Abstract
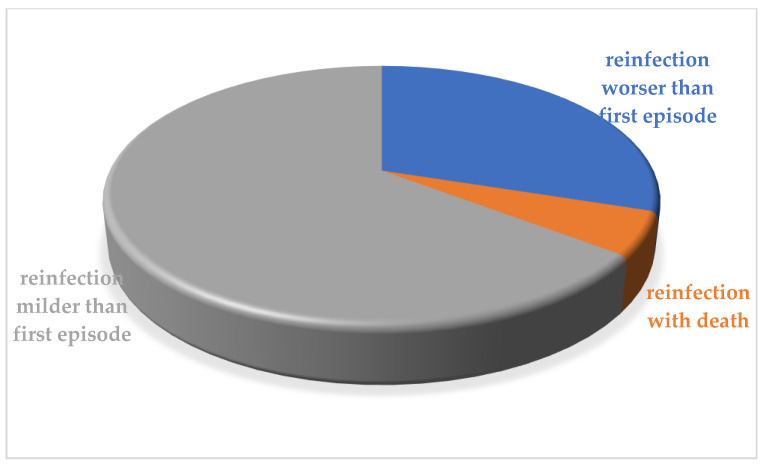
Reinfection with SARS-CoV-2 seems to be a rare phenomenon. The objective of this study is to carry out a systematic search of literature on the SARS-CoV-2 reinfection in order to understand the success of the global vaccine campaigns. A systematic search was performed. Inclusion criteria included a positive RT-PCR test of more than 90 days after the initial test and the confirmed recovery or a positive RT-PCR test of more than 45 days after the initial test that is accompanied by compatible symptoms or epidemiological exposure, naturally after the confirmed recovery. Only 117 articles were included in the final review with 260 confirmed cases. The severity of the reinfection episode was more severe in 92/260 (35.3%) with death only in 14 cases. The observation that many reinfection cases were less severe than initial cases is interesting because it may suggest partial protection from disease. Another interesting line of data is the detection of different clades or lineages by genome sequencing between initial infection and reinfection in 52/260 cases (20%). The findings are useful and contribute towards the role of vaccination in response to the COVID-19 infections. Due to the reinfection cases with SARS-CoV-2, it is evident that the level of immunity is not 100% for all individuals. These data highlight how it is necessary to continue to observe all the prescriptions recently indicated in the literature in order to avoid new contagion for all people after healing from COVID-19 or becoming asymptomatic positive.
Keywords: coronavirus, reinfection, COVID-19, SARS-CoV-2, systematic review
1. Introduction
The novel coronavirus (SARS-CoV-2) outbreak since December 2019 has continued to exhibit devastating consequences, and was declared as a pandemic by the World Health Organization in early 2020 [1,2,3]. To date, as of 17 October 2021, 240,421,359 infections have been confirmed, with 4,895,034 deaths [4]. In many countries, the vaccination campaign has started with the use of various vaccines recently put on the market and the total number of vaccine doses administered is 6,609,632,994. However, a new problem is emerging with regard to the evolution of the behavior of SARS-CoV-2: the possibility of reinfection of healed subjects after the first infection. On 25 August 2020, the first case of reinfection of SARS-CoV-2 was reported in international literature [5]. This event pointed out that infection by this virus does not uniformly confer protective immunity to all infected individuals [6]. Therefore, several critical questions are intriguing the researchers. Is SARS-CoV-2 reinfection a widespread phenomenon or is it limited to few subjects with immune deficits or specific comorbidities [6]? Can this phenomenon be due to a too weak, too short, or too narrow natural immune response to SARS-CoV-2, that is unable to protect from subsequent exposure [6]? What is the clinical behavior, in regard to the evolution of the reinfections? Can these reinfected patients transmit the viruses? This important problem needs to be addressed, because the possibility of reinfection could drastically reduce the effectiveness of the vaccination campaigns in progress. Protective, sustainable and long-lasting immunity following COVID-19 infection is uncertain, but it is essential for the efficacity of vaccine strategy.
For some viruses, the first infection can provide lifelong immunity, for seasonal coronaviruses protective immunity is short-lived [7]. Over the years, other viruses responsible for various infectious respiratory diseases have been able to present reinfection in the originally cured subjects, such as the coronavirus HCoV-NL63 (NL63) [8] and the human respiratory syncytial virus (hRSV) [9].
The SARS-CoV-2 pandemic poses a challenge regarding the follow-up of recovered patients and the question of the reinfection risk. Several reports confirmed that most patients with SARS-CoV-2 produce antibodies against spike and N-proteins of the virus within 30 days after the infection [10,11]. In fact, an outbreak of the virus on a fishery vessel showed that fishers with prior neutralizing antibodies against SARS-CoV-2 were not reinfected [12]. The potential mechanisms that mediate immunity post-COVID-19 are not yet fully understood. COVID-19 typically follows a course similar to other respiratory viral illnesses, and it is self-limiting in more than 80% of cases [13]. An innate immune response involving T cells and B cells is activated, leading to the production of neutralizing antiviral antibodies [13]. The specific IgM antibody response starts to peak within the first 7 days [13]. Specific IgG and IgA antibodies develop a few days after IgM and are hypothesized to persist at low levels, conferring lifelong protective antibodies [14]. While this hypothesis may hold true for symptomatic patients, emerging data have revealed negative IgM and IgG during the early convalescent phase in asymptomatic patients [15] and 40% of asymptomatic patients became seronegative for IgG 8 weeks after discharging compared with 12.9% who were seronegative for the symptomatic group [15]. A seronegative status could leave open the possibility of reinfection. Immunosuppression and comorbid diseases can be other risk factors for a reinfection [16].
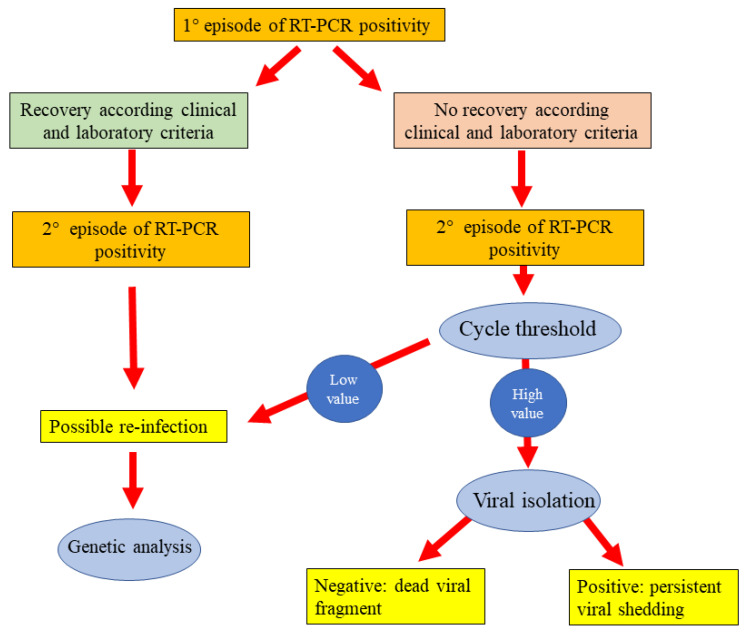
However, a distinction must be made between prolonged shedding/reactivation and true reinfection [17], in fact one of the features of SARS-CoV-2 infection is prolonged virus shedding. Several studies reported persistent or recurrent elimination of viral RNA in nasopharyngeal samples starting from first contact with a positive subject [18,19,20]. For this reason, recently the Center for Disease Control and Prevention (CDC) released a guidance protocol designed to identify cases of real SARS-CoV-2 reinfection [21]. This guidance defines some criteria about sequencing parameters, epidemiological data and laboratory diagnostic data (Table 1). Specifically, investigative criteria include a positive RT-PCR test more than 90 days after the initial test in healed patients or a positive RT-PCR test more than 45 days after the initial test that is accompanied by compatible symptoms or epidemiological exposure, after confirmed healing.
Table 1.
Protocol of Center for Disease Control and Prevention for investigating suspected SARS-CoV-2 reinfection.
| Investigative Criteria | Laboratory Evidence |
|---|---|
|
Best evidence Differing clades as defined in Nextstrain and GISAID of SARS-CoV-2 between the first and second infection, ideally coupled with other evidence of actual infection (e.g., high viral titers in each sample or positive for subgenomic mRNA, and culture) |
with a symptomatic second episode and no obvious alternate etiology for COVID-19-like symptoms or close contact with a person known to have laboratory-confirmed COVID-19 |
Moderate evidence >2 nucleotide differences per month * in consensus between sequences that meet quality metrics above, ideally coupled with other evidence of actual infection (e.g., high viral titers in each sample or positive for subgenomic mRNA, and culture) |
|
Poor evidence but possible ≤2 nucleotide differences per month * in consensus between sequences that meet quality metrics above or >2 nucleotide differences per month * in consensus between sequences that do not meet quality metrics above, ideally coupled with other evidence of actual infection (e.g., high viral titers in each sample or positive for subgenomic mRNA, and culture) |
* The mutation rate of SARS-CoV-2 is estimated at 2 nucleotide differences per month, therefore if suspected reinfection occurs 90 days after initial infection, moderate evidence would require >6 nucleotide differences.
Another emerging problem that can influence the possibility of reinfection and the vaccination efficacity is the new variants of SARS-CoV-2, such us alpha, beta, gamma and delta. A recent study on 9119 patients with SAS-CoV-2 infection identified reinfection in 63 cases (0.7%, 95% confidence interval 0.5–0.9%) [22]. The mean period between two positive tests was 116 ± 21 days [22]. There were no significant differences based on age or sex, while nicotine dependence/tobacco use, asthma were higher in patients with reinfection [22]. There was a significantly lower rate of pneumonia, heart failure, and acute kidney injury during reinfection compared with primary infection [22]. There were two deaths (3.2%) associated with reinfection [22].
Another study conducted in Switzerland reported five cases of reinfection (1%) in 498 seropositive individuals followed for 35 weeks [23]. Breathnach et al. examined data of 10,727 patients with COVID-19 in the first wave and individuated eight reinfection cases (0.07%), all in female patients, and only one was admitted in hospital [24]. Bongiovanni et al. examined 677 subjects with at least a positive nasopharyngeal swab, 328 during the first wave and 349 during the second individuating 13 (1.9%) cases of reinfection [25]. Vitale et al. examined a cohort of 1579 patients and reported five reinfections (0.31%, 95% CI, 0.03–0.58%), of whom only one was hospitalized and the mean (SD) interval between primary infection and reinfection was longer than 230 (90) days [26].
The understanding of COVID-19 reinfection will be key in guiding government and public health policy decisions in the coming months.
A systematic review of literature was performed in order to individuate cases of reinfection for SARS-CoV-2. To date there are more than 300 reported cases of COVID-19 reinfection from different countries such as United States [27], Ecuador [28], Hong Kong [5], and Belgium [29]. It is necessary to understand if all these cases are really reinfection.
2. Materials and Methods
This systematic review of literature on reinfections of SARS-CoV-2 was conducted in August 2021. Our study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist to ensure the reliability and validity of this study and results.
2.1. Data Sources
By application of a systematic search and using the keywords in the online databases including PubMed, Scopus, Web of Science, Science Direct, EMBASE, and preprint servers (MedRxiv, BioRxiv, and SSRN) on 31 July 2021, we extracted all the papers published in English from December 2019 to July 2021. We included several combinations of keywords in the following orders to conduct the search strategy: (1) “CoVID-19” or “SARS-CoV-2” or “2019-nCoV” [all field]; (2) “Reinfection” or “Re-infection” [all field].
2.2. Study Selection
Three independent investigators retrieved the studies that were the most relevant by titles and abstracts (ELM, LLM, MA). Subsequently, the full text of the retrieved papers was reviewed, and the most relevant papers were chosen according to the eligibility criteria. Then, we extracted the relevant data and organized them in tables. The original papers that were peer-reviewed and published in English and fulfilled the eligibility criteria were included in the final report, together with two works not reviewed at the time of preparation of this report [30,31].
The following inclusion criteria was used: a positive RT-PCR test carried out more than 90 days after the initial test in healed patients or a positive RT-PCR test carried out more than 45 days after the initial test that is accompanied by compatible symptoms or epidemiological exposure, after confirmed healing. This criteria corresponds to the CDC protocol designed to identify cases of real SARS-CoV-2 reinfection (Table 1) [32].
We considered the exclusion criteria for this study as follows: (1) papers conveying non-human studies including in vitro observations or articles focusing on animal experiments; (2) papers in which their full text were out of access; (3) any suspicious and duplicated results in the databases.
2.3. Data Extraction
After summarizing, we transferred the information of the authors, type of article (e.g., case reports), publication date, country of origin, age, gender, and clinical symptoms to a data extraction sheet. Three independent investigators collected this information and subsequently organized them in the tables. Finally, to ensure no duplications or overlap existed in the content, all the selected articles were cross-checked by other authors.
2.4. Quality and Risk of Bias Assessment
As aforementioned, we applied the PRISMA checklist to ensure the quality and reliability of selected articles. Two independent researchers evaluated the consistency and quality of the articles and the risk of bias. In either case of discrepancy in viewpoints, a third independent researcher resolved the issue. The full text of selected articles was read, and the key findings were extracted.
Included studies underwent quality check and risk of bias assessment. This qualitative analysis was performed according Murad’s quality checklist of case series and case report [33]. As reported, the scale consists of four parameters, to evaluate the (a) patient selection; (b) exposure ascertainment; (c) causality; (d) reporting. Each section contains one to four question to be addressed. As it is suggested we performed an overall judgement about methodological quality since questions 4, 5 and 6 are mostly relevant to cases of adverse drug events. Each requested field will be considered as adequate, inadequate or not evaluable. The table showing this tool for evaluating the methodological quality of case reports and case series, is reported in the original manuscript [33].
3. Results
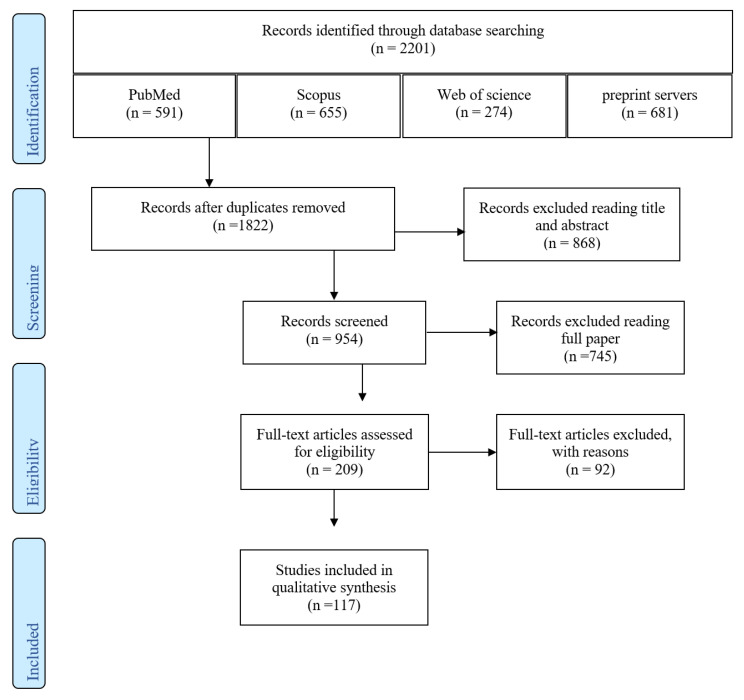
In this study, 117 documents were identified using the systematic search strategy. After a primary review of 2201 retrieved articles, 379 duplicates were removed, and the title and abstract of the remaining 1822 resources were reviewed. After applying the selection criteria, only 117 articles met the inclusion criteria and were included in the final review (Figure 1). Therefore, the cases confirmed according to these parameters were 260 (Table 2).
Figure 1.
Flow diagram for the selection process of identified articles.
Table 2.
Cases of SARS-CoV2 reinfection in the international literature (all cases were again positive for SARS-CoV-2 after complete symptomatic recovery in addition to negative RT-PCR test for SARS-CoV-2, according to WHO recommendations [34]).
| Authors | Year | Patient Country | Patient | Interval Time between 1 Infection and Reinfection | Viral Genome Sequence | COVID-19 | Symptoms | Antibody after First Infection or Reinfection |
|---|---|---|---|---|---|---|---|---|
|
2021 | Qatar | 25–29-year-old man | 46 | 9 SNVs compared to initial infection strain, including D614G | Mild | N/A | N/A |
| Mild | N/A | |||||||
|
2021 | Qatar | 40–44-year-old man | 71 | 11 SNVs compared to initial infection strain, including D614G | Mild | N/A | N/A |
| Mild | N/A | |||||||
|
2021 | Qatar | 45–49-year-old woman | 88 | 3 SNVs compared to initial infection strain, including D614G | Mild | N/A | ROCHE elecsys antiSARS-CoV-2 negative at time of reinfection |
| Mild | N/A | |||||||
|
2021 | Qatar | 25–29-year-old woman | 55 | 1 SNVs compared to initial infection strain, including D614G | Mild | N/A | N/A |
| Mild | N/A | |||||||
|
2021 | Brazil | 44-year-old healthcare man with systemic arterial hypertension, obesity | 53 | 20A | Mild | Dry cough, dyspnea, dysgeusia, diarrhea, asthenia, sneezing/runny nose | N/A |
| Clade B.1.1.28 | Worse | Dry cough, dyspnea, fever, myalgia, asthenia, arthralgia, headache, nausea/vomiting, sneezing/runny nose, severe respiratory symptoms and was admitted to ICU, dying after 20 days of symptoms | ||||||
|
2021 | Spain | 39-year-old healthcare man | 290 | N/A | Mild | Sore throat, fever, general malaise, nasal congestion, tachycardia, chest pain, loss of smell and taste | Rapid antibody test: positive |
| 201/501Y.V1.Britain variant B.1.17 | Milder | Sore throat, slight general malaise, nasal congestion, tiredness | Rapid antibody test: positive | |||||
|
2021 | Iran | 36-year-old healthcare man | 60 | N/A | Mild | Lethargy, fatigue, shortness of breath, headache, fever, chills | N/A |
| Milder | Eye infection, fever, fatigue, shortness of breath, muscle pain | |||||||
|
2021 | Pakistan | Healthcare worker man | 118 | N/A | Mild | Arthralgia, weakness, anosmia, ageusia | N/A |
| Milder | Fever, sore throat, dry cough | |||||||
|
2021 | Pakistan | Healthcare worker man | 86 | N/A | Mild | Fever, sore throat | N/A |
| Milder | Sinusitis | |||||||
|
2021 | Pakistan | 40-year-old male | 94 | N/A | Mild | Fever | N/A |
| Worse | Sore throat, cough, diarrhea | |||||||
|
2021 | Bahrain | 47-year-old woman without comorbidities | 60 | N/A | Mild | Mild respiratory tract symptoms | N/A |
| Worse | Abdominal pain, fulminant hepatic failure > death | |||||||
|
2020 | Iran | 20s year age range, male | 89 ** | N/A | Mild | Fever, myalgia | 6.7 IgG (s/ca) after recovery |
| Worse | Fever, myalgia, cough, loss of taste, loss of smell | |||||||
|
2020 | Iran | 30s year age range, female | 55 ** | N/A | Mild | Fever, myalgia | 10.3 IgG (s/ca) after recovery |
| Worse | Fever, loss of taste and smell, myalgia, cough | |||||||
|
2020 | Iran | 40s year age range, male | 55 ** | N/A | Mild | Fever, myalgia | 15.5 IgG (s/ca) after recovery |
| Mild | Fever, myalgia, cough | |||||||
|
2020 | Iran | 50s year age range, male | 46 ** | N/A | Mild | Fever, myalgia | 10.3 IgG (s/ca) after recovery |
| Worse | Fever, loss of taste and smell, myalgia, cough | |||||||
|
2020 | Iran | 50s year age range, female | 53 ** | N/A | Mild | Fever, loss of taste and smell | 5.35 IgG (s/ca) after recovery |
| Milder | Fever, myalgia, cough | |||||||
|
2020 | Iran | 40s year age range, male | 76 ** | N/A | Mild | Fever, myalgia | 7.22 IgG (s/ca) after recovery |
| Worse | Fever, loss of taste and smell, myalgia, cough | |||||||
|
2020 | Iran | 40s year age range, female | 45 ** | N/A | Mild | Fever, myalgia | 11.2 IgG (s/ca) after recovery |
| Worse | Fever, loss of taste and smell, myalgia, cough | |||||||
|
2020 | Iran | 40s year age range, male | 50 ** | N/A | Mild | Fever, loss of taste and smell, myalgia | 12.51 IgG (s/ca) after recovery |
| Mild | Fever, loss of taste and smell, myalgia, cough | |||||||
|
2020 | Iran | 40s year age range, male | 62 ** | N/A | Mild | Fever, cough | 7.11 IgG (s/ca) after recovery |
| Worse | Fever, loss of taste and smell, myalgia, cough | |||||||
|
2020 | Iran | 40s year age range, female | 49 ** | N/A | Mild | Fever | 8.37 IgG (s/ca) after recovery |
| Worse | Fever, loss of taste and smell, myalgia | |||||||
|
2020 | Iran | 40s year age range, male | 72 ** | N/A | Mild | Fever | 5.11 IgG (s/ca) after recovery |
| Worse | Fever, loss of taste and smell, myalgia, cough | |||||||
|
2020 | Iran | 30s year age range, male | 59 ** | N/A | Mild | Fever, loss of taste and smell, myalgia | 6.3 IgG (s/ca) after recovery |
| Mild | Fever, loss of taste and smell, myalgia, cough | |||||||
|
2020 | Iran | 50s year age range, male | 53 ** | N/A | Mild | Fever, myalgia | 9.3 IgG (s/ca) after recovery |
| Worse | Fever, loss of taste and smell, myalgia, cough | |||||||
|
2020 | Iran | 20s year age range, male | 49 ** | N/A | Mild | Fever, myalgia | 7.25 IgG (s/ca) after recovery |
| Worse | Fever, loss of taste and smell, myalgia, cough | |||||||
|
2020 | Iran | 40s year age range, female | 52 ** | N/A | Mild | Fever, myalgia | 6.21 IgG (s/ca) after recovery |
| Worse | Loss of taste and smell, myalgia | |||||||
|
2020 | Iran | 20s year age range, female | 54 ** | N/A | Mild | Fever | 11.9 IgG (s/ca) after recovery |
| Mild | Fever, cough | |||||||
|
2020 | Iran | 30s year age range, male | 138 ** | N/A | Moderate | Fever, loss of taste and smell, myalgia, cough | 2.08 IgG (s/ca) after recovery |
| Asymptomatic | Asymptomatic | |||||||
|
2020 | Qatar | 46-year-old woman with mild asthma | 80 | N/A | Mild | Sore throat | N/A |
| Moderate | Chest pain, fever, sore throat, body pain, cough, mild dyspnea | |||||||
|
2021 | Saudi Arabia | 51-year-old woman with autologous hematopoietic stem cell transplantation for follicular non-Hodgkin lymphoma | 160 | 19B | Mild | Fever, cough, malaise, and headache | Negative COVID-19 serology after 1st infection and reinfection |
| 20B | Mild | Fever and dyspnea | ||||||
|
2021 | Turkey | 34-year-old man with chronic glomerulonephritis | >150 | N/A | Mild | Asymptomatic | N/A |
| Worse | Cough, fever, bilateral infiltrates at computed chest tomography | |||||||
|
2021 | Brazil | 35-year-old healthcare worker woman | 55 | N/A | Mild | Fever, headache, chills, sneezing, coryza, myalgia | N/A |
| Mild | Headache, nasal congestion, odynophagia, ageusia, anosmia | |||||||
|
2021 | Brazil | 61-year-old healthcare worker woman with chronic bronchitis | 170 | N/A | Mild | Headache, cough, myalgia, odynophagia, coryza, diarrhea, ageusia | N/A |
| Mild | Cough, myalgia, odynophagia, anosmia, diarrhea | |||||||
|
2021 | Brazil | 40-year-old healthcare worker woman | 131 | N/A | Mild | Nasal congestion, coryza, cough, ageusia | N/A |
| Mild | Odynophagia, sneezing, coryza, diarrhea, ageusia, anosmia | |||||||
|
2021 | Brazil | 40-year-old healthcare worker woman | 148 | N/A | Mild | Fever, headache, myalgia, coryza, dry cough, vomiting, malaise | N/A |
| Mild | Odynophagia, dry cough, myalgia, malaise, coryza, headache | |||||||
|
2021 | Peru | 42-year-old healthcare worker woman | 107 | N/A | Mild with home management | Odynophagia, headache, malaise, rhinorrhea, ageusia, anosmia, cough | IgM and IgG+ |
| Worse with home management | Chest pain, productive cough, anosmia, pneumonia | |||||||
|
2021 | Turkey | 46-year-old healthcare worker man | 114 | N/A | Moderate | Fever, sore throat, headache, cough, weakness, nausea and diarrhea, bilateral ground glass opacities and peribronchial thickening predominating on the right lung |
N/A |
| Mild | Sore throat, fever, headache, myalgia, weakness and nausea | |||||||
|
2021 | Turkey | 47-year-old healthcare worker woman | 128 | N/A | Mild | Myalgia, headache and abdominal pain started without fever and cough | N/A |
| Worse | Sore throat, headache and myalgia, fever, cough and mild respiratory symptoms, ground glass opacities and subpleural nodule on the left lung base consistent with COVID-19 on chest CT imagine | |||||||
|
2021 | Lebanon | 27-year-old man | 56 | N/A | Mild | Fever, chills, diffuse arthralgia, myalgia, headache, back pain | N/A |
| Milder | Fever, headache | |||||||
|
2021 | USA | 73-year-old man with obesity, chronic obstructive pulmonary disease, pancreatic insufficiency, type II diabetes mellitus | 60 | N/A | Mild | Shortness of breath | N/A |
| Worse | Dyspnea, fevers, confusion with worsening clinical situation and intubation | |||||||
|
2021 | USA | 28-year-old male with diabetes mellitus type 1, hypertension, and end-stage renal disease on hemodialysis with multiple past admissions for diabetic ketoacidosis and uncontrolled hypertension | 122 | N/A | Mild | Nausea and vomiting | N/A |
| Worse | Headaches and altered mental status, left-hand weakness. The patient became unresponsive and was intubated for airway protection > cerebrovascular accident | |||||||
|
2021 | Brazil | 76-year-old female with end-stage kidney disease related to lambda light chain multiple myeloma | 126 | N/A | Moderate | Hip pain, confusion, respiratory distress | N/A |
| Worse | Dyspnea, acute respiratory failure, hypoxemia > death | |||||||
|
2020 | Italy | 48-year-old nurse female | 90 | N/A | Mild | Dry cough, mild fever | LIASON ® SARS-CoV-2 S1/S2 IgG+ 30 Au/mL |
| Asymptomatic | Asymptomatic | IgG+ 102.9 Au/mL | ||||||
|
2020 | Brazil | 24-year-old white female without comorbidities | 76 | N/A | Mild with complete resolution at home within 10 days | Headache, malaise, adynamia, feverish sensation, sore throat, nasal congestion | N/A |
| Worse with home resolution in 12 days, headache and hyposmia for 63 days | Malaise, myalgia, severe headache, fatigue, weakness, feverish sensation, sore throat, anosmia, dysgeusia, diarrhea, coughing | IgG/IgM– at NAAT+IgG/IgM+ 28 days after NAAT+ | ||||||
|
2021 | Italy | 52-year-old man with transitional cell carcinoma of the renal pelvis | 110 | Clade 20B and Pangolin lineage B.1.1 | Mild | Cough, fever | |
| Clade 20A and Pangolin lineage B.1 | Milder | Fever | Very low levels of IgG anti-SARS-CoV-2 Spike protein, positive IgG anti-SARS-CoV-2 N protein | |||||
|
2021 | Germany | 27-year-old female nurse | 282 | HH-24.I (19A) |
Mild | Fever, chills, dyspnea | IgG anti-SARS-CoV-2 Spike protein: 40 AU/mL in July 2020, 15 AU/mL in September 2020 |
| HH-24.II (20EU1) with differences in 21 positions, including 2 typical variations in spike proteins A222V and D614G | Milder | Dry cough, mild rhinorrhea | IgG anti-SARS-CoV-2 Spike protein: 97 AU/mL on 29 December | |||||
|
2021 | The Netherlands | 16-year-old girl | 390 | Classic | Moderate | High fever, mild conjunctivitis, malaise, chest pain, coughing, abdominal pain and diarrhea. She was diagnosed with myocarditis, shock and had high inflammatory parameters. | IgG SARS-CoV-2 was negative (Abbott SARS-CoV-2 IgG; Abbott Laboratories) |
| B.1.1.7 variant (UK variant), | Mild | Mild respiratory symptoms | ||||||
|
2021 | USA | 60 with diabetes | 72 | N/A | Mild | Acute renal failure | |
| Milder | Fatigue | |||||||
|
2021 | USA | 27 with psoriatic arthritis | 79 | N/A | Mild | Fever, flu-like | IgG+ |
| Milder | Fatigue, loss taste | |||||||
|
2021 | USA | 33 year-old woman with allergic rhinitis | 172 | N/A | Mild | Fever, cough, diarrhea | IgG+ |
| Milder | Fever headache | |||||||
|
2021 | USA | 71 with renal/liver transplant HIV, diabetes | 93 | N/A | Moderate | Fever, pneumonia, respiratory insufficiency | |
| Asymptomatic | Asymptomatic | |||||||
|
2021 | USA | 72 with pulmonary/cardiac sarcoidosis | 111 | N/A | Mild | Dyspnea, fatigue, headache | |
| Milder | Fatigue | |||||||
|
2021 | USA | M (80–89 years old) | 101 | N/A | Asymptomatic | asymptomatic | N/A |
| Mild | Lethargy, decreased appetite, dry cough for 14 days | |||||||
|
2021 | USA | F (80–89 years old) | 103 | N/A | Asymptomatic | asymptomatic | N/A |
| Worse | Congestion, respiratory failure and death | |||||||
|
2021 | USA | F (60–69 years old) | 109 | N/A | Mild | nausea | N/A |
| Mild | Cough, sore throat, loss of appetite, malaise, muscle aches for 17 days | |||||||
|
2021 | USA | F (70–79 years old) | 109 | N/A | Mild | Gastrointestinal symptoms for 17 days | N/A |
| Milder | Loss of appetite, malaise for 12 days | |||||||
|
2021 | USA | Female (90–99 years old) | 110 | N/A | Asymptomatic | asymptomatic | N/A |
| Mild | Cough, loss of appetite, malaise, muscle aches for 6 days | |||||||
|
2021 | France | 70-year-old man | 105 | Clade 20A | Moderate | Fever, cough | IgG+ on D26 |
| 20A.E2, 34 nucleotide differences | Asymptomatic, during a systematic screening | Asymptomatic | ||||||
|
2021 | Bangladesh | A 35–49-year-old man with hypertension | 98 | N/A | Mild | Fever, cough | |
| Milder | Fever, cough, cold | |||||||
|
2021 | Bangladesh | A 35–49-year-old researcher woman | 92 | N/A | Mild | Malaise | |
| Milder | Sore throat, fever, cough, headache | |||||||
|
2021 | Bangladesh | 35–49 hypertensive physician | 94 | N/A | Mild | Fever, headache, sore throat | |
| Mild | Fever, cold, low oxygen saturation | |||||||
|
2021 | Bangladesh | 35–49 man with asthma | 93 | N/A | Mild | Fever | |
| Mild | Fever, cough | |||||||
|
2021 | Bangladesh | 35–49-year-old health worker woman with hypertension, hypothyroidism | 131 | N/A | Mild | Fever, cough | |
| Worse | Chest pain, headache, sore throat, hospitalized | |||||||
|
2021 | Libya | 52-year-old healthy male | 72 | N/A | Mild | Cough, sore throat, fever, myalgias, headache | N/A |
| Worse | Fever, cough, shortness of breath, gastrointestinal symptoms | |||||||
|
2020 | Brazil | 40-year-old male doctor | 46 | N/A | Moderate | Fever, cough, sore throat, fatigue, myalgia, headache, diarrhea | IgG and IgM– 42 days after 1 infection |
| Moderate | Fever, cough, sore throat, fatigue, myalgia, headache, diarrhea, anosmia and dysgeusia | IgG and IgM– | ||||||
|
2021 | Panama | 36-year-old man without comorbidities | 181 | A.2.4 | Mild | Myalgia, chest pain, fever, cephalea, rhinorrhea, hyposmia, ageusia | |
| A.2.5 containing Spike mutations D614G and L452R | Milder | Cephalea, myalgia, rhinorrhea | ||||||
|
2021 | France | 25-year-old female healthcare worker | >90 | N/A | Asymptomatic | Asymptomatic | No neutralizing antibodies |
| Moderate | Fever, rhinorrhea, dyspnea, chest pain, dysgeusia, anosmia, asthenia, myalgia, eye pain, pharyngitis; not hospitalized | Yes, neutralizing antibodies | ||||||
|
2021 | France | 40-year-old female healthcare worker | >90 | N/A | Asymptomatic | Asymptomatic | No neutralizing antibodies |
| Asymptomatic | Asymptomatic | No neutralizing antibodies | ||||||
|
2021 | France | 46-year-old female healthcare worker | >90 | N/A | Moderate | Fever, rhinorrhea, cough, dyspnea, chest pain, intestinal disorders, dysgeusia, anosmia, asthenia, headache, myalgia, not hospitalized | Yes, neutralizing antibodies |
| Mild | Fever, cough, dyspnea, chest pain, headache, asthenia, myalgia, pharyngitis; not hospitalized | Yes, neutralizing antibodies | ||||||
|
2021 | France | 31-year-old male healthcare worker | >90 | N/A | Mild | Anosmia; not hospitalized | Yes, neutralizing antibodies |
| Asymptomatic | Asymptomatic | Yes, neutralizing antibodies | ||||||
|
2021 | France | 50-year-old female healthcare worker | >90 | N/A | Asymptomatic | Asymptomatic | Yes, neutralizing antibodies |
| Mild | Cough, headache; not hospitalized | Yes, neutralizing antibodies | ||||||
|
2021 | Spain | 29-year-old female healthcare worker | 212 | N/A | Mild | 60 days | Seronegative after 1st infection, seroconverted after re-infection |
| Mild | 70 days | |||||||
|
2021 | Spain | 41-year-old female healthcare worker | 154 | N/A | Mild | 61 days | Seronegative after 1st infection, seroconverted after re-infection |
| Milder | ||||||||
|
2021 | Spain | 58-year-old female healthcare worker | 58 | N/A | Mild | 3 days | Unknow after 1st infection, seropositive after reinfection |
| Mild | 3 days | |||||||
|
2021 | Spain | 44-year-old female healthcare worker | 211 | N/A | Mild | 11 days | Seropositive after 1st infection with antibody low-level |
| Asymptomatic | Asymptomatic | |||||||
|
2020 | USA | 82-year-old male with Parkinson, insulin-dependent diabetes, chronic kidney disease, hypertension | 48 | N/A | Severe with intubation | Fever, shortness of breath, hypoxia, pneumonia | N/A |
| Severe without intubation | Fever, hypoxia, hypotension, tachycardia, pneumonia | |||||||
|
2021 | Saudi Arabia | 51-year-old man without comorbidities | 58 | Asymptomatic | Asymptomatic | 7.04 SARS-CoV-2 IgG (Abbot) during second admission | |
| Worse | Fever, cough, generalized weakness, and shortness of breath, bilateral diffuse patchy airspace disease while a CT scan revealed bilateral patchy 4 central and peripheral ground glass opacities most likely related to COVID-19 | |||||||
|
2021 | Saudi Arabia | 55-year-old man with relapsed NHL | 31 | Mild | Mild | 0.01 SARS-CoV-2 IgG (Abbot) index negative during second admission | |
| Worse | High grade fever, dry cough, sore throat, tachycardia and (SPO2) 93% on room air | |||||||
|
2021 | Saudi Arabia | 60-year-old man with diabetes mellitus, hypertension, ischemic heart disease | 27 | Mild | Mild | N/A | |
| Milder | Cough, shortness of breath | |||||||
|
2021 | Saudi Arabia | 48-year-old woman with metastatic breast cancer | 85 | Moderate | Pneumonia | N/A | |
| Mild | Fever, shortness of breath | |||||||
|
2021 | Saudi Arabia | 24-year-old male dental student | 90 | N/A | Mild | Sore throat, cough, headache, nausea, diarrhea, loss of taste and smell, insomnia, loss of appetite, and fatigue, fear and anxiety, increased insomnia, and increased body ache | N/A |
| Mild | Coughing, body ache, loss of taste and smell, and diarrhea symptoms were slightly less severe, the patient was less anxious and slept well. Fever | |||||||
|
2021 | Czech Republic | 60-year-old man with diabetes | 177 | N/A | Mild | Mild—long term care facility | N/A |
| Moderate | Mild—hospitalized | |||||||
|
2021 | Czech Republic | 75-year-old man with diabetes, cardiovascular disease | 102 | N/A | Mild | Mild—long term care facility | N/A |
| Severe | Mild—hospitalized | |||||||
|
2021 | Czech Republic | 72-year-old man with malignity | 205 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 62-year-old woman with asthma | 137 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 57-year-old woman without comorbidities | 203 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 56-year-old woman without comorbidities | 216 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 55-year-old man without comorbidities | 212 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 53-year-old man without comorbidities | 214 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 50-year-old woman with malignity | 197 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 49-year-old woman without comorbidities | 195 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 49-year-old woman without comorbidities | 200 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 47-year-old man without comorbidities | 141 | N/A | Mild | Mild—home | N/A |
| Moderate | Mild—home | |||||||
|
2021 | Czech Republic | 47-year-old man without comorbidities | 206 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 46-year-old man without comorbidities | 154 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 46-year-old woman without comorbidities | 231 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 45-year-old woman without comorbidities | 101 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 45-year-old woman with diabetes, chronic pulmonary disease, allergy | 196 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 45-year-old woman with cardiovascular disease | 211 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 44-year-old woman with hypertension | 169 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 44-year-old man without comorbidities | 224 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 42-year-old woman without comorbidities | 206 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 39-year-old woman without comorbidities | 229 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 34-year-old man without comorbidities | 158 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 30-year-old woman without comorbidities | 219 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 29-year-old woman without comorbidities | 139 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 27-year-old woman without comorbidities | 172 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 27-year-old woman without comorbidities | 215 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Czech Republic | 25-year-old man without comorbidities | 222 | N/A | Mild | Mild—home | N/A |
| Mild | Mild—home | |||||||
|
2021 | Portugal | 28-year-old man with asthma | 285 | N/A | Mild | Fever, chills, sneezing | N/A |
| Worse | Fever, tiredness, productive cough, frontal headache, dizziness, dark urine, dysuria | |||||||
|
2021 | Brazil | 24-year-old woman without comorbidities | 109 | N/A | Asymptomatic | Asymptomatic | No IgG antibodies after first infection |
| P1 variant | Worse | Headache, sore throat, odynophagia, nasal congestion, tiredness, fatigue, chest pain, lack of appetite, hypertension, tachycardia | ||||||
|
2021 | Brazil | 54-year-old man without comorbidities | 65 | N/A | Mild | Headache | IgM, IgA, IgG detected <1:4 |
| Clade 20B | Worse | Fever, dry cough, tiredness, body ache, anosmia, ageusia | IgM, IgA, IgG detected 1:128 | |||||
|
2021 | Brazil | 57-year-old woman with discoid lupus erythematous | 61 | Clade 19A | Mild | Mild diarrhea | IgM, IgA, IgG detected <1:4 |
| Clade 20B | Worse | Fever, diarrhea, headache, body ache, anosmia, ageusia | IgM, IgA, IgG detected 1:32 | |||||
|
2021 | Brazil | 34-year-old man without comorbidities | 64 | Clade 20B | Mild | Asymptomatic | IgM, IgA, IgG detected <1:4 |
| Clade 20B | Worse | Fever, nausea, tiredness, headache, body ache | IgM, IgA, IgG detected 1.64 | |||||
|
2021 | Brazil | 34-year-old woman without comorbidities | 60 | N/A | Mild | Mild diarrhea | IgM, IgA, IgG detected <1:4 |
| Clade 20B | Worse | Dry cough, diarrhea, tiredness, headache, body ache, anosmia, ageusia | IgM, IgA, IgG detected 1:64 | |||||
|
2021 | Brazil | 29-year-old health care worker man without comorbidities | 225 | B.1.1.28 Spike D614G |
Mild | Fever, myalgia cough, sore throat, diarrhea | IgG negative 180 days after the 1st infection |
| B,1,2 Spike D614G |
Mild | Again symptoms | ||||||
|
2021 | Mexico | 40-year-old healthcare worker woman with hypertension, smoking | 134 | N/A | Moderate | Fever, dry cough, nasal drainage, dyspnea, myalgia, arthralgia, headache, anosmia, dysgeusia, decreased oxygen saturation up to 84%, maculopapular rash on the upper and lower limbs, chest, face, neck | |
| Worse | Sneezing, runny nose, myalgia, arthralgia, fever, dry cough, headache, dyspnea, emphysema of the right lung | |||||||
|
2021 | Mexico | 49-year-old health care worker woman with hypothyroidism | 129 | N/A | Mild | Nasal congestion, myalgia, arthralgia, chills, headache, dry cough, dysgeusia, anosmia, maculopapular exanthema, insomnia | |
| Mild | Headache, dry cough, odynophagia, myalgia, dyspnea, conjunctivitis | |||||||
|
2021 | Mexico | 53-year-old health care worker man without comorbidities | 107 | N/A | Mild | Fever, dyspnea, pneumonia | |
| Mild | Fever, chills, anosmia, dysgeusia dry cough, rhinorrhea, general malaise, chest pain, | |||||||
|
2021 | Mexico | 52-year-old health care worker man without comorbidities | 82 | N/A | Mild | Odynophagia, dry cough, nasopharyngeal exudate | |
| Worse | Myalgias, arthralgias, dry cough, dyspnea, odynophagia, pneumonia> intensive care for hypoxia | |||||||
|
2021 | India | 30-year-old health care worker man without comorbidities | 90 | N/A | Mild | Fever | 30 days after initial diagnosis IgG antibody negativity |
| Worse | Fever, severe myalgia, anosmia, loss of taste | 30 days after reinfection diagnosis IgG antibody positivity | ||||||
|
2021 | UK | 92-year-old man with dementia | 207 | 1st wave | Moderate | Pyrexia, dry cough, shortness of breath, bilateral pneumonia | |
| B.1.177 (Spain variant) | Moderate | Lethargy, persistent cough, pyrexia, pneumonia | ||||||
|
2021 | UK | 84-year-old man with dementia and Paget’s disease | 224 | 1st wave | Mild | Lethargy, confusion, headache, fatigue | |
| B.1.177 (Spain variant) | Mild | Positive | ||||||
|
2021 | UK | 59-year-old man with end stage renal failure | 236 | 1st wave | Mild | Cough, fluctuating temperature | |
| B.1.1.7 (Kent variant) | none | None | ||||||
|
2021 | USA | 59-year-old man with end stage renal failure and hemodialysis | 59 | N/A | Moderate | Cough, fever, pneumonia > hospitalization | |
| Milder | Cough, shortness of breath, >hospitalization | SARS-CoV-2 IgG antibody positive after re-infection | ||||||
|
2020 | USA (Washington) | Sexagenarian (age between 60 and 69) with emphysema and hypertension | 140 | Clade 19B | Severe | Fever, chills, productive cough, dyspnea, chest pain | |
| Clade 20A harboring the spike variant D614G | Severe, but milder than first | Dyspnea, dry cough, weakness | RBD, spike and NC IgG, spike IgM, NC IgA+ on D14 of reinfection | |||||
|
2021 | UK | 61-year-old south Asian with immunosuppression for ANCA-associated vasculitis | 180 | N/A | Severe | Dry cough, dyspnea, fever, myalgia, kidney dysfunction, pneumonia | N/A |
| Moderate | Fever, myalgia, dyspnea, pneumonia | |||||||
|
2020 | India | 25-year-old male healthcare worker | 108 | 9 SNVs compared to initial infection (19A first infection–20A second infection) | Asymptomatic | Asymptomatic | N/A |
| Asymptomatic | Asymptomatic with higher viral load | |||||||
|
2020 | India | 28-year-old female healthcare worker | 111 | 10 SNVs compared to initial infection; mutation 22882T > G (S:N440K) within the receptor binding domain found in the second episode | Asymptomatic | Asymptomatic | N/A |
| Asymptomatic | Asymptomatic with higher viral load | |||||||
|
2021 | SAU | 44-year-old woman healthcare worker | 108 | N/A | Moderate | Fever, chills, severe sore throat, fatigue | N/A |
| Moderate | Severe persistent productive cough, runny nose, loss of smell, partial loss of taste | |||||||
|
2021 | SAU | 35-year-old heavy male smoker | 94 | N/A | Asymptomatic | Asymptomatic | N/A |
| Worse | Fever, cough, body ache, abdominal pain, loss of taste | |||||||
|
2020 | Pakistan | 58-year-old cardiac surgeon male without comorbidities | 55 | N/A | Hospitalized for 30 days | Fatigue, headache, sore throat, pneumonia | N/A |
| Hospitalized for 14 days | Fever >39 °C, headache, muscle aches | |||||||
|
2021 | UK | 78-year-old man with type 2 diabetes mellitus, diabetic nephropathy, chronic obstructive pulmonary diseases, sleep apnea, ischemic heart disease | 250 | Lineage B.2 with no mutations in the S region | Discharged home | Mild illness | SARS-CoV-2 antibodies (using the Roche anti-SARS-CoV-2 IgM/IgG assay detecting antibodies targeting viral nucleocapsid “N” antigen) were detectable on 6 occasions between 4 June 2020 and 13 November 2020 with no evidence of antibody waning seen |
| Variant VOC-20201/01 of lineage B.1.1.7 with 18 amino acid replacement and deletions in the S region | Emergency intubation, worse | Shortness breath, severe hypoxia, pneumonia, myocardial infarction | ||||||
|
2021 | USA | 30-year-old female healthcare worker with idiopathic thrombocytopenic purpura, pancreatitis, GERD, anxiety, recurrent pneumonia | 183 | N/A | Mild | Fever, fatigue, sore throat, nasal congestion, dry cough, chest tightness | After 1st infection anti-SARS-CoV-2 IgG were negative |
| Mild | Headaches, fever, sinus congestion | After 2nd infection anti-SARS-CoV-2 IgG were positive | ||||||
|
2021 | USA | 81-year-old woman with immunosuppression for rheumatoid arthritis | 62 | N/A | Mild | Altered mental status, | N/A |
| Moderate | Cough, shortness of breath, oxygen requirement | |||||||
|
2021 | Iraq | 39-year-old man with hypertension | 112 | N/A | Moderate | Fever, dry cough, hypoxemia | SARS-CoV-2 2 months after discharge |
| Mild | Fever, not hypoxemia | |||||||
|
2021 | Iraq | 32-year-old man | 82 | N/A | Mild | Myalgia, fever | N/A |
| Mild | Myalgia | |||||||
|
2021 | Iraq | 40-year-old man | 50 | N/A | Severe | Fever, loss of smell, myalgia, dyspnea | N/A |
| Mild | Fever, sore throat | |||||||
|
2021 | Iraq | 46-year-old man | 74 | N/A | Mild | Fever, dry cough | N/A |
| Moderate | Fever, sore throat, loss of taste and smell | |||||||
|
2021 | Iraq | 39-year-old man | 122 | N/A | Severe | Fever, dry cough, dyspnea | N/A |
| Mild | Fever, sore throat | |||||||
|
2021 | Iraq | 32-year-old woman | 174 | N/A | Mild | Fever, dry cough, loss of smell, sore throat | N/A |
| Mild | Fever, sore throat, myalgia | |||||||
|
2021 | Iraq | 44-year-old man with colon cancer | 51 | N/A | Mild | Fever, myalgia | N/A |
| Mild | Myalgia | |||||||
|
2021 | Iraq | 26-year-old woman | 84 | N/A | Mild | Headache, sweating, loss of taste | N/A |
| Mild | Headache, myalgia | |||||||
|
2021 | Iraq | 26-year-old woman | 84 | N/A | Mild | Headache, loss of taste | N/A |
| Moderate | Myalgia, cough, dyspnea | |||||||
|
2021 | Iraq | 36-year-old woman with diabetes | 51 | N/A | Mild | Sore throat, fever | N/A |
| Severe | Fever, myalgia, cough, dyspnea | |||||||
|
2021 | Iraq | 34-year-old man | 49 | N/A | Mild | Headache, fever | N/A |
| Severe | Myalgia, fever, headache, anorexia | |||||||
|
2021 | Iraq | 79-year-old woman with heart failure and hypertension | 58 | N/A | Severe | Fever, dyspnea | N/A |
| Severe | Cough, anorexia, fever | |||||||
|
2021 | USA | 59-year-old Caucasian male with Hodgkin lymphoma | 150 | N/A | Moderate | Shortness of breath, dry cough, tachycardia, oxygen desaturation to 85% | N/A |
| Moderate | Chills, worsening shortness of breath, productive cough, fever, tachycardia, hypoxemia | |||||||
|
2021 | Japan | 58-year-old with mild dyslipidemia | 105 | N/A | Moderate | Fever, bilateral pneumonia | After 1st episode IC50 of neutralizing antibodies anti-SARS-CoV-2 was 50.0 microg/mL |
| Asymptomatic | Asymptomatic | After 2nd episode IC50 of neutralizing antibodies anti-SARS-CoV-2 was 14.8 microg/mL | ||||||
|
2020 | India | 21-year-old female | 50 | N/A | Asymptomatic | Asymptomatic | N/A |
| mild | Complete loss of smell for 2 weeks | |||||||
|
2021 | India | 39-year-old male with multiple myeloma | 84 | N/A | Asymptomatic | Asymptomatic | N/A |
| Severe | High grade fever, chills, shortness of breath, bilateral pneunomia | |||||||
|
2021 | India | 33-year-old male with T cell acute lymphoblastic leukemia | 60 | N/A | Severe | Fever, cough, pneumonia | N/A |
| Severe | Headache, vomiting, high grade fever, pneumonia | |||||||
|
2021 | India | 26-year-old male with Philadelphia chromosome positive acute lymphoblastic leukemia | 91 | N/A | Asymptomatic | Asymptomatic | N/A |
| Moderate | Fever | |||||||
|
2021 | USA | 70-year-old man with hypertension, diabetes mellitus, coronary artery disease | 45 | N/A | Asymptomatic | Asymptomatic | COVID-19 IgG positive after 1st infection |
| Worse | Shortness of breath, cough, chest pain, myalgias | |||||||
|
2021 | USA | Late 50s woman with hypertension, hepatitis C, heart failure | 75 | N/A | Asymptomatic | Asymptomatic | N/A |
| Worse | Fever, myalgias, sore throat | |||||||
|
2021 | USA | 66-year-old man with bipolar disorder, end-stage renal disease due to lithium toxicity and renal transplantation | 210 | Clade B.1 | Mild | Fever, fatigue, dry cough | Failure of humoral immunity with defective response of the neutralizing antibodies after primary infection |
| Clade B.1.280 | Milder | Fatigue and nonproductive cough | ||||||
|
2021 | India | 61-year-old male healthcare worker | 75 | 20B clade | Asymptomatic | Asymptomatic | N/A |
| 20B clade with 10 variations | Mild | Cough, weakness | ||||||
|
2020 | USA (Virginia) | 42-year-old man military healthcare provider | 64 | Lineage B.1.26 | Moderate, clinical resolution in 10 days | Cough, fever, myalgias | |
| Lineage B.1.26 with several potential variations | Severe, worse | Fever, cough, shortness of breath, gastrointestinal symptoms, pneumonia | Spike IgG+ on D8 of reinfection | |||||
|
2020 | France | 42-year-old Parisian male | 7 months | N/A | Home-managed | Dyspnea, fever, headache, diarrhea, abdominal pain, ageusia, total less of smell | IgG 2 months after |
| Milder | Fever, nasal burning, total loss of taste and smell | |||||||
|
2020 | Spain | 38-year-old Spanish health care worker female | 6 months | N/A | Moderate—hospitalized for 7 days | Dyspnea, fever, headache, diarrhea, loss of smell | N/A |
| Milder | Fever, headache, new total loss of smell and taste | |||||||
|
2020 | South Korea | 21-year-old healthy woman | 26 | Clade V—found in Asia and Europe | Hospitalized with few symptoms | Sore throat | |
| Clade G—found in south Korea | Mild | Cough, sore throat | IgG+ | |||||
|
2021 | Italy | 41-year-old healthcare worker woman | 289 | 20B | Mild | Fever, arthralgia, headache, diarrhea, anosmia, ageusia | IgG positive after 1st infection and after 2nd infection |
| 20E (EU1) | Mild | Headache, sore throat, diarrhea | ||||||
|
2021 | UK | 55-year-old man with X-linked agammaglobulinemia | 56 | N/A | Moderate | Purulent sputum, fever, breathlessness, fever, headache, myalgia, chest tightness | N/A |
| Worse | Short of breath, fevers > death | |||||||
|
2020 | Italy | 69-year-old man, heavy smoker with classic Hodgkin’s lymphoma with mixed cellularity | 131 | N/A | Moderate with 3 months of hospitalization | Pneumonia, fever, diarrhea | IgG+ 50 days after hospitalization |
| Moderate with 64 days of hospitalization | Fever, dyspnea, anemia, leukopenia, pneumonia | N/A | ||||||
|
2021 | India | 33-year-old man | 90 | N/A | Mild | Sore throat | N/A |
| Worse | Influenza like Illness symptoms with breathing difficulty | |||||||
|
2021 | India | 27-year-old man | 69 | N/A | Asymptomatic | Asymptomatic | N/A |
| Worse | Fever, cough, myalgia | |||||||
|
2021 | India | 48-year-old woman | 97 | N/A | Mild | Myalgia | N/A |
| Mild | Myalgia | |||||||
|
2021 | India | 26-year-old woman | 55 | N/A | Mild | Fever, myalgia | N/A |
| Mild | Fever, sore throat, myalgia | |||||||
|
2021 | India | 25-year-old man | 89 | N/A | Mild | Fever, sore throat, myalgia and loss of smell and taste | N/A |
| Mild | Fever | |||||||
|
2021 | India | 31-year-old man | 70 | N/A | Asymptomatic | Asymptomatic | N/A |
| Worse | Myalgia | |||||||
|
2021 | India | 51-year-old woman | 157 | N/A | Asymptomatic | Asymptomatic | N/A |
| Worse | Myalgia, headache, pneumonia (25% lung involvement) | |||||||
|
2021 | USA | 16-year-old woman with end-stage renal disease | 90 | B.1.2 | Mild | Sore throat, fatigue, nasal congestion, rhinorrhea, dry cough | IgM+ and IgG− after the 2nd infection |
| B.1.1.7 | Milder | Leg pain, fatigue, swelling leg, fever | ||||||
|
2021 | Spain | 62-year-old male healthcare worker with previous history of mild asthma, hypertension, dyslipidemia, liver steatosis, hyperuricemia, and overweight (body mass index ≥ 30 kg/m2) | 158 | Mild | Fever of 38 °C, diarrhea, anosmia, dysgeusia, cough, intense asthenia, and arthromyalgia | After reinfection weak immune response, with marginal humoral and specific T-cell responses against SARS-CoV-2. All antibody isotypes tested as well as SARS-CoV-2 neutralizing antibodies increased sharply after day 8 post symptoms. A slight increase of T-cell responses was observed at day 19 after symptom onset | |
| B.1.79 (G) | Worse | Intense arthromyalgias, headache, fever, cough, and dyspnea > admitted to the emergency room for worsening dyspnea, cough, chills, fever 39 °C, myalgias, anosmia, and ageusia. His respiratory rate was 36 breaths/minute, his heart rate was 100 beats/minute, and he had bilateral inspiratory crackles. The chest radiograph showed bilateral alveolar-interstitial infiltrates | ||||||
|
2021 | USA | 53-year-old female with liver transplant in 2010 due to alcoholic cirrhosis, hypertension, hypothyroidism, anxiety, and chronic kidney disease | 90 | N/A | Severe | Encephalopathy due to her COVID-19 | N/A |
| Mild | Nausea, vomiting, diarrhea, and myalgias | |||||||
|
2020 | Denmark | 89-year-old immunocompromised woman (Waldestrom macroglobulinemia) | 59 | The 2 strains differed at 10 nucleotide positions in ORF1a (4), ORF1b (2), spike (2), ORF3A (1), M (1) genes | Hospitalized for 5 days | Fever, severe cough, persisting fatigue | IgM- |
| Worse | Fever, cough, dyspnea > death after 2 weeks | N/A | ||||||
|
2020 | USA | 51-year-old African American male with hypertension and hemodialysis history | 2 months | N/A | Asymptomatic | Positive for NAAT and IgG at a routine control during hemodialysis | IgM−, IgG+ |
| Severe, hospitalized with non-invasive positive pressure mechanical ventilation | Fever 38.3 °C, severe dyspnea, pneumonia | IgG+, IgM+, IgA+ | ||||||
|
2020 | Israel | 22-year-old woman without comorbidities | 111 | N/A | Mild with home back after 23 days | Fever, cough | |
| Asymptomatic | Tachycardia | IgG+ | ||||||
|
2021 | Brazil | 29-year-old | 281 | 20A | Mild | Fever, myalgia, cough, sore throat, nausea, and back pain | |
| 20J (P.1) | Mild | Fever, cough, sore throat, diarrhea, anosmia, ageusia, headache, runny nose, and resting pulse oximetry of 97% | ||||||
|
2021 | Brazil | 50-year-old | 153 | 20B | Mild | Fever, cough, and tiredness | |
| 20J (P.1) | Mild | Cough, headache, and runny nose | ||||||
|
2021 | Brazil | 40-year-old woman | 282 | 20A | Mild | Fever, headache, chest pain, and weakness | |
| 20J (P.1) | Mild | Sore throat and running nose | ||||||
|
2020 | India | 26-year-old man healthcare worker | 97 | N/A | Asymptomatic | Asymptomatic | N/A |
| Asymptomatic | Asymptomatic | |||||||
|
2021 | USA | 46-year-old man with hypertension, gastroesophageal reflux disease, plantar fasciitis | >90 | N/A | Mild | Fever, myalgias, sore throat, chills, headaches, nausea, shortness of breath | SARS-CoV−2 IgG testing 1st test: 1:4096 (BCM laboratory) |
| Asymptomatic | Asymptomatic | SARS-CoV−2 IgG testing 2nd test: 1:2048 (BCM laboratory) | ||||||
|
2021 | USA | 27-year-old woman | >90 | N/A | Mild | Congestion, fatigue, loss of taste, loss of smell, headache | N/A |
| Milder | Fever, chills, fatigue | |||||||
|
2021 | USA | 53-year-old man with hypertension, sleep apnea | >90 | N/A | Mild | Cough, congestion, loss of taste, loss of smell | SARS-CoV−2 IgG testing 1st test: 1:2048 (BCM laboratory) |
| Asymptomatic | Asymptomatic | SARS-CoV−2 IgG testing 2nd test: 1:1024 (BCM laboratory) | ||||||
|
2021 | USA | 66-year-old woman with diabetes mellitus, rheumatoid arthritis, systemic lupus erythematosus, congestive heart failure, renal disease, gout, hypertension | >90 | N/A | Mild | Fatigue | N/A |
| Asymptomatic | Asymptomatic | |||||||
|
2021 | USA | 73-year-old woman with hypertension, hyperlipidemia, depression | >90 | N/A | Mild | Congestion, sore throat, headache | N/A |
| Mild | Cough, shortness of breath, congestion, abdominal pain, nausea, vomiting, headache | |||||||
|
2021 | USA | 42-year-old woman with breast cancer | >90 | N/A | Mild | Cough, shortness of breath, fatigue, loss of taste, loss of smell, headache, fever | SARS-CoV−2 IgG testing 1st test: 1:4096 (BCM laboratory) |
| Asymptomatic | Asymptomatic | |||||||
|
2021 | USA | 36-year-old man | >90 | N/A | Mild | Cough, fatigue, nausea, loss of smell, fever | SARS-CoV−2 IgG testing 1st test: 1:4096 (BCM laboratory), 2nd test: 1:4096 (BCM laboratory) |
| Asymptomatic | Asymptomatic | |||||||
|
2021 | Brazil | 45-year-old woman | 147 | Lineage B.1.1.33 with S:G1219C mutation | Mild | Diarrhea, myalgia, asthenia, odynophagia for 7 days | N/A |
| Lineage P.2 (or B.1.1.28.2) with S:E484K mutation | Moderate | Headache, malaise, ageusia, muscle fatigue, insomnia, mild dyspnea, shortness of breath | ||||||
|
2021 | Colombia | 44-year-old male, healthcare worker | 103 | N/A | Asymptomatic | Asymptomatic | N/A |
| Moderate | Malaise, chills, headache, fever, odynophagia | |||||||
|
2020 | Turkey | 23-year-old woman | 116 | N/A | Hospitalized | Fever >39 °C, chills, fatigue, cough, headache, sore throat, muscle and joint pain | N/A |
| Recovered in 10 days | Fever 28.7 °C, chills, fatigue, loss of appetite, taste and smell loss, muscle and joint pain | IgG slightly positive | ||||||
|
2021 | USA | 40-year-old man | 89 | N/A | Mild | Fever, cough | N/A |
| Worse | Dyspnea, tachycardia > death | |||||||
|
2020 | Ecuador | 46-year-old man | 63 | Nextstrain 20A/GISAID B1.p9 lineage | Mild | Intense headache, drowsiness | IgM+ IgG− on D7 of initial infection |
| Nextstrain 19B/GISAID A.1.1 lineage; 18 mutations difference | Moderate | Odynophagia, nasal congestion, fever 39 °C, back pain, productive cough, dyspnea | IgM+ IgG+ on D28 | |||||
|
2021 | Spain | 60-year-old male, with chronic kidney disease (CKD) due to focal and segmental glomerulosclerosis that received his first kidney transplant 2004 |
149 | N/A | Mild | Cough and low-grade fever | Antibodies (IgM and IgG) for SARS-CoV2 resulted negative after reinfection |
| Worse | Respiratory fever and acute injury of the allograft function. A chest X-ray showed bilateral infiltrates with unilateral pleural effusion > death | |||||||
|
2021 | Colombia | 54-year-old woman with hypertension, gastritis, arthrosis | 33 | B.1 | Mild | Fever, cough, odynophagia, fatigue | N/A |
| B.1.1.269 | Milder | Fever, odynophagia | ||||||
|
2021 | India | 47-year-old man | 46 | 15 genetic variants with 22882T > G (Spike N440K) | Asymptomatic | Asymptomatic | N/A |
| 17 genetic variants with 22882T > G (Spike N440K) | Worse | Fever, cough, malaise | ||||||
|
2021 | Brazil | 37-year-old healthcare worker woman | 116 | B.1.1.33 | Mild | Headache, runny nose, diarrhea, myalgia | IgG+ after re-infection |
| VOI P.2 with mutation S-E484K | Mild | Headache, ageusia, anosmia, fatigue | ||||||
|
2021 | Spain | 76-year-old man with hypertension, biological aortic heart valve replacement, and end-stage kidney disease secondary to autosomal dominant polycystic kidney disease | 58 | Asymptomatic | Asymptomatic | IgG and IgM to SARS-CoV-2 tested negative after 1st and 2nd episode | |
| Worse | Fever, cough, and shortness of breath, bilateral pneumonia > death 18 days after admission | |||||||
|
2021 | Brazil | 26-year-old woman | 128 | Non-VOC virus | Mild | Dry cough, dizziness, headache, fatigue, stuffy nose, back pain, loss of taste, nausea, diarrhea | |
| VOC-virus P.1 variant | Mild | Dry cough, dizziness, headache, fatigue, diarrhea, joint pain legs, difficult breathing | ||||||
|
2021 | USA | 62-year-old woman with hypertension, hypothyroidism, chronic lower back pain | 90 | N/A | Hospitalized | Worsening shortness of breath, cough, hypoxia | N/A |
| Worse with intubation twice | Tachypnea, hypoxia, pneumonia | |||||||
|
2021 | Iran | 42-year-old man | 128 | 20G with 11 mutations | Mild | Cough, headache, severe diarrhea | IgG and IgM negative |
| 20G with 17 mutations | Mild | Body pain, shortness of breath, headache, anosmia | IgG and IgM negative | |||||
|
2021 | Iran | 32-year-old woman | 63 | N/A | Mild | Headache, sore throat, cough, fever | The antibody titration was achieved positive by the rapid test (sensitivity 72%, specificity: 76%) for IgM (At the time of second infection, IgG titration was assessed as 4.89 AU/mL which after two months turned to a significant raise (over ELISA reader standard range). |
| D614G mutation | Worse | Severe cough, fever, fatigue | ||||||
|
2021 | Iran | 54-year-old man | 156 | L139L non-synonymous mutation | Mild | Fatigue, anxiety, chest pain, cough, fever | IgM and IgG were detected in the first incidence, and he was being followed up to the second virus presentation. In the whole duration between two incidences, IgG test was positive. Antibody titration at the time of second infection showed that IgG level was 5.25 IU/mL which increased to 27.5 IU/mL after about 2 weeks. |
| L139L non-synonymous mutation | Mild | Milder fatigue, chest pain, dizziness, diarrhea | ||||||
|
2021 | Iran | 42-year-old man | 111 | N/A | Mild | Shortness of breath, sore throat, shaking chills, pain, diarrhea | The IgG titration was 17.5 IU/mL which decreased to 6.5 IU/mL after almost 2 weeks. |
| D614G mutation | Mild | Similar to the first infection with severe diarrhea | ||||||
|
2021 | Austria | 95-year old man with dementia, hypertension, total thyroidectomy | 124 | N/A | Mild | Fever, leukopenia | N/A |
| Severe | Pneumonia | |||||||
|
2021 | Gambia | 31-year-old woman without comorbidities | 145 | B1 | Mild | Mild | |
| B1.1.74 | Mild | Mild | ||||||
|
2021 | Gambia | 36-year-old woman without comorbidities | 184 | B.1.235 | Asymptomatic | Asymptomatic | |
| B.1 | Worse | Mild | ||||||
|
2021 | Italy | 63-year-old healthcare man with type II diabetes, atrial fibrillation, chronic obstructive pulmonary disease | 299 | Clade 20A | Asymptomatic | Asymptomatic | |
| Clade 20E | Worse | Shortness of breath with rapid worsening of clinical presentation and recovering in intensive care unit > death | ||||||
|
2020 | Belgium | 39-year-old female immunocompetent healthcare worker | 185 | Different clades: 19A | Mild | Cough, dyspnea, headache, fever, general malaise | IgG+ |
| 20A | Milder | Dyspnea | IgM and IgG+ | |||||
|
2020 | USA | 70-year-old male with obesity, neuropathy, asthma, obstructive sleep apnea, hypertension | 7 months | N/A | Hospitalized | Worsening shortness of breath, tachypneic, mild, patchy mid and lower lung airspace disease bilaterally | SARS-CoV-2 IgG− |
| Hospitalized | Shortness of breath, fever, body aches, nausea, malaise | |||||||
|
2020 | India | 78-year-old man with coronary artery disease | 57 | N/A | Mild | Fever, cough for 2 days | N/A |
| Mild | Fever, cough, dyspnea for 1 day | |||||||
|
2021 | Ecuador | 28-year-old man | 102 | B.1.1 | Mild | Sore throat, cough, headache, nausea, diarrhea, anxiety, panic attack | IgM and IgG negative after 1st infection |
| Different in 27 nucleotides | Mild | Anosmia, ageusia, fever, headache | IgM and IgG negative after 2nd infection | |||||
|
2020 | Qatar | 57-year-old male with diabetes mellitus | 86 | N/A | Asymptomatic | Asymptomatic, screening for exposition to an infected work colleague | N/A |
| Symptomatic | Fever, myalgia, headache, productive cough | IgM and IgG+ | ||||||
|
2021 | India | 27-year-old male doctor | 66 | Lineage B.1 | Mild, 2 days of symptoms | Sore throat, nasal congestion, rhinitis | N/A |
| Lineage B with 7 differences | Mild, worse than initial (1 week) | Myalgia, fever, non-productive cough, fatigue | Abbott anti-NC IgG− on D5 of reinfection | |||||
|
2021 | India | 31-year-old male doctor | 65 | Lineage B.1.1 | Asymptomatic | Nothing | N/A |
| Lineage B.1.1 with 8SPSs in initial strain compared to reference not present in reinfection strain including D614G | Mild, worse than initial (2 days) | Myalgia, malaise | Abbott NC IgG− on D7 of reinfection | |||||
|
2021 | India | 27-year-old male doctor | 19 | Lineage B.1.1 | Asymptomatic | Asymptomatic—screening prior going home to visit parents | N/A |
| Lineage B.1.1 with 9 SNPs compared to reference not present in initial infection strain including D614G | Mild | Fever, headache, myalgia not productive cough | IgG/IgM/IgA− | |||||
|
2021 | India | 24-year-old woman nurse | 55 | Lineage B.1.1 | Mild, 5 days | Sore throat, rhinitis, myalgia | N/A |
| Lineage B.1.1 with 10SNPs compared to reference not present in initial infection strain including D614G | Mild, worse than initial—3 weeks | Fever, myalgia, rhinitis, sore throat, not productive cough, fatigue | IgG/IgM/IgA− | |||||
|
2021 | USA | 31-year-old healthcare worker man | 79 | N/A | Severe | Malaise, cough, shortness of breath, anosmia, =2 saturation to 88%, pneumonia | N/A |
| Milder | Malaise, aphthous gingival ulcer, desquamating palmar lesion, fever, myalgia | |||||||
|
2021 | USA | 69-year-old woman with asthma, hypercholesteremia, hypertension, OSA (obstructive sleep apnea) | 70 | N/A | Mild | Shortness of breath, dry cough, headache, fatigue, fevers | N/A |
| Moderate | Cough, fever, ageusia | |||||||
|
2021 | Brazil | 76-year-old woman with chronic renal failure and renal squamous cell carcinoma | 104 | 9 single nucleotide variations (SNVs) | Severe | Cough, fever, pneumonia | N/A |
| Worse | Cough, fever, pneumonia > death | |||||||
|
2021 | Brazil | 39-year-old man with chronic cardiovascular disease, diabetes mellitus | 101 | P.1 | Not reported | Not reported | N/A |
| P.2 | Worse | Dyspnea, fatigue, respiratory distress > intubated > death 12 days after the onset of symptoms | ||||||
|
2021 | France | Mid-20s healthcare worker man without comorbidities | >83 | November N/A | Asymptomatic | Asymptomatic | N/A |
| B1.351—identified in December 2020 in South Africa | Worse | Cough | ||||||
|
2021 | France | Mid-20s healthcare worker woman without comorbidities | 288 | April 2020—N/A | Mild | Fever, headache, chills, diarrhea, loss of taste and smell | N/A |
| B1.351 | Milder | Fever, headache, chills | ||||||
|
2021 | France | Late-20s healthcare worker woman without comorbidities | 90 | November 2020—N/A | Mild | Fever, muscle pain, headache, loss of taste and smell | N/A |
| B1.351 | Milder | Cough, muscle pain | ||||||
|
2020 | Brazil | 29-year-old man healthcare professional without comorbidities | 53 | N/A | Mild | Myalgia, fever | N/A |
| Mild | Fever, anosmia, loss of taste | |||||||
|
2020 | Brazil | 63-year-old man healthcare professional without comorbidities | 58 | N/A | Mild | Diarrhea, fever | N/A |
| Mild | Hypoxemia, fever | |||||||
|
2020 | Brazil | 40-year-old woman healthcare professional with ankylosing spondylitis and asthma | 70 | N/A | Moderate | Fever, Pneumonia, myalgia | Not specified |
| Mild | Anosmia, fever | |||||||
|
2020 | Brazil | 67-year-old man healthcare professional with obesity, apnea syndrome, rhinitis | 54 | N/A | Mild | Coryza, arthralgia | Not specified |
| Hospitalized with high-flow oxygen therapy | Hypoxia | |||||||
|
2020 | Brazil | 47-year-old man healthcare professional without comorbidities | 56 | N/A | Mild | Myalgia, fever | Not specified |
| Mild | Fever | |||||||
|
2020 | Brazil | 31-year-old man healthcare professional without comorbidities | 57 | N/A | Moderate | Hypoxemia, myalgia, diarrhea, fever | Not specified |
| Moderate | Hypoxemia, fever | |||||||
|
2021 | USA | Female in 20s with asthma, obesity, anxiety, depression | 19 | PANGOLIN A.3 lineage | Mild | Cough, chills, exertional dyspnea, sore throat, dizziness, rhinorrhea, fever | N/A |
| PANGOLIN B.1.1 lineage | Milder | |||||||
| Cough, fatigue, dyspnea | ||||||||
|
2021 | Iran | 15-year-old boy with acute myeloid leukemia M3 | 43 | N/A | Moderate | Cough, dyspnea, patchy infiltration in the left lung | IgG+ IgM− |
| Severe | Fever, neutropenia, cough, myalgia and shivering, O2 saturation at 75%, pneumonia | IgG− | ||||||
|
2021 | Libya | 18-year-old man | 80 | N/A | Mild | Fever, headache, sore throat, cough, shortness of breath, anosmia | IgG positive after re-infection |
| Worse | Fever, cough, muscle pain, dyspnea, hypoxia | |||||||
|
2020 | USA (Nevada) | 25-year-old man without comorbidities | 48 | Clade 20C | Mild | Sore throat, cough, headache, nausea, diarrhea | N/A |
| Clade 20C with 11SNP mutation | Severe with hospitalization | Fever, headache, dizziness, cough, nausea, diarrhea, hypoxia, shortness of breath | Roche Elecsys Anrti-SARS-CoV-2 IgM/IgG+ on D8 of reinfection | |||||
| 2020 | Hong Kong | A 33-year-old male | 142 | Nextstrain 19A/GISAID V/Pangolin lineage B.2 | Mild—hospitalized | Fever, headache, cough, sore throat | IgG negativity by ELISA or microsphere based antibody assay 10 days post symptom onset; IgG positivity but IgM negativity by indirect immunofluoresence assay; neutralizing antibody presence 10 days post-symptom onset with conventional and pseudovirus-based neutralization tests (VNTs) | |
| Nextstrain 20A/GISAID G/Rambout B.1.79; 24 nucleotides difference | Asymptomatic, systematic screening | Asymptomatic | IgG negativity by ELISA or microsphere based antibody assay 1 day post-hospitalization, but positivity at day 5; absence of neutralizing antibodies by VNTs and IgM negativity by IFI assay and CLIA 1 day post-hospitalization; then positivation on day 3; neutralizing antibody detection on day 3; IgG detection by IFon day 3; high affinity IgG | |||||
|
2021 | USA | 61-year-old man with liver transplant due to chronic hepatitis B and C infections | 111 | Genome of 2nd episode differed by 11 to 12 single base substitutions | Mild | Fever, nausea, vomiting, cough | |
| Worse | Confusion, hallucination, lethargy, hypoxia | Anti-SARS-CoV-2 assay positive after 2nd episode | ||||||
|
2021 | UK | 93-year-old British male with multiple myeloma, cognitive impairment | 55 | N/A | 14 days—hospitalized | Lethargy, reduced appetite, diarrhea | |
| Cough, fever, dyspnea | Abbott Architect SARS-CoV-2 IgG+ on D58 | |||||||
|
2021 | UK | 82-year-old British male with atrial fibrillation, congestive cardiac failure, abdominal aortic aneurism, lung cancer, diabetes | 87 | N/A | Mild—hospitalized | Fever, cough, sore throat, dyspnea, hemoptysis, hypoxia | |
| Milder | Fever, cough, dyspnea | Abbott Architect SARS-CoV-2 IgG+ on D88. 92 | ||||||
|
2020 | Brazil | 36-year-old female medical doctor without comorbidities | 87 | N/A | Moderate | Rhinorrhea, sore throat, low fever, diarrhea, asthenia, mild headache, erythematous vesicles on her right calf, severe musculoskeletal pain of the lower limbs, hyperesthesia | IgG− 23 days after the onset, IgM/IgG− after 33 and 67 days from onset |
| Worse | Nasal obstruction, hyaline rhinorrhea, sudden and complete anosmia and ageusia, frontal headache and asthenia, pneumonia | IgG+ at the 20th day | ||||||
|
2021 | USA | 44-year-old Hispanic man with type 2 diabetes mellitus, obesity | 4 months | N/A | Severe with tracheostomy | Dyspnea, stridor, difficulty at breath, | IgG+ |
| Mild | Fever, respiratory decompensation | |||||||
|
2020 | Pakistan | 41-year-old healthcare worker man | 133 | N/A | Mild | Fever, oxygen saturation of 90–92%, bilateral lung infiltrates, mild shortness of breath, loss of taste, severe restlessness, insomnia, body-aches | SARS-CoV-2 antibodies: 1.97 |
| Milder | Fever, moderate shortness of breath, loss of smell, moderate restlessness, insomnia, body aches | SARS-CoV-2 antibodies: 0.08 | ||||||
|
2020 | Belgium | 51-year-old woman with asthma | 93 | Pangolin Lineage B.1.1 | Moderate with self-quarantine for 2 weeks | Headache, myalgia, fever, cough, chest pain, dyspnea; some persisting symptoms for 5 weeks | N/A |
| Lineage A; 11 nucleotide differences | Milder with resolution in 1 week | Headache, cough, fatigue, rhinitis | Roche nucleocapsid IgG+ on D7 of reinfection | |||||
|
2021 | Switzerland | 36-year-old female physician | 205 | Clade 20A | Mild | Asthenia, headache, slight memory loss | Positivity for anti-S1 IgG and anti-N Ig at 14th and at 30th days |
| Clade 20A.EU2 with non-synonymous mutation in the S (S477N) | Mild | Asthenia, shivering, rhinorrhea, anosmia, arthralgia, headache, exertional dyspnea for 10 days | Positivity for anti-S1 IgG and anti-N Ig | |||||
|
2021 | India | 58-year-old woman with hypertension, hypothyroidism | 120 | N/A | Mild | Fever, generalized body ache, running nose, soreness of throat | Total antibody and immunoglobulin G antibody test for COVID-19 were negative after first infection |
| Milder | Fever, generalized body ache, dry cough, throat pain | |||||||
|
2021 | India | 58-year-old woman with hypertension and hypothyroidism | 91 | N/A | Mild | Low-grade intermittent fever, generalized body ache, running nose and soreness of throat | N/A |
| Mild | Intermittent fever, generalized body ache, dry cough, and throat pain | |||||||
|
2021 | UK | 25-year-old male UK doctor | 17 | N/A | Mild | High-grade fevers, headache of 3-day duration, severe fatigue lasting 3 weeks | N/A |
| Milder | Fatigue, coryzal symptoms for 4 days | Rest at home | ||||||
|
2021 | USA | 25-year-old female medical student with vitiligo | 120 | N/A | Asymptomatic | Asymptomatic | IgG+ |
| Severe | Fever, abdominal pain, fatigue, vomiting and fulminant myocarditis with co-infection of parvovirus and SARS-CoV-2 | N/A | ||||||
|
2021 | Brazil | 41-year-old woman with gastroplasty history | 146 | B.1.1.33 lineage | Mild | Headache, myalgia, fatigue, fever, dry cough, shortness of breath, anosmia, loss of taste | N/A |
| B.1.1.28 lineage | Mild | Headache, myalgia, fatigue, fever, dry cough, shortness of breath, anosmia, loss of taste, diarrhea, loss of appetite, dizziness | ||||||
|
2021 | Brazil | 34-year-old healthcare worker woman with chronic respiratory disease | 173 | B.1.1.28 lineage | Mild | Fever, cough, odynophagia, dyspnea | N/A |
| P2 | Mild | Headache, running nose, fever, sore throat | ||||||
|
2021 | Iran | 50-year-old man | 230 | N/A | N/A | N/A | N/A |
|
2021 | Iran | 81-year-old woman | 234 | N/A | Moderate | N/A | N/A |
| Worse | Death for COVID-19 | |||||||
|
2021 | Iran | 42-year-old woman | 107 | N/A | N/A | N/A | N/A |
|
2021 | Iran | 27-year-old man | 115 | N/A | N/A | N/A | N/A |
|
2021 | Iran | 79-year-old man | 150 | N/A | Moderate | N/A | N/A |
| Worse | Death for COVID-19 | |||||||
|
2021 | Iran | 86-year-old man | 164 | N/A | Moderate | N/A | N/A |
| Worse | Death for COVID-19 | |||||||
|
2021 | Iran | 90-year-old woman | 130 | N/A | N/A | N/A | N/A |
|
2021 | Iran | 13-year-old woman | 124 | N/A | N/A | N/A | N/A |
|
2020 | China | 33-year-old female | 59 | N/A | Moderate—hospitalized for 16 days | Reduction of IgG+ to − | |
| Moderate | IgG+ | |||||||
|
2020 | China | 33-year-old female | 86 | N/A | Severe—hospitalized for 38 days | Reduction of IgG+ to weak+ | |
| Moderate | IgM+ and IgG+ | |||||||
|
2021 | South African | 58-year-old male with asthma | 120 | N/A | Mild | Dyspnea, fever | IgG+ |
| South African variant 501Y.V2 | Severe with intubation and mechanical ventilation | Dyspnea, fever, severe acute respiratory distress syndrome |
* data from papers which are not certified by peer review, medRxiv or Research Square preprints. ** days from recovery not from 1 infection. NAAT: nasopharyngeal nucleic acid amplification test; AT: antibody test.
3.1. Demographic and Clinical Features of Reinfection Cases
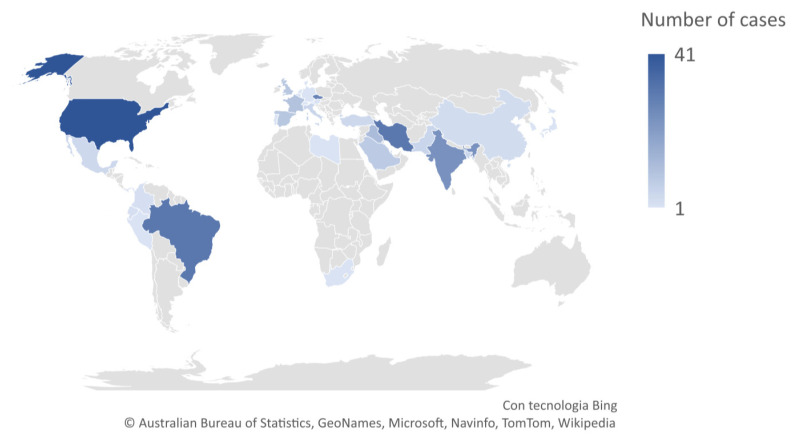
Reinfection occurred across the world: 1 case from Austria, 1 from Bahrain, 5 from Bangladesh, 2 from Belgium, 31 from Brazil, 3 from China including 1 from Hong Kong, 2 from Colombia, 28 from the Czech Republic, 1 from Denmark, 2 from Ecuador, 10 from France, 2 from Gambia, 1 from Germany, 24 from India, 31 from Iran, 12 from Iraq, 1 from Israel, 5 from Italy, 1 from Japan, 1 from Lebanon, 1 from Libya, 4 from Mexico, 5 from Pakistan, 1 from Panama, 1 from Peru, 1 from Portugal, 6 from Qatar, 1 from South Korea, 1 from Switzerland, 8 from Saudi Arabia, 1 from South Africa, 9 from Spain, 1 from the Netherlands, 4 from Turkey, 9 from the United Kingdom, 42 from the United States of America (Figure 2).
Figure 2.
Distribution of cases worldwide.
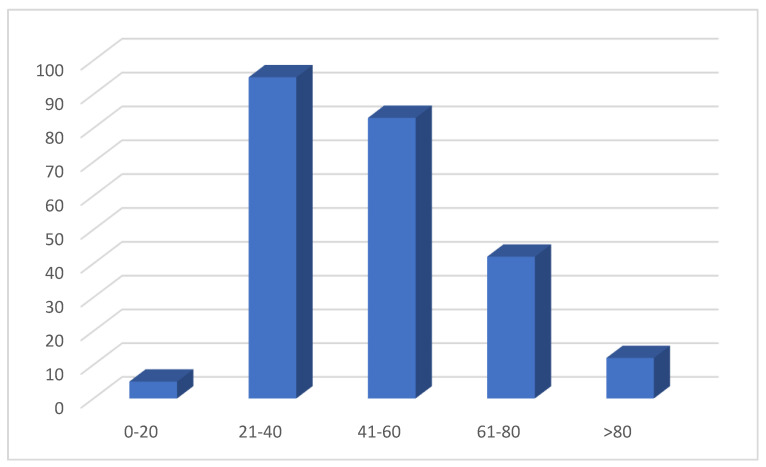
Age was reported in 237 cases: 5/237 patients (2.1%) were between 0 and 20 years old, 95/237 (40%) between 21 and 40 years old, 83/237 (35%) between 41 and 60, 42/237 (17%) between 61 and 80, and 12/237 (5%) > 80 years old (Figure 3).
Figure 3.
Distribution of cases according age.
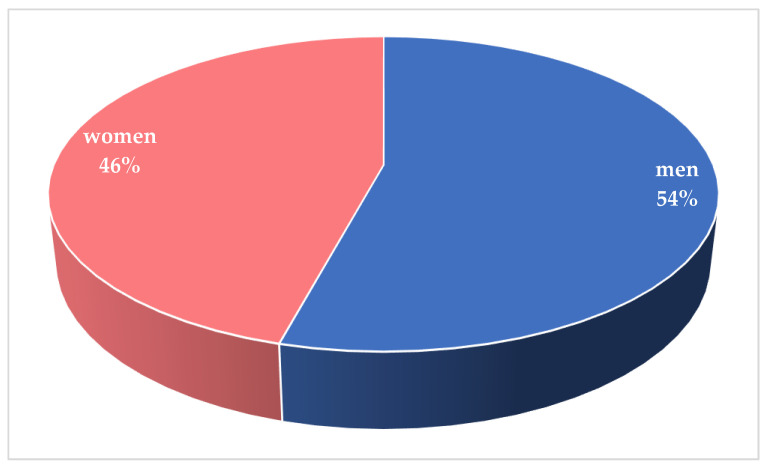
Gender was reported in 251/260 cases, among which 115/251 patients (45.8%) were female and 136/251 (54.2%) were male (Figure 4).
Figure 4.
Distribution of cases according sex.
The main risk groups were healthcare workers and patients with comorbidities. In total, 66/260 cases (2.3%) occurred among high risk groups, including healthcare workers (HCWs), doctors, students and nursing resident. A total of 91 cases (35%) occurred among patients with comorbidities, 48 in men and 38 in woman (Figure 5).
Figure 5.
In total, 60% of reinfection involved patients in risk groups.
The evolution of the reinfection episode itself was more severe in 92/260 (35.3%) cases with the death only in 14/260 cases (5.3%), 7/260 male (2.65%) and 7/260 females (2.65%); 8 of these had a neoplastic immune system diseases, or transplant or other important comorbidities and 3 were over 80 years old (Figure 6).
Figure 6.
The evolution of the reinfection episode was more severe in 35.3% of cases.
Notably, reinfection occurred among patients whose initial infections were both asymptomatic/mild, 80% (207/260), and moderate/severe, 20% (53/260). The demonstration that moderate/severe initial infections do not necessarily provide enhanced protection against reinfection is important because patients with more severe infection have been found to have higher neutralizing antibody titers, which may be expected to confer protection. Additionally of note, the severity of the reinfection episode itself was less in 21/53 cases (40%). The observation that many reinfection cases were less severe than initial cases is interesting because it may suggest partial protection from disease [152] and argues against antibody-dependent immune enhancement, which can be seen with other viral pathogens. In the absence of routine surveillance, we would have expected a bias toward detection of symptomatic reinfection, underscoring the importance of prospective screening.
Another interesting datapoint is the detection of different clades or lineages detected by genome sequencing between initial infection and reinfection in 52/260 cases (20%). The current gold standard for identifying reinfection is detection of a distinct virus by genome sequencing. Detection of reinfection is most straightforward when viruses belong to a different clade or lineage, as this provides clear evidence of infection by a different virus [6]. Although reinfection is most apparent when viruses are different enough to distinguish by genome sequencing, it remains unclear whether these viral genomic differences play a causative role in reinfection. That is, does reinfection occur when viral genomic differences permit escape from an existing, but narrow, immune response to the initial infection? Answering this question will require detailed mapping of the relationship between virus substitutions and immune escape.
3.2. Quality and Risk of Bias Assessment
Briefly, only 14 studies fulfilled the quality checklist. “Selection—Does the patient(s) represent(s) the whole experience of the investigator (center) or is the selection method unclear to the extent that other patients with similar presentation may not have been reported?” checklist resulted unclear in most of the studies, because the patient selection method was unclear. In general, overall quality was satisfactory in all included studies.
4. Discussion
Since the first cases, a question has haunted all researchers: can a patient recovered from COVID-19 get sick again? The first confirmed case of reinfection occurred in a 33-year-old Caucasian man of Hong Kong, that was admitted to the hospital for COVID-19 on 23 March 2020 [5]. After two negative tests by RT-PCR on days 21 and 22 he was discharged from the hospital and resumed his usual work [5]. Serological controls after the first infection showed that he did not produce virus neutralizing antibodies [139]. On 15 August 2020 after a 1-week trip in Spain, the patient returned to Hong Kong and was submitted to a collection of a deep throat saliva sample for RT-PCR as border surveillance and resulted positive [5]. The patient was asymptomatic until the new negative test. The viruses from the first and the second infection were phylogenetically distinct and the virus of first infection had a truncation in the 58AA open reading frame 8 gene, that could be responsible immune evasion [138]. However T cells and mucosal immunity might have played an important role in resolving the second infection, even if there was the absence of primary neutralizing antibodies [139].
In October 2020, Tillett et al. reported the first confirmed case of SARS-CoV-2 reinfection in the USA [27]. A 25-year-old man from Nevada, without known immune disorders, had PCR-confirmed SARS-CoV-2 infection in April, 2020 (cycle threshold (Ct) value 35·24; specimen A) [27]. He recovered in quarantine, testing negative by RT-PCR at two consecutive timepoints thereafter [27]. However, 48 days after the initial test, the patient tested positive again by RT-PCR (Ct value 35·31; specimen B) [27]. Viral genome sequencing showed that both specimens A and B belonged to clade 20C, a predominant clade seen in northern Nevada [27]. The genome sequences of isolates from the first infection (specimen A) and reinfection (specimen B) differed significantly, making the chance of the virus being from the same infection very small [27]. The particularity of this report is that SARS-CoV-2 reinfection resulted in worse disease than the first infection, requiring oxygen support and hospitalization [27]. The patient had positive antibodies after the reinfection, but whether he had pre-existing antibody after the first infection is unknown [27]. Both cases reported from Nevada and Hong Kong seem to confirm the possibility that the reinfections are due to a different variant of SARS-CoV-2.
The first important question to be answered is: are all cases reported in the literature as reinfection by SARS-COV-2 true reinfections?
A distinction must be made between true reinfection, relapsed infection, recurrence of positive (re-positive) nucleic acid detection [17,153], in fact one of the features of SARS-CoV-2 infection is prolonged virus shedding. Several studies reported persistent or recurrent elimination of viral RNA in nasopharyngeal samples starting from first contact with a positive subject [18,19,20]. Several explanations can exist in order to explain this phenomenon without it being a true reinfection. One possible explanation for testing positive after a previously negative result could be that the negative results after patient recovery were really false-negative results [154]. Literature reported that false-negative rates can be as high as 30% for SARS-CoV-2 PCR testing [155]. However, actually the KCDC (Korean Control Disease Center) determined recovery as two separate negative PCR results within 24 h [156]. In this way, patients positive after having two consecutive negative results would be positive for an increase in viral genetic material due to reinfection [156]. It is difficult to have two previous consecutive false-negative results [156]. Another possible explanation could be the contamination of the samples, but most testing centers are requiring testers to change personal protective equipment (e.g., gloves, gowns and masks) [156]. However, surely one of the main points to consider is the basis of PCR testing: the test is able to amplify nucleic acid in the sample, not fully active viral particles. The genetic material (RNA and DNA) left behind degrades over time [157]. Thus, positive PCR results after recovery may not necessarily signify reinfection, but rather the presence of leftover genetic material from previously active infection [156]. Therefore, a patient who retests positive for virus might not necessarily be experiencing a second, new SARS-CoV-2 infection [158]. True reinfection has criteria that must be considered, including isolation of the complete genome of the virus (and not just genomic fragments) in the second episode, identification of two different virus strains in two episodes of infection based on phylogenetic analysis; proof of virus infectivity in the second episode by virus isolation and evaluation of its cytopathic effect in cell culture; investigation of immune responses and their comparison in two episodes; epidemiologic data such as re-exposure history to COVID-19 patient in the second event and timing between episodes, with a longer time interval between two episodes favoring the reinfection hypothesis [17,159]. To date, positive retesting more than 83 days after the first positive test, along with other criteria, favors confirmation of reinfection, even if Turner et al. recently reported a patient with prolonged viral RNA shedding lasting 87 days after the initial positive clinical PCR test and 97 days after the onset of symptoms, probably due to the poor CD8+ T cell response during the first three months of his illness [160]. In addition to the abovementioned reasons, the disease clinical data are also useful in confirming the second episode, although the second episode may be asymptomatic [17]. A time interval where the patient is free of clinical signs between the two episodes is also necessary. In conclusion, only cases with clinical symptoms and RT-PCR positivity after negative tests following recovery from COVID-19 could be considered true SARS-CoV-2 reinfections. Recently Raveendran et al. suggested an interesting approach in order to individuate the reasons for a persistent RT-PCR positivity (Figure 7) [161]. According to this flow chart it is possible to individuate cases of persistent RT-PCR positivity due to reinfection or to presence of dead viral fragment or to persistent viral shedding.
Figure 7.
Flow diagram in order to determine the cause of persistent RT-PCR positivity for SARS-CoV-2, modified by Raveendran, A.V. et al. [161].
The second important question to be answered is: can SARS-CoV-2 re-infect a patient after recovery?
When any unwanted virus comes into contact with our body, also in the case of SARS-CoV-2 infection, most patients are able to develop specific antibodies neutralizing the spike proteins of this virus [5]. A recent study of Pilz et al. pointed out that the relatively low tentative reinfection rate (40 cases in 14,840 COVID-19 survivors of first wave—0.27%) ensures a good protection after natural infection for SARS-CoV-2 [162]. However there are three main mechanisms for reinfection: the immune response can be ineffective, strain-specific, or short-lived [156].
Monoclonal antibodies formed against the SARS-CoV-2 virus target the Spike (S) glycoprotein component, the receptor-binding domain of the virion [156]. SARS-CoV-2, however, has been shown to develop “escape mutants,” or alterations, in the epitope of the S protein that contribute to host tropism and viral virulence [156]. Sui et al. reported that major variations exist in the S protein at positions 360, 479, and 487 [163]. They found that altering 1–2 amino acids at those positions led previously efficacious neutralizing antibodies to SARS-CoV-2 to a 20–50% reduction in binding capacity [163]. Theoretically, if SARS-CoV-2 is also able to form “escape mutants” in the S protein, IgG antibodies formed in patients may be less ineffective, though not completely, in neutralizing the virus [156]. This could mean that patients remain resistant to SARS-CoV-2 infection even after mutations, with antibody responses that are 50–80% efficacious [156].
Another possibility that could allow the reinfection of a patient is the duration of the body immune response [156]. Recent findings suggested that protective immunity does not occur in all infected individuals [164], supporting the possibility of reinfection [103], even if 93% of the infected produce neutralizing antibodies [165]. Their function is to prevent the virus from entering cells between 6 and 20 days after infection [166] with this mechanism: after the infection, B lymphocytes are activated and produce IgM, IgG and IgA antibodies. A subset of them (IgG and IgA) then manage to make the new viral particles harmless. The neutralizing antibodies, in turn, are accompanied by the activation of killer cells (T lymphocytes), specialized in recognizing and destroying the virus [167].
Seroconversion of IgM and IgG antibodies occurs the first week after onset of symptoms, seroconversion rates rise until the fourth week and decline thereafter, by the seventh week IgM antibodies are not detected in most cases, even if some reports showed IgM antibodies to persist for up to 8 months post-COVID-19 [168], whereas IgG antibodies persist longer for a period of time yet unknown [169]. Immunoglobulins alone are not truly sufficient to confer long-term immunity to coronavirus [156]. CD4+ T-cells and memory CD8+ T-cells with their products, such as effector cytokines and IFN-γ, are important in providing protection from coronavirus [170]. In fact, when the infection is over, in the following weeks or months, the antibodies drop: the virus is no longer there, they are no longer needed. However, the memory cells remain in the body, ready to intervene in case of need. All the studies so far show that a long-lasting immune response occurs. A very recent study carried out in collaboration between the Policlinico San Matteo in Pavia and the Karolinska Institute in Stockholm quantifies this “time” more precisely: memory cells persist for at least 6–8 months after infection [171]. Considering that the disease erupted just under a year ago, this is the maximum observation time possible to date, but it could be much longer [171]. Previous studies showed that virus-specific memory CD8+ T-cells were found to persist for up to 6 years after a SARS associated coronavirus infection, but memory B-cells and accompanying antibodies were undetectable at that time [172]. However Vetter et al. hypothesized that reinfection can be due to a loss of protection elicited after the first episode for a progressive reduction of protective antibody titers [144,173].
We can conclude that antibody formation and longevity of immunity in a subject could be dependent by the strain of virus, its severity and age of subject [174].
Khoshkam et al. tried to classify the recovered and immunized subjects in four categories:
-
(1)
Infected cases with very mild symptoms or asymptomatic without any humoral immune response or elicited memory.
-
(2)
Infected cases with mild to moderate symptoms with low humoral immunity and low cellular immunity.
-
(3)
Infected cases with moderate or severe symptoms with highly activated humoral immunity and elicited memory.
-
(4)
Infected cases with moderate or severe symptoms with highly activated humoral immunity and low cellular immunity [175].
They hypothesized that reinfection may happen in groups 1 and 2, which may also develop the severe disease in the future due to the absence or low levels of acquired immunity [175]. Individuals in group 3 are more protective against further exposures and they may show long-term immunity since they develop increased elicited memory in defense of SARS-CoV-2 [175]. The last group may show rapid response against reinfection; they may not be safe for longer periods because of the non-imprinted memory of immunity [175].
The question to be solved is whether these antibodies can neutralize each SARS-CoV-2 clade and guarantee immunity to subsequent contact. Reinfection from SARS-CoV-2 with a genetically distinct strain of SARS-CoV-2 is, in theory, possible in patients immediately after recovery from COVID-19. SARS-CoV-2 infection may not confer immunity against a different SARS-CoV-2 strain, so more research is needed. SARS-CoV-2, even if it is a virus similar to that of the flu, seems to have a more stable genome and the response that the immune system generates is towards several fragments of the viral proteins and not just one. In fact, the mutations observed so far (and, perhaps, also the new English variant, at least until proven otherwise) are not associated with a change in the severity of the disease.
The new variants are accumulating mutations in different spike domains, such as the alpha variant or B.1.1.7 lineage (also known as 501Y.V1 or VOC202012/01), the beta variant or B.1.351 lineage (501Y.V2), the gamma variant or P.1 lineage (501Y.V3) and the delta variant or B.1.617.2 lineage [176]. All these variants have cumulated at least nine non-synonymous mutations/deletions throughout the Spike coding region. For example, the case reported by Harrington et al. showed that anti-SARS-CoV-2 antibodies were still present shortly before onset of reinfection, with no evidence of antibody waning [82]. This may raise some concerns about immune evasion by the alpha variant, which is a concern with the high number of spike region mutations seen. However, the study has a bias: there were no assays for SARS-CoV-2 antibodies recognizing spike antigen in the second reinfection, while the tested antibodies recognized “N” antigen, so it is difficult to point out an evident role of antibodies in the reinfection. The 501Y.V2 variant, or beta variant, is characterized by eight mutations in the spike protein-coding sequences that can improve its ability to transmission [151]. The case reported by Zucman et al. showed that beta variant can be more aggressive than non-VOC SARS-CoV-2 [151]. The last, the delta variant, is characterized by P681R and L452R mutations that can help the delta variant spread. For all these reasons it is necessary to investigate urgently the possibility of these new variants to escape the vaccine action. The immune responses generated by mRNA and adenoviral vector-based vaccines are restricted to the Spike glycoprotein, so new variants with big antigenic drift could reduce their efficiency and determine a growing number of reinfections.
Another possibility that could allow the reinfection of a patient is the reactivation of dormant virus which is commonly seen in immunosuppressed patients with some viruses, such as Epstein Barr, cytomegalovirus and herpes groups [90], but it is necessary to sequence viral genome for differential diagnosis between viral reactivation or reinfection with a different strain.
For all these reasons, it is important to identify cases of reinfection to understand if the “immunological memory” affects the symptoms during a second infection, a crucial fact, in particular, to predict the effectiveness of the vaccination campaign. If in the second time the symptoms are generally reduced, as in the Hong Kong [5], Belgium and the Netherlands [29] patients, this suggests that the immune system is responding as it should. However, if symptoms are consistently more severe during a second COVID-19 attack, as in the case of the Nevada [27] or Ecuador [28] subjects, it may be that the immune system makes matters worse. The mechanisms that could account for a more severe secondary infection can only be speculated. First, a very high dose of virus might have led to the second instance of infection and induced more severe disease [177]. Second, it is possible that reinfection was caused by a more virulent variant of the virus, or more virulent in this patient’s context [27]. Third, a mechanism of antibody-dependent enhancement might be the cause, a means by which specific Fc-bearing immune cells become infected with virus by binding to specific antibodies [27]. In fact, the clinical course of some severe COVID-19 cases has been worsened by abnormal immune responses that damage healthy tissue. Patients who experienced that problem during a first infection may have immune cells that are induced to respond disproportionately the second time too. Sometimes antibodies produced in response to SARS-CoV-2 can facilitate the virus during a second infection rather than fight it [178,179,180,181,182,183,184]. The phenomenon [185,186,187,188,189] is rare, but researchers have found worrying signs of it while trying to develop vaccines against the coronaviruses responsible for severe acute respiratory syndrome and Middle East respiratory syndrome [190] and against SARS-CoV-2 [191,192,193,194].
As researchers accumulate more examples of reinfection, the situation should become clearer. Depending on the criteria used, rates of reinfection can vary widely [195]. There are some reports about retrospective observational study such as that of Pilz et al. that reported 40 cases of tentative reinfection in Austria, but these data are limited by the lack of detailed clinical characteristics [162]. For this reason, in November 2020 the Centers for Disease Control and Prevention pointed out the following criteria to define reinfection with SARS-CoV-2: detection of SARS-CoV-2 RNA (with Ct values < 33 if detected by RT-PCR) >90 days after the first detection of viral RNA whether or not symptoms were present and paired respiratory specimens from each episode that belong to different clades of virus or have genomes with >2 nucleotide differences per month [32]. Cases in which detection of SARS-CoV-2 RNA is present >45 days to 89 days apart are considered reinfections if the second symptomatic episode had no obvious alternate explanation for the COVID-19-like symptoms or there was close contact with a person known to have laboratory diagnosed COVID-19 and paired specimens are available with the Ct values and sequence diversity noted above.
However, the ability to re-infect does not mean that a SARS-CoV-2 vaccine cannot be effective. Some vaccines, for example, require a “booster” dose to maintain protection. Learning more about reinfection could help researchers in developing truly effective vaccines by showing them which immune responses are important for maintaining immunity. For example, researchers may find that people become vulnerable to reinfection after antibodies drop below a certain level, and so they can modify vaccination strategies accordingly using a booster dose to maintain that level of antibodies. At a time when health authorities are grappling with the dizzying logistical difficulties of vaccinating the world population against SARS-CoV-2, the need for a booster injection is a necessity that complicates the management of the vaccination campaign, but it does not make long-term immunity from SARS-CoV-2 impossible. However, some researchers fear that vaccines will only reduce symptoms during a second infection, rather than prevent it altogether. While giving some advantages, this possibility could turn vaccinated individuals into asymptomatic carriers of SARS-CoV-2, putting vulnerable populations at risk. The elderly, for example, are among the most affected by COVID-19, but they tend not to respond well to vaccines. For all these reasons, it would be interesting to see data on how much virus SARS-CoV-2 reinfected individuals spread.
The real problem to be solved is, therefore, the duration of immunity conferred by a COVID-19 episode. There is evidence in the literature that the COVID-19 immune response is variable and patient-specific with respect to the development of antibodies and to antibody persistence in serum over time [146]. In considering the protective effect of antibodies against a reinfection, the evidence is still inadequate, and more research is necessary in order to clarify the interplay between the roles of adaptive and innate immunity. A recent study of Gudbjartsson et al. reported that Icelandic humoral response to SARS-CoV-2 infection was persistent within the 120-day timeframe used with a modest decline in antibody titers after 120 days [196]. Iyer et al. observed declining antibody titers over 90 days, with “median times to sero-reversion of 71 and 49 days following symptom onset” [197].
The genetic analysis of all the new cases reported as reinfection would help in understanding if the reinfection would be due to a new infection by a different SARS-CoV-2 or a reinfection by the same virus for a decline of immune response, but unfortunately genomic analysis is not available for some of these cases.
5. Conclusions
All these findings are useful and contribute towards the role of vaccination in response to the COVID-19 infections. Collected data show a wide range of situations: spanning a broad distribution of ages, risk groups, baseline health status and reinfection severity compared to the initial infection. Reinfection occurred as early as 45 days or >300 days after the initial infection. Common explanations for reinfection can be either waning SARS-CoV-2 antibodies or the presence of viral escape mutations [198]. While several cases of SARS-CoV-2 reinfection did involve infection with a different clade, it is noteworthy that mutations were identified throughout the genomes and the frequency of mutations within the S gene was not elevated relative to the rest of the genome [199]. In addition, individuals with more severe reinfections did not have significantly greater frequency of S gene mutations [199]. Finally, the presence of rare mutations was uncommon in the re-infecting virus, which largely mirrored the contemporaneously circulating variants in the region of infection, as reported by Choudhary et al. [199]. Concerning the problem of recognizing reinfection and persistent infection, two factors generally differentiated them. First, reinfections have so far been largely described in immunocompetent individuals while the majority of persistent COVID cases have been in immunosuppressed patients [199]. Secondly, phylogenetic analysis can generally differentiate between reinfection and persistent infection, especially in cases where persistent infection allowed the longitudinal collection of >2 sequences [199]. Due to the reinfection cases with SARS-CoV-2, it is evident that the level of immunity is not 100% for all individuals. Reinfection with SARS-CoV-2 is a possibility in both vaccinated and unvaccinated individuals, because vaccines to the virus may not translate to total immunity [199]. Recently breakthrough infections were reported following mRNA vaccination in healthy subjects [200,201], despite evidence of effective immune response among the breakthrough subjects [202]. Another study reported that eight symptomatic SARS-CoV-2 infections occurred in fully vaccinated healthcare workers (incidence rate 4.7 per 100,000 person-days adjusted) [203]. This type of challenge was also observed during the process of vaccine preparation for influenza [204]. Even though several vaccines are ready, the presence of more than 80 genotypical variants of the virus, possibility of reinfection, and short duration of seropositivity for neutralizing antibodies raise the concern that vaccination may not result in an effective and long-term immunity against SARS-CoV-2. Furthermore, immunoglobulin levels may not correlate with viral shedding and risk of transmissibility of SARS-CoV-2 [205]. Additionally, the short duration of immunity against the virus may not allow for increasing homogeneity of affected populations in a non-specific time frame. These factors raise concerns that eliminating the COVID-19 pandemic may not be as feasible as once assumed and that we must rely more on prevention of transmission until more aspects of the virus and its pathogenicity are discovered. A recent study suggested that among persons with previous SARS-CoV-2 infection, full vaccination provides additional protection against reinfection [206]. In fact, among previously infected Kentucky residents, those who were not vaccinated were more than twice as likely to be reinfected compared with those with full vaccination [206]. Data from literature are comforting: out of hundreds of millions of people infected with the virus and then cured, only a few are reported cases of confirmed reinfection [199]. Despite the appearance of different variants of the virus, vaccines seem to help us for the near future. However, the presence of immunosuppressed or transplanted subjects requires us to continue to observe the precautionary rules useful to prevent the spread of the virus. In fact, it is imperative that all individuals, whether previously diagnosed with COVID-19 or not should take identical precautions to avoid reinfection with SARS-CoV-2 till the time when community immunity had been achieved [207]. All eligible persons should be offered vaccination, including those with previous SARS-CoV-2 infection, to reduce their risk for future infection [206].
This report highlights how it is necessary to continue to observe all the prescriptions recently indicated in the literature [208,209,210] in order to avoid new contagion for all patients after healed from COVID-19 or asymptomatic positive, since the infection does not ensure complete immunity in 100% of cases.
Author Contributions
Conceptualization, L.L.M.; methodology, L.L.M.; validation, E.L.M. and M.A.; formal analysis, M.A.; investigation, M.F.A.Q.; resources, L.L.M.; data curation, L.L.M.; writing—original draft preparation, L.L.M., M.A., E.L.M.; writing—review and editing, M.F.A.Q.; visualization, M.A.; supervision, L.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Rolling Updates on Corona Virus Disease (COVID-19). Health Emergencies. 2020. [(accessed on 1 October 2021)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen.
- 4.John Hopkins University COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) [(accessed on 17 October 2021)]. Available online: https://coronavirus.jhu.edu/map.html.
- 5.To K.K., Hung I.F., Ip J.D., Chu A.W., Chan W.M., Tam A.R., Fong C.H., Yuan S., Tsoi H.W., Ng A.C., et al. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin. Infect. Dis. 2020:ciaa1275. doi: 10.1093/cid/ciaa1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babiker A., Marvil C.E., Waggoner J.J., Collins M.H., Piantadosi A. The Importance and Challenges of Identifying SARS-CoV-2 Reinfections. J. Clin. Microbiol. 2021;59:e02769-20. doi: 10.1128/JCM.02769-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edridge A.W.D., Kaczorowska J., Hoste A.C.R., Bakker M., Klein M., Loens K., Jebbink M.F., Matser A., Kinsella C.M., Rueda P., et al. Seasonal coronavirus protective immunity is short-lasting. Nat. Med. 2020;26:1691–1693. doi: 10.1038/s41591-020-1083-1. [DOI] [PubMed] [Google Scholar]
- 8.Kiyuka P.K., Agoti C.N., Munywoki P.K., Njeru R., Bett A., Otieno J.R., Otieno G.P., Kamau E., Clark T.G., van der Hoek L., et al. Human Coronavirus NL63 Molecular Epidemiology and Evolutionary Patterns in Rural Coastal Kenya. J. Infect. Dis. 2018;217:1728–1739. doi: 10.1093/infdis/jiy098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glezen W.P., Taber L.H., Frank A.L., Kasel J.A. Risk of primary infection and reinfection with respiratory syncytial virus. Am. J. Dis. Child. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 10.Perera R.A., Mok C.K., Tsang O.T., Lv H., Ko R.L., Wu N.C., Yuan M., Leung W.S., Chan J.M., Chik T.S., et al. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Eurosurveill. 2020;25:2000421. doi: 10.2807/1560-7917.ES.2020.25.16.2000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hachim A., Kavian N., Cohen C.A., Chin A.W.H., Chu D.K.W., Mok C.K.P., Tsang O.T.Y., Yeung Y.C., Perera R., Poon L.L.M., et al. ORF8 and ORF3b antibodies are accurate serological markers of early and late SARS-CoV-2 infection. Nat. Immunol. 2020;21:1293–1301. doi: 10.1038/s41590-020-0773-7. [DOI] [PubMed] [Google Scholar]
- 12.Addetia A., Crawford K.H.D., Dingens A., Zhu H., Roychoudhury P., Huang M.L., Jerome K.R., Bloom J.D., Greninger A.L. Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. J. Clin. Microbiol. 2020;58:e02107-20. doi: 10.1128/JCM.02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma R., Sardar S., Mohammad Arshad A., Ata F., Zara S., Munir W. A Patient with Asymptomatic SARS-CoV-2 Infection Who Presented 86 Days Later with COVID-19 Pneumonia Possibly Due to Reinfection with SARS-CoV-2. Am. J. Case Rep. 2020;21:e927154. doi: 10.12659/AJCR.927154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azkur A.K., Akdis M., Azkur D., Sokolowska M., van de Veen W., Bruggen M.C., O’Mahony L., Gao Y., Nadeau K., Akdis C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75:1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J., Hu J.L., Xu W., Zhang Y., Lv F.J., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 16.Amikishiyev S., Demir E., Aghamuradov S., Garayeva N., Artan A.S., Gul A., Turkmen A. Reinfection with SARS-CoV-2 in a kidney transplant recipient. Transpl. Infect. Dis. 2021;23:e13695. doi: 10.1111/tid.13695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falahi S., Kenarkoohi A. COVID-19 reinfection: Prolonged shedding or true reinfection? New Microbes New Infect. 2020;38:100812. doi: 10.1016/j.nmni.2020.100812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao A.T., Tong Y.X., Gao C., Zhu L., Zhang Y.J., Zhang S. Dynamic profile of RT-PCR findings from 301 COVID-19 patients in Wuhan, China: A descriptive study. J. Clin. Virol. 2020;127:104346. doi: 10.1016/j.jcv.2020.104346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lan L., Xu D., Ye G., Xia C., Wang S., Li Y., Xu H. Positive RT-PCR Test Results in Patients Recovered From COVID-19. JAMA. 2020;323:1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gombar S., Chang M., Hogan C.A., Zehnder J., Boyd S., Pinsky B.A., Shah N.H. Persistent detection of SARS-CoV-2 RNA in patients and healthcare workers with COVID-19. J. Clin. Virol. 2020;129:104477. doi: 10.1016/j.jcv.2020.104477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention Common Investigation Protocol for Investigating Suspected SARS-CoV-2 Reinfection. [(accessed on 30 December 2020)]; Available online: https://www.cdc.gov/coronavirus/2019-ncov/php/reinfection.html.
- 22.Qureshi A.I., Baskett W.I., Huang W., Lobanova I., Naqvi S.H., Shyu C.R. Re-infection with SARS-CoV-2 in Patients Undergoing Serial Laboratory Testing. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leidi A., Koegler F., Dumont R., Dubos R., Zaballa M.E., Piumatti G., Coen M., Berner A., Darbellay Farhoumand P., Vetter P., et al. Risk of reinfection after seroconversion to SARS-CoV-2: A population-based propensity-score matched cohort study. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breathnach A.S., Riley P.A., Cotter M.P., Houston A.C., Habibi M.S., Planche T.D. Prior COVID-19 significantly reduces the risk of subsequent infection, but reinfections are seen after eight months. J. Infect. 2021;82:e11–e12. doi: 10.1016/j.jinf.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bongiovanni M., Marra A.M., Bini F., Bodini B.D., Carlo D.D., Giuliani G. COVID-19 reinfection in healthcare workers: A case series. J. Infect. 2021;82:e4–e5. doi: 10.1016/j.jinf.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vitale J., Mumoli N., Clerici P., De Paschale M., Evangelista I., Cei M., Mazzone A. Assessment of SARS-CoV-2 Reinfection 1 Year After Primary Infection in a Population in Lombardy, Italy. JAMA Intern. Med. 2021;181:1407–1408. doi: 10.1001/jamainternmed.2021.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tillett R.L., Sevinsky J.R., Hartley P.D., Kerwin H., Crawford N., Gorzalski A., Laverdure C., Verma S.C., Rossetto C.C., Jackson D., et al. Genomic evidence for reinfection with SARS-CoV-2: A case study. Lancet Infect. Dis. 2021;21:52–58. doi: 10.1016/S1473-3099(20)30764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prado-Vivar B., Becerra-Wong M., Guadalupe J.J., Marquez S., Gutierrez B., Rojas-Silva P., Grunauer M., Trueba G., Barragan V., Cardenas P. A case of SARS-CoV-2 reinfection in Ecuador. Lancet Infect. Dis. 2021;21:e142. doi: 10.1016/S1473-3099(20)30910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Elslande J., Vermeersch P., Vandervoort K., Wawina-Bokalanga T., Vanmechelen B., Wollants E., Laenen L., Andre E., Van Ranst M., Lagrou K., et al. Symptomatic Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Reinfection by a Phylogenetically Distinct Strain. Clin. Infect. Dis. 2021;73:354–356. doi: 10.1093/cid/ciaa1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldman J.D., Wang K., Roltgen K., Nielsen S.C.A., Roach J.C., Naccache S.N., Yang F., Wirz O.F., Yost K.E., Lee J.Y., et al. Reinfection with SARS-CoV-2 and Failure of Humoral Immunity: A case report. medRxiv. 2020 doi: 10.1101/2020.09.22.20192443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein J., Brito A.F., Trubin P., Lu P., Wong P., Alpert T., Pena-Hernandez M.A., Haynes W., Kamath K., Liu F., et al. Case Study: Longitudinal immune profiling of a SARS-CoV-2 reinfection in a solid organ transplant recipient. medRxiv. 2021 doi: 10.1101/2021.03.24.21253992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention Investigative Criteria for Suspected Cases of SARS-CoV-2 Reinfection (ICR) [(accessed on 2 July 2021)]; Available online: https://www.cdc.gov/coronavirus/2019-ncov/php/invest-criteria.html.
- 33.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid. Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization Clinical Management of Severe Acute Respiratory Infection When COVID-19 Is Suspected: Interim Guidance. Mar 13, 2020. [(accessed on 26 May 2020)]. Available online: https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf?sfvrsn=bc7da517_2.
- 35.Abu-Raddad L.J., Chemaitelly H., Malek J.A., Ahmed A.A., Mohamoud Y.A., Younuskunju S., Ayoub H.H., Al Kanaani Z., Al Khal A., Al Kuwari E., et al. Assessment of the risk of SARS-CoV-2 reinfection in an intense re-exposure setting. Clin. Infect. Dis. 2020;73:e1830–e1840. doi: 10.1093/cid/ciaa1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adrielle Dos Santos L., Filho P.G.G., Silva A.M.F., Santos J.V.G., Santos D.S., Aquino M.M., de Jesus R.M., Almeida M.L.D., da Silva J.S., Altmann D.M., et al. Recurrent COVID-19 including evidence of reinfection and enhanced severity in thirty Brazilian healthcare workers. J. Infect. 2021;82:399–406. doi: 10.1016/j.jinf.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aguilar-Shea A.L., Gutierrez-Martin-Arroyo J., Vacas-Cordoba M., Gallardo-Mayo C. Reinfection by SARS-CoV-2: The first one in a family reported in Spain. Med. Clin. 2021 doi: 10.1016/j.medcli.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmadian S., Fathizadeh H., Shabestari Khiabani S., Asgharzadeh M., Kafil H.S. COVID-19 reinfection in a healthcare worker after exposure with high dose of virus: A case report. Clin. Case Rep. 2021;9:e04257. doi: 10.1002/ccr3.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmed A., Sana F., Ikram A., Yousaf S., Khan A. Reinfection or relapse of COVID-19 in health care workers; case series of 2 patients from Pakistan. New Microbes New Infect. 2021;42:100896. doi: 10.1016/j.nmni.2021.100896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ak R., Yilmaz E., Seyhan A.U., Doganay F. Recurrence of COVID-19 Documented with RT-PCR. J. Coll. Physicians Surg. Pak. 2021;30:S26–S28. doi: 10.29271/jcpsp.2021.01.S26. [DOI] [PubMed] [Google Scholar]
- 41.Aldossary B., Hassan A., Moussa M., Alsaif H.S., Alfaraj D. Fulminant hepatic failure in a patient testing re-positive for SARS-CoV-2: A case report. Int. J. Emerg. Med. 2021;14:24. doi: 10.1186/s12245-021-00349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali A.M., Ali K.M., Fatah M.H., Tawfeeq H.M., Rostam H.M. SARS-CoV-2 Reinfection in Patients Negative for Immunoglobulin G Following Recovery from COVID-19. New Microbes New Infect. 2021;43:100926. doi: 10.1016/j.nmni.2021.100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.AlFehaidi A., Ahmad S.A., Hamed E. SARS-CoV-2 re-infection: A case report from Qatar. J. Infect. 2021;82:414–451. doi: 10.1016/j.jinf.2020.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alshukairi A.N., El-Kafrawy S.A., Dada A., Yasir M., Yamani A.H., Saeedi M.F., Aljohaney A., AlJohani N.I., Bahaudden H.A., Alam I., et al. Re-infection with different SARS-CoV-2 clade and prolonged viral shedding in a patient with hematopoietic stem cell transplantation: SARS-CoV-2 Re-infection with different clade. Int. J. Infect. Dis. 2021;110:267–271. doi: 10.1016/j.ijid.2021.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amorim M.R., Souza W.M., Barros A.C.G., Jr., Toledo-Teixeira D.A., Dos-Santos K.B., Simeoni C.L., Parise P.L., Vieira A., Forato J., Claro I.M., et al. Respiratory Viral Shedding in Healthcare Workers Reinfected with SARS-CoV-2, Brazil, 2020. Emerg. Infect. Dis. 2021;27:1737–1740. doi: 10.3201/eid2706.210558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arteaga-Livias K., Panduro-Correa V., Pinzas-Acosta K., Perez-Abad L., Pecho-Silva S., Espinoza-Sanchez F., Damaso-Mata B., Rodriguez-Morales A.J. COVID-19 reinfection? A suspected case in a Peruvian patient. Travel Med. Infect. Dis. 2021;39:101947. doi: 10.1016/j.tmaid.2020.101947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atici S., Ek O.F., Yildiz M.S., Sikgenc M.M., Guzel E., Soysal A. Symptomatic recurrence of SARS-CoV-2 infection in healthcare workers recovered from COVID-19. J. Infect. Dev. Ctries. 2021;15:69–72. doi: 10.3855/jidc.14305. [DOI] [PubMed] [Google Scholar]
- 48.Awada H., Nassereldine H., Hajj Ali A. Severe acute respiratory syndrome coronavirus 2 reinfection in a coronavirus disease 2019 recovered young adult: A case report. J. Med. Case Rep. 2021;15:382. doi: 10.1186/s13256-021-02965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bader N., Khattab M., Farah F. Severe reinfection with severe acute respiratory syndrome coronavirus 2 in a nursing home resident: A case report. J. Med. Case Rep. 2021;15:392. doi: 10.1186/s13256-021-02958-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baiswar S., Mittal R., Tiwary T., Jinnur P. Re-Positive SARS-CoV-2 With Respiratory Failure and Cerebrovascular Accident: Is This a Reinfection? Cureus. 2021;13:e15825. doi: 10.7759/cureus.15825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bellesso M., Bruniera F.R., Trunkel A.T., Nicodemo I.P. Second COVID-19 infection in a patient with multiple myeloma in Brazil—Reinfection or reactivation? Hematol. Transfus. Cell Ther. 2021;43:109–111. doi: 10.1016/j.htct.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bongiovanni M. COVID-19 re-infection in an healthcare worker. J. Med. Virol. 2020;93:4058–4059. doi: 10.1002/jmv.26565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonifacio L.P., Pereira A.P.S., Araujo D., Balbao V., Fonseca B., Passos A.D.C., Bellissimo-Rodrigues F. Are SARS-CoV-2 reinfection and Covid-19 recurrence possible? a case report from Brazil. Rev. Soc. Bras. Med. Trop. 2020;53:e20200619. doi: 10.1590/0037-8682-0619-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borgogna C., De Andrea M., Griffante G., Lai A., Bergna A., Galli M., Zehender G., Castello L., Ravanini P., Cattrini C., et al. SARS-CoV-2 reinfection in a cancer patient with a defective neutralizing humoral response. J. Med. Virol. 2021;93:6444–6446. doi: 10.1002/jmv.27200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brehm T.T., Pfefferle S., von Possel R., Kobbe R., Norz D., Schmiedel S., Grundhoff A., Olearo F., Emmerich P., Robitaille A., et al. SARS-CoV-2 Reinfection in a Healthcare Worker Despite the Presence of Detectable Neutralizing Antibodies. Viruses. 2021;13:661. doi: 10.3390/v13040661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buddingh E.P., Vossen A., Lamb H.J., van der Palen R.L.F., Brinkman D.M.C. Reinfection With Severe Acute Respiratory Syndrome Coronavirus 2 Without Recurrence of Multisystem Inflammatory Syndrome in Children. Pediatr. Infect. Dis. J. 2021 doi: 10.1097/INF.0000000000003280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caralis P. Case Reports of COVID 19 Recurrence. J. Prim. Care Community Health. 2021;12:2150132720982752. doi: 10.1177/2150132720982752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cavanaugh A.M., Thoroughman D., Miranda H., Spicer K. Suspected Recurrent SARS-CoV-2 Infections Among Residents of a Skilled Nursing Facility During a Second COVID-19 Outbreak—Kentucky, July-November 2020. MMWR Morb. Mortal. Wkly. Rep. 2021;70:273–277. doi: 10.15585/mmwr.mm7008a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colson P., Finaud M., Levy N., Lagier J.C., Raoult D. Evidence of SARS-CoV-2 re-infection with a different genotype. J. Infect. 2021;82:84–123. doi: 10.1016/j.jinf.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Das P., Satter S.M., Ross A.G., Abdullah Z., Nazneen A., Sultana R., Rimi N.A., Chowdhury K., Alam R., Parveen S., et al. A Case Series Describing the Recurrence of COVID-19 in Patients Who Recovered from Initial Illness in Bangladesh. Trop. Med. Infect. Dis. 2021;6:41. doi: 10.3390/tropicalmed6020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daw M.A., Daw A.M., Miftah M.M., El-Bouzedi A., Ahmed M.O., Libyan Study Group of COVID-19 Familial Clustering and Reinfection With 2019 Novel Coronavirus (COVID-19, SARS-CoV-2) in the Libyan Community. Disaster Med. Public Health Prep. 2021:1–3. doi: 10.1017/dmp.2021.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Brito C.A.A., Lima P.M.A., de Brito M.C.M., de Oliveira D.B. Second Episode of COVID-19 in Health Professionals: Report of Two Cases. Int. Med. Case Rep. J. 2020;13:471–475. doi: 10.2147/IMCRJ.S277882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Diaz Y., Ortiz A., Weeden A., Castillo D., Gonzalez C., Moreno B., Martinez-Montero M., Castillo M., Vasquez G., Saenz L., et al. SARS-CoV-2 reinfection with a virus harboring mutation in the Spike and the Nucleocapsid proteins in Panama. Int. J. Infect. Dis. 2021;108:588–591. doi: 10.1016/j.ijid.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dimeglio C., Herin F., Miedouge M., Martin-Blondel G., Soulat J.M., Izopet J. Protection of healthcare workers against SARS-CoV-2 reinfection. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dobano C., Ramirez-Morros A., Alonso S., Vidal-Alaball J., Ruiz-Olalla G., Vidal M., Rubio R., Cascant E., Parras D., Rodrigo Melero N., et al. Persistence and baseline determinants of seropositivity and reinfection rates in health care workers up to 12.5 months after COVID-19. BMC Med. 2021;19:155. doi: 10.1186/s12916-021-02032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duggan N.M., Ludy S.M., Shannon B.C., Reisner A.T., Wilcox S.R. Is novel coronavirus 2019 reinfection possible? Interpreting dynamic SARSCoV-2 test results through a case report. Am. J. Emerg. Med. 2020;39:256.e1–256.e3. doi: 10.1016/j.ajem.2020.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elzein F., Ibrahim A., Alshahrani F., Mahrous M., Murshid E., Aldhehyan T., Almutiri G., Altowairqi M., Ahmed M., Alsaeed M., et al. Reinfection, recurrence, or delayed presentation of COVID-19? Case series and review of the literature. J. Infect. Public Health. 2021;14:474–477. doi: 10.1016/j.jiph.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fageeh H., Alshehri A., Fageeh H., Bizzoca M.E., Lo Muzio L., Quadri M.F.A. Re-infection of SARS-CoV-2: A case in a young dental healthcare worker. J. Infect. Public Health. 2021;14:685–688. doi: 10.1016/j.jiph.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fabianova K., Kyncl J., Vlckova I., Jirincova H., Kostalova J., Liptakova M., Orlikova H., Sebestova H., Limberkova R., Mackova B., et al. COVID-19 reinfections. Epidemiol. Mikrobiol. Imunol. 2021;70:62–67. [PubMed] [Google Scholar]
- 70.Fernandes A.C., Figueiredo R. SARS-CoV-2 reinfection: A case report from Portugal. Rev. Soc. Bras. Med. Trop. 2021;54:e0002-2021. doi: 10.1590/0037-8682-0002-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferrante L., Livas S., Steinmetz W.A., Almeida A.C.L., Leao J., Vassao R.C., Tupinambas U., Fearnside P.M., Duczmal L.H. The First Case of Immunity Loss and SARS-CoV-2 Reinfection by the Same Virus Lineage in Amazonia. J. Racial Ethn. Health Disparities. 2021;8:821–823. doi: 10.1007/s40615-021-01084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fintelman-Rodrigues N., da Silva A.P.D., Dos Santos M.C., Saraiva F.B., Ferreira M.A., Gesto J., Rodrigues D.A.S., Vale A.M., de Azevedo I.G., Soares V.C., et al. Genetic Evidence and Host Immune Response in Persons Reinfected with SARS-CoV-2, Brazil. Emerg. Infect. Dis. 2021;27:1446–1453. doi: 10.3201/eid2705.204912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fonseca V., de Jesus R., Adelino T., Reis A.B., de Souza B.B., Ribeiro A.A., Guimaraes N.R., Livorati M., Neto D.F.L., Kato R.B., et al. Genomic evidence of SARS-CoV-2 reinfection case with the emerging B.1.2 variant in Brazil. J. Infect. 2021:5126. doi: 10.1016/j.jinf.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garduno-Orbe B., Sanchez-Rebolledo J.M., Cortes-Rafael M., Garcia-Jimenez Y., Perez-Ortiz M., Mendiola-Pastrana I.R., Lopez-Ortiz E., Lopez-Ortiz G. SARS-CoV-2 Reinfection among Healthcare Workers in Mexico: Case Report and Literature Review. Medicina (Kaunas) 2021;57:442. doi: 10.3390/medicina57050442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garg J., Agarwal J., Das A., Sen M. Recurrent COVID-19 infection in a health care worker: A case report. J. Med. Case Rep. 2021;15:363. doi: 10.1186/s13256-021-02881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garvey M.I., Casey A.L., Wilkinson M.A.C., Ratcliffe L., McMurray C., Stockton J., Holden E., Osman H., Loman N.J. Details of SARS-CoV-2 reinfections at a major UK tertiary centre. J. Infect. 2021;82:e29–e30. doi: 10.1016/j.jinf.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goel N., Jain D., Haddad D.B. Coronavirus Disease-19 and Re-infection: Unknown of the Unknown. Saudi J. Kidney Dis. Transpl. 2021;32:261–264. doi: 10.4103/1319-2442.318536. [DOI] [PubMed] [Google Scholar]
- 78.Gulati K., Prendecki M., Clarke C., Willicombe M., McAdoo S. COVID-19 Reinfection in a Patient Receiving Immunosuppressive Treatment for Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis Rheumatol. 2021;73:1091–1092. doi: 10.1002/art.41671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gupta V., Bhoyar R.C., Jain A., Srivastava S., Upadhayay R., Imran M., Jolly B., Divakar M.K., Sharma D., Sehgal P., et al. Asymptomatic reinfection in two healthcare workers from India with genetically distinct SARS-CoV-2. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Habadi M.I., Balla Abdalla T.H., Hamza N., Al-Gedeei A. COVID-19 Reinfection. Cureus. 2021;13:e12730. doi: 10.7759/cureus.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hanif M., Haider M.A., Ali M.J., Naz S., Sundas F. Reinfection of COVID-19 in Pakistan: A First Case Report. Cureus. 2020;12:e11176. doi: 10.7759/cureus.11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harrington D., Kele B., Pereira S., Couto-Parada X., Riddell A., Forbes S., Dobbie H., Cutino-Moguel T. Confirmed Reinfection with SARS-CoV-2 Variant VOC-202012/01. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hayes B., Stanley J., Peppers B.P. COVID-19 Recurrence Without Seroconversion in a Patient With Mannose-Binding Lectin Deficiency. Allergy Rhinol. (Providence) 2021;12:21526567211024140. doi: 10.1177/21526567211024140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hunsinger D.H.P., Kutti Sridharan D.G., Rokkam D., Fantry D.L.E. COVID-19 Reinfection in An Immunosuppressed Patient Without An Antibody Response. Am. J. Med. Sci. 2021;362:103. doi: 10.1016/j.amjms.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hussein N.R., Musa D.H., Saleem Z.S.M., Naqid I.A., Ibrahim N. Possible COVID-19 reinfection case in Duhok City, Kurdistan: A case report. J. Fam. Med. Prim. Care. 2021;10:2035–2037. doi: 10.4103/jfmpc.jfmpc_2396_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hussein N.R., Rashad B.H., Almizori L.A., Yousif S.S., Sadeeq A.T., Abdulkareem Y.R., Mahmood A.M., Salih Z.K. The Risk of SARS-CoV-2 Reinfection in Duhok city, Kurdistan Region of Iraq. Mediterr. J. Hematol. Infect. Dis. 2021;13:e2021035. doi: 10.4084/MJHID.2021.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ibrahim M., Vegel A., Niu A., Panse K., Chen R., Safah H., Socola F., Luk A., Saba N.S. Reinfection versus failure of viral clearance in a COVID-19 patient with hematologic malignancy. Leuk. Res. 2021;101:106514. doi: 10.1016/j.leukres.2021.106514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Inada M., Ishikane M., Terada M., Matsunaga A., Maeda K., Tsuchiya K., Miura K., Sairenji Y., Kinoshita N., Ujiie M., et al. Asymptomatic COVID-19 re-infection in a Japanese male by elevated half-maximal inhibitory concentration (IC50) of neutralizing antibodies. J. Infect. Chemother. 2021;27:1063–1067. doi: 10.1016/j.jiac.2021.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jain A., Kaur J., Rai A.K., Pandey A.K. Anosmia: A Clinical Indicator of COVID-19 Reinfection. Ear Nose Throat J. 2021;100:180S–181S. doi: 10.1177/0145561320978169. [DOI] [PubMed] [Google Scholar]
- 90.Kapoor R., Nair R.K., Nayan N., Bhalla S., Singh J. Reinfection or Reactivation of Coronavirus-19 in Patients with Hematologic Malignancies: Case Report Series. SN Compr. Clin. Med. 2021;3:670–674. doi: 10.1007/s42399-021-00790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krishna V.N., Ahmad M., Overton E.T., Jain G. Recurrent COVID-19 in Hemodialysis: A Case Report of 2 Possible Reinfections. Kidney Med. 2021;3:447–450. doi: 10.1016/j.xkme.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kulkarni O., Narreddy S., Zaveri L., Kalal I.G., Tallapaka K.B., Sowpati D.T. Evidence of SARS-CoV-2 reinfection without mutations in Spike protein. Clin. Infect. Dis. 2021;73:e1239–e1241. doi: 10.1093/cid/ciab136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Larson D., Brodniak S.L., Voegtly L.J., Cer R.Z., Glang L.A., Malagon F.J., Long K.A., Potocki R., Smith D.R., Lanteri C., et al. A Case of Early Re-infection with SARS-CoV-2. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lechien J.R., Chiesa-Estomba C.M., Vaira L.A., Saussez S., Hans S. COVID-19 Reinfection and Second Episodes of Olfactory and Gustatory Dysfunctions: Report of First Cases. Ear Nose Throat J. 2020:145561320970105. doi: 10.1177/0145561320970105. [DOI] [PubMed] [Google Scholar]
- 95.Lee J.S., Kim S.Y., Kim T.S., Hong K.H., Ryoo N.H., Lee J., Park J.H., Cho S.I., Kim M.J., Kim Y.G., et al. Evidence of Severe Acute Respiratory Syndrome Coronavirus 2 Reinfection After Recovery from Mild Coronavirus Disease 2019. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leung S., Hossain N. Recurrence and Recovery of COVID-19 in an Older Adult Patient with Multiple Comorbidities: A Case Report. Gerontology. 2021;67:445–448. doi: 10.1159/000514675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luciani M., Bentivegna E., Spuntarelli V., Lamberti P.A., Cacioli G., Del Porto F., Sesti G., Martelletti P., De Biase L. Recurrent COVID-19 pneumonia in the course of chemotherapy: Consequence of a weakened immune system? J. Med. Virol. 2021;93:1882–1884. doi: 10.1002/jmv.26701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mahajan N.N., Gajbhiye R.K., Lokhande P.D., Bahirat S., Modi D., Mathe A.M., Bharmal R., Rathi S., Mohite S.C., Tilve A. Clinical Presentation of Cases with SARS-CoV-2 Reinfection/ Reactivation. J. Assoc. Physicians India. 2021;69:16–18. [PubMed] [Google Scholar]
- 99.Marquez L., Koy T., Spinler J.K., Luna R.A., Tocco L., Fasciano L., Dunn J., Campbell J.R. Reinfection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) B.1.1.7 variant in an immunocompromised adolescent. Infect. Control. Hosp. Epidemiol. 2021:1–2. doi: 10.1017/ice.2021.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Massanella M., Martin-Urda A., Mateu L., Marin T., Aldas I., Riveira-Munoz E., Kipelainen A., Jimenez-Moyano E., Rodriguez de la Concepcion M.L., Avila-Nieto C., et al. Critical Presentation of a Severe Acute Respiratory Syndrome Coronavirus 2 Reinfection: A Case Report. Open Forum Infect. Dis. 2021;8:ofab329. doi: 10.1093/ofid/ofab329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mohseni M., Albus M., Kaminski A., Harrison M.F. A Case of COVID-19 Re-Infection in a Liver Transplant Patient. Cureus. 2021;13:e14916. doi: 10.7759/cureus.14916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mulder M., van der Vegt D., Oude Munnink B.B., GeurtsvanKessel C.H., van de Bovenkamp J., Sikkema R.S., Jacobs E.M.G., Koopmans M.P.G., Wegdam-Blans M.C.A. Reinfection of SARS-CoV-2 in an immunocompromised patient: A case report. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Munoz Mendoza J., Alcaide M.L. COVID-19 in a patient with end-stage renal disease on chronic in-center hemodialysis after evidence of SARS-CoV-2 IgG antibodies. Reinfection or inaccuracy of antibody testing. IDCases. 2020;22:e00943. doi: 10.1016/j.idcr.2020.e00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nachmias V., Fusman R., Mann S., Koren G. The first case of documented Covid-19 reinfection in Israel. IDCases. 2020;22:e00970. doi: 10.1016/j.idcr.2020.e00970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Naveca F., da Costa C., Nascimento V., Souza V., Corado A., Nascimento F., Costa A., Duarte D., Silva G., Mejía M., et al. Three SARS-CoV-2 reinfection cases by the new Variant of Concern (VOC) P.1/501Y.V3. Res. Sq. 2021 doi: 10.21203/rs.3.rs-318392/v1. [DOI] [Google Scholar]
- 106.Nazir N., Ahirwar A., Jain S. Reinfection in a healthcare worker with COVID-19 in a hospital in North India. Anaesth. Pain Intensive Care. 2020;24:572–573. doi: 10.35975/apic.v24i5.1369. [DOI] [Google Scholar]
- 107.Nicholson E.G., Avadhanula V., Fragoso S., Stroh R., Ye X., Bond N., Santarcangelo P., Stroh J., Piedra P.A. SARS-CoV-2 re-infection versus prolonged shedding: A case series. Influenza Other Respir Viruses. 2021 doi: 10.1111/irv.12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nonaka C.K.V., Franco M.M., Graf T., de Lorenzo Barcia C.A., de Avila Mendonca R.N., de Sousa K.A.F., Neiva L.M.C., Fosenca V., Mendes A.V.A., de Aguiar R.S., et al. Genomic Evidence of SARS-CoV-2 Reinfection Involving E484K Spike Mutation, Brazil. Emerg. Infect. Dis. 2021;27:1522–1524. doi: 10.3201/eid2705.210191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Novoa W., Miller H., Mattar S., Faccini-Martinez A.A., Rivero R., Serrano-Coll H. A first probable case of SARS-CoV-2 reinfection in Colombia. Ann. Clin. Microbiol. Antimicrob. 2021;20:7. doi: 10.1186/s12941-020-00413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ozaras R., Ozdogru I., Yilmaz A.A. Coronavirus disease 2019 re-infection: First report from Turkey. New Microbes New Infect. 2020;38:100774. doi: 10.1016/j.nmni.2020.100774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pow T., Allen S., Brailovsky Y., Darki A. Acute submassive pulmonary embolism after SARS-CoV-2 infection: A case report of reinfection or prolonged hypercoagulable state. Eur. Heart J. Case Rep. 2021;5:ytab103. doi: 10.1093/ehjcr/ytab103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Quiroga B., Fernandez Ramos A., Delgado Arroyo A. COVID-19 reinfection in a kidney transplant recipient, time for rethinking? Nefrologia. 2021 doi: 10.1016/j.nefro.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ramirez J.D., Munoz M., Ballesteros N., Patino L.H., Castaneda S., Rincon C.A., Mendez C., Oliveros C., Perez J., Marquez E.K., et al. Phylogenomic Evidence of Reinfection and Persistence of SARS-CoV-2: First Report from Colombia. Vaccines. 2021;9:282. doi: 10.3390/vaccines9030282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rani P.R., Imran M., Lakshmi J.V., Jolly B., Jain A., Surekha A., Senthivel V., Chandrasekhar P., Divakar M.K., Srinivasulu D., et al. Symptomatic reinfection of SARS-CoV-2 with spike protein variant N440K associated with immune escape. J. Med. Virol. 2021;93:4163–4165. doi: 10.1002/jmv.26997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Resende P.C., Bezerra J.F., Teixeira Vasconcelos R.H., Arantes I., Appolinario L., Mendonca A.C., Paixao A.C., Duarte A.C., Silva T., Rocha A.S., et al. Severe Acute Respiratory Syndrome Coronavirus 2 P.2 Lineage Associated with Reinfection Case, Brazil, June-October 2020. Emerg. Infect. Dis. 2021;27:1789–1794. doi: 10.3201/eid2707.210401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rodriguez-Espinosa D., Broseta Monzo J.J., Casals Q., Pineiro G.J., Rodas L., Vera M., Maduell F. Fatal SARS-CoV-2 reinfection in an immunosuppressed patient on hemodialysis. J. Nephrol. 2021;34:1041–1043. doi: 10.1007/s40620-021-01039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Romano C.M., Felix A.C., Paula A.V., Jesus J.G., Andrade P.S., Candido D., Oliveira F.M., Ribeiro A.C., Silva F.C.D., Inemami M., et al. SARS-CoV-2 reinfection caused by the P.1 lineage in Araraquara city, Sao Paulo State, Brazil. Rev. Inst. Med. Trop. Sao Paulo. 2021;63:e36. doi: 10.1590/s1678-9946202163036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Salcin S., Fontem F. Recurrent SARS-CoV-2 infection resulting in acute respiratory distress syndrome and development of pulmonary hypertension: A case report. Respir. Med. Case Rep. 2021;33:101314. doi: 10.1016/j.rmcr.2020.101314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Salehi-Vaziri M., Omrani M.D., Pouriayevali M.H., Fotouhi F., Banifazl M., Farahmand B., Sadat Larijani M., Ahmadi Z., Fereydouni Z., Tavakoli M., et al. SARS-CoV-2 presented moderately during two episodes of the infection with lack of antibody responses. Virus Res. 2021;299:198421. doi: 10.1016/j.virusres.2021.198421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Salehi-Vaziri M., Jalali T., Farahmand B., Fotouhi F., Banifazl M., Pouriayevali M.H., Sadat Larijani M., Afzali N., Ramezani A. Clinical characteristics of SARS-CoV-2 by re-infection vs. reactivation: A case series from Iran. Eur J. Clin. Microbiol. Infect. Dis. 2021;40:1713–1719. doi: 10.1007/s10096-021-04221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Salzer H.J.F. Emerging COVID-19 reinfection four months after primary SARS-CoV-2 infection. Wien. Med. Wochenschr. 2021 doi: 10.1007/s10354-021-00813-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sanyang B., Kanteh A., Usuf E., Nadjm B., Jarju S., Bah A., Bojang A., Grey-Johnson M., Jones J.C., Gai A., et al. COVID-19 reinfections in The Gambia by phylogenetically distinct SARS-CoV-2 variants-first two confirmed events in west Africa. Lancet Glob. Health. 2021;9:e905–e907. doi: 10.1016/S2214-109X(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Scarpati G., Piazza O., Pagliano P., Rizzo F. COVID-19: A confirmed case of reinfection in a nurse. BMJ Case Rep. 2021;14:e244507. doi: 10.1136/bcr-2021-244507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Selhorst P., Van Ierssel S., Michiels J., Marien J., Bartholomeeusen K., Dirinck E., Vandamme S., Jansens H., Arien K.K. Symptomatic SARS-CoV-2 reinfection of a health care worker in a Belgian nosocomial outbreak despite primary neutralizing antibody response. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Selvaraj V., Herman K., Dapaah-Afriyie K. Severe, Symptomatic Reinfection in a Patient with COVID-19. R I Med. J. 2020;103:24–26. [PubMed] [Google Scholar]
- 126.Sen M.K., Gupta N., Yadav S.R., Kumar R., Singh B., Ish P. Contentious Issue in Recurrent COVID-19 Infection: Reactivation or Reinfection. Turk. Thorac. J. 2020;21:463–466. doi: 10.5152/TurkThoracJ.2020.20164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sevillano G., Ortega-Paredes D., Loaiza K., Zurita-Salinas C., Zurita J. Evidence of SARS-CoV-2 reinfection within the same clade in Ecuador: A case study. Int. J. Infect. Dis. 2021;108:53–56. doi: 10.1016/j.ijid.2021.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shastri J., Parikh S., Agrawal S., Chatterjee N., Pathak M., Chaudhary S., Sharma C., Kanakan A., Vivekanand A., Srinivasa Vasudevan J., et al. Clinical, Serological, Whole Genome Sequence Analyses to Confirm SARS-CoV-2 Reinfection in Patients From Mumbai, India. Front. Med. (Lausanne) 2021;8:631769. doi: 10.3389/fmed.2021.631769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shoar S., Khavandi S., Tabibzadeh E., Khavandi S., Naderan M., Shoar N. Recurrent coronavirus diseases 19 (COVID-19): A different presentation from the first episode. Clin. Case Rep. 2021;9:2149–2152. doi: 10.1002/ccr3.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sicsic I., Jr., Chacon A.R., Zaw M., Ascher K., Abreu A., Chediak A. A case of SARS-CoV-2 reinfection in a patient with obstructive sleep apnea managed with telemedicine. BMJ Case Rep. 2021;14:e240496. doi: 10.1136/bcr-2020-240496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Siqueira J.D., Goes L.R., Alves B.M., da Silva A.C.P., de Carvalho P.S., Cicala C., Arthos J., Viola J.P.B., Soares M.A. Distinguishing SARS-CoV-2 bonafide re-infection from pre-existing minor variant reactivation. Infect. Genet. Evol. 2021;90:104772. doi: 10.1016/j.meegid.2021.104772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Silva M.S.D., Demoliner M., Hansen A.W., Gularte J.S., Silveira F., Heldt F.H., Filippi M., Pereira V., Silva F.P.D., Mallmann L., et al. Early detection of SARS-CoV-2 P.1 variant in Southern Brazil and reinfection of the same patient by P.2. Rev. Inst. Med. Trop. Sao Paulo. 2021;63:e58. doi: 10.1590/s1678-9946202163058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Staub T., Arendt V., Lasso de la Vega E.C., Braquet P., Michaux C., Kohnen M., Tsobo C., Abdelrahman T., Wienecke-Baldacchino A., Francois J.H. Case series of four re-infections with a SARS-CoV-2 B.1.351 variant, Luxembourg, February 2021. Euro Surveill. 2021;26:2100423. doi: 10.2807/1560-7917.ES.2021.26.18.2100423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Takeda C.F.V., de Almeida M.M., Gomes R.G.D., Souza T.C., Mota M.A.D., Cavalcanti L.P.D., Colares J.K.B. Case Report: Recurrent Clinical Symptoms of COVID-19 in Healthcare Professionals: A Series of Cases from Brazil. Am. J. Trop. Med. Hyg. 2020;103:1993–1996. doi: 10.4269/ajtmh.20-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tang C.Y., Wang Y., McElroy J.A., Li T., Hammer R., Ritter D., Lidl G.M., Webby R., Hang J., Wan X.F. Reinfection with two genetically distinct SARS-CoV-2 viruses within 19 days. J. Med. Virol. 2021;93:5700–5703. doi: 10.1002/jmv.27154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tuan J., Spichler-Moffarah A., Ogbuagu O. A new positive SARS-CoV-2 test months after severe COVID-19 illness: Reinfection or intermittent viral shedding? BMJ Case Rep. 2021;14:e240531. doi: 10.1136/bcr-2020-240531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Teka I.A., BenHasan M.H., Alkershini A.A., Alatresh O.K., Abulifa T.A., Lembagga H.A., Alhudiri I.M., Elzagheid A. Reinfection with SARS-CoV-2: A case report from Libya. Travel Med. Infect. Dis. 2021;41:102040. doi: 10.1016/j.tmaid.2021.102040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.To K.K., Hung I.F., Chan K.H., Yuan S., To W.K., Tsang D.N., Cheng V.C., Chen Z., Kok K.H., Yuen K.Y. Serum Antibody Profile of a Patient With Coronavirus Disease 2019 Reinfection. Clin. Infect. Dis. 2021;72:e659–e662. doi: 10.1093/cid/ciaa1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chan P.K.S., Lui G., Hachim A., Ko R.L.W., Boon S.S., Li T., Kavian N., Luk F., Chen Z., Yau E.M., et al. Serologic Responses in Healthy Adult with SARS-CoV-2 Reinfection, Hong Kong, August 2020. Emerg. Infect. Dis. 2020;26:3076–3078. doi: 10.3201/eid2612.203833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tomkins-Tinch C.H., Daly J.S., Gladden-Young A., Theodoropoulos N.M., Madaio M.P., Yu N., Vanguri V.K., Siddle K.J., Adams G., Krasilnikova L.A., et al. SARS-CoV-2 Reinfection in a Liver Transplant Recipient. Ann. Intern. Med. 2021;174:1178–1180. doi: 10.7326/L21-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tomassini S., Kotecha D., Bird P.W., Folwell A., Biju S., Tang J.W. Setting the criteria for SARS-CoV-2 reinfection—Six possible cases. J. Infect. 2021;82:282–327. doi: 10.1016/j.jinf.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Torres D.A., Ribeiro L., Riello A., Horovitz D.D.G., Pinto L.F.R., Croda J. Reinfection of COVID-19 after 3 months with a distinct and more aggressive clinical presentation: Case report. J. Med. Virol. 2020;93:1857–1859. doi: 10.1002/jmv.26637. [DOI] [PubMed] [Google Scholar]
- 143.Ul-Haq Z., Khan A., Fazid S., Noor F., Yousafzai Y.M., Sherin A. First documented reinfection of SARS-COV-2 in second wave from Pakistan. J. Ayub. Med. Coll. Abbottabad. 2020;32((Suppl. 1)):S704–S705. [PubMed] [Google Scholar]
- 144.Vetter P., Cordey S., Schibler M., Vieux L., Despres L., Laubscher F., Andrey D.O., Martischang R., Harbarth S., Cuvelier C., et al. Clinical, virological and immunological features of a mild case of SARS-CoV-2 re-infection. Clin. Microbiol. Infect. 2021;27:791.e1–791.e4. doi: 10.1016/j.cmi.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vora T., Vora P., Vora F., Sharma K., Desai H.D. Symptomatic reinfection with COVID-19: A first from Western India. J. Fam. Med. Prim. Care. 2021;10:1496–1498. doi: 10.4103/jfmpc.jfmpc_2002_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.West J., Everden S., Nikitas N. A case of COVID-19 reinfection in the UK. Clin. Med. (Lond) 2021;21:e52–e53. doi: 10.7861/clinmed.2020-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yeleti R., Guglin M., Saleem K., Adigopula S.V., Sinha A., Upadhyay S., Everett J.E., Ballut K., Uppuluri S., Rao R.A. Fulminant myocarditis: COVID or not COVID? Reinfection or co-infection? Future Cardiol. 2021 doi: 10.2217/fca-2020-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yu A.L.F., Liphaus B.L., Ferreira P.M., Tanamachi A.T., Masuda E.T., Trevisan C.M., Lucas P.C.C., Bugno A., Carvalhanas T. SARS-CoV-2 reinfection: Report of two cases in Southeast Brazil. Rev. Inst. Med. Trop. Sao Paulo. 2021;63:e50. doi: 10.1590/s1678-9946202163050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zare F., Teimouri M., Khosravi A., Rohani-Rasaf M., Chaman R., Hosseinzadeh A., Jamali Atergeleh H., Binesh E., Emamian M.H. COVID-19 re-infection in Shahroud, Iran: A follow-up study. Epidemiol. Infect. 2021;149:e159. doi: 10.1017/S095026882100087X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang K., Yiu-Nam Lau J., Yang L., Ma Z. SARS-CoV-2 reinfection in two patients who have recovered from COVID-19. Precis. Clin. Med. 2020;3:292–293. doi: 10.1093/pcmedi/pbaa031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zucman N., Uhel F., Descamps D., Roux D., Ricard J.D. Severe reinfection with South African SARS-CoV-2 variant 501Y.V2: A case report. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tirado S.M., Yoon K.J. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003;16:69–86. doi: 10.1089/088282403763635465. [DOI] [PubMed] [Google Scholar]
- 153.Yahav D., Yelin D., Eckerle I., Eberhardt C.S., Wang J., Cao B., Kaiser L. Definitions for coronavirus disease 2019 reinfection, relapse and PCR re-positivity. Clin. Microbiol. Infect. 2021;27:315–318. doi: 10.1016/j.cmi.2020.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xiao A.T., Tong Y.X., Zhang S. False negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: Rather than recurrence. J. Med. Virol. 2020;92:1755–1756. doi: 10.1002/jmv.25855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chaturvedi R., Naidu R., Sheth S., Chakravarthy K. Efficacy of Serology Testing in Predicting Reinfection in Patients With SARS-CoV-2. Disaster Med. Public Health Prep. 2020;15:e29–e31. doi: 10.1017/dmp.2020.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Atkinson B., Petersen E. SARS-CoV-2 shedding and infectivity. Lancet. 2020;395:1339–1340. doi: 10.1016/S0140-6736(20)30868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Anonymous. COVID-19 reinfection: Are we ready for winter? EBioMedicine. 2020;62:103173. doi: 10.1016/j.ebiom.2020.103173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gousseff M., Penot P., Gallay L., Batisse D., Benech N., Bouiller K., Collarino R., Conrad A., Slama D., Joseph C., et al. Clinical recurrences of COVID-19 symptoms after recovery: Viral relapse, reinfection or inflammatory rebound? J. Infect. 2020;81:816–846. doi: 10.1016/j.jinf.2020.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Turner J.S., Day A., Alsoussi W.B., Liu Z., O’Halloran J.A., Presti R.M., Patterson B.K., Whelan S.P.J., Ellebedy A.H., Mudd P.A. SARS-CoV-2 Viral RNA Shedding for More Than 87 Days in an Individual With an Impaired CD8+ T Cell Response. Front. Immunol. 2020;11:618402. doi: 10.3389/fimmu.2020.618402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Raveendran A.V. COVID-19 re-infection: Diagnostic challenges and proposed diagnostic criteria. Diabetes Metab. Syndr. 2021;15:645–648. doi: 10.1016/j.dsx.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pilz S., Chakeri A., Ioannidis J.P., Richter L., Theiler-Schwetz V., Trummer C., Krause R., Allerberger F. SARS-CoV-2 re-infection risk in Austria. Eur. J. Clin. Investig. 2021;51:e13520. doi: 10.1111/eci.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sui J., Li W., Roberts A., Matthews L.J., Murakami A., Vogel L., Wong S.K., Subbarao K., Farzan M., Marasco W.A. Evaluation of human monoclonal antibody 80R for immunoprophylaxis of severe acute respiratory syndrome by an animal study, epitope mapping, and analysis of spike variants. J. Virol. 2005;79:5900–5906. doi: 10.1128/JVI.79.10.5900-5906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., Wang X., Yuan J., Li T., Li J., et al. Antibody Responses to SARS-CoV-2 in Patients With Novel Coronavirus Disease 2019. Clin. Infect. Dis. 2020;71:2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Del Fante C., Franchini M., Baldanti F., Percivalle E., Glingani C., Marano G., Mengoli C., Mortellaro C., Viarengo G., Perotti C., et al. A retrospective study assessing the characteristics of COVID-19 convalescent plasma donors and donations. Transfusion. 2020;61:830–838. doi: 10.1111/trf.16208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K., Liao P., Qiu J.F., Lin Y., Cai X.F., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 167.Cassaniti I., Novazzi F., Giardina F., Salinaro F., Sachs M., Perlini S., Bruno R., Mojoli F., Baldanti F., Members of the San Matteo Pavia C.-T.F. Performance of VivaDiag COVID-19 IgM/IgG Rapid Test is inadequate for diagnosis of COVID-19 in acute patients referring to emergency room department. J. Med. Virol. 2020;92:1724–1727. doi: 10.1002/jmv.25800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bichara C., Amoras E., Vaz G., Bichara C., Amaral I., Vallinoto A. Persistence of Anti-SARS-CoV-2 IgM Antibody up to 8 Months Post-COVID-19. Case Rep. Clin. Med. 2021;10:227–231. doi: 10.4236/crcm.2021.109029. [DOI] [Google Scholar]
- 169.Sethuraman N., Jeremiah S.S., Ryo A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA. 2020;323:2249–2251. doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 170.Channappanavar R., Fett C., Zhao J., Meyerholz D.K., Perlman S. Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J. Virol. 2014;88:11034–11044. doi: 10.1128/JVI.01505-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sherina N., Piralla A., Du L., Wan H., Kumagai-Braesh M., Andréll J., Braesch-Andersen S., Cassaniti I., Percivalle E., Sarasini A., et al. Persistence of SARS-CoV-2 specific B- and T-cell responses in convalescent COVID-19 patients 6-8 months after the infection. BioRxiv. 2020 doi: 10.1101/2020.11.06.371617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Enjuanes L., Zuniga S., Castano-Rodriguez C., Gutierrez-Alvarez J., Canton J., Sola I. Molecular Basis of Coronavirus Virulence and Vaccine Development. Adv. Virus Res. 2016;96:245–286. doi: 10.1016/bs.aivir.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Figueiredo-Campos P., Blankenhaus B., Mota C., Gomes A., Serrano M., Ariotti S., Costa C., Nunes-Cabaco H., Mendes A.M., Gaspar P., et al. Seroprevalence of anti-SARS-CoV-2 antibodies in COVID-19 patients and healthy volunteers up to 6 months post disease onset. Eur. J. Immunol. 2020;50:2025–2040. doi: 10.1002/eji.202048970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Krishna E., Pathak V.K., Prasad R., Jose H., Kumar M.M. COVID-19 reinfection: Linked Possibilities and future outlook. J. Fam. Med. Prim. Care. 2020;9:5445–5449. doi: 10.4103/jfmpc.jfmpc_1672_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Khoshkam Z., Aftabi Y., Stenvinkel P., Paige Lawrence B., Rezaei M.H., Ichihara G., Fereidouni S. Recovery scenario and immunity in COVID-19 disease: A new strategy to predict the potential of reinfection. J. Adv. Res. 2021;31:49–60. doi: 10.1016/j.jare.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Prevost J., Finzi A. The great escape? SARS-CoV-2 variants evading neutralizing responses. Cell Host Microbe. 2021;29:322–324. doi: 10.1016/j.chom.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Guallar M.P., Meirino R., Donat-Vargas C., Corral O., Jouve N., Soriano V. Inoculum at the time of SARS-CoV-2 exposure and risk of disease severity. Int. J. Infect. Dis. 2020;97:290–292. doi: 10.1016/j.ijid.2020.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Arvin A.M., Fink K., Schmid M.A., Cathcart A., Spreafico R., Havenar-Daughton C., Lanzavecchia A., Corti D., Virgin H.W. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature. 2020;584:353–363. doi: 10.1038/s41586-020-2538-8. [DOI] [PubMed] [Google Scholar]
- 179.Negro F. Is antibody-dependent enhancement playing a role in COVID-19 pathogenesis? Swiss Med. Wkly. 2020;150:w20249. doi: 10.4414/smw.2020.20249. [DOI] [PubMed] [Google Scholar]
- 180.Peron J.P.S., Nakaya H. Susceptibility of the Elderly to SARS-CoV-2 Infection: ACE-2 Overexpression, Shedding, and Antibody-dependent Enhancement (ADE) Clinics (Sao Paulo) 2020;75:e1912. doi: 10.6061/clinics/2020/e1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ulrich H., Pillat M.M., Tarnok A. Dengue Fever, COVID-19 (SARS-CoV-2), and Antibody-Dependent Enhancement (ADE): A Perspective. Cytom. A. 2020;97:662–667. doi: 10.1002/cyto.a.24047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wan Y., Shang J., Sun S., Tai W., Chen J., Geng Q., He L., Chen Y., Wu J., Shi Z., et al. Molecular Mechanism for Antibody-Dependent Enhancement of Coronavirus Entry. J. Virol. 2020;94:e02015-19. doi: 10.1128/JVI.02015-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wen J., Cheng Y., Ling R., Dai Y., Huang B., Huang W., Zhang S., Jiang Y. Antibody-dependent enhancement of coronavirus. Int. J. Infect. Dis. 2020;100:483–489. doi: 10.1016/j.ijid.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yager E.J. Antibody-dependent enhancement and COVID-19: Moving toward acquittal. Clin. Immunol. 2020;217:108496. doi: 10.1016/j.clim.2020.108496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bournazos S., Gupta A., Ravetch J.V. The role of IgG Fc receptors in antibody-dependent enhancement. Nat. Rev. Immunol. 2020;20:633–643. doi: 10.1038/s41577-020-00410-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Eroshenko N., Gill T., Keaveney M.K., Church G.M., Trevejo J.M., Rajaniemi H. Implications of antibody-dependent enhancement of infection for SARS-CoV-2 countermeasures. Nat. Biotechnol. 2020;38:789–791. doi: 10.1038/s41587-020-0577-1. [DOI] [PubMed] [Google Scholar]
- 187.Fleming A.B., Raabe V. Current studies of convalescent plasma therapy for COVID-19 may underestimate risk of antibody-dependent enhancement. J. Clin. Virol. 2020;127:104388. doi: 10.1016/j.jcv.2020.104388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kumar R., Gupta N., Kodan P., Mittal A., Soneja M., Wig N. Is there antibody-dependent enhancement in SARS Coronavirus 2? J. Fam. Med. Prim. Care. 2020;9:2589–2590. doi: 10.4103/jfmpc.jfmpc_540_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nechipurenko Y.D., Anashkina A.A., Matveeva O.V. Change of Antigenic Determinants of SARS-CoV-2 Virus S-Protein as a Possible Cause of Antibody-Dependent Enhancement of Virus Infection and Cytokine Storm. Biophysics (Oxford) 2020;65:703–709. doi: 10.1134/S0006350920040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yip M.S., Leung N.H., Cheung C.Y., Li P.H., Lee H.H., Daeron M., Peiris J.S., Bruzzone R., Jaume M. Antibody-dependent infection of human macrophages by severe acute respiratory syndrome coronavirus. Virol. J. 2014;11:82. doi: 10.1186/1743-422X-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lee W.S., Wheatley A.K., Kent S.J., DeKosky B.J. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat. Microbiol. 2020;5:1185–1191. doi: 10.1038/s41564-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kabir K.M.A., Tanimoto J. Cost-efficiency analysis of voluntary vaccination against n-serovar diseases using antibody-dependent enhancement: A game approach. J. Theor. Biol. 2020;503:110379. doi: 10.1016/j.jtbi.2020.110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kadkhoda K. Severe acute respiratory syndrome coronavirus 2, original antigenic sin, and antibody-dependent enhancement: Ménage à trois. Curr. Opin. Rheumatol. 2020;32:458–461. doi: 10.1097/BOR.0000000000000728. [DOI] [PubMed] [Google Scholar]
- 194.Karthik K., Senthilkumar T.M.A., Udhayavel S., Raj G.D. Role of antibody-dependent enhancement (ADE) in the virulence of SARS-CoV-2 and its mitigation strategies for the development of vaccines and immunotherapies to counter COVID-19. Hum. Vaccines Immunother. 2020;16:3055–3060. doi: 10.1080/21645515.2020.1796425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cohen J.I., Burbelo P.D. Reinfection with SARS-CoV-2: Implications for Vaccines. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gudbjartsson D.F., Norddahl G.L., Melsted P., Gunnarsdottir K., Holm H., Eythorsson E., Arnthorsson A.O., Helgason D., Bjarnadottir K., Ingvarsson R.F., et al. Humoral Immune Response to SARS-CoV-2 in Iceland. N. Engl. J. Med. 2020;383:1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Iyer A.S., Jones F.K., Nodoushani A., Kelly M., Becker M., Slater D., Mills R., Teng E., Kamruzzaman M., Garcia-Beltran W.F., et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci. Immunol. 2020;5:eabe0367. doi: 10.1126/sciimmunol.abe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Weisblum Y., Schmidt F., Zhang F., DaSilva J., Poston D., Lorenzi J.C., Muecksch F., Rutkowska M., Hoffmann H.H., Michailidis E., et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. Elife. 2020;9:e61312. doi: 10.7554/eLife.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Choudhary M.C., Crain C.R., Qiu X., Hanage W., Li J.Z. SARS-CoV-2 Sequence Characteristics of COVID-19 Persistence and Reinfection. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ulhaq Z.S., Soraya G.V., Indriana K. Breakthrough COVID-19 case after full-dose administration of CoronaVac vaccine. Indian J. Med. Microbiol. 2021 doi: 10.1016/j.ijmmb.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hacisuleyman E., Hale C., Saito Y., Blachere N.E., Bergh M., Conlon E.G., Schaefer-Babajew D.J., DaSilva J., Muecksch F., Gaebler C., et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N. Engl. J. Med. 2021;384:2212–2218. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Dyer O. Covid-19: Chinese vaccines may need changes to improve efficacy, admits official. BMJ. 2021;373:n969. doi: 10.1136/bmj.n969. [DOI] [PubMed] [Google Scholar]
- 203.Angel Y., Spitzer A., Henig O., Saiag E., Sprecher E., Padova H., Ben-Ami R. Association Between Vaccination With BNT162b2 and Incidence of Symptomatic and Asymptomatic SARS-CoV-2 Infections Among Health Care Workers. JAMA. 2021;325:2457–2465. doi: 10.1001/jama.2021.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Osterholm M.T., Kelley N.S., Sommer A., Belongia E.A. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect. Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 205.Roy S. COVID-19 Reinfection: Myth or Truth? SN Compr. Clin. Med. 2020;2:710–713. doi: 10.1007/s42399-020-00335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cavanaugh A.M., Spicer K.B., Thoroughman D., Glick C., Winter K. Reduced Risk of Reinfection with SARS-CoV-2 After COVID-19 Vaccination—Kentucky, May-June 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:1081–1083. doi: 10.15585/mmwr.mm7032e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Al-Tawfiq J.A., Rabaan A.A., Al-Omari A., Al Mutair A., Al-Qahtani M., Tirupathi R. Learning from SARS and MERS: COVID-19 reinfection where do we stand? Travel Med. Infect. Dis. 2021;41:102024. doi: 10.1016/j.tmaid.2021.102024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bizzoca M.E., Campisi G., Lo Muzio L. An innovative risk-scoring system of dental procedures and safety protocols in the COVID-19 era. BMC Oral Health. 2020;20:301. doi: 10.1186/s12903-020-01301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bizzoca M.E., Campisi G., Lo Muzio L. Covid-19 Pandemic: What Changes for Dentists and Oral Medicine Experts? A Narrative Review and Novel Approaches to Infection Containment. Int. J. Environ. Res. Public Health. 2020;17:3793. doi: 10.3390/ijerph17113793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Campisi G., Bazzano M., Mauceri R., Panzarella V., Di Fede O., Bizzoca M.E., Lo Muzio L. The patient-doctor relationship: New insights in light of the current Ministerial recommendations regarding Phase 2 of the COVID-19 pandemic. Minerva Stomatol. 2020;69:251–255. doi: 10.23736/S0026-4970.20.04396-4. [DOI] [PubMed] [Google Scholar]