Abstract
The emergence of a nucleosome-based chromatin structure accompanied the evolutionary transition from prokaryotes to eukaryotes. In this scenario, histones became the heart of the complex and precisely timed coordination between chromatin architecture and functions during adaptive responses to environmental influence by means of epigenetic mechanisms. Notably, such an epigenetic machinery involves an overwhelming number of post-translational modifications at multiple residues of core and linker histones. This review aims to comprehensively describe old and recent evidence in this exciting field of research. In particular, histone post-translational modification establishing/removal mechanisms, their genomic locations and implication in nucleosome dynamics and chromatin-based processes, as well as their harmonious combination and interdependence will be discussed.
Keywords: histone post-translational modifications, chromatin, nucleosome, epigenetics
1. Introduction
Chromatin is a polymeric nucleoprotein complex composed primarily of DNA closely associating with positively charged histone proteins [1]. The basic structural repeating unit is the nucleosome core particle, consisting of 147 base pairs of DNA wrapped nearly twice in a left-handed toroidal supercoil around a core histone octamer [2]. The latter exhibits a tripartite organization, which comprises a tetramer containing two copies of H3 and H4, coupled to two H2A-H2B dimers [3]. Each core histone contains a globular histone fold domain, which mediates heterodimeric interactions, and two amino- and carboxy-terminal unstructured extensions protruding from the surface of the nucleosome [4]. These flexible domains, commonly known as the histone tails, provide additional stabilization effects through their contacts with the nucleosomal DNA backbone and acidic patch of adjacent nucleosomes [5]. As an additional hierarchical level of chromatin compaction, core particles are further stabilized by the binding of histone H1 at the two linker DNA arms entering and exiting on the surface of each octamer [6,7]. Molecular processes establishing such a histone stoichiometry determines the basic chromatin fiber structure [8,9].
Key mechanisms contributing to the dynamic regulation of the degree of chromatin compaction to allow the harmonious utilization of the genetic information include covalent post-translational modifications (PTMs) of histones [10,11]. In this regard, histone tails represent preferential, but not exclusive, targets of a myriad of PTMs that correlate with changes in gene activity. In fact, the recent improvement of the sensitivity of mass spectrometry-based methods allowed the discovery of more than two hundred histone modification sites so far (Figure 1), although no functional significance has been assigned to the majority of them [12,13]. These modifications are imposed, and can be reversed as well, by an assortment of histone-modifying enzymes, so that they represent a major mechanism of cellular adaptation to the varying environmental conditions [14,15,16]. Broadly speaking, the presence of histone PTMs alters the stereochemical environment of the nucleosomes and their binding affinity for regulatory complexes that can be recruited or turned away from chromatin, thereby eliciting functional outcomes.
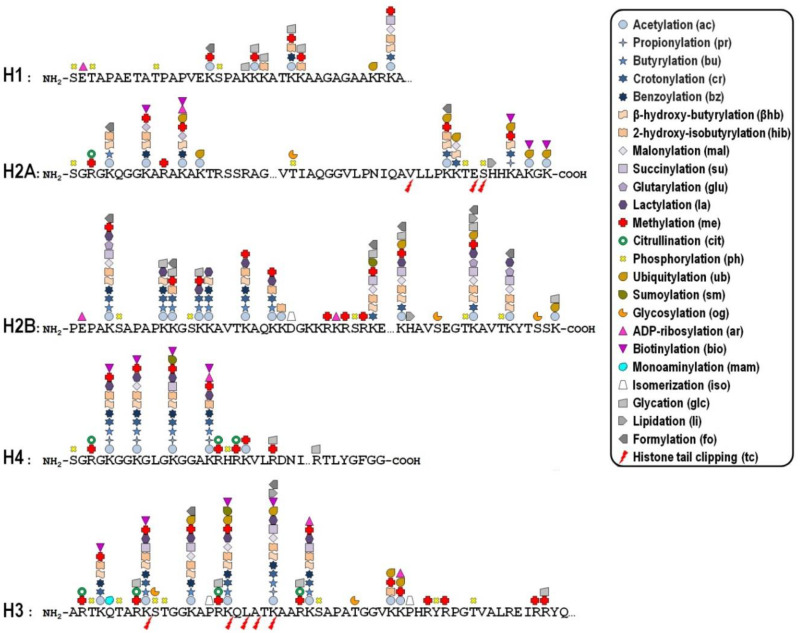
Figure 1.
Diagrammatic representation showing the distribution of post-translational modifications within the N- and C-terminal portions of the five canonical histones. The first amino acid methionine was omitted from the sequences. Key for each histone PTM is included in diagram. Reference sequences of histone proteins were retrieved from the NCBI protein database (https://www.ncbi.nlm.nih.gov/protein?cmd=retrieve; accessed on 2 October 2021) using the following accession numbers: NP_005313.1 (H1), NP_003504.2 (H2A), NP_066406.1 (H2B), NP_003520.1 (H3), and NP_003529.1 (H4).
Whether a given histone PTM correlates with gene expression in a positive or negative fashion most likely depends on its genomic location. To make matters more complicated, it is now becoming clear that the combinatorial nature of histone PTMs implies both the coalescence of multiple modifications on the same nucleosome and the possibility of functional promiscuity induced by synergistic or antagonistic cross-talk among them. The simplest example of such a cross-talk is the direct competition, on the same histone residue, among alternative PTMs associated with functionally opposite transcriptional readouts. In many other cases, one modification promotes/inhibits the generation/removal of another modification “in cis”, viz within a distinct site in the same histone, or “in trans”, between distinct histones either within the same nucleosome or across nucleosomes, as well as between histones and DNA methylation [17]. In addition, different histone PTMs may cooperate in order to recruit specific complexes efficiently [18].
This review aims to provide a comprehensive summary of the ever-growing number of histone PTMs that have been studied over the last years. The molecular mechanisms involving histone PTMs, their genomic locations and implication in chromatin-based processes, as well as their interdependence will be discussed. Given the expanding dictionary of histone PTMs, in the following paragraphs I adopt the standard notation that has been conceived to represent them in a consistent and coherent format [19]. More particularly, the histone protein is first defined in uppercase letters, followed by a two-syllable word univocally indicating the individual modified residue and an abbreviation, in lowercase letters, conventionally indicating the type of modification.
2. Acetylation
In the early ‘60s, Phillips pioneered the field of histone PTMs originally reporting the N-acetylation of lysine residues in histones extracted from calf thymus [20]. Curiously, many authors attributed the achievement of this discovery to Allfrey and collaborators, although they clearly gave credit to Phillips in their first paper on this topic [21]. Undoubtedly, acetylation became the most studied histone PTM, so that nowadays we know that selected lysines are acetylated during specific biological processes, and that the overall histone acetylation level is dynamically modulated by the opposing action of two families of enzymes, namely the histone acetyltransferases (HATs) and the histone deacetylases (HDACs). This nomenclature, albeit currently used, is actually inappropriate because these enzymes can respectively transfer or remove a wide repertory of acyl species, besides the acetyl group, to both histone and non-histone target proteins (see below). In the case of acetylation, the HATs require acetyl-CoA as a cofactor to catalyse the transfer of an acetyl group to the ε-amino group of lysine side chains [22]. Based on their subcellular localization, HATs have been traditionally divided in two distinct types: type-A HATs are directly involved in histone acetylation in the context of nucleosomes, while type-B HATs acetylate free newly synthesized histones in the cytoplasm to facilitate their incorporation into the newly replicated chromatin [23,24]. HDAC enzymes reverse the activity of HATs, restoring the original unacetylated state of histone lysines. There are four classes of HDACs, whose members typically associate into multiprotein complexes showing rather low substrate specificity [25]. Collectively, both the mentioned enzyme families modify numerous, but not all, histone lysines in vivo [26]. Importantly, the HAT activity, differential substrate specificity and recruitment are modulated through multisubunit complex formation [27]. For example, yeast Gcn5 alone does acetylate free histones in vitro, but only when it is combined with the Ada2 and Ada3 subunits within the ADA and SAGA complexes, it can preferentially modify H2B enclosed into nucleosomes [28].
In principle, the observation that acetylation of lysine residues neutralizes their positive charge would suggest that HATs may reduce chromatin compaction by weakening the stabilizing influence of histone-DNA and inter-nucleosomal electrostatic interactions [29]. Accordingly, acetylation of H4K16 has been demonstrated to directly impact on chromatin structure by charge neutralization and loss of H4 tail flexibility, collectively reducing interaction with the neighbouring nucleosome [30,31,32]. In support of this evidence, ChIP-seq studies on a genome-wide scale revealed that site-specific acetylation is frequently associated with enhancers and promoters of actively transcribed genes [33,34,35,36]. However, there is some variation in the exact distribution of individual acetylation sites along the gene structure. For instance, H3K9ac peaks at the transcription start site, while H4K16ac shows a broader localization across gene bodies [36]. In sharp contrast, structural studies highlighted that lysine acetylation itself generally does not provoke major reorganization of DNA or core histones at the nucleosome level [37], leading to reasonably suppose that histone acetylation could generate recognition sites for effector proteins involved in the local regulation of chromatin organization and transcriptional activity.
3. Non-Acetyl Acylation
Besides acetylation, the ε-amino group of histone lysines can be alternatively modified by a growing repertoire of acyl moieties. These modifications deal with the transfer of at least four types of acyl groups: (1) short-chain hydrophobic groups, such as propionyl, butyryl, crotonyl, and the aromatic group benzoyl, proportionally increase the size and hydrophobicity of the lysine side chain compared to acetylation [38,39,40], (2) long-chain fatty acids, including hexanoyl, octanoyl, decanoyl, dodecanoyl, myristoyl, palmitoyl and stearoyl, have larger molecular volume and stronger hydrophobicity compared to the above mentioned short-chain groups, (3) polar groups, including β-hydroxy-butyryl and 2-hydroxy-isobutyryl, have branched hydrocarbon chains and provide a hydroxyl group implementing the hydrogen bond capability of the modified lysine [41,42], (4) negatively charged groups, including malonyl, succinyl, glutaryl, and lactoyl, switch the net electrostatic charge of the modified residue from +1 to −1 at a physiological pH [43,44,45].
All these acyl groups are derived from cellular metabolites and their acyl-CoA counterparts represent the source of diverse histone marks accompanying feedback and feedforward mechanisms of gene regulation and chromatin structure modulation. Notably, although the possible competition and functional interplay between distinct acylations on histone lysine residues require further investigation, these PTMs appear to be not redundant with each other and with acetylation.
For example, compared to histone acetylation, the transcriptional output associated with histone butyrylation is similar, while those associated with crotonylation and 2-hydroxy-isobutyrylation are greater and lesser, respectively [46,47,48].
The high reactivity of the various acyl-CoA types may lead to histones acylation independently of enzymatic involvement [49]. On the other hand, recent kinetic analysis confirmed promiscuous acylase/deacylase catalytic activities for the previously described HATs and HDACs, respectively [50]. Surprisingly, no enzyme showing specificity for only a given acylation has been discovered so far. Therefore, assuming that HATs interchangeably use different acyl-CoAs to modify histone lysine substrates, it could be reasoned that the relative abundance of the numerous acyl-CoA types would globally affect the differential histone acylation state, thereby connecting cell metabolism and gene regulation [51]. This hypothesis is supported by the fact that most of the available ChIP-seq datasets concordantly reveal the genomic co-occurrence of alternative acylation marks at distinctive proportional mixtures that correlate with specific functional outputs [46].
Site-specific acylation of core histones, such as H4K91glu, H3K122suc and H4K77suc, generally weakens histone-DNA interactions, thereby promoting nucleosome sliding [52,53,54]. Intriguingly, specific acylation patterns involving crotonylation, butyrylation and 2-hydroxy-isobutyrylation have been observed in the post-meiotic chromatin of murine spermatogenic lineage [39,46,55]. In addition, site-specific propionylation and butyrylation have been implicated in the expression of genes involved in lipid metabolism pathways in liver of fasted mice, while β-hydroxy-butyrylation was associated to activated transcription of genes involved in the starvation response [42,56]. Similarly, lysine benzoylation predominantly occurs on the N-terminal tails of H3 and H2B, specifically marking promoters of selected genes involved in phospholipase D signaling, glycerophospholipid metabolism, ovarian steroidogenesis and serotonergic synapse [40]. The unique physiological relevance of this epigenetic mark is revealed by the positive correlation between transcriptional upregulation of the mentioned genes and specific enrichment of benzoylation, but not other acylations, in their promoters. Moreover, while acetylated lysines are normally recognized by bromodomain-containing proteins, histone benzoyl-lysines preferentially recruit chromatin interactors bearing YEATS or DPF domains [57]. Among the known HDAC enzymes, only SIRT2 exhibited significant lysine debenzoylation activity in a catalytic screening, suggesting that it efficiently operates the reversal of benzoylation in vivo [40].
Lactylation of lysines strictly associates with the glycolysis pathway, relying upon the intracellular production of D-lactate in a dose-proportional fashion [58]. The HAT p300 has been implicated in the histone lactylation, and ChIP-seq analysis on samples derived from macrophages revealed that lysine lactylation specifically accumulates in the proximal promoter region of transcribed genes, being indicative of steady-state mRNA abundance [58,59]. Lysine lactylation can also occur via a non-enzymatic acyl transfer from S,D-lactoylglutathione, which is generated by the glyoxalase1 enzyme from the glycolytic by-product methylglyoxal [45,60].
In striking contrast to all these examples of positive correlation between histone acylation and gene expression, crotonylation at H3K9 has been associated to decreased transcription of growth genes during low-nutrient periods in Saccharomyces cerevisiae, most probably due to dynamic recruitment or repulsion of specific interactors not yet characterized [61].
4. Methylation
Unlike acetylation, histone methylation does not alter the net charge of modified residues and involves additional layers of complexity, because the side chains of two distinct amino acids can be modified at a different extent. More specifically, the ε-amino group of lysines can be mono-, di- or tri-methylated, while the ω-guanidine group of arginines can be mono- or di-methylated either symmetrically or asymmetrically [62,63].
Histone methyltransferases (HMTs) and demethylases (HDMs) enzymes carry out fine-tuning of the methylation state. Based on the specificity towards lysine or arginine, two major classes of HMTs can be distinguished, each of them containing a distinctive catalytic domain that transfers a methyl group from the S-adenosylmethionine donor. In contrast to other histone modifying enzymes, members of both classes are devoted to methylation of highly specific residues to an appropriate degree. For example, PRMT7 catalyses the formation of only mono-methyl-arginine, while PRMT5 electively determines symmetrical di-methylation of arginine [64,65]. Similarly, DIM5 univocally targets H3K9 for tri-methylation, while SET7/9 can only mono-methylate H3K4 in Neurospora crassa [66,67]. Notably, unlike acetylation, the different methylated histone sites may associate with diametrically opposed functional outcomes [68]. For instance, H3K4me3 and H3K36me3 have been primarily linked to a transcriptionally permissive chromatin environment [69], H3K4me1 is considered a predictive hallmark of active enhancers [70], while H3K27me3 and H4K20me3 have been generally associated with heterochromatin formation and gene silencing [71,72]. Interestingly, H3K9 provides an emblematic example of the dualistic association of methylation with repressed and active states of chromatin. Indeed, several studies concordantly showed that H3K9me3 represents a docking site for the α isoform of the heterochromatin protein 1 (HP1α), which assists the progress of chromatin condensation in various organisms [73,74]. Other studies came to apparently conflicting conclusions, demonstrating that H3K9me3 and the HP1γ isoform co-localize in mammalian euchromatic regions, where they are involved in transcriptional elongation and are rapidly lost upon cessation of transcription [75].
Analogously to other epigenetic mechanisms, the histone methylation status varies widely during adaptive responses to environmental influence. For example, H3K14me3 is not normally found in human chromatin but it is distinctively generated following infection of lung epithelial cells with the intracellular pathogen Legionella pneumophila [76].
As with the HMTs, HDM enzymes display a high grade of target specificity, being sensitive to the degree of methylation. For example, JMJD2A can reverse H3K9me3 to H3K9me2 but not to H3K9me1 [77]. Removal of methyl groups from mono-methylated and asymmetrically di-methylated arginines is most probably carried out by JMJD6 [78]. To date, this is the one and only enzyme possessing arginine demethylase activity directed toward histones, although this function has been questioned by contradictory evidence [79,80].
5. Citrullination
Indirect reversal of histone arginine methylation can be achieved through citrullination, which is the demethyl-imination modification of the primary monomethyl-imine group to a ketone group, yielding methylamine as a side-product. The conversion of methyl-arginine into citrulline is catalysed by the Ca2+-dependent peptidylarginine deiminase (PADI) enzymes [81,82]. In fact, PADIs exert a dual enzymatic activity, as they convert methylated arginine and unmodified arginine into citrulline via demethyl-imination and deamination, respectively [81,82]. Whatever is the case, PADI intervention determines the loss of the positive charge of the arginine side chains, which in turn promotes chromatin decondensation by affecting histone-DNA interactions [83]. In particular, citrullination of the single arginine residue at position 54 within the nucleosomal DNA binding region of H1 was found to lead to extensive chromatin decondensation [84]. Despite this, citrullination may be a repressive epigenetic mark on selected genes. For instance, in MCF-7 cells exposed to estradiol stimulation, level of histone citrulline on the estrogen-responsive pS2 gene promoter was found to be inversely correlated to methyl-arginine level and pS2 gene transcription [81,82]. Based on these findings it could be argued that the PADI4 enzyme not only controls the methylation status of multiple arginine residues in histone H3 and H4 by converting them into citrulline, but it also acts as a transcriptional co-repressor [81,82]. Given that the occurrence of an inverse demethyl-imination reaction catalysed by specific enzymes appears questionable, possibly histone replacement is required to regenerate unmodified arginine residues from citrulline.
6. Phosphorylation
Histone phosphorylation was first reported more than fifty years ago, yet this is among the more intensively investigated histone PTMs [85]. The overall level of phosphorylation in the chromatin depends upon the combined action of a plethora of kinases and phosphatases, which impose and remove the modification, respectively [86]. As usual, these enzymes are specifically recruited to their target sites on chromatin in the form of larger holoenzyme complexes, although for some kinases a direct binding activity has been described. This is the case of the mammalian MAPK1 kinase, which tethers to chromatin by means of an intrinsic DNA-binding domain [87].
Both the linker and core histones endure a large number of phosphorylation events on the hydroxyl group situated on the side chain of serine, threonine, and tyrosine residues [88]. The presence at these sites of a phosphate group, derived from the ATP donor, confers a heavy negative charge that undoubtedly induces relevant changes in the chromatin structure. A pertinent example is given by the phosphorylation of Tetrahymena histone H1 residues, which increases the dissociation rate of the linker histone from chromatin in vivo, through a so-called charge patch effect [89]. As a consequence, phosphorylation-dependent H1 ejection promotes chromatin relaxation, which in turn differentially affects the expression of some genes [89].
More generally speaking, phosphorylation of specific histone residues has been involved in several biological processes such as mitosis, meiosis, DNA damage response, apoptosis, and of course also gene expression [90,91,92]. For example, H3S10ph, which is the most thoroughly characterized histone phosphorylation site, is a highly dynamic modification showing an extremely rapid turnover during the cell cycle in plants, lower eukaryotes and mammal chromatin [93,94]. During mitosis and meiosis, this peculiar modification is deposited by the Aurora-B kinase and it is involved in chromosomal condensation by a so called phospho-methyl switch mechanism. In particular, the HP1 family proteins that normally bind to H3K9me3 show a significantly reduced affinity for H3 N-tails exhibiting the dual H3K9me3S10ph phospho-methylation mark, thus allowing the access of factors needed for proper condensation and segregation of the chromosomes [95,96]. In interphase, phosphorylation of H3S10 is instead mediated by two distinct kinases, namely Rak2 and Msk1, and it is associated with active transcription of numerous genes [97]. Contrarily, dephosphorylation of H3S10ph is carried out by the PP2A phosphatase and associates with transcriptional repression [98].
7. Ubiquitylation
Ubiquitylation involves only a single Ubiquitin moiety linked through an isopeptide bond between its C-terminal exposed glycine residue and the ε-amino group of specific target lysines within histones. Thus, mono-ubiquitylation peculiarly results in the addition of a relatively large polypeptide of about 8.5 kDa, rather than the small chemical groups described for many other histone PTMs. Unlike poly-ubiquitylation, which targets proteins to proteasome-dependent degradation, mono-ubiquitylation of histones is associated with the regulation of gene transcription and DNA damage response [99]. The site-specific attachment of Ubiquitin requires a sequential catalytic cascade consisting of activation, conjugation, and ligation, performed by the E1, E2, and E3 enzymes, respectively, while specialized isopeptidases attain the removal of Ubiquitin [99]. Comprehensive mapping of histone PTMs in the adult mouse brain revealed that ubiquitylation covers ~16% of total PTMs in core histones, localizing primarily on H2A and H2B [100]. The most extensively studied histone residues undergoing mono-ubiquitylation in higher eukaryotes are H2AK119 and H2BK120, both lying in the C-terminal tails of the two core histones [101]. More specifically, H2AK119Ub1 is generally associated with gene silencing and chromatin compaction, by stimulating deposition of the repressive mark H3K27me3 and stabilizing the association of linker histone H1 [102]. By contrast, H2BK120 is situated at the interface of adjacent nucleosomes, and its mono-ubiquitylation results in the inhibition of inter-nucleosome interactions to allow a more accessible conformation of the chromatin fiber [103]. Moreover, in the mammalian genome H2BK120Ub1 is largely confined within gene bodies, rather than promoters, where it directly stimulates histone methyltransferase activity of DOT1L and SET1 on H3K79 and H3K4, respectively, leading to a local enrichment of epigenetic marks closely correlated with the transcriptional elongation process [104,105,106,107].
Deciphering the intrinsic effects of ubiquitylation on the nucleosome structure and dynamics is considerably challenging due to the lack of a defined in vitro assay system. Nonetheless, it could be reasonably presumed that such a bulky modification is generally irreconcilable with the canonical nucleosome structure due to steric hindrances of the massive ubiquitin protein. For example, H2BK34 resides between two superhelical DNA gyres, and its mono-ubiquitilyation leads to drastic eviction of a H2A-H2B heterodimer from the nucleosome [108]. The resulting histone hexasome is quite stable and inefficiently incorporate a second, even unmodified, heterodimer, thus facilitating nucleosomal DNA breathing and accessibility [108]. Notably, a similar effect, although to a lesser extent, has been observed when ubiquitylation embraces histone residues residing at the nucleosome periphery, such as H2BK120 [108]. In addition, mono-ubiquitylation of the C-terminal H3K121, H3K122 and H3K125 residues has been reported to occur on histones that are not yet incorporated into yeast chromatin, outlining a ubiquitylation-dependent mechanism that control nucleosome assembly [109].
8. Sumoylation
Sumoylation involves the covalent attachment of either a single or multiple protein units among the small Ubiquitin-like modifier (SUMO) family members to histone lysines [110]. In principle, this modification could appear to be closely related and somehow redundant with respect to ubiquitylation, principally because Ubiquitin and SUMO proteins share comparable molecular size and three-dimensional structure, and both are managed by E1, E2, and E3 enzymes [111]. However, the two modifications are not related at all, at least not at an epigenetic level. Notably, sumoylation does not functionally replace ubiquitylation at H2BK120, strongly supporting the notion that the above-mentioned effects on gene expression cannot be explained merely by steric effects [112]. Sumoylation has been detected on all four nucleosomal histones at many genomic locations, where it has been involved in distinct functions. Mechanistically, histone sumoylation may inhibit chromatin compaction by affecting inter-nucleosomal interactions and by cross-regulating other histone PTMs. For example, sumoylated H2B and H3 in nucleosome occupying the yeast gal1 gene were specifically associated with transcriptional repression and histone hypo-acetylation [113]. It has been suggested that transient sumoylation of H4 stimulates the local recruitment of repressive complexes bearing the HDAC1 deacylase and the H3K4-specific demethylase LSD1, allowing spatially restricted transcriptional repression by erasure of epigenetic marks that would otherwise lead to gene expression [114]. However, other studies reported that nucleosomes containing sumoylated H2B are the preferential binding platform for the ATP-dependent nucleosome remodeler RSC, which stimulates gene expression by altering the position, occupancy, and composition of nucleosomes [115].
Histone sumoylation may also play a relevant role in DNA repair, since persistent DNA double strand breaks incorporate nucleosomes containing sumoylated H2A.Z, which is absolutely required for DNA-lesion tethering to the nuclear periphery [116]. Furthermore, sumoylation of the yeast centromeric H3 variant Cse4 at K215 and K216 sites is required for normal kinetochore assembly and faithful chromosome segregation, while sumoylation at K65 prevents the aberrant spread of Cse4 into euchromatin [117,118].
9. Glycosylation
O-glycosylation, hereinafter referred to as glycosylation, relates to the addition of a single β-D-N-acetylglucosamine (GlcNAc) monosaccharide unit to the hydroxyl group of selected serine and threonine residues of the four core histones [119,120]. In contrast to most histone PTMs, there appear to be only two enzymes involved in the control of the glycosylation levels in chromatin, a transferase (OGT) that employs the donor substrate UDP-GlcNAc, and an antagonistic hydrolase (OGA) [121,122]. Intriguingly, OGA possesses an additional catalytic domain exhibiting HAT activity in vitro, suggesting that OGA would be able to simultaneously regulate the glycosylation and acetylation of histone residues [123,124]. On the other hand, OGA is not able to bind acetyl-CoA directly, suggesting that an ancillary partner would be required to ensure the putative HAT activity in vivo [124,125].
Histone glycosylation level normally fluctuates in response to variation in temperature and extracellular glucose abundance, as well as along cell cycle, decreasing during mitosis [120].
In most cases, glycosylation maps residues located in strategic positions of the nucleosome particle, including the loop 1 within the histone-fold domain, the lateral surface of the histone octamer stuck to the nucleosomal DNA, and the docking domain at the interface between the H2A/H2B dimer and H3/H4 tetramer, suggesting that this PTM could entail chromatin opening [119]. Structural effects induced by histone glycosylation has been investigated employing a site-selective chemoenzymatic assay, which allows installing GlcNAc onto mutagenically introduced cysteine residues on recombinant histones H2A and H2B, to generate semisynthetic assembled nucleosomes [126,127]. This approach confirmed that glycosylation of H2AT101, which lies at the dimer–tetramer interface of the nucleosome, indeed affects chromatin remodelling by destabilizing the H2A/H2B dimers and thus reducing nucleosome stability [128]. In the case of residues occupying nucleosome regions remote from any critical interface, rather than modulating nucleosome structure, histone glycosylation may favour the recruitment of protein complexes to mediate specific functional outputs. This is the case of H2BS112og, which impacts chromatin remodelling through direct recruitment of FACT, a pivotal histone chaperone complex, and the ubiquitin ligase BRE1A, which catalyses mono-ubiquitylation at H2BK120 [129,130].
Expectedly, genome-wide profiling of histone glycosylation by ChIP assays revealed that this modification is typically linked to transcriptional activation of selected genes [131]. Enrichment of O-glycosylation in these specific chromosomal sites likely depends on the stable association of OGT with prominent interactors, such as the Hcf1 transcriptional co-regulator, the Ten-eleven translocation proteins involved in the reversal of DNA methylation, and the Ubinuclein1 component of the histone chaperone HIRA complex [132,133,134].
10. ADP-Ribosylation
ADP-ribosylation is characterized by the synthesis of negatively charged monomers or polymers of ADP-ribose at specific acceptor amino acid residues of histone proteins [135]. The catalytic process requires nicotinamide adenine dinucleotide (NAD+) as donor substrate and is mediated by members of the ADP-ribosyltransferase family, while reversal of the modification is performed by specific hydrolases [136]. The number of studies regarding mono-ADP-ribosylation of histones remains very low at present. Only a small number of mono-ADP-ribosyltransferases, mainly ARTD-1, -3 and -10, have been reported to modify specific residues of linker and core histones in vertebrates [137,138,139]. Intriguingly, the SIRT-6 HDAC has been also implicated in histone mono-ADP-ribosylation by a dedicated catalytic domain [140]. This epigenetic modification occurs during DNA damage and repair, predominantly involving histone residues located on the nucleosomal surface [141]. Moreover, measurement of the histone ADP-ribosylation level in lysates derived from quiescent and dividing cells revealed a negative correlation between histone mono-ADP-ribosylation and cell proliferation [142]. Of note, poly-ADP-ribosylation exhibits a diametrically opposed trend, suggesting that the interconversion of mono- and poly-ADP-ribosylated histones may play a regulatory role in cell proliferation.
A few glutamate residues in H1 and H2B histones are the preferred acceptor sites for poly-ADP-ribosylation in native chromatin [143,144]. None of them, however, have been ultimately confirmed by mass spectrometry, probably due to the extremely reduced fraction of ADP-ribosylated histones, which is less than 1% of the total amount [145,146]. In principle, due to the bulky size and high negative electrostatic charge of poly-ADP-ribose, this PTM could diminish the local level of chromatin compaction. Indeed, high-resolution electron microscopy confirmed that poly-ADP-ribosylated chromatin reversibly adopts a more relaxed structure than its native counterpart, without dissociation of H1 and histone octamers from DNA [147,148]. Accordingly, specific enrichment in histone poly-ADP-ribosylation has been reported in transcriptionally active chromatin regions [149,150].
11. Biotinylation
Biotinylation is a reversible process relying on the covalent attachment of a B8 vitamin moiety to the ε-amino group of lysine residues of either linker or core histones [151,152]. Homeostasis of histone biotinylation is maintained by two biotinyl transferases, namely biotinidase (BTD) and holocarboxylase synthetase (HCS), which require biotinyl-ε-lysine and biotinyl-5′-AMP, respectively, as donor substrates [153,154]. Independent studies suggest that HCS plays a prominent role in histone biotinylation, while distinct enzymatic forms of BTD derived by alternative splicing seem to be involved in catalysing either biotinylation or debiotinylation of histones [155,156]. Globally, the human epigenome exhibits very low levels of histone biotinylation, in the order of attomoles of biotin incorporated into histones isolated from a million of cells [157,158]. However, the relative abundance of biotinylation marks depends on dietary biotin and may vary in response to cell proliferation [159,160]. Moreover, biotinylated histones are locally enriched at distinct repressed loci, including pericentromeric heterochromatin, telomeric repeats, and long tandem repeats [161,162,163,164]. In these chromatin regions, histone biotinylation generally co-localizes with the well-established repressive epigenetic mark H3K9me2, most probably as a consequence of the physical interaction between HCS and the H3K9-specific histone methyltransferase EHMT1 [165]. The involvement of histone biotinylation in gene repression has been corroborated by atomic force microscopy studies showing that H4K12bio and H4K16bio substantially facilitates chromatin condensation by increasing of almost 0.2 nucleosomal turns the length of DNA wrapped around the histone core octamer [166,167]. However, it should be emphasized that the authors of these studies employed recombinant histone H4 in which either K12 or K16 residues were mutated to cysteine for subsequent sulfhydryl-specific chemical reaction with maleimide-PEG-biotin to create biotinylated histones. Being this chemical modification quite different from the in vivo biotinylation, it somehow could have contributed to the observed changes on the nucleosome structure.
12. Monoaminylation
Recent evidence highlighted that transglutaminase enzymes can crosslink various monoamines to different protein targets, including histones, suggesting that neurotransmitters might be directly implicated in chromatin-associated processes. In particular, histone H3 but no other core histones can be modified by monoamines, namely serotonin and dopamine, in response to fluctuations in intracellular availability [168,169]. Transglutaminase-2 can covalently transfer both the mentioned monoamines to the carboxamide group of glutamine at position 5 on histone H3, yielding either serotonylated or dopaminylated nucleosomes. Transglutaminases can also remove monoamines from histones through deamidation reactions, indicating that monoaminylation is a reversible epigenetic modification [170,171].
While H3Q5 serotonylation plays a critical role for neurogenesis in mammals, dopaminylation on the same histone residue has been implicated in cocaine-induced transcriptional plasticity in dopamine neurons of the ventral tegmental area [169]. The H3Q5ser mark was found to coexist with adjacent H3K4me3 on the same histone tail at euchromatic regions of active gene expression in differentiating serotonergic neurons [168]. In this chromatin context, the TAF3 subunit of TFIID, normally involved in the recognition of H3K4me3, exhibits stronger binding preference for the H3K4me3Q5ser dual modification [172,173,174]. Concurrently, the H3K4me2/3 demethylases LSD1 and KDM5B/C are profoundly inhibited by the presence of the neighbouring H3Q5ser [174]. Most notably, deposition of the H3K4me3 mark is hampered upon disruption of the interaction between H3Q5ser and WDR5, which is a core subunit of the H3K4-specific MLL1 methyltransferase complex [174,175]. Taken together, these findings suggest that H3Q5ser is required to impose H3K4me3 and to stabilize it from dynamic turnover, enhancing its physical readout by downstream effectors.
13. Isomerization
Isomerization is the paradigm of non-covalent histone PTMs. It is the chemical conversion of an amino-acid residue into any of its isomeric forms, which possess the same chemical composition but exhibit different structure or configuration of atoms in space. In the case of proline, two possible isomeric conformations, either cis or trans, can be described depending on their dihedral angle referred to the peptide bond preceding the proline residue [176]. Although the two isomeric forms slowly switch one another in a spontaneous equilibrium state, enzymes belonging to the peptidyl-prolyl cis-trans isomerase (PPIase) superfamily accelerate such an interconversion process [177]. For example, in S. cerevisiae the PPIase Fpr4 specifically governs the cis-trans equilibrium of P38 on the histone H3 N-terminal tail [178]. In particular, the conformational status adopted by H3P38 disposes the histone tail either farther or closer to nucleosomal DNA, thereby affecting the opportunity for histone-DNA interaction and nucleosome stability, as well as the formation of higher-order chromatin structures [178].
Interestingly, Fpr4-dependent H3P38 isomerization regulates the methylation of the neighbouring H3K36 on the same histone tail. In particular, the trans conformation of H3P38 displaces the H3 tail far away from the nucleosome surface, creating favourable conditions for efficient recognition of H3K36 by the Set2 methyltransferase [178]. Reciprocally, in the presence of the cis H3P38 isomer, H3K36 no longer fits the active site of Set2 and it is more accessible for the demethylase JMJD2 [178]. The human ortholog of yeast Fpr4, hFKBP25, has been found to stably interact with HDAC-1 and -2, suggesting that an epigenetic crosstalk between lysine acetylation and proline isomerization could occur in vivo [179]. A confirmation for this hypothesis came from a more recent study showing that acetylation of H3K14 stimulates the trans-isomerization ratio of the adjoining H3P16, which in turn disfavours methylation of H3K4 [180].
Beyond the epigenetic control of chromatin plasticity at selected loci, histone proline isomerization can epigenetically define chromosome segregation fidelity in S. pombe [181]. In particular, a proline-rich heptapeptide motif of the N-terminal domain of the centromere-specific histone H3 variant CenPA is essential for ensuring precise chromosome segregation. Within this short amino acid stretch, cis-trans isomerization of P15 is carried out by the two FKBP-like PPIases encoded by the SPBC1347.02 and SPAC27F1.06c loci, leading to accurate propagation of centromeric integrity in fission yeast.
Similar to proline, aspartic acid isomerization can occur spontaneously or can be catalysed by protein-L-isoaspartyl/D-aspartyl O-methyltransferase (PIMT) enzymes [182]. PIMTs catalyse the transfer of a methyl group from SAM to a carboxyl group of either an L-isoaspartyl or a D-aspartyl atypical residues. The methyl ester formed by this reaction is then converted into a succinimidyl residue, which is spontaneously hydrolyzed to produce a mixture of the physiologically dominant L-aspartate form and L-isoaspartate or occasionally D-aspartate [182]. Selective accumulation of L-isoaspartate has been observed at H2BD25, but not in other core histones, in chromatin derived from brain of PIMT knockout mice, indicating that rigorous control of H2BD25iso levels take place in neurons [183]. Intriguingly, in mouse brain a D-aspartate form of H2BD25 is present in approximately 12% of the overall H2B amount, and it is preferentially associated with active chromatin [184,185].
14. Glycation
Amongst the most prevalent non-enzymatic histone PTMs, glycation follows the Maillard reaction consisting in the condensation of reducing monosaccharides (especially glucose and ribose), their derivatives or glycolytic by-products with reactive nucleophile groups of amino acid residues, forming Schiff base adducts [186]. For example, both the amine functional groups of arginine and lysine side chains are susceptible to adduction by the glycolytic by-product methylglyoxal, resulting in N7-carboxymethyl-arginine and Nε-carboxymethyl-lysine, respectively [187]. The resulting adducts undergo rapid oxidization and rearrangement to form a plethora of more stable species generally referred to as advanced glycation end-products [188].
Recent reports revealed that methylglyoxal-mediated glycation sites mostly map on the lateral surface of the histone octamer, in close proximity to DNA, suggesting that they may lead to disruption of nucleosomal stability and global chromatin architecture by altering histone-DNA electrostatic interactions in a mechanism similar to acetylation [189,190]. Indeed, partial loss of secondary protein structure and incomplete nucleosome formation has been observed following glycation of both core and linker histones [191,192]. Accordingly, hyperglycemia or glyoxalase1 gene knockout may allow accumulation of glycation adducts in mouse chromatin at the expense of canonical histone PTMs, leading to increased gene accessibility and global transcriptome alteration [189]. On the other hand, over-accumulation of adducts through high concentration or prolonged exposure to glycation agents results in robust cross-linking and hyper-compaction of the chromatin fiber, leading to the abrogation in DNA accessibility especially at transcription start sites [190].
Enzymatic reversion of Schiff base adducts is catalysed by the DJ-1 deglicase at lysine and arginine histone residues, while PADI4 shows deglycating activity solely towards arginines [190,193]. In this latter situation, PADI4-mediated citrullination not merely antagonizes histone glycation, but it shields citrullinated histones from undergoing glycation. Unfortunately, however, advanced glycation end-products cannot be reversed by both the mentioned enzymes.
15. Lipidation
As a result of oxidative stress in the cell, the fragmentation of polyunsaturated fatty acids yields numerous highly reactive α,β-unsaturated alkenals, including 4-hydroxy-2-nonenal (4-HNE) and 4-oxo-2-nonenal (4-ONE) [194,195]. Although sharing a very similar chemical backbone, the two mentioned species appear to arise independently, they do not interconvert metabolically and differ markedly in reactivity towards histones. In particular, 4-HNE primarily reacts with histidines via Michael addition, while 4-ONE forms ketoamide adducts via 1,2 addition to lysine residues [196,197]. A few 4-HNE-sensitive histidine residues have been identified exclusively in H2A, H2B and H4, lying in the globular and C-terminal domains of the histone proteins, and their adduction disrupts chromatin compaction by both 4-HNE steric bulk and neutralization of DNA-histone charge interactions [197,198]. Conceivably, these effects may make DNA more vulnerable to oxidative damage in Alzheimer disease [198,199]. All four core histones are instead modified by 4-ONE, being H2B the most heavily adducted species, and the adducted lysine and histidine residues mainly reside on the lateral surface of the nucleosome [196]. Unlike 4-HNE, adduction by 4-ONE elicits minor alterations in chromatin structure, although it can prevent de novo assembly of nucleosomes [196].
16. Formylation
In the last few years, formylation has been identified as a non-enzymatic histone PTM occurring on the ε-amino group of lysine residues under oxidative and nitrosative stress [200]. The formyl moiety predominantly derives from endogenous formaldehyde generated by oxidative demethylation of histones and nucleic acids, although it can occasionally arise from the 3′-formylphosphate intermediate in the 5′-oxidation of 2-deoxyribose in DNA [200,201]. In striking contrast to most of PTMs, the formyl-lysine adducts have been found to be rather equally distributed among different classes of histones from human lymphoblastoid cells TK6, suggesting that formylation is a fortuitous modification [202]. In spite of the chemical similarity between formyl and acetyl groups, formyl-lysines are not appreciably recognized by anti-acetyl-lysine antibodies, and they appear to be refractory to reversal by HDACs, with only the SIRT1 enzyme showing a minimum detectable activity value less than 10% in vitro [202,203].
Persistence of formyl-lysine adducts in individual histone proteins, coupled to the observation that they often mark residues that could be otherwise modified [204], strongly suggest that formylation can prevents the establishment of conventional histone PTMs. Additionally, formylation of residues involved in critical electrostatic interactions, such as H3K64, H4K77, H4K79, and H2BK34, may also impair organization and stability of the nucleosome particle [204].
17. Histone Tail Clipping
Numerous old and new studies reported that limited proteolysis of core and linker histone tails, referred to as histone tail clipping, is an evolutionarily conserved mechanism mediated by nuclear aminopeptidases and endopeptidases [205,206,207]. Although each study described distinct cleavage sites recognized by biochemically unrelated enzymes with clipping properties, these findings collectively suggest that the occurrence of histone tail clipping is crucial in many organisms and cell types for the regulation of various biological processes such as yeast sporulation and starvation, Tetrahymena conjugation, malaria parasite infection, mammalian embryonic stem cell differentiation, osteoclastogenesis, and senescence [208,209,210,211,212,213,214].
The occurrence of this irreversible epigenetic modification heavily impacts on chromatin plasticity and function. For instance, truncation of the N-terminal tails of core histones and/or the C-terminal tail of H2A destabilizes both intra- and inter-nucleosomal interactions, thereby increasing the breathability of chromatin [215,216,217,218]. Moreover, histone clipping could be thought as the most radical way to permanently reverse whatsoever PTM and to concomitantly abolish docking sites for chromatin-binding regulators mapping on histone tails. On the other hand, histone PTMs themselves might regulate the clipping incidence. For example, the JMJD5 and JMJD7 proteases specifically recognize and cleave at all possible degrees of arginine methylation on H2A, H3 and H4 histone tails through endopeptidase activity, progressively continuing to trim histone tails with aminopeptidase activity to generate tail-less nucleosomes [219]. Importantly, JMJD5 operates on nucleosomes at position +1, allowing RNA polII-dependent transcription elongation to occur smoothly [220]. In the case of histone H3, tail clipping can take place in the nucleosome or in the free pool of histones, preceding their deposition into specific genomic loci, which will integrate nucleosomes with unique properties both structurally and functionally [210]. An additional layer of complexity relies on the fact that the resected N-terminal H3 tail peptide may also directly bind the H3 mRNA, regulating its own translation [221].
A peculiar form of clipping without enzymatic engagement has been observed for the H2A C-terminal tail. In particular, Ni2+ ions are capable of forming a complex of octahedral geometry by binding the tetrapeptide sequence ESHH located at the C-terminal tail of histone H2A, through the carboxylate group on the side chain of glutamic acid and imidazole nitrogens on both of the histidine residues [222]. The particular steric arrangement of this square-planar complex is essential to assist Ni2+-dependent hydrolytic attack of the E121-S122 peptide bond, resulting in the removal of the C-terminal SHHKAKGK octapeptide from H2A [222,223,224]. Since the C-terminal tail normally interacts with histone H2B and linker DNA [225], the Ni2+-dependent truncation of H2A likely destabilizes the formation of higher-order structures of chromatin, thereby affecting gene transcription. Accordingly, several genes involved in neoplastic transformation exhibited either reduced or enhanced expression following H2A C-tail clipping, providing an epigenetic explanation for the mechanism of Ni2+-induced carcinogenesis [226].
Regardless of the enzymatic or non-enzymatic mechanism involved, as the cleaved tails cannot be re-ligated to the original histones, histone tail clipping can only be reversed through histone turnover or replacement.
18. Conclusions
At the present time, additional new types of histone PTMs have been identified, including asparagine deamidation [170,227], arginine and lysine carbonylation [228], tyrosine nitrosylation and hydroxylation [39,229], lysine 5′-hydroxylation [230], cysteine glutathionylation and sulfonylation [231,232], glutamine methylation [233], and H3K4me3 oxidation [234]. Intriguingly, although less well studied than those detailed in this review, most of them appear to be reversible and highly dynamic in a context-dependent fashion. However, the mechanisms by which these modifications can be imposed and reversed on the various classes of histones, as well as their structural and functional effects on chromatin are still largely unexplored.
There is a strong conceptual relationship between the combinatorial complexity of histone PTMs and the popular assumption of a “histone code”, whereby the sequential placement and/or removal of diverse histone PTMs on nucleosomes would univocally specify spatiotemporal patterns of gene expression [235]. From the perspective of this paradigm, histone PTMs are usually considered to be activating or repressive marks with respect to gene transcription, thus implying direct functional causality. Moreover, the histone-modifying enzymes are often referred to as writers and erasers, based on their respective capability of processing the forward or reverse modification reactions, while the so-called readers correspond to the protein complexes specifically recruited on chromatin by the various PTMs. Although the adoption of these metaphors became a commonplace in publications on this field, the mere existence of a histone code has been the subject of controversies and a conclusive one-to-one correspondence between histone PTMs and gene expression did not emerge [236]. In this regard, it should be emphasized that the alleged histone code lacks of the univocal information that should characterize each unit of a code. In fact, a same histone residue can potentially endure alternative types of PTM, and each of them may recruit multiple effector complexes in a context-dependent fashion, not to say that some enzymes can operate more than a single modification [237]. In addition, the two copies of each histone are not necessarily modified identically within a single nucleosome, giving rise to asymmetrically modified or bivalent nucleosomes, especially in the chromatin of stem cells or developing embryos [238,239]. Worth mentioning, the causal correlation between histone PTMs and downstream transcriptional outputs is formally questionable as well, since there are several cases in which a given histone PTM has found to be associated with antipodal effects on transcription activity. In this respect, it should be emphasized that nucleosomes are not static targets of modification enzymes within actively transcribed chromatin, which is instead characterized by a dynamic histone replacement process [240]. Finally, such a hypothetical code might not pertain specifically to histones, since proteins in general display complex patterns of covalent post-translational modifications.
Given all of that, probably it would be more appropriate to talk about a histone language, in which the different categories of PTM represent individual letters that will allow us to compose and/or decipher complex epigenetic words in the years to come.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kornberg R.D. Chromatin structure: A repeating unit of histones and DNA. Science. 1974;184:868–871. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- 2.Kornberg R.D., Lorch Y. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell. 1999;98:285–294. doi: 10.1016/S0092-8674(00)81958-3. [DOI] [PubMed] [Google Scholar]
- 3.Arents G., Burlingame R.W., Wang B.C., Love W.E., Moudrianakis E.N. The nucleosomal core histone octamer at 3.1 Å resolution: A tripartite protein assembly and a left-handed superhelix. Proc. Natl. Acad. Sci. USA. 1991;88:10148–10152. doi: 10.1073/pnas.88.22.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luger K., Mäder A.W., Richmond R.K., Sargent D.F., Richmond T.J. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 5.Ausio J., Dong F., van Holde K.E. Use of selectively trypsinized nucleosome core particles to analyze the role of the histone “tails” in the stabilization of the nucleosome. J. Mol. Biol. 1989;206:451–463. doi: 10.1016/0022-2836(89)90493-2. [DOI] [PubMed] [Google Scholar]
- 6.Bednar J., Horowitz R.A., Grigoryev S.A., Carruthers L.M., Hansen J.C., Koster A.J., Woodcock C.L. Nucleosomes, linker DNA, and linker histone form a unique structural motif that directs the higher-order folding and compaction of chromatin. Proc. Natl. Acad. Sci. USA. 1998;95:14173–14178. doi: 10.1073/pnas.95.24.14173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bednar J., Garcia-Saez I., Boopathi R., Cutter A.R., Papai G., Reymer A., Syed S.H., Lone I.N., Tonchev O., Crucifix C., et al. Structure and Dynamics of a 197 bp Nucleosome in Complex with Linker Histone H1. Mol. Cell. 2017;66:384–397. doi: 10.1016/j.molcel.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavalieri V., Melfi R., Spinelli G. Promoter activity of the sea urchin (Paracentrotus lividus) nucleosomal H3 and H2A and linker H1 {alpha}-histone genes is modulated by enhancer and chromatin insulator. Nucleic Acids Res. 2009;37:7407–7415. doi: 10.1093/nar/gkp859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavalieri V., Melfi R., Spinelli G. The Compass-like locus, exclusive to the Ambulacrarians, encodes a 874 chromatin insulator binding protein in the Sea Urchin embryo. PLoS Genet. 2013;9:e1003847. doi: 10.1371/journal.pgen.1003847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavalieri V., Spinelli G. Histone-mediated transgenerational epigenetics. In: Tollefsbol T.O., editor. Transgenerational Epigenetics. 2nd ed. Elsevier; Amsterdam, The Netherlands: 2019. pp. 157–183. [Google Scholar]
- 11.Cavalieri V. Histones, Their Variants and Post-translational Modifications in Zebrafish Development. Front. Cell Dev. Biol. 2020;8:456. doi: 10.3389/fcell.2020.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su X., Ren C., Freitas M.A. Mass spectrometry-based strategies for characterization of histones and their posttranslational modifications. Expert Rev. Proteom. 2007;4:211–225. doi: 10.1586/14789450.4.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y., Garcia B.A. Comprehensive Catalog of Currently Documented Histone Modifications. Cold Spring Harb. Perspect. Biol. 2015;7:a025064. doi: 10.1101/cshperspect.a025064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith E., Shilatifard A. The chromatin signaling pathway: Diverse mechanisms of recruitment of histone-modifying enzymes and varied biological outcomes. Mol. Cell. 2010;40:689e701. doi: 10.1016/j.molcel.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavalieri V., Spinelli G. Environmental epigenetics in zebrafish. Epigenetics Chromatin. 2017;10:46. doi: 10.1186/s13072-017-0154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavalieri V. Model organisms and their application in environmental epigenetics. In: Fry R., editor. Environmental Epigenetics in Toxicology and Public Health. Elsevier; Amsterdam, The Netherlands: 2020. pp. 67–87. [Google Scholar]
- 17.Fischle W., Wang Y., Allis C.D. Binary switches and modification cassettes in histone biology and beyond. Nature. 2003;425:475–479. doi: 10.1038/nature02017. [DOI] [PubMed] [Google Scholar]
- 18.Oey N.E., Leung H.W., Ezhilarasan R., Zhou L., Beuerman R.W., VanDongen H.M., VanDongen A.M. A Neuronal Activity-Dependent Dual Function Chromatin-Modifying Complex Regulates Arc Expression. eNeuro. 2015;2:ENEURO.0020-14.2015. doi: 10.1523/ENEURO.0020-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner B.M. Reading signals on the nucleosome with a new nomenclature for modified histones. Nat. Struct. Mol. Biol. 2005;12:110–112. doi: 10.1038/nsmb0205-110. [DOI] [PubMed] [Google Scholar]
- 20.Phillips D.M.P. The presence of acetyl groups in histones. Biochem. J. 1963;87:258–263. doi: 10.1042/bj0870258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allfrey V.G., Faulkner R., Mirsky A.E. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc. Natl. Acad. Sci. USA. 1964;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berndsen C.B., Denu J.M. Catalysis and substrate selection by histone/protein lysine acetyltransferases. Curr. Opin. Struct. Biol. 2008;18:682–689. doi: 10.1016/j.sbi.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richman R., Chicoine L.G., Collini M.P., Cook R.G., Allis C.D. Micronuclei and the cytoplasm of growing Tetrahymena contain a histone acetylase activity which is highly specific for free histone H4. J. Cell Biol. 1988;106:1017–1026. doi: 10.1083/jcb.106.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parthun M.R., Widom J., Gottschling D.E. The major cytoplasmic histone acetyltransferase in yeast: Links to chromatin replication and histone metabolism. Cell. 1996;87:85–94. doi: 10.1016/S0092-8674(00)81325-2. [DOI] [PubMed] [Google Scholar]
- 25.Seto E., Yoshida M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 2014;6:a018713. doi: 10.1101/cshperspect.a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorne A.W., Kmiciek D., Mitchelson K., Sautiere P., Crane-Robinson C. Patterns of histone acetylation. Eur. J. Biochem. 1990;193:701–713. doi: 10.1111/j.1432-1033.1990.tb19390.x. [DOI] [PubMed] [Google Scholar]
- 27.Kimura A., Horikoshi M. How do histone acetyltransferases select lysine residues in core histones? FEBS Lett. 1998;431:131–133. doi: 10.1016/S0014-5793(98)00752-2. [DOI] [PubMed] [Google Scholar]
- 28.Grant P.A., Duggan L., Côté J., Roberts S.M., Brownell J.E., Candau R., Ohba R., Owen-Hughes T., Allis C.D., Winston F. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: Characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 1997;11:1640–1650. doi: 10.1101/gad.11.13.1640. [DOI] [PubMed] [Google Scholar]
- 29.Workman J.L., Kingston R.E. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu. Rev. Biochem. 1998;67:545–579. doi: 10.1146/annurev.biochem.67.1.545. [DOI] [PubMed] [Google Scholar]
- 30.Shogren-Knaak M. Histone H4–K16 acetylation controls chromatin structure and protein interactions. Science. 2006;311:844–847. doi: 10.1126/science.1124000. [DOI] [PubMed] [Google Scholar]
- 31.Allahverdi A., Yang R., Korolev N., Fan Y., Davey C.A., Liu C.F., Nordenskiöld L. The effects of histone H4 tail acetylations on cation-induced chromatin folding and self-association. Nucleic Acids Res. 2011;39:1680–1691. doi: 10.1093/nar/gkq900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collepardo-Guevara R., Portella G., Vendruscolo M., Frenkel D., Schlick T., Orozco M. Chromatin unfolding by epigenetic modifications explained by dramatic impairment of internucleosome interactions: A multiscale computational study. J. Am. Chem. Soc. 2015;137:10205–10215. doi: 10.1021/jacs.5b04086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schubeler D., MacAlpine D.M., Scalzo D., Wirbelauer C., Kooperberg C., van Leeuwen F., Gottschling D.E., O’Neill L.P., Turner B.M., Delrow J., et al. The histone modification pattern of active genes revealed through genome-wide chromatin analysis of a higher eukaryote. Genes Dev. 2004;18:1263–1271. doi: 10.1101/gad.1198204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cavalieri V., Spinelli G. Ectopic hbox12 Expression Evoked by Histone Deacetylase Inhibition Disrupts Axial Specification of the Sea Urchin Embryo. PLoS ONE. 2015;10:e0143860. doi: 10.1371/journal.pone.0143860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Caro V., Cavalieri V., Melfi R., Spinelli G. Constitutive promoter occupancy by the MBF-1 activator and chromatin modification of the developmental regulated sea urchin alpha-H2A histone gene. J. Mol. Biol. 2007;365:1285–1297. doi: 10.1016/j.jmb.2006.10.098. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z.B., Zang C.Z., Rosenfeld J.A., Schones D.E., Barski A., Cuddapah S., Cui K., Roh T.Y., Peng W., Zhang M.Q., et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat. Genet. 2008;40:897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brower-Toland B., Wacker D.A., Fulbright R.M., Lis J.T., Kraus W.L., Wang M.D. Specific contributions of histone tails and their acetylation to the mechanical stability of nucleosomes. J. Mol. Biol. 2005;346:135–146. doi: 10.1016/j.jmb.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y., Sprung R., Tang Y., Ball H., Sangras B., Kim S.C., Falck J.R., Peng J., Gu W., Zhao Y. Lysine propionylation and butyrylation are novel post-translational modifications in histones. Mol. Cell Proteom. 2007;6:812–819. doi: 10.1074/mcp.M700021-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan M., Luo H., Lee S., Jin F., Yang J.S., Montellier E., Buchou T., Cheng Z., Rousseaux S., Rajagopal N., et al. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell. 2011;146:1016–1028. doi: 10.1016/j.cell.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang H., Zhang D., Wang Y., Perez-Neut M., Han Z., Zheng Y.G., Hao Q., Zhao Y. Lysine benzoylation is a histone mark regulated by SIRT2. Nat. Commun. 2018;9:3374. doi: 10.1038/s41467-018-05567-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai L., Peng C., Montellier E., Lu Z., Chen Y., Ishii H., Debernardi A., Buchou T., Rousseaux S., Jin F., et al. Lysine 2-hydroxyisobutyrylation is a widely distributed active histone mark. Nat. Chem. Biol. 2014;10:365–370. doi: 10.1038/nchembio.1497. [DOI] [PubMed] [Google Scholar]
- 42.Xie Z., Zhang D., Chung D., Tang Z., Huang H., Dai L., Qi S., Li J., Colak G., Chen Y., et al. Metabolic Regulation of Gene Expression by Histone Lysine β-Hydroxybutyrylation. Mol. Cell. 2016;62:194–206. doi: 10.1016/j.molcel.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie Z., Dai J., Dai L., Tan M., Cheng Z., Wu Y., Boeke J.D., Zhao Y. Lysine succinylation and lysine malonylation in histones. Mol. Cell Proteom. 2012;11:100–107. doi: 10.1074/mcp.M111.015875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan M., Peng C., Anderson K.A., Chhoy P., Xie Z., Dai L., Park J., Chen Y., Huang H., Zhang Y., et al. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab. 2014;19:605–617. doi: 10.1016/j.cmet.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaffney D.O., Jennings E.Q., Anderson C.C., Marentette J.O., Shi T., Schou Oxvig A.M., Streeter M.D., Johannsen M., Spiegel D.A., Chapman E., et al. Non-enzymatic Lysine Lactoylation of Glycolytic Enzymes. Cell Chem. Biol. 2020;27:206–213.e6. doi: 10.1016/j.chembiol.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goudarzi A., Zhang D., Huang H., Barral S., Kwon O.K., Qi S., Tang Z., Buchou T., Vitte A.L., He T., et al. Dynamic Competing Histone H4 K5K8 Acetylation and Butyrylation Are Hallmarks of Highly Active Gene Promoters. Mol. Cell. 2016;62:169–180. doi: 10.1016/j.molcel.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sabari B.R., Tang Z., Huang H., Yong-Gonzalez V., Molina H., Kong H.E., Dai L., Shimada M., Cross J.R., Zhao Y., et al. Intracellular crotonyl-CoA stimulates transcription through p300-catalyzed histone crotonylation. Mol. Cell. 2015;58:203–215. doi: 10.1016/j.molcel.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang H., Tang S., Ji M., Tang Z., Shimada M., Liu X., Qi S., Locasale J.W., Roeder R.G., Zhao Y., et al. EP300-mediated lysine 2-hydroxyisobutyrylation regulates glycolysis. Mol. Cell. 2018;70:663–678.e6. doi: 10.1016/j.molcel.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trub A.G., Hirschey M.D. Reactive acyl-CoA species modify proteins and induce carbon Stress. Trends Biochem. Sci. 2018;43:369–379. doi: 10.1016/j.tibs.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaczmarska Z., Ortega E., Goudarzi A., Huang H., Kim S., Márquez J.A., Zhao Y., Khochbin S., Panne D. Structure of p300 in complex with acyl-CoA variants. Nat. Chem. Biol. 2017;13:21–29. doi: 10.1038/nchembio.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sabari B.R., Zhang D., Allis C.D., Zhao Y. Metabolic regulation of gene expression through histone acylations. Nat. Rev. Mol. Cell Biol. 2017;18:90–101. doi: 10.1038/nrm.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bao X., Liu Z., Zhang W., Gladysz K., Fung Y.M.E., Tian G., Xiong Y., Wong J.W.H., Yuen K.W.Y., Li X.D. Glutarylation of histone H4 lysine 91 regulates chromatin dynamics. Mol. Cell. 2019;76:660–675.e9. doi: 10.1016/j.molcel.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 53.Zorro Shahidian L., Haas M., Le Gras S., Nitsch S., Mourao A., Geerlof A., Margueron R., Michaelis J., Daujat S., Schneider R. Succinylation of H3K122 destabilizes nucleosomes and enhances transcription. EMBO Rep. 2021;22:e51009. doi: 10.15252/embr.202051009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jing Y., Ding D., Tian G., Kwan K.C.J., Liu Z., Ishibashi T., Li X.D. Semisynthesis of site-specifically succinylated histone reveals that succinlation regulates nucleosome unwrapping rate and DNA accessibility. Nucleic Acids Res. 2020;48:9538–9549. doi: 10.1093/nar/gkaa663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montellier E., Rousseaux S., Zhao Y., Khochbin S. Histone crotonylation specifically marks the haploid male germ cell gene expression program: Post-meiotic male-specific gene expression. Bioessays. 2012;34:187–193. doi: 10.1002/bies.201100141. [DOI] [PubMed] [Google Scholar]
- 56.Kebede A.F., Nieborak A., Shahidian L.Z., Le Gras S., Richter F., Gomez D.A., Baltissen M.P., Meszaros G., Magliarelli H.D.F., Taudt A., et al. Histone propionylation is a mark of active chromatin. Nat. Struct. Mol. Biol. 2017;24:1048–1056. doi: 10.1038/nsmb.3490. [DOI] [PubMed] [Google Scholar]
- 57.Ren X., Zhou Y., Xue Z., Hao N., Li Y., Guo X., Wang D., Shi X., Li H. Histone benzoylation serves as an epigenetic mark for DPF and YEATS family proteins. Nucleic Acids Res. 2021;49:114–126. doi: 10.1093/nar/gkaa1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang D., Tang Z., Huang H., Zhou G., Cui C., Weng Y., Liu W., Kim S., Lee S., Perez-Neut M., et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574:575–580. doi: 10.1038/s41586-019-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cui H., Xie N., Banerjee S., Ge J., Jiang D., Dey T., Matthews Q.L., Liu R.M., Liu G. Lung Myofibroblasts Promote Macrophage Profibrotic Activity through Lactate-induced Histone Lactylation. Am. J. Respir. Cell Mol. Biol. 2021;64:115–125. doi: 10.1165/rcmb.2020-0360OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rabbani N., Xue M., Thornalley P.J. Activity, regulation, copy number and function in the glyoxalase system. Biochem. Soc. Trans. 2014;42:419–424. doi: 10.1042/BST20140008. [DOI] [PubMed] [Google Scholar]
- 61.Gowans G.J., Bridgers J.B., Zhang J., Dronamraju R., Burnetti A., King D.A., Thiengmany A.V., Shinsky S.A., Bhanu N.V., Garcia B.A., et al. Recognition of histone crotonylation by Taf14 links metabolic state to gene expression. Mol. Cell. 2019;76:909–921. doi: 10.1016/j.molcel.2019.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ng S.S., Yue W.W., Oppermann U., Klose R.J. Dynamic protein methylation in chromatin biology. Cell Mol. Life Sci. 2009;66:407–422. doi: 10.1007/s00018-008-8303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bedford M.T., Clarke S.G. Protein arginine methylation in mammals: Who, what, and why. Mol. Cell. 2009;33:1–13. doi: 10.1016/j.molcel.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zurita-Lopez C.I., Sandberg T., Kelly R., Clarke S.G. Human protein arginine methyltransferase 7 (PRMT7) is a type III enzyme forming ω-NG-monomethylated arginine residues. J. Biol. Chem. 2012;287:7859–7870. doi: 10.1074/jbc.M111.336271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang M., Xu R.M., Thompson P.R. Substrate specificity, processivity, and kinetic mechanism of protein arginine methyltransferase 5. Biochemistry. 2013;52:5430–5440. doi: 10.1021/bi4005123. [DOI] [PubMed] [Google Scholar]
- 66.Tamaru H., Zhang X., McMillen D., Singh P.B., Nakayama J., Grewal S.I., Allis C.D., Cheng X., Selker E.U. Trimethylated lysine 9 of histone H3 is a mark for DNA methylation in Neurospora crassa. Nat. Genet. 2003;34:75–79. doi: 10.1038/ng1143. [DOI] [PubMed] [Google Scholar]
- 67.Xiao B., Jing C., Wilson J.R., Walker P.A., Vasisht N., Kelly G., Howell S., Taylor I.A., Blackburn G.M., Gamblin S.J. Structure and catalytic mechanism of the human histone methyltransferase SET7/9. Nature. 2003;421:652–656. doi: 10.1038/nature01378. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y., Reinberg D. Transcription regulation by histone methylation: Interplay between different covalent modifications of the core histone tails. Genes Dev. 2001;15:2343–2360. doi: 10.1101/gad.927301. [DOI] [PubMed] [Google Scholar]
- 69.Schneider R., Bannister A.J., Myers F.A., Thorne A.W., Crane-Robinson C., Kouzarides T. Histone H3 lysine 4 methylation patterns in higher eukaryotic genes. Nat. Cell Biol. 2004;6:73–77. doi: 10.1038/ncb1076. [DOI] [PubMed] [Google Scholar]
- 70.Heintzman N.D., Stuart R.K., Hon G., Fu Y., Ching C.W., Hawkins R.D., Barrera L.O., Van Calcar S., Qu C., Ching K.A. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat. Genet. 2007;39:311–318. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 71.Noma K., Allis C.D., Grewal S.I. Transitions in distinct histone H3 methylation patterns at the heterochromatin domain boundaries. Science. 2001;293:1150–1155. doi: 10.1126/science.1064150. [DOI] [PubMed] [Google Scholar]
- 72.Schotta G., Lachner M., Sarma K., Ebert A., Sengupta R., Reuter G., Reinberg D., Jenuwein T. A silencing pathway to induce H3-K9 and H4-K20 trimethylation at constitutive heterochromatin. Genes Dev. 2004;18:1251–1262. doi: 10.1101/gad.300704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lachner M., O’Carroll D., Rea S., Mechtler K., Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410:116–120. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- 74.Maison C., Almouzni G. HP1 and the dynamics of heterochromatin maintenance. Nat. Rev. Mol. Cell Biol. 2004;5:296–304. doi: 10.1038/nrm1355. [DOI] [PubMed] [Google Scholar]
- 75.Vakoc C.R., Mandat S.A., Olenchock B.A., Blobel G.A. Histone H3 lysine 9 methylation and HP1gamma are associated with transcription elongation through mammalian chromatin. Mol. Cell. 2005;19:381–391. doi: 10.1016/j.molcel.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 76.Rolando M., Sanulli S., Rusniok C., Gomez-Valero L., Bertholet C., Sahr T., Margueron R., Buchrieser C. Legionella pneumophila effector RomA uniquely modifies host chromatin to repress gene expression and promote intracellular bacterial replication. Cell Host Microbe. 2013;13:395–405. doi: 10.1016/j.chom.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 77.Whetstine J.R., Nottke A., Lan F., Huarte M., Smolikov S., Chen Z., Spooner E., Li E., Zhang G., Colaiacovo M., et al. Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell. 2006;125:467–481. doi: 10.1016/j.cell.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 78.Chang B., Chen Y., Zhao Y., Bruick R.K. JMJD6 is a histone arginine demethylase. Science. 2007;318:444–447. doi: 10.1126/science.1145801. [DOI] [PubMed] [Google Scholar]
- 79.Webby C.J., Wolf A., Gromak N., Dreger M., Kramer H., Kessler B., Nielsen M.L., Schmitz C., Butler D.S., Yates J.R., 3rd, et al. Jmjd6 catalyses lysyl-hydroxylation of U2AF65, a protein associated with RNA splicing. Science. 2009;325:90–93. doi: 10.1126/science.1175865. [DOI] [PubMed] [Google Scholar]
- 80.Mantri M., Krojer T., Bagg E.A., Webby C.A., Butler D.S., Kochan G., Kavanagh K.L., Oppermann U., McDonough M.A., Schofield C.J. Crystal structure of the 2-oxoglutarate- and Fe(II)-dependent lysyl hydroxylase JMJD6. J. Mol. Biol. 2010;401:211–222. doi: 10.1016/j.jmb.2010.05.054. [DOI] [PubMed] [Google Scholar]
- 81.Cuthbert G.L., Daujat S., Snowden A.W., Erdjument-Bromage H., Hagiwara T., Yamada M., Schneider R., Gregory P.D., Tempst P., Bannister A.J., et al. Histone deimination antagonizes arginine methylation. Cell. 2004;118:545–553. doi: 10.1016/j.cell.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y., Wysocka J., Sayegh J., Lee Y.H., Perlin J.R., Leonelli L., Sonbuchner L.S., McDonald C.H., Cook R.G., Dou Y., et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science. 2004;306:279–283. doi: 10.1126/science.1101400. [DOI] [PubMed] [Google Scholar]
- 83.Leshner M., Wang S., Lewis C., Zheng H., Chen X.A., Santy L., Wang Y. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front. Immunol. 2012;3:307. doi: 10.3389/fimmu.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Christophorou M.A., Castelo-Branco G., Halley-Stott R.P., Oliveira C.S., Loos R., Radzisheuskaya A., Mowen K.A., Bertone P., Silva J.C., Zernicka-Goetz M., et al. Citrullination regulates pluripotency and histone H1 binding to chromatin. Nature. 2014;507:104–108. doi: 10.1038/nature12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ord M.G., Stocken L.A. Metabolic properties of histones from rat liver and thymus gland. Biochem. J. 1966;98:888–897. doi: 10.1042/bj0980888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rossetto D., Avvakumov N., Côté J. Histone phosphorylation: A chromatin modification involved in diverse nuclear events. Epigenetics. 2012;7:1098–1108. doi: 10.4161/epi.21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu S., Xie Z., Onishi A., Yu X., Jiang L., Lin J., Rho H.S., Woodard C., Wang H., Jeong J.S., et al. Profiling the human protein-DNA interactome reveals ERK2 as a transcriptional repressor of interferon signaling. Cell. 2009;139:610–622. doi: 10.1016/j.cell.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Banerjee T., Chakravarti D. A peek into the complex realm of histone phosphorylation. Mol. Cell Biol. 2011;24:4858–4873. doi: 10.1128/MCB.05631-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dou Y., Gorovsky M.A. Phosphorylation of linker histone H1 regulates gene expression in vivo by creating a charge patch. Mol. Cell. 2000;6:225–231. doi: 10.1016/S1097-2765(00)00024-1. [DOI] [PubMed] [Google Scholar]
- 90.Cheung P., Allis C.D., Sassone-Corsi P. Signaling to chromatin through histone modifications. Cell. 2000;103:263–271. doi: 10.1016/S0092-8674(00)00118-5. [DOI] [PubMed] [Google Scholar]
- 91.Cook P.J., Ju B.G., Telese F., Wang X., Glass C.K., Rosenfeld M.G. Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions. Nature. 2009;58:591–596. doi: 10.1038/nature07849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singh R.K., Gunjan A. Histone tyrosine phosphorylation comes of age. Epigenetics. 2011;6:153–160. doi: 10.4161/epi.6.2.13589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wei Y., Yu L., Bowen J., Gorovsky M.A., Allis C.D. Phosphorylation of histone H3 is required for proper chromosome condensation and segregation. Cell. 1999;97:99–109. doi: 10.1016/S0092-8674(00)80718-7. [DOI] [PubMed] [Google Scholar]
- 94.Ivaldi M.S., Karam C.S., Corces V.G. Phosphorylation of histone H3 at Ser10 facilitates RNA polymerase II release from promoter-proximal pausing in Drosophila. Genes Dev. 2007;21:2818–2831. doi: 10.1101/gad.1604007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wei Y., Mizzen C.A., Cook R.G., Gorovsky M.A., Allis C.D. Phosphorylation of histone H3 at serine 10 is correlated with chromosome condensation during mitosis and meiosis in Tetrahymena. Proc. Natl. Acad. Sci. USA. 1998;95:7480–7484. doi: 10.1073/pnas.95.13.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fischle W., Tseng B.S., Dormann H.L., Ueberheide B.M., Garcia B.A., Shabanowitz J., Hunt D.F., Funabiki H., Allis C.D. Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature. 2005;438:1116–1122. doi: 10.1038/nature04219. [DOI] [PubMed] [Google Scholar]
- 97.Hartzog G.A., Tamkun J.W. A new role for histone tail modifications in transcription elongation. Genes Dev. 2007;21:3209–3213. doi: 10.1101/gad.1628707. [DOI] [PubMed] [Google Scholar]
- 98.Nowak S.J., Corces V.G. Phosphorylation of histone H3: A balancing act between chromosome condensation and transcriptional activation. Trends Genet. 2004;20:214–220. doi: 10.1016/j.tig.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 99.Du H.N. Transcription, DNA damage and beyond: The roles of histone ubiquitination and deubiquitination. Curr. Protein Pept. Sci. 2012;13:447–466. doi: 10.2174/138920312802430617. [DOI] [PubMed] [Google Scholar]
- 100.Tweedie-Cullen R.Y., Reck J.M., Mansuy I.M. Comprehensive mapping of post-translational modifications on synaptic, nuclear, and histone proteins in the adult mouse brain. J. Proteome Res. 2009;8:4966–4982. doi: 10.1021/pr9003739. [DOI] [PubMed] [Google Scholar]
- 101.Wright D.E., Wang C.Y., Kao C.F. Histone ubiquitylation and chromatin dynamics. Front. Biosci. 2012;17:1051–1078. doi: 10.2741/3973. [DOI] [PubMed] [Google Scholar]
- 102.Zhao J., Wang M., Chang L., Yu J., Song A., Liu C., Huang W., Zhang T., Wu X., Shen X., et al. RYBP/YAF2-PRC1 complexes and histone H1-dependent chromatin compaction mediate propagation of H2AK119ub1 during cell division. Nat. Cell Biol. 2020;22:439–452. doi: 10.1038/s41556-020-0484-1. [DOI] [PubMed] [Google Scholar]
- 103.Fierz B., Chatterjee C., McGinty R.K., Bar-Dagan M., Raleigh D.P., Muir T.W. Histone H2B ubiquitylation disrupts local and higher-order chromatin compaction. Nat. Chem. Biol. 2011;7:113–119. doi: 10.1038/nchembio.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dover J., Schneider J., Tawiah-Boateng M.A., Wood A., Dean K., Johnston M., Shilatifard A. Methylation of histone H3 by COMPASS requires ubiquitination of histone H2B by Rad6. J. Biol. Chem. 2002;277:28368–28371. doi: 10.1074/jbc.C200348200. [DOI] [PubMed] [Google Scholar]
- 105.Ng H.H., Xu R.M., Zhang Y., Struhl K. Ubiquitination of histone H2B by Rad6 is required for efficient Dot1-mediated methylation of histone H3 lysine 79. J. Biol. Chem. 2002;277:34655–34657. doi: 10.1074/jbc.C200433200. [DOI] [PubMed] [Google Scholar]
- 106.Minsky N., Shema E., Field Y., Schuster M., Segal E., Oren M. Monoubiquitinated H2B is associated with the transcribed region of highly expressed genes in human cells. Nat. Cell Biol. 2008;10:483–488. doi: 10.1038/ncb1712. [DOI] [PubMed] [Google Scholar]
- 107.Kim J., Guermah M., McGinty R.K., Lee J.S., Tang Z., Milne T.A., Shilatifard A., Muir T.W., Roeder R.G. RAD6-Mediated transcription-coupled H2B ubiquitylation directly stimulates H3K4 methylation in human cells. Cell. 2009;137:459–471. doi: 10.1016/j.cell.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krajewski W.A., Li J., Dou Y. Effects of histone H2B ubiquitylation on the nucleosome structure and dynamics. Nucleic Acids Res. 2018;46:7631–7642. doi: 10.1093/nar/gky526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Han J., Zhang H., Zhang H., Wang Z., Zhou H., Zhang Z. A Cul4 E3 ubiquitin ligase regulates histone hand-off during nucleosome assembly. Cell. 2013;155:817–829. doi: 10.1016/j.cell.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ryu H.Y., Hochstrasser M. Histone sumoylation and chromatin dynamics. Nucleic Acids Res. 2021;49:6043–6052. doi: 10.1093/nar/gkab280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meulmeester E., Melchior F. Cell biology: SUMO. Nature. 2008;452:709–711. doi: 10.1038/452709a. [DOI] [PubMed] [Google Scholar]
- 112.Chandrasekharan M.B., Huang F., Sun Z.W. Ubiquitination of histone H2B regulates chromatin dynamics by enhancing nucleosome stability. Proc. Natl. Acad. Sci. USA. 2009;106:16686–16691. doi: 10.1073/pnas.0907862106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nathan D., Ingvarsdottir K., Sterner D.E., Bylebyl G.R., Dokmanovic M., Dorsey J.A., Whelan K.A., Krsmanovic M., Lane W.S., Meluh P.B., et al. Histone sumoylation is a negative regulator in Saccharomyces cerevisiae and shows dynamic interplay with positive-acting histone modifications. Genes Dev. 2006;20:966–976. doi: 10.1101/gad.1404206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dhall A., Weller C.E., Chu A., Shelton P.M.M., Chatterjee C. Chemically sumoylated histone H4 stimulates intranucleosomal demethylation by the LSD1-CoREST complex. ACS Chem. Biol. 2017;12:2275–2280. doi: 10.1021/acschembio.7b00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jain N., Tamborrini D., Evans B., Chaudhry S., Wilkins B.J., Neumann H. Interaction of RSC Chromatin Remodeling Complex with Nucleosomes Is Modulated by H3 K14 Acetylation and H2B SUMOylation In Vivo. iScience. 2020;23:101292. doi: 10.1016/j.isci.2020.101292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kalocsay M., Hiller N.J., Jentsch S. Chromosome-wide Rad51 spreading and SUMO-H2A.Z-dependent chromosome fixation in response to a persistent DNA double-strand break. Mol. Cell. 2009;33:335–343. doi: 10.1016/j.molcel.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 117.Ohkuni K., Levy-Myers R., Warren J., Au W.C., Takahashi Y., Baker R.E., Basrai M.A. N-terminal sumoylation of centromeric histone H3 variant Cse4 regulates Its proteolysis to prevent mislocalization to non-centromeric chromatin. G3. 2018;8:1215–1223. doi: 10.1534/g3.117.300419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ohkuni K., Suva E., Au W.C., Walker R.L., Levy-Myers R., Meltzer P.S., Baker R.E., Basrai M.A. Deposition of centromeric histone H3 variant CENP-A/Cse4 into chromatin is facilitated by its C-terminal sumoylation. Genetics. 2020;214:839–854. doi: 10.1534/genetics.120.303090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sakabe K., Wang Z., Hart G.W. Beta-N-acetylglucosamine (O-GlcNAc) is part of the histone code. Proc. Natl. Acad. Sci. USA. 2010;107:19915–19920. doi: 10.1073/pnas.1009023107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang S., Roche K., Nasheuer H.P., Lowndes N.F. Modification of histones by sugar β-N-acetylglucosamine (GlcNAc) occurs on multiple residues, including histone H3 serine 10, and is cell cycle-regulated. J. Biol. Chem. 2011;286:37483–37495. doi: 10.1074/jbc.M111.284885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kreppel L.K., Blomberg M.A., Hart G.W. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J. Biol. Chem. 1997;272:9308–9315. doi: 10.1074/jbc.272.14.9308. [DOI] [PubMed] [Google Scholar]
- 122.Gao Y., Wells L., Comer F.I., Parker G.J., Hart G.W. Dynamic O-glycosylation of nuclear and cytosolic proteins: Cloning and characterization of a neutral, cytosolic beta-N-acetylglucosaminidase from human brain. J. Biol. Chem. 2001;276:9838–9845. doi: 10.1074/jbc.M010420200. [DOI] [PubMed] [Google Scholar]
- 123.Toleman C., Paterson A.J., Whisenhunt T.R., Kudlow J.E. Characterization of the histone acetyltransferase (HAT) domain of a bifunctional protein with activable O-GlcNAcase and HAT activities. J. Biol. Chem. 2004;279:53665–53673. doi: 10.1074/jbc.M410406200. [DOI] [PubMed] [Google Scholar]
- 124.Rao F.V., Schüttelkopf A.W., Dorfmueller H.C., Ferenbach A.T., Navratilova I., van Aalten D.M. Structure of a bacterial putative acetyltransferase defines the fold of the human O-GlcNAcase C-terminal domain. Open Biol. 2013;3:130021. doi: 10.1098/rsob.130021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.He Y., Roth C., Turkenburg J.P., Davies G.J. Three-dimensional structure of a Streptomyces sviceus GNAT acetyltransferase with similarity to the C-terminal domain of the human GH84 O-GlcNAcase. Acta Crystallogr. D Biol. Crystallogr. 2014;70:186–195. doi: 10.1107/S1399004713029155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chalker J.M., Gunnoo S.B., Boutureira O., Gerstberger S.C., Fernandez-Gonzalez M., Bernardes G.J.L., Griffin L., Hailu H., Schofield C.J., Davis B.G. Methods for converting cysteine to dehydroalanine on peptides and proteins. Chem. Sci. 2011;2:1666–1676. doi: 10.1039/c1sc00185j. [DOI] [Google Scholar]
- 127.Fernández-González M., Boutureira O., Bernardes G.J.L., Chalker J.M., Young M.A., Errey J.C., Davis B.G. Site-selective chemoenzymatic construction of synthetic glycoproteins using endoglycosidases. Chem. Sci. 2010;1:709–715. doi: 10.1039/c0sc00265h. [DOI] [Google Scholar]
- 128.Lercher L., Raj R., Patel N.A., Price J., Mohammed S., Robinson C.V., Schofield C.J., Davis B.G. Generation of a synthetic GlcNAcylated nucleosome reveals regulation of stability by H2A-Thr101 GlcNAcylation. Nat. Commun. 2015;6:7978. doi: 10.1038/ncomms8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Raj R., Lercher L., Mohammed S., Davis B.G. Synthetic Nucleosomes Reveal that GlcNAcylation Modulates Direct Interaction with the FACT Complex. Angew. Chem. Int. Ed. Engl. 2016;55:8918–8922. doi: 10.1002/anie.201603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fujiki R., Hashiba W., Sekine H., Yokoyama A., Chikanishi T., Ito S., Imai Y., Kim J., He H.H., Igarashi K., et al. GlcNAcylation of histone H2B facilitates its monoubiquitination. Nature. 2011;480:557–560. doi: 10.1038/nature10656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen Q., Chen Y., Bian C., Fujiki R., Yu X. TET2 promotes histone O-GlcNAcylation during gene transcription. Nature. 2013;493:561–564. doi: 10.1038/nature11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Deplus R., Delatte B., Schwinn M.K., Defrance M., Méndez J., Murphy N., Dawson M.A., Volkmar M., Putmans P., Calonne E., et al. TET2 and TET3 regulate GlcNAcylation and H3K4 methylation through OGT and SET1/COMPASS. EMBO J. 2013;32:645–655. doi: 10.1038/emboj.2012.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vella P., Scelfo A., Jammula S., Chiacchiera F., Williams K., Cuomo A., Roberto A., Christensen J., Bonaldi T., Helin K., et al. Tet proteins connect the O-linked N-acetylglucosamine transferase Ogt to chromatin in embryonic stem cells. Mol. Cell. 2013;49:645–656. doi: 10.1016/j.molcel.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 134.Lee J.S., Zhang Z. O-linked N-acetylglucosamine transferase (OGT) interacts with the histone chaperone HIRA complex and regulates nucleosome assembly and cellular senescence. Proc. Natl. Acad. Sci. USA. 2016;113:E3213–E3220. doi: 10.1073/pnas.1600509113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Smith J.A., Stocken L.A. Identification of poly (ADP-ribose) covalently bound to histone F1 in vivo. Biochem. Biophys. Res. Commun. 1973;54:297–300. doi: 10.1016/0006-291X(73)90922-4. [DOI] [PubMed] [Google Scholar]
- 136.Glowacki G., Braren R., Firner K., Nissen M., Kühl M., Reche P., Bazan F., Cetkovic-Cvrlje M., Leiter E., Haag F., et al. The family of toxin-related ecto-ADP-ribosyltransferases in humans and the mouse. Protein Sci. 2002;11:1657–1670. doi: 10.1110/ps.0200602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Messner S., Altmeyer M., Zhao H., Pozivil A., Roschitzki B., Gehrig P., Rutishauser D., Huang D., Caflisch A., Hottiger M.O. PARP1 ADP-ribosylates lysine residues of the core histone tails. Nucleic Acids Res. 2010;38:6350–6362. doi: 10.1093/nar/gkq463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rulten S.L., Fisher A.E., Robert I., Zuma M.C., Rouleau M., Ju L., Poirier G., Reina-San-Martin B., Caldecott K.W. PARP-3 and APLF function together to accelerate nonhomologous end-joining. Mol. Cell. 2011;41:33–45. doi: 10.1016/j.molcel.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 139.Kleine H., Poreba E., Lesniewicz K., Hassa P.O., Hottiger M.O., Litchfield D.W., Shilton B.H., Lüscher B. Substrate-assisted catalysis by PARP10 limits its activity to mono-ADP-ribosylation. Mol. Cell. 2008;32:57–69. doi: 10.1016/j.molcel.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 140.Liszt G., Ford E., Kurtev M., Guarente L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J. Biol. Chem. 2005;280:21313–21320. doi: 10.1074/jbc.M413296200. [DOI] [PubMed] [Google Scholar]
- 141.Karch K.R., Langelier M.F., Pascal J.M., Garcia B.A. The nucleosomal surface is the main target of histone ADP-ribosylation in response to DNA damage. Mol. Biosyst. 2017;13:2660–2671. doi: 10.1039/C7MB00498B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Boulikas T. Poly(ADP-ribosylated) histones in chromatin replication. J. Biol. Chem. 1990;265:14638–14647. doi: 10.1016/S0021-9258(18)77350-X. [DOI] [PubMed] [Google Scholar]
- 143.Burzio L.O., Riquelme P.T., Koide S.S. ADP ribosylation of rat liver nucleosomal core histones. J. Biol. Chem. 1979;254:3029–3037. doi: 10.1016/S0021-9258(17)30178-3. [DOI] [PubMed] [Google Scholar]
- 144.Huletsy A., de Murcia G., Muller S., Hengartner M., Ménard L., Lamarre D., Poirier G.G. The effect of poly(ADP-ribosyl)ation on native and H1-depleted chromatin. A role of poly(ADP-ribosyl)ation on core nucleosome structure. J. Biol. Chem. 1989;264:8878–8886. doi: 10.1016/S0021-9258(18)81875-0. [DOI] [PubMed] [Google Scholar]
- 145.Stone P., Lorimer W., Kidwell W. Properties of the complex between histone H1 and poly(ADP-ribose synthesised in HeLa cell nuclei. Eur. J. Biochem. 1977;81:9–18. doi: 10.1111/j.1432-1033.1977.tb11921.x. [DOI] [PubMed] [Google Scholar]
- 146.Boulikas T. DNA strand breaks alter histone ADP-ribosylation. Proc. Natl. Acad. Sci. USA. 1989;86:3499–3503. doi: 10.1073/pnas.86.10.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Poirier G.G., de Murcia G., Jongstra-Bilen J., Niedergang C., Mandel P. Poly(ADP-ribosyl)ation of polynucleosomes causes relaxation of chromatin structure. Proc. Natl. Acad. Sci. USA. 1982;79:3423–3427. doi: 10.1073/pnas.79.11.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.De Murcia G., Huletsky A., Lamarre D., Gaudreau A., Pouyet J., Daune M., Poirier G.G. Modulation of chromatin superstructure induced by poly(ADP-ribose) synthesis and degradation. J. Biol. Chem. 1986;261:7011–7017. doi: 10.1016/S0021-9258(19)62715-8. [DOI] [PubMed] [Google Scholar]
- 149.Petesch S.J., Lis J.T. Activator-induced spread of poly(ADP-ribose) polymerase promotes nucleosome loss at Hsp70. Mol. Cell. 2012;45:64–74. doi: 10.1016/j.molcel.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lehmann M., Pirinen E., Mirsaidi A., Kunze F.A., Richards P.J., Auwerx J., Hottiger M.O. ARTD1-induced poly-ADP-ribose formation enhances PPARγ ligand binding and co-factor exchange. Nucleic Acids Res. 2015;43:129–142. doi: 10.1093/nar/gku1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hymes J., Fleischhauer K., Wolf B. Biotinylation of histones by human serum biotinidase: Assessment of biotinyl-transferase activity in sera from normal individuals and children with biotinidase deficiency. Biochem. Mol. Med. 1995;56:76–83. doi: 10.1006/bmme.1995.1059. [DOI] [PubMed] [Google Scholar]
- 152.Kothapalli N., Camporeale G., Kueh A., Chew Y.C., Oommen A.M., Griffin J.B., Zempleni J. Biological functions of biotinylated histones. J. Nutr. Biochem. 2005;16:446–448. doi: 10.1016/j.jnutbio.2005.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Brenner C. Catalysis in the nitrilase superfamily. Curr. Opin. Struct. Biol. 2002;12:775–782. doi: 10.1016/S0959-440X(02)00387-1. [DOI] [PubMed] [Google Scholar]
- 154.Narang M.A., Dumas R., Ayer L.M., Gravel R.A. Reduced histone biotinylation in multiple carboxylase deficiency patients: A nuclear role for holocarboxylase synthetase. Hum. Mol. Genet. 2004;13:15–23. doi: 10.1093/hmg/ddh006. [DOI] [PubMed] [Google Scholar]
- 155.Bao B., Pestinger V., Hassan Y.I., Borgstahl G.E., Kolar C., Zempleni J. Holocarboxylase synthetase is a chromatin protein and interacts directly with histone H3 to mediate biotinylation of K9 and K18. J. Nutr. Biochem. 2011;22:470–475. doi: 10.1016/j.jnutbio.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ballard T.D., Wolff J., Griffin J.B., Stanley J.S., van Calcar S., Zempleni J. Biotinidase catalyzes debiotinylation of histones. Eur. J. Nutr. 2002;41:78–84. doi: 10.1007/s003940200011. [DOI] [PubMed] [Google Scholar]
- 157.Bailey L.M., Ivanov R.A., Wallace J.C., Polyak S.W. Artifactual detection of biotin on histones by streptavidin. Anal. Biochem. 2008;373:71–77. doi: 10.1016/j.ab.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 158.Kuroishi T., Rios-Avila L., Pestinger V., Wijeratne S.S., Zempleni J. Biotinylation is a natural, albeit rare, modification of human histones. Mol. Genet. Metab. 2011;104:537–545. doi: 10.1016/j.ymgme.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stanley J.S., Griffin J.B., Zempleni J. Biotinylation of histones in human cells: Effects of cell proliferation. Eur. J. Biochem. 2001;268:5424–5429. doi: 10.1046/j.0014-2956.2001.02481.x. [DOI] [PubMed] [Google Scholar]
- 160.Smith E.M., Hoi J.T., Eissenberg J.C., Shoemaker J.D., Neckameyer W.S., Ilvarsonn A.M., Harshman L.G., Schlegel V.L., Zempleni J. Feeding Drosophila a biotin-deficient diet for multiple generations increases stress resistance and lifespan and alters gene expression and histone biotinylation patterns. J. Nutr. 2007;137:2006–2012. doi: 10.1093/jn/137.9.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pestinger V., Wijeratne S.S., Rodriguez-Melendez R., Zempleni J. Novel histone biotinylation marks are enriched in repeat regions and participate in repression of transcriptionally competent genes. J. Nutr. Biochem. 2011;22:328–333. doi: 10.1016/j.jnutbio.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chew Y.C., West J.T., Kratzer S.J., Ilvarsonn A.M., Eissenberg J.C., Dave B.J., Klinkebiel D., Christman J.K., Zempleni J. Biotinylation of histones represses transposable elements in human and mouse cells and cell lines and in Drosophila melanogaster. J. Nutr. 2008;138:2316–2322. doi: 10.3945/jn.108.098673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Camporeale G., Oommen A.M., Griffin J.B., Sarath G., Zempleni J. K12-biotinylated histone H4 marks heterochromatin in human lymphoblastoma cells. J. Nutr. Biochem. 2007;18:760–768. doi: 10.1016/j.jnutbio.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wijeratne S.S., Camporeale G., Zempleni J. K12-biotinylated histone H4 is enriched in telomeric repeats from human lung IMR-90 fibroblasts. J. Nutr. Biochem. 2010;21:310–316. doi: 10.1016/j.jnutbio.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li Y., Hassan Y.I., Moriyama H., Zempleni J. Holocarboxylase synthetase interacts physically with euchromatic histone-lysine N-methyltransferase, linking histone biotinylation with methylation events. J. Nutr. Biochem. 2013;24:1446–1452. doi: 10.1016/j.jnutbio.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Filenko N.A., Kolar C., West J.T. Smith SA, Hassan YI, Borgstahl GE, Zempleni, J., Lyubchenko YL. The role of histone H4 biotinylation in the structure of nucleosomes. PLoS ONE. 2011;6:e16299. doi: 10.1371/journal.pone.0016299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Singh M.P., Wijeratne S.S., Zempleni J. Biotinylation of lysine 16 in histone H4 contributes toward nucleosome condensation. Arch. Biochem. Biophys. 2013;529:105–111. doi: 10.1016/j.abb.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Farrelly L.A., Thompson R.E., Zhao S., Lepack A.E., Lyu Y., Bhanu N.V., Zhang B., Loh Y.E., Ramakrishnan A., Vadodaria K.C., et al. Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3. Nature. 2019;567:535–539. doi: 10.1038/s41586-019-1024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lepack A.E., Werner C.T., Stewart A.F., Fulton S.L., Zhong P., Farrelly L.A., Smith A.C.W., Ramakrishnan A., Lyu Y., Bastle R.M., et al. Dopaminylation of histone H3 in ventral tegmental area regulates cocaine seeking. Science. 2020;368:197–201. doi: 10.1126/science.aaw8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lindner H., Sarg B., Hoertnagl B., Helliger W. The microheterogeneity of the mammalian H1(0) histone. Evidence for an age-dependent deamidation. J. Biol. Chem. 1998;273:13324–13330. doi: 10.1074/jbc.273.21.13324. [DOI] [PubMed] [Google Scholar]
- 171.Lorand L., Graham R.M. Transglutaminases: Crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- 172.Vermeulen M., Mulder K.W., Denissov S., Pijnappel W.W., van Schaik F.M., Varier R.A., Baltissen M.P., Stunnenberg H.G., Mann M., Timmers H.T. Selective anchoring of TFIID to nucleosomes by trimethylation of histone H3 lysine 4. Cell. 2007;131:58–69. doi: 10.1016/j.cell.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 173.Lauberth S.M., Nakayama T., Wu X., Ferris A.L., Tang Z., Hughes S.H., Roeder R.G. H3K4me3 interactions with TAF3 regulate preinitiation complex assembly and selective gene activation. Cell. 2013;152:1021–1036. doi: 10.1016/j.cell.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhao J., Chen W., Pan Y., Zhang Y., Sun H., Wang H., Yang F., Liu Y., Shen N., Zhang X., et al. Structural insights into the recognition of histone H3Q5 serotonylation by WDR5. Sci. Adv. 2021;7:eabf4291. doi: 10.1126/sciadv.abf4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wysocka J., Swigut T., Milne T.A., Dou Y., Zhang X., Burlingame A.L., Roeder R.G., Brivanlou A.H., Allis C.D. WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development. Cell. 2005;121:859–872. doi: 10.1016/j.cell.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 176.Wedemeyer W.J., Welker E., Scheraga H.A. Proline cis-trans isomerization and protein folding. Biochemistry. 2002;41:14637–14644. doi: 10.1021/bi020574b. [DOI] [PubMed] [Google Scholar]
- 177.Göthel S.F., Marahiel M.A. Peptidyl-prolyl cis-trans isomerases, a superfamily of ubiquitous folding catalysts. Cell Mol. Life Sci. 1999;55:423–436. doi: 10.1007/s000180050299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nelson C.J., Santos-Rosa H., Kouzarides T. Proline isomerization of histone H3 regulates lysine methylation and gene expression. Cell. 2006;126:905–916. doi: 10.1016/j.cell.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 179.Yang W.M., Yao Y.L., Seto E. The FK506-binding protein 25 functionally associates with histone deacetylases and with transcription factor YY1. EMBO J. 2001;20:4814–4825. doi: 10.1093/emboj/20.17.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Howe F.S., Boubriak I., Sale M.J., Nair A., Clynes D., Grijzenhout A., Murray S.C., Woloszczuk R., Mellor J. Lysine acetylation controls local protein conformation by influencing proline isomerization. Mol. Cell. 2014;55:733–744. doi: 10.1016/j.molcel.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tan H.L., Lim K.K., Yang Q., Fan J.S., Sayed A.M.M., Low L.S., Ren B., Lim T.K., Lin Q., Mok Y.K., et al. Prolyl isomerization of the CENP-A N-terminus regulates centromeric integrity in fission yeast. Nucleic Acids Res. 2018;46:1167–1179. doi: 10.1093/nar/gkx1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Furuchi T., Sakurako K., Katane M., Sekine M., Homma H. The role of protein L-isoaspartyl/D-aspartyl O-methyltransferase (PIMT) in intracellular signal transduction. Chem. Biodivers. 2010;7:1337–1348. doi: 10.1002/cbdv.200900273. [DOI] [PubMed] [Google Scholar]
- 183.Young A.L., Carter W.G., Doyle H.A., Mamula M.J., Aswad D.W. Structural integrity of histone H2B in vivo requires the activity of protein L-isoaspartate O-methyltransferase a putative protein repair enzyme. J. Biol. Chem. 2001;276:37161–37165. doi: 10.1074/jbc.M106682200. [DOI] [PubMed] [Google Scholar]
- 184.Young G.W., Hoofring S.A., Mamula M.J., Doyle H.A., Bunick G.J., Hu Y., Aswad D.W. Protein L-isoaspartyl methyltransferase catalyzes in vivo racemization of Aspartate-25 in mammalian histone H2B. J. Biol. Chem. 2005;280:26094–26098. doi: 10.1074/jbc.M503624200. [DOI] [PubMed] [Google Scholar]
- 185.Qin Z., Zhu J.X., Aswad D.W. The D-isoAsp-25 variant of histone H2B is highly enriched in active chromatin: Potential role in the regulation of gene expression? Amino Acids. 2016;48:599–603. doi: 10.1007/s00726-015-2140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Maillard L.C. Action des acidesamines sur les sucres: Formation des melanoidines par voie methodique. CR Acad. Sci. Paris. 1912;154:66–68. [Google Scholar]
- 187.Ansari N.A., Chaudhary D.K., Dash D. Modification of histone by glyoxal: Recognition of glycated histone containing advanced glycation adducts by serum antibodies of type 1 diabetes patients. Glycobiology. 2018;28:207–213. doi: 10.1093/glycob/cwy006. [DOI] [PubMed] [Google Scholar]
- 188.Hellwig M., Henle T. Baking, ageing, diabetes: A short history of the Maillard reaction. Angew. Chem. Int. Ed. Engl. 2014;53:10316–10329. doi: 10.1002/anie.201308808. [DOI] [PubMed] [Google Scholar]
- 189.Galligan J.J., Wepy J.A., Streeter M.D., Kingsley P.J., Mitchener M.M., Wauchope O.R., Beavers W.N., Rose K.L., Wang T., Spiegel D.A., et al. Methylglyoxal-derived posttranslational arginine modifications are abundant histone marks. Proc. Natl. Acad. Sci. USA. 2018;115:9228–9233. doi: 10.1073/pnas.1802901115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zheng Q., Omans N.D., Leicher R., Osunsade A., Agustinus A.S., Finkin-Groner E., D’Ambrosio H., Liu B., Chandarlapaty S., Liu S., et al. Reversible histone glycation is associated with disease-related changes in chromatin architecture. Nat. Commun. 2019;10:1289. doi: 10.1038/s41467-019-09192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ashraf J.M., Rabbani G., Ahmad S., Hasan Q., Khan R.H., Alam K., Choi I. Glycation ofH1 histone by 3-deoxyglucosone: Effects on protein structure and generation of different advanced glycation end products. PLoS ONE. 2015;10:e0130630. doi: 10.1371/journal.pone.0130630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rahmanpour R., Bathaie S.Z. Histone H1 structural changes and its interaction with DNA in the presence of high glucose concentration in vivo and in vitro. J. Biomol. Struct. Dyn. 2011;28:575–586. doi: 10.1080/07391102.2011.10508596. [DOI] [PubMed] [Google Scholar]
- 193.Zheng Q., Osunsade A., David Y. Protein arginine deiminase 4 antagonizes methylglyoxal-induced histone glycation. Nat. Commun. 2020;11:3241. doi: 10.1038/s41467-020-17066-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Uchida K. 4-Hydroxy-2-nonenal: A product and mediator of oxidative stress. Prog. Lipid Res. 2003;42:318–343. doi: 10.1016/S0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 195.Lee S.H., Blair I.A. Characterization of 4-oxo-2-nonenal as a novel product of lipid peroxidation. Chem. Res. Toxicol. 2000;13:698–702. doi: 10.1021/tx000101a. [DOI] [PubMed] [Google Scholar]
- 196.Galligan J.J., Rose K.L., Beavers W.N., Hill S., Tallman K.A., Tansey W.P., Marnett L.J. Stable histone adduction by 4-oxo-2-nonenal: A potential link between oxidative stress and epigenetics. J. Am. Chem. Soc. 2014;136:11864–11866. doi: 10.1021/ja503604t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Geib T., Iacob C., Jribi R., Fernandes J., Benderdour M., Sleno L. Identification of 4-hydroxynonenal-modified proteins in human osteoarthritic chondrocytes. J. Proteom. 2021;232:104024. doi: 10.1016/j.jprot.2020.104024. [DOI] [PubMed] [Google Scholar]
- 198.Drake J., Petroze R., Castegna A., Ding Q., Keller J.N., Markesbery W.R., Lovell M.A., Butterfield D.A. 4-Hydroxynonenal oxidatively modifies histones: Implications for Alzheimer’s disease. Neurosci. Lett. 2004;356:155–158. doi: 10.1016/j.neulet.2003.11.047. [DOI] [PubMed] [Google Scholar]
- 199.Pellicanò M., Picone P., Cavalieri V., Carrotta R., Spinelli G., Di Carlo M. The sea urchin embryo: A model to study Alzheimer’s beta amyloid induced toxicity. Arch. Biochem. Biophys. 2009;483:120–126. doi: 10.1016/j.abb.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 200.Jiang T., Zhou X., Taghizadeh K., Dong M., Dedon P.C. N-formylation of lysine in histone proteins as a secondary modification arising from oxidative DNA damage. Proc. Natl. Acad. Sci. USA. 2007;104:60–65. doi: 10.1073/pnas.0606775103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Walport L.J., Hopkinson R.J., Schofield C.J. Mechanisms of human histone and nucleic acid demethylases. Curr. Opin. Chem. Biol. 2012;16:525–534. doi: 10.1016/j.cbpa.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 202.Edrissi B., Taghizadeh K., Dedon P.C. Quantitative analysis of histone modifications: Formaldehyde is a source of pathological n(6)-formyllysine that is refractory to histone deacetylases. PLoS Genet. 2013;9:e1003328. doi: 10.1371/journal.pgen.1003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wang T., Zhou Q., Li F., Yu Y., Yin X., Wang J. Genetic Incorporation of N(ε)-Formyllysine, a New Histone Post-translational Modification. Chembiochem. 2015;16:1440–1442. doi: 10.1002/cbic.201500170. [DOI] [PubMed] [Google Scholar]
- 204.Wisniewski J.R., Zougman A., Mann M. Nepsilon-formylation of lysine is a widespread post-translational modification of nuclear proteins occurring at residues involved in regulation of chromatin function. Nucleic Acids Res. 2008;36:570–577. doi: 10.1093/nar/gkm1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Eickbush T.H., Watson D.K., Moudrianakis E.N. A chromatin-bound proteolytic activity with unique specificity for histone H2A. Cell. 1976;9:785–792. doi: 10.1016/0092-8674(76)90141-0. [DOI] [PubMed] [Google Scholar]
- 206.Allis C.D., Bowen J.K., Abraham G.N., Glover C.V., Gorovsky M.A. Proteolytic processing of histone H3 in chromatin: A physiologically regulated event in Tetrahymena micronuclei. Cell. 1980;20:55–64. doi: 10.1016/0092-8674(80)90234-2. [DOI] [PubMed] [Google Scholar]
- 207.Azad G.K., Swagatika S., Kumawat M., Kumawat R., Tomar R.S. Modifying chromatin by histone tail clipping. J. Mol. Biol. 2018;430:3051–3067. doi: 10.1016/j.jmb.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 208.Santos-Rosa H., Kirmizis A., Nelson C., Bartke T., Saksouk N., Cote J., Kouzarides T. Histone H3 tail clipping regulates gene expression. Nat. Struct. Mol. Biol. 2009;16:17–22. doi: 10.1038/nsmb.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Allis C.D., Wiggins J.C. Proteolytic processing of micronuclear H3 and histone phosphorylation during conjugation in Tetrahymena thermophila. Exp. Cell Res. 1984;153:287–298. doi: 10.1016/0014-4827(84)90601-3. [DOI] [PubMed] [Google Scholar]
- 210.Herrera-Solorio A.M., Vembar S.S., MacPherson C.R., Lozano-Amado D., Meza G.R., Xoconostle-Cazares B., Martins R.M., Chen P., Vargas M., Scherf A., et al. Clipped histone H3 is integrated into nucleosomes of DNA replication genes in the human malaria parasite Plasmodium falciparum. EMBO Rep. 2019;20:e46331. doi: 10.15252/embr.201846331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Duncan E.M., Muratore-Schroeder T.L., Cook R.G., Garcia B.A., Shabanowitz J., Hunt D.F., Allis C.D. Cathepsin L proteolytically processes histone H3 during mouse embryonic stem cell differentiation. Cell. 2008;135:284–294. doi: 10.1016/j.cell.2008.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kim K., Punj V., Kim J.M., Lee S., Ulmer T.S., Lu W., Rice J.C., An W. MMP-9 facilitates selective proteolysis of the histone H3 tail at genes necessary for proficient osteoclastogenesis. Genes Dev. 2016;30:208–219. doi: 10.1101/gad.268714.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Duarte L.F., Young A.R., Wang Z., Wu H.A., Panda T., Kou Y., Kapoor A., Hasson D., Mills N.R., Ma’ayan A., et al. Histone H3.3 and its proteolytically processed form drive a cellular senescence programme. Nat. Commun. 2014;5:5210. doi: 10.1038/ncomms6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cavalieri V., Bernardo M.D., Spinelli G. Regulatory sequences driving expression of the sea urchin Otp homeobox gene in oral ectoderm cells. Gene Expr. Patterns. 2007;7:124–130. doi: 10.1016/j.modgep.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 215.Biswas M., Voltz K., Smith J.C., Langowski J. Role of histone tails in structural stability of the nucleosome. PLoS Comput. Biol. 2011;7:e1002279. doi: 10.1371/journal.pcbi.1002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Guyon J.R., Narlikar G.J., Sif S., Kingston R.E. Kingston, Stable remodeling of tailless nucleosomes by the human SWI– SNF complex. Mol. Cell Biol. 1999;19:2088–2097. doi: 10.1128/MCB.19.3.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Vogler C., Huber C., Waldmann T., Ettig R., Braun L., Izzo A., Daujat S., Chassignet I., Lopez-Contreras A.J., Fernandez-Capetillo O., et al. Histone H2A C-terminus regulates chromatin dynamics, remodeling, and histone H1 binding. PLoS Genet. 2010;6:e1001234. doi: 10.1371/journal.pgen.1001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Iwasaki W., Miya Y., Horikoshi N., Osakabe A., Taguchi H., Tachiwana H., Shibata T., Kagawa W., Kurumizaka H. Contribution of histone N-terminal tails to the structure and stability of nucleosomes. FEBS Open Bio. 2013;3:363–369. doi: 10.1016/j.fob.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Liu H., Wang C., Lee S., Deng Y., Wither M., Oh S., Ning F., Dege C., Zhang Q., Liu X., et al. Clipping of arginine-methylated histone tails by JMJD5 and JMJD7. Proc. Natl. Acad. Sci. USA. 2017;114:E7717–E7726. doi: 10.1073/pnas.1706831114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Liu H., Wang C., Lee S., Ning F., Wang Y., Zhang Q., Chen Z., Zang J., Nix J., Dai S., et al. Specific Recognition of Arginine Methylated Histone Tails by JMJD5 and JMJD7. Sci. Rep. 2018;8:3275. doi: 10.1038/s41598-018-21432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Studitsky V.M., Kassavetis G.A., Geiduschek E.P., Felsenfeld G. Mechanism of transcription through the nucleosome by eukaryotic RNA polymerase. Science. 1997;278:1960–1963. doi: 10.1126/science.278.5345.1960. [DOI] [PubMed] [Google Scholar]
- 222.Bal W., Lukszo J., Bialkowski K., Kasprzak K.S. Interactions of Nickel(II) with histones: Interactions of Nickel(II) with CH3CO-Thr-Glu-Ser-His-His-Lys-NH2, a peptide modeling the potential metal binding site in the "C-Tail" region of histone H2A. Chem. Res. Toxicol. 1998;11:1014–1023. doi: 10.1021/tx980051y. [DOI] [PubMed] [Google Scholar]
- 223.Bal W., Liang R., Lukszo J., Lee S.H., Dizdaroglu M., Kasprzak K.S. Ni(II) specifically cleaves the C-terminal tail of the major variant of histone H2A and forms an oxidative damage-mediating complex with the cleaved-off octapeptide. Chem. Res. Toxicol. 2000;13:616–624. doi: 10.1021/tx000044l. [DOI] [PubMed] [Google Scholar]
- 224.Karaczyn A.A., Bal W., North S.L., Bare R.M., Hoang V.M., Fisher R.J., Kasprzak K.S. The octapeptidic end of the C-terminal tail of histone H2A is cleaved off in cells exposed to carcinogenic nickel(II) Chem. Res. Toxicol. 2003;16:1555–1559. doi: 10.1021/tx0300277. [DOI] [PubMed] [Google Scholar]
- 225.Usachenko S.I., Bavykin S.G., Gavin I.M., Bradbury E.M. Rearrangement of the histone H2A C-terminal domain in the nucleosome. Proc. Natl. Acad. Sci. USA. 1994;91:6845–6849. doi: 10.1073/pnas.91.15.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Karaczyn A.A., Cheng R.Y., Buzard G.S., Hartley J., Esposito D., Kasprzak K.S. Truncation of histone H2A’s C-terminal tail, as is typical for Ni(II)-assisted specific peptide bond hydrolysis, has gene expression altering effects. Ann. Clin. Lab. Sci. 2009;39:251–262. [PMC free article] [PubMed] [Google Scholar]
- 227.Lindner H., Sarg B., Grunicke H., Helliger W. Age-dependent deamidation of H1(0) histones in chromatin of mammalian tissues. J. Cancer Res. Clin. Oncol. 1999;125:182–186. doi: 10.1007/s004320050261. [DOI] [PubMed] [Google Scholar]
- 228.Wondrak G.T., Cervantes-Laurean D., Jacobson E.L., Jacobson M.K. Histone carbonylation in vivo and in vitro. Biochem. J. 2000;351:769–777. doi: 10.1042/bj3510769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Dixit K., Khan M.A., Sharma Y.D., Moinuddin, Alam K. Physicochemical studies on peroxynitrite-modified H3 histone. Int. J. Biol. Macromol. 2010;46:20–26. doi: 10.1016/j.ijbiomac.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 230.Unoki M., Masuda A., Dohmae N., Arita K., Yoshimatsu M., Iwai Y., Fukui Y., Ueda K., Hamamoto R., Shirakawa M., et al. Lysyl 5-hydroxylation, a novel histone modification, by Jumonji domain containing 6 (JMJD6) J. Biol. Chem. 2013;288:6053–6062. doi: 10.1074/jbc.M112.433284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Paulsen C.E., Carroll K.S. Cysteine-mediated redox signaling: Chemistry, biology, and tools for discovery. Chem. Rev. 2013;113:4633–4679. doi: 10.1021/cr300163e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.De Luca A., Moroni N., Serafino A., Primavera A., Pastore A., Pedersen J.Z., Petruzzelli R., Farrace M.G., Pierimarchi P., Moroni G., et al. Treatment of doxorubicin-resistant MCF7/Dx cells with nitric oxide causes histone glutathionylation and reversal of drug resistance. Biochem. J. 2011;440:175–183. doi: 10.1042/BJ20111333. [DOI] [PubMed] [Google Scholar]
- 233.Tessarz P., Santos-Rosa H., Robson S.C., Sylvestersen K.B., Nelson C.J., Nielsen M.L., Kouzarides T. Glutamine methylation in histone H2A is an RNA-polymerase-I-dedicated modification. Nature. 2014;505:564–568. doi: 10.1038/nature12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Cebrià-Costa J.P., Pascual-Reguant L., Gonzalez-Perez A., Serra-Bardenys G., Querol J., Cosín M., Verde G., Cigliano R.A., Sanseverino W., Segura-Bayona S., et al. LOXL2-mediated H3K4 oxidation reduces chromatin accessibility in triple-negative breast cancer cells. Oncogene. 2020;39:79–121. doi: 10.1038/s41388-019-0969-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Strahl B.D., Allis C.D. The language of covalent histone modifications. Nature. 2000;403:41–44. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 236.Henikoff S., Shilatifard A. Histone modification: Cause or cog? Trends Genet. 2011;27:389–396. doi: 10.1016/j.tig.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 237.Ernst J., Kellis M. Discovery and characterization of chromatin states for systematic annotation of the human genome. Nat. Biotechnol. 2010;28:817–825. doi: 10.1038/nbt.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Voigt P., LeRoy G., Drury W.J., 3rd, Zee B.M., Son J., Beck D.B., Young N.L., Garcia B.A., Reinberg D. Asymmetrically modified nucleosomes. Cell. 2012;151:181–193. doi: 10.1016/j.cell.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Voigt P., Tee W.W., Reinberg D. A double take on bivalent promoters. Genes Dev. 2013;27:1318–1338. doi: 10.1101/gad.219626.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Dion M., Kaplan T., Friedman N., Rando O.J. Dynamics of replication-independent histone turnover in budding yeast. Science. 2007;315:1405–1408. doi: 10.1126/science.1134053. [DOI] [PubMed] [Google Scholar]