Abstract
Voriconazole is a new triazole antifungal agent that has potent activity against many isolates of Candida, including Candida krusei and Candida glabrata. In this work, we studied the impact of glucose supplementation, incubation time, agitation of the plates prior to reading, endpoint determination rule, visual versus spectrophotometric reading, Candida species, and fluconazole MIC on the MIC of voriconazole for Candida isolates tested by using the microdilution format assay of the National Committee for Clinical Laboratory Standards (NCCLS) M27-A antifungal susceptibility testing methodology. For both voriconazole and fluconazole, a spectrophotometric endpoint of 50% reduction in turbidity relative to the growth control correlated most closely with the NCCLS-defined visual endpoint of “prominent decrease in turbidity.” Correlation was generally better after 24 h of incubation than after 48 h. Supplementation of the medium to contain 20 g of glucose/liter did not alter the MIC significantly but did enhance growth and simplify visual readings. All Candida species appeared potentially susceptible to voriconazole, including isolates of C. krusei. For some isolates for which fluconazole MICs were markedly elevated voriconazole MICs were also elevated, but the clinical significance of these observations remains to be determined.
Voriconazole is a new triazole antifungal agent developed for the treatment of mycotic infections. The drug has been shown to have potent activity in vitro (3, 5, 14, 18, 22, 28, 34) and in vivo (11, 13, 17, 19) against a variety of organisms, including both molds and yeasts. In addition to its excellent antifungal activity, it has also been shown to inhibit the interaction of Candida krusei with endothelial cells (8).
As new agents of this type are introduced, it is important that laboratory methods for estimating the likelihood of in vivo response be developed. Susceptibility testing of fungi has been extensively examined recently, and the importance of factors such as endpoint definition, buffer concentration, inoculum size and preparation, incubation time and temperature, and test media is now well known (1, 6, 9, 31). A new level of reproducibility has been achieved with the recent publication by the National Committee for Clinical Laboratory Standards (NCCLS) of document M27-A (20). This document describes a reproducible macro- and microdilution methodology for the testing of Candida spp. and Cryptococcus neoformans against amphotericin B, flucytosine, ketoconazole, itraconazole, and fluconazole. However, use of this methodology is not problem-free: current issues include proper interpretation of the trailing growth of azoles (30, 32), detection of resistance to amphotericin B (29), and poor growth of C. neoformans with the recommended RPMI 1640 medium (12). In addition, there is no certainty that new agents will not require modified methods for optimal results. Thus, we have explored in this study the impact of endpoint selection, glucose supplementation, time of incubation, and the mechanism of reading on the MIC of voriconazole when testing is done in the microdilution format.
(This work was presented in part at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, 1997 [16a].)
MATERIALS AND METHODS
Isolates.
A total of 209 pathogenic bloodstream and oral Candida isolates were tested and included C. albicans (125 isolates), C. tropicalis (26 isolates), C. parapsilosis (25 isolates), C. glabrata (20 isolates), C. lusitaniae (9 isolates), C. krusei (3 isolates), and C. lipolytica (1 isolate). All organisms were identified to the species level by use of standard biochemical testing with the API 20C system (BioMérieux Vitek, Hazelwood, Mo.). Included with these organisms was a collection of putatively amphotericin B-resistant and -susceptible isolates (29, 35). All the isolates were kept at −70°C and were passaged at least twice on Sabouraud dextrose agar at 35°C prior to being tested.
Test media.
RPMI 1640, RPMI 1640 supplemented with 2% glucose (RPMI 1640–2%), and antibiotic medium 3 were used as test media. RPMI 1640 was obtained from Sigma (lot 85H46331; Sigma Chemical Co., St. Louis, Mo.), and antibiotic medium 3 was obtained from BBL (lot JD4ZSG; Becton Dickinson Microbiology Systems, Cockeysville, Md.). Preparation of RPMI 1640 followed NCCLS M27-A guidelines (20). RPMI 1640–2% was prepared by supplementing RPMI 1640 with 18 g of d-glucose/liter as described by Rodriguez-Tudela and Martinez-Suarez (32). As RPMI 1640 already contains 2 g of glucose/liter, the resulting medium contains 20 g/liter as its final glucose concentration. Both plain RPMI 1640 and RPMI 1640–2% were buffered to pH 7.0 with 0.165 mol of 3-(N-morpholino) propanesulfonic acid (lot 75H5734; Sigma Chemical Co.) per liter. Antibiotic medium 3 was supplemented with glucose to achieve a final glucose concentration of 2% (20 g/liter). The buffering capacity of the medium was further increased by adding 0.0044 mol of dipotassium monophosphate/liter and 0.0073 mol of monopotassium monophosphate/liter, and the pH was adjusted to 7.0 by using NaOH. All three media were filter sterilized by passage through a 0.22-μm-pore-size filter system (Corning Inc., Corning, N.Y.).
Susceptibility testing and determination of MICs.
Activities of voriconazole (Pfizer Pharmaceuticals, Inc., New York, N.Y.), fluconazole (Pfizer), and amphotericin B (Bristol-Myers Squibb, Wallingford, Conn.) against Candida spp. were studied. Voriconazole and amphotericin B were dissolved in 100% dimethyl sulfoxide, and fluconazole was dissolved in sterile distilled water. Following the M27-A guidelines, dilution series were prepared in the same solvent.
The tested drug concentration ranges were 0.125 to 64 μg/ml for fluconazole and 0.0078 to 4 μg/ml for voriconazole and amphotericin B. All susceptibility tests were performed by using the microdilution version of the NCCLS M27-A method (20). Dilution plates were arranged in order to contain 100 μl of the desired culture medium plus the study drug, both at a concentration double the desired final concentration. Testing was carried out by adding 100 μl of the test organism adjusted to a concentration double the desired final concentration, yielding the final concentrations of both drug and organism (5.0 × 102 to 2.5 × 103 cells per ml). Since dimethyl sulfoxide was used to dissolve voriconazole and amphotericin B, the result was a final concentration of the solvent of 1% in all test wells. All determinations were done in duplicate.
Readings were performed visually and spectrophotometrically after 24 and 48 h of incubation at 35°C. With the aid of a reading mirror, visual readings for all three drugs were made before and after agitation of the plates (1). Visual endpoints were determined as described in the M27-A document (20) and were recorded as a visual reading of MIC-0 (wells optically clear or showing complete inhibition of growth), MIC-1 (slightly hazy turbidity), or MIC-2 (prominent decrease in turbidity). The NCCLS-recommended endpoint for azoles is the lowest drug concentration at which a score of 2 is observed, while the MIC for amphotericin B is the lowest drug concentration at which a score of 0 is observed (20). Labels of BA (before agitation) and AA (after agitation) were used. An example of the combined notation would be BA-2, which would correspond to a visual reading of MIC-2 before shaking.
Spectrophotometric readings for all three drugs were performed after plate agitation with the aid of a plate reader (model EL-310; Bio-Tek, Burlington, Vt.) by measuring optical density (OD) at 530 nm. No readings without agitation were made with the spectrophotometer. The percentage of control growth was computed as follows: [sample OD/(sample OD − materials blank OD)] × 100. Three spectrophotometric endpoints were defined as Spec-95, Spec-80, and Spec-50, corresponding to the lowest drug concentration reducing control growth by 95, 80, and 50%, respectively. Reduction in growth by 95% was found to correspond to optically clear wells, and a mathematically determined endpoint of 95% reduction rather than 100% was used to compensate for minor reading variations and plate imperfections. Finally, in order to assess the influence of glucose supplementation on the MIC of voriconazole, we tested 67 isolates in RPMI 1640 without glucose and compared the results to those obtained with glucose.
RESULTS
Influence of glucose on voriconazole MIC.
To determine the effect of glucose concentration on voriconazole MIC, 67 Candida isolates (35 C. albicans, 9 C. glabrata, 4 C. lusitaniae, 12 C. parapsilosis, and 7 C. tropicalis isolates) were tested in parallel in RPMI 1640 and RPMI 1640–2%. Supplementation of the culture medium to a final concentration of 2% glucose has been shown to simplify endpoint determination without affecting fluconazole MIC (32). After 24 h of incubation, 99 and 94% (using Spec-50 and Spec-80 as endpoints, respectively) of the MICs obtained in RPMI 1640 without glucose were within 1 dilution of those obtained in the presence of glucose. After 48 h of incubation, the corresponding percentages were 95 and 93%. Likewise, when the readings were performed visually, the percentages at 24 h were 97% (AA-2) and 90% (AA-1). After 48 h, the percentages fell to 87 and 77%, respectively. Isolates had better overall growth in RPMI 1640–2% than in RPMI 1640, with a median increase in OD at 530 nm of the growth control wells of approximately twofold. This seemed to contribute to an increase in the consistency of the visual readings but was not a factor for the mechanically generated spectrophotometric readings. For example, the AA-2 and Spec-50 readings were within 1 dilution for 99 and 95% of the readings done with glucose at 24 and 48 h, respectively. Without glucose, these MICs were within 1 dilution for 97% of the readings at 24 h but only for 77% of the readings done at 48 h. Finally, some isolates grew so poorly without glucose that an MIC could not be determined. However, the MIC was readily apparent when the isolate was cultured in RPMI 1640–2%. Based on these observations, RPMI 1640–2% was used for all subsequent testing.
Correlation between visual and spectrophotometric readings of voriconazole and fluconazole MICs.
The restrictive MIC-0, AA-0, and BA-0 endpoints were found to produce many off-scale values for voriconazole and fluconazole (data not shown). Results for the less restrictive endpoints are shown in Table 1. BA and AA readings tended to be similar, especially for MIC-2 readings. When visual and spectrophotometric readings were compared for both voriconazole and fluconazole, AA-1 most closely matched Spec-80 and AA-2 most closely matched Spec-50 at both 24 and 48 h. The most consistent agreement between visual and spectrophotometric readings was found between AA-2 and Spec-50, where there was 99% agreement for both drugs at 24 h and ≥91% agreement at 48 h.
TABLE 1.
Percents agreement between endpoints for voriconazole and fluconazolea
| Endpoints compared | % Agreement for the following drug at the indicated time
|
|||
|---|---|---|---|---|
| Voriconazole
|
Fluconazole
|
|||
| 24 h | 48 h | 24 h | 48 h | |
| BA-1 vs AA-1 | 90 | 94 | 83 | 95 |
| BA-2 vs AA-2 | 97 | 88 | 94 | 96 |
| AA-1 vs Spec-50 | 91 | 86 | 92 | 85 |
| AA-1 vs Spec-80 | 96 | 94 | 95 | 97 |
| AA-2 vs Spec-50 | 99 | 95 | 99 | 91 |
| AA-2 vs Spec-80 | 96 | 90 | 97 | 87 |
Testing was performed in RPMI 1640–2%, and the paired readings on the 209 isolates were said to be in agreement if they were within 1 dilution of each other. The microtiter plates were visually read before and after agitation. Spectrophotometric readings were made after agitation of the plates. Spec-80 and Spec-50 corresponded to the lowest drug concentrations reducing control growth by 80 and 50%, respectively.
In vitro activities of voriconazole and fluconazole.
Table 2 shows the activities of voriconazole and fluconazole after 24 and 48 h of incubation. The results are generally similar to those seen in recent surveys with these drugs (3, 14, 23, 26, 34). Comparison of Spec-50 and Spec-80 results further highlights the impact of endpoint choice for spectrophotometric MICs. Off-scale readings were common with both drugs at the Spec-80 endpoint, especially when the incubation was continued to 48 h. The Spec-50 MICs reduced the discrepancy between the two reading times for both drugs and produced modal MICs that did not change or that rose by the single twofold dilution that had generally characterized the difference between 24- and 48-h readings in other studies (30, 31). Thus, the Spec-50 MIC appears to minimize the influence of trailing and to produce increasingly consistent MICs.
TABLE 2.
Activities of voriconazole and fluconazole by Candida species
| Time and species (no. of isolates) | MIC (μg/ml) of the following drug at the indicated endpointa
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Voriconazole
|
Fluconazole
|
|||||||
| Spec-50
|
Spec-80
|
Spec-50
|
Spec-80
|
|||||
| Modal | Range | Modal | Range | Modal | Range | Modal | Range | |
| 24 h | ||||||||
| C. albicans (125) | 0.008 | 0.008–0.5 | 0.008 | 0.008–1 | 0.125 | 0.125–32 | 0.25 | 0.125–64 |
| C. glabrata (20) | 0.0625 | 0.008–4 | 0.125 | 0.031–4 | 8 | 0.125–>64 | 8 | 0.125–>64 |
| C. krusei (3) | 0.008 | 0.008–0.125 | 0.008 | 0.008–0.25 | 16 | 8.0–16.0 | 16 | 16 |
| C. lipolytica (1) | 0.125 | 0.125 | 8 | 16 | ||||
| C. lusitaniae (9) | 0.008 | 0.008 | 0.008 | 0.008–0.016 | 0.5 | 0.125–1 | 0.5 | 0.125–2 |
| C. parapsilosis (25) | 0.008 | 0.008–0.03 | 0.008 | 0.008–0.016 | 0.5 | 0.125–2 | 0.5 | 0.125–2 |
| C. tropicalis (26) | 0.008 | 0.008–0.06 | 0.008 | 0.008–>4 | 0.25 | 0.125–2 | 0.25 | 0.125–>64 |
| 48 h | ||||||||
| C. albicans (125) | 0.008 | 0.008–1 | 0.008 | 0.008–>4 | 0.25 | 0.125–64 | 0.25 | 0.125–>64 |
| C. glabrata (20) | 0.25 | 0.008–4 | 0.5 | 0.063–>4 | 16 | 0.125–>64 | 32 | 8–>64 |
| C. krusei (3) | 0.016 | 0.016–0.25 | 0.25 | 0.25–>4 | 32 | 32 | 64 | 32–64 |
| C. lipolytica (1) | 0.25 | 0.25 | 32 | 32 | ||||
| C. lusitaniae (9) | 0.008 | 0.008–0.03 | 0.008 | 0.008–0.06 | 0.5 | 0.125–4 | 0.5 | 0.25–4 |
| C. parapsilosis (25) | 0.008 | 0.008–0.03 | 0.0625 | 0.008–>4 | 0.5 | 0.125–4 | 1 | 0.25–64 |
| C. tropicalis (26) | 0.016 | 0.008–4 | >4 | 0.016–>4 | 0.5 | 0.125–2 | 0.5 | 0.125–>64 |
Activities of voriconazole and fluconazole against 209 isolates in RPMI 1640–2%. The analysis determined two endpoints, Spec-50 and Spec-80, representing 50 and 80% inhibition, respectively, as measured spectrophotometrically. Modal, modal MIC; range, MIC range.
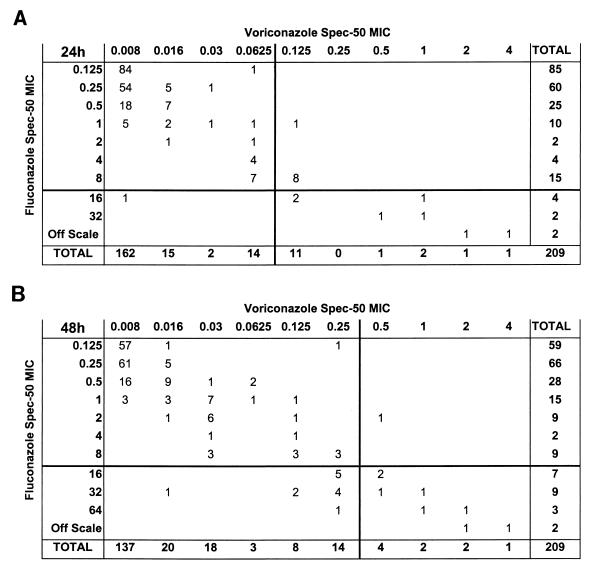
Using Spec-50 MICs, Fig. 1 shows the relationship between fluconazole MIC and voriconazole activity against the 209 strains. Voriconazole MICs are seen to rise with fluconazole MICs but not in all cases: for isolates for which fluconazole MICs were ≥32 μg/ml, voriconazole MICs were consistently elevated (0.5 μg/ml), but for isolates for which fluconazole Spec-50 MICs were 16 μg/ml at 24 h, voriconazole MICs were 0.008 to 1 μg/ml.
FIG. 1.
Cross-tabulation of voriconazole and fluconazole MICs. The data are the number of isolates for which the indicated MICs of voriconazole and fluconazole were obtained. MICs were determined as 50% inhibition (Spec-50) spectrophotometrically after 24 h (A) and 48 h (B) of incubation. The lines indicate the MIC at which 90% of the isolates tested were inhibited.
In vitro activity of amphotericin B.
The susceptibility of 209 Candida spp. isolates to amphotericin B in antibiotic medium 3 was also tested. Spec-95, Spec-80, Spec-50, AA-0, AA-1, AA-2, BA-0, BA-1, and BA-2 MICs had a concordance of ≥98% for all paired readings. As noted before, the putatively amphotericin B-resistant group of isolates was also identified by using all three endpoints after 24 h of incubation but not at 48 h. There was no evidence for cross-resistance between amphotericin B and either fluconazole or voriconazole (data not shown).
DISCUSSION
The recently published NCCLS standard method for susceptibility testing of yeasts (20) is a noteworthy advance in the field of in vitro testing of antifungal agents. Not surprisingly, however, publications documenting possible improvements to the method have appeared (1, 2, 12, 15, 16, 21, 25, 29, 30, 32). We have now explored the impact of these factors, some of which are mentioned as possible modifications in the NCCLS M27-A document (20), on the susceptibility of Candida species to the new triazole antifungal agent voriconazole.
Supplementation of the culture medium with glucose to a final concentration of 20 g/liter has been reported to simplify endpoint determination with fluconazole (32). Although this modification is not part of the formal M27-A methodology, it is now widely used for susceptibility testing of fungi both in broth and on agar (4, 10, 27, 30, 33). In this work, we found that supplementation of RPMI 1640 with glucose had only a modest effect on the MIC, especially when the MIC was determined mechanically with a spectrophotometer. On the other hand, we did note, as have others (32, 33), that the increased growth produced in glucose-supplemented media simplified visual endpoint determinations made by human observers and increased the correlation between these readings and mechanically generated readings. Furthermore, glucose supplementation was required for adequate growth of a small number of isolates. Thus, we elected to supplement our media with glucose for most of our studies.
Visual determination of the MIC is the most commonly used method in the field of antifungal susceptibility. In this regard, the NCCLS M27-A document (20) specifies visual endpoints derived from the work of Espinel-Ingroff et al. (6, 7) in which a visual endpoint for azoles was defined as a turbidity less than or equal to the turbidity of a fivefold dilution of the drug-free growth control (an 80% reduction). Spectrophotometric readings, on the other hand, have the advantage of permitting automated MIC determination. Our comparisons of visual and spectrophotometric MICs demonstrated differences between these approaches and also showed that an endpoint definition that works visually may not translate directly into an appropriate spectrophotometric endpoint. The best agreement (≥99%) was obtained when the NCCLS M27-A MIC-2 reading was compared with the Spec-50 reading after 24 h of incubation. Other authors have found endpoints with reduced stringency to be of value (1, 12, 24) and have also shown that these specific reading conditions tend to be concordant (25). The importance of this concept is further reinforced by our recent demonstration (30) that the Spec-50 MIC at 24 h is associated with an increased correlation with the outcome in vivo. In that study, readings after 48 h and Spec-80 MICs both failed to correlate with the outcome for some isolates. Based on these data, we feel that use of the Spec-50 MIC at 24 h has numerous advantages when both voriconazole and fluconazole are being tested against Candida spp.
The NCCLS M27-A document permits dispersion of the yeast suspension by vortexing, pipetting, or other techniques, as this has been helpful in some studies (1, 25). Agitation of the microdilution plates prior to visual readings has likewise been found to be valuable (1, 2, 12, 32) and is of course required for spectrophotometric readings. In these studies, however, agitation had little effect on the visual MIC, perhaps due to the use of glucose-enriched media.
Our results also provide a survey of the activity of voriconazole against a large collection of recently obtained pathogenic bloodstream and oral Candida isolates. Ninety percent of all the tested isolates were inhibited by voriconazole at concentrations ranging from 0.008 to 0.0625 μg/ml. These results are comparable to those obtained by others. For example, Ruhnke et al. (34) found that 90% of 105 isolates from a collection containing both fluconazole-susceptible and -resistant C. albicans isolates obtained from HIV patients had an MIC of ≤0.19 μg/ml. Voriconazole, as opposed to fluconazole, had consistent and significant activity against isolates of both C. krusei and C. glabrata, although MICs of both voriconazole and fluconazole for some Candida isolates were very high. The clinical meaning of these observations is unknown for voriconazole, but these data raise the possibility of cross-resistance between voriconazole and fluconazole.
For amphotericin B, there was almost complete agreement (98%) between visual and spectrophotometric readings, even when the strictest endpoints (AA-0 and Spec-95) were used. Unlike the agreement for azoles, the agreement remained constant after 48 h of incubation (99%). When we analyzed the three spectrophotometric endpoints used (Spec-50, Spec-80, and Spec-95), the modal MIC for each endpoint remained stable and did not change throughout the duration of the study. The ability to identify the putatively amphotericin B-resistant isolates (29, 35) was present at all endpoints studied. Although the modal MICs of the endpoints remained the same after both times of incubation, reliable identification of resistant isolates was achieved only after 24 h of incubation, as previously reported (15).
In conclusion, voriconazole is a new triazole antifungal agent that has potent in vitro activity against many isolates of Candida. Voriconazole MIC rises with fluconazole MIC but not for every isolate. When tested in vitro, it is possible to assay voriconazole with or without glucose supplementation of the culture medium. Glucose supplementation does, however, increase growth and simplify visual readings for some isolates. There is a strong correlation between the NCCLS visual MIC-2 and MIC-1 endpoints and the spectrophotometric Spec-50 and Spec-80 endpoints, respectively. Specifically, AA-2 and Spec-50 were almost identical (99% of paired readings were within 1 dilution) after 24 h of incubation, and Spec-50 was associated with an increased correlation with the in vivo response for fluconazole (30). Further comparisons of the MIC obtained by using these rules with the official NCCLS M27-A MIC are needed, so that the implications of discordant results may be better understood.
ACKNOWLEDGMENTS
This work was supported by a grant to M. Lozano-Chiu from the Dirección General de Investigación Científica y Enseñanza Superior of Spain and by a grant from Pfizer Pharmaceuticals, Inc.
REFERENCES
- 1.Anaissie E, Paetznick V, Bodey G P. Fluconazole susceptibility testing of Candida albicans: microtiter method that is independent of inoculum size, temperature, and time of reading. Antimicrob Agents Chemother. 1991;35:1641–1646. doi: 10.1128/aac.35.8.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anaissie E J, Paetznick V L, Ensign L G, Espinel-Ingroff A, Galgiani J N, Hitchcock C A, LaRocco M, Patterson T, Pfaller M A, Rex J H, Rinaldi M G. Microdilution antifungal susceptibility testing of Candida and Cryptococcus neoformans with and without agitation: an eight-center collaborative study. Antimicrob Agents Chemother. 1996;40:2387–2391. doi: 10.1128/aac.40.10.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barry A L, Brown S D. In vitro studies of two triazole antifungal agents (voriconazole [UK-109,496] and fluconazole) against Candida species. Antimicrob Agents Chemother. 1996;40:1948–1949. doi: 10.1128/aac.40.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dronda F, Alonso-Sanz M, Laguna F, Chaves F, Martinez-Suarez J V, Rodriguez-Tudela J L, Gonzalez-Lopez A, Valencia E. Mixed oropharyngeal candidiasis due to Candida albicans and non-albicans Candida strains in HIV-infected patients. Eur J Clin Microbiol Infect Dis. 1996;15:446–452. doi: 10.1007/BF01691310. [DOI] [PubMed] [Google Scholar]
- 5.Espinel-Ingroff A. In vitro activity of the new triazole voriconazole (UK-109,496) against opportunistic filamentous and dimorphic fungi and common and emerging yeast pathogens. J Clin Microbiol. 1998;36:198–202. doi: 10.1128/jcm.36.1.198-202.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff A, Kish C W, Kerkering T M, Fromtling R A, Bartizal K, Galgiani J N, Villareal K, Pfaller M A, Gerarden T, Rinaldi M G, Fothergill A. Collaborative comparison of broth macrodilution and microdilution antifungal susceptibility tests. J Clin Microbiol. 1992;30:3138–3145. doi: 10.1128/jcm.30.12.3138-3145.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espinel-Ingroff A, Steele-Moore L, Galgiani J N. Evaluation of 80% inhibition standards for the determination of fluconazole minimum inhibitory concentrations in three laboratories. Diagn Microbiol Infect Dis. 1994;20:81–86. doi: 10.1016/0732-8893(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 8.Fratti R A, Belanger P H, Ibrahim A S, Sanati H, Ghannoum M A. Abstracts of the 36th Interscience Conference on Antimicrobials Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. The new triazole, voriconazole (UK-109,496) inhibits the growth of Candida krusei and its interactions with endothelial cells, abstr. C66; p. 46. [Google Scholar]
- 9.Fromtling R A, Galgiani J N, Pfaller M A, Espinel-Ingroff A, Bartizal K F, Bartlett M S, Body B A, Frey C, Hall G, Roberts G D, Nolte F B, Odds F C, Rinaldi M G, Sugar A M, Villareal K. Multicenter evaluation of a macrobroth antifungal susceptibility test for yeasts. Antimicrob Agents Chemother. 1993;37:39–45. doi: 10.1128/aac.37.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gadea I, Cuenca M, Gegundez M I, Zapardiel J, Valero M L, Soriano F. Effect of pH and buffer system on the in-vitro activity of five antifungals against yeasts. J Antimicrob Chemother. 1997;39:453–459. doi: 10.1093/jac/39.4.453. [DOI] [PubMed] [Google Scholar]
- 11.George D, Miniter P, Andriole V T. Efficacy of UK-109,496, a new azole antifungal agent, in an experimental model of invasive aspergillosis. Antimicrob Agents Chemother. 1996;40:86–91. doi: 10.1128/aac.40.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghannoum M A, Ibrahim A S, Fu Y, Shafiq M C, Edwards J E, Criddle R S. Susceptibility testing of Cryptococcus neoformans: a microdilution technique. J Clin Microbiol. 1992;30:2881–2886. doi: 10.1128/jcm.30.11.2881-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girmenia C, Luzi G, Monaco M, Martino P. Use of voriconazole in treatment of Scedosporium apiospermum infection: case report. J Clin Microbiol. 1998;36:1436–1438. doi: 10.1128/jcm.36.5.1436-1438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kauffman C A, Zarins L T. In vitro activity of voriconazole against Candida species. Diagn Microbiol Infect Dis. 1998;31:297–300. doi: 10.1016/s0732-8893(98)00005-4. [DOI] [PubMed] [Google Scholar]
- 15.Lozano-Chiu M, Nelson P W, Lancaster M, Pfaller M A, Rex J H. Lot-to-lot variability of antibiotic medium 3 used for testing susceptibility of Candida isolates to amphotericin B. J Clin Microbiol. 1997;35:270–272. doi: 10.1128/jcm.35.1.270-272.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lozano-Chiu M, Paetznick V L, Ghannoum M A, Rex J H. Detection of resistance to amphotericin B among clinical isolates of Cryptococcus neoformans: performance of three different media. J Clin Microbiol. 1998;36:2817–2822. doi: 10.1128/jcm.36.10.2817-2822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Lozano-Chiu M, Nelson P W, Paetznick V, Rex J H. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Activity of voriconazole (VORI) vs. Candida: effects of incubation time, Candida species, and fluconazole (FLU) susceptibility, abstr. E-87; p. 129. [Google Scholar]
- 17.Martin M V, Yates J, Hitchcock C A. Comparison of voriconazole (UK-109,496) and itraconazole in prevention and treatment of Aspergillus fumigatus endocarditis in guinea pigs. Antimicrob Agents Chemother. 1997;41:13–16. doi: 10.1128/aac.41.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGinnis M R, Pasarell L, Sutton D A, Fothergill A W, Cooper C R, Rinaldi M G. In vitro evaluation of voriconazole against some clinically important fungi. Antimicrob Agents Chemother. 1997;41:1832–1834. doi: 10.1128/aac.41.8.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy M, Bernard E M, Ishimaru T, Armstrong D. Activity of voriconazole (UK-109,496) against clinical isolates of Aspergillus species and its effectiveness in an experimental model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 1997;41:696–698. doi: 10.1128/aac.41.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 21.Nguyen M H, Clancy C J, Yu V L, Yu Y C, Morris A J, Snydman D R, Sutton D A, Rinaldi M G. Do in vitro susceptibility data predict the microbiologic response to amphotericin B? Results of a prospective study of patients with Candida fungemia. J Infect Dis. 1998;177:425–430. doi: 10.1086/514193. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen M H, Yu C Y. In vitro comparative efficacy of voriconazole and itraconazole against fluconazole-susceptible and -resistant Cryptococcus neoformans isolates. Antimicrob Agents Chemother. 1998;42:471–472. doi: 10.1128/aac.42.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen M H, Yu C Y. Voriconazole against fluconazole-susceptible and resistant candida isolates: in-vitro efficacy compares with that of itraconazole and ketoconazole. J Antimicrob Chemother. 1998;42:253–256. doi: 10.1093/jac/42.2.253. [DOI] [PubMed] [Google Scholar]
- 24.Odds F C, Vranckx L, Woestenborghs F. Antifungal susceptibility testing of yeasts: evaluation of technical variables for test automation. Antimicrob Agents Chemother. 1995;39:2051–2060. doi: 10.1128/aac.39.9.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfaller M A, Messer S A, Coffmann S. Comparison of visual and spectrophotometric methods of MIC endpoint determinations by using broth microdilution methods to test five antifungal agents, including the new triazole D070. J Clin Microbiol. 1995;33:1094–1097. doi: 10.1128/jcm.33.5.1094-1097.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaller M A, Messer S A, Hollis R J, Jones R N, Doern G V, Brandt M E, Hajjeh R A. In vitro susceptibilities of Candida bloodstream isolates to the new triazole antifungal agents BMS-207147, Sch 56592, and voriconazole. Antimicrob Agents Chemother. 1998;42:3242–3244. doi: 10.1128/aac.42.12.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quereda C, Polanco A M, Giner C, Sanchez-Sousa A, Pereira E, Navas E, Fortun J, Guerrero A, Baquero F. Correlation between in vitro resistance to fluconazole and clinical outcome of oropharyngeal candidiasis in HIV-infected patients. Eur J Clin Microbiol Infect Dis. 1996;15:30–37. doi: 10.1007/BF01586182. [DOI] [PubMed] [Google Scholar]
- 28.Radford S A, Johnson E M, Warnock D W. In vitro studies of activity of voriconazole (UK-109,496), a new triazole antifungal agent, against emerging and less-common mold pathogens. Antimicrob Agents Chemother. 1997;41:841–843. doi: 10.1128/aac.41.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rex J H, Cooper C R, Jr, Merz W G, Galgiani J N, Anaissie E J. Detection of amphotericin B-resistant Candida isolates in a broth-based system. Antimicrob Agents Chemother. 1995;39:906–909. doi: 10.1128/aac.39.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rex J H, Nelson P W, Paetznick V L, Lozano-Chiu M, Espinel-Ingroff A, Anaissie E J. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing critical isolates in a murine model of invasive candidiasis. Antimicrob Agents Chemother. 1998;42:129–134. doi: 10.1128/aac.42.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rex J H, Pfaller M A, Rinaldi M G, Polak A, Galgiani J N. Antifungal susceptibility testing. Clin Microbiol Rev. 1993;6:367–381. doi: 10.1128/cmr.6.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Tudela J L, Martinez-Suarez J V. Improved medium for fluconazole susceptibility testing of Candida albicans. Antimicrob Agents Chemother. 1994;38:45–48. doi: 10.1128/aac.38.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Tudela J L, Martinez-Suarez J V, Dronda F, Chaves F, Valencia E. Correlation of in vitro susceptibility test results with in vivo response: a study of azole therapy in AIDS patients. J Antimicrob Chemother. 1995;35:793–804. doi: 10.1093/jac/35.6.793. [DOI] [PubMed] [Google Scholar]
- 34.Ruhnke M, Schmidt-Weshausen A, Trautmann M. In vitro activities of voriconazole (UK-109,496) against fluconazole-susceptible and -resistant Candida albicans isolates from oral cavities of patients with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1997;41:575–577. doi: 10.1128/aac.41.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wanger A, Mills K, Nelson P W, Rex J H. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for antifungal susceptibility testing: enhanced ability to detect amphotericin B-resistant Candida isolates. Antimicrob Agents Chemother. 1995;39:2520–2522. doi: 10.1128/aac.39.11.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]