Significance
Many animals depend on maternally transmitted symbiotic bacteria that provide nutrients or other benefits. The evolution of these symbionts is complicated: natural selection can act on hosts, favoring symbionts that increase host reproduction, or on symbionts, favoring symbionts that spread within hosts. Furthermore, transmission bottlenecks can facilitate the spread of mutations deleterious to both. By measuring changes in frequencies of symbiont genotypes within individual insect matrilines, we estimated within-host selection and transmission bottleneck size. Results revealed surprisingly strong selection, with some symbiont genotypes more successful in colonizing progeny, as well as a severe transmission bottleneck, consistent with observations of deleterious mutation accumulation in symbiont genomes. Findings elucidate the forces driving evolution of heritable symbionts and generating their distinctive genomic features.
Keywords: endosymbiosis, within-host selection, levels of selection, mutualism, maternal transmission
Abstract
Numerous animal lineages have maternally inherited symbionts that are required for host reproduction and growth. Endosymbionts also pose a risk to their hosts because of the mutational decay of their genomes through genetic drift or to selfish mutations that favor symbiont fitness over host fitness. One model for heritable endosymbiosis is the association of aphids with their obligate bacterial symbiont, Buchnera. We experimentally established heteroplasmic pea aphid matrilines containing pairs of closely related Buchnera haplotypes and used deep sequencing of diagnostic markers to measure haplotype frequencies in successive host generations. These frequencies were used to estimate the effective population size of Buchnera within hosts (i.e., the transmission bottleneck size) and the extent of within-host selection. The within-host effective population size was in the range of 10 to 20, indicating a strong potential for genetic drift and fixation of deleterious mutations. Remarkably, closely related haplotypes were subject to strong within-host selection, with selection coefficients as high as 0.5 per aphid generation. In one case, the direction of selection depended on the thermal environment and went in the same direction as between-host selection. In another, a new mutant haplotype had a strong within-host advantage under both environments but had no discernible effect on host-level fitness under laboratory conditions. Thus, within-host selection can be strong, resulting in a rapid fixation of mutations with little impact on host-level fitness. Together, these results show that within-host selection can drive evolution of an obligate symbiont, accelerating sequence evolution.
Eukaryotic hosts often depend on symbiotic microorganisms for survival, growth, and reproduction. The most ancient of such symbionts, the mitochondria, are central to metabolism of almost all eukaryotic cells. A myriad of other bacterial symbionts have evolved intimate partnerships with protozoans, fungi, plants, and animals. Obligate symbionts, including mitochondria and many others, are often maternally transmitted to offspring, a feature that ensures the short-term benefit of reliable inoculation of progeny but incurs the long-term drawback of enforcing strict clonality and small genetic population size on symbionts, leading to increased fixation of deleterious mutations (1, 2). In the long term, exclusively uniparental transmission results in degraded symbiont genomes featuring fewer intact genes and reduced thermal stability of remaining gene products (1, 3). Furthermore, selection on symbiont populations within individual hosts potentially leads to the spread of selfish symbiont mutations that benefit symbionts but reduce host fitness (4). In human mitochondria, for example, mutations favored at the organelle level can increase in frequency in the mother’s germline, causing deleterious effects in progeny (5).
The spread of symbiont alleles harmful to hosts, whether broadly deleterious or selfish, will be countered by selection on individual hosts. The effectiveness of host-level selection depends on several parameters. First, the host effective population size (NHOST) affects efficacy of host-level selection (and for maternally transmitted symbionts only the female population size is relevant). Multicellular hosts (i.e., animals and plants) typically have small population sizes compared to bacteria, so asexual genomic components, such as maternally transmitted symbionts or Y chromosomes, will usually accumulate deleterious mutations (6, 7).
A second factor that influences the effectiveness of host-level selection on symbiont alleles is the size of the genetic bottleneck (NSYM) imposed by the transmission cycle (8–10). NSYM reflects both the size of the inoculum transferred from mother to daughter and also the packaging of symbionts into host cells during development. A large NSYM enables more effective selection on symbionts within hosts, with contradictory effects depending on the type of symbiont mutations: it will enhance purifying selection against mutations deleterious to both symbiont and host but will promote the spread of selfish mutations that harm hosts. Examples of such mutations include any that increase symbiont replication rate and deplete host resources.
The symbiosis of the pea aphid, Acyrthosiphon pisum, with its mutualistic bacterial symbiont Buchnera aphidicola is a model for maternally transmitted obligate symbiosis (11). Buchnera has a small genome encoding only 583 genes but retains pathways for synthesis of essential amino acids needed by hosts (12). Buchnera is packaged within specialized aphid cells, bacteriocytes, and is transmitted to progeny within the mother’s body. Most aphid reproduction is asexual and viviparous, with female embryos developing prenatally and born in order as they descend the maternal ovariole. Buchnera colonizes through exocytosis from a maternal bacteriocyte and endocytosis by the syncytial cell of a nearby early-stage embryo (13). Buchnera replicates within the embryo where it is packaged into about 80 bacteriocytes (14). Each embryo is colonized by symbionts from one, or possibly two, maternal bacteriocyte(s), so the inoculum is not randomly drawn from the entire maternal population (13). Thus, NSYM is a function of the number of Buchnera cells transferred and also of the packaging that occurs during development.
In this study, we address three questions regarding Buchnera evolution within host matrilines: 1) Does within-host selection occur? 2) What is the effective bottleneck size (NSYM), imposed by a transmission cycle within a matriline? 3) Does within-host selection oppose or support selection at the host level; that is, do symbionts evolve to be selfish, neutral, or beneficial? To address these questions, we generated A. pisum clones heteroplasmic for closely related Buchnera haplotypes using a modification of a previously established microinjection method (15). We then measured haplotype frequencies over five generations in mothers and daughters sampled at the same developmental stage using deep sequencing of a diagnostic nucleotide difference to obtain precise estimates of frequencies. We measured intergenerational changes in frequencies in order to estimate NSYM and sSYM, the magnitude of within-host selection on symbionts. We demonstrate that within-host selection can be surprisingly strong. Drift due to transmission bottlenecks is also substantial, as NSYM is far smaller than the number of Buchnera cells transferred to daughters. Finally, in order to address whether a mutation strongly favored within hosts affects fitness of host individuals, we assessed selection on aphids fixed for Buchnera haplotypes that differed strongly in within-host fitness.
Results
Visualizing Haplotype Frequencies within Individual Aphids over Generations.
Using microinjection, we established aphid matrilines with intermediate frequencies of two Buchnera haplotypes, then sampled successive female generations at the same developmental stage (adults at initiation of reproduction) in the absence of host-level selection (Fig. 1). The Buchnera haplotypes were near identical (SI Appendix, Fig. S1 and Table S1). We performed two experiments corresponding to two haplotype pairs, all within a single background aphid genotype. One experiment included Buchnera genotypes (5AY and LSR1) that differ at 19 single base indels or changes (12 intergenic or silent) including a single base difference known to sharply affect thermal tolerance. A second experiment included Buchnera genotypes (5A and LSR1) that differ at 17 locations (10 intergenic or silent) not predicted to affect fitness. Both experiments were carried out under two thermal environments: constant cool conditions (20 °C) or daily 4-h exposure to heat (33 °C). We obtained accurate estimates of haplotype frequencies in each aphid using high-throughput sequencing of amplicons across a diagnostic polymorphic site. In all, we sampled 732 aphids, which gave 165, 165, 182, and 175 mother–daughter pairs for the four combinations of Buchnera haplotypes and environments.
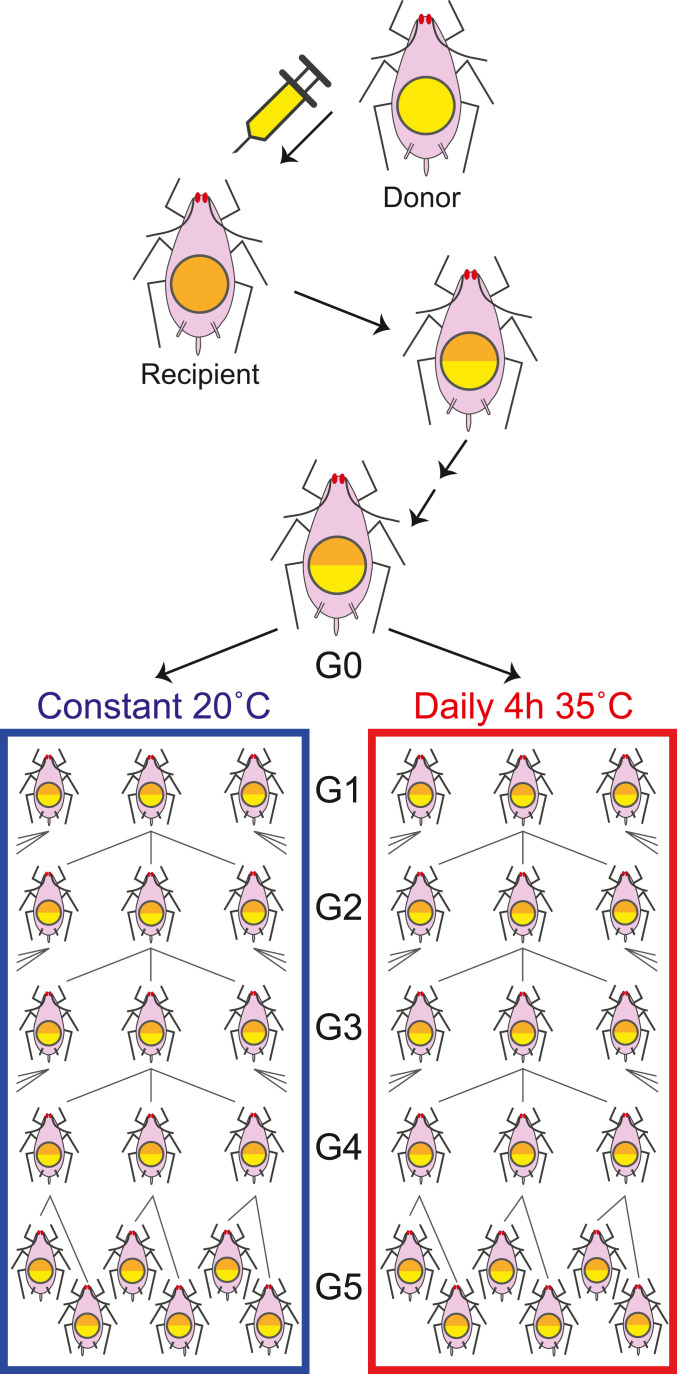
Fig. 1.
Experimental design for measuring within-host selection and drift of Buchnera. A single female was injected with Buchnera from a donor line to establish a clonal aphid line heteroplasmic for two Buchnera haplotypes. After three to four generations, a founder female (G0) was used to initiate experiments in which successive female generations were allowed to reach adulthood and reproduce for 1 d and then harvested to estimate Buchnera haplotype frequencies. Three progeny were allowed to develop in each generation, and all aphids were sampled 1 d after beginning to reproduce. Experiments were performed under two thermal environments: constant 20 °C (“Cool”) and constant 20 °C interrupted daily by 4 h of exposure to 33 °C (“Heat”).
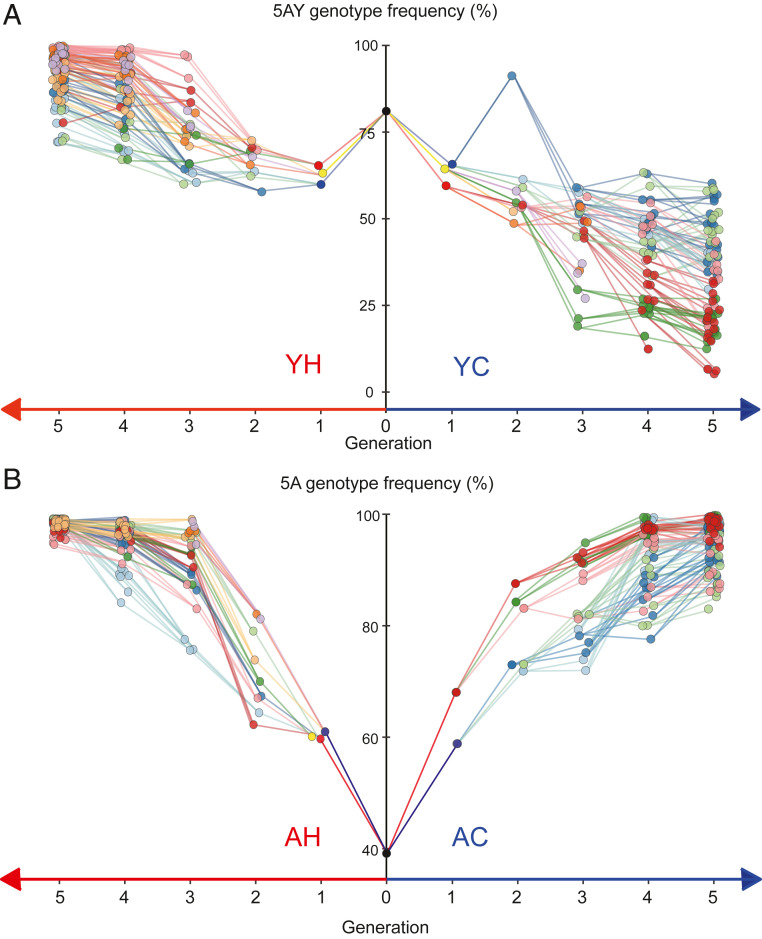
Haplotype frequencies for each aphid are presented in two formats: changes in frequencies within individual matrilines over five aphid generations (Fig. 2) and haplotype frequencies in mothers versus their daughters for each mother–daughter pair (Fig. 3). Visual inspection suggests strong within-host selection for both haplotype combinations and for both temperature conditions. As expected, Buchnera 5AY increases under heat exposure, where it approaches fixation in all lines after five generations and has a disadvantage at constant 20 °C, although all matrilines are still polymorphic at the end of the experiment (Fig. 2A). For the pair for which no difference in fitness was predicted, we found instead that Buchnera 5A increases relative to Buchnera LSR1 under both environments, with daughters having higher Buchnera 5A frequencies than their mothers (Fig. 3B). Buchnera 5A is fixed or nearly fixed after five generations in every matriline and at both temperatures (Fig. 2B).
Fig. 2.
Buchnera haplotype frequencies within individual mothers and daughters, over five generations. (A) Frequencies starting with a single female heteroplasmic for Buchnera haplotypes 5AY and LSR1. (B) Frequencies in aphids starting with a single female heteroplasmic for Buchnera haplotypes 5A and LSR1. (A and B, Right) Lines kept at constant 20 °C (“Cool” treatment), and (Left) lines subjected daily to 4 h at 33 °C (“Heat” treatment). Lines connect the mother–daughter pairs and are colored to enable visualization of individual matrilines within experiments. For Generations 0 to 3, each mother produced three sampled daughters, each of which became mothers for the next generation. Generation 4 mothers each produced two daughters, and Generation 5 was the last one sampled. In some cases, an aphid died, ending that subline prematurely. The overall death rate was 8.0%.
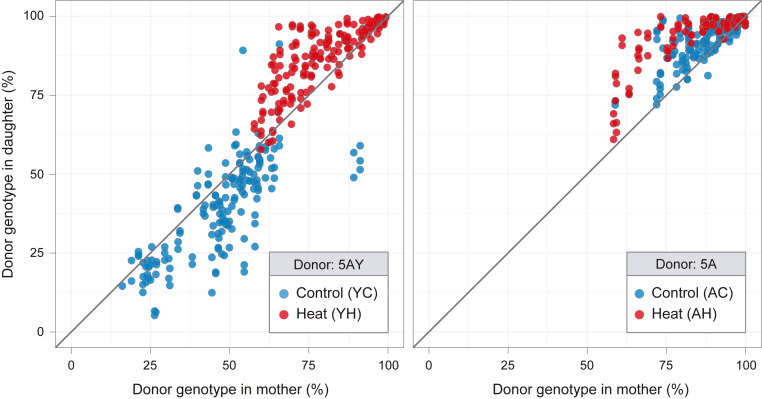
Fig. 3.
Buchnera haplotype frequencies in mothers and their daughters. Frequencies in mothers are compared to frequencies in daughters for (A) 5AY-LSR1 heteroplasmic lines and (B) 5A-LSR1 heteroplasmic lines. The diagonal line corresponds to equal frequencies in mother and daughter, sampled at the same age. Samples for 5AY-Heat, 5AY-Cool, 5A-Heat, and 5A-Cool included 165, 165, 182, and 175 mother–daughter pairs, respectively.
To understand the dynamics of haplotype frequencies within hosts, we estimated the effective bottleneck size (NSYM) and the selection coefficient for the introduced haplotype (sSYM) using a maximum likelihood approach. The estimated parameters summarize the net effects of drift and selection through a full generation cycle, with NSYM and sSYM assumed constant. For example, sSYM is assumed to be frequency independent. We identified the values of NSYM and sSYM that maximize the likelihood of our observed frequencies and estimated the 95% confidence regions for both parameters (Fig. 4).
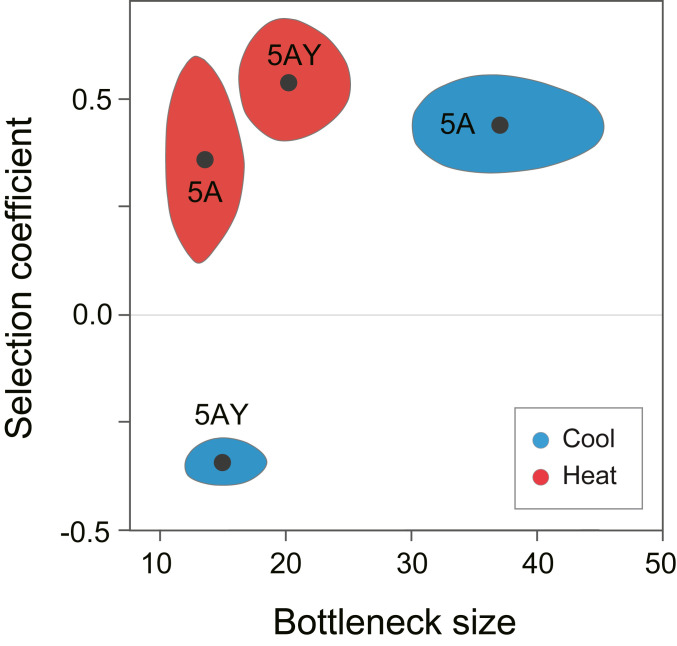
Fig. 4.
Bottleneck size and selection coefficient estimates. The black dots are maximum likelihood estimates of NSYM and sSYM for the two combinations of Buchnera haplotypes and the two temperature treatments. The shaded areas correspond to the 95% confidence regions for the estimates.
Within-Host Selection: Near-Identical Buchnera Haplotypes Differing in Thermal Tolerances.
As expected, Buchnera 5AY has a clear advantage within matrilines when aphids are exposed to heat as juveniles: the maximum likelihood estimate for sSYM = 0.54 (95% CI: ∼0.41 to 0.67) (Fig. 4). This result is consistent with previous evidence that the responsive ibpA heat-shock promoter, present in Buchnera 5AY but absent in Buchnera LSR1, improves survivorship of Buchnera within aphid hosts following heat exposure (15, 16). In contrast, Buchnera LSR1 has the advantage under constant 20 °C, with the highest likelihood sSYM = −0.36 (95% CI: ∼ −0.30 to −0.41). Previously, the nonresponsive Buchnera ibpA promoter was shown to benefit aphid fitness under constant 20 °C (16). Our results suggest that the host-level advantage at each temperature reflects better survivorship of Buchnera corresponding to that condition. Thus, for this haplotype combination, within-host selection acts in the same direction as between-host selection but in opposite directions depending on temperature.
Unexpectedly Strong Within-Host Selection on Near-Identical Buchnera Haplotypes Expected to Be Neutral.
Unexpectedly, Buchnera 5A had a strong selective advantage, under both the cool and heat environments. We estimated sSYM for Buchnera 5A versus Buchnera LSR1 at 0.45 (95% CI: ∼0.23 to 0.61) and 0.37 (95% CI: ∼0.33 to 0.54) for the two environments (Fig. 4). To verify that we had a complete catalog of genetic differences, we resequenced representative lines at the end of the experiment and were surprised to discover four new mutations in the Buchnera 5A main chromosome and two mutations on the plasmid encoding trpEG. A haplotype with these new mutations had become fixed by the end of the experiment; we named the derived line 5AE. Chromosomal mutations included a 13-nucleotide duplication within an intergenic spacer plus three single-nucleotide substitutions (one intergenic, one synonymous, and one nonsynonymous); plasmid mutations included one synonymous and one single base deletion in an intergenic spacer (SI Appendix, Table S1). We designed PCR primers to investigate the time of origin of the new mutations and discovered that the heteroplasmic female initiating the experiment (Generation 0) contained a mixture of Buchnera 5A and Buchnera 5AE, along with Buchnera LSR1. Buchnera 5AE increased in frequency over time, such that both the original Buchnera 5A and Buchnera LSR1 were eliminated or nearly eliminated by Generation 5. Although our diagnostic polymorphism for distinguishing Buchnera 5A from Buchnera LSR1 did not distinguish 5A from 5AE, the fitness advantage must have been one of the new mutations in Buchnera 5AE, which eliminated or nearly eliminated the original Buchnera 5A haplotype.
Our model for estimating NSYM and sSYM assumes that these parameters are constant during our experiment. However, for the Buchnera 5A/Buchnera LSR1 experiment, this assumption is likely violated since Buchnera 5A was itself a mixture of haplotypes (5A and 5AE) of changing frequencies. Clearly, selection strongly favors Buchnera 5AE, but the maximum likelihood estimates of sSYM and NSYM may be inaccurate for the Buchnera 5A-Buchnera LSR1 combination.
Measuring the Transmission Genetic Bottleneck (NSYM).
We expected NSYM to be similar across experimental trials, reflecting mechanisms for symbiont packaging and transmission in this aphid species. Indeed, NSYM estimates fell into a fairly narrow range for most trials, with maximum likelihood estimates of 15 and 20 for Buchnera 5AY under the two conditions and 37 and 13 for Buchnera 5A under the two conditions (Fig. 4). The values for the Buchnera 5A combination are less reliable since the magnitude of selection on the Buchnera 5A+5AE combined haplotype changed over time as the 5AE haplotype increased in frequency. In contrast, no new mutations occurred in the Buchnera 5AY trials (in either haplotype), so the assumption of a constant selective coefficient is reasonable. Thus, the value of NSYM appears to be in the range of 10 to 20. Effectively, this means that the level of drift is equivalent to drawing 10 to 20 Buchnera genomes at random from the maternal population for inoculation of each progeny.
Between-Host Selection on Buchnera Haplotypes Differing in Within-Host Fitness.
Because Buchnera 5AE had a strong within-host fitness advantage under both thermal environments, we wondered whether this haplotype was beneficial or neutral at the host level or whether it was selfish (i.e., harmful to hosts). We performed experiments to measure whether aphid fitness differed between aphids with the same host genotype (A. pisum LSR1) but fixed for the two Buchnera haplotypes (5AE versus LSR1). First, we compared three fitness-related parameters—time from birth to first reproduction, adult mass, and fecundity—for the two matrilines. Aphids fixed for Buchnera 5AE developed faster, with average first reproduction of 9.5 versus 10.2 d for aphids fixed for Buchnera LSR1 (P < 0.001, analysis of covariance (ANCOVA); SI Appendix, Fig. S2). They were also heavier at an average of 4.34 mg as compared to 3.98 mg (P < 0.001, ANCOVA; SI Appendix, Fig. S2). However, greater body mass did not correspond to greater fecundity, measured as the number of progeny in first 7 d of reproduction; in fact, the trend was in the opposite direction [i.e., higher fecundity for the line bearing Buchnera LSR1 (P = 0.077 by ANCOVA) (SI Appendix, Fig. S2)]. These measures of aphid fitness parameters showed considerable variation.
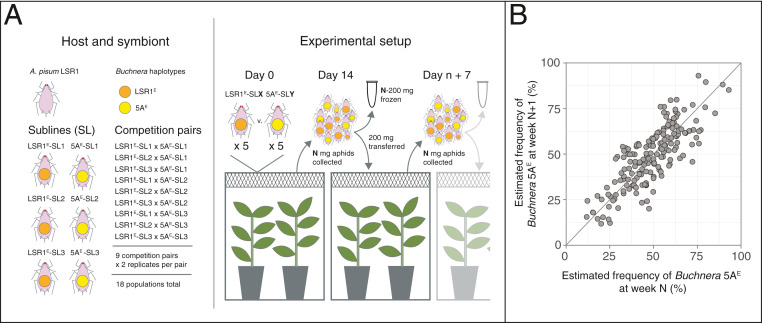
To obtain a direct measure of selection on aphid lines fixed for the two Buchnera haplotypes, we allowed the lines to compete directly in larger cages, transferring a large number of randomly sampled, mixed-age aphids to new plants weekly to avoid population bottlenecks. This experiment to estimate sHOST paralleled those for within-host selection to measure sSYM, but the selection interval was 1 wk rather than one aphid generation (Fig. 5A). We used weekly pooled aphid samples to estimate frequencies of the two Buchnera haplotypes in the sample and verified this approach with a standard curve based on known ratios of aphids from the two lines (SI Appendix, Fig. S3). We thus obtained frequencies in weekly population samples for the 18 replicate competition experiments (SI Appendix, Fig. S4) and used these to calculate pairwise frequencies for successive weeks for each replicate, over 12 wk. We used SI Appendix, Eq. 1 to calculate the selection coefficient at the host level, sHOST, as applicable to the weekly sampling time. We obtained a maximum likelihood sHOST = 0.0016 (95% CI: ∼−0.061 to 0.063). Thus, the Buchnera 5AE haplotype appears neutral or near neutral at the host level, despite its strong within-host advantage over Buchnera LSR1.
Fig. 5.
Measuring host-level selection on Buchnera haplotypes 5AE and LSR1 in a common aphid genotype background. (A) Experimental design included first growing each of the two lines in sublines to control for maternal effects due to variable rearing conditions. Aphids fixed for alternative Buchnera haplotypes were then competed in cages with plants, sampled and transferred weekly for 12 wk, in 18 replicate populations. (B) Buchnera haplotype frequencies in pooled population samples in successive weeks. The diagonal line corresponds to equal frequencies in successive samples. n = 204 population samples.
Recombination.
We resequenced fixed haplotypes at the end of the experiments and found no recombinants. Thus, even when Buchnera haplotypes reside in the same host, they do not recombine, at least not often enough to appear in our experiments. The polymorphisms separating the haplotypes are scattered around the Buchnera chromosome, so any longer-range recombinants would likely have been detected.
Discussion
Within-Host Selection and Consequences for Host Fitness.
The most surprising result from these experiments was the occurrence of very strong within-host selection in every trial: some Buchnera haplotypes are much better able than others to compete within heteroplasmic mothers for transmission to the next generation. In the case of the 5AY–LSR1 heteroplasmy (Figs. 2 and 3), within-host selection is in the same direction as between-host selection, and the direction of selection depends on the thermal environment. Previous studies, based on Buchnera haplotypes in their native hosts, showed that the ibpA promoter mutation had a strong effect on the heat-shock response of IbpA, a small heat-shock protein, and that the single base deletion increased or decreased aphid survival, developmental time, and fecundity depending on heat exposure (16, 17). We found that haplotypes that differed in this mutation were selected within hosts and in the same direction as between hosts in each environment. In this case, within-host selection benefits hosts, enabling each mother to produce daughters with fitness higher than her own, provided they live under the same thermal conditions. Since hosts require Buchnera for nutrient provisioning and normal development, it is logical that some mutations that affect Buchnera fitness within hosts would affect aphid fitness in the same direction. This within-host selection is similar to germline selection within individuals, in the sense that the large number of genes needed for basic cellular functions undergo purifying selection during replication within hosts (18). Such germline selection also appears to operate on human mitochondrial haplotypes in heteroplasmic human mothers (5).
In the case of the Buchnera 5AE–5A–LSR1 heteroplasmy of the second experiment, we expected no selection, given the near identity of the haplotypes (SI Appendix, Table S1). Surprisingly, we found strong within-host selection favoring Buchnera 5AE over both Buchnera 5A and Buchnera LSR1, under both temperature conditions. Using a long-term selection experiment on aphids fixed for alternative Buchnera haplotypes, we found that the 5AE haplotype was neutral or close to neutral at the host level.
The absence of a host-level cost raises the question of why the advantageous mutation (or combination of mutations) of Buchnera 5AE has not arisen and become fixed through within-host selection in natural populations. A possible explanation is that neutrality at the host level reflects the favorable and invariant conditions of our laboratory selection experiment: these include a constant supply of nutrient-rich seedling host plants, absence of natural enemies, and absence of some life stages (winged females or sexual generations). The Buchnera 5AE haplotype might be deleterious to hosts during nutrient limitation or during the sexual stages.
Our experiments did not identify the nature of the advantage of Buchnera 5AE over Buchnera LSR1 within hosts. Potentially, Buchnera 5AE replicates faster during some or all stages of host development; alternatively, Buchnera 5AE may have some advantage in colonizing embryos. None of the five observed mutations of Buchnera 5AE (SI Appendix, Table S1) has an obvious functional implication. One speculation is that one of the two mutations on the Trp plasmid reduces tryptophan provisioning, a host-beneficial contribution of Buchnera to hosts; the energy savings might thereby enable these Buchnera cells to replicate faster.
Buchnera 5AE arose from Buchnera 5A at the beginning of our second selection experiment and featured six mutations in the founding females. This sudden appearance of multiple mutations is curious, as previous deep sequencing of Buchnera genomes of lines descending from a single female and maintained separately in the laboratory revealed only two single base substitutions during 14 y of evolution in the laboratory (19). Our resequencing of Buchnera haplotypes is otherwise consistent with this low rate [e.g., three base substitutions occurred during 18 y in the lineage leading to Buchnera 5AY (SI Appendix, Fig. S1)]. We verified that the Buchnera 5AE haplotype arose from the Buchnera 5A haplotype, as these share several unique polymorphic sites in common (SI Appendix, Table S1). Some mutagenic condition seems to have impacted Buchnera 5AE, potentially during the microinjection protocol, which may impose cellular stress when Buchnera cells are released from bacteriocytes of their original host and subjected to artificial conditions.
Within-Host Genetic Drift.
Another surprising result from these experiments is the small estimate for NSYM, at 10 to 20. Previously published direct counts of Buchnera cells entering developing A. pisum embryos show that ∼200 Buchnera cells are transferred, although this number is difficult to ascertain as the cells are actively dividing as they enter the embryo (20). Flow cytometry–based counts indicate that Buchnera multiplies rapidly after being packaged into bacteriocytes, with numbers increasing by 460-fold from the late embryo stage to the adult stage (21). A smaller NSYM is likely enforced by developmental packaging; the inoculum for each embryo is drawn from one or sometimes two bacteriocytes (13). Buchnera replicates actively within bacteriocytes; therefore, in heteroplasmic mothers, Buchnera haplotypes are expected to be nonrandomly sorted into bacteriocytes, lowering NSYM. However, results also imply that single bacteriocytes contain both haplotypes, since we would otherwise expect complete loss of one or the other haplotype during a single transmission cycle.
The low estimate of NSYM implies that many mutations that are deleterious within hosts can be fixed, since such deleterious mutations will be effectively neutral if the selection coefficient is less than 1/NSYM (22). A large proportion of new mutations are likely deleterious for both symbiont and host; these would include any mutations that reduce the function or stability of essential genes. Essential genes make up most of the Buchnera genome (11, 12) and thus present a large mutational target. Buchnera and many other obligate symbiont lineages exhibit a distinctive genomic syndrome of accelerated sequence evolution and reduction in genome size and number of genes. This syndrome is attributed to increased rates of fixation of slightly deleterious mutations, leading to increased ratios of nonsynonymous to synonymous changes in protein-coding genes (higher dN/dS) and to inactivation and loss of many genes (23). Small values of NSYM are likely a major basis of this genomic syndrome. Variation in NSYM among systems of maternally transmitted symbioses, due to differences in transmission mechanisms or symbiont packaging within hosts, may contribute to variation in the extent of this genomic degradation. Within-host selection leading to rapid fixation of mutations, as we observed for Buchnera 5AE, could also contribute to the observed high rates of sequence evolution in symbiont genomes.
A small NSYM also leads to low within-host polymorphism, consistent with observations that obligate symbionts are almost always genetically homogeneous. For example, with NSYM of 10, the time to fixation for a completely neutral mutation is about 20 aphid generations, about the length of one season (24). Mutations under selection are fixed more rapidly. These estimates apply to dynamics within a single matriline and not to the aphid population: drift may fix different Buchnera mutations in different matrilines.
The estimate of NSYM for Buchnera is close to the estimated bottleneck size of 10 to 20 for a transmission cycle of human mitochondria, in which drift can lead to high frequencies of deleterious haplotypes in offspring (5, 25). In humans, drift in heteroplasmic mothers can elevate frequencies of deleterious mitochondrial haplotypes, such that offspring exhibit fitness deficits when mothers were not affected.
Lack of Recombination within Hosts.
Recombination will only make a difference if at least two loci are polymorphic within a host, but this situation is likely rare in Buchnera given the general lack of within-host polymorphism. A study using deep sequencing of Buchnera genomes of several A. pisum lines revealed no sites with intermediate frequency polymorphisms (19). Thus, Buchnera is effectively clonal, because haplotypes diverging at more than one locus are almost always segregated into separate matrilines. In our experiments, we had the opportunity to test for the occurrence of recombination within hosts because we generated heteroplasmic matrilines that contained Buchnera haplotypes differing at several sites around the genome. We resequenced lines that became fixed for a haplotype and observed no recombinants, implying that recombination does not occur within hosts or occurs rarely enough that we did not observe it. Buchnera has a very stable genome architecture, with almost no genomic rearrangements or gene acquisition across more than 100 million y of evolution (26, 27). Both the absence of recombination and the stability of the genome may reflect the loss of recombination pathways. In particular, Buchnera is one of the few bacteria known to lack the recA pathway, which is required in Escherichia coli for homologous recombination.
Implications of Within-Host Selection and Drift for the Evolution of Symbiosis.
The clonality and small population size of obligate endosymbiont genomes results in rapid evolution of both DNA and protein sequences and loss of many ancestral genes (6, 7). These features are characteristic of all obligately maternally transmitted symbionts for which genomes have been sequenced and has resulted in the tiniest known bacterial genomes (23). The extent of within-host selection has not been quantified for any obligate endosymbionts, except for mitochondria (5). We observed strong evidence of within-host selection in every trial, often resulting in fixation within a few generations. Positively selected mutations are of course part of Buchnera evolution, and our results suggest they may be frequent. However, these likely do not cause selective sweeps that affect other loci: multiple Buchnera loci are rarely polymorphic within a host.
For a maternally transmitted symbiont, the optimal NSYM from the host perspective is a compromise between increasing NSYM to control spread of uniformly deleterious mutations and decreasing NSYM to control spread of selfish mutations. Inoculation appears to be a largely host-controlled process (e.g., ref. 13), but selection on hosts to optimize NSYM may be ineffective if deleterious consequences of symbiont evolution are mostly long term. We found that NSYM is very small but that within-host selection was nonetheless effective due to large sSYM values. Potentially, within-host selection has both positive and negative effects on aphid fitness in this symbiosis. Mutations deleterious to both symbiont and host are almost certainly frequent, as most mutations in coding genes will destabilize or inactivate the encoded protein; thus, within-host selection benefits hosts. However, selfish symbiont mutations also might be common: symbionts generally have low replication rates adjusted to host development, and mutations that release this control will spread within hosts, despite host fitness costs. Frank (28) suggested that in some maternally inherited symbioses in insects, hosts sequester a population of symbionts to serve as inoculum, thereby limiting within-host selection, but separation of germline and somatic symbionts is not apparent from detailed studies of transmission in the aphid/Buchnera symbiosis (13).
A somewhat parallel case of strong within-host selection has been documented for facultative symbionts within Drosophila melanogaster. Wolbachia haplotypes with increased copy numbers of a 21-kb region called “Octomom” proliferate more, with negative consequences for host survivorship (29). However, in the presence of viral pathogens, the higher titers can give an enhanced protective effect thereby increasing fly survivorship. Thus, the consequences of a selfish symbiont for host-level fitness can be dependent on the environmental conditions. The aphid/Buchnera symbiosis shows that within-host selection can occur even in all-female generations within an obligate symbiosis, in which host and symbiont fitness are expected to be tightly linked due to shared reproductive interests.
Our results show that, in the aphid/Buchnera symbiosis, a new mutation can undergo strong positive selection within hosts and quickly spread to fixation during a time span much shorter than an annual seasonal cycle. For the case we studied under laboratory conditions, no strong consequences for host fitness were observed. However, we did observe effects on host phenotypes (body mass and time to first reproduction), and this host-level selective neutrality may not apply to all mutations favored within hosts. Thus, attention to within-host dynamics is critical to understanding evolution of maternally transmitted symbioses.
Materials and Methods
A detailed account of methods is given in SI Appendix; only the general experimental framework is described here.
Buchnera haplotypes used in this study (LSR1, 5A, and 5AY) are closely related, with a maximum of 21 nucleotide differences genome wide (19) (SI Appendix, Table S1). A single aphid nuclear genotype, A. pisum LSR1, was used for all experiments. Buchnera LSR1 was the “resident” haplotype, and two foreign haplotypes, Buchnera 5A and Buchnera 5AY, were “donor” haplotypes injected to establish two heteroplasmic lines (LSR1-5AY and LSR1-5A) using a method similar to that previously reported (15). Prior to the experiment, A. pisum LSR1, 5A, and 5AY were screened for the presence of additional bacterial symbionts using universal bacterial primers (559F and 35R) that amplify essentially all bacteria excluding Buchnera (30). These aphid lines were found to be singly infected with Buchnera. We also resequenced representative lines at the end of the experiments to identify any new Buchnera mutations, and no other bacterial sequences were retrieved in screens of these datasets.
To generate aphid lines heteroplasmic for Buchnera haplotypes, reproductive A. pisum LSR1 were placed on seedlings and allowed to deposit nymphs for 24 h to generate age-controlled recipient aphids. At 4 d old, recipient aphids were exposed to 35 °C for 4 h to reduce resident Buchnera LSR1 titer and were returned to 20 °C for ∼24 h before injections were performed. On the day of the injections, adult donor aphids possessing Buchnera 5A or 5AY were ground individually in 30 µL Buffer A (25 mM KCl, 10 mM MgCl2, 250 mM sucrose, 35 mM Tris HCl, adjusted to pH 7.5). Aphid homogenate was injected into the ventral abdominal segments nearest to the posterior legs, delivering ∼0.1 μL homogenate per injection. Injections of Buchnera 5A and 5AY were performed on separate days.
Injected aphids were placed on fava leaves in Petri dishes for 24 h and survivors transferred to seedlings. Offspring born 9 to 12 d after injection were transferred to individual dishes containing a single fava leaf in 1.5% agar, where they were allowed to mature to adulthood and reproduce for 1 d. Mothers were then screened for Buchnera 5A and 5AY using PCR and a diagnostic restriction digest, as previously described (15). Offspring from mothers that tested positive for both recipient Buchnera LSR1 and donor Buchnera 5A or 5AY were retained, including 3 LSR1-5A mothers (of 148 screened) and 5 LSR1-5AY mothers (of 152 screened). After three generations, we began experiments using individual aphids from matrilines in which sampled aphids had intermediate haplotype frequencies, based on band intensities following PCR and restriction digest. For both combinations (5A-LSR1 and 5AY-LSR1), a single female (G0) was used to initiate the experiment. G0 females were allowed to reproduce for several days and were then sampled; haplotype proportions were later determined to be 38.0% 5A in the LSR1-5A G0 female and 81.0% 5AY in the LSR1-5AY G0 female (as shown in Fig. 2). Because they were older when harvested, G0 females were not included in later analyses. All further generations were sampled after 1 d of reproduction.
We conducted two within-host selection experiments, each initiated with a single female. The LSR1-5AY haplotypes differed in a single base deletion in the promoter for a small heat-shock gene ibpA, and these haplotypes were expected to be under temperature-dependent selection, favoring Buchnera 5AY under heat and Buchnera LSR1 under cool conditions. The LSR1-5A haplotypes showed a few differences that were expected to be neutral, so we predicted no within-host selection. In each experiment, selection occurred under two environments: cool (constant 20 °C) and heat (20 °C interrupted by 4 h at 33 °C during each 24-h cycle). We estimated Buchnera haplotype frequencies in individual females over five generations by sampling females at the same developmental stage in each generation. In all, we estimated haplotype frequencies for 330 and 357 mother–daughter pairs for the LSR1-5AY and LSR1-5A experiments, respectively. Frequencies in individual females were estimated using PCR across a single-nucleotide polymorphism, then using Illumina sequencing of amplicons to compute the frequency of each type.
We used a likelihood approach to quantify selection and drift acting on Buchnera. Drift reflects the bottleneck that occurs during inoculation and during developmental packaging into bacteriocytes, and so we refer to this population size as the “bottleneck size,” NSYM. NSYM can be regarded as an effective population size describing the drift acting on symbionts that occurs during a single aphid generation. Likewise, selection acts on Buchnera while it inhabits its host and during transmission to progeny, so sSYM can be regarded as a selection coefficient describing the cumulative selective forces that occur throughout a single aphid generation. Details of the likelihood model are given in SI Appendix.
We resequenced Buchnera genomes at the end of the experiment using Illumina iSeq to verify all polymorphisms, including any that arose during the experiment. We discovered that the Buchnera 5A haplotype had produced a descendant with several mutations, and we named this haplotype Buchnera 5AE.
To determine whether the fitness advantage observed within hosts had an effect on between-host selection, we assessed fitness parameters of individual aphids with the same nuclear genotype (LSR1) but with different Buchnera haplotypes: Buchnera 5AE and Buchnera LSR1. We also used these aphid lines in a long-term competition experiment in which we transferred aphids to new plants each week for 12 wk, using a large transfer population to minimize chance sampling effects. We sampled the population at each transfer date and estimated frequencies of the two aphid types by extracting DNA from these population samples, which pooled many individuals. Both transferred and harvested samples consisted of randomly selected, mixed-age aphids from the total population. For each sample, we performed PCR over a polymorphic 13 base pair insertion and sequenced amplicons in a single run of Illumina iSeq (as for the within-host experiment). We constructed a standard curve with known proportions of aphids of the two lines to verify this method of estimating population frequencies (SI Appendix, Fig. S3). We applied a similar maximum likelihood approach to that used for the within-host experiment, to obtain an estimate of the between-host selection coefficient, sHOST.
Supplementary Material
Acknowledgments
Funding came from NSF Award 1551092 to N.A.M., a Provost’s Graduate Excellence Fellowship to J.P., NIH Grant R01-GM116853 to M.K., and the China Scholarship Council and the Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences to B.Z. We thank Kim Hammond for maintaining aphids, plants, and laboratory supplies and for work on figures, Eli Powell for technical assistance, and Yiyuan Li for sharing bioinformatic scripts.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2102467118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.McCutcheon J. P., Boyd B. M., Dale C., The life of an insect endosymbiont from the cradle to the grave. Curr. Biol. 29, R485–R495 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Moran N. A., McCutcheon J. P., Nakabachi A., Genomics and evolution of heritable bacterial symbionts. Annu. Rev. Genet. 42, 165–190 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Wernegreen J. J., Ancient bacterial endosymbionts of insects: Genomes as sources of insight and springboards for inquiry. Exp. Cell Res. 358, 427–432 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Bennett G. M., Moran N. A., Heritable symbiosis: The advantages and perils of an evolutionary rabbit hole. Proc. Natl. Acad. Sci. U.S.A. 112, 10169–10176 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaidi A. A., et al., Bottleneck and selection in the germline and maternal age influence transmission of mitochondrial DNA in human pedigrees. Proc. Natl. Acad. Sci. U.S.A. 116, 25172–25178 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charlesworth B., Charlesworth D., The degeneration of Y chromosomes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355, 1563–1572 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moran N. A., Accelerated evolution and Muller’s rachet in endosymbiotic bacteria. Proc. Natl. Acad. Sci. U.S.A. 93, 2873–2878 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rispe C., Moran N. A., Accumulation of deleterious mutations in endosymbionts: Muller’s ratchet with two levels of selection. Am. Nat. 156, 425–441 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Pettersson M. E., Berg O. G., Muller’s ratchet in symbiont populations. Genetica 130, 199–211 (2007). [DOI] [PubMed] [Google Scholar]
- 10.O’Fallon B., Population structure, levels of selection, and the evolution of intracellular symbionts. Evolution 62, 361–373 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Shigenobu S., Wilson A. C. C., Genomic revelations of a mutualism: The pea aphid and its obligate bacterial symbiont. Cell. Mol. Life Sci. 68, 1297–1309 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shigenobu S., Watanabe H., Hattori M., Sakaki Y., Ishikawa H., Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature 407, 81–86 (2000). [DOI] [PubMed] [Google Scholar]
- 13.Koga R., Meng X.-Y., Tsuchida T., Fukatsu T., Cellular mechanism for selective vertical transmission of an obligate insect symbiont at the bacteriocyte-embryo interface. Proc. Natl. Acad. Sci. U.S.A. 109, E1230–E1237 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braendle C., et al., Developmental origin and evolution of bacteriocytes in the aphid-Buchnera symbiosis. PLoS Biol. 1, E21 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moran N. A., Yun Y., Experimental replacement of an obligate insect symbiont. Proc. Natl. Acad. Sci. U.S.A. 112, 2093–2096 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunbar H. E., Wilson A. C. C., Ferguson N. R., Moran N. A., Aphid thermal tolerance is governed by a point mutation in bacterial symbionts. PLoS Biol. 5, e96 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang B., Leonard S. P., Li Y., Moran N. A., Obligate bacterial endosymbionts limit thermal tolerance of insect host species. Proc. Natl. Acad. Sci. U.S.A. 116, 24712–24718 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otto S. P., Hastings I. M., Mutation and selection within the individual. Genetica 102-103, 507–524 (1998). [PubMed] [Google Scholar]
- 19.Moran N. A., McLaughlin H. J., Sorek R., The dynamics and time scale of ongoing genomic erosion in symbiotic bacteria. Science 323, 379–382 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Mira A., Moran N. A., Estimating population size and transmission bottlenecks in maternally transmitted endosymbiotic bacteria. Microb. Ecol. 44, 137–143 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Simonet P., et al., Direct flow cytometry measurements reveal a fine-tuning of symbiotic cell dynamics according to the host developmental needs in aphid symbiosis. Sci. Rep. 6, 19967 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akashi H., Osada N., Ohta T., Weak selection and protein evolution. Genetics 192, 15–31 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCutcheon J. P., Moran N. A., Extreme genome reduction in symbiotic bacteria. Nat. Rev. Microbiol. 10, 13–26 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Kimura M., Ohta T., The average number of generations until fixation of a mutant gene in a finite population. Genetics 61, 763–771 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rebolledo-Jaramillo B., et al., Maternal age effect and severe germ-line bottleneck in the inheritance of human mitochondrial DNA. Proc. Natl. Acad. Sci. U.S.A. 111, 15474–15479 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamas I., et al., 50 million years of genomic stasis in endosymbiotic bacteria. Science 296, 2376–2379 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Chong R. A., Park H., Moran N. A., Genome evolution of the obligate endosymbiont Buchnera aphidicola. Mol. Biol. Evol. 36, 1481–1489 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Frank S. A., Perspective: Repression of competition and the evolution of cooperation. Evolution 57, 693–705 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Chrostek E., Teixeira L., Mutualism breakdown by amplification of Wolbachia genes. PLoS Biol. 13, e1002065 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell J. A., Latorre A., Sabater-Muñoz B., Moya A., Moran N. A., Side-stepping secondary symbionts: Widespread horizontal transfer across and beyond the Aphidoidea. Mol. Ecol. 12, 1061–1075 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.