Abstract
Accumulating evidence suggests that phosphatases play an important role in regulating a variety of signal transduction pathways that have a bearing on cancer. The kinase-associated phosphatase (KAP) is a human dual-specificity protein phosphatase that was identified as a Cdc2- or Cdk2-interacting protein by a yeast two-hybrid screening, yet the biological significance of these interactions remains elusive. We have identified the KAP gene as an overexpressed gene in breast and prostate cancer by using a phosphatase domain-specific differential-display PCR strategy. Here we report that breast and prostate malignancies are associated with high levels of KAP expression. The sublocalization of KAP is variable. In normal cells, KAP is primarily found in the perinuclear region, but in tumor cells, a significant portion of KAP is found in the cytoplasm. Blocking KAP expression by antisense KAP in a tetracycline-regulatable system results in a reduced population of S-phase cells and reduced Cdk2 kinase activity. Furthermore, lowering KAP expression led to inhibition of the transformed phenotype, with reduced anchorage-independent growth and tumorigenic potential in athymic nude mice. These findings suggest that therapeutic intervention might be aimed at repression of KAP gene overexpression in human breast and prostate cancer.
Human cancer development is a multistage process that results from the stepwise acquisition of genetic alterations. These alterations may involve the dysregulation of a variety of normal cellular functions, leading to the initiation and progression of a tumor. Among normal cellular functions, regulatory control of the cell cycle plays an important role in normal cell proliferation, and genetic alterations that affect cell cycle control have been shown to be associated with tumor progression (reviewed in references 26, 28, 30, 32, 44, and 46). The transition from one stage of the cell cycle to another is regulated by the transcription of a number of cyclin genes, the degradation of cyclin proteins, and the modification of the cyclin-dependent kinase proteins by phosphorylation (reviewed in references 11, 29, and 30). These controls play important roles in preventing tumorigenesis (26, 28, 30, 31).
Cell cycle progression in mammals requires multiple cyclin-dependent kinases (Cdks) (44). The activity of these kinases depends on their association with a family of positive regulatory protein subunits known as cyclins during the cell cycle. Proteins that interact with Cdks play distinct and specific roles in cell cycle regulation. Among these, the mammalian G1 Cdk inhibitors have been shown to be involved in diverse processes such as repair of DNA damage, differentiation, tumor suppression, and cellular senescence (12, 22, 24, 25, 37, 52). The identification of these negative regulators of Cdks has provided key insights into how the cell cycle can be controlled.
A Cdk-interacting protein called KAP/Cdi1 was first identified as a novel G1- and S-phase dual-specificity phosphatase that associates with Cdk2 and/or Cdc2 (22, 24). Further studies demonstrated that kinase-associated phosphatase (KAP) binds to Cdk2 and dephosphorylates Thr160 when the associated cyclin subunit is degraded or dissociated (2, 42). However, the biological significance of the interactions remains to be elucidated. It has been reported that KAP may inactivate a specific protein kinase, probably Cdk2 or Cdc2, by removing phosphates from the cyclin complexes, and this may contribute to cell cycle control (2, 24, 42). However, the physiological substrate(s) for tyrosine dephosphorylation of KAP has not yet been identified.
Accumulating evidence suggests that deregulation of protein phosphorylation is a key event in neoplastic transformation. Phosphatases have also been shown to play an important role in regulating a variety of signal transduction pathways that have a bearing on cancer (reviewed in references 5, 9, 29, 31, 36, and 39). In an effort to search for candidate genes whose function or expression is altered causally in carcinogenesis, we identified the KAP gene as an overexpressed gene in human breast and prostate cancer using differential screening. We report that breast and prostate malignancies are associated with high levels of KAP expression. We demonstrate further that selective inhibition of KAP overexpression by an antisense approach in the HeLa and LNCaP cancer cell lines decreased their transformed phenotype in vitro and their tumorigenicity in nude mice. In addition, blocking KAP overexpression resulted in a decreased population of S-phase cells during cell cycle progression and reduced Cdk2 kinase activity. Together, these data suggest that KAP plays a role in normal growth control and that deregulated KAP expression may contribute to the malignant phenotypes.
MATERIALS AND METHODS
Ds-DD.
The quality of total RNA from each normal and tumor cell was tested by Northern blot analysis before the reverse transcription. Total RNA (1 μg) was reverse transcribed in a final volume of 20 μl using oligo(dT) or random primers, 20 μM deoxynucleoside triphosphates, and 200 U of Super Script II reverse transcriptase (Life Technologies, Inc.) and incubated for 60 min at 37°C. The cDNA products were used for display PCRs. Domain-specific differential display (Ds-DD) was performed by modifying the conventional differential-display (DD) technique, which uses random artificial primers for the PCR (34). Instead of using a short, arbitrary 5′ primer and 3′ one- or two-base anchored deoxyribosylthymine primers for PCR, primers representing the tyrosine phosphatase catalytic domain motif (VHCSAG) (21, 40) and an arbitrary primer (OPA1; Operon Inc.) were used in the display PCRs (1 μM each). Each cDNA reaction mixture (2 μl) was amplified by PCR in the presence of 35S-dATP for labeling. The PCR cycling parameters were as follows: 94°C for 20 s, 40°C for 1 min, and 72°C for 45 s (30 cycles) and then 72°C for 5 min. The PCR products were denatured at 80°C for 2 min and then electrophoresed through a 6% polyacrylamide denaturing gel at 80 W for 2.5 h. The gels were then dried and exposed to autoradiography overnight at −80°C. cDNAs of interest were cut out of the gel and boiled in 100 μl of double-distilled water. Three to five microliters was reamplified by PCR using the same primers as those described above but without the radioactive nucleotide. The PCR products were run onto an agarose gel, purified, labeled with a 32P-labeled random primer, and used as a probe for Northern blot analysis. The candidate clones were selected for further experiments. The positive clones whose expression was differentially regulated in normal and cancer cells were sequenced by the dideoxynucleotide chain termination method with Sequenase (USB Inc.).
Cell lines and cultures.
Primary human normal mammary epithelial cells (hNMECs) were established from reduction mammoplasties obtained through the Cooperative Human Tissue Network (CHTN) and were designated 12N, 15N, and 17N, as described previously (1, 13, 23). These cells were grown in DFCI-1 medium (D complete) as described previously (1) and were used at early to mid-passage, i.e., 5 to 10 population doublings. Human breast cancer cells (HBL100, BT20, T47D, SKBR3, ZR75-1, MDAMB231, MDAMB435, MDAMB436, MDAMB157) were obtained from the American Type Culture Collection (ATCC). 21MT-2 was provided by V. Band. MCF10 and MCF7 were gifts from S. Ethier (University of Michigan). Benzo[a]pyrene-immortalized mammary epithelial cell line 184B5 was also obtained from ATCC. Growth medium for these mammary cancer cells was Dulbecco modified Eagle medium (DMEM)–10% fetal bovine serum (FBS). Primary human normal prostate cells were derived from normal adjacent tumor tissue biopsies received from CHTN and were designated NPrEC-1 and -2. These cultures were grown in Clonetics prostate epithelial media. The human prostate carcinoma cell lines PC3, LNCaP, and DU145 were purchased from the ATCC and maintained in DMEM–10% FBS. ND1 was kindly provided by P. Narayan. HeLa-tTA cells, which contain the transactivator for tetracycline (tet)-regulated expression, were obtained from Clontech Inc. pTet-AS-KAP, pTet-Sense-KAP, or pTet-luciferase plasmid DNA was transfected into HeLa-tTA cells using the standard Lipofectin (Lipofectamine; GIBCO-BRL) methods. Transfectants were double selected in the presence of hygromycin (150 μg/ml) and G418 (500 μg/ml). Individual clones of stable transfectants, designated HeLa/AS-KAP or HeLa/luciferase, were selected for further analysis. Stable transfectants from the pTet-Sense-KAP transfection were selected, and resistant colonies were pooled for further analysis. All the stable clones were cultured in the presence or absence of tet (2 μg/ml) in DMEM–10% FBS. To induce the expression of antisense KAP, sense KAP, or luciferase in HeLa cells, cells were washed three times with phosphate-buffered saline (PBS) and fresh medium without tet was added.
Soft-agar clonogenic assay.
For soft-agar assays, cells were plated at a density of 104 in 35-mm-diameter tissue culture plates containing 0.33% top low-melt agarose–0.6% bottom low-melt agarose. After the first 4 days, cells were fed every 3 days. Foci or colonies were counted and measured after 2 weeks.
Immunohistoperoxidase staining.
Immunohistologic studies were performed on 5-μm-thick frozen sections cut from tissues embedded in OCT. Tissue blocks (breast and prostate) were obtained from the CHTN, Eastern Division. Immunostaining of sections was performed by the avidin-biotin peroxidase complex (ABC) method using the Vectastain Elite ABC kit (Vector Laboratories) as follows. Tissues were treated to remove endogenous peroxidase activity, blocked with goat serum for 30 min at room temperature, and then incubated with anti-KAP monoclonal antibodies (Transduction Labs and Santa Cruz Biotechnology Inc.; 10 μg/ml) for 2 h. Sections were washed three times in PBS–0.1% Tween 20 (PBST) and then incubated with a secondary anti-rabbit antibody conjugated to horseradish peroxidase (HRP) for 30 min. Following PBS washes, the sections were incubated with ABC Elite reagent for 30 min at room temperature and washed three times in PBS. The bound HRP complexes were developed using diaminobenzidine tetrahydrochloride (Fastdab; Sigma) according to the manufacturer's instructions. The sections were counterstained with hematoxylin, dehydrated, and mounted with glass coverslips.
KAP immunofluorescence.
Cells were plated onto chamber slides (Lab-Tek) and, when 50% confluent, were fixed with 3.7% paraformaldehyde in PBS. Cells were permeabilized with 0.1% Triton X-100 for 5 min prior to immunostaining. Staining was carried out as follows: cells were blocked with PBST–5% goat serum–3% milk powder and then incubated with KAP antibody (K32120; Transduction Labs) at a concentration of 5 μg/ml for 2 h, followed by incubation with secondary anti-mouse fluorescein isothiocyanate (FITC) antibody for 1 h.
Western and Northern blot analysis.
For Western blot analysis, samples were adjusted for equal protein levels and separated on sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis (SDS–12% PAGE) gels under reducing conditions. Gels were transferred to a nitrocellulose membrane, and blots were probed with antibodies to KAP (Transduction Labs and Santa Cruz Biotechnology), Cdc2 and Cdk2 (Santa Cruz Biotechnology), and β-actin (clone AC-15; Sigma). Bands were detected using the ECL chemiluminescence detection method (Amersham) and exposed to X-ray film. For Northern blot analysis, total RNA was extracted, denatured, and electrophoresed through a 1% agarose–formaldehyde gel (20 μg of total RNA/lane). The gel was transferred to a nylon membrane (Bio-Rad) and hybridized as follows. Human KAP, histone H4, and 36B4 (loading control) probes were 32P-labeled by using randomly primed DNA labeling techniques. Blots were exposed on X-ray film after washing. In all cases, films were scanned (ScanJet IIcs; Hewlett-Packard) and analyzed using Adobe Photoshop software.
FACS analysis.
Subconfluent cultures with and without tet were pulse-labeled for 30 min with 10 μM bromodeoxyuridine (BrdU; Sigma), harvested, fixed in 70% ethanol, and then double stained with FITC-conjugated anti-BrdU antibody (Becton Dickinson) and 5 μg of propidium iodide (Sigma)/ml. Cell cycle analysis was performed on a fluorescence-activated cell sorter (FACS; FACScan; Coulter). Data were analyzed using Elite software (Coulter).
In vitro kinase assay.
Following lysis, 500 μg of total cell protein per sample was precleared with 20 μl of protein A-triacryl GF-2000 beads (Pierce) for 30 min at 4°C. Immunoprecipitation of Cdk2 was performed overnight with 2 μl of anti-Cdk2 (M2; Santa Cruz Biotechnology). Immunoprecipitates were washed three times with NET-N (20 mM Tris [pH 8.0], 100 mM NaCl, 1 mM EDTA, 0.5% NP-40, 100 mM sodium fluoride, 2 mM sodium vanadate, 2 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin/ml) and twice in kinase buffer (50 mM Tris [pH 7.4], 10 mM MgCl, 1 mM dithiothreitol). For the kinase reaction, immunocomplexes were incubated in kinase buffer supplemented with 50 μM ATP, 5 μCi of [γ-32P]ATP (Amersham), and 10 μg of histone H1 (Boehringer Mannheim) for 30 min at 30°C, and the reaction was terminated by adding sample buffer. Samples were then separated on an SDS–10% PAGE gel, and the phosphorylated H1 was visualized by autoradiography.
Tumorigenicity assay of nude mice.
HeLa cells were harvested and washed twice in PBS. Cells were resuspended in 0.25 ml of cold serum-free DMEM, and the suspension was mixed with an equal volume of cold Matrigel (10 mg/ml; Becton Dickinson) at a final concentration of 2 × 106 cells/ml. The cell suspension was injected subcutaneously (bilaterally; 0.5 ml per site, 106 cells) into 5- to 6-week-old nude athymic mice [Taconic; Cr:(NCr)-nu fBR]. Tumors were harvested 3 weeks after injection and weighed. Some mice were fed with water containing 500 μg of doxycycline/ml in 1% sucrose.
Mitotic index determination.
Tumors were harvested, weighed, fixed in formalin, and processed for routine histology and hematoxylin and eosin staining. Tumor sections were examined for mitotic figures by counting mitotic figures from 10 × 40 fields per tumor. Each group contained six tumors.
RESULTS
Identification of the KAP gene as an overexpressed gene in breast cancer through a modified DD-PCR method.
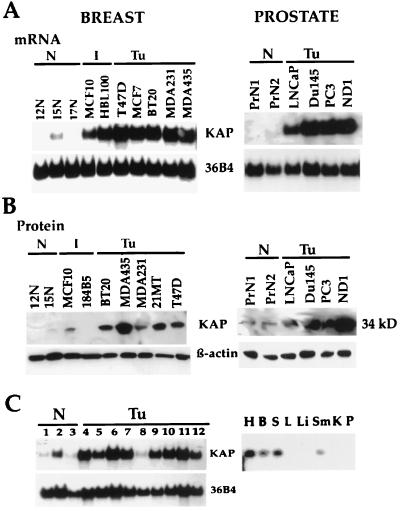
Differential hybridization reactions were performed to compare the mRNA expression profiles of normal (12N and 15N) and cancerous (MCF7 and MDAMB435) human breast epithelial cells. Degenerate PCR primers based on the sequences of the regions exhibiting the well-conserved catalytic domains of protein tyrosine phosphatases (PTP) were designed to amplify cDNA fragments for DD-PCR. A reverse 3′ primer corresponding to a portion of the PTP catalytic domains (5, 9, 21) (the VHCSAG motif) and an arbitrary primer (OPA1; Operon, Inc.) as the forwarding 5′ primer were used for the DD-PCR experiments. This PCR-based DD using a conserved domain primer of known proteins from the PTP catalytic domain produced a less banded but more clearly resolved pattern on the gel than conventional DD-PCR. Potentially differentially expressed clones were selected for further characterization by Northern blot analysis and DNA sequencing. A PCR product, named initially PTP-2, was identified from the display gel. The PTP-2 clone was strongly overexpressed in human breast cancer cells. Initial DNA sequencing of a PTP-2 cDNA partial clone revealed that it encoded a protein phosphatase which was previously identified as KAP/Cdi1, a dual-specificity phosphatase (22, 24). Using the full-length KAP cDNA as a probe, we examined expression levels in normal and tumor human mammary epithelial cells as well as normal and tumor prostate cells by Northern and Western blot analyses. The breast and prostate cancer cell lines examined had levels of KAP mRNA (Fig. 1A) and protein expression (Fig. 1B) that were variable but that were substantially higher, regardless of estrogen receptor status, than the levels of expression detected in any of the human normal mammary and prostate epithelial cells analyzed (Fig. 1A and B). KAP was also overexpressed in a majority of human tumor cells of epithelial origin tested (Fig. 1C, left). KAP mRNA expression in various human tissues was also examined (Fig. 1C, right). Longer exposure of a Northern blot demonstrated the presence of KAP mRNA in normal human tissues. It was expressed at relatively low levels in heart, brain, spleen, and skeletal muscle and was barely detectable in the lung, liver, kidney, and pancreas. This broad expression pattern may imply an important role in many cell types.
FIG. 1.
Overexpression of KAP in human mammary and prostate carcinoma cell lines. (A) Northern blot analysis showed overexpression of KAP mRNA in human mammary and prostate cancer cell lines (Tu) compared to normal human breast (12N, 15N, 17N) and prostate (PrN1, PrN2) epithelial cells. Twenty micrograms of total RNA, isolated from exponentially growing cells (70 to 80% confluence), was hybridized to a 32P-labeled KAP cDNA probe. A 36B4 cDNA probe was used as a loading control. I, immortal. (B) Western blot analysis was used to determine the level of KAP protein expression in several normal breast (12N and 15N) and prostate (PrN1 and PrN2) cell lines and in a number of immortal and cancerous mammary cell lines. Aliquots of cell lysates obtained from the indicated cell lines were loaded in each lane. Blots were probed with antibodies to KAP and β-actin. Immunoblotting for β-actin was used to achieve equal loading for protein samples. (C) KAP mRNA expression patterns in a variety of human normal and tumor cell lines (left) and in normal human tissues (right). Lanes 1 to 3, human normal cells; lanes 4 to 12, human cancer cell lines. Lane 1, hNMECs; lane 2, human normal urothelium; lane 3, human normal keratinocytes; lane 4, neuroblastoma cell line (SK-M-MC); lane 5, HeLa cells; lane 6, Jurkat cells; lane 7, 293 cells; lane 8, a renal carcinoma cell line (UMRC); lane 9, small-cell lung carcinoma cell line (H747); lane 10, melanoma (SK-MEL5); lane 11, osteosarcoma (HOS); lane 12, colon carcinoma cell line (HT29). Right, expression of KAP in human normal tissues. H, heart; B, brain; S, spleen; L, lung; Li, liver; Sm, skeletal muscle; K, kidney; P, pancreas.
Tumor-specific overexpression of KAP in human breast and prostate cancer patient specimens.
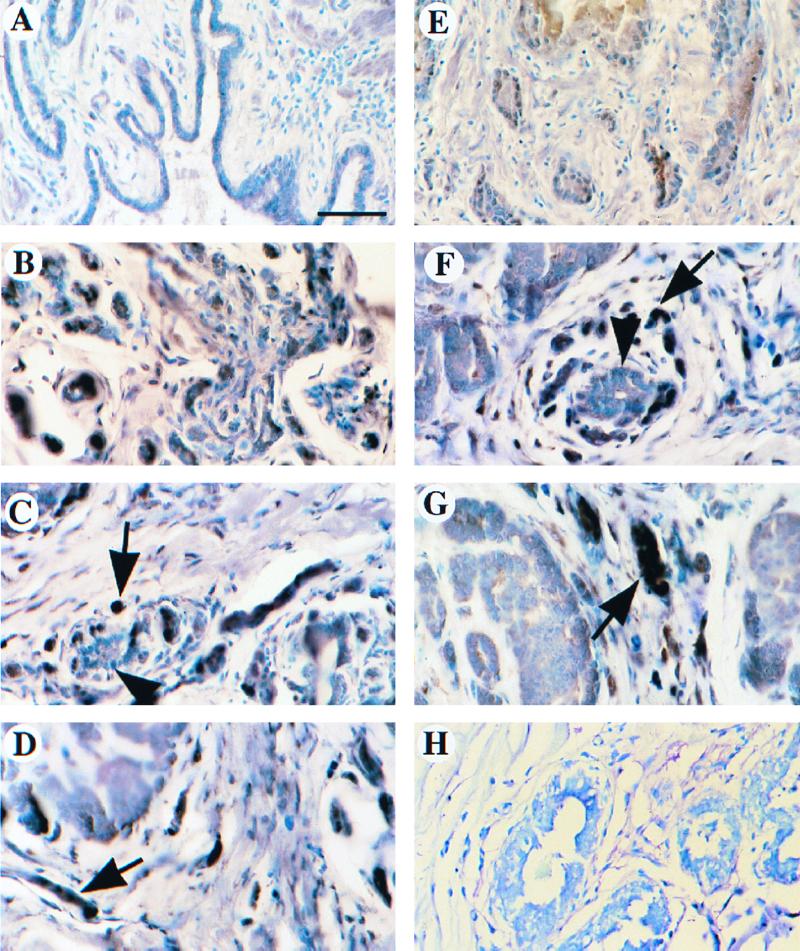
The expression of the KAP protein was evaluated using immunohistochemistry in a number of normal and tumorous tissue specimens of human breast and prostate to further define the relationship between levels of KAP expression in normal and cancer cells. A total of 32 different breast tissue samples were analyzed, including 13 normal biopsies and 19 tumor biopsies (10 in situ carcinomas and 9 infiltrating ductal carcinomas). In addition, expression of the KAP protein in 12 matched (total of 24 samples) normal and tumorous human prostate specimens was evaluated. There was little or no detectable expression of KAP either in the epithelial or stromal cells of ductal units of normal breast or prostate tissue (Fig. 2A and E). However, many tumors (15 of 19 breast and 9 of 12 prostate tumor specimens) showed intense immunostaining for the KAP protein in the majority of tumor cells (Fig. 2B, C, and D [prostate cancer], and Fig. 2F and G [breast cancer]).
FIG. 2.
Tumor-specific overexpression of KAP in breast and prostate cancer patient samples. Expression of the KAP protein was evaluated using immunohistochemistry for a number of human normal and tumor tissue specimens of prostate (A to D) and breast (E to H). (A) Normal prostate gland had no noticeable staining of KAP protein. (B) Weakly invasive carcinoma of the prostate stained with high levels of KAP in epithelial cells. (C and D) Invasive carcinoma of the prostate showed intense cytoplasmic and nuclear immunoreactivity (arrow). Note normal prostate gland (arrowhead) with low or no KAP expression. (E) Normal breast epithelium had low expression of KAP. (F) Invasive carcinoma cells of the breast showed strong staining for KAP protein (arrow). Normal ducts with lower KAP expression are also shown in the same field (arrowhead). (G) KAP expression led to stronger staining in an invasive carcinoma than in a preinvasive ductal carcinoma in situ (arrow). (H) Negative control with normal immunoglobulin G showed no immunoreactivity in both invasive and ductal carcinomas in situ. Bar, 75 μm. Tissues were fixed in 3% paraformaldehyde and embedded in paraffin, and 5-μm-thick sections were processed for detection of the KAP protein. Immunocomplexes were identified by an ABC procedure, and 1% toluidine blue was used as a counterstain. There was little or no detectable expression of KAP either in the epithelial or stromal cells of ductal units of normal breast or prostate. However, in many tumors, large numbers of KAP proteins were displayed in a majority of cells.
As shown in Fig. 2A, normal prostate gland had no detectable expression of the KAP protein. However, a weakly invasive carcinoma of the prostate stained with relatively high levels of KAP in epithelial cells (Fig. 2B). An invasive carcinoma of the prostate showed intense cytoplasmic and nuclear immunoreactivity (Fig. 2C and D), whereas normal prostate tissue shown in the same field had little KAP expression. Normal mammary gland epithelium also stained very weakly for KAP (Fig. 2E). In contrast, invasive carcinoma cells of the breast (Fig. 2F) showed significant expression of the KAP protein. A normal ductal unit having lower KAP expression is also shown in the same field (Fig. 2F). In breast cancer samples, KAP expression led to staining that was more intense in an invasive carcinoma than in a preinvasive ductal carcinoma in situ (Fig. 2G). These findings indicate that KAP overexpression is a frequent occurrence in breast and prostate cancer.
Differential cellular localization of KAP between normal and tumor mammary epithelial cells.
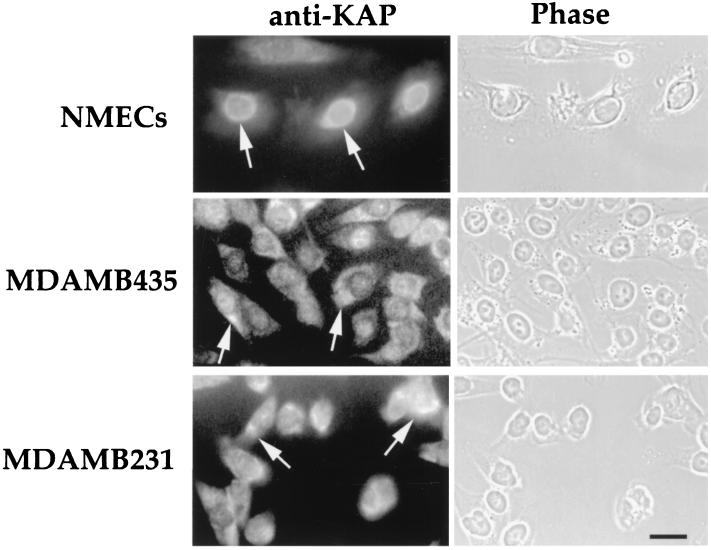
The cellular distribution of the KAP protein was next analyzed by immunofluorescence (Fig. 3). In hNMECs, KAP staining was predominantly perinuclear, while breast tumor cells (MDAMB435 and MDAMB231) exhibited staining in both the cytoplasm and perinuclear area. Moreover, the pattern of KAP localization in human cancer cells was more diffuse than that observed in normal cells. The cytoplasmic localization of the KAP protein as well as its overexpression might be involved in KAP dysfunction in breast cancer cells.
FIG. 3.
Subcellular localization of KAP in hNMECs (15N) and tumor mammary epithelial cells (MDAMB435 and MDAMB231). The cultures were fixed with 3.7% paraformaldehyde in PBS. Cells were permeabilized with 0.1% Triton X-100 prior to KAP antibody staining. Notice that the staining was restricted to the perinuclear region in hNMECs, while breast tumor cells (MDAMB435 and MDAMB231) had staining in both the cytoplasm and perinuclear area (arrows). The corresponding phase-contrast photograph is shown at the right of each fluorescence image. Bar, 10 μm.
Effects of antisense inhibition of KAP overexpression on cell cycle progression in human cancer cells.
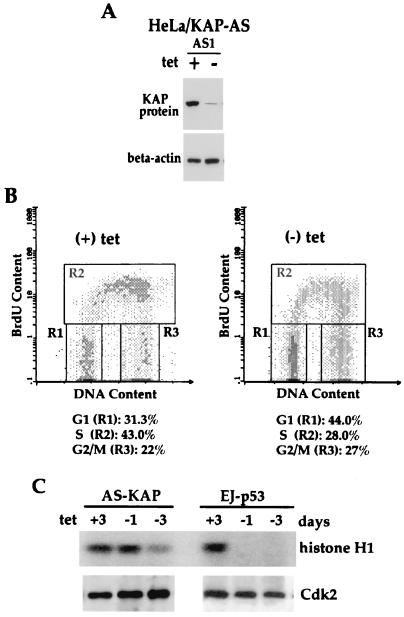
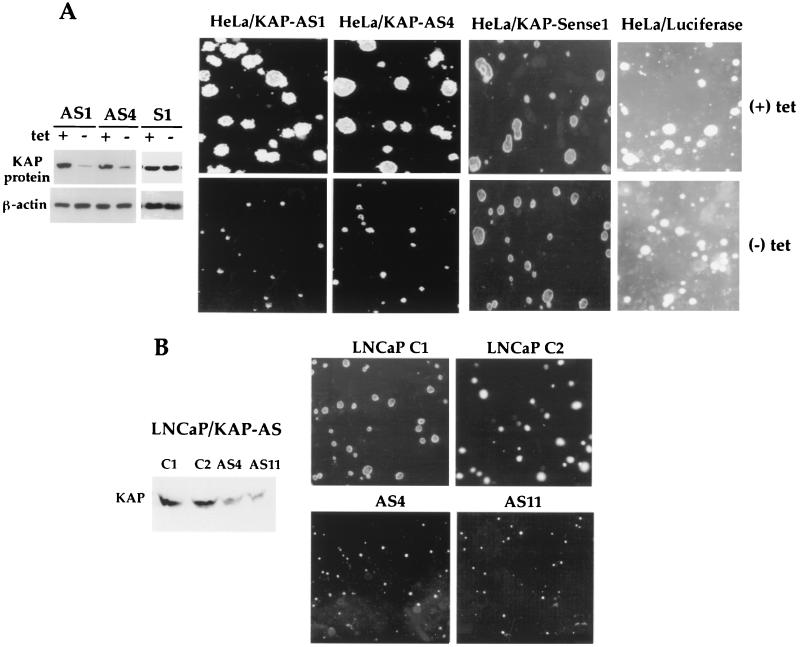
In order to elucidate the role of KAP overexpression in tumor cells, we introduced antisense KAP expression into HeLa and LNCaP cells. In HeLa cells a tet-regulatable expression system (20) was utilized. A BamHI fragment consisting of approximately 550 bp of partial KAP cDNA encoding the N-terminal region and a 5′ untranslated sequence was inserted into a tet-regulated plasmid in the antisense (pTet-AS-KAP) or sense (pTet-S-KAP) orientation. HeLa-tTA cells, which have high KAP expression, were transfected with pTet-AS-KAP, pTet-S-KAP, or pTet-luciferase plasmids. Several stably transfected clones with antisense or sense KAP constructs (HeLa/KAP-sense1) or the luciferase (HeLa/luciferase) expression plasmid were recovered, and the inhibition of KAP protein expression was determined by Western blot analysis. Two clones, designated HeLa/KAP-AS1 (Fig. 4A) and HeLa/KAP-AS4 (Fig. 5A, left), showed reduced levels of KAP proteins within 48 h after removal of tet compared to the level of KAP proteins from the same cells grown in the presence of 2 μg of tet/ml. However, no change in pTet-S-KAP- (sense KAP) or pTet-luciferase-transfected cells with or without tet was observed (Fig. 5A, left).
FIG. 4.
Cell cycle analysis and inhibition of Cdk2-associated histone kinase activity in HeLa/AS-KAP cells following antisense KAP induction. (A) Western blot analysis of KAP in antisense KAP cDNA-transfected HeLa cells (HeLa/KAP-AS1) in the presence or absence of tet (2 μg/ml). (B) Cell cycle analysis was performed on a FACS (FACScan; Becton Dickinson). Cells were maintained with tet (+) or without tet (−) for 3 days. y axis, BrdU uptake, as measured by FITC fluorescence; x axis, DNA content, as measured by propidium iodide fluorescence. Populations of cells in different phases of the cell cycle are gated as shown. The percentage of cells in each gate is indicated for each sample. (C) Upper gel, level of phosphorylation of histone H1 by Cdk2; lower gel, Western blot analysis of Cdk2 protein level at different times after induction of antisense KAP. HeLa/AS-KAP cells were grown in medium with or without tet for the indicated times. In vitro kinase assays were carried out using cell cycle lysates as described in Materials and Methods.
FIG. 5.
Effects of antisense (AS) KAP on the in vitro-transformed phenotype. (A) Suppression of KAP expression by antisense KAP resulted in reduced anchorage-independent growth in soft agarose in tet-regulated HeLa cells. Left, Western blot analysis of KAP in antisense or sense KAP cDNA-transfected HeLa cells in the presence or absence of tet (2 μg/ml); right, anchorage-independent growth of antisense KAP-transfected cells (HeLa/KAP-AS1 and -AS4) as well as of two control lines (HeLa/KAP-sense1 and HeLa/luciferase) in soft agarose for 2 weeks with or without tet. Two stable antisense KAP clones that contained reduced levels of KAP protein exhibited inhibition of colony formation in soft agarose in the absence of tet compared to the same cells in the presence of tet. In addition, colony sizes of HeLa/KAP-sense1 and HeLa/luciferase cells grown in the absence of tet were similar to those of cells grown in the presence of tet. (B) Reduced KAP expression in LNCaP cells resulted in smaller colonies in soft agarose. Western blot analysis shows that antisense KAP-expressing cells (PC3/KAP-AS4 and -AS11) had relatively lower KAP expression than controls. Right, reduced anchorage-independent growth of antisense KAP-expressing LNCaP cells in soft agarose.
Studies were performed to determine if the induction of antisense KAP modifies the kinetics of cell cycle progression. HeLa/KAP-AS1 cells were maintained in the presence or absence of tet for 3 days, followed by analysis using simultaneous flow cytometry for both DNA content and DNA synthesis, with propidium iodide staining and BrdU labeling, respectively. Following tet removal, cells exhibited a reduction of BrdU incorporation, with the population of S-phase cells declining from 43.0% with tet to 28.0% without tet (Fig. 4B). Conversely, the percentages of cells in G1 and G2/M phases increased from 31.3 and 22.0%, respectively, with tet to 44.0 and 27%, respectively, without tet by 3 days. Next, we investigated the effect of decreased KAP expression on Cdk kinase activity with and without antisense KAP induction. HeLa/KAP-AS1 cells cultured in the presence or absence of tet were lysed, and Cdk2 immunocomplexes were assessed for in vitro kinase activity using histone H1 as the substrate. As shown in Fig. 4C, Cdk2 kinase activity was reduced after 3 days of antisense KAP induction by ∼60% as measured by the phosphorylation of histone H1 in the absence of any detectable change in Cdk2 protein level. In contrast, Cdk2 kinase activity was reduced to an undetectable level as early as 24 h after wild-type p53 induction in p53-null human bladder cancer cells (EJ), as expected from the known potent inhibitory activity of p53-induced p21. Inhibition of the cell cycle was consistent with the level of Cdk2 kinase reduction by antisense KAP.
Effects of antisense KAP on the transformed phenotype.
We next evaluated whether inhibition of KAP overexpression had an effect on the in vitro- and in vivo-transformed phenotype. First, a soft-agar colony-forming assay showed that HeLa/KAP-AS1 and -AS4 cells formed smaller colonies in agarose in the absence of tet than in the presence of 2 μg of tet/ml in soft agarose (Fig. 5A, right). However, colony sizes of HeLa/KAP-sense1 and HeLa/luciferase cells grown in the absence of tet were similar to those of cells grown in the presence of tet (Fig. 5A, right). In addition, mammalian expression vector pcDNA3 containing an antisense construct of KAP cDNA (a 550-bp BamHI fragment) driven by a cytomegalovirus promoter was transfected into LNCaP cells. Stable clones were selected, and expression levels of KAP were evaluated by Western blot analysis. Two clones, AS4 and AS11, had reduced levels of KAP protein expression and were further characterized. Consistent with HeLa cells containing antisense KAP, LNCaP/AS4 and -AS11 cells formed smaller colonies in soft agarose (Fig. 5B). Together, these results suggest that antisense inhibition of KAP expression led to suppression of anchorage-independent growth.
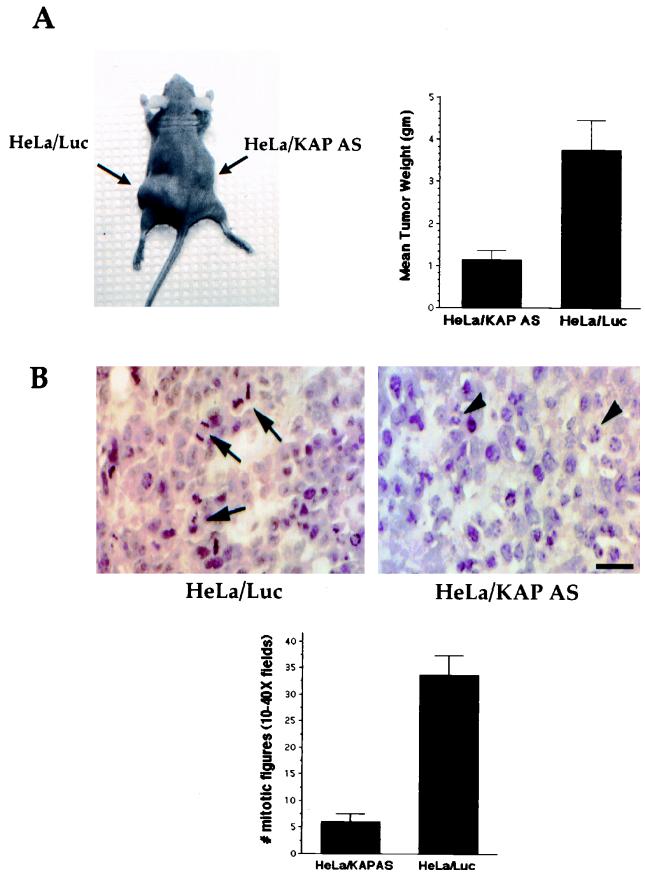
To determine whether inhibition of KAP overexpression reduced tumorigenicity in vivo, xenograft studies were conducted using HeLa cells expressing antisense KAP mRNA and control cells expressing luciferase in the absence of tet. Six mice were injected with each cell population (106 cells mixed with Matrigel) in two different sites. As shown in Fig. 6A, HeLa cells expressing antisense KAP mRNA showed a reduced overall tumor size when injected into nude athymic mice (HeLa-KAP tumors), compared with that shown by HeLa cells expressing a control luciferase vector (HeLa-luciferase tumors). The mean size of HeLa-KAP tumors was 1.15 g, significantly less than that of HeLa-luciferase tumors, which were 3.76 g on average (P < 0.008).
FIG. 6.
Inhibition of KAP overexpression reduces tumorigenicity in nude mice. (A) Antisense (AS) KAP expression inhibited the tumorigenic potential of HeLa cells. Athymic nude mice were injected with 2 × 106 HeLa/KAP-AS1 cells or HeLa/luciferase cells/ml mixed with an equal volume of Matrigel. The cell suspension was injected subcutaneously (bilaterally; 0.5 ml per site) into nude mice. Results are average tumor sizes for six animals in two different sites per experimental condition 3 weeks after injection. The mean tumor size of AS KAP-expressing HeLa cells was significantly less than that of the HeLa-luciferase tumor. Similar relative tumor sizes were obtained from mice injected with HeLa/KAP-AS1 cells and fed with water containing 500 μg of doxycycline/ml (data not shown), compared to sizes of HeLa-luciferase tumors. (B) HeLa/KAP-AS cells showed reduced mitotic index. AS KAP and control tumors harvested after 3 weeks of growth were processed for histological studies (hematoxylin and eosin sections). Top, representative hematoxylin- and eosin-stained section of control (HeLa/Luc) and AS KAP tumors (HeLa/KAP-AS). Mitotic figures are shown as arrows, and apoptotic cells are shown with arrowheads.
Antisense KAP and control KAP tumors harvested after 3 weeks of growth were processed for histological studies. Hematoxylin and eosin sections were examined for mitotic figures (10 × 40 fields for every tumor), and significant differences were observed. HeLa control tumors had a mean of 33.6 mitotic figures, whereas HeLa-antisense KAP tumors showed a significant reduction and on average had 6 mitotic figures, indicating that antisense KAP has an antiproliferative effect. Figure 6B shows a representative hematoxylin and eosin section of a control tumor, displaying several mitotic figures (arrows), whereas a similar section of an antisense KAP tumor shows no mitotic figures (Fig. 6B). In addition, close examination of the antisense KAP tumors (HeLa/KAP-AS) revealed many cells undergoing apoptosis, as evidenced by the condensed, fragmented nuclei, characteristic of cells undergoing early stages of apoptosis (Fig. 6B, right).
DISCUSSION
There is emerging evidence that the altered regulation of protein phosphorylation can directly contribute to multistep carcinogenesis. The link between cancer and protein phosphorylation was established by an understanding of the contribution of kinases to cancer. It is now well established that phosphatases also have important roles in regulating a variety of signal transduction pathways that have bearing on human cancer (29, 39, 49). In the present study, we describe the identification and characterization of KAP, a dual-specificity phosphatase, whose gene is a potential oncogene and whose overexpression is a frequent occurrence in breast and prostate cancer. To date, several phosphatases have been implicated in the etiology of tumors, including protein phosphatase 2A (39, 40), PTEN/MMAC1 (33, 42), and CDC25A and -B (15). The serine-threonine phosphatase PP2A suppresses growth factor proliferative signals by interaction with the mitogen-activated protein kinase family members ERK1 and ERK2 (45). PP2A is also known to bind to viral oncogenes such as those of the simian virus 40 and polyoma small-t antigens (35, 38, 45). The PTEN gene is a tumor suppressor gene implicated in many types of human cancer, including breast cancer, prostate cancer, melanoma, glioblastoma, and endometroid ovarian cancer (33, 42). Overexpression of PTEN can suppress colony formation, growth in soft agar, and tumor formation in nude mice (7, 14). It has been suggested that PTEN may function, at least in part, through regulation of focal adhesion kinase and the subsequent inhibition of adhesion and migration (47, 48). However, several recent reports suggest that PTEN might regulate cell cycle progression by blocking activation of downstream targets of phosphatidylinositol 3-kinase such as the Akt proto-oncogene (4, 27, 51). In the mouse, loss of PTEN leads to hyperplasia and dysplasia in the skin, gastrointestinal tract, and prostate and increased tumor formation (10).
Several lines of evidence suggest a role for genes of the dual-specificity phosphatases CDC25A and -B as oncogenes (16, 18). CDC25A and -B genes have been shown to cooperate with the Ha-ras oncogene in oncogenic transformation (16). In addition, CDC25A alone was sufficient enough to induce tumor formation in Rb-null fibroblasts (16). CDC25A and -B are significantly overexpressed in some cancer cell lines and in several human cancers including breast and prostate cancer (16, 18), suggesting that deregulated expression of CDC25A and -B may play an important role in the development of a number of human cancers.
Our efforts to discover candidate genes whose function or expression is altered during breast carcinogenesis led to the identification of KAP whose expression is significantly upregulated in breast and prostate cancer cells both in vitro and in vivo. Of note, KAP is also a dual-specificity phosphatase and was previously identified as a protein interacting with Cdk2 or Cdc2, suggesting that KAP may play a role in cell cycle regulation (3, 22, 24). Overexpression of KAP was demonstrated by us in the majority of in situ and invasive ductal carcinomas examined, while there is little or no detectable expression of KAP either in the epithelial or stromal ductal units of normal breast or prostate. The predominant pattern of KAP overexpression in transformed cells in vitro and in vivo indicates a potential diagnostic and prognostic value for breast and prostate cancer development.
The KAP gene previously mapped at 14q22 (8) at which chromosome abnormalities linked to several neoplasms have been localized (19, 43, 50). Of note, a comparative genomic hybridization study demonstrated an amplification of the band 14q22 region in the PC3 prostate cancer cell line (2). Whether overexpression of KAP is due to gene amplification or other mechanisms remains to be elucidated.
To assess the functional significance of KAP overexpression in tumorgenic phenotypes of human cancer cells, we used antisense approaches to selectively suppress KAP expression. We established tet-regulated expression of antisense KAP in HeLa cells and stable antisense KAP expression in LNCaP cells. Suppression of KAP expression in HeLa cells caused a reduction of the population of S-phase cells. In these cells, repression of KAP protein expression also led to suppression of anchorage-independent growth in soft agar and a significant reduction in the growth rates of tumors in nude mice. These findings argue that KAP overexpression may not merely be a consequence of, but instead might contribute to, the tumorigenesis.
In the initial characterization of KAP/Cdi1, Gyuris et al. (22) presented evidence of cell cycle-dependent variation in RNA expression, with highest levels in late G1 phase and early S phase. In our studies, overexpression of KAP RNA and protein did not show significant cell cycle variation in MCF7 tumor cells (data not shown). Gyuris et al. (22) also presented evidence that transfection of HeLa cells with a KAP expression vector led to no reduction in colony formation but that selected transfectants showed a reduction in the S-phase fraction. These findings led to the suggestion that KAP overexpression may retard passage through these phases of the HeLa cell cycle (22). How both exogenous overexpression and decreased KAP expression might contribute to retardation in cell cycle progression remains to be resolved.
The phosphorylation of Cdk2 on Thr160 is catalyzed by Cdk-activating protein kinase CAK, while its dephosphorylation is catalyzed by KAP in a cyclin A-dependent manner in vitro (3, 41). In the presence of cyclin A, phosphorylated Thr160 is resistant to dephosphorylation by KAP. However, given its phosphatase activity toward substrates containing phosphotyrosine as well as phosphoserine residues, KAP might act as a positive regulator for specific Cdks by activating tyrosine-phosphorylated Cdks. In addition, KAP/Cdi1 interacts with Cdks with different affinities. KAP interacts strongly with Cdc2 and Cdk3 and, to a lesser extent, with Cdk2 (22). It remains to be elucidated whether it dephosphorylates Cdc2- or Cdk3-associated proteins such as cyclins, thus indirectly affecting Cdk activity. It is also possible that excess amounts of KAP in transformed cells affect the levels of dephosphorylation of Cdk2 or Cdc2 and their association with their cyclin partners, which results in dysregulation of cell division.
Overexpression of CDC25A and -B can be induced by Myc overexpression, and CDC25A and -B are known to be direct transcriptional targets of c-myc (17). Thus, it is possible that KAP overexpression might also be associated with the overexpression of a specific oncogene in breast and prostate cancer. By whatever mechanism KAP is overexpressed during tumorigenesis, our studies imply that it can play an important role in the proliferation of tumor cells. Given its overexpression in a high percentage of breast and prostate tumors, we hypothesize that the KAP gene may behave as an oncogene, and as such could have importance not only as a marker but as a therapeutic target.
ACKNOWLEDGMENTS
We thank P. Arizti, S. Kurdistani, C. Adra, and J. Lawler for useful suggestions and D. Campbell and M. Tang for technical help. We are also grateful to D. Beach for the KAP constructs.
C.L.R. and L.F. contributed equally to this work.
This work was supported by NIH grants CA66271 and AG13314-01.
REFERENCES
- 1.Band V, Sager R. Human papilloma virus DNAs immortalize normal human mammary epithelial cells and reduce their growth factor requirements. Proc Natl Acad Sci USA. 1990;86:1249–1253. doi: 10.1073/pnas.87.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernardino J, Bourgeois C A, Muleris M, Dutrillaux A M, Malfoy B, Dutrillaux B. Characterization of chromosome changes in two human prostatic carcinoma cell lines (PC-3 and DU145) using chromosome painting and comparative genomic hybridization. Cancer Genet Cytogenet. 1997;96:123–128. doi: 10.1016/s0165-4608(96)00258-0. [DOI] [PubMed] [Google Scholar]
- 3.Brown N, Noble M, Lawrie A, Morris M C, Tunnah P, Divita G, Johnson L N, Endicott J A. Effects of phosphorylation of threonine 160 on cyclin-dependent kinase 2 structure and activity. J Biol Chem. 1999;174:8746–8756. doi: 10.1074/jbc.274.13.8746. [DOI] [PubMed] [Google Scholar]
- 4.Carson J P, Kulik G, Weber M J. Antiapoptotic signaling in LNCaP prostate cancer cells: a survival signaling pathway independent of phosphatidylinositol 3′-kinase and Akt/protein kinase B. Cancer Res. 1999;59:1449–1453. [PubMed] [Google Scholar]
- 5.Charbonneau H, Tonks N K, Kumar S, Diltz C D, Harrylock M, Cool D E, Krebs E G, Fischer E H, Walsh K A. Human placenta protein-tyrosine-phosphatase: amino acid sequence and relationship to a family of receptor-like proteins. Proc Natl Acad Sci USA. 1989;86:5252–5256. doi: 10.1073/pnas.86.14.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charbonneau H, Tonks N K. 1002 protein phosphatases? Annu Rev Cell Biol. 1992;8:463–493. doi: 10.1146/annurev.cb.08.110192.002335. [DOI] [PubMed] [Google Scholar]
- 7.Cheney I W, Johnson D E, Vaillancourt M T, Avazini J, Morimoto A, Demers G W, Wills K N, Shabram P W, Bolen J B, Tavtigian S V, Bookstein R. Suppression of tumorigenicity of glioblastoma cells by adenovirus-mediated MMAC1/PTEN gene transfer. Cancer Res. 1998;58:2331–2334. [PubMed] [Google Scholar]
- 8.Demetrick D J, Matsumoto S, Hannon G J, Okamoto K, Xiong Y, Zhang H, Beach D H. Chromosomal mapping of the genes for the human cell cycle proteins cyclin C (CCNC), cyclin E (CCNE), p21 (CDKN1) and KAP (CDKN3) Cytogenet Cell Genet. 1995;69:190–192. doi: 10.1159/000133960. [DOI] [PubMed] [Google Scholar]
- 9.Denu J M, Stuckey M A, Saper M, Dixon J E. Form and function in protein dephosphorylation. Cell. 1996;87:361–364. doi: 10.1016/s0092-8674(00)81356-2. [DOI] [PubMed] [Google Scholar]
- 10.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi P P. Pten is essential for embryonic development and tumor suppression. Nat Genet. 1988;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 11.Draetta G F. Mammalian G1 cyclins. Curr Opin Cell Biol. 1994;6:842–846. doi: 10.1016/0955-0674(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 12.El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 13.Ethier S P, Mahacek M L, Gullick W J, Frank T S, Weber B L. Differential isolation of normal luminal mammary epithelial cells and breast cancer cells from primary and metastatic sites using selective media. Cancer Res. 1993;53:627–635. [PubMed] [Google Scholar]
- 14.Furnari F B, Lin H, Huang H S, Cavenee W K. Growth suppression of glioma cells by PTEN requires a functional phosphatase catalytic domain. Proc Natl Acad Sci USA. 1997;94:12479–12484. doi: 10.1073/pnas.94.23.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galaktionov K, Beach D. Specific activation of cdc25 tyrosine phosphatases by B-type cyclins: evidence for multiple roles of mitotic cyclins. Cell. 1991;67:1181–1194. doi: 10.1016/0092-8674(91)90294-9. [DOI] [PubMed] [Google Scholar]
- 16.Galaktionov K, Lee A K, Eckstein J, Draetta G, Meckler J, Loda M, Beach D. CDC25 phosphatases as potential human oncogenes. Science. 1995;269:1575–1577. doi: 10.1126/science.7667636. [DOI] [PubMed] [Google Scholar]
- 17.Galaktionov K, Chen X, Beach D. Cdc25 cell cycle phosphatase as a target of c-myc. Nature. 1996;382:511–517. doi: 10.1038/382511a0. [DOI] [PubMed] [Google Scholar]
- 18.Gasparotto D, Maestro R, Piccinin S, Vukosavljevic T, Barzan L, Sulfaro S, Boiocchi M. Overexpression of CDC25A and CDC25B in head and neck cancers. Cancer Res. 1997;57:2366–2368. [PubMed] [Google Scholar]
- 19.Gilladoga A D, Edelhoff S, Blackwood E M, Eisenman R N, Disteche C M. Mapping of MAX to human chromosome 14 and mouse chromosome 12 by in situ hybridization. Oncogene. 1992;7:1249–1251. [PubMed] [Google Scholar]
- 20.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan K, Deschenes R J, Qiu H, Dixon J E. Cloning and expression of a yeast protein tyrosine phosphatase. J Biol Chem. 1991;266:12964–12970. [PubMed] [Google Scholar]
- 22.Gyuris J, Golemis E, Chertkov H, Brent R. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1994;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- 23.Hammond S L, Ham R C, Stampfer M R. Serum-free growth of human mammary epithelial cells: rapid clonal growth in defined medium and extended serial passage with pituitary extract. Proc Natl Acad Sci USA. 1984;81:5435–5439. doi: 10.1073/pnas.81.17.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hannon G J, Casso D, Beach D. KAP: a dual specificity phosphatase that interacts with cyclin-dependent kinases. Proc Natl Acad Sci USA. 1994;91:1731–1735. doi: 10.1073/pnas.91.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harper J W, Adami G R, Wei N, Keomarsi K, Elledge S J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 26.Hartwell L H, Kastan M B. Cell cycle control and cancer. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 27.Hopkin K. A surprising function for the PTEN tumor suppressor. Science. 1998;282:1027–1030. doi: 10.1126/science.282.5391.1027. [DOI] [PubMed] [Google Scholar]
- 28.Hunter T, Pines J. Cyclins and cancer. Cell. 1994;79:573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 29.Hunter T. Protein kinases and phosphatases. The yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 30.Jacks T, Weinberg R A. The expanding role of cell cycle regulators. Science. 1998;280:1035–1036. doi: 10.1126/science.280.5366.1035. [DOI] [PubMed] [Google Scholar]
- 31.Kinzler K W, Vogelstein B. Landscaping the cancer terrain. Science. 1998;280:1036–1037. doi: 10.1126/science.280.5366.1036. [DOI] [PubMed] [Google Scholar]
- 32.Leake R. The cell cycle and regulation of cancer cell growth. Ann N Y Acad Sci. 1996;784:252–262. doi: 10.1111/j.1749-6632.1996.tb16240.x. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I, Pue J, Miliaresis C, Rogers L, McCombie R, Bigner S H, Giovanella B C, Ittmann M, Tycko B, Hibshoosh H, Wigler M H, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 34.Liang P, Pardee A B. Differential display of eukaryotic mRNA by means of the polymerase chain reaction. Science. 1992;257:967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- 35.Mungre S, Enderle K, Turk B, Porras A, Wu Y, Mumby M C, Rundell K. Mutations which affect the inhibition of protein phosphatase 2A by simian virus 40 small t antigen in vitro decrease viral transformation. J Virol. 1994;68:1675–1681. doi: 10.1128/jvi.68.3.1675-1681.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neel B G, Tonks N K. Protein tyrosine phosphatases in signal transduction. Curr Opin Cell Biol. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- 37.Noda A, Ning Y, Venable S F, Pereira-Smith O M, Smith J R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994;211:90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- 38.Pallas D, Shahrik L K, Martin B L, Jasper S, Miller T B, Brautigan D L, Roberts T M. Polyoma small and middle t antigens and sv40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990;60:167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- 39.Parsons R. Phosphatases and tumorigenesis. Curr Opin Oncol. 1998;10:88–91. doi: 10.1097/00001622-199801000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Plutzky J, Neel B, Rosenberg R D. Isolation of a src homology 2-containing tyrosine phosphatase. Proc Natl Acad Sci USA. 1992;89:1123–1127. doi: 10.1073/pnas.89.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poon R Y C, Hunter T. Dephosphorylation of Cdk2 thr160 by the cyclin-dependent kinase-interacting phosphatase KAP in the absence of cyclin. Science. 1995;270:90–93. doi: 10.1126/science.270.5233.90. [DOI] [PubMed] [Google Scholar]
- 42.Robertson G P, Furnari F B, Miele M E, Glendening M J, Welch D R, Fountain J W, Lugo T G, Huang H J, Cavenee W K. In vitro loss of heterozygosity targets the PTEN/MMAC1 gene in melanoma. Proc Natl Acad Sci USA. 1998;95:9418–9423. doi: 10.1073/pnas.95.16.9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roulston D, Espinosa R, Nucifora G, Larson R A, Le Beau M M, Rowley J D. CBFA2(AML1) translocations with novel partner chromosomes in myeloid leukemias: association with prior therapy. Blood. 1998;92:2879–2885. [PubMed] [Google Scholar]
- 44.Sherr C J. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 45.Sontag E, Federov S, Kamibayashi C, Robbins D, Cobb M, Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell. 1993;75:887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- 46.Strauss M, Lukas J, Bartek J. Unrestricted cell cycling and cancer. Nat Med. 1995;1:1245–1246. doi: 10.1038/nm1295-1245. [DOI] [PubMed] [Google Scholar]
- 47.Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada K M. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614–1617. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- 48.Tamura M, Gu J, Takino T, Yamada K M. Tumor suppressor PTEN inhibition of cell invasion, migration, and growth: differential involvement of focal adhesion kinase and p130Cas. Cancer Res. 1999;59:442–449. [PubMed] [Google Scholar]
- 49.Tonks N K, Neel B G. From form to function: signaling by protein tyrosine phosphatases. Cell. 1996;87:365–368. doi: 10.1016/s0092-8674(00)81357-4. [DOI] [PubMed] [Google Scholar]
- 50.Turc-Carel C, Dal Cin P, Boghosian L, Terk-Zakarian J, Sandberg A A. Consistent breakpoints in region 14q22-q24 in uterine leiomyoma. Cancer Genet Cytogenet. 1988;32:25–31. doi: 10.1016/0165-4608(88)90307-x. [DOI] [PubMed] [Google Scholar]
- 51.Wu X, Senechal K, Neshat N S, Whang Y E, Sawyers C L. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1998;95:15587–15591. doi: 10.1073/pnas.95.26.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiong Y, Hannon G J, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;66:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]