Abstract
Introduction
Non-pharmaceutical interventions that have been implemented in southern hemisphere countries because of COVID-19 pandemic declaration in March 2020, have evidenced some unexpected changes in the way of spreading of many other viruses. This study as a part of ECEALHBA’s Project, reports the consequences of COVID-19 pandemic over 2020–2021 bronchiolitis epidemic period in the Central and Eastern regions of Spain.
Method
Multicenter, observational, descriptive and ambispective study of admitted infants with the diagnosis of bronchiolitis in 16 Spanish hospitals involved in the investigation project. Five epidemic periods previous to COVID-19 pandemic, from 2015 to 2020, were compared with the current one, 2020–2021, in both a qualitative and quantitative manner.
Results
Total of 4643 infants were admitted to the participating hospitals along the study period. Pandemic season hospital admissions for bronchiolitis were 94.1% lower than in pre-pandemic period. December and January were peak months for bronchiolitis admissions during pre-pandemic period, but September was the peak month during pandemic year. There was a progressive decrease of admissions from this moment until the end of the follow-up, in April 2021. Rhinovirus has been the commonest etiology for bronchiolitis in 2020–2021 epidemic period of bronchiolitis.
Conclusions
Some of the non-pharmaceutical interventions initiated because of COVID-19 pandemic are probably related to the dramatic decrease of bronchiolitis cases in 2020–2021 season. It would be rewarding to purpose novel research to clarify how these simple interventions can be useful, close to vaccines and antiviral drugs, to achieve the goal of avoiding the spread of respiratory viruses in pediatric population.
Keywords: COVID-19, Bronchiolitis, Non-pharmaceutical interventions, Epidemic period, Pandemic
Abstract
Introducción
Las intervenciones no farmacológicas puestas en marcha en territorios del hemisferio sur tras la declaración de la pandemia por COVID-19 en marzo de 2020, han puesto de manifiesto toda una serie de cambios inesperados en la propagación de otros virus. Dentro del proyecto Estudio Colaborativo Español para la Atención de Lactantes Hospitalizados por Bronquiolitis Aguda (ECEALHBA) presentamos este estudio cuyo objetivo ha sido constatar las repercusiones de la pandemia sobre la temporada epidémica de bronquiolitis de 2020 a 2021 en el centro y este de España.
Material y métodos
Estudio multicéntrico, observacional, descriptivo y ambispectivo de una muestra de lactantes ingresados por bronquiolitis aguda en alguno de los 16 hospitales españoles participantes en la investigación. Se compararon desde el punto de vista cualitativo y cuantitativo las cinco temporadas epidémicas previas a la pandemia, de 2015 a 2020, con la última de 2020 a 2021.
Resultados
Un total de 4.643 lactantes ingresaron en alguno de los hospitales participantes durante el periodo de estudio. Se describe un descenso porcentual de ingresos del 94,1% entre el periodo previo y la temporada epidémica coincidente con la pandemia. Septiembre pasó a ser el mes pico de ingresos en lugar de diciembre y enero como era habitual, con un descenso progresivo de los ingresos a partir de ese momento hasta el final del seguimiento en abril de 2021. El rinovirus, de forma atípica, ha sido el agente responsable de la mayoría de las bronquiolitis en esta última temporada de 2020 a 2021.
Conclusiones
Las intervenciones no farmacológicas puestas en marcha como consecuencia de la COVID-19 han hecho desaparecer de su ubicación cronológica habitual la temporada epidémica de bronquiolitis aguda de 2020 a 2021. Cabe plantearse investigaciones específicas para dilucidar el lugar que ocupan estas medidas junto a las vacunas y fármacos antivirales para el control de las infecciones respiratorias de etiología viral en la población pediátrica.
Palabras clave: COVID-19, Bronquiolitis, Intervenciones no farmacológicas, Temporada epidémica, Pandemia
Introduction
At the end of 2019, cases of pneumonia caused by a yet unknown virus were detected in patients in Wuhan, China.1 At the time, nobody knew the outbreak was to become a global health disaster with important public health, social and economic repercussions. On March 11, 2020, the World Health Organization officially declared the epidemic of novel coronavirus disease (COVID-19) caused by SARS-CoV-2 a pandemic. This was around the time that nations in the southern hemisphere were preparing for the winter season of viral infectious diseases, between the months of June and August, in which the most important pathogens tend to be respiratory syncytial virus (RSV) and influenza viruses.2 A concurrence of these and other viruses was expected that could overwhelm national health systems.2 However, the collapse was not brought by the expected source, but instead was due exclusively to SARS-CoV-2, causing a global pandemic in the midst of which all other viruses, including RSV, the main virus responsible for acute bronchiolitis (AB), played a negligible role.2, 3, 4
In clinical terms, AB is defined as a first episode of wheezing preceded by cold symptoms in children aged less than 2 years, and it is the most frequent reason for hospital admission in infants aged up to 12 months of age.5 Respiratory syncytial virus is the causative agent in more than half of the cases of AB, although other virus, such as rhinovirus, bocavirus, adenovirus, metapneumovirus, influenza and parainfluenzavirus, enterovirus and coronavirus6 (probably in that order of frequency7, 8) can also cause it.9 In countries with a temperate climate in the northern hemisphere, such as Spain, the incidence of AB exhibits seasonal peaks in the form of annual outbreaks between October and April of the following year, with the bulk of related hospital admissions clustering between December and February, resulting in an increased demand for health care services and sometimes saturating inpatient neonatal and infant care units.10
Since March 2020, the COVID-19 pandemic has led to the adoption at the population level of nonpharmacological preventive interventions, such as hand hygiene, the use of face masks and different restrictions on free movement with the aim of containing the spread of the virus.11 These public health measures, implemented continuously and for an extended period of time, have achieved a decrease not only in infectious communicable diseases typically found in the paediatric population,12, 13, 14, 15, 16 but also in hospital admissions and the use of health care resources by the general paediatric population,17, 18, 19 while changes have also been made to the way that care is delivered.20 Independently, before all of this took place, in January 2019 a group of Spanish paediatricians thought of conducting a collaborative study to assess differences between hospitals in the care provided to infants admitted to hospital with a diagnosis of AB. This idea came to fruition during 2019 with the development of a research project known as ECEALHBA (Appendix A). The first study within this project aimed at providing a qualitative and quantitative description of AB seasons in terms of related hospital admissions from 2015 to 2020 and comparing them with the 2020–2021 season in the context of COVID-19 to assess the impact of the pandemic on the 2020–2021 AB epidemic season.
Material and methods
Study design and universe
We conducted a prospective and retrospective multicentre, observational and descriptive study in a sample of infants aged up to 12 months admitted due to AB to any of the 16 hospitals that participate in the ECEALHBA project.
Sample selection, inclusion and exclusion criteria
We sent a memorandum with an invitation to participate in the project to all public hospitals in the regions of Castilla La Mancha and the Valencian Community. For the period between September 1, 2020 and April 15, 2021, we included prospectively every infant admitted with a diagnosis of AB based on the McConnochie criteria21 adapted for age. We chose April 15, 2021 as the date to end data collection because based on the published data for the month of April of different years, epidemic seasons are practically over by this date. For the period ranging from September 1, 2015 to August 31, 2020, which encompasses 5 AB seasons, we included retrospectively all infants with a discharge diagnosis of AB, documented with International Classification of Diseases (ICD) codes 466.11 or 466.19 (ICD-9) or J21.0, J21.8 or J21.9 (ICD-10), with no other restrictions or exclusion criteria.
Data collection
We collected data for the same variables for the entire period under study. In the retrospective phase, we retrieved anonymised data by sending a request to the Health Records Coding Unit and the Department of Microbiology of each participating hospital. The data for each participating hospital was collected in an Excel spreadsheet and transferred in the form of anonymised aggregated data to the coordinating hospital that was responsible for the global analysis of the data. In the prospective phase, data were collected in a form designed for the purpose after obtaining informed consent for the inclusion of each patient. The variables under study were: number of patients admitted due to AB, date of birth, date of admission, date of discharge, sex, age, nationality, length of stay (LOS), RSV testing and need of admission to the intensive care unit (ICU). When it came to the aetiology of AB, and only in the prospective phase of the study, we analysed whether other diagnostic tests had been done to try to identify the virus that caused AB in patients with negative RSV test results.
Statistical analysis
We have summarised qualitative variables as absolute frequencies and percentages of the total. We summarised quantitative variables as mean and standard deviation (SD) if they followed a normal distribution, and otherwise as median and interquartile range (IQR). The statistical analysis was performed with the software SPSS for Windows, version 21.0 (SPSS Inc, Chicago, IL, USA).
Ethical considerations
The study, in the framework of the ECEALHBA project, was approved by the ethics committees of the coordinating hospital and the rest of participating hospitals. Every process related to the collection, transfer and analysis of data and any other circumstance requiring interaction with the family or the patient for the purpose of the study adhered to the principles of the Declaration of Helsinki and Organic Law 3/2018 of December 5 on the Protection of Personal Data and Guarantee of Digital Rights, and we obtained informed consent of the parents or legal guardians of patients for their participation in the study and for accessing, if needed for research purposes, health records of the patient.
Results
A total of 4643 infants were admitted to any of the 16 hospitals in the 6 epidemic seasons under study. The predominant profile was an infant of male sex and Spanish nationality, aged 3 months or younger, hospitalised for 4 to 7 days without requiring admission to the ICU, and the most frequent causative agent of the viral illness was RSV, as can be seen in Table 1. Most of the participating hospitals are of small to medium size and do not have an ICU. Together, participating hospitals serve a total catchment population of 2 664 520 inhabitants, of who 373 247 (14.0%) are children aged 0 to 14 years (both included). These hospitals manage a mean of 937 childbirths and 211 infant admissions a year, and their staff includes 4 (smallest hospital) to 43 (largest hospital) paediatricians. Table 2 presents a detailed breakdown of indicators allowing comparison of the participating hospitals.
Table 1.
Main characteristics of the patients included in the study (N = 4643).
| Female sex, 1936 (41.7%) |
| Spanish citizenship, 3900 (84.6%) |
| Age group distribution |
| Neonate, 539 (11.6%) |
| Infant ≤ 3 months, 2214 (47.7%) |
| Infant/toddler > 3 months, 1889 (40.7%) |
| Median age, 2.4 months (IQR, 1.4–4.5) |
| LOS interval distribution |
| ≤1 day, 358 (7.7%) |
| 2-3 days, 1687 (36.3%) |
| 4-7 days, 2070 (44.6%) |
| >7 days, 527 (11.4%) |
| Mean LOS, 4.4 days (SD, 2.7) |
| RSV+, 2849 (64.1%)* |
| ICU admission, 367 (7.9%) |
ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; RSV, respiratory syncytial virus; SD, standard deviation.
RSV test performed in 4442 patients (95.6% of the total sample).
Table 2.
Characteristics of participating hospitals
| Hospital | TCP | TPCP | Annual births | DP (BW/WG) | Inf/Tod admissions/year | Paed. and inf. beds | NB beds | NICU incubators | PICU beds | Paediatricians on staff | Paediatrics residents |
|---|---|---|---|---|---|---|---|---|---|---|---|
| H. de Tomelloso | 62 353 | 8520 | 405 | 2000/36 | 96 | 6 | – | – | – | 4 | – |
| H. de Puertollano | 70 578 | 9178 | 270* | 2000/36 | 76 | 6 | 2 | – | – | 5 | – |
| H. de Manzanares | 41 671 | 5594 | 342 | No threshold/35 | 98 | 9 | 3 | – | – | 5 | – |
| H. G. de Albacete | 273 000 | 35 981 | 1769 | No threshold | 320 | 18 | 18 | 6 | 3 | 24,5** | 16 |
| H. de Villarrobledo | 61 019 | 7447 | 372 | 1900/35 | 68 | 6 | 2 | – | – | 5 | – |
| H. de Cuenca | 135 541 | 16 619 | 672 | 1500/32 | 179 | 16 | 7 | – | – | 9 | – |
| H. de Sagunto | 151 394 | 21 846 | 654 | 2000/35 | 140 | 14 | 4 | – | – | 7 | – |
| H. de Xátiva | 194 799 | 26 165 | 926 | 1500/32 | 182 | 20 | 7 | – | – | 8 | 4 |
| H. de La Ribera | 246 264 | 34 937 | 1362 | 1500/34 | – | 24 | 14 | – | – | 14 | 8 |
| H. de Gandía | 177 464 | 27 300 | 1091 | 1500/32 | 236 | 18 | 6 | – | – | 9 | 8 |
| H. G. de Castellón | 284 183 | 40 071 | 1339 | No threshold | 443 | 34 | 18 | 8 | 4 | 21 | 16 |
| H. de La Plana | 187 616 | 28 452 | 1225 | 1500/34 | 231 | 9 | 6 | – | – | 8 | – |
| H. de Vinarós | 90 364 | 13 073 | 476 | 2000/35 | 204 | 8 | 6 | – | – | 6 | – |
| H. G. de Alicante | 274 913 | 41 587 | 2037 | No threshold | 538 | 42 | 32 | 12 | 5 | 43 | 24 |
| H. de Alcoy | 136 081 | 18 581 | 917 | 1500/32 | 96 | 11 | 6 | 1 | – | 8 | 4 |
| H. Dr. Peset | 277 280 | 37 896 | 1132 | 32 | 262 | 22 | 10 | – | – | 13 | 4 |
| Total | 2664 520 | 373 247 | 14 989 | – | 3169 | 263 | 141 | 27 | 12 | 191 | 82 |
| Mean or range | 166 533 | 23 328 | 937 | – | 211 | 6–42 | 2–32 | 1–12 | 3–12 | 4–43 | 2–24 |
BW, birth weight; DP, degree of prematurity; H., Hospital; H.G., Hospital General; paed, paediatric; NB, newborn; NICU, neonatal intensive care unit; PICU, paediatric intensive care unit; TCP, total catchment population (number of individual health cards assigned to hospital); TPCP, total paediatric catchment population (number of paediatric individual health cards assigned to hospital); Inf/Tod, infant/toddler; WG, weeks of gestation.
Degree of prematurity (DP): weight and gestational age thresholds above which newborn infants are admitted for care and treatment in the corresponding hospital.
Paediatric individual health card: public health care system card of patients aged 0 to 14 years + 364 days as of December 31, 2020.
Birth frequencies refer to year 2020.
Infant/toddler admissions: admission of patients aged 24 months or less, excluding neonates (birth to day 27 post birth); data for 2019.
An isolated hyphen as the sole content of a cell means that the variable does not apply to the hospital (does not exist in hospital or does not make sense to apply to hospital) or that data could not be collected.
This hospital did not manage child births between March and May 2020, which were referred to the Hospital de Ciudad Real.
One of the paediatricians is employed on a half-time basis.
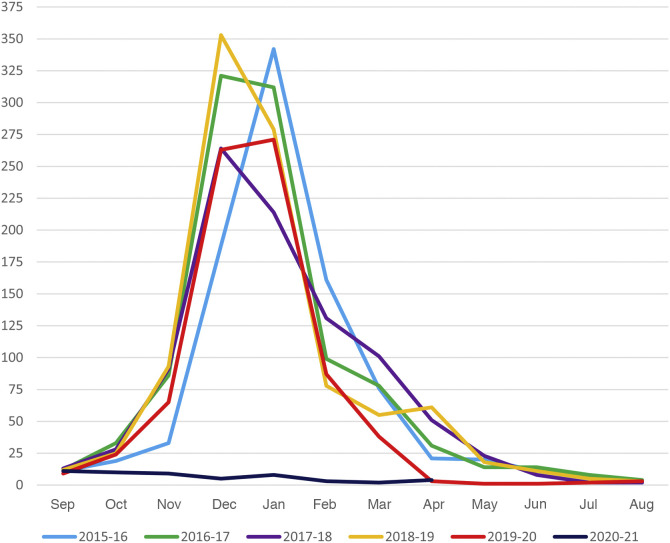
Between September 1, 2015 and April 15, 2020, a period encompassing 5 epidemic seasons, with these specific date limits established for data collection to allow correct comparison, the overall frequency of hospital admission due to AB was 4436, with an average of 887.2 admissions per season, compared to only 52 admissions in the same interval in the 2020–2021 season. This corresponds to a 94.1% decrease in admissions. Looking at the total admissions from September of one year through August of the following year, in 4 of the 5 seasons before the pandemic the number of admissions ranged from nearly 900 to slightly over 1000 per season. However, in the 2019–2020 season before the pandemic, the number of admissions dropped substantially to 767. The descent can be explained by the decrease in the number of admissions due to AB from March 2020. Fig. 1 and Table 3 illustrate these temporal trends. In the 5 seasons preceding the pandemic, admissions peaked in December or January, and the months when the season started and ended in most seasons were November and April or May, respectively, with the exception, once again, of the 2019–2020 season, which ended in March with only 38 admissions. The 2020–2021 season differed significantly, with admissions peaking in September and a gradual decline through December, with a resurgence in January 2021 that decreased again to 3 admissions in February, 2 in March and 4 in April (through the 15th). Fig. 1 provides a graphic representation of these data.
Figure 1.
Total admissions in participating hospitals per month and epidemic season.
Table 3.
Cases of acute bronchiolitis requiring admission by hospital and epidemic season.
| Centre/season | 2015-16 | 2016-17 | 2017-18 | 2018-19 | 2019-20 | 2020-21 | Total |
|---|---|---|---|---|---|---|---|
| H. de Tomelloso | 26 (8/16/2) | 17 (8/4/5) | 26 (12/10/4) | 13 (6/6/1) | 27 (15/12/0) | 7 (1/6/0) | 116 (50/54/12) |
| H. de Puertollano | 21 (11/10/0) | 25 (18/7/0) | 23 (7/16/0) | 26 (13/13/0) | 11 (7/4/0) | 0 (0/0/0) | 106 (56/50/0) |
| H. de Manzanares | 23 (8/12/3) | 28 (10/16/2) | 31 (10/21/0) | 20 (9/9/2) | 28 (18/10/0) | 0 (0/0/0) | 130 (55/68/7) |
| H. G. de Albacete | 63 (44/19/0) | 99 (68/31/0) | 97 (62/35/0) | 100 (72/28/0) | 77 (63/14/0) | 5 (0/5/0) | 441 (309/132/0) |
| H. de Villarrobledo | 10 (7/3/0) | 16 (13/3/0) | 20 (14/5/1) | 21 (13/7/1) | 5 (4/1/0) | 0 (0/0/0) | 72 (51/19/2) |
| H. de Cuenca | 17 (10/6/1) | 29 (9/17/3) | 35 (11/16/8) | 24 (11/9/4) | 21 (2/2/17) | 4 (0/4/0) | 130 (43/54/33) |
| H. de Sagunto | 18 (7/3/8) | 54 (31/19/4) | 32 (25/5/2) | 45 (26/18/1) | 28 (21/6/1) | 4 (1/3/0) | 181 (111/54/16) |
| H. de Xátiva | 41 (28/10/3) | 48 (39/7/2) | 46 (35/10/1) | 58 (42/12/4) | 40 (33/7/0) | 2 (0/2/0) | 235 (177/48/10) |
| H. de La Ribera | 132 (69/62/1) | 140 (79/59/2) | 93 (46/47/0) | 136 (98/38/0) | 101 (73/25/3) | 6 (2/2/2) | 608 (367/233/8) |
| H. de Gandía | 43 (21/20/2) | 41 (26/15/0) | 42 (18/23/1) | 63 (36/25/2) | 50 (39/11/0) | 2 (1/1/0) | 241 (141/95/5) |
| H. G. de Castellón | 140 (91/47/2) | 139 (96/42/1) | 131 (95/36/0) | 119 (99/19/1) | 95 (67/27/1) | 7 (0/7/0) | 631 (448/178/5) |
| H. La Plana | 77 (59/16/2) | 94 (54/38/2) | 71 (40/27/4) | 72 (55/16/1) | 49 (40/9/0) | 2 (0/2/0) | 365 (248/108/9) |
| H. de Vinarós | 41 (5/2/34) | 48 (12/16/20) | 27 (11/12/4) | 42 (15/13/14) | 32 (23/9/0) | 2 (0/2/0) | 192 (66/54/72) |
| H. G. de Alicante | 175 (66/102/7) | 177 (105/72/0) | 157 (114/42/1) | 176 (128/43/5) | 130 (101/29/0) | 6 (0/6/0) | 821 (514/294/13) |
| H. de Alcoy | 22 (19/2/1) | 17 (12/2/3) | 19 (13/4/2) | 27 (17/7/3) | 20 (15/5/0) | 1 (0/1/0) | 106 (76/21/9) |
| H. Dr. Peset | 42 (21/21/0) | 40 (23/17/0) | 78 (44/34/0) | 51 (21/30/0) | 53 (28/25/0) | 4 (0/4/0) | 268 (137/131/0) |
| Totals | 891 (474/351/66) | 1.012 (603/365/44) | 928 (557/343/28) | 993 (661/293/39) | 767 (549/196/22) | 52 (5/45/2) | 4.643 (2.849/1.593/201) |
H., Hospital; H.G., Hospital General.
The results in each cell refer to total cases per hospital per season: (RSV + cases/RSV–cases/cases without RSV testing).
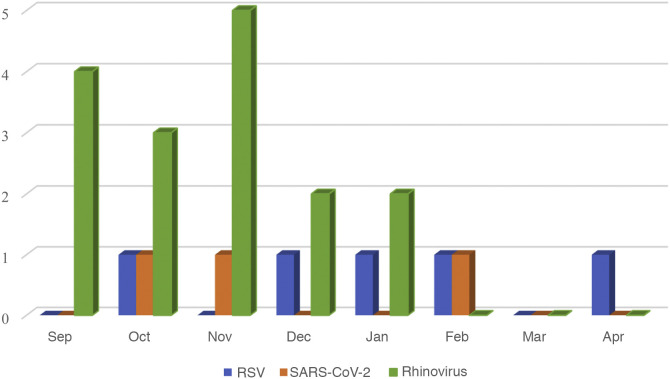
In the period before the pandemic, RSV was responsible for 73.0% of cases of AB that required admission between November and February in which tests were performed for aetiological diagnosis, while during the rest of the year RSV, was only responsible for 26.5% of cases of AB. In contrast, in the 2020–2021 season, of the 50 cases out of 52 in which RSV testing was performed, only 5 (10%) turned out positive. Testing for detection of SARS-CoV-2 was performed in 51 of the 52 patients and was positive in 3 (5.9%). Only 18 of the 52 total patients with AB underwent testing for detection of rhinovirus. Sixteen of the 18 patients (88.8%) tested positive. There were no detected cases of viral coinfection. Thus, 24 cases of AB were positive for one of these 3 viruses and the most frequently involved was rhinovirus, detected in 16 of these cases (66.6%). Fig. 2 provides a graphical representation of these data. Table 3 presents the distribution of admissions per hospital and season based on RSV testing and results.
Figure 2.
Aetiology of bronchiolitis requiring admission from September 2020 to April 2021. The figure only shows the 24 cases with a known aetiology out of the total 52 cases.
Testing for RSV, SARS-CoV-2 and rhinovirus was performed in 50, 51 and 18 cases, respectively, out of the total of 52.
Discussion
Expectations based on the trends observed in the southern hemisphere turned out to be accurate, as evinced by the findings of our study. The nonpharmacological interventions implemented to contain the COVID-19 pandemic were at least partly responsible for the containment of RSV, all but eliminating the expected AB outbreak in the 2020–2021 season in Spain. There have also been other reports in areas of the northern hemisphere, including Spain, describing a sharp decline in paediatric health care resources in general,17, 22, 23, 24, 25, 26 and specifically in the demand for health care for management of communicable diseases of a viral aetiology, such as AB, acute otitis media, common cold, laryngitis, influenza, pneumonia, gastroenteritis and asthma exacerbations,22, 23, 24, 25 but not for other infectious but noncommunicable diseases, such as skin or urinary tract infections.23
In the literature focused specifically on the European continent and the impact of the COVID-19 pandemic on AB, we have only found 2 studies with similar findings.27, 28 Their authors described decreases in the frequency of admission due to AB of 92.5%27 and 82.5%,28 similar to the 94.1% decrease observed in our study, although of lesser magnitude. Due to the early cessation of data collection in those 2 studies (on the 4th week of December 2020), while the authors reported that the peak in admissions due to AB had not started as expected,27, 28 they could only speculate whether the 2020-2021 AB outbreak was going to be delayed or, on the contrary, was going to disappear or at least shift from its usual position in the calendar between October of one year and April of the following year. Since we continued the collection of admissions data through April 2021, we can assert that the 2020–2021 AB season did not take place in the expected time frame.
The likely explanation to our findings has already been described in other studies going back several decades29 and warrants a reflection on the potential benefits of the correct implementation at the population level of nonpharmacological interventions in terms of health care efficiency and public health. These benefits would include a reduction of lost workdays for parents, childcare workers and businesses and corporations and of morbidity and mortality in vulnerable individuals, avoiding crowding in emergency departments and the need for adding third beds in inpatient units, and disease prevention in the general population, all of which could, if handled correctly, make our health care system more efficient in future epidemic seasons. On the other hand, the implementation of these interventions may increase in the susceptibility of the population to other infectious agents, which could give rise to more severe and prolonged outbreaks in the future, possibly outside the expected time frame.30 We also need to take into account the impact of restrictive measures on the mental health of children, adolescents31 and parents,32 which calls for correct management and implementation of these measures in the general population.
In the last epidemic season documented in our study, RSV all but disappeared as a causative agent of AB, with a concurrent emergence of rhinovirus. The latter was the causative agent in 16 of the 24 cases of AB of known aetiology, corresponding to 66.6% of cases, even though testing for rhinovirus was only performed in 18 of the total 52 cases of AB. The proportion of cases of rhinovirus among cases of AB requiring hospital admission followed a pattern reminiscent of RSV in previous epidemic seasons. This had not been described before and is a striking phenomenon given that previous studies consider it only the second leading cause of AB following RSV, accounting for less than 20% of cases.8 It is known that rhinovirus is viable in the community throughout the year and causes outbreaks in spring and autumn. Rhinovirus does not have a lipid bilayer envelope, unlike RSV and coronavirus, so handwashing may be less effective in preventing colonization of the host compared to these other viruses.3 There is also evidence that interaction of rhinovirus with other viruses, such as influenzavirus or even SARS-CoV-2, could impair replication of these viruses by triggering interferon secretion by host respiratory epithelial cells.33 These and other factors could explain why the implemented measures achieved containment of RSV and SARS-CoV-2 transmission with the opposite effect on rhinovirus.
We ought to comment on the new developments and reports that have emerged in the southern hemisphere after the lifting of COVID-19-related restrictions. For instance, in South New Wales, Australia, there has been a resurgence in cases of AB associated with an increase in the circulation of RSV that started in October 2020 and continued through at least January 2021, which corresponds to the summer months in this area of the world.34 A similar phenomenon could also take place in countries in the northern hemisphere, like Spain, which would confirm that the outbreak of AB caused by RSV was not eliminated, but delayed.
Collection of longitudinal data starting from April 2021 in a multicentre, collaborative study with a long-term follow-up would allow us to draw conclusions on this subject.
Our study evinces the temporal association between the implementation of measures like hand hygiene, the use of face masks and the isolation of infected individuals with the aim of containing the spread of SARS-CoV-2 and the decreased spread of RSV in the population of 2 autonomous communities in Spain. In light of these findings, we propose performance of studies specifically aimed at establishing with greater precision the role that these measures could play in combination with vaccination and antiviral drugs in the approach of the health care system to not only future pandemics, but also the annual outbreaks caused by other viruses, such as RSV, influenzavirus, parainfluenzavirus and rhinovirus.
Funding
This research did not receive any external funding.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Please cite this article as: Rius-Peris JM, Lucas-García J, García-Peris M, Escrivá Tomás P, Sequí-Canet JM, González de Dios J, en representación resto de autores grupo ECEALHBA. Pandemia por COVID-19 y su repercusión sobre las hospitalizaciones por bronquiolitis en el Centro y Este de España An Pediatr (Barc). 2021;95:345–353.
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.anpede.2021.06.006.
Appendix A. MEMBERS OF THE ECEALHBA PROJECT RESEARCH GROUP
Molini Menchón N2; Olmos García JM4; Silvestre Beneyto R4; Felipe Almira E5; Sánchez-Sánchez A6; Vicent Castelló MC6; Marcilla Vázquez C7; Pareja León M7; García Maset L8; Coret Sinisterra A8; Campayo Losa F9; Castillo Ochando F9; Pantoja Martínez J10; Povo Martín S10; Caballero Mora FJ11; Edo Tena A12; Rabasco Álvarez L12; Moya Díaz-Pintado MT13; Amat Madramani A14; Cardete Pascual I14; Moreno López M15; Pons Morales S16.
2Hospital General Universitario de Castellón, Castellón, Spain.
4Hospital Universitario Virgen de los Lirios, Alcoy, Alicante, Spain.
5Hospital Universitario Francisco Borja, Gandía, Valencia, Spain.
6Hospital General Universitario de Alicante, Alicante, Spain.
7Hospital General Universitario de Albacete, Albacete, Spain.
8Hospital Universitario de Sagunto, Sagunto, Valencia, Spain.
9Hospital General de Villarrobledo, Villarrobledo, Albacete, Spain.
10Hospital Universitario de La Plana, Crta. Villarreal, Castellón, Spain.
11Hospital Santa Bárbara, Puertollano, Ciudad Real, Spain.
12Hospital de Vinarós, Vinarós, Castellón, Spain.
13Hospital Virgen de Altagracia, Manzanares, Ciudad Real, Spain.
14Hospital Universitario de La Ribera, Alcira, Valencia, Spain.
15Hospital General de Tomelloso, Tomelloso, Ciudad Real, Spain.
16Hospital Universitario Dr, Peset, Valencia, España.
Appendix B. Supplementary data
The following is Supplementary data to this article:
References
- 1.WHO. Novel coronavirus (2019-nCoV) situation report 5. [Consulted 1 April 2021]. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200125-sitrep-5-2019ncov.pdf?sfvrsn=429b143d_8.
- 2.Sullivan S.G., Carlson S., Cheng A.C., Chilver M.B., Dwyer D.E., Irwin M., et al. Where has all the influenza gone? The impact of COVID-19 on the circulation of influenza and other respiratory viruses, Australia, March to September 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.47.2001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Britton P.N., Hu N., Saravanos G., Shrapnel J., Davis J., Snelling T., et al. COVID-19 public health measures and respiratory syncytial virus. Lancet Child Adolesc Health. 2020;4:e42–e43. doi: 10.1016/S2352-4642(20)30307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Q.S., Wood T., Jelley L., Jennings T., Jefferies S., Daniells K., et al. Impact of the COVID-19 nonpharmaceutical interventions on influenza and other respiratory viral infections in New Zealand. MedRxiv. 2020;13 doi: 10.1038/s41467-021-21157-9. 2020.11.11.20228692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Florin T.A., Plint A.C., Zorc J.J. Viral bronchiolitis. Lancet. 2017;389:211–224. doi: 10.1016/S0140-6736(16)30951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvo C., Alcolea S., Casas I., Pozo F., Iglesias M., González-Esguevillas M., et al. A 14-year prospective study of human coronavirus infections in hospitalized children: comparison with other respiratory viruses. Pediatr Infect Dis J. 2020;39:653–657. doi: 10.1097/INF.0000000000002760. [DOI] [PubMed] [Google Scholar]
- 7.Salvador García C., Moreno Docón A., Piñero J.A., Alfayate Miguélez S., Iborra Bendicho M.A. Etiología de bronquiolitis en niños hospitalizados en el sureste de España. An Pediatr (Barc) 2012;77:386–390. doi: 10.1016/j.anpedi.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kenmoe S., Kengne-Nde C., Ebogo-Belobo J.T., Mbaga D.S., Fatawou Modiyinji A., Njouom R. Systematic review and meta-analysis of the prevalence of common respiratory viruses in children < 2 years with bronchiolitis in the pre-COVID-19 pandemic era. PLoS One. 2020;15 doi: 10.1371/journal.pone.0242302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meissner H.C. Viral bronchiolitis in children. N Engl J Med. 2016;374:62–72. doi: 10.1056/NEJMra1413456. [DOI] [PubMed] [Google Scholar]
- 10.Bloom-Feshbach K., Alonso W.J., Charu V., Tamerius J., Simonsen L., Miller M.A., et al. Latitudinal variations in seasonal activity of influenza and respiratory syncytial virus (RSV): a global comparative review. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fricke L.M., Glöckner S., Dreier M., Lange N. Impact of non-pharmaceutical interventions targeted at COVID-19 pandemic on influenza burden - a systematic review. J Infect. 2021;82:1–35. doi: 10.1016/j.jinf.2020.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trenholme A., Webb R., Lawrence S., Arrol S., Taylor S., Ameratunga S., et al. COVID-19 and infant hospitalizations for seasonal respiratory virus infections, New Zealand, 2020. Emerg Infect Dis. 2021;27:641–643. doi: 10.3201/eid2702.204041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huh K., Jung J., Hong J., Kim M., Ahn J.G., Kim J.H., Kang J.M. Impact of non-pharmaceutical interventions on the incidence of respiratory infections during the Coronavirus Disease 2019 (COVID-19) outbreak in Korea: A nationwide surveillance study. Clin Infect Dis. 2021;72:e184–e191. doi: 10.1093/cid/ciaa1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nascimento M.S., Baggio D.M., Fascina L.P., do Prado C. Impact of social isolation due to COVID-19 on the seasonality of pediatric respiratory diseases. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedrich F., Ongaratto R., Scotta M.C., Veras T.N., Stein R., Lumertz M.S., et al. Early impact of social distancing in response to COVID-19 on hospitalizations for acute bronchiolitis in infants in Brazil. Clin Infect Dis. 2021;72:2071–2075. doi: 10.1093/cid/ciaa1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeoh D.K., Foley D.A., Minney-Smith C.A., Martin A.C., Mace A.O., Sikazwe C.T., et al. The impact of COVID-19 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin Infect Dis. 2020;(September) doi: 10.1093/cid/ciaa1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molina Gutiérrez M.Á., Ruiz Domínguez J.A., Bueno Barriocanal M., de Miguel Lavisier B., López López R., Martín Sánchez J., et al. Impacto de la pandemia COVID-19 en urgencias: primeros hallazgos de un hospital de Madrid. An Pediatr (Barc) 2020;93:313–322. doi: 10.1016/j.anpedi.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bover-Bauza C., Rosselló Gomila M.A., Díaz Pérez D., Millán Pons A.R., Gil Sánchez J.A., Peña-Zarza J.A., et al. The Impact of the SARS-CoV-2 pandemic on the emergency department and management of the pediatric asthmatic patient. J Asthma Allergy. 2021;14:101–108. doi: 10.2147/JAA.S284813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang F., Yu L., Qin D., Hua F., Song G. Online consultation and emergency management in paediatric dentistry during the COVID-19 epidemic in Wuhan: a retrospective study. Int J Paediatr Dent. 2021;31:5–11. doi: 10.1111/ipd.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Somekh I., Somech R., Pettoello-Mantovani M., Somekh E. Changes in routine pediatric practice in light of Coronavirus 2019 (COVID-19) J Pediatr. 2020;224:190–193. doi: 10.1016/j.jpeds.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McConnochie K.M. Bronchiolitis. What’s in the name? Am J Dis Child. 1983;137:11–13. [PubMed] [Google Scholar]
- 22.Wilder J.L., Parsons C.R., Growdon A.S., Toomey S.L., Mansbach J.M. Pediatric hospitalizations during the COVID-19 pandemic. Pediatrics. 2020;146 doi: 10.1542/peds.2020-005983. e2020005983. [DOI] [PubMed] [Google Scholar]
- 23.Hatoun J., Correa E.T., Donahue S.M.A., Vernacchio L. Social distancing for COVID-19 and diagnoses of other infectious diseases in children. Pediatrics. 2020;146 doi: 10.1542/peds.2020-006460. e2020006460. [DOI] [PubMed] [Google Scholar]
- 24.Gavish R., Krause I., Goldberg L., Bilavsky E., Kadmon G., Livni G., et al. A drop in number of hospitalizations among children with bacterial infections during the COVID-19 pandemic. Pediatr Infect Dis J. 2021;40:e39–e41. doi: 10.1097/INF.0000000000002963. [DOI] [PubMed] [Google Scholar]
- 25.Chavasse R.J. Covid-19: reduced asthma presentations in children. BMJ. 2020;370(July) doi: 10.1136/bmj.m2806. [DOI] [PubMed] [Google Scholar]
- 26.Gavish R., Levinsky Y., Dizitzer Y., Bilavsky E., Livni G., Pirogovsky A., et al. The COVID-19 pandemic dramatically reduced admissions of children with and without chronic conditions to general paediatric wards. Acta Paediatr. 2021;4 doi: 10.1111/apa.15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Brusselen D., De Troeyer K., Ter Haar E., Vander Auwera A., Poschet K., Van Nuijs S., et al. Bronchiolitis in COVID-19 times: a nearly absent disease? Eur J Pediatr. 2021;180:1969–1973. doi: 10.1186/s13031-020-00281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guedj R., Lorrot M., Lecarpentier T., Leger P.L., Corvol H., Carbajal R. Infant bronchiolitis dramatically reduced during the second French COVID-19 outbreak. Acta Paediatr. 2021;110:1297–1299. doi: 10.1111/apa.15780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jefferson T., Foxlee R., Del Mar C., Dooley L., Ferroni E., Hewak B., et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ. 2008;336:77–80. doi: 10.1136/bmj.39393.510347.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker R.E., Park S.W., Yang W., Vecchi G.A., Metcalf C.J.E., Grenfell B.T. The impact of COVID-19 nonpharmaceutical interventions on the future dynamics of endemic infections. Proc Natl Acad Sci U S A. 2020;117:30547–30553. doi: 10.1073/pnas.2013182117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt S.J., Barblan L.P., Lory I., Landolt M.A. Age-related effects of the COVID-19 pandemic on mental health of children and adolescents. Eur J Psychotraumatol. 2021;12 doi: 10.1080/20008198.2021.1901407. 1901407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Osta A., Alaa A., Webber I., Riboli Sasco E., Bagkeris E., Millar H., et al. How is the COVID-19 lockdown impacting the mental health of parents of school-age children in the UK? A cross-sectional online survey. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-043397. e043397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dee K., Goldfarb D.M., Haney J., Amat J.A.R., Herder V., Stewart M., et al. Human rhinovirus infection blocks SARS-CoV-2 replication within the respiratory epithelium: implications for COVID-19 epidemiology. J Infect Dis. 2021;224:31–38. doi: 10.1093/infdis/jiab147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.COVID-19 weekly surveillance in NSWE. NSW GOVERNMENT. [Consulted April 2021]. Available at: https://www.health.nsw.gov.au/Infectious/covid-19/Documents/covid-19-surveillance-report-20210102.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.