Abstract
Introduction
Biliary cystadenomas (BCAs) are rare benign tumors with malignant potential. They are most commonly found in middle-aged women and are quite infrequently reported in children. Even with advanced imaging, diagnosing and distinguishing BCA from other cystic liver lesions remain challenging.
Case presentation
A 5-year-old boy was brought in by his parents to our hospital with abdominal swelling that had been persistent for a year, along with loss of appetite and weight loss. On examination, the abdomen was distended and dull on percussion. We considered mesenchymal hamartoma of the liver (MHL) as the top differential after an abdominal CT scan with contrast showed a multi-loculated cystic tumour. For both definitive diagnosis and therapy, the patient underwent exploratory laparotomy with excision of the cystic mass. Surprisingly, histopathology examination of the resected specimen revealed biliary mucinous cystadenoma (BCA).
Conclusion
Since conservative methods are associated with high recurrence rates, biliary mucinous cystic neoplasms require a high index of suspicion and should be handled with total surgical resection. In the post-operative phase, periodic surveillance imaging is recommended due to the risk of recurrence and malignant transformation.
Keywords: Biliary mucinous cystadenoma, Mesenchymal Hamartoma, Liver cysts, Pediatrics, Case report, Ethiopia
Highlights
-
•
Biliary mucinous cystadenomas are cystic benign tumors that are extremely rare in children.
-
•
Pathologic evaluation of resected specimen is important for an accurate diagnosis.
1. Introduction
Biliary cystadenomas (BCA), also known as hepatic mucinous cystadenomas are uncommon benign tumors with a risk for malignant degeneration. They are more common in women in their fourth and fifth decade and are rarely discovered in children [1], [2], [3]. The tumour is detected in the majority of patients during workup for nonspecific abdominal complaints such as abdominal discomfort, pain, and swelling. Because hepatic mucinous cystadenomas appear on preoperative imaging to be similar to a range of cystic lesions of the liver, definitive diagnosis and identification of benign and malignant cystadenomas always necessitate histological study after formal resection [4]. Hepatic mucinous cystadenomas are susceptible to recurrence and malignant changes when surgical excisions are inadequate [3].
Below we present a 5-year-old male child with a giant hepatic mucinous cystadenoma mimicking mesenchymal hamartoma of the liver. This case is reported in line with the SCARE 2020 criteria [5]. We believe that by sharing this report, surgeons and paediatricians will be better informed about a rare cystic liver mass that they may encounter in practice. We will go through the role of imaging and histology in mucinous cystadenoma diagnosis, treatment, and routine surveillance in affected patients.
2. Case presentation
The patient is a 5-year-old male child who was brought by his family to a surgical referral clinic of Debremarkos Comprehensive Specialized Hospital in December 2020 with a one-year history of abdominal swelling. The swelling began modest, in the epigastrium, and gradually became larger, eventually filling the entire abdomen. Associated with this he had a loss of appetite and un-quantified weight loss. Otherwise, he didn't experience other symptoms such as vomiting, jaundice, abdominal pain, diarrhoea, or rectum bleeding. Otherwise, he has no any history of childhood illness, no family history of similar illness. Except for gross abdominal distension and low weight for his age other physical examinations were normal.
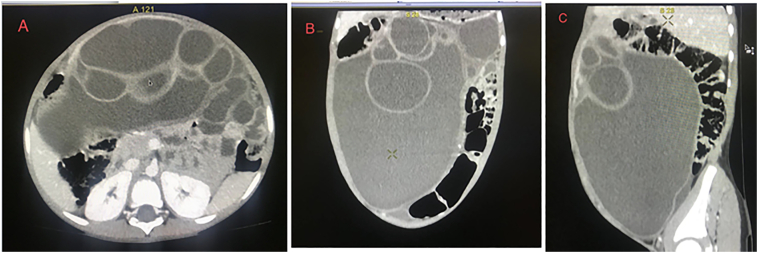
His complete blood count (CBC) and liver function tests (LFT) were within the normal limits. His electrolytes were normal except for moderate hyponatremia (Na+ = 127.2 mmol/L). The tests for HBsAg and HCV antibodies were also negative. We were unable to determine tumour markers such as CEA, AFP, and Ca19-9 since they were not available in our setting. Abdominopelvic ultrasonography discovered a large multiloculated anechoic mass lesion in the peritoneal cavity. Because of the difficulty in establishing the origin of the mass, a huge mesenteric cyst was entertained as a differential diagnosis and our radiologists recommended a CT scan. Abdominal CT with contrast revealed multiloculated cystic mass with enhancing thick wall and septations measuring 26 cm × 23 cm × 18 cm and occupying the abdominopelvic cavity. The mass was attached with the inferior surface of the left lobe of the liver and bowel loops were dilated and displaced posteriorly and to the left (Fig. 1A, B, C). After discussing with the radiology team, hepatic mesenchymal hamartoma was considered as a working diagnosis preoperatively because of the patient's age and imaging findings. Following discussions with the family, we performed an exploratory laparotomy to treat the symptoms while also confirming the diagnosis of mesenchymal hamartoma using excisional biopsy. The patient was operated in a Referral Hospital by a general surgeon who has three years of experience.
Fig. 1.
Contrast enhanced abdominopelvic CT; axial (A), coronal (B), and sagittal (C) scan demonstrate huge multiloculated cystic mass with enhancing thick wall and septations.
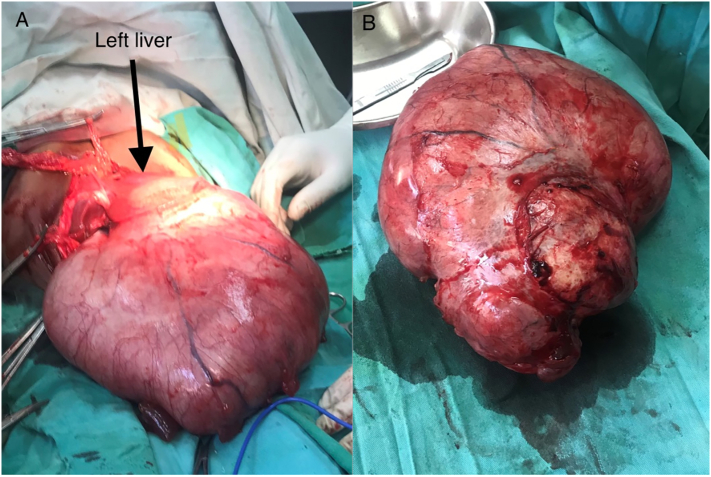
After the patient's hyponatremia was corrected, two units of cross-matched blood were prepared and the patient was taken for a laparotomy. Under general anesthesia and endotracheal intubation, he was explored through a longitudinal mid-line abdominal incision and intra-operatively, we found a 25 cm × 19 cm, lobulated cystic mass that originated from the left lateral section (segment II/III) of the liver and occupied the entire abdominopelvic cavity (Fig. 2A). There was no apparent attachment or adhesion between the mass and other intra-peritoneal structures. The cyst was completely enucleated without rupture or spillage (Fig. 2B). One unit of blood was transfused to the patient during his post-operative care. He was discharged home four days following surgery in a stable condition.
Fig. 2.
Intraoperative view; cystic mass which arises from the left lobe of the liver (arrow) before excision (A); enoculated specimen of a huge cystic hepatic mass (B).
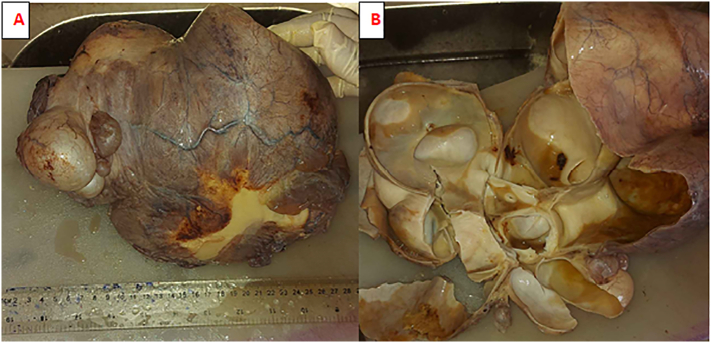
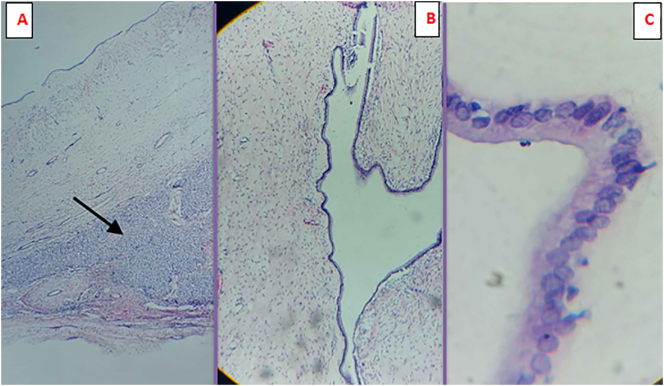
On gross appearance, the cystic specimen had a smooth, glistening surface and measured 26x16x10cm (Fig. 3A). On cut surface, a multi-locular cavity filled with seromucinous and turbid fluid was seen (Fig. 3B). There was no wall thickening or surface excrescence. Microscopic section revealed cyst wall fragments composed of single layer of cuboidal to low columnar biliary type epithelial cells with apical mucin resting on loosely collagenized fibromyxoid stroma. There were no features of cytological atypia, dysplasia or stratification. The adjacent hepatic tissue was unremarkable. Foci of calcification and bile pigment laden foamy histocytes were seen. The specimen did not contain ovarian-type stroma. Hepatic mucinous cystadenoma without ovarian-type stroma was put as the final histopathologic diagnosis (Fig. 4A, B, C).
Fig. 3.
Mucinous cystadenoma of the liver, gross (A) Cystic specimen with smooth glistening surface; Cut surface (B) revealing multilocular cavity filled with seromucinous and turbid fluid.
Fig. 4.
Microscopy (A) Low power shows cyst wall fragment with an adjacent normal appearing hepatic parenchyma (arrow); (B) Cyst lining composed of a single layer of mucinous epithelium appearing (C) cuboidal to low columnar with apical mucin on High power field.
The patient was examined three weeks following surgery in the surgical referral clinic, and he was able to tolerate oral feeds, with no abdominal pain, swelling, or discomfort. Eight months following the surgery, the patient returned to our surgical referral clinic for a follow-up ultrasound, which revealed no evidence of a liver lesion. The results of his liver function tests were similarly within the normal limits.
3. Discussion
Biliary mucinous cyst neoplasms are rare cystic lesions that account for fewer than 5% of non-parasitic liver cysts [1], [3]. Although they mostly develop in the liver from the intrahepatic biliary system, they can also arise in the extra-hepatic biliary tree. BCAs have a slight predilection for the right side of the liver [6]. They are usually slow-growing tumors that range in size from 1.5 cm to 35 cm in diameter. Biliary mucinous cyst neoplasms are most commonly found in middle-aged women and they are quite uncommon in children with few cases reports in the literature [2], [3]. Here, we reported a giant intra-peritoneal cystic mass in a 6-year-old male child. The mass was initially considered as hepatic mesenchymal hamartoma but pathologic examination revealed it to be a biliary cystadenoma arising from the left lobe of the liver. Hepatic mesenchymal hamartoma is nearly exclusively found in infants and toddlers under the age of 2-years and it occurs in only 5% of children beyond the age of five, and it is extremely rare in adults. Most (75%) of MHL arises from the right lobe of the liver [7].
Because they grow slowly, a large percentage of benign mucinous cystadenomas are asymptomatic. Symptomatic ones, on the other hand, are more likely to manifest with abdominal swelling and, in some cases abdominal pain or discomfort due to pressure effects, as in this case. An abdominal mass can occasionally be detected on physical examination [8], [9]. Most patients with BCA have normal laboratory results, while serum bilirubin and liver enzyme levels may occasionally be slightly increased. In asymptomatic patients, the greater use of abdominal imaging in clinical practice has led to an increase in the identification of hepatic cysts [9], [10]. Similarly, children with MHL present with a slow-growing asymptomatic right upper quadrant palpable mass or with nonspecific symptoms such as abdominal pain, fatigue, and fever. Although laboratory results may be normal, an increased alpha-fetoprotein may be found in some cases of MHL [7], [11].
Biliary cystadenomas are anechoic on ultrasonography, with thicker irregular walls and internal septations. On CT, they appear as a multiseptated cystic mass with an enhanced wall [9]. The radiologic distinction of cystadenoma from cystadenocarcinoma is still difficult [12]. Although a mural nodule and irregular cyst wall thickening may indicate a higher risk of malignancy, these characteristics are not pathognomonic for cystadenocarcinoma of the liver [13]. Radiologic appearance of hepatic mesenchymal hamartoma in children can show a wide variety of characteristics, ranging from a multiseptated cystic tumour to mixed solid and cystic tumour, and rarely even a solid tumour [14]. Because the radiologic findings of cystic liver lesions tend to overlap, it is difficult to tell the difference between BCA and other cystic lesions of the liver before surgery. When a multiloculated cystic liver lesion is discovered, there are numerous disease entities to consider. Hydatid disease, abscesses, complicated congenital hepatic cysts, cystic metastatic disease, and as in this case, mesenchymal hamartoma are all liver lesions that can mimic BCA on imaging [1], [12].
Biliary cystadenomas are solitary, multi-locular cystic lesions having fluid contents on gross pathological examination and they can have fibrosis and calcific cyst walls. BCA is divided into two histologic sub-types: BCA with ovarian stroma and BCA without ovarian stroma [1], [15]. On microscopic section, typical BCA has a single layer of cuboidal to columnar epithelium, as well as an ovarian-type stroma that expresses estrogen and progesterone receptors, which only affects women in their forties. BCA without ovarian stroma is frequently seen in men in their forties and fifties [1], [15]. Our patient is a young male child who had BCA without ovarian stroma. BCA with ovarian stroma has a better prognosis, whereas those lacking ovarian stroma is more prone to malignant transformation and have a poorer prognosis.
Biliary cystadenocarcinoma (BCAC) is distinguished from BCA by the presence of multilayered epithelium, numerous mitotic figures, loss of polarity, and nuclear pleiomorphism in proliferating cytologically malignant epithelium. Mucin production and immunoreactivity to cytokeratins (CAM5.2, AE1, and AE3), CA 19-9, epithelial membrane antigen, and CEA are found in both BCA and BCAC epithelial cells. Carcinoma embryonic antigen is found on the luminal surface of BCA epithelial cells, whereas it is found in the cytoplasm of BCAC epithelial cells diffusely [4], [9], [13]. Due to the lack of such investigations in our setting we were unable to determine the above-mentioned tumour markers.
Histologic examination of the cyst is necessary for accurate diagnoses of BCA [12]. Various options ranging from simple fenestration and marsupialization to inoculation and formal hepatic resection have been reported in the surgical treatment of BCA. Because of the potential of recurrence and malignant change, complete surgical excision is the standard approach for both diagnosis and management of BCA [2], [4]. In the case of MHL complete surgical excision is advised, although malignant transformation and recurrence are rare events. A few cases of MHL have shown spontaneous regression [7].
Biliary cystadenoma has a favourable prognosis if it is completely resected [15]. Recurrence is almost always associated with incomplete therapies such as aspiration, percutaneous drainage, ablation, marsupialization, and internal drainage [1], [2], [9], [13]. In most cases of MHL, total resection is associated with an excellent prognosis, just as it is in BCA [7], [11].
4. Conclusion
Biliary cystadenomas are rare hepatic tumors in children that can mimic several cystic lesions of the liver like MHL like in our case. Preoperative diagnosis of BCA based on radiologic features is difficult. However, when radiologic imaging reveals a multilocular cystic hepatic lesion, BCA should be considered as a differential. Therefore, we recommend complete surgical excision when BCA is highly suspected because incomplete resections are associated with a higher recurrence rate. Post-resection periodic follow using ultrasound or CT-scan and tumour markers such as CA 19-9 and CEA is recommended because of the possibility of recurrence and malignant transformation.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Ethical approval was obtained for the publication of this case report from the Research Ethical Review Committee of School of Medicine, Debre Markos University. IRB file number is S/R/C/03/01/2021.
Consent
Written informed consent was obtained from the patient's mother for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Research registration
N/A
Guarantor
Yoseph Solomon Bezabih
Provenance and peer review
Not commissioned, externally peer-reviewed.
CRediT authorship contribution statement
The authors contributed equally to this work.
Declaration of competing interest
None.
References
- 1.Ahanatha Pillai S. Biliary cystadenomas: a case for complete resection. HPB Surg. 2012;2012 doi: 10.1155/2012/501705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartolome M.A.H. Biliary cystadenoma. World J. Gastroenterol. 2009;15(28):3573. doi: 10.3748/wjg.15.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferraguti D.A. Mucinous cystadenoma: a rare hepatic tumor in a child. Front. Pediatr. 2017;5:215. doi: 10.3389/fped.2017.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Poggio P., Buonocore M. Cystic tumors of the liver: a practical approach. World J. Gastroenterol. 2008;14(23):3616. doi: 10.3748/wjg.14.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agha R.A. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Fragulidis G.P. Diagnostic and therapeutic challenges of intrahepatic biliary cystadenoma and cystadenocarcinoma: a report of 10 cases and review of the literature. Int. Surg. 2015;100(7–8):1212–1219. doi: 10.9738/INTSURG-D-15-00025.1. [DOI] [PubMed] [Google Scholar]
- 7.Grubor N.M. Giant biliary mucinous cystadenoma of the liver. Ann. Hepatol. 2013;12(6):979–983. [PubMed] [Google Scholar]
- 8.Joel J.M., Jeyasingh S.D., Kalyanaraman S. Biliary cystadenoma: a case report. J. Clin. Diagn. Res. 2016;10(2):p. ED19. doi: 10.7860/JCDR/2016/16368.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S.H. Radiological spectrum of hepatic mesenchymal hamartoma in children. Korean J. Radiol. 2007;8(6):498–505. doi: 10.3348/kjr.2007.8.6.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tholomier C. Biliary mucinous cystic neoplasm mimicking a hydatid cyst: a case report and literature review. BMC Gastroenterol. 2019;19(1):1–8. doi: 10.1186/s12876-019-1001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao W. A 4 and a half years old boy with mesenchymal hamartomas in the left lateral lobe of the liver: a case report and literature review. Medicine. 2017;96(31) doi: 10.1097/MD.0000000000007281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Unal E. Mesenchymal hamartoma of the liver mimicking hepatoblastoma. J. Pediatr. Hematol. Oncol. 2008;30(6):458–460. doi: 10.1097/MPH.0b013e318169171b. [DOI] [PubMed] [Google Scholar]
- 13.Pitchaimuthu M. Outcome following resection of biliary cystadenoma: A single centre experience. HPB. 2016;18:e681–e682. doi: 10.1155/2015/382315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soares K.C. Cystic neoplasms of the liver: biliary cystadenoma and cystadenocarcinoma. J. Am. Coll. Surg. 2014;218(1):119–128. doi: 10.1016/j.jamcollsurg.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang F.-B. Preoperative differential diagnosis between intrahepatic biliary cystadenoma and cystadenocarcinoma: a single-center experience. World J. Gastroenterol. 2014;20(35):12595. doi: 10.3748/wjg.v20.i35.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]