Graphical abstract
Keywords: Ultrasound drying, Blackberry, Phenolic acid co-pigmentation, Anthocyanins, Soluble phenolics, Bound phenolics
Highlights
-
•
Co-pigmentation pretreatment protected blackberry anthocyanins during drying.
-
•
Ethanol pretreatment with phenolic acids only enhanced water effective diffusivity.
-
•
Non-anthocyaninic soluble phenolics were better preserved under contact sonication.
-
•
Bound phenolics were less affected by pretreatments and ultrasound-assisted drying.
Abstract
In this work, the spraying of ethanol solution containing phenolic acid (ferulic acid or caffeic acid) was performed before subjecting to contact ultrasound-assisted air drying of blackberry. The mass transfer modeling results revealed that sonication intensified both internal water diffusion and external water exchange during drying, and ethanol pretreatment enhanced the effective diffusivity of water. Compared with air drying alone, the drying time for sequential ferulic acid pretreatment and drying with sonication was shortened by 89.2%. Owing to the co-pigmentation between phenolic acid and anthocyanins, the retention of anthocyanins was significantly enhanced after dehydration. At the end of drying, the total anthocyanin contents in the ultrasound-dried samples pretreated with ferulic acid and caffeic acid were 25.3% and 10.5% higher than the sonicated samples without pretreatments, respectively. Furthermore, drying simultaneously with sonication promoted the preservation of non-anthocyaninic soluble phenolics including catechin, phloretic acid, rutin in blackberry compared to air drying alone. Besides, bound phenolics in blackberry were less influences by the applied dehydration treatments. This study demonstrates that the combination of phenolic acid co-pigmentation pretreatment and ultrasound drying could be a promising method to protect anthocyanin pigments during dehydration of berry fruits.
1. Introduction
Blackberry (Rubus ursinus Cham.), native to North America, is a tasty and nutritious Rosaceae plant fruit [1]. However, fresh blackberries are liable to rot and deteriorate due to high water content and soft outer skin. Drying is a promising method to process dehydrated blackberries and extend their shelf-life. Among all the existing drying technologies, ultrasound-incorporated air drying has been successfully demonstrated as an effective method to produce quality dried blackberry products within a short drying period [2]. Although simultaneous air drying and sonication have several advantages over conventional air drying alone, the loss of heat-sensitive components still cannot be ignored under ultrasound-intensified air drying [3], [4], [5]. Regarding ultrasound drying of blackberries, more efforts are needed to avoid or alleviate the degradation of anthocyanins.
The degradation of anthocyanins is common during the processing and storage of many fruits and vegetables containing anthocyanin pigments. Many studies assert that exogenous anthocyanins and other phenolics can form complexes, improving the stability of anthocyanins. This phenomenon is well-known as the co-pigmentation based on the molecular interactions [6], [7], in which anthocyanins and phenolics interact to form complex mainly through the non-covalent bond, such as π-π stacking, hydrogen bonding and van der Waals interaction [8]. In the literature, Fan et al. reported that ferulic acids showed preferable co-pigmentation ability on blackberry wine residue anthocyanin solutions with the enhanced color intensity and thermostability [8]. Also, Zhang et al. demonstrated that the addition of high-concentration gallic acid enhanced the retention of anthocyanins in blueberry juice from 52% to 66% after 10-days storage at 25 °C [9]. Considering the effectiveness of co-pigmentation treatment to protect anthocyanins in liquid foods [8], [10], it is also of interest to supplement exogenous phenolics to potentially alleviate the loss of anthocyanins during the processing of solid foods. No published studies have reported the influence of exogenous phenolic treatment on anthocyanin stability under food drying. The exogenous phenolics capable of reacting with anthocyanins include caffeic acid, ferulic acid, gallic acid, rutin, etc. [8], [9].
Phenolics are important bioactive in fruits and vegetables. Phenolics usually exist in plant cells in both soluble and bound forms, depending on their locations. Soluble phenolics are mainly located in the vacuoles of plant cells, which can be easily extracted [11]. Meanwhile, bound phenolics mostly bind with the cell wall matrix of plant cells through covalent bonds, such as pectin, cellulose, arabinoxylan and structural proteins [11]. Both soluble and bound phenolics can benefit human health. Soluble phenolics possess higher bioavailability than bound phenolics during digestion due to the easier absorption into the body's circulation [12]. Bound phenolics can survive the stomach and intestinal digestion before reaching the colon and then be utilized by gut microbes [13]. To date, the changes of soluble phenolics during drying of various fruits and vegetables have been extensively studied, and the reasons for the observed variations in the soluble phenolics are also explored in depth [14], [15]. However, only a limited number of studies are focusing on bound phenolics under drying [16]. Moreover, increasing evidence shows that the covalent and non-covalent interactions between phenolics and cell walls can affect the stability of phenolics during processing [16], [17], [18]. To produce nutritive dried blackberry products and investigate the interactions between phenolics and cell walls, it is of significance to understand the fates of both types of phenolics during dehydration.
In this context, the spraying of ethanol solution containing exogenous phenolic acid (ferulic acid or caffeic acid) was exerted before subjecting to contact ultrasound-assisted air drying of blackberry. On the one hand, the moisture transport mechanism under ultrasound drying was investigated through phenomenological modeling. On the other hand, the profiles of both soluble phenolics (including anthocyanins and non-anthocyaninic phenolics) and bound phenolics in blackberry at different drying stages were analyzed. This study provides a reference for future studies of producing dehydrated blackberries with high retention of anthocyanins and offers some preliminary guidance on the binding between phenolics and cell walls under drying.
2. Materials and methods
2.1. Materials and chemicals
Fresh blackberry fruits (Rubus americanus Britton, cultivar: Hull) were provided by the plantation located in Lishui, Nanjing belonging to the Institute of Botany, Jiangsu Province and Chinese Academy of Science. Samples were stored in a freezer at −20 °C after reaching the laboratory in July 2020.
The standards of ferulic acid, caffeic acid, p-coumaric acid, phloretic acid, p-hydroxybenzoic acid, gallic acid, catechin, myricetin, rutin and quercitrin were purchased from Yuanye Bio-Technology Co., Ltd. (Shanghai, China). All other chemicals employed were analytical grade reagents.
2.2. Drying pretreatments
Before each experiment, blackberries were thawed at 4 °C for 12 h. After thawing, blackberry pellets with a diameter of 1.5 ± 0.1 cm and a weight of 1.00 g ± 0.01 g were prepared. It should be noted that the difference in phenolic contents of different blackberry fruits cannot be ignored in this study. Blackberry pellets were used instead of the whole blackberry fruits to avoid the experimental error due to variations in the sample properties. Then, each blackberry pellet was evenly sprayed with 1 mL of ethanol solution containing caffeic acid or ferulic acid (2 g/L). For comparison, blackberry pellets sprayed with pure ethanol solution and without any pretreatments were used as the control. The samples were wrapped in parafilm and equilibrated at room temperature in the dark for 10 h. Afterwards, blackberry pellets were sent to drying.
2.3. Drying treatment
Drying treatment was performed in a self-assembly hybrid dryer involving ultrasound irradiation and hot air flow inside (Fig. 1). A 20-kHz ultrasound probe system made with titanium alloy (5 cm in diameter) was inserted from the top of this dryer. The detailed configuration of this dryer has been reported in our previous work [19]. For each drying treatment, approximately 3.0 g of blackberry pellets were placed on the plate underneath the ultrasonic probe, and the samples were made to touch the probe gently by adjusting the height of the plate. During drying, the samples were kept in contact with the ultrasound probe gently by adjusting the height of the plate periodically.
Fig. 1.
Air dryer coupled with ultrasound device used for blackberry drying.
The probe’s actual emitted ultrasound intensity was 180.1 W/dm2 measured by the calorimetric method [2]. The ultrasound device was employed in an intermittent mode (5 s on, 5 s off). The air temperature and velocity from the air inlet were 65 °C and 2.0 m/s, respectively. The ambient temperature was controlled in the range between 20 and 25 °C by an air condition (KFR-35GW/(35570)Aa-2, GREE Electric Appliances Inc., China) in the experiment period, and the air was continuously dehumidified by a dehumidifier (DH20EE, GREE Electric Appliances Inc., China). The weight of blackberry pellets was monitored throughout drying and the water content was then calculated as the mass of water in the sample divided by the mass of dry matter (DM). The drying process was terminated when the water content decreased below 2 kg water/kg dry matter, which is a representative value for the water content of commercial dried blackberry products. Drying without contact sonication was also performed as a control. Simultaneously with air drying, the temperature inside the blackberry pellet was recorded by a K-type thermocouple connected with a datalogger (RDXL12SD, Omega Engineering Inc. USA). All the treatments were conducted in triplicate. All the dehydration treatments in this study are listed in Table 1.
Table 1.
The followed dehydration treatments for blackberry pellets.
| Treatment | Abbreviation |
|---|---|
| Air drying | AD |
| Spraying with ethanol solution + air drying | E + AD |
| Spraying with ethanol solution containing ferulic acid + air drying | FA + AD |
| Spraying with ethanol solution containing caffeic acid + air drying | CA + AD |
| Contact ultrasound-assisted with air drying | AD-US |
| Spraying with ethanol solution alone + ultrasound-assisted air drying | E + AD-US |
| Spraying with ethanol solution containing ferulic acid + ultrasound-assisted air drying | FA + AD-US |
| Spraying with ethanol solution containing caffeic acid + ultrasound-assisted air drying | CA + AD-US |
2.4. Drying kinetic model
The drying kinetic curves were modeled using the diffusion theory that considers both internal and external mass transfer. In this study, blackberry pellets with a radius of 7.5 mm were considered as an approximate spherical geometry. Under the mass transfer modeling, the radius of the blackberry sphere was discretized into 50 parts after optimization. Before drying, the water content and temperature are distributed equally within the samples. Water molecules moved along the radial direction of blackberry pellet by liquid diffusion and evaporate only on the surface [2]. Moreover, the influence of changes in temperature on the water effective diffusivity was tentatively neglected [20]. Based on the above, the one-dimensional diffusion model based on Fick's second law specifies the moisture transfer for sphere under air drying as follows [21], [22], [23]:
| (1) |
where w (x, t) is the water content (kg water/kg DM) at time t (s) and a given position x (m) within the samples and De is the water effective diffusion coefficient (m2/s).
The initial condition is written as:
| (2) |
where w0 is the water content before drying (kg water/kg DM).
The boundary conditions are expressed as:
| (3) |
| (4) |
where ρDM is the dry solid density of blackberry (kg/m3), k is the external mass transfer coefficient on blackberry surface (kg water/m2 s), aw(x, t) is the water activity on the surface of blackberry pellet and φair is the relative humidity of surrounding air.
In the current model, the volume shrinkage due to water loss was not incorporated [20]. The value of aw(x, t) on the blackberry surface was estimated from the adsorption isotherm curve of the blackberry at 65 °C, as reported in our previous study [2]. The model was solved numerically by the “pdepe” function in Matlab, R2009a (The MathWorks, Inc., USA). Before solving the model, already known values were given to De and k. Both values were then optimized until the absolute average deviation (AAD) value between experimental and calculated water contents in blackberry reached the minimum:
| (5) |
At the same time, the coefficient of determination (R2) value is also calculated:
| (6) |
where wp and we are the predicted and experimental water contents (kg water/kg DM), wave is the average value of experimental water contents (kg water/kg DM).
2.5. Extraction of phenolics from blackberry
2.5.1. Extraction of soluble phenolics including anthocyanins
Soluble phenolics were extracted using the method described by Tao et al. [24] with some modifications. Aqueous ethanol was selected as a solvent for extraction. First, blackberry pellets were mixed with 50% aqueous ethanol (pH 3.5) at 20:1 (mL:g) solvent-to-solid ratio and mashed in a mortar by hand. Then, the solid–liquid mixture was moved to an Erlenmeyer flask and incubated under orbital agitation at 150 rpm and 35 °C for 1 h. The supernatant was collected through centrifugation at 8000 rpm for 15 min. The procedures mentioned above were repeated to extract phenolics in the residues using fresh 50% aqueous ethanol at a solvent-to-solid ratio of 10:1 (mL:g). Lastly, the two supernatants were mixed and stored in a refrigerator at 4 °C before being subjected to further analysis.
2.5.2. Extraction of bound phenolics
Bound phenolics were extracted by alkaline hydrolysis following the method of Tang et al. [25] with some modifications. The blackberry residues left after the extraction of soluble phenolics were mixed with 4 M NaOH at a solvent-to-solid ratio of 15:1 (mL:g), hydrolyzed for 4 h at room temperature, acidified to pH 2.0 with 6 M HCl, and then centrifuged at 8000 rpm for 15 min. Then, phenolics in the supernatant were extracted three times with ethyl acetate at a liquid-to-liquid ratio of 1:1 (mL:mL). The extracts were mixed and vacuum-evaporated to remove ethyl acetate in a rotary evaporator (Xiande Experimental Instrument Co., Ltd., Shanghai, China) at 35 °C. The residues were subsequently dissolved in 3 mL of 70% methanol, filtered with a 0.45-μm membrane and stored at 4 °C until analysis.
2.6. Scanning of visible absorbance spectra of soluble phenolic extracts after phenolic acid pretreatments
The soluble phenolic extracts after phenolic acid pretreatments were transferred into cuvettes with a pathlength of 1 cm. Then, the absorbance from 400 nm to 700 nm was scanned in a TU-1900 UV–vis spectrophotometer (Persee General Instruments Co., Ltd, Beijing, China).
2.7. Identification of phenolic components in soluble and bound phenolic extracts
The composition of phenolic components in both soluble and bound phenolic extracts was analyzed by HPLC-QTOF-MS/MS [8]. The UHPLC system (Nexera X2, Shimadzu, Japan) was coupled to a time-of-flight mass-mass spectrometer (AB SCIEX TripleTOF 4600–1, Shimadzu, Japan) with DuoSpray Ion Source. An Ultimate XB-C18 analytical column (100 × 2.1 mm) with a particle size of 3.0 μm was used. The mobile phases consisted of phase A (deionized water with 0.1% formic acid) and phase B (acetonitrile). An elution gradient with a flow rate of 0.4 mL/min was applied: 0.0 min, 5% B; 2.0 min, 5% B; 19.0 min, 70% B; 21.0 min, 90% B; 25.0 min, 90% B; 25.1 min, 5% B; and 30.0 min, 5% B. The injection volume and column temperature were 2 μL and 40 °C, respectively. The calibration of HPLC-QTOF-MS/MS was followed as reported by Tao et al [24]. The identification was carried out using an electrospray source operating in positive or negative ionization mode under the following conditions: full-scan mass range, 50–1500; temperature, 550 °C; pulse frequency, 12.891 kHz; curtain gas, 35.00 PSI; ion source gas 1, 55.00 PSI; ion source gas 2, 55.00 PSI; ion spray voltage in negative ionization mode, 4500 V; collision voltage in negative ionization mode, −40 V; collision voltage error in negative ionization mode, 20 V; ion spray voltage in positive ionization mode, 5500 V; collision voltage in positive ionization mode, 40 V; collision voltage error in positive ionization mode, 20 V. Herein, the MS information about all the identified blackberry phenolics are listed in Supplementary Table 1 and Supplementary Table 2.
2.8. Measurements of total anthocyanin contents
Total anthocyanin contents were measured using the spectrophotometric method of Ivanova et al. [26]. The detailed procedures can be found in this literature. The results are expressed as mg malvidin-3-O-glucoside equivalent/g DM.
2.9. Quantification of contents of individual phenolic compounds
2.9.1. Anthocyanins
The contents of individual anthocyaninic compounds were determined following the HPLC method of Tao et al. and Cui et al. [2], [27]. The analysis was performed in a Shimadzu HPLC system (LC-2010A, Shimadzu Corporation, Japan) coupled with an Agilent TC-C18 column (250 × 4.6 mm, 5 μm). Mobile phases A (0.5% trifluoroacetate water solution) and mobile phases B (100% acetonitrile) were applied following an elution gradient at a flow rate of 0.6 mL/min: 0–5 min, 85%-82% A; 5–11 min, 82%-79% A; 11–13 min, 79%-78% A; 13–15 min, 78%-77% A; 15–19 min, 77%-76% A; 19–22 min, 76%-75% A; 22–35 min, 75%-70% A; and 35–45 min, 85% A. The injection volume was set to 10 μL, and the column temperature was maintained at 30 °C. The detection wavelength was 520 nm. The standard of each anthocyanin was used to construct the calibration curve. The results are expressed as mg each anthocyanin/g DM. A representative chromatographic plot for blackberry anthocyanins is shown in Supplementary Fig. 1a.
2.9.2. Non-anthocyaninic phenolics
The contents of other phenolic compounds except anthocyanins were determined following the HPLC method of Li et al. [28]. The analysis was performed in the same Shimadzu HPLC system equipped with an Inertsil ODS-3 C18 column (250 × 4.6 mm, 5 μm). Mobile phase A was milli-Q water with 1% acetic acid and mobile phase B was methanol with 1% acetic acid. The flow rate was 0.6 mL/min. The chromatographic conditions were: 0–10 min, 90%-74% A; 10–25 min, 74%-60% A; 25–45 min, 60%-35% A; 45–55 min, 35%-5% A; 55–58 min, 5–90% A; and 58–65 min, 90% A. The detection wavelengths for ferulic acid, caffeic acid, p-coumaric acid, phloretic acid, p-hydroxybenzoic acid, gallic acid, catechin were 280 nm, and the detection wavelengths for myricetin, rutin and quercitrin were 350 nm. The column temperature was 25 °C and the injection volume was 20 μL. The results are expressed as mg of each phenolic compound/g DM. The representative chromatographic plots for blackberry non-anthocyaninic phenolics are shown in Supplementary Fig. 1b and c.
2.10. Data analysis
One-way analysis of variance (ANOVA) was conducted in Statistix 9.0 (Analytical Software, Tallahassee, FL, USA) to compare the means of quality data of blackberry under different treatments. The least Significant Difference (Fisher’s LSD) test was applied and the significance was set at p < 0.05. Heatmap plot was generated in Morpheus (https://software.broadinstitute.org/morpheus/) by Hierarchical Clustering Cluster analysis. Correlations between the studied data determined by Pearson's correlation method were conducted in SPSS 11.5 (SPSS Inc., USA).
The changes in the total anthocyanin content during drying were fitted using a Weibull model as shown below [29]:
| (7) |
where Ct is the content of total anthocyanins (mg/g DM) during drying at time (h), k is the degradation rate constant of total anthocyanins during drying, C0 is the initial anthocyanin content (mg/g DM), and β (dimensionless) is the shape constant for Weibull model. The data fitting process was implemented in Matlab, R2009a (The MathWorks, Inc., USA).
3. Results and discussion
3.1. Drying kinetics of blackberry
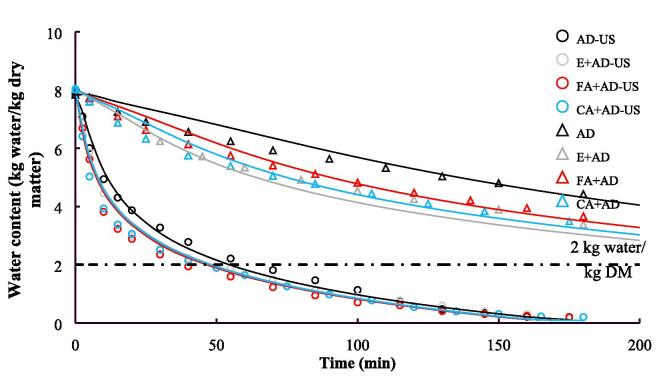
The initial water content in the blackberry pellet was 7.83 ± 0.82 kg water/kg DM. After all the three ethanol spraying pretreatments, no significant changes (p ≥ 0.05) in the water content were observed. The drying kinetic curves of the blackberry are plotted in Fig. 2. As expected, the water content of blackberry pellets decreased faster under contact ultrasound-assisted air drying than under air drying alone due to the cycles of compression and rarefaction created by ultrasound waves on blackberry [19], [30], [31]. Meanwhile, in the group of air drying in the absence of ultrasonic irradiation, the samples pretreated by ethanol spraying got dried faster than the control samples. However, the differences in the drying rates for samples pretreated by different ethanol solutions were ignorable. Da Cunha et al. [32] and Rojas et al. [33] reported similar results, where ethanol pretreatment accelerated the air drying of melon and pumpkin. Spraying with ethanol may change the permeability of fruit cell membrane and cause the cells to lose water, thus speeding up the drying process [33]. On the other hand, in the groups of ultrasound-intensified drying, the drying kinetic curves of the samples with and without ethanol pretreatments almost overlapped, indicating that sonication attenuated the promotive effect of ethanol pretreatment on the subsequent water removal under air drying. Considering a water content of 2 kg water/kg DM as the drying ending point, it took approximately 480 min for AD, 330 min for E + AD, 390 min for FA + AD, 360 min for CA + AD, 65 min for AD-US, 55 min for E + AD-US, 55 min for CA + AD-US, and 52 min for FA + AD-US to achieve this point.
Fig. 2.
Drying kinetic curves of blackberry under different treatments. The points are the experimentally determined data and the lines refer to the modeled results.
Besides, the temperature changes under drying are plotted in Supplementary Fig. 2. In the early stage of drying, the temperature inside the blackberry increased faster in the presence of sonication. The highest temperatures for all the treatments were close to each other.
3.2. Mass transfer modeling
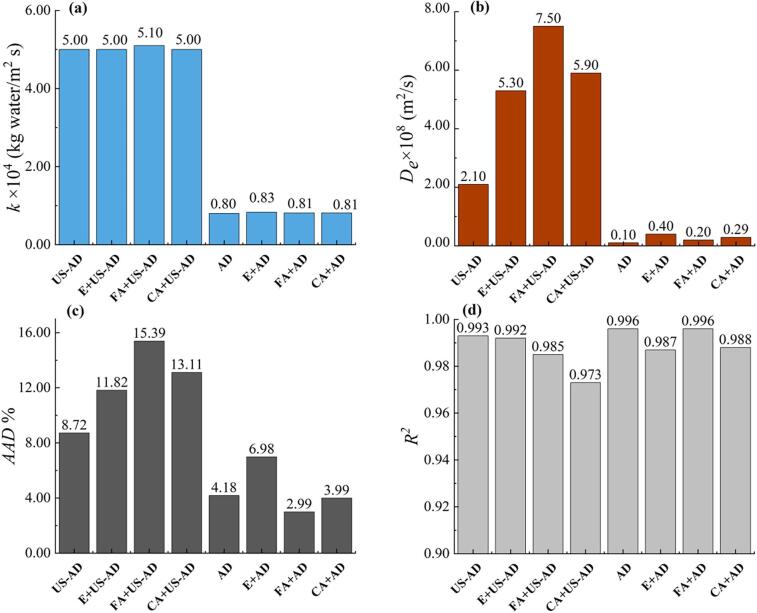
In Fig. 2, the predicted curves about the decline of water content in the blackberry match well with the experimental data. The statistical indices including AAD% and R2 also imply that the simulated results are accurate (Fig. 3).
Fig. 3.
External mass transfer coefficient (a), water effective diffusion coefficient (b) of blackberry during drying, and AAD% (c) and R2 (d) values for the assessment of the modeling quality.
Following the diffusion modeling, the mass transfer parameters including De and k under different drying treatments are plotted in Fig. 3. Both De and k values under the applied drying conditions stayed within the common ranges for convective drying of many fruits and vegetables (10-10-10-7 m2/s for De, 10-5-10-3 kg water/m2 s for k) [20], [34], [35].
Among all the treatments, k and De values under ultrasound-assisted air drying were approximately one order of magnitude higher than air drying without sonication, agreeing with the drying kinetic results. Many studies also report that ultrasound can reduce the internal and external mass transfer resistances to promote drying [36], [37], [38]. On the other hand, ethanol spraying pretreatments did not significantly affect the water exchange on the blackberry surface, since the k values for the samples with and without ethanol pretreatment were very close in both cases of drying with and without contact sonication.
In both groups of ultrasound-incorporated drying and air drying alone, the De values for drying coupled with ethanol spraying pretreatments were higher than without any pretreatments, demonstrating that the spraying of pure ethanol solution or phenolic acid in ethanol solvent facilitates the movement of water molecules inside blackberry [32]. For instance, the De value for E + AD (0.4 × 10-8 m2/s) was 3.0-fold greater than that for AD (0.1 × 10-8 m2/s), and the De value for FA + AD-US (7.5 × 10-8 m2/s) was 257% higher than AD-US (2.1 × 10-8 m2/s).
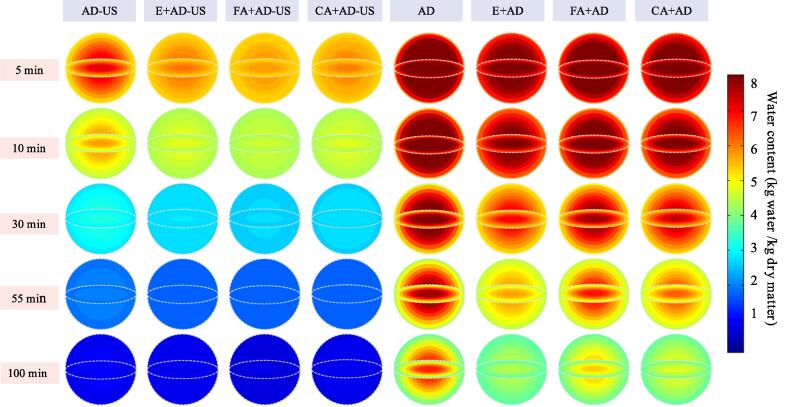
3.2.1. Moisture distribution within blackberry under different drying treatments
The data matrix from numerical simulation was coded to visualize the moisture distribution within the blackberry pellet at various drying stages (Fig. 4). As can be seen, the water content gradually decreased from the center of sphere to the outer surface. This is a common phenomenon, since the outer surface surrounded by hot air always gets dried first, and then the concentration gradient drives water to move from the interior to the surface [39]. Meanwhile, the interior water concentration gradient was attenuated with drying, implying that the forces driving the water movement got weakened with the loss of water. As a result, the drying rate usually decreases gradually and the falling rate period is dominant throughout the drying process. At the same drying stage, the interior water distributions of ethanol-pretreated samples were similar to the counterparts without ethanol pretreatment. However, the internal water contents in ultrasound-treated samples were obviously lower than in non-ultrasound processed samples. This result further proves that ultrasound treatment can speed up the drying process and significantly shorten drying time. Furthermore, the water distribution in ultrasound-treated samples tended to be uniform faster than that in samples dried by air drying alone.
Fig. 4.
Spatial distribution of water content within blackberry along with the radial direction at different drying stages.
3.3. Qualitative evaluation of the co-pigmentation between anthocyanins and phenolic acids after pretreatments
The absorbance spectra of the blackberry soluble phenolic extract after phenolic acid pretreatments in the visible range (400–700 nm) are plotted in Fig. 5. In all the spectra, a peak always exists in the range of 500–600 nm, representing the presence of anthocyanins [29]. The λmax for samples without pretreatment, pretreated by ethanol alone, pretreated by ferulic acid in ethanol, and pretreated by caffeic acid in ethanol, are located in 530, 531, 534 and 535 nm, respectively. The phenolic acid pretreatment shifted the maximum visible absorption of anthocyanins to longer wavelengths, referred to as the bathochromic effect [40]. Similarly, Zhao et al. reported that the maximum absorption wavelength of cyanidin-3-O-glucoside in red wine was shifted from 513 nm to 517 nm in the presence of gallic acid, owing to the co-pigmentation of anthocyanins with phenolic acid [41]. Thus, it can be speculated that the co-pigmentation reaction might have taken place between blackberry anthocyanins and exogenous phenolic acids after the applied pretreatments.
Fig. 5.
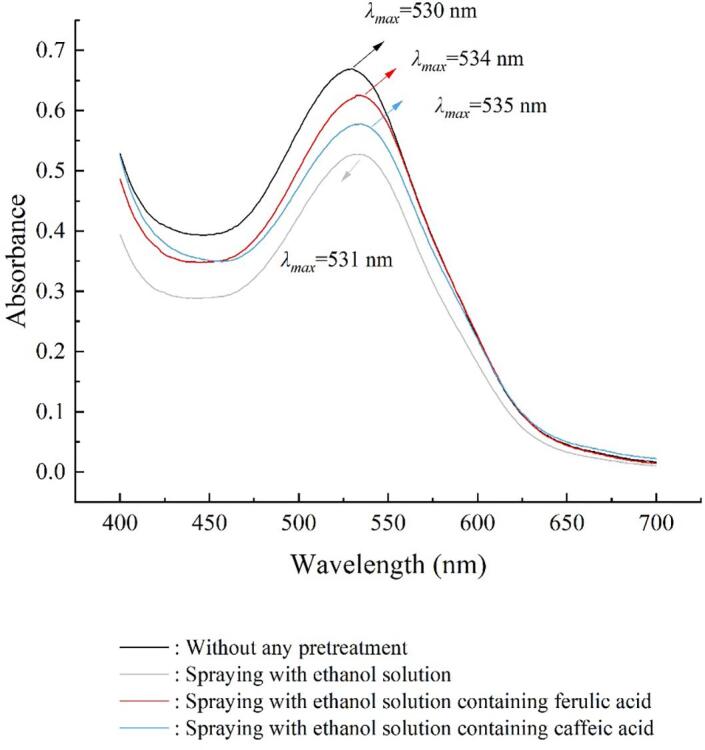
Visible absorbance spectra of the soluble phenolic extract from blackberry after pretreatments.
On the other hand, the intensity of maximum absorption peaks attenuated for samples receiving the pretreatments, reflecting a decline in the anthocyanin content after the pretreatments. The influences of spraying ethanol containing phenolic acids on blackberry anthocyanin have been discussed in the following section.
3.4. Phenolic profile in soluble phenolic portion
3.4.1. Anthocyanins
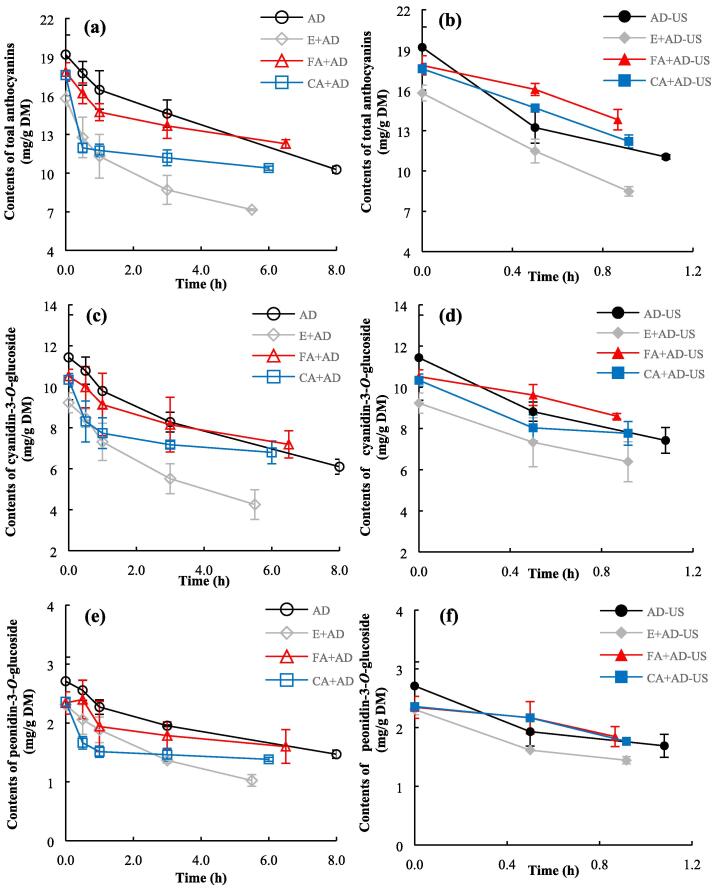
Anthocyanins are mainly located in the vacuole, belonging to the portion of soluble phenolics [42]. The variations in the contents of total anthocyanins and individual anthocyanins in blackberry pellets under drying are plotted in Fig. 6. Based on Fig. 6a and b, there is a slight loss of anthocyanins (7.0–17.7%) after subjecting to the three ethanol spraying pretreatments, owing to the dissolution of anthocyanins in ethanol and the washing effect of ethanol [43], [44]. Total anthocyanin contents under all the dehydration treatments were decreased with air drying, as anthocyanins are heat-sensitive [45], [46]. Meanwhile, the sprayings of ethanol solutions containing ferulic acid or caffeic acid, simultaneous sonication with drying, and their combinations all alleviated the loss of anthocyanins compared to direct drying without any pretreatments and sonication. At the end of drying, the retentions of anthocyanins in the samples dried by E + AD, E + AD-US, AD, CA + AD, AD-US, CA + AD-US, FA + AD, FA + AD-US were 37.3%, 44.2%, 53.5%, 54.1%, 57.5%, 63.5%, 64.0%, and 72.0%, respectively. Generally, in the ultrasound-treated samples, high retention of anthocyanins has been noted as the samples are exposed to hot air for a short time only [2], [46], while the protective effects of both ferulic acid and caffeic acid on anthocyanins can be attributed to the co-pigmentation reaction between exogenous phenolic acid molecules and anthocyanins, causing a hyperchromic effect and subsequently enhance anthocyanin stability [9], [47], [48]. Moreover, ferulic acid pretreatment is more effective than caffeic acid pretreatment to preserve blackberry anthocyanins during drying, since the total anthocyanin content in only air dried samples with ferulic acid pretreatment (12.28 ± 0.32 mg/g DM) was significantly (p < 0.05) higher than only air dried samples with caffeic acid pretreatment (10.39 ± 0.15 mg/g DM). Besides, the combination of phenolic acid spraying and contact sonication provided a stronger protective effect on anthocyanins than individual treatments, implying a synergistic effect between phenolic acid co-pigmentation pretreatment and contact sonication under drying to protect anthocyanins. In the literature, the co-pigmentation treatments using phenolic acids as reagents have been widely proved as an effective method to protect anthocyanins in liquids, such as berry wines and juices [8], [9]. Our current results also demonstrate that the co-pigmentation pretreatment can protect anthocyanin pigments during air drying of berry fruits.
Fig. 6.
Changes in the contents of total anthocyanins and individual anthocyanins during drying. (a): total anthocyanins under air drying alone; (b): total anthocyanins under ultrasound-assisted air drying; (c): cyanidin-3-O-glucoside under air drying alone; (d): cyanidin-3-O-glucoside under ultrasound-assisted air drying; (e): peonidin-3-O-glucoside under air drying alone; (f): peonidin-3-O-glucoside under ultrasound-assisted air drying.
The kinetic data about total anthocyanin degradation exhibited in Fig. 6 were well fitted to Eq. (7)with satisfactory accuracy (Supplementary Table 3). The parameter k reflects the degradation rate constant of anthocyanins under each dehydration treatment [49]. It can be seen that the k values under ultrasound-incorporated drying were always higher than under air drying alone, indicating that the degradation of blackberry anthocyanins got intensified under ultrasound field. Similarly, Tiwari et al. reported that the concentration of cyanidin-3-O-glucoside in grape juice treated with ultrasound at an amplitude of 61 μm (11.83 mg/100 mL) was significantly lower than without ultrasonic treatment (13.39 mg/100 mL) [50]. However, since the drying time was markedly shortened by contact ultrasound, the anthocyanin retention after ultrasound-assisted drying was still higher than at the end of air drying alone. On the other hand, among all the ultrasound treatments, k values for coupled phenolic acid co-pigmentation pretreatments and contact sonication (0.323 for FA + AD-US, 0.406 for CA + AD-US) were lower than drying without any pretreatments (0.535 for AD-US). Also, under air drying in the absence of sonication, k values for AD were even lower than FA + AD and CA + AD. Thus, ultrasound and the selected phenolic acid co-pigmentation pretreatments demonstrate synergy to protect blackberry anthocyanins again.
Apart from total anthocyanins, the changes in the two individual anthocyanins in blackberry pellets under drying are plotted in Fig. 6c-f. According to the mass spectrometry results (Supplementary Table 1), cyanidin-3-O-glucoside and peonidin-3-O-glucoside are two major anthocyanins in the studied blackberry variety. Fresh blackberries were rich in cyanidin-3-O-glucoside (11.44 ± 2.07 mg/g DM), accounting for about 60% of total anthocyanin content. Under exposure to hot air, the patterns for the changes in the contents of both cyanidin-3-O-glucoside and peonidin-3-O-glucoside in blackberry pellets are similar to the total anthocyanin content. Both phenolic acid co-pigmentation pretreatments and contact sonication enhanced the retentions of cyanidin-3-O-glucoside and peonidin-3-O-glucoside after drying. Moreover, the loss of peonidin-3-O-glucoside is comparable to cyanidin-3-O-glucoside under each dehydration treatment. Taking contact ultrasound-intensified air drying without pretreatment for example, the magnitudes for the losses of peonidin-3-O-glucoside and cyanidin-3-O-glucoside at the end of drying remains in the range between 35.1% and 37.6% (from 2.71 ± 0.41 mg/g DM to 1.69 ± 0.19 mg/g DM for peonidin-3-O-glucoside, from 11.44 ± 2.07 mg/g DM to 7.42 ± 0.63 mg/g DM for cyanidin-3-O-glucoside).
3.4.2. Non-anthocyaninic phenolics
Besides anthocyanins, another 4 flavonoids (catechin, rutin, myricetin and quercitrin) and 4 phenolic acids (p-hydroxybenzoic acid, phloretic acid, p-coumaric acid and ferulic acid) have been identified in the portion of soluble phenolic extract from blackberry following the HPLC-QTOF-MS/MS results (Supplementary Table 1). In the fresh blackberry sample, the amounts of soluble catechin (22.24 ± 3.63 mg/g DM) and phloretic acid (6.29 ± 1.12 mg/g DM) were significantly higher than other soluble phenolic compounds. The amounts of other soluble phenolics in fresh blackberry are in the range from 0.02 ± 0.00 to 0.92 ± 0.09 mg/g DM. This result is consistent with the studies of Schulz, who reported that flavonoids are the main phenolics of blackberry [51].
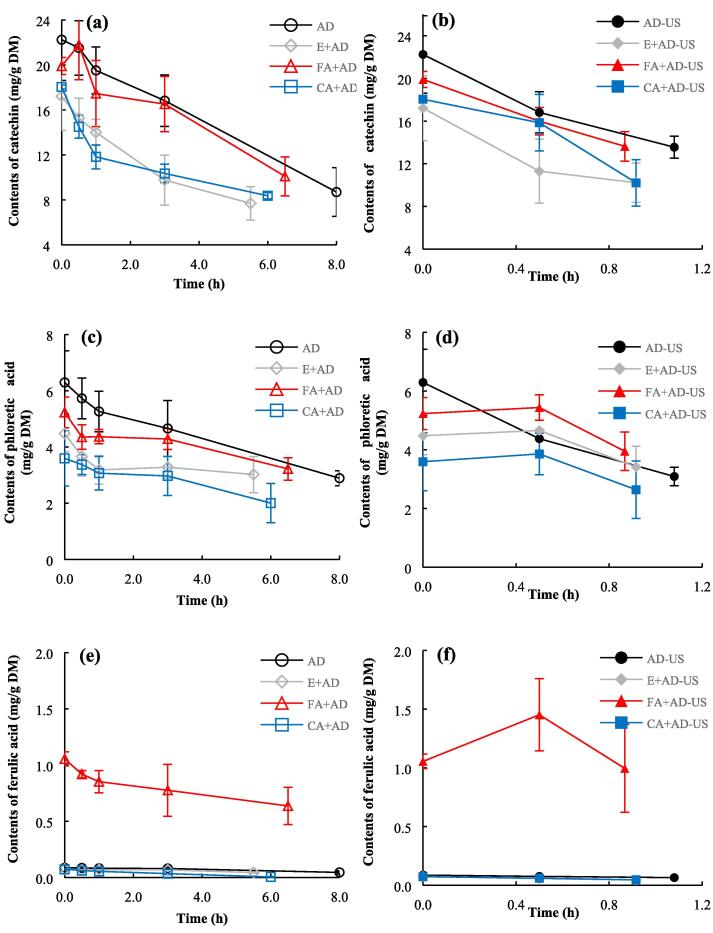
The changes in the contents of soluble catechin, phloretic acid and ferulic acid during drying are plotted in Fig. 7. Meanwhile, a heatmap coupled with Hierarchical Clustering analysis is also generated to visualize the variations arising from other soluble phenolics at different drying periods (Fig. 8). The original data in Fig. 7 and Fig. 8 are summarized in Supplementary Table 4. It must be explained that catechin and phloretic acid were excluded from the heatmap, since their inclusions can visually attenuate the influences of drying treatments on other soluble phenolics. Meanwhile, caffeic acid and ferulic acid contents were also analyzed individually, since exogenous caffeic acid and ferulic acid were added before drying. Similar to anthocyanins mentioned above, the spraying pretreatment of ethanol solution containing phenolic acid led to the losses of catechin and phloretic acid due to the liquid washing effect (Fig. 7a-d). For example, the catechin contents decreased from 22.24 ± 3.63 mg/g DM to 17.21 ± 3.03, 19.90 ± 0.75, and 18.04 ± 0.18 mg/g DM after the spraying pretreatments of ethanol alone, ethanol containing ferulic acid, and ethanol containing caffeic acid, respectively. Also, the spraying of exogenous caffeic acid or ferulic acid markedly increased their contents in blackberry pellets. The amounts of ferulic acid and caffeic acid were enhanced from 0.09 ± 0.02 to 1.06 ± 0.06 mg/g DM and from 0 to 1.95 ± 0.33 mg/g DM, respectively. Generally, the catechin content exhibited a continuous declining trend during the whole air-drying process with and without sonication. The loss of catechin could be attributed to the enzymatic degradation with the participation of peroxidase and polyphenol oxidase, non-enzymatic degradation, as well as both [16], [52]. At the end of drying, the catechin contents in the samples dried by E + AD, CA + AD, AD, FA + AD, CA + AD-US, E + AD-US, AD-US and FA + AD-US were 7.69 ± 1.48, 8.37 ± 0.29, 8.70 ± 2.14, 10.10 ± 1.73, 10.22 ± 2.18, 10.23 ± 1.84, 13.55 ± 1.03 and 13.63 ± 1.38 mg/g DM, respectively. Thus, it can be concluded that contact sonication alleviated the loss of catechin due to the shortened exposure to hot air [2], [53], [54]. In contrast, phenolic acid co-pigmentation pretreatment did not provide any protective effect on catechin.
Fig. 7.
Changes in the contents of soluble phenolics during drying. (a): catechin under air drying alone; (b): catechin under ultrasound-assisted air drying; (c): phloretic acid during under air drying alone; (d): phloretic acid under ultrasound-assisted air drying; (e): ferulic acid under air drying alone; (f): ferulic acid under ultrasound-assisted air drying.
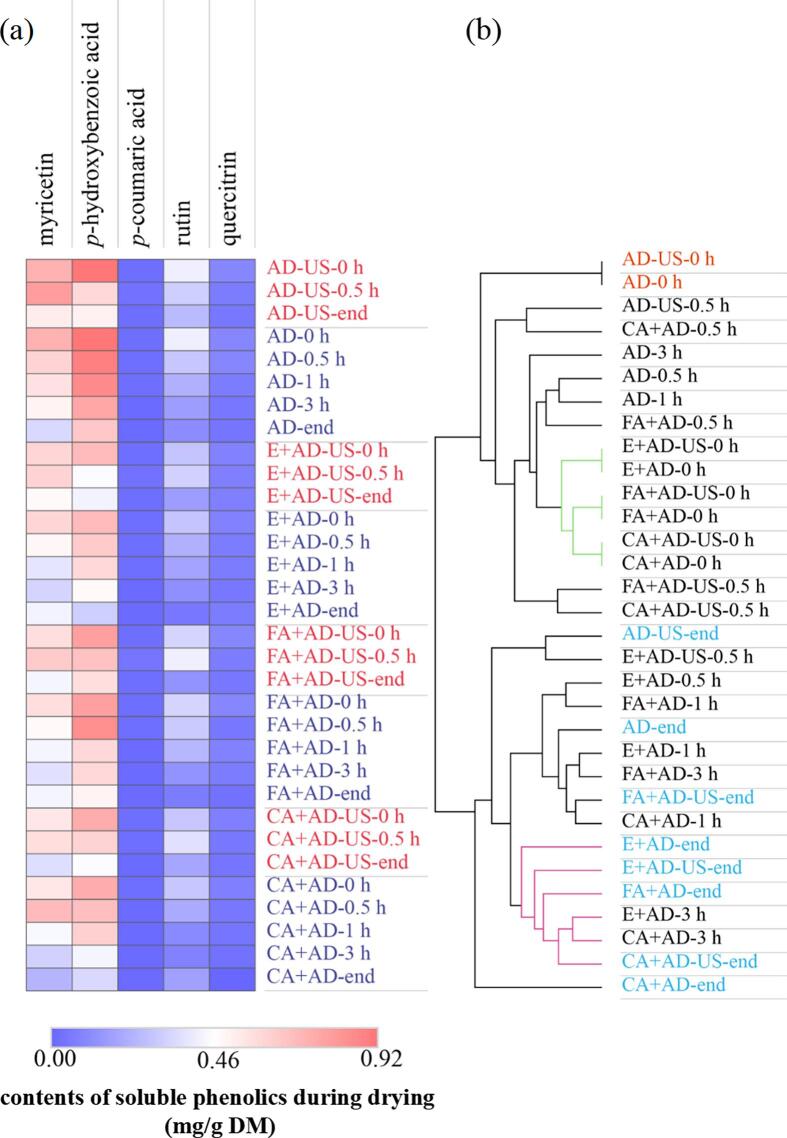
Fig. 8.
Heatmap (a) and cluster analysis (b) of soluble phenolic components (excluding anthocyanins) in blackberry during drying. The time followed by the treatment means the sampling time.
Regarding phloretic acid, their contents in non-ultrasound treated samples declined gradually throughout the drying process (Fig. 7c and d). At the same time, the contents of phloretic acid in blackberries which received both the applied pretreatments and sonication were stable in the first 0.5 h of drying, followed by a decreasing trend. No significant positive effects of ultrasound and phenolic acid co-pigmentation pretreatment on soluble phloretic acid were found, since the differences in phloretic acid content among all the samples after drying were negligible. Furthermore, the losses of soluble ferulic acid were observed in all the dehydration treatments (Fig. 7e and f). However, the soluble ferulic acid content in the samples pretreated with exogenous ferulic acid spraying increased significantly from 1.06 ± 0.06 to 1.45 ± 0.31 mg/g DM within the first 0.5 h of contact ultrasound-intensified drying. Several researchers have also reported increasing soluble phenolic components in fruits and vegetables under air drying [15], [55]. Contact ultrasound-intensified drying may benefit the release of bound phenolics from the cell matrix [2], [15]. The increase in phenolic acid content may also be due to synthesis reactions and the transformation (degradation) of anthocyanins to phenolic acids during the drying process [56].
Regarding other minor soluble phenolic components, losses of rutin, myricetin, quercitrin, and p-hydroxybenzoic acid were noted at the end of all the dehydration treatments. In contrast, the changes of p-coumaric acid contents were insignificant (Fig. 8a). Myricetin and rutin exhibited an increase at first and subsequently a decline during ultrasound-enhanced air drying periods. The plot from cluster analysis also verified the losses of soluble phenolics during drying, since the distance between fresh and dried samples generally increased with drying time (Fig. 8b). According to the distance between samples before and after drying, it can be found that the amounts of indicated soluble phenolics in the AD-US processed samples were higher compared to other samples at the end of drying (Fig. 8b).
Considering the amounts of all the detected soluble phenolics (excluding anthocyanins and ferulic acid), the sum of phenolic contents in FA + AD-US, AD-US, E + AD-US, FA + AD, CA + AD-US, AD, E + AD and CA + AD treated samples were 19.78, 18.00, 14.79, 14.36, 13.91, 12.74, 11.56 and 11.12 mg/g DM. These results further confirm that contact ultrasound could enhance the retention of the indicated soluble phenolic components in the blackberry pellets during air drying. Also, the spraying of the phenolic acid solution provided a moderate positive effect on non-anthocyanin soluble phenolics.
3.5. Phenolic profile in bound phenolic portion
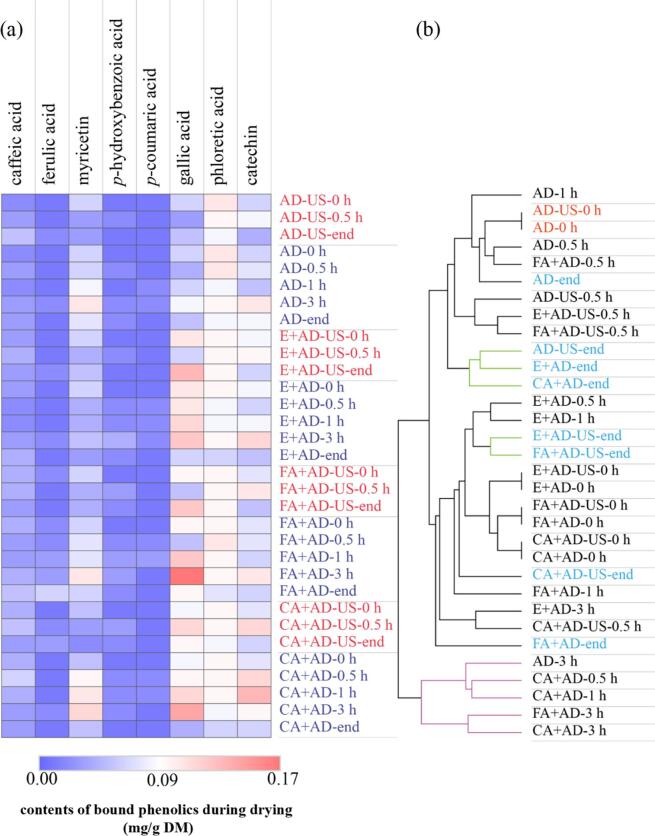
Theoretically, the interactions between phenolics and blackberry cell walls can affect the stability of phenolics during drying [57]. Thus, the bound phenolics in blackberry at different drying stages were removed from the cell walls and analyzed quantitatively. Based on Supplementary Table 2, 8 bound phenolic compounds were identified by HPLC-QTOF-MS/MS, including six phenolic acids (caffeic acid, phloretic acid, gallic acid, p-hydroxybenzoic acid, p-coumaric acid and ferulic acid) and two flavonoids (catechin and myricetin). The original data in Fig. 9 are prepared in Supplementary Table 5. Among them, the content of phloretic acid was the highest (0.10 ± 0.01 mg/g DM), followed by catechin (0.06 ± 0.01 mg/g DM), myricetin (0.06 ± 0.02 mg/g DM), gallic acid (0.06 ± 0.01 mg/g DM) and caffeic acid (0.02 ± 0.01 mg/g DM). Overall, blackberry possessed higher amounts of soluble phenolics than insoluble phenolics.
Fig. 9.
Heatmap (a) and cluster analysis (b) of bound phenolic components in blackberry during drying. The time followed by the treatment means the sampling time.
The evolution of bound phenolics during drying is plotted in Fig. 9a. No obvious declines in the amounts of bound phenolics after phenolic acid co-pigmentation pretreatment could be observed. During all the dehydration treatments, the changes in the contents of bound p-hydroxybenzoic acid, p-coumaric acid are negligible. At the end of drying, the amounts of phloretic acid in the samples dried by E + AD, FA + AD and CA + AD, gallic acid in the samples dried by CA + AD and E + AD and myricetin in the samples dried by E + AD and CA + AD-US were significantly lower than the counterparts in the samples before drying. For example, CA + AD-US treatment resulted in decreased myricetin from 0.05 ± 0.01 to 0.02 ± 0.01 mg/g DM and E + AD treatment decreased phloretic acid from 0.09 ± 0.00 to 0.06 ± 0.01 mg/g DM. The loss of these bound phenolics could be attributed to their transformations to soluble phenolics by the disruption of covalent bond between bound phenolics and cell walls during drying [58]. Besides, the contents of caffeic acid in CA + AD-US processed sample, gallic acid in CA + AD-US processed sample, and catechin in AD-US, FA + AD, CA + AD-US and CA + AD processed samples were even increased at the beginning of drying. After drying, the content of ferulic acid in FA + AD processed samples was 200% higher than before drying. An increment of the bound phenolics in blackberry pellets might be due to the experimental error, soluble phenolics being converted to bound phenolics by condensation reactions [58] and other unknown reasons. Due to the lack of literature, more studies are needed to explore the changes of the bound phenolics during the dehydration of various fruits.
At the end of drying, the sums of all the bound phenolics in the samples dried by FA + AD (0.439 mg/g DM), E + AD-US (0.387 mg/g DM) and FA + AD-US (0.373 mg/g DM) were higher than the total amount of bound phenolics in fresh blackberry (0.342 mg/g DM). Instead, the samples dried by AD (0.335 mg/g DM), CA + AD-US (0.330 mg/g DM), AD-US (0.292 mg/g DM), E + AD (0.288 mg/g DM) and CA + AD (0.278 mg/g DM) possessed less amounts of total bound phenolics than fresh blackberry. In Fig. 9b, the dried samples treated by AD, AD-US, E + AD and CA-AD were clustered, implying that the composition of bound phenolics in these samples is similar and can be differentiated from other samples after drying. The sample dried by FA + AD is located underneath all the dried blackberry pellets, confirming that this sample contains a higher amount of bound phenolics than other samples. However, no obvious effect of phenolic acid co-pigmentation pretreatment and contact sonication on the bound phenolic profile can be observed.
3.6. Correlation analysis between drying parameters and blackberry phenolic composition
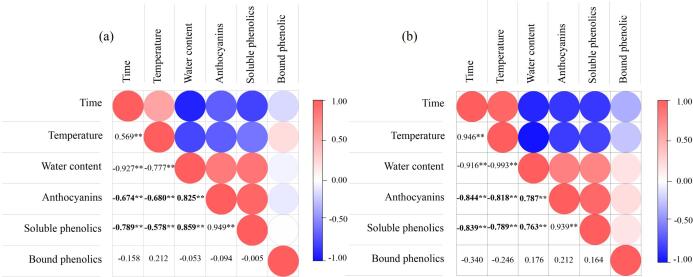
The above-exhibited results indicate that the stability of phenolics in blackberry varies with the applied drying methods. The dehydration treatments, especially contact sonication, also affected the water content and temperature inside blackberry profoundly. Since the retention of phenolics in blackberry is dependent on the environment (water content and temperature) and drying time, a correlation analysis was performed in Fig. 10 to explore the relationship between the processing variables (water, temperature and time) and phenolic variables (contents of total anthocyanins, total soluble phenolics excluding anthocyanins, and total bound phenolics). It can be found under drying both with and without contact sonication, the time and temperature are negatively correlated with the amounts of soluble phenolics with significance (p < 0.01), and the water content is positively correlated with soluble phenolics. These results confirmed that the soluble phenolics in blackberry could degrade with the increased drying time and temperature, and the environment with high water activity can also facilitate the degradation reactions. The absolute correlation coefficient values for soluble phenolics-temperature (0.789) and soluble phenolics-time (0.839) under drying with sonication were higher than the values for soluble phenolics-temperature (0.578) and soluble phenolics-time under air drying alone (0.789). Instead, the correlation coefficients between water content and soluble phenolics under sonication (0.787 for anthocyanins-water, 0.763 for soluble phenolics-water) were lower than the counterparts in the absence of sonication (0.825 for anthocyanins-water, 0.859 for soluble phenolics-water). Based on these data and high retention of soluble phenolics under sonication, it can be deduced that contact sonication mainly inhibits the loss of soluble phenolics in blackberry through speeding up the loss of water instead of shortening the time exposed to hot air. Besides, the fast loss of water under sonication can weaken the destructive effect of temperature increase on the stability of phenolics.
Fig. 10.
Correlation analysis between drying processing variables and blackberry phenolic composition. (a): Data from air drying without sonication; (b): Data from contact ultrasound-assisted air drying. Time: drying time; Temperature: internal temperature of blackberry pellets; Water content: water content of blackberry pellets during drying; Anthocyanins: total anthocyanin content; Soluble phenolics: sum of contents of non-anthocyaninic soluble phenolics; Bound phenolics: sum of contents of bound phenolics. **: significant correlation (p < 0.01).
On the other hand, the correlation relationships between bound phenolics and the processing variables were all insignificant, indicating that drying with and without sonication exhibited an insignificant influence on the stability of bound phenolics.
4. Conclusion
This study demonstrates that the combination of phenolic acid co-pigmentation pretreatment and ultrasonication during drying effectively shortens the drying time of blackberry and enhances the retention of anthocyanins in dehydrated blackberry. From the mass transfer perspective, ultrasound can exert positive influences on De and k during drying, while ethanol pretreatment containing phenolic acids merely led to the increment of De. Co-pigmentation reactions between blackberry anthocyanins and exogenous phenolic acids occurred after the applied pretreatment, thus providing an extra protective effect on blackberry anthocyanins under exposure to hot air. The obtained results verify that the phenolic acid co-pigmentation pretreatment can be utilized to preserve anthocyanins during the processing of solid foods. On the other hand, this study is also concerned with the evolutions of soluble and bound phenolics during novel drying of blackberry. Sonication alleviated the loss of soluble phenolics under air drying, whereas phenolic acid co-pigmentation pretreatment had no significant effect on non-anthocyaninic soluble phenolics during drying. All the dehydration treatments exerted weak influence on the bound phenolics during drying. The contents of some soluble and bound phenolics changed irregularly throughout the whole drying process or in a certain drying period. The cell wall is an important matrix to separate soluble and bound phenolics. The plant cell walls can combine phenolics through covalent and non-covalent bonds, affecting phenolic stability. Thus, the interactions between phenolics and plant cell walls at different drying stages is an interesting topic and will be the focus of our next study.
CRediT authorship contribution statement
Wenjin Gong: Investigation, Methodology, Validation, Writing – original draft, Formal analysis. Dandan Li: Investigation, Methodology, Writing – review & editing. Yue Wu: Writing – review & editing. Sivakumar Manickam: Writing – review & editing. Xun Sun: Writing – review & editing. Yongbin Han: Conceptualization, Supervision, Writing – review & editing. Yang Tao: Conceptualization, Investigation, Validation, Data curation, Supervision, Funding acquisition, Writing – review & editing. Xiaoli Liu: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was sponsored by National Natural Science Foundation of China (No. 32072351 and 31701616), Jiangsu Agricultural Science and Technology Innovation Fund (No. CX (18)2017), and Priority Academic Program Development of Jiangsu Higher Education Institutions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105788.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References:
- 1.Kaume L., Howard L.R., Devareddy L. The blackberry fruit: A review on its composition and chemistry, metabolism and bioavailability, and health benefits. J. Agr. Food Chem. 2012;60:5716–5727. doi: 10.1021/jf203318p. [DOI] [PubMed] [Google Scholar]
- 2.Tao Y., Li D.D., Chai W.S., Show P.L., Yang X.H., Manickam S., Xie G.J., Han Y.B. Comparison between airborne ultrasound and contact ultrasound to intensify air drying of blackberry: Heat and mass transfer simulation, energy consumption and quality evaluation. Ultrason. Sonochem. 2021;72 doi: 10.1016/j.ultsonch.2020.105410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruz L., Clemente G., Mulet A., Ahmad-Qasem M.H., Barrajon-Catalan E., Garcia-Perez J. Air-borne ultrasonic application in the drying of grape skin: Kinetic and quality considerations. J. Food Eng. 2016;168:251–258. [Google Scholar]
- 4.Tao Y., Zhang J.L., Jiang S.R., Xu Y.Q., Show P.L., Han Y.B., Ye X.S., Ye M.R. Contacting ultrasound enhanced hot-air convective drying of garlic slices: Mass transfer modeling and quality evaluation. J. Food Eng. 2018;235:79–88. [Google Scholar]
- 5.Wu B.G., Guo X.Y., Guo Y.T., Ma H.L., Zhou C.S. Enhancing jackfruit infrared drying by combining ultrasound treatments: Effect on drying characteristics, quality properties and microstructure. Food Chem. 2021;358 doi: 10.1016/j.foodchem.2021.129845. [DOI] [PubMed] [Google Scholar]
- 6.Sari P., Wijaya C.H., Sajuthi D., Supratman U. Colour properties, stability, and free radical scavenging activity of jambolan (Syzygium cumini) fruit anthocyanins in a beverage model system: Natural and copigmented anthocyanins. Food Chem. 2012;132(4):1908–1914. [Google Scholar]
- 7.Castañeda-Ovando A., Pacheco-Hernández M.d.L., Páez-Hernández M.E., Rodríguez J.A., Galán-Vidal C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009;113(4):859–871. [Google Scholar]
- 8.Fan L.L., Wang Y., Xie P.J., Zhang L.X., Li Y.H., Zhou J.Z. Copigmentation effects of phenolics on color enhancement and stability of blackberry wine residue anthocyanins: Chromaticity, kinetics and structural simulation. Food Chem. 2019;275:299–308. doi: 10.1016/j.foodchem.2018.09.103. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L., Wang W., Yue X., Wu G., Yue P., Gao X. Gallic acid as a copigment enhance anthocyanin stabilities and color characteristics in blueberry juice. J. Food Sci. Technol. 2020;57:1405–1414. doi: 10.1007/s13197-019-04175-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markovic J.M.D., Petranovic N.A., Baranac J.M. A spectrophotometric study of the copigmentation of malvin with caffeic and ferulic acids. J. Agr. Food Chem. 2000;48:5530–5536. doi: 10.1021/jf000038v. [DOI] [PubMed] [Google Scholar]
- 11.Shahidi F., Yeo J. Insoluble-bound phenolics in food. Molecules. 2016;21:1091216. doi: 10.3390/molecules21091216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang B., Zhang Y.J., Li H.Y., Deng Z.Y., Tsao R. A review on insoluble-bound phenolics in plant-based food matrix and their contribution to human health with future perspectives. Trends Food Sci. Tech. 2020;105:347–362. [Google Scholar]
- 13.Bourvellec C.L., Priscilla B., Lepercq P., Comtet-Marre S., Auffret P., Ruiz P., Bott R., Renard C., Dufour C., Chatel J.M. Procyanidin—cell wall interactions within apple matrices decrease the metabolization of procyanidins by the human gut microbiota and the anti-inflammatory effect of the resulting microbial metabolome in vitro. Nutrients. 2019;11:664. doi: 10.3390/nu11030664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birtic S., Regis S., Le Bourvellec C., Renard C. Impact of air-drying on polyphenol extractability from apple pomace. Food Chem. 2019;296:142–149. doi: 10.1016/j.foodchem.2019.05.131. [DOI] [PubMed] [Google Scholar]
- 15.Tan S., Ke Z.L., Chai D., Miao Y.W., Luo K., Li W.F. Lycopene, polyphenols and antioxidant activities of three characteristic tomato cultivars subjected to two drying methods. Food Chem. 2021;338 doi: 10.1016/j.foodchem.2020.128062. [DOI] [PubMed] [Google Scholar]
- 16.Nunes J.C., Lago M.G., Castelo-Branco V.N., Oliveira F.R., Torres A.G., Perrone D., Monteiro M. Effect of drying method on volatile compounds, phenolic profile and antioxidant capacity of guava powders. Food Chem. 2016;197:881–890. doi: 10.1016/j.foodchem.2015.11.050. [DOI] [PubMed] [Google Scholar]
- 17.D. Ferreira, S. Guyot, N. Marnet, I. Delgadillo, C.M.G.C. Renard, M.A. Coimbra, Composition of phenolic compounds in a Portuguese pear (Pyrus communis L. var. S. Bartolomeu) and changes after sun-drying, J. Agr. Food Chem., 50 (2002) 4537-4544. [DOI] [PubMed]
- 18.Liu X., Le Bourvellec C., Renard C.M.G.C. Interactions between cell wall polysaccharides and polyphenols: Effect of molecular internal structure. Compr. Rev. Food Sci. F. 2020;19(6):3574–3617. doi: 10.1111/1541-4337.12632. [DOI] [PubMed] [Google Scholar]
- 19.Tao Y., Han M.F., Gao X.G., Han Y.B., Show P.L., Liu C.Q., Ye X.S., Xie G.J. Applications of water blanching, surface contacting ultrasound-assisted air drying, and their combination for dehydration of white cabbage: Drying mechanism, bioactive profile, color and rehydration property. Ultrason. Sonochem. 2019;53:192–201. doi: 10.1016/j.ultsonch.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Magalhaes M.L., Cartaxo S.J.M., Gallao M.I., Garcia-Perez J.V., Carcel J.A., Rodrigues S., Fernandes F.A.N. Drying intensification combining ultrasound pre-treatment and ultrasound-assisted air drying. J. Food Eng. 2017;215:72–77. [Google Scholar]
- 21.Dinani S.T., Hamdami N., Shahedi M., Havet M. Mathematical modeling of hot air/electrohydrodynamic (EHD) drying kinetics of mushroom slices, Energ. Convers. Manage. 2014;86:70–80. [Google Scholar]
- 22.Kowalski S.J., Rybicki A. Ultrasound in wet biological materials subjected to drying. J. Food Eng. 2017;212:271–282. [Google Scholar]
- 23.Castro A.M., Mayorga E.Y., Moreno F.L. Mathematical modelling of convective drying of feijoa (Acca sellowiana Berg) slices. J. Food Eng. 2019;252:44–52. [Google Scholar]
- 24.Tao Y., Wang Y.L., Pan M.S., Zhong S.R., Wu Y., Yang R.Q., Han Y.B., Zhou J.Z. Combined ANFIS and numerical methods to simulate ultrasoundassisted extraction of phenolics from chokeberry cultivated in China and analysis of phenolic composition. Sep. Purif. Technol. 2017;178:178–188. [Google Scholar]
- 25.Tang Y., Zhang B., Li X.H., Chen P.X., Zhang H., Liu R.H., Tsao R. Bound phenolics of quinoa seeds released by acid, alkaline, and enzymatic treatments and their antioxidant and α-glucosidase and pancreatic lipase inhibitory effects. J. Agr. Food Chem. 2016;64:1712–1719. doi: 10.1021/acs.jafc.5b05761. [DOI] [PubMed] [Google Scholar]
- 26.Ivanova V., Dornyei A., Mark L., Vojnoski B., Stafilov T., Stefova M., Kilar F. Polyphenolic content of Vranec wines produced by different vinification conditions. Food Chem. 2011;124:316–325. [Google Scholar]
- 27.Cui C., Zhang S.M., You L.J., Ren J.Y., Luo W., Chen W.F., Zhao M.M. Antioxidant capacity of anthocyanins from Rhodomyrtus tomentosa (Ait.) and identification of the major anthocyanins. Food Chem. 2013;139:1–8. doi: 10.1016/j.foodchem.2013.01.107. [DOI] [PubMed] [Google Scholar]
- 28.Li S., Tao Y., Li D., Wen G., Zhou J., Manickam S., Han Y., Chai W.S. Fermentation of blueberry juices using autochthonous lactic acid bacteria isolated from fruit environment: Fermentation characteristics and evolution of phenolic profiles. Chemosphere. 2021;276 doi: 10.1016/j.chemosphere.2021.130090. [DOI] [PubMed] [Google Scholar]
- 29.Tao Y., Sun D.-W., Górecki A., Błaszczak W., Lamparski G., Amarowicz R., Fornal J., Jeliński T. Effects of high hydrostatic pressure processing on the physicochemical and sensorial properties of a red wine. Innov. Food Sci. Emerg. 2012;16:409–416. doi: 10.1016/j.foodchem.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 30.Schossler K., Jager H., Knorr D. Novel contact ultrasound system for the accelerated freeze-drying of vegetables. Innov. Food Sci. Emerg. 2012;16:113–120. [Google Scholar]
- 31.Taha A., Ahmed E., Ismaiel A., Ashokkumar M., Xu X., Pan S., Hu H. Ultrasonic emulsification: An overview on the preparation of different emulsifiers-stabilized emulsions. Trends Food Sci. Tech. 2020;105:363–377. [Google Scholar]
- 32.da Cunha R.M.C., Brandao S.C.R., de Medeiros R.A.B., Junior E.V.D., da Silva J.H.F., Azoubel P.M. Effect of ethanol pretreatment on melon convective drying. Food Chem. 2020;333 doi: 10.1016/j.foodchem.2020.127502. [DOI] [PubMed] [Google Scholar]
- 33.Rojas M.L., Augusto P.E.D. Ethanol pre-treatment improves vegetable drying and rehydration: Kinetics, mechanisms and impact on viscoelastic properties. J. Food Eng. 2018;233:17–27. [Google Scholar]
- 34.Gamboa-Santos J., Montilla A., Cárcel J.A., Villamiel M., Garcia-Perez J.V. Air-borne ultrasound application in the convective drying of strawberry. J. Food Eng. 2014;128:132–139. [Google Scholar]
- 35.Puig A., Perez-Munuera I., Carcel J.A., Hernando I., Garcia-Perez J.V. Moisture loss kinetics and microstructural changes in eggplant (Solanum melongena L.) during conventional and ultrasonically assisted convective drying. Food Bioprod. Process. 2012;90:624–632. [Google Scholar]
- 36.Fan K., Zhang M., Mujumdar A.S. Application of airborne ultrasound in the convective drying of fruits and vegetables: A review. Ultrason. Sonochem. 2017;39:47–57. doi: 10.1016/j.ultsonch.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Kiani H., Sun D.W., Zhang Z.H. The effect of ultrasound irradiation on the convective heat transfer rate during immersion cooling of a stationary sphere. Ultrason. Sonochem. 2012;19:1238–1245. doi: 10.1016/j.ultsonch.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Szadzinska J., Lechtanska J., Kowalski S.J., Stasiak M. The effect of high power airborne ultrasound and microwaves on convective drying effectiveness and quality of green pepper. Ultrason. Sonochem. 2017;34:531–539. doi: 10.1016/j.ultsonch.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 39.Tzempelikos D.A., Mitrakos D., Vouros A.P., Bardakas A.V., Filios A.E., Margaris D.P. Numerical modeling of heat and mass transfer during convective drying of cylindrical quince slices. J. Food Eng. 2015;156:10–21. [Google Scholar]
- 40.Sigurdson G.T., Robbins R.J., Collins T.M., Giusti M.M. Evaluating the role of metal ions in the bathochromic and hyperchromic responses of cyanidin derivatives in acidic and alkaline pH. Food Chem. 2016;208:26–34. doi: 10.1016/j.foodchem.2016.03.109. [DOI] [PubMed] [Google Scholar]
- 41.Zhao X., Ding B.W., Qin J.W., He F., Duan C.Q. Intermolecular copigmentation between five common 3-O-monoglucosidic anthocyanins and three phenolics in red wine model solutions: The influence of substituent pattern of anthocyanin B ring. Food Chem. 2020;326 doi: 10.1016/j.foodchem.2020.126960. [DOI] [PubMed] [Google Scholar]
- 42.Quatrin A., Pauletto R., Maurer L.H., Minuzzi N., Nichelle S.M., Carvalho J.F.C., Maróstica M.R., Rodrigues E., Bochi V.C., Emanuelli T. Characterization and quantification of tannins, flavonols, anthocyanins and matrix-bound polyphenols from jaboticaba fruit peel: A comparison between Myrciaria trunciflora and M. jaboticaba. J. Food Compos. Anal. 2019;78:59–74. [Google Scholar]
- 43.Zuleta-Correa A., Chinn M.S., Alfaro-Cordoba M., Truong V.D., Yencho G.C., Bruno-Barcena J.M. Use of unconventional mixed Acetone-Butanol-Ethanol solvents for anthocyanin extraction from Purple-Fleshed sweetpotatoes. Food Chem. 2020;314 doi: 10.1016/j.foodchem.2019.125959. [DOI] [PubMed] [Google Scholar]
- 44.Silva S., Costa E.M., Calhau C., Morais R.M., Pintado M.E. Anthocyanin extraction from plant tissues: A review. Crit. Rev. Food Sci. 2017;57(14):3072–3083. doi: 10.1080/10408398.2015.1087963. [DOI] [PubMed] [Google Scholar]
- 45.Mendez-Lagunas L., Rodriguez-Ramirez J., Cruz-Gracida M., Sandoval-Torres S., Barriada-Bernal G. Convective drying kinetics of strawberry (Fragaria ananassa): Effects on antioxidant activity, anthocyanins and total phenolic content. Food Chem. 2017;230:174–181. doi: 10.1016/j.foodchem.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 46.Charmongkolpradit S., Somboon T., Phatchana R., Sang-aroon W., Tanwanichkul B. Influence of drying temperature on anthocyanin and moisture contents in purple waxy corn kernel using a tunnel dryer. Case Stud. Therm. Eng. 2021;25 [Google Scholar]
- 47.Liu J.N., Zhuang Y.H., Hu Y.H., Xue S., Li H., Chen L., Fei P. Improving the color stability and antioxidation activity of blueberry anthocyanins by enzymatic acylation with p-coumaric acid and caffeic acid. LWT-Food Sci. Technol. 2020;130 [Google Scholar]
- 48.Zhao C.L., Yu Y.Q., Chen Z.J., Wen G.S., Wei F.G., Zheng Q., Wang C.D., Xiao X.L. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chem. 2017;214:119–128. doi: 10.1016/j.foodchem.2016.07.073. [DOI] [PubMed] [Google Scholar]
- 49.Tiwari B.K., Donnell C.P.O., Muthukumarappan K., Cullen P.J. Ascorbic acid degradation kinetics of sonicated orange juice during storage and comparison with thermally pasteurised juice, LWT-Food. Sci. Technol. 2009;42:700–704. [Google Scholar]
- 50.Tiwari B.K., Patras A., Brunton N., Cullen P.J., O'Donnell C.P. Effect of ultrasound processing on anthocyanins and color of red grape juice. Ultrason. Sonochem. 2010;17:598–604. doi: 10.1016/j.ultsonch.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 51.Schulz M., Seraglio S.K.T., Della Betta F., Nehring P., Valese A.C., Daguer H., Gonzaga L.V., Costa A.C.O., Fett R. Blackberry (Rubus ulmifolius Schott): Chemical composition, phenolic compounds and antioxidant capacity in two edible stages. Food Res. Int. 2019;122:627–634. doi: 10.1016/j.foodres.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 52.do Nascimento E.M.G.C., Mulet A., Ascheri J.L.R., de Carvalho C.W.P., Carcel J.A. Effects of high-intensity ultrasound on drying kinetics and antioxidant properties of passion fruit peel. J. Food Eng. 2016;170:108–118. [Google Scholar]
- 53.Fonteles T.V., Leite A.K., Silva A.R., Carneiro A.P., Miguel Ede C., Cavada B.S., Fernandes F.A., Rodrigues S. Ultrasound processing to enhance drying of cashew apple bagasse puree: Influence on antioxidant properties and in vitro bioaccessibility of bioactive compounds. Ultrason. Sonochem. 2016;31:237–249. doi: 10.1016/j.ultsonch.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 54.Wang J., Ma H., Pan Z., Qu W. Sonochemical effect of flat sweep frequency and pulsed ultrasound (FSFP) treatment on stability of phenolic acids in a model system. Ultrason. Sonochem. 2017;39:707–715. doi: 10.1016/j.ultsonch.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 55.Kroehnke J., Szadzinska J., Stasiak M., Radziejewska-Kubzdela E., Bieganska-Marecik R., Musielak G. Ultrasound- and microwave-assisted convective drying of carrots - Process kinetics and product's quality analysis. Ultrason. Sonochem. 2018;48:249–258. doi: 10.1016/j.ultsonch.2018.05.040. [DOI] [PubMed] [Google Scholar]
- 56.Wu Y., Han Y.B., Tao Y., Li D.D., Xie G.J., Show P.L., Lee S.Y. In vitro gastrointestinal digestion and fecal fermentation reveal the effect of different encapsulation materials on the release, degradation and modulation of gut microbiota of blueberry anthocyanin extract. Food Res. Int. 2020;132 doi: 10.1016/j.foodres.2020.109098. [DOI] [PubMed] [Google Scholar]
- 57.Xiong X., Cao X.J., Zeng Q.Z., Yang X.Q., Wang Y.L., Zhang R.F., Huang F., Dong L.H., Zhang M.W., Su D.X. Effects of heat pump drying and superfine grinding on the composition of bound phenolics, morphology and microstructure of lychee juice by-products. LWT-Food Sci. Technol. 2021;144 [Google Scholar]
- 58.Li M., Chen X., Deng J., Ouyang D., Wang D., Liang Y., Chen Y., Sun Y. Effect of thermal processing on free and bound phenolic compounds and antioxidant activities of hawthorn. Food Chem. 2020;332 doi: 10.1016/j.foodchem.2020.127429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.