Significance
A critical, but not well characterized, step in bacterial cell division is the activation of the FtsWI septal peptidoglycan (sPG) synthase at the Z-ring. In Escherichia coli, activation depends upon FtsN, which acts indirectly by activating FtsQLB in the periplasm and FtsA in the cytoplasm. Here, we show that an activated FtsA rescues FtsW recruitment and activation when the hierarchical pathway of recruitment is disrupted. Furthermore, an ftsW mutant was isolated that responds to a hyperactive FtsQLB but not to a hyperactive FtsA. Our results indicate an activated FtsA acts through FtsW and synergizes with an activated FtsL acting on FtsI to activate FtsWI within the divisome to synthesize sPG, resulting in cytokinesis.
Keywords: FtsW, FtsA, FtsQ, cytokinesis, septal PG
Abstract
In Escherichia coli, FtsQLB is required to recruit the essential septal peptidoglycan (sPG) synthase FtsWI to FtsA, which tethers FtsZ filaments to the membrane. The arrival of FtsN switches FtsQLB in the periplasm and FtsA in the cytoplasm from a recruitment role to active forms that synergize to activate FtsWI. Genetic evidence indicates that the active form of FtsQLB has an altered conformation with an exposed domain of FtsL that acts on FtsI to activate FtsW. However, how FtsA contributes to the activation of FtsW is not clear, as it could promote the conformational change in FtsQLB or act directly on FtsW. Here, we show that the overexpression of an activated FtsA (FtsA*) bypasses FtsQ, indicating it can compensate for FtsQ’s recruitment function. Consistent with this, FtsA* also rescued FtsL and FtsB mutants deficient in FtsW recruitment. FtsA* also rescued an FtsL mutant unable to deliver the periplasmic signal from FtsN, consistent with FtsA* acting on FtsW. In support of this, an FtsW mutant was isolated that was rescued by an activated FtsQLB but not by FtsA*, indicating it was specifically defective in activation by FtsA. Our results suggest that in response to FtsN, the active form of FtsA acts on FtsW in the cytoplasm and synergizes with the active form of FtsQLB acting on FtsI in the periplasm to activate FtsWI to carry out sPG synthesis.
Cell division in Escherichia coli starts with the assembly of the Z-ring, which forms when FtsZ filaments, tethered to the membrane by FtsA and ZipA, coalesce at midcell (1–3). FtsEX joins the ring as it is formed and acts on FtsA to initiate the recruitment of the late cell division proteins (4). Various studies indicate a hierarchy in the recruitment of the late proteins with the order FtsK, FtsQ, FtsLB, FtsW, FtsI, and FtsN (Fig. 1A) (5–7). Despite this hierarchy, there is a web of interactions among these proteins and FtsQ, FtsL, and FtsB, each a bitopic membrane protein, are in a complex, even in the absence of the Z-ring (8). FtsQ is responsible for the localization of the complex to the Z-ring, and FtsL is required to recruit FtsWI (9, 10), which synthesizes septal peptidoglycan (sPG) (11–13). FtsW is a glycosyltransferase and a member of the SEDS family (septation, elongation, division, and sporulation) that functions with its cognate transpeptidase (FtsI [PBP3]) to carry out the two enzymatic steps necessary to incorporate peptidoglycan precursors into the cell wall.
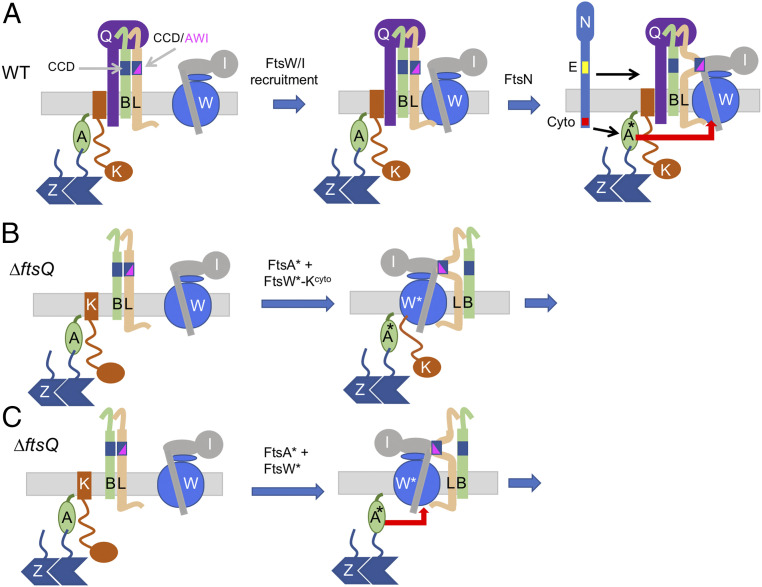
Fig. 1.
Recruitment and activation of FtsWI in E. coli. (A) The hierarchical divisome assembly pathway and activation mechanism. The FtsQLB complex lies between FtsK and FtsWI in the pathway. FtsWI is recruited to the Z-ring in an FtsQ-dependent manner with the cytoplasmic domain of FtsL being required to recruit FtsWI. The arrival of FtsN (E domain in yellow and cyto domain in red) switches FtsA in the cytoplasm and FtsQLB in the periplasm to the “on” state, which synergize to activate FtsWI to carry out sPG synthesis. Part of this activation is due to a conformational change in FtsQLB, which exposes a domain of FtsL (AWI) that interacts with FtsI that affects FtsW. The work presented in this study indicates that FtsA* acts directly on FtsW. Note that some cell division proteins are not depicted, including ZipA and FtsEX. Also, the deletion of FtsQ, loss of FtsLcyto or disruption of the FtsB–FtsQ interaction prevents the recruitment of FtsWI. (B) Bypassing ftsQ with the FtsW–FtsKcyto fusion and FtsA*. The FtsW–FtsKcyto fusion was used to bypass the requirement for FtsQ for the recruitment to the Z-ring. This fusion bypasses ftsQ, provided it carries an ftsW* mutation and ftsA* is present. It is likely that FtsL and FtsB are back recruited by the FtsWI complex since they cannot be deleted. (C) Bypass of ftsQ by overexpression of ftsA* in the presence of ftsW*. The results presented herein indicate that, in the absence of FtsQ, FtsA* rescues the recruitment of FtsW by interacting with it in the cytoplasm. FtsA* can also activate FtsWI in the absence of the signal from FtsLAWI.
The FtsQLB complex is conserved in peptidoglycan containing bacteria and, in addition to a role in recruitment, is also involved in regulating sPG synthesis (10). FtsQLB, along with FtsA and FtsWI, is required to recruit FtsN, which leads to the activation of FtsWI to synthesize sPG, which is essential for cell division (14–16). FtsN may also stimulate PBP1b, which contributes to sPG synthesis, but this activity is not essential (17–20). In the current model, the arrival of FtsN switches both FtsQLB and FtsA to an active state, which synergize to activate FtsWI (21, 22). This step requires two domains of FtsN; FtsNcyto acts on FtsA in the cytoplasm and FtsNE, a short sequence of ∼20 amino acts in the periplasmic domain, likely acts on FtsQLB (21) (Fig. 1A). How FtsA contributes to the activation is not clear.
Although signaling by both domains of FtsN is normally required, hyperactivation of either signal alone is sufficient for viability. Such cells are filamentous in rich media however, suggesting that activation is not fully restored (21, 22). The periplasmic signal can be hyperactivated by overexpressing FtsNE in the periplasm or by “activation (superfission)” mutations in ftsB or ftsL (4, 21). These activation mutations appear to mimic FtsN action, causing a region of FtsL (called AWI) to contact FtsI, which activates FtsW (23). The cytoplasmic signal can be hyperactivated by the overexpression of certain “activated” ftsA alleles (ftsAE124A and ftsAI143L), which may act through the FtsQLB complex or act directly on FtsWI (21, 24). A mutant of FtsEX that is unable to hydrolyze ATP blocks the cytoplasmic signal, but this can be overcome by the hyperactivation of the periplasmic signal (4, 25) or a mutation in ftsW, which is thought to produce a constitutively active FtsW that is less dependent upon FtsN (4).
Beckwith’s group provided evidence that the cytoplasmic signal generated by FtsN does not go through FtsQ. They isolated FtsAI143L as a suppressor of an FtsQ mutant (V92D) defective in localization, and surprisingly, it restored localization, even in the absence of the cytoplasmic domain of FtsQ (the only region of FtsQ that could contact FtsA) (9). Although FtsAI143L was unable to bypass ftsQ, it rescued another FtsQ mutant (FtsQA252P) unable to bind FtsB and therefore recruit FtsW. Another FtsA allele (FtsAR286W) also suppressed these deficiencies but to different degrees. As a result, it was concluded that these activated FtsA mutants do not interact with FtsQ but somehow stabilize the divisome, possibly by interacting with another late division protein. Many additional activated FtsA mutants have been isolated, and most show a reduction in self-interaction (26).
Other attempts to bypass ftsQ in E. coli have been unsuccessful, although it has been bypassed in several gram-positive bacteria (27–30). Overexpression of ftsAR286W (hereafter ftsA*), which bypasses ftsK, the gene immediately upstream of ftsQ in the recruitment pathway (Fig. 1A), did not bypass ftsQ (25). Attempts to bypass ftsL or ftsB using the same conditions that bypass ftsK also failed (27). Nonetheless, a ZapA–FtsL fusion, which targets FtsL directly to the Z-ring, recruits FtsB in the absence of FtsQ and also recruits FtsWI, indicating that FtsL and FtsB retain some function in the absence of FtsQ as in gram-positive bacteria (14).
Although FtsQLB is involved in activating FtsWI in response to FtsN, how an activated FtsA (FtsA*) contributes to this activation is not clear (21, 22). In vitro reconstitution confirmed that FtsWI was a PG synthase and was activated by FtsQLB; however, the system did not fully recapitulate the in vivo regulation, as FtsN had no effect (12, 13). Furthermore, FtsA was not included. In this study, we set out to explore the role of FtsA in activation. Our results indicate that an FtsA* contributes to the activation of FtsWI by acting on FtsW.
Results
Depletion of ftsQ Is Suppressed by FtsA* or an FtsW*–FtsKcyto Fusion.
As a first step to determine if the signal from an active form of FtsA acts directly on FtsWI or goes through the FtsQLB complex, we tested various conditions to see if FtsQ could be depleted without compromising growth. For FtsQ to be bypassed, one has to compensate for its role in recruitment, as well as the activation, of FtsWI. If successful, it would argue against the signal going through FtsQLB. We used a depletion strain with the chromosomal ftsQ gene disrupted [ftsQ14::kan contains the kan resistance gene in place of codons 14 to 80 of ftsQ, disrupting the gene (31)] and a ftsQ provided by a plasmid under arabinose promoter control. Compatible plasmids carrying different genes were then introduced to see if they allowed colony formation in the absence of arabinose. One plasmid contained ftsA* under an IPTG-inducible promoter, since it, like other ftsA*-like alleles, suppresses various cell division deficiencies, including various FtsQ mutants (4, 9, 32–34). We previously observed that the overexpression of ftsA* improved its ability to suppress various division defects (34) and decided to reevaluate its ability to bypass ftsQ (25). Another plasmid constitutively expressed an ftsW–ftsKcyto fusion, which targets FtsW directly to the Z-ring, bypassing the need for the FtsQLB complex for recruitment, so only activation is required (35) (Fig. 1B). We also used a version of this fusion containing the ftsWM269I (hereafter ftsW*) mutation, which is classified as an “activation” mutation, since it reduces the amount of FtsN required for growth (4). This fusion might bypass FtsQ since it is targeted to the Z-ring and contains an activation mutation.
Initial spot tests were done on Luria-Broth (LB) plates supplemented with 0.5% NaCl, and weak suppression was observed with ftsA* at high-IPTG concentrations (SI Appendix, Fig. S1A, Top, fourth row). Suppression was dramatically improved when LB plates were supplemented with 1.0% NaCl (SI Appendix, Fig. S1A, Lower, fourth row), so 1.0% NaCl was used for the remaining experiments. The ftsW–ftsKcyto fusion was unable to suppress the ftsQ depletion, suggesting that it is not activated in the absence of FtsQ. Consistent with this, the ftsW*–ftsKcyto fusion (containing an activating mutation) suppressed the ftsQ depletion, indicating that bypassing both the recruitment and, to a large extent, the activation of FtsW [ftsW* weakly bypasses ftsN (4)] allowed ftsQ to be bypassed. Importantly, ftsW* was unable to suppress the ftsQ depletion, emphasizing it had to be fused to FtsKcyto so that it is recruited to the Z-ring (SI Appendix, Fig. S1B).
We also detected synergism between our two constructs as the overexpression of ftsA* enhanced the suppression by the ftsW*–ftsKcyto fusion (larger colonies at highest dilutions). Moreover, the presence of the ftsW–ftsKcyto enhanced colony formation over that seen with ftsA* alone (SI Appendix, Fig. S1A; larger colonies at the highest dilution with 25 and 50 μM IPTG). Although these results were obtained with W3110, we observed similar results with another laboratory strain (MC4100), indicating that this behavior is a general characteristic and not strain specific (SI Appendix, Fig. S2). These results suggested that the overexpression of FtsA* can compensate for FtsQ’s role in the recruitment of FtsW.
Deletion of ftsQ Is Supported by the ftsW*–ftsK Fusion in the Presence of ftsA*.
Residual FtsQ is likely present in the depletion studies because of basal expression from the arabinose promoter. Furthermore, it has been shown that less ftsQ is required when an ftsA* allele is present (9). To see if ftsQ could be completely bypassed, we transduced ftsQ14::kan into W3110, carrying the plasmids used in the depletion of ftsQ suppressed by FtsA* or FtsW*–FtsK fusion. Transductants were readily obtained in the presence of a control plasmid expressing ftsQ or plasmids expressing the ftsW*–ftsKcyto fusion along with ftsA* (Fig. 2). In a strain expressing just the ftsW*–ftsKcyto fusion colonies that appeared, however, their delayed appearance, variation in size, and the outgrowth of larger colonies suggested the emergence of suppressor mutations, and they were not studied further. The inability of this activated fusion to bypass FtsQ is likely due to ftsW*’s failure (not fully activated) to completely bypass FtsN (4). In a strain expressing just ftsA*, homogeneous, sized colonies appeared in the presence of IPTG after 2 d (SI Appendix, Fig. S3), indicating that FtsA* partially compensated for FtsQ’s role in the recruitment and activation of FtsWI. However, such transductants grew poorly in liquid culture, and cells were filamentous, so they were not studied further.
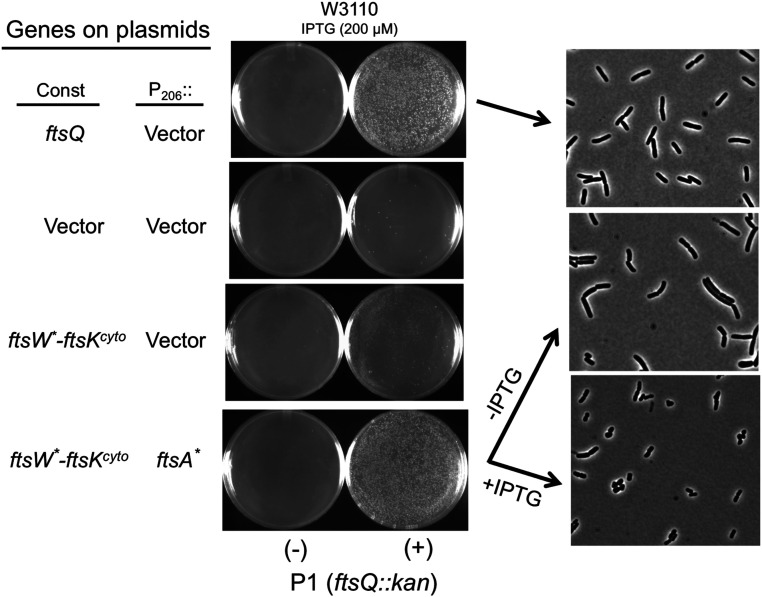
Fig. 2.
Deletion of ftsQ in the presence of ftsW*–ftsKcyto and ftsA*. P1 grown on JOE417 (ftsQ14::kan/pBAD33-ftsQ) was used to transduce W3110 to kanamycin resistance. The strain carried plasmids that constitutively expressed ftsQ (pSEB468) or ftsW*–ftsKcyto (pND16*) and either ftsA* inducible with IPTG (pSEB306*[P206::ftsA*]) or a vector. Transductants were selected at 30 °C on plates containing 200 µM IPTG. Colonies from the Top and Bottom were purified and then grown in liquid medium of the same composition. The strain containing plasmids expressing ftsW*–ftsKcyto and ftsA* was centrifuged, washed, and resuspended in LB with or without IPTG. Samples were taken 2 h later for photography. Colonies also arose on plates expressing just ftsW*–ftsKcyto but grew slower and were of variable size. Colonies also arose on plates expressing just ftsA*, but cells displayed elongated and chaining phenotypes (SI Appendix, Fig. S3). Upon restreaking, growth of these colonies was poor, and when they were cultured in LB broth, cells were filamentous and not studied further. Note that sporadic colonies appeared on plates of the strain with just the vector plasmids, suggesting they arose because of suppressor mutations.
To assess how well combining ftsW*–ftsKcyto and ftsA* bypassed ftsQ, we examined the morphology of ftsQ14::kan transductants. In the absence of IPTG, cells had a mild chaining phenotype; however, in the presence of IPTG, cells were actually shorter than the control (i.e., with the ftsQ plasmid), although some cells had a distorted rod shape (Fig. 2). The results were similar with strain MC4100 (SI Appendix, Fig. S4), and PCR analysis of the transductants confirmed the presence of the ftsQ14::kan allele and the absence of an intact ftsQ gene (SI Appendix, Fig. S5).
Combining FtsA* and FtsW* Efficiently Bypasses ftsQ but Not ftsB or ftsL.
As shown in SI Appendix, Fig. S1B, the overexpression of ftsA* alone weakly bypassed ftsQ (slow growing colonies on LB plates containing 1% NaCl, following the transduction of ftsQ14::kan; SI Appendix, Fig. S3). This is consistent with ftsA* providing an activation signal in the cytoplasm for sPG synthesis, as suggested (21), but also indicates it compensates for FtsQ’s recruitment function, possibly directly recruiting FtsWI. If so, the coexpression of ftsA* and ftsW* (without being fused to FtsKcyto) may allow the bypass of FtsQ, as the activated alleles may have stronger interactions (Fig. 1C). To test this, we transduced ftsQ::E14 into a strain that had ftsW* on the chromosome and contained a plasmid with ftsA* under an IPTG-inducible promoter. Surprisingly, ftsQ14::kan could be readily transduced into such a strain in the presence of IPTG (Fig. 3). In liquid culture, the transductants were filamentous in the absence of IPTG; however, they were not much longer than the control (with ftsQ) in the presence of IPTG, although some cells displayed a mild chaining phenotype (Fig. 3). It should be noted that the overexpression of ftsA* was required to obtain this cell morphology (at least 25 μM IPTG was required, leading to approximately fivefold increase in FtsA*). This result raises the possibility that FtsA* recruits FtsW* in the absence of ftsQ and contributes to its activation (FtsW* requires less FtsN but is unable to bypass ftsN at the chromosomal level) (4).
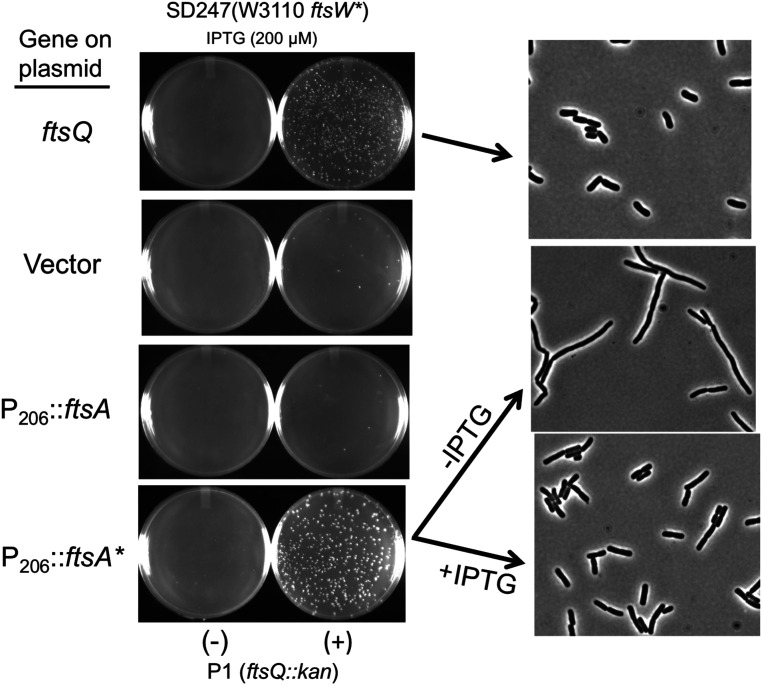
Fig. 3.
FtsA* and FtsW* synergize to bypass ftsQ. P1 grown on JOE417 (ftsQ14::kan/pBAD33-ftsQ) was used to transduce SD247 (ftsW*) to kanamycin resistance. The strain also contained plasmids (pSEB306 [P206::ftsA] or pSEB306* [P206::ftsA*]) containing ftsA alleles inducible with IPTG. Kanamycin-resistant transductants were selected at 30 °C in the presence of 200 µM IPTG (to induce ftsA or ftsA*). Transductants obtained from the strain containing ftsA* were restreaked under the same conditions. Colonies were grown in LB in the presence of 200 µM IPTG, centrifuged, and grown with or without IPTG. Samples were taken for photography, as described in the legend to Fig. 2. At least 25 µM IPTG was required to have a nonfilamentous morphology.
Since ftsQ can be bypassed, we tested whether ftsL and ftsB could also be bypassed using the same strategy we employed to bypass ftsQ. However, we were unable to obtain transductants that inactivated ftsB or ftsL in a strain that contained ftsW*–ftsK and in which ftsA* was overexpressed (SI Appendix, Fig. S7 A and B) or in a strain that contained ftsW* on the chromosome and ftsA* was overexpressed (SI Appendix, Fig. S7 A and B). This result indicates that FtsL and FtsB have a function, even in the absence of FtsQ.
FtsA*’s Ability to Bypass ftsQ Does Not Depend upon ftsN.
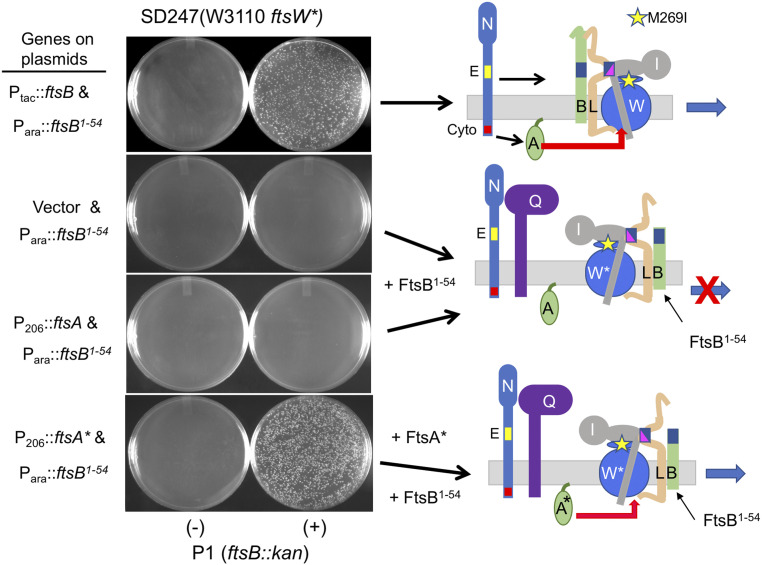
A possible explanation for FtsA*’s ability to bypass FtsQ is that it recruits FtsN, which back recruits and activates FtsW. Some genes (zipA, ftsEX, and ftsK) in the recruitment pathway (Fig. 1A) can be bypassed by the overexpression of ftsN. This bypass requires that FtsN interact with FtsA and that a functional FtsNE domain be connected to FtsNcyto (4, 27, 36). Thus, the overexpression of ftsN might bypass ftsQ, as FtsK would be recruited normally while FtsLB and FtsWI could be back recruited and activated. To test this, we introduced pKD140 (PftsN::ftsN), which overexpresses ftsN about 10-fold and is sufficient to bypass zipA, ftsEX, or ftsK (4, 33, 36). However, we were unable to obtain ftsQ14::kan transductants (SI Appendix, Fig. S8). Thus, the overexpression of ftsN cannot bypass ftsQ, suggesting that either recruitment or activation (or both) of FtsW was not occurring.
As an additional test to see whether FtsA* acts through FtsQLB by recruiting FtsN, we examined if the overexpression of ftsA* could suppress ftsLL86F/E87K (23). This allele of ftsL is a dominant-negative mutant that assembles into an FtsQLB complex and recruits FtsWI but fails to rescue division because it cannot interact with FtsI in response to FtsN to activate FtsW. As previously reported (23), ftsLL86F/E87K is not rescued by the overexpression of ftsN but is rescued by ftsW* (Fig. 4). FtsW* rescues because it is recruited and is less dependent upon the periplasmic activation signal from FtsN (23). Importantly, the overexpression of ftsA*, but not ftsA, also rescued ftsLL86F/E87K, indicating that FtsW was being activated in the absence of the periplasmic signal. Other ftsA*-like alleles (ftsAI143L and ftsAE124A) also displayed some ability to rescue ftsLL86F/E87K but were not as strong as ftsA* (SI Appendix, Fig. S9). Since the overexpression of ftsN is unable to rescue ftsLL86F/E87K, it argues that the rescue by ftsA* is not through the recruitment of FtsN but is consistent with FtsA* acting directly on the FtsWI complex in the cytoplasm.
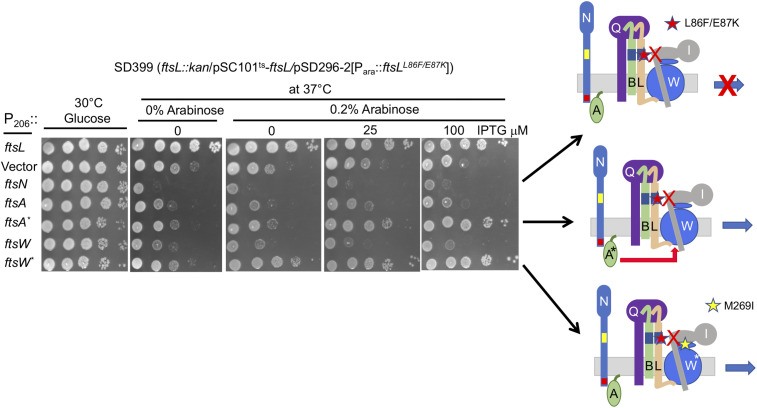
Fig. 4.
Overexpression of ftsA* or ftsW* but not ftsN rescues an ftsL allele deficient in activating ftsW. SD399 (ftsL::kan/pSC101ts-ftsL) containing pSD296-2 (Para::ftsLL86F/E87K) and pSEB417 (P204::ftsN), pSEB306* (P206::ftsA*), pSEB429 (P204::ftsW), pSEB429-1 (P204::ftsW*), or pKTP100 (Ptac::ftsL) was spotted at 37 °C to deplete WT ftsL. Arabinose was added to induce ftsLL86F/E87K, and IPTG was added to induce ftsL, ftsN, ftsA, ftsA*, ftsW, or ftsW*. The control plate lacked arabinose, whereas the test plates contained 0.2% arabinose to induce ftsLL86F/E87K and increasing concentrations of IPTG to induce the other genes. Note that the basal expression of ftsL from pKTP100 is sufficient for complementation in the absence of IPTG.
Bypass of ftsN by ftsA* Is Enhanced by ftsW*.
If FtsA* activates the FtsWI complex, it may bypass ftsN. In fact, two ftsA*-like alleles (ftsAE124A and ftsAI143L) have been shown to bypass ftsN when overexpressed; however, such strains are filamentous in LB with 0.5% NaCl, although less so in minimal medium (21). Since minimal medium has higher ionic strength, and we observed that the bypass of ftsQ was enhanced by increased NaCl (SI Appendix, Fig. S1A), we tested the effect of increased NaCl on bypassing ftsN. To do this, ftsN::kan was transduced into W3110 containing a plasmid with ftsA* inducible by IPTG. On LB plates with 0.5% NaCl and 200 μM IPTG, colonies slowly emerged but were of variable size; however, on plates with 1.0% NaCl and 200 μM IPTG, colonies were homogeneous, although cells were filamentous in liquid culture (SI Appendix, Fig. S10). This suggests that ftsA*, like ftsAE124A and ftsAI143L, weakly bypasses ftsN and at least partially activates FtsW; however, the presence of filamentous cells indicates that the bypass is inefficient.
Since ftsW* is synergistic with the overexpression of ftsA* in bypassing ftsQ, and is less dependent upon FtsN, it should enhance the ability of overexpressed ftsA* to bypass ftsN. This was indeed the case, as ftsN::kan could be readily introduced into a strain containing ftsW* on the chromosome and a plasmid with an IPTG-inducible copy of ftsA* (Fig. 5A). Such cells had a fairly normal morphology, even in the absence of IPTG, indicating that the basal expression of ftsA* from the plasmid was sufficient. Thus, ftsA* (in the presence of ftsW*) readily promotes the bypass of ftsN, whereas the bypass of ftsQ requires ftsA* to be overexpressed and, even then, a wild-type (WT) morphology was not obtained. A major difference in bypassing FtsQ versus FtsN is that, to bypass FtsQ, FtsW* recruitment has to be restored, whereas FtsW* is recruited normally in the absence of FtsN (7, 37). This result suggests that FtsA* recruits FtsW in the absence of FtsQ.
Fig. 5.
FtsA* and FtsW* synergize to bypass ftsN and ftsEX. (A) Bypass of ftsN. To examine conditions for bypassing ftsN, P1 prepared on CH34 (ftsN::kan/pCH201 [Plac:: gfp-ftsN]) was used to transduce SD247 (ftsW*) containing plasmids pSEB306 (P206::ftsA), pSEB306* (P206::ftsA*), or pSEB417 (P204::ftsN) to kanamycin resistance. Transductants were selected at 30 °C on LB agar in the presence of 200 µM IPTG. A transductant growing exponentially in LB with 200 μM IPTG was centrifuged and resuspended in LB with or without 200 µM IPTG, and samples were taken for photography, as described in the legend to Fig. 2. (B) Bypass of ftsN and ftsEX. To see if ftsN and ftsEX could be bypassed simultaneously, a kanamycin-resistant transductant from part A, PK7 (ftsW* ftsN::kan/p306*[P206::ftsA*]), was transduced to chloramphenicol resistance with P1 grown on SD205 (ftsEX::cat). A transductant was grown for photography, as described in the legend to Fig. 2.
To further examine the possibility that FtsA* recruits and activates FtsWI, we transduced ftsEX::cat into a strain (containing ftsW* and overexpressing ftsA*) in which ftsN was already deleted. As expected from the ftsQ bypass results shown earlier, ftsEX could be readily deleted (Fig. 5B). The ftsN and ftsEX double-deletion strain was filamentous in the absence of IPTG; however, upon the overexpression of ftsA*, it was less filamentous, indicating that FtsW* was being recruited.
FtsA* Rescues Alleles of ftsB and ftsL That Disrupt Recruitment of FtsWI.
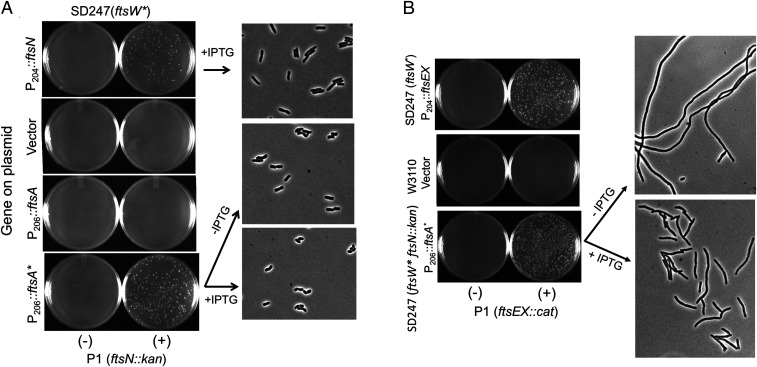
The results so far suggest that FtsA*, when overexpressed, can recruit FtsW. To further test this, we examined if the overexpression of ftsA* could rescue conditions that disrupt the hierarchical pathway. A C-terminally truncated FtsB (FtsB1–54) forms a complex with FtsL, but it is not recruited to the division site because it lacks the region that interacts with FtsQ (38–40). As a result, FtsWI does not localize to the Z-ring. If FtsA* acts directly on FtsWI, it may rescue such an FtsB mutant by restoring FtsWI recruitment. To test this, we transduced ftsB::kan into a strain containing ftsW* on the chromosome, along with plasmids expressing ftsA* or ftsA under an IPTG-inducible promoter and ftsB1–-54 under an arabinose inducible promoter. Transductants were readily obtained in the presence of arabinose when ftsA*, but not ftsA, was overexpressed (Fig. 6). Thus, the overexpression of ftsA* rescued the recruitment of FtsW in a strain in which the hierarchical pathway had been disrupted by uncoupling FtsQ from FtsLB. This result is similar to ftsA* (and FtsAI143L) suppressing FtsQA252P, which disrupts the recruitment of FtsLB and FtsWI because of its defect in interacting with FtsB (9).
Fig. 6.
Overexpression of ftsA* rescues an ftsB allele that disrupts the hierarchical recruitment pathway. To see if FtsA* could rescue a strain in which the hierarchical recruitment pathway was disrupted (because of the lack of FtsQ–FtsB interaction), P1 phage prepared on SD368 (ftsB::kan/pSD255 [pSC101ts/Psyn135::ftsB]) was used to transduce SD247 (ftsW*) containing plasmids expressing ftsB1–54 (pSD295-54/Para::ftsB1–54) and ftsA* (pSEB306*/P206::ftsA*) to kanamycin resistance. FtsB1–54 is a C-terminal–truncated FtsB mutant that interacts with FtsL but not FtsQ and cannot support the hierarchical pathway of divisome assembly. Transductants were selected on kanamycin plates with 0.2% arabinose (to induce ftsB1–54) and 200 µM IPTG (to induce ftsA*). No transductants were obtained with SD247 (ftsW*) expressing ftsB1–54 or ftsB1–54 and pSEB306 (P206::ftsA).
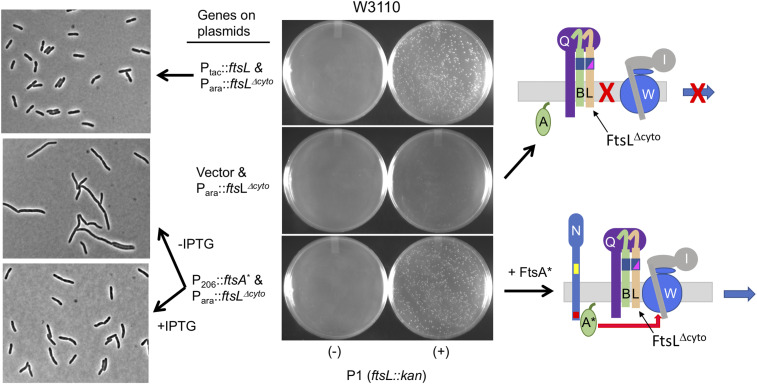
Next, we examined whether FtsA* could rescue FtsLΔcyto, which assembles into a complex with FtsQB and localizes to the Z-ring but fails to recruit FtsW because it lacks the cytoplasmic domain of FtsL (10). To do this, a plasmid carrying an IPTG-inducible ftsA* was introduced into a strain carrying the WT ftsW allele on the chromosome. We then introduced a plasmid expressing ftsLΔcyto under arabinose promoter control and tested whether ftsL could be deleted from the chromosome. In contrast to the test in Fig. 6 with FtsB, this test was done in the presence of WT ftsW, so rescue requires the activation of FtsW in addition to restoration of its recruitment. Indeed, we were able to readily transduce ftsL::kan into this strain in the presence of IPTG and arabinose (Fig. 7). The growth of these transductants was comparable to the positive control in which ftsL was provided by a high copy plasmid [ftsL from this plasmid is in excess to suppress the dominant-negative effect of ftsLΔcyto, which blocks FtsW recruitment (23)]. In addition, since the rescue by FtsA* occurred in the absence of the cytoplasmic domain of FtsL, it rules out FtsA* acting through FtsL.
Fig. 7.
Overexpression of ftsA* rescues an ftsL allele deficient in recruitment FtsW. To investigate whether FtsA* recruits FtsW, we tested whether the overexpression of ftsA* rescues ftsLΔcyto, which cannot recruit FtsW. To do this, P1 prepared on SD439 (ftsL::kan/pSD296 [Para::ftsL]) was used to transduce W3110 containing plasmid pKTP107 (Para::ftsLΔcyto]) or pSEB306* (P206::ftsA*) inducible with IPTG to kanamycin resistance. For a control, W3110 contained pKTP100 (Ptac::ftsL), which expresses ftsL at a high level to overcome the dominant-negative effect caused by ftsLΔcyto. Kanamycin-resistant transductants were selected at 30 °C on plates containing 0.2% arabinose, 200 µM IPTG, chloramphenicol, and ampicillin. Transductants were grown in LB with 200 µM IPTG and photographed, as in Fig. 2.
Cytoplasmic ftsW Mutations Rescued by ftsL* but Not ftsA*.
Both the periplasmic and cytoplasmic signals generated by FtsN are required to activate sPG synthesis; however, hyperactivation of either signal allows survival in the absence of the other (21). Thus, as shown earlier, FtsA* was able to rescue ftsLL86F/E87K, which blocks the periplasmic signal. We reasoned that if the cytoplasmic signal acts on FtsW, it should be possible to isolate ftsW mutations that can be rescued by a hyperactive periplasmic signal (ftsL*) but not by a hyperactive cytoplasmic signal (ftsA*). The identification of such mutants would indicate a domain of FtsW needed for responsiveness to FtsA*. To do this, we investigated FtsW residues that are exposed to the cytoplasm.
FtsW has four intracellular loops, and both the N and C termini are in the cytoplasm (Fig. 8A) (41). Since the N-terminal region is quite long but lacks conserved amino acid residues, as does the C-terminal region, we focused on the two larger intracellular loops. About 10 mutations, most of which were in intracellular loop 2 (it is more conserved), were generated by site-directed mutagenesis. Of these 10, six no longer complemented an ftsW depletion strain, indicating that they were loss-of-function mutations (SI Appendix, Fig. S11A and Table S3). We focused on four of the six that displayed various degrees of a dominant-negative phenotype, indicating that the mutant proteins were stable (SI Appendix, Fig. S11B and Table S3). Although it is possible that these mutants do not localize to the division site because they no longer interact with the cytoplasmic domain of FtsL, this is unlikely, as such mutants would not be expected to be dominant negative. Nonetheless, we checked the localization of FtsWR172D, the mutant with the strongest dominant-negative phenotype. GFP-FtsWR172D localized in cross-bands in filamentous cells following the depletion of WT FtsW, confirming it did not have a defect in localization (SI Appendix, Fig. S12).
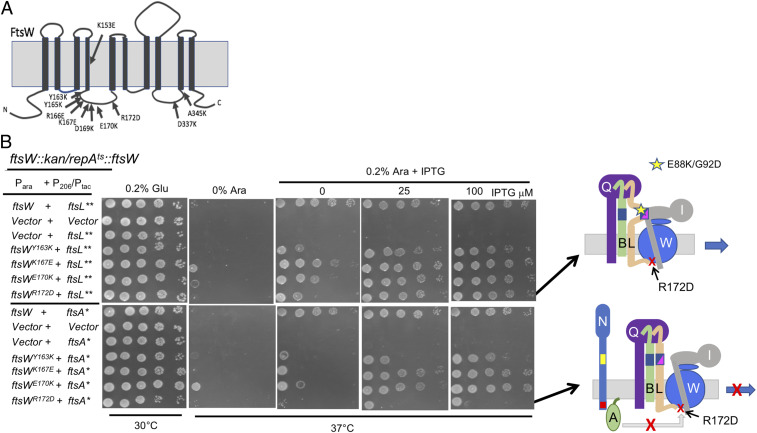
Fig. 8.
The effect of hyperactive periplasmic and cytoplasmic signals on the rescue of FtsW cytoplasmic mutants. (A) Diagram indicating FtsW residues targeted for site-directed mutagenesis. Conserved residues in cytoplasmic loops 2 and 4 of FtsW were altered by site-directed mutagenesis and tested for loss of function and dominant-negative phenotypes (SI Appendix, Fig. S11). (B) Four FtsW loss-of-function mutants that are also dominant negative were tested to see if they could be rescued by the expression of ftsL** (hyperactive periplasmic signal, Top) or ftsA* (hyperactive cytoplasmic signal, Bottom). To do this, SD295 (ftsW::kan/pSD257 [repATS ftsW]) was transformed with plasmids carrying various ftsW alleles under the arabinose promoter control (pDSW406/Para::ftsW) along with plasmids expressing ftsA* (pSEB306*[P206::ftsA*]) or ftsLE88K/G92D (pKTP100* [Ptac::ftsLE88K/G92D]) under IPTG control. Plates were incubated at 37 °C to deplete WT ftsW, and 0.2% arabinose (to induce ftsW alleles) and IPTG (to induced ftsA* or ftsL**) were added.
The four dominant-negative FtsW mutants were then screened for their ability to be rescued by active alleles of ftsL and ftsA. The ftsL allele we used was ftsLE88K/G92D (ftsL**), as other tests indicated it is a stronger activation allele than ftsLE88K (23). For the test, the ftsW alleles contained on a plasmid were induced with arabinose, and ftsL** or ftsA* (on a compatible plasmid) were induced with IPTG in a strain depleted for FtsW. As shown in Fig. 8B, all four mutants were rescued by ftsL**, demonstrating that they responded to FtsL. Two were rescued by ftsA*; however, ftsWY163K was only weakly rescued by ftsA*, and ftsWR172D was not rescued. Since, ftsWR172D and ftsWY163K mutations are readily rescued by a hyperactive periplasmic signal (ftsL**) but not by a hyperactive cytoplasmic signal (ftsA*), it indicates they are specifically defective in activation by FtsA*.
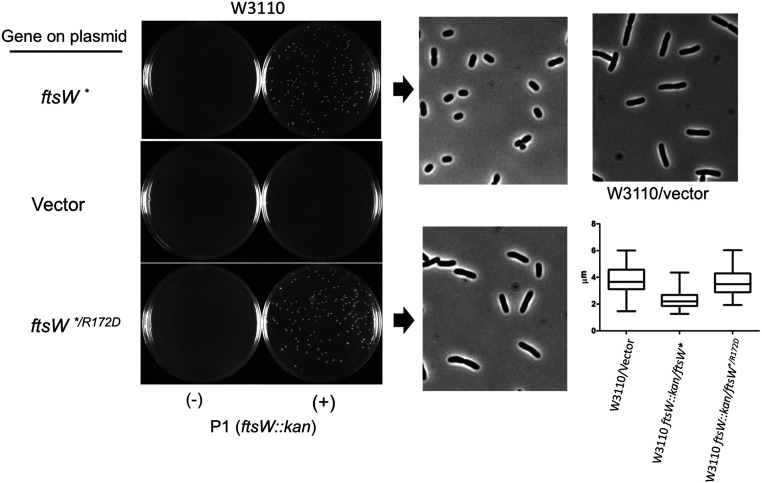
FtsWR172D is rescued by ftsL**, indicating that it retains the ability to respond to FtsLAWI in the periplasm. Since FtsL** acts on FtsI, causing FtsW to take on the active state, the ftsW* mutation, which alters a residue (M269I) in the large periplasmic loop of FtsW (ECL4), should also rescue FtsWR172D. Tests showed that ftsWR172D/* complemented a depletion strain, confirming that ftsW* was an intragenic suppressor of ftsWR172D (SI Appendix, Fig. S13). To further explore the effects of the ftsWR172D mutation, we knocked out ftsW in W3110 containing derivatives of pSEB429, carrying ftsWR172D/* or ftsW* by P1 transduction (Fig. 9). In liquid culture, the transductants obtained in the presence of ftsW* had a short cell phenotype, typical of a strain with an activation mutation [such as those in ftsA, ftsB, or ftsL (21, 22, 32)]. In contrast, transductants obtained in the presence of ftsWR172D/* were similar in length to a WT strain, indicating that the short cell phenotype, characteristic of an activation mutation, is negated by the ftsWR172D mutation.
Fig. 9.
An ftsW activation mutation (ftsW*) is an intragenic suppressor of ftsWR172D. To test if ftsW* was an intragenic suppressor of ftsWR172D, we used P1 transduction. P1 grown on EC912 (ftsW::kan/pDSW406 [Para::ftsW]) was used to transduce W3110 to kanamycin resistance. W3110 contained derivatives of pSEB439 (P204::ftsW) carrying either ftsW* or ftsW*/R172D. Colonies from the plates were grown to the exponential phase in liquid culture and photographed. The panel to the extreme Right shows the cells of W3110, containing a vector for comparison. Cell lengths were measured and plotted.
To further assess the impact of the ftsWR172D mutation, we tested if we could delete ftsEX. At low to moderate osmolarity, deletion of ftsEX disrupts the recruitment of the late arriving division proteins, as it is needed to act on FtsA to start their recruitment (4). However, activation mutations in ftsA, ftsW, or ftsB suppress this requirement. As expected, ftsEX::cat could be transduced into a strain expressing ftsW*; however, it could not be transduced into a strain expressing ftsW*/R172D (SI Appendix, Fig. S14). This result suggests that activation mutations (in ftsA, ftsW, ftsB, or ftsL) may bypass ftsEX because they enhance the interaction between FtsA and FtsWI.
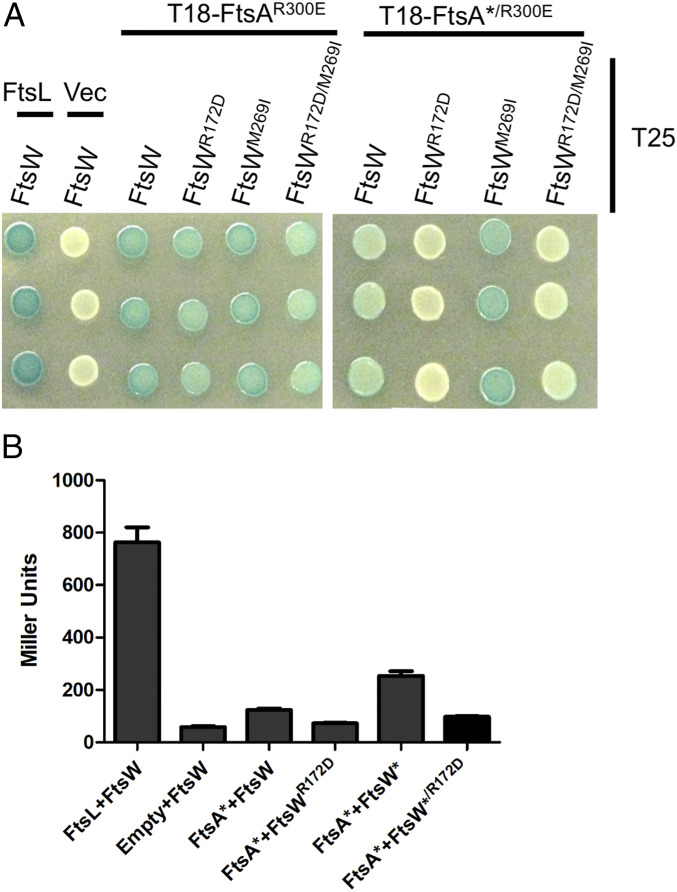
So far, the genetic results suggest an interaction between FtsA and FtsW, which is reduced by the ftsWR172D mutation. Since the bacterial adenylate cyclase-based two-hybrid (BACTH) is prone to false positives, when testing two proteins that localize to the Z-ring (42), we used an allele of FtsA (FtsAR300E), which abrogates binding to FtsZ and reduces the ability of FtsA to localize to the Z-ring (36, 43). Using this FtsA allele, we detected an interaction between FtsAR300E and FtsW or FtsW* and found that this interaction was reduced by the FtsWR172D mutation (Fig. 10). However, there was still considerable interaction. Since FtsAR300E still self-interacts, it can copolymerize with the endogenous FtsA, and this may be the reason for the nonspecific interaction. We therefore added the ftsA* mutation, which reduces self- interaction and the toxicity of FtsAR300E (44). We observed that FtsA*/R300E interacts with FtsW and even more strongly with FtsW* (Fig. 10 A and B). In both cases, however, the interaction was decreased by the addition of ftsWR172D, supporting our interpretation of the genetic results.
Fig. 10.
ftsWR172D reduces the interaction between FtsA and FtsW. (A) Plasmids carrying various alleles of FtsA and FtsW were transformed into BTH101. Three colonies from each transformation were picked into LB and spotted onto indicator plates that were incubated for 24 to 36 h at 30 °C. (B) The β-galactosidase activity was quantitated in liquid cultures from the four strains containing T18–FtsA*/R300E (the SE is indicated). The controls contained FtsL and FtsW or an empty vector (Vec) and FtsW.
Discussion
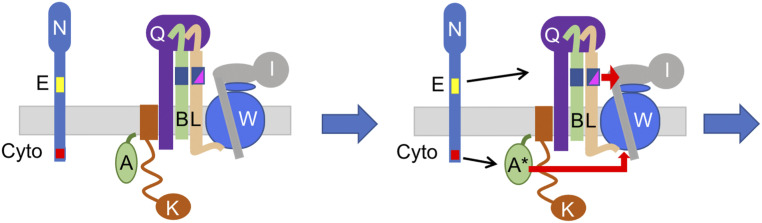
FtsN delivers signals on both sides of the cytoplasmic membrane that synergize to activate the FtsWI synthase to carry out sPG synthesis (21, 22). In an earlier study, we found that the periplasmic signal (FtsNE) goes through FtsQLB, leading to a model in which a domain of FtsL (AWI) becomes available to contact FtsI, which activates FtsW (23). Here, we find that FtsA* does not act through FtsQLB but acts on FtsW. This result, combined with our earlier result (23), suggests a model for how FtsN signals converge to activate FtsWI. Upon FtsN arrival at the divisome the following occurs: 1) FtsQLB changes from a recruitment complex to an activation complex, in which the AWI domain of FtsL is available to interact with FtsI in the periplasm and 2) FtsA is converted to an FtsA*-like state that contacts FtsW in the cytoplasm (Fig. 11). Previously, we suggested that FtsL serves as a clamp for the FtsWI synthase: FtsLcyto contacts FtsW during the recruitment phase of divisome assembly and FtsLperi (AWI) contacts FtsI in the periplasm during the activation step (23). Here, we find that the cytoplasmic signal delivered by FtsN causes FtsA to contact FtsW in the cytoplasm during the activation step, further strengthening the interaction between FtsQLB–FtsA–FtsWI.
Fig. 11.
Updated model for the activation of FtsW for sPG synthesis. In the activation model, the arrival of FtsN switches FtsA (cytoplasm) and FtsQLB (periplasm) to the “on” state, which activates FtsWI. Our earlier study indicated that the FtsNE domain alters the FtsQLB conformation so that a domain of FtsL is exposed and contacts FtsI. In this study, we find that FtsNcyto, which induces the FtsA*-like state, causes FtsA* to act on FtsW.
FtsA* Recruits and Activates FtsW When the Hierarchical Pathway Is Disrupted.
The ftsA* gain-of-function allele was isolated as a suppressor of ΔzipA and was subsequently shown to suppress ΔftsEX, weakly suppress ΔftsK, and also suppress an FtsQ mutant deficient in localization (4, 9, 27, 32). Thus, FtsA* suppresses various conditions that have in common a failure to recruit the late division proteins. Here, we observed that the overexpression of FtsA* weakly bypassed ftsQ and, in the presence of FtsW*, readily bypassed ftsQ, indicating that FtsA* compensates for FtsQ’s role in FtsW’s recruitment. FtsA* also suppressed ftsB and ftsL alleles with distinct defects in FtsW recruitment; ftsB1–54 lacks the domain that interacts with ftsQ, so FtsB1–54/FtsL is uncoupled from FtsQ and, therefore, FtsWI is not recruited (40), whereas FtsLΔcyto localizes to the divisome but is unable to recruit FtsW (10). Since the rescue of FtsLΔcyto by FtsA* occurred in the presence of WT FtsW, it indicated that FtsA* promoted the activation of FtsW in addition to its recruitment. Consistent with this, FtsA* suppressed FtsLL86F/E87K, which blocks FtsL from delivering the periplasmic signal generated by FtsN, arguing that FtsA* delivers an activation signal distinct from FtsL.
Our results are consistent with the findings by Goehring et al. (9) who found two ftsA alleles (ftsAI143L and ftsA*), rescued FtsQ mutants that disrupt the hierarchical recruitment pathway. One of these (FtsQV92D) is deficient in localization, and its rescue was found to be independent of cytoFtsQ. In contrast, the other mutant (ftsQA252P) localized but was defective in interaction with FtsB, so it was unable to recruit FtsBL and FtsWI. The rescue of FtsQA252P, like the rescue of FtsLΔcyto studied here, occurred in the presence of WT FtsW, consistent with FtsA*-like alleles, contributing to the recruitment and activation of FtsW. Since the ftsA alleles were on the chromosome, they did not bypass ftsQ, but their study demonstrated that they displayed activity at the physiological level.
Among the FtsW cytoplasmic mutants we isolated, we focused on one with the strongest dominant-negative phenotype. It was rescued by a hyperactive periplasmic signal (ftsL**) but not by a hyperactive cytoplasmic signal (ftsA*), indicating that this mutant was receptive to the periplasmic but not the cytoplasmic signal. The residue altered in this FtsW mutant is in intracellular loop 2, which is connected to the transmembrane domain (TM4) that contains charged residues that are likely a part of the active site. Interestingly, an ftsW activation mutation in loop 2 (F145L in Cc corresponding to L163 in Ec) implicated this loop in the regulation of FtsW (45). The ftsW* mutation, which occurs in ECL4 and mimics the action of FtsL**, also rescued FtsWR172D, consistent with FtsWR172D being receptive to a hyperactive periplasmic signal.
The genetic evidence suggesting that FtsA* interacts with FtsW is supported by our BACTH results. An interaction was observed between FtsA* and FtsW or FtsW* and was disrupted by the ftsWR172D mutation. The specificity of this interaction was enhanced by using an FtsA mutant (FtsAR300E/*), which decreases nonspecific interactions because it cannot localize to the division site. Thus, we propose that FtsA* interacts with FtsW through loop 2, contributing to the activation of the FtsWI septal synthase. This interaction also likely contributes to the stabilization of FtsWI in the divisome.
There is a well-established, but enigmatic, delay between the assembly of the Z-ring, consisting of FtsZ, FtsA, ZipA, and FtsEX, and the recruitment of the remaining (late) proteins, such as FtsWI (6). Interestingly, activation mutations in ftsA, ftsL, ftsB, and ftsW, as well as the overexpression of ftsN, result in a short cell phenotype (4, 21, 22, 32, 46). Where it has been studied, this phenotype is largely due to the elimination of the delay—the late proteins arrive as the Z-ring forms and immediately commence sPG synthesis (21, 22). Since the short cell phenotype of FtsW* is prevented by the addition of the ftsWR172D mutation, it suggests that, at least in the case of FtsW* but possibly with all activation alleles, the short cell phenotype requires the FtsA–FtsW interaction. Furthermore, the lack of a recruitment delay observed with an activated synthase (FtsQLBWI with a * mutation in FtsL, FtsB, or FtsW) suggests it has a higher affinity for FtsA and that FtsA* has a higher affinity for the synthase. This would explain why activation mutations in these genes bypass FtsEX (4), which is otherwise required to recruit the late proteins.
FtsL and FtsB Cannot Be Bypassed.
Although combining ftsW* with the overexpression of ftsA* bypassed ftsQ or ftsN, we were unable to bypass ftsL, even though ftsA* was able to bypass ftsL’s known functions in the recruitment (loss of the FtsLcyto domain) and activation of FtsW (dominant-negative mutations in the FtsLAWI domain) (Fig. 1A). We could also suppress an ftsB allele deficient in interaction with FtsQ (and therefore the recruitment of FtsW), but we could not bypass ftsB. This suggests that the transmembrane and/or the transmembrane proximal regions (putative coiled coil regions) of FtsB and FtsL have an essential function. They may play a structural role in stabilizing the FtsWI complex. Another possibility, that is not mutually exclusive, is that they are needed for DedD signaling. DedD is an additional activator of sPG synthesis that acts in parallel with FtsN and is essential when FtsN is impaired (47).
FtsN Is Readily Bypassed by FtsA* and FtsW*.
Previous studies demonstrated that the overexpression of two ftsA*-like alleles (ftsAE124A and ftsAI143L) weakly bypass FtsN, as cells are filamentous in rich media (21). Similarly, we found that the overexpression of ftsA* weakly bypasses ftsN, suggesting that this is a general property of ftsA*-like alleles and that, in the absence of FtsN, ftsA*-like alleles indeed activate FtsW. The bypass of ftsN was further enhanced by the addition of ftsW*, as normal-sized cells were produced even without overexpressing ftsA*. In contrast, ftsA* overexpression was required to rescue the morphology of an ftsW* strain deleted for ftsQ. This difference is likely due to the different requirements to bypass these genes. In the absence of ftsN, FtsW* is recruited by the hierarchical pathway (Fig. 1A), and ftsA* makes some contribution so that it is fully activated. In contrast, in the absence of ftsQ, the normal recruitment pathway is abolished, and ftsA* has to recruit FtsW*.
Recently, it was reported that the E domain of FtsN stimulated the glycosyl transferase activity of PBP1b (19). Since activation mutations in ftsL, ftsB, or ftsA only weakly bypass FtsN (cells are filamentous in rich media), it suggested that either FtsW was not fully activated, or the ability of the E domain to stimulate PBP1b was missing (21). Since we observed that FtsN is readily bypassed by a combination of FtsA* and FtsW*, and cells displayed a short cell phenotype, it indicates that the stimulation of PBP1b by FtsN is not required for efficient cell division under these conditions.
Updated Model and Comparison to the Elongasome.
Our results support and extend the current model (21, 23) for divisome activation, which posits that FtsN 1) generates a cytoplasmic signal via FtsNcyto, which FtsA*-like mutants such as FtsA*, FtsAE124A, and FtsAI143L typify; 2) acts on FtsQLB via FtsNE to generate a periplasmic signal, which exposes the AWI domain of FtsL to interact with FtsI to activate FtsW; and 3) causes the two signals to cooperate to activate FtsWI for sPG synthesis (23). Although hyperactivation of either signal makes the other dispensable, under normal conditions, both are needed. Our evidence indicates that FtsNE signaling in the periplasm requires FtsQ, and presumably an intact FtsQLB complex, and that the FtsA–FtsN signal (FtsA*) is transmitted directly to FtsW in the cytoplasm through loop 2 of FtsW to promote its activation (Fig. 8). Although the FtsA*–FtsW interaction is weak, under normal circumstances, FtsA* acts on FtsW that is already part of a complex with FtsQLB because of the hierarchical recruitment pathway (Fig. 1A). When this pathway is abolished (by deletion of ftsQ or loss-of-function mutations in ftsL or ftsB), however, the overexpression of FtsA* is able to compensate.
While FtsEX acts on FtsA to promote the recruitment of downstream division proteins, it needs to hydrolyze ATP for sPG synthesis to proceed (4). Thus, it is likely that ATP hydrolysis by FtsEX allows the signal from FtsN to be transmitted from FtsA to FtsW. However, how the ATPase activity of FtsEX (25) is stimulated and the detailed molecular mechanism of the FtsA*–FtsW interaction and how it leads to the activation of FtsWI remain to be elucidated. With so many proteins within the divisome interacting with FtsA, it is clear that FtsA acts as a hub for divisome assembly and activation by FtsN. Furthermore, single-molecule tracking suggests that FtsN dislodges FtsWI from FtsZ filaments and promotes its activation (48). We suggest that FtsQLB–FtsA–FtsWI, along with FtsN, constitute the active synthetase making sPG.
Although this work is focused on the activation of FtsWI for sPG synthesis, there are similarities with the elongasome, which mediates PG synthesis to maintain a rod shape (49). Both systems contain an SEDS family member polymerase (FtsW versus RodA) and a cognate PBP (FtsI versus PBP2) with a similar activation mechanism (50). These complexes are linked to an actin-like cytoskeleton (FtsA versus MreB). In addition to the RodA–PBP2 pair, the elongasome complex has additional components (MreCD and RodZ), which serve as a link to the MreB cytoskeleton. Among these, RodZ is a bitopic membrane protein with an N-terminal cytoplasmic domain that binds MreB and is required for the rod shape (51–53). Activation mutations in the gene-encoding PBP2 are able to suppress the loss of RodZ and MreCD; however, MreB is still required (54). This implies that the RodA–PBP2 complex also interacts with MreB analogous to FtsA interacting with FtsW. In addition, the elongasome, when activated by mutation, results in an increased number of shorter MreB filaments, suggesting that the activated elongasome modulates MreB polymerization. It is possible that an activated elongasome has a higher affinity for MreB, which may be analogous to an activated divisome synthase having a higher affinity for FtsA.
Materials and Methods
Bacterial Strains and Growth Conditions.
All E. coli strains are K12 derivatives. A complete list of strains is in SI Appendix, Table S1. JS238 [MC1061, araD Δ(ara leu) galU galK hsdS rpsL Δ(lacIOPZYA)X74 malP::lacIQ srlC::Tn10 recA1] was used for construction, amplification, and maintenance of plasmids. W3110 [F− lambda− IN (rrnD-rrnE)1 rph-1] and MC4100 [F− araD139Δ (argF-lac) U169 rspL150 relA1 flbB5301 fruA25 deoC1 ptsF25] were used for the deletion of ftsQ by P1 transduction. JOE417 [JOE309 (MC4100 ara+) ftsQE14::kan/pBAD33‐ftsQ] and SD247 (ftsW*I) were previously described (4, 9). To create PK3116 (W3110 ftsQ::kan/pBAD33-ftsQ), P1 phage grown on JOE417 was transduced into W3110/pBAD33-ftsQ, and transductants were selected for kan resistance on LB agar plates containing 0.2% arabinose, 25 µg/mL kanamycin, 10 µg/mL chloramphenicol, and 8 mM sodium citrate at 30 °C. For the deletion of ftsQ, P1 was grown on JOE417 (ftsQE14::kan) and was introduced into W3110 and MC4100 containing various plasmids. Transductants were selected on LB agar plates containing 25 µg/mL kanamycin (or other appropriate antibiotics, as needed) and 8 mM sodium citrate for 32 h or 2 d (for slow growing colonies) at 30 °C. BTH101 (F− cya-99, araD139, galE15, galK16, rpsL1 [Strr], hsdR2, mcrA1, and mcrB1) was used for bacterial two-hybrid assay (55).
CH34 (ftsN::kan/pCH201) was described previously (15) and was used to grow P1 for ftsN::kan transduction (15). SD295 (ftsW::kan/pSD257 [pSC101 syn135 repAts::ftsW] recA::Tn10) was created by transduction with P1 grown on PB143 (ftsZ0 recA::Tn10/pCX41 (cat repAts ftsZ+) into SD292 (ftsW::kan/pSD257 [repAts Psyn135::ftsW]) and selecting on LB containing 25 µg/mL kanamycin, 50 µg/mL spectinomycin, 10 µg/mL tetracycline, and 8 mM sodium citrate at 30 °C. Colonies were further subcloned and tested for temperature sensitivity at 42 °C. To construct SD399 (ftsL::kan/pSD256 [pSC101 repAtssyn135::ftsL]), P1 phage grown on BL156/pBL195 (TB28, ftsL::kan/pJH2 [Psyn135::ftsL]) was used to transduce W3110/pSD256 (pSC101ts Psyn135::ftsL). Transductants were selected on LB plates containing 25 µg/mL kanamycin, 50 µg/mL spectinomycin, and 8 mM sodium citrate at 30 °C. Colonies were subcloned on LB plates containing the same antibiotics at 30 °C and were further screened for temperature sensitivity at 42 °C. SD439 was created by transforming SD399 with pSD296 (Para::ftsL) and selecting for transformants that grew at 42 °C in the presence of 10 µg/mL chloramphenicol and 0.2% arabinose.
LB medium containing 1% NaCl was used as indicated and at the indicated temperatures. Preliminary experiments were carried out using LB medium containing 0.5% NaCl. For selection on LB agar and in LB broth, the following antibiotics and reagents were added at the indicated final concentrations when required (ampicillin, 100 μg/mL; spectinomycin, 50 μg/mL; kanamycin, 25 µg/mL; chloramphenicol, 10 µg/mL; tetracycline, 10 µg/mL; IPTG, 0 to 200 µM; glucose, 0.2%; and arabinose, 0.2%).
Plasmids.
A complete list of plasmids is in SI Appendix, Table S2. Plasmid pND16 [pGB2 PftsK::ftsW-ftsK179-1329] constitutively expresses the FtsW–FtsKcyto fusion (33) and pND16* expresses FtsW*–FtsKcyto. pDSW406 ([Para::ftsW]) was previously described (56). Plasmids for the overexpression of FtsA, FtsA*, FtsN, MalG–FtsN, and FtsW are pSEB306 (Ptrc/206::ftsA), pSEB306* (Ptrc/206::ftsA*), pSEB417 (Ptrc/204::ftsN) or pKD140 (PftsN::ftsN), pSEB453 (PftsN::malG1-33-ftsN46-319), and pSEB429 (Ptrc/204:: ftsW), respectively, and were described earlier (13, 28, 31). To create pSEB468 (Psyn135-ftsQ), the ftsQ open reading frame, including the endogenous ribosome binding site, was PCR amplified using genomic DNA from W3110 as a template, digested with XbaI, and HindIII followed by ligation into pSC101 (Psyn135) that carries compatible cohesive ends. Plasmid pSEB453 was generated by PCR amplification of malG1–33–ftsN46–319 using pKD146 (57) as a template. The fragment was digested with AflII and AgeI and ligated into the same sites in pKD140, replacing ftsN (which was excised with the AflII and AgeI enzymes). For construction of pSD256 (pSC101 repts [Psyn135::ftsL]), the ftsL ORF was PCR amplified using primers that target 250 bp upstream and downstream of ftsL. The PCR fragment was digested with HindIII and EcoRI and ligated into the pSC101ts vector. To create pSD257 (repts Psyn135::ftsW), the ftsW ORF and its 250 bp flanking regions were PCR amplified and cloned into the pSC101ts vector. Construction of plasmids pKT100, pKTP107, and pDSW311 were previously described (23, 48). Various mutations were introduced into these plasmids by site-directed mutagenesis. For example, pDSW311-1 contains the ftsWR172D mutation. Also, the ftsB gene was truncated at codon 54 in plasmid pSD295 by introducing two stop codons in tandem to make pSD295-54. Plasmids pUT18C-ftsA, pUT18C-ftsAR300E, pUT18C-ftsA*/R300E, pUT18C-ftsL, pKT25-ftsW, and pKT25-ftsW* were previously described (23, 58).
Site-Directed Mutagenesis.
Point mutations were introduced in ftsA, ftsB, ftsL, ftsN, and ftsW, contained on such plasmids as pSEB306, pSD295, pKTP100, pSEB417, and pND16 by using the QuikChange site-directed mutagenesis kit according to the manufacturer’s instructions (Agilent Technologies). Primers are listed in SI Appendix, Table S4.
Analysis of ftsQ Deletion.
To confirm the deletion of ftsQ following P1 transduction, genomic DNA from MC4100 and derivatives carrying pSEB468 alone or pND16* or in combination with pSEB306*, respectively, were isolated and subjected to PCR with two sets of primers. One pair of primers amplifies a region that includes 65 bp upstream (primer—ftsQ+65) of the translation start site and 55 bp downstream (primer—ftsQ-55) of the ftsQ translation stop codon. Another pair of primers targets the inside of the ftsQ ORF (ftsQ-L6E FW and ftsQ-HindII). PCR products were analyzed by 1% agarose gel electrophoresis.
Microscopy.
To determine the effect of ftsQ or ftsN on cell morphology, overnight cultures of E. coli strains were diluted 1:200 to 1:500 in fresh LB media, containing 1% NaCl, appropriate antibiotics, and inducers such as arabinose (0 to 0.2%) and IPTG (0 to 200 µM), and were incubated at 30 °C until the optical density at 540 nm ∼0.3 to 0.4. To monitor GFP–FtsW localization, overnight cultures of EC912 (ftsW::kan/pDSW406 [Para::ftsW]) containing pDSW311 or pDSW311-1 were diluted 1:500 to 1:1,000 and grown to exponential phase in 0.2% arabinose at 30 °C. After removal of arabinose, cells were cultured for 2 h, and IPTG (0.5 to 1 mM) was added. After an additional 1 h, cells were taken for photography. Cells were immobilized on an LB agarose pad that contained 1% agarose and 50% LB medium. Morphological phenotypes were examined by phase-contrast microscopy, and images were recorded with CoolSNAP HQ2 CCD camera (Photometrics) and processed using Metamorph software (Molecular Devices).
Bacterial Two-Hybrid Assay.
The cya null strain BTH101 (F-, cya-99, araD139, galE15, galK16, rpsL1 (Strr), hsdR2, mcrA1, and mcrB1) was simultaneously transformed with plasmids pKT25-ftsW and pUT18C-ftsA (carrying WT or mutant ftsW and ftsA alleles) and grown overnight at 30 °C on LB plates containing 0.2% glucose, 25 μg/mL kanamycin, and 100 μg/mL ampicillin. pUT18C-ftsL (or pUT18C vector) and pKT25-ftsW were used as a positive (or negative) control (23). Colonies from the LB plates were picked into LB broth and spotted onto fresh LB plates, supplemented with 25 μg/mL kanamycin, 100 μg/mL ampicillin, 40 μg/mL 5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside (X-Gal), and 0.5 mM IPTG. The color changes were recorded after overnight incubation at 30 °C and room temperature for 1 d. The assay was repeated three times.
For quantitative analysis of BACTH, three colonies were picked from transformations and cultured overnight at 30 °C in LB broth containing 0.2% glucose, 100 µg/mL ampicillin, and 25 µg/mL kanamycin. The cultures were diluted 1:100 into fresh LB medium containing 0.5 mM IPTG, 100 µg/mL ampicillin, and 25 µg/mL kanamycin and cultured for 3 to 4 h at 25 °C. At an optical density at 600 nm around 0.3 to 0.35, cells were permeabilized by vortexing, following the addition of 0.0016% SDS (weight/volume) and 2.5% chloroform (volume/volume). Thereafter, 0.4 mL permeabilized cells were mixed with 0.6 mL Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4 [pH 7.5], 1 mM MgSO4, and 50 mM β-mercaptoethanol) and 0.25 mL ONPG (o-nitrophenyl β-D-galactopyranoside [4 mg/mL]) was added. The reactions were incubated for 20 min at 30 °C and stopped with 400 mM Na2CO3. The absorbance at 420 nm and the optical density at 540 nm were recorded and converted into Miller activity units, as described (23).
Supplementary Material
Acknowledgments
We are grateful to Tom Bernhardt, David Weiss, Piet de Boer, Francois-Xavier Barre, and Bill Margolin for sending strains and plasmids. This work was supported by Grant GM029764 from the NIH.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2107210118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Du S., Lutkenhaus J., At the heart of bacterial cytokinesis: The Z ring. Trends Microbiol. 27, 781–791 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker B. E., Männik J., Männik J., Transient membrane-linked FtsZ assemblies precede Z-ring formation in Escherichia coli. Curr. Biol. 30, 499–508.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pichoff S., Lutkenhaus J., Unique and overlapping roles for ZipA and FtsA in septal ring assembly in Escherichia coli. EMBO J. 21, 685–693 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du S., Pichoff S., Lutkenhaus J., FtsEX acts on FtsA to regulate divisome assembly and activity. Proc. Natl. Acad. Sci. U.S.A. 113, E5052–E5061 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goehring N. W., Beckwith J., Diverse paths to midcell: Assembly of the bacterial cell division machinery. Curr. Biol. 15, R514–R526 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Du S., Henke W., Pichoff S., Lutkenhaus J., How FtsEX localizes to the Z ring and interacts with FtsA to regulate cell division. Mol. Microbiol. 112, 881–895 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Addinall S. G., Cao C., Lutkenhaus J., FtsN, a late recruit to the septum in Escherichia coli. Mol. Microbiol. 25, 303–309 (1997). [DOI] [PubMed] [Google Scholar]
- 8.Buddelmeijer N., Beckwith J., A complex of the Escherichia coli cell division proteins FtsL, FtsB and FtsQ forms independently of its localization to the septal region. Mol. Microbiol. 52, 1315–1327 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Goehring N. W., Petrovska I., Boyd D., Beckwith J., Mutants, suppressors, and wrinkled colonies: Mutant alleles of the cell division gene ftsQ point to functional domains in FtsQ and a role for domain 1C of FtsA in divisome assembly. J. Bacteriol. 189, 633–645 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez M. D., Akbay E. A., Boyd D., Beckwith J., Multiple interaction domains in FtsL, a protein component of the widely conserved bacterial FtsLBQ cell division complex. J. Bacteriol. 192, 2757–2768 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meeske A. J., et al., SEDS proteins are a widespread family of bacterial cell wall polymerases. Nature 537, 634–638 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taguchi A., et al., FtsW is a peptidoglycan polymerase that is functional only in complex with its cognate penicillin-binding protein. Nat. Microbiol. 4, 587–594 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marmont L. S., Bernhardt T. G., A conserved subcomplex within the bacterial cytokinetic ring activates cell wall synthesis by the FtsW-FtsI synthase. Proc. Natl. Acad. Sci. U.S.A. 117, 23879–23885 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goehring N. W., Gonzalez M. D., Beckwith J., Premature targeting of cell division proteins to midcell reveals hierarchies of protein interactions involved in divisome assembly. Mol. Microbiol. 61, 33–45 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Gerding M. A., et al., Self-enhanced accumulation of FtsN at division sites and roles for other proteins with a SPOR domain (DamX, DedD, and RlpA) in Escherichia coli cell constriction. J. Bacteriol. 191, 7383–7401 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutkenhaus J., FtsN—Trigger for septation. J. Bacteriol. 191, 7381–7382 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jorgenson M. A., et al., Simultaneously inhibiting undecaprenyl phosphate production and peptidoglycan synthases promotes rapid lysis in Escherichia coli. Mol. Microbiol. 112, 233–248 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boes A., Olatunji S., Breukink E., Terrak M., Regulation of the peptidoglycan polymerase activity of PBP1b by antagonist actions of the core divisome proteins FtsBLQ and FtsN. MBio 10, e01912-18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boes A., et al., The bacterial cell division protein fragment (E)FtsN binds to and activates the major peptidoglycan synthase PBP1b. J. Biol. Chem. 295, 18256–18265 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller P., et al., The essential cell division protein FtsN interacts with the murein (peptidoglycan) synthase PBP1B in Escherichia coli. J. Biol. Chem. 282, 36394–36402 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Liu B., Persons L., Lee L., de Boer P. A., Roles for both FtsA and the FtsBLQ subcomplex in FtsN-stimulated cell constriction in Escherichia coli. Mol. Microbiol. 95, 945–970 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsang M.-J., Bernhardt T. G., A role for the FtsQLB complex in cytokinetic ring activation revealed by an ftsL allele that accelerates division. Mol. Microbiol. 95, 925–944 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park K.-T., Du S., Lutkenhaus J., Essential role for FtsL in activation of septal peptidoglycan synthesis. MBio 11, e03012-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss D. S., Last but not least: New insights into how FtsN triggers constriction during Escherichia coli cell division. Mol. Microbiol. 95, 903–909 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Du S., Pichoff S., Lutkenhaus J., Roles of ATP hydrolysis by FtsEX and interaction with FtsA in regulation of septal peptidoglycan synthesis and hydrolysis. MBio 11, e01247-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pichoff S., Shen B., Sullivan B., Lutkenhaus J., FtsA mutants impaired for self-interaction bypass ZipA suggesting a model in which FtsA’s self-interaction competes with its ability to recruit downstream division proteins. Mol. Microbiol. 83, 151–167 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geissler B., Margolin W., Evidence for functional overlap among multiple bacterial cell division proteins: Compensating for the loss of FtsK. Mol. Microbiol. 58, 596–612 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Gouëllec A., et al., Roles of pneumococcal DivIB in cell division. J. Bacteriol. 190, 4501–4511 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daniel R. A., Errington J., Intrinsic instability of the essential cell division protein FtsL of Bacillus subtilis and a role for DivIB protein in FtsL turnover. Mol. Microbiol. 36, 278–289 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Beall B., Lutkenhaus J., Nucleotide sequence and insertional inactivation of a Bacillus subtilis gene that affects cell division, sporulation, and temperature sensitivity. J. Bacteriol. 171, 6821–6834 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J. C., Minev M., Beckwith J., Analysis of ftsQ mutant alleles in Escherichia coli: Complementation, septal localization, and recruitment of downstream cell division proteins. J. Bacteriol. 184, 695–705 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geissler B., Elraheb D., Margolin W., A gain-of-function mutation in ftsA bypasses the requirement for the essential cell division gene zipA in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 100, 4197–4202 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy M., Role of FtsEX in cell division of Escherichia coli: Viability of ftsEX mutants is dependent on functional SufI or high osmotic strength. J. Bacteriol. 189, 98–108 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pichoff S., Du S., Lutkenhaus J., Disruption of divisome assembly rescued by FtsN-FtsA interaction in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 115, E6855–E6862 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubarry N., Possoz C., Barre F.-X., Multiple regions along the Escherichia coli FtsK protein are implicated in cell division. Mol. Microbiol. 78, 1088–1100 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Pichoff S., Du S., Lutkenhaus J., The bypass of ZipA by overexpression of FtsN requires a previously unknown conserved FtsN motif essential for FtsA-FtsN interaction supporting a model in which FtsA monomers recruit late cell division proteins to the Z ring. Mol. Microbiol. 95, 971–987 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J. C., Beckwith J., FtsQ, FtsL and FtsI require FtsK, but not FtsN, for co-localization with FtsZ during Escherichia coli cell division. Mol. Microbiol. 42, 395–413 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Choi Y., et al., Structural insights into the FtsQ/FtsB/FtsL complex, a key component of the divisome. Sci. Rep. 8, 18061 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kureisaite-Ciziene D., et al., Structural analysis of the interaction between the bacterial cell division proteins FtsQ and FtsB. MBio 9, e01346-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez M. D., Beckwith J., Divisome under construction: Distinct domains of the small membrane protein FtsB are necessary for interaction with multiple cell division proteins. J. Bacteriol. 191, 2815–2825 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lara B., Ayala J. A., Topological characterization of the essential Escherichia coli cell division protein FtsW. FEMS Microbiol. Lett. 216, 23–32 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Galli E., Gerdes K., Spatial resolution of two bacterial cell division proteins: ZapA recruits ZapB to the inner face of the Z-ring. Mol. Microbiol. 76, 1514–1526 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Pichoff S., Lutkenhaus J., Identification of a region of FtsA required for interaction with FtsZ. Mol. Microbiol. 64, 1129–1138 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Herricks J. R., Nguyen D., Margolin W., A thermosensitive defect in the ATP binding pocket of FtsA can be suppressed by allosteric changes in the dimer interface. Mol. Microbiol. 94, 713–727 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Modell J. W., Hopkins A. C., Laub M. T., A DNA damage checkpoint in Caulobacter crescentus inhibits cell division through a direct interaction with FtsW. Genes Dev. 25, 1328–1343 (2011). Correction in: Genes Dev. 25, 1662 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Modell J. W., Kambara T. K., Perchuk B. S., Laub M. T., A DNA damage-induced, SOS-independent checkpoint regulates cell division in Caulobacter crescentus. PLoS Biol. 12, e1001977 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu B., Hale C. A., Persons L., Phillips-Mason P. J., de Boer P. A. J., Roles of the DedD protein in Escherichia coli cell constriction. J. Bacteriol. 201, e00698-18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang X., et al., A two-track model for the spatiotemporal coordination of bacterial septal cell wall synthesis revealed by single-molecule imaging of FtsW. Nat. Microbiol. 6, 584–593 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szwedziak P., Löwe J., Do the divisome and elongasome share a common evolutionary past? Curr. Opin. Microbiol. 16, 745–751 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Li Y., et al., Genetic analysis of the septal peptidoglycan synthase FtsWI complex supports a conserved activation mechanism for SEDS-bPBP complexes. PLoS Genet. 17, e1009366 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van den Ent F., Johnson C. M., Persons L., de Boer P., Löwe J., Bacterial actin MreB assembles in complex with cell shape protein RodZ. EMBO J. 29, 1081–1090 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shiomi D., et al., Mutations in cell elongation genes mreB, mrdA and mrdB suppress the shape defect of RodZ-deficient cells. Mol. Microbiol. 87, 1029–1044 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bendezú F. O., Hale C. A., Bernhardt T. G., de Boer P. A., RodZ (YfgA) is required for proper assembly of the MreB actin cytoskeleton and cell shape in E. coli. EMBO J. 28, 193–204 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rohs P. D. A., et al., A central role for PBP2 in the activation of peptidoglycan polymerization by the bacterial cell elongation machinery. PLoS Genet. 14, e1007726 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karimova G., Dautin N., Ladant D., Interaction network among Escherichia coli membrane proteins involved in cell division as revealed by bacterial two-hybrid analysis. J. Bacteriol. 187, 2233–2243 (2005). Correction in: J. Bacteriol. 190, 8248 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mercer K. L. N., Weiss D. S., The Escherichia coli cell division protein FtsW is required to recruit its cognate transpeptidase, FtsI (PBP3), to the division site. J. Bacteriol. 184, 904–912 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dai K., Xu Y., Lutkenhaus J., Topological characterization of the essential Escherichia coli cell division protein FtsN. J. Bacteriol. 178, 1328–1334 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Busiek K. K., Eraso J. M., Wang Y., Margolin W., The early divisome protein FtsA interacts directly through its 1c subdomain with the cytoplasmic domain of the late divisome protein FtsN. J. Bacteriol. 194, 1989–2000 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.