Abstract
INTRODUCTION:
Binge drinking is a deadly pattern of alcohol abuse. Evidence suggests that genetic variation in clock genes is strongly associated with alcohol abuse, however, the neuroanatomical basis for such a relationship is unknown. The shell region of nucleus accumbens (NAcSh) is well-known for its role in binge drinking. Hence, we ask if clock genes in the NAcSh regulate binge drinking.
METHODS:
To address this question, two experiments were performed on male C57BL/6J mice. In the first experiment, mice, exposed to alcohol or sucrose under the 4-day drinking-in-the-dark (DID) paradigm, were euthanized at two different time-points on Day 4 [7 hours after light (pre-binge drinking) or dark (post-binge drinking) onset]. The brains were processed for RT-PCR to examine the expression of circadian clock genes (Clock, Per1, and Per2) in the NAcSh and suprachiasmatic nucleus (SCN). In the second experiment, mice were exposed to alcohol, sucrose or water as described above. On Day 4, one hour prior to the onset of alcohol exposure, mice were bilaterally infused with either a mixture of circadian clock genes antisense oligodeoxynucleotides (AS-ODNs; Antisense group) or nonsense/random ODNs (R-ODNs; Control group) through surgically implanted cannulas above the NAcSh. Alcohol/sucrose/water consumption was measured for 4 hours. Blood alcohol concentration was measured to confirm binge drinking. Microinfusion sites were histologically verified using cresyl violet staining.
RESULTS:
As compared to sucrose, mice euthanized post-binge drinking (not pre-binge drinking) on Day 4 displayed an increased expression of circadian genes in the NAcSh but not in the SCN. Knockdown of clock genes in the NAcSh caused a significant reduction in the amount of alcohol consumed on Day 4 as compared to the control treatment. No differences were found in sucrose and water consumption.
CONCLUSIONS:
Our results suggest that clock genes in the NAcSh play a crucial role in binge drinking.
Keywords: Clock, Per1, Per2, Nucleus accumbens, Binge drinking
INTRODUCTION
Binge drinking, a highly prevalent and harmful pattern of alcohol consumption, has serious health and economic consequences. According to NIAAA, binge drinking is a pattern of drinking that brings blood alcohol concentration (BAC) levels to 0.08 g/dl (Gowin et al., 2017). It is responsible for more than 50% of alcohol-related deaths with an economic burden of $191 billion in the United States (Sacks et al., 2015, Esser et al., 2014).
Clinical and preclinical studies suggest that genetic variation in clock genes is strongly associated with alcohol abuse (Logan et al., 2014). For example, single nucleotide polymorphism in clock genes [such as circadian locomotor output cycles kaput (Clock), period genes (Per1, Per2 and Per3)] is associated with alcohol use disorder (AUD) in humans (Spanagel et al., 2005b, Dong et al., 2011). In addition, shift workers and travelers moving across multiple time zones are at high risk for alcohol abuse (Trinkoff and Storr, 1998, Rogers and Reilly, 2002). Similarly, humans with an evening circadian preference or eveningness [preference for evening activities and for late bedtime (Preckel et al., 2020)] report greater alcohol consumption, especially binge drinking (Hasler and Clark, 2013, Prat and Adan, 2011). Higher amounts of alcohol consumption are observed in both men and women during the early evening (Gibson and Shirreffs, 2013).
Preclinical studies also suggest a role of circadian rhythm in alcohol consumption. For example, in rodents, circadian active (dark) period or experimental shift work is associated with increased alcohol consumption and/or increased Per1 expression in the brain (Gauvin et al., 1997, Gamsby and Gulick, 2015, Colombo et al., 2014, Resendiz-Flores and Escobar, 2019). Transgenic animals that display high alcohol consumption exhibit a disruption in Per2 expression in the nucleus accumbens (NAc) (Ruby et al., 2014, Gamsby and Gulick, 2015). Mice and rats selectively bred for high alcohol or binge-like alcohol consumption have altered circadian phenotypes (Hofstetter et al., 2003, McCulley et al., 2013, Rosenwasser et al., 2005). Overall, the above clinical and preclinical studies strongly suggest that circadian clock disruption increases alcohol drinking, however, very little is known about the neuroanatomical substrates mediating the effects of circadian clock genes on alcohol consumption.
There is strong and convincing evidence implicating the nucleus accumbens shell region (NAcSh) in binge alcohol consumption (Lei et al., 2019, Kasten and Boehm, 2014, Gimenez-Gomez et al., 2018, Cozzoli et al., 2012, Balla et al., 2018). Hence, we sought to determine if circadian genes in the NAcSh play a role in the regulation of binge drinking. We hypothesized that a) binge alcohol consumption is associated with an increase in the expression of major circadian genes (Clock, Per1 and Per2) in the NAcSh, and b) antisense-induced downregulation of these genes (Clock, Per1 and Per2) in the NAcSh will reduce binge drinking. To test our hypothesis, C57BL/6J mice were exposed to binge alcohol drinking under Drinking-in-the-Dark (DID) paradigm and the effect of binge alcohol consumption on the expression of circadian genes (Per1, Per2, and Clock) in the SCN and NAcSh was examined. Next, we determined the effect of antisense-induced downregulation of circadian genes in the NAcSh on binge drinking.
MATERIALS AND METHODS
Animals:
Male C57BL/6J mice (7 to 8 weeks old; RRID:IMSR_JAX:000664) were kept four per cage at the Harry S. Truman Memorial Veterans Hospital’s vivarium with an ambient room temperature (25 ± 2°C), reverse 12:12 hour light/dark cycle (lights on at 10:00 PM) and ad libitum access to food and water. Animals were allowed to recover from transportation stress and habituate to the new environment (including dark-light cycle) for at least two weeks before any experimental procedure. All experiments were in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care policies and the Guide for the Care and Use of Laboratory Animals. All protocols were approved by local committees (Subcommittee for Animals Safety Protocol #163).
Chemicals and Drugs:
A 20% alcohol solution (v/v) was prepared by mixing ethanol (EtOH; 200 proof; Fisher Scientific, Pittsburgh, PA) in tap water. A 10% solution (w/v) of sucrose (D-sucrose; Fisher Scientific) was prepared in tap water. The sequences for Clock, Per1 and Per2 antisense (Table 1) were obtained from previous studies (Sellix et al., 2006, Poletini et al., 2007). Phosphorothioate antisense (AS-ODNs) specific for 5’ transcription initiation site (5’INI) and 3’ cap site of Per1, Per2 and Clock mRNA and random sequence (RS-ODNs) were synthesized by Integrated DNA Technologies (Coralville, IA, USA). Once received, each of the AS or RS-ODNs were reconstituted to obtain a stock concentration (10X). All working solutions were prepared fresh before administration.
Table 1:
Antisense and random oligodeoxynucleotide (ODN) sequences
| Gene Accession # | ||
|---|---|---|
| Antisense Sequence | ||
| Per1 – 5’INI | CCT*TCTAGGGGACCACT*CAT | NM 011065 |
| Per1 – 3’CAP | GGT*GCTGTTTTCTTCTG*CAG | |
| Per2 – 5’INI | TAT*CCATTCATGTCGGG*CTC | NM 011066 |
| Per2 – 3’CAP | GAC*ACAAGCAGTCAAC*AAA | |
| Clock – 5’INI | CAG*CTTTACGGTAAACAA*CAT | NM 007715 |
| Clock – 3’CAP | AAG*GGTCAGTCAGGCT*GTC | |
| Random Sequence | ||
| Per1 – RS | GCT*CTGGTCTAGTACC*CTA | |
| Per2 – RS | ATC*TGCTACTAGGTTC*GTC | |
| Clock – RS | ACC*GTACTACTTCGGCT*GTC | |
Indicates phosphorothioate linkage within the oligonucleotide sequence. Per, Period gene; Clock, circadian locomotor output cycles kaput gene; INI, transcription start site.
Binge Drinking:
To facilitate binge drinking in non-stressful (home cage) environment, a well-established four-day drinking-in-the-dark (DID) procedure was used (Sharma et al., 2014a, Rhodes et al., 2005, Thiele and Navarro, 2014). In brief, starting 2.5 hours after dark onset, water bottles were removed from each mouse cage. 30 min later, 20% (v/v) alcohol or 10% (w/v) sucrose was dispensed in a pre-weighed bottle (identical to water-bottle) to each mouse in place of water-bottle. Sucrose was used as a control for the taste. Mice were allowed to consume alcohol (or sucrose/water) for 2 hours. On completion, alcohol/sucrose bottles were removed and weighed, followed by weighing the animals to calculate the amount of alcohol/sucrose consumed (g/kg or ml/kg of the bodyweight). Subsequently, mice were re-provided with the original water bottle and left undisturbed. The same procedure was repeated on Days 2, and 3.
On day 4, binge alcohol (or sucrose/water) consumption was performed as described above except the alcohol (or sucrose/water) exposure was continued for 4 hours.
Experimental Design:
All experiments were performed during the dark period between ZT14 (2 hours after dark onset) and ZT19 (7 hours after dark onset). To achieve rigor and reproducibility, all groups (controls and experimental) were run in parallel and repeated at least twice. Random group assignments were performed as follows: The animals were first numbered using ear tags and were randomly assigned to each group using the Online GraphPad randomization calculator (weblink: https://www.graphpad.com/quickcalcs/randomselect1/), just before beginning the experiment. In addition, the experimenters who examined binge drinking were blinded for antisense treatment. The data obtained from preliminary studies (N=3/group) was used to perform a priori power analysis [α =0.05; power ≥ 0.9; G*Power (Faul et al., 2007)] to calculate effect of and sample size.
Experiment 1:
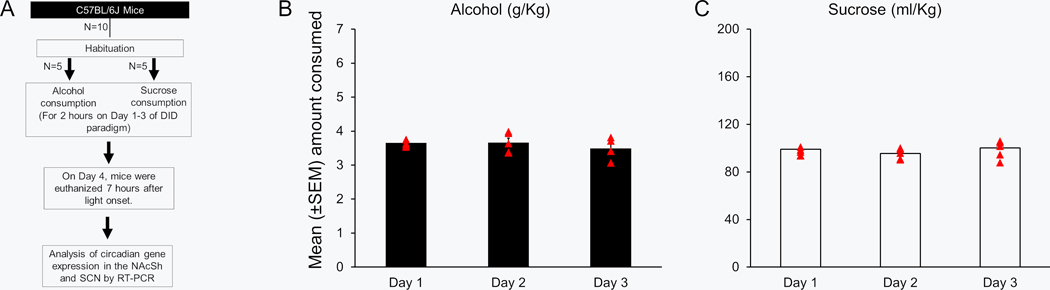
Effect of binge drinking on the expression of the circadian genes in the NAcSh and suprachiasmatic nucleus (SCN). In this experiment, animals (N = 20) were divided in two groups: Alcohol (N = 10) and Sucrose (N = 10). Using DID procedure (described above), animals were exposed to either alcohol (Alcohol group) or sucrose (Sucrose group) for three days. On Day 4, animals were euthanized by decapitation at two different time points: A) After 7 hours of light onset (8 hours prior to 4 hours of alcohol consumption; N = 5 mice/group; Figure 1A). B) After 7 hours of dark onset (immediately after 4 hours of alcohol consumption; N = 5 mice/group; Figure 3A). The brains were rapidly removed and placed into cryogenic vials containing embedding matrix (O.C.T. compound, Fisher Scientific) and snap-frozen in super-cooled isopentane, maintained on dry ice. Once frozen, the brains were stored at −80 °C until sectioning. The cryostat (Thermo Fisher Scientific, Kalamazoo, MI) was used to obtain the brain sections (0.5 mm thickness). Sections containing NAcSh and SCN were separated, mounted on a slide (SuperFrost Plus, Fisher Scientific). The NAcSh and SCN regions were punched (~500 μm) out using stainless steel metal tubes (23 G; Small Parts, Miami Lakes, FL) and placed into 100 μl RNALater™ (Sigma, St. Louis, MO), frozen on dry ice, and stored at −80 C. RNA isolation followed by RT-PCR was performed to examine the gene expression of Clock, Per1 and Per2 as previously described (Sharma et al., 2010, Sharma et al., 2018).
Figure 1:
The graphical timeline of the experimental procedure is described (Panel A). The amount of alcohol (Panel B; N = 5) and sucrose (Panel C; N = 5) consumption during 2 hours duration on Days 1 to 3 is described. AS-ODNs = Antisense oligodeoxynucleotides; R-ODNs = random oligodeoxynucleotides; NAcSh = Shell region of nucleus accumbens; SCN = Suprachiasmatic nucleus.
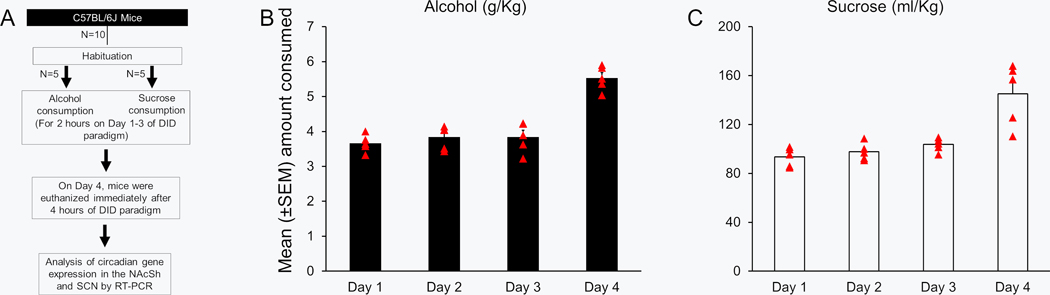
Figure 3:
The graphical timeline of the experimental procedure is described (Panel A). The amount of alcohol (Panel B; N = 5) and sucrose (Panel C; N = 5) consumption during 2 hours duration on Days 1 to 3 and 4 hours on Day 4 is described. AS-ODNs = Antisense oligodeoxynucleotides; R-ODNs = random oligodeoxynucleotides; NAcSh = Shell region of nucleus accumbens; SCN = Suprachiasmatic nucleus.
Experiment 2:
Effect of circadian genes knockdown in the NAcSh on binge alcohol drinking
Surgery:
Bilateral guide cannula were implanted as previously described (Thakkar et al., 2010, Sharma et al., 2014a, Sharma et al., 2015). Maintaining sterile conditions and under inhalation anesthesia (2% isoflurane), mice were stereotaxically implanted with bilateral stainless-steel guide cannulas (27 G), 2.0 mm above the NAcSh. The coordinates for the NAcSh were: Anteroposterior = +1.4 mm; Mediolateral = ±0.5 mm and dorsoventral = −4.8 mm, with reference to the bregma and the skull surface (Franklin and Paxinos, 2008). The guide cannulas were anchored to the skull via implantation of two stainless steel screws followed by covering the entire assembly with dental cement. A stylet (31-gauge stainless steel) was inserted in the guide cannulas to maintain patency. After surgery, mice were removed from stereotaxic frame and administered with flunixin (2.5 mg/kg/12 h for 1 day) to alleviate pain. The animals were then monitored until fully ambulatory (30–60 min). Each individual mouse was then housed singly in the experimental cage (similar to normal mouse cage with one grommeted hole on the shorter side for dispensing water/alcohol) and allowed to recover for 5 to 7 days.
Microinfusions:
The microinfusions were performed as described previously (Thakkar et al., 2010, Sharma et al., 2014a, Sharma et al., 2015). Briefly, each mouse was gently removed from the cage, held carefully and the stylus was removed. The injector cannula [connected to a 0.5 μl Hamilton syringe (Hamilton, Reno, NV) via FEP connector (Eicom, San Diego, CA)] was bilaterally inserted into the guide cannulas. Once the injector was in place, 300 nL of RS-ODNs or AS-ODNs was microinjected at a rate of 100 nL/min using an infusion pump (Pump 11 Pico Plus Elite; Harvard Apparatus, Holliston, MA). On completion, the injector cannulas were left in place for two more minutes before retracting. The entire microinfusion procedure was completed in approximately 5 minutes. On completion, the animal was returned to its cage.
Habituation to restrain, handling and microinfusions:
Binge drinking was performed as described above. To reduce handling stress caused due to microinfusions, mice were habituated to the bilateral microinfusions procedure by performing sham microinfusions on drinking days 1, 2, and 3. The sham microinfusions procedure was identical to the ODN microinfusions (described below) and performed at the same time (one hour before alcohol exposure) except that the sham-injector was shorter (remained 1.5 mm above the target site; to avoid damaging target sites) and no fluid was infused. On completion, the animal was returned to its cage.
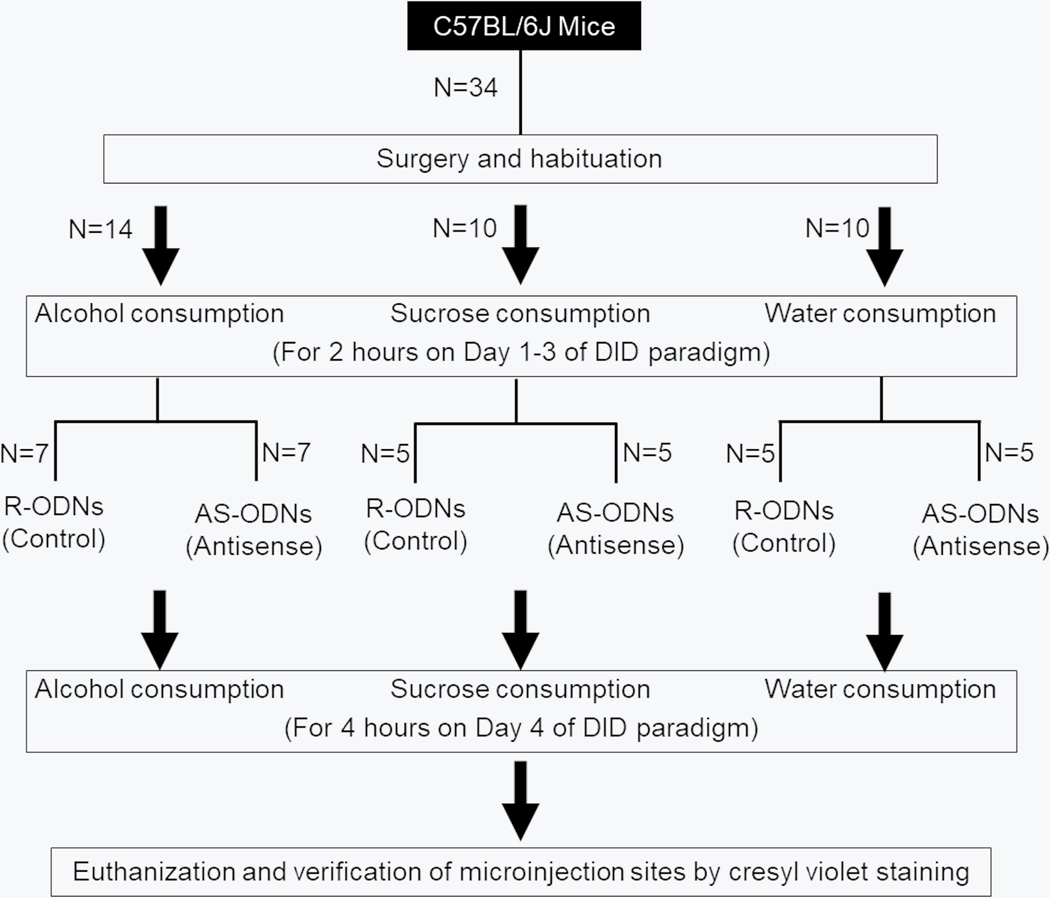
On day 4, one hour prior to initiating binge drinking, mice (N = 14) were randomly divided into two groups: Control (N = 7) and Antisense (N = 7). Mice in the Control group were bilaterally microinjected with RS-ODNs [300nl; 100nl (6 nmoles) each of Clock, Per1 and Per2], whereas mice in the Antisense group were bilaterally infused with a mixture of AS-ODNs [300nl; 100nl (6 nmoles) of Clock, Per1 and Per2 AS] into the NAcSh (details described below). Binge drinking was performed for 4 hours as described above.
Blood Alcohol Concentration:
Blood alcohol concentration (BAC) was measured immediately after 4 hours of alcohol consumption on Day 4 as described previously (Sharma et al., 2014b). Briefly, the mice were removed from their cages and a small amount (25 μl) of blood was collected from the tail vein and centrifuged to separate plasma. The BAC was measured from the plasma using an alcohol measurement kit as per the manufacturer’s instructions (Sekisui, Burlington, MA, USA).
In separate group of animals, the effects of circadian gene knock down was determined on sucrose (as a control for calorie and taste; N = 10; 5 mice/group) or water (as a control for general consummatory behavior; N = 10; 5 mice/group) consumption using the same DID paradigm as described above.
Localization of microinfusion sites:
After completion of the experiments, mice were deeply anesthetized using CO2 and then perfused transcardially with ice-cold normal saline followed by 10% buffered formalin (fixative; Fisher Scientific). The brains were removed, post-fixed with the same fixative overnight, immersed in 20% sucrose (w/v) in 0.1 M phosphate-buffered saline; pH 7.4) until equilibrated and serially sectioned (30 μm; three series) with a freezing microtome. One series was used for cresyl violet staining to localize the injection site in the NAcSh (Sharma et al., 2010, Sharma et al., 2014c).
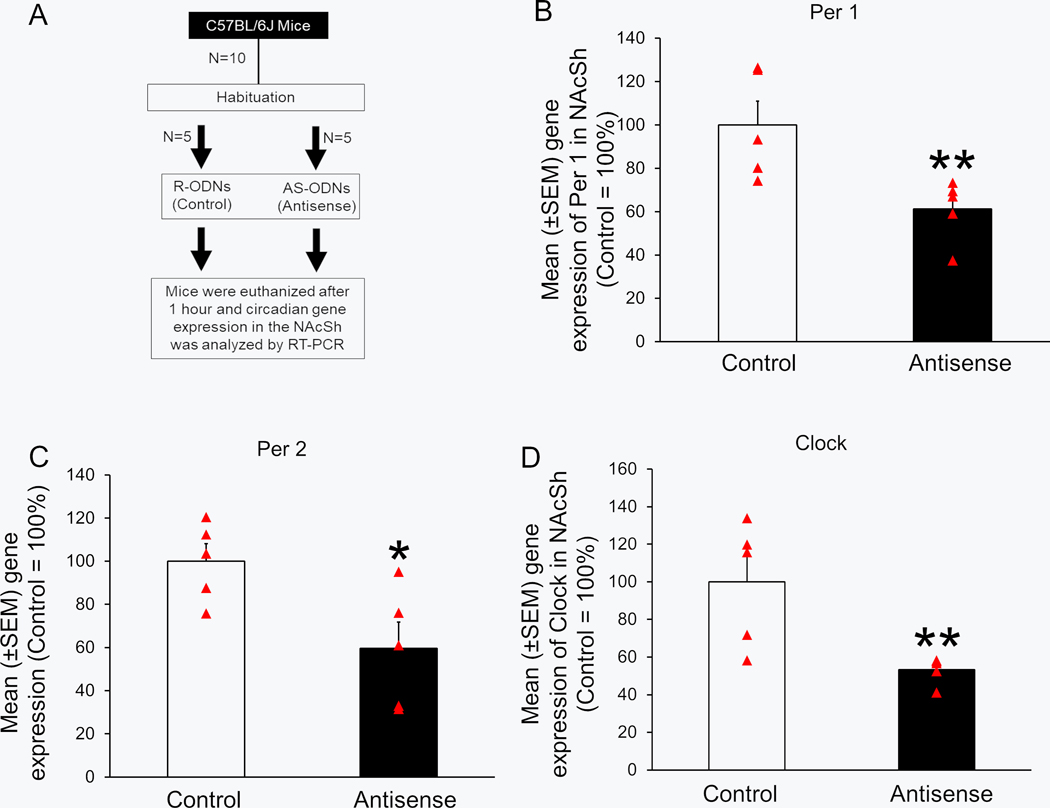
Next, we verified the downregulation of circadian clock genes expression in the NAcSh of animals administered with either RS-ODNs (Control; N = 5) or AS-ODNs (N = 5) in a separate group of animals. The animals (N = 10) were divided in two groups: Control (N = 5) and Antisense (N = 5). The experimental design is described in Figure 9A. Mice in the Control group were bilaterally microinjected with RS-ODNs [300nl; 100nl (6 nmoles) each of Clock, Per1 and Per2], whereas mice in the Antisense group were bilaterally infused with a mixture of AS-ODNs [300nl; 100nl (6 nmoles) of Clock, Per1 and Per2 AS] into the NAcSh as described above. After the completion of the injections in all animals, the animals were left undisturbed for one hour. Subsequently, mice were euthanized by decapitation, brains were processed to determine the gene expression of Clock, Per1 and Per2 as described above.
Figure 9:
Bilateral administration of a mixture of clock genes antisense (Antisense group; N = 5) significantly downregulated the expression of Per1 (Panel A), Per2 (Panel B) and Clock (Panel C) genes into the shell region of nucleus accumbens (NAcSh) as compared to the controls (N = 5). *P < 0.05 vs Control.
Statistics:
All statistical analysis were performed by GraphPad Prism Software (La Jolla, CA). The possible outlier was determined using the online Graphpad’s Outlier calculator (Grubb’s). Mann-Whitney U-test was performed to determine a) the effect of antisense-induced knockdown of clock genes on alcohol, water and sucrose consumption, b) antisense-induced downregulation of circadian genes in the NAcSh and c) binge drinking induced changes in the expression of circadian genes in the NAcSh and SCN. Kruskal-Wallis test with Dunn’s post hoc test was performed to determine the changes in alcohol/sucrose consumption during days 1 to 3 of DID paradigm. Friedman test with Dunn’s post hoc test was performed to determine differences in alcohol/sucrose/water consumption during days 1 to 3. The level of significance (α) was maintained at 0.05.
RESULTS
Experiment 1: Binge drinking is associated with an increase in the expression of major circadian genes in the NAcSh but not in the SCN.
Pre-drinking Day 4 (Light Period)
Alcohol/sucrose consumption:
No significant change was observed in alcohol consumption during Days 1 to 3 (p > 0.05; Figure 1B). No significant change was observed in sucrose consumption during three days (p > 0.05; Figure 1C).
NAcSh:
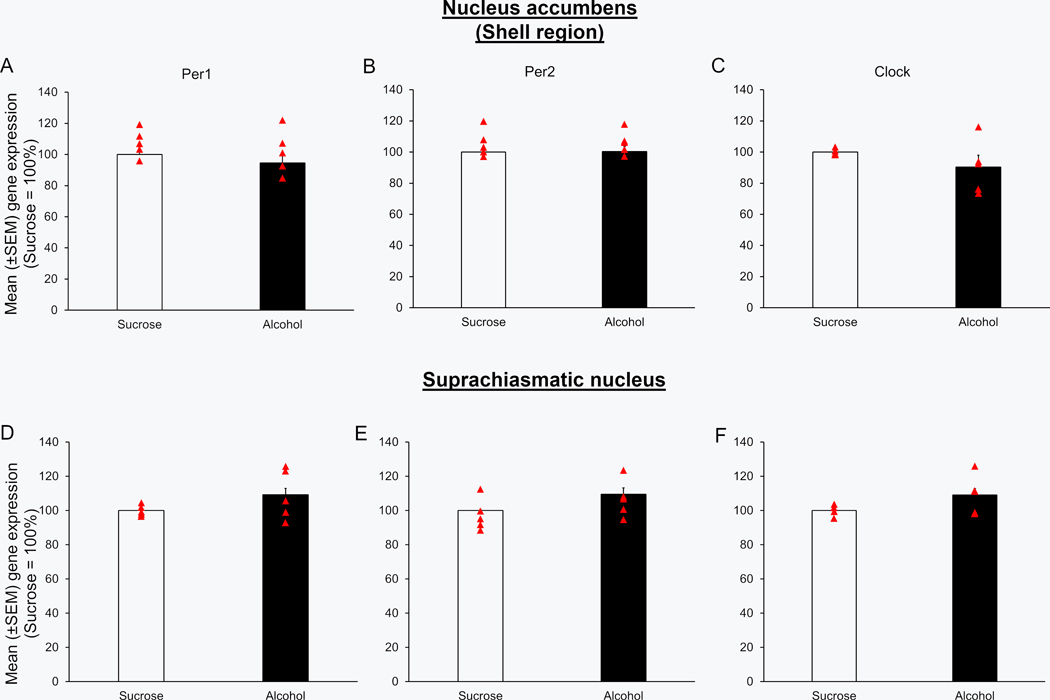
No significant change was observed in the expression of Per1 (U = 7.5; p > 0.05; Figure 2A), Per2 (U = 11.0; p > 0.05; Figure 2B) and Clock (U = 5.0; p > 0.05; Figure 2C) genes in the NAcSh of mice in the alcohol group (N = 5) as compared to mice in the Sucrose group (N = 5) during the light period prior to Day 4 alcohol consumption.
Figure 2:
The circadian genes expression was normal during the light period on Day 4 of DID paradigm. Mice exposed to three days of binge alcohol consumption (N = 5; euthanized 7 hours after the light onset on Day 4 of binge drinking) displayed no significant changes in the expression of Per1 (Panel A & D), Per2 (Panel B & E) and Clock (Panel C & F) genes into the shell region of nucleus accumbens (NAcSh) and suprachiasmatic nucleus (SCN) as compared to the mice exposed to two hours of sucrose consumption on Days 1 to 3 (N = 5).
SCN:
No significant change was observed in the expression of Per1 (U = 8.5; p > 0.05; Figure 2D), Per2 (U = 8.0; p > 0.05; Figure 2E) and Clock (U = 5.0; p > 0.05; Figure 2F) genes in the NAcSh of mice in the alcohol group (N = 5) as compared to mice in the Sucrose group (N = 5) during the light period prior to Day 4 alcohol consumption.
Post-drinking Day 4 (Dark Period)
Alcohol/sucrose consumption:
No significant change was observed in alcohol consumption during 2 hours on Days 1 to 3 (p > 0.05; Figure 3B). No significant change was observed in sucrose consumption during 2 hours on Days 1 to 3 (p > 0.05; Figure 3C). The BAC level at the end of alcohol drinking session (4 hours) on Day 4 was 108.8 ± 7.84 mg/dl (N = 5) in mice exposed to alcohol suggesting binge drinking in this group of mice.
NAcSh:
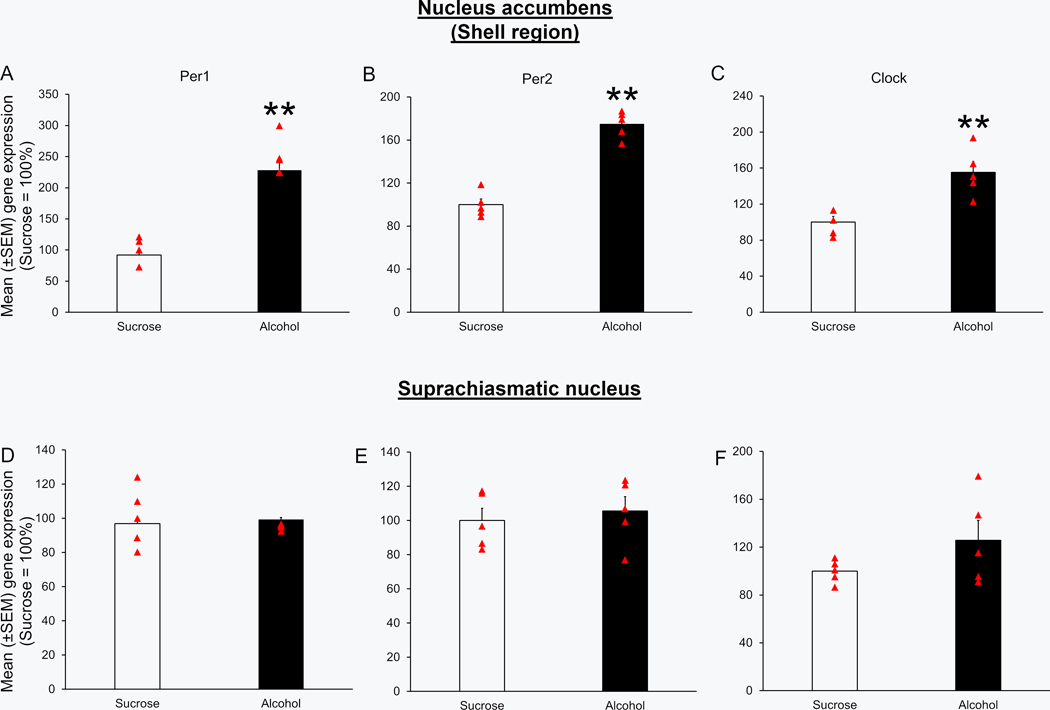
Mice in the alcohol group (N = 5) showed a significant increase in the expression of Per1 (U = 0.0; p < 0.01; Figure 4A), Per2 (U = 0.0; p < 0.01; Figure 4B) and clock (U = 0.0; p < 0.01; Figure 4C) genes in the NAcSh as compared to mice in the Sucrose group (N = 5).
Figure 4:
Alcohol consumption on Day 4 was associated with an increase in the expression of circadian genes in the NAcSh. Mice exposed to four days of binge alcohol consumption (N = 5) displayed a significant increase in the expression of Per1 (Panel A & D), Per2 (Panel B & E) and Clock (Panel C & F) genes into the nucleus accumbens (NAcSh) and suprachiasmatic nucleus (SCN) as compared to the mice exposed to sucrose (N = 5). **p < 0.01 and ***p<0.001 vs Sucrose.
SCN:
No significant difference was observed in the expression of Per1 (U = 11.0; p > 0.05; Figure 4D), Per2 (U = 9.0; p > 0.05; Figure 4E) and clock (U = 8.0; p > 0.05; Figure 4F) genes in the SCN of mice exposed to binge alcohol consumption (N = 5) as compared to mice in the Sucrose group (N = 5).
Experiment 2:
Knockdown of major circadian genes in the NAcSh reduces binge drinking in mice.
A. Statistical power and Sample Size.
G*Power analysis (t-test; α = 0.05; power = 0.95) conducted after preliminary experiments suggested a total of 10 mice (5/Group) with an effect size of 2.7. The experimental design is described in Figure 5.
Figure 5:
Effects of circadian clock genes antisense into the shell region of nucleus accumbens on alcohol consumption. The graphical timeline is described. AS-ODNs = Antisense oligodeoxynucleotides; R-ODNs = random oligodeoxynucleotides; NAcSh = Shell region of nucleus accumbens; SCN = Suprachiasmatic nucleus.
B. Knockdown of clock genes in the NAcSh reduced binge alcohol consumption.
Localization of injection sites:
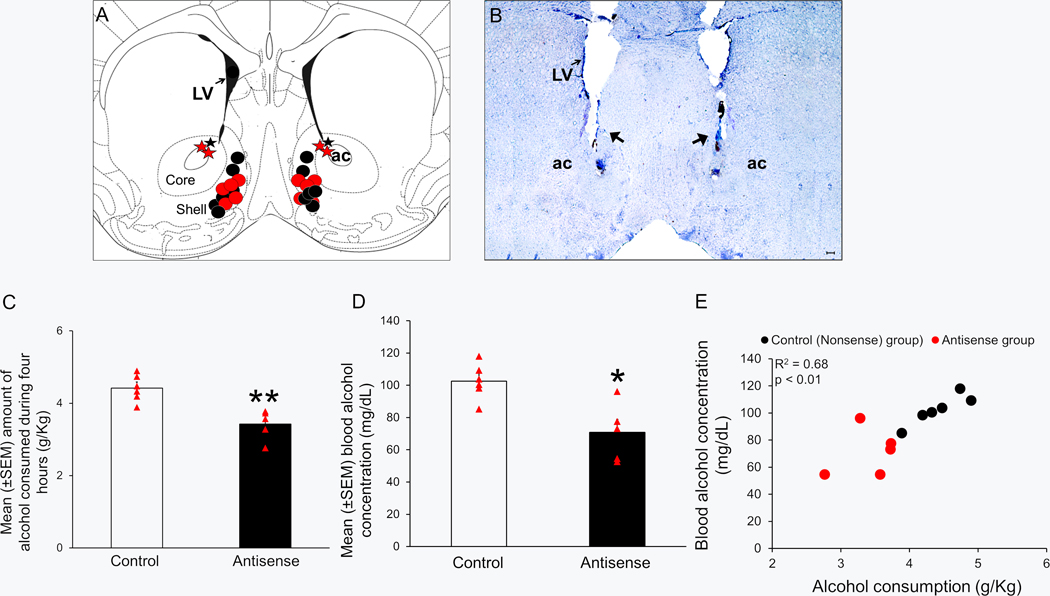
A total of 14 mice were used in this study. All bilateral microinfusion sites (N = 14) are described in a single coronal schematic (AP = 1.2) in Figure 6A [adapted from figure 21; see Franklin and Paxinos (2008)]. Histological analysis revealed that while microinfusion sites in 11 animals (5 in Antisense group denoted by red circles; 6 in Control group denoted by black circles) were on target and localized in the medial NAcSh region [between AP = 1.5 and 1.1], injection sites in three mice (two in Antisense group, denoted by the red star; one in Control group, denoted by the black star) were off-target. The data from these three “off-target animals” were excluded from analysis but described in Table 2. A representative photomicrograph depicting the bilateral injection sites in the NAcSh in Figure 6B.
Figure 6:
Bilateral infusion of a mixture of clock genes antisense into the shell region of nucleus accumbens (NAcSh) attenuates binge drinking.
Panel A: Bilateral localization of the target sites in the NAcSh (localized between AP levels 1.5 and 1.1) of the mice infused with a mixture of clock genes antisense oligodeoxynucleotides (Antisense Group; N = 5; Red circles) and random-oligodeoxynucleotides (Control Group; N = 6; black circles) is shown in one coronal schematic (Figure 21, AP = 1.2 mm, adapted from Franklin and Paxinos, (2008). Missed target sites are described as red (Antisense Group) and black (Control Group) stars. ac = anterior commissure, LV = lateral ventricles, AP = anteroposterior.
Panel B: A representative photomicrograph illustrating bilateral lesions (black arrows) caused by microinjector cannula in the shell region of nucleus accumbens. ac = anterior commissure, LV = lateral ventricles. Scale bar = 100 μm.
Panel C: A graphical representation showing that as compared to the Controls (N = 6), mice infused with a mixture of clock genes antisense oligodeoxynucleotide (Antisense group; N = 5), bilaterally in the NAcSh, displayed a significant reduction in alcohol consumption during four hours of binge drinking period on day 4 of drinking-in-the-dark paradigm. **P < 0.01.
Panel D: A graph showing that as compared to the Controls (N = 6), mice infused with a mixture of clock genes antisense oligodeoxynucleotide (Antisense group; N = 5) bilaterally in the NAcSh displayed a significant reduction in blood alcohol concentration measured at the end of four hours of alcohol consumption on day 4 of drinking-in-the-dark paradigm. **P < 0.01 vs Control.
Panel E: A significant correlation (R2 = 0.68; p < 0.01) was observed between the amount of alcohol consumption (g/kg) and the blood alcohol concentration (mg/dl) during 4 hours of DID paradigm on Day 4.
Table 2:
Alcohol consumption during four days of Drinking-in-the-Dark procedure in each animal with off-target microinfusion sites.
| Group | Day 1 | Day 2 | Day 3 | Day 4 |
|---|---|---|---|---|
| Control (N =1) | 2.81 | 2.63 | 2.81 | 4.56 |
| Antisense (N = 2) | 2.67 | 2.15 | 2.84 | 4.74 |
| 2.39 | 2.65 | 2.74 | 4.36 |
The microinfusions (targeted towards shell region of nucleus accumbens) were performed on Day 4, one hour prior to alcohol exposure. Control = Random oligodeoxynucleotides were infused; Antisense = Antisense oligodeoxynucleotides were infused.
Alcohol consumption:
Alcohol consumption, during 2 hours of exposure, on days 1, 2 and 3, was comparable between Control and Antisense groups as there was no significant (p > 0.05) main effect of treatment observed. The mean ± SEM alcohol consumption on Days 1, 2 and 3 was 2.50 ± 0.23, 2.61 ± 0.13 and 2.81 ± 0.13 g/Kg in mice in the Antisense group whereas 2.71 ± 0.14, 2.49 ± 0.23 and 2.99 ± 0.08 g/Kg in mice in the Control group. However, on day 4, during 4 hours of alcohol exposure, as compared to mice in the Control group (4.42 ± 0.16 g/Kg; N = 6), mice in the Antisense group (3.41 ± 0.18 g/Kg; N = 5) displayed a significant (U = 0.0; p < 0.01) reduction in the amount of alcohol consumed (Figure 6C).
BAC:
As compared to the Control group (102.5 ± 4.9 mg/dl; N = 6), BAC was significantly (U = 1.0; p < 0.05) reduced in Antisense group (71.2 ± 7.8 mg/dl; N = 5) suggesting that the reduced binge drinking due to clock genes knockdown in the NAcSh is not due to altered alcohol metabolism (Figure 6D). The BAC levels were significantly (Pearson r = 0.90; p < 0.001) correlated with the amount of alcohol consumption on day 4 (Figure 6E).
C. Knockdown of major clock genes in the NAcSh had no effect on sucrose consumption
Localization of injection sites:
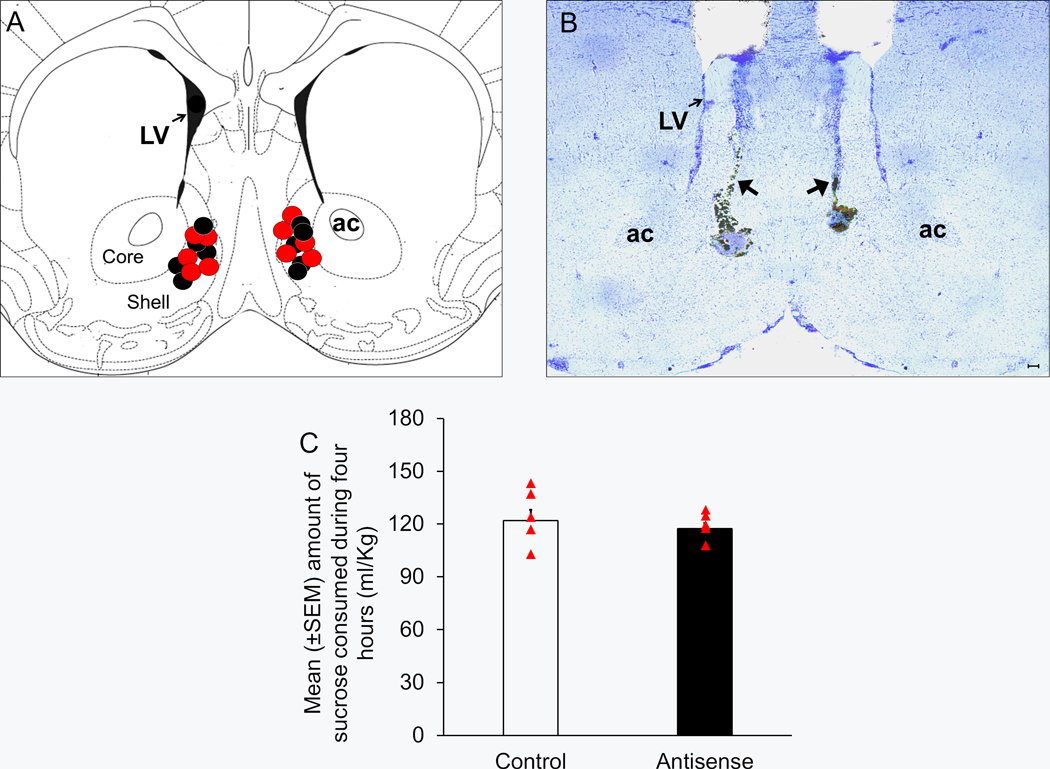
All bilateral microinfusion sites [N = 10 (5/group); indicated as black (Control) and red (Antisense) circles] are described in a single coronal schematic (AP = 1.2) in Figure 7A [adapted from figure 21; Franklin and Paxinos (2008)]. A representative photomicrograph depicting bilateral injection sites in the NAcSh is shown in Figure 7B.
Figure 7:
Bilateral infusion of a mixture of clock genes antisense oligodeoxynucleotide into the shell region of nucleus accumbens (NAcSh) did not produce any significant effect on sucrose consumption.
Panel A: A Coronal schematic [Figure 21, AP = 1.2 mm, adapted from Franklin and Paxinos, (2008)] showing target sites of the bilateral microinjector cannulas localized in the NAcSh (localized between anteroposterior levels 1.5 and 1.1) of the mice infused with antisense oligodeoxynucleotides (Antisense Group; N = 5; Red circles) and random oligodeoxynucleotides (Control Group; N = 5; black circles). ac = anterior commissure, LV = lateral ventricles, AP = antero-posterior.
Panel B: A representative photomicrograph showing lesions caused by bilateral microinfusions (black arrows) in the NAcSh. ac = anterior commissure, LV = lateral ventricles. Scale bar = 100 μm.
Panel C: A graphical representation depicting no significant change in sucrose consumption during four hours on Day 4 of drinking-in-the-dark paradigm in mice infused with a mixture of clock genes antisense oligodeoxynucleotide into the NAcSh (Antisense group; N = 5) as compared to the Controls (N = 5).
Sucrose consumption:
No significant main effect of treatment (p > 0.05) was observed on sucrose consumption on Days 1, 2 and 3 (Data not shown). Also, on Day 4, there was no significant (U = 11.0; p > 0.05) difference in the sucrose consumption between Antisense (117.3 ± 3.0 ml/Kg; N = 5) and Control (121.9 ± 6.3 ml/Kg; N = 5) groups (Figure 7C).
D. Knockdown of major clock genes in the NAcSh had no effect on water consumption
Localization of injection sites:
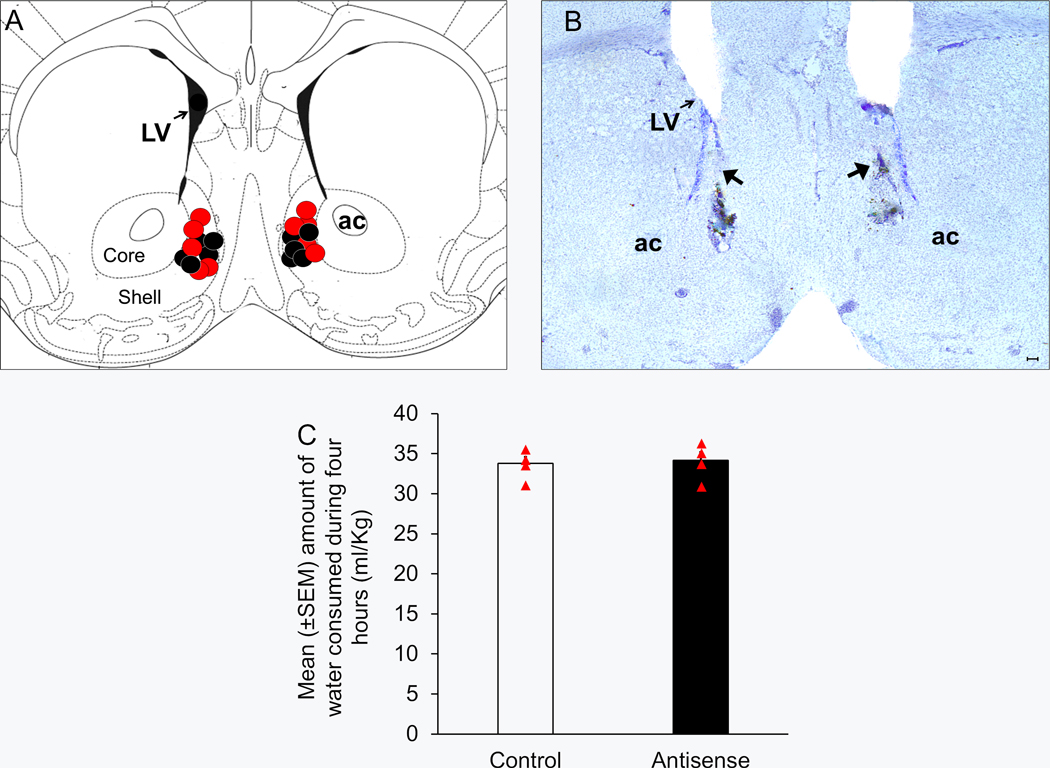
All bilateral microinfusion sites (N = 10 (5 mice/group); indicated as circles) are described in a single coronal schematic (AP = 1.2) in Figure 8A [adapted from figure 21; see Franklin and Paxinos (2008)]. A representative photomicrograph depicting the bilateral injection sites in the NAcSh is shown in Figure 8B.
Figure 8:
Bilateral administration of a mixture of clock genes antisense into the shell region of nucleus accumbens (NAcSh) did not produce any significant change on water consumption.
Panel A: The target sites of the bilateral microinjector cannulas localized in the NAcSh (localized between anteroposterior levels 1.5 and 1.1) of the mice infused with antisense oligodeoxynucleotide (Antisense Group; N = 5; Red circles) and random-ODNs (Control Group; N = 5; black circles) is shown in one coronal schematic [Figure 21, AP = 1.2 mm, adapted from Franklin and Paxinos, (2008)]. ac = anterior commissure, LV = lateral ventricles, AP = anteroposterior.
Panel B: A representative photomicrograph showing lesions caused by bilateral microinfusion (black arrows) in the NAcSh. ac = anterior commissure, LV = lateral ventricles. Scale bar = 100 μm.
Panel C: A graphical representation suggesting no significant change in water consumption in mice infused with a mixture of clock genes antisense oligodeoxynucleotide into the NAcSh (Antisense group; N = 5) as compared to the controls (N = 5).
Water consumption:
No significant main effect of treatment (p > 0.05) was observed on water consumption on Days 1, 2 and 3 (Data not shown). Also, on Day 4, no significant (U = 9.5; p > 0.05) difference in the water consumption between Antisense (34.2 ± 1.1 ml/Kg; N = 5) and Control (33.8 ± 0.9 ml/Kg; N = 5) groups was observed (Figure 8C).
E. Infusion of major clock gene antisense downregulates clock genes expression in the NAcSh.
Gene expression:
Mice in the Antisense group (N = 5) showed a significant downregulation of Per1 (U = 0.0; p < 0.01; Figure 9B), Per2 (U = 2.0; p < 0.05; Figure 9C) and clock (U = 0.0; p < 0.01; Figure 9D) genes as compared to mice in the Control group (N = 5).
DISCUSSION
This is the first study to show that a) mice exposed to 4-Day DID protocol displayed a significant increase in the expression of circadian clock genes (Clock. Per1 and Per2) in the NAcSh on Day 4 after four hours of alcohol consumption while no changes were observed in the SCN and b) antisense-induced downregulation of clock genes in the NAcSh caused a significant reduction in the binge alcohol administration in C57BL/6J mice without affecting sucrose (intake of calorie or tastant) and water (general consummatory behavior) intake.
A ‘binge’ is defined as a risky pattern of alcohol consumption in humans that produces BAC greater than 0.08% (80 mg/dL) within a few hours (NIAAA, 2004). To investigate the role of circadian genes in the NAcSh in the regulation of binge drinking, we used the DID paradigm in C57BL/6J mice, a very well-known model with a high face validity in terms of mimicking human binge drinking. This method involves daily access to 20% alcohol, for a limited duration and selectively during the dark (active) phase of the circadian light cycle. In this paradigm, C57BL/6J mice consume large amounts of alcohol voluntarily, without any prior training, resulting in a pharmacologically relevant BAC within a short period of time. Moreover, the DID method is an ideal tool for studying the neurobiology underlying binge drinking (Rhodes et al., 2005, Rhodes et al., 2007, Sprow and Thiele, 2012, Thiele and Navarro, 2014).
The NAc is a terminal reward center of mesolimbic dopaminergic reward circuitry which originates from the ventral tegmental area (VTA) (Blum et al., 2012). Anatomically, the NAc consists of the central core (NAcC) and the NAcSh regions. The NAcSh is considered a part of the extended amygdala. It has a major role in motivation for rewarding stimuli and facilitates reinforcement of rewards, whereas the NAcC resembles the dorsal striatum and has a critical role in cognitive processing of motor function (McBride et al., 1999, Clarke and Adermark, 2015, Di Chiara, 2002). Within the NAcSh, the medial NAcSh plays a crucial role in binge drinking (Lei et al., 2019, Kasten and Boehm, 2014, Gimenez-Gomez et al., 2018, Cozzoli et al., 2012, Balla et al., 2018). In addition, neuronal activation along with the expression of clock genes and markers of dopaminergic activity (tyrosine hydroxylase and dopamine transporters) display diurnal rhythm in the NAcSh with peaks during the active (dark) period (Baltazar et al., 2013, Sleipness et al., 2007, Webb et al., 2009, Li et al., 2009, Falcon et al., 2013).
As described in the introduction, strong evidence from clinical studies clearly suggests that circadian genes are associated with hazardous or binge drinking. However, the causal relationship is not well understood. We hypothesized that the downregulation of major clock genes (Clock, Per1 and Per2) expression in the NAcSh will reduce binge alcohol drinking in mice. Our hypothesis was based on previous observations suggesting that C57BL/6J mice exhibit an association between increased clock genes expression in the NAc and binge drinking during the dark (active) period (Thiele and Navarro, 2014, Rhodes et al., 2005, Falcon et al., 2013, Li et al., 2009).
Although the suprachiasmatic nucleus is the master clock, the rhythmicity of circadian oscillations and its association with clock genes (Clock, Per1 and Per2) expression is present in every cell, including in the neurons of the NAcSh (Parekh et al., 2015). Thus, to test our hypothesis, we used the antisense technology to downregulate clock genes in the medial NAcSh. Previous studies have demonstrated that infusion of AS ODNs against Clock, Per1 and Per2 genes in the suprachiasmatic nucleus or lateral ventricles attenuated their expression and disrupted the associated circadian functions within a relatively short time (Akiyama et al., 1999, Poletini et al., 2007, Sellix et al., 2006).
The result of our study suggests that binge drinking was significantly reduced in animals following antisense-induced downregulation of clock genes in the NAcSh suggesting that a causal relationship exist between circadian genes in the NAcSh and binge drinking in C57BL/6J mice. Supporting our results, previous study has demonstrated that knockdown of Per2 gene selectively in the dorsal and ventral striatum reduced alcohol consumption (Zavalia et al., 2020). Additionally, the involvement of these circadian genes was also investigated in regards to other substance of abuse. For example, the Clock (in the VTA, but not in the NAc) and NPAS2, functionally similar to clock (in the NAc), have been shown to mediate the rewarding effects of drugs such as cocaine (Parekh et al., 2015). It is becoming increasingly evident that alcohol consumption, both in humans and laboratory rodents may be regulated by clock genes (Clock, Bmal1/Arntl, Per1 or Per2) (Kovanen et al., 2010, Dong et al., 2011, Spanagel et al., 2005a, Ozburn et al., 2013). While the majority of rodent studies have used constitutive global knockouts, we have used a restricted approach “antisense-induced selectively knockdown of clock genes in the medial NAcSh region”. Thus, while constitutive knockout may affect central circadian functioning (altered sleep-wake regulation, glutamatergic levels and/or stress-induced cortisol levels), our approach selectively attenuated the expression of clock genes in the medial region of the NAcSh (Shiromani et al., 2004, Spanagel et al., 2005a). Interestingly, we also found that the expression of circadian genes (Clock, Per1 and Per2) was upregulated after binge drinking in the NAcSh, but not in the SCN, on Day 4. Additionally, we did not find any significant change in the expression of any of the circadian gene in either NAcSh or SCN of mice (exposed to 4-day DID protocol) euthanized in the light period (ZT = 7), 8 hours prior to Day 4 of binge drinking which further supports our hypothesis that these circadian genes are crucial for binge drinking in mice exposed to DID paradigm. Similarly, majority of rodent studies investigating the role of circadian genes in alcohol consumption have used the circadian neutral, 24 hours, continuous access, alcohol consumption paradigm. In contrast, the current study examined the effects of acute knockdown of Clock, Per1, and Per2 genes in the NAcSh on binge drinking during the dark period, the circadian active period, when mice are known to consume a high amount of alcohol (Resendiz-Flores and Escobar, 2019). The data obtained from animals with missed target sites (localized in the core region with small Ns) suggest that the effects of clock genes knockdown on binge drinking are specific for NAcSh region.
One limitation of the study is that we did not monitor locomotor activity because previous studies have shown that knock-down of clock genes in the NAc did not produce any effect on locomotor activity (Ozburn et al., 2015). Furthermore, it is unlikely that reduction in alcohol consumption after antisense injection in the NAcSh is due to reduced activity since antisense treatment did not reduce water and sucrose consumption. In addition, since Clock is a positive regulator of the circadian molecular loop while Per is a negative regulator, their individual involvement in the regulation of binge drinking warrants further investigations.
In summary, our study is the first to show that acute knockdown of clock genes in the NAcSh, significantly reduced binge alcohol administration in C57BL/6J mice suggesting a positive influence of these clock genes in the NAcSh on binge drinking.
ACKNOWLEDGEMENTS:
This work was supported by grants from the Department of Veterans Affairs Merit Research Award (I01BX002661) and NIH (AA028175–01). The authors would like to thank Research Services, Harry S. Truman Memorial Veterans Hospital for excellent research facilities; Robert Crawford and Jennifer Couch for administrative support; Carrie Harris for animal care; Abhilasha Sharma, Imran Rice, Jacques Du Plessis and Dylan Clemmons for their help with experiments.
Footnotes
CONFLICT OF INTERESTS: None
REFERENCES
- Akiyama M, Kouzu Y, Takahashi S, Wakamatsu H, Moriya T, Maetani M, Watanabe S, Tei H, Sakaki Y, Shibata S (1999) Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J Neurosci 19:1115–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla A, Dong B, Shilpa BM, Vemuri K, Makriyannis A, Pandey SC, Sershen H, Suckow RF, Vinod KY (2018) Cannabinoid-1 receptor neutral antagonist reduces binge-like alcohol consumption and alcohol-induced accumbal dopaminergic signaling. Neuropharmacology 131:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltazar RM, Coolen LM, Webb IC (2013) Diurnal rhythms in neural activation in the mesolimbic reward system: critical role of the medial prefrontal cortex. Eur J Neurosci 38:2319–2327. [DOI] [PubMed] [Google Scholar]
- Blum K, Gardner E, Oscar-Berman M, Gold M (2012) “Liking” and “wanting” linked to Reward Deficiency Syndrome (RDS): hypothesizing differential responsivity in brain reward circuitry. Curr Pharm Des 18:113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R, Adermark L (2015) Dopaminergic Regulation of Striatal Interneurons in Reward and Addiction: Focus on Alcohol. Neural Plasticity 2015:814567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Maccioni P, Acciaro C, Lobina C, Loi B, Zaru A, Carai MA, Gessa GL (2014) Binge drinking in alcohol-preferring sP rats at the end of the nocturnal period. Alcohol 48:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Caruana AL, Miller BW, Greentree DI, Thompson AB, Wroten MG, Zhang PW, Xiao B, Hu JH, Klugmann M, Metten P, Worley PF, Crabbe JC, Szumlinski KK (2012) Nucleus accumbens mGluR5-associated signaling regulates binge alcohol drinking under drinking-in-the-dark procedures. Alcohol Clin. Exp Res 36:1623–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G (2002) Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res 137:75–114. [DOI] [PubMed] [Google Scholar]
- Dong L, Bilbao A, Laucht M, Henriksson R, Yakovleva T, Ridinger M, Desrivieres S, Clarke TK, Lourdusamy A, Smolka MN, Cichon S, Blomeyer D, Treutlein J, Perreau-Lenz S, Witt S, Leonardi-Essmann F, Wodarz N, Zill P, Soyka M, Albrecht U, Rietschel M, Lathrop M, Bakalkin G, Spanagel R, Schumann G (2011) Effects of the circadian rhythm gene period 1 (per1) on psychosocial stress-induced alcohol drinking. The American journal of psychiatry 168:1090–1098. [DOI] [PubMed] [Google Scholar]
- Esser MB, Hedden SL, Kanny D, Brewer RD, Gfroerer JC, Naimi TS (2014) Prevalence of alcohol dependence among US adult drinkers, 2009–2011. Prev Chronic Dis 11:E206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon E, Ozburn A, Mukherjee S, Roybal K, McClung CA (2013) Differential regulation of the period genes in striatal regions following cocaine exposure. PLoS One 8:e66438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior research methods 39:175–191. [DOI] [PubMed] [Google Scholar]
- Franklin KB, Paxinos G (2008) The mouse brain in stereotaxic coordinates. 3 ed., Academic Press, New York, NY. [Google Scholar]
- Gamsby JJ, Gulick D (2015) Chronic shifts in the length and phase of the light cycle increase intermittent alcohol drinking in C57BL/6J mice. Front Behav Neurosci 9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvin DV, Baird TJ, Vanecek SA, Briscoe RJ, Vallett M, Holloway FA (1997) Effects of time-of-day and photoperiod phase shifts on voluntary ethanol consumption in rats. Alcohol Clin Exp Res 21:817–825. [PubMed] [Google Scholar]
- Gibson S, Shirreffs SM (2013) Beverage consumption habits “24/7” among British adults: association with total water intake and energy intake. Nutr J 12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Gomez P, Perez-Hernandez M, Gutierrez-Lopez MD, Vidal R, Abuin-Martinez C, O’Shea E, Colado MI (2018) Increasing kynurenine brain levels reduces ethanol consumption in mice by inhibiting dopamine release in nucleus accumbens. Neuropharmacology 135:581–591. [DOI] [PubMed] [Google Scholar]
- Gowin JL, Sloan ME, Stangl BL, Vatsalya V, Ramchandani VA (2017) Vulnerability for Alcohol Use Disorder and Rate of Alcohol Consumption. The American journal of psychiatry 174:1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Clark DB (2013) Circadian misalignment, reward-related brain function, and adolescent alcohol involvement. Alcohol Clin Exp Res 37:558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter JR, Grahame NJ, Mayeda AR (2003) Circadian activity rhythms in high-alcohol-preferring and low-alcohol-preferring mice. Alcohol 30:81–85. [DOI] [PubMed] [Google Scholar]
- Kasten CR, Boehm SL 2nd, (2014) Intra-nucleus accumbens shell injections of R(+)- and S(−)-baclofen bidirectionally alter binge-like ethanol, but not saccharin, intake in C57Bl/6J mice. Behav Brain Res 272:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanen L, Saarikoski ST, Haukka J, Pirkola S, Aromaa A, Lonnqvist J, Partonen T (2010) Circadian clock gene polymorphisms in alcohol use disorders and alcohol consumption. Alcohol Alcohol 45:303–311. [DOI] [PubMed] [Google Scholar]
- Lei K, Kwok C, Darevsky D, Wegner SA, Yu J, Nakayama L, Pedrozo V, Anderson L, Ghotra S, Fouad M, Hopf FW (2019) Nucleus Accumbens Shell Orexin-1 Receptors Are Critical Mediators of Binge Intake in Excessive-Drinking Individuals. Front Neurosci 13:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SX, Liu LJ, Jiang WG, Lu L (2009) Morphine withdrawal produces circadian rhythm alterations of clock genes in mesolimbic brain areas and peripheral blood mononuclear cells in rats. J Neurochem 109:1668–1679. [DOI] [PubMed] [Google Scholar]
- Logan RW, Williams WP, McClung CA (2014) Circadian rhythms and addiction: Mechanistic insights and future directions. Behav. Neurosci 128:387–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Ikemoto S (1999) Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav. Brain Res 101:129–152. [DOI] [PubMed] [Google Scholar]
- McCulley WD 3rd, , Ascheid S, Crabbe JC, Rosenwasser AM (2013) Selective breeding for ethanol-related traits alters circadian phenotype. Alcohol 47:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA (2004) NIAAA council approves definition of binge drinking, in Series NIAAA council approves definition of binge drinking, pp 3. [Google Scholar]
- Ozburn AR, Falcon E, Mukherjee S, Gillman A, Arey R, Spencer S, McClung CA (2013) The role of clock in ethanol-related behaviors. Neuropsychopharmacology 38:2393–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozburn AR, Falcon E, Twaddle A, Nugent AL, Gillman AG, Spencer SM, Arey RN, Mukherjee S, Lyons-Weiler J, Self DW, McClung CA (2015) Direct regulation of diurnal Drd3 expression and cocaine reward by NPAS2. Biol Psychiatry 77:425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh PK, Ozburn AR, McClung CA (2015) Circadian clock genes: effects on dopamine, reward and addiction. Alcohol (Fayetteville, N.Y.) 49:341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletini MO, McKee DT, Kennett JE, Doster J, Freeman ME (2007) Knockdown of clock genes in the suprachiasmatic nucleus blocks prolactin surges and alters FRA expression in the locus coeruleus of female rats. Am J Physiol Endocrinol Metab 293:E1325–1334. [DOI] [PubMed] [Google Scholar]
- Prat G, Adan A (2011) Influence of circadian typology on drug consumption, hazardous alcohol use, and hangover symptoms. Chronobiol Int 28:248–257. [DOI] [PubMed] [Google Scholar]
- Preckel F, Fischbach A, Scherrer V, Brunner M, Ugen S, Lipnevich AA, Roberts RD (2020) Circadian preference as a typology: Latent-class analysis of adolescents’ morningness/eveningness, relation with sleep behavior, and with academic outcomes. Learning and Individual Differences 78:101725. [Google Scholar]
- Resendiz-Flores M, Escobar C (2019) Circadian disruption favors alcohol consumption and differential DeltaFosB accumulation in Corticolimbic structures. Addict Biol 24:1179–1190. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC (2005) Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav 84:53–63. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T Jr., , Crabbe JC (2007) Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav 6:1–18. [DOI] [PubMed] [Google Scholar]
- Rogers HL, Reilly SM (2002) A survey of the health experiences of international business travelers. Part One--Physiological aspects. AAOHN J 50:449–459. [PubMed] [Google Scholar]
- Rosenwasser AM, Fecteau ME, Logan RW, Reed JD, Cotter SJ, Seggio JA (2005) Circadian activity rhythms in selectively bred ethanol-preferring and nonpreferring rats. Alcohol 36:69–81. [DOI] [PubMed] [Google Scholar]
- Ruby CL, Vadnie CA, Hinton DJ, Abulseoud OA, Walker DL, O’Connor KM, Noterman MF, Choi DS (2014) Adenosinergic regulation of striatal clock gene expression and ethanol intake during constant light. Neuropsychopharmacology 39:2432–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD (2015) 2010 National and State Costs of Excessive Alcohol Consumption. Am J Prev Med 49:e73–e79. [DOI] [PubMed] [Google Scholar]
- Sellix MT, Egli M, Poletini MO, McKee DT, Bosworth MD, Fitch CA, Freeman ME (2006) Anatomical and functional characterization of clock gene expression in neuroendocrine dopaminergic neurons. American journal of physiology. Regulatory, integrative and comparative physiology 290:R1309–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Engemann S, Sahota P, Thakkar MM (2010) Role of adenosine and wake-promoting basal forebrain in insomnia and associated sleep disruptions caused by ethanol dependence. J. Neurochem 115:782–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Lodhi S, Sahota P, Thakkar MM (2015) Nicotine administration in the wake-promoting basal forebrain attenuates sleep-promoting effects of alcohol. J Neurochem. [DOI] [PubMed] [Google Scholar]
- Sharma R, Sahota P, Thakkar MM (2014a) Nicotine Administration in the Cholinergic Basal Forebrain Increases Alcohol Consumption in C57BL/6J Mice. Alcohol Clin. Exp Res 38:1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Sahota P, Thakkar MM (2014b) Rapid tolerance development to the NREM sleep promoting effect of alcohol. Sleep 37:821–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Sahota P, Thakkar MM (2014c) Role of adenosine and the orexinergic perifornical hypothalamus in sleep-promoting effects of ethanol. Sleep 37:525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Sahota P, Thakkar MM (2018) A single episode of binge alcohol drinking causes sleep disturbance, disrupts sleep homeostasis and downregulates equilibrative nucleoside transporter 1. J Neurochem. [DOI] [PubMed] [Google Scholar]
- Shiromani PJ, Xu M, Winston EM, Shiromani SN, Gerashchenko D, Weaver DR (2004) Sleep rhythmicity and homeostasis in mice with targeted disruption of mPeriod genes. American journal of physiology. Regulatory, integrative and comparative physiology 287:R47–57. [DOI] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, Jansen HT (2007) Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: dependence on the suprachiasmatic nucleus. Brain Res 1129:34–42. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U (2005a) The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med 11:35–42. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Rosenwasser AM, Schumann G, Sarkar DK (2005b) Alcohol consumption and the body’s biological clock. Alcohol Clin. Exp Res 29:1550–1557. [DOI] [PubMed] [Google Scholar]
- Sprow GM, Thiele TEs (2012) The neurobiology of binge-like ethanol drinking: Evidence from rodent models. Physiol Behav 106:325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM, Engemann SC, Sharma R, Sahota P (2010) Role of wake-promoting basal forebrain and adenosinergic mechanisms in sleep-promoting effects of ethanol. Alcohol Clin. Exp. Res 34:997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Navarro M (2014) “Drinking in the dark” (DID) procedures: a model of binge-like ethanol drinking in non-dependent mice. Alcohol 48:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkoff AM, Storr CL (1998) Work schedule characteristics and substance use in nurses. Am J Ind Med 34:266–271. [DOI] [PubMed] [Google Scholar]
- Webb IC, Baltazar RM, Wang X, Pitchers KK, Coolen LM, Lehman MN (2009) Diurnal variations in natural and drug reward, mesolimbic tyrosine hydroxylase, and clock gene expression in the male rat. J Biol Rhythms 24:465–476. [DOI] [PubMed] [Google Scholar]
- Zavalia ND, Schoettner K, Goldsmith JA, Solis P, Ferraro S, Parent G, Amir S, (2020) Sexually dimorphic influence of the circadian clock gene Bmal1 in the striatum on alcohol intake. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]