Abstract
Most studies of coronavirus disease 2019 (COVID-19) progression have focused on the transfer of patients within secondary or tertiary care hospitals from regular wards to intensive care units. Little is known about the risk factors predicting the progression to severe COVID-19 among patients in community isolation, who are either asymptomatic or suffer from only mild to moderate symptoms. Using a multivariable competing risk survival analysis, we identify several important predictors of progression to severe COVID-19—rather than to recovery—among patients in the largest community isolation center in Wuhan, China from 6 February 2020 (when the center opened) to 9 March 2020 (when it closed). All patients in community isolation in Wuhan were either asymptomatic or suffered from mild to moderate COVID-19 symptoms. We performed competing risk survival analysis on time-to-event data from a cohort study of all COVID-19 patients (n = 1753) in the isolation center. The potential predictors we investigated were the routine patient data collected upon admission to the isolation center: age, sex, respiratory symptoms, gastrointestinal symptoms, general symptoms, and computed tomography (CT) scan signs. The main outcomes were time to severe COVID-19 or recovery. The factors predicting progression to severe COVID-19 were: male sex (hazard ratio (HR) = 1.29, 95% confidence interval (CI) 1.04–1.58, p = 0.018), young and old age, dyspnea (HR = 1.58, 95% CI 1.24–2.01, p < 0.001), and CT signs of ground-glass opacity (HR = 1.39, 95% CI 1.04–1.86, p = 0.024) and infiltrating shadows (HR = 1.84, 95% CI 1.22–2.78, p = 0.004). The risk of progression was found to be lower among patients with nausea or vomiting (HR = 0.53, 95% CI 0.30–0.96, p = 0.036) and headaches (HR = 0.54, 95% CI 0.29–0.99, p = 0.046). Our results suggest that several factors that can be easily measured even in resource-poor settings (dyspnea, sex, and age) can be used to identify mild COVID-19 patients who are at increased risk of disease progression. Looking for CT signs of ground-glass opacity and infiltrating shadows may be an affordable option to support triage decisions in resource-rich settings. Common and unspecific symptoms (headaches, nausea, and vomiting) are likely to have led to the identification and subsequent community isolation of COVID-19 patients who were relatively unlikely to deteriorate. Future public health and clinical guidelines should build on this evidence to improve the screening, triage, and monitoring of COVID-19 patients who are asymtomatic or suffer from mild to moderate symptoms.
Keywords: COVID-19, Asymptomatic and mild, Community isolation, Fangcang shelter hospital, Competing risk survival analysis
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic is inflicting an enormous toll on societies around the world. To deal with the pandemic, countries have adopted two types of isolation strategies for asymptomatic or mildly ill COVID-19 patients who do not require hospitalization: home isolation or community isolation. Home isolation has been adopted by many countries in the Americas and in Europe [1], [2], [3], [4]. However, a major disadvantage of home isolation is that COVID-19 patients, even when asymptomatic or mildly ill, can still transmit the virus to their family or community members. Community isolation of COVID-19 patients in dedicated community isolation centers is an alternative isolation strategy that has been adopted by many East Asian countries, such as China, Republic of Korea, Singapore, and Vietnam [5], [6], [7], [8], [9], [10]. A crucial advantage of community isolation is that it provides asymptomatic and mildly ill COVID-19 patients with a safe space to isolate outside their homes until they are no longer infectious [5], [8], [11], [12].
For all isolation strategies, it is important to identify which COVID-19 patients are likely to progress to severe disease and require specialized hospital care [13], [14]. The ability to identify such patients could guide decisions whether to isolate or hospitalize a patient and the design of routine monitoring and medical care of COVID-19 patients who are isolating [12], [14], [15].
Predicting the progression to severe disease is particularly important for COVID-19 because the disease often progresses rapidly [14], [15], [16], [17]. Indeed, one major reason for the large differences in COVID-19 case–fatality ratios across countries may be the varying ability of national health systems to rapidly provide high-level care to patients [18], [19], [20]. While several studies have explored the risk factors of COVID-19 progression, most of these studies have focused on the transfer of COVID-19 patients within secondary or tertiary care hospitals from regular wards to intensive care units [19], [21], [22], [23], [24], [25], [26], [27], [28], [29]. Our study focuses on progression from asymptomatic or mild to moderate symptoms—which can be treated at the community level—to more severe symptoms that require hospital care.
For this study, we used the clinical and health systems data collected from all patients in the largest of the community isolation centers—so-called Fangcang shelter hospitals—that were built in Wuhan, China during February and March 2020 [5]. The Fangcang shelter hospitals were an important component of the COVID-19 response in Wuhan, providing community isolation and care for COVID-19 patients who were either asymptomatic or suffered from mild to moderate symptoms. These community isolation centers were constructed within existing public infrastructures, such as stadiums and exhibition centers [5]. While the centers were developed and deployed for the first time during February and March 2020 in Wuhan, several other Asian communities have used this model of isolation and care for COVID-19 since then, as an alternative to home isolation [6], [7], [30].
The nurses and physicians working in the Fangcang shelter hospitals regularly monitored the disease progression of their patients and decided on a daily basis whether a patient needed to be referred to a higher level hospital because she/he developed more severe symptoms or signs indicating a deteriorating health status [5]. We used the clinical data that was routinely collected among all the patients in community isolation during the admission process to predict deterioration from asymptomatic, mild, or moderate COVID-19 to severe COVID-19. Our two competing outcomes in this survival analysis were deterioration (referral to a higher level hospital) and recovery (leading to discharge).
Our study provides important information for health policymakers and clinicians concerned with designing policies and systems for long-term COVID-19 control following the easing of lockdown policies in many countries [31], [32], [33], [34]. In particular, our findings can inform the initial decision on whether to isolate and care for a patient who has newly tested positive for COVID-19 at home, in community facilities, or in higher level hospitals. Our findings can also inform the design of routine clinical monitoring and care for patients with no, mild, or moderate COVID-19 symptoms in home, community, or hospital isolation.
2. Methods
2.1. Study population
Our study population consisted of all patients with no, mild, or moderate COVID-19 symptoms who were admitted to the first and largest of the 16 Fangcang shelter hospitals that were constructed in Wuhan, China in February 2020 [5]. Our observation period lasted from 6 February 2020 (when the first patients were admitted to this community isolation center) to 9 March 2020 (when this center suspended operations because the number of new COVID-19 cases had substantially declined in Wuhan). Polymerase chain reaction (PCR) tests were given to people who had come into close contact with COVID-19 patients or who presented with symptoms. The following official community isolation center admission criteria, which were adhered to across all of the 16 community isolation centers in Wuhan were then applied to determine the eligibility for admission [5]: ① a positive nucleic acid test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); ② either asymptomatic or mild signs or symptoms (mild clinical symptoms and imaging showing no signs of pneumonia) or moderate signs or symptoms (fever, respiratory tract symptoms, and imaging showing pneumonia); ③ ability to walk and live independently; ④ absence of severe chronic diseases, including hypertension, diabetes, coronary heart disease, malignancy, structural lung disease, pulmonary heart disease, and immunosuppression; ⑤ no history of mental health conditions; ⑥ ≥ 15 and ≤ 65 years old (with the exception of patients who had family members in the same community isolation center); ⑦ a negative influenza test; and ⑧ blood oxygen saturation (SpO2) > 93% and respiratory rate < 30 breaths per minute. Patients were admitted to the Fangcang shelter hospital as a result of care seeking, contact tracing, or population screening for COVID-19. In Fig. 1 , we show the distribution of the number of symptoms the patients in our sample suffered from upon admission to the Fangcang shelter hospital.
Fig. 1.
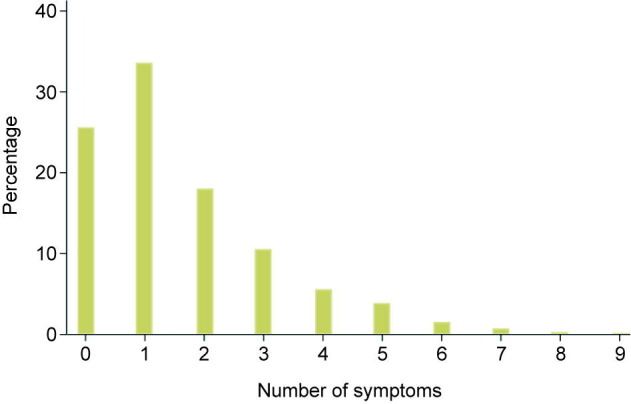
Distribution of symptom counts among patients upon admission to the Fangcang shelter hospital. 0 refers to patients who had no symptoms.
2.2. Ethics
The study was approved by the Ethics Commission of the Union Hospital affiliated to Tongji Medical College of Huazhong University of Science and Technology (KY-2020–01.01), and the requirement for informed consent was waived by the Ethics Commission.
2.3. Study location
Located in the Jianghan district of Wuhan near the Yangtze River, the community isolation center that is the location of our study was the first and largest of the 16 Fangcang shelter hospitals in Wuhan, the capital of Hubei Province in China. It was built within the Wuhan International Conference and Exhibition Center. The distance from the community isolation center to the nearest higher level hospital designated for COVID-19 care was approximately 1 km.
2.4. Outcomes
Upon admission to the community isolation center, all COVID-19 patients had no, mild, or moderate symptoms. Patients with deteriorating health status were quickly transferred from the community isolation center to higher level hospitals. Not a single COVID-19 patient died in the community isolation center during the observation period of this study. All patients in our study could thus develop only one of two mutually exclusive outcomes: deterioration (leading to referral) or recovery (leading to discharge).
2.4.1. Deterioration
If COVID-19 patients in the community isolation centers met any of the following clinical criteria, they were judged to have deteriorated and were rapidly referred to higher level hospitals designated for COVID-19 care [5], [35]: ① respiratory rate ≥ 30 breaths per minute; ② SpO2 ≤ 93%; ③ ratio of partial pressure arterial oxygen and fraction of inspired oxygen (PaO2/FiO2) ≤ 300 mmHg (1 mmHg = 133 Pa); ④ lung imaging showing a greater than 50% progression of lesions within 24–48 hours; or ⑤ development of severe chronic diseases, including hypertension, diabetes, coronary heart disease, cancer, structural lung disease, pulmonary heart disease, or immunosuppression.
2.4.2. Recovery
If patients met any of the following clinical criteria, they were discharged from the community isolation centers because they were judged to have recovered: ① negative nucleic acid tests results for COVID-19 at two consecutive times with a sampling interval of at least one day; ② normal body temperature for more than three days; ③ significant improvement of respiratory symptoms; or ④ lung imaging showing obvious absorption of inflammation.
2.5. Predictors
We used three broad categories of predictors in our analysis. The choice of these predictor categories was based on data availability in the routine Fangcang shelter hospital database—all predictors used in this study were routinely collected from all patients upon admission to the community isolation center—as well as on usefulness for the routine monitoring of COVID-19 patients in a variety of settings. The first category comprises demographic characteristics (i.e., age and sex). The second category comprises clinical symptoms and consists of the following three subcategories: ① respiratory symptoms (cough or sputum, stuffy or runny nose, sore throat, and dyspnea); ② gastrointestinal symptoms (nausea or vomiting, and abdominal pain or diarrhea); and ③ general symptoms (fatigue, headache, myalgia, and fever). The third and final category comprises CT scan results (ground-glass opacity, infiltrating shadows, consolidation, pleural effusion, and pulmonary interstitial changes). Fever was defined as an axillary temperature of at least 37.5 °C. The CT signs were read and coded by radiologists.
2.6. Statistical analysis
We used a competing risk survival analysis to measure the predictors of progression from asymptomatic, mild, or moderate COVID-19 to severe COVID-19, because our outcomes data included the time to either of two mutually exclusive events: deterioration or recovery. For each patient with asymptomatic, mild, or moderate COVID-19, our observation period began upon admission to the community isolation center and ended when one of the following events occurred: ① the patient was transferred to a designated hospital for higher level clinical care because his or her clinical symptoms had deteriorated (outcome “deterioration”); ② the patient was discharged because he or she had recovered from COVID-19 (competing outcome “recovery”); or ③ neither ① nor ② had occurred before the community isolation center suspended operation (right-censored data). In the case of ③, the patients were transferred to higher level hospitals for continued isolation and care. In this case, the transfer took place because isolation and care in the community isolation center was no longer possible. We computed sub-distribution hazard ratios (HRs) in the competing risk survival analysis [36] to identify predictors of the absolute risk of deterioration during the time the patients stayed in community isolation [37], [38], [39].
We conducted both univariable and multivariable competing risk survival analyses. We adopted competing risk survival analyses because they are a valid, well-established procedure for estimating the cumulative recovery rate curve across a study period and are recommended by statisticians [40]. In our multivariable analyses, we sequentially added categories of potential predictors in nested regression models: demographic characteristics, symptoms, and CT signs. We assessed the proportional hazards assumption of the competing risk survival analysis using interaction terms between time and all other variables [41]. We used Stata version 15 to conduct the statistical analyses.
3. Results
3.1. Sample characteristics
There were 1806 asymptomatic, mild, or moderate COVID-19 patients in community isolation who had a valid registration record in the database as of 9 March 2020 (the date on which the community isolation center was suspended). After excluding 53 patients with missing or invalid dates of admission (e.g., the date of admission was missing or was before the opening or after the closing of the Fangcang shelter hospital) or missing or invalid outcome data (e.g., the date of transfer or recovery was before the opening or after the closing of the Fangcang shelter hospital, or before the date of admission), we included 1753 inpatients in the final analysis. Out of these patients, 382 (21.8%) were transferred to designated higher level hospitals, 1270 (72.4%) were discharged, and 101 (5.8%) had right-censored outcomes data. The deterioration rate was slightly higher in our sample than in another study focusing on Fangcang shelter hospitals, in which 10% were transferred to designated hospitals due to deterioration [28].
The median time from admission to deterioration and transfer to designated hospitals was 16 days (interquartile range (IQR): 12–20), with a range of 0–30 days. The median time from admission to recovery was 14 days (IQR: 9–18), with a range of 0–32 days. The median age of the 1753 patients was 50 years (IQR: 40–57), with a range of 15–73 years. Male patients accounted for 43% of the patients.
As shown in Fig. 1, most patients had one symptom (33.6%), while about one quarter (25.6%) of patients were asymptomatic. Patients who had four symptoms or less accounted for 93.4%. Patients with seven or more symptoms accounted for only 1.2% of all patients. The most common symptoms on admission were cough or fever, followed by fatigue and dyspnea. The most common CT sign was ground-glass opacity (Table 1 ).
Table 1.
Patients’ demographic characteristics, symptoms, and radiographic signs upon admission by outcome.
| Demographic characteristics, symptoms, and radiographic signs | Full sample (n = 1753) |
Recovery (n = 1270) |
Deterioration (n = 382) |
Censored (n = 101) |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Agea (year) | 48.7 | 48.4 | 50.0 | 48.5 |
| (11.3) | (11.2) | (11.5) | (11.3) | |
| Age groups (years) | ||||
| < 40 | 405 | 301 | 81 | 23 |
| (23.1%) | (23.7%) | (21.2%) | (22.8%) | |
| 40–45 | 187 | 144 | 30 | 13 |
| (10.7%) | (11.3%) | (7.9%) | (12.9%) | |
| 45–49 | 216 | 167 | 40 | 9 |
| (12.3%) | (13.2%) | (10.5%) | (8.9%) | |
| 50–54 | 278 | 195 | 64 | 19 |
| (15.9%) | (15.4%) | (16.8%) | (18.8%) | |
| 55–59 | 321 | 230 | 74 | 17 |
| (18.3%) | (18.1%) | (19.4%) | (16.8%) | |
| ≥ 60 | 346 | 233 | 93 | 20 |
| (19.7%) | (18.4%) | (24.4%) | (19.8%) | |
| Sex | ||||
| Male | 758 | 527 | 186 | 45 |
| (43.2%) | (41.5%) | (48.7%) | (44.6%) | |
| Respiratory symptoms | ||||
| Coughb | 809 | 564 | 201 | 44 |
| (46.2%) | (44.4%) | (52.6%) | (43.6%) | |
| Stuffy or runny nose | 81 | 58 | 20 | 3 |
| (4.6%) | (4.6%) | (5.2%) | (3.0%) | |
| Sore throat | 98 | 68 | 27 | 3 |
| (5.6%) | (5.4%) | (7.1%) | (3.0%) | |
| Dyspnea | 365 | 226 | 109 | 30 |
| (20.8%) | (17.8%) | (28.5%) | (30.0%) | |
| Gastrointestinal symptoms | ||||
| Nausea or vomiting | 84 | 57 | 17 | 10 |
| (4.8%) | (4.5%) | (4.5%) | (9.9%) | |
| Abdominal pain or diarrhea | 150 | 104 | 38 | 8 |
| (8.6%) | (8.2%) | (10.0%) | (7.9%) | |
| General symptoms | ||||
| Fatigue | 439 | 309 | 103 | 27 |
| (25.0%) | (24.3%) | (27.0%) | (26.7%) | |
| Headache | 82 | 66 | 12 | 4 |
| (4.7%) | (5.2%) | (3.1%) | (4.0%) | |
| Myalgia | 152 | 91 | 44 | 17 |
| (8.7%) | (7.2%) | (11.5%) | (16.8%) | |
| Fever | 568 | 377 | 123 | 68 |
| (32.4%) | (29.7%) | (32.2%) | (67.3%) | |
| CT signs | ||||
| Ground-glass opacity | 1402 | 1020 | 322 | 60 |
| (80.0%) | (80.3%) | (84.3%) | (59.4%) | |
| Infiltrating shadows | 69 | 40 | 25 | 4 |
| (3.9%) | (3.2%) | (6.5%) | (4.0%) | |
| Consolidation | 22 | 15 | 7 | 0 |
| (1.3%) | (1.2%) | (1.8%) | (0) | |
| Pleural effusion | 11 | 8 | 3 | 0 |
| (0.6%) | (0.6%) | (0.8%) | (0) | |
| Pulmonary interstitial changes | 26 | 20 | 6 | 0 |
| (1.5%) | (1.6%) | (1.6%) | (0) | |
This table shows the demographic characteristics; respiratory, gastrointestinal, and general symptoms; and CT signs for all patients and for patients with deterioration, recovery, or right-censored outcome data.
Values in the parentheses represent standard deviation.
Cough includes cough with and without sputum production.
Fig. 2 presents the cumulative incidence curves for deterioration and recovery for all patients. The cumulative incidence for deterioration at days 5, 10, 15, 20, 25, and 30 was 1.5% (95% confidence interval (CI) 1.0%–2.2%), 3.6% (95% CI 2.8%–4.5%), 9.8% (95% CI 8.5%–11.3%), 16.8% (95% CI 15.1%–18.7%), 21.2% (95% CI 19.3%–23.2%), and 22.7% (95% CI 20.7%–24.7%), respectively. The cumulative incidence for recovery at days 5, 10, 15, 20, 25, and 30 was 5.3% (95% CI 4.3%–6.4%), 19.9% (95% CI 18.1%–21.9%), 41.8% (95% CI 39.5%–44.2%), 59.2% (95% CI 56.9%–61.5%), 71.1% (95% CI 68.9%–73.2%), and 73.3% (95% CI 71.1%–75.4%), respectively.
Fig. 2.
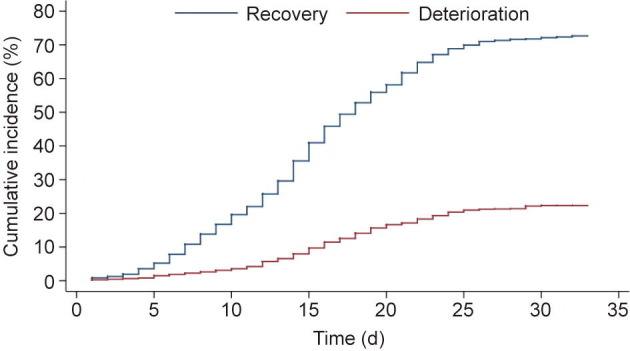
Cumulative incidence of deterioration and recovery.
3.2. Univariable competing risk regression
We show the cumulative incidence of progression to severe COVID-19 for the factors that are significant in multivariable competing risk survival analyses and are well-known risk factors of progression (Fig. 3 (a)), CT scan signs (Fig. 3(b)), and common and unspecific disease symptoms (Fig. 3(c)). Fig. S1 in Appendix A provides the cumulative incidence curves for deterioration from the univariable regressions for other variables. In the univariable analysis, the HR of deterioration was higher in men and older patients (Fig. S2 in Appendix A). The symptoms at admission that significantly increased the cumulative incidence of deterioration were cough or sputum production (HR = 1.34, 95% CI 1.09–1.64, p = 0.004), dyspnea (HR = 1.61, 95% CI 1.29–2.02, p = 0.002), and myalgia (HR = 1.39, 95% CI 1.01–1.91, p < 0.001). CT signs that significantly increased the cumulative incidence of deterioration were ground-glass opacity (HR = 1.39, 95% CI 1.05–1.85, p < 0.001) and infiltrating shadows (HR = 1.83, 95% CI 1.23–2.71, p < 0.001). Fig. S3 in Appendix A presents the cumulative incidence curves for recovery from univariable regressions.
Fig. 3.
Cumulative incidence of progression to severe COVID-19 for factors that are significant in multivariable competing risk survival analyses and are (a) well-known risk factors of progression, (b) CT scan signs, or (c) common and unspecific disease symptoms.
3.3. Multivariable competing risk regression
Table 2 shows the main results for the cumulative incidence of COVID-19 deterioration. In the multivariable model including all risk factors, deterioration was higher among men than women (HR = 1.29, 95% CI 1.04–1.58, p = 0.018), implying that male sex is associated with a 29% increase in the sub-distribution hazard of deterioration—that is, the instantaneous rate of occurrence of deterioration in subjects who have not yet experienced an event of deterioration [42]. Deterioration was lower among middle-aged adults in comparison with younger adults and the elderly (Wald test of joint significance, p = 0.0943). Compared with patients aged below 40, the estimated risks of deterioration for the age group 40–44 and the age group 45–49 were lower (age group 40–44: HR = 0.74, 95% CI 0.48–1.14, p = 0.170; age group 45–49: HR = 0.94, 95% CI 0.64–1.37, p = 0.746), and the estimated risks of deterioration were higher for age groups older than 50 (age group 50–54: HR = 1.08, 95% CI 0.77–1.51, p = 0.672; age group 55–59: HR = 1.14, 95% CI 0.82–1.59, p = 0.426; and age group ≥ 60: HR = 1.33, 95% CI 0.98–1.81, p = 0.063).
Table 2.
Cumulative incidence of COVID-19 deterioration.
| Risk factors | Cumulative incidence (HR, 95%CI, p)c |
||||
|---|---|---|---|---|---|
| Model 1 (n = 1701) | Model 2 (n = 1701) | Model 3 (n = 1701) | Model 4 (n = 1701) | Model 5 (n = 1701) | |
| Demographic characteristics | |||||
| Age groupsa | |||||
| 40–44 | 0.78, 0.51–1.20, 0.258 | 0.77, 0.50–1.17, 0.215 | 0.74, 0.48–1.13, 0.167 | 0.73, 0.48–1.13, 0.160 | 0.74, 0.48–1.14, 0.170 |
| 45–49 | 0.92, 0.63–1.35, 0.679 | 0.91, 0.62–1.33, 0.623 | 0.92, 0.63–1.34, 0.648 | 0.92, 0.63–1.35, 0.677 | 0.94, 0.64–1.37, 0.746 |
| 50–54 | 1.16, 0.83–1.61, 0.382 | 1.12, 0.80–1.57, 0.499 | 1.12, 0.80–1.56, 0.514 | 1.10, 0.79–1.54, 0.569 | 1.08, 0.77–1.51, 0.672 |
| 55–59 | 1.17, 0.85–1.60, 0.345 | 1.15, 0.84–1.59, 0.386 | 1.13, 0.82–1.56, 0.463 | 1.16, 0.84–1.61, 0.368 | 1.14, 0.82–1.59, 0.426 |
| ≥ 60 | 1.43, 1.06–1.93, 0.019 | 1.38, 1.02–1.87, 0.035 | 1.37, 1.01–1.85, 0.041 | 1.37, 1.01–1.86, 0.040 | 1.33, 0.98–1.81, 0.063 |
| Sex | |||||
| Male | 1.25, 1.02–1.53, 0.033 | 1.31, 1.06–1.60, 0.011 | 1.29, 1.05–1.59, 0.015 | 1.28, 1.04–1.58, 0.018 | 1.29, 1.04–1.58, 0.018 |
| Respiratory symptoms | |||||
| Coughb, d | — | 1.27, 1.03–1.56, 0.028 | 1.27, 1.03–1.57, 0.024 | 1.26, 1.02–1.57, 0.033 | 1.22, 0.98–1.51, 0.077 |
| Stuffy or runny nose | — | 0.89, 0.55–1.42, 0.616 | 0.97, 0.59–1.58, 0.899 | 1.02, 0.62–1.67, 0.952 | 1.01, 0.61–1.66, 0.978 |
| Sore throat | — | 1.20, 0.79–1.81, 0.396 | 1.23, 0.82–1.86, 0.315 | 1.28, 0.85–1.93, 0.239 | 1.33, 0.88–2.01, 0.180 |
| Dyspnea | — | 1.52, 1.20–1.93, 0.001 | 1.57, 1.24–2.00, < 0.001 | 1.58, 1.24–2.01, < 0.001 | 1.58, 1.24–2.01, < 0.001 |
| Gastrointestinal symptoms | |||||
| Nausea or vomiting | — | — | 0.57, 0.32–0.99, 0.046 | 0.55, 0.31–0.99, 0.046 | 0.53, 0.30–0.96, 0.036 |
| Abdominal pain or diarrhea | — | — | 1.11, 0.78–1.56, 0.571 | 1.13, 0.80–1.60, 0.497 | 1.09, 0.77–1.55, 0.631 |
| General symptoms | |||||
| Fatigue | — | — | — | 0.94, 0.73–1.21, 0.626 | 0.94, 0.73–1.21, 0.613 |
| Headache | — | — | — | 0.53, 0.29–0.98, 0.042 | 0.54, 0.29–0.99, 0.046 |
| Myalgia | — | — | — | 1.39, 0.96–2.03, 0.084 | 1.36, 0.93–1.98, 0.109 |
| Feverb | — | — | — | 1.07, 0.85–1.34, 0.573 | 1.14, 0.91–1.44, 0.255 |
| CT signs | |||||
| Ground-glass opacity | — | — | — | — | 1.39, 1.04–1.86, 0.024 |
| Infiltrating shadows | — | — | — | — | 1.84, 1.22–2.78, 0.004 |
| Consolidation | — | — | — | — | 1.17, 0.56–2.42, 0.678 |
| Pleural effusion | — | — | — | — | 1.20, 0.37–3.94, 0.761 |
| Pulmonary interstitial changes | — | — | — | — | 0.83, 0.37–1.83, 0.642 |
Age group younger than 40 is the reference group.
Evidence of violation of proportional hazards assumption in models 3 and 5 for cough, and in models 4 and 5 for fever. Estimates should be interpreted as the weighted average over the follow-up period.
The regressions excluded 52 patients who were transferred immediately after admission. In all regressions, the total number of patients transferred, discharged, and censored were 372, 1228, and 101, respectively.
Cough includes cough with and without sputum production.
The hazard of deterioration was higher among patients with dyspnea (HR = 1.58, 95% CI 1.24–2.01, p < 0.001) and lower among patients with nausea or vomiting (HR = 0.53, 95%CI 0.30–0.96, p = 0.036) than for patients without these symptoms at admission. Patients with CT signs of ground-glass opacity (HR = 1.39, 95%CI 1.04–1.86, p = 0.024) and infiltrating shadows (HR = 1.84, 95%CI 1.22–2.78, p = 0.004) were significantly more likely to be transferred than to be discharged.
4. Discussion
Our study provides answers to an important public health research question: Which factors predict progression to severe COVID-19 requiring hospitalization for intensive or complex care? Most previous studies elucidating the risk factors for COVID-19 progression have used data from COVID-19 patients that were already hospitalized with relatively severe symptoms [19], [21], [22], [23], [24], [25], [26], [27], [28], [29]. However, most COVID-19 patients never require hospitalization and can be isolated and cared for at home or in community facilities. We used data from COVID-19 patients in community isolation in Wuhan, China, to determine the factors predicting COVID-19 progression. Most COVID-19 patients in our sample had mild to moderate symptoms; about one quarter of patients were asymptomatic. One in five patients in community isolation eventually needed the intensive or complex treatments that were only available in higher level hospitals designated for COVID-19 care. Another important advantage of this study is that we tried to include a list of potential risk factors based on low-cost diagnostic information, such as demographic and social characteristics, as well as easily identifiable symptoms. In low-income countries or settings where expensive CT scans are difficult to perform, this low-cost information is crucial for designing triage and isolation strategies and minimizing the economic harm of COVID-19 [43], [44].
The most common clinical symptoms among the COVID-19 patients that were monitored and cared for in the community center in Wuhan were cough and fever, followed by fatigue and dyspnea. The standard protocol for community isolation in Wuhan included CT scans to rule out signs of severe disease. The most common CT sign among patients in the community center was ground-glass opacity.
Male sex, dyspnea, and ground-glass opacities and infiltrating shadows in CT scans were important predictors of progression to severe COVID-19 rather than recovery. These findings demonstrate that several important risk factors of progression to severe COVID-19 are similar among patients in community isolation and among patients already needing hospitalization [45], [46], [47], [48], [49], [50]. For example, increasing evidence shows that COVID-19 produces more severe symptoms and higher mortality among men than women, which is likely due to sex differences in immune responses during the course of SARS-CoV-2 infection [51]. Knowledge gained among hospitalized patients may thus be at least partially generalizable to milder cases of COVID-19 in patients in community or home isolation. Our findings also indicate that two easily identifiable risk factors—namely, male sex and dyspnea—should guide public health and clinical decisions regarding the intensity of monitoring and referral to designated hospitals. The strong associations of two specific CT symptoms with a deteriorating course of COVID-19 further suggest that countries where mass CT scanning is technologically feasible and affordable should consider adding CT scans to the routine clinical work-up of COVID-19 patients whose symptoms do not already indicate a need for hospitalization. Such decisions should be supported by future cost-effectiveness analyses of CT scans for screening COVID-19 patients to decide whether they should be hospitalized or not. It is possible that CT scans will indeed be cost-effective for the routine screening of COVID-19 patients, because most patients in most pandemic situations cannot be hospitalized, while patients that experience a deteriorating course of the disease often do so rapidly and with a relatively high risk of mortality if they do not quickly receive higher level care [18], [19], [20]. CT scan results may substantially and cost-effectively improve the outcomes of triage decisions that separate patients into those requiring immediate hospitalization and those that can be isolated in community centers or at home.
Headaches and nausea or vomiting were significant factors associated with a reduced risk of severe COVID-19 among our sample of patients in community isolation. The reason for these findings is unlikely to be biological. Headaches and nausea or vomiting are not specific symptoms of COVID-19, but occur in people suffering from a large number of other diseases and conditions. For example, previous research has found that, during pandemic, stress, mask wearing, social isolation, and taking medicines can all trigger headaches, of which nausea is an accompanying symptom [52]. The likely explanation for these "protective" associations is thus that increased stress induced by people’s fear of COVID-19 or coincidental diseases other than COVID-19 led patients to visit COVID-19 testing facilities or general outpatient healthcare, where they received COVID-19 tests [53]. These tests, in turn, led to COVID-19 diagnosis and subsequent isolation in community centers. Because the unspecific symptoms that brought these patients to the community center were likely caused by mostly harmless diseases (such as migraine or viral gastroenteritis)—rather than by COVID-19—these patients were less likely to progress to severe COVID-19 than other patients in the center, who were more likely to have presented with symptoms already indicating a degree of COVID-19 progression, which, in turn, increases the risk of further deterioration. Another study in China also reported on the “protective” association of nausea on disease progression among COVID-19 patients [54]. This finding has implications for the routine monitoring of patients in community and home isolation. Patients with unspecific symptoms occuring in many harmless diseases may face a similar overall risk of COVID-19 deterioration as patients with asymptomatic COVID-19. Both groups are likely to be relatively safe in home or community isolation and have lower priority for monitoring and triage to higher level care than patients suffering from symptoms specific to COVID-19.
Our finding that common unspecific symptoms can induce selection effects in triage and prioritization systems has public health relevance far beyond the COVID-19 pandemic. Future research should test alternative approaches to guard against inefficiencies in triage and prioritization that can occur because patients suffering harmless comorbidities are more likely to seek health care, which then leads to diagnostic detection and health system prioritization for an unrelated disease. Such knowledge could be actively incorporated into triage algorithms, for example, by adding further screening tests with prognostic value for those patients who are considered for prioritization for one potentially dangerous disease, but suffer from common unspecific symptoms indicative of many other harmless diseases.
Our study has a few important limitations. First, the public health guidelines for community isolation in China stipulated that COVID-19 patients with important comorbidities [55], including cardiovascular diseases, diabetes, chronic respiratory diseases, and mental health conditions, should be hospitalized rather than receive care in a community center [5]. We were thus unable to investigate comorbidities as risk factors for COVID-19 deterioration. Second, we did not have access to other risk factors that could predict disease progression. For example, smoking was associated with COVID-19 disease progression in hospitals [56]. In China, about half of adult men smoke, which is much higher than the prevalence of smoking among women [57], [58]. It is possible that smoking may partially explain the elevated risk in men in this study. Third, we did not have access to laboratory tests and other biomarkers that could be important predictors of COVID-19 deterioration. Therefore, we cannot rule out the possibility that other factors could be important for early COVID-19 triage decisions. These limitations, however, do not reduce the usefulness of the findings on which factors predict COVID-19 deterioration and which do not. Our findings are broadly relevant for all contexts, including resource-poor communities, because they relate to symptoms and signs—that is, factors whose values can be measured at low cost and with simple and robust technologies. These findings are also relevant for resource-rich health systems in which CT capacity is widely available and CT use is generally affordable.
In sum, our study finds that male sex, old age, dyspnea, and ground-glass opacity and infiltrating shadows in CT scans were strong predictors of severe COVID-19 deterioration among patients in community isolation. We also find that common and unspecific symptoms—namely, headaches and nausea or vomiting—have likely induced a selection of COVID-19 patients for community isolation who are relatively unlikely to experience severe disease deterioration. Future public health and clinical guidelines should build on this evidence to design better screening and triage systems, as well as monitoring and care pathways, to ensure effective, safe, and efficient community isolation.
Acknowledgments
Acknowledgments
We thank all health workers working in the Fangcang shelter hospitals. Simiao Chen was supported by the Alexander von Humboldt Foundation in Germany and the Bill & Melinda Gates Foundation (Project INV-006261). Pascal Geldsetzer was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (KL2TR003143). Till Bärnighausen was supported by the Alexander von Humboldt Foundation through the Alexander von Humboldt Professor award, funded by the German Federal Ministry of Education and Research, the European Union’s Research and Innovation Programme Horizon 2020, and the European & Developing Countries Clinical Trials Partnership (EDCTP). Simiao Chen, Till Bärnighausen, Chen Wang, Juntao Yang, Zhuoran Wang, and Peixin Wu were also supported by the Sino-German Center for Research Promotion (Project C-0048), which is funded by the German Research Foundation (DFG) and the National Natural Science Foundation of China (NSFC).
Compliance with ethics guidelines
Simiao Chen, Hui Sun, Mei Heng, Xunliang Tong, Pascal Geldsetzer, Zhuoran Wang, Peixin Wu, Juntao Yang, Yu Hu, Chen Wang, and Till Bärnighausen declare that they have no conflict of interest or financial conflicts to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eng.2021.07.021.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Nelson B. Too little or too much? Missing the Goldilocks zone of hospital capacity during COVID-19. BMJ. 2020;369 doi: 10.1136/bmj.m2332. [DOI] [PubMed] [Google Scholar]
- 2.Haroon S., Chandan J.S., Middleton J., Cheng K.K. COVID-19: breaking the chain of household transmission. BMJ. 2020;370 doi: 10.1136/bmj.m3181. [DOI] [PubMed] [Google Scholar]
- 3.Wilder-Smith A., Cook A.R., Dickens B.L. Institutional versus home isolation to curb the COVID-19 outbreak—authors’ reply. Lancet. 2020;396(10263):1632–1633. doi: 10.1016/S0140-6736(20)32171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S., Chen Q., Yang J., Lin L., Li L., Jiao L., et al. Curbing the COVID-19 pandemic with facility-based isolation of mild cases: a mathematical modeling study. J Travel Med. 2020;28(2):taaa226. doi: 10.1093/jtm/taaa226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen S., Zhang Z., Yang J., Wang J., Zhai X., Bärnighausen T., et al. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. 2020;395(10232):1305–1314. doi: 10.1016/S0140-6736(20)30744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Atlantic. What’s behind South Korea’s COVID-19 exceptionalism [Internet]. The Atlantic Monthly Group; [updated 2020 May 6; cited 2020 May 14]. Available from: https://www.theatlantic.com/ideas/archive/2020/05/whats-south-koreas-secret/611215/.
- 7.Her M. Repurposing and reshaping of hospitals during the COVID-19 outbreak in South Korea. One Health. 2020;10 doi: 10.1016/j.onehlt.2020.100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chia M.L., Him Chau D.H., Lim K.S., Yang Liu C.W., Tan H.K., Tan Y.R. Managing COVID-19 in a novel, rapidly deployable community isolation quarantine facility. Ann Intern Med. 2021;174(2):247–251. doi: 10.7326/M20-4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Towards Data Science. COVID-19—what do we know about the situation in Vietnam? [Internet]. [updated 2020 May 2; cited 2020 Nov 13]. Available from: https://towardsdatascience.com/covid-19-what-do-we-know-about-the-situation-in-vietnam-82c195163d7e.
- 10.Chen S., Yang J., Yang W., Wang C., Bärnighausen T. COVID-19 control in China during mass population movements at New Year. Lancet. 2020;395(10226):764–766. doi: 10.1016/S0140-6736(20)30421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Operational considerations for community isolation centers for COVID-19 in low-resource settings [Internet]. Washington, DC: US Department of Health & Human Services; [updated 2020 Aug 19; cited 2020 Sep 29]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/global-covid-19/operational-considerations-isolation-centers.html.
- 12.World Health Organization Operational considerations for case management of COVID-19 in health facility and community. Interim guidance. Pediatria i Med Rodz. 2020;16(1):27–32. [Google Scholar]
- 13.Lipsitch M., Swerdlow D.L., Finelli L. Defining the epidemiology of COVID-19—studies needed. N Engl J Med. 2020;382(13):1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 14.Goh K.J., Choong M.C., Cheong E.H., Kalimuddin S., Wen S.D., Phua G.C., et al. Rapid progression to acute respiratory distress syndrome: review of current understanding of critical illness from COVID-19 infection. Ann Acad Med Singap. 2020;49(1):1–9. [PubMed] [Google Scholar]
- 15.Greenhalgh T., Koh G.C.H., Car J. COVID-19: a remote assessment in primary care. BMJ. 2020;368 doi: 10.1136/bmj.m1182. [DOI] [PubMed] [Google Scholar]
- 16.Li M., Lei P., Zeng B., Li Z., Yu P., Fan B., et al. Coronavirus disease (COVID-19): spectrum of CT findings and temporal progression of the disease. Acad Radiol. 2020;27(5):603–608. doi: 10.1016/j.acra.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yousefzadegan S., Rezaei N. Case report: death due to novel coronavirus disease (COVID-19) in three brothers. Am J Trop Med Hyg. 2020;102(6):1203–1204. doi: 10.4269/ajtmh.20-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395(10231):1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent J.L., Taccone F.S. Understanding pathways to death in patients with COVID-19. Lancet Respir Med. 2020;8(5):430–432. doi: 10.1016/S2213-2600(20)30165-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J., Qi T., Liu L., Ling Y., Qian Z., Li T., et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80(5):e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L., He W., Yu X., Hu D., Bao M., Liu H., et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80(6):639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang W., Liang H., Ou L., Chen B., Chen A., Li C., et al. China Medical Treatment Expert Group for COVID-19. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1081–1089. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phua J., Weng L., Ling L., Egi M., Lim C.M., Divatia J.V., et al. Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu W., Tao Z.W., Wang L., Yuan M.L., Liu K., Zhou L., et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J. 2020;133(9):1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang W.H., Guan W.J., Li C.C., Li Y.M., Liang H.R., Zhao Y., et al. Clinical characteristics and outcomes of hospitalised patients with COVID-19 treated in Hubei and outside Hubei: a nationwide analysis of China. Eur Respir J. 2020;55(6):2000562. doi: 10.1183/13993003.00562-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X., Fang J., Zhu Y., Chen L., Ding F., Zhou R., et al. Clinical characteristics of non-critically ill patients with novel coronavirus infection (COVID-19) in a Fangcang hospital. Clin Microbiol Infect. 2020;26(8):1063–1068. doi: 10.1016/j.cmi.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao Y., Feng Y., Wang B., Wang H., Huang J., Wu Y., et al. Clinical characteristics and prognostic factors of COVID-19 patients progression to severe: a retrospective, observational study. Aging. 2020;12(19):18853–18865. doi: 10.18632/aging.103931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X. Mainland medics banking on experience to help HK fight COVID-19 [Internet]. Beijing: China Daily Information Co.; c1995–2021 [updated 2020 Aug 3; cited 2020 Aug 4]. Available online: https://www.chinadaily.com.cn/a/202008/03/WS5f27ad72a31083481725dd2e.html.
- 31.The Lancet Sustaining containment of COVID-19 in China. Lancet. 2020;395(10232):1230. doi: 10.1016/S0140-6736(20)30864-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilbert M., Dewatripont M., Muraille E., Platteau J.P., Goldman M. Preparing for a responsible lockdown exit strategy. Nat Med. 2020;26(5):643–644. doi: 10.1038/s41591-020-0871-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen E., Wasserman S., Lee S.S., Go U., Holmes A.H., Al-Abri S., et al. COVID-19—we urgently need to start developing an exit strategy. Int J Infect Dis. 2020;96(96):233–239. doi: 10.1016/j.ijid.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen S., Chen Q., Yang W., Xue L., Liu Y., Yang J., et al. Buying time for an effective epidemic response: the impact of a public holiday for outbreak control on COVID-19 epidemic spread. Engineering. 2020;6(10):1108–1114. doi: 10.1016/j.eng.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Health Commission of the People’s Republic of China. Manual for working in Fangcang shelter hospitals (3rd edition) [Internet]. Beijing: National Health Commission of the People’s Republic of China; [updated 2020 Feb 22; cited 2020 Feb 24]. Available from: https://mp.weixin.qq.com/s/va9vs4HuP8wRQM5fALQcrg.Chinese.
- 36.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 37.Wolbers M., Koller M.T., Stel V.S., Schaer B., Jager K.J., Leffondré K., et al. Competing risks analyses: objectives and approaches. Eur Heart J. 2014;35(42):2936–2941. doi: 10.1093/eurheartj/ehu131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lau B., Cole S.R., Gange S.J. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hosmer D.W., Lemeshow S., May S. Wiley; New York: 2002. Applied survival analysis: regression modeling of time to event data. [Google Scholar]
- 40.McCaw Z.R., Tian L., Vassy J.L., Ritchie C.S., Lee C.C., Kim D.H., et al. How to quantify and interpret treatment effects in comparative clinical studies of COVID-19. Ann Intern Med. 2020;173(8):632–637. doi: 10.7326/M20-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–241. [Google Scholar]
- 42.Austin P.C., Fine J.P. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017;36(27):4391–4400. doi: 10.1002/sim.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prettner K, Chen S, Kuhn M, Bloom DE. Effective pandemic management that minimises economic harm [Internet]. [updated 2021 Jan 4; cited 2021 Jan 22]. Available from: https://voxeu.org/article/effective-pandemic-management-minimises-economic-harm.
- 44.Chen S., Prettner K., Kuhn M., Bloom D.E. The economic burden of COVID-19 in the United States: estimates and projections under an infection-based herd immunity approach. J Econ Ageing. 2021;20 doi: 10.1016/j.jeoa.2021.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palaiodimos L., Kokkinidis D.G., Li W., Karamanis D., Ognibene J., Arora S., et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108 doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie J., Covassin N., Fan Z., Singh P., Gao W., Li G., et al. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. 2020;95(6):1138–1147. doi: 10.1016/j.mayocp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cecconi M., Piovani D., Brunetta E., Aghemo A., Greco M., Ciccarelli M., et al. Early predictors of clinical deterioration in a cohort of 239 patients hospitalized for COVID-19 infection in Lombardy, Italy. J Clin Med. 2020;9(5):1548. doi: 10.3390/jcm9051548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan M., Yin W., Tao Z., Tan W., Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS ONE. 2020;15(3) doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gallo Marin B., Aghagoli G., Lavine K., Yang L., Siff E.J., Chiang S.S., et al. Predictors of COVID-19 severity: a literature review. Rev Med Virol. 2021;31(1):1–10. doi: 10.1002/rmv.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi T., Ellingson M.K., Wong P., Israelow B., Lucas C., Klein J., et al. Yale IMPACT Research Team. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588(7837):315–320. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uygun Ö., Ertaş M., Ekizoğlu E., Bolay H., Özge A., Kocasoy Orhan E., et al. Headache characteristics in COVID-19 pandemic—a survey study. J Headache Pain. 2020;21(1):121. doi: 10.1186/s10194-020-01188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu F., Geldsetzer P., Meierkord A., Yang J., Chen Q., Jiao L., et al. Knowledge about coronavirus disease 2019 among adults in China: a cross-sectional online survey. J Med Internet Res. 2021;23(4) doi: 10.2196/26940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li J., Chen Z., Nie Y., Ma Y., Guo Q., Dai X. Identification of symptoms prognostic of COVID-19 severity: multivariate data analysis of a case series in Henan Province. J Med Internet Res. 2020;22(6) doi: 10.2196/19636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pan XF, Yang J, Wen Y, Li N, Chen S, Pan A. Non-communicable diseases during the COVID-19 pandemic and beyond. Engineering 2021;7(7):899–902. [DOI] [PMC free article] [PubMed]
- 56.Reddy R.K., Charles W.N., Sklavounos A., Dutt A., Seed P.T., Khajuria A. The effect of smoking on COVID-19 severity: a systematic review and meta-analysis. J Med Virol. 2021;93(2):1045–1056. doi: 10.1002/jmv.26389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen S., Kuhn M., Prettner K. Tobacco control policies: the authors reply. Health Aff. 2020;39(2):346. doi: 10.1377/hlthaff.2019.01661. [DOI] [PubMed] [Google Scholar]
- 58.Chen S., Kuhn M., Prettner K., Bloom D.E. Noncommunicable diseases attributable to tobacco use in China: macroeconomic burden and tobacco control policies. Health Aff. 2019;38(11):1832–1839. doi: 10.1377/hlthaff.2019.00291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



