Abstract
The Covid-19 pandemic has spread rapidly across the globe, resulting in more than 3 million deaths worldwide. The symptoms of Covid-19 are usually mild and non-specific, however in some cases patients may develop acute respiratory distress syndrome (ARDS) and systemic inflammation. Individuals with inflammatory or immunocompromising illnesses, such as cancer, are more susceptible to develop ARDS and have higher rates of mortality. This is mediated through an initial hyperstimulated immune response which results in elevated levels of pro-inflammatory cytokines and a subsequent cytokine storm. This potentiates positive feedback loops which are unable to be balanced by anti-inflammatory mediators. Therefore, elevated levels of IL-1β, as a result of NLRP3 inflammasome activation, as well as IL-6 and TNF-α amongst many others, contribute to the progression of various cancer types. Furthermore, Covid-19 progression is associated with the depletion of CD8+ and CD4+ T cells, B cell and natural killer cell numbers. Collectively, a Covid-19-dependent pro-inflammatory profile and immune suppression promotes the optimal microenvironment for tumourigenesis, initiation and immune evasion of malignant cells, tumour progression and metastasis as well as cancer recurrence. There are, however, therapeutic windows of opportunity that may combat both Covid-19 and cancer to improve patient outcomes.
Keywords: Covid-19, Cancer, Inflammation, NLRP3 inflammasome
Graphical Abstract
Nomenclature
- ACE
angiotensin converting-enzyme
- ACE2R
angiotensin converting-enzyme 2 receptor
- APC
antigen presenting cell
- ARDS
acute respiratory distress syndrome
- CCL
chemokine ligand
- DAMP
damage associated molecular pattern
- DCC
dormant cells
- DMV
double membrane vesicle
- ECM
extracellular matrix
- GSDMD
Gasdermin D
- HIF
hypoxia-inducible factor
- HMGB1
high mobility group box1
- IFN
interferon
- IFNAR
IFN-α-receptor subunit
- IKK
IκB kinase
- IRF
interferon regulatory factor
- JAK
Janus kinase
- MAPK
mitogen-activated protein kinase
- MCP
monocyte chemoattractant protein
- MERS-COV
Middle East respiratory syndrome corona virus
- MMP
matrix metallopeptidase
- mTOR
mammalian target of rapamycin
- mtROS
mitochondrial ROS
- NET
neutrophil extracellular traps
- NFκβ
nuclear factor kappa beta
- NLRP3
nod-like receptor family, pyrin containing 3
- NO
nitric oxide
- NOD2
nucleotide-binding oligomerization domain-containing protein 2
- ORF
open reading frame
- PI3K
phosphoinositide 3-kinases
- PRR
pattern recognition receptor
- RIG
retinoic acid-inducible gene
- RIP
receptor-interacting protein
- ROS
reactive oxygen species
- SARS-COV-2
severe acute respiratory syndrome coronavirus 2
- SIRS
systemic inflammatory response syndrome
- STAT
signal transducer and activator of transcription
- TLR
toll like receptor
- TME
tumour microenvironment
- TMPRSS2
transmembrane serine protease 2
- TNF
tumour necrosis factor
- TNFR
tumour necrosis factor receptor
- TRADD
TNFR1-asssociated death domain protein
- TRAF
TNFR associated factor
- VEGF
vascular endothelial growth factor
1. Background
The severe acute respiratory syndrome coronavirus-2 (SARS-Cov-2) is responsible for the rapidly spreading Covid-19, and is the fifth betacoronavirus to infect humans. As of 2021, the virus resulted in more than 100 million cases and 3 million deaths (World Health Organization). The majority of individuals infected only display mild symptoms, such as low-grade fever, cough and fatigue/malaise [162]. However, severe cases are characterized by clinical features consistent with lower respiratory tract infection, microembolic disease and multiple organ failure. Acute respiratory distress syndrome (ARDS) is the most severe clinical manifestation of systemic Covid-19 infection secondary to the increased expression of pro-inflammatory cytokines [75].
Therefore, Covid-19 is a systemic inflammatory syndrome associated with changes in immune patterns and dysregulated pro-inflammatory cytokine cascades, both of which is triggered by an intense, rapid activation of the innate immune response followed by lymphopenia and hypercoagulability [137]. As such, individuals with diabetes, hypertension, cardiovascular disease and cancer are at a higher risk for severe infection and mortality [2], [53], [125], [156]. Furthermore, cancer patients have an attenuated immune capacity in most cases, as a result of chemotherapeutic agents and steroid treatments [40]. The pro-inflammatory nature of Covid-19 may contribute to tumourigenesis by providing pre-malignant, malignant and dormant cancer cells with the optimal microenvironment for tumourigenesis. Therefore, this review will investigate the possible interplay between the systemic effects of Covid-19 and the development and progression of cancer.
2. Covid-19 results in a shift from STAT1 to STAT3 signaling
Covid-19 has been reported to induce a change from signal transducer and activator of transcription 1 (STAT1) to STAT3 signalling. The janus kinase (JAK)/STAT3 signaling pathway is constitutively activated in most cancers and is associated with prolonged or amplified inflammatory signaling, proliferation and survival [79], [148]. The STAT1 signaling pathway enhances adaptive and innate immunity, is a major component of IFN signaling and is a well-known tumour suppressor. The activation of STAT3 and its resulting pro-inflammatory response is a common feature of Covid-19 infection [67], [102].
Covid-19 activates membrane-bound or intracellular PRRs, including toll-like receptors (TLR) and retinoic acid-inducible gene I (RIG-I) [118]. The activation of these PRRs results in the activation of the nuclear factor-kappa B (NFκβ) transcription factors, promoting the transcription of IFN-I-III. These factors alert surrounding cells to the viral attack and elicits a host immune response. However, Covid-19 antagonizes the antiviral activities of IFNs and the downstream JAK-STAT1 signaling pathways [17]. This is dependent on the Covid-19 NSP protein family. NSP1 blocks STAT1 phosphorylation, while NSP3, −4 and −6 establish double-membrane vesicles (DMV) [150]. DMVs create a platform to separate the replication site of Covid-19, including the replicase proteins, viral genome and host replication proteins, from the cytoplasmic sensors of the innate immune response [7], [47]. Covid-19 inhibits the activity of IFNs through nonstructural accessory proteins, which are coded by open reading frames (ORFs) dispersed throughout the viral genome [96], [159]. The ORFa protein induces the serine phosphorylation of the IFN-α-receptor subunit 1 (IFNAR1) degradation motif and promotes its ubiquitination. The ORF3b protein antagonizes IFNs and inhibits the phosphorylation of interferon regulatory factor 3 (IRF3). The SARS-CoV-2 family often display a larger ORF3b variant with increased anti-INF-I activity [86], [87]. Furthermore, the ORF6 in SARS-CoV-1 is a strong antagonist to the IFN-I response by inhibiting the IFN-β promoter activity and IFN signaling [87], [159]. STAT1 inhibition by Covid-19 also attenuates the transcription if IFN stimulated genes in macrophages [102], [155]. Consequently, the antagonistic effects of Covid-19 on IFN and STAT1 result in the shift to a STAT3-dependent transcriptional profile.
It has been proposed that STAT3, in turn, inhibits STAT1-mediated IFN-I responses [140], [145]. STAT3 inhibits the dimerization of STAT1 by forming a heterodimer of STAT3-STAT1. Furthermore, changes in STAT1/3 signaling may promote tumourigenesis. STAT1 signaling is proportional to IFN-γ sensitivity in cancer cells. The knockout of STAT1 in mice resulted in accelerated tumour growth and defective IFN-γ driven immune response of NK and T cells, impairing their ability to kill cancer cells [28], [106]. Furthermore, the loss of STAT1 in breast cancer cells have been linked to HER2/neu-driven tumourigenesis [85], [117]. The direct activation of the JAK/STAT3 signaling can also drive the production of pro-inflammatory cytokines and contributes to the Covid-19 associated cytokine storm.
3. The cytokine storm
Approximately 20% of all cancers arise in association with chronic inflammation. Cancers that do not arise with inflammation often display inflammatory infiltrates and high levels of cytokine expression in the tumour microenvironment during the later stages of the disease [61], [62]. Covid-19 has been reported to induce a cytokine storm, a term which is used to describe unchecked systemic overproduction of cytokines. As such, the cytokine storm results in increased plasma concentrations and local upregulation of the following cytokines, IL-1β, IL-2, IL-4, IL-6, IL-7, IL-8, IL-10, IFN- α/β/γ, MCP-1, TNF-α, interferon-γ (IFN-γ)-induced protein 10 (IP-10 or CXCL-10), CCL3, CCL5 and CCL2 [31], [32], [91], [105]. The cytokine storm, also known as cytokine release syndrome, has also been reported for certain cancer types such as lung, pancreatic and liver cancer. The cytokines are released as a result of the over-activation of resident tissue macrophages and other immune cells. Furthermore, the activation of pulmonary capillary endothelium can contribute to the cycle of an amplified immune response and the cytokine storm [138].
The cytokine storm results in a feedback loop that is unable to be mediated by anti-inflammatory factors, resulting in systemic inflammation. The main drivers of this process are TNF-α and IL-6, which activate the oncogenic transcription factors NFκβ, AP-1 and STAT3 [3], [10], [25], [60]. Therefore, TNF-α and IL-6 are considered as the best characterized inflammatory drivers of cancer progression. Additionally, elevated expression levels of IL-6 and IL-1β have been detected in the autopsy tissues from SARS-CoV patients and increased subsets of CD14+ -IL-1β producing monocytes in the peripheral blood of these patients have also been observed.
3.1. IL-6 and cancer
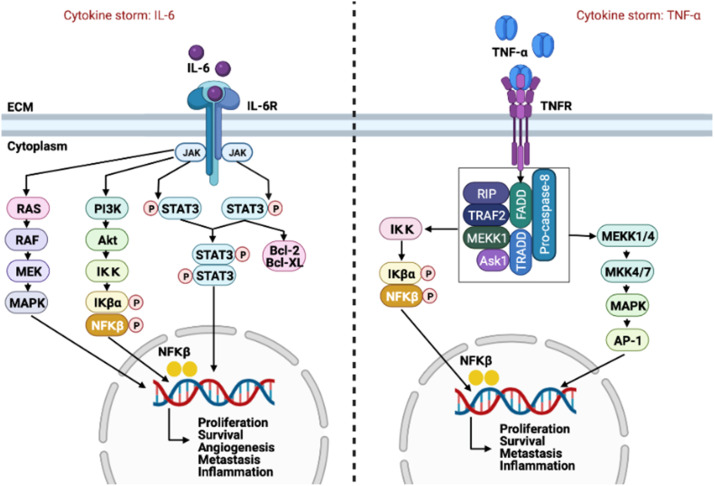
IL-6 expression is associated with disease severity during Covid-19 infection and cancer. IL-6 is one of the major dysregulated pro-inflammatory cytokines present in the tumour microenvironment (TME) and is overexpressed in a variety of cancers including breast, prostate, ovarian, lung and cervical cancer [36], [42], [82]. High levels of IL-6 are associated with poor prognosis, drives cancer progression and can serve as a clinical biomarker for cancer [10], [83], [143]. IL-6 regulates three main signaling pathways, including JAK/STAT3, Ras/MAPK and PI3K/Akt. These signaling pathways results in the expression of various gene products that promote proliferation, differentiation, apoptosis, angiogenesis and metastasis of cancer cells [78].
The IL-6/JAK/STAT3 axis is a major driver of tumourigenesis [76]. STAT3 is an oncogenic transcription factor and a signal transducer [95]. IL-6 receptor binding results in the activation of JAK, which phosphorylates STAT3 on active sites. Activated STAT3 inhibits the pro-apoptotic proteins Bcl-2, Bcl-xl and survivin, and promotes gene expression of anti-apoptotic proteins, mcl-1 and XIAP, thereby inhibiting apoptosis and promoting cancer cell survival [147], [163]. STAT3 upregulates the expression of the matrix metalloproteinase (MMP) −2 and −9, which are responsible for the degradation and remodeling of the extracellular matrix (ECM) and basement membranes. Changes in MMP expression enable metastatic cells to migrate to distant tissues and organs [58], [88], [111], [152]. Furthermore, the activation of STAT3 and MAPK through JAK signaling promotes the formation of new blood vessels throughout the tumour by inducing the expression of the vascular endothelial growth factor (VEGF), a key mediator of angiogenesis [157].
The activation of the PI3K/Akt signaling pathway promotes proliferation and survival of cancer cells by inhibiting the activity of p53, a tumour suppressor, as well as p27 and p130, inhibitors of G1 cyclin-dependent kinases [139]. Furthermore, the PI3K/Akt pathway promotes proliferation through the increased induction of cyclin D1, a regulatory protein of the cell cycle [94]. The activation of PI3K/Akt results in the downstream activation of the mammalian target of rapamycin (mTOR), which inhibits autophagy, a catabolic process responsible for the degradation of intracellular components, by preventing the formation of the initiation complex. The induction of autophagy is associated with decreased proliferation, therefore the downstream inhibition of autophagy may promote proliferation in cancer cells. Lastly, the PI3K/Akt pathway activates NFκβ, which promotes cell survival, angiogenesis and metastasis [23], [154].
IL-6 signaling through a JAK/STAT3-dependent manner results in a pro-inflammatory arsenal by inducing the expression of other pro-inflammatory cytokines [141]. STAT3 activation mediates the production of TGF-β in a positive feedback loop with PAI-1, facilitating the TLR4 inflammatory response [89], [166]. Additionally, exhausted CD8+ T cells are a source of IL-6 production in squamous cells and small-cell carcinoma, which may also be the case during Covid-19 infection ( Fig. 1) [108].
Fig. 1.
The role of TNF-α and IL-6 in cancer progression. The Covid-19 infection initiates the cytokine storm, characterized by the excessive production of pro-inflammatory cytokines. IL-6 receptor binding results in the activation of JAK/STAT3 signaling. JAK mediates the activation of the Ras/Raf/MEK signaling cascade. Furthermore, JAK activates the PI3K/Akt signaling pathway, which activates NFκβ. NFκβ translocate to the nucleus where it promotes the expression of gene products responsible for tumourigenesis. STAT3 activation results in dimer formation which translocate to the nucleus where it mediates gene expression. STAT3 also promotes cell survival by activating the anti-apoptotic proteins Bcl-2 and Bcl-XL. TNFR binding activates the effector proteins FADD, TRADD, RIP, TRAF2, MEKK1, Ask1 and pro-caspase-8. This results in the activation of NFκβ and the activation of the AP-1 protein through MEKK1/4, MKK4/7 and MAPK signaling. Collectively, the activation of MAPK, NFκβ, STAT3 dimerisation and Ap-1 promotes the expression of target genes that promotes cancer proliferation, survival, angiogenesis, metastasis and inflammation.
3.2. TNF-α and cancer
TNF-α is a pro-inflammatory cytokine which plays a major role during adaptive immunity. However, TNF-α is also one of the major dysregulated pro-inflammatory cytokines present in the TME [68]. The role of TNF-α in cancer is considered to be a double-edged sword. TNF-α can act as an effector molecule in cell-mediated killing of cancer cells or it can promote tumour survival through cancer-promoting inflammation [10]. However, increasing evidence suggests that TNF-α is involved in all stages of tumourigenesis and elevated levels of TNF-α in the TME is an indicator of poor prognosis.
TNF-α can activate two receptors, TNF-receptor 1 (TNFR1) and TNF-receptor 2 (TNFR2) [4]. TNFR1 is expressed on almost all cell types, while TNFR2 is specific to endothelial and immune cells. TNFR1 binding results in receptor trimerization with the recruitment of the TNFR1-asssociated death domain protein (TRADD) that binds to the death domain of the TNFR1. TRADD recruits TNFR associated factor (TRAF2) and activates IkB kinase (IKK) through receptor-interacting protein (RIP), a crucial factor involved in NFκβ activation. The IKKB components phosphorylates IKB, resulting in its ubiquitination and degradation, this results in free NFκβ that translocate to the nucleus where it induces gene transcription [70]. NFκβ is responsible for the gene expression of many pro-inflammatory cytokines and it promotes the activation of anti-apoptotic proteins, such as Bcl-2 and XIAP, thereby promoting inflammation and inhibiting apoptosis [149]. TNFR1 activates two MAPK’s, JNK and p38, and the PI3K/Akt signaling pathway [35]. These signaling pathways converge to activate c-Jun, inducing the expression of MMP9 and subsequent angiogenesis and metastasis. TRAF2 binding activates the MAPK signaling pathway and AP-1, promoting the activation of VEGF which induces angiogenesis [27], [63], [103]. NFκβ activation by TRAF2 regulates metastasis by inducing the expression of the metastatic transcription factors Snail, Slug and Twist [153]. These transcription factors induces a phenotypic switch from an epithelial, non-invasive phenotype to a mesenchymal, invasive phenotype.
TNF-α-dependent activation of PI3K/Akt signaling and downstream activation of NFκβ promotes the expression of cyclin D1 which phosphorylates and deactivates the tumor suppressor protein retinoblastoma (pRb). Therefore, TNF-α promotes tumourigenesis through the regulation of inflammation, proliferation and survival [127].
Therefore, elevated levels of systemic and local pro-inflammatory cytokines, including IL-6 and TNF-α, generates a TME that is ideal for cancer survival, establishes a pro-metastatic niche, promotes resistance to treatment and can promote cancer recurrence (Fig. 1) [46], [49], [57], [129]. Furthermore, the cytokine storm promotes the release other pro-inflammatory cytokines, such as IL-2, IL-12 and MCP-1, which perpetuates a pro-inflammatory and pro-invasive positive feedback loop in the TME [77]. Covid-19 infection may also induce inflammasome activation, which is a major contributor to the pro-inflammatory profile seen during disease progression.
3.3. The NLRP3 inflammasome: priming and activation
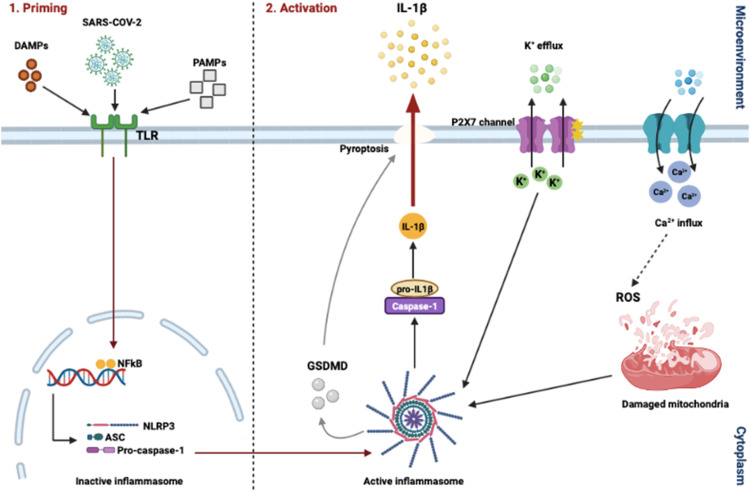
Several studies have implicated the Nod-like receptor family, pyrin containing 3 (NLRP3) inflammasome and cytokine release/storm in the pathogenesis of the Covid-19 virus [54]. One of the main functions of the NLRP3 inflammasome is to activate the innate immune system to recognize pathogens, including viral infections. The NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) inflammasome is characterized by a sensor (NLRP3), an adaptor (ASC or PYCARD) and an effector (caspase 1) [136]. NLRP3 contains an amino-terminal pyrin domain (PYD), a central NACHT domain and a carboxyl-terminal leucine-rich repeat domain (LRR domain).
Inflammasome activation is highly regulated and mediated by a two-step process, namely priming and activation [13], [164]. During the priming step, NLRP3 genes are transcriptionally upregulated in response to the recognition of PAMPs (e.g., LPS and viral DNA) or damage associated molecular patterns (DAMPs) (e.g., ATP and ROS) through purine sensing receptors like P2RX7 [128]. PAMPs/DAMPs can also activate PRRs like TLRs or nucleotide-binding oligomerization domain-containing protein 2 (NOD2). Binding of PAMPs/DAMPs to these receptors results in the activation of NFκβ and gene transcription [14]. Once the priming step is completed, activation of the inflammasome can occur in response to several pathogens or endogenous DAMPs. This activation is associated with multiple cellular signaling events including the efflux of K+, Ca2+ or Cl- ions, lysosomal disruption, mitochondrial dysfunction, trans-Golgi disassembly and metabolic changes. DAMPs such as high mobility group box 1 (HMGB1) can also be released after inflammasome activation, however, these DAMPs have a dual function. During normal immune responses, they induce co-stimulatory activation of antigen-presenting cells (APCs), but also play a role in resolution and tissue regeneration. When hyperactivation of the inflammasome occurs, DAMPs are released in high concentrations and can result in pyroptosis, macrophage activation, reduced apoptosis, neutrophil infiltration and a significant increase in pro-inflammatory cytokine production. As mentioned above, pyroptosis can also occur as a result of NLRP3 activation, which is an inflammatory programmed cell death pathway that occurs in T-lymphocytes. This form of cell death is activated through gasdermin D (GSDMD) cleavage by caspase 1, 4, 5 and 11, which could result in cytoplasm swelling, rupturing of the plasma membrane and release of cytoplasmic contents into the extracellular space [109]. Additionally, GSDMD also mediates IL-1β and IL-18 secretion and can occur dependently or independently of NLRP3 signaling ( Fig. 2).
Fig. 2.
NLRP3 inflammasome activation and priming. Inflammasomes are cytosolic multiprotein oligomers of the innate immune system, and their main function is to activate inflammatory responses. The Nod-like receptor family, pyrin containing 3 (NLRP3) inflammasome pathway is activated by priming and activation, respectively. Binding of PAMPs/DAMPs to TLRs results in the activation of nuclear factor kappa beta (NFκβ), caspase-1 activation and gene transcription. Once the priming step is completed, activation of the inflammasome can occur in response to several pathogens or endogenous DAMPs. Similarly, SARS-CoV-2 can bind to these receptors to induce an inflammatory response.
Several studies have implicated the NLRP3 inflammasome and IL-1β production in mediating inflammation during ARDS and lung injury. When compared to healthy controls, patients with ARDS have increased IL-1β levels in bronchoalveolar fluid and plasma which is associated with a worse clinical outcome [21]. In coronavirus infections (MERSCoV and SARS-CoV), patients with ARDS had increased levels of IL-6, IL-8 and IL-1β [71]. Similar to these findings, patients with influenza, which is also a respiratory viral infection, had high levels of IL-1β. Furthermore, mice with influenza infection and which are deficient in inflammasome components (AIM2 (-/-), NLRP3 (-/-)) have reduced lung injury and enhanced survival [161]. The authors observed that viral infection significantly increased the abundance of cleaved caspase-1 and cleaved IL-1β in these mice.
Taking these findings into consideration, it is plausible that IL-1β plays a major role in acute lung injury induced by respiratory viral infections, therefore pharmacologic targeting of this pathway may have beneficial effects.
3.4. NLRP3 inflammasome and Covid-19
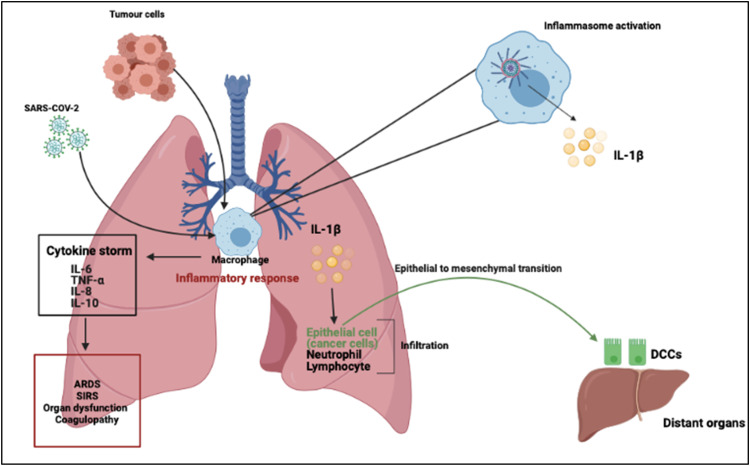
It has been observed that injury of type II alveolar epithelial cells that express ACE2 results in inflammasome activation [164]. The acute immune response to SARS-CoV-2 infection is mainly driven by inflammatory alveolar and monocyte-derived macrophages, which is activated by PAMPs/DAMPs released by infected and apoptotic pneumocytes [56]. Alveolar macrophages secrete TNF-α and IL-1β which initiates an acute pro-inflammatory cascade after infection. These cytokines secreted by the macrophages then induce cell death and damage, PAMP/DAMP production, recruitment of immune cells and NLRP3 activation, thereby creating a positive feedback cascade. Blanco-Melo and co-authors have reported that primary human bronchial epithelial cells infected with SARS-CoV-2 resulted in the expression of several cytokines and chemokines including TNF-α, IL-1β and IL-6. The authors proposed that reduced innate antiviral defenses coupled with excessive inflammatory cytokine production are the defining and driving features of Covid-19 [20]. The onset of this pathological immune response is therefore characterized by a hyperinflammatory microenvironment localized at the site of tissue injury. As the inflammatory cascade progresses, TNF-α and IL-1β induce the secretion of other NLRP3 cytokines such as IL-6, which is then observed in the peripheral blood of patients due to the loss of vascular integrity [55]. IL-6 can therefore be useful to predict immunomodulatory treatment decisions. The rapid decline in health of Covid-19 patients is generally associated with an abrupt shift from the NLRP3 cytokine storm to a compensatory immunosuppressive state [105]. This phase is characterized by IL-10 production, suppression of NLRP3, polarization of macrophages to the anti-inflammatory M2 state and the recruitment of fibroblasts and platelets. This accumulation of fibroblasts and macrophages in the lung is responsible for collagen deposition and constriction of the extracellular matrices that are characteristic of ARDs fibrosis. Liao and authors found that pro-inflammatory monocyte-derived macrophages were abundant in the bronchoalveolar fluid from patients with severe Covid-19 and moderate cases were characterized by highly clonally expanded CD8+ T-cells ( Fig. 3) [93].
Fig. 3.
Covid-19 induced inflammation affects tumor cells and their microenvironment. Once inhaled, SARS-CoV-2 infection activates the Nod-like receptor family, pyrin containing 3 (NLRP3) inflammasome in macrophages to mediate lung inflammation. In cancer patients however, alongside SARS-CoV-2 infection, tumor cells can also activate the inflammasome, which drives the secretion of IL-1β and IL-18. Activation of immune cell subsets, largely through activated macrophages, results in a cascade of massive inflammatory cytokine activation including IL-6, tumor necrosis factor-α (TNF-α), IL-8 and IL-10, that leads to acute lung injury with acute respiratory distress syndrome, systemic inflammatory response syndrome (SIRS), shock and multiorgan dysfunction, and coagulopathy. The significant upregulation of IL-1β can result in the metastasis of dormant cancer cells to distant organs, potentially resulting in cancer relapse and recurrence in these patients.
The SARS-CoV genome encodes three ion channel proteins namely: E, open reading frame 3a (ORF3a) and ORF8a, where E and ORF3 are required for replication and virulence. Apart from canonical activation of the NLRP3 inflammasome by PAMPs and DAMPs, these proteins of SARS-CoV function as NLRP3 agonists [39], [131] where they activate both signaling mechanisms for inflammasome activation independently of ion channel activity. It has been observed that SARS-CoV ORF8 interacts with the LRR domain of NLRP3 to activate the inflammasome, resulting in increased levels of IL-1β and IL-18. Taking this into consideration, the NLRP3 inflammasome plays an important role in antiviral host defenses and its aberrant activation and induction of downstream mediators can lead to pathological tissue injury during infection. The inflammasome is therefore a core inflammatory component involved in the innate immune response, once induction with various stimuli has occurred. Since the activation of the NLRP3 inflammasome has also been reported in several types of cancer (e.g., breast, colon, lung and cervical cancer), the consequences of inflammasome activation in cancer patients with Covid-19 infection can have far reaching implications [110].
3.5. Covid-19, the NLRP3 inflammasome and cancer
The SARS-CoV-2 pandemic is spreading in a world where cancer prevalence is also rapidly growing, which raises questions as to how they could potentially interact with one another. As mentioned previously, the main risk factors of severe Covid-19 in the general population are gender, advanced age, obesity and diseases such as coronary heart disease, diabetes, hypertension and cancer. Cancer type, staging and therapies are additional risk factors for severe Covid-19 in this patient population. SARS-CoV-2 recruits several proteins involved in cellular replication, DNA damage, epigenetics and metabolism, which is also implicated in cancer [142].
The manifestation of severe forms of Covid-19 in a proportion of patients highlights the inability of the immune system to counteract the viral infection. These patients develop hyperinflammation with high levels of pro-inflammatory cytokines, like IL-6 (normally present at low levels in the blood), which is associated with the cytokine storm, blood hypercoagulability and reduced cell-mediated immunity. IL-6, TNF-α and IL-1 have been demonstrated by Maccio and authors to be correlated with impaired T-cell responses in women with advanced epithelial ovarian cancer [99]. This was also associated with the onset of systemic symptoms like anemia, anorexia and cardiac and respiratory muscle wasting [98]. IL-6 is therefore also an important role player in the pathogenesis of cancer. The IL-6-JAK-STAT3 pathway is hyperactivated in several cancers, which drives the proliferation, survival and invasiveness of tumor cells and suppresses anti-tumor immunity [151]. Oxidative stress in these patients can also be induced by chronic inflammation and macrophage activation. It has previously been shown that high levels of IL-6 in patients with advanced cancer was correlated with high levels of reactive oxygen species (ROS) and reduced antioxidant enzymes [97]. ROS overproduction can also be sensed by the NLRP3 inflammasome, which can further contribute to the prolonged and excessive inflammatory response in Covid-19 patients.
Inflammation and persistent infection contribute to several malignancies by promoting cancer initiation, development, progression, angiogenesis and invasion. Furthermore, IL-1β contributes to carcinogenesis, possibly via the NLRP3 pathway. In NLRP3-deficient mice, lung tumor cells were significantly decreased when compared to control animals. Although there is no direct evidence to support NLRP3 activation in breast cancer, it has been suggested that IL-1β promotes breast tumor development. Guo et al. reported that the inflammasome as well as IL-1β play important roles in the promotion of tumor growth and metastasis in breast cancer [65]. In this study they showed that in both NLRP3-deficient and caspase-1-deficient tumor mouse models, primary tumor growth was significantly reduced when compared to the wild type controls which had high circulating IL-1β levels. Furthermore, by blocking IL-1R (with an IL-1R antagonist), tumor growth and spontaneous metastasis were reduced in caspase-1-deficient mice, while wild type tumor-bearing mice treated with IL-1Ra had a reduced number of tumor nodules in the lungs. Taken together, these results indicate that inflammasome activation and IL-1β production may create favourable microenvironments for tumor metastasis.
Covid-19-induced inflammation can also affect tumor cells and their microenvironment ( Fig. 4). In breast cancer, the effects of Covid-19 remain to be elucidated, but it has been reported that patients with lung, haematological and breast cancer are at greater risk [41]. Furthermore, evidence suggests that Covid-19 may affect a particular stage in the tumor’s life cycle, which is represented by dormant cancer cells (DCCs). DCCs can survive after treatment of primary tumors and localize in specific microanatomical compartments of metastasis-prone organs [37]. These cells can reside in a quiescent state for a clinically asymptomatic period, referred to as metastatic dormancy. At some point, these cells may reactivate in response to microenvironmental cues like inflammatory or immune-mediated signaling thereby promoting metastasis of these cells.
Fig. 4.
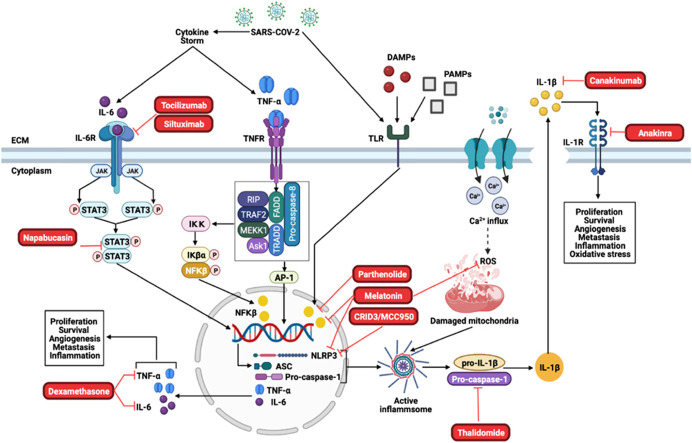
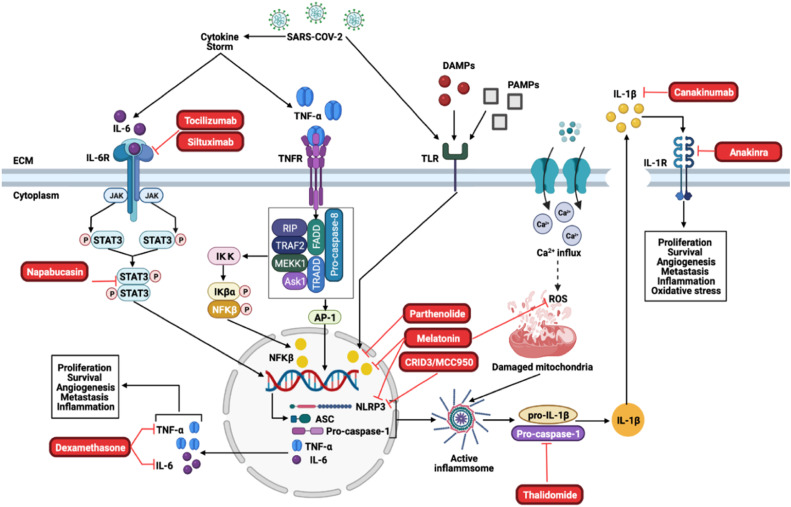
Proposed treatment strategies for Covid-19 and cancer. Tocilizumab and Siltuximab are inhibitors of the IL-6R, which will inhibit downstream activation of JAK/STAT3. Napabucasin is an inhibitor of STAT3 and prevents the STAT3 dimer to translocate to the nucleus where it induces the transcription of the inflammasome components and pro-inflammatory cytokines, IL-6 and TNF-α. Dexamethasone inhibits TNF-α and IL-6. Furthermore, Parthenolide and Melatonin inhibits NFκβ, which prevents the transcription of the inflammasome components as well as IL-6 and TNF-α. CRIDs/MCC950 and melatonin inhibits the NLRP3 components of the inflammasome, while melatonin additionally inhibits the production of ROS which contributes to inflammsome activation. Thalidomide is an inhibitor of the caspase-1 component, which prevents the activation of the NLRP3-inflammasome. Canakinumab inhibits the NLRP3-inflammsome product, IL-1β, while Anakinra inhibits the IL-1β receptor (IL-1R).
As stated previously, profound immune system alterations occur during SARS-CoV-2 infection which include decreases in natural killer cells and T-cells in the peripheral blood as well as the dysregulated activation of monocytes, neutrophils and tissue macrophages. Activated neutrophils release several tissue damaging products including web-like structures of proteins and DNA, referred to as neutrophil extracellular traps (NETs) [12], [52]. NETs entrap pathogens and thereby provides a high local concentration of anti-microbial components and creates a physical barrier that obstructs local access to immune cells. Although several factors involved in Covid-19 may play a role in the reawakening of dormant tumor cells, the strongest evidence points to NETs and neutrophils, which are emerging as important role players in the pathogenesis of this virus. It has been shown that acute lung inflammation and NETs have been respectively shown to trigger the exit from dormancy of breast DCCs, resulting in metastasis [52]. Albrengues and authors reported that NETs formed by neutrophils during LPS- or tobacco smoke-induced lung inflammation are required to awaken dormant cancer cells and cause metastasis in mice [6]. The authors proposed that NETs concentrate neutrophil proteases, neutrophil elastase and MMP-9 at their substrate, laminin, which allowed for the cleavage and generation of an epitope that triggered cancer cell awakening. Furthermore, laminin destruction by NET-associated proteases activated integrin signaling in lung-resident DCCs, which induced proliferation and lung metastasis. It is therefore plausible that NET generation that occurs during Covid-19 could trigger DCCs reawakening as well as other pro-inflammatory factors, like cytokine production. Similar to other findings, elevated levels of IL-6 during severe Covid-19 result in a widespread activation of NFκβ in immune and non-immune cells, possibly via the NLRP3 inflammasome pathway. Once NFκβ activation is induced, pre-metastatic niches may contribute to the reawakening of DCCs either directly by stimulating cancer cell proliferation or indirectly by inducing the formation of a pro-metastatic environment [38].
Following the cytokine storm and strong pro-inflammatory response, immune cells display exhaustion phenotypes during Covid-19 disease progression. As such, the functional diversity of these cells is also reduced, which indicates that Covid-19 triggers an excessive, non-effective host immune response, where a pro-inflammatory positive feedback loop persists following the exhaustion of the immune cells [121].
4. Immunosuppression
Intracellular mechanisms, including cell-cycle check points and tumour-suppressor proteins, strongly inhibit the development of malignant cells [22], [146]. Extracellularly, the innate and adaptive immune mechanisms are involved in the recognition and destruction of malignant cells. The NK cells destroy the initial transformed or malignant cells by detecting specific ligands on the cell surface, this process is known as immuno-surveillance [51]. Subsequently, macrophages and dendritic cells are recruited for the uptake and processing of the remaining fragments of the tumour cells and secrete inflammatory cytokines which recruits activated T and B cells. These cells activate the innate immune response which promotes the production of antibodies. This sequence of events leads to the destruction of any remaining tumour cells and the generation of tumour-specific antibodies [46], [51].
Additionally, some tumours have a genetic instability, namely microsatellite instability, where defects in DNA mismatch repair mechanisms result in the duplication or deletion of short repeated sequences of DNA, also known as microsatellites [26], [64]. These tumours have a high rate of mutation, resulting in the expression of unique tumour antigens. The antigens presented on the major histocompatibility complex (MHC) class 1 molecules can be recognized and is targeted by B cells and CD4+ and CD8+ T cells, resulting in the eventual destruction of the tumour. Consequently, the infiltration of CD4+ and CD8+ T cells and NK cells in some cancer types are associated with improved prognosis, including colorectal and medullary cancer. However, these cells often have increased expression of cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1), which interacts with PD-ligand1 (PD-L1). CTLA-4 and PD-1, which interacts with PD-L1, are the two main immune checkpoints that are triggered as shutdown mechanisms following T lymphocyte activation. Increased expression of CTLA-4 and PD-L1 results in an impaired immune response to these tumour-specific antigens [130]. Cancer cells promote CTLA-4 induction and induces PD-L1 expression as a mechanism of immune evasion. Furthermore, TNF-α upregulates CTLA-4 and PD-L1 expression in cancer, promoting tumourigenesis. Therefore, patients who are immunosuppressed have an increased risk of developing malignant cells with higher instances of spontaneous tumour regression. In line with this, it has been reported that mice lacking T cell and NK cell cytotoxic effector pathways develop spontaneous tumours. For example, mice that lack perforin, a cytotoxic molecule employed by CD8+ T cells and NK cells to form membrane pores in target cells, have an increased susceptibility to develop lymphomas [132], [134], [135].
Covid-19 infection induces a large-scale activation of lymphocytes, including CD8+ and CD4+ T cells, due to high levels of IFN-γ and TNF-α. However, these cells display exhaustion phenotypes during disease progression [43], [165]. T cell exhaustion is characterized by the stepwise and progressive loss of T-cell effector functions and can result in the physical deletion of these cells. Exhausted T cells also display metabolic dysregulation, poor memory recall and homeostatic self-renewal and changes in epigenetic programs [104]. This is also supported by the increased expression of programmed cell death protein 1 and T-cell immunoglobulin domain and mucin domain-3 levels on CD8+ cells [43], [156]. As such, the functional diversity of these cells are reduced. Therefore, Covid-19 results in an altered immune function in patients, also known as lymphopenia, as evident in decreased levels of CD4+ and CD8+ T cells, natural killer cells and B cell number [30], [75]. A decrease of up to 80% of these lymphocytes are observed in severe cases of the disease. As such, the possibility exists that lymphopenia and the exhaustion of immune cells during Covid-19 can contribute to the development of spontaneous tumours, cancer progression and cancer recurrence. Therefore, there is a need to identify treatments that target the common pathways implicated in both Covid-19 and cancer to improve patient outcomes.
5. Treatment strategies
5.1. Immunotherapy: anti-CTLA-4 and Anti-PD-1
Immunotherapy is a promising treatment modality for cancer, and targets cancer-associated inflammation. Two common modulators of the immune system are anti-PD-L1 and anti-CTLA-4 [130]. There are 7 drugs available that target CTLA-4 and PD-1, these agents have been approved for the treatment of various cancer types, including melanoma, lung cancer, breast cancer, cervical cancer, gastric cancers and B cell lymphomas. The CTLA4 blockers include ipilimumab, nivolumab, pembrolizumab and cemiplimab. The PD-L1 blockers include atezolizumab, avelumab and durvalumab, while ipilimumab and nivolumab can be administered as a combination therapy [126]. The comined inhibition of CTLA4 and PD-1 results in effector T-cell and NK cell activation in peripheral tissues and in induction of Treg cell differentiation which elicits an anti-tumour immune response. Therefore, the inhibition of CTLA-4 and PD-1/PD-L1 in Covid-19 and cancer patients may improve patient outcomes and prevent immune cell depletion following Covid-19 infection (Fig. 4) [11].
5.2. IL-6 and STAT3 inhibitors
IL-6 inhibitors may benefit cancer and Covid-19 patients due to the dual role that IL-6 plays in these diseases. Tocilizumab and Siltuximab are monoclonal antibodies that target the IL-6 receptor (IL-R). Tocilizumab has been approved for the treatment of rheumatoid arthritis patients, with no off-target effects, and may dampen the cytokine storm [19]. Siltuximab has been used for the treatment of cancer patients and inhibits STAT3 and MAPKs, which resulted in significant antitumor effects. Furthermore, Siltuximab has been tested in Covid-19 patients, and successfully decreased levels of IL-6 and CRP in these patients while improving disease outcomes. The use of the corticosteroid, dexamethasone, in Covid-19 patients resulted in reduced mortality risk. Furthermore, corticosteroids have been reported to inhibit the production of IL-6 and TNF-α-mediated IL-6 production [141]. The effects of corticosteroids on cancer remains uncertain, however is has been shown that glucocorticoids inhibit the development of lymphoids and has anti-inflammatory effects [74].
Napabucasin is a well-known inhibitor of cancer cell stemness and has been reported to inhibit STAT3 and ROS-mediated angiogenesis [17], [113]. Furthermore, the use of melatonin as an adjuvant treatment to cancer has been well established and it has recently been suggested as an adjuvant treatment for Covid-19 patients. Melatonin has indirect anti-viral effects by inhibiting the excessive production of pro-inflammatory cytokines, ROS production and the over activation of the immune system [162]. Therefore, the anti-inflammatory, antioxidant and immunomodulatory effects may benefit patients with cancer and Covid-19 (Fig. 4).
5.3. NLRP3 inhibitors
Excessive inflammation induced by the NLRP3 inflammasome in many diseases, including cancer, can be detrimental and the inhibition thereof is a promising approach for cancer prevention and treatment. Based on these above-mentioned studies, it could also alleviate the devastating effects of Covid-19 in these patients. By taking advantage of the complex signaling cascade of the NLRP3 inflammasome, a diverse range of targets can be used for its inhibition. This includes but is not limited to: inhibiting NLRP3 inflammasome activation, suppressing upstream signals, blocking inflammasome assembly, inhibiting caspase-1 activation and neutralizing inflammatory cytokines produced by the inflammasome, like IL-1β.
Drugs already used in clinical applications include thalidomide, a drug that targets and inhibits the activation of caspase-1, which is mainly used as a sedative to treat anxiety, insomnia and gastritis [107]. For the treatment of multiple myeloma, thalidomide has been approved for first-line therapy in combination with other chemotherapeutic drugs, which exerts anti-tumour effects [24]. It has been suggested that the mechanism of malignancy control with thalidomide is attributed to its anti-angiogenic activity, by reducing angiogenic factors like fibroblast growth factor (FGF) and vascular endothelial factor (VEGF) [48]. However, in a randomized, double-blinded, placebo-controlled trial, patients with advanced non-small lung cell cancer were treated with thalidomide in combination with gemcitabine and carboplatin. The study showed that the treatment regime did not improve the survival rate of these patients. However, it did increase the risk of thrombotic events [92].
Anakinra is a recombinant form of interleukin-1 receptor antagonist (IL-1Ra), which is commonly utilized in the treatment of autoinflammatory diseases and rheumatoid arthritis [44]. Previous studies with myeloma cells have shown that anakinra significantly reduces IL-6 levels but does not increase myeloma cell death. In a breast cancer mouse model, anakinra inhibited tumour growth to the bone and reduced the number of mice with metastasis. Previous studies have shown that IL-1β elevates the expression of VEGF and that the inhibition of IL-1β by anakinra could suppress the activity of VEGF, resulting in an anti-angiogenic effect [115].
Parthenolide is a sesquiterpene lactone component found in feverfew, a herb generally used as anti-inflammatory medicine which is considered as a potential anti-tumour drug that functions by inhibiting NFκβ signaling pathways [90], [114]. In gastric cancer, parthenolide significantly inhibits tumour cell growth and downregulates the phosphorylation of NFκβ [133]. The anti-tumour activity mechanisms of parthenolide, which include NFκβ inhibition, JNK activation, p53 activation and suppression of STAT3, have attracted great interest in the field of cancer.
Canakinumab is a human monoclonal antibody that targets IL-1β but not IL-1α. The use of canakinumab has not been studied significantly in cancer, but during a randomized, double-blinded, placebo-controlled trial of patients with lung cancers and atherosclerosis, researchers found that canakinumab significantly decreased lung cancer mortality by targeting the IL-1β innate immunity pathway [124]. The use of canakinumab as an anti-tumour drug has mainly focused on lung cancer and is being studied in phase III clinical trials in non-small lung cancer.
The cytokine release inhibitory drug, CRID3, also known as MCC950, is a diarylsulfonylurea-containing compound that inhibits the activation of the NLRP3 inflammasome, in mice in vivo and in human cells in vitro [33]. In a spontaneous chronic colitis mouse model, MCC950 significantly suppressed IL-1β secretion and caspase-1 activation, which indicates its potential use as an anti-tumour therapeutic agent [119].
Taking these inhibitors into consideration, developing or utilizing drugs that downregulate the functions of pro-inflammatory cytokines (like IL-1β and IL-6) could be beneficial for cancer patients with Covid-19. Several studies have shown that inflammasome inhibitors attenuate the proliferation and invasion of cancer cells, however, their anti-tumour activities are limited to specific types of cancer (Fig. 4).
6. Conclusion
Covid-19 infection is a serious and often deadly condition and significantly more so for patients with underlying conditions such as cancer. The unique inflammatory cascades associated with Covid-19 including excessive inflammation, inflammasome activation, exhaustion of immune function and the aberrant activation of several signalling pathways creates an optimal environment for malignant transformation and progression. This could also be true for dormant cancer cells and pre-malignant cell transformation. These effects may also contribute to resistance of cancer cells to cancer therapies. We have discussed various treatments that may prove effective against the pro-inflammatory effects of Covid-19 as well as cancer. These treatments may contribute to the resolution of Covid-19 infection and may protect against the development, progression and recurrence of cancerous cells during and following Covid-19 infection.
Ethical approval and consent to participate
Not applicable.
Funding
The financial assistance of the National Research Foundation (NRF) of South Africa (118566), South African Medical Research Counsil (SAMRC) and the Cancer Association of South Africa (CANSA) towards this research is hereby acknowledged. Opinions expressed and conclusions arrived at, are those of the authors and are not necessarily to be attributed to the NRF, SAMRC or CANSA.
Authors contributions
Each author has substantially contributed to conducting the underlying research and drafting this manuscript.
Declaration of Competing Interest
To the best of our knowledge, we have no conflicts of interest to disclose. All authors confirm that this work is original and has not been published elsewhere, nor is it currently under consideration for publication elsewhere.
Acknowledgements
Not applicable.
Consent for publication
All authors have approved the contents of this paper and have agreed to the submission policies.
Availability of supporting data
Not applicable.
Biographies
 Ms Manisha du Plessis, Department of Physiological Sciences, Stellenbosch University, South Africa, I am part of the Cancer Research Group in the Department of Physiological Sciences at the University of Stellenbosch, with a specific focus on breast cancer research. I am a PhD student under the supervision of Prof Anna-Mart Engelbrecht, with Dr Tanja Davis and Prof WJS de Villiers as my co-supervisors. My primary area of interest is the signaling mechanisms involved in tumor initiation and cancer progression. The goal for my PhD is to determine which role Serum amyloid A (SAA) plays in autophagy induction or inhibition in breast cancer cells, and how the modulation of autophagy by SAA regulates metastasis.
Ms Manisha du Plessis, Department of Physiological Sciences, Stellenbosch University, South Africa, I am part of the Cancer Research Group in the Department of Physiological Sciences at the University of Stellenbosch, with a specific focus on breast cancer research. I am a PhD student under the supervision of Prof Anna-Mart Engelbrecht, with Dr Tanja Davis and Prof WJS de Villiers as my co-supervisors. My primary area of interest is the signaling mechanisms involved in tumor initiation and cancer progression. The goal for my PhD is to determine which role Serum amyloid A (SAA) plays in autophagy induction or inhibition in breast cancer cells, and how the modulation of autophagy by SAA regulates metastasis.
 Ms Carla Fourie, Department of Physiological Sciences, Stellenbosch University, South Africa. I am part of the Cancer Research Group in the Physiological Sciences department at the University of Stellenbosch. I am currently busy with my PhD, with Professor Anna-mart Engelbrecht as my supervisor. I am mostly interested in signaling mechanisms and pathways that influence breast cancer growth and development. The aim for my PhD is to determine the role of Serum amyloid A (SAA) and inflammasome activation in breast cancer progression as well as understanding the interaction between cancer cells and other cell types in the tumour microenvironment, such as cancer associated fibroblasts.
Ms Carla Fourie, Department of Physiological Sciences, Stellenbosch University, South Africa. I am part of the Cancer Research Group in the Physiological Sciences department at the University of Stellenbosch. I am currently busy with my PhD, with Professor Anna-mart Engelbrecht as my supervisor. I am mostly interested in signaling mechanisms and pathways that influence breast cancer growth and development. The aim for my PhD is to determine the role of Serum amyloid A (SAA) and inflammasome activation in breast cancer progression as well as understanding the interaction between cancer cells and other cell types in the tumour microenvironment, such as cancer associated fibroblasts.
 Dr Johann Riedemann is a Clinical Radiation and Molecular Oncologist at Cancercare Cape Gate and Panorama Oncology Centres. He completed his MBChB degree at the Stellenbosch University and his internship and community service at Karl Bremer and Western Cape Rehabilitation hospitals respectively. Prior to becoming a clinician, he obtained BSc, HonsBSc and MSc (Molecular, cum laude) also from Stellenbosch University. He holds a PhD (Molecular Oncology) from the Weatherall Institute of Molecular Medicine (Oxford, Cancer Research UK) and was a fellow in precision oncology at the University of Oxford. He worked as clinical research fellow and registrar at Groote Schuur and Red Cross Children's Hospitals and completed the fellowship of radiation oncologists (CMSA) in 2020. His primary fields of interest include urology, breast, sarcoma, neuro-oncology and pediatric solid tumour malignancies. He is passionate about multidisciplinary team management and is a proponent of precision oncology that includes advanced radiotherapy techniques and implementation of targeted- and immunotherapies. He has published in numerous peer reviewed journals and presented at both national and international congresses.
Dr Johann Riedemann is a Clinical Radiation and Molecular Oncologist at Cancercare Cape Gate and Panorama Oncology Centres. He completed his MBChB degree at the Stellenbosch University and his internship and community service at Karl Bremer and Western Cape Rehabilitation hospitals respectively. Prior to becoming a clinician, he obtained BSc, HonsBSc and MSc (Molecular, cum laude) also from Stellenbosch University. He holds a PhD (Molecular Oncology) from the Weatherall Institute of Molecular Medicine (Oxford, Cancer Research UK) and was a fellow in precision oncology at the University of Oxford. He worked as clinical research fellow and registrar at Groote Schuur and Red Cross Children's Hospitals and completed the fellowship of radiation oncologists (CMSA) in 2020. His primary fields of interest include urology, breast, sarcoma, neuro-oncology and pediatric solid tumour malignancies. He is passionate about multidisciplinary team management and is a proponent of precision oncology that includes advanced radiotherapy techniques and implementation of targeted- and immunotherapies. He has published in numerous peer reviewed journals and presented at both national and international congresses.
 Prof Willem JS de Villiers, Department of Physiological Sciences, Science Faculty, Stellenbosch University, Stellenbosch, South Africa. Department of Internal Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University, Tygerberg Campus, South Africa. Rector and Vice-Chancellor of South Africa's Stellenbosch University, Professor Willem de Villiers is committed to supporting students to achieve their potential and has worked in the UK, America and South Africa to support them. In the 1990's Wim travelled to the UK and completed a DPhil in Immunology at Oxford University in 1995. He became Head of Gastroenterology at the University of Kentucky and Administrative Head of the Good Samaritan Hospital in Lexington. Working as a gastroenterologist, he became a respected medical researcher and was featured in the Best Doctors in America publication. He has shown the importance of macrophages (and their pattern recognition receptors) in many murine models of gut inflammation. He then contributed significantly to the clinical development and wide-spread application of targeted monoclonal antibody therapies in numerous patients with complicated inflammatory bowel disease. This work was published in top medical journals such as the New England Journal of Medicine and Gastroenterology. Moving back to South Africa in 2013, he became the Dean of Health Sciences at the University of Cape Town, before moving to his current position at Stellenbosch in December 2014.
Prof Willem JS de Villiers, Department of Physiological Sciences, Science Faculty, Stellenbosch University, Stellenbosch, South Africa. Department of Internal Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University, Tygerberg Campus, South Africa. Rector and Vice-Chancellor of South Africa's Stellenbosch University, Professor Willem de Villiers is committed to supporting students to achieve their potential and has worked in the UK, America and South Africa to support them. In the 1990's Wim travelled to the UK and completed a DPhil in Immunology at Oxford University in 1995. He became Head of Gastroenterology at the University of Kentucky and Administrative Head of the Good Samaritan Hospital in Lexington. Working as a gastroenterologist, he became a respected medical researcher and was featured in the Best Doctors in America publication. He has shown the importance of macrophages (and their pattern recognition receptors) in many murine models of gut inflammation. He then contributed significantly to the clinical development and wide-spread application of targeted monoclonal antibody therapies in numerous patients with complicated inflammatory bowel disease. This work was published in top medical journals such as the New England Journal of Medicine and Gastroenterology. Moving back to South Africa in 2013, he became the Dean of Health Sciences at the University of Cape Town, before moving to his current position at Stellenbosch in December 2014.
 Prof Anna-Mart Engelbrecht, Department of Physiological Sciences, Stellenbosch University, Stellenbosch. Anna-Mart Engelbrecht is currently professor in the Department of Physiological Sciences at Stellenbosch University. She received several prestigious awards which include the Dean’s and Senate’s Medals as well as the Gencor Bronze Medal, the Rector’s award for Excellence in Research and the Vice-Rector’s Research Award for exceptional achievement from Stellenbosch University as well as the Lasec Award for Excellence in Physiology Research from the Physiological Society of Southern Africa (PSSA). Seventeen MSc and fifteen PhD students completed the degrees under her supervision; she currently serves as promotor and co-promotor for four PhD students. She has published 86 peer reviewed, research articles and presented invited lectures at national and international conferences. She established the Cancer Research Group (CRG) where they investigate chemo-resistance and mechanisms to counteract chemotherapy-induced damage to the heart and skeletal muscle; as well as metabolic pathways in the cancer micro-environment.
Prof Anna-Mart Engelbrecht, Department of Physiological Sciences, Stellenbosch University, Stellenbosch. Anna-Mart Engelbrecht is currently professor in the Department of Physiological Sciences at Stellenbosch University. She received several prestigious awards which include the Dean’s and Senate’s Medals as well as the Gencor Bronze Medal, the Rector’s award for Excellence in Research and the Vice-Rector’s Research Award for exceptional achievement from Stellenbosch University as well as the Lasec Award for Excellence in Physiology Research from the Physiological Society of Southern Africa (PSSA). Seventeen MSc and fifteen PhD students completed the degrees under her supervision; she currently serves as promotor and co-promotor for four PhD students. She has published 86 peer reviewed, research articles and presented invited lectures at national and international conferences. She established the Cancer Research Group (CRG) where they investigate chemo-resistance and mechanisms to counteract chemotherapy-induced damage to the heart and skeletal muscle; as well as metabolic pathways in the cancer micro-environment.
References
- 2.Addeo A., Friedlaender A. Cancer and COVID-19: unmasking their ties. Cancer Treatment Rev. 2020;88 doi: 10.1016/j.ctrv.2020.102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aggarwal B.B., Vijayalekshmi R.V., Sung B. Targeting infl ammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin. Cancer Res. 2009;15:425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal B.B. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 2003;3(9):745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 6.Albrengues J., Shields M.A., Ng D., Park C.G., Ambrico A., Poindexter M.E., Upadhyay P., Uyeminami D.L., Pommier A., Küttner V., Bružas E., Maiorino L., Bautista C., Carmona E.M., Gimotty P.A., Fearon D.T., Chang K., Lyons S.K., Pinkerton K.E., Trotman L.C., Goldberg M.S., Yeh J.T., Egeblad M. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361(6409) doi: 10.1126/science.aao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angelini M.M., Akhlaghpour M., Neuman B.W., Buchmeier M.J. Severe acute respiratory syndrome coronavirus nonstructural proteins 3, 4, and 6 induce double-membrane vesicles. mBio. 2013;4:4. doi: 10.1128/mBio.00524-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balkwill F. Tumour necrosis factor and cancer. Nat. Rev. Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 11.Barber D.L., Wherry E.J., Masopust D., Zhu B., Allison J.P., Sharpe A.H., Freeman G.J., Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;9:439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 12.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., Daßler-Plenker J., Guerci P., Huynh C., Knight J.S., Loda M., Looney M.R., McAllister F., Rayes R., Renaud S., Rousseau S., Salvatore S., Schwartz R.E., Spicer J.D., Yost C.C., Weber A., Zuo Y., Egeblad M. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020;217(6) doi: 10.1084/jem.20200652. e20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauernfeind F., Ablasser A., Bartok E., Kim S., Schmid-Burgk J., Cavlar T., Hornung V. Inflammasomes: current understanding and open questions. Cell. Mol. Life Sci. 2011;68:765–783. doi: 10.1007/s00018-010-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauernfeind F.G., Horvath G., Stutz A., Alnemri E.S., MacDonald K., Speert D., Fernandes-Alnemri T., Wu J., Monks B.G., Fitzgerald K.A., Hornung V., Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bharadwaj U., Kasembeli M.M., Robinson P., Tweardy D.J. Targeting janus kinases and signal transducer and activator of transcription 3 to treat inflammation, fibrosis, and cancer: rationale, progress, and caution. Pharm. Rev. 2020;72:486–526. doi: 10.1124/pr.119.018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biran N., Ip A., Ahn J., Go R.C., Wang S., Mathura S., Sinclaire B.A., Bednarz U., Marafelias M., Hansen E., Siegel D.S., Goy A.H., Pecora A.L., Sawczuk I.S., Koniaris L.S., Simwenyi M., Varga D.W., Tank L.K., Stein A.A., Allusson V., Lin G.S., Oser W.F., Tuma R.A., Reichman J., Brusco L Jr, Carpenter K.L., Costanzo E.J., Vivona V., Goldberg S.L. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;2:e603–e612. doi: 10.1016/S2665-9913(20)30277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenOever B.R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouros D., Alexandrakis M.G., Antoniou K.M., Agouridakis P., Pneumatikos I., Anevlavis S., Pataka A., Patlakas G., Karkavitsas N., Kyriakou D. The clinical significance of serum and bronchoalveolar lavage inflammatory cytokines in patients at risk for acute respiratory distress syndrome. BMC Pulmon. Med. 2004;4:6. doi: 10.1186/1471-2466-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bower J.J., Vance L.D., Psioda M., Smith-Roe S.L., Simpson D.A., Ibrahim J.G., Hoadley K.A., Perou C.M., Kaufmann W.K. Patterns of cell cycle checkpoint deregulation associated with intrinsic molecular subtypes of human breast cancer cells. NPJ Breast Cancer. 2017;3:9. doi: 10.1038/s41523-017-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boye K., Grotterod I., Aasheim H.C., Hovig E., Mælandsmo G.M. Activation of NF-κB by extracellular S100A4: analysis of signal transduction mechanisms and identification of target genes. Int. J. Cancer. 2008;123(6):1301–1310. doi: 10.1002/ijc.23617. [DOI] [PubMed] [Google Scholar]
- 24.Breitkreutz I., Anderson K.C. Thalidomide in multiple myeloma--clinical trials and aspects of drug metabolism and toxicity. Expert Opin. Drug Metab. Toxicol. 2008;4(7):973–985. doi: 10.1517/17425255.4.7.973. [DOI] [PubMed] [Google Scholar]
- 25.Bromberg J.F., Wrzeszczynska M.H., Devgan G., Zhao Y., Pestell R.G., Albanese C., Darnell J.E., J.r Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 26.Buckowitz A., Knaebel H.P., Benner A., Bläker H., Gebert J., Kienle P., von Knebel Doeberitz M., Kloor M. Microsatellite instability in colorectal cancer is associated with local lymphocyte infiltration and low frequency of distant metastases. Br. J. Cancer. 2005;92:1746–1753. doi: 10.1038/sj.bjc.6602534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai X., Cao C., Li J., Chen F., Zhang S., Liu B., Zhang W., Zhang X., Ye L. Inflammatory factor TNF-alpha promotes the growth of breast cancer via the positive feedback loop of TNFR1/NF-kappaB (and/or p38)/p-STAT3/HBXIP/TNFR1. Oncotarget. 2017;8:58338–58352. doi: 10.18632/oncotarget.16873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castro F., Cardoso A.P., Gonçalves R.M., Serre K., Oliveira M.J. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front. Immunol. 2018;9:847. doi: 10.3389/fimmu.2018.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S01406736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheung C.Y., Poon L.L., Ng I.H., Luk W., Sia S.F., Wu M.H., Chan K.H., Yuen K.Y., Gordon S., Guan Y., Peiris J.S. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J. Virol. 2005;79:7819–7826. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu H., Zhou J., Wong B.H., Li C., Chan J.F., Cheng Z.S., Yang D., Wang D., Lee A.C., Li C., Yeung M.L., Cai J.P., Chan I.H., Ho W.K., To K.K., Zheng B.J., Yao Y., Qin C., Yuen K.Y. Middle East respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J. Infect. Dis. 2016;213:904–914. doi: 10.1093/infdis/jiv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coll R.C., Robertson A.A., Chae J.J., Higgins S.C., Muñoz-Planillo R., Inserra M.C., Vetter I., Dungan L.S., Monks B.G., Stutz A., Croker D.E., Butler M.S., Haneklaus M., Sutton C.E., Núñez G., Latz E., Kastner D.L., Mills K.H., Masters S.L., Schroder K., Cooper M.A., O’Neill L.A. A small molecule inhibitior of the NLRP3 inflammasome is a potential therapeutic for inflammatory diseases. Nat. Med. 2015;21(3):248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cruceriu D., Baldasici O., Balacescu O., Berindan-Neagoe I. The dual role of tumour necrosis factor-alpha (TNF-a) in breast cancer: molecular insights and therapeutic approaches. Cell. Oncol. 2020;43:1–18. doi: 10.1007/s13402-019-00489-1. [DOI] [PubMed] [Google Scholar]
- 36.Culig Z., Puhr M. Interleukin-6: a multifunctional targetable cytokine in human prostate cancer. Mol. Cell Endocrinol. 2012;360(1–2):52–58. doi: 10.1016/j.mce.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Angelis M.L., Francescangeli F., Zeuner A. Breast cancer stem cells as drivers of tumor chemoresistance, dormancy and relapse: new challenges and therapeutic opportunities. Cancers. 2019;11(10):1569. doi: 10.3390/cancers11101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Cock J.M., Shibue T., Dongre A., Keckesova Z., Reinhardt F., Weinberg R.A. Inflammation triggers Zeb1-dependent escape from tumor latency. Cancer Res. 2016;76(23):6778–6784. doi: 10.1158/0008-5472.CAN-16-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Diego M.L., Nieto-Torres J.L., Jimenez-Guardeño J.M., Regla-Nava J.A., Castaño-Rodriguez C., Fernandez-Delgado R., Usera F., Enjuanes L. Coronavirus virulence genes with main focus on SARS-CoV envelope gene. Virus Res. 2014;194:124–137. doi: 10.1016/j.virusres.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Della Corte C.M., Morgillo F. Early use of steroids affects immune cells and impairs immunotherapy efficacy. ESMO Open. 2019;4(1) doi: 10.1136/esmoopen-2018-000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Derosa L., Melenotte C., Griscelli F., Gachot B., Marabelle A., Kroemer G., Zitvogel L. The immuno-oncological challenge of COVID-19. Nat. Cancer. 2020;1:946–964. doi: 10.1038/s43018-020-00122-3. [DOI] [PubMed] [Google Scholar]
- 42.Dethlefsen C., Hojfeldt G., Hojman P. The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res. Treat. 2013;138(3):657–664. doi: 10.1007/s10549-013-2488-z. [DOI] [PubMed] [Google Scholar]
- 43.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., Yuan Z., Feng Z., Zhang Y., Wu Y., Chen Y. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dinarello C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 46.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 47.Emmanuelle Blanchard P.R. Virus-induced double-membrane vesicles. Cell Microbiol. 2015;17:45–50. doi: 10.1111/cmi.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Figg W.D., Kruger E.A., Price D.K., Kim S., Dahut W.D. Inhibition of angiogenesis: treatment options for patients with metastatic prostate cancer. Invest New Drugs. 2002;20(2):183–194. doi: 10.1023/a:1015626410273. [DOI] [PubMed] [Google Scholar]
- 49.Filippou P.S., Karagiannis G.S. Cytokine storm during chemotherapy: a new companion diagnostic emerges? Oncotarget. 2020;11(3):213–215. doi: 10.18632/oncotarget.27442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finn O.J. Immuno-onology: understanding the function and dysfunction of the immune system in cancer. Ann. Oncol. 2012;23:viii6–viii9. doi: 10.1093/annonc/mds356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Francescangeli F., De Angelis M.L., Zeuner A. COVID-19: a potential driver of immune-mediated breast cancer recurrence. Breast Cancer Res. 2020;22:117. doi: 10.1186/s13058-020-01360-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Franceschi C., Bonafè M., Valensin S., Olivieri F., de Luca M., Ottaviani E., De Benedictis G. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 54.Freeman T.L., Swartz T.H. Targeting the NLRP3 inflammasome in severe covid-19. Front. Immunol. 2020;11:1518. doi: 10.3389/fimmu.2020.01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol. Sin. 2020;35(3):266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fung S.Y., Yuen K.S., Ye Z.W., Chan C.P., Jin D.Y. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg. Microbes Infect. 2020;9:558–570. doi: 10.1080/22221751.2020.1736644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gartung A., Yang J., Sukhatme V.P., Bielenberg D.R., Fernandes D., Chang J., Schmidt B.A., Hwang S.H., Zurakowski D., Huang S., Kieran M.W., Hammock B.D., Panigrahy D. Suppression of chemotherapy-induced cytokine/lipid mediator surge and ovarian cancer by a dual COX-2/sEH inhibitor. Proc. Natl. Acad. Sci. U. S. A. 2019;116:1698–1703. doi: 10.1073/pnas.1803999116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gatta L.B., Melocchi L., Bugatti M., Missale F., Lonardi S., Zanetti B., Cristinelli L., Belotti S., Simeone C., Ronca R., Grillo E., Licini S., Bresciani D., Tardanico R., Chan S.R., Giurisato E., Calza S., Vermi W. Hyper-activation of STAT3 sustains progression of non-papillary basal-type bladder cancer via FOSL1 regulome. Cancers. 2019;11:1219. doi: 10.3390/cancers11091219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grivennikov S.I., Greten F.R., Karin M. Immunity, infl ammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grivennikov S.I., Karin M. Inflammation and oncogenesis: a vicious connection. Curr. Opin. Genet. Dev. 2010;20:65–71. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grivennikov S.I., Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin take the stage. Ann. Rheum. Dis. 2010;70:i104–i108. doi: 10.1136/ard.2010.140145i104. [DOI] [PubMed] [Google Scholar]
- 63.Grivennikov S.I., Kuprash D.V., Liu Z.G., Nedospasov S.A. Intracellular signals and events activated by cytokines of the tumor necrosis factor superfamily: from simple paradigms to complex mechanisms. Int. Rev. Cytol. 2006;252:129–161. doi: 10.1016/S0074-7696(06)52002-9. [DOI] [PubMed] [Google Scholar]
- 64.Guidoboni M., Gafà R., Viel A., Doglioni C., Russo A., Santini A., Del Tin L., Macrì E., Lanza G., Boiocchi M., Dolcetti R. Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis. Am. J. Pathol. 2001;159:297–304. doi: 10.1016/S0002-9440(10)61695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo B., Fu S., Zhang J., Liu B. Targeting inflammasome/IL-1 pathways for cancer immunotherapy. Scientific Reports. 2016;6 doi: 10.1038/srep36107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., Péré H., Charbit B., Bondet V., Chenevier-Gobeaux C., Breillat P., Carlier N., Gauzit R., Morbieu C., Pène F., Marin N., Roche N., Szwebel T.A., Merkling S.H., Treluyer J.M., Veyer D., Mouthon L., Blanc C., Tharaux P.L., Rozenberg F., Fischer A., Duffy D., Rieux-Laucat F., Kernéis S., Terrier B. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ham B., Fernandez M.C., D’Costa Z., Brodt P. The diverse roles of the TNF axis in cancer progression and metastasis. Trends Cancer Res. 2016;11(1):1–27. [PMC free article] [PubMed] [Google Scholar]
- 70.Hayden M.S., Ghosh S. Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 2014;26(3):253–266. doi: 10.1016/j.smim.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He L., Ding Y., Zhang Q., Che X., He Y., Shen H. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J. Pathol. 2006;210:288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. New Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [Google Scholar]
- 75.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang M., Zhang W.W., Liu P., Yu W., Liu T., Yu J. Dysregulation of SOCS-mediated negative feedback of cytokine signaling in carcinogenesis and its significance in cancer treatment. Front. Immunol. 2017;8:70. doi: 10.3389/fimmu.2017.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson B.S., Laloraya M. A cytokine super cyclone in COVID-19 patients with risk factors: the therapeutic potential of BCG immunization. Cytokine Growth Factor Rev. 2020;54:32–42. doi: 10.1016/j.cytogfr.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnston P.A., Grandis J.R. STAT3 signaling: anticancer strategies and challenges. Mol. Interv. 2011;11(1):18–26. doi: 10.1124/mi.11.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kamran M.Z., Patil P., Gude R.P. Role of STAT3 in cancer metastasis and translational advances. Biomed. Res. Int. 2013;2013 doi: 10.1155/2013/421821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaur S., Bansal Y., Kumar R., Bansal G. A panoramic review of IL-6: structure, pathophysiological roles and inhibitors. Bioorg. Med. Chem. 2020;28 doi: 10.1016/j.bmc.2020.115327. [DOI] [PubMed] [Google Scholar]
- 83.Kim S., Keku T.O., Martin C., Galanko J., Woosley J.T., Schroeder J.C., Satia J.A., Halabi S., Sandler R.S. Circulating levels of infl ammatory cytokines and risk of colorectal adenomas. Cancer Res. 2008;68:323–328. doi: 10.1158/0008-5472.CAN-07-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Klover P.J., Muller W.J., Robinson G.W., Pfeiffer R.M., Yamaji D., Hennighausen L. Loss of STAT1 from mouse mammary epithelium results in an increased neu-induced tumor burden. Neoplasia. 2010;12:899–905. doi: 10.1593/neo.10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Konno Y., Kimura I., Uriu K., Fukushi M., Irie T., Koyanagi Y. et al. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is further increased by a naturally occurring elongation variant. bioRxiv. 2020. 10.1016/j.celrep.2020.108185. [DOI] [PMC free article] [PubMed]
- 87.Kopecky-Bromberg S.A., Martínez-Sobrido L., Frieman M., Baric R.A., Palese P. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J. Virol. 2007;81:548–557. doi: 10.1128/JVI.01782-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kujawski M., Kortylewski M., Lee H., Herrmann A., Kay H., Yu H. STAT3 mediates myeloid cell–dependent tumor angiogenesis in mice. J. Clin. Invest. 2008;118(10):3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kutz S.M., Hordines J., McKeown-Longo P.J., Higgins P.J. TGFbeta1-induced PAI-1 gene expression requires MEK activity and cell-to-substrate adhesion. J. Cell Sci. 2001;114:3905–3914. doi: 10.1242/jcs.114.21.3905. [DOI] [PubMed] [Google Scholar]
- 90.Kwok B.H., Koh B., Ndubuisi M.I., Elofsson M., Crews C.M. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem. Biol. 2001;8(8):759–766. doi: 10.1016/s1074-5521(01)00049-7. [DOI] [PubMed] [Google Scholar]
- 91.Law H.K., Cheung C.Y., Ng H.Y., Sia S.F., Chan Y.O., Luk W., Nicholls J.M., Peiris J.S., Lau Y.L. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood. 2005;106:2366–2374. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee S.M., Rudd R., Woll P.J., Ottensmeier C., Gilligan D., Price A., Spiro S., Gower N., Jitlal M., Hackshaw A. Randomized double-blind placebo-controlled trial of thalidomide in combination with gemcitabine and Carboplatin in advanced non-small-cell lung cancer. J. Clin. Oncol. 2009;27(31):5248–5254. doi: 10.1200/JCO.2009.21.9733. [DOI] [PubMed] [Google Scholar]
- 93.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F., Liu L., Amit I., Zhang S., Zhang Z. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 94.Liu W., Zhou Y., Reske S.N., Shen C. PTEN mutation: many birds with one stone in tumorigenesis. Anticancer Res. 2008;28(6A):3613–3619. 〈https://www.ncbi.nlm.nih.gov/pubmed/19189642?dopt=Abstract〉 [PubMed] [Google Scholar]
- 95.Lokau J., Schoeder V., Haybaeck J., Garbers C. Jak-stat signaling induced by interleukin-6 family cytokines in hepatocellular carcinoma. Cancers. 2019;11:1704. doi: 10.3390/cancers11111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lokugamage K.G., Hage A., Schindewolf C., Rajsbaum R., Menachery V.D. SARS-CoV-2 is sensitive to type I interferon pretreatment. bioRxiv. 2020 doi: 10.1101/2020.03.07.982264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Macciò A., Madeddu C. Inflammation and ovarian cancer. Cytokine. 2012;58:133–147. doi: 10.1016/j.cyto.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 98.Macciò A., Madeddu C. The role of interleukin-6 in the evolution of ovarian cancer: clinical and prognostic implications--a review. J. Mol. Med. 2013;91:1355–1368. doi: 10.1007/s00109-013-1080-7. [DOI] [PubMed] [Google Scholar]
- 99.Macciò A., Oppi S., Madeddu C. COVID-19 and cytokine storm syndrome: can what we know about interleukin-6 in ovarian cancer be applied? J. Ovarian Res. 2021;14:28. doi: 10.1186/s13048-021-00772-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Matsuyama T., Kubli S.P., Yoshinaga S.K., Pfeffer K., Mak T.W. An abberant STAT pathway is central to COVID-19. Cell Death Diff. 2020;27:3209–3225. doi: 10.1038/s41418-020-00633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Matthews C.P., Colburn N.H., Young M.R. AP-1 a target for cancer prevention. Curr. Cancer Drug Targets. 2007;7:317–324. doi: 10.2174/156800907780809723. [DOI] [PubMed] [Google Scholar]
- 104.McLane L.M., Abdel-Hakeem M.S., Wherry E.J. CD8 T cell exhaustion during chronic viral infection and cancer. Annu. Rev. Immunol. 2019;37:457–495. doi: 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- 105.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH Across Speciality Collaboration U. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Meissl K., Macho-Maschler S., Müller M., Strobl B. The good and the bad faces of STAT1 in solid tumours. Cytokine. 2017;89:12–20. doi: 10.1016/j.cyto.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 107.Miller M.T. Thalidomide embryopathy: a model for the study of congenital incomitant horizontal strabismus. Trans. Am. Ophthalmol. Soc. 1991;89:623–674. [PMC free article] [PubMed] [Google Scholar]
- 108.Mondal A.M., Horikawa I., Pine S.R., Fujita K., Morgan K.M., Vera E., Mazur S.J., Appella E., Vojtesek B., Blasco M.A., Lane D.P., Harris C.C. p53 isoforms regulate aging- and tumor-associated replicative senescence in T lymphocytes. J. Clin. Invest. 2013;123:5247–5257. doi: 10.1172/JCI70355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Monteleone M., Stanley A.C., Chen K.W., Brown D.L., Bezbradica J.S., von Pein J.B., Holley C.L., Boucher D., Shakespear M.R., Kapetanovic R., Rolfes V., Sweet M.J., Stow J.L., Schroder K. Interleukin-1b maturation triggers its relocation to the plasma membrane for gasdermin-D-dependent and independent secretion. Cell Rep. 2018;24:1425–1433. doi: 10.1016/j.celrep.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 110.Moossavi M., Parsamanesg N., Bahrami A., Atkin S.L., Sahebkar A. Role of the NLRP3 inflammasome in cancer. Mol. Cancer. 2018;17:158. doi: 10.1186/s12943-018-0900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mori K., Shibanuma M., Nose K. Invasive potential induced under long-term oxidative stress in mammary epithelial cells. Cancer Res. 2004;64:7464–7472. doi: 10.1158/0008-5472.CAN-04-1725. [DOI] [PubMed] [Google Scholar]
- 113.Nagaraju G.P., Farran B., Farren M., Chalikonda G., Wu C., Lesinski G.B., El-Rayes B.F. Napabucasin (BBI 608), a potent chemoradiosensitizer in rectal cancer. Cancer. 2020;126:3360–3371. doi: 10.1002/cncr.32954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nennig S.E., Schank J.R. The role of NFkB in drug addiction: beyond inflammation. Alcohol Alcohol. 2017;52(2):172–179. doi: 10.1093/alcalc/agw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nie L., Lyros O., Medda R., Jovanovic N., Schmidt J.L., Otterson M.F., Johnson C.P., Behmaram B., Shaker R., Rafiee P. Endothelial-mesenchymal transition in normal human esophageal endothelial cells cocultured with esophageal adenocarcinoma cells: role of IL-1beta and TGF-beta2. Am. Physiol. Soc. 2014;307(9):C859–C877. doi: 10.1152/ajpcell.00081.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Owen K.L., Brockwell N.K., Parker B.S. JAK-STAT signaling: a double edged sword of immune regulation and cancer progression. Cancers. 2019;11:2002. doi: 10.3390/cancers11122002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Park A., Iwasaki A., Type I. and type III interferons—induction, signaling, evasion, and application to combat COVID-19. Cell Host Microb. 2020;27:870–878. doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Perera A.P., Fernando R., Shinde T., Gundamaraju R., Southam B., Sohal S.S., Robertson A., Schroder K., Kunde D., Eri R. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci. Rep. 2018;8(1):8618. doi: 10.1038/s41598-018-26775-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Qin C. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Lancet. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ridker P.M., MacFadyen J.G., Thuren T., Everett B.M., Libby P., Glynn R.J. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10105):1833–1842. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]
- 125.Robilotti E.V., Babady N.E., Mead P.A., Rolling T., Perez-Johnston R., Bernardes M., Bogler Y., Caldararo M., Figueroa C.J., Glickman M.S., Joanow A., Kaltsas A., Lee Y.J., Lucca A., Mariano A., Morjaria S., Nawar T., Papanicolaou G.A., Predmore J., Redelman-Sidi G., Schmidt E., Seo S.K., Sepkowitz K., Shah M.K., Wolchok J.D., Hohl T.M., Taur Y., Kamboj M. Deter,inants of COVID-19 disease severity in patients with cancer. Nat. Med. Lett. 2020;26:1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J. Exp. Clin. Cancer Res. 2019;38:255. doi: 10.1186/s13046-019-1259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rubio M.F., Werbajh S., Cafferata E.G., Quaglino A., Coló G.P., Nojek I.M., Kordon E.C., Nahmod V.E., Costas M.A. TNF-alpha enhances estrogen-induced cell proliferation of estrogen dependent breast tumor cells through a complex containing nuclear factor-kappa B. Oncogene. 2006;25:1367–1377. doi: 10.1038/sj.onc.1209176. [DOI] [PubMed] [Google Scholar]
- 128.Samways D.S., Li Z., Egan T.M. Principles and properties of ion flow in P2X receptors. Front. Cell Neurosci. 2014;8:6. doi: 10.3389/fncel.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sanchez L.R., Borriello L., Entenberg D., Condeelis J.S., Oktay M.H., Karagiannis G.S. The emerging roles of macrophages in cancer metastasis and response to chemotherapy. J. Leukoc. Biol. 2019;106:259–274. doi: 10.1002/JLB.MR0218-056RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Seidel J.A., Otsuka A., Kabashima K. Anti-PD-1 and Anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front. Oncol. 2018;8:86. doi: 10.3389/fonc.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Siu K.L., Yuen K.S., Castaño-Rodriguez C., Ye Z.W., Yeung M.L., Fung S.Y., Yuan S., Chan C.P., Yuen K.Y., Enjuanes L., Jin D.Y. Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J. 2019;33:8865–8877. doi: 10.1096/fj.201802418R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Smyth M.J., Thia K.Y., Street S.E., MacGregor D., Godfrey D.I., Trapani J.A. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J. Exp. Med. 2000;192:755–760. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sohma I., Fujiwara Y., Sugita Y., Yoshioka A., Shirakawa M., Moon J.H., Takiguchi S., Miyata H., Yamasaki M., Mori M., Doki Y. Parthenolide, an NF-kappaB inhibitor, suppresses tumor growth and enhances response to chemotherapy in gastric cancer. Cancer Genomics Proteomics. 2011;8(1):39–47. [PubMed] [Google Scholar]
- 134.Street S.E., Trapani J.A., MacGregor D., Smyth M.J. Suppression of lymphoma and epithelial malignancies effected by interferon gamma. J. Exp. Med. 2002;2002(196):129–134. doi: 10.1084/jem.20020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Swann J.B., Smyth M.J. Immune surveillance of tumours. J. Clin. Investig. 2007;117(5):1137–1146. doi: 10.1172/JCI31405. 〈http://www.jci.org〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Swanson K.V., Deng M., Ting J.P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.Q., Wang Q., Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct. Target Ther. 2020;5:33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Teijaro J.R., Walsh K.B., Cahalan S., Fremgen D.M., Roberts E., Scott F., Martinborough E., Peach R., Oldstone M.B., Rosen H. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146 doi: 10.1016/j.cell.2011.08.015. 980991-980991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Trotman L.C., Pandolfi P.P. PTEN and p53: who will get the upperhand. Cancer Cell. 2003;3(2):97–99. doi: 10.1016/S1535-6108(03)00022-9. [DOI] [PubMed] [Google Scholar]
- 140.Tsai M.H., Pai L.M., Lee C.K. Fine-tuning of type I interferon response by STAT3. Front. Immunol. 2019;10:1448. doi: 10.3389/fimmu.2019.01448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Turnquist C., Ryan B.M., Horikawa I., Harris B.T., Harris C.C. Cytokine storms in cancer and covid-19. Cancer Cell. 2020;38:598–601. doi: 10.1016/j.ccell.2020.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tutuncuoglu B., Cakir M., Batra J., Bouhaddou M., Eckhardt M., Gordon D.E., Krogan N.J. The landscape of human cancer proteins targeted by SARS-CoV-2. Cancer Discov. 2020;10(7):916–921. doi: 10.1158/2159-8290.CD-20-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Waldner M.J., Neurath M.F. Cytokines in colitis associated cancer: potential drug targets? Inflamm. Allergy Drug Targets. 2008;7:187–194. doi: 10.2174/187152808785748137. [DOI] [PubMed] [Google Scholar]
- 145.Wang H., Yuan M., Wang S., Zhang L., Zhang R., Zou X., Wang X., Chen D., Wu Z. STAT3 regulates the type I IFN-mediated antiviral response by interfering with the nuclear entry of STAT1. Int. J. Mol. Sci. 2019;20:4870. doi: 10.3390/ijms20194870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang L.H., Wu C.F., Rajasekaran N., Shin Y.K. Loss of tumour suppressor gene function in human cancer: an overview. Cell. Physiol. Biochem. 2018;51:2647–2693. doi: 10.1159/000495956. [DOI] [PubMed] [Google Scholar]
- 147.Wang X.H., Liu B.R., Qu B., Xing H., Gao S.L., Yin J.M., Wang X.F., Cheng Y.Q. Silencing STAT3 may inhibit cell growth through regulating signalling pathway, telomerase, cellcycle, apoptosis and angiogenesis in hepatocellular carcinoma: potential uses for gene therapy. Neoplasma. 2011;58(2):158–171. doi: 10.4149/neo_2011_02_158. [DOI] [PubMed] [Google Scholar]
- 148.Wang Y., van Boxel-Dezaire A.H.H., Cheon H., Yang J., Stark G.R. STAT3 activation in response to IL-6 is prolonged by the binding of IL-6 receptor to EGF receptor. Proc. Natl. Acad. Sci. U. S. A. 2013;110(42):16975–16980. doi: 10.1073/pnas.1315862110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang Y., Wang X., Zhao H., Liang B., Du Q. Clusterin confers resistance to TNF-alpha-induced apoptosis in breast cancer cells through NF-kappaB activation and Bcl-2 overexpression. J. Chemother. 2012;24:348–357. doi: 10.1179/1973947812Y.0000000049. [DOI] [PubMed] [Google Scholar]
- 150.Wathelet M.G., Orr M., Frieman M.B., Baric R.S. Severe acute respiratory syndrome coronavirus evades antiviral signaling: role of nsp1 and rational design of an attenuated strain. J. Virol. 2007;81:11620–11633. doi: 10.1128/JVI.00702-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Weiss T.W., Arnesen H., Seljeflot I. Components of the interleukin-6 transsignalling system are associated with the metabolic syndrome, endothelial dysfunction and arterial stiffness. Metabolism. 2013;62:1008–1013. doi: 10.1016/j.metabol.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 152.Westermarck J., Kahari V.M. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- 153.Wu Y., Zhou B.P. TNF-α/NF-kB/Snail pathway in cancer cell migration and invasion. Br. J. Cancer. 2010;102:639–644. doi: 10.1038/sj.bjc.6605530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wullschleger S., Loewith R., Hall M.N. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 155.Yang D., Chu H., Hou Y., Chai Y., Shuai H., Lee A.C.-Y., Zhang X., Wang Y., Hu B., Huang X., Yuen T.T.T., Cai J.P., Zhou J., Yuan S., Zhang A.J., Chan J.F.W., Yuen K.Y. Attenuated interferon and pro-inflammatory response in SARSCoV-2-infected human dendritic cells is associated with viral antagonism of STAT1 phosphorylation. J. Infect. Dis. 2020;222:734–745. doi: 10.1093/infdis/jiaa356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yang L., Liu S., Liu J., Zhang Z., Wan X., Huang B., Chen Y., Zhang Y. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduc. Targeted Ther. 2020;5:128. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang X.M., Wang Y.S., Zhang J., Li Y., Xu J.F., Zhu J., Zhao W., Chu D.K., Wiedemann P. Role of PI3K/Akt and MEK/ERK in mediating hypoxia-induced expression of HIF-1α and VEGF in laser-induced rat choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 2009;50(4):1873–1879. doi: 10.1167/iovs.08-2591. [DOI] [PubMed] [Google Scholar]
- 159.Yuen C.K., Lam J.Y., Wong W.M., Mak L.F., Wang X., Chu H., Cai J.P., Jin D.Y., To K.K., Chan J.F., Yuen K.Y., Kok K.H. SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists. Emerg. Microbes Infect. 2020;9:1–29. doi: 10.1080/22221751.2020.1780953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang H., Luo J., Alcorn J.F., Chen K., Fan S., Pilewski J., Liu A., Chen W., Kolls J.K., Wang J. AIM2 inflammasome is critical for influenza-induced lung injury and mortality. J. Immunol. 2017;198:4383–4393. doi: 10.4049/jimmunol.1600714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang R., Wang X., Ni L., Di X., Ma B., Niu S., Liu C., Reiter R.J. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020;250 doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang X., Zhang J., Wei H., Tian Z. STAT3-decoyoligodeoxynucleotide inhibits the growth of human lung cancer via down-regulating its target genes. Oncol. Rep. 2007;17(6):1377–1382. doi: 10.3892/or.17.6.1377. [DOI] [PubMed] [Google Scholar]
- 164.Zhao C., Zhao W. NLRP3 inflammasome-a key player in antiviral responses. Front. Immunol. 2020;11:211. doi: 10.3389/fimmu.2020.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zheng H.Y., Zhang M., Yang C.X., Zhang N., Wang X.C., Yang X.P., Dong X.Q., Zheng Y.T. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020;17:541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhu C., Shen H., Zhu L., Zhao F., Shu Y. Plasminogen activator inhibitor 1 promotes immunosuppression in human non-small cell lung cancers by enhancing TGF-β1 expression in macrophage. Cell Physiol. Biochem. 2017;44:2201–2211. doi: 10.1159/000486025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.