Abstract
Fgf8 is expressed transiently during embryogenesis at the midbrain-hindbrain border, an area that gives rise to a variety of neuronal populations including the dorsal raphe nucleus (DR). Using an inducible Fgf8-cre allele we identified the populations of neurons defined by Fgf8 lineage at different stages of development. When Fgf8-cre expression is induced at embryonic day 7.5 (T-E7.5), in the adult the entire DR and part of the median raphe (MnR) has Fgf8 lineage. When induced at later timepoints, Fgf8 lineage progressively ebbs from the caudal and ventral aspect of this domain, particularly on the midline. Successively excluded from Fgf8- lineage at T-E9.5 are serotonin neurons in the MnR and caudal-intrafascicular DR, followed at T-E11.5 by ventral-middle and caudal-dorsal DR. The last to show Fgf8 lineage are those serotonin neurons in the lateral wings and those at the rostral-dorsal pole of dorsal raphe nucleus. Thus the temporal succession of Fgf8 lineage correlates with organizational features of serotonin neurons in these nuclei.
Keywords: Serotonin, development, Pax5, isthmus, dopamine, acetylcholine, hindbrain
Graphical Abstract
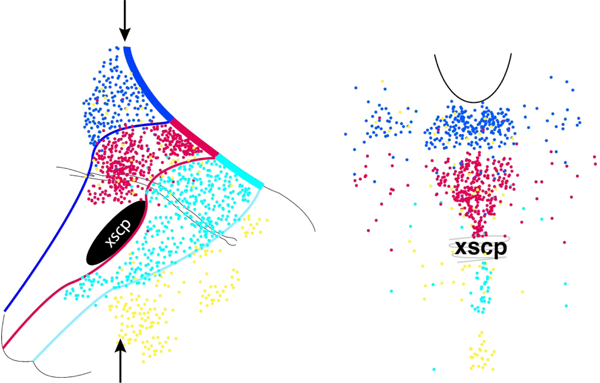
Serotonin neurons (dots) depicted in sagittal section (left) and coronal section (right), color-coded by Fgf8-lineage captured at different time-points in development. The arrows indicate the approximate level of the coronal section. Serotonin neurons arising from progenitors expressing Fgf8 at later ages in development progressively populate more rostral and dorsal locations in the dorsal raphe, with serotonin neurons arising from the latest Fgf8 expressers also located in the lateral wings.
Introduction
The dorsal raphe nucleus (DR) contains the largest cohort of neurons providing serotonin innervation to the forebrain. The DR and other ascending serotonin nuclei are located in the most rostral part of the hindbrain, the prepontine hindbrain. However, they are often referred to archaically and inaccurately as the ‘midbrain raphe nuclei’. That is, the definition of midbrain, hindbrain and pons has changed since the initial description of serotonin neurons with the advent of a molecular understanding of development (Watson et al., 2019). Currently the midbrain-hindbrain border is defined as the developmental meeting point between the expression of Otx2 in the midbrain and Gbx2 in the hindbrain. The dorsal and median raphe, as well as B9 serotonin neurons all arise caudal to this border.
Within the hindbrain, development is organized in transverse neuromeric units named rhombomeres (Nieuwenhuys, 2011; Watson et al., 2019). The first rhombomere (r1), as defined by En1 lineage, gives rise to a particularly large and complex area (Aroca and Puelles, 2005). During development a rostral part of r1 has unique characteristics. The most rostral portion of the Gbx2 expression area abutting Otx2 expressing cells is transiently a morphologically distinct constriction of the neural tube called the isthmus (Wassarman et al., 1997; Joyner et al., 2000). The cells of the isthmus secret fibroblast growth factor-8 (Fgf8), which serves as a key signaling molecule directing the development of both the neighboring midbrain and hindbrain and thus this functionally important area is known as the isthmus organizer (Crossley et al., 1996; Lee et al., 1997; Partanen, 2007). Given these features during development combined with observations on the adult anatomy, it has been argued that it may be useful to view the isthmus and coinciding Fgf8 expression as defining a distinct rhombomeric unit, namely r0. As a consequence, the En1 lineage domain would be considered to be encompassing both r0 and the remaining part of r1, sometimes called r1 proper.
The difficulty in studying r0 is that the definition of its’ caudal limit is particularly vague because Fgf8 expression is not static during development. Fgf8 expression is initially broad and with developmental time it is progressively refined to a smaller and smaller domain at the rostral cusp of the Gbx2-expressing hindbrain, only transiently defining the proposed r0 (Hidalgo-Sanchez et al., 2005). Both the spatial and temporal patterns of Fgf8 signaling seem essential for normal development (Sato and Joyner, 2009), and without Fgf8 signaling serotonin neurons in the rostral hindbrain do not develop (Ye et al., 1998). Thus while the conceptual construct of r0 may be useful, to some extent it applies a quantal model over a dynamic process.
All of the serotonin neurons of the DR arise from the r0/r1 domain (Jensen et al., 2008). The DR has a rather complicated cytoarchitecture that can be divided into multiple subregions. Likewise transcriptional analysis of DR serotonin neurons can define many subtypes of neurons that seem to correlate roughly with cytoarchitecture (Huang et al., 2019; Ren et al., 2019; Okaty et al., 2020a). How these neurons arise developmentally remains poorly understood. All DR neurons have Fgf8 lineage as determined using a mouse with constitutive Fgf8-Cre-LacZ expression (Watson et al., 2017). Yet because Fgf8 expression is dynamic, it is likely that different subsets of serotonin neurons within the DR are associated with early vs. late Fgf8 expression.
METHODS
All animal procedures were reviewed and approved by the Institutional Care and Use Committee at Boston Children’s Hospital, in agreement with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. We used Fgf8CreER heterozygous knock-in mice (CD1;B6-Fgf8tm1(cre/ert2)Jlr/Mmucd;) developed and characterized previously (Hoch et al., 2015) obtained from MMRRC Repository at the University of California at Davis (Cat# 037634-UCD, RRID:MMRRC_037634-UCD). These mice were bred with mice carrying a cre-dependent tdTomato (TDT) reporter, Ai14, B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J Jackson Laboratory (Cat# JAX:007914, RRID:IMSR_JAX:007914) to produce Fgf8-CreER/TD-tomato positive mice. Female mice pregnant with pups of this genotype were treated with tamoxifen at one of these embryonic days (E) 7.5, E9.5, E10.5 and E11.5. In timed matings, noon on the day of the vaginal plug was designated E0.5. Tamoxifen treatment (T) was administered to pregnant dams by oral gavage (7.5 mg at 20 mg/ml in corn oil) to induce Cre-reporter recombination. Original characterization of the Fgf8CreER allele suggested that tamoxifen exposure caused activation that was sparse at 6 hours, pervasive at 24 hours and negligible at 48 hours after treatment (Hoch et al., 2015).
Brains were collected at postnatal day 21. Mice were heavily anesthetized and perfused transcardially with solution 4% paraformaldehyde in 0.1 M phosphate buffer. After the dissection, brains remained in the same fixative for 24 h and where then equilibrated in 30% sucrose overnight. Brains were frozen on dry ice and sectioned 40 µm thick using a microtome with a frozen chuck either in the coronal plane or sagittal plane.
Immunofluorescence processing of the tissue were performed on free-floating sections (Ehlinger and Commons, 2017). After sectioning, tissue was rinsed in 0.1 M phosphate-buffered saline (PBS) incubated in primary antibody diluted in PBS with 0.3% Triton X-100, 0.1% bovine serum albumin and 0.01% sodium azide for 48–72 hours at 4°C. After the tissue was washed in the PBS, incubated in secondary antibody 1 to 2 hours at room temperature, it was rinsed and mounted on glass slides. The following primary antibodies were used, all diluted at 1:1000. Anti-tryptophan hydroxylase (TPH) raised in sheep from Millipore Corporation (Bedford, MA USA) Millipore Cat# AB1541, RRID:AB_90754. The immunogen was recombinant rabbit TPH and the affinity-purified sheep antibody recognizes both peripheral and central forms of TPH, (TPH1 and TPH2). Characterization of this antibody in our laboratory shows complete identification of serotonin neurons as detected with antibodies to serotonin, but also slight and variable cross-reactivity to tyrosine hydroxylase (TH) particularly evident in mouse. Anti-tyrosine hydroxylase (TH) raised in mouse from ImmunoStar (Hudson, WI, USA) Cat# 22941, RRID:AB_572268. The antigen was TH purified from rat PC12 cells. This monocolonal antibody does not cross react with related enzymes according to manufacturer’s specifications. In our characterization, it yields a pattern of labeling consistent with identification of all catecholaminergic neurons. Anti-DsRed from Clontech, now Takara Bio Cat# 632496, RRID:AB_10013483. This antibody was raised in rabbit against recombinant full-length Zoanthus species yellow fluorescent protein and recognizes a variety of related flourescent proteins including TDT. In our characterization, immunolabeling with this antibody can boost fluorescence of TDT but has no effect in tissue sections lacking TDT. Anti-nitric oxide synthase (NOS) raised from Santa Cruz Biotechnology Cat# sc-5302, RRID:AB_626757. This is a mouse monoclonal antibody raised against amino acids 2–300 of NOS1 of human origin, but recognizes NOS1 of mouse and rat as well. Manufacturer’s characterization shows detection of a single band via western blot of mouse brain. Characterization in our laboratory indicates this antibody yields labeling as predicted by the expression pattern of Nos1. anti-serotonin from ImmunoStar Cat# 20080, RRID:AB_572263. This antibody was raised in rabbit against serotonin coupled to bovine serum albumin with paraformaldehyde and does not cross react with related chemicals. Immunolabeling with this antibody yields a pattern indicative of serotonin. Anti-PAX5 from Cell Signaling Technology Cat# 8970, RRID:AB_10950222. This monoclonal rabbit antibody was raised against residues surrounding Gln350 of human PAX5 protein and positively recognizes human and mouse forms of PAX5 from various cell lines. The pattern of immunolabeling with this antibody is consistent with known gene expression pattern for Pax5. Finally we used anti-vesicular acetyl choline transporter (VAChT) from Millipore Cat# AG260, RRID:AB_10000324. This antibody was raised in goat to a 20 amino acid fragment of rat VAChT (511–530) that yields a pattern of labeling that perfectly colocalizes with other antibodies to the same protein (Siembab et al., 2010).
The following combinations of antibodies were used (1) sheep-anti-TPH with mouse-anti-TH, with or without rabbit-anti-DsRed; (2) mouse-anti-Nos with rabbit 5-HT; (3) mouse-anti-Nos with rabbit-anti-DsRed; (4) rabbit-anti-PAX5 with sheep-anti-TPH. With these combinations, TDT was either visualized directly or detected with using the DsRed primary antibody with a secondary conjugated to Cy3 from Jackson Immunoresearch, West Grove, PA. Other antigens were detected with Alexa Flour-488 or Alexa Flour-647 conjugated secondary antibodies from Invitrogen, ThermoFisher Scientific. All secondary antibodies were raised in donkey and had limited cross reactivity to other species. Images were captured using an Olympus IX-81 fluorescence microscope with a 10×−20x objective, a Hamamatsu Orca ER camera and Olympus cellSens software. Fate mapping was completed in at least three mice from at least 2 separate litters, except for T-E10.5 was conducted using one litter.
Results
When Fgf8-cre expression was induced at E7.5 (T-E7.5), the TDT-labeling pattern observed in the adult recapitulated the distribution described for the constitutively expressed Fgf8-Cre-LacZ lineage analysis (Watson et al., 2017). Specifically, labeling was primarily restricted to a tissue band consisting of both neurons and glia with occasional spurious labeling in adjacent areas. This band in sagittal section is seen as a contiguous rostro-caudal segment (Figs. 1, 2). However because coronal sections are skewed to the developmental rostro-caudal axis, this appears as a horizontal band that moves dorsally in more caudal coronal sections (Fig.3). The band of TDT labeling extended from the ventral tegmental area, (VTA, A10) and the retrorubral field (RRF, A8) rostrally to the locus coeruleus (LC) caudally and included the rostral interpeduncular nucleus (IPR) ventrally and the entire DR dorsally, as depicted in both sagittal (panels A and B of Figs 1, 2) and coronal sections (Fig. 3).
Figure 1.
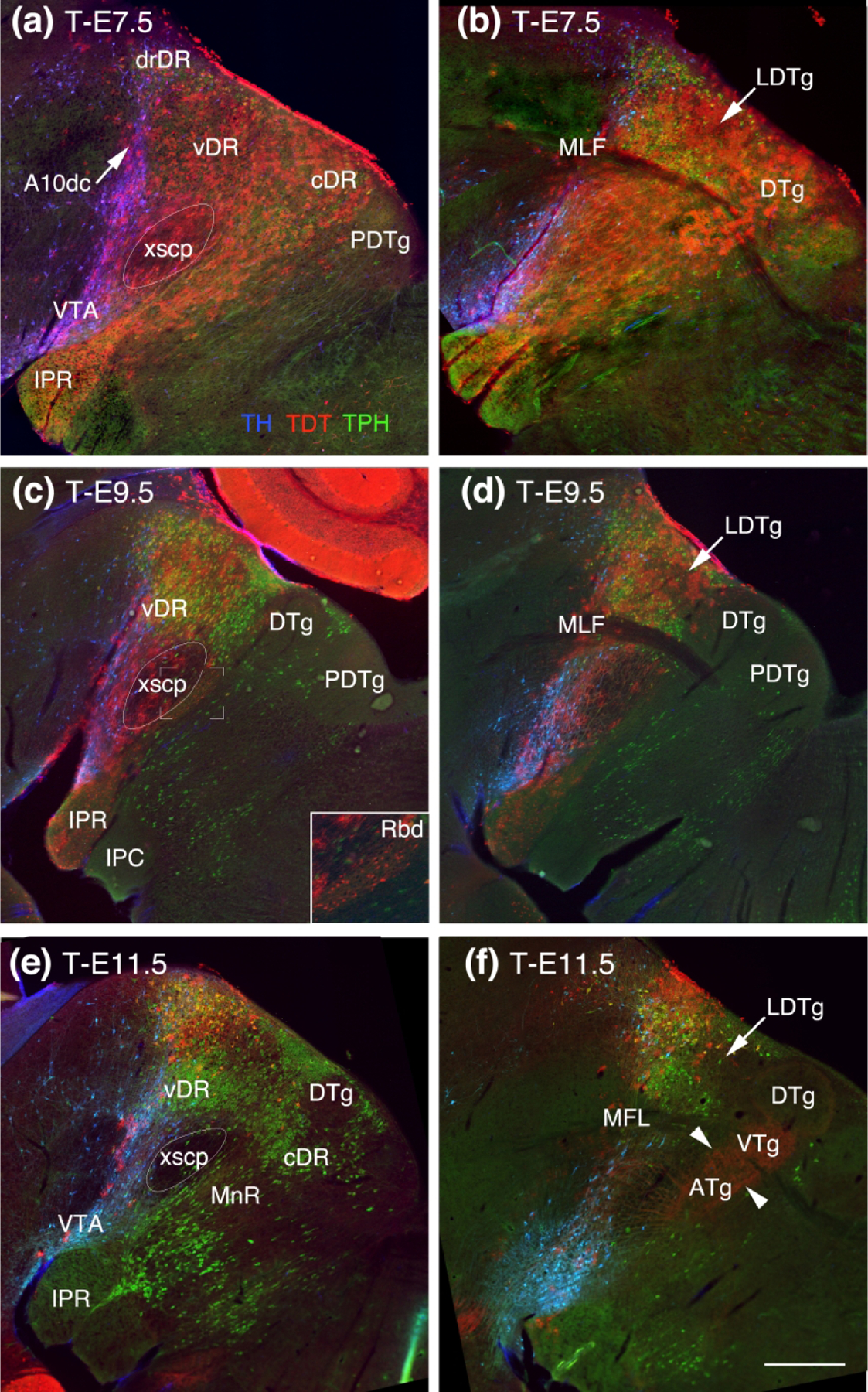
Adult sagittal midline (a, c, e) and paramedial sections (b, d, f) showing the Ffg8 lineage map (red, TDT) labeled at different developmental ages with respect to TH (blue) and TPH2 (green). (a) At T-E7.5, rostral most TDT labeled neurons surround dopaminergic neurons in the VTA and A10dc (blue, arrow). TDT extends past the decussation of the superior cerebellar peduncle (xscp). Ventrally the rostral interpeduncular nucleus (IPR) contains TDT as does the entire DR dorsally. (b) In a section just off the midline that captures the medial longitudinal fasciculus (MLF), TDT labeling can be seen extending as far caudally as to encompass Gudden’s dorsal tegmental nucleus (DTg). (c, d) At T-E-9.5 the rostral extent of TDT is largely unchanged. Caudally there is a slight difference between TDT labeled astrocytes and neurons. TDT labeled astrocytes are within the xscp and the most rostral fraction of the IPR whereas TDT labeled neurons are found throughout the IPR, behind the xscp in the rhabdoid nucleus (Rbd, inset of c) and the cortical DTg. (e, f) At T-E11.5 just a wisp of TDT labeled cells remain in the vicinity of the VTA and dorsally located serotonin neurons with the most prominent TDT labeling encompassing the dorsorostral DR (drDR) (E.). (f) A red haze caused by TDT labeled axons (arrowheads), probably from the mammillary nucleus, can be seen coursing perpendicular to the xscp to innervate the Gudden’s columnar trio of tegmental nuclei, the anterior, ventral and dorsal tegmental nuclei (ATg, VTg and DTg). All panels same scale, bar in (f) = 0.75 mm.
Figure 2.
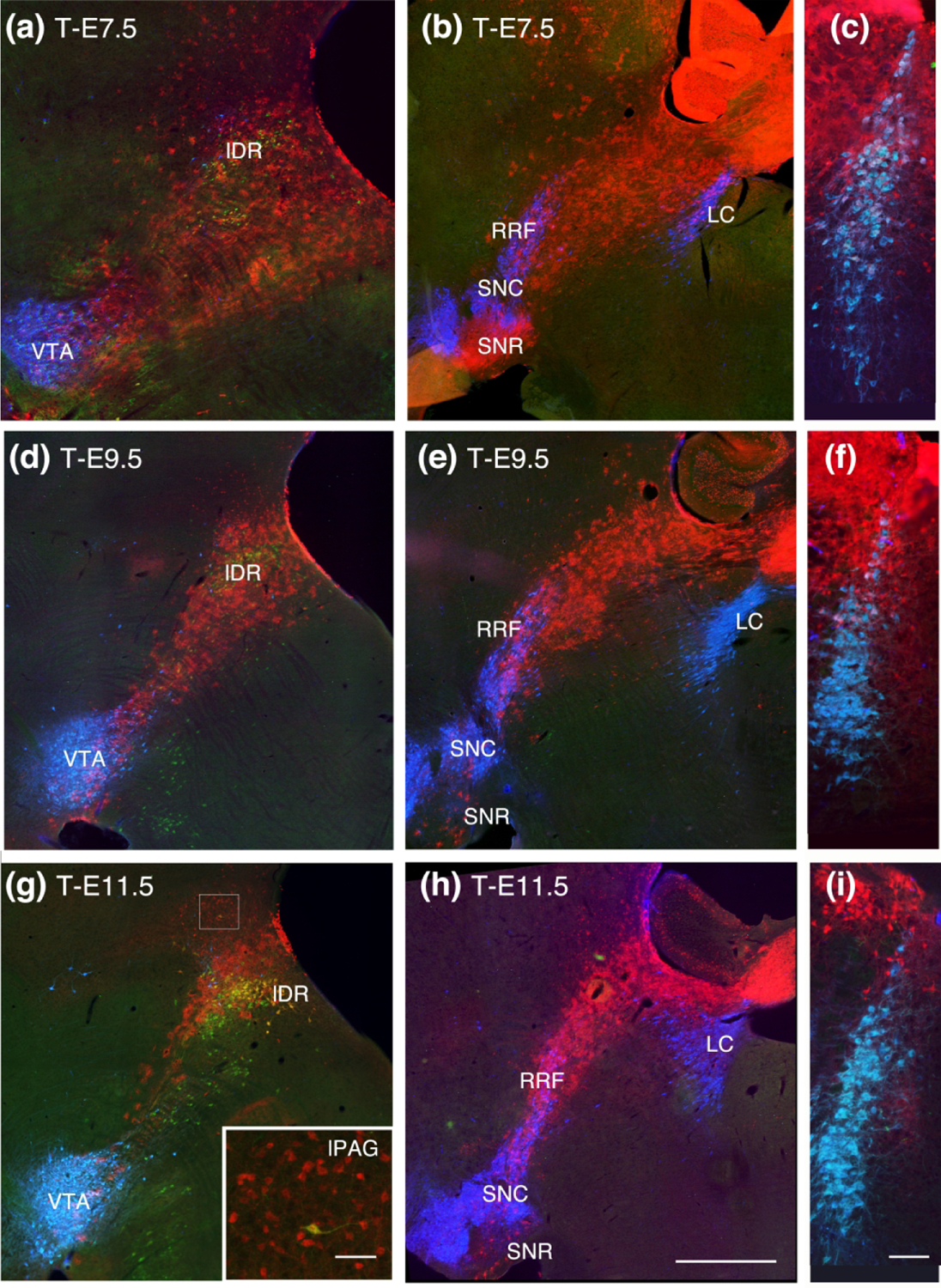
Sagittal sections progressively lateral to the midline, continuing the progression as shown in Fig 1. and coronal sections through the locus coeruleus (LC). (a, b, c) At T-E7.5 TDT surrounds serotonin neurons in the lateral wings of the DR (lDR) and extends caudally to include TH neurons in the rostral and dorsal LC. (d, e, f) Although there is considerable thinning of the TDT band at T-E9.5, the lateral DR and dorsal tip of the LC retain TDT labeling. (g) At T-E11.5 TDT remains dorsally located in lDR serotonin neurons and migrated neurons can be seen in the lateral PAG (inset in G). (h, i) The rostral extent of TDT is similar to previous timepoints and just dorsal to the LC. Sagittal sections show at the scale in (h), bar = 1 mm; inset in (g) bar = 100 um; (c, f, i) scale show in (i) bar = 200 um.
Figure 3.
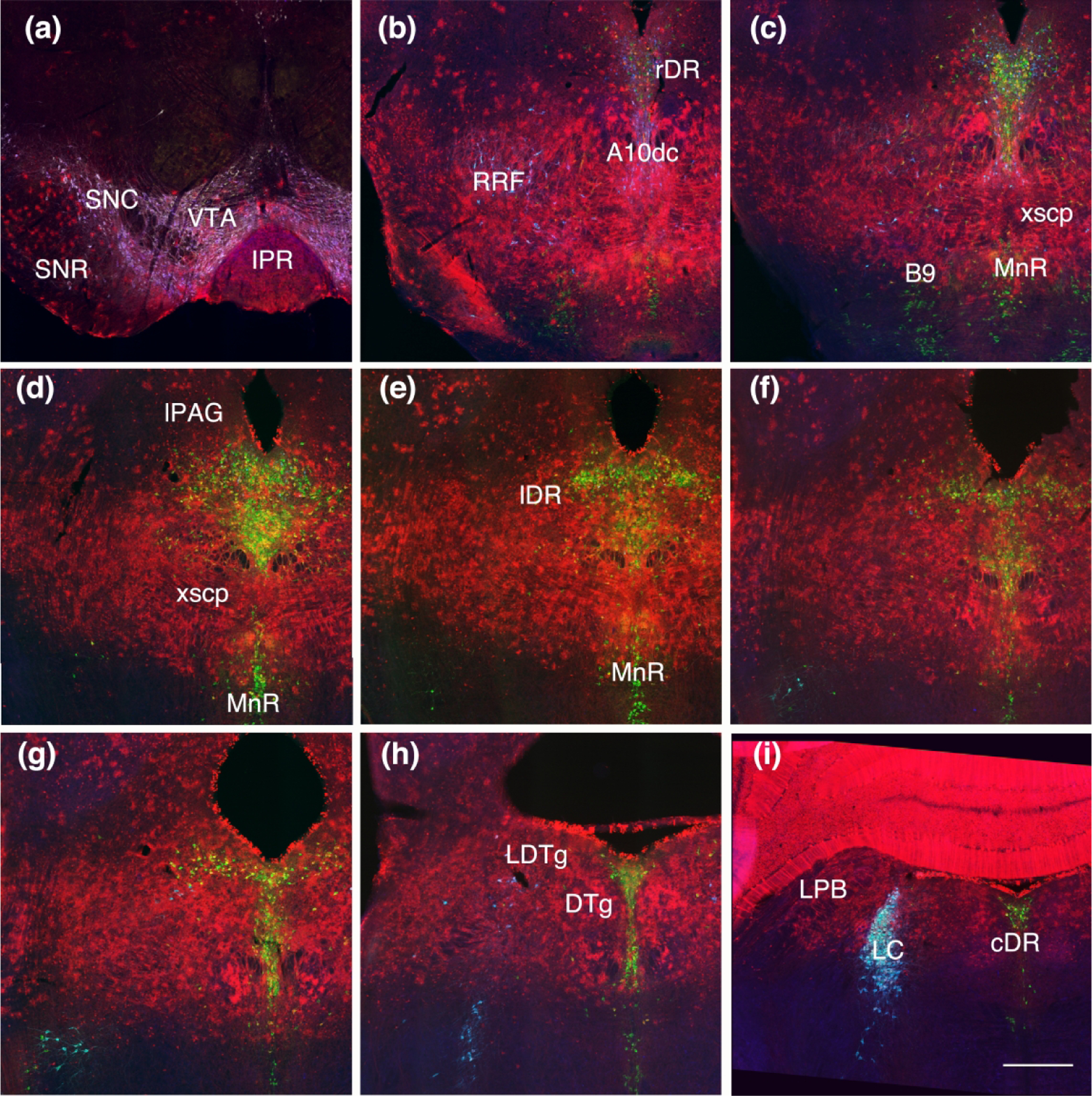
Coronal sections showing Fgf8 fate map at T-E7.5 (red, TDT) with immunolabeling for TH (blue) and TPH (green). (a) TDT is intermixed with the midbrain DA cell groups. (b) The retrorubral field (RRF) and A10 dorsocaudal (A10dc) groups contain TDT, although only a minority of DA neurons have detectable dual labeling. (c, d, e, f) TDT labeling extends ventral to the xscp and includes part of the MnR and B9 area. (g, h, i) TDT is found in the entire DR, including the most caudal extent medial to the DTg. Scale bar in (i) = 250 um.
At T-E7.5 TDT-containing neurons and astrocytes were very abundant in dopaminergic nuclei including the substantia nigra, retrorubral field, and ventral tegmental area. In addition TDT was found in the area of dopamine neurons in and near the rostral DR, the group that is sometimes referred to as A10 dorsocaudal (A10dc). However, in all of these areas only an occasional TH neuron also contained TDT. TDT extended behind the decussation of the superior cerebellar peduncle (xscp) into areas occupied by the anterior, ventral and dorsal tegmental nuclei (ATg, VTg and DTg). The ATg, VTg and DTg are a trio of adjacent and related nuclei that are innervated by the mammillotegmental tract and are also known as Gudden’s tegmental nuclei. Serotonin neurons in the most rostral and dorsal aspects of the MnR also contained TDT (Fig. 1, 3). TH neurons were found dually labeled for TDT in the dorsal part of the LC (Fig. 2,3), consistent with Watson et al. 2017.
The comparison between T-E7.5 and T-E9.5 shows a shift in the character of the labeling in that TDT was more specific to a contiguous band of labeling at T-E9.5 (Figs. 1, 2). The most rostral extent of TDT labeling maintained a similar distribution where it overlaid the dopaminergic ventral tegmental area and retrorubral field (Fig. 1, 2 and 4). However, at T-E9.5 it was difficult to identify TH-positive neurons in any dopaminergic cell group that also contained TDT. Thus only the earliest tamoxifen treatment captured any dopaminergic neurons. While the rostral extent of labeling was generally unchanged, there was a considerable retraction of the caudal extent of TDT labeling comparing T-E9.5 to T-E7.5.
Figure 4.
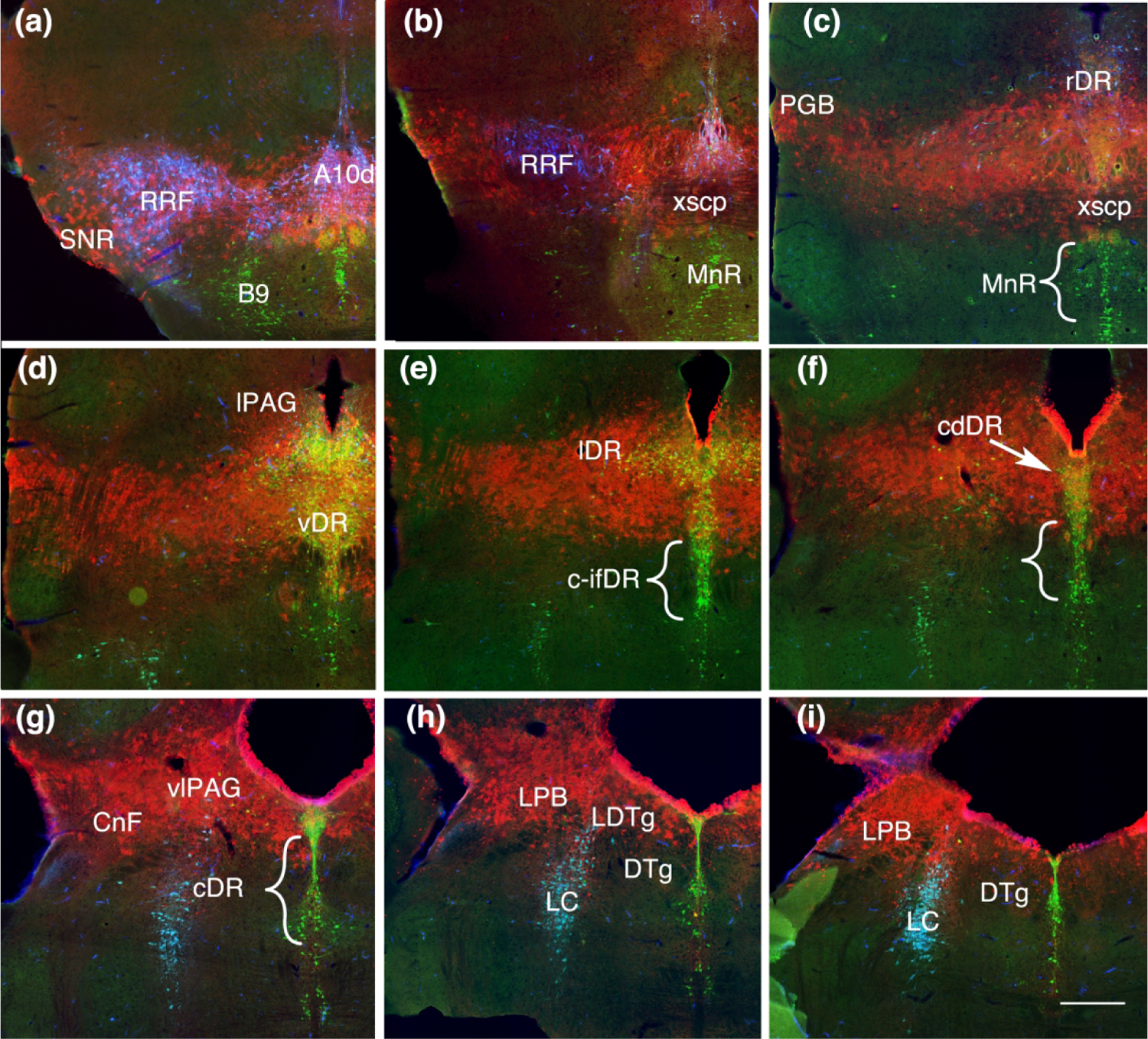
Coronal sections showing Fgf8 fate map at T-E9.5 (red, TDT) with immunolabeling for TH (blue) and TPH (green). (a) Rostrally TDT remains associated with the dopaminergic nuclei, but is not present within dopamine neurons. (b, c) TDT is within the xscp but not in serotonin neurons ventral to the xscp in the MnR. (d) All of the middle levels of the DR containing TDT. (e, i) Lateral wings (lDR) and caudal-dorsal DR (cdDR) contain TDT however serotonin neurons ventral in the caudal and caudal intrafasicular DR (c-DR c-ifDR) and areas medial to the DTg lack TDT. Scale bar in (i) = 250 um
We also noticed, particularly along the caudal edge of labeling, instances where TDT was present only in neurons, in contrast to most of the area containing TDT where both neurons and astrocytes had TDT. We interpret areas with only neuronal labeling as arising from instances of postmitotic migration. Specifically, while labeled astrocytes were confined to the rostral tip of the rostral interpeduncular nucleus, labeled neurons were more fully dispersed in this area. While non-neuronal TDT labeling was present within the xscp, TDT neurons alone were observed caudal to this point, including within in the rhabdoid nucleus (RbN) and Gudden’s columnar trio of tegmental nuclei, ATg, VTg and DTg. Neurons and astrocytes overlapped the dorsal-rostral tip of the LC where a few TH neurons retained TDT labeling, and some of the neurons seem more ventral than the astrocytes in this nucleus. Laterally, the microcellular tegmental nucleus (MiTg) and the parabigeminal nucleus (PBG) contained TDT labeled neurons and astrocytes at T-E9.5. (Fig. 4).
With respect to serotonin, at T-E9.5 MnR neurons lacked TDT. Likewise, DR neurons that were located ventral and/or caudal to the xscp in the caudal intrafascicular DR, as well as serotonin neurons medial to the DTg, lacked TDT (Fig. 4). Neurons packed at the base of the aqueduct in the dorsal part of the caudal DR mostly maintained TDT labeling at T-E9.5 (Fig. 4).
Comparing T-E9.5 to T-E11.5 the rostral extent of TDT labeling again appeared little changed (Fig. 1,2 and 5). Due to overall less TDT labeling at this timepoint, apparently migrated neurons rostral to the band of TDT labeling were obvious. These included serotonin neurons in the rostral-dorsal extent of the DR and non-serotonergic neurons in the lateral periaqueductal gray (PAG) (Fig 2G inset and 5C). The caudal limit of TDT retracted notably along the midline and to a lesser extent laterally. That is, there was less labeling within substantia nigra pars compacta (SNc) and retrorubral field, but a more substantial loss from the ventral tegmental area (VTA). In these areas the shift in labeling was caused by changes in non-dopaminergic neurons, as dopaminergic neurons lacked TDT at T-E9.5. In addition, considerably fewer serotonin neurons contained TDT throughout the ventral DR. However, retaining TDT at T-E11.5 are serotonin neurons that populate the rostral DR, dorsal DR and the lateral wings throughout its rostro-caudal extent (Fig. 5).
Figure 5.
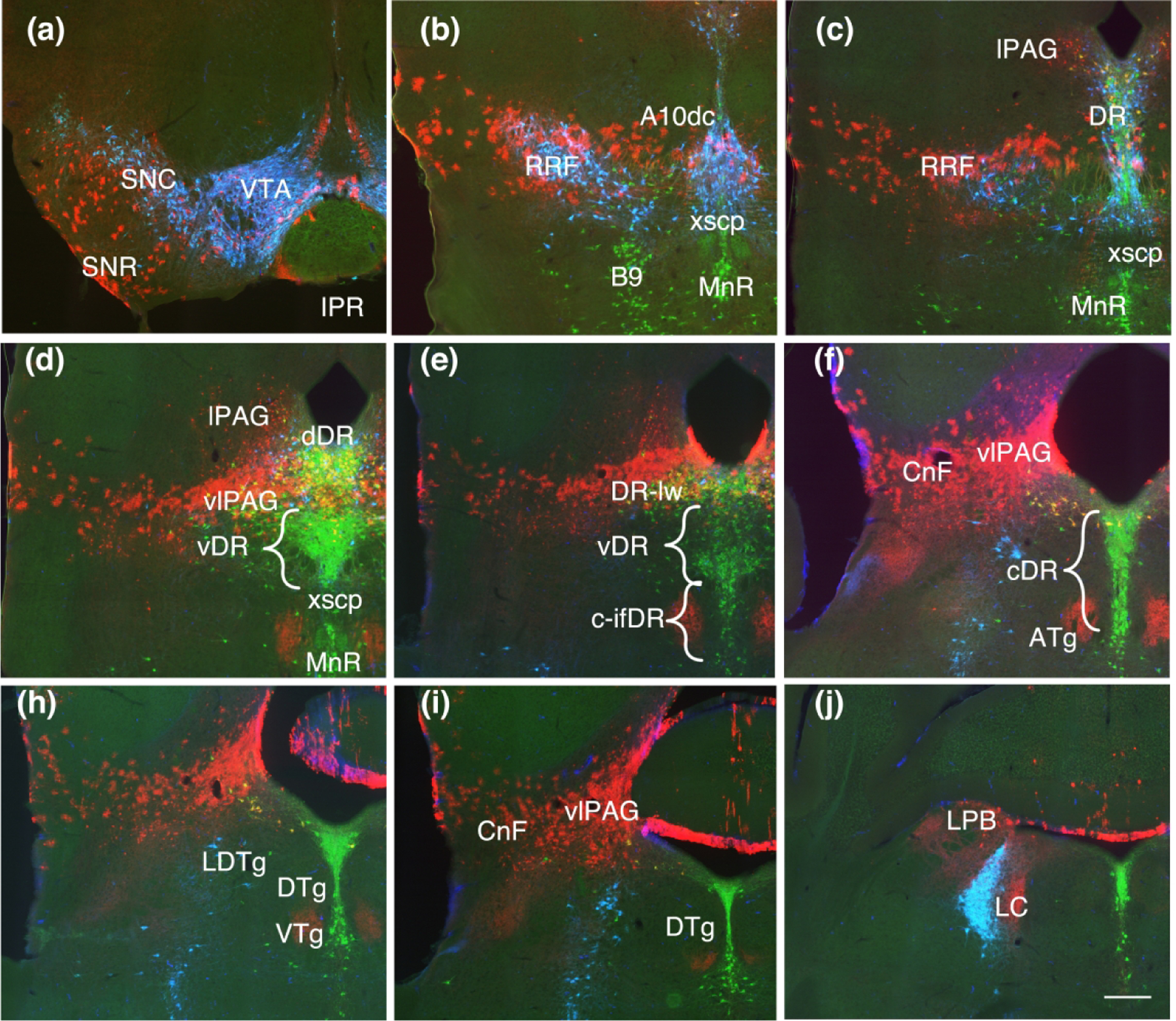
Coronal sections showing Fgf8 fate map at T-E11.5 (red, TDT) with immunolabeling for TH (blue) and TPH (green). (a, b, c) TDT labeling in the dopaminergic nuclei follow the same pattern as with earlier timepoints but the band of labeling appears sparser and thinner, with a reduced ventral extent. (d) At this point, TDT is largely absent from the ventral DR (vDR) where serotonin neurons extend laterally in a triangular shape above the xscp. (e, f) Except for the caudal lateral DR (DR-lw) which retains TDT, the remaining caudal DR both dorsal and intrafasicular (c-DR, c-ifDR) is excluded from the TDT domain except for a few scattered neurons. (h, i, j) TDT labeling persists in the ventrolateral PAG (vlPAG) and cuneiform nucleus (CnF). Scale bar in (i) = 250 um
We took a closer look at the ebbing of TDT through the DR by comparing T-E9.5, T-E10.5 and T-E11.5 (Fig. 6). At T-E10.5 the caudal dorsal DR lacks TDT as does a fraction of the neurons located ventrally, in the middle of the DR. It’s not until T-E11.5 that the large middle-ventral group of serotonin neurons is excluded more completely from the TDT domain. The temporal progression of Fgf8-lineage through serotonin neurons is summarized in Figure 7.
Figure 6.
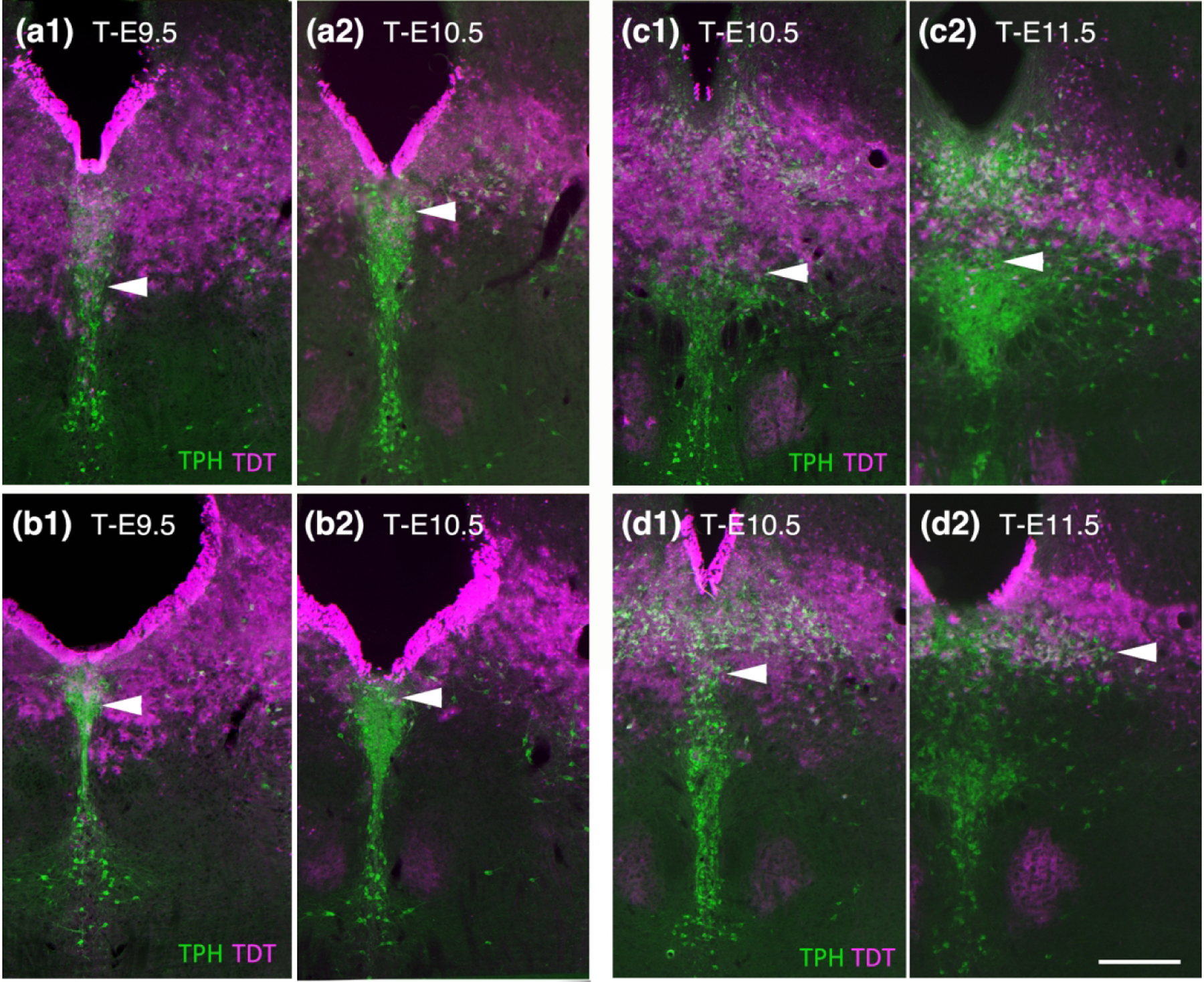
A more detailed look at the ebbing of Fgf8 lineage through serotonin neurons in the DR comparing T-E9.5 to T-E10.5 (a, b) and T-E10.5 to T-E11.5 (c, d). (a1 and b1) At T-E9.5 neurons in the caudal dorsal DR packed at the base of the aqueduct contain TDT while at T-E10.5 this area has considerably less TDT (a2 and b2). (c1 and d1) At T-E10.5 TDT dips into the ventral cluster of neurons and is pronounced at the midline. (c2 and d2). At T-E11.5 the ventral DR is largely free of TDT and there is less TDT dorsal particularly on the midline, however although the lateral wings retain TDT. Scale bar in (d2) = 250 um.
Figure 7.
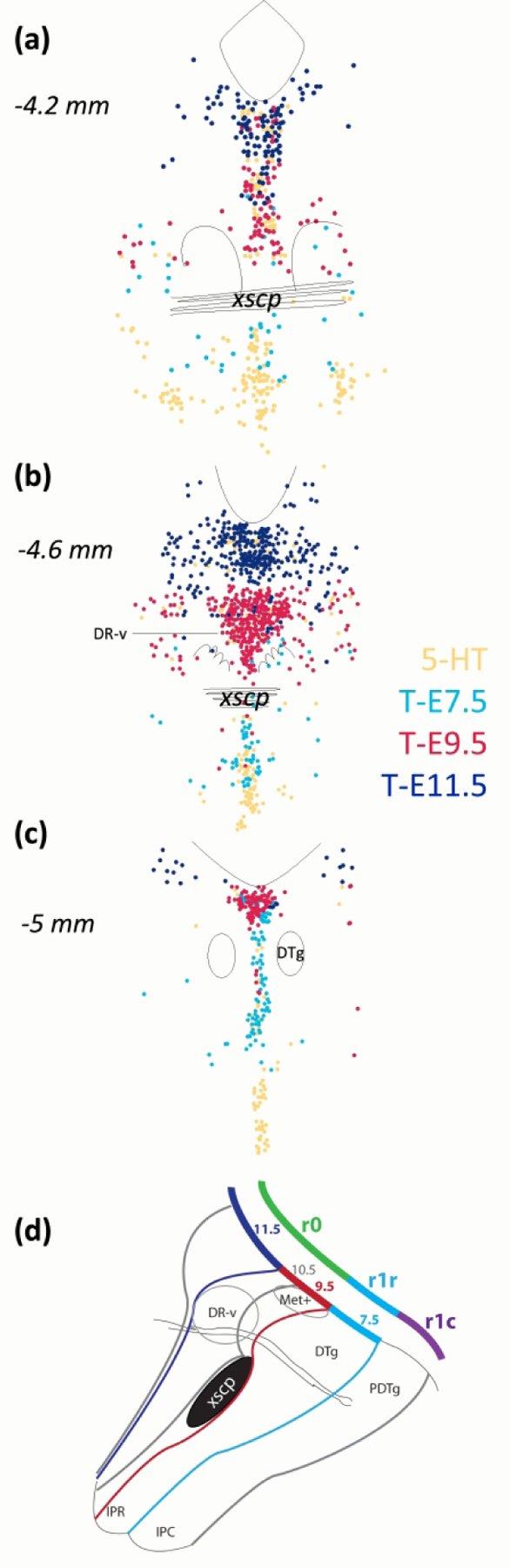
Summary schematics showing the differential location of serotonin neurons captured with Fgf8 lineage at different time-points of tamoxifen treatment. (a, b, c) Serotonin neurons in locations uniquely captured by T-E7.5 in light blue can be seen in locations ventral and caudal to the decussation of the superior cerebellar peduncle (xscp). Serotonins uniquely captured by T-E9.5 in red dominate the largest cluster of serotonin neurons in the DR-ventral (DR-v) depicted in (b). The last group of serotonin neurons captured by T-E11.5 in dark blue is located rostral, dorsal and lateral. There are always some serotonin neurons lacking Fgf8 lineage (yellow), and incomplete recombination with a single drug treatment may contribute to this. (d) Illustrated in sagittal section, where the relationship to developmental organization is more evident, the areas captured by T-E9.5 (red), inclusive of those captured at T-E11.5 (dark blue), correspond to predicted r0 derivatives. This border of this area is marked by the xscp. Fgf8 lineage as captured at T-E7.5 (light blue) extends into r1, but includes only a rostral part of r1 (r1r). A caudal part of r1 (r1c) would contain posterior dorsal tegmental nucleus (PDTg) and the caudal interpeduncular nucleus (IPC).
Next we studied the distribution of TDT with respect to nitric oxide synthase (Nos) in the DR. Nos is present in a subpopulation of serotonin neurons that are generally located rostral and ventral in the DR as well as in the middle of the nucleus (Fig. 8 A.B.). In examining the rostral ventral population at three different timepoints we found that most Nos cells in the DR were double-labeled with TDT at T-E 7.5 and T-E9.5, however this area was almost clear of TDT at T-E11.5.
Figure 8.
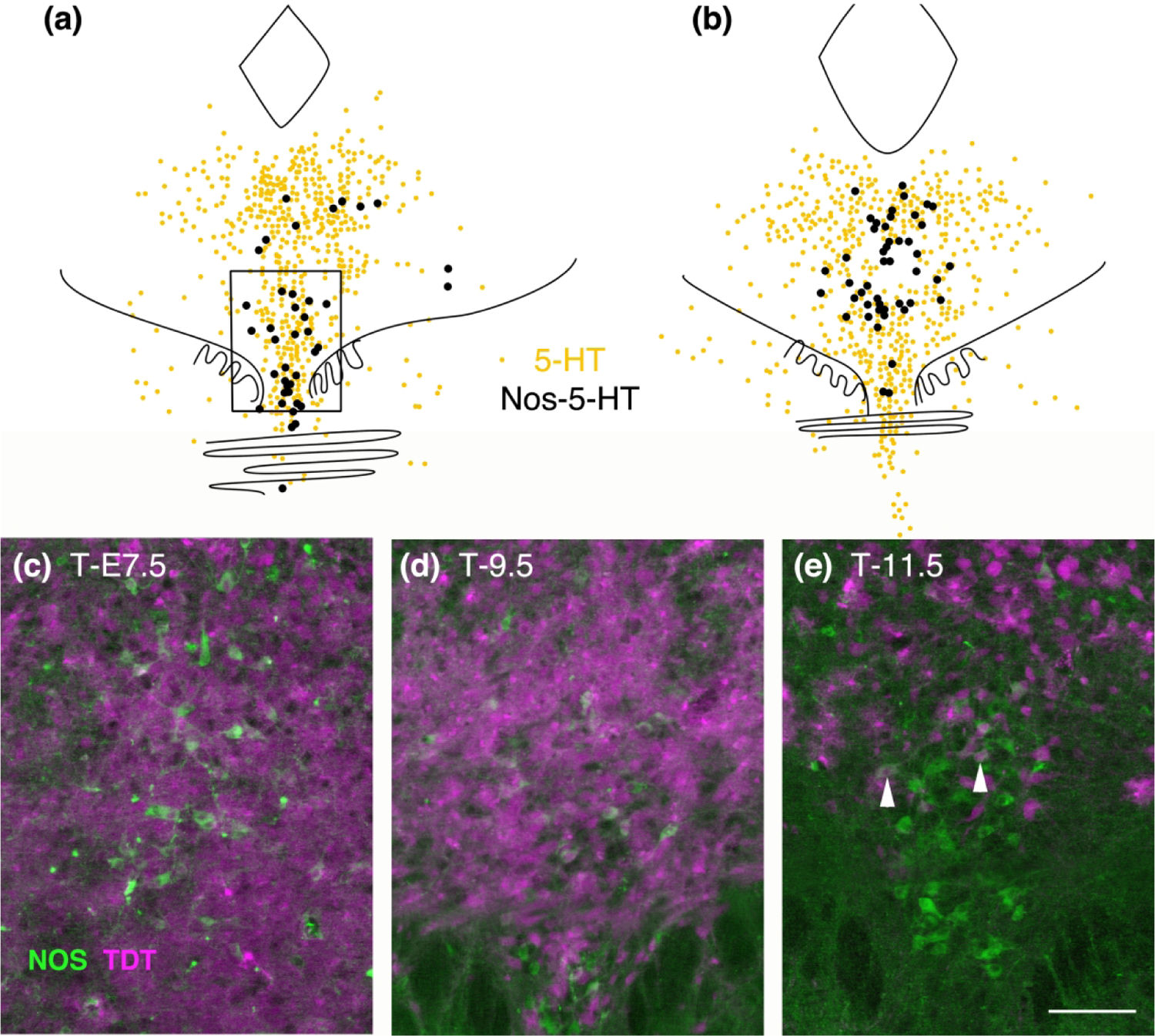
(a, b) Map of the location of Nos-immunolabeled serotonin neurons at rostral (a) and middle (b) levels of the DR. Nos neurons located in the rostral ventral DR (boxed area in a) dually labeled for TDT at T-E7.5, 9.5 and 11.5 (c, d, e) shows that TDT only excludes these neurons at T-E11.5, although a few retain TDT at that timepoint as well (arrowheads).
We likewise mapped TDT with respect to serotonin neurons that contained immunolabeling for Pax5. Pax5 labeled serotonin neurons tend to be located rostral and dorsal in the nucleus as well as in the lateral wings along the entire rostral-caudal extents of these cells (Fig. 9). With triple immunolabeling we found that regardless of the location (rostral-dorsal or lateral wing), most Pax5 serotonin neurons had TDT at T-E11.5. These showed a slightly different relationship to the band of TDT-containing glia or mixed neurons and glia. That is, rostral-dorsal neurons were above the band whereas caudal-lateral neurons were below the band. Intermixed with these triple-labeled neurons are serotonin neurons, with and without TDT, that lacked detectable Pax5 suggesting heterogeneity of these areas.
Figure 9.
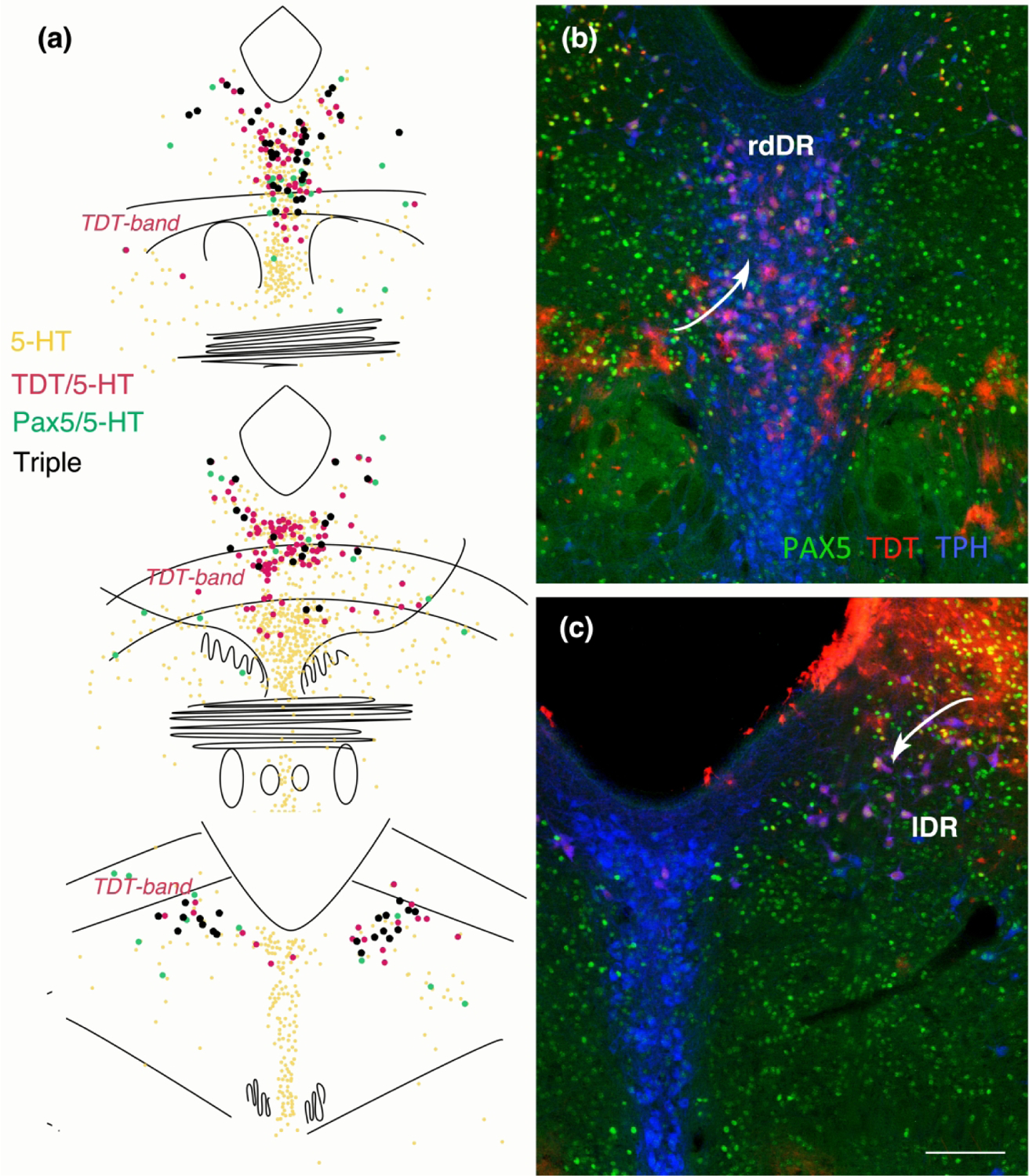
(a) Map of Pax-5 immunolabeled serotonin neurons with and without TDT at T-E11.5 from rostral (top) to caudal (bottom). The majority of serotonin neurons with Pax5 also contain TDT and tend to be located rostral and dorsal as well as in the lateral wings. (b) Dorsal rostral DR (drDR) serotonin neurons (labeled with TPH, blue) are often dually labeled for TDT (red) and are located above the band of TDT containing astrocytes (arrow). Many of these serotonin-TDT labeled neurons also contain Pax5 (green). (c) Caudally in the lateral wings of the DR (lDR) many TDT-containing serotonin neurons are located ventral to the band of astrocytic TDT labeling (arrow), and those neurons also often contain Pax5. (b, c) same scale bar in (c) = 200 um.
Lastly, we examined the distribution of TDT in cholinergic neurons in the lateral dorsal tegmental nucleus and the pedunculopontine tegmental nucleus (LDTg and PPTg Fig. 10). At T-E7.5 a few cholinergic neurons contained TDT while at T-E9.5 it was difficult to detect dually labeled cells, even though at its’ rostral extent the LDTg appeared surrounded by TDT cells suggesting a post-mitotic migration of cholinergic neurons to this location
Figure 10.
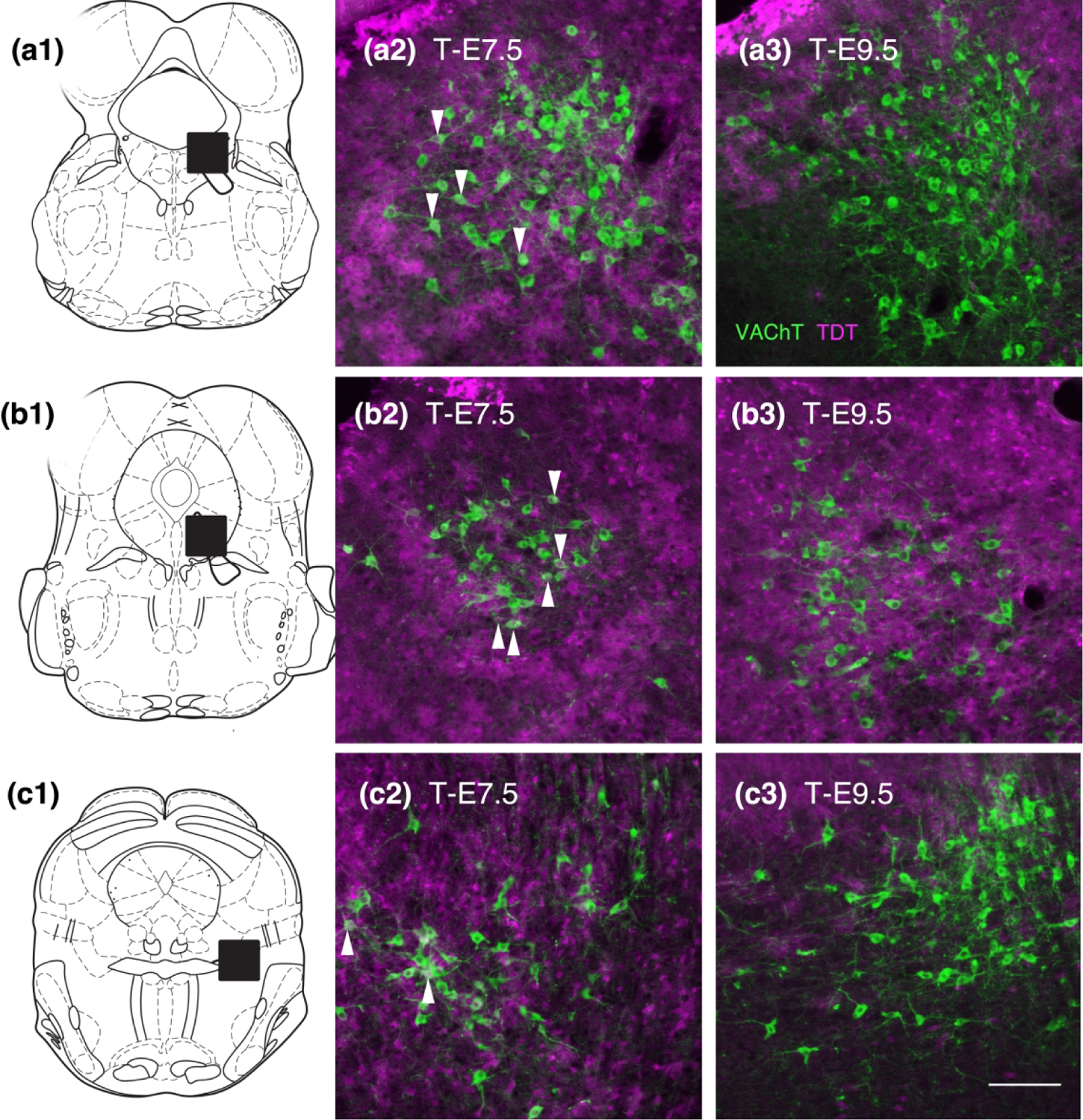
Distribution of TDT (magenta) with respect to cholinergic neurons (VAChT, green) in the lateral dorsal and pedunculopontine tegmental nuclei (LDTg and PPTg). ‘a’ series shows the caudal LDTg, ‘b’ series shows rostral LDTg and ‘c’ series shows PPTg, boxed areas on schematics. In all of these areas at T-E7.5 a minority of cholinergic neurons (arrows) are dually labeled for TDT (a2, b2, c2). In contrast at T-E9.5 (a3, b3, c3) it is difficult to find cholinergic neurons with clear TDT content, although in (b3) cholinergic neurons are surrounded and overlie TDT labeled structures they lack clear nuclear labeling indicative of cellular content seen at T-E7.5. Bar in (c3) = 250 um.
Discussion
Fgf8 lineage temporally recedes across the r0/r1 domain in a way that seems ideal to set up a diversity of descendent neurons. Although this process is gradual, it also seems useful to conceptualize it in quantal terms. This is because captured or excluded by Fgf8 lineage at different timepoints are groups of serotonin neurons that tend to have related characteristics. These divide the DRN roughly into thirds: a caudal third that with the MnR is associated with septohippocampal serotonin, a middle third correlating with the source of cortical serotonin and a rostral and lateral third where projections are known to be more subcortical than cortical (Muzerelle et al., 2014; Commons, 2016; Ren et al., 2019). The differential epochs of Fgf8 lineage in these groups of neurons not only correlate with projection patterns but also to some extent with transcriptional profile in the adult (Huang et al., 2019; Ren et al., 2019; Okaty et al., 2020a). This organization should not be viewed rigidly however as it is one formed by trends and correlations.
Since Fgf8 expression is temporally dynamic, the border between r0 and r1 is defined only by indirect evidence in analogy to the development of the remaining hindbrain (Aroca and Puelles, 2005). What has been proposed to constitute r0 seems captured by Fgf8-lineage at T-E9.5. Specifically, this would include areas such as the parabigeminal nucleus and a portion of the rostral interpeduncular nucleus (Moreno-Bravo et al., 2014; Watson et al., 2017). Likewise predicted migratory contributions of r0 to Gudden’s trio of tegmental nuclei, ATg, VTg and DTg, are visible at this timepoint (Moreno-Bravo et al., 2014). Working on the assumption that T-E9.5 Fgf8 lineage defines r0 we would conclude from the current data that almost no dopamine or cholinergic neurons are r0 derived, consistent with the literature (Achim et al., 2012; Yang et al., 2013; Veenvliet and Smidt, 2014). Although there are a few noradrenergic neurons in the LC that are captured at this timepoint (Figure 2F).
The T-E9.5-defined isthmus territory differs from the area described by Watson et al., 2017 using a constitutive Fgf8-cre, which was more closely replicated by our T-E7.5 group. Specifically in these two latter cases, some dopaminergic and cholinergic neurons are captured and lineage tracer is found within areas caudal to the predicted r0, such as within the MnR and caudal extent of the DR (Watson et al., 2017). We would argue that the Fgf8 lineage captured with the constitutively expressed Fgf8-Cre or T-E7.5 group includes r0 plus a rostral part of the remaining r1 proper. The cause of this may be that at the earliest states of the hindbrain, Fgf8 expression is slightly broader than the anlage of the r0 territory, to which it is subsequently refined before ebbing to it’s most rostral cusp. However the T-E7.5 Fgf8-lineage does not seem to include the entire r0/r1 as would be defined by En1-lineage in that the caudal interpeduncular nucleus and the posterior dorsal tegmental nucleus are excluded from this domain (Aroca and Puelles, 2005). Considering r1 as it exists caudal to r0 (‘r1 proper’) there are no clear developmental landmarks that divide this region into rostral and caudal portions, but based upon the adult organization such a construct has been argued (Aroca and Puelles, 2005). The partial extension of the earliest Fgf8 lineage through the rostral part of r1 proper, coinciding with the duplicative or twin-like organization of the rostral and caudal parts of the interpeduncular nucleus on the ventral surface and the dorsal and posterior dorsal tegmental nuclei at dorsal locations would be consistent with the utility of modeling the area caudal to r0 as encompassing both r1-rostral and r1-caudal zones (Fig. 7).
Many axon pathways organize with respect to the area defined by T-E9.5 Fgf8-lineage, supporting the utility of the r0 scheme. While cranial nerves mark several rhombomeric borders, the r0-r1 border appears to be described by the decussation of the superior cerebellar peduncle (xscp). In addition several axon pathways travel perpendicular to the xscp to innervate the column of tegmental nuclei ATg, VTg and DTg, including axons from the mammillary nucleus, which can be seen in Fig. 1F, and serotonergic axons originating in r2 (Bang et al., 2012). Likewise, quantitative analysis of the distribution pattern of 26 different cases of anterograde tract-tracing into DRN showed that afferents tend to exhibit one of two general patterns (Commons, 2015). That is, either they have a propensity to ramify to serotonin neurons that are either captured or excluded by Fgf8-lineage tracing at T-E9.5.
Specifically the caudal intrafascicular (IF)-DR and MnR neurons, both excluded by Fgf8 lineage at T-E9.5, receive more innervation from the anterior cingulate and retrosplenial cortices, medial septum and the lateral habenula (Commons, 2015). At the same time, neurons in the IF-DR and MnR neurons are similar in their efferent projections in that they both contribute to serotonin innervation of the septohippocampal system (Commons, 2016). Thus, like Gudden’s DTg, these serotonin neurons are associated with regulating hippocampal theta. These patterns of connections coupled with the propensity of serotonin neurons in the caudal DR to exhibit markers of activation after stressful conditions have given rise to the idea that these areas could be involved in learning, particularly under aversive conditions (Commons, 2016).
In contrast serotonergic neurons included in Fgf8 lineage domain at T-E9.5 in the remaining rostral DR are preferentially innervated by the insular and prelimbic cortices, ventral tegmental area, lateral hypothalamus and the amygdala (Commons, 2016). Therefore it has been proposed that these areas are associated with more emotional and motivational functions (Commons, 2016; Ren et al., 2019). Other structures associated with Fgf8 lineage at this timepoint including the ventrolateral PAG, cuneiform and parabigemenal nuclei, all have some relationship to the amygdala (Price and Amaral, 1981; Paredes et al., 2000; Usunoff et al., 2007). These latter areas share a functional theme in that they are associated with coordinating response to proximal threat (Dielenberg et al., 2001).
Caudal dorsal DR neurons, densely clustered together at the base of the aqueduct are not excluded from Fgf8 lineage completely until T-E10.5 thus developmentally they seem similar to the rostral interpeduncular nucleus, which has a ‘multisegmental origin’ (Lorente-Canovas et al., 2012). This border location is consistent with the ambiguous analysis of Alonso et al., where they depict an r1 origin in coronal section and r0 origin in sagittal section for this area (Alonso et al., 2013). Although related in some features of connectivity to the IF-DR, serotonin neurons in this area are known to have several unique characteristics. Their transcriptional profile is distinct from other serotonin neurons, characterized by high levels of Met expression (Kast et al., 2017). In addition they notably innervate the ventricular walls and may be important for proliferation within the subventricular zone (Tong et al., 2014). Thus these neurons may be best considered somewhat independently from the remainder of the DR.
At T-E11.5 two additional groups of serotonin neurons are defined by the presence or absence of Fgf8 lineage. Those that retain Fgf8 lineage at T-E11.5 are those located both rostral in the DR and in the lateral wings, including those located lateral at considerably caudal levels. The developmental kinship of the lateral and rostral neurons and their correlation with Pax5 expression shifts understanding of the lateral wing population. Previously these neurons were seen as either distinct from midline serotonin neurons or else binned with midline neurons that appeared in the same coronal section. Pax5 expression is characteristic of transcriptionally distinct subsets of serotonin neurons that also tend to have low Vglut3 expression (Ren et al., 2019; Okaty et al., 2020a; Okaty et al., 2020b). Our finding that the latest Fgf8-expressing lineage accounts for many Pax5-positive serotonin neurons supports the likelihood of a causative relationship between development and transcriptional profile. Supporting this possibility, at T-E11.5 the serotonin neurons that are newly devoid of Fgf8 lineage primarily populate the ventral DR, an area that is also preferentially populated by serotonin neurons that are transcriptionally distinguishable, in part by relatively higher levels of Vglut3 expression.
While there is a pronounced organization of axon pathways with respect to the areas included and excluded by the T-E9.5 Fgf8-lineage, there are more subtle differences in the organization of axons correlating with these last two groups of serotonin neurons that are included or newly excluded by T-E11.5. In our analysis of 26 anterograde tract-tracing experiments, there was a faint correlation in afferent innervation between lateral and dorsal areas, which seemed to be preferential targets of the amygdala while ventral areas had more association with the mesoaccumbal system. Clearer are the differences in efferent projection patterns of these areas. The ventral DR has long been known to provide the bulk of serotonin to the cortex (O’Hearn and Molliver, 1984; Waterhouse et al., 1986). In contrast serotonin neurons rostral and lateral in the DR that are the last to show Fgf8 lineage tend to project subcortically (Muzerelle et al., 2014; Ren et al., 2018).
Here we show that different groups of serotonin neurons are associated with early vs. late Fgf8 expression using conditional genetic linage analysis. We note a general relationship between different groups and the projections with the earliest serotonin neurons free of Fgf8 lineage associated with septohippocampal function, the next with cortical serotonin and the last with subcortical serotonin. These observations shed light on organization and the developmental origin of distinct subregions of the dorsal raphe nucleus.
Acknowledgements:
Thanks to Dr. Susan Dymecki for advice on the project and to Dr. Ben O’katy for comments on the manuscript. Funding from DA021801.
Footnotes
Conflict of interest: KGC received consulting fee from Zogenix, Inc.
Ethics approval: Animal procedures were approved by Boston Children’s Hospital’s Institutional Animal Care and Use Committee.
Data availability:
The data are available upon request from the corresponding author.
References
- Achim K, Peltopuro P, Lahti L, Li J, Salminen M, Partanen J. 2012. Distinct developmental origins and regulatory mechanisms for GABAergic neurons associated with dopaminergic nuclei in the ventral mesodiencephalic region. Development 139(13):2360–2370. [DOI] [PubMed] [Google Scholar]
- Alonso A, Merchan P, Sandoval JE, Sanchez-Arrones L, Garcia-Cazorla A, Artuch R, Ferran JL, Martinez-de-la-Torre M, Puelles L. 2013. Development of the serotonergic cells in murine raphe nuclei and their relations with rhombomeric domains. Brain Struct Funct 218(5):1229–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroca P, Puelles L. 2005. Postulated boundaries and differential fate in the developing rostral hindbrain. Brain research Brain research reviews 49(2):179–190. [DOI] [PubMed] [Google Scholar]
- Bang SJ, Jensen P, Dymecki SM, Commons KG. 2012. Projections and interconnections of genetically defined serotonin neurons in mice. Eur J Neurosci 35(1):85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG. 2015. Two major network domains in the dorsal raphe nucleus. J Comp Neurol 523(10):1488–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG. 2016. Ascending serotonin neuron diversity under two umbrellas. Brain Struct Funct 221(7):3347–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley PH, Martinez S, Martin GR. 1996. Midbrain development induced by FGF8 in the chick embryo. Nature 380(6569):66–68. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Hunt GE, McGregor IS. 2001. “When a rat smells a cat”: the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience 104(4):1085–1097. [DOI] [PubMed] [Google Scholar]
- Ehlinger DG, Commons KG. 2017. Altered Cav1.2 function in the Timothy syndrome mouse model produces ascending serotonergic abnormalities. The European journal of neuroscience 46(8):2416–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo-Sanchez M, Martinez-de-la-Torre M, Alvarado-Mallart RM, Puelles L. 2005. A distinct preisthmic histogenetic domain is defined by overlap of Otx2 and Pax2 gene expression in the avian caudal midbrain. J Comp Neurol 483(1):17–29. [DOI] [PubMed] [Google Scholar]
- Hoch RV, Clarke JA, Rubenstein JL. 2015. Fgf signaling controls the telencephalic distribution of Fgf- expressing progenitors generated in the rostral patterning center. Neural Dev 10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang KW, Ochandarena NE, Philson AC, Hyun M, Birnbaum JE, Cicconet M, Sabatini BL. 2019. Molecular and anatomical organization of the dorsal raphe nucleus. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P, Farago AF, Awatramani RB, Scott MM, Deneris ES, Dymecki SM. 2008. Redefining the serotonergic system by genetic lineage. Nat Neurosci 11(4):417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner AL, Liu A, Millet S. 2000. Otx2, Gbx2 and Fgf8 interact to position and maintain a mid-hindbrain organizer. Curr Opin Cell Biol 12(6):736–741. [DOI] [PubMed] [Google Scholar]
- Kast RJ, Wu HH, Williams P, Gaspar P, Levitt P. 2017. Specific Connectivity and Unique Molecular Identity of MET Receptor Tyrosine Kinase Expressing Serotonergic Neurons in the Caudal Dorsal Raphe Nuclei. ACS chemical neuroscience 8(5):1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Danielian PS, Fritzsch B, McMahon AP. 1997. Evidence that FGF8 signalling from the midbrain-hindbrain junction regulates growth and polarity in the developing midbrain. Development 124(5):959–969. [DOI] [PubMed] [Google Scholar]
- Lorente-Canovas B, Marin F, Corral-San-Miguel R, Hidalgo-Sanchez M, Ferran JL, Puelles L, Aroca P. 2012. Multiple origins, migratory paths and molecular profiles of cells populating the avian interpeduncular nucleus. Dev Biol 361(1):12–26. [DOI] [PubMed] [Google Scholar]
- Moreno-Bravo JA, Perez-Balaguer A, Martinez-Lopez JE, Aroca P, Puelles L, Martinez S, Puelles E. 2014. Role of Shh in the development of molecularly characterized tegmental nuclei in mouse rhombomere 1. Brain Struct Funct 219(3):777–792. [DOI] [PubMed] [Google Scholar]
- Muzerelle A, Scotto-Lomassese S, Bernard JF, Soiza-Reilly M, Gaspar P. 2014. Conditional anterograde tracing reveals distinct targeting of individual serotonin cell groups (B5-B9) to the forebrain and brainstem. Brain Struct Funct. [DOI] [PMC free article] [PubMed]
- Nieuwenhuys R 2011. The structural, functional, and molecular organization of the brainstem. Front Neuroanat 5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hearn E, Molliver ME. 1984. Organization of raphe-cortical projections in rat: a quantitative retrograde study. Brain Res Bull 13(6):709–726. [DOI] [PubMed] [Google Scholar]
- Okaty BW, Sturrock N, Escobedo Lozoya Y, Chang Y, Senft RA, Lyon KA, Alekseyenko OV, Dymecki SM. 2020a. A single-cell transcriptomic and anatomic atlas of mouse dorsal raphe Pet1 neurons. eLife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okaty BW, Sturrock N, Lozoya YE, Chang Y, Senft R, Lyon K, Alekseyenko OV, Dymecki SM. 2020b. A single-cell transcriptomic and anatomic atlas of mouse dorsal raphe Pet1 neurons. eLife in press. [DOI] [PMC free article] [PubMed]
- Paredes J, Winters RW, Schneiderman N, McCabe PM. 2000. Afferents to the central nucleus of the amygdala and functional subdivisions of the periaqueductal gray: neuroanatomical substrates for affective behavior. Brain Res 887(1):157–173. [DOI] [PubMed] [Google Scholar]
- Partanen J 2007. FGF signalling pathways in development of the midbrain and anterior hindbrain. J Neurochem 101(5):1185–1193. [DOI] [PubMed] [Google Scholar]
- Price JL, Amaral DG. 1981. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci 1(11):1242–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Friedmann D, Xiong J, Liu CD, Ferguson BR, Weerakkody T, DeLoach KE, Ran C, Pun A, Sun Y, Weissbourd B, Neve RL, Huguenard J, Horowitz MA, Luo L. 2018. Anatomically Defined and Functionally Distinct Dorsal Raphe Serotonin Sub-systems. Cell 175(2):472–487 e420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Isakova A, Friedmann D, Zeng J, Grutzner SM, Pun A, Zhao GQ, Kolluru SS, Wang R, Lin R, Li P, Li A, Raymond JL, Luo Q, Luo M, Quake SR, Luo L. 2019. Single-cell transcriptomes and whole-brain projections of serotonin neurons in the mouse dorsal and median raphe nuclei. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Joyner AL. 2009. The duration of Fgf8 isthmic organizer expression is key to patterning different tectal-isthmo-cerebellum structures. Development 136(21):3617–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siembab VC, Smith CA, Zagoraiou L, Berrocal MC, Mentis GZ, Alvarez FJ. 2010. Target selection of proprioceptive and motor axon synapses on neonatal V1-derived Ia inhibitory interneurons and Renshaw cells. J Comp Neurol 518(23):4675–4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong CK, Chen J, Cebrian-Silla A, Mirzadeh Z, Obernier K, Guinto CD, Tecott LH, Garcia-Verdugo JM, Kriegstein A, Alvarez-Buylla A. 2014. Axonal control of the adult neural stem cell niche. Cell Stem Cell 14(4):500–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usunoff KG, Schmitt O, Itzev DE, Rolfs A, Wree A. 2007. Efferent connections of the parabigeminal nucleus to the amygdala and the superior colliculus in the rat: a double-labeling fluorescent retrograde tracing study. Brain Res 1133(1):87–91. [DOI] [PubMed] [Google Scholar]
- Veenvliet JV, Smidt MP. 2014. Molecular mechanisms of dopaminergic subset specification: fundamental aspects and clinical perspectives. Cell Mol Life Sci 71(24):4703–4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman KM, Lewandoski M, Campbell K, Joyner AL, Rubenstein JL, Martinez S, Martin GR. 1997. Specification of the anterior hindbrain and establishment of a normal mid/hindbrain organizer is dependent on Gbx2 gene function. Development 124(15):2923–2934. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Mihailoff GA, Baack JC, Woodward DJ. 1986. Topographical distribution of dorsal and median raphe neurons projecting to motor, sensorimotor, and visual cortical areas in the rat. J Comp Neurol 249(4):460–476, 478–481. [DOI] [PubMed] [Google Scholar]
- Watson C, Bartholomaeus C, Puelles L. 2019. Time for Radical Changes in Brain Stem Nomenclature-Applying the Lessons From Developmental Gene Patterns. Front Neuroanat 13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C, Shimogori T, Puelles L. 2017. Mouse Fgf8-Cre-LacZ lineage analysis defines the territory of the postnatal mammalian isthmus. J Comp Neurol 525(12):2782–2799. [DOI] [PubMed] [Google Scholar]
- Yang J, Brown A, Ellisor D, Paul E, Hagan N, Zervas M. 2013. Dynamic temporal requirement of Wnt1 in midbrain dopamine neuron development. Development 140(6):1342–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W, Shimamura K, Rubenstein JL, Hynes MA, Rosenthal A. 1998. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell 93(5):755–766. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available upon request from the corresponding author.


