Abstract
Objectives
The use of antibiotics was common in some countries during the early phase of the coronavirus disease 2019 (COVID-19) pandemic, but adequate evaluation remains lacking. This study aimed to evaluate the effect of early antibiotic use in patients with non-severe COVID-19 admitted without bacterial infection.
Methods
This multi-centre retrospective cohort study included 1,373 inpatients with non-severe COVID-19 admitted without bacterial infection. Patients were divided into two groups according to their exposure to antibiotics within 48 h of admission. The outcomes were progression to severe COVID-19, length of stay >15 days and mortality rate. A mixed-effect Cox model and random effect logistic regression were used to explore the association between early antibiotic use and outcomes.
Results
During the 30-day follow-up period, the proportion of patients who progressed to severe COVID-19 in the early antibiotic use group was almost 1.4 times that of the comparison group. In the mixed-effect model, the early use of antibiotics was associated with higher probability of developing severe COVID-19 and staying in hospital for >15 days. However, there was no significant association between early use of antibiotics and mortality. Analysis with propensity-score-matched cohorts displayed similar results. In subgroup analysis, patients receiving any class of antibiotic were at increased risk of adverse health outcomes. Azithromycin did not improve disease progression and length of stay in patients with COVID-19.
Conclusions
It is suggested that antibiotic use should be avoided unless absolutely necessary in patients with non-severe COVID-19, particularly in the early stages.
Keywords: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ID, identification; EAU, early antibiotic use; NEAU, non-early antibiotic use; IQR, interquartile range; COPD, chronic obstructive pulmonary disease; NSAIDs, non-steroidal anti-inflammatory drugs; DID, difference-in-difference; SD, standard deviation; HR, hazard ratio; CI, confidence interval; OR, odds ratio; FEU, fibrinogen equivalent units
Keywords: COVID-19, Antibiotics, Progression, Length of stay, Mortality
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has spread rapidly worldwide since December 2019 [1]. This pandemic has brought a major challenge to global health systems [2]. However, as for severe acute respiratory syndrome and Middle East respiratory syndrome, there are no specific medications for COVID-19 other than supportive and adjunctive therapies [3].
Although antibiotic therapy is not recommended for patients with virus infections [4], the use of antibiotics was common in some countries during the early phase of the COVID-19 pandemic. A recent meta-analysis showed that the prevalence of antibiotic prescribing for patients with COVID-19 was 74.6% in the first 6 months of the pandemic [5]. A possible explanation for this is that the clinical symptoms of COVID-19 are similar to those of bacterial pneumonia, such as coughing, fever and fatigue [6]. When these disease diagnoses cannot be identified effectively, clinicians usually treat patients with empirical or prophylactic antibiotics. In addition, most patients with COVID-19 have mild clinical symptoms in the early stages. A report of 72,314 cases by the Chinese Centre for Disease Control and Prevention showed that 81% of patients with COVID-19 were classified as non-severe cases [7]. When non-severe patients were admitted to hospital, their specific symptoms for COVID-19 were not obvious, and laboratory confirmation could not be obtained quickly due to the limited ability of nucleic acid testing. Moreover, antigen and antibody detection reagents based on immunochromatographic techniques were still in the development stage during the early phase of the COVID-19 pandemic [8,9]. It is anticipated that an increased number of non-severe patients will be prescribed with antibiotics for empirical or prophylactic therapy during the COVID-19 pandemic. Therefore, it is necessary to evaluate the effect of early antibiotic use in patients with COVID-19.
Few studies have been conducted to evaluate the efficacy of antibiotics in patients with COVID-19 [10]. Existing research has mainly focused on specific drugs, particularly azithromycin [11], [12], [13], [14]. However, the effectiveness of azithromycin remains uncertain. Gautret et al. reported that the virological cure rate at day 5–6 post-inclusion among patients treated with a combination of hydroxychloroquine and azithromycin was significantly higher compared with patients treated with hydroxychloroquine alone and other control groups [15,16]. However, in some studies, azithromycin did not reduce in-hospital mortality [11] and was even associated with increased risk of lethal arrhythmias [17]. In these studies, azithromycin was evaluated for therapeutic efficacy as a potential drug to treat SARS-CoV-2 infection [12,13]. Few studies have evaluated the impact of empirical antibiotic use on clinical outcomes in patients with COVID-19 without bacterial infection. Thus, proper evaluation of the effect of the use of antibiotics among patients with COVID-19 is still needed.
Based on the data of patients admitted with non-severe COVID-19, this study used a retrospective cohort design to analyse the effects of antibiotic use within 48 h of admission on disease progression, length of stay and mortality rate to provide clinical evidence for the formulation of prescription and management strategies of antibiotic therapy for patients with COVID-19.
2. Materials and methods
2.1. Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and approved by the Medical Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (Ref. 2020IECA252). The requirement for informed consent was waived by the Ethics Committee. Only pseudonymized data with no risk of identification were used for the analyses. The authors only had access to anonymized data, and had no interaction with patients or patient samples.
2.2. Study design and patients
This multi-centre retrospective cohort study analysed information on hospitalized patients with COVID-19 admitted to four hospitals in Hubei Province, China.
The diagnosis of COVID-19 was based on the interim guidance of the World Health Organization, and the Diagnosis and Treatment Protocol for COVID-19 Patients released by the National Health Commission of China [18,19]. All confirmed cases of COVID-19 included in this study were diagnosed by physicians based on epidemiological history, clinical symptoms, laboratory examination, aetiology, serological examination and chest imaging. In total, 2,501 patients with COVID-19 were admitted to four hospitals in Hubei Province from 31 December 2019 to 31 March 2020. Patients were followed-up until 5 April 2020. The treatments received by the patients were standard of care in accordance with the Diagnosis and Treatment Protocol for COVID-19 Patients. This study aimed to analyse the effects of early antibiotic use on disease progression, length of stay and mortality rate among patients with COVID-19 without bacterial co-infection. In this study, early antibiotic use was defined as antibiotic use within 48 h of admission with a course of medication ≥3 days. Based on the definition of the Diagnosis and Treatment Protocol for COVID-19 Patients, there were four clinical classifications of patients with COVID-19: (1) mild cases: mild clinical symptoms and no evidence of pneumonia on chest radiology; (2) moderate cases: fever and respiratory symptoms and chest radiology suggestive of pneumonia; (3) severe cases: cases meeting any of the following criteria – respiratory rate ≤30 breaths per min, oxygen saturation ≤93% in a resting state, ratio of arterial partial pressure of oxygen and oxygen concentration ≤300 mmHg, and >50% lesion progression in lung imaging within 24–48 h; and (4) critical cases: cases meeting any of the following criteria – respiratory failure requiring mechanical ventilation, other organ failure, shock or death. For this study, patients were grouped into two categories: severe (severe and critical cases) and non-severe (mild and moderate cases). Bacterial infection was defined using both clinical and laboratory data [20], [21], [22]; three physicians involved in the treatment of COVID-19 performed a chart review of the electronic medical records of patients, and determined bacterial infection using the majority rule. Indicators of bacterial infection were either significant positive microbiological cultures (e.g. blood, sputum, urine, stool, tracheal secretions) or a clinical picture that strongly suggested bacterial infection (e.g. radiological or laboratory tests).
The inclusion criteria were patients hospitalized with COVID-19 and admitted to the four hospitals in Hubei, China from 31 December 2019 to 31 March 2020. Exclusion criteria were: (1) patients discharged within 24 h of admission; (2) cases that became severe within 48 h of admission; (3) patients with bacterial infection within 48 h of admission; and (4) patients who received antibiotics within 48 h of admission, but whose medication course was <3 days.
Demographic information, clinical symptoms, medical history, in-hospital medication and clinical outcomes were obtained from the electronic medical system. Laboratory data (white blood cell count, procalcitonin, C-reactive protein, aspartate aminotransferase, alanine aminotransferase, albumin, albumin/globulin ratio, serum creatinine, blood urea, uric acid and D-dimer) were collected from the laboratory information system. Personal identification (ID) information including name and ID was anonymized, and a new study ID was generated for each patient.
2.3. Antibiotic exposure and outcomes
Antibiotic exposure was defined as commencing antibiotics within 48 h of hospital admission, with a course of duration ≥3 days; patients in this group were classified as the early antibiotic use group (EAU group). Otherwise, patients were classified as the non-early antibiotic use group (NEAU group). To further compare the efficacy of different classes of antibiotics, the following four situations of antibiotic administration were analysed in the subgroup analysis: (1) fluoroquinolones (J01M) alone; (2) cephalosporins (J01D) alone; (3) other classes of antibiotics alone, including penicillins (J01C) alone, macrolides (J01FA) alone, nitroimidazoles (J01XD) alone, aminoglycosides (J01G) alone, oxazolidinones (J01XX) alone, and lincosamides (J01FF) alone; and (4) a combination of two or more classes of antibiotics. Patients administrated penicillins alone, macrolides alone, nitroimidazoles alone, aminoglycosides alone, oxazolidinones alone and lincosamides alone were grouped together because of the small number of cases in each group. As azithromycin had been recommended as a therapeutic regimen for cases of COVID-19 [12,14], the effect of azithromycin was also analysed in a subgroup analysis. The study outcomes were: (1) progression from non-severe COVID-19 to severe COVID-19; (2) length of stay >15 days; and (3) all-cause mortality during 30 days of in-hospital follow-up.
2.4. Statistical analyses
Data are presented as median and interquartile range (IQR), or number and percentage, as appropriate. Comparisons of parameters between the two groups were conducted with the Wilcoxon–Mann–Whitney test for continuous variables. For categorical variables, Pearson's χ2 test or Fisher's exact test was used. The risk of outcomes of interest was calculated using the Cox proportional hazard model if hazard curves for the EAU and NEAU groups were proportional (determined by the Kaplan–Meier curve) or logistic regression. Site was modelled as a random effect in the mixed-effect Cox model and random effect logistic regression. Factors associated with disease severity were adjusted in the multi-variate analysis, including basic demographic characteristics (age and gender), symptoms (cough and fever), comorbidities [hypertension, diabetes, malignancy, chronic obstructive pulmonary disease (COPD) and coronary heart disease] and treatments [antiviral drugs, non-steroidal anti-inflammatory drugs (NSAIDs), steroids, immunomodulators and biologics]. The following variables were included for propensity score matching: basic demographic characteristics (age and gender), symptoms (cough and fever), vital signs (diastolic blood pressure and respiratory rate), comorbidities (hypertension, diabetes, malignancy and COPD), treatments (antiviral drugs, NSAIDs, steroids, immunomodulators and biologics) and laboratory data on admission (white blood cell count, lymphocyte percentage and C-reactive protein). Two cohorts were matched at a ratio of 1:1 with a caliper width of 0.15. The balance among covariates was evaluated by estimating the standardized differences between two groups before and after matching. Only those with absolute value <0.1 were considered as qualified matching. A sensitivity analysis was conducted to evaluate the robustness of propensity-score-matched cohort analyses. This additional analysis of the full dataset included the propensity score as a continuous variable in the model to adjust for differences in patient characteristics. For all comparisons, differences were tested using two-tailed tests, and P<0.05 was considered to indicate statistical significance.
The difference-in-difference (DID) methodology was employed to evaluate the impact of antibiotic use on liver function, kidney function and fibrinolytic activity, while controlling for confounding factors in linear regression analysis. The model was also adjusted for basic demographic characteristics (age and gender), symptoms (cough and fever), comorbidities (hypertension, diabetes, malignancy, COPD and coronary heart disease) and treatments (antiviral drugs, NSAIDs, steroids, immunomodulators and biologics). All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and R 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
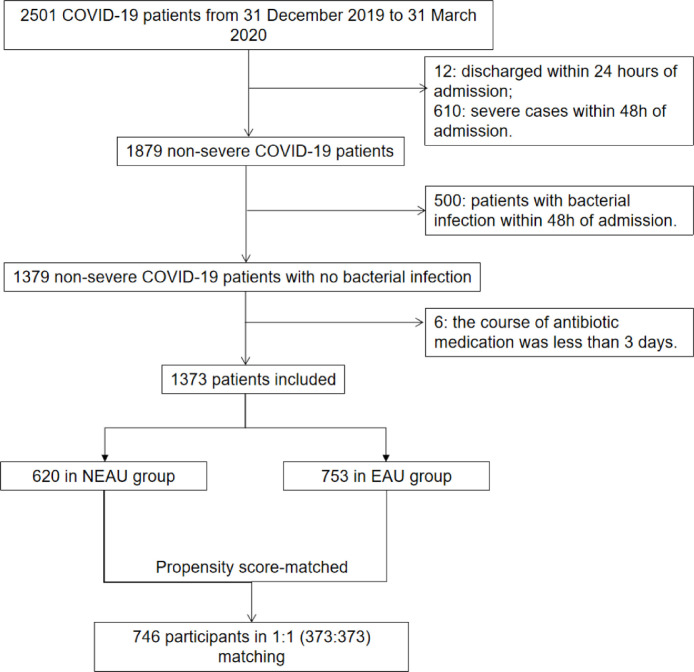
Overall, 2,501 patients with COVID-19 were admitted to four hospitals in Hubei Province, China. Of these, 1,128 patients were excluded, so 1,373 patients with non-severe COVID-19 without bacterial infection were included in the analysis (Figure 1 ). There were 753 patients in the EAU group and 620 patients in the NEAU group. In the EAU group, 438 patients received fluoroquinolones alone, 124 patients received cephalosporins alone, 38 patients received other antibiotics alone, and 153 patients received a combination of antibiotics. The mean ages of patients in the NEAU and EAU groups were 55.1 [standard deviation (SD) 17.4] and 49.0 (SD 16.7) years, respectively (P<0.0001). Over 50% of the patients were female in both groups. Compared with the NEAU group, the EAU group had higher prevalence rates of cough and fever, but lower prevalence rates of hypertension, diabetes and coronary heart disease. A higher proportion of the EAU group received antiviral therapies (95.35% vs 81.13%; P<0.0001). After propensity score matching, the two cohorts were balanced with no significant difference (Table 1 ). The standard mean differences shrank within the range of ± 0.1, indicating good balance (Figure S1, see online supplementary material). Among the patients in the EAU group, eight different antibiotic classes were administered. The most frequently prescribed class of antibiotics was fluoroquinolones (n=573, 76.10%), followed by cephalosporins (n=191, 25.37%) and penicillins (n=89, 11.82%) (Table S1, see online supplementary material). The frequencies of individual antibiotics prescribed are shown in Table S2 (see online supplementary material); the single most commonly prescribed antibiotic was moxifloxacin, accounting for 37.84% of prescriptions.
Figure 1.
Flowchart of patient enrolment. Patients with non-severe coronavirus disease 2019 (COVID-19) include mild cases and moderate cases. Patients with severe COVID-19 include severe cases and critical cases. Patients with the following two conditions were designated as bacterial infections in this study: (i) patients with positive microbiological cultures, including blood, sputum, urine, stool and tracheal secretions; and (ii) patients with no evidence of positive microbiological cultures, but in whom the clinical picture radiological evidence laboratory tests strongly suggest bacterial infection. NEAU, non-early antibiotic use; EAU, early antibiotic use.
Table 1.
Characteristics of patients in early antibiotic use (EAU) and non-early antibiotic use (NEAU) groups before and after propensity score matching.
| Parameters | Unmatched (n=1373) |
Matched (N=746)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NEAU | EAU | P-valueb | NEAU | EAU | P-valueb | |||||
| n | 620 | 753 | 373 | 373 | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Age (years) | 55.1 | 17.4 | 49.0 | 16.7 | <0.0001 | 53.3 | 16.8 | 51.5 | 17.8 | 0.1596 |
| Length of stay (days) | 14.6 | 7.2 | 17.8 | 7.8 | <0.0001 | 15.2 | 7.3 | 16.7 | 7.8 | 0.0059 |
| Laboratory test | ||||||||||
| White blood cell count (109/L) | 5.7 | 1.6 | 5.2 | 2.1 | <0.0001 | 5.6 | 1.6 | 5.5 | 2.0 | 0.4127 |
| Lymphocyte percentage (%) | 30.1 | 9.2 | 28.3 | 10.5 | 0.0009 | 29.8 | 9.4 | 29.8 | 10.9 | 0.9834 |
| Procalcitonin (ng/L) | 0.1 | 0.2 | 0.1 | 0.3 | 0.6396 | 0.1 | 0.2 | 0.1 | 0.4 | 0.6687 |
| C-reactive protein (mg/L) | 5.2 | 13.9 | 12.0 | 17.6 | <0.0001 | 6.1 | 13.9 | 7.0 | 9.9 | 0.3103 |
| Vital signs | ||||||||||
| Pulse | 83.7 | 13.5 | 85.9 | 13.5 | 0.0346 | 84.0 | 13.8 | 86.0 | 13.8 | 0.1275 |
| SBP | 131.6 | 17.3 | 125.2 | 14.1 | <0.0001 | 129.1 | 16.8 | 126.5 | 15.1 | 0.0970 |
| DBP | 79.3 | 10.8 | 76.9 | 9.0 | 0.0016 | 78.5 | 11.1 | 77.7 | 9.5 | 0.4487 |
| Respiratory rate | 19.5 | 1.8 | 19.7 | 2.0 | 0.0493 | 19.5 | 1.8 | 19.7 | 2.0 | 0.1173 |
| n | % | n | % | n | % | n | % | |||
| Gender | 0.0154 | 0.7649 | ||||||||
| Female | 331 | 53.39 | 451 | 59.89 | 222 | 59.52 | 226 | 60.59 | ||
| Male | 289 | 46.61 | 302 | 40.11 | 151 | 40.48 | 147 | 39.41 | ||
| Symptom | ||||||||||
| Cough | 247 | 39.84 | 383 | 50.86 | <0.0001 | 167 | 44.77 | 162 | 43.43 | 0.7124 |
| Fever | 279 | 45.00 | 501 | 66.53 | <0.0001 | 207 | 55.50 | 199 | 53.35 | 0.5565 |
| Comorbidity | ||||||||||
| Hypertension | 212 | 34.19 | 169 | 22.44 | <0.0001 | 100 | 26.81 | 101 | 27.08 | 0.9342 |
| Malignancy | 25 | 4.03 | 44 | 5.84 | 0.1264 | 16 | 4.29 | 16 | 4.29 | 1.0000 |
| Diabetes | 98 | 15.81 | 76 | 10.09 | 0.0015 | 44 | 11.80 | 46 | 12.33 | 0.8221 |
| COPD | 37 | 5.97 | 45 | 5.98 | 0.9948 | 24 | 6.43 | 25 | 6.70 | 0.8825 |
| Coronary heart disease | 60 | 9.68 | 47 | 6.24 | 0.0181 | 27 | 7.24 | 28 | 7.51 | 0.8886 |
| Treatment | ||||||||||
| Antivirus drugs | 503 | 81.13 | 718 | 95.35 | <0.0001 | 341 | 91.42 | 341 | 91.42 | 1.0000 |
| NSAIDs | 137 | 22.10 | 226 | 30.01 | 0.0009 | 73 | 19.57 | 92 | 24.66 | 0.0937 |
| Steroids | 89 | 14.35 | 317 | 42.10 | <0.0001 | 71 | 19.03 | 91 | 24.40 | 0.0757 |
| Immunomodulators | 30 | 4.84 | 233 | 30.94 | <0.0001 | 27 | 7.24 | 32 | 8.58 | 0.4976 |
| Biologics | 140 | 22.58 | 340 | 45.15 | <0.0001 | 90 | 24.13 | 100 | 26.81 | 0.4006 |
| All-cause death (30 days) | 11 | 1.77 | 12 | 1.59 | 0.7953 | 6 | 1.61 | 4 | 1.07 | 0.5243 |
| Progression to severe disease (30 days) | 136 | 21.94 | 239 | 31.74 | <0.0001 | 87 | 23.32 | 115 | 30.83 | 0.0211 |
SD, standard deviation; SBP, systolic blood pressure; DBP, diastolic blood pressure; COPD, chronic obstructive pulmonary disease; NSAIDs, non-steroidal anti-inflammatory drugs.
Propensity score matching was conducted to adjust for basic demographic characteristics (age and gender), symptoms (cough and fever), vital signs (DBP and respiratory rate), comorbidities (hypertension, diabetes, malignancy and COPD), treatments (antiviral drugs, NSAID, steroids, immunomodulators and biologics) and laboratory data on admission (white blood cell count, lymphocyte percentage and C-reactive protein).
P-values were calculated by Chi-squared test or Fisher's exact test.
3.1. Progression to severe COVID‐19
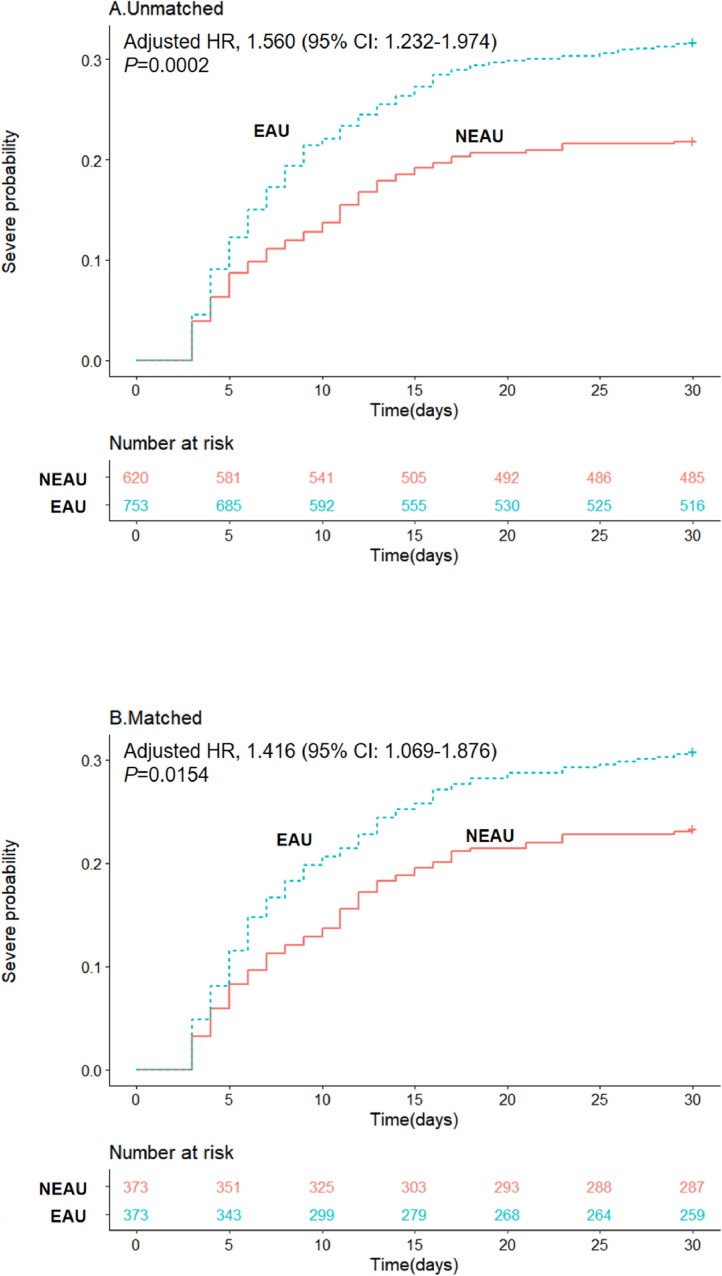
During the 30-day follow-up period, 375 of the 1,373 patients admitted with non-severe COVID-19 progressed to severe disease. The proportion of patients who progressed to severe COVID-19 was higher in the EAU group compared with the NEAU group (31.74% vs 21.94%; P<0.0001) (Table 1). In the mixed-effect Cox model treating site as a random effect, the early use of antibiotics was associated with higher probability of progression to severe COVID-19 [adjusted hazard ratio (HR) 1.555, 95% confidence interval (CI) 1.227–1.970] (Figure 2 A and Table 2 ).
Figure 2.
Kaplan–Meier curves for cumulative probability of progression to severe coronavirus disease 2019 (COVID-19) during the 30-day follow-up period in the early antibiotic use (EAU) and non-early antiobiotic use (NEAU) groups among 1373 patients in unmatched cohorts and 746 patients in matched cohorts. HR, hazard ratio; CI, confidence interval.
Table 2.
Relative risks for outcomes in early antibiotic use (EAU) or non-early antibiotic use (NEAU) groups under the mixed-effect model before and after propensity score matching.
| Unmatched (n=1373) | Matched (n=746)a |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crudeb |
Mixed-effect modelc |
Crudeb |
Mixed-effect modelc |
|||||||||||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |||||
| Progression to severe disease | 1.560 | 1.232 | 1.974 | 0.0002 | 1.555 | 1.227 | 1.970 | 0.0003 | 1.416 | 1.069 | 1.876 | 0.0154 | 1.416 | 1.069 | 1.876 | 0.0154 |
| OR | 95% CI | P-value | OR | 95%CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |||||
| Length of stay>15 days | 1.711 | 1.308 | 2.240 | <0.0001 | 1.827 | 1.389 | 2.403 | <0.0001 | 1.684 | 1.218 | 2.328 | 0.0016 | 1.784 | 1.279 | 2.487 | 0.0007 |
| All-cause mortality (30 days) | 3.070 | 1.008 | 9.348 | 0.0484 | 3.051 | 1.001 | 9.301 | 0.0498 | 1.182 | 0.237 | 5.909 | 0.8385 | 1.190 | 0.237 | 5.969 | 0.8324 |
OR, odds ratio; CI, confidence interval; HR, hazard ratio; COPD, chronic obstructive pulmonary disease; NSAIDs, non-steroidal anti-inflammatory drugs.
Propensity score matching was conducted to adjust for basic demographic characteristics (age and gender), symptoms (cough and fever), vital signs (diastolic blood pressure and respiratory rate), comorbidities (hypertension, diabetes, malignancy and COPD), treatments (antiviral drugs, NSAIDs, steroids, immunomodulators and biologics) and laboratory data on admission (white blood cell count, lymphocyte percentage and C-reactive protein).
Crude estimations were adjusted for basic demographic characteristics (age and gender), symptoms (cough and fever), comorbidities (hypertension, diabetes, and coronary heart disease, malignancy and COPD) and treatments (antiviral drugs, NSAIDs, steroids, immunomodulators and biologics).
Site (hospital) was modelled as a random effect in the multi-variate analyses.
Further analysis was conducted with propensity-score-matched datasets, in which 746 patients were included. In total, 373 patients in the NEAU group were matched with 373 patients in the EAU group at a ratio of 1:1. The results remained consistent, showing higher risk of progression to severe COVID-19 in the EAU group (adjusted HR 1.416, 95% CI 1.069–1.876) (Figure 2B and Table 2). The sensitivity analysis of the full dataset by including the propensity score as a continuous variable showed consistent results with the results of propensity-score-matched cohorts (adjusted HR 1.449, 95% CI 1.128–1.861) (Table 3 ).
Table 3.
Relative risks for outcomes in early antibiotic use (EAU) and non-early antibiotic use (NEAU) groups while including the variable propensity as a covariate in the mixed-effect model (n=1373).
| Crudea |
Mixed-effect modelb |
|||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |||
| Progression to severe disease | 1.444 | 1.126 | 1.853 | 0.0038 | 1.449 | 1.128 | 1.861 | 0.0037 |
| OR | 95% CI | P-value | OR | 95% CI | P-value | |||
| Length of stay >15 days | 1.621 | 1.217 | 2.159 | 0.0010 | 1.664 | 1.244 | 2.227 | 0.0006 |
| All-cause mortality (30 days) | 2.710 | 0.725 | 10.136 | 0.1384 | 2.733 | 0.732 | 10.206 | 0.1345 |
OR, odds ratio; CI, confidence interval; HR, hazard ratio.
Crude estimations were adjusted for basic demographic characteristics (age and gender), symptoms (cough and fever), comorbidities (hypertension, diabetes, coronary heart disease, malignancy and chronic obstructive pulmonary disease) and treatment (antiviral drugs, non-steroidal anti-inflammatory drugs, steroids, immunomodulators and biologics).
Site (hospital) was modelled as a random effect in the multi-variate analyses.
3.2. Length of stay
The average length of stay was 17.8 days (SD 7.8) for the EAU group and 14.6 days (SD 7.2) for the NEAU group (P<0.0001) (Table 1). In the mixed-effect model, antibiotic use was associated with higher risk of staying in hospital for >15 days [adjusted odds ratio (OR) 1.827, 95% CI 1.389–2.403]. In propensity-score-matched cohort analysis, higher risk was also observed for patients administered antibiotics within 48 h of admission (adjusted OR 1.784, 95% CI 1.279–2.487) (Table 2). The sensitivity analysis of the full dataset showed consistent results with the results of propensity-score-matched cohorts (adjusted OR 1.664, 95% CI 1.244–2.227) (Table 3).
3.3. All‐cause mortality during 30 days of in‐hospital follow-up
During the 30-day follow-up period, 23 of 1,373 patients with COVID-19 died. There was no significant difference in the risk of all-cause mortality during the 30-day follow-up period between the EAU group and the NEAU group [1.59% (12/753) vs 1.77% (11/620); P=0.7953] (Table 1). The mixed-effect model (adjusted OR 3.051, 95% CI 1.001–9.301) and analysis with propensity-score-matched cohorts (adjusted OR 1.190, 95% CI 0.237–5.969) displayed similar results (Table 2).
3.4. Subgroup analysis
There was no evidence of improved health outcomes in patients who received any class of antibiotics (Tables S3 and S4, see online supplementary material). Of particular note, there was no significant difference in progression to severe COVID-19 (adjusted HR 2.225, 95% CI 0.862–5.745) or length of stay (adjusted HR 1.515, 95% CI 0.441–5.201) between patients who received azithromycin and those who did not receive antibiotics after propensity score matching (Table S4, see online supplementary material). The case number was too low for assessment of the effect of azithromycin on survival.
3.5. Liver function, kidney function and fibrinolytic activity
After adjusting for covariates, the DID estimator was negative and statistically significant at the 1% level for albumin, and it was positive and statistically significant for D-dimer. Albumin decreased by 2.5 g/L in the EAU group and by 1.4 g/L in the NEAU group. D-dimer increased by 1.7 μg/mL fibrinogen equivalent units (FEU) in the EAU group and decreased by 0.1 μg/mL FEU in the NEAU group (Table S5A, see online supplementary material). However, the effect of early antibiotic use on blood examination indicators was not significant in the propensity-score-matched cohorts (Table S5B, see online supplementary material).
4. Discussion
This study found that early use of antibiotics in patients with non-severe COVID-19 without bacterial infection was associated with progression to severe COVID-19 and longer length of hospital stay, and had no effect on 30-day survival. Subgroup analysis showed that this held for all antibiotic types.
Antibiotic use was common among patients with COVID-19 in the early phase of the pandemic [5]. One possible explanation for the adverse impact of antibiotics is dysbiosis, which occurs within 4–7 days of antibiotic use [23,24]. Considering that the length of stay of patients in the EAU group was 17.8 days, dysbiosis is certainly temporally feasible. Microbial balance has a positive impact on lung immunity. The gut microbiome can activate G-protein-coupled receptors to inhibit inflammation in the lung via its metabolisms, such as short-chain fatty acids to reduce lung injury [25], [26], [27]. By affecting the composition and function of the microbiome, antibiotic use has been proven to undermine the ability of the lungs to clear pathogens and make the lungs more vulnerable to viral infection [28], [29], [30], [31].
Previous studies have suggested that antibiotic treatment without bacterial infection could cause a cytokine storm and a septic-shock-like picture [32], [33], [34], [35], [36]. This release of pro-inflammatory cytokines may promote immune dysfunction and tissue damage, and increase susceptibility to infection [37]. In the context of COVID-19, antibiotic exposure – by adding to the release of pro-inflammatory cytokines – could be associated with higher risk of progression from non-severe to severe disease.
The finding that azithromycin was not associated with lower risk of progression to severe COVID-19 or reduced length of stay is consistent with a multi-centre retrospective cohort study in New York [11]. A Spanish observational study also found no significant association between azithromycin and length of stay in patients with COVID-19 [38]. Moreover, a recent meta-analysis of randomized clinical trials showed no benefit of azithromycin on length of stay [39].
Average changes in the decrease of albumin and the increase in D-dimer were greater in the EAU group compared with the NEAU group. This may be due to increased release of pro-inflammatory factors by antibiotics [33], [34], [35]. Several pro-inflammatory factors such as interleukin-6 and tumour necrosis factor-α can inhibit the synthesis of albumin in the liver [40], [41], [42]. A decreased level of albumin is involved in the regulation of coagulation function [43]. Pro-inflammatory factors can also lead to systemic inflammatory response syndrome and subsequent damage to the vascular system and extensive microthrombosis [44], [45], [46]. Elevation of D-dimer indicates a hypercoagulable state in patient with COVID-19 [47]. However, the differences in the changes of albumin and D-dimer were not significant in the propensity-score-matched cohorts. Further investigation in large clinical trials is needed to assess the influence of antibiotics on liver/renal function and coagulation among patients with COVID-19.
This study does have some limitations. First, it was not possible to retrieve details of pre-admission self-medication with antibiotics. Second, due to limited test availability during the early stages of the pandemic, not all patients in this study had their diagnosis of COVID-19 confirmed virologically. Third, the sample size was too small to determine reliably whether there was any association between antibiotic use and survival. Fourth, as a single-locality study, the possibility of regional or ethnic differences cannot be excluded. Fifth, obesity is known to be associated with increased risk of unfavourable clinical outcomes, including death, in patients with COVID-19 [48], [49], [50], [51]. Although body mass index was not addressed directly in this study, obesity-related comorbidities such as diabetes were adjusted in the analysis to partially mitigate the effect of obesity on outcome. Sixth, although a commonly used official definition of severe cases was adopted in this study, some previous studies used admission to an intensive care unit as a standard [52,53]. Seventh, although propensity score matching and additional propensity score matching analysis were used to balance disease severity between the EAU and NEAU groups, it was only possible to adjust for known and measurable confounders. Thus, it was not possible to eliminate selection bias completely caused by unidentified confounders that affected severity of disease. Further randomized clinical trials are needed to verify the impact of early antibiotic use in patients with non-severe COVID-19.
5. Conclusion
This study found that early use of antibiotics among patients with non-severe COVID-19 was significantly associated with the risk of progression to severe disease, and prolonged hospitalization. Azithromycin use was not associated with better clinical outcomes. It is suggested that antibiotic use should be avoided in patients with non-severe COVID-19 in the absence of evidence of bacterial infection.
Author contributions
XY, NX and YG were responsible for the conception, design and writing of the manuscript. XY, XX and NX were responsible for the acquisition of data and literature research. XX, YG, HL, NJ, JW and ZL were responsible for the analysis and interpretation of data. All authors reviewed and revised the manuscript and approved the final version.
Declaration of Competing Interest
None declared.
Acknowledgments
Funding
This work was supported by the Fundamental Research Funds for the Central Universities (Grant No. 2020kfyXGYJ073), Huazhong University of Science and Technology.
Ethical approval
This study was approved by the Medical Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (Ref. 2020IECA252).
Editor by Dr Jim Gray
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijantimicag.2021.106462.
Appendix. Supplementary materials
References
- 1.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . WHO; Geneva: 2020. Are antibiotics effective in preventing or treating COVID-19?https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19 Available at: [accessed 15 May 2020] [Google Scholar]
- 5.Langford BJ, So M, Raybardhan S, Leung V, Soucy JR, Westwood D, et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect. 2021;27:520–531. doi: 10.1016/j.cmi.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 8.Li H, Pan J, Su Y, Wang B, Ge J. SARS-CoV-2 lgM/lgG antibody detection confirms the infection after three negative nucleic acid detection. J Cell Mol Med. 2020;24:8262–8265. doi: 10.1111/jcmm.15275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petherick A. Developing antibody tests for SARS-CoV-2. Lancet. 2020;395:1101–1102. doi: 10.1016/S0140-6736(20)30788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buetti N, Mazzuchelli T, Lo Priore E, Balmelli C, Llamas M, Pallanza M, et al. Early administered antibiotics do not impact mortality in critically ill patients with COVID-19. J Infect. 2020;81:e148–e149. doi: 10.1016/j.jinf.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323:2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sultana J, Cutroneo PM, Crisafulli S, Puglisi G, Caramori G, Trifirò G. Azithromycin in COVID-19 patients: pharmacological mechanism, clinical evidence and prescribing guidelines. Drug Saf. 2020;43:691–698. doi: 10.1007/s40264-020-00976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derwand R, Scholz M, Zelenko V. COVID-19 outpatients – early risk-stratified treatment with zinc plus low dose hydroxychloroquine and azithromycin: a retrospective case series study. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.106214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parra-Lara LG, Martínez-Arboleda JJ, Rosso F. Azithromycin and SARS-CoV-2 infection: where we are now and where we are going. J Glob Antimicrob Resist. 2020;22:680–684. doi: 10.1016/j.jgar.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Sevestre J, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med Infect Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramireddy A, Chugh H, Reinier K, Ebinger J, Park E, Thompson M, et al. Experience with hydroxychloroquine and azithromycin in the coronavirus disease 2019 pandemic: implications for QT interval monitoring. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.017144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization . WHO; Geneva: 2020. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. Interim guidance.https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117 Available at: [accessed 1 May 2020] [Google Scholar]
- 19.National Health Commission of the People's Republic of China. Diagnosis and treatment protocol for COVID-19 patients. Tentative 8th edition. Available at: http://regional.chinadaily.com.cn/pdf/DiagnosisandTreatmentProtocolforCOVID-19Patients(Tentative8thEdition).pdf [accessed 10 May 2020].
- 20.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 21.Hanna WJ, Berrens Z, Langner T, Lahni P, Wong HR. Interleukin-27: a novel biomarker in predicting bacterial infection among the critically ill. Crit Care. 2015;19:378. doi: 10.1186/s13054-015-1095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seigerman D, Lutsky K, Banner L, Fletcher D, Leinberry C, Lucenti L, et al. The reliability of determining the presence of surgical site infection based on retrospective chart review. J Hand Surg Am. 2020;45:1181. doi: 10.1016/j.jhsa.2020.05.016. e1–4. [DOI] [PubMed] [Google Scholar]
- 23.Mikkelsen KH, Frost M, Bahl MI, Licht TR, Jensen US, Rosenberg J, et al. Effect of antibiotics on gut microbiota, gut hormones and glucose metabolism. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voigt AY, Costea PI, Kultima JR, Li SS, Zeller G, Sunagawa S, et al. Temporal and technical variability of human gut metagenomes. Genome Biol. 2015;16:73. doi: 10.1186/s13059-015-0639-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAleer JP, Kolls JK. Contributions of the intestinal microbiome in lung immunity. Eur J Immunol. 2018;48:39–49. doi: 10.1002/eji.201646721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marsland BJ, Trompette A, Gollwitzer ES. The gut–lung axis in respiratory disease. Ann Am Thorac Soc. 2015;12(Suppl. 2):S150–S156. doi: 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Jin S, Wang C, Jiang R, Wan J. Histone deacetylase inhibitors attenuate acute lung injury during cecal ligation and puncture-induced polymicrobial sepsis. World J Surg. 2010;34:1676–1683. doi: 10.1007/s00268-010-0493-5. [DOI] [PubMed] [Google Scholar]
- 28.Wypych TP, Marsland BJ. Antibiotics as instigators of microbial dysbiosis: implications for asthma and allergy. Trends Immunol. 2018;39:697–711. doi: 10.1016/j.it.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Feng Y, Huang Y, Wang Y, Wang P, Song H, Wang F. Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy. PLoS One. 2019;14 doi: 10.1371/journal.pone.0218384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim S, Covington A, Pamer EG. The intestinal microbiota: antibiotics, colonization resistance, and enteric pathogens. Immunol Rev. 2017;279:90–105. doi: 10.1111/imr.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fagundes CT, Amaral FA, Vieira AT, Soares AC, Pinho V, Nicoli JR, et al. Transient TLR activation restores inflammatory response and ability to control pulmonary bacterial infection in germfree mice. J Immunol. 2012;188:1411–1420. doi: 10.4049/jimmunol.1101682. [DOI] [PubMed] [Google Scholar]
- 32.Hantoushzadeh S, Norooznezhad AH. Possible cause of inflammatory storm and septic shock in patients diagnosed with COVID-19. Arch Med Res. 2020;51:347–348. doi: 10.1016/j.arcmed.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hantoushzadeh S, Anvari Aliabad R, Norooznezhad AH. Antibiotics, pregnancy, and fetal mental illnesses: where is the link? Am J Obstet Gynecol. 2020;222:639–640. doi: 10.1016/j.ajog.2020.01.050. [DOI] [PubMed] [Google Scholar]
- 34.Alkharfy KM, Kellum JA, Frye RF, Matzke GR. Effect of ceftazidime on systemic cytokine concentrations in rats. Antimicrob Agents Chemother. 2000;44:3217–3219. doi: 10.1128/aac.44.11.3217-3219.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cecconi M EL, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392:75–87. doi: 10.1016/S0140-6736(18)30696-2. [DOI] [PubMed] [Google Scholar]
- 36.Goncalves Mendes Neto A, Lo KB, Wattoo A, Salacup G, Pelayo J, DeJoy R 3rd, et al. Bacterial infections and patterns of antibiotic use in patients with COVID-19. J Med Virol. 2021;93:1489–1495. doi: 10.1002/jmv.26441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Picchianti Diamanti A, Rosado MM, Pioli C, Sesti G, Laganà B. Cytokine release syndrome in COVID-19 patients, a new scenario for an old concern: the fragile balance between infections and autoimmunity. Int J Mol Sci. 2020;21:3330. doi: 10.3390/ijms21093330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodríguez-Molinero A, Pérez-López C, Gálvez-Barrón C, Miñarro A, Macho O, López GF, et al. Observational study of azithromycin in hospitalized patients with COVID-19. PLoS One. 2020;15 doi: 10.1371/journal.pone.0238681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamel AM, Monem MSA, Sharaf NA, Magdy N, Farid SF. Efficacy and safety of azithromycin in COVID-19 patients: a systematic review and meta-analysis of randomized clinical trials. Rev Med Virol. 2021 doi: 10.1002/rmv.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith Jeffrey T.L. Inventor; Alderbio Holdings LLC (US) Vitaeris Inc., assignee. Antagonists of IL-6 to raise albumin and/or lower CRP. United States patent US. 2009;26 20090291082. [Google Scholar]
- 41.Buck M, Zhang L, Halasz NA, Hunter T, Chojkier M. Nuclear export of phosphorylated C/EBPbeta mediates the inhibition of albumin expression by TNF-alpha. EMBO J. 2001;20:6712–6723. doi: 10.1093/emboj/20.23.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doweiko JP, Nompleggi DJ. Role of albumin in human physiology and pathophysiology. J Parenter Enteral Nutr. 1991;15:207–211. doi: 10.1177/0148607191015002207. [DOI] [PubMed] [Google Scholar]
- 43.Blanloeil Y, Trossaërt M, Rigal JC, Rozec B. Effects of plasma substitutes on hemostasis. Ann Fr Anesth Reanim. 2002;21:648–667. doi: 10.1016/s0750-7658(02)00695-0. [DOI] [PubMed] [Google Scholar]
- 44.Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39:517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 45.Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, SY Xiao. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joob B, Wiwanitkit V. Pulmonary pathology of early phase 2019 novel coronavirus pneumonia. J Thorac Oncol. 2020;15:e67. doi: 10.1016/j.jtho.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, et al. D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bienvenu LA, Noonan J, Wang X, Peter K. Higher mortality of COVID-19 in males: sex differences in immune response and cardiovascular comorbidities. Cardiovasc Res. 2020;116:2197–2206. doi: 10.1093/cvr/cvaa284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Földi M, Farkas N, Kiss S, Váncsa S, Szakó L, Dembrovszky F, et al. Obesity is a risk factor for developing critical condition in COVID-19 patients: a systematic review and meta-analysis. Obes Rev. 2020;21:e13095. doi: 10.1111/obr.13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smati S, Tramunt B, Wargny M, Caussy C, Gaborit B, Vatier C, et al. Relationship between obesity and severe COVID-19 outcomes in patients with type 2 diabetes: results from the CORONADO study. Diabetes Obes Metab. 2021;23:391–403. doi: 10.1111/dom.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108 doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rochwerg B, Agarwal A, Zeng L, Leo YS, Appiah JA, Agoritsas T, et al. Remdesivir for severe COVID-19: a clinical practice guideline. BMJ. 2020;370:m2924. doi: 10.1136/bmj.m2924. [DOI] [PubMed] [Google Scholar]
- 53.Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, et al. Renin-angiotensin-aldosterone system inhibitors and risk of COVID-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.