Abstract
RNA viruses have high rate of replication and mutation that help them adapt and change according to their environmental conditions. Many viral mutants are the cause of various severe and lethal diseases. Vaccines, on the other hand have the capacity to protect us from infectious diseases by eliciting antibody or cell-mediated immune responses that are pathogen-specific. While there are a few reviews pertaining to the use of artificial intelligence (AI) for SARS-COV-2 vaccine development, none focus on peptide vaccination for RNA viruses and the important role played by AI in it. Peptide vaccine which is slowly coming to be recognized as a safe and effective vaccination strategy has the capacity to overcome the mutant escape problem which is also being currently faced by SARS-COV-2 vaccines in circulation.Here we review the present scenario of peptide vaccines which are developed using mathematical and computational statistics methods to prevent the spread of disease caused by RNA viruses. We also focus on the importance and current stage of AI and mathematical evolutionary modeling using machine learning tools in the establishment of these new peptide vaccines for the control of viral disease.
Keywords: Artificial intelligence, Machine learning, Peptide, RNA-Virus, Vaccine
1. Introduction
RNA viruses use the host's cellular mechanism for their rapid growth and mass-scale transmission and are responsible for the spread of many serious and lethal diseases in humans and many other animal species [1,2]. It has been difficult to develop vaccines for many RNA viruses due to the high mutation rate in replication such as Dengue virus (2.64 × 10−5), Influenza H3N2 (1.35 × 10−5), and HIV-1 (4 × 10−5) [[3], [4], [5], [6]]. DNA sequencing techniques have produced extensive data on the genetic diversity of these viruses. The integration and analysis of these large data sets provide valuable information which, when combined with mathematical and computational statistic methods, can become a powerful tool in predicting future vaccine candidates [7]. In the present scenario of the COVID 19 pandemic, the therapeutic approaches based on computational biology and machine-learning algorithms provide faster and better outcomes in vaccine development and other therapeutics such as drug repurposing, plasma therapy, and drug discovery [[8], [9], [10], [11], [12]]. Machine-learning tools help to predict components that generate an immune response in humans against RNA viruses. These tools enable researchers to understand the virus and its structure, tracks the virus's genetic mutations over time, information that will determine any vaccine's value in the years to come [13]. Different bioinformatics tools have been established to identify potential immunogenic regions in viral antigenic protein sequences that helped in generation of peptide vaccine some of which are now licensed and in use while many are in the pipeline [14].
The surge in coronavirus 2 (SARS-CoV-2)variants of concern (VOC) in the ongoing pandemic i.e AlphaB.1.1.7, BetaB.1.351, GammaP.1, Delta B.1.617.2, Kappa*B.1.617.1 is a major international public health concern as these altered genomes has led to an increase in the viral transmissibility, virulence, and ability to evade host response. The vaccines introduced are based on introducing the spike protein either in mRNA, Adenovirus vectors, recombinant protein form using the early isolates or as the inactivated whole virus as the Indian vaccine BBV152. Studies suggest that BNT162b2 (mRNA) vaccines were able to neutralize Alpha and Gamma variants but less significantly against Betavariant [[15], [16], [17]]. mRNA-1273 (mRNA) was able to neutralize the Alpha variant but to a lower extent against the Beta variant [15,18,19].Ad26.COV2.S (Adenovirus vector based)was shown to have low efficacies to the Beta (64%), the Gamma (61%) and the Alpha (72%) [20]. ChAdOx1 nCoV-19 (Oxford) was 74.6% efficacious against Alpha and 10.4% against Beta [21,22]. BBV152 (Bharat Biotech International Limited) vaccinated human serum is able to neutralize the Alpha variant [23].The phase 3 clinical trial data fact sheet of Novavax (recombinant protein) shows its vaccine to have 90.4% overall efficacy as well as 93.2% efficacy against VOC(https://ir.novavax.com).Peptide vaccination could be a possible strategy which could protect against VOC. Hence this review brings forward the advancement in the process of peptide vaccine design due to AI along with the advantages and drawbacks.
2. Peptide vaccine for RNA virus: how far have they come?
The rapid evolution of RNA viruses makes it difficult to develop robust vaccine candidates.
Vaccine development deals with the four major attributes of viral evolution: diversity, virulence, dynamics, and fitness [24]. Each one of these parameters has been exposed to enormous studies but still remains a problem to predict robust vaccine candidates, probably due to complexity of each attributes and the intrinsic randomness of the evolutionary process [24]. The earlier vaccines were either made by killed viruses that are relatively safe but sometimes ineffective, or by live, attenuated viruses that constitute safety risks [25]. Recently developed vaccines target only immunogenic components of a virus including specific viral protein domains or peptides [14] and are believed to be safe and effective [26]. The progress and increased use of Next-Generation Sequencing (NGS) helped create a large database of the viral genomic sequences for identifying putative vaccine targets, thus enabling a rationalized peptide vaccine design [27].
Viral peptide vaccines are composed of one or more viral antigenic sites that, when administered, elicit protective immunity against the targeted viral pathogen. The design of a peptide vaccine includes initially the identification of immuno-dominant domains of epitopes that can induce protective immune response in terms of humoral or cell mediated immunity against a specific viral antigen [28]. Peptide vaccines can create immunity against multiple strains of a specific virus due to the presence of multiple non-contiguous immunodominant conserved epitopes of different genotypes/serotypes/immunotypes of the virus. While peptide vaccines could restrict notably the chances for allergenic and/or reactogenic complications, they need carriers and adjuvants to increase the low efficacy of oligopeptides [29,30]. Peptide vaccine manufacturing is safe and cost effective in comparison to conventional vaccines.
Unlike replication-incompetent or live and attenuated viral vaccines, peptide vaccines include those epitopes of the virus genome that could develop high levels of both B cell and T cell responses against the virus [31,32]. Various B and T cell epitope prediction tools developed using machine learning (ML) algorithms are in use for identification of the right epitope(s) or peptide(s) that can activate a good amount of T cells is the next challenge to confer protective immunity [33]. Various tools used in vaccine design are listed in Table 1 .
Table 1.
The bioinformatics prediction tools used in vaccine designing.
| Tools for B-cell epitope prediction | Tools for T-helper epitope prediction | Tools for prediction of MHC Class I binders | Tools for prediction MHC Class II binders | Tools for prediction of endogenous antigen processing | Tools for prediction of Allergenicity of peptides | Tools for prediction of Antigenicity |
|---|---|---|---|---|---|---|
| ABCpred | IFNepitope | ProPred1 | Propred | CTLPred | AllerHunter | SVMTriP, |
| Bcepred | IL4pred | NetMHCcons | Consensus | NetTepi | Algpred | VaxiJen, |
| BepiPred | nHLAPred | EpiDOCK | FRED | Toxinpred | ANTIGENpro | |
| SVMTriP | MMBPred | EpiTOP | TAPPred | PREAL | ||
| COBEpro | NetCTLpan | MHC2Pred | TAPhunter | Hemolytik | ||
| EPMLR | RANKPEP | HLA DR4Pred | TAPreg | AHTPDB | ||
| Pep-3DSearch | MULTIPRED2 | Pcleavage | AllergenFP | |||
| IgPred | MARIA | NetChop | Allertop | |||
| Lbtope | ||||||
| BEpro | ||||||
| CBTOPE | ||||||
| CEP | ||||||
| PEASE | ||||||
| DiscoTope | ||||||
| Epitopia | ||||||
| SEPPA | ||||||
| BEST | ||||||
| EPCES | ||||||
| BEPro (PEPITO) | ||||||
| EpiSearch | ||||||
| MimoPro | ||||||
| MIMOX | ||||||
| Pep-3D-Search | ||||||
| PepSurf | ||||||
| ElliPro |
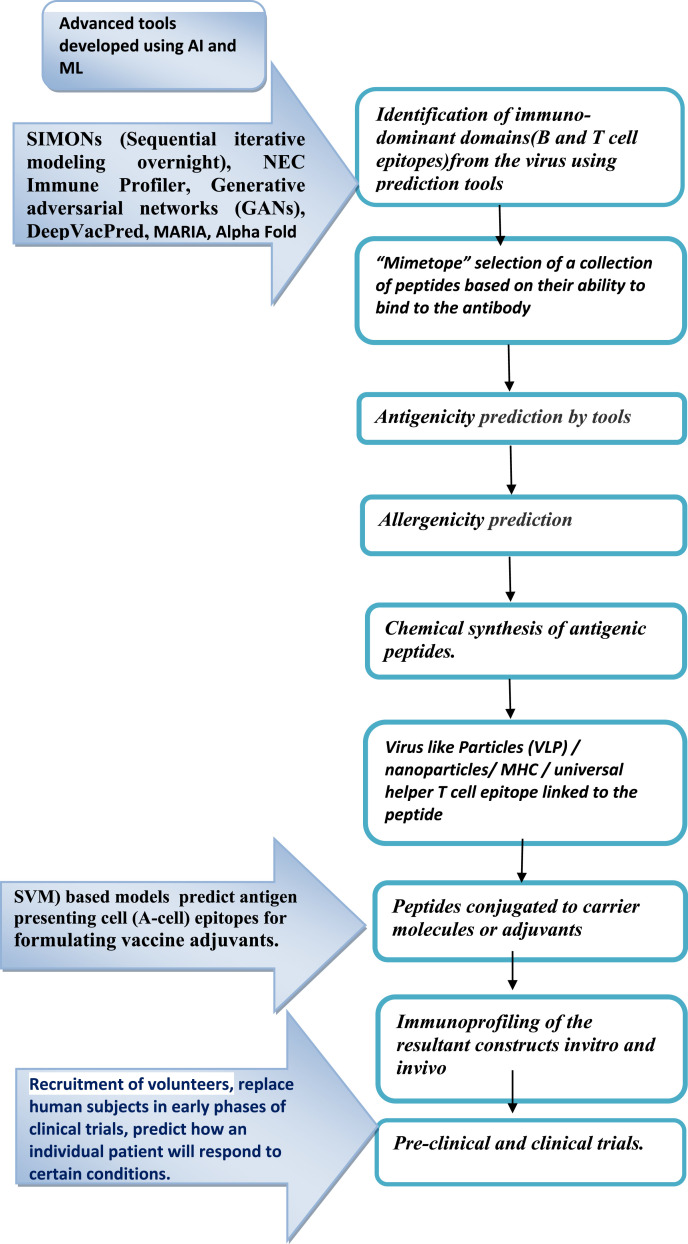
For development of B-Cell responses, Virus like Particles (VLP) or nanoparticles are linked to the selected peptide so that the presentation of ordered, multivalent epitopes can efficiently cross-link B cell receptors (BCRs), required for improved antibody response [34]. Antibodies can target the specific epitopes in conformation dependent or independent manners (Linear epitopes) [35]. Protein or peptide engineering is used to mimic the “structural epitope” or conformation dependent larger epitopes [36,37]. “Mimetope” selection is when a collection of peptides is selected based on their ability to bind to the antibody by phage display or other display methods [38,39]. For development of T Cell responses, peptide epitope is fused to the major histocompatibility complex (MHC) using a polypeptide linker to recognize particular T cell receptors(TCRs) on cells or sometimes a universal helper T cell epitope which stimulates the antibody response is fused to the peptide or protein sequences [28,29]. Examples of immunodominance peptide vaccines are HIV-1, influenza and dengue viruses where the most conserved epitopes on the virus genomes that are also sites of susceptibility for virus neutralization have been used as the respective vaccine candidates [36,37]. In some cases, the epitope structure in the antigen-antibody complex is important for the activity of the antibody [40], the presentation of peptide vaccines in a relevant manner becomes very crucial for vaccine design [41]. For development of a peptide vaccine for respiratory syncytial virus (RSV), the epitope for protective antibody (motavizumab) targeting the F protein was determined by computational methods [25].
The identification, selection, and construction of candidate epitope(s) or peptide vaccine antigen(s) is followed by chemical synthesis of antigenic peptides [14]. The optimal length for peptide-MHC affinity should be approximately 18–20 amino acids as it has been observed that the relationship between peptide length and MHC class II affinity has important consequences for the binding and functioning of antigenic peptides as vaccines [[42], [43], [44], [45]].Hence, peptides of 20–30 amino acids length, which are highly immunogenic and capable of triggering a protective immune response, are synthesized in vitro [14]. The peptides are subsequently conjugated to carrier molecules or adjuvants, as needed. Immunoprofiling of the resultant constructs is carried-out in vitro as well as in appropriate animal models for ascertainment of efficacy and safety, followed by pre-clinical and clinical trials.A few critical bottlenecks for peptide vaccines are (1) only a single peptide epitope can be presented as vaccine candidate, (2) vaccines that are able to evade immune system as the exogenously administered peptide will not necessarily follow the same pathway of processing as the native pathogen [46], (3) a peptide may be unable to generate a prolonged immune response, (4) lack of efficacy may be the result of degradation and conformational instability [46],(5) the improper programming of clinical trials, which includes protocol design, execution, and successive trial planning [47], and (6) safety issues related to the use of adjuvants and particulate peptide vaccine delivery systems [14].
Peptides being short, the epitopes in these vaccines tend to be small, linear and non-conformational. However, the antibodies produced in response to a peptide vaccine targeting B cell epitopes (which are mostly conformational in nature) may offer only restricted protection against natural infection [24]. In order to overcome these shortcomings, support vector machine (SVM) based models have been developed for prediction of antigen presenting cell (A-cell) epitopes that could be used to formulate vaccine adjuvants, which is likely to have the potential to provide protection against viruses and other pathogens [48]. To cover the criteria of population coverage, a subunit vaccine with a set of peptides is incorporated, which can encode a three-dimensional epitope to stimulate neutralizing antibody production by B cells thus improving its efficacy and population coverage [49]. The translation of somatic mutations into neoantigens in the development of neoantigen vaccines by the use of ML helps to overcome the time limitation in the development of potential vaccines for cancer patients thus allowing better selection of therapeutically efficient antitumor immunity [50,51]. To speed up the process of vaccine designing, rapid prediction of potential vaccine candidates (PVCs) reverse vaccinology (genome to vaccine) is helpful (Fig. 1 ). This utilizes bioinformatics tools to design vaccines based on the genetic sequences of the organism [52]. Multi epitope based peptide (MEBP) vaccine candidates have been designed recently using this Immunoinformatics approach for RNA viruses such as zika, nipah and SARS-COV-2 [[53], [54], [55]] by studying immunogenetics and immunology data using bioinformatics.
Fig. 1.
Schematic of key steps involved in peptide vaccine development and the application of AI in various stages.
Promising results for peptide vaccine development have been obtained with the following viruses: Influenza virus [56], hepatitis B virus [57], respiratory syncytial virus [58,59], bovine leukemia virus [60,61], feline immunodeficiency virus [62,63] and hepatitis C virus [64]. Lately, many predictions of peptide vaccine candidates for RNA viruses have been made due to progress in bioinformatics, for e.g.: corona virus [[65], [66], [67], [68]], rotavirus [[69], [70], [71]], foot-and-mouth disease [72,73], Hepatitis C [[74], [75], [76]], influenza [77,78]. These are predicted using bioinformatics tools, which are based on statistics, AI and ML application to the known data sets in immunology [79]. Improvised therapeutic strategies with the help of ML applications in structure prediction, docking, molecular dynamics simulation are being used for the development of a peptide vaccine for covid-19 [8]. Peptide vaccines for human immune-deficiency virus (HIV) [80], hepatitis-C virus (HCV) [81], malaria [82], foot-and-mouth disease [83], swine fever [84], influenza [49], anthrax [85], human papilloma virus (HPV) [86], therapeutic anti-cancer vaccines [[87], [88], [89], [90], [91]] for pancreatic cancer, melanoma, lung cancer, advanced hepatocellular carcinoma cutaneous T cell lymphoma and B Cell chronic lymphocytic leukemia are under development.
3. Role of AI in peptide vaccine development
ML is an AI tool that has the capacity to process large amount of data and generate models for data so that similar data can fit in. This allows AI to classify the inputs and predict the outcomes. AI and mathematical evolutionary modeling using ML tools will pave the road for the development of these new peptide vaccines and the control of viral disease [7]. AI helps in identifying genomic regions that are under selective pressure, selection of vaccine strains and estimating the efficacy and efficiency of therapeutic vaccines by determining epitope hotspots and conserved regions among different strains of the virus, hence, designing universal vaccines. Progress in studying how these factors interact depends on quantitative descriptions and predictive models [92]. AI applications work best when the data set is large and clean. Ironically, most of the known data sets in immunology are very small and of low quality [[93], [94], [95], [96]]. At present, AI is in the nascent stage of its use in the field of vaccines. The computational algorithms developed are trained on in vitro binding data which is insufficient and contains high noise and outliers with algorithmic constraints. To get accurate results, these tools require larger good quality data sets, which unfortunately are very limited [97,98]. In the current pandemic scenario, ML tools and computational analyses have shown its importance in keeping a real-time track of the pattern in spread of the SARSCoV-2 infection, management of the large amount of big data generated, improving diagnostic speed and accuracy, and developing candidate drugs and vaccines in a short time [99,100]. A new approach in ML applications such as SIMONs (Sequential iterative modeling overnight), an automated ML system that compares results from different clinical datasets to increase the predictive accuracy from heterogeneous biological datasets, can provide new targets for the development of the next generation of vaccines [101]. Computationally optimized broadly reactive antigens with consensus sequences, phylogenetic model-based ancestral sequence reconstruction, and immunomics to compute conserved cross-reactive T cell epitopes are some of the algorithms under development for the application of ML in vaccine development [102]. Random Forest (RF) [103], an ensemble learning tool for classification, regression and other tasks, which operate by constructing many decision trees at training time and gives an output of the class in the mode of the classes (classification) or mean/average prediction (regression) of the individual trees. SVM [104] finds a hyperplane in an N-dimensional space (N — the number of features) that distinctly classifies the data points and Recursive Feature Selection (RFE) [105], selects those features in a training dataset that are most relevant in predicting the target variable. Deep Convolutional Neural Networks (DCNN) [106] are neural networks that process data in complex ways by using sophisticated math modeling. LSTM networks [107] classify, process and make predictions based on time series data, since there can be lags of unknown duration between important events in a time series. NEC Immune Profiler (http://www.oncoimmunity.com/products/1-nec-immune-profiler.htm) integrates patent pending modules of HLA binding, processing, and antigen presentation in an integrated system. Immune Epitope Database (IEDB) (https://www.iedb.org/) has tools which assist in the prediction and analysis of epitopes. BepiPred [108] is a web server for predicting B cell epitopes from antigen sequences, which is based on a random forest algorithm trained on epitopes annotated from antibody-antigen protein structures. The state machine model can be used to compare different viral clades and detect mutations separating the clades without the need for alignment, the temporal characteristics of viral evolution can be captured and can be used to characterize the evolution within a clade and in the identification of the actively mutating regions that could lead to the emergence of a new viral clade [109]. Generative adversarial networks (GANs) detect unknown genetic mutations and classifies genes while maintaining a high performance level when facing unseen data with unknown patterns and providing explain-ability capabilities [110].The AI-based framework DeepVacPred, skips 95% of unnecessary predictions to directly predict the potential vaccine subunit sequence without the need to use numerous in silico vaccine design tools which address only one of the several predictions at a time manually [111]. AI and ML applications have the limitation that they cannot overcome the time-consuming but most critical aspect of a successful vaccine development, the animal model studies and human clinical trials [13].
One of the important molecular interactions for vaccine design and prediction algorithms is the ability to determine whether a peptide can be presented by the MHC molecules. This algorithm is developed using raw data from mass spectrometry (MS) techniques [112]. It can be trained by the current data on human immune-peptidome and predict whether a given peptide can be presented by MHC-I molecules. Data acquired from in vitro studies provide information on peptide-MHC affinity. At present, the MHC-I binding predictors have been found to be very efficient and have a wide allelic coverage with a prediction accuracy of 90–95% [[113], [114], [115]]. Epitope prediction for MHC-II molecules is even more challenging due to the differences in peptide length. AI based algorithms are good at predicting MHC-II epitopes based on the amino acid sequence and for designing vaccines that target the MHC-II immunopeptidome [116,117]. Since these models are based on in vitro experimental data, they are limited in predicting in vivo interactions with the same accuracy. The MHC-II binding prediction accuracy is low in comparison to that for MHC- I binding peptide prediction. The performance of the current prediction tools for assessing binding stability is dependent on the MHC allele association. The poor prediction is due to the low quality training data or the difficulty of modeling the allele. In a recent study on SARS-CoV2, improved performance of the prediction tool PrdX 1.0 on allele HLA-A*02:01 of the SARS-CoV2 was obtained using in-house generated stability data [118]. Recently, scientists have listed a number of targets or epitopes on the corona virus responsible for immune response using the neural-network algorithms NetMHCpan-4.0, MARIA and DiscoTope [13]. NetMHCpan-4.0 is a new framework for the training of prediction methods for MHC–peptide presentation prediction integrating information from two data sources (mass spectrometry eluted ligand and peptide binding affinity) [119] and DiscoTope server predicts discontinuous B cell epitopes from the three dimensional structures of proteins. Peptide presentation by MHC and accurate protein structure prediction being invaluable for vaccine design, MARIA and Alpha Fold are two new improved programs developed for MHC-II binding peptide prediction and protein folding, respectively, that show better results as they are based on better training data and better ML algorithms. MARIA (major histocompatibility complex analysis) is a multimodal recurrent neural network used for predicting antigen presentation by specific HLA class II alleles that have been trained on data generated by mass spectrometry, antigenic genes expression and protease cleavage information [120], where as Alpha fold is trained on protein data bank [3]. Positive-unlabeled Learning using AuTOml (PLATO) is a general semi-supervised approach to improving accuracy of model-based classifiers, which generates a set of high confidence positive calls by applying a stringent filter to model-based predictions. It has improved performance compared to model-based approaches for two key steps in tumor rejection mediating neoepitopes (TRMNs) prediction, namely somatic variant calling from exome sequencing data and peptide identification from MS/MS data [121]. AI has an important role to play in the clinical trial phase of vaccine candidates. It can analyze a large amount of clinical records of patients to determine their eligibility for a given study. Recruitment of volunteers is one of the many bottlenecks in conducting clinical trials [122]. It is often the most time-consuming and expensive step. Natural language processing (NLP) is a branch of AI that trains computers to analyze the written and spoken words [123]. This allows algorithms to search doctors’ notes and pathology reports to check for the people eligible to participate in a particular clinical trial [124]. An open-source web tool called Criteria2Query uses NLP to search databases without requiring knowing a database query language [125]. With AI it is possible to replace human subjects in early phases of clinical trials. The company Novadiscovery (https://www.novadiscovery.com/) aims at creating virtual patients by running the trials first in-silico. It helps clinical trials directly from phase I to phase III and also reduces the size of the phase III trial by focusing only on profiles of responders who give optimum response. The technology Jinkō (https://www.novadiscovery.com/jinko-platform.html ) uses real data collected from scientific studies regarding disease pathobiology and then uses drug data from existing studies to model its effect on patients. This predicts the clinical trial outcome. Computer models of disease progression and treatment response can represent each physical individual (digital twin) or a hypothetical individual whose key characteristics (described by the inputs of the model) are sampled from the joint distribution of a representative population (digital trials). Digitalizing clinical trials can be used to predict how an individual patient will respond to certain conditions.
T.N Chan School of public health at Harvard University and the Human Vaccines project have started a joint venture HII (Human Immunomics Initiative) to improve the understanding of the human immune system and speed up the process of vaccine development. They are developing AI assisted Immunological models based on huge data that has been collected from clinical research. Now with advances in computing, AI, genomics, systems biology and bioinformatics, HII plans to understand the mechanisms that govern the human immune system's fight against disease. This would accelerate the design and testing of vaccines for many diseases in the near future.
4. Concluding remark
Increase in the amount of experimentally validated data along with further development of better algorithms trained on better data sets will lead to better vaccine design strategies. The fact that the machine learning algorithms need to be trained on larger and reliable data sets of in vitro and in vivo experimental data requires collaborative projects to be designed for AI scientists and vaccinologists to work together. The advanced technologies such as the development of peptide presenting nanoparticles have shown to have the potential to overcome the challenge of weak immune response elicited by peptides, therefore making use of AI in developing peptide vaccines against RNA viruses, hold a great promise.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We thank Dr Preeti chhabra and Dr Sandeep Kumar for useful discussions and guidance.We also thank Catherine Demos, Biomedical Engineering Department, Emory University and Georgia Institute of Technology, Atlanta GA.) for refining the English grammatical structure and phraseology.
References
- 1.Flint S.J., Enquist L.W., Racaniello V.R., Skalka A.M. ASM Press; Washington, D.C.: 2004. Principles of virology. Molecular biology, pathogenesis, and control of animal viruses. second. [Google Scholar]
- 2.Domingo E. In: Knipe D.M., Howley P.M., editors. vol. 12. Lappincott Williams & Wilkins; Philadelphia: 2007. pp. 389–421. (Virus evolution. Fields virology). [Google Scholar]
- 3.Duffy S. Why are RNA virus mutation rates so damn high? PLoS Biol. 2018 Aug 13;16(8) doi: 10.1371/journal.pbio.3000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett S.N., Holmes E.C., Chirivella M., Rodriguez D.M., Beltran M., Vorndam V., Gubler D.J., McMillan W.O. Selection-driven evolution of emergent dengue virus. Mol Biol Evol. 2003;20:1650–1658. doi: 10.1093/molbev/msg182. [DOI] [PubMed] [Google Scholar]
- 5.Herlocher M.L., Elias S., Truscon R., Harrison S., Mindell D., Simon C., Monto A.S. Ferrets as a transmission model for influenza: sequence changes in HA1 of type A (H3N2) virus. J Infect Dis. 2001 Sep 1;184(5):542–546. doi: 10.1086/322801. [DOI] [PubMed] [Google Scholar]
- 6.Mansky L.M. Forward mutation rate of human immunodeficiency virus type 1 in a T lymphoid cell line. AIDS Res Hum Retrovir. 1996 Mar 1;12(4):307–314. doi: 10.1089/aid.1996.12.307. [DOI] [PubMed] [Google Scholar]
- 7.Ojosnegros S., Beerenwinkel N. Models of RNA virus evolution and their roles in vaccine design. Immunome Res. 2010 Nov 1;6(S2):S5. doi: 10.1186/1745-7580-6-S2-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kabra R., Singh S. Evolutionary artificial intelligence based peptide discoveries for effective Covid-19 therapeutics. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2021 Jan 1;1867(1):165978. doi: 10.1016/j.bbadis.2020.165978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalita P., Padhi A.K., Zhang K.Y.J., Tripathi T. Design of a peptide-based subunit vaccine against novel coronavirus SARS-CoV-2. Microb Pathog. 2020 Aug;145:104236. doi: 10.1016/j.micpath.2020.104236. Epub 2020 May 4. PMID: 32376359; PMCID: PMC7196559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padhi A.K., Tripathi T. Targeted design of drug binding sites in the main protease of SARS-CoV-2 reveals potential signatures of adaptation. Biochem Biophys Res Commun. 2021 May 28;555:147–153. doi: 10.1016/j.bbrc.2021.03.118. Epub 2021 Mar 26. PMID: 33813274; PMCID: PMC7997393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padhi A.K., Tripathi T. Can SARS-CoV-2 accumulate mutations in the S-protein to increase pathogenicity? ACS Pharmacol Transl Sci. 2020 Sep 8;3(5):1023–1026. doi: 10.1021/acsptsci.0c00113. PMID: 33073197; PMCID: PMC7551720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padhi A.K., Shukla R., Saudagar P., Tripathi T. High-throughput rational design of the remdesivir binding site in the RdRp of SARS-CoV-2: implications for potential resistance. iScience. 2020 Dec 26;24(1) doi: 10.1016/j.isci.2020.101992. PMID: 33490902; PMCID: PMC7807151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waltz e. What AI can-and can't-do in the race for a coronavirus vaccine AI takes its best shot. ieee spectrum. 2020 oct 1;57(10):25–+. [Google Scholar]
- 14.Li W., Joshi M.D., Singhania S., Ramsey K.H., Murthy A.K. Peptide vaccine: progress and challenges. Vaccines. 2014 Sep;2(3):515–536. doi: 10.3390/vaccines2030515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang P., Liu L., Iketani S., Luo Y., Guo Y., Wang M., et al. Cold Spring Harbor Laboratory; 2021. Increased resistanceof SARS-CoV-2 variants B.1.351 and B.1.1.7 to antibody Neutralization.bioRxiv prepr serv biol [internet]http://www.ncbi.nlm.nih.gov/pubmed/33532778 cited 2021 Mar 15];2021.01.25.428137. [Google Scholar]
- 16.Liu Y., Liu J., Xia H., Zhang X., Fontes-Garfias C.R., Swanson K.A., et al. Neutralizing activity of BNT162b2-elicited serum - preliminary report. Nav Eng J. 2021 doi: 10.1056/NEJMc2102017. http://www.ncbi.nlm.nih.gov/pubmed/33596352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muik A., Wallisch A.-K., Sänger B., Swanson K.A., Mühl J., Chen W., et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine–elicited human sera. Science. 2021 doi: 10.1101/2020.12.30.20249034. 80- 371:eabg6105., [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu K., Werner A.P., Koch M., Choi A., Narayanan E., Stewart-Jones G.B.E., et al. Serum neutralizing activity elicited by mRNA-1273 vaccine—preliminary report. N Engl J Med. 2021 doi: 10.1056/NEJMc2102179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu K., Werner A.P., Moliva J.I., Koch M., Choi A., Steward-Jones G.B.E., et al. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. bioRxiv. 2021 [Google Scholar]
- 20.Vaccines and related biological products advisory committee february 26, 2021 meeting announcement. FDA; 2021. https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-february-26-2021-meeting-announcement#event-materials 02/26/2021 - 02/26. [Google Scholar]
- 21.Emary K.R.W., Golubchik T., Aley P.K., Ariani C.V., Angus B.J., Bibi S., et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 VOC202012/01 (B.1.1.7) Lancet Prepr. 2021 [Google Scholar]
- 22.Madhi S.A., Baillie V., Cutland C.L., Voysey M., Koen A.L., Fairlie L., et al. Efficacy of the ChAdOx1 nCoV-19 covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021 doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sapkal G.N., Yadav P.D., Ella R., Deshpande G.R., Sahay R.R., Gupta N., et al. Neutralizationof UK-variant VUI-202012/01 with COVAXIN vaccinated human serum. bioRxiv. 2021 doi: 10.1101/2021.01.26.426986. [Internet] cited 2021 Mar 17]; [DOI] [Google Scholar]
- 24.Ojosnegros Samuel, NikoBeerenwinkel Models of RNA virus evolution and their roles in vaccine design. Immunome Res. 2010;6(Suppl 2):S5. doi: 10.1186/1745-7580-6-S2-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaccine safety basics manual . 2013. who. [Google Scholar]
- 26.Murin C.D., Wilson I.A., Ward A.B. Antibody responses to viral infections: a structural perspective across three different enveloped viruses. Nat Microbiol. 2019 May;4(5):734–747. doi: 10.1038/s41564-019-0392-y. Epub 2019 Mar 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luciani F., Bull R.A., Lloyd A.R. Next generation deep sequencing and vaccine design: today and tomorrow. Trends Biotechnol. 2012 Sep;30(9):443–452. doi: 10.1016/j.tibtech.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Correia B.E., Bates J.T., Loomis R.J., Baneyx G., Carrico C., Jardine J.G., Rupert P., Correnti C., Kalyuzhniy O., Vittal V., Connell M.J. Proof of principle for epitope-focused vaccine design. Nature. 2014 Mar;507(7491):201–206. doi: 10.1038/nature12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Purcell A.W., McCluskey J., Rossjohn J. More than one reason to rethink the use of peptides in vaccine design. Nat Rev Drug Discov. 2007 May;6(5):404–414. doi: 10.1038/nrd2224. [DOI] [PubMed] [Google Scholar]
- 30.Aguilar J.C., Rodriguez E.G. Vaccine adjuvants revisited. Vaccine. 2007 May 10;25(19):3752–3762. doi: 10.1016/j.vaccine.2007.01.111. [DOI] [PubMed] [Google Scholar]
- 31.Testa J.S., Philip R. Role of T-cell epitope-based vaccine in prophylactic and therapeutic applications. Future Virol. 2012 Nov;7(11):1077–1088. doi: 10.2217/fvl.12.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanier J.G., Newman M.J., Lee E.M., Sette A., Ahmed R. Peptide vaccination using nonionic block copolymers induces protective anti-viral CTL responses. Vaccine. 1999 Oct 14;18(5–6):549–557. doi: 10.1016/s0264-410x(99)00220-0. [DOI] [PubMed] [Google Scholar]
- 33.Soria-Guerra R.E., Nieto-Gomez R., Govea-Alonso D.O., Rosales-Mendoza S. An overview of bioinformatics tools for epitope prediction: implications on vaccine development. J Biomed Inf. 2015 Feb 1;53:405–414. doi: 10.1016/j.jbi.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Perotti M., Perez L. Virus-like Particles and nanoparticles for vaccine development against HCMV. Viruses. 2019 Dec 28;12(1):35. doi: 10.3390/v12010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolesanova E.F., Sanzhakov M.A., Kharybin O.N. Development of the schedule for multiple parallel “difficult” Peptide synthesis on pins. International journal of peptides. 2013;2013 doi: 10.1155/2013/197317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwong P.D., Wilson I.A. HIV-1 and influenza antibodies: seeing antigens in new ways. Nat Immunol. 2009 Jun;10(6):573–578. doi: 10.1038/ni.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frei J.C., Wirchnianski A.S., Govero J., Vergnolle O., Dowd K.A., Pierson T.C., Kielian M., Girvin M.E., Diamond M.S., Lai J.R. Engineered dengue virus domain III proteins elicit cross-neutralizing antibody responses in mice. J Virol. 2018 Sep 15;(18):92. doi: 10.1128/JVI.01023-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khondee S., Piyawattanametha W. Targeting peptides derived from phage display for clinical imaging. InBacteriophages-Perspectives and Future. 2019 Mar 18 [IntechOpen] [Google Scholar]
- 39.Saphire E.O., Montero M., Menendez A., van Houten N.E., Irving M.B., Pantophlet R., Zwick M.B., Parren P.W., Burton D.R., Scott J.K., Wilson I.A. Structure of a high-affinity “mimotope” peptide bound to HIV-1-neutralizing antibody b12 explains its inability to elicit gp120 cross-reactive antibodies. J Mol Biol. 2007 Jun 8;369(3):696–709. doi: 10.1016/j.jmb.2007.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sela-Culang I., Kunik V., Ofran Y. The structural basis of antibody-antigen recognition. Front Immunol. 2013 Oct 8;4:302. doi: 10.3389/fimmu.2013.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graham B.S., Gilman M.S., McLellan J.S. Structure-based vaccine antigen design. Annu Rev Med. 2019 Jan 27;70:91–104. doi: 10.1146/annurev-med-121217-094234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Brien C., Flower D.R., Feighery C. Peptide length significantly influences in vitro affinity for MHC class II molecules. Immunome Res. 2008;4:6. doi: 10.1186/1745-7580-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malcherek G., Gnau V., Stevanovic S., Rammensee H.G., Jung G., Melms A. Analysis of allele-specific contact sites of natural HLA-DR17 ligands. J Immunol. 1994 Aug 1;153(3):1141–1149. PMID: 8027545. [PubMed] [Google Scholar]
- 44.Vogt A.B., Kropshofer H., Kalbacher H., Kalbus M., Rammensee H.G., Coligan J.E., Martin R. Ligand motifs of HLA-DRB5*0101 and DRB1*1501 molecules delineated from self-peptides. J Immunol. 1994 Aug 15;153(4):1665–1673. PMID: 7519208. [PubMed] [Google Scholar]
- 45.Chang Stewart T., Ghosh Debashis, Kirschner Denise E., Linderman Jennifer J. Peptide length-based prediction of peptide–MHC class II binding. Bioinformatics. 15 November 2006;22(Issue 22):2761–2767. doi: 10.1093/bioinformatics/btl479. [DOI] [PubMed] [Google Scholar]
- 46.Chen S.W., Van Regenmortel M.H., Pellequer J.L. Structure-activity relationships in peptide-antibody complexes: implications for epitope prediction and development of synthetic peptide vaccines. Curr Med Chem. 2009 Mar 1;16(8):953–964. doi: 10.2174/092986709787581914. [DOI] [PubMed] [Google Scholar]
- 47.Fogel D.B. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: a review. Contemporary clinical trials communications. 2018 Sep 1;11:156–164. doi: 10.1016/j.conctc.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagpal G., Chaudhary K., Agrawal P., Raghava G.P. Computer-aided prediction of antigen presenting cell modulators for designing peptide-based vaccine adjuvants. J Transl Med. 2018 Jul 3;16(1):181. doi: 10.1186/s12967-018-1560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu G., Carter B., Gifford D.K. Predicted cellular immunity population coverage gaps for SARS-CoV-2 subunit vaccines and their augmentation by compact peptide sets. Cell systems. 2021 Jan 20;12(1):102–107.e4. doi: 10.1016/j.cels.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou W.J., Qu Z., Song C.Y., Sun Y., Lai A.L., Luo M.Y., Ying Y.Z., Meng H., Liang Z., He Y.J., Li Y.H. NeoPeptide: an immunoinformatic database of T-cell-defined neoantigens. Database. 2019 doi: 10.1093/database/baz128. 2019 Jan 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith C.C., Chai S., Washington A.R., Lee S.J., Landoni E., Field K., Garness J., Bixby L.M., Selitsky S.R., Parker J.S., Savoldo B. Machine-learning prediction of tumor antigen immunogenicity in the selection of therapeutic epitopes. Cancer immunology research. 2019 Oct;7(10):1591–1604. doi: 10.1158/2326-6066.CIR-19-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D'Mello A., Ahearn C.P., Murphy T.F., Tettelin H. ReVac: a reverse vaccinology computational pipeline for prioritization of prokaryotic protein vaccine candidates. BMC Genom. 2019 Dec 16;20(1):981. doi: 10.1186/s12864-019-6195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.PrativaMajee Neha Jain, Amit Kumar. Designing of a multi-epitope vaccine candidate against Nipah virus by insilicoapproach: a putative prophylactic solution for the deadly virus. J Biomol Struct Dyn. 2021;39(4):1461–1480. doi: 10.1080/07391102.2020.1734088. [DOI] [PubMed] [Google Scholar]
- 54.Shahid F., Ashfaq U.A., Javaid A., et al. Immunoinformaticsguided rational design of a next generation multi epitope based peptide (MEBP) vaccine by exploring Zika virus proteome. Infect Genet Evol. 2020 Jun;80 doi: 10.1016/j.meegid.2020.104199. [DOI] [PubMed] [Google Scholar]
- 55.Sanami Samira, Alizadeh Morteza, Nosrati Masoud, Ashrafi Dehkordid Korosh, Azadegan-Dehkordi Fatemeh, Tahmasebian Shahram, Nosrati Hamed, Mohammad-Hassan Arjmand, Ghasemi-Dehnoo Maryam, Rafiei Ali, Bagheri Nader. Exploring SARS-COV-2 structural proteins to design a multi-epitope vaccine using immunoinformatics approach: an in silico study. Comput Biol Med. 2021 June;133 doi: 10.1016/j.compbiomed.2021.104390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stanekova Z., Kiraly J., Stropkovska A., Mikušková T., Mucha V., Kostolanský F., Varečková E. Heterosubtypic protective immunity against influenza A virus induced by fusion peptide of the hemagglutinin in comparison to ectodomain of M2 protein. Acta Virol. 2011 Jan 1;55(1):61–67. doi: 10.4149/av_2011_01_61. [DOI] [PubMed] [Google Scholar]
- 57.World Health Organization Hepatitis B vaccines. Weekly Epidemiological Record= Relevéépidémiologiquehebdomadaire. 2009;84(40):405–419. [Google Scholar]
- 58.Steward M.W. The development of a mimotope-based synthetic peptide vaccine against respiratory syncytial virus. Biologicals. 2001 Sep 1;29(3–4):215–219. doi: 10.1006/biol.2001.0291. [DOI] [PubMed] [Google Scholar]
- 59.Yusibov V., Mett V., Mett V., Davidson C., Musiychuk K., Gilliam S., Farese A., MacVittie T., Mann D. Peptide-based candidate vaccine against respiratory syncytial virus. Vaccine. 2005 Mar 18;23(17–18):2261–2265. doi: 10.1016/j.vaccine.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 60.Ohishi K., Kabeya H., Amanuma H., Onuma M. Peptide-based bovine leukemia virus (BLV) vaccine that induces BLV-Env specific Th-1 type immunity. Leukemia. 1997;11(Suppl 3):223–226. [PubMed] [Google Scholar]
- 61.Gutiérrez G., Rodríguez S.M., De Brogniez A., Gillet N., Golime R., Burny A., Jaworski J.P., Alvarez I., Vagnoni L., Trono K., Willems L. Vaccination against δ-retroviruses: the bovine leukemia virus paradigm. Viruses. 2014 Jun;6(6):2416–2427. doi: 10.3390/v6062416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hosie M.J., Dunsford T.H., de Ronde A., Willett B.J., Cannon C.A., Neil J.C., Jarrett O. Suppression of virus burden by immunization with feline immunodeficiency virus Env protein. Vaccine. 1996 Apr 1;14(5):405–411. doi: 10.1016/0264-410x(95)00193-5. [DOI] [PubMed] [Google Scholar]
- 63.Hosie M.J., Flynn J.N., Rigby M.A., Cannon C., Dunsford T., Mackay N.A., Argyle D., Willett B.J., Miyazawa T., Onions D.E., Jarrett O. DNA vaccination affords significant protection against feline immunodeficiency virus infection without inducing detectable antiviral antibodies. J Virol. 1998 Sep 1;72(9):7310–7319. doi: 10.1128/jvi.72.9.7310-7319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Firbas C., Boehm T., Buerger V., Schuller E., Sabarth N., Jilma B., Klade C.S. Immunogenicity and safety of different injection routes and schedules of IC41, a Hepatitis C virus (HCV) peptide vaccine. Vaccine. 2010 Mar 11;28(12):2397–2407. doi: 10.1016/j.vaccine.2009.12.072. [DOI] [PubMed] [Google Scholar]
- 65.Lee C.H., Koohy H. In silico identification of vaccine targets for 2019-nCoV. F1000Research. 2020;9 doi: 10.12688/f1000research.22507.1. PMID: 32269766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ul Qamar M.T., Saleem S., Ashfaq U.A., Bari A., Anwar F., Alqahtani S. Epitope‐based peptide vaccine design and target site depiction against Middle East Respiratory Syndrome Coronavirus: an immune-informatics study. J Transl Med. 2019 Dec 1;17(1):362. doi: 10.1186/s12967-019-2116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharmin R., Islam A.B. A highly conserved WDYPKCDRA epitope in the RNA directed RNA polymerase of human coronaviruses can be used as epitope-based universal vaccine design. BMC Bioinf. 2014 Dec 1;15(1):161. doi: 10.1186/1471-2105-15-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhattacharya M., Sharma A.R., Patra P., Ghosh P., Sharma G., Patra B.C., Lee S.S., Chakraborty C. Development of epitope‐based peptide vaccine against novel coronavirus 2019 (SARS‐COV‐2): immunoinformatics approach. J Med Virol. 2020 Jun;92(6):618–631. doi: 10.1002/jmv.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Namjoshi G.S., Mitra M., Lalwani S.K., Sachdeva A., Balasubramanian S., Babji S., Ghosh A., Pandey S., Kulkarni S., Goyal V.K. Rotavirus gastroenteritis among children less than 5 years of age in private outpatient setting in urban India. Vaccine. 2014 Aug 11;32:A36–A44. doi: 10.1016/j.vaccine.2014.03.070. [DOI] [PubMed] [Google Scholar]
- 70.Mohanty E., Dehury B., Satapathy A.K., Dwibedi B. Design and testing of a highly conserved human Rotavirus VP8* immunogenic peptide with potential for vaccine development. J Biotechnol. 2018 Sep 10;281:48–60. doi: 10.1016/j.jbiotec.2018.06.306. [DOI] [PubMed] [Google Scholar]
- 71.Jafarpour S., Ayat H., Ahadi A.M. Design and antigenic epitopes prediction of a new trial recombinant multiepitopic rotaviral vaccine: in silico analyses. Viral Immunol. 2015 Jul 1;28(6):325–330. doi: 10.1089/vim.2014.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang C.Y., Chang T.Y., Walfield A.M., Ye J., Shen M., Chen S.P., Li M.C., Lin Y.L., Jong M.H., Yang P.C., Chyr N. Effective synthetic peptide vaccine for foot-and-mouth disease in swine. Vaccine. 2002 Jun 7;20(19–20):2603–2610. doi: 10.1016/s0264-410x(02)00148-2. [DOI] [PubMed] [Google Scholar]
- 73.Cañas-Arranz R., Forner M., Defaus S., de León P., Bustos M.J., Torres E., Sobrino F., Andreu D., Blanco E. A single dose of dendrimer B2T peptide vaccine partially protects pigs against foot-and-mouth disease virus infection. Vaccines. 2020 Mar;8(1):19. doi: 10.3390/vaccines8010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roccasecca R., Folgori A., Ercole B.B., Puntoriero G., Lahm A., Zucchelli S., Tafi R., Pezzanera M., Galfre G., Tramontano A., Mondelli M.U. Mimotopes of the hyper variable region 1 of the hepatitis C virus induce cross-reactive antibodies directed against discontinuous epitopes. Mol Immunol. 2001 Dec 1;38(6):485–492. doi: 10.1016/s0161-5890(01)00084-0. [DOI] [PubMed] [Google Scholar]
- 75.Filskov J., Andersen P., Agger E.M., Bukh J. HCV p7 as a novel vaccine-target inducing multifunctional CD4+ and CD8+ T-cells targeting liver cells expressing the viral antigen. Sci Rep. 2019 Oct 1;9(1):1–3. doi: 10.1038/s41598-019-50365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dawood R.M., Moustafa R.I., Abdelhafez T.H., El-Shenawy R., El-Abd Y., El Din N.G., Dubuisson J., El Awady M.K. A multiepitope peptide vaccine against HCV stimulates neutralizing humoral and persistent cellular responses in mice. BMC Infect Dis. 2019 Dec 1;19(1):932. doi: 10.1186/s12879-019-4571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nachbagauer R., Palese P. Development of next generation hemagglutinin-based broadly protective influenza virus vaccines. Curr Opin Immunol. 2018 Aug 1;53:51–57. doi: 10.1016/j.coi.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 78.Rosendahl Huber S.K., Camps M.G., Jacobi R.H., Mouthaan J., van Dijken H., van Beek J., Ossendorp F., de Jonge J. Synthetic long peptide influenza vaccine containing conserved T and B cell epitopes reduces viral load in lungs of mice and ferrets. PLoS One. 2015 Jun 5;10(6) doi: 10.1371/journal.pone.0127969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bagherian M., Sabeti E., Wang K., Sartor M.A., Nikolovska-Coleska Z., Najarian K. Machine learning approaches and databases for prediction of drug-target interaction: a survey paper. Briefings Bioinf. 2021 Jan 18;22(1):247–269. doi: 10.1093/bib/bbz157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu Y., McNevin J., Zhao H., Tebit D.M., Troyer R.M., McSweyn M., Ghosh A.K., Shriner D., Arts E.J., McElrath M.J., Mullins J.I. Evolution of human immunodeficiency virus type 1 cytotoxic T-lymphocyte epitopes: fitness-balanced escape. J Virol. 2007 Nov 15;81(22):12179–12188. doi: 10.1128/JVI.01277-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burioni R., Perotti M., Mancini N., Clementi M. Perspectives for the utilization of neutralizing human monoclonal antibodies as anti-HCV drugs. J Hepatol. 2008 Aug 1;49(2):299–300. doi: 10.1016/j.jhep.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 82.Epstein J.E., Giersing B., Mullen G., Moorthy V., Richie T.L. Malaria vaccines: are we getting closer? Curr Opin Mol Therapeut. 2007 Feb;9(1):12. [PubMed] [Google Scholar]
- 83.Volpina O.M., Gelfanov V.M., Yarov A.V., Surovoy A.Y., Chepurkin A.V., Ivanov V.T. New virus‐specific T‐helper epitopes of foot‐and‐mouth disease viral VP1 protein. FEBS Lett. 1993 Oct 25;333(1–2):175–178. doi: 10.1016/0014-5793(93)80399-f. [DOI] [PubMed] [Google Scholar]
- 84.Tarradas J., Monsó M., Muñoz M., Rosell R., Fraile L., Frías M.T., Domingo M., Andreu D., Sobrino F., Ganges L. Partial protection against classical swine fever virus elicited by dendrimeric vaccine-candidate peptides in domestic pigs. Vaccine. 2011 Jun 10;29(26):4422–4429. doi: 10.1016/j.vaccine.2011.03.095. [DOI] [PubMed] [Google Scholar]
- 85.Oscherwitz J., Yu F., Cease K.B. A synthetic peptide vaccine directed against the 2ß2-2ß3 loop of domain 2 of protective antigen protects rabbits from inhalation anthrax. J Immunol. 2010;185(6):3661–3668. doi: 10.4049/jimmunol.1001749. [DOI] [PubMed] [Google Scholar]
- 86.Solares A.M., Baladron I., Ramos T., Valenzuela C., Borbon Z., Fanjull S., Gonzalez L., Castillo D., Esmir J., Granadillo M., Batte A. International scholarly research network. ISRN Obstetrics and Gynecology; 2011. Safety and immunogenicity of a human papillomavirus peptide vaccine (CIGB-228) in women with high-grade cervical intraepithelial neoplasia: first-in-human, proof-of-concept trial. 2011. 9 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bernhardt S.L., Gjertsen M.K., Trachsel S., Møller M., Eriksen J.A., Meo M., Buanes T., Gaudernack G. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: a dose escalating phase I/II study. Br J Cancer. 2006 Dec;95(11):1474–1482. doi: 10.1038/sj.bjc.6603437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brunsvig P.F., Kyte J.A., Kersten C., Sundstrøm S., Møller M., Nyakas M., Hansen G.L., Gaudernack G., Aamdal S. Telomerase peptide vaccination in NSCLC: a phase II trial in stage III patients vaccinated after chemoradiotherapy and an 8-year update on a phase I/II trial. Clin Cancer Res. 2011 Nov 1;17(21):6847–6857. doi: 10.1158/1078-0432.CCR-11-1385. [DOI] [PubMed] [Google Scholar]
- 89.Kyte J.A., Gaudernack G., Dueland S., Trachsel S., Julsrud L., Aamdal S. Telomerase peptide vaccination combined with temozolomide: a clinical trial in stage IV melanoma patients. Clin Cancer Res. 2011 Jul 1;17(13):4568–4580. doi: 10.1158/1078-0432.CCR-11-0184. [DOI] [PubMed] [Google Scholar]
- 90.Greten T.F., Forner A., Korangy F., N'Kontchou G., Barget N., Ayuso C., Ormandy L.A., Manns M.P., Beaugrand M., Bruix J. A phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMC Cancer. 2010 Dec 1;10(1):209. doi: 10.1186/1471-2407-10-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kyte J.A., Trachsel S., Risberg B., thorStraten P., Lislerud K., Gaudernack G. Unconventional cytokine profiles and development of T cell memory in long-term survivors after cancer vaccination. Cancer immunology. Immunotherapy. 2009 Oct 1;58(10):1609–1626. doi: 10.1007/s00262-009-0670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Malone B., Simovski B., Moliné C., Cheng J., Gheorghe M., Fontenelle H., Vardaxis I., Tennøe S., Malmberg J.A., Stratford R., Clancy T. Artificial intelligence predicts the immunogenic landscape of SARS-CoV-2 leading to universal blueprints for vaccine designs. Sci Rep. 2020 Dec 23;10(1):1–4. doi: 10.1038/s41598-020-78758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jeon J., Nim S., Teyra J., Datti A., Wrana J.L., Sidhu S.S., Moffat J., Kim P.M. A systematic approach to identify novel cancer drug targets using machine learning, inhibitor design and high-throughput screening. Genome Med. 2014 Jul 30;6(7):57. doi: 10.1186/s13073-014-0057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ferrero E., Dunham I., Sanseau P. In silico prediction of novel therapeutic targets using gene-disease association data. J Transl Med. 2017 Aug 29;15(1):182. doi: 10.1186/s12967-017-1285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Riniker S., Wang Y., Jenkins J.L., Landrum G.A. Using information from historical high-throughput screens to predict active compounds. J Chem Inf Model. 2014 Jul 28;54(7):1880–1891. doi: 10.1021/ci500190p. [DOI] [PubMed] [Google Scholar]
- 96.Vamathevan J., Clark D., Czodrowski P., Dunham I., Ferran E., Lee G., Li B., Madabhushi A., Shah P., Spitzer M., Zhao S. Applications of machine learning in drug discovery and development. Nat Rev Drug Discov. 2019 Jun;18(6):463–477. doi: 10.1038/s41573-019-0024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Naudé W. Artificial intelligence vs COVID-19: limitations, constraints and pitfalls. AI Soc. 2020 Sep;35(3):761–765. doi: 10.1007/s00146-020-00978-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nanni L., Brahnam S., Lumini A. Artificial intelligence systems based on texture descriptors for vaccine development. Amino Acids. 2011 Feb 1;40(2):443–451. doi: 10.1007/s00726-010-0654-8. [DOI] [PubMed] [Google Scholar]
- 99.Bharadwaj K.K., Srivastava A., Panda M.K., Singh Y.D., Maharana R., Mandal K., Singh B.M., Singh D., Das M., Murmu D., Kabi S.K. Computational intelligence in vaccine design against COVID-19. Studies in Computational Intelligence. 2021;923:311–329. [Google Scholar]
- 100.Alimadadi A., Aryal S., Manandhar I., Munroe P.B., Joe B., Cheng X. Artificial intelligence and machine learning to fight COVID-19. 2020 Apr 1;52(4):200–202. doi: 10.1152/physiolgenomics.00029.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tomic A., Tomic I., Rosenberg-Hasson Y., Dekker C.L., Maecker H.T., Davis M.M. SIMON, an automated machine learning system, reveals immune signatures of influenza vaccine responses. J Immunol. 2019 Aug 1;203(3):749–759. doi: 10.4049/jimmunol.1900033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qiu X., Duvvuri V.R., Bahl J. Computational approaches and challenges to developing universal influenza vaccines. Vaccines. 2019 Jun;7(2):45. doi: 10.3390/vaccines7020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Breiman L. Random forests. Mach Learn. 2001 Oct;45(1):5–32. [Google Scholar]
- 104.Gandhi R. Support vector machine—introduction to machine learning algorithms. Data Sci. 2018 Jun [Google Scholar]
- 105.Brownlee J. Recursive feature elimination (RFE) for feature selection in Python. Machine Learning Mastery. 2020 https://machinelearningmastery.com/rfe-feature-selection-in-python/ [Google Scholar]
- 106.Han Y., Kim D. Deep convolutional neural networks for pan-specific peptide-MHC class I binding prediction. BMC Bioinf. 2017 Dec;18(1):1–9. doi: 10.1186/s12859-017-1997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jain Siddharth, Xiao Xiongye, Bogdan Paul, Bruck1 Jehoshua. Predicting the emergence of SARS-CoV-2 clades. bioRxiv. 2020 07.26. [Google Scholar]
- 108.Cheng M., Li Y., Nazarian S., Bogdan P. From rumor to genetic mutation detection with explanations: a GAN approach. Sci Rep. 2021 Mar 12;11(1) doi: 10.1038/s41598-021-84993-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang Z., Bogdan P., Nazarian S. An in silico deep learning approach to multi-epitope vaccine design: a SARS-CoV-2 case study. Sci Rep. 2021 Feb 5;11(1) doi: 10.1038/s41598-021-81749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hochreiter S. JA1 4 rgenSchmidhuber (1997).“Long short-term memory”. Neural Comput.;9(8): 1735–1780. [DOI] [PubMed]
- 111.Ovsyannikova I.G., Johnson K.L., Bergen H.R., III, Poland G.A. Mass spectrometry and peptide‐based vaccine development. Clin Pharmacol Ther. 2007 Dec;82(6):644–652. doi: 10.1038/sj.clpt.6100389. [DOI] [PubMed] [Google Scholar]
- 112.He Y., Rappuoli R., De Groot A.S., Chen R. Emerging vaccine informatics. J Biomed Biotechnol. 2010:1–26. doi: 10.1155/2010/218590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hoof I., Peters B., Sidney J., Pedersen L.E., Sette A., Lund O., et al. NetMHCpan, a method for MHC class I binding prediction beyond humans. Immunogenetics. 2009;61:1–13. doi: 10.1007/s00251-008-0341-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lundegaard C., Lund O., Nielsen M. Accurate approximation method for prediction of class I MHC affinities for peptides of length 8, 10 and 11 using prediction tools trained on 9mers. Bioinformatics. 2008;24:1397–1398. doi: 10.1093/bioinformatics/btn128. [DOI] [PubMed] [Google Scholar]
- 115.Moghram B.A., Nabil E., Badr A. Ab-initio conformational epitope structure prediction using genetic algorithm and SVM for vaccine design. Comput Methods Progr Biomed. 2018 Jan 1;153:161–170. doi: 10.1016/j.cmpb.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 116.Degoot A.M., Chirove F., Ndifon W. Trans-allelic model for prediction of peptide: MHC-II interactions. Front Immunol. 2018 Jun 20;9 doi: 10.3389/fimmu.2018.01410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Prachar M., Justesen S., Steen-Jensen D.B., Thorgrimsen S., Jurgons E., Winther O., Bagger F.O. Identification and validation of 174 COVID-19 vaccine candidate epitopes reveals low performance of common epitope prediction tools. Sci Rep. 2020 Nov 24;10(1):1–8. doi: 10.1038/s41598-020-77466-4. 20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jurtz V., Paul S., Andreatta M., Marcatili P., Peters B., Nielsen M. NetMHCpan-4.0: improved peptide–MHC class I interaction predictions integrating eluted ligand and peptide binding affinity data. J Immunol. 2017 Nov 1;199(9):3360–3368. doi: 10.4049/jimmunol.1700893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen B., Khodadoust M.S., Olsson N., Wagar L.E., Fast E., Liu C.L., Muftuoglu Y., Sworder B.J., Diehn M., Levy R., Davis M.M. Predicting HLA class II antigen presentation through integrated deep learning. Nat Biotechnol. 2019 Nov;37(11):1332–1343. doi: 10.1038/s41587-019-0280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sherafat E., Force J., Măndoiu Semi-supervised learning for somatic variant calling and peptide identification in personalized cancer immunotherapy. BMC Bioinf. 2020 Dec 30;21(Suppl 18):498. doi: 10.1186/s12859-020-03813-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jespersen M.C., Peters B., Nielsen M., Marcatili P. BepiPred-2.0: improving sequence-based B-cell epitope prediction using conformational epitopes. Nucleic Acids Res. 2017 Jul 3;45(W1):W24–W29. doi: 10.1093/nar/gkx346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chaudhari Nayan, Ravi Renju, Nithya J., Gogtay, Urmila M. Thatte. Recruitment and retention of the participants in clinical trials: challenges and solutions. Perspect Clin Res. 2020 Apr-Jun;11(2):64–69. doi: 10.4103/picr.PICR_206_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.KeshavarziArshadi A., Webb J., Salem M., Cruz E., Calad-Thomson S., Ghadirian N., Collins J., Diez-Cecilia E., Kelly B., Goodarzi H., Yuan J.S. Artificial intelligence for COVID-19 drug discovery and vaccine development. Front. Artif. Intell. 2020 Aug 18;3:65. doi: 10.3389/frai.2020.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Woo M. An AI boost for clinical trials. Nature. 2019 Sep 1;573(7775):S100. doi: 10.1038/d41586-019-02871-3. [DOI] [PubMed] [Google Scholar]
- 125.Yuan C., Ryan P.B., Ta C., Guo Y., Li Z., Hardin J., Makadia R., Jin P., Shang N., Kang T., Weng C. Criteria2Query: a natural language interface to clinical databases for cohort definition. J Am Med Inf Assoc. 2019 Apr;26(4):294–305. doi: 10.1093/jamia/ocy178. [DOI] [PMC free article] [PubMed] [Google Scholar]