Abstract
Background
Young adults are now considered major spreaders of coronavirus disease 2019 (COVID-19) disease. Although most young individuals experience mild to moderate disease, there are concerns of long-term adverse health effects. The impact of COVID-19 disease and to which extent population-level immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) exists in young adults remain unclear.
Objective
We conducted a population-based study on humoral and cellular immunity to SARS-CoV-2 and explored COVID-19 disease characteristics in young adults.
Methods
We invited participants from the Swedish BAMSE (Barn [Children], Allergy Milieu, Stockholm, Epidemiology) birth cohort (age 24-27 years) to take part in a COVID-19 follow-up. From 980 participants (October 2020 to June 2021), we here present data on SARS-CoV-2 receptor-binding domain–specific IgM, IgA, and IgG titers measured by ELISA and on symptoms and epidemiologic factors associated with seropositivity. Further, SARS-CoV-2–specific memory B- and T-cell responses were detected for a subpopulation (n = 108) by ELISpot and FluoroSpot.
Results
A total of 28.4% of subjects were seropositive, of whom 18.4% were IgM single positive. One in 7 seropositive subjects was asymptomatic. Seropositivity was associated with use of public transport, but not with sex, asthma, rhinitis, IgE sensitization, smoking, or body mass index. In a subset of representative samples, 20.7% and 35.0% had detectable SARS-CoV-2 specific B- and T-cell responses, respectively. B- and T-cell memory responses were clearly associated with seropositivity, but T-cell responses were also detected in 17.2% of seronegative subjects.
Conclusions
Assessment of IgM and T-cell responses may improve population-based estimations of SARS-CoV-2 infection. The pronounced surge of both symptomatic and asymptomatic infections among young adults indicates that the large-scale vaccination campaign should be continued.
Key words: SARS-CoV-2, COVID-19 disease, IgM, IgA, IgG, memory T cells, memory B cells, young adults, population-based cohort, asthma, risk factors
Abbreviations used: BAMSE, Barn (Children), Allergy Milieu, Stockholm, Epidemiology; COVID-19, Coronavirus disease 2019; PBMC, Peripheral blood mononuclear cell; RBD, Receptor-binding domain; S N M O, Spike protein (S), nucleoprotein (N), membrane protein (M), and open reading frame (ORF)-3a and ORF-7a proteins (O) peptide pool; S1, Spike 1 scanning peptide pool; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), poses a severe threat to public health worldwide. In Sweden, more than 1 million cases and 14,729 deaths have been confirmed up to September 15, 2021. Recently the death rate has decreased, mainly as a result of the vaccination campaign. Still, the number of patients in intensive care units remains at relatively high levels, which may reflect an increased general spread of the infection that is potentially due to more transmissible new variants. Young adults have been considered to be major spreaders of disease since last autumn.1 Even though the risk for severe illness and mortality increases with age, it is feared that the number of younger adults requiring intensive care may increase. Furthermore, there are also concerns for long-term adverse health effects in infected individuals, including young adults. The impact of COVID-19 disease and to what extent population-level immunity against SARS-CoV-2 exists in young adults are currently unclear—and are of particular interest because restrictions and recommendations suggested by the World Health Organization (WHO) to reduce disease spread was implemented rather late in Sweden, so throughout the pandemic, schools have remained open, and no formal lockdown was enforced.
SARS-CoV-2 infection elicits robust humoral and cellular adaptive immune responses to 4 major structural proteins encoded in the viral genome—the spike (S) protein, nucleoprotein (N), membrane (M) protein, and envelope (E) protein—as well as in several other minor proteins.2, 3, 4 T-cell responses against SARS-CoV-2 have been described in mild or asymptomatic cases, also without seroconversion.5 , 6 However, the prevalence of SARS-CoV-2 cellular memory responses at the population level is not well studied, especially among young adults.
We therefore conducted a population-based study on the presence of humoral and cellular immunity to SARS-CoV-2 and explored disease characteristics in young adults (age 24-27 years). We invited participants from the ongoing BAMSE (Swedish acronym for Barn [Children], Allergy Milieu, Stockholm, Epidemiology) birth cohort study7 , 8 to participate in a COVID-19 follow-up. We here present data on SARS-CoV-2 receptor-binding domain (RBD)-specific IgM, IgA, and IgG titers and on symptoms and epidemiologic factors associated with seropositivity in all unvaccinated participants (October 2020 to June 2021; n = 980). In addition, we present data on memory B- and T-cell responses in 108 unvaccinated participants (October 2020 to January 2021).
Methods
Study population, study design, and variables
The study population included participants from the prospective birth cohort BAMSE, which originally included 4089 newborns born between 1994 and 1996.8 A 24-year follow-up was conducted from 2016 to 2019, with a total of 2271 participants attending a clinical examination.7 These participants were invited to an ongoing COVID-19 follow-up for which a phase 1 web-based questionnaire was answered August to November 2020 (n = 1645). A total of 1453 of 1645 subjects were invited to the study’s phase 2 (start October 6, 2020), which included a clinical examination and a new web-based questionnaire.
From the phase 1 and phase 2 questionnaires, we extracted self-reported data on COVID-19–related symptoms, SARS-CoV-2 PCR and/or antibody tests, household members with COVID-19, use of public transport, face mask use, interactions at work, asthma diagnosis, and rhinitis.9 The presence of symptoms was evaluated by the question “Have you had symptoms of suspected COVID-19” and follow-up questions including type and duration of symptoms and being bedbound or hospitalized. Weight and body fat percentage were measured by an MC 780 body composition monitor (Tanita, Tokyo, Japan), and body mass index was calculated from measured weight and height.
PCR and antibody tests were reported through the questions “When was the last time you performed a nose- or throat test” with the follow-up question “What was the result?” (PCR); and “When was the last time you performed a blood test” with the follow-up question “What was the result?” (antibody test). Positive PCR tests (primarily) and positive antibody tests (secondary) were used to estimate the time in months that elapsed between presumed COVID-19 disease and clinical examination.
Data on IgE sensitization was obtained from the 24-year cohort follow-up. Further details are available in the Methods section in this article’s Online Repository at www.jacionline.org.
The present study included all 1028 participants who completed the clinical examination (October 6, 2020, to June 23, 2021), resulting in a study population of 1011 subjects after exclusion of subjects with insufficient sample material (n = 17). A total of 980 unvaccinated subjects were included in the main study population and 31 vaccinated subjects in a subanalysis (see Fig E1 in this article’s Online Repository at www.jacionline.org). The study was approved by the Swedish ethical review authority (approval 2020-02922). Participants provided written informed consent.
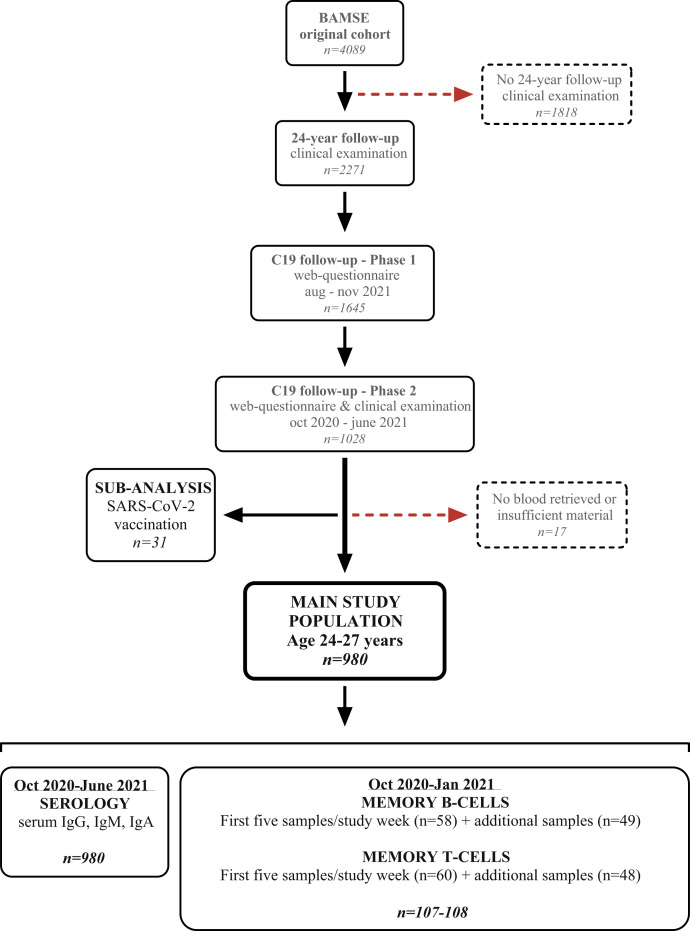
Fig E1.
Flowchart of the study protocol. From the BAMSE original cohort (n = 4089), we invited all subjects who participated in the clinical phase of the 24-year follow-up to the COVID-19 follow-up (n = 2270). Of these, 1644 participants answered the phase 1 web-based questionnaire and were then invited to the clinical examination. Of the 1026 participants who attended the COVID-19 clinical examination, 980 subjects constituted the main study population, whereas 32 vaccinated subjects were grouped for subanalysis. Fourteen subjects were excluded after the clinical examination as a result of lack of or insufficient sample material.
Sample preparation
Venous blood was collected in serum and sodium heparin tubes (BD Vacutainer; Becton Dickinson, San Diego, Calif) at the site of clinical examination (Södersjukhuset, Stockholm, Sweden). Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood samples by density centrifugation with Lymphoprep (STEMCELL Technologies, Vancouver, British Columbia, Canada). PBMCs were cryopreserved in 90% fetal bovine serum + 10% dimethyl sulfoxide and stored in liquid nitrogen. Serum was stored at −20°C.
Detection of anti–SARS-CoV-2 antibodies
Serum titers of anti–SARS-CoV-2 antibodies were measured for the whole study population (980 unvaccinated subjects and 31 vaccinated subjects). Serum samples were diluted 1:400, and levels of anti-RBD IgM, IgA, and IgG antibodies were determined by an in-house ELISA as previously described.4 In-house standards made by pooled highly positive serum were calibrated by using the WHO international standard for anti–SARS-CoV-2 immunoglobulin (NIBSC, 20/136). One arbitrary unit (AU) per milliliter of in-house serum standard equaled 7.55 AU/mL IgM, 46.4 AU/mL IgA, and 1.03 AU/mL IgG of WHO international standards, respectively. Cutoff values for antibody positivity were determined on the basis of receiver operating characteristic curves with data from convalescent COVID-19 patients and 108 negative historical control samples (outside BAMSE).4 The value with the highest sensitivity and a specificity of at least 99% was selected as the cutoff for each isotype: 14.42 AU/mL for IgM, 2.61 AU/mL for IgA, and 25.09 AU/mL for IgG.
Detection of SARS-CoV-2–specific memory B- and T-cell responses
SARS-CoV-2–specific memory B- and T-cell responses were analyzed for the first 5 samples collected per study week (n = 58-60, October 2020 to January 2021) and for an additional 49 subjects from the same time period to increase group sizes. The number of B cells secreting SARS-CoV-2 RBD-specific IgG was measured using the Human IgG SARS-CoV-2 RBD ELISpotPLUS kit (Mabtech AB, Cincinnati, Ohio), and the numbers of spike 1 scanning peptide pool (S1) and spike protein/nucleo protein/membrane protein/open reading frame (ORF-3a and ORF-7a) proteins (S N M O) peptide pool–specific IFN-γ– and IL-2–secreting T cells were detected using the Human IFN-γ/IL-2 SARS-CoV-2 FluoroSpotPLUS kit (Mabtech AB) (see this article’s Online Repository for details).4 The results are expressed as the number of spots per 300,000 cells, after subtracting the background. The cutoff values were set at the highest number of spots detected in 11 prepandemic PBMC control samples.4
Statistical analyses and graphical presentation
The chi-square test, the Fisher exact test, or multiple logistic regression was used for categorical data and the Mann-Whitney U test or the Kruskal-Wallis test was used for continuous data. For multiple comparisons, the Dunn test with Benjamini-Hochberg correction was used. Categorical data are presented as numbers and percentages, while continuous data are presented as medians and interquartile ranges. Spearman rank correlation was used for associations between 2 continuous variables. Statistical analysis was conducted by Stata 16.0 software (StataCorp, College Station, Tex). P < .05 was considered statistically significant. Graphical presentations were made by GraphPad Prism 9.1.0 (GraphPad Software, La Jolla, Calif) and RStudio 1.3.1093 (https://rstudio.com/) software.
Results
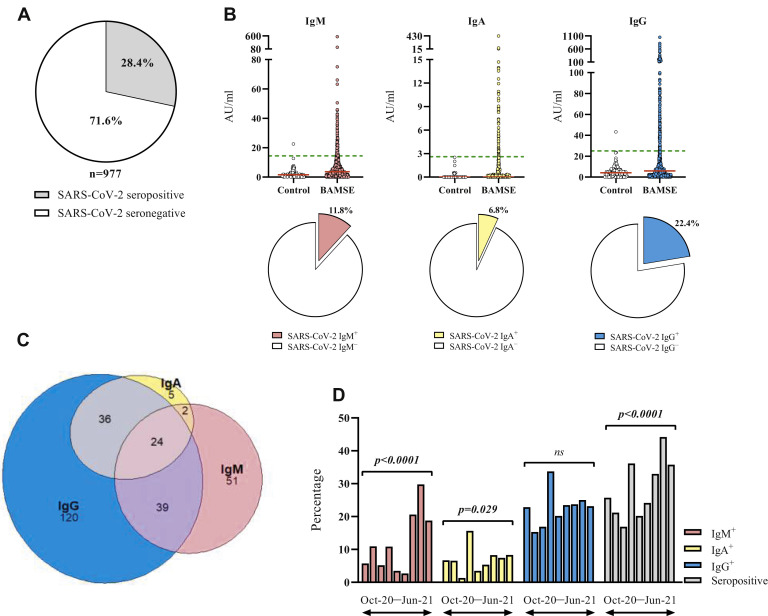
The study flowchart is presented in Fig E1. A total of 277 serum samples (28.4%) were positive for at least 1 SARS-CoV-2 anti-RBD antibody isotype (seropositive), while 700 samples (71.6%) were triple negative (Fig 1 , A). IgM, IgA, and IgG antibodies were detected in 11.8%, 6.8%, and 22.4% of samples, respectively (Table I ). Individual data from BAMSE participants and prepandemic control samples4 are displayed in Fig 1, B. Among seropositive samples, 8.7% were triple positive and 27.8% were positive for 2 isotypes (14.1% IgM+IgG+, 13.0% IgA+IgG+, 0.7% IgM+IgA+). The remaining samples were single positive (18.4% IgM+, 1.8% IgA+, 43.3% IgG+) (Table I, Fig 1, C). Analysis of seroprevalence by month revealed a notable increase in the proportion of IgG+ and IgA+ subjects in January 2021 (33.7% and 15.7%), which is also reflected in a corresponding peak in the overall seropositivity (Fig 1, D). The proportion of IgM+ subjects notably increased during April to June 2021, and the overall seropositivity per month increased during the study period (Fig 1, D). Taken together, more than 1 in 4 young adult participants were seropositive, and assessment of IgM in addition to IgG can better estimate the prevalence of infection.
Fig 1.
SARS-CoV-2 anti-RBD IgM, IgA, and IgG prevalence and titers. (A) The proportions of SARS-CoV-2 seropositive and seronegative subjects. (B) The titers of SARS-CoV-2 anti-RBD IgM, IgA, and IgG in samples from historical controls collected before the pandemic (n = 108) and BAMSE participants (n = 980), expressed in arbitrary units, and prevalence of IgM, IgA, and IgG displayed as pie charts for the BAMSE participants. (C) Venn diagram showing the overlap of IgM, IgA, and IgG seropositivity. (D) The percentages of IgM+, IgA+, and IgG+ subjects for each study month. The chi-square test was used for statistical analysis. Red lines indicate median values; green lines, assay cutoff values.
Table I.
Proportions of SARS-CoV-2 anti-RBD IgM-, IgA-, and IgG-positive and -negative subjects
| Antibody | Result | No. (%) or n/N (%) | Antibody titer (AU/mL), median (IQR) |
|---|---|---|---|
| Anti-RBD | Positive | 277 (28.4) | NA |
| Negative | 700 (71.6) | ||
| Anti-RBD IgM | Positive | 116 (11.8) | 21.7 (14.5-568.4) |
| Negative | 864 (88.2) | 3.1 (0.0-14.4) | |
| Anti-RBD IgA | Positive | 67 (6.8) | 5.3 (2.7-429.4) |
| Negative | 913 (93.2) | 0.0 (0.0-2.6) | |
| Anti-RBD IgG | Positive | 219 (22.4) | 60.3 (25.9-1407.4) |
| Negative | 758 (77.6) | 3.7 (0.0-24.8) | |
| IgG+IgM+IgA+ | Positive/total | 24/277 (8.7) | NA |
| IgG+IgM+IgA− | Positive/total | 39/277 (14.1) | NA |
| IgG+IgM−IgA+ | Positive/total | 36/277 (13.0) | NA |
| IgG−IgM+IgA+ | Positive/total | 2/277 (0.7) | NA |
| IgG−IgM+IgA− | Positive/total | 51/277 (18.4) | NA |
| IgG−IgM−IgA+ | Positive/total | 5/277 (1.8) | NA |
| IgG+IgM−IgA− | Positive/total | 120/277 (43.3) | NA |
IQR, Interquartile range; NA, not applicable.
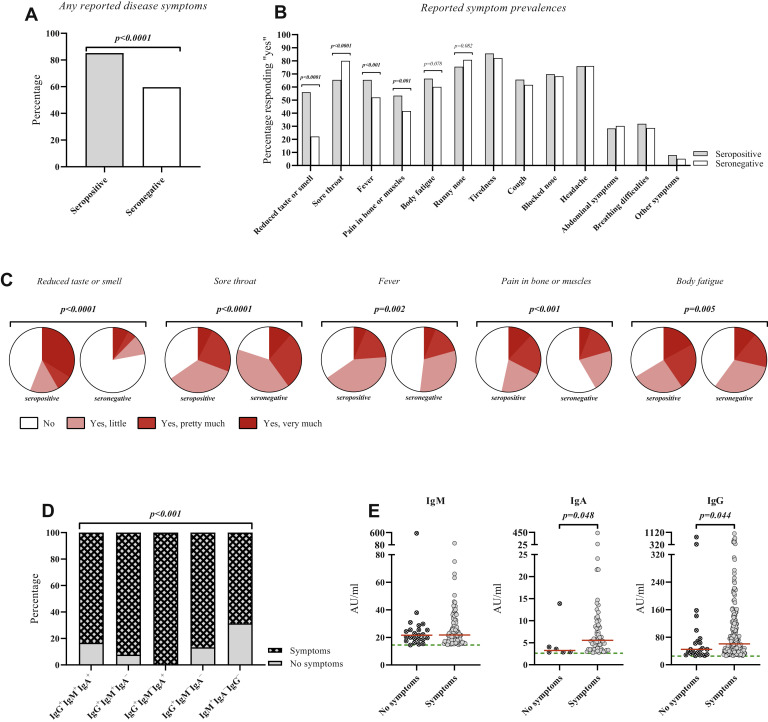
The seropositive and seronegative groups did not differ significantly by age, sex, asthma diagnosis, rhinitis, IgE sensitization, smoking, snuff use, body mass index, or body fat percentage (Table II ). A higher proportion of seropositive subjects reported suspected/confirmed COVID-19 disease in the household (P < .001), and small but significant differences were noted for regular use of public transport and face mask use (P < .05), while the frequencies of regular interactions with people at work was rather similar (Table II). The seropositive group more often reported having taken a PCR or antibody test (P < .0001, P = .010), and this group more often reported positive results from these tests (P < .0001) (Table II). Among seropositive and seronegative subjects, 85.2% and 59.6% reported at least 1 occasion with possible COVID-19–related symptoms between February 2020 and the clinical visit (P < .0001, Table III , Fig 2 , A). A total of 14.8% of seropositive subjects were asymptomatic during the whole study period. When seropositive subjects were divided into IgG+ and IgM+IgA−IgG−, we noticed a higher prevalence of asymptomatic subjects (P < .001) and a shorter duration of symptoms (P = .047) in the latter group (see Table E1 in this article’s Online Repository at www.jacionline.org).
Table II.
Background characteristics of SARS-CoV-2 seropositive and seronegative subjects
| Characteristic | Seropositive |
Seronegative |
P value∗ | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Age in years | 25.8 | 23.9-27.3 | 25.8 | 24.0-27.2 | ns |
| BMI | 22.9 | 15.2-48.3 | 22.5 | 14.8-59.9 | ns |
| Body fat percentage | 23.9 | 18.9-28.4 | 22.8 | 17.7-27.2 | ns |
| Seropositive |
Seronegative |
P value† | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Sex | |||||
| Female | 177 | 63.9 | 428 | 61.2 | ns |
| Male | 100 | 36.1 | 271 | 38.8 | |
| Asthma | |||||
| Yes | 37 | 13.4 | 100 | 14.3 | ns |
| No | 240 | 86.6 | 600 | 85.7 | |
| Rhinitis | |||||
| Yes | 45 | 16.2 | 103 | 14.8 | ns |
| No | 232 | 83.8 | 595 | 85.2 | |
| IgE-sensitization | |||||
| Yes | 111 | 40.2 | 313 | 45.0 | ns |
| No | 165 | 59.8 | 382 | 55.0 | |
| Smoking | |||||
| Yes | 39 | 14.2 | 78 | 11.2 | ns |
| No | 236 | 85.8 | 619 | 88.8 | |
| Snuff | |||||
| Yes | 67 | 24.4 | 157 | 22.5 | ns |
| No | 208 | 75.6 | 541 | 77.5 | |
| BMI categories‡ | |||||
| Overweight/obese | 75 | 28.2 | 157 | 23.1 | ns |
| No overweight | 191 | 71.8 | 526 | 76.9 | |
| COVID-19 in household§ | |||||
| Feb 2020-Jul 2020 | |||||
| Yes/No/Not sure | 94/167/14 | 34.2/60.7/5.1 | 125/519/56 | 17.9/74.1/8.0 | <.0001 |
| Aug 2020-June 2021 | |||||
| Yes/No/Not sure | 84/181/8 | 30.8/66.3/2.9 | 103/578/17 | 14.8/82.8/2.4 | <.0001 |
| Do you use face mask? | |||||
| Yes, often | 35 | 12.7 | 102 | 14.6 | .031 |
| Yes, sometimes | 162 | 57.9 | 346 | 49.6 | |
| No | 78 | 28.4 | 249 | 35.7 | |
| Regular interactions with people at work | |||||
| Feb 2020-Jul 2020 | |||||
| Yes/No | 204/73 | 73.6/26.4 | 497/203 | 71.0/29.0 | ns |
| Aug 2020-June 2021 | |||||
| Yes/No | 211/64 | 76.7/23.3 | 491/207 | 70.3/29.7 | .045 |
| Regular use of public transport to work | |||||
| Feb 2020-Jul 2020 | |||||
| Yes/No | 90/187 | 32.5/67.5 | 176/524 | 25.1/74.9 | .020‖ |
| Aug 2020-June 2021 | |||||
| Yes/No | 91/184 | 33.1/66.9 | 174/523 | 25.0/75.0 | .010¶ |
| PCR-test performed prior to clinical examination (self-reported) | |||||
| No | 112 | 40.4 | 378 | 54.0 | <.0001 |
| Yes | 165 | 59.6 | 322 | 460 | |
| Positive | 86 | 52.1 | 14 | 4.4 | <.0001 |
| Negative | 79 | 47.9 | 308 | 95.6 | |
| Antibody-test performed prior to clinical examination (self-reported) | |||||
| No | 171 | 61.7 | 492 | 70.3 | .010 |
| Yes | 106 | 38.3 | 208 | 29.7 | |
| Positive | 59 | 55.7 | 16 | 7.7 | <.0001 |
| Negative | 47 | 44.3 | 192 | 92.3 | |
BMI, Body mass index; IQR, interquartile range.
Mann-Whitney U-test.
Chi-square test or Fisher exact test.
Overweight/obese defined as BMI ≥25 kg/m2, no overweight defined as BMI <25 kg/m2.
Suspected or confirmed.
Corresponding odds ratio 1.5 (95% CI, 1.1-2.0) for regular use of public transport to work during phase 1 after adjustment for age, sex, smoking, having regular interactions at work and occupation.
Corresponding odds ratio 1.4 (95% CI, 1.0-1.9) for regular use of public transport to work during phase 2 after adjustment for age, sex, smoking, face mask usage, having regular interactions at work and occupation.
Table III.
Self-reported symptom and disease characteristics reported by SARS-CoV-2 seropositive and seronegative subjects
| Characteristic | Result | Seropositive |
Seronegative |
P value∗ | ||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Any COVID-19–related symptoms between February 2020 and June 2021 | Yes | 236 | 85.2 | 415 | 59.6 | <.0001 |
| No | 41 | 14.8 | 281 | 40.4 | ||
| Disease symptoms experienced more than once between February 2020 and June 2021 | More than once | 78 | 33.1 | 139 | 33.5 | NS |
| Once | 158 | 66.9 | 276 | 66.5 | ||
| Total number of occasions with symptoms | 314 | 554 | NA | |||
| Bedbound during disease | Yes, ≥7 days | 25 | 8.0 | 26 | 4.7 | .001 |
| Yes, <7 days | 137 | 43.8 | 191 | 34.6 | ||
| No | 151 | 48.2 | 335 | 60.7 | ||
| Hospitalization | Yes | 3 | 1.0 | 3 | 0.5 | NS |
| No | 310 | 99.0 | 549 | 99.5 | ||
| When were symptoms experienced? | Feb 20 | 13 | 4.2 | 52 | 9.4 | NA |
| Mar 20 | 56 | 18.0 | 124 | 22.5 | ||
| Apr 20 | 38 | 12.2 | 51 | 9.2 | ||
| May 20 | 24 | 7.7 | 9 | 1.6 | ||
| Jun 20 | 16 | 5.1 | 14 | 2.5 | ||
| Jul 20 | 7 | 2.3 | 22 | 4.0 | ||
| Aug 20 | 7 | 2.3 | 41 | 7.4 | ||
| Sep 20 | 19 | 6.1 | 56 | 10.1 | ||
| Oct 20 | 30 | 9.6 | 52 | 9.4 | ||
| Nov 20 | 31 | 10.0 | 45 | 8.2 | ||
| Dec 20 | 23 | 7.4 | 31 | 5.6 | ||
| Jan 21 | 11 | 3.5 | 19 | 3.4 | ||
| Feb 21 | 7 | 2.3 | 14 | 2.5 | ||
| Mar 21 | 17 | 5.5 | 12 | 2.2 | ||
| Apr 21 | 9 | 2.9 | 8 | 1.5 | ||
| May 21 | 3 | 1.0 | 2 | 0.4 | ||
| Duration of symptoms | <1 week | 82 | 26.2 | 160 | 28.9 | NS |
| 1 week | 89 | 28.4 | 164 | 29.7 | ||
| 2 weeks | 84 | 26.8 | 138 | 25.0 | ||
| 3 weeks | 27 | 8.6 | 44 | 8.0 | ||
| 4 weeks | 11 | 3.5 | 19 | 3.4 | ||
| 5-7 weeks | 8 | 2.6 | 9 | 1.6 | ||
| ≥8 weeks | 12 | 3.8 | 19 | 3.4 | ||
NA, Not applicable; NS, not statistically significant.
Chi-square test or Fisher exact test.
Fig 2.
Symptom prevalence and characteristics. (A) The proportions of SARS-CoV-2 seropositive and seronegative subjects who reported at least 1 occasion with symptoms throughout the study. (B and C) The proportions of SARS-CoV-2 seropositive and seronegative subjects reporting to have experienced the indicated symptoms (B) and grading of symptom severity (C). (D) The proportion of seropositive subjects who reported at least 1 occasion with symptoms throughout the study stratified by antibody profiles. (E) The titers of IgM, IgA, and IgG, expressed in arbitrary units, in symptomatic and asymptomatic SARS-CoV-2 seropositive subjects. Fisher exact test, chi-square test, or Mann-Whitney U test was used for statistical analysis. Red lines indicate median values; green lines, assay cutoff values.
One-third of seronegative and seropositive symptomatic subjects reported symptoms more than once, resulting in a total of 314 and 554 occasions with symptoms, respectively. The seropositive group more often reported having been bedbound because of their symptoms (51.8% vs 39.3%) (P < .001), while hospitalization was rare in both groups (Table III). Symptom duration did not differ (Table III), while seropositive subjects significantly more often reported reduced taste and smell (P < .0001), fever (P < .001), and pain in bone and muscles (P = .001), but less often sore throat (P < .0001) (Fig 2, B; see Table E2 in this article’s Online Repository at www.jacionline.org). After rating the symptoms, the most notable observation was the high impact on taste and smell in the seropositive group (Fig 2, C, see Table E3 in this article’s Online Repository). The proportion of subjects who reported at least 1 occasion with symptoms significantly differed among seropositive subjects with different antibody profiles (P < .001) (Fig 2, D). Among seropositive subjects, positive titers of IgA and IgG, but not IgM, were significantly higher in the symptomatic group (P = .048, P = .044) (Fig 2, E). Overall, seropositive subjects reported significantly more often to have been bedbound; they reported substantial effects on taste and smell; and 1 in 7 was asymptomatic.
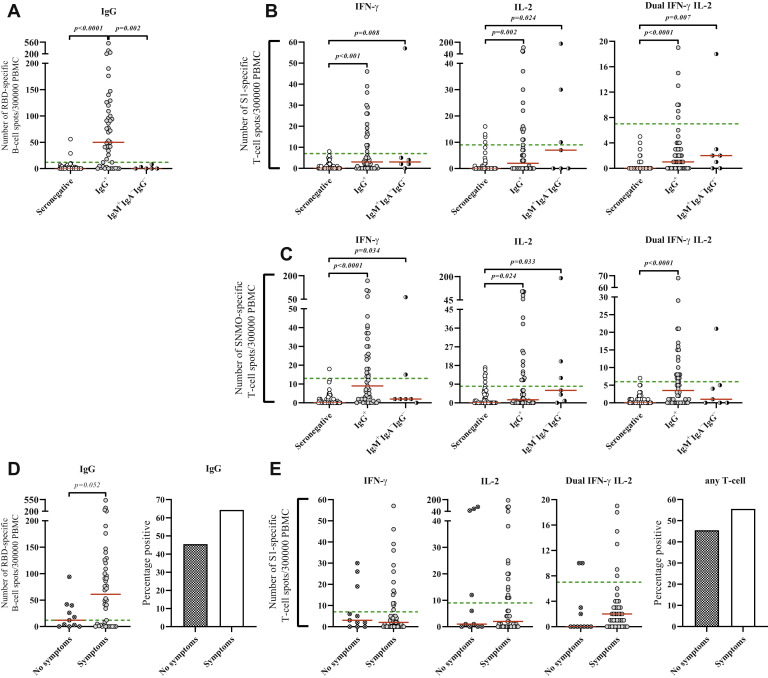
We then investigated SARS-CoV-2–specific memory B- and T-cell responses by ELISpot and FluoroSpot assays, respectively. We first analyzed cellular immunity on a population-based level by restricting the analyses to the first 5 samples collected during each week of the first 4 months of the study period. Among these 60 samples, 30.0%, 20.7%, and 35.0% were positive for specific antibodies and B- and T-cell responses, respectively. The proportions of subjects with positive memory T-cell responses were 66.7% and 21.4% among seropositive and seronegative subjects, respectively, and for memory B-cell responses, the corresponding numbers were 58.8% and 4.9%.
To increase sample size, we included additional samples from seropositive (n = 31) and seronegative (n = 17) subjects, resulting in a total of 107 and 108 samples analyzed for B- and T-cell responses, respectively. The IgG+ group had a significantly higher proportion of subjects with positive memory B-cell response (68.1%), a higher median number of IgG-producing B cells (50 spot-forming cells per 300,000 PBMC), and a higher proportion of individuals with at least 1 S1- or S N M O-specific IFN-γ and/or IL-2 T-cell response (55.1%) compared to the IgM+IgA−IgG− group (positive B cells, 0; positive T cells, 42.9%) and the seronegative group (positive B cells, 3.7%; positive T cells, 17.3%) (P < .05 for all parameters) (Table IV , Fig 3 , A). Further investigation of S1- or S N M O-specific IFN-γ T-cell responses revealed similar patterns regarding both the proportion of positive subjects and the median number of spot-forming cells (P < .05 for all parameters) (Table IV, Fig 3, B and C). Of note, IgM+IgA−IgG− and seronegative subjects who were positive for memory T-cell responses mainly produced IL-2 (Table IV, Fig 3, B and C). The number of IgG-producing B cells and the proportion of subjects with a detectable memory B-cell response were higher in the symptomatic group, although this was not statistically significant (Fig 3, D). T-cell responses were not associated with the presence of symptoms (Fig 3, E; similar results obtained for S N M O are not shown).
Table IV.
SARS-Cov-2 memory B- and T-cell responses among SARS-CoV-2 IgG+, IgM+IgA−IgG−, and seronegative subjects
| Characteristic | IgG+ |
IgM+IgA−IgG− |
Seronegative |
P value† | |||
|---|---|---|---|---|---|---|---|
| N/n (%) | Median (IQR)∗ | n/N (%) | Median (IQR)∗ | n/N (%) | Median (IQR)∗ | ||
| B-cell response | 32/47 (68.1) | 50 (4-109) | 0/6 (0.0) | 0 (0-3) | 2/54 (3.7) | 0 (0-2) | <.0001/<.001 |
| T-cell response (any) | 27/49 (55.1) | NA | 3/7 (42.9) | NA | 9/52 (17.3) | NA | <.0001/NA |
| T-cell response S1 | 21/49 (42.9) | NA | 3/7 (42.9) | NA | 5/52 (9.6) | NA | .001/NA |
| IFN-γ | 16/49 (32.7) | 3 (0-11) | 1/7 (14.3) | 2 (2-5) | 1/52 (1.9) | 0 (0-1) | <.0001/<.001 |
| IL-2 | 14/49 (28.6) | 1 (0-11) | 3/7 (42.9) | 7 (0-30) | 4/52 (7.7) | 0 (0-0.5) | .008/.010 |
| IFN-γ/IL-2 | 7/49 (14.3) | 1 (0-3) | 1/7 (14.3) | 2 (0-3) | 0/52 (0.0) | 0 (0-0) | .018/.0001 |
| T-cell response S N M O | 26/49 (53.1) | NA | 3/7 (42.9) | NA | 7/52 (13.5) | NA | <.0001/NA |
| IFN-γ | 23/49 (44.9) | 9 (2-26) | 2/7 (28.6) | 2 (2-15) | 1/52 (1.9) | 0 (0-2) | <.0001/<.001 |
| IL-2 | 17/49 (34.7) | 1 (0-14) | 3/7 (42.9) | 6 (1-20) | 6/52 (11.5) | 0 (0-4.5) | .012/.028 |
| IFN-γ/IL-2 | 17/49 (34.7) | 3 (0-8) | 1/7 (14.2) | 1 (0-5) | 1/52 (1.9) | 0 (0-1) | <.0001/<.001 |
IQR, Interquartile range; NA, not applicable.
Median number of positive cells.
Chi-square test/Kruskal-Wallis test.
Fig 3.
SARS-CoV-2 anti-RBD memory B-cell responses and anti-S1 and anti-S N M O memory T-cell responses. (A-C) The numbers of RBD-specific IgG+ B cells (A), S1-specific IFN-γ+, IL-2+, and IFN-γ+IL-2+ T cells (B), and S N M O-specific IFN-γ+, IL-2+ and IFN-γ+IL-2+ T cells (C) per 300,000 PBMCs within the SARS-CoV-2 IgG+, IgM+IgA−IgG−, and seronegative groups. (D and E) The numbers of RBD-specific IgG+ B cells (D) and S1-specific IFN-γ+, IL-2+, and IFN-γ+IL-2+ T cells (E). (F) The percentages of B-cell– and T-cell–positive subjects within the SARS-CoV-2 seropositive symptomatic and asymptomatic groups. Dunn test, Mann-Whitney U test, or Fisher exact test was used for statistical analysis. Red lines indicate median values; green lines, assay cutoff values.
Ten subjects had positive memory B- and/or T-cell responses within the seronegative group. Among these, 2 had a positive B-cell response, 1 of whom was borderline positive for all 3 antibody isotypes, was T-cell positive, reported a positive PCR test, and suspected COVID-19 in the household. Among the remaining 8 subjects with T-cell responses, 7 had IgG and/or IgM titers exceeding the median value in the seronegative group, none reported a positive PCR test, 3 reported suspected/confirmed COVID-19 in the household, and 5 reported a history of COVID-19–related symptoms (see Table E4 in this article’s Online Repository at www.jacionline.org). Taken together, SARS-CoV-2 B- and T-cell memory responses were detected in 68.1% and 55.1% of seropositive individuals, respectively. A memory B-cell response was almost exclusively associated with seropositivity, while memory T-cell responses were found in 1 in 5 seronegative subjects.
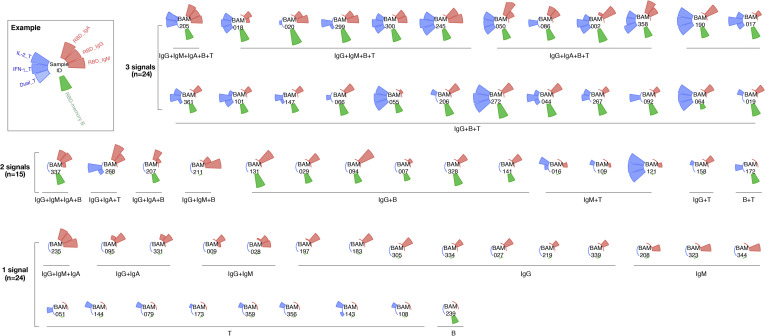
Data on all 3 arms of adaptive immunity (virus-specific antibodies, and memory B and T cells) were available for 104 subjects. Among these, 60.6% were positive for at least 1 investigated immune parameter with highly variable patterns, suggesting a high heterogeneity of adaptive immune responses in young adults (see Fig E2 in this article’s Online Repository at www.jacionline.org).
Fig E2.
Polar bar plot of SARS-CoV-2–specific adaptive immune responses for subjects with available data on all 3 arms of adaptive immunity (virus-specific antibodies, memory B and T cells). From top in a clockwise direction, the bars indicate the serum anti-RBD IgA, IgG, and IgM titers (red bar), the number of RBD-specific memory B cells (green bar), and the number of T cells (blue bar) specific for the virus protein–derived peptide pools S1 and S N M O that produce IFN-γ, IL-2, or both (Dual). The axis was scaled from 0 to 1 using minimum and maximum log-normalized values. The headers shown in the center of each plot are unrelated to the study participants’ identification numbers used within the BAMSE cohort and cannot be connected to any individual.
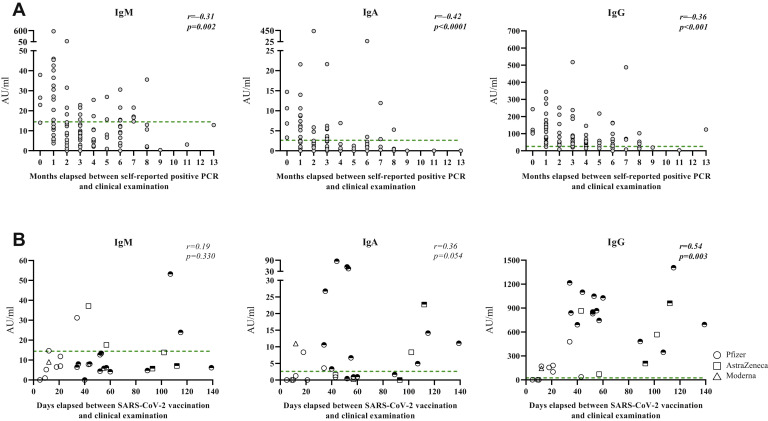
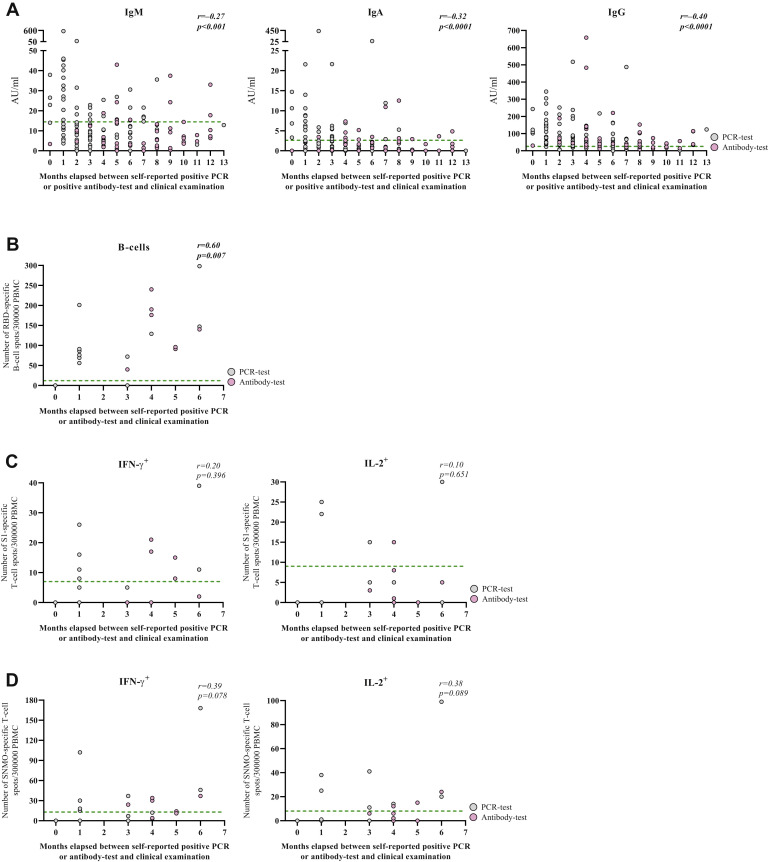
We correlated measured antibody titers and cellular responses at the time of clinical examination with the time in months that elapsed between presumed COVID-19 disease primarily on the basis of self-reported positive PCR test (n = 99 for antibody titers and n = 12-13 for cellular responses) and secondarily self-reported positive antibody test (additional n = 56 for antibody titers and n = 6-8 for cellular responses). Of note, the estimation that was based on self-reported positive antibody tests gives a minimum number of elapsed months. IgM, IgA, and IgG titers negatively correlated with time elapsed since presumed disease, regardless of whether the estimation was solely based on positive PCR test (Fig 4 , A, IgM: r = −0.31, P = .002; IgA: r = −0.42, P < .0001, IgG: r = −0.36, P < .001) or when combining positive PCR and antibody tests (see Fig E3, A, in this article’s Online Repository at www.jacionline.org). The number of IgG-producing B cells positively correlated with time elapsed since presumed disease (Fig E3, B, P = .007), while T-cell responses did not show clear associations (Fig E3, C and D); however, all investigated cellular responses, except S1-specific IL-2, were measurable 6 months after presumed disease.
Fig 4.
Correlation of antibody titers and elapsed time since presumed COVID-19 disease or SARS-CoV-2 vaccination. (A) Correlation between the number of months that elapsed between self-reported positive PCR test to clinical examination and the titers of anti–SARS-CoV-2 IgM, IgA, or IgG. (B) Correlation between the number of days that elapsed since the date of receiving dose 1 of a SARS-CoV-2 vaccine and the titers of anti–SARS-CoV-2 IgM, IgA, or IgG. Open symbols indicate 1 dose received; half-filled symbols, 2 doses received. Spearman rank correlation was used for statistical analysis. Green lines represent assay cutoff values.
Fig E3.
Correlation of antibody titer or cellular responses with the time in months that elapsed since presumed COVID-19 disease. (A) Correlation between the number of months that elapsed between self-reported positive PCR test or antibody test to clinical examination and the titers of anti–SARS-CoV-2 IgM, IgA or IgG. Gray circles indicate PCR test; purple circles, antibody test. (B-D) Correlation between the number of months that elapsed since presumed COVID-19 disease among seropositive subjects based on self-reported positive PCR test (gray circles) or antibody test (pink circles) and the numbers of RBD-specific IgG+ B cells (A), the numbers of S1-specific IFN-γ+ or IL-2+ T cells (B), and the numbers of S N M O-specific IFN-γ+ or IL-2+ T cells (C). Spearman rank correlation was used for statistical analysis. Green lines represent assay cutoff values.
Finally, we investigated antibody levels among 31 subjects who had received the first dose of a SARS-CoV-2 vaccine (AstraZeneca, n = 6; Moderna, n = 1; Pfizer, n = 24), 18 of 31 of whom had also received a second vaccine dose. Few vaccinated subjects were IgM+, while IgA and IgG responses were robust after the second dose (Fig 4, B).
Discussion
In this population-based study, we show that more than 1 in 4 young adults were SARS-CoV-2 seropositive, with a significant proportion of subjects being IgM single positive. Seropositivity was associated with COVID-19 disease in the household and weakly with use of public transport. Memory B- and T-cell responses were observed in the majority of IgG+ subjects, but T-cell responses were also observed in 1 in 5 seronegative subjects.
The proportion of seropositive subjects remained relatively stable October to December 2020, increased to over 30% in January 2021, declined during early spring, and increased again to above 40% close to the summer of 2021. The latter increase was mostly due to an increased number of IgM+ subjects, suggesting that we detected more cases of recent or ongoing infections during the late spring. These subjects may have been asymptomatic at the time of clinical examination, considering that the participants were asked not to attend if symptomatic. Whether this reflects a higher degree of asymptomatic infections due to virus variants during this time period or less cautiousness is not known. Data from the Swedish Public Health Agency collected late May to early June 2021 report the national IgG seroprevalence to be 52% for subjects aged 20 to 64 years; however, this heterogenous age group also includes a large proportion of vaccinated subjects.10 Our data suggest that the spread of infection was, and still is, substantial in young adults.
Our results indicate that measuring IgM likely captures a significant proportion of recent or ongoing SARS-CoV-2 infections. However, anti-RBD IgM was less frequently observed in acute and convalescent COVID-19 patients compared to IgG,11 and studies of anti–SARS-CoV-2 IgM indicate the longevity to be 4 months or less among symptomatic individuals.4 , 12 Nevertheless, our result suggests that using only IgG as a readout underestimates the prevalence of SARS-CoV-2 infection in the population, especially when the epidemic is resurgent.
In this cohort, seropositive subjects had more frequently used public transport and more often had household members with suspected or confirmed COVID-19 disease compared to seronegative subjects. These associations have been described by others.13 , 14 Notably, even though public transport areas are likely to contribute to the virus’s spread,15 we cannot conclude that participants were infected while using the public transport system because we do not have detailed data on virus spread. Also, we adjusted for potential confounders in the regression model (Table II), but we cannot exclude residual confounding effects (eg, contact with confirmed cases at work or at social events). In Sweden, public face mask use was not recommended until the beginning of 2021. We found a weak but significant inverse association between face mask use and seropositivity, although other observational studies have found no association.16 , 17 Of note, our study was not designed to evaluate the effect of face mask use or the use of public transport systems. Seroprevalence was not associated with asthma diagnosis—a finding similar to other reports.18 We observed mostly mild COVID-19 disease in young adults defined as not requiring hospital care, and fewer than 1 in 10 seropositive subjects experienced symptoms for more than 4 weeks. A high degree of lost taste and/or smell was the most evident symptom among seropositive subjects, in accordance with other data.19
SARS-CoV-2–specific cellular responses develop in most subjects with confirmed infection.3 , 4 We observed memory B- and T-cell responses in 68.1% and 55.1% of IgG+ subjects, respectively. This is somewhat different from other studies showing that memory B- and T-cell responses occur in more or less all subjects with confirmed COVID-19 infection.3 , 4 , 11 , 20 , 21 Of note, our participants were younger than included subjects in the abovementioned studies, had mild disease, and were sampled in a population-based manner. We have not investigated CD4 and CD8 T-cell responses separately, analyzed additional modes of activation besides cytokine expression, or performed intracellular cytokine staining.
One in 10 IgG+ subjects and 1 in 3 IgM single-positive subjects were reported to have been asymptomatic throughout the study; these results suggest that undetected infection may be a major public health issue in young adults. Data regarding associations between the magnitude and duration of immune responses and symptom severity during COVID-19 disease are conflicting.11 , 22, 23, 24 A connection between mild symptoms and lower T-cell responses has been suggested,20 while other studies have failed to find this association,4 , 5 , 22 and we and others have shown that specific T-cell responses are similar in symptomatic and asymptomatic subjects.25 , 26 Furthermore, IgG titers were significantly higher and the median number of memory B cells tended to be higher in symptomatic subjects—results also reported elsewhere.22 Others have shown that individuals with mild or severe COVID-19 disease mount comparable and equally durable memory B-cell responses.27
We found that almost 1 in 5 seronegative subjects tested positive in at least 1 T-cell assay. Studies report that 40% to 60% of nonexposed prepandemic subjects had SARS-CoV-2–reactive T cells, most likely as a result of cross-reactivity,20 and that cross-reactive T cells exist to a higher extent in young adults.28 However, the protective effect of such cross-reactive memory T cells to newly encountered infections like SARS-CoV-2 is unknown. Our data show that most seronegative subjects with positive T-cell responses have higher titers (though still below the cutoffs) of anti–SARS-CoV-2 antibodies than seronegative subjects without T-cell responses, suggesting that some subjects mount low antibody responses that are not detected by available assays. Because the full dynamics of the SARS-CoV-2 immune response are not understood, a missing overlap between seropositivity and cellular memory responses may be due to sampling timing. One recent review highlights an urgent need for the development of SARS-CoV-2 T-cell assays,29 and Sweden is now one of the first countries to offer T-cell tests to the community. Still, it is unclear how broadscale T-cell testing can increase the understanding of population immunity, guide restrictions, and behavioral recommendations or evaluate the vaccine response. Finally, the question on which T-cell assay would be the most appropriate to choose for broadscale testing of a variety of age groups remains to be addressed.
Several studies have assessed the maintenance of protective levels of antibodies and the durability of cellular responses to SARS-CoV-2,3 , 4 , 20 partly as a result of concerns regarding reinfections. Some studies suggest that antibody titers wane within a few months,22 , 30 while others show that titers are maintained at a relatively high level for at least 6 months after onset of symptoms.3 , 4 , 31 We used the results of self-reported PCR and antibody tests to make an assumption about when participants had COVID-19 disease. Our results suggest that even though the levels of antibodies declined, they were measurable up until 8 months after infection, and in some cases longer for IgG. For the 14 subjects who were seronegative at clinical examination but who reported a previous positive PCR test, 7 subjects had ≥6 months, while 1 subject had <1 month, elapse between presumed disease and clinical examination, and their IgG or IgM levels were measurable but below the assay cutoffs. Four subjects had 3 to 5 months in between presumed disease and clinical examination. Our data indicate that antibody titers decline more rapidly in some individuals, which may be worth commenting on in light of vaccination.
The strengths of this study are the population-based approach targeting young adults and the lengthy consecutive follow-up. We measured IgM and IgA in addition to IgG, as well as specific B- and T-cell responses. We included only unvaccinated subjects in our main analyses to gain knowledge about virus spread and risk factors, as well as knowledge about the level of adaptive immunity among young adults. Vaccination campaigns for this target age group are ongoing in Sweden, and our pilot analyses of 31 vaccinated participants showed adequate serologic responses. Limitations of this study include that we were only able to study mild COVID-19 disease, we lack information regarding exact disease dates for some subjects, and we investigated cellular memory responses in a relatively small group.
In summary, to our knowledge, this is the first population-based cohort study to investigate different arms of adaptive immunity against SARS-CoV-2 among young adults. Our study provides new information regarding COVID-19 disease characteristics and protective immunity among young, nonhospitalized adults.
Clinical implications.
In young adults, characterizing COVID-19 disease and measuring IgM and memory T-cell responses in addition to IgG improve estimations of SARS-CoV-2–specific immunity and create awareness of disease spread.
Acknowledgments
BAMSE COVID-19 study group members include Catarina Almqvist, Niklas Andersson, Natalia Ballardini, Anna Bergström, Sophia Björkander, Petter Brodin, Anna Castel, Sandra Ekström, Antonios Georgelis, Lennart Hammarström, Qiang Pan-Hammarström, Jenny Hallberg, Christer Jansson, Maura Kere, Inger Kull, André Lauber, Alexandra Lövquist, Erik Melén, Jenny Mjösberg, Ida Mogensen, Lena Palmberg, Göran Pershagen, Niclas Roxhed, and Jochen Schwenk.
We thank the children and parents participating in the BAMSE cohort and all staff involved in the study through the years.
Footnotes
The first 2 authors contributed equally to this article, and both should be considered first author. The last 2 authors contributed equally to this article, and both should be considered senior author.
The study was funded by the Swedish Research Council, the Swedish Heart-Lung Foundation, the European Union’s Horizon 2020 research and innovation program (ATAC, 101003650 and ERC no. 757919 TRIBAL), the Karolinska Institutet, and Region Stockholm (ALF project, and for cohort and database maintenance).
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Contributor Information
BAMSE COVID-19 study group:
Catarina Almqvist, Niklas Andersson, Natalia Ballardini, Anna Bergström, Sophia Björkander, Petter Brodin, Anna Castel, Sandra Ekström, Antonios Georgelis, Lennart Hammarström, Qiang Pan-Hammarström, Jenny Hallberg, Christer Jansson, Maura Kere, Inger Kull, André Lauber, Alexandra Lövquist, Erik Melén, Jenny Mjösberg, Ida Mogensen, Lena Palmberg, Göran Pershagen, Niclas Roxhed, and Jochen Schwenk
Methods
Extended definition of variables obtained from the questionnaires
From the phase 1 and phase 2 web-based questionnaires, we obtained information regarding type of symptoms: fever, cough, reduced taste or smell, sore throat, running nose, blocked nose, headache, joint or muscle pain, abdominal symptoms, breathing difficulties, tiredness, and body fatigue from the questions: “Has anyone in your household had suspected or confirmed COVID-19 during the pandemic/since August 1, 2020?,” “Do you use face mask?” (asked only during phase 2, when national face mask recommendations were issued), “Have you regularly met other people at work?,” and “What is your main use of transport to your daily work?” PCR and antibody tests were reported through the questions “When was the last time you performed a nose or throat test” with the follow-up question “What was the result?” (PCR); and “When was the last time you performed a blood test” with the follow-up question “What was the result?” (antibody test). Asthma was defined as doctor’s diagnosis (ever) together with symptoms of breathing difficulties or asthma medication occasionally or regularly in the last 12 months.E1 Rhinitis was defined as doctor’s diagnosis (ever) together with nasal or eye problems without having a cold in the last 12 months. The phase 1 questionnaire covers February 2020 until November 2020, and the phase 2 questionnaire covers August 1, 2020, until the clinical examination.E2
IgE sensitization was analyzed during the 24-year follow-up and was defined as ≥0.35 kU/L of soluble serum IgE to at least 1 of the tested allergens using ImmunoCAP System (Thermo Fisher Scientific, Waltham, Mass).E3
Detection of SARS-CoV-2–specific memory B- and T-cell responses
To detect SARS-CoV-2 RBD-specific IgG-secreting memory B cells, PBMCs were cultured for 4 days in RPMI 1640 medium with 10% fetal bovine serum and 1% penicillin–streptomycin, supplemented with 1 μg/mL R848 (resiquimod; a TLR7/8 agonist) and 10 ng/mL recombinant human IL-2. A total of 300,000 cells per well were then loaded onto ELISpot plates precoated with monoclonal anti-human IgG antibodies (Human IgG SARS-CoV-2 RBD ELISpotPLUS kit, Mabtech AB). IFN-γ– and IL-2–secreting T cells were detected using the Human IFN-γ/IL-2 SARS-CoV-2 FluoroSpotPLUS kit (Mabtech AB). A total of 300,000 PBMCs per well were added to FluoroSpot plates precoated with monoclonal anti–IFN-γ and anti–IL-2 antibodies together with the S1 scanning pool containing 16 peptides (#3629-1, Mabtech AB) or the S N M O–defined peptide pool containing 47 synthetic peptides binding to human HLA (#3620-1, Mabtech AB) and 100 ng/mL anti-CD28. The plates were incubated overnight and developed the following day according to the manufacturer’s protocol. ELISpot and FluoroSpot images and spot counts were obtained using an IRIS plate reader. The results are expressed as number of spots per 300,000 cells, after subtracting the background. The cutoff value was set at the highest number of spots detected in 11 prepandemic PBMC control samples.E4
Table E1.
Self-reported symptom and disease characteristics reported by SARS-CoV-2 IgG+ and IgM+IgA−IgG− subjects
| Characteristic | Variable | IgG+ |
IgM+IgA−IgG− |
P value∗ | ||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Any COVID-19 related symptoms between Feb 20 and June-21 | Yes | 196 | 89.5 | 35 | 68.6 | <.0001 |
| No | 23 | 10.5 | 16 | 31.4 | ||
| Disease symptoms experienced more than once between Feb 20 and June-21 | More than once | 64 | 32.7 | 14 | 40.0 | NS |
| Once | 132 | 67.3 | 21 | 60.0 | ||
| Total number of occasions with symptoms | 260 | 49 | NA | |||
| Bedbound during disease | Yes, ≥7 days | 23 | 8.8 | 1 | 2.1 | NS |
| Yes, 1-6 days | 114 | 43.8 | 21 | 43.8 | ||
| No | 123 | 47.3 | 26 | 54.2 | ||
| Hospitalization | Yes | 3 | 1.2 | 0 | 0.0 | NS |
| No | 257 | 98.8 | 48 | 100.0 | ||
| When were symptoms experienced? | Feb 20 | 12 | 4.7 | 1 | 2.1 | <.0001 |
| Mar 20 | 47 | 18.2 | 7 | 14.6 | ||
| Apr 20 | 31 | 12.0 | 6 | 12.5 | ||
| May 20 | 20 | 7.8 | 3 | 6.3 | ||
| Jun 20 | 13 | 5.0 | 3 | 6.3 | ||
| Jul 20 | 4 | 1.6 | 3 | 6.3 | ||
| Aug 20 | 4 | 1.6 | 3 | 6.3 | ||
| Sep 20 | 15 | 5.8 | 3 | 6.3 | ||
| Oct 20 | 20 | 7.8 | 10 | 20.8 | ||
| Nov 20 | 31 | 12.0 | 0 | 0.0 | ||
| Dec 20 | 22 | 8.5 | 1 | 2.1 | ||
| Jan-21 | 10 | 3.9 | 1 | 2.1 | ||
| Feb-21 | 7 | 2.7 | 0 | 0.0 | ||
| Mar-21 | 13 | 5.0 | 4 | 8.3 | ||
| Apr-21 | 6 | 2.3 | 3 | 6.3 | ||
| May-21 | 3 | 1.2 | 0 | 0.0 | ||
| Duration of symptoms | <1 week | 60 | 23.1 | 21 | 43.8 | NS |
| 1 week | 75 | 28.8 | 12 | 25.0 | ||
| 2 weeks | 73 | 28.1 | 10 | 20.8 | ||
| 3 weeks | 24 | 9.2 | 2 | 4.2 | ||
| ≥4 weeks | 28 | 10.8 | 3 | 6.3 | ||
NA, Not applicable.
Chi-square test or Fisher exact test.
Table E2.
Prevalence of symptoms for all disease occasions reported by SARS-CoV-2 seropositive and seronegative subjects
| Characteristic | Variable | Seropositive |
Seronegative |
P value∗ | ||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Reduced taste or smell | Yes | 169 | 56.0 | 118 | 22.1 | <.0001 |
| No | 133 | 44.0 | 416 | 77.9 | ||
| Sore throat | Yes | 202 | 65.4 | 440 | 80.0 | <.0001 |
| No | 107 | 34.6 | 110 | 20.0 | ||
| Fever | Yes | 200 | 65.4 | 279 | 52.0 | <.001 |
| No | 106 | 34.6 | 258 | 48.0 | ||
| Pain in bone or muscles | Yes | 164 | 53.4 | 225 | 41.6 | .001 |
| No | 143 | 46.6 | 316 | 58.4 | ||
| Body fatigue | Yes | 205 | 66.3 | 330 | 60.1 | .078 |
| No | 104 | 33.7 | 219 | 39.9 | ||
| Runny nose | Yes | 234 | 75.5 | 442 | 80.7 | .082 |
| No | 76 | 24.5 | 106 | 19.3 | ||
| Tiredness | Yes | 262 | 85.6 | 453 | 82.1 | NS |
| No | 44 | 14.4 | 99 | 17.9 | ||
| Cough | Yes | 204 | 65.6 | 338 | 61.6 | NS |
| No | 107 | 34.4 | 211 | 38.4 | ||
| Blocked nose | Yes | 216 | 69.7 | 370 | 68.1 | NS |
| No | 94 | 30.3 | 173 | 31.9 | ||
| Headache | Yes | 235 | 75.8 | 417 | 76.1 | NS |
| No | 75 | 24.2 | 131 | 23.9 | ||
| Abdominal symptoms: stomach pain, nausea, vomiting, diarrhea | Yes | 87 | 28.3 | 164 | 30.3 | NS |
| No | 220 | 71.7 | 378 | 69.7 | ||
| Breathing difficulties | Yes | 98 | 31.9 | 158 | 28.7 | NS |
| No | 209 | 68.1 | 392 | 71.3 | ||
| Other symptoms | Yes | 22 | 7.9 | 26 | 5.1 | NS |
| No | 257 | 92.1 | 484 | 94.9 | ||
Chi-square test or Fisher exact test.
Table E3.
Symptom rating for all disease occasions reported by SARS-CoV-2 seropositive and seronegative subjects
| Symptom | Rating | Seropositive |
Seronegative |
P value∗ | ||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Reduced taste or smell | Yes, very much | 102 | 33.8 | 43 | 8.1 | <.0001 |
| Yes, pretty much | 24 | 8.0 | 23 | 4.3 | ||
| Yes, a little | 43 | 14.2 | 52 | 9.7 | ||
| No | 133 | 44.0 | 416 | 77.9 | ||
| Sore throat | Yes, very much | 23 | 7.4 | 63 | 11.5 | <.0001 |
| Yes, pretty much | 72 | 23.3 | 157 | 28.5 | ||
| Yes, a little | 107 | 34.6 | 220 | 40.0 | ||
| No | 107 | 34.6 | 110 | 20.0 | ||
| Fever | Yes, very much | 20 | 6.5 | 32 | 6.0 | .002 |
| Yes, pretty much | 53 | 17.3 | 79 | 14.7 | ||
| Yes, a little | 127 | 41.5 | 168 | 31.3 | ||
| No | 106 | 34.6 | 258 | 48.0 | ||
| Pain in bone or muscles | Yes, very much | 37 | 12.1 | 32 | 5.9 | <.001 |
| Yes, pretty much | 63 | 20.5 | 79 | 14.6 | ||
| Yes, a little | 64 | 20.8 | 114 | 21.1 | ||
| No | 143 | 46.6 | 316 | 58.4 | ||
| Body fatigue | Yes, very much | 53 | 17.2 | 61 | 11.1 | .005 |
| Yes, pretty much | 72 | 23.3 | 96 | 17.5 | ||
| Yes, a little | 80 | 25.9 | 173 | 31.5 | ||
| No | 104 | 33.7 | 219 | 39.9 | ||
| Runny nose | Yes, very much | 31 | 10.0 | 69 | 12.6 | NS |
| Yes, pretty much | 78 | 25.2 | 162 | 29.6 | ||
| Yes, a little | 125 | 40.3 | 211 | 38.5 | ||
| No | 76 | 24.5 | 106 | 19.3 | ||
Chi-square test or Fisher exact test.
Table E4.
Characterization of 10 seronegative subjects with SARS-CoV-2 memory B- and T-cell responses
| Characteristic | 1A | 2B | 3C | 4D | 5E | 6F | 7G | 8H | 9I | 10J |
|---|---|---|---|---|---|---|---|---|---|---|
| Cellular memory immunity | ||||||||||
| Memory B-cell response | No | Yes | No | No | No | No | No | No | Yes | No |
| Memory T-cell response∗ | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Antibody titers†‡ (cutoff/borderline/median) | ||||||||||
| IgG (25.1/22.5/6.5 AU/mL) | 9.7‡ | 6.6‡ | 8.6‡ | 7.5‡ | 3.1 | 11.5‡ | 9.2‡ | 16.2‡ | 23.2† | 9.6‡ |
| IgM (14.4/7.8/3.0 AU/mL) | 3.7‡ | 7.0‡ | 4.3‡ | 4.6‡ | 2.5 | 6.5‡ | 2.2 | 11.2† | 10.5† | 3.7‡ |
| IgA (2.6/1.4/0.1 AU/mL) | 0 | 0 | 2.1† | 0 | 0 | 0 | 1.1‡ | 0.5‡ | 2.1† | 0.0 |
| Symptoms | ||||||||||
| Any symptoms Feb 20 to Jan 21 | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | No |
| Time from symptoms to clinical visit§ | 0 | NA | 0 | NA | NA | 2 | 8 | 10 | 1 | NA |
| Other factors | ||||||||||
| Self-reported positive PCR test | No | No | No | No | No | No | No | No | Yes | No |
| COVID-19 in household‖ | No | No | Yes | No | No | No | Yes | No | Yes | Yes |
Participant cohort identification numbers are renamed by 1 number and 1 letter that cannot be connected to original identification numbers. NA, Not applicable.
Positive in at least 1 T-cell assay.
Bordeline positive antibody titer.
Titer exceeding median antibody titer value in seronegative group.
Time in months between latest episode of reported symptoms and clinical visit.
Suspected or confirmed COVID-19 disease in household.
References
- 1.Monod M., Blenkinsop A., Xi X., Hebert D., Bershan S., Tietze S., et al. Age groups that sustain resurging COVID-19 epidemics in the United States. Science. 2021;371:eabe8372. doi: 10.1126/science.abe8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naqvi A.A.T., Fatima K., Mohammad T., Fatima U., Singh I.K., Singh A., et al. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim Biophys Acta Mol Basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2020.165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371:eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherina N., Piralla A., Du L., Wan H., Kumagai-Braesch M., Andréll J., et al. Persistence of SARS-CoV-2–specific B and T cell responses in convalescent COVID-19 patients 6-8 months after the infection. Med (N Y) 2021;2:281–295.e4. doi: 10.1016/j.medj.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sekine T., Perez-Potti A., Rivera-Ballesteros O., Strålin K., Gorin J.B., Olsson A., et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168.e14. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallais F., Velay A., Nazon C., Wendling M.J., Partisani M., Sibilia J., et al. Intrafamilial exposure to SARS-CoV-2 associated with cellular immune response without seroconversion, France. Emerg Infect Dis. 2021;27:113–121. doi: 10.3201/eid2701.203611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melén E, Bergström A, Kull I, Almqvist C, Andersson N, Asarnoj A, et al. Male sex is strongly associated with IgE-sensitization to airborne but not food allergens: results up to age 24 years from the BAMSE birth cohort. Clin Transl Allergy 2020 May 25;10:15. 10.1186/s13601-020-00319-w. eCollection 2020. [DOI] [PMC free article] [PubMed]
- 8.Westman M., Åberg K., Apostolovic D., Lupinek C., Gattinger P., Mittermann I., et al. Sensitization to grass pollen allergen molecules in a birth cohort—natural Phl p 4 as an early indicator of grass pollen allergy. J Allergy Clin Immunol. 2020;145:1174–1181.e6. doi: 10.1016/j.jaci.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Ekström S., Andersson N., Lövquist A., Lauber A., Georgelis A., Kull I., et al. COVID-19 among young adults in Sweden: self-reported long-term symptoms and associated factors. Scand J Public Health. 2021 doi: 10.1177/14034948211025425. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Public Health Agency of Sweden Antikroppar mot COVID-19 ökar i alla grupper. June 20, 2021. https://www.folkhalsomyndigheten.se/nyheter-och-press/nyhetsarkiv/2021/juli/antikroppar-mot-COVID-19-okar-i-alla-grupper Available at:
- 11.Rydyznski Moderbacher C., Ramirez S.I., Dan J.M., Grifoni A., Hastie K.M., Weiskopf D., et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piccoli L., Park Y.J., Tortorici M.A., Czudnochowski N., Walls A.C., Beltramello M., et al. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell. 2020;183:1024–1042.e21. doi: 10.1016/j.cell.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa S.F., Giavina-Bianchi P., Buss L., Mesquita Peres C.H., Rafael M.M., dos Santos L.G.N., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) seroprevalence and risk factors among oligo/asymptomatic healthcare workers: estimating the impact of community transmission. Clin Infect Dis. 2021;73:e1214–e1218. doi: 10.1093/cid/ciaa1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martischang R., Iten A., Arm I., Abbas M., Meyer B., Yerly S., et al. Severe acute respiratory coronavirus virus 2 (SARS-CoV-2) seroconversion and occupational exposure of employees at a Swiss university hospital: a large longitudinal cohort study. Infect Control Hosp Epidemiol. 2021:1–8. doi: 10.1017/ice.2021.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo K., Lei Z., Hai Z., Xiao S., Rui J., Yang H., et al. Transmission of SARS-CoV-2 in public transportation vehicles: a case study in Hunan Province, China. Open Forum Infect Dis. 2020;7:ofaa430. doi: 10.1093/ofid/ofaa430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng O.T., Marimuthu K., Koh V., Pang J., Linn K.Z., Sun J., et al. SARS-CoV-2 seroprevalence and transmission risk factors among high-risk close contacts: a retrospective cohort study. Lancet Infect Dis. 2021;21:333–343. doi: 10.1016/S1473-3099(20)30833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marks M., Millat-Martinez P., Ouchi D., Roberts C.H., Alemany A., Corbacho-Monné M., et al. Transmission of COVID-19 in 282 clusters in Catalonia, Spain: a cohort study. Lancet Infect Dis. 2021;21:629–636. doi: 10.1016/S1473-3099(20)30985-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skevaki C., Karsonova A., Karaulov A., Xie M., Renz H. Asthma-associated risk for COVID-19 development. J Allergy Clin Immunol. 2020;146:1295–1301. doi: 10.1016/j.jaci.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudberg A.S., Havervall S., Månberg A., Jernbom Falk A., Aguilera K., Ng H., et al. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020;11:5064. doi: 10.1038/s41467-020-18848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuo J., Dowell A.C., Pearce H., Verma K., Long H.M., Begum J., et al. Robust SARS-CoV-2–specific T cell immunity is maintained at 6 months following primary infection. Nat Immunol. 2021;22:620–626. doi: 10.1038/s41590-021-00902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abayasingam A., Balachandran H., Agapiou D., Hammoud M., Rodrigo C., Keoshkerian E., et al. Long-term persistence of RBD+ memory B cells encoding neutralizing antibodies in SARS-CoV-2 infection. Cell Reports Med. 2021;2:100228. doi: 10.1016/j.xcrm.2021.100228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 23.Long Q.-X., Jia Y.-J., Wang X., Deng H.-J., Cao X.-X., Yuan J., et al. Immune memory in convalescent patients with asymptomatic or mild COVID-19. Cell Discov. 2021;7:18. doi: 10.1038/s41421-021-00250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cervia C., Nilsson J., Zurbuchen Y., Valaperti A., Schreiner J., Wolfensberger A., et al. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J Allergy Clin Immunol. 2021;147:545–557.e9. doi: 10.1016/j.jaci.2020.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Bert N., Clapham H.E., Tan A.T., Chia W.N., Tham C.Y.L., Lim J.M., et al. Highly functional virus-specific cellular immune response in asymptomatic SARS-CoV-2 infection. J Exp Med. 2021;218:e20202617. doi: 10.1084/jem.20202617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z., Yang X., Zhong J., Zhou Y., Tang Z., Zhou H., et al. Exposure to SARS-CoV-2 generates T-cell memory in the absence of a detectable viral infection. Nat Commun. 2021;12:1724. doi: 10.1038/s41467-021-22036-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogega C.O., Skinner N.E., Blair P.W., Park H.S., Littlefield K., Ganesan A., et al. Durable SARS-CoV-2 B cell immunity after mild or severe disease. J Clin Invest. 2021;131:e145516. doi: 10.1172/JCI145516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saletti G., Gerlach T., Jansen J.M., Molle A., Elbahesh H., Ludlow M., et al. Older adults lack SARS-CoV-2 cross-reactive T lymphocytes directed to human coronaviruses OC43 and NL63. Sci Rep. 2020;10:21447. doi: 10.1038/s41598-020-78506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ameratunga R., Woon S.-T., Jordan A., Longhurst H., Leung E., Steele R., et al. Perspective: diagnostic laboratories should urgently develop T cell assays for SARS-CoV-2 infection. Expert Rev Clin Immunol. 2021;17:421–430. doi: 10.1080/1744666X.2021.1905525. [DOI] [PubMed] [Google Scholar]
- 30.Ibarrondo F.J., Fulcher J.A., Goodman-Meza D., Elliott J., Hofmann C., Hausner M.A., et al. Rapid decay of anti–SARS-CoV-2 antibodies in persons with mild COVID-19. N Engl J Med. 2020;383:1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaebler C., Wang Z., Lorenzi J.C.C., Muecksch F., Finkin S., Tokuyama M., et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- Wang G., Hallberg J., Bergström P.U., Janson C., Pershagen G., Gruzieva O., et al. Assessment of chronic bronchitis and risk factors in young adults: results from BAMSE. Eur Respir J. 2021;57:2002120. doi: 10.1183/13993003.02120-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekström S., Andersson N., Lövquist A., Lauber A., Georgelis A., Kull I., et al. COVID-19 among young adults in Sweden: self-reported long-term symptoms and associated factors. Scand J Public Health. 2021 doi: 10.1177/14034948211025425. 14034948211025425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MelénE, Bergström A., Kull I., Almqvist C., Andersson N., Asarnoj A., et al. Male sex is strongly associated with IgE-sensitization to airborne but not food allergens: results up to age 24 years from the BAMSE birth cohort. Clin Transl Allergy. 2020;10:15. doi: 10.1186/s13601-020-00319-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherina N., Piralla A., Du L., Wan H., Kumagai-Braesch M., Andréll J., et al. Persistence of SARS-CoV-2–specific B and T cell responses in convalescent COVID-19 patients 6-8 months after the infection. Med (N Y) 2021;2:281–295.e4. doi: 10.1016/j.medj.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]