuming alone and combined with hot water and lactic acid sprays. J. Food Prot. 1999;62:146–151. doi: 10.4315/0362-028X-62.2.146. [DOI] [PubMed] [Google Scholar]
- 41.Yoder S.F., Henning W.R., Mills E.W., Doores S., Ostiguy N., Cutter C.N. Investigation of chemical rinses suitable for very small meat plants to reduce pathogens on beef surfaces. J. Food Prot. 2012;75:14–21. doi: 10.4315/0362-028X.JFP-11-084. [DOI] [PubMed] [Google Scholar]
- 42.Elmali M., Yaman H., Tekinsen K.K., Öner S., Çekin E. Inhibitory Effects of Different Decontamination Agents on the Levels of Listeria monocytogenes in the Experimentally Inoculated Raw Beef Samples in the Laboratory Conditions. J. Fac. Vet. Med. 2012;18:763–768. doi: 10.9775/kvfd.2012.6371. [DOI] [Google Scholar]
- 43.Mani-López E., García H., López-Malo A. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res. Int. 2012;45:713–721. doi: 10.1016/j.foodres.2011.04.043. [DOI] [Google Scholar]
- 44.De Martinez Y.B., Ferrer K., Salas E.M. Combined effects of lactic acid and nisin solution in reducing levels of microbiological contamination in red meat carcasses. J. Food Prot. 2002;65:1780–1783. doi: 10.4315/0362-028X-65.11.1780. [DOI] [PubMed] [Google Scholar]
- 45.Gill A., Gill C. Microbial Decontamination in the Food Industry. Elsevier; Amsterdam, The Netherlands: 2012. Microbial decontamination of raw and ready-to-eat meats; pp. 30–59. [Google Scholar]
- 46.Chen J., Ren Y., Seow J., Liu T., Bang W., Yuk H. Intervention technologies for ensuring microbiological safety of meat: Current and future trends. Compr. Rev. Food Sci. Food Saf. 2012;11:119–132. doi: 10.1111/j.1541-4337.2011.00177.x. [DOI] [Google Scholar]
- 47.Avens J.S., Albright S.N., Morton A.S., Prewitt B.E., Kendall P.A., Sofos J.N. Destruction of microorganisms on chicken carcasses by steam and boiling water immersion. Food Control. 2002;13:445–450. doi: 10.1016/S0956-7135(01)00073-1. [DOI] [Google Scholar]
- 48.Stopforth J.D., Yoon Y., Belk K., Scanga J., Kendall P., Smith G., Sofos J. Effect of simulated spray chilling with chemical solutions on acid-habituated and non–acid-habituated Escherichia coli O157: H7 cells attached to beef carcass tissue. J. Food Prot. 2004;67:2099–2106. doi: 10.4315/0362-028X-67.10.2099. [DOI] [PubMed] [Google Scholar]
- 49.Rovira R.F., Bermudo F.M., Cameán A.M., Fernández A.C.S., Álvarez M.D., Marteache A.H., Toledano F.L., de Santos M.R.M., de Victoria Muñoz E.M., Larrañaga M.R.M. Report of the Scientific Committee of the Spanish Agency for Food Safety and Nutrition (AESAN) on the Control Strategies to Reduce the Burden of Campylobacter spp. in Fresh Poultry Meat (Broiler) AESAN; Madrid, Spain: 2012. Report number: 2012-005. [Google Scholar]
- 50.Koolman L., Whyte P., Meade J., Lyng J., Bolton D. Use of chemical treatments applied alone and in combination to reduce Campylobacter on raw poultry. Food Control. 2014;46:299–303. doi: 10.1016/j.foodcont.2014.05.041. [DOI] [Google Scholar]
- 51.Menconi A., Shivaramaiah S., Huff G., Prado O., Morales J., Pumford N., Morgan M., Wolfenden A., Bielke L., Hargis B. Effect of different concentrations of acetic, citric, and propionic acid dipping solutions on bacterial contamination of raw chicken skin. Poult. Sci. 2013;92:2216–2220. doi: 10.3382/ps.2013-03172. [DOI] [PubMed] [Google Scholar]
- 52.Burin R.C.K., Silva A., Jr., Nero L.A. Influence of lactic acid and acetic acid on Salmonella spp. growth and expression of acid tolerance-related genes. Food Res. Int. 2014;64:726–732. doi: 10.1016/j.foodres.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 53.Milios K., Drosinos E., Zoiopoulos P. Carcass decontamination methods in slaughterhouses: A review. J. Hell. Vet. Med Soc. 2014;65:65–78. doi: 10.12681/jhvms.15517. [DOI] [Google Scholar]
- 54.Madushanka D., Jayaweera T., Jayasinghe J., Ruwandeepika H. Effect of organic acids (citric, acitic, lactic) and natural compounds (nutmeg, mace, cardemom) on Salmonella typhimurium in broiler chicken meat; Proceedings of the ISAE 2014—International Symposium on Agriculture and Environment 2014; Ruhuna, Sri Lanka. 27 November 2014; pp. 273–277. [Google Scholar]
- 55.Zhao T., Zhao P., Chen D., Jadeja R., Hung Y.-C., Doyle M.P. Reductions of Shiga toxin–producing Escherichia coli and Salmonella Typhimurium on beef trim by lactic acid, levulinic acid, and sodium dodecyl sulfate treatments. J. Food Prot. 2014;77:528–537. doi: 10.4315/0362-028X.JFP-13-335. [DOI] [PubMed] [Google Scholar]
- 56.Pothakos V., Devlieghere F., Villani F., Björkroth J., Ercolini D. Lactic acid bacteria and their controversial role in fresh meat spoilage. Meat Sci. 2015;109:66–74. doi: 10.1016/j.meatsci.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 57.Jones R.J., Hussein H.M., Zagorec M., Brightwell G., Tagg J.R. Isolation of lactic acid bacteria with inhibitory activity against pathogens and spoilage organisms associated with fresh meat. Food Microbiol. 2008;25:228–234. doi: 10.1016/j.fm.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 58.Nesbakken T. Advances in Microbial Food Safety. Elsevier; Amsterdam, The Netherlands: 2015. Update on Yersinia as a foodborne pathogen: Analysis and control; pp. 33–58. [Google Scholar]
- 59.Delmore L.G., Sofos J., Schmidt G., Smith G. Decontamination of inoculated beef with sequential spraying treatments. J. Food Sci. 1998;63:890–900. doi: 10.1111/j.1365-2621.1998.tb17921.x. [DOI] [Google Scholar]
- 60.Álvarez-Ordóñez A., Fernández A., Bernardo A., López M. Acid tolerance in Salmonella typhimurium induced by culturing in the presence of organic acids at different growth temperatures. Food Microbiol. 2010;27:44–49. doi: 10.1016/j.fm.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 61.Bullard B., Geornaras I., Delmore R., Woerner D., Martin J., Belk K. Validation of Antimicrobial Interventions Including the Use of 1, 3-Dibromo-5, 5-Dimethylhydantoin Applied in a Final Carcass Wash in a Commercial Beef Harvest Operation. Meat Muscle Biol. 2017;1:121–122. doi: 10.22175/rmc2017.117. [DOI] [Google Scholar]
- 62.Rodríguez-Melcón C., Alonso-Calleja C., Capita R. Lactic acid concentrations that reduce microbial load yet minimally impact colour and sensory characteristics of beef. Meat Sci. 2017;129:169–175. doi: 10.1016/j.meatsci.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 63.Warriner K., Namvar A. New Aspects of Meat Quality. Elsevier; Amsterdam, The Netherlands: 2017. Current Challenges in Enhancing the Microbiological Safety of Raw Meat; pp. 191–222. [Google Scholar]
- 64.Saad S.M., Hassanin F.S., Salem A.M., Saleh E.A.E. Efficiency of some organic acids as decontaminants in sheep carcasses. Benha Vet. Med J. 2020;38:116–119. [Google Scholar]
- 65.Yeh Y., De Moura F., Van Den Broek K., De Mello A. Effect of ultraviolet light, organic acids, and bacteriophage on Salmonella populations in ground beef. Meat Sci. 2018;139:44–48. doi: 10.1016/j.meatsci.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 66.Beier R.C., Harvey R.B., Hernandez C.A., Hume M.E., Andrews K., Droleskey R.E., Davidson M.K., Bodeis-Jones S., Young S., Duke S.E. Interactions of organic acids with Campylobacter coli from swine. PLoS ONE. 2018;13:e0202100. doi: 10.1371/journal.pone.0202100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sumner J., Kiermeier A., Jolley J. Microbiological Food Safety and Storage Life of Australian Red Meat. AMPC; Sidney, Australia: 2018. Report number 2018-1086. [Google Scholar]
- 68.Barlow R.S., Mellor G.E. Prevalence of enterohemorrhagic Escherichia coli serotypes in Australian beef cattle. Foodborne Pathog. Dis. 2010;7:1239–1245. doi: 10.1089/fpd.2010.0574. [DOI] [PubMed] [Google Scholar]
- 69.Sallam K.I., Abd-Elghany S.M., Hussein M.A., Imre K., Morar A., Morshdy A.E., Sayed-Ahmed M.Z. Microbial decontamination of beef carcass surfaces by lactic acid, acetic acid, and trisodium phosphate sprays. Bio. Med. Res. Int. 2020;2020:2324358. doi: 10.1155/2020/2324358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van den Honert M., Gouws P., Hoffman L. Importance and implications of antibiotic resistance development in livestock and wildlife farming in South Africa: A Review. S. Af. J. Anim. Sci. 2018;48:401–412. doi: 10.4314/sajas.v48i3.1. [DOI] [Google Scholar]
- 71.Van den Honert M.S., Gouws P.A., Hoffman L.C. Escherichia coli Antibiotic Resistance Patterns from Co-Grazing and Non-Co-Grazing Livestock and Wildlife Species from Two Farms in the Western Cape, South Africa. Antibiotics. 2021;10:618. doi: 10.3390/antibiotics10060618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van den Honert M.S., Gouws P.A., Hoffman L.C. A Preliminary Study: Antibiotic Resistance of Escherichia coli and Staphylococcus aureus from the Meat and Feces of Various South African Wildlife Species. Food Sci. Anim. Resour. 2021;41:135. doi: 10.5851/kosfa.2020.e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neethling J., Hoffman L., Muller M. Factors influencing the flavour of game meat: A review. Meat Sci. 2016;113:139–153. doi: 10.1016/j.meatsci.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 74.Van Schalkwyk D.L., Hoffman L.C. Guidelines for the Harvesting & Processing of Wild Game in Namibia 2016. Ministry of Environment & Tourism; Windhoek, Namibia: 2016. [Google Scholar]
- 75.Zhang L., Ben Said L., Diarra M.S., Fliss I. Inhibitory activity of natural synergetic antimicrobial consortia against Salmonella enterica on broiler chicken carcasses. Front. Microbiol. 2021;12:972. doi: 10.3389/fmicb.2021.656956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Viator C.L., Cates S.C., Karns S.A., Muth M.K. Food safety practices in the US meat slaughter and processing industry: Changes from 2005 to 2015. J. Food Prot. 2017;80:1384–1392. doi: 10.4315/0362-028X.JFP-16-378. [DOI] [PubMed] [Google Scholar]
- 77.Chen H., Liu S., Chen Y., Chen C., Yang H., Chen Y. Food safety management systems based on ISO 22000: 2018 methodology of hazard analysis compared to ISO 22000: 2005. Accredit. Qual. Assur. 2020;25:23–37. doi: 10.1007/s00769-019-01409-4. [DOI] [Google Scholar]
- 78.Da Silva S., Farag K. The impact of lamb cleanliness and line speed on the effectiveness of steam vacuum and carcass wash as decontamination methods after slaughter. Meat Sci. 2021;171:108276. doi: 10.1016/j.meatsci.2020.108276. [DOI] [PubMed] [Google Scholar]
- 79.Castro V.S., Mutz Y.d.S., Rosario D.K.A., Cunha-Neto A., Figueiredo E.E.d.S., Conte-Junior C.A. Inactivation of Multi-Drug Resistant Non-Typhoidal Salmonella and Wild-Type Escherichia coli STEC Using Organic Acids: A Potential Alternative to the Food Industry. Pathogens. 2020;9:849. doi: 10.3390/pathogens9100849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Keeton J., Ricke S., Anderson R., Miller D., Azefor N. Application of Novel Hurdle Technologies to Meat Carcasses and Trimmings for Reduction of Pathogens. University of Arkansas; Fayetteville, AR, USA: 2008. FSIS-C-14-2004. [Google Scholar]
- 81.Dias-Morse P., Pohlman F., Pinidiya S., Coffman C. Microbial characteristics of ground beef processed from beef trimmings decontaminated by peroxyacetic acid alone or followed by organic acids interventions. Anim. Sci. Ark. Anim. Sci. 2013;12:105–109. [Google Scholar]
- 82.Shebs E., Lukov M., Giotto F., Torres E., de Mello A. Efficacy of bacteriophage and organic acids in decreasing STEC O157: H7 populations in beef kept under vacuum and aerobic conditions: A simulated High Event Period scenario. Meat Sci. 2020;162:108023. doi: 10.1016/j.meatsci.2019.108023. [DOI] [PubMed] [Google Scholar]
- 83.Projahn M., Sachsenroeder J., Correia-Carreira G., Becker E., Martin A., Thomas C., Hobe C., Reich F., Robé C., Roesler U. Impact of On-Farm Interventions against CTX-Resistant Escherichia coli on the Contamination of Carcasses before and during an Experimental Slaughter. Antibiotics. 2021;10:228. doi: 10.3390/antibiotics10030228. [DOI] [PMC free article] [PubMed] [Google Scholar]