Significance
Globally, wheat farming is a major source of nitrogen pollution. Rapid generation of soil nitrates cause nitrogen leakage and damage ecosystems and human health. Here, we show the 3NsbS chromosome arm in wild grass (Leymus racemosus) that controls root nitrification inhibitor production can be transferred into elite wheat cultivars, without disrupting the elite agronomic features. Biological nitrification inhibition (BNI)–enabled wheats can improve soil ammonium levels by slowing down its oxidation and generate significant synergistic benefits from assimilating dual nitrogen forms and improving adaptation to low N systems. Deploying BNI-enabled wheat on a significant proportion of current global wheat area (ca. 225 M ha) could be a powerful nature-based solution for reducing N fertilizer use and nitrogen losses while maintaining productivity.
Keywords: BNI, nitrogen pollution, nitrification inhibition, genetic improvement, wheat
Abstract
Active nitrifiers and rapid nitrification are major contributing factors to nitrogen losses in global wheat production. Suppressing nitrifier activity is an effective strategy to limit N losses from agriculture. Production and release of nitrification inhibitors from plant roots is termed “biological nitrification inhibition” (BNI). Here, we report the discovery of a chromosome region that controls BNI production in “wheat grass” Leymus racemosus (Lam.) Tzvelev, located on the short arm of the “Lr#3Nsb” (Lr#n), which can be transferred to wheat as T3BL.3NsbS (denoted Lr#n-SA), where 3BS arm of chromosome 3B of wheat was replaced by 3NsbS of L. racemosus. We successfully introduced T3BL.3NsbS into the wheat cultivar “Chinese Spring” (CS-Lr#n-SA, referred to as “BNI-CS”), which resulted in the doubling of its BNI capacity. T3BL.3NsbS from BNI-CS was then transferred to several elite high-yielding hexaploid wheat cultivars, leading to near doubling of BNI production in “BNI-MUNAL” and “BNI-ROELFS.” Laboratory incubation studies with root-zone soil from field-grown BNI-MUNAL confirmed BNI trait expression, evident from suppression of soil nitrifier activity, reduced nitrification potential, and N2O emissions. Changes in N metabolism included reductions in both leaf nitrate, nitrate reductase activity, and enhanced glutamine synthetase activity, indicating a shift toward ammonium nutrition. Nitrogen uptake from soil organic matter mineralization improved under low N conditions. Biomass production, grain yields, and N uptake were significantly higher in BNI-MUNAL across N treatments. Grain protein levels and breadmaking attributes were not negatively impacted. Wide use of BNI functions in wheat breeding may combat nitrification in high N input–intensive farming but also can improve adaptation to low N input marginal areas.
Nitrification and denitrification are critical soil biological processes, which, left unchecked, can accelerate generation of harmful reactive nitrogen (N) forms (NO3 −, N2O, and NOx) that trigger a “nitrogen cascade,” damaging ecosystems, water systems, and soil fertility (1 –8). Excessive nitrifier activity and a rapid generation of soil nitrates plague modern cereal production systems. This has led to shifting crop N nutrition toward an “all nitrate form,” which is largely responsible for N losses and a decline in agronomic nitrogen-use efficiency (NUE) (6, 7, 9 –11).
Wheat, one of the three founding crops for food security (12), consumes nearly a fifth of factory-produced N fertilizers, and it has an average NUE of 33%, which has remained unchanged for the last two decades (13 –15). Regulating soil nitrifier activity to slow the rate of soil nitrate formation should provide more balanced N forms (NH4 + and NO3 −) for plant uptake (rather than nearly “all NO3 −” at present), reduce N losses, and facilitate the assimilation of dual N forms. This optimizes the utilization of biochemical machinery for N assimilation, improving stability and possibly enhancing yield potential (16). In addition, the assimilation of NH4 + is energetically more efficient (requiring 40% less metabolic energy) than NO3 − assimilation (16). Often, a stimulatory growth response is observed in wheat, when 15 to 30% of NO3 − is replaced with NH4 + in nutrient solutions (17, 18).
Synthetic nitrification inhibitors (SNIs) have been shown to suppress N2O emissions, reduce N losses, and improve agronomic NUE in several cereal crops including wheat (6, 19 –21). However, the lack of cost effectiveness, inconsistency in field performance, inability to function in tropical environments, and the concerns related to the entering of SNIs into food chains have limited their adoption in production agriculture (6, 7, 19, 20).
Biological nitrification inhibition (BNI) is a plant function whereby nitrification inhibitors (BNIs) are produced from root systems to suppress soil nitrifier activity (22 –26). Earlier, we reported that the BNI capacity in the root systems of cultivated wheat lack adequate strength to effectively suppress soil nitrifier activity in the rhizosphere (24, 25). Leymus racemosus (hereafter referred to as “wild grass”), a perennial Triticeae evolutionarily related to wheat, produces extensive root systems ( SI Appendix, Fig. S1) and was discovered to have a high BNI capacity several times higher than cultivated wheat. It was also effective in suppressing soil nitrifier activity and in reducing soi -nitrate formation ( SI Appendix, Fig. S2) (25). Subsequently, the chromosome Lr#n = 3Nsb was found to be controlling a major part of BNI capacity in wild grass, and it is the focus of our current research (25, 27, 28). Earlier, we reported that Lr#I and Lr#J had a minor impact on BNI capacity, but they are not the focus of this research (25).
We transferred the Lr#n chromosome (Lr#n-SA = T3BL.3NsbS) controlling BNI capacity (hereafter referred to as BNI trait) into the cultivated wheat, Chinese Spring (CS). The results of the transfer of this BNI trait into several elite wheat types with a grain-yield (GY) potential >10 t ha−1, resulting in substantial improvements of BNI capacity in root systems, are reported in this paper.
Results
BNI Capacity Has Not Increased over Five Decades of Wheat Breeding.
We evaluated 20 International Maize and Wheat Improvement Center (CIMMYT)–derived wheat varieties released between 1950 and 2010 (belonging to both pre-Green Revolution [GR] and post-GR era wheat varieties) to determine the impact of five decades of breeding under high nitrogen input conditions on the BNI capacity of wheat root systems ( SI Appendix, Study 1). We observed no clear trend in the 20 varieties’ BNI capacity ( SI Appendix, Fig. S3). There were significant differences (P < 0.001) in the BNI capacities of wheat varieties ( SI Appendix, Table S1), but none showed higher BNI capacity than the standard wheat genetic stock, CS, an old landrace from China ( SI Appendix, Table S1). Some elite varieties released in 2000 (such as ROELFS) as well as “SONORA-64” (an early GR era variety released in 1964) have BNI capacity akin to CS (ranging from 70 to 90 allylthiourea unit [ATU] ⋅ g−1 root dryweight d−1). Also, these results indicate that wheat breeding had no directional impact (i.e., neither positive nor negative) on the BNI capacity of root systems (i.e., either selected “for” or “against”) ( SI Appendix, Fig. S3). This, in turn, could be due to a lack of adequate allelic variation for this trait (i.e., BNI strength necessary to suppress soil nitrifier activity) in wheat or close wheat relatives used in breeding.
Genetic Sources for BNI Capacity Identified in Perennial Wild Grasses.
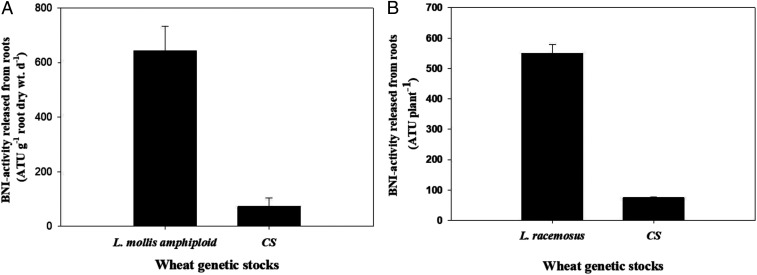
Of 20 amphiploids (generated by crossing various wild wheat species and wild grasses with cultivated wheat) evaluated for BNI capacity ( SI Appendix, Study 2a and b), only the Leymus mollis (Trin.) Pilger (4× = 28, genomes NsNsXmXm) amphiploid (generated by crossing with Triticum turgidum) showed BNI capacity several times (around seven times) higher than CS (Fig. 1A and SI Appendix, Table S2 A–C ). This is akin to what was reported earlier for another wild grass species L. racemosus (Lam.) Tzvelev (4× = 28, genomes NsNsXmXm) and from the results reported here (Fig. 1B ). Both perennial wild grass relatives are part of the tertiary gene pool of wheat (i.e., distant relatives of wheat), posing major practical challenges for the transfer of BNI genes to wheat. No other wild relatives tested in this study showed higher BNI capacity than CS ( SI Appendix, Table S2C ). Negative BNI activity was detected in the root exudates of most amphiploids derived from wild wheats, implying that they are likely to stimulate nitrifying bacteria, thus accelerating soil nitrification ( SI Appendix, Table S2C ). So far, only two wild grasses (L. racemosus and L. mollis) have been identified as genetic sources for improving the BNI capacity in the root systems of cultivated wheat.
Fig. 1.
BNI activity in L. mollis and L. racemosus compared to CS. (A) Nearly sevenfold higher BNI activity is released from L. mollis amphiploid (L. mollis × T. turgidum) compared to cultivated wheat cv. CS. Values are means ± SE of four replications. Plants were grown hydroponically for 60 d in a walk-in growth chamber before collecting root exudates to determine BNI release ( SI Appendix, Study 2b). (B) About eightfold higher BNI activity is released from roots of wild grass (L. racemosus) compared to cultivated wheat cv. CS. For L. racemosus, plants were grown in pots filled with soil (Andosol; 13 kg soil pot−1) for 1 y before using them for root exudate collection (see SI Appendix, Fig. S1 for pictures of root systems of wild grass and field-grown cultivated wheat, MUNAL root systems). For cultivated wheat (CS), plants were grown hydroponically for 60 d in a walk-in growth chamber as described in Materials and Methods , and root exudates were collected from intact plant root systems to determine BNI activity released (data from results of experiment 5a). Values are means ± SE of four replications ( SI Appendix, Study 2a).
Identification of Chromosomal Region Controlling BNI Capacity in L. racemosus.
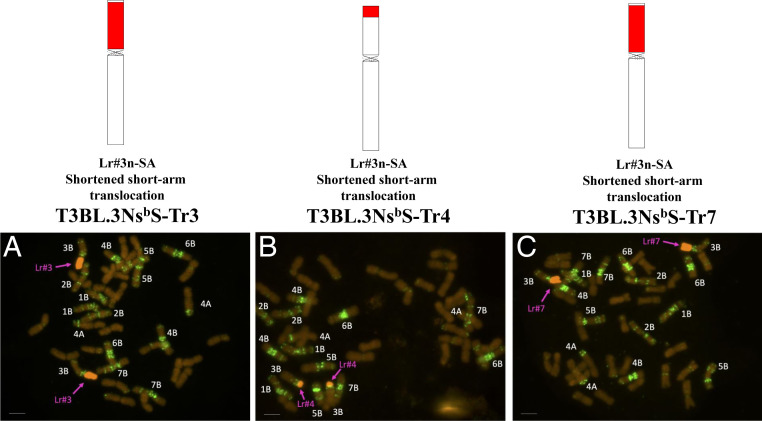
We reported earlier that the high BNI capacity of L. racemosus was controlled by chromosome Lr#n (25). The BNI capacity is conferred by the short arm of Lr#n; this was evident from the doubling (P < 0.001) of BNI capacity when the short arm was introduced into CS but not with long-arm translocation (Table 1 and SI Appendix, Study 4a and Fig. S4 A and B ). A previous study established that the short arm of Lr#n corresponds to a Leymus chromosome from homoeologous group 3 (3Ns) (28). In addition, we tested two independent translocations of the complete 3NsbS arm to chromosomes 7B and 3B to determine which translocation position maximizes BNI trait expression ( SI Appendix, Fig. S4C ). The BNI trait from Lr#n fully expressed (P < 0.001) only on wheat 3B translocation (T3BL.3NsbS), not on 7B translocation (T7BL.3NsbS) (Table 1). Three 3NsbS recombinant chromosomes with reduced 3Nsb arm sizes (T3BL.3NsbS-Tr-3; T3BL.3NsbS-Tr-4; T3BL.3NsbS-Tr-7) were developed in CS using the ph1b mutation (Fig. 2 and SI Appendix, Study 4b and Fig. S5). BNI activity release rates in two recombinant chromosomes were 20 to 40% higher (P < 0.001) compared to T3BL.3NsbS (Table 2), suggesting that by reducing the size of the 3NsbS arm, BNI trait expression can be further improved by minimizing negative impacts from other wild genes accompanying the BNI trait.
Table 1.
BNI capacity of wheat Leymus genetic stocks ( SI Appendix, Study 3a)
| Serial no. | Genetic stock details | BNI activity released from intact plant roots (ATU ⋅ g−1 root dry wt. ⋅ d−1) | |
| Mean | SE | ||
| 1 | CS | 77.7a | 2.9 |
| 2 | Lr#n-addition (CS*2/LE.RA) | 162.9b | 17.7 |
| 3 | Lr#n-Short-arm Tr.-7B | 80.0a | 4.2 |
| 4 | Lr#n-Short-arm Tr.-3B (T3BL.3NsbS) | 168.6b | 7.4 |
| 5 | Lr#n-Long-Arm Tr. (CS*2/LE.RA//2*WBLL1/3/CS) | 79.3a | 3.7 |
| SEM (P < 0.05) (based on two-way analysis General Linear Model using SYSTAT 14.0) | 9.34 (P < 0.05) | ||
Holm–Sidak method—letters represent values that are significantly different (P < 0.05). Values are means ± SE of four replications.
Fig. 2.
Lr#n short-arm recombinants with shorter fragment size than Lr#n-SA in CS. Chromosome analysis of three recombinants. (A) Lr-n-SA-Tr3 (red: L. racemosus genomic DNA, green: mixture of AAG and Tail probes). (B) Lr-n-SA-Tr4 (red: L. racemosus genomic DNA, green: mixture of AAG and Tail probes). (C) Lr-n-SA-Tr7 (red: L. racemosus genomic DNA, green: mixture of AAG and Tail probes). (Scale bar, 10 μm.)
Table 2.
BNI capacity of Lr#n short-arm translocations (with shortened short arm of chromosome Lr#n-SA) in CS genetic background along with CS ( SI Appendix, Study 4b)
| Serial no. | Genetic stock details | Genetic stock explanation | BNI activity released from intact plant roots (ATU ⋅ g−1 root dry wt. ⋅ d−1) | Percent of CS control | |
| Mean | SE | ||||
| 1 | CS | 57.4a | 3.8 | 100 | |
| 2 | CSMONO3B//CS*2/LE.RA Lr#n-SA) (T3BL.3NsbS) | N complete short arm | 113.0b | 4.5 | 196 |
| 3 | CSMONO3B//CS*2/LE. RA Shortened short-arm translocation-3 (T3BL.3NsbS-Tr3) | N shortened SA Tr-3 | 137.4c | 2.6 | 239 |
| 4 | CSMONO3B//CS*2/LE.RA/3/CS ph ph 1b Shortened short-arm translocation-4 (T3BL.3NsbS-Tr4) | N shortened SA Tr-4 | 97.6b | 9.3 | 170 |
| 5 | CSMONO3B//CS*2/LE.RA/3/CS ph ph 1b Shortened short-arm translocation-7 (T3BL.3NsbS-Tr7) | N shortened SA Tr-7 | 163.9c | 5.9 | 286 |
| SE of Least Square Mean (P < 0.001) (based on two-way analysis General Linear Model using SYSTAT 14.0) | 6.86 (P < 0.001) | ||||
Holm–Sidak method—letters represent values that are significantly different (P < 0.001). Values are means ± SE of four replications.
Transfer of T3BL.3NsbS into Elite Wheats Conferring BNI Capacity.
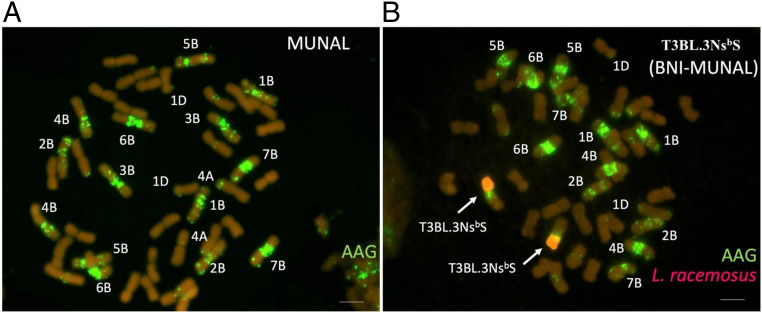
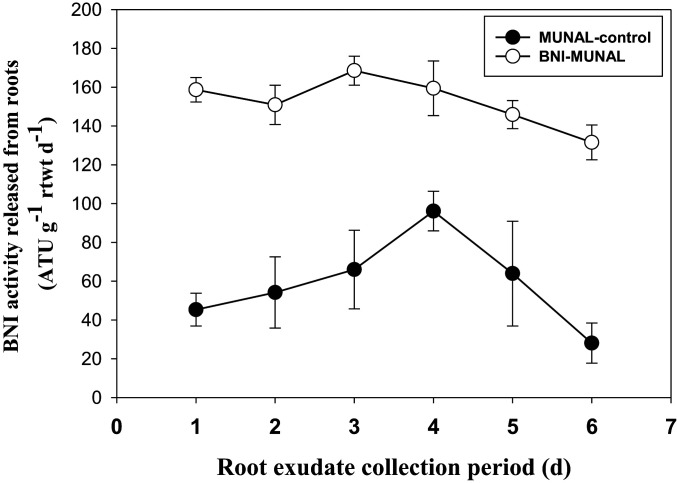
The wheat L.racemosus T3BL.3NsbS chromosome was successfully transferred from CS into several elite hexaploid wheat cultivars: “ROELFS,” “MUNAL,” “NAVOJOA,” and “QUAIU,” with a GY potential of >10 t ⋅ ha−1 ( SI Appendix, Tables S2D and S3 and https://www.orderseed.cimmyt.org/iwin/iwin-results-1.php). This was achieved by utilizing at least four backcrosses and selection for T3BL.3NsbS using fluorescence in situ hybridization following Kishii et al. (29) (Fig. 3 A and B and SI Appendix, Fig. S6 A and B ). We conformed an enhanced BNI capacity, as there were significant improvements (P < 0.001) in BNI activity release from the root systems of the most BNI elite wheats (Table 3 and SI Appendix, Study 5a). We also observed a near doubling (P < 0.001) in the release of BNI activity from root systems of BNI-MUNAL and BNI-ROELFS (compared to MUNAL control and ROELFS control). For “BNI-QUAIU,” we observed only a 50% increase (P < 0.001) in BNI activity release. For “BNI-NAVOJOA,” there was no significant improvement in BNI release compared to “NAVOJOA control” (Table 3), indicating that BNI trait expression is wheat genetic background dependent. Subsequent studies ( SI Appendix, Study 5b) with BNI-MUNAL revealed that BNI release rates were between two and five times higher than in MUNAL control (monitored over 6 d, during which time root exudates were collected using different trap solutions), indicating enormous plasticity in the phenotypic expression of BNI trait (Fig. 4). Such plasticity in the magnitude of BNI release is needed to deliver the required dosage of BNIs (determined by amounts of NH4 + available at soil sites) for suppressing and/or moderating nitrifier activity (23, 26); root systems constantly face the challenge of temporal and spatial variation in rhizosphere environment.
Fig. 3.
Karyotype analysis of BNI isogenic wheat lines. (A) Wheat line MUNAL. (B) BNI-MUNAL carrying Lr#n-SA translocation (complete short arm) on wheat chromosome 3B (T3BL.3NsbS) Genomic in situ hybridization (GISH)/Florescence in situ hybridization (FISH) (red: L. racemosus genomic DNA, green AAG probe).
Table 3.
BNI capacity of elite wheat genetic stocks and BNI elite wheats ( SI Appendix, Study 5a)
| Serial no. | Wheat genetic stock | BNI activity released from intact plant roots (ATU ⋅ g−1 root dry wt. ⋅ d−1) | |
| Mean | SE | ||
| 1 | ROELFS | 86.5a | 12.8 |
| 2 | BNI-ROELFS (CSMONO3B/3/CS/LE.RA/CS/4/CSph/5/5*ROELFS(N) | 162.2b | 16.8 |
| SE of Least Square Mean (P < 0.001) (based on two-way analysis General Linear Model using SYSTAT 14.0) | 6.99 (P < 0.005) | ||
| 3 | MUNAL | 92.7a | 12.1 |
| 4 | BNI-MUNAL (CSMONO3B/3/CS/LE.RA/CS/4/CS/ph/5/5*MUNAL(N) | 181.7b | 22.3 |
| SE of Least Square Mean (P < 0.001) (based on two-way analysis General Linear Model using SYSTAT 14.0) | 17.9 (P < 0.05) | ||
| 5 | NAVOJOA | 91.2a | 22.4 |
| 6 | BNI-NAVOJOA(CSMONO3B/3/CS/LE.RA/CS/4/CS/ph/4/4*NAVAJOA(N) | 119.2a | 14.2 |
| SE of Least Square Mean (P < 0.001) (based on two-way analysis General Linear Model using SYSTAT 14.0) | 22.42ns | ||
| 7 | QUAIU | 70.2a | 4.9 |
| 8 | BNI-QUAIU CSMONO3B/3/CS/LE.RA/CS/4/CSph/5/5*Quaiu(N) | 126.4b | 11.8 |
| SE of Least Square Mean (P < 0.001) (based on two-way analysis General Linear Model using SYSTAT 14.0) | 11.19 (P < 0.05) | ||
Holm–Sidak method—letters represent values that are significantly different (P < 0.05). Values are means ± SE of four replications.
Fig. 4.
Two- to fivefold higher BNI activity is released from BNI-MUNAL (i.e., MUNAL carrying T3BL.3NsbS) compared to MUNAL control (BNI isogenic lines) during a 6-d monitoring period using various root exudate trap solutions ( SI Appendix, Study 5b). 1) RE-NH4-1 (1.8 L aerated solutions of 0.5 mM NH4Cl + 200 μM CaCl2 for 24 h—first day collection); 2) RE-nutr-NH4-1 (1.8 L aerated solutions of one-quarter strength nutrient solution with 0.5 mM NH4Cl for 24 h—second day collection); 3) RE-water-1 (1.8 L aerated solutions of 200 μM CaCl2 for 24 h—third day collection); 4) RE-NH4-2 (1.8 L aerated solutions of 1.0 mM NH4Cl + 200 μM CaCl2 for 24 h—fourth day collection); 5) RE-nutr-NH4-2 (1.8 L aerated solutions of one-quarter strength nutrient solution with 1.0 mM NH4Cl for 24 h—fifth day collection); and 6) RE-water-2 (1.8 L aerated solutions of 200 μM CaCl2 for 24 h—sixth day collection). Values are means ± SE from four replications.
BNI Trait (T3BL.3NsbS) Suppresses Nitrification and Improves N Uptake, Biomass Production, and GY in a Range of Nitrogen Inputs under Field Conditions—Proof of Concept.
Based on conservative estimates of root biomass being 1.95 Mg ⋅ ha−1 (assuming that 10% of the total aboveground biomass measured is allocated to roots) with maximum BNI activity release rates of 182 ATU ⋅ g−1 root dry weight d−1 (Table 3 and Fig. 4), we estimate that 354.9 × 106 ATU ⋅ ha−1 ⋅ d−1 can potentially be released from the root systems of BNI-MUNAL at its peak (i.e., booting stage—GS51, Zadoks scale), measured in hydroponics (30). This estimate amounts to an inhibitory potential equivalent to the application of 212.9 g nitrapyrin ha−1 ⋅ d−1 [based on 1 ATU being equivalent to 0.6 μg of nitrapyrin (26, 31)]. Such high levels of BNI release may not be sustained over extended periods under field conditions. Nevertheless, this is large enough to have significant suppressive effect on nitrifier populations.
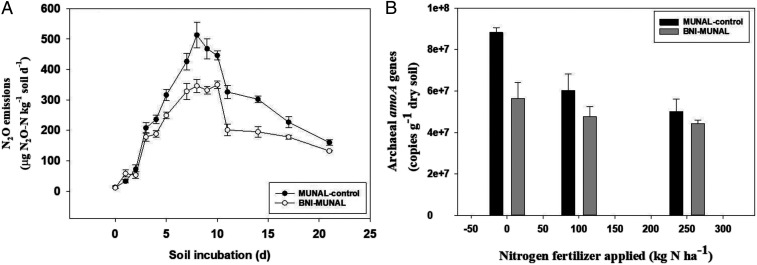
Field studies on acidic soils (soil pH 5.0 to 5.5) at Japan International Research Center for Agricultural Sciences (JIRCAS; Tsukuba, Japan; SI Appendix, Study 6a) indicated a 30% reduction in soil nitrate levels (P < 0.05) and substantial improvements in soil ammonium levels (P < 0.001) (in core soil samples taken at a 20-cm depth near plant roots) ( SI Appendix, Table S4A ) (compared to MUNAL control field plots), indicating the expression of BNI function in BNI-MUNAL root systems. In root-zone soils (defined as “soil that is in close proximity to roots”) ( SI Appendix, Fig. S7A, 1), the nitrate percentage of inorganic N pool (%) declined by 26% (P < 0.001), potential net nitrification rates declined by 17% (P < 0.05) (Table 4), and potential nitrification declined by 28% ( SI Appendix, Fig. S7A ). The slopes of regression lines are significantly different (P < 0.001) based on analysis of covariance (ANCOVA). Also, N2O emissions based on laboratory incubation studies declined by 25% (P < 0.01) (Fig. 5A ; SI Appendix, Fig. S7B ) and soil archaea (AOA) populations declined by 20 to 36% (P < 0.005) (Fig. 5B ). BNI function had a stronger inhibitory effect on archaea compared to ammonium oxidizing bacteria (AOB) populations, as AOBs did not show significant decline in BNI-MUNAL ( SI Appendix, Fig. S7C ). This lends support to recent reports of BNIs being more potent on AOAs (32, 33), whereas SNIs are more effective on AOBs (34). Furthermore, soil microcosm studies ( SI Appendix, Study 6b) with alkaline soils suggested a 45% decline (P < 0.05) in AOBs with BNI-MUNAL but did not influence AOAs ( SI Appendix, Table S4B ).
Table 4.
Nitrate percentage of inorganic N pool and potential net nitrification rates in root-zone soils of field-grown plants of BNI isogenic lines, MUNAL control versus BNI-MUNAL after 21-d incubation period ( SI Appendix, Study 6a)
| Wheat genetic stocks | Soil ammonium (μg ⋅ g−1 soil) | Soil nitrate (μg ⋅ g−1 soil) | Total inorganic N (NH4 + + NO3 −) μg ⋅ g−1 soil | Nitrate percentage of inorganic N pool (%) | Potential net nitrification rate (μg ⋅ NO3 − ⋅ g−1 soil ⋅ d−1) | |||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| MUNAL control | 86.4 | 3.2 | 505.0 | 31.1 | 591.4 | 28.2 | 85.4 | 1.2 | 24.05 | 0.74 |
| BNI-MUNAL | 245.0 | 23.0 | 422.5 | 22.2 | 667.5 | 15.0 | 63.3 | 3.2 | 20.10 | 0.52 |
| SE of LS mean (based on two-way analysis GLM using SYSTAT 14.0) | 8.9** | 18.1* | 1.44** | 0.87* | ||||||
The nitrate percentage of inorganic N pool is calculated as [soil − NO3 −/(soil NO3 −+ soil NH4 +)] × 100], that is, proportion of nitrate to total inorganic N. Initial soil inorganic nitrogen levels (NO3 − and NH4 +) are deducted before calculating net change in soil inorganic N forms during the incubation period. Potential net nitrification rate = [soil nitrate levels at the end of incubation period (i.e., at 21 d) – soil nitrate levels at the beginning of incubation period (i.e., time “0”)]/21. Based on Holm–Sidak test; **significant at P < 0.001; *significant at P < 0.05. Values are means ± SE of four replications.
Fig. 5.
BNI function impact on N2O emissions and nitrifying populations in root-zone soils of field-grown wheat lines ( SI Appendix, Study 6a). (A) N2O emissions from root-zone soils of BNI isogenic lines, MUNAL control versus BNI-MUNAL. The root-zone soils used in this study were collected from MUNAL control and BNI-MUNAL (from 250 kg ⋅ N ⋅ ha−1 field plots). A total of 5 g air-dried soil was incubated with 250 ppm N [as (NH4)2SO4] using a 100-mL glass vial at 20 °C with 80% relative humidity in the incubator; soil moisture levels were maintained at 60% water-filled pore space during the incubation period. Values are means ± SE of four replications (see SI Appendix, Fig. S7B for cumulative N2O emissions over the 21-d period and for a statistical analysis of these results). (B) Influence of BNI-MUNAL on AOA populations in root-zone soils of field-grown plants. These results suggest that BNI-MUNAL suppressed AOA ranging from 20 to 36% depending on the nitrogen treatment of field plots. Root-zone soil samples were taken 16 d after the application of the second split nitrogen fertilizer. Values are means ± SE of four replications. Based on a three-way analysis of data using a General Linear Model model with SYSTAT 14.0; significant (P < 0.005) genetic stock effect on AOA; significant (P < 0.005) nitrogen treatment effect on AOA populations in rhizosphere soils.
However, this requires additional studies because most available evidence indicates that BNI function is mostly effective in soils that are acidic or neutral (5, 6, 23 –26). Functionally, AOAs are most active and dominant in acid soils (34), whereas AOBs are active and dominate in neutral alkaline soils (35 –37). The possibility for BNI trait expression under a wide range of soil pH conditions can potentially expand the scope for soil types in which BNI wheats can be deployed. The above observations were, however, based on laboratory incubation studies using root-zone soils from field-grown plants. The magnitude of BNI impact on bulk soils remains unknown, as is the BNI pathway that could influence nitrifier populations beyond the rhizosphere root zone.
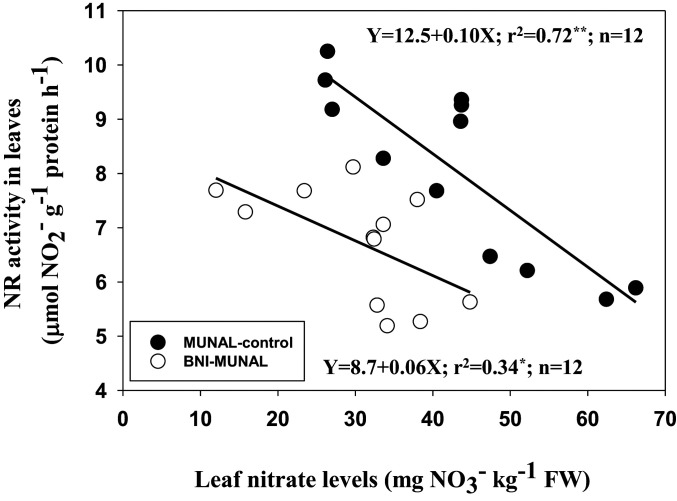
In addition, nitrogen metabolism in BNI-MUNAL was fundamentally altered. This is evident from radical changes in the relationship between leaf nitrate levels and nitrate reductase activity (NRA) in BNI-MUNAL (compared to MUNAL control) ( SI Appendix, Study 6a). The slopes of regression lines are significantly different (P < 0.001) based on ANCOVA (Fig. 6). Further, BNI trait introduction led to a substantial decline in leaf nitrate levels (about 30 to 40%; P < 0.001; SI Appendix, Table S5) and leaf NRA (around 20%; P < 0.001) ( SI Appendix, Table S6) and an increase (about 15%; P < 0.001) in glutamine synthetase activity (GSA) in leaves; GSA is an enzyme that is at the forefront of ammonium assimilation ( SI Appendix, Table S6). Likely due to enhanced ammonium nutrition (uptake and assimilation), the root-zone soil pH was consistently lower (about 0.1 to 0.2 unit; P < 0.001) in BNI-MUNAL ( SI Appendix, Fig. S7D ).
Fig. 6.
Relationship between leaf nitrate levels and NRA activity in field-grown BNI isogenic lines, MUNAL control, and BNI-MUNAL ( SI Appendix, Study 6a). The slopes of regression lines are significantly different (P < 0.001) based on ANCOVA. Leaf sample data from all three nitrogen treatments are used in this presentation; first sampling data are used. NRA and leaf nitrate analysis from leaf samples collected from four plants for each experimental plot represent each data point. Leaf nitrate levels and NRA levels were substantially lower in BNI-MUNAL compared to MUNAL control. The relationship between NRA and leaf nitrate levels is fundamentally different in BNI-MUNAL compared to MUNAL control. Also, see SI Appendix, Tables S5 and S6 for detailed results on NRA and leaf nitrate levels and for the statistical analysis of results.
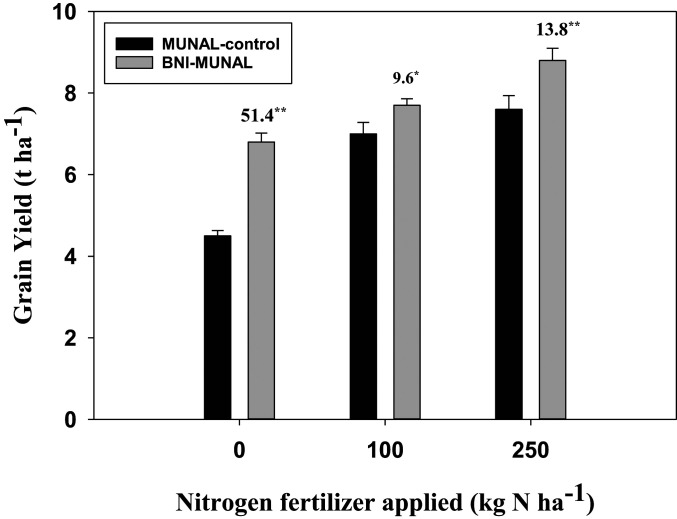
BNI-MUNAL had improved total biomass production (P < 0.001) and GY (P < 0.001) across treatments (Fig. 7 and SI Appendix, Study 6a and Table S7) based on field evaluations at the JIRCAS experimental station in 2019, with improved (P < 0.001) agronomic attributes: harvest index, tiller numbers, and 100 seed wt. ( SI Appendix, Table S7 ). The biggest impact from introducing a BNI trait is evident in no N application field plots in which N deficiency symptoms are visible (also based on Soil Plant Analysis Development chlorophyll meter readings that reflect chlorophyll and nitrogen content in leaves) only in MUNAL control but not in BNI-MUNAL ( SI Appendix, Fig. S8 A–C ). Its biomass production and GYs were 50% higher (P < 0.001) than MUNAL control (Fig. 7 and SI Appendix, Table S7 ; SI Appendix, Fig. S8D ). With N fertilization (100 to 250 kg ⋅ N ⋅ ha−1), BNI-MUNAL yielded about 10 to 14% (P < 0.001) higher than MUNAL control (Fig. 7). This is possibly due to improved NH4 + assimilation, which is energetically more efficient than NO3 − assimilation (16) and can have a synergistic impact on growth and GYs. Supplemental NH4 + in nutrient solutions has been reported to stimulate growth in wheat and maize (15, 17).
Fig. 7.
GY of BNI isogenic lines, MUNAL control, and BNI-MUNAL under various nitrogen fertilizer applications in the field ( SI Appendix, Study 6a). Three-way ANOVA using General Linear Model with SYSTAT 14.0; SE of Least Square mean (genetic stock) 0.164 (P < 0.001); SE of LS mean (N-Tr) 0.201 (P < 0.001); values are means ± SE of four replications. **P < 0.001; *P < 0.05.
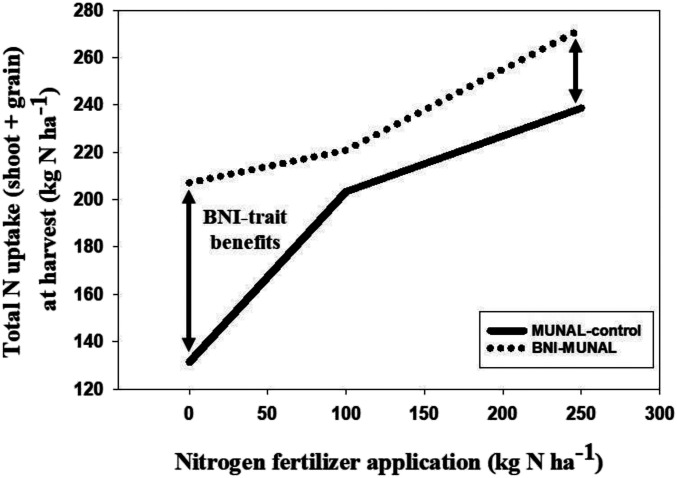
Nitrogen uptake (aboveground biomass that includes grain) improved by about 28% (P < 0.001) (ranging from 9 to 58% depending on N fertilizer treatment) in BNI-MUNAL (Fig. 8 and SI Appendix, Study 6a and Fig. S9 A and B ). It is likely that the root systems of perennial wild grass have the ability to mineralize N more efficiently from soil organic matter (SOM) than cultivated wheat, as they are highly adapted to low fertility and low N environments (38). Efficient N uptake from SOM can be part of the adaptation to low N environments. The exceptional performance of BNI-MUNAL under low N conditions (58% higher N uptake; P < 0.001) supports the hypothesis that T3BL.3NsbS, in addition to the BNI trait, is also carrying genes that improve the uptake of native soil N by efficient SOM mineralization, introduced as part of the BNI trait package. SOM mineralization rates were nearly doubled (P < 0.05) in root-zone soils of BNI-MUNAL compared to MUNAL control within low to medium N treatments but not under high N treatment (250 kg ⋅ N ⋅ ha−1) ( SI Appendix, Fig. S9C ), further supporting such a hypothesis. Nevertheless, the potential impact of BNI function on SOM mineralization beyond root-zone soils (i.e., bulk soils) remains unknown at this stage.
Fig. 8.
Total nitrogen uptake (based on aboveground dry matter that includes grain) in wheat BNI isogenic lines (MUNAL control and BNI-MUNAL) under various nitrogen fertilizer applications in the field ( SI Appendix, Study 6a). Introduction of BNI trait (T3BL.3NsbS) resulted in substantial improvements in nitrogen uptake in BNI-MUNAL compared to MUNAL control. Three-way ANOVA using General Linear Model with SYSTAT 14.0; SE of Least Square mean (genetic stock) 5.79 (P < 0.001); SE of LS of mean (N-Tr) 7.10 (P < 0.001)—see SI Appendix, Fig. S9B for detailed results on total N uptake with a statistical analysis of results and their significance). Values are means ± SE of four replications.
Enhanced BNI production from BNI-MUNAL root systems decreased soil NO3 − levels and improved soil NH4 + availability in field plots. Also, the enhanced GSA in leaves and lower natural abundance (δ15N in ‰) values (P < 0.002) in wheat grain ( SI Appendix, Fig. S9D ) indicated the likelihood of higher ammonium assimilation. Lower δ15N values indicate reduced soil microbial nitrification, a process known for strong isotopic N fractionation, which results in 15N enrichment of source N (soil NH4 +) (39, 40). Brachiaria grass genotypes that have a high BNI capacity in root systems show lower δ15N in leaf tissues compared to low BNI capacity genotypes (41). Lower grain δ15N observed in BNI-MUNAL, 16% lower (P < 0.002) than in MUNAL control ( SI Appendix, Fig. S9D ), suggested reduced nitrification and consequently lower NO3 − availability and improved NH4 + assimilation during the growth period. In addition to yield, the introduction of T3BL.3NsbS into MUNAL and ROELFS did not affect the grain protein profile, protein levels, or breadmaking attributes ( SI Appendix, Table S8 A and B and Fig. S10) and therefore should facilitate a broader deployment of BNI traits in wheat production.
BNI Trait Introduction Did Not Negatively Impact Wheat Yield Potential.
GYs of BNI-MUNAL were either akin to MUNAL control (field trials of Obregon-1 and Obregon-2 in 2020 and in Obregon-1 in 2019 in which soils are of coarse sandy clay type, with a soil pH of 7.7) or significantly (P < 0.05) higher (in Obregon-2 in 2019) ( SI Appendix, Study 6c and Table S9) under high N input systems (≥250 kg ⋅ N ⋅ ha−1). However, for BNI-ROELFS, GYs were significantly (P < 0.05) lower than ROELFS control in three of the Obregon trials and were not significantly different in one trial ( SI Appendix, Table S9). It should be noted that BNI-ROELFS is at the BC5 stage, whereas BNI-MUNAL is at the BC6 stage. These results suggest that introduction of a BNI trait into these elite wheats did not negatively impact yield potential under high N inputs, even though soil conditions (i.e., because of alkaline soil pH) were not best suited for optimum BNI trait expression (5, 6, 23 –26).
Discussion
The last five decades of wheat breeding did not result in improvements in the BNI capacity of root systems, thus necessitating the use of wild grasses to source this trait. Two perennial wild grasses, L. racemosus and L. mollis, were identified as potential donors for a BNI trait. During this study, we showed the feasibility for transferring a chromosome arm controlling BNI traits (T3BL.3NsbS) into modern wheats without disrupting their agronomic performance, grain protein content, or breadmaking quality ( SI Appendix, Tables S7 and S8 A and B and Fig. S10 ). Introducing a BNI trait into MUNAL resulted in substantial improvements in N uptake and significant yield gains across N inputs, particularly under low nitrogen inputs. The potential for further genetic gains in enhancing BNI capacity is evident from reducing the size of T3BL.3NsbS (e.g., T3BL.3NsbS-Tr-3 and T3BL.3NsbS-Tr-7). Also, L. mollis can provide an additional genetic platform for introducing BNI traits into cultivated wheat. These field studies are part of establishing a proof of concept of trait expression in the field and potential beneficial impacts. Further studies are needed in agricultural settings, using multilocations with varying N inputs, to assess elite BNI wheats’ productivity gains. Similarly, future research should investigate how the BNI trait interacts with diverse environmental conditions (e.g., soil moisture, soil texture, SOM levels) as part of identifying farmlands suitable for deploying BNI wheats.
A major shift to “all nitrate” nutrition is the hallmark of modern production systems (6, 7) and is largely responsible for N pollution reaching the present crisis (42, 43). By inhibiting nitrifier activity, BNI wheats can facilitate dual N form availability in the soil root zone, which is more conducive for plant growth and can possibly enhance yield potential. Dual N forms provide better optimization of internal N assimilation pathways compared to all NO3 − nutrition (15 –17, 44). In addition, BNIs may promote mycorrhizal associations (45, 46), which can further improve N and P uptake. The enhanced NH4 + assimilation and the resulting rhizosphere acidification can bring additional benefits, such as improving BNI release further through positive autofeedback regulation and enhanced micronutrient availability (23, 26, 47, 48). Interplay between these cascading sequences of interconnected rhizosphere processes and their accrued synergistic interactions can result in improved plant growth and productivity (as observed in BNI-MUNAL across N treatments of the field study in Japan 2019).
In principle, BNI traits can be introduced into elite wheat varieties that fit into various production systems globally, although it is likely that light-textured soils with a pH < 6.0 are more suited than alkaline heavy clay soils for BNI trait expression (23 –26, 47, 48). Both spring and winter wheat types can benefit from BNI trait introduction. Assuming that the expression of a BNI trait is similar in winter wheats, the longer growing season may generate a higher inhibitory impact and be more effective. Large-scale yield trials with BNI wheats suggest no yield penalty from BNI trait introduction in high N input systems. Thus, T3BL.3NsbS can be transferred into wheat varieties adapted to diverse geographical regions to improve the BNI capacity of cultivated wheats. In addition, the increasing CO2 levels in the atmosphere favors assimilation of NH4 + over NO3 − in wheat (49), underlining the importance of shifting N nutrition in wheat systems away from NO3 − and toward more NH4 +.
Conclusions and Perspectives
We have demonstrated the feasibility of enhancing BNI capacity in elite wheats by transferring a chromosome arm 3NsbS controlling BNI traits from wild grass as a wheat L. racemosus translocation chromosome (T3BL.3NsbS). The enhanced BNI release from root systems of T3BL.3NsbS (about two- to fivefold increase) resulted in the suppression of soil nitrifier activity, reduced levels of soil nitrates, enhanced soil NH4 + availability, and accelerated SOM mineralization, which led to substantial gains in N uptake and GY. Also, the introduction of BNI traits led to major changes to N metabolism, indicating a shift toward more NH4 + assimilation. The newly developed BNI elite wheats retained their yield potential with an enhanced ability to uptake N from SOM mineralization (as evident from 58% higher N uptake in BNI-MUNAL under no N treatment), which has implications for the adaptation to low N input systems.
Nearly 60% of wheat area is in the Global South (50), mostly rainfed and highly variable in soil N. With an anticipated shortage of irrigation water, the likelihood of wheat being grown in more marginally productive areas in the future is high. Considering current and future food security hotspots, strong BNI performance in low N growing environments would become even more valuable. It remains to be seen how the newly developed BNI wheats perform in real production environments, particularly under low to moderate N inputs but also under intensive farming with high N inputs because also here, BNI traits may facilitate productivity gains (51). Most developed countries agreed to implement the Paris Climate Accord, committing to reduce agricultural greenhouse gas emissions by 30% by 2050. They could be early adopters of BNI wheats in intensive production systems (52). From an agronomic perspective, the aim is to minimize nitrogen leakage, allowing for lower N fertilization without losing productivity. The success of BNI wheats will be judged on their ability to maintain or improve productivity with reduced N inputs. This will drive their adoption.
The positive impact from BNI wheats on soil N cycling processes (e.g., reduced NO3 − leaching, lower N2O emissions, and improved soil N retention) needs to be validated and quantified in field settings closer to farmer conditions. A comparative advantage of BNI wheats needs to be assessed based on operational-scale field plots in multilocations (representing wheat production environments) to understand the potential value (i.e., productivity gains while reducing N losses) of BNI trait introduction. Like most production technologies or plant traits, it is likely that BNI traits will function only in certain agro-ecosystems and soil types. Based on current knowledge, BNI function is best expressed under mild acidic soil conditions, which potentially limits areas where BNI wheats can be deployed with current technology. If BNI traits could, in the future, be sourced from other wild grasses that can express well under alkaline soils, BNI wheat deployment could be expanded.
The genetic exploitation of BNI capacity in root systems of staple crops has the potential to facilitate the development of low-nitrifying and low N2O–emitting production systems. Being a seed-based technology and representing a nature-based solution, the potential for BNI wheats’ greater scalability and wider adoption can be high (51). An integrated approach involving BNI-enabled crops with a strategic deployment of SNIs may be the most effective approach to make farming more N efficient and less N leaky. It is likely that SNIs will be more effective on BNI crops due to the complementary nature of these inhibiting compounds (i.e., BNIs and SNIs), which suppress different segments of nitrifier populations (e.g., BNIs are more effective on AOAs, while SNIs are more effective on AOBs). The results presented here may have the potential to usher in a new era of BNI-enabled elite wheats based on genetic resources of wild grass. Realizing the full potential of BNI wheats, however, depends on a successful transfer of BNI trait (T3BL.3NsbS) into elite varieties adapted to diverse agro-climatic conditions representative of wheat growing areas. In our view, this requires a major initiative by the global wheat research community, private sector, policy makers, and research funders (51, 52).
Materials and Methods
Several growth-chamber experiments ( SI Appendix, Study 1, Study 2a and b, Study 4, and Study 5) were conducted to characterize wheat genetic stocks for BNI capacity using hydroponically grown plants. Plants are grown for about 50 to 60 d, and root exudates are collected using aerated trap solutions (e.g., 0.5 mM NH4Cl + 200 mM CaCl2) for 24 h to collect BNI activity. Root exudates are evaporated to dryness, and BNI activity is extracted and determined using luciferous recombinant Nitrosomonas assay described earlier (22). The BNI activity of root exudates is expressed in ATU ⋅ g−1 root dwt. ⋅ d−1 (see SI Appendix, Materials and Methods for details of each experiment and genetic stocks involved). Detailed methodology involved in developing wheat alien addition/translocation lines are described in SI Appendix, Study 3 ( SI Appendix, Materials and Methods ). During 2019, two BNI isogenic MUNAL genetic stocks (MUNAL control and BNI-MUNAL) were evaluated at JIRCAS experimental station in Tsukuba, Japan, to validate the effectiveness of BNI traits in suppressing soil nitrification, nitrogen uptake, and productivity gains in a range of nitrogen inputs ( SI Appendix, Study 6a and Materials and Methods for detailed description of this field study). A soil microcosm study was undertaken with BNI isogenic MUNAL genetic stocks at the University of the Basque Country, Spain ( SI Appendix, Study 6b) to validate BNI impact on soil nitrifier populations using plants grown in pots in a greenhouse ( SI Appendix, Materials and Methods for details). The yield potential of BNI isogenic lines of MUNAL control, BNI-MUNAL, ROELFS control, and BNI-ROELFS were measured in the CIMMYT field station in Obregon, Mexico, during 2018 and 2019 ( SI Appendix, Study 6c and Materials and Methods for details).
Supplementary Material
Acknowledgments
We gratefully acknowledge funding support from Japanese Ministry of Agriculture, Forestry and Fisheries, CGIAR Research Program on WHEAT during the execution of the research presented in this study. Also, we thank Drs. Y. Ando and P.S Sarr for research assistance related to molecular analysis of soil samples for AOB and AOA from the field study in Japan. Also, we thank Dr. Terasava, National Agricultural Research Center, Hokkaido for the technical assistance related to the grain protein analysis of samples from the JIRCAS field study. We acknowledge the discussions and feedback from several anonymous colleagues from US institutions who reviewed earlier drafts of this manuscript and provided insightful feedback and helpful and constructive comments.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This paper is a winner of the 2021 Cozzarelli Prize.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2106595118/-/DCSupplemental.
Data Availability
The genetic stocks developed and presented in this study are deposited with CIMMYT and available upon request subjected to consent from JIRCAS.
References
- 1. Bremner J. M., Blackmer A. M., Nitrous oxide: Emission from soils during nitrification of fertilizer nitrogen. Science 199, 295–296 (1978). [DOI] [PubMed] [Google Scholar]
- 2. Vitousek P. M., Mooney H. A., Lubchenco J., Melillo J. M., Human domination of Earth’s ecosystems. Science 277, 494–499 (1997). [Google Scholar]
- 3. Schlesinger W. H., On the fate of anthropogenic nitrogen. Proc. Natl. Acad. Sci. U.S.A. 106, 203–208 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Galloway J. N., Cowling E. B., Reflections on 200 years of nitrogen, 20 years later. Ambio 50, 745–749 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Subbarao G. V., et al., A paradigm shift towards low-nitrifying production systems: The role of biological nitrification inhibition (BNI). Ann. Bot. 112, 297–316 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Subbarao G. V., et al., Suppression of soil nitrification by plants. Plant Sci. 233, 155–164 (2015). [DOI] [PubMed] [Google Scholar]
- 7. Subbarao G. V., et al., Genetic mitigation strategies to tackle agricultural GHG emissions: The case for biological nitrification inhibition technology. Plant Sci. 262, 165–168 (2017). [DOI] [PubMed] [Google Scholar]
- 8. Billen G., Garnier J., Lassaletta L., The nitrogen cascade from agricultural soils to the sea: Modelling nitrogen transfers at regional watershed and global scales. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20130123 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salsac L., et al., Nitrate and ammonium nutrition in plants. Plant Physiol. Biochem. 25, 805–812 (1987). [Google Scholar]
- 10. Dinnes D. L., et al., Nitrogen management strategies to reduce nitrate leaching in tile drained Midwestern soils. Agron. J. 94, 153–171 (2002). [Google Scholar]
- 11. Bellamy P. H., Loveland P. J., Bradley R. I., Lark R. M., Kirk G. J. D., Carbon losses from all soils across England and Wales 1978-2003. Nature 437, 245–248 (2005). [DOI] [PubMed] [Google Scholar]
- 12. Galloway J. N., et al., Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 320, 889–892 (2008). [DOI] [PubMed] [Google Scholar]
- 13. Cassman K. G., Ecological intensification of cereal production systems: Yield potential, soil quality, and precision agriculture. Proc. Natl. Acad. Sci. U.S.A. 96, 5952–5959 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raun W. R., Johnson G. V., Improving nitrogen-use efficiency for cereal production. Agron. J. 91, 357–363 (1999). [Google Scholar]
- 15. Ju X. T., et al., Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc. Natl. Acad. Sci. U.S.A. 106, 3041–3046 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang P., et al., Interaction effect of nitrogen form and planting density on plant growth and nutrient uptake in maize seedlings. J. Integr. Agric. 17, 60345–60347 (2018). [Google Scholar]
- 17. Cox W. J., Reisenauer H. M., Growth and ion uptake by wheat supplied nitrogen as nitrate or ammonium or both. Plant Soil 38, 363–380 (1973). [Google Scholar]
- 18. Torralbo F., González-Moro M. B., Baroja-Fernández E., Aranjuelo I., González-Murua C., Differential regulation of stomatal conductance as a strategy to cope with ammonium fertilizer under ambient versus elevated CO2 . Front Plant Sci 10, 597 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Subbarao G. V., et al., Scope and strategies for regulation of nitrification in agricultural systems – Challenges and opportunities. Crit. Rev. Plant Sci. 25, 303–335 (2006). [Google Scholar]
- 20. Kanter D. R., Searchinger T. D., A technology-forcing approach to reduce nitrogen pollution. Nat. Sustain. 1, 544–552 (2018). [Google Scholar]
- 21. Corrochano-Monsalve M., et al., Relationship between tillage management and DMPSA nitrification inhibitor efficiency. Sci. Total Environ. 718, 134748 (2020). [DOI] [PubMed] [Google Scholar]
- 22. Subbarao G. V., et al., A bioluminescence assay to detect nitrification inhibitors released from plant roots: A case study with Brachiaria humidicola . Plant Soil 288, 101–112 (2006). [Google Scholar]
- 23. Subbarao G. V., Wang H. Y., Ito O., Nakahara K., Berry W. L., NH4 + triggers the synthesis and release of biological nitrification inhibition compounds in Brachiaria humidicola roots. Plant Soil 290, 245–257 (2007). [Google Scholar]
- 24. Subbarao G. V., et al., Biological nitrification inhibition (BNI) – Is it a widespread phenomenon? Plant Soil 294, 5–18 (2007). [Google Scholar]
- 25. Subbarao G. V., et al., Can biological nitrification inhibition (BNI) genes from perennial Leymus racemosus (Triticeae) combat nitrification in wheat farming? Plant Soil 299, 55–64 (2007). [Google Scholar]
- 26. Subbarao G. V., et al., Evidence for biological nitrification inhibition in Brachiaria pastures. Proc. Natl. Acad. Sci. U.S.A. 106, 17302–17307 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zahn L. M., A boost from wild wheat. Science 318, 171 (2007). [Google Scholar]
- 28. Edet O. U., et al., Efficient anchoring of alien chromosome segments introgressed into bread wheat by new Leymus racemosus genome-based markers. BMC Genet. 19, 18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kishii M., Yamada T., Sasakuma T., Tsujimoto H., Production of wheat-Leymus racemosus chromosome addition lines. Theor. Appl. Genet. 109, 255–260 (2004). [DOI] [PubMed] [Google Scholar]
- 30. Tottman D. R., Makepeace R. J., Broad H., An explanation of the decimal code for the growth stages of cereals, with illustrations. Ann. Appl. Biol. 93, 221–234 (1979). [Google Scholar]
- 31. Subbarao G. V., et al., Free fatty acids from the pasture grass Brachiaria humidicola and one of their methyl esters as inhibitors of nitrification. Plant Soil 313, 89–99 (2008). [Google Scholar]
- 32. Sarr P. S., Ando Y., Nakamura S., Deshmpande S., Subbarao G. V., Sorgoleone release from sorghum roots shapes the composition of nitrifying populations, total bacteria, and archaea and determines the level of nitrification. Biol. Fertil. Soils 56, 145–166 (2020). [Google Scholar]
- 33. Kaur-Bhambra J., Wardak D. L. R., Prosser J. I., Gubry-Rangin C., Revisiting plant biological nitrification inhibition efficiency using multiple archaeal and bacterial ammonia-oxidizing cultures. Biol. Fertil. Soils 10.1007/s00374-020-01533-1 (2021). [DOI] [Google Scholar]
- 34. Shen T., Stieglmeier M., Dai J., Urich T., Schleper C., Responses of the terrestrial ammonia-oxidizing archaeon Ca. Nitrososphaera viennensis and the ammonia-oxidizing bacterium Nitrosospira multiformis to nitrification inhibitors. FEMS Microbiol. Lett. 344, 121–129 (2013). [DOI] [PubMed] [Google Scholar]
- 35. Di H. J., Cameron K. C., Inhibition of nitrification to mitigate nitrate leaching and nitrous oxide emissions in grazed grassland: A review. J. Soils Sediments 16, 1401–1420 (2016). [Google Scholar]
- 36. Hink L., Gubry-Rangin C., Nicol G. W., Prosser J. I., The consequences of niche and physiological differentiation of archaeal and bacterial ammonia oxidisers for nitrous oxide emissions. ISME J. 12, 1084–1093 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fabian C., et al., GEMAS: Spatial distribution of the pH of European agricultural and grazing land soil. Appl. Geochem. 48, 207–216 (2014). [Google Scholar]
- 38. Bell L. W., Wade L. J., Ewing M. A., Perennial wheat: A review of environmental and agronomic prospects for development in Australia. Crop Pasture Sci. 61, 679–690 (2010). [Google Scholar]
- 39. Robinson D., δ(15)N as an integrator of the nitrogen cycle. Trends Ecol. Evol. 16, 153–162 (2001). [DOI] [PubMed] [Google Scholar]
- 40. Jones A. R., Dalal R. C., Enrichment of natural 15N abundance during soil N losses under 20 years of continuous cereal cropping. Sci. Total Environ. 574, 282–287 (2017). [DOI] [PubMed] [Google Scholar]
- 41. Karwat H., et al., Low 15N natural abundance in shoot tissue of Brachiaria humidicola is an indicator of reduced N losses due to biological nitrification inhibition (BNI). Front. Microbiol. 9, 2383 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rockström J., et al., A safe operating space for humanity. Nature 461, 472–475 (2009). [DOI] [PubMed] [Google Scholar]
- 43. Gilbert N., Summit urged to clean up farming. Nature 479, 279 (2011). [DOI] [PubMed] [Google Scholar]
- 44. Britto D., Kronzucker H., NH4 + toxicity in higher plants: A critical review. J. Plant Physiol. 159, 567–584 (2002). [Google Scholar]
- 45. Teutscherova N., et al., Differences in arbuscular mycorrhizal colonization and P acquisition between genotypes of the tropical Brachiaria grasses: Is there a relation with BNI activity? Biol. Fertil. Soils 55, 325–337 (2019). [Google Scholar]
- 46. Sarr P. S., Nakamura S., Ando Y., Iwasaki S., Subbarao G. V., Sorgoleone production enhances mycorrhizal association and reduces soil nitrification in sorghum. Rhizosphere 17, 1–12 (2021). [Google Scholar]
- 47. Zhu Y., Zeng H., Shen Q., Ishikawa T., Subbarao G. V., Interplay among NH4 + uptake, rhizosphere pH and plasma membrane H+-ATPase determine the release of BNIs in sorghum roots - possible mechanisms and underlying hypothesis. Plant Soil 358, 131–141 (2012). [Google Scholar]
- 48. Di T., et al., Further insights into underlying mechanisms for the release of biological nitrification inhibitors from sorghum roots. Plant Soil 423, 99–110 (2018). [Google Scholar]
- 49. Bloom A., Burger M., Kimball B. A., Pinter P., Nitrate assimilation is inhibited by elevated CO2 in field-grown wheat. Nat. Clim. Chang. 4, 477–480 (2014). [Google Scholar]
- 50. Lantican M. A., et al., Impacts of International Wheat Improvement Research, 1994-2014 (CIMMYT, D.F., Mexico, 2016). [Google Scholar]
- 51. Subbarao G. V., Searchinger T. D., Opinion: A “more ammonium solution” to mitigate nitrogen pollution and boost crop yields. Proc. Natl. Acad. Sci. U.S.A. 118, e2107576118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Searchinger T. D., et al., "A pathway to carbon neutral agriculture in Denmark, WRI report" (World Resources Institute, Washington DC, 2021, pp. 61–95; 10.46830/wrirpt.20.00006). [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genetic stocks developed and presented in this study are deposited with CIMMYT and available upon request subjected to consent from JIRCAS.