Abstract
Selenium (Se) deficiency can seriously affect the small intestine of swine, and cause diarrhea in swine. However, the specific mechanism of Se deficiency-induced swine diarrhea has rarely been reported. Here, to explore the damage of Se deficiency on the calcium homeostasis and autophagy mechanism of swine, in vivo and in vitro models of swine intestinal Se deficiency were established. Twenty-four pure line castrated male Yorkshire pigs (45 d old, 12.50 ± 1.32 kg, 12 full-sibling pairs) were divided into 2 equal groups and fed Se-deficient diet (0.007 mg Se/kg) as the Se-deficiency group, or fed Se-adequate diet (0.3 mg Se/kg) as the control group for 16 weeks. The intestinal porcine enterocyte cell line (IPEC-J2) was divided into 2 groups, and cultured by Se-deficient medium as the Se-deficient group, or cultured by normal medium as the control group. Morphological observations showed that compared with the control group, intestinal cells in the Se-deficiency group were significantly damaged, and autophagosomes increased. Autophagy staining and cytoplasmic calcium staining results showed that in the Se-deficiency group, autophagy increased and calcium homeostasis was destroyed. According to the reactive oxygen species (ROS) staining results, the percentage of ROS in the Se-deficiency group was higher than that in the control group in the in vitro model. Compared with the control group, the protein and mRNA expressions of autophagy-calcium-related genes including Beclin 1, microtubule-associated proteins 1A (LC3-1), microtubule-associated proteins 1B (LC3-2), autophagy-related protein 5 (ATG5), autophagy-related protein 12 (ATG12), autophagy-related protein 16 (ATG16), mammalian target of rapamycin (mTOR), calmodulin-dependent protein kinase kinase β (CAMKK-β), adenosine 5′-monophosphate-activated protein kinase (AMPK), sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA), and calpain in the Se-deficiency group were significantly increased which was consistent in vivo and in vitro (P < 0.05). Altogether, our results indicated that Se deficiency could destroy the calcium homeostasis of the swine small intestine to trigger cell autophagy and oxidative stress, which was helpful to explain the mechanism of Se deficiency-induced diarrhea in swine.
Keywords: Se deficiency swine, Small intestine, Intestinal porcine enterocyte cell line, Autophagy, Ca overload
1. Introduction
Selenium (Se) is an indispensable nutrient element for human and animal organisms, which has physiological effects such as being anti-inflammatory, antioxidant (Rayman, 2000), and anti-mutation (Peng et al., 2016) and it also impacts immunity (Amantana et al., 2002). Selenium plays an important role in immune function (Zhang et al., 2020a, Zhang et al., 2020b). There are studies that show that selenium deficiency can lead to immune injury in the trachea of chickens (Qin et al., 2020). Studies have also shown that Se deficiency can cause Keshan disease in humans (Loscalzo, 2014), heart failure in swine (Bomer et al., 2020), white muscle disease of calves, lambs, ponies, and other animals, and vitamin E/selenium deficiency syndrome of swine (Hosnedlova et al., 2017), even affecting maternal thyroid metabolism and oxidative stress, leading to weight loss (Hofstee et al., 2019). These impacts have brought significant economic losses to the swine industry. Selenium can have a positive effect on intestinal function in swine, such as alleviating diarrhea-induced colitis injury (Teige et al., 1984). As the small intestine is the main organ that absorbs Se (Speckmann and Steinbrenner, 2014), the two are closely linked. Swine can be used as a good model to study the potential risks and related mechanisms of human Se intake (Lu et al., 2019). Compared to rodent cell lines, intestinal porcine enterocyte cell line (IPEC-J2) play an important role in the study of zoonotic infections, and are often used as an in vitro model for microbial research (Brosnahan and Brown, 2012). Regarding the intestinal tract, past studies have found that Se deficiency can cause intestinal eosinophilic inflammation (Hong and Chow, 1988), but the specific mechanism of Se deficiency leading to damage is still unclear.
Oxidative stress is a state of imbalance between oxidation and antioxidation, which tends to oxidize and produces a large number of oxidation intermediates. It is considered to be an important inducing factor leading to aging and disease. Selenium has a good antioxidant function that acts as a scavenger of free radicals and other reactive oxygen species (ROS) (Estevam et al., 2015). Therefore, Se deficiency can contribute to oxidative stress and damage to various tissues (Zhong et al., 2011). As a stimulus point for oxidative stress, ROS is an intracellular chemical capable of triggering various biological responses (Glasauer and Chandel, 2013). Intestinal exposure to adverse environments triggers oxidative stress (Wang et al., 2019). Under the condition of Se deficiency, ROS can trigger the nuclear factor kappa beta (NF-kB) inflammation signaling pathway and the intrinsic apoptosis pathway to cause the apoptosis of duodenal villi cells (Wang et al., 2018). Moreover, ROS can mediate autophagy during nutrient deficiency (Filomeni et al., 2015). ROS induces autophagic expression in the nucleus by triggering endoplasmic reticulum (ER) stress. Intracytoplasmic ROS may also influence autophagy by modulating autophagy-related protein 4 (ATG4) activity (Li et al., 2015). Our latest study demonstrated that excessive accumulation of ROS can trigger inflammation and aggravate small intestinal injury (Chen et al., 2021). Our previous experiments have demonstrated that Se deficiency can activate the ROS mediated mitogen-activated protein kinase (MAPK) pathways to regulate autophagy (Cai et al., 2019).
Autophagy is a cellular process that occurs in eukaryotic cells, degrades cytoplasmic content by lysosomal phagocytosis, and recycles large molecules in the cytoplasm (Feng et al., 2014). Some studies have shown that differentially expressed genes caused by Se deficiency can be enriched in the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling pathway (Zhang et al., 2020a, Zhang et al., 2020b), and the mTOR gene is closely related to the autophagy pathway (Wang et al., 2020a, Wang et al., 2020b). and (Lian et al., 2020) have reported that the PI3K/AKT/mTOR pathway promotes autophagy to protect hepatocytes and proximal tubular cells in rats. Autophagy plays a vital role in cell physiology, including adapting to metabolic stress, clearing dangerous goods, renewing cells during differentiation and development, and preventing damage to the genome (Levine and Kroemer, 2019). It has been proven that Se deficiency induces autophagy in cardiomyocytes (Yang et al., 2021). Moreover, the change of calcium ion (Ca2+) homeostasis is one of the main factors affecting autophagy (Kania et al., 2015). The increase of free Ca2+ activated by calmodulin-dependent protein kinase kinase β (CAMKK-β) and adenosine 5′-monophosphate-activated protein kinase (AMPK) in the cytoplasm can be used as an effective inducer of autophagy and also become an ER target of B-cell lymphoma-2 (Bcl-2) against autophagy (Høyer-Hansen et al., 2007). In addition, as the Ca2+-ATPase pump, sarco (endo) plasmic reticulum Ca2+ ATPase (SERCA) plays an important role in regulating cellular Ca2+ homeostasis, and calpain is activated under the influence of a high concentration of Ca2+ (Toral-Ojeda et al., 2016). Also, studies have confirmed the interaction between calpain and SERCA through immunoprecipitation (Moldoveanu et al., 2002). Experimental results of cancer cell lines show that CAMKK-β and AMPK could induce autophagy, and even apoptosis, and this result has also been verified in the experiment of carp (Vara et al., 2011; Hu et al., 2015). Selenium deficiency causes symptoms in the digestive system of swine and triggers intestinal cell damage. However, it is unclear what role Ca2+ homeostasis and autophagy play in Se-deficient intestinal damage.
Consequently, we established an in vivo experiment of Se deficiency in swine small intestine and an in vitro experiment of Se deficiency in IPEC-J2 of swine to explore the mechanism of the autophagy-calcium pathway in Se-deficient intestinal injury.
2. Materials and methods
All procedures used in this study were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University (SRM-11).
2.1. Animals and treatments
A total of 24 healthy pure line big white emasculated piglets of similar weight (chosen from 12 nests, 2 piglets per nest) were weaned for 1 week. Then the piglets were randomly assigned to 2 groups of 12 (adopting the full sibling pairing test): the Se-deficient and control groups. There are 2 repetitions in each group, and 6 piglets in each repetition. The piglets were maintained on either a Se-deficient diet (the Se-deficient group) containing Se at 0.007 mg/kg or a normal selenite diet (the control group) containing Se at 0.300 mg/kg for 16 weeks. The detailed feed ingredients are shown in Table 1. All the piglets were housed under the same conditions from the start of the experiment, and the piglets were fed in their pens. The experimental piglets were fed 3 times each day and provided with free access to drinking water. Small intestine tissue samples were collected and promptly frozen at the 16th week of the experiment. Part of the clean tissue was sliced and immersed in 10% neutral buffered formalin solution and electron microscopy solution and stored at 4 °C, and other parts of the small intestine tissue frozen at −80 °C for future use.
Table 1.
Composition and nutrient levels of the basal diet (DM basis, %).
| Item | Phase 1: |
Phase 2: |
|---|---|---|
| Body weight <25 kg | Body weight >25 kg | |
| Ingredients | ||
| Corn | 66 | 75 |
| Bean cake | 26.4 | 20 |
| Soybean oil | 3.5 | 1.5 |
| Common salt | 0.5 | 0.3 |
| Stone powder | 0.9 | 0.9 |
| Calcium hydrogen phosphate | 1 | 0.9 |
| Premix1 | 1 | 1 |
| Nutrient levels | ||
| Digestive energy, kcal/kg | 3,494.41 | 3,406.7 |
| Crude protein | 15.96 | 14.66 |
| Ca | 0.72 | 0.67 |
| Effective P | 0.29 | 0.27 |
| Lys | 1.23 | 0.98 |
| Met | 0.36 | 0.28 |
| Thr | 0.73 | 0.59 |
| Try | 0.2 | 0.17 |
Premix provided per kilogram of diet: copper (5 mg for phase 1; 4 mg for phase 2), iodine (0.14 mg for both phases), iron (100 mg for phase 1; 60 mg for phase 2), manganese (3 mg for phase 1; 2 mg for phase 2), Zinc (80 mg for phase 1; 60 mg for phase 2); vitamin A (1,750 IU for phase 1; 1,300 IU for phase 2), vitamin D3 (200 IU for phase 1; 150 IU for phase 2), vitamin E (11 IU for both phases), vitamin K3 (0.5 mg for both phases), biotin (0.05 mg for both phases), choline (0.4 g for phase 1; 0.3 g for phase 2), folic acid (0.3 mg for both phases), nicotinic acid (30 mg for both phases), pantothenic acid (9 mg for phase 1; 8 mg for phase 2), riboflavin (3 mg for phase 1; 2.5 mg for phase 2), thiamine (1 mg for both phases), vitamin B6 (3 mg for phase 1; 1 mg for phase 2), vitamin B12 (15 μg for phase 1; 10 μg for phase 2).
2.2. Cell culture and treatment
The IPEC-J2 cell line was obtained from the College of animal science, Northeast Agricultural University and cultured using Dulbecco's modified Eagle medium DMEM-high glucose (GIBCO, NY, USA) medium as a liquid environment which contained 10% fetal bovine serum (FBS) (GIBCO, NY, USA), and 1% penicillin-streptomycin (GIBCO, NY, USA). DMEM-High Glucose medium, FBS, and penicillin-streptomycin were all sterilized through a 0.22-μm Millipore filter to remove any contaminants. Cells were fed once a day and subcultured once every 2 to 3 d until the cell density reached 70% to 80%. When passaging, the cells were first inoculated in a culture flask, then incubated for 12 h, to ensure that the cells attached to the culture flask. The original medium was then discarded and the cells were cultured in other mediums. For the Se-deficient group, the cells were cultured in DMEM-high glucose medium with 1% FBS, 1% penicillin-streptomycin, 10 μg/mL insulin, and 5 μg/mL transferrin resulting in Se depletion for at least 5 d (Yan et al., 2013). IPEC-J2 cells in the Se-deficient group needed to be changed every day to remove dead cells. But IPEC-J2 cells in the control group were still cultured on the normal medium, passaged, and allowed to grow for 5 d. The cells were cultured at 37 °C and 5% CO2 and collected for analysis after 5 d.
2.3. Morphological examination of swine small intestine and IPEC-J2 cells
The small intestine tissues and IPEC-J2 cells were fixed in 2.5% glutaraldehyde phosphate-buffered saline, post-fixed with 1% osmium tetroxide, stained with 4.8% uranyl acetate, and finally dehydrated in a graded ethanol series. The ultra-thin sections were cut, incubated with uranyl acetate and lead citrate. The small intestine specimens were visualized using transmission electron microscopy (GEM-1200ES, Japan).
The treatment method of IPEC-J2 cells electron microscope observation was the same as that of tissues.
2.4. Cell autophagy detection
Autophagic staining was measured using a cell autophagy detection assay kit (Beijing Solarbio Science & Technology, Beijing, China). The 10 μmol/L monodansylcadaverine (MDC) staining agent (Dansylcadaverine) was added to the medium containing enterocytes, which was incubated in a constant temperature incubator (37 °C) for 25 min. The medium was discarded and the cells washed with phosphate saline buffer (PBS) (37 °C preheat) 3 times. Finally, cells were collected using a fluorescence microscope at an excitation wavelength of 355 nm and an emission wavelength of 512 nm for observation of fluorescence.
2.5. ROS activities detection
ROS activities were measured using a ROS assay kit (Nanjing Jiancheng Bioengineering Institute, China). And then, 10 μmol/L DCFH-DA (2,7-dichlorofluorescin diacetate) was added in the culture medium, where there were cell samples to be tested, and this was incubated in a constant temperature incubator (37 °C) for 45 min. The medium was discarded and PBS (37 °C preheat) was used to wash the cells 3 times. Finally, the cells were collected for detection of the activities of ROS in excitation wavelength 500 ± 15 nm and emission wavelength 530 ± 20 nm. Enterocytes were visualized using fluorescence microscopy.
2.6. Intracellular Ca2+ concentration detection
The fluo-3-pentaacetoxymethyl ester (Fluo-3AM) assay kit (Beijing Solarbio Science & Technology Co., Ltd) was used to detect intracellular Ca2+ concentration. After 4 d of treatment, the cells were cultured in 6-well plates and were digested with trypsin digestion solution (0.1%), then the cells were resuspended and plated in 12-well plates for 24 h. The Fluo-3AM mother liquor was diluted with PBS until the concentration reached 1 μmol/L for use. The cells were washed with PBS before they were covered with the diluted working fluid. After incubating at 37 °C for 40 min, the cells were cleaned and observed under a fluorescence microscope.
2.7. Total RNA extraction and determination of the mRNA expression of the autophagy-calcium homeostasis related genes
Total RNA was isolated from small intestine tissues and enterocytes using Trizol reagent according to the manufacturer's instructions (Invitrogen, Shanghai China). The dried RNA pellets were resuspended in 50 μL of diethyl-pyrocarbon-ate-treated water. The concentration and purity of the total RNA were determined by a spectrophotometer. cDNA was synthesized from 5 μg of the total RNA using oligo dT primers and Superscript II reverse transcriptase according to the manufacturer's instructions (Promega, Beijing, China), and cDNA was stored at −80 °C (Shi et al., 2018).
Specific primers including Beclin 1, GABA(A) receptor-associated protein 1 (LC3-1), GABA(A) receptor-associated protein 2 (LC3-2), autophagy-related protein 5 (ATG5), autophagy-related protein 12 (ATG12), autophagy-related protein 16 (ATG16), mammalian target of Rapamycin (mTOR), calmodulin-dependent protein kinase kinase β (CAMKK-β), adenosine 5′-monophosphate-activated protein kinase (AMPK), sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA), calpain for target genes (Table 2) were designed based on known sequences using Primer-BLAST at the National Center for Biotechnology Information (NCBI). Quantitative real-time PCR (qRT-PCR) was performed with a BIOER detection system (China, Hangzhou). Reactions were performed in a 10 μL reaction mixture containing 5 μL of 2 × SYBR Green I PCR Master Mix (R),1 μL of cDNA, 0.3 μL of each primer (10 μmol/L), and 3.4 μL of PCR-grade water. The relative abundance of each mRNA was calculated according to the 2−ΔΔCt method and normalized to the mean expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Table 2.
Gene-special primers used in the quantitative real-time PCR.
| Gene | Primer sequence (5′→3′) |
|---|---|
| GAPDH | Forward: GTGAACGGGTGAGTTAGGGG |
| Reverse: CGATGCGGCCAAATCTTGAG | |
| Beclin1 | Forward: GCAGGTGAGCTTCGTGTGTCAG |
| Reverse: CCTGGGCTGTGGCAAGTAATGG | |
| LC3-1 | Forward: CCAGTCCTGGACAAGACCAAGTTC |
| Reverse: GGTTCACCAGCAGGAAGAAGGC | |
| LC3-2 | Forward: TTCCTGGTGCCTGATCATGTCAAC |
| Reverse: ACTCACCATGCTATGTCCGTTCAC | |
| ATG5 | Forward: AGAAACCTAGAGAGGGCCACA |
| Reverse: TCTTCCTAGTCAAACAACGTCA | |
| ATG12 | Forward: CAACTGCTGCTGAGGGCGATG |
| Reverse: CACCGGCAGGTTCTTCTGTTCC | |
| ATG16 | Forward: TCGCAGAAGCAGCAAAGGAACC |
| Reverse: TGATGGCTCGCACGGGAGAG | |
| mTOR | Forward: GCACGTCAGCACCATCAACCTC |
| Reverse: GCCTCAGCCATTCCAACCAGTC | |
| DIO1 | Forward: GCTGAAGGTCCGATGGCAACG |
| Reverse: AGATGTGGCACCTCTGTCCTGAC | |
| DIO2 | Forward: TGGTGGAGGAGTTCTCATCAGTGG |
| Reverse: GCACATCGGTCTTCCTGGTTCTG | |
| DIO3 | Forward: CAACAGTGATGGCGACGAGGTG |
| Reverse: CGAGGATGTGCTGGTTCTGGAAG | |
| TXNRD1 | Forward: GCTCAAGTGCGGACTGACCAAG |
| Reverse: AGCAACCGGCCTGGAGGATG | |
| TXNRD2 | Forward: ACGGTCTTCACGCCACTGGAG |
| Reverse: CGCATCTCGTTCAGGCACTGTG | |
| TXNRD3 | Forward: ATGTCACTCAGAAGGGCTGC |
| Reverse: TCCAGGGACGATGGATGAGT | |
| GPX1 | Forward: GCGTCGCTCTGAGGCACAAC |
| Reverse: GGTCGGACGTACTTGAGGCAATTC | |
| GPX2 | Forward: CTCGCTCTGAGGCACAACCAC |
| Reverse: GCGGACGTACTTGAGGCTGTTC | |
| GPX3 | Forward: AGAACGCCTGTCCTCCTACTTCG |
| Reverse: GTTGATGGTGGTGCGGTGGTAC | |
| GPX4 | Forward: GCCTGTTCCGCCTGCTGAAG |
| Reverse: GGTGACGATGCACACGTAGCC | |
| GPX7 | Forward: GGCAAGTTGGTGTCGCTGGAG |
| Reverse: GCGGTAGTGCTGGTCTGTGAAG | |
| SELT | Forward: CGATGCCTCGGCCAACATGG |
| Reverse: CTCTCCTTCAATGCGGATGTCTGG | |
| SELS | Forward: GGCTGGTACATCGTCTTCTGCTG |
| Reverse: TCCAGGTGCCTCTGCCTCAAG | |
| SELM | Forward: GCTCGGCTTCTATCGCAAGGC |
| Reverse: TACAGGTCAGCGCGGTCAGG | |
| SELO | Forward: GCCGACTTCACCAACACCTTCTAC |
| Reverse: AGCCAGGAACTCAGCCAGGTC | |
| SELH | Forward: GCTTCGAGGTGACGCTGATGC |
| Reverse: CAGCTCCTCCACCACCTCTTGAG | |
| SELI | Forward: TTCAGCACCAGGTCACAAGCATG |
| Reverse: TCCACACCATCCAGAGTGTAGGC | |
| SELK | Forward: GCGTTGGACAGCAGGAGTCAG |
| Reverse: GGAATCAGATGAGCCTCCATAGCC | |
| SELW | Forward: GCCGTTCGAGTCGTCTATTGTGG |
| Reverse: GGAGTGAACCAACTTCCTGCTACC | |
| SELP | Forward: CATCACCATCGCCATCACCATCAC |
| Reverse: GGCTGCTCAGGAGCGTCTGG | |
| SEPX1 | Forward: ACCGAGACCATCCACGCTGAC |
| Reverse: GTTGCCACATCTGCCACAGGAC | |
| SPS2 | Forward: CTCCTCAGCGTCAGCCAGAATATG |
| Reverse: CCACTCCGCCGATGATAATCCAAG | |
| AMPK | Forward: CACCAGGACCCTTTGGCAGTTG |
| Reverse: TCTTTCAGGATGGGGCCGAGTC | |
| CAMKK | Forward: GAGTCGCACCATGTCTCCATCAC |
| Reverse: GCCAACTTGACCACGCCATAGG | |
| Calpain | Forward: AACACAAAGACCTGCGGACCAAG |
| Reverse: CCAGCTTCCCGTTGCCATCAC | |
| SERCA | Forward: CCTTACCAGTCATCGGGCTC |
| Reverse: GACACGGTTCAAAGAGGTGGA |
GAPDH = glyceraldehyde-3-phosphate dehydrogenase; LC3-1 = microtubule-associated proteins 1A; LC3-2 = microtubule-associated proteins 1B; ATG = autophagy-related protein; mTOR = mammalian target of rapamycin; DIO = deiodinase; TXNRD = thioredoxin reductase; GPX = glutathione peroxidase; SELT = selenoprotein T; SELS = selenoprotein S; SELM = selenoprotein M; SELO = selenoprotein O; SELH = selenoprotein H; SELI = selenoprotein I; SELK = selenoprotein K; SELW = selenoprotein W; SELP = selenoprotein P; SEPX1 = recombinant selenoprotein X1; SPS2 = selenophosphohate synthase 2; AMPK = adenosine 5′-monophosphate-activated protein kinase; CAMKK = calmodulin-dependent protein kinase; SERCA = sarco(endo)plasmic reticulum Ca2+-ATPase.
2.8. Detection of selenoproteins
Detection of selenoprotein content in small intestine tissue and IPEC-J2 cells was performed by qRT-PCR, whose method was the same as the detection of autophagy-calcium target genes, and the specific primers for selenoprotein-related genes including glutathione peroxidase (GPX) 1 to 4 and 7, thioredoxin reductase (TXRND) 1 to 3, deiodinase (DIO) 1 to 3, selenoprotein H (SELH), selenoprotein O (SELO), selenoprotein M (SELM), selenoprotein S (SELS), selenoprotein 1 (SEP1S), selenoprotein W (SEPW), selenophosphohate synthase 2 (SPS2), recombinant selenoprotein X1 (SEPX1), selenoprotein T (SELT), selenoprotein I (SELI), selenoprotein P (SEPP) (Table 2) were designed based on known sequences using Primer-BLAST at the NCBI.
2.9. Total protein extraction and determination of the protein expression of autophagy-calcium homeostasis related genes
Total protein was extracted from small intestine tissues and enterocytes by lysis buffer for Western blotting with phenylmethanesulfonyl fluoride (PMSF) (100 mmol/L). These extracts were subjected to Sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions. Separated proteins were transferred to nitrocellulose membranes in Tris-glycine buffer containing 20% methanol at 4 °C. The membranes were blocked with 5% skim milk for 2 h and incubated overnight with diluted primary antibodies against Beclin 1 (1:500, Wanleibio, China), LC3 (1:500, ABclonal, China), mTOR (1:500, Wanleibio, China), AMPK (1:800, the polyclonal antibody produced by our lab), CAMKK-β (1:500, Proteintech, China), SERCA (1:500, the polyclonal antibody produced by our lab) and β-actin (1:10,000, ABclonal, China) followed by goat anti-rabbit IgG (H + L) (1:10,000, Immuno Way, China). The signal was detected using an enhanced chemiluminescence system (Zheng et al., 2020).
2.10. Ions’ detection of swine small intestine tissue
Inductively coupled plasma mass spectroscopy (ICP-MS) method was used to detect the levels of 23 ions in the small intestine tissue of the control group and the Se-deficiency group. This was started and measured under optimized instrument working conditions.
2.11. Statistical analysis
Each group in the in vivo experiment consisted of 6 single observation replications (n = 6), and 2 parallel experiments were performed to ensure the accuracy of the experimental data. And, each group in the in vitro experiment consisted of 3 single observation replications (n = 3), and 3 parallel experiments were performed to ensure the accuracy of the experimental data. The data are expressed as the mean ± standard deviation (SD), and GraphPad Prism v8.0 software was used for all the statistical analyses and the Single-sample Kolmogorov–Smirnov test t-tests showed that the data were normally distributed. The data were compared using a t-test analysis of variance to determine the difference between the control group and the Se-deficient group. An asterisk (∗) denotes a significant difference from the corresponding control (P < 0.05).
3. Results
3.1. Selenium deficiency induced swine intestinal autophagy
3.1.1. Ultrastructural observation of autophagosomes in swine small intestine
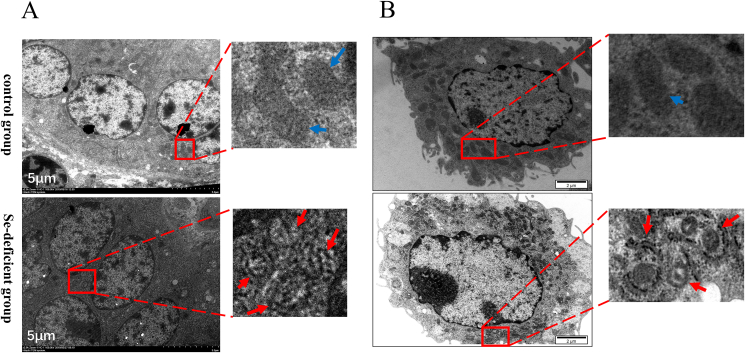
The ultrastructure of the swine small intestine tissue (Fig. 1A) of the control group and the Se-deficient group was observed by a transmission electron microscope. In the control group, normal mitochondria and a few lysosomes were observed. However, a large number of autophagic vesicles with a double-layer membrane structure and seldom lysosomes appeared in the Se-deficient group.
Fig. 1.
Ultrastructural observation of swine small intestine tissues and IPEC-J2 cells. (A) Swine small intestine tissues (scale bar, 5 μm) and (B) IPEC-J2 cells were small intestine tissues observed using TEM (scale bar, 2 μm). Blue arrows pointing to normal mitochondria. Red arrows pointing to autophagosome. IPEC-J2 = intestinal porcine enterocyte cell line; TEM = transmission electron microscope.
3.1.2. Ultrastructural observation of autophagosomes in IPEC-J2 cells
The ultrastructure of the control group and the Se-deficient group of IPEC-J2 cells (Fig. 1B) was observed by a transmission electron microscope. In the control group of IPEC-J2 cells, a large number of normal mitochondria could be observed. There were a small number of autophagy lysosomal vesicles characterized by incomplete boundary membrane, intact intima, and amorphous substances. There were also a small number of autophagic vesicles that did not contain substances. In the Se-deficient group, the cytoplasm mainly consisted of autophagic lysosome vesicles, which contained mitochondria. There were also a large number of secondary lysosomes and autophagic vesicles, as well as a small number of mitochondria with normal morphology.
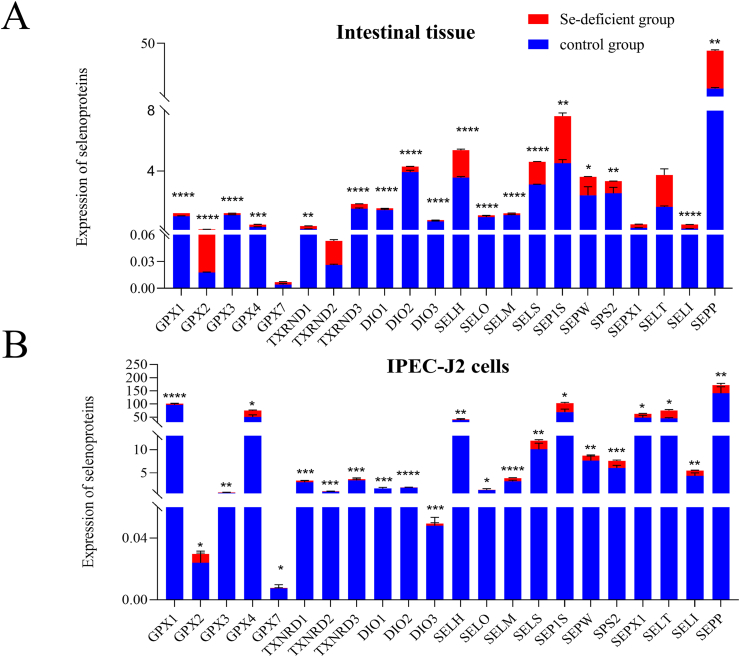
3.2. Effects of selenium deficiency on the selenium content of swine small intestine tissue and IPEC-J2 cells
In small intestine tissue and IPEC-J2 cells, the expression of 22 selenoproteins in the control group was higher than that in the Se-deficient group (Fig. 2). It should be noted that the expression of GPX2 in the tissue Se-deficiency group was slightly higher than that of the control group.
Fig. 2.
The mRNA expressions of 22 selenoproteins in IPEC-J2 cells and swine small intestine tissues. (A) Selenoproteins expressions in small intestine tissues on mRNA level. (B) Selenoproteins expressions in IPEC-J2 cells on mRNA level. The ∗ indicates significant differences (∗P < 0.05, ∗∗P < 0.002, ∗∗∗P < 0.0002, ∗∗∗∗P < 0.0001) between the 2 groups. Results were expressed as means ± SD (n = 6). IPEC-J2 = intestinal porcine enterocyte cell line; GPX = glutathione peroxidase; TXRND = thioredoxin reductase; DIO = deiodinase; SELT = selenoprotein T; SELS = selenoprotein S; SELM = selenoprotein M; SELO = selenoprotein O; SELH = selenoprotein H; SELI = selenoprotein I; SELK = selenoprotein K; SELW = selenoprotein W; SELP = selenoprotein P; SEPX1 = recombinant selenoprotein X1; SPS2 = selenophosphohate synthase 2.
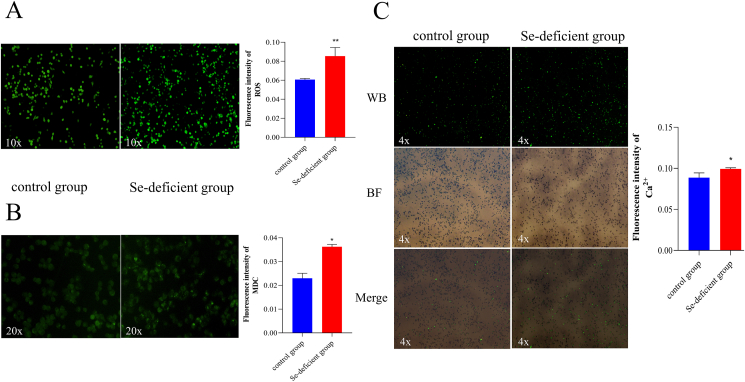
3.3. Effects of selenium deficiency on ROS viability in IPEC-J2 cells
Affected by the lack of Se, the ROS activities of the 2 groups are shown in Fig. 3A. The ROS activity of the Se-deficient group had a significant increase compared to the control group (P < 0.01).
Fig. 3.
Cell staining results. (A) ROS fluorescence of IPEC-J2 cells was detected by fluorescence microscope (10× magnification). And the ROS fluorescence intensity quantitative analysis by Image J (20× magnification). (B) Autophagy fluorescence of IPEC-J2 cells was detected by fluorescence microscope (4 × magnification). And the autophagy fluorescence intensity quantitative analysis by Image J. (C) The fluorescence of cytoplasm Ca2+ in IPEC-J2 cells was detected by the fluorescence microscope in WB, BF, and merged in 4 times mirror. Green fluorescence is cytoplasmic calcium. The Ca2+ fluorescence intensity quantitative analysis by Image J. The ∗ indicates significant differences (∗P < 0.05, ∗∗P < 0.002, ∗∗∗P < 0.0002, ∗∗∗∗P < 0.0001) between the 2 groups, and the data are presented as the mean ± SD (n = 6). ROS = reactive oxygen species; IPEC-J2 = intestinal porcine enterocyte cell line; MDC = monodansylcadaverine; WB = blue excitation light; BF = bright field.
3.4. Effects of selenium deficiency on autophagy in IPEC-J2 cells
Dansylcadaverine-MDC is a fluorescent pigment that stains normal cells into a uniform yellowish-green, and autophagy becomes bright green. The IPEC-J2 cells in the control group were yellowish-green with few green highlights (Fig. 3B). Compared with the control group, the bright green spots of the Se-deficiency group were dense, which meant that the Se-deficient group had more autophagosome accumulation (P < 0.01).
3.5. Effects of selenium deficiency on the concentration of Ca2+ in IPEC-J2 cells
The lack of Se caused an increase in the cytoplasmic Ca2+ concentration of the Se-deficiency group (Fig. 3C). Compared with the control group, the Se-deficiency group had more bright green spots, and calcium overload occurred significantly (P < 0.05).
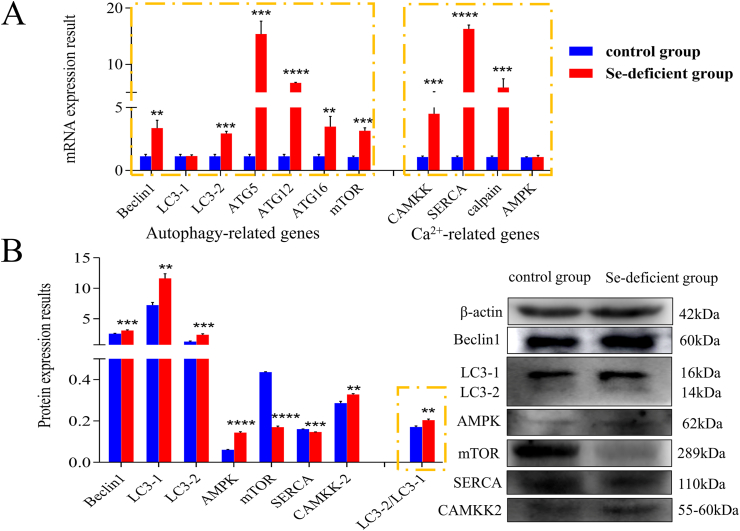
3.6. Protein and mRNA expression of autophagy-Ca2+ related genes in swine small intestine tissues
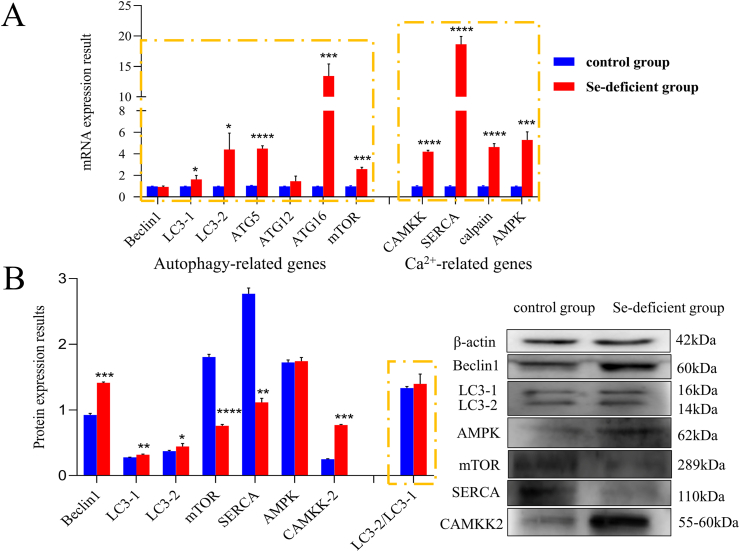
Affected by Se deficiency, protein and mRNA expression abundance of autophagy-related genes in small intestine tissues are shown in Fig. 4.
Fig. 4.
Detection of autophagy-Ca2+ homeostasis-related genes in small intestine tissue. The expression of autophagy-Ca2+ homeostasis related genes at (A) mRNA and (B) protein levels in small intestine tissue of the control and selenium-deficient groups, respectively. The ∗ indicates significant differences (∗P < 0.05, ∗∗P < 0.002, ∗∗∗P < 0.0002, ∗∗∗∗P < 0.0001) between the 2 groups, and the data are presented as the mean ± SD (n = 6). LC3 = microtubule-associated proteins; ATG = autophagy-related protein; mTOR = mammalian target; CAMKK-β = calmodulin-dependent protein kinase kinase β; AMPK = adenosine 5′-monophosphate-activated protein kinase; SERCA = sarco(endo)plasmic reticulum Ca2+-ATPase.
Quantitative real-time PCR results revealed that mRNA expression of autophagy-related genes (Beclin1, LC3-1, LC3-2, ATG5, ATG12, ATG16, mTOR) significantly increased (P < 0.05) in the Se-deficient group, compared with the control group. Similarly, the mRNA expression of Ca2+ pathway-related genes (CAMKK, SERCA, and calpain) in the Se-deficiency group also increased significantly compared to the control group. But the mRNA expression of LC3-1 and AMPK increased slightly, they did not show significant differences (P > 0.05). Meanwhile, the protein expression of Beclin1, LC3-1, LC3-2, AMPK, CAMKK-β significantly increased (P < 0.05) in the Se-deficient group compared with the control group respectively. The protein expression of SERCA and mTOR in the Se-deficiency group was significantly lower than that in the control group (P < 0.01). The expression results of the above genes indicated that Se deficiency caused Ca2+ overload and autophagy accumulation in the swine small intestine.
3.7. Protein and mRNA expression of autophagy-Ca2+ related genes in IPEC-J2 cells
Affected by Se deficiency, protein and mRNA expression abundance of autophagy-related genes in IPEC-J2 cells are shown in Fig. 5. Similar to the expression in small intestine tissue, compared with the control group, autophagy-related genes (LC3-1, LC3-2, ATG5, ATG12, ATG16, mTOR) were significantly increased in the mRNA expression of Se-deficient group IPEC-J2 (Fig. 5A). The mRNA expression of Beclin1 was not significantly different in the 2 groups. The mRNA expression of Ca2+ related genes (CAMKK, SERCA, calpain, AMPK) had the same trend as that of the main autophagy-related genes, and the expression in the Se-deficiency group was significantly higher than that in the control group (P < 0.01).
Fig. 5.
Detection of autophagy-Ca2+ homeostasis-related genes in IPEC-J2 cells. The expression of autophagy-Ca2+ homeostasis related genes at (A) mRNA and (B) protein levels in IPEC-J2 cells of the control and selenium-deficient groups, respectively. The ∗ in indicates significant differences (∗P < 0.05, ∗∗P < 0.002, ∗∗∗P < 0.0002, ∗∗∗∗P < 0.0001) between the 2 groups, and the data are presented as the mean ± SD (n = 6). IPEC-J2 = intestinal porcine enterocyte cell line; LC3 = microtubule-associated proteins; ATG = autophagy-related protein; mTOR = mammalian target; CAMKK = calmodulin-dependent protein kinase kinase; AMPK = adenosine 5′-monophosphate-activated protein kinase; SERCA = sarco(endo)plasmic reticulum Ca2+-ATPase.
The change in protein expressions of autophagy-Ca2+ related genes is shown in Fig. 5B. As detected, compared with the control group, the protein expressions of Beclin1, LC3-1, LC3-2, and ATG16 were significantly increased. However, the protein expression of SERCA in the Se-deficiency group was significantly lower than that in the control group. The results of gene expression in IPEC-J2 cells are the same as in vivo, showing that Se deficiency can lead to Ca2+ overload and autophagy accumulation in cells.
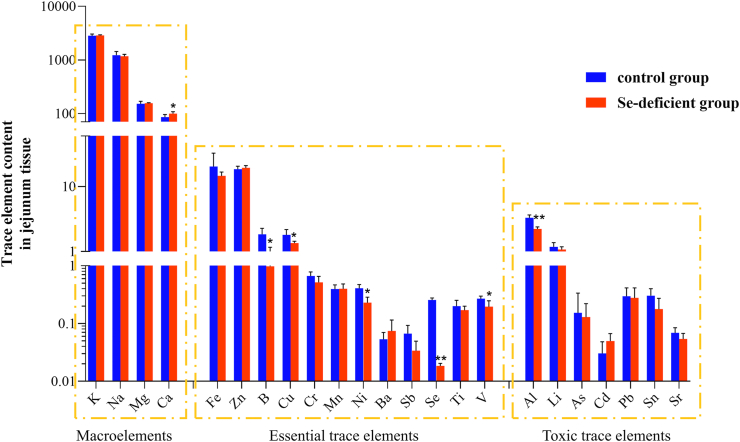
3.8. Results of detection and analysis of multiple elements in small intestine tissue
The detection results of 23 elements in swine small intestine tissue are shown in Fig. 6. It can be roughly divided into 3 groups of main elements: macroelements (sodium [Na], magnesium [Mg], potassium [K], calcium [Ca]), essential trace elements (ferrum [Fe], zinc [Zn], boron [B], copper [Cu], manganese [Mn], nickel [Ni], barium [Ba], antimony [Sb], selenium [Se], titanium [Ti], vanadium [V]), and toxic trace elements (aluminum [Al], lithium [Li], arsenic [As], cadmium [Cd], palladium [Pd], tin [Sn], strontium [Sr]). The results showed that Se deficiency caused an increase in the major element Ca (P < 0.05), and the levels of other major elements were basically not affected (P > 0.05). The content of essential trace elements B, Cu, Ni, V was significantly reduced (P < 0.05), and other essential trace elements were not affected (P > 0.05). At the same time, Se deficiency led to a decrease in the content of toxic trace element Al (P < 0.05). The results showed that the lack of Se caused the imbalance of the ion level in the swine small intestine tissue, the compensation of Ca increased, and the loss of B, Cu, Ni, V, and Al.
Fig. 6.
Using ICP-MS technology detected 23 kinds of trace element ion levels, roughly divided into 3 groups of main elements: macroelements (Na, Mg, K, Ca), essential trace elements (Fe, Zn, B, Cu, Mn, Ni, Ba, Sb, Se, Ti, and V), and toxic trace elements (Al, Li, As, Cd, Pd, Sn, and Sr). The ∗ indicates significant differences (∗P < 0.05, ∗∗P < 0.002, ∗∗∗P < 0.0002, ∗∗∗∗P < 0.0001) between the 2 groups, and the data are presented as the mean ± SD (n = 6). ICP-MS = inductively coupled plasma mass spectroscopy.
4. Discussion
Selenium is an indispensable trace element in the body and affects organisms' health. Selenium deficiency can cause swine mulberry heart disease and has a particularly significant effect on the digestive system of swine (Teige et al., 1982), which can lead to enteritis (Smith et al., 2011) and even death. Although different forms of Se supplements are convenient methods to treat these diseases, Se can be supplemented to the host by adding it to a daily diet. This method has been proven to be effective in reducing the incidence and mortality in mouse models and human colon cancers (Irons et al., 2006). However, there are still many factors that affect its effectiveness, such as the chemical form of Se additives and the health of animals (Hefnawy and Tórtora-Pérez, 2010).
In collaboration with the Chinese Academy of Agricultural Sciences, we constructed a selenium deficiency model for swine. The production performance including feed intake, weight, and feed utilization rate showed that there was a significant difference between the control group and the Se-deficient group. The production performance of the Se-deficient group was significantly lower than that in the control group (Tang et al., 2020). The clinical manifestations of the Se-deficient group were normal body temperature, but mental retardation, gray skin, muscle weakness, mild edema in abdomen and buttocks, swing of hind limbs during the walking, tremor, and mild diarrhea during forced exercise. The swine in the control group were healthy and had no obvious clinical symptoms, and the data are shown in an unpublished study of our collaborators.
Some studies have shown that dietary Se affects the host's intestinal flora balance and gastrointestinal colonization, thereby affecting the host's Se status and the expressions of selenoproteins (Kasaikina et al., 2011). Selenoproteins are involved in the regulation of cellular redox homeostasis, and the protection of oxidative stress (Schomburg and Schweizer, 2009). Besides, the study has shown that Se deficiency can change the distribution and steady-state of other minerals, which had been confirmed again in our experiment (Zhang et al., 2021). In this study, we established the Se deficiency model in swine and IPEC-J2 cells, in which results showed that Se deficiency caused a downward trend in the overall selenoproteins expressions and disrupted the balance of intestinal trace elements. Selenium deficiency induced the occurrence of oxidative stress, the imbalance of Ca2+ homeostasis in the small intestine and in vitro, and ultimately promoted autophagy.
Selenoproteins are mainly divided into 3 categories: GPX, TXNRD, and DIO. Most selenoproteins are widely found in various tissues and organs of the body, and a few selenoproteins only exist or express in specific tissues. Because of the wide variety, wide distribution, and different subcellular locations of selenoproteins, they have various functions (Zhu et al., 2017). For example, studies have shown that GPX has an antioxidant effect (Guillin et al., 2019). TXNRD families are widely distributed in cytoplasm and organs (St Germain, 1988), which is very important for cell proliferation, differentiation, development, and death (Amantana et al., 2002). The function of DIO family selenoproteins is mainly through the influence of thyroxine metabolism (Kuiper et al., 2005), and then on growth and development and energy metabolism (St Germain et al., 2009). SELT can also regulate the intracellular Ca2+ homeostasis through the redox mechanism, and overexpression of SELT can significantly increase the Ca2+ concentration in cells (Grumolato et al., 2008). In the IPEC-J2 cells results, all selenoproteins were lower in the Se-deficient group than in the control group. The expression of GPX1, GPX3, GPX4, TXRND1, TXRND3, DIO1, DIO2, DIO3, SELH, SELO, SELM, SELS, SEP1S, SEPW, SPS2, SEPX1, SELT, SELI, and SEPP in the Se-deficient group was decreased. However, the expression of GPX2 was increased in the Se-deficient group, and there was no significant difference between GPX7 and TXRND2. GPX2 is distributed in the gastrointestinal epithelium, which is a barrier to the gastrointestinal tract against endogenous H2O2. It is reported that the expression of GPX2 can be decreased or increased by knocking down and activating nuclear factor erythroid-2 related factor 2 (NRF2) in rat lung gland epithelial cells (Bianco et al., 2002). Therefore, the increase of GPX2 expression in Se-deficient intestinal tissues may be due to the influence of other organs in vivo.
Selenium is involved in regulating ROS levels and redox balance in all tissues (Bizerea et al., 2018), a large number of studies have shown that ROS is an important target for tissue damage caused by Se deficiency (Gao et al., 2019). found that Se deficiency could stimulate ROS-induced inflammation. Selenium can be used as a ROS scavenger to exert additive effects on the proliferation and paracrine in human amniotic fluid-derived mesenchymal stem cells (Park et al., 2018). Due to the combined effect of Se deficiency and low protein intake, the levels of ROS in the serum and myocardial tissue defects of rats are significantly increased. Eventually, this causes myocardial oxidative stress and induces apoptosis through mitochondrial-mediated pathways (Zhang et al., 2019a, Zhang et al., 2019b). The imbalance between ROS production and the elimination of protective mechanisms may lead to chronic inflammation (Hussain et al., 2016). Also, our previous research showed that Se deficiency did cause inflammation in swine intestinal tissue and IPEC-J2 cells (Zhang et al., 2020a, Zhang et al., 2020b). In our present experiment, it was observed that ROS production in IPEC-J2 cells increased due to Se deficiency stimulation. Similar to the specific expansion of myeloid cells that causes excessive ROS and promotes intestinal pathology (El-Kenawi and Ruffell, 2017), it is believed that the swine intestinal damage caused by Se deficiency is also closely related to the accumulation of ROS. Besides, studies have shown that the addition of trace element chelating agents can improve antioxidant capacity and immune function (Liu et al., 2015). But excessive cadmium can promote oxidative stress (Zhang et al., 2017; Chi et al., 2020). Therefore, we speculate that the destruction of the homeostasis of trace elements in our experiment may also be one of the reasons for the accumulation of ROS.
A large number of reports indicate that ROS is an early inducer of autophagy during nutritional deficiencies (Murphy, 2009). NADPH oxidase 2 (NOX2), which produces ROS, is the key for phagocytes to kill microorganisms and recruit LC3 into phagocytes (Huang et al., 2009). ROS can induce autophagy by activating the mucolipin 1-lysosome Ca2+-TFEB pathway to eliminate damaged mitochondria and excess ROS (Zhang et al., 2016). Our previous research found that Se deficiency could inhibit the expression of troponin T (TNNT2), thereby activating the channel for Ca2+ to flow outside the cell, and inhibiting the channel for Ca2+ to flow inward (Yang et al., 2019). AMPK is an important signaling molecule for Ca2+-dependent autophagy activation (Ji et al., 2018). Silver nanoparticles (AgNP) induces ER stress and autophagy through the Ca2+/CAMKK/AMPK/mTOR pathway. The specific mechanism is that CAMKK and AMPK are activated by the increase of Ca2+ levels in the cytoplasm after being stimulated by AgNP, which leads to the downregulation of mTOR and the upregulation of Beclin1, eventually activating autophagy (Li et al., 2019a, Li et al., 2019b). In our experiment, Se deficiency also caused the accumulation of autophagy in the swine small intestine by increasing the expression of CAMKK-β and AMPK in the Ca2+/CAMKK-β/AMPK/mTOR pathway and decreasing the expression of mTOR. However, it is worth noting that the mRNA expression level of Beclin1 in IPEC-J2 cells did not show a significant difference between the control and the Se-deficient groups (Fig. 5), and the protein expression of mTOR was not consistent with the mRNA expression results. The mRNA expression level of mTOR was higher in the Se-deficient group than in the control group, and the protein expression level was lower in the Se-deficient group than in the control group. There are many reasons for the inconsistent levels of mRNA and protein expression, as follows: mRNA itself is regulated by various molecules such as miRNA; the degradation rate of mRNA is faster than that of protein; and post-transcriptional mRNA is not expressed as protein until it is processed through multiple levels of complexity including post-transcriptional processing, translation, and post-translational modification. We believe that there may be other pathway genes that are stimulated by Se deficiency at the mRNA level and inhibit the expressions of Beclin1 and mTOR. For example, Bcl-2, an anti-apoptotic protein, which interacts with Beclin1 to regulate autophagy, keeps cells alive rather than dead (Pattingre et al., 2005). Growth factor/RTK/PI3K signaling can activate mTOR to regulate cell survival and metabolism through protein kinase A, G, and C family (AGC) kinases (Kim and Guan, 2015). But the protein determines the phenotype to a greater extent, so the expression level of the protein is more informative. Both suppressed mTOR protein expression and elevated Beclin1 protein expression in the Se-deficiency group indicated that selenium deficiency promoted autophagy.
Autophagy can prevent cell damage and promote cell survival in the absence of nutrition, and can also respond to cytotoxic damage (Dikic and Elazar, 2018). Therefore, autophagy is sensitive to Se deficiency. The study has shown that Se deficiency can cause increased expression of autophagy in the chicken spleen, bursa, and thymus and cause damage to chicken immune organs (Khoso et al., 2017). Our previous investigation on Se nutrition has shown that Se deficiency can cause autophagy in cardiomyocytes (Yang et al., 2017). Autophagy can antagonize apoptosis mechanisms in cardiomyocytes knocked out of the glutathione peroxidase 3 (GPX3) gene, a kind of selenoproteins (Gong et al., 2019). Autophagy plays a key role in regulating the interaction between the gut microbiota and innate, and adaptive immunity and the host's defense against intestinal pathogens, maintaining intestinal homeostasis (Mizushima, 2018). Starvation allows autophagy to selectively reduce epithelial tight junction permeability to ions and small molecules to enhance intestinal epithelial tight junction barrier function (Nighot et al., 2015). Our results suggest that due to the stimulation of Se deficiency, Ca2+ homeostasis and ROS homeostasis were destroyed, which resulted in the overload of Ca2+ and ROS in cytoplasm, leading to increased autophagy. Autophagy plays an active role in maintaining the function in IPEC-J2 cells and protecting the small intestine from injury under Se deficiency. Blocking the healthy autophagy pathway increases the risk of enteritis (Hu et al., 2015).
However, it is too early to say that autophagy effectively protects swine small intestine injury caused by Se deficiency. Because we observed the apoptotic bodies such as the formation of apoptotic bodies and the shrinkage of the nucleus while observing the Se-deficient group autophagic vesicles, we suspected that Se deficiency may also trigger apoptosis in small intestines. It may be due to excessive autophagy or damage that has exceeded cell death caused by autophagic protection. The study has shown that autophagy maintains the metabolism and vitality of tumor cells during the period of lack of nutrition, but chronic nutritional deficiency will lead to cell death (Jin et al., 2007). Moreover, oxidative stress is also a key factor in triggering apoptosis (Liu et al., 2020; Wang et al., 2020a, Wang et al., 2020b, 2021).
Attention should also be paid to the destruction of element balance. In our experiment, the contents of B, V, and Cu were reduced in Se-deficiency, which could increase the risk of dementia, autism, and depression (Janka, 2019). Decreased levels of Ni and Al affect fasting blood glucose (Li et al., 2019a, Li et al., 2019b). Increased Ca content can exacerbate the risk of diabetes, even accumulation of Ca2+ in mitochondria and promote apoptosis (Kahya et al., 2017). Calcium accumulation is often regarded as one of the signs of injury (Wang et al., 2020b).
5. Conclusions
Our research showed that the lack of Se nutrition could destroy the Ca2+ homeostasis of the swine small intestine and trigger the overload of ROS, which ultimately leads to the accumulation of autophagy. The key mechanism for promoting autophagy is the CAMKK-β-AMPK-mTOR pathway. Selenium deficiency also destroys the balance of other elements, increasing the risk of physical and even psychological diseases. However, the specific relationship and mechanism of crosstalk between autophagy and apoptosis will be explored in the next experiments. Although many questions remain, these insights open a new line of investigation concerning how Se deficiency modulates intestinal autophagy.
Author contributions
Ziwei Zhang conceived and designed the experiments, and critically reviewed the manuscript. Yingying Zheng performed the experiments, analyzed the data and wrote the article. Haoyue Guan, Jie Yang, Jingzeng Cai, and Qi Liu assisted in analyzing the data and reviewing the manuscript.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgment
This study was supported by the Natural Science Foundation of Heilongjiang Province of China (YQ2021C021).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Amantana Adams, Vorachek William R., Butler Judith A., Costa Nicholas D., Whanger Philip D. Effect of copper, zinc and cadmium on the promoter of selenoprotein W in glial and myoblast cells. J Inorg Biochem. 2002;91:356–362. doi: 10.1016/s0162-0134(02)00453-1. [DOI] [PubMed] [Google Scholar]
- Bianco Antonio C., Domenico Salvatore, Balázs Gereben, Berry Marla J., Reed Larsen P. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- Bizerea Teofana O., Dezsi Stefana G., Otilia Marginean, Ramona Stroescu, Alexandru Rogobete, Otilia Bizerea-Spiridon. The link between selenium, oxidative stress and pregnancy induced hypertensive disorders. Clin Lab. 2018;64:1593–1610. doi: 10.7754/Clin.Lab.2018.180307. [DOI] [PubMed] [Google Scholar]
- Bomer Nils, Grote Beverborg Niels, Hoes Martijn F., Streng Koen W., Mathilde Vermeer, Dokter Martin M. Selenium and outcome in heart failure. Eur J Heart Fail. 2020;22:1415–1423. doi: 10.1002/ejhf.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnahan Amanda J., Brown David R. Porcine IPEC-J2 intestinal epithelial cells in microbiological investigations. Vet Microbiol. 2012;156:229–237. doi: 10.1016/j.vetmic.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Jingzeng, Yang Jie, Qi Liu, Gong Yafan, Zhang Yuan, Zheng Yingying. Mir-215-5p induces autophagy by targeting PI3K and activating ROS-mediated MAPK pathways in cardiomyocytes of chicken. J Inorg Biochem. 2019;193:60–69. doi: 10.1016/j.jinorgbio.2019.01.010. [DOI] [PubMed] [Google Scholar]
- Chen Xiaoming, Mingyu Bi, Yang Jie, Cai Jingzeng, Zhang Haoran, Zhu Yue. Cadmium exposure triggers oxidative stress, necroptosis, Th1/Th2 imbalance and promotes inflammation through the TNF-α/NF-κB pathway in swine small intestine. J Hazard Mater. 2021;20:421. doi: 10.1016/j.jhazmat.2021.126704. 126704. [DOI] [PubMed] [Google Scholar]
- Chi Xin, Shi Guangliang, Zhang Qing, Liu Qingqing, Yin Hang, Zhang Yiming. Astilbin protects chicken peripheral blood lymphocytes from cadmium-induced necroptosis via oxidative stress and the PI3K/Akt pathway. Ecotoxicol Environ Saf. 2020;190:110064. doi: 10.1016/j.ecoenv.2019.110064. [DOI] [PubMed] [Google Scholar]
- Dikic Ivan, Elazar Zvulun. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19:349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- El-Kenawi Asmaa, Ruffell Brian. Inflammation, ROS, and mutagenesis. Cancer Cell. 2017;32:727–729. doi: 10.1016/j.ccell.2017.11.015. [DOI] [PubMed] [Google Scholar]
- Estevam Ethiene Castellucci, Witek Karolina, Faulstich Lisa, Nasim Muhammad Jawad, Latacz Gniewomir, Domínguez-Álvarez Enrique. Aspects of a distinct cytotoxicity of selenium salts and organic selenides in living cells with possible implications for drug design. Molecules. 2015;20:13894–13912. doi: 10.3390/molecules200813894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Yuchen, He Ding, Yao Zhiyuan, Klionsky Daniel J. The machinery of macroautophagy. Cell Res. 2014;24:24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filomeni G., De Zio D., Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22:377–388. doi: 10.1038/cdd.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Xuejiao, Tang Bin, Liang Huihuang, Li Yi, Zigong Wei. Selenium deficiency inhibits micRNA-146a to promote ROS-induced inflammation via regulation of the MAPK pathway in the head kidney of carp. Fish Shellfish Immunol. 2019;91:284–292. doi: 10.1016/j.fsi.2019.05.039. [DOI] [PubMed] [Google Scholar]
- Glasauer Andrea, Chandel Navdeep S. ROS. Curr Biol. 2013;23:R100–R102. doi: 10.1016/j.cub.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Gong Yafan, Yang Jie, Cai Jingzeng, Qi Liu, Zhang Jun Min, Zhang Ziwei. Effect of Gpx3 gene silencing by siRNA on apoptosis and autophagy in chicken cardiomyocytes. J Cell Physiol. 2019;234:7828–7838. doi: 10.1002/jcp.27842. [DOI] [PubMed] [Google Scholar]
- Grumolato Luca, Ghzili Hafida, Montero-Hadjadje Maité, Gasman Stéphane, Lesage Jean, Tanguy Yannick. Selenoprotein T is a PACAP-regulated gene involved in intracellular Ca2+ mobilization and neuroendocrine secretion. FASEB J. 2008;22:1756–1768. doi: 10.1096/fj.06-075820. [DOI] [PubMed] [Google Scholar]
- Guillin Olivia M., Caroline Vindry, Théophile Ohlmann, Laurent Chavatte. Selenium, selenoproteins and viral infection. Nutrients. 2019;11:2101. doi: 10.3390/nu11092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefnawy Abd El Ghany, Tórtora-Pérez J.L. The importance of selenium and the effects of its deficiency in animal health. Small Rumin Res. 2010;89:185–192. [Google Scholar]
- Hofstee Pierre, Bartho Lucy A., McKeating Daniel R., Filip Radenkovic, Georgia McEnroe, Fisher Joshua J. Maternal selenium deficiency during pregnancy in mice increases thyroid hormone concentrations, alters placental function and reduces fetal growth. J Physiol. 2019;597:5597–5617. doi: 10.1113/JP278473. [DOI] [PubMed] [Google Scholar]
- Hong Chuenbin, Chow Chingkuang. Induction of eosinophilic enteritis and eosinophilia in rats by vitamin E and selenium deficiency. Exp Mol Pathol. 1988;48:182–192. doi: 10.1016/0014-4800(88)90055-x. [DOI] [PubMed] [Google Scholar]
- Hosnedlova Bozena, Marta Kepinska, Sylvie Skalickova, Carlos Fernandez, Branislav Ruttkay-Nedecky, Donald Thembinkosi Malevu. A summary of new findings on the biological effects of selenium in selected animal species—a critical review. Int J Molec Ences. 2017;18:2209. doi: 10.3390/ijms18102209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høyer-Hansen Maria, Lone Bastholm, Piotr Szyniarowski, Michelangelo Campanella, György Szabadkai, Farkas Thomas. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Hu Chien-An A., Hou Yongqing, Yi Dan, Qiu Yinsheng, Wu Guoyao, Kong Xiangfeng. Autophagy and tight junction proteins in the intestine and intestinal diseases. Anim Nutr. 2015;1:123–127. doi: 10.1016/j.aninu.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Ju, Canadien Veronica, Lam Grace Y., Steinberg Benjamin E., Dinauer Mary C., Magalhaes Marco A.O. Activation of antibacterial autophagy by NADPH oxidases. Proc Natl Acad Sci USA. 2009;106:6226–6231. doi: 10.1073/pnas.0811045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain Tarique, Tan Bie, Yin Yulong, Francois Blachier, Tossou Myrlene C.B., Najma Rahu. Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Long. 2016;2016:7432797. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons Robert, Carlson Bradley A., Hatfield Dolph L., Davis Cindy D. Both selenoproteins and low molecular weight selenocompounds reduce colon cancer risk in mice with genetically impaired selenoprotein expression. J Nutr. 2006;136:1311–1317. doi: 10.1093/jn/136.5.1311. [DOI] [PubMed] [Google Scholar]
- Janka Zoltán. Tracing trace elements in mental function. Ideggyogyaszati Szle. 2019;72:367–379. doi: 10.18071/isz.72.0367. [DOI] [PubMed] [Google Scholar]
- Ji Tianrong, Zhang Chengwei, Ma Linlin, Wang Qin, Zou Li, Meng Kexin. TRPC6-Mediated Ca2+ signaling is required for hypoxia-induced autophagy in human podocytes. Cell Physiol Biochem. 2018;48:1782–1792. doi: 10.1159/000492351. [DOI] [PubMed] [Google Scholar]
- Jin Shengkan, DiPaola Robert S., Robin Mathew, White Eileen. Metabolic catastrophe as a means to cancer cell death. J Cell Sci. 2007;120:379–383. doi: 10.1242/jcs.03349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahya Mehmet Cemal, Nazıroğlu Mustafa, Övey İshak Suat. Modulation of diabetes-induced oxidative stress, apoptosis, and Ca(2+) entry through TRPM2 and TRPV1 channels in dorsal root ganglion and Hippocampus of diabetic rats by melatonin and selenium. Mol Neurobiol. 2017;54:2345–2360. doi: 10.1007/s12035-016-9727-3. [DOI] [PubMed] [Google Scholar]
- Kania Elżbieta, Pająk Beata, Orzechowski Arkadiusz. Calcium homeostasis and ER stress in control of autophagy in cancer cells. BioMed Res Int. 2015;2015:352794. doi: 10.1155/2015/352794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasaikina Marina V., Kravtsova Marina A., Lee Byung Cheon, Javier Seravalli, Peterson Daniel A., Walter Jens. Dietary selenium affects host selenoproteome expression by influencing the gut microbiota. FASEB J. 2011;25:2492–2499. doi: 10.1096/fj.11-181990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoso Pervez Ahmed, Pan Tingr, Wan Na, Yang Zijiang, Liu Ci, Shu Li. Selenium deficiency induces autophagy in immune organs of chickens. Biol Trace Elem Res. 2017;177:159–168. doi: 10.1007/s12011-016-0860-7. [DOI] [PubMed] [Google Scholar]
- Kim Young Chul, Kun-Liang Guan. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125:25–32. doi: 10.1172/JCI73939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper George G.J.M., Harry Kester, Peeters Robin, Visser Theo J. Biochemical mechanisms of thyroid hormone deiodination. Thyroid. 2005;15:787–798. doi: 10.1089/thy.2005.15.787. [DOI] [PubMed] [Google Scholar]
- Levine Beth, Kroemer Guido. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176:11–42. doi: 10.1016/j.cell.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Lulu, Tan Jin, Miao Yuyang, Lei Ping, Zhang Qiang. ROS and autophagy: interactions and molecular regulatory mechanisms. Cell Mol Neurobiol. 2015;35:615–621. doi: 10.1007/s10571-015-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Lin, Li Lu, Zhou Xuejiao, Yu Yang, Li Zengqiang, Zuo Daiying. Silver nanoparticles induce protective autophagy via Ca/CaMKKβ/AMPK/mTOR pathway in SH-SY5Y cells and rat brains. Nanotoxicology. 2019;13:369–391. doi: 10.1080/17435390.2018.1550226. [DOI] [PubMed] [Google Scholar]
- Li Zhaoyang, Xu Yali, Huang Zhijun, Yue Wei, Hou Jian, Long Tengfei. Association between exposure to arsenic, nickel, cadmium, selenium, and zinc and fasting blood glucose levels. Environ Pollut. 2019;255:113325. doi: 10.1016/j.envpol.2019.113325. [DOI] [PubMed] [Google Scholar]
- Lian CaiYu, Yang Heng, Zhai ZhenZhen, Li ZiFa, Han DianGang, Wang Lin. mTORC1 activation contributes to autophagy inhibition via its recruitment to lysosomes and consequent lysosomal dysfunction in cadmium-exposed rat proximal tubular cells. J Inorg Biochem. 2020;212:111231. doi: 10.1016/j.jinorgbio.2020.111231. [DOI] [PubMed] [Google Scholar]
- Liu Ran, Jin Cong, Wang Zhenyong, Wang Zhaojun, Wang Jian, Wang Lin. Effects of manganese deficiency on the microstructure of proximal tibia and OPG/RANKL gene expression in chicks. Vet Res Commun. 2015;39:31–37. doi: 10.1007/s11259-015-9626-5. [DOI] [PubMed] [Google Scholar]
- Liu Qingqing, Wang Wei, Zhang Yiming, Cui Yuan, Xu Shiwen, Shu Li. Bisphenol A regulates cytochrome P450 1B1 through miR-27b-3p and induces carp lymphocyte oxidative stress leading to apoptosis. Fish Shellfish Immunol. 2020;102:489–498. doi: 10.1016/j.fsi.2020.05.009. [DOI] [PubMed] [Google Scholar]
- Loscalzo Joseph. Keshan disease, selenium deficiency, and the selenoproteome. N Engl J Med. 2014;370:1756–1760. doi: 10.1056/NEJMcibr1402199. [DOI] [PubMed] [Google Scholar]
- Lu Zhuang, Wang Pengzu, Teng Teng, Shi Baoming, Shan Anshan, Gen Lei Xin. Effects of dietary selenium deficiency or excess on selenoprotein gene expression in the spleen tissue of pigs. Animals. 2019;9:1122. doi: 10.3390/ani9121122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima Noboru. A brief history of autophagy from cell biology to physiology and disease. Nat Cell Biol. 2018;20:521–527. doi: 10.1038/s41556-018-0092-5. [DOI] [PubMed] [Google Scholar]
- Moldoveanu Davies Tudor, Hosfield Christopher M., Lim Daniel, Elce John S., Jia Zongchao, Davies Peter L. A Ca2+ switch aligns the active site of calpain. Cell. 2002;108:649–660. doi: 10.1016/s0092-8674(02)00659-1. [DOI] [PubMed] [Google Scholar]
- Murphy Michael P. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nighot Prashant K., Hu Chien-An Andy, Ma Thomas Y. Autophagy enhances intestinal epithelial tight junction barrier function by targeting claudin-2 protein degradation. J Biol Chem. 2015;290:7234–7246. doi: 10.1074/jbc.M114.597492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Junghyun, Lee Jung Han, Yoon Byung Sun, Eun Kyoung Jun, Lee Gilju, Kim In Yong. Additive effect of bFGF and selenium on expansion and paracrine action of human amniotic fluid-derived mesenchymal stem cells. Stem Cell Res Ther. 2018;9:293. doi: 10.1186/s13287-018-1058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattingre Sophie, Tassa Amina, Qu Xueping, Garuti Rita, Liang Xiao Huan, Mizushima Noboru. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Peng Fan, Guo Xin, Li Zhihong, Li Changzheng, Wang Changdong, Lv Weiran. Antimutagenic effects of selenium-enriched polysaccharides from pyracantha fortuneana through suppression of cytochrome P450 1A subfamily in the mouse liver. Molecules. 2016;21:1731. doi: 10.3390/molecules21121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Linqian, Zhang Yiming, Wan Chunyan, Wang Zhu, Cong Yimei, Shu Li. MiR-196-5p involvement in selenium deficiency-induced immune damage via targeting of NFκBIA in the chicken trachea. Metallomics. 2020;12:1679–1692. doi: 10.1039/d0mt00164c. [DOI] [PubMed] [Google Scholar]
- Rayman M.P. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- Schomburg Lutz, Schweizer Ulrich. Hierarchical regulation of selenoprotein expression and sex-specific effects of selenium. Biochim Biophys Acta. 2009;1790:1453–1462. doi: 10.1016/j.bbagen.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Shi Qunxiang, Wang Wei, Chen Menghao, Zhang Hongfu, Xu Shiwen. Ammonia induces Treg/Th1 imbalance with triggered NF-κB pathway leading to chicken respiratory inflammation response. Ence Total Environ. 2018;659:354–362. doi: 10.1016/j.scitotenv.2018.12.375. [DOI] [PubMed] [Google Scholar]
- Smith Allen D., Sebastian Botero, Terez Shea-Donohue, Urban Joseph F. The pathogenicity of an enteric Citrobacter rodentium Infection is enhanced by deficiencies in the antioxidants selenium and vitamin E. Infect Immun. 2011;79:1471–1478. doi: 10.1128/IAI.01017-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speckmann Bodo, Steinbrenner Holger. Selenium and selenoproteins in inflammatory bowel diseases and experimental colitis. Inflamm Bowel Dis. 2014;20:1110–1119. doi: 10.1097/MIB.0000000000000020. [DOI] [PubMed] [Google Scholar]
- St Germain Donald L. The effects and interactions of substrates, inhibitors, and the cellular thiol-disulfide balance on the regulation of type II iodothyronine 5’-deiodinase. Endocrinology. 1988;122:1860–1868. doi: 10.1210/endo-122-5-1860. [DOI] [PubMed] [Google Scholar]
- St Germain Donald L., Galton Valerie Anne, Hernandez Arturo. Minireview: defining the roles of the iodothyronine deiodinases: current concepts and challenges. Endocrinology. 2009;150:1097–1107. doi: 10.1210/en.2008-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Chaohua, Li Shuang, Zhang Kai, Jing Li, Han Yunsheng, Zhan Tengfei. Selenium deficiency-induced redox imbalance leads to metabolic reprogramming and inflammation in the liver. Redox Biol. 2020;36:101519. doi: 10.1016/j.redox.2020.101519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teige Jon, Jr., Larsen Hans Jorgen, Tollersrud Sverre. Swine dysentery: the influence of dietary selenium on clinical and pathological effects of Treponema hyodysenteriae infection. Res Vet Sci. 1982;32:95–100. [PubMed] [Google Scholar]
- Teige Jon, Jr., Larsen Hans Jørgen, Tollersrud Sverre. Swine dysentery: the influence of dietary selenium on clinical and pathological effects of Treponema hyodysenteriae infection. Acta Vet Scand. 1984;25:1–9. doi: 10.1186/BF03547273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toral-Ojeda Ivan, Aldanondo Garazi, Lasa-Elgarresta Jaione, Lasa-Fernández Haizpea, Fernández-Torrón Roberto, López de Munain Adolfo. Calpain 3 deficiency affects SERCA expression and function in the skeletal muscle. Expet Rev Mol Med. 2016;18:e7. doi: 10.1017/erm.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vara D., Salazar M., Olea-Herrero N., Guzmán M., Velasco G., Díaz-Laviada I. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: role of AMPK-dependent activation of autophagy. Cell Death Differ. 2011;18:1099–1111. doi: 10.1038/cdd.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Jianfa, Liu Zhe, He Xianjing, Lian Shuai, Yu Debin, Liang Jianbin. Selenium deficiency induces duodenal villi cells apoptosis via oxidative stress-induced mitochondrial apoptosis pathway and inflammatory signaling-induced death receptor pathway. Metallomics. 2018;10:1390–1400. doi: 10.1039/c8mt00142a. [DOI] [PubMed] [Google Scholar]
- Wang Shengchen, Li Xiaojing, Wang Wei, Zhang Hongfu, Xu Shiwen. Application of transcriptome analysis: oxidative stress, inflammation and microtubule activity disorder caused by ammonia exposure may be the primary factors of intestinal microvilli deficiency in chicken. Ence Total Environ. 2019;696:134035. doi: 10.1016/j.scitotenv.2019.134035. [DOI] [PubMed] [Google Scholar]
- Wang Lanqiao, Wang Lanxi, Xu Shi, Xu Shiwen. Chlorpyrifos induces the apoptosis and necroptosis of L8824 cells through the ROS/PTEN/PI3K/AKT axis. J Hazard Mater. 2020;398:122905. doi: 10.1016/j.jhazmat.2020.122905. [DOI] [PubMed] [Google Scholar]
- Wang Yu, Zhao Hongjing, Guo Menghao, Fei Dongxue, Zhang Lina, Xing Mingwei. Targeting the miR-122/PKM2 autophagy axis relieves arsenic stress. J Hazard Mater. 2020;383:121217. doi: 10.1016/j.jhazmat.2019.121217. [DOI] [PubMed] [Google Scholar]
- Wang Yu, Zhao Hongjing, Mu Mengyao, Guo Menghao, Xing Mingwei. Zinc offers splenic protection through suppressing PERK/IRE1-driven apoptosis pathway in common carp (Cyprinus carpio) under arsenic stress. Ecotoxicol Environ Saf. 2021;208:111473. doi: 10.1016/j.ecoenv.2020.111473. [DOI] [PubMed] [Google Scholar]
- Yan Jidong, Zheng Yuewen, Min Zixin, Ning Qilan, Lu Shemin. Selenium effect on selenoprotein transcriptome in chondrocytes. BioMetals. 2013;26:285–296. doi: 10.1007/s10534-013-9610-x. [DOI] [PubMed] [Google Scholar]
- Yang Jie, Zhang Yuan, Hamid Sattar, Cai Jingzeng, Qi Liu, Li Hao. Interplay between autophagy and apoptosis in selenium deficient cardiomyocytes in chicken. J Inorg Biochem. 2017;170:17–25. doi: 10.1016/j.jinorgbio.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Yang Jie, Gong Yafan, Cai Jingzeng, Qi Liu, Zhang Ziwei. lnc-3215 suppression leads to calcium overload in selenium deficiency-induced chicken heart lesion via the lnc-3215-miR-1594-TNN2 pathway. Mol Ther Nucleic Acids. 2019;18:1–15. doi: 10.1016/j.omtn.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Jie, Shi Guangliang, Gong Yafan, Cai Jingzeng, Zheng Yingying, Zhang Ziwei. LncRNA 0003250 accelerates heart autophagy and binds to miR-17-5p as a competitive endogenous RNA in chicken induced by selenium deficiency. J Cell Physiol. 2021;236:157–177. doi: 10.1002/jcp.29831. [DOI] [PubMed] [Google Scholar]
- Zhang Xiaoli, Lu Yu, Xu Haoxing. Lysosome calcium in ROS regulation of autophagy. Autophagy. 2016;12:1954–1955. doi: 10.1080/15548627.2016.1212787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Ziwei, Zheng Zhi, Cai Jingzeng, Qi Liu, Yang Jie, Gong Yafan. Effect of cadmium on oxidative stress and immune function of common carp (Cyprinus carpio L.) by transcriptome analysis. Aquat Toxicol. 2017;192:171–177. doi: 10.1016/j.aquatox.2017.09.022. [DOI] [PubMed] [Google Scholar]
- Zhang Liwei, Gao Yanhui, Feng Hongqi, Zou Ning, Wang Kewei, Sun Dianjun. Effects of selenium deficiency and low protein intake on the apoptosis through a mitochondria-dependent pathway. J Trace Elem Med Biol. 2019;56:21–30. doi: 10.1016/j.jtemb.2019.06.019. [DOI] [PubMed] [Google Scholar]
- Zhang Qiaojian, Zheng Shufang, Wang Shengchen, Wang Wei, Xing Houjuan, Xu Shiwen. Chlorpyrifos induced oxidative stress to promote apoptosis and autophagy through the regulation of miR-19a-AMPK axis in common carp. Fish Shellfish Immunol. 2019;93:1093–1099. doi: 10.1016/j.fsi.2019.07.022. [DOI] [PubMed] [Google Scholar]
- Zhang Yiming, Liu Qingqing, Yin Hang, Min Yahong, Shu Li. Selenium deficiency causes immune damage by activating the DUSP1/NF-κB pathway and endoplasmic reticulum stress in chicken spleen. Food Function. 2020;11:6467–6475. doi: 10.1039/d0fo00394h. [DOI] [PubMed] [Google Scholar]
- Zhang Ziwei, Qi Liu, Yang Jie, Yao Haidong, Fan Ruifeng, Cao Changyu. The proteomic profiling of multiple tissue damage in chickens for a selenium deficiency biomarker discovery. Food Function. 2020;11:1312–1321. doi: 10.1039/c9fo02861g. [DOI] [PubMed] [Google Scholar]
- Zhang Yuan, Zhang Jiuki, Bao Jun, Tang Chaohua, Zhang Ziwei. Selenium deficiency induced necroptosis, Th1/Th2 imbalance, and inflammatory responses in swine ileum. J Cell Physiol. 2021;236:222–234. doi: 10.1002/jcp.29836. [DOI] [PubMed] [Google Scholar]
- Zheng Yingying, Shi Guangliang, Cai Jingzeng, Yang Jie, Zhang Yuan, Gong Yafan. Di-(2-ethyl hexyl) phthalate induces necroptosis in chicken cardiomyocytes by triggering calcium overload. J Hazard Mater. 2020;387:121696. doi: 10.1016/j.jhazmat.2019.121696. [DOI] [PubMed] [Google Scholar]
- Zhong Ran, Li Fenglan, Zhang Xuefeng, Cui Jian, Chu Erfu, Chen Deqing. Oxidative stress and mitochondrial DNA D-Loop mutation in different tissues of selenium deficiency mice. J Harbin Med Univ. 2011 [Google Scholar]
- Zhu Shiyong, Li Xuenan, Sun Xiaochen, Jia Lin, Li Wei, Zhang Cong. Biochemical characterization of the selenoproteome in Gallus gallus via bioinformatics analysis: structure-function relationships and interactions of binding molecules. Metallomics. 2017;9:124–131. doi: 10.1039/c6mt00254d. [DOI] [PubMed] [Google Scholar]