Summary
Objective:
Neuroinflammation associated with anti-N-methyl-D-aspartate receptor encephalitis may facilitate seizures. We previously showed that intraventricular administration of CSF from patients with anti-NMDAR encephalitis to mice precipitates seizures, thereby confirming that antibodies are directly pathogenic. To determine if interleukin (IL)-1-mediated inflammation exacerbates autoimmune seizures, we asked if blocking the effects of IL-1 by anakinra, a selective IL-1 receptor antagonist, blunts antibody-induced seizures.
Methods:
We infused C57BL/6 mice intraventricularly with purified serum IgG from patients with anti-NMDAR encephalitis or monoclonal anti-NMDAR IgG; subdural EEG was continuously recorded. After a 6-day interval, mice received anakinra (25 mg/kg s.c., twice daily) or vehicle for five days. Following a 4-day washout period, we performed behavioral tests to assess motor function, anxiety, and memory, followed by hippocampus tissue analysis to assess astrocytic (GFAP) and microglial (Iba-1) activation.
Results:
Of 31 mice infused with purified patient NMDAR-IgG (n = 17) or monoclonal NMDAR-IgG (n = 14), 81% developed seizures. Median baseline daily seizure counts during exposure to antibodies were 3.9; most seizures were electrographic. Median duration of seizures during the baseline was 82.5 sec. Anakinra administration attenuated daily seizure frequency by 60% (p = 0.02). Anakinra reduced seizure duration; however, the effect was delayed and become apparent only after the cessation of treatment (p = 0.04). Anakinra improved novel object recognition in mice with antibody-induced seizures (p = 0.03) but did not alter other behaviors. Anakinra reduced the expression of GFAP and Iba-1 in the hippocampus of mice with seizures, indicating decreased astrocytic and microglial activation.
Significance:
Our evidence supports a role for IL-1 in the pathogenesis of seizures in anti-NMDAR encephalitis. These data are consistent with therapeutic effects of anakinra in other severe autoimmune and inflammatory seizure syndromes. Targeting inflammation via blocking IL-1 receptor mediated-signaling may be promising for developing novel treatments for refractory autoimmune seizures.
Keywords: neuroinflammation, IL-1, cytokines, anti-NMDA receptor encephalitis, autoimmune seizures, autoantibodies
1. Introduction
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is an autoimmune disease that manifests with acute confusion, memory loss, and severe seizures in previously healthy people.1 Status epilepticus is present in 45% of patients treated in intensive care settings, and seizures that do not respond to treatment develop in two-thirds of these patients.2 Seizures subside in some patients after immunotherapies aimed to remove antibodies, thereby suggesting that antibody proteins were linked to clinical symptoms.3 We recently established the antibodies’ pathogenic role in seizures, in that intraventricular administration of cerebrospinal fluid (CSF) from affected patients, or purified patient anti-NMDAR antibodies, or commercial antibodies directed to the N-terminal domain of GluN1, into mice was shown to precipitate seizures, thereby confirming that antibodies are directly pathogenic for seizures.4
Along with modulation of NMDAR channels possibly resulting in increased overall excitability of the seizure network in autoimmune encephalitis, the innate immune system in patients with this condition has been increasingly considered as a potential target for new anticonvulsive therapies.5-7 Seizures or brain injury can trigger neurogenic inflammation.8 In particular, both brief and prolonged seizures in mice rapidly induce cyclooxygenase-2 (Cox-2) in forebrain glutamatergic neurons.9-11 The resulting release of prostaglandins from these neurons exacerbates ongoing inflammatory processes and may further perpetuate seizures.12, 13 Proinflammatory cytokines (e.g., interleukin (IL-6, IL-17A and IL-2) are persistently elevated in the CSF and serum of anti-NMDAR encephalitis patients.14 Furthermore, the levels of C-X-C motif chemokine 13 (CXCL-13) are increased in the CSF of patients with anti-NMDAR encephalitis and are positively correlated with intrathecal anti-NMDAR antibody titer, suboptimal response to treatment and higher rates of disease relapse.15 Corticosteroids, broad spectrum anti-inflammatory agents, are the first line of therapy for anti-NMDAR encephalitis; however, only half of patients show improvement leaving the remaining patients to be approached with other limited treatment modalities.3 Identifying the role of specific inflammatory pathways in the development of autoimmune epilepsy would uncover new therapeutic targets for seizure attenuation.8
One potential target for novel anticonvulsant therapies is IL-1 receptor-mediated signaling8, 11. In inflammatory states following the recruitment of the Toll-like receptor (TLR) system, microglial cells release IL-1β, a potent proconvulsant.5 Similarly, the sustained seizures in status epilepticus cause neuroinflammation partly via the IL-1β system, thereby promoting epileptogenesis.8, 16-18 Anakinra (Kineret®, Swedish Orphan Biovitrum, Sobi), a recombinant and modified version of the human IL-1 receptor antagonist protein currently approved for the treatment of rheumatoid arthritis, has been regarded as a promising therapy for inflammatory epilepsies.19 It has been used effectively in a few patients with severe autoimmune seizures, both in the acute and chronic phases.20-23 Anakinra is a hydrophilic protein that penetrates the blood-brain barrier and has a relatively short half-life, which decreases the possibility of side effects when used in affected patients.24, 25 To establish the role of IL-1 proteins fundamentally involved in epileptogenesis in autoimmune seizures, we asked if blocking the effects of IL-1 by anakinra, a selective antagonist of IL-1 receptor 26, would blunt seizures induced by anti-NMDAR antibodies.
2. Materials and Methods
All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Nebraska Medical Center (UNMC). The principles outlined in the ARRIVE guidelines27 and the Basel declaration, including the 3R concept, were followed during experimental planning.
2.1. Animal care
Male C57BL/6N mice (8-10 weeks, 25-30 g from Charles River, Roanoke, IL) were housed in groups of five and maintained on a 12 h light cycle (light on/off at 7 a.m. / 7 p.m.) with ad libitum access to food and water. Following the implantation of the guide cannula targeting the lateral ventricle and the EEG mount, mice were housed individually in the EEG recording chambers until the completion of EEG monitoring. All functional measures were acquired in a blinded manner.
2.2. Drugs and experimental antibodies
Human serum IgG fraction containing anti-NMDAR antibodies (provided by A.Z and S.J. P. at Mayo Clinic) were purified from the pooled serum of 12 patients with anti-NMDAR encephalitis (approved by the Mayo Clinic IRB) and the activity against NMDAR-IgG was confirmed using indirect immunofluorescence on human embryonic kidney (HEK) 293 cells transfected with a plasmid encoding the GluN1 subunit of the NMDAR (Euroimmun Lübeck, Germany) and by tissue-indirect immunofluorescence, confirming NMDAR-IgG-specific pattern of staining as previously described.4 The serum tested negative for other neural autoantibodies, including anti-neuronal nuclear antibody types 1 and 2 (anti-Hu and anti-Ri, respectively), anti-neuronal antibody type 3, anti-glial neuronal antibody type 1 (anti-SOX1), PCA2 (anti-MAP1B), Purkinje cell cytoplasmic antibody (PCA) types 1 and 2 (anti-Yo and anti-MAP1B, respectively), PCA-Tr (anti-DNER), amphiphysin, collapsing response mediator protein 5, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, gamma amino butyric acid B receptor, leucine glioma inactivated 1 and Caspr 2 protein antibodies, antibodies to metabotropic glutamate receptors 1, 2, dipeptidyl aminopeptidase-like protein 6, and glial fibrillary acidic protein. The IgG protein was dissolved in phosphate-buffered saline (PBS) (0.02 μg/μl).
A human monoclonal GluN1 antibody (5F5) specific for GluN1 was derived from memory B cells of a patient with anti-NMDAR encephalitis and seizures.28 Specificity for NMDAR-IgG was confirmed using indirect immunofluorescence on HEK 293 cells.28 The 5F5 antibody binds in the extracellular amino terminal domain of the GluN1 and requires the N368 site.28 Proteins were dissolved in PBS (0.02 μg/μl).
Anakinra was a gift from Sobi (Stockholm, Sweden). The drug was dissolved in normal saline and administered subcutaneously (25 mg/kg) twice daily.
2.3. Stereotactic surgery, osmotic minipump insertion, and administration of anakinra
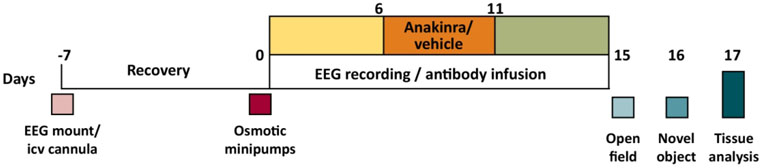
Mice were anesthetized with isoflurane and implanted with a unilateral injector guide cannula into the lateral ventricle, the 2 EEG/1 EMG head mount (Pinnacle Technology Inc., Lawrence, KS), and two cortical screw EEG electrodes to derive signals from the parietal cortex overlying the hippocampus and ipsilateral frontal cortex as previously described.4 Seven days later (day 0) mice were re-anesthetized with isoflurane, implanted with subcutaneous micro-osmotic infusion pumps (Alzet, Cupertino, CA) containing purified serum IgG or monoclonal IgG (Fig. 1); head mounts were connected to the EEG acquisition system. IgG solution was continuously perfused at a flow rate of 0.25 μl/h. Following a 6-day assessment of baseline seizure counts, mice were injected with anakinra or vehicle twice daily for five days (Fig. 1). The EEG was continued for an additional four days. Upon completion of the experiments, the contents of the pumps were examined for residual IgG solution, and the residual volumes were negligible. The mean amount of protein (± SEM) delivered intracerebroventicular (i.c.v.) to mice was 5.7 ± 0.09 ng/kg/day.
Figure 1.
Experimental protocol to assess the role of anakinra in the persistence of seizures induced by anti-NMDAR antibodies, followed by behavioral and histological analysis.
2.4. EEG acquisition and analysis of seizure patterns
Continuous video EEG acquisition (Pinnacle Technology, Inc.) was started on day 0, immediately following the implantation of the infusion pumps (Fig. 1). EEG analysis was carried out retrospectively as previously described (Sirenia Seizure Pro 1.7.6, Pinnacle Technology, Inc.) and verified visually without knowledge of the treatment status.4 Briefly, seizures were defined as rhythmic activity for 5 sec or longer that exceeds the baseline amplitude by at least 3-fold. A modified Racine scale was applied to characterize the following behavioral signs: nonconvulsive (0), freezing (1), facial tremor (2), head shaking (3), isolated generalized jerks (4), sustained clonic activity (5), or unclear behavior (6).4 All EEGs were analyzed by two investigators with one of them being unaware of the treatment status.
2.5. Open field
An open field test was performed using a video tracking system (Noldus, Leesburg, VA) as previously described.4 Briefly, mice were habituated in the testing room for 30 min prior to being placed into the custom-made acrylic chamber (41 L x 41 W x 35 H cm) and were allowed to move freely for 20 min. The motor behavior was measured as the total distance travelled during the trial. Anxiety-related behavior was measured based on the percent time animals spent in the center of the arena (25% of the total area) during a 20-min trial.
2.6. Novel object
A novel object recognition test was performed as previously described4. The exploration behavior was defined as orientation of the nose to the object at a distance of ≤2 cm. The time spent with familiar object (FO) and novel object (NO) was measured and compared for each treatment group. Mice that explored both objects for a total time of < 4.5 sec were excluded. Additionally, to account for normalization on total exploration time, a novel object index (NOI) was calculated as time spent exploring the novel object divided by the sum of times spent exploring both novel and familiar objects.
2.7. Histological evaluations
Upon completion of experiments, animals were deeply anesthetized with isoflurane and transcardially perfused with PBS followed by 4% paraformaldehyde in PBS. Frozen coronal sections (50 μm; at least 4 sections per mice) were processed for immunohistochemistry for GFAP (astrocytes) and Iba-1 (microglia) as previously described.4, 29 Briefly, sections were incubated at 4 °C overnight with polyclonal rabbit anti-GFAP antibodies (1:500, N 1506, Dako, Carpentaria, CA) or anti-Iba-1 antibodies (1:500, 019-19741, Fujifilm Wako Chemicals, Richmond VA). Following extensive washing, goat anti-rabbit biotinylated secondary antibodies (1:300, Vector, Burlingame, CA) were used for signal detection.
2.8. Imaging acquisition and processing
Slide specimens of the CA1 region of hippocampus from both hemispheres between bregma −1.55 and −2.03 were scanned with a Nuance Multi-Spectral Imaging System (Cambridge Research Instruments, Woburn, MA) fitted to a Nikon ECLIPSE 55i microscope (Nikon, Tokoyo, Japan) with a 20X objective (1392 X 1040 pixels, 0.498 μm2/pixel). The absorbance spectrum for each pixel was scanned from 530 nm to 620 nm at 10 nm increments; the absorbance profile of immunostaining for GFAP or Iba-1 was input to the system; and the quantitative greyscale image corresponding to the GFAP or Iba-1 profile was extracted from the scanned images. Greyscale images were transferred to the Nuance environment for quantification of the intensities (grey scale units, gru) and areas (μm2) as previously described.30 The GFAP or Iba-1 abundances per the positivity event were computed as the product of mean pixel intensity and area (gsu ● μm2). The immunopositivity in the hippocampal CA1 region was taken as the sum of abundances of all events in the CA1 region scanned. The same area of the CA1 region was analyzed for the specimens from anakinra-treated and control mice; therefore, abundances were normalized to the fixed scanned area. Duplicate slides from each mouse were averaged. The analyses were performed by the investigators who were unaware of the treatment assignment.
2.9. Statistical analysis
Seizure counts and seizure duration were compared between two treatment groups using two-way analysis of variance (ANOVA) followed by Sidak’s multiple comparison tests to assess the change from the corresponding baseline in each treatment group (GraphPad Prizm 8.4, San Diego, CA). The time course of seizure counts was assessed using repeated measures ANOVA. The times spent with familiar and novel objects in the novel object assay were compared for each treatment group using paired t-tests while NOIs were compared using one-sample t-tests with the assumption that random presence of the animal at the objects would lead to a NOI index of 50%. The distance travelled and percent time spent in the middle in the open field assay were compared between the treatment groups using Student’s t-tests. The expressions of GFAP- and Iba-1-immunoreactivity in hippocampus were compared using Student’s t-tests.
3. Results
3.1. Anakinra attenuates the frequency and duration of antibody-induced seizures
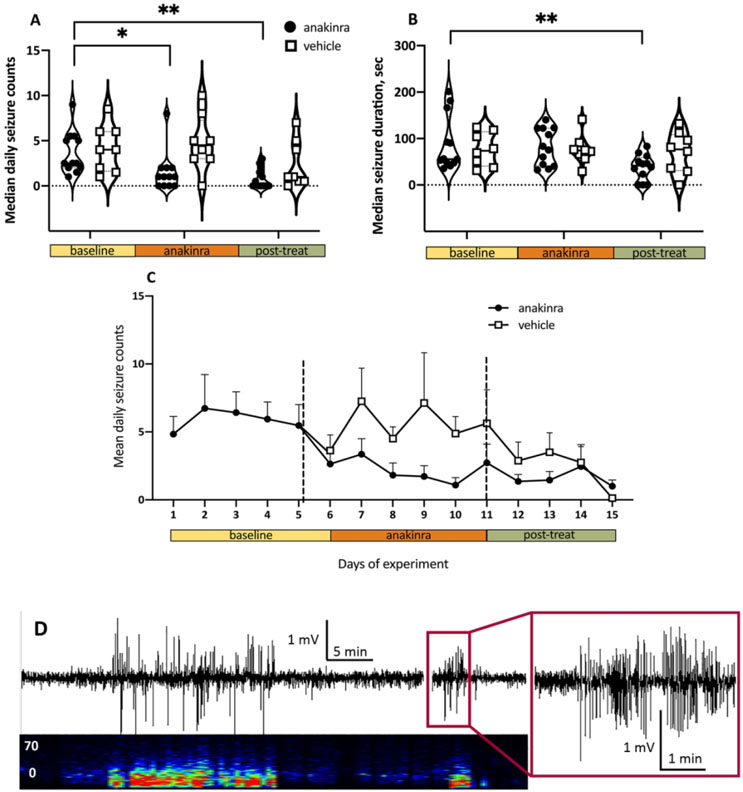
Thirty-one mice were infused with purified patient anti-NMDAR antibodies (n = 17) or the 5F5 anti-NMDAR monoclonal antibodies (n = 14). Consistent with our previous report4, 25 mice (81%) developed seizures following the exposure to antibodies. Six mice that did not develop seizures and five mice with median daily seizure counts of < 0.5 were excluded; the remaining 20 mice were randomized to receive anakinra (n = 12) or vehicle (n = 8; Fig. 1). The median baseline seizure counts were not significantly different in mice infused with purified patient antibodies (n=10) or monoclonal antibodies (n=10; p=0.86, unpaired t-test) and therefore these groups were combined.. The median daily seizure count during the baseline period in all mice combined was 3.9 (25-75% Interquartile range, IQR 3.7-4.1). At two weeks from the initiation of antibody infusion (9 days after initiation of anakinra treatment), anakinra reduced the number of seizures by 60% compared to the corresponding baseline counts in anakinra-treated mice (p = 0.02; F 2, 36=7.40, two-way ANOVA, Fig. 2A). Specifically, in post-hoc comparisons, seizure frequency in anakinra-treated mice decreased during the treatment period and remained low upon cessation of treatment (p = 0.02 and p = 0.002 vs. baseline, respectively; Sidak’s multiple comparison tests, Fig. 2A). When examining the temporal distribution of seizures, the daily counts decreased starting at 24-48 h following the initiation of anakinra (p = 0.02, F 14, 238 = 2.0, repeated measures ANOVA; Fig. 2C). There was no change in the seizure counts during the treatment or after washout in the vehicle-treated groups (p = 0.88 and p = 0.19 vs. baseline, respectively; Sidak’s multiple comparison tests, Fig. 2A). Of note, seizure responses to anakinra were not different in the groups of mice infused with patient-derived antibodies or monoclonal antibodies (p=0.65, unpaired t-test). Of 1096 seizures recorded during the study in both treatment groups, the majority (97.4%) were electrographic only (Racine’s score 0; Fig. 2D) while the remaining were accompanied by behavioral arrest (score 1, 0.8%) or their behavioral pattern was unclear (score 6, 1.7%).
Figure 2.
Administration of anakinra reduced seizures induced by anti-NMDAR antibodies. Data are daily medians and 25-75% interquartile ranges (IQR). Solid and dotted horizontal lines indicate median values and IQRs, respectively *, p < 0.05; **, p < 0.01, ANOVA with Sidak multiple comparisons tests. (A) Daily seizure counts in anakinra-treated mice (n = 12) were reduced during the treatment (orange bar) and post-treatment periods (green bar) compared to the corresponding baseline (yellow bar). The seizure counts were unchanged in the vehicle treated mice (n = 8). (B) Delayed effect of anakinra on seizure duration. The duration of seizures was decreased in the washout phase following anakinra. (C) Effects of anakinra on seizure counts were apparent 24-48 h following the initiation of treatment. Each time point represents the mean and SEM at the completion of a 24-h of recording. (D) Representative 60-min EEG recording and the corresponding spectrogram showing clusters of electrographic seizures in the parietal cortex of mice during the continuous intracerebroventicular (i.c.v.) infusion of anti-NMDAR antibodies. The vertical axis of the spectrogram represents frequency from 0 to 70 Hz and the horizontal axis represents time in min. The trace of one seizure is expanded to demonstrate the characteristic pattern of high amplitude sharp rhythmic activity.
The median seizure duration during baseline period in all mice was 82.5 sec (IQR, 76.5-88.7). Anakinra shortened the duration of epileptic activity by 25.2 % compared to the corresponding control (p = 0.04, F 2, 36 = 3.60, two-way ANOVA; Fig. 2B). However, reduction of the seizure duration was not apparent during the treatment period but instead was delayed until after the cessation of treatment (p = 0.96 and p = 0.004 vs. baseline, respectively; Sidak’s multiple comparison tests; Fig. 2B). The median duration of seizures remained unchanged in mice from the vehicle-treated group (p = 0.99 and p = 0.97 vs. baseline, respectively; Sidak’s multiple comparison tests).
3. 2. Anakinra improves memory in mice with seizures induced by antibodies
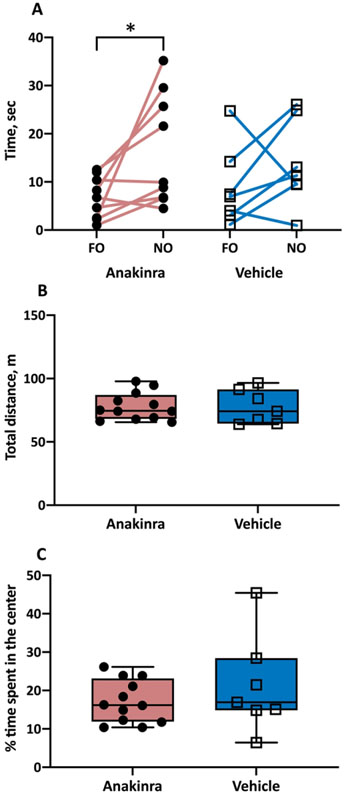
Since there were no differences in the vehicle-treated mice infused with patient and monoclonal antibodies with respect to times spent exploring the familiar or novel objects (p=0.31 and p=0.88, respectively), these two groups were pooled together. The time spent with FO and NO in the vehicle treated group were (mean ± SEM) 8.8 ± 3.1 and 13.6 ± 3.4 sec, respectively (Fig. 3A). There was no significant difference between these latencies (p = 0.29, paired t- tests). There were no differences in the anakinra-treated mice infused with patient and monoclonal antibodies with respect to times spent exploring the familiar or novel objects (p = 0.47 and p= 0. 50, respectively) and the two groups of animals were combined. The times for FO and NO in the anakinra-groups were 6.7 ± 1.5 and 16.5 ± 3.9 sec, respectively. Anakinra induced a significant improvement in the recognition of NO by allowing the prolongation of the time spent with NO by 9.8 sec, compared to that spent with FO (p = 0.03, paired t test; Fig. 3A). Sham mice and vehicle-treated mice that had no seizures or developed very low seizure counts (i.e., median daily seizure counts of < 0.5) in response to the antibody infusion did not demonstrate memory impairment in the novel object paradigm (Supplemental data).
Figure 3.
Behavioral phenotype of mice with autoimmune seizures treated with anakinra. (A) Anakinra rescued an ability to discriminate between familiar object (FO) and novel object (NO) in mice with seizures induced by anti-NMDAR antibodies. N = 9 (anakinra-treated), n = 7 (vehicle-treated). (B) Anakinra had no effect on locomotor activity in mice with seizures. N = 12 (anakinra- treated), n = 7 (vehicle-treated). (C) Anakinra did not affect the anxiety scores in mice with seizures. *, p<0.05, paired t-tests. Error bars represent mean ± SEM.
The NOIs in the vehicle- and anakinra-treated groups were 0.60 ± 0.27 and 0.69 ± 0.18, respectively (mean ± SEM). The direct comparison of the NOIs between the two groups failed to reach statistical significance (p= 0.45, unpaired t test). However, the NOI is expected to be higher than 50% when novelty is recognized and NOI was in fact significantly different from chance in the anakinra-treated mice (p=0.01, one sample t-test) but not in the vehicle-treated mice (p = 0.36, one sample t-test).
When exposed to the open field, the total distance traversed by mice (mean ± SEM) in the vehicle- and anakinra- treated groups was 77.9 ± 3.2 and 75.5 ± 5.0 m, respectively (Fig. 3B). Furthermore, the proportion of time spent in the inner zone of the arena in the same groups was 17.1 ± 1.6 and 20 ± 4.7 percent, respectively (Fig. 3C). Administration of anakinra did not affect the locomotor activity and emotion-related behavior in mice (p = 0.94 and p = 0.33, respectively, t-tests).
3.3. Anakinra reduces the expression of astrocytic and microglial markers of inflammation in the hippocampus
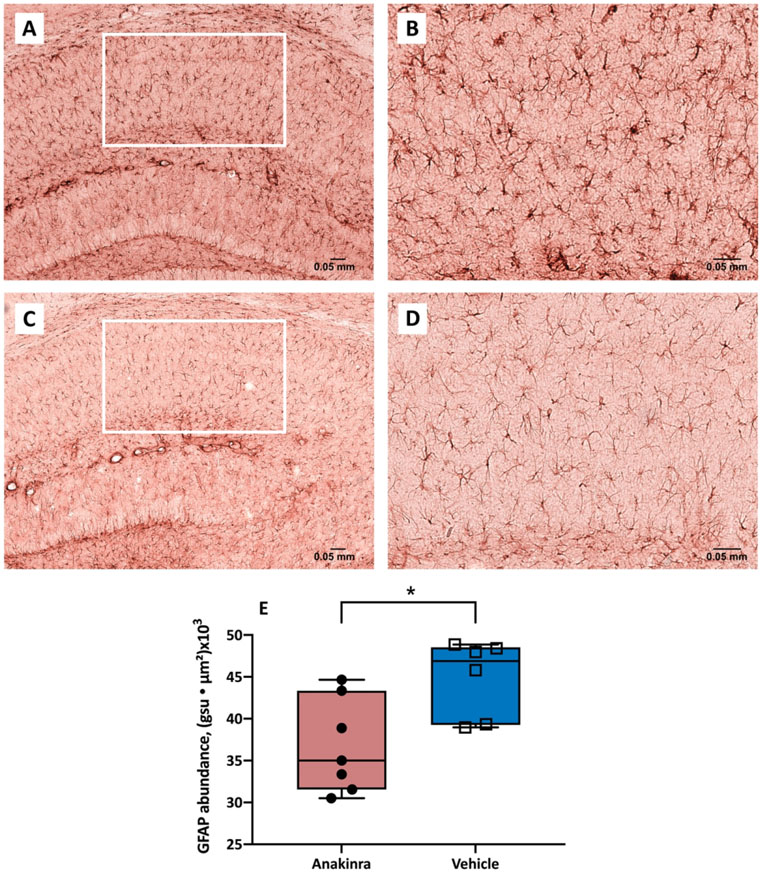
Sections of CA1 region of the hippocampus stained for GFAP immunoreactivity demonstrated a characteristic pattern of immunostaining and the glial cells had a typical morphological appearance (Fig. 4A-D). The expression of GFAP in the CA1 region of hippocampus (i.e., GFAP abundance) was computed as the sum of abundances of all positivity events in the region while the GFAP abundance per event was determined as the product of mean pixel intensity and area (gsu ● μm2). The expression of GFAP was (mean ± SEM) 44.9 ± 1.9 and 36.8 ± 2.1 gsu ● μm2 x (103) in the vehicle- and anakinra treated mice, respectively (Fig. 4E). Anakinra significantly reduced the expression of GFAP in CA1 region of mice with antibody-induced seizures (p = 0.02, Student’s t-test).
Figure 4.
Abundance of GFAP-positive astrocytes in the CA1 region of hippocampus of mice with autoimmune seizures. (A-D) Representative GFAP immunostaining of the CA1 region in vehicle-treated (upper panel) and anakinra-treated (lower panel) mice with seizures induced by continuous infusion of anti-NMDAR antibodies at 10 X (A, C) and 20 X (B, D). (E) Anakinra reduced the expression of GFAP in the CA1 region of hippocampus in mice with seizures. The abundance of GFAP labeling in the CA1 region was determined as the sum of the products of mean pixel intensity (grey scale units, gsu) and area of each event (μ2) in a fixed scan area. N = 7 (anakinra-treated), n = 6 (vehicle-treated). * p<0.05, Student’s t-test. Error bars represent mean ± SEM.
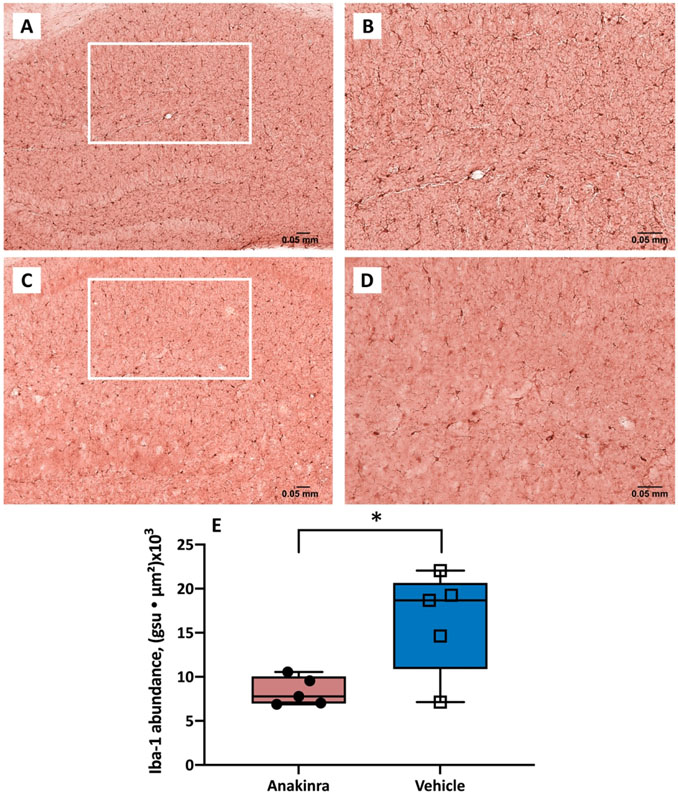
To assess the extent of Iba-1 expression in the hippocampal CA1 sections (i.e., Iba-1 abundance), we measured the sum of abundances of all events in the region; the Iba-1 abundance per event was determined as the product of mean pixel intensity and area (gsu ● μm2; Fig. 5A-D). The expression of Iba-1 immunoreactivity in the CA1 region of hippocampus was 16.3 ± 2.5 and 8.4 ± 0.7 gsu ● μm2 (x103) in the vehicle- and anakinra-treated groups, respectively Anakinra reduced the area occupied by microglia in mice with autoimmune seizures (Fig. 5E, p = 0.02, Student’s t-test).
Figure 5.
Expression of Iba-1 immunoreactivity in the CA1 region of hippocampus in mice with autoimmune seizures. (A-D) Representative Iba-1 immunostaining images of the CA1 region of vehicle-treated (upper panel) and anakinra-treated (lower panel) mice with seizures induced by anti-NMDAR antibodies at 10 X (A, C) and 20 X (B, D). (E) Anakinra reduced the expression of Iba-1 in the CA1 region of hippocampus in mice with seizures. The abundance of Iba-1 labeling in the CA1 region was determined as the sum of the products of mean pixel intensity (gsu) and area of each event (μ2) in a fixed scan area. N=5 (anakinra-treated), n=5 (vehicle-treated). * p<0.05, Student’s t-test. Error bars represent mean ± SEM.
4. Discussion
In the present study, we examined the role of IL-1 receptor-mediated inflammation in the persistence of autoimmune seizures using the mouse model of anti-NMDAR encephalitis developed in our laboratory.4 We showed that repeated systemic administration of anakinra, a potent and selective IL-1 receptor antagonist, significantly attenuated the frequency and duration of seizures induced by anti-NMDAR antibodies and improved memory of mice with seizures without affecting other behaviors. Furthermore, anakinra reduced the hippocampal expression of markers of activated microglia and astrogliosis in mice with autoimmune seizures. These findings suggest that targeting IL-1 signaling may be useful for treatment of seizures associated with anti-NMDAR encephalitis.
Neurogenic inflammation and proinflammatory cytokines, including IL-1β, are increasingly recognized as contributing to the pathogenesis of seizures.8, 11, 31 IL-1β synthesis in the brain takes place in astrocytes, microglia, and a subset of neurons.32, 33 The baseline levels of IL-1β are low but increase robustly in neurons and glia following the induction of status epilepticus by electrical stimulation of hippocampus or during chemically induced seizures in rodents.33-36 IL-1β levels were elevated in serum of patients with autoimmune epilepsy presenting with new-onset refractory status epilepticus (NORSE) and in the CSF and serum of patients with anti-NMDAR encephalitis; notably the decline of cytokine concentration in response to anti-inflammatory therapies correlated with clinical improvement.6, 37-39 These data suggest that autoimmune encephalitis and other acute devastating epileptic encephalopathies are accompanied by sustained disruption of cytokine-mediated signaling; and thus, targeted attenuation of IL-1β signaling may provide new therapeutic opportunities for seizures. We did not measure cytokine expression in mice with antibody-induced seizures in this study; however, our preliminary results from related experiments showed that mice with seizures induced by monoclonal anti-NMDAR antibodies showed signs of hippocampal inflammation with increased levels of inflammatory markers, including IL-1β (unpublished).
Pharmacological blockade of IL-1 receptors in rodent models of limbic epilepsy attenuates chemically- and electrically-induced status epilepticus and reduces inflammation, further supporting the premise that IL-1 receptor-mediated signaling contributes to the onset and persistence of seizures.12, 34 Anakinra, a recombinant IL-1 receptor antagonist, has a plasma half-life of 4-6 hours after subcutaneous administration in humans and blocks the activity of IL-1β and IL-1α, the product of a related gene.25, 40 In case reports, administration of anakinra to patients with recurrent seizures in FIRES and epilepsy associated with autoinflammatory conditions attenuated epileptic activity and improved encephalopathy; this occurred in parallel to the reduction of serum IL-1β levels.20-22 Furthermore, anakinra was beneficial in combatting relapsing seizures in the late stages of prolonged febrile infection-related encephalopathy syndrome (FIRES), suggesting that targeting IL-1 receptor may also be effective in chronic seizures and may interfere with epileptogenesis.23 We showed that anakinra reduced seizure burden in mice with antibody-induced seizures by attenuating both frequency and duration of seizures. Taken collectively with previous reports in patients, these data suggest that anti-inflammatory therapy may be an effective approach to reduce autoimmune seizures. This anticonvulsant activity could be due to a direct effect of anakinra on the IL-1 receptor and interruption of the downstream effects triggered by IL-1β, including blood-brain barrier breakdown, which occurs during seizures.11, 12, 41 Interestingly, a transient interruption of IL-1 function in our studies had a long-lasting effect on seizure activity as evidenced by decreased seizure duration following the removal of treatment. The latter suggests the intriguing possibility that anakinra not only reduces acute neuroinflammation but may also have a disease-modifying effect on antibody-induced seizures.
A daily dose of 50 mg/kg anakinra administered to mice in our study corresponds to a daily equivalent of 285 mg in an adult human42, which is higher than that recommended for the treatment of systemic rheumatological conditions 26. However, we anticipate that anakinra will be administered for a much shorter period of time to patients with autoimmune encephalitis and NORSE compared to those with other chronic autoinflammatory conditions26. Furthermore, previous studies in healthy volunteers treated with 700-800 mg of anakinra did not find any clinically significant differences between the drug and placebo-treated groups in physical examination, complete blood counts, mononuclear cell phenotypes, blood chemistry profiles or serum cortisol levels 43. In our study, mice were continuously monitored with video; they showed no overt behavioral signs of toxicity.
The mechanism of seizure reduction achieved by IL-1 receptor blockade is not completely understood. Exogenous application of IL-1β into the hippocampus increases cytokine production in glial cells and prolongs kainate-induced seizures likely by enhancing glutamatergic neurotransmission.35 The potentiation of NMDA responses occurs via increased Ca2+ entry through receptor-associated ion channels, which remains sustained following a transient exposure to IL-1β.44 On the other hand, the release of IL-1β can cause a reduction of inhibitory tone via attenuation of GABA-evoked currents as observed in tissue from patients with refractory temporal epilepsy.45 Anakinra might attenuate epileptic activity by restoring the balance between the inhibitory and excitatory circuits in hippocampus. Since the drug was administered systemically and there is evidence of peripheral inflammatory response in autoimmune encephalitis6, 29, it is possible that in addition to blocking the central effects of the IL-1-mediated signaling, anakinra also attenuated peripheral proinflammatory factors that may have contributed to promoting seizure activity.
Patients with anti-NMDAR encephalitis demonstrate memory impairment that correlates with the loss of functional connectivity in the hippocampus.1, 46 In animal studies, mice infused with anti-NMDA-IgG-positive CSF or immunized with NMDA-like holoreceptors develop progressive memory deficits and a corresponding increase of tissue-bound antibodies in the hippocampus.47, 48 Consistent with these previous reports from preclinical studies and observations in patients, mice with autoimmune seizures in our study showed impaired hippocampal-dependent memory. 1, 47, 48 The restoration of memory with anakinra opens an intriguing possibility that the blockade of IL-1 receptor-mediated signaling could repair functional connectivity in the hippocampus and improve memory in patients. In a case series of children with FIRES, anakinra promoted resolution of seizures and cognitive improvement; however, the role of improved seizure control in the neurological recovery could not be teased apart.49 We cannot determine from our data if the improved memory is a consequence of the reduced seizure burden or an anti-inflammatory effect of anakinra itself. The effects of persistent ictal and interictal activity are well documented in patients with epilepsy along with the improvement in cognition upon seizure control achieved with surgery or conventional anti-seizure medications. 50, 51 IL-1 blockade with anakinra was also effective to reduce cognitive impairment in other acute seizure models and in a mouse model of traumatic brain injury.52, 53 Of note, in our previous study, we found insignificant memory deficit in mice with seizures induced by infusion of CSF or purified IgG from patients with anti-NMDAR encephalitis.4 Our ability to demonstrate memory decline in the present study could be supported by the use of monoclonal anti-NMDAR antibodies that bind to a single epitope and possibly contributes to a more consistent behavioral phenotype as well as technical improvement of the previously used assay to assess memory.4, 28, 54
Histopathology of anti-NMDAR encephalitis in patients demonstrated neuroinflammation, infiltrates of lymphocytes or macrophages and rare neuronal loss.55, 56 We previously found morphological changes in glial cells of the CA1 region in the same mouse model, suggestive of inflammation; however, there was no increase in the number of GFAP-positive cells in mice with seizures.4 Using a different method of quantification of immunoreactivity in the present study, we showed that anakinra reduced the expression of markers of microgliosis and astrogliosis in the CA1 region of hippocampus. The GFAP and Iba-1 immunoreactivity in hippocampus of mice with experimentally-induced anti-NMDAR encephalitis in another mouse model did not differ from that in control mice after three weeks of florid behavioral presentations of encephalitis but was significantly increased at the fulminant stages of the disease at six weeks.48 The authors proposed that the detection of inflammatory changes with these markers in mice with autoimmune encephalitis may depend on the time point at which the tissue is examined.48
Targeting IL-1 receptor-mediated signaling has tremendous potential for treating refractory autoimmune seizures and other drug-resistant epilepsies57. Thus, the introduction of anakinra in 1993 was followed by the development of canakinumab, a human IL-1β monoclonal antibody that has a long plasma half-life, and binds selectively with high affinity to IL-1β.25 Gevokizumab, a novel IgG2 humanized monoclonal antibody, targets a single epitope of IL-1β and modulates the interaction of the cytokine with the receptor complex.25 While these agents are currently in clinical trials for peripheral autoimmune and inflammatory disorders, their success may catalyze their use for relapsing autoimmune seizures25, if they are brain-penetrant. Based on the existing literature, our present findings and previously published data from our laboratory4, we propose that seizures resulted from exposure to antibodies induce neuroinflammation and facilitate the release of IL-1, which exacerbates both seizures and cognitive deficits.
5. Conclusions
In summary, we demonstrated that the IL-1 receptor antagonist anakinra attenuated seizures induced by antibodies against NMDA receptors and improved memory in mice as tested in the novel object recognition assay. Furthermore, anakinra reduced the expression of the inflammatory astrocytic and microglial markers in the hippocampus of mice with autoimmune seizures. These findings suggest that targeting IL-1 signaling may be a promising novel approach for the treatment of clinical conditions that present with autoimmune seizures and associated encephalopathy.
6. Limitations
In this study, we used a mouse model of anti-NMDAR antibody-induced seizures developed in our laboratory.4 While the seizures in mice were frequent, they did not recapitulate the full extent of epileptic activity that occurs in patients with NORSE, FIRES, and other acute devastating seizure encephalopathies. In addition to neuroinflammation and autoantibodies, other currently unidentified insults may perpetuate seizures in these patients and these factors might not be recounted in the present model. Therefore, it remains unclear which population of patients will benefit from blockade of IL-1 receptors. Furthermore, the EEG recordings in mice were limited to two brain areas; thus, the full extent of seizure propagation could not be ascertained. Therefore, the findings may not be generalizable to predict the effects of anakinra in multifocal or generalized seizures, which occur in autoimmune status epilepticus. As acknowledged in other studies on inflammation and seizures 12, 58 it is difficult to discern the effects of reduced seizures from unrelated anti-inflammatory effects of anakinra on behavior and the expression of astrogliosis and microgliosis.
Supplementary Material
Key Points.
Anakinra, an antagonist of interleukin-1 (IL-1) receptor, attenuates seizures in mice induced by neuronal antibodies from patients with autoimmune encephalitis.
Anakinra also attenuates memory impairment and reduces inflammation in the hippocampus of mice with autoimmune seizures.
In addition to encouraging clinical data, these findings suggest that IL-1 plays a role in pathogenesis of autoimmune seizures.
Acknowledgments
The authors would like to thank Dr. Larisa Poluektova and Mr. Edward Makarov for their expert advice and technical assistance with image processing, and Ms. Robin Taylor for excellent editorial support. We thank Swedish Orphan Biovitrum (Stockholm, Sweden) for providing the supply of anakinra as a gift. SKD holds the Joseph and Ray Gordon Chair for Clinical Oncology and Research at the Lankenau Institute for Medical Research. O.T. received grant support from the American Epilepsy Society Junior Investigator Research Award, and R.D. from NIH award R01NS112308. The work described herein is consistent with the Journal’s guidelines for ethical publication.
Footnotes
Disclosures
The authors have declared that no conflict of interest exists.
References
- 1.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011. January;10(1):63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Montmollin E, Demeret S, Brule N, Conrad M, Dailler F, Lerolle N, Navellou JC, Schwebel C, Alves M, Cour M, et al. Anti-N-methyl-d-aspartate receptor encephalitis in adult patients requiring intensive care. Am J Respir Crit Care Med 2017. February 15;195(4):491–9. [DOI] [PubMed] [Google Scholar]
- 3.Titulaer MJ, McCracken L, Gabilondo I, Armangue T, Glaser C, Iizuka T, Honig LS, Benseler SM, Kawachi I, Martinez-Hernandez E, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: An observational cohort study. Lancet Neurol 2013. February;12(2):157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taraschenko O, Fox H, Pittock S,Zekeridou A, Gafurova M, Eldridge E, Liu J, Dravid S, Dingledine R A mouse model of seizures in anti-N-methyl D-aspartate receptor encephalitis. Epilepsia 2019;60(3):452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matin N, Tabatabaie O, Falsaperla R, Lubrano R, Pavone P, Mahmood F, Gullotta M, Serra A, Di Mauro P, Cocuzza S, et al. Epilepsy and innate immune system: A possible immunogenic predisposition and related therapeutic implications. Hum Vaccin Immunother 2015;11(8):2021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kothur K, Wienholt L, Brilot F, Dale RC. CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: A systematic review. Cytokine 2016. January;77:227–37. [DOI] [PubMed] [Google Scholar]
- 7.Toledano M, Britton JW, McKeon A, Shin C, Lennon VA, Quek AM, So E, Worrell GA, Cascino GD, Klein CJ, et al. Utility of an immunotherapy trial in evaluating patients with presumed autoimmune epilepsy. Neurology 2014. May 6;82(18):1578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aronica E, Bauer S, Bozzi Y, Caleo M, Dingledine R, Gorter JA, Henshall DC, Kaufer D, Koh S, Loscher W, et al. Neuroinflammatory targets and treatments for epilepsy validated in experimental models. Epilepsia 2017. July;58 Suppl 3:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang J, Quan Y, Ganesh T, Pouliot WA, Dudek FE, Dingledine R. Inhibition of the prostaglandin receptor EP2 following status epilepticus reduces delayed mortality and brain inflammation. Proc Natl Acad Sci U S A 2013. February 26;110(9):3591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang J, Yang MS, Quan Y, Gueorguieva P, Ganesh T, Dingledine R. Therapeutic window for cyclooxygenase-2 related anti-inflammatory therapy after status epilepticus. Neurobiol Dis 2015. April;76:126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wesselingh R, Butzkueven H, Buzzard K, Tarlinton D, O'Brien TJ, Monif M. Seizures in autoimmune encephalitis: Kindling the fire. Epilepsia 2020. June;61(6):1033–44. [DOI] [PubMed] [Google Scholar]
- 12.Marchi N, Fan Q, Ghosh C, Fazio V, Bertolini F, Betto G, Batra A, Carlton E, Najm I, Granata T, et al. Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol Dis 2009. February;33(2):171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vezzani A, Friedman A, Dingledine RJ. The role of inflammation in epileptogenesis. Neuropharmacology 2013. June;69:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byun JI, Lee ST, Moon J, Jung KH, Sunwoo JS, Lim JA, Kim TJ, Shin YW, Lee KJ, Jun JS, et al. Distinct intrathecal interleukin-17/interleukin-6 activation in anti-N-methyl-d-aspartate receptor encephalitis. J Neuroimmunol 2016. August 15;297:141–7. [DOI] [PubMed] [Google Scholar]
- 15.Leypoldt F, Hoftberger R, Titulaer MJ, Armangue T, Gresa-Arribas N, Jahn H, Rostasy K, Schlumberger W, Meyer T, Wandinger KP, et al. Investigations on CXCL13 in anti-N-methyl-D-aspartate receptor encephalitis: A potential biomarker of treatment response. JAMA Neurol 2015. February;72(2):180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popkirov S, Ismail FS, Gronheit W, Kapauer M, Wellmer J, Bien CG. Progressive hippocampal sclerosis after viral encephalitis: Potential role of NMDA receptor antibodies. Seizure 2017. July 19;51:6–8. [DOI] [PubMed] [Google Scholar]
- 17.Turrin NP, Rivest S. Innate immune reaction in response to seizures: Implications for the neuropathology associated with epilepsy. Neurobiol Dis 2004. July;16(2):321–34. [DOI] [PubMed] [Google Scholar]
- 18.Spatola M, Dalmau J. Seizures and risk of epilepsy in autoimmune and other inflammatory encephalitis. Curr Opin Neurol 2017. June;30(3):345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bialer M, Johannessen SI, Koepp MJ, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: A summary of the fourteenth eilat conference on new antiepileptic drugs and devices (EILAT XIV). I. drugs in preclinical and early clinical development. Epilepsia 2018. October;59(10):1811–41. [DOI] [PubMed] [Google Scholar]
- 20.Kenney-Jung DL, Vezzani A, Kahoud RJ, LaFrance-Corey RG, Ho ML, Muskardin TW, Wirrell EC, Howe CL, Payne ET. Febrile infection-related epilepsy syndrome treated with anakinra. Ann Neurol 2016. December;80(6):939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jyonouchi H, Geng L. Intractable epilepsy (IE) and responses to anakinra, a human recombinant IL-1 receptor agonist (IL-1ra): Case reports. J Clin Cell Immunol 2016;7:5. [Google Scholar]
- 22.DeSena AD, Do T, Schulert GS. Systemic autoinflammation with intractable epilepsy managed with interleukin-1 blockade. J Neuroinflammation 2018. February 9;15(1):38,018-1063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dilena R, Mauri E, Aronica E, Bernasconi P, Bana C, Cappelletti C, Carrabba G, Ferrero S, Giorda R, Guez S, et al. Therapeutic effect of anakinra in the relapsing chronic phase of febrile infection-related epilepsy syndrome. Epilepsia Open 2019. March 27;4(2):344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galea J, Ogungbenro K, Hulme S, Greenhalgh A, Aarons L, Scarth S, Hutchinson P, Grainger S, King A, Hopkins SJ, et al. Intravenous anakinra can achieve experimentally effective concentrations in the central nervous system within a therapeutic time window: Results of a dose-ranging study. J Cereb Blood Flow Metab 2011. February;31(2):439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Federici S, Martini A, Gattorno M. The central role of anti-IL-1 blockade in the treatment of monogenic and multi-factorial autoinflammatory diseases. Front Immunol 2013. October 31;4:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 2012. August;11(8):633–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, NC3Rs Reporting Guidelines Working Group. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br J Pharmacol 2010. August;160(7):1577–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma R, Al-Saleem FH, Panzer J, Lee J, Puligedda RD, Felicori LF, Kattala CD, Rattelle AJ, Ippolito G, Cox RH, et al. Monoclonal antibodies from a patient with anti-NMDA receptor encephalitis. Ann Clin Transl Neurol 2018. July 5;5(8):935–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Yang X, Zhou Y, Fox H, Xiong H. Direct contacts of microglia on myelin sheath and ranvier's node in the corpus callosum in rats. J Biomed Res 2019. June 4;33(3):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mietus CJ, Lackner TJ, Karvelis PS, Willcockson GT, Shields CM, Lambert NG, Koutakis P, Fuglestad MA, Hernandez H, Haynatzki GR, et al. Abnormal microvascular architecture, fibrosis, and pericyte characteristics in the calf muscle of peripheral artery disease patients with claudication and critical limb ischemia. J Clin Med 2020. August 8;9(8): 10.3390/jcm9082575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T. IL-1 receptor/toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun 2011. October;25(7):1281–9. [DOI] [PubMed] [Google Scholar]
- 32.Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA. Glia and epilepsy: Excitability and inflammation. Trends Neurosci 2013. March;36(3):174–84. [DOI] [PubMed] [Google Scholar]
- 33.Rojas A, Wang J, Glover A, Dingledine R. Urethane attenuates early neuropathology of diisopropylfluorophosphate-induced status epilepticus in rats. Neurobiol Dis 2020. July;140:104863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Simoni MG, Perego C, Ravizza T, Moneta D, Conti M, Marchesi F, De Luigi A, Garattini S, Vezzani A. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur J Neurosci 2000. July;12(7):2623–33. [DOI] [PubMed] [Google Scholar]
- 35.Vezzani A, Conti M, De Luigi A, Ravizza T, Moneta D, Marchesi F, De Simoni MG. Interleukin-1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: Functional evidence for enhancement of electrographic seizures. J Neurosci 1999. June 15;19(12):5054–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arisi GM, Foresti ML, Katki K, Shapiro LA. Increased CCL2, CCL3, CCL5, and IL-1beta cytokine concentration in piriform cortex, hippocampus, and neocortex after pilocarpine-induced seizures. J Neuroinflammation 2015. July 2;12:129,015-0347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jun JS, Lee ST, Kim R, Chu K, Lee SK. Tocilizumab treatment for new onset refractory status epilepticus. Ann Neurol 2018. December;84(6):940–5. [DOI] [PubMed] [Google Scholar]
- 38.Zeng C, Chen L, Chen B, Cai Y, Li P, Yan L, Zeng D. Th17 cells were recruited and accumulated in the cerebrospinal fluid and correlated with the poor prognosis of anti-NMDAR encephalitis. Acta Biochim Biophys Sin (Shanghai) 2018. December 1;50(12):1266–73. [DOI] [PubMed] [Google Scholar]
- 39.Byun JI, Lee ST, Jung KH, Sunwoo JS, Moon J, Lim JA, Lee DY, Shin YW, Kim TJ, Lee KJ, et al. Effect of immunotherapy on seizure outcome in patients with autoimmune encephalitis: A prospective observational registry study. PLoS One 2016. January 15;11(1):e0146455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braddock M, Quinn A. Targeting IL-1 in inflammatory disease: New opportunities for therapeutic intervention. Nat Rev Drug Discov 2004. April;3(4):330–9. [DOI] [PubMed] [Google Scholar]
- 41.Librizzi L, Noe F, Vezzani A, de Curtis M, Ravizza T. Seizure-induced brain-borne inflammation sustains seizure recurrence and blood-brain barrier damage. Ann Neurol 2012. July;72(1):82–90. [DOI] [PubMed] [Google Scholar]
- 42.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. Faseb J 2008. March;22(3):659–61. [DOI] [PubMed] [Google Scholar]
- 43.Granowitz EV, Porat R, Mier JW, Pribble JP, Stiles DM, Bloedow DC, Catalano MA, Wolff SM, Dinarello CA. Pharmacokinetics, safety and immunomodulatory effects of human recombinant interleukin-1 receptor antagonist in healthy humans. Cytokine 1992. September;4(5):353–60. [DOI] [PubMed] [Google Scholar]
- 44.Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the src family of kinases. J Neurosci 2003. September 24;23(25):8692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roseti C, van Vliet EA, Cifelli P, Ruffolo G, Baayen JC, Di Castro MA, Bertollini C, Limatola C, Aronica E, Vezzani A, et al. GABAA currents are decreased by IL-1beta in epileptogenic tissue of patients with temporal lobe epilepsy: Implications for ictogenesis. Neurobiol Dis 2015. October;82:311–20. [DOI] [PubMed] [Google Scholar]
- 46.Finke C, Kopp UA, Scheel M, Pech LM, Soemmer C, Schlichting J, Leypoldt F, Brandt AU, Wuerfel J, Probst C, et al. Functional and structural brain changes in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol 2013. August;74(2):284–96. [DOI] [PubMed] [Google Scholar]
- 47.Planaguma J, Leypoldt F, Mannara F, Gutierrez-Cuesta J, Martin-Garcia E, Aguilar E, Titulaer MJ, Petit-Pedrol M, Jain A, Balice-Gordon R, et al. Human N-methyl D-aspartate receptor antibodies alter memory and behaviour in mice. Brain 2015. January;138(Pt 1):94–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones BE, Tovar KR, Goehring A, Jalali-Yazdi F, Okada NJ, Gouaux E, Westbrook GL. Autoimmune receptor encephalitis in mice induced by active immunization with conformationally stabilized holoreceptors. Sci Transl Med 2019. July 10;11(500): 10.1126/scitranslmed.aaw0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shukla N, Risen S, Erklauer J, Lai Y, Riviello J, Muscal E. Anakinra (IL-1 blockade) use in children wiht suspected FIRES: A single institution experience. Neurology 2018;90 (15 Supplement):P4.346. [Google Scholar]
- 50.Novelly RA, Augustine EA, Mattson RH, Glaser GH, Williamson PD, Spencer DD, Spencer SS. Selective memory improvement and impairment in temporal lobectomy for epilepsy. Ann Neurol 1984. January;15(1):64–7. [DOI] [PubMed] [Google Scholar]
- 51.Nariai H, Duberstein S, Shinnar S. Treatment of epileptic encephalopathies: Current state of the art. J Child Neurol 2018. January;33(1):41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newell EA, Todd BP, Mahoney J, Pieper AA, Ferguson PJ, Bassuk AG. Combined blockade of interleukin-1alpha and −1beta signaling protects mice from cognitive dysfunction after traumatic brain injury. eNeuro 2018. April 13;5(2): 10.1523/ENEURO.0385,17.2018. eCollection 2018 Mar-Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han T, Qin Y, Mou C, Wang M, Jiang M, Liu B. Seizure induced synaptic plasticity alteration in hippocampus is mediated by IL-1beta receptor through PI3K/akt pathway. Am J Transl Res 2016. October 15;8(10):4499–509. [PMC free article] [PubMed] [Google Scholar]
- 54.Ly LT, Kreye J, Jurek B, Leubner J, Scheibe F, Lemcke J, Wenke NK, Reincke SM, Pruss H. Affinities of human NMDA receptor autoantibodies: Implications for disease mechanisms and clinical diagnostics. J Neurol 2018. November;265(11):2625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bien CG, Vincent A, Barnett MH, Becker AJ, Blumcke I, Graus F, Jellinger KA, Reuss DE, Ribalta T, Schlegel J, et al. Immunopathology of autoantibody-associated encephalitides: Clues for pathogenesis. Brain 2012. May;135(Pt 5):1622–38. [DOI] [PubMed] [Google Scholar]
- 56.Camdessanche JP, Streichenberger N, Cavillon G, Rogemond V, Jousserand G, Honnorat J, Convers P, Antoine JC. Brain immunohistopathological study in a patient with anti-NMDAR encephalitis. Eur J Neurol 2011. June;18(6):929–31. [DOI] [PubMed] [Google Scholar]
- 57.Vezzani A, Balosso S, Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol 2019. August;15(8):459–72. [DOI] [PubMed] [Google Scholar]
- 58.Wesselingh R, Butzkueven H, Buzzard K, Tarlinton D, O'Brien TJ, Monif M. Innate immunity in the central nervous system: A missing piece of the autoimmune encephalitis puzzle? Front Immunol 2019. September 10;10:2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.