Abstract
Angiogenesis is crucial in tissue repair and prevents scar tissue formation following an ischemic event such as myocardial infarction. The ischemia induces formation of new capillaries, which have high expression of integrin αvβ3. [68Ga]Ga-NODAGA-E[(cRGDyK)]2 ([68Ga]Ga-RGD) is a promising PET-radiotracer reflecting angiogenesis by binding to integrin αvβ3. A Göttingen mini-pig underwent transient catheter-induced left anterior descending artery (LAD) occlusion for 120 min, and after 8 weeks was imaged on a Siemens mMR 3T PET/MR system. A large antero-septal infarction was evident by late gadolinium enhancement (LGE) on the short axis and 2–4 chamber views. The infarcted area corresponded to the area with high [68Ga]Ga-RGD uptake on the fused PET/MR images, with no uptake in the healthy myocardium. To support the hypothesis that [68Ga]Ga-RGD uptake reflects angiogenesis, biopsies were sampled from the infarct border and healthy myocardium. Expression of αvβ3 was evaluated using immunohistochemistry. The staining showed higher αvβ3 expression in the capillaries of the infarct border compared to those in the healthy myocardium. These initial data confirm in vivo detection of angiogenesis using [68Ga]Ga-RGD PET in a translational model, which overall support the method applicability when evaluating novel cardio-protective therapies.
Keywords: positron emission tomography, angiogenesis, myocardial infarction, magnetic resonance imaging, late gadolinium enhancement, large animal model, cardiac imaging, PET/MRI
To evaluate angiogenesis following transient occlusion of the LAD artery, a Göttingen minipig underwent PET/MR examination [1,2]. A bolus injection of approximately 100 MBq [68Ga]Ga-RGD was administered through a central venous catheter. A 15 min PET acquisition was performed 30 min after the injection. After the PET acquisition, gadolinium was injected in order to examine the final infarct size.
Figure 1.
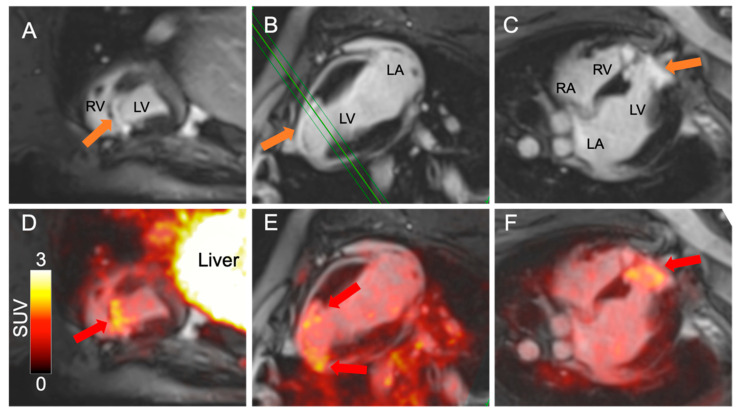
Anatomical cardiac MR images of a Göttingen minipig following gadolinium injection in short axis (A), two-chamber (B) and four-chamber view (C). The animal had undergone 120 min invasive LAD occlusion 8 weeks prior to the scan [3]. The green line in (B) illustrates the placement of the short axis slice shown in (A). A large antero-septal infarction is evident by late gadolinium enhancement (LGE) on all views (orange arrows). On the midventricular short axis image (A), the LGE appears subendocardial, while towards the apex of the heart, the infarct is transmural, indicating a large infarction. All images were obtained using a Siemens mMR 3T PET/MR system and flex body coils. The infarcted area corresponded to the area with high [68Ga]Ga-RGD uptake on the fused PET/MR images ((D–F), red arrows), with no [68Ga]Ga-RGD uptake observed in the healthy parts of the myocardium. RV: Right ventricle, LV: Left ventricle, LA: Left atrium, RA: Right atrium, SUV: Standardized uptake value.
Figure 2.
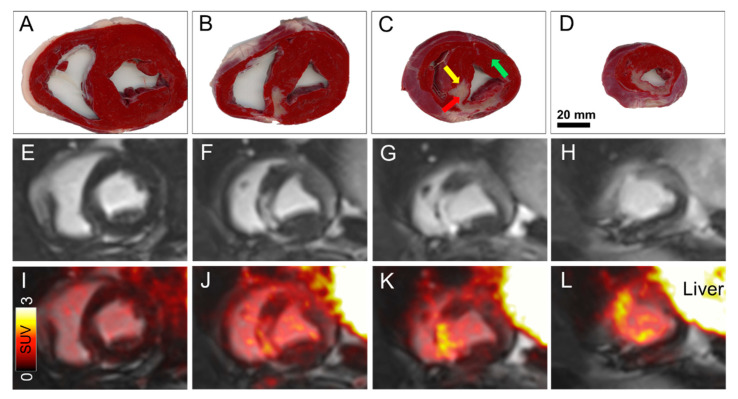
To confirm infarct severity ex-vivo, the excised heart was divided into 4 axial slices and stained using 1% triphenyl-tetrazolium-chloride (TTC) solution (A–D). The TTC staining in (B) shows a subendocardial infarction, while in (C,D), the TTC staining is transmural. The TTC staining corresponds to patterns seen by LGE on the corresponding MRI images (E–H) and fused PET/MR images (I–L). The red, yellow and green arrows in (C) mark the infarct zone, infarct border zone and healthy myocardium, respectively. These areas correspond to the immunohistochemical images in Figure 3. Scale bar (20 mm) is shown in (D) for size reference, SUV: Standardized uptake value.
This study was performed as a hypothesis-generating exploration of the evaluation of integrin αvβ3 expression following transient occlusion of the LAD artery. The use of [68Ga]Ga-RGD as a prognostic marker for functional outcome following MI has been explored in both animal and human studies [4]. The [68Ga]Ga-RGD tracer in blocking studies shows a high sensitivity towards αvβ3 [5], indicating the potential in future studies with a radiotracer. However, the minipig was euthanized following the PET/MR scan in order to examine histology and immunohistochemistry. This study shows promising PET images of [68Ga]Ga-RGD, but further studies need to be performed in order to evaluate the prognostic value of the tracer.
Figure 3.
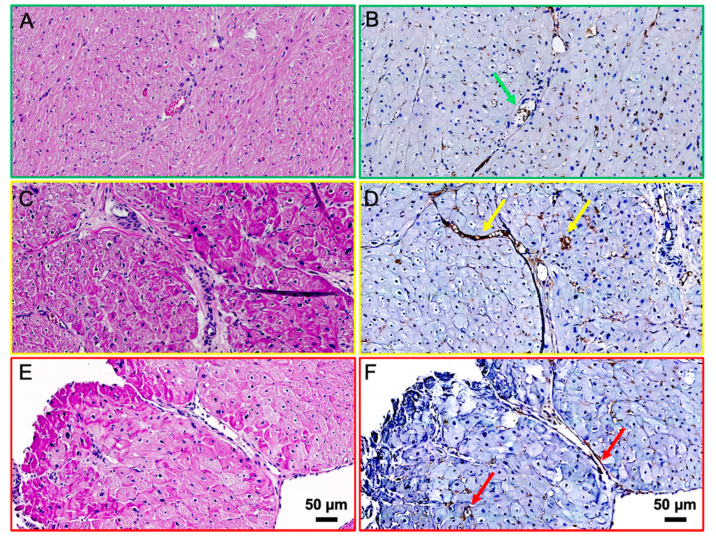
To further support the hypothesis that [68Ga]Ga-RGD PET uptake reflects angiogenesis, biopsies were sampled from the healthy myocardium (A,B), infarct border (C,D) and infarct area (E,F). Classic H&E staining was performed (A,C,E). The H&E staining of the infarcted area (E), shows a high degree of atypical nuclei and an amyloid-like substance, most likely collagen compared to the infarct boder zone (C) or healthy myocardium (A). The expression of αvβ3 was evaluated using immunohistochemistry (B,D,F) (αvβ3, absolute antibody; the antibody was diluted 1:100 and antigen retrieval was performed with proteinase K). The staining indicated a higher αvβ3 expression in the capillaries of the infarct border ((D), yellow arrows), compared to those in the healthy myocardium ((B), green arrow) and in the infarcted area ((F), red arrows). Scale bar (50 µm) is shown in (E,F) for size reference.
Acknowledgments
The authors thank nuclear medicine technologist Marianne Federspiel (Rigshospitalet), molecular biologist Katrine Qvist (University of Copenhagen) as well as laboratory technicians Susanne Juul Rasmussen, Anja Benfeldt, Pernille Pedersen and Inge Rubach (Novo Nordisk A/S) for their invaluable assistance.
Author Contributions
Conceptualization, S.B., A.C., M.L., M.F., T.P.L., J.K., K.P.D., L.H.O., C.B.L., T.J., J.E.M. and A.K.; methodology, S.B., A.C., M.L., T.L.A., H.H.J., M.F., T.P.L., J.K., K.P.D., L.H.O., C.B.L., T.J., J.E.M. and A.K.; software, T.L.A., H.H.J.; formal analysis, S.B., A.C., M.L., M.F., K.P.D., A.K.; investigation, S.B., A.C., M.L., M.F., T.P.L., J.K., K.P.D., C.B.L. and J.E.M.; resources, L.H.O., T.J., J.E.M., A.K.; writing—original draft preparation, S.B., A.C., M.L., M.F., A.K.; writing—review and editing, all authors; visualization, S.B., A.C., M.L.; supervision, L.H.O., T.J., A.K.; project administration, M.F., H.H.J., A.K.; funding acquisition, L.H.O., T.J., A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This project received funding from the Danish National Research Foundation (grants 124 and 126) the European Union’s Horizon 2020 research and innovation programme under grant agreements no. 670261 (ERC Advanced Grant) and 668532 (Click-It), the Lundbeck Foundation, the Novo Nordisk Foundation, the Innovation Fund Denmark, the Danish Cancer Society, Arvid Nilsson Foundation, Svend Andersen Foundation, the Neye Foundation, the Research Foundation of Rigshospitalet, the Research Council of the Capital Region of Denmark, the Danish Health Authority, Danish Research Council for Independent Research (Grant Numbers 12-127232 and 1331-00259A), the LIFEPHARM In Vivo Pharmacology Centre and Novo Nordisk A/S.
Institutional Review Board Statement
This study was approved by the Danish Animal Experiment Inspectorate (Permit No. 2018-15-0201-01414, approved 23 Febuary 2018). All animal procedures performed were in accordance with the guidelines in Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
Andreas Kjaer is listed as the inventor on patents covering the PET tracer used (EP3706809A1 and US16/762,873).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dobrucki L.W., Sinusas A.J. Imaging angiogenesis. Curr. Opin. Biotechnol. 2007;18:90–96. doi: 10.1016/j.copbio.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Kiugel M., Dijkgraaf I., Kytö V., Helin S., Liljenbäck H., Saanijoki T., Yim C.-B., Oikonen V., Saukko P., Knuuti G., et al. Dimeric [(68)Ga]DOTA-RGD peptide targeting alphavbeta3 integrin reveals extracellular matrix alterations after myocardial infarction. Mol. Imaging Biol. 2014;16:793–801. doi: 10.1007/s11307-014-0752-1. [DOI] [PubMed] [Google Scholar]
- 3.Schuleri K.H., Boyle A., Centola M., Amado L.C., Evers R., Zimmet J.M., Evers K.S., Ostbye K.M., Scorpio D.G., Hare J.M., et al. The Adult Gottingen Minipig as a Model for Chronic Heart Failure After Myocardial Infarction: Focus on Cardiovascular Imaging and Regenerative Therapies. Comp. Med. 2008;58:568–579. [PMC free article] [PubMed] [Google Scholar]
- 4.Chen H., Niu G., Wu H., Chen X. Clinical Application of Radiolabeled RGD Peptides for PET Imaging of Integrin alphavbeta3. Theranostics. 2016;6:78–92. doi: 10.7150/thno.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oxboel J., Schjoeth-Eskesen C., El-Ali H.H., Madsen J., Kjaer A. (64)Cu-NODAGA-c(RGDyK) Is a Promising New Angiogenesis PET Tracer: Correlation between Tumor Uptake and Integrin alpha(V)beta(3) Expression in Human Neuroendocrine Tumor Xenografts. Int. J. Mol. Imaging. 2012;2012:379807. doi: 10.1155/2012/379807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.