Abstract
On May 24, 2019, the FDA granted regular approval to alpelisib in combination with fulvestrant for postmenopausal women, and men, with hormone receptor (HR)-positive, HER2-negative, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA)-mutated, advanced or metastatic breast cancer as detected by an FDA-approved test following progression on or after an endocrine-based regimen. Approval was based on the SOLAR-1 study, a randomized, double-blind, placebo-controlled trial of alpelisib plus fulvestrant versus placebo plus fulvestrant. The primary endpoint was investigator-assessed progression-free survival (PFS) per RECIST v1.1 in the cohort of trial participants whose tumors had a PIK3CA mutation. The estimated median PFS by investigator assessment in the alpelisib plus fulvestrant arm was 11.0 months (95% CI: 7.5, 14.5) compared with 5.7 months (95% CI: 3.7, 7.4) in the placebo plus fulvestrant arm (HR 0.65; 95% CI: 0.50, 0.85; two-sided p=0.001). The median overall survival (OS) was not yet reached for the alpelisib plus fulvestrant arm (95% CI: 28.1, NE) and was 26.9 months (95% CI: 21.9, NE) for the fulvestrant control arm. No PFS benefit was observed in trial participants whose tumors did not have a PIK3CA mutation (HR = 0.85; 95% CI: 0.58, 1.25).The most common adverse reactions, including laboratory abnormalities, on the alpelisib plus fulvestrant arm were increased glucose, increased creatinine, diarrhea, rash, decreased lymphocyte count, increased gamma glutamyl transferase, nausea, increased alanine aminotransferase, fatigue, decreased hemoglobin, increased lipase, decreased appetite, stomatitis, vomiting, decreased weight, decreased calcium, decreased glucose, prolonged activated partial thromboplastin time, and alopecia.
Introduction
In the United States (US), breast cancer is the most common cancer in women, with more than 270,000 new cases and 40,000 deaths annually (1). Breast cancer is rare in men, with only 2,600 cases per year (2). Metastatic breast cancer is categorized into different histopathological subtypes based on expression of hormone receptor (HR, estrogen receptor [ER] and/or progesterone receptor [PR]), and human epidermal growth factor receptor 2 (HER2). Hormone receptor-positive, HER2-negative breast cancer is the most common subtype in both women and men. The majority of patients with HR-positive, HER2-negative breast cancer are initially diagnosed and treated at an early stage with a combination of surgery with or without radiation and adjuvant endocrine therapy with or without adjuvant chemotherapy. Even after completion of 5 years of adjuvant endocrine therapy, 10-41% of women will experience distant recurrence by 20 years, depending on tumor diameter, nodal status and tumor grade (3). Male patients with breast cancer tend to present at a higher stage, likely due to the lack of public awareness and mammographic screening in men. Therefore, there remains an unmet need for further effective therapies for these patients.
Current FDA approved therapies for patients with HR-positive, HER2-negative advanced or metastatic breast cancer include hormonal-based (aromatase inhibitor [AI], fulvestrant) therapies in combination with cyclin-dependent kinase (CDK) 4/6 inhibitors (abemaciclib, palbociclib, ribociclib), everolimus with exemestane, hormonal monotherapy (AI, fulvestrant, tamoxifen), and chemotherapy (capecitabine, eribulin, ixabepilone, paclitaxel protein-bound, gemcitabine, etc.)(4). Metastatic HR-positive, HER2-negative breast cancer is currently incurable and has a 5-year survival rate of approximately 30% (5). PIK3CA mutations are present in approximately 40% of patients with HR-positive, HER2-negative advanced breast cancer and is a negative prognostic factor that potentially mediates resistance to endocrine therapy (6). Treatment options for all patients, including male patients, remains an area of significant unmet need. This article summarizes the FDA rationale for granting regular approval in May 2019 to alpelisib in combination with fulvestrant for postmenopausal women and men with HR-positive, HER2-negative, PIK3CA-mutated, advanced or metastatic breast cancer (7).
Chemistry, Manufacturing, and Control
The alpelisib drug substance is a white to almost white powder that is practically insoluble in water. The chemical name of alpelisib is (2S)-N1-[4-Methyl-5-[2-(2,2,2-trifluoro-1,1-dimethylethyl)-4-pyridinyl]-2-thiazolyl]-1,2-pyrrolidinedicarboxamide, with the chemical structure shown in Figure 1 (8). The molecular formula for alpelisib is C19H22F3N5O2S with a relative molecular mass is 441.47 g/mol.
Figure 1:
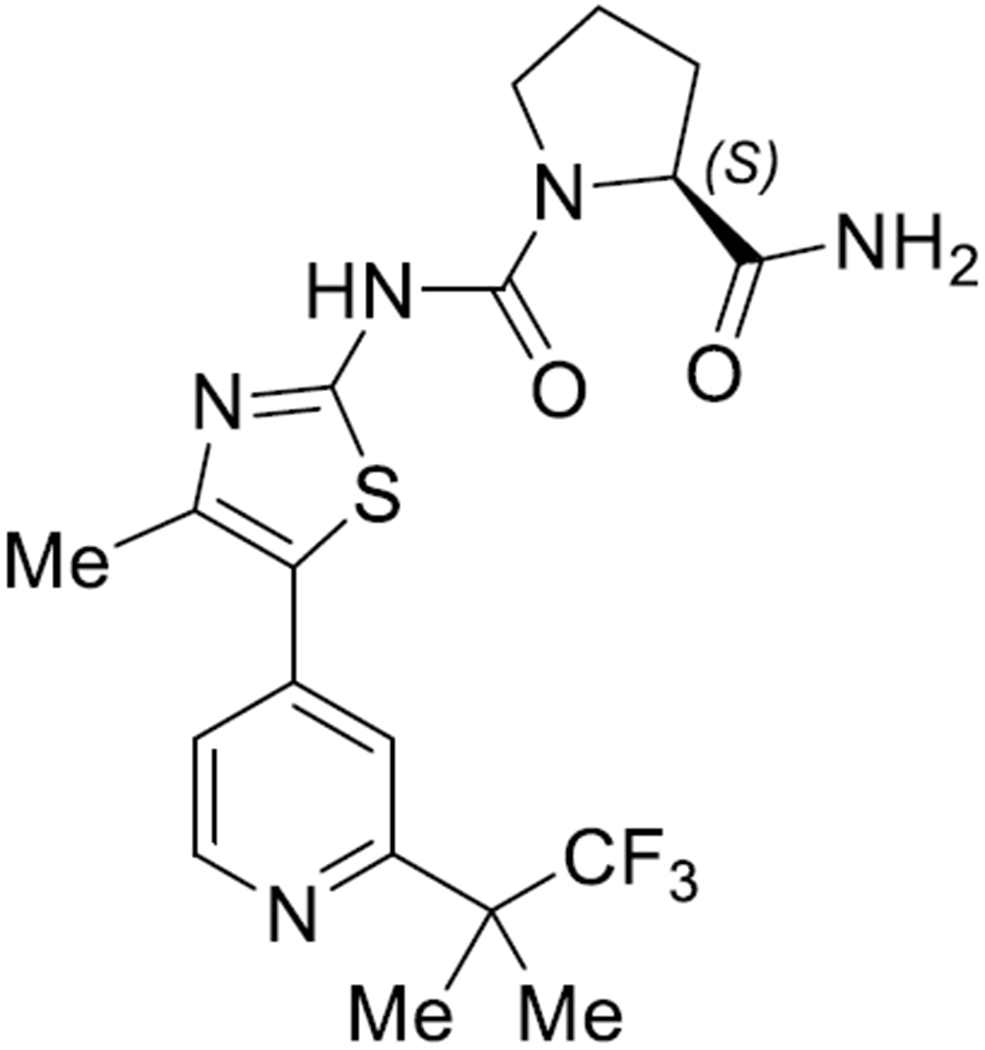
Chemical Structure of Alpelisib (8)
The film-coated tablets are supplied in blister packs for oral administration with three strengths that contain 50 mg, 150 mg and 200 mg of alpelisib. These tablets are composed of hypromellose, magnesium stearate, mannitol, microcrystalline cellulose, and sodium starch glycolate, with the film-coating consisting of hypromellose, iron oxide black, iron oxide red, macrogol/polyethylene glycol (PEG) 4000, talc, and titanium dioxide. The tablets are to be stored at 20°C to 25°C (68°F to 77°F), with excursions permitted between 15°C and 30°C (59°F and 86°F) [USP Controlled Room Temperature].
Nonclinical Pharmacology and Toxicology
Alpelisib is a kinase inhibitor with inhibitory activity predominantly against the α-isoform of class I phosphatidylinositol-3-kinase (PI3K). Alpelisib showed higher kinase inhibitory activity in cell lines harboring mutations of the catalytic α-subunit of PI3K (PIK3CA) compared to wildtype cell lines. Cell viability assays showed that a higher percentage of PIK3CA mutant cancer cell lines were sensitive to alpelisib treatment compared with PIK3CA wildtype cell lines tested. In mouse xenograft models of breast cancer, including ER-positive breast cancer models with PIK3CA mutations, single agent alpelisib showed anti-tumor activity, and the combination of alpelisib and fulvestrant demonstrated an increase in anti-tumor activity compared to fulvestrant or alpelisib alone. The observed anti-tumor activity correlated with inhibition of the PI3K/Akt pathway. In repeat-dose toxicity studies, administration of alpelisib to rats and dogs for up to 3 months resulted in adverse effects in the GI tract, hemolymphoid system, skin, metabolic, and reproductive system. Alpelisib increased blood insulin and glucose levels, which correlated with histopathologic changes in the pancreas (vacuolation or hyperplasia of endocrine cells). The toxicity profile in animals was similar to that in trial participants treated with alpelisib. In embryo-fetal development studies, administration of alpelisib to pregnant rats and rabbits resulted in embryo-fetal mortality, reduced fetal weights, and fetal malformations at ≥ 0.8 times the human AUC at the recommended clinical dose. Based on findings in animals and mechanism of action, alpelisib can cause fetal harm when administered to a pregnant woman.
Clinical Pharmacology
The proposed alpelisib 300 mg QD dosage was found to be acceptable for approval based on the efficacy results and clinically manageable safety profile demonstrated in the SOLAR-1 trial. Due to treatment-related adverse events, such as hyperglycemia, diarrhea, and rash, 72% of trial participants required at least one dose interruption and 59% required at least one dose reduction. Overall, 21% of trial participants discontinued treatment with alpelisib alone in the alpelisib plus fulvestrant arm. After the second treatment cycle, most trial participants remained on a stable dose with roughly 40%, 30% and 30% receiving 300, 250, and 200 mg, respectively.
In cancer patients, alpelisib demonstrated linear pharmacokinetics (PK) across the dose range 30 to 450 mg following a single dose and multiple doses. After approximately 3 once daily administrations, the exposure of alpelisib reached steady state with an approximately 1.3 to 1.5-fold accumulation ratio. In healthy subjects, both high-fat high-calorie meal and low-fat low-calorie meal boost alpelisib systemic exposure by 75% compared to fasting condition. Therefore, alpelisib should be taken immediately after food. Alpelisib can be co-administered with acid-reducing agents, given that alpelisib should be taken with food, as alpelisib systemic exposure decreased by 21% with ranitidine in the presence of low-fat low-calorie meal.
Based on analysis of specific populations such as age (21 to 87 years), sex, race/ethnicity (Japanese or Caucasian), body weight (37 to 181 kg), mild to moderate renal impairment (CrCl 30 to < 90 mL/min based on the Cockcroft-Gault formula), or mild to severe hepatic impairment (Child-Pugh Class A, B, and C), there were no clinically significant differences in the pharmacokinetics of alpelisib that were predicted. As there was no dedicated study in patients with severe renal impairment, (CrCl < 30 mL/min), the effect on the pharmacokinetics of alpelisib is unknown in this setting.
The metabolism of alpelisib involves primarily chemical and enzymatic hydrolysis with a lesser contribution of CYP3A4. Two post-marketing commitment studies are requested to evaluate the effect of repeated doses of a strong CYP3A4 inducer on the PK of alpelisib, and to evaluate the effect of repeat doses of alpelisib on the single dose PK of sensitive substrates of CYP2B6, CYP3A4 and CYP2C-family enzymes (CYP2C9, CYP2C19 and/or CYP2C8) in order to determine the magnitude of exposure change for sensitive substrates of the above CYP enzymes.
Clinical Trial Design:
SOLAR-1 was a randomized, double-blind, placebo-controlled, international, multicenter study to determine the efficacy and safety of treatment with alpelisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal women, and men, with HR-positive, HER2-negative advanced breast cancer following progression on or after AI treatment. Alpelisib (300 mg) or placebo was administered orally daily and fulvestrant was administered at a dose of 500 mg via intramuscular (IM) injection on Days 1 and 15 of cycle 1 and on Day 1 of each subsequent cycle, every 28 days. The primary endpoint was PFS by investigator assessment per RECIST 1.1. On March 9, 2016 the protocol was amended to limit the primary analysis population to the cohort with a PIK3CA tumor mutation, and PFS in the cohort with wildtype PIK3CA was changed to a secondary proof-of-concept endpoint. OS for trial participants with a PIK3CA tumor mutation was a key secondary endpoint. Randomization was stratified by the presence of lung and/or liver metastases, and by previous treatment with any CDK 4/6 inhibitor within each of the PIK3CA tumor mutation and PIK3CA wildtype cohorts. The two-sided alpha of 0.05 was split to allocate 0.04 to the PIK3CA tumor mutation cohort and 0.01 to the PIK3CA wildtype cohort, respectively. Crossover from the placebo plus fulvestrant arm after documented progression was not permitted in the SOLAR-1 study. Trial participants received treatment until radiographic progression or unacceptable toxicity, with tumor assessments performed every 8 weeks for the first 18 months and every 12 weeks thereafter.
Results:
Efficacy:
A total of 572 trial participants (341 with PIK3CA mutated tumors and 231 with wildtype tumors) across 33 countries in North and Latin America, Europe, and Asia were randomized between the two arms. Baseline demographics of the intention to treat (ITT) population are shown in Table 1 and baseline disease characteristics are shown in Table 2. Trial participant characteristics were generally similar between the two treatment groups. Of note, among the cohort with a PIK3CA tumor mutation, only 9 trial participants randomized to the alpelisib arm and 11 trial participants randomized to the placebo arm had previously received a CDK 4/6 inhibitor. Overall, 60% of trial participants had tumors with a PIK3CA mutation, as determined by tissue-based testing, and were balanced between the control and experimental arm.
Table 1:
SOLAR-1 Demographics, PIK3CA Tumor Mutated and Wildtype Cohorts (7)
| PIK3CA mutated tumors | PIK3CA wildtype tumors | |||||
|---|---|---|---|---|---|---|
| Alpelisib + fulvestrant | Placebo + fulvestrant | All patients | Alpelisib + fulvestrant | Placebo + fulvestrant | All patients | |
| N = 169 (%) | N = 172 (%) | N = 341 (%) | N = 115 (%) | N = 116 (%) | N = 231 (%) | |
| Sex | ||||||
| Female | 168 (99.4) | 172 (100) | 340 (99.7) | 115 (100) | 116 (100) | 231 (100) |
| Male | 1 (0.6) | 0 | 1 (0.3) | 0 | 0 | 0 |
| Age (years) | ||||||
| Median | 63 | 64 | 63 | 62 | 63 | 62 |
| Range | 25 - 87 | 38 - 92 | 25 - 92 | 39 - 82 | 32 - 88 | 32 - 88 |
| Age category (years) | ||||||
| 18 to <65 | 95 (56) | 89 (52) | 184 (54) | 72 (63) | 65 (56) | 137 (59) |
| 65 to <85 | 73 (43) | 80 (47) | 153 (45) | 43 (37) | 50 (43) | 93 (40) |
| ≥ 85 | 1 (<1) | 3 (2) | 4 (1) | 0 | 1 (1) | 1 (<1) |
| Race | ||||||
| White | 117 (69) | 109 (63) | 226 (66) | 82 (71) | 69 (60) | 151 (65) |
| Asian | 34 (20) | 40 (23) | 74 (22) | 25 (22) | 26 (22) | 51 (22) |
| Black or African American | 1 (1) | 3 (2) | 4 (1) | 1 (1) | 3 (3) | 4 (2) |
| American Indian or Alaska Native | 1 (1) | 2 (1) | 3 (1) | 0 | 2 (2) | 2 (1) |
| Other | 8 (5) | 10 (6) | 18 (5) | 1 (1) | 7 (6) | 8 (4) |
| Unknown | 8 (5) | 8 (5) | 16 (5) | 6 (5) | 9 (8) | 15 (7) |
| ECOG | ||||||
| 0 | 112 (66) | 113 (66) | 225 (66) | 84 (73) | 79 (68) | 163 (71) |
| 1 | 56 (33) | 58 (34) | 114 (33) | 30 (26) | 37 (32) | 67 (29) |
| Missing | 1 (1) | 1 (1) | 2 (1) | 1 (1) | 0 | 1 (<1) |
Percentages in the table for each category may not sum to 100% due to rounding
Table 2:
Baseline Disease Characteristics, SOLAR-1 (7)
| PIK3CA mutant tumors | PIK3CA non-mutant tumors | |||||
|---|---|---|---|---|---|---|
| Alpelisib + fulvestrant | Placebo + fulvestrant | All patients | Alpelisib + fulvestrant | Placebo + fulvestrant | All patients | |
| N = 169 (%) | N = 172 (%) | N = 341 (%) | N = 115 (%) | N = 116 (%) | N = 231 (%) | |
| Sites of metastases | ||||||
| Breast | 1 (1) | 3 (2) | 4 (1) | 5 (4) | 4 (3) | 9 (4) |
| Bone | ||||||
| Any | 131 (78) | 121 (70) | 252 (74) | 79 (69) | 89 (77) | 168 (73) |
| Only | 42 (25) | 35 (20) | 77 (23) | 26 (23) | 23 (20) | 49 (21) |
| Visceral | ||||||
| Any | 93 (55) | 100 (58) | 193 (57) | 66 (57) | 74 (64) | 140 (61) |
| Liver | 49 (29) | 54 (31) | 103 (30) | 41 (36) | 36 (31) | 77 (33) |
| Lung | 57 (34) | 68 (40) | 125 (37) | 37 (32) | 55 (47) | 92 (40) |
| Number of metastatic sites | ||||||
| 0 | 0 | 1 (1) | 1 (<1) | 0 | 0 | 0 |
| 1 | 63 (37) | 52 (30) | 115 (34) | 44 (38) | 33 (29) | 77 (33) |
| 2 | 58 (34) | 60 (35) | 118 (35) | 35 (30) | 38 (33) | 73 (32) |
| ≥3 | 48 (28) | 59 (34) | 107 (31) | 36 (31) | 45 (39) | 81 (35) |
| Prior treatment | ||||||
| Any CDK 4/6 inhibitor | 9 (5) | 11 (6) | 20 (6) | 7 (6) | 8 (7) | 15 (7) |
| Tamoxifen | 59 (35) | 62 (36) | 121 (36) | 37 (32) | 50 (43) | 87 (37) |
| Chemotherapy | 101 (60) | 107 (62) | 208 (61) | 78 (68) | 72 (62) | 150 (65) |
| Neoadjuvant | 25 (15) | 29 (17) | 54 (16) | 20 (17) | 23 (20) | 43 (19) |
| Adjuvant | 78 (42) | 86 (50) | 164 (48) | 64 (56) | 58 (50) | 122 (53) |
| Line of treatment in advanced disease | ||||||
| First line | 88 (52) | 89 (52) | 177 (52) | 72 (63) | 61 (53) | 133 (58) |
| Second line | 79 (47) | 82 (48) | 161 (47) | 44 (38) | 52 (45) | 96 (42) |
| Endocrine status | ||||||
| Primary resistance | 23 (14) | 22 (13) | 45 (13) | 31 (27) | 26 (22) | 57 (25) |
| Secondary resistance | 120 (71) | 127 (74) | 247 (72) | 66 (57) | 65 (56) | 131 (57) |
| Sensitive | 20 (12) | 19 (11) | 39 (11) | 16 (14) | 20 (17) | 36 (16) |
Percentages in the table for each category may not sum to 100% due to rounding
In the primary efficacy population of trial participants whose tumors had a PIK3CA mutation, SOLAR-1 demonstrated a statistically significant improvement in PFS for the alpelisib plus fulvestrant arm (HR 0.65; 95% CI: 0.50, 0.85; p=0.001), with a median PFS of 11.0 months (95% CI: 7.5, 14.5) compared to 5.7 months (95% CI: 3.7, 7.4) in the placebo plus fulvestrant arm. Figure 2 shows the Kaplan-Meier curve for PFS in the cohort with PIK3CA tumor mutation.
Figure 2:
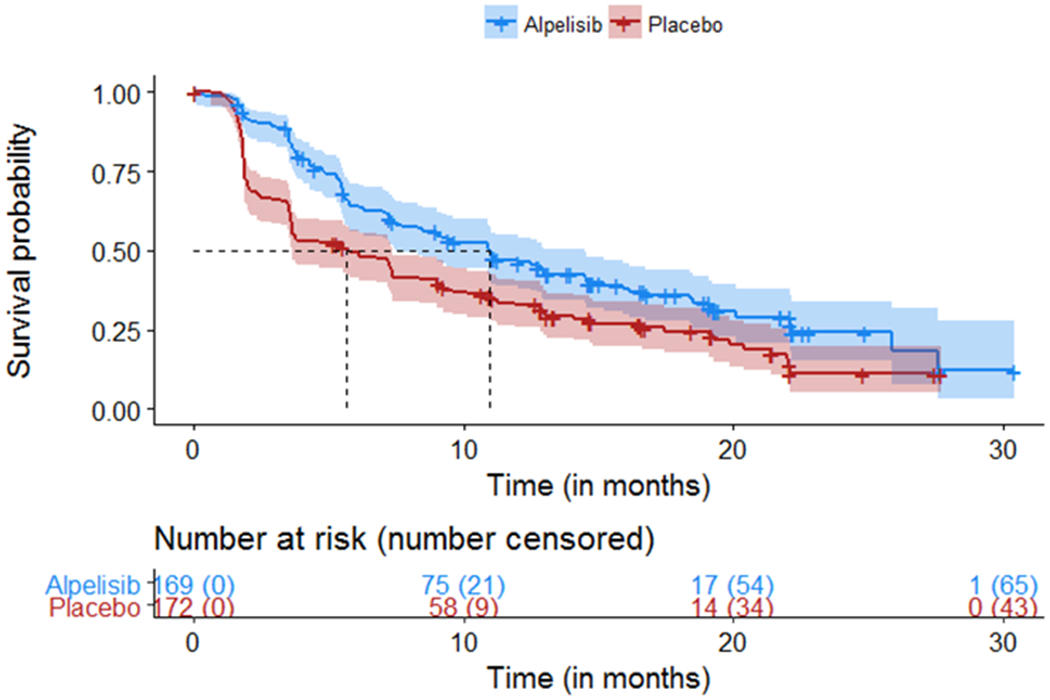
Kaplan-Meier Plot of Progression-Free Survival in SOLAR-1 (Per Investigator Assessment of Patients with a PIK3CA Tumor Mutation)(7)
OS was a key secondary endpoint, and results were immature at the time of the first interim analysis. In the PIK3CA cohort, 92 deaths were reported by the data cut-off date for the regulatory submission to the FDA (23.7% in the alpelisib plus fulvestrant arm and 30.2% in the placebo plus fulvestrant arm), corresponding to a 51.7% information fraction of the targeted 178 events for the final OS analysis. The median OS was not yet reached for the alpelisib plus fulvestrant arm (95% CI: 28.1, NE) and was 26.9 months (95% CI: 21.9, NE) for the fulvestrant control arm.
Safety:
The assessment of safety was based on a total of 571 trial participants who were treated with at least one dose of study medication (340 with PIK3CA mutated tumors and 231 with wildtype tumors). There were a high number of dose modifications and discontinuations reported in the SOLAR-1 trial. Dose interruptions occurred in 188/284 (66%) of those in the alpelisib plus fulvestrant arm versus 61/287 (21%) in the placebo plus fulvestrant arm. Dose reductions due to adverse events occurred in 156/284 (55%) versus 13/287 (4.5%) of trial participants, respectively. Discontinuations due to AEs were reported in 71/284 (25%) versus 13/287 (4.5%) of trial participants, respectively (these percentages included discontinuations of either alpelisib or placebo, fulvestrant, or both drugs in each arm). The most frequent adverse reactions resulting in discontinuation of alpelisib were hyperglycemia, rash, diarrhea, and fatigue. While the majority of trial participants were able to continue alpelisib plus fulvestrant with supportive care medications, the high percentage of dose modifications and discontinuations indicate that the alpelisib dose of 300 mg daily was too high for many trial participants. The safety of alpelisib plus fulvestrant in the cohort with PIK3CA tumor mutation did not differ from that of the overall SOLAR-1 population.
Alpelisib plus fulvestrant demonstrated acceptable tolerability for the indicated population with a serious and life-threatening disease. Adverse reactions were common and, except for hyperglycemia and rash, predominantly grade 1-2 in severity. The most common adverse reactions observed in the alpelisib plus fulvestrant arm were hyperglycemia (65%), diarrhea (58%), and rash (52%). Hypersensitivity reactions, severe cutaneous reactions, hyperglycemia, pneumonitis, diarrhea, and embryo-fetal toxicity are labeled as Warnings and Precautions. Additional common adverse reactions with alpelisib plus fulvestrant ≥ 20% included diarrhea, nausea, fatigue, anemia, decreased appetitie, stomatitis, vomiting, anorexia, and alopecia.
Companion Diagnostic (CDx) Development
The therascreen PIK3CA RGQ PCR Kit was developed as a companion diagnostic of 11 defined mutations in the phosphatidylinositol 3-kinase catalytic subunit alpha (PIK3CA) gene (Exon 7: C420R; Exon 9: E542K, E545A, E545D [1635G>T only], E545G, E545K, Q546E, Q546R; and Exon 20: H1047L, H1047R, H1047Y) using genomic DNA (gDNA) extracted from formalin-fixed, paraffin-embedded (FFPE) tumor tissue or circulating tumor DNA (ctDNA) isolated from plasma (9). The safety and effectiveness of the therascreen PIK3CA RGQ PCR Kit was demonstrated through prospectively and retrospectively testing of tissue and plasma specimens from trial participants enrolled in SOLAR-1.
There were 341 trial participants enrolled in the PIK3CA mutation cohort (using tumor tissue) and 231 enrolled in the PIK3CA wildtype cohort. Of the 341 trial participants in the cohort with a PIK3CA mutation, almost all (n=336, 99%) had confirmed PIK3CA mutations in tumor tissue using the FDA-approved therascreen® PIK3CA RGQ PCR Kit. Three hundred and seventeen trial participants with PIK3CA mutations confirmed in tumor tissue had a plasma specimen available for testing and of these, 177 trial participants (56%) had PIK3CA mutations identified in plasma specimen, and 140 trial participants (44%) did not. The data provide reasonable assurance of safety and effectiveness of the test kit when used in accordance with the indications for use.
Concordance of the plasma results to tumor tissue results were determined using plasma samples collected at baseline from 328 trial participantswith therascreen PIK3CA RGQ PCR Kit tissue-positive results and 215 trial participants with therascreen PIK3CA RGQ PCR Kit tissue-negative. The positive percent agreement (PPA) of the plasma test for 11 mutations compared to the tissue test was 179/328 (54.6%) in the concordance analysis, which may be due to low tumor shedding into the blood. Notably, 5 of the 11 mutations targeted by the therascreen PIK3CA RGQ PCR Kit (H1047Y, Q546R, Q546E, E545D, and E545A) were not detected when testing plasma from trial participants enrolled in SOLAR-1 whose tissue was also tested. The negative percent agreement (NPA) was 97.2% (209/215). Taking into consideration the convenience of plasma testing, as well as the risk of false negative results in plasma and the low PPA between the plasma and tissue, the approved product labeling recommends a reflex approach, with plasma testing to be followed by tissue testing for those in whom plasma is negative for PIK3CA mutation.
Patient-Reported Outcomes (PRO):
In this trial, the European Organization for Research and Treatment of Cancer Quality of Life Core 30 (EORTC QLQ-C30) was collected at baseline and every second cycle until the end of treatment. This PRO measure captures functioning, symptoms, and overall quality of life. The completion rates were >95% at baseline and > 84% for assessments on both arms during the first 12 months of therapy regardless of treatment arm. Prespecified COA and PRO analyses were exploratory without any control of Type I error and included time to 10% deterioration in the global health status/quality of life (Qol) scale score of the EORTC QLQ-C30 instrument and change from baseline in the GHS/Qol scale score of the EORTC QLQ-C30.
For trial participants who remained on therapy, patient responses to both physical and role functioning scales were not indicative of large decrements for either treatment arm. The descriptive distributions of trial participants reporting key gastrointestinal side effects that were captured using PRO measures reveal a similar pattern to what was reported in the adverse reaction table, i.e. more trial participants in the alpelisib arm experienced worsening diarrhea, nausea, and vomiting.
The overall review of the PRO results by the FDA did not identify a large decrement in symptoms or function that would materially alter the net favorable risk-benefit determination. Therefore, no information on patient-reported outcomes was included in the US prescribing information (USPI) for alpelisib.
Regulatory Insights
This is the first FDA approval for treatment of patients with PIK3CA-mutated advanced or metastatic breast cancer (10). The SOLAR-1 trial met its primary endpoint in the intended use population of patients with HR-positive, HER2-negative PIK3CA tumor-mutated advanced or metastatic breast cancer. Superior efficacy was not demonstrated for alpelisib plus fulvestrant in the PIK3CA-wildtype cohort, and therefore use of alpelisib plus fulvestrant was limited to the PIK3CA-mutated population, as reflected in the indication for which regular approval was granted.
The current standard of care for first-line treatment of postmenopausal women with HR-positive, HER2-negative metastatic breast cancer in the United States is a combination of endocrine therapy and a CDK 4/6 inhibitor, irrespective of PIK3CA mutation status. Only 6% of trial participants on the SOLAR-1 trial had previously received an aromatase inhibitor plus CDK 4/6 inhibitor combination and no trial participants had previously received fulvestrant plus a CDK 4/6 inhibitor. The drug company noted that some trial participants with metastatic disease previously treated with a CDK 4/6 inhibitor had been enrolled in SOLAR-1, and that the PFS results in this subset similarly favored the alpelisib plus fulvestrant arm (HR 0.48; 95% CI: 0.17, 1.36). This observation was based on a very small sample size of 20 trial participants, of whom only nine were treated with alpelisib plus fulvestrant, with correspondingly wide confidence intervals. However, activation of PI3K signaling is a known mechanism of resistance in patients whose tumors have progressed on CDK 4/6 inhibitors, and therefore a strategy of combining endocrine therapy plus a PI3K inhibitor after progression on CDK 4/6 inhibitor-based regimens is a possible approach (11, 12).
At the American Society for Clinical Oncology (ASCO) Annual Meeting in 2020, results were presented from a cohort of the BYLieve study, a multicohort phase 2 study with a cohort of patients with PIK3CA-mutated HR-positive, HER2-negative advanced breast cancer treated with alpelisib plus fulvestrant who previously received CDK 4/6 inhibitor and AI as immediate prior treatment (13). The primary endpoint of the proportion of patients alive without progression of disease at 6 months was met in this cohort at 50.4% (95% CI: 41.2, 59.6). This data is supportive of the use of alpelisib plus fulvestrant in the post-CDK4/6 inhibitor setting and supports the findings seen in the SOLAR-1 study.
As breast cancer in men is rare, male patients have historically been excluded from clinical trials and their clinical management is often based on extrapolation of data from female patients enrolled in trials. The FDA has encouraged the inclusion of male patients in breast cancer trials and has released a guidance on this topic, which in part details that even when male enrollment is limited it may be possible to extrapolate findings in cases when no difference between males and females is anticipated based on the mechanism of action of the drug (14). Male patients were eligible and included in the SOLAR-1 study, and the current indication for alpelisib includes men.
Premenopausal patients were not eligible for, nor enrolled in the SOLAR-1 trial and therefore the indication granted only included postmenopausal women. However, similar to male patients, in the clinical setting data in postmenopausal patients is typically extrapolated to the treatment of premenopausal patients in combination with ovarian suppression. FDA encourages the inclusion of premenopausal and postmenopausal patients into future clinical trials to increase the data and knowledge regarding the use of drugs for this important group of patients.
Alpelisib was the first new molecular entity (NME) reviewed using the Real Time Oncology Review (RTOR) program, which allows the FDA to begin analyzing key efficacy and safety datasets prior to the official submission of an application, aiding the review team to begin their analysis and communicate with the submitting drug company (referred to as the applicant) earlier on for important review issues prior to the official filing of the application (15, 16). This new drug application (NDA) also utilized the Assessment Aid (AAid), a multidisciplinary review template that is a voluntary submission from the applicant with the goal to focus the FDA’s written review on critical thinking and analysis and decrease the time spent on repeating data already presented by the applicant (17). Both the RTOR and AAid are initiatives by the Oncology Center of Excellence (OCE) intended to streamline the FDA’s review process that may lead to patients’ earlier access to therapies that serve unmet needs in oncology. Using these programs, this application was approved on May 24, 2019, approximately 3 months ahead of the prescription drug user fee act (PDUFA) VI deadline of August 18, 2019.
Conclusions
In summary, the addition of alpelisib to fulvestrant demonstrates a favorable benefit-risk profile (Table 3) for the treatment of postmenopausal women and men with hormone receptor (HR)-positive, HER2-negative, PIK3CA-mutated, advanced or metastatic breast cancer. The safety profile of alpelisib plus fulvestrant showed acceptable tolerability for this population of patients with a life-threatening disease. Therefore, the results from the SOLAR-1 study supported a regular approval for this indication.
Table 3:
FDA Risk-Benefit Analysis, SOLAR-1 (7)
| Dimension | Evidence and Uncertainties | Conclusions and Reasons |
|---|---|---|
| Analysis of Condition | Breast cancer is the most common cancer in women, with more than 260,000 new cases and 40,000 deaths annually. Breast cancer is rare in men, and limited data are available from clinical trials on its treatment. Advanced or metastatic breast cancer is incurable. |
Advanced or metastatic breast cancer is a serious and life-threatening condition with ongoing unmet medical need in both female and male patients. |
| Current Treatment Options | Metastatic breast cancer is not presently curable. Treatment goals are palliative in nature and include delay of disease progression, prolongation of survival, and reduction of cancer-related symptoms. FDA approved therapies for patients with HR-positive, HER2-negative advanced or metastatic breast cancer include endocrine therapy (aromatase inhibitor [AI], fulvestrant) in combination with CDK 4/6 inhibitors (abemaciclib, palbociclib, ribociclib), everolimus with exemestane, endocrine monotherapy (AI, fulvestrant, tamoxifen), and chemotherapy (multiple agents including taxanes, capecitabine, eribulin, vinorelbine, ixabepilone, and gemcitabine.). |
All currently available treatment options are palliative. There is an unmet medical need to improve outcomes of female and male patients with HR-positive, HER2-negative advanced or metastatic breast cancer. |
| Benefit | SOLAR-1 enrolled 572 postmenopausal women and men with HR-positive, HER2-negative advanced or metastatic breast cancer whose disease had progressed or recurred on or after an aromatase inhibitor, with or without a CDK 4/6 inhibitor. In patients whose tumors had a PIK3CA tumor mutation, the estimated median PFS by investigator assessment in the alpelisib plus fulvestrant arm was 11.0 months (95% CI: 7.5, 14.5) compared to 5.7 months (95% CI: 3.7, 7.4) in the placebo plus fulvestrant arm (HR 0.65; 95% CI: 0.50, 0.85; two-sided p=0.001). The overall response rate (ORR) in patients with a PIK3CA tumor mutation, measurable disease at baseline, and confirmed response was higher in the alpelisib plus fulvestrant arm (36% versus 16%). |
The SOLAR-1 trial met its primary endpoint with a statistically significant and clinically meaningful improvement in PFS. This is also the first drug approved specifically for the treatment of patients with PIK3CA tumor mutated advanced breast cancer, which represents a new molecular subset in breast cancer. The companion diagnostic test therascreen® PIK3CA RGQ PCR Kit, (QIAGEN Manchester, Ltd.) will be used to select patients who have PIK3CA mutations in tumor tissue specimens and/or in circulating tumor DNA (ctDNA) isolated from plasma specimens. If the test is negative for PIK3CA mutations in plasma, tumor tissue should be tested. |
| Risk and Risk Management | Adverse reactions were common and, except for hyperglycemia and rash, predominantly grade 1-2 in severity. The majority of adverse reactions were managed with dose reductions, temporary treatment discontinuations, supportive care treatments, and/or standard therapy but 21% of patients discontinued alpelisib due to adverse events Severe hypersensitivity, severe cutaneous reactions, hyperglycemia, pneumonitis, diarrhea, and embryo-fetal toxicity are labeled as Warnings and Precautions. The most common adverse reactions on the alpelisib plus fulvestrant arm were glucose increased (79%), creatinine increased (67%), diarrhea (58%), rash (52%), lymphocyte count decreased (52%), gamma glutamyl transferase (GGT) increased (52%), nausea (45%), alanine aminotransferase (ALT) increased (44%), lipase increased (42%), and fatigue (42%). Serious adverse reactions occurred in 35% of patients who received alpelisib plus fulvestrant, including hyperglycemia, rash, diarrhea, acute kidney injury, abdominal pain, and anemia. Twenty-one percent of patients permanently discontinued alpelisib alone due to adverse reactions, and 4.6% permanently discontinued both alpelisib and fulvestrant. |
Alpelisib plus fulvestrant can be used safely with appropriate labeling. No REMS is indicated. |
Footnotes
Note: This is a U.S. Government work. There are no restrictions on its use.
Disclosure of Potential Conflicts of Interest: The authors report no financial interests or relationships with the commercial sponsors of any products discussed in this report.
References
- 1.National Cancer Institute. Surveillance, Epidemiology and End Results Program (SEER). Female Breast Cancer. [cited 2020 Jun 2]. Available from: https://seer.cancer.gov/statfacts/html/breast.html
- 2.American Cancer Society. Key Statistics for Breast Cancer in Men. [cited 2020 Jun 2]. Available from: https://www.cancer.org/cancer/breast-cancer-in-men/about/key-statistics.html
- 3.Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, Peto R, et al. 20year risk of breast cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 2017; 377:1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network (NCCN) Guidelines. Breast Cancer [about 4 screens]. [cited 2020 June 2]. Available from https://www.nccn.org/professionals/physician_gls/default.aspx.
- 5.National Cancer Institute. Surveillance, Epidemiology and End Results Program (SEER). Female Breast Cancer Subtypes. [cited 2020 Jun 2]. Available from: https://seer.cancer.gov/statfacts/html/breast-subtypes.html
- 6.Mosele F, Stefanovska B, Lusque A, Tran Dien A, Garberis I, Droin N, et al. Outcome and molecular landscape of patients with PIK3CA-mutated metastatic breast cancer. Annals of Oncology 2020; 31: 377–386. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Food and Drug Administration: Drug Approval Package: PIQRAY. Multi-Discipline Review [about 2 screens].[cited 2020 Oct 17]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212526Orig1s000TOC.cfm
- 8.U.S. Food and Drug Administration: Drugs@FDA: FDA-Approved Drugs. PIQRAY Label for NDA 212526 [about 4 screens]. [cited 2020 Oct 17]. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process
- 9.U.S. Food and Drug Administration: Premarket Approval Therascreen PIK3CA RGQ PCR Kit [about 2 screens]. [cited 2020 Jun 5]. Available from:
- 10.U.S. Food and Drug Administration: FDA approves first PI3K inhibitor for breast cancer [about 3 screens]. [cited 2020 Jun 2]. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P190001
- 11.Vora SR, Juric D, Kim N, Mino-Kenudson M, Huynh T, Costa C, et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell 2014; 26: 136–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michaloglou C, Crafter C, Siersbaek R, Delpuech O, Curwen JO, Carnevalli LS, et al. Combined inhibition of mTOR and CDK4/6 is required for optimal blockade of E2F function and long-term growth inhibition in estrogen receptor-positive breast cancer. Molecular Cancer Therapeutics 2018; 17: 908–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ASCO Meeting Library: 2020 ASCO Virtual Scientific Program. Alpelisib + fulvestrant in patients with PIK3CA-mutated hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) advanced breast cancer previously treated with cyclin-dependent kinase 4/6 inhibitor + aromatase inhibitor: BYLieve study results. [cited 2020 Jun 2]. Available from: https://meetinglibrary.asco.org/record/186927/abstract
- 14.U.S. Food and Drug Administration: Male Breast Cancer: Developing Drugs for Treatment [about 2 screens]. [cited 2020 Jun 5]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/male-breast-cancer-developing-drugs-treatment
- 15.U.S. Food and Drug Administration: Real-Time Oncology Review Pilot Program [about 4 screens]. [cited 2020 Jun 5]. Available from: https://www.fda.gov/about-fda/oncology-center-excellence/real-time-oncology-review-pilot-program
- 16.de Claro RA, Gao JJ, Kim T, Kluetz PG, Theoret MR, Beaver JA, et al. U.S. Food and Drug Administration: Initial experience with the real-time oncology review. Clinical Cancer Research August 19, 2020. (online ahead of print). DOI: 10.1158/1078-0432.CCR-20-2220. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Food and Drug Administration: Assessment Aid [about 3 screens]. [cited 2020 Jun 5]. Available from: https://www.fda.gov/about-fda/oncology-center-excellence/assessment-aid


