Abstract
Diseases that result in retinal pigment epithelium (RPE) degeneration, such as age-related macular degeneration (AMD), are among the leading causes of blindness worldwide. Atrophic (dry) AMD is the most prevalent form of AMD and there are currently no effective therapies to prevent RPE cell death or restore RPE cells lost from AMD. An intriguing approach to treat AMD and other RPE degenerative diseases is to develop therapies focused on stimulating endogenous RPE regeneration. For this to become feasible, a deeper understanding of the mechanisms underlying RPE development, injury responses and regenerative potential is needed. In mammals, RPE regeneration is extremely limited; small lesions can be repaired by the expansion of adjacent RPE cells, but large lesions cannot be repaired as remaining RPE cells are unable to functionally replace lost RPE tissue. In some injury paradigms, RPE cells proliferate but do not regenerate a morphologically normal monolayer, while in others, proliferation is pathogenic and results in further disruption to the retina. This is in contrast to non-mammalian vertebrates, which possess tremendous RPE regenerative potential. Here, we discuss what is known about RPE formation during development in mammalian and non-mammalian vertebrates, we detail the processes by which RPE cells respond to injury, and we describe examples of RPE-to-retina and RPE-to-RPE regeneration in non-mammalian vertebrates. Finally, we outline barriers to RPE-dependent regeneration in mammals that could potentially be overcome to stimulate a regenerative response from the RPE.
Keywords: retinal pigment epithelium (RPE), regeneration, development, age-related macular degeneration (AMD), zebrafish
1. INTRODUCTION
The retinal pigment epithelium (RPE) is a monolayer of cells located at the posterior of the eye, between the retina and choroid, which serves a number of important roles in vision (reviewed in Strauss, 2005; Lakkaraju et al., 2020; Fig. 1). The RPE is a major component of the blood-retinal-barrier (BRB) due to tight cell-cell junctions that form between RPE cells. On their apical side, RPE cells interdigitate with photoreceptor outer segments (POS) and play a crucial role in the function and maintenance of photoreceptor cells. For example, transport of oxygen, nutrients and ions from the choroid to the retina and back is mediated by the RPE, and the RPE functions in degrading or recycling components of the visual cascade to maintain the visual cycle, amongst other critical functions in supporting the retina. Evolutionarily, the association between pigmentation and vision also appears to be essential; across phyla, image-forming visual organs contain either a pigmented organelle located within a photoreceptive cell, or a pigmented cell tightly associated with a photoreceptive cell, underscoring the likely importance of pigmentation/RPE function and the evolution of visual function (reviewed in Koenig and Gross, 2020).
Figure 1. RPE biology and generalized functions.
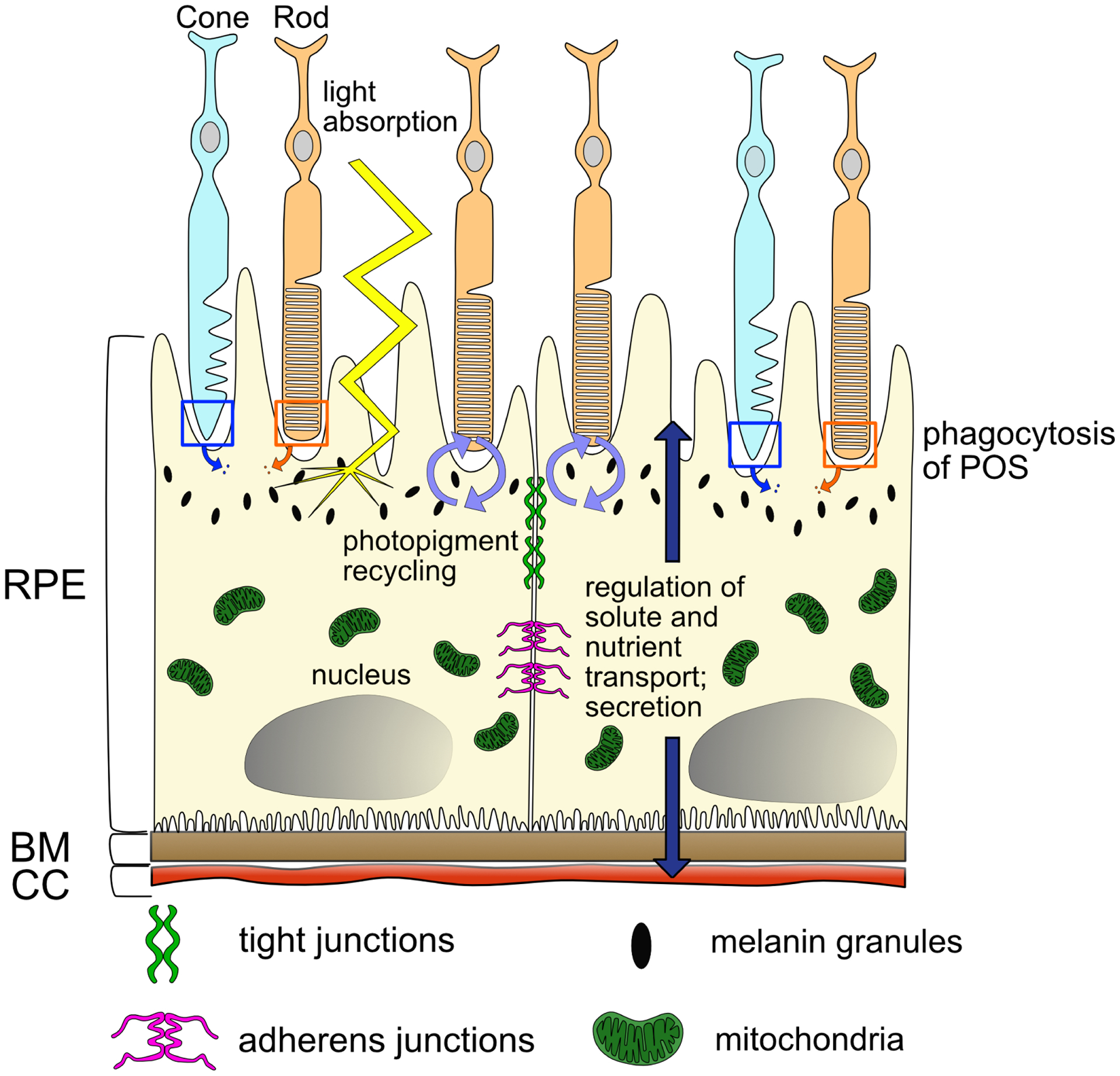
Cartoon illustrating the mature, polarized RPE monolayer and its interactions with the rod and cone PRs, BM, and CC, as well as the diversity of functions mediated by the RPE. Abbreviations: PRs, photoreceptors; RPE, retinal pigment epithelium; CC, choriocapillaris; BM, Bruch’s membrane; POS, photoreceptor outer segments.
Not surprisingly, diseases that affect the RPE also severely affect vision. Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in developed countries (Klein et al., 1999; Congdon et al., 2004), and primarily affects the RPE. Other RPE-associated diseases include Stargardt disease and some forms of retinitis pigmentosa. Atrophic (dry) AMD is the most prevalent form of AMD, affecting approximately 90% of patients. In atrophic AMD, parafoveal RPE first degenerates and over time, RPE degeneration progresses centrally to the fovea, resulting in death of foveal cones and consequent loss of high-acuity vision (Scholl et al., 2004; Holz et al., 2007; Steinberg et al., 2015; Curcio et al., 1996; Lim et al., 2012; Jackson et al., 2002). There are currently no FDA-approved therapies for atrophic AMD and with incidences of AMD projected to continue to rapidly rise globally (Wong et al., 2014), new strategies must be developed to curb RPE cell death and subsequent retinal degeneration associated with AMD progression. A number of such strategies are in various phases of development and testing, and these include: pharmacological interventions (reviewed in Ammar et al., 2020); cell-based therapies (reviewed in Ben M’Barek et al., 2019); gene therapy (reviewed in Bordet and Behar-Cohen, 2019); stem cell therapies (reviewed in Nazari et al., 2015; Leach and Clegg, 2015); and stem cell-derived transplantation of RPE patches or sheets (reviewed in Sharma et al., 2020), amongst others. While all of these approaches are exciting and some may yield effective therapies, a complementary approach is to stimulate intrinsic regenerative pathways in RPE cells such that tissue damage can be repaired endogenously. Indeed, such a strategy has gained traction over the last few years as a possible treatment for retinal degenerations and optic neuropathies, which have been the focus of several National Eye Institute initiatives (Vetter and Hitchcock, 2017; Burns and Stevens, 2018). Non-mammalian vertebrates possess remarkable regenerative abilities to repair and regenerate ocular tissues after injury (reviewed in Ail and Perron, 2017; Wilken and Reh, 2016; Wan and Goldman, 2016; Barbosa-Sabanero, 2012; Chiba, 2014), and identification of the molecular and cellular mechanisms underlying these regenerative strategies can serve as the foundation for developing approaches to potentially stimulate such responses in humans to treat currently intractable diseases like AMD.
In this review, we first summarize what is known about RPE development in mammals as well as non-mammalian vertebrate systems used for RPE development, injury and regeneration studies. We then define the molecular and cellular responses to acute and chronic RPE injury in these systems and discuss how they can contribute to further pathology in mammals, which cannot regenerate tissues from their RPE, and are therefore termed regeneration-incompetent. Further, we discuss pro-regenerative pathways in non-mammalian vertebrate systems like chickens, newts, frogs and zebrafish, which are regeneration-competent and possess the ability to regenerate retinal and/or RPE cells from native RPE after RPE or retinal injury. Finally, we delve into potential barriers to RPE reprogramming and regeneration. Our focus in this review is also on in vivo studies, whenever possible; while in vitro work on RPE cells has most certainly contributed to our understanding of RPE disease and injury responses, cultured systems do not adequately recapitulate the in vivo physiology of the RPE after injury, nor do they model the three-dimensional architecture of the eye, where RPE cells are intimately associated with photoreceptors, Bruch’s membrane, the choroid, as well as other cell types (e.g. components of the immune system) that collectively modulate their behavior after injury and during regeneration.
2. RPE DEVELOPMENT
Here, we highlight key phases of RPE development and known molecular regulators of RPE specification, differentiation and morphogenesis, with an eye towards their reiterative deployment during RPE injury responses in mammalian systems and injury/regenerative responses in non-mammalian systems. There are a number of additional, excellent reviews on RPE development to which we direct interested readers (e.g. Martinez-Morales and Wittbrodt, 2009; Fuhrmann, 2010; Fuhrmann et al., 2014; Moreno-Marmol et al., 2018).
2.1. Eye Field Specification, Optic Vesicle, and Optic Cup Formation
RPE, retina, and optic stalk progenitors are derived from neuroepithelial tissue of the diencephalon (reviewed in Chow and Lang, 2001). Eye field transcription factors including Rx, Six3, Lhx2, and Pax6, along with cell-cell signaling pathways, collectively initiate eye field formation in mice, frogs and other vertebrates (Mathers et al., 1997; Lagutin et al., 2003; Porter et al., 1997; Grindley et al., 1995; Zuber et al., 2003). In all vertebrates, bilateral optic primordia evaginate from the diencephalon, forming the optic vesicles (OVs). The distal portion of the OV elongates outward, while the proximal portion constricts to form the optic stalk. Once the distal OV reaches the surface ectoderm, the OV invaginates to form a bilayered optic cup (OC) (reviewed in Fuhrmann, 2010). The distal layer of the OC forms the retina, and the proximal layer wraps around the retina to form the RPE (reviewed in Fuhrmann et al., 2014). Teleosts (e.g. zebrafish and medaka) have become increasingly useful in studies of RPE development and regeneration in the last decade and importantly, the stages of OC morphogenesis in teleosts are largely similar to those in mammals and birds; however, each OV is flattened, as compared to the spherical presumptive OC in mammals, and extends posteriorly during elongation stages (Kwan et al., 2012; reviewed in Martinez-Morales and Wittbrodt, 2009). OC formation in teleosts also includes unique cell migration patterns not observed during mammalian OC formation; however, RPE progenitors in teleosts ultimately occupy the same region as RPE progenitors in amniotes, in the dorsal region of the eye behind the presumptive retina (Kwan et al., 2012). A comparison of eye development between teleosts and amniotes is summarized in Fig. 2.
Figure 2. Eye morphogenesis in amniotes and teleosts.
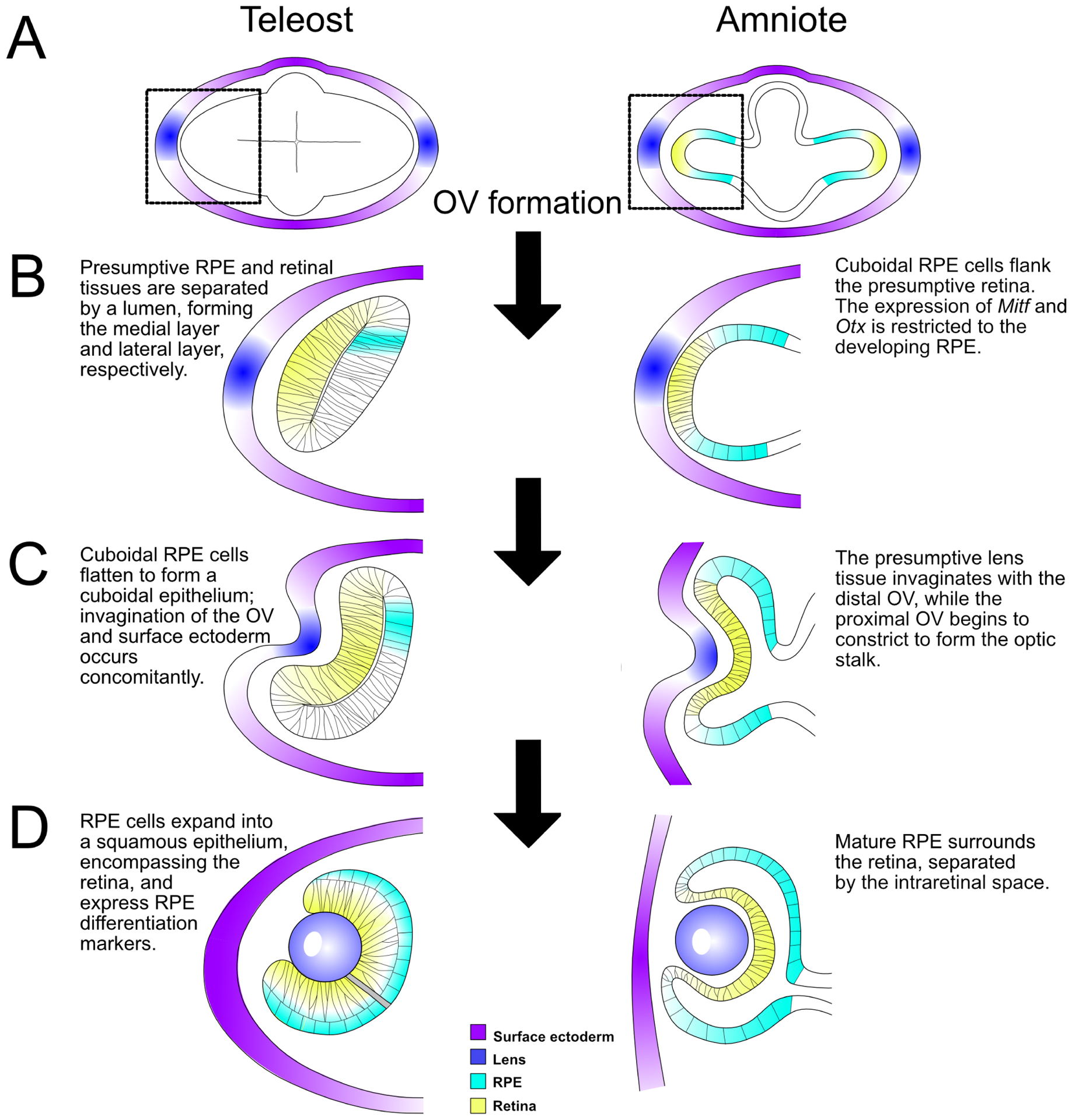
(A) In teleosts (e.g. zebrafish and medaka) and amniotes (e.g. mammals, birds), the OVs evaginate from the presumptive eye field in the forebrain. Enlargement of the left OV (black dashed boxes, A) to highlight subsequent steps of eye development (B-D). (B) OV elongation occurs posteriorly in teleosts, where RPE progenitors occupy a small portion of the medial (dorsal) layer as a pseudostratified epithelium. In amniotes, the OV extends outward, and the future RPE cells are cuboidal and occupy the region surrounding the presumptive retina. (C) Invagination of the surface ectoderm and distal OV occurs simultaneously; RPE in both teleosts and amniotes are cuboidal epithelial cells. (D) Eye morphogenesis concludes with the formation of a bilayered optic cup: RPE cells in teleosts are squamous and directly surround/interdigitate with the retina with no intervening space, while RPE cells in amniotes remain cuboidal and interdigitate apically with the retina, but remain separated from it by the intraretinal space, which disappears before birth. Abbreviations: OV, optic vesicle; RPE; retinal pigment epithelium.
2.2. RPE Specification/Differentiation
RPE specification begins upon OV evagination and is marked by the expression of microphthalmia-associated transcription factor (Mitf) and orthodenticle homeobox 2 (Otx2) (Hodgkinson et al., 1993; Bovolenta et al., 1997; Ma et al., 2019). Mitf is initially expressed throughout the early eye field, but later restricted to the presumptive RPE through the action of Vsx2 (Chx10) (Horsford et al., 2005; Rowan et al., 2004) and Otx2 (Martinez-Morales et al., 2003; Martinez-Morales et al., 2001), the latter of which promotes RPE fate over retina. Both Mitf and Otx2 are directly required for normal RPE formation in mice (Matsuo et al., 1995; Martinez-Morales et al., 2001; Nguyen and Arnheiter, 2000; Hodgkinson et al., 1993; Hero et al., 1991). Similarly, Otx paralogs are required for RPE formation in zebrafish; however, the two zebrafish Mitf paralogs (mitfa and mitfb) are dispensable for RPE formation (Lane and Lister, 2012). It is thought that loss of mitfa/b function in zebrafish is compensated for by the expression of a related MITF family member, tfec (Lister et al., 2011; Sinagoga et al., 2020). Once the OV reaches the overlying surface ectoderm, Mitf and Otx2 expression is identifiable solely in the dorsal OV, where the RPE begins to develop (Nguyen and Arnheiter, 2000; Bovolenta et al., 1997). Otx2 remains as a marker of the RPE throughout the lifespan of many animals (Bovolenta et al., 1997; Baas et al., 2000; Martinez-Morales et al., 2001), while Mitf is only expressed during early development in the chick and mouse RPE (Mochii et al., 1998; Nakayama et al., 1998). Mitf exists in at least nine isoforms in mice, with MITF-D and MITF-H being the dominant isoforms required for normal RPE development (Bharti et al., 2008; Hallsson et al., 2000; Takeda et al., 2002), whereas MITF-M, MITF-H, and MITF-A predominate in adult human RPE tissues (Maruotti et al., 2012). In addition to Otx2 and Vsx2, known regulators of Mitf expression in mice and humans include: Lhx2, Pax2 and Pax6 (Martinez-Morales et al., 2003; Yun et al., 2009; Bharti et al., 2012; Bharti et al., 2008; Bäumer et al., 2003). Pax6 encodes a multifunctional transcription factor specifying both the eye field and specific tissues within the eye, including the RPE, retina, lens placode, iris, and cornea (Bharti et al., 2012; Singh et al., 2002; Ashery-Padan et al., 2000; Bäumer et al., 2003). Pax6, Mitf, and Tfec interact to promote RPE development through down-regulation of the Wnt inhibitor, Dkk13, and the proretinogenic growth factor, Fgf15 (Bharti et al., 2012). Six6, Lhx2 and Vsx2 activities also stimulate Pax6 expression in the prospective retina where it facilitates a variety of events during retina formation (e.g. Zuber et al., 2003; Marquardt et al., 2001; Philips et al., 2005; Grindley et al., 1995; Oron-Karni et al., 2008), highlighting the context-dependent and spatially-restricted roles that Pax6 plays in driving development of both the RPE and retina. Canonical Wnt/β-catenin signaling also modulates Mitf expression and more broadly, RPE development (Westenskow et al., 2009, Fujimura et al., 2009). Like Mitf and Otx2, β-catenin is expressed in the presumptive RPE and further modulates Mitf-D and Otx2 through TCF/LEF binding within respective enhancer regions (Westenskow et al., 2009). An additional regulator of RPE-specific expression is the Hippo pathway, whose activity is modulated by the cellular localization of the transcriptional co-activators, Yap and Taz (Yu and Guan, 2013). Loss of Yap and Taz activity results in aberrant RPE specification and development in zebrafish and mice (Miesfeld et al., 2015; Kim et al., 2016). Moreover, Neurofibromin 2 (NF2) is an upstream activator of the Hippo pathway that is strongly expressed in the RPE and required for normal RPE development in a Yap/Taz-dependent manner (Moon et al., 2018).
After invagination of the OVs and early RPE specification events, the RPE becomes distinguishable through increased pigmentation and transition from a pseudostratified epithelium to a cuboidal monolayer (reviewed in Moreno-Marmol et al., 2018). Here, studies in a variety of animal models have contributed to our understanding of RPE development, including in non-human primates. For example, in rhesus macaques, the morphology of RPE differs remarkably between peripheral and central RPE at embryonic day (E) 45. The peripheral RPE appears multi-stratified, while the central RPE forms a mono-stratified layer of cuboidal epithelium, and it is thought that this change in morphology proceeds in a central to peripheral fashion (Rappaport et al., 1995). RPE cell proliferation occurs from ~E27 to E85; postnatally, RPE proliferation continues, but to a far more limited extent (Rapaport, et al., 1995). While multiple histological studies of RPE formation have been performed like the above in other animals models, our understanding of RPE morphogenesis in mammalian systems remains quite limited and much of our knowledge on this topic has come from in vivo and fixed sample imaging studies in zebrafish (Kwan et al., 2012; reviewed in Moreno-Marmol, 2018). In zebrafish, RPE morphogenesis occurs in two phases as the OV invaginates to form the OC. In the first phase, the presumptive RPE progenitor domain expands anteriorly and RPE progenitors initially exhibit a cuboidal shape, concomitant with increased cell number caused by de novo specification. This is followed by a second phase in which the cuboidal RPE cells elongate to adopt an RPE- like morphology and stretch around the retina along the dorsoventral and posterior axes (Li et al., 2000; Cechmanek and McFarlane, 2017). The molecular and cellular mechanisms underlying RPE cell flattening/stretching are not well-understood in any system and need to be further studied.
Finally, the RPE does not develop independently; rather, it interacts with surrounding extraocular mesenchyme, which sends signals that are required for RPE development (Fuhrmann et al., 2000). The transforming growth factor-beta (TGF-β) superfamily member, activin A, is a candidate signaling molecule mediating extraocular mesenchyme-dependent RPE formation. In chick RPE explants lacking extraocular mesenchyme, addition of activin A resulted in an upregulation of RPE markers and a downregulation of retinogenic factors, demonstrating that it was sufficient to enable RPE formation (Fuhrmann et al., 2000). While these data are exciting, this remains to be demonstrated in vivo. Bone morphogenetic proteins (BMPs), another critical member of the TGF-β superfamily, are also involved in RPE development. In chick, BMPs released from the surface ectoderm inhibit retina and optic stalk development and specify the RPE in dorsal OV cells by inducing Mitf expression (Müller et al., 2000). Wnt proteins are also released from the surface ectoderm and stabilize BMP signaling to initiate RPE specification via a Wnt/β-catenin-independent signaling pathway (Steinfeld et al., 2013). Surface ectoderm signals to the developing OC via fibroblast growth factor (FGF) to promote retinogenesis rather than RPE fates (Nguyen and Arnheiter, 2000), and therefore the extraocular mesenchyme appears to be the primary source of pro-RPE signals during early eye development. Finally, retinoic acid (RA), a derivative from vitamin A, is synthesized from multiple sites in and around the developing eye, including the RPE, and contributes to OC morphogenesis and retinal development (Prabhudesai et al., 2005, Hyatt et al., 1997; Molotkov et al., 2006). In human RPE cells in vitro, exposure to exogenous RA prevented cellular overgrowth and maintained a mature RPE-like morphology, suggesting a possible role for RA in the development and/or maintenance of RPE in vivo (Campochiaro et al., 1991).
2.3. RPE maturation
After early stages of specification, the retina and RPE are separated by the interphotoreceptor matrix (IPM), which also contributes to RPE maturation. The IPM acts as the intermediary between the two tissues during development, as well as throughout life (reviewed in Strauss, 2005; Halilagic et al., 2007; Ishikawa et al., 2015). Pigmentation, achieved through synthesis of melanin, is an obvious readout of differentiated RPE tissue (Beermann et al., 1992). Cell biologically, during maturation, the RPE attains a near-complete polarization along the apical-basal axis, as apical microvilli emerge and rudimentary basal infoldings make contact with the developing Bruch’s membrane (Fig. 1). The outer aspect of the RPE basal membrane becomes incorporated into the developing Bruch’s membrane, which matures into a pentalaminar structure upon incorporation of the basement membrane of the choriocapillaris and deposition of collagen and elastin proteins, regulated by the choroid and RPE (reviewed in Strauss, 2005; Lakkaraju et al., 2020; Booij et al., 2010). The RPE starts to express mature markers, including the adhesion molecule, N-CAM-140 (Gunderson et al., 1993), and apical microvilli protein, Ezrin, which promotes elongation of microvilli (Bonilha et al., 1999). Junctional proteins form tight connections between adjacent RPE cells, contributing to the completion of RPE apical-basal polarization and formation of the BRB (reviewed in Strauss 2005; Williams and Rizzolo, 1997). Indeed, the RPE tightly controls both basal nutrient entry from the fenestrated choriocapillaris and apical growth factor secretion to the retina (reviewed in Rizzolo, 2007), and these functions require the acquisition of precise apical-basal cell polarity during RPE development and maturation. During ocular morphogenesis, junctional protein composition in RPE cells is dynamic with protein expression changing over time as a function of retinal secretions (Williams and Rizzolo, 1997; Rahner et al., 2004). Initially, leaky tight junctions form discontinuities around developing RPE cells, permitting paracellular transport (Williams and Rizzolo, 1997). Later developmental stages elicit minimal transport between cells and ion selectivity, indicating mature barrier formation (Williams and Rizzolo, 1997; Ban and Rizzolo, 1999). Any remaining leaks between cells are resolved, resulting in a functional, continuous barrier (reviewed in Rizzolo et al., 2007).
As the RPE matures, its ability to regulate and secrete growth factors, extracellular matrix (ECM), and ECM-associated proteins is critical for maintaining the functional integrity of adjacent tissues. For example, pigment epithelium- derived factor (PEDF) is secreted apically from the RPE and provides neurotrophic support to photoreceptors (Becerra et al., 2004; Barnstable and Tombran-Tink, 2004). PEDF also acts as an angiogenic inhibitor (Dawson et al., 1999), which is important because pro-angiogenic vascular endothelial growth factor (VEGF) is secreted basally from RPE cells and acts as a paracrine signal to the underlying choroid (Campochiaro et al., 1989, Blaauwgeers et al., 1999, Marneros et al., 2005; Saint-Geniez et al., 2009). Indeed, VEGF secretion from the RPE is required for choroidal vasculature development (Goto et al., 2018), highlighting the importance of RPE-derived PEDF in modulating VEGF activity to prevent retinal neovascularization. TGF-β1 and TGF-β2 are secreted by cultured human RPE cells, with TGF-β2 as the dominant isoform (Hirsch et al., 2015). Apical secretion of active TGF-β might play a role in maintaining the RPE immune microenvironment (Hirsch et al., 2015, Sugita et al., 2006). Studies in cultured RPE also suggest functions for RPE-derived FGFs in mitogenesis, angiogenesis and cell survival. For example, bFGF (FGF2) stimulates mitogenesis, possibly in an autocrine fashion (Schwegler et al., 1997), while FGF5 is secreted basally and serves to maintain choroid function (Dunn et al., 1998). Various other proteins including brain-derived growth factor (BDNF) (Kolomeyer et al., 2011), ciliary neurotrophic factor (CNTF) (Li et al., 2011), insulin-like growth factor-I (IGF-I) (Moriarty et al.,1994), nerve growth factor (NGF) (Kolomeyer et al., 2011), tissue inhibitor of matrix metalloproteinase (TIMP-1) (Padgett et al., 1997), and adhesion molecules (Aisenbrey et al., 2006) are also secreted from the RPE, some in a directional manner, further highlighting the importance of correct RPE polarization as the RPE matures and assumes its functional roles (reviewed in Kay et al., 2013).
As mentioned above, RPE polarity is plastic and dependent on interactions with the developing retina. For example, the elongation of apical microvilli is related to the extension of photoreceptor outer segments and the expression of adhesion proteins, including N-CAM and certain integrins, in the basolateral membranes of RPE that change progressively as the neural retina develops (reviewed in Rizzolo, 1997). As intercellular permeability decreases as a result of tight junction development, the RPE expresses GLUT transporters to supply the retina with glucose, which is critical for maintaining the health of the retina (Ban and Rizzolo, 2000), as well as other solutes and nutrients (reviewed in Strauss, 2005). The RPE also functions as a key metabolic component of a succinate shuttle that serves to transfer electrons from the hypoxic retina to the oxygen-rich RPE (Bisbach et al., 2020). In the final step of maturation, the RPE and retina interdigitate permanently, as the apical microvilli extend to interact with the POS in the subretinal space; this process is accompanied by the formation of complete basal infoldings (reviewed in Strauss, 2005). Mature RPE markers include: visual cycle proteins, RPE65 (Mata et al., 2004) and CRALBP (Xue et al., 2015); integrin aVb5 (Anderson et al., 1995); the basolateral RPE protein, bestrophin (Bakall et al., 2003); PEDF (Wu et al., 1995); MerTK (Feng et al., 2002); pre-melanosomal protein PMEL17 (Raposo et al., 2001); tyrosinase (Dryja et al., 1978); apically localized Na+ K+-ATPase (Hu et al., 1994); extracellular matrix metalloproteinase inducer (EMMPRIN) (Marmostein et al., 1998); in addition to N-CAM-140 (Gundersen et al., 1993) and Ezrin (Bonilha et al., 1999). Gene expression profiling and single-cell sequencing of fetal and adult human RPE have also been completed, identifying a suite of RPE-enriched genes (Strunnikova et al., 2010; Hu et al., 2019; Schumacker et al., 2020; Voigt et al., 2019).
3. RPE INJURY RESPONSES
Once developed, the RPE becomes a structure vital to maintaining the health of photoreceptors and the function of the retina as a whole. Given the prevalence of RPE-related diseases that have deleterious effects on visual function, RPE injury paradigms have been developed in mammalian and non-mammalian systems to identify aspects of the injury response and to determine whether modulation of injury-responsive pathways might be leveraged to develop therapies to treat RPE disorders (Table 1).
Table 1.
RPE injury responses
| Injury type | Model System | Biological Response | References |
|---|---|---|---|
| NaIO3 injection | Mice | Loss of pigmentation, RPE cell enlargement, vacuolization, loss of cell-to-cell contact | Hanus et al., 2016 |
| NaIO3 injection | MRL/MPJ mice | Loss of RPE65 expression | Xia et al., 2011 |
| NaIO3 injection | Rabbit | Loss of pigmentation, apical microvilli, basal infolds | Korte et al., 1984 |
| NaIO3 injection | Rat | Loss of apical microvilli, basal infoldings, and tight junctions | Liu et al., 2019a |
| Genetic ablation | Zebrafish | Loss of pigmentation, shortening of apical microvilli, BM thinning and degradation | Hanovice et al., 2019 |
| Genetic ablation | Mice | RPE cell enlargement, abnormal shape | Longbottom et al., 2009 |
| Selective photocoagulation of RPE | Rabbit | Loss of cell-to-cell contact, RPE cell enlargement, vacuolization | Roider et al., 1992 |
| Adeno-associated viral injection of Rz432 | Mice | RPE cell enlargement, multinucleation, vacuolization | Seo et al., 2012 |
| APOE-4 targeted replacement with high-fat, cholesterol-enriched diet | Mice | RPE cell enlargement, multinucleation, vacuolization | Ding et al., 2011 |
| Ceruloplasmin/Hephaestin knock-out (KO) | Mice | Multinucleation | Hadziahmetovic et al., 2008 |
| RPE debridement | Pig | Abnormal cell morphology, loss of pigmentation, formation of mono- or multi-layered epithelium apical to BM | Del Priore et al., 1995 |
| Photic maculopathy | Monkey | Morphologically disrupted pigment granules, vacuolization, RPE pyknosis, migration to subretinal space, loss of pigmentation, loss of basal infoldings, RPE proliferation, abnormal morphology | Tso, 1973 |
| Retinectomy | Chick | Loss of epithelial characteristics and pigmentation, proliferation | Coulombre & Coulombre, 1965 |
| Retinectomy | Frog | Partial loss of pigmentation and BM detachment | Yoshii et al., 2007 |
| Retinectomy | Newt | Loss of epithelial characteristics and pigmentation, BM detachment, RPE cells gain multipotency | Chiba et al., 2006 |
| Traction retinal detachment | Rabbit | Pigmented granules on retinal surface | Cleary & Ryan, 1979a |
| Traction retinal detachment | Rabbit | RPE proliferation, formation of epiretinal membrane, partial loss of pigmentation, abnormal morphology | Cleary & Ryan, 1979b |
Abbreviations: RPE, retinal pigment epithelium; BM, Bruch’s membrane; NaIO3, sodium iodate
Despite biological similarities, many different experimental methods have been utilized to reproduce degenerative ocular phenotypes, including, but not limited to, induction of oxidative stress, genetic ablation, and laser-induced choroidal neovascularization (CNV), which adds a level of complexity to studying RPE injury responses. Many traditional RPE injury studies have been conducted in mammalian model systems through the use of sodium iodate (NaIO3 ) injection, which induces oxidative stress in RPE cells that results in cell death (Noell, 1953; Grignolo et al., 1966). Depending on the dosage, animal, and method of administration, RPE degeneration and injury responses vary. For example, high-dose NaIO3 injections have been widely characterized and implicated in primary degeneration of RPE, followed by secondary photoreceptor dysfunction (Kiuchi et al., 2002). However, low-dose administration of NaIO3 in rats causes retinal disruption prior to any RPE degeneration, suggesting that NaIO3 is not entirely specific to RPE (Wang et al., 2014). Other methods include AAV injection of a Sod2 ribozyme and Ceruloplasmin/Hephaestin knockout mouse models to elevate reactive oxygen species (ROS), resulting in acute destruction of RPE cells (Seo et al., 2012; Hadziahmetovic et al., 2008). On the other hand, genetic ablation models utilize transgenic cassettes targeting a variety of proteins that stimulate programmed cell death as a means to recapitulate the loss of RPE cells that occurs during AMD. Like models increasing oxidative stress, genetic ablation causes acute damage to the RPE with secondary damage to the retina (Hanovice et al., 2019; Longbottom et al., 2009). Similarly, laser photocoagulation injures the RPE and/or retina, providing instant damage and stimulation of rapid wound healing responses (Roider et al., 1992; Tso et al., 1973). Targeted induction of CNV is also an established methodology that utilizes laser burns to model exudative AMD (reviewed in Grossniklaus et al., 2010; Ryan, 1979). Here, immediately post-injury, the RPE and Bruch’s membrane lose association, which leads to vascular invasion and leukocyte infiltration. Subsequently, fibrovascular tissue and proliferative RPE repair the disrupted BRB (Zhou et al., 2017; Sakurai et al., 2003; Miller et al., 1990). These models seek to emulate common late stages of AMD when choroidal vessels invade the retina (reviewed in Das and McGuire, 2003).
As the aforementioned systems provide insight into the biological response after acute injury, other models are used to study the responses following chronic RPE dysfunction/death in degenerative disease. Aged mice with human APOE-4 targeted replacement that were provided with a high fat diet to stimulate amyloid beta (Aβ) accumulation showed an AMD-like phenotype as Aβ is present in drusen (Anderson et al., 2004) and co-localizes with complement proteins (Wang et al., 2009). These data suggest that Aβ contributes to pathology by perpetuating chronic inflammation (Ding et al., 2011). This model demonstrates chronic presentations of the disease (e.g. basal RPE deposits, thickening of Bruch’s membrane, and loss of visual function); however, acute presentations still remained (e.g. vacuole formation, multinucleate and enlarged RPE, and variable pigmentation) (Ding et al., 2011). Thus, although AMD progresses chronically, some stages of the disease are acute, highlighting the complexity of pathogenesis and a need to develop stage-specific therapeutic options.
Despite the existence of numerous methods to recapitulate RPE degenerative diseases, common injury responses suggest a trend in cellular behavior. Here, we discuss RPE injury responses, with a particular emphasis on those pathways that are also involved in RPE development and/or regeneration.
3.1. Acute injury responses
Cellular responses immediately following RPE injury have been widely characterized in mammalian and non-mammalian vertebrate model systems, although for many of these responses, how they lead to wound healing, RPE cell proliferation or repair remains ambiguous. RPE injury studies demonstrate a trend in biological responses that includes: loss of pigmentation (Hanovice et al., 2019; Hanus et al., 2016; Korte et al., 1984; Tso, 1973), loss of apical microvilli (Liu et al., 2019a; Korte et al., 1984) and basal infoldings (Liu et al., 2019a; Korte et al., 1984; Tso, 1973); followed by RPE cell enlargement (Hanus et al., 2016; Seo et al., 2012; Longbottom et al., 2009; Roider et al., 1992), the formation of multinucleate RPE cells (Seo et al., 2012; Ding et al., 2011; Hadziahmetovic et al., 2008), accumulation of intracellular vacuoles (Seo et al., 2012; Ding et al., 2011; Roider et al., 1992); collectively culminating in overall disruption of the RPE monolayer and photoreceptor disorganization (Hanovice et al., 2019; Moriguchi et al., 2018).
One common RPE phenotype resulting from injury, regardless of injury type, is cell enlargement, which could occur in an attempt to preserve the association between surrounding RPE cells, as well as RPE cells and underlying photoreceptors, to maintain physiological function. Multinucleate RPE exist physiologically in the human eye, specifically in the perifovea and near-periphery regions (Starnes et al., 2016). Multinucleation and cell enlargement becomes more pronounced after injury in rodent models (Seo et al., 2012; Ding et al., 2011; Hadziahmetovic et al., 2008). It is unknown if multinucleate RPE exist in non-mammalian systems. The function, if any, of these multinucleate cells in mammals is also not clear; however, it is possible that RPE re-enter the cell cycle in an attempt to proliferate and restore the monolayer, but are unable to complete cytokinesis, resulting in enlarged multinucleate cells. As the RPE is responsible for managing oxidative stress in the eye, targeted oxidative damage also results in enlargement and damage to mitochondria (Seo et al., 2012). Bruch’s membrane breaks down in response to selective RPE insult in several animal model systems (Hanovice et al., 2019; Seo et al., 2012) and in AMD patients (Chong et al., 2005). Atrophy of the choriocapillaris following RPE degeneration (Korte et al., 1984) suggests the integrity of the RPE layer is necessary to maintain structure and function of the underlying blood supply. Unlike biological responses observed in mammalian models, targeted ablation of zebrafish RPE resulted in degradation of Bruch’s membrane, with gaps permeating the normally confluent structure, resulting in physical breakage in the BRB (Hanovice et al., 2019).
Typically, the confluent monolayer of RPE cells is held together through cadherins and epithelial junctional proteins, resulting in a rigid structure. Post-injury, there is a loss of the mature RPE marker, RPE65, as well as decreased cadherin and junctional protein, ZO-1, expression (Moriguchi et al., 2018; Hanus et al., 2016; Longbottom et al., 2009). These changes are often accompanied by a visible loss of cell-cell contact (Roider et al., 1992), dismantling the anchored monolayer. Most epithelial cells predominantly express E-cadherin (Halbieb and Nelson, 2006), but the dominant cadherin in RPE cells is disputed, as results differ between species and tissue origin (e.g. in vivo vs. in vitro). Recently, P-cadherin was found to be the dominant cadherin in physiological conditions in human and mouse RPE cells in situ (Yang et al., 2018). Following targeted oxidative stress, P-cadherin distribution decreases at RPE cell junctions and increases intracellularly. The localization of β-catenin follows that of P-cadherin, decreasing at cell-cell junctions and increasing in the nucleus, suggesting potential activation of the Wnt signaling pathway (Yang et al., 2018). Canonical Wnt/β-signaling has been implicated in injury responses and tissue regeneration in a variety of contexts (reviewed in Whyte et al., 2012) and may be activated post-RPE injury in attempts to repair the epithelial layer. As opposed to the typical hexagonal phenotype, RPE cells become rounded post-injury, which might signify functional changes, such as epithelial-mesenchymal-transition (EMT) (see the following section on chronic injury responses for further discussion of EMT). Several other signaling pathways have been proposed to be activated rapidly after an RPE insult, including PI3K/Akt (Liao et al., 2018; Yang et al., 2006), but more data are needed to understand the roles that these pathways play post-injury. Similarly, RPE cells responding to various modes of injury secrete signaling factors that can further modulate RPE or retinal physiology. One striking example of this is from experiments in the Royal College of Surgeons (RCS) rat model of photoreceptor degeneration, where subretinal injection of bFGF (FGF2) was shown to rescue photoreceptor survival (Faktorovich et al., 1990). However, in these experiments, the surgical needle control or injection of phosphate-buffered saline (PBS) also rescued photoreceptors, albeit to a lesser extent, suggesting that acute injury responses in the RPE triggered the release of trophic factors that affected surrounding tissues. In addition to FGF, other known RPE-derived factors, including PEDF, BDNF, and CNTF (discussed in section 2.3), have the potential to preserve photoreceptors when added exogenously to animal models of acute injury and inherited degeneration (Faktorovich et al., 1990; Cayouette et al., 1999; Imai et al., 2005; LaVail et al., 1992; LaVail et al., 1998; reviewed in Kolomeyer and Zarbin, 2014). Some of these factors are being pursued clinically as possible treatments for human patients with ocular disease (reviewed in Kolomeyer and Zarbin, 2014).
In physiological conditions, the apically-localized RPE receptors, integrin aVb5 and MerTK, promote phagocytosis of POS and recycling of their components; critically, this is achieved without initiating an inflammatory response (Finneman and Nandrot, 2006; Finneman and Rodriguez-Boulan, 1999). Likewise, the space between the RPE and the photoreceptors, called the subretinal space (SRS), is a zone normally devoid of immune cells due to the stable resident microglia niche in the neural parenchyma, the presence of an outer BRB, and immunosuppressive factors secreted by the RPE (Karlstetter et al., 2015). There is evidence showing that accumulation of mononuclear phagocytes (MPs), including resident microglia and systemic monocyte-derived macrophages, occurs in several animal models of retinal and/or RPE damage (Sennlaub et al., 2013; Combadière et al., 2007; Leach et al., 2020; Moriguchi et al., 2018; O’Koren et al., 2019); however, the protective and/or pathogenic contribution of subretinal MPs appears to be context dependent. In the NaIO3 -induced mouse injury model, pro-inflammatory macrophages have been shown to accumulate in the RPE layer, and depletion of macrophages by intraperitoneal injection of clodronate liposomes attenuated ONL thinning; however, macrophage depletion alone did not protect the RPE from damage (Moriguchi et al., 2018). While these observations may be specific to the NaIO3 injury and/or macrophage depletion paradigms, variations in cell-specific requirements for macrophages and/or microglia post-injury are also possible. For instance, a recent study in mice revealed a potential RPE-protective feature of MPs in the SRS. Genetic depletion of microglia, which relocate toward the RPE layer in a model of photoreceptor degeneration, resulted in RPE disease-associated morphological defects, such as dysmorphic microvilli and disrupted interdigitation with retinal photoreceptors (O’Koren et al., 2019). Similarly, we have shown pharmacological and genetic perturbation of macrophages/microglia impaired RPE regeneration post-ablation in zebrafish (Leach et al., 2020). Collectively, these studies and others highlight the complex role subretinal MPs play in different ocular injury contexts. MicroRNAs (miRNAs) might also feed into the crosstalk between the immune system and injury/regenerative responses. Aging RPE appear to possess intrinsic protective mechanisms to maintain homeostasis; for example, miR-146a, was found to be upregulated in aged mouse RPE (Hao et al., 2016) and has been shown to repress pro-inflammatory signals (Kutty et al., 2013; Taganov et al., 2006). miRNAs could be potential targets to harness and deliver to damaged RPE to enhance repair and/or stimulate a regenerative response in mammals. Further understanding how signals from infiltrating MPs and miRNAs impact disease progression or attenuation will be critical for harnessing the therapeutic potential of the immune response in treating diseases involving the RPE.
Finally, the mechanism underlying RPE cell death can also provide insight into inflammatory responses and how they affect the RPE post-injury and conversely, how the inflammatory environment post-RPE injury could shape the degree of tissue damage and cell death. In a zebrafish RPE ablation model (see section 5.2 for details of this model), TUNEL staining revealed that programmed cell death began at 3 hours post-injury (hpi), peaking at 24hpi (Hanovice et al., 2019) and that macrophages and microglia infiltrated the injury site post-ablation (Leach et al., 2020), pointing to a potential reparative role of the immune system. Apoptosis is classically thought to be immunologically quiescent as the microenvironment surrounding apoptotic cells remains immunosuppressive through the release of anti-inflammatory cytokines, packaging of intracellular contents, and phagocytic cleanup of debris (reviewed in Campisi et al., 2014). However, other forms of cell death such as necroptosis and pyroptosis are associated with robust inflammation and diminish the regenerative potential of damaged cells. For example, in a model of RPE photooxidative damage where primary RPE cells were exposed to lipid-modified POS then irradiated with blue light, inflammasome priming resulted in increased cell death by pyroptosis (Brandstetter et al., 2016), which is characterized by caspase 1 activation, membrane swelling, splitting and vesicle formation, and the release of pro-inflammatory cytokines (reviewed in Lamkanfi, 2011). Inflammatory cascades could easily counteract mechanisms that facilitate tissue regeneration and thereby stimulate fibrosis, scarring, or additional pathology. A recent study in mice demonstrated that RPE cells die through a regulated form of necrosis, or necroptosis, following damage from NaIO3 injection, as demonstrated through the release of HMGB1, a necroptosis specific cytokine, and elevated RIPK3 expression, which is a downstream effector of a necroptotic response (Hanus et al., 2016). Although necroptosis shares features of apoptosis and necrosis, necroptosis results in the release of pro-inflammatory cytokines, priming a potent immune response (Dhuriya and Sharma, 2018). Other studies have suggested that there are additional mechanisms through which RPE cell death occurs, such as autophagy (Mitter et al., 2014). Indeed, it has been shown that autophagy might play an important role in physiological RPE cell death, as inhibition of autophagy results in accumulation of inflammatory monocytes contributing to pathology (Liu et al., 2016). It is important to note that the process of autophagy also has complex interactions with the immune system, where it contributes to leukocyte development and cytokine production, and may playing a critical role in perpetuating the pathogenesis of chronic inflammatory diseases (reviewed in Qian and Fang, 2017) Overall, the mechanisms underlying RPE cell death in human patients with ocular disease remain uncertain and are probably influenced by the mode of RPE injury or nature of RPE disease, amongst other variables. Regardless, the mechanism of RPE death could also contribute variable inflammatory responses and immune cell infiltration, which could then further affect disease progression as well as modulate the ability of the RPE to repair itself or regenerate.
3.2. Chronic injury responses
Failure to resolve inflammation and responses to RPE insult invariably results in functional deficits in vision, depending on the severity of the injury, and can lead to further degeneration and pathology. In the case of AMD, disease etiology is thought to be rooted in chronic, unresolved inflammation (reviewed in Hageman et al., 2001). Inflammation is multifaceted and characterized by complement activation, inflammasome assembly/activation, pro-inflammatory cytokine secretion, and MP recruitment, among other things (reviewed in Akhtar-Schäfer et al., 2018, Hageman et al., 2001; Ardeljan and Chan, 2013). Some of these characteristics (e.g. production of complement factors and MP accumulation and activation) are a consequence of normal homeostatic and aging processes (reviewed in Chen et al., 2019; Datta et al., 2017; Ardeljan and Chan, 2013). Another result of normal aging is the accumulation of extracellular drusen deposits between the RPE and Bruch’s membrane; however, depending on their size and morphology, drusen are also a primary indicator of AMD (reviewed in Ardeljan and Chan, 2013). Complement components have been identified in drusen by proteomic and histological studies (Mullins et al., 2000; Crabb et al., 2002; Anderson et al., 2002; Johnson et al., 2000; Johnson et al., 2001; Hageman et al., 2005; reviewed in Toomey et al., 2018), and may exacerbate inflammation and expedite pathology by serving as a signal for MP recruitment, inflammasome activation, and subsequent secretion of pro-inflammatory cytokines (reviewed in Akhtar-Schafer et al., 2018). Indeed, there is strong evidence showing that MPs accumulate in AMD lesions (reviewed in Guillonneau et al., 2017). As outlined in the acute injury responses (section 3.1), MP recruitment can be protective in some contexts, however, many studies have reported MPs contribute to pathology in AMD. For example, in donor eyes with AMD- and non-AMD-related CNV, stronger VEGF expression was found in lesions showing evidence of active inflammation (e.g. increased presence of macrophages); and some CNV-associated macrophages were the source of VEGF (Grossniklaus et al., 2002). It is thought that macrophages do not serve a reparative function in this context and instead may contribute to pathological blood vessel growth (Grossniklaus et al., 2002). Another study from humans showed activated microglia were present in the SRS and also the outer nuclear layer (ONL) of adult donor eyes presenting with a range of retinal degenerative diseases, including AMD (Gupta et al., 2003). Activated microglia contained membrane-bound rhodopsin, suggesting phagocytosis of rod photoreceptor debris; however, given the localization of microglia to regions of rod photoreceptor cell death, coupled with their capacity to produce cytotoxic secretions, the authors speculated that effects of activated microglia were likely deleterious, leading to further photoreceptor degeneration (Gupta et al., 2003). While evidence for the pathological role of immune-related components and signaling pathways is abundant, protective effects have also been shown. In a mouse model of laser-induced CNV, MP-specific genetic perturbation of interferon (IFN) signaling resulted in increased MP activation and angiogenic growth at the lesion site, while mice treated intravenously with IFN-β showed improvements in resolution of laser lesions when compared to controls (Lückoff et al., 2016). IFN-β-dependent neuroprotection has been shown in other disease contexts and may work through several downstream pathways to resolve inflammation (reviewed in Rashid et al., 2019). These immune-related components, some of which appear to exacerbate the progression of AMD, are likely inextricably linked (reviewed in Akhtar-Schafer et al., 2018), thus therapeutic intervention, like the roots of the disease, may require a multifaceted approach.
Proliferative responses are critical for tissue repair, but, similar to inflammation, can become pathological when left unresolved. This is the case in proliferative vitreoretinopathy (PVR), where damaged RPE cells lose contact with their neighbors and undergo EMT, a reversible process initiated by TGF-β in which cells lose their epithelial morphology and shift to a mesenchymal-like phenotype (Miettinen et al., 1994; reviewed in Xu et al., 2009). Although EMT is necessary in a variety of contexts during early development and tissue formation, in PVR, RPE cells undergo type II EMT, where cell injury triggers RPE proliferation and fibrosis (reviewed in Dongre and Weinberg., 2019). EMT is a common sequela in other RPE diseases like AMD, where it is thought that RPE-derived mesenchymal cells contribute to subretinal fibrotic lesions in late stages of the disease (reviewed in Shu et al., 2020; Zhou et al., 2020). Disruption of cell-cell contact occurs during EMT to permit morphological changes and enable cell migration (Ozdamar et al., 2005). During EMT, the transcription factor Snail is often upregulated, which results in decreased expression of junctional proteins like occludins and claudin (Ozdamar et al., 2005; Ikenouchi et al., 2003). These changes lead to loss of apical-basal polarity within RPE cells and breakdown of the confluent RPE monolayer. The repression of E-cadherin is necessary for the onset of EMT and modulated through Zeb-1, Snail family transcription factors, and E47 (Liu et al., 2008; Cano et al., 2000; Perez-Moreno et al., 2001). However, instances of type II EMT in PVR might require the repression of P-cadherin rather than E-cadherin as low levels of E-cadherin exist in human RPE cells (Yang et al., 2018). The repression of epithelial markers is accompanied by an increase in mesenchymal and myofibroblast gene expression, including N-cadherin, vimentin, and alpha-smooth muscle actin (α-SMA) (Islam et al., 1996; Kim et al., 2000; Gilles et al., 1999; Kim et al., 2006).
During EMT, cells progressively reorganize intracellular actin filaments and front-rear polarity develops to enable migration (Ridley et al., 2003). These rounded cells migrate through the retina and invade the vitreous, where they settle, proliferate, and phenotypically appear as fibroblasts (Tamiya et al., 2010). Migratory RPE cells also deposit collagen and other ECM proteins that contribute to the formation of contractile scar tissue, leading to retinal detachment and vision loss (Morino et al., 1990). Basement membrane destruction and changes in ECM composition accompany the cellular changes, likely facilitating the events leading to pathology. After retinal injury or detachment, and during PVR, RPE cells can undergo EMT and contribute to the formation of an epiretinal membrane, which is a thin layer of fibrotic tissue overlying the retina (Wang et al., 2015; Tamiya and Kaplan, 2016; Boles et al., 2020). Numerous components of the immune system, including macrophages, microglia, thrombin, and T-helper cells contribute to the large inflammatory response that continues in the later phases of the RPE injury response (reviewed in Chaudhary et al., 2020). Downstream of these, cytokines, chemokines, and growth factors (including FGF and TGF-β) are released (reviewed in Chaudhary et al., 2020). Multiple in vitro studies highlight the role of TGF-β signaling on EMT and promoting proliferation of the fibroblast-like RPE cells, and several recent studies have identified a novel role for miRNAs, including miR-124, miR-302d, and miR-93, in inhibiting TGF-β-mediated EMT and the maintenance of fibroblastic phenotypes in cultured RPE cells (Jun and Joo, 2016; Fuchs et al., 2020). Importantly, transfection of miR-302d and miR-93 retransformed fibroblast-like RPE cells back to an epithelial-like state, regardless of TGF-β exposure, as well as inhibited the TGF-β-mediated secretion of VEGF (Fuchs et al., 2020). Beyond PVR, TGF-β-mediated EMT also contributes to the pathogenesis of exudative AMD and VEGF-stimulated neovascularization. Thus, miRNAs could be novel therapeutic targets for PVR and AMD treatments. Finally, as discussed above, RA might also contribute to RPE proliferation and morphological changes during EMT. The lack of retinoid input due to retinal detachment could induce additional RPE cells to undergo EMT, or exacerbate EMT phenotypes (Campochiaro et al., 1991).
RPE proliferation holds many implications for understanding disease pathogenesis as well as the potential for RPE repair (reviewed in Stern and Temple, 2015). In vitro studies have extensively characterized RPE proliferation in a variety of contexts; however, support for the physiological relevance of many of these pathways in vivo is limited. Although adult RPE cells are known to be post-mitotic, RPE cells have the intrinsic capacity to re-enter the cell cycle in both injury and non-injury contexts (Hanovice et al., 2019; Grierson et al., 1994; von Leithner et al., 2010; Al-Hussaini et al., 2016; reviewed in Stern and Temple, 2015). Breakdown of Bruch’s membrane is implicated in facilitating a proliferative response in RPE (Grierson et al., 1994). Lesions created in Bruch’s membrane result in choroidal neovascularization (CNV), followed by RPE proliferation and a corresponding regression of blood vessel growth, which suggests a possible link between RPE proliferation and amelioration of CNV (Miller et al., 1990; reviewed in Stern and Temple, 2015). RPE cell location within the eye might also be critical in modulating proliferative potential; for example, selective RPE ablation by low-energy laser-photocoagulation results in peripheral RPE re-entering the cell cycle to a larger extent than central RPE, regardless of the location of the lesion (von Leithner et al., 2010). Indeed, it has been demonstrated that peripheral RPE have a higher proliferative capacity then central RPE in a variety of contexts (Al-Hussaini et al., 2008; Kiilgaard et al., 2007; Kokkinopoulos et al., 2011). It could be that contact inhibition in central RPE prevents cell-cycle reentry, so peripheral RPE, which are more able to migrate to repair damaged tissue, become proliferative in response to injury. Co-localization of RPE-specific markers and cell-cycle markers also demonstrate that peripheral and equatorial RPE cells have the capacity to divide in contrast to central RPE (Al-Hussaini et al., 2008). In a zebrafish RPE ablation and regeneration model, proliferative cells were present in the injury site, first appearing in the peripheral RPE, and at later time-points, in the central RPE (Hanovice et al., 2019). Conceivably, protein expression levels also differ between peripheral and central RPE, as markers for cell proliferation, Wnt and BMP signaling, and cell cycle modulators are all upregulated in peripheral RPE cells under physiological conditions (Al-Hussaini et al., 2016). These and other studies highlight the dual significance of RPE proliferation: it can be detrimental to vision by contributing to EMT or it can potentially be beneficial, by contributing to the capacity to endogenously repair damaged RPE tissue.
4. RPE AGING
Age-related changes of the RPE are physiological and on their own, do not denote pathogenesis; however, age-related changes might contribute to the development of disease states in the RPE (reviewed in Ardeljan and Chan, 2013). Aged rat RPE cells proliferate, form vacuoles, and possess abnormal basal infoldings in the peripheral eye in vivo (Fan et al., 1996; Lai et al., 1978). These changes, although not pathological, could contribute to increased susceptibility to developing ocular diseases, such as AMD (reviewed in Bonilha, 2008; Sarks, 1976). Lipofuscin is a lipid-derived pigment characteristic of aged RPE and exists as autofluorescent residual molecules from POS phagocytosis (reviewed in Bonilha, 2008; Kennedy et al., 1995). Clumping of lipofuscin bodies results in intracellular accumulation, increased oxidative stress, and melanin depletion through the disruption of antioxidation (reviewed in Bonila, 2008). Lipofuscin can contribute to autofluorescence visible in unconventional subretinal drusenoid deposits (Lee and Ham, 2014). Conventional drusen, discussed above, are located between the RPE and Bruch’s membrane and are composed of lipoprotein deposits and complement factors that collect with age (reviewed in Khan et al., 2016); these are not shown to directly cause AMD, but rather pose a risk factor as extracellular deposits accumulate. Regardless, progressive build-up of debris can lead to RPE cell detachment and disruption of RPE cell junctions (reviewed in Al-Hussaini et al., 2008). While drusen and lipofuscin are often present in pathological contexts, both are visible in aged mouse eyes and considered a physiological component of the aging process (Liu et al., 2019b; Xu et al., 2008). Expression of the tight junction protein, ZO-1, decreases in aged mouse RPE (Chen et al., 2019), suggesting that RPE undergo morphological changes throughout aging. Microglia also accumulate in the SRS of aging mice suggesting that they may be involved in aging or the progression of age-related diseases that affect the RPE (Xu et al., 2008; Combadière et al., 2007),
Management of oxidative stress could be compromised in aging RPE. Differential protein expression exists between young and aged rats, and fewer proteins that reduce oxidative stress are detected in aged RPE (Gu et al., 2012). RPE mitochondria in aged mice have elevated levels of glycolytic intermediates and decreased lipid levels, suggesting a decrease in mitochondrial efficiency and transition to fatty-acid metabolism (Wang et al., 2018). RPE cells rely on oxidative metabolism (reviewed in Lakkaraju et al., 2020), so any imbalance could affect normal function. Interestingly, aged monkey RPE have fewer mitochondria, and the ones that remain are abnormally elongated (Gouras et al., 2016). It has been previously shown that mitochondrial enlargement/elongation is associated with senescence and oxidative stress (Mai et al., 2010; Yoon et al., 2006), as aged RPE cells produce a high amount of reactive oxygen species (ROS) (reviewed in Bellezza, 2018). While ROS are required for redox signaling that is vital to RPE health, physiological levels can become pathogenic in aged RPE, although the transition point between maintaining RPE health and pathogenesis has yet to be determined (reviewed in Datta et al., 2017). Critical antioxidative enzymes in the RPE include members of the superoxide dismutase (SOD) family, catalase, and glutathione enzymes (reviewed in Newsome et al., 1994).
Results from rodent model systems corroborate many phenotypes observed in aged human RPE: accumulation of lipofuscin and drusen and enlarged RPE cell cytoplasm have all been reported (Feeney-Burns et al., 1984). In vivo studies also highlight the characteristic increase in Bruch’s membrane thickness in older patients (Harris et al., 2017). Bruch’s membrane thickens as a result of increased protein and lipid deposition and decreased degradation of ECM (reviewed in Zarbin, 2004). Bruch’s membrane thickening leads to decreased permeability and flow of nutrients from the choriocapillaris to RPE, and decreased export of RPE waste, contributing to RPE stress. This phenotype seems to be largely limited to aging RPE (reviewed in Ardeljan and Chan., 2013) and has been reported in aging mouse and rat models (Ivert et al., 2005, Fan et al., 1996). However, some mammalian models of AMD have also reported increased thickness of Bruch’s membrane as a result of disease progression (Seo et al., 2012; Ding et al., 2011). Nonetheless, Bruch’s membrane thickening remains an aging phenotype, as advanced stages of AMD involve choroidal vasculature invasion through Bruch’s membrane, resulting in Bruch’s membrane thinning and deterioration (Bird et al., 1995).
Increased ratios of multinucleate RPE are observed in aged mice (Chen et al., 2016) and aged human eyes, specifically in the perifoveal region of the latter (Starnes et al., 2016). As discussed previously, the implications of multinucleation in human eyes are still under debate; multinucleation could contribute to enlarged cytoplasmic space to compensate for RPE cell loss. Together, these data suggest that mature mammalian RPE might maintain some proliferative potential, and if this ability could be harnessed, endogenous tissue repair might be possible.
5. REGENERATIVE RESPONSES IN RPE
As discussed above, a range of cellular and systemic responses are triggered by RPE injury, including signaling cascades (e.g. leading to EMT), changes in the cell biology of RPE and surrounding tissues, activation of an immune response, and RPE proliferation, among others. Resolution of these injury responses is critical to restoring tissue homeostasis, thus many of the same injury responses also contribute to RPE repair and regeneration. Here, we discuss what is known about RPE repair and regenerative responses in mammals and non-mammalian vertebrates, some of which possess remarkable abilities to regenerate retina and/or RPE after injury (Fig. 3).
Figure 3. RPE-dependent repair and regeneration in mammalian and non-mammalian systems.

Models of repair and regenerative responses in mammals (A); the newt, Cynops pyrrhogaster (B); the frog, Xenopus laevis (C); and zebrafish (D). (A) In mammals, after transgenic ablation or pharmacological injury of the RPE, remaining RPE cells are unable to regenerate. (B) In adult C. pyrrhogaster, upon retinectomy RPE cells undergo reprogramming, re-enter the cell cycle, and convert into a multipotent state through the regulation of MEKERK/β-catenin and Pax6 signaling after retinectomy. The multipotent cells segregate into two layers; the outer layer renews the RPE while the inner layer regenerates NR. (C) In adult X. laevis, upon retinectomy, with RVM present, RPE cells detach from each other and BM, express Pax6, and migrate towards the RVM to regenerate NR. RPE cells that remain attached to BM replenish the RPE layer. (D) In zebrafish, rpe65a:nfsB-eGFP-mediated transgenic ablation of large swathes of RPE results in the proliferation of injury-adjacent RPE cells that subsequently regenerate lost RPE tissue in a peripheral to central fashion. Abbreviations: NR, neural retina; BM, Bruch’s Membrane; RVM, retinal vascular membrane; RPE, retinal pigment epithelium; pRPE, peripheral retinal pigment epithelium; dRPE: dedifferentiated retinal pigment epithelium; TZ: transition zone. (B, modified from Chiba, 2014; D, modified from Hanovice et al., 2019).
5.1. RPE repair and regeneration in mammals
Mammalian RPE is normally non-self-renewing, but as discussed above, peripheral RPE cells do have intrinsic proliferative capacity in mice, rats, and pigs (Al-Hussaini et al., 2008; Kokkinopoulos et al., 2011; von Leithner et al., 2010; Kiilgaard et al., 2007). Indeed, RPE repair in mammals depends much on the severity of injury (Table 2). The RPE heals after debridement in animal models like pig and rabbit, but data suggest that only small lesions can be repaired by migration and proliferation of injury-adjacent RPE (Del Priore et al., 1995, Grierson et al., 1994, Lopez et al., 1995). In particular, in MRL/MpJ mice, a mammalian model with extraordinary regeneration abilities (Clark et al., 1998), robust RPE regeneration and full recovery of retinal function were observed one month after NaIO3 -induced RPE injury at low-dose NaIO3 injection, while little RPE regeneration occurred after high-dose NaIO3 injection (Xia et al., 2011). Intravenous, low-dose NaIO3 treatments have been shown to induce patchy RPE loss or central RPE damage with preservation of the peripheral RPE, while high-dose treatments cause complete RPE ablation and progressive retinal degeneration (Franco et al., 2009, Machalińska et al., 2010). This suggests that peripheral RPE are vital for repair and regeneration, but the roles that they play in this process remain to be defined. Consistently, Machalińska et al. (2014) observed complete RPE regeneration in mice 3 months post-injury in low-dose NaIO3 -treated animals, while irreversible degeneration occurred at high doses (Machalińska et al., 2014). Transgene-mediated RPE ablation in mice has also been utilized to study RPE degeneration, repair, and regeneration. In adult RPECreER/DTA mice, two weeks post-tamoxifen administration, surrounding RPE cells enlarged to fill space vacated by lost RPE but no proliferation or regeneration was observed (Longbottom et al., 2009). The RPE does have the potential to proliferate and regenerate, however, as subretinal injection of a viral vector containing E2F2 induced limited RPE proliferation and regeneration after genetic ablation in adult RPECreER/DTA mice, indicating that mammalian RPE are capable of overcoming intrinsic barriers to regeneration (Kampik et al., 2017).
Table 2.
RPE-dependent repair and regenerative responses
| Injury Type | Model System | Age | Regenerative Ability | RPE regenerative Responses | References | |
|---|---|---|---|---|---|---|
| RPE-to-RPE | RPE-to-Retina | |||||
| Genetic RPE ablation | Zebrafish | Larvae and adult | yes | no | Lose epithelia characteristic; RPE proliferates in a peripheral to central fashion to regenerate a functional RPE layer | Hanovice et al., 2019 |
| Mouse | Infants and adult | no | no | Regenerative capacity in RPECreER/DTA mice was not observed. | Longbottom et al., 2009 | |
| NaIO3 injection | Mouse | Adult | yes | no | Low-dose NaIO3 treated mice display RPE regeneration. | Machalinska et al., 2014; Machalinska et al., 2010 |
| Mouse | Adult | yes | no | RPE regenerates with complete retinal function upon low dose of NaIO3 in MRL/MpJ mice. | Xia et al., 2011; | |
| Retinectomy | Newt | Juvenile and adult | yes | yes | Lose epithelial characteristic; RPE cells enter a multipotent state, form “pro-RPE layer” and “pro-NR layer” and subsequently regenerate NR and RPE | Chiba et al., 2006; Islam et al., 2014 |
| Frogs | Adult | yes | yes | Transdifferentiated RPE cells migrate onto RVM to generate NR, RPE cells that remain attached to BM renew RPE layer | Yoshii et al., 2007; Kuriyama et al., 2009 | |
| Chicken | E4–4.5 | no | yes | Transdifferentiated RPE does not produce RPE cells | Coulombre & Coulombre, 1965 | |
| E2F2 injection using lentiviral vector | Mouse | Juvenile and adult | limited | no | Limited RPE proliferation and regeneration | Kampik et al., 2017 |
Abbreviations: RPE, retinal pigment epithelium; NR, neural retina; DTA, diphtheria toxin subunit A, NaIO3, sodium iodate; BM, Bruch’s membrane
Interestingly, a study investigating the efficacy of human central nervous system stem cell (HuCNS-SC) replacement therapy in the RCS rat model of retinal degeneration found that host RPE proliferated when in proximity to transplanted HuCNS-SCs (McGill et al., 2012). Subsequent investigations demonstrated that proliferation occurred in RPE cells adjacent to HuCNS-SCs that were injected into the SRS of a rat model of retinitis pigmentosa, further supporting the ability of HuCNS-SCs to stimulate host RPE proliferation (McGill et al., 2019). In humans, under physiological conditions, the RPE remains non-proliferative and RPE loss cannot be compensated by cellular regeneration. Nevertheless, when human cadaver-derived adult RPE cells were cultured in vitro with appropriate growth conditions, RPE cells proliferated and were able to generate an RPE monolayer, in addition to neural and mesenchymal cells types, suggesting the presence of an RPE stem cell in the human eye (Salero et al., 2012; reviewed in Saini et al., 2016).
5.2. RPE regeneration and RPE-dependent retinal regeneration in non-mammalian systems
The regenerative capacity of RPE has been studied in amniotes (mammals and birds) and anamniotes (amphibians and fish). Unlike in mammals, there is a range of regenerative responses in the RPE of non-mammalian systems. Most studies, however, have experimentally characterized the ability of the RPE to transdifferentiate into retina after retinal injury or retinectomy, so-called RPE-to-retina regeneration. By contrast, examples of intrinsic RPE regeneration (RPE-to-RPE) have thus far been limited to studies in zebrafish (Hanovice et al., 2019). Here we discuss what is known about RPE-dependent regenerative responses, both RPE-to-RPE and RPE-to-retina; for the latter, we focus on how it relates to the ability of RPE cells to reprogram, transdifferentiate, and repair retinal damage. Indeed, the inability of RPE to reprogram and transdifferentiate could be an intrinsic barrier to endogenous RPE regeneration in mammals. We also point interested readers to several excellent reviews that delve further into RPE-to-retina regeneration in non-mammalian systems (reviewed in Ail and Perron, 2017; Grigoryan, 2016; Araki, 2007; Chiba, 2014; Wang et al., 2010; Barbosa-Sabanero, 2012).
Amniotes (Birds)
RPE-to-retina regeneration has been extensively studied in the chicken, a non-mammalian amniote. Unlike the adult chicken, embryonic chicks can regenerate all of the retinal layers after retinectomy during a limited window in their development, around E4 (Hamburger and Hamilton stages 22–24.5) (Coulombre and Coulombre, 1965). The sources of regenerated retina are transdifferentiated RPE and the ciliary body/ciliary marginal zone (CB/CMZ) at the periphery of the retina (Coulombre and Coulombre, 1965, Spence et al., 2004). Members of the FGF family stimulate transdifferentiation of RPE cells through MEK–ERK signaling to induce retinal regeneration after retinectomy, while Shh and activin signaling may impair the transdifferentiation process (Park and Hollenberg, 1989; Park and Hollenberg, 1991; Spence et al., 2004; Spence et al., 2007; Sakami et al., 2008). During transdifferentiation, chick RPE cells lose their epithelial characteristics, depigment, and proliferate. RPE-derived cells form a neuroepithelial layer that then differentiates into retinal tissue (Spence et al., 2007; Spence et al., 2004). The transdifferentiated RPE regenerates a retina with reversed polarity which occurs primarily in the posterior part of the OC (Coulombre and Coulombre, 1965). As the retinal layers are reversed, the rod and cone photoreceptors are located in the innermost layer of the retina, closest to the lens. Thus, the RPE transdifferentiates to form a retina, but does not self-regenerate, resulting in an eye that lacks the RPE layer (Coulombre and Coulombre, 1965). However, regenerated retinal tissue derived from the CB/CMZ develops with correct polarity and in the presence of a pigmented RPE layer (Luz-Madrigal et al., 2014; Luz-Madrigal et al., 2020; Spence et al., 2004). Based on these studies, intrinsic RPE-to-RPE regeneration cannot be studied in chicks, at least using current models and injury paradigms.
Anamniotes (Amphibians and Fish)
Amphibians:
Urodele (Newt) –
Newts are capable of regenerating a completely functional retina after traumatic eye injury or retinectomy. In adult newts, the primary source of regenerating retina is RPE cells while retinal stem cells and retinal progenitor cells present in the CMZ regenerate only peripheral aspects of the retina (Chiba et al., 2006, Ikegami et al., 2002). In the adult Japanese fire-bellied newt, Cynops pyrrhogaster, RPE cells reprogram after retinectomy to produce RPE cells and all cell types of the retina (Chiba et al., 2006; Islam et al., 2014). While definitive lineage tracing has not been performed in this system, the source of regenerating retinal cells in the posterior eye of C. pyrrhogaster is likely to be the RPE as the peripheral retina is removed during retinectomy.
The molecular mechanisms underlying the ability of RPE cells to transdifferentiate and re-enter the cell cycle after retinal injury have been studied in vitro and in vivo and are beginning to be understood. Upon retinectomy, RPE cells reprogram and transdifferentiate in a process that involves detachment from each other and from Bruch’s membrane, loss of epithelial characteristics, and reentry into the cell cycle (Stage E-1; between days 5 and 10 post-retinectomy) (Chiba et al., 2006). In amphibians, it is thought that upon injury, mature RPE cells re-enter the cell cycle due to loss of cell-cell contacts (reviewed in Chiba, 2014). As discussed above, in humans and other mammalian models, loss of RPE cell-cell contact contributes to EMT and subsequent pathology, suggesting that events downstream of cell-cell contact attenuation could also contribute to the different RPE repair/regenerative responses in amphibians and mammals. The MEK–ERK pathway has also been shown to play a role in re-entry of mitotically quiescent RPE cells into the cell-cycle both in vivo and in vitro. MEK–ERK pathway activity is rapidly upregulated (within 30 min) after retinectomy in RPE cells (Mizuno et al., 2012).
During RPE reprogramming and transdifferentiation, RPE cells enter a multipotent state (defined as RPE stem cells; RPESC) (Islam et al., 2014). RPESCs uniformly express the neuronal stem cell marker, Musashi-1 (Kaneko and Chiba, 2009), multipotency factors c-Myc and Klf4, and early eye development markers such as Sox2, Pax6, and Mitf (Islam et al., 2014); however, RPESCs maintain RPE65 protein expression (Chiba et al., 2006). Transcriptome analyses of early transdifferentiating RPE cells (Stage E-0 to E-2) also demonstrated that the expression of genes associated with differentiated RPE cells such as RPE65, CRALBP/RLBP1, ZO1, Otx2, and Musashi1a/c gradually decrease between E-0 and E-2 post-retinectomy, while genes associated with cell-cycle progression such as Cyclin D1, CDK4, Histone H3, and those associated with growth factor signaling like FGFR1 and FGFR3 are upregulated (Nakamura et al., 2014). RPESCs subsequently re-differentiate to form two epithelial layers that have correct polarity and partial pigmentation, a “pro-RPE layer” and “pro-retina layer”, which renew the RPE and regenerate the retina, respectively (Islam et al., 2014). Pax6 appears to play a critical role during RPE cell reprogramming. RPESCs from juvenile newts (around 7 months old) in which Pax6 was knocked down were unable to self-organize into two epithelial layers and did not differentiate into pro-retina or pro-RPE layers; instead, RPESCs underwent EMT and formed myofibroblasts, similar to what occurs in RPE injury and disease in mammals (Casco-Robles et al., 2016; Chaudhary et al., 2020; Yang et al., 2018). The pro-RPE layer exits the cell cycle and reinitiates pigmentation, while the pro-retina layer continues to proliferate (Stage E-3; day 19 post retinectomy). RPE65 immunoreactivity decreases in both layers at Stage E-3 but increases as the pro-RPE layer matures. Between days 45 and 65 after retinectomy, regeneration is complete and the regenerated retina and RPE have identical morphology and characteristics to those in intact eyes (Islam et al., 2014).
The mechanisms underlying RPE cell proliferation and cell cycle re-entry have also been studied in vitro using a retina-less eyecup (RLEC) culture system (Susaki and Chiba, 2007). In the RLEC system, under serum-free culture, the retina is removed from the posterior half of the eyeball and after 10 days in culture, RPE cells become mitotically active and behave as they do in vivo during retinal regeneration (Yoshikawa et al., 2012). In RLEC, MEK–ERK signaling is activated within 1 hour post-retinectomy (Yoshikawa et al., 2012) and β-catenin-positive nuclei increase in RPE cells when cell–cell contacts are disrupted by incision or treatment with ethylene glycol tetraacetic acid (EGTA) (Yasumuro et al., 2017), similar to what is observed in mouse RPE after NaIO3 injection (Yang et al., 2018). Presumably, β-catenin nuclear translocation contributes to transcriptional regulation of injury-responsive genes in RPE cells, but this has not yet been addressed experimentally. In vitro studies also demonstrate that interaction between the RPE and choroid are likely to play a critical role during RPE-to-retina regeneration. Under organotypic culture conditions, explanted adult RPE and surrounding connective tissue, including the choroid, were co-cultured to understand mechanisms involving RPE proliferation and transdifferentiation (Ikegami et al., 2002). When the choroid was included in these organ cultures, RPE transdifferentiated into retinal tissue, but not when the choroid was absent (Mitsuda et al., 2005). FGF2 and IGF-1, amongst other factors, were shown to be involved in this inductive process.
Anurans (Frogs) –
Adult Xenopus laevis (3 to 9 months after metamorphosis), regenerate their retina after retinectomy through RPE-to-retina transdifferentiation, like newts (Yoshii et al., 2007; reviewed in Ail and Perron, 2017). RPE-to-retina transdifferentiation during regeneration is not uniform across frog species; however, as Xenopus tropicalis regenerates its retina after retinectomy from the CMZ through a process that does not involve RPE transdifferentiation (Miyake and Araki, 2014). In X. laevis, RPE transdifferentiation only occurs if the retinal vascular membrane (RVM) (consisting of capillary rich basal membrane bounding the inner margin of the retina) is retained after retinectomy (Yoshii et al., 2007). After retinectomy, RPE cells migrate to the RVM, which facilitates RPE transdifferentiation, although the inducing factors and molecular mechanisms present in the RVM are not fully understood. However, retinectomy that also includes removal of the RVM, followed by FGF2 induction, successfully results in a regenerated retina indicating that FGF2 is sufficient in this regard (Vergara and Del Rio-Tsonis, 2009). FGF2 activates the MEK-ERK pathway and similar to newt RPE-to-retina regeneration (Yoshikawa et al., 2012), the MEK-ERK pathway is activated during the early stages of the regenerative response. Moreover, inhibition of MEK signaling significantly impairs RPE-to-retina regeneration in frogs (Vergara and Del Rio-Tsonis, 2009), again highlighting the potential importance of MEK-ERK activity in modulating RPE-derived injury and regenerative responses.
Upon retinectomy, it is Pax6-positive pigmented RPE cells that detach from Bruch’s membrane and migrate towards the RVM (Kuriyama et al., 2009; Yoshii et al., 2007). At the RVM, RPE-derived cells undergo transdifferentiation, become stem-cell-like, and form a neuroepithelium (Kuriyama et al., 2009). Loss of cell-cell contact between RPE cells and underlying Bruch’s membrane results in upregulation of Rax expression followed by Pax6 expression (Martinez-De Luna et al., 2011; Nabeshima et al., 2013). The Pax6-positive RPE-derived epithelial layer transdifferentiates into retinal tissue along the RVM. RPE cells that remain attached to Bruch’s membrane are Pax6-negative and subsequently repair and renew the RPE itself; however, the molecular mechanisms underlying this process are not known (Kuriyama et al., 2009; Yoshii et al., 2007). Loss of contact between RPE cells and the choroid appears to play a role in triggering Pax6 expression. RPE cells become Pax6-positive once they migrate away from choroid, although the choroid could also be inductive in this regard (Kuriyama et al., 2009). FGF2 is essential for sustained Pax6 expression, as it promotes differentiation of RPE-derived proliferating progenitors into retina (Kuriyama et al., 2009). In comparison, in humans and other mammals, loss of contact between RPE cells and the choroid triggers EMT, leading to PVR (reviewed in Chiba, 2014).
Zebrafish –
Zebrafish have a robust intrinsic potential to regenerate multiple tissue and organ types after damage, including the retina (reviewed in Goldman, 2014; Karl and Reh, 2010; Gao et al., 2021). Recent work demonstrates that both larval and adult zebrafish are also able to fully regenerate RPE after widespread RPE injury (Hanovice et al., 2019). Experimentally, a transgenic system was developed in zebrafish through which large swathes of mature RPE could be ablated and regeneration assessed (Fig. 4). RPE ablation resulted in rapid apoptosis of RPE, followed by degeneration of photoreceptors (Fig. 5) and Bruch’s membrane, and loss of visual function (Hanovice et al., 2019). After ablation, the RPE regenerates in a peripheral-to-central manner (Fig. 6), driven by proliferative cells that first appear in the periphery and over the duration of the regenerative response are detected more centrally as the injury site is repopulated (Fig. 7). The cellular source of the regenerated RPE is not yet known but two candidates are peripheral RPE that is spared from ablation or cells of the CMZ, which are known to generate both retina and RPE progenitor cells (Reinhardt et al., 2015).
Figure 4. RPE ablation paradigm in zebrafish:
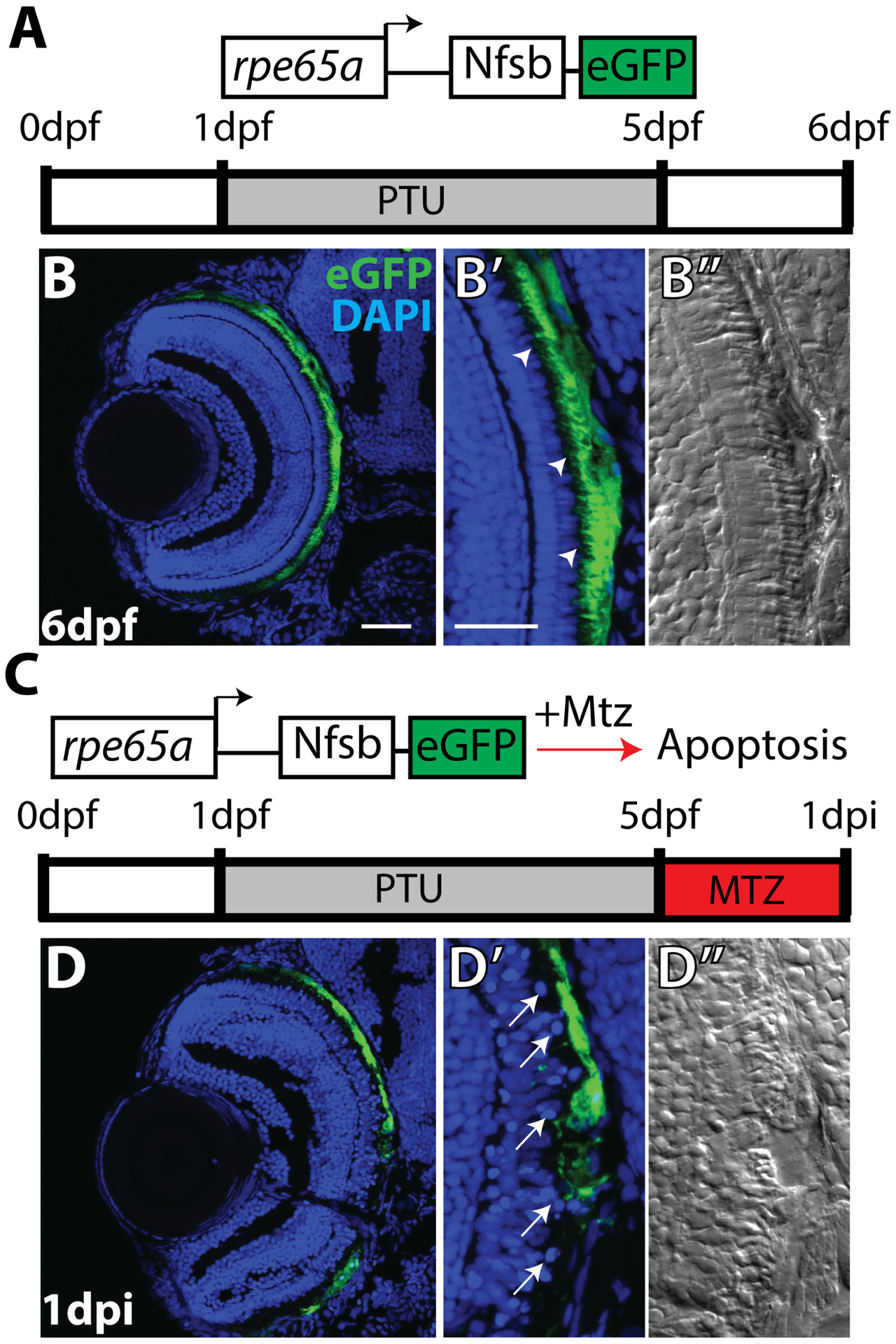
(A) Cartoon depicting the rpe65a:nfsB-eGFP transgene and treatment course of unablated embryos. (B) Transverse cryosections of an unablated 6dpf larva. (B,B’) After exposure to PTU between 1–5dpf, transgene expression is specifically restricted to mature RPE cells, with the brightest expression confined to the central two-thirds of the RPE. Arrowheads indicate apical microvilli. (B”) DIC images reveal RPE repigmentation and normal photoreceptor layer architecture. (C) Cartoon depicting the nitroreductase-mediated ablation paradigm: after washing out PTU, larvae were treated with MTZ for 24 hours. Within cells expressing the transgene, nfsB converts MTZ into a potent DNA crosslinking agent and induces cell death. (D,D’) Transverse cryosections of a 1dpi larva reveal significant disruption of eGFP+ cell morphology and disorganization in INL nuclear lamination. Arrows indicate delaminated and pyknotic nuclei. (D”) DIC images reveal a lack of RPE pigmentation and the marked disruption of photoreceptor layer architecture. Abbreviations: dpf, days post-fertilization; PTU, phenylthiourea; RPE, retinal pigment epithelium; MTZ, metronidazole; nfsB, nitroreductase; dpi, days post-injury; INL, inner nuclear layer. Green=eGFP, blue=nuclei. Dorsal is up and distal is left. Scale bar = 40mm. Figure taken from Hanovice et al., 2019.
Figure 5. Ablation of the RPE in zebrafish leads to degeneration of underlying photoreceptors.
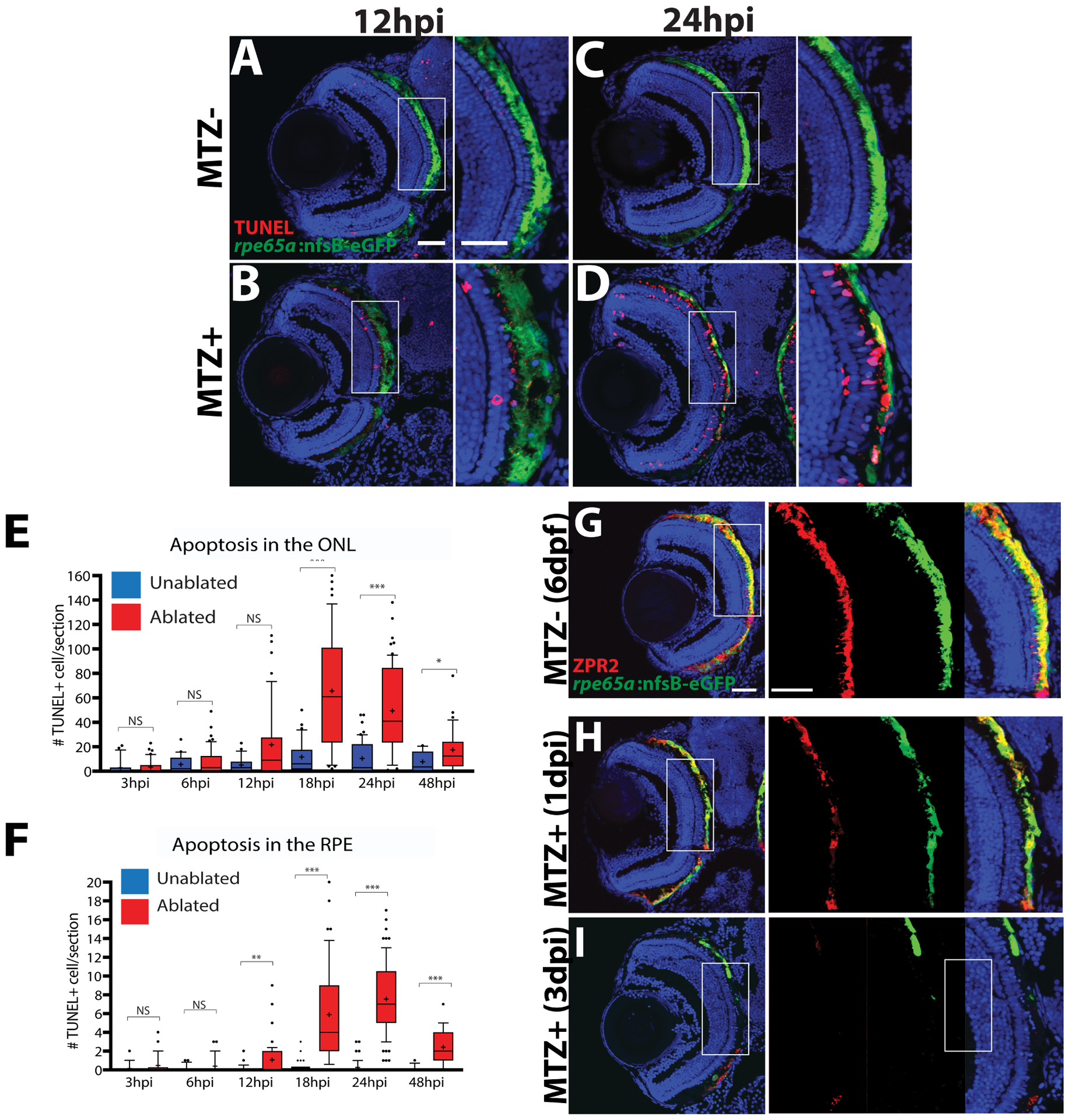
(A-D) Transverse cryosections stained for TUNEL (red). Compared to untreated (A,C) larvae, ablated RPE were disrupted by 12hpi (B), and TUNEL+ cells appeared throughout the RPE and ONL at 24hpi (D). (E, F) Quantification of TUNEL+ cells/section in the RPE (E) and ONL (F) revealed a significant increase in the RPE by 12hpi and in the ONL by 18hpi. Significance determined using Mann-Whitney U test. * p≤0.05, ** p<0.005, *** p<0.0005. (G-I) Transverse sections of unablated 6dpf larvae stained for ZPR2, an RPE marker (red, G). By 1dpi, ZPR2 is disrupted in a similar manner to eGFP (H). By 3dpi, ZPR2 signal is absent from the central injury site (I). Abbreviations: RPE, retinal pigment epithelium; hpi, hours post-injury; dpf, days post-fertilization; ONL, outer nuclear layer; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling. Green=eGFP, blue=nuclei. Dorsal is up and distal is left. Scale bar = 40mm. Figure modified from Hanovice et al., 2019.
Figure 6. RPE regeneration in zebrafish initiates in the periphery and proceeds inward.
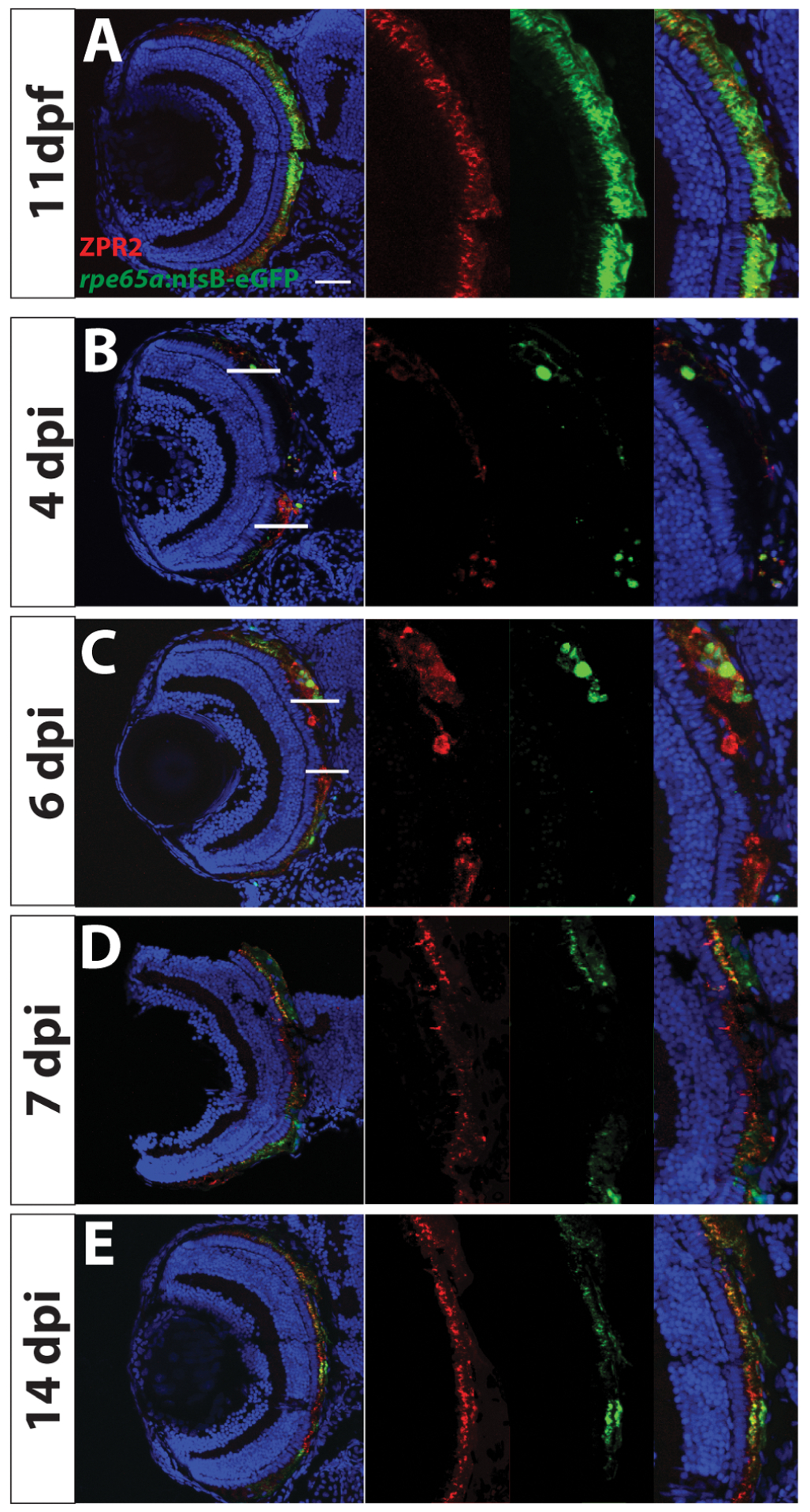
Transverse sections of unablated larvae stained for the RPE marker ZPR2 (A) at 11dpf. Ablated eyes stained for ZPR2 at 4, 6, 7 and 14dpi (B-E). Green=eGFP, blue=nuclei, red=ZPR2. eGFP+ RPE appears in the periphery at 4dpi (marked by lines at dorsal and ventral margins in B,C). As regeneration proceeds, eGFP+ RPE extends further toward the eye center, and the leading tip of the regenerated monolayer often consists of both immature and mature RPE (ZPR2+/eGFP− cells in C). By 7dpi, ZPR2+ RPE is present throughout the RPE (D). By 14dpi, mature eGFP+/ZPR2+ RPE cells are present throughout the RPE (E). Dorsal is up and distal is left. Abbreviations: RPE, retinal pigment epithelium; dpf, days post-fertilization; dpi, days post-injury. Scale bar = 40mm. Figure modified from Hanovice et al., 2019.
Figure 7. RPE regeneration in zebrafish involves a robust proliferative response.
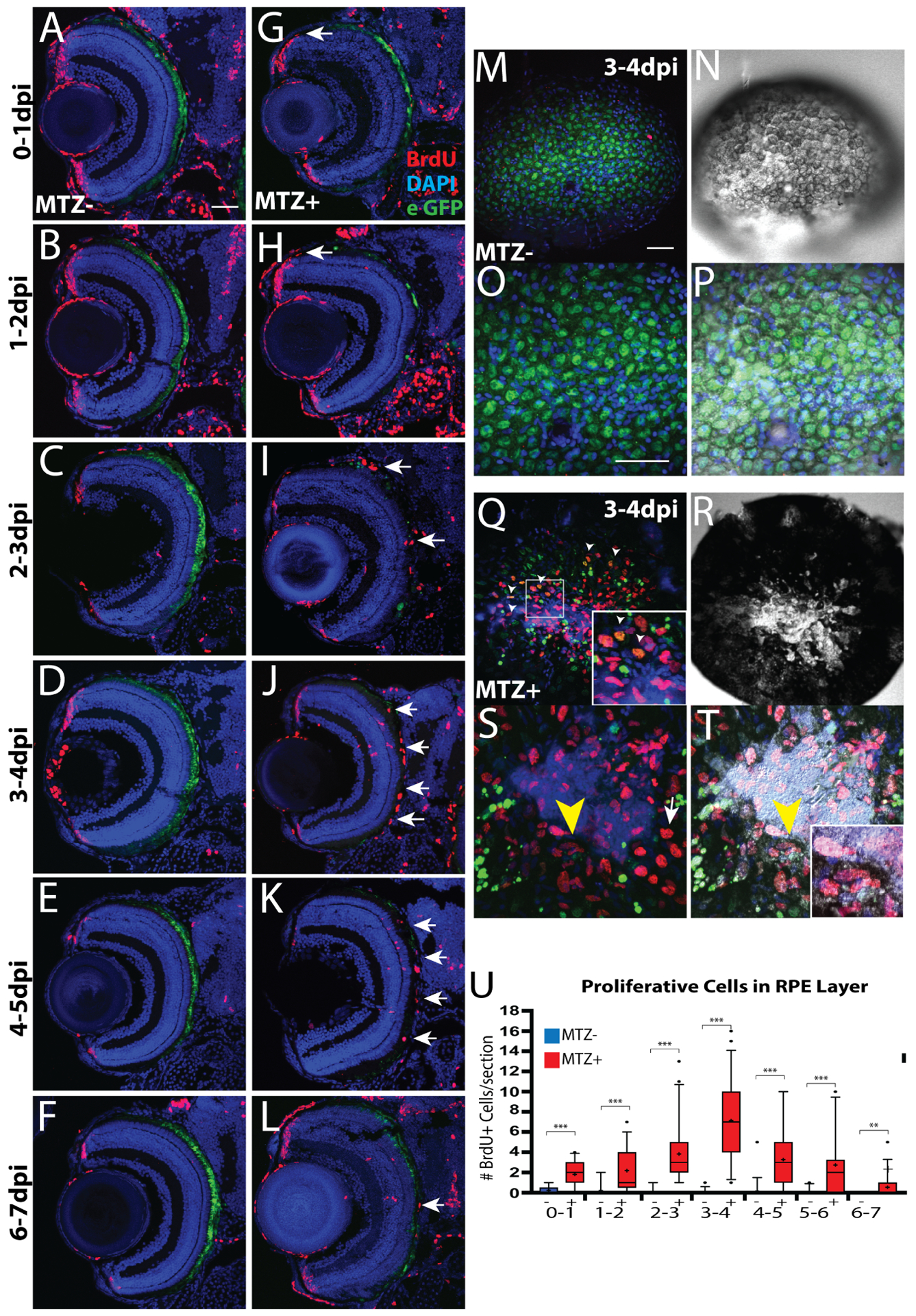
(A-L) Transverse retinal sections of unablated (A-F) and ablated (G-L) larvae exposed to 24-hour BrdU pulses at various days post-injury. BrdU+ cells first appear in the periphery as early as 0–1dpi (arrow, G), and 1–2dpi (arrow, H). As regeneration proceeds, BrdU+ cells appear closer to the central injury site and at the inner tip of the regenerating RPE layer (arrows, I). BrdU+ cells then populate the injury site (arrows, J-L). (M-T) en face wholemount images of unablated (M-P) and ablated (Q-T) eyes from larvae exposed to BrdU between 3–4dpi. White arrowheads in (Q) and (inset, Q) indicate BrdU+/eGFP+ cells near the injury site. Yellow arrowhead in (S) and (T) indicate BrdU+ cells proximal to the injury site that are beginning to become pigmented. (Inset, T) Magnified image of BrdU+, pigmented cells. (U) Quantification of total number of BrdU+ cells/section in the RPE reveals an increase of BrdU+ cells in the RPE starting at 0–1dpi and peaking at 3–4dpi. Mann-Whitney U Test, * p<0.05, ** p<0.005, *** p<0.0005. Dorsal is up and distal is left. Abbreviations: dpi, days post-injury; RPE, retinal pigment epithelium; BrdU, bromodeoxyuridine. Scale bar = 40mm. Figure modified from Hanovice et al., 2019.
This zebrafish RPE ablation and regeneration paradigm is a powerful in vivo system through which the molecular underpinnings of endogenous RPE regeneration can be identified. Wnt signaling was shown to play a role in RPE regeneration as the Wnt target gene lef1 was upregulated post-ablation and disruption of Wnt pathway activity reduced proliferation post-injury and impaired the regenerative response (Fig. 8; Hanovice et al., 2019). Interestingly, Wnt/β-catenin signaling was activated after laser photocoagulation in RPE cells of the mouse eye and the Wnt targets, Otx2 and Mitf, were upregulated (Han et al., 2015). Moreover, inhibition of Wnt pathway activity impaired RPE cell proliferation and EMT, suggesting that Wnt activity may be a common mechanism involved in RPE proliferation and repair. Indeed, nuclear β-catenin accumulates in RPE cells in mouse and newt models when RPE cell-cell adhesions are perturbed supporting a potential role in the injury and RPE-to-retina transdifferentiation pathways, respectively, in these systems (Han et al., 2015, Yasumuro et al., 2017). Results in the chicken, however, indicate that β-catenin in the RPE suppresses the ability of RPE to regenerate retinal tissue after retinectomy (Zhu et al., 2014), so there are also context-dependent roles for Wnt/β-catenin activity in various models of RPE injury and regeneration that could modulate cellular responses. Finally, recent genomic analyses of the regenerative response in zebrafish identified a number of immune-related signaling pathways activated in RPE after injury and a direct role for macrophages and microglia as key mediators of intrinsic RPE regeneration (Leach et al., 2020). While much more needs to be done in this system, these early results point to an interplay between components of the immune system and RPE after injury and during regeneration, a link that has also been made during retinal regeneration (Silva et al., 2020; White et al., 2017) as well as regeneration in other tissues and organs (reviewed in Mescher, 2017; Mescher et al., 2017).
Figure 8. Pharmacological inhibition of Wnt pathway activity using IWR-1 impairs RPE regeneration in zebrafish.
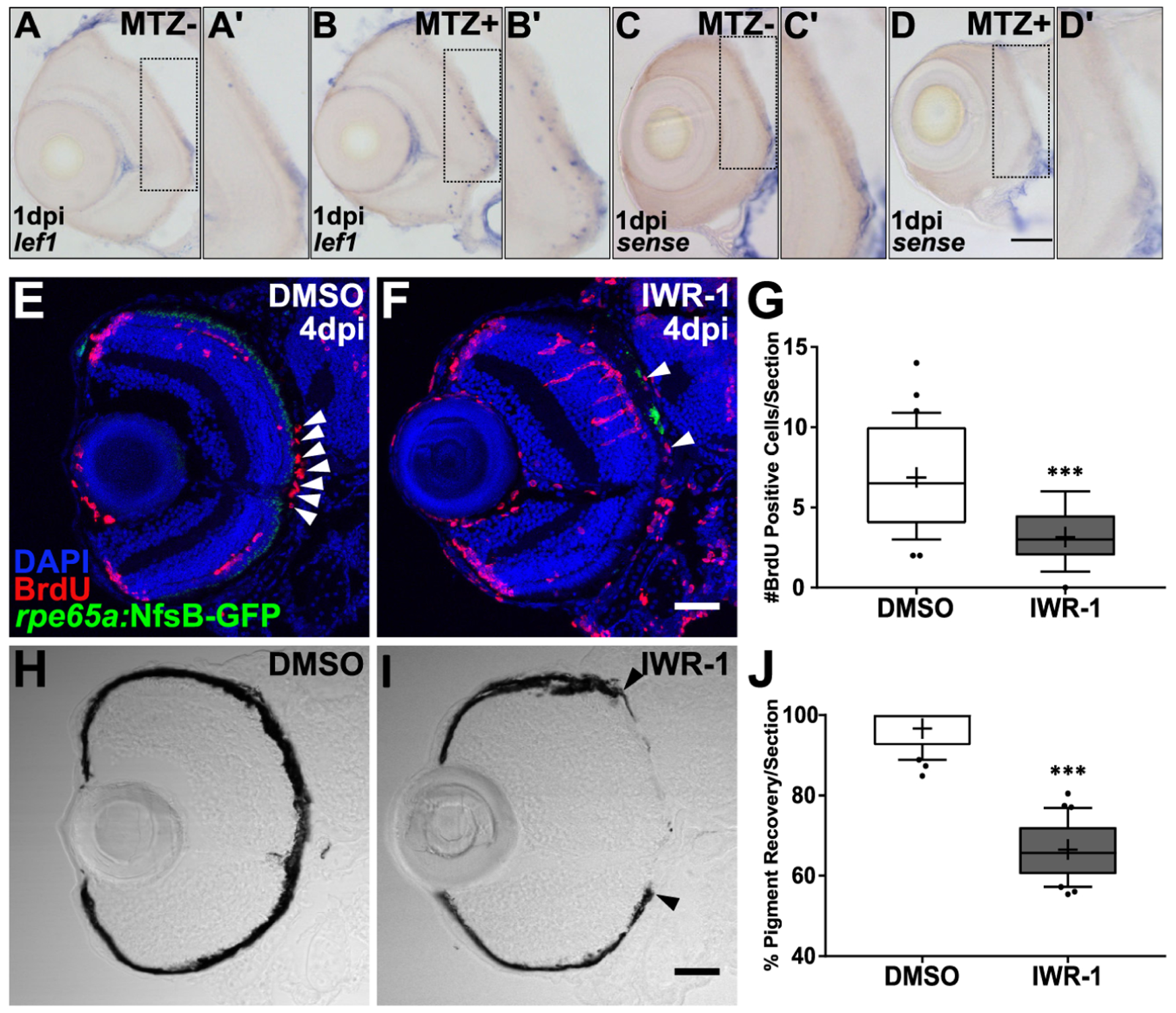
(A-D) Transverse sections of lef1, a marker of Wnt pathway activity, or sense RNA expression in unablated 6dpf (MTZ−) and ablated 1dpi (MTZ+) larvae. lef1 is detected in and around the RPE in MTZ+ (B’) but not MTZ- larvae (A’). lef1: n>5; lef1 sense: n=4. (E-J) Transverse sections of 4dpi ablated DMSO- (E,H; n=10) and 15μM IWR-1-treated (F,I; n=11) larvae exposed to a 24-hour pulse of BrdU from 3–4dpi. (E,F) Green=eGFP, blue=DNA, red=BrdU; white arrowheads highlight BrdU+ cells in the RPE. (G) Quantification of BrdU+ cells/section reveals that IWR-1 treatment significantly decreases the number of proliferative cells in the RPE at 4dpi (Student’s unpaired t-test, *** p<0.0001). Brightfield images (H,I) and quantification of percent RPE recovery/section (J) shows a significant delay in recovery of a pigmented monolayer in IWR-1 treated larvae (Student’s unpaired t-test, *** p<0.0001). (I) Black arrowheads indicate the central-most edge of the regenerating RPE. Abbreviations: dpf, days post-fertilization; MTZ, metronidazole; dpi, days post-injury; RPE, retinal pigment epithelium; BrdU, bromodeoxyuridine; DMSO, dimethylsulfoxide. Dorsal is up and distal is left. Scale bars = 40μm. Figure modified from Hanovice et al., 2019.
6. MECHANISMS INVOLVED IN REPROGRAMMING AND REGENERATION
A common theme in non-mammalian systems that possess the capacity to regenerate lost or damaged tissues is the reprogramming of differentiated cells to a progenitor-like state, which then proliferate and regenerate lost tissue (Tai et al., 2020; Fang et al., 2018). Reprogramming refers to the process through which a differentiated somatic cell is converted into a multipotent or pluripotent state, so called induced pluripotent stem cells (iPSCs) (Gurdon, 1962; Takahashi and Yamanaka, 2006), or converted directly into an unrelated terminally differentiated cell type (reviewed in Srivastava and DeWitt, 2016; Fang et al., 2018). The reprogramming process has been studied extensively in many cell types and organs (reviewed Chen et al., 2020), including the retina, where Müller glia of zebrafish and Xenopus are able to regenerate damaged retinal neurons (reviewed in Lahne et al., 2020; Garcia-Garcia et al., 2020). Comparatively less is known about reprogramming in the RPE, but recent studies in the chicken have begun to shed some light on this process during RPE-to-retina regeneration (Luz-Madrigal, et al., 2014; Luz-Madrigal, et al., 2020). Here, the differential expression of genes associated with epigenetic modification, histone markers, and DNA methylation/demethylation in reprogramming-competent RPE before injury and at different phases post-retinectomy was quantified. Significant changes of bivalent chromatin marks (decrease of H3K27me3 and increase of H3K4me3) and in DNA demethylation activity during early phase of reprogramming of intact RPE to transient reprogrammed RPE were detected, as well as during late phases of reprogramming of RPE to neuroepithelium in response to retinectomy and FGF2 addition. Ten-eleven translocation 3 (TET3), one of the TET family of enzymes that catalyze DNA hydroxymethylation, and potentially contributing to DNA demethylation, was found to be significantly upregulated at 24hpi in RPE cells. Overexpression of TET3 was sufficient to reprogram RPE by 3 days post-retinectomy without the addition of exogenous FGF2, suggesting that DNA hydroxymethylation and/or demethylation might play a role in the reprogramming of RPE cells during RPE-to-retina transdifferentiation (Luz-Madrigal, et al., 2014; Luz-Madrigal, et al., 2020). Consistently in zebrafish, DNA demethylation events predominate at early times during MG reprogramming and fin regeneration after retinal injury or fin amputation, although the functional importance of these changes remains unknown (Hirose et al., 2013, Powell et al., 2013).
7. BARRIERS TO RPE-DEPENDENT REGENERATION
As discussed above, newts, X. laevis, zebrafish, and chickens are regeneration-competent non-mammalian systems in which RPE have the capacity to regenerate lost retina or RPE tissue. In newts, X. laevis, and chickens, RPE undergo reprogramming and transdifferentiation after retinal injury to regenerate lost retinal tissue. However, RPE-to-RPE regeneration either does not occur (in chickens) or is limited to solely replenishing RPE cells that undergo transdifferentiation to regenerate retinal tissue (in X. laevis and newts). Zebrafish do not regenerate retinal tissue from the RPE after injury, instead doing so through Müller glia, but they are able to intrinsically regenerate RPE through what appears to be an RPE-to-RPE dependent process. Reprogramming and transdifferentiation of RPE are not observed in mammalian systems and RPE-to-RPE or RPE-to-retina regeneration do not occur, with the exception of in the MRL/MPJ mouse when peripheral RPE cells are spared from injury and are therefore able to repair minor RPE lesions (Xia et al., 2011). What then can we learn about RPE regeneration from these regeneration-competent systems that could identify potential barriers to RPE-to-RPE or RPE-to-retina regeneration in mammals and, most importantly, in humans suffering from ocular degenerative diseases? Three potential barriers to regeneration in mammals are: i) differences in the activities or functions of pro-regenerative cell-cell signaling pathways and proliferative potential of RPE cells; ii) epigenetic barriers to reprogramming; and iii) differing pro- and anti-regenerative functions of the immune system.
7.1. Differences in cell-cell signaling pathway activities and proliferative potential
MEK-ERK signaling is required for RPE-to-retina regeneration in newts (Mizuno et al., 2012) and chickens (Spence et al., 2007). MEK-ERK signaling is involved in other regenerative processes in non-mammalian vertebrates, such as cardiac regeneration after heart resection in zebrafish (Liu and Zhong, 2017), blastema formation after limb amputation in X. laevis (Suzuki et al., 2007), and muscle (myotube) formation in cultured salamander cells (Yun et al., 2014). In the salamander in vitro myotube culture system, MEK-ERK signaling was activated in response to serum stimulation, and sustained ERK activity was found to be necessary for reprogramming and cell cycle re-entry of salamander myotubes. In contrast, only transient activation of ERK signaling, was observed and no change of proliferative genes like cyclins and PCNA were found in a murine myogenic cell line, which was unable to dedifferentiate or regenerate upon serum stimulation (Yun et al., 2014). These data suggest that the duration or magnitude of MEK-ERK activation could influence the regenerative process and that this differs between mammals and regeneration-competent animals like salamanders. In the eye, retina, or RPE, injury may stimulate MEK-ERK activity and thereby enable or enhance the reprogramming and/or proliferative responses in RPE cells in these regeneration-competent animals, but not in mammals, which do not regenerate.
FGF and Wnt/β-catenin signaling also play roles in RPE-dependent regenerative responses in regeneration-competent non-mammalian vertebrates. FGF is known to function in maintaining pluripotency in a variety of stem cells (reviewed in Mossahebi-Mohammadi et al., 2020) and in facilitating regenerative responses in a number of organ systems and animal models (reviewed in Maddaluno et al., 2017). In the eye, pro-regenerative roles include during Müller glia-derived retinal regeneration in chickens (Fischer et al., 2002) and zebrafish (Wan et al., 2014), rod regeneration in zebrafish (Qin et al., 2011), and lens regeneration in X. laevis (Fukui and Henry, 2011), amongst others, in addition to its role during the RPE-to-retina transdifferentiation process. In mammals, FGF2 secretion is elevated in human iPSC-derived RPE cells in an in vitro scratch assay (Greene et al., 2016) and FGFs are upregulated in mouse and rat retina after mechanical injury (Cao et al., 1997; Cao et al., 2001). Interestingly, FGF2 addition to culture medium enables human RPE stem cell self-renewal in vitro (Salero et al., 2012). Although the ability of FGF activation to stimulate human RPE stem cell proliferation in vivo, or in response to injury, has yet to be established, given the role of FGF in facilitating regenerative responses in the eyes of many regeneration-competent non-mammalian systems, this is an interesting avenue for future studies. Wnt/β-catenin signaling is required for zebrafish RPE-to-RPE regeneration (Hanovice et al., 2019); however, in mammals, Wnt/β-catenin activity is linked to pathological proliferation and EMT in PVR (Han et al., 2015; reviewed in Tamiya and Kaplan, 2016). Moreover, in chicken, β-catenin negatively regulates RPE-dependent retina regeneration (Zhu et al., 2014), so β-catenin roles in modulating RPE-dependent repair and regenerative processes are also context dependent, differing between regeneration-competent non-mammalian systems and mammals, which do not regenerate.
Taken together, these data suggest species-specific differences in signaling pathway activity during the injury and regenerative responses. One common thread in these pathways is the downstream activation of proliferative responses in RPE cells. Some of these pathways are also activated in mammalian RPE after injury; however, they do not stimulate proliferative responses that contribute to repair or regeneration. Rather, RPE cells that do proliferate after injury often undergo EMT and lead to additional pathology. Thus, the cellular response to signaling pathway activity also differs between regeneration-competent and regeneration-incompetent animals. With that in mind, one approach towards enhancing the regenerative ability of mammalian RPE might be to modulate pro-regenerative signaling pathways that are either inactive in injured mammalian RPE or only modestly activated upon injury, or to somehow modulate the proliferative responses of mammalian RPE cells in such a way that pushes them away from EMT and towards a pro-regenerative response. While this strategy may be appealing, it is however not likely to stimulate a regenerative response de novo after injury and would most certainly have to be combined with parallel strategies to reprogram RPE cells towards a pro-regenerative state.
7.2. Epigenetic barriers to reprogramming:
Direct reprogramming of somatic cells has gained traction as a potential mechanism to stimulate regenerative responses in non-regenerating cells and tissues, including the retina (Yao et al., 2018). RPE-to-retina transdifferentiation in chickens and newts is an in vivo exemplar of the direct reprogramming process whereby differentiated RPE cells reprogram after retinectomy and differentiate into retinal neurons; as such, it can serve as a useful model through which physiologically relevant mechanisms that modulate cell reprogramming can be identified. One such mechanism is the epigenetic regulation of gene expression, through which genes required for reprogramming and/or repair and regeneration are held in an epigenetically silenced state in mammals and therefore unable to respond to injury. Studies in the chicken support this notion and identified significant changes in bivalent chromatin marks and in DNA demethylation activity in RPE cells during RPE-to-retina regeneration post-retinectomy (Luz-Madrigal, et al., 2014; Luz-Madrigal, et al., 2020). Beyond this, no studies have been reported that identify changes in the expression of genes associated with epigenetic modifications or the modifications themselves during RPE-to-RPE or RPE-to-retina regeneration in other regenerating systems. However, a recent study characterized the epigenetic profile of adult mouse RPE cells using ChIP-seq and whole-genome bisulfite sequencing, and data therein provide some insight into potential epigenetic constraints in mammals that might limit the regenerative capacity of RPE (Dvoriantchikova et al., 2019). In this study, a large portion of genes associated with optic vesicle development, retinal progenitor cells, and Müller glia possessed chromatin in an open/active state in adult RPE and their surrounding genomic regions were unmethylated or hypomethylated. Genes associated with retinal neuron functions were in a repressed chromatin state, with the exception of photoreceptor genes, which were in a permissive state, but associated with methylated regions of the genome. Taken together, these data suggest that RPE cells could be stimulated to express retinal neuron genes, but this would still require them to first reprogram and transdifferentiate.
As mentioned above, direct reprogramming of somatic cells is an exciting approach for stimulating regenerative responses in normally non-regenerative tissues, but there are as-yet no reports of the efficacy of this approach in the RPE. However, also as noted above, viral delivery of E2F2 can induce limited RPE proliferation and regeneration after genetic ablation in mice, supporting the notion that mammalian RPE are capable of overcoming intrinsic barriers to regeneration (Kampik et al., 2017). As noted by Dvoriantchikova et al., (2019), it could be that the addition of specific pioneer transcription factors to RPE cells that open the repressive chromatin state, and/or mechanisms that promote epigenetic changes at pro-regenerative loci after injury, could be an effective strategy to stimulate a regenerative response in mammals. Indeed, such a strategy has proven effective based on knowledge of the regenerative response in zebrafish Müller glia where a number of key regulators of reprogramming have been identified, including Ascl1, a master regulator of neuron specification (Lahne et al., 2020; Wan and Goldman, 2016). Ascl1, when expressed in mammalian Müller glia in culture or in vivo, stimulates the production of new neurons from the Müller glia (Pollak et al., 2013; Ueki et al., 2015). Moreover, this effect can be dramatically enhanced in vivo when combined with a histone deacetylase inhibitor (Jorstad et al., 2017), highlighting the potential role for epigenetic regulation of the regenerative process, as well as the likelihood that combinatorial approaches will be needed to simulate such responses from normally non-regenerative cell types. The ability of zebrafish to endogenously regenerate RPE after widespread injury also presents a unique opportunity to identify epigenetic factors that regulate this process, which may also be potential targets for modulation in non-regenerating mammals. Here, the process may be even simpler in the sense that no transdifferentiation is required; RPE need only to be stimulated to proliferate again, differentiate, and re-establish epithelial architecture. It will be of interest to identify the epigenetic signature of RPE cells in zebrafish and mammals before and after injury, as well as to screen for potential epigenetic regulators whose expression changes in response to injury, as these factors could be good targets for further studies.
7.3. Differences in immune responses:
It has been postulated that the evolutionary complexity of the immune system is a direct contributor to the capacity for an organism to regenerate tissues; wherein animals with primitive immune responses (e.g. teleosts and amphibians) are robust regenerators and phylogenetic acquisition of a sophisticated immune system (as in mammals) comes at the cost of losing the ability to regenerate damaged tissues (reviewed in Aurora and Olson, 2014; Mescher and Neff, 2005; Mescher et al., 2017). Some immune-related elements thought to contribute to these differences include the composition of immune cells at the injury site and the duration, strength, and specificity of the immune/inflammatory response (reviewed in Aurora and Olson, 2014; Mescher and Neff, 2005; Mescher et al., 2017). A stunning example of this exists in X. laevis, where larvae are able to undergo epimorphic regeneration (where new tissues regrow from a blastema) of limbs and tails at early developmental stages, but regenerative capacity decreases as animals approach metamorphosis (reviewed in Mescher and Neff, 2005; Mescher et al., 2017). Importantly, in this context, anuran amphibians at metamorphosis undergo a complete restructuring of the immune system, which increases in complexity and mature lymphocyte pools when compared to the immune system present at larval stages (reviewed in Rollins-Smith, 1998). Adult anurans (e.g. Xenopus) are part of the earliest evolutionary class of vertebrates capable of canonical antibody class switching (CSR), a DNA recombination mechanism also present in mammals that improves the diversity and effectiveness of antibody responses; this and other components of post-metamorphic immunity are considered analogous to the mammalian response, but like mammals, these animals are unable to achieve epimorphic regeneration (reviewed in Flajnik, 2018; Robert and Ohta, 2009; Stavnezer and Amemiya, 2004; Stavnezer et al., 2008). Pre-metamorphic larval anurans possess epimorphic regeneration capacity but have poor CSR and weaker lymphocyte responses than their adult counterparts (reviewed in Robert and Ohta, 2009; Mescher and Neff, 2005). Comparatively, zebrafish (which are phylogenetically “lower” than amphibians) are epimorphic regeneration-competent as adults, for example, regeneration of the tail fin (Poss et al., 2000), but are incapable of canonical CSR and may be more reliant on innate rather than adaptive immunity (reviewed in Iwanami, 2014; Lewis et al., 2014). In X. laevis, the switch from epimorphic regeneration competence to incompetence occurs at a stage in development (stage 57) where T cell markers become expressed; likewise, at stage 57 post-hindlimb amputation, markers of inflammation resolution persist, and markers indicative of tissue patterning are decreased in comparison to younger (stage 53) epimorphic regeneration-competent larvae (Mescher et al., 2013). Indeed, chemical induction of inflammation in stage 53 larvae recapitulated stage 57 expression patterns (e.g. persistent inflammatory markers and loss of blastema patterning markers) and epimorphic regeneration capacity was compromised (Mescher et al., 2013). Similarly, X. laevis possess a transient loss of regenerative capacity during development, termed the “refractory period” (stages 45–47), and this capacity can be restored by chemical immune suppression (Fukazawa et al., 2009), indicating that dampening the immune/inflammatory response in some contexts may be permissive to regeneration.
The above examples demonstrate a link between modulating the immune response to affect regenerative capacity, and also highlight how acute inflammation may present a barrier to regeneration in animals with highly evolved immune responses. Other examples exist in fetal mammalian tissues, such as skin, which is able to undergo scar-free wound healing early in development and the transition to fibrotic wound healing (scarring) appears to occur with maturation of elements of the immune response (reviewed in Mescher and Neff, 2005; Colwell et al., 2005). In the adult human eye, chronic inflammation has been linked to AMD as leukocytes (Gupta et al., 2003) and immune-related components have been identified in patient tissues (reviewed in Guillonneau et al., 2017). Indeed, immunosuppression has been assessed as an adjuvant therapy for AMD treatment (Nussenblatt et al., 2010). As onset of RPE degenerative diseases such as AMD can be difficult to pinpoint and tissue loss may be significant by the time of diagnosis, dampening inflammation in conjunction with stimulating pro-regenerative factors may be a feasible therapeutic approach to restoring RPE in mammals.
8. FUTURE DIRECTIONS
Herein we have summarized known mechanisms involved in RPE injury, regenerative capacity of the RPE in mammalian and non-mammalian model systems, and discussed likely barriers to RPE regeneration in mammals. Additionally, we have highlighted an existing theme in regenerative biology, which is that utilizing regeneration-competent non-mammalian organisms to understand and identify pro-regenerative cues is a viable translational strategy for treating degenerative diseases, like AMD, in mammalian systems incapable of robust tissue regeneration. In the context of RPE-based treatments, specifically, this approach is feasible and may only require stimulating the intrinsic regenerative capacity of a subset of RPE to greatly impact severity of vision loss and quell disease progression. Indeed, with each foveal RPE cell supporting many cones in the human fovea (e.g. ~16:1 (Granger et al., 2018), ~19:1 (Liu et al., 2017), and ~24:1 (Gao and Hollyfield, 1992) reported cone-to-RPE ratios), minimal RPE regeneration or even maintenance of existing RPE without further degeneration could lead to sufficient photoreceptor preservation to, in turn, preserve central visual acuity and patient quality of life. Looking to the future, modulating pro-RPE regenerative cues in humans could be complementary to stem cell-based RPE and/or retina replacement therapies in patients with advanced disease and tissue loss, or used in combination with other therapeutic approaches, such as immune suppression or anti-VEGF treatments (in the case of exudative AMD), which may help promote a regeneration-permissive microenvironment in the eye.
ACKNOWLEDGEMENTS:
Research on RPE regeneration in J.M.G’s laboratory is supported by the National Institutes of Health (T32-EY17271 to L.L.L., RO1-EY29410 to J.M.G, and NIH CORE Grant P30-EY08098 to the Department of Ophthalmology); the UPMC Immune Transplant & Therapy Center (to L.L.L. and J.M.G.); the Pennsylvania Lions Sight Conservation and Eye Research Foundation (to L.L.L. and J.M.G.); the Charles and Louella Snyder Retinal Regeneration Fund (to J.M.G.); the Macular Degeneration Research Program of the BrightFocus Foundation (M2016067 to J.M.G); and the E. Ronald Salvitti Chair in Ophthalmology Research (to J.M.G.). Additional support was received from the Martha Wandrisco Neff Research Award in Macular Degeneration (to L.L.L.), the Wiegand Fellowship in Ophthalmology (to L.L.L), an Honors College HEAL Research Fellowship (to S.M.G), the Eye & Ear Foundation of Pittsburgh, and an unrestricted grant from Research to Prevent Blindness, New York, NY. We also wish to thank Rachel Nagy for editorial assistance and Drs. Emeline Nandrot and Debasish Sinha for helpful comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
L.L.L. is co-inventor on a US Patent (#9,458,428) related to deriving RPE from pluripotent stem cells in vitro; while not directly related to the content herein, we wish to disclose this patent. All other authors declare no conflicts of interest.
LITERATURE CITED:
- Ail D, Perron M, 2017. Retinal Degeneration and Regeneration-Lessons From Fishes and Amphibians. Curr Pathobiol Rep 5, 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisenbrey S, Zhang M, Bacher D, Yee J, Brunken WJ, Hunter DD, 2006. Retinal Pigment Epithelial Cells Synthesize Laminins, Including Laminin 5, and Adhere to Them through α3- and α6-Containing Integrins. Investigative Ophthalmology & Visual Science 47, 5537–5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar-Schäfer I, Wang L, Krohne TU, Xu H, Langmann T, 2018. Modulation of three key innate immune pathways for the most common retinal degenerative diseases. EMBO Mol Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hussaini H, Kam JH, Vugler A, Semo M, Jeffery G, 2008. Mature retinal pigment epithelium cells are retained in the cell cycle and proliferate in vivo. Mol Vis 14, 1784–1791. [PMC free article] [PubMed] [Google Scholar]
- Al-Hussaini H, Kilarkaje N, Shahabi G, Al-Mulla F, 2016. Proliferation and Migration of Peripheral Retinal Pigment Epithelial Cells Are Associated with the Upregulation of Wingless-Related Integration and Bone Morphogenetic Protein Signaling in Dark Agouti Rats. Med Princ Pract 25, 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammar MJ, Hsu J, Chiang A, Ho AC, Regillo CD, 2020. Age-related macular degeneration therapy: a review. Curr Opin Ophthalmol 31, 215–221. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Johnson LV, Hageman GS, 1995. Vitronectin receptor expression and distribution at the photoreceptor-retinal pigment epithelial interface. J Comp Neurol 360, 1–16. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Mullins RF, Hageman GS, Johnson LV, 2002. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol 134, 411–431. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Talaga KC, Rivest AJ, Barron E, Hageman GS, Johnson LV, 2004. Characterization of β amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Experimental Eye Research 78, 243–256. [DOI] [PubMed] [Google Scholar]
- Araki M, 2007. Regeneration of the amphibian retina: Role of tissue interaction and related signaling molecules on RPE transdifferentiation. Dev Growth Differ 49, 109–120. [DOI] [PubMed] [Google Scholar]
- Ardeljan D, Chan CC, 2013. Aging is not a disease: distinguishing age-related macular degeneration from aging. Prog Retin Eye Res 37, 68–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P, 2000. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes & development 14, 2701–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurora AB, Olson EN, 2014. Immune modulation of stem cells and regeneration. Cell Stem Cell 15, 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas D, Bumsted KM, Martinez JA, Vaccarino FM, Wikler KC, Barnstable CJ, 2000. The subcellular localization of Otx2 is cell-type specific and developmentally regulated in the mouse retina. Brain Res Mol Brain Res 78, 26–37. [DOI] [PubMed] [Google Scholar]
- Bakall B, Marmorstein LY, Hoppe G, Peachey NS, Wadelius C, Marmorstein AD, 2003. Expression and localization of bestrophin during normal mouse development. Invest Ophthalmol Vis Sci 44, 3622–3628. [DOI] [PubMed] [Google Scholar]
- Ban Y, Rizzolo LJ, 2000. Regulation of glucose transporters during development of the retinal pigment epithelium. Developmental Brain Research 121, 89–95. [DOI] [PubMed] [Google Scholar]
- Barbosa-Sabanero K, Hoffmann A, Judge C, Lightcap N, Tsonis Panagiotis A., Del Rio-Tsonis K, 2012. Lens and retina regeneration: new perspectives from model organisms. Biochemical Journal 447, 321–334. [DOI] [PubMed] [Google Scholar]
- Barnstable CJ, Tombran-Tink J, 2004. Neuroprotective and antiangiogenic actions of PEDF in the eye: molecular targets and therapeutic potential. Prog Retin Eye Res 23, 561–577. [DOI] [PubMed] [Google Scholar]
- Bäumer N, Marquardt T, Stoykova A, Spieler D, Treichel D, Ashery-Padan R, Gruss P, 2003. Retinal pigmented epithelium determination requires the redundant activities of Pax2 and Pax6. Development 130, 2903–2915. [DOI] [PubMed] [Google Scholar]
- Becerra SP, Fariss RN, Wu YQ, Montuenga LM, Wong P, Pfeffer BA, 2004. Pigment epithelium-derived factor in the monkey retinal pigment epithelium and interphotoreceptor matrix: apical secretion and distribution. Exp Eye Res 78, 223–234. [DOI] [PubMed] [Google Scholar]
- Beermann F, Schmid E, Schütz G, 1992. Expression of the mouse tyrosinase gene during embryonic development: recapitulation of the temporal regulation in transgenic mice. Proc Natl Acad Sci U S A 89, 2809–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellezza I, 2018. Oxidative Stress in Age-Related Macular Degeneration: Nrf2 as Therapeutic Target. Frontiers in Pharmacology 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben M’Barek K, Habeler W, Regent F, Monville C, 2019. Developing Cell-Based Therapies for RPE-Associated Degenerative Eye Diseases. Adv Exp Med Biol 1186, 55–97. [DOI] [PubMed] [Google Scholar]
- Bharti K, Gasper M, Ou J, Brucato M, Clore-Gronenborn K, Pickel J, Arnheiter H, 2012. A regulatory loop involving PAX6, MITF, and WNT signaling controls retinal pigment epithelium development. PLoS Genet 8, e1002757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti K, Liu W, Csermely T, Bertuzzi S, Arnheiter H, 2008. Alternative promoter use in eye development: the complex role and regulation of the transcription factor MITF. Development 135, 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD, de Jong PTVM, Klaver CCW, Klein BEK, Klein R, Mitchell P, Sarks JP, Sarks SH, Soubrane G, Taylor HR, Vingerling JR, 1995. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Survey of Ophthalmology 39, 367–374. [DOI] [PubMed] [Google Scholar]
- Bisbach CM, Hass DT, Robbings BM, Rountree AM, Sadilek M, Sweet IR, Hurley JB, 2020. Succinate Can Shuttle Reducing Power from the Hypoxic Retina to the O(2)-Rich Pigment Epithelium. Cell Rep 31, 107606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaauwgeers HG, Holtkamp GM, Rutten H, Witmer AN, Koolwijk P, Partanen TA, Alitalo K, Kroon ME, Kijlstra A, van Hinsbergh VW, Schlingemann RO, 1999. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. The American journal of pathology 155, 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles NC, Fernandes M, Swigut T, Srinivasan R, Schiff L, Rada-Iglesias A, Wang Q, Saini JS, Kiehl T, Stern JH, Wysocka J, Blenkinsop TA, Temple S, 2020. Epigenomic and Transcriptomic Changes During Human RPE EMT in a Stem Cell Model of Epiretinal Membrane Pathogenesis and Prevention by Nicotinamide. Stem Cell Reports 14, 631–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha VL, 2008. Age and disease-related structural changes in the retinal pigment epithelium. Clin Ophthalmol 2, 413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha VL, Finnemann SC, Rodriguez-Boulan E, 1999. Ezrin promotes morphogenesis of apical microvilli and basal infoldings in retinal pigment epithelium. The Journal of cell biology 147, 1533–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij JC, Baas DC, Beisekeeva J, Gorgels TG, Bergen AA, 2010. The dynamic nature of Bruch’s membrane. Prog Retin Eye Res 29, 1–18. [DOI] [PubMed] [Google Scholar]
- Bordet T, Behar-Cohen F, 2019. Ocular gene therapies in clinical practice: viral vectors and nonviral alternatives. Drug Discov Today 24, 1685–1693. [DOI] [PubMed] [Google Scholar]
- Bovolenta P, Mallamaci A, Briata P, Corte G, Boncinelli E, 1997. Implication of OTX2 in pigment epithelium determination and neural retina differentiation. J Neurosci 17, 4243–4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstetter C, Patt J, Holz FG, Krohne TU, 2016. Inflammasome priming increases retinal pigment epithelial cell susceptibility to lipofuscin phototoxicity by changing the cell death mechanism from apoptosis to pyroptosis. Journal of Photochemistry and Photobiology B: Biology 161, 177–183. [DOI] [PubMed] [Google Scholar]
- Burns ME, Stevens B, 2018. Report on the National Eye Institute’s Audacious Goals Initiative: Creating a Cellular Environment for Neuroregeneration. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi L, Cummings RJ, Blander JM, 2014. Death-defining immune responses after apoptosis. Am J Transplant 14, 1488–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campochiaro PA, Hackett SF, Conway BP, 1991. Retinoic acid promotes density-dependent growth arrest in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 32, 65–72. [PubMed] [Google Scholar]
- Campochiaro PA, Sugg R, Grotendorst G, Hjelmeland LM, 1989. Retinal pigment epithelial cells produce PDGF-like proteins and secrete them into their media. Experimental Eye Research 49, 217–227. [DOI] [PubMed] [Google Scholar]
- Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA, 2000. The transcription factor Snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nature Cell Biology 2, 76–83. [DOI] [PubMed] [Google Scholar]
- Cao W, Li F, Steinberg RH, Lavail MM, 2001. Development of normal and injury-induced gene expression of aFGF, bFGF, CNTF, BDNF, GFAP and IGF-I in the rat retina. Exp Eye Res 72, 591–604. [DOI] [PubMed] [Google Scholar]
- Cao W, Wen R, Li F, Lavail MM, Steinberg RH, 1997. Mechanical injury increases bFGF and CNTF mRNA expression in the mouse retina. Exp Eye Res 65, 241–248. [DOI] [PubMed] [Google Scholar]
- Casco-Robles MM, Islam MR, Inami W, Tanaka HV, Kunahong A, Yasumuro H, Hanzawa S, Casco-Robles RM, Toyama F, Maruo F, Chiba C, 2016. Turning the fate of reprogramming cells from retinal disorder to regeneration by Pax6 in newts. Sci Rep 6, 33761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayouette M, Smith SB, Becerra SP, Gravel C, 1999. Pigment epithelium-derived factor delays the death of photoreceptors in mouse models of inherited retinal degenerations. Neurobiol Dis 6, 523–532. [DOI] [PubMed] [Google Scholar]
- Cechmanek PB, McFarlane S, 2017. Retinal pigment epithelium expansion around the neural retina occurs in two separate phases with distinct mechanisms. Developmental Dynamics 246, 598–609. [DOI] [PubMed] [Google Scholar]
- Chaudhary R, Scott RAH, Wallace G, Berry M, Logan A, Blanch RJ, 2020. Inflammatory and Fibrogenic Factors in Proliferative Vitreoretinopathy Development. Translational Vision Science & Technology 9, 23–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, 2020. Perspectives on somatic reprogramming: spotlighting epigenetic regulation and cellular heterogeneity. Curr Opin Genet Dev 64, 21–25. [DOI] [PubMed] [Google Scholar]
- Chen M, Luo C, Zhao J, Devarajan G, Xu H, 2019. Immune regulation in the aging retina. Progress in Retinal and Eye Research 69, 159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Rajapakse D, Fraczek M, Luo C, Forrester JV, Xu H, 2016. Retinal pigment epithelial cell multinucleation in the aging eye - a mechanism to repair damage and maintain homoeostasis. Aging Cell 15, 436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba C, 2014. The retinal pigment epithelium: An important player of retinal disorders and regeneration. Experimental Eye Research 123, 107–114. [DOI] [PubMed] [Google Scholar]
- Chiba C, Hoshino A, Nakamura K, Susaki K, Yamano Y, Kaneko Y, Kuwata O, Maruo F, Saito T, 2006. Visual cycle protein RPE65 persists in new retinal cells during retinal regeneration of adult newt. Journal of Comparative Neurology 495, 391–407. [DOI] [PubMed] [Google Scholar]
- Chong NHV, Keonin J, Luthert PJ, Frennesson CI, Weingeist DM, Wolf RL, Mullins RF, Hageman GS, 2005. Decreased thickness and integrity of the macular elastic layer of Bruch’s membrane correspond to the distribution of lesions associated with age-related macular degeneration. The American journal of pathology 166, 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RL, Lang RA, 2001. Early Eye Development in Vertebrates. Annual Review of Cell and Developmental Biology 17, 255–296. [DOI] [PubMed] [Google Scholar]
- Clark LD, Clark RK, Heber-Katz E, 1998. A New Murine Model for Mammalian Wound Repair and Regeneration. Clin Immunol Immunopathol 88, 35–45. [DOI] [PubMed] [Google Scholar]
- Colwell AS, Longaker MT, Lorenz HP, 2005. Mammalian fetal organ regeneration. Adv Biochem Eng Biotechnol 93, 83–100. [DOI] [PubMed] [Google Scholar]
- Combadière C, Feumi C, Raoul W, Keller N, Rodéro M, Pézard A, Lavalette S, Houssier M, Jonet L, Picard E, Debré P, Sirinyan M, Deterre P, Ferroukhi T, Cohen SY, Chauvaud D, Jeanny JC, Chemtob S, Behar-Cohen F, Sennlaub F, 2007. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest 117, 2920–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon N, O’Colmain B, Klaver CC, Klein R, Muñoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P, 2004. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 122, 477–485. [DOI] [PubMed] [Google Scholar]
- Coulombre JL, Coulombre AJ, 1965. Regeneration of neural retina from the pigmented epithelium in the chick embryo. Dev Biol 12, 79–92. [DOI] [PubMed] [Google Scholar]
- Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG, 2002. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A 99, 14682–14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Medeiros NE, Millican CL, 1996. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci 37, 1236–1249. [PubMed] [Google Scholar]
- Das A, McGuire PG, 2003. Retinal and choroidal angiogenesis: pathophysiology and strategies for inhibition. Progress in Retinal and Eye Research 22, 721–748. [DOI] [PubMed] [Google Scholar]
- Datta S, Cano M, Ebrahimi K, Wang L, Handa JT, 2017. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Progress in retinal and eye research 60, 201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, Bouck NP, 1999. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science 285, 245–248. [DOI] [PubMed] [Google Scholar]
- Del Priore LV, Hornbeck R, Kaplan HJ, Jones Z, Valentino TL, Mosinger-Ogilvie J, Swinn M, 1995. Débridement of the pig retinal pigment epithelium in vivo. Archives of Ophthalmology 113, 939–944. [DOI] [PubMed] [Google Scholar]
- Dhuriya YK, Sharma D, 2018. Necroptosis: a regulated inflammatory mode of cell death. Journal of Neuroinflammation 15, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J-D, Johnson LV, Herrmann R, Farsiu S, Smith SG, Groelle M, Mace BE, Sullivan P, Jamison JA, Kelly U, Harrabi O, Bollini SS, Dilley J, Kobayashi D, Kuang B, Li W, Pons J, Lin JC, Bowes Rickman C, 2011. Anti-amyloid therapy protects against retinal pigmented epithelium damage and vision loss in a model of age-related macular degeneration. Proc Natl Acad Sci USA 108, E279–E287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongre A, Weinberg RA, 2019. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nature Reviews Molecular Cell Biology 20, 69–84. [DOI] [PubMed] [Google Scholar]
- Dryja TP, O’Neil-Dryja M, Pawelek JM, Albert DM, 1978. Demonstration of tyrosinase in the adult bovine uveal tract and retinal pigment epithelium. Invest Ophthalmol Vis Sci 17, 511–514. [PubMed] [Google Scholar]
- Dunn KC, Marmorstein AD, Bonilha VL, Rodriguez-Boulan E, Giordano F, Hjelmeland LM, 1998. Use of the ARPE-19 cell line as a model of RPE polarity: basolateral secretion of FGF5. Invest Ophthalmol Vis Sci 39, 2744–2749. [PubMed] [Google Scholar]
- Dvoriantchikova G, Seemungal RJ, Ivanov D, 2019. The epigenetic basis for the impaired ability of adult murine retinal pigment epithelium cells to regenerate retinal tissue. Scientific Reports 9, 3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM, 1990. Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature 347, 83–86. [DOI] [PubMed] [Google Scholar]
- Fan WEI, Lin N, Sheedlo HJ, Turner JE, 1996. Müller and RPE Cell Response to Photoreceptor Cell Degeneration in Aging Fischer Rats. Experimental Eye Research 63, 9–18. [DOI] [PubMed] [Google Scholar]
- Fang L, El Wazan L, Tan C, Nguyen T, Hung SSC, Hewitt AW, Wong RCB, 2018. Potentials of Cellular Reprogramming as a Novel Strategy for Neuroregeneration. Front Cell Neurosci 12, 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney-Burns L, Hilderbrand ES, Eldridge S, 1984. Aging human RPE: morphometric analysis of macular, equatorial, and peripheral cells. Invest Ophthalmol Vis Sci 25, 195–200. [PubMed] [Google Scholar]
- Feng W, Yasumura D, Matthes MT, LaVail MM, Vollrath D, 2002. Mertk triggers uptake of photoreceptor outer segments during phagocytosis by cultured retinal pigment epithelial cells. J Biol Chem 277, 17016–17022. [DOI] [PubMed] [Google Scholar]
- Finnemann SC, Nandrot EF, 2006. MerTK activation during RPE phagocytosis in vivo requires alphaVbeta5 integrin. Adv Exp Med Biol 572, 499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC, Rodriguez-Boulan E, 1999. Macrophage and retinal pigment epithelium phagocytosis: apoptotic cells and photoreceptors compete for alphavbeta3 and alphavbeta5 integrins, and protein kinase C regulates alphavbeta5 binding and cytoskeletal linkage. J Exp Med 190, 861–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, McGuire CR, Dierks BD, Reh TA, 2002. Insulin and fibroblast growth factor 2 activate a neurogenic program in Müller glia of the chicken retina. J Neurosci 22, 9387–9398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajnik MF, 2018. A cold-blooded view of adaptive immunity. Nat Rev Immunol 18, 438–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco LM, Zulliger R, Wolf-Schnurrbusch UEK, Katagiri Y, Kaplan HJ, Wolf S, Enzmann V, 2009. Decreased Visual Function after Patchy Loss of Retinal Pigment Epithelium Induced by Low-Dose Sodium Iodate. Investigative Ophthalmology & Visual Science 50, 4004–4010. [DOI] [PubMed] [Google Scholar]
- Fuchs HR, Meister R, Lotke R, Framme C, 2020. The microRNAs miR-302d and miR-93 inhibit TGFB-mediated EMT and VEGFA secretion from ARPE-19 cells. Exp Eye Res 201, 108258. [DOI] [PubMed] [Google Scholar]
- Fuhrmann S, 2010. Eye morphogenesis and patterning of the optic vesicle. Curr Top Dev Biol 93, 61–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann S, Levine EM, Reh TA, 2000. Extraocular mesenchyme patterns the optic vesicle during early eye development in the embryonic chick. Development 127, 4599–4609. [DOI] [PubMed] [Google Scholar]
- Fuhrmann S, Zou C, Levine EM, 2014. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Experimental eye research 123, 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura N, Taketo MM, Mori M, Korinek V, Kozmik Z, 2009. Spatial and temporal regulation of Wnt/beta-catenin signaling is essential for development of the retinal pigment epithelium. Dev Biol 334, 31–45. [DOI] [PubMed] [Google Scholar]
- Fukazawa T, Naora Y, Kunieda T, Kubo T, 2009. Suppression of the immune response potentiates tadpole tail regeneration during the refractory period. Development 136, 2323–2327. [DOI] [PubMed] [Google Scholar]
- Fukui L, Henry JJ, 2011. FGF signaling is required for lens regeneration in Xenopus laevis. Biol Bull 221, 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, A L, Huang X, Chen X, Xu H, 2021. Müller Glia-Mediated Retinal Regeneration. Mol Neurobiol. [DOI] [PubMed] [Google Scholar]
- Gao H, Hollyfield JG, 1992. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 33, 1–17. [PubMed] [Google Scholar]
- García-García D, Locker M, Perron M, 2020. Update on Müller glia regenerative potential for retinal repair. Current Opinion in Genetics & Development 64, 52–59. [DOI] [PubMed] [Google Scholar]
- Gilles C, Polette M, Zahm JM, Tournier JM, Volders L, Foidart JM, Birembaut P, 1999. Vimentin contributes to human mammary epithelial cell migration. J Cell Sci 112 ( Pt 24), 4615–4625. [DOI] [PubMed] [Google Scholar]
- Goldman D, 2014. Müller glial cell reprogramming and retina regeneration. Nat Rev Neurosci 15, 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto S, Onishi A, Misaki K, Yonemura S, Sugita S, Ito H, Ohigashi Y, Ema M, Sakaguchi H, Nishida K, Takahashi M, 2018. Neural retina-specific Aldh1a1 controls dorsal choroidal vascular development via Sox9 expression in retinal pigment epithelial cells. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P, Ivert L, Neuringer M, Nagasaki T, 2016. Mitochondrial elongation in the macular RPE of aging monkeys, evidence of metabolic stress. Graefes Arch Clin Exp Ophthalmol 254, 1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger CE, Yang Q, Song H, Saito K, Nozato K, Latchney LR, Leonard BT, Chung MM, Williams DR, Rossi EA, 2018. Human Retinal Pigment Epithelium: In Vivo Cell Morphometry, Multispectral Autofluorescence, and Relationship to Cone Mosaic. Invest Ophthalmol Vis Sci 59, 5705–5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene WA, Burke TA, Por ED, Kaini RR, Wang HC, 2016. Secretion Profile of Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium During Wound Healing. Invest Ophthalmol Vis Sci 57, 4428–4441. [DOI] [PubMed] [Google Scholar]
- Grierson I, Hiscott P, Hogg P, Robey H, Mazure A, Larkin G, 1994. Development, repair and regeneration of the retinal pigment epithelium. Eye (Lond) 8 ( Pt 2), 255–262. [DOI] [PubMed] [Google Scholar]
- Grignolo A, Orzalesi N, Calabria GA, 1966. Studies on the fine structure and the rhodopsin cycle of the rabbit retina in experimental degeneration induced by sodium iodate. Exp Eye Res 5, 86–97. [DOI] [PubMed] [Google Scholar]
- Grigoryan EN, Markitantova YV, 2016. Cellular and Molecular Preconditions for Retinal Pigment Epithelium (RPE) Natural Reprogramming during Retinal Regeneration in Urodela. Biomedicines 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindley JC, Davidson DR, Hill RE, 1995. The role of Pax-6 in eye and nasal development. Development 121, 1433–1442. [DOI] [PubMed] [Google Scholar]
- Grossniklaus HE, Kang SJ, Berglin L, 2010. Animal models of choroidal and retinal neovascularization. Progress in retinal and eye research 29, 500–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossniklaus HE, Ling JX, Wallace TM, Dithmar S, Lawson DH, Cohen C, Elner VM, Elner SG, Sternberg P Jr., 2002. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol Vis 8, 119–126. [PubMed] [Google Scholar]
- Gu X, Neric NJ, Crabb JS, Crabb JW, Bhattacharya SK, Rayborn ME, Hollyfield JG, Bonilha VL, 2012. Age-related changes in the retinal pigment epithelium (RPE). PloS one 7, e38673–e38673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillonneau X, Eandi CM, Paques M, Sahel JA, Sapieha P, Sennlaub F, 2017. On phagocytes and macular degeneration. Prog Retin Eye Res 61, 98–128. [DOI] [PubMed] [Google Scholar]
- Gundersen D, Powell SK, Rodriguez-Boulan E, 1993. Apical polarization of N-CAM in retinal pigment epithelium is dependent on contact with the neural retina. J Cell Biol 121, 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Brown KE, Milam AH, 2003. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Experimental Eye Research 76, 463–471. [DOI] [PubMed] [Google Scholar]
- Gurdon JB, 1962. The transplantation of nuclei between two species of Xenopus. Dev Biol 5, 68–83. [DOI] [PubMed] [Google Scholar]
- Hadziahmetovic M, Dentchev T, Song Y, Haddad N, He X, Hahn P, Pratico D, Wen R, Harris ZL, Lambris JD, Beard J, Dunaief JL, 2008. Ceruloplasmin/hephaestin knockout mice model morphologic and molecular features of AMD. Investigative ophthalmology & visual science 49, 2728–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R, 2005. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A 102, 7227–7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF, 2001. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res 20, 705–732. [DOI] [PubMed] [Google Scholar]
- Halbleib JM, Nelson WJ, 2006. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev 20, 3199–3214. [DOI] [PubMed] [Google Scholar]
- Halilagic A, Ribes V, Ghyselinck NB, Zile MH, Dollé P, Studer M, 2007. Retinoids control anterior and dorsal properties in the developing forebrain. Dev Biol 303, 362–375. [DOI] [PubMed] [Google Scholar]
- Hallsson JH, Favor J, Hodgkinson C, Glaser T, Lamoreux ML, Magnúsdóttir R, Gunnarsson GJ, Sweet HO, Copeland NG, Jenkins NA, Steingrímsson E, 2000. Genomic, transcriptional and mutational analysis of the mouse microphthalmia locus. Genetics 155, 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JW, Lyu J, Park Y.j., Jang SY, Park TK, 2015. Wnt/β-Catenin Signaling Mediates Regeneration of Retinal Pigment Epithelium After Laser Photocoagulation in Mouse Eye. Investigative Ophthalmology & Visual Science 56, 8314–8324. [DOI] [PubMed] [Google Scholar]
- Hanovice NJ, Leach LL, Slater K, Gabriel AE, Romanovicz D, Shao E, Collery R, Burton EA, Lathrop KL, Link BA, Gross JM, 2019. Regeneration of the zebrafish retinal pigment epithelium after widespread genetic ablation. PLOS Genetics 15, e1007939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus J, Anderson C, Sarraf D, Ma J, Wang S, 2016. Retinal pigment epithelial cell necroptosis in response to sodium iodate. Cell Death Discov 2, 16054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Zhou Q, Ma J, Zhao Y, Wang S, 2016. miR-146a is upregulated during retinal pigment epithelium (RPE)/choroid aging in mice and represses IL-6 and VEGF-A expression in RPE cells. J Clin Exp Ophthalmol 7, 562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J, Subhi Y, Sørensen TL, 2017. Effect of aging and lifestyle on photoreceptors and retinal pigment epithelium: cross-sectional study in a healthy Danish population. Pathobiol Aging Age Relat Dis 7, 1398016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hero I, Farjah M, Scholtz CL, 1991. The prenatal development of the optic fissure in colobomatous microphthalmia. Invest Ophthalmol Vis Sci 32, 2622–2635. [PubMed] [Google Scholar]
- Hirose K, Shimoda N, Kikuchi Y, 2013. Transient reduction of 5-methylcytosine and 5-hydroxymethylcytosine is associated with active DNA demethylation during regeneration of zebrafish fin. Epigenetics 8, 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch L, Nazari H, Sreekumar PG, Kannan R, Dustin L, Zhu D, Barron E, Hinton DR, 2015. TGF-β2 secretion from RPE decreases with polarization and becomes apically oriented. Cytokine 71, 394–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson CA, Moore KJ, Nakayama A, Steingrímsson E, Copeland NG, Jenkins NA, Arnheiter H, 1993. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell 74, 395–404. [DOI] [PubMed] [Google Scholar]
- Holz FG, Bindewald-Wittich A, Fleckenstein M, Dreyhaupt J, Scholl HP, Schmitz-Valckenberg S, 2007. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol 143, 463–472. [DOI] [PubMed] [Google Scholar]
- Horsford DJ, Nguyen MT, Sellar GC, Kothary R, Arnheiter H, McInnes RR, 2005. Chx10 repression of Mitf is required for the maintenance of mammalian neuroretinal identity. Development 132, 177–187. [DOI] [PubMed] [Google Scholar]
- Hu JG, Gallemore RP, Bok D, Lee AY, Frambach DA, 1994. Localization of NaK ATPase on cultured human retinal pigment epithelium. Invest Ophthalmol Vis Sci 35, 3582–3588. [PubMed] [Google Scholar]
- Hu Y, Wang X, Hu B, Mao Y, Chen Y, Yan L, Yong J, Dong J, Wei Y, Wang W, Wen L, Qiao J, Tang F, 2019. Dissecting the transcriptome landscape of the human fetal neural retina and retinal pigment epithelium by single-cell RNA-seq analysis. PLOS Biology 17, e3000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt GA, Dowling JE, 1997. Retinoic acid. A key molecule for eye and photoreceptor development. Invest Ophthalmol Vis Sci 38, 1471–1475. [PubMed] [Google Scholar]
- Ikegami Y, Mitsuda S, Araki M, 2002. Neural cell differentiation from retinal pigment epithelial cells of the newt: An organ culture model for the urodele retinal regeneration. Journal of Neurobiology 50, 209–220. [DOI] [PubMed] [Google Scholar]
- Ikenouchi J, Matsuda M, Furuse M, Tsukita S, 2003. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. Journal of Cell Science 116, 1959. [DOI] [PubMed] [Google Scholar]
- Imai D, Yoneya S, Gehlbach PL, Wei LL, Mori K, 2005. Intraocular gene transfer of pigment epithelium-derived factor rescues photoreceptors from light-induced cell death. J Cell Physiol 202, 570–578. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Sawada Y, Yoshitomi T, 2015. Structure and function of the interphotoreceptor matrix surrounding retinal photoreceptor cells. Experimental Eye Research 133, 3–18. [DOI] [PubMed] [Google Scholar]
- Islam MR, Nakamura K, Casco-Robles MM, Kunahong A, Inami W, Toyama F, Maruo F, Chiba C, 2014. The newt reprograms mature RPE cells into a unique multipotent state for retinal regeneration. Scientific Reports 4, 6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam S, Carey TE, Wolf GT, Wheelock MJ, Johnson KR, 1996. Expression of N-cadherin by human squamous carcinoma cells induces a scattered fibroblastic phenotype with disrupted cell-cell adhesion. J Cell Biol 135, 1643–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivert L, Keldbye H, Gouras P, 2005. Age-related changes in the basement membrane of the retinal pigment epithelium of Rpe65 −/− and wild-type mice. Graefes Arch Clin Exp Ophthalmol 243, 250–256. [DOI] [PubMed] [Google Scholar]
- Iwanami N, 2014. Zebrafish as a model for understanding the evolution of the vertebrate immune system and human primary immunodeficiency. Exp Hematol 42, 697–706. [DOI] [PubMed] [Google Scholar]
- Jackson GR, Owsley C, Curcio CA, 2002. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res Rev 1, 381–396. [DOI] [PubMed] [Google Scholar]
- Johnson LV, Leitner WP, Staples MK, Anderson DH, 2001. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp Eye Res 73, 887–896. [DOI] [PubMed] [Google Scholar]
- Johnson LV, Ozaki S, Staples MK, Erickson PA, Anderson DH, 2000. A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res 70, 441–449. [DOI] [PubMed] [Google Scholar]
- Jorstad NL, Wilken MS, Grimes WN, Wohl SG, VandenBosch LS, Yoshimatsu T, Wong RO, Rieke F, Reh TA, 2017. Stimulation of functional neuronal regeneration from Müller glia in adult mice. Nature 548, 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JH, Joo CK, 2016. MicroRNA-124 Controls Transforming Growth Factor β1-Induced Epithelial-Mesenchymal Transition in the Retinal Pigment Epithelium by Targeting RHOG. Invest Ophthalmol Vis Sci 57, 12–22. [DOI] [PubMed] [Google Scholar]
- Kampik D, Basche M, Luhmann UFO, Nishiguchi KM, Williams JAE, Greenwood J, Moss SE, Han H, Azam S, Duran Y, Robbie SJ, Bainbridge JWB, Larkin DF, Smith AJ, Ali RR, 2017. In situ regeneration of retinal pigment epithelium by gene transfer of E2F2: a potential strategy for treatment of macular degenerations. Gene Ther 24, 810–818. [DOI] [PubMed] [Google Scholar]
- Kaneko J, Chiba C, 2009. Immunohistochemical analysis of Musashi-1 expression during retinal regeneration of adult newt. Neuroscience Letters 450, 252–257. [DOI] [PubMed] [Google Scholar]
- Karl MO, Reh TA, 2010. Regenerative medicine for retinal diseases: activating endogenous repair mechanisms. Trends Mol Med 16, 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM, Langmann T, 2015. Retinal microglia: just bystander or target for therapy? Progress in Retinal and Eye Research 45, 30–57. [DOI] [PubMed] [Google Scholar]
- Kay P, Yang YC, Paraoan L, 2013. Directional protein secretion by the retinal pigment epithelium: roles in retinal health and the development of age-related macular degeneration. J Cell Mol Med 17, 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy CJ, Rakoczy PE, Constable IJ, 1995. Lipofuscin of the retinal pigment epithelium: A review. Eye 9, 763–771. [DOI] [PubMed] [Google Scholar]
- Khan KN, Mahroo OA, Khan RS, Mohamed MD, McKibbin M, Bird A, Michaelides M, Tufail A, Moore AT, 2016. Differentiating drusen: Drusen and drusen-like appearances associated with ageing, age-related macular degeneration, inherited eye disease and other pathological processes. Prog Retin Eye Res 53, 70–106. [DOI] [PubMed] [Google Scholar]
- Kiilgaard JF, Prause JU, Prause M, Scherfig E, Nissen MH, la Cour M, 2007. Subretinal posterior pole injury induces selective proliferation of RPE cells in the periphery in in vivo studies in pigs. Invest Ophthalmol Vis Sci 48, 355–360. [DOI] [PubMed] [Google Scholar]
- Kim JB, Islam S, Kim YJ, Prudoff RS, Sass KM, Wheelock MJ, Johnson KR, 2000. N-Cadherin extracellular repeat 4 mediates epithelial to mesenchymal transition and increased motility. J Cell Biol 151, 1193–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Park R, Lee JHJ, Shin J, Nicklas J, Kim S, Cho S, 2016. Yap is essential for retinal progenitor cell cycle progression and RPE cell fate acquisition in the developing mouse eye. Developmental Biology 419(2), 336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA, 2006. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A 103, 13180–13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiuchi K, Yoshizawa K, Shikata N, Moriguchi K, Tsubura A, 2002. Morphologic characteristics of retinal degeneration induced by sodium iodate in mice. Current Eye Research 25, 373–379. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Cruickshanks KJ, 1999. The prevalence of age-related maculopathy by geographic region and ethnicity. Prog Retin Eye Res 18, 371–389. [DOI] [PubMed] [Google Scholar]
- Koenig KM, Gross JM, 2020. Evolution and development of complex eyes: a celebration of diversity. Development 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkinopoulos I, Shahabi G, Colman A, Jeffery G, 2011. Mature peripheral RPE cells have an intrinsic capacity to proliferate; a potential regulatory mechanism for age-related cell loss. PLoS One 6, e18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolomeyer AM, Sugino IK, Zarbin MA, 2011. Characterization of conditioned media collected from cultured adult versus fetal retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 52, 5973–5986. [DOI] [PubMed] [Google Scholar]
- Kolomeyer AM, Zarbin MA, 2014. Trophic factors in the pathogenesis and therapy for retinal degenerative diseases. Surv Ophthalmol 59, 134–165. [DOI] [PubMed] [Google Scholar]
- Korte GE, Reppucci V, Henkind P, 1984. RPE destruction causes choriocapillary atrophy. Invest Ophthalmol Vis Sci 25, 1135–1145. [PubMed] [Google Scholar]
- Kuriyama F, Ueda Y, Araki M, 2009. Complete reconstruction of the retinal laminar structure from a cultured retinal pigment epithelium is triggered by altered tissue interaction and promoted by overlaid extracellular matrices. Dev Neurobiol 69, 950–958. [DOI] [PubMed] [Google Scholar]
- Kutty RK, Nagineni CN, Samuel W, Vijayasarathy C, Jaworski C, Duncan T, Cameron JE, Flemington EK, Hooks JJ, Redmond TM, 2013. Differential regulation of microRNA-146a and microRNA-146b-5p in human retinal pigment epithelial cells by interleukin-1β, tumor necrosis factor-α, and interferon-γ. Mol Vis 19, 737–750. [PMC free article] [PubMed] [Google Scholar]
- Kwan KM, Otsuna H, Kidokoro H, Carney KR, Saijoh Y, Chien CB, 2012. A complex choreography of cell movements shapes the vertebrate eye. Development 139, 359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagutin OV, Zhu CC, Kobayashi D, Topczewski J, Shimamura K, Puelles L, Russell HRC, McKinnon PJ, Solnica-Krezel L, Oliver G, 2003. Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes & development 17, 368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahne M, Nagashima M, Hyde DR, Hitchcock PF, 2020. Reprogramming Müller Glia to Regenerate Retinal Neurons. Annu Rev Vis Sci 6, 171–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YL, Jacoby RO, Jonas AM, 1978. Age-related and light-associated retinal changes in Fischer rats. Invest Ophthalmol Vis Sci 17, 634–638. [PubMed] [Google Scholar]
- Lakkaraju A, Umapathy A, Tan LX, Daniele L, Philp NJ, Boesze-Battaglia K, Williams DS, 2020. The cell biology of the retinal pigment epithelium. Prog Retin Eye Res, 100846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, 2011. Emerging inflammasome effector mechanisms. Nature Reviews Immunology 11, 213–220. [DOI] [PubMed] [Google Scholar]
- Lane BM, Lister JA, 2012. Otx but not Mitf transcription factors are required for zebrafish retinal pigment epithelium development. PloS one 7, e49357–e49357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM, Unoki K, Yasumura D, Matthes MT, Yancopoulos GD, Steinberg RH, 1992. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci USA 89, 11249–11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM, Yasumura D, Matthes MT, Lau-Villacorta C, Unoki K, Sung CH, Steinberg RH, 1998. Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophthalmol Vis Sci 39, 592–602. [PubMed] [Google Scholar]
- Leach LL, Clegg DO, 2015. Concise Review: Making Stem Cells Retinal: Methods for Deriving Retinal Pigment Epithelium and Implications for Patients With Ocular Disease. Stem Cells 33, 2363–2373. [DOI] [PubMed] [Google Scholar]
- Leach LL, Hanovice NJ, George SM, Gabriel AE, Gross JM, 2020. The immune response is a critical regulator of zebrafish retinal pigment epithelium regeneration. bioRxiv, 2020.2008.2014.250043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MY, Ham DI, 2014. Subretinal drusenoid deposits with increased autofluorescence in eyes with reticular pseudodrusen. Retina 34, 69–76. [DOI] [PubMed] [Google Scholar]
- Lewis KL, Del Cid N, Traver D, 2014. Perspectives on antigen presenting cells in zebrafish. Developmental and comparative immunology 46, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Wen R, Banzon T, Maminishkis A, Miller SS, 2011. CNTF mediates neurotrophic factor secretion and fluid absorption in human retinal pigment epithelium. PLoS One 6, e23148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Joseph NM, Easter SS Jr., 2000. The morphogenesis of the zebrafish eye, including a fate map of the optic vesicle. Dev Dyn 218, 175–188. [DOI] [PubMed] [Google Scholar]
- Liao R, Yan F, Zeng Z, Wang H, Qiu K, Xu J, Zheng W, 2018. Insulin-like growth factor-1 activates PI3K/Akt signalling to protect human retinal pigment epithelial cells from amiodarone-induced oxidative injury. Br J Pharmacol 175, 125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY, 2012. Age-related macular degeneration. Lancet 379, 1728–1738. [DOI] [PubMed] [Google Scholar]
- Lister JA, Lane BM, Nguyen A, Lunney K, 2011. Embryonic expression of zebrafish MiT family genes tfe3b, tfeb, and tfec. Developmental Dynamics 240, 2529–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Copland DA, Theodoropoulou S, Chiu HA, Barba MD, Mak KW, Mack M, Nicholson LB, Dick AD, 2016. Impairing autophagy in retinal pigment epithelium leads to inflammasome activation and enhanced macrophage-mediated angiogenesis. Sci Rep 6, 20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Zhong TP, 2017. MAPK/ERK signalling is required for zebrafish cardiac regeneration. Biotechnology Letters 39, 1069–1077. [DOI] [PubMed] [Google Scholar]
- Liu T, Jung H, Liu J, Droettboom M, Tam J, 2017. Noninvasive near infrared autofluorescence imaging of retinal pigment epithelial cells in the human retina using adaptive optics. Biomed Opt Express 8, 4348–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, El-Naggar S, Darling DS, Higashi Y, Dean DC, 2008. Zeb1 links epithelial-mesenchymal transition and cellular senescence. Development 135, 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li Y, Wang C, Zhang Y, Su G, 2019a. Morphologic and histopathologic change of sodium iodate-induced retinal degeneration in adult rats. International journal of clinical and experimental pathology 12, 443–454. [PMC free article] [PubMed] [Google Scholar]
- Liu Y-H, Mölzer C, Milne GC, Kuffová L, Forrester JV, 2019b. Transmission Electron Microscopy Data on drusen-like deposits in the retinal degeneration sTg-IRBP: HEL mouse model. Data in Brief 22, 140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longbottom R, Fruttiger M, Douglas RH, Martinez-Barbera JP, Greenwood J, Moss SE, 2009. Genetic ablation of retinal pigment epithelial cells reveals the adaptive response of the epithelium and impact on photoreceptors. Proc Natl Acad Sci U S A 106, 18728–18733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez PF, Yan Q, Kohen L, Rao NA, Spee C, Black J, Oganesian A, 1995. Retinal Pigment Epithelial Wound Healing In Vivo. Archives of Ophthalmology 113, 1437–1446. [DOI] [PubMed] [Google Scholar]
- Lückoff A, Caramoy A, Scholz R, Prinz M, Kalinke U, Langmann T, 2016. Interferon-beta signaling in retinal mononuclear phagocytes attenuates pathological neovascularization. EMBO Mol Med 8, 670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz-Madrigal A, Grajales-Esquivel E, McCorkle A, DiLorenzo AM, Barbosa-Sabanero K, Tsonis PA, Del Rio-Tsonis K, 2014. Reprogramming of the chick retinal pigmented epithelium after retinal injury. BMC Biol 12, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz-Madrigal A, Grajales-Esquivel E, Tangeman J, Kosse S, Liu L, Wang K, Fausey A, Liang C, Tsonis PA, Del Rio-Tsonis K, 2020. DNA demethylation is a driver for chick retina regeneration. Epigenetics, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Li H, Chen Y, Yang J, Chen H, Arnheiter H, Hou L, 2019. The transcription factor MITF in RPE function and dysfunction. Prog Retin Eye Res 73, 100766. [DOI] [PubMed] [Google Scholar]
- Machalińska A, Lejkowska R, Duchnik M, Kawa M, Rogińska D, Wiszniewska B, Machaliński B, 2014. Dose-Dependent Retinal Changes Following Sodium Iodate Administration: Application of Spectral-Domain Optical Coherence Tomography for Monitoring of Retinal Injury and Endogenous Regeneration. Current Eye Research 39, 1033–1041. [DOI] [PubMed] [Google Scholar]
- Machalińska A, Lubiński W, Kłos P, Kawa M, Baumert B, Penkala K, Grzegrzółka R, Karczewicz D, Wiszniewska B, Machaliński B, 2010. Sodium Iodate Selectively Injuries the Posterior Pole of the Retina in a Dose-Dependent Manner: Morphological and Electrophysiological Study. Neurochemical Research 35, 1819–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddaluno L, Urwyler C, Werner S, 2017. Fibroblast growth factors: key players in regeneration and tissue repair. Development 144, 4047. [DOI] [PubMed] [Google Scholar]
- Mai S, Klinkenberg M, Auburger G, Bereiter-Hahn J, Jendrach M, 2010. Decreased expression of Drp1 and Fis1 mediates mitochondrial elongation in senescent cells and enhances resistance to oxidative stress through PINK1. Journal of Cell Science 123, 917–926. [DOI] [PubMed] [Google Scholar]
- Marmorstein AD, Gan YC, Bonilha VL, Finnemann SC, Csaky KG, Rodriguez-Boulan E, 1998. Apical polarity of N-CAM and EMMPRIN in retinal pigment epithelium resulting from suppression of basolateral signal recognition. J Cell Biol 142, 697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marneros AG, Fan J, Yokoyama Y, Gerber HP, Ferrara N, Crouch RK, Olsen BR, 2005. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol 167, 1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P, 2001. Pax6 is required for the multipotent state of retinal progenitor cells. Cell 105, 43–55. [DOI] [PubMed] [Google Scholar]
- Martinez-De Luna RI, Kelly LE, El-Hodiri HM, 2011. The Retinal Homeobox (Rx) gene is necessary for retinal regeneration. Dev Biol 353, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Morales JR, Dolez V, Rodrigo I, Zaccarini R, Leconte L, Bovolenta P, Saule S, 2003. OTX2 activates the molecular network underlying retina pigment epithelium differentiation. J Biol Chem 278, 21721–21731. [DOI] [PubMed] [Google Scholar]
- Martinez-Morales JR, Signore M, Acampora D, Simeone A, Bovolenta P, 2001. Otx genes are required for tissue specification in the developing eye. Development 128, 2019–2030. [DOI] [PubMed] [Google Scholar]
- Martinez-Morales JR, Wittbrodt J, 2009. Shaping the vertebrate eye. Curr Opin Genet Dev 19, 511–517. [DOI] [PubMed] [Google Scholar]
- Maruotti J, Thein T, Zack DJ, Esumi N, 2012. MITF-M, a ‘melanocyte-specific’ isoform, is expressed in the adult retinal pigment epithelium. Pigment Cell Melanoma Res 25, 641–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata NL, Moghrabi WN, Lee JS, Bui TV, Radu RA, Horwitz J, Travis GH, 2004. Rpe65 is a retinyl ester binding protein that presents insoluble substrate to the isomerase in retinal pigment epithelial cells. J Biol Chem 279, 635–643. [DOI] [PubMed] [Google Scholar]
- Mathers PH, Grinberg A, Mahon KA, Jamrich M, 1997. The Rx homeobox gene is essential for vertebrate eye development. Nature 387, 603–607. [DOI] [PubMed] [Google Scholar]
- Matsuo I, Kuratani S, Kimura C, Takeda N, Aizawa S, 1995. Mouse Otx2 functions in the formation and patterning of rostral head. Genes Dev 9, 2646–2658. [DOI] [PubMed] [Google Scholar]
- McGill TJ, Cottam B, Lu B, Wang S, Girman S, Tian C, Huhn SL, Lund RD, Capela A, 2012. Transplantation of human central nervous system stem cells – neuroprotection in retinal degeneration. European Journal of Neuroscience 35, 468–477. [DOI] [PubMed] [Google Scholar]
- McGill TJ, Osborne L, Lu B, Stoddard J, Huhn S, Tsukamoto A, Capela A, 2019. Subretinal Transplantation of Human Central Nervous System Stem Cells Stimulates Controlled Proliferation of Endogenous Retinal Pigment Epithelium. Translational Vision Science & Technology 8, 43–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher AL, 2017. Macrophages and fibroblasts during inflammation and tissue repair in models of organ regeneration. Regeneration (Oxf) 4, 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher AL, Neff AW, 2005. Regenerative capacity and the developing immune system. Adv Biochem Eng Biotechnol 93, 39–66. [DOI] [PubMed] [Google Scholar]
- Mescher AL, Neff AW, King MW, 2013. Changes in the inflammatory response to injury and its resolution during the loss of regenerative capacity in developing Xenopus limbs. PLoS One 8, e80477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher AL, Neff AW, King MW, 2017. Inflammation and immunity in organ regeneration. Dev Comp Immunol 66, 98–110. [DOI] [PubMed] [Google Scholar]
- Miesfeld JB, Gestri G, Clark BS, Flinn MA, Poole RJ, Bader JR, Besharse JC, Wilson SW, Link BA, 2015. Yap and Taz regulate retinal pigment epithelial cell fate. Development 142, 3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen PJ, Ebner R, Lopez AR, Derynck R, 1994. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol 127, 2021–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H, Miller B, Ishibashi T, Ryan SJ, 1990. Pathogenesis of laser-induced choroidal subretinal neovascularization. Investigative Ophthalmology & Visual Science 31, 899–908. [PubMed] [Google Scholar]
- Mitsuda S, Yoshii C, Ikegami Y, Araki M, 2005. Tissue interaction between the retinal pigment epithelium and the choroid triggers retinal regeneration of the newt Cynops pyrrhogaster. Developmental Biology 280, 122–132. [DOI] [PubMed] [Google Scholar]
- Mitter SK, Song C, Qi X, Mao H, Rao H, Akin D, Lewin A, Grant M, Dunn W Jr., Ding J, Bowes Rickman C, Boulton M, 2014. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 10, 1989–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Araki M, 2014. Retinal stem/progenitor cells in the ciliary marginal zone complete retinal regeneration: A study of retinal regeneration in a novel animal model. Dev Neurobiol 74, 739–756. [DOI] [PubMed] [Google Scholar]
- Mizuno A, Yasumuro H, Yoshikawa T, Inami W, Chiba C, 2012. MEK-ERK signaling in adult newt retinal pigment epithelium cells is strengthened immediately after surgical induction of retinal regeneration. Neurosci Lett 523, 39–44. [DOI] [PubMed] [Google Scholar]
- Mochii M, Mazaki Y, Mizuno N, Hayashi H, Eguchi G, 1998. Role of Mitf in differentiation and transdifferentiation of chicken pigmented epithelial cell. Dev Biol 193, 47–62. [DOI] [PubMed] [Google Scholar]
- Molotkov A, Molotkova N, Duester G, 2006. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development 133, 1901–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KH, Kim H-T, Lee D, Rao MB, Levine EM, Lim D-S, Kim JW, 2018. Differential Expression of NF2 in Neuroepithelial Compartments Is Necessary for Mammalian Eye Development. Developmental cell 44, 13–28.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Marmol T, Cavodeassi F, Bovolenta P, 2018. Setting Eyes on the Retinal Pigment Epithelium. Front Cell Dev Biol 6, 145–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty P, Boulton M, Dickson A, McLeod D, 1994. Production of IGF-I and IGF binding proteins by retinal cells in vitro. Br J Ophthalmol 78, 638–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi M, Nakamura S, Inoue Y, Nishinaka A, Nakamura M, Shimazawa M, Hara H, 2018. Irreversible Photoreceptors and RPE Cells Damage by Intravenous Sodium Iodate in Mice Is Related to Macrophage Accumulation. Invest Ophthalmol Vis Sci 59, 3476–3487. [DOI] [PubMed] [Google Scholar]
- Morino I, Hiscott P, McKechnie N, Grierson I, 1990. Variation in epiretinal membrane components with clinical duration of the proliferative tissue. Br J Ophthalmol 74, 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossahebi-Mohammadi M, Quan M, Zhang J-S, Li X, 2020. FGF Signaling Pathway: A Key Regulator of Stem Cell Pluripotency. Front Cell Dev Biol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F, Rohrer H, Vogel-Höpker A, 2007. Bone morphogenetic proteins specify the retinal pigment epithelium in the chick embryo. Development 134, 3483–3493. [DOI] [PubMed] [Google Scholar]
- Mullins RF, Russell SR, Anderson DH, Hageman GS, 2000. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. Faseb j 14, 835–846. [PubMed] [Google Scholar]
- Nabeshima A, Nishibayashi C, Ueda Y, Ogino H, Araki M, 2013. Loss of cell-extracellular matrix interaction triggers retinal regeneration accompanied by Rax and Pax6 activation. Genesis 51, 410–419. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Islam MR, Takayanagi M, Yasumuro H, Inami W, Kunahong A, Casco-Robles RM, Toyama F, Chiba C, 2014. A Transcriptome for the Study of Early Processes of Retinal Regeneration in the Adult Newt, Cynops pyrrhogaster. PLOS ONE 9, e109831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama A, Nguyen MT, Chen CC, Opdecamp K, Hodgkinson CA, Arnheiter H, 1998. Mutations in microphthalmia, the mouse homolog of the human deafness gene MITF, affect neuroepithelial and neural crest-derived melanocytes differently. Mech Dev 70, 155–166. [DOI] [PubMed] [Google Scholar]
- Nazari H, Zhang L, Zhu D, Chader GJ, Falabella P, Stefanini F, Rowland T, Clegg DO, Kashani AH, Hinton DR, Humayun MS, 2015. Stem cell based therapies for age-related macular degeneration: The promises and the challenges. Prog 48, 1–39. [DOI] [PubMed] [Google Scholar]
- Newsome DA, Miceli MV, Liles MR, Tate DJ, Oliver PD, 1994. Antioxidants in the Retin Eye Res retinal pigment epithelium. Progress in Retinal and Eye Research 13, 101–123. [Google Scholar]
- Nguyen M, Arnheiter H, 2000. Signaling and transcriptional regulation in early mammalian eye development: a link between FGF and MITF. Development 127, 3581–3591. [DOI] [PubMed] [Google Scholar]
- Noell WK, 1953. Experimentally induced toxic effects on structure and function of visual cells and pigment epithelium. Am J Ophthalmol 36, 103–116. [DOI] [PubMed] [Google Scholar]
- Nussenblatt RB, Byrnes G, Sen HN, Yeh S, Faia L, Meyerle C, Wroblewski K, Li Z, Liu B, Chew E, Sherry PR, Friedman P, Gill F, Ferris F 3rd, 2010. A randomized pilot study of systemic immunosuppression in the treatment of age-related macular degeneration with choroidal neovascularization. Retina 30, 1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Koren EG, Yu C, Klingeborn M, Wong AYW, Prigge CL, Mathew R, Kalnitsky J, Msallam RA, Silvin A, Kay JN, Bowes Rickman C, Arshavsky VY, Ginhoux F, Merad M, Saban DR, 2019. Microglial Function Is Distinct in Different Anatomical Locations during Retinal Homeostasis and Degeneration. Immunity 50, 723–737.e727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oron-Karni V, Farhy C, Elgart M, Marquardt T, Remizova L, Yaron O, Xie Q, Cvekl A, Ashery-Padan R, 2008. Dual requirement for Pax6 in retinal progenitor cells. Development 135, 4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdamar B, Bose R, Barrios-Rodiles M, Wang H-R, Zhang Y, Wrana JL, 2005. Regulation of the Polarity Protein Par6 by TGFß Receptors Controls Epithelial Cell Plasticity. Science 307, 1603. [DOI] [PubMed] [Google Scholar]
- Padgett LC, Lui GM, Werb Z, LaVail MM, 1997. Matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 in the retinal pigment epithelium and interphotoreceptor matrix: vectorial secretion and regulation. Exp Eye Res 64, 927–938. [DOI] [PubMed] [Google Scholar]
- Park CM, Hollenberg MJ, 1989. Basic fibroblast growth factor induces retinal regeneration in vivo. Developmental Biology 134, 201–205. [DOI] [PubMed] [Google Scholar]
- Park CM, Hollenberg MJ, 1991. Induction of retinal regeneration in vivo by growth factors. Dev Biol 148, 322–333. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno MA, Locascio A, Rodrigo I, Dhondt G, Portillo F, Nieto MA, Cano A, 2001. A new role for E12/E47 in the repression of E-cadherin expression and epithelial-mesenchymal transitions. J Biol Chem 276, 27424–27431. [DOI] [PubMed] [Google Scholar]
- Philips GT, Stair CN, Young Lee H, Wroblewski E, Berberoglu MA, Brown NL, Mastick GS, 2005. Precocious retinal neurons: Pax6 controls timing of differentiation and determination of cell type. Dev Biol 279, 308–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak J, Wilken MS, Ueki Y, Cox KE, Sullivan JM, Taylor RJ, Levine EM, Reh TA, 2013. ASCL1 reprograms mouse Müller glia into neurogenic retinal progenitors. Development 140, 2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter FD, Drago J, Xu Y, Cheema SS, Wassif C, Huang SP, Lee E, Grinberg A, Massalas JS, Bodine D, Alt F, Westphal H, 1997. Lhx2, a LIM homeobox gene, is required for eye, forebrain, and definitive erythrocyte development. Development 124, 2935. [DOI] [PubMed] [Google Scholar]
- Poss KD, Shen J, Keating MT, 2000. Induction of lef1 during zebrafish fin regeneration. Dev Dyn 219, 282–286. [DOI] [PubMed] [Google Scholar]
- Powell C, Grant AR, Cornblath E, Goldman D, 2013. Analysis of DNA methylation reveals a partial reprogramming of the Müller glia genome during retina regeneration. Proceedings of the National Academy of Sciences 110, 19814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhudesai SN, Cameron DA, Stenkamp DL, 2005. Targeted effects of retinoic acid signaling upon photoreceptor development in zebrafish. Developmental biology 287, 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian M, Fang X, Wang X, 2017. Autophagy and inflammation. Clin Transl Med 6, 24–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Kidd AR 3rd, Thomas JL, Poss KD, Hyde DR, Raymond PA, Thummel R, 2011. FGF signaling regulates rod photoreceptor cell maintenance and regeneration in zebrafish. Exp Eye Res 93, 726–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahner C, Fukuhara M, Peng S, Kojima S, Rizzolo LJ, 2004. The apical and basal environments of the retinal pigment epithelium regulate the maturation of tight junctions during development. Journal of Cell Science 117, 3307. [DOI] [PubMed] [Google Scholar]
- Rapaport DH, Rakic P, Yasamura D, LaVail MM, 1995. Genesis of the retinal pigment epithelium in the macaque monkey. J Comp Neurol 363, 359–376. [DOI] [PubMed] [Google Scholar]
- Raposo G, Tenza D, Murphy DM, Berson JF, Marks MS, 2001. Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J Cell Biol 152, 809–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid K, Akhtar-Schaefer I, Langmann T, 2019. Microglia in Retinal Degeneration. Front Immunol 10, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt R, Centanin L, Tavhelidse T, Inoue D, Wittbrodt B, Concordet J-P, Martinez-Morales JR, Wittbrodt J, 2015. Sox2, Tlx, Gli3, and Her9 converge on Rx2 to define retinal stem cells in vivo. The EMBO Journal 34, 1572–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR, 2003. Cell Migration: Integrating Signals from Front to Back. Science 302, 1704. [DOI] [PubMed] [Google Scholar]
- Rizzolo LJ, 1997. Polarity and the development of the outer blood-retinal barrier. Histol Histopathol 12, 1057–1067. [PubMed] [Google Scholar]
- Rizzolo LJ, 2007. Development and Role of Tight Junctions in the Retinal Pigment Epithelium, International Review of Cytology. Academic Press, pp. 195–234. [DOI] [PubMed] [Google Scholar]
- Robert J, Ohta Y, 2009. Comparative and developmental study of the immune system in Xenopus. Dev Dyn 238, 1249–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roider J, Michaud NA, Flotte TJ, Birngruber R, 1992. Response of the retinal pigment epithelium to selective photocoagulation. Arch Ophthalmol 110, 1786–1792. [DOI] [PubMed] [Google Scholar]
- Rollins-Smith LA, 1998. Metamorphosis and the amphibian immune system. Immunol Rev 166, 221–230. [DOI] [PubMed] [Google Scholar]
- Rowan S, Chen CM, Young TL, Fisher DE, Cepko CL, 2004. Transdifferentiation of the retina into pigmented cells in ocular retardation mice defines a new function of the homeodomain gene Chx10. Development 131, 5139–5152. [DOI] [PubMed] [Google Scholar]
- Ryan SJ, 1979. The development of an experimental model of subretinal neovascularization in disciform macular degeneration. Trans Am Ophthalmol Soc 77, 707–745. [PMC free article] [PubMed] [Google Scholar]
- Saini JS, Temple S, Stern JH, 2016. Human retinal pigment epithelium stem cell (RPESC). Adv Exp Med Biol 854, 557–562. [DOI] [PubMed] [Google Scholar]
- Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, Amore PA, 2009. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proceedings of the National Academy of Sciences 106, 18751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakami S, Etter P, Reh TA, 2008. Activin signaling limits the competence for retinal regeneration from the pigmented epithelium. Mechanisms of development 125, 106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J, 2003. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci 44, 3578–3585. [DOI] [PubMed] [Google Scholar]
- Salero E, Blenkinsop TA, Corneo B, Harris A, Rabin D, Stern JH, Temple S, 2012. Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell 10, 88–95. [DOI] [PubMed] [Google Scholar]
- Sarks SH, 1976. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol 60, 324–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl HP, Bellmann C, Dandekar SS, Bird AC, Fitzke FW, 2004. Photopic and scotopic fine matrix mapping of retinal areas of increased fundus autofluorescence in patients with age-related maculopathy. Invest Ophthalmol Vis Sci 45, 574–583. [DOI] [PubMed] [Google Scholar]
- Schumacker ST, Coppage KR, Enke RA, 2020. RNA sequencing analysis of the human retina and associated ocular tissues. Scientific Data 7, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwegler JS, Knorz MC, Akkoyun I, Liesenhoff H, 1997. Basic, not acidic fibroblast growth factor stimulates proliferation of cultured human retinal pigment epithelial cells. Mol Vis 3, 10. [PubMed] [Google Scholar]
- Sennlaub F, Auvynet C, Calippe B, Lavalette S, Poupel L, Hu SJ, Dominguez E, Camelo S, Levy O, Guyon E, Saederup N, Charo IF, Rooijen NV, Nandrot E, Bourges J-L, Behar-Cohen F, Sahel J-A, Guillonneau X, Raoul W, Combadiere C, 2013. CCR2(+) monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice. EMBO Mol Med 5, 1775–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo SJ, Krebs MP, Mao H, Jones K, Conners M, Lewin AS, 2012. Pathological consequences of long-term mitochondrial oxidative stress in the mouse retinal pigment epithelium. Exp Eye Res 101, 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Bose D, Maminishkis A, Bharti K, 2020. Retinal Pigment Epithelium Replacement Therapy for Age-Related Macular Degeneration: Are We There Yet? Annual Review of Pharmacology and Toxicology 60, 553–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu DY, Butcher E, Saint-Geniez M, 2020. EMT and EndMT: Emerging Roles in Age-Related Macular Degeneration. International journal of molecular sciences 21, 4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva NJ, Nagashima M, Li J, Kakuk-Atkins L, Ashrafzadeh M, Hyde DR, Hitchcock PF, 2020. Inflammation and matrix metalloproteinase 9 (Mmp-9) regulate photoreceptor regeneration in adult zebrafish. Glia 68, 1445–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinagoga KL, Larimer-Picciani AM, George SM, Spencer SA, Lister JA, Gross JM, 2020. Mitf-family transcription factor function is required within cranial neural crest cells to promote choroid fissure closure. Development 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Mishra R, Arango NA, Deng JM, Behringer RR, Saunders GF, 2002. Iris hypoplasia in mice that lack the alternatively spliced Pax6(5a) isoform. Proc Natl Acad Sci U S A 99, 6812–6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JR, Aycinena J-C, Del Rio-Tsonis K, 2007. Fibroblast growth factor–hedgehog interdependence during retina regeneration. Developmental Dynamics 236, 1161–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JR, Madhavan M, Ewing JD, Jones DK, Lehman BM, Del Rio-Tsonis K, 2004. The hedgehog pathway is a modulator of retina regeneration. Development 131, 4607–4621. [DOI] [PubMed] [Google Scholar]
- Srivastava D, DeWitt N, 2016. In Vivo Cellular Reprogramming: The Next Generation. Cell 166, 1386–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starnes AC, Huisingh C, McGwin G Jr., Sloan KR, Ablonczy Z, Smith RT, Curcio CA, Ach T, 2016. Multi-nucleate retinal pigment epithelium cells of the human macula exhibit a characteristic and highly specific distribution. Vis Neurosci 33, e001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavnezer J, Amemiya CT, 2004. Evolution of isotype switching. Semin Immunol 16, 257–275. [DOI] [PubMed] [Google Scholar]
- Stavnezer J, Guikema JE, Schrader CE, 2008. Mechanism and regulation of class switch recombination. Annu Rev Immunol 26, 261–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg JS, Fitzke FW, Fimmers R, Fleckenstein M, Holz FG, Schmitz-Valckenberg S, 2015. Scotopic and Photopic Microperimetry in Patients With Reticular Drusen and Age-Related Macular Degeneration. JAMA Ophthalmology 133, 690–697. [DOI] [PubMed] [Google Scholar]
- Steinfeld J, Steinfeld I, Coronato N, Hampel M-L, Layer PG, Araki M, Vogel-Höpker A, 2013. RPE specification in the chick is mediated by surface ectoderm-derived BMP and Wnt signalling. Development 140, 4959. [DOI] [PubMed] [Google Scholar]
- Stern J, Temple S, 2015. Retinal pigment epithelial cell proliferation. Exp Biol Med (Maywood) 240, 1079–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss O, 2005. The retinal pigment epithelium in visual function. Physiological Reviews 85, 845–881. [DOI] [PubMed] [Google Scholar]
- Strunnikova NV, Maminishkis A, Barb JJ, Wang F, Zhi C, Sergeev Y, Chen W, Edwards AO, Stambolian D, Abecasis G, Swaroop A, Munson PJ, Miller SS, 2010. Transcriptome analysis and molecular signature of human retinal pigment epithelium. Hum Mol Genet 19, 2468–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Futagami Y, Smith SB, Naggar H, Mochizuki M, 2006. Retinal and ciliary body pigment epithelium suppress activation of T lymphocytes via transforming growth factor beta. Exp Eye Res 83, 1459–1471. [DOI] [PubMed] [Google Scholar]
- Susaki K, Chiba C, 2007. MEK mediates in vitro neural transdifferentiation of the adult newt retinal pigment epithelium cells: Is FGF2 an induction factor? Pigment Cell Research 20, 364–379. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Satoh A, Ide H, Tamura K, 2007. Transgenic Xenopus with prx1 limb enhancer reveals crucial contribution of MEK/ERK and PI3K/AKT pathways in blastema formation during limb regeneration. Developmental Biology 304, 675–686. [DOI] [PubMed] [Google Scholar]
- Taganov KD, Boldin MP, Chang K-J, Baltimore D, 2006. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA 103, 12481–12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai W, Xu X-M, Zhang C-L, 2020. Regeneration Through in vivo Cell Fate Reprogramming for Neural Repair. Frontiers in Cellular Neuroscience 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S, 2006. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 126, 663–676. [DOI] [PubMed] [Google Scholar]
- Takeda K, Yasumoto K, Kawaguchi N, Udono T, Watanabe K, Saito H, Takahashi K, Noda M, Shibahara S, 2002. Mitf-D, a newly identified isoform, expressed in the retinal pigment epithelium and monocyte-lineage cells affected by Mitf mutations. Biochim Biophys Acta 1574, 15–23. [DOI] [PubMed] [Google Scholar]
- Tamiya S, Kaplan HJ, 2016. Role of epithelial–mesenchymal transition in proliferative vitreoretinopathy. Experimental Eye Research 142, 26–31. [DOI] [PubMed] [Google Scholar]
- Tamiya S, Liu L, Kaplan HJ, 2010. Epithelial-mesenchymal transition and proliferation of retinal pigment epithelial cells initiated upon loss of cell-cell contact. Invest Ophthalmol Vis Sci 51, 2755–2763. [DOI] [PubMed] [Google Scholar]
- Toomey CB, Johnson LV, Bowes Rickman C, 2018. Complement factor H in AMD: Bridging genetic associations and pathobiology. Prog Retin Eye Res 62, 38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso MOM, 1973. Photic maculopathy in rhesus monkey : A light and electron microscopic study. Investigative Ophthalmology & Visual Science 12, 17–34. [PubMed] [Google Scholar]
- Ueki Y, Wilken MS, Cox KE, Chipman L, Jorstad N, Sternhagen K, Simic M, Ullom K, Nakafuku M, Reh TA, 2015. Transgenic expression of the proneural transcription factor Ascl1 in Müller glia stimulates retinal regeneration in young mice. Proceedings of the National Academy of Sciences 112, 13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara MN, Del Rio-Tsonis K, 2009. Retinal regeneration in the Xenopus laevis tadpole: a new model system. Mol Vis 15, 1000–1013. [PMC free article] [PubMed] [Google Scholar]
- Vetter ML, Hitchcock PF, 2017. Report on the National Eye Institute Audacious Goals Initiative: Replacement of Retinal Ganglion Cells from Endogenous Cell Sources. Transl Vis Sci Technol 6, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt AP, Mulfaul K, Mullin NK, Flamme-Wiese MJ, Giacalone JC, Stone EM, Tucker BA, Scheetz TE, Mullins RF, 2019. Single-cell transcriptomics of the human retinal pigment epithelium and choroid in health and macular degeneration. Proceedings of the National Academy of Sciences 116, 24100–24107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Leithner PL, Ciurtin C, Jeffery G, 2010. Microscopic mammalian retinal pigment epithelium lesions induce widespread proliferation with differences in magnitude between center and periphery. Mol Vis 16, 570–581. [PMC free article] [PubMed] [Google Scholar]
- Wan J, Goldman D, 2016. Retina regeneration in zebrafish. Curr Opin Genet Dev 40, 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Zhao X-F, Vojtek A, Goldman D, 2014. Retinal Injury, Growth Factors, and Cytokines Converge on β-Catenin and pStat3 Signaling to Stimulate Retina Regeneration. Cell Reports 9, 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Iacovelli J, Spencer C, Saint-Geniez M, 2014. Direct effect of sodium iodate on neurosensory retina. Investigative ophthalmology & visual science 55, 1941–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ohno-Matsui K, Yoshida T, Shimada N, Ichinose S, Sato T, Mochizuki M, Morita I, 2009. Amyloid-beta up-regulates complement factor B in retinal pigment epithelial cells through cytokines released from recruited macrophages/microglia: Another mechanism of complement activation in age-related macular degeneration. J Cell Physiol 220, 119–128. [DOI] [PubMed] [Google Scholar]
- Wang L-C, Hung K-H, Hsu C-C, Chen S-J, Li W-Y, Lin T-C, 2015. Assessment of retinal pigment epithelial cells in epiretinal membrane formation. Journal of the Chinese Medical Association 78. [DOI] [PubMed] [Google Scholar]
- Wang S-Z, Ma W, Yan R-T, Mao W, 2010. Generating retinal neurons by reprogramming retinal pigment epithelial cells. Expert Opinion on Biological Therapy 10, 1227–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Grenell A, Zhong F, Yam M, Hauer A, Gregor E, Zhu S, Lohner D, Zhu J, Du J, 2018. Metabolic signature of the aging eye in mice. Neurobiol Aging 71, 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenskow P, Piccolo S, Fuhrmann S, 2009. Beta-catenin controls differentiation of the retinal pigment epithelium in the mouse optic cup by regulating Mitf and Otx2 expression. Development 136, 2505–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DT, Sengupta S, Saxena MT, Xu Q, Hanes J, Ding D, Ji H, Mumm JS, 2017. Immunomodulation-accelerated neuronal regeneration following selective rod photoreceptor cell ablation in the zebrafish retina. Proceedings of the National Academy of Sciences 114, E3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte JL, Smith AA, Helms JA, 2012. Wnt signaling and injury repair. Cold Spring Harb Perspect Biol 4, a008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilken MS, Reh TA, 2016. Retinal regeneration in birds and mice. Curr Opin Genet Dev 40, 57–64. [DOI] [PubMed] [Google Scholar]
- Williams CD, Rizzolo LJ, 1997. Remodeling of junctional complexes during the development of the outer blood-retinal barrier. Anat Rec 249, 380–388. [DOI] [PubMed] [Google Scholar]
- Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY, 2014. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2, e106–116. [DOI] [PubMed] [Google Scholar]
- Wu YQ, Notario V, Chader GJ, Becerra SP, 1995. Identification of Pigment Epithelium-Derived Factor in the Interphotoreceptor Matrix of Bovine Eyes. Protein Expression and Purification 6, 447–456. [DOI] [PubMed] [Google Scholar]
- Xia H, Krebs MP, Kaushal S, Scott EW, 2011. Enhanced retinal pigment epithelium regeneration after injury in MRL/MpJ mice. Experimental Eye Research 93, 862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Chen M, Manivannan A, Lois N, Forrester JV, 2008. Age-dependent accumulation of lipofuscin in perivascular and subretinal microglia in experimental mice. Aging Cell 7, 58–68. [DOI] [PubMed] [Google Scholar]
- Xu J, Lamouille S, Derynck R, 2009. TGF-beta-induced epithelial to mesenchymal transition. Cell research 19, 156–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Shen SQ, Jui J, Rupp AC, Byrne LC, Hattar S, Flannery JG, Corbo JC, Kefalov VJ, 2015. CRALBP supports the mammalian retinal visual cycle and cone vision. J Clin Invest 125, 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Peairs JJ, Tano R, Jaffe GJ, 2006. Oxidant-mediated Akt activation in human RPE cells. Invest Ophthalmol Vis Sci 47, 4598–4606. [DOI] [PubMed] [Google Scholar]
- Yang X, Chung JY, Rai U, Esumi N, 2018. Cadherins in the retinal pigment epithelium (RPE) revisited: P-cadherin is the highly dominant cadherin expressed in human and mouse RPE in vivo. PLoS One 13, e0191279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K, Qiu S, Wang YV, Park SJH, Mohns EJ, Mehta B, Liu X, Chang B, Zenisek D, Crair MC, Demb JB, Chen B, 2018. Restoration of vision after de novo genesis of rod photoreceptors in mammalian retinas. Nature 560, 484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumuro H, Sakurai K, Toyama F, Maruo F, Chiba C, 2017. Implications of a Multi-Step Trigger of Retinal Regeneration in the Adult Newt. Biomedicines 5, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon YS, Yoon DS, Lim IK, Yoon SH, Chung HY, Rojo M, Malka F, Jou MJ, Martinou JC, Yoon G, 2006. Formation of elongated giant mitochondria in DFO-induced cellular senescence: involvement of enhanced fusion process through modulation of Fis1. J Cell Physiol 209, 468–480. [DOI] [PubMed] [Google Scholar]
- Yoshii C, Ueda Y, Okamoto M, Araki M, 2007. Neural retinal regeneration in the anuran amphibian Xenopus laevis post-metamorphosis: Transdifferentiation of retinal pigmented epithelium regenerates the neural retina. Developmental Biology 303, 45–56. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Mizuno A, Yasumuro H, Inami W, Vergara MN, Del Rio-Tsonis K, Chiba C, 2012. MEK-ERK and heparin-susceptible signaling pathways are involved in cell-cycle entry of the wound edge retinal pigment epithelium cells in the adult newt. Pigment Cell Melanoma Res 25, 66–82. [DOI] [PubMed] [Google Scholar]
- Yu FX, Guan KL, 2013. The Hippo pathway: regulators and regulations. Genes Dev 27, 355–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun MH, Gates PB, Brockes JP, 2014. Sustained ERK activation underlies reprogramming in regeneration-competent salamander cells and distinguishes them from their mammalian counterparts. Stem Cell Reports 3, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun S, Saijoh Y, Hirokawa KE, Kopinke D, Murtaugh LC, Monuki ES, Levine EM, 2009. Lhx2 links the intrinsic and extrinsic factors that control optic cup formation. Development 136, 3895–3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbin MA, 2004. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol 122, 598–614. [DOI] [PubMed] [Google Scholar]
- Zhou M, Geathers JS, Grillo SL, Weber SR, Wang W, Zhao Y, Sundstrom JM, 2020. Role of Epithelial-Mesenchymal Transition in Retinal Pigment Epithelium Dysfunction. Front Cell Dev Biol 8, 501–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Yoshida S, Kubo Y, Yoshimura T, Kobayashi Y, Nakama T, Yamaguchi M, Ishikawa K, Oshima Y, Ishibashi T, 2017. Different distributions of M1 and M2 macrophages in a mouse model of laser-induced choroidal neovascularization. Mol Med Rep 15, 3949–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Luz-Madrigal A, Haynes T, Zavada J, Burke AK, Rio-Tsonis KD, 2014. β-Catenin Inactivation Is a Pre-Requisite for Chick Retina Regeneration. PLOS ONE 9, e101748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber ME, Gestri G, Viczian AS, Barsacchi G, Harris WA, 2003. Specification of the vertebrate eye by a network of eye field transcription factors. Development 130, 5155–5167. [DOI] [PubMed] [Google Scholar]


