Abstract
The first-line treatment for Leishmania donovani-induced cutaneous leishmaniasis (CL) in Sri Lanka is intra-lesional sodium stibogluconate (IL-SSG). Antimony failures in leishmaniasis is a challenge both at regional and global level, threatening the ongoing disease control efforts. There is a dearth of information on treatment failures to routine therapy in Sri Lanka, which hinders policy changes in therapeutics. Laboratory-confirmed CL patients (n = 201) who attended the District General Hospital Hambantota and Base Hospital Tangalle in southern Sri Lanka between 2016 and 2018 were included in a descriptive cohort study and followed up for three months to assess the treatment response of their lesions to IL-SSG. Treatment failure (TF) of total study population was 75.1% and the majority of them were >20 years (127/151,84%). Highest TF was seen in lesions on the trunk (16/18, 89%) while those on head and neck showed the least (31/44, 70%). Nodules were least responsive to therapy (27/31, 87.1%) unlike papules (28/44, 63.6%). Susceptibility to antimony therapy seemed age-dependant with treatment failure associated with factors such as time elapsed since onset to seeking treatment, number and site of the lesions. This is the first detailed study on characteristics of CL treatment failures in Sri Lanka. The findings highlight the need for in depth investigations on pathogenesis of TF and importance of reviewing existing treatment protocols to introduce more effective strategies. Such interventions would enable containment of the rapid spread of L.donovani infections in Sri Lanka that threatens the ongoing regional elimination drive.
Introduction
Leishmaniasis is a vector borne disease caused by Leishmania parasites and transmitted by the phlebotomine sand flies [1]. Leishmaniasis in Sri Lanka is caused by Leishmania donovani zymodeme MON-37, with the disease manifesting predominantly as cutaneous leishmaniasis (CL) [2, 3]. CL has different clinical sub types such as papules, nodules, plaques and ulcers. First indigenous case of CL in Sri Lanka was reported in 1992, with a significant number of cases reported during an outbreak in the northern part of the country from 2001 to 2003 [4, 5]. A subsequent rise was seen in reported cases with two hot spots in the northern and southern parts of the country. By 2018, eight out of 25 districts in Sri Lanka had a recorded leishmaniasis incidence rate of more than 10 cases per 100,000 population with an estimate of nearly one third of the Sri Lankan population living with the risk of acquiring leishmaniasis [6].
As per the standard guidelines used by local clinicians, CL in Sri Lanka is treated with local infiltration of sodium stibogluconate (SSG) or cryotherapy with application of liquid nitrogen [7]. Sri Lanka has used intra-lesional SSG (IL-SSG) as a first-line treatment for CL over the past 2 decades. The average number of IL-SSG injections required for healing is estimated as 10 injections given at weekly intervals [8, 9]. SSG is used as a mainstay of treatment for leishmaniasis in many parts of the world [10, 11]. Observed in toto, drug resistance is a major emerging problem world over in chemotherapy of leishmaniasis, including visceral leishmaniasis (VL) in the Indian subcontinent (with L. donovani as the causative agent) [12–15]. Similar observations have been made in Sri Lanka with increasing numbers of CL cases failing to respond to regular treatment with IL-SSG [16, 17].
Sri Lanka is confronted with an atypical, predominantly dermotropic variant of L. donovani, which has the potential to visceralise, if the parasites by-pass the local tissue immune responses [2, 3, 18]. The parasite is genetically and biochemically closely related to VL-causing parasite in India (L. donovani MON-2) [3, 18]. Sand flies that belong to the P. argentipes complex are the vectors of leishmaniasis in Sri Lanka, and similar to a large extent to those reported from India, Nepal and Bangladesh [19–21]. Presence of genetically-related Leishmania parasites, Phlebotomine vectors, close geographical and social ties between Sri Lanka and other South Asian countries (with frequent travelling in between) are important factors to consider when decisions are made on patient management, disease control and elimination of leishmaniasis [22]. Many attempts have been made to achieve sustained elimination of VL in the Indian subcontinent but with limited success [23]. In Sri Lanka, there is a steady expansion in spatial spread of leishmaniasis over the past 2 decades with hotspots in transmission [24]. Persistent CL lesions despite treatment, due to poor-response or treatment failure to IL-SSG might be a contributory factor for such disease spread. The situation remains a challenge for the national public health sector, which also may be a threat for the elimination program in the South Asian region [5, 6, 25]. Such threats may further escalate with the ongoing national plans to make Sri Lanka a hub of tourism in the post-COVID era (https://www.sltda.gov.lk/en).
Treatment failure is a complex phenomenon and it is not synonymous with parasite drug resistance [26]. Multiple factors such as parasite drug resistance, drug related factors, host factors (viz. co-morbidities, host immune response, pharmacokinetics, nutritional status) and environmental factors can result in treatment failure [26, 27]. Cases of poor response to antimony have been previously reported from disease hotspots both in the north and the south of the country, particularly from the Anuradhapura and Hambantota districts respectively [16, 17]. Even though clinico-epidemiological characteristics of general CL patient populations have been described in the past, treatment failure in CL patients have caught only little attention in Sri Lanka [8, 28, 29]. Therefore, this study aimed to fill that void.
The aims of this study were to identify the clinical response pattern of CL patients to regular IL-SSG therapy, to describe the characteristics of treatment failed patients, their lesions and to understand possible factors associated with treatment failure in a selected cohort of patients in southern coastal region of Sri Lanka. The findings of this study provided baseline information on therapeutic response of L. donovani causing CL in southern Sri Lanka following treatment with IL-SSG and possible factors associated with treatment failure that are important in making policy decisions related to treatment modalities recommended for CL patients.
Materials & methods
Ethics statement
This study has been approved by the Ethics Review Committee, Faculty of Medicine, University of Colombo, Sri Lanka (EC-16-080). Written, informed consent was obtained during prospective data collection. Data were analysed anonymously.
Study sites, design and participants
A descriptive cohort study was conducted in Hambantota District, in Southern Province, Sri Lanka, at the District General Hospital Hambantota (6.12680N, 81.1260E) and Base Hospital Tangalle (6.022780N, 80.79750E), from April 2016 to October 2018. Data of CL patients who attended the dermatology clinic during the study period were obtained both retrospectively through their clinic records and prospectively through follow-ups. The southern region was selected as the study area as it forms the biggest hotspot of leishmaniasis transmission in the country [6]. The two selected hospitals are located in the area (Hambantota district) that accounts for the majority of CL patients reported from the southern region to the central database, maintained by the Ministry of Health (www.epid.gov.lk).
A total of 201 laboratory-confirmed CL patients were recruited to the study, adhering to the selection criteria: 1) patients who were treated with IL-SSG and were aged between 1 to 70 years, 2) with less than 5 lesions 3) longest diameter of the lesion less than 5 cm and 4) sought treatment within 1 year of lesion onset. Patients were treated by the clinicians of the study sites with IL-SSG [StibovitaTM, Vital Healthcare Pvt. Ltd., India], each 1ml containing sodium stibogluconate BP 330mg (which is equivalent to 100mg of pentavalent Antimony)]. Treatment schedule: similar schedule was used on all patients irrespective of the age categories in accordance with the standard guidelines in Sri Lanka and carried out by the same clinicians in the two study sites. One to 3 ml of SSG (i.e., maximum dose of 300mg of pentavalent Antimony per week), as intra-lesional injections until the lesion was fully infiltrated, was given once a week. The lesions were assessed for clinical signs of healing as per the study definition, after 1 week of the last IL-SSG injection, weekly until the patient achieved complete healing or completed 10 IL-SSG injections, after which the patients were relieved from the study. Case definitions used in this study were: 1) ‘Completely healed by IL-SSG’: A patient who has been treated only with IL-SSG weekly injections and has achieved complete cure as per established criteria, i.e.: complete flattening of papules, nodules or plaques and no open ulcer or induration or any sign of inflammation, with treatment of 10 or a lesser number of IL-SSG weekly injections, 2) ‘Treatment failure’: A patient whose CL lesion was not completely healed following at least 10 injections of weekly IL-SSG or if the patient has had a relapse of the lesion after previous complete course of IL-SSG treatment (10 or more weekly injections) [17]. Treatment failures were managed at the clinic as decided by the treating clinicians.
The age, gender, number of lesions on a patient, site of lesion, type of lesion at the time of first visit to the dermatology clinic and the time duration since the onset of the lesion to starting treatment with IL-SSG were recorded. Tissue scrapings, tissue aspirates and/or tissue biopsies were obtained from active edges of skin lesions from new clinic attendees and used for laboratory confirmation of CL by microscopic examination of Leishmania amastigotes and/or in-vitro culturing of Leishmania promastigotes and/or by histopathology. Laboratory confirmation reports were traced for those who have been diagnosed previously.
Statistical methods
Patients’ data were expressed as ‘mean ± standard deviation’ or as a percentage with respect to the total number. Two association tests, Kruskal-Wallis test for scale variables and Chi-squared test for categorical variables, were carried out to check the association between the response variable ‘Response to IL-SSG’ and the predictor variables (H0: There is an association between the two variables, H1:There is no association between the two variables). Binary logistic regression analysis was carried out to find the factors associated with treatment failure (S1 Table). A ‘Final regression model’ was formulated as a binary logistic regression with backward Wald, using the variables: categorised time since onset (TSO), categorised age, other variables in their natural form and taking treatment failure as the base category. The ‘Final regression model’ was fitted for each of the age categories 1–30 years (65 cases), 31–50 years (75 cases) and 51–70 years (61 cases), where the classes were approximately balanced.
Results
Total study population was 201 patients and the selected variables viz. age, time since onset to seeking treatment and number of lesions on a single patient, were comparable between the ‘completely healed’ and ‘treatment failure’ groups (p-values > 0.05) (Table 1, S1 Datasets).
Table 1. Characteristics of cutaneous leishmaniasis patients recruited for the study and comparison between the ‘completely healed’ and ‘treatment failure’ groups.
| Factor | Total study population (n = 201) | Treatment outcome after 10 weekly IL-SSG injections | Completely healed group versus Treatment failures | |
|---|---|---|---|---|
| Completely healed (n = 50) | Treatment failures (n = 151) | p-value# | ||
| Age (years)* | 38.1±17.5 | 35.6±19.2 | 39.0±16.9 | 0.240 |
| Age category (years) * | ||||
| 1–10 | 6.5±3.2 | 6.2±4.4 | 6.6±2.2 | 0.806 |
| 11–20 | 14.9±2.8 | 14.8±3.0 | 14.9±2.9 | 0.937 |
| 21–30 | 27.4±2.4 | 27.6±2.5 | 27.3±2.5 | 0.812 |
| 31–40 | 35.9±3.2 | 34.7±3.4 | 36.2±3.2 | 0.208 |
| 41–50 | 45.0±3.1 | 44.3±2.3 | 45.2±3.4 | 0.399 |
| 51–60 | 55.6±3.0 | 56.8±3.4 | 55.3±2.9 | 0.227 |
| 61–70 | 65.4±3.0 | 66.2±3.5 | 65.2±3.0 | 0.555 |
| Time since onset (months)* | 4.1±2.8 | 4.3±3.2 | 4.0±2.6 | 0.612 |
| Time since onset categories (months) * | ||||
| 0–2.0 | 1.5±0.5 | 1.7±0.5 | 1.5±0.5 | 0.633 |
| 2.1–4.0 | 3.3±0.5 | 3.4±0.6 | 3.2±0.5 | 0.331 |
| 4.1–6.0 | 5.5±0.5 | 5.6±0.5 | 5.5±0.5 | 0.716 |
| 6.1–8.0 | 7.5±0.6 | 7.0±0.0 | 7.6±.6 | 0.181 |
| 8.1–10.0 | 9.7±0.6 | 10±0.0 | 9.5±0.7 | 0.667 |
| 10.1–12.0 | 11.9±0.3 | 11.8±0.4 | 12.0±0.0 | 0.255 |
| Number of lesions on a single patient* | 1.2±0.6 | 1.4±0.7 | 1.2±0.5 | 0.052 |
Note
*Data expressed as ‘mean ± standard deviation’.
# Independent-Samples t test was performed to calculate the p-values.
The total study population was predominantly males (63.7%), most lesions were on shoulder and upper limbs (43.3%) and most patients had sought treatment 2.1 to 4.0 months since the onset of the lesions with the majority (65.7%) obtaining treatment within the first 4 months since the onset (Table 2, S1 Datasets). Most study participants were adults viz. 31–40 years = 21.4%, 41-50years = 18.4%, 51-60years = 18.4% with a lesser proportion of young adults 21-30years (13.9%) in that category. Most common lesion type was ulcers (96/201, 47.8%).
Table 2. Characteristics and frequencies of laboratory-confirmed CL patients who were treated with IL-SSG weekly injections (n = 201).
| Factor | Number (%) of study subjects (Total n = 201) | Number (%) study subjects in study groups | Dummy variable names used for the regression analysis | |
|---|---|---|---|---|
| Completely healed (Total n = 50) | Treatment failures (Total n = 151) | |||
| Age category (years) ** | ||||
| 1–10 | 19 (9.5%) | 8 (16.0%) | 11 (7.3%) | Age 1 |
| 11–20 | 18 (9.0%) | 5 (10.0%) | 13 (8.6%) | Age 2 |
| 21–30 | 28 (13.9%) | 5 (10.0%) | 23 (15.2%) | Age 3 |
| 31–40 | 43 (21.4%) | 9 (18.0%) | 34 (22.5%) | Age 4 |
| 41–50 | 37 (18.4%) | 11 (22.0%) | 26 (17.2%) | Age 5 |
| 51–60 | 37 (18.4%) | 8 (16.0%) | 29 (19.2%) | Age 6 |
| 61–70 | 19 (9.5%) | 4 (8.0%) | 15 (9.9%) | |
| Time since onset categories (months) ** | ||||
| 0–2.0 | 58 (28.9%) | 16 (32.0%) | 42 (27.8%) | TSO 1 |
| 2.1–4.0 | 74 (36.8%) | 18 (36.0%) | 56 (37.1%) | TSO 2 |
| 4.1–6.0 | 42 (20.9%) | 8 (16.0%) | 34 (22.5%) | TSO 3 |
| 6.1–8.0 | 12 (6.0%) | 2 (4.0%) | 10 (6.6%) | TSO 4 |
| 8.1–10.0 | 3 (1.5%) | 1 (2.0%) | 2 (1.3%) | TSO 5 |
| 10.1–12.0 | 12 (6.0%) | 5 (10.0%) | 7 (4.6%) | |
| Gender** | Gender | |||
| Male | 128 (63.7%) | 31 (62.0%) | 97 (64.2%) | |
| Female | 73 (36.3%) | 19 (38.0%) | 54 (35.8%) | |
| Lesion site** | ||||
| Head or neck | 44 (21.9%) | 13 (26.0%) | 31 (20.5%) | Site 1 |
| Trunk | 18 (9.0) | 2 (4.0%) | 16 (10.6%) | Site 2 |
| Shoulder & upper limbs | 87 (43.3%) | 19 (38.0%) | 68 (45.0%) | Site 3 |
| Lower limbs | 40 (19.9%) | 10 (20.0%) | 30 (19.9%) | Site 4 |
| Multiple sites | 12 (6.0%) | 6 (12.0%) | 6 (4.0%) | |
| Lesion type** | ||||
| Papule | 44 (21.9%) | 16 (32.0%) | 28 (18.5%) | Type 1 |
| Nodule | 31 (15.4%) | 4 (8.0%) | 27 (17.9%) | Type 2 |
| Plaque | 27 (13.4%) | 6 (12.0%) | 21 (13.9%) | Type 3 |
| Ulcer | 96 (47.8%) | 23 (46.0%) | 73 (48.3%) | Type 4 |
| Multiple types | 3 (1.5%) | 1 (2%) | 2 (1.3%) | |
Note: Data expressed as ‘number of cases (percentage with respect to the total number n)’
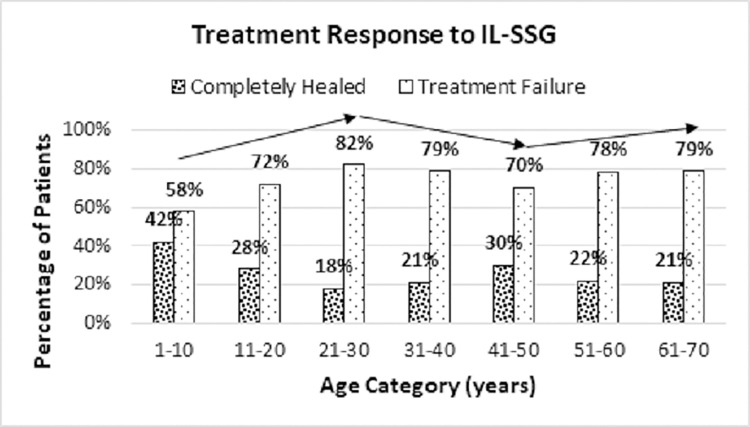
In the total patient group, 151 patients (151/201, 75.1%) failed treatment with IL-SSG with persistent lesions, unlike the balance whose lesions healed completely (50/201, 24.9%). Treatment failure (TF) was more in the age group 21–70 years (127/164 = 77%) than in the 1–20 year olds (24/37 = 65%). Lesions on trunk showed highest TF (16/18, 89%) while those on head and neck showed the least (31/44, 70%). Of the four clinical types, nodules were least sensitive to treatment (TF: 27/31, 87.1%) while papules were the most sensitive (TF: 28/44, 63.6%). The proportion of patients in the 61–70 age group of study population was comparatively low (9.5%) and the proportion of TF in this age group was higher (15/19, 9.9%) than those who have healed completely (4/19, 8.0%).
Application of association tests in statistics showed a statistically significant association only between the number of lesions and the treatment response (p-value = 0.044) and all other factors had p-values greater than 0.05 (Table 3).
Table 3. Results of association tests for predictor factors and the treatment response to IL-SSG.
| *Age | #Age category | *Time since onset | #Time since onset category | *No of lesions on a patient | #Gender | #Lesion site | #Lesion type | |
|---|---|---|---|---|---|---|---|---|
| p-value | 0.361 | 0.541 | 0.968 | 0.644 | 0.044 | 0.775 | 0.146 | 0.219 |
Note
*Kruskal–Wallis test.
# Chi-squared test.
Since more treatment failures were seen among adults (21–70 years) than in children and adolescents aged 1 to 20 years (77% versus 65%), the direction of the analysis was shifted towards age category-wise treatment response (Fig 1).
Fig 1. Treatment response to IL-SSG with age.
There were 3 trends noted in treatment failure viz. 1) increase in treatment failure from age 1–30 years, 2) decrease in treatment failure from age 31–50 years and 3) increase in treatment failure from 51–70 years (Fig 1, S1 Datasets).
When the ‘Final regression model’ was fitted for each of the above three age groups, the equation of the fitted final regression model for the age group 1–30 years was as follows:
The outcome of the modelling experiment indicated that the time since onset ≤ 6 months was significantly associated with increase in treatment failure while multiple lesions and location of lesion on head or neck were significantly associated with decrease in treatment failure. For the other two age categories, none of the variables were preserved in the final model. Considering the order of variables removed from the model and their coefficients, the inference was that in the age group 31–50 years, nodules and location of lesions on sites other than the head and neck would create an impact on treatment response to increase treatment failure, while time since onset ≤ 2 months would have the opposite effect to decrease treatment failure. In the age group 51–70 years, only the time since onset ≤ 4 months seemed to reduce the likelihood of treatment failure (S1 Table).
Discussion
Leishmaniases is a group of diseases which includes several clinical forms. To understand and counter the emerging drug resistance, it is essential to investigate into the different disease forms based on their clinical and serological features, to enable prompt diagnosis and effective management (Table 4) [30–42].
Table 4. Characteristics of common clinical forms of Leishmaniases.
|
|
Clinical form, the causative parasite/s and prevalence | Clinical profile and serological diagnosis | Ref |
|---|---|---|---|
| (1) |
Visceral leishmaniasis (also known as kala-azar) Leishmania donovani complex: • Indian subcontinent and Africa—L. donovani • Mediterranean basin, Central and South America—L. infantum (also known as L. chagasi) Prevalence • Mostly reported from Brazil, East Africa and India • More than 90% of global VL cases in 2019 occurred in 10 countries: Brazil, India, Ethiopia, Eritrea, Iraq, Kenya, Nepal, Somalia, South Sudan, Sudan |
Systemic and most severe form. Fatal if untreated. Irregular, long-term fever often associated with rigor and chills. Splenomegaly. Hepatomegaly. Lymphadenopathy. Pancytopenia. Anaemia. Weight loss. Serological diagnosis • Strong humoral response • Antibody detection by—Direct Agglutination Test (DAT), Enzyme-linked Immunosorbent Assay (ELISA), Indirect Immunofluorescence Antibody Test (IFAT), rapid immunochromatographic rK39 dip-stick testing • rK39 appears to be more sensitive in Asia than in Africa because the humoral response is stronger in most Asian countries resulting in higher antibody levels. • Antibody testing cannot differentiate between active, past, sub-clinical, relapse and re-infections. Antigen testing: Antigen-based latex agglutination test (KAtex) has shown good specificity in detecting antigen in urine with low to moderate sensitivity in Asia and Africa. |
[30, 31, 35–38] |
| (2) |
Cutaneous leishmaniasis • Old World (the Eastern Hemisphere): L. aethiopica, L. donovani, L. infantum, L. major, and L. tropica. • New World (the Western Hemisphere): Two major subgenera, Leishmania Leishmania and Leishmania Viannia Prevalence • CL is more widely distributed • About 95% of cases in 2019 were from the Americas, Central Asia, Mediterranean basin and Middle East. |
• Most common form. Cause skin lesions. • Old World CL: Starts as a papule leading to an ulcer, usually 1–2 lesions but rarely multiple, heal spontaneously with disfiguring scars. • New World CL: localized skin lesions • A rare, chronic CL called lupoid/ recidivans/ relapsing CL is caused by L. tropica Serological diagnosis • Poor humoral response. • rK39 rapid kit–negative • Old world: serological testing not useful • New world: IFA and ELISA with L. amazonensis antigen shown to be effective in diagnosing L. braziliensis and L. guyanensis infections • Serology cannot differentiate past and active infections. |
[30, 31] |
| (3) |
Mucocutaneous leishmaniasis Commonly caused by New World species • New World: L. braziliensis, L. panamensis, and L. guyanensis • Old World: L. donovani, L. major, and L. infantum Prevalence 90% of cases reported from Brazil, Peru, Bolivia and Ethiopia |
Destructive lesions of nasopharyngeal mucosa Serological diagnosis • rK39 rapid kit: positive band • Serological testing may aid the diagnosis in the presence of other indicators such as clinical and histopathologcal features • A rising titre of antibodies indicate a relapse. |
[30, 31, 39] |
| (4) |
Diffuse cutaneous leishmaniasis • Old World: L. aethiopica, to a lesser extent L. major • New World: Species of subgenus Leishmania: L. mexicana and L. amazonensis • No reports of Viannia causing diffuse-CL to date Prevalence • Old World: Uncommon • New World: South and Central American countries |
A rare syndrome. Chronic, multiple, non-ulcerative, lepromatous lesions spread over the whole body except on scalp, axillae, inguinal folds, palms and soles. Plaques are common but papules, nodules, macules and erythema may also be seen. No mucosal involvement. Do not heal spontaneously. Poorly respond to treatment and frequently relapse after treatments. Serological diagnosis • Strong humoral response • rK39 rapid kit: strong positive band |
[30] |
| (5) |
Disseminated cutaneous leishmaniasis New World: L. brazilliensis, L. Mexicana Prevalence Mainly found in the New World and rarely from Old World |
Co-existence of different types of lesions such as papules, nodules & ulcers. Ulceration is common. Plaques are rare. May have mucosal involvement. Not chronic. Better response to treatment than diffuse-CL. Serological diagnosis • Strong humoral response • rK39 rapid kit: strong positive band |
[40, 41] |
| (6) |
Post-kala-azar dermal leishmaniasis (PKDL) Late complication of VL caused by L. donovani. Can be seen rarely in patients infected with L. infantum Prevalence Predominantly occur in South Asia and East Africa. |
• Majority occur after recovering from VL but may occur without previous VL or simultaneously with VL. Interval between VL and PKDL in Asia is more (0 to 3 or more years) than in Africa (0 to 13 months). Clinical features differ in Asia and Africa. • Asia: Macular rash is more common, and treatment is indicated. • Africa: Papular rash is more common, spontaneously heal and treatment is indicated only for chronic or severe cases Serological diagnosis • rk39, DAT, ELISA are usually positive but is of limited value in diagnosis due to remaining serological response due to VL • Serology is helpful in differentiating from other diseases such as leprosy |
[30, 31, 40] |
| (7) |
Leishmania-HIV coinfection Different causative species have been reported and some have shown atypical presentations such as dermotropic species causing VL in HIV-infected patients. Prevalence Highest prevalence had been reported in South-western Europe, but the numbers have been increasing in sub-Saharan Africa, South Asia and Brazil. |
Leishmaniasis is an important opportunistic infection in HIV-infected patients. VL is commonly associated with HIV but association of other clinical forms such CL and MCL have also been reported. Clinical features could be similar to the classical clinical form, more severe or atypical. HIV and Leishmania infection reinforce each other. Serological diagnosis • Antibody based tests are less sensitive and less reliable in HIV co-infected persons, due to low humoral response • VL-HIV co-infected patients have shown high sensitivity to detection of antigen in urine by the latex agglutination test. |
[30–34, 40, 42] |
Antimony has been extensively used for the treatment of CL and VL for more than half a century. Emerging antimony resistance of CL (the most common clinical type) and VL (the most severe form) is a challenge against successful implementation of strategies for the control and elimination of leishmaniasis. Antimony failure has been observed more following its parenteral use in VL patients than when used intra-lesionally in CL patients or when combination of systemic and intralesional treatment is used (though the difference was not statistically significant) [43]. The extensive antimony resistance in VL patients in Bihar, India has even led to changes in treatment policy [43]. However, pharmacokinetics of antimony administered either IV or IM are similar [44]. Thus, the mechanisms of antimony resistance appear multifaceted, the knowledge of which remains important to combat treatment failure. Particularly important are the molecular mechanisms which are important in discovering targets for new drugs. Commonly discussed antimony resistant mechanisms are summarised in Table 5.
Table 5. Mechanisms of the antimony resistance.
| Mechanism | Comment | |
|---|---|---|
| 1 | Reduced uptake of the drug by the parasite [45–47]. | Aquaglyceroporin1 (AQP1) is known to facilitate the uptake of SbIII by the parasite |
| Downregulation of AQP1 was seen drug resistant parasites. | ||
| 2 | Increased intracellular thiol levels [47–49]. | In drug sensitive strains, SbIII disrupts the thiol homeostasis by: - a) inducing outflow of thiols and from parasites: • Intracellular thiols such as trypanothione (TSH), glutathione (GSH) and cysteine, in Leishmania maintain the thiol redox homeostasis, protecting the parasite from chemical and oxidative stress. • The γ-GCS gene encodes an enzyme catalyzing the rate limiting step of glutathione (GSH) biosynthesis and the ODC gene encodes for an enzyme regulating the biosynthesis of polyamines. Polyamines are the precursor metabolites of trypanothione. • Antimony resistant strains have shown non-consistent upregulation of γ-GCS and over expression ODC genes, increasing the intra-cellular thiol-dependent antioxidant capacity, resulting in resistance to antimony. and b) inhibiting the reduction of trypanothione: Trypanothione reductase gene is amplified in antimony resistant isolates. This leads to high intracellular trypanothione levels and increased resistance to SbIII. |
| 3 | sequestration and rapid drug efflux [13, 45, 49, 50]. | ATP-binding cassette (ABC) transporters efflux drug out of the parasite or sequestrate the drugs in intracellular vesicles. |
| Eg: The two classes of ABC transporters, P-glycoprotein (eg: MRPA) and multi-drug resistance-related protein (eg: MRP1) known to lead to multi drug resistance. Genes encoding these transports have been amplified in antimony resistant parasites. | ||
| 4 | Altered membrane fluidity [51]. | Changes in membrane fluidity have been demonstrated in resistance to antimony combinations |
| 5 | Heat shock proteins and cell death related proteins [46, 52–55]. | Heat shock protein (eg: HSP83 and HSP70) associated modulation of cell death has been reported in resistant parasites. Cell death related protein tyrosine phosphate (PTP), proliferating cell nuclear antigen (PCNA) were upregulated and mitogen-activated protein kinase (MAPK) was downregulated in antimony resistant strains. |
| 6 | Modulation of host-pathogen interaction the host immune response [36, 56–58]. | Leishmania modulates signaling pathways of the host macrophages. |
| Drug resistant parasites have modulated the host pathogen interaction and there by the host immune response in previous studies. | ||
| 7 | Differentially expressed 8proteins associated with antimony resistance [59–62]. | Some proteins have been shown to be differentially expressed in with antimony response. Eg: Proteins such as the histone1, H2A, H4 and leucine-rich repeat protein are over expressed in antimony resistant parasites while proteins such as the kinetoplastid membrane protein (KMP-11) are under-expressed. |
| 8 | Misuse of the antimony drugs [30]. | Practices such as inadequate dose, inappropriate regimes, free availability, management of patients by unqualified persons and not completing treatment have led to development of subtherapeutic levels of antimony in blood causing development of parasite tolerance to antimony. |
Participants of this study were from Hambantota district, in the southern leishmaniasis hotspot which has recorded the highest caseload from 2001 to 2018 [6]. A high proportion of the study population (75.1%) failed to achieve complete healing with standard treatment of IL-SSG. Proportion of treatment failures in the age group 21–70 years (127/164 = 77%) was higher than in the 1–20 year olds (24/37 = 65%). Diverse factors associated with or having an impact on treatment failure were observed among the age groups 1–30 years, 31–50 years and 51–70 years.
Treatment response is largely dependent on host’s immune response [63] with the latter influenced by the age of the patient. Immune ageing, which occur with increasing age, would result in decreased activation of macrophages to kill intracellular parasites and reduce direct cytotoxic killing of parasitized cells by the NK cells [64, 65]. Additionally, the effective immune response that supplements the anti-leishmanial properties of SSG is likely to be reduced with age. These would contribute to the increase in treatment failure with advancing age as observed in this study. Furthermore, the increasing trend in treatment failure from children to adolescents and the reversed trend in adults from 31–50 years (Fig 1), could be due to the percentage decrease of CD4+ T cells from infancy until adolescence and rising trend observed thereafter [64]. CD4+ T cells are known to be important for Th1 proinflammatory response which promotes parasite clearance and healing.
Age-related thickening of dermis in children and adolescents, in contrast to its thinning in adults (with dermal atrophy occurring beyond 50 years of age) have been documented earlier [66]. Such age-related phenomena could reduce the establishment of parasites in dermal macrophages that may explain the low frequency of CL in older people. Similarly, reduce the number of macrophages available for converting SSG to its active form leading to higher treatment failures among them.
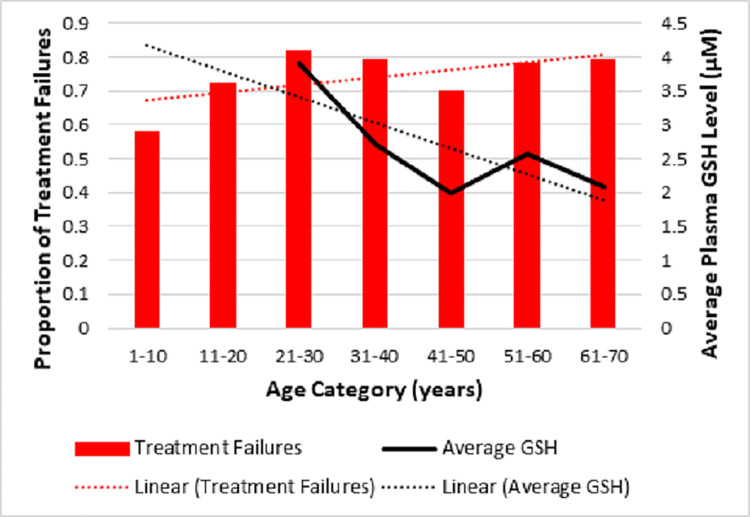
Decrease in plasma glutathione (GSH), which is an important antioxidant, can affect both the immune response of the host and the mechanism of action of SSG with resultant increase in treatment failure with advancing age [67, 68]. When the proportion of treatment failures with age was tabulated with average plasma GSH levels that was adapted from Giustarini et al, 2006 (Fig 2, S1 Datasets) showed an interesting relationship.
Fig 2. Proportion of treatment failures versus age category with average plasma glutathione (GSH) levels adapted from Giustarini et al, 2006 [69].
In Fig 2, the linear forecast trend line of proportion of patients with treatment failure (red dotted line) increased in contrast to the decreasing linear forecast trendline of the average plasma glutathione (GSH) levels (black dotted line) with advancing age. The considerable drop seen in treatment failure and the corresponding drop in the GSH level in the age group 41–50 years as seen in Fig 2, could be related to menopause/andropause. The mean age of perimenopause is 46.1 (±3.7) years in Sri Lankan females while andropause begins by about 40 years, which would lead to oxidative stress enhancing parasite clearance and the drop in treatment failure seen in the same age group in this study [68].
Higher treatment failure seen in lesions on trunk (16/18, 89%) as opposed to those on head and neck (31/44, 70%) could also be explained by haemodynamic features such as the rich blood supply in scalp and face (as opposed to the trunk) which aids in healing. Furthermore, blood vessels with atherosclerotic plaques leading to less blood supply and interference of inflammatory cell migration through capillaries to the lesions and dermal atrophy could also account for higher treatment failure in elderly than in children.
The noticeably high treatment failure percentage in the study group (75.1%) is a matter of concern which calls for further research. In addition to the host and parasite related factors, extraneous factors such as chemicals might play a role in treatment response. Hambantota is a coastal district known for its agriculture and consumer preference for tuna. Even though the levels of arsenic in agrochemicals and fish seem to be much lower than the maximum contaminant levels and may not be significant or toxic when considered individually, it might be postulated that study participants may have been chronically exposed to sub-lethal levels of arsenic with the development of arsenic-resistant parasite strains that might be cross-resistant to antimony therapy [46, 68, 70–75]. Whether such environmental factors affect the treatment response in CL calls for further studies in different regions of the country. Excluding patients over 70 years of age, which accounts for approximately 4.7% of Sri Lankan population (www.statistics.gov.lk) may be viewed as a limitation.
According to estimates, nearly one third of the Sri Lankan population in 2018 live at risk of leishmaniasis, with the incidence rate of leishmaniasis in the Southern Province (the study location) showing an alarming increase from 1.2 cases / 100,000 in 2001 to 117.2 cases / 100,000 in 2018 [6]. High rates of treatment failures as reported here may explain this rapid disease spread that threatens the plans for control and elimination of leishmaniasis in the region.
Of the two standard therapeutic measures (IL-SSG and cryotherapy) used in Sri Lanka, IL-SSG given until cure is the widely used first line treatment and the drug resistance is an emerging problem. Even though cryotherapy is also used to a lesser extent, it has limitations such as inability to use on areas such as the face, scarring, keloid formation, ulceration and post inflammatory depigmentation specially in skin of colour [7, 76]. Intramuscular injection of SSG (IM-SSG) is not widely used for treatment of CL in Sri Lanka due to more side effects when compared with local infiltration. However, IM-SSG is used when the lesion is very large, many in number or when It’s difficult to administer IL-SSG due to the location of the lesion. Even though cryotherapy is an accepted, relatively cheap standard therapy in Sri Lanka, it has limitations such as inability to apply on sites as such as face, disfigurement/scarring, keloid formation, depigmentation specially in skin of colour and smear positivity for long periods following treatment [76–78]. Therefore, it is extremely important to review the current treatment protocols and introduce safe and efficacious alternative treatment methods. A recent study has proven thermotherapy to be safe and efficacious for persons not healed by IL-SSG in Sri Lanka [17]. With further feasibility studies such treatment methods may be incorporated to the local guidelines as the first line treatment methods or alternative methods for treatment failures.
Conclusions
In conclusion, this study characterises in detail the leishmaniasis treatment failures in Sri Lanka initiating and encouraging further studies focusing on emerging drug resistance. Nearly three fourths (75.1%) of the study population failed treatment with IL-SSG and majority of treatment failures were > 20 years of age (84%). Lesions located on trunk and nodules seemed least sensitive to antimony therapy. Factors associated with or having an impact on treatment failure varied among different age groups, which warrants further investigations. It would be timely to revisit and revise the current treatment strategies of CL in Sri Lanka. Understanding and combating CL treatment failure will aid the containment of the escalating CL spread in Sri Lanka, with favourable impact on the regional and even global-level disease control and elimination efforts.
Supporting information
(PDF)
(XLSX)
Acknowledgments
We are thankful to the Directors, Medical Officers, Nursing Officers and staff members of Dermatology Clinics of BH Tangalle and DGH Hambantota and the study participants. We thank Mr.Sudath Weerasingha and Ms.Yasasmi Gange for technical assistance. We are grateful to the Head and Staff of Department of Parasitology, Faculty of Medicine, University of Colombo.
Data Availability
All the relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the University of Colombo (https://cmb.ac.lk/) under Grant Number AP/3/2/2017/PG/31 to HS; the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH), USA (https://www.niaid.nih.gov/) under Award Number U01AI136033 to NK. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID, NIH or the University of Colombo. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5). doi: 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karunaweera ND, Pratlong F, Siriwardane HVYD, Ihalamulla RL, Dedet JP. Sri Lankan cutaneous leishmaniasis is caused by Leishmania donovani zymodeme MON-37. Trans R Soc Trop Med Hyg. 2003. Jan;97(4):380–1. doi: 10.1016/s0035-9203(03)90061-7 [DOI] [PubMed] [Google Scholar]
- 3.Kariyawasam KKGDUL, Selvapandiyan A, Siriwardana HVYD, Dube A, Karunanayake P, Senanayake SASC, et al. Dermotropic Leishmania donovani in Sri Lanka: Visceralizing potential in clinical and preclinical studies. Parasitology. 2018;145(4):443–52. doi: 10.1017/S003118201700169X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Athukorale D. N., Seneviratne J. K., Ihalamulla R. L., & Premaratne UN. Locally acquired cutaneous leishmaniasis in Sri Lanka. J Trop Med Hyg. 1992;6:432–3. [PubMed] [Google Scholar]
- 5.Karunaweera ND. Leishmania donovani causing cutaneous leishmaniasis in Sri Lanka: a wolf in sheep’s clothing? Trends Parasitol. 2009. Oct;25(10):458–63. doi: 10.1016/j.pt.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 6.Karunaweera ND, Ginige S, Senanayake S, Silva H, Manamperi N, Samaranayake N, et al. Spatial epidemiologic trends and hotspots of leishmaniasis, Sri Lanka, 2001–2018. Emerg Infect Dis. 2020;26(1):1–10. doi: 10.3201/eid2601.190971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sri Lanka College of Dermatologists. Guidelines on Management of Leishmaniasis. 2013. [Google Scholar]
- 8.Siriwardana Y, Deepachandi B, Gunasekara C, Warnasooriya W, Karunaweera ND. Leishmania donovani induced cutaneous leishmaniasis: An insight into atypical clinical variants in sri lanka. J Trop Med. 2019;2019. doi: 10.1155/2019/4538597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Senanayake S, Somaratne K, Munidasa U, Kotalawala H, Karunaweera N. Responsiveness to sodium stibogluconate in cutaneous leishmaniasis in a cohort of patients in Sri Lanka. Proceedings of the Annual Research Symposium. 2014;128. [Google Scholar]
- 10.Croft SL, Olliaro P. Leishmaniasis chemotherapy-challenges and opportunities. Clin Microbiol Infect. 2011;17(10):1478–83. doi: 10.1111/j.1469-0691.2011.03630.x [DOI] [PubMed] [Google Scholar]
- 11.Cordeiro LV. Antimony Resistance in Leishmania Parasites may be Related to an Increased Virulence. Biomed J Sci Tech Res. 2019;21(1):15620–1. [Google Scholar]
- 12.García-Hernández R, Gómez-Pérez V, Castanys S, Gamarro F. Fitness of Leishmania donovani Parasites Resistant to Drug Combinations. PLoS Negl Trop Dis. 2015;9(4):1–12. doi: 10.1371/journal.pntd.0003704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croft SL, Sundar S, Fairlamb AH. Drug Resistance in Leishmaniasis. Clin Microbiol Rev. 2006;19(1):111–26. doi: 10.1128/CMR.19.1.111-126.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumetz F, Cuypers B, Imamura H, Zander D, D’Haenens E, Maes I, et al. Molecular Preadaptation to Antimony Resistance in Leishmania donovani on the Indian Subcontinent. mSphere. 2018;3(2):e00548–17. doi: 10.1128/mSphere.00548-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selvapandiyan A, Croft SL, Rijal S, Nakhasi HL, Ganguly NK. Innovations for the elimination and control of visceral leishmaniasis. PLoS Negl Trop Dis. 2019;13(9):1–5. doi: 10.1371/journal.pntd.0007616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Refai FW, Madarasingha NP, Fernandopulle R, Karunaweera N. Nonresponsiveness to standard treatment in cutaneous leishmaniasis: A case series from Sri Lanka. Trop Parasitol. 2016;6(2):155–8. doi: 10.4103/2229-5070.190835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva H, Liyanage A, Deerasinghe T, Sumanasena B, Munidasa D, de Silva H, et al. Therapeutic Response to Thermotherapy in Cutaneous Leishmaniasis Treatment Failures for Sodium Stibogluconate: A Randomized Controlled Proof of Principle Clinical Trial. Am J Trop Med Hyg. 2021;00(0):1–6. doi: 10.4269/ajtmh.20-0855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siriwardana H, Noyes H, Beeching N, Chance M, Karunaweera N, Bates P. Leishmania donovani and cutaneous leishmaniasis in Sri Lanka. 2007;13(3):1–3. doi: 10.3201/eid1303.060242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gajapathy K, Peiris LB, Goodacre SL, Silva A, Jude PJ, Surendran SN. Molecular identification of potential leishmaniasis vector species within the Phlebotomus (Euphlebotomus) argentipes species complex in Sri Lanka. Parasites and Vectors. 2013;6(1):1–9. doi: 10.1186/1756-3305-6-302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Senanayake SASC, Abeyewicreme W, Dotson EM, Karunaweera N. Characteristics of Phlebotomine sandflies in selected areas of Sri Lanka. Southeast Asian J Trop Med Public Heal. 2015;46(6):994–1004. [PMC free article] [PubMed] [Google Scholar]
- 21.Lane RP, Pile MM, Amerasinghe FP. Anthropophagy and aggregation behaviour of the sandfly Phlebotomus argentipes in Sri Lanka. Med Vet Entomol. 1990;4(1):79–88. doi: 10.1111/j.1365-2915.1990.tb00263.x [DOI] [PubMed] [Google Scholar]
- 22.Sri Lanka Tourism Development Authority. Annual Statistical Report—2019. 2019. 42 p. 32744812 [Google Scholar]
- 23.Rijal S, Sundar S, Mondal D, Das P, Alvar J, Boelaert M. Eliminating visceral leishmaniasis in South Asia: The road ahead. BMJ. 2019;364. doi: 10.1136/bmj.k5224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karunaweera ND, Senanayake S, Ginige S, Silva H, Manamperi N, Samaranayake N, et al. Spatiotemporal distribution of cutaneous leishmaniasis in Sri Lanka and future case burden estimates. PLoS Negl Trop Dis. 2021;15(4):1–16. doi: 10.1371/journal.pntd.0009346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karunaweera N. Leishmaniasis: Path toward elimination from the Indian subcontinent. Trop Parasitol. 2016;6(1):2–4. doi: 10.4103/2229-5070.175023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Regli IB, Fernández OL, Martínez-Salazar B, Gómez MA, Saravia NG, Tacchini-Cottier F. Resistance of Leishmania (Viannia) Panamensis to Meglumine Antimoniate or Miltefosine Modulates Neutrophil Effector Functions. Front Immunol. 2018;9(December):3040. doi: 10.3389/fimmu.2018.03040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorlo TPC, Huitema ADR, Beijnen JH, De Vries PJ. Optimal dosing of miltefosine in children and adults with visceral leishmaniasis. Antimicrob Agents Chemother. 2012;56(7):3864–72. doi: 10.1128/AAC.00292-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iddawela D, Vithana SMP, Atapattu D, Wijekoon L. Clinical and epidemiological characteristics of cutaneous leishmaniasis in Sri Lanka. BMC Infect Dis. 2018;18(1):1–9. doi: 10.1186/s12879-017-2892-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galgamuwa LS, Sumanasena B, Yatawara L, Wickramasinghe S, Iddawela D. Clinico-epidemiological patterns of cutaneous leishmaniasis patients attending the Anuradhapura teaching hospital, Sri Lanka. Korean J Parasitol. 2017;55(1):1–7. doi: 10.3347/kjp.2017.55.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundar S, Chakravarty J. Drug Resistance in Leishmaniasis. Antimicrob Drug Resist Leishmaniasis Antimicrob Drug Resist. 2017;1293–304. [Google Scholar]
- 31.WHO Fact Sheet, 2021. WHO Fact Sheet on Leishmaniasis. Geneva, Switzerland, World Health Organization. Available from: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. Accessed July 10, 2021. [Google Scholar]
- 32.Desjeux P, Alvar J. Leishmania/HIV co-infections: Epidemiology in Europe. Ann Trop Med Parasitol. 2003;97(SUPPL. 1):3–15. doi: 10.1179/000349803225002499 [DOI] [PubMed] [Google Scholar]
- 33.Alvar J, Aparicio P, Aseffa A, Den Boer M, Cañavate C, Dedet JP, et al. The relationship between leishmaniasis and AIDS: The second 10 years. Clin Microbiol Rev. 2008;21(2):334–59. doi: 10.1128/CMR.00061-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.da Silva GAR, Sugui D, Nunes RF, de Azevedo K, de Azevedo M, Marques A, et al. Mucocutaneous Leishmaniasis/HIV Coinfection Presented as a Diffuse Desquamative Rash. Case Rep Infect Dis. 2014;2014:1–5. doi: 10.1155/2014/293761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhor R, Rafati S, Pai K. Cytokine saga in visceral leishmaniasis. Cytokine. 2021;147(September):155322. doi: 10.1016/j.cyto.2020.155322 [DOI] [PubMed] [Google Scholar]
- 36.Lockwood DNJ, Sundar S. Serological tests for visceral leishmaniasis. Br Med J. 2006;333(7571):711–2. doi: 10.1136/bmj.38989.567083.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sundar S, Agrawal S, Pai K, Chance M, Hommel M. Detection of leishmanial antigen in the urine of patients with visceral leishmaniasis by a latex agglutination test. Am J Trop Med Hyg. 2005;73(2):269–71. [PubMed] [Google Scholar]
- 38.Kumar R, Pai K, Pathak K, Sundar S. Enzyme-linked immunosorbent assay for recombinant K39 antigen in diagnosis and prognosis of Indian visceral leishmaniasis. Clin Diagn Lab Immunol. 2001;8(6):1220–4. doi: 10.1128/CDLI.8.6.1220-1224.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Lima CMF, Magalhães AS, Costa R, Barreto CC, Machado PRL, Carvalho EM, et al. High Anti-Leishmania IgG Antibody Levels Are Associated With Severity of Mucosal Leishmaniasis. Front Cell Infect Microbiol. 2021;11(April):1–6. doi: 10.3389/fcimb.2021.652956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashiguchi Y, Gomez EL, Kato H, Martini LR, Velez LN, Uezato H. Diffuse and disseminated cutaneous leishmaniasis: Clinical cases experienced in Ecuador and a brief review. Trop Med Health. 2016;44(1):1–9. doi: 10.1186/s41182-016-0002-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turetz ML, Machado PR, Ko AI, Alves F, Bittencourt A, Almeida RP, et al. Disseminated leishmaniasis: A new and emerging form of leishmaniasis observed in Northeastern Brazil. J Infect Dis. 2002;186(12):1829–34. doi: 10.1086/345772 [DOI] [PubMed] [Google Scholar]
- 42.Lindoso JAL, Cunha MA, Queiroz IT, Moreira CHV. Leishmaniasis–HIV coinfection: Current challenges. HIV/AIDS—Res Palliat Care. 2016;8:147–56. doi: 10.2147/HIV.S93789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaffary F, Nilforoushzadeh MA, Abdellahi L, Poor HT. Antimonial treatment failure rate in patients with cutaneous leishmaniasi. Tehran Univ Med J. 2018;76(3):197–203. [Google Scholar]
- 44.Kip AE, Schellens JHM, Beijnen JH, Dorlo TPC. Clinical Pharmacokinetics of Systemically Administered Antileishmanial Drugs. Clin Pharmacokinet. 2018;57(2):151–76. doi: 10.1007/s40262-017-0570-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sundar S, Chakravarty J, Meena LP. Leishmaniasis: treatment, drug resistance and emerging therapies. Expert Opin Orphan Drugs. 2019;7(1):1–10. [Google Scholar]
- 46.Hefnawy A, Berg M, Dujardin JC, De Muylder G. Exploiting Knowledge on Leishmania Drug Resistance to Support the Quest for New Drugs. Trends Parasitol. 2017;33(3):162–74. doi: 10.1016/j.pt.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 47.Rai S, Bhaskar, Goel SK, Nath Dwivedi U, Sundar S, Goyal N. Role of Efflux Pumps and Intracellular Thiols in Natural Antimony Resistant Isolates of Leishmania donovani. PLoS One. 2013;8(9):1–16. doi: 10.1371/journal.pone.0074862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carter KC, Hutchison S, Henriquez FL, Légaré D, Ouellette M, Roberts CW, et al. Resistance of Leishmania donovani to sodium stibogluconate is related to the expression of host and parasite γ-glutamylcysteine synthetase. Antimicrob Agents Chemother. 2006;50(1):88–95. doi: 10.1128/AAC.50.1.88-95.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haldar AK, Sen P, Roy S. Use of Antimony in the Treatment of Leishmaniasis: Current Status and Future Directions. Mol Biol Int. 2011;2011:1–23. doi: 10.4061/2011/571242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ashutosh, Sundar S, Goyal N. Molecular mechanisms of antimony resistance in Leishmania. J Med Microbiol. 2007;56(PART 2):143–53. doi: 10.1099/jmm.0.46841-0 [DOI] [PubMed] [Google Scholar]
- 51.Berg M, García-Hernández R, Cuypers B, Vanaerschot M, Manzano JI, Poveda JA, et al. Experimental resistance to drug combinations in leishmania donovani: Metabolic and phenotypic adaptations. Antimicrob Agents Chemother. 2015;59(4):2242–55. doi: 10.1128/AAC.04231-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeddi F, Piarroux R, Mary C. Antimony resistance in Leishmania, focusing on experimental research. J Trop Med. 2011;2011. doi: 10.1155/2011/695382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vergnes B, Gourbal B, Girard I, Sundar S, Drummelsmith J, Ouellette M. A proteomics screen implicates HSP83 and a small kinetoplastid calpain-related protein in drug resistance in Leishmania donovani clinical field isolates by modulating drug-induced programmed cell death. Mol Cell Proteomics. 2007;6(1):88–101. doi: 10.1074/mcp.M600319-MCP200 [DOI] [PubMed] [Google Scholar]
- 54.Tandon R, Chandra S, Baharia RK, Das S, Misra P, Kumar A, et al. Characterization of the proliferating cell nuclear antigen of leishmania donovani clinical isolates and its association with antimony resistance. Antimicrob Agents Chemother. 2014;58(6):2997–3007. doi: 10.1128/AAC.01847-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kazemi-Rad E, Mohebali M, Erfan MBK, Saffari M, Raoofian R, Hajjaran H, et al. Identification of antimony resistance markers in Leishmania tropica field isolates through a cDNA-AFLP approach. Exp Parasitol. 2013;135(2):344–9. doi: 10.1016/j.exppara.2013.07.018 [DOI] [PubMed] [Google Scholar]
- 56.Haldar AK, Yadav V, Singhal E, Bisht KK, Singh A, Bhaumik S, et al. Leishmania donovani isolates with antimony-resistant but not -sensitive phenotype inhibit sodium antimony gluconate-induced dendritic cell activation. PLoS Pathog. 2010;6(5):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mukhopadhyay R, Mukherjee S, Mukherjee B, Naskar K, Mondal D, Decuypere S, et al. Characterisation of antimony-resistant Leishmania donovani isolates: Biochemical and biophysical studies and interaction with host cells. Int J Parasitol. 2011;41(13–14):1311–21. doi: 10.1016/j.ijpara.2011.07.013 [DOI] [PubMed] [Google Scholar]
- 58.Ouakad M, Vanaerschot M, Rijal S, Sundar S, Speybroeck N, Kestens L, et al. Increased metacyclogenesis of antimony-resistant Leishmania donovani clinical lines. Parasitology. 2011;138(11):1392–9. doi: 10.1017/S0031182011001120 [DOI] [PubMed] [Google Scholar]
- 59.El Fadili K, Drummelsmith J, Roy G, Jardim A, Ouellette M. Down regulation of KMP-11 in Leishmania infantum axenic antimony resistant amastigotes as revealed by a proteomic screen. Exp Parasitol. 2009;123(1):51–7. doi: 10.1016/j.exppara.2009.05.013 [DOI] [PubMed] [Google Scholar]
- 60.Das S, Shah P, Tandon R, Yadav NK, Sahasrabuddhe AA, Sundar S, et al. Over-expression of cysteine leucine rich protein is related to SAG resistance in clinical isolates of leishmania donovani. PLoS Negl Trop Dis. 2015;9(8):1–18. doi: 10.1371/journal.pntd.0003992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh R, Kumar D, Duncan RC, Nakhasi HL, Salotra P. Overexpression of histone H2A modulates drug susceptibility in Leishmania parasites. Int J Antimicrob Agents. 2010;36(1):50–7. doi: 10.1016/j.ijantimicag.2010.03.012 [DOI] [PubMed] [Google Scholar]
- 62.Brotherton MC, Bourassa S, Leprohon P, Légaré D, Poirier GG, Droit A, et al. Proteomic and genomic analyses of antimony resistant Leishmania infantum mutant. PLoS One. 2013;8(11):23–7. doi: 10.1371/journal.pone.0081899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cummings HE, Tuladhar R, Satoskar AR. Cytokines and their STATs in cutaneous and visceral leishmaniasis. J Biomed Biotechnol. 2010;2010. doi: 10.1155/2010/294389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Valiathan R, Ashman M, Asthana D. Effects of Ageing on the Immune System: Infants to Elderly. Scand J Immunol. 2016;83(4):255–66. doi: 10.1111/sji.12413 [DOI] [PubMed] [Google Scholar]
- 65.Alexander J, Satoskar AR, Russell DG. Leishmania species: Models of intracellular parasitism. J Cell Sci. 1999;112(18):2993–3002. [DOI] [PubMed] [Google Scholar]
- 66.Derraik JGB, Rademaker M, Cutfield WS, Pinto TE, Tregurtha S, Faherty A, et al. Effects of age, gender, BMI, and anatomical site on skin thickness in children and adults with diabetes. PLoS One. 2014;9(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murata Y, Shimamura T, Hamuro J. The polarization of Th1/Th2 balance is dependent on the intracellular thiol redox status of macrophages due to the distinctive cytokine production. Int Immunol. 2002;14(2):201–12. doi: 10.1093/intimm/14.2.201 [DOI] [PubMed] [Google Scholar]
- 68.Frézard F, Demicheli C, Ferreira CS, Costa MAP. Glutathione-induced conversion of pentavalent antimony to trivalent antimony in meglumine antimoniate. Antimicrob Agents Chemother. 2001;45(3):913–6. doi: 10.1128/AAC.45.3.913-916.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Giustarini D, Dalle-Donne I, Lorenzini S, Milzani A, Rossi R. Age-related influence on thiol, disulfide, and protein-mixed disulfide levels in human plasma. Journals Gerontol—Ser A Biol Sci Med Sci. 2006;61(10):1030–8. [DOI] [PubMed] [Google Scholar]
- 70.Paddy statistics-2016/2017 Maha Season [Internet]. Department of Census and Statistics, Ministry of National Policies and Economic Affairs; 2017. Available from: http://www.statistics.gov.lk
- 71.D.O.S. Statistical Handbook 2010. Stat Handb. 2017;(August):2010.
- 72.Marine small-scale fisheries of Sri Lanka: A general description. 1984.
- 73.Jayasumana M a. CS, Paranagama P a., Amarasinghe MD, Wijewardane KMRC, Dahanayake KS, Fonseka SI, et al. Possible link of Chronic arsenic toxicity with Chronic Kidney Disease of unknown etiology in Sri Lanka. J Nat Sci Res. 2013;3(1):64–73. [Google Scholar]
- 74.Jayasumana C, Fonseka S, Fernando A, Jayalath K, Amarasinghe M, Siribaddana S, et al. Phosphate fertilizer is a main source of arsenic in areas affected with chronic kidney disease of unknown etiology in Sri Lanka. Springerplus. 2015;4(1):1–8. doi: 10.1186/s40064-015-0868-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wijayawardhana D, Herath V, Weerasinghe A. Heavy Metal Pollution in Sri Lanka with Special Reference to Agriculture: A Review of Current Research Evidence. Rajarata Univ J. 2016;4(1):52–6. [Google Scholar]
- 76.Amarasinghe A, Wickramasinghe S. A Comprehensive Review of Cutaneous Leishmaniasis in Sri Lanka and Identification of Existing Knowledge Gaps. Acta Parasitol. 2020;65(2):300–9. doi: 10.2478/s11686-020-00174-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Siriwardena H V., Udagedara CU, Karunaweera ND. Clinical features, risk factors and efficacy of cryotherapy in cutaneous leishmaniasis in Sri Lanka. Vol. 48, The Ceylon medical journal. 2003. p. 10–2. doi: 10.4038/cmj.v48i1.3386 [DOI] [PubMed] [Google Scholar]
- 78.Ranawaka RR, Weerakoon HS, Opathella N. Liquid nitrogen cryotherapy on Leishmania donovani cutaneous leishmaniasis. J Dermatolog Treat. 2011;22(4):241–5. doi: 10.3109/09546631003762654 [DOI] [PubMed] [Google Scholar]