Abstract
Implantable neural interfaces are important tools to accelerate neuroscience research and translate clinical neurotechnologies. The promise of a bidirectional communication link between the nervous system of humans and computers is compelling, yet important materials challenges must be first addressed to improve the reliability of implantable neural interfaces. This perspective highlights recent progress and challenges related to arguably two of the most common failure modes for implantable neural interfaces: (1) compromised barrier layers and packaging leading to failure of electronic components; (2) encapsulation and rejection of the implant due to injurious tissue–biomaterials interactions, which erode the quality and bandwidth of signals across the biology–technology interface. Innovative materials and device design concepts could address these failure modes to improve device performance and broaden the translational prospects of neural interfaces. A brief overview of contemporary neural interfaces is presented and followed by recent progress in chemistry, materials, and fabrication techniques to improve in vivo reliability, including novel barrier materials and harmonizing the various incongruences of the tissue–device interface. Challenges and opportunities related to the clinical translation of neural interfaces are also discussed.
Introduction
Contemporary neural interfaces
Neural interfaces are important tools for neuroscience and compelling medical devices for potential applications in clinical rehabilitation. Implantable microdevices for neural recording have increased in both complexity and recording capability in recent decades. Brain-penetrating microelectrode technologies for recording and stimulation have been commercially available to researchers for a decade or more.1 These consist of a biocompatible and conductive material fashioned into one or more microscale electrodes that can be placed in close proximity to neurons to obtain extracellular recordings that provide information on neural activity. Many early iterations of neural recording technologies used microscale wires. Individual microwires can record single channels, while bundling many wires together could be used for multichannel recording.2 Microwire technologies include metal microwire arrays (e.g., commercially available from Microprobes, TDT, and Plexon), single-channel silicon microwire arrays (e.g., from Blackrock Microsystems), and silicon multi-electrode probe arrays (e.g., from NeuroNexus, Atlas Neuroengineering, Cambridge NeuroTech, and Neuropixel). Advances in neural recording technology leverage new materials and microfabrication technology to improve performance and increase channel count.
Silicon-based microtechnologies have advanced neural recording capabilities primarily by facilitating insertion, simplifying cabling and connectors, and increasing channel count. Utah (silicon microwire array) and Michigan (silicon multi-electrode probe array) are prominent examples of leveraging silicon-based microdevices for neural recording. The bandwidth of multichannel recording capabilities has also increased. Leveraging silicon manufacturing techniques from the microelectronics industry have produced neural recording implants with >1000 channels and on-chip amplification, thereby greatly improving the bandwidth and quality of data. For example, the Neuropixel uses complementary metal oxide semiconductor (CMOS) circuitry for signal condition and digitization which allows it to fit 960 recording sites within a 10-mm long, 70-mm wide shank and select 384 of these for simultaneous recording.3 The Neuropixel device has been used to great effect for in vivo recording from animal subjects,4,5
Chronic recordings are often limited by the immune response to the implant, which leads to fouling and encapsulation of recording sites by proteins and cells.6 These phenomena are associated with acute damage to the vasculature of the brain during insertion along with the downstream immune response that is generated from the continuous presence of rigid implants comprised of foreign materials.7,8 The fundamental challenge of interfacing silicon-based microdevices with excitable tissue in the brain motivates the investigation of flexible implants to reduce the modulus mismatch at the tissue–device interface and miniaturized form factors to minimize the risk of damage to the vasculature and tissue loss altogether.9,10 Recent examples flexible devices include carbon fibers and polymer-based systems.11–18 At present, these devices typically lack integrated CMOS circuitry and have comparatively low channel counts compared to silicon-based counterparts. Innovations in low-temperature processing and photolithography will improve feature resolution and therefore increase channel count.
There is great interest in developing more sophisticated electrode-based brain interfaces for use in human subjects. The Utah Array from Blackrock Microsystems has been the only intracortical microelectrode array approved for human use by the US Food and Drug Administration (FDA).17 Neuralink is a private neurotechnology company that is developing thin-film polymer electrode arrays integrated into fine threads that can be inserted into the cortex of humans.20 This concept, which will be described in more detail later, is notable because of the unique robotic-assisted insertion technology that can insert multiple threads while avoiding blood vessels.
Electrical coupling between neural interfaces and excitable tissues
Voltage-mediated signals between neurons and implanted electrical sensors remains the gold standard for bidirectional communication between the natural nervous system and human-made neural interfaces. However, there are now numerous possible alternative modes of communication between neurons and synthetic recording/stimulation devices. Neurons can be manipulated using many exogenous signals, including external electric fields, ultrasound, light, or even magnetic fields. At present, voltage-mediated communication between neurons and human-made electronics using implantable devices is the most likely candidate for widespread clinical adoption because it is the most mature technology and has a well-documented history of safety.
Modulating neural activity using electric fields originated from advances in deep brain stimulation (DBS), a procedure first approved in 1997.19 Voltage signals from the flow of ions are intrinsic information currency of excitable tissues such as neurons in the brain. Therefore, no genetic manipulation is required for signal transduction across these subsystems. Implantable neural interfaces must achieve an appropriate balance between invasiveness, specificity, and information density (Figure 1). Implantable neural interfaces often require invasive procedures and carry potential infection risk. However, anatomically precise positioning of devices in the body also confers target specificity.
Figure 1.
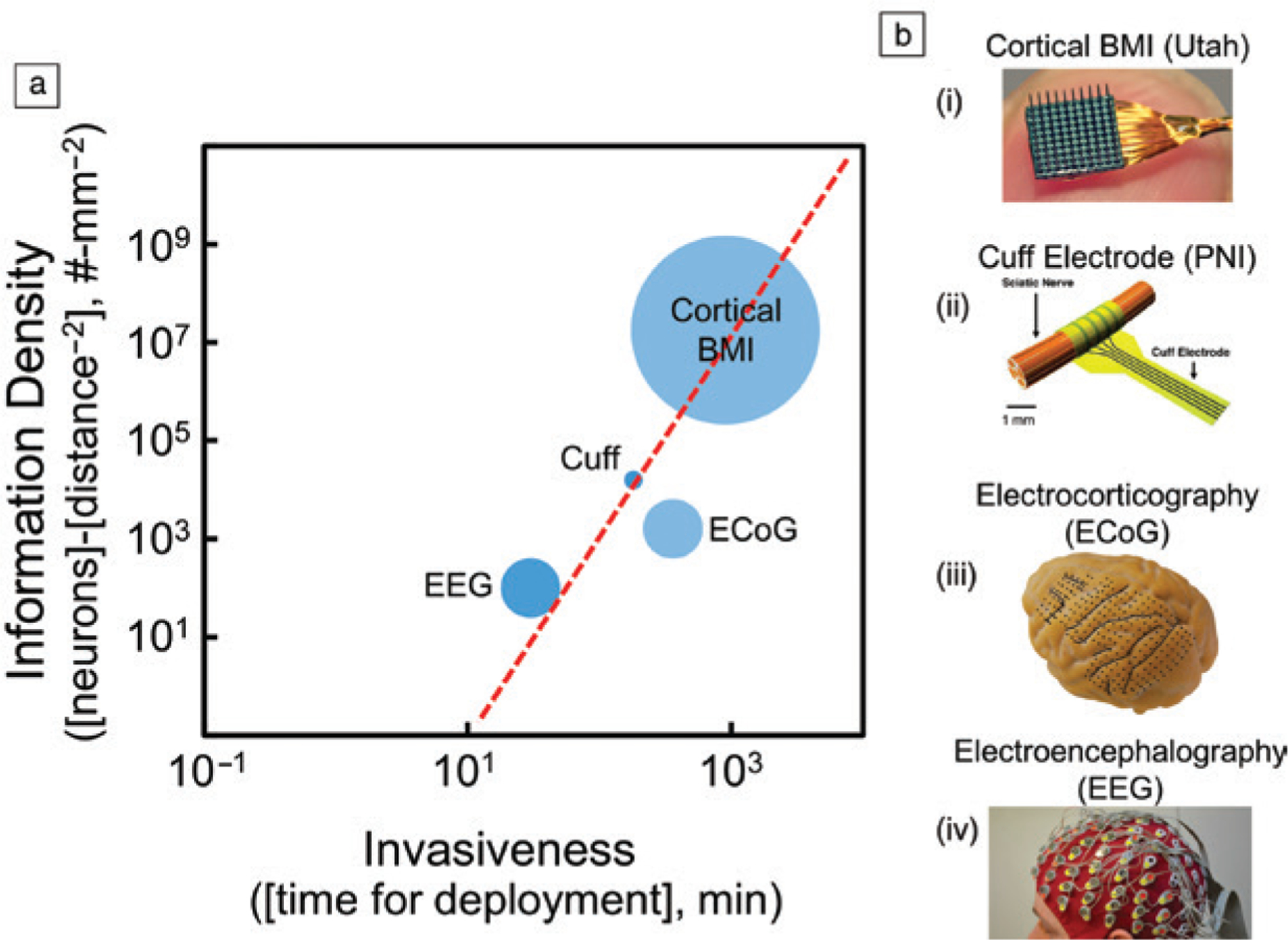
(a) Log–log plot of information density versus invasiveness for representative examples from various classes of brain–machine interfaces (BMIs). Information density is an estimate of neurons that can be accessed using a given tissue integration strategy normalized by the square of the approximate distance between the neuron and the electrodes owing to the r−2 decay in electric fields. Invasiveness is quantified by the approximate number of minutes to integrate the device with the intended target tissue. The approximate total number of neurons accessible is represented by the radius of the circle. The tradeoff and limitations between information density and invasiveness are apparent using (b) examples of devices from various classes of implantable neural interfaces: (i) Utah arrays for cortical BMIs; (ii) cuff electrodes for peripheral nerve interfaces (PNIs); (iii) grid electrodes for electrocorticography (ECoG); (iv) electrode cap for electroencephalography (EEG).
Materials-related challenges for improving performance of neural interfaces
The ideal neural interface will be able to measure neural activity for years, if not decades, in awake, behaving subjects, including humans. The ultimate vision of the ideal neural interface varies according to intended application and even the specific experiment. Such an achievement could broaden the impact of neural interfaces by advancing applications ranging from rehabilitation and assistive technologies to implants that could augment or enhance cognition. The overall utility of a generalized neural interface can broadly be improved by addressing one or more of the following: increasing signal quality and bandwidth, reducing the invasive of implantation or integration, expanding the recording volume, and extending the in vivo lifetime of the device. The design of the ideal neural interface is guided by the desire to extract as much spatiotemporal information throughout the usable lifetime of the device.
Many of these parameters are interdependent and inextricably linked by fundamental tradeoffs in performance.20 Increasing bandwidth, reducing invasiveness, and expanding the recording volume are compelling and meritorious pursuits that can be addressed in part by exploring new recording paradigms, implementing new device architectures, and exploring novel form factors.
There are two commonly observed failure modes in implanted neural interfaces: (1) compromised packaging leading to failure of electronic components; (2) deterioration of recording quality at the tissue–device interface. These canonical failure modes underscore two important technical challenges to improve the scientific and clinical utility of implanted neural interfaces: (1) improving barrier layers and packaging to increase the lifetime of in vivo devices, and (2) improving implant biocompatibility and supporting neuronal health. These challenges are primarily governed by limitations in materials properties and, as a corollary, can be addressed by discovering and implementing new materials into device architectures.
Materials to improve hermeticity in neural interfaces
Designing barrier layers for neural interfaces is challenging because these implantable medical devices have sensitive miniaturized electronic components linked together with sensitive connectors and operate in complex biological environments. A typical neural interface consists of a front-end implantable array with micron-scale sensors that are connected to back-end processors using insulated wires, cabling, and connectors. Heterogenous integration of micron-scale sensors with laboratory-scale electroncs presents many challenges. The components of the device that are most susceptible to failure from permeable species may not be the front-end multielectrode arrays, but rather the back-end connectors, flex circuits, and cabling. Each of these components can tolerate varying amounts of contaminants that permeate from the body into the device such as water or ions from body fluids. Comprehensive and application specific packaging solutions are therefore critical when using devices in “real-world” applications such as recording neural activity in freely behaving animals.
The ideal barrier layer for neural interfaces would be electrically insulating, impermeable to liquid water and ions, chemically stable, processable into thin conformal films with low defect densities, low flexural rigidity, and robust adhesion to any substrate material. This wish list of properties, while challenging to achieve in composite, contextualizes performance limitations of existing materials and provides guidance for designing new barrier materials, especially for flexible and stretchable devices. High-performance barrier layers also play important roles in established industries such as food packaging and storage, consumer electronics, and other types of implantable medical devices. Materials advances in other domains can be translated to the field of neural interfaces.
Mature industries also provide standardized testing methods and establish key figures of merit that can help compare the performance of barrier layers. Water vapor transmission rate (WVTR), while not the only measure of device hermeticity, is an important and ubiquitous figure of merit for barrier layers of medical devices. The WVTR is an extensive property that measures the steady state flux of water vapor through a film with a certain thickness. WVTR is also easily measured and therefore able to be compared across various materials and form factors. Benchmarking WVTR values can be informed (in part) by other industries. For example, visual displays that use organic light-emitting diodes (OLEDs) are highly sensitive to moisture and therefore provide a convenient benchmark for barrier layer performance. To achieve a device lifetime of ~10 years, barrier layers for OLEDs must achieve an overall WVTR of <10−6 g m−2 day−1.21 Other potentially useful figures of merit include gravimetric water sorption and, in the case of hydro-lytically labile materials, etch rates of films due to hydrolysis.
Inorganic barrier layers
Silicon-based packaging materials
Silicon-based ceramics are workhorse packaging materials and dielectrics for the microelectronics industry. Silicon dioxide (SiO2) is an attractive barrier layer material because of the vast foundational knowledge of structure–property–processing relationships. SiO2 is a compelling barrier layer owing to excellent dielectric properties, electronic insulation, near-zero water vapor transmission rates, and low ionic permeability compared to polymer-based counterparts. However, conformal SiO2 films produced by plasma-enhanced chemical vapor deposition (PECVD) or thermal evaporation have relatively high defect densities. SiO2 films with high defect densities are susceptible to hydrolysis, producing water-soluble silicic acid at rates that can impact the function of chronic implants.22 SiO2 films are also permeable to sodium and potassium ions, which accumulate at interfaces and ultimately limit the in vivo lifetime of implanted electronic devices by compromising device function. Thermally grown SiO2 have minimal defects and are more resistant to hydrolysis but are difficult to integrate with flexible electronics. A recently described fabrication technique can integrate 1-μm-thick films of thermally grown SiO2 with flexible electronic structures.23 Hydrolysis rates of ~10−2 nm day−1 at 37°C suggest that these films have a projected lifetime of >70 years. Thermally grown SiO2 barrier layers have been combined with thin-film processing techniques to create a high-resolution multiplexed electrode arrays for in vivo recording called the NeuroMatrix.29 The barrier layers permit long-term in vivo recordings in various animal models (including nonhuman primates) for up to one year with predicted operational lifetimes of up to six years. This impressive technological demonstration represents a confluence of materials research and development combined with non-conventional thin-film processing strategies to greatly improve the longevity of in vivo recording thus addressing one of the foremost challenges in neural interfaces.
Other ceramics and carbon-based barrier layers
Ceramics are attractive barrier layers because they are electronically insulating, resist water sorption, and can be deposited as thin conformal films using reliable manufacturing processes. For example, aluminum oxide (Al2O3) is an attractive barrier layer since it can be deposited as thin conformal films by atomic layer deposition (ALD) using commercially available equipment. Al2O3 films produced by ALD are chemically stable in aqueous conditions and can achieve a WVTR of approximately ~10−10 g mm m−2 day−1, making them attractive options as barrier layers. Like silicon oxide, aluminum oxide surfaces can be easily modified to bind self-assembled monolayers, peptides, proteins, or other polymers to improve in vivo biocompatibility.
Silicon oxides films can be combined with ion diffusion barriers to reduce the permeability to cations in body fluid such as sodium and calcium.24 Devices packaged with SiO2/SiNx bilayers exhibit dramatically extended lifetimes when incubated in phosphate buffered saline (PBS). Furthermore, silicon nitride25 and silicon carbide26 have been used as packaging materials in neural interfaces.
Combining SiO2 with hafnium oxide produce thin-film composites that resist hydrolysis and reduce ionic diffusion. Thin films of hafnium oxide have been used as gate dielectric layers in high-performance silicon-based microelectronics. HfO2 films can be processed using ALD and have exceptional electronic properties such as high electric constants and low leakage currents.27 HfO2 films ~100-nm thickness were recently incorporated into SiO2/HfO2 bilayers to encapsulate flexible electronic implants.28 SiO2/HfO2 bilayers exhibit hydrolysis rates that are significantly smaller compared to barrier layers of comparable thickness comprise of SiO2 alone. Devices encapsulated using HfO2/SiO2 bilayers versus SiO2 monofilms incubated in PBS at 37°C have a projected lifetime of more than 40 years and 30 h, respectively.23
Devices encapsulated with SiO2/HfO2 bilayers and incubated in aqueous solutions of Na+ or Ca2+ exhibit lifetimes >10× longer compared to devices encapsulated in SiO2 mono-layers owing to the improved resistance to cation diffusion. Barrier films composed of SiO2/HfO2 bilayers can therefore address the two most common failure modes of silicon-based films: hydrolysis and ionic diffusion. Composite barrier layers have been combined with thin-film processing techniques to create high-resolution multiplexed electrode arrays for in vivo recording called the NeuroMatrix.29 The efficacy of the barrier layers is evident by long-term in vivo recordings in various animal models (including nonhuman primates) for up to one year with predicted operational lifetimes of up to six years. This impressive technological demonstration represents a confluence of materials research and development combined with nonconventional thin-film processing strategies to greatly improve the longevity of in vivo recording thus addressing one of the foremost challenges in neural interfaces.
Diamond-based coatings are compelling alternatives to ceramic films because they are mechanically robust, chemically inert, and have enjoyed recent improvements in manufacturability and processing for use in implantable biomedical devices for various applications, including prospective use in neural interfaces.30 The properties of polycrystalline diamond can be controlled through doping, which allows the same base material to be used for multiple components thus greatly improving the prospects of hermeticity for the overall device.31 For example, boron-doped nanocrystalline diamond coatings have been used successfully as an electrode material for implantable neural interfaces.32
Polymer-based barrier layers
Parylene-based materials
Parylenes are hydrophobic films prepared by the chemical vapor deposition (CVD) of aromatic precursors to produce poly-(p-xylene). Parylene has many different compositions that are defined by the substituents on precursors. Parylene C is formed from a chlorinated precursor, which confers exceptional performance as a barrier layer. Parylene C is widely used as a polymeric encapsulation layer for neural interfaces owing to its long history as a coating material for medical implants.33 Conformal films of parylene C can be prepared using CVD and etched using oxygen plasma to create photolithographically defined structures. Film thicknesses can vary from <100 nm to >50 μm.
The WVTR of parylene C varies with film thickness and processing conditions. However, typical values for normalized WVTR are on the order of 15 g m−2 day−1 25 μm−1. The WVTR for a 25-μm-thick parylene C film is orders of magnitude larger than that for inorganic counterparts such as Al2O3. Diverse manufacturing capabilities have motivated the use of parylene C as a barrier layer for flexible electronic implants, including neural interfaces. Polymer-based encapsulation layers such as parylene C are attractive for mechanically compliant implants. Parylene C is an intrinsically nontoxic material and resists moisture uptake with equilibrium water sorption of <2 wt% for films >40 nm in thickness.34 Neural interfaces with parylene C encapsulation layers can preserve suitable signal-to-noise ratios of chronic in vivo recordings.35 While certainly a promising material, there are consistent technical challenges that limit long-term performance, including high defect density and low crystallinity in thin films, challenges with cohesive bonding, and poor interfacial adhesion to many substrate materials.36
Polyimides
Polyimides are commodity polymers that have been used extensively in flexible microelectronics for decades. These polymers are most often used as substrates for flex circuits because of their robust chemical and thermal stability. In addition, their electrical and elastic properties can be tuned by altering the monomer composition pendant to the imide and in the polymer backbone. The size of polyimide-based structures can be controlled by spin coating and photolithography. Polyimide films can also be etched and patterned using laser ablation, oxygen plasma or deep reactive ion etching (DRIE). Most types of polyimides require high-temperature post-bake processes, which typically restricts their use to substrates and other structural materials.12–37 The Young’s modulus of polyimides is in the range of 1–10 GPa. Therefore polyimide films with typical thicknesses of 10–50 μm exhibit low flexural rigidity compared to many ceramics and metals.38 While attractive from a potential tissue-biocompatibility standpoint, the low flexural rigidity complicates probe insertion. Thus polyimide-based probes often employ a rigid implantation shuttle, which eases insertion, but may also cause excess local damage to the tissue.
Polyimides offer significant advantages as an encapsulation material for neural interfaces. Some of the first demonstrations of flexible neural interfaces fabricated microelectrodes arrays on a specific type of polyimide (PYRALIN PI 2611) by optimizing metallization procedures and interconnect design.39 Since these early demonstrations, flexible polyimide neural interfaces have been multiplexed into arrays that can record brain activity in a freely moving rodent model.40 However, polyimides have challenges for chronic applications owing in particular to large equilibrium water sorption (0.4–4 wt%).41,42
Dielectric elastomers
Dielectric elastomers are compelling for packing flexible electronic implants because this class of materials combines mechanical compliance, extensibility, and electronic insulation. Many of these elastomers are commercially available, used widely in various mature industries, and their properties are well characterized. Perhaps the most common class of dielectric elastomers are silicones, including the well-known poly(dimethylsiloxane) (PDMS). Silicones are widely used as structural materials in medical devices, including recent demonstrations as flexible neural interfaces.43 PDMS and other silicon-containing polymer networks are suitable for both packaging materials and substrates because they are electronically insulating, hydrophobic, and therefore resistant to liquid water. However, silicones are highly permeable to water vapor, which is problematic for achieving hermeticity in chronic implants.
The WVTR of silicones varies widely across precursor composition, processing, and form factor, however a typical range of normalized WVTR is 100–2000 15 g m−2 day−1 25 μm−1. Silicone alkyds have WVTR closer to 100 g m−2 day−1 25 μm−1 while room-temperature vulcanizing (RTV) silicones have WVTR closer to 2000 g m−2 day−1 25 μm−1. In general, the WVTR of silicones is up to 1000× larger than those for Parylene C films of comparable thickness. Other widely available dielectric elastomers that have potential as packaging materials include styrene-ethylene-butylene-styrene copolymer (SEBS) and polyisobutylene (PIB),44–46 though these have been used sparingly in neural interfaces to date.
One of the persistent challenges with using dielectric elastomers as encapsulation materials for chronic implants is the relatively high permeability to water vapor compared to their inorganic counterparts. The critical challenge in advancing dielectric elastomers as encapsulation materials for implantable neural interfaces is the strong correlation between extensibility and permeability. Elastomeric properties are achieved by creating networks of amorphous polymers while superior barrier properties are achieved by forming polymer layers with large crystalline domains and low defect densities. These properties are fundamentally anticorrelated at the molecular level, which makes it difficult to employ engineering solutions to achieve both extensibility and superior barrier properties. Breakthroughs in the performance of dielectric elastomers for applications in flexible neural interfaces will likely have to originate from advanced polymers that are engineered at the macromolecular level. The materials design challenge is further confounded by application-specific needs with multiple figures of merit and established tradeoffs in performance. For example, neural interfaces recording from dynamic environments for short periods of time may value extensibility over barrier properties. These are open questions that require a continuous dialogue between end-users and materials scientists.
Multilayer and composite strategies
Composite barrier layers contain multiple layers that can combine orthogonal properties, including high dielectric constant, low water vapor transport, low water sorption, and low permeabilities for ions found in biological fluids. Barrier layers composed of multilayer composites are advantageous for two principle reasons. First, stacks of multilayers can mitigate the negative impact of high defect densities in a single layer. Defects are critical to barrier performance because they can potentially short-circuit the transport of water, gases, and ions directly across the film. However, stacking multiple thin-film barrier layers can reduce the likelihood that defects overlap thereby improving overall barrier performance relative to a single film of equal overall thickness to the multilayer. Second, each material of the composite can contain orthogonal chemistries that provide a specific function. Multilayer composites can therefore combine the barrier properties of each individual layer.
Utah arrays have been encapsulated in multilayer composites of aluminum oxide and parylene C. Al2O3 films on the order of ~50-nm thickness deposited by ALD are combined with parylene C films 6 μm in thickness deposited by CVD. Devices coated with Al2O3/parylene C bilayers retained critical device function when incubated in vitro for >1000 days. Al2O3/parylene C bilayers outperformed films composed only of parylene C, which preserved device function for ~100 days. This dramatic improvement is attributed to the much improved WVTR of Al2O3 compared to that of parylene ; WVTRparylene C ~0.2 g mm m−2 day−1.
Al2O3 films can be prepared into multilayer composites when combined with polymers such as the perfluoropolymer CYTOP,47 PDMS, and photoresists such as SU-8.21 Al2O3/polymer thin-film composites achieve WVTR of 1.23 × 10−6 g m−2 day−1 and 1.05 × 10−6 g m−2 day−1 when combined with PDMS and CYTOP, respectively. The performance of these composite multilayers is significantly improved compared to both Al2O3/SU-8 composite films (7.94 × 10−4 g m−2 day−1) and control films without polymers (5.43 × 10−5 g m−2 day−1).
Opportunities for improving barrier layer performance
The ideal encapsulation material for implantable neural interfaces would be easily processable into thin films with low defect densities, have exceptional barrier properties, and accommodate large cyclic strains while maintaining performance. In general, it is advantageous to combine the moisture barrier and electrical insulation properties of ceramics with other encapsulation materials that impart chemical resistance to reactions such as hydrolysis. While sufficiently thin ceramic-based barrier layers can accommodate some degree of out-of-plane bending, these brittle materials have poor in-plane extensibility. Therefore, any barrier layer strategy using ceramics will have limitations in performance for certain deformation modes. Ceramic films that are microstructured or integrated with corrugated substrates may have the potential to increase extensibility in some applications.48,49 Novel ceramics or ceramic hybrids, possibly engineered at the molecular level, that are both extensible and exhibit high-performance barrier properties would be of broad interest to the neural interface community.
Another opportunity in barrier layer design is to achieve the advantages of increasing the robustness of barrier layers while maintaining device sensitivity and minimizing flexural rigidity. Full encapsulation of sensing elements with conformable ultrathin barriers, as described in the Neural Matrix device29 extends in vivo recording lifetime at the expense of sensitivity. Any intermediary layer between the excitable tissue and the recording site could potentially reduce the signal-to-noise ratio. Increasing the thickness of barrier layers reduces permeability but increases flexural rigidity. The he ideal film thickness to both minimize flexural rigidity thereby preserving biocompatibility while also maintaining suitable barrier properties. The flexural rigidity D of a film of thickness t scales as D ~ t3 while the steady state flux of species through a film Φi scales with film thickness as Φi ~ t−1. Assuming the dimensions of the implant are dictated by the structural materials and barrier layers, as opposed to the microelectronics and metallization, there is an implicit tradeoff between these critical device properties. The optimization exercise should ideally be informed by many considerations including the anticipated tissue reaction of the implant site, recording volume, and desired in vivo device lifetime.
Experiments to determine key figures of merit (e.g., water vapor transmission rate and water sorption) for barrier layer properties use ideal test structures and operating environments. However, implants often use these materials in more challenging contexts. For example, barrier layers are often patterned, processed on diverse substrates, and are subject to bending and flexion during handling. As such, functional device lifetime of implants is often much shorter compared to the lifetime of ideal test structures. Oftentimes, barrier layers do not fail within the continuum film construct but rather are compromised at materials interfaces such as recording/stimulation windows or coating for packaging and connectors. Total encapsulation of the device across all components is critical to translating fundamental discoveries in barrier layer design to practical improvements for improving in vivo device lifetime. Complex geometries complicate material deposition processes and can lead to poor uniformity across the entire device.
Materials strategies to improve implant biocompatibility and supporting neuronal health
Biomaterials challenges at the tissue–device interface
High spatiotemporal resolution electrical, optical, and chemical signals rapidly fall off in the body with increasing distance from the source.20–51 In turn, this necessitates that the sensing components of brain–machine interfaces (BMI) must be implanted close to the target sources (typically neurons) and maintain proximity throughout recording. This has historically been true because neuronal activity generally elicits membrane depolarizing action potentials that can be detected by a nearby microelectrode as an electrical signal. Consequently, neuroscience research and clinical neuromodulation paradigms largely assume that neurocomputation is a purely neuronal process governed by electrically excitable cells. However, non-neuronal cells make up the majority of cells in the brain and are responsible for modulating signal transduction across the biology–technology interface.52
Early biocompatibility studies used post-mortem histology to examine neurons together with non-neuronal cells in the brain (e.g., astrocytes and microglia) since the latter form insular scar tissue around the implanted interface.53 This led to the dogma that any non-neuronal cell activity negatively affected neuronal activity and compromised BMI function in vivo. The assumption was that “inactive” glial cells “activated” in response to implantation injury, and this “activation” led to an upregulation of pro-inflammatory cytokine release, which are injury- and stress-related signaling molecules that ultimately promote neurodegeneration. When the BMI failed to record action potentials, it was assumed that the glial activation led to the apoptosis (cell death) of nearby neurons around the electrode within the recording radius. However, recent advances in subcellular level resolution in vivo high-speed two-photon microscopy (TPM) and the development of new transgenic approaches have enabled researchers to examine non-neuronal cell activity in real time.54–56 In turn, this has enabled new studies that challenge many of the old assumptions.
A recent study showed examples of a Michigan-style silicon intracortical microelectrode with sufficient material integrity, electrical impedance, neuronal density, and lack of a glial scar.54 Other studies confirmed that histological outcomes remain poor predictors of actual recording performance.57,58 In vivo TPM, mesoscale calcium imaging and blood-oxygenation level dependent optical intrinsic imaging (BOLD-OIS) further elucidated the nature of this unexpected outcome. Following intracortical microelectrode insertion, tissue around the probe can receive less blood supply based on the degree of vascular injury during implantation.56 In turn, this leads to decreased oxygen and nutrient supply to the nearby tissue.54 Ultimately, this leads to decreased metabolic support, increased metabolic stress, upregulation of pro-inflammatory cytokines by nearby glia, loss of nearby oligodendrocytes (myelin forming glia), decrease in neurotrophic support by glia, and ultimately the silencing of nearby neurons (Figure 2).52–59 However, restoring metabolic support during the critical period may allow for recovery of nearby neurons.54
Figure 2.
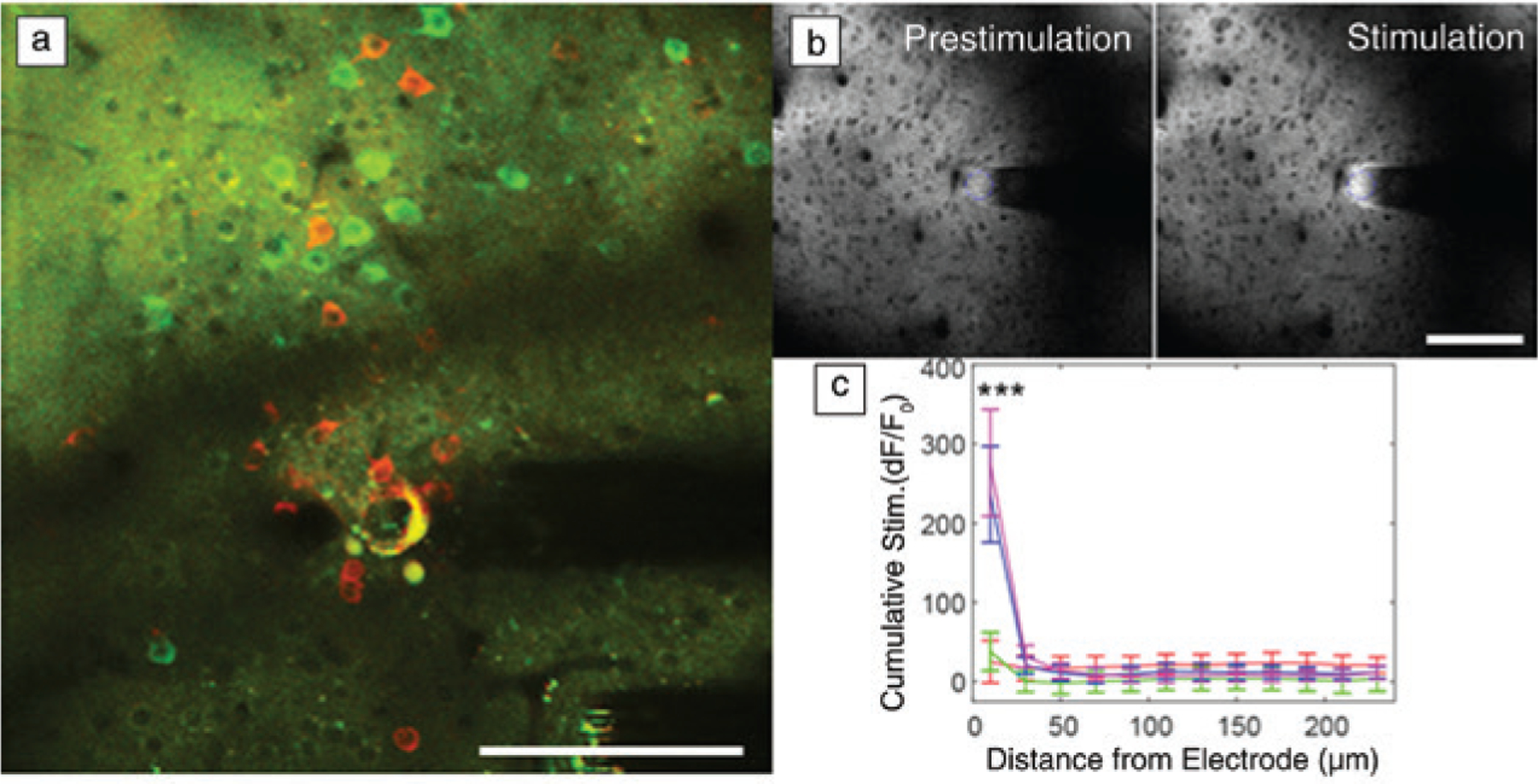
Functional neural activity near implanted recording and stimulation interfaces are sensitive to distance. (a) In vivo two-photon microscopy (TPM) of neurons in the visual cortex show GCaMP activity (green; a chimera protein of green fluorescent protein [GFP], calmodulin, and M13, a peptide sequence from myosin light chain kinase) at a distance (>60 μm) a month after chronic implantation. However, there are silenced neurons (red) near the electrode that can only be driven by strong microstimulation. (b) In vivo TPM of extracellular glutamate measured by iGluSnFr (intensity-based glutamate sensing fluorescent reporter) before and after electrical stimulation shows that significant glutamate release from electrical stimulation is limited to the first 20 μm. (c) These studies indicate that biomaterial selection and probe designs for maintaining a tightly coupled functional interface are crucial for both recording and stimulating interfaces. Scale bar = 100 μm. (b, c) Reprinted with permission from Reference 65. © 2020 Elsevier.
For stimulation interfaces, a long-standing dogma has been that there is less concern for mitigating variability and performance impact on neurostimulation, since stimulation amplitude can simply be increased up to the Shannon–McCreery limit, which is an empirically derived rule for estimating possible tissue damage caused by electrical stimulation.60 However, recent studies suggest that dramatic performance variabilities do, in fact, exist with stimulation technologies.61–65 In vivo TPM studies with implanted interfaces in the chronic immune response phase show that even when stimulation parameters were well below the safety limit, electrolysis and gas evolution occurred on some electrodes and altered neural network activity well after stimulation ended.66 Moreover, in vivo studies with genetically encoded extracellular glutamate sensors demonstrated that significant glutamate release from microstimulation was limited to the first 20 μm from the electrode site (Figure 2b–c).65 These findings suggest that material choice and electrode parameters are equally important when designing interfaces for both stimulation and recording.
Taken together, these early results highlight unexplored dimensions to biocompatibility and new avenues to materials-based strategies for improving the integration of the brain and technology. For BMI, it is particularly important to consider that compatibility of the tissue (not producing a toxic or immunological response) is an insufficient definition of biocompatibility.67 Instead, BMI biocompatibility needs to also consider the fidelity with which signals from nearby cells can be detected by the BMI or the ability of the BMI to modulate the activity of nearby cells in the desired manner.54–68 As such, it is important to explore novel materials to improve the integration of the tissue and interface technology,69 including coatings and miniaturized form factors.70
Lastly, as technology improves, and the sizes of the interfaces decrease and channel counts increase, it is important to weigh the tradeoffs of multiple material properties during material selection and design. In turn, recent studies have highlighted that non-neuronal cells are normally active and play crucial roles in homeostasis. Following implantation injury, cell activity changes such that pro-inflammatory cytokines are upregulated, and some resting-state activity is down-regulated. Therefore, materials and interfaces need to consider minimally impacting non-neuronal cell activity or restoring the original activity rather than using pharmaceuticals that completely suspend non-neuronal cell activity.
Dynamically softening polymers
The merits of using implants with mechanical flexibility have been etched into the cannon of the BMI research community in recent decades. However, soft or flexible penetrating probes must be initially mechanically resilient to facilitate insertion and tissue integration. One strategy to resolve this inherent dilemma is the use of self-softening polymers as substrates for neural interfaces (Figure 3). These polymers are initially mechanically robust to withstand insertion forces during the implantation, but then soften upon hydration to reduce the modulus mismatch at the tissue–device interface.71–77 The compliance to tissue is expected to reduce foreign body responses and therefore improve the long-term recording capabilities of devices due to minimized scar tissue formation. The first generation of softening polymeric substrates was comprised of thiol-ene and thiol-ene/acrylate polymers that contain ester groups in their backbone and are therefore susceptible to hydrolytic degradation. In order to reliably record neural signals over decades however, softening polymers must also be chemically stable at physiological conditions. Next-generation dynamically softening polymers are ester-free to limit hydrolysis and exhibit greatly improved durability over first-generation polymers.78 Neural interfaces can benefit by designing systems that employ non-degradable self-softening polymers as substrates.
Figure 3.
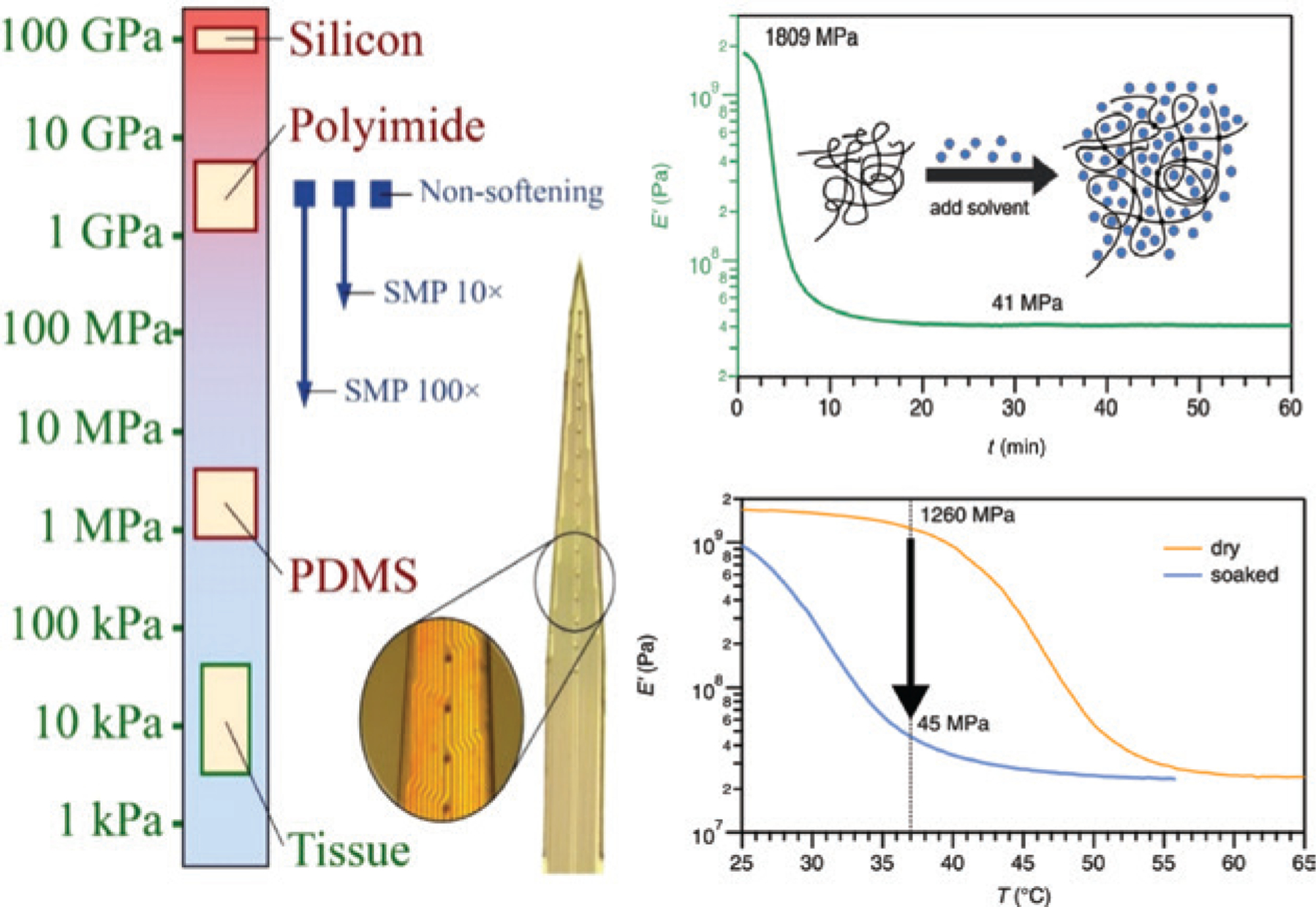
Schematic displays the stiffness of various materials as compared to tissue (left) and the softening of polymers under physiological conditions due to plasticization as measured by dynamic mechanical analysis (right).71 The top right shows the effect of solvent-induced plasticization. The elastic modulus of the polymer decreases within minutes after immersion in 37°C phosphate buffered saline (PBS). The bottom right displays the elastic modulus of the polymer before (orange, dry) and after immersion (blue, soaked) in PBS. The glass-transition temperature of the polymer shifts toward lower temperatures due to plasticization and therefore the properties of the material at body temperature change from glassy (dry) to rubbery (hydrated).
Transient and self-adhesive substrate materials
It is advisable to design neural interfaces with materials and form factors that limit implant micro-motion and reduce mechanical mismatch at the biology–technology interface. This guidance is most often cited in the context of the cortical interface, which often uses high-density silicon-based multi-electrode arrays implanted into highly compliant tissue. Recent studies suggest a limit to the efficacy of mechanical matching between the implant and the resident tissue.79 At least in the context of tissue response, the marginal benefits of reducing the mechanical modulus becomes vanishingly small for implants with an effective Young’s modulus of E ~ 1 MPa. These data combined with previous findings53 suggest that the persistent presence of a material is largely responsible for degradation of signal transduction across the biology–technology interface.
Transient substrate materials with elastomeric properties could therefore serve as an emerging approach to neural interface design.80,81 Biodegradable elastomers have been explored in the context of regenerative medicine,82–84 but may also have applications as substrates in transient electronics.81 This class of materials may facilitate device implantation and then erode in a controllable way to allow tissue to integrate with the underlying electronics. Biodegradable elastomers offer significant advantages, including tunable mechanical properties, controllable and well-characterized degradation mechanisms,85 intrinsic biocompatibility,86 and unique properties such as shape memory.87 Biodegradable elastomers can be processed into diverse form factors using widely available manufacturing processes such as laser cutting,88 photolithography,89 and replica-molding.81–84,90–92 Therefore, there may be new opportunities to integrate electronics with transient support materials that can facilitate tissue integration and then biodegrade into benign components that can be metabolized by cells near the implant site.
Neural interfaces with self-adhesive substrates offer another approach to harmonizing the biotechnology–interface. Insertional probes can benefit from dynamically softening and transient substrate materials. However, laminating electrodes to the surface of dynamic tissues and organs are beneficial in many prospective applications. For example, large area paddle electrodes can be used for recording from the dorsal root ganglia of the peripheral nervous system. However, the hydrated and mechanically dynamic environment can shift the electrode location thereby losing spatial registry. Biomimetic adhesive hydrogels have the potential to anchor electrodes in place while also serving as an ultracompliant substrate material (Figure 4).93,94 The composition of catechol-bearing hydrogels can be tuned to optimize transfer printing of microelectronic structures.95 Furthermore, materials-specific process flows can fabricate ultracompliant adhesive peripheral nerve electrodes for recording from the dorsal root ganglia (as has been demonstrated for feline subjects). The combination of mechanical compliance and robust underwater adhesion enables acute recordings while also maintaining spatial registry during the session.96 Ultracompliant adhesive hydrogels have also been leveraged for recording from other ultrafine structures in the peripheral nervous system.97
Figure 4.
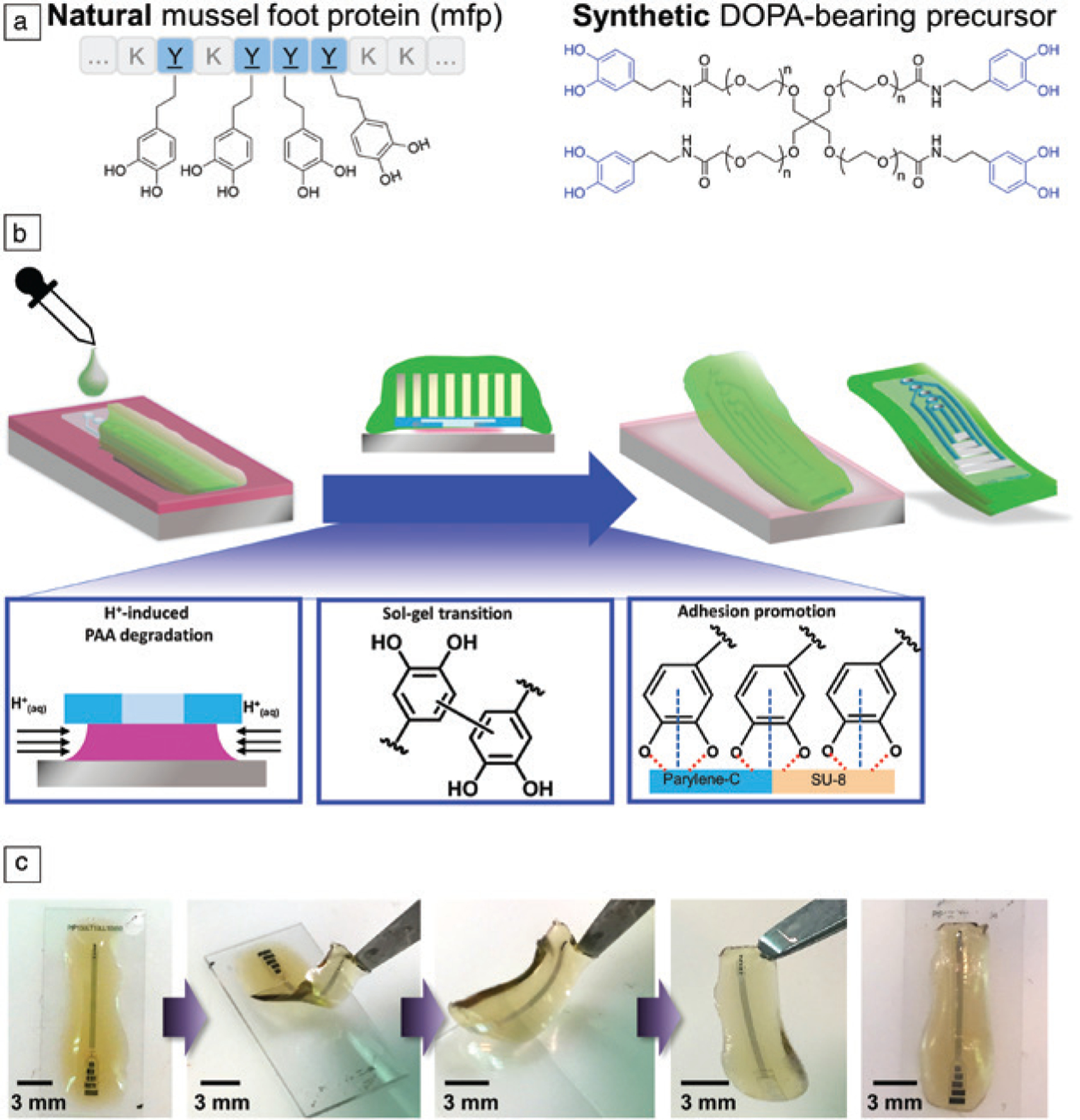
Hydrogel-mediated aqueous-phase transfer printing. (a) The adhesive elements in mussel foot proteins (mfps) can be recapitulated in synthetic hydrogel precursors containing L-3,4-dihydroxyphenylalanine (DOPA) groups. (b) (Top) Schematic representation and (bottom) experimental observation illustrating the mechanisms composed of three processes that enable one-step transfer printing multielectrode structure to catechol-bearing adhesive hydrogels. (c) Photographs of hydrogel-based electrodes during sol-gel phase transitions and integration with microelectronics. Reprinted with permission from Reference 96. © 2018 Wiley.
Emerging concepts for tissue integration
The material requirements to maintain fidelity of signal transduction across the biology–technology interface and device packaging of multielectrode arrays depend on the device form factor, implantation site, and procedure for tissue integration. Tissue-penetrating monolithic probes with multiple recording sites inserted into excitable tissue remains the gold standard for neural interfaces. Prominent examples include Utah and Michigan multielectrode arrays, as mentioned earlier. However, there have been many exciting innovations in device architecture in recent years. This section briefly highlights some emerging concepts for tissue integration that could dictate future materials requirements for maintaining chronic device biocompatibility and hermeticity in vivo.
Highly parallelized implantable fiber arrays
Recording over large tissue volumes can provide great value for both neuroscientists and clinicians. Increasing the recording volume often requires increased device size and subsequently more damage to tissue upon device integration. Individual recording microwires can reduce tissue damage, but often scale poorly since it is difficult to bundle microwires into larger arrays. A novel concept by Melosh et al. and Paradromics fabricates and packages large microwire arrays into easy-to-handle systems that enable bidirectional recording in large tissue volumes.98 Briefly, the device uses techniques from the textile industry to bundle as many as ~10,000 insulated microwires in a collet to create a multielectrode arrays.99 The microwires are trimmed and sacrificial layers are removed to define the device dimensions and electrode spacing. Finally, the microwire arrays are bonded to a CMOS pixel array stochastically fusing microwires with recording elements. This clever design affords numerous advantages, including modular device dimensions that are defined by choosing the microwire length, sacrificial layer thickness (interwire spacing), and the total number of microwires in the array. Highly parallelized microwire arrays have the potential for long-term high-density recording because of the miniaturization of each individual recording site. The device lifetime is therefore likely governed by tissue biocompatibility and barrier properties of the insulation material on the individual microwires.100 These microwire arrays also simplify packaging challenges because the packaging, connectors, and back-end electronics can be safely positioned far from the recording site.
Robotic-assisted insertion of high-bandwidth devices: “Neural lace”
Another iteration of highly parallelized microscale recording devices is the “neural lace” developed by Neuralink.18–101 The recording device in this concept consists of multiple polyimide fibers that are ~20-mm long, between 4–6-μm thick, and between 5–50-μm wide (Figure 5). Each fiber contains up to 32 independent passive electrodes with coatings to improve charge injection and metallized traces. Each array contains up to 96 threads and the overall device contains ~3000 individual recording electrodes. The device contains the following onboard electronics: individually programmable amplifiers, on-chip analog-to-digital converters, and peripheral control circuitry for serializing the digitized outputs. Critically, the back-end hardware also ultimately interfaces flexible polyimide arrays to a USB-C connector, which could accelerate broad adoption by a diverse user base. While the device fabrication and hardware are impressive, perhaps the most innovative aspect of the “neural lace” concept is not the device itself, but the tissue integration strategy. Specifically, a robotic system uses optical tracking and precise movements to rapidly and reliably insert threads into the surface of excitable tissue such as the cortex. Light modules combined with software predetermine insertion sites to avoid rupturing the neuro-vasculature and thus maximizing the likelihood for chronic high-fidelity recordings. Devices with more than 3000 independent electrodes have recorded neural activity in freely moving rats. The clinical prospects of this device are bolstered using well-characterized materials such as gold and polyimide. Future challenges include ensuring barrier layer integrity long-term and reducing the effect of micro-motion artifacts. The “neural lace” is a compelling concept that could help usher in the era of high-bandwidth devices.
Figure 5.
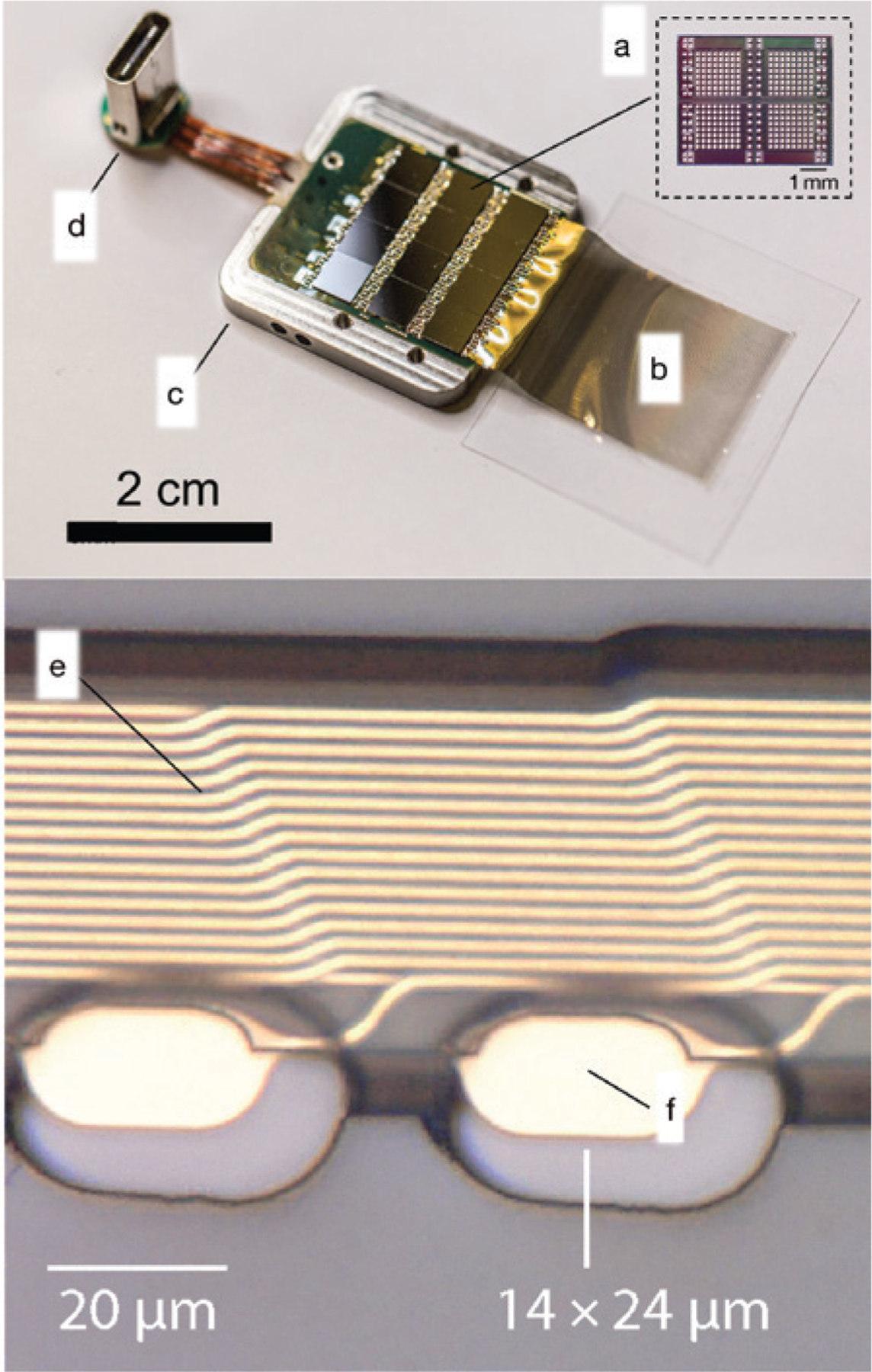
(Top) Photograph of the neural lace technology.18 This device features the following components: (a) 12 application-specific integrated circuits for processing; (b) arrays of electrode arrays on polymer threads on a parylene C handling layer; (c) titanium enclosure with lid removed; (d) USB-C connector. (Bottom, e) Close-up of two recording sites (f) with gold traces.
Minimally invasive neural interfaces: Stentrode
Tissue-penetrating implants can provide access to high-quality electrophysiological recordings of unitary activities and local field potentials when placed within the brain. However, insertional probes require highly invasive craniotomies, risk damage to the brain regions and vasculature along the implantation track and have well-documented challenges with long-term in vivo biocompatibility. These limitations motivated the use of the blood vessels as a minimally invasive route to access the inner regions of the brain.102 A recent example is the Stentrode led by Oxley et al. along with partners at Synchron. This bidirectional interface is essentially an endovascular stent with ~10 electrode discs 750 μm in diameter that are mounted radially about the external surface of the device (Figure 6).103 The Stentrode can be introduced into blood vessels measuring approximately 3 mm in diameter.104 Multielectrode arrays on these devices can record local field potentials (LFPs), which can be used for decoding speech.
Figure 6.
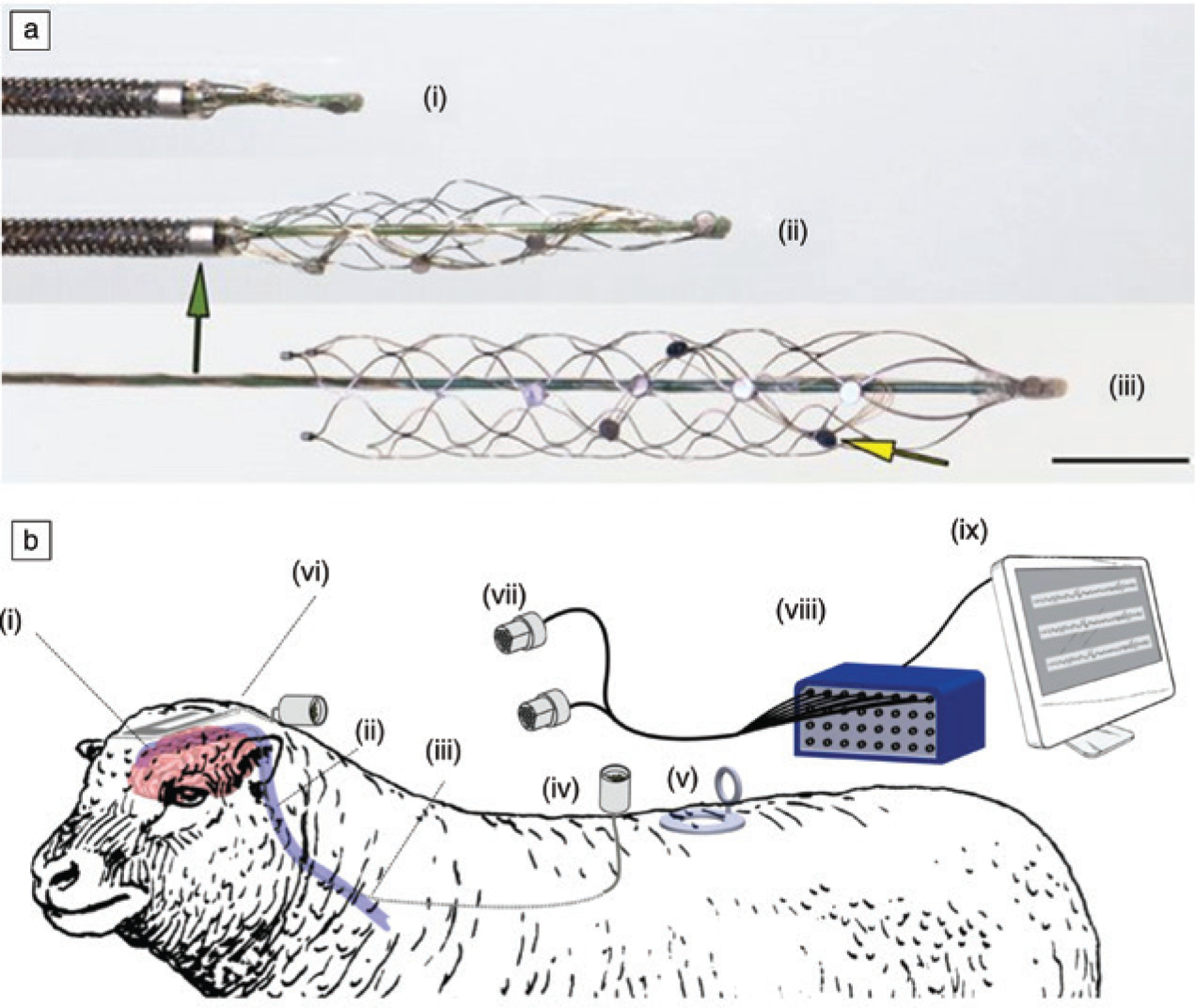
(a) Stentrode with 8 × 750 μm electrode discs (yellow arrow) self-expanding during deployment from a 4 French (4F) catheter (green arrow); (i) retracted device; (ii) partially deployed device; (iii) fully deployed device. Scale bar = 3 mm. (b) Schematic of recording setup for the endovascular stentrode (i) implanted in the brain of sheep. (ii) Leads exit the brain via the internal jugular vein and (iii) protrude through the wall of the common jugular vein tunneling subcutaneously to (iv) custom-made connectors secured to a muscle. (v, vi) Stainless steel and platinum ground electrodes. (vi) Electrode lead wires and ground electrodes are linked to custom connectors (vii) that are then attached to a (viii) data acquisition system (ix) and computer for recording.
Stentrodes have been implanted into both sheep (>180 days) and more recently in humans.104,105 Devices are eventually incorporated into the neointima the scar tissue that forms within blood vessels, and achieve stable recordings while preserving vessel patency during the testing period. Avoiding costly and invasive surgeries by routing neural interfaces through the vasculature is compelling. Although the number of recording sites and the possible use cases will likely increase with time, the set of potential recording sites is ultimately dictated by the anatomy of the neuro-vasculature. Furthermore, there are potential biocompatibility concerns not with the device, but with the blood-contacting catheter that is necessary for transmitting data from the central nervous system to devices outside of the body. Nevertheless, this creative approach and impressive demonstration could inspire new types of minimally invasive approaches to integrate neural interfaces with the body.
Conclusions and future directions
Challenges to clinical translation of neural interfaces
The developments previously described highlight concerted efforts to leverage novel materials to improve the performance and utility of neural interfaces. The same conclusion can be drawn by the increasing number of bioelectronics-themed talks and symposia at the Materials Research Society (MRS) meetings and other scientific conferences. A large amount of work, for example, concentrates on the development of new materials for neural electrodes. This effort, however, seems to be disproportionate to the number of new materials introduced in implantable electronic medical devices (IEMDs), which is limited. Indeed, there has been little innovation and adoption of new materials in these devices: The electrode array of the cochlear implant, for example, has changed little since the device was first developed in the late 1960s.
This raises the question as to why the efforts of a large research community are not valorized, when they can potentially lead to improvements in healthcare and quality of life for many patients. The answer lies in the high costs and high risks associated with the introduction of new materials in medical devices. When a new material is introduced, device biocompatibility must be reevaluated. While the device may pass biocompatibility tests, it can fail after several years in the human body, which increases legal liability and could result in litigation. Thus, key material suppliers from the chemical industry are reluctant to provide novel materials to medical device manufacturers for use in implants intended for human use.
Finally, the cost associated with the introduction of new materials is borne disproportionately by the first manufacturer who innovates and ends up lowering the regulatory barrier for their competitors. As a result, there must be compelling advantages arising from the use of new materials to make a sound business case. A new electrode coating that significantly lowers electrode impedance leading to longer battery life, might be such an example. When it comes to more disruptive concepts, such as devices with novel form factors that enable less invasive surgery, or devices employing novel transduction/actuation mechanisms, the path to the clinic would, in general, be even longer.
Perhaps a semiconductor manufacturing technology-(SEMATECH) style consortium106 could bring together stake-holders from industry, government, academia, and healthcare to help establish a model for sharing the risks-rewards of innovations in invasive neural interfaces. Still, a great deal of progress can be achieved within academia by collaborations between technology-based and clinical groups. An example is the NeuroGrid,107 a flexible microelectrode array for electrocorticography (ECoG), a technique that records electrical activity in the brain by placing electrodes in direct contact with the surface. The electrodes are coated with the conducting polymer PEDOT:PSS that lowers impedance,108 allowing the fabrication of electrodes with diameters down to tens of micrometers. These are placed on a thin plastic foil that ensures conformal contact to the brain.109 The combination of small electrodes and conformal contact leads to high-resolution corticograms that capture single neuron signals without penetrating the brain. This is compelling advantage compared to traditional ECoGs, and the device was translated to the clinic where it is used today to explore the human brain at unprecedented spatial resolution.110
Future materials challenges and opportunities
Materials innovations will fundamentally drive advances in the performance of implantable neural interfaces in the coming decades. Two prominent materials challenges highlighted here, including reliable device packaging and managing the tissue–device interface. For designing and testing of novel packaging materials, our collective scientific understanding of the problem is likely sufficient, the technical challenges are properly framed, the figures of merit to define performance are well defined, and the testing platforms are standardized across laboratories. Creative engineering solutions, bounded only by fundamental limits on materials performance, will forge the path to improved barrier layer performance in neural interfaces.
Materials innovations to better manage the tissue–device interface have a potentially more tortuous path because many scientific questions remain. The neural interface community and biomaterials scientists have advanced our knowledge of the biological mechanisms that underpin the tissue response to implants. However, critical knowledge gaps remain and must be first addressed with additional detailed fundamental studies. Furthermore, it is difficult, but not impossible to make global comparisons of results related to in vivo device performance conducted by different laboratories. Heterogeneity in animal models, data acquisition, data analysis, insertion methods, and post-operative care make it difficult to compare results across studies. Challenges and opportunities for improving materials for implantable neural interfaces are currently by defined de facto roadmaps. It is possible that the neural interface of the future may avoid implantation altogether or may use alternative signal transduction mechanisms without the need for genetic manipulation. For now, however, materials limitations establish device criticalities that must be addressed with the mindset of >10× improvement to realize the full potential of implantable neural interfaces.
Acknowledgments
The authors would like to acknowledge the members of the biomaterials and neural interfaces communities whose work could not be included due to space limitations. C.J.B. acknowledges financial support provided by the following organizations: The Kavli Foundation; National Institutes of Health (R21NS095250; R21EB026073); the Defense Advanced Research Projects Agency (D14AP00040); the National Science Foundation (DMR1542196). E.M. acknowledges financial support provided by the following organizations: National Science Foundation (CBET1343193) and the National Institutes of Health (U24NS113647; U01NS099703). T.D.Y.K. acknowledges financial support provided by the following organizations: the National Institutes of Health (R01NS094396, R21NS108098, R01NS105691); the Defense Advanced Research Projects Agency (DARPA-BAA-16-09-NESD-FP-001); the National Science Foundation (CAREER 1943906). G.G.M. acknowledges support from the European Union’s Horizon 2020 Research and Innovation Program under Grant Agreement No. 732032 (BrainCom) and from the King Abdullah University of Science and Technology Office of Sponsored Research under Award No. OSR-2016-CRG5-3003.
Biographies

Christopher Bettinger is a professor in the Department of Materials Science and Engineering and Biomedical Engineering at Carnegie Mellon University. He received his SB degree in chemical engineering, his MEng degree in biomedical engineering, and his PhD degree in materials science as a Charles Stark Draper Fellow, all from the Massachusetts Institute of Technology. He completed a postdoctoral fellowship at Stanford University in the Department of Chemical Engineering as a National Institutes of Health Ruth L. Kirschstein Fellow. His current research includes designing new materials for flexible electronics in medicine. Bettinger can be reached by email at cbetting@andrew.cmu.edu.

Melanie Ecker is an assistant professor in the Department of Biomedical Engineering at the University of North Texas. She earned her PhD degree in natural sciences from Freie Universität Berlin, Germany, in 2015. She conducted her postdoctoral studies at The University of Texas at Dallas from 2015 to 2019. Her research interests focus on polymer science and biomedical engineering, and include combining both fields to develop the next generation of biomedical devices, such as conformal and biocompatible neural devices to study the electrophysiology of the enteric nervous system. Ecker can be reached by email at Melanie.Ecker@unt.edu.

Takashi Kozai is an assistant professor of bioengineering at the University of Pittsburgh. He received a BA degree in molecular, cellular, and developmental biology, and a BA degree in biochemistry from the University of Colorado Boulder, in 2005, and his PhD in BME from the University of Michigan–Ann Arbor, in 2011. His research interests include understanding the role of neuroimmune cells in neuronal damage and regeneration, and improving long-term performance of implanted electrodes and integrating technology with the brain for the purpose of studying normal and injured/diseased nervous systems. Kozai can be reached by email at tk.kozai@pitt.edu.

George Malliaras is the Prince Philip Professor of Technology in the Electrical Engineering Division at the University of Cambridge, UK. He received his PhD degree from the University of Groningen, The Netherlands. He was a professor at Cornell University and École des Mines, France. His awards include the New York Academy of Sciences, the National Science Foundation, and DuPont. Malliaras received an honorary doctorate from the University of Linköping, Sweden. He is a Fellow of the Materials Research Society and the Royal Society of Chemistry, and he serves as deputy editor of Science Advances. Malliaras can be reached by email at gm603@cam.ac.uk.

Ellis Meng is a professor of biomedical engineering and electrical and computer engineering in the Viterbi School of Engineering, and the Vice Dean for Technology Innovation and Entrepreneurship at the University of Southern California. She also directs the Biomedical Microsystems Laboratory. She received her BS degree in engineering and applied science and her MS and PhD degrees in electrical engineering from the California Institute of Technology in 1997, 1998, and 2003, respectively. Her research focuses on advancing medicine and science through enabling micro-machining, microsensor and actuator, microfluidic, and microsystems technologies. Meng can be reached by email at ellismen@usc.edu.

Walter Voit is an associate professor of mechanical engineering with a joint appointment in materials science and engineering at The University of Texas at Dallas. He received his PhD degree in materials science and engineering from the Georgia Institute of Technology in 2009, his MS degree in computer science–intelligent systems in 2006, and his BS degree in computer science in 2005, both from The University of Texas at Dallas. His research interests include shape-memory polymers, polymer manufacturing, ionizing radiation, thermomechanical properties, and biopolymer mechanics. Voit can be reached by email at walter.voit@utdallas.edu.
Footnotes
This article is based on the Materials Research Society/Kavli Future of Materials workshop: Brain–Machine Interfaces: Materials to Clinical Translation, presented at the 2019 MRS Spring Meeting in Phoenix, Ariz.
Contributor Information
Christopher J. Bettinger, Department of Materials Science and Engineering, and Department of Biomedical Engineering, Carnegie Mellon University, USA
Melanie Ecker, Department of Biomedical Engineering, University of North Texas, USA.
Takashi Daniel Yoshida Kozai, Department of Bioengineering, University of Pittsburgh, USA.
George G. Malliaras, University of Cambridge, UK
Ellis Meng, Department of Electrical and Computer Engineering, University of Southern California, Los Angeles, USA.
Walter Voit, Department of Mechanical Engineering, The University of Texas at Dallas, USA.
References
- 1.Rousche PJ, Normann RA, J. Neurosci. Methods 82, 1 (1998). [DOI] [PubMed] [Google Scholar]
- 2.Kollo M, Racz R, Hanna M, Obaid A, Angle MR, Wray W, Kong Y, Hierlemann A, Müller J, Melosh NA, Schaefer AT, bioRxiv 570069 (2019), 10.1101/570069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jun JJ, Steinmetz NA, Siegle JH, Denman DJ, Bauza M, Barbarits B, Lee AK, Anastassiou CA, Andrei A, Aydın Ç, Barbic M, Blanche TJ, Bonin V, Couto J, Dutta B, Gratiy SL, Gutnisky DA, Häusser M, Karsh B, Ledochowitsch P, Lopez CM, Mitelut C, Musa S, Okun M, Pachitariu M, Putzeys J, Rich PD, Rossant C, Sun W, Svoboda K, Carandini M, Harris KD, Koch C, O’Keefe J, Harris TD, Nature 551, 232 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juavinett AL, Bekheet G, Churchland AK, eLife 8, e47188 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Putzeys J, Raducanu BC, Carton A, De Ceulaer J, Karsh B, Siegle JH, Van Helleputte N, Harris TD, Dutta B, Musa S, Lopez CM, IEEE Trans. Biomed. Circuits Syst 13, 1 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Golabchi A, Wu B, Cao B, Bettinger CJ, Cui XT, Biomaterials 225, 119519 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polikov VS, Tresco PA, Reichert WM, Neurosci J, Methods 148, 1 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Biran R, Martin DC, Tresco PA, Exp. Neurol 195, 115 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Nolta NF, Christensen MB, Crane PD, Skousen JL, Tresco PA, Biomaterials 53, 753 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Patel PR, Na K, Zhang H, Kozai TDY, Kotov NA, Yoon E, Chestek CA, J. Neural Eng. 12, 046009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee K, Singh A, He J, Massia S, Kim B, Raupp G, Sens. Actuators B 102, 67 (2004). [Google Scholar]
- 12.Mercanzini A, Cheung K, Buhl DL, Boers M, Maillard A, Colin P, Bensadoun J-C, Bertsch A, Renaud P, Sens. Actuators A 143, 90 (2008). [Google Scholar]
- 13.Castagnola V, Descamps E, Lecestre A, Dahan L, Remaud J, Nowak LG, Bergaud C, Biosens. Bioelectron 67, 450 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Hara SA, Kim BJ, Kuo JTW, Lee CD, Meng E, Pikov V, J. Neural Eng. 13, 066020 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Du ZJ, Kolarcik CL, Kozai TDY, Luebben SD, Sapp SA, Zheng XS, Nabity JA, Cui XT, Acta Biomater. 53, 46 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu H, Hirschberg AW, Scholten K, Berger TW, Song D, Meng E, J. Neural Eng. 15, 016017 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smalley E, Nat. Biotechnol 37, 978 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Musk Neuralink E, bioRxiv 703801 (2019), 10.1101/703801. [DOI] [Google Scholar]
- 19.Miocinovic S, Somayajula S, Chitnis S, Vitek JL, JAMA Neurol. 70, 163 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Wellman SM, Eles JR, Ludwig KA, Seymour JP, Michelson NJ, McFadden WE, Vazquez AL, Kozai TDY, Adv. Funct. Mater 28, 1701269 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Ruan C, Li M, Zou J, Tao H, Peng J, Xu M, J. Mater. Chem. C 5, 4017 (2017). [Google Scholar]
- 22.Yin L, Farimani AB, Min K, Vishal N, Lam J, Lee YK, Aluru NR, Rogers JA, Adv. Mater (2015), 10.1002/adma.201404579. [DOI] [PubMed] [Google Scholar]
- 23.Fang H, Zhao J, Yu KJ, Song E, Farimani AB, Chiang C-H, Jin X, Xue Y, Xu D, Du W, Seo KJ, Zhong Y, Yang Z, Won SM, Fang G, Choi SW, Chaudhuri S, Huang Y, Alam MA, Viventi J, Aluru NR, Rogers JA, Proc. Natl. Acad. Sci. U.S.A 113, 11682 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song E, Fang H, Jin X, Zhao J, Jiang C, Yu KJ, Zhong Y, Xu D, Li J, Fang G, Du H, Zhang J, Park JM, Huang Y, Alam MA, Mei Y, Rogers JA, Adv. Electron. Mater 3, 1700077 (2017). [Google Scholar]
- 25.Cogan SF, Edell DJ, Guzelian AA, Liu YP, Edell R, J. Biomed. Mater. Res. A 67A, 856 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Diaz-Botia CA, Luna LE, Chamanzar M, Carraro C, Sabes PN, Maboudian R, Maharbiz MM, 8th Int. IEEE/EMBS Conf. Neural Engr. (NER) (2017), pp. 170–173. [DOI] [PubMed] [Google Scholar]
- 27.Lee BH, Kang L, Qi W-J, Nieh R, Jeon Y, Onishi K, Lee JC, IEDM 1999, Tech. Dig (Cat.N0.99CH36318) (1999), pp. 133–136. [Google Scholar]
- 28.Song E, Lee YK, Li R, Li J, Jin X, Yu KJ, Xie Z, Fang H, Zhong Y, Du H, Zhang J, Fang G, Kim Y, Yoon Y, Alam MA, Mei Y, Huang Y, Rogers JA, Adv. Funct. Mater 28, 1702284 (2018). [Google Scholar]
- 29.Chiang C-H, Won SM, Orsborn AL, Yu KJ, Trumpis M, Bent B, Wang C, Xue Y, Min S, Woods V, Yu C, Kim BH, Kim SB, Huq R, Li J, Seo KJ, Vitale F, Richardson A, Fang H, Huang Y, Shepard K, Pesaran B, Rogers JA, Viventi J, Sci. Transl. Med 12, eaay4682 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy RK, Lee K-R, J. Biomed. Mater. Res. B 83B, 72 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Ganesan K, Garrett DJ, Ahnood A, Shivdasani MN, Tong W, Turnley AM, Fox K, Meffin H, Prawer S, Biomaterials 35, 908 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Alcaide M, Taylor A, Fjorback M, Zachar V, Pennisi CP, Front. Neurosci 10 (2016), 10.3389/fnins.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu J-M, Rieth L, Normann RA, Tathireddy P, Solzbacher F, IEEE Trans. Biomed. Eng 56, 23 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Huang H, Xu Y, Low HY, Polymer 46, 5949 (2005). [Google Scholar]
- 35.Noh H, Kyoung-sik Moon A Cannon PJ Hesketh CP Wong, Proc. 54th Electron. Comp. Technol. Conf (IEEE Cat. N0.04CH37546) (Las Vegas, NV, 2004), pp. 924–930. [Google Scholar]
- 36.Hassler C, von Metzen RP, Ruther P, Stieglitz T, J. Biomed. Mater. Res. B 93B, 266 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Chen Y-Y, Lai H-Y, Lin S-H, Cho C-W, Chao W-H, Liao C-H, Tsang S, Chen Y-F, Lin S-Y, Neurosci J. Methods 182, 6 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi S, Suzuki T, Mabuchi K, Fujita H, J. Micromech. Microeng 14, 104 (2003). [Google Scholar]
- 39.Stieglitz T, Beutel H, Schuettler M, Meyer J-U, Biomed. Microdevices 2, 283 (2000). [Google Scholar]
- 40.van Daal RJJ, Sun J-J, Ceyssens F, Michon F, Kraft M, Puers R, Kloosterman F, J. Neural Eng. 17, 016046 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Noh H, Moon K, Cannon A, Hesketh PJ, Wong CP, J. Micromech. Microeng 14, 625 (2004). [Google Scholar]
- 42.Huang J, Cranford RJ, Matsuura T, Roy C, J. Appl. Polym. Sci 87, 2306 (2003). [Google Scholar]
- 43.Minev IR, Musienko P, Hirsch A, Barraud Q, Wenger N, Moraud EM, Gandar J, Capogrosso M, Milekovic T, Asboth L, Torres RF, Vachicouras N, Liu Q, Pavlova N, Duis S, Larmagnac A, Vörös J, Micera S, Suo Z, Courtine G, Lacour SP, Science 347, 159 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Vocke C, Anttila U, Seppälä J, J. Appl. Polym. Sci 72, 1443 (1999). [Google Scholar]
- 45.Yam KL, The Wiley Encyclopedia of Packaging Technology (Wiley, New York, 2010). [Google Scholar]
- 46.Kolarcik CL, Luebben SD, Sapp SA, Hanner J, Snyder N, Kozai TDY, Chang E, Nabity JA, Nabity ST, Lagenaur CF, Cui XT, Soft Matter 11, 4847 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Granstrom J, Swensen JS, Moon JS, Rowell G, Yuen J, Heeger AJ, Appl. Phys. Lett 93, 193304 (2008). [Google Scholar]
- 48.Wu H, Kustra S, Gates EM, Bettinger CJ, Org. Electron 14, 1636 (2013). [Google Scholar]
- 49.Le Floch P, Meixuanzi S, Tang J, Liu J, Suo Z, ACS Appl. Mater. Interfaces 10, 27333 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Kozai TDY, Vazquez AL, J. Mater. Chem. B 3, 4965 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kozai TDY, Jaquins-Gerstl AS, Vazquez AL, Michael AC, Cui XT, ACS Chem. Neurosci 6, 48 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salatino JW, Ludwig KA, Kozai TDY, Purcell EK, Nat. Biomed. Eng 1, 862 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Biran R, Martin DC, Tresco PA, Exp. Neurol 195, 115 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Michelson NJ, Vazquez AL, Eles JR, Salatino JW, Purcell EK, Williams JJ, Cui XT, Kozai TDY, J. Neural Eng. 15, 033001 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wellman SM, Kozai TDY, Biomaterials 164, 121 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kozai TDY, Marzullo TC, Hooi F, Langhals NB, Majewska AK, Brown EB, Kipke DR, J. Neural Eng 7, 046011 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCreery D, Cogan S, Kane S, Pikov V, J. Neural Eng 13, 036012 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kozai TDY, Li X, Bodily LM, Caparosa EM, Zenonos GA, Carlisle DL, Friedlander RM, Cui XT, Biomaterials 35, 9620 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wellman SM, Li L, Yaxiaer Y, McNamara I, Kozai TDY, Front. Neurosci 13 (2019), 10.3389/fnins.2019.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merrill DR, Bikson M, Jefferys JGR, Neurosci J. Methods 141, 171 (2005). [DOI] [PubMed] [Google Scholar]
- 61.Romo R, Hernández A, Zainos A, Salinas E, Nature 392, 387 (1998). [DOI] [PubMed] [Google Scholar]
- 62.Gittis A, Science 361, 462 (2018). [DOI] [PubMed] [Google Scholar]
- 63.Armenta Salas M, Bashford L, Kellis S, Jafari M, Jo H, Kramer D, Shanfield K, Pejsa K, Lee B, Liu CY, Andersen RA, eLife 7, e32904 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Callier T, Brantly NW, Caravelli A, Bensmaia SJ, Proc. Natl. Acad. Sci. U.S.A 117, 1191 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eles JR, Kozai TDY, Biomaterials 234, 119767 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eles JR, Vazquez AL, Kozai TDY, Cui XT, Biomaterials 174, 79 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR, Science 264, 1102 (1994). [DOI] [PubMed] [Google Scholar]
- 68.Stocking KC, Vazquez AL, Kozai TDY, IEEE Trans. Biomed. Eng 66, 2402 (2019). [DOI] [PubMed] [Google Scholar]
- 69.Eles JR, Vazquez AL, Snyder NR, Lagenaur C, Murphy MC, Kozai TDY, Cui XT, Biomaterials 113, 279 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferguson M, Sharma D, Ross D, Zhao F, Adv. Healthc. Mater 8, 1900558 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ecker M, Joshi-Imre A, Modi R, Frewin CL, Garcia-Sandoval A, Maeng J, Gutierrez-Heredia G, Pancrazio JJ, Voit WE, Multifunct. Mater 2, 012001 (2018). [Google Scholar]
- 72.Stiller AM, Usoro J, Frewin CL, Danda VR, Ecker M, Joshi-Imre A, Musselman KC, Voit W, Modi R, Pancrazio JJ, Black BJ, Micromachines 9, 500 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shoffstall AJ, Ecker M, Danda V, Joshi-Imre A, Stiller A, Yu M, Paiz JE, Mancuso E, Bedell HW, Voit WE, Pancrazio JJ, Capadona JR, Micromachines 9, 486 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Do D-H, Ecker M, Voit WE, ACS Omega 2, 4604 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ware T, Simon D, Liu C, Musa T, Vasudevan S, Sloan A, Keefer EW, Rennaker RL, Voit W, J. Biomed. Mater. Res. B 102, 1 (2014). [DOI] [PubMed] [Google Scholar]
- 76.Simon DM, Charkhkar H, John CS, Rajendran S, Kang T, Reit R, Arreaga-Salas D, McHail DG, Knaack GL, Sloan A, Grasse D, Dumas TC, Rennaker RL, Pancrazio JJ, Voit WE, J. Biomed. Mater. Res. A 105, 159 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hosseini SM, Voit WE, Ecker M, J. Vis. Exp e59209 (2019), 10.3791/59209. [DOI] [PubMed] [Google Scholar]
- 78.Hosseini SM, Rihani R, Batchelor B, Stiller AM, Pancrazio JJ, Voit WE, Ecker M, Front. Mater 5 (2018), 10.3389/fmats.2018.00066. [DOI] [Google Scholar]
- 79.Lee HC, Ejserholm F, Gaire J, Currlin S, Schouenborg J, Wallman L, Bengtsson M, Park K, Otto KJ, J. Neural Eng. 14, 036026 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Bettinger J, Bao Z, Adv. Mater 22, 651 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bettinger CJ, Bao Z, Polym. Int 59, 563 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bettinger CJ, Bruggeman JP, Borenstein JT, Langer RS, Biomaterials 29, 2315 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bruggeman JP, de Bruin B-J, Bettinger CJ, Langer R, Biomaterials 29, 4726 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bettinger CJ, Macromol. Biosci 11, 467 (2011). [DOI] [PubMed] [Google Scholar]
- 85.Bettinger CJ, Bruggeman JP, Borenstein JT, Langer R, J. Biomed. Mater. Res. A 91A, 1077 (2009). [DOI] [PubMed] [Google Scholar]
- 86.Bruggeman JP, Bettinger CJ, Langer R, J. Biomed. Mater. Res. A 95A, 92 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ninh C, Bettinger CJ, Biomacromolecules 14, 2162 (2013). [DOI] [PubMed] [Google Scholar]
- 88.Engelmayr GC, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE, Nat. Mater 7, 1003 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nijst CLE, Bruggeman JP, Karp JM, Ferreira L, Zumbuehl A, Bettinger CJ, Langer R, Biomacromolecules 8, 3067 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bettinger CJ, Orrick B, Misra A, Langer R, Borenstein JT, Biomaterials 27, 2558 (2006). [DOI] [PubMed] [Google Scholar]
- 91.Bettinger CJ, Kulig KM, Vacanti JP, Langer R, Borenstein JT, Tissue Eng. A 15, 1321 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu C, Kustra SR, Bettinger CJ, Acta Biomater. 9, 7362 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang W-C, Ali F, Zhao J, Rhee K, Mou C, Bettinger CJ, Biomacromolecules 18, 1162 (2017). [DOI] [PubMed] [Google Scholar]
- 94.Mou C, Ali F, Malaviya A, Bettinger CJ, J. Mater. Chem. B 7, 1690 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu H, Sariola V, Zhu C, Zhao J, Sitti M, Bettinger CJ, Adv. Mater 27, 3398 (2015). [DOI] [PubMed] [Google Scholar]
- 96.Huang W-C, Ong XC, Kwon IS, Gopinath C, Fisher LE, Wu H, Fedder GK, Gaunt RA, Bettinger CJ, Adv. Funct. Mater 28, 1801059 (2018). [Google Scholar]
- 97.Ong XC, Huang W, Kwon IS, Gopinath C, Wu H, Fisher LE, Gaunt RA, Bettinger CJ, Fedder GK, presented at the 31st International Conference on Micro Electro Mechanical Systems (MEMS), Belfast, Ireland, 2018, pp. 376–379. [Google Scholar]
- 98.Angle MR, Kong Y, “Neural-Interface Probe and Methods of Packaging the Same,” US Patent 10327655B2, (2019). [Google Scholar]
- 99.Obaid A, Hanna M-E, Wu Y-W, Kollo M, Racz R, Angle MR, Müller J, Brackbill N, Wray W, Franke F, Chichilnisky EJ, Hierlemann A, Ding JB, Schaefer AT, Melosh NA, Sci. Adv 6, eaay2789 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kozai TDY, Langhals NB, Patel PR, Deng X, Zhang H, Smith KL, Lahann J, Kotov NA, Kipke DR, Nat. Mater 11, 1065 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Musk E, Med J. Internet Res. 21 (2019), 10.2196/16194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Driller J, Hilal SK, Michelsen WJ, Sollish B, Katz B, Konig W, Med. Res. Eng 8, 11 (1969). [PubMed] [Google Scholar]
- 103.Oxley TJ, Opie NL, John SE, Rind GS, Ronayne SM, Wheeler TL, Judy JW, McDonald AJ, Dornom A, Lovell TJH, Steward C, Garrett DJ, Moffat BA, Lui EH, Yassi N, Campbell BCV, Wong YT, Fox KE, Nurse ES, Bennett IE, Bauquier SH, Liyanage KA, van der Nagel NR, Perucca P, Ahnood A, Gill KP, Yan B, Churilov L, French CR, Desmond PM, Horne MK, Kiers L, Prawer S, Davis SM, Burkitt AN, Mitchell PJ, Grayden DB, May CN, O’Brien TJ, Nat. Biotechnol 34, 320 (2016). [DOI] [PubMed] [Google Scholar]
- 104.Opie NL, van der Nagel NR, John SE, Vessey K, Rind GS, Ronayne SM, Fletcher EL, May CN, O’Brien TJ, Oxley TJ, IEEE Trans. Biomed. Eng 64, 928 (2017). [DOI] [PubMed] [Google Scholar]
- 105.DiMilia T, “Synchron Achieves First Successful Human Implantation of Brain Computer Interface,” Synchron (2019), https://www.synchronmed.com/media/2019/09/synchron-achieves-first-successful-human-implantation-of-brain-computer-interface (accessed April 9, 2020). [Google Scholar]
- 106.Hof RD, “Lessons from Sematech,” MIT Technology Review (2011), https://www.technologyreview.com/2011/07/25/192832/lessons-from-sematech (accessed April 9, 2020). [Google Scholar]
- 107.Khodagholy D, Gelinas JN, Thesen T, Doyle W, Devinsky O, Malliaras GG, Buzsáki G, Nat. Neurosci 18, 310 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martin DC, Malliaras GG, ChemElectroChem 3, 686 (2016). [Google Scholar]
- 109.Khodagholy D, Doublet T, Gurfinkel M, Quilichini P, Ismailova E, Leleux P, Herve T, Sanaur S, Bernard C, Malliaras GG, Adv. Mater 23, H268 (2011). [DOI] [PubMed] [Google Scholar]
- 110.Khodagholy D, Gelinas JN, Zhao Z, Yeh M, Long M, Greenlee JD, Doyle W, Devinsky O, Buzsáki G, Sci. Adv 2, e1601027 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


