Abstract
We aimed to analyze the risk factors of positive peripherally inserted central catheter (PICC)-related fungal colonization in preterm infants. This retrospective study collected data from 2018 to 2020. The enrolled infants who underwent PICC insertion were born at < 32 weeks’ gestation or birth weight < 1500 g. The demographics, PICC-related characteristics, and treatment information were collected. Univariate and multivariate analyses were performed to investigate risk factors for PICC-related fungal colonization. The receiver operating characteristic (ROC) curve was used to determine the optimal cut-off values for the duration of antibiotics and parenteral nutrition. In total, 124 premature infants underwent PICC insertion. Among them, 19 patients had positive results of fungi on the PICC tips. The duration of antibiotics (odds ratio [OR] 1.16, 95% confidence interval [CI] 1.02–1.31), parenteral nutrition infusion (OR 1.27, 95% CI 1.05–1.54), and postnatal glucocorticoid exposure (OR 9.48, 95% CI 1.06–84.98) were independent risk factors for fungal colonization in PICCs. The ROC curves showed that the risk increased after 15 days of antibiotic use and 28 days of parenteral nutrition infusion. Appropriate clinical management should be used to prevent fungal colonization and fungemia.
Subject terms: Paediatric research, Preterm birth
Introduction
Peripherally inserted central catheters (PICCs) are widely used in neonatal intensive care units (NICUs), especially for the treatment of extremely preterm infants and very low birth weight (VLBW) infants to provide secure venous access or safe administration of hyperosmolar solutions1. However, the use of PICCs increases the risk of central line-associated bloodstream infection (CLABSI), one of the most common nosocomial infections related to PICCs2,3. CLABSI has been associated with several life-threatening complications, including necrotizing enterocolitis, intraventricular hemorrhage, bronchopulmonary dysplasia, and retinopathy of prematurity, extended hospital stay, and increased mortality or morbidity of premature infants1,4. Bacteria were the most important pathogen of CLABSI5. However, in premature infants, an increasing trend has been shown in fungal infections6, and the fungi were another common pathogen of CLABSI7,8.
Fungal infection is a severe complication during PICC placement and is often fatal in very premature infants9. The colonization of fungi in the catheters is the onset step of PICC-related fungal infections10,11. Invasive Candida infections can be prevented with antifungal prophylaxis targeted when the central venous catheter is in place in preterm infants12–14. However, we still have observed a high positive fungal culture from the PICC tips in our NICU over the past few years. Thus, in the present study, we aimed to explore the risk factors of PICC-related positive catheter tip cultures in very premature infants.
Results
Demographics
During the study period, 124 premature infants underwent PICC insertion. Among them, 21 patients were excluded for the following reasons: 1 had accidental detachment, 5 had an ectopic catheter, 6 had the catheter inserted twice, 9 died within the first week of life. The remaining 103 cases of catheter tips were cultured, of whom 28 patients were positive including 19 for fungi and 9 for bacteria, and 75 cases were negative. The most commonly isolated microorganism was fungi (67.9%, 19/28), especially Candida albicans and Candida glabrata. The followed positive pathogen was bacteria (32.1%, 9/28) (Table 1). None of the blood cultures obtained during the time of the catheter tip cultures showed positive results.
Table 1.
Microorganisms cultured from the catheter tip (n = 28).
| Microorganism | Year | |||
|---|---|---|---|---|
| 2018 | 2019 | 2020 | Total | |
| Gram-positive bacteria | ||||
| Streptococcus viridans | 1 | – | – | 1 (3.6%) |
| Gram-negative bacteria | ||||
| Pseudomonas aeruginosa | – | 1 | 5 | 6 (21.4%) |
| Morganella morganii | – | – | 1 | 1 (3.6%) |
| Acinetobacter haemolyticus | 1 | – | – | 1 (3.6%) |
| Fungi | ||||
| Candida albicans | 5 | 4 | 4 | 13 (46.4%) |
| Candida glabrata | 2 | 2 | 2 | 6 (21.4%) |
Univariate analysis of fungal colonization at peripherally inserted central catheter tips
We compared the medical parameters between positive (n = 19) and negative cases (n = 75) for fungal colonization. As shown in Table 2, the positive fungal colonized PICC group had a significantly longer duration of PICC dwelling, antibiotic use, parenteral nutrition (PN) infusion, and postnatal glucocorticoid exposure than the negative fungal colonized PICC group.
Table 2.
Univariate analysis of fungal colonization at the catheter tips.
| Parameter | Total (n = 94) | Positive (n = 19) | Negative (n = 75) | χ2/t/Z | p |
|---|---|---|---|---|---|
| Demographic | |||||
| Sex (n) | 0.00* | 0.96 | |||
| Male | 50 | 10 (52.6%) | 40 (53.3%) | ||
| Female | 44 | 9 (47.4%) | 35 (46.7%) | ||
| Gestational age (weeks) | 29.5 (28.3, 31.4) | 29 (27.6, 31.4) | 29.6 (28.4, 31.4) | 1.05◆ | 0.29 |
| Birth weight (grams) | 1224 ± 190 | 1270 ± 192 | 1213 ± 189 | 1.18★ | 0.24 |
| Mode of delivery (n) | 0.52* | 0.47 | |||
| Vaginal | 68 | 15 (78.9%) | 53 (70.7%) | ||
| Caesarean section | 26 | 4 (21.1%) | 22 (29.3%) | ||
| Assisted reproductive technology (n) | 0.08* | 0.78 | |||
| Yes | 13 | 3 (15.8%) | 10 (13.3%) | ||
| No | 81 | 16 (84.2%) | 65 (86.7%) | ||
| Twin (n) | 0.18* | 0.67 | |||
| Yes | 26 | 6 (31.6%) | 20 (26.7%) | ||
| No | 68 | 13 (68.4%) | 55 (73.3%) | ||
| Premature rupture of membranes (n) | 2.20* | 0.14 | |||
| Yes | 22 | 2 (10.5%) | 20 (26.7%) | ||
| No | 72 | 17 (89.5%) | 55 (73.3%) | ||
| PICC-related characteristic | |||||
| Age at catheter insertion (days) | 4 (2, 6) | 4 (2, 6) | 4 (2, 6) | 0.31◆ | 0.75 |
| PICC dwelling time (days) | 30 (24, 35.3) | 37 (33, 44) | 28 (22, 33) | 4.50◆ | < 0.001 |
| Tip position (n) | 0.00* | 0.99 | |||
| Superior vena cava | 89 | 18 (94.7%) | 71 (94.7%) | ||
| Inferior vena cava | 5 | 1 (5.3%) | 4 (5.3%) | ||
| Treatment | |||||
| Duration of antibiotic (days) | 11 (7.75, 20) | 22 (20, 29) | 10 (5, 15) | 5.71◆ | < 0.001 |
| Duration of PN (days) | 27 (20, 31.3) | 35 (31, 39) | 24 (18, 30) | 5.41◆ | < 0.001 |
| Postnatal glucocorticoid exposure (n) | 18.96* | < 0.001 | |||
| Yes | 38 | 16 (84.2%) | 22 (29.3%) | ||
| No | 56 | 3 (15.8%) | 53 (70.7%) | ||
*Chi-square test, ★t test, and ◆rank-sum test.
PICC peripherally inserted central catheter, PN parenteral nutrition.
Multivariate logistic regression analysis of fungal colonization at peripherally inserted central catheter tips
Multivariate logistic regression analysis was performed to identify the risk factors for fungal colonization. As shown in Table 3, all those factors increased the risk of fungal colonization. Logistic analyses demonstrated that with each additional day of antibiotic use and PN infusion, the risk of fungal colonization in PICC tips was increased by 16% and 27%, respectively. Postnatal exposure to the glucocorticoid also increased the risk by 9.48 times.
Table 3.
Logistic regression analysis for fungal colonization at the catheter tips.
| Parameter | OR | 95% CI | Adjusted OR | 95% CI |
|---|---|---|---|---|
| PICC dwelling time (by each day) | 1.21 | 1.09, 1.35 | 1.07 | 0.93, 1.23 |
| Antibiotic use (by each day) | 1.23 | 1.11, 1.35 | 1.16 | 1.02, 1.31 |
| PN use (by each day) | 1.35 | 1.16, 1.58 | 1.27 | 1.05, 1.54 |
| Postnatal glucocorticoid exposure (yes or no) | 12.91 | 3.37, 49.51 | 9.48 | 1.06, 84.98 |
The duration of PN and PICC dwelling time were entered into the logistic regression model only one parameter at a time.
OR odds ratio, CI confidence interval, PICC peripherally inserted central catheter, PN parenteral nutrition.
Optimal cut-off values for the duration of antibiotics and parenteral nutrition
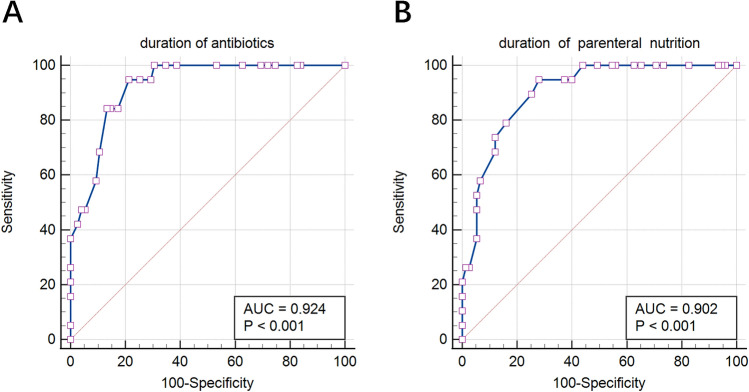
The calculated optimal cut-off values for the duration of antibiotics and PN were 15 days and 28 days, respectively. Both the area under the receiver operating characteristic (ROC) curve values were larger than 0.9, which showed a high diagnostic value (Fig. 1). The Youden index, sensitivity, and specificity are shown in Table 4.
Figure 1.
Receiver operating characteristic curve for the duration of antibiotics (A) and parenteral nutrition (B). AUC, area under the receiver operating characteristic curve.
Table 4.
Optimal cut-off value for the duration of antibiotics and PN.
| Duration of antibiotics | Duration of PN | |
|---|---|---|
| Youden index | 0.73 | 0.67 |
| Criterion (days) | > 15 | > 28 |
| Sensitivity | 94.7 | 94.7 |
| Specificity | 78.6 | 72.0 |
PN parenteral nutrition.
Discussion
In recent decades, the incidence of premature infants has increased. Very premature infants or VLBW infants have had long hospitalizations. Because of poor peripheral vascular conditions, the use of PICC has increased substantially. However, exogenous catheters are associated with an increased risk of CLABSI15,16. Previous studies have shown that coagulase-negative Staphylococcus was the predominant pathogen of CLABSI in the NICU17,18. However, recently, it seems that the proportion of fungi has increased in PICC-associated bloodstream infections19. A previous study20 showed that the distribution of neonatal fungal culture-positive strains has gradually shifted to non-C. albicans strains. Candida parapsilosis has emerged as a main cause of candidemia worldwide among non-albicans species21. However, the composition of pathogens varies by hospital, with C. albicans ranked first, followed by C. glabrata; no case of C. parapsilosis colonization was found in the present study.
Nosocomial infections caused by Candida species are a public health problem because of their severity and high morbidity and mortality rates22. To prevent the occurrence of PICC-associated fungemia, the risk factors of fungal colonization at PICC tips were analyzed in the current study. Multivariate logistic regression analysis identified significant risk factors: the duration of antibiotics, PN infusion, and postnatal glucocorticoid exposure.
A high proportion (66/94, 70%) of premature infants were suspected to have early-onset sepsis and were empirically treated with antibiotics if any of the following were present within 3 days of age: abnormal clinical manifestations, the mother had chorioamnionitis, and premature rupture of membranes exceeded 18 h23. However, the long-term use of broad-spectrum antibiotics will cause a flora imbalance in the body, induce drug-resistant bacteria, cause refractory sepsis, and even trigger the outbreak of nosocomial infection24. In addition, long-term use of antibiotics carries various side effects, such as damage to liver and kidney function, further decline of immunity, and increased probability of fungal infection25. Therefore, risk assessment methods should be used to optimize early antibiotic practice26. Antibiotics should also be withdrawn as soon as possible without indication to reduce fungi colonization. In particular, clinicians have a high suspicion for fungal infections in infants who have been receiving antibiotics for a long time. Especially, if the duration of antibiotics exceeds 15 days, the infant’s risk of fungal colonization will be greatly increased.
Furthermore, the present study determined that the duration of PN was a risk factor for fungal colonization at PICC tips. Fungi, especially Candida spp., which are notoriously adept at forming drug-resistant biofilm structures, are strongly adherent to surfaces and easily colonize in the PICC catheter. PN solutions contain electrolytes, micronutrients, and the macronutrients dextrose, amino acids, and lipid emulsions. Hypertonic glucose in the PN solutions is ideal for Candida growth, while fat emulsions are suitable for the growth of various microorganisms27. These conditions increase the risk of fungal colonization in PICCs.
We determined that the optimal cut-off value for the duration of PN was 28 days. However, in clinical practice, it is common for extremely preterm infants to need PN for more than 28 days, which means that the PICC is still required. Additionally, based on other literature28, replacing the PICC with one at a new site can be considered to reduce the risk of CLABSI. On the other hand, it has also been suggested that clinicians improve nutritional management and use measures including breastfeeding, early micro-feeding, and oral massage to improve infants’ feeding tolerance and reduce the duration of PN29–31.
Glucocorticoids are widely used in very preterm infants to minimise the magnitude and duration of ventilatory support and decrease pulmonary morbidity. At the same time, glucocorticoids can increase the risk of fungal sepsis32. Our study showed similar results that postnatal glucocorticoid exposure increased the risk of catheter fungal colonization by 9.48 times. This might be related to dexamethasone therapy, which has side effects on neutrophils and lymphocytes and thus increases the risk of fungal infection in preterm infants32. Hence, strict guidelines should be implemented for the use of glucocorticoids in clinical practice. Patients should be monitored for fungal infections, especially those exposed to postnatal glucocorticoids. Additionally, medical personnel should improve the prevention of fungal infections. Fluconazole prophylaxis has demonstrated significant efficacy in preventing invasive Candida infections in preterm infants. It is safe and resistance has not emerged when using 3–6 mg/kg twice a week12–14,33. This is the guideline for the prophylactic use of fluconazole in the clinical setting.
The present study indicates risk factors for positive PICC tip cultures in preterm infants. The findings may shed some light on preventing fungal colonization in premature infants in future clinical practice. However, our study also has some limitations. First, this was a retrospective study, and some bias might exist in the patient collection. Second, only a small number of patients from a single centre were enrolled in this study, which might affect the reliability of our study, and no significance was observed in some potential risk factors. Therefore, large sample multi-centre prospective cohort studies should be performed to validate our findings. In addition, it also should take into account that some risk factors may be affected by receiving 5 mg/kg of fluconazole prophylaxis twice a week while PICC line was in place in infants < 32 weeks or 1500 g.
In conclusion, in our study population, fungal colonization of the PICC tips was affected by the duration of antibiotic use, PN infusion, and postnatal glucocorticoid exposure. To prevent fungal colonization and further fungemia, clinical management strategies for the above risk factors should be improved.
Materials and methods
Study design and patient selection
This retrospective study was conducted between 1 January 2018 and 31 December 2020 at the Affiliated Hospital of Southwest Medical University. The enrolled infants who underwent ultrasound-guided PICC insertion during their hospitalization were born at < 32 weeks' gestation or birth weight < 1500 g. While the PICC line was in place, these enrolled infants were received 5 mg/kg of fluconazole prophylaxis twice a week. The blood culture and catheter tip culture were performed at the same time when the PICC catheters were removed. Exclusive criteria included accidental detachment, ectopic catheters, two or more catheterizations, and death before catheter removal.
The Institutional Review Board of the Affiliated Hospital of Southwest Medical University approved the study protocol (KY2021074) and waived informed consent because of the retrospective study design. We confirm that all methods were performed in accordance with the relevant guidelines and regulations.
Procedural management of peripherally inserted central catheters
A 1.9-French silica gel PICC catheter was inserted into neonates by qualified nurses using ultrasound guidance. An X-ray was obtained to confirm the catheter tip's position in a central vein outside the cardiac silhouette. The catheterization room was a separate room, and all instruments including the air were disinfected after each catheterization. Dressings were changed 24 h after catheterization and once a week afterwards. However, if the dressings were wet, loose, or both, they were changed immediately. After removing the previous dressing, a skin disinfectant was used to disinfect the 10-cm2 area around the puncture site. The skin disinfectant comprised iodine of 0.2% ± 0.02%, chlorhexidine acetate of 0.45% ± 0.045%, and ethanol of 65% ± 5%. The catheter connector was replaced every week. Only syringes larger than 10 mL were used for drug infusion through the PICC. Aseptic technology was used for any puncture, fluid preparation and connection. The PICC was not used for blood draws or transfusions. Tubes were flushed with 2 mL of only saline every day before infusions were performed and sealed with 1 IU/mL of a heparin solution34. Health care workers continued to review infants’ needs for a PICC daily. The PICC was withdrawn when parenteral nutrient or hypertonic fluids were no longer required, or when CLABSI was highly suspected34.
Definitions
Catheter colonization was defined as the distal part of the catheter having pathogens amounting to ≥ 15 colony-forming units (CFU)/tablet, with semiquantitative cultures or pathogens amounting to ≥ 1000 CFU on the quantitative culture. Five cm of the catheter tip was kept for pathogen culture after the PICC was removed immediately. At the time of PICC removal, 1–2 mL of blood was taken from the contralateral side of the PICC insertion limb for blood culture.
CLABSI was diagnosed using the following definition from the National Health Safety Net work: a laboratory-confirmed bloodstream infection in patients wherein an eligible bloodstream infection-causing organism was identified and an eligible central venous catheter was present on or one day before the infection date35.
Early-onset sepsis was defined as the presence of clinical symptoms and a positive culture from blood or cerebrospinal fluid samples drawn within 72 h of birth36.
Data collection
Relevant clinical data from the hospital information system were separately collected using EpiData (version 3.02, EpiData Association) by two researchers. The collected data were as follows: demographics including sex, gestational age, birth weight, mode of delivery, assisted reproductive technology, twin pregnancy, and premature rupture of membranes; PICC-related characteristics including age at catheter insertion, indwelling time, tip position, and results of the PICC tip cultures; and treatment information including the duration of antibiotics, PN infusion, and glucocorticoid administration.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 (IBM Corp.). The categorical data were described as a percentage (%) and compared using the chi-square test. The continuous data with normal distribution were described as mean ± standard deviation and compared using the Student t test. The continuous data with abnormal distribution were described as a quartile and analysed using the rank-sum test. The variables with significant statistical differences were selected and included in the multivariate model. Because they had a strong collinear relationship, the PICC dwelling time and duration of PN were entered into the logistic regression model only one parameter at a time. Gestational age and birth weight were mentioned as important influencing factors in many works of literature37–39, so they were included in the model for statistical analysis. Then multivariate logistic regression was performed to calculate the adjusted odds ratios and 95% confidence intervals. The ROC curve was used to determine the optimal cut-off value for the duration of antibiotics and PN by calculating the Youden index.
Acknowledgements
Thanks to Southwest Medical University's financial support of the research project (2019ZQ165).
Author contributions
X.P.L. and W.B.D. conceived the study, L.Y.Z conducted the PICC insertion, X.L.L. collected the data, L.P.Z. and L.Y. analyzed the data and wrote the manuscript. All authors reviewed the manuscript.
Data availability
We state that the study data is available to readers.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Lingping Zhang and Liu Yang.
Contributor Information
Xiaoping Lei, Email: leixiaopingde@126.com.
Lianyu Zhang, Email: 460256916@qq.com.
References
- 1.Liang H, Zhang L, Guo X, Sun L. Vancomycin-lock therapy for prevention of catheter-related bloodstream infection in very low body weight infants. BMC Pediatr. 2021;21:3. doi: 10.1186/s12887-020-02482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenthal VD, et al. International Nosocomial Infection Control Consortium report, data summary of 50 countries for 2010–2015: Device-associated module. Am. J. Infect. Control. 2016;44:1495–1504. doi: 10.1016/j.ajic.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Soares BN, Pissarra S, Rouxinol-Dias AL, Costa S, Guimarães H. Complications of central lines in neonates admitted to a level III Neonatal Intensive Care Unit. J. Matern. Fetal Neonatal. Med. 2018;31:2770–2776. doi: 10.1080/14767058.2017.1355902. [DOI] [PubMed] [Google Scholar]
- 4.Lai, N. M., Taylor, J. E., Tan, K., Choo, Y. M., Ahmad Kamar, A. & Muhamad, N. A. Antimicrobial dressings for the prevention of catheter-related infections in newborn infants with central venous catheters. Cochrane Database Syst. Rev.3, CD011082 (2016). [DOI] [PMC free article] [PubMed]
- 5.Bulbul A, Okan F, Nuhoglu A. Percutaneously inserted central catheters in the newborns: a center's experience in Turkey. J. Matern. Fetal Neonatal. Med. 2010;23:529–535. doi: 10.3109/14767050903214582. [DOI] [PubMed] [Google Scholar]
- 6.Yang YC, Mao J. Value of platelet count in the early diagnosis of nosocomial invasive fungal infections in premature infants. Platelets. 2018;29:65–70. doi: 10.1080/09537104.2017.1293810. [DOI] [PubMed] [Google Scholar]
- 7.Wen J, Yu Q, Chen H, Chen N, Huang S, Cai W. Peripherally inserted central venous catheter-associated complications exert negative effects on body weight gain in neonatal intensive care units. Asia Pac. J. Clin. Nutr. 2017;26:1–5. doi: 10.6133/apjcn.112015.07. [DOI] [PubMed] [Google Scholar]
- 8.Njere I, Islam S, Parish D, Kuna J, Keshtgar AS. Outcome of peripherally inserted central venous catheters in surgical and medical neonates. J. Pediatr. Surg. 2011;46:946–950. doi: 10.1016/j.jpedsurg.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 9.Ma XL, Sun W, Liu T. Clinical characteristics of Candida septicemia seen in a neonatal intensive care unit: Analysis of 9 cases. Zhonghua Er Ke Za Zhi. 2006;44:694–697. [PubMed] [Google Scholar]
- 10.Yu Y, Du L, Yuan T, Zheng J, Chen A, Chen L, Shi L. Risk factors and clinical analysis for invasive fungal infection in neonatal intensive care unit patients. Am. J. Perinatol. 2013;30:589–594. doi: 10.1055/s-0032-1329688. [DOI] [PubMed] [Google Scholar]
- 11.Spiliopoulou A, Dimitriou G, Jelastopulu E, Giannakopoulos I, Anastassiou ED, Christofidou M. Neonatal intensive care unit candidemia: Epidemiology, risk factors, outcome, and critical review of published case series. Mycopathologia. 2012;173:219–228. doi: 10.1007/s11046-011-9498-3. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Donowitz LG. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N. Engl. J. Med. 2001;345:1660–1666. doi: 10.1056/NEJMoa010494. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Grossman LB. Twice weekly fluconazole prophylaxis for prevention of invasive Candida infection in high-risk infants of < 1000 grams birth weight. J. Pediatr. 2005;147:172–179. doi: 10.1016/j.jpeds.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 14.Ericson JE, et al. Fluconazole prophylaxis for the prevention of candidiasis in premature infants: A meta-analysis using patient-level data. Clin. Infect. Dis. 2016;63:604–610. doi: 10.1093/cid/ciw363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassetti, M. et al. Clinical and therapeutic aspects of candidemia: A five year single centre study. PLoS One10, e0127534 (2015). [DOI] [PMC free article] [PubMed]
- 16.Pfaller, M. A., Castanheira, M., Messer, S. A., Moet, G. J. & Jones, R. N. Variation in Candida spp. distribution and antifungal resistance rates among bloodstream infection isolates by patient age: report from the SENTRY Antimicrobial Surveillance Program (2008–2009). Diagn. Microbiol. Infect. Dis.68, 278–283 (2010). [DOI] [PubMed]
- 17.Mularoni, A., Madrid, M., Azpeitia, A. & Valls i Soler, A. The role of coagulase-negative staphylococci in early onset sepsis in a large European cohort of very low birth weight infants. Pediatr. Infect. Dis. J33, e121–125 (2014). [DOI] [PubMed]
- 18.Seale AC, Obiero CW, Berkley JA. Rational development of guidelines for management of neonatal sepsis in developing countries. Curr. Opin. Infect. Dis. 2015;28:225–230. doi: 10.1097/QCO.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Ru XF, Wang Y, Li X, Sang T, Feng Q. Clinical characteristics of neonatal fungal sepsis in neonatal intensive care unit. Beijing Da Xue Xue Bao Yi Xue Ban. 2017;49:789–793. [PubMed] [Google Scholar]
- 20.Hundalani S, Pammi M. Invasive fungal infections in newborns and current management strategies. Expert Rev. Anti. Infect. Ther. 2013;11:709–721. doi: 10.1586/14787210.2013.811925. [DOI] [PubMed] [Google Scholar]
- 21.Herek, T. C., Menegazzo, V. R., Ogaki, M. B., Perini, H. F., Maia, L. F. & Furlaneto, M. C. Biofilm formation by blood isolates of Candida parapsilosis sensu stricto in the presence of a hyperglycidic solution at comparable concentrations of total parenteral nutrition. Rev. Soc. Bras. Med. Trop.52, e20180182 (2019). [DOI] [PubMed]
- 22.Canela, H., Cardoso, B., Vitali, L. H., Coelho, H. C., Martinez, R. & Ferreira, M. Prevalence, virulence factors and antifungal susceptibility of Candida spp. isolated from bloodstream infections in a tertiary care hospital in Brazil. Mycoses61, 11–21 (2018). [DOI] [PubMed]
- 23.[Expert consensus on the diagnosis and management of neonatal sepsis (version 2019)]. Zhonghua Er Ke Za Zhi57, 252–257 (2019). [DOI] [PubMed]
- 24.Kim YK, et al. Bloodstream infections by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in children: Epidemiology and clinical outcome. Antimicrob. Agents Chemother. 2002;46:1481–1491. doi: 10.1128/AAC.46.5.1481-1491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conde-Rosa A, et al. Candidemia distribution, associated risk factors, and attributed mortality at a university-based medical center. Proc. Roy. Health Sci. J. 2010;29:26–29. [PMC free article] [PubMed] [Google Scholar]
- 26.Kuzniewicz, M. W. & Puopolo, K. M. Antibiotic stewardship for early-onset sepsis. Semin. Perinatol.44, 151325 (2020). [DOI] [PubMed]
- 27.Uko S, et al. Targeted short-term fluconazole prophylaxis among very low birth weight and extremely low birth weight infants. Pediatrics. 2006;117:1243–1252. doi: 10.1542/peds.2005-1969. [DOI] [PubMed] [Google Scholar]
- 28.Sengupta A, Lehmann C, Diener-West M, Perl TM, Milstone AM. Catheter duration and risk of CLA-BSI in neonates with PICCs. Pediatrics. 2010;125:648–653. doi: 10.1542/peds.2009-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Section of Breastfeeding. Breastfeeding and the use of human milk. Pediatrics129, e827–841 (2012). [DOI] [PubMed]
- 30.Dutta S, et al. Guidelines for feeding very low birth weight infants. Nutrients. 2015;7:423–442. doi: 10.3390/nu7010423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greene, Z., O’Donnell, C. P. & Walshe, M. Oral stimulation for promoting oral feeding in preterm infants. Cochrane Database Syst. Rev.9, CD009720 (2016). [DOI] [PMC free article] [PubMed]
- 32.Pera A, Byun A, Gribar S, Schwartz R, Kumar D, Parimi P. Dexamethasone therapy and Candida sepsis in neonates less than 1250 grams. J Perinatol. 2002;22:204–208. doi: 10.1038/sj.jp.7210699. [DOI] [PubMed] [Google Scholar]
- 33.Luparia, M. et al. Fungal ecology in a tertiary neonatal intensive care unit after 16 years of routine fluconazole prophylaxis: No emergence of native fluconazole-resistant strains. Am. J. Perinatol.36, S126–126S133 (2019). [DOI] [PubMed]
- 34.[Operation and management guidelines for peripherally inserted central catheter in neonates (2021)]. Zhongguo Dang Dai Er Ke Za Zhi23, 201–212 (2021). [DOI] [PMC free article] [PubMed]
- 35.Johansson E, Hammarskjöld F, Lundberg D, Arnlind MH. Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: A systematic review of the literature. Acta Oncol. 2013;52:886–892. doi: 10.3109/0284186X.2013.773072. [DOI] [PubMed] [Google Scholar]
- 36.Jiang S, Hong L, Gai J, Shi J, Yang Y, Lee SK, Cao Y. Early-onset sepsis among preterm neonates in China, 2015 to 2018. Pediatr. Infect. Dis. J. 2019;38:1236–1241. doi: 10.1097/INF.0000000000002492. [DOI] [PubMed] [Google Scholar]
- 37.Lindquist S, Hentz E, Tessin I, Elfvin A. Very low birthweight infants face an increased risk of bloodstream infections following the removal of umbilical catheters. Acta Paediatr. 2016;105:391–396. doi: 10.1111/apa.13240. [DOI] [PubMed] [Google Scholar]
- 38.Perlman SE, Saiman L, Larson EL. Risk factors for late-onset health care-associated bloodstream infections in patients in neonatal intensive care units. Am. J. Infect. Control. 2007;35:177–182. doi: 10.1016/j.ajic.2006.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furuichi M, Miyairi I. Risk factors for persistent bacteremia in infants with catheter-related bloodstream infection due to coagulase-negative Staphylococcus in the neonatal intensive care unit. J Infect Chemother. 2016;22:785–789. doi: 10.1016/j.jiac.2016.08.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We state that the study data is available to readers.