Abstract
Background: Mycosis fungoides (MF) and Sezary Syndrome (SS) are the most common cutaneous T-cell lymphomas. It has been hypothesized that the interaction between the immune system, cutaneous cells, and neoplastic elements may play a role in MF/SS pathogenesis and progression. Methods: This paper aims to revise in a narrative way our current knowledge of the microenvironment’s role in MF/SS. Results and Conclusions: Literature data support a possible implication of microenvironment cells in MF/SS pathogenesis and progression, opening up new therapeutic avenues.
Keywords: cutaneous, lymphomas, cutaneous T-cell lymphomas (CTCLs), Sezary syndrome
1. Introduction
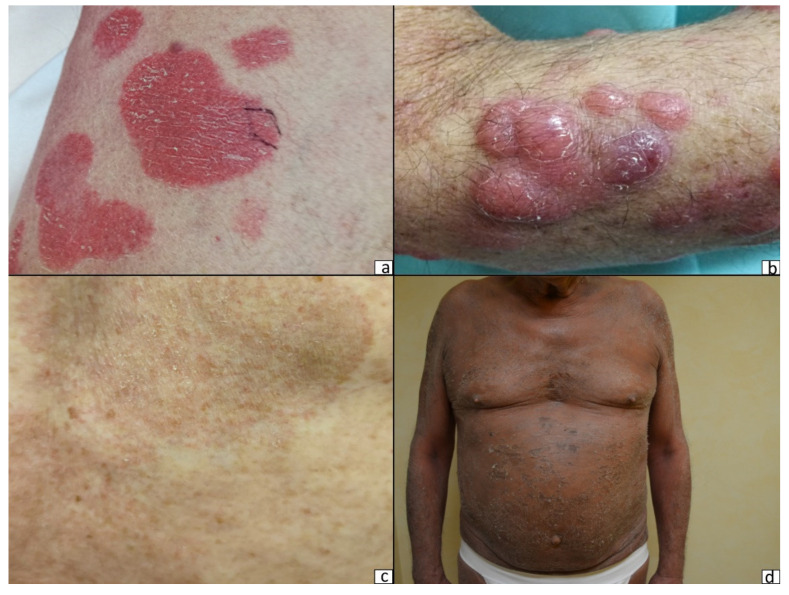
Mycosis fungoides (MF) and Sézary syndrome (SS) are the most common cutaneous T-cell lymphomas (CTCLs) [1]. Although MF and SS are closely related neoplasms, they are considered separate entities on the basis of differences in clinical behavior and the cells of origin. While Sézary syndrome (SS) is defined by the triad of erythroderma, generalized lymphadenopathy, and the presence of clonally related neoplastic T-cells with cerebriform nuclei (Sézary cells) in skin, lymph nodes, and peripheral blood, MF is characterized by a long-standing history of erythematous and scaly patch and plaque lesions, eventually evolving into erythroderma or tumor lesions (Figure 1 and Figure 2a–d). Such an evolution is fascinating from a clinical and therapeutic perspective owing to the different clinical outcomes (indolent in the early phases, aggressive in the advanced ones) and therapeutic approaches (skin-directed vs. systemic therapies). Although new treatment modalities have recently been proposed both for early [2] and advanced phases [3,4,5,6], the mechanisms involved in MF progression remain a matter of debate. It has been hypothesized that different players may be involved: aberrant molecular expression, genetic mutations, microRNA overexpression, changes in cytokine release, and different compositions of microenvironment cells [6].
Figure 1.
Different presentations of mycosis fungoides disease: (a) plaque stage, (b) tumor stage, (c) patch stage, and (d) erythroderma in a Sezary syndrome patient.
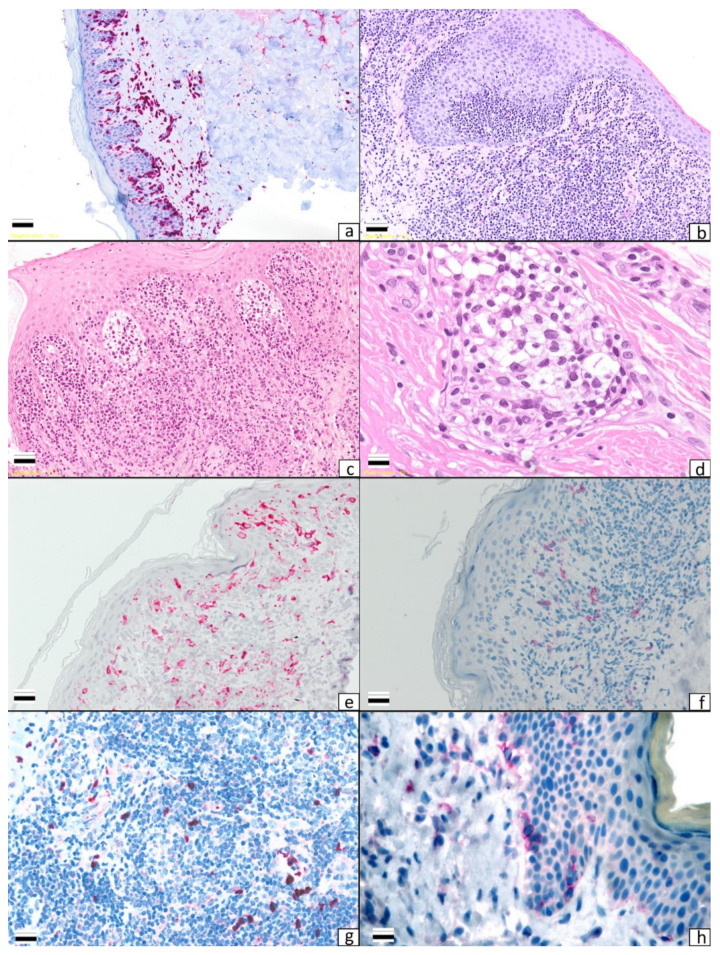
Figure 2.
Different histological patterns in mycosis fungoides: (a) patch stage (×10), (b) plaque stage (×10), (c) tumor stage (×10), (d) Sezary cells within a blood vessel in a Sezary syndrome patient (×40), (e) a high number of Langerin-positive cells in an early stage of mycosis fungoides (×10), (f) CD303-positive cells in the plaque stage of mycosis fungoides (×10), (g) a high number of myeloid-derived suppressor cells (Arginase+) in the tumor stage of mycosis fungoides (×10), (h) a high number of VEGF-A-positive cells in a patch stage case (×10).
The very first hypothesis that the immune system may reverse tumorigenesis and the spread of cancer cells to internal organs was proposed by Burnet et al. [7] in the 1950s. Since then, the immune system’s role has been investigated in many cancers. The tumor microenvironment can be defined as a complex system including cells and molecules that under certain conditions promote tumor growth and spread. Microenvironment cells can be defined as any type of cell that interacts with cancer cells to gain a specific phenotype and functions. Dunn et al. [8], in the 2000s, by proposing the so-called ‘immunoediting theory’, described three steps of interaction between neoplastic and microenvironment cells: elimination, equilibrium, and immune suppression. In the first step, the immune system reverses neoplasia in cells by inducing their apoptosis, leading to tumor destruction. In the case of failure, a sort of “equilibrium” between tumorigenic and anti-tumor actions can be observed. Such a balance between anti-tumor and tumorigenic actions will be lost later when the tumor cells acquire the ability to spread via the lymphatic and blood vessels. Under such circumstances, immunosuppression is predominant and induced by neoplastic cells by secreting immunosuppressive cytokines and recruiting immunosuppressive cells. An increased number of immature antigen-presenting cells within the microenvironment will be observed, leading to immune system anergy, the depletion of anti-tumor cells, and the accumulation of exhausted anti-tumor cells. Microenvironment changes in CTCLs have been the subject of several studies, and the aim of the present paper—which mainly focuses on MF—is to analyze the state of the art by reviewing the role of all microenvironment cells and analyzing all possible therapeutic approaches capable of reversing the microenvironment’s role from a tolerogenic to an anti-tumor one.
1.1. Dendritic Cells’ Role and Regulation in Anti-Tumor Immunity
First described by Langerhans in the late nineteenth century, the function of dendritic cells (DCs) has not yet been completely elucidated owing to the presence of different subsets featuring different functions. DCs act as the “sentinel” of the immune system and, as professional antigen-presenting cells (APCs), they activate naïve T-cells, orchestrating the innate and adaptive immune response [9]. However, DCs also induce tolerance by deleting self-reactive thymocytes, mediating the anergy of mature T-lymphocytes, and generating regulatory (Treg) cells [10]. The different actions exerted upon the immune system (activation/inhibition) are due to different DC subsets and different activation states. Immature DCs, by presenting antigens to T-cells in the absence of co-stimulatory signals (present on mature DCs), induce the development of Treg cells, eventually leading to tolerance [11]. Defective DC function has been related to many pathological conditions, such as autoimmune diseases, allergies, and cancers. In tumors, it has been hypothesized that the maturation state and location of DC infiltrates may be related to a different clinical outcome [10,12]. In humans, two main DC subsets can be observed: CD11c+, CD123−/low-myeloid DCs (mDCs) and CD11c-, CD123+ plasmacytoid DCs (pDCs). mDCs form the main group of professional APCs and can be observed in peripheral tissues, secondary lymphoid organs, and circulating blood. Two main populations of mDCs can commonly be observed in the skin: epidermal Langerhans cells (LCs) and dermal dendritic cells (DDCs). The former express the CD207/Langerin marker and the latter express the DC-DIGN/CD209 molecule [13]. pDCs are a unique cell population capable of producing large amounts of type I interferon (IFN) in the case of a viral infection. Type I IFN blocks viral replication, plays a pivotal role in linking the innate and adaptative immune system, and is fundamental to mDC activation. The pDC immunophenotype is characterized by the expression of CD123 and BDCA-2 (CD303) molecules [14].
1.2. Myeloid-Derived Suppressor Cells’ Role and Regulation in Anti-Tumor Immunity
Myeloid-derived suppressor cells (MDSCs) are a newly proposed cell population [15] whose nature and biological role have recently been clarified. They play a role as a regulator of the immune system response in many pathologic conditions. Two groups of MDSCs can be distinguished: granulocytic or polymorphonuclear MDSCs (PMN-MDSCs) and monocytic MDSCs (M-MDSCs). The former are phenotypically and morphologically similar to neutrophils, while the latter are more similar to monocytes [15]. Under chronic conditions and in cancers, there is a large accumulation of MDSCs featuring an immature morphology, weak phagocytic activity, and increased levels of arginase, nitric oxide (NO), and anti-inflammatory cytokines [16], leading to the inhibition of adaptative immunity and promoting tumor progression and spread. The most prominent factors implicated in MDSCs’ suppressive activity include high arginase and NO levels, upregulation of ROS, and the production of prostaglandin E2 (PGE2) [16]. MDSCs are thought to play an important role in cancer progression and high MDSC levels have been related to a worse clinical outcome in many cancers [17,18].
1.3. LCs, DDCs, and MDSCs in MF and SS
DCs’ role in MF has been the object of several studies posing the question as to whether DCs may play a crucial role in MF progression [10,19,20,21,22]. DCs are thought to attract tumor cells to the skin [23,24], eventually mediating the cross-presentation of tumor-related antigens within regional lymph nodes (Figure 2e). As a consequence, an expansion of effector T-cells will be observed. After expansion, anti-tumor lymphocytes will move to the skin as tumor-infiltrating lymphocytes (TILs) and will provide an anti-MF response after activation induced by costimulatory signals provided by infiltrating mature DCs. It has been postulated that the absence of or a reduction in mature DCs may lead to a dramatic defect in the above-described anti-tumor response, leading to tolerance and advantages in MF progression.
It has been hypothesized that LCs may play a role in MF and SS pathogenesis and progression. In 1976, Goos et al. [23] first proposed that LCs may play a role in MF pathogenesis owing to the detection of LCs in MF infiltrates, a finding later reported by two different groups [24,25]. In 2002, Luftl et al. [19], by comparing patch and plaque-stage MF to tumor-stage MF, found that equal numbers of immature and mature DCs were present in patch and plaque-stage MF. Most of the immature DCs were LCs observed within the epidermis, while equal numbers of mature and immature (CD1a+, CD1c+) DCs were interspersed between the lymphocytic infiltrates both in early and in advanced lesions. The authors’ conclusion was that DCs may play a double role, i.e., an anti-tumor role in early phases and a tumorigenic role in advanced ones. The induction of tolerance to tumor advantages may be due to the production of immunosuppressive cytokines, such as IL-10. Moving on, Schlapback et al. [10], by comparing MF tissue samples to healthy skin, found an increased level of DCs in MF patients. DCs interspersed within MF cells were mostly at an immature state (CD209/DC-SIGN1 DCs) and in close contact with tumor cells. The authors hypothesized that the accumulation of immature DCs may play a role in MF progression. Indeed, the accumulation of immature DCs may produce an immunosuppressive environment eventually facilitating tumor growth and spread. Moreover, by releasing chemotactic cytokines, MF cells may be able to recruit immature DCs from the blood, empowering the tumor immune escape response mechanisms. A further study [21] reached the same conclusions after analyzing the maturation state and distribution of DCs in 25 MF cases. A predominance of immature DCs over mature ones, as well as a positive correlation between the tumor infiltrate and DC numbers, was found. The authors [21] proposed that the inability of immature DCs to switch to a mature (and anti-tumor) state may be suppressed by MF cells. On the other hand, a defect in DCs’ activation may be the cause per se of an inefficient immune response and may explain the long course of the disease. The first finding of a positive correlation between the accumulation of immature DCs and MF progression was provided in 2014 [18]. The study described an increase in immature DC-SIGN+ DCs in tumor-stage MF compared with patch or plaque lesions. The authors’ conclusion was that immature DC-SIGN+ DCs induce immune suppression in the late stage of MF and may be a potential therapeutic target. The same conclusions have been further supported by other investigations [22] on the distribution of LCs, pDCs, and MDSCs in MF/SS tissue samples. A decrease in mature DCs (LCs in particular) was observed as well as an accumulation of immature DCs (pDCs) by comparing patch/plaque lesions to tumor lesions. Moreover, for the first time in the literature, in the same analysis an increase in MDSCs was detected. Taken together, these findings may reflect that the accumulation of immunosuppressive cells may be crucial in MF/SS progression (Figure 2f–g). This finding opened a debate on MDSCs’ role in MF/SS. Geskin et al. [26] observed a decrease in MDSC activity in IFN-responder patients, stressing the hypothesis of a possible role of MDSCs in MF/SS progression as well as MDSCs being a marker of response. The same conclusion was advanced by Argyropoulos et al. [27]. The authors observed that the presence of a high number of MDSCs featuring a granulocytic morphology could serve as a parameter for aggressive clinical behavior and, therefore, clinicians should provide aggressive treatment in such cases.
1.4. Regulatory B-Cells (Breg Cells)
Peripheral B-cells are a large category of cells featuring different phenotypic markers and functions [28]. B-cells are classically known to prompt an immune response and inflammation by promoting T-cell activation and proliferation as well as antibody production. In recent years, the presence of B-cells characterized by the ability to suppress the immune response has been postulated. The physiological role of those cells should be to avoid autoimmune diseases [29]. In analogy with regulatory T-cells (Tregs), regulatory B cells have been named Breg cells. Breg cells have the capacity to maintain immune tolerance via the release of immunosuppressive cytokines, such as IL-10, or by the expression of PD-L1 molecules [30,31,32]. Despite the efforts to analyze in detail the role and function of Bregs, the presence of Breg cells with different phenotypes remains a matter of debate. Different groups have described Breg cells with different phenotypes or roles [28,33,34,35,36]; however, it remains unclear whether Bregs should be regarded as a specific B-cell subset or whether, under unknown stimuli, B-cells acquire a suppressive function. However, a crucial indicator of Bregs seems to be the production of a large number of IL-10 cytokines.
1.5. Brigs in MF
It has been described that the presence of CD20+ tumor-infiltrating B-cells negatively modulates tumor growth in many different cancers and correlates to a better clinical outcome [37]. Other studies have shown that the presence of B-cells may be related to a worse clinical outcome and an empowerment of tumor angiogenesis [28]. Indeed, the presence of CD19+ infiltrating B-cells was related to a poor clinical outcome in ovarian cancer [38]. Other studies have shown that the presence of CD20+ B-cells and CD138+ plasma cells also had the same negative prognostic impact on ovarian neoplasms [39]. Such contrasting findings on B-cells’ prognostic impact in cancer remain a matter of debate. In MF, little is known and only a few reports are available. Akatsuka et al. [40] observed a decrease in CD19+ CD24hiCD27+ B-cells, CD19+ CD38hi B-cells, and IL-10-producing B-cells related to advanced MF phases. Their conclusion was that the decrease in IL-10-producing Bregs may play an important role in MF progression. Some case reports of MF patients with the CD20+ phenotype with a good response to rituximab (an anti-CD20 monoclonal antibody) are available in the literature [41,42], suggesting that Bregs may play a role in MF progression and encouraging further studies to shed light on their role.
1.6. Regulatory T-Cells (Treg Cells)
Regulatory T-cells (Tregs) are a T-cell population capable of negatively modulating the activity of other lymphoid cells. Tregs represent approximately 5%–10% of peripheral T-cells and express a CD4+ CD25+ phenotype, while their physiological role is to avoid autoimmune diseases and induce tolerance to self-antigens [43]. Tregs express the transcriptional repressor Forkhead box P3 (FOXP3), which is considered to be a specific marker of this T-cell subpopulation. However, Tregs play an important role in immune evasion by neoplastic cells, empowering tumor immune escape response mechanisms. In several types of cancer, an increase in Tregs is related to a worse clinical outcome, while in many hematologic diseases high numbers of FOXP3+ cells are related to a good prognosis with improved survival rates [44].
1.7. Tregs in MF and SS
Contrasting results on Tregs’ role in MF are present in the literature. At first, Berger et al. [45] proposed that MF cells may have a Treg phenotype, and so MF may be regarded as Treg neoplasia. Such a theory was further supported by others who observed an expression of FOXP3 in five MF cases with a large cell transformation [46]. However, the availability of a more specific FOXP3 antibody was crucial for refuting the initial speculation. Indeed, several groups demonstrated that FOXP3 expression is rare in neoplastic cells, while FOXP3+ cells are mainly non-neoplastic cells [47,48,49,50,51,52,53,54]. Most of the studies provided evidence of the presence of a high number of Treg cells in the early patch/plaque stage, while in advanced MF phases the number of FOXP3+ cells was lower [47,48,49,50,51,52,53,54,55]. In addition, Gjerdum et al. [49] related an increased Treg number to a better clinical outcome. Another study found low numbers of FOXP3+ cells both in tissue samples and in blood samples of Sézary syndrome (SS) patients [47], supporting the evidence provided by Gjerdum et al. [49]. The same findings at the molecular level were made by Johnson et al. [56] by observing a decreased expression of FOXP3 mRNA in skin samples in advanced MF stages. However, some studies provided criticism of the prognostic role of Treg cells in MF. Indeed, Fried et al. [54] found a non-stage-dependent expression of FOXP3 in 14 patients with sequential biopsies, evidence not confirmed by Zhang et al. [57]. However, the theory that Treg cells may not only suppress the anti-tumor response, as observed in solid malignancies, but may also negatively modulate MF cell growth contrasts with the finding that some treatments, such as mogamulizumab or lenalidomide, may decrease the numbers of FOXP3+ cells in treated patients [58,59,60]. Geskin et al. [26] added to our current knowledge the finding that Treg counts in SS patients may be underestimated. Indeed, by analyzing CD4+ CD25+ T-cells isolated from blood samples, the authors found a high level in Treg cells. The authors speculated whether in previous reports Treg counts may have been miscalculated due to the expansion of the malignant clone. Moreover, in their analysis the American group [26] found a connection between the Treg cell number and MDSC activity, suggesting crosstalk between the two populations. Taking all the studies together, it is clear that Tregs’ role in MF as well as in SS is yet to be fully elucidated.
1.8. Macrophages
Macrophages play important roles in inflammation (cytokine release, phagocytosis) and tissue repair (stem cell proliferation, angiogenesis, fibrosis). So-called “macrophage polarization” is a concept that explains how macrophages can be directed towards inflammatory or reparative functions by different stimuli from their microenvironments. Macrophages are a component of the innate immunity and are one of the major players in the leukocyte infiltrate [61]. Macrophages involved in tumor development are called tumor-associated macrophages (TAMs) and play a critical role in the biology of various types of cancers. Based on the response to various stimuli from tumor cells, macrophages are polarized into two main categories: M1 (classically activated) and M2 (alternately activated) [62]. M1 macrophages are induced by interferon (IFN)γ and have anti-tumor activity, producing inflammatory cytokines such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α, IL-6, and IL-23. M2 macrophages are induced by IL4 and promote a Th2 immune response and tumor growth and progression [62,63]. These two phenotypes are not stable and, considering the plasticity of macrophages, several in vivo studies have demonstrated that the polarization can change over time [64]. A high number of TAMs in the tumor microenvironment correlates to poor survival in patients with several cancer types, including hematological malignancies, such as follicular lymphoma, angioimmunoblastic T-cell lymphoma, Hodgkin’s lymphoma, and diffuse large B-cell lymphoma [65]. In particular, M2 TAMs are involved in immune suppression, tumor migration, invasion, and angiogenesis by releasing vascular endothelial growth factor (VEGF), matrix metalloproteinase (MMP)-7, MMP-9, IL-12, high levels of IL-10, TGF-β, hepatocyte growth factor and basic fibroblast growth factor, adrenomedullin, urokinase-type plasminogen activator, thymidine phosphorylase, prostaglandin E2, and semaphoring 4D [66]. Moreover, the subpopulation M2a, by IL4 and IL13 induction, promotes the expression of chemokine ligand (CCL) 24, CCL17, and CCL22, favoring the recruitment of eosinophils, basophils, and Th2 cells. The M2b subpopulation secretes CCL1, a chemokine that, with its receptor CCR1, promotes the infiltration of eosinophils, Th2 cells, and T regulatory (Treg) cells. Finally, the M2c subpopulation, by IL10 stimulation, expresses chemokine ligand (CXCL) 13, CCL16, and CCL18, which with their receptors CXCR5, CCR1, and CCR8 induce the accumulation of eosinophils and naïve T-cells with T-cell anergy [64].
1.9. Tumor-Associated Macrophages (TAMs) in MF and SS
The role of TAMs in the cutaneous T-cell lymphoma (CTCL) microenvironment has been also reported, showing that M2 TAMs are involved in the development and progression of CTCL. In their xenograft human CTCL cell model, Wu et al. [67] demonstrated an important role of macrophages in tumor development and lymph angiogenesis. The authors injected MBL2 T lymphoma cells into the ears of mice and induced lymphoma development by application of di-nitro-fluorobenzene. After checking for the presence of numerous M2 macrophages in the lesions, they used a clodronate-encapsulated liposome to selectively remove the macrophages and showed a strong reduction in skin tumors. Moreover, in the group of clodronate-treated mice, STAT3 was found to be down-modulated by podoplanin and CD31, which stained lymphatic and vascular vessels. By the expression of CD163, a marker of M2 macrophages, Sugaya et al. [65] demonstrated that M2 cells were significantly more numerous in CTCL lesional skin compared with normal skin, and their number increased as more tumor cells infiltrated. These data also correlated with a worse prognosis. Similarly, the serum of CTCL patients showed significantly higher levels of CD163 than those of normal controls, correlating also with serum IL-2R levels. M2 TAMs have also been correlated to lymph-node staging [68]. Indirectly, the role of macrophages in CTCL development was also demonstrated by the evaluation of CCL18, a chemokine involved in inflammatory skin reactions, by the recruitment of Th2 cells with CCL17 and CCL26. Previous in vitro studies showed the contradictory effects of CCL18 on CTCL cell lines [69]. Instead, Miyagaki et al. [70] showed high in vivo serum and skin levels of CCL18 in CTCL compared with controls and these data significantly correlated to modified severity-weighted assessment scores, serum sIL-2R, and a poor prognosis. The involvement of macrophages in CTCL has also been studied in early stages. Furudate S et al. [71] evaluated the activity of macrophages in each stage of MF, from the early stage to the tumor stage, and the role of periostin, an extracellular matrix protein that is known to be involved in the recruitment of Th2 cells and the polarization of M2 macrophages in the tumor microenvironment [72,73]. The authors found that in plaque-stage MF, periostin-stimulated macrophages are the dominant factor in the formation of the tumor mass and, after the plaque stage, M2-like macrophages are dominant in maintaining an immunosuppressive tumor microenvironment [71].
1.10. Keratinocytes
Keratinocytes represent 90% of the cell types present in the epidermis. Their function is to provide a barrier against external agents. However, keratinocytes play a role in immune system activation [74]. Epidermal keratinocytes express several tool-like receptors (TLRs). TLR expression by keratinocytes may be crucial for promoting skin immune responses, as the activation of these receptors on human keratinocytes leads to a predominant Th1-type immune response and to the production of type I interferon (IFN). Moreover, by expressing MHC class II molecules, keratinocytes act as non-professional APCs and it has been supposed that keratinocytes may display features of APCs with the potential for both antigen-specific tolerization and activation.
1.11. Keratinocytes in MF and SS
It has been hypothesized that interaction and cross-signaling between keratinocytes, stromal cells, and malignant T-cells may lead to MF and SS progression [74]. Different studies have highlighted the existence of a complex loop of continuous signaling between keratinocytes, fibroblasts, and malignant T-cells that eventually leads to the permanent activation of STAT proteins and induces the expression of tumorigenic (Th2) molecules [74]. Takahashi et al. demonstrated that a feedback loop between keratinocytes, stromal cells, and malignant T-cells leads to the activation of the STAT gene, resulting in a Th2 polarization of the inflammatory milieu. The same change within the microenvironment’s composition has the consequence of reinforcing STAT pathway expression [75]. STAT overexpression can also be induced by periostin, a molecule expressed by fibroblast elements. Different groups have observed high levels of periostin and thymic stromal lymphopoietin (TSLP) in both the serum and the lesional skin of CTCL patients [70,75,76]. Theoretically, high Th2 cytokine levels can induce periostin expression in dermal fibroblasts, stimulating epidermal keratinocytes to produce TSLP [75]. TSLP can induce STAT5 activation in malignant T-cells, promoting their survival and proliferation via IL-4 and IL-13 overexpression [75]. Furthermore, STAT5 overexpression downregulates STAT4 and SATB1 expression through the induction of microRNA-155 (miR-155) [77,78,79], empowering tumor immune response escape mechanisms and decreasing the secretion of anti-tumor molecules, such as IFNγ. Moreover, high periostin, IL-4, and IL-13 levels may stimulate the production of other immunosuppressive molecules, such as IL-25 [80,81]. High IL-25 levels have been observed in advanced MF and SS. Theoretically, high IL-25 levels may empower the expression of immunosuppressive cytokines, such as IL-13, via activation of the STAT6 pathway [81]. In conclusion, all of the above-mentioned findings illustrate that, after an initial increase in Th2 cytokines induced by neoplastic elements, the crosstalk between keratinocytes, fibroblasts, stromal cells, and malignant T-cells can start a complex loop of continuous signals that sustain and empower tumorigenic and depauperate anti-tumor action in CTCL [82].
1.12. Endothelial Cells
Angiogenesis is the process of formation of new blood vessels from existing ones and the same process involving lymphatic vessels is called lymph angiogenesis. Development of new vessels is complex and consists of different steps: migration, proliferation, and differentiation of endothelial cells, extracellular matrix degradation, and formation/stabilization of new vessels. All these steps are regulated by growth factors, cytokines, and other proteins. Neo-angiogenesis and lymph angiogenesis can be analyzed in different ways: by investigating micro-vessel density (characterized by MMP-2, MMP-9, and CD34 expression) or by analyzing vascular endothelial growth factor (VEGF) expression (VEGF-A for angiogenesis and VEGF-C for lymph angiogenesis).
1.13. Endothelial Cells in MF and SS
It has been speculated whether an increase in both angiogenesis and lymph angiogenesis may be related to MF progression. Angiogenesis in MF has been extensively investigated over the last three decades (Figure 2h). In 1997, Vacca et al. [83] found that the micro-vessel density (MVD) was higher in MF than in healthy controls by analyzing MMP-2 and MMP-9 mRNA levels. Moreover, the highest MVD was observed in tumor lesions. The same findings were later reported by Rasheed et al. [84] and Mazur et al. [85]. The latter [85] found that the mean number of CD34+ endothelial cells was significantly higher in MF than in normal skin samples. The increase in microvascular density levels were corroborated by further studies [86] demonstrating an overexpression in VEGF-A levels both at the protein and molecular level. The role of lymph angiogenetic markers has been analyzed by different authors. Karpova et al. [87] investigated lymph angiogenic marker expression (CD31, podoplanin, LYVE-1, VEGF-C, and VEGFR-3) in MF and SS cases. The authors found high levels of the selected markers, speculating a possible role in MF and SS pathogenesis. Jankowska-Konsur et al. [88,89] reported that an increase in density in lymphatic vessels was related to an increased possibility of lymph node metastases. The authors analyzed podoplanin and VEGF-C expression in MF and SS. Podoplanin, a glycoprotein expressed in the lymphatic endothelium, is related to the expression of vascular endothelial growth factor C (VEGF-C), a molecule that is considered to be a key stimulator of lymph angiogenesis [89,90]. Due to the presence of high podoplanin and VEGF-C levels in advanced MF and SS lesions, the authors proposed those markers as being related to aggressive clinical behavior. The same conclusion has recently been reached by other investigations [91] focused on lymphotoxin α (LTα)’s role in the formation of lymphatic vasculature and secondary lymphoid structures in CTCL. LT-α is involved in the regulation of cell survival, proliferation, differentiation, and apoptosis, can exert an anti-tumor or a tumorigenic function, and, as a pro-cancerogenic molecule, can play a role in angiogenesis. It has been observed that the expression of in situ LTα in CTCL cells is driven by an aberrantly activated JAK3/STAT5 pathway. LTα may act as an autocrine factor by stimulating the expression of IL-6 in malignant cells. Theoretically, LTα, IL-6, and VEGF may promote angiogenesis, inducing an increase in endothelial cells and, thus, promoting tumor growth and spread [92].
1.14. Tumor-Infiltrating Lymphocytes (TILs)
Tumor-infiltrating lymphocytes (TILs) represent a heterogeneous population of T-, NK, and B-cells activated against tumor cells. Infiltration of immune cells, particularly infiltration of anti-tumor type 1 lymphocytes, is associated with an improved prognosis in many different tumor types. However, due to immunosuppressive factors within the tumor microenvironment (TME), their tumor-killing ability is inhibited. Therefore, TILs exert a major role in the response of the immune system to tumor cells.
1.15. Tumor-Infiltrating Lymphocytes in MF and SS
An unusual and, therefore, fascinating feature of MF is that TILs have to control a malignant population from within their own lineage [93]. Unfortunately, studying the TILs in the MF microenvironment is technically challenging. The main problem, apart from the rarity of the condition, is that no single positive surface biomarker is able to accurately separate MF cells from the reactive, benign CD4+ T lymphocytes. Hence, all the methods used so far to distinguish MF cells from reactive CD4+ cells, except possibly for single-cell DNA analysis paired with a TCR repertoire, are hampered by a selection bias, sometimes potentially collecting only some of cells from a more heterogeneous tumor population, or by mislabeling a some of the reactive cells as cancerous. Taking into account these important technical limitations, multiple studies have highlighted the progressive shift from a Th1-enriched TME, as observed in early stage MF skin lesions, towards a Th2-oriented TME in the advanced stages, with the loss of Th1 markers and activated CD8+ cells and increased expression of Th2 markers, such as GATA3, IL-4, IL-5, and IL-13 [56,77,82,94,95,96,97,98]. Hence, these discoveries drove great expectations for IL-4 inhibitors as promising therapies in CTCL. Unfortunately, the use of the IL-4 inhibitor dupilumab (Dupixent) in patients with MF or SS may lead to rapid disease progression [99,100]. Hence, the “Th1 to Th2 disease progression” theory is likely an oversimplification. More data are needed, especially from early stage MF samples, to better understand, and potentially exploit, cytokine modulation in CTCL. More recent studies have proposed that the leading modification occurring in TILs of the MF TME is immune exhaustion. T-cell immune exhaustion is a dysfunctional status of T lymphocytes defined, classically, by the coexistence of a reduced cytotoxic activity, a decreased ability to secrete cytokines, increased expression of inhibitory receptors, reduced proliferation, and a reduced survival rate [101]. More recently, it has been proven that the exhausted phenotype is best described by its unique transcriptomic signature. In particular, the key role of thymocyte-selection-associated high-motility group box protein (TOX) in regulating the exhausted phenotype of CD8+ T-cells [102], and likely of CD4+ T-cells, has been postulated. TOX is a nuclear binding protein that plays a fundamental role in in the maturation of T-cells and NK cells but is also critical to the differentiation of tumor-specific T-cells [103]. Chronic TCR signaling is the main driver of immune exhaustion. During this process, TOX has been proven to induce immune exhaustion by increasing the expression of several exhaustion-related genes and suppression of effector-related ones, and it has been demonstrated that exhausted T-cells do not form in its absence [104]. Multiple studies focused on the genomic and transcriptomic profile of CTCL by WES, and WGS and bulk RNA-Seq have highlighted recursive perturbations on multiple points in the TCR-signaling machinery [105,106,107,108,109,110,111]. Most of these alterations converge into an abnormal chronic activation status. In particular, there is a growing body of literature showing the increased expression of TOX in CTCL both by tumor cells and by CD4+ and CD8+ reactive T-cells in skin and blood samples [112,113,114]. Perhaps not surprisingly, TOX is a good biomarker on immunohistochemistry to differentiate CTCL from benign inflammatory dermatoses. [107,115]. It has been identified that CD4 and CD8+ TILs and tumor cells obtained from lesional skin samples in MF cases show increased co-expression of the typical exhausted T cell surface markers PD-1, TIGIT, and TIM-3 [93]. This increase was not shared with paired T-cells from PBMCs, suggesting a local modulation rather than a generalized immune exhaustion. The degree of expression of these markers was broadly uniform among patients and among stages, suggesting that this immune exhaustion may occur as an early event in MF biology and as a commonly affected pathway.
1.16. NK Cells
NK cells work as the effector branch of the innate immune system and undergo activation in response to a reduction or complete abrogation of the self-human leukocyte antigen (HLA-I) alleles on tumor cells, with the release of granzyme and perforin alongside the production of IFN-gamma and TNF alpha [116]. NK cells are the best-studied elements among the innate immune system, and their anti-tumor role has been extensively studied. They are the most abundant innate immune cells, and their numbers correlate directly with a better prognosis and a reduced risk of metastasis [117,118].
1.17. NK Cells in MF and SS
NK cells may strongly express PD-1 (in a KIR+ NKG2A-CD57+ subpopulation) in PBMCs in healthy donors, and their count is stable over time, regardless of the donor’s age [119]. Their increase in the TME (but not in the paired PBMCs) has been proven to be associated with a worse prognosis in several solid and hematologic tumors, but not yet in MF or SS. [120,121,122] These NK cells have shown increased anti-tumor activity against PD-1/PD-L2+ tumor cells and may be relevant to the treatment of tumors showing a T-cell-resistant phenotype. Hence, one approach to improve the responses would be to combine an anti PD-1 or PD-1L agent with a checkpoint inhibitor related to NK cells. NK cells also express Killer Immunoglobulin Receptors (KIRs), which can be functionally divided into inhibitory (iKIRs) and activating (aKIRs). In particular, NK cells recognize and kill cells lacking or poorly expressing the ligand of their iKIR (“missing self-hypothesis”). Hence, these cells are extremely important in recognizing tumors downregulating HLA-I molecules. On the other hand, if a tumor does not downregulate its HLA-I, iKIRs aid the immune evasion; thus, blocking the iKIR–HLA interaction can boost the immune response against HLA-I+ tumors. [123] NKG2A is an inhibitory receptor co-expressed with CD94 in about 50% of NK cells in the peripheral blood [124]. CD94 and NGK2A work by recognizing HLA-E, which is ubiquitously expressed on normal human tissue, and its interaction with CD94/NGK2A determines a strong inhibition of the activating receptor NKG2C [125]. Hence, this is another “self-signaling” mechanism inducing self-tolerance and preventing NK-mediated autoimmunity. Unfortunately, tumor cells might upregulate the expression of HLA-E, effectively reducing the anti-tumoral NK and other cytotoxic lymphocyte-mediated responses, leading to a worse prognosis [126,127,128,129,130,131]. Hence, targeting the NGK2A axis seems to be a clever approach to reversing cytotoxic inhibition in several malignancies. However, there is a lack of data on NK cells’ role in the TME and progression in MF. Sako et al. [132] observed an increase in expression of the NK receptor KIR3DL2 (CD158k) in MF, proposing the hypothesis that MF cells may originate from a subset of NK cells expressing CD160 and KIR3DL2.
1.18. Eosinophils
Eosinophils are innate immune cells involved in the protective immune response of the host against helminths and viral and microbial pathogens. Human eosinophils derive from CD34+ CD117+ pluripotent hematopoietic stem cells in the bone marrow, where they complete their maturation and subsequently enter the bloodstream [133]. Phenotypically, eosinophils are characterized as CD11b+/Gr-1lo/F4-80+ cells. These markers are also found on macrophages, but eosinophils can be distinguished due to their high granularity, lack of expression of MHC-II, and expression of the sialic-acid-binding lectin Siglec-F [134]. Eosinophils are recruited from the blood into the sites of inflammation where, upon activation, they can release an array of inflammatory mediators, such as cationic proteins (major basic protein (MBP) and eosinophil cationic protein (ECP)), eosinophil peroxide (EPX), and eosinophil-derived neurotoxins (EDNs), that are unique to eosinophils and are important in the defense against parasitic infections [135]. Worth noting is that IL-5, IL-3, and GM-CSF are crucial for supporting the maturation of human eosinophils in the bone marrow [136] and mediate their survival by NF-κB-induced Bcl-xL, which inhibits apoptosis.
1.19. Eosinophils’ Role in MF and SS
Evidence indicates the presence of eosinophils in the TME of several human hematological and solid tumors, even if the mechanisms responsible for the infiltration of eosinophils into the tumors are not completely known [137,138,139]. However, some data show that the high-mobility group box 1 protein (HMGB1), IL-1α, and IL-33 potentially trigger eosinophil recruitment [140]. Moreover, macrophages and MCs can recruit eosinophils via the production of VEGFs [141,142] and/or the release of histamine and prostaglandin D2 (PGD2) through the activation of the chemoattractant-homologous receptors expressed on Th2 cells (CRTH2) [143] and H4 receptors [144], respectively. In the TME, eosinophils influence other leukocytes, such as T-cells, NK cells, DCs, and macrophages. In particular, they are able to recruit and activate T-cells through CXCL9, CXCL10, and CCL5, attract NK cells by IL-6, IL-12, and CXCL10 production, and induce M1 polarization [145]. Therefore, the presence of eosinophils in the tumor or in the bloodstream is a favorable prognostic factor for many cancers, although evidence of a pro-tumorigenic role for eosinophils has been reported [146]. Recent findings revealed that eosinophils display regulatory functions towards other immune cell subsets in the TME or direct cytotoxic functions against tumor cells, leading to either anti-tumor or pro-tumor effects. This paradoxical role of eosinophils was suggested to be dependent on the different factors in the TME [145]. Usually, in CTCLs eosinophils are rare within the infiltrate. Some authors have described a significantly higher number of eosinophils in the advanced stages than in the early stages, while other studies did not find correlations between the number of these cells and the stage of the disease. In 2016, Iliadis et al. observed a virtual absence of eosinophils in the early MF stage. In the study, there was no statistically significant correlation between the number of eosinophils and the stage of the disease, nor between the number of cells and the treatment response [147]. Other authors did not find significant correlations between the number of infiltrating eosinophils and the disease stage in MF [148]. However, many studies proposed an active role for eosinophils within the infiltrate in CTCL [149,150]. Ionescu et al. suggested that both the density and activation of tissue eosinophils were significantly related to disease progression in 26 primary CTCLs (including MF cases) with blood eosinophilia [151]. Theoretically, an accumulation of eosinophils in the TME may be related to disease progression, indicating a pro-tumor role of eosinophils in CTCLs or, at least, no anti-tumor actions. A suggested mechanism in which STAT3 activation in neoplastic T-cells leads to eosinophil accumulation in the TME through IL-5 production by malignant T-cells was hypothesized by Fredholm et al. The authors speculated as to whether malignant T-cells may “trap” inactivated eosinophils by a high secretion of cyclooxygenase 2 and prostaglandin E2 (PGE2) [152]. Finally, a massive presence of eosinophils in the tumor infiltrate was observed in erythrodermic mycosis fungoides (E-MF) [153] and in follicular mycosis fungoides (F-MF) [154,155].
1.20. Fibroblasts
Fibroblasts (FBs) are cells that synthesize and integrate structural proteins, such as collagen and elastin, into the extracellular matrix (ECM) of most mesenchymal tissues. Furthermore, FBs play an essential role in maintaining the structural integrity of most tissues. As a consequence, it is not surprising that cancer-associated fibroblasts (CAFs) are the most abundant type of cells within the tumor microenvironment (TME). They are activated fibroblasts that share several similarities with fibroblasts found in fibrotic tissues or during the healing phase of a wound [156]. Their presence in a tumor is associated with a poor prognosis in several types of cancer [157,158,159]. The recruitment of activated fibroblasts in many cancers is dependent on transforming growth factor beta (TGFβ) [160,161]. Local CAF proliferation and invasion is stimulated by TGFβ secretion by TME cells. Moreover, in the TME, CAF-derived TGFβ plays a key paracrine role in controlling epithelial carcinogenesis. More specifically, TGFβ secreted by CAFs promotes the epithelial-to-mesenchymal transition (EMT) process by weakening intercellular epithelial adhesion [162]. Furthermore, CAF-derived TGFβ stimulates the EMT in the adjacent cancer cells in various types of cancer [163,164]. Activation of fibroblasts could reflect a host defense mechanism to restrain cancer progression and potentially eliminate cancer [165,166,167,168]. Nevertheless, in solid tumors, the ability of CAFs to influence tumor growth was partly dependent on their ability to induce angiogenesis by CXCL12 and to recruit bone-marrow-derived endothelial cells [169]. Theoretically, in the early stages of neoplasia, inflammatory cues, emerging from pathological tissue remodeling, may initiate pro-inflammatory and tumor-promoting functions in fibroblasts. IL 1β secretion by immune cells in early lesions emerges as a potential initiator of nuclear factor κB (NF κB) signaling in fibroblasts, instructing them to produce a pro-tumorigenic secretome [170].
1.21. Fibroblasts’ Role in MF and SS
In CTCLs, the presence and role of CAFs remain unknown. In a recent study, Aronovich et al. sought to characterize CAFs in MF using primary fibroblast cultures from punch biopsies of patients with early stage MF. They found increased levels of CAF-associated genes and proteins, particularly CXCL12, collagen XI, and MMP2. MyLa cells cocultured with MF-derived fibroblasts reduced their sensitivity to doxorubicin and enhanced their migration. The authors speculated whether CAFs may protect MF cells from doxorubicin-induced cell death and may increase their migration through secretion of CXCL12. Finally, the authors proposed that targeting CAFs in MF may improve the efficiency of anti-cancer therapy [171].
1.22. Cytokines’ Influence on the Tumor Microenvironment’s Composition in MF/SS
Changes in the composition of the tumor microenvironment are driven by the release of cytokines from neoplastic cells. Although in early phases MF cells are few and interspersed between inflammatory milieu, they can acquire the ability to shift the immune system response from an anti-tumor (Th1) to a tumorigenic (Th2) one [172]. In brief, the shift from a Th1 to a Th2 inflammatory response will lead to an increase in immunosuppressive cytokine release (IL-2, IL-4, IL-7, IL-13, and IL-15) by neoplastic elements and tumor-associated cells, sustaining tumor growth and spread. As a consequence, there will be an accumulation of immature and immune-suppressive DCs and a depletion of mature (anti-tumor) ones as well as a recruitment of immunosuppressive cells (Treg cells and MDSCs) from blood vessels. Such a cascade of events will eventually empower tolerance and immune suppression by neoplastic cells, providing advantages to tumor growth and spread that are also driven by an increase in angiogenetic growth factor [87,172]. The overexpression of Th2 cytokines will also determine an overexpression of STAT3 and STAT5 pathways, which may be cytokine-dependent in early stages [173]. Changes in cytokine secretion in early and advanced MF/SS phases may pave the way to new target drugs able to restore an anti-tumor response and reverse the accumulation of immune-suppressive cells within the tumor microenvironment.
2. Materials and Methods
All of the literature concerning mycosis fungoides and microenvironment cells between 1950 and 2021 was examined. In particular, the keywords “mycosis fungoides” and “dendritic cells”, “mycosis fungoides” and “keratinocytes”, “mycosis fungoides” and “fibroblast”, “mycosis fungoides” and “endothelial cells”, “mycosis fungoides” and “tumor infiltrating lymphocytes”, “mycosis fungoides” and “eosinophils”, “mycosis fungoides” and “NK cells”, “mycosis fungoides” and “macrophages”, “mycosis fungoides” and “regulatory T and B cells”, and “mycosis fungoides” and “tumor associated macrophages” were searched for on PubMed. Data collected on the study were examined and elaborated in a narrative way.
3. Conclusions
The role of the microenvironment in MF progression is fascinating. Indeed, apart from genetic mutations [174,175,176], one alternative player involved in the progression from early to advanced lesions, the microenvironment may play a crucial role in MF and SS progression. Overall, microenvironment changes lead to immunosuppression given the presence of immature APCs or by the recruitment of immunosuppressive cells. Such a finding can pave the way to new therapeutic approaches focused on reversing the role of the microenvironment from an immunosuppressive to an anti-tumor one. From this point of view, some well-known drugs acting on the empowerment of the anti-tumor response are available: interferon-α (IFN- α) and bexarotene [177]. IFN-α directly enhances cell-mediated cytotoxicity and suppresses Th2 cytokine production by malignant T-cells. Geskin et al. observed a reduction in Treg cell and MDSC activity [26]. Bexarotene, by inducing malignant T-cell apoptosis and suppression of IL-4 production, may stimulate an anti-tumor response in responder patients [178]. A modest effect can also be obtained by retinoic acid receptor-specific (RAR-specific) administration (i.e., acitretin) by an increase in IL-12-dependent IFN- α production [177]. Another possible strategy to re-awaken the immune system is vaccination. Kim et al. [179] obtained systemic clinical responses in one-third of enrolled patients in a clinical trial based on the administration of intra-tumoral injection of a TLR9 agonist after a radiotherapeutic procedure. Most of the patients were highly treated non-responder patients. Currently, the tool-like receptor agonist seems to be a promising treatment focused on immune system empowerment. Imiquimod (a TLR7 agonist) works by inducing the release of a massive number of local cytokines, including type I IFNs, against MF. Anecdotal evidence seems quite promising, with a response rate ranging from 50% to 100% [180]. Other TLR agonists, such as topical resiquimod (a TLR7/8 agonist), have produced higher response rates (9 PR and 2 CR in 12 patients). Other experiences with the use of vaccines in MF are few, despite the attractive mechanism of action of sensitizing the host immune system against MF and SS neoantigens. Maier et al. [181] observed quite promising results (an ORR of 50%) with a vaccine of tumor-antigen-specific dendritic cells (generated by pulsing the cells in an autologous tumor lysate). Similar results were obtained by the use of attenuated virus after IFN-α treatment [182]. Extracorporeal photopheresis (ECP) is another treatment option that predominantly acts through immunomodulation, and its efficacy has been emphasized by 19 trials with over 400 patients [183]. By shifting from a Th2 to a Th1 cytokine pattern release, ECP plays a role in the anti-tumor host response. However, ECP is highly effective in SS owing to its ability to induce the apoptosis of circulating neoplastic cells. Another promising therapeutic scenario is represented by immunotherapy, although limited data are available. A phase I study of nivolumab demonstrated a 15% ORR in 13 patients [184]. Clinical trials on pembrolizumab in advanced-stage, highly pre-treated CTCLs showed a 56% and 27% ORR in MF and SS patients, respectively [185]. Only a case report of the application of ipilimumab to treat melanoma in a patient with concurrent MF is present in the literature [186]. There is an urgent unmet clinical need to identify the best candidates for immune therapy and also try to increase the response rates. Chimeric Antigen Receptor T-cells (CAR-T cells) may be another possible option. Indeed, CAR can be engineered to target specific antigens and to be inserted into T-cells to eliminate cells expressing those antigens. The principal problem in a T-cell malignancy is related to distinguishing between normal and tumor CAR. Two major problems are related to CAR-T cell use: mutual killing of CAR-T cells (fratricide) and T-cell aplasia induced by the destruction of normal T-cells. Another possible risk is contamination with circulating tumor T-cells when autologous T-cells are harvested to develop CAR-T cells [187]. The widely available evidence that TAMs are involved in CTCL development and associated with a poor prognosis suggests that macrophages can be a potential therapeutic target. Pharmacological drugs such as thalidomide, lenalidomide, pentoxifylline, and genistein are able to inhibit macrophage infiltration and reduce the tumor’s size, while other drugs can work against macrophage-induced angiogenesis, such as anti-VEGF-A and avastin/bevacizumab [64]. The therapeutic effects of bexarotene, a third-generation X receptor retinoid, are partially attributable to suppressive effects on the production of CCL22 by M2 TAMs [188]. Evidence of strong expression of CD30 on TAMs in MF and SS patients suggests that depletion of macrophages is one of the possible targets of the anti-CD30 monoclonal antibody Brentuximab vedotin [189]. TAMs also express PD-L1 and this expression negatively correlates with their phagocytic activity, so monoclonal antibodies that block the PD1/PDL1 axis were found to reduce tumor growth in macrophage-dependent fashion using in vitro and in vivo colon cancer models and improve macrophage-mediated T-cell activation in in vivo hepatocellular cancer studies [64,190,191]. Converting M2 macrophages into M1 macrophages could be a good goal for therapy and the use of activators of toll-like receptors has been proposed as a therapeutic strategy [64,192]. Moreover, paclitaxel, a plant-derived diterpenoid, can stimulate M1 macrophages, enhancing tumor cell cytotoxicity [64]. Due to MF’s indolent course and the frequency of relapses in MF and SS, treatments focused on the host immune system’s role are warranted. A recent finding showed that genetic aberrations and microenvironment cells may promote a transcriptional response fostering rapid malignant expansion, potentially influencing the response to scheduled treatments [193]. Knowledge of the role of the microenvironment and its interaction with neoplastic cells is an unmet need in the light of developing new treatment approaches.
Author Contributions
Conceptualization, A.P., A.G., V.G., S.A.V., D.F., and P.F.; Methodology, A.P., A.G., V.G., S.A.V., D.F., and P.F.; Validation, A.P., A.G., V.G., S.A.V., D.F., and P.F.; Writing—Original Draft Preparation, A.P., A.G., V.G., S.A.V., D.F., and P.F.; Writing—Review & Editing, A.P., C.A., P.Q., E.B., and N.P.; Visualization, C.A., P.Q., E.B., and N.P.; Supervision, C.A., P.Q., E.B., and N.P.; Funding Acquisition, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bradford P.T., Devesa S.S., Anderson W.F., Toro J.R. Cutaneous Lymphoma Incidence Patterns in the United States: A Population-Based Study of 3884 Cases. Blood. 2009;113:5064–5073. doi: 10.1182/blood-2008-10-184168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lessin S.R., Duvic M., Guitart J., Pandya A.G., Strober B.E., Olsen E.A., Hull C.M., Knobler E.H., Rook A.H., Kim E.J., et al. Topical Chemotherapy in Cutaneous T-Cell Lymphoma: Positive Results of a Randomized, Controlled, Multi-Center Trial Testing the Efficacy and Safety of a Novel 0.02% Mechlorethamine Gel in Mycosis Fungoides. JAMA Dermatol. 2013;149:25–32. doi: 10.1001/2013.jamadermatol.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quaglino P., Maule M., Prince H.M., Porcu P., Horwitz S., Duvic M., Talpur R., Vermeer M., Bagot M., Guitart J., et al. Global Patterns of Care in Advanced Stage Mycosis Fungoides/Sezary Syndrome: A Multicenter Retrospective Follow-up Study from the Cutaneous Lymphoma International Consortium. Ann. Oncol. 2017;28:2517–2525. doi: 10.1093/annonc/mdx352. [DOI] [PubMed] [Google Scholar]
- 4.Prince H.M., Kim Y.H., Horwitz S.M., Dummer R., Scarisbrick J., Quaglino P., Zinzani P.L., Wolter P., Sanches J.A., Ortiz-Romero P.L., et al. Brentuximab Vedotin or Physician’s Choice in CD30-Positive Cutaneous T-Cell Lymphoma (ALCANZA): An International, Open-Label, Randomised, Phase 3, Multicentre Trial. Lancet. 2017;390:555–566. doi: 10.1016/S0140-6736(17)31266-7. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y.H., Bagot M., Pinter-Brown L., Rook A.H., Porcu P., Horwitz S.M., Whittaker S., Tokura Y., Vermeer M., Zinzani P.L., et al. Mogamulizumab versus Vorinostat in Previously Treated Cutaneous T-Cell Lymphoma (MAVORIC): An International, Open-Label, Randomised, Controlled Phase 3 Trial. Lancet Oncol. 2018;19:1192–1204. doi: 10.1016/S1470-2045(18)30379-6. [DOI] [PubMed] [Google Scholar]
- 6.Quaglino P., Fava P., Pileri A., Grandi V., Sanlorenzo M., Panasiti V., Guglielmo A., Alberti-Violetti S., Novelli M., Astrua C., et al. Phenotypical Markers, Molecular Mutations, and Immune Microenvironment as Targets for New Treatments in Patients with Mycosis Fungoides and/or Sézary Syndrome. J. Investig. Dermatol. 2021;141:484–495. doi: 10.1016/j.jid.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 7.Burnet Cancer: A Biological Approach. III. Viruses Associated with Neoplastic Conditions. IV. Practical Applications. Abstract —Europe PMC. [(accessed on 12 June 2021)]. Available online: http://europepmc.org/article/PMC/1973618. [DOI] [PMC free article] [PubMed]
- 8.Dunn G.P., Old L.J., Schreiber R.D. The Immunobiology of Cancer Immunosurveillance and Immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Ni X. Dendritic Cells and Cutaneous T-Cell Lymphomas. Giorn. Ital. Dermatol. Venereol. 2011;146:103–113. [PubMed] [Google Scholar]
- 10.Schlapbach C., Ochsenbein A., Kaelin U., Hassan A.S., Hunger R.E., Yawalkar N. High Numbers of DC-SIGN+ Dendritic Cells in Lesional Skin of Cutaneous T-Cell Lymphoma. J. Am. Acad. Dermatol. 2010;62:995–1004. doi: 10.1016/j.jaad.2009.06.082. [DOI] [PubMed] [Google Scholar]
- 11.Kaiko G.E., Horvat J.C., Beagley K.W., Hansbro P.M. Immunological Decision-Making: How Does the Immune System Decide to Mount a Helper T-Cell Response? Immunology. 2008;123:326–338. doi: 10.1111/j.1365-2567.2007.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prokopi A., Tripp C., Tummers B., Hornsteiner F., Spoeck S., Crawford J.C., Clements D., Efremova M., Hutter K., Bellmann L., et al. Skin Dendritic Cells in Melanoma Are Key for Successful Checkpoint. Blockade Therapy. J. Immunother. Cancer. 2021;9:e000832. doi: 10.1136/jitc-2020-000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valladeau J., Saeland S. Cutaneous Dendritic Cells. Semin. Immunol. 2005;17:273–283. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Siegal F.P. The Nature of the Principal Type 1 Interferon-Producing Cells in Human Blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 15.Gabrilovich D.I., Nagaraj S. Myeloid-Derived Suppressor Cells as Regulators of the Immune System. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Umansky V., Blattner C., Gebhardt C., Utikal J. The Role of Myeloid-Derived Suppressor Cells (MDSC) in Cancer Progression. Vaccines. 2016;4:36. doi: 10.3390/vaccines4040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan K.R., Amaria R.N., Ramirez O., Callihan E.B., Gao D., Borakove M., Manthey E., Borges V.F., McCarter M.D. Myeloid-Derived Suppressor Cells Are Associated with Disease Progression and Decreased Overall Survival in Advanced-Stage Melanoma Patients. Cancer Immunol. Immunother. 2013;62:1711–1722. doi: 10.1007/s00262-013-1475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S., Wu K., Liu Y., Lin Y., Zhang X., Zhou J., Zhang H., Pan T., Fu Y. Finasteride Enhances the Generation of Human Myeloid-Derived Suppressor Cells by Up-Regulating the COX2/PGE2 Pathway. PLoS ONE. 2016;11:e0156549. doi: 10.1371/journal.pone.0156549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lüftl M., Feng A., Licha E., Schuler G. Dendritic Cells and Apoptosis in Mycosis Fungoides. Br. J. Dermatol. 2002;147:1171–1179. doi: 10.1046/j.1365-2133.2002.04994.x. [DOI] [PubMed] [Google Scholar]
- 20.Der-Petrossian M., Valencak J., Jonak C., Klosner G., Dani T., Müllauer L., Pehamberger H., Knobler R., Trautinger F. Dermal Infiltrates of Cutaneous T-Cell Lymphomas with Epidermotropism but Not Other Cutaneous Lymphomas Are Abundant with Langerin+ Dendritic Cells. J. Eur. Acad. Dermatol. Venereol. 2011;25:922–927. doi: 10.1111/j.1468-3083.2010.03882.x. [DOI] [PubMed] [Google Scholar]
- 21.Schwingshackl P., Obermoser G., Nguyen A., Fritsch P., Sepp N., Romani N. Distribution and Maturation of Skin Dendritic Cell Subsets in Two Forms of Cutaneous T-Cell Lymphoma: Mycosis Fungoides and Sézary Syndrome. Acta Derm.-Venereol. 2012;92:269–275. doi: 10.2340/00015555-1220. [DOI] [PubMed] [Google Scholar]
- 22.Pileri A., Agostinelli C., Sessa M., Quaglino P., Santucci M., Tomasini C., Grandi V., Fava P., Astrua C., Righi S., et al. Langerhans, Plasmacytoid Dendritic and Myeloid-Derived Suppressor Cell Levels in Mycosis Fungoides Vary According to the Stage of the Disease. Virchows Arch. 2017;470:575–582. doi: 10.1007/s00428-017-2107-1. [DOI] [PubMed] [Google Scholar]
- 23.Goos M., Kaiserling E., Lennert K. Mycosis Fungoides: Model for T-Lymphocyte Homing to the Skin? Br. J. Dermatol. 1976;94:221–222. doi: 10.1111/j.1365-2133.1976.tb04374.x. [DOI] [PubMed] [Google Scholar]
- 24.Pimpinelli N., Santucci M., Romagnoli P., Giannotti B. Dendritic Cells in T- and B-Cell Proliferation in the Skin. Dermatol. Clin. 1994;12:255–270. doi: 10.1016/S0733-8635(18)30173-6. [DOI] [PubMed] [Google Scholar]
- 25.Nestle F.O., Turka L.A., Nickoloff B.J. Characterization of Dermal Dendritic Cells in Psoriasis. Autostimulation of T Lymphocytes and Induction of Th1 Type Cytokines. J. Clin. Investig. 1994;94:202–209. doi: 10.1172/JCI117308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geskin L.J., Akilov O.E., Kwon S., Schowalter M., Watkins S., Whiteside T.L., Butterfield L.H., Falo L.D. Therapeutic Reduction of Cell-Mediated Immunosuppression in Mycosis Fungoides and Sézary Syndrome. Cancer Immunol. Immunother. 2018;67:423–434. doi: 10.1007/s00262-017-2090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Argyropoulos K.V., Pulitzer M., Perez S., Korkolopoulou P., Angelopoulou M., Baxevanis C., Palomba M.L., Siakantaris M. Tumor-Infiltrating and Circulating Granulocytic Myeloid-Derived Suppressor Cells Correlate with Disease Activity and Adverse Clinical Outcomes in Mycosis Fungoides. Clin. Transl. Oncol. 2020;22:1059–1066. doi: 10.1007/s12094-019-02231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarvaria A., Madrigal J.A., Saudemont A. B Cell Regulation in Cancer and Anti-Tumor Immunity. Cell Mol. Immunol. 2017;14:662–674. doi: 10.1038/cmi.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DiLillo D.J., Yanaba K., Tedder T.F. B Cells Are Required for Optimal CD4+ and CD8+ T Cell Tumor Immunity: Therapeutic B Cell Depletion Enhances B16 Melanoma Growth in Mice. J. Immunol. 2010;184:4006–4016. doi: 10.4049/jimmunol.0903009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blair P.A., Chavez-Rueda K.A., Evans J.G., Shlomchik M.J., Eddaoudi A., Isenberg D.A., Ehrenstein M.R., Mauri C. Selective Targeting of B Cells with Agonistic Anti-CD40 Is an Efficacious Strategy for the Generation of Induced Regulatory T2-like B Cells and for the Suppression of Lupus in MRL/Lpr Mice. J. Immunol. 2009;182:3492–3502. doi: 10.4049/jimmunol.0803052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blair P.A., Noreña L.Y., Flores-Borja F., Rawlings D.J., Isenberg D.A., Ehrenstein M.R., Mauri C. CD19(+)CD24(Hi)CD38(Hi) B Cells Exhibit Regulatory Capacity in Healthy Individuals but Are Functionally Impaired in Systemic Lupus Erythematosus Patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Khan A.R., Hams E., Floudas A., Sparwasser T., Weaver C.T., Fallon P.G. PD-L1hi B Cells Are Critical Regulators of Humoral Immunity. Nat. Commun. 2015;6:5997. doi: 10.1038/ncomms6997. [DOI] [PubMed] [Google Scholar]
- 33.Yanaba K., Bouaziz J.-D., Haas K.M., Poe J.C., Fujimoto M., Tedder T.F. A Regulatory B Cell Subset with a Unique CD1dhiCD5+ Phenotype Controls T Cell-Dependent Inflammatory Responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Yanaba K., Bouaziz J.-D., Matsushita T., Tsubata T., Tedder T.F. The Development and Function of Regulatory B Cells Expressing IL-10 (B10 Cells) Requires. Antigen Receptor Diversity and TLR Signals. J. Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshizaki A., Miyagaki T., DiLillo D.J., Matsushita T., Horikawa M., Kountikov E.I., Spolski R., Poe J.C., Leonard W.J., Tedder T.F. Regulatory B Cells Control T-Cell Autoimmunity through IL-21-Dependent Cognate Interactions. Nature. 2012;491:264–268. doi: 10.1038/nature11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shalapour S., Font-Burgada J., Di Caro G., Zhong Z., Sanchez-Lopez E., Dhar D., Willimsky G., Ammirante M., Strasner A., Hansel D.E., et al. Immunosuppressive Plasma Cells Impede T-Cell-Dependent Immunogenic Chemotherapy. Nature. 2015;521:94–98. doi: 10.1038/nature14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen J.S., Sahota R.A., Milne K., Kost S.E., Nesslinger N.J., Watson P.H., Nelson B.H. CD20+ Tumor-Infiltrating Lymphocytes Have an Atypical CD27- Memory Phenotype and Together with CD8+ T Cells Promote Favorable Prognosis in Ovarian Cancer. Clin. Cancer Res. 2012;18:3281–3292. doi: 10.1158/1078-0432.CCR-12-0234. [DOI] [PubMed] [Google Scholar]
- 38.Dong H.P., Elstrand M.B., Holth A., Silins I., Berner A., Trope C.G., Davidson B., Risberg B. NK- and B-Cell Infiltration Correlates with Worse Outcome in Metastatic Ovarian Carcinoma. Am. J. Clin. Pathol. 2006;125:451–458. doi: 10.1309/15B66DQMFYYM78CJ. [DOI] [PubMed] [Google Scholar]
- 39.Lundgren S., Berntsson J., Nodin B., Micke P., Jirström K. Prognostic Impact of Tumor-Associated B Cells and Plasma Cells in Epithelial Ovarian Cancer. J. Ovarian Res. 2016;9:21. doi: 10.1186/s13048-016-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akatsuka T., Miyagaki T., Nakajima R., Kamijo H., Oka T., Takahashi N., Suga H., Yoshizaki A., Asano Y., Sugaya M., et al. Decreased IL-10-Producing Regulatory B Cells in Patients with Advanced Mycosis Fungoides. Eur. J. Dermatol. 2018;28:314–319. doi: 10.1684/ejd.2018.3319. [DOI] [PubMed] [Google Scholar]
- 41.Nikolaou V., Iliakis T., Marinos L., Voudouri D., Sidiropoulou P., Rigopoulos D., Stratigos A.J. Another Window into Tumor Microenvironment: A Case of Β-Cell Rich Folliculotropic Mycosis Fungoides Responding to Rituximab. Australas. J. Dermatol. 2020;61:e226–e228. doi: 10.1111/ajd.13217. [DOI] [PubMed] [Google Scholar]
- 42.Tschetter A.J., Zafar F., Moye M.S., Ghahramani G.K., Swick B.L., Link B.K., Liu V. CD20+ Cutaneous T-Cell Lymphoma with Phenotypic Shift after Treatment with Rituximab: Case Report and Review of the Literature. JAAD Case Rep. 2020;6:308–310. doi: 10.1016/j.jdcr.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zou W. Regulatory T Cells, Tumor Immunity and Immunotherapy. Nat. Rev. Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 44.Park Y.-H., Koo S.-K., Kim Y., Kim H.-M., Joe I.-Y., Park C.-S., Kim S.-C., Han D.-J., Lim D.-G. Effect of in Vitroexpanded CD4(+)CD25(+)Foxp3(+) Regulatory T Cell Therapy Combined with Lymphodepletion in Murine Skin Allotransplantation. Clin. Immunol. 2010;135:43–54. doi: 10.1016/j.clim.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 45.Berger C.L., Tigelaar R., Cohen J., Mariwalla K., Trinh J., Wang N., Edelson R.L. Cutaneous T-Cell Lymphoma: Malignant Proliferation of T-Regulatory Cells. Blood. 2005;105:1640–1647. doi: 10.1182/blood-2004-06-2181. [DOI] [PubMed] [Google Scholar]
- 46.Hallermann C., Niermann C., Schulze H.-J. Regulatory T-Cell Phenotype in Association with Large Cell Transformation of Mycosis Fungoides. Eur. J. Haematol. 2007;78:260–263. doi: 10.1111/j.1600-0609.2006.00809.x. [DOI] [PubMed] [Google Scholar]
- 47.Klemke C.-D., Fritzsching B., Franz B., Kleinmann E.V., Oberle N., Poenitz N., Sykora J., Banham A.H., Roncador G., Kuhn A., et al. Paucity of FOXP3+ Cells in Skin and Peripheral Blood Distinguishes Sézary Syndrome from Other Cutaneous T-Cell Lymphomas. Leukemia. 2006;20:1123–1129. doi: 10.1038/sj.leu.2404182. [DOI] [PubMed] [Google Scholar]
- 48.Tiemessen M.M., Mitchell T.J., Hendry L., Whittaker S.J., Taams L.S., John S. Lack of Suppressive CD4+CD25+FOXP3+ T Cells in Advanced Stages of Primary Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2006;126:2217–2223. doi: 10.1038/sj.jid.5700371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gjerdrum L.M., Woetmann A., Odum N., Burton C.M., Rossen K., Skovgaard G.L., Ryder L.P., Ralfkiaer E. FOXP3+ Regulatory T Cells in Cutaneous T-Cell Lymphomas: Association with Disease Stage and Survival. Leukemia. 2007;21:2512–2518. doi: 10.1038/sj.leu.2404913. [DOI] [PubMed] [Google Scholar]
- 50.Capriotti E., Vonderheid E.C., Thoburn C.J., Wasik M.A., Bahler D.W., Hess A.D. Expression of T-Plastin, FoxP3 and Other Tumor-Associated Markers by Leukemic T-Cells of Cutaneous T-Cell Lymphoma. Leuk. Lymphoma. 2008;49:1190–1201. doi: 10.1080/10428190802064917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solomon G.J., Magro C.M. Foxp3 Expression in Cutaneous T-Cell Lymphocytic Infiltrates. J. Cutan. Pathol. 2008;35:1032–1039. doi: 10.1111/j.1600-0560.2007.00969.x. [DOI] [PubMed] [Google Scholar]
- 52.Wada D.A., Wilcox R.A., Weenig R.H., Gibson L.E. Paucity of Intraepidermal FoxP3-Positive T Cells in Cutaneous T-Cell Lymphoma in Contrast with Spongiotic and Lichenoid Dermatitis. J. Cutan. Pathol. 2010;37:535–541. doi: 10.1111/j.1600-0560.2009.01381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alcántara-Hernández M., Torres-Zárate C., Pérez-Montesinos G., Jurado-Santacruz F., Domínguez-Gómez M.A., Peniche-Castellanos A., Ferat-Osorio E., Neri N., Nambo M.J., Alvarado-Cabrero I., et al. Overexpression of Hypoxia-Inducible Factor 1 Alpha Impacts FoxP3 Levels in Mycosis Fungoides—Cutaneous T-Cell Lymphoma: Clinical Implications. Int. J. Cancer. 2014;134:2136–2145. doi: 10.1002/ijc.28546. [DOI] [PubMed] [Google Scholar]
- 54.Fried I., Cerroni L. FOXP3 in Sequential Biopsies of Progressive Mycosis Fungoides. Am. J. Dermatol. 2012;34:263–265. doi: 10.1097/DAD.0b013e31823062db. [DOI] [PubMed] [Google Scholar]
- 55.Shareef M.M., Elgarhy L.H., Wasfy R.E.-S. Expression of Granulysin and FOXP3 in Cutaneous T Cell Lymphoma and Sézary Syndrome. Asian Pac. J. Cancer Prev. 2015;16:5359–5364. doi: 10.7314/APJCP.2015.16.13.5359. [DOI] [PubMed] [Google Scholar]
- 56.Johnson V.E., Vonderheid E.C., Hess A.D., Eischen C.M., McGirt L.Y. Genetic Markers Associated with Progression in Early Mycosis Fungoides. J. Eur. Acad. Dermatol. Venereol. 2014;28:1431–1435. doi: 10.1111/jdv.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Q.-A., Chen Z.-Q., Chen M.-H., Xu Z.-D. The Number of Regular T Cells and Immature Dendritic Cells Involved in Mycosis Fungoides Is Linked to the Tumor Stage. Eur. Rev. Med. Pharmacol. Sci. 2014;18:553–558. [PubMed] [Google Scholar]
- 58.Querfeld C., Rosen S.T., Guitart J., Duvic M., Kim Y.H., Dusza S.W., Kuzel T.M. Results of an Open-Label Multicenter Phase 2 Trial of Lenalidomide Monotherapy in Refractory Mycosis Fungoides and Sézary Syndrome. Blood. 2014;123:1159–1166. doi: 10.1182/blood-2013-09-525915. [DOI] [PubMed] [Google Scholar]
- 59.Ni X., Jorgensen J.L., Goswami M., Challagundla P., Decker W.K., Kim Y.H., Duvic M.A. Reduction of Regulatory T Cells by Mogamulizumab, a Defucosylated Anti-CC Chemokine Receptor 4 Antibody, in Patients with Aggressive/Refractory Mycosis Fungoides and Sézary Syndrome. Clin. Cancer Res. 2015;21:274–285. doi: 10.1158/1078-0432.CCR-14-0830. [DOI] [PubMed] [Google Scholar]
- 60.Shiue L.H., Couturier J., Lewis D.E., Wei C., Ni X., Duvic M. The Effect of Extracorporeal Photopheresis Alone or in Combination Therapy on Circulating CD4(+) Foxp3(+) CD25(-) T Cells in Patients with Leukemic Cutaneous T-Cell Lymphoma. Photodermatol. Photoimmunol. Photomed. 2015;31:184–194. doi: 10.1111/phpp.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mantovani A., Bottazzi B., Colotta F., Sozzani S., Ruco L. The Origin and Function of Tumor-Associated Macrophages. Immunol. Today. 1992;13:265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- 62.Parisi L., Gini E., Baci D., Tremolati M., Fanuli M., Bassani B., Farronato G., Bruno A., Mortara L. Macrophage Polarization in Chronic Inflammatory Diseases: Killers or Builders? J. Immunol. Res. 2018;2018:8917804. doi: 10.1155/2018/8917804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gordon S., Martinez F.O. Alternative Activation of Macrophages: Mechanism and Functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 64.Hao N.-B., Lü M.-H., Fan Y.-H., Cao Y.-L., Zhang Z.-R., Yang S.-M. Macrophages in Tumor Microenvironments and the Progression of Tumors. Clin. Dev. Immunol. 2012;2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sugaya M., Miyagaki T., Ohmatsu H., Suga H., Kai H., Kamata M., Fujita H., Asano Y., Tada Y., Kadono T., et al. Association of the Numbers of CD163+ Cells in Lesional Skin and Serum Levels of Soluble CD163 with Disease Progression of Cutaneous T Cell Lymphoma. J. Dermatol. Sci. 2012;68:45–51. doi: 10.1016/j.jdermsci.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 66.Labani-Motlagh A., Ashja-Mahdavi M., Loskog A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front. Immunol. 2020;11:940. doi: 10.3389/fimmu.2020.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu X., Schulte B.C., Zhou Y., Haribhai D., Mackinnon A.C., Plaza J.A., Williams C.B., Hwang S.T. Depletion of M2-Like Tumor-Associated Macrophages Delays Cutaneous T-Cell Lymphoma Development In Vivo. J. Investig. Dermatol. 2014;134:2814–2822. doi: 10.1038/jid.2014.206. [DOI] [PubMed] [Google Scholar]
- 68.Tada K., Hamada T., Asagoe K., Umemura H., Mizuno-Ikeda K., Aoyama Y., Otsuka M., Yamasaki O., Iwatsuki K. Increase of DC-LAMP+ Mature Dendritic Cell Subsets in Dermatopathic Lymphadenitis of Mycosis Fungoides. Eur. J. Dermatol. 2014;24:670–675. doi: 10.1684/ejd.2014.2437. [DOI] [PubMed] [Google Scholar]
- 69.Günther C., Zimmermann N., Berndt N., Großer M., Stein A., Koch A., Meurer M. Up-Regulation of the Chemokine CCL18 by Macrophages Is a Potential Immunomodulatory Pathway in Cutaneous T-Cell Lymphoma. Am. J. Pathol. 2011;179:1434–1442. doi: 10.1016/j.ajpath.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miyagaki T., Sugaya M., Suga H., Ohmatsu H., Fujita H., Asano Y., Tada Y., Kadono T., Sato S. Increased CCL18 Expression in Patients with Cutaneous T-Cell Lymphoma: Association with Disease Severity and Prognosis. J. Eur. Acad. Dermatol. Venereol. 2013;27:e60–e67. doi: 10.1111/j.1468-3083.2012.04495.x. [DOI] [PubMed] [Google Scholar]
- 71.Furudate S., Fujimura T., Kakizaki A., Kambayashi Y., Asano M., Watabe A., Aiba S. The Possible Interaction between Periostin Expressed by Cancer Stroma and Tumor-Associated Macrophages in Developing Mycosis Fungoides. Exp. Dermatol. 2016;25:107–112. doi: 10.1111/exd.12873. [DOI] [PubMed] [Google Scholar]
- 72.Ando T., Xiao W., Gao P., Namiranian S., Matsumoto K., Tomimori Y., Hong H., Yamashita H., Kimura M., Kashiwakura J., et al. Critical Role for Mast Cell Stat5 Activity in Skin Inflammation. Cell Rep. 2014;6:366–376. doi: 10.1016/j.celrep.2013.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou W., Ke S.Q., Huang Z., Flavahan W., Fang X., Paul J., Wu L., Sloan A.E., McLendon R.E., Li X., et al. Periostin Secreted by Glioblastoma Stem Cells Recruits M2 Tumor-Associated Macrophages and Promotes Malignant Growth. Nat. Cell Biol. 2015;17:170–182. doi: 10.1038/ncb3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nestle F.O., Di Meglio P., Qin J.-Z., Nickoloff B.J. Skin Immune Sentinels in Health and Disease. Nat. Rev. Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takahashi N., Sugaya M., Suga H., Oka T., Kawaguchi M., Miyagaki T., Fujita H., Sato S. Thymic Stromal Chemokine TSLP Acts through Th2 Cytokine Production to Induce Cutaneous T-Cell Lymphoma. Cancer Res. 2016;76:6241–6252. doi: 10.1158/0008-5472.CAN-16-0992. [DOI] [PubMed] [Google Scholar]
- 76.Tuzova M., Richmond J., Wolpowitz D., Curiel-Lewandrowski C., Chaney K., Kupper T., Cruikshank W. CCR4+T Cell Recruitment to the Skin in Mycosis Fungoides: Potential Contributions by Thymic Stromal Lymphopoietin and Interleukin-16. Leuk. Lymphoma. 2015;56:440–449. doi: 10.3109/10428194.2014.919634. [DOI] [PubMed] [Google Scholar]
- 77.Litvinov I.V., Cordeiro B., Fredholm S., Ødum N., Zargham H., Huang Y., Zhou Y., Pehr K., Kupper T.S., Woetmann A., et al. Analysis of STAT4 Expression in Cutaneous T-Cell Lymphoma (CTCL) Patients and Patient-Derived Cell Lines. Cell Cycle. 2014;13:2975–2982. doi: 10.4161/15384101.2014.947759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fredholm S., Willerslev-Olsen A., Met Ö., Kubat L., Gluud M., Mathiasen S.L., Friese C., Blümel E., Petersen D.L., Hu T., et al. SATB1 in Malignant T Cells. J. Investig. Dermatol. 2018;138:1805–1815. doi: 10.1016/j.jid.2018.03.1526. [DOI] [PubMed] [Google Scholar]
- 79.Herrera M., Mezheyeuski A., Villabona L., Corvigno S., Strell C., Klein C., Hölzlwimmer G., Glimelius B., Masucci G., Sjöblom T., et al. Prognostic Interactions between FAP+ Fibroblasts and CD8a+ T Cells in Colon Cancer. Cancers. 2020;12:3238. doi: 10.3390/cancers12113238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu L., Shi Y., Zhuang S., Liu N. Recent Advances on Uric Acid Transporters. Oncotarget. 2017;8:100852–100862. doi: 10.18632/oncotarget.20135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nakajima R., Miyagaki T., Hirakawa M., Oka T., Takahashi N., Suga H., Yoshizaki A., Fujita H., Asano Y., Sugaya M., et al. Interleukin-25 Is Involved in Cutaneous T-Cell Lymphoma Progression by Establishing a T Helper 2-Dominant Microenvironment. Br. J. Dermatol. 2018;178:1373–1382. doi: 10.1111/bjd.16237. [DOI] [PubMed] [Google Scholar]
- 82.Geskin L.J., Viragova S., Stolz D.B., Fuschiotti P. Interleukin-13 Is Overexpressed in Cutaneous T-Cell Lymphoma Cells and Regulates Their Proliferation. Blood. 2015;125:2798–2805. doi: 10.1182/blood-2014-07-590398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vacca A., Moretti S., Ribatti D., Pellegrino A., Pimpinelli N., Bianchi B., Bonifazi E., Ria R., Serio G., Dammacco F. Progression of Mycosis Fungoides Is Associated with Changes in Angiogenesis and Expression of the Matrix Metalloproteinases 2 and 9. Eur. J. Cancer. 1997;33:1685–1692. doi: 10.1016/S0959-8049(97)00186-X. [DOI] [PubMed] [Google Scholar]
- 84.Rasheed H., Tolba Fawzi M.M., Abdel-Halim M.R.E., Eissa A.M., Mohammed Salem N., Mahfouz S. Immunohistochemical Study of the Expression of Matrix Metalloproteinase-9 in Skin Lesions of Mycosis Fungoides. Am. J. Dermatol. 2010;32:162–169. doi: 10.1097/DAD.0b013e3181b72678. [DOI] [PubMed] [Google Scholar]
- 85.Mazur G., Woźniak Z., Wróbel T., Maj J., Kuliczkowski K. Increased Angiogenesis in Cutaneous T-Cell Lymphomas. Pathol. Oncol. Res. 2004;10:34–36. doi: 10.1007/BF02893406. [DOI] [PubMed] [Google Scholar]
- 86.Pileri A., Agostinelli C., Righi S., Fuligni F., Bacci F., Sabattini E., Patrizi A., Pileri S.A., Piccaluga P.P. Vascular Endothelial Growth Factor A (VEGFA) Expression in Mycosis Fungoides. Histopathology. 2015;66:173–181. doi: 10.1111/his.12445. [DOI] [PubMed] [Google Scholar]
- 87.Karpova M.B., Fujii K., Jenni D., Dummer R., Urosevic-Maiwald M. Evaluation of Lymphangiogenic Markers in Sézary Syndrome. Leuk. Lymphoma. 2011;52:491–501. doi: 10.3109/10428194.2010.517877. [DOI] [PubMed] [Google Scholar]
- 88.Jankowska-Konsur A., Kobierzycki C., Grzegrzolka J., Piotrowska A., Gomulkiewicz A., Glatzel-Plucinska N., Olbromski M., Podhorska-Okolow M., Szepietowski J.C., Dziegiel P. Expression of CD31 in Mycosis Fungoides. Anticancer Res. 2016;36:4575–4582. doi: 10.21873/anticanres.11006. [DOI] [PubMed] [Google Scholar]
- 89.Jankowska-Konsur A., Kobierzycki C., Reich A., Piotrowska A., Gomulkiewicz A., Olbromski M., Podhorska-Okołów M., Dzięgiel P., Szepietowski J.C. Expression of SOX18 in Mycosis Fungoides. Acta Derm.-Venereol. 2017;97:17–23. doi: 10.2340/00015555-2466. [DOI] [PubMed] [Google Scholar]
- 90.Matsumoto M., Roufail S., Inder R., Caesar C., Karnezis T., Shayan R., Farnsworth R.H., Sato T., Achen M.G., Mann G.B., et al. Signaling for Lymph angiogenesis via VEGFR-3 Is Required for the Early Events of Metastasis. Clin. Exp. Metastasis. 2013;30:819–832. doi: 10.1007/s10585-013-9581-x. [DOI] [PubMed] [Google Scholar]
- 91.El-Ashmawy A.A., Shamloula M.M., Elfar N.N. Podoplanin as a Predictive Marker for Identification of High-Risk Mycosis Fungoides Patients: An Immunohistochemical Study. Indian J. Dermatol. 2020;65:500–505. doi: 10.4103/ijd.IJD_269_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lauenborg B., Christensen L., Ralfkiaer U., Kopp K.L., Jønson L., Dabelsteen S., Bonefeld C.M., Geisler C., Gjerdrum L.M.R., Zhang Q., et al. Malignant T Cells Express Lymphotoxin α and Drive Endothelial Activation in Cutaneous T Cell Lymphoma. Oncotarget. 2015;6:15235–15249. doi: 10.18632/oncotarget.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murray D., McMurray J.L., Eldershaw S., Pearce H., Davies N., Scarisbrick J.J., Moss P. Progression of Mycosis Fungoides Occurs through Divergence of Tumor Immunophenotype by Differential Expression of HLA-DR. Blood Adv. 2019;3:519–530. doi: 10.1182/bloodadvances.2018025114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vowels B.R., Lessin S.R., Cassin M., Jaworsky C., Benoit B., Wolfe J.T., Rook A.H. Th2 Cytokine MRNA Expression in Skin in Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 1994;103:669–673. doi: 10.1111/1523-1747.ep12398454. [DOI] [PubMed] [Google Scholar]
- 95.Hoppe R.T., Medeiros L.J., Warnke R.A., Wood G.S. CD8-Positive Tumor-Infiltrating Lymphocytes Influence the Long-Term Survival of Patients with Mycosis Fungoides. J. Am. Acad. Dermatol. 1995;32:448–453. doi: 10.1016/0190-9622(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 96.Vermeer M.H., van Doorn R., Dukers D., Bekkenk M.W., Meijer C.J., Willemze R. CD8+ T Cells in Cutaneous T-Cell Lymphoma: Expression of Cytotoxic Proteins, Fas Ligand, and Killing Inhibitory Receptors and Their Relationship with Clinical Behavior. J. Clin. Oncol. 2001;19:4322–4329. doi: 10.1200/JCO.2001.19.23.4322. [DOI] [PubMed] [Google Scholar]
- 97.Hahtola S., Tuomela S., Elo L., Häkkinen T., Karenko L., Nedoszytko B., Heikkilä H., Saarialho-Kere U., Roszkiewicz J., Aittokallio T., et al. Th1 Response and Cytotoxicity Genes Are Down-Regulated in Cutaneous T-Cell Lymphoma. Clin. Cancer Res. 2006;12:4812–4821. doi: 10.1158/1078-0432.CCR-06-0532. [DOI] [PubMed] [Google Scholar]
- 98.Hsi A.C., Lee S.J., Rosman I.S., Carson K.R., Kelley A., Viele V., Pang X., Musiek A., Schaffer A. Expression of Helper T Cell Master Regulators in Inflammatory Dermatoses and Primary Cutaneous T-Cell Lymphomas: Diagnostic Implications. J. Am. Acad. Dermatol. 2015;72:159–167. doi: 10.1016/j.jaad.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 99.Miyashiro D., Vivarelli A.G., Gonçalves F., Cury-Martins J., Sanches J.A. Progression of Mycosis Fungoides after Treatment with Dupilumab: A Case Report. Dermatol. Ther. 2020;33:e13880. doi: 10.1111/dth.13880. [DOI] [PubMed] [Google Scholar]
- 100.Russomanno K., Carver DeKlotz C.M. Acceleration of Cutaneous T-Cell Lymphoma Following Dupilumab Administration. JAAD Case Rep. 2021;8:83–85. doi: 10.1016/j.jdcr.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wherry E.J., Kurachi M. Molecular and Cellular Insights into T Cell Exhaustion. Nat. Rev. Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zeng Z., Wei F., Ren X. Exhausted T Cells and Epigenetic Status. Cancer Biol. Med. 2020;17:923–936. doi: 10.20892/j.issn.2095-3941.2020.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Scott A.C., Dündar F., Zumbo P., Chandran S.S., Klebanoff C.A., Shakiba M., Trivedi P., Menocal L., Appleby H., Camara S., et al. TOX Is a Critical Regulator of Tumor-Specific T Cell Differentiation. Nature. 2019;571:270–274. doi: 10.1038/s41586-019-1324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khan O., Giles J.R., McDonald S., Manne S., Ngiow S.F., Patel K.P., Werner M.T., Huang A.C., Alexander K.A., Wu J.E., et al. TOX Transcriptionally and Epigenetically Programs CD8+ T Cell Exhaustion. Nature. 2019;571:211–218. doi: 10.1038/s41586-019-1325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Choi J., Goh G., Walradt T., Hong B.S., Bunick C.G., Chen K., Bjornson R.D., Maman Y., Wang T., Tordoff J., et al. Genomic Landscape of Cutaneous T Cell Lymphoma. Nat. Genet. 2015;47:1011–1019. doi: 10.1038/ng.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Da Silva Almeida A.C., Abate F., Khiabanian H., Martinez-Escala E., Guitart J., Tensen C.P., Vermeer M.H., Rabadan R., Ferrando A., Palomero T. The Mutational Landscape of Cutaneous T Cell Lymphoma and Sézary Syndrome. Nat. Genet. 2015;47:1465–1470. doi: 10.1038/ng.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McGirt L.Y., Degesys C.A., Johnson V.E., Zic J.A., Zwerner J.P., Eischen C.M. TOX Expression and Role in CTCL. J. Eur. Acad. Dermatol. Venereol. 2016;30:1497–1502. doi: 10.1111/jdv.13651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ungewickell A., Bhaduri A., Rios E., Reuter J., Lee C.S., Mah A., Zehnder A., Ohgami R., Kulkarni S., Armstrong R., et al. Genomic Analysis of Mycosis Fungoides and Sézary Syndrome Identifies Recurrent Alterations in TNFR2. Nat. Genet. 2015;47:1056–1060. doi: 10.1038/ng.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang L., Ni X., Covington K.R., Yang B.Y., Shiu J., Zhang X., Xi L., Meng Q., Langridge T., Drummond J., et al. Genomic Profiling of Sézary Syndrome Identifies Alterations of Key T Cell Signaling and Differentiation Genes. Nat. Genet. 2015;47:1426–1434. doi: 10.1038/ng.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Woollard W.J., Pullabhatla V., Lorenc A., Patel V.M., Butler R.M., Bayega A., Begum N., Bakr F., Dedhia K., Fisher J., et al. Candidate Driver Genes Involved in Genome Maintenance and DNA Repair in Sézary Syndrome. Blood. 2016;127:3387–3397. doi: 10.1182/blood-2016-02-699843. [DOI] [PubMed] [Google Scholar]
- 111.Iyer A., Hennessey D., O’Keefe S., Patterson J., Wang W., Wong G.K.-S., Gniadecki R. Branched Evolution and Genomic Intratumor Heterogeneity in the Pathogenesis of Cutaneous T-Cell Lymphoma. Blood Adv. 2020;4:2489–2500. doi: 10.1182/bloodadvances.2020001441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dulmage B.O., Akilov O., Vu J.R., Falo L.D., Geskin L.J. Dysregulation of the TOX-RUNX3 Pathway in Cutaneous T-Cell Lymphoma. Oncotarget. 2019;10:3104–3113. doi: 10.18632/oncotarget.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moerman-Herzog A.M., Acheampong D.A., Brooks A.G., Blair S.M., Hsu P.-C., Wong H.K. Transcriptome Analysis of Sézary Syndrome and Lymphocytic-Variant Hypereosinophilic Syndrome T Cells Reveals Common and Divergent Genes. Oncotarget. 2019;10:5052–5069. doi: 10.18632/oncotarget.27120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lefrançois P., Xie P., Wang L., Tetzlaff M.T., Moreau L., Watters A.K., Netchiporouk E., Provost N., Gilbert M., Ni X., et al. Gene Expression Profiling and Immune Cell-Type Deconvolution Highlight Robust Disease Progression and Survival Markers in Multiple Cohorts of CTCL Patients. Oncoimmunology. 2018;7:e1467856. doi: 10.1080/2162402X.2018.1467856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schrader A.M.R., Jansen P.M., Willemze R. TOX Expression in Cutaneous T-Cell Lymphomas: An Adjunctive Diagnostic Marker That Is Not Tumor Specific and Not Restricted to the CD4(+) CD8(-) Phenotype. Br. J. Dermatol. 2016;175:382–386. doi: 10.1111/bjd.14508. [DOI] [PubMed] [Google Scholar]
- 116.Vivier E., Raulet D.H., Moretta A., Caligiuri M.A., Zitvogel L., Lanier L.L., Yokoyama W.M., Ugolini S. Innate or Adaptive Immunity? The Example of Natural Killer Cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Simoni Y., Fehlings M., Kløverpris H.N., McGovern N., Koo S.-L., Loh C.Y., Lim S., Kurioka A., Fergusson J.R., Tang C.-L., et al. Human Innate Lymphoid Cell Subsets Possess Tissue-Type Based Heterogeneity in Phenotype and Frequency. Immunity. 2018;48:1060. doi: 10.1016/j.immuni.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 118.López-Soto A., Gonzalez S., Smyth M.J., Galluzzi L. Control of Metastasis by NK Cells. Cancer Cell. 2017;32:135–154. doi: 10.1016/j.ccell.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 119.Pesce S., Greppi M., Tabellini G., Rampinelli F., Parolini S., Olive D., Moretta L., Moretta A., Marcenaro E. Identification of a Subset of Human Natural Killer Cells Expressing High Levels of Programmed Death 1: A Phenotypic and Functional Characterization. J. Allergy Clin. Immunol. 2017;139:335–346.e3. doi: 10.1016/j.jaci.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 120.Beldi-Ferchiou A., Lambert M., Dogniaux S., Vély F., Vivier E., Olive D., Dupuy S., Levasseur F., Zucman D., Lebbé C., et al. PD-1 Mediates Functional Exhaustion of Activated NK Cells in Patients with Kaposi Sarcoma. Oncotarget. 2016;7:72961–72977. doi: 10.18632/oncotarget.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vari F., Arpon D., Keane C., Hertzberg M.S., Talaulikar D., Jain S., Cui Q., Han E., Tobin J., Bird R., et al. Immune Evasion via PD-1/PD-L1 on NK Cells and Monocyte/Macrophages Is More Prominent in Hodgkin Lymphoma than DLBCL. Blood. 2018;131:1809–1819. doi: 10.1182/blood-2017-07-796342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tumino N., Martini S., Munari E., Scordamaglia F., Besi F., Mariotti F.R., Bogina G., Mingari M.C., Vacca P., Moretta L. Presence of Innate Lymphoid Cells in Pleural Effusions of Primary and Metastatic Tumors: Functional Analysis and Expression of PD-1 Receptor. Int. J. Cancer. 2019;145:1660–1668. doi: 10.1002/ijc.32262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Moretta A., Bottino C., Vitale M., Pende D., Biassoni R., Mingari M.C., Moretta L. Receptors for HLA Class-I Molecules in Human Natural Killer Cells. Annu. Rev. Immunol. 1996;14:619–648. doi: 10.1146/annurev.immunol.14.1.619. [DOI] [PubMed] [Google Scholar]
- 124.Godal R., Bachanova V., Gleason M., McCullar V., Yun G.H., Cooley S., Verneris M.R., McGlave P.B., Miller J.S. Natural Killer Cell Killing of Acute Myelogenous Leukemia and Acute Lymphoblastic Leukemia Blasts by Killer Cell Immunoglobulin-like Receptor-Negative Natural Killer Cells after NKG2A and LIR-1 Blockade. Biol. Blood Marrow Transpl. 2010;16:612–621. doi: 10.1016/j.bbmt.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Braud V.M., Allan D.S., O’Callaghan C.A., Söderström K., D’Andrea A., Ogg G.S., Lazetic S., Young N.T., Bell J.I., Phillips J.H., et al. HLA-E Binds to Natural Killer Cell Receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 126.De Kruijf E.M., Sajet A., van Nes J.G.H., Natanov R., Putter H., Smit V.T.H.B.M., Liefers G.J., van den Elsen P.J., van de Velde C.J.H., Kuppen P.J.K. HLA-E and HLA-G Expression in Classical HLA Class I-Negative Tumors Is of Prognostic Value for Clinical Outcome of Early Breast Cancer Patients. J. Immunol. 2010;185:7452–7459. doi: 10.4049/jimmunol.1002629. [DOI] [PubMed] [Google Scholar]
- 127.Sun C., Xu J., Huang Q., Huang M., Wen H., Zhang C., Wang J., Song J., Zheng M., Sun H., et al. High NKG2A Expression Contributes to NK Cell Exhaustion and Predicts a Poor Prognosis of Patients with Liver Cancer. Oncoimmunology. 2017;6:e1264562. doi: 10.1080/2162402X.2016.1264562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Seliger B., Jasinski-Bergner S., Quandt D., Stoehr C., Bukur J., Wach S., Legal W., Taubert H., Wullich B., Hartmann A. HLA-E Expression and Its Clinical Relevance in Human Renal Cell Carcinoma. Oncotarget. 2016;7:67360–67372. doi: 10.18632/oncotarget.11744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gooden M., Lampen M., Jordanova E.S., Leffers N., Trimbos J.B., van der Burg S.H., Nijman H., van Hall T. HLA-E Expression by Gynecological Cancers Restrains Tumor-Infiltrating CD8+ T Lymphocytes. Proc. Natl. Acad. Sci. USA. 2011;108:10656–10661. doi: 10.1073/pnas.1100354108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Levy E.M., Bianchini M., Von Euw E.M., Barrio M.M., Bravo A.I., Furman D., Domenichini E., Macagno C., Pinsky V., Zucchini C., et al. Human Leukocyte Antigen-E Protein Is Overexpressed in Primary Human Colorectal Cancer. Int. J. Oncol. 2008;32:633–641. doi: 10.3892/ijo.32.3.633. [DOI] [PubMed] [Google Scholar]
- 131.Spaans V.M., Peters A.A.W., Fleuren G.J., Jordanova E.S. HLA-E Expression in Cervical Adenocarcinomas: Association with Improved Long-Term Survival. J. Transl. Med. 2012;10:184. doi: 10.1186/1479-5876-10-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sako N., Schiavon V., Bounfour T., Dessirier V., Ortonne N., Olive D., Ram-Wolff C., Michel L., Sicard H., Marie-Cardine A., et al. Membrane Expression of NK Receptors CD160 and CD158k Contributes to Delineate a Unique CD4+ T-Lymphocyte Subset in Normal and Mycosis Fungoides Skin. Cytom. A. 2014;85:869–882. doi: 10.1002/cyto.a.22512. [DOI] [PubMed] [Google Scholar]
- 133.Mattei F., Andreone S., Marone G., Gambardella A.R., Loffredo S., Varricchi G., Schiavoni G. Eosinophils in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020;1273:1–28. doi: 10.1007/978-3-030-49270-0_1. [DOI] [PubMed] [Google Scholar]
- 134.Carretero R., Sektioglu I.M., Garbi N., Salgado O.C., Beckhove P., Hämmerling G.J. Eosinophils Orchestrate Cancer Rejection by Normalizing Tumor Vessels and Enhancing Infiltration of CD8(+) T Cells. Nat. Immunol. 2015;16:609–617. doi: 10.1038/ni.3159. [DOI] [PubMed] [Google Scholar]
- 135.Woschnagg C., Rubin J., Venge P. Eosinophil Cationic Protein (ECP) Is Processed during Secretion. J. Immunol. 2009;183:3949–3954. doi: 10.4049/jimmunol.0900509. [DOI] [PubMed] [Google Scholar]
- 136.Varricchi G., Bagnasco D., Borriello F., Heffler E., Canonica G.W. Interleukin-5 Pathway Inhibition in the Treatment of Eosinophilic Respiratory Disorders: Evidence and Unmet Needs. Curr. Opin. Allergy Clin. Immunol. 2016;16:186–200. doi: 10.1097/ACI.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xie F., Liu L.-B., Shang W.-Q., Chang K.-K., Meng Y.-H., Mei J., Yu J.-J., Li D.-J., Li M.-Q. The Infiltration and Functional Regulation of Eosinophils Induced by TSLP Promote the Proliferation of Cervical Cancer Cell. Cancer Lett. 2015;364:106–117. doi: 10.1016/j.canlet.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 138.Onesti C.E., Josse C., Boulet D., Thiry J., Beaumecker B., Bours V., Jerusalem G. Blood Eosinophilic Relative Count Is Prognostic for Breast Cancer and Associated with the Presence of Tumor at Diagnosis and at Time of Relapse. Oncoimmunology. 2020;9:1761176. doi: 10.1080/2162402X.2020.1761176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Harbaum L., Pollheimer M.J., Kornprat P., Lindtner R.A., Bokemeyer C., Langner C. Peritumoral Eosinophils Predict Recurrence in Colorectal Cancer. Mod. Pathol. 2015;28:403–413. doi: 10.1038/modpathol.2014.104. [DOI] [PubMed] [Google Scholar]
- 140.Bertheloot D., Latz E. HMGB1, IL-1α, IL-33 and S100 Proteins: Dual-Function Alarmins. Cell Mol. Immunol. 2017;14:43–64. doi: 10.1038/cmi.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Granata F., Frattini A., Loffredo S., Staiano R.I., Petraroli A., Ribatti D., Oslund R., Gelb M.H., Lambeau G., Marone G., et al. Production of Vascular Endothelial Growth Factors from Human Lung Macrophages Induced by Group IIA and Group X Secreted Phospholipases A2. J. Immunol. 2010;184:5232–5241. doi: 10.4049/jimmunol.0902501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Detoraki A., Staiano R.I., Granata F., Giannattasio G., Prevete N., de Paulis A., Ribatti D., Genovese A., Triggiani M., Marone G. Vascular Endothelial Growth Factors Synthesized by Human Lung Mast Cells Exert Angiogenic Effects. J. Allergy Clin. Immunol. 2009;123:1142–1149.e5. doi: 10.1016/j.jaci.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 143.Schratl P., Royer J.F., Kostenis E., Ulven T., Sturm E.M., Waldhoer M., Hoefler G., Schuligoi R., Lippe I.T., Peskar B.A., et al. The Role of the Prostaglandin D2 Receptor, DP, in Eosinophil Trafficking. J. Immunol. 2007;179:4792–4799. doi: 10.4049/jimmunol.179.7.4792. [DOI] [PubMed] [Google Scholar]
- 144.Capelo R., Lehmann C., Ahmad K., Snodgrass R., Diehl O., Ringleb J., Flamand N., Weigert A., Stark H., Steinhilber D., et al. Cellular Analysis of the Histamine H4 Receptor in Human Myeloid Cells. Biochem. Pharm. 2016;103:74–84. doi: 10.1016/j.bcp.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 145.Simon S.C.S., Utikal J., Umansky V. Opposing Roles of Eosinophils in Cancer. Cancer Immunol. Immunother. 2019;68:823–833. doi: 10.1007/s00262-018-2255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Varricchi G., Galdiero M.R., Loffredo S., Lucarini V., Marone G., Mattei F., Marone G., Schiavoni G. Eosinophils: The Unsung Heroes in Cancer? Oncoimmunology. 2018;7:e1393134. doi: 10.1080/2162402X.2017.1393134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Iliadis A., Koletsa T., Patsatsi A., Georgiou E., Sotiriadis D., Kostopoulos I. The Cellular Microenvironment and Neoplastic Population in Mycosis Fungoides Skin Lesions: A Clinicopathological Correlation. Eur. J. Dermatol. 2016;26:566–571. doi: 10.1684/ejd.2016.2847. [DOI] [PubMed] [Google Scholar]
- 148.Bahalı A.G., Su O., Cengiz F.P., Emiroğlu N., Ozkaya D.B., Onsun N. Prognostic Factors of Patients with Mycosis Fungoides. Postepy Derm. Alergol. 2020;37:796–799. doi: 10.5114/ada.2020.100491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kural Y.B., Su O., Onsun N., Uras A.R. Atopy, IgE and Eosinophilic Cationic Protein Concentration, Specific IgE Positivity, Eosinophil Count in Cutaneous T Cell Lymphoma. Int. J. Dermatol. 2010;49:390–395. doi: 10.1111/j.1365-4632.2010.04228.x. [DOI] [PubMed] [Google Scholar]
- 150.Tancrède-Bohin E., Ionescu M.A., de La Salmonière P., Dupuy A., Rivet J., Rybojad M., Dubertret L., Bachelez H., Lebbé C., Morel P. Prognostic Value of Blood Eosinophilia in Primary Cutaneous T-Cell Lymphomas. Arch. Dermatol. 2004;140:1057–1061. doi: 10.1001/archderm.140.9.1057. [DOI] [PubMed] [Google Scholar]
- 151.Ionescu M.A., Rivet J., Daneshpouy M., Briere J., Morel P., Janin A. In Situ Eosinophil Activation in 26 Primary Cutaneous T-Cell Lymphomas with Blood Eosinophilia. J. Am. Acad. Dermatol. 2005;52:32–39. doi: 10.1016/j.jaad.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 152.Fredholm S., Gjerdrum L.M.R., Willerslev-Olsen A., Petersen D.L., Nielsen I.Ø., Kauczok C.-S., Wobser M., Ralfkiaer U., Bonefeld C.M., Wasik M.A., et al. STAT3 Activation and Infiltration of Eosinophil Granulocytes in Mycosis Fungoides. Anticancer Res. 2014;10:5277–5286. [PubMed] [Google Scholar]
- 153.Vonderheid E.C., Kantor G.R., Telang G.H., Bujanouskas P., Kadin M.E. A Histo-Immunopathologic and Prognostic Study of Erythrodermic Cutaneous T-Cell Lymphoma. J. Cutan. Pathol. 2019;46:913–924. doi: 10.1111/cup.13564. [DOI] [PubMed] [Google Scholar]
- 154.Terada T. Mycosis Fungoides in Plaque Stage with Pronounced Eosinophilic Infiltration, Folliculotropism, and Concomitant Invasive Squamous Cell Carcinoma. Int. J. Clin. Exp. Pathol. 2013;6:749–756. [PMC free article] [PubMed] [Google Scholar]
- 155.Boone S.L., Guitart J., Gerami P. Follicular Mycosis Fungoides: A Histopathologic, Immunohistochemical, and Genotypic Review. G. Ital. Derm. Venereol. 2008;143:409–414. [PubMed] [Google Scholar]
- 156.Chang N.-S. Transforming Growth Factor-Β1 Blocks the Enhancement of Tumor Necrosis Factor Cytotoxicity by Hyaluronidase Hyal-2 in L929 Fibroblasts. BMC Cell Biol. 2002;3:8. doi: 10.1186/1471-2121-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Owusu B.Y., Vaid M., Kaler P., Klampfer L. Prognostic and Predictive Significance of Stromal Fibroblasts and Macrophages in Colon Cancer: Supplementary Issue: Biomarkers for Colon Cancer. Biomark. Cancer. 2015;7:29–37. doi: 10.4137/BIC.S25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Surowiak P., Suchocki S., GyĂśrffy B., Gansukh T., Wojnar A., Maciejczyk A., Pudełko M., Zabel M. Stromal Myofibroblasts in Breast Cancer: Relations between Their Occurrence, Tumor Grade and Expression of Some Tumor Markers. Folia Histochem. Cytobiol. 2006;44:111–116. [PubMed] [Google Scholar]
- 159.Cheng Y., Wang K., Ma W., Zhang X., Song Y., Wang J., Wang N., Song Q., Cao F., Tan B., et al. Cancer-Associated Fibroblasts Are Associated with Poor Prognosis in Esophageal Squamous Cell Carcinoma after Surgery. Int. J. Clin. Exp. Med. 2015;8:1896–1903. [PMC free article] [PubMed] [Google Scholar]
- 160.Lohr M., Schmidt C., Ringel J., Kluth M. Transforming Growth Factor-β1 Induces Desmoplasia in an Experimental Model of Human Pancreatic Carcinoma. Cancer Res. 2001;61:550–555. [PubMed] [Google Scholar]
- 161.Aoyagi Y., Oda T., Kinoshita T., Nakahashi C., Hasebe T., Ohkohchi N., Ochiai A. Overexpression of TGF-β by Infiltrated Granulocytes Correlates with the Expression of Collagen MRNA in Pancreatic Cancer. Br. J. Cancer. 2004;91:1316–1326. doi: 10.1038/sj.bjc.6602141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cirillo N., Hassona Y., Celentano A., Lim K.P., Manchella S., Parkinson E.K., Prime S.S. Cancer-Associated Fibroblasts Regulate Keratinocyte Cell–Cell Adhesion via TGF-β-Dependent Pathways in Genotype-Specific Oral Cancer. Carcinogenesis. 2017;38:76–85. doi: 10.1093/carcin/bgw113. [DOI] [PubMed] [Google Scholar]
- 163.Kim S.-A., Lee E.K., Kuh H.-J. Co-Culture of 3D Tumor Spheroids with Fibroblasts as a Model for Epithelial–Mesenchymal Transition in Vitro. Exp. Cell Res. 2015;335:187–196. doi: 10.1016/j.yexcr.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 164.Caja L., Dituri F., Mancarella S., Caballero-Diaz D., Moustakas A., Giannelli G., Fabregat I. TGF-β and the Tissue Microenvironment: Relevance in Fibrosis and Cancer. Int. J. Mol. Sci. 2018;19:1294. doi: 10.3390/ijms19051294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kalluri R., Zeisberg M. Fibroblasts in Cancer. Nat. Rev. Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 166.Dumont N., Liu B., DeFilippis R.A., Chang H., Rabban J.T., Karnezis A.N., Tjoe J.A., Marx J., Parvin B., Tlsty T.D. Breast Fibroblasts Modulate Early Dissemination, Tumorigenesis, and Metastasis through Alteration of Extracellular Matrix Characteristics. Neoplasia. 2013;15:249–262. doi: 10.1593/neo.121950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Elenbaas B., Weinberg R.A. Heterotypic Signaling between Epithelial Tumor Cells and Fibroblasts in Carcinoma Formation. Exp. Cell Res. 2001;264:169–184. doi: 10.1006/excr.2000.5133. [DOI] [PubMed] [Google Scholar]
- 168.Ishii G., Ochiai A., Neri S. Phenotypic and Functional Heterogeneity of Cancer-Associated Fibroblast within the Tumor Microenvironment. Adv. Drug Deliv. Rev. 2016;99:186–196. doi: 10.1016/j.addr.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 169.Orimo A., Gupta P.B., Sgroi D.C., Arenzana-Seisdedos F., Delaunay T., Naeem R., Carey V.J., Richardson A.L., Weinberg R.A. Stromal Fibroblasts Present in Invasive Human Breast Carcinomas Promote Tumor Growth and Angiogenesis through Elevated SDF-1/CXCL12 Secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 170.Erez N., Truitt M., Olson P., Arron S.T., Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-KappaB-Dependent Manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 171.Aronovich A., Moyal L., Gorovitz B., Amitay-Laish I., Naveh H.P., Forer Y., Maron L., Knaneh J., Ad-El D., Yaacobi D., et al. Cancer-Associated Fibroblasts in Mycosis Fungoides Promote Tumor Cell Migration and Drug Resistance through CXCL12/CXCR4. J. Investig. Dermatol. 2021;141:619–627.e2. doi: 10.1016/j.jid.2020.06.034. [DOI] [PubMed] [Google Scholar]
- 172.Krejsgaard T., Lindahl L.M., Mongan N.P., Wasik M.A., Litvinov I.V., Iversen L., Langhoff E., Woetmann A., Odum N. Malignant Inflammation in Cutaneous T-cell Lymphoma—a Hostile Takeover. Semin. Immunopathol. 2017;39:269–282. doi: 10.1007/s00281-016-0594-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Durgin J.S., Weiner D.M., Wysocka M., Rook A.H. The Immunopathogenesis and Immunotherapy of Cutaneous T Cell Lymphoma: Pathways and Targets for Immune Restoration and Tumor Eradication. J. Am. Acad. Dermatol. 2021;84:587–595. doi: 10.1016/j.jaad.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jones C.L., Degasperi A., Grandi V., Amarante T.D., Genomics England Research Consortium. Mitchell T.J., Nik-Zainal S., Whittaker S.J. Spectrum of Mutational Signatures in T-Cell Lymphoma Reveals a Key Role for UV Radiation in Cutaneous T-Cell Lymphoma. Sci. Rep. 2021;11:3962. doi: 10.1038/s41598-021-83352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rojansky R., Fernandez-Pol S., Wang E., Rieger K.E., Novoa R.A., Zehnder J.L., Kunder C.A., Kim Y.H., Khodadoust M.S., Brown R.A. Cutaneous T-Cell Lymphomas with Pathogenic Somatic Mutations and Absence of Detectable Clonal T-Cell Receptor Gene Rearrangement: Two Case Reports. Diagn. Pathol. 2020;15:122. doi: 10.1186/s13000-020-01022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mirza A.-S., Horna P., Teer J.K., Song J., Akabari R., Hussaini M., Sokol L. New Insights Into the Complex Mutational Landscape of Sézary Syndrome. Front. Oncol. 2020;10:514. doi: 10.3389/fonc.2020.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kim E.J., Hess S., Richardson S.K., Newton S., Showe L.C., Benoit B.M., Ubriani R., Vittorio C.C., Junkins-Hopkins J.M., Wysocka M., et al. Immunopathogenesis and Therapy of Cutaneous T Cell Lymphoma. J. Clin. Investig. 2005;115:798–812. doi: 10.1172/JCI24826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Budgin J.B., Richardson S.K., Newton S.B., Wysocka M., Zaki M.H., Benoit B., Rook A.H. Biological Effects of Bexarotene in Cutaneous T-Cell Lymphoma. Arch. Dermatol. 2005;141:315–321. doi: 10.1001/archderm.141.3.315. [DOI] [PubMed] [Google Scholar]
- 179.Kim Y.H., Gratzinger D., Harrison C., Brody J.D., Czerwinski D.K., Ai W.Z., Morales A., Abdulla F., Xing L., Navi D., et al. In Situ Vaccination against Mycosis Fungoides by Intratumoral Injection of a TLR9 Agonist Combined with Radiation: A Phase 1/2 Study. Blood. 2012;119:355–363. doi: 10.1182/blood-2011-05-355222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sivanand A., Surmanowicz P., Alhusayen R., Hull P., Litvinov I.V., Zhou Y., Gniadecki R. Immunotherapy for Cutaneous T-Cell Lymphoma: Current Landscape and Future Developments. J. Cutan. Med. Surg. 2019;23:537–544. doi: 10.1177/1203475419867610. [DOI] [PubMed] [Google Scholar]
- 181.Maier T., Tun-Kyi A., Tassis A., Jungius K.-P., Burg G., Dummer R., Nestle F.O. Vaccination of Patients with Cutaneous T-Cell Lymphoma Using Intranodal Injection of Autologous Tumor-Lysate-Pulsed Dendritic Cells. Blood. 2003;102:2338–2344. doi: 10.1182/blood-2002-08-2455. [DOI] [PubMed] [Google Scholar]
- 182.Heinzerling L., Künzi V., Oberholzer P.A., Kündig T., Naim H., Dummer R. Oncolytic Measles Virus in Cutaneous T-Cell Lymphomas Mounts Antitumor Immune Responses in Vivo and Targets Interferon-Resistant Tumor Cells. Blood. 2005;106:2287–2294. doi: 10.1182/blood-2004-11-4558. [DOI] [PubMed] [Google Scholar]
- 183.Zic J.A. The Treatment of Cutaneous T-Cell Lymphoma with Photopheresis. Dermatol. Ther. 2003;16:337–346. doi: 10.1111/j.1396-0296.2003.01646.x. [DOI] [PubMed] [Google Scholar]
- 184.Lesokhin A.M., Ansell S.M., Armand P., Scott E.C., Halwani A., Gutierrez M., Millenson M.M., Cohen A.D., Schuster S.J., Lebovic D., et al. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J. Clin. Oncol. 2016;34:2698–2704. doi: 10.1200/JCO.2015.65.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Khodadoust M.S., Rook A.H., Porcu P., Foss F., Moskowitz A.J., Shustov A., Shanbhag S., Sokol L., Fling S.P., Ramchurren N., et al. Pembrolizumab in Relapsed and Refractory Mycosis Fungoides and Sézary Syndrome: A Multicenter Phase II Study. J. Clin. Oncol. 2020;38:20–28. doi: 10.1200/JCO.19.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bar-Sela G., Bergman R. Complete Regression of Mycosis Fungoides after Ipilimumab Therapy for Advanced Melanoma. JAAD Case Rep. 2015;1:99–100. doi: 10.1016/j.jdcr.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Alcantara M., Tesio M., June C.H., Houot R. CAR T-Cells for T-Cell Malignancies: Challenges in Distinguishing between Therapeutic, Normal, and Neoplastic T-Cells. Leukemia. 2018;32:2307–2315. doi: 10.1038/s41375-018-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tanita K., Fujimura T., Sato Y., Lyu C., Kambayashi Y., Ogata D., Fukushima S., Miyashita A., Nakajima H., Nakamura M., et al. Bexarotene Reduces Production of CCL22 From Tumor-Associated Macrophages in Cutaneous T-Cell Lymphoma. Front. Oncol. 2019;9:907. doi: 10.3389/fonc.2019.00907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kim Y.H., Tavallaee M., Sundram U., Salva K.A., Wood G.S., Li S., Rozati S., Nagpal S., Krathen M., Reddy S., et al. Phase II Investigator-Initiated Study of Brentuximab Vedotin in Mycosis Fungoides and Sézary Syndrome With Variable CD30 Expression Level: A Multi-Institution Collaborative Project. J. Clin. Oncol. 2015;33:3750–3758. doi: 10.1200/JCO.2014.60.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gao Q., Wang X.-Y., Qiu S.-J., Yamato I., Sho M., Nakajima Y., Zhou J., Li B.-Z., Shi Y.-H., Xiao Y.-S., et al. Overexpression of PD-L1 Significantly Associates with Tumor Aggressiveness and Postoperative Recurrence in Human Hepatocellular Carcinoma. Clin. Cancer Res. 2009;15:971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 191.Suda K. Tumor-Associated Macrophages-Additional Effectors at Anti-PD-1/PD-L1 Therapy? J. Thorac. Dis. 2017;9:4197–4200. doi: 10.21037/jtd.2017.10.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Krieg A.M. Therapeutic Potential of Toll-like Receptor 9 Activation. Nat. Rev. Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 193.Herrera A., Cheng A., Mimitou E.P., Seffens A., George D.D., Bar-Natan M., Heguy A., Ruggles K.V., Scher J.U., Hymes K., et al. Multimodal Single-Cell Analysis of Cutaneous T Cell Lymphoma Reveals Distinct Sub-Clonal Tissue-Dependent Signatures. Blood. 2021 doi: 10.1182/blood.2020009346. [DOI] [PMC free article] [PubMed] [Google Scholar]