Significance
Lipid organization of the plasma membrane is known to be important for facilitating protein interactions in transmembrane signaling. However, the orchestration of these interactions in live cells remains elusive. We employed imaging fluorescence correlation spectroscopy (ImFCS) to systemically investigate the interplay of lipids and proteins during mast cell signaling, initiated as phosphorylation of antigen-crosslinked immunoglobulin E–receptor (IgE-FcεRI) complexes by lipid-anchored Lyn kinase. We found that stabilized liquid ordered-like nanodomains around clustered FcεRI—which can be accessed by Lyn kinase but not by transmembrane phosphatase—provide essential spatial filtering that augments Lyn’s binding and phosphorylating FcεRI while suppressing dephosphorylation by phosphatase. ImFCS provides quantitative evidence of the functional link between lipid-based membrane organization and transmembrane signaling in live cells.
Keywords: transmembrane signaling, plasma membrane domains, rafts, Imaging FCS, FcεRI
Abstract
Antigen (Ag) crosslinking of immunoglobulin E–receptor (IgE-FcεRI) complexes in mast cells stimulates transmembrane (TM) signaling, requiring phosphorylation of the clustered FcεRI by lipid-anchored Lyn tyrosine kinase. Previous studies showed that this stimulated coupling between Lyn and FcεRI occurs in liquid ordered (Lo)-like nanodomains of the plasma membrane and that Lyn binds directly to cytosolic segments of FcεRI that it initially phosphorylates for amplified activity. Net phosphorylation above a nonfunctional threshold is achieved in the stimulated state but not in the resting state, and current evidence supports the hypothesis that this relies on Ag crosslinking to disrupt a balance between Lyn and tyrosine phosphatase activities. However, the structural interactions that underlie the stimulation process remain poorly defined. This study evaluates the relative contributions and functional importance of different types of interactions leading to suprathreshold phosphorylation of Ag-crosslinked IgE-FcεRI in live rat basophilic leukemia mast cells. Our high-precision diffusion measurements by imaging fluorescence correlation spectroscopy on multiple structural variants of Lyn and other lipid-anchored probes confirm subtle, stimulated stabilization of the Lo-like nanodomains in the membrane inner leaflet and concomitant sharpening of segregation from liquid disordered (Ld)-like regions. With other structural variants, we determine that lipid-based interactions are essential for access by Lyn, leading to phosphorylation of and protein-based binding to clustered FcεRI. By contrast, TM tyrosine phosphatase, PTPα, is excluded from these regions due to its Ld-preference and steric exclusion of TM segments. Overall, we establish a synergy of lipid-based, protein-based, and steric interactions underlying functional TM signaling in mast cells.
Transmembrane (TM) signaling stimulated by antigen (Ag) occurs through cell surface immunoreceptors that lack a cytosolic kinase module, thus requiring tyrosine phosphorylation mediated by intermolecular coupling with a separate, plasma membrane–localized kinase (1). Effective coupling corresponds to a suprathreshold level of receptor phosphorylation that surmounts dephosphorylation by proximal tyrosine phosphatases. Orchestrated modulation of interactions among the signaling proteins (i.e., receptor, kinase, and phosphatase) (2, 3) and with other proteins [e.g., actin cytoskeleton (4)] are key to Ag-stimulated TM signaling. Although signaling studies have tended to focus on protein–protein interactions, contributions by collective lipid-based interactions are increasingly appreciated. In particular, phase-like organization of the plasma membrane provides capacity for colocalizing receptor and kinase while segregating phosphatase, according to their phase preferences (5). However, the relative importance remains a subject of debate (6–11), largely because it is experimentally difficult to separate the signaling contributions of lipid-based interactions from those of protein-based interactions in live cells.
Our group has worked to develop biophysical approaches that systematically delineate signaling interactions in the context of a prototypical immunoreceptor signaling system: the high-affinity receptor for immunoglobulin E (IgE), FcεRI, in rat basophilic leukemia (RBL) mast cells (12, 13). Crosslinking of IgE-FcεRI by soluble, multivalent Ag creates FcεRI nanoclusters (14, 15) that are phosphorylated by Lyn, a src-family tyrosine kinase anchored to the inner leaflet of the plasma membrane by saturated acyl chains (Fig. 1A). Phosphorylated tyrosines on cytosolic β- and γ-subunits of FcεRI provide direct binding sites for Lyn’s SH2 module to amplify the phosphorylation activity and create a binding site for Syk kinase and consequent assembly of a protein-based signaling platform that incorporates LAT scaffold and links to activation of phospholipase C-γ and attachment to the actin cytoskeleton (16). In mast cells, stimulated coupling of clustered FcεRI with Lyn initiates the cascade of cellular signaling and responses that underlie allergy and inflammation (12).
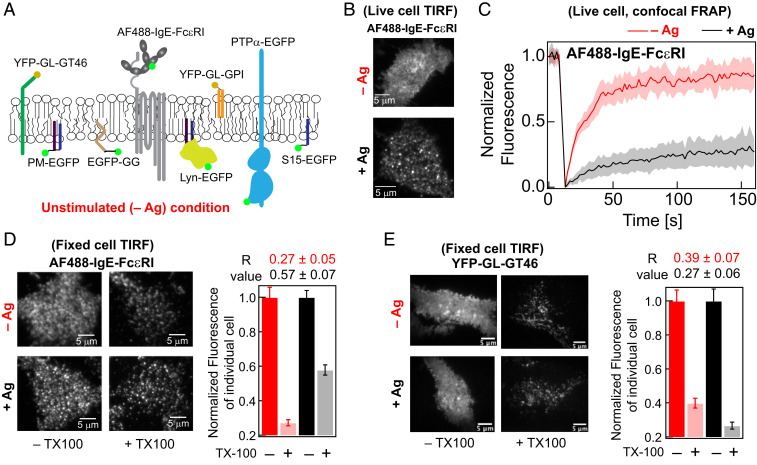
Fig. 1.
AF488-IgE-FcεRI is clustered, partially immobilized, and exhibits elevated detergent resistance after crosslinking by soluble Ag (DNP-BSA) in RBL cells. (A) Plasma membrane localization in resting cells (−Ag) of AF488-IgE-FcεRI and other probes evaluated in this study. (B) Representative TIRF images of AF488-IgE-FcεRI on the ventral plasma membrane in live cells before (−Ag) and after (+Ag) stimulation by Ag. (C) Normalized FRAP curves of AF488-IgE-FcεRI obtained from individual cells are overlaid in −Ag (pink) and +Ag (gray) conditions. The solid red and black curves are average of the pink and gray curves, respectively. SI Appendix, Fig. S1A shows representative fitted FRAP data and box plots of recovery time and mobile fraction of all cells evaluated. (D) Representative fixed-cell TIRF images of AF488-IgE-FcεRI without (−TX100) and with (+0.04% TX100) treatment of both −Ag and +Ag conditions. Fluorescence retained after +TX100 treatment is normalized against corresponding −TX100 sample. The R values, corresponding to level of detergent resistance, are calculated from the ratio of median fluorescence of multiple cells in +TX100 to −TX100 samples (SI Appendix, Eq. S2). The error of R values was determined by bootstrapping as described in SI Appendix. (E) Representative fixed cell TIRF images under −/+Ag and −/+TX100 conditions and R values for YFP-GL-GT46. For each condition, 60 to 90 cells were imaged from at least two independent sample preparations for both AF488-IgE-FcεRI and YFP-GL-GT46. Box plots of fluorescence values for individual cells under −/+Ag and −/+TX100 conditions for both probes in representative experiments are provided in SI Appendix, Fig. S2.
The role of lipids in stimulated Lyn/FcεRI coupling has been scrutinized in experimental (15, 17–19) and theoretical (20) studies over two decades, yielding the view that Ag crosslinking stabilizes liquid ordered (Lo)-like nanodomains around the clustered IgE-FcεRI. Referred to commonly (and roughly) as “rafts,” these proteolipid organizational features of plasma membranes have variable properties that depend on cellular circumstance but generally resemble Lo domains that coexist with liquid disordered (Ld) regions at equilibrium in model membranes of defined composition (21). Experiments in cells show that Lyn kinase preferentially partitions into Lo-like nanodomains in the inner leaflet of the plasma membrane as mediated by its saturated lipid (palmitoyl/myristoyl, PM) anchor (15, 22, 23). By contrast, a TM tyrosine phosphatase, PTPα, and an inner-leaflet Ld-preferring lipid probe (geranylgeranyl, GG) preferentially localize to Ld-like regions, away from the Ag-clustered FcεRI (15, 23, 24). In early studies, the functional relevance of this lipid-driven spatial partitioning of the signaling components was shown by appearance of phosphorylated IgE-FcεRI in the Lo-like, detergent-resistant membrane (DRM) fraction only after Ag stimulation (24–26). Further functional evidence came from showing abrogated FcεRI phosphorylation after pharmacological depletion of cholesterol, a key component of Lo-like nanodomain formation (26). However, both DRM isolation and cholesterol depletion have known limitations (27), and they do not directly show whether lipid-based partitioning of Lyn is necessary or sufficient for functional coupling with clustered FcεRI.
In addition to the lipid-based partitioning into regions of clustered FcεRI, Lyn interacts directly with FcεRI-β subunit by its cytosolic protein modules (SH3, SH2, and kinase modules). We showed previously that full-length Lyn is recruited to μm-scale Ag-patterned features more readily than its minimal PM lipid anchor (17), indicating that protein binding stabilizes this interaction. Soluble Ag creates IgE-FcεRI nanoclusters, and their colocalization with Lyn (somewhat less with a PM lipid probe and not with a GG lipid probe) is observed using superresolution fluorescence microscopy and cross-correlation analysis (15). The relative low level of cross-correlation observed for Lyn and IgE-FcεRI clusters points to both lipid- and protein-based interactions being weak and dynamic. In general, the difference in phase-like organization of the plasma membrane between resting and stimulated states appears to be subtle and difficult to detect by conventional fluorescence microscopy and spectroscopy (15, 28, 29). Single-particle tracking (SPT) can be successful in picking up small changes. For example, Kusumi and colleagues developed high-speed SPT analysis built around a microscope dedicated to delineating transient interactions at single-molecule level (30, 31). Both superresolution and SPT approaches are technically demanding and typically require special fluorescent tags. As a complementary approach, we recently demonstrated that imaging fluorescence correlation spectroscopy (ImFCS) quantifies subtle differences in the diffusion properties of structurally distinct probes and subtle changes in the diffusion of a particular probe under different cell treatments (32). ImFCS images conventional fluorophores with a diffraction-limited total internal reflection fluorescence (TIRF) microscope (32, 33). These measurements yield probe diffusion coefficients (D) in hundreds of pixel units simultaneously, and these measurements can be further extended over multiple cells to thousands of data points. Such large data statistics permit precise evaluation of D, thereby enabling detection of small changes in diffusion of a given probe that arises from the change in membrane organization. For example, by comparing Lo- and Ld-preferring probes, we previously detected changes in plasma membrane phase-like organization after inhibition of actin polymerization (32).
Faced with the challenge of delineating contributions of protein- and lipid-based interaction and subtle changes that occur in the plasma membrane after Ag-crosslinking of IgE-FcεRI, we have now improved the robustness of ImFCS data analysis, such that small changes are unambiguously determined. We evaluate ImFCS results, together with those from fluorescence recovery after photobleaching (FRAP) and a modified, image-based DRM assay (34), to compare biophysical properties of multiple probes (e.g., Fig. 1A), which, as a composite, represent membrane organization under resting (−Ag) and stimulated (+Ag) steady states of RBL cells. By evaluating diffusion properties of passive, inner-leaflet lipid probes with variable Lo-preference, we first characterize a relatively stable Lo-like environment around Ag-crosslinked FcεRI nanoclusters in the stimulated steady state. We construct Lyn variants to modify Lyn’s lipid-based partitioning interactions, cytosolic protein–based interactions, and kinase activity. Comparative evaluation of variants reveals individual contribution of different interaction modes toward Lyn’s coupling with Ag-clustered FcεRI. We show that the lipid-based partitioning of Lyn in the Lo-like nanodomains proximal to the Ag-clustered FcεRI increases the probability of the cytosolic protein–based interaction, thereby synergizing an efficient kinase/receptor coupling. We confirm that Lyn’s Lo-preference is essential for stimulated phosphorylation of IgE-FcεRI in a reconstituted system. In contrast, our results provide evidence that TM tyrosine phosphatase, PTPα, is excluded from the region of Ag-clustered FcεRI because of the inherent Ld preference of PTPα and by steric hindrance of its TM segments. Overall, this study provides key experimental evidence to explain how cells utilize subtle changes in the phase-like lipid organization to enable protein–protein interactions necessary for initiating stimulated TM signaling.
Results
Ag-crosslinking of IgE-FcεRI in RBL mast cells stimulates new interactions within the plasma membrane to facilitate suprathreshold FcεRI phosphorylation by Lyn tyrosine kinase as the first step of TM signaling. To delineate comprehensively the functional redistributions of lipids and proteins, we build on previous studies and use complementary approaches to monitor Ag-stimulated changes in diffusion and other properties over a range of selected probes (Fig. 1A and following figures). We find that shifts in distribution curves of diffusion coefficients derived from ImFCS provide an exceptionally sensitive representation of membrane changes that occur to initiate signaling.
ImFCS Readily Detects Subtle Changes in Membrane Heterogeneity Caused by Ag Clustering of IgE-FcεRI.
Crosslinking of AlexaFluor488-labeled IgE-FcεRI (AF488-IgE-FcεRI; Fig. 1A) by Ag (DNP-BSA) in RBL plasma membranes forms distributed nanoclusters within 15 min that are visible by diffraction-limited TIRF microscopy (Fig. 1B). Our previous superresolution imaging showed that these individual nanoclusters have an average radius of ∼80 nm (14). As previously measured by FRAP (35, 36) and confirmed here, 70% of the crosslinked AF488-IgE-FcεRI are immobile, and the 30% mobile fraction diffuse slower (i.e., longer fluorescence recovery time) than monomeric AF488-IgE-FcεRI present in resting cells (85% mobile; Fig. 1C and SI Appendix, Fig. S1A). By comparison, the yellow fluorescent protein (YFP)–tagged, Ld-preferring TM probe, YFP-GL-GT46 (comprising the TM segment of the low-density lipoprotein receptor and the cytoplasmic tail of CD46) (37–39), has a mobile fraction of 84% with an insignificant shift in that value or the fluorescence recovery time after Ag crosslinking of IgE-FcεRI (SI Appendix, Fig. S1B).
Changes in resistance to detergent solubilization provided early evidence that crosslinked IgE-FcεRI nanoclusters associate with and stabilize Lo-like nanodomains (5, 15, 22, 24), as similarly documented for B and T cell receptors (34, 40–43). DRMs are Lo-like in lipid composition (44, 45) and retain coassociating proteins after solubilizing cells with 0.04% Triton X 100 (TX100) and floating on sucrose gradients (25). We recently adapted this basic methodology for evaluation of single cells by fluorescence microscopy (46), and we quantify the detergent resistance of a particular probe in the plasma membrane by a characteristic retention (R) value. R is taken as the ratio of median fluorescence per cell after treatment with 0.04% TX100 to that of untreated cells (SI Appendix, Eq. S2). A larger R value reflects stronger interaction of the probe with membrane constituents that are not released under these conditions. We find that the R value of AF488-IgE-FcεRI increases from 0.3 to 0.6 after Ag crosslinking (Fig. 1D and SI Appendix, S2A). By comparison, the Ld-preferring TM probe YFP-GL-GT46 shows a slightly smaller R value after crosslinking IgE-FcεRI (0.4 to 0.3; Fig. 1E and SI Appendix, S2B). These distinctive behaviors are consistent with crosslinked AF488-IgE-FcεRI stabilizing Lo-like regions.
We recently established that diffusion properties of mobile membrane probes and their subtle changes after pharmacological treatments can be quantified precisely by TIRF-based ImFCS (32). This approach uses a fast camera and autocorrelates fluorescence fluctuations to determine diffusion coefficients (D) of a particular probe at several hundreds of diffraction-limited spatial locations (Px unit = 320 nm × 320 nm) in single cells (Fig. 2 A and B). The D value determined for an individual Px unit represents all nanoscopic environments that the probe moves through within that Px unit during the data acquisition time (280 s). Nanoscopic environments with more interactions (e.g., higher effective viscosity) yield a slower D value, and less-interactive environments yield a faster D value (Fig. 2 B and C). By reflecting the interactions that structurally distinct probes experience, distinctive diffusion properties provide information about membrane organization. Pooling data from multiple cells yields ∼10,000 D values for a given probe, and the resulting high level of precision enables subtle differences in diffusion properties among probes to be discerned [(32); SI Appendix, Table S1]. As one measure of precision, the arithmetic average of pooled D values, Dav, has an SEM of less than 1% for every probe evaluated by ImFCS in this study (SI Appendix, Table S1).
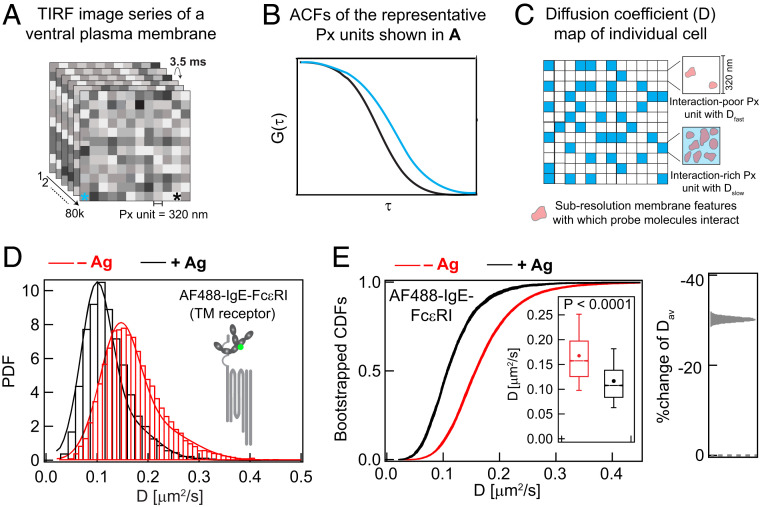
Fig. 2.
Large datasets of ImFCS precisely characterize spatially heterogeneous diffusion of plasma membrane probes in both unstimulated (−Ag) and stimulated (+Ag) cells. (A and B) In a typical ImFCS recording, 80,000 TIRF microscopy images of fluorescently labeled ventral plasma membrane are collected at 3.5 ms/frame. The autocorrelation function (ACF) from a given Px unit (320 nm × 320 nm) decays faster if probes diffuse faster within that Px unit. The ACFs, corresponding to the Px units designated with asterisks of same color, illustrate probes diffusing slower (cyan) and faster (black). (C) Schematic diffusion coefficient (D) map, obtained after ACF analyses of all Px units contains some Px units with relatively slower (Dslow, cyan) or faster (Dfast, white) diffusion coefficient, interpreted as interaction-rich and interaction-poor units, respectively. (D) Histograms of experimental D values (>10,000; SI Appendix, Table S1) and PDF for AF488-IgE-FcεRI at −Ag (red) and +Ag (black) steady states. PDFs are fitted using parameters derived from bin-independent CDFs (SI Appendix, Eq. A2). (E) CDFs of the same D values as in part (D). Pooled D values are resampled 30 times by bootstrapping with 50% of all data each time, and individual bootstrapped CDFs are fitted for Dslow, Dfast, and Fslow (SI Appendix, Table S1). Individual raw bootstrapped CDFs of D values of AF488-IgE-FcεRI at each condition are overlaid and shown (red: −Ag and black: +Ag). (Inset) Box plots of all D values. Box height corresponds to 25th to 75th percentile; error bars represent 9th to 91st percentile of entire dataset; mean and median values are represented as solid circle and bar, respectively; notches signify 95% CI of the median. Right shows the stimulated %change of Dav: Effect change distribution is calculated from the bootstrapped mean values at each condition.
The probability distribution functions (PDFs) of D values determined by ImFCS for mobile AF488-IgE-FcεRI from resting cells (red) and from cells stimulated with Ag (black) show a clear shift to slower D values after Ag is added (Fig. 2D), consistent with FRAP measurements (Fig. 1C and SI Appendix, S1A). To quantify possible subpopulations, we convert the pooled D values into cumulative distribution functions (CDFs; Fig. 2E)—a mathematically equivalent but bin-independent alternative to PDFs—which can be precisely resolved into one or two Gaussian components, parameterized as Dfast, Dslow, and Fslow [SI Appendix, Eqs. S3 and S4 (32)]. If a probe diffuses distinctively in subpopulations of Px units, the D CDF for this probe cannot be fitted by single Gaussian component (Dfast = Dslow). Roughly interpreted, Dfast and Dslow represent the average diffusion coefficient of a particular probe in, respectively, interaction-poor and -rich subpopulations of Px units in the plasma membrane (Fig. 2C), and Fslow is the fraction of the interaction-rich population of Px units. To test whether CDF curves are overly influenced by outliers and to provide a curve thickness (related to level of uncertainty), we routinely resample the D values by bootstrapping 30 times (with 50% of all data each time), and the corresponding bootstrapped CDFs are fitted individually (SI Appendix). For all probes tested in this study, we found narrowly distributed values of fitting parameters Dfast, Dslow, and Fslow, confirming the reliability and robustness of our analysis (SI Appendix, Table S1). The Dav determined from ImFCS measurements of D across all Px units and the profile of the D CDFs reveal how structurally distinct probes sense changes in local environments caused by Ag-mediated clustering of IgE-FcεRI to stimulate TM signaling. Visually striking are the distinctive shifts in the bootstrapped CDF curves of D values that accompany Ag stimulation (Figs. 2–6). Comparing these shifts in CDF curves and their representative Dav values for a panel of probes with defined structural features allows us to evaluate contributions of individual structural features to stimulated changes in diffusion properties. In this manner, we can infer how Ag-stimulation changes lipid- or protein-based interactions in the plasma membrane and corresponding changes in membrane organization.
Fig. 6.
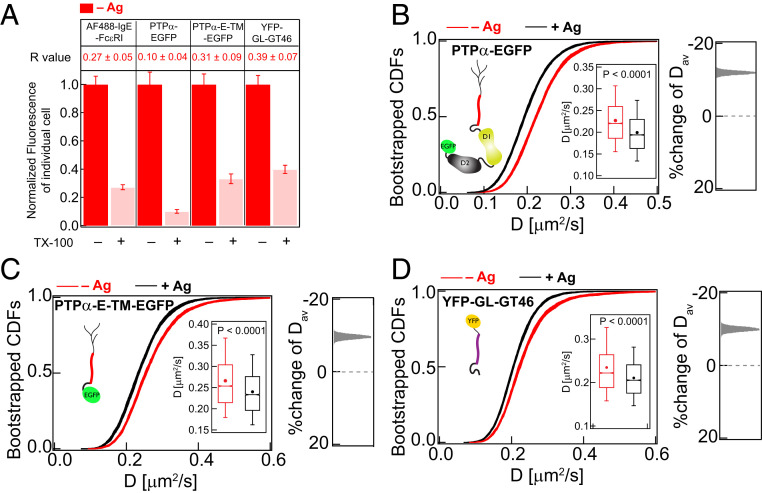
TM probes are strongly detergent soluble but show relatively slower diffusion in stimulated cells. (A) Detergent resistance of AF488-IgE-FcεRI, PTPα-EGFP, PTPα-E-TM-EGFP, and YFP-GL-GT46 as represented by relative loss of fluorescence after TX100 treatment and corresponding R values in unstimulated (−Ag) RBL cells. Box plots of fluorescence values of individual cells for −/+TX100 and −/+Ag conditions for these probes are provided in SI Appendix, Figs. S2 (AF488-IgE-FcεRI and YFP-GL-GT46) and S8A (PTPα-EGFP and PTPα-E-TM-EGFP). (B–D) A total of 30 bootstrapped CDFs of D values from ImFCS measurements are overlaid for specified probes and conditions (−/+Ag). Box plots of all D values and stimulated %change of Dav are shown as described for Fig. 2E. SI Appendix, Table S1 shows number of autocorrelation function and cells measured for ImFCS analyses.
After Ag-crosslinking AF488-IgE-FcεRI to form nanoclusters (14, 47), ImFCS measurements show the mobile fraction (30% as measured by FRAP; Fig. 1C and SI Appendix, S1A) has ∼35% smaller Dav (0.117 ± 0.0005 μm2/s) than monomeric AF488-IgE-FcεRI in resting cells (0.168 ± 0.0004 μm2/s) (SI Appendix, Table S1). Correspondingly, the PDFs and bootstrapped CDFs clearly shift to lower D values after stimulation with Ag (Fig. 2 D and E). The effect size distribution of Dav further quantifies the amount and direction of diffusion change based on the bootstrapped CDF curves (Fig. 2E, Right). Mobile IgE-FcεRI in the stimulated steady state are likely to include small oligomers, which have been shown to be signaling competent (48). We find that Ag crosslinking of IgE-FcεRI imparts biophysical changes across and on both sides of the membrane. Stimulated modulation in diffusion of structurally distinct probes reflects these changes.
Evaluating Ag-stimulated changes in the lipid phase-like properties of the plasma membrane requires consideration of compositional differences in the outer- and inner-membrane leaflets. As recently characterized in erythrocyte plasma membranes, outer-leaflet lipids are more Lo-like, whereas inner-leaflet lipids are more Ld-like (49). Similarly, the outer leaflet of resting RBL cells was found to be more ordered when compared to the inner leaflet. We might then expect that Ag-stimulated changes in phase-like properties may manifest differently for the inner and outer leaflets. Because stimulated coupling of Lyn kinase with Ag-clustered FcεRI occurs in the inner leaflet of the plasma membrane, we first focused on stimulated changes in the Lo/Ld-like properties in this leaflet. For this purpose, we evaluated properties of inner-leaflet lipid probes that are phase selective but otherwise passive in the signaling process. We employed three genetically encoded probes that distinctively anchor enhanced green fluorescent protein (EGFP) to the inner leaflet [Fig. 1A (23, 50)]: PM-EGFP, with palmitoyl and myristoyl acyl chains [Lo-preferring (23)], EGFP-GG, with a GG chain and a polybasic protein sequence [Ld preferring (23)] and S15-EGFP, with a myristoyl chain and a polybasic protein sequence [Ld preferring (51)]. Our previous superresolution imaging demonstrated that the PM construct coclusters with crosslinked IgE-FcεRI, but the GG construct does not (15), and consistent results were obtained with clustered B cell receptors (41). Diffraction-limited TIRF imaging reveals no visible changes in the distribution of any of these probes after Ag addition (SI Appendix, Fig. S5), and we examined their membrane interactions using DRM imaging, FRAP, and ImFCS.
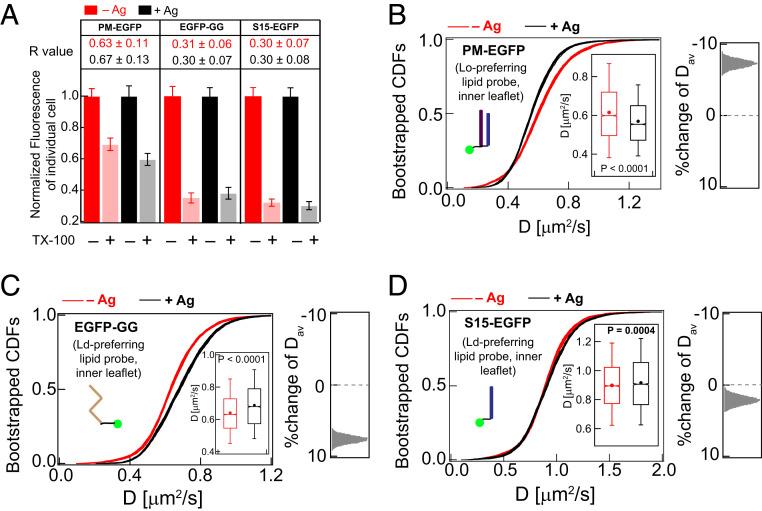
For phase-selective lipid probes, a larger R value in the DRM assay indicates stronger partitioning into Lo-like nanodomains. In resting cells, we found R values to be consistent with their phase preferences in membranes as reported previously (50–52): PM-EGFP (R = 0.6) > EGFP-GG (0.3) = S15-EGFP (0.3) (Fig. 3A and SI Appendix, Fig.S3, red/pink). Although these R values are useful for monitoring Lo- versus Ld-phase preference of these lipid probes, we found the method to be insufficiently sensitive for detecting significant differences before and after Ag-crosslinking of IgE-FcεRI (Fig. 3A and SI Appendix, Fig. S3, compare red/pink to black/gray). Similarly, FRAP measurements do not resolve significant differences for any of these probes before and after stimulation (SI Appendix, Fig. S4). In contrast, Ag-crosslinking of IgE-FcεRI causes distinctive shifts in the ImFCS CDF curves for each of these probes (Fig. 3 B–D): Lo-preferring probe PM-EGFP shifts to slower D values, and Ld-preferring probes EGFP-GG and S15-EGFP shift to faster D values. In terms of Dav values (SI Appendix, Table S1), the shifts are 8% slower for PM-EGFP (0.62 ± 0.002 to 0.57 ± 0.002 μm2/s), 8% faster for EGFP-GG (0.64 ± 0.002 to 0.69 ± 0.002 μm2/s), and 2% faster for S15-EGFP (0.90 ± 0.003 to 0.92 ± 0.003 μm2/s). These contrasting behaviors of Lo- and Ld-preferring lipid probes are consistent with the Lo-like phase in the inner leaflet of the plasma membrane becoming more ordered and stabilized against surrounding Ld-like regions, which become more disordered after Ag addition. These ImFCS measurements on inner-leaflet lipid probes before and after stimulation are consistent with and extend quantitative results from previous superresolution imaging (15). Collectively, they characterize and quantify stabilization of a Lo-like environment encompassing Ag-crosslinked IgE-FcεRI nanoclusters in the membrane inner leaflet.
Fig. 3.
ImFCS but not DRM detects subtle stabilization of Lo-like regions in Ag-stimulated RBL cells. (A) Degree of detergent resistance for PM-EGFP, EGFP-GG, and S15-EGFP and R values for −Ag (red) and +Ag (black) conditions. Box plots of fluorescence values of individual cells for −/+TX100 and −/+Ag conditions for these probes are provided in SI Appendix, Fig. S3. (B–D) A total of 30 bootstrapped CDFs of D values from ImFCS measurements are overlaid for specified probes and conditions (−/+Ag). Box plots of all D values and distribution of stimulated %change of Dav as described for Fig. 2E. SI Appendix, Table S1 shows number of autocorrelation function and cells measured for ImFCS analyses.
Lo-Preference of Lipid Anchor Is Necessary for Lyn’s Functional Coupling with Crosslinked IgE-FcεRI.
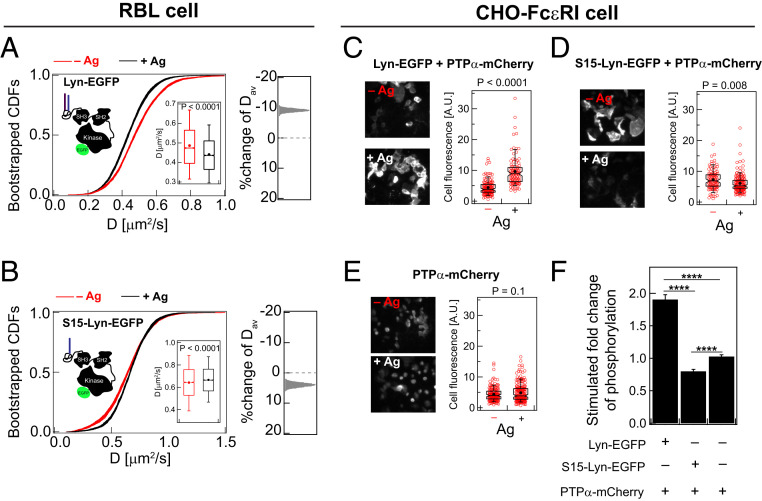
Nanoscopic colocalization of Lyn with crosslinked IgE-FcεRI nanoclusters was observed by superresolution cross-correlation imaging as a threefold enhancement in Lyn density in these regions compared with other parts of the membrane (15). This low level of stimulated modulation in Lyn interactions is not detectable in TIRF images, DRM R values, or FRAP curves (SI Appendix, Fig. S6 A–C). However, ImFCS sensitively detects the subtle change by resolving a shift of the CDF curves to lower D values (Fig. 4A), corresponding to 10% reduction of Dav of Lyn-EGFP after Ag addition (from 0.49 ± 0.001 to 0.44 ± 0.001 μm2/s; SI Appendix, Table S1). Lyn-EGFP is highly detergent resistant [R = 0.8; Fig. 5A (28)], as expected for the Lo-preference of its PM membrane anchor and consistent with the stimulated shift in this probe’s diffusion properties being the consequence of more stable Lo-like nanodomains after Ag crosslinking of IgE-FcεRI. However, the degree of shifts in the CDF curves are different for Lyn-EGFP (Fig. 4A) compared to PM-EGFP (Fig. 3B), as quantified by the effect size distributions of Dav for these two probes (Right panels in Figs. 3 B and 4 A). These differences indicate that some of Lyn-EGFP interactions in stimulated cells occur directly with the crosslinked FcεRI, in addition to those with the surrounding stabilized Lo-like regions into which the PM lipid anchor partitions favorably.
Fig. 4.
Lipid-driven Lo-preference of Lyn is necessary for its functional coupling with Ag-crosslinked IgE-FcεRI. A total of 30 bootstrapped CDFs of D values from ImFCS measurements in RBL cells are overlaid for WT Lyn-EGFP (A) and the Ld-preferring Lyn chimera S15-Lyn-EGFP (B) in −/+Ag conditions. Box plots of all D values and stimulated %change of Dav are shown as described for Fig. 2E. SI Appendix, Table S1 shows number of autocorrelation function and cells measured for ImFCS analyses. (C–E) Representative images of immunostained phosphorylation for CHO cells stably transfected with FcεRI and transiently transfected with specified Lyn variant and PTPα. Red circles are fluorescence of individual cells for a representative biological replica, and the box plots are defined as described in Fig. 2E. (F) Quantification of stimulated changes of phosphorylation for each condition. ****: P < 10−4 (unpaired Student’s t test).
Fig. 5.
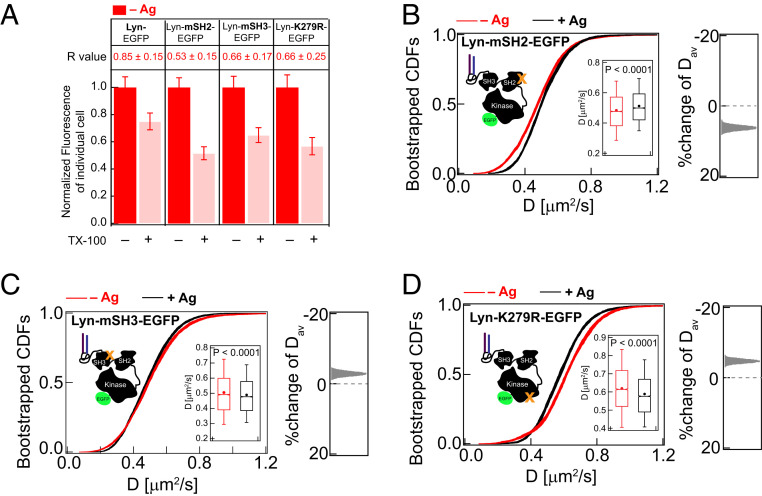
Cytosolic protein modules of Lyn-EGFP contribute to detergent-resistance and reduction of diffusion caused by Ag-crosslinking of IgE-FcεRI. (A) Detergent resistance of Lyn-EGFP compared to variants Lyn-mSH2-EGFP, Lyn-mSH3-EGFP, and Lyn-K279R-EGFP as represented by relative loss of fluorescence after TX100 treatment and corresponding R values in unstimulated (−Ag) RBL cells. Box plots of fluorescence values of individual cells for −/+TX100 and −/+Ag conditions for these probes are provided in SI Appendix, Figs. S6B (Lyn-EGFP) and S7A (Lyn-EGFP variants). (B–D) 30 bootstrapped CDFs of D values from ImFCS measurements are overlaid for specified probes and conditions (−/+Ag). Box plots of all D values and stimulated %change of Dav are shown as described for Fig. 2E. SI Appendix, Table S1 shows number of autocorrelation function and cells measured for ImFCS analyses.
To deconvolve lipid- and protein-based interactions, we compared diffusion modes of Lyn-EGFP in both resting and stimulated states to a Lyn chimera. S15-Lyn-EGFP was created by replacing the first 15 amino acids of wild-type (WT) Lyn, which possesses Lo-targeting palmitoylation and myristoylation sites, with the first 15 amino acids of Ld-preferring lipid probe S15-EGFP containing a single myristoyl site and a polybasic region (51). Unlike Lyn-EGFP, S15-Lyn-EGFP exhibits little detergent-resistance (R = 0.2; SI Appendix, Fig. S6B) and has a faster Dav in resting cells (Dav = 0.64 ± 0.002 μm2/s; Fig. 4B and SI Appendix, Table S1). S15-Lyn-EGFP serves as a Lyn probe that anchors to the inner leaflet by an Ld-preferring lipid but possesses Lyn’s functional protein modules. If protein–protein interactions primarily drive stimulated reduction of Lyn-EGFP diffusion, we expect to see similar net change of diffusion of S15-Lyn-EGFP after Ag-crosslinking of IgE-FcεRI. However, in sharp contrast to Lyn-EGFP (Fig. 4A), the Dav value of S15-Lyn-EGFP (Fig. 4B) does not decrease after Ag addition but rather increases to 0.67 ± 0.002 μm2/s (5% faster), similar to the behavior of Ld-preferring lipid probes, EGFP-GG and S15-EGFP (Fig. 3 C and D and SI Appendix, Table S1). Although the trend appears similar, the data statistics of FRAP are not sufficient to resolve these differences between Lyn and S15-Lyn (SI Appendix, Fig. S6 C and D).
The slower diffusion of Lyn-EGFP and faster diffusion of S15-Lyn-EGFP after crosslinking IgE-FcεRI revealed by ImFCS represent a subtle but distinguishable change in the phase-like organization that is sensed by Lo- and Ld-preferring probes. These ImFCS results for Lyn-EGFP and S15-Lyn-EGFP are consistent with the view that Lo-preference is necessary for Lyn’s interaction with clustered IgE-FcεRI. Changes in diffusion for Lyn-EGFP compared to PM-EGFP points to a role for protein-based interactions for optimal coupling leading to receptor phosphorylation and downstream signaling.
Lyn-EGFP but not S15-Lyn-EGFP Facilitates Ag-Dependent Tyrosine Phosphorylation.
To test directly a functional outcome inferred from our ImFCS results, we compared the capacities of Lyn-EGFP and S15-Lyn-EGFP to facilitate stimulated phosphorylation in cells. We used a reconstitution approach for this purpose: FcεRI stably expressed in Chinese hamster ovary cells (CHO-FcεRI) that are transiently cotransfected with Lyn and an Ld-preferring TM tyrosine phosphatase α (PTPα). With this experimental system, we found previously that PTPα suppresses Lyn kinase activity and minimizes its spontaneous phosphorylation of FcεRI, while facilitating Ag-stimulated FcεRI phosphorylation (24). This previous study also showed that a PTPα chimera anchored to the inner leaflet by Lo-preferring lipids (PM) fails to reconstitute Ag-dependent FcεRI phosphorylation. In the current study, we used immunostaining to monitor tyrosine phosphorylation in CHO-FcεRI cells transiently transfected with mCherry tagged WT PTPα (PTPα-mCherry) and either Lyn-EGFP or S15-Lyn-EGFP before and after addition of Ag. As shown in Fig. 4 C–F, cotransfection with Lyn-EGFP causes a nearly twofold enhancement of tyrosine phosphorylation, compared to no Lyn construct, whereas cotransfection with S15-Lyn-EGFP results in a somewhat reduced level of tyrosine phosphorylation. Results from this functional assay are consistent with ImFCS diffusion measurements and show that Lo-preference is essential for Lyn’s colocalization with clustered IgE-FcεRI but leaving open the possibility that protein-based interactions are also involved.
Intact Protein Modules of Lyn Are Necessary for Its Effective Coupling with Crosslinked IgE-FcεRI.
The cytosolic segments of Lyn comprise an SH2 module that docks on phosphotyrosine (pY) sites, an SH3 module which interacts with polyproline (PxxP) motifs, and a kinase module (53). To test the importance of direct interactions with FcεRI in stimulated cells, we evaluated the diffusion properties of two point mutants (54): Lyn-mSH2-EGFP (Arg to Ala at position 135) and Lyn-mSH3-EGFP (Trp to Ala at position 78), with intact PM lipid anchor but disabled binding capabilities through SH2 and SH3 modules, respectively. In resting cells, the detergent resistance of both mutants (R = ∼0.6; SI Appendix, Fig. S7A) is less than Lyn-EGFP (R = 0.8; Fig. 5A), similar to PM-EGFP (R = 0.6; Fig. 3A) and greater than Ld-preferring S15-Lyn-EGFP (R = 0.2; SI Appendix, Fig. S6B). Changes in diffusion properties of Lyn variants after Ag crosslinking of IgE-FcεRI are not resolved by FRAP (SI Appendix, Fig. S7 B–D) but revealed by ImFCS (Fig. 5 B and C). ImFCS further reveals clear differences for Lyn-mSH2-EGFP and Lyn-mSH3-EGFP compared to Lyn-EGFP in terms of stimulated shifts in CDF curves. After Ag addition, the Dav values for Lyn-EGFP decrease (Fig. 4A), increase slightly for Lyn-mSH2-EGFP (Fig. 5B), and decrease slightly for Lyn-mSH3-EGFP (Fig. 5C). Also, Lyn-mSH2-EGFP responds differently from PM-EGFP (Fig. 3B) to stimulated stabilization of Lo-like nanodomains, even though it is similarly detergent resistant. One possible explanation for differences is cytosolic steric hindrance due to scaffold proteins and cytoskeleton components that are recruited to Ag-crosslinked IgE-FcεRI (13, 55, 56). Lo-preferring lipid, PM-EGFP, with no Lyn protein modules in the cytoplasm, is likely to be less sensitive to these steric factors. Unlike Lyn-EGFP, Lyn-mSH2-EGFP and Lyn-mSH3-EGFP cannot efficiently overcome steric hindrance by docking at the pY219 site on clustered FcεRI. It appears that Ag-induced changes in Lyn diffusion compared to these two Lyn mutants depend on interactions with FcεRI rather than entirely on their Lo-preference.
We measured the stimulated diffusion change of kinase-inactive Lyn mutant, Lyn-K279R-EGFP (57), to test whether phosphorylation of FcεRI by Lyn stabilizes the coupling of these two proteins. In resting cells, similar to Lyn-mSH2-EGFP and Lyn-mSH3-EGFP, Lyn-K279R-EGFP shows weaker detergent resistance (R = 0.6) compared to WT Lyn-EGFP (Fig. 5A and SI Appendix, S7A). This consistent difference suggests that kinase activity and resulting protein-based interactions contribute to Lyn’s greater tendency to localize in a Lo-like environment. Lyn-K279R-EGFP, unlike Lyn-mSH2-EGFP and Lyn-mSH3-EGFP, is expected to undergo proper SH2/pY219 intermolecular docking after FcεRI phosphorylation by the endogenous Lyn present in these cells (57). However, the shift in the CDF curve to lower diffusion coefficients is less for Lyn-K279R-EGFP (5% decrease of Dav: 0.62 ± 0.002 to 0.59 ± 0.002 μm2/s; Fig. 5D) compared to Lyn-EGFP (10% decrease of Dav: 0.49 ± 0.001 to 0.44 ± 0.001 μm2/s; Fig. 4A), suggesting competitive advantage of endogenous Lyn over Lyn-K279R in associating with phosphorylated FcεRI.
Collectively, the shifts in CDF curves (Figs. 4 A and B and 5 B–D) of Lyn-EGFP compared to the Lyn variants we tested support the following view: the Lo-preference of Lyn, as mediated by saturated lipid anchors, is essential for its coupling with Ag-crosslinked FcεRI; Lyn’s kinase activity and FcεRI-docking capacities secondarily serve to stabilize the interaction.
TM PTPα Exclusion from Ag-Crosslinked FcεRI Nanoclusters Is Accompanied by Its Slowed Diffusion.
Raising the phosphorylated state of Ag-crosslinked FcεRI above the stimulation threshold requires that access by phosphatases be minimized. Previous studies (5, 22, 24, 58) support the hypothesis that tyrosine phosphorylation of FcεRI by Lyn kinase prior to Ag engagement is counterbalanced by TM phosphatase–mediated dephosphorylation: Transient nanodomains present in the resting steady state do not sufficiently coconfine Lo-preferring Lyn and monomeric FcεRI nor prevent access by phosphatase to disrupt the balance. Nanodomains that are stabilized around the Ag-clustered FcεRI preferentially include kinase, exclude phosphatase, and tip the balance to exceed the phosphorylation threshold. We examined participation of the TM phosphatase within this mechanism by measuring stimulated changes in diffusional and other properties of EGFP-tagged PTPα variants.
Consistent with our previous observations that PTPα prefers an Ld-like environment (24), we found that PTPα-EGFP exhibits little detergent resistance in resting cells (R = 0.1; Fig. 6A and SI Appendix, S8A). ImFCS measurements of this probe in resting cells yields Dav = 0.227 ± 0.0006 μm2/s. This value is similar to other single-pass TM probes reported previously (32, 59) and slower than inner- or outer-leaflet lipid probes (32, 38) or inner-leaflet lipid–anchored proteins such as Lyn kinase (0.49 ± 0.001 μm2/s; SI Appendix, Table S1). This is not surprising because the diffusion of TM probes is influenced by their interactions within both membrane leaflets and with extracellular and cytosolic components. After Ag addition, PTPα-EGFP is expected to be excluded from regions of crosslinked IgE-FcεRI because of its Ld preference. Interestingly, however, we found that Dav for PTPα-EGFP decreases by 12% (to 0.199 ± 0.0005 μm2/s) after Ag addition (Fig. 6B and SI Appendix, Table S1). This direction in stimulated change of Dav is same as for inner-leaflet, Lo-preferring lipid probes (PM-EGFP and Lyn-EGFP) and opposite to that of inner-leaflet, Ld-preferring probes (EGFP-GG, S15-EGFP, and S15-Lyn) (Fig. 7A). These comparisons raise intriguing questions and indicate minimally that the stimulated diffusion change of TM PTPα-EGFP is not controlled by modulation of lipid organization in the membrane inner leaflet.
Fig. 7.
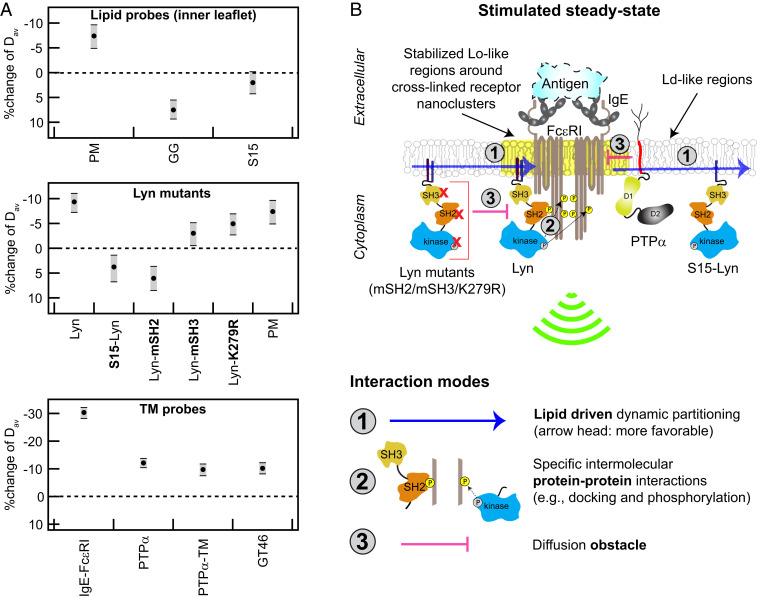
Ag-crosslinking of IgE-FcεRI stabilizes surrounding Lo-like nanodomains, causing dynamic lipid- and protein-based interactions that shift diffusion properties of signaling components and lead to suprathreshold phosphorylation by Lyn. (A) Stimulated changes in Dav for inner leaflet lipid probes, Lyn variants, and TM probes, including IgE-FcεRI and PTPα. Values and error bars represent effect change distributions shown in Figs. 2–6. (B) Proposed interaction modes leading to functional coupling of Lyn with clustered FcεRI: Stabilized Lo-like environment preferentially includes Lo-preferring Lyn and excludes Ld-preferring S15-Lyn and PTPα (interaction mode 1). Preferentially proximal Lyn (interaction mode 1) phosphorylates clustered FcεRI via its kinase module and then binds to pTyr via its SH2 module as facilitated by its SH3 module (interaction mode 2); these cumulative interactions stabilize the coupling. Lyn variants with impaired kinase, SH2, or SH3 modules are sterically hindered by cytoplasmic segments of clustered FcεRI (interaction mode 3). PTPα, which is preferentially excluded from Lo-like environments (interaction mode 1), is further limited in access to FcεRI-pTyr due to steric hindrance by clustered FcεRITMDs (interaction mode 3).
To consider whether diffusion of TM probes is influenced by Ag-stimulated modulation of outer-leaflet phase-like properties, we evaluated YFP-tagged glycosylphosphatidylinositol (YFP-GL-GPI), a Lo-preferring lipid probe. Although neither DRM assays (R = ∼1) nor FRAP resolve differences between resting and Ag-stimulated cells for YFP-GL-GPI (SI Appendix, Fig. S9 A and B), ImFCS revealed a stimulated decrease in Dav (0.328 ± 0.0008 μm2/s to 0.276 ± 0.0009 μm2/s, 16%; SI Appendix, Fig. S9C and Table S1). This might be expected if Ag clustering of IgE-FcεRI stabilizes more ordered lipids around these clusters in the outer leaflet. However, the relative decrease in Dav for YFP-GL-GPI is similar to that of Ld-preferring PTPα-EGFP, which suggests another possibility: If the outer leaflet is primarily Lo-like in resting cells (49), stimulated reduction in diffusion observed for both probes may point to globally increased ordering in this leaflet that occurs with Ag crosslinking of IgE-FcεRI and stimulated signaling.
Another, nonexclusive explanation for slowed diffusion of PTPα-EGFP arises from considering other effects caused by Ag, which crosslink and immobilize IgE-FcεRI (Fig. 1 B and C). Theoretical studies considering hydrodynamic and steric interactions between membrane components indicate that the presence of immobile objects, even at very low surface coverage (5%), decreases diffusion of other mobile objects (60–62). These predictions led us to test whether the diffusion of TM probes generally decrease after Ag stimulation creates new diffusion obstacles, minimally those in the form of immobilized TM domains within the FcεRI nanoclusters. To consider this possibility, we created a PTPα construct, PTPα-E-TM-EGFP, which includes the extracellular module and TM domain (TMD) of PTPα and is C terminally fused to EGFP (as illustrated in Fig. 6C). Because this probe does not contain the catalytic or other cytosolic modules of PTPα, any Ag-stimulated change of its diffusion is not due to functional interactions with FcεRI. PTPα-E-TM-EGFP in resting cells has low detergent resistance (R = 0.3, Fig. 6A) and FRAP similar to PTPα-EGFP (SI Appendix, Fig. S8 B and C). ImFCS determined Dav (0.266 ± 0.0008 μm2/s) for PTPα-E-TM-EGFP in resting cells is slightly greater than that for PTPα-EGFP (SI Appendix, Table S1), possibly due to eliminated cytosolic interactions. After Ag crosslinking of IgE-FcεRI, Dav for PTPα-E-TM-EGFP decreases by 11%, and the shift in ImFCS CDF curves is strikingly similar to the shift for PTPα-EGFP (Fig. 6 B and C). These results indicate that Ag-stimulated reduction of PTPα diffusion is not due to functional interactions with other proteins. We observed a similar 13% stimulated decrease in Dav for YFP-GL-GT46, another Ld-preferring but passive TM probe (Fig. 6D). These experimental results are consistent with the possibility that immobilization of FcεRI after Ag-clustering creates new diffusion obstacles that slow diffusion of other TM proteins on the length scale measured by ImFCS. They also provide evidence that exclusion of PTPα-EGFP from Ag-clustered FcεRI is due in part to steric hindrance.
Discussion
Nanoclustering of TM receptors by extracellular ligands followed by local reorganization in the plasma membrane to facilitate kinase coupling and receptor phosphorylation above a stimulation threshold has become a general paradigm of signaling through cell surface immunoreceptors. A large body of evidence supports the view that Ag-mediated nanoclustering of sensitized mast cell receptors, IgE-FcεRI, coalesces proteolipid nanodomains that have Lo-like character (5, 6, 63). However, the functional significance of lipid phase-like behavior in TM signaling mediated by this and other immunoreceptors continues to be debated (21, 64, 65), and some have taken the position that stimulated protein–protein interactions are sufficient (3). Although protein-based interactions may increasingly dominate as signaling proceeds, the initial upshift in receptor phosphorylation appears to depend on disrupting the balance between kinase and phosphatase access, and this is mediated by membrane lipids. Simply stated, stabilization of Lo-like nanodomains around Ag-crosslinked immunoreceptors serves to colocalize Lo-preferring lipid-anchored kinase (e.g., Lyn), while Ld-preferring TM phosphatases (e.g., PTPα) are excluded. In this study, we used ImFCS measurements that build on other imaging approaches to quantify systematically the subtle shift in plasma membrane organization that accompanies Ag-mediated stimulation. We confirm the primacy of lipid-based interactions and unveil how these synergize with protein-based interactions among IgE-FcεRI, Lyn kinase, and PTPα phosphatase. We evaluate particular contributions of these different types of interactions for functional assembly of TM signaling components.
We previously established ImFCS as a statistically robust approach for quantifying differences in the diffusion properties of selected probes that collectively sense the organization of the plasma membrane under specified conditions (32). The sampling provided by our ImFCS measurements (∼10,000 D values for each probe) yields extremely precise CDF curves and values for Dav. As we demonstrate herein, ImFCS measurements of 14 independent, structurally distinct probes sensitively reveal subtle changes in plasma membrane organization that result from Ag-stimulation. These changes are simply represented by Dav values and distinguished by the shifts in CDF curves of all D values (Figs. 2–6 and summarized in Fig. 7A). Ag-stimulated differences are quantified by effect size distributions of Dav and by detailed changes in the fit parameters of the CDF curves (SI Appendix, Table S1). By systematically evaluating key structural features among the selected probes and corresponding differences in their diffusion properties measured before and after Ag stimulation, we can minimally reconstruct participation of different types of interactions experienced by the initial signaling components as illustrated in Fig. 7B.
Ag Crosslinking Creates Nanoclusters of IgE-FcεRI and Stabilizes Surrounding Lo-Like Domains in the Membrane Inner Leaflet.
Because the plasma membrane is asymmetric in lipid composition, we focused primarily on the inner leaflet, where membrane-anchored Lyn phosphorylates FcεRI after its clustering by Ag. In general, the phase-like behavior of the plasma membrane in resting cells can be approximated by the tendency of Lo-preferring probes to be more detergent resistant than Ld-preferring probes. Consistent with previous results, we found in resting cells that Lo-preferring PM-EGFP and Lyn-EGFP to be more detergent resistant than Ld-preferring EGFP-GG and S15-EGFP (Fig. 3A and SI Appendix, Fig. S6B). However, the Lo-like domains in this resting state appear to be small and transient (21), with relative weak distinction from Ld-like regions, as suggested by ImFCS measurement of small differences in Dav values for PM-EGFP and EGFP-GG [(32); SI Appendix, Table S1]. This relatively weak phase-like heterogeneity in the cell’s resting state would allow access to monomeric FcεRI by both Lyn kinase and TM phosphatase and thereby limit net phosphorylation (66). That Ag crosslinking of IgE-FcεRI leads to proximal coalescence and stabilization of Lo-like domains in the inner leaflet was clearly indicated previously by reconstitution studies (24) and by superresolution imaging of coclustering with IgE-FcεRI by PM probes but not GG probes (15). However, this level of stabilization is not detected within the limited sensitivity of DRM imaging (Fig. 3) and FRAP measurements (SI Appendix, Fig. S4). In contrast, statistically robust ImFCS measurements show subtle but distinctive shifts in D CDF curves and Dav values for the Lo-preferring probes compared to Ld-preferring probes. The lipid-anchored probes are driven by their intrinsic partitioning preferences: PM-EGFP and Lyn-EGFP shift to slower diffusion in modulated Lo-like domains, whereas EGFP-GG and S15-EGFP shift to faster diffusion in proximal Ld-like regions (Figs. 3, 4, and 7A). Stabilization of a Lo-like environment that encompasses the clustered FcεRI on the membrane inner leaflet may represent a thermodynamic adjustment, such as overcoming a hydrophobic mismatch of the collected TMDs and surrounding lipids (67) or possibly biasing critical fluctuations (68). We and others used a two-dimensional (2D)-Ising model to show that, when exogenously clustered, membrane components with Lo-preference weakly coalesce with other Lo-preferring components (15, 20, 41, 43). This lipid rearrangement establishes a more distinctive, phase-like membrane organization in the Ag-stimulated steady state (15, 18, 19). Such remodeling of membrane organization on the inner leaflet would facilitate lipid-driven sorting of Lo- (e.g., Lyn) from Ld-preferring components as previously proposed (66).
The smaller decrease in diffusion for PM-EGFP compared to Lyn-EGFP is similar to the trend observed with superresolution imaging: the Lyn probe coclusters with Ag-crosslinked IgE-FcεRI somewhat more than PM (15). The differences indicate interactions of Lyn’s cytosolic protein modules, in addition to its membrane anchor. To derive additional insight from shifts in the ImFCS CDF curves after Ag-stimulation, we can consider their precise fit parameters, which are based on a Gaussian model. This simple model is limited in providing physical information because quantified changes in diffusion (measured on the Px unit length scale) may involve multiple lipid-based factors (e.g., changes in lipid ordering, probe partitioning, and domain coverage), in addition to any protein-based interactions. All contributing factors are averaged together and represented as changes in populations of interaction-rich (Dslow) or -poor (Dfast) Px units [Fig. 2C (32)]. However, a reasonable interpretation can be made for inner-leaflet probes, which are localized primarily by the phase preference of their lipid anchors. For example, a quantitative comparison of CDF curve shifts for Lyn-EGFP and PM-EGFP indicates that Lyn’s cytosolic protein modules (SH2, SH3, and kinase) participate in its slowed diffusion. We find that both fit parameters Dfast and Dslow decrease for Lyn-EGFP, while only Dslow decreases for PM-EGFP after Ag stimulation (SI Appendix, Table S1). This comparison suggests that IgE-FcεRI clusters in the interaction-poor Px units do not adequately stabilize the Lo-like nanodomains to have an impact on the diffusion of PM-EGFP, which undergoes only lipid-based interactions. However, even relatively weak stabilization of Lo-like nanodomains in the interaction-poor Px units is sufficient to decrease the diffusion of Lyn-EGFP in these units due to its additional protein-based interactions with clustered IgE-FcεRI. We are currently exploring other fitting models to further exploit the quantitatively precise D CDF shapes and shifts, thereby to extract more detailed physical information about stimulated changes in membrane organization.
Lyn Access to Ag-Crosslinked IgE-FcεRI on the Inner Leaflet Requires Lipid-Based Filtering and is Enhanced by Protein Binding.
Comparing Lyn-EGFP (Lo-preferring) to S15-Lyn-EGFP (Ld-preferring) confirms that lipid-based sorting into stabilized Lo-like domains is the primary requirement for Lyn’s capacity to couple functionally with IgE-FcεRI. Unlike Lyn-EGFP and PM-EGFP, Dav values and CDF curves for S15-Lyn-EGFP shift to faster diffusion after Ag addition, similarly to EGFP-GG and S15-EGFP (Figs. 3, 4, and 7 A). This comparison shows that interactions mediated by Lyn’s cytosolic protein modules do not serve to slow diffusion unless Lyn’s PM anchor steers it into stabilized Lo-like domains. We infer that the slowed diffusion of Lyn-EGFP corresponds to Lyn first partitioning into the Lo-like domains that surround Ag-crosslinked IgE-FcεRI [Fig. 7 B, interaction mode 1 (15)]. Then, in this proximal location, Lyn phosphorylates tyrosines in cytosolic segments of FcεRI (pTyr) and transiently binds to these pTyr via its SH2 module [Fig. 7 B, interaction mode 2 (69)]. In comparison, S15-Lyn does not appreciably undergo interaction mode 1, and PM lipid anchor does not undergo interaction mode 2 (Fig. 7B). These interaction differences, which produce contrasting diffusion shifts after Ag addition (Fig. 7A), also manifest functionally: Ag stimulates tyrosine phosphorylation mediated by Lyn-EGFP but not by S15-Lyn-EGFP (Fig. 4F).
ImFCS measurements on Lyn probes with variations in cytosolic protein modules further show that protein interactions participate in appropriate coupling with Ag-crosslinked IgE-FcεRI. We found that if SH2-mediated binding to phosphorylated FcεRI is prevented by a mutation in this module (54), then the diffusion of this Lyn-mSH2-EGFP variant shifts faster (rather than slower) after Ag addition (Figs. 5 B and 7 A). These results suggest that the clustered cytosolic segments of Ag-crosslinked FcεRI sterically hinder the cytosolic protein modules of this Lyn variant (Fig. 7 B, interaction mode 3) to counteract lipid-based partitioning of its PM anchor. This interpretation is consistent with results for other Lyn variants. Unlike Lyn-mSH2-EGFP, the D CDFs and Dav values for Lyn-K279R-EGFP and Lyn-mSH3-EGFP shift to slightly slower diffusion after Ag-crosslinking of IgE-FcεRI, but the extent of this negative shift is less than that for Lyn-EGFP and PM-EGFP (Figs. 4, 5, and 7 A). We expect kinase-inactive Lyn-K279R-EGFP to be steered to stabilized Lo-domains (via PM anchor; Fig. 7 B, interaction mode 1) resulting in slower diffusion, and this variant has the capacity to bind to FcεRI tyrosines that are phosphorylated by endogenous Lyn in these cells (Fig. 7 B, interaction mode 2). However, endogenous Lyn is likely to be more competitive for proximal binding to the tyrosines it phosphorylates, and accordingly, Lyn-K279R-EGFP is probably more sensitive to steric hindrance by the clustered cytosolic segments of FcεRI (Fig. 7 B, interaction mode 3).
The Lyn-mSH3-EGFP variant has a PM anchor, kinase activity, and an intact SH2 module. However, the cytosolic SH3 module, which connects the PM anchor to SH2 and kinase modules, has been found to provide conformational plasticity of Lyn cytosolic segments for optimal catalytic activity and subsequent binding to pTyr in FcεRI (70). The impaired SH3 domain in Lyn-mSH3-EGFP is expected to limit this optimizing effect, rendering this variant more susceptible to steric hindrance by the clustered FcεRI. These ImFCS results are consistent with our previous observations that Lyn-mSH2-EGFP is not recruited, and Lyn-mSH3-EGFP is only weakly recruited to μm-scale IgE-FcεRI clusters that form when cells are placed on Ag-micropatterned surfaces (54). In contrast, both Lyn-EGFP and PM-EGFP are recruited to these micropatterned features (17, 54). Overall, the balance among interaction modes 1, 2, and 3 (Fig. 7B) after Ag addition results in slower diffusion for Lyn-K279R-EGFP and Lyn-mSH3-EGFP but distinctively smaller shifts than for Lyn-EGFP (Fig. 7 A and B). We conclude that after Ag crosslinking of IgE-FcεRI, the primary coupling interaction is Lo-preference of Lyn’s PM anchor to facilitate its phosphorylation of FcεRI cytosolic segments followed by binding of its SH2 module to stabilize further the interaction. Despite these stabilizing effects, the interactions are dynamic and relatively weak, such that the overall slowing of Lyn-EGFP diffusion is subtle (10% reduction in Dav; Fig. 7A). The small magnitude of this change is consistent with the degree of coclustering of Lyn and Ag-crosslinked IgE-FcεRI observed with superresolution imaging (15).
Slowed Diffusion of PTPα that Accompanies Exclusion from Ag-Clustered IgE-FcεRI Reflects Increased Ordering of the Outer Leaflet and Steric Hindrance.
Modulation of PTPα-EGFP’s biophysical properties after Ag addition is consistent with exclusion from Ag-clustered FcεRI and points to involvement of multiple factors. That PTPα-EGFP has very low detergent resistance (Fig. 6A) agrees with our previous reconstitution studies (24) showing that this TM phosphatase prefers an Ld-like environment, thereby limiting its access to the stabilized Lo-like environment surrounding Lyn-phosphorylated FcεRI after Ag addition (Fig. 7 B, interaction mode 1). More recently, we observed that PTPα is preferentially excluded from subclusters of IgE-FcεRI when cells are placed on Ag-patterned surfaces (71). For both studies, the effects were reversed when the extracellular and TM modules of PTPα were replaced by a Lo-preferring PM membrane anchor. Consistent with our results, superresolution imaging and cross-correlation analysis showed that Ld-preferring TM phosphatase CD45 is depleted from the vicinity of Ag-crosslinked B cell receptor clusters (43).
It is interesting that the D CDF curves (Fig. 6B) and Dav (Fig. 7A) for PTPα-EGFP shift to slower diffusion after Ag addition. This trend differs from stimulated changes in the diffusion of Ld-preferring lipid probes in the inner leaflet, showing that the diffusion of TM PTPα-EGFP is not controlled by the phase-like properties of the membrane inner leaflet. The membrane outer leaflet in resting RBL cells was found to be more ordered than the inner leaflet (49), and this differential lipid organization is consistent with our previously published result that outer-leaflet Lo-preferring probe YFP-GL-GPI diffuses much slower than the inner-leaflet Lo-preferring probe PM-EGFP in unstimulated cells (32). Our ImFCS measurements allow the possibility that Ag crosslinking of IgE-FcεRI increases the overall ordering in the outer leaflet through a mechanism that may involve the cytoskeleton but has not yet been established. For example, predominant Lo-like domains may become more ordered and connected, whereas Ld-like domains become more disordered and isolated. However, Ag-stimulated slowing of YFP-GL-GPI (SI Appendix, Fig. S9C) could also be explained by stabilization of more highly ordered nanodomains around Ag-clustered FcεRI in the outer leaflet, as suggested in our early imaging of DiIC16 colocalizing with patches of highly crosslinked IgE-FcεRI (72). Recently, high-speed single-particle tracking revealed transient arrest in the lateral diffusion of outer-leaflet, Lo-preferring sphingomyelin around Ag-clustered FcεRI, whereas outer leaflet, Ld-preferring dioleoylphosphatidylcholine shows no arrest (30). Our ImFCS measurements leave open the possibility that FcεRI clustering increases the ordering of outer-leaflet lipids overall, thereby slowing both Ld- and Lo-preferring probes that experience this leaflet.
Exclusion of an Ld-preferring TM phosphatase from Ag-clustered FcεRI probably involves stabilizing a more ordered environment proximally and steric hindrance by FcεRI TM segments (Fig. 7 B, interaction modes 1 and 3, respectively). The steric hindrance factor may also participate in PTPα’s slowed diffusion, considering that Ag addition leads to 70% immobilization of IgE-FcεRI. These immobilized FcεRI nanoclusters would be expected to obstruct the diffusion of PTPα and other mobile TM proteins. From superresolution imaging, we estimate that Ag-clustered FcεRI (dimension about 80 nm) would occupy about 5% of the membrane surface area (14), which would be an increase over any diffusion obstacles present in the membrane, prior to or created by Ag stimulation. Theoretical studies show that even a small level of immobile objects can significantly slow the diffusion of mobile objects in 2D systems (60, 61). In particular, the model of Singh et al. (62) predicts that an increase in the surface coverage of immobile objects by 5% reduces diffusion of a mobile object by amounts consistent with our measurements: Diffusion of PTPα-EGFP, a PTPα variant without cytosolic protein modules (PTPα-E-TM-EGFP), and a passive TM probe (YFP-GL-GT46) are all slowed by about 10% (Fig. 7A). Hence, it seems likely that immobilization of Ag-clustered FcεRI contributes to both the exclusion and slowed diffusion of PTPα, although other explanations cannot be ruled out. Overall, our ImFCS measurements of modulated diffusion point to the importance of both lipid-based and steric exclusion processes in protecting Ag-crosslinked IgE-FcεRI, phosphorylated by Lyn, from dephosphorylation from a TM phosphatase.
Conclusion
As depicted by interaction modes in Fig. 7B, a coordinated synergy of lipid- and protein-based interactions explains how Ag-crosslinking of IgE-FcεRI leads to its suprathreshold tyrosine phosphorylation, both by facilitating access by Lyn kinase and limiting access by a TM phosphatase. Based on many studies in our and other laboratories, we take the view that the resting cell is poised to respond to a specific stimulus and that the change in membrane organization to initiate signaling is subtle (15). The subtlety of the change has made detection challenging, requiring superresolution imaging, SPT, and other technically difficult approaches. The strength of the mechanism proposed in Fig. 7B rests on precise ImFCS measurements of small but distinctive diffusion shifts stimulated by Ag for multiple structural variants of the key signaling components and passive probes. Although this suggested mechanism is based primarily on diffusion measurements, these are both internally consistent and consistent with previous studies cited herein. In particular, we showed directly and provided the strongest evidence to date that Lyn’s lipid-based steering is necessary to initiate tyrosine phosphorylation. Overall, we demonstrated the relative ease of applying ImFCS, using multiple probes and conventional fluorophores, to dissect contributions of structural features to weak interactions that collectively have decisive impact in stimulated TM signaling. We expect that ImFCS and the experimental strategies described herein will be widely applicable to advance understanding of TM signaling where plasma membrane organization is likely to play an integral role.
Materials and Methods
Images for ImFCS, DRM, and immunostaining were collected using a home-built TIRF microscope equipped with an electron multiplying charge coupled device (EMCCD) camera (14). The raw images were further processed using ImageJ/FIJI (73) and Igor Pro (version 8; WaveMetrics) routines to determine diffusion coefficient (D) values (ImFCS) and R values (DRM). The FRAP experiments were conducted on a Zeiss 710 confocal microscope. Detailed descriptions of instrumental setups, image processing, data fitting, and error analyses are provided in SI Appendix. The source of all materials including chemicals and plasmids as well as preparation protocols of fixed and live cell samples are also provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank Prof. Jeremy Baskin (Cornell University) for the access of the confocal microscope and Alex Batrouni for helping with the imaging especially during pandemic restrictions. We thank Henry Phan and Boyu Yin for discussions on the DRM preparations. This work is supported by National Institute of General Medical Sciences (NIGMS) Grant R01GM117552. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIGMS or NIH.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2026583118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Sigalov A., Multi-chain immune recognition receptors: Spatial organization and signal transduction. Semin. Immunol. 17, 51–64 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Su X., et al., Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 352, 595–599 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douglass A. D., Vale R. D., Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell 121, 937–950 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews N. L., et al., Actin restricts FcepsilonRI diffusion and facilitates antigen-induced receptor immobilization. Nat. Cell Biol. 10, 955–963 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holowka D., Baird B., Roles for lipid heterogeneity in immunoreceptor signaling. Biochim. Biophys. Acta 1861 (8 Pt B), 830–836 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holowka D., et al., Lipid segregation and IgE receptor signaling: A decade of progress. Biochim. Biophys. Acta 1746, 252–259 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Bugajev V., Bambousková M., Dráberová L., Dráber P., What precedes the initial tyrosine phosphorylation of the high affinity IgE receptor in antigen-activated mast cell? FEBS Lett. 584, 4949–4955 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Goyette J., Nieves D. J., Ma Y., Gaus K., How does T cell receptor clustering impact on signal transduction? J. Cell Sci. 132, jcs226423 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Pierce S. K., Lipid rafts and B-cell activation. Nat. Rev. Immunol. 2, 96–105 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Bálint Š., Dustin M. L., Localizing order to boost signaling. eLife 6, e25375 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Resh M. D., Regulation of cellular signalling by fatty acid acylation and prenylation of signal transduction proteins. Cell. Signal. 8, 403–412 (1996). [DOI] [PubMed] [Google Scholar]
- 12.Rivera J., Gilfillan A. M., Molecular regulation of mast cell activation. J. Allergy Clin. Immunol. 117, 1214–1225, quiz 1226 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Rivera J., Fierro N. A., Olivera A., Suzuki R., New insights on mast cell activation via the high affinity receptor for IgE. Adv. Immunol. 98, 85–120 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shelby S. A., Holowka D., Baird B., Veatch S. L., Distinct stages of stimulated FcεRI receptor clustering and immobilization are identified through superresolution imaging. Biophys. J. 105, 2343–2354 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shelby S. A., Veatch S. L., Holowka D. A., Baird B. A., Functional nanoscale coupling of Lyn kinase with IgE-FcεRI is restricted by the actin cytoskeleton in early antigen-stimulated signaling. Mol. Biol. Cell 27, 3645–3658 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kambayashi T., Koretzky G. A., Proximal signaling events in Fc epsilon RI-mediated mast cell activation. J. Allergy Clin. Immunol. 119, 544–552, quiz 553–554 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Wu M., Holowka D., Craighead H. G., Baird B., Visualization of plasma membrane compartmentalization with patterned lipid bilayers. Proc. Natl. Acad. Sci. U.S.A. 101, 13798–13803 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davey A. M., Krise K. M., Sheets E. D., Heikal A. A., Molecular perspective of antigen-mediated mast cell signaling. J. Biol. Chem. 283, 7117–7127 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Davey A. M., Walvick R. P., Liu Y., Heikal A. A., Sheets E. D., Membrane order and molecular dynamics associated with IgE receptor cross-linking in mast cells. Biophys. J. 92, 343–355 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitra E. D., Whitehead S. C., Holowka D., Baird B., Sethna J. P., Computation of a theoretical membrane phase diagram and the role of phase in lipid-raft-mediated protein organization. J. Phys. Chem. B 122, 3500–3513 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sezgin E., Levental I., Mayor S., Eggeling C., The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 18, 361–374 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Field K. A., Holowka D., Baird B., Fc epsilon RI-mediated recruitment of p53/56lyn to detergent-resistant membrane domains accompanies cellular signaling. Proc. Natl. Acad. Sci. U.S.A. 92, 9201–9205 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pyenta P. S., Holowka D., Baird B., Cross-correlation analysis of inner-leaflet-anchored green fluorescent protein co-redistributed with IgE receptors and outer leaflet lipid raft components. Biophys. J. 80, 2120–2132 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young R. M., Zheng X., Holowka D., Baird B., Reconstitution of regulated phosphorylation of FcepsilonRI by a lipid raft-excluded protein-tyrosine phosphatase. J. Biol. Chem. 280, 1230–1235 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Field K. A., Holowka D., Baird B., Structural aspects of the association of FcepsilonRI with detergent-resistant membranes. J. Biol. Chem. 274, 1753–1758 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Sheets E. D., Holowka D., Baird B., Critical role for cholesterol in Lyn-mediated tyrosine phosphorylation of FcepsilonRI and their association with detergent-resistant membranes. J. Cell Biol. 145, 877–887 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goñi F. M., “Rafts”: A nickname for putative transient nanodomains. Chem. Phys. Lipids 218, 34–39 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Sengupta P., et al., Probing protein heterogeneity in the plasma membrane using PALM and pair correlation analysis. Nat. Methods 8, 969–975 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veatch S. L., et al., Correlation functions quantify super-resolution images and estimate apparent clustering due to over-counting. PLoS One 7, e31457 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinoshita M., et al., Raft-based sphingomyelin interactions revealed by new fluorescent sphingomyelin analogs. J. Cell Biol. 216, 1183–1204 10.1083/jcb.201607086. (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koyama-Honda I., et al., High-speed single-molecule imaging reveals signal transduction by induced transbilayer raft phases. J. Cell Biol. 219, e202006125 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bag N., Holowka D. A., Baird B. A., Imaging FCS delineates subtle heterogeneity in plasma membranes of resting mast cells. Mol. Biol. Cell 31, 709–723 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kannan B., et al., Spatially resolved total internal reflection fluorescence correlation microscopy using an electron multiplying charge-coupled device camera. Anal. Chem. 79, 4463–4470 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Lingwood D., Simons K., Detergent resistance as a tool in membrane research. Nat. Protoc. 2, 2159–2165 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Menon A. K., Holowka D., Webb W. W., Baird B., Clustering, mobility, and triggering activity of small oligomers of immunoglobulin E on rat basophilic leukemia cells. J. Cell Biol. 102, 534–540 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pyenta P. S., Schwille P., Webb W. W., Holowka D., Baird B., Lateral diffusion of membrane lipid-anchored probes before and after aggregation of cell surface IgE-receptors. J. Phys. Chem. A 107, 8310–8318 (2003). [Google Scholar]
- 37.Pralle A., Keller P., Florin E.-L., Simons K., Hörber J. K., Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J. Cell Biol. 148, 997–1008 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kenworthy A. K., et al., Dynamics of putative raft-associated proteins at the cell surface. J. Cell Biol. 165, 735–746 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sengupta P., et al., A lipid-based partitioning mechanism for selective incorporation of proteins into membranes of HIV particles. Nat. Cell Biol. 21, 452–461 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Sohn H. W., Tolar P., Jin T., Pierce S. K., Fluorescence resonance energy transfer in living cells reveals dynamic membrane changes in the initiation of B cell signaling. Proc. Natl. Acad. Sci. U.S.A. 103, 8143–8148 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stone M. B., Shelby S. A., Núñez M. F., Wisser K., Veatch S. L., Protein sorting by lipid phase-like domains supports emergent signaling function in B lymphocyte plasma membranes. eLife 6, e19891 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zech T., et al., Accumulation of raft lipids in T-cell plasma membrane domains engaged in TCR signalling. EMBO J. 28, 466–476 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Núñez M. F., Wisser K., Veatch S. L., Synergistic factors control kinase-phosphatase organization in B-cells engaged with supported bilayers. Mol. Biol. Cell 31, 667–682 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fridriksson E. K., et al., Quantitative analysis of phospholipids in functionally important membrane domains from RBL-2H3 mast cells using tandem high-resolution mass spectrometry. Biochemistry 38, 8056–8063 (1999). [DOI] [PubMed] [Google Scholar]
- 45.Pathak P., London E., Measurement of lipid nanodomain (raft) formation and size in sphingomyelin/POPC/cholesterol vesicles shows TX-100 and transmembrane helices increase domain size by coalescing preexisting nanodomains but do not induce domain formation. Biophys. J. 101, 2417–2425 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holowka D., Thanapuasuwan K., Baird B., Short chain ceramides disrupt immunoreceptor signaling by inhibiting segregation of Lo from Ld Plasma membrane components. Biol. Open 7, bio034702 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larson D. R., Gosse J. A., Holowka D. A., Baird B. A., Webb W. W., Temporally resolved interactions between antigen-stimulated IgE receptors and Lyn kinase on living cells. J. Cell Biol. 171, 527–536 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andrews N. L., et al., Small, mobile FcepsilonRI receptor aggregates are signaling competent. Immunity 31, 469–479 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lorent J. H., et al., Plasma membranes are asymmetric in lipid unsaturation, packing and protein shape. Nat. Chem. Biol. 16, 644–652 10.1038/s41589-020-0529-6. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sengupta P., Hammond A., Holowka D., Baird B., Structural determinants for partitioning of lipids and proteins between coexisting fluid phases in giant plasma membrane vesicles. Biochim. Biophys. Acta 1778, 20–32 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodgers W., Making membranes green: Construction and characterization of GFP-fusion proteins targeted to discrete plasma membrane domains. Biotechniques 32, 1044–1046, 1048, 1050–1051 (2002). [DOI] [PubMed] [Google Scholar]
- 52.Baumgart T., et al., Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc. Natl. Acad. Sci. U.S.A. 104, 3165–3170 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ingley E., Functions of the Lyn tyrosine kinase in health and disease. Cell Commun. Signal. 10, 21 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hammond S., Wagenknecht-Wiesner A., Veatch S. L., Holowka D., Baird B., Roles for SH2 and SH3 domains in Lyn kinase association with activated FcepsilonRI in RBL mast cells revealed by patterned surface analysis. J. Struct. Biol. 168, 161–167 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torres A. J., Vasudevan L., Holowka D., Baird B. A., Focal adhesion proteins connect IgE receptors to the cytoskeleton as revealed by micropatterned ligand arrays. Proc. Natl. Acad. Sci. U.S.A. 105, 17238–17244 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wakefield D. L., Holowka D., Baird B., The FcεRI signaling cascade and integrin trafficking converge at patterned ligand surfaces. Mol. Biol. Cell 28, 3383–3396 10.1091/mbc.E17-03-0208. (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kovárová M., et al., Structure-function analysis of Lyn kinase association with lipid rafts and initiation of early signaling events after Fcepsilon receptor I aggregation. Mol. Cell. Biol. 21, 8318–8328 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Field K. A., Holowka D., Baird B., Compartmentalized activation of the high affinity immunoglobulin E receptor within membrane domains. J. Biol. Chem. 272, 4276–4280 (1997). [DOI] [PubMed] [Google Scholar]
- 59.Bag N., Huang S., Wohland T., Plasma membrane organization of epidermal growth factor receptor in resting and ligand-bound states. Biophys. J. 109, 1925–1936 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalay Z., Fujiwara T. K., Otaka A., Kusumi A., Lateral diffusion in a discrete fluid membrane with immobile particles. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 89, 022724 (2014). [DOI] [PubMed] [Google Scholar]
- 61.Oppenheimer N., Diamant H., In-plane dynamics of membranes with immobile inclusions. Phys. Rev. Lett. 107, 258102 (2011). [DOI] [PubMed] [Google Scholar]
- 62.Singh R. R., Sangani A. S., Daniel S., Koch D. L., The combined hydrodynamic and thermodynamic effects of immobilized proteins on the diffusion of mobile transmembrane proteins. J. Fluid Mech. 877, 648–681 (2019). [Google Scholar]
- 63.Holowka D., Baird B., Nanodomains in early and later phases of FcεRI signalling. Essays Biochem. 57, 147–163 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Honigmann A., Pralle A., Compartmentalization of the Cell Membrane. J. Mol. Biol. 428 (24 Pt A), 4739–4748 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Kenworthy A. K., Have we become overly reliant on lipid rafts? Talking Point on the involvement of lipid rafts in T-cell activation. EMBO Rep. 9, 531–535 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown D. A., Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology (Bethesda) 21, 430–439 (2006). [DOI] [PubMed] [Google Scholar]
- 67.Lee A. G., Lipid-protein interactions. Biochem. Soc. Trans. 39, 761–766 (2011). [DOI] [PubMed] [Google Scholar]
- 68.Machta B. B., Veatch S. L., Sethna J. P., Critical Casimir forces in cellular membranes. Phys. Rev. Lett. 109, 138101 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Turner H., Kinet J.-P., Signalling through the high-affnity IgE receptor FCeRI. Nature 402, B24–B30 (1999). [DOI] [PubMed] [Google Scholar]
- 70.Ingley E., Src family kinases: Regulation of their activities, levels and identification of new pathways. Biochim. Biophys. Acta 1784, 56–65 (2008). [DOI] [PubMed] [Google Scholar]
- 71.Mohr J. D., “Characterizing membrane receptor signaling: New roles for electrostatic residues in EGFR regulation and actin in FcεRI signaling,” PhD dissertation, Biomedical and Biological Sciences (2019).
- 72.Thomas J. L., Holowka D., Baird B., Webb W. W., Large-scale co-aggregation of fluorescent lipid probes with cell surface proteins. J. Cell Biol. 125, 795–802 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schindelin J., et al., Fiji: An open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.