Abstract
Bioprinting is an emerging additive manufacturing technique which shows an outstanding potential for shaping customized functional substitutes for tissue engineering. Its introduction into the clinical space in order to replace injured organs could ideally overcome the limitations faced with allografts. Presently, even though there have been years of prolific research in the field, there is a wide gap to bridge in order to bring bioprinting from “bench to bedside”. This is due to the fact that bioprinted designs have not yet reached the complexity required for clinical use, nor have clear GMP (good manufacturing practices) rules or precise regulatory guidelines been established. This review provides an overview of some of the most recent and remarkable achievements for skin, heart, pancreas and cartilage bioprinting breakthroughs while highlighting the critical shortcomings for each tissue type which is keeping this technique from becoming widespread reality.
Keywords: bioprinting, skin, heart, pancreas, cartilage, tissue engineering, regenerative medicine, clinical applications
1. Introduction
Three-dimensional bioprinting is an additive manufacturing technology, which applies the principles of 3D printing to the biomedical field. Its goal is to create in vitro viable and functional biological constructs with complex architectures which are able to mimic the native organs, and to provide powerful platforms for studying tissue development and homeostasis and for modeling diseases in pharmaceutical testing [1].
The bioprinter shapes the tissue through a layer-by-layer controlled deposition of a bioink consisting of growth factors (optional), a peculiar cell type and a biomaterial fluid matrix in which cells are embedded and interspersed. Thanks to its interdisciplinary nature, the main areas of bioprinting application involve drug testing, disease modeling and tissue engineering [2,3,4]. Despite the critical issues that currently preclude an in vivo application, in vitro personalized disease modeling and drug screening remain the closest reality to clinical application.
Bioprinted tissues have not yet reached the structural and functional organicity necessary to make them a valid substitute for allografts, despite 10 years of research and progress on this topic. Yet while a routine clinical application of bioprinted materials may be rather far off, many important advancements have been made in this field. The greatest challenges lay in the complexity of reproducing a viable tissue with all its biological features which is able to integrate within the host tissue and to mechanically and physiologically support the self-renewal of the damaged organ. For these reasons, an ideally engineered bioprinted design should have a degradation rate that fits the timing for endogenous regenerative processes, simultaneously undergoing vascularization and innervation [5]. In addition, ethical questions and proper regulatory aspects should be considered [6]. In this review, we provide an overview of the latest major achievements in bioprinting tissue prototypes and discuss recent developments, current challenges and the future prospects for the design and realization of 3D bioprinting for complex tissues such as skin, heart, pancreas and cartilage.
2. Methods
We conducted a scoping review following the method of Arksey and O’Malley (2005): (i) identifying the research question and the relevant studies; (ii) selecting studies; (iii) summarizing the data and reporting the results.
2.1. Identifying the Research Question
We identified three research questions: (i) What evidence exists on bioprinting as to clinical aspects? (ii) What are the limitations of this new technique? (iii) What are the future challenges?
2.2. Identifying Relevant Studies
The present scoping review identified, retrieved and evaluated information from peer-reviewed articles that examined the impacts of bioprinting on clinical practice in regard to four different tissues: cardiac tissue, skin, cartilage and pancreatic tissue.
We focused on studies published between 1 January 2016 and 31 December 2020 and consulted three databases (PubMed, Google Scholar and Medline) using the following search strings: “bioprinting AND cardi*”, “bioprinting AND skin”, “bioprinting AND cartilage”, “bioprinting AND pancreatic*”. This period was selected because the bioprinting technique and its related applications are relatively recent and most of the bioprinting studies have been published in these years.
2.3. Selecting Studies
Only empirical papers with an English language abstract were included. We considered all types of research design. We applied the following exclusion criteria at two stages of the study selection: screening by title, abstract and full text.
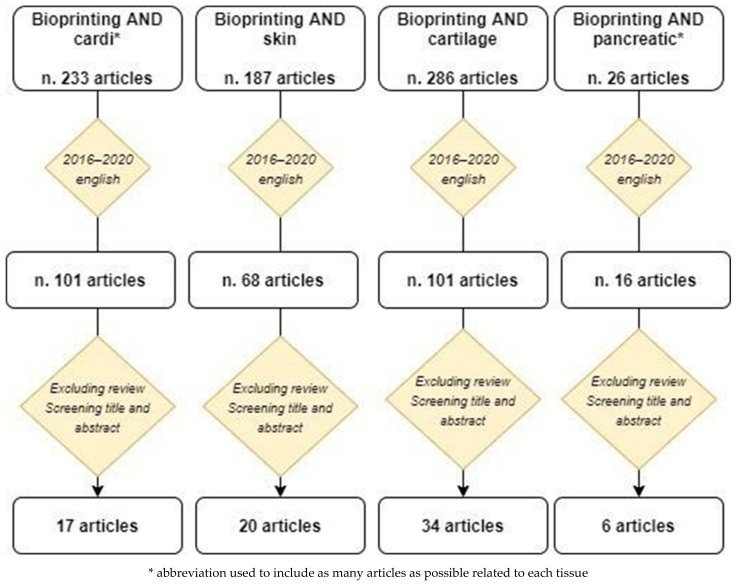
Figure 1 summarizes the selection process. Using the specific search string, the search yielded 233 articles for cardiovascular tissue, 187 articles for skin, 26 articles for pancreatic tissue and 286 articles for cartilage. When restricted to the articles published in the past five years and written in the English language, the search yielded 101 articles for cardiovascular tissue, 68 articles for skin, 101 articles for cartilage and 16 articles for pancreatic tissue. Based on the screening of the title and the abstract and excluding the reviews, 17 articles for cardiovascular tissue, 20 articles for skin, 34 articles for cartilage and 6 articles for pancreatic tissue were included in this review.
Figure 1.
Article selection process.
The selected articles by type of tissue are reported in Table 1, Table 2, Table 3 and Table 4.
Table 1.
Remarkable achievements in 3D bioprinting of skin tissue. Articles from 2016 to 2018 and from 2019 to 2020.
| Tissue | Cell Type | Biomaterial | Printed Model | Outcome | Reference |
|---|---|---|---|---|---|
| Skin | |||||
| 2016–2018 | |||||
| Human dermal endothelial cells | Sodium alginate, chitosan, gelatin, gellan gum, collagen I (core materials), pure alginate (shell material) | Core/shell construct |
|
Akkineni et al. 2016 | |
| Human fibroblasts (hFB) and keratinocytes (hKC) obtained from skin biopsies of healthy donors | Plasma-derived fibrin scaffold | Skin: dermis + epidermis |
|
Cubo et al. 2016 | |
| Human primary dermal fibroblasts, human primary epidermal keratinocytes | Newly-developed ECM-like bioInk | Skin model |
|
Rimann et al. 2016 | |
| Amniotic fluid-derived stem (AFS) cells | Photo-cross-linkable heparin-conjugated hyaluronic acid (HA-HP) hydrogel | In situ skin graft |
|
Skardal et al. 2016 | |
| Fibroblasts | Collagen hydrogel | Dermal construct |
|
Hou et al. 2017 | |
| Human fibroblasts and human keratinocytes | Unique bioink of alginate, gelatin, fibrinogen | Skin: dermis + epidermis |
|
Pourchet et al. 2017 | |
| Human primary skin cells (fibroblasts and keratinocytes) | Collagen, gelatin. PCL (prevents collagen shrinkage) | Skin: dermis + epidermis |
|
Kim et al. 2017 | |
| Keratinocytes (KCs), melanocytes (MCs) and fibroblasts (FBs) (from three different Caucasian skin donors) | Collagen, PVP (Polyvinylpyrrolidone) polymer | Pigmented skin: bioprinting vs. manual-cast approach |
|
Ng et al. 2018 | |
| Endothelial progenitor cells (EPCs) and adipose-derived stem cells (ASCs) added to HDF (human dermal fibroblast) and HEK (human epidermal keratinocyte) | Skin-derived extracellular matrix (S-dECM) bioink, collagen I matrix (as a control) | Skin patch |
|
Kim et al. 2018 | |
| Fibroblasts, melanocytes and keratinocytes | Collagen hydrogel | Pigmented skin model (dermal + epidermal layer) |
|
Min et al. 2018 | |
| Human melanocytes (HEM), human keratinocytes (HaCat) and human dermal fibroblasts (HDF) | Gelatin methacrylamide (GelMA) and collagen (Col)+ tyrosinase (Ty) | Living skin model |
|
Shi et al. 2018 | |
| 2019–2020 | |||||
| Fibroblasts and keratinocytes | Hydrogel (fibrinogen, collagen I, trombin) | In situ skin bioprinting |
|
Albanna et al. 2019 | |
| Neonatal human dermal fibroblasts and neonatal normal human epithelial keratinocytes | Gelatin, fibrinogen, collagen, elastin (dermal hydrogel) | Skin equivalent |
|
Derr et al. 2019 | |
| Human fibroblasts, keratinocytes, human umbilical vein endothelial cells (HUVECs), preadipocytes | dECM-based bioinks, gelatin hydrogel. PCL transwell system (supportive mesh) | A vascularized tri-layered skin model (epidermis, dermis, and hypodermis) |
|
Kim et al. 2019 | |
| Human amniotic epithelial cells (AECs), Wharton’s jelly-derived mesenchymal stem cells (WJMSCs) | Alginate/gelatin composite hydrogels | Skin bilayered construct |
|
Liu et al. 2019 | |
| No cells | PCL and silk sericin for epidermis + CS_SA hydrogel for dermis (CS, chitosan; SA, sodium alginate) | Composite skin construct: three-dimensional skin asymmetric construct (3D_SAC) |
|
Miguel et al. 2019 | |
| Human dermal fibroblasts (HDFs) | Skin decellularized extracellular matrix (dECM) | Bioprinted 3D construct |
|
Won et al. 2019 | |
| Neonatal human dermal fibroblasts (NHDFs), immortalized human keratinocyte cell line (HaCaT) and human umbilical vein endothelial cells (HUVECs) | Methacrylated gelatin (GelMA) and succinylated chitosan/dextran aldehyde | Prevascularized core/shell construct for wound healing |
|
Turner et al. 2020 | |
| Human-derived skin fibroblasts (hSF) | Bioink made of nanofibrillated cellulose (NFC), alginate (ALG) and carboxymethyl cellulose (CMC) | Dermal construct |
|
Zidaric et al. 2020 | |
Table 2.
Remarkable achievements in 3D bioprinting of cardiovascular tissue. Articles from 2016 to 2018 and from 2019 to 2020.
| Tissue | Cell Type | Biomaterial | Printed Model | Outcome | Reference |
|---|---|---|---|---|---|
| Heart | |||||
| 2016–2018 | |||||
| HUVECs, neonatal rat CMs/ hiPSCs-CMs | Alginate and GelMa | Endothelialized-myocardium-on-a-chip model |
|
Zhang et al. 2016 | |
| Human adipose derived mesenchymal stem cells (HADMSC), aortic valve interstitial cells (HAVIC) and aortic valve sinus smooth muscle cells (HASSMC) | Mixture of methacrylated gelatin/polyethylene glycol diacrylate/alginate (MEGEL/PEGDA 3350/alginate) | 3D-bioprinted hydrogels for cardiac valve |
|
Kang et al. 2017 | |
| Human coronary artery endothelial cells | Sodium alginate | Cardiac constructs (different architectures) |
|
Izadifar et al. 2017 | |
| hiPSC-CMs, FBs, ECs | Scaffold-free | Patch |
|
Ong et al. 2017 | |
| hiPSC-CMs, human dermal FB and EC (HUVECs) | Scaffold-free | Tubular cardiac constructs made of cardiac spheroid |
|
Arai et al. 2018 | |
| Rat primary cardiomyocytes | Fibrin cell-laden hydrogel, sacrificial hydrogel and a PCL supporting frame | Patch |
|
Wang et al. 2018 | |
| hCPCs, cECM | Decellularized cardiac extracellular matrix hydrogel (cECM) and gelatin methacrylate (GelMA) | Patch |
|
Bejleri et al. 2018 | |
| Bone marrow- derived human mesenchymal stem cell (hMSCs), neonatal rat CMs | Gelatin hydrogel | Patterned gelatin hydrogel 3D bioprinted grid |
|
Tijore et al. 2018 | |
| Human coronary artery endothelial cells (HCAECs) | Carboxyl functionalized carbon nanotubes (CNTs) incorporated alginate framework and cell-laden methacrylated collagen (MeCol) | Nanoreinforced hybrid cardiac patch |
|
Izadifar et al. 2018 | |
| 2019–2020 | |||||
| iPSCs-derived CMs and ECs (patient-specific) | Patient-specific hydrogel (collagen/ECM) | Patient-specifically designed patch |
|
Noor et al. 2019 | |
| hESC-CMs and cardiac FBs | Collagen | Left ventricle model, tricuspid heart valve |
|
Lee et al. 2019 | |
| hiPSC-CMs, FB and EC | Scaffold-free | Patch |
|
Yeung et al. 2019 | |
| hiPSC-CMs | Photo-cross-linkable cardiac dECM | Biomimetically patterned construct |
|
Yu et al. 2019 | |
| Wnt-activated hiPSC-cardiomyocytes, hiPSC-cardiomyocytes | GelMA and 0.3% (wt/vol) lithium phenyl-2 4 6-trimethylbenzoylphosphinate (LAP) | hPSC-derived cardiomyocyte mini hearts |
|
Ren et al. 2019 | |
| hiPSC-CM and normal human cardiac fibroblasts (NHCFs) coated with fibronectin and gelatin | Fibrinogen and hyaluronic acid | Layer-by-layer heart construct hiPSC-CM-derived |
|
Chikae et al. 2019 | |
| iPSCs-derived CMs, ECs | Photo-cross-linkable bioink of ECM proteins and GelMa | Chambered cardiac pump |
|
Kupfer et al. 2020 | |
Table 3.
Remarkable achievements in 3D bioprinting of pancreatic tissue.
| Tissue | Cell Type | Biomaterial | Printed Model | Outcome | Reference |
|---|---|---|---|---|---|
| Pancreas | |||||
| Human pancreatic islet cells | Polylactic acid functionalized with growth factor-enriched platelet gel | 3D printed construct |
|
Farina et al. 2017 | |
| Pancreatic cancer cells (Patu8902) and activated pancreatic fibroblast cells (PS1) | Nanoshuttle (NS) composed of iron oxide, poly L-lysine and gold nanoparticles | In vitro pancreatic tumor model |
|
Noel et al. 2017 | |
| Human colorectal adenocarcinoma cell line HT-29, human pancreatic epithelial carcinoma cell line PANC-1 | Nanoshuttle (NS) composed of iron oxide, poly L-lysine and gold nanoparticles | Primary pancreatic organoid tumor models |
|
Hou et al. 2018 | |
| Pancreatic cancer cell lines, i.e., MIA PaCa-2 and PANC-1 | NanoShuttle nanoparticles (Nano3D Biosciences Inc., Houston, TX, USA) | Spheroids from MIA PaCa-2 and PANC-1 cells, mixed with human fibroblasts in a ratio of 1:1, and incubated with NanoShuttle nanoparticles |
|
Daunys et al. 2019 | |
| Human primary pancreatic stellate cells (PSCs), human umbilical vein endothelial cells (HUVECs), HMF, subcutaneous preadipocytes(SPA), and MCF-7 cells | Alginate-containing hydrogel |
|
Langer et al. 2019 | ||
| AR42J-B-13 rat acinar cell line | Methacrylated gelatin (GELMA) | Laser-assisted bioprinted 3D pancreatic cell spheroid arrays |
|
Hakobyan et al. 2020 |
Table 4.
Remarkable achievements in 3D bioprinting of cartilage tissue. Articles from 2016 to 2018 and from 2019 to 2020.
| Tissue | Cell Type | Biomaterial | Printed Model | Outcome | Reference |
|---|---|---|---|---|---|
| Cartilage | |||||
| 2016–2018 | |||||
| Rabbit ear chondrocytes | PCL, gelatin, fibrinogen, HA (hyaluronic acid) | PCL and chondrocytes laden scaffold |
|
Kang HW et al. 2016 | |
| hMSC | Nanocrystalline hydroxyapatite | Scaffold |
|
Nowicki et al. 2016 | |
| Human mesenchymal stem cell | 2D nanosilicate reinforced kappa-carrageenan (κCA) hydrogels | Hydrogel scaffold |
|
Thakur et al. 2016 | |
| Human mesenchymal stem cells (hMSCs) | Poly(ethylene) glycol diacrylate (PEGDA)/acrylated peptides/I-2959 photoinitiator | bioprinted 3D construct |
|
Gao et al. 2016 | |
| Human adipose stem cells |
Gelatin–methacrylamide/hyaluronic acid–methacrylate (GelMa/HAMa) hydrogel |
Hand-made 3D Scaffold |
|
O’Connell et al. 2016 | |
| hMSCs and human nasal chondrocytes | Nanofibrillated cellulose and alginate |
5 × 5 × 1.2 m biological construct |
|
Apelgren P. et al. 2017 | |
| iPSCs | Nanofibrillated cellulose and alginate (NFC/A) or hyaluronic acid (NFC/HA) | Scaffold |
|
Nguyen D. et al. 2017 | |
| Chondrocytes | Methacrylated hyaluronic acid (HAMA) + methacrylated poly[N-(2-hydroxypropyl) methacrylamide mono/dilactate] (pHPMA-lac)/polyethylene glycol (PEG) + polycaprolactone (PCL) | Scaffold |
|
Mouser et al. 2017 | |
| Human and equine mesenchymal stem cells (hMSCs) | Hyaluronic acid/poly(glycidol) and poly(ε-caprolactone) | Bioprinted 3D construct |
|
Stichler et al. 2017 | |
| Human embryonic kidney (HEK) cells and ovine mesenchymal stem cells (oMSCs) | 8:1 v:v mixture of ULGT-agarose solution to Fmoc-dipeptide solution, with or without collagen | High-resolution patterned 3D cellular constructs |
|
Graham et al. 2017 | |
| MSCs | GelMA + PEGDA + TGF-β1 embedded in nanospheres | Stereolithography Scaffold |
|
Zhu et al. 2018 | |
| hADSCs | GelMa + hyaluronic acid methacrylate | Scaffold with Biopen |
|
Onofrillo et al. 2018 | |
| hMSCs | Poly (l-lactide-co-caprolactone) + poly (lactic-co-glycolic acid) + Aggrecan | Scaffold |
|
Guo et al. 2018 | |
| Human chondrocyte | Gelatin methacryloyl bioink | Three-dimensional disks |
|
Gu et al. 2018 | |
| Mesenchymal stem cells (MSCs) | Gelatin methacrylamide (GelMa) and hyaluronic acid methacrylate (HAMA) hydrogel | Hand-made 3D Scaffold |
|
Di Bella et al. 2018 | |
| 2019–2020 | |||||
| Human chondrocytes | Polylactic acid | Reticular layered scaffold |
|
Baena J.M. et al. 2019 | |
| BM-MSCs | Scaffold-free | Spheroid |
|
Breathwaite E.K. et al. 2019 | |
| Human mesenchymal stem cells (hMSCs) | Polycaprolactone (PCL) | Bioprinted 3D tracheal shape construct |
|
Dongxu et al. 2019 | |
| hMSCs and hACs (human artificial chromosomes) |
GelMa + CS-AEMA (chondroitin sulfate amino ethyl methacrylate) Hyaluronic acid (HC) + TCP (tricalcium phosphate) microparticles |
Reticular layered scaffold |
|
Idaszek J. et al. 2019 | |
| hMSC | PCL | Scaffold |
|
Ke et al. 2019 | |
| ATDC5 cells | Oxidized hyaluronate (OHA), oxidized hyaluronate (OHA), oxidized hyaluronate (OHA) | Scaffold |
|
Kim et al. 2019 | |
| BM-MSCs | β-tricalcium phosphate (TCP) | 3D biomimetic hydrogel scaffold |
|
Kosik-Kozioł et al. 2019 | |
| hADSCs | hydroxybutyl chitosan (HBC) + oxidized chondroitin sulfate (OCS) Hydrogel | Macroporous hydrogel scaffold |
|
Li et al. 2019 | |
| Human cartilage cells + human fibroblasts + human umbilical vein endothelial cells + human mesenchymal stem cells | Culture medium | Spheroid |
|
Machino et al. 2019 | |
| MSCs | Cartilage extracellular matrix (cECM)-functionalized alginate bioink | Scaffold |
|
Rathan et al. 2019 | |
| hMSCs | PEGDA | GDF5-conjugated BMSC laden scaffold |
|
Sun et al. 2019 | |
| No cells used | CTGF (connective tissue growth factor) + TGFβ3 (transforming growth factor beta 3) + BMP2 (Bone Morphogenetic protein 2). All these growth factors are encapsulated in PLGA—poly(lactic-co-glycolic acid) scaffold |
Thin membrane-like scaffold |
|
Tarafder S et al. 2019 | |
| Human auricular chondrocytes (hACs) | poly(2-ethyl-2-oxazoline) (PEOXA)-peptide conjugates + sortase A (SA) + alginate + cellulose nanofibrils (CNF) | Scaffold |
|
Trachsel et al. 2019 | |
| ADSCs | Alginate support | Spheroid |
|
Ayan et al. 2020 | |
| Ovine fetal chondrocytes | Collagen I + fibrin glue | Scaffold |
|
Dasargyri et al. 2020 | |
| hADSCs | 10%GelMa/2%HAMa Hydrogel | Core/shell bioscaffold |
|
Duchi et al. 2020 | |
| hMSC | Calcium phosphate cement (CPC) + alginate-methylcellulose (algMC) | Scaffold |
|
Kilian et al. 2020 | |
| Human adipose tissue-derived mesenchymal stem cells (hADMSCs) | Photo-cross-linkable alginate + gelatin and chondroitin sulfate + graphene oxide | Scaffold |
|
Olate Moya et al. 2020 | |
| ADSCs | GelMA + PEGDA coated with lysine-rosette nanotubes (RNTK) | Lysine-functionalized rosette nanotubes scaffold |
|
Zohu et al. 2020 | |
3. Main Bioprinting Techniques
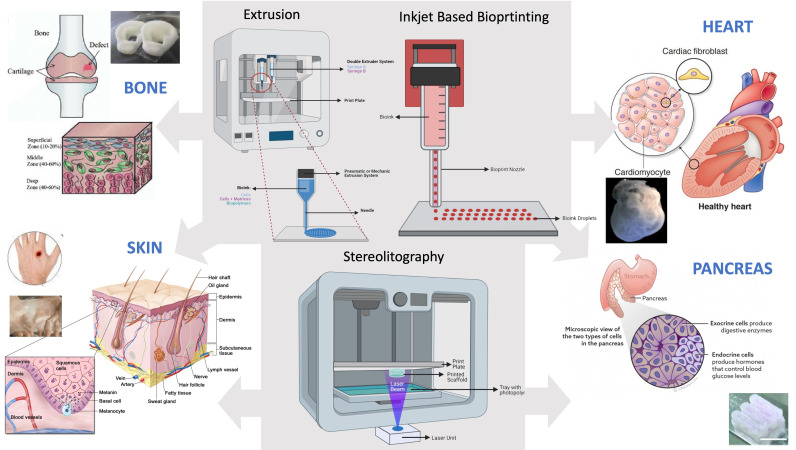
Bioprinting is based on technologies used in mechanical and biomedical engineering. There are different types of bioprinting techniques based on different types of 3D printers and on the deposition system of the biomaterial on the print plate, as schematized in Figure 2, where the applications for the four tissues considered in this review paper are evidenced. The most important bioprinting techniques are extrusion, inkjet and laser printing, but other currently used techniques include microvalve-based [7] and stereolithography (SLA) printing.
Figure 2.
Main bioprinting techniques for different tissue-specific applications.
3.1. Extrusion Printing
One of the most relevant bioprinting technologies is extrusion printing [7] that derives from FFF (fused filament fabrication) technology normally used by conventional 3D printers for printing thermoplastic materials. This type of printing is based on the use of 3D printers formed by pressurized syringes loaded with cells embedded in a bioink, and is particularly suitable for extrusion of viscous gel materials.
The main advantage of extrusion 3D printing is the possibility of extruding cells, extracellular matrices and thermo-biopolymers both individually and simultaneously with more than one extrusion system. This is a bioprinting technique widely used in the bone tissue regeneration field [8] and in drug release studies [9]. The extrusion technique also allows the printing of scaffolds with thermo-biopolymers such as PCL (polycaprolactone) [10,11], PLA (polylactic acid) [11] and PVA (polyvinyl alcohol) [11]. The disadvantage of this technology is the poor print resolution depending on the needle’s size, making it difficult to print very small objects.
3.2. Inkjet—Based Bioprinting
Inkjet is a technology that uses bioink microdroplets which are deposited on culture plates or various substrates. Microdroplets are deposited from a bioink-filled extrusor by positive pressure that causes the drops to escape from the printing nozzle. The pressure which causes droplets to leak can be generated by a heat source in the syringe that creates a positive compression bubble with which the bioink lets it out, or by a piezoelectric system. In the case of the thermal system, the obtained droplets are made up of different sizes and frequently cause the nozzle to clog, which results in the printing of objects that are not particularly smooth. In the case of the piezoelectric system, the cells can be damaged by lysis of the cell membrane.
The inkjet technology has been successfully used in the printing of DNA molecules [12], and of ovarian cells in hamster and rat motor neurons [13]. In 2015, heart valves were printed with this technology although their functional properties have to be still unraveled and studied [14].
One of the main criticisms of the inkjet printing is the poor mechanical consistency of the bioinks which makes the constructs poorly manipulated. It is necessary to develop bioinks that allow for the production of more solid and structurally defined biological objects.
3.3. Laser—Assisted Bioprinting (LAB)
Laser-assisted bioprinting (LAB) is a technique that allows printing cells and liquid materials with a micrometer resolution. Laser-assisted bioprinters consist of three components: (1) a pulsed laser source, (2) a target to which a biological material is printed, and (3) a receiving substrate that collects the printed material. The ribbon is made of a thin absorbing layer of metal (such as gold or titanium) coated onto a laser transparent support (i.e., glass). Organic material (molecules or cells) is prepared in a liquid solution (i.e., culture media), and deposited on the surface of the metal film. The laser pulse induces vaporization of the metal substrate, resulting in the production of a jet of liquid solution which is deposited onto the facing substrate. LAB is an effective tool for in situ printing of a bone substitute, due to its high printing resolution and precision [15,16].
3.4. Microvalve—Based Bioprinting
Microvalve-based bioprinting is a system comprised of a movable robotic platform and an array of multiple electromechanical microvalve print-heads. The bioink ejection is controlled by the pneumatic pressure applied through a gas regulator and a system of micro-valves, which offers a controlled deposition of materials through a layer-by-layer fabrication approach [7]. This technique has some main advantages: it allows the synchronized ejection of biomaterials and cells from different print-heads and the deposition of a thin material layer with a precise cellular positioning and high throughput printing. However, it prints only hydrogels within a limited range of viscosity (∼1 to 200 mPa s) and cell concentrations of up to 106 cells per ml due to the clogging issues in the small nozzle orifice (100–250 μm) [7,17]. The material deposition is highly dependent on the nozzle diameter, the viscosity and surface tension of the bioink, the pneumatic pressure and the valve opening time.
3.5. Stereolithography (SLA)
Stereolithography (SLA) is an early and widely used 3D printing technology. In the early 1980s, a Japanese researcher invented the modern layered approach to stereolithography by using ultraviolet light to cure photosensitive polymers. Stereolithography works by focusing an ultraviolet (UV) laser onto a vat of photopolymer resin [18]. With the help of computer-aided manufacturing or computer-aided design (CAM/CAD) software, the UV laser is used to draw a pre-programmed design or shape on the surface of the photopolymer vat. Photopolymers are sensitive to ultraviolet light, so the resin is photochemically solidified and forms a single layer of the desired 3D object. The liquid materials used for SLA printing are commonly referred to as “resins” and are thermoset polymers. Stereolithographic models have been used in medicine since the 1990s, and they are used as an aid for diagnosis, preoperative planning as well as implant design and manufacture. An example of stereolithography’s application is in rehearsing osteotomies, but also in the production of prototypes and models to help plan surgeries. The main advantage of this technique is its speed, since some functional parts can be manufactured within a day, but the cost represents a limit to its diffusion.
4. 3D Bioprinting of Skin
4.1. Background
Human skin is a multi-layered tissue in which a complex system of cells, glands, nerves and blood vessels cooperate to fulfill several important functions in our body [19,20].
Skin wounds due to trauma, ulcers, burns or other causes represent a social and economic burden, being quite common [21]. Nowadays, the gold standard clinical treatment for these injuries is skin autograft, but its application is strictly related to the extension of the wound [22]. Other common strategies include allografts, liquid formulation, wound dressing and even cell spray techniques [23,24]; more often, traditional graft approaches are combined with skin substitutes. However, despite their great benefits in clinical practice, these substitutes do not fully recapitulate the complexity of native skin, and they display some side effects according to their composition, together with a high cost of production [22]. This disadvantage may be overcome by bioprinting.
4.2. Current Applications and Future Perspectives
Bioprinting represents an emerging tool that could overcome the gap between grafts and skin substitutes.
In a notable study, Cubo et al. bioprinted human skin (dermal and epidermal layers) in less than 35 min, making an important step towards the needs of clinical reality (Table 1). The authors bioprinted primary human fibroblasts (hFBs) within a plasma-derived hydrogel and then seeded primary keratinocytes: clear stratification and differentiation of dermis and epidermis occurred, as shown by the presence of rete ridges (peculiar to human skin) in some regions [25].
Pourchet et al. developed a unique bioink combination of alginate, fibrinogen and gelatin with hFBs for dermal matrix, and then layered keratinocytes on the top (Table 1). A complete evaluation of bioprinted skin morphology was performed: epithelium and dermis maturation, ECM production and the formation of desmosomes and hemidesmosomes demonstrated structural similarity to human normal skin [26].
In the work of Kim et al., the skin substitute was realized using a novel hybrid printing system (integrated composite tissue/organ building system) in which extrusion and inkjet printing are combined in a single-step process (Table 1). A polycaprolactone (PCL) mesh supported the printing of the dermal layer (collagen + fibroblasts), preventing collagen shrinkage; then, the inkjet printer uniformly seeded keratinocytes on the dermal matrix. After 14 days, 3D printed layers displayed a good degree of maturation, as shown by the cellular spatial distribution and the expression of dermal and epidermal markers [27]. One year later, the authors tested the properties of porcine decellularized extracellular matrix (dECM), processing it and using it as a bioink. The dECM was demonstrated to mostly retain its composition after processing and also proved to be a biomimicking substrate, setting the proper conditions for cell growth and differentiation. Moreover, the authors succeeded in bioprinting a pre-vascularized skin patch (made of adipose-derived stem cells and endothelial progenitor cells in dECM), which resulted in a significantly improved neovascularization and re-epithelialization after a graft in an athymic nude mouse model [28]. This represents a remarkable issue for engineered constructs, since they must go through a perfect integration with the host endogenous tissues, in addition to being vascularized and innervated. Other approaches to induce angiogenesis in tissue remodeling involve the fabrication of scaffolds soaked with growth factors, or absorbing pro-angiogenic molecules within the bioink [29].
Melanocytes reside in the basal layer of the epidermis and they shield skin surface from UV radiation through melanin production, thus playing an important protective role [30]. Some authors tried to produce skin models including also this cell type, with different outcomes [31,32,33] (Table 1). Recently, to overcome poor printability and long cross-linking duration of collagen biomaterials, Ng et al. reported the use of polyelectrolyte gelatin-chitosan (PGC) hydrogel for 3D bioprinting of skin. They optimized a co-culture medium for human melanocytes, keratinocytes and hFBs, and they bioprinted a uniformly pigmented skin, with human melanocytes anchored at the basement membrane and well stratified and mature layers of dermis and epidermis [34] (Table 1). This could represent a promising starting point for further improvements in co-culture techniques.
All these latest remarkable achievements bring us closer everyday to the clinical application of bioprinting in the treatment of skin defects, but currently this is not yet a therapeutic option since there are several key points that represent critical limitations to overcome.
First of all, bioprinted skin should have the proper thickness, texture and permeability, and the biological construct should achieve the right internal porosity to grow and integrate in vivo [35,36]. Unfortunately, the proper permeability is often not reached in the printed constructs [24]. On the other hand, an essential requirement concerns vascularization and innervation: indeed, these goals are far from being fully achieved for bioprinted skin, despite the progress in the field [3].
Another critical issue is the integration within the construct of skin appendages and different cell types: no current bioprinted prototype of human skin fully recapitulates all cells and appendages present in native skin [24], even if there is active research in the field. Finally, the importance of GMP (good manufacturing practices) requirements should be considered: all the cells, the biomaterials and the products destined for clinical use should undergo prior validation of the production process, quality assessments and regulatory approval [4,24,37]. Furthermore, the intrinsic time- and cost-effectiveness of such technology have an impact on its everyday use in clinical practice.
5. 3D Bioprinting in Cardiovascular Disease
5.1. Background
Cardiovascular diseases are a leading cause of mortality, affecting a lot of people worldwide [38]. In China and the USA, cardiovascular diseases are recognized as a major national health concern; their burden grows over time and it [39,40] is estimated that one in three deaths are due to cardiovascular diseases.
Myocardium is a very complex tissue, with a hierarchical structure [41], made of a thick weave of highly specialized cells (cardiomyocytes, CMs), fibroblasts (FBs) and blood vessels. It has poor regenerative capacities and an electrical activity that must be well coordinated and responsive to physiological and pharmacological stimuli; one of the main risks of implanting a cardiac patch is the unsuccessful integration with the host cardiac frequency, thus generating arrhythmias [42,43]. A myocardial injury (such as an infarction) causes CMs death and the formation of scarring tissue, with possible heart failure in the case of extensive damage [44]. Currently, the main therapeutic option for severe heart failure is organ transplantation, but it presents two major limitations: the lack/scarcity of donors and the possible rejection by the immune system [45]. Thus, a promising future option could be represented by the tissue engineering approach, associated with the bioprinting technique [46].
Notably, Lee et al. bioprinted a contractile left ventricle model seeded with human stem cell-derived cardiomyocytes (hESC-CMs) and cardiac fibroblastsin the core region, using a novel bioprinting procedure (“FRESH—Freeform Reversible Embedding of Suspended Hydrogels system) (Table 2). They also bioprinted a tricuspid heart-valve and a heart of neonatal size, achieving scalability, high printing reliability and accuracy [47]. In a recent study, Noor et al. bioprinted a vascularized cardiac patch and a mini heart-like structure using a “personalized” bioink, made of a hydrogel derived from human omental tissue and of CMs + endothelial cells differentiated from human iPSCs (Table 2). The patch showed sarcomeric organization, contractile potential and also a prototype of a vascular network [48]. Kupfer et al. also bioprinted iPSCs-derived CMs and endothelial cells in a chambered mini-sized heart, endowed with thick-walls and electromechanical function [49] (Table 2); this could represent a promising model for in vitro studies. Similarly, in another study, a bioprinted cardiac patch physiologically responded to cardiac drugs (carbachol and epinephrine), reversibly modulating the beating frequency, thus showing potential relevance in drug testing [50].
Among the most used bioinks in the cardiac field are dECM and its components [51,52,53]; several new compositions have been/are being investigated [54] and even scaffold-free approaches, involving the bioprinting of cellular spheroids on a micro-needles platform as a support [55]. An interesting example of this scaffold-free technology is described by Ong et al., who co-cultured iPSC-CMs, FBs and endothelial cells in different ratios to form cardiospheres (Table 2). Then cardiospheres were selectively picked up by a robotic arm and very precisely bioprinted on a needle array. Spheroid fusion formed cardiac patches electrically well integrated and able to engraft in vivo, but they showed poor mechanical properties and arrhythmias were detected in the regions at higher FBs concentrations [56]. In vivo analysis was performed in a successive work, with grafted patches increasing survival rate in infarcted rats and slightly improving cardiac function [57]. However, the short follow-up period and a lack of coordination between endogenous and iPSCs-CMs contraction rate have to be considered in this study [57].
5.2. Current Applications and Future Perspectives
Three-dimensional stem cell bioprinting approaches can have huge implications in regenerative medicine, for the modeling and treatment of heart disease and failure. One of the most dynamic research topics of bioprinting is actually focusing on cardiac valve and myocardial regeneration, with a specific interest in creating a prototype of a biofunctional cardiac patch [58,59].
The first aspect to consider in bioprinting myocardial tissue is the cell source. Adult cardiomyocytes are difficult to expand in vitro [60], and their availability is scarce. For this reason, several alternative cell types have been considered, including MSCs (mesenchymal stem cells), human cardiac progenitor cells (hCPCs) [61], ESCs (embryonic stem cells) and iPSCs (induced pluripotent stem cells) [48,62]. However, all these cell types have some important limitations: unlike ESCs, iPSCs-derived cardiomyocytes do not trigger any immune reactions in the patient since they are endogenous cells, but on the other hand they often display an incomplete differentiation into cardiomyocytes [63]. As with stem cells, use of both iPSCs and ESCs raises concerns about safety because of their teratogenic potential [64]. Finally, the clinical use of ESCs implies some ethical questions, since they can come from the disruption of an embryo [65]. Another aspect to consider is that, independently of the cells used, a damaged microenvironment—such as the one of the host after an infarction—can impinge on the survival rate, differentiation capabilities and functionality of the transplanted cells [65].
The scaffold-free 3D bioprinting approach could be the technique which mostly mimics the native tissue physiology, but currently the bioengineering and fabrication techniques are not advanced enough to guarantee an adequate fit for clinical purposes [66].
Nowadays, there is no clinical application of bioprinting for heart repair: engineered myocardium grafts are currently at the preclinical study level and may very well represent economical and efficient solutions to myocardial infarction in the future [67,68].
Besides the limitations related to the type of cells employed in the fabrication of a patch, another critical shortcoming of all the bioprinted grafts is that the implant should be highly vascularized to allow cell survival. Indeed, the construct has to reach an in vivo degree of vascularization not limited to capillaries, but implying an organized and hierarchical network of blood vessels, able to support and nourish the implanted cells. This level of vascularization for bioprinted patches has not been fully achieved [34].
Further studies are needed for printing adequately vascularized heart tissue of clinically-relevant thickness that can appropriately respond to electrical impulses and maintain a synchronous beating pattern [68], since a synchronous and host-integrated contractility rate should be reached by the implant. To improve this aspect, several kinds of biomaterials have been optimized, such as silicon-nanowire field-effect transistors integrated with collagen, alginate and PLGA (poly(lactic-co-glycolic acid)) scaffolds that have been used to monitor the electrical activities of seeded cardiomyocytes [69]. Further efforts need to be undertaken in the use of nanoelectronic scaffolds [70] to provide electrical and mechanical stimulation to the cells in order to promote cardiomyocyte growth and stimulation. Additionally, next generation scaffolds should facilitate spontaneous beating of engineered cardiac tissue such as through the incorporation of carbon nanotubes in hydrogels [71], which has been shown to enhance viability and phenotypical features of rat ventricular myocytes [69].
Finally, some critical roadblocks—common for all bioprinted organs—have to be overcome to bring cardiac bioprinting into clinical practice: scalability, cost-effectiveness, and the establishment of GMP production rules and precise regulatory guidelines [42,72].
6. 3D Bioprinting of Pancreatic Tissue
6.1. Background
In vitro 3D models have been developed to investigate the in vivo tumor biology, microenvironment and growth conditions of pancreatic cancer cells and to identify new therapeutic approaches for diabetes [73,74]. Diabetes is a major health concern worldwide with a significant burden in terms of clinical and socioeconomic impact [75]. Its prevalence is increasing worldwide, with an estimate of approximately 1 in 300 by 18 years of age in the United States [76]. Type 1 diabetes mellitus (T1DM) is a chronic autoimmune disease caused by a dysfunction of pancreatic beta cells with a loss of insulin secretion [77]. Most beta cells are destroyed before clinical onset and this leads to persistently high levels of glucose in the blood [78]. Longstanding hyperglycemia leads to several complications including neuropathy, cardiovascular disease, nephropathy and retinopathy. T1DM management requires a high patient compliance with multiple daily blood glucose measurements and subcutaneous insulin injections [79].
New approaches providing cellular replacement strategies could radically change the management of the disease and the quality of life of diabetic patients. Much progress has been made regarding the in vitro culturing methods, also in studying the cancer microenvironment. Compared to growth in 2D systems, cancer cells grown in these 3D environments have shown different gene expression and phenotypes, highlighting the importance of these cellular interactions to oncogenesis [80]. Moreover, 3D cancer models showed high potential to simulate the tumor microenvironment and to help better understand in vivo tumor features, such as toxicity and therapeutic resistance [81,82].
6.2. Current Applications and Future Perspectives
Developing therapies for pancreatic diseases, such as diabetes and cancer, is hampered by a limited access to pancreatic tissue in vivo. The only curative cell therapy for type 1 diabetes mellitus is actually pancreatic islet cell transplantation [78]. However, its potential to treat many more patients is limited by several challenges. The emergence of 3D bioprinting technology from recent advances in 3D printing, biomaterials and cell biology has provided the means to overcome these challenges.
Mouse models allowed the study of environmental factors necessary for tumor growth, progression and therapeutic response [83]. Through magnetic bioprinting, 3D spheroids were obtained using a co-culture of pancreatic cancer cells and activated pancreatic fibroblasts and they have been subjected to metabolic assay [84] (Table 3). These spheroids provide an in vitro tumor model with stromal cells, which are missing in 3D models. This model can be improved and replicated for other cell types and it provides an important tool to generate functional spheroids that contain the two major cell types found in most tumor tissues, cancer cells and cancer-associated fibroblasts, which can be employed for other analysis, such as drug screening [84]. Other authors evaluated pancreatic cancer cells’ ability to form spheroids or organoids and demonstrated that this ability is influenced by the expression levels of adhesion molecules, such as β1-integrin and E-cadherin, and the interaction of β1-integrin with extracellular matrix proteins (ECM), similar to what has been demonstrated in other cancers such as hepatoma, and breast cancer [85] (Table 3).
Hakobian et al., using laser-assisted bioprinting, generated 3D pancreatic cell spheroid arrays, composed of both acinar and ductal cells; they characterized their phenotypic evolution over time and showed that they can mimic the initial stage of pancreatic ductal adenocarcinoma (PDAC) (Table 3). The analysis of internal and external factors that contribute to the formation of precursor PDAC lesions and to cancer progression can shed light on future PDAC therapy strategies [86].
Even though human models are needed to better understand the interaction between tumor and stroma, Langer et al. demonstrated that bioprinting allows for modeling patient-derived tissues in a complex microenvironment and that bioprinting- generated tissues represent the most accurate in vivo models relevant to evaluate the therapeutic response [83] (Table 3).
An important promise for diabetes treatment is islets transplantation on porous biomaterials. Some authors created a device to maintain pancreatic islets close to blood vessels in a growth factor-enriched environment which facilitates cell delivery subcutaneously [87]. In experimental studies, islets have also been implanted in the renal capsule, which also has a rich blood supply or in the omento through a fibrin scaffold. However, both technologies require invasive surgeries [88]. Further studies are needed in humans to obtain the creation of artificial pancreas for the treatment of diabetic patients. This being said, a bioprinted pancreas-on-a-chip model is possible in the near future. Bioprinting technologies may be crucial in the organization of diverse cell types and complete organ manifestation.
7. 3D Bioprinting of Cartilage
7.1. Background
Cartilage is a flexible, aneural and avascular connective tissue with poor regenerating capacities [89]. It constitutes a very important covering tissue of articular surfaces as well as a constituent of the intervertebral discs, the intra-articular menisci and the auricle. It also has a role in providing support to some organs of the respiratory system.
Cartilage is a tissue that does not feed through the blood supply (at least not directly) but mainly through the synovial fluid, which contains nutrients and proteins capable of nourishing the cartilage tissue. On its own, cartilage is unable to repair itself following traumatic or degenerative injuries.
In the orthopedic field, there have been many attempts to regenerate cartilage (especially in joints) through the transplantation of either autologous human chondrocytes or mesenchymal cells. All these attempts, however, mainly led to the reduction of painful symptoms for the patient but not to a biologically “exact” regeneration of the native tissue. In fact, the new “generated” fibrous or fibro-cartilage tissue is very different from native cartilage. Thus, articular cartilage injuries are scarcely repaired and represent an outstanding healthcare problem, since the lesions can often progress to osteoarthritis [90]. Current strategies to treat articular cartilage defects include microfracture, mosaicplasty and cell-based techniques [91], but they have several limitations, such as the lack of effectiveness in recreating the native architecture of the tissue, the costs and the short-term resolution [92]. The fact that cartilage tissue is not a vascularized tissue, not innervated and only indirectly fed by blood makes it one of the most “easily” tackled tissues in terms of tissue regeneration, bioprinting and tissue engineering, also taking into account the difficulty of recreating neo-angiogenesis with 3D printers and, in general, with tissue engineering approaches.
Mesenchymal stem cells are shown to be the most suitable cell type to avoid the expansion of mature chondrocytes with all the negative aspects described above. The first attempt was in 1970 by Friedenstein et al., who cultured in vitro cells isolated from bone marrow, producing cartilaginous and bone sketches. In 1998 Johnstone et al. gave rise to “micromasses” with cells put in test tubes, centrifuged, and with addition of various growth factors to increase the chondrogenic possibilities of mesenchymal stem cells and to prevent their dedifferentiation (which, as reported above, occurs for in vitro expansion of mature chondrocytes).
7.2. Current Applications and Future Perspectives
For the lower airways defects, such as tracheobronchial malformations, the treatment options are few and they include surgical resection and subsequent end-to-end anastomosis, or the placement of splints/stents to maintain the patency of the respiratory tracts [93].
For this reason, there is a need for alternative therapeutic approaches that could bring significant benefits to patients, minimizing the side effects. There are some major hurdles in bioprinting constructs for airway defects, including the lumen patency, the mechanical stability, the necessity of long-term preclinical studies [94], the exposure to air contaminants which could impair the proper epithelium maturation [94] and the risk of granulation/inflammatory tissue formation when there is no epithelial layer covering the scaffold lumen-side [95,96,97].
Despite many important achievements in the bioprinting of joint cartilage, several major roadblocks must be overcome. Articular cartilage can be divided into four different zones which appear heterogenous for cell density, composition and mechanical properties. Reproducing this spatial organization and structural complexity through bioprinting has not been achieved yet [98]; moreover, the cartilage implant has to fully integrate with the underlying articular subchondral bone [3,99]. Further problems deal with the long-term effectiveness of engineered cartilage, the optimum of cell density related to defect size, and the concerns about laboratory procedures, the serum-based media and biomaterials containing products of animal origin [100].
Actually, there are not many clinical applications of mesenchymal stem cells in the regeneration of cartilage tissue in humans. Considering the existing clinical trials, (ClinicalTrials.gov) we found eight projects aimed at experimenting with the regeneration of cartilage in humans, especially in the case of osteoarthritis. Very good results were achieved: histological examinations in some trials showed cartilage regeneration, a significant decrease in pain and a return to normal post-implantation activities in 6 months.
The possibility to obtain chondrocytes starting from mesenchymal stem cells isolated from adipose tissue which, unlike stem cells from bone marrow, seem to confer a certain degree of protection to other cell types and have a lower inflammatory activity, has recently been taken into consideration [101,102].
A promising option for articular cartilage repair could be represented by the in situ bioprinting approach, which takes advantage of the possibility to precisely shape the construct directly on the defect site at the time of the surgical operation [103]. Indeed, O’Connell et al. developed a hand-held in situ printing device, called “Biopen”, endowed with two ink chambers and exploiting a coaxial extrusion-based printing mechanism [104] (Table 4). They optimized the core/shell printing method of a cell-laden bioink for this device [26], and then Biopen was tested in vivo on six sheep with chondral defects, showing some promising preliminary results [35]. In another work, Ma et al. proposed an in situ 3D printing approach, in which a robotic-assisted printing technology was applied to repair osteochondral defects in rabbits; after 12 weeks, the defect was filled with newly-formed hyaline cartilage [105] (Table 4).
To address the issue of osteochondral integration for engineered joints implants, some authors perfected biomaterials enhancing calcified cartilage differentiation. You et al. optimized a hydrogel combining alginate and broadly-distributed hydroxyapatite particles: this bioink supported the production of a mineralized matrix from embryonic chick chondrocytes [106] (Table 4). Another research group printed hMSCs in a bioink enriched with β-tricalcium phosphate, then characterized the hydrogel from the mechanical and rheological points of view, and finally assessed the expression of several chondrogenic markers, including some related to mineralization [107].
Park et al. bioprinted autologous rabbit chondrocytes and epithelial cells in a construct, alternating five layers of alginate cell-laden hydrogel and PCL (Table 4). Epithelium regenerated after three months, while mature cartilage formation was not yet achieved at one year of follow-up [108]. In another recent interesting experiment, the authors employed a PCL supportive scaffold and two hMSCs-laden hydrogels to bioprint a tracheal construct with a cartilage and a smooth muscle region, thus mimicking the structural composition and mechanical properties of human trachea [109].
8. Hurdles and Promises of Bioprinting in the Clinical Routine
Three-dimensional bioprinting is an emerging technique with a striking potential in solving those clinical issues which generally require a tissue/organ allograft. The major strengths of this technology lie in the possibility of tailoring patient-specific constructs through last-generation bioprinters and, most of all, in the potential use of autologous biological material to shape personalized constructs in regenerative medicine applications.
However, to take the step “from bench to bedside” in bioprinting is still challenging, even if the research trend of the past few years is highly focused on finding new solutions to the current problems which prevent the clinical use of this technique in tissue engineering.
In this context, the main limitations are due to the biological complexity of human tissues and organs, together with the biophysical and rheological properties of biomaterials and their actual “printability”. One of the major roadblocks to overcome resides in reproducing a functional vascular network within complex biological structures shaped in vitro: nowadays, this aspect is still an open challenge in the bioprinting field [110]. Actually, the intrinsic morphological and structural features of vasculature, which is made by a hollow and perfusable lumen enclosed by three layers with different physical properties, requires an appropriate combination of biomaterials and printing conditions which is not easy to achieve in vitro [110].
Despite this, some human tissues are certainly more easily printable than others both for their biochemical characteristics and for their actual physical consistency. Bone tissue and cartilage tissue are among the most studied tissues that have reached a good level of maturation, so it is highly probable that they can reach the patient’s bedside before the others. In the case of bone tissue, dentistry is the most interested and active medical discipline in the in vivo experimentation on humans for the implantation of 3D printed bone substitutes, with the use of biopolymers [111] functionalized with growth factors and proangiogenic factors [112]
To date, there are bone substitutes on the market obtained through the combination of bioceramic materials capable of performing an osteogenic and osteoinductive function [112].
Cartilage is another tissue whose 3D bioprinting can be tackled more easily than other tissues [113].
Indeed, cartilage tissue is not vascularized and has a general consistency favorable to in vitro bioprinting. The rheological characteristics of collagen (especially if highly concentrated) treated with molecular cross-linking techniques are favorable to 3D printing in the laboratory, and this opens the way to important future developments considering, above all, the increasing demand for cartilage substitutes due to trauma or pathologies from tissue degeneration as a result of the aging of the population.
In some specific biomedical contexts, bioprinting has reached the stage of human experimentation, as for example in skin generation, a pressing need in the dermatological sector and plastic surgery due to the complexity of the treatment of skin lesions and burns. A clinical trial is currently under way (NCT04925323) which involves the recruitment of 25 subjects from which to extract epidermal tissue to be used for the creation of 3D printed skin substitutes.
However, the clinical applicability of bioprinting should not be understood as simply oriented towards tissue regeneration. It is in fact important to remember that, to date, there are important and relevant applications of bioprinting for the in vitro study of human models.
In vitro tissue bioprinting is essential for drug screening and for the study of chemotherapeutic sensitivity of tissues in medical oncology. In the oncology field, its concrete application is to recreate the tumor microenvironment in vitro in order to study drug combinations for the treatment of cancer [114]. In this regard, numerous clinical trials are under way to bioprint patient-derived organoids for the study of the chemosensitivity of myeloma (NCT03890614) and for the study of the chemotherapy response of colorectal cancer with and without liver metastases (NCT04755907). Finally, clinical trials are also active in the study of myocardial infarction by reproducing in vitro the thromboembolic microenvironment from patient-derived biological material through the construction of 3D organoids (NCT03832153).
9. Conclusions
Bioprinting represents a research topic of great interest and has the potential to change the future of medical science. Its application in tissue engineering could totally subvert our concept of transplantation, eliminating all the issues linked to immunocompatibility and organ waiting lists, and ultimately creating a completely tailor-made and patient-specific tissue substitute. As aforementioned, striking results have been obtained and are continuing each day in the realm of bioprinting of heart, cartilage, skin, pancreas and many other tissues. The real challenge for the future of bioprinting is the development of printing techniques and materials which are able to provide suitable mechanical resistance before, during, and after printing as well as to simulate real anatomical tissues as much as possible. Today, the most important critical issue is the difficulty in printing soft materials such as those that make up human tissues. The poor physical consistency of these materials makes the printing process difficult and often unable to achieve complex 3D structures. Thus, the future of bioprinting hinges on the need for improvements in printing techniques and especially in the choice and formulation of proper biological materials for the construction of fabrics and organ parts. This will be only possible with a multidisciplinary approach involving complementary disciplines such as materials engineering, bioengineering and biology.
Abbreviations
(h)FBs: (human) fibroblasts; (d)ECM: (de-cellularized) extracellular matrix; PCL: polycaprolactone; (h)CMs: (human) cardiomyocytes; (h)MSCs: (human) mesenchymal stromal/stem cells; (h)ESCs: (human) embryonic stem cells; (h)iPSCs: (human) induced pluripotent stem cells; GMP: good medical practices.
Author Contributions
E.D.P.: literature review, design of the work, writing the article; E.P.: writing the article, literature review and reference reviewing; I.C.: writing the article and reference reviewing; A.D.F.: literature and reference reviewing; A.E.T.: article and literature reviewing; A.S.: writing the article, article reviewing; L.B.: design of the work, writing the article, literature review. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Conflicts of Interest
The authors declare the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Matai I., Kaur G., Seyedsalehi A., McClinton A., Laurencin C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226:119536. doi: 10.1016/j.biomaterials.2019.119536. [DOI] [PubMed] [Google Scholar]
- 2.Ma X., Liu J., Zhu W., Tang M., Lawrence N., Yu C., Goud M., Chen S. 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling. Adv. Drug Deliv. Rev. 2018;132:235–251. doi: 10.1016/j.addr.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vijayavenkataraman S., Yan W.-C., Lu W.F., Wang C.-H., Fuh J.Y.H. 3D bioprinting of tissues and organs for regenerative medicine. Adv. Drug Deliv. Rev. 2018;132:296–332. doi: 10.1016/j.addr.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Ozbolat I.T., Peng W., Ozbolat V. Application areas of 3D bioprinting. Drug Discov. Today. 2016;21:1257–1271. doi: 10.1016/j.drudis.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Murphy S.V., Atala A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 6.Hospodiuk M., Dey M., Sosnoski D., Ozbolat I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017;35:217–239. doi: 10.1016/j.biotechadv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Ng W.L., Lee J.M., Yeong W.Y., Win Naing M. Microvalve-based bioprinting—Process, bio-inks and applications. Biomater. Sci. 2017;5:632–647. doi: 10.1039/C6BM00861E. [DOI] [PubMed] [Google Scholar]
- 8.Tiberio F., Cacciotti I., Frassanito P., Nocca G., Tamburrini G., Arcovito A., Lattanzi W. Personalized Bone Reconstruction and Regeneration in the Treatment of Craniosynostosis. [(accessed on 1 September 2021)];Appl. Sci. 2021 11:2649. doi: 10.3390/app11062649. Available online: https://www.mdpi.com/2076-3417/11/6/2649. [DOI] [Google Scholar]
- 9.Seoane-Viaño I., Januskaite P., Alvarez-Lorenzo C., Basit A.W., Goyanes A. Semi-solid extrusion 3D printing in drug delivery and biomedicine: Personalised solutions for healthcare challenges. J. Control. Release. 2021;332:367–389. doi: 10.1016/j.jconrel.2021.02.027. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Z., Diggle B., Tan M.L., Viktorova J., Bennett C.W., Connal L.A. Extrusion 3D Printing of Polymeric Materials with Advanced Properties. Adv. Sci. 2020;7:2001379. doi: 10.1002/advs.202001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sodupe Ortega E., Sanz-Garcia A., Pernia-Espinoza A., Escobedo-Lucea C. Efficient Fabrication of Polycaprolactone Scaffolds for Printing Hybrid Tissue-Engineered Constructs. Materials. 2019;12:613. doi: 10.3390/ma12040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nurdillayeva R.N., Oshido A.B., Bamford T.A., El-Zubir O., Houlton A., Hedley J., Pike A.R., Horrocks B.R. Inkjet printing and electrical characterisation of DNA-templated cadmium sulphide nanowires. Nanotechnology. 2018;29:135704. doi: 10.1088/1361-6528/aaa92f. [DOI] [PubMed] [Google Scholar]
- 13.Tse C.C.W., Smith P.J. Inkjet Printing for Biomedical Applications. Methods Mol. Biol. 2018;1771:107–117. doi: 10.1007/978-1-4939-7792-5_9. [DOI] [PubMed] [Google Scholar]
- 14.Capulli A.K., Emmert M.Y., Pasqualini F.S., Kehl D., Caliskan E., Lind J.U., Sheehya S.P., Park S.J., Ahn S., Weber B., et al. JetValve: Rapid manufacturing of biohybrid scaffolds for biomimetic heart valve replacement. Biomaterials. 2017;133:229–241. doi: 10.1016/j.biomaterials.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakobyan D., Kerouredan O., Remy M., Dusserre N., Medina C., Devillard R., Fricain J.-C., Oliveira H. Laser-Assisted Bioprinting for Bone Repair. Methods Mol. Biol. 2020;2140:135–144. doi: 10.1007/978-1-0716-0520-2_8. [DOI] [PubMed] [Google Scholar]
- 16.Kérourédan O., Hakobyan D., Rémy M., Ziane S., Dusserre N., Fricain J.-C., Delmond S., Thébaud N.B., Devillard R. In situ prevascularization designed by laser-assisted bioprinting: Effect on bone regeneration. Biofabrication. 2019;11:045002. doi: 10.1088/1758-5090/ab2620. [DOI] [PubMed] [Google Scholar]
- 17.Shi P., Tan Y.S.E., Yeong W.Y., Li H.Y., Laude A. A bilayer photoreceptor-retinal tissue model with gradient cell density design: A study of microvalve-based bioprinting. J. Tissue Eng. Regen. Med. 2018;12:1297–1306. doi: 10.1002/term.2661. [DOI] [PubMed] [Google Scholar]
- 18.Melchels F.P.W., Feijen J., Grijpma D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010;31:6121–6130. doi: 10.1016/j.biomaterials.2010.04.050. [DOI] [PubMed] [Google Scholar]
- 19.Wickett R.R. and Visscher, M.O. Structure and Function of the Epidermal Barrier. [(accessed on 1 September 2021)];Am. J. Infect. Control. 2006 34:S98–S110. doi: 10.1016/j.ajic.2006.05.295. doi: 10.1016/j.ajic.2006.05.295. Available online: [DOI] [Google Scholar]
- 20.Abdo J., Ortman H. Biologic and Synthetic Cellular and/or Tissue-Based Products and Smart Wound Dressings/Coverings. Surg. Clin. N. Am. 2020;100:741–756. doi: 10.1016/j.suc.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Coyer F., Gardner A., Doubrovsky A., Cole R., Ryan F.M., Allen C., McNamara G. Reducing pressure injuries in critically ill patients by using a patient skin integrity care bundle (InSPiRE) Am. J. Crit. Care. 2015;24:199–209. doi: 10.4037/ajcc2015930. [DOI] [PubMed] [Google Scholar]
- 22.Chocarro-Wrona C., López-Ruiz E., Perán M., Gálvez-Martín P., Marchal J.A. Therapeutic strategies for skin regeneration based on biomedical substitutes. J. Eur. Acad. Dermatol. Venereol. 2019;33:484–496. doi: 10.1111/jdv.15391. [DOI] [PubMed] [Google Scholar]
- 23.Peirce S.C., Carolan-Rees G. ReCell® Spray-On Skin System for Treating Skin Loss, Scarring and Depigmentation after Burn Injury: A NICE Medical Technology Guidance. Appl. Health Econ. Health Policy. 2019;17:131–141. doi: 10.1007/s40258-018-00457-0. [DOI] [PubMed] [Google Scholar]
- 24.Yan W.-C., Davoodi P., Vijayavenkataraman S., Tian Y., Ng W.C., Fuh J.Y.H., Robinson K.S., Wang C.H. 3D bioprinting of skin tissue: From pre-processing to final product evaluation. Adv. Drug Deliv. Rev. 2018;132:270–295. doi: 10.1016/j.addr.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Cubo N., Garcia M., Del Cañizo J.F., Velasco D., Jorcano J.L. 3D bioprinting of functional human skin: Production and in vivo analysis. Biofabrication. 2016;9:015006. doi: 10.1088/1758-5090/9/1/015006. [DOI] [PubMed] [Google Scholar]
- 26.Pourchet L.J., Thepot A., Albouy M., Courtial E.J., Boher A., Blum L.J., Marquette C.A. Human Skin 3D Bioprinting Using Scaffold-Free Approach. Adv. Healthc. Mater. 2017;6:1601101. doi: 10.1002/adhm.201601101. [DOI] [PubMed] [Google Scholar]
- 27.Kim J.H., Yoo J.J., Lee S.J. Three-dimensional cell-based bioprinting for soft tissue regeneration. Tissue Eng. Regen. Med. 2016;13:647–662. doi: 10.1007/s13770-016-0133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim B.S., Kwon Y.W., Kong J.-S., Park G.T., Gao G., Han W., Kim M.-B., Lee H., Kim J.H., Choa D.-W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials. 2018;168:38–53. doi: 10.1016/j.biomaterials.2018.03.040. [DOI] [PubMed] [Google Scholar]
- 29.Xiong R., Zhang Z., Chai W., Chrisey D.B., Huang Y. Study of gelatin as an effective energy absorbing layer for laser bioprinting. Biofabrication. 2017;9:024103. doi: 10.1088/1758-5090/aa74f2. [DOI] [PubMed] [Google Scholar]
- 30.Hossain M.R., Ansary T.M., Komine M., Ohtsuki M. Diversified Stimuli-Induced Inflammatory Pathways Cause Skin Pigmentation. Int. J. Mol. Sci. 2021;22:3970. doi: 10.3390/ijms22083970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jorgensen A.M., Varkey M., Gorkun A., Clouse C., Xu L., Chou Z., Murphy S.V., Molnar J., Lee S.J., Yoo J.J., et al. Bioprinted Skin Recapitulates Normal Collagen Remodeling in Full-Thickness Wounds. Tissue Eng. Part A. 2020;26:512–526. doi: 10.1089/ten.tea.2019.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Min D., Lee W., Bae I.-H., Lee T.R., Croce P., Yoo S.-S. Bioprinting of biomimetic skin containing melanocytes. Exp. Dermatol. 2018;27:453–459. doi: 10.1111/exd.13376. [DOI] [PubMed] [Google Scholar]
- 33.Shi Y., Xing T.L., Zhang H.B., Yin R.X., Yang S.M., Wei J., Zhang W.J. Tyrosinase-doped bioink for 3D bioprinting of living skin constructs. Biomed. Mater. 2018;13:035008. doi: 10.1088/1748-605X/aaa5b6. [DOI] [PubMed] [Google Scholar]
- 34.Ng H.Y., Lee K.-X.A., Kuo C.-N., Shen Y.-F. Bioprinting of artificial blood vessels. Int. J. Bioprinting. 2018;4:140. doi: 10.18063/ijb.v4i2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Bella C.D., Duchi S., O’Connell C.D., Blanchard R., Augustine C., Yue Z., Thompson F., Richards C., Beirne S., Onofrillo C., et al. In situ handheld three-dimensional bioprinting for cartilage regeneration. J. Tissue Eng. Regen. Med. 2018;12:611–621. doi: 10.1002/term.2476. [DOI] [PubMed] [Google Scholar]
- 36.Park S.-H., Jung C.S., Min B.-H. Advances in three-dimensional bioprinting for hard tissue engineering. Tissue Eng. Regen. Med. 2016;13:622–635. doi: 10.1007/s13770-016-0145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Research C for DE and Current Good Manufacturing Practice (CGMP) Regulations. [(accessed on 1 September 2021)];2020 Available online: https://www.fda.gov/drugs/pharmaceutical-quality-resources/current-good-manufacturing-practice-cgmp-regulations.
- 38.Duan B. State-of-the-Art Review of 3D Bioprinting for Cardiovascular Tissue Engineering. Ann. Biomed. Eng. 2016;45:195–209. doi: 10.1007/s10439-016-1607-5. [DOI] [PubMed] [Google Scholar]
- 39.Liu S., Li Y., Zeng X., Wang H., Yin P., Wang L., Liu Y., Liu J., Qi J., Ran S., et al. Burden of Cardiovascular Diseases in China, 1990–2016: Findings From the 2016 Global Burden of Disease Study. JAMA Cardiol. 2019;4:342–352. doi: 10.1001/jamacardio.2019.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.UN General Assembly General Assembly United Nations. Political Declaration of the High-Level Meeting of the General Assembly on the Prevention and Control of Non-Communicable Diseases. 2021. [(accessed on 1 September 2021)]. Available online: https://documents-dds-ny.un.org/doc/UNDOC/LTD/N11/497/77/PDF/N1149777.pdf?OpenElement.
- 41.Zhang Y.S., Arneri A., Bersini S., Shin S.-R., Zhu K., Goli-Malekabadi Z., Aleman J., Colosi C., Busignani F., Dell’Erba V., et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. 2016;110:45–59. doi: 10.1016/j.biomaterials.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madonna R., Van Laake L.W., Botker H.E., Davidson S.M., De Caterina R., Engel F.B., Eschenhagen T., Fernandez-Aviles F., Hausenloy D.J., Hulot J.S., et al. ESC Working Group on Cellular Biology of the Heart: Position paper for Cardiovascular Research: Tissue engineering strategies combined with cell therapies for cardiac repair in ischaemic heart disease and heart failure. Cardiovasc. Res. 2019;115:488–500. doi: 10.1093/cvr/cvz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao G., Cui X. Three-dimensional bioprinting in tissue engineering and regenerative medicine. Biotechnol. Lett. 2016;38:203–211. doi: 10.1007/s10529-015-1975-1. [DOI] [PubMed] [Google Scholar]
- 44.Richardson W.J., Clarke S.A., Quinn T.A., Holmes J.W. Physiological Implications of Myocardial Scar Structure. Compr. Physiol. 2015;5:1877–1909. doi: 10.1002/cphy.c140067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seymour D.G., Green M., Vaz F.G., Coles E.C. Risk prediction in medicine and surgery: Ethical and practical considerations. J. R. Coll. Physicians Lond. 1990;24:173–177. [PMC free article] [PubMed] [Google Scholar]
- 46.Bishop E.S., Mostafa S., Pakvasa M., Luu H.H., Lee M.J., Wolf J.M., Ameer G.A., He T.-C., Reid R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017;4:185–195. doi: 10.1016/j.gendis.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee A., Hudson A.R., Shiwarski D.J., Tashman J.W., Hinton T.J., Yerneni S., Bliley J.M., Campbell P.G., Feinberg A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science. 2019;365:482–487. doi: 10.1126/science.aav9051. [DOI] [PubMed] [Google Scholar]
- 48.Noor N., Shapira A., Edri R., Gal I., Wertheim L., Dvir T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019;6:1900344. doi: 10.1002/advs.201900344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kupfer M.E., Lin W.-H., Ravikumar V., Qiu K., Wang L., Gao L., Bhuiyan D.B., Lenz M., Ai J., Mahutga R.R., et al. In Situ Expansion, Differentiation, and Electromechanical Coupling of Human Cardiac Muscle in a 3D Bioprinted, Chambered Organoid. Circ. Res. 2020;127:207–224. doi: 10.1161/CIRCRESAHA.119.316155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Z., Lee S.J., Cheng H.-J., Yoo J.J., Atala A. 3D bioprinted functional and contractile cardiac tissue constructs. Acta Biomater. 2018;70:48–56. doi: 10.1016/j.actbio.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Das S., Kim S.-W., Choi Y.-J., Lee S., Lee S.-H., Kong J.-S., Park H.J., Cho D.W., Jang J. Decellularized extracellular matrix bioinks and the external stimuli to enhance cardiac tissue development in vitro. Acta Biomater. 2019;95:188–200. doi: 10.1016/j.actbio.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 52.Jang J., Park H.-J., Kim S.-W., Kim H., Park J.Y., Na S.J., Kim H.J., Park M.N., Choi S.H., Park S.H., et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials. 2017;112:264–274. doi: 10.1016/j.biomaterials.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 53.Pati F., Cho D.-W. Bioprinting of 3D Tissue Models Using Decellularized Extracellular Matrix Bioink. Methods Mol. Biol. 2017;1612:381–390. doi: 10.1007/978-1-4939-7021-6_27. [DOI] [PubMed] [Google Scholar]
- 54.Zhu K., Shin S.R., van Kempen T., Li Y.-C., Ponraj V., Nasajpour A., Mandla S., Hu N., Liu X., Leijten J., et al. Gold Nanocomposite Bioink for Printing 3D Cardiac Constructs. Adv. Funct. Mater. 2017;27:1605352. doi: 10.1002/adfm.201605352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moldovan N.I., Hibino N., Nakayama K. Principles of the Kenzan Method for Robotic Cell Spheroid-Based Three-Dimensional Bioprinting. Tissue Eng. Part B Rev. 2017;23:237–244. doi: 10.1089/ten.teb.2016.0322. [DOI] [PubMed] [Google Scholar]
- 56.Ong C.S., Fukunishi T., Zhang H., Huang C.Y., Nashed A., Blazeski A., DiSilvestre D., Vricella L., Conte J., Tung L., et al. Biomaterial-Free Three-Dimensional Bioprinting of Cardiac Tissue using Human Induced Pluripotent Stem Cell Derived Cardiomyocytes. Sci. Rep. 2017;7:4566. doi: 10.1038/s41598-017-05018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeung E., Fukunishi T., Bai Y., Bedja D., Pitaktong I., Mattson G., Jeyaram A., Lui C., Ong C.S., Inoue T., et al. Cardiac regeneration using human-induced pluripotent stem cell-derived biomaterial-free 3D-bioprinted cardiac patch in vivo. J. Tissue Eng. Regen. Med. 2019;13:2031–2039. doi: 10.1002/term.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu N., Ye X., Yao B., Zhao M., Wu P., Liu G., Zhuang D., Jiang H., Chen X., He Y., et al. Advances in 3D bioprinting technology for cardiac tissue engineering and regeneration. Bioact. Mater. 2021;6:1388–1401. doi: 10.1016/j.bioactmat.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bajaj P., Schweller R.M., Khademhosseini A., West J.L., Bashir R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 2014;16:247–276. doi: 10.1146/annurev-bioeng-071813-105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y., Nuglozeh E., Touré F., Schmidt A.M., Vunjak-Novakovic G. Controllable expansion of primary cardiomyocytes by reversible immortalization. Hum. Gene Ther. 2009;20:1687–1696. doi: 10.1089/hum.2009.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bejleri D., Streeter B.W., Nachlas A.L.Y., Brown M.E., Gaetani R., Christman K.L., Davis M.E. A Bioprinted Cardiac Patch Composed of Cardiac-Specific Extracellular Matrix and Progenitor Cells for Heart Repair. Adv. Healthc. Mater. 2018;7:e1800672. doi: 10.1002/adhm.201800672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park S.-J., Kim R.Y., Park B.-W., Lee S., Choi S.W., Park J.-H., Choi J.J., Kim S.-W., Jang J., Cho D.-W., et al. Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nat. Commun. 2019;10:3123. doi: 10.1038/s41467-019-11091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Birla R.K., Williams S.K. 3D bioprinting and its potential impact on cardiac failure treatment: An industry perspective. APL Bioeng. 2020;4:010903. doi: 10.1063/1.5128371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doss M.X., Sachinidis A. Current Challenges of iPSC-Based Disease Modeling and Therapeutic Implications. Cells. 2019;8:403. doi: 10.3390/cells8050403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roche C.D., Brereton R.J.L., Ashton A.W., Jackson C., Gentile C. Current challenges in three-dimensional bioprinting heart tissues for cardiac surgery. Eur. J. Cardio-Thorac. Surg. 2020;58:500–510. doi: 10.1093/ejcts/ezaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ruiz-Alonso S., Villate-Beitia I., Gallego I., Lafuente-Merchan M., Puras G., Saenz-Del-Burgo L., Pedraz J. Current Insights Into 3D Bioprinting: An Advanced Approach for Eye Tissue Regeneration. Pharmaceutics. 2021;13:308. doi: 10.3390/pharmaceutics13030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jakab K., Norotte C., Marga F., Murphy K., Vunjak-Novakovic G., Forgacs G. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication. 2010;2:022001. doi: 10.1088/1758-5082/2/2/022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xin Y., Chai G., Zhang T., Wang X., Qu M., Tan A., Bogari M., Zhu M., Lin L., Hu Q., et al. Analysis of multiple types of human cells subsequent to bioprinting with electrospraying technology. Biomed. Rep. 2016;5:723–730. doi: 10.3892/br.2016.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pina S., Ribeiro V.P., Marques C.F., Maia F.R., Silva T.H., Reis R.L., Oliveira J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials. 2019;12:1824. doi: 10.3390/ma12111824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tian B., Liu J., Dvir T., Jin L., Tsui J.H., Qing Q., Suo Z., Langer R., Kohane D.S., Lieber C.M. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat. Mater. 2012;11:986–994. doi: 10.1038/nmat3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shin S.R., Jung S.M., Zalabany M., Kim K., Zorlutuna P., Kim S.B., Nikkhah M., Khabiry M., Azize M., Kong J. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano. 2013;7:2369–2380. doi: 10.1021/nn305559j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mason J., Visintini S., Quay T. CADTH Issues in Emerging Health Technologies. Canadian Agency for Drugs and Technologies in Health; Ottawa, ON, Canada: 2016. [(accessed on 1 September 2021)]. An Overview of Clinical Applications of 3-D Printing and Bioprinting. Available online: http://www.ncbi.nlm.nih.gov/books/NBK542711/ [PubMed] [Google Scholar]
- 73.Nyga A., Cheema U., Loizidou M. 3D tumour models: Novel in vitro approaches to cancer studies. J. Cell Commun. Signal. 2011;5:239–248. doi: 10.1007/s12079-011-0132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ware M.J., Keshishian V., Law J.J., Ho J.C., Favela C.A., Rees P., Smith B., Mohammad S., Hwang R.F., Rajapakshe K., et al. Generation of an in vitro 3D PDAC stroma rich spheroid model. Biomaterials. 2016;108:129–142. doi: 10.1016/j.biomaterials.2016.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim J., Kang K., Drogemuller C.J., Wallace G.G., Coates P.T. Bioprinting an Artificial Pancreas for Type 1 Diabetes. Curr. Diab. Rep. 2019;19:53. doi: 10.1007/s11892-019-1166-x. [DOI] [PubMed] [Google Scholar]
- 76.Gan M.J., Albanese-O’Neill A., Haller M.J. Type 1 diabetes: Current concepts in epidemiology, pathophysiology, clinical care, and research. Curr. Probl. Pediatr. Adolesc. Health Care. 2012;42:269–291. doi: 10.1016/j.cppeds.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 77.Cibulskis R.E., Alonso P., Aponte J., Aregawi M., Barrette A., Bergeron L., Fergus C.A., Knox T., Lynch M., Patouillard E., et al. Malaria: Global progress 2000–2015 and future challenges. Infect. Dis. Poverty. 2016;5:61. doi: 10.1186/s40249-016-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ravnic D.J., Leberfinger A.N., Ozbolat I.T. Bioprinting and Cellular Therapies for Type 1 Diabetes. Trends Biotechnol. 2017;35:1025–1034. doi: 10.1016/j.tibtech.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 79.Holstein A., Plaschke A., Egberts E.-H. Clinical characterisation of severe hypoglycaemia––A prospective population—Based study. Exp. Clin. Endocrinol. Diabetes. 2003;111:364–369. doi: 10.1055/s-2003-42728. [DOI] [PubMed] [Google Scholar]
- 80.Fong E.L.S., Harrington D.A., Farach–Carson M.C., Yu H. Heralding a new paradigm in 3D tumor modeling. Biomaterials. 2016;108:197–213. doi: 10.1016/j.biomaterials.2016.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Imamura Y., Mukohara T., Shimono Y., Funakoshi Y., Chayahara N., Toyoda M., Kiyota N., Takao S., Kono S., Nakatsura T., et al. Comparison of 2D- and 3D-culture models as drug—Testing platforms in breast cancer. Oncol. Rep. 2015;33:1837–1843. doi: 10.3892/or.2015.3767. [DOI] [PubMed] [Google Scholar]
- 82.Jiang L., Shestov A.A., Swain P., Yang C., Parker S.J., Wang Q.A., Terada L.S., Adams N.D., McCabe M.T., Pietrak B., et al. Reductive carboxylation supports redox homeostasis during anchorage–independent growth. Nature. 2016;532:255–258. doi: 10.1038/nature17393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Langer E.M., Allen–Petersen B.L., King S.M., Kendsersky N.D., Turnidge M.A., Kuziel G.M., Riggers R., Samatham R., Amery T.S., Jacques S.L., et al. Modeling Tumor Phenotypes In Vitro with Three–Dimensional Bioprinting. Cell Rep. 2019;26:608–623.e6. doi: 10.1016/j.celrep.2018.12.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Noel P., Muñoz R., Rogers G.W., Neilson A., Von Hoff D.D., Han H. Preparation and Metabolic Assay of 3-dimensional Spheroid Co-cultures of Pancreatic Cancer Cells and Fibroblasts. J. Vis. Exp. JoVE. 2017;126:56081. doi: 10.3791/56081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hou S., Tiriac H., Sridharan B.P., Scampavia L., Madoux F., Seldin J., Souza G.R., Watson D., Tuveson D., Spicer T.P., et al. Advanced Development of Primary Pancreatic Organoid Tumor Models for High-Throughput Phenotypic Drug Screening. SLAS Discov. Adv. Life. Sci. R D. 2018;23:574–584. doi: 10.1177/2472555218766842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hakobyan D., Médina C., Dusserre N., Stachowicz M.-L., Handschin C., Fricain J.-C., Guillermet-Guibert J., Oliveira H. Laser-assisted 3D bioprinting of exocrine pancreas spheroid models for cancer initiation study. Biofabrication. 2020;12:035001. doi: 10.1088/1758-5090/ab7cb8. [DOI] [PubMed] [Google Scholar]
- 87.Farina M., Ballerini A., Fraga D.W., Nicolov E., Hogan M., Demarchi D., Scaglione F., Sabek O.M., Horner P., Thekkedath U., et al. 3D Printed Vascularized Device for Subcutaneous Transplantation of Human Islets. Biotechnol. J. 2017;12:1700169. doi: 10.1002/biot.201700169. [DOI] [PubMed] [Google Scholar]
- 88.Baidal D.A., Ricordi C., Berman D.M., Alvarez A., Padilla N., Ciancio G., Linetsky E., Pileggi A., Alejandro R. Bioengineering of an Intraabdominal Endocrine Pancreas. N. Engl. J. Med. 2017;376:1887–1889. doi: 10.1056/NEJMc1613959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xiongfa J., Hao Z., Liming Z., Jun X. Recent advances in 3D bioprinting for the regeneration of functional cartilage. Regen. Med. 2018;13:73–87. doi: 10.2217/rme-2017-0106. [DOI] [PubMed] [Google Scholar]
- 90.Murray I.R., Benke M.T., Mandelbaum B.R. Management of knee articular cartilage injuries in athletes: Chondroprotection, chondrofacilitation, and resurfacing. Knee Surg. Sports Traumatol. Arthrosc. 2016;24:1617–1626. doi: 10.1007/s00167-015-3509-8. [DOI] [PubMed] [Google Scholar]
- 91.Wu Y., Kennedy P., Bonazza N., Yu Y., Dhawan A., Ozbolat I. Three-Dimensional Bioprinting of Articular Cartilage: A Systematic Review. Cartilage. 2021;12:76–92. doi: 10.1177/1947603518809410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dhawan A., Kennedy P.M., Rizk E.B., Ozbolat I.T. Three-dimensional Bioprinting for Bone and Cartilage Restoration in Orthopaedic Surgery. J. Am. Acad. Orthop. Surg. 2019;27:e215–e226. doi: 10.5435/JAAOS-D-17-00632. [DOI] [PubMed] [Google Scholar]
- 93.Kamran A., Jennings R.W. Tracheomalacia and Tracheobronchomalacia in Pediatrics: An Overview of Evaluation, Medical Management, and Surgical Treatment. Front. Pediatr. 2019;7:512. doi: 10.3389/fped.2019.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Galliger Z., Vogt C.D., Panoskaltsis-Mortari A. 3D bioprinting for lungs and hollow organs. Transl. Res. 2019;211:19–34. doi: 10.1016/j.trsl.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jeong H.-J., Nam H., Jang J., Lee S.-J. 3D Bioprinting Strategies for the Regeneration of Functional Tubular Tissues and Organs. Bioengineering. 2020;7:32. doi: 10.3390/bioengineering7020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frejo L., Grande D.A. 3D-bioprinted tracheal reconstruction: An overview. Bioelectron. Med. 2019;5:15. doi: 10.1186/s42234-019-0031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kaye R., Goldstein T., Grande D.A., Zeltsman D., Smith L.P. A 3-dimensional bioprinted tracheal segment implant pilot study: Rabbit tracheal resection with graft implantation. Int. J. Pediatr. Otorhinolaryngol. 2019;117:175–178. doi: 10.1016/j.ijporl.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 98.Ng W.L., Chua C.K., Shen Y.-F. Print Me An Organ! Why We Are Not There Yet. [(accessed on 1 September 2021)];Prog. Polym. Sci. 2019 97:101145. doi: 10.1016/j.progpolymsci.2019.101145. Available online: https://www.sciencedirect.com/science/article/pii/S007967001930156X. [DOI] [Google Scholar]
- 99.Favreau H., Pijnenburg L., Seitlinger J., Fioretti F., Keller L., Scipioni D., Adriaensen H., Kuchler-Bopp S., Ehlinger M., Mainard D., et al. Osteochondral repair combining therapeutics implant with mesenchymal stem cells spheroids. Nanomed. Nanotechnol. Biol. Med. 2020;29:102253. doi: 10.1016/j.nano.2020.102253. [DOI] [PubMed] [Google Scholar]
- 100.Francis S.L., Di Bella C., Wallace G.G., Choong P.F.M. Cartilage Tissue Engineering Using Stem Cells and Bioprinting Technology-Barriers to Clinical Translation. Front. Surg. 2018;5:70. doi: 10.3389/fsurg.2018.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fellows C.R., Matta C., Zakany R., Khan I.M., Mobasheri A. Adipose, Bone Marrow and Synovial Joint-Derived Mesenchymal Stem Cells for Cartilage Repair. Front. Genet. 2016;7:213. doi: 10.3389/fgene.2016.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen F.H., Tuan R.S. Mesenchymal stem cells in arthritic diseases. Arthritis Res. Ther. 2008;10:223. doi: 10.1186/ar2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Singh S., Choudhury D., Yu F., Mironov V., Naing M.W. In situ bioprinting—Bioprinting from benchside to bedside? Acta Biomater. 2020;101:14–25. doi: 10.1016/j.actbio.2019.08.045. [DOI] [PubMed] [Google Scholar]
- 104.O’Connell C.D., Di Bella C., Thompson F., Augustine C., Beirne S., Cornock R., Richards C.J., Chung J., Gambhir S., Yue Z., et al. Development of the Biopen: A handheld device for surgical printing of adipose stem cells at a chondral wound site. Biofabrication. 2016;8:015019. doi: 10.1088/1758-5090/8/1/015019. [DOI] [PubMed] [Google Scholar]
- 105.Ma K., Zhao T., Yang L., Wang P., Jin J., Teng H., Xia D., Zhu L., Li L., Jiang Q., et al. Application of robotic-assisted in situ 3D printing in cartilage regeneration with HAMA hydrogel: An in vivo study. J. Adv. Res. 2020;23:123–132. doi: 10.1016/j.jare.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.You F., Chen X., Cooper D.M.L., Chang T., Eames B.F. Homogeneous hydroxyapatite/alginate composite hydrogel promotes calcified cartilage matrix deposition with potential for three-dimensional bioprinting. Biofabrication. 2018;11:015015. doi: 10.1088/1758-5090/aaf44a. [DOI] [PubMed] [Google Scholar]
- 107.Kosik-Kozioł A., Costantini M., Mróz A., Idaszek J., Heljak M., Jaroszewicz J., Kijeńska-Gawrońska E., Szöke K., Frerker N., Barbetta A., et al. 3D bioprinted hydrogel model incorporating β-tricalcium phosphate for calcified cartilage tissue engineering. Biofabrication. 2019;11:035016. doi: 10.1088/1758-5090/ab15cb. [DOI] [PubMed] [Google Scholar]
- 108.Park J.-H., Yoon J.-K., Lee J.B., Shin Y.M., Lee K.-W., Bae S.-W., Lee J., Yu J., Jung C.-R., Youn Y.-N., et al. Experimental Tracheal Replacement Using 3-dimensional Bioprinted Artificial Trachea with Autologous Epithelial Cells and Chondrocytes. Sci. Rep. 2019;9:2103. doi: 10.1038/s41598-019-38565-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ke D., Yi H., Est-Witte S., George S., Kengla C., Lee S.J., Atala A., Murphy S.V. Bioprinted trachea constructs with patient-matched design, mechanical and biological properties. Biofabrication. 2019;12:015022. doi: 10.1088/1758-5090/ab5354. [DOI] [PubMed] [Google Scholar]
- 110.Jafarkhani M., Salehi Z., Aidun A., Shokrgozar M.A. Bioprinting in Vascularization Strategies. Iran. Biomed. J. 2019;23:9–20. doi: 10.29252/ibj.23.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao R., Yang R., Cooper P.R., Khurshid Z., Shavandi A., Ratnayake J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. [(accessed on 1 September 2021)];Molecules. 2021 26:3007. doi: 10.3390/molecules26103007. Available online: https://www.mdpi.com/1420-3049/26/10/3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bisht B., Hope A., Mukherjee A., Paul M.K. Advances in the Fabrication of Scaffold and 3D Printing of Biomimetic Bone Graft. Ann. Biomed. Eng. 2021;49:1128–1150. doi: 10.1007/s10439-021-02752-9. [DOI] [PubMed] [Google Scholar]
- 113.Roseti L., Cavallo C., Desando G., Parisi V., Petretta M., Bartolotti I., Grigolo B. Three-Dimensional Bioprinting of Cartilage by the Use of Stem Cells: A Strategy to Improve Regeneration. [(accessed on 1 September 2021)];Materials. 2018 11:1749. doi: 10.3390/ma11091749. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6164915/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Datta P., Dey M., Ataie Z., Unutmaz D., Ozbolat I.T. 3D bioprinting for reconstituting the cancer microenvironment. NPJ Precis Oncol. 2020;4:18. doi: 10.1038/s41698-020-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]