Highlights
-
•
KFAC and PC NMES induced similar relative submaximal and maximum evoked-torque and perceived discomfort for a given phase duration.
-
•
Regardless of NMES-type, the wider phase duration resulted in higher current NMES-efficiency in generating evoked-torque.
-
•
For different types of current, intensity was not related to the maximum perceived discomfort to produce submaximal contractions.
Keywords: Neuromuscular electrical stimulation, Physical therapy, Rehabilitation
Abstract
Background
Neuromuscular electrical stimulation (NMES) is an important therapeutic tool for rehabilitation. However, best stimulation parameters remain to be determined.
Objective
To determine the influence of different electrical stimulation currents and phase durations on torque, efficiency, and discomfort.
Methods
Using a cross-over design, kHz frequency alternating currents (KFAC) and pulsed currents (PC) with narrow (200 µs) or wide (500 µs) phase durations were randomly applied on knee extensor muscles of healthy participants with a minimum of seven days between sessions. The NMES-evoked torque, NMES-efficiency, and discomfort (visual 0−10 cm analogue scale) were measured for each stimulation intensity increments (10 mA). Statistics were conducted using a three-way analysis of variances (phase duration x current x intensity), followed by Tukey post-hoc.
Results
Twenty-four males (age 22.3 ± 3.5years) were included. No effect of NMES current was observed for torque, efficiency, and discomfort. For wide phase durations (500 µs), torque significantly increased for all stimulation intensities. For narrow phase durations (200 µs) evoked torque significantly increased only after 40% of maximal stimulation intensity. Phase durations of 500 µs produced greater torque than 200 µs. Discomfort was greater with 500 µs when compared to 200 µs. Submaximal relative torque, for example 40% of maximum voluntary contraction (MVC), was obtained with ∼ 60% and ∼ 80% of the maximal current intensity for 500 µs and 200 µs, respectively.
Conclusion
KFAC and PC current applied with the same phase duration induced similar relative submaximal and maximum evoked-torque, efficiency, and perceived discomfort. However, currents with 500 µs induced higher evoked-torque, current efficiency, and perceived discomfort.
Introduction
Neuromuscular electrical stimulation (NMES) is an important therapeutic tool for rehabilitation.1, 2, 3 Current intensity is one of the main limiting factors due to the high levels of perceived sensorial discomfort,2, 4 which could potentially limit NMES effectiveness.5 In clinical practice, gradually increasing the intensity of the current has been recommended to achieve a higher degree of muscle NMES-evoked torque and strength adaptions.6, 7 In addition, it is important to consider NMES-efficiency, i.e. producing the highest torque output with the lowest current intensity.8 Submaximal evoked torque levels, between 5% and 50% of the maximum voluntary contraction (MVC), have been reported to be effective for strengthening for orthopedic, pulmonary, and intensive care patients.9, 10, 11, 12
Different NMES waveforms have been used to evoke contractions. Conventional NMES consists of an electrical pulsed current (PC) with phase/pulse durations <500 µs delivered between 20−100 Hz.13, 14, 15 Kilohertz frequency alternating current (KFAC) is delivered with frequencies ranging from 1 kHz to 10 kHz, applied at a burst frequency between 1 Hz and 100 Hz, and a burst duty cycle of 10% or more.16 KFAC has been proposed to minimize perceived discomfort during stimulation.17, 18, 19
Previous studies have found that KFAC generated similar or less torque than PC,4, 20, 21 while two systematic reviews with meta-analysis have shown that PC and KFAC currents have similar effects on quadriceps evoked-torque and self-reported discomfort in healthy individuals.3, 15 However, the authors concluded that the weakness in study designs prevented a strong conclusion.3, 15 Only two studies investigated submaximal electrically elicited contractions with PC and KFAC. Liebano and Alves22 demonstrated no differences between KFAC (2500 Hz – Russian current) and PC in perceived discomfort after minimal evoked torque. In contrast, Vaz et al.23 concluded that PC required significantly lower current intensity and induced lower discomfort level (∼50%) to achieve 10% of MVC compared to KFAC. Moreover, NMES with wide pulse (500 µs) induces higher NMES-evoked torque compared to a narrow phase duration (250−200 µs)24, 25 regardless of KFAC frequency.20 To date, to our knowledge, no study has concomitantly assessed submaximal and maximal evoked-torque related to perceived discomfort with different NMES currents and phase durations.
The purpose of this study was to compare the effects of two types of KFAC (2500 Hz/Russian current and 1000 Hz/Aussie current with 200 µs and 500 µs phase duration, respectively) and PC (200 µs and 500 µs phase duration) on submaximal and maximum NMES-evoked torque, NMES-efficiency, and NMES-induced discomfort on healthy participants. To avoid some previous methodological bias, we used an increment approach that allows a more rigorous assessment of submaximal and maximum evoked-torque.
Methods
Trial design
This study was a randomized, cross-over, double-blind study comparing a protocol of gradual current intensity increase over time for four different NMES conditions. Participants were informed about the purposes, benefits, and risks before enrollment, and all agreed to participate and signed a consent form. Approval was obtained (protocol number 52685516.6.0000.0030) from the Research Ethics Committee of the Universidade de Brasília (Faculdade de Ciências da Saúde, Brasília, DF, Brazil) in accordance with the Helsinki Declaration of 1975.
Participants
To be included in the study, participants had to be male, aged 18–30 years old, and physically active. In addition, participants had to present with normal knee range of motion and function and report no previous experience with NMES. Participants who were using non-steroidal anti-inflammatory drugs or nutritional supplements, presented with skin lesions or skin allergies, had been previously diagnosed with neuromuscular diseases, or had external fixation or metal implants in the lower limbs were also not included in the study. Participants who did not tolerate NMES (due to fear or hypersensitivity to the stimulus) were also excluded.
Randomization and allocation concealment
Four conditions were randomly applied: (1) 500 µs phase duration (PC 500), (2) 200 µs phase duration (PC 200), (3) 500 µs phase duration and low carrier frequency (KFAC 500: 1 kHz/Aussie current), and (4) 200 µs phase duration and high carrier frequency (KFAC 200: 2.5 kHz/Russian current). Computer-generated randomization lists were prepared using the website www.random.org, which sequentially distributed the participants into the four conditions. One researcher (KAM) prepared sealed, opaque, and numbered envelopes containing the order of the NMES conditions.
Blinding
The researcher (NLPD) who applied the NMES and the participants were blinded to treatment allocation. This was achieved by another researcher (KAM) programming the NMES unit and placing an opaque cover on the NMES device panel to hide the parameters from the researcher (NLPD) and participants, except for current intensity.
Interventions
Participants participated in five sessions, each of which lasted approximately 2 h and were at least seven days apart. A different type of NMES was tested at each session. Each participant was assessed at the same time of day in a physical therapy research laboratory by the same assessor. Participants were asked to avoid stimulants (e.g., alcohol, caffeine, chocolate) and performing exercises on the testing days. The first visit served to familiarize participants with the measurement of MVC and the four types of NMES current. Two MVCs and two evoked contractions were randomly performed for each NMES condition to verify that participants tolerated enough current amplitude to generate maximum induced evoked contraction. Participants were also asked about health conditions and anthropometric assessments were performed. Participants self-reported their activity level using the International Physical Activity Questionnaire (IPAQ) and all reported participating in vigorous aerobic-type exercise.26
Each of the four experimental sessions was preceded by a warm-up of performing 10 submaximal isokinetic knee flexions and extensions at 180°/s. Then, peak voluntary torque for knee extension was assessed at 60° of knee flexion (0° = full knee extension). Participants were requested to perform three 10-second MVC separated by a 2-minute rest interval. During each voluntary contraction, participants received verbal and visual torque feedback. The greatest peak torque achieved was recorded and used for further analysis. After these measurements, the protocol to assess NMES-evoked torque was performed. MVC and NMES-evoked torque were obtained through an isokinetic dynamometer (System 3; Biodex Medical Systems, Shirley, New York). All procedures were performed on the dominant (kicking) side. The four types of NMES currents are described in Table 1. All currents were delivered with an “on” time of 10 s (8 s of constant time with 1 s of decay and 1 s of ramp up) and “off” time of 60 s for recovery.27
Table 1.
Description of the physical parameters for the 4 types of electrical stimulations.
| Current type | Current frequency (Hz) | Phase duration (μs) | Burst frequency (Hz) | Burst/interburst duration (ms) | Waveform |
|---|---|---|---|---|---|
| PC 500 | 50 | 500 | Square | ||
| PC 200 | 50 | 200 | Square | ||
| KFAC 500 | 1000 | 500 | 50 | 2/18 | Sine |
| KFAC 200 | 2500 | 200 | 50 | 10/10 | Sine |
Note: PC, Pulsed Current; KFAC, kHz frequency alternating current.
All electrical currents had an on-time of 10 s (1 s rise and 1 s of decline) and 60 s off-time.
Outcomes
Primary outcome was NMES-evoked torque. Secondary outcomes were NMES-efficiency and NMES-induced discomfort.
NMES-evoked torque assessment
Participants were instructed to relax the leg to avoid any voluntary contraction and were seated upright on the isokinetic dynamometer. The lateral epicondyle of the knee was aligned with the equipment’s rotational axis, and the back was reclined at approximately 100°. Stabilizing belts were positioned across the chest and around the hips and dominant ankle. Stimulation was produced using a neuromuscular electrical stimulator (version 2.0; Neurodyn, Ibramed, Amparo, SP, Brazil), connected to two pairs of 25 cm2 self-adhesive electrodes (Valutrode, São Paulo, Brazil). The skin was trichotomized and cleansed with alcohol, and one pair of electrodes was positioned on motor points of the vastus medialis and vastus lateralis muscles according to locations found by pen-type electrodes, as previously described.28 The other pair was positioned close to the proximal insertion of the quadriceps muscle, 3–5 cm below the inguinal ligament.20
The intensity of the stimulation was gradually increased from 10 mA, with 10 mA increments until the participants reported having attained their maximal tolerated current intensity. The NMES-evoked torque was measured during each contraction. A 60-second rest interval was used between each increase in intensity to minimize the effect of muscle fatigue.29, 30, 31, 32 Evoked torque was normalized to the MVC using the following equation [(NMES evoked torque/MVC) × 100].
NMES-efficiency assessment
NMES-efficiency was calculated as NMES-evoked torque/current intensity (Nm/mA).20, 33
Discomfort assessment
The discomfort level induced by the different forms of NMES was measured using a Visual Analog Scale (VAS). The scale was placed horizontally, ranging from 0 to 10 cm, 0 being the absence of discomfort and 10 being the maximum discomfort level tolerated by the participant.
Statistical analysis
Values for evoked-torque, VAS, and efficiency are reported as mean and confidence intervals (95% CI). We used parametric tests based on normal distribution (Shapiro-Wilk test) and homogeneous variances (Levene’s test) of the data. Statistics were performed using categorized values. NMES-evoked torque was categorized according to the percentage of MVC (close to each 20, 40, 60, 80, and 100% of MVC). Stimulation intensity was also categorized according to the percentage of maximal intensity (close to each 20, 40, 60, 80, and 100% of maximal stimulation intensity). A three-way mixed-model ANOVA with repeated measures [two levels: duration (500 and 200 µs) × currents (two levels: PC and KFAC) x stimulation intensity (five levels: 20 × 40 × 60 × 80 × 100%)] was performed. One-way ANOVA was performed for maximum values for MVC, absolute torque, relative torque, perceived discomfort, and intensity. In case of a significant main effect or interaction, a Tukey post-hoc test was used. All statistical analyses and graphics design were performed using GraphPad Prism 6.0 software (San Diego, CA, USA). The sample size was determined a priori using G*Power (version 3.1.3; University of Trier, Trier, Germany) with the level of significance set at p = 0.05 and power (1-β) = 0.80 to detect a large effect (f2 > 0.5). We conducted a pilot study with 5 participants to evaluate the effect size to detect a significant difference of 23.97 (N.m) on the evoked-torque (primary outcome) and a standard deviation of 18.39 (N.m). Based on these variables, a final sample size of 24 participants was determined.
Results
General observation
Baseline characteristics (mean ± SD) of the 24 participants were: age 22.3 ± 3.5 years, body mass 76.2 ± 11.4 kg, height 174 ± 5.5 cm, and body mass index 24.8 ± 2.9 kg/m2. There was no skin or other types of injury caused by NMES. KFAC and PC NMES waveforms types induced similar maximum values between all stimulation conditions for MVC and perceived discomfort (P > .05; Table 2). Phase duration of 500 µs produced greater absolute and maximum evoked torque than phase duration of 200 µs (P < .01); however, higher values of current intensity were obtained with 200 µs phase duration (P < .01). Table 3 shows mean and 95% CI of all outcomes for each current intensity percentage.
Table 2.
Results of different NMES currents and phase durations.
| PC 500 | PC 200 | KFAC 500 | KFAC 200 | |
|---|---|---|---|---|
| MVC max (Nm) | 269 (244, 294) | 265 (243, 288) | 262 (235, 289) | 262 (236, 288) |
| Absolute torque max (Nm) | 171a (153, 188) | 122 (102, 142) | 172a (148, 197) | 144 (125, 163) |
| Relative torque max (% MVC) | 71a (62, 79) | 56 (44, 67) | 72a (60, 84) | 65 (52, 77) |
| Discomfort (0, 10) | 9.8 (9.5, 10.0) | 9.0 (8.4, 9.7) | 9.2 (8.7, 9.8) | 9.3 (8.9, 9.8) |
| Intensity max (mA) | 128 (94, 175) | 155b (118, 193) | 131 (104, 159) | 150b (128, 188) |
Values are reported as mean (95% confidence interval). KFAC, kHz frequency alternating currents; NMES, neuromuscular electrical stimulation; MVC, maximal voluntary contraction; PC, pulsed current.
Phase durations of 500 µs produced greater absolute and evoked torque maximum than 200 µs.
Phase durations of 200 µs displayed higher values of intensity when compared 500 µs.
Table 3.
Results of different NMES currents and phase durations for each stimulation intensity.
| Outcome | PC 500 | PC 200 | KFAC 500 | KFAC 200 | |
|---|---|---|---|---|---|
| Absolute torque (Nm) | 20% | 7.1 (3.7, 10.6) | 3.4 (2.3, 4.6) | 6.1 (2.3, 10) | 3.9 (2.6, 5.3) |
| 40% | 48.1 (33.7, 62.3) | 13.8 (6.5, 21.2) | 45.9 (27.2, 64.6) | 19.2 (9.1, 29.3) | |
| 60% | 105.5 (84.1, 126.9) | 48.8 (32.8, 64.9) | 100.1 (76.6, 123.6) | 63.9 (46.5, 81.2) | |
| 80% | 149.1 (129.1, 169.1) | 84.4 (64.8, 104.1) | 146.8 (120.1, 172.7) | 111.8 (90.6, 133.0) | |
| 100% | 170.7 (153.1, 188.3) | 122.1 (101.9, 142.3) | 172.3 (147.9, 96.6) | 144.1 (125.1, 163.2) | |
| Evoked torque (Nm) | 20% | 3.1 (1.5, 4.5) | 1.2 (0.8, 1.6) | 2.9 (1.1, 4.8) | 1.5 (0.8, 2.1) |
| 40% | 18.7 (13.1, 24.3) | 5.9 (2.5, 9.4) | 23.9 (15.1, 32.7) | 7.8 (3.4, 12.1) | |
| 60% | 42.1 (32.5, 51.5) | 22.1 (14.1, 30.1) | 47.6 (36.5, 58.6) | 27.4 (19.5, 35.3) | |
| 80% | 58.3 (47.6, 68.9) | 36.4 (26.3, 46.5) | 64.9 (52.1, 77.7) | 45.6 (34.9, 56.2) | |
| 100% | 70.5 (62, 79.) | 55.6 (43.9, 67.2) | 72. (59.9, 84.1) | 64.5 (51.7, 77.4) | |
| Discomfort (0, 10) | 20% | 1.6 (1, 2.2) | 0.7 (0.5, 1.) | 1.1 (0.8, 1.3) | 0.8 (0.6, 1.) |
| 40% | 3.1 (2.5, 3.6) | 2.3 (1.8, 2.8) | 2.9 (2.5, 3.4) | 2.3 (1.9, 2.7) | |
| 60% | 5.3 (4.7, 5.8) | 4.6 (4.0, 5.2) | 5.2 (4.7, 5.7) | 4.8 (4.3, 5.3) | |
| 80% | 7.8 (7.4, 8.2) | 6.8 (6.1, 7.5) | 7.8 (7.2, 8.3) | 7.3 (6.9, 7.7) | |
| 100% | 9.7 (9.5, 10) | 9. (8.3, 9.6) | 9.2 (8.6, 9.7) | 9.3 (8.8, 9.7) | |
| NMES efficiency (Nm/mA) | 20% | 14.8 (5.3, 24.3) | 6.2 (4.1, 8.3) | 13.3 (7.9, 22.6) | 13.3 (6.9, 19.7) |
| 40% | 59.8 (27.8, 91.7) | 14.9 (6.3, 23.5) | 46.9 (33.2, 60.6) | 7.5 (4.4, 10.7) | |
| 60% | 79.3 (60.9, 97.8) | 36.8 (23.4, 50.2) | 70.1 (54.5, 85.5) | 19.5 (8.6, 30.4) | |
| 80% | 81.1 (65.1, 97.1) | 45.5 (32.9, 58.1) | 72.8 (59.8, 85.9) | 45.7 (32.5, 58.9) | |
| 100% | 72 (59.9, 84.1) | 55.6 (43.9, 67.2) | 70.5 (62.2, 78.8) | 64.5 (51.7, 77.4) |
Data are reported as mean (95% CI).
KFAC, kHz frequency alternating currents; NMES, neuromuscular electrical stimulation; MVC, maximal voluntary contraction; PC, pulsed current.
The P value for the factor “Phase Duration” is <.01 for all variables.
The P value for the factor “Current Type” is >.05 for all variables.
The P value for the factor “Current Intensity” is <.01 for all variables.
NMES-evoked torque
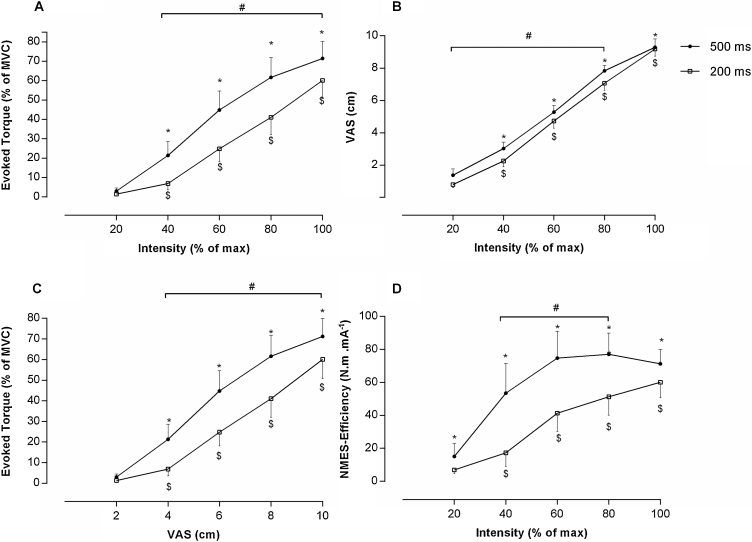
There was a significant phase duration x intensity interaction for both absolute and relative evoked-torque (P = .001 for both). No current effect (KFAC versus PC) was observed for absolute and relative evoked-torque (P = 0.42 and P = 0.2, respectively). Accordingly, data were pooled across phase duration and intensity (Fig. 1). With the longer phase duration (500 µs), evoked-torque increased as current intensity increased (P < .001). For the short phase duration (200 µs), a similar increase was observed except when comparing 20 and 40% of stimulation intensity (P < .001). With the exception of the lowest stimulation intensity (20%), a long phase duration produced greater torque than a short phase duration (P < .01). In addition, evoked torque at 40% of MVC, was obtained with ∼ 60% and ∼ 80% of the maximal tolerated current intensity for 500 µs and 200 µs, respectively (P < .001; Fig. 1A).
Figure 1.
(A) Evoked Torque (% MVC) versus Intensity (% of max); (B) Discomfort (VAS) versus Intensity (% of max); (C) Evoked Torque (% MVC) versus VAS (scale nº); (D) Relative Efficiency versus Intensity (%). Values are Mean and 95% CI. Statistically significant differences compared to: $ 20% of intensity (200 µs); * 20% of intensity (500 µs); # between 500 µs and 200 µs, p ≤ 0.05.
Discomfort
The VAS value was similar between all stimulation patterns for the maximum stimulation intensity (Table 2). There was a significant phase duration and current intensity effect (P = .003 and P = .001, respectively; Fig. 1B). No current effect was observed (P > .05). Except for the maximal stimulation intensity, VAS scores were greater with long phase durations (500 µs) compared to short phase durations (200 µs) (P = .001). As expected, VAS scores increased with increasing stimulation intensity (P < .001).
Relative evoked-torque revealed a significant duration x VAS interaction (P < .001, Fig. 1). No current effect (KFAC versus PC) was observed (P = .82). The results revealed that the greater the relative evoked-torque, the greater the VAS (P < .001). Moreover, for a given VAS score, wide phase durations produced greater relative evoked-torque (P < .001). Finally, at ∼40% of the NMES-evoked torque, for example, there was a perceived discomfort of 6 cm and 8 cm for the 500 µs and 200 µs phase duration, respectively (P < .001; Fig. 1C).
NMES-efficiency
Regarding NMES-efficiency, a significant interaction was found for phase duration and intensity (P < 0.001 and P < 0.001), respectively; Fig. 1D). No current effect was observed (P = 0.94). Results indicated that NMES-efficiency was greater with a wider phase duration (500 µs versus 200 µs; P < .001)). In addition, NMES-efficiency increased with increasing stimulation intensity (Fig. 1D; P < 0.001).
Discussion
The effectiveness of NMES is proportional to the evoked torque expressed here as a percentage of MVC. Several studies have shown that, to evoke acceptable contractions to induce muscle adaptations, a high NMES intensity is needed, which is limited by the amount of discomfort tolerated by the participants.4, 5, 9, 16,20, 34, 35 Clinically, the amount of evoked-torque is key to induce strength gains and counteract muscle wasting in different clinical populations. The desired evoked torque has been suggested to be between ∼5% and 50% of MVC for patients with orthopedic problems (leg immobilization, anterior cruciate ligament reconstruction, and total knee arthroplasty 9, 11, 36), between 15% and 25% of MVC for patients with chronic obstructive pulmonary disease,10 and less than 25% of the MVC for patients in the intensive care unit.12
In the present study, independently of the NMES-types, current intensity did not appear to have a similar relationship related to the maximum perceived discomfort in an attempt to produce submaximal contractions (5–50% of evoked-torque). For example, we reached the tolerable NMES-efficiency of stimulation (∼40% of evoked-torque) using a moderate intensity (∼60% of maximum intensity) and discomfort range (VAS ∼6) for 500 µs of phase duration. Interestingly, to reach the same NMES-efficiency of stimulation (∼40% of evoked-torque) it was necessary to use higher current intensity (∼80% of maximum intensity) and higher perceived discomfort (VAS ∼8) for 200 µs of phase duration. This percentage of torque induced by NMES in this example (∼40%) has been recommended for strength gains.2, 11, 12, 37,38 Considering that the key factor for optimizing NMES effectiveness has been related to the level of evoked-torque with a low perception of discomfort,2, 38 our results clearly demonstrated that it is more suitable to evoke torque with lower current intensities mainly for wider phase duration.
The present study also analyzed the NMES-efficiency of a given stimulation condition through the relationship between torque and current intensity, which means higher torque output when applying the lowest current intensity.20, 25 The NMES-efficiency was similar for both types of currents (KFAC × PC) when using matched phase duration and phase charge. Our results indicate no difference in NMES-evoked torque, NMES-efficiency, and NMES-induced discomfort between KFAC and PC. This is consistent with the results of a well-conducted systematic review by Vaz and Frasson3 who concluded that KFAC generates equal or less force, equal or more fatigue, and equal or more discomfort compared to PC for quadriceps stimulation. Taking the current evidence together, physical therapists could choose KFAC or PC and would expect similar force generation as well as similar perceived discomfort.
Interestingly, some studies demonstrated that different burst duration waveforms can affect NMES-evoked torque. Bellew et al.24 demonstrated that interferential current (2500 Hz and burst duration of 20 ms) and burst-modulated pulsed current (1765 Hz and burst duration of 1.7 ms) generated higher evoked torque than Russian current (2500 Hz and burst duration of 1.7 ms). They also demonstrated that burst-modulated pulsed current in a lower frequency of KFAC induced less discomfort when compared to the higher frequency of KFAC.24 Those results are in agreement with our results that revealed a dependency on burst duration for KFAC with different phase duration. KFAC with burst duration of 2 ms induced higher evoked torque compared to the KFAC with burst duration of 10 ms. While Bellew et al.24, 39 did not compare KFAC with different burst duration with PC, it is reasonable to expect that KFAC modulated with long phase duration or lower burst is more suitable for higher NMES-induced torque.
Curiously, no participants were excluded due to NMES intolerance, and this may be related to the incremental intensity method, as well as the familiarization performed in the first session. In previous studies that reported this information, 20% of volunteers, on average, were excluded due to intolerance to NMES.4, 16 It seems that current accommodation increases during each NMES session, as well as during the treatment session. This fact could also be related to the decreasing apprehension as the participants become familiar with the NMES device and sessions.40
Study limitations
The present study has some limitations. Only healthy young male participants of relatively low body mass index were assessed. Between 13 and 16 NMES induced muscle contractions were performed within a single testing session. Despite an interval of 60 s between contractions,29, 30, 31, 32 it is possible that muscle fatigue may have influenced the results. Aldayel et al.34 evaluated 40 contractions in a single session and observed that the best current analysis occurs on average in the 10th to 17th NMES contractions. Thus, it is possible to suggest that our NMES protocol did not generate sufficient muscle fatigue to reduce muscle performance.
Conclusion
KFAC and PC applied with the same phase duration induced similar relative submaximal and maximum evoked-torque, efficiency, and discomfort. However, currents with wide pulse evoked greater torques, higher current NMES-efficiency, and higher perceived discomfort compared to the narrow phase duration currents, both in submaximal and maximum conditions.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This project was supported by the Fundação de Amparo à Pesquisa do Distrito Federal (FAPDF; Process number: 193.000.862/2014 and 00193.0000168/2019-8); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq Process numbers: 447529/2014-5/312136/2018-8); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil (CAPES; Process number: 88881.068106/2014-01; and Finance Code 001), and PVEX (Programa de professor visitante no exterior - 88881.172234/2018-01). The authors are grateful to the Electrical Engineering Department from the University of Brasília for the support in checking the calibration of the electrical stimulation device.
References
- 1.Bax L., Staes F., Verhagen A. Does neuromuscular electrical stimulation strengthen the quadriceps femoris? A systematic review of randomised controlled trials. Sports Med. 2005;35(3):191–212. doi: 10.2165/00007256-200535030-00002. [DOI] [PubMed] [Google Scholar]
- 2.Maffiuletti N.A., Minetto M.A., Farina D., Bottinelli R. Electrical stimulation for neuromuscular testing and training: State-of-the art and unresolved issues. Eur J Appl Physiol. 2011;111(10):2391–2397. doi: 10.1007/s00421-011-2133-7. [DOI] [PubMed] [Google Scholar]
- 3.Vaz M.A., Frasson V.B. Low-frequency pulsed current versus kilohertz-frequency alternating current: A scoping literature review. Arch Phys Med Rehabil. 2018;99(4):792–805. doi: 10.1016/j.apmr.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Dantas L.O., Vieira A., Siqueira A.L., Jr., Salvini T.F., Durigan J.L. Comparison between the effects of 4 different electrical stimulation current waveforms on isometric knee extension torque and perceived discomfort in healthy women. Muscle Nerve. 2015;51(1):76–82. doi: 10.1002/mus.24280. [DOI] [PubMed] [Google Scholar]
- 5.Laufer Y., Elboim M. Effect of burst frequency and duration of kilohertz-frequency alternating currents and of low-frequency pulsed currents on strength of contraction, muscle fatigue, and perceived discomfort. Phys Ther. 2008;88(10):1167–1176. doi: 10.2522/ptj.20080001. [DOI] [PubMed] [Google Scholar]
- 6.Vanderthommen M., Duchateau J. Electrical stimulation as a modality to improve performance of the neuromuscular system. Exerc Sport Sci Rev. 2007;35(4):180–185. doi: 10.1097/jes.0b013e318156e785. [DOI] [PubMed] [Google Scholar]
- 7.Maffiuletti N.A. Physiological and methodological considerations for the use of neuromuscular electrical stimulation. Eur J Appl Physiol. 2010;110(2):223–234. doi: 10.1007/s00421-010-1502-y. [DOI] [PubMed] [Google Scholar]
- 8.Lieber R.L., Kelly M.J. Factors influencing quadriceps femoris muscle torque using transcutaneous neuromuscular electrical stimulation. Phys Ther. 1991;71(10):715–721. doi: 10.1093/ptj/71.10.715. [DOI] [PubMed] [Google Scholar]
- 9.Gibson J.N., Smith K., Rennie M.J. Prevention of disuse muscle atrophy by means of electrical stimulation: Maintenance of protein synthesis. Lancet. 1988;2(8614):767–770. doi: 10.1016/s0140-6736(88)92417-8. [DOI] [PubMed] [Google Scholar]
- 10.Maddocks M., Nolan C.M., Man W.D. Neuromuscular electrical stimulation to improve exercise capacity in patients with severe COPD: A randomised double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:27–36. doi: 10.1016/S2213-2600(15)00503-2. [DOI] [PubMed] [Google Scholar]
- 11.Laufer Y., Snyder-Mackler L. Response of male and female subjects after total knee arthroplasty to repeated neuromuscular electrical stimulation of the quadriceps femoris muscle. Am J Phys Med Rehabil. 2010;89(6):464–472. doi: 10.1097/PHM.0b013e3181dd8c0e. [DOI] [PubMed] [Google Scholar]
- 12.Dirks M.L., Hansen D., Van Assche A., Dendale P., Van Loon L.J. Neuromuscular electrical stimulation prevents muscle wasting in critically ill comatose patients. Clin Sci. 2015;128(6):357–365. doi: 10.1042/CS20140447. [DOI] [PubMed] [Google Scholar]
- 13.Ward A.R., Robertson V.J. The variation in fatigue rate with frequency using kHz frequency alternating current. Med Eng Phys. 2000;22(9):637–646. doi: 10.1016/s1350-4533(00)00085-0. [DOI] [PubMed] [Google Scholar]
- 14.Ward A.R., Lucas-Toumbourou S., McCarthy B. A comparison of the analgesic efficacy of medium-frequency alternating current and TENS. Physiotherapy. 2009;95(4):280–288. doi: 10.1016/j.physio.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 15.da Silva V.Z., Durigan J.L., Arena R., de Noronha M., Gurney B., Cipriano G., Jr. Current evidence demonstrates similar effects of kilohertz-frequency and low-frequency current on quadriceps evoked torque and discomfort in healthy individuals: A systematic review with meta-analysis. Physiother Theory Pract. 2015;31(8):533–539. doi: 10.3109/09593985.2015.1064191. [DOI] [PubMed] [Google Scholar]
- 16.Aldayel A., Muthalib M., Jubeau M., McGuigan M., Nosaka K. Muscle oxygenation of vastus lateralis and medialis muscles during alternating and pulsed current electrical stimulation. Eur J Appl Physiol. 2011;111(5):779–787. doi: 10.1007/s00421-010-1699-9. [DOI] [PubMed] [Google Scholar]
- 17.Kots Y.M. Electrostimulation (Canadian-Soviet exchange symposium on electrostimulation of skeletal muscles, Concordia University, Montreal, Quebec, Canada, December 6–15, 1977). Quoted in: Kramer J, Mendryk SW. Electrical stimulation as a strength improvement technique. J Orthop Sports Phys Ther. 1982;4:91–98. doi: 10.2519/jospt.1982.4.2.91. [DOI] [PubMed] [Google Scholar]
- 18.Ward A.R., Shkuratova N. Russian electrical stimulation: The early experiments. Phys Ther. 2002;82(10):1019–1030. [PubMed] [Google Scholar]
- 19.Ward A.R., Robertson V.J., Ioannou H. The effect of duty cycle and frequency on muscle torque production using kilohertz frequency range alternating current. Med Eng Phys. 2004;26(7):569–579. doi: 10.1016/j.medengphy.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Medeiros F.V., Bottaro M., Vieira A. Kilohertz and low-frequency electrical stimulation with the same pulse duration have similar efficiency for inducing isometric knee extension torque and discomfort. Am J Phys Med Rehabil. 2017;96(6):388–394. doi: 10.1097/PHM.0000000000000631. [DOI] [PubMed] [Google Scholar]
- 21.Ward A.R., Oliver W.G., Buccella D. Wrist extensor torque production and discomfort associated with low-frequency and burst-modulated kilohertz-frequency currents. Phys Ther. 2006;86(10):1360–1367. doi: 10.2522/ptj.20050300. [DOI] [PubMed] [Google Scholar]
- 22.Liebano R.E., Alves L.M. Comparação do índice de desconforto sensorial durante a estimulação elétrica neuromuscular com correntes excitomotoras de baixa e média frequência em mulheres saudáveis. Rev Bras Med Esporte. 2009;15(1):50–53. [Google Scholar]
- 23.Vaz M.A., Aragão F.A., Boschi ÉS., Fortuna R., Melo Mde O. Effects of Russian current and low-frequency pulsed current on discomfort level and current amplitude at 10% maximal knee extensor torque. Physiother Theory Pract. 2012;28(8):617–623. doi: 10.3109/09593985.2012.665984. [DOI] [PubMed] [Google Scholar]
- 24.Bellew J.W., Beiswanger Z., Freeman E., Gaerte C., Trafton J. Interferential and burst-modulated biphasic pulsed currents yield greater muscular force than Russian current. Physiother Theory Pract. 2012;28:384–390. doi: 10.3109/09593985.2011.637286. [DOI] [PubMed] [Google Scholar]
- 25.Scott W., Adams C., Cyr S. Electrically elicited muscle torque: Comparison between 2500-Hz burst-modulated alternating current and monophasic pulsed current. J Orthop Sports Phys Ther. 2015;45(12):1035–1041. doi: 10.2519/jospt.2015.5861. [DOI] [PubMed] [Google Scholar]
- 26.Pardini R., Matsudo S.M.M., Araújo T., Matsudo V.K.R., Andrade E., Braggion G. Validação do Questionário Internacional de Nível de Atividade Física (IPAQ – Versão 6): Estudo-piloto em adultos jovens brasileiros. Rev Bras Ciên e Mov. 2001;9:45–51. [Google Scholar]
- 27.Holcomb W.R., Golestani S., Hill S.A. Comparison of knee-extension torque production with biphasic versus Russian current. J Sport Rehabil. 2000:229–239. [Google Scholar]
- 28.Botter A., Oprandi G., Lanfranco F., Allasia S., Maffiuletti N.A., Minetto M.A. Atlas of the muscle motor points for the lower limb: Implications for electrical stimulation procedures and electrode positioning. Eur J Appl Physiol. 2011;111:2461–2471. doi: 10.1007/s00421-011-2093-y. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida T., Abe D., Fukuoka Y. Phosphocreatine resynthesis during recovery in different muscles of the exercising leg by 31 P‐MRS. Scand J Med Sci Sports. 2013;23(5):e313–e319. doi: 10.1111/sms.12081. [DOI] [PubMed] [Google Scholar]
- 30.McMahon S., Jenkins D. Factors affecting the rate of phosphocreatine resynthesis following intense exercise. Sports Med. 2002;32(12):761–784. doi: 10.2165/00007256-200232120-00002. [DOI] [PubMed] [Google Scholar]
- 31.Harris R.C., Edwards R.H.T., Hultman E., Nordesjo L.O., Nylind B., Sahlin K. The time course of phosphocreatine resynthesis during the recovery of quadriceps muscle in man. Pflugers Arch. 1976;97:392–397. doi: 10.1007/BF00585149. [DOI] [PubMed] [Google Scholar]
- 32.Nevill A.M., Jones D.A., McIntyre D., Bogdanis G.C., Nevill A.M.E. A model for phosphocreatine resynthesis. J Appl Physiol. 1997;82(1):329–335. doi: 10.1152/jappl.1997.82.1.329. [DOI] [PubMed] [Google Scholar]
- 33.Petrofsky J., Prowse M., Bain M. Estimation of the distribution of intramuscular current during electrical stimulation of the quadriceps muscle. Eur J Appl Physiol. 2008;103(3):265–273. doi: 10.1007/s00421-008-0700-3. [DOI] [PubMed] [Google Scholar]
- 34.Aldayel A., Jubeau M., McGuigan M., Nosaka K. Comparison between alternating and pulsed current electrical muscle stimulation for muscle and systemic acute responses. J Appl Physiol (1985) 2010;109(3):735–744. doi: 10.1152/japplphysiol.00189.2010. [DOI] [PubMed] [Google Scholar]
- 35.Szecsi J., Fornusek C. Comparison of torque and discomfort produced by sinusoidal and rectangular alternating current electrical stimulation in the quadriceps muscle at variable burst duty cycles. Am J Phys Med Rehabil. 2014;93(2):146–159. doi: 10.1097/PHM.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 36.Snyder-Mackler L., Delitto A., Stralka S.W., Bailey S.L. Use of electrical stimulation to enhance recovery of quadriceps femoris muscle force production in patients following anterior cruciate ligament reconstruction. Phys Ther. 1994;74:901–907. doi: 10.1093/ptj/74.10.901. [DOI] [PubMed] [Google Scholar]
- 37.Lieber R.L., Kelly M.J. Factors influencing quadriceps femoris muscle torque using transcutaneous neuromuscular electrical stimulation. Phys Ther. 1991;71(10):715–721. doi: 10.1093/ptj/71.10.715. discussion 722-713. [DOI] [PubMed] [Google Scholar]
- 38.Miyamoto N., Fukutani A., Yanai T., Kawakami Y. Twitch potentiation after voluntary contraction and neuromuscular electrical stimulation at various frequencies in human quadriceps femoris. Muscle Nerve. 2012;45(1):110–115. doi: 10.1002/mus.22259. [DOI] [PubMed] [Google Scholar]
- 39.Bellew J.W., Sanders K., Schuman K., Barton M. Muscle force production with low and medium frequency burst modulated biphasic pulsed currents. Physiother Theory Pract. 2014;30(2):105–109. doi: 10.3109/09593985.2013.823582. [DOI] [PubMed] [Google Scholar]
- 40.Owens J., Malone T.R. Treatment parameters of high frequency electrical stimulation as established on the Electro-Stim 180. J Orthop Sports Phys Ther. 1983;4:162–168. doi: 10.2519/jospt.1983.4.3.162. [DOI] [PubMed] [Google Scholar]