A recent article in this journal by Cohen et al.,1 ‘Looking beyond the face area: lesion network mapping of prosopagnosia’, concluded that prosopagnosia was caused not only by lesions to core face processing areas—such as the face fusiform area (FFA)—but also observed when other nodes within a distributed brain network were damaged. They sought to identify these nodes using ‘lesion network mapping’, which indirectly estimates functional connections that would be ‘lost’ due to localized brain lesions. The locations of lesions were used as seeds for resting state functional connectivity analysis with data from healthy participants. Cohen et al.1 report that in 44 cases with acquired prosopagnosia, all the lesion locations outside the right FFA were functionally connected in healthy controls to this region and were also functionally connected (albeit through negative correlation) with regions in the left frontal cortex. While generating compelling hypotheses, the indirect nature of lesion network mapping requires confirmation by other methods, as discussed in recent articles in Brain,2,3 including direct mapping of residual structural and functional connections in the patients.4
The purpose of this letter is to present evidence from a case of prosopagnosia (Subject EP) based on both indirect and direct structural/functional connectivity mapping, that suggests a modification of the network proposed by Cohen et al.1 In this case, the disruption of connections between ventro-temporal cortex (outside of the FFA) and the dorsal-visual/parietal areas was found to be an important factor in his face recognition deficits.
Subject EP is a 46-year-old male who suffered two consecutive strokes within a 1-day interval (see Fig. 1A for CT image less than 24 h after the strokes). The major cognitive sequelae consisted of difficulties in recognizing familiar faces, including those of his spouse and close relatives. Severe facial identity recognition impairments were found with the Benton Facial Recognition Test (score = 10/27) and the Facial Expressive Action Stimulus Test (score on Facial Identity Matching Test = 46/64). Famous face recognition was also severely impaired with only 5/14 celebrity faces being visually recognized (despite established familiarity with the posers). Subject EP reported that his residual recognition ability was based on non-facial features like hairstyle. Low-level aspects of visual perception were intact, as revealed by average scores on orientation, length, and size matching tasks of the Birmingham Object Recognition Battery. However, intermediate level tasks such as face and house part-to-whole matching tasks (dependent on configural information) were also impaired.
Figure 1.
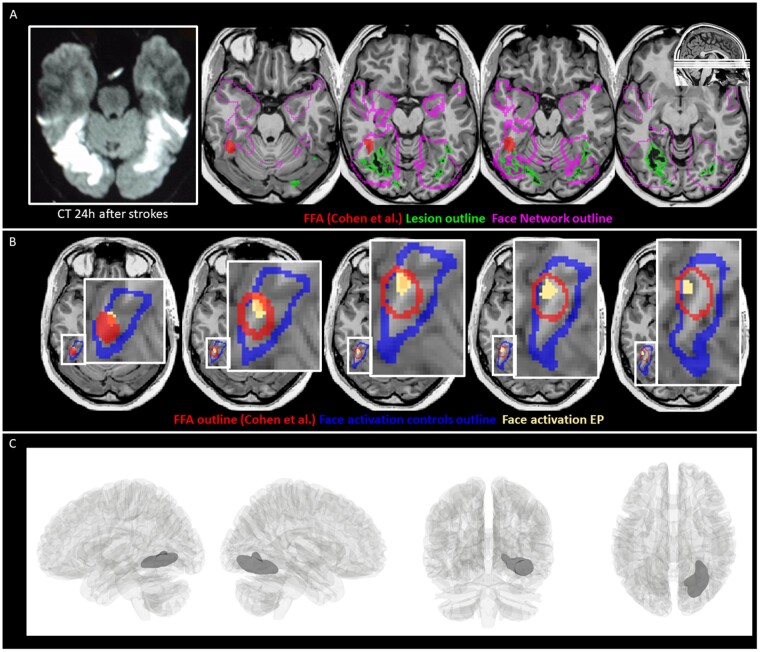
Lesion and face processing topography. (A) On the left, axial slices from a CT within 24 h following stroke; on the right, T1-weighted MRI slices taken 1 month after the strokes with the segmented lesion (outlined in green) superimposed as well as the face network (outlined in pink) and FFA defined by Cohen et al.1 (in red). (B) Face-specific (faces versus tools) activation in Subject EP (yellow) in the ventral occipito-temporal cortex (P < 0.005), relative to the FFA defined in the meta-analysis by Cohen et al.1 (outlined in red) and face-responsive activation (faces versus fixation) of the control subjects (outlined in blue), defined by means of an object-category localizer performed in six age-matched healthy control subjects (three male, mean age = 46.3, age range = 39–56 years) following a fixed-effect analysis (q < 0.01, FDR-corrected). (C) Lesion estimated by voxel-based morphometry (thresholded at FDR = 0.01) overlaid on a 3D glass brain.
A T1-weighted MRI (Fig. 1A) performed 1 month after the strokes revealed a lesion in the right hemisphere that partially overlapped the lingual, medial fusiform, and a small part of the inferior temporal gyrus. This lesion extended dorsally into white matter, slightly affecting the ventral part of the optic radiation (consistent with a small scotoma in the left upper visual quadrant). A much smaller lesion was found in the left hemisphere, confined to the middle and posterior fusiform gyrus. To assess the relationship of the lesion with the right FFA, we overlaid a region of interest mask of this area as defined in Cohen et al.,1 as well as one of their face network on Subject EP’s T1 image. This revealed that the lesion did not intersect the FFA (Fig. 1A) but did fall within locations in reported face network in more medial portions of the ventral-temporal cortex (Fig. 1B).
We also assessed face-selective activation with functional MRI in Subject EP and in six age-matched healthy control subjects using methods described elsewhere.5 As expected, face-selective activation in the control subjects overlapped the FFA region of interest. Interestingly, Subject EP showed face-specific activation within this FFA region of interest, as well as within the face-sensitive activation region of the controls (Fig. 1B). Thus, no damage to FFA was not found in Subject EP.
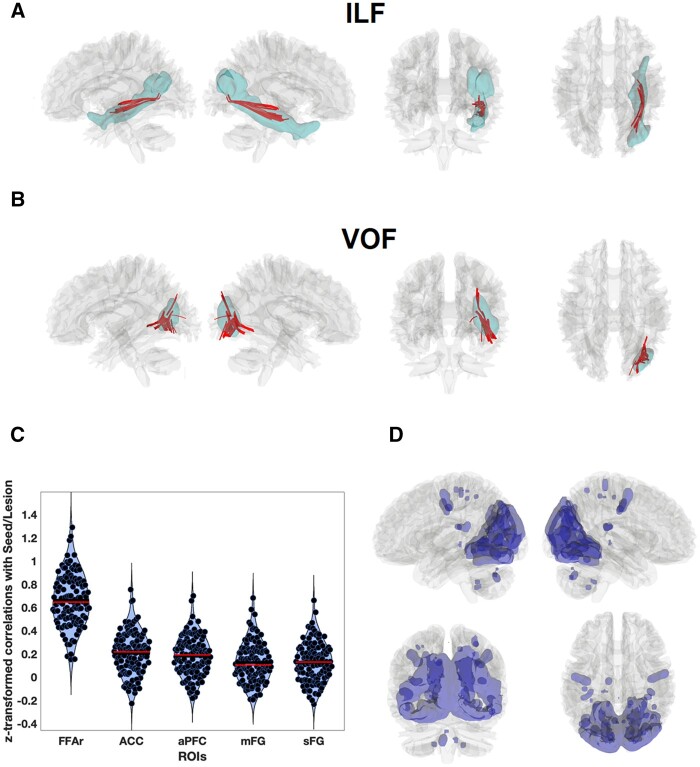
After transforming Subject EP’s T1 into MNI space using enantiomorphic normalization,6 the voxels containing the right-sided lesion were identified with single case4 voxel-based morphometry, using 15 sex- and age-matched volunteers as controls (Fig. 1C). We used the overlap of the lesion with probabilistic maps of trajectories of 80 white matter tracks (http://dsi-studio.labsolver.org/, based on diffusion-weighted imaging tractography of healthy subjects) as an indirect estimation of white matter disconnection. Only the trajectories of the ventral occipital fasciculus (VOF) and the inferior longitudinal fasciculus (ILF) were intersected by the lesion. A direct estimation of structural disconnection was obtained comparing Subject EP with control subjects using individual connectometry analysis with DSI-Studio,7 which evinced that 3 cm3 of the total volume of the VOF and 2.3 cm3 of the ILF were affected in Subject EP (Fig. 2A and B). This represents damage to 60% of the VOF and 23% of the IFL total volume. None of the VOF or the ILF streamlines were traced back into the FFA, although they did reach more medial ventro-temporal cortex. The latter result is important as it precludes—in this case—an established cause of prosopagnosia: the disconnection of core face areas from the anterior temporal lobe8 via ILF. Note that VOF connects ventral-temporal cortex with dorsal visual areas.
Figure 2.
Lesion effect on connectivity. The trajectories of (A) inferior longitudinal fasciculus (ILF) and (B) inferior fronto-occipital fasciculus (IFOF) in the HCP841 atlas (blue) in the right hemisphere. The lesioned tracts in Subject EP identified by the individual connectometry analysis are plotted in red. (C) Violin plots of Fisher transformed correlations between the seed region of interest (lesion) and four regions of interest from Cohen et al.1: the right FFA (FFAr), the left anterior prefrontal cortex (aPFC), the left middle frontal gyrus (mFG), the dorsal anterior cingulate cortex (ACC), and the left superior frontal gyrus (sFG), for 119 healthy subjects. Horizontal red lines represent the median, blue the mean. (D) Seed-to-voxel connectivity map for the lesion. The map was thresholded at q = 0.001, FDR-corrected, equivalent to t = 15.25. Significant negative correlations were not found. ROIs = regions of interest.
We also applied the indirect lesion network mapping, as used by Cohen et al.1 This analysis was based on resting state data from 119 healthy control subjects.9 Consistent with their findings, we found that functional MRI time series from a seed at the lesion location were positively correlated with those from the right FFA, with a large effect size [P < 0.0001, mean r = 0.0.660, t(118) = 32.032]. Nevertheless, we were unable to replicate the negative correlation the lesion seed and from the frontal regions of interest described by Cohen et al.1 All of these correlations tended to be slightly positive (Fig. 2C) although with small effect sizes, thus indicating that this is not a necessary condition for producing prosopagnosia. In fact, the seed-to-whole brain (Fig. 2D) mapping showed that the lesion site was connected (with positive correlations) with other visual areas including dorsal components, and with portions of fronto-parietal attentional control areas (https://neurosynth.org/analyses/terms/attentional%20control/, accessed 31 August 2021). Following the logic of Salvalaggio et al.,2 we directly compared the strength of functional connectivity between the right FFA and other face-related regions of interest in the patient and in 15 normal control subjects. Fifteen regions of interest were identified in a face/house localizer task in the controls (defined as clusters exceeding the threshold of P = 0.001 uncorrected with sizes > 20 voxels). In this direct measurement of functional disconnection (assessed against sex- and age-matched controls), the correlation between the right FFA and the left parietal areas was significantly reduced (P < 0.01).
In summary, our results indicate that the structural interruption of ventro-temporal to dorsal visual areas via VOF, accompanied by a concomitant functional disconnection to parietal components of the frontoparietal attentional control system, also play a central role in face and visual object recognition. Frontoparietal subnetworks are involved in multiple tasks, playing an important role in visual processing. Cohen et al.1 focused on the idea that these disconnections to the frontal components of this frontoparietal subnetwork contribute to prosopagnosia. Interestingly, the figures of Cohen et al.1 suggest a parietal involvement in their network of areas where lesions can cause prosopagnosia, although they do not discuss this possibility. This raises the questions of the nature of the information contributed by the ventro-temporal areas in Subject EP (that are outside of, and medial, to FFA) as well as why they are so necessary for face recognition. Of course, prosopagnosia is an heterogeneous disorder and the specific pattern of grey matter lesions and disconnections in Subject EP may be related to his apperceptive dysfunction. The possible role of the smaller ILF disruption found in Subject EP also needs to be further explored.
These results reinforce calls to supplement2 the indirect lesion network mapping method with other techniques. If we had only used this indirect method, we would have noted a partial congruence with results by Cohen and colleagues, but we would have missed the important ventral-temporal-dorsal visual/parietal disconnection found in Subject EP.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request. Some data may be unavailable to preserve patient confidentiality.
Funding
M.A.B. and M.V.-S. are supported by the National Fund for Science and Innovation of Cuba. J.V.d.S. is supported by the Sequoia Funds for Research on Ageing and Mental Health and by KU Leuven (C24/18/095). B.d.G. was supported by the European Research Council (ERC) FP7-IDEAS-ERC (Grant agreement number 295673; Emobodies), by the ERC Synergy grant (Grant agreement 856495; Relevance), by the Future and Emerging Technologies (FET) Proactive Program H2020-EU.1.2.2 (Grant agreement 824160; EnTimeMent) and by the Industrial Leadership Program H2020-EU.1.2.2 (Grant agreement 825079; MindSpaces).
Competing interests
The authors report no competing interests.
References
- 1. Cohen AL, Soussand L, Corrow SL, Martinaud O, Barton JJS, Fox MD.. Looking beyond the face area: Lesion network mapping of prosopagnosia. Brain. 2019;142(12):3975–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Salvalaggio A, Grazia MD, Schotten MD, Corbetta M, Zorzi M.. Post-stroke deficit prediction from lesion and indirect structural and functional disconnection. Brain. 2020;143(7):2173–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boes AD. Lesion network mapping: Where do we go from here? Brain. 2021;144(1):e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Valdés-Sosa M, Bobes MA, Quiñones I, et al. Covert face recognition without the fusiform-temporal pathways. Neuroimage. 2011;57(3):1162–1176. [DOI] [PubMed] [Google Scholar]
- 5. Van den Stock J, de Gelder B, De Winter FL, Van Laere K, Vandenbulcke M.. A strange face in the mirror. Face-selective self-misidentification in a patient with right lateralized occipito-temporal hypo-metabolism. Cortex. 2012;48(8):1088–1090. [DOI] [PubMed] [Google Scholar]
- 6. Nachev P, Coulthard E, Jäger HR, Kennard C, Husain M.. Enantiomorphic normalization of focally lesioned brains. Neuroimage. 2008;39(3):1215–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yeh FC, Tang PF, Tseng WYI.. Diffusion MRI connectometry automatically reveals affected fiber pathways in individuals with chronic stroke. NeuroImage Clin. 2013;2(1):912–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y, Metoki A, Alm KH, Olson IR.. White matter pathways and social cognition. Neurosci Biobehav Rev. 2018;90:350–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mendes N, Oligschläger S, Lauckner ME, . et al. A functional connectome phenotyping dataset including cognitive state and personality measures. Sci Data. 2019;6:180307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request. Some data may be unavailable to preserve patient confidentiality.