Highlights
-
•
PSP patients can show marked ventricular enlargement (PSP-vd).
-
•
PSP-vd patients had increased FA and AxD in CST regions close to lateral ventricles.
-
•
CST alterations in PSP-vd are due to CST compression by the enlarged ventricles.
-
•
CST alterations in PSP-vd are similar to those found in NPH but of lesser degree.
-
•
Higher ventriculomegaly is associated with higher severity of the CST alterations.
Keywords: Ventricular dilatation, Progressive supranuclear palsy, Idiopathic normal pressure hydrocephalus, Corticospinal tract profile, DTI
Abstract
Background
Microstructural alterations of corticospinal tract (CST) have been found in idiopathic normal pressure hydrocephalus (iNPH). No study, however, investigated the effect of ventricular dilatation on CST in Progressive Supranuclear Palsy (PSP).
Objective
The aim of this study was to investigate CST diffusion profile in a large cohort of PSP patients with and without ventricular dilatation.
Methods
Twenty-three iNPH patients, 87 PSP patients and 26 controls were enrolled. Evans index (EI) and ventricular volume (VV) were measured in all patients. CST tractography was performed to calculate FA, MD, AxD and RD in six different anatomical regions: medulla oblungata (MO), pons (P), cerebral peduncle (CP), posterior limb of internal capsule (PLIC), corona radiata (CR), subcortical white matter (SWM). ANCOVA was used for comparing CST diffusion profiles between the groups and association between CST microstructural metrics and measures of ventricular dilatation (EI and VV) was assessed.
Results
Thirty-three PSP patients had ventricular dilatation (EI > 0.30, PSP-vd) while 54 PSP patients had normal ventricular system (EI ≤ 0.30, PSP-wvd). iNPH patients had the most marked FA and AxD increase in PLIC and CR of CST followed by PSP-vd, PSP-wvd and controls; RD was altered only in iNPH. A strong correlation was found between CST diffusion metrics and EI or VV.
Conclusions
Our findings confirm the microstructural changes of CST in iNPH patients and demonstrate for the first time similar alterations in PSP-vd patients, suggesting a crucial role of ventricular dilatation in the mechanical compression of CST.
1. Introduction
Idiopathic normal pressure hydrocephalus (iNPH) (Starr et al., 2014), is a syndrome characterized by the triad of gait disturbance, cognitive impairment and urinary incontinence (Mori et al., 2012). Generally, the diagnosis of iNPH is based on clinical evidence supported by structural imaging features characterizing the ventriculomegaly typical of this disease, such as the “disproportionately enlarged subarachnoid space hydrocephalus” (DESH), the Evans Index (EI), the Callosal Angle and the ventricular volume (VV) (Miskin et al., 2017, MORI et al., 2012). The ventriculomegaly of iNPH had been shown to produce microstructural alterations of the white matter (WM), where the corticospinal tract (CST) resulted to be the most affected brain structure by the mechanical pressure produced by the abnormal ventricular enlargement (Ades-Aron et al., 2018, Hattori et al., 2011, Nakanishi et al., 2013). These abnormal alterations of the CST due to the ventricle enlargement have been hypothesized to contribute to gait disturbance in iNPH patients (Bradley, 2000, Hattingen et al., 2010, KOYAMA et al., 2013, KOYAMA et al., 2012, Marumoto et al., 2012, Tarnaris et al., 2009), although the etiology of gait disturbances is not entirely understood.
Progressive Supranuclear Palsy (PSP) is a neurodegenerative disorder which shares several clinical findings with NPH (parkinsonism, disturbance of gait balance, cognitive impairment and urinary incontinence), and can show marked ventricular dilation, making the differentiation between the two diseases often challenging (Cucca et al., 2018, Quattrone et al., 2020, Quattrone et al., 2021). A very recent work (Quattrone et al., 2020), demonstrated that quantitative MRI biomarkers such as automated ventricular volumetry (AVV) and Magnetic Resonance Hydrocephalic Index (MRHI) were able to accurately distinguish between iNPH and PSP patients. However, the possible alteration of the CST due to the ventricular enlargement detected in iNPH has not been explored yet in PSP.
The aim of the present work is to assess the microstructural integrity of the CST in PSP patients without and with ventricular dilatation in comparison with iNPH patients and healthy control subjects. In these patients, we used DTI deterministic tractography and the tract profile technique (Sarica et al., 2017, Yeatman et al., 2012) for exploring the possible regional changes in FA, MD, AxD and RD. Furthermore, we conducted a study of the association between the diffusion properties of the CST and some measures of ventricular dilatation, such as EI and VV.
2. Methods
2.1. Participants
The patients were recruited consecutively between 2010 and 2020 among those referred to the Institute of Neurology at the University of Catanzaro, Italy. Clinical diagnosis of PSP and iNPH were established according to international diagnostic criteria by a neurologist with > 10 years of experience in movement disorders and dementia (Höglinger et al., 2017, MORI et al., 2012). All PSP patients enrolled before 2017 were diagnosed according to previous diagnostic criteria (Litvan et al., 1996, Williams et al., 2005) and expert guidelines and were reclassified according to the recent diagnostic criteria as probable PSP-Richardson’s syndrome or probable PSP-Parkinsonism (Höglinger et al., 2017).
Diagnosis of possible iNPH with MRI support was established according to Japanese guidelines (Mori et al., 2012), requiring the presence of at least two clinical symptoms of the classical triad (gait disturbance, urinary incontinence and cognitive impairment) associated with ventricular dilation (defined by an Evans index > 0.3) and supported by the presence of DESH on MR images. All MRI data of the iNPH patients analyzed in this study were obtained before shunt procedures (Sarica et al., 2021). For each patient, a clinical assessment was performed, including Unified Parkinson’s Disease Rating Scale – pars III (UPDRS-III), the Mini-Mental State Examination (MMSE), and the iNPH grading scale (iNPHGS) in iNPH patients.
Exclusion criteria for patients were: history of neuroleptic use within the previous six months, clinical features suggestive of other diseases, and MRI abnormalities such as vascular lesions in the basal ganglia. In addition, none of the iNPH patients had a history of subarachnoid hemorrhage, meningitis, head injury or congenital hydrocephalus, or radiological evidence of aqueductal stenosis.
No control subject had a history of neurological, psychiatric, or other major medical illnesses.
All participants gave written informed consent, and all study procedures and ethical aspects were approved by the institutional review board (Magna Graecia University review board, Catanzaro, Italy), according to the Helsinki Declaration.
2.2. MRI acquisition
Brain MRI was performed according to routine protocol (Sarica et al., 2014) by a 3 T scanner with an 8-channel head coil (Discovery MR-750, GE, Milwaukee, WI, USA). The protocol included: (a) whole-brain T1-weighted scan MRI (SPGR; TE/TR = 3.7/9.2 msec, flip angle 12°, voxel- size 1 × 1 × 1 mm3); (b) T2‐weighted fast spin echo and T2‐weighted FLAIR sequences; (c) diffusion-weighted volumes, acquired by using spin-echo echo-planar imaging (TE/TR = 87/10,000 msec, bandwidth 250KHz, matrix size 128 × 128, 80 axial slices, voxel size 2.0 × 2.0 × 2.0 mm3) with 27 equally distributed orientations, b-value 1,000 s/mm2. Particular care was taken to restrain subject’s movements with cushions and adhesive medical tape. A rater, blinded to diagnoses, visually checked the images to exclude all scans with artefacts.
2.3. Image processing
The FMRIB’s v6 (Oxford Centre for Functional MRI of the Brain) Diffusion Toolbox (Jenkinson et al., 2012) was used for correcting eddy currents and motion distortions. The non-diffusion-weighted images were skull stripped using the FMRIB’s brain extraction tool (bet) and used to mask all diffusion-weighted image. A diffusion-tensor model was fitted at each voxel using dtifit, generating fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AxD) and radial diffusivity (RD) maps, used for following processing (Sarica et al., 2019a).
The Automated Fiber Quantification (AFQ version 1.2, MATLAB R2018a) tool (Yeatman et al., 2012) was used for reconstructing the CST and for evaluating diffusion metrics along the tract length. AFQ builds the main white matter tracts, for both hemispheres, and measures FA, MD, AxD and RD along their trajectories (100 nodes). In particular, AFQ uses a three-step procedure to identify 24 major fiber tracts, based on a combination of methods (Yeatman et al., 2012) consisting of: (1) whole-brain fiber tractography, (2) waypoint ROI-based fiber tract segmentation and (3) cleaning and refinement of fiber tracts based on a probabilistic tracts atlas. For the CST, the first ROI was defined at the cerebral peduncle in axial plane at the level of the decussation of the superior cerebellar peduncle, while the second ROI is placed in the central sulcus, right after the bifurcation to the motor and sensory cortex. More details could be found elsewhere (Sarica et al., 2017, Sarica et al., 2019b, Yeatman et al., 2012). Visual inspection was performed by an expert neuroradiologist for assuring the correct coregistration of the T1 and DTI images and for checking the placement of the CST fibers after performing the tractography, and no manual correction was needed.
An anatomical subdivision of the corticospinal tract was performed by computing the first order derivatives of FA along each node of the mean of the control group (Ho et al., 2017). Then, we found the local maxima of the FA first order derivative and used their location to define the voxel interval corresponding to six anatomical regions: the medulla oblungata (MO), the pons (P), the cerebral peduncle (CP), the posterior limb of internal capsule (PLIC), the corona radiata (CR) and the subcortical white matter (SWM). The mean of left and right CST for each diffusion metric was then evaluated. EI and MRHI were measured on T1-weighted images in all study participants according to previously described procedures (Miskin et al., 2017, Quattrone et al., 2020).
Freesurfer (v.6) (Dale et al., 1999) and its standard reconstruction pipeline were used for obtaining from the T1 images the ventricles volume (VV) as the sum of the lateral and the inferior lateral ventricle of the left and the right hemisphere, third ventricle and fourth ventricle. The VV was then normalized for the total intracranial volume.
2.4. Statistical analysis
The statistical analysis was performed with a home-made script in Python (v. 3.8.6). An analysis of variance (ANOVA) was employed for comparing age, age at onset, disease duration and UPDRS-III. Differences in the gender distribution were assessed with pairwise Pearson Chi-square tests (p < 0.05). An analysis of covariance (ANCOVA) was used with age and gender as covariates for comparing the MMSE, EI, VV, MRHI and the diffusion metrics in the six anatomical regions of the corticospinal tract among the four diagnostic groups. Moreover, we performed an ANCOVA with age, sex and UPDRS-III as covariates for comparing the diffusion metrics among PSP-wvd, PSP-vd and iNPH patients.
All the multiple comparisons were accounted by post-hoc Tukey’s honest significant difference tests (p < 0.05). Spearman’s partial correlation (with age and gender as control variables) was performed for exploring the association between the EI, VV and the diffusion metrics per anatomical region of the CST. The multiple comparisons in the partial correlation were accounted with Bonferroni’s correction (p < 0.5/24 = 0.002).
3. Results
3.1. Participants
Twenty-three possible iNPH patients with MRI support (mean age 74.5 ± 7.87, 2 females, EI 0.379 ± 0.034), 54 probable PSP without ventricular dilatation (PSP-wvd, mean age 70.7 ± 6, 25 females, EI 0.267 ± 0.026), 33 PSP with ventricular dilatation (PSP-vd, 8 females, mean age 74.9 ± 9.49, EI 0.327 ± 0.015), and 26 control subjects (CTRL, mean age 72.4 ± 4.48, 7 females, EI 0.262 ± 0.046) were enrolled in the current study. According to the recent diagnostic criteria (Höglinger et al., 2017), PSP patients were reclassified as probable PSP-Richardson’s syndrome (PSP-RS, n = 57) or probable PSP-Parkinsonism (PSP-P, n = 30). Fourteen of 23 iNPH patients subsequently underwent shunt procedure with clinical benefit and were reclassified as definite iNPH.
Thirty-three PSP patients (21 PSP-RS and 12 PSP-P) had ventricular dilatation (EI > 0.30, PSP-vd) while the remaining 54 PSP patients (36 PSP-RS and 18 PSP-P) had normal ventricular system (EI ≤ 0.30, PSP-wvd).
3.2. Statistical analysis
The results of the statistical analysis for the demographic and clinical variables are reported in Table 1. No differences in age existed among the four groups (Table 1), while the gender distribution was different only between iNPH and PSP-wvd. The age at onset was higher in iNPH patients than PSP-wvd, while the disease duration was not different among the three pathological groups. The UPDRS-III was lower in iNPH patients than in both PSP groups. The analysis of the UPDRS-III gait score (item 29) revealed that iNPH patients had lower values than the PSP patients, while PSP-wvd and PSP-vd had not significant differences. The MMSE score was higher in healthy controls than iNPH, PSP-wvd and PSP-vd. Regarding the EI, VV and MRHI, PSP-vd patients had lower values than iNPH patients, while they showed significant higher values than PSP-wvd patients and CTRL subjects.
Table 1.
Clinical and demographics values per diagnostic group. Mean ± standard deviation were reported.
| CTRL (26) |
PSP-wvd (54) |
PSP-vd (33) |
iNPH (23) |
P-value | Post-hoc | |
|---|---|---|---|---|---|---|
| Age | 72.4 ± 4.5 | 70.7 ± 6.0 | 74.9 ± 9.5 | 74.5 ± 7.9 | 0.20a | N.Ab |
| Female, n | 7 | 25 | 8 | 2 | N.A. | iNPH ≠ PSP-wvdc |
| Age at onset | N.A. | 65.7 ± 6.2 | 67.4 ± 7.4 | 70.4 ± 8.4 | <0.001a | iNPH > PSP-wvdb |
| Disease duration | N.A. | 4.87 ± 3.0 | 5.73 ± 3.2 | 4.00 ± 3.7 | 0.24a | N.A.b |
| UPDRS-III | N.A. | 37.9 ± 9.3 | 40.4 ± 7.1 | 13.1 ± 11.1 | <0.001a | iNPH < PSP-wvd, PSP-vde |
| UPDRS gait (item 29) | N.A. | 2.65 ± 0.9 | 2.91 ± 0.9 | 1.89 ± 1.3 | 0.02a | iNPH < PSP-wvd, PSP-vdb |
| iNPHGS | N.A. | N.A. | N.A. | 5.0 ± 3.2 | N.A. | N.A. |
| MMSE | 27.1 ± 1.2 | 22.2 ± 5.8 | 22.1 ± 5.7 | 21.9 ± 7.3 | 0.012d | CTRL > PSP-wvd, PSP-vd, iNPHe |
| EI | 0.262 ± 0.05 | 0.267 ± 0.03 | 0.327 ± 0.01 | 0.379 ± 0.03 | <0.001d | CTRL < PSP-vd, iNPHe PSP-wvd < PSP-vd, iNPHe PSP-vd < iNPHe |
| VVf | 0.002 ± 0.004 | 0.016 ± 0.01 | 0.030 ± 0.01 | 0.062 ± 0.03 | <0.001d | CTRL < PSP-vd, iNPHe PSP-wvd < PSP-vde iNPH > PSP-wvd, PSP-vde |
| MRHI | 0.50 ± 0.02 | 0.51 ± 0.02 | 0.54 ± 0.02 | 0.61 ± 0.02 | <0.001d | CTRL < PSP-vd, iNPHe PSP-wvd < PSP-vd, iNPHe PSP-vd < iNPHe |
Abbreviations: CTRL = controls; PSP-wvd = Progressive Supranuclear Palsy without ventricular dilatation; PSP-vd = Progressive Supranuclear Palsy with ventricular dilatation; iNPH = Normal Pressure Hydrocephalus; UPDRS-III = Unified Parkinson’s Disease Rating Scale – pars III, MMSE = Mini-Mental State Examination, iNPHGS = iNPH grading scale; EI = Evans Index; VV = Ventricle Volume; N.A. = Not applicable.
ANOVA p-value, significant at < 0.05.
Post-Hoc of ANOVA corrected for multiple comparisons with Tukey’s, significant at < 0.05.
Pairwise Chi-squared, significant at < 0.05.
ANCOVA with age and gender in covariates, significant at < 0.05.
Post-Hoc of ANCOVA with age and gender in covariates, corrected for multiple comparisons with Tukey’s HSD test, significant at < 0.05.
Normalized for the total intracranial volume.
3.3. Diffusion metrics
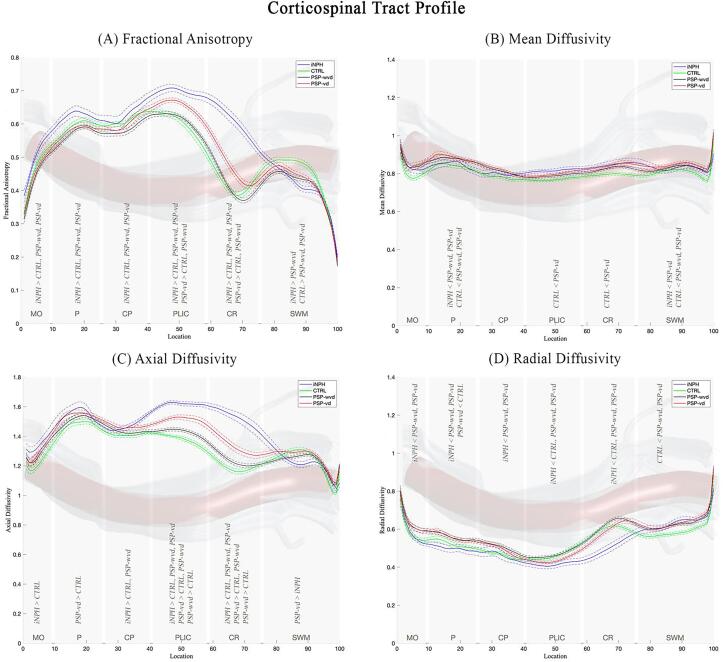
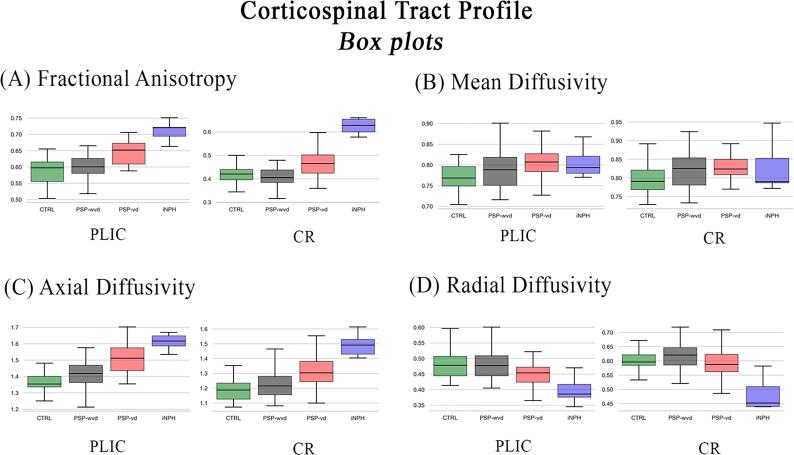
Fig. 1 shows the CST profile along its 100 nodes in all patient groups. The Fig. 1A-1D show the analyzed diffusion metrics in all patient groups for each anatomical region of CST. The results of ANCOVA and its post-hoc for comparing the diffusion metrics between the anatomical regions of CST in the different patient groups were reported in Table 2. iNPH patients had higher FA (Fig. 1A and Table 2) and AxD (Fig. 1C and Table 2) and lower RD (Fig. 1D and Table 2) values than the other three groups in PLIC and CR. PSP-vd patients had higher FA, and AxD in PLIC and CR than PSP-wvd and controls (Fig. 1A, Fig. 1C and Table 2). RD was significantly altered only in iNPH patients, while the other two diagnostic groups (PSP-wvd and PSP-vd) had no differences in RD compared with healthy controls in the region between the cerebral peduncle and corona radiata of the CST (Fig. 1D and Table 2). The PSP-wvd had significant alterations only in the PLIC and CR with the AxD higher than the controls (Fig. 1C and Table 2). Fig. 2 reports the box plots of the four diffusion metrics for the most involved CST regions (PLIC and CR) per group (supplementary material for the other ROIs, Figure S1). The findings of ANCOVA with age, gender and UPDRS-III as covariates were not substantially changed.
Fig. 1.
Tract profile of CST along its 100 nodes divided per anatomical region (in gray), numbered from inferior (0) to superior (1 0 0). The significant (p < 0.05) ANCOVA post-hoc results are also reported. (A) Fractional Anisotropy; (B) Mean Diffusivity; (C) Axial Diffusivity; (D) Radial Diffusivity. Abbreviations: MO = Medulla Oblungata; P = Pons; CP = Cerebral Peduncle; PLIC = Posterior Limb of the Internal Capsule; CR = Corona Radiata; SWM = Subcortical White Matter; CTRL = controls (in green); PSP-wvd = Progressive Supranuclear Palsy without ventricular dilatation (in black); PSP-vd = PSP with ventricular dilatation (in red); iNPH = Idiopathic Normal Pressure Hydrocephalus (in blue). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Results of the CST tractography comparison among the four diagnostic groups. Mean ± standard deviation of the four diffusion metrics, FA, MD, AxD and RD are reported for each of the six anatomical regions and for the whole CST.
| CTRL (26) |
PSP-wvd (54) |
PSP-vd (33) |
iNPH (23) |
P-value a | Post-hoc b | ||
|---|---|---|---|---|---|---|---|
| FA | MO | 0.489 ± 0.0363 | 0.468 ± 0.0429 | 0.479 ± 0.0447 | 0.54 ± 0.0333 | <0.001 | iNPH > CTRL, PSP-wvd, PSP-vd |
| P | 0.598 ± 0.0391 | 0.577 ± 0.0363 | 0.59 ± 0.0331 | 0.645 ± 0.05 | <0.001 | iNPH > CTRL, PSP-wvd, PSP-vd | |
| CP | 0.619 ± 0.0312 | 0.595 ± 0.0563 | 0.614 ± 0.0608 | 0.667 ± 0.0604 | <0.001 | iNPH > CTRL, PSP-wvd, PSP-vd | |
| PLIC | 0.587 ± 0.0538 | 0.598 ± 0.0391 | 0.644 ± 0.0401 | 0.708 ± 0.0361 | <0.001 | iNPH > CTRL, PSP-wvd, PSP-vd; PSP-vd > CTRL, PSP-wvd | |
| CR | 0.415 ± 0.0431 | 0.409 ± 0.0437 | 0.465 ± 0.064 | 0.615 ± 0.0459 | <0.001 | iNPH > CTRL, PSP-wvd, PSP-vd; PSP-vd > CTRL, PSP-wvd | |
| SWM | 0.447 ± 0.0208 | 0.413 ± 0.0318 | 0.422 ± 0.0357 | 0.433 ± 0.023 | <0.001 | iNPH > PSP-wvd; CTRL > PSP-wvd, PSP-vd | |
| whole CST | 0.526 ± 0.0236 | 0.510 ± 0.0290 | 0.535 ± 0.0266 | 0.601 ± 0.0320 | <0.001 | iNPH > CTRL, PSP-wvd, PSP-vd; PSP-vd > PSP-wvd | |
| MD | MO | 0.816 ± 0.0593 | 0.861 ± 0.0814 | 0.868 ± 0.0789 | 0.844 ± 0.0463 | 0.06 | N.A. |
| P | 0.83 ± 0.0429 | 0.869 ± 0.0625 | 0.875 ± 0.0467 | 0.822 ± 0.0518 | <0.001 | iNPH < PSP-wvd, PSP-vd; CTRL < PSP-wvd, PSP-vd | |
| CP | 0.775 ± 0.038 | 0.801 ± 0.0662 | 0.807 ± 0.055 | 0.784 ± 0.0378 | 0.056 | N.A. | |
| PLIC | 0.772 ± 0.0307 | 0.79 ± 0.0459 | 0.803 ± 0.0352 | 0.805 ± 0.0355 | 0.027 | CTRL < PSP-vd | |
| CR | 0.795 ± 0.0402 | 0.825 ± 0.0532 | 0.842 ± 0.0627 | 0.822 ± 0.0597 | 0.024 | CTRL < PSP-vd | |
| SWM | 0.809 ± 0.0333 | 0.834 ± 0.0453 | 0.850 ± 0.038 | 0.815 ± 0.0481 | <0.001 | iNPH < PSP-vd; CTRL < PSP-wvd, PSP-vd | |
| whole CST | 0.799 ± 0.0275 | 0.830 ± 0.0431 | 0.841 ± 0.0380 | 0.815 ± 0.0334 | <0.001 | iNPH < PSP-vd; CTRL < PSP-wvd, PSP-vd; | |
| AxD | MO | 1.29 ± 0.0858 | 1.33 ± 0.114 | 1.36 ± 0.0908 | 1.41 ± 0.0972 | 0.003 | iNPH > CTRL |
| P | 1.47 ± 0.0627 | 1.51 ± 0.0859 | 1.53 ± 0.0805 | 1.53 ± 0.0754 | 0.005 | PSP-vd > CTRL | |
| CP | 1.41 ± 0.0638 | 1.42 ± 0.0942 | 1.47 ± 0.0634 | 1.51 ± 0.0443 | <0.001 | iNPH > CTRL, PSP-wvd | |
| PLIC | 1.36 ± 0.0573 | 1.41 ± 0.0875 | 1.51 ± 0.107 | 1.61 ± 0.0326 | <0.001 | iNPH > CTRL, PSP-wvd, PSP-vd; PSP-vd > CTRL, PSP-wvd; PSP-wvd > CTRL |
|
| CR | 1.19 ± 0.0706 | 1.23 ± 0.0942 | 1.33 ± 0.129 | 1.5 ± 0.0946 | <0.001 | iNPH > CTRL, PSP-wvd, PSP-vd; PSP-vd > CTRL, PSP-wvd; PSP-wvd > CTRL |
|
| SWM | 1.23 ± 0.0483 | 1.22 ± 0.0574 | 1.25 ± 0.0318 | 1.21 ± 0.0642 | 0.009 | PSP-vd > iNPH | |
| whole CST | 1.32 ± 0.0335 | 1.35 ± 0.0614 | 1.41 ± 0.0517 | 1.46 ± 0.0194 | <0.001 | iNPH > CTRL, PSP-wvd, PSP-vd; PSP-vd > CTRL, PSP-wvd; PSP-wvd > CTRL |
|
| RD | MO | 0.58 ± 0.0574 | 0.625 ± 0.076 | 0.623 ± 0.0865 | 0.559 ± 0.0373 | 0.001 | iNPH < PSP-wvd, PSP-vd |
| P | 0.509 ± 0.0512 | 0.55 ± 0.0611 | 0.545 ± 0.0447 | 0.466 ± 0.0639 | <0.001 | iNPH < PSP-wvd, PSP-vd; PSP-wvd < CTRL | |
| CP | 0.456 ± 0.0372 | 0.489 ± 0.0727 | 0.477 ± 0.0731 | 0.422 ± 0.0659 | 0.001 | iNPH < PSP-wvd, PSP-vd | |
| PLIC | 0.477 ± 0.0523 | 0.479 ± 0.0438 | 0.450 ± 0.031 | 0.4 ± 0.0484 | <0.001 | iNPH < CTRL, PSP-wvd, PSP-vd | |
| CR | 0.599 ± 0.0418 | 0.624 ± 0.0502 | 0.599 ± 0.0634 | 0.486 ± 0.0618 | <0.001 | iNPH < CTRL, PSP-wvd, PSP-vd | |
| SWM | 0.60 ± 0.0313 | 0.64 ± 0.0465 | 0.647 ± 0.0479 | 0.617 ± 0.0432 | <0.001 | CTRL < PSP-wvd, PSP-vd | |
| whole CST | 0.537 ± 0.0316 | 0.568 ± 0.0427 | 0.557 ± 0.0395 | 0.492 ± 0.0446 | <0.001 | iNPH < CTRL, PSP-wvd, PSP-vd; CTRL < PSP-wvd |
Abbreviations: CTRL = controls; PSP-wvd = Progressive Supranuclear Palsy without ventricular dilatation; PSP-vd = Progressive Supranuclear Palsy with ventricular dilatation; iNPH = Normal Pressure Hydrocephalus; FA = Fractional Anisotropy; MD = Mean Diffusivity; AxD = Axial Diffusivity; RD = Radial Diffusivity; N.A. = Not applicable.
ANCOVA with age and gender as covariates, significant at < 0.05.
Post-Hoc of ANCOVA with age and gender as covariates, corrected for multiple comparisons with Tukey’s HSD test, significant at < 0.05.
Fig. 2.
Box plots of the four diffusion metrics for the most involved CST region per groups. Abbreviations: PLIC = Posterior Limb of the Internal Capsule; CR = Corona Radiata; CTRL = controls (in green); PSP-wvd = Progressive Supranuclear Palsy without ventricular dilatation (in black); PSP-vd = PSP with ventricular dilatation (in red); iNPH = Idiopathic Normal Pressure Hydrocephalus (in blue). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Partial correlation in the whole group (Table 3) revealed that EI had a strong positive association with FA and AxD and a strong negative association with RD in the region between the cerebral peduncle and the corona radiata. Similarly to EI, the ventricle volume was positively significantly correlated with FA and AxD, and negatively correlated with RD in the posterior limb of internal capsule and corona radiata (Table 3). The plots of Linear Regression between the diffusion metrics (FA, AD and RD) and EI and VV in the most representative CST regions (PLIC and CR) are reported in Supplementary Material (Figure S2).
Table 3.
Results of the partial correlation between EI and VV and diffusion metrics per anatomical region of the CST. The correlation coefficients r and the p-values were reported. In bold significant results corrected for Bonferroni’s multiple comparisons (p < 0.5/24 = 0.002).
| Partial Correlation | EI (r, p-value) |
VV a (r, p-value) |
|
|---|---|---|---|
| FA | MO | 0.285, <0.001 | 0.197, 0.07 |
| P | 0.229, 0.07 | 0.305, 0.004 | |
| CP | 0.315, <0.001 | 0.190, 0.08 | |
| PLIC | 0.664, <0.001 | 0.632, <0.001 | |
| CR | 0.720, <0.001 | 0.635, <0.001 | |
| SWM | 0.132, 0.12 | 0.275, 0.13 | |
| MD | MO | 0.013, 0.88 | 0.175, 0.10 |
| P | −0.077, 0.37 | −0.018, 0.87 | |
| CP | −0.104, 0.22 | 0.029, 0.79 | |
| PLIC | 0.107, 0.21 | 0.152, 0.15 | |
| CR | −0.007, 0.93 | 0.105, 0.34 | |
| SWM | −0.032, 0.71 | 0.090, 0.41 | |
| AxD | MO | 0.199, 0.019 | 0.344, 0.01 |
| P | 0.196, 0.02 | 0.320, 0.003 | |
| CP | 0.305, <0.001 | 0.314, 0.003 | |
| PLIC | 0.635, <0.001 | 0.666, <0.001 | |
| CR | 0.614, <0.001 | 0.628, <0.001 | |
| SWM | 0.005, 0.95 | −0.022, 0.69 | |
| RD | MO | −0.205, 0.01 | −0.015, 0.89 |
| P | −0.161, 0.05 | −0.146, 0.18 | |
| CP | −0.261, 0.002 | −0.128, 0.24 | |
| PLIC | −0.511, <0.001 | −0.485, <0.001 | |
| CR | −0.522, <0.001 | −0.389, <0.001 | |
| SWM | −0.054, 0.52 | 0.173, 0.11 | |
Abbreviations: EI = Evans Index; VV = Ventricle Volume; FA = Fractional Anisotropy; MD = Mean Diffusivity; AxD = Axial Diffusivity; RD = Radial Diffusivity; MO = Medulla Oblungata; P = Pons; CP = Cerebral Peduncle; PLIC = Posterior Limb of the Internal Capsule; CR = Corona Radiata; SWM = Subcortical White Matter; CTRL = controls; PSP-wvd = Progressive Supranuclear Palsy without ventricular dilatation; PSP-vd = PSP with ventricular dilatation; iNPH = Idiopathic Normal Pressure Hydrocephalus.
Normalized for the total intracranial volume.
A schematic representation of the relationship between the ventricular dilatation assessed by EI and the DTI CST changes was reported in Fig. 3. The Fig. 3A represents the absence of ventricular dilatation (controls and PSP-wvd), while the Fig. 3B shows a moderate ventricular enlargement (PSP-vd), which was accompanied by a moderate increase of FA and AxD. Finally, the Fig. 3C represents the case of iNPH patients with marked ventricular dilatation, in which the FA and AxD strongly increased, and the RD decreased.
Fig. 3.
Schematic representation of three models of the CST fibers (in red) compressed by the ventricular dilatation (real MRI ventricles in coronal view). (A) Normal EI, no CST alterations (CTRL and PSP-wvd patients); (B) EI > 0.31, increment of FA and AxD (PSP-vd patients); (C) EI > 0.37, increment of FA and AxD and decrement of RD (iNPH patients). The brightest color of the CST fibers represents the most affected regions, the internal capsule, and the corona radiata. Abbreviations: CTRL = controls; PSP-wvd = Progressive Supranuclear Palsy without ventricular dilatation; PSP-vd = PSP with ventricular dilatation; iNPH = Idiopathic Normal Pressure Hydrocephalus; FA = Fractional Anisotropy; AxD = Axial Diffusivity; RD = Radial Diffusivity. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
In the present work, we found microstructural alterations of the CST in PSP patients with ventricular dilatation (PSP-vd) and iNPH patients, in comparison with PSP patients without ventricular dilatation (PSP-wvd) and healthy controls. iNPH and PSP-vd patients had a marked increase in FA and AxD in CST, especially in the posterior limb of internal capsule and in the corona radiata. Interestingly, the RD alterations in the PLIC and CR were found only in iNPH patients, who showed significantly lower RD values than controls and PSP patients.
Our findings confirm the pathological alterations of the CST in iNPH patients (Hattingen et al., 2010, Hattori et al., 2011, Nakanishi et al., 2013). The increment of the FA and AxD implies a rise of the diffusion along the longitudinal axis of CST fibers, parallel to axon orientation (Ades-Aron et al., 2018) with an increase of the fiber density (Nakanishi et al., 2013). On the contrary, the decrease of RD, found in iNPH patients, reflects a decrease of the diffusion along the radial direction. It has been hypothesized that this compression and deformation of the CST in iNPH patients may result in frontal lobe dysfunction, alterations of the circuit cortico-basal ganglia-thalamus and affect the supplementary motor cortex producing the well-known gait disturbances (Siasios et al., 2016).
In this study, we also evaluated the corticospinal diffusion tract profile in two distinct cohorts of PSP patients, without and with ventricular dilatation as assessed by the EI, in comparison with iNPH patients and healthy controls.
Our PSP-vd patients showed a similar pattern of CST alterations in comparison with iNPH patients, with higher FA and AxD values than PSP-wvd and healthy controls. These CST alterations of PSP-vd patients were particularly evident in the regions of the posterior limb of internal capsule and in the corona radiata, which are the two regions closer to the head of lateral ventricles. On the contrary, our PSP-wvd had in these regions only a mild increment of the AxD, without any significant alterations in the FA. Thus, we can speculate that the ventricular enlargement in our PSP-vd patients, produced a stretching on the neural fibers along the axonal direction, similarly, though of lesser degree, to what occurring in iNPH patients, (Ades-Aron et al., 2018, Hattingen et al., 2010, Hattori et al., 2011, Nakanishi et al., 2013). The lack of alterations of the RD in the PLIC and CR of PSP patients may be explained by the less marked ventricular enlargement in these patients than iNPH, indicating that this metric was less sensitive than FA and AxD to the increase of ventricles volume. Our findings are supported by correlation data showing a strong association between DTI metrics and EI and VV values, thus demonstrating that the graded severity of ventriculomegaly was strictly related to the graded severity of the CST microstructural alterations.
The involvement of the pyramidal system in PSP patients was previously demonstrated in clinical, imaging and neuropathological studies (Barbagallo et al., 2019, Stejskalova et al., 2019). Considering the whole CST, our PSP patients, both with and without ventricular dilatation, showed the increment of MD, AxD and RD, while no differences were found in the FA in comparison with controls. These findings are in accordance with previous tractography studies (Agosta et al., 2012, Piattella et al., 2015, Tessitore et al., 2014, Worker et al., 2014, Zanigni et al., 2017) showing that PSP patients had diffusion alterations of the CST, characterized by increased MD, AxD and RD compared with healthy controls. Regarding the FA, it was decreased in some studies (Agosta et al., 2012, Tessitore et al., 2014), while it was not different from controls in other reports (Kvickstrom et al., 2011, Potrusil et al., 2020). The heterogeneity of the FA findings in CST of PSP patients could be related to the coexistence of two different pathological processes: the neurodegenerative aspect, which could produce a decrease of the FA, and the compression effect due to the ventricular enlargement in PSP with ventricular dilatation, which could increase the FA of CST. In the present study, we also analyzed regionally the CST, and we found lower FA in the subcortical WM in both PSP groups without and with ventricular dilatation compared with controls, probably reflecting a neurodegenerative process, while we found a higher FA in the internal capsule and corona radiata in PSP-vd patients, in comparison with both PSP-wvd and controls, indicating the compression effect of ventricular dilatation in these CST regions. Taken together, these findings suggest that in PSP patients FA values can decrease in some CST regions, probably affected by the neurodegeneration, and can be increased in others due to the ventricle enlargement.
Thus, we can hypothesize that regional anatomical division of the CST could be more powerful than the whole CST analysis in distinguishing diffusion alterations caused by the neurodegenerative and compressive processes, or in detecting subtle diffusion abnormalities that may be otherwise lost.
The present study has two main strengths. First, to evaluate the effect of ventricular enlargement in PSP on corticospinal fibers, we compared PSP patients with and without ventricular dilation, thus separating the possible effect of neurodegeneration on CST from the mechanical effect of the ventricular compression on the CST fibers. Second, in addition to the whole CST analysis, we also explored the tract profile in six different anatomical regions, which gave the specific localization of the diffusion changes in PSP patients.
One limitation of this study is that PSP patients did not undergo pathological examination. However, PSP diagnosis was performed by expert clinicians, according to international diagnostic criteria with high sensitivity and specificity (Ali et al., 2019). The second limitation is the small sample size of iNPH cohort, however most iNPH underwent shunt surgery with clinical benefit, and our findings in these patients were in accordance with the existing literature.
5. Conclusions
In conclusion, this study demonstrates that the ventricular dilatation has a compressive effect against the corticospinal fibers in patients with PSP, similarly to what occurs in iNPH. The graded severity of the ventriculomegaly was associated with the graded severity of the microstructural alterations of the CST. The tract profile technique based on analysis of different anatomical regions of CST provides new insights on the focal effect of ventricular dilation against corticospinal fibers at the level of internal capsule and corona radiata with a better understanding of the DTI alterations of CST in PSP.
CRediT authorship contribution statement
Alessia Sarica: Conceptualization, Methodology, Software, Validation, Formal analysis, Writing – original draft, Writing – review & editing, Visualization. Andrea Quattrone: Conceptualization, Investigation, Resources, Writing – original draft, Writing – review & editing, Visualization. Alessandro Mechelli: Resources, Writing – review & editing. Maria Grazia Vaccaro: Resources, Writing – review & editing. Maurizio Morelli: Resources, Writing – review & editing. Aldo Quattrone: Conceptualization, Investigation, Writing – original draft, Writing – review & editing, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ades-Aron B., Yeager S., Miskin N., Fieremans E., George A., Golomb J. Diffusional Kurtosis along the Corticospinal Tract in Adult Normal Pressure Hydrocephalus. AJNR Am J Neuroradiol. 2018;39(12):2218–2223. doi: 10.3174/ajnr.A5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F., Pievani M., Svetel M., Ječmenica Lukić M., Copetti M., Tomić A., Scarale A., Longoni G., Comi G., Kostić V.S., Filippi M. Diffusion tensor MRI contributes to differentiate Richardson's syndrome from PSP-parkinsonism. Neurobiol Aging. 2012;33(12):2817–2826. doi: 10.1016/j.neurobiolaging.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Ali F., Martin P.R., Botha H., Ahlskog J.E., Bower J.H., Masumoto J.Y., Maraganore D., Hassan A., Eggers S., Boeve B.F., Knopman D.S., Drubach D., Petersen R.C., Dunkley E.D., Gerpen J., Uitti R., Whitwell J.L., Dickson D.W., Josephs K.A. Sensitivity and Specificity of Diagnostic Criteria for Progressive Supranuclear Palsy. Mov Disord. 2019;34(8):1144–1153. doi: 10.1002/mds.27619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbagallo G., Morelli M., Quattrone A., Chiriaco C., Vaccaro M.G., Gullà D., Rocca F., Caracciolo M., Novellino F., Sarica A., Arabia G., Sabatini U., Quattrone A. In vivo evidence for decreased scyllo-inositol levels in the supplementary motor area of patients with Progressive Supranuclear Palsy: A proton MR spectroscopy study. Parkinsonism Relat D. 2019;62:185–191. doi: 10.1016/j.parkreldis.2018.12.008. [DOI] [PubMed] [Google Scholar]
- Bradley W.G. Normal pressure hydrocephalus: new concepts on etiology and diagnosis. AJNR Am J Neuroradiol. 2000;21:1586–1590. [PMC free article] [PubMed] [Google Scholar]
- Cucca A., Biagioni M.C., Sharma K., Golomb J., Gilbert R.M., Di Rocco A., Fleisher J.E. Comorbid Normal Pressure Hydrocephalus with Parkinsonism: A Clinical Challenge and Call for Awareness. Case Rep Neurol Med. 2018;2018:1–8. doi: 10.1155/2018/2513474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Hattingen E., Jurcoane A., Melber J., Blasel S., Zanella F.E., Neumann-Haefelin T., Singer O.C. Diffusion tensor imaging in patients with adult chronic idiopathic hydrocephalus. Neurosurgery. 2010;66(5):917–924. doi: 10.1227/01.NEU.0000367801.35654.EC. [DOI] [PubMed] [Google Scholar]
- Hattori T., Yuasa T., Aoki S., Sato R., Sawaura H., Mori T., Mizusawa H. Altered microstructure in corticospinal tract in idiopathic normal pressure hydrocephalus: comparison with Alzheimer disease and Parkinson disease with dementia. AJNR Am J Neuroradiol. 2011;32(9):1681–1687. doi: 10.3174/ajnr.A2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.C., King L.S., Leong J.K., Colich N.L., Humphreys K.L., Ordaz S.J., Gotlib I.H. Effects of sensitivity to life stress on uncinate fasciculus segments in early adolescence. Soc Cogn Affect Neurosci. 2017;12(9):1460–1469. doi: 10.1093/scan/nsx065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglinger G.U., Respondek G., Stamelou M., Kurz C., Josephs K.A., Lang A.E., Mollenhauer B., Müller U., Nilsson C., Whitwell J.L., Arzberger T., Englund E., Gelpi E., Giese A., Irwin D.J., Meissner W.G., Pantelyat A., Rajput A., van Swieten J.C., Troakes C., Antonini A., Bhatia K.P., Bordelon Y., Compta Y., Corvol J.-C., Colosimo C., Dickson D.W., Dodel R., Ferguson L., Grossman M., Kassubek J., Krismer F., Levin J., Lorenzl S., Morris H.R., Nestor P., Oertel W.H., Poewe W., Rabinovici G., Rowe J.B., Schellenberg G.D., Seppi K., van Eimeren T., Wenning G.K., Boxer A.L., Golbe L.I., Litvan I. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord. 2017;32(6):853–864. doi: 10.1002/mds.26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. Fsl. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- KOYAMA T., MARUMOTO K., DOMEN K., MIYAKE H. White matter characteristics of idiopathic normal pressure hydrocephalus: a diffusion tensor tract-based spatial statistic study. Neurol Med Chir (Tokyo) 2013;53(9):601–608. doi: 10.2176/nmc.oa2012-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOYAMA T., MARUMOTO K., DOMEN K., OHMURA T., MIYAKE H. Diffusion tensor imaging of idiopathic normal pressure hydrocephalus: a voxel-based fractional anisotropy study. Neurol Med Chir (Tokyo) 2012;52(2):68–74. doi: 10.2176/nmc.52.68. [DOI] [PubMed] [Google Scholar]
- Kvickstrom P., Eriksson B., van Westen D., Latt J., Elfgren C., Nilsson C. Selective frontal neurodegeneration of the inferior fronto-occipital fasciculus in progressive supranuclear palsy (PSP) demonstrated by diffusion tensor tractography. BMC Neurol. 2011;11:13. doi: 10.1186/1471-2377-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I., Agid Y., Calne D., Campbell G., Dubois B., Duvoisin R.C., Goetz C.G., Golbe L.I., Grafman J., Growdon J.H., Hallett M., Jankovic J., Quinn N.P., Tolosa E., Zee D.S. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47(1):1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- Marumoto K., Koyama T., Hosomi M., Kodama N., Miyake H., Domen K. Diffusion tensor imaging in elderly patients with idiopathic normal pressure hydrocephalus or Parkinson's disease: diagnosis of gait abnormalities. Fluids Barriers CNS. 2012;9(1):20. doi: 10.1186/2045-8118-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskin N., Patel H., Franceschi A.M., Ades-Aron B., Le A., Damadian B.E., Stanton C., Serulle Y., Golomb J., Gonen O., Rusinek H., George A.E., Neuroimaging A.D., I. Diagnosis of Normal-Pressure Hydrocephalus: Use of Traditional Measures in the Era of Volumetric MR Imaging. Radiology. 2017;285:197–205. doi: 10.1148/radiol.2017161216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, E., Ishikawa, M., Kato, T., Kazui, H., Miyake, H., Miyajima, M., Nakajima, M., Hashimoto, M., Kuriyama, N., Tokuda, T., Ishii, K., Kaijima, M., Hirata, Y., Saito, M., Arai, H., Japanese Society of Normal Pressure, H., 2012. Guidelines for management of idiopathic normal pressure hydrocephalus: second edition. Neurol Med Chir (Tokyo) 52, 775-809. [DOI] [PubMed]
- Nakanishi A., Fukunaga I., Hori M., Masutani Y., Takaaki H., Miyajima M., Aoki S. Microstructural changes of the corticospinal tract in idiopathic normal pressure hydrocephalus: a comparison of diffusion tensor and diffusional kurtosis imaging. Neuroradiology. 2013;55(8):971–976. doi: 10.1007/s00234-013-1201-6. [DOI] [PubMed] [Google Scholar]
- Piattella M.C., Upadhyay N., Bologna M., Sbardella E., Tona F., Formica A., Petsas N., Berardelli A., Pantano P. Neuroimaging evidence of gray and white matter damage and clinical correlates in progressive supranuclear palsy. J Neurol. 2015;262(8):1850–1858. doi: 10.1007/s00415-015-7779-3. [DOI] [PubMed] [Google Scholar]
- Potrusil T., Krismer F., Beliveau V., Seppi K., Müller C., Troger F., Göbel G., Steiger R., Gizewski E.R., Poewe W., Scherfler C. Diagnostic potential of automated tractography in progressive supranuclear palsy variants. Parkinsonism Relat Disord. 2020;72:65–71. doi: 10.1016/j.parkreldis.2020.02.007. [DOI] [PubMed] [Google Scholar]
- Quattrone A., Sarica A., La Torre D., Morelli M., Mechelli A., Arcuri P.P., Quattrone A. Progressive supranuclear palsy with marked ventricular dilatation mimicking normal pressure hydrocephalus. Neurol Sci. 2021:1–8. doi: 10.1007/s10072-021-05594-4. In press. [DOI] [PubMed] [Google Scholar]
- Quattrone A., Sarica A., La Torre D., Morelli M., Vescio B., Nigro S., Barbagallo G., Nisticò R., Salsone M., Arcuri P.P., Novellino F., Bianco M.G., Arabia G., Cascini G., Quattrone A. Magnetic Resonance Imaging Biomarkers Distinguish Normal Pressure Hydrocephalus From Progressive Supranuclear Palsy. Mov Disord. 2020;35(8):1406–1415. doi: 10.1002/mds.28087. [DOI] [PubMed] [Google Scholar]
- Sarica A., Cerasa A., Valentino P., Yeatman J., Trotta M., Barone S., Granata A., Nisticò R., Perrotta P., Pucci F., Quattrone A. The corticospinal tract profile in amyotrophic lateral sclerosis. Hum Brain Mapp. 2017;38(2):727–739. doi: 10.1002/hbm.23412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarica A., Cerasa A., Vasta R., Perrotta P., Valentino P., Mangone G., Guzzi P.H., Rocca F., Nonnis M., Cannataro M., Quattrone A. Tractography in amyotrophic lateral sclerosis using a novel probabilistic tool: a study with tract-based reconstruction compared to voxel-based approach. J Neurosci Methods. 2014;224:79–87. doi: 10.1016/j.jneumeth.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Sarica A., Curcio M., Rapisarda L., Cerasa A., Quattrone A., Bono F. Periventricular white matter changes in idiopathic intracranial hypertension. Ann Clin Transl Neurol. 2019;6(2):233–242. doi: 10.1002/acn3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarica A., Quattrone A., Quarantelli M., Arcuri P.P., Mechelli A., La Torre D., Vaccaro M.G., Cascini G.L., Quattrone A. Reduced Striatal DAT Uptake Normalizes After Shunt in Normal-Pressure Hydrocephalus. Mov Disord. 2021;36:261–262. doi: 10.1002/mds.28336. [DOI] [PubMed] [Google Scholar]
- Sarica A., Valentino P., Nisticò R., Barone S., Pucci F., Quattrone A., Cerasa A., Quattrone A. Assessment of the Corticospinal Tract Profile in Pure Lower Motor Neuron Disease: A Diffusion Tensor Imaging Study. Neurodegener Dis. 2019;19(3-4):128–138. doi: 10.1159/000503970. [DOI] [PubMed] [Google Scholar]
- Siasios I., Kapsalaki E.Z., Fountas K.N., Fotiadou A., Dorsch A., Vakharia K., Pollina J., Dimopoulos V. The role of diffusion tensor imaging and fractional anisotropy in the evaluation of patients with idiopathic normal pressure hydrocephalus: a literature review. Neurosurg Focus. 2016;41(3):E12. doi: 10.3171/2016.6.FOCUS16192. [DOI] [PubMed] [Google Scholar]
- Starr B.W., Hagen M.C., Espay A.J. Hydrocephalic Parkinsonism: lessons from normal pressure hydrocephalus mimics. J Clin Mov Disord. 2014;1:2. doi: 10.1186/2054-7072-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stejskalova Z., Rohan Z., Rusina R., Tesar A., Kukal J., Kovacs G.G., Bartos A., Matej R. Pyramidal system involvement in progressive supranuclear palsy - a clinicopathological correlation. BMC Neurol. 2019;19:42. doi: 10.1186/s12883-019-1270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnaris A., Kitchen N.D., Watkins L.D. Noninvasive biomarkers in normal pressure hydrocephalus: evidence for the role of neuroimaging. J Neurosurg. 2009;110(5):837–851. doi: 10.3171/2007.9.17572. [DOI] [PubMed] [Google Scholar]
- Tessitore A., Giordano A., Caiazzo G., Corbo D., De Micco R., Russo A., Liguori S., Cirillo M., Esposito F., Tedeschi G. Clinical correlations of microstructural changes in progressive supranuclear palsy. Neurobiol Aging. 2014;35(10):2404–2410. doi: 10.1016/j.neurobiolaging.2014.03.028. [DOI] [PubMed] [Google Scholar]
- Williams D.R., de Silva R., Paviour D.C., Pittman A., Watt H.C., Kilford L., Holton J.L., Revesz T., Lees A.J. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson's syndrome and PSP-parkinsonism. Brain. 2005;128(6):1247–1258. doi: 10.1093/brain/awh488. [DOI] [PubMed] [Google Scholar]
- Worker A., Blain C., Jarosz J., Chaudhuri K.R., Barker G.J., Williams S.C.R., Brown R.G., Leigh P.N., Dell’Acqua F., Simmons A., Kassubek J. Diffusion tensor imaging of Parkinson's disease, multiple system atrophy and progressive supranuclear palsy: a tract-based spatial statistics study. PLoS One. 2014;9(11):e112638. doi: 10.1371/journal.pone.0112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman J.D., Dougherty R.F., Myall N.J., Wandell B.A., Feldman H.M., Beaulieu C. Tract profiles of white matter properties: automating fiber-tract quantification. PLoS One. 2012;7(11):e49790. doi: 10.1371/journal.pone.0049790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanigni S., Evangelisti S., Testa C., Manners D.N., Calandra-Buonaura G., Guarino M., Gabellini A., Gramegna L.L., Giannini G., Sambati L., Cortelli P., Lodi R., Tonon C. White matter and cortical changes in atypical parkinsonisms: A multimodal quantitative MR study. Parkinsonism Relat Disord. 2017;39:44–51. doi: 10.1016/j.parkreldis.2017.03.001. [DOI] [PubMed] [Google Scholar]