Abstract
Nitrogen (N) is an essential macronutrient, which contributes substantially to the growth and development of plants. In the soil, nitrate (NO3) is the predominant form of N available to the plant and its acquisition by the plant involves several NO3 transporters; however, the mechanism underlying their involvement in the adaptive response under abiotic stress is poorly understood. Initially, we performed an in silico analysis to identify potential binding sites for the basic leucine zipper 62 transcription factor (AtbZIP62 TF) in the promoter of the target genes, and constructed their protein–protein interaction networks. Rather than AtbZIP62, results revealed the presence of cis-regulatory elements specific to two other bZIP TFs, AtbZIP18 and 69. A recent report showed that AtbZIP62 TF negatively regulated AtbZIP18 and AtbZIP69. Therefore, we investigated the transcriptional regulation of AtNPF6.2/NRT1.4 (low-affinity NO3 transporter), AtNPF6.3/NRT1.1 (dual-affinity NO3 transporter), AtNRT2.1 and AtNRT2.2 (high-affinity NO3 transporters), and AtGLU1 and AtGLU2 (both encoding glutamate synthase) in response to drought stress in Col-0. From the perspective of exploring the transcriptional interplay of the target genes with AtbZIP62 TF, we measured their expression by qPCR in the atbzip62 (lacking the AtbZIP62 gene) under the same conditions. Our recent study revealed that AtbZIP62 TF positively regulates the expression of AtPYD1 (Pyrimidine 1, a key gene of the de novo pyrimidine biosynthesis pathway know to share a common substrate with the N metabolic pathway). For this reason, we included the atpyd1-2 mutant in the study. Our findings revealed that the expression of AtNPF6.2/NRT1.4, AtNPF6.3/NRT1.1 and AtNRT2.2 was similarly regulated in atzbip62 and atpyd1-2 but differentially regulated between the mutant lines and Col-0. Meanwhile, the expression pattern of AtNRT2.1 in atbzip62 was similar to that observed in Col-0 but was suppressed in atpyd1-2. The breakthrough is that AtNRT2.2 had the highest expression level in Col-0, while being suppressed in atbzip62 and atpyd1-2. Furthermore, the transcript accumulation of AtGLU1 and AtGLU2 showed differential regulation patterns between Col-0 and atbzip62, and atpyd1-2. Therefore, results suggest that of all tested NO3 transporters, AtNRT2.2 is thought to play a preponderant role in contributing to NO3 transport events under the regulatory influence of AtbZIP62 TF in response to drought stress.
Keywords: nitrogen use efficiency, nitrate transporters, nitrogen assimilation, AtbZIP62 transcription factor, AtPYD1, drought stress, Arabidopsis
1. Introduction
Nitrogen (N) is an essential macronutrient, which contributes substantially to the growth and development of plants [1]. The primary step of N acquisition by roots is the active transport across the plasma membrane of root epidermal and cortical cells, in the form of nitrate (NO3) and ammonium (NH4), with NO3 being the major source of N. The acquisition of N from the soil occurs mostly through the combined activities of low- and high-affinity NO3 transporters [2,3]. In higher plants, NO3 transporters are found within five (5) protein families, including NO3 transporter 1 (NRT1), NO3 transporter 2 (NRT2), chloride channel (CLC), and slow anion channel-associated/slow anion channel-associated homologs (SLAC/SLAH) [4,5]. In Arabidopsis thaliana (herein referred to as Arabidopsis), the NO3 transporter 1/peptide transporter (NRT1/PTR) gene family (NPF) and NRT2 have fifty-three (53) and seven (7) members, respectively. Of this number, a few genes functionally characterized were selected for this study, including AtNPF6.2/NRT1.4 (low-affinity NO3 transporter [6]), AtNPF6.3/NRT1.1 (dual-affinity NO3 transporter [7,8]), AtNRT2.1 and AtNRT2.2 (major components of the high-affinity uptake system) [9,10]. Members of the NRT1/PTR family (NPF) are not only involved in the uptake and transport of NO3 and its translocation and sensing; they also transport other biological compounds, such as peptides, amino acids, dicarboxylates, glucosinolates, indole-3-acetic acid (IAA) and abscisic acid (ABA) [11].
Nitrogen is transported from the roots to the shoot via the xylem as NO3, dissolved NH3, and amino acids. Usually [12], but not always [13], most NO3 reduction is carried out in the shoot, while the roots reduce only a fraction of the absorbed NO3 to NH3. After N acquisition by the roots, NO3 reductase (NR) and nitrite reductase (NiR) convert the exogenous NO3 to NH4, which is assimilated by glutamine synthase and glutamate synthase into amino acids [14]. Tcherkez and Hodges [15] indicated that glutamine synthase incorporates ammonia (NH3) as the amide group of glutamine using glutamate as a substrate in the chloroplasts. Glutamate synthase (Fd-GOGAT and NADH-GOGAT) transfers the amide group onto a 2-oxoglutarate molecule producing two glutamates. In the process, transaminations take place to make other amino acids (most commonly asparagine) from glutamine. The enzyme glutamate dehydrogenase (GDH) protects the mitochondrial functions during periods of high N metabolism and takes part in N remobilization [16]. To maintain an ionic balance, every NO3 molecule taken into the roots must be accompanied by either the uptake of a cation or the excretion of an anion. Plants take up metal ions like potassium (K+), sodium (Na+), calcium (Ca2+), and magnesium (Mg2+) to exactly match every NO3 taken up and store these as the salts of organic acids like malate and oxalate [17].
However, external fluctuations of N supply to plants caused by abiotic stress such as drought hinder NO3 acquisition events due to water scarcity. As a result, the reduced water availability affects the whole N use necessary for plants to complete their life cycle [18], including N uptake and transport, translocation, assimilation, and redistribution (also referred to as nitrogen use efficiency, NUE). Under the same conditions, a transcriptional reprogramming takes place, resulting in the activation or suppression of abiotic stress-responsive genes, as well as the induction of various signaling networks to optimize N uptake and utilization [19]. Ashoub, et al. [20] reported that the activity of a large number of enzymes involved in N assimilation decreases under long-term abiotic stress conditions, while Wendelboe-Nelson and Morris [21] supported that the activity of other enzymes involved in the same process increases under short-term exposure.
Protein–DNA and protein–protein interactions are fundamental to nearly all biological processes in all biological systems [22]. The joint actions of transcription factors (TFs) regulate the expression of genes in biological systems. TFs bind to cis-regulatory elements present in the promoter region of genes [23]. TFs operate either alone or in complex with other molecules to activate or repress the recruitment of the basal transcriptional machinery to specific genes [24], thereby determining when and where the target genes are transcribed, and the level of protein synthesis, as well as the resulting phenotype [22]. Basic leucine zipper proteins (bZIP) are transcription factors (TFs) involved in various developmental processes, including seeds maturation, plant growth, flower development, and signaling during abiotic and biotic stresses [25].
Recently, a study attempting to characterize a member of the bZIP TF family, AtbZIP62 TF, proposed this gene as a positive regulator of drought stress response in Arabidopsis [26]. Thus, from the perspective of exploring the transcriptional regulation of NO3 transporters and assimilation-related genes under drought stress, we performed an in silico analysis, which helped to identify cis-regulatory elements specific to two members of the bZIP family (AtbZIP18 and AtbZIP69 earlier suggested to be regulated by AtbZIP62 TF), in the promoter of the target genes. Similarly, AtbZIP62 was proposed to interact with AtbZIP18 or AtbZIP69 TFs to regulate the expression of AtPYD1 (Pyrimidine 1, a gene catalyzing the step-limiting factor of the de novo pyrimidine biosynthesis pathway, which in turn is proposed to have a crosstalk with the N metabolism) under drought stress [26].
Therefore, in the present study, we investigated the role of AtbZIP62 TF in the regulation of the expression of four (4) NO3 transporters and two glutamate synthase-encoding genes involved in N assimilation in Arabidopsis in response to drought stress. Furthermore, we were interested to see how the proposed transcriptional interplay of AtbZIP62 with AtPYD1 would contribute to elucidating the mechanism underlying the regulation of NO3 transporters and assimilation-related genes by AtbZIP62 TF. To achieve that, the Arabidopsis Col-0 (wild type, WT), and the atbzip62 and atpyd1-2 mutant lines lacking AtbZIP62 and AtPYD1, respectively, were used as genetic materials.
2. Results
2.1. In Silico Transcription Factor Binding Sites Analysis Identified Cis-Regulatory Elemenents for bZIP TFs in NO3 Transporters and Glutamate Synthase-Encoding Genes
In our previous studies, AtbZIP18 and AtbZIP69 were proposed to be co-expressed, while being negatively regulated by AtbZIP62 TF under salinity [27] and drought stress [26]. In this study, the results in Table 1 show the presence of two binding sites specific to AtbZIP18 and AtbZIP69 TFs in the promoter of AtbZIP62 TF; while in that of AtPYD1, one binding site for AtbZIP69 TF was found. In addition, two (2) binding sites for AtbZIP18 and three (3) for AtbZIP69 were identified in the promoter of AtNPF6.2/NRT1.4. In the same way, two binding sites for AtbZIP18 and AtbZIP69 were detected in the promoter of AtNRT2.2. Furthermore, a single binding site for AtbZIP18 was found in the promoter of AtGLU1. However, no binding sites for AtbZIP18 or AtbZIP69 were identified in the promoter of AtNPF6.3, AtNRT2.1, and AtGLU2.
Table 1.
Identified transcription factor binding sites in the promoter of target genes.
| Gene Locus ID | TF Name | Target Genes | Position | Strand | p-Value | q-Value | Matched Sequence |
|---|---|---|---|---|---|---|---|
| AtbZIP62 TF | |||||||
| AT1G06070 | AtbZIP69 | AT1G19490 | 1528–1538 | 3.77 10−5 | 0.154 | AACAACTGGCC | |
| AT2G40620 | AtbZIP18 | AT1G19490 | 1912–1922 | + | 1.31 10−5 | 0.0546 | ATGAGCTGGCA |
| AtPYD1 | |||||||
| AT1G06070 | AtbZIP69 | AT3G17810 | 427–437 | + | 2.8 10−5 | 0.115 | TGCAGCTGGAG |
| AT1G06070 | AtbZIP69 | AT3G17810 | 427–437 | 6.13 10−5 | 0.126 | TGCAGCTGTTG | |
| AtNPF6.2/NRT1.4 | |||||||
| AT1G06070 | AtbZIP69 | AT2G26690 | 2934–2944 | 1.04 10−5 | 0.079 | ACCAGCTGGGA | |
| AT1G06070 | AtbZIP69 | AT2G26690 | 2935–2945 | + | 4.24 10−5 | 0.109 | CCCAGCTGGTT |
| AT1G06070 | AtbZIP69 | AT2G26690 | 1096–1106 | 4.34 10−5 | 0.109 | GACAACTGGTA | |
| AT2G40620 | AtbZIP18 | AT2G26690 | 2983–2993 | + | 7.12 10−5 | 0.34 | TCTAGCTGTCT |
| AT2G40620 | AtbZIP18 | AT2G26690 | 3469–3479 | + | 8.91 10−5 | 0.34 | GATGGCTGGCT |
| AtNPF6.3/NRT1.1 | |||||||
| * | * | AT1G12110 | * | * | * | * | * |
| * | * | AT1G12110 | * | * | * | * | * |
| AtNRT2.1 | |||||||
| * | * | AT1G08090 | * | * | * | * | * |
| * | * | AT1G08090 | * | * | * | * | * |
| AtNRT2.2 | |||||||
| AT1G06070 | AtbZIP69 | AT1G08100 | 1761–1771 | 9.28 10−5 | 0.388 | GACAACTGTAT | |
| AtGLU1 | |||||||
| AT2G40620 | AtbZIP18 | AT5G04140 | 459–469 | + | 8.32 10−5 | 0.348 | TAGGGCTGGCT |
| AtGLU2 | |||||||
| * | * | AT2G41220 | * | * | * | * | * |
| * | * | AT2G41220 | * | * | * | * | * |
(*) the asterisk indicates that the binding sites for the AtbZIP18 and/or AtbZIP69 associated with AtbZIP62 TF were not detected.
2.2. Prediction of Protein–Protein Interaction Network of Nitrate Transporters and Assimilation
To further understand the mechanism underlying the transcriptional regulation of the NO3 transport and assimilation-related genes, as well as their possible interaction networks, we employed the STRING database (version 11.5, https://string-db.org, accessed on 9 September 2021), which proposes protein–protein interactions in plants. Because AtNRT2.2 (a high-affinity NO3 transporter) exhibited the highest transcriptional level (upregulated by 94.2-fold change) in response to drought stress, we selected the protein sequence of this gene to unveil the identity of proteins predicted to interact with NRT2.2.
At first, we were interested to see the possible functional interactome involving NRT2.2 and other NO3 transporters. The results of the protein–protein interaction prediction revealed that NRT2.2 has been experimentally determined to functionally interact with WR3 (AT5G0200, a high-affinity NO3 transporter 3.1 acting as a dual component transporter with NRT2.1, and said to be involved in targeting NRT2 proteins to the plasma membrane) (Figure 1, Table 2). What is common in the predicted interactome is that NRT2-encoding genes (NRT2.1 and NRT2.2) do not show direct functional interactions or synergy, but both are predicted to have a relationship with WR3 (would-responsive gene 3, encoding a high-affinity nitrate transporter), NIR1, NIA1 and NIA2 (Figure 1C,D). Similarly, NFP6.3/NRT1.1 is predicted to interact with NRT2.2, while NFP6.2 is proposed to be functionally associated with NRT2.2 and WR3. It was observed that NIA1 and NIA2, as well as WR3 appear to functionally interact with both dual- and high-affinity NO3 transporter (NRT6.3/NRT1.1, NRT2.1 and NRT2.2), except NPF6.2 (a low-affinity NO3 transporter) (Figure 1A–D).
Figure 1.
Prediction of the functional protein–protein interactome of selected nitrate transporters and assimilation-related genes. Predicted protein–protein interaction network involving the Arabidopsis (A) NPF6.2, (B) NFP6.3/NRT1.1, (C) NRT2.1, (D) NRT2.2, (E) GLU1, (F) GLU2, and (G) PYD1. The prediction was done using the protein sequence of each target gene obtained from the Arabidopsis Information Resource (TAIR, www.arabidopsis.org, accessed on 9 September 2021) and uploaded to STRING protein database version 11.5 (https://string-db.org/, accessed on 9 September 2021). Red nodes represent the gene of interest, while other nodes show the interactome of a different nature indicated by connecting lines. The nature of the interaction is indicated by specific line colors and the source of information (from curated databases, experimentally determined, predicted interactions, and others) as explained in the figure legend next to the panel (G).
Table 2.
Predicted protein–protein interactions involving AtNRT2.2 (topmost upregulated by drought stress).
| Target Proteins | Locus | Description | Reference |
|---|---|---|---|
| NIR1 | AT2G15620 | Ferredoxin—nitrite reductase, chloroplastic; Involved in the second step of nitrate assimilation. | [28] |
| NIA1 | AT1G12110 | Nitrate reductase [NADH] 1; Encodes the cytosolic minor isoform of nitrate reductase (NR). Involved in the first step of nitrate assimilation, it contributes about 15% of the nitrate reductase activity in shoots. | [29] |
| NIA2 | AT1G37130 | Nitrate reductase [NADH] 2; Identified as a mutant resistant to chlorate. Encodes nitrate reductase structural gene. Involved in nitrate assimilation. Has nitrate reductase activity. | [29] |
| WR3 | AT1G08100 | High-affinity nitrate transporter 3.1; Acts as a dual component transporter with NTR2.1. Required for high-affinity nitrate transport. May be involved in targeting NRT2 proteins to the plasma membrane. | [30] |
| NRT1.1 | AT1G12110 | Protein NRT1/PTR family 6.3; Dual affinity nitrate transporter. Involved in proton- dependent nitrate uptake and in the regulation of the nitrate transporter NRT2.1. Acts also as a nitrate sensor that trigger a specific signaling pathway stimulating lateral root growth and seed germination. The uptake activity is not required for sensor function. | [31] |
| NRT1.5 | AT1G32450 | Protein NRT1/PTR FAMILY 7.3; Transmembrane nitrate transporter. Involved in xylem transport of nitrate from root to shoot. Induced in response to nitrate. Not involved in nitrate uptake. Belongs to the PTR2/POT transporter (TC 2.A.17) family | [33] |
| NRT1.2 | AT1G69850 | Protein NRT1/PTR family 4.6; Low-affinity proton-dependent nitrate transporter. Involved in constitutive nitrate uptake. Involved in (+)-abscisic acid (ABA) transport. Mediates cellular ABA uptake. Belongs to the PTR2/POT transporter (TC 2.A.17) family | [32] |
| NRT1.7 | AT1G69870 | Protein NRT1/PTR family 2.13; Low-affinity proton-dependent nitrate transporter. Involved in phloem loading and nitrate remobilization from the older leaves to other tissues; Belongs to the PTR2/POT transporter (TC 2.A.17) family | [34] |
In Arabidopsis, N assimilation is mediated by a variety of enzymes, including GLU1 and GLU2 known for their important roles during the early steps of the process. GLU1 is a chloroplastic/mitochondrial ferredoxin-dependent glutamate synthase 1 (Fd-GOGAT), proposed to be involved in photorespiration and N assimilation, while GLU2 is a chloroplastic ferredoxin-dependent glutamate synthase. These proteins have the ability to supply a constitutive level of glutamate to maintain a basal level of protein synthesis, and are suggested to play a role in primary N assimilation in roots. Here, the STRING results proposed that both GLU1 and GLU2 would have a functional protein–protein interaction with same protein counterparts, including two cytosolic glutamine synthase (GLN1.4, AT5G16570; GSR2, AT1G66200) and a chloroplastic/mitochondrial glutamine synthase (GS2, AT5G35630) identified as being responsible for the reassimilation of the NH3 generated by photorespiration. In addition, a set of three glutamate dehydrogenase-encoding genes (AtGDH1, AT5G18170; AtGDH2, AT5G07440; AtGDH3, AT3G03910, associated with N assimilation) were suggested (experimentally determined as indicated by the purple connecting line) to have common functional target protein interactions, with a few exceptions like NIR1 (AT2G15620, identified as a chloroplastic ferredoxin-nitrite reductase involved in the second step of NO3 assimilation) only linked with GLU2 (Figure 1E,F).
2.3. KEGG Analysis Suggests a Crosstalk between De Novo Pyrimidine Biosynthesis and the Nitrogen Metabolic Pathways
A possible crosstalk between the de novo pyrimidine biosynthesis pathway and the nitrogen metabolism was explored. In this study, we included AtPYD1, a gene catalyzing the step-limiting factor of the de novo pyrimidine biosynthesis pathway. As indicated in the panels A and B of Figure 2, de novo pyrimidine biosynthesis pathway is shown to have a crosstalk with the nitrogen metabolic pathway, and carbamoyl phosphate is proposed to serve as a common substrate for both pathways.
Figure 2.
Interplay between pyrimidine, arginine nitrogen metabolism. (A) Shows Carbamoyl phosphate as the major substrate linking the pyrimidine pathway, arginine pathway and the nitrogen metabolism. (B) Displays the Arginine biosynthesis pathway highlighting the crosstalk between nitrogen metabolism and pyrimidine biosynthesis pathway (see dotted read circle). We generated this metabolic pathway using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (https://www.genome.jp/pathway/map01100+M00052, accessed on 11 September 2021).
2.4. AtNPF6.2/NRT1.4, AtNPF6.3/NRT1.1 and AtNRT2.2 Were Similarly Regulated in atbzip62 and atpyd1-2 Mutants, While ANRT2.1 Showed Differential Expression Pattern under Drought Stress
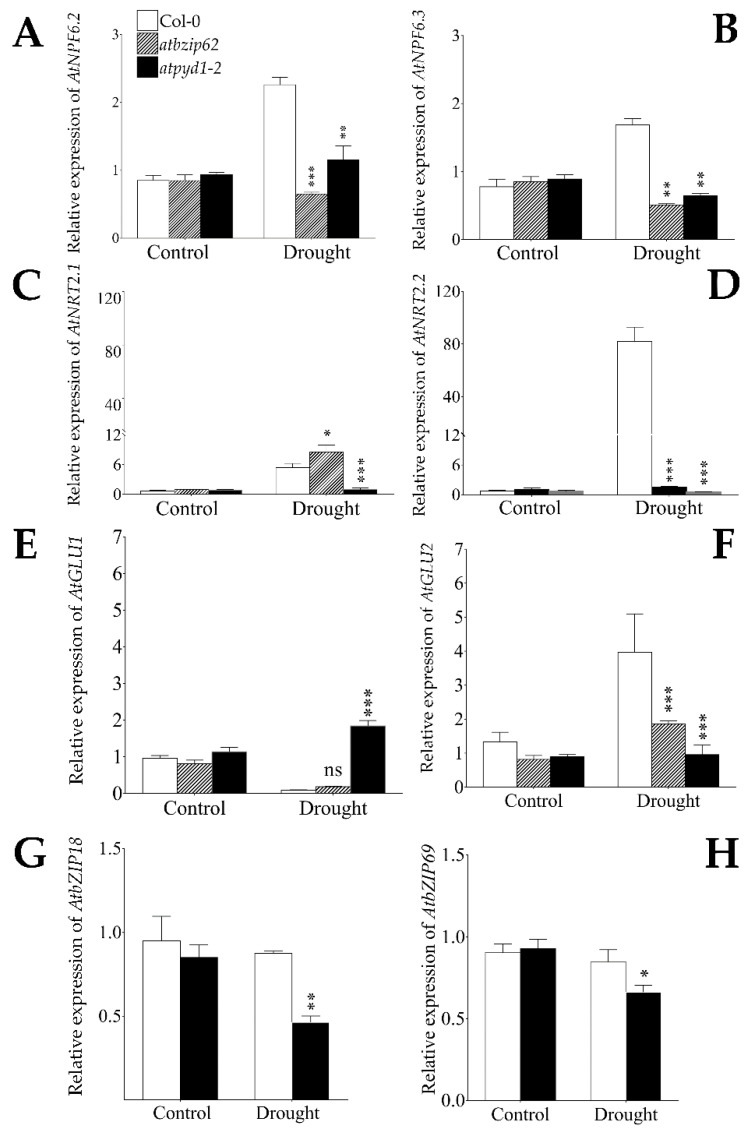
Nitrate uptake, transport and assimilation are strongly affected when plants experience reduced water potential as well as physical water unavailability. Here, data show that the low-affinity NO3 transporter ANPF6.2/NRT1.1 as well as the dual-affinity (AtNPF6.3/NRT1.1) and the high-affinity (AtNRT2.1 and AtNRT2.2) NO3 transporter-encoding genes were significantly upregulated by drought stress in Col-0 WT (Figure 3A–D), with AtNRT2.2 having the highest transcript accumulation level (upregulated by about a 94.2-fold change). Under the same conditions, the expression of AtNPF6.2/NRT1.4, AtNPF6.3/NRT1.1, and AtNRT2.2 was downregulated in the atbzip62 mutant (Figure 3A,B,D). Meanwhile, AtNRT2.1 showed a similar transcript accumulation pattern with Col-0 (Figure 3C).
Figure 3.
Transcript accumulation of low-, dual-, and high-affinity nitrate transporters and glutamate synthase-encoding genes in response to drought stress monitored in Arabidopsis Col-0, atbzip62 and atpyd1-2 mutant lines lacking AtbZIP62 and AtPYD1, respectively, under drought stress. (A) Expression levels of AtNFP6.2, (B) AtNPF6.3, (C) AtNRT2.1, (D) AtNRT2.2, (E) AtGLU1, (F) AtGLU2, (G) AtbZIP18, and (H) AtbZIP69 upon drought stress induction. Bars are mean values of triplicate ± SD from non-stressed and drought-stressed plants. White bars are Col-0 WT, bars with hatch lines represent the atbzip62, and black bars indicate the atpyd1-2. The relative expression values of each target gene were normalized to that of the housekeeping gene Actin2. For statistical significance, the normalized transcript accumulation level of target genes in the mutant lines was compared with that recorded in Col-0 wild type. The statistical significance is indicated on the top of each bar. * p < 0.05, ** p < 0.01, *** p < 0.001, ns non-significant.
In addition, AtNPF6.2/NRT1.4 transcript accumulation decreased significantly in atpyd1-2 compared to that of Col-0. However, the expression of AtNPF6.3/NRT1.1 was significantly downregulated in a similar manner with the one observed in atbzip62. Similarly, the expression of AtNRT2.2 in atpyd1-2 showed a downregulation pattern (Figure 3D). However, AtNRT2.1 transcript accumulation was not affected by drought stress in atpyd1-2 (Figure 3C). The AtbZIP62 TF was recently proposed to be involved in the regulation of the expression events of AtPYD1, when investigating their transcriptional interplay under drought stress [26].
2.5. Drought Stress Differentially Regulated AtGLU1 and AtGLU2 Genes
Glutamate synthase enzymes are key enzymes identified as substrates for glutamine synthase in the initial steps of N assimilation in plants under optimal growth conditions. Here, our data show that the expression of AtGLU1 and AtGLU2, two genes encoding glutamate synthase, was differentially regulated by drought stress, with AtGLU1 being downregulated and AtGLU2 upregulated in both Col-0 and atbzip62 (Figure 3E,F). Meanwhile, the transcript accumulation of AtGLU1 increased significantly in atpyd1-2. In contrast, the expression of AtGLU2 reduced significantly in atbzip62 and atpyd1-2 compared to that recorded in Col-0.
Previous studies suggested that AtbZIP18 and AtbZIP69 would co-express under salinity [27] and drought stress [26], and AtbZIP62 TF is identified as a negative regulator of both genes. In the same studies, the authors proposed that AtbZIP62 TF would interact with AtbZIP69 TF to regulate the expression of AtPYD1. As show in Table 1, in silico transcription factor binding sites analysis detected cis-regulatory elements specific to AtbZIP69 in the proximal promoter of AtPYD1 and that of AtGLU1. Therefore, we measured the expression of AtbZIP18 and AtbZIP69 in atpyd1-2. Data in the panels G and H of Figure 1 reveal that the transcript accumulation of both AtbZIP18 and AtbZIP69 decreased significantly in atpyd1-2 compared to that observed in Col-0.
Because AtNRT2.2 recorded the highest transcript accumulation (94.2-fold change) upon drought stress induction in Col-0 WT, we were interested in investigating in detail the possible interactome underlying its regulatory network. The results in Table 2 give insights into the possible functional protein–protein interactions that AtNRT2.2 (protein) may have; these include the NIR1 (a ferredoxin-nitrate reductase involved in the second step of nitrate assimilation) [28], NIA1 and NIA2 (encoding NO3 reductase, and involved in nitric oxide (NO) biosynthesis) [29], WR3 (known as having a high-affinity for NO3 transport, acting as a dual component with NRT2.1) [30], NPF6.3/NRT1.1 (a dual-affinity NO3 transporter involved in the regulation of NRT2.1) [31], and NRT1.2 (a low-affinity NO3 transporter mediating constitutive nitrate uptake) [32]. This may imply that AtNRT2.2 would play a key role during N acquisition and transport when plants experience drought stress conditions, while interacting with a set of other NO3 transporters and NO3 reductase-encoding genes.
2.6. Proposed Signaling Model of AtbZIP62 TF and NO3 Transporters and Glutamate Synthase under Drought Stress
We have summarized the data and proposed a signaling model primarily using the recorded gene expression data of the analyzed NO3 transporters and glutamate synthase-encoding genes in Col-0 (WT), and the atbzip62 and atpyd1-2 mutant lines, in response to drought stress (Figure 4). Here, AtbZIP62 is shown to positively regulate the expression of AtPYD1 under drought stress, as previously suggested [26]. Then, based on the transcript accumulation patterns of AtNPF6.2/NRT1.1, AtNPF6.3/NRT1.1 (low- and dual-affinity NO3 transporters), AtNRT2.1 and AtNRT2.2 (high-affinity NO3 transporters), and AtGLU1 and AtGLU2 (encoding glutamate synthase) in the mutants compared with the wild type, we proposed a positive or negative regulation by AtbZIP62 TF. This signaling model also considered the known and predicted interactions between the target genes in the present study to draw a possible linkage between genes, based on protein–protein interaction by STRING and the literature. Nitrate transporters are widely known for their roles in mediating the mobility, sensing, and translocation of nitrogen (N), while the assimilation of N by plants in the form of amino acids involves a glutamine–glutamate synthase complex, interacting with other compounds. Our results give new insights into the transcriptional regulation mechanism of some of the key NO3 transporters, while highlighting the regulatory role of AtbZIP62 TF and AtPYD1 under drought stress.
Figure 4.
Signaling model of the transcriptional regulation of nitrate transporters as well as glutamate synthase-encoding genes in response to drought stress. When plants experience adverse environmental conditions, they activate signaling cascades and the appropriate defense system, which includes the induction of a wide range of drought-responsive genes, of which the transcript accumulation events are mediated by transcription factors, and their interplay determines the degree of the defense. The basic leucine zipper (bZIP) transcription factor AtbZIP62 TF is here suggested to interact with other proteins or DNA to regulate the expression of AtNPF6.2 (low-affinity NO3 transporter), AtNPF6.3/NRT1.1 (dual-affinity NO3 transporter), AtNRT2.1 and AtNRT2.2 (both having a high affinity for NO3 transport), as well as AtGLU1 and AtGLU2 (encoding glutamate synthase) under drought stress conditions in Arabidopsis. Arrows with continuous lines indicate a positive regulation (of gene expression); whereas, continuous lines with a perpendicular bar (blunt end) show a negative regulation of gene expression by our studies (current and previous). Dotted lines with an arrow at their tips suggest a possible functional interaction as predicted through in silico transcription factor binding sites analysis and protein–protein interaction, coupled with the literature. Dotted lines with arrows indicate a proposed interaction between NO3 transport and assimilation in the literature.
3. Discussion
3.1. AtbZIP62 Regulates Nitrate Transporter-Encoding Genes in Response to Drought Stress
Studies describing the physiology and biochemistry aspects of plant nutrition have established that macronutrients such as nitrogen (N) are acquired from the soil, through the combined activities of low- and high-affinity NO3 transport systems across the plasma membrane of epidermal and cortical cells of roots [2,3]. In aerobic soils where nitrification can occur, NO3 is usually the predominant form of available N that is absorbed [35,36]. Nitrate is taken up by several NO3 transporters, which use a proton gradient to power the transport [37,38]. Currently, only a few NO3 transporters have been associated with the coping mechanism under limited N availability caused by adverse environmental conditions [31,34,39,40,41,42,43] in Arabidopsis and rice. For instance, AtNPF7.2/NRT1.8, a low-affinity NO3 transporter, is believed to be actively involved in NO3 uploading from xylem vessels in response to cadmium (Cd) stress [44]. Another essential component in the regulation of NO3 reallocation, AtNRT1.5 (a xylem NO3-loading transporter) was proposed to be downregulated by salt and Cd stress in Arabidopsis [45]. The authors supported that AtNPF7.2 regulates AtNRT1.5. Léran, et al. [46] reported that AtNPF6.3/NRT1.1 is involved in NO3 influx and translocation to the shoot [7,47]. In the same way, AtNPF6.3/NRT1.1 (also known as CHL1, chlorate resistance 1) was reported to act as an NO3 sensor [48,49,50,51], and participates in the signaling pathway triggering root colonization of NO3-rich patches [52].
In this study, the recorded downregulation patterns of AtNPF6.2/NRT1.4, AtNPF6.3/NRT1.1 (low- and dual-affinity NO3 transporters), and AtNRT2.2 (the high-affinity NO3 transporter) between Col-0 WT and atbzip62 and atpyd1-2 in response to drought stress would suggest a positive regulation by AtbZIP62 TF and AtPYD1. AtNRT2.2 exhibited the highest transcript accumulation level in response to drought stress (about a 94.2-fold change) in Col-0. Table 2 identified NRT1.5 (AT1G32450) and NRT1.7 (AT1G69870) as potential targets for AtNRT2.2 during NO3 transport. Meanwhile, the expression of AtNRT2.1 showed a similar transcript accumulation pattern with Col-0 WT (Figure 3C), which may imply that AtbZIP62 TF may not be involved in the transcriptional regulation events of AtNRT2.1 under drought stress. However, the significant decrease in the transcript accumulation of AtNRT2.1 in atpyd1-2 would imply that AtPYD1 might be involved in the positive regulation of AtNRT2.1 under drought stress.
Under these conditions, AtbZIP62 TF, known as a positive regulator of drought stress response, would play a key role in promoting NO3 transport activity. This event may involve AtPYD1, considering their regulatory records as well as the crosstalk between the de novo pyrimidine biosynthesis pathway and the nitrogen metabolism (Figure 2A,B), supported by the predicted functional protein–protein interaction between PYD1 (Pyrimidine 1) and CARB (carbamoyl phosphate) (Figure 1G). Therefore, AtbZIP62 TF and AtPYD1 are proposed to positively regulate the transcriptional events of NO3 transporter-encoding genes in response to drought stress. In a converse approach, Zhong, et al. [53] supported that a member of the bZIP transcription factor family, AtTGA4/AtbZIP57 (AT5G10030) confers drought tolerance by promoting NO3 transport and assimilation.
Owing to these results, we could speculate that upon drought stress induction, the availability of NO3 may be affected significantly due to water scarcity. Under these conditions, AtNRT2.2 is believed to play a preponderant role in NO3 transport when plants experience drought stress, under the regulatory influence of AtbZIP62 TF, while interacting with other NO3 transporters as proposed in Table 2. This may be part of the adaptive response mechanism towards drought stress-mediated N deficiency.
3.2. AtPYD1 May Play a Role in Mediating Nitrogen Assimilation through Regulation of Glutamate Synthase Encoded Genes under Drought Stress
Abiotic stress such as drought deprives plants not only from water availability but also from acquiring essential nutrients, including nitrogen (N) and N-containing compounds, which are necessary for efficient plant growth and development [18]. It has been established that glutamate synthase (protein) plays a key role as the substrate for glutamine synthase in the initial steps of N assimilation by plants [54]. In this study, we observed that the two genes encoding glutamate synthase in Arabidopsis were differentially regulated by drought stress. AtGLU1 was downregulated, while AtGLU2 exhibited an opposite pattern in Col-0 and atbzip62, and atpyd1-2 (Figure 3E,F). We could then say that AtGLU2 would prevail over AtGLU1 during NO3 assimilation events when plants experience drought stress. In addition, we observed that AtGLU1 and AtGLU2 were similarly regulated in Col-0 and atbzip62, which may imply that AtbZIP62 TF would not be required in the regulation of the expression of glutamate synthase-encoding genes. The KEGG analysis revealed that carbamoyl phosphate serves a precursor for de novo pyrimidine biosynthesis and the N metabolic pathways [55]. In the same way, the panel G of Figure 1 proposes that a protein-protein interaction would exist between PYD1 and CARB. Therefore, the significant increase in the transcript accumulation of AtGLU1 and the decrease of AtGLU2 recorded in atpyd1-2 would suggest that AtPYD1 might be involved in the regulation of glutamate synthase-mediated NO3 assimilation in plants under drought stress.
4. Materials and Methods
4.1. Plant Materials, Growth Conditions, and Drought Stress Induction
Seeds of Arabidopsis Col-0 (wild type, WT), mutant lines atbzip62 (AT1G19490: SALK_053908C) and atpyd1-2 (AT3G17810: SALK_083897C) were acquired from the Arabidopsis Biological Resource Center (ABRC) (https://abrc.osu.edu/, accessed on 20 March 2019). All Arabidopsis mutant lines were from a Col-0 genetic background.
Plants were challenged with drought stress at the rosette stage by employing the water withholding mehtod as described earlier by Harb and Pereira [56], with slight modifications. Briefly, soil moisture content (MC) was evaluated by monitoring the weight of each pot for each Arabidopsis genotype (in triplicate) regularly in order to track water loss. The percentage (about 30% soil MC) was calculated as a percentage of the actual weight loss to the initial weight of the saturated soil considered as 100% MC. Leaf samples were collected nine (9) days after water withholding (when symptoms of loss of turgidity and wilting of leaves were apparent). The target genes used in the study for qPCR (real-time quantitative polymerase chain reaction) analysis were selected based on their affinity for NO3 transport in plants. These included AtNPF6.2/NRT1.4 (low-affinity NO3 transporter [6]), AtNPF6.3/NRT1.1 (dual-affinity NO3 transporter [7,8]), AtNRT2.1 and AtNRT2.2 (considered as major components of the high-affinity uptake system) [9,10]. In addition, two genes encoding glutamate synthase were included to investigate their role as key substrates for glutamine synthase during N assimilation.
4.2. In silico Transcritpion Factor Binding Sites Analysis, Protein–Protein Interaction, and KEGG Pathways Analysis
To understand the relationship between the de novo pyrimidine biosynthesis pathway and the N metabolism in plants, the Kyoto Encyclopedia of Genes and Genomes (KEGG) was employed (https://www.genome.jp/kegg/, accessed on 11 September 2021). In addition, we were interested to see how the target NO3 transporters and glutamate synthase would interact with each other. Therefore, we conducted an in silico functional protein association network analysis using the STRING database (https://string-db.org/, accessed on 9 September 2021). Furthermore, the transcription regulation prediction was performed using the PlantRegMap feature within the Plant Transcription Regulatory Map (http://plantregmap.gao-lab.org/binding_site_prediction_result.php, accessed on 9 September 2021). The DNA sequences (coding sequence, CDS, FASTA format: “>ATxGxxxxx” proceeds the sequence) of target genes used for the prediction of binding sites specific to bZIP TFs were downloaded from the Arabidopsis Information Resource database (TAIR, https://www.arabidopsis.org/index.jsp, accessed on 9 September 2021).
4.3. Total RNA Isolation, cDNA Synthesis, and qPCR Analysis
Total RNA was isolated from leaf tissue samples using the TRI-SolutionTM Reagent (Cat. No: TS200-001, Virginia Tech Bio-Technology, Lot: 337871401001, Blacksburg, VA, USA) as described by the manufacturer’s protocol. Thereafter, the complementary DNA (cDNA) was synthesized as described earlier by Mun, et al. [57]. Briefly, 1 µg of RNA was used to synthesize cDNA using BioFACTTM RT-Kit (BioFACTTM, Daejeon, Korea) according to the manufacturer’s protocol. The cDNA was then used as a template in qPCR to study the transcript accumulation of selected genes (Table 3).
Table 3.
Arabidopsis mutant lines and set of qPCR primers for the expression of target genes used in the study.
| Gene Name/ Genotype |
Locus/SALK | 5′-Forwad Primer-3′ | 5′-Reverse Primer-3′ | GC Content (%) F/R | Amplicon Size (bp) |
|---|---|---|---|---|---|
| Genotyping primers (Left border and right border) | |||||
| atbzip62 | SALK_053908C | TGGCACTTTTAACTTTGTGCC | TACGTTTCCATCGAGTGAACC | - | atbzip62 mutant |
| atpyd1-2 | SALK_083897C | TTGGGTGGCAGAACATAGAAC | ATGAATTCAGCGGCATCATAG | - | atpyd1-2 mutant |
| Nitrate transporters and assimilation genes in Arabidopsis | |||||
| AtNPF6.2 | AT2G26690 | TGGAGAGCAAAGGGAGTTGG | AATGAGAGCGGCAGTGATCC | 55.0/55.0 | 102 |
| AtNPF6.3 | AT1G12110 | ATGAAAGGGATGAGCACGGG | CATGGATGAGCTTTCCCGGT | 55.0/55.0 | 110 |
| AtNRT2.1 | AT1G08090 | GGCTACGCATCTGACTTTGC | AACGGCAGTTACAAGGGTGT | 55.0/50.0 | 132 |
| AtNRT2.2 | AT1G08100 | CTCCGTCTCGGGGAGTATCT | TCATGGAGAACACCGTTGGG | 60.0/55.0 | 119 |
| AtGLU1 | AT5G04140 | CTTCTGCATGGGCGACGATA | CCTAAGGGGGTCAATGGCAG | 55.0/60.0 | 118 |
| AtGLU2 | AT2G41220 | GCAGCATTTAGCCAACCGTC | AGGCTCAACCTTCCCAACAG | 55.0/55.0 | 94 |
| AtActin2 | AT3G18780 | CGCTGACCGTATGAGCAAAG | GGAACCACCGATCCAGACAC | 55.0/60.0 | 106 |
The expression of genes was monitored by qPCR under the following conditions: we prepared a reaction mixture composed of SYBR green 2X Master Mix (BioFACT, Daejeon, Korea) along with 100 ng of template DNA and 10 nM of each forward and reverse primers in a final reaction of 20 µL volume. A no-template control (NTC) was used as a control. A 2-step reaction including polymerase activation at 95 °C for 15 min, following denaturation at 95 °C for 5 s, annealing and extension at 65 °C for 30 s was performed in a real-time PCR machine (Eco™ Illumina). The number of total reaction cycles was 40. Prior to assessing the changes in the transcript accumulation of the target genes, their relative expression values were normalized to that of the housekeeping gene AtActin2.
4.4. Statistical Analysis
All the experiments were conducted using a CRD design. The data were collected in triplicate and analyzed statistically with GraphPad Prism software (Version 7.00, ©1992–2016 GraphPad Software, Inc., San Diego, CA, USA). The analysis of variance (ANOVA) for Completely Randomized Design was performed, and the Turkey’s multiple comparisons test was employed at a significance level of 0.05. To assess the statistical significance level of the observed changes in the expression of target genes between Arabidopsis genotypes, we compared the normalized relative expression values in the mutant lines atbzip62 or atpyd1-2 with those recorded in Col-0 upon drought stress.
5. Conclusions
A growing interest in plant biosciences-related studies in investigating nitrogen (N) metabolism in response to abiotic stress has marked the last two decades. The use of N in Agriculture is indispensable for efficient food production and quality. However, under abiotic stress conditions, N availability to plants is compromised, which may lead to crop failure.
This study investigated the role of AtbZIP62 encoding transcription factor and AtPYD1 in the regulation of genes encoding low-, dual- and high-affinity NO3 transporters as well as glutamate synthase in response to drought stress. The results revealed that the transcript accumulation of AtNPF6.2/NRT1.4, AtNPF6.3/NRT1.1, and AtNRT2.2 decreased significantly in atbzip62 and atpyd1-2 compared to Col-0 (WT). Meanwhile, AtNRT2.1 showed a similar expression pattern in Col-0 and atbzip62, while having an opposite pattern in atpyd1-2. In the same way, glutamate synthase-encoding genes AtGLU1 and AtGU2 were differentially regulated by drought stress, with AtGLU2 being upregulated. Of all NO3 transporter-related genes, AtNRT2.2, which exhibited the highest transcript accumulation level, while being differentially regulated between Col-0 and atbzip62, may prevail over other test genes under drought stress, under the regulatory influence of AtbZIP62 TF. Furthermore, AtbZIP62 may require AtbZIP18 and/or AtbZIP69 to regulate the expression of the analyzed NO3 transporters under drought stress conditions. Future studies using NO3 transporter-specific mutant lines, coupled with advanced physiological, biochemical and molecular analyses would help to elucidate the mechanism underlying NO3 transport during abiotic stress to optimize the nitrogen use efficiency in plants.
Author Contributions
Conceptualization, B.-W.Y.; methodology, B.-W.Y. and N.K.R.; software, N.K.R.; validation, B.-W.Y.; formal analysis, N.K.R.; investigation, N.K.R.; resources, B.-W.Y.; data curation, N.K.R.; writing—original draft preparation, N.K.R.; writing—review and editing, B.-W.Y.; visualization, B.-W.Y.; supervision, B.-W.Y.; project administration, B.-W.Y.; funding acquisition, B.-W.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant number 2020R1I1A3073247), Republic of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Krapp A. Plant nitrogen assimilation and its regulation: A complex puzzle with missing pieces. Curr. Opin. Plant Biol. 2015;25:115–122. doi: 10.1016/j.pbi.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Lea P.J. Primary nitrogen metabolism. Plant Biochem. 1997;7:273–313. [Google Scholar]
- 3.Forde B.G. Nitrate transporters in plants: Structure, function and regulation. Biochim. Bioph. Acta Biom. 2000;1465:219–235. doi: 10.1016/S0005-2736(00)00140-1. [DOI] [PubMed] [Google Scholar]
- 4.Fan X., Naz M., Fan X., Xuan W., Miller A.J., Xu G. Plant nitrate transporters: From gene function to application. J. Exp. Bot. 2017;68:2463–2475. doi: 10.1093/jxb/erx011. [DOI] [PubMed] [Google Scholar]
- 5.Krapp A., David L.C., Chardin C., Girin T., Marmagne A., Leprince A.-S., Chaillou S., Ferrario-Méry S., Meyer C., Daniel-Vedele F. Nitrate transport and signalling in Arabidopsis. J. Exp. Bot. 2014;65:789–798. doi: 10.1093/jxb/eru001. [DOI] [PubMed] [Google Scholar]
- 6.Huang N.-C., Liu K.-H., Lo H.-J., Tsay Y.-F. Cloning and functional characterization of an Arabidopsis nitrate transporter gene that encodes a constitutive component of low-affinity uptake. Plant Cell. 1999;11:1381–1392. doi: 10.1105/tpc.11.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsay Y.-F., Schroeder J.I., Feldmann K.A., Crawford N.M.J.C. The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell. 1993;72:705–713. doi: 10.1016/0092-8674(93)90399-B. [DOI] [PubMed] [Google Scholar]
- 8.Liu K.-H., Huang C.-Y., Tsay Y.-F. CHL1 is a dual-affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate uptake. Plant Cell. 1999;11:865–874. doi: 10.1105/tpc.11.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filleur S., Dorbe M.-F., Cerezo M., Orsel M., Granier F., Gojon A., Daniel-Vedele F. An Arabidopsis T-DNA mutant affected in Nrt2 genes is impaired in nitrate uptake. FEBS Lett. 2001;489:220–224. doi: 10.1016/S0014-5793(01)02096-8. [DOI] [PubMed] [Google Scholar]
- 10.Cerezo M., Tillard P., Filleur S., Munos S., Daniel-Vedele F., Gojon A. Major alterations of the regulation of root NO3− uptake are associated with the mutation of Nrt2.1 and Nrt2.2 genes in Arabidopsis. Plant Physiol. 2001;127:262–271. doi: 10.1104/pp.127.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Léran S., Varala K., Boyer J.-C., Chiurazzi M., Crawford N., Daniel-Vedele F., David L., Dickstein R., Fernandez E., Forde B., et al. A unified nomenclature of NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family members in plants. Trends Plant Sci. 2014;19:5–9. doi: 10.1016/j.tplants.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Scheurwater I., Koren M., Lambers H., Atkin O. The contribution of roots and shoots to whole plant nitrate reduction in fast-and slow-growing grass species. J. Exp. Bot. 2002;53:1635–1642. doi: 10.1093/jxb/erf008. [DOI] [PubMed] [Google Scholar]
- 13.Stewart G.R., Popp M., Holzapfel I., Stewart J.A., Dickie-Eskew A. Localization of nitrate reduction in ferns and its relationship to environment and physiological characteristics. New Phytol. 1986;104:373–384. doi: 10.1111/j.1469-8137.1986.tb02905.x. [DOI] [Google Scholar]
- 14.Wang X., Cai X., Xu C., Wang Q., Dai S. Drought-responsive mechanisms in plant leaves revealed by proteomics. Int. J. Mol. Sci. 2016;17:1706. doi: 10.3390/ijms17101706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tcherkez G., Hodges M. How stable isotopes may help to elucidate primary nitrogen metabolism and its interaction with (photo) respiration in C3 leaves. J. Exp. Bot. 2007;59:1685–1693. doi: 10.1093/jxb/erm115. [DOI] [PubMed] [Google Scholar]
- 16.Lea P.J., Miflin B.J. Glutamate synthase and the synthesis of glutamate in plants. Plant Physiol. Biochem. 2003;41:555–564. doi: 10.1016/S0981-9428(03)00060-3. [DOI] [Google Scholar]
- 17.Kirkby E.A., Knight A.H. Influence of the level of nitrate nutrition on ion uptake and assimilation, organic acid accumulation, and cation-anion balance in whole tomato plants. Plant Physiol. 1977;60:349–353. doi: 10.1104/pp.60.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mundim F.M., Pringle E.G. Whole-plant metabolic allocation under water stress. Front. Plant Sci. 2018;9:852. doi: 10.3389/fpls.2018.00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xuan W., Beeckman T., Xu G. Plant nitrogen nutrition: Sensing and signaling. Curr. Opin. Plant Biol. 2017;39:57–65. doi: 10.1016/j.pbi.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Ashoub A., Baeumlisberger M., Neupaertl M., Karas M., Brüggemann W. Characterization of common and distinctive adjustments of wild barley leaf proteome under drought acclimation, heat stress and their combination. Plant Mol. Biol. 2015;87:459–471. doi: 10.1007/s11103-015-0291-4. [DOI] [PubMed] [Google Scholar]
- 21.Wendelboe-Nelson C., Morris P.C. Proteins linked to drought tolerance revealed by DIGE analysis of drought resistant and susceptible barley varieties. Proteomics. 2012;12:3374–3385. doi: 10.1002/pmic.201200154. [DOI] [PubMed] [Google Scholar]
- 22.Alberts B., Johnson A., Lewis J., Raff M., Roberts K., Walter P. Molecular Biology of the Cell 4th edn (New York: Garland Science) Ann. Bot. 2002;91:401. [Google Scholar]
- 23.Watson D.K., Kitching R., Vary C., Kola I., Seth A. Transcription Factor Protocols. Springer; Berlin/Heidelberg, Gemany: 2000. Isolation of target gene promoter/enhancer sequences by whole genome PCR method; pp. 1–11. [Google Scholar]
- 24.Perry S.E. Plant Transcription Factors: Methods and Protocols. Humana Press; Totowa, NJ, USA: 2011. [Google Scholar]
- 25.Ali Z., Sarwat S.S., Karim I., Faridi R., Jaskani M.J., Khan A.A. Functions of plant’s bZIP transcription factors. Pak. J. Agric. Sci. 2016;53:303–314. [Google Scholar]
- 26.Rolly N.K., Imran Q.M., Shahid M., Imran M., Khan M., Lee S.-U., Hussain A., Lee I.-J., Yun B.-W. Drought-induced AtbZIP62 transcription factor regulates drought stress response in Arabidopsis. Plant Physiol. Biochem. 2020;156:384–395. doi: 10.1016/j.plaphy.2020.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Rolly N.K., Imran Q.M., Lee I.-J., Yun B.-W. Salinity stress-mediated suppression of expression of salt overly sensitive signaling pathway genes suggests negative regulation by AtbZIP62 transcription factor in Arabidopsis thaliana. Int. J. Mol. Sci. 2020;21:1726. doi: 10.3390/ijms21051726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa-Broseta Á., Castillo M., León J. Nitrite reductase 1 is a target of nitric oxide-mediated post-translational modifications and controls nitrogen flux and growth in Arabidopsis. Int. J. Mol. Sci. 2020;21:7270. doi: 10.3390/ijms21197270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neill S., Barros R., Bright J., Desikan R., Hancock J., Harrison J., Morris P., Ribeiro D., Wilson I. Nitric oxide, stomatal closure, and abiotic stress. J. Exp. Bot. 2008;59:165–176. doi: 10.1093/jxb/erm293. [DOI] [PubMed] [Google Scholar]
- 30.Ma H., Zhao J., Feng S., Qiao K., Gong S., Wang J., Zhou A. Heterologous expression of nitrate assimilation related-protein DsNAR2.1/NRT3.1 affects uptake of nitrate and ammonium in nitrogen-starved Arabidopsis. Int. J. Mol. Sci. 2020;21:4027. doi: 10.3390/ijms21114027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang X.Z., Fang S.Q., Ye Z.Q., Liu D., Zhao K.L., Jin C.W. NRT1.1 Dual-Affinity Nitrate Transport/Signalling and its Roles in Plant Abiotic Stress Resistance. Front. Plant Sci. 2021:1817. doi: 10.3389/fpls.2021.715694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Babst B.A., Gao F., Acosta-Gamboa L.M., Karve A., Schueller M.J., Lorence A. Three NPF genes in Arabidopsis are necessary for normal nitrogen cycling under low nitrogen stress. Plant Physiol. Biochem. 2019;143:1–10. doi: 10.1016/j.plaphy.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe S., Takahashi N., Kanno Y., Suzuki H., Aoi Y., Takeda-Kamiya N., Toyooka K., Kasahara H., Hayashi K.-i., Umeda M., et al. The Arabidopsis NRT1/PTR FAMILY protein NPF7.3/NRT1.5 is an indole-3-butyric acid transporter involved in root gravitropism. Proc. Natl. Acad. Sci. USA. 2020;117:31500–31509. doi: 10.1073/pnas.2013305117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W., Sun Q., Wang K., Du Q., Li W.X. Nitrogen Limitation Adaptation (NLA) is involved in source-to-sink remobilization of nitrate by mediating the degradation of NRT 1.7 in Arabidopsis. New Phytol. 2017;214:734–744. doi: 10.1111/nph.14396. [DOI] [PubMed] [Google Scholar]
- 35.Xu G., Fan X., Miller A.J. Plant nitrogen assimilation and use efficiency. Ann. Rev. Plant Biol. 2012;63:153–182. doi: 10.1146/annurev-arplant-042811-105532. [DOI] [PubMed] [Google Scholar]
- 36.Nadelhoffer K.J., Aber J.D., Melillo J.M. Seasonal patterns of ammonium and nitrate uptake in nine temperate forest ecosystems. Plant Soil. 1984;80:321–335. doi: 10.1007/BF02140039. [DOI] [Google Scholar]
- 37.Sorgona A., Lupini A., Mercati F., Di Dio L., Sunseri F., Abenavoli M.R. Nitrate uptake along the maize primary root: An integrated physiological and molecular approach. Plant Cell Environ. 2011;34:1127–1140. doi: 10.1111/j.1365-3040.2011.02311.x. [DOI] [PubMed] [Google Scholar]
- 38.Tischner R. Nitrate uptake and reduction in higher and lower plants. Plant Cell Environ. 2000;23:1005–1024. doi: 10.1046/j.1365-3040.2000.00595.x. [DOI] [Google Scholar]
- 39.Zhang G.-B., Meng S., Gong J.-M. The expected and unexpected roles of nitrate transporters in plant abiotic stress resistance and their regulation. Int. J. Mol. Sci. 2018;19:3535. doi: 10.3390/ijms19113535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan S.-C., Lin C.-S., Hsu P.-K., Lin S.-H., Tsay Y.-F. The Arabidopsis nitrate transporter NRT1.7, expressed in phloem, is responsible for source-to-sink remobilization of nitrate. Plant Cell. 2009;21:2750–2761. doi: 10.1105/tpc.109.067603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu B., Wang W., Ou S., Tang J., Li H., Che R., Zhang Z., Chai X., Wang H., Wang Y., et al. Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat. Genet. 2015;47:834–838. doi: 10.1038/ng.3337. [DOI] [PubMed] [Google Scholar]
- 42.Kiba T., Krapp A.J.P., Physiology C. Plant nitrogen acquisition under low availability: Regulation of uptake and root architecture. Plant Cell Physiol. 2016;57:707–714. doi: 10.1093/pcp/pcw052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiba T., Feria-Bourrellier A.-B., Lafouge F., Lezhneva L., Boutet-Mercey S., Orsel M., Bréhaut V., Miller A., Daniel-Vedele F., Sakakibara H., et al. The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants. Plant Cell. 2012;24:245–258. doi: 10.1105/tpc.111.092221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J.-Y., Fu Y.-L., Pike S.M., Bao J., Tian W., Zhang Y., Chen C.-Z., Zhang Y., Li H.-M., Huang J., et al. The Arabidopsis nitrate transporter NRT1.8 functions in nitrate removal from the xylem sap and mediates cadmium tolerance. Plant Cell. 2010;22:1633–1646. doi: 10.1105/tpc.110.075242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen C.-Z., Lv X.-F., Li J.-Y., Yi H.-Y., Gong J.-M. Arabidopsis NRT1.5 is another essential component in the regulation of nitrate reallocation and stress tolerance. Plant Physiol. 2012;159:1582–1590. doi: 10.1104/pp.112.199257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Léran S., Muños S., Brachet C., Tillard P., Gojon A., Lacombe B. Arabidopsis NRT1.1 is a bidirectional transporter involved in root-to-shoot nitrate translocation. Mol. Plant. 2013;6:1984–1987. doi: 10.1093/mp/sst068. [DOI] [PubMed] [Google Scholar]
- 47.Wang W., Hu B., Yuan D., Liu Y., Che R., Hu Y., Ou S., Liu Y., Zhang Z., Wang H., et al. Expression of the nitrate transporter gene OsNRT1.1A/OsNPF6.3 confers high yield and early maturation in rice. Plant Cell. 2018;30:638–651. doi: 10.1105/tpc.17.00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ho C.-H., Lin S.-H., Hu H.-C., Tsay Y.-F. CHL1 functions as a nitrate sensor in plants. Cell. 2009;138:1184–1194. doi: 10.1016/j.cell.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Krouk G., Lacombe B., Bielach A., Perrine-Walker F., Malinska K., Mounier E., Hoyerova K., Tillard P., Leon S., Ljung K., et al. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev. Cell. 2010;18:927–937. doi: 10.1016/j.devcel.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 50.Bouguyon E., Gojon A., Nacry P. Seminars in Cell & Developmental Biology. Volume 23. Academic Press; London, UK: 2012. Nitrate sensing and signaling in plants; pp. 648–654. [DOI] [PubMed] [Google Scholar]
- 51.Mounier E., Pervent M., Ljung K., Gojon A., Nacry P. Auxin-mediated nitrate signalling by NRT 1.1 participates in the adaptive response of A rabidopsis root architecture to the spatial heterogeneity of nitrate availability. Plant Cell Environ. 2014;37:162–174. doi: 10.1111/pce.12143. [DOI] [PubMed] [Google Scholar]
- 52.Remans T., Nacry P., Pervent M., Filleur S., Diatloff E., Mounier E., Tillard P., Forde B.G., Gojon A. The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches. Plant Cell Environ. 2006;103:19206–19211. doi: 10.1073/pnas.0605275103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhong L., Chen D., Min D., Li W., Xu Z., Zhou Y., Li L., Chen M., Ma Y. AtTGA4, a bZIP transcription factor, confers drought resistance by enhancing nitrate transport and assimilation in Arabidopsis thaliana. Biochem. Bioph. Res. Commun. 2015;457:433–439. doi: 10.1016/j.bbrc.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Temple S.J., Vance C.P., Gantt J.S. Glutamate synthase and nitrogen assimilation. Trends Plant Sci. 1998;3:51–56. doi: 10.1016/S1360-1385(97)01159-X. [DOI] [Google Scholar]
- 55.Charlier D., Le Minh P.N., Roovers M. Regulation of carbamoylphosphate synthesis in Escherichia coli: An amazing metabolite at the crossroad of arginine and pyrimidine biosynthesis. Amino Acids. 2018;50:1647–1661. doi: 10.1007/s00726-018-2654-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harb A., Pereira A. Plant Reverse Genetics. Springer; Berlin/Heidlelberg, Germany: 2011. Screening Arabidopsis genotypes for drought stress resistance; pp. 191–198. [DOI] [PubMed] [Google Scholar]
- 57.Mun B.-G., Lee S.-U., Hussain A., Kim H.-H., Rolly N.K., Jung K.-H., Yun B.-W. S-nitrosocysteine-responsive genes modulate diverse regulatory pathways in Oryza sativa: A transcriptome profiling study. Funct. Plant Biol. 2018;45:630–644. doi: 10.1071/FP17249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not Applicable.