Abstract
Topoisomerases are enzymes that play essential roles in DNA replication, transcription, chromosome segregation, and recombination. All cells have two major forms of DNA topoisomerases: type I enzymes, which make single-stranded cuts in DNA, and type II enzymes, which cut and decatenate double-stranded DNA. DNA topoisomerases are important targets of approved and experimental anti-cancer agents. Provided in this unit are protocols to assess activities of topoiosomerases and their inhibitors. Included are an assay for topoisomerase I activity based on relaxation of supercoiled DNA; an assay for topoisomerase II based on the decatenation of double-stranded DNA; and approaches for enriching and quantifying DNA-protein covalent complexes formed as obligatory intermediates in the reactions of type I and II topoisomerases with DNA; and assays for measuring DNA cleavage in vitro. Topoisomerases are not the only proteins that form covalent adducts with DNA in living cells, and the approaches described here are likely to find use in characterizing other protein-DNA adducts and exploring their utility as targets for therapy.
Basic Protocol 1: Assay of Topoisomerase I Activity
Basic Protocol 2: Assay of Topoisomerase II Activity
Basic Protocol 3: In Vivo Determination of Topoisomerase Covalent Complexes using the In Vivo Complex of Enzyme (ICE) Assay
Support Protocol 1: Preparation of Mouse Tissue for Determination of Topoisomerase Covalent Complexes using the (ICE) Assay
Support Protocol 2: Using Recombinant Topoisomerase Standard for Absolute Quantification of Cellular TOP2CC
Basic Protocol 4: Quantification of Topoisomerase-DNA Covalent Complexes by RADAR/ ELISA: The Rapid Approach to DNA Adduct Recovery (RADAR) Combined with the Enzyme-Linked Immunosorbent Assay (ELISA)
Basic Protocol 5: Analysis of Protein-DNA Covalent Complexes by RADAR/Western
Support Protocol 3: Adduct-Seq to Characterize Adducted DNA
Support Protocol 4: Nuclear Fractionation and RNase Treatment to Reduce Sample Complexity
Basic Protocol 6: Determination of DNA Cleavage by Purified Topoisomerase I
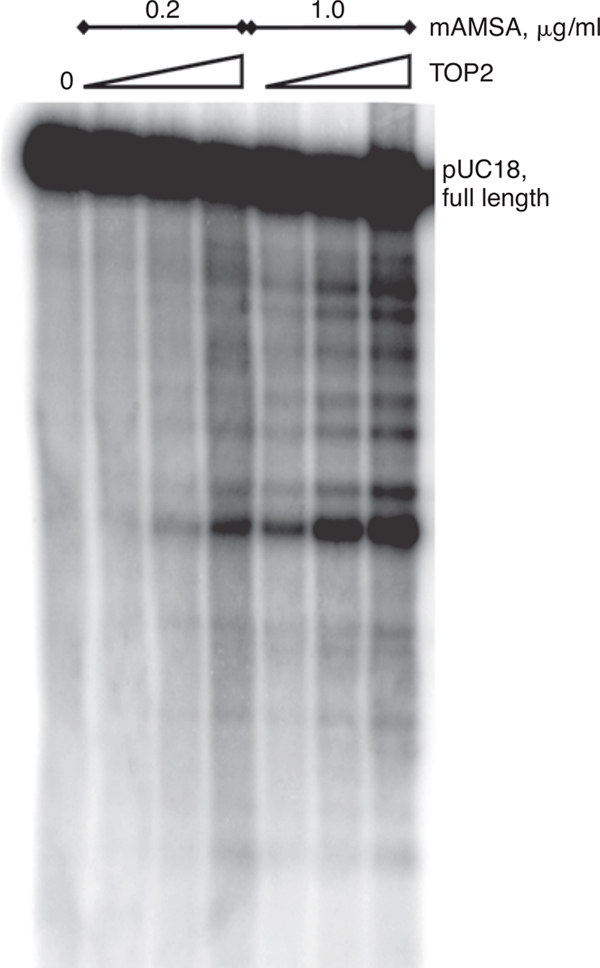
Basic Protocol 7: Determination of Inhibitor Effects on DNA Cleavage by Topoisomerase II using a Plasmid Linearization Assay
Alternate Protocol 1: Gel Electrophoresis Determination of Topoisomerase II Cleavage
Keywords: topoisomerase, topoisomerase I, topoisomerase II, camptothecin, etoposide, topoisomerase poison, ICE asay, RADAR enrichment, DNA cleavage, protein-DNA adduct
INTRODUCTION
Topoisomerases (Table 1) play critical roles in replication, transcription, and chromosome structure by altering the topological state of DNA. These enzymes are capable of relaxing supercoiled DNA and of “decatenating” sister chromatids that have become interlocked or “catenated” in the course of semiconservative DNA replication (Fig. 1) (J. L. Nitiss, 2009a; Pommier, Sun, Huang, & Nitiss, 2016; Postow, Crisona, Peter, Hardy, & Cozzarelli, 2001; Vos, Tretter, Schmidt, & Berger, 2011). All cells contain two major forms of topoisomerase: type I (EC 5.99.1.2), which makes single-stranded cuts in DNA, and type II (EC 5.99.1.3), which cuts and decatenates double-stranded DNA (Leppard & Champoux, 2005; McKie, Neuman, & Maxwell, 2021; Pommier, 2013; Pommier et al., 2016; Schoeffler & Berger, 2005; Vos et al., 2011). Type I topoisomerases are further subdivided into two mechanistically distinct subgroups: type IA enzymes, which are homologous to E. coli topoisomerase I, and type IB enzymes, which are homologous to human topoisomerase I. There are two bacterial counterparts of eukaryotic DNA topoisomerase II, DNA gyrase and DNA topoisomerase IV (Vos et al., 2011; J.C. Wang, 1996; J. C. Wang, 1998). While eukaryotic topoisomerases can relax supercoiled DNA, bacterial DNA gyrase can also introduce negative supercoils (Schoeffler & Berger, 2008).
Table 1.
Eukaryotic Topoisomerases
| Enzyme | Type | GenBank accession no. | Inhibitors |
|---|---|---|---|
|
| |||
| Topoisomerase Ia | IB | K03077 (S. cerevisiae) L20632 (mouse) J03250 (H. sapiens) AF349017 (H. sapiens Mitochondrial) | Camptothecin, topotecan, irinotecan, actinomycin Db, aclarubicinb |
| Topoisomerase IIc,d | II | M13814 (S. cerevisiae) D12513 (mouse II α) D38046 (mouse II β) J04088 (H. sapiens II α) X68060 (H. sapiens IIβ) | Doxorubicin, daunomycin, etoposide, mitoxantrone |
| Topoisomerase IIIa | IA | M24939 (S. cerevisiae) U43431 (H. sapiens III α) AF017146 (H. sapiens III β) | No known inhibitors |
The International Enzyme Commission functional designation EC 5.99.1.2 includes all bacterial and eukaryotic type I topoisomerases—e.g., eukaryotic topoisomerase I and topoisomerase III.
Actinomycin D and aclarubicin are examples of compounds that inhibit both topoisomerase I and topoisomerase II.
Lower eukaryotes such as yeast and Drosophila appear to have only a single type II topoisomerase; mammals have at least two type II isozymes, termed topoisomerase II α and topoisomerase II β.
The International Enzyme Commission functional designation EC 5.99.1.3 includes all type II topoisomerases—e.g., bacterial DNA gyrase, yeast topoisomerase II, and mammalian topoisomerase II α and topoisomerase II β
Figure 1.

Reactions of DNA topoisomerases. DNA topoisomerases catalyze the interconversion of different topological forms of DNA, such as the knotting and unknotting of DNA and catenation and decatenation of DNA rings. Type I topoisomerases are able to unknot or decatenate single-stranded knots and catenanes, but are unable to carry out these reactions on intact double-stranded DNA. (A) Both type I and type II topoisomerases can relax supercoiled DNA. Type II topoisomerases are able to catalize (B) the knotting/unknotting of intact double-stranded DNA and (C) the catenation/decatenation of intact double-stranded DNA.
Topoisomerases bind to DNA and cleave it in a reaction in which a covalent topoisomerase-DNA complex forms as an obligatory intermediate (see Background Information). DNA breakage is required to effect topological changes in DNA. Normally, the bound topoisomerase reseals the DNA break, but as noted below, a variety of conditions can compromise the enzyme-mediated religation.
Topoisomerases are important targets for many antibiotics and chemotherapeutic agents. Fluoroquinolones that inhibit prokaryotic type II topoisomerases (e.g., ciprofloxacin and levofloxacin) are commonly employed broad-spectrum antibiotics. Drugs that target eukaryotic DNA topoisomerase I (camptothecin, irinotecan, topotecan) or II (doxorubicin and etoposide) are clinically useful anticancer agents (Albright & Slatko, 2001; Bax, Murshudov, Maxwell, & Germe, 2019; Bush, Diez-Santos, Abbott, & Maxwell, 2020; Cinelli, 2019; Drlica et al., 2009; Marchand et al., 2006; McClendon & Osheroff, 2007; Pommier et al., 2016).
The DNA topoisomerase drugs in current clinical use are termed “topoisomerase poisons”. They employ a similar mechanism of action regardless of whether the target is a bacterial or eukaryotic topoisomerase. Normally, topoisomerase reseals the DNA break upon release of the covalent adduct. However, treatment with a topoisomerase poisons results in formation of a drug-enzyme-DNA complex, inhibiting religation of the topoisomerase-induced break and in effect converting the adducted topoisomerase into a DNA-damaging agent. Quantification of DNA-protein covalent complexes thus provides a critical measure of activity of compounds that target topoisomerases.
Although topoisomerases provide a paradigm for analysis of formation and resolution of DNA-protein covalent complexes, they are not the only proteins that form DNA adducts in living cells. Methyltransferases, tyrosyl-DNA phosphodiesterases, DNA glycosylases, polymerases, and repair proteins with AP lyase activity also form adducts as reaction intermediates (Ide, Shoulkamy, Nakano, Miyamoto-Matsubara, & Salem, 2011; Verdine & Norman, 2003). In addition, a much wider spectrum of proteins become crosslinked to nucleic acid in response to treatment of cells with ultraviolet radiation or chemicals such as formaldehyde [e.g. (Feng et al., 2019; F. C. Y. Lee & Ule, 2018; Trendel et al., 2019)]. Just as topoisomerases can be weaponized by treatment with topoisomerase inhibitors, other protein-DNA adducts can be cytotoxic if not resolved, and robust pathways repair persistent adducts in bacterial and eukaryotic cells (Ide et al., 2011; Stingele, Bellelli, & Boulton, 2017; Vaz, Popovic, & Ramadan, 2017).
The protocols for analysis of topoisomerase activity presented below are designed to be of use to those experienced with topoisomerase biochemistry and to those just entering the field of DNA adduct formation and resolution. In vitro assays are presented for quantification of topoisomerase I activity based on relaxation of supercoiled DNA (Basic Protocol 1), and for topoisomerase II based on the decatenation of double-stranded DNA (Basic Protocol 2). Two different approaches are presented for quantifying DNA-protein covalent complexes in vivo. An updated protocol is described for the ICE (In Vivo Complex Of Enzyme) assay, which relies on CsCl buoyant density gradient centrifugation to separate free protein and protein-DNA adducts (Basic Protocol 3). Also covered is the newer RADAR (Rapid Approach to DNA Adduct Recovery) assay, which depends on alcohol precipitation to recover adducts enriched by cell lysis in chaotropic salts and detergent, and which is suitable for quantification of adducts by ELISA (Basic Protocol 4) as well as adduct characterization by Western blot and genomic mapping (Basic Protocol 5). Approaches for measuring DNA cleavage caused by topoisomerase I (Basic Protocol 6) and for studying topoisomerase II cleavage in vitro and mapping topoisomerase cleavage sites (Basic Protocol 7 and Alternate Protocol) are also described. All of these protocols can be readily adapted to study formation and resolution of topoisomerase-DNA adducts and other covalent adducts in a variety of different cell types. These assays can also be extended to characterize the response to known inhibitors and to identify compounds with novel inhibitory activities.
BASIC PROTOCOL 1
ASSAY OF TOPOISOMERASE I ACTIVITY
A principal reaction of topoisomerase I is the relaxation of supercoiled DNA, which is readily distinguished from relaxed (not supercoiled) DNA based on electrophoretic mobility. Plasmid DNA isolated from most natural sources is negatively supercoiled, and any plasmid isolated from E. coli can be used to assay DNA relaxation by topoisomerase I. Unlike other eukaryotic topoisomerase activities, topoisomerase I is an ATP-independent enzyme and does not require a divalent cation (e.g., Mg2+) for activity, although Mg2+ stimulates activity ~3- to 5-fold (see Background Information). The assay detailed below is designed for eukaryotic topoisomerase I and uses a reaction buffer lacking ATP, conditions under which neither topoisomerase II nor topoisomerase III is active.
Materials
10× topoisomerase I reaction buffer (see recipe)
Substrate: plasmid DNA (any common plasmid such as pUC18 or pBR322 can be used
Purified topoisomerase I or cell extract (see Support Protocol)
5× loading dye (see recipe)
0.8% agarose gel (Voytas, 2001)
Additional reagents and equipment for agarose gel electrophoresis, ethidium bromide staining, and gel photography (Voytas, 2001).
Add 2 μl of 10× topoisomerase I reaction buffer and 200 ng plasmid DNA (e.g., 10 μl of a 20 μg/ml stock) to each of a series of 1.5-ml microcentrifuge tubes on ice. Adjust volumes with distilled water so that the final reaction volume in each tube, including that of the protein or extract added in step 2, is 20 μl.
-
Add various amounts of purified topoisomerase I protein or cell extract to the tubes, then incubate for 30 min at 37°C.
For crude extracts, it is important to assay a range of protein concentrations. An appropriate range for intial assays is 5–250 μg/ml (0.1–5.0 μg protein per 20 μl reaction). If necessary, the extract may be diluted, but the diluted extract should be used promptly.
This assay can also be used for studying inhibition of relaxation catalyzed by topoisomerase I. The assay will be most robust if conducted within the linear range of enzyme concentration, as determined in the initial assay. The test agent under examination should normally be added before the enzyme. A solvent control must be included if the test agent is dissolved in a solvent other than water (commonly DMSO). If the solvent interferes with the reaction, it may be useful to reduce the solvent concentration by increasing the total reaction volume. The amount of enzyme added typically need not be changed if the reaction volume is increased slightly (up to 50 μl).
Add 5 μl of 5× loading dye to each tube and load contents on an 0.8% agarose gel. Run gel 2 to 3 hr at 5 to 10 V/cm (all procedures described in Voytas, 2001).
-
Stain gel with ethidium bromide, destain briefly with water, and photograph the gel illuminated with a UV transilluminator (Voytas, 2001).
CAUTION: As ethidium bromide is a mutagen it must be handled with gloves, and solutions disposed of according to institutional guidelines.
-
Determine the amount of topoisomerase I needed to completely relax a supercoiled plasmid under standard conditions.
See Figure 2 for an example of a topoisomerase I assay performed with purified protein. Also shown on Figure 2 are data demonstrating how an intercalating agent can interfere with topoisomerase I assays performed using the conditions described here. See Anticipated Results for interpretation of the findings.
Figure 2.

Gels obtained from topoisomerase I activity assays. Yeast topoisomerase I was purified from yeast cells expressing wild-type yeast topoisomerase I from the Gal1 promoter. (A) Lane λ, λ HindIII molecular weight markers; lane S, 0.1 μg pUC18 plasmid with no added protein; other lanes contain decreasing amounts of purified protein. Samples were electrophoresed on a 1.0% agarose gel. (B) The same conditions as in panel A, lane 4, were used, but increasing concentrations of ethidium bromide were added (from 100 ng/ml to 2 μg/ml ethidium bromide). Ethidium bromide is not an inhibitor of topoisomerase I, but because it intercalates and unwinds DNA, carrying out the reaction in the presence of ethidium bromide introduces positive supercoils in the DNA, which can be relaxed by topoisomerase I. Therefore, one might erroneously conclude that ethidium bromide inhibits topoisomerase I. Approaches to distinguish effects of intercalation are discussed in detail by Bailly (Bailly, 2001).
BASIC PROTOCOL 2
ASSAY OF TOPOISOMERASE II ACTIVITY
Topoisomerase II catalyzes relaxation of supercoiled DNA and the decatenation of intact double-stranded DNA, a property that allows the enzyme to separate replicated DNA molecules at mitosis (Chen, Chan, & Hsieh, 2013; McKie et al., 2021; Pommier et al., 2016). Unlike topoisomerase I, topoisomerase II requires ATP and a divalent cation. DNA decatenation is readily assayed utilizing kinetoplast DNA from Crithidia fasciculata as the substrate. These molecules form a large network of interlocked (catenated) circles (Marini, Miller, & Englund, 1980) which are unable to enter an agarose gel. Topoisomerase II decatenates the circles from the network, releasing free circles that migrate as a discrete band on the gel (or as singly catenated DNA, as described in a new variation of this assay discussed in Anticipated Results). Although topoisomerase I is fully active in the topoisomerase II reaction buffer, the former cannot decatenate circular substrates, making this assay selective for topoisomerase II.
Materials
10× topoisomerase II reaction buffer (see recipe)
Substrate: kinetoplast DNA (Topogen, http://www.topogen.com)
Purified topoisomerase II (1–5 units) (see Critical Parameters) or cell extract
5× loading dye (see recipe)
0.8% agarose gel (Voytas, 2001)
Additional reagents and equipment for agarose gel electrophoresis, ethidium bromide staining, and gel photography (Voytas, 2001)
Add 2 μl of 10× topoisomerase II reaction buffer and 200 ng kinetoplast DNA (e.g., 10 μl of a 20 μg/ml stock) to each of a series of 1.5-ml microcentrifuge tubes. Adjust volumes with distilled water so that the final reaction volume in each tube, including that of the protein or extract added in step 2, is 20 μl.
-
Add purified topoisomerase II protein or cell extract to the tubes, then incubate for 30 min at 37°C.
Topoisomerases from commercial suppliers are typically supplied based on units. Initial experiments should test a range of 1–5 units of enzyme for the conditions described here.
For cellular extracts it is important to use a range of enzyme concentrations. An appropriate range for intial assays is 5–250 μg/ml (0.1–5.0 μg protein per 20 μl reaction). At high enzyme concentrations, topoisomerase II will catalyze the catenation of circular DNA.
This assay can also be applied to studying inhibition of decatenation by experimental agents. Test agents are typically added before the enzyme. Inclusion of a solvent control is critical of the test agent is dissolved in a solvent (commonly DMSO) other than water. If the solvent interferes with the reaction, it may be useful to increase the reaction volume. The amount of enzyme added need not be changed if the reaction volume is increased slightly (up to 50 μl).
Place 5 μl of 5× loading dye into each tube and load contents on a 0.8% agarose gel. Run gel 2 to 3 hr at 5 to 10 V/cm (Voytas, 2001).
Stain gel with ethidium bromide, destain briefly with water, and photograph the gel illuminated with a UV transilluminator (Voytas, 2001).
-
Determine the amount of topoisomerase II needed to fully decatenate kinetoplast DNA under standard conditions.
See Figure 3 for an example of a decatenation reaction using kinetoplast DNA and purified topoisomerase II. See Anticipated Results for interpretation of the findings.
Figure 3.

Topoisomerase II decatenation assay. Different amounts of purified yeast topoisomerase II were added to reactions that contained 0.2 μg kinetoplast DNA as a substrate. Lane M, λ HindIII molecular weight markers; lane S, substrate DNA; lane 1, substrate and 5 ng purified yeast topoisomerase II; lane 2, substrate and 50 ng purified yeast topoisomerase II; lane 3, substrate and 100 ng purified yeast topoisomerase II; lane 4, substrate and 200 ng purified yeast topoisomerase II; lane 5, substrate and 500 ng purified yeast topoisomerase II; lane 6, substrate and 1 μg purified yeast topoisomerase II. The fluorescence just below the well in lane S is due to the catenated kinetoplast DNA that fails to enter the gel; the decatenated product is clearly seen in lanes 1 to 5. Samples were electrophoresed on a 0.8% agarose gel.
BASIC PROTOCOL 3
IN VIVO DETERMINATION OF TOPOISOMERASE COVALENT COMPLEXES USING THE IN VIVO COMPLEX OF ENZYME (ICE) ASSAY
Many drugs affecting DNA topoisomerases act by stabilizing the covalent complex, an intermediate state in the enzyme reaction (Delgado, Hsieh, Chan, & Hiasa, 2018; J. L. Nitiss, 2009c; Pommier, Leo, Zhang, & Marchand, 2010). Treatment with such agents increases the amount of topoisomerase covalently bound to DNA. The In Vivo Complex of Enzyme (ICE) protocol allows for the quantification of covalent protein–DNA complexes in cells, making it a useful assay for topoisomerase inhibitors. The ICE assay depends on the separation of free topoisomerase protein from topoisomerase bound to DNA in a cesium chloride gradient. The high concentrations of CsCl in the gradient (> 8M) promote dissociation of non-covalently bound protein from DNA. Because the buoyant density of DNA or of the DNA:topoisomerase covalent complexes is greater than that of free protein, during centrifugation covalently bound DNA-protein complexes migrate into the gradient while free protein remains near the top of the centrifuge tube. Under the conditions described below, free DNA, as well as topoisomerase-DNA covalent complexes, are pelleted by the centrifugation, while the free protein remains near the top of the centrifuge tube. As detection of the bound protein relies on antibodies, this assay can be used to assess either topoisomerases I or II, or to distinguish complexes formed by topoisomerase II isoforms α or β.
The experimental procedures described below can be used to determine whether a test agent stabilizes covalent complexes that include topoisomerases I and II. The results will reveal whether an agent is selective for a topoisomerase II isoforms in vivo. It can also be used to characterize cells treated with a topoisomerase-targeting drug to demonstrate alterations in drug action, such as characterizing resistance of a cell line to a class of topoisomerase targeting agents. A further enhancement of the ICE assay is described in a support protocol that involves the use of purified topoisomerases to generate standard curves that allow for determination of the numbers of covalent complexes per cell under a variety of conditions.
Two important shortcomings of the ICE assay should be noted. First, an ultracentrifuge and appropriate rotors are required, and the assay can only be applied to the number of samples matching the ultracentrifuge rotor capacity. Second, the assay requires approximately 1–2 ×106 mammalian cells per sample. Investigators lacking access to an ultracentrifuge, needing to analyze a large number of samples or fewer cells per sample should consider the RADAR/ELISA assay described in Basic Protocol 4 (see Commentary).
Materials
Cells of interest: e.g., HeLa (ATCC #CCL-2.2) or K562 (ATCC #HB-84)
Test compound(s): Camptothecin is a suitable Top1 poison, and etoposide is an appropriate Top2 poison. If an unknown compound is being tested, camptothecin or etoposide are suitable positive controls.
1% (w/v) Sarkosyl in 1× TE buffer (see TE buffer)
CsCl solution: add 75 g of CsCl (molecular biology grade) to 50 ml H2O; if necessary, warm to completely dissolve the CsCl (stable indefinitely at room temperature)
70% ethanol
1× TE buffer, pH 7.5
25 mM sodium phosphate buffer, pH 6.5
PBS-T: 1× phosphate-buffered saline (see recipe) containing 0.01% (v/v) Tween 20
Blocking solution: 5% (w/v) instant non-fat dry milk (Nestle, Carnation) in PBS-T
Primary antibody, e.g.:
Rabbit anti- human TOPIIα IgG antibody (Bethyl Laboratories, cat. no. BL 983)
Mouse anti-human TOPIIβ IgG antibody (BD Transduction Laboratories, cat. no. 611493)
Secondary antibodies:
For rabbit primary antibody: ECL anti-rabbit IgG, horse peroxidase-linked antibody from donkey (Amersham, cat. no. NA934V)
For mouse primary antibody: ECL anti-mouse IgG, horse peroxidase-linked antibody from donkey (Amersham, cat. no. NA934V)
Amersham ECL Plus Western Blotting Detection Reagents (GE Healthcare, cat. no. RPN2132)
100-mm culture dishes
14-ml polypropylene round-bottom tubes
1-ml latex-free syringe with 25-G 5/8 in. needle (Becton Dickinson)
3-ml latex-free syringe with a 16-G 1/2 in. precision-glide needle (Becton Dickinson)
4.9 ml OptiSeal tubes for ultracentrifugation (Beckman Coulter)
Beckman ultracentrifuge with NVT65.1rotor, or equivalent
Sharp blade
65°C water bath
Trans Blot nitrocellulose membrane (BioRad, cat. no. 162–0115)
Slot-format filter paper (BioRad, cat. no. 162–0161)
Slot blotting apparatus (Bio-Dot SF apparatus, BioRad, cat. no. 170–6542)
Hoefer Red orbital shaker, or equivalent
Additional reagents and equipment for quantitation of DNA (Desjardins and Conklin, 2011; Gallagher, 2011)
Day 1: Prepare cells for assay
-
1
Grow cells to 1–2 × 106 cells per 100-mm culture dish for analysis. Use one plate for each condition to be examined (i.e., control, plus one plate for each test compound concentration).
If a higher volume of cells is used, or if more than one plate needs to be combined to obtain the required cell number, then the amount of Sarkosyl buffer used in the later steps to collect cells can be increased from 1.5 to 2 ml. Using less than 5 × 105 cells, or more than 2 × 106 cells/ml, may compromise the reproducibility of the results. If using less than a million cells, then the pellet obtained at step 7 will be small, and there is a greater chance of losing the pellet while washing with 70% ethanol. The large pellet obtained when using a large number of cells can also be dislodged easily and can be lost while washing with 70% ethanol.
Day 2: Test compound treatment, harvesting of samples, preparation of cell lysate, and ultracentrifugation
-
2
If assessing covalent complexes with a small molecule, add test compound to plates after 24 hr cell growth/attachment. Incubate in the presence of the test compound for the desired length of time.
The length of time for test compound incubation depends on experimental goals. Typically, a 1-hr exposure is sufficient to obtain a good signal with standard agents such as camptothecin or etoposide. Longer compound exposure times may reduce the signal because of degradation of topoisomerases (Zhang et al., 2006; Nitiss, 2009c) or induction of apoptosis (Kaufmann, 1998).
-
3
After test compound exposure, aspirate the growth medium using suction, then add 1.5 ml of 1% Sarkosyl solution to the plates. Gently tap the plates from the sides to allow the Sarkosyl to reach all the cells on the plate. Leave the plates tilted on the bench at an angle of approximately 40° to allow the cell lysate to be collected on the edge of the culture plate. Using a 100- to 1000-μl pipet, collect the lysate into a 14-ml polypropylene round-bottom tube.
It is important to add the Sarkosyl solution as soon as possible after removing the medium.
-
4
The solution containing DNA is sheared to reduce its viscosity. Using a 1-ml latex-free syringe with 25-G 5/8-in. needle, shear 1 ml of lysate at a time by passing the lysate through the syringe ten times. After shearing, increase the final volume of the lysate to 3 ml using the 1% Sarkosyl solution.
Pull the solution into the syringe slowly to minimize detergent foaming.
If necessary, the lysate can be stored at −70°C for up to 24 hr. Longer periods of storage may be acceptable but have not been attempted by us.
-
5
After adding 2 ml of CsCl solution to a 4.9 ml Beckman OptiSeal tube, carefully layer the lysate on top of the CsCl using a 3-ml latex-free syringe with a 16-G 1/2 -in. precision glide needle. If necessary, top off the tube with the 1% Sarkosyl solution as ultracentrifuge tubes need to be completely full. Use a Kimwipe to wipe the top of the tube, then seal the tubes according to the manufacturer’s instructions.
Tubes should be completely full, as suggested by the manufacturer.
-
6
Place the sealed tubes in an NVT 65.1 rotor (Beckman), making sure to balance the tubes evenly if all slots are not filled. Add appropriate caps as recommended by the manufacturer. Mount the rotor in the ultracentrifuge chamber and centrifuge the tubes for 20 hr at 121,900 × g (42,000 rpm for the NVT 65.1 rotor) at 25°C.
Rotors other than the NVT 65. rotor can be used, although centrifugation times will likely need to be adjusted. We have had some trials with tabletop ultracentrifuges, and it is likely that they would be suitable for this protocol, with appropriate adjustments. Use of a fixed-angle rotor will likely require significantly longer centrifugation times. Be certain to follow all manufacturers instructions when using ultracentrifuges. Ultracentrifuge failure can result in severe injury.
Day 3: Recover DNA and apply the resuspended DNA solution to a membrane
-
7
Carefully remove the tubes from the ultracentrifuge rotor. To retrieve the pellet, first remove about 2 ml of the top layer. Using a sharp blade, cut approximately 1 cm from the neck of the tube. Carefully remove the rest of the supernatant either by decanting or using a pipet. Carefully remove any remaining liquid with a laboratory wipe.
The pellet is colorless and can be difficult to see. Take care not to dislodge the pellet when removing the supernatant. When all the solution is removed, a glossy oval viscous pellet is noticeable and can be marked easily.
-
8
Wash the pellet once with 500 μl of 70% ethanol. Gently remove the ethanol with suction, being careful to avoid dislodging the pellet. Allow any residual ethanol to evaporate by resting the tube containing the pellet for at least 15 sec prior to subsequent steps.
-
9
Dissolve the pellet by adding 500 μl of TE buffer, pH 7.5, and vortexing. Make certain the volume added is sufficient for the pellet to be completely immersed in TE buffer. Incubate overnight at 4°C to complete the dissolution of the DNA.
-
10
After overnight incubation in TE buffer for resuspension, incubate the solution in a water bath at 65°C for 5 min. After cooling to room temperature, transfer the solution to 1.5-ml microcentrifuge tubes.
-
11
Measure the DNA concentration of the samples (e.g., by absorbance at 260 nm; Desjardins and Conklin, 2011; Gallagher, 2011).
A typical DNA concentration under these conditions is 100 to 200 ng/μl.
Day 4: Apply DNA solution to a nitrocellulose membrane and measure associated topoisomerase protein by immunoblotting
-
12
Dilute the DNA solution with 25 mM sodium phosphate buffer, pH 6.5, so that the total volume to be applied is 200 μl. Typically, 2 to 20 μg of DNA will be applied per sample.
-
13
Prepare the nitrocellulose membrane by equilibrating it in 25 mM sodium phosphate buffer, pH 6.5, for 15 min before placing the membrane on the slot blotting apparatus. Immerse two slot-format filter paper sheets in 25 mM sodium phosphate buffer, pH 6.5, for 15 min as well. Afterwards, place the wetted filter paper sheets on the slot blot apparatus, and place the nitrocellulose membrane over the filter paper.
-
14
Assemble the slot blot apparatus according to the manufacturer’s instructions. Wash all the membrane slots with 200 μl of sodium phosphate buffer, pH 6.5, using a p200 multichannel pipetor, and apply vacuum. Release the vacuum and apply the DNA solution to the slots. Apply vacuum until the samples have passed through the filter. Make certain all of the samples have passed through the membrane. Release vacuum and add 200 μl sodium phosphate buffer, pH 6.5, to all the slots. Apply vacuum until the wash has passed through the membrane. Open the slot blot apparatus and carefully remove the membrane.
-
15
Place the membrane in 50 ml PBS-T and incubate for 5 min to wash the membrane. Discard the wash and block the membrane using 10 ml 5% non-fat dry milk prepared with PBS-T for 1 hr at room temperature on a shaker at medium speed (Hoefer Red orbital shaker or equivalent).
-
16
Discard the blocking solution, then add an appropriate dilution of primary antibody in the blocking solution. Incubate overnight with shaking at 4°C.
The appropriate dilution of antibody is determined empirically. Dilutions suitable for immunoblot analysis are a good starting point for detection of topoisomerases.
The list of materials above provide suggestions for topoisomerase IIα and topoisomerase IIβ antibodies. There are a very large number of antibodies available that target topoisomerases, and suppliers frequently discontinue antibodies with little notice. If a large series of experiments is anticipated, and a well-performing antibody is available, it is recommended that a sufficient stock of antibody be procured to ensure that there will be no issues with future availability.
Day 5: Treat membrane with secondary antibody and detect immune complex
-
17
After overnight incubation with primary antibody, discard the solution. Rinse with PBS-T and pour off the solution.
-
18
Proceed with treatment with appropriately diluted secondary antibody in blocking solution (typically 1 hr at room temperature with shaking).
-
19
Rinse with PBS-T and pour off the solution. Wash with PBS-T for 20 min and then discard the buffer. Wash the membrane again three times with PBS-T, 5 min for each wash at room temperature with shaking.
-
20
Detect immune complexes using any conventional method such as ECL (using a kit and following manufacturer’s recommended conditions).
See Figure 4 for an example of an ICE assay detecting topoisomerase IIα complexes in HeLa cells treated with etoposide. See Anticipated Results for interpretation of findings.
Figure 4.

ICE assay of topoisomerase II covalent complexes. HeLa cells were treated with various etoposide concentrations for 1 hr. The ICE assay was performed as described in Basic Protocol 3, and the slot blots of DNA recovered from the pellet after CsCl centrifugation, probed with an antibody directed against topoisomerase IIα, are shown in panel (A). The signal arises from topoisomerase II that covalently associates with DNA. Panel (B) shows a quantification of the signal from the blot shown in panel A.
SUPPORT PROTOCOL 1
PREPARATION OF MOUSE TISSUE FOR DETERMINATION OF TOPOISOMERASE COVALENT COMPLEXES USING THE IN VIVO COMPLEX OF ENZYME (ICE) ASSAY
The procedure below describes the initial processing required for mouse tissue samples before conducting the ICE assay (Basic Protocol 3). The assay has been used to assess the roles of repair functions in mouse neuronal development (Katyal et al., 2014).
Materials
Mouse tissue sample. This will typically be obtained from animals generated by the investigator. There is no current experimental evidence using samples obtained commercially or from tissue banks.
1% (w/v) Sarkosyl in 1× TE buffer (see TE buffer)
2-ml tissue grinder (Wheaton)
14-ml polypropylene round-bottom tube
1-ml latex-free syringe with 25-G 5/8 in. needle (Becton Dickinson)
Additional reagents and equipment for ICE Assay (Basic Protocol 3)
Place 50 to 100 mg of rinsed mouse tissue in ice-cold tissue grinder containing 1.5 ml 1% Sarkosyl.
-
Homogenize the tissue samples a minimum of 10 times. Collect the homogenized samples in a 14-ml polypropylene round-bottom tube.
Depending upon the viscosity of the solution, additional homogenization or additional volumes of 1% Sarkosyl buffer may be required.
-
Further shear the homogenized samples to reduce viscosity as follows: shear 1 ml of lysate at a time using a 1-ml latex-free syringe with 25-G 5/8-in. needle. Pass the lysate through the syringe ten times. After shearing, increase the final volume of the lysate to 3 ml using 1% Sarkosyl solution.
Pull the solution into the syringe slowly to minimize detergent foaming.
If necessary, the lysate can be stored at −70°C for up to 24 hr. Longer periods of storage may be acceptable, they have yet to be attempted.
If it is required to get rid of cellular debris, the sheared lysate may be centrifuged for 15 min at 300 × g at 4°C.
Follow Basic Protocol 3 from step 5 onwards, applying the Sarkosyl buffer lysate to a CsCl gradient to isolate the covalent complexes for later detection using the slot blot assay.
SUPPORT PROTOCOL 2
USING RECOMBINANT TOPOISOMERASE STANDARDS FOR ABSOLUTE QUANTIFICATION OF CELLULAR TOP2CC
An important issue with the ICE assay is expressing the overall level of topoisomerase covalent complexes. Typically, the signal obtained in the absence of inhibitor is low. An unbiased way to express the results from ICE assays is to relate the signal to the total level of covalently bound topoisomerase. Because purified topoisomerases are readily available from multiple commercial sources (see Commentary) it is easy to prepare a standard curve and express the signal obtained as ng of covalently bound topoisomerase. While the example presented quantifies TOP2α and TOP2β, it can be readily adopted for TOPOISOMERASE I.
Materials
purified recombinant TOP2α and TOP2β proteins
25 mM sodium phosphate buffer, pH 6.5
PBS-T: 1× phosphate-buffered saline (see recipe) containing 0.01% (v/v) Tween 20
Blocking solution: 5% (w/v) instant non-fat dry milk (Nestle, Carnation) in PBS-T
Primary antibody, e.g.:
Rabbit anti- human TOP2α IgG antibody (Bethyl Laboratories, cat. no. BL 983)
Mouse anti-human TOP2β IgG antibody (BD Transduction Laboratories, cat. no. 611493)
Secondary antibodies:
For rabbit primary antibody: ECL anti-rabbit IgG, horseradish peroxidase-linked antibody (Amersham, cat. no. NA934V)
65°C water bath
Orbital shaker
Trans Blot nitrocellulose membrane (BioRad, cat. no. 162–0115)
Slot-format filter paper (BioRad, cat. no. 162–0161)
Slot blotting apparatus (Bio-Dot SF apparatus, BioRad, cat. no. 170–6542)
SuperSignal™ West Femto Maximum Sensitivity Substrate (Thermo Scientific, cat. no. 34094)
Bio-Rad ChemiDoc™ Imaging System
ImageJ2 software (Rueden et al., 2017)
Day 1: Apply purified recombinant topoisomerase protein to a nitrocellulose membrane for slot blotting
-
1
Dilute the recombinant topoisomerase II protein with 25 mM sodium phosphate buffer (pH 6.5) in a 1.5 ml Eppendorf tube so that the total volume to be applied is 200 μl. Two-fold serial dilutions of topoisomerase II proteins (0.5, 1, 2, 4 ng) will be applied to slot blot.
-
2
Denature the samples in a 95°C water bath for 5 min. After cooling to room temperature, run the recombinant proteins along with ICE assay samples on a slot blotting apparatus as described above.
-
3
Rinse the slot blot membrane in PBS-T then transfer the membrane to the blocking solution (5% non-fat dry milk prepared with PBS-T) for 30 min at room temperature on a shaker at medium speed (Hoefer Red orbital shaker or equivalent).
-
4
Discard the blocking solution, then add primary antibody in blocking solution (1: 1,000 dilution). Incubate samples overnight with shaking at 4°C.
Day 2: Treat membrane with secondary antibody and detect recombinant topoisomerase
-
5
Discard the solution after overnight incubation with primary antibody. Wash the membrane one time for 15 mins and three times with PBS-T, 5 min for each wash at room temperature with shaking.
-
6
Proceed with treatment with secondary antibody in blocking solution (1; 10,000 dilution) for 1 hr at room temperature with shaking.
-
7
Wash the membrane three times with PBS-T, 5 min for each wash at room temperature with shaking.
-
8
Incubate the membrane with SuperSignal™ West Femto Maximum Sensitivity Substrate or other alternative ECL substrates following manufacturer’s instructions
-
9
Detect the recombinant topoisomerase II protein using ChemiDoc Imager or alternative imaging system following manufacturer’s instructions.
Day 3: Densitometric analysis of the slot blot image of recombinant topoisomerase
-
10
Export the acquired images as TIFF files and open the files in ImageJ.
-
11
Under the “Image” menu select “Type”. Change the image to 8 bit.
-
12
Under the “Analysis” menu select “Set Measurements”. Check only the “Integrated Density” in the check boxes.
-
13
Under the “Analysis” menu select “Set Scale”. Click the button “Click to Remove Scale”.
-
14
Under the “Process” menu select “Subtract Background”. Set rolling ball radius to 50 pixels and select “light background”.
-
15
Under the “Edit” menu select “Invert”.
-
16
Use the rectangular selection tool to outline the slot and select “Measure” under the “Analysis” menu.
-
17
Quantify the proteins by calculating the ratio of each slot relative to the slot of 0.5 ng recombinant topoisomerase II protein (the lowest amount).
-
18
Plot the weight of the recombinant protein (x) versus the relative density (y) for linear regression.
-
19
Determine the absolute amount of Topcc from the ICE assay by substituting its relative density to the linear equation determined in step 18.
-
20
An example is shown in Figure 5.
Figure 5.

Generation of a standard curve for quantification of ICE assays. A. Slot blot of 2 μg ICE assay samples from RH30 cells treated with etoposide of indicated concentrations and purified recombinant topoisomerase IIα protein of indicated weights, followed by immunodetection using anti-topoisomerase IIα antibody. B. Densitometric quantification of the slot blot of purified recombinant topoisomerase IIα protein in panel A by ImageJ. C. Slot blot of 2 μg ICE assay samples from RH30 cells treated with etoposide of indicated concentrations and purified recombinant topoisomerase IIβ protein of indicated weights, followed by immunodetection using anti-topoisomerase IIβ antibody. D. Densitometric quantification of the slot blot of purified recombinant topoisomerase IIβ protein in panel C by ImageJ.
BASIC PROTOCOL 4
QUANTIFICATION OF TOPOISOMERASE-DNA COVALENT COMPLEXES BY RADAR/ELISA: THE RAPID APPROACH TO DNA RECOVERY (RADAR) COMBINED WITH THE ENZYME-LINKED IMMUNOABSORBENT ASSAY (ELISA)
The Rapid Approach to DNA Adduct Recovery (RADAR) assay accommodates samples of small and large volume and allows for many samples to be assayed in parallel within a single day. RADAR extraction relies on stringent cell lysis in a combination of chaotropic salt and detergents to dissociate non-covalently bound protein from DNA, followed by alcohol precipitation to separate DNA-protein complexes from free protein (Kiianitsa & Maizels, 2013, 2014), Figure 6. This overcomes two limitations of the ICE assay (Basic Protocol 3): dependence on ultracentrifugation and the requirement for relatively large amounts of sample.
Figure 6.

RADAR enrichment followed by ELISA or characterization by Western blotting. The steps prior to analysis are illustrated in the top three lines of the schematic: the free protein (circles), DNA (double lines), and DNA bearing covalent bound protein are first enriched by RADAR lysis and isopropanol precipitation; recovered DNA and DNA carrying adducts are digested with Benzonase to release adducted protein; and proteins analyzed by ELISA or characterized by Western blotting. ELISA (left) relies on spontaneous binding to activated polystyrene to immobilize recovered protein on plastic wells, which is then detected by probing with specific antibody. Western blot (right) detects adducted proteins with specific antibody.
As described here, specific proteins in samples prepared by RADAR fractionation can be quantified and characterized by ELISA (Kiianitsa & Maizels, 2014; Sinha, Kiianitsa, Sherman, & Maizels, 2020), Basic Protocol 4, Figure 7; by slot blot (Kiianitsa & Maizels, 2013); or by Western blot (Kiianitsa, Zhang, & Maizels, 2020; Sun et al., 2020), Basic Protocol 5, Figure 8. DNA fragments recovered from the final purification step can also be used for genomic mapping of sites of adduct formation (Adduct-Seq) (Husain et al., 2016; Kiianitsa et al., 2020), Basic Protocol 5 Support Protocol. RADAR extracts are also suitable for unbiased characterization of adducted proteins by mass spectrometry (Kiianitsa & Maizels, 2020) and for monitoring global adduct burden (Hu et al., 2020).
Figure 7.

RADAR/ELISA assay of topoisomerase I-DNA adducts in cells treated with camptothecin. GM639 fibroblasts or HCT116 colon carcinoma cells were seeded in plates at 4 × 10^4 cells/well were cultured overnight, then briefly further cultured for 15 min in fresh medium containing no drug or 5 μM camptothecin, after which cells were harvested, RADAR extracts generated, and samples resuspended in 200 μl final volume. A. To quantify DNA yield, 5% of each sample was aliquoted to a new plate and DNA concentration determined with PicoGreen. B. To quantify induction of topoisomerase I in response to drug treatment by ELISA, 10% of each sample was aliquoted, treated with Benzonase to digest nucleic acids, and transferred to an ELISA plate for immunodetection. Diagram on left illustrates hypothetical signals from analysis of control samples containing no DNA (column 2), or cells treated with (columns 3,5,7,9) or without (columns 4,6,8,10) camptothecin. Graph on right shows Topoisomerase I signal determined by ELISA assay.
Figure 8.

RADAR/Western characterization of PARP1-DNA adducts formed in GM639 cells treated with 5-aza-deoxycytidine (5-aza-dC). PARP1 is a DNA repair factor that migrates to the nucleus in response to DNA damage and forms covalent adducts with DNA (Kiianitsa et al., 2020; Prasad et al., 2014; Prasad et al., 2019). In apoptotic cells, PARP1 undergoes regulated proteolysis that removes the N-terminus, converting the 113 kD polypeptide to an 89 kD fragment, a hallmark of apoptosis. Patients with acute myelogenous leukemia (AML) are treated with 5-aza-deoxycytidine (Estey, 2016), making it of interest to determine whether this treatment affects the integrity of PARP1 and PARP1-DNA adducts. RADAR fractions were generated from nuclear pellets of GM639 cells treated with 5-aza-dC (20 μg DNA equivalent per lane), then probed with antibodies to intact PARP1 or caspase-cleaved PARP1.
Left: RADAR/Western blots probed with antibody that recognize intact PARP1 (Enzo Life Sciences ALX- 210− 302-R100; 1:4000 dilution; secondary BioLegend #406401, 1:5000 dilution) revealed induction of 113 kD PARP1 covalent adducts in response to 48 hr treatment with 1 μM 5-aza-dC. Right: RADAR/Western blots probed with a monoclonal antibody specific to the neoepitope generated upon caspase cleavage of PARP1 (BD Biosciences #552596, 1:1000 dilution; secondary (BioLegend, #405306, 1:5000 dilution) revealed that adducts containing cleaved PARP1 appeared at 72–96 hr of drug treatment. Smaller PARP1 fragments are also evident at late times, suggesting that adducted PARP1 undergoes further degradation following apoptotic cleavage.
The RADAR assay is readily adapted for studies of specific adducts and cell types for diverse applications, including analyses of the roles of SUMO (Sun et al., 2020) and SPRTN (Fielden et al., 2020) in resolution of topoisomerase I-DNA adducts; detection of endogenous adducts resolved by GCNA (Bhargava et al., 2020); characterization of covalent DNA and RNA adducts formed by TOP3B (Saha et al., 2020); identification of roles for adducts formed by HMCES in protection of abasic sites in single-stranded DNA (Mohni et al., 2019); and establishment of cell cycle-dependent crosslinking of the Epstein-Barr virus EBNA1 regulatory protein to viral episomes (Dheekollu et al., 2021).
RADAR/ELISA of cells cultured in microplate format (2–4×104 cells/well)
The approach described below, and outlined in Figure 7, was designed for applications using 96 well microplates containing 75–150 μl of culture medium and 2–4 × 104 cells per well. It is compatible with robotic liquid handling stations and work in semi-automatic mode, except for transfer to the centrifuge and precipitation steps. It can be scaled down for analysis of cells cultured in 384 well microplates without loss of sensitivity.
Materials
Test compound(s): Camptothecin or etoposides are appropriate poisons for topoisomerases I and II, respectively. If an unknown compound is being tested, camptothecin or etoposide may be suitable positive controls.
Cells of interest: e.g. K562 multipotential, hematopoietic malignant cells (ATCC CCL-243), CCRF-CEM acute T cell lymphoblastic leukemia (ATCC CRM-CCL-119), HeLa cervical carcinoma cells (ATCC CCL-2.2), GM00639 SV40-transformed fibroblasts (RRID:CVCL 7299); HCT116 colorectal carcinoma (ATCC CCL-247).
Cell culture medium, composition determined by cell type studied
Ultrapure™ Guanidine Isothiocyanate (Thermo-Fisher, #15535016)
Silica gel fines, 4% (Sigma #288519)
Sarkosyl (Reagent grade)
100% ethanol, 75% ethanol
isopropanol
Sodium-Acetate; 3 M, pH 5.3
Tris-HCl; 1 M, pH 8.0
Sodium-EDTA; 0.5 M, pH 8.0
Dithiothreitol (DTT); 200 mg/ml solution, maintained as frozen stock
5 N NaOH (made fresh every 3 months, stored in plastic container)
12 M LiCl in dd H2O
PBS-T: 1× phosphate-buffered saline (see reagents and solutions) containing 0.01% (v/v) Tween 20
Lysis Solution (LS): See reagents and solutions.
Microplates for cell culture
Heat-resistant adhesive film for microtiter plates (Qiagen Tape Pads or similar)
V-Shaped bottom polypropylene microtiter plates (250 μl capacity)
Nunc MaxiSorp™ flat-bottom microtiter plates (ThermoFisher Scientific, Waltham, MA)
Benzonase nuclease (Millipore-Sigma 103773, St. Louis, MO)
ELISA Coating Buffer (BioLegend, San Diego, CA), used to block plates coated with RADAR extract
ELISA Assay Diluent (BioLegend, San Diego, CA), used to dilute primary and secondary antibodies
TMB High Sensitivity Substrate Solution (BioLegend, San Diego, CA), the ELISA substrate
TMB Stop Solution (BioLegend, San Diego, CA), stops the ELISA reaction
Rabbit polyclonal IgG anti-topoisomerase I antibodies (ab28432, Abcam, Waltham, MA) Secondary anti-rabbit HRP-conjugated antibodies (HRP Donkey anti-rabbit IgG (minimal cross-reactivity) antibody (BioLegend, #406401)
Equipment
For shaking microplates: ThermoFisher Scientific (Waltham, MA) or Eppendorf F-series ThermoMixer with thermoblock for microplates (Eppendorf USA, Framingham, MA)
For quantifying dsDNA: PicoGreen™ reagent (ThermoFisher P7589); or NanoDrop 2000 or Qubit 4 fluorometer (ThermoFisher)
For quantifying both ELISA signal and dsDNA: plate reader with fluorometer capacity (SpectraMax ABS absorbance reader, Molecular Devices, Silicon Valley, CA)
For quantifying ELISA signal but not dsDNA: plate reader, range 450–570 nm
RADAR/ELISA protocol
Extracts are best prepared from cells which have been actively dividing but not reached confluence. Determine growth rate and confluence density in a pilot experiment using the same medium, culture plates and culture conditions as will be used for the assay.
Day 1: Seed cultures for RADAR extraction from 2–4 × 10^4 cells
-
1
Seed culture plates with 0.5–1.0 × 10^4 cells per well. Set up triplicate samples for each control and culture condition (e.g drug concentration, time point) to ensure results are statistically robust. Culture 24–36 hr. Culture adherent cells in flat-bottom wells, suspension cells in U-bottom wells.
To obtain several-fold larger samples, cells can be grown, drug-treated and lysed in multiplicate, then lysates combined for downstream steps in adduct recovery.
Day 2–3: Treat cells with drug and prepare RADAR extracts
-
2
Add compound in desired concentration range. Typically, cells should be treated with test agent in mid- to late-log stage, at densities of approximately 5 × 105 cells/ml. At least one cell doubling should occur before test compound addition (18–24 hr culture).
-
3
Incubate cells in the presence of the test compound for the desired length of time.
The length of time for test compound incubation depends on the compound and the cells. Typically, 1 hr is sufficient to obtain a good signal from topoisomerases following treatment with standard agents such as camptothecin or etoposide. Extended treatment may reduce the signal because topoisomerases undergo proteolytic degradation (Zhang et al., 2006; Nitiss, 2009c) or because the test compound induces apoptosis and cell lysis (Kaufmann, 1998).
-
4
Harvest cells by centrifugation at RCF 2,740 × g (3,500 RPM), 5 min, room temperature. Remove medium by careful aspiration, leaving cells in well.
-
5
To each well add 100 μl LS, then seal plates with heat-resistant adhesive film, shake 5 min on a plate shaker at room temperature, transfer to 55°C incubator for 15 min, then shake an additional 15 min at room temperature. Add 20 μl 12 M LiCl (2M final concentration) and briefly mix (1–2 min) on plate shaker.
-
6
Prepare a v-bottom polypropylene plate (250 μl capacity) containing 3–6 mg/well silica fines by adding 75–150 μl of 4% silica gel fines suspended in LS per well to a plate, then pelleting silica by brief centrifugation and removing liquid by aspiration.
-
7
To precipitate nucleic acids and covalently bound proteins, transfer 120 μl RADAR lysates to plate containing silica fines. Incubate lysates with silica for 15 min on shaker at room temperature, then add an equal volume of isopropanol (120 μl). (Alternatively, one may first transfer 120 μl RADAR lysate to a v-bottom polypropylene plate; 4% silica gel fines are prepared in isopropanol and 120 μl suspension is added as the last component). Seal plate, mix by inversion, and immediately centrifuge at RCF 2,740 × g and aspirate supernatants. Wash pellets with 200 μl 75% ethanol, centrifuge for 5 min at RCF 2,740 × g, then aspirate supernatant. Re-wash pellets with 100 μl 100% ethanol, centrifuge for 5 min at RCF 2,740 × g, then aspirate supernatant. Air dry pellets at room temperature for 5–15 min, until they become whitish.
-
8
To elute nucleic acids and covalently bound proteins, add 100–200 μl of 8 mM NaOH (made fresh from 5 M NaOH stock), shake for 15 min on a plate shaker and immediately centrifuge for 5 min at RCF 2,740 × g.
-
9
Transfer eluted material (supernatant) to a new microplate for storage (e.g. 200 μl capacity polypropylene plates suitable for PCR). Neutralize samples by addition of 1:50 volume 1 M HEPES (20 mM final concentration). These samples may be stored at −20°C for up to 2 months prior to further analysis
Day 3: Quantify dsDNA to assess yield of RADAR extracts and quantify ELISA signal
-
10
To determine dsDNA concentration, aliquot a fixed volume of the eluted sample to a new microplate containing 50 μl TE/well and quantifying dsDNA per well with PicoGreen™, and assay following manufacturer’s instructions.
Yields following RADAR extraction are expressed in terms of dsDNA content, because adducts are covalently bound to DNA and DNA recovery per well is usually quite reproducible. In contrast, yields of RNA and protein may vary considerably among samples, reflecting natural differences in RNA and protein content due to differences in size and nucleus/cytoplasm ratio among different cell types. The typical yield of dsDNA is 7–8 μg per 106 human diploid cells, which corresponds to 75 ng genomic DNA per 104 cells. dsDNA yield in RADAR extracts may vary if cells are undergoing lysis in response to drug treatment, so it is important to quantify dsDNA in each sample.
Depending upon availability of equipment, dsDNA concentration may alternatively be measured using the Qubit fluorometric assay, or by reading A260 on a plate reader or with a NanoDrop spectrophotometer.
If extracts have been prepared from sonicated cells, or undergo extended exposure to alkaline conditions, they may contain ssDNA which is undetectable in a dsDNA specific fluorescent assay. In these cases it may be useful to determine UV absorbance of RADAR samples.
The alcohol precipitation step in RADAR fractionation separates free protein from DNA. Reduction in protein content can be used to quality control fractionation, by monitoring protein levels using the BCA assay.
-
11
Coat and block ELISA plates. RADAR/ELISA takes advantage of the intrinsic affinity of proteins for activated polystyrene. Nucleic acids may interfere with this, so they are first eliminated by treatment with Benzonase nuclease. Supplement samples containing 20–100 ng ds DNA with 2 mM MgCl2 and treat with 12.5 U per sample Benzonase nuclease for 30 min at 37°C to digest DNA and RNA. Adjust sample to 1× ELISA Coating Buffer (Biolegend), apply 50–100 μl to a new, untreated 96 well ELISA plate (Nunc MaxiSorp™ or similar), and incubate on a plate shaker for 1–2 hr at room temperature or overnight at 4°C. Wash plates 3 times with 150–200 μl 1x PBS, then block for 1 hr with 100 μl of 1x ELISA Assay Diluent (Biolegend).
Include control wells that contain the same buffer but no sample. Additional controls may contain the protein to be detected in the assay, provided as purified protein or in cell extracts.
ELISA plates have limited capacity to absorb protein, which is approximately 100 ng total protein per well in a 96 well plate. Thus it is important that the total protein added per well does not exceed the protein absorption capacity. When adducts are at low abundance it may be necessary to load relatively high amounts of sample per well. In this circumstance, care must be taken to ensure the amount loaded does not exceed the linear range of the ELISA signal. Control assays on serially diluted samples should be conducted to determine the signal linearity range.
-
12
Detect bound protein. To detect the protein of interest, incubate plates with 60 μl primary antibody, wash 4 times with 150 μl 1x ELISA Wash Buffer (BioLegend), incubate with 60 μl secondary antibodies, then wash again 4 times with 150 μl 1x ELISA Wash Buffer. Add 100 μl of TMB High Sensitivity Substrate Solution (Biolegend), incubate plates in the dark for 20 min at room temperature. Terminate the reaction by adding 50 μl TMB Stop Solution (BioLegend).
TMB Stop Solution reagent is diluted sulfuric acid! Take necessary safety precautions when handling it.
-
13
Quantify ELISA signal. Use a plate reader to determine ELISA signal. First, determine signals at A450 (specific) and at A570 (non-specific, reflecting contributions from plastic, debris. Correct by subtraction: A450-A570 = ELISA signal. Next, correct for the assay background by subtracting averaged signal from control wells that contained no DNA from averaged signals from experimental samples.
An example is provided in Figure 7 of detection of topoisomerase I-DNA adducts in cells treated with 5 μM camptothecin, a topoisomerase I inhibitor, for 15 min at 37°C, using anti-topoisomerase I antibodies (polyclonal rabbit IgG, ab28432, Abcam) at 1:2,000 dilution and secondary anti-rabbit HRP-conjugated antibodies (BioLegend) at 1:5,000 dilution for 30–45 min.
The sensitivity of immunodetection can be enhanced by using biotinylated secondary antibodies followed by multivalent streptavidin-HRP conjugates for detection. Alternatively, “sandwich” ELISA assays can be employed to measure antigen between two layers of antibodies, one for capture and one for detection (Sinha et al., 2020).
Proteins enriched as above can be characterized not only by ELISA but also by slot blots, using the procedure described in detail for slot blot analysis of ICE samples (Basic Protocol 3).
Quantification of adducts in RADAR fractions by ELISA is at least as convenient as a slot blot, if not more so. Moreover, assays of replicate samples at a series of dilutions ensure that the data are reliable and facilitate quantitative comparison of samples that contain very different amounts of the target protein.
It may be useful on occasion to quantify adducts in the culture medium as well as in intact cells. Provided that the cell culture medium is compatible with the RADAR lysis solution, this can be accomplished by conducting the assay on the entire culture, without pelleting cells prior to lysis. Compatibility of media can be tested by mixing medium alone with RADAR lysis solution at a concentration sufficient to lyse washed cells. If an insoluble precipitate forms, it will be necessary to adjust the components of the culture medium to avoid formation of the precipitate.
BASIC PROTOCOL 5
ANALYSIS OF PROTEIN-DNA COVALENT COMPLEXES BY RADAR/WESTERN
This procedure, outlined in Figure 8, uses many of the same reagents, materials and equipment as the RADAR/ELISA protocol, but as more cells are processed it requires different plastic consumables, as cells are cultured on plates (adherent cells) or in flasks (suspension cells) and extracts prepared in tubes rather than microtiter plates. The protocol below can be used to generate RADAR extracts from 2–4 × 106 mammalian cells, and may be scaled up for larger cell numbers. Perform manipulations at room temperature unless stated otherwise.
Materials
Flasks or plates for cell culture
TrypLE Select Enzyme (ThermoFisher #12563011)
Corning polypropylene 15 ml and 50 ml for cell recovery and extract preparation (Sigma-Aldrich, Tewksbury, MA)
Polystyrene 1.5 ml sonication tubes with caps (Active Motif #53071, Carlsbad, CA)
Low protein binding 1.5 ml tubes (Thermo Scientific #904010)
Protease inhibitor cocktail (e.g. Halt protease inhibitor, ThermoFisher #87786)
14-ml Falcon polypropylene round-bottom tubes (Fisher Scientific #352059)
Precast 4–12% gradient protein gels (Bolt, Invitrogen)
Nitrocellulose membrane (0.45 μM pore, Invitrogen)
Blocking solution: 0.5% alkali-soluble casein (Novagen) in PBS-T
SuperSignal™ West Dura Extended Duration Substrate (Thermo-Fisher)
Primary and secondary antibodies
Cup horn sonicator (QSonica Q800R3 or similar) for high efficiency indirect sonication of multiple samples; or standard sonicator
Day 1: Seed cultures for RADAR extraction from 2–4 × 10^6 cells
-
1
Seed wells by dispensing cells at 10–20% the desired final density. Culture 24–48 hr.
Extracts are best prepared from cells which have been actively dividing but not reached confluence. Determine growth rate and confluence density in a pilot experiment using the same medium, culture plates and culture conditions as will be used for the assay.
Day 2–3: Harvest, wash and count cells
-
2
Harvest cells.
For suspension cells: harvest by centrifugation, wash pellet once in 1 ml PBS and resuspend in 1 ml PBS.
-
For adherent cells: transfer 10 ml of medium from each 100 mm culture dish to a 15 ml tube, detach remaining cells with 2 ml TrypLE Select Enzyme, transfer suspension to the tube containing medium, pellet cells and resuspend in 1 ml PBS.
Centrifugation at RCF 2,740 × g (3,500 RPM in a standard tissue culture centrifuge) for 10 min is appropriate for small cells, such as T cell leukemia cells, while larger cells, such as HeLa or SV40 transformed fibroblasts, may be harvested at lower speeds.
The PBS wash step is especially important for samples containing more than 107 cells and for applications sensitive to the components of serum used for cell culture, such as silver staining of protein gels or proteomic analysis. For adducts that can resolve rapidly in the absence of drug, as often occurs with topoisomerases, the test substance should be present in the washing buffer. Alternatively, omit the washing step if cells will be lysed immediately after harvest.
-
3
Count washed cells.
-
4
Transfer cells to a 1.5 ml polystyrene tube suitable for sonication. Pellet cells by centrifugation for 5 min in a microcentrifuge. Remove supernatant.
-
5
Lyse cells. Add 400 μl RADAR lysis reagent (LS). Resuspend the pellet by gentle vortexing or by shaking on a ThermoMixer for 5–10 min at 1,200 rpm at room temperature. Dispense 400 μl aliquots of lysate into sonication tubes.
If processing more than 1 × 107 cells, resuspend pellets in 1 ml LS per 20 × 106 cells, then reduce viscosity by passing 8–12 times through a 22G 1½ inch needle.
-
6
Sonicate cells. Pulse ten times, 30 sec/pulse at amplitude 100, with a 60 sec cool-off period between pulses. Note: It is essential to use an ice water bath for effective cooling during this process.
Sonication improves efficiency of lysis, so analysis can be carried out on fewer cells or the same lysate volume can accommodate more cells. Sonicated LS lysates are clear and non-viscous, with no insoluble particulates evident following clearing. They are superior for Western blots and other downstream applications including slot blots and Adduct-Seq. Multiple samples can be subject to indirect sonication using a Cup horn sonicator in an ice bath. Sonication can also be performed on a small number of samples with a standard sonicator, keeping samples cold during sonication and allowing a cooling off period between pulses.
Sonicators are subtly different from one another and it is important to follow manufacturer’s instructions for each specific instrument. Sonication yields DNA of average size 500 bp.
-
7
Clarify lysate. Transfer sonicated lysate (400 μl) to a 1.5 ml polypropylene tube, add 80 μl 12 M LiCl (final concentration 2 M LiCl), mix and incubate for 10 min at 37°C on a Thermomixer at 1,600 RPM. Centrifuge at 14,000 rpm for 10 min, then recover the supernatant conservatively by aspiration.
-
8
Recover DNA-protein adducts. Transfer lysate (approximately 450 μl) to a new, 1.5 ml low protein binding tube.
-
9
Add an equal volume of isopropanol, mix, and precipitate nucleic acids and covalently bound protein by centrifugation at 14,000 × g for 10 min.
-
10
Wash pellet thrice with 1 ml 75% ethanol, followed by centrifugation at 14,000 × g for 2 min after each wash.
-
11
Resuspend nucleic acid pellet. Add 100 μl of freshly prepared 8 mM NaOH to the cell pellet, and then shake on a Thermomixer for 15–30 min at 1,600–2,000 RPM at room temperature. Neutralize by addition of 2 μl of 1 M HEPES, pH 7.5 (final concentration 20 mM).
These samples, referred to as the R1 fraction, contain dsDNA and variable amounts of RNA and protein, depending on the cell type. Samples can be stored at −80°C for at least 2 months prior to analysis.
Characterize adducted proteins by Western blotting
-
12
Treat neutralized samples with Benzonase nuclease (EMD Millipore; 0.5 units per μg DNA) in the presence of 2 mM magnesium chloride at 37°C for 30 min to digest DNA and RNA.
Digestion of nucleic acids will improve the resolution of the proteins in the subsequent western blot. To verify the completeness of nuclease digestion, assay dsDNA using the Qubit assay as outlined in Basic Protocol 4, step 10.
-
13
Carry out a Western blot as outlined in basic Protocol 3, steps 12–20.
Western blotting provides valuable insights into the variety of proteins that form adducts in specific treatment conditions and can also be used to determine whether specific proteins are targets of regulated proteolysis. Figure 8 provides an example of the use of RADAR/Western blots to characterize PARP1 covalent adducts formed in response to treatment with 5-aza-deoxycytidine.
SUPPORT PROTOCOL 3: ADDUCT-SEQ TO CHARACTERIZE ADDUCTED DNA
“Adduct-Seq” was developed to identify genomic locations of adducted proteins (Kiianitsa et al., 2020). Adduct-Seq is analogous to ChIP-Seq but depends upon biological adduction rather than formaldehyde-induced crosslinking to form stable protein-DNA complexes. This approach was validated by genomic mapping of adducts formed by PARP1 protein (Kiianitsa et al., 2020). A similar approach was used to interrogate genomic locations of DNA-topoisomerase I adducts by ChIP qPCR (Husain et al., 2016).
Materials
ChromaFlash High-Sensitivity ChIP kit (Epigentek, East Farmingdale, NY)
ChIP DNA Clean & Concentrator kit (Zymo Research, Irvine, CA)
proteinase K (ThermoFisher #EO0491)
-
Prepare adducts. Culture and harvest approximately 1.5 × 107 treated or untreated cells and prepare adducts from them, as described in Basic Protocol 5, steps 1–11, but with the following modifications to maintain the duplex structure of the DNA, essential for ligation of adapters for sequencing.
Step 6: It is critical that the ice/water bath used to cool DNA during sonication is at 4°C, to avoid DNA denaturation by heating.
Step 11: Resuspend DNA in 100 μl of 5 mM Tris-HCl (pH 8.5), not 8 mM NaOH.
Capture adducts of interest. Capture the DNA adducts of interest using ChIP Kit (Epigentek), following the manufacturer’s instructions. In brief, a solid support is precoated with antibody specific to the protein of interest, then washed. For protein capture, the support is incubated at 4°C overnight with 100 μl of the RADAR fraction, containing the equivalent of approximately 40 μg of dsDNA; then washed with three times with 100 μl of 5 mM Tris-HCl, pH 8.5; treated with RNase A; and re-washed.
Elute DNA. Treat with 400 μg/ml proteinase K (ThermoFisher) in 10 mm Tris, 1 mM EDTA, pH 7.9. This digests the antibody-protein complex and releases DNA.
Purify DNA. Use a ChIP DNA Clean & Concentrator kit (Zymo) to recover dsDNA. The yield will depend upon the number of adducts formed by the protein of interest. Recovery of PARP1/DNA adducts by this method yielded 1– 2 ng DNA/107 cells (Kiianitsa et al., 2020).
Sequence the DNA adducts. Sequence samples containing 1.0–1.5 ng DNA by Illumina paired-end sequencing, 100 nt per read, with ~40 million read pairs per sample sequencing depths. For further information and guidance on appropriate platforms, see the Illumina website: https://www.illumina.com/techniques/sequencing/dna-sequencing/whole-genome-sequencing.html?scid=2021–269PPC3922&catt=platforms_ppc
Identify sites of adduct formation. Map the bound DNA fragments to genomic regions of interest as described by (Kiianitsa et al., 2020).
SUPPORT PROTOCOL 1
NUCLEAR FRACTIONATION AND RNASE TREATMENT TO REDUCE SAMPLE COMPLEXITY
Materials
12 M LiCl
Thermomixer
Isopropanol
M-PER reagent (ThermoFisher #78501)
Halt™ protease inhibitor cocktail (ThermoFisher #78430)
0.1 M DTT
Nuclear fractionation
Resuspend the washed cell pellet (Basic Protocol 5, step 3) in 1 ml of ice-cold M-PER reagent supplemented with Halt protease inhibitor cocktail and 1 mM DTT.
Homogenize the pellet by pipeting up and down 8–10 times. Recover nuclei by pelleting for 2 min at 14,000 × g at room temperature or 4°C.
-
Lyse nuclear pellets in LS and process as described above in steps 1–5.
Following nuclear fractionation, samples contain approximately 70 μg DNA per 107 cells, along with some nuclear RNA. Nuclear proteins such as topoisomerase I, PARP1 and DNMT1 are exclusively in the pellet, as is essentially all genomic DNA. The DNA:protein mass ratio in these samples is 100:3 or higher, which is comparable to adducts prepared by ultracentrifugation in a CsCl gradient (Kiianitsa & Maizels, 2020). Samples resuspend readily in non-denaturing buffers such as 10 mM Tris-HCl pH 8.0, with virtually no insoluble matter.
RNase A treatment
Incubate 150 μl R1 fraction (Basic Protocol 5, step 11) with 50 μg/ml at 37°C for 30 min.
Add 350 μl LS reagent and 100 μl 12 M LiCl and mix on a Thermomixer at 37°C for 10 min. Precipitate nucleic acids by addition of an equal volume (600 μl) of isopropanol.
Wash pellet.
-
Resuspend sample in 100 μl freshly prepared 8 mM NaOH, and neutralize by addition of 1 μl 1 M HEPES to a final concentration of 20 mM.
The obvious drawback of this fractionation step is that it eliminates adducts formed by RNA binding proteins, which are of interest in some biological contexts.
The presence of residual RNase A in a sample does not interfere with antibody detection, it does increase retention of dsDNA on a nitrocellulose membrane. If this is a concern, a second LS extraction and alcohol precipitation is performed to remove RNase A.
BASIC PROTOCOL 6
DETERMINATION OF DNA CLEAVAGE BY PURIFIED TOPOISOMERASE I
Topoisomerase poisons stabilize a covalent complex between the topoisomerase protein and DNA. The level of covalent complexes may be quantified by measuring the amount of cleaved DNA formed in the presence of test compound and enzyme. Although the covalent complexes formed by an active topoisomerase are reversible, protein denaturation inactivates enzymatic activity and traps the protein on the DNA, enabling the measurement of DNA strand breaks. The procedure detailed below quantifies topoisomerase I–mediated strand breaks in the presence of a topoisomerase I poison, taking advantage of a DNA sequence identified by Westergaard and co-workers (Bonven et al., 1985) that is a primary site for topoisomerase I cleavage. For the assay, a double-stranded oligonucleotide is synthesized that includes the primary topoisomerase I cleavage site (arrow on Fig. 5). The oligonucleotide substrate is end labeled with [32P]-cordycepin and incubated in the presence of topoisomerase I and camptothecin. Treatment with SDS denaturates the topoisomerase, generating a single-strand break with inactive protein bound to its 3’ end in samples in which DNA cleavage has occurred. Separation of the DNA strands reveals a unique, labeled oligonucleotide that is shorter and therefore readily separated from the starting substrate by gel electrophoresis. This procedure is suitable for assaying test agents as topoisomerase I poisons (Tanizawa et al., 1995). It can also be employed to characterize the sensitivity of topoisomerase I derived from many different sources to a variety of test agents. A schematic if the assay is shown in Figure 9.
Figure 9.

Schematic representation of in vitro cleavage of a double-stranded oligonucleotide by topoisomerase I. The annealed oligonucleotides are shown; “*L” indicates the cordycepin label. Note that only the top strand is labeled. Upon addition of topoisomerase I and camptothecin, a specific cleavage by topoisomerase I occurs. The oligonucleotide is designed so that cleavage occurs specifically at the site indicated by the arrow. The topoisomerase I protein is denatured and trapped on the DNA upon addition of SDS, and the strands are separated by the addition of formamide. If no topoisomerase I cleavage occurs, the only product observed on a sequencing gel is the 37-nucleotide labeled substrate (the top strand). Topoisomerase I cleavage results in a smaller oligonucleotide. Note that the oligonucleotide that has protein covalently bound is unlabeled, so it will not be detected.
Materials
Oligonucleotides (custom-synthesized):
5′-GATCTAAAAGACTTGGAAAAATTTTTAAAAAAGATC-3′ (upper strand)
5′-GATCTTTTTTAAAAATTTTTCCAAGTCTTTTAGATC-3′ (lower strand)
[α−32P]-cordycepin 5000 Ci/mmol (Perkin-Elmer Product BLU26250UC)
Terminal deoxynucleotidyl transferase (TdT) labeling kit (Stratagene)
10× topoisomerase I cleavage buffer (see recipe)
50 U/μl purified topoisomerase I (Topogen; also see Critical Parameters; 1 U topoisomerase I is the quantity of enzyme that will relax 200 ng pUC18 DNA in 30 min)
Compounds to be tested [*Copy editor – adjust the the test compounds from basic protocol 3 – which includes more details]
5% (w/v) SDS
Formamide loading buffer (see recipe)
30- to 40-cm denaturing 16% polyacrylamide/7 M urea sequencing gel prepared as described in Slatko and Albright (1992); also see Albright and Slatko (2001); electrophoresis of DNA on sequencing gels requires a large vertical gel apparatus, a high-voltage power supply, and a darkroom for developing autoradiograms (or a phosphoimager)
Sephadex G-25 spin columns (GE Healthcare)
Scintillation vials, scintillation fluid, and counter
95°C heating block
400-μl microcentrifuge tubes
25°C water bath
Whatman 3MM filter paper
Additional reagents and equipment for sequencing by denaturing polyacrylamide gel electrophoresis (Slatko and Albright, 1992; Albright and Slatko, 2001)
CAUTION: Radioactive materials require special handling. All supernatants must be considered radioactive waste and disposed of accordingly.
Prepare substrate
-
1
End label 10 pmol of the upper strand of the oligonucleotide in a total volume of 40 μl with [α−32P]-cordycepin using a terminal deoxynucleotidyl transferase labeling kit according to the manufacturer’s instructions.
-
2
Remove unincorporated [α−32P]-cordycepin using a Sephadex G-25 spin column. Transfer a 1 μl portion of the purified oligonucleotide to a scintillation vial with scintillation fluid and quantify radioactivity. Adjust volume to yield ~100,000 cpm/μl.
-
3
Anneal the labeled upper strand with an equimolar amount of unlabeled lower strand by heating the mixture 5 min at 95°C in a heat block. Turn off the heat block and allow the samples to cool to room temperature, and let them stand overnight.
Perform topoisomerase I reactions
-
4
Assemble reactions in 1.6 ml microcentrifuge tubes, adding ingredients to each tube in the following order:
~50 fmol (25,000 to 50,000 cpm) labeled oligonucleotide substrate (from step 3)
1 μl 10× topoisomerase I cleavage buffer
H2O for final volume of 10 μl (taking into consideration volumes of protein and test compound to be added)
1 μl (50 U) purified topoisomerase I protein
1 μl test compound solution (or solvent alone for controls)
It is also possible to use a crude extract containing topoisomerase I for the cleavage reaction. The amount of topoisomerase I activity will vary depending on the cell line from which the extract was obtained. A reasonable range of protein concentrations for testing is 1 to 20 ng protein per reaction.
-
5
Incubate for 15 min at 25°C, then terminate the reaction by adding 1 μl of 5% SDS.
Analyze cleavage products by gel electrophoresis
-
6
Prepare samples for gel electrophoresis by adding 4 vol (44 μl) formamide loading buffer to each reaction mixture. Load a 5 μl portion from each reaction onto a 30- to 40 cm denaturing 16% polyacrylamide/7 M urea DNA sequencing gel.
Slatko and Albright (1992) and Albright and Slatko (2001) provide detailed protocols for pouring, running, and processing sequencing gels, including autoradiography.
An alternative procedure is to precipitate the oligonucleotide by adding 3 vol of 100% ethanol and incubating in the microcentrifuge tube for 30 min on dry ice. The sample is then microcentrifuged 15 min at maximum speed at 4°C. The ethanol is then removed, and the pellet resuspended in 5 μl formamide loading buffer. This additional step increases the sensitivity of the assay.
-
7
Run the gel 2 to 3 hr at 40 V/cm at 50°C. After electrophoresis, transfer the gel to Whatman 3MM paper and dry in a gel dryer. Appose the dried gel to film for autoradiography (Albright and Slatko, 2001).
Alternately, visualize the radioactive bands using a phosphoimager, which allows quantitative determination of the cleaved band.
BASIC PROTOCOL 7
DETERMINATION OF INHIBITOR EFFECTS ON DNA CLEAVAGE BY TOPOISOMERASE II USING A PLASMID LINEARIZATION ASSAY
The protocol detailed below uses a plasmid linearization assay. Simple and rapid, this assay is somewhat less sensitive than that described in the Alternate Protocol and uses relatively large amounts of purified enzyme. The assay is derived in large part from one described by Osheroff and co-workers (Anderson et al., 1999; Burden et al., 2001).
CAUTION: Ethidium bromide is a mutagen. Wear gloves when handling, and properly dispose of all solutions containing ethidium bromide.
Materials
10× topoisomerase II reaction buffer
Supercoiled plasmid DNA (see Critical Parameters)
Test compound(s)
Positive control (e.g., etoposide)
4 to 20 U/μl purified topoisomerase II (see Critical Parameters)
10% (w/v) SDS
250 mM tetrasodium EDTA, pH 8.0
4 mg/ml stock solution of proteinase K: dilute to 0.8 mg/ml before use
20 mM ATP (diluted with H2O from purchased stock solution; store up to 6 months in small aliquots at –20°C)
5× loading dye (see recipe)
0.8% agarose gel (Voytas, 2001)
Restriction enzyme appropriate for cutting plasmid once, to linearize plasmid
10 mg/ml ethidium bromide stock solution: store at room temperature protected from light
Gel densitometer
Additional reagents and equipment for performing agarose gel electrophoresis, ethidium bromide staining, and gel photography (Voytas, 2001)
Protocol Steps:
-
Place the following in the order listed into 1.5-ml microcentrifuge tubes (on ice):
2 μl 10× topoisomerase II reaction buffer
5 nM supercoiled plasmid DNA
1 to 2 μl of test agent or positive control
H2O to bring the final reaction volume to 20 μl (including the volume of enzyme to be added in step 2).
It is desirable to add ATP to the topoisomerase II reaction buffer just before use. An appropirate dilution of a 20 mM ATP solution is used for this purpose.
-
Add 1 to 2 μl of 4 to 20 U/μl topoisomerase II (0.1 to 1 μg purified protein). Incubate the reaction mixture 15 to 30 min at 37°C.
The optimal incubation temperature depends on how rapidly enzyme/DNA cleavage occurs. Incubation times as short as 5 min are often adequate.
-
Terminate the reaction by adding 2 μl of 10% SDS.
It is important to add the SDS stop solution to tubes in the 37°C water bath. Do not remove the tubes to ice before adding SDS. It is especially critical to add SDS before adding the EDTA solution. Addition of EDTA prior to SDS will result in complete reversal of DNA cleavage.
-
Add 1.5 μl of 250 mM tetrasodium EDTA, pH 8.0, and 2 μl of 0.8 mg/ml proteinase K solution. Incubate 1 to 2 hr at 55°C.
While the proteinase K incubation time is not critical, sufficient time must be allowed for complete digestion of the enzyme covalently bound to DNA. Some prefer to incubate the proteinase K reaction at 55°C overnight.
It is frequently desirable to precipitate the samples using ethanol after the proteinase K digestion and prior to gel electrophoresis.
Add 2 μl of 5× loading dye to each sample.
-
Load the sample on a 0.8% agarose gel and electrophorese for 2 to 4 hr at 5 V/cm. As a control, include a lane of linearized plasmid cut with a restriction enzyme that will only cut the plasmid a single time.
The concentration of agarose is not critical. Gels from 0.8% to 1% agarose can be used, with 1% agarose preferable for small plasmids such as pUC18.
-
Stain the gel for 30 to 60 min in 1 μg/ml ethidium bromide (diluted from 10 mg/ml stock), followed by destaining in water for 5 to 10 min.
CAUTION: Ethidium bromide is a mutagen. Wear gloves when handling, and properly dispose of all solutions containing ethidium bromide.
Visualize lanes using a UV transilluminator and photograph the gel (Voytas, 2001).
-
Using densitometry, quantify the concentration of linear DNA formed, typically by comparing to quantities of linear DNA concentrations formed in the presence of specific concentrations of the positive control (e.g., ciprofloxacin).
See Figure 6 for an example of a cleavage with purified topoisomerase II in the presence of etoposide. See Anticipated Results for interpretation of findings.
ALTERNATE PROTOCOL 1
GEL ELECTROPHORESIS DETERMINATION OF TOPOISOMERASE II CLEAVAGE
This protocol is conceptually similar to Basic Protocol 6. However, because it uses radioactively labeled DNA as a substrate for DNA cleavage it provides a highly sensitive and accurate cleavage assay. After cleavage, the covalently bound protein is removed by proteinase K digestion, and the DNA samples separated by electrophoresis. While this protocol employs agarose gel electrophoresis, higher resolution of smaller fragments can be obtained using polyacrylamide gel electrophoresis.
The first part of the protocol describes preparation of a linear end-labeled substrate for DNA cleavage. Other approaches to generating the substrate include PCR amplification of a specific DNA followed by labeling (Dexheimer and Pommier, 2008).
CAUTION: Radioactive materials require special handling. All supernatants must be considered radioactive waste and disposed of appropriately.
Materials
Substrate: plasmid DNA (e.g., pUC18 or pBluescript)
EcoRI restriction endonuclease (additional restriction endonuclease, e.g., BamHI is optional)
Formamide loading buffer (see recipe)
0.8% agarose gels (Voytas, 2001)
TE buffer, pH 8.0
5 mM dCTP, dGTP, and dTTP
5 U/μl Klenow fragment of DNA polymerase I and 10× Klenow buffer
10 mCi/ml [α−32P] dATP (800 Ci/mmol; Perkin-Elmer)
10× topoisomerase II reaction buffer (see recipe)
Compounds to be tested
20 to 40 U/μl purified topoisomerase II
5% (w/v) SDS containing 1 mg/ml proteinase K
5× loading dye (see recipe)
Sephadex G-25 spin column (GE Healthcare)
Additional reagents and equipment for agarose gel electrophoresis (Voytas, 2001), purification of DNA by phenol/chloroform/isoamyl alcohol extraction and ethanol precipitation and autoradiography (Voytas and Ke, 2001)
Prepare substrate
-
1
Digest DNA substrate (e.g., plasmid pUC18) with EcoRI or other enzyme as appropriate.
-
2
Ensure that the restriction enzyme digestion is complete by adding a small aliquot (100 ng of DNA) to formamide loading buffer in a microcentrifuge tube, electrophorese on a small 0.8% agarose gel, and visualize with ethidium bromide (Voytas, 2001). Include a lane of uncut plasmid to use as a comparison
The electrophoresis product will depend upon the substrate. For pUC18, a single band will be seen.
-
3
Purify digested plasmid from the restriction digest reaction by phenol/chloroform/isoamyl alcohol extraction and ethanol precipitation. Resuspend DNA at 0.35 μg/μl in TE buffer, pH 8.0.
Alternatively, restriction enzyme clean-up kits such as Qiagen ID 28204 can be used in place of the extraction and precipitation.
-
4
Prepare the following labeling mixture for 20 reactions (may be scaled as necessary), adding ingredients in the indicated order:
20 μl (7 μg) digested DNA
15 μl H2O
1 μl 5 mM dCTP
1 μl 5 mM dGTP
1 μl 5 mM dTTP
5 μl 10× Klenow buffer
5 μl 10 mCi/ml [α−32P]-dATP
2 μl 5 U/μl Klenow fragment of DNA polymerase I.
-
5
Incubate reaction mixture for 30 min at 37°C.
-
6
Purify the labeled substrate from unincorporated nucleotides using a Sephadex G-25 spin column or other appropriate method.
For detailed mapping of cleavage sites, the DNA should be uniquely end labeled. For pUC18, this can be accomplished by digestion with another enzyme such as BamHI. It is convenient to perform the second digestion after labeling and before the spin column. Note that this removes 50% of the incorporated label. The digestion can also be performed after the cleavage reaction if desired.
Perform cleavage assay
-
7
Assemble the following reactants (add ingredients in the order listed):
2 μl 10× topoisomerase II reaction buffer
350 ng labeled DNA substrate (step 6)
1 to 2 μl test compound solution
H2O for final volume of 20 μl
20 U topoisomerase II (0.5 to 1 μg purified protein).
-
8
Terminate the reaction by adding 0.5 ml of 5% SDS containing 1 mg/ml proteinase K and incubating for 1 hr at 37°C.
-
9
Add 5× loading dye to each reaction and load on an 0.8% agarose gel. Electrophorese 2 to 4 hr at 5 V/cm (Voytas, 2001).
It is also possible to use polyacrylamide gel electrophoresis to separate the cleavage products.
-
10
Dry the gel, wrap in plastic wrap, and expose to film for 24 to 48 hr at −80°C (Voytas and Ke, 2001).
See Figure 7 for an example of a cleavage with purified topoisomerase II in the presence of the intercalating agent mAMSA. See Anticipated Results for interpretation of findings.
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps. For common stock solution.
Formamide loading buffer
80% (v/v) formamide
1 mM EDTA
10 mM NaOH
1 mg/ml xylene cyanol
1 mg/ml bromphenol blue
Store up to 1 year at −20°C
Loading dye, 5×
30% (v/v) glycerol
0.25 mg/ml bromphenol blue
Store up to 1 year at 4°C
As an alternative, almost any commonly used loading dye can be used for topoisomerase I and topoisomerase II assays.
Low-salt extraction buffer
20 mM Tris∙Cl, pH 7.5
5 mM KCl
1 mM MgCl2
10% (v/v) glycerol
Store indefinitely at room temperature
Immediately before use, add DTT to a final concentration of 1 mM and phenylmethylsulfonyl fluoride (PMSF) from a 0.1 M solution in isopropyl alcohol (store up to 6 months at −20°C) to a final concentration of 1 mM.
PMSF should be added just before the buffer is used, as it is very unstable in aqueous solution.
Phosphate-buffered saline (PBS)
8 g NaCl
0.2 g KCl
1.44 g Na2HPO4
0.24 g KH2PO4
H2O to 1 liter
Adjust pH to 7.4 with 1 M HCl
Store indefinitely at room temperature
Topoisomerase I cleavage buffer, 10×
100 mM Tris∙Cl, pH 7.5
500 mM KCl
50 mM MgCl2
1 mM EDTA
150 μg/ml BSA
Store up to 1 year at room temperature
Topoisomerase I reaction buffer, 10×
500 mM Tris∙Cl, pH 7.5
1 M KCl
10 mM dithiothreitol
100 mM EDTA
50 μg/ml acetylated bovine serum albumin (Life Technologies)
Store up to 1 year at −20°C
Topoisomerase II reaction buffer, 10×
200 mM Tris∙Cl, pH 7.5
100 mM MgCl2
10 mM ATP
10 mM EDTA
10 mM dithiothreitol (DTT)
1.5 M KCl
300 μg/ml acetylated bovine serum albumin (Life Technologies)
Store up to 2 months at −20°C
Lysis Solution (LS)
5 M guanidinium isothiocyanate (GTC)
20 mM Tris-HCl, 20 mM EDTA
0.1 M sodium acetate
2% Sarkosyl
10 mg/ml DTT
pH 6.5
To prepare 200 ml of LS: dissolve 118.4 gm GTC in 60 ml ddH2O which has been pre-heated to 65°C. Then add:
4 ml 1 M Tris-HCl (pH 8.0)
8 ml 0.5 M EDTA (pH 8.0)
6.67 ml 3 M sodium acetate (pH 5.3)
4 g Sarkosyl
Adjust pH to 6.5 by addition of 5 M NaOH (approximately 2.4 ml)
Adjust final volume to 200 ml by addition of ddH2O
Pass through a 0.1 μm (VWR) or 0.22 μM (Corning, 250 ml filtration unit) PES membrane to remove small particulates, which may be evident as yellowish sulphur particles. Store at room temperature protected from light.
Add 1/20 volume of 200 mg/ml DTT immediately before use.
COMMENTARY
Background Information
The procedures detailed in this unit deal specifically with the two principal eukaryotic topoisomerases: topoisomerase I (EC 5.99.1.2) and topoisomerase II (EC 5.99.1.3). Type I is IB and II represents both isozymes of type II topoisomerase. The full complement of human topoisomerases, along with their genes, is displayed on Table 1. Mammalian cells contain two type IB topoisomerases, a nuclear enzyme and a mitochondrial enzyme (Goffart, Hangas, & Pohjoismaki, 2019; Zhang et al., 2001). The assays described for topoisomerase I in Basic Protocols 1, 3, and 4 should be applicable to both nuclear and mitochondrial enzymes.
In mammalian cells, there are two isozymes of topoisomerase II: a 170-kD form (termed p170 or α) (Tsai-Pflugfelder et al., 1988) and a 180-kD form (termed p180 or β) (Austin et al., 2018; Drake et al., 1987). These two isoforms are the products of different genes, located in human cells on chromosomes 17q and 3q, respectively (Wang, 1996). These isoforms are expressed differentially through the cell cycle, with topoisomerase IIα preferentially expressed in proliferating cells and topoisomerase IIβ expressed throughout the cell cycle (J. H. Lee & Berger, 2019). DNA cleavage by topoisomerase IIβ is proposed to stimulate gene expression at specific loci, including targets of the estrogen and adrogen receptor and neuronal genes (Haffner et al., 2010; Ju et al., 2006; Madabhushi et al., 2015). This aspect of topoisomerase activity that has been addressed in several reviews (Madabhushi, 2018; Pommier et al., 2016; Puc, Aggarwal, & Rosenfeld, 2017). Nonetheless, the two isozymes of topoisomerase II appear to have similar biochemical properties, with no major differences in their sensitivities to current anti-topoisomerase agents. The assays described for topoisomerase II herein are applicable to both isozymes.
An additional nuclear topoisomerase, termed topoisomerase III, is found in the yeast S. cerevisiae as well as all other eukaryotes and is homologous to the bacterial type I enzyme but not to other eukaryotic topoisomerases (Wallis, Chrebet, Brodsky, Rolfe, & Rothstein, 1989). Topoisomerase III is a type IA topoisomerase that is active only on negatively supercoiled DNA. Its ability to convert supercoiled DNA to a completely relaxed form is considerably lower than that of topoisomerase I (Kim and Wang, 1992).
Human cells encode two type IA enzymes, topoisomerase IIIα (Hanai, Caron, & Wang, 1996; W. Li & Wang, 1998) and topoisomerase IIIβ (Bizard & Hickson, 2020). As topoisomerase III absolutely requires a divalent cation for activity, its presence does not interfere with the topoisomerase I assay described in Basic Protocol 1. As there are as yet no small molecules that selectively inhibit eukaryotic type IA topoisomerases, detailed protocols for studying inhibitors of these enzymes have not been developed. It is noteworthy that topoisomerase III, especially topoisomerase IIIβ, has been shown to act on RNA as well as DNA (M. Ahmad, Xu, & Wang, 2017; Nott & Tsai, 2013). Assays for this activity have not yet reached sufficient maturity to be included in this unit, although several assays for topoisomerase action on RNA have been described (M. Ahmad, Xu, & Wang, 2018).
Both type I and type II topoisomerases cleave DNA by forming a covalent complex with it (Chen et al., 2013; Vos et al., 2011; J. C. Wang, 1998). A key tyrosine residue attacks the phosphodiester backbone of DNA, forming a phosphotyrosine bond. The enzyme then passes DNA strands through the break, resulting in relaxation of DNA supercoiling or decatenation DNA. After strand passage, the phosphotyrosine bond reverses, and the energy of the phosphotyrosine bond is used to reform the phosphodiester backbone of DNA. There are several key characteristics of this complex: it includes protein covalently bound to DNA as well as a strand break in the DNA substrate, and it is freely reversible.
Human topoisomerases are critical targets for antitumor agents, most notably the topoisomerase poisons which cause the protein-DNA covalent adduct to persist, inhibiting release of the bound protein and religation of the DNA (Kreuzer & Cozzarelli, 1979; J. Nitiss & Wang, 1988). This is the mechanism of cell killing by chemotherapeutics such as topotecan and its analogs topotecan and irinotecan, as well as a series of non-camptothecin compounds such as certain indenoisoquinolines (Bjornsti & Kaufmann, 2019; J. L. Nitiss, 2009b; J. L. Nitiss & Beck, 1996; Pommier, 2013). Agents that inhibit topoisomerase II including the epipodophyllotoxins etoposide and teniposide, the anthracyclines doxorubicin and daunorubicin, and other chemically diverse compounds (Pommier et al., 2010; Walker & Nitiss, 2002). An analogous mechanism is employed by the fluoroquinolone antibiotics, such as ciprofloxacin, moxifloxacin, levofloxacin, and ofloxacin, which target the prokaryotic type II topoisomerases DNA gyrase and Topo IV (Aldred, Kerns, & Osheroff, 2014). Efforts to identify potent small molecule inhibitors of bacterial Type IA enzymes have met with only limited success (Nagaraja, Godbole, Henderson, & Maxwell, 2017). Screens of large compound libraries using high throughput RADAR assays may enable identification of such compounds in the relatively near future (Sinha et al., 2020).
Identification and characterization of novel chemotherapeutics and antibiotics is an important component of current research on DNA topoisomerases. It remains an open question whether inhibiting topoisomerase catalytic activity without stimulating DNA cleavage represents a viable anticancer strategy. Selective and potent catalytic inhibitors of eukaryotic type IA and type IB topoisomerases have not yet been described, although several novel compounds that catalytically inhibit type II enzymes have recently been reported (P. Ahmad et al., 2016; J. H. Lee, Wendorff, & Berger, 2017; Selvakumar, Chandrasekaran, Doss, & Kumar, 2019). While the biochemical effects of catalytic inhibitors have been discussed in detail in several reviews (J. L. Nitiss, 2009b; Vos et al., 2011), there has been limited analysis of their activities in vivo. In contrast, a large body of evidence indicates that stabilization of covalent complexes is a critical factor in the action of topoisomerase poisons, and that most clinically active anti-topoisomerase drugs do not kill cells merely by inhibiting enzyme activity. Because a complete discussion of this topic is beyond the scope of this commentary, interested readers are referred to other sources (Bax et al., 2019; Cinelli, 2019; T. K. Li & Liu, 2001; McClendon & Osheroff, 2007; Pommier, 2006; Pommier et al., 2016). In general, determination of the ability of a compound to trap topoisomerase-DNA covalent complexes is a key step in characterizing the action of an antitopoisomerase agent.
The procedures detailed in this unit enable quantitification of the two key intermediates in the topoisomerase reaction, topoisomerase-DNA covalent complexes (Basic Protocols 3 and 4) and DNA strand breaks (Basic Protocols 5 and 6). The DNA cleavage and formation of the covalent complex are normal steps in the topoisomerase reaction, and these intermediates can be detected (albeit at low levels) in normally proliferating cells even in the absence of a topoisomerase poison. Treatment with a topoisomerase poison causes levels of cleavage complexes to increase. These procedures thus provide the essential foundation for optimization of derivatives of topoisomerase drugs in current use and for discovery and characterization of new compounds.
Critical Parameters and Troubleshooting
Described on Table 3 are some problems commonly encountered using the assays described in this unit, along with possible reasons for their occurance and suggestions for overcoming or avoiding them.
Table 3.
Troubleshooting for Topoisomerase Assays
| Problem | Possible cause | Solution |
|---|---|---|
|
| ||
| Basic Protocol 1 | ||
| No detected relaxation of supercoiled DNA in samples lacking drug | Loss of enzyme activity | Use a fresh aliquot of enzyme |
| Basic Protocol 2 | ||
| No detected decatenation in samples lacking drug | Loss of enzyme activity | Use a fresh aliquot of enzyme |
| No detected decatenation in samples lacking drug | Degradation of ATP (a common issue with these assays) | Use a new ATP aliquot |
| Basic Protocol 3 | ||
| No detected enzyme covalent complexes in positive control | Antibody issues | Use a new aliquot of antibody. Verify antibody activity using western analysis. |
| No detected enzyme covalent complexes in positive control | Lack of expression of target enzyme | Verify that the target enzyme (e.g., topoisomerase IIα) is expressed under the growth conditions used (e.g., by immunoblot analysis) |
| Basic Protocol 4 | ||
| Inconcistency between repeat samples | Different amounts of DNA applied to membrane | Carefully check DNA concentrations. In some cases it may be useful to re-precipitate the DNA with ethanol and re-check DNA concentrations. |
| Failure to detect covalent complexes | Poor recovery from alcohol precipitation | Take care not to dislodge pellet following precipitation. |
| Retain supernatants, re-precipitate and re-assay. | ||
| Basic Protocol 5 | ||
| Regulatory proteolysis of the target adduct in vivo | Determine whether polypeptide is intact by western blotting whole cell extracts. Use antibodies that detect a a variety of epitopes. Vary concentrations of primary and secondary antibodies for immunodetection to minimize background and maximize signal. | |
| Basic Protocol 6 | ||
| No detected cleavage of DNA in samples with positive control | Loss of enzyme activity | Use a fresh aliquot of enzyme |
| Low levels of cleavage in samples with positive control | Inaccurate determination of enzyme activity | Measure enzyme activity using Basic Protocol 1 |
| Basic Protocol 7 | ||
| No detected cleavage of DNA in samples with positive control | Loss of enzyme activity | Use a fresh aliquot of enzyme |
| Low levels of cleavage in samples with positive control | Insufficient enzyme activity | Increase added enzyme quantity |
| Low levels of cleavage in samples with positive control | Degradation of ATP | Use a new ATP aliquot |
Sources of purified enzymes for Basic Protocols 1, 2, 6, and the Alternate Protocol
Many of the protocols in this unit require purified topoisomerases, although the assays can be conducted with crude lysates albeit with reduced sensitivity. Most investigators will conduct experiments with human topoisomerases, although there is an extensive literature with the yeast enzymes (see protocol volumes under Key references for more information on topoisomerase purification). Experimental success depends on reliable enzyme preparations. Two specialized popular commercial sources of purified topoisomerase I and topoisomerase II are TopoGen (http://www.topogen.com) and Inspiralis (http://www.inspiralis.com). The assay buffers recommended by the manufacturer may differ from those described in this unit, in which case it is best to use the manufacturer’s assay buffers with the enzymes they provide.
Regardless of the source, enzymes must be handled appropriately for the assay to work properly. In general, purified enzymes are stored in small aliquots at −80°C, and multiple freeze-thaw cycles avoided. Enzymes maintain activity best when stored at relatively high concentrations, as recommended by the manufacturer. Enzyme dilution buffers are usually provided by the supplier. For the sake of consistency, it is important to be wary of results obtained with preparations that have markedly different enzyme activities.
A number of compounds are available for use as positive controls for the experiments described in this unit. The standard control for topoisomerase I poisons is camptothecin. Topotecan is currently used clinically, but it is substantially less potent than camptothecin. Irinotecan is also used clinically but is a prodrug with minimal activity until it is activated by carboxylesterases. While the active compound, termed SN38, is quite potent, it is poorly soluble. Easily available topoisomerase II poisons include etoposide, teniposide, mAMSA, mitoxantrone, ellipticine, amonafide, and doxorubicin. Etoposide is commonly used as a control for topoisomerase II poisons. If an intercalating topoisomerase II poison is required, mAMSA is frequently employed because of its potency and selectivity. Although doxorubicin and mitoxantrone are more potent, they have non-topoisomerase II targets as well.
Supercoiled plasmid DNA for Basic Protocols 1 and 6 and the Alternate Protocol
Basic Protocols 1 and 5 require purified supercoiled plasmid DNA. The plasmid can be chosen for the convenience of the investigator. High-copy plasmids, such as pUC18, are typically a good choice. Standard plasmid preparation assays, or kits that provide good-quality DNA suitable for subcloning, are also appropriate for the assays described here. However, care must be taken to use plasmid DNA with low levels of nicked circles, especially when assaying relaxation of supercoiling. Typically, plasmid preparations that are >90% supercoiled will yield reliable results.
Recovery and detection of adducts from RADAR extracts
It is important to determine the amount of material recovered in a RADAR-fractionated sample prior to further quantification or characterization of adducts by ELISA, slot blot or Western blot. This provides quality control on adduct recovery, prevents overloading of microtiter wells, gels and membranes used for subsequent analysis, and enables comparison of adduct levels among samples following immunodetection (Basic Protocol 4). DNA constitutes more than 95% of the sample following RADAR extraction, and sample load normalization based on dsDNA recovery per sample is the most appropriate approach. dsDNA content may be determined by measuring A260 absorbance using a Nanodrop or measuring dsDNA fluorescence using PicoGreen or the Qubit assay. dsDNA can also be quantified with anti-dsDNA antibodies, but this is not recommended for slot blots because nitrocellulose does not retain dsDNA.
Note that endogenous proteins cannot provide a “loading control” for antibody-based detection of proteins in a RADAR extract, because free proteins are removed during RADAR extraction, and adduct-forming proteins have not been sufficiently characterized to rely on any of them as a standard for total protein-DNA adduct recovery.
Recovery of DNA is usually around 90% of the maximum yield predicted based on the number of cells in the starting fraction. Poor yield of DNA most likely results from sample loss during alcohol precipitation due to the pellet becoming dislodged during removal of the supernatant. This is especially likely to occur when nucleic acids are precipitated from small numbers of cells (<106). Several procedural modifications help address this problem. To facilitate focused precipitation at the bottom of a tube, leave LS lysates mixed with isopropanol for 20–30 min at −20°C prior to centrifugation. Remove supernatants by aspiration, not by inverting a tube or plate. Using superfine gel loading tips to aspirate supernatants provides an extra level of care at this step. DNA grade glycogen (e.g. GlycoBlue, Thermo-Fisher) may also be added as a carrier to improve RADAR sample recovery. While this does not interfere with proteomic applications, it should be tested for compatibility with immunodetection.
High viscosity of the initial LS extract may prevent efficient extraction of adducts, impairing sample loading and increasing background in subsequent steps. High viscosity may result from inclusion of too many cells in the first extraction steps. This is readily addressed by reducing the number of cells per sample or increasing the volume of LS used. Alternatively, a sonication step can be added to reduce sample viscosity. Sonication reduces dsDNA to a limit size of approximately 500 bp, accompanied by a noticeable loss in viscosity. Sonication may be most efficient if a sample has been homogenized by shearing by forcing it through an 18–22G needle several times. A sample containing too little or too much solution will not be efficiently sonicated. As these parameters vary from instrument to instrument, it is important to read sonicator instructions before sonicating. Completeness of sonication can be monitored by assessing viscosity by gentle pipetting or, more quantitatively, by monitoring dsDNA fragment size by gel electrophoresis.
Proteolytic processing of adducts in the cell may interfere with immune detection, even when all steps in sample preparation have been optimized. Adducts formed by topoisomerases and other proteins undergo proteolytic degradation via a variety of pathways, with adduct half-life dependent upon the type of adducted protein, crosslinking agent, cellular context and drug treatment. For example, endogenous topoisomerase I represents one of the major adducted species, as measured by mass-spectrometry (Kiianitsa & Maizels, 2020), but topoisomerase I adducts cannot be detected by antibodies in RADAR extracts of untreated cells because they are metabolized by the ubiquitin proteasome system (UPS) and Sumo (Sun et al., 2020). Adducts formed by topoisomerases and by other proteins, such as DNMT1, POLB and PARP1, can be partially protected from proteolysis by inhibition of the UPS (Ghoshal et al., 2005; Prasad, Horton, Dai, & Wilson, 2019; Quinones et al., 2015). However, the utility of this strategy for adduct quantification is limited by the high cytotoxicity of UPS inhibitors.
In vitro cleavage assays
As it is crucial that a known amount of topoisomerase activity be used in any of the in vitro cleavage assays, it is advisable to assay topoisomerase activity (see Basic Protocols 1 and 2) shortly before an enzyme preparation is used in this way.
Most topoisomerase II poisons and some topoisomerase I poisons bind to DNA and intercalate in the DNA helix. Such agents yield an unusual dose-response curve. At low concentrations, the compound increases DNA cleavage in a concentration-dependent manner, whereas there is a decrease in cleavage at higher concentrations of test agent, with the level of cleavage falling even below that observed in the absence of drug when the agent is added at very high levels. The most likely explanation for this pattern of responses is that high levels of intercalated compound completely block cleavage (Tewey et al., 1984). For example, mitoxantrone, a strong intercalating agent, yields a six-fold increase in precipitated counts at a concentration of 0.1 μg/ml using Basic Protocol 6. Thus, it is important that a wide range of compound concentrations be tested when examining test agents.
Anticipated Results
Topoisomerase assays
Shown on Figure 2A is a sample topoisomerase I reaction, with lane S representing the DNA substrate alone. Under the electrophoresis conditions used here (i.e., absence of ethidium bromide), the supercoiled form of the DNA migrates most rapidly. Addition of topoisomerase I produces a ladder of more slowly migrating DNA bands. A potential issue is agents that intercalate in DNA. As shown on Figure 2B, it appears that ethidium bromide inhibits topoisomerase I. In fact, the intercalation leads to supercoils that are relaxed by topoisomerase I. When the compound is removed, the net result is a positively supercoiled plasmid that migrates like negatively supercoiled DNA. There are a variety of ways to distinguish the effects of intercalation (Bailly, 2001).
Shown on Figure 3 is a gel from a sample topoisomerase II reaction. Although no bands are present in the substrate lane, there is fluorescence just below the well. Displayed on lane 1 is a single DNA band, which represents the decatenated rings from the kinetoplast network. Because faint fluorescence is still seen in the well, the sample in lane 1 has incomplete decatenation. Care should be taken in interpreting the fluorescence in the well as catenated substrate when a crude extract is used because DNA-binding proteins and other cellular components may also contribute to fluorescence in the wells. The proteins in the extract can bind DNA and prevent it from entering the gel. From Figure 3 it is apparent that a maximum amount of decatenated product is formed in the sample in lane 2, indicating it is a concentration that completely decatenates the substrate. In lane 6 there is an almost complete disappearance of the decatenated product, with the DNA remaining catenated as a result of the high concentration of enzyme in this sample.
Topoisomerase II assays continue to be challenging because quantification of products from kDNA decatenation can be complex. An assay has been described that generates singly catenated DNA. An example of this is shown on Figure 12. The generation of the substrate for this assay has been described (Waraich et al., 2020), with Inspiralis (http://www.inspiralis.com) indicating it plans to make the substrate commercially available.
Figure 12.
Decatenation of kDNA and bis-cat DNA by human topoisomerase IIα. DNA decantation assays to directly compare kDNA (top) and bis-cat DNA (bottom) as substrates for decatenation by human topoisomerase IIa. Concentration of enzyme in pM above each lane; kDNA: kinetoplast DNA substrate network (Inspiralis); BC: bis-cat DNA (Waraich et al., 2020). Demonstration of the conversion of substrates to products as illustrated from left to right of gels. Note that with the bis-cat substrate, topoisomerase IIa first converts the catenated substrate to the supercoiled product, which is relaxed at higher enzyme concentrations. (Courtesy of Nidda Waraich, John Innes Centre, Norwich, UK.)
The In vivo Complex of Enzyme (ICE) assay is a simple, specific and flexible test for demonstrating that an agent stimulates topoisomerase-DNA covalent complexes in vivo. The data on Figure 4 show sensitive detection of topoisomerase IIα covalent complexes by etoposide. Similar results can be obtained when an antibody directed specifically against topoisomerase IIβ is used. The assay is also applicable for studying topoisomerase I agents using an antibody directed against topoisomerase I. In proliferating cells, the levels of topoisomerase IIα typically are greater than the levels of topoisomerase IIβ. Therefore, the signal with topoisomerase IIβ complexes will often be lower than that illustrated on Figure 4. However, nonproliferating cells usually express little topoisomerase IIα, with topoisomerase IIβ being the predominant species in covalent complexes. This assay is particularly useful for laboratories examining aspects of agents targeting topoisomerases, but which do not have the expertise or need to examine purified proteins. This assay is available in kit form from Topogen (http://www.topogen.com).
The greatest shortcoming of the ICE assay (Basic Protocol 3) is the relatively small number of samples that can be easily processed.
Displayed on Figure 7 is the results of a RADAR/ELISA assay of topoisomerase I-DNA adducts in cells treated with camptothecin that was conducted in 96 well format microtiter plates. This is convenient for parallel RADAR extraction and adduct immunoassay of multiple samples in microplate format. Applications may include dose-response and kinetic analyses, as well as compound library screening. Adherent cells, such as GM639 and HCT116 shown here, must be seeded and cultured to the desired confluence in test plates. Non-adherent cells may be grown in a larger capacity flask and apportioned into test plates immediately before the experiment. The typical yield of dsDNA following a single step of RADAR fractionation (the R1 fraction) is 700 ng per 105 human diploid cells. Because less than 100 ng DNA of RADAR-fractionated sample is required for ELISA detection of topoisomerase I adducts in camptothecin treated human cells, culture in a standard 96 well microtiter plate produces sufficient material for several such assays. Up to 500 ng of DNA may be required to detect topoisomerase IIα adducts in etoposide treated cells (Kiianitsa & Maizels, 2014). Although DNA yield is relatively constant among cell types, the signal from adducts in cells treated identically with drug can differ, as is evident in the experiment shown. This may be determined by several factors, including the ability of the drug to enter the cells, the stability of the adducts once formed, and the effect of drug on these adducts. Immunodetection of adducts by direct ELISA is more sensitive and less labor-intensive than slot blotting and has the potential for automation. ELISA can be used to anlalyze samples fractionated in microplates as well as samples prepared by other variations of the RADAR assay.
Shown on Figure 8 is a RADAR/Western analysis characterization of PARP1-DNA adducts in cells treated with 5-aza-dC. Intact PARP1 is a 113 kD polypeptide, which migrates heterogeneously on gels due to post-translational PARylation. In apoptotic cells, PARP1 is caspase-cleaved to generate an 89 kD C-terminal fragment that is the hallmark of apoptosis and is readily detected by a monoclonal antibody specific to the neo-epitope on the N-terminus of the cleaved polypeptide. As shown in the panel on the right, in RADAR extracts of drug-treated cells, this antibody recognizes caspase-cleaved PARP1 polypeptide of 89 kD in length, as well as smaller fragments, presumably products of further proteolysis. The RADAR/Western provides information on both adduct formation and processing that would not be evident by slot blot or ELISA assay.
An alternate approach to cleavage assays is shown in diagram form in Figure 9, and Figures 10 and 11 display results from two different types of cleavage assays. Figure 10 shows results from a plasmid linearization assay. A high concentration of purified protein was used for this experiment. Similar results are obtained if lower amounts of protein are added. However, it is important to remember that DNA cleavage is stoichiometric. It is therefore desirable to add (at least) approximately equimolar concentrations of plasmid and enzyme. Assays like those shown on Figure 10 are frequently used because the detection of cleavage is quite sensitive. Frequently, the most important aspect of this analysis is visualization of cleavage, rather than the location of specific cleavage sites. However, drugs of different classes frequently give differences in the specific cleavage sites, and the demonstration of differences in cleavage patterns can be used to suggest that test agents may belong to different mechanistic classes.
Figure 10.
Plasmid DNA cleavage with purified topoisomerase II. The plasmid cleavage assay was conducted with 2 μg of purified yeast topoisomerase II (a very high concentration). Lanes: λ HindIII markers; Sc, substrate with no added protein; L, pUC18 linearized with a restriction enzyme; and increasing concentrations of etoposide as indicated on the figure. Note that a band that has the same electrophoretic mobility as linear DNA is absent without etoposide, but clearly seen when 1 μg/ml of etoposide is added. The linear DNA is seen with 3 μg/ml etoposide, and decreases in intensity as more etoposide is added. Samples containing ≥ 3 μg/ml of etoposide show smearing of DNA that arises from cleaving the DNA at multiple sites. This smearing is readily interpretable when purified topoisomerase II is used. However, care must be taken with crude enzyme preparations that may contain contaminating nuclease activity.
Figure 11.
DNA cleavage with purified topoisomerase II. DNA cleavage assays were conducted using 32P-end-labeled pUC18 DNA. Cleavage was conducted in the absence of inhibitor (lane marked 0), in the presence of 0.2 μg/ml mAMSA or 1.0 μg/ml mAMSA. Enzyme concentrations added ranged from 2 units (50 ng) to 25 units (1.25 μg).
Time Considerations
Basic Protocol 1: Assay of Topoisomerase I Activity
The assays for both topoisomerase I and topoisomerase II (Basic Protocols 1 and 2) can be accomplished in 4–6 hours (20 samples and 2 gels).
The ICE assay (Basic Protocol 3) requires approximately 5 days, with days 2–4 demanding the greatest time commitment. The time commitment for Support protocol 1 does not substially change the time required to complete the ICE assay.
Using purified topoisomerases to generate a standard curve for quantifying absolute levels of protein (Support protocol 2) does not change the time required to complete Basic Protocol 3.
RADAR/ELISA (Basic Protocol 4) requires approximately 24 hr once cells are grown.
Substrates for the DNA cleavage assays (Basic Protocols 4 and 5 and the Alternate Protocol) can be prepared in ~3 to 4 hr the day before the cleavage reactions are performed. The reactions themselves take <1 hr to perform. Subsequent proteinase K treatment and analysis of topoisomerase cleavage products by gel electrophoresis requires ~4 to 6 hr.
Support protocol 3 requires an additional day beyond that required for basic protocol 5. However, the rate determining step is likely the turnaround time required for the DNA sequencing.
Support protocol 4 can be comfortably accomplished in 1 day.
Basic protocol 6 can be carried out over one day, although additional time (typoically overnight) is required for image analysis.
Basic Protocol 7. If the proteinase K digestion is carried out overnight, then the assays can be accomplished in two half days. If a shorter proteinase K digestion (2 hrs) is used, then the complete protocol can be accomplished in on day, with several breaksfor longer incubation times.
Detailed protocols for many different aspects of DNA topoisomerases.
Introduction and current status of the biology and biochemistry of DNA topoisomerases.
Table 2.
Human Proteins that form Covalent DNA Adducts
| Enzyme Activity | Gene Name |
|---|---|
|
| |
| Topoisomerase | SPO11, TOP1, TOP1MT, TOP2A, TOP2B, TOP3A, TOP3B |
| Methyltransferase | DNMT1, DNMT3A, DNMT3B, NSUN2, TRDMT1 |
| AP/5’dRP lyase | ALKBH1, GAPDH, HMCES, MRE11A, NEIL1, NEIL2, NEIL3, NME2, NTHL1, OGG1, PARP1, PARP2, POLB, POLH, POLI, POLK, POLQ, XRCC5, XRCC6 |
| Other/unknown | C1D, MGMT, PTMA, TDP1 |
Acknowledgments
Basic Protocol 6 was developed in the laboratory of Dr. Yves Pommier. Basic Protocol 7 follows closely on techniques and protocols from Dr. Neil Osheroff. Drs. Nidda Waraich and Anthony Maxwell, John Innes Centre, Norwich, UK, developed the assay shown in Figure 12, and we thank them for providing Figure 12. We gratefully acknowledge the contributions of our co-authors from the previous version of these protocols: Aman Seth and Dr. Eroica Soans, St Jude Children’s Research Hospital, Dr. Anna Rogojina, Greehey Children’s Cancer Research Institute, and Margarita Mishina, Centers for Disease Control and Prevention. This work was supported by grants R01 CA183967, R21 AI123501 and P01 CA077852 to NM, and grant R03 NS116666 to KCN.
Footnotes
The authors affirm no conflicts of interest relevant to this paper.
Key References
Detailed protocols for many different aspects of DNA topoisomerases.
The key references listed below refer to other volumes that describe a wide range of topoisomerase related protocols.
Bjornsti, M.A. and Osheroff, N (eds.). DNA Topoisomerase Protocols: Volume I: DNA Topology and Enzymes Methods in Molecular Biology, Vol. 94, 1999 Print ISBN: 978-0-89603-444-0. Humana Press, Totowa, N.J.
Bjornsti, M.A. and Osheroff, N (eds.). DNA Topoisomerase Protocols: Volume II: Enzymology and Drugs Methods in Molecular Biology, Vol. 95, 1999 Print ISBN: 978-0-89603-512-6. Humana Press, Totowa, N.J.
Nitiss, J. L. (2001). Topoisomerase assays. Current protocols in pharmacology, Chapter 3, Unit3 3. doi:10.1002/0471141755.ph0303s00. The first version of this chapter.
Nitiss, J. L., Soans, E., Rogojina, A., Seth, A., & Mishina, M. (2012). Topoisomerase assays. Current protocols in pharmacology, Chapter 3, Unit 3 3. doi:10.1002/0471141755.ph0303s57. Th second version of this chapter.
Clarke, D.J. (ed) DNA Topoisomerases Methods and Protocols, Methods in Molecular Biology, Vol. 562, 2009, Softcover ISBN: 978-1-61779-644-9, Humana Press, Totowa, N.J.
Drolet, M. (ed) DNA Topoisomerases Methods and Protocols, Methods in Molecular Biology, 2018 ISBN 978-1-4939-7459-7, , Humana Press, Totowa, N.J.
Detailed protocols for many different aspects of DNA topoisomerases.
Introduction and current status of the biology and biochemistry of DNA topoisomerases.
McKie, S. J., Neuman, K. C., & Maxwell, A. (2021). DNA topoisomerases: Advances in understanding of cellular roles and multi-protein complexes via structure-function analysis. Bioessays, 43(4), e2000286. doi:10.1002/bies.202000286
Dasgupta, T., Ferdous, S., & Tse-Dinh, Y. C. (2020). Mechanism of Type IA Topoisomerases. Molecules, 25(20). doi:10.3390/molecules25204769
Delgado, J. L., Hsieh, C. M., Chan, N. L., & Hiasa, H. (2018). Topoisomerases as anticancer targets. Biochem J, 475(2), 373–398. doi:10.1042/bcj20160583
Pommier, Y., Sun, Y., Huang, S. N., & Nitiss, J. L. (2016). Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nature reviews. Molecular cell biology, 17(11), 703–721. doi:10.1038/nrm.2016.111
Vos, S. M., Tretter, E. M., Schmidt, B. H., & Berger, J. M. (2011). All tangled up: how cells direct, manage and exploit topoisomerase function. Nat Rev Mol Cell Biol, 12(12), 827–841. doi:10.1038/nrm322
Literature Cited
- Ahmad M, Xu D, & Wang W (2017). Type IA topoisomerases can be “magicians” for both DNA and RNA in all domains of life. RNA Biol, 14(7), 854–864. doi: 10.1080/15476286.2017.1330741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M, Xu D, & Wang W (2018). An Assay for Detecting RNA Topoisomerase Activity. Methods Mol Biol, 1703, 161–172. doi: 10.1007/978-1-4939-7459-7_12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad P, Woo H, Jun KY, Kadi AA, Abdel-Aziz HA, Kwon Y, & Rahman AF (2016). Design, synthesis, topoisomerase I & II inhibitory activity, antiproliferative activity, and structure-activity relationship study of pyrazoline derivatives: An ATP-competitive human topoisomerase IIalpha catalytic inhibitor. Bioorg Med Chem, 24(8), 1898–1908. doi: 10.1016/j.bmc.2016.03.017 [DOI] [PubMed] [Google Scholar]
- Albright LM, & Slatko BE (2001). Denaturing polyacrylamide gel electrophoresis. Curr Protoc Nucleic Acid Chem. doi: 10.1002/0471142700.nca03bs00 [DOI] [PubMed] [Google Scholar]
- Aldred KJ, Kerns RJ, & Osheroff N (2014). Mechanism of quinolone action and resistance. Biochemistry, 53(10), 1565–1574. doi: 10.1021/bi5000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin CA, Lee KC, Swan RL, Khazeem MM, Manville CM, Cridland P, . . . Cowell IG (2018). TOP2B: The First Thirty Years. Int J Mol Sci, 19(9). doi: 10.3390/ijms19092765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bax BD, Murshudov G, Maxwell A, & Germe T (2019). DNA Topoisomerase Inhibitors: Trapping a DNA-Cleaving Machine in Motion. J Mol Biol, 431(18), 3427–3449. doi: 10.1016/j.jmb.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava V, Goldstein CD, Russell L, Xu L, Ahmed M, Li W, . . . Buszczak M (2020). GCNA Preserves Genome Integrity and Fertility Across Species. Dev Cell, 52(1), 38–52 e10. doi: 10.1016/j.devcel.2019.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizard AH, & Hickson ID (2020). The many lives of type IA topoisomerases. J Biol Chem, 295(20), 7138–7153. doi: 10.1074/jbc.REV120.008286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsti MA, & Kaufmann SH (2019). Topoisomerases and cancer chemotherapy: recent advances and unanswered questions. F1000Res, 8. doi: 10.12688/f1000research.20201.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush NG, Diez-Santos I, Abbott LR, & Maxwell A (2020). Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules, 25(23). doi: 10.3390/molecules25235662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Chan NL, & Hsieh TS (2013). New mechanistic and functional insights into DNA topoisomerases. Annu Rev Biochem, 82, 139–170. doi: 10.1146/annurev-biochem-061809-100002 [DOI] [PubMed] [Google Scholar]
- Cinelli MA (2019). Topoisomerase IB poisons: Over a half-century of drug leads, clinical candidates, and serendipitous discoveries. Med Res Rev, 39(4), 1294–1337. doi: 10.1002/med.21546 [DOI] [PubMed] [Google Scholar]
- Delgado JL, Hsieh CM, Chan NL, & Hiasa H (2018). Topoisomerases as anticancer targets. Biochem J, 475(2), 373–398. doi: 10.1042/bcj20160583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dheekollu J, Wiedmer A, Ayyanathan K, Deakyne JS, Messick TE, & Lieberman PM (2021). Cell-cycle-dependent EBNA1-DNA crosslinking promotes replication termination at oriP and viral episome maintenance. Cell, 184(3), 643–654 e613. doi: 10.1016/j.cell.2020.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake FH, Zimmerman JP, McCabe FL, Bartus HF, Per SR, Sullivan DM, . . . et al. (1987). Purification of topoisomerase II from amsacrine-resistant P388 leukemia cells. Evidence for two forms of the enzyme. J Biol Chem, 262(34), 16739–16747. [PubMed] [Google Scholar]
- Drlica K, Hiasa H, Kerns R, Malik M, Mustaev A, & Zhao X (2009). Quinolones: action and resistance updated. Curr Top Med Chem, 9(11), 981–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estey E (2016). Acute Myeloid Leukemia - Many Diseases, Many Treatments. N Engl J Med, 375(21), 2094–2095. doi: 10.1056/NEJMe1611424 [DOI] [PubMed] [Google Scholar]
- Feng H, Bao S, Rahman MA, Weyn-Vanhentenryck SM, Khan A, Wong J, . . . Zhang C (2019). Modeling RNA-Binding Protein Specificity In Vivo by Precisely Registering Protein-RNA Crosslink Sites. Mol Cell, 74(6), 1189–1204 e1186. doi: 10.1016/j.molcel.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielden J, Wiseman K, Torrecilla I, Li S, Hume S, Chiang SC, . . . Ramadan K (2020). TEX264 coordinates p97- and SPRTN-mediated resolution of topoisomerase I-DNA adducts. Nat Commun, 11(1), 1274. doi: 10.1038/s41467-020-15000-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal K, Datta J, Majumder S, Bai S, Kutay H, Motiwala T, & Jacob ST (2005). 5-Aza-deoxycytidine induces selective degradation of DNA methyltransferase 1 by a proteasomal pathway that requires the KEN box, bromo-adjacent homology domain, and nuclear localization signal. Mol Cell Biol, 25(11), 4727–4741. doi: 10.1128/MCB.25.11.4727-4741.2005 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Goffart S, Hangas A, & Pohjoismaki JLO (2019). Twist and Turn-Topoisomerase Functions in Mitochondrial DNA Maintenance. Int J Mol Sci, 20(8). doi: 10.3390/ijms20082041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner MC, Aryee MJ, Toubaji A, Esopi DM, Albadine R, Gurel B, . . . Yegnasubramanian S (2010). Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nature Genetics, 42(8), 668–675. doi: 10.1038/ng.613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanai R, Caron PR, & Wang JC (1996). Human TOP3: a single-copy gene encoding DNA topoisomerase III. Proc Natl Acad Sci U S A, 93(8), 3653–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Klages-Mundt N, Wang R, Lynn E, Kuma Saha L, Zhang H, . . . Li L (2020). The ARK Assay Is a Sensitive and Versatile Method for the Global Detection of DNA-Protein Crosslinks. Cell Rep, 30(4), 1235–1245 e1234. doi: 10.1016/j.celrep.2019.12.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain A, Begum NA, Taniguchi T, Taniguchi H, Kobayashi M, & Honjo T (2016). Chromatin remodeller SMARCA4 recruits topoisomerase I and suppresses transcription-associated genomic instability. Nat Commun, 7, 10549. doi: 10.1038/ncomms10549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide H, Shoulkamy MI, Nakano T, Miyamoto-Matsubara M, & Salem AM (2011). Repair and biochemical effects of DNA-protein crosslinks. Mutat Res, 711(1–2), 113–122. doi: 10.1016/j.mrfmmm.2010.12.007 [DOI] [PubMed] [Google Scholar]
- Ju BG, Lunyak VV, Perissi V, Garcia-Bassets I, Rose DW, Glass CK, & Rosenfeld MG (2006). A topoisomerase IIbeta-mediated dsDNA break required for regulated transcription. Science, 312(5781), 1798–1802. doi: 10.1126/science.1127196 [DOI] [PubMed] [Google Scholar]
- Katyal S, Lee Y, Nitiss KC, Downing SM, Li Y, Shimada M, . . . McKinnon PJ (2014). Aberrant topoisomerase-1 DNA lesions are pathogenic in neurodegenerative genome instability syndromes. Nature Neuroscience, 17(6), 813–821. doi: 10.1038/nn.3715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiianitsa K, & Maizels N (2013). A rapid and sensitive assay for DNA-protein covalent complexes in living cells. Nucleic Acids Res, 41(9), e104. doi:gkt171 [pii] 10.1093/nar/gkt171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiianitsa K, & Maizels N (2014). Ultrasensitive isolation, identification and quantification of DNA-protein adducts by ELISA-based RADAR assay. Nucleic Acids Res, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiianitsa K, & Maizels N (2020). The “adductome”: A limited repertoire of adducted proteins in human cells. DNA Repair (Amst), 89, 102825. doi: 10.1016/j.dnarep.2020.102825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiianitsa K, Zhang Y, & Maizels N (2020). Treatment of human cells with 5-aza-dC induces formation of PARP1-DNA covalent adducts at genomic regions targeted by DNMT1. DNA Repair (Amst), 96, 102977. doi: 10.1016/j.dnarep.2020.102977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer KN, & Cozzarelli NR (1979). Escherichia coli mutants thermosensitive for deoxyribonucleic acid gyrase subunit A: effects on deoxyribonucleic acid replication, transcription, and bacteriophage growth. J Bacteriol, 140(2), 424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FCY, & Ule J (2018). Advances in CLIP Technologies for Studies of Protein-RNA Interactions. Mol Cell, 69(3), 354–369. doi: 10.1016/j.molcel.2018.01.005 [DOI] [PubMed] [Google Scholar]
- Lee JH, & Berger JM (2019). Cell Cycle-Dependent Control and Roles of DNA Topoisomerase II. Genes (Basel), 10(11). doi: 10.3390/genes10110859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Wendorff TJ, & Berger JM (2017). Resveratrol: A novel type of topoisomerase II inhibitor. J Biol Chem, 292(51), 21011–21022. doi: 10.1074/jbc.M117.810580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppard JB, & Champoux JJ (2005). Human DNA topoisomerase I: relaxation, roles, and damage control. Chromosoma, 114(2), 75–85. doi: 10.1007/s00412-005-0345-5 [DOI] [PubMed] [Google Scholar]
- Li TK, & Liu LF (2001). Tumor cell death induced by topoisomerase-targeting drugs. Annu Rev Pharmacol Toxicol, 41, 53–77. [DOI] [PubMed] [Google Scholar]
- Li W, & Wang JC (1998). Mammalian DNA topoisomerase IIIalpha is essential in early embryogenesis. Proc Natl Acad Sci U S A, 95(3), 1010–1013. doi: 10.1073/pnas.95.3.1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madabhushi R (2018). The Roles of DNA Topoisomerase IIbeta in Transcription. International journal of molecular sciences, 19(7). doi: 10.3390/ijms19071917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madabhushi R, Gao F, Pfenning AR, Pan L, Yamakawa S, Seo J, . . . Tsai LH (2015). Activity-Induced DNA Breaks Govern the Expression of Neuronal Early-Response Genes. Cell, 161(7), 1592–1605. doi: 10.1016/j.cell.2015.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand C, Antony S, Kohn KW, Cushman M, Ioanoviciu A, Staker BL, . . . Pommier Y (2006). A novel norindenoisoquinoline structure reveals a common interfacial inhibitor paradigm for ternary trapping of the topoisomerase I-DNA covalent complex. Molecular cancer therapeutics, 5(2), 287–295. doi: 10.1158/1535-7163.MCT-05-0456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini JC, Miller KG, & Englund PT (1980). Decatenation of kinetoplast DNA by topoisomerases. J Biol Chem, 255(11), 4976–4979. [PubMed] [Google Scholar]
- McClendon AK, & Osheroff N (2007). DNA topoisomerase II, genotoxicity, and cancer. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis, 623, 83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKie SJ, Neuman KC, & Maxwell A (2021). DNA topoisomerases: Advances in understanding of cellular roles and multi-protein complexes via structure-function analysis. Bioessays, 43(4), e2000286. doi: 10.1002/bies.202000286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohni KN, Wessel SR, Zhao R, Wojciechowski AC, Luzwick JW, Layden H, . . . Cortez D (2019). HMCES Maintains Genome Integrity by Shielding Abasic Sites in Single-Strand DNA. Cell, 176(1–2), 144–153 e113. doi: 10.1016/j.cell.2018.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraja V, Godbole AA, Henderson SR, & Maxwell A (2017). DNA topoisomerase I and DNA gyrase as targets for TB therapy. Drug Discov Today, 22(3), 510–518. doi: 10.1016/j.drudis.2016.11.006 [DOI] [PubMed] [Google Scholar]
- Nitiss J, & Wang JC (1988). DNA topoisomerase-targeting antitumor drugs can be studied in yeast. Proceedings of the National Academy of Sciences of the United States of America, 85(20), 7501–7505. doi: 10.1073/pnas.85.20.7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitiss JL (2009a). DNA topoisomerase II and its growing repertoire of biological functions. Nature reviews. Cancer, 9(5), 327–337. doi: 10.1038/nrc2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitiss JL (2009b). Targeting DNA topoisomerase II in cancer chemotherapy. Nature Reviews Cancer, 9(5), 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitiss JL (2009c). Targeting DNA topoisomerase II in cancer chemotherapy. Nature reviews. Cancer, 9(5), 338–350. doi: 10.1038/nrc2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitiss JL, & Beck WT (1996). Antitopoisomerase drug action and resistance. Eur J Cancer, 32A(6), 958–966. [DOI] [PubMed] [Google Scholar]
- Nott A, & Tsai LH (2013). The Top3beta way to untangle RNA. Nat Neurosci, 16(9), 1163–1164. doi: 10.1038/nn.3506 [DOI] [PubMed] [Google Scholar]
- Pommier Y (2006). Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer, 6(10), 789–802. doi:nrc1977 [pii] 10.1038/nrc1977 [DOI] [PubMed] [Google Scholar]
- Pommier Y (2013). Drugging topoisomerases: lessons and challenges. ACS chemical biology, 8(1), 82–95. doi: 10.1021/cb300648v [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y, Leo E, Zhang H, & Marchand C (2010). DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol, 17(5), 421–433. doi:S1074–5521(10)00161–4 [pii] 10.1016/j.chembiol.2010.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y, Sun Y, Huang SN, & Nitiss JL (2016). Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nature reviews. Molecular cell biology, 17(11), 703–721. doi: 10.1038/nrm.2016.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow L, Crisona NJ, Peter BJ, Hardy CD, & Cozzarelli NR (2001). Topological challenges to DNA replication: Conformations at the fork. Proceedings of the National Academy of Sciences of the United States of America, 98(15), 8219–8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad R, Horton JK, Chastain PD 2nd, Gassman NR, Freudenthal BD, Hou EW, & Wilson SH (2014). Suicidal cross-linking of PARP-1 to AP site intermediates in cells undergoing base excision repair. Nucleic Acids Res, 42(10), 6337–6351. doi: 10.1093/nar/gku288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad R, Horton JK, Dai DP, & Wilson SH (2019). Repair pathway for PARP-1 DNA-protein crosslinks. DNA Repair (Amst), 73, 71–77. doi: 10.1016/j.dnarep.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puc J, Aggarwal AK, & Rosenfeld MG (2017). Physiological functions of programmed DNA breaks in signal-induced transcription. Nat Rev Mol Cell Biol, 18(8), 471–476. doi: 10.1038/nrm.2017.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones JL, Thapar U, Yu K, Fang Q, Sobol RW, & Demple B (2015). Enzyme mechanism-based, oxidative DNA-protein cross-links formed with DNA polymerase beta in vivo. Proc Natl Acad Sci U S A, 112(28), 8602–8607. doi: 10.1073/pnas.1501101112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, & Eliceiri KW (2017). ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics, 18(1), 529. doi: 10.1186/s12859-017-1934-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Sun Y, Huang SN, Baechler SA, Pongor LS, Agama K, . . . Pommier Y (2020). DNA and RNA Cleavage Complexes and Repair Pathway for TOP3B RNA- and DNA-Protein Crosslinks. Cell Rep, 33(13), 108569. doi: 10.1016/j.celrep.2020.108569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeffler AJ, & Berger JM (2005). Recent advances in understanding structure-function relationships in the type II topoisomerase mechanism. Biochem Soc Trans, 33(Pt 6), 1465–1470. doi: 10.1042/bst20051465 [DOI] [PubMed] [Google Scholar]
- Schoeffler AJ, & Berger JM (2008). DNA topoisomerases: harnessing and constraining energy to govern chromosome topology. Q Rev Biophys, 41(1), 41–101. doi: 10.1017/S003358350800468X [DOI] [PubMed] [Google Scholar]
- Selvakumar JN, Chandrasekaran SD, Doss GPC, & Kumar TD (2019). Inhibition of the ATPase Domain of Human Topoisomerase IIa on HepG2 Cells by 1, 2-benzenedicarboxylic Acid, Mono (2-ethylhexyl) Ester: Molecular Docking and Dynamics Simulations. Curr Cancer Drug Targets, 19(6), 495–503. doi: 10.2174/1568009619666181127122230 [DOI] [PubMed] [Google Scholar]
- Sinha D, Kiianitsa K, Sherman DR, & Maizels N (2020). Rapid, direct detection of bacterial topoisomerase I-DNA adducts by RADAR/ELISA. Anal Biochem, 608, 113827. doi: 10.1016/j.ab.2020.113827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingele J, Bellelli R, & Boulton SJ (2017). Mechanisms of DNA-protein crosslink repair. Nat Rev Mol Cell Biol, 18(9), 563–573. doi: 10.1038/nrm.2017.56 [DOI] [PubMed] [Google Scholar]
- Sun Y, Miller Jenkins LM, Su YP, Nitiss KC, Nitiss JL, & Pommier Y (2020). A conserved SUMO pathway repairs topoisomerase DNA-protein cross-links by engaging ubiquitin-mediated proteasomal degradation. Sci Adv, 6(46). doi: 10.1126/sciadv.aba6290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trendel J, Schwarzl T, Horos R, Prakash A, Bateman A, Hentze MW, & Krijgsveld J (2019). The Human RNA-Binding Proteome and Its Dynamics during Translational Arrest. Cell, 176(1–2), 391–403 e319. doi: 10.1016/j.cell.2018.11.004 [DOI] [PubMed] [Google Scholar]
- Tsai-Pflugfelder M, Liu LF, Liu AA, Tewey KM, Whang-Peng J, Knutsen T, . . . Wang JC (1988). Cloning and sequencing of cDNA encoding human DNA topoisomerase II and localization of the gene to chromosome region 17q21–22. Proc Natl Acad Sci U S A, 85(19), 7177–7181. doi: 10.1073/pnas.85.19.7177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz B, Popovic M, & Ramadan K (2017). DNA-Protein Crosslink Proteolysis Repair. Trends Biochem Sci, 42(6), 483–495. doi: 10.1016/j.tibs.2017.03.005 [DOI] [PubMed] [Google Scholar]
- Verdine GL, & Norman DP (2003). Covalent trapping of protein-DNA complexes. Annu Rev Biochem, 72, 337–366. doi: 10.1146/annurev.biochem.72.121801.161447 [DOI] [PubMed] [Google Scholar]
- Vos SM, Tretter EM, Schmidt BH, & Berger JM (2011). All tangled up: how cells direct, manage and exploit topoisomerase function. Nat Rev Mol Cell Biol, 12(12), 827–841. doi: 10.1038/nrm3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytas D (2001). Agarose gel electrophoresis. Curr Protoc Mol Biol, Chapter 2, Unit2 5A. doi: 10.1002/0471142727.mb0205as51 [DOI] [PubMed] [Google Scholar]
- Walker JV, & Nitiss JL (2002). DNA topoisomerase II as a target for cancer chemotherapy. Cancer Invest, 20(4), 570–589. [DOI] [PubMed] [Google Scholar]
- Wallis JW, Chrebet G, Brodsky G, Rolfe M, & Rothstein R (1989). A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell, 58(2), 409–419. [DOI] [PubMed] [Google Scholar]
- Wang JC (1996). DNA topoisomerases. Annual Review of Biochemistry, 65, 635–692. [DOI] [PubMed] [Google Scholar]
- Wang JC (1998). Moving one DNA double helix through another by a type II DNA topoisomerase: the story of a simple molecular machine. Q Rev Biophys, 31(2), 107–144. [DOI] [PubMed] [Google Scholar]
- Waraich NF, Jain S, Colloms SD, Stark WM, Burton NP, & Maxwell A (2020). A novel decatenation assay for DNA topoisomerases using a singly-linked catenated substrate. Biotechniques, 69(5), 356–362. doi: 10.2144/btn-2020-0059 [DOI] [PubMed] [Google Scholar]
- Zhang H, Barcelo JM, Lee B, Kohlhagen G, Zimonjic DB, Popescu NC, & Pommier Y (2001). Human mitochondrial topoisomerase I. Proc Natl Acad Sci U S A, 98(19), 10608–10613. doi: 10.1073/pnas.191321998191321998 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]