Abstract
Introduction
COVID-19 is a pandemic with significant mortality and it is important to differentiate severe and non-severe cases. We conducted a study to evaluate hematologic profiles with inflammation markers in COVID-19 patients and to determine the correlation of neutrophil-lymphocyte ratio (NLR) with disease severity.
Methods
A cross-sectional study involving hospitalized COVID-19 patients confirmed with a positive SARS-CoV-2 PCR test in Dr. Cipto Mangunkusumo Hospital. Lymphocyte count, NLR, C-reactive protein (CRP) and ferritin were evaluated in severe and non-severe COVID-19 cases at hospital admission. Data was analyzed using Spearman correlation.
Results
There were 41 patients aged 20 to 79 years with COVID-19; 33 (80.5%) were non-severe, and 8 (19.5%) were severe cases. There is a statistically significant difference in WBC, relative neutrophils and lymphocytes, NLR, and CRP between non-severe and severe cases. There is a strong correlation between NLR and CRP (r = 0.738; p < 0.001). Our findings show that NLR and absolute lymphocyte count, but not ferritin, play a role in differentiating between non-severe and severe COVID-19 cases.
Conclusion
In COVID-19 cases, a strong correlation between NLR and CRP might suggest the use of NLR to differentiate between non-severe and severe cases, especially in a remote healthcare facility.
Keywords: hematologic profiles, lymphocyte, NLR, inflammation, COVID-19
Introduction
Coronavirus 2019 (COVID-19) is an infectious respiratory disease caused by the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection. The World Health Organization stated this disease as a global pandemic on March 12th, 2020.1,2 By August 2021, global confirmed cases have reached more than two hundred million people with more than 4.2 million deaths.3
As described by a vast array of case reports and reviews, clinical manifestations of COVID-19 might vary. Patients develop variable signs and symptoms, ranging from asymptomatic to acute respiratory distress syndrome, leading to death, so it is important to differentiate between severe and non-severe cases.4 A systematic review by Lippi et al5 shows that in severe COVID-19 cases, several laboratory abnormalities may occur, such as leukocytosis, neutrophilia, lymphopenia, increase in lactate dehydrogenase (LDH) and C-reactive protein (CRP).
A haematological profile of COVID-19 patients has initially been reported in a study of 41 cases and it is found that leukopenia is found in 25% of the cases, while lymphopenia is found in 63%. Only a few patients showed thrombocytopenia.6,7 Another study in Mexico by Lagunas-Rangel,8 provides additional evidence that an increase in neutrophil to lymphocyte ratio is related to more severe inflammation which implies a poorer prognosis in COVID-19. Similarly, other inflammation markers to predict COVID-19 severity are being studied.9–11
The neutrophil to lymphocyte ratio (NLR) parameter has been widely used to assess systemic inflammation in a variety of diseases.12 In both acute and chronic illness, NLR could predict disease severity regardless of the aetiology, such as infection, autoimmune or malignancy. In this study, we would like to evaluate hematologic profiles and their correlation with markers of inflammation in hospitalized COVID-19 and to determine whether NLR might provide useful information regarding COVID-19 disease severity.
Materials and Methods
Patient Selection
This is a cross-sectional study involving hospitalized COVID-19 in Cipto Mangunkusumo National Hospital. Diagnosis of COVID-19 is based on clinical and laboratory data confirmed with SARS-CoV-2 PCR microbiology data. Patients were informed about this study and appropriate consent was obtained. The exclusion criteria are patients with haematological malignancies, advanced liver cirrhosis, stage IV–V kidney failure, autoimmune diseases, HIV infection. Stratification of patients into severe and non-severe categories was based on the National Guidelines of The Management of COVID-19. The sample size of this study was calculated using the estimation formula for bivariate correlation which yielded a minimum number of 30 subjects.
Baseline Data Collection
The baseline demographic and clinical data were obtained and recorded on both study data and patient’s electronic medical record from all eligible subjects. Complete blood count, LDH, and CRP were collected at hospital admission and the analysis was made using Sysmex® and Architect® machines.
Statistical Analysis
All data were analysed using Statistical Package for the Social Sciences (SPSS) software. Data with normal distribution were analysed using a parametric test, while those without normal distribution were analysed using an appropriate non-parametric test. In those with a normal distribution, means were compared using Student’s t-test and for nonparametric data, central tendencies were compared using the Mann–Whitney test. Correlation studies were performed using Spearman’s test.
Ethics
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of The Faculty of Medicine, University of Indonesia-Cipto Mangunkusumo Hospital with ethical approval number: KET-647/UN2.F1/ETIK/PPM.00.02/2020. Informed consent was obtained from all subjects involved in the study.
Results
There were 41 patients aged 20 to 79 years old with COVID-19. Of the 41 patients with COVID-19, 33 (80.5%) were categorized as non-severe, and 8 (19.5%) were severe cases. Baseline subjects’ characteristics can be seen in Table 1. Only 14 patients (34.1%) were without co-morbidity, while others had at least one comorbidity present.
Table 1.
Study Subject's Characteristics
| Variables | Result |
|---|---|
| Number of patients (N) | 41 |
| Age (years), mean (SD) | 48.44 (SD 14.76) |
| Sex (n, %) | |
| • Male | 23 (56.1) |
| • Female | 18 (43.9) |
| COVID-19 Severity (n, %) | |
| • Non severe | 33 (80.5) |
| • Severe | 8 (19.5) |
| Comorbidities (n, %) | |
| • No comorbidity | 14 (34.1) |
| • Hypertension | 7 (17.1) |
| • Type 2 diabetes | 6 (14.6) |
| • Hypertension and type 2 diabetes | 5 (12.2) |
| • Obese/overweight | 3 (7.3) |
| • Chronic heart failure | 3 (7.3) |
| • Prior lung tuberculosis | 2 (4.9) |
| • Atrial fibrillation | 1 (2.4) |
Abbreviation: SD, standard deviation.
Hematologic profiles of both non-severe and severe patients are depicted in Table 2. There is a statistically significant difference in white blood cells count, relative neutrophils, relative lymphocytes, NLR, and CRP between non-severe and severe COVID-19 cases. C-reactive protein levels were markedly elevated in severe cases. Despite a higher ferritin value being found in severe COVID-19, there is no statistically significant difference between non-severe and severe cases.
Table 2.
Hematologic Profiles Between Non-Severe and Severe COVID-19 Patients
| Variables | Units | Non-Severe (n = 33) | Severe (n = 8) | p value |
|---|---|---|---|---|
| Age (years), mean (SD) | Years | 46.8 (SD 14.6) | 55.3 (SD 14.1) | 0.15 |
| Sex (n,%) | ||||
| • Male | 18 (54.5) | 5 (62.5) | ||
| • Female | 15 (45.5) | 3 (37.5) | ||
| Comorbidities (n,%) | ||||
| • No comorbidity | 12 (36.4) | 2 (25) | ||
| • One or more comorbidities | 21 (63.6) | 6 (75) | ||
| Haemoglobin | g/dL | Mean 13.94 (SD 1.31) | Mean 13.06 (SD 2.09) | 0.143 |
| Haematocrit | % | Mean 40.06 (SD 3.55) | Mean 37.55 (SD 6.14) | 0.298 |
| White blood cells count | cells/μL | Median 5510 (IQR 3210) | Median 9040 (IQR 4903) | 0.049* |
| Platelet count | cells/μL | Mean 255,576 (SD 79,807.6) | Mean 376,875 (SD 171,719.4) | 0.088 |
| WBC relative differential count | ||||
| • Basophils | % | Median 0.3 (IQR 0.2) | Median 0.2 (IQR 0.4) | 0.176 |
| • Eosinophils | % | Median 0.1 (IQR 0.9) | Median 0.05 (IQR 0.2) | 0.391 |
| • Neutrophils | % | Mean 66.38 (SD 10.67) | Mean 78.8 (SD 9.42) | 0.005* |
| • Lymphocytes | % | Mean 23.75 (SD 8.59) | Mean 13.1 (SD 7.99) | 0.003* |
| • Monocytes | % | Mean 8.82 (SD 3.44) | Mean 7.8 (SD 2.44) | 0.424 |
| Neutrophil to lymphocyte ratio (NLR) | Median 2.62 (IQR 2.12) | Median 7.06 (IQR 6.41) | 0.004* | |
| Absolute lymphocyte count | cells/μL | Median 1380 (IQR 390) | Median 1020 (IQR 875) | 0.146 |
| C-reactive protein | mg/L | Median 14 (IQR 50.7) | Median 84.6 (IQR 86.9) | 0.011* |
| Lactate dehydrogenase | U/L | Median 280 (IQR 80) | Median 360.5 (IQR 180) | 0.128 |
| Ferritin | ng/mL | Median 513.84 (IQR 612,75) | Median 772.44 (IQR 271.42) | 0.073 |
Note: *p<0.05.
Abbreviation: SD, standard deviation.
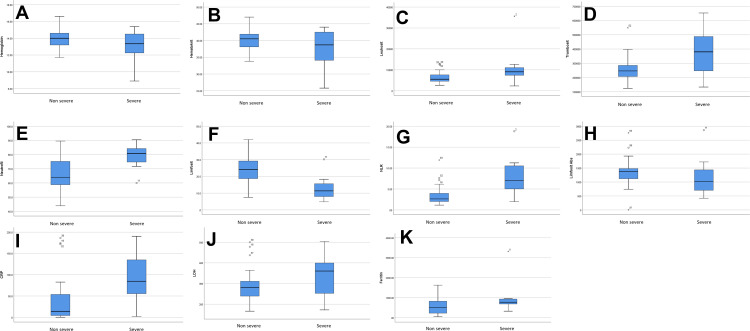
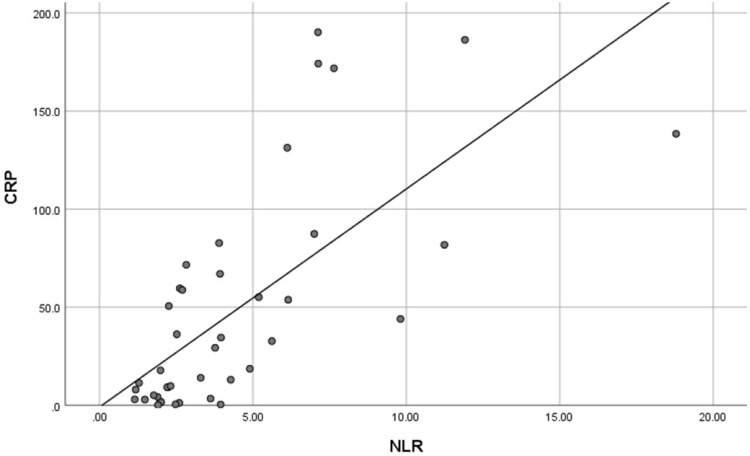
Figure 1 shows a comparison of hematologic parameters between both groups. In addition to the aforementioned difference, there is a difference of LDH values between non-severe and severe COVID-19 patients although not statistically significant. A strong correlation (r = 0.738, p < 0.001) was found between NLR and CRP (shown in Figure 2).
Figure 1.
Comparison of hematologic profiles between non-severe and severe COVID-19 patients. (A) Haemoglobin; (B) haematocrit; (C) white blood cells count; (D) platelet count; (E) relative neutrophils (%); (F) relative lymphocytes (%); (G) neutrophil to lymphocyte ratio (NLR); (H) absolute lymphocyte count; (I) C-reactive protein; (J) lactate dehydrogenase; (K) ferritin. ºDenotes outlier values outside the Q1-Q3 range. *Denotes extreme outlier values which is defined as distance to the median >1.5 times the IQR.
Figure 2.
Scatter plot showing correlation between neutrophil to lymphocyte ratio and C-reactive protein.
We found a moderate correlation (r = −0.414, p = 0.004) between NLR and absolute lymphocyte count (Table 3). Other correlations can be seen in Table 3. In addition, a moderate correlation is observed between NLR and absolute lymphocytes, absolute lymphocyte and LDH, CRP and LDH, and CRP and ferritin.
Table 3.
Correlation Between NLR, Absolute Lymphocyte Count, CRP, LDH and Ferritin in Hospitalized COVID-19 Patients
| Variables | r | p value | Remarks |
|---|---|---|---|
| NLR and absolute lymphocytes | −0.414 | 0.004* | Moderate correlation |
| NLR and CRP | 0.738** | <0.001* | Strong correlation |
| NLR and LDH | 0.349 | 0.013* | Fair correlation |
| NLR and ferritin | 0.324 | 0.019* | Fair correlation |
| Absolute lymphocytes and CRP | −0.386 | 0.006* | Fair correlation |
| Absolute lymphocytes and LDH | −0.502 | <0.001* | Moderate correlation |
| Absolute lymphocytes and CRP | −0.038 | 0.407 | No correlation |
| CRP and LDH | 0.568 | <0.001* | Moderate correlation |
| CRP and ferritin | 0.427 | 0.003* | Moderate correlation |
| LDH and ferritin | 0.358 | 0.011* | Fair correlation |
Notes: *p<0.05, **Strong correlation.
Abbreviations: NLR, neutrophil to lymphocyte ratio; CRP, C-reactive protein; LDH, lactate dehydrogenase.
Discussion
The haematological profiles of patients with COVID-19 showed differences between severe and non-severe cases. While haemoglobin, haematocrit and platelet count did not differ between these two groups, white blood cells and their differential counts seemed to play an important role in terms of disease severity. We found that in severe cases, white blood cells were significantly increased compared to non-severe cases. More specifically, subjects with severe COVID-19 showed higher neutrophil percentage and lower lymphocyte percentage.
Lymphopenia is found to be a characteristic of COVID-19 and was found to be useful in differentiating between COVID-19 pneumonia and non-COVID-19 pneumonia.13 Studies show that the decrease in lymphocyte is mainly caused by depletion of T-lymphocyte subsets, mainly T-helper and T-suppressor cells, and the presence of lymphopenia in COVID-19 patients suggests significant inflammation and tissue damage. Our findings show that absolute lymphocyte count is lower in severe cases, although not as markedly distinct as the difference in NLR.
The relationship between LDH and mild COVID-19 illness has been reported by Shi et al.9 In severe COVID-19, LDH is also found to be beneficial in predicting disease progression, as reported by Han et al.10 Henry et al11 has published a meta-analysis involving nine studies in mild, moderate and severe COVID-19 patients, and the meta-analysis shows that an increase in LDH levels is correlated to disease severity (OR = 6.53) and mortality (OR = 16.64).11
The NLR of the COVID-19 patients in this study is significantly higher in severe patients (median value 7.06; IQR 6.41). The use of NLR provides clinicians with rapid, easy, and economical means to determine the degree of inflammation. However, there is no consensus on the cutoff of NLR value. Several studies showed a cutoff of elevated NLR of 0.8, 2.9, 3.17, and even as high as 11.75 as described in a metanalysis.13,14 In this study, we found that non-severe patients had a median NLR of 2.62, whereas those with severe disease had a median NLR of 7.06. We analyzed the numerical value of NLR and correlated the variable with CRP. These provide valuable information on the usefulness of NLR regardless of the cutoff level.
In this study, we found a strong correlation between NLR and CRP (r = 0.738; p < 0.001). Similar results were also obtained in patients in China and Pakistan.13,15 These data suggest NLR can be used to predict the disease severity of COVID-19 in remote areas where the examination of the other laboratory inflammatory markers are not available. A study by Yang, et al13 which involved 69 non-severe and 24 severe COVID-19 patients found that NLR is an independent factor for a poor clinical outcome of COVID-19. In a south Asian population, Imran, et al15 found that among 63 patients (32 non-severe and 31 severe cases), NLR is a valuable tool for early detection of worsening severe COVID-19.
In severe cases, the inflammation markers will be markedly elevated. CRP, LDH and ferritin were found to be elevated in severe cases of COVID-19. A moderate correlation is observed between absolute lymphocyte and LDH, CRP and LDH, and CRP and ferritin. In this study, among those inflammatory markers, CRP showed a stronger and more significant difference than LDH or ferritin between non-severe and severe COVID-19. In contrast to other studies, we did not find ferritin to be useful in differentiating between severe and non-severe cases.
There are some limitations of this study: 1. Relatively small number of subjects. 2. We did not measure the cutoff of NLR in our subjects. 3. We did not obtain the serological status of the patients because the samples were taken during acute infection, before the serological conversion. This study was done during the first months of the pandemic when vaccines had not been distributed, hence the subjects had no vaccine-induced immunity response which could alter the inflammatory markers in this study. This study could provide valuable information regarding haematologic profiles, especially NLR, and inflammatory markers in COVID-19 patients.
Conclusion
In severe COVID-19 cases, NLR is significantly higher, and a strong correlation between NLR and CRP might suggest that healthcare professionals could utilize NLR to differentiate between non-severe and severe cases.
Acknowledgments
We thank Rumah Sakit Dr. Cipto Mangunkusumo (RSCM) for providing the research grant for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organization [homepage on the Internet]. Novel coronavirus (2019-nCoV) situation report-522020; 2020. Available from: https://apps.who.int/iris/bitstream/handle/10665/331476/nCoVsitrep12Mar2020-eng.pdf?sequence=1&isAllowed=y. Accessed January 2, 2021.
- 2.World Health Organization [homepage on the Internet]. Novel coronavirus (2019-nCoV) situation report-742020; 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200403-sitrep-74-covid-19-mp.pdf?sfvrsn=4e043d03_4. Accessed January 2, 2021.
- 3.World Health Organization [homepage on the Internet]. Weekly operational update on COVID-19; 2021. Available from: https://www.who.int/publications/m/item/weekly-operational-update-on-covid-19---9-august-2021. Accessed August 14, 2021.
- 4.Han R, Huang L, Jiang H, Dong J, Peng H, Zhang D. Early clinical and CT manifestations of coronavirus disease 2019 (COVID-19) pneumonia. Am J Roentgenol. 2020;215:338–343. [DOI] [PubMed] [Google Scholar]
- 5.Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med. 2020;58(7):1131–1134. doi: 10.1515/cclm-2020-0198 [DOI] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lagunas-Rangel FA. Neutrophil-to-Lymphocyte ratio and Lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J Med Virol. 2020;92(10):1733–1734. doi: 10.1002/jmv.25819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi J, Li Y, Zhou X, et al. Lactate dehydrogenase and susceptibility to deterioration of mild COVID-19 patients: a multicenter nested case-control study. BMC Med. 2020;18(1):168. doi: 10.1186/s12916-020-01633-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han Y, Zhang H, Mu S, et al. Lactate dehydrogenase, a risk factor of severe COVID-19 patients: a retrospective and observational study. Aging. 2020;12(12):11245–11258. doi: 10.18632/aging.103372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry BM, Aggarwal G, Wong J, et al. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am J Emerg Med. 2020;38(9):1722–1726. doi: 10.1016/j.ajem.2020.05.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isaac V, Wu CY, Huang CT, Baune BT, Tseng CL, McLachlan CS. Elevated neutrophil to lymphocyte ratio predicts mortality in medical inpatients with multiple chronic conditions. Medicine. 2016;95(23):e3832. doi: 10.1097/MD.0000000000003832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang A, Liu J, Tao W, Li H. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504. doi: 10.1016/j.intimp.2020.106504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simadibrata DM, Calvin J, Wijaya AD, Ibrahim NAA. Neutrophil-to-lymphocyte ratio on admission to predict the severity and mortality of COVID-19 patients: A meta-analysis. The Am J Emerg Med. 2021;42:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imran MM, Ahmad U, Usman U, Ali M, Shaukat A, Gul N. Neutrophil/lymphocyte ratio-a marker of COVID-19 pneumonia severity. Int J Clin Pract. 2021;75(4):e13698. doi: 10.1111/ijcp.13698 [DOI] [PubMed] [Google Scholar]