Abstract
In low-income countries, prospective data on combined effects of in utero teratogen exposure are lacking and necessitates new research. The aim of the present study was to explore the effect of in utero teratogen exposure on the size of the kidneys and pancreas 5 years after birth in a low-income paediatric population. Data was collected from 500 mother–child pairs from a low-income setting. Anthropometric measurements included body weight, (BW) body height, mid-upper arm and waist circumference (WC). Clinical measurements included blood pressure (BP), mean arterial pressure and heart rate. Ultrasound measurements included pancreas, and kidney measurements at age 5 years. The main outcome of interest was the effect of maternal smoking and alcohol consumption on ultrasound measurements of organ size at age 5 years. Left and right kidney length measurements were significantly lower in smoking exposed children compared to controls (p = 0.04 and p = 0.03). Pancreas body measurements were significantly lower in smoking exposed children (p = 0.04). Multiple regression analyses were used to examine the associations between the independent variables (IDVs), maternal age, body mass index (BMI), mid-upper arm circumference (MUAC) and BW of the child, on the dependent variables (DVs) kidney lengths and kidney volumes. Also, the association between in utero exposure to alcohol and nicotine and pancreas size. WC was strongest (r = 0.28; p < 0.01) associated with pancreas head [F (4, 454) = 13.44; R2 = 0.11; p < 0.01] and tail (r = 0.30; p < 0.01) measurements at age 5 years, with in utero exposure, sex of the child and BMI as covariates. Kidney length and pancreas body measurements are affected by in utero exposure to nicotine at age 5 years and might contribute to cardiometabolic risk in later life. Also, findings from this study report on ultrasound reference values for kidney and pancreas measurements of children at age 5 years from a low-income setting.
Keywords: Alcohol, body mass index, intrauterine teratogen exposure, kidney size, low birth weight, low-income setting, nicotine, pancreas size, paediatrics, waist circumference
Introduction
High prevalence of alcohol consumption and cigarette smoking during pregnancy in association with low socio-economic status (SES) and poverty, may result in a poor maternal nutritional status and magnify the cardiometabolic risk experienced by offspring in early childhood.1–5 Both maternal alcohol consumption and cigarette smoking during pregnancy are teratogens and two of the leading preventable causes of birth defects and impaired fetal development. These teratogens constitute micronutrient deficiencies, which is especially evident in females of reproductive age, and impairs optimal fetal development.6–8 Maternal smoking or exposure to secondary smoking during pregnancy is strongly associated with fetal growth restriction through two possible mechanisms. First, the nicotine in cigarette smoking causes vasoconstriction of the uteroplacental blood vessels leading to a decrease in blood flow to the placenta, which will result in a decreased delivery of oxygen and micronutrients to the fetus. Second, the fetal circulation is also compromised as the nicotine concentration increases to higher levels in the fetal circulation compared to the mother’s circulation. In addition, the carbon monoxide caused by maternal smoking influences the development of the placenta and it leads to an increase in fetal carboxyhaemoglobinaemia with a subsequent decrease in oxygenation of the developing organs and tissue.9 Ultimately, the impaired fetal blood flow to the abdomen of the fetus may lead to smaller kidneys.10 Excessive maternal alcohol consumption during pregnancy has similar somatic growth restriction effects on the fetus. In excess, alcohol affects fetal growth and development through DNA methylation and by interfering with folate metabolism and availability and accordingly affects organ development in the fetus. The intricacies of in utero teratogen exposure in low-income countries and the association with cardiometabolic diseases in later life remain to be elucidated.
Both alcohol and nicotine in cigarette smoking are teratogens associated with epigenetic responses in the fetus.11,12 These agents affect the gene expression with or without directly affecting the gene sequence of DNA. Teratogenic effects may depend on several factors such as the timing and duration of the exposure, the distribution across the placenta, the concentration amount in the amniotic fluid, as well as the ability of the teratogenic agent to hinder the specific developmental processes and organogenesis.4,6,11 Given the above, in utero exposure to teratogens impacts on fetal growth through epigenetic alteration of DNA methylation processes13,14 and may result in adult cardiometabolic diseases.15 Children exposed to maternal cigarette smoking during pregnancy are not only shorter in body length (BL), but animal and human studies confirm they may be predisposed to abnormal glucose tolerance, hypertension and dyslipidaemia by the time they reach reproductive age.7,11 Other effects of maternal smoking are higher adiposity,6 lower fat-free mass16 and increased appetite as part of hypothalamus–pituitary–adrenal axis involvement and associated obesity in later life.6,12,17
Thus, this constellation of teratogenic effects on various organs not only affect an individual exposed to it but also a society, communities, health systems as well as the well-being of future generations.4,18,19 Therefore, early childhood and post-puberty are critical time periods to start addressing cardiometabolic risk factors, especially in females.20
The in utero effects of alcohol consumption and cigarette smoking exposure is most commonly reported in low-and-middle-income countries (LMIC)8,21 but the scarcity of prospective data charting cardiometabolic risk, in relation to the in utero teratogen exposure from childhood into adulthood, necessitate future research. To map the trajectory of these metabolic risk factors, such as diet, passive smoking exposure and physical inactivity in children needs consideration earlier rather than later. Determining those cardiometabolic risk factors present in school-aged children, and to map the trajectories of these risk factors are essential.22 Also, through regular and early screening and intervention, the burden of cardiometabolic complications experienced in adult life, be might lessen. 20 We hypothesised the effect of in utero exposure is associated with smaller visceral organ size, i.e. kidneys and pancreas at age 5 years and thus associated with cardiometabolic risk. The aim of the present study was to explore the effect of in utero teratogen exposure on the size of the kidneys and pancreas 5 years after birth. Towards this aim, we included 500 children from a low-income setting and we compared the in utero exposure effects between controls and exposed children. Anthropometric measurements at birth, anthropometric measurements at age 5 years, blood pressure (BP) measurements and ultrasound measurements of the kidneys and pancreas at age 5 years were compared between controls and exposed children.
Materials and methods
Study design
The present study was conducted at SU, Obstetrics and Gynaecology SPS unit at Tygerberg Hospital, Cape Town over an 18-month period (June 2016–December 2017). It was a follow-up study of 500 of the children born to the Safe Passage Study (SPS), a prospective longitudinal study, to investigate the role of exposure to alcohol during pregnancy on stillbirths and sudden infant deaths.23 Maternal data was documented for the SPS during dedicated antenatal clinic visits. A modified timeline follow-back method was used to assess alcohol intake during pregnancy and group-based trajectories were used to categorise smoking and drinking patterns during pregnancy.23 Information on paediatric health was obtained from assessments at birth and again at 5 years of age.
Selection of study participants
Pregnant women were recruited from the Belhar antenatal clinic or Bishop Lavis Midwife Obstetric Unit (MOU) and had prenatal follow-up visits at Tygerberg Hospital. All pregnant women booking for antenatal care with their children born from June 2011 through to December 2012 were invited to be part of the study. Five-hundred mother–child pairs were selected for this sub-study. For infant follow-up, twins and children with congenital abnormalities at birth were excluded from the present study23 and 500 infants born to these mothers were included in the study.
Participant assessments
Maternal assessments
Socio-demographic information including data on nutrition, pregnancy history, as well as alcohol and tobacco use was documented using a study questionnaire. Body mass index (BMI) was calculated from measurements collected at the first antenatal visit and calculated as the body weight (BW) in kilograms divided by the height in metres squared. Mid-upper arm circumference (MUAC) was measured as the circumference of the right upper arm measured at the midpoint between the tip of the shoulder and the tip of the elbow (olecranon process and the acromion) using a tape measure. Gestational age (GA) at enrolment, as determinant by an early ultrasound examination, was recorded.
Paediatric assessments
Measurements at birth included weight, length and MUAC. BW and length were used to calculate BMI. GA at delivery was recorded. At year 5, BW, length and BMI calculations were measured again in all children. BW was measured using an electronic scale to the nearest 0.01 kg. For body height, children removed their shoes and were measured using a mechanical stadiometer fixed to a wall. Waist circumference (WC) was measured using a tape measure around the waist at the midpoint between the last rib and the iliac crest. Each child was measured three times and mean values were obtained from the three measurements. Systolic and diastolic BP, and heart rate were measured at 5 years of age. BP was measured from the right upper arm in a sitting position using a validated CAS 740 MAXNIBP automated digital sphygmomanometer. A size-appropriate BP cuff was used and all measurements were repeated three times.
Ultrasound measurements were obtained from children at age 5 years using a Voluson E8 ultrasound machine (GE Healthcare). Measurements were taken with the child in a supine position and fasting approximately 4 h prior to the examination. Some children were asked to turn into an oblique or prone position in cases with poor visualisation of the kidneys. Imaging of the pancreas and kidneys were attained on held inspiration or in some cases with the abdomen extended/pushed out. For the measurement of the pancreas, the transducer was placed transversely in the midline of the upper abdomen (high in the epigastrium). The pancreas was visualised in a coronal section. The transducer was angled heel-toe for optimal visualisation of the maximal pancreas length. The head of the pancreas was measured in the mediolaterally and anteroposteriorly, whereas the body and tail was measured anteroposteriorly.
The kidneys were visualised in the longitudinal and transverse views. The right liver lobe and spleen were used as acoustic windows, respectively. The transducer was placed perpendicular, just inferior to the lateral edge of the right costal margin in the sagittal plane and moved medially until the kidney was optimally visualised in the coronal plane (long axis). The maximum length of the kidney was measured from the upper pole to the lower pole of the kidney. The transducer was rotated 90° into the transverse plane, the maximum measurements of the transverse (W) and anteroposterior (T) dimensions of the kidney was taken. The same was repeated on the left side. The kidney volumes were calculated using V = LxWxTx(∏/6).24,25
Statistical analysis
Statistical analysis was performed using SPSS® software (version 21.0 for Windows; SPSS, Inc., Chicago, IL, USA). Quantitative data was described as the means along with SD, minimum and maximum values with 95% confidence intervals (CIs). Categorical variables were presented as percentages. Intergroup differences were determined using independent samples t-tests and one-way analysis of variance (ANOVA) to evaluate the differences amongst in utero exposure groups. Post hoc Tukey comparisons for in-between group differences. Pearson correlation coefficients were used to describe linear relationships between continuous variables. Chi-square analysis was conducted for categorical variables to explore the likelihood of mothers with gestational hypertension to fall in the exposure groups. Partial correlations were conducted to determine the relationship between kidney, pancreas measurements and BP, WC whilst controlling for the BMI of the child. Multiple linear regression analyses were performed to illustrate the independent association between independent variables (IDVs), in utero exposure to alcohol and nicotine, maternal age, maternal BMI, maternal MUAC or sex, weight and WC of the child and dependent variables (DVs), kidney and pancreas measurements. Data was analysed per exposure group. The control group constitutes of the children born to non-smoking mothers who abstained from consuming alcohol, the alcohol and nicotine (ALCNIC) group was formed by the children born to mothers who both smoked cigarettes and consumed alcohol during pregnancy. The alcohol-only (ALC) group was formed by children born to alcohol-consuming mothers, but no smoking and the nicotine-only (NIC) group was formed by the children born to smoking mothers who did not consume alcohol. Statistical significance was set at p < 0.05.
Results
Maternal and birth outcomes of infants
The participant characteristics at baseline are described in Tables 1 and 2. Demographic characteristics for the mothers forming the control group differed significantly from the mothers forming the exposure groups, both ALCNIC, ALC and NIC (Table 1). Maternal age was not significantly different (p = 0.50), but maternal weight was significantly lower for ALCNIC (63.7 ± 14.5 kg) and NIC (63.5 ± 14.8 kg) compared to the controls (68.9 ± 18.3 kg) at p < 0.01. In addition, maternal BMI and MUAC were significantly lower for ALCNIC mothers (25.1 ± 5.6 kg/m2, 27.2 ± 4.4 cm) and NIC mothers (25.1 ± 5.6 kg/m2 and 27.4 ± 4.6 cm) compared to controls (27.4 ± 7.1 kg/m2 and 27.2 ± 4.4 cm) at p < 0.01. GA at enrollment was also significantly (p < 0.01) lower for ALCNIC (142.7 ± 49.4 days) and for ALC (154.5 ± 240 49.1 days) compared to controls (126.2 ± 44.7 days) (Table 1). Gestational diabetes mellitus (GDM) was observed in only 1.4% of mothers, hypertension during pregnancy affected 10.7% and anaemia during pregnancy affected 41.7% of mothers. Neither maternal hypertension nor anaemia during pregnancy affected birth or 5-year outcomes.
Table 1.
Maternal characteristics at baseline
| Maternal characteristic | Controls | ALCNIC | ALC only | NIC only | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | M (SD) | 95 % CI | N | M (SD) | 95 % CI | N | M (SD) | 95% CI | N | M (SD) | 95% CI | |
| Maternal age in years | 147 | 25.6 (6.1) | 24.6–26.6 | 154 | 24.6 (5.9) | 23.6–25.5 | 33 | 24.5 (5.0) | 22.7–26.3 | 167 | 24.9 (6.2) | 24.0–25.8 |
| Body weight in kg | 145 | 68.9 (18.3) | 65.9–71.9 | 154 | 63.7 (14.5) | 61.4–66.0 | 33 | 67.2 (15.6) | 61.6–72.7 | 166 | * 63.5 (14.8) | 61.2–65.8 |
| Body length in cm | 146 | 158.2 (6.9) | 157.1–159.4 | 150 | 159.6 (6.5) | 158.6–160.7 | 31 | 156.4 (7.2) | 153.8–159.1 | 165 | 158.7 (6.2) | 157.8–159.7 |
| BMI in kg/m2 | 143 | 27.4 (7.1) | 26.3–28.6 | 150 | * 25.1 (5.6) | 24.2–26.0 | 31 | 27.3 (6.0) | 25.1–29.6 | 165 | * 25.1 (5.6) | 24.3–26.0 |
| MUAC in cm | 145 | 29.2 (5.4) | 28.3–30.1 | 152 | * 27.2 (4.4) | 26.5–27.9 | 32 | 28.2 (4.5) | 26.5–29.8 | 164 | * 27.4 (4.6) | 26.7–28.1 |
| GA at enrolment in days | 147 | 126.2 (44.7) | 119.0–133.5 | 154 | * 142.7 (49.4) | 134.8–150.5 | 33 | * 154.5 (49.1) | 137.0–171.9 | 167 | 134.6 (46.2) | 127.5–141.6 |
| GDM | 4 | 0 | 0 | 3 | ||||||||
| HPT | 19 | 12 | 8 | 16 | ||||||||
| Anaemia | 60 | 61 | 20 | 68 | ||||||||
ALCNIC, smoking and alcohol-consuming mothers; ALC only, mothers-only consuming alcohol without smoking; NIC only, smoking mothers without consuming alcohol; BMI, body mass index; MUAC, mid-upper arm circumference; GA, gestational age; N, number of participants; M, mean value; SD, standard deviation.
The values in bold represent and highlight the significant differences between the different groups.
significance at p-value <0.05 versus Controls.
Table 2.
Neonatal characteristics at baseline according to the four exposure groups
| Characteristic | Controls | ALCNIC | ALC only | NIC only | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | M (SD) | 95 % CI | N | M (SD) | 95 % CI | N | M (SD) | 95% CI | N | M (SD) | 95% CI | |
| Birth weight in kg | 147 | 3.06 (0.52) | 2.98–3.14 | 154 | 2.98 (0.57) | 2.89–3.07 | 33 | 3.06 (0.59) | 2.85–3.27 | 166 | 2.98 (0.59) | 2.89–3.07 |
| Birth length in cm | 126 | 49.06 (2.09) | 48.69–49.43 | 125 | 48.31 (2.41) | 47.88–48.73 | 24 | 48.50 (2.16) | 47.59–49.41 | 140 | 48.62 (2.12) | 48.44–48.87 |
| Birth BMI in kg/m2 | 126 | 12.60 (1.94) | 12.26–12.95 | 125 | 12.62 (1.81) | 12.30–12.94 | 24 | 12.87 (1.81) | 12.10–13.63 | 139 | 12.69 (2.07) | 12.35–13.04 |
| MUAC in cm | 124 | 10.50 (0.95) | 10.34–10.67 | 124 | 10.40 (1.03) | 10.22–10.59 | 24 | 10.62 (0.97) | 10.21–11.03 | 140 | 10.56 (0.96) | 10.40–10.72 |
| GA at birth in days | 147 | 274.14 (13.78) | 271.90–276.39 | 154 | 271.73 (14.16) | 269.48–273.99 | 33 | 275.76 (13.15) | 271.10–280.42 | 167 | * 269.87 (15.47) | 267.51–272.24 |
| Low birth weight | 16 | 27 | 6 | 32 | ||||||||
BMI, body mass index; MUAC, mid-upper arm circumference; GA, gestational age; N, number of participants; M, mean value; SD, standard deviation.
The values in bold represent and highlight the significant differences between the different groups.
significance at p-value < 0.05 versus Controls.
A total of 15.9% of children were born with low birth weight (LBW), 70.2% of children were exposed to alcohol and nicotine whilst 64.1% were exposed to cigarette smoking during pregnancy. Paediatric neonatal data at birth showed significantly lower GA at delivery for NIC compared to the controls (p = 0.03). All other measurements at birth did not differ significantly between the four exposure groups (Table 2).
Paediatric outcomes at age 5 years
Paediatric participant characteristics at end point are described in Table 3. When paediatric characteristics were compared between the four exposure groups, significantly lower mean BW and BL values were observed between the NIC group (17.3 ± 2.3 kg and 108.2 ± 4.5 cm, respectively, p = 0.03) and controls (18.7 ± 3.6 kg and 110.0 ± 5.2 cm, respectively, p = 0.01) (Table 3).
Table 3.
Paediatric characteristics at age 5 years, according to control and exposure groups
| Characteristic | Controls | ALCNIC | ALC only | NIC only | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | M (SD) | 95% CI | N | M (SD) | 95% CI | N | M (SD) | 95% CI | N | M (SD) | 95% CI | |
| Body weight in kg | 147 | * 18.7 (3.6) | 18.1–18.3 | 154 | 18.2 (2.7) | 17.7–18.6 | 33 | 17.8 (2.7) | 16.9–18.8 | 167 | * 17.7 (2.3) | 17.3–18.0 |
| Body length in cm | 147 | * 110.0 (5.2) | 10.9.2–110.9 | 154 | 109.0 (5.2) | 108.2–109.8 | 33 | 108.5 (4.6) | 106.9–110.1 | 167 | * 108.2 (4.5) | 107.5–108.9 |
| BMI in kg/m2 | 147 | 15.4 (2.1) | 15.0–15.7 | 154 | 15.2 (1.5) | 15.0–15.4 | 33 | 15.1 (1.7) | 14.5–15.7 | 167 | 15.1 (1.4) | 14.9–15.3 |
| Waist circumference in cm | 132 | 51.0 (5.2) | 51.0–52.8 | 150 | 51.4 (3.9) | 50.8–52.1 | 30 | 51.2 (3.7) | 49.8–52.6 | 158 | 50.7 (3.5) | 50.1–51.2 |
| Systolic blood pressure in mmHg | 146 | 106.5 (10.7) | 104.7–108.2 | 154 | 107.1 (10.0) | 105.6–108.7 | 33 | 105.8 (11.7) | 101.6–109.9 | 167 | 104.3 (9.3) | 102.8–105.7 |
| Diastolic blood pressure in mmHg | 146 | 65.3 (9.1) | 63.8–66.7 | 154 | 65.7 (9.1) | 64.3–67.2 | 33 | 65.6 (11.4) | 61.6–69.7 | 167 | 63.9 (9.0) | 62.5–65.3 |
| Mean arterial pressure in mmHg | 146 | 78.8 (9.5) | 77.3–80.4 | 154 | 79.4 (9.3) | 78.0–80.9 | 33 | 79.3 (11.3) | 75.3–83.3 | 167 | 77.3 (9.3) | 75.9–78.7 |
| Heart rate in beats per minute | 146 | 91.7 (12.4) | 89.7–93.8 | 154 | 92.5 (14.4) | 90.2–94.8 | 33 | 91.8 (8.7) | 88.7–94.9 | 167 | 91.4 (13.3) | 89.3–93.4 |
| LT K length in mm | 146 | * 74.2 (6.1) | 73.2–75.2 | 153 | 72.7 (5.8) | 71.7–73.6 | 33 | 72.9 (4.9) | 71.2–74.7 | 165 | * 72.6 (5.6) | 71.8–73.5 |
| LT K width in mm | 146 | 35.0 (3.8) | 34.4–35.7 | 153 | 35.4 (3.8) | 34.8–36.0 | 33 | 35.1 (3.7) | 33.8–36.5 | 165 | 34.5 (3.6) | 33.9–35.0 |
| LT K height in mm | 146 | 35.5 (3.7) | 34.9–36.1 | 153 | 35.4 (3.8) | 34.8–36.0 | 33 | 35.5 (3.6) | 34.2–36.8 | 165 | 35.1 (3.7) | 34.6–35.7 |
| LT K volume in mm3 | 146 | 48697.1 (10102.6) | 47044.6–50349.6 | 153 | 48116.3 (10731.5) | 46402.2–49830.4 | 33 | 47954.8 (9824.4) | 44471.2–51438.4 | 165 | 46493.7 (10449.0) | 44887.5–48099.9 |
| RT K length in mm | 146 | * 73.3 (5.9) | 72.4–74.3 | 153 | 72.0 (5.6) | 71.1–72.9 | 33 | 73.2 (4.6) | 71.5–74.8 | 165 | * 71.5 (5.7) | 70.6–72.4 |
| RT K width in mm | 146 | 32.1 (3.4) | 31.6–32.7 | 153 | 33.1 (3.7) | 32.5–33.7 | 33 | 31.5 (2.2) | 30.8–32.3 | 165 | 32.3 (3.2) | 31.8–32.8 |
| RT K height in mm | 146 | 38.4 (3.9) | 37.7–39.0 | 153 | 38.7 (4.4) | 37.9–39.4 | 33 | 38.0 (3.4) | 36.8–39.2 | 165 | 37.8 (4.1) | 37.1–38.4 |
| RT K volume in mm3 | 146 | 47629.7 (9546.0) | 46068.3–49191.2 | 153 | 48829.3 (11451.9) | 47000.1–50658.5 | 33 | 46099.3 (6980.8) | 43624.0–48574.6 | 165 | 46127.6 (10132.7) | 44570.0–47685.2 |
| Pancreas head in mm | 141 | 18.3 (2.6) | 17.8–18.7 | 151 | 17.8 (2.5) | 17.4–18.3 | 33 | 18.4 (2.8) | 17.5–19.4 | 164 | 17.6 (2.5) | 17.2–18.0 |
| Pancreas body in mm | 141 | * 7.2 (1.6) | 6.9–7.4 | 151 | 7.0 (1.6) | 6.8–7.3 | 33 | 7.2 (1.7) | 6.6–7.8 | 164 | * 6.7 (1.5) | 6.5–6.9 |
| Pancreas tail in mm | 141 | 12.8 (2.2) | 12.5–13.2 | 151 | 13.0 (2.1) | 12.7–13.3 | 33 | 13.3 (3.0) | 12.3–14.4 | 164 | 12.7 (2.2) | 12.3–13.0 |
BMI, body mass index; WC, waist circumference; LT, left; RT, right; K, kidney; Min, minimum value; Max, maximum value; N, number of participants; M, mean value; SD, standard deviation.
The values in bold represent and highlight the significant differences between the different groups.
significance at p-value <0.05. Data is expressed as mean (SD), minimum and maximum values.
For the kidney measurements, mean left and right kidney length measurements were significantly lower for the children from NIC-only group (72.6 ± 5.6 and 71.5 ± 5.7 mm) compared to controls (74.2 ± 6.1 and 73.3 ± 5.9 mm) at p = 0.04 and p = 0.03, respectively (Fig. 1). However, all other kidney measurements including width, height and volume, did not differ significantly between the four exposure groups.
Fig. 1.
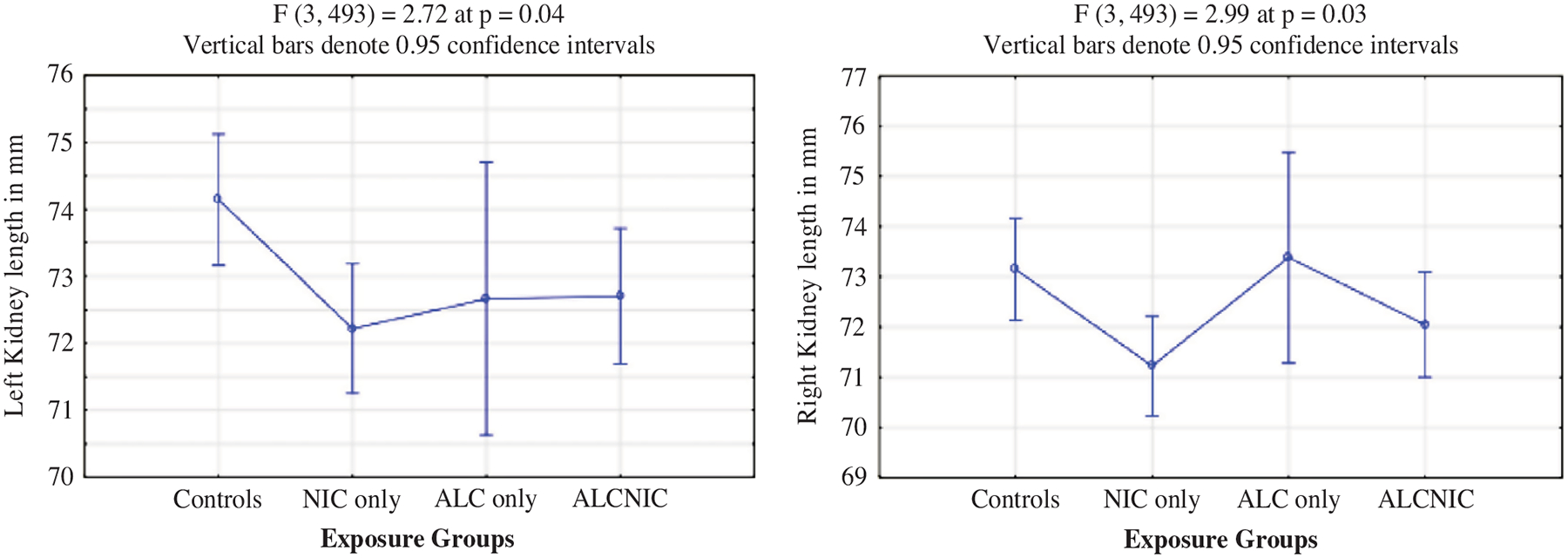
Left and right kidney length as per exposure groups.
Also, mean pancreas body measurements were significantly lower for NIC (6.7 ± 1.5 mm) compared to controls (7.2 ± 1.6 mm) at p = 0.04 (Table 3 and Fig. 2). Mean values for the pancreas head (p = 0.08) and tail (p = 0.33) for the children in the exposure groups did not differ significantly from the controls.
Fig. 2.
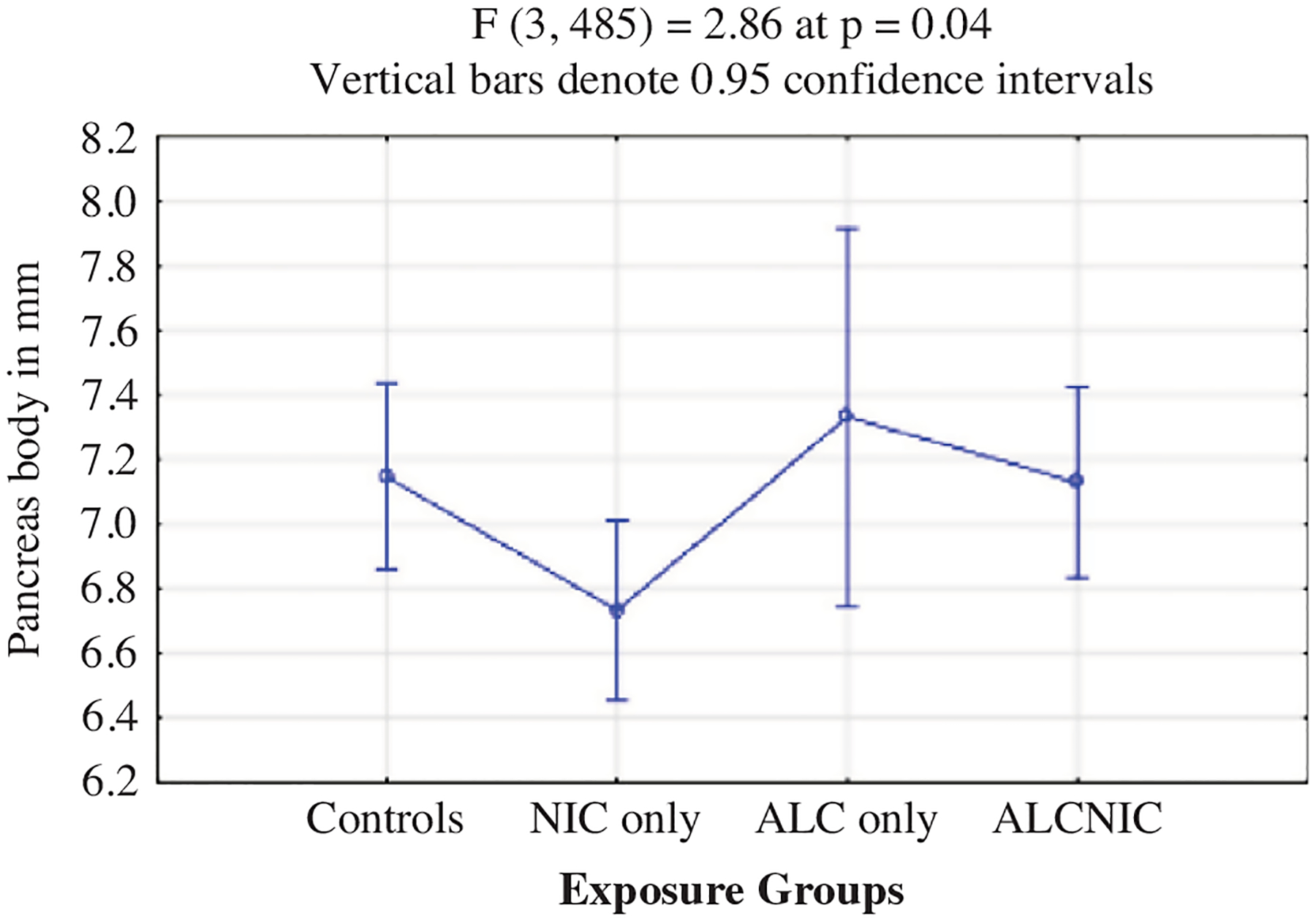
Pancreas body values as per exposure groups.
Furthermore, significant positive correlations were found between all anthropometric measurements including BW, BL and WC with all kidney measurements for both the controls and the exposure groups except for left kidney width of the controls (Tables 4 and 5). Significant positive correlations were also found between anthropometric measurements and pancreas measurements. After controlling for the BMI of the child, the relationship between WC and the pancreas head and tail still existed (r = 0.13, p < 0.01 and r = 0.24, p < 0.01, respectively) but not for pancreas body.
Table 4.
Correlations between mean kidney values and anthropometric measurements of the controls
| Controls N146 | Body weight (kg) | Body length (cm) | BMI (kg/m2) | WC (cm) | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| LT K length | 0.45 | <0.01 | 0.51 | <0.01 | 0.26 | <0.01 | 0.35 | <0.01 |
| RT K length | 0.41 | <0.01 | 0.48 | <0.01 | 0.24 | <0.01 | 0.31 | <0.01 |
| LT K width | 0.15 | 0.07 | 0.21 | 0.01 | 0.06 | 0.49 | 0.09 | 0.29 |
| RT K width | 0.20 | 0.02 | 0.26 | <0.01 | 0.10 | 0.23 | 0.19 | 0.03 |
| LT K height | 0.25 | <0.01 | 0.31 | <0.01 | 0.13 | 0.11 | 0.19 | 0.03 |
| RT K height | 0.34 | <0.01 | 0.29 | <0.01 | 0.27 | 0.01 | 0.29 | <0.01 |
| LT K volume | 0.38 | <0.01 | 0.46 | <0.01 | 0.20 | 0.02 | 0.28 | 0.01 |
| RT K volume | 0.45 | <0.01 | 0.47 | <0.01 | 0.30 | <0.01 | 0.38 | <0.01 |
LT, left; RT, right; K, kidney; BMI, body mass index; WC, waist circumference; p, p-value/significance.
The values in bold represent and highlight the significant differences between the different groups.
Table 5.
Correlations between mean kidney values and anthropometric measurements of the exposed group
| Exposed N354 | Body weight (kg) | Body length (cm) | BMI (kg/m2) | WC (cm) | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| LT K length | 0.45 | <0.01 | 0.45 | <0.01 | 0.26 | <0.01 | 0.34 | <0.01 |
| RT K length | 0.42 | <0.01 | 0.43 | <0.01 | 0.23 | <0.01 | 0.31 | <0.01 |
| LT K width | 0.28 | <0.01 | 0.22 | <0.01 | 0.22 | <0.01 | 0.26 | <0.01 |
| RT K width | 0.28 | <0.01 | 0.19 | <0.01 | 0.24 | <0.01 | 0.29 | <0.01 |
| LT K height | 0.33 | <0.01 | 0.27 | <0.01 | 0.23 | <0.01 | 0.28 | <0.01 |
| RT K height | 0.39 | <0.01 | 0.30 | <0.01 | 0.30 | <0.01 | 0.32 | <0.01 |
| LT K volume | 0.45 | <0.01 | 0.39 | <0.01 | 0.31 | <0.01 | 0.38 | <0.01 |
| RT K volume | 0.47 | <0.01 | 0.38 | <0.01 | 0.35 | <0.01 | 0.41 | <0.01 |
LT, left; RT, right; K, kidney; BMI, body mass index; WC, waist circumference; p, p-value/significance.
The values in bold represent and highlight the significant differences between the different groups.
Table 6 summarises the correlations between BP measurements, SBP, DBP, MAP HR and kidney length, kidney volume as well as BMI. BP was not significantly associated with kidney length nor volume at this age (p > 0.01) instead, BP significantly and positively correlated with BMI (p < 0.01). Heart rate correlated significantly but negatively with right kidney volume (r = −0.10 at p = 0.02).
Table 6.
Associations between kidney measurements, BMI and blood pressure measurements at age 5 years expressed as Pearson correlation coefficients (r)
| LT K length | LT K volume | RT K length | RT K volume | BMI (kg/m2) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | n | r | p | n | r | p | n | r | p | n | r | p | n | |
| SBP | 0.01 | 0.92 | 496 | −0.03 | 0.45 | 496 | −0.05 | 0.26 | 496 | −0.06 | 0.17 | 496 | 0.16 | <0.01 | 496 |
| DBP | 0.62 | 0.75 | 496 | −0.01 | 0.88 | 496 | −0.04 | 0.35 | 496 | −0.05 | 0.29 | 496 | 0.18 | <0.01 | 496 |
| MAP | 0.01 | 0.84 | 496 | −0.04 | 0.40 | 496 | −0.05 | 0.29 | 496 | −0.07 | 0.14 | 496 | 0.17 | <0.01 | 496 |
| HR | 0.01 | 0.87 | 496 | −0.04 | 0.40 | 496 | −0.03 | 0.57 | 496 | −0.10 | * 0.02 | 496 | 0.03 | 0.48 | 500 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate; LT K length, left kidney length; LT K volume, left kidney volume; RT K length, right kidney length; RT K volume, right kidney volume; BMI, body mass index; p, p-value/significance.
The values in bold represent and highlight the significant differences between the different groups.
significance at p-value <0.05.
To explore associations between IDVs, maternal age, maternal BMI, maternal MUAC as well as the weight of the child at age 5 years, and DVs, right and left kidney lengths and volumes, multiple linear regression analyses were used (Table 7). For all the exposure groups, BW of the child played a significant role. Strong positive associations (p < 0.01) between higher BW of the child and bigger kidney length and volume, with maternal age, maternal BMI and maternal MUAC as covariates. In addition, for the NIC group, maternal age (r = 0.18 at p = 0.03) was significantly associated with left kidney volume with all other maternal factors as covariates. Children born to older smoking mothers had higher left kidney volumes compared to children born to non-smoking or alcohol-consuming mothers. Also, in utero exposure to alcohol and nicotine, male sex, BMI and WC of the child were associated with pancreas measurements using multiple linear regression models (Table 8).
Table 7.
Linear models incorporating maternal age, BMI, MUAC as well as the body weight of the child in association with kidney lengths and volumes at age 5 years
| Dependent variables | Controls N147 | ALCNIC N154 | ALC only N33 | NIC only N164 |
|---|---|---|---|---|
| LT K length | p < 0.01 | p < 0.01 | p = 0.25 | p < 0.01 |
| R2 = 0.17 | R2 = 0.25 | R2 = 0.19 | ||
| IDV: weight of the child r = 0.40, p < 0.01 | IDV: weight of the child r = 0.47, p < 0.01 | IDV: weight of the child r = 0.39, p < 0.01 | ||
| Covariates: Mat. Age, Mat. BMI, Mat. MUAC | Covariates: Mat. Age, Mat. BMI, Mat. MUAC | Covariates: Mat. Age, Mat. BMI, Mat. MUAC | ||
| LT K volume | p < 0.01 | p < 0.01 | p = 0.08 | p < 0.01 |
| R2 = 0.11 | R2 = 0.30 | R2 = 0.24 | ||
| IDV: weight of the child r = 0.30 at p < 0.01 | IDV: weight of the child r = 0.53 at p < 0.01 | IDV: Mat Age r = −0.18 at p = 0.03; Weight of the child r = 0.45 at p < 0.01 | ||
| Covariates: Mat. Age, Mat. BMI, mat. MUAC | Covariates: Mat. Age, Mat. BMI, Mat. MUAC | Covariates: Mat. BMI and Mat. MUAC | ||
| RT K length | p < 0.01 | p <0.01 | p =0.02 | p <0.01 |
| R2 = 0.24 | R2 = 0.13 | R2 = 0.39 | R2 = 0.15 | |
| IDV: Mat. MUAC r = 0.63 at p = 0.01; Mat. BMI r = −0.49 at p = 0.04; weight of the child r = 0.40 at p < 0.01 | IDV: weight of the child r = 0.35 at p < 0.01 | IDV: Weight of the child r = 0.45 at p = 0.02 | IDV: Weight of the child r = 0.39 at p < 0.01 | |
| Covariates: Mat. Age | Covariates: Mat. Age, Mat. BMI, Mat. MUAC | Covariates: Mat. Age, Mat. BMI, Mat. MUAC | Covariates: Mat. Age, Mat. BMI, Mat. MUAC | |
| RT K volume | p < 0.01 | p < 0.01 | p = 0.19 | p <0.01 |
| R2 = 0.23 | R2 = 0.34 | R2 = 0.23 | R2 = 0.14 | |
| IDV: Weight of the child r = 0.46 at p < 0.01 | IDV: weight of the child r = 0.56 at p < 0.01 | IDV: weight of the child r = 0.41 at p = 0.04 | IDV: weight of the child r = 0.37 at p < 0.01 | |
| Covariates: Mat. Age, Mat. BMI, Mat. MUAC | Covariates: Mat. Age, Mat. BMI, Mat. MUAC | Covariates: Mat. Age, Mat. BMI, Mat. MUAC | Covariates: Mat. Age, Mat. BMI, Mat. MUAC |
LT K length, left kidney length; LT K volume, left kidney volume; RT K length, right kidney length; RT K volume, right kidney volume; Mat. Age, maternal age; Mat. BMI, maternal body mass index; Mat. MUAC, maternal mid-upper arm circumference; ALCNIC, group of children exposed to both alcohol and nicotine during pregnancy; ALC only, group of children exposed to alcohol but no smoking during pregnancy; NIC only, group of children exposed to nicotine but no alcohol during pregnancy.
significance at p-value <0.05.
It is important to note that only significant findings are reported in Table 7.
Table 8.
Linear models incorporating in utero exposure, sex, BMI and WC in association with pancreas measurements at age 5 years
| F | df | p-value | r squared | beta | p-value | |
|---|---|---|---|---|---|---|
| Model 1: pancreas head | 13.44 | 4, 454 | <0.01 | 0.11 | ||
| IDV: exposure | −0.05 | 0.28 | ||||
| Sex | −0.13 | <0.01 | ||||
| WC | 0.28 | <0.01 | ||||
| BMI | 0.02 | 0.83 | ||||
| Model 2: pancreas body | 3.56 | 4, 454 | 0.01 | 0.03 | ||
| IDV: exposure | −0.07 | 0.12 | ||||
| Sex | −0.03 | 0.58 | ||||
| WC | 0.15 | 0.13 | ||||
| BMI | −0.01 | 0.98 | ||||
| Model 3: pancreas tail | 6.60 | 4, 454 | <0.01 | 0.06 | ||
| IDV: exposure | 0.04 | 0.42 | ||||
| Sex | −0.06 | 0.22 | ||||
| WC | 0.31 | <0.01 | ||||
| BMI | −0.10 | 0.34 |
BMI, body mass index; WC, waist circumference.
significance at p-value <0.05.
For pancreas head, WC (r = 0.28, p < 0.01) and male sex (r = 0.13, p < 0.01) were significantly associated with pancreas head values [F (4,454) = 13.4; p < 0.01, R2 = 0.11] with in utero exposure and BMI as covariates. WC (r = 0.31, p < 0.01) was significantly and independently associated with pancreas tail values [F (4,545) = 6.60; p < 0.01, R2 = 0.06] with in utero exposure, male sex and BMI as covariates (Table 8).
Discussion
In 5-year-old children exposed in utero to alcohol and or nicotine, the nicotine exposed children had significantly lower BWs and BLs compared to the controls or the other two exposure groups. However, BMI for the nicotine exposed children was not significantly lower. Furthermore, the smoking mothers were also significantly lighter in BW compared to the control mothers. Consequently, smaller children born to smaller mothers are expected although these smoking exposed children were not significantly smaller than the controls at birth. The main results from the present study are significantly lower values for both left and right kidney length for the in utero nicotine exposed children, compared to controls. Also, significantly lower pancreas body measurements were observed for in utero nicotine exposed children compared to controls. In contrast, in the present study, kidney width, height and volume as well as pancreas head and tail measurements were not significantly lower amongst the in utero nicotine exposed children. These findings might be explained by the smaller body size of the nicotine exposed children and the fact that they are born to smaller mothers when compared to the controls. The BW to organ size ratios were the same for all the exposure groups, but it does not explain why only the kidney length and pancreas body measurements were significantly lower. A possible explanation might be kidney length and pancreas body are regions within those organs most sensitive to in utero teratogen exposure in this population. In the present study, smoking mothers were significantly smaller, shorter stature and lower BMI, compared to the controls or AIC-consuming mothers. Short maternal stature might be a result of LBW and the childhood environment of the mother.15,26 Heavy smoking mothers may consume less nutrient-rich diets compared to controls and the compounds of cigarette smoking may interfere with the bioavailability of micronutrients to the developing baby.19,27 These findings are supported by those of other studies where smoking mothers were smaller.5,28 As described, in the nicotine exposed children smaller BW, BL as well as organ size might be expected.19 Confirmed by results from animal studies, maternal nicotine exposure predisposes an increased appetite, increased adipose tissue, as well as impaired glucose metabolism in their offspring in later life.6,29,30 The child’s diet during the postnatal life plays an equally important role. Accelerated weight gain and high BMIs early in life are d etrimental to the individual’s adult health.31,32 High fat combined with high carbohydrate and low protein diet is associated with a higher BMI and development of obesity-related cardiometabolic diseases.11,27, 32–34
According to the literature, smaller pancreas size is associated with T2DM,35,36 through beta-cell destruction and an increased pancreas size is caused by fat infiltration37 due to obesity. Accordingly, we found significant positive correlations between pancreas measurements and WC, a strong indication of abdominal adiposity, was observed. After controlling for the BMI of the child, WC remained significantly associated with all pancreas measurements. Because WC is a fairly easy measurement to perform, it may be helpful to identify prepubertal children at risk of cardiometabolic diseases.38 Furthermore, literature confirm WC, which may be a reflection of central obesity, is an important cardiometabolic risk indicator.39 In the present study, maternal BMI and MUAC were also significantly associated with the pancreas head and body measurements, indicating a genetic association with adiposity. However, after controlling for the BMI and WC of the child, these associations were no longer significant. Illustrating the important contribution of postnatal weight gain, BMI and WC compared to the genetic contribution of maternal adiposity.14 WC remained the strongest independent indicator for pancreatic head and tail as well as kidney measurements. In the present study, using a novel comprehensive assessment of visceral organ size (kidneys and pancreas) by ultrasound in association with WC, we found a significant positive correlation between WC and kidney and pancreas size measurements. These findings are supported by previous studies using ultrasound measurements of abdominal adipose tissue.40 Ultimately, findings from this present study, are in line with other studies from low-income countries where maternal age, education level and SES remained the strongest independent predictors of health and well-being for both mother and child.41,42 Females exposed to in utero nicotine are more likely to develop gestational diabetes and obesity,6,30 and have higher BMI and central adiposity20,31,39 in later life. Furthermore, LBW, associated with maternal smoking during pregnancy, is an important driver of cardiometabolic risk in low-income settings.13,18,19, 43–45
In this paediatric population, an in-normal-range mean, BMI of 15.2 kg/m2 was observed and might be explained by the age of the children as well as the current living conditions and nutritional status of these children. Literature reports overweight and obesity, a modifiable risk factor, as high as 32% and 8.1%, respectively, amongst primary school-aged children, adding to the cardiometabolic risk experienced in later life.20,31 Contrasting to other South African low-income settings where high obesity rates are due to nutritional transition from poverty to affluence.26 Moreover, the age of adiposity rebound (AR), the lowest BMI values just before an increase in BMI, is usually observed after the age of 5 years.22,46,47 Children from our study population might very well be experiencing an AR, which explains the in-normal-range BMI. Challenging living conditions, extreme poverty and suboptimal diet may also explain the normal mean BMI at this age. Other factors such as passive smoking, physical activity and genetics might be equally important explanatory factors.
However, BMI might not be the most sensitive measurement of adiposity (Rerksuppaphol et al., 2014). Instead, WC measurement is easy to perform and a more sensitive measurement of central obesity compared to BMI and should ideally be done routinely for paediatric patients as part of screening for cardiometabolic risk.38,39,48
Also, in the present study, BP was not significantly associated with kidney length nor kidney volume at this age. Confirmed by results from various other studies, BP is not associated with kidney size at age 5 years.10,49 Instead, in the present study, BP correlated significantly with BMI at age 5 years. Authors from the Leningrad siege study found similar results confirming the association of BP and obesity instead of an association between BP and in utero malnutrition.15 Other literature confirms a stronger association between the early development of adiposity and elevated BP.31 Fetal programming studies confirm an association of elevated BP and intrauterine growth restriction (IUGR) in both sexes but, only males remained hypertensive in later life.44,50 In utero malnutrition was also associated with the development of obesity-related hypertension.11,13,15 However, in the present study, as in other studies, kidney size correlated significantly with anthropometric measurements.51 Other authors found an association between LBW, smaller kidney size, low nephron numbers and development of hypertension.10,13,44,45
In a previous study of the same 500 mother–child pairs from this low-income community, we demonstrated the role of dual in utero exposure of alcohol and nicotine and the association with higher intima–media thickness (IMT) in children aged 5 years.53 In the present study, we sought to build on these existing findings by exploring the effects of maternal alcohol consumption and smoking during pregnancy and the association with kidney and pancreas size as part of the temporal evolution of cardiometabolic risk factors in children over the first 5 years of life.
This prospective follow-up study of 500 mother–child pairs extends to the existing body of evidence of maternal lifestyle choices including smoking and alcohol consumption during pregnancy impacting on a population’s cardiometabolic health.5,6,11,14 From a public health perspective, the novel approach of measuring organ size, using ultrasound, in conjunction with routine WC measurement, for central obesity screening, in primary school-aged children may be used as a preventative tool.
Strength and limitations
The present study had a few strengths and limitations worth mentioning. Perhaps, the main limitation of the present study was the inability to measure pancreatic mass and compare it with measures of pancreatic beta-cell functioning, i.e. insulin sensitivity. However, measurement of pancreas and kidney functions was not part of the scope of the present study. Also, our main aim of the study was to assess in utero exposure to alcohol and nicotine on the growth and development of visceral organs such as the pancreas and kidneys in a low-income setting paediatric population, we opted for the non-invasive, radiation free and relative ease of ultrasound. Therefore, a huge strength of the present study was the ability to obtain reference values for pancreas and kidney parameters measured by ultrasound.
These values obtained by ultrasound may serve as a screening tool to identify and follow-up at-risk children from similar low-income settings in South Africa.
Conclusions
In utero exposure to alcohol and nicotine was significantly associated with lower values for both left and right kidney length. WC, an indicator of abdominal adiposity, was independently associated with pancreas and kidney size. Perhaps, the most important conclusion of the present study is the correlation between WC and all the pancreas measurements, independently of the weight, height or sex of the child. This study confirms the harmful effects of in utero teratogen exposure on visceral organ size in a paediatric population 5 years after birth from a low-income setting in South Africa. Pancreas and kidney size by ultrasound could be used as a screening tool for diabetes and hypertension risk development.
Acknowledgements.
We would like to thank all the participants and the mothers/caregivers of the research study for their participation. Second, we would like to thank the sonographer, Heidi Nolan, for collecting the ultrasound data and lastly, Lucy Brink, for data management.
Financial support.
The Safe Passage Study was funded by the National Institute on Alcohol Abuse and Alcoholism, Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Institute of Deafness and other Communication Disorders: U01 HD055154, U01 HD045935, U01 HD055155, U01 HD045991 and U01 AA016501.
South African Sugar Association.
Footnotes
Ethical standards. Ethics approval was obtained from the Health and Research Ethics Committee (HREC) of Stellenbosch University (SU) and Biomedical Research Ethics Committee (BMREC) of the University of the Western Cape (UWC).
Conflict of interest. No potential conflict of interest relevant to this article was reported.
References
- 1.Hales CN, Barker DJP. Type 2 (non-insulin-dependant) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992; 35(7), 595–601. [DOI] [PubMed] [Google Scholar]
- 2.Hales CN, Barker DJP. The thrifty phenotype hypothesis. Br Med Bull. 2001; 60, 5–10. [DOI] [PubMed] [Google Scholar]
- 3.Vorster HH, Kruger A. Poverty, malnutrition, underdevelopment and cardiovascular disease: a South African perspective. Cardiovasc J Africa. 2007; 18(5), 321–324. [PMC free article] [PubMed] [Google Scholar]
- 4.Nakhoul MR, Seif KE, Haddad N, Haddad GE. Foetal alcohol exposure: the common toll. J Alcohol Drug Depend. 2017; 5(1), 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamulka J, Zieliñska MA, Chadzyñska K. The combined effects of alcohol and tobacco use during pregnancy on birth outcomes. National Institute of Public Health. Rocz Panstw Zakl Hig. 2018; 69(1), 45–54. [PubMed] [Google Scholar]
- 6.Capra L, Tezza G, Mazzei F, Boner AL. The origins of health and disease: the influence of maternal diseases and lifestyle during gestation. Ital J Paediatrics. 2013; 39(7), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monterio LJ, Norman JE, Rice GE, Illanes SE. Foetal programming and gestational diabetes mellitus. Placenta Trophoblast Res. 2015; 30(48) (Supplement), S54–S60. [DOI] [PubMed] [Google Scholar]
- 8.May PA, Hamrick KJ, Corbin KD, et al. Maternal nutritional status as a contributing factor for the risk of foetal alcohol spectrum disorders. Reprod Toxicol. 2016; 59, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone WL, Bailey B, Khraisha N. The pathophysiology of smoking during pregnancy: a systems biology approach. Front Biosci. 2014; E6, 318–328. [DOI] [PubMed] [Google Scholar]
- 10.Kooijman MN, Bakker H, Van der Heijden AJ, et al. Childhood kidney outcomes in relation to foetal blood flow and kidney size. J Am Soc Nephrol. 2014; 25, 2616–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lunde ER, Washburn SE, Golding MC, Bake S, Miranda RC, Ramadoss J. Alcohol-induced developmental origin of adult diseases. Alcohol Clin Exp Res. 2016; 40(7). doi: 10.1111/acer.13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucendo-Villarin B, Fillis P, Swortwood MJ, et al. Modelling foetal exposure to maternal smoking using hepatoblasts from pluripotent stem cells. 2017. doi: 10.1007/s00204-017-1983-0 [DOI] [PMC free article] [PubMed]
- 13.Koleganova N, Benza K, Piecha G, Ritz E, Amann K. Renal, cardiovascular and metabolic effect of foetal programming. Nephrol Dialysis Transp. 2012; 27, 3003–3007. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein D, Golson ML, Kaestner KH. Epigenetic control of ß-cell function and failure. Diabetes Res. Clin Pract 2017; 123, 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanner SA, Bulmer K, Andrès C, et al. Does malnutrition in-utero determine diabetes and coronary heart disease in adulthood? Results from the Leningrad siege study, a cross sectional study. Br Med J. 1997; 315(7119), 1342–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindsay CA, Thomas AJ, Catalano PM. The effect of smoking tobacco on neonatal body composition. Am J Obstetrics Gynaecology. 1997; 177(5), 1124–1128. [DOI] [PubMed] [Google Scholar]
- 17.Xiong F, Zhang L. Role of the hypothalamic-pituitary-adrenal axis in developmental programming of health and disease. Front Neuroendocrinol. 2013; 34, 27–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakker H, Jaddoe VWV. Cardiovascular and metabolic influences of foetal smoke exposure. Eur J Epidemiol. 2011; 26, 763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anblagan D, Jones NW, Costigan C, et al. Maternal smoking during pregnancy and foetal organ growth. PLoS ONE. 2014; 87, 7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundeen FA, Norris SA, Adair LS, Richter LM, Stein AD. Sex difference in obesity incidence: 20-year Prospective cohort is South Africa. Paediatric Obesity. 2015; 11, 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forray A Substance use during pregnancy. F1000 Res. 2016; 5, 887–896. [Google Scholar]
- 22.Boyer BP, Nelson JA, Holub SC. Childhood BMI trajectories predicting cardiovascular risk in adolescence. J Adolesc Health. 2015; 56(6), 599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dukes KA, Burd L, Elliott AJ, et al. The safe passage study: design, methods, recruitment, and follow-up approach. Pediatr Perinatal Epidemiol. 2014; 28, 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merz E, Benoit B, Blaas HG, Baba K, Kratochwil A, Nelson TR. Standardization of three-dimensional images in obstetrics and gynecology: consensus statement. Ultrasound Obstet Gynecol. 2007; 29(6), 697–703. [DOI] [PubMed] [Google Scholar]
- 25.Babcock DS, Patriquin HB. Diagnostic Ultrasound: Volume 2. 4th ed. (eds. Rumack CM, Wilson SR, Charboneau JW, Levine D), 2011; pp. 1845–1890. Elsevier, Philadelphia. [Google Scholar]
- 26.Kimani-Murage EW, Kahn K, Pettifor JM, et al. The prevalence of stunting, overweight and obesity, and metabolic disease risk in rural South African children. BMC Public Health. 2010; 10, 158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navarro E, Funtikova AN, Schroder H. Prenatal nutrition and the risk of adult obesity: long-term effects of nutrition on epigenetic mechanisms regulating gene expression. J Nutr Biochem. 2017; 39, 1–14. [DOI] [PubMed] [Google Scholar]
- 28.Caleyachetty R, Tait Ch A, Kengne AP, Corvalan C, Uauny R, Echouffo-Tcheugui JB. Tobacco use in pregnant women: analysis of data from demographic and health surveys from 54 low-income and middle income countries. Lancet Global Health. 2014; 2, e513–e520. [DOI] [PubMed] [Google Scholar]
- 29.Cornelius MD, Day NL. The effects of tobacco use during and after pregnancy on exposed children. Relevance of findings for alcohol research. Alcohol Res Health. 2000; 24(4), 242–249. [PMC free article] [PubMed] [Google Scholar]
- 30.Mattsson K, Källén K, Longnecker MP, Rignell-Hydbom A, Rylander L. Maternal smoking during pregnancy and daughters’ risk of gestational diabetes and obesity. Diabetologia. 2013; 56(8), 1689–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munthali R, Kagura J, Lombard Z, Norris SA. Childhood adiposity trajectories are associated with late adolescent blood pressure: birth to twenty cohort. BMC Public Health. 2016; 16, 665–675. doi: 10.1186/s12889-016-3337-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geserick M, Vogel M, Gausche R, et al. Acceleration of BMI in early childhood and risk of sustained obesity. N Engl J Med. 2018; 379, 1303–1312. [DOI] [PubMed] [Google Scholar]
- 33.Steyn NP, Ochse R. “Enjoy a variety of foods”: as a food-based dietary guideline for South Africa. S Afr J Clin Nutr. 2013; 26(3) (Supplement), S13–S17. [Google Scholar]
- 34.Balaji S, Napolitano T, Silvano S, et al. Epigenetic control of pancreatic regeneration in diabetes. Genes. 2018; 9, 448–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saisho Y, Butler AE, Meier JJ, et al. Pancreas voumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type-2 diabetes. Clin Anat. 2007; 20, 993–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agabi JO, Akhigbe AO. Comparative sonographic evaluation of the anteroposterior dimensions of the pancreas in diabetic and nondiabetics. Niger J Clin Pract. 2016; 19, 175–181. [DOI] [PubMed] [Google Scholar]
- 37.Sakai N. Obesity, metabolic disease and the pancreas: quantitative imaging of the pancreatic fat. Br J Radiol. 2018; 91(1089), 20180267. doi: 10.1259/6jr20180267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maffeis C, Pietrobelli A, Grezzani A, Provera S, Tató L. Waist circumference and cardiovascular risk factors in pre-pubertal children. Obesity Res. 2001; 9(3), 179–188. [DOI] [PubMed] [Google Scholar]
- 39.Rerksuppaphal S, Rerksuppaphol L. Waist circumference, waist-to-height ratio and body mass index of Thai children: secular changes and updated reference standards. J Clin Diagn Res. 2014; 8(11), 05–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mook-Kanamori DO, Holzhauer S, Hollestein LM, et al. Abdominal fat in children measured by ultrasound and computed tomography. Ultrasound Med Biol. 2009; 35(12), 1938–1946. [DOI] [PubMed] [Google Scholar]
- 41.Victoria CG, Adair L, Fall C, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008; 371, 340–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rasiah R, Yusoff K, Mohammadreza A, et al. Cardiovascular disease risk factors and socioeconomic variables in a nation undergoing epidemiological transition. BMC Public Health. 2013; 13, 886–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zarén B, Lindmark G, Bakketeig L. Maternal smoking affects foetal growth more in the male foetus. Paediatr Perinatal Epidemiol. 2000; 14, 118–126. [DOI] [PubMed] [Google Scholar]
- 44.Jones JE, Jurgens JA, Erans SA, Ennis RC, Villar VAM, Jose PA. Mechanisms of foetal programming in hypertension. Int J Paediatr. 2011; 2012, Article id 584831, 7 p. 10.1155/2012/58483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luyckx VA, Bertram JF, Brenner BM, et al. Effect of foetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet. 2013; 382(9888), 273–283. [DOI] [PubMed] [Google Scholar]
- 46.Berends LM, Ozanne SE. Early determinants of type-2 diabetes. Best Pract Res Clin Endocrinol Metab. 2012; 26, 569–580. [DOI] [PubMed] [Google Scholar]
- 47.Koyama S, Ichikawa G, Kojima M, Shimura N, Sairenchi T, Arisaka O. Adiposity rebound and the development of metabolic syndrome. Paediatrics. 2014; 133(1), 114–119. [DOI] [PubMed] [Google Scholar]
- 48.Siega-Riz AM. Modification of lifestyle behaviour during pregnancy for prevention of childhood obesity. Lancet Child Adolesc Health. 2018; 2(11), 770–772. [DOI] [PubMed] [Google Scholar]
- 49.Gurusinghe S, Palvano A, Bittman ME, et al. Kidney volumes and ambulatory blood pressure in children. J Clin Hypertens. 2017; 19, 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ojeda NB, Grigore D, Alexander BT. Developmental programming of hypertension: insights from animal models of nutritional manipulations. Hypertension. 2008; 52(1), 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jovanovic D, Gasic B, Pavlovic S, Naumovic R. Correlation of kidney size with kidney function and anthropometric parameters in healthy subjects and patients with chronic kidney diseases. Renal Fail. 2013; 35(6), 896–900. [DOI] [PubMed] [Google Scholar]
- 52.Parquette K, Fernandes R, Xie LF, et al. Kidney size, renal function, angiotensin, peptides and blood pressure in young adults born preterm. The Hapi study. Hypertension. 2018; 72, 918–928. [DOI] [PubMed] [Google Scholar]
- 53.De Smidt JJA, Odendaal HJ, Nel DG, et al. In utero teratogen exposure and cardiometabolic risk in 5 year-old children: a prospective study. J Maternal-Fetal Neonatal Med. 2019. doi: 10.1080/14767058.2019.1692337. [DOI] [PMC free article] [PubMed] [Google Scholar]


