Abstract
Currently, ocular inserts and nanoparticles have received much attention due to the limited bioavailability of conventional eye preparations and the toxicity problems of systemic drug administration. The current systematic review aims to present recent studies on the use of electrospun nanofiber-based ocular inserts to improve the bioavailability of drugs used for different ophthalmic diseases. A systematic search was performed in PubMed, Ovid Medline, Web of Science, ScienceDirect, Scopus, Reaxys, Google Scholar, and Google Patents/Espacenet taking “drug-loaded”, “nanofibers”, and “ophthalmic inserts” and their equivalent terms as keywords. The search was limited to original and peer-reviewed studies published in 2011–2021 in English language. Only 13 out of 795 articles and 15 out of 197 patents were included. All results revealed the success of nanofiber-based ocular inserts in targeting and improved bioavailability. Ocular inserts based on nanofibers can be used as safe, efficient carriers for the treatment of anterior and posterior eye diseases.
Keywords: ophthalmic inserts, drug-loaded, nanofibers, electrospinning
1. Introduction
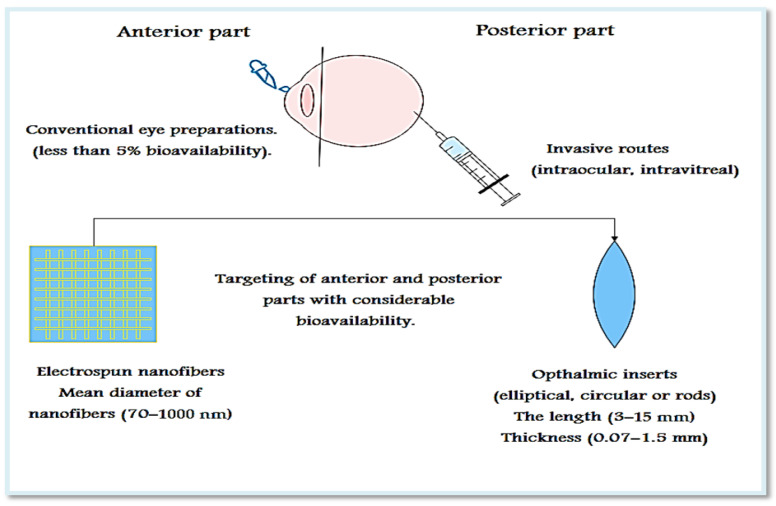
The unique anatomy and physiology of the eye, because of the physical and blood barriers, makes the targeting of ocular diseases a very difficult and challenging process [1,2]. These barriers interfere with drug absorption and diminish the drug concentration in the eye due to dilution and early drainage through lacrimation and poor permeation due to the effect of blood-retinal and blood-aqueous barriers [3,4,5]. Drugs used for the treatment of ocular disorders are administered via different routes (Figure 1): systemic, topical, subconjunctival, intrastromal, intracameral, intrascleral, intravitreal, suprachoroidal, and subretinal [6,7]. Oral administration of most drugs is subjected to kidney clearance, hepatic clearance, and failure of absorption due to physical and blood barriers. Likewise, absorption of drugs with the use of parenteral administration may subjected to retardation by blood-aqueous and blood-retinal barriers [8,9]. The most frequently used method is administration of drugs through the conventional topical route, which is characterized by being non-invasive, easy to self-administer, and more acceptable to patients. Nevertheless, it can only be used for the treatment of diseases affecting the surface and anterior segment of the eye, because it has poor bioavailability (less than about 5% of the drug is retained on the ocular surface). In addition, this method is not suitable for hydrophobic drugs or drugs that are unstable at a pH level tolerable to the eye and require frequent administration in order to overcome the barriers and nasolacrimal duct drainage [10,11]. Suitable for administration of up to 500 μL and relatively less invasive, subconjunctival administration is a highly convenient route and can be successfully used for targeting anterior or posterior segments [6,7]. Direct administration of the drug to the site of action with lower bioavailability can be done invasively based on intraocular routes [6], with intravitreal injection being the most promising for targeting posterior diseases, but it can be accompanied by retinal detachment, increased intraocular pressure, intravitreal hemorrhage, cataract, or endophthalmitis, which results in poor patient adherence [2,12].
Figure 1.
The most common routes of targeting different parts of the eye.
Apart from the conventional and parenteral routes, many alternatives, such as permeation enhancers, viscosity modifiers, pro-drug strategies, nanoparticles, long-term or permanent punctal inserts, corneal shields, contact lenses, mini-tablets, disposable lenses, biodegradable polymer systems, hydrogels, implants, and inserts, have been investigated to overcome the ocular barrier problem and increase bioavailability [12,13,14,15,16]. They act by increasing the contact time and slowing down the elimination of the drug from the eye [14]. A very promising approach for ocular targeting is the use of novel drug delivery systems based on nanotechnology; consisting of colloidal particles with sizes ranging from 1–1000 nm [17], nano-carrier drug delivery systems easily penetrate different barriers and have a lower chance of causing eye irritation. Moreover, they offer targeted and sustained release effects [18,19]. They include, but are not limited to, nanocapsules, liposomes, nanomicelles, lipid nanoparticles, niosomes, dendrimers, nanosuspensions, nanoemulsions, and nanocrystals [20,21].
Among the novel alternative approaches, ophthalmic inserts are sterile solid or semi-solid devices consisting of polymeric material with or without medicament, whose suitable size and shape are designed to be placed into the conjunctival sac [22]. Ocular inserts have received attention, as they increase the residence time of the drug on the eye surface, allow slow and controlled drug release, and reduce the overall dose and dose frequency. Moreover, they are stable and eliminate the need for preservatives, thereby reducing the possible side effects and increasing the shelf life of the drug compared to liquid formulations. They are classified into soluble, insoluble, and bio-erodible inserts. Soluble and erodible inserts undergo slow dissolution and do not need to be removed, and insoluble inserts should be removed from the eye when they are free of drugs [23].
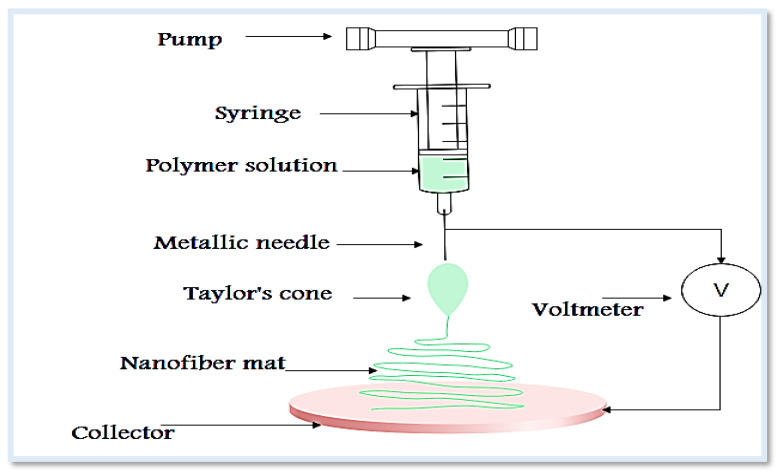
Drug release from ocular inserts takes place by three mechanisms: diffusion, osmosis, and erosion. Ocular inserts are prepared using solvent casting, glass substrate, and melt extrusion [24] techniques, and electrospinning (Figure 2) has been used as a simple, versatile technique for the production of nanofibrous film with unique properties [25]. The process of electrospinning is highly flexible and provides the chance to fabricate a wide range of natural and synthetic polymers or drugs; the technique relies on the application of a high voltage between the metallic needle and a grounded collector [26,27]. When the applied voltage exceeds certain critical value, the liquid is ejected from the needle, forming a conical droplet known as Taylor’s cone, followed by elongation, thinning and precipitation on the surface of the collector [27]. The resulted fibers are randomly deposited, but for more structured and aligned fibers rotating mandrel or a wheel-like collector are used [28]. The electrospun nanofibers with diameters of 100 nm [29] are constructed from a wide range of biodegradable and biocompatible polymers. The process is carried out simply by applying a high voltage to a drug or mixture of a drug and polymer solution or melts. The drugs can be incorporated through different methods such as blending, surface immobilization, and emulsion [28]. A wide range of drug materials can be encapsulated ranging from small inorganic molecule to large molecules of biological drugs such as protein and nucleic acids. In addition, the technique enables delivery of more than one drug in one step. The produced fibers have unique properties making them a suitable candidate for variety of pharmaceutical and biomedical [26,28,30]. Their large surface area enables ease of surface engineering and modification using different materials, such as permeation enhancers and mucoadhesive agents, and with the proper polymer selection, systems with controlled, sustained, and targeted release can be produced [31,32,33]. Furthermore, the produced fibers are homogeneous and highly reproducible [34], and due to their characteristic size and texture, they also represent good candidates for tissue reconstitution and promotion of human astrocytoma cell growth [35] and cell migration and proliferation [36]. A wide range of polymers are used for the fabrication of nanofibers for ocular uses, they are classified as natural, semisynthetic, and synthetic [37]. The final properties and quality of fibers are determined by many factors of which the type and concentration of polymers are of paramount importance. Some of the suitable polymers for the electro-spinning technique include polyesters (e.g., poly-glycolic acid- (PGA), poly-lactic acid- (PLA), polycaprolactone- (PCL)) [33]. These polymers are characterized by possessing sufficient mechanical strength to be electrospun, biocompatible, and useful for cell adhesion [34]. In some cases, a blend of the polymers is used together to control the drug release. Other cases include surface modification such as coating with mucoadhesive polymers or complexation with solubility or permeability enhancers [33]. According to the general rule, like dissolves like, hydrophilic polymers are a suitable candidate to encapsulate hydrophilic drugs without sustaining the release, while hydrophobic polymers can modulate sustained effects [37]. Natural polymers are widely used for biomedical applications [38], but synthetic polymers are used to fabricate products with superior quality; therefore, a combination of polymers from different origins is more advantageous for added functional properties [37]. Chitosan is a polysaccharide with a cationic amino group with wide pharmaceutical and biomedical applications [39]. The presence of an amino group leads to extensive protonation in aqueous media due to electrostatic interaction with a solvent molecule, which alters the solubility, viscosity, and mucoadhesive properties of the polymer [40]. Chitosan has gained significant attention in ophthalmic formulations due to its mucoadhesive properties, biocompatibility, and biodegradability and can increase drug permeability [19,41,42]. Nevertheless, it has several limitations include low stability in acidic media and poor mechanical strength. Complexation with cyclodextrins has been developed to strengthen the chitosan structure [43]. Another polymer relatively similar to chitosan is hyaluronic acid; it is a mucoadhesive polymer, forming a hydrogen bond with mucin; hence, it can successfully be used to prolong the drug release rate [37]. Gelatin as biopolymer is a natural protein of an animal origin that contains arginine-glycine-aspartic acids and metalloproteinase residues. Being widely available with lower antigenicity, possessing good bioadhesive properties, and promoting tissue regeneration renders it a good candidate for several biomedical and drug delivery applications. However, it has poor aqueous stability and low mechanical strength [44]. Integration of carbon nanotubes, graphene oxide, and carbon nano-onion into gelatin base improves mechanical strength and enables surface modification [45]. The drug loading and release kinetics are affected by molecular weight and cross-linking of the gelatin [46]. Poly-caprolactone (PCL) is a semi-crystalline hydrophobic biopolymer characterized by being biocompatible, possess significant toughness, soluble in most organic solvents; therefore, it is suitable for biomedical and drug delivery purposes [33]. In addition, it has a slow degradation rate, thereby can extend the drug release [25]. However, PCL has some limitations, such as insufficient strength, which necessitates additional reinforcement, such as integrating carbon nano-onions [47,48]. Bovine serum albumin (BSA) can be used as a nanocarrier for various drug molecules, incorporated through electrostatic interactions and covalent or non-covalent conjugation. It has many advantages, including being affordable, producing no immunogenic response, biodegradable, and modulating for different release patterns. Although it is very difficult to produce electrospun nanofibers from BSA alone, they can be combined with other polymers such as polyvinyl alcohol (PVA) and polyethylene oxide (PEO) [49]. Zein is a water-insoluble plant protein that has been used in drug delivery and coating. It requires physical and chemical treatment to improve its low mechanical strength and increase its water stability. Poly 4-mercaptophenyl methacrylate-carbon nano-onions can be incorporated within zein for enforcement of the mechanical strength [50].
Figure 2.
Schematic diagram of electrospinning process.
Although there are many published reviews describing ocular inserts, few of them cover the formulation of ocular inserts based on nanofibers; therefore, the current systematic review aims to describe the use of ocular inserts in the treatment of various eye diseases by shedding light on nanofiber-based ocular inserts as a promising, non-invasive method for targeting the posterior segment of the eye and improving bioavailability.
2. Materials and Methods
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) guidelines were followed to search for the relevant studies and report construction.
2.1. Eligibility Criteria
The following criteria were set for articles to be eligible for inclusion in this systematic review: Original research studies published in peer-reviewed journals and published patents were included, while review articles, conference papers, editorials, and commentaries were not. The study was limited to articles published in 2011–2021 in the English language. In addition, only ocular inserts or products to be placed in the conjunctival sac or cul-de-sac were considered, and all other dosage forms, including contact lenses and ocular implants, were excluded. The study included only nanofibrous ocular inserts fabricated via electrospinning technique; other techniques for fiber production were not considered. No clinical trial limits were set, and all in vitro and in vivo studies were eligible. Studies comparing electrospinning with other techniques were also included.
2.2. Search Strategy
To find the relevant articles and patents of drug-loaded electrospun nanofibrous ocular inserts, a systematized search was performed in PubMed, Ovid Medline, Web of Science, ScienceDirect, Scopus, Reaxys, Google Scholar, Google Patents, and Espacenet using the specified keywords together with their equivalent synonyms. We built up our search queries as: (Drug-loaded) AND (electrospinning) AND (Nanofibers OR nanofibrous) AND (ocular inserts OR ophthalmic inserts). The results were synthesized and tabulated accordingly.
2.3. Data Collection and Extraction
We used a PRISMA 2020 flow diagram to extract the most relevant data essential for synthesizing the results. First, all results obtained from all databases were exported to the Mendeley reference manager, and duplicate studies were removed. The rest of the articles underwent two successive screening processes: Irrelevant articles based on title were immediately excluded, then the abstracts and full texts of eligible articles were reviewed and analyzed. The relevant articles were double-checked by the reviewers based on the inclusion criteria, and the required information was extracted and tabulated into the following variables: polymer base, loaded drug/concentration, dimensions of inserts used in the study, diameter of nanofibers, in vivo animal model, and effects/properties of presented system.
3. Results
3.1. Database Search and Included Studies
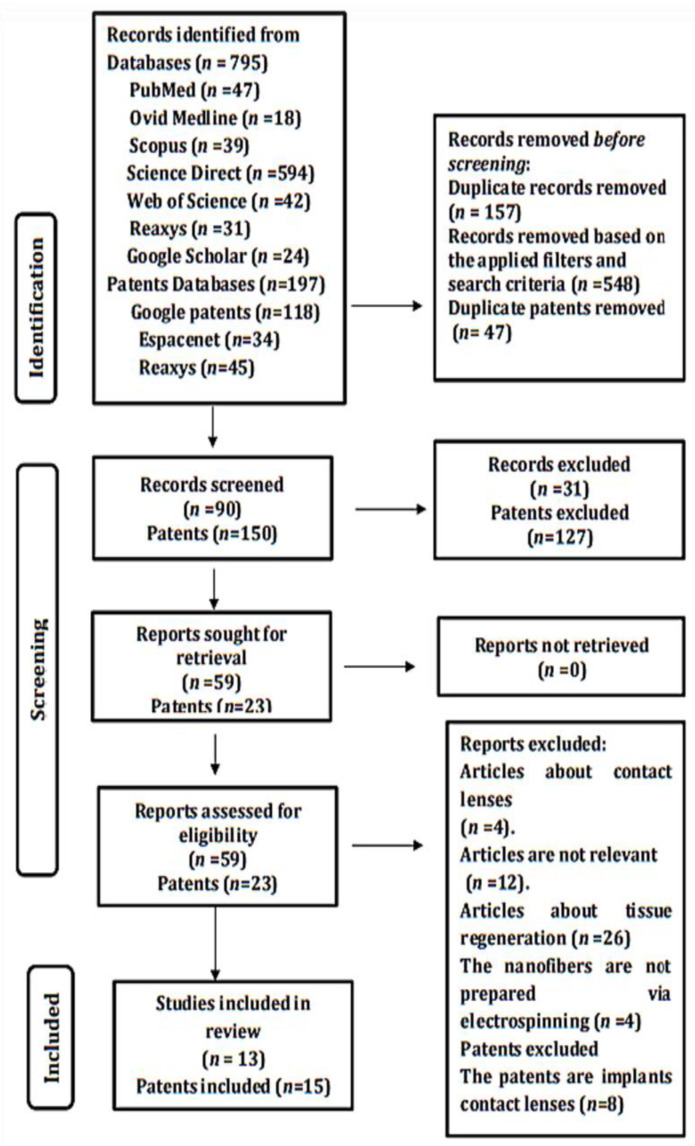
A total of 795 articles and 197 patents were obtained from all database searches, among which 594 were from Science Direct, 47 from PubMed, 42 from Web of Science, 39 from Scopus, 31 from Reaxys, 24 from Google Scholar, and 18 from Ovid Medline. Of the total patents obtained, 118 were from Google Patents, 34 from Espacenet, and 45 from Reaxys. Only 13 articles and 15 patents were included in the review, and they were selected based on the specified inclusion criteria. The process of identification and screening is presented in Figure 3.
Figure 3.
PRISMA-2020 flow diagram showing relevant articles and patents included in the study.
3.2. Results of Studies
According to the specified criteria, all relevant articles were thoroughly reviewed, and are summarized in Table 1. According to the extracted relevant information, New Zealand albino rabbits and some in vitro models are used to study eye toxicity, and the results revealed no or lower eye toxicity. The main focus was on increased residence time and bioavailability. The results showed the possibility of modulating the drug release from a few minutes up to a month based on the polymer base used and the properties of nanofibers, and the majority of studies showed controlled drug release. The formulations were compatible with the eye and the particle size showed no signs of ocular irritation.
Table 1.
Summary of nanofiber-based ocular insert studies.
| No | Polymer Base | Loaded Drug/Concentration | Dimensions of Inserts Used | Diameter of Nanofibers | In Vivo/Animal Model | Effects/Properties | References |
|---|---|---|---|---|---|---|---|
| 1 | Polycaprolactone (PCL), polyethylene glycol (PEG), sodium alginate (SA), thiolated sodium alginate (TSA) | Besiloxacin HCl (BH) (40 μg per 1 cm2) |
3.5 mm2 (thickness: 0.66 ± 0.004; diameter: 6.7 ± 0.012) | Less than 1057 nm | Yes, New Zealand albino rabbits |
|
[33] |
| |||||||
| |||||||
| 2 | Poly-lactic-co-glycolic acid (PLGA), polyvinylpyrrolidone (PVP) | Moxifloxacin HCl (1% w/v) pirfenidone (2% w/v) | 0.5 cm × 0.5 cm | Drug-loaded fibers were 630 ± 300 nm | Yes, New Zealand male albino rabbits, |
|
[36,51] |
| |||||||
| 3 | Polylactic acid (PLA), poly(vinyl alcohol) (PVA) | Dexamethasone (1, 5, and 10% w/w) | Thickness of fibers ranged from 50 to 93 μm | Within nanometer size | No in vivo studies have been done |
|
[52] |
| |||||||
| |||||||
| 4 | Polycaprolactone, poly (lactic-co-glycolic acid), polyvinyl alcohol | Gentamicin (GNT) (10% w/w), methylprednisolone (MP) (6% w/w) | NA | Mean range was 70–650 nm | No in vivo studies have been done |
|
[53] |
| |||||||
| 5 | Hyaluronan (HA), polyvinylpyrrolidone (PVP) | Ferulic acid (FA) (5.7 ± 0.2% w/w) | Mean thickness of 270 ± 21 μm | Approx. 100 nm to 1 μm | No in vivo studies have been done |
|
[54] |
| |||||||
| |||||||
| 6 | Poly(1,4-butylene succinate) (PBS) | Triamcinolone acetonide (TA) (2 mg/cm2) | Scaffold disk: 0.4 cm diameter | Range of 1–3 μm |
No in vivo studies have been done |
|
[34] |
| |||||||
| |||||||
| 7 | Chitosan/polyvinyl alcohol/polyvinyl pyrrolidone (CS/PVA-PVP) | Azithromycin (AZM)(10% w/w) | Diameter: 6 mm; thickness: 0.108 ± 0.012 to 0.121 ± 0.002 mm | Mean range of 119.01 ± 29.77 to 171.61 ± 39.40 nm | Yes, New Zealand rabbits |
|
[55] |
| |||||||
| |||||||
| |||||||
| |||||||
| 8 | Polycaprolactone (PCL) | Fluocinolone acetonide (1–5% w/w) | Average range of 350–400 nm |
Yes, New Zealand white rabbits |
|
[25] | |
| |||||||
| |||||||
| |||||||
| |||||||
| 9 | Poly(lactic-co-glycolic acid) copolymer/pluronic polyvinylpyrrolidone | Azithromycin (10 m/1 cm2) | Range of 200–550 nm | Yes, albino rabbits |
|
[56] | |
| |||||||
| |||||||
| 10 | Polycaprolactone (PCL),polyvinyl alcohol (PVA) | Timolol maleate (0.5% w/v), and dorzolamide hydrochloride (0.2% w/v) | Range of 200–400 nm | Yes, New Zealand white albino rabbits |
|
[57] | |
| |||||||
| |||||||
| |||||||
| |||||||
| 11 | Chitosan/polyvinyl alcohol (CS/PVA), Eudragit RL100 | Ofloxacin (OFX) (0.6% w/v) | Thickness range: 0.075 ± 0.002 to 0.095 ± 0.002 mm | Average 123 ± 23 to 159 ± 30 nm | Yes, New Zealand white albino rabbits |
|
[58] |
| |||||||
| |||||||
| |||||||
| 12 | Chitosan, polyvinyl alcohol (PVA), polyvinyl pyrrolidone (PVP), Eudragit S100,Zein | Triamcinolone acetonide (1% w/v) | NA | Range of 120 ± 30 to 172 ± 48 nm | No in vivo studies have been done |
|
[38] |
|
Here is a summary of the published studies based on the use of electrospinning technology for the fabrication of nanofiber-based ocular inserts and formulations designed to be used in the cul-de-sac: Besifloxacin HCl loaded inserts were prepared by electrospinning and investigated in in vitro, ex vivo, and in vivo studies as a potential alternative to commercial formulations. The formulations were subjected to modification with the addition of mucoadhesive polymer (sodium alginate (SA) or thiolated sodium alginate (TSA) as coating material) or corneal permeation enhancer (HP-β-CD) to the base polymer system of poly-caprolactone (PCL) and polyethylene glycol (PEG) in a 2:1 ratio of PCL/PEG. The in vitro studies, including thickness, diameter, degradation, and encapsulation, were within acceptable limits. The studies showed that the formulations produced no cytotoxic effect, with notable activity against bacteria. Corneal keratitis was significantly reduced by treatment with TSA-coated and HP-β-CD drug complex inserts [33].
Core-shell electrospinning was successfully used to fabricate a nanofibrous mat of pirfenidone-PLGA/moxifloxacin-PVP for the treatment of corneal abrasion. The results showed non-porous and smooth fibers with a diameter of 630 ± 220 nm that were capable of sustaining the release of both drugs and suitable for once-daily use [36]. The same results from pharmacokinetics studies showed the possibility of extended release over 24 h, while a Draize test showed mild irritation to the eye. Furthermore, antimicrobial and anti-scarring effects obtained were comparable to those of free solution [51].
Electrospun nanofiber inserts were fabricated and compared to inserts prepared by solvent casting employing poly-lactic acid (PLA) and poly-vinyl alcohol (PVA) as the polymeric system. The results showed thin, uniform inserts of nanometer size. In addition, the drug content was found to be more uniform in the inserts prepared by electrospinning, and it was concluded that electrospun nanofiber inserts could be a potential alternative to conventional eye drops [52]. Electrospun nanofibers loaded with gentamicin and methylprednisolone were prepared in different structures (single-jet electrospinning, sandwich structure using single-jet electrospinning, and core-shell electrospinning). All formulations were evaluated for physicochemical and antibacterial properties against S. aureus. The formulations showed acceptable mechanical and antimicrobial activity, with diameters of 70–350 nm, and the core-shell preparation showed the best drug release [53].
New nanofiber-based ocular inserts were prepared using hyaluronan (HA) and polyvinylpyrrolidone (PVP) for dual delivery of ferulic acid (FA) as antioxidant and ε-polylysine (ε-PL), an antimicrobial peptide. Two series of inserts were prepared, blank, and FA-loaded. The prepared inserts were subjected to physical, morphological, compatibility, release, and antimicrobial studies. The results showed acceptable thickness of 270 ± 21 μm to 273 ± 41 μm with fiber diameter of approx. 100 nm to 1 μm; in addition, they demonstrated adequate drug release, with antimicrobial activity against Pseudomonas aeruginosa and Staphylococcus aureus [54].
Ocular polymeric inserts loaded with triamcinolone acetonide (TA) have been developed in two steps: electrospinning of a solution of poly-butylene succinate (PBS), followed by surface modification through plasma activation and reaction with inulin, heparin, and α,β-poly(N-2-hydroxyethyl)-d,l-aspartamide. The morphological results showed a flexible non-porous scaffold with fibers ranging from 1 to 3 μm in diameter. The surface modification allowed stable, non-erodible, mucoadhesive, and highly loaded inserts that were compatible with human cells; it also allowed extended drug release for up to 30 days [34]. Nanofiber inserts loaded with azithromycin were prepared using a mixture of chitosan, polyvinyl alcohol, and polyvinyl pyrrolidone via electrospinning. The formulated inserts were subjected to physicochemical, morphological, in vitro, and in vivo release, antibacterial activity, cytotoxicity, and ocular irritation evaluation. The results showed acceptable hardness and uniform weight and thickness, with fiber diameter ranging from 119 ± 29 to 171 ± 39 nm. The inserts were also found to be nontoxic and non-irritating to the rabbits’ eyes. The drug release was extended up to 6–8 days [55].
In an attempt to target retinal inflammatory disease, nanofibrous ocular inserts loaded with fluocinolone acetonide were prepared and evaluated for permeability, in vivo pharmacokinetic, and in vivo release as well as other mechanical and chemical characteristics. Preclinical results revealed the possibility of retinal delivery with no cytotoxicity. The obtained fibers were smooth, non-woven, and homogeneous. Degradation and release studies confirmed extended drug release up to 12 days [25].
Nanoparticles loaded with azithromycin were incorporated into electrospun nanofibers to obtain ocular inserts that were mucoadhesive and biodegradable. The formulations were characterized in vitro, ex vivo, and in vivo. The results showed improved bioavailability, low risk of toxicity, and prolonged drug release over 10 days [56]. Two polymer series, polycaprolactone (PCL) and polyvinyl alcohol (PVA), were used to formulate biodegradable polymeric patches containing timolol maleate and dorzolamide hydrochloride for cul-de-sac insertion. The formulations were characterized in terms of morphology, folding endurance, drug release, ocular irritation, and in vivo efficacy. The fibers were uniform and smooth with sufficient mechanical strength. An in vitro release study for up to 24 h confirmed a single daily dose. Ocular irritation results showed minor irritation with PCL formulation and no comparable effect with PVA patches. The products successfully reduced the induced intraocular pressure and maintained for up to 72 h [57].
An internal layer of hydrophilic chitosan/polyvinyl alcohol (CS/PVA) and an outer layer of hydrophobic Eudragit RL100 were used in the fabrication of ocular inserts for delivery of ofloxacin (OFX) to increase the residence time on the eye. All parameters related to strength, thickness, and morphology were within acceptable limits. The formulations showed significant in vitro antimicrobial activity against S. aureus and E. coli. The formulations allowed prolonged release for up to 95 h with no signs of ocular irritation as demonstrated by in vivo studies [58]. Four formulations were used to prepare nanofibrous ocular inserts; three of them were chitosan-based and the fourth was composed of Eudragit S100 and Zein for sustained delivery of triamcinolone acetonide. All fibers were smooth and fibrous except the formulation containing a mixture of PVP, PVA, and chitosan. In vitro release studies demonstrated sustained drug release (zero-order rate), and no in vivo studies have been done [38].
Based on the results summarized in Table 1, it is obvious that the formulation of these ocular inserts depends on the use of natural and/or synthetic polymers. These polymers are involved in determining the final quality of products. The most widely used polymers include polyglycolic acid (PGA), polylactic acid (PLA), polycaprolactone (PCL), polyvinyl alcohol (PVA), polyacrylic acid (PAA), polyvinyl pyrrolidone, hyaluronic acid, chitosan (CS), polyethylene oxide (PEO), polymethacrylate, and cellulose derivates [25,33,37,41]. The majority of these polymers are biodegradable and classified as being soluble, insoluble, or bioerodible. With the proper base selection, a wide range of ocular inserts with different forms of drug release and targeting can be obtained. The results of many studies based on polymer type are given in Table 2.
Table 2.
Ocular inserts formulations and base types.
| Drug (Concentration) | Applied Polymer Base | Type of Base (Soluble/Insoluble/Erodible) | Effect/Aim | References |
|---|---|---|---|---|
| Azithromycin (20% w/w) | Hydroxypropylmethyl cellulose (HPMC) and Eudragit RL100 | Erodible |
|
[10] |
| ||||
| Triamcinolone acetonide (0.5% w/w) | Poly(1,4-butylene succinate) extended with 1,6-diisocyanatohexane (PBS) | Insoluble |
|
[34] |
| ||||
| Azithromycin (10% w/w) | Hydroxypropyl methylcellulose (HPMC) or hydroxyethyl cellulose (HEC). | Soluble and erodible |
|
[59] |
| ||||
| Cetirizine (7.5% w/v) | Hydroxypropyl methylcellulose (HPMC) and polyvinyl alcohol (PVA) | Soluble and erodible |
|
[60] |
| ||||
| Ofloxacin (1.5 and 6% w/w) | Poly(ethylene oxide) (PEO 400 or PEO 900) | Erodible |
|
[61] |
| Besifloxacin HCl (40 μg/) | Poly(caprolactone)/polyethylene glycol (PLC/PEG) (2:1) | Erodible |
|
[33] |
| ||||
| Dorzolamide HCl (0.37% w/v) | Polyvinyl alcohol, poloxamer 407 | Soluble |
|
[62] |
| Timolol maleate (TM) (0.5% w/v) | Hyaluronic acid (HA) and hydroxypropyl methylcellulose (HPMC) | Erodible |
|
[63] |
| Moxifloxacin hydrochloride (5% w/w) | Eudragit™ FS-100 (FS) and propylene glycol (PG) | Soluble |
|
[64] |
| Gatifloxacin (2.4 mg/78.5 mm2) | Thiolated sodium alginate (TSA), sodium alginate (SA), and acrylates: ERL:ERS (75:25) | Erodible |
|
[65] |
| Dexamethasone (1%, 5% and 10% w/w) | Polylactic acid (PLA) and poly-vinyl alcohol (PVA) | Erodible |
|
[52] |
| Curcumin (1% w/w) | Carboxymethylcellulose (CMC), polyvinyl alcohol, hydroxypropyl methylcellulose | Soluble |
|
[66] |
| Lysozyme (3% w/w) | Hydroxypropyl methylcellulose (HPMC) | Erodible |
|
[67] |
| Gentamicin sulfate (25.0% w/w) | Mixture of hydroxypropyl cellulose, ethyl cellulose, poly(acrylic) acid | Soluble |
|
[68] |
| Indomethacin (IN; 10% w/w), prednisolone sodium phosphate (PSP; 10% w/w), ciprofloxacin hydrochloride (CIP; 10% w/w) | Polyethylene oxide (PEO) N10 | Erodible |
|
[69] |
| ||||
| ||||
| Valacyclovir HCl (20% w/w) | HPC and HPMC | Soluble and erodible |
|
[70] |
| ||||
| Fluorescein (3% w/w), lysozyme (3% w/w), albumin (3% w/w) | HPMC | Erodible |
|
[71] |
| Gentamicin sulfate (25.0% w/w), dexamethasone phosphate (5.0 and 25% w/w) | Hydroxypropyl cellulose (HPC) | Soluble |
|
[72] |
| Ketorolac tromethamine (KT; 1% w/v) | Eudragit S100 and Zein | Erodible |
|
[38] |
| ||||
| Ofloxacin (3–9% w/w) | Chitosan/polyvinyl alcohol (CS/PVA), Eudragit RL100. | Erodible |
|
[58] |
| ||||
| Brimonidine tartrate (≈10–30% w/w) | PEO with Eudragit (RL 100/RS 100) | Erodible |
|
[73] |
| Linezolid (LNZ; 0.2% w/v) | Modified sodium alginate-grafted poly (butyl methacrylate) and sodium alginate-grafted poly (lauryl methacrylate) | Erodible |
|
[74] |
3.3. Ocular Insert Patents Studies (2011–2021)
Ocular inserts have recently received attention and many patents have been published demonstrating conventional and nanofiber-based formulations. Some examples of interesting and promising systems are presented here along with summaries, shown in Table 3. An Australian patent describes a nanostructured biocompatible wafer for placement in the conjunctival cul-de-sac. The wafer contains a tissue-reactive mucoadhesive polymer, and provides a method for treating glaucoma or infection on the eye surface. The thickness of the wafer is between 0.05 and 0.5 mm, preferably between 0.05 and 0.1 mm, but it is not limited to hydrophobic polymers or any combination of biodegradable polymers, with the polymers available commercially and approved for human use being the best choice [75].
Table 3.
Summary of ocular insert patents studies (2011–2021).
| Insert Base | Targeted Diseases | Model Drug Used for Study | Applicant /Manufacturer/Assignee | Patent Number | Publication Date | Reference |
|---|---|---|---|---|---|---|
| Biodegradable, hydrophobic polymer | Tissue regeneration, glaucoma, infections on eye surface (all are possible) | Travoprost | Integral Biosystems LLC | AU 2019250153 A1 | 31 October 2019 | [75] |
| Biodegradable, hydrophobic, and/or amphiphilic polymer | Eye infections | Azithromycin | Zewail City of Science and Technology, Egypt | GB 2570113 A | 17 July 2019 | [76] |
| Hydrophilic, biodegradable, bioabsorbable, or bioerodible polymer | Glaucoma | Timolol maleate | Valeant International (Barbados) SRL | US2012215184A1 | 23 August 2012 | [77] |
| Thermoplastic polymer such as acrylonitrile butadiene styrene and ethylene-vinyl acetate | Glaucoma | Bimatoprost | ForSight VISION5 Inc. (US) | US2016022695A1 | 28 January 2016 | [78] |
| Polyvinyl-alcohol (PVA) | Bacterial infections (tuberculosis (TB)) | Rifampicin | Tongling Wutongshu Agricultural Dev. Co. Ltd. | CN104116722A | 29 October 2014 | [79] |
| Polymer such as poly-lactic/glycolic acid | Ocular inflammation associated with inflammatory diseases or following ocular surgery | Steroids | Interface Biologics Inc. (CA) | WO2019148291A1 | 08 August 2019 | [80] |
| Arylborono-containing hydrophilic copolymer | Anterior and posterior segment diseases | Optional | Alcon Inc. (CH) | US2021077385A1 | 18 March 2021 | [81] |
| Hydroxypropyl methylcellulose, polyvinylpyrrolidone, sodium carboxymethyl cellulose, and gelatin | Myopia | Atropine sulfate | Univ. Shenyang Pharmaceutical | CN111358771A | 07 July 2020 | [82] |
| Hydroxypropylcellulose and polyethylene oxide block copolymer | Glaucoma | Timolol maleate | Univ. Witwatersrand JHB (ZA) | WO2014041485A1 | 20 March 2014 | [83] |
| Thermoplastic polymer such as acrylonitrile butadiene styrene and thermosetting polymer such as silicone and polyesters | Glaucoma | Bimatoprost | ForSight VISION5 Inc. (US) | US2016296532A1 | 13 October 2016 | [84] |
| Polycaprolactone/polyethelyneglycol | Bacterial infections | Moxifloxacin | Univ. De Coimbra (PT) | WO2017137934A1 | 17 August 2017 | [85] |
| Poly-vinyl-alcohol | Fungal infections | Voriconazole | Univ. Zhejiang | CN105726517A | 06 July 2016 | [86] |
| Hyaluronic acid, polyvinyl-pyrrolidone, hydroxypropyl guar, and polyethyleneglycol | Dry eye | - | Alcon Inc. (CH) | WO2021116907A1 WO2020222195A1 US2021169781A1 | 17 June 2021 05 November 2020 10 June 2021 |
[87,88,89] |
Another patent describes an ocular delivery system that consists of a nanofibrous matrix containing drug-loaded nanoparticles as a candidate for ocular inserts. In addition, the method of preparation (electrospinning) and medical uses of the nanofibrous ocular system are demonstrated, which include anti-microbial, anti-glaucoma, anti-inflammatory, analgesic, anesthetic, or combined effects. Biodegradable hydrophobic polymer and/or biodegradable amphiphilic polymer can be used. The system can also contain a mucoadhesive polymer to provide controlled release of drug over a period of at least 3 days [76].
Another patent describes various shapes of ocular inserts together with methods of preparation and potential uses. Hydrophilic polymers with biodegradable, bioabsorbable, or bioerodible properties are used. In addition to active ingredients, it may contain dyes, lubricant, emollient, and jelling agent. The system can be loaded with thermo-labile, poorly soluble, soluble, micronized, and nanoparticle substances. The pharmaceutically active agents include anti-bacterial, steroidal and non-steroidal anti-inflammatory, anti-allergy, anti-viral, anti-cholinergic, and mydriatic drugs, or any suitable combination [77]. Another patent discusses the bimatoprost composition, preparation, and devices comprising these compositions. These formulations are responsible for sustained release of bimatoprost to the eye. The composition of this invention comprises stable amorphous bimatoprost with a thermoplastic polymer matrix, such as acrylonitrile butadiene styrene (ABS), celluloid, cellulose acetate, ethylene-vinyl acetate (EVA), ethylene vinyl alcohol (EVOH), polyacrylate (acrylic), or polyacrylonitrile (PAN or acrylonitrile). It may contain thermosetting polymers. This medical device can serve as an ocular insert with a ring shape (ring diameter can be about 10 to 40 mm or about 20 to 30 mm) [78].
A patent describes a method for preparing rifampicin film to overcome uneven drug content by using poly-vinyl alcohol (PVA) via a simple method with temperature, pressure, and time control [79]. The patent pertains to ocular inserts for sustained release of steroids to the eye. The steroids are considered as articles from D1-L-D2 (A-I), where D1 and D2 are steroid radicals and L is linker that is covalent to D1 and D2. The article can be fiber, fiber mesh, nanoparticles, microparticles, or woven or non-woven fabric. The linker can be carbonate or carbamate ester [80].
Another patent describes soft polymeric hydrogel ocular inserts that are readily available and comfortable for use to release drug and lubricant to the anterior and posterior segments of the eye in a controlled manner. The hydrogel material is derived from at least one arylborono-containing hydrophilic copolymer and at least one mucoadhesive polymer, and cyclic boronic ester. The patent also describes the method for preparation [81]. Another patent describes atropine sulfate ocular inserts and the method of preparation to enhance stability and bioavailability by utilizing hydroxypropyl methylcellulose, polyvinylpyrrolidone, sodium carboxymethyl cellulose, and gelatin or any suitable combination [82]. Another patent describes the delivery of at least one drug to the desired site of action of the human or animal eye. The system contains at least two polymers, preferably from the class of polyethylene oxide block copolymers and cellulose derived polymers, such as hydroxpropyl cellulose. It may also contain an anti-collapsing agent, such as amino acid. The active ingredients include prostaglandin, beta blockers, alpha agonists, carbonic anhydrous inhibitors, or any suitable combination [83].
Another patent describes polymeric ocular inserts composed of a pharmaceutically active semi-crystalline or crystalline agent dispersed in a polymer matrix in order to provide a formulation that is more stable and has fewer impurities. The method of preparation is also described. The composition includes for example, bimatoprost as a pharmaceutically active ingredient, a polymeric matrix, which is a thermoplastic polymer such as acrylonitrile butadiene styrene, and a thermosetting polymer such as silicone and polyesters [84].
Another patent describes an ocular insert that is considered as a new biocompatible polymer-based controlled drug delivery system for the release of suitable drug to the eye for up to 300 days. The inserts are prepared with different shapes and are suitable for self-administration, and can be inserted in the lower or upper fornix conjunctiva. At least one drug can be incorporated, including antibiotics, antibacterials such as sulfonamides, antivirals, anti-allergy, anti-inflammatories such as hydrocortisone or hydrocortisone acetate, decongestants such as tetrahydrozoline, miotics and anticholinesterase, sympathomimetics such as epinephrine, immunological drugs such as vaccines and immune stimulants, hormonal agents, growth factors, or carbonic anhydrase inhibitors. The polymers used are polycaprolactone (PCL), polyethylene glycol (PEG), or co-polymers PEG-PCL, or a mixture of these [85].
Another intervention aims to provide voriconazole-loaded ocular film for continuous release of the drug. The ocular film consists of nanopolymer fiber and film-forming and other excipients. Electrospinning is used to prepare the film in order to obtain nanopolymer fibers with uniform morphology. Polyvinyl alcohol, acrylic resin, polyvinylpyrrolidone, polyvinylpyrrolidone derivative, cellulose, cellulose derivative, and chitosan individually or in combination are considered to be the best materials for film preparation [86]. Another patent describes dissolvable polymeric eye inserts with a biodegradable polymer for sustained release of drugs and lubricants to the anterior and posterior segments of the eye. They provide a way to treat different eye disorders by incorporating different active pharmaceutical ingredients. The inserts are comfortable for patients and the thickness of the film ranges from 50–250 μm, and preferably from 70–150 μm. The polymers used include hyaluronic acid, hydroxypropyl guar (HP guar), and a plasticizer, such as polyethylene glycol (PEG). The inserts are suitable for insertion in the lower eyelid. The composition is rapidly wetted by tears to release the lubricant. The active ingredients may be added to the same polymeric base [87,88,89].
4. Discussion
It is a very difficult process to deliver drugs to the eye, especially the posterior segment, due to the presence of physical barriers as well as blood retinal barriers, particularly when using conventional topical formulations. Therefore, scientists have performed many studies on eye diseases in an attempt to deliver drugs to the target site of action with sufficient bioavailability. Nanotechnology-based drug carriers have been investigated for their potential technological and therapeutic advantages for ocular delivery. Nanocarriers are used to deliver drugs for local or systemic effect by being localized to a specific site in the eye and releasing the required drug concentration through diffusion or as a response to external stimuli [18,90]. They offer numerous advantages; for example, the surface of the nanocarrier can be modified using different polymers for different purposes, such as mucoadhesive properties, by increasing the residence time, thus tailoring the drug release to reach controlled or sustained delivery. Furthermore, enhanced bioavailability with a lower risk of adverse effects and eye irritation can be obtained to increase patients’ adherence to medications [28,91,92,93,94,95].
The current systematic review demonstrates the efficiency and superiority of nanoformulation, particularly nanofibrous webs, as a promising alternative to conventional methods regarding site targeting and improved bioavailability. All toxicity studies proved their safety in animal models and in vitro alternative models. Pharmacokinetic studies have shown sustained drug release through different models such as zero-order and Higuchi models, which allows the possibility for single-dose administration for up to 30 days [34]. Combining the advantages of nanofibers with the advantages of ocular inserts will increase the bioavailability by many times [58] and reduce or even eliminate the disadvantages of using normal macro-size ocular inserts.
5. Future Perspective
Many people have ocular diseases, which interfere with quality of life, and the number is increasing by 7 million per year [96]. Even though many published studies have demonstrated that ocular inserts are a successful and non-invasive method of delivering drugs to the eye, only a small number of ocular inserts are available on the market, for example, Lacrisert®, a topical insert approved by the U.S. Food and Drug Administration [64] and Mydriasert© [97]. A significant amount of research on tailoring the delivery of various drugs to different parts of the eye using ocular inserts has provided very promising results, such as delivery of antimicrobial peptides to the pre-corneal area [98] and ocular inserts for delivery of thermolabile therapeutics [71].
Since nanotechnology has received a great deal of attention in recent years, nanofibers are expected to become an integral part of frequently used dosage forms in the near future, as they are able to penetrate and target different sites, including posterior segments of the eye [38] and the vitreous cavity for the treatment of retinal diseases [99]. From a technical point of view, nanofibers can be used directly as films [58] or after further processing into suitable ocular inserts or other surface modifications [100]. In addition, nanofibers can be formulated using a simple, versatile electrospinning technique [31]. The process enables encapsulation of more than one drug in one step [38], which will result in decreased multiple-drug regimens and increased patient compliance [27,101]. It also enables encapsulation and delivery of macromolecules such as genes and proteins, and this will open the door for research in this area, especially since there are not many studies on macromolecules [102]. In addition it can be scaled up for large-scale production to meet the requirements and sustainability of pharmaceutical companies [103].
Obviously, ocular inserts have also been intensively investigated because of their unique advantages as an alternative way to target eye disease, considering the possibility of fabricating them using different methods and polymeric materials. To meet the needs of the patients and minimize their suffering, a new non-invasive drug delivery system that offers good bioavailability and has the potential to deliver medicaments to posterior parts of the eye is expected to be investigated and introduced to the market in the near future. As many studies have proven the success of ocular inserts, the application of electrospun nanofibers as film or as part of ocular inserts is expected to result in a great advancement in the targeting of eye diseases, if these studies find a place in human clinical trials to confirm their efficiency and nontoxicity.
6. Conclusions
Eye problems are increasing daily; therefore, a smart approach is needed. It has been proven that drug delivery to the eye involves many problems that result from the barriers present in the eye, including the retinal blood barrier, lacrimation, eye blinking, and dilution, which is eventually reflected in poor drug bioavailability, particularly when using conventional topical drug delivery systems such as eye drops. In order to overcome such barriers, it is important to increase the contact time of the eye formulation to increase absorption and decrease the frequency of drug administration.
Different unconventional approaches are available, but every approach comes with some limitations. Nanofibers have gained attention recently, since they have superior advantages compared to other available systems; they are less irritating to the eye due to their small size and can be fabricated using different polymeric systems, therefore sustained release formulations could be applied, resulting in lower administration frequency. In addition, many studies have demonstrated successful posterior eye targeting.
An interesting characteristic of nanoparticulates is their large surface area, which allows the fabrication of different structures, including ocular inserts. Ocular inserts have been found to have many advantages: they provide increased bioavailability through the prolonged residence time of the drug on the conjunctival surface, can be formulated using different polymeric materials and methods, and allow the preparation of preservative-free formulations, thus resulting in lower eye sensitivity or even no sensitivity or irritation. In the light of the advantages of nanofibers and ocular inserts, a synergistic effect could be obtained by formulating ocular inserts with a nanofibrous architecture. Many published studies confirm the possibility of using such systems as an alternative to conventional topical formulations with better results.
Author Contributions
Conceptualization, S.O. and R.Z.; methodology, S.O., R.Z.; writing—original draft preparation, S.O.; writing—review and editing, R.Z. Both authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mehta P., Al-Kinani A.A., Arshad M.S., Chang M.W., Alany R.G., Ahmad Z. Development and Characterisation of Electrospun Timolol Maleate-Loaded Polymeric Contact Lens Coatings Containing Various Permeation Enhancers. Int. J. Pharm. 2017;532:408–420. doi: 10.1016/j.ijpharm.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 2.Tsai C.H., Wang P.Y., Lin I.C., Huang H., Liu G.S., Tseng C.L. Ocular Drug Delivery: Role of Degradable Polymeric Nanocarriers for Ophthalmic Application. Int. J. Mol. Sci. 2018;19:2830. doi: 10.3390/ijms19092830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maharjan P., Cho K.H., Maharjan A., Shin M.C., Moon C., Min K.A. Pharmaceutical Challenges and Perspectives in Developing Ophthalmic Drug Formulations. J. Pharm. Investig. 2019;49:215–228. doi: 10.1007/s40005-018-0404-6. [DOI] [Google Scholar]
- 4.Lynch C.R., Kondiah P.P.D., Choonara Y.E., du Toit L.C., Ally N., Pillay V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020;8:1–18. doi: 10.3389/fbioe.2020.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal P., Scherer D., Günther B., Rupenthal I.D. Semifluorinated Alkane Based Systems for Enhanced Corneal Penetration of Poorly Soluble Drugs. Int. J. Pharm. 2018;538:119–129. doi: 10.1016/j.ijpharm.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y.C., Chiang B., Wu X., Prausnitz M.R. Ocular Delivery of Macromolecules. J. Control Release. 2014;190:172–181. doi: 10.1016/j.jconrel.2014.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen J., Lu G.W., Hughes P. Targeted Ocular Drug Delivery with Pharmacokinetic/Pharmacodynamic Considerations. Pharm. Res. 2018;35:1–20. doi: 10.1007/s11095-018-2498-y. [DOI] [PubMed] [Google Scholar]
- 8.Jervis L.P. A Summary of Recent Advances in Ocular Inserts and Implants. J. Bioequiv. Bioavailab. 2016;9:320–323. doi: 10.4172/jbb.1000318. [DOI] [Google Scholar]
- 9.Gorantla S., Rapalli V.K., Waghule T., Singh P.P., Dubey S.K., Saha R.N., Singhvi G. Nanocarriers for Ocular Drug Delivery: Current Status and Translational Opportunity. RSC Adv. 2020;10:27835–27855. doi: 10.1039/D0RA04971A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thakur R., Swami G., Rahman M. Development and Optimization of Controlled Release Bioerodable Anti Infective Ophthalmic Insert. Curr. Drug Deliv. 2014;11:2–10. doi: 10.2174/15672018113106660060. [DOI] [PubMed] [Google Scholar]
- 11.Mudgil M., Pawar P.K. Preparation and In Vitro/Ex Vivo Evaluation of Moxifloxacin-Loaded PLGA Nanosuspensions for Ophthalmic Application. Sci. Pharm. 2013;81:591–606. doi: 10.3797/scipharm.1204-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tundisi L.L., Mostaço G.B., Carricondo P.C., Petri D.F.S. Hydroxypropyl Methylcellulose: Physicochemical Properties and Ocular Drug Delivery Formulations. Eur. J. Pharm. Sci. 2021;159:105736. doi: 10.1016/j.ejps.2021.105736. [DOI] [PubMed] [Google Scholar]
- 13.Park C.G., Kim Y.K., Kim S.N., Lee S.H., Huh B.K., Park M.A., Won H., Park K.H., Choy Y.B. Enhanced Ocular Efficacy of Topically-Delivered Dorzolamide with Nanostructured Mucoadhesive Microparticles. Int. J. Pharm. 2017;522:66–73. doi: 10.1016/j.ijpharm.2017.02.035. [DOI] [PubMed] [Google Scholar]
- 14.Rahić O., Tucak A., Omerović N., Sirbubalo M., Hindija L., Hadžiabdić J., Vranić E. Novel Drug Delivery Systems Fighting Glaucoma: Formulation Obstacles and Solutions. Pharmaceutics. 2021;13:28. doi: 10.3390/pharmaceutics13010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie J., Wang C., Ning Q., Gao Q., Gao C., Gou Z., Ye J. A New Strategy to Sustained Release of Ocular Drugs by One-Step Drug-Loaded Microcapsule Manufacturing in Hydrogel Punctal Plugs. Graefes Arch. Clin. Exp. Ophthalmol. 2017;255:2173–2184. doi: 10.1007/s00417-017-3755-1. [DOI] [PubMed] [Google Scholar]
- 16.Asim M.H., Ijaz M., Mahmood A., Knoll P., Jalil A., Arshad S., Bernkop-Schnürch A. Thiolated Cyclodextrins: Mucoadhesive and Permeation Enhancing Excipients for Ocular Drug Delivery. Int. J. Pharm. 2021;599:120451. doi: 10.1016/j.ijpharm.2021.120451. [DOI] [PubMed] [Google Scholar]
- 17.Khames A., Khaleel M.A., El-Badawy M.F., El-Nezhawy A.O.H. Natamycin Solid Lipid Nanoparticles-Sustained Ocular Delivery System of Higher Corneal Penetration against Deep Fungal Keratitis: Preparation and Optimization. Int. J. Nanomed. 2019;14:2515–2531. doi: 10.2147/IJN.S190502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barar J., Aghanejad A., Fathi M., Omidi Y. Advanced Drug Delivery and Targeting Technologies for the Ocular Diseases. BioImpacts. 2016;6:49–67. doi: 10.15171/bi.2016.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taghe S., Mirzaeei S. Preparation and Characterization of Novel, Mucoadhesive of Loxacin Nanoparticles for Ocular Drug Delivery. Brazilian J. Pharm. Sci. 2019;55:1–12. doi: 10.1590/s2175-97902019000117105. [DOI] [Google Scholar]
- 20.Omerovi N., Vrani E. Application of Nanoparticles in Ocular Drug Delivery Systems. Health Technol. 2020;10:61–78. doi: 10.1007/s12553-019-00381-w. [DOI] [Google Scholar]
- 21.Chetoni P., Burgalassi S., Monti D., Tampucci S., Tullio V., Cuffini A.M., Muntoni E., Spagnolo R., Zara G.P., Cavalli R. Solid Lipid Nanoparticles as Promising Tool for Intraocular Tobramycin Delivery: Pharmacokinetic Studies on Rabbits. Eur. J. Pharm. Biopharm. 2016;109:214–223. doi: 10.1016/j.ejpb.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Kaul S., Kumar G., Kothiyal P. An Insight into Ocular Insert. Int. J. Pharm. Sci. Res. 2012;3:1905–1912. [Google Scholar]
- 23.Rozi M.F., Mohmad Sabere A.S. Review on Conventional and Novel Topical Ocular Drug Delivery System. J. Pharm. 2021;1:19–26. doi: 10.31436/jop.v1i1.32. [DOI] [Google Scholar]
- 24.Dabral K., Uniyal Y. Ocular Inserts: Novel Approach for Drug Delivery into Eyes. GSC Biol. Pharm. Sci. 2019;7:001–007. doi: 10.30574/gscbps.2019.7.3.0087. [DOI] [Google Scholar]
- 25.Singla J., Bajaj T., Goyal A.K., Rath G. Development of Nanofibrous Ocular Insert for Retinal Delivery of Fluocinolone Acetonide. Curr. Eye Res. 2019;44:541–550. doi: 10.1080/02713683.2018.1563196. [DOI] [PubMed] [Google Scholar]
- 26.Sofi H.S., Abdal-hay A., Ivanovski S., Zhang Y.S., Sheikh F.A. Electrospun Nanofibers for the Delivery of Active Drugs through Nasal, Oral and Vaginal Mucosa: Current Status and Future Perspectives. Mater. Sci. Eng. C. 2020;111:110756. doi: 10.1016/j.msec.2020.110756. [DOI] [PubMed] [Google Scholar]
- 27.Krstić M., Radojević M., Stojanović D., Radojević V., Uskoković P., Ibrić S. Formulation and Characterization of Nanofibers and Films with Carvedilol Prepared by Electrospinning and Solution Casting Method. Eur. J. Pharm. Sci. 2017;101:160–166. doi: 10.1016/j.ejps.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Luraghi A., Peri F., Moroni L. Electrospinning for Drug Delivery Applications: A Review. J. Control Release. 2021;334:463–484. doi: 10.1016/j.jconrel.2021.03.033. [DOI] [PubMed] [Google Scholar]
- 29.Fadil F., Affandi N.D.N., Misnon M.I., Bonnia N.N., Harun A.M., Alam M.K. Review on Electrospun Nanofiber-Applied Products. Polymers. 2021;13:2087. doi: 10.3390/polym13132087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vass P., Szabó E., Domokos A., Hirsch E., Galata D., Farkas B., Démuth B., Andersen S.K., Vigh T., Verreck G., et al. Scale-up of Electrospinning Technology: Applications in the Pharmaceutical Industry. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020;12:1–24. doi: 10.1002/wnan.1611. [DOI] [PubMed] [Google Scholar]
- 31.Arthanari S., Mani G., Jang J.H., Choi J.O., Cho Y.H., Lee J.H., Cha S.E., Oh H.S., Kwon D.H., Jang H.T. Preparation and Characterization of Gatifloxacin-Loaded Alginate/Poly (Vinyl Alcohol) Electrospun Nanofibers. Artif. Cells Nanomed. Biotechnol. 2016;44:847–852. doi: 10.3109/21691401.2014.986676. [DOI] [PubMed] [Google Scholar]
- 32.Dadashi S., Boddohi S., Soleimani N. Preparation, Characterization, and Antibacterial Effect of Doxycycline Loaded Kefiran Nanofibers. J. Drug Deliv. Sci. Technol. 2019;52:979–985. doi: 10.1016/j.jddst.2019.06.012. [DOI] [Google Scholar]
- 33.Polat H.K., Bozdağ Pehlivan S., Özkul C., Çalamak S., Öztürk N., Aytekin E., Fırat A., Ulubayram K., Kocabeyoğlu S., İrkeç M., et al. Development of Besifloxacin HCl Loaded Nanofibrous Ocular Inserts for the Treatment of Bacterial Keratitis: In Vitro, Ex Vivo and In Vivo Evaluation. Int. J. Pharm. 2020;585:119552. doi: 10.1016/j.ijpharm.2020.119552. [DOI] [PubMed] [Google Scholar]
- 34.Di Prima G., Licciardi M., Carfì Pavia F., Lo Monte A.I., Cavallaro G., Giammona G. Microfibrillar Polymeric Ocular Inserts for Triamcinolone Acetonide Delivery. Int. J. Pharm. 2019;567:118459. doi: 10.1016/j.ijpharm.2019.118459. [DOI] [PubMed] [Google Scholar]
- 35.Chou S.F., Luo L.J., Lai J.Y., Ma D.H.K. Role of Solvent-Mediated Carbodiimide Cross-Linking in Fabrication of Electrospun Gelatin Nanofibrous Membranes as Ophthalmic Biomaterials. Mater. Sci. Eng. C. 2017;71:1145–1155. doi: 10.1016/j.msec.2016.11.105. [DOI] [PubMed] [Google Scholar]
- 36.Tawfik E.A., Craig D.Q.M., Barker S.A. Dual Drug-Loaded Coaxial Nanofibers for the Treatment of Corneal Abrasion. Int. J. Pharm. 2020;581:119296. doi: 10.1016/j.ijpharm.2020.119296. [DOI] [PubMed] [Google Scholar]
- 37.Imperiale J.C., Acosta G.B., Sosnik A. Polymer-Based Carriers for Ophthalmic Drug Delivery. J. Control Release. 2018;285:106–141. doi: 10.1016/j.jconrel.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 38.Mirzaeei S., Berenjian K., Khazaei R. Preparation of the Potential Ocular Inserts by Electrospinning Method to Achieve the Prolong Release Profile of Triamcinolone Acetonide. Adv. Pharm. Bull. 2018;8:21–27. doi: 10.15171/apb.2018.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashri L.Y., Abou El Ela A.E.S.F., Ibrahim M.A., Alshora D.H., Naguib M.J. Optimization and Evaluation of Chitosan Buccal Films Containing Tenoxicam for Treating Chronic Periodontitis: In Vitro and In Vivo Studies. J. Drug Deliv. Sci. Technol. 2020;57:101720. doi: 10.1016/j.jddst.2020.101720. [DOI] [Google Scholar]
- 40.Wani T.U., Pandith A.H., Sheikh F.A. Polyelectrolytic Nature of Chitosan: Influence on Physicochemical Properties and Synthesis of Nanoparticles. J. Drug Deliv. Sci. Technol. 2021;65:102730. doi: 10.1016/j.jddst.2021.102730. [DOI] [Google Scholar]
- 41.Almeida H., Amaral M.H., Lobão P., Lobo J.M.S. In Situ Gelling Systems: A Strategy to Improve the Bioavailability of Ophthalmic Pharmaceutical Formulations. Drug Discov. Today. 2014;19:400–412. doi: 10.1016/j.drudis.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Mehta P., Al-Kinani A.A., Arshad M.S., Singh N., van der Merwe S.M., Chang M.W., Alany R.G., Ahmad Z. Engineering and Development of Chitosan-Based Nanocoatings for Ocular Contact Lenses. J. Pharm. Sci. 2019;108:1540–1551. doi: 10.1016/j.xphs.2018.11.036. [DOI] [PubMed] [Google Scholar]
- 43.Mamidi N., Delgadillo R.M.V. Design, Fabrication and Drug Release Potential of Dual Stimuli-Responsive Composite Hydrogel Nanoparticle Interfaces. Colloids Surf. B Biointerfaces. 2021;204:111819. doi: 10.1016/j.colsurfb.2021.111819. [DOI] [PubMed] [Google Scholar]
- 44.Mamidi N., Villela Castrejón J., González-Ortiz A. Rational Design and Engineering of Carbon Nano-Onions Reinforced Natural Protein Nanocomposite Hydrogels for Biomedical Applications. J. Mech. Behav. Biomed. Mater. 2020;104:103696. doi: 10.1016/j.jmbbm.2020.103696. [DOI] [PubMed] [Google Scholar]
- 45.Mamidi N., Velasco Delgadillo R.M., Barrera E.V. Covalently Functionalized Carbon Nano-Onions Integrated Gelatin Methacryloyl Nanocomposite Hydrogel Containing γ-Cyclodextrin as Drug Carrier for High-Performance Ph-Triggered Drug Release. Pharmaceuticals. 2021;14:291. doi: 10.3390/ph14040291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmady A., Abu Samah N.H. A Review: Gelatine as a Bioadhesive Material for Medical and Pharmaceutical Applications. Int. J. Pharm. 2021;608:121037. doi: 10.1016/j.ijpharm.2021.121037. [DOI] [PubMed] [Google Scholar]
- 47.Mamidi N., Zuníga A.E., Villela-Castrejón J. Engineering and Evaluation of Forcespun Functionalized Carbon Nano-Onions Reinforced Poly (ε-Caprolactone) Composite Nanofibers for PH-Responsive Drug Release. Mater. Sci. Eng. C. 2020;112:110928. doi: 10.1016/j.msec.2020.110928. [DOI] [PubMed] [Google Scholar]
- 48.Mahmoud Salehi A.O., Heidari Keshel S., Sefat F., Tayebi L. Use of Polycaprolactone in Corneal Tissue Engineering: A Review. Mater. Today Commun. 2021;27:102402. doi: 10.1016/j.mtcomm.2021.102402. [DOI] [Google Scholar]
- 49.Mamidi N., Delgadillo R.M.V., González-Ortiz A. Engineering of Carbon Nano-Onion Bioconjugates for Biomedical Applications. Mater. Sci. Eng. C. 2021;120:111698. doi: 10.1016/j.msec.2020.111698. [DOI] [PubMed] [Google Scholar]
- 50.Mamidi N., González-Ortiz A., Romo I.L., Barrera E.V. Development of Functionalized Carbon Nano-Onions Reinforced Zein Protein Hydrogel Interfaces for Controlled Drug Release. Pharmaceutics. 2019;11:621. doi: 10.3390/pharmaceutics11120621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tawfik E.A., Alshamsan A., Abul Kalam M., Raish M., Alkholief M., Stapleton P., Harvey K., Craig D.Q.M., Barker S.A. In Vitro and In Vivo Biological Assessment of Dual Drug-Loaded Coaxial Nanofibers for the Treatment of Corneal Abrasion. Int. J. Pharm. 2021;604:120732. doi: 10.1016/j.ijpharm.2021.120732. [DOI] [PubMed] [Google Scholar]
- 52.Bhattarai R.S., Das A., Alzhrani R.M., Kang D., Bhaduri S.B., Boddu S.H.S. Comparison of Electrospun and Solvent Cast Polylactic Acid (PLA)/Poly(Vinyl Alcohol) (PVA) Inserts as Potential Ocular Drug Delivery Vehicles. Mater. Sci. Eng. C. 2017;77:895–903. doi: 10.1016/j.msec.2017.03.305. [DOI] [PubMed] [Google Scholar]
- 53.Mirzaeei S., Barfar D. Design and Development of Antibacterial/Anti-Inflammatory Dual Drug-Loaded Nanofibrous Inserts for Ophthalmic Sustained Delivery of Gentamicin and Methylprednisolone: In Vitro Bioassay, Solvent, and Method Effects’ Evaluation. Adv. Pharm. Bull. 2021 doi: 10.34172/apb.2022.056. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grimaudo M.A., Concheiro A., Alvarez-Lorenzo C. Crosslinked Hyaluronan Electrospun Nanofibers for Ferulic Acid Ocular Delivery. Pharmaceutics. 2020;12:274. doi: 10.3390/pharmaceutics12030274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taghe S.H., Mehrandish S., Mirzaeei S. Preparation of Azithromycin Nanofibers as Controlled Release Ophthalmic Drug Carriers Using Electrospinning Technique: In- Vitro and In- Vivo Characterization. Adv. Pharm. Bull. 2021 doi: 10.34172/apb.2022.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khalil I.A., Ali I.H., El-sherbiny I.M. Noninvasive Biodegradable Nanoparticles-in-Nanofibers Single-Dose Ocular Insert: In Vitro, Ex Vivo and In Vivo Evaluation. Nanomedicine. 2018;14:33–55. doi: 10.2217/nnm-2018-0297. [DOI] [PubMed] [Google Scholar]
- 57.Gagandeep, Garg T., Malik B., Rath G., Goyal A.K. Development and Characterization of Nano-Fiber Patch for the Treatment of Glaucoma. Eur. J. Pharm. Sci. 2014;53:10–16. doi: 10.1016/j.ejps.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 58.Mirzaeei S., Taghe S., Asare-Addo K., Nokhodchi A. Polyvinyl Alcohol/Chitosan Single-Layered and Polyvinyl Alcohol/Chitosan/Eudragit RL100 Multi-Layered Electrospun Nanofibers as an Ocular Matrix for the Controlled Release of Ofloxacin: An In Vitro and In Vivo Evaluation. AAPS PharmSciTech. 2021;22:1–13. doi: 10.1208/s12249-021-02051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taghe S., Mirzaeei S., Alany R.G., Nokhodchi A. Polymeric Inserts Containing Eudragit® L100 Nanoparticle for Improved Ocular Delivery of Azithromycin. Biomedicines. 2020;8:466. doi: 10.3390/biomedicines8110466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shah S.N.H., Nawaz A., Javed H., Rafiq M., Riaz R., Sadaquat H., Akhtar M. Preparation and In Vitro/In Vivo Evaluation of Antihistaminic Ocular Inserts. Pharm. Chem. J. 2018;52:615–622. doi: 10.1007/s11094-018-1870-x. [DOI] [Google Scholar]
- 61.Di Colo G., Zambito Y. A Study of Release Mechanisms of Different Ophthalmic Drugs from Erodible Ocular Inserts Based on Poly(Ethylene Oxide) Eur. J. Pharm. Biopharm. 2002;54:193–199. doi: 10.1016/S0939-6411(02)00086-3. [DOI] [PubMed] [Google Scholar]
- 62.Patil S.S., Bade A., Tagalpallewar A. Design, Optimization and Pharmacodynamic Comparison of Dorzolamide Hydrochloride Soluble Ocular Drug Insert Prepared by Using 32 Factorial Design. J. Drug Deliv. Sci. Technol. 2018;46:138–147. doi: 10.1016/j.jddst.2018.05.010. [DOI] [Google Scholar]
- 63.Tighsazzadeh M., Mitchell J.C., Boateng J.S. Development and Evaluation of Performance Characteristics of Timolol-Loaded Composite Ocular Films as Potential Delivery Platforms for Treatment of Glaucoma. Int. J. Pharm. 2019;566:111–125. doi: 10.1016/j.ijpharm.2019.05.059. [DOI] [PubMed] [Google Scholar]
- 64.Thakkar R., Komanduri N., Dudhipala N., Tripathi S., Repka M.A., Majumdar S. Development and Optimization of Hot-Melt Extruded Moxifloxacin Hydrochloride Inserts, for Ocular Applications, Using the Design of Experiments. Int. J. Pharm. 2021;603:120676. doi: 10.1016/j.ijpharm.2021.120676. [DOI] [PubMed] [Google Scholar]
- 65.Aher N.D., Nair H.A. Bilayered Films Based on Novel Polymer Derivative for Improved Ocular Therapy of Gatifloxacin. Sci. World J. 2014;2014 doi: 10.1155/2014/297603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abdelkader H., Wertheim D., Pierscionek B., Alany R.G. Curcumin in Situ Gelling Polymeric Insert with Enhanced Ocular Performance. Pharmaceutics. 2020;12:1158. doi: 10.3390/pharmaceutics12121158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Everaert A., Broeckx G., Fransen E., Ludwig A., Kiekens F., Weyenberg W. Evaluation of a Newly Developed HPMC Ophthalmic Insert with Sustained Release Properties as a Carrier for Thermolabile Therapeutics. Int. J. Pharm. 2015;481:37–46. doi: 10.1016/j.ijpharm.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 68.Gurtler F., Kaltsatos V., Boisramé B., Gurny R. Long-Acting Soluble Bioadhesive Ophthalmic Drug Insert (BODI) Containing Gentamicin for Veterinary Use: Optimization and Clinical Investigation. J. Control Release. 1995;33:231–236. doi: 10.1016/0168-3659(94)00096-D. [DOI] [Google Scholar]
- 69.Balguri S.P., Adelli G.R., Tatke A., Janga K.Y., Bhagav P., Majumdar S. Melt-Cast Noninvasive Ocular Inserts for Posterior Segment Drug Delivery. J. Pharm. Sci. 2017;106:3515–3523. doi: 10.1016/j.xphs.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shadambikar G., Marathe S., Patil A., Joshi R., Bandari S., Majumdar S., Repka M. Novel Application of Hot Melt Extrusion Technology for Preparation and Evaluation of Valacyclovir Hydrochloride Ocular Inserts. AAPS PharmSciTech. 2021;22:1–7. doi: 10.1208/s12249-020-01916-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Everaert A., Wouters Y., Melsbach E., Zakaria N., Ludwig A., Kiekens F., Weyenberg W. Optimisation of HPMC Ophthalmic Inserts with Sustained Release Properties as a Carrier for Thermolabile Therapeutics. Int. J. Pharm. 2017;528:395–405. doi: 10.1016/j.ijpharm.2017.06.047. [DOI] [PubMed] [Google Scholar]
- 72.Baeyens V., Kaltsatos V., Boisramé B., Varesio E., Veuthey J.L., Fathi M., Balant L.P., Gex-Fabry M., Gurny R. Optimized Release of Dexamethasone and Gentamicin from a Soluble Ocular Insert for the Treatment of External Ophthalmic Infections. J. Control Release. 1998;52:215–220. doi: 10.1016/S0168-3659(97)00212-5. [DOI] [PubMed] [Google Scholar]
- 73.Bhagav P., Trivedi V., Shah D., Chandran S. Sustained Release Ocular Inserts of Brimonidine Tartrate for Better Treatment in Open-Angle Glaucoma. Drug Deliv. Transl. Res. 2011;1:161–174. doi: 10.1007/s13346-011-0018-2. [DOI] [PubMed] [Google Scholar]
- 74.Sadeghi A.M., Farjadian F., Alipour S. Sustained Release of Linezolid in Ocular Insert Based on Lipophilic Modified Structure of Sodium Alginate. Iran. J. Basic Med. Sci. 2021;24:331–340. doi: 10.22038/ijbms.2021.49866.11385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barman S.P., Liu M., Barman K., Ward K.L., Hackett B. Methods and Biocompatible Compositions to Achieve Sustained Drug Release in the Eye. Application No. AU 2019250153 A1. Patent. 2019 October 16;
- 76.El-Sherbiny I.M., Ali I.H., Khalil I.A. Ocular Drug Delivery System. Application No. GB 2570113 A. Patent. 2019 July 17;
- 77.Johnson LIM, Edison, N. (US). Cylndrical Ocular Inserts. Application No. US2012215184A1. Patent. 2012 August 23;
- 78.Reich C., Schuler C., Ye Q., Stark L., Jain R. Bimatoprost Ocular Silicone Inserts and Methods of Use Thereof. Application No. US2016022695A1. Patent. 2016 January 28;
- 79.Hongzhen F. Method for Preparing Rifampicin Ocular Inserts. Application No. CN104116722A. Patent. 2014 October 29;
- 80.Parrag I.C., Statham M.A.J., Battiston K., Naimark W.A., Fischer H.C. Ocular Inserts Comprising A Covalently Linked Steroid Dimer. Application No. WO2019148291A1. Patent. 2019 August 8;
- 81.Ge J., Zhang S.Y., Wu D., Cheng J. Wet-Packed Soft Hydrogel Ocular Inserts. Application No. US2021077385A1. Patent. 2021 March 25;
- 82.Jingxin G., Haibing H., Muse J., Xing T., Tian Y., Yu Z. Atropine Sulfate Ocular Inserts and Preparation Method Thereof. Application No. CN111358771A. Patent. 2020 July 7;
- 83.Choonara Y.E., Du Toit L.C., Kumar P., Pillay V., Moosa R.M., Jhetam R. Fast Dissolving Ocular Insert. Application No. WO2014041485A1. Patent. 2014 March 20;
- 84.Stark L., Jain R., Srinivasan R., Reich C.J., Schuler C. Ocular Insert Composition of a Sem-Crystalline or Crystalline Pharmaceutically Active Agent. Application No. US2016296532A1. Patent. 2016 October 20;
- 85.Mota Leite Machado Mariz M.J., Nunes Ferreira Calvinho P.C., Mendes Gil M.H., Neto Murta J.C. Non-Invasive Ocular Drug Delivery Insert Technology. Application No. WO2017137934A1. Patent. 2017 August 17;
- 86.Xiaoyi S., Lingyan Y., Zhenwei Y. Ocular Insert Containing Voriconazole. Application No. CN105726517A. Patent. 2016 July 6;
- 87.Allen K.H., Rekha R. Dissolvable Polymeric Eye Inserts with A Biodegradable Polymer. Application No. WO2021116907A1. Patent. 2021 June 17;
- 88.Ketelson H.A., Rangarajan R., Laredo W.R., John C.S. Dissolvable Polymeric Eye Inserts and Method of Using Same. Application No. WO2020222195A1. Patent. 2020 November 5;
- 89.Ketelson H.A., Rangarajan R. Dissolvable Polymeric Eye Inserts with a Biodegradable Polymer and Method of Using Same. Application No. US2021169781A1. Patent. 2021 June 10;
- 90.Fang G., Yang X., Wang Q., Zhang A., Tang B. Hydrogels-Based Ophthalmic Drug Delivery Systems for Treatment of Ocular Diseases. Mater. Sci. Eng. C. 2021;127:112212. doi: 10.1016/j.msec.2021.112212. [DOI] [PubMed] [Google Scholar]
- 91.Brahamdutt M.C., Kumar S., Bhatia M., Budhwar V. Formulation and In-Vitro Evaluation of Sustained Release Tropicamide Loaded Chitosan Nanoparticles for Ocular Drug Delivery. Int. Res. J. Pharm. 2016;7:27–35. doi: 10.7897/2230-8407.0710118. [DOI] [Google Scholar]
- 92.Bachu R.D., Chowdhury P., Al-Saedi Z.H.F., Karla P.K., Boddu S.H.S. Ocular Drug Delivery Barriers—Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics. 2018;10:28. doi: 10.3390/pharmaceutics10010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen H., Jin Y., Sun L., Li X., Nan K., Liu H., Zheng Q., Wang B. Recent Developments in Ophthalmic Drug Delivery Systems for Therapy of Both Anterior and Posterior Segment Diseases. Colloids Interface Sci. Commun. 2018;24:54–61. doi: 10.1016/j.colcom.2018.03.008. [DOI] [Google Scholar]
- 94.El Hoffy N.M., Abdel Azim E.A., Hathout R.M., Fouly M.A., Elkheshen S.A. Glaucoma: Management and Future Perspectives for Nanotechnology-Based Treatment Modalities. Eur. J. Pharm. Sci. 2021;158:105648. doi: 10.1016/j.ejps.2020.105648. [DOI] [PubMed] [Google Scholar]
- 95.Mahaling B., Katti D.S. Physicochemical Properties of Core-Shell Type Nanoparticles Govern Their Spatiotemporal Biodistribution in the Eye. Nanomed. Nanotechnol. Biol. Med. 2016;12:2149–2160. doi: 10.1016/j.nano.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 96.Pascolini D., Mariotti S.P. Global Estimates of Visual Impairment: 2010. Br. J. Ophthalmol. 2012;96:614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 97.Bertens C.J.F., Gijs M., van den Biggelaar F.J.H.M., Nuijts R.M.M.A. Topical Drug Delivery Devices: A Review. Exp. Eye Res. 2018;168:149–160. doi: 10.1016/j.exer.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 98.Terreni E., Burgalassi S., Chetoni P., Tampucci S., Zucchetti E., Fais R., Ghelardi E., Lupetti A., Monti D. Development and Characterization of a Novel Peptide-Loaded Antimicrobial Ocular Insert. Biomolecules. 2020;10:664. doi: 10.3390/biom10050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Da Silva G.R., Lima T.H., Fernandes-Cunha G.M., Oréfice R.L., Da Silva-Cunha A., Zhao M., Behar-Cohen F. Ocular Biocompatibility of Dexamethasone Acetate Loaded Poly(ɛ-Caprolactone) Nanofibers. Eur. J. Pharm. Biopharm. 2019;142:20–30. doi: 10.1016/j.ejpb.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 100.Yan D., Yao Q., Yu F., Chen L., Zhang S., Sun H., Lin J., Fu Y. Surface Modified Electrospun Poly(Lactic Acid) Fibrous Scaffold with Cellulose Nanofibrils and Ag Nanoparticles for Ocular Cell Proliferation and Antimicrobial Application. Mater. Sci. Eng. C. 2020;111:110767. doi: 10.1016/j.msec.2020.110767. [DOI] [PubMed] [Google Scholar]
- 101.Baskakova A., Awwad S., Jiménez J.Q., Gill H., Novikov O., Khaw P.T., Brocchini S., Zhilyakova E., Williams G.R. Electrospun Formulations of Acyclovir, Ciprofloxacin and Cyanocobalamin for Ocular Drug Delivery. Int. J. Pharm. 2016;502:208–218. doi: 10.1016/j.ijpharm.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 102.Wen P., Zong M.H., Linhardt R.J., Feng K., Wu H. Electrospinning: A Novel Nano-Encapsulation Approach for Bioactive Compounds. Trends Food Sci. Technol. 2017;70:56–68. doi: 10.1016/j.tifs.2017.10.009. [DOI] [Google Scholar]
- 103.Omer S., Forgách L., Zelkó R., Sebe I. Scale-up of Electrospinning: Market Overview of Products and Devices for Pharmaceutical and Biomedical Purposes. Pharmaceutics. 2021;13:286. doi: 10.3390/pharmaceutics13020286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.