Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a pandemic as of March 2020, creating a global crisis and claiming millions of lives. To halt the pandemic and alleviate its impact on society, economy, and public health, the development of vaccines and antiviral agents against SARS-CoV-2 was a dire need. To date, various platforms have been utilized for SARS-CoV-2 vaccine development, and over 200 vaccine candidates have been produced, many of which have obtained the United States Food and Drug Administration (FDA) approval for emergency use. Despite this successful development and licensure, concerns regarding the safety and efficacy of these vaccines have arisen, given the unprecedented speed of vaccine development and the newly emerging SARS-CoV-2 strains and variants. In this review, we summarize the different platforms used for Coronavirus Disease 2019 (COVID-19) vaccine development, discuss their strengths and limitations, and highlight the major safety concerns and potential risks associated with each vaccine type.
Keywords: SARS-CoV-2, COVID-19, vaccine platforms, challenges, safety, strengths, limitations
1. Introduction
The coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first reported in late 2019 in Wuhan, the capital of Hubei province in Central China, has become a global pandemic with devastating effects worldwide [1]. Since then, and until 29 June 2021, this newly emerging disease caused by the enveloped SARS-CoV-2 virus, which belongs to the Coronaviridae family and the lineage B of the betacoronavirus (β-CoV) genera, has brought over 181 million confirmed cases and claimed the lives of about 4 million people worldwide [1]. SARS-CoV-2 has a positive-sense, single-stranded genome that encodes a large non-structural polyprotein (ORF1a/b) proteolytically cleaved to generate proteins, four of which are structural proteins including spike (S), envelope (E), membrane (M), and nucleocapsid (N) (Figure 1a) [2,3]. Among these proteins, the S surface glycoprotein plays a critical role in receptor recognition and attachment to host cells [4]. The S protein also induces T-cell responses and is the main target of highly potent neutralizing antibodies (nAbs) against the virus, presenting it as the major antigenic pick out for vaccine design [5]. The structure of SARS-CoV-2 is similar to other β-CoVs, including the severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle Eastern respiratory syndrome-related coronavirus (MERS-CoV), the causative agents of SARS and MERS, two previously reported viral pneumonia disease outbreaks, respectively [6]. Compared to SARS-CoV and MERS-CoV; however, SARS-CoV-2 has higher infectivity and transmissibility due to its high-affinity binding to the host cell receptors and high viral shedding levels during the early stage of infection, contributing to the vastly infectious nature of asymptomatic and mildly symptomatic patients [7,8,9]. As initial measures to control the disease spread, the COVID-19 pandemic was primarily withstood through social distancing, hygiene measures, and repurposed drugs [10]. Some countries’ implemented measures were relatively emollient and particularly designated to control the disease by achieving herd immunity following natural infection [11,12].
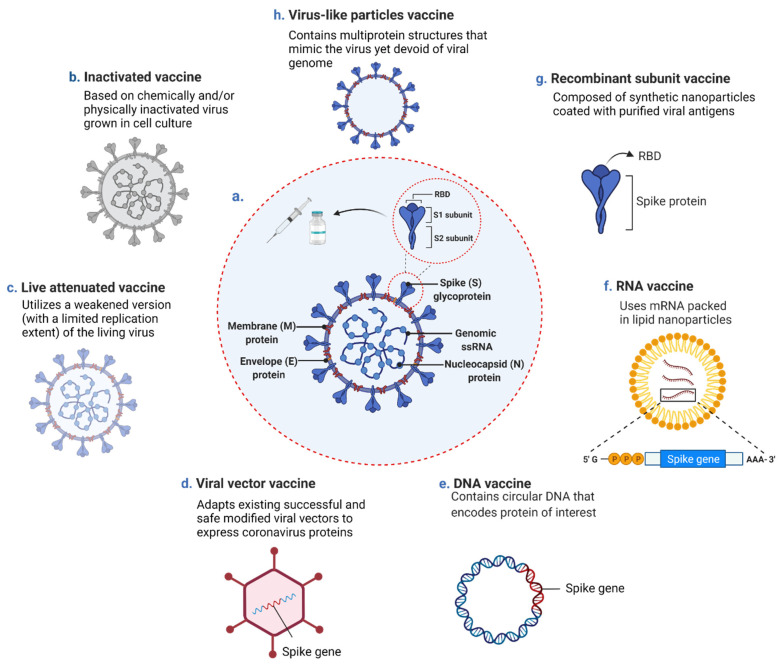
Figure 1.
SARS-CoV-2 structure and contemporary COVID-19 vaccine platforms. (a) Schematic diagram of SARS-CoV-2 structure including the single-stranded RNA (ssRNA) genome and the four structural proteins: spike protein (S), envelope protein (E), membrane protein (M), and nucleocaspid protein (N). Diverse vaccine platforms including (b) inactivated vaccine (c) live attenuated vaccine (d) viral vector vaccine (e) DNA vaccine (f) RNA vaccine (g) recombinant subunit vaccine (h) virus-like particles vaccine. mRNA: messenger RNA, RBD: receptor-binding domain. The diagram was created with BioRender.com.
Therefore, despite the taken measures and as a consequence of not implementing immediate lockdown, the COVID-19 death toll increased [13,14]. This necessitated the development of an effective and safe vaccine as an imperative solution to control the pandemic and prevent future outbreaks [13,15]. As such, and since the release of the SARS-CoV-2 genome sequence in January 2020, all efforts have been directed towards COVID-19 vaccines development [16,17]. The hope and hype placed on vaccines to prevail over the disease stand up from the success of previously developed vaccines to control other infectious diseases [13]. The route for vaccine development; however, was not always paved, and several historical attempts of vaccines production were doomed with defeats [18]. Until today, and despite all the knowledge and technology at one’s disposal, scientists are still unable to conclude the safest and most effective vaccine platform [18]. Back in time, particularly following the outbreak of SARS-CoV in 2002, vaccines against the emerging virus were also developed, a few of which reached phase I clinical trials; yet, did not achieve the final stages and obtain the United States Food and Drug Administration (FDA) approval as the virus was eradicated from the human population in 2004 [16,19,20,21]. Similarly, several vaccines against MERS-CoV were under development, none of which have obtained FDA approved thus far [21]. Within the same notion, and in relay for safe and effective COVID-19 vaccine production, censorious steps are currently followed in all phases of COVID-19 vaccine development, including manufacturing, dispersal, and vaccination [22]. For the time being, many of the newly developed COVID-19 vaccines are undergoing clinical evaluation and have reached phase III of clinical trials. A few of which have been approved for emergency use [13] (Figure 2a, Table 1), with the research and discovery phase being skipped [21,23]. Several approaches, including traditional platforms (inactivated and live attenuated virus vaccines), and newly established ones (replicating and non-replicating viral vector vaccines, nucleic acid (DNA and RNA) vaccines, recombinant subunit vaccines, and peptide-based/virus-like particles vaccines), have been adopted for COVID-19 vaccine development (Figure 1b–h) [16,24,25]. As of 29 June 2021, and according to the World Health Organization (WHO), out of the 293 total COVID-19 vaccine candidates, 105 are currently in the clinical phase of development and 184 are still in the pre-clinical phase (Figure 2a) [26].Presently, and besides the FDA consideration of the possibility of booster vaccine shots, several standpoints are now advocating the notion that “hybrid immunity” and “the mix and match of different vaccines strategy” could provide an even stronger immune boost, presenting such approaches, if supported by data, as plausible pandemic game-changers. In this review, we detail the different COVID-19 vaccine platforms and highlight their strengths, limitations, and major risks and safety concerns associated with each type, particularly those relevant to the fast-track pace taken for their production. We also summarize all candidate COVID-19 vaccines currently in the clinical phase of development and categorize them according to the platform used for their development.
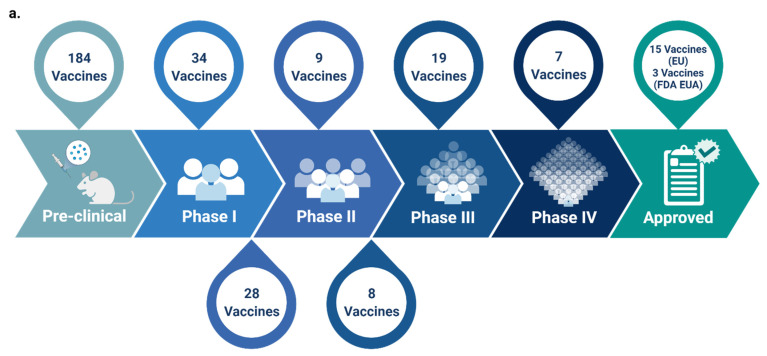
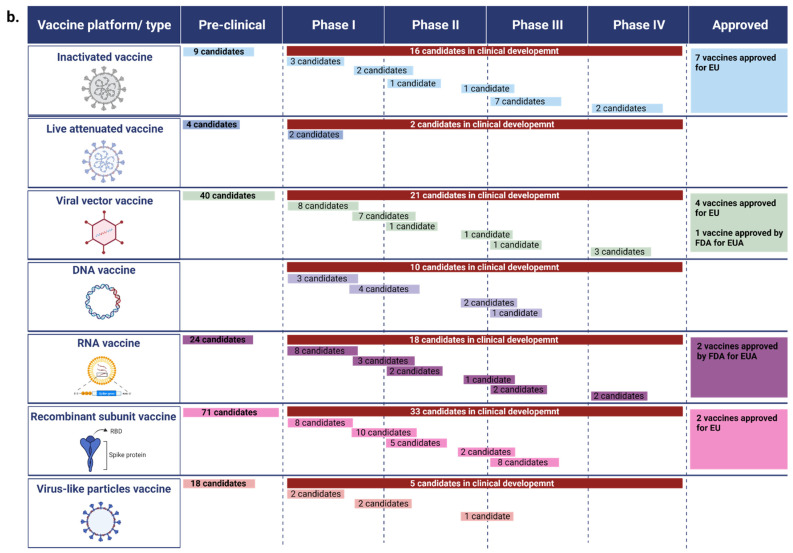
Figure 2.
Phases of COVID-19 vaccine development. (a) The total number of currently available COVID-19 vaccine candidates in the pre-clinical and clinical phases of development. (b) Number of developed COVID-19 vaccine candidates per vaccine platform. Data was retrieved from the World Health Organization (WHO) vaccine tracker and landscape website [26] on 29 June 2021. N.B. The reported numbers are subject to change with time given the current efforts and pace of COVID-19 vaccines development. EU: emergency use, FDA: food and drug administration, EUA: emergency use authorization. The figure and tablewere created with BioRender.com.
Table 1.
SARS-CoV-2 Vaccine Candidates in Clinical Development Stages.
| Platform/Vaccine Type | No. | Vaccine Name | Number of Doses (Dosage) | Dosing Schedule | Route of Administration | Developer/Manufacturer | Construct and/or Targeted SARS-CoV-2 Protein | Current Stage of Clinical Trial (Recruitment Status) | Efficacy * | Current Approvals/Authorizations | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inactivated virus | 1 | CoronaVac | 2 doses (3 μg) | Day 0 + 14 | IM | Sinovac Research and Development Co., Ltd. | Whole inactivated SARS-CoV-2 with aluminum hydroxide adjuvant | Phase IV (Not yet recruiting) |
Efficacy from clinical trials: Brazil: 50.7% against symptomatic disease ≥14 d after 2 doses. Turkey: 83.5% against symptomatic disease ≥14 d after 2 doses. Indonesia: 65.3% against symptomatic disease ≥14 d after 2 doses. Efficacy/effectiveness against variants: Chile (predominant circulation of P.1 and B.1.1.7.): 67% against symptomatic disease ≥28 d after 2 doses. Brazil (predominant circulation of P.2 and P.1 lineages): 50.7% and 36.8% against symptomatic disease ≥14 d after 2 doses, respectively. |
WHO EUL Approved in 37 countries 1 |
[26,36,37,38,109,110,111,112] |
| Inactivated virus | 2 | BBIBP-CorV | 2 doses (4 μg) | Day 0 + 21 | IM | Sinopharm + China National Biotec Group Co + Beijing Institute of Biological Products | Whole inactivated SARS-CoV-2 | Phase IV (Recruiting) | Efficacy from clinical trials in UAE, Bahrain, Egypt, and Jordan: 78.1% against symptomatic disease ≥14 d after 2 doses, and 79% against hospitalization. | WHO EUL Approved in 56 countries 2 |
[26,34,39,41,110,113] |
| Inactivated virus | 3 | Inactivated SARS-CoV-2 vaccine (Vero cell) | 2–3 doses (5 μg) | Day 0 + 21 + 42 or 111 or 171 | IM | Sinopharm + China National Biotec Group Co + Wuhan Institute of Biological Products | Whole inactivated SARS-CoV-2 with aluminum hydroxide adjuvant | Phase III (Completed) | Efficacy from clinical trials in UAE, Bahrain, Egypt, and Jordan: 72.8% against symptomatic disease ≥14 d after 2 doses, and 79% against hospitalization. | WHO EUL (Approval pending) China |
[26,40,110,114,115] |
| Inactivated virus | 4 | Inactivated SARS-CoV-2 vaccine (Vero cell) | 2 doses (50, 100, or 150 EU) | Day 0 + 14 | IM | Institute of Medical Biology + Chinese Academy of Medical Sciences | Whole inactivated SARS-CoV-2 with Al(OH)3 adjuvant | Phase III (Enrolling by invitation) | NR | Not yet approved in any country | [26,116,117] |
| Inactivated virus | 5 | QazCovid-in | 2 doses | Day 0 + 21 | IM | Research Institute for Biological Safety Problems, Rep of Kazakhstan | Whole inactivated SARS-CoV-2 | Phase III (Active, not recruiting) | Efficacy from clinical trials in the Republic of Kazakhstan: 96% | Republic of Kazakhstan | [26,118,119] |
| Inactivated virus | 6 | BBV152 (COVAXIN) | 2 doses (3 or 6 μg) | Day 0 + 14 | IM | Bharat Biotech International Limited | Whole inactivated SARS-CoV-2 with Algel-IMDG adjuvant | Phase III (Active, not recruiting) |
Efficacy from clinical trials: 77.8% against symptomatic disease, 93.4% against severe disease, 63.6% against asymptomatic disease. Efficacy/effectiveness against variants: 65.2% against disease caused by Delta (B.617.2) variant. |
WHO EUL (Approval pending) Approved in 9 countries 3 |
[26,110,120,121,122,123] |
| Inactivated virus | 7 | Inactivated SARS-CoV-2 vaccine (Vero cell) | 2 doses | Day 0 + 28 | IM | Shenzhen Kangtai Biological Products Co., Ltd. | Whole inactivated SARS-CoV-2 | Phase III (Not yet recruiting) | NR | China | [26,124] |
| Inactivated virus | 8 | VLA2001 | 2 doses | Day 0 + 21 | IM | Valneva, National Institute for Health Research, United Kingdom | Whole inactivated SARS-CoV-2 with high S-protein density, in combination with two adjuvants, alum and CpG 1018 | Phase III (Not yet recruiting) | NR | Not yet approved in any country | [26,125] |
| Inactivated virus | 9 | ERUCOV-VAC (TURKOVAC) | 2 doses (3 μg) | Day 0 + 28 | IM | Erciyes University + Health Institutes of Turkey | Whole inactivated SARS-CoV-2 | Phase III (Recruiting) | NR | Not yet approved in any country | [26,126] |
| Inactivated virus | 10 | COVID-19 inactivated vaccine | 2 doses (5 μg) | Day 0 + 28 | IM | Shifa Pharmed Industrial Co | Whole inactivated SARS-CoV-2 | Phase II–III (Recruitment complete) | NR | Iran | [26,127] |
| Inactivated virus | 11 | FAKHRAVAC (MIVAC) | 2 doses (10 µg) | Day 0 + 14 | IM | Organization of Defensive Innovation and Research | Whole inactivated SARS-CoV-2 | Phase II (Recruiting) | NR | Not yet approved in any country | [26,128] |
| Inactivated virus | 12 | Inactivated (NDV-based) chimeric vaccine | 2 doses | Day 0 + 28 | IM | The Government Pharmaceutical Organization (GPO) + PATH + Dynavax | Whole inactivated NDV chimera stably expressing membrane-anchored SARS-CoV-2 S protein +/− CpG 1018 adjuvant | Phase I–II (NR) | NR | Not yet approved in any country | [26,129] |
| Inactivated virus | 13 | KD-414 | 2 doses | Day 0 + 28 | IM | KM Biologics Co., Ltd. | Whole inactivated SARS-CoV-2 | Phase I–II (Not Recruiting) | NR | Not yet approved in any country | [26,130] |
| Inactivated virus | 14 | Koçak-19 | 2 doses (4 or 6 µg) | Day 0 + 21 | IM | Kocak Farma, Turkey | Whole inactivated SARS-CoV-2 with adjuvant | Phase I (Recruiting) | NR | Not yet approved in any country | [26,131] |
| Inactivated virus | 15 | Adjuvanted inactivated vaccine | 2 doses (10 µg-3M or 20 µg-6M) | Day 0 + 20 | SC | The Scientific and Technological Research Council of Turkey (TÜBITAK) | Whole inactivated SARS-CoV-2 with CpG ODN adjuvant | Phase I (Recruiting) | NR | Not yet approved in any country | [26,132] |
| Inactivated virus | 16 | Live recombinant (rNDV) vector vaccine | 2 doses | Day 0 + 21 | IM or IN | Laboratorio Avi-Mex | Live recombinant NDV vector expressing SARS-CoV-2 S protein | Phase I (Recruiting) | NR | Not yet approved in any country | [26,133] |
| Live-attenuated virus | 1 | COVI-VAC | 1–2 doses | Day 0 or Day 0 + 28 | IN | Codagenix, Inc + Serum Institute of India | Whole SARS-CoV-2 with all viral proteins | Phase I (Active, not recruiting) | NR | Not yet approved in any country | [26,134] |
| Live-attenuated virus | 2 | MV-014-212 | 1 dose | Day 0 | IN | Meissa Vaccines, Inc. | RSV expressing SARS-CoV-2 S protein | Phase I (Recruiting) | NR | Not yet approved in any country | [26,55,135] |
| Viral vector (non-replicating) | 1 | ChAdO x 1 AZD1222 | 2 doses (standard dose: 5 × 1010 viral particles, low dose: 2.2 × 1010 viral particles) | Day 0 + 28 | IM | AstraZeneca + University of Oxford | Chimpanzee adenovirus-vectored vaccine (ChAdOx1) expressing S protein | Phase IV (Recruiting) |
Efficacy from clinical trials in UK, Brazil, and South Africa: 66.7%–70.4% overall efficacy ≥14 d after 2 doses, 62.1% after 2 standard doses76.0% after single low dose within 20–90 d, 90.0% after one low dose and one standard dose. Real-world effectiveness: England: 60–75% after 1 dose. Scotland: 88% against hospitalization 28–34 d after 1 dose. U.S: 76% in adults, and 85% in elderly (≥65 y). Efficacy/effectiveness against variants: UK: 70.4% against Alpha (B.1.1.7) variant, 81.5% against non-B.1.1.7 lineages. South Africa: 10.4% against Beta (B.1.351) variant. England: 76.0% after 1 dose, 86.0% after 2 doses against Beta variant. 71.0% after 1 dose, 92.0% after 2 doses against Delta variant. Canada: 68% ≥ 14 d after dose 1 against symptomatic infection caused by Alpha variant. 48% ≥ 14 d after 1 dose against symptomatic infection caused by Beta or Gamma (P.1) variants. 67% ≥ 14 d after 1 dose against symptomatic infection caused by Delta variant. |
WHO EUL Approved in 118 countries 4 and issued an Endorsed by ART CARPHA EU recommendation EMA approved |
[67,72,73,74,93,110,136,137,138,139,140,141,142,143,144,145] |
| Viral vector (non-replicating) | 2 | Convidicea (Ad5-nCoV) | 1 dose (5 × 1010 viral particles per dose) | Day 0 | IM | CanSino Biological Inc. + Beijing Institute of Biotechnology | Recombinant replication-defective human type 5 adenovirus (Ad5) expressing S protein | Phase IV (Active, not recruiting) | Efficacy from clinical trials in Pakistan, Russia, Argentina, Mexico, and Chile: 68.8% and 65.7% against symptomatic disease ≥14 d and ≥28 d after vaccination, respectively. 95.5% and 91.0% against severe disease ≥14 d and ≥28 d after vaccination, respectively. | WHO EUL (Approval pending) Approved in 8 countries 5 |
[26,110,146,147,148,149,150,151] |
| Viral vector (non-replicating) | 3 | Ad26.COV2.S | 1 dose (5 × 1010 viral particles per dose) | Day 0 | IM | Janssen Pharmaceutical | Recombinant replication-incompetent adenovirus serotype 26 (Ad26) vector encoding full-length and stabilized S protein | Phase IV (NR) |
Efficacy from clinical trials in Argentina, Brazil, Chile, Colombia, Mexico, Peru, South Africa, and the U.S: 66.3-76.3% and 65.5-83.5% against moderate to severe/critical disease ≥14 d and ≥28d after vaccination, respectively. Real-world efficacy: U.S. and India: 76.7% against infection ≥14 d after vaccination. Efficacy/effectiveness against variants: South Africa (95% predominant B.1.351 variant): 52.0–73.1% and 64.0–81.7% against moderate to severe/critical disease ≥14 d and ≥28 d after vaccination, respectively. Brazil (69% predominant P.2 lineages): 66.2–68.1% and 81.9–87.6% against moderate to severe/critical disease ≥14 d and ≥28 d after vaccination, respectively. |
FDA EUA WHO EUL Approved in 55 countries 6 Endorsed by ART EMA approved |
[26,69,71,110,145,152,153] |
| Viral vector (non-replicating) | 4 | Gam-COVID-Vac (Sputnik V) | 2 doses (1 × 1011 viral particles per dose) | Day 0 + 21 (first: rAd26-S; second: rAd5-S) | IM | Gamaleya Research Institute + Health Ministry of the Russian Federation | Recombinant Ad26 and recombinant Ad5 encoding full-length S protein (rAd26-S and rAd5-S) | Phase III (Active, not recruiting) |
Efficacy from clinical trials: 91.6% overall efficacy against symptomatic disease, 100% against moderate-severe disease, 73.1% after 1 dose, 91.1% after 2 doses. Efficacy/effectiveness against variants: 90% against Delta variant. |
WHO EUL (Approval pending) Approved in 69 countries 7 |
[26,110,154,155,156,157] |
| Viral vector (non-replicating) | 5 | GRAd-COV2 | 1–2 doses (1 × 1011 viral particles per dose) | Day 0 + 21 | IM | ReiThera + Leukocare + Univercells | Replication defective Simian Adenovirus (GRAd) encoding S protein | Phase II–III (Active, not recruiting) | NR | Not yet approved in any country | [26,158,159,160] |
| Viral vector (non-replicating) | 6 | LV-SMENP-DC | 1 dose (5 × 106 cells of LV-DC vaccine and 1 × 108 antigen-specific CTLs) | Day 0 | SC (LV-DC vaccine) and IV (antigen-specific CTLs) | Shenzhen Geno-Immune Medical Institute | Modified dendritic cells (DC) with lentivirus vectors (LV) expressing minigenes SMENP and immune-modulatory genes. Cytotoxic T-cells (CTLs) are activated by LV-DC, presenting specific viral antigens | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,161] |
| Viral vector (non-replicating) | 7 | hAd5-S-Fusion + N-ETSD vaccine | 1 dose (5 × 1010 IU/ dose SC, 1 × 1010 IU/ dose SL) | Day 0 | SC, oral, or SL | ImmunityBio, Inc. + NantKwest, Inc. | Human second-generation adenovirus 5 (hAd5) encoding S and N antigens | Phase I–II (Not yet recruiting) | NR | Not yet approved in any country | [26,162,163,164] |
| Viral vector (non-replicating) | 8 | AdCLD-CoV19 | 1 dose (2.5 × 1010, 5 × 1010, or 1 × 1011 virus particles per dose) | Day 0 | IM | Cellid Co., Ltd. | Replication-defective human adenovirus type 5/35 vector expressing S protein | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,165] |
| Viral vector (non-replicating) | 9 | COVIVAC | 2 doses (1 × 107 IU, 5 × 107 IU, or 1 × 108 IU per dose) | Day 0 + 28 | IM | Institute of Vaccines and Medical Biologicals, Vietnam | NDV expressing membrane-anchored pre-fusion-stabilized trimeric S protein +/− CpG 1018 adjuvant | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,166] |
| Viral vector (non-replicating) | 10 | MVA-SARS-2-ST | 2 doses (1 × 107 IU, or 1 × 108 IU per dose) | Day 0 + 28 | IM | Universitätsklinikum Hamburg-Eppendorf + German Center for Infection Research | MVA vector expressing stabilized S protein | Phase I–II (Not yet recruiting) | NR | Not yet approved in any country | [26,167] |
| Viral vector (non-replicating) | 11 | MVA-SARS-2-S | 2 doses (1 × 107 IU, or 1 × 108 IU per dose) | Day 0 + 28 | IM | University of Munich (Ludwig-Maximilians) | MVA vector expressing S protein | Phase I (Recruiting) | NR | Not yet approved in any country | [26,168] |
| Viral vector (non-replicating) | 12 | VXA-CoV2-1 | 1–2 doses (1 × 1010 IU, or 1 × 1011 IU per dose) | Day 0 or Day 0 + 28 | Oral | Vaxart | Non-replicating adenovirus vector expressing viral antigens and dsRNA adjuvant | Phase I (Active, not recruiting) | NR | Not yet approved in any country | [26,169,170] |
| Viral vector (non-replicating) | 13 | AdCOVID, | 1–2 doses | Day 0 + NR | IN | Altimmune, Inc. | Adenovirus expressing the RBD of S protein | Phase I (Recruiting) | NR | Not yet approved in any country | [26,171] |
| Viral vector (non-replicating) | 14 | COH04S1 (MVA-SARS-2-S) | 2 doses (1 × 107, 1 × 108, or 2.5 × 108 PFU per dose) | Day 0 + 28 | IM | City of Hope Medical Center + National Cancer Institute | Synthetic MVA carrying small pieces of SARS-CoV-2 DNA (the chemical form of genes) | Phase I (Recruiting) | NR | Not yet approved in any country | [26,172] |
| Viral vector (non-replicating) | 15 | ChAdV68-S ChAdV68-S-TCE (Homologous and heterologous prime-boost schedule) |
2–3 doses (5 × 1010 or 1 × 1011 viral particles of ChAdV68-S, 10 µg or 30 µg SEM) | Day 0 + 28, or Day 0 + 56, or Day 0 + 112, or Day 0 + 56 + 112 | IM | Gritstone Oncology | Chimpanzee Adenovirus serotype 68 (ChAd) and self-amplifying mRNA (SAM) vectors expressing either S protein alone, or S protein with additional T-cell epitopes (TCE) | Phase I (Recruiting) | NR | Not yet approved in any country | [26,173] |
| Viral vector (non-replicating) | 16 | SC-Ad6-1 | 1–2 doses | Day 0 or Day 0 + 21 | IM | Tetherex Pharmaceuticals Corporation | Adenovirus vector vaccine | Phase I (Not yet recruiting) | NR | Not yet approved in any country | [26,174] |
| Viral vector (non-replicating) | 17 | BBV154 | 1–2 doses (1 × 1010 viral particles per dose) | Day 0 or Day 0 + 28 | IN | Bharat Biotech International Limited | S protein | Phase I (Active, not recruiting) | NR | Not yet approved in any country | [26,175] |
| Viral vector (replicating) | 18 | DelNS1-2019-nCoV-RBD-OPT1 | 2 doses (1 × 107 EID50 and 1 × 107.7 EID50) | Day 0 + 28 | IN | University of Hong Kong, Xiamen University + Beijing Wantai Biological Pharmacy | Genetically engineered live attenuated influenza virus vector expressing the RBD of S protein | Phase II (Recruiting) | NR | Not yet approved in any country | [26,176,177] |
| Viral vector (replicating) | 19 | rVSV-SARS-CoV-2-S Vaccine | 2 doses (1 × 105, 1 × 106, 1 × 107, or 1 × 108 PFU/mL) | Day 0 + 28 | IM | Institute for Biological Research | cDNA vector encoding the sequence of the N, P, M, and L genes of the VSV genome, and SARS-CoV-2 S protein | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,178] |
| Viral vector (replicating) | 20 | AV-COVID-19 | 1 dose (0.1, 0.33, or 1.0 mg) | Day 0 | IM | Aivita Biomedical, Inc. + National Institute of Health Research and Development + Ministry of Health Republic of Indonesia | Autologous dendritic cells loaded with antigens from SARS-CoV-2 +/− GM-CSF | Phase I–II (Not yet recruiting) | NR | Not yet approved in any country | [26,179] |
| Viral vector (replicating) | 21 | Covid-19/aAPC vaccine | 3 doses | Day 0 + 14 + 28 | SC | Shenzhen Geno-Immune Medical Institute | Lentivirus vector system expressing viral minigenes to the artificial antigen-presenting cells (aAPCs) | Phase I (Recruiting) | NR | Not yet approved in any country | [26,180] |
| DNA based vaccine | 1 | nCov vaccine (ZyCoV-D) | 3 doses (1 or 2 mg) | Day 0 + 28 + 56 | ID | Zydus Cadila | S protein | Phase III (Not recruiting) | Efficacy from clinical trials in India: 66.6% | Not yet approved in any country | [26,81,181,182] |
| DNA based vaccine | 2 | INO-4800+ electroporation | 2 doses (1 mg) | Day 0 + 28 | ID | Inovio Pharmaceuticals + International Vaccine Institute + Advaccine Biopharmaceutical Co., Ltd. | S1 and S2 subunits of SARS-CoV-2 S protein | Phase II–III (Active, not recruiting) | NR | Not yet approved in any country | [26,183,184] |
| DNA based vaccine | 3 | AG0301-COVID19 | 2 doses (2 mg) | Day 0 + 14 | IM | AnGes + Takara Bio + Osaka University | S protein | Phase II–III (Active, not recruiting) | NR | Not yet approved in any country | [26,185] |
| DNA based vaccine | 4 | GX-19 | 2 doses | Day 0 + 28 | IM | Genexine Consortium | S protein | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,186] |
| DNA based vaccine | 5 | Covigenix VAX-001 | 2 doses | Day 0 + 14 | IM | Entos Pharmaceuticals Inc. | Full-length S protein | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,187] |
| DNA based vaccine | 6 | GLS-5310 | 2 doses (0.6 or 1.2 mg) | Day 0 + 56 or Day 0 + 84 | ID | GeneOne Life Science, Inc. | S protein and a second antigenic target of SARS-CoV-2 | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,188,189] |
| DNA based vaccine | 7 | COVID-eVax | 2 doses (0.5, 1, or 2 mg) | Day 0 + 28 | IM | Takis + Rottapharm Biotech | RBD of S protein | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,190] |
| DNA based vaccine | 8 | CORVax | 2 doses | Day 0 + 14 | ID | Providence Health and Services | S protein +/− the combination of electroporated IL-12p70 plasmid | Phase I (Active, not recruiting) | NR | Not yet approved in any country | [26,191] |
| DNA based vaccine | 9 | bacTRL | 1–2 doses | Day 0 or Day 0 + 28 | Oral | Symvivo Corporation | S protein | Phase I (Active, not recruiting) | NR | Not yet approved in any country | [26,192] |
| DNA based vaccine | 10 | COVIGEN (COVALIA) | 2 doses (0.8, 2, or 4 mg) | Day 0 + 28 | IM or ID | University of Sydney, Bionet Co., Ltd. | S protein | Phase I (Not yet recruiting) | NR | Not yet approved in any country | [26,193] |
| RNA vaccine | 1 | mRNA-1273 | 2 doses (100 μg) | Day 0 + 28 | IM | Moderna + National Institute of Allergy and Infectious Diseases (NIAID) | Full-length S protein with proline substitutions | Phase IV (Recruiting) |
Efficacy from clinical trials in the U.S.: 92.1% against symptomatic disease ≥14 d after 1 dose, 94.1% ≥ 14 d after 2 doses, and 100% against severe disease. Real-world efficacy: U.S.: 80% ≥ 14 d after 1 dose and 90% ≥ 14 d after 2 doses. 83% ≥ 14 d after 1 dose and 82% after 2 doses. 88.7% against infection ≥ 36 d after 1 dose. Canada: 72% against infection after 1 dose and 94% after 2 doses. Efficacy/ effectiveness against variants: Qatar: 88.1% ≥ 14 d after 1 dose, 100% after 2 doses against Alpha variant. 61.3% ≥ 14 d after 1 dose, 96.4% after 2 doses against Betavariant. Canada: 83% ≥ 14 d after 1 dose and 92% ≥ 7 d after 2 doses against symptomatic infection caused by Alpha variant. 77% ≥ 14 d after 1 dose against symptomatic infection caused by Beta or Gammavariants. 72% ≥ 14 d after 1 dose against symptomatic infection caused by Delta variant. |
FDA EUAWHO EUL Approved in 57 countries 9EMA approved |
[26,73,88,92,94,110,137,145,194,195,196,197,198] |
| RNA vaccine | 2 | BNT162b2 (3 LNP-mRNAs), also known as “Comirnaty” | 2 doses (30 μg) | Day 0 + 21 | IM | Pfizer/BioNTech + Fosun Pharma | Full-length S protein with proline substitutions | Phase IV (Recruiting) |
Efficacy from clinical trials: 52.4% after 1 dose and 94.6% ≥ 7 d after 2 doses in adults. Real-world efficacy: England: 60–70% against infection after 1 dose, 85–90% after 2 doses in elderly (≥80 y). 72% against infection ≥21 d after 1 dose, and 86% ≥ 7 d after 2 doses. 91% against infection 15–28 d after 1 dose. UK: 70% ≥ 21 d after 1 dose, 85% ≥ 7 d after 2 doses. Denmark: 17% ≥ 14 d after 1 dose, 64–90% ≥ 7 d after 2 doses. Scotland: 91% against hospitalization 28–34 d after 1 dose. U.S.: 80% ≥ 14 d after 1 dose, 93% ≥ 14 d after 2 doses. 88.7% against infection ≥ 36 d after 1 dose. Sweden: 42% against infection ≥ 14 d after 1 dose, 86% ≥ 7 d after 2 doses. Canada: 59% ≥ 14 d after 1 dose and 91% after 2 doses. Qatar: 39.4% against disease after 1 dose and 97.4% ≥ 14 d after 2 doses. Efficacy/ effectiveness against variants: England: 83.0% against hospitalization after 1 dose, 95.0% after 2 doses against Alpha variant. 94.0% against hospitalization after 1 dose, 96.0% after 2 doses against Deltavariant. Canada: 89% ≥ 7 d after 2 doses against symptomatic infection caused by Alpha variant. 60% ≥ 14 d after 1 dose and 84% ≥ 7 d against symptomatic infection caused by Beta or Gammavariants. 56% ≥ 14 d after 1 dose and 87% ≥ 7 d against symptomatic infection caused by Delta variant. Qatar: 29.5% after 1 dose and 89.5% ≥ 14 d after 2 doses against infection caused by Alpha variant. 16.9% after 1 dose and 75.0% after 2 doses against infection caused by Beta variant. |
FDA EUA WHO EUL Approved in 93 countries 10 CARPHA EU recommendation EMA approved |
[26,73,89,92,93,110,141,142,145,196,197,199,200,201,202,203,204,205,206,207,208,209] |
| RNA vaccine | 3 | CVnCoV (CureVac) | 2 doses (12 μg) | Day 0 + 28 | IM | CureVac AG | LNP-encapsulated mRNA vaccine encoding the full-length, pre-fusion stabilized S protein | Phase III (Active, not recruiting) | Efficacy from clinical trials conducted in 10 countries in Latin America and Europe: 47% against symptomatic disease across all age groups and 15 variants, 53% against any disease severity, 77% against moderate and severe disease. | WHO EUL (Pending approval) Not yet approved in any country |
[26,110,210,211,212] |
| RNA vaccine | 4 | ARCoV or ARCoVax | 1 dose (15 μg) | Day 0 | IM | Academy of Military Science (AMS), Walvax Biotechnology and Suzhou Abogen Biosciences | LNP-encapsulated mRNA vaccine encoding the RBD of S protein | Phase III (Not yet recruiting) | NR | Not yet approved in any country | [26,213,214] |
| RNA vaccine | 5 | mRNA-1273.211 | 1 dose (50 μg) | Day 0 | IM | ModernaTX, Inc. | A multivalent booster candidate combining mRNA-1273 + mRNA-1273.351 | Phase II-III (Active, not recruiting) | NR | Not yet approved in any country | [26,215] |
| RNA vaccine | 6 | mRNA-1273.351 | 1–2 doses (20 or 50 μg) | Day 0, or Day 0 + 28, or Day 56 after 2nd dose of mRNA-1273 | IM | Moderna + NIAID | Full-length prefusion stabilized S protein of SARS-CoV-2 B.1.351 variant | Phase II (Active, not recruiting) | NR | Not yet approved in any country | [26,216,217,218] |
| RNA vaccine | 7 | ARCT-021 | 1–2 doses ± booster dose (5 or 7.5 μg) | Day 0, or Day 0 + 28, or Day 0 + 28 ± 208 (booster) | IM | Arcturus Therapeutics | S protein | Phase II (Two trials: one is recruiting, and the other is active, not recruiting) | NR | Not yet approved in any country | [26,219,220,221] |
| RNA vaccine | 8 | MRT5500 | 2 doses (15, 45, or 135 µg) | Day 0 + 21 | IM | Sanofi Pasteur and Translate Bio | S protein | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,222,223,224] |
| RNA vaccine | 9 | DS-5670a | 2 doses (10, 30, 60 or 100 µg) | Day 0 + 21 | IM | Daiichi Sankyo Co., Ltd. | NR | Phase I–II (Active, not recruiting) | NR | Not yet approved in any country | [26,225,226] |
| RNA vaccine | 10 | EXG-5003 | 1 dose | Day 0 | ID | Elixirgen Therapeutics, Inc | Temperature-sensitive ssRNA vaccine expressing the RBD of S protein | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,227] |
| RNA vaccine | 11 | LNP-nCoVsaRNA (COVAC1) | 2 doses (0.1–10.0 µg) | ND | IM | Imperial College London | S protein | Phase I (No longer recruiting) | NR | Not yet approved in any country | [26,228,229] |
| RNA vaccine | 12 | ChulaCov19 mRNA vaccine | 2 doses (10, 25, 50, or 100 µg) | Day 0 + 21 | IM | Chulalongkorn University | S protein | Phase I (Not yet recruiting) | NR | Not yet approved in any country | [26,230,231] |
| RNA vaccine | 13 | PTX-COVID19-B | 2 doses (16, 40, or 100 μg) | Day 0 + 28 | IM | Providence Therapeutics | Full-length membrane-anchored S protein | Phase I (Active, not recruiting) | NR | Not yet approved in any country | [26,232,233] |
| RNA vaccine | 14 | CoV2 SAM (LNP) | 2 doses (1.0 μg) | Day 0 + 30 | IM | GSK | S protein | Phase I (Active, not recruiting) | NR | Not yet approved in any country | [26,234] |
| RNA vaccine | 15 | HDT-301 | 2 doses (1, 5, or 25 μg) | Day 0 + 28 | IM | SENAI CIMATEC | Full-length S protein | Phase I (Not yet recruiting) | NR | Not yet approved in any country | [26,235] |
| RNA vaccine | 16 | mRNA-1283 | 1–2 doses (10, 30, or 100 μg) | Day 0 or Day 0 + 28 | IM | ModernaTX, Inc. | RBD and NTD of S protein | Phase I (Recruiting) | NR | Not yet approved in any country | [26,236,237] |
| RNA vaccine | 17 | SW-0123 | 2 doses | NR | IM | Shanghai East Hospital + Stemirna Therapeutics | NR | Phase I (Recruiting) | NR | Not yet approved in any country | [26,238,239] |
| RNA vaccine | 18 | LNP-nCOV saRNA-02 (COVAC-Uganda) | 2 doses (5.0 µg) | Day 0 + 28 | IM | MRC/UVRI and LSHTM Uganda Research Unit | S protein | Phase I (Not yet recruiting) | NR | Not yet approved in any country | [26,240] |
| Protein subunit | 1 | NVX-CoV2373 | 2 doses (5 µg) | Day 0 + 21 | IM | Novavax | S protein with Matrix-M adjuvant | Phase III (Recruiting) |
Efficacy from clinical trials: UK: 89.7% against symptomatic disease ≥7 d after 2 doses. Real-world efficacy: U.S.: 100% against mild and severe disease. Efficacy/effectiveness against variants: UK: 86.2% against Alpha variant, 96.4% against non-B.1.1.7 variants. South Africa: 51.0% against Beta variant after 2 doses. 85.6% against symptomatic disease caused by Alpha variant. 60% against any disease severity in predominantly circulating Beta variant. U.S.: 93% against Alpha, Beta, and other VOCs/ VOIs. |
WHO EUL (Approval pending) Not yet approved in any country |
[26,102,103,110,241,242,243] |
| Protein subunit | 2 | ZF2001 (Recombinant SARS-CoV-2 vaccine) | 3 doses (25 µg) | Day 0 + 30 + 93 | IM | Anhui Zhifei Longcom Biopharmaceutical + Institute of Microbiology, Chinese Academy of Sciences | RBD-Dimer with alum adjuvant | Phase III (Recruiting) | NR | China (EUA), Uzbekistan | [26,244,245] |
| Protein subunit | 3 | VAT00008 | 2 doses | Day 0 + 21 | IM | Sanofi Pasteur + GSK | Monovalent and bivalent S protein with adjuvant | Phase III (Not yet recruiting) | NR | Not yet approved in any country | [26,246,247] |
| Protein subunit | 4 | FINLAY-FR-2 | 2 doses (25 μg) + booster dose (FINLAY-FR-1A, 50 μg)) | Day 0 + 28 Day 56 (booster dose) |
IM | Instituto Finlay de Vacunas | FINLAY-FR-2: chemically conjugated RBD to tetanus toxoid plus adjuvant FINLAY-FR-1A: dimeric RBD + alum adjuvant |
Phase III (Pending) | 62% | Not yet approved in any country | [26,248,249,250] |
| Protein subunit | 5 | Recombinant SARS-CoV-2 vaccine (Sf9 Cell) | 3 doses | Day 0 + 28 + 42 | IM | West China Hospital + Sichuan University | RBD with alum adjuvant | Phase III (Enrolling by invitation) | NR | Not yet approved in any country | [26,251] |
| Protein subunit | 6 | EpiVacCorona | 2 doses | Day 0 + 21 | IM | Federal Budgetary Research Institution State Research Center of Virology and Biotechnology | Peptide antigens of SARS-CoV-2 proteins with alum adjuvant | Phase III (Active, not recruiting) | Efficacy from clinical trials: 100% | Russia, Turkmenistan | [26,252,253] |
| Protein subunit | 7 | CIGB-66 | 3 doses (50 µg RBD + 0.3 mg aluminum hydroxide) | Day 0 + 14 + 28 or Day 0 + 28 + 56 | IM | Center for Genetic Engineering and Biotechnology (CIGB) | RBD with aluminum hydroxide adjuvant | Phase III (Pending) | Efficacy from clinical trials: 91.6% | Not yet approved in any country | [26,254,255] |
| Protein subunit | 8 | NanoCovax | 2 doses (25 µg) | Day 0 + 28 | IM | Nanogen Pharmaceutical Biotechnology | Recombinant S protein with alum adjuvant | Phase III (Recruiting) | NR | Not yet approved in any country | [26,256] |
| Protein subunit | 9 | SCB-2019 | 2 doses (30 μg) | Day 0 + 21 | IM | Clover Biopharmaceuticals Inc. + GSK + Dynavax | Trimeric S protein with CpG 1018 and Alum adjuvants | Phase II–III (Not yet recruiting) | NR | Not yet approved in any country | [26,257,258,259] |
| Protein subunit | 10 | UB-612 | 2 doses (100 µg) | Day 0 + 28 | IM | Vaxxinity, Inc. + Diagnósticos da América S/A (DASA) | RBD of S protein | Phase II–III (Not yet recruiting) | NR | Not yet approved in any country | [26,260] |
| Protein subunit | 11 | FINLAY-FR-1 | 2 doses (10 or 20 μg) | Day 0 + 28 | IM | Instituto Finlay de Vacunas | RBD with adjuvant | Phase II (Pending) | NR | Not yet approved in any country | [26,261] |
| Protein subunit | 12 | COVAX-19 | 2 doses (25 μg) | Day 0 + 21 | IM | Vaxine Pty Ltd. + CinnaGen Co. | Recombinant S protein with Advax-CpG adjuvant | Phase II (Recruiting) | NR | Not yet approved in any country | [26,262] |
| Protein subunit | 13 | MVC-COV1901 | 2 doses (5, 15, or 25 μg) | Day 0 + 28 | IM | Medigen Vaccine Biologics + Dynavax + NIAID | Recombinant S protein with CpG 1018 and alum adjuvants | Phase II (Active, not recruiting for adults, recruiting for elderly) | NR | Not yet approved in any country | [26,263,264,265] |
| Protein subunit | 14 | Razi Cov Pars | 3 doses | Day 0 + 21 (IM) + 51 (IN) | IM and IN | Razi Vaccine and Serum Research Institute | Recombinant S protein | Phase II (Complete) | NR | Not yet approved in any country | [26,266] |
| Protein subunit | 15 | V-01 | 2 doses (10 or 25 μg) | Day 0 + 21 | IM | Guangdong Provincial Center for Disease Control and Prevention/ Gaozhou Center for Disease Control and Prevention | Recombinant S protein | Phase II (Not yet recruiting) | NR | Not yet approved in any country | [26,267] |
| Protein subunit | 16 | CIGB-669 | 3 doses (50 µg RBD + 40 µg AgnHB) | Day 0 + 14 + 28 or Day 0 + 28 + 56 | IN | Center for Genetic Engineering and Biotechnology (CIGB) | Recombinant RBD with AgnHB | Phase I–II (Pending) | NR | Not yet approved in any country | [26,268] |
| Protein subunit | 17 | KBP-COVID-19 | 2 doses (15 μg in phase I, 45 μg in phase II) | Day 0 + 21 | IM | Kentucky Bioprocessing Inc. | RBD of S protein | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,269,270] |
| Protein subunit | 18 | BECOV2 | 2 doses | Day 0 + 28 | IM | Biological E. Limited | Recombinant RBD | Phase I–II (Closed) | NR | Not yet approved in any country | [26,271] |
| Protein subunit | 19 | S-268019 | 2 doses | Day 0 + 21 | IM | Shionogi | Recombinant S protein | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,272] |
| Protein subunit | 20 | AKS-452 | 1–2 doses (22.5, 45, or 90 µg) | NR | SC or IM | University Medical Center Groningen + Akston Biosciences Inc. | RBD-Fc fusion protein | Phase I–II (Enrolling by invitation) | NR | Not yet approved in any country | [26,273] |
| Protein subunit | 21 | COVAC-1 and COVAC-2 | 2 doses (25, 50, or 100 µg) | Day 0 + 28 | IM | University of Saskatchewan | S1 protein with SWE adjuvant | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,274] |
| Protein subunit | 22 | GBP510 | 2 doses (10, or 25 µg) | Day 0 + 28 | IM | SK Bioscience Co., Ltd. And CEPI | Recombinant RBD with AS03 aluminum hydroxide adjuvant | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,275] |
| Protein subunit | 23 | QazCoVac-P | 1–2 doses | Day 0 + 21 | IM | Research Institute for Biological Safety Problems | Phase I–II (Active, not recruiting) | NR | Not yet approved in any country | [26,276] | |
| Protein subunit | 24 | EuCorVac-19 | 2 doses | Day 0 + 21 | IM | POP Biotechnologies and EuBiologics Co., Ltd | Recombinant S protein with an adjuvant | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,277] |
| Protein subunit | 25 | Recombinant SARS-CoV-2 Vaccine (CHO cell) | 3 doses | Day 0 + 30 + 60 | IM | National Vaccine and Serum Institute, China | Recombinant SARS-CoV-2 | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,278] |
| Protein subunit | 26 | SARS-CoV-2 Sclamp vaccine | 2 doses (5, 15, or 45 μg) | Day 0 + 28 | IM | University of Queensland + Syneos Health + CEPI | Recombinant S protein with MF59 adjuvant | Phase I (Recruiting) | NR | Not yet approved in any country | [26,279,280,281] |
| Protein subunit | 27 | IMP CoVac-1 | 1 dose (500 µL) | Day 0 | SC | University Hospital Tuebingen | SARS-CoV-2 HLA-DR peptides | Phase I (Recruiting) | NR | Not yet approved in any country | [26,282] |
| Protein subunit | 28 | AdimrSC-2f | NR | NR | NR | Adimmune Corporation | Recombinant RBD with alum adjuvant | Phase I (Recruiting) | NR | Not yet approved in any country | [26,283] |
| Protein subunit | 29 | NBP2001 | 2 doses (30 or 50 μg) | Day 0 + 28 | IM | SK Bioscience Co., Ltd. | Recombinant RBD protein with alum adjuvant | Phase I (Active, not recruiting) | NR | Not yet approved in any country | [26,284] |
| Protein subunit | 30 | ReCOV | 2 doses (20 or 40 μg) | Day 0 + 21 | IM | Jiangsu Rec-Biotechnology | Recombinant two-component S and RBD protein | Phase I (Not yet recruiting) | NR | Not yet approved in any country | [26,285] |
| Protein subunit | 31 | Spike-Ferritin-Nanoparticle (SpFN) | 2–3 doses (25 or 50 μg) | Day 0 + 28 + 180 | IM | Walter Reed Army Institute of Research (WRAIR) | S proteins with a liposomal formulation QS21 (ALFQ) adjuvant | Phase I (Recruiting) | NR | Not yet approved in any country | [26,286,287,288] |
| Protein subunit | 32 | CoVepiT | 1–2 doses | Day 0 or Day 0 + 21 | SC | OSE Immunotherapeutics | Target 11 viral protein (S, M, N, and several non-structural proteins) | Phase I (Recruiting) | NR | Not yet approved in any country | [26,289] |
| Protein subunit | 33 | CoV2-OGEN1 | 1–2 doses (50, 100, or 200 μg) | Day 0 or Day 0 + 14 | Oral | VaxForm | Recombinant RBD protein | Phase I (Not yet recruiting) | NR | Not yet approved in any country | [26,290] |
| Virus-like particle | 1 | CoVLP | 2 doses (3.75 µg) | Day 0 + 21 | IM | Medicago Inc. | Trimeric S protein with AS03 adjuvant | Phase II–III (Recruiting) | NR | Not yet approved in any country | [26,291,292] |
| Virus-like particle | 2 | RBD SARS-CoV-2 HBsAg VLP | 2 doses (5 or 25 µg) | Day 0 + 28 | IM | Serum Institute of India + Accelagen Pty + SpyBiotech | RBD conjugated to the hepatitis B surface antigen | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,293] |
| Virus-like particle | 3 | VBI-2902a | 2 doses (5 or 10 μg) | Day 0 + 28 | IM | VBI Vaccines Inc. | Enveloped S glycoprotein with aluminum phosphate adjuvant | Phase I–II (Active, not recruiting) | NR | Not yet approved in any country | [26,294] |
| Virus-like particle | 4 | SARS-CoV-2 VLP Vaccine | 2 doses | NR | SC | The Scientific and Technological Research Council of Turkey | SARS-CoV-2 VLP adjuvanted with alum and CpG ODN-K3 | Phase I (Recruiting) | NR | Not yet approved in any country | [26,295] |
| Virus-like particle | 5 | ABNCoV2 | 2 doses | Day 0 + 28 | IM | Radboud University | capsid virus-like particle (cVLP) +/− adjuvant MF59 | Phase I (Recruiting) | NR | Not yet approved in any country | [26,296] |
Abbreviations: IM: Intramuscular, IN: Intranasal, IV: Intravascular, SC: Subcutaneous, ID: Intradermal, SL: Sublingual, NR: Not reported, d: days, FDA: Food and Drug Administration, WHO: World Health Organization, EUA: Emergency Use Authorization, EUL: Emergency Use Listing, ART: Africa Regulatory Taskforce, CRS: Caribbean Regulatory System, EMA: European Medicines Agency, EU: Equivalent units, IU: Infectious unit, PFU: Plaque-forming unit, S: Spike, RBD: Receptor-binding domain, N: nucleocapsid, M: membrane, NTD: N-terminal domain, Al(OH)3: aluminum hydroxide, Algel-IMDG: chemosorbed imidazoquinoline onto aluminum hydroxide gel, CpG 1018: cytosine phosphoguanine 1018, CpG ODN: CpG oligodeoxynucleotide, NVD: Newcastle Disease Virus, RSV: Respiratory syncytial virus, MVA: Modified vaccinia virus Ankara, VSV: Vesicular stomatitis virus, GM-CSF: Granulocyte-macrophage colony-stimulating factor, ssRNA: Self-amplifying ribonucleic acid, LNP: Lipid nanoparticles, AgnHBL antigen of Hepatitis B, VOCs: variants of concern, VOIs: variants of interest.
* Efficacy against COVID-19 varies by age and time after vaccinations.1 Albania, Armenia, Azerbaijan, Bangladesh, Benin, Brazil, Cambodia, Chile, China, Colombia, Dominican Republic, Ecuador, Egypt, El Salvador, Georgia, Hong Kong, Indonesia, Kazakhstan, Lao People’s Democratic Republic, Malaysia, Mexico, Nepal, Oman, Pakistan, Panama, Paraguay, Philippines, South Africa, Tajikistan, Thailand, Timor-Leste, Togo, Tunisia, Turkey, Ukraine, Uruguay, and Zimbabwe. 2 Angola, Argentina, Bahrain, Bangladesh, Belarus, Belize, Bolivia, Brazil, Brunei Darussalam, Cambodia, Cameroon, China, Comoros, Egypt, Equatorial Guinea, Gabon, Gambia, Georgia, Guyana, Hungary, Indonesia, Iran, Iraq, Jordan, Kyrgyzstan, Lao People’s Democratic Republic, Lebanon, Maldives, Mauritania, Mauritius, Mongolia, Montenegro, Morocco, Mozambique, Namibia, Nepal, Niger, North Macedonia, Pakistan, Paraguay, Peru, Philippines, Republic of the Congo, Senegal, Serbia, Seychelles, Sierra Leone, Solomon Islands, Somalia, Sri Lanka, Thailand, Trinidad and Tobago, United Arab Emirates, Venezuela (Bolivarian Republic of Venezuela), Vietnam, and Zimbabwe. 3 Guyana, India, Iran, Mauritius, Mexico, Nepal, Paraguay, Philippines, and Zimbabwe. 4 Albania, Angola, Argentina, Armenia, Australia, Austria, Azerbaijan, Belgium, Belize, Benin, Bermuda, Bosnia and Herzegovina, Botswana, Brazil, Brunei Darussalam, Bulgaria, Burkina Faso, Cambodia, Canada, Central African Republic, Chile, Colombia, Costa Rica, Croatia, Cyprus, Czechia, Côte d’Ivoire, Democratic Republic of the Congo, Dominican Republic, Ecuador, Egypt, El Salvador, Estonia, Eswatini, Fiji, Finland, France, Gambia, Georgia, Germany, Ghana, Greece, Grenada, Guatemala, Guinea-Bissau, Guyana, Haiti, Hungary, Iceland, India, Indonesia, Iran, Iraq, Ireland, Italy, Jamaica, Japan, Jordan, Kenya, Kosovo, Kuwait, Latvia, Lesotho, Libya, Liechtenstein, Lithuania, Luxembourg, Malawi, Malaysia, Mali, Malta, Mauritius, Mexico, Mongolia, Morocco, Nauru, Netherlands, Niger, Nigeria, North Macedonia, Oman, Pakistan, Panama, Papua New Guinea, Paraguay, Peru, Philippines, Poland, Portugal, Republic of Korea, Republic of Moldova, Romania, Rwanda, Sao Tome and Principe, Saudi Arabia, Senegal, Serbia, Sierra Leone, Slovakia, Slovenia, South Sudan, Spain, Sudan, Sweden, Taiwan, Tajikistan, Thailand, Timor-Leste, Togo, Tunisia, Uganda, United Arab Emirates, United Kingdom of Great Britain and Northern Ireland, Uzbekistan, Vanuatu, Viet Nam, Yemen, and Zambia. 5 Argentina, Chile, China, Ecuador, Hungary, Malaysia, Mexico, and Pakistan. 6 Austria, Bahrain, Bangladesh, Belgium, Brazil, Bulgaria, Canada, Chile, Colombia, Croatia, Cyprus, Czechia, Denmark, Estonia, Faroe Islands, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Kuwait, Latvia, Libya, Liechtenstein, Lithuania, Luxembourg, Malaysia, Maldives, Malta, Mexico, Netherlands, New Zealand, Nigeria, Norway, Philippines, Poland, Portugal, Republic of Korea, Romania, Saint Vincent and the Grenadines, Slovakia, Slovenia, South Africa, Spain, Sweden, Switzerland, Thailand, Tunisia, Ukraine, United Kingdom of Great Britain and Northern Ireland, United States of America, and Zambia. 7 Albania, Algeria, Angola, Antigua and Barbuda, Argentina, Armenia, Azerbaijan, Bahrain, Bangladesh, Belarus, Bolivia, Brazil, Cameroon, Djibouti, Ecuador, Egypt, Gabon, Ghana, Guatemala, Guinea, Guyana, Honduras, Hungary, India, Iran, Iraq, Jordan, Kazakhstan, Kenya, Kyrgyzstan, Lao People’s Democratic Republic, Lebanon, Libya, Maldives, Mali, Mauritius, Mexico, Mongolia, Montenegro, Morocco, Myanmar, Namibia, Nepal, Nicaragua, North Macedonia, Oman, Pakistan, Panama, Paraguay, Philippines, Republic of Moldova, Republic of the Congo, Russian Federation, Saint Vincent and the Grenadines, San Marino, Serbia, Seychelles, Slovakia, Sri Lanka, Syrian Arab Republic, Tunisia, Turkey, Turkmenistan, United Arab Emirates, Uzbekistan, Venezuela, Vietnam, West Bank, and Zimbabwe. 8 Afghanistan, Antigua and Barbuda, Argentina, Bahrain, Bangladesh, Barbados, Bhutan, Bolivia, Botswana, Brazil, Cabo Verde, Canada, Côte d’Ivoire, Dominica, Egypt, Ethiopia, Ghana, Grenada, Honduras, Hungary, India, Jamaica, Lebanon, Maldives, Morocco, Myanmar, Namibia, Nepal, Nicaragua, Nigeria, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadines, Seychelles, Solomon Islands, Somalia, South Africa, Sri Lanka, Suriname, The Bahamas, Togo, Tonga, Trinidad and Tobago, Ukraine. 9 Austria, Bangladesh, Belgium, Botswana, Bulgaria, Canada, Croatia, Cyprus, Czechia, Denmark, Estonia, Faroe Islands, Finland, France, Germany, Greece, Greenland, Guatemala, Honduras, Hungary, Iceland, India, Ireland, Italy, Kuwait, Latvia, Libya, Liechtenstein, Lithuania, Luxembourg, Maldives, Mongolia, Netherlands, Norway, Philippines, Poland, Portugal, Qatar, Republic of Korea, Romania, Rwanda, Saint Vincent and the Grenadines, Seychelles, Singapore, Slovakia, Slovenia, Spain, Sweden, Switzerland, Taiwan, Thailand, United Arab Emirates, United Kingdom of Great Britain and Northern Ireland, United States of America, Viet Nam, and West Bank. 10 Albania, Argentina, Australia, Austria, Azerbaijan, Bahrain, Bangladesh, Belgium, Bermuda, Bosnia and Herzegovina, Botswana, Brazil, Brunei Darussalam, Bulgaria, Cabo Verde, Canada, Chile, Colombia, Costa Rica, Croatia, Cyprus, Czechia, Denmark, Dominican Republic, Ecuador, El Salvador, Estonia, Faroe Islands, Finland, France, Georgia, Germany, Greece, Greenland, Hong Kong, Hungary, Iceland, Iraq, Ireland, Italy, Japan, Jordan, Kuwait, Latvia, Lebanon, Libya, Liechtenstein, Lithuania, Luxembourg, Malaysia, Maldives, Malta, Mexico, Monaco, Mongolia, Netherlands, New Zealand, North Macedonia, Norway, Oman, Pakistan, Panama, Paraguay, Peru, Philippines, Poland, Portugal, Qatar, Republic of Korea, Republic of Moldova, Romania, Rwanda, Saint Vincent and the Grenadines, Saudi Arabia, Serbia, Singapore, Slovakia, Slovenia, South Africa, Spain, Sri Lanka, Sweden, Switzerland, Tunisia, Turkey, Ukraine, United Arab Emirates, United Kingdom of Great Britain and Northern Ireland, United States of America, Uruguay, Vatican, Viet Nam, and West Bank.
2. Contemporary COVID-19 Vaccine Platforms and Allied Safety and Efficacy Concerns
2.1. Inactivated Vaccine
Purified inactivated viruses have been widely used for over a century in vaccine development against various emerging infectious diseases, including influenza, polio, rabies, and hepatitis A [27,28,29,30,31]. Today, inactivated vaccines are typically produced by propagating the virus in cell culture systems, followed by purification, concentration, and chemical and/or physical inactivation to demolish infectivity while retaining immunogenicity (Figure 1b) [32,33]. This type of vaccine is notably featured by its highly efficient proliferation and genetic stability [34]; yet, limited by the viral yield in a cell culture setting, the requirement of a biosafety level 3 facility, and the short duration of the elicited immune response, possibly making the vaccines less effective in preventing viral entry [33,35]. Up to date, 16 inactivated SARS-CoV-2 vaccines have been developed and are currently in clinical trial phases (Figure 2b) [26]. One of which, for example, is the Sinovac’s CoronaVac vaccine candidate which has demonstrated sufficient safety and efficacy in phase III of clinical trials in Brazil, Turkey, and Indonesia and is currently in phase IV of clinical trials (Table 1) [26,34,36,37,38,39]. Another is the BBIBP-CorV vaccine candidate, which showed adequate humoral immune responses in adults aged 18 years and above and currently stands in phase IV of clinical trials (Table 1) [39,40,41]. Both vaccines have been listed by the WHO for COVID-19 Emergency Use (EUL) and are presently being adopted by several countries worldwide. Despite these promising data, concerns of using inactivated virus vaccines platforms against COVID-19 still reside, some of which relate to the difficulty of confirming a complete virus inactivation status, a risk that could translate into a scenario similar to the 1955 Cutter incident where children receiving the polio vaccine were infected with the inactivated poliovirus [33,42]. Into the bargain, although several developed inactivated SARS-CoV vaccines have been reported to induce nAbs, vaccinated animals still display significant disease upon challenge, which could explain why no vaccines are currently licensed for SARS-CoV [43]. Further, previous studies on animal models have shown that immunizations with inactivated SARS-CoV and MERS-CoV vaccines are associated with hypersensitive-type lung pathology post-challenges with the infectious virus [32,44,45,46]. Similarly, respiratory syncytial virus (RSV) formalin-inactivated vaccine has been reported to cause enhanced pulmonary disease after live RSV infection [47,48]. In addition, it was suggested that treating the vaccine with formalin could have altered the epitopes, inducing functional antibodies, causing the immune system to produce antibodies against non-protective epitopes [33,49]. It is worth noting here that none of these concerns and/or complications of using inactivated virus vaccines have been thus far reported from the use of recently developed COVID-19 inactivated vaccines.
2.2. Live Attenuated Vaccine
Live attenuated vaccines, which embody a weakened version of the live virus with reduced virulence, are considered one of the oldest and most effective immunization approaches to elicit life-long immune responses (Figure 1c) [32,50]. A remarkable advantage of such a vaccine type is its relatively low production and delivery costs, given that the attenuated virus can replicate and propagate within the host. As such, a relatively small dose of the virus can be enough to induce immunity [51]. Moreover, live attenuated vaccines can be given intranasally, allowing the attenuated virus to replicate in the mucosal tissue of the upper respiratory tract, a major portal for coronaviruses entry into the host [52]. For the time being, only six SARS-CoV-2 live attenuated virus vaccines have been developed, four of which are in the pre-clinical phase, and two are in phase I of clinical trials (Figure 2b, Table 1) [26]. Both COVI-VAC and MV-014-212 vaccines are attenuated via codon pair deoptimization, a strategy that involves synthetic recoding of the viral genome by amending the positions of synonymous codons, thereby raising the number of suboptimal codon pairs and cytosine phosphoguanine (CpG) dinucleotides in the recoded genome [25,53,54,55]. In parallel to live attenuated SARS-CoV-2 vaccine studies, ongoing studies on other live attenuated virus vaccines such as the RSV vaccine have shown success in using the codon pair deoptimization strategy in vaccine production evidenced by the robust humoral and cellular immune responses triggered in non-human primates [56].
Despite the aforementioned advantages and the pulled off accomplishments of using live attenuated virus vaccine in combating different infectious diseases, the overt risk of using such a type of vaccine still resides in the use of a live replicating virus, which can revert under any condition to its pathologic phenotype, causing disease after vaccination, especially in immunocompromised individuals [57,58]. Although this anticipated scenario is considered relatively rare, the degree of unpredictability regarding the virus stability and the arising safety considerations after that should never be ruled out [59]. Further, live attenuated vaccines could result in viral shedding into the environment, posing a potential risk to the unvaccinated community [60]. It also goes without saying that these highlighted disadvantages are acquainted with time consumption and technical difficulties associated with the virus modification approaches if such a vaccine platform is to be implemented [16].
2.3. Viral Vector Vaccine
Viral vector vaccines, in both replicating and non-replicating forms, utilize modified viruses such as adenoviruses or poxviruses as the vector to deliver the genetic material coding for a viral antigen of interest into the host cell (Figure 1d) [57,61]. In self-replicating (replication-competent) viral vector-based vaccines, and through the host cell machinery used by the virus vector, new viral particles are produced in infected cells, which then infect other new cells, resulting in additional vaccine antigen production [62]. On the contrary, non-replicating (replication-incompetent or deficient) viral vector-based vaccines cannot produce new viral particles, and the host cell machinery is used to produce the vaccine antigens, after which the viral vector gets cleared [61,62]. Both viral vector vaccine forms do not cause infection from neither the loaded virus nor the viral vector as the delivered genetic material does not become integrated into the host genome [61,63]. Typically, the advantage of this type of vaccine lies in promoting the expression of viral antigens within infected host cells for efficient major histocompatibility complex (MHC) class I and class II presentation [61]. Moreover, viral vectors are characterized by their high gene transduction efficiency, high specificity of genes delivered to target cells, and the immune response they elicit with increased cellular response [64]. Further, although viral vector vaccines are generally considered less robust than traditional vaccine types, the fact that they persist as genetic material in the host, directly infect antigen-presenting cells, and possess a strong inherent adjuvant activity triggering innate and adaptive immune responses and generating high titers of nAbs, could suffice a single vaccine dose for adequate immunization as in the case of the vesicular-stomatitis virus -(VSV)-based Ervebo vaccine against Ebola virus [62,63,65]. In COVID-19 vector-based vaccine production, replicating and non-replicating vectors have been utilized to deliver genes encoding for either the SARS-CoV-2 S glycoprotein or the receptor-binding domain (RBD) [16,26]. Thus far, vaccinia and adenovirus are the predominantly used virus vectors for vectored vaccines development [64]. The adenovirus, for example, has been previously utilized in developing SARS-CoV vaccines expressing the S and N proteins [32,43,66]. Currently, it is also being used for developing COVID-19 vector-based vaccines. Up to date, 4 replicating and 17 non-replicating COVID-19 vector-based vaccines have been developed, of which 2 have reached phase III clinical trials, and 3 are currently in phase IV (Table 1, Figure 2b) [26]. All five vaccines are adenovirus-based non-replicating vaccines containing the gene encoding for SARS-CoV-2 S glycoprotein [67,68,69,70]. Among these vaccines, Janssen’s (Ad26.COV2.S) vaccine has recently received the FDA EUA for use in in 18 years old and elder individuals after showing good efficacy data in phase III of clinical trials [71]. Although the Ad26.COV2.S vaccine showed around 65–66% efficacy in moderate to severe/critical and around 76–83% in severe/critical COVID-19 patients, its efficacy dropped to 52 and 64% against the Beta (B.1.351) variant in moderate to severe/critical disease conditions, respectively [69] (Table 1). Low efficacy data were also reported for AstraZeneca vaccine against the Beta variant, with an efficiency of 10.4% only reported in South Africa and 48% in Canada [72,73], contrarily to the 70.4% retained efficacy against the Alpha (B.1.1.7) variant as reported in a study conducted in the UK [74]. The other three viral vector vaccines at stages II/III–IV of clinical development are CanSino’s adenovirus type-5 (Ad5) vectored vaccine, Gamaleya Research Institute’s Gam-COVID-Vac vaccine, and ReiThera’s GRAd-COV2 (Table 1). Although clinical trials have revealed that these vaccines are tolerable and immunogenic, age and the presence of high pre-existing anti-adenovirus immunity were shown to partly diminish vaccination-induced specific antibody and T-cell responses [68]. To overcome pre-existing immunity to the adenovirus in vaccinated individuals, a plausible approach could be using a heterologous recombinant vector as in the Gam-COVID-Vac (Sputnik V) vaccine, the only heterologous COVID-19 vaccine that uses both adenovirus 26 (Ad26) and adenovirus 5 (Ad5) as vectors to express the SARS-CoV-2 S protein [70,75]. Of note, the general principle of prime-boost with two distinct vectors was not exclusively used in recent COVID-19 vaccine platforms but has been largely implemented experimentally and was also previously used in developing the GamEvac-Combi Ebola virus vaccine [76].
2.4. Nucleic Acid (DNA and RNA)-Based Vaccine
In nucleic acid-based vaccines, only the genetic material (DNA or RNA), but not the recombinant/live virus, is taken up by host cells and translated into the protein to elicit an immune response (Figure 1e,f) [77]. Although various messenger RNA (mRNA) vaccines, including those against influenza, Zika, and rabies viruses, have been thus far developed, this vaccine development platform is still considered relatively new [78]. The pronounced advantage of some types of nucleic acid vaccines generally lies in the large-scale production pace and cost [16]. DNA vaccines, for example, are based on the use of highly stable plasmid DNA that can be easily propagated at a large scale in bacteria, as the plasmid DNA typically encloses mammalian expression promoters and the gene encoding the protein of interest [16]. On the other hand, presenting mRNA vaccines as promising alternatives for conventional vaccines mainly lies in the ability to produce the vaccine completely in vivo, along with their high potency, cost-effectiveness, rapid development, and safe delivery [16,78,79]. Currently, lipid nanoparticles (LNPs) are among the most commonly used in vivo RNA delivery vectors, protecting the mRNA from enzymatic degradation and facilitating endocytosis and endosomal escape [80]. Contrarily to the highlighted recognition of mRNA vaccines, the physiochemical properties of the mRNA that may impact its cellular and organ dispersal, the questioned safety and efficacy of mRNA vaccine use in humans, them being unlikely to induce strong mucosal immunity due to their intramuscular administration, and the uncertainty from what could arise with large-scale production, storage, and stability are among the alarming concerns tailored to mRNA vaccines production [16,57,80]. Likewise, potential disadvantages also relate to DNA vaccines, particularly those relevant to their low immunogenicity and to the need of DNA molecules to traverse the nuclear membrane to be transcribed, necessitating complicated delivery systems such as electroporators for better efficiency [16,57]. In addition, introducing mutation and dysregulated gene expression by the plausible stable integration of transfected DNA into the somatic or germline host cells genome is another arising concern [81] though unconventional as per relevant follow-up studies [82,83,84,85]. Up to date, 28 nucleic acids (10 DNA and 18 mRNA)-based COVID-19 vaccines have been developed and are currently in the clinical stages, and 24 mRNA vaccines are in the pre-clinical stage (Figure 2b, Table 1) [26]. Two mRNA-based vaccines, developed by Pfizer/BioNTech and Moderna, are currently in phase IV clinical trials and have received the FDA EUA for protection against COVID-19 [26,86,87]. Preliminary results showed astoundingly 94–95% efficacy for both vaccines [88,89]. Though promising, a major concern relevant to mRNA vaccines resides in their rapid pace of development and the uncertainty of potential long-term adverse effects associated with them, particularly because these are the first approved mRNA vaccines with no other FDA-approved mRNA vaccines to date [90]. Another concern is the efficacy of these vaccines against the newly emerging SARS-CoV-2 variants with mutations in the S protein, the main target in COVID-19 vaccines development [91]. As of yet, Pfizer/BioNTech COVID-19 vaccine was reported to protect against four variants of concern (VOCs), including Alpha, Beta, Gamma, and Delta (Table 1) [91,92,93,94]. Interestingly, a recent study by Zakhartchouk et al. reported that combining DNA vaccine and whole killed virus vaccines augments immune responses to SARS-CoV [95], a propitious tactic worth considering in ongoing COVID-19 vaccine development approaches [95].
2.5. Protein Subunit and Virus-Like Particles Vaccine
As compared to the whole-pathogen vaccine platform, a protein subunit vaccine is composed of in vitro harvested and highly purified viral protein antigens carefully chosen for their ability to elicit an immune response (Figure 1g) [96]. Being incapable of causing disease, the protein subunit vaccine platform is considered safer than the whole-virus (live attenuated and inactivated) platforms [97]. Not displaying the full antigenic complexity of the virus and enclosing small antigens deficient of pathogen-associated molecular patterns (PAMPs); however, it may promote skewed immune responses, bringing the immunogenicity potential and protective efficacy of protein subunit vaccines into question [57,97]. Subunit vaccine design and production could be also costly and might necessitate specific adjuvants to boost the immune response [98], in addition to the potential occurrence of antigen denaturation, which could lead to non-specific binding [99]. Examples of developed subunit vaccines include the recombinant RBD subunit vaccine, which was reported to elicit partial protective immunity in rhesus macaques against MERS-CoV challenge [100], and S protein-based subunit vaccines against SARS-CoV infection with potency to induce nAbs and protect against SARS-CoV intranasal infection in mice [32,101]. Up to date, 33 COVID-19 protein subunit vaccines based on the S protein or the RBD have been developed and are in the clinical stages. Of which, 10 vaccines, including Novavax’s (NVX-CoV2373) are in phase III [26,102]. Recent reports showed that a two-dose regimen of the NVX-CoV2373 vaccine exhibited 89.7% efficacy against SARS-CoV-2 infection, with high efficacy against the Alpha, Beta, and other VOCs [102,103] (Table 1). Virus-like particles (VLPs) vaccine is another type of protein-based vaccine composed of proteins from the viral capsid only with no viral genetic material (Figure 1h) [57,104]. In addition to being safe, VLPs elicit potent immune responses due to their repetitive structures [104]. VLP vaccines against many viruses, including Hepatitis B virus, Human papillomaviruses, and Influenza A virus, do exist [104,105,106,107]. Likewise, VLP vaccines against MERS-CoV and SARS-CoV infection have been also developed, with eosinophilic pulmonary immunopathology detected after viral challenge in some cases [21,46,108]. For the COVID-19 status quo particularly, five VLPs vaccines in different phases of clinical trials are thus far available (Figure 2b, Table 1) [26].
3. Conclusions
With the ongoing SARS-CoV-2 pandemic, safe and effective vaccines could be the major aid in retrenching this outbreak and probably the best bet to return us to ‘normal life’. The impulse of an accelerated vaccine development process, though needed, is faced with a broad spectrum of challenges that necessitates collective strives from both the public and the private sectors to fully understand the potential utility of these vaccines not only for overcoming the current pandemic but also for preventing future waves.
Author Contributions
H.T.A.-J., M.N.A., S.A. and L.K. conceptualized the review. H.T.A.-J., M.N.A. and S.A. wrote the first draft of the review. H.T.A.-J., H.N., A.Q. and L.K. wrote the second draft, edited, and revised the final version of the review. H.T.A.-J. created Figure 1 and Figure 2 and Table 1. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data used in the review were retrieved from the World Health Organization (WHO) vaccine tracker and landscape website (https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines) (accessed on 15 June 2021) and/or other publicly available resources as detailed through in-text citation and the references section of the review. Figures were created with BioRender.com.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO WHO Coronavirus Disease (COVID-19) Dashboard. [(accessed on 1 July 2020)]. Available online: https://covid19.who.int/
- 2.Mariano G., Farthing R.J., Lale-Farjat S.L.M., Bergeron J.R.C. Structural Characterization of SARS-CoV-2: Where We Are, and Where We Need to Be. Front. Mol. Biosci. 2020;7:605236. doi: 10.3389/fmolb.2020.605236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naqvi A.A.T., Fatima K., Mohammad T., Fatima U., Singh I.K., Singh A., Atif S.M., Hariprasad G., Hasan G.M., Hassan M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866:165878. doi: 10.1016/j.bbadis.2020.165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.V’kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021;19:155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tortorici M.A., Veesler D. Structural insights into coronavirus entry. Adv. Virus Res. 2019;105:93–116. doi: 10.1016/bs.aivir.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu T., Liu Y., Zhao M., Zhuang Q., Xu L., He Q. A comparison of COVID-19, SARS and MERS. PeerJ. 2020;8:e9725. doi: 10.7717/peerj.9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z., Harrich D., Li Z., Hu D., Li D. The unique features of SARS-CoV-2 transmission: Comparison with SARS-CoV, MERS-CoV and 2009 H1N1 pandemic influenza virus. Rev. Med. Virol. 2021;31:e2171. doi: 10.1002/rmv.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen E., Koopmans M., Go U., Hamer D.H., Petrosillo N., Castelli F., Storgaard M., Al Khalili S., Simonsen L. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis. 2020;20:e238–e244. doi: 10.1016/S1473-3099(20)30484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cyprian F., Sohail M.U., Abdelhafez I., Salman S., Attique Z., Kamareddine L., Al-Asmakh M. SARS-CoV-2 and immune-microbiome interactions: Lessons from respiratory viral infections. Int. J. Infect. Dis. 2021;105:540–550. doi: 10.1016/j.ijid.2021.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Güner R., Hasanoğlu I., Aktaş F. COVID-19: Prevention and control measures in community. Turk. J. Med. Sci. 2020;50:571–577. doi: 10.3906/sag-2004-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontanet A., Cauchemez S. COVID-19 herd immunity: Where are we? Nat. Rev. Immunol. 2020;20:583–584. doi: 10.1038/s41577-020-00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spellberg B., Nielsen T.B., Casadevall A. Antibodies, Immunity, and COVID-19. JAMA Intern. Med. 2021;181:460–462. doi: 10.1001/jamainternmed.2020.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma O., Sultan A.A., Ding H., Triggle C.R. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front. Immunol. 2020;11:585354. doi: 10.3389/fimmu.2020.585354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung F., Krieger V., Hufert F., Küpper J.-H. Herd immunity or suppression strategy to combat COVID-19. Clin. Hemorheol. Microcirc. 2020;75:13–17. doi: 10.3233/CH-209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Randolph H.E., Barreiro L.B. Herd Immunity: Understanding COVID-19. Immunity. 2020;52:737–741. doi: 10.1016/j.immuni.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 17.National Institutes of Health Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) [(accessed on 17 April 2020)]; Available online: https://www.nih.gov/research-training/medical-research-initiatives/activ#:~:text=On%20April%2017%2C%202020%20the,most%20promising%20treatments%20and%20vaccines.
- 18.Forni G., Mantovani A., Forni G., Mantovani A., Moretta L., Rappuoli R., Rezza G., Bagnasco A., Barsacchi G., Bussolati G., et al. COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021;28:626–639. doi: 10.1038/s41418-020-00720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin J.E., Louder M.K., Holman L.A., Gordon I.J., Enama M.E., Larkin B.D., Andrews C.A., Vogel L., Koup R.A., Roederer M., et al. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine. 2008;26:6338–6343. doi: 10.1016/j.vaccine.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin J.T., Zhang J.S., Su N., Xu J.G., Wang N., Chen J.T., Chen X., Liu Y.X., Gao H., Jia Y.P., et al. Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antivir. Ther. 2007;12:1107–1113. [PubMed] [Google Scholar]
- 21.Li Y.-D., Chi W.-Y., Su J.-H., Ferrall L., Hung C.-F., Wu T.C. Coronavirus vaccine development: From SARS and MERS to COVID-19. J. Biomed. Sci. 2020;27:104. doi: 10.1186/s12929-020-00695-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J., Peng Y., Xu H., Cui Z., Williams R.O., 3rd The COVID-19 Vaccine Race: Challenges and Opportunities in Vaccine Formulation. AAPS PharmSciTech. 2020;21:225. doi: 10.1208/s12249-020-01744-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lurie N., Saville M., Hatchett R., Halton J. Developing Covid-19 Vaccines at Pandemic Speed. N. Engl. J. Med. 2020;382:1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 24.Clem A.S. Fundamentals of vaccine immunology. J. Glob. Infect. Dis. 2011;3:73–78. doi: 10.4103/0974-777X.77299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poland G.A., Ovsyannikova I.G., Crooke S.N., Kennedy R.B. SARS-CoV-2 Vaccine Development: Current Status. Mayo Clin. Proc. 2020;95:2172–2188. doi: 10.1016/j.mayocp.2020.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO Draft Landscape of COVID-19 Candidate Vaccines. 2020. [(accessed on 29 June 2021)]. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- 27.Murdin A.D., Barreto L., Plotkin S. Inactivated poliovirus vaccine: Past and present experience. Vaccine. 1996;14:735–746. doi: 10.1016/0264-410X(95)00211-I. [DOI] [PubMed] [Google Scholar]
- 28.Vellozzi C., Burwen D.R., Dobardzic A., Ball R., Walton K., Haber P. Safety of trivalent inactivated influenza vaccines in adults: Background for pandemic influenza vaccine safety monitoring. Vaccine. 2009;27:2114–2120. doi: 10.1016/j.vaccine.2009.01.125. [DOI] [PubMed] [Google Scholar]
- 29.Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., Li Y., Zhu L., Wang N., Lv Z., et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Briggs D.J., Nagarajan T., Rupprecht C.E. Chapter 13—Rabies Vaccines. In: Jackson A.C., editor. Rabies. 3rd ed. Academic Press; Boston, MA, USA: 2013. pp. 497–526. [Google Scholar]
- 31.André F., Van Damme P., Safary A., Banatvala J. Inactivated hepatitis A vaccine: Immunogenicity, efficacy, safety and review of official recommendations for use. Expert Rev. Vaccines. 2002;1:9–23. doi: 10.1586/14760584.1.1.9. [DOI] [PubMed] [Google Scholar]
- 32.Graham R.L., Donaldson E.F., Baric R.S. A decade after SARS: Strategies for controlling emerging coronaviruses. Nat. Rev. Microbiol. 2013;11:836–848. doi: 10.1038/nrmicro3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanders B., Koldijk M., Schuitemaker H. Vaccine Analysis: Strategies, Principles, and Control. Springer; Berlin/Heidelberg, Germany: 2014. Inactivated Viral Vaccines; pp. 45–80. [DOI] [Google Scholar]
- 34.Wang H., Zhang Y., Huang B., Deng W., Quan Y., Wang W., Xu W., Zhao Y., Li N., Zhang J., et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell. 2020;182:713–721.e9. doi: 10.1016/j.cell.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burrell C.J., Howard C.R., Murphy F.A. Fenner and White’s Medical Virology. 5th ed. Academic Press; London, UK: 2017. Chapter 11—Vaccines and Vaccination; pp. 155–167. [Google Scholar]
- 36.NIH . A Study to Assess the Safety and Immunogenicity of the Coronavac Vaccine Against COVID-19. NIH; Bethseda, MD, USA: 2021. [(accessed on 18 February 2021)]. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04756830?term=NCT04756830&draw=2&rank=1. [Google Scholar]
- 37.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., Han W., Chen Z., Tang R., Yin W., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palacios R., Batista A.P., Albuquerque C.S.N., Patiño E.G., Santos J.D.P., Conde M.T.R.P., Piorelli R.D., Júnior L.C.P., Raboni S.M., Ramos F., et al. Efficacy and Safety of a COVID-19 Inactivated Vaccine in Healthcare Professionals in Brazil: The PROFISCOV Study. SSRN; Amsterdam, The Netherlands: 2021. [Google Scholar]
- 39.Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., Tan W., Wu G., Xu M., Lou Z., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al Kaabi N., Zhang Y., Xia S., Yang Y., Al Qahtani M.M., Abdulrazzaq N., Al Nusair M., Hassany M., Jawad J.S., Abdalla J., et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA. 2021;326:35–45. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.NIH A Immuno-bridging and Immunization Schedules Study of COVID-19 Vaccine (Vero Cell), Inactivated (COVID-19) [(accessed on 29 June 2021)];2021 Available online: https://clinicaltrials.gov/ct2/show/NCT04863638.
- 42.Nathanson N., Langmuir A.D. The cutter incident. poliomyelitis following formaldehyde- inactivated poliovirus vaccination in the united states during the spring of 1955. II. Relationship of poliomyelitis to cutter vaccine. Am. J. Hyg. 1963;78:29–60. doi: 10.1093/oxfordjournals.aje.a120328. [DOI] [PubMed] [Google Scholar]
- 43.Roper R.L., Rehm K.E. SARS vaccines: Where are we? Expert Rev. Vaccines. 2009;8:887–898. doi: 10.1586/erv.09.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agrawal A.S., Tao X., Algaissi A., Garron T., Narayanan K., Peng B.H., Couch R.B., Tseng C.T. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum. Vaccines Immunother. 2016;12:2351–2356. doi: 10.1080/21645515.2016.1177688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bolles M., Deming D., Long K., Agnihothram S., Whitmore A., Ferris M., Funkhouser W., Gralinski L., Totura A., Heise M., et al. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J. Virol. 2011;85:12201–12215. doi: 10.1128/JVI.06048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tseng C.-T., Sbrana E., Iwata-Yoshikawa N., Newman P.C., Garron T., Atmar R.L., Peters C.J., Couch R.B. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS ONE. 2012;7:e35421. doi: 10.1371/annotation/2965cfae-b77d-4014-8b7b-236e01a35492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castilow E.M., Olson M.R., Varga S.M. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunol. Res. 2007;39:225–239. doi: 10.1007/s12026-007-0071-6. [DOI] [PubMed] [Google Scholar]
- 48.Kapikian A.Z., Mitchell R.H., Chanock R.M., Shvedoff R.A., Stewart C.E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 49.Murphy B.R., Walsh E.E. Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. J. Clin. Microbiol. 1988;26:1595–1597. doi: 10.1128/jcm.26.8.1595-1597.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vignuzzi M., Wendt E., Andino R. Engineering attenuated virus vaccines by controlling replication fidelity. Nat. Med. 2008;14:154–161. doi: 10.1038/nm1726. [DOI] [PubMed] [Google Scholar]
- 51.Mak T.W., Saunders M.E. 23—Vaccines and Clinical Immunization. In: Mak T.W., Saunders M.E., editors. The Immune Response. Academic Press; Burlington, NJ, USA: 2006. pp. 695–749. [Google Scholar]
- 52.Tiboni M., Casettari L., Illum L. Nasal vaccination against SARS-CoV-2: Synergistic or alternative to intramuscular vaccines? Int. J. Pharm. 2021;603:120686. doi: 10.1016/j.ijpharm.2021.120686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Groenke N., Trimpert J., Merz S., Conradie A.M., Wyler E., Zhang H., Hazapis O.-G., Rausch S., Landthaler M., Osterrieder N., et al. Mechanism of Virus Attenuation by Codon Pair Deoptimization. Cell Rep. 2020;31:107586. doi: 10.1016/j.celrep.2020.107586. [DOI] [PubMed] [Google Scholar]
- 54.Coleman J.R., Papamichail D., Skiena S., Futcher B., Wimmer E., Mueller S. Virus Attenuation by Genome-Scale Changes in Codon Pair Bias. Science. 2008;320:1784–1787. doi: 10.1126/science.1155761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.News G.E.B. Meissa Vaccines—MV-014-212. [(accessed on 30 June 2021)]. Available online: https://www.genengnews.com/covid-19-candidates/meissa-vaccines-mv-014-212/
- 56.Mueller S., Stauft C.B., Kalkeri R., Koidei F., Kushnir A., Tasker S., Coleman J.R. A codon-pair deoptimized live-attenuated vaccine against respiratory syncytial virus is immunogenic and efficacious in non-human primates. Vaccine. 2020;38:2943–2948. doi: 10.1016/j.vaccine.2020.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong Y., Dai T., Wei Y., Zhang L., Zheng M., Zhou F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct. Target. Ther. 2020;5:237. doi: 10.1038/s41392-020-00352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yadav D.K., Yadav N., Khurana S.M.P. Chapter 26—Vaccines: Present Status and Applications. In: Verma A.S., Singh A., editors. Animal Biotechnology. Academic Press; San Diego, CA, USA: 2014. pp. 491–508. [Google Scholar]
- 59.WHO MODULE 2 Types of Vaccine and Adverse Reactions. [(accessed on 14 February 2021)]. Available online: https://vaccine-safety-training.org/live-attenuated-vaccines.html.
- 60.Levin M.J., Song L.-Y., Fenton T., Nachman S., Patterson J., Walker R., Kemble G., Allende M., Hultquist M., Yi T., et al. Shedding of live vaccine virus, comparative safety, and influenza-specific antibody responses after administration of live attenuated and inactivated trivalent influenza vaccines to HIV-infected children. Vaccine. 2008;26:4210–4217. doi: 10.1016/j.vaccine.2008.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dudek T., Knipe D.M. Replication-defective viruses as vaccines and vaccine vectors. Virology. 2006;344:230–239. doi: 10.1016/j.virol.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 62.Robert-Guroff M. Replicating and non-replicating viral vectors for vaccine development. Curr. Opin. Biotechnol. 2007;18:546–556. doi: 10.1016/j.copbio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Henao-Restrepo A.M., Camacho A., Longini I.M., Watson C.H., Edmunds W.J., Egger M., Carroll M.W., Dean N.E., Diatta I., Doumbia M., et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: Final results from the Guinea ring vaccination, open-label, cluster-randomised trial. Lancet. 2017;389:505–518. doi: 10.1016/S0140-6736(16)32621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ura T., Okuda K., Shimada M. Developments in Viral Vector-Based Vaccines. Vaccines. 2014;2:624–641. doi: 10.3390/vaccines2030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Riel D., de Wit E. Next-generation vaccine platforms for COVID-19. Nat. Mater. 2020;19:810–812. doi: 10.1038/s41563-020-0746-0. [DOI] [PubMed] [Google Scholar]
- 66.Gao W., Tamin A., Soloff A., D’Aiuto L., Nwanegbo E., Robbins P.D., Bellini W.J., Barratt-Boyes S., Gambotto A. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet. 2003;362:1895–1896. doi: 10.1016/S0140-6736(03)14962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S., Bittaye M., Clutterbuck E.A., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu F.C., Li Y.H., Guan X.H., Hou L.H., Wang W.J., Li J.X., Wu S.P., Wang B.S., Wang Z., Wang L., et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., Goepfert P.A., Truyers C., Fennema H., Spiessens B., et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jones I., Roy P. Sputnik V COVID-19 vaccine candidate appears safe and effective. Lancet. 2021;397:642–643. doi: 10.1016/S0140-6736(21)00191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.U.S. Food and Drug Administration Janssen COVID-19 Vaccine. [(accessed on 30 June 2021)]; Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/janssen-covid-19-vaccine.
- 72.Madhi S.A., Baillie V., Cutland C.L., Voysey M., Koen A.L., Fairlie L., Padayachee S.D., Dheda K., Barnabas S.L., Bhorat Q.E., et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021;384:1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nasreen S., He S., Chung H., Brown K.A., Gubbay J.B., Buchan S.A., Wilson S.E., Sundaram M.E., Fell D.B., Chen B., et al. Effectiveness of COVID-19 vaccines against variants of concern, Canada. medRxiv. 2021 doi: 10.1101/2021.06.28.21259420. [DOI] [Google Scholar]
- 74.Emary K.R.W., Golubchik T., Aley P.K., Ariani C.V., Angus B., Bibi S., Blane B., Bonsall D., Cicconi P., Charlton S., et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): An exploratory analysis of a randomised controlled trial. Lancet. 2021;397:1351–1362. doi: 10.1016/S0140-6736(21)00628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barouch D.H., Kik S.V., Weverling G.J., Dilan R., King S.L., Maxfield L.F., Clark S., Ng’ang’a D., Brandariz K.L., Abbink P., et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203–5209. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dolzhikova I.V., Zubkova O.V., Tukhvatulin A.I., Dzharullaeva A.S., Tukhvatulina N.M., Shcheblyakov D.V., Shmarov M.M., Tokarskaya E.A., Simakova Y.V., Egorova D.A., et al. Safety and immunogenicity of GamEvac-Combi, a heterologous VSV- and Ad5-vectored Ebola vaccine: An open phase I/II trial in healthy adults in Russia. Hum. Vaccines Immunother. 2017;13:613–620. doi: 10.1080/21645515.2016.1238535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Restifo N.P., Ying H., Hwang L., Leitner W.W. The promise of nucleic acid vaccines. Gene Ther. 2000;7:89–92. doi: 10.1038/sj.gt.3301117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang F., Kream R.M., Stefano G.B. An Evidence Based Perspective on mRNA-SARS-CoV-2 Vaccine Development. Med. Sci. Monit. 2020;26:e924700. doi: 10.12659/MSM.924700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reichmuth A.M., Oberli M.A., Jaklenec A., Langer R., Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016;7:319–334. doi: 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dey A., Chozhavel Rajanathan T.M., Chandra H., Pericherla H.P.R., Kumar S., Choonia H.S., Bajpai M., Singh A.K., Sinha A., Saini G., et al. Immunogenic Potential of DNA Vaccine candidate, ZyCoV-D against SARS-CoV-2 in Animal Models. bioRxiv. 2021;30:4108–4116. doi: 10.1101/2021.01.26.428240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hobernik D., Bros M. DNA Vaccines-How Far From Clinical Use? Int. J. Mol. Sci. 2018;19:3605. doi: 10.3390/ijms19113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolff J.A., Ludtke J.J., Acsadi G., Williams P., Jani A. Long-term persistence of plasmid DNA and foreign gone expression in mouse muscle. Hum. Mol. Genet. 1992;1:363–369. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- 84.Wang Z., Troilo P., Wang X., Griffiths T., Pacchione S., Barnum A., Harper L., Pauley C., Niu Z., Denisova L. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 2004;11:711–721. doi: 10.1038/sj.gt.3302213. [DOI] [PubMed] [Google Scholar]
- 85.Manam S., Ledwith B.J., Barnum A.B., Troilo P.J., Pauley C.J., Harper L.B., Griffiths II T.G., Niu Z., Denisova L., Follmer T.T. Plasmid DNA vaccines: Tissue distribution and effects of DNA sequence, adjuvants and delivery method on integration into host DNA. Intervirology. 2000;43:273–281. doi: 10.1159/000053994. [DOI] [PubMed] [Google Scholar]
- 86.U.S. Food and Drug Administration Pfizer-BioNTech COVID-19 Vaccine. [(accessed on 30 June 2021)]; Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine.
- 87.U.S. Food and Drug Administration Moderna COVID-19 Vaccine. [(accessed on 30 June 2021)]; Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine.
- 88.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2020;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Skowronski D.M., de Serres G. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2021;384:1576–1577. doi: 10.1056/NEJMc2036242. [DOI] [PubMed] [Google Scholar]
- 90.Conversation T. 4 Things about mRNA COVID Vaccines Researchers Still Want to Find out. 2021. [(accessed on 3 July 2021)]. Available online: https://theconversation.com/4-things-about-mrna-covid-vaccines-researchers-still-want-to-find-out-154160.
- 91.Xie X., Liu Y., Liu J., Zhang X., Zou J., Fontes-Garfias C.R., Xia H., Swanson K.A., Cutler M., Cooper D., et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat. Med. 2021;27:620–621. doi: 10.1038/s41591-021-01270-4. [DOI] [PubMed] [Google Scholar]
- 92.Abu-Raddad L.J., Chemaitelly H., Butt A.A. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N. Engl. J. Med. 2021;385:187–189. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.PHE Effectiveness of COVID-19 Vaccines against Hospital Admission with the Delta (B.1.617.2) Variant. [(accessed on 14 June 2021)]. Available online: https://khub.net/web/phe-national/public-library/-/document_library/v2WsRK3ZlEig/view_file/479607329?_com_liferay_document_library_web_portlet_DLPortlet_INSTANCE_v2WsRK3ZlEig_redirect=https%3A%2F%2Fkhub.net%3A443%2Fweb%2Fphe-national%2Fpublic-library%2F-%2Fdocument_library%2Fv2WsRK3ZlEig%2Fview%2F479607266.
- 94.Chemaitelly H., Yassine H.M., Benslimane F.M., Al Khatib H.A., Tang P., Hasan M.R., Malek J.A., Coyle P., Ayoub H.H., Al Kanaani Z., et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat. Med. 2021;27:1614–1621. doi: 10.1038/s41591-021-01446-y. [DOI] [PubMed] [Google Scholar]
- 95.Zakhartchouk A.N., Liu Q., Petric M., Babiuk L.A. Augmentation of immune responses to SARS coronavirus by a combination of DNA and whole killed virus vaccines. Vaccine. 2005;23:4385–4391. doi: 10.1016/j.vaccine.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Du L., He Y., Jiang S., Zheng B.-J. Development of subunit vaccines against severe acute respiratory syndrome. Drugs Today. 2008;44:63–74. doi: 10.1358/dot.2008.44.1.1131830. [DOI] [PubMed] [Google Scholar]
- 97.Enjuanes L., Zuñiga S., Castaño-Rodriguez C., Gutierrez-Alvarez J., Canton J., Sola I. Molecular Basis of Coronavirus Virulence and Vaccine Development. Adv. Virus Res. 2016;96:245–286. doi: 10.1016/bs.aivir.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lidder P., Sonnino A. Chapter 1—Biotechnologies for the Management of Genetic Resources for Food and Agriculture. In: Goodwin S.F., Friedmann T., Dunlap J.C., editors. Advances in Genetics. Volume 78. Academic Press; Cambridge, MA, USA: 2012. pp. 1–167. [DOI] [PubMed] [Google Scholar]
- 99.WHO WHO Vaccine Safety Basics. [(accessed on 30 June 2021)]. Available online: https://vaccine-safety-training.org/subunit-vaccines.html.
- 100.Lan J., Yao Y., Deng Y., Chen H., Lu G., Wang W., Bao L., Deng W., Wei Q., Gao G.F., et al. Recombinant Receptor Binding Domain Protein Induces Partial Protective Immunity in Rhesus Macaques Against Middle East Respiratory Syndrome Coronavirus Challenge. EBioMedicine. 2015;2:1438–1446. doi: 10.1016/j.ebiom.2015.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bisht H., Roberts A., Vogel L., Subbarao K., Moss B. Neutralizing antibody and protective immunity to SARS coronavirus infection of mice induced by a soluble recombinant polypeptide containing an N-terminal segment of the spike glycoprotein. Virology. 2005;334:160–165. doi: 10.1016/j.virol.2005.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heath P.T., Galiza E.P., Baxter D.N., Boffito M., Browne D., Burns F., Chadwick D.R., Clark R., Cosgrove C., Galloway J., et al. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N. Engl. J. Med. 2021;385:1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Medicine N. Novavax Vaccine Results: How Effective is it against Variants? 2021. [(accessed on 12 July 2021)]. Available online: https://www.nebraskamed.com/COVID/novavax-vaccine-results-how-effective-is-it-against-variants.
- 104.Fuenmayor J., Gòdia F., Cervera L. Production of virus-like particles for vaccines. New Biotechnol. 2017;39 (Pt B):174–180. doi: 10.1016/j.nbt.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cai X., Zheng W., Pan S., Zhang S., Xie Y., Guo H., Wang G., Li Z., Luo M. A virus-like particle of the hepatitis B virus preS antigen elicits robust neutralizing antibodies and T cell responses in mice. Antiviral. Res. 2018;149:48–57. doi: 10.1016/j.antiviral.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bright R.A., Carter D.M., Daniluk S., Toapanta F.R., Ahmad A., Gavrilov V., Massare M., Pushko P., Mytle N., Rowe T., et al. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine. 2007;25:3871–3878. doi: 10.1016/j.vaccine.2007.01.106. [DOI] [PubMed] [Google Scholar]
- 107.Harper D.M., Franco E.L., Wheeler C., Ferris D.G., Jenkins D., Schuind A., Zahaf T., Innis B., Naud P., De Carvalho N.S., et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: A randomised controlled trial. Lancet. 2004;364:1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 108.Lokugamage K.G., Yoshikawa-Iwata N., Ito N., Watts D.M., Wyde P.R., Wang N., Newman P., Kent Tseng C.T., Peters C.J., Makino S. Chimeric coronavirus-like particles carrying severe acute respiratory syndrome coronavirus (SCoV) S protein protect mice against challenge with SCoV. Vaccine. 2008;26:797–808. doi: 10.1016/j.vaccine.2007.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.WHO Interim Recommendations for Use of the Inactivated COVID-19 Vaccine, CoronaVac, Developed by Sinovac. [(accessed on 24 May 2021)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/341454/WHO-2019-nCoV-vaccines-SAGE-recommendation-Sinovac-CoronaVac-2021.1-eng.pdf.
- 110.WHO Status of COVID-19 Vaccines within WHO EUL/PQ Evaluation Process. [(accessed on 2 July 2021)]. Available online: https://extranet.who.int/pqweb/sites/default/files/documents/Status_COVID_VAX_02July2021.pdf.
- 111.WHO Background Document on the Inactivated Vaccine Sinovac-CoronaVac against COVID-19. [(accessed on 1 June 2021)]. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-Sinovac-CoronaVac-background-2021.1.
- 112.Ramasamy M.N., Jessop L.J. CoronaVac: More data for regulators and policy makers. Lancet. 2021;398:186–188. doi: 10.1016/S0140-6736(21)01543-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.WHO Interim Recommendations for Use of the Inactivated COVID-19 Vaccine BIBP Developed by China National Biotec Group (CNBG), Sinopharm. [(accessed on 7 May 2021)]. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-BIBP-2021.1.
- 114.Xia S., Duan K., Zhang Y., Zhao D., Zhang H., Xie Z., Li X., Peng C., Zhang Y., Zhang W., et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. JAMA. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Registry C.C.T. A Phase III Clinical Trial for Inactivated Novel Coronavirus Pneumonia (COVID-19) Vaccine (Vero Cells) [(accessed on 4 July 2021)]. Available online: http://www.chictr.org.cn/showprojen.aspx?proj=56651.
- 116.Pu J., Yu Q., Yin Z., Zhang Y., Li X., Li D., Chen H., Long R., Zhao Z., Mou T., et al. An in-depth investigation of the safety and immunogenicity of an inactivated SARS-CoV-2 vaccine. medRxiv. 2020 doi: 10.1101/2020.09.27.20189548. [DOI] [Google Scholar]
- 117.NIH The Efficacy, Safety and Immunogenicity Study of Inactivated SARS-CoV-2 Vaccine for Preventing Against COVID-19. [(accessed on 10 February 2021)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04659239?term=vaccination&cond=covid&draw=3.
- 118.NIH Immunogenicity, Efficacy and Safety of QazCovid-in® COVID-19 Vaccine. [(accessed on 4 May 2021)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04691908?id=NCT04639466+OR+NCT04659941+OR+NCT04691947+OR+NCT04651790+OR+NCT04659239+OR+NCT04648800+OR+NCT04691908+OR+NCT04656613+OR+NCT04672395+OR+NCT04673149+OR+NCT04671017+OR+NCT04685603+OR+NCT04664309+OR+NCT04686773+OR+NCT04681092+OR+NCT04662697+OR+NCT04652102+OR+NCT04665258+OR+NCT04649021+OR+NCT04686409+OR+NCT04690387+OR+NCT04666012+OR+NCT04649151+OR+NCT04655625+OR+NCT04684446+OR+NCT04668339+OR+NCT04683224+OR+NCT04674189+OR+NCT04690816+OR+NCT04679909&draw=2&rank=2&load=cart.
- 119.Institute E.R. QazCovid-in. [(accessed on 20 December 2020)]. Available online: https://economy.kz/en/Novosti_ekonomiki_Kazahstana/id=1588.
- 120.Ella R., Vadrevu K.M., Jogdand H., Prasad S., Reddy S., Sarangi V., Ganneru B., Sapkal G., Yadav P., Abraham P., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: A double-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021;21:637–646. doi: 10.1016/S1473-3099(20)30942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Biotech B. COVAXIN®—India’s First Indigenous COVID-19 Vaccine. [(accessed on 4 July 2021)]. Available online: https://www.bharatbiotech.com/covaxin.html.
- 122.NIH An Efficacy and Safety Clinical Trial of an Investigational COVID-19 Vaccine (BBV152) in Adult Volunteers. [(accessed on 19 March 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04641481.
- 123.Ella R., Reddy S., Blackwelder W., Potdar V., Yadav P., Sarangi V., Aileni V.K., Kanungo S., Rai S., Reddy P., et al. Efficacy, safety, and lot to lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): A, double-blind, randomised, controlled phase 3 trial. medRxiv. 2021 doi: 10.1101/2021.06.30.21259439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.NIH A Study to Evaluate the Efficacy, Safety and Immunogenicity of SARS-CoV-2 Vaccine (Vero Cells), Inactivated in Healthy Adults Aged 18 Years and Older (COVID-19) [(accessed on 22 April 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04852705?term=vaccine&recrs=abdf&cond=COVID-19&phase=0123&sort=nwst&draw=2.
- 125.NIH Study To Compare the Immunogenicity Against COVID-19, of VLA2001 Vaccine to AZD1222 Vaccine (COV-COMPARE) [(accessed on 8 June 2021)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04864561#contacts.
- 126.NIH Efficacy, Immunogenicity, and Safety of the Inactivated COVID-19 Vaccine (TURKOVAC) Versus the CoronaVac Vaccine. [(accessed on 28 June 2021)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04942405.
- 127.IRCT A Double-Blind, Randomized, Placebo-Controlled Phase II/III Clinical Trial to Evaluate the Safety and Efficacy of COVID-19 Inactivated Vaccine (Shifa-Pharmed) in a Population Aged 18 to 75 Years. [(accessed on 4 July 2021)]. Available online: https://en.irct.ir/trial/54881.
- 128.IRCT Phase 2 Trial of Safety and Immunogenicity of 10 Micro Gram Inactivated SARS-CoV-2 Vaccine (FAKHRAVAC), Two Doses Two Weeks Apart in Adults Aged 18–70 Years: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial. [(accessed on 5 July 2021)]. Available online: https://en.irct.ir/trial/56027.
- 129.Sun W., McCroskery S., Liu W.-C., Leist S.R., Liu Y., Albrecht R.A., Slamanig S., Oliva J., Amanat F., Schäfer A., et al. A Newcastle Disease Virus (NDV) Expressing a Membrane-Anchored Spike as a Cost-Effective Inactivated SARS-CoV-2 Vaccine. Vaccines. 2020;8:711. doi: 10.3390/vaccines8040771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.JRCT Placebo-Controlled, Multicenter, Double-Blind, Randomized, Parallel-Group, Comparative Study to Evaluate the Safety and Immunogenicity of KD-414, a Vaccine Against COVID-19, in Healthy Adults Aged ≥20 Years to <65 Years, and Healthy Elderly Subjects Aged ≥65 Years. [(accessed on 5 July 2021)]. Available online: https://jrct.niph.go.jp/en-latest-detail/jRCT2071200106.
- 131.NIH Safety and Immunogenicity of the Inactivated Koçak-19 Inaktif Adjuvanlı COVID-19 Vaccine Compared to Placebo. [(accessed on 13 April 2021)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04838080?term=NCT04838080&draw=2&rank=1.
- 132.NIH Study of a Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) Adjuvanted Inactivated Vaccine in Healthy Adults (COVID-19) [(accessed on 6 May 2021)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04866069.
- 133.NIH Study of a Live rNDV Based Vaccine against COVID-19. [(accessed on 27 May 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04871737.
- 134.NIH Safety and Immunogenicity of COVI-VAC, a Live Attenuated Vaccine against COVID-19. [(accessed on 6 November 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04619628.
- 135.NIH Safety and Immunogenicity of an Intranasal RSV Vaccine Expressing SARS-CoV-2 Spike Protein (COVID-19 Vaccine) in Adults. [(accessed on 21 May 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04798001?term=covid-19+vaccine&draw=2.
- 136.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.NIH National Cohort Study of Effectiveness and Safety of SARS-CoV-2/COVID-19 Vaccines (ENFORCE) (ENFORCE) [(accessed on 26 February 2021)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04760132?term=vaccine%2C+phase+4&cond=Covid19&draw=2.
- 138.Africa CDC Africa Regulatory Taskforce Has Endorsed the Emergency Used Listing for Two Versions of the AstraZeneca-Oxford Vaccine. [(accessed on 7 June 2021)]. Available online: https://africacdc.org/download/africa-regulatory-taskforce-has-endorsed-the-emergency-used-listing-for-two-versions-of-the-astrazeneca-oxford-vaccine-astrazeneca-skbio-in-south-korea-and-serum-institute-of-india/
- 139.CARPHA CARPHA COVID-19 Vaccine Update. [(accessed on 22 March 2021)]. Available online: https://carpha.org/Portals/0/Documents/COVID-19%20Vaccine%20Updates/CARPHA%20COVID-19%20Vaccine%20Update%20011%20March%2022,%202021.pdf.
- 140.Voysey M., Costa Clemens S.A., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lopez Bernal J., Andrews N., Gower C., Robertson C., Stowe J., Tessier E., Simmons R., Cottrell S., Roberts R., O’Doherty M., et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vasileiou E., Simpson C.R., Shi T., Kerr S., Agrawal U., Akbari A., Bedston S., Beggs J., Bradley D., Chuter A., et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: A national prospective cohort study. Lancet. 2021;397:1646–1657. doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.BBC Covid vaccine: AstraZeneca Updates US Vaccine Efficacy Results. [(accessed on 25 March 2021)]. Available online: https://www.bbc.com/news/world-us-canada-56521166.
- 144.Madhi S.A., Baillie V., Cutland C.L., Voysey M., Koen A.L., Fairlie L., Padayachee S.D., Dheda K., Barnabas S.L., Bhorat Q.E., et al. Safety and efficacy of the ChAdOx1 nCoV-19 (AZD1222) Covid-19 vaccine against the B.1.351 variant in South Africa. medRxiv. 2021 doi: 10.1101/2021.02.10.21251247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.EMA COVID-19 Vaccines. [(accessed on 6 July 2021)]. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-vaccines.
- 146.NIH Study on Sequential Immunization of Inactivated SARS-CoV-2 Vaccine and Recombinant SARS-CoV-2 Vaccine (Ad5 Vector) [(accessed on 30 June 2021)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04892459?term=NCT04892459&draw=2&rank=1.
- 147.NIH Clinical Trial of Recombinant Novel Coronavirus Vaccine (Adenovirus Type 5 Vector) Against COVID-19. [(accessed on 16 February 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04540419.
- 148.REUTERS CanSinoBIO’s COVID-19 Vaccine 65.7% Effective in Global Trials, Pakistan Official Says. [(accessed on 8 February 2021)]. Available online: https://www.reuters.com/article/us-health-coronavirus-vaccine-pakistan-idUSKBN2A81N0.
- 149.Wu S., Zhong G., Zhang J., Shuai L., Zhang Z., Wen Z., Wang B., Zhao Z., Song X., Chen Y., et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020;11:4081. doi: 10.1038/s41467-020-17972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhu F.-C., Guan X.-H., Li Y.-H., Huang J.-Y., Jiang T., Hou L.-H., Li J.-X., Yang B.-F., Wang L., Wang W.-J., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vaccinations P. Convidicea Vaccine. [(accessed on 30 June 2021)]. Available online: https://www.precisionvaccinations.com/vaccines/convidicea-vaccine.
- 152.Africa CDC African Union and the Africa CDC’s Africa Regulatory Taskforce Has Endorsed the Emergency Used Authorization for Janssen COVID-19 Vaccine. [(accessed on 7 June 2021)]. Available online: https://africacdc.org/download/african-union-and-the-africa-centers-for-disease-control-and-preventions-africa-regulatory-taskforce-has-endorsed-the-emergency-used-authorization-for-janssen-covid-19-vaccine-2/
- 153.Corchado-Garcia J., Puyraimond-Zemmour D., Hughes T., Cristea-Platon T., Lenehan P., Pawlowski C., Bade S., O’Horo J.C., Gores G.J., Williams A.W., et al. Real-world effectiveness of Ad26.COV2.S adenoviral vector vaccine for COVID-19. medRxiv. 2021 doi: 10.1101/2021.04.27.21256193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Logunov D.Y., Dolzhikova I.V., Zubkova O.V., Tukhvatulin A.I., Shcheblyakov D.V., Dzharullaeva A.S., Grousova D.M., Erokhova A.S., Kovyrshina A.V., Botikov A.G., et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.NIH Clinical Trial of Efficacy, Safety, and Immunogenicity of Gam-COVID-Vac Vaccine Against COVID-19 (RESIST) [(accessed on 22 January 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04530396?term=vaccine&cond=covid-19&draw=3.
- 156.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., Lubenets N.L., Grousova D.M., Erokhova A.S., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.World News . Hindustan Times. World News; New Delhi, India: 2021. Sputnik V Gives 90% Protection against Delta Strain of Covid-19: Scientist. [Google Scholar]
- 158.NIH Study of GRAd-COV2 for the Prevention of COVID-19 in Adults (COVITAR) [(accessed on 14 April 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04791423.
- 159.Capone S., Raggioli A., Gentile M., Battella S., Lahm A., Sommella A., Contino A.M., Urbanowicz R.A., Scala R., Barra F., et al. Immunogenicity of a new gorilla adenovirus vaccine candidate for COVID-19. Mol. Ther. 2021;29:2412–2423. doi: 10.1016/j.ymthe.2021.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lanini S., Capone S., Antinori A., Milleri S., Nicastri E., Camerini R., Agrati C., Castilletti C., Mori F., Sacchi A., et al. GRAd-COV2, a gorilla adenovirus based candidate vaccine against COVID-19, is safe and immunogenic in young and older adults. medRxiv. 2021 doi: 10.1101/2021.04.10.21255202. [DOI] [PubMed] [Google Scholar]
- 161.NIH Immunity and Safety of Covid-19 Synthetic Minigene Vaccine. [(accessed on 19 March 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04276896.
- 162.NIH COVID-19 Supplemental Vaccine Boost to Enhance T Cell Protection in Those Who Have Already Received EUA S-Based Vaccines. [(accessed on 10 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04843722.
- 163.ImmunityBio Fighting a war on two fronts: ImmunityBio targets cancer and COVID-19. [(accessed on 8 June 2021)];Biopharma Deal. 2021 Available online: https://www.nature.com/articles/d43747-020-00963-y. [Google Scholar]
- 164.Rice A., Verma M., Shin A., Zakin L., Sieling P., Tanaka S., Adisetiyo H., Taft J., Patel R., Buta S., et al. A Next Generation Bivalent Human Ad5 COVID-19 Vaccine Delivering Both Spike and Nucleocapsid Antigens Elicits Th1 Dominant CD4+, CD8+ T-cell and Neutralizing Antibody Responses. bioRxiv. 2020 doi: 10.1101/2020.07.29.227595. [DOI] [Google Scholar]
- 165.NIH Safety and Immunogenicity Study of AdCLD-CoV19: A COVID-19 Preventive Vaccine in Healthy Volunteers. [(accessed on 29 March 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04666012.
- 166.NIH A Phase 1/2 Safety and Immunogenicity Trial of COVID-19 Vaccine COVIVAC. [(accessed on 5 April 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04830800.
- 167.NIH Safety, Tolerability and Immunogenicity of the Candidate Vaccine MVA-SARS-2-ST Against COVID-19 (MVA-SARS-2-ST) [(accessed on 20 May 2021)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04895449?term=NCT04895449&draw=2&rank=1.
- 168.NIH Safety, Tolerability and Immunogenicity of the Candidate Vaccine MVA-SARS-2-S Against COVID-19. [(accessed on 1 December 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04569383?term=vaccine&cond=covid-19&draw=5.
- 169.NIH Safety and Immunogenicity Trial of an Oral SARS-CoV-2 Vaccine (VXA-CoV2-1) for Prevention of COVID-19 in Healthy Adults. [(accessed on 8 April 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04563702.
- 170.Moore A.C., Dora E.G., Peinovich N., Tucker K.P., Lin K., Cortese M., Tucker S.N. Pre-clinical studies of a recombinant adenoviral mucosal vaccine to prevent SARS-CoV-2 infection. bioRxiv. 2020 doi: 10.1101/2020.09.04.283853. [DOI] [Google Scholar]
- 171.NIH Safety and Immunogenicity of AdCOVID in Healthy Adults (COVID-19 Vaccine Study) [(accessed on 24 February 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04679909.
- 172.NIH A Synthetic MVA-Based SARS-CoV-2 Vaccine, COH04S1, for the Prevention of COVID-19. [(accessed on 7 January 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04639466.
- 173.NIH Chimpanzee Adenovirus and Self-Amplifying mRNA Prime-Boost Prophylactic Vaccines against SARS-CoV-2 in Healthy Adults. [(accessed on 2 July 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04776317.
- 174.NIH A Phase 1, First-in-Human Study of the Investigational COVID-19 Vaccine SC-Ad6-1 in Healthy Volunteers. [(accessed on 27 April 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04839042.
- 175.NIH Safety and Immunogenicity of an Intranasal SARS-CoV-2 Vaccine (BBV154) for COVID-19. [(accessed on 22 June 2021)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04751682?term=vaccine&cond=Coronavirus&sfpd_s=01%2F01%2F2021&draw=2.
- 176.ChiCTR The Nasal Spray Influenza Virus Vector New Coronary Pneumonia (COVID-19) Vaccine (DelNS1-2019-nCoV-RBD-OPT1) Phase II Clinical Trial. 2020. [(accessed on 7 August 2021)]. Available online: http://www.chictr.org.cn/showproj.aspx?proj=63754.
- 177.NIH A Study to Evaluate Safety and Immunogenicity of DelNS1-nCoV-RBD LAIV for COVID-19. [(accessed on 3 May 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04809389.
- 178.Yahalom-Ronen Y., Tamir H., Melamed S., Politi B., Shifman O., Achdout H., Vitner E.B., Israeli O., Milrot E., Stein D., et al. A single dose of recombinant VSV-ΔG-spike vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020;11:6402. doi: 10.1038/s41467-020-20228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.NIH Phase I-II Trial of Dendritic Cell Vaccine to Prevent COVID-19 in Adults. [(accessed on 8 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04386252.
- 180.NIH Safety and Immunity of Covid-19 aAPC Vaccine. [(accessed on 9 March 2021)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT04299724.
- 181.CTRI A Prospective, Randomized, Adaptive, Phase I/II Clinical Study to Evaluate the Safety and Immunogenicity of Novel Corona Virus -2019-nCov Vaccine Candidate of M/s Cadila Healthcare Limited by Intradermal Route in Healthy Subjects. [(accessed on 8 November 2020)]. Available online: http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=45306&EncHid=&userName=Zydus.
- 182.Das S. Business Standard. Fiancial Press; New Delhi, India: 2021. Zydus’ Covid-19 vaccine shows 66.6% efficacy, seeks DCGI approval. [Google Scholar]
- 183.Mammen M.P., Tebas P., Agnes J., Giffear M., Kraynyak K.A., Blackwood E., Amante D., Reuschel E.L., Purwar M., Christensen-Quick A., et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: A preliminary report of a randomized, blinded, placebo-controlled, Phase 2 clinical trial in adults at high risk of viral exposure. medRxiv. 2021 doi: 10.1101/2021.05.07.21256652. [DOI] [Google Scholar]
- 184.NIH Safety, Immunogenicity, and Efficacy of INO-4800 for COVID-19 in Healthy Seronegative Adults at High Risk of SARS-CoV-2 Exposure. [(accessed on 29 March 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04642638.
- 185.NIH Phase II/III Study of COVID-19 DNA Vaccine (AG0302-COVID19) [(accessed on 8 April 2021)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04655625?term=vaccination&cond=covid&draw=1.
- 186.NIH Safety and Immunogenicity Study of GX-19, a COVID-19 Preventive DNA Vaccine in Healthy Adults. [(accessed on 28 July 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04445389?term=vaccine&cond=covid-19&draw=3.
- 187.NIH A Clinical Trial of a Prophylactic Plasmid DNA Vaccine for COVID-19 [Covigenix VAX-001] in Adults. [(accessed on 27 April 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04591184.
- 188.Sciences G.L. Nucleic Acid Vaccines. [(accessed on 1 September 2021)]. Available online: http://www.genels.com/en/sub/technology/vaccine.asp.
- 189.NIH GLS-5310 Vaccine for the Prevention of SARS-CoV-2 (COVID-19) [(accessed on 24 December 2020)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04673149?term=NCT04673149&draw=2&rank=1.
- 190.NIH Safety and Immunogenicity of COVID-eVax, a Candidate Plasmid DNA Vaccine for COVID-19, in Healthy Adult Volunteers. [(accessed on 16 March 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04788459.
- 191.NIH CORVax12: SARS-CoV-2 Spike (S) Protein Plasmid DNA Vaccine Trial for COVID-19 (SARS-CoV-2) (CORVax12) [(accessed on 3 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04627675.
- 192.NIH Evaluating the Safety, Tolerability and Immunogenicity of bacTRL-Spike Vaccine for Prevention of COVID-19. [(accessed on 4 March 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04334980.
- 193.NIH The Safety and Immunogenicity of a DNA-based Vaccine (COVIGEN) in Healthy Volunteers (COVALIA) [(accessed on 8 February 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04742842.
- 194.Food and Drug Administration (FDA) Vaccines and Related Biological Products Advisory Committee Meeting December 17, 2020. Food and Drug Administration (FDA); White Oak, MD, USA: 2020. [Google Scholar]
- 195.Thompson M.G., Burgess J.L., Naleway A.L., Tyner H.L., Yoon S.K., Meece J., Olsho L.E.W., Caban-Martinez A.J., Fowlkes A., Lutrick K., et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers—Eight U.S. Locations, December 2020–March 2021. MMWR Morb. Mortal. Wkly Rep. 2021;70:495–500. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Thompson M.G., Burgess J.L., Naleway A.L., Tyner H., Yoon S.K., Meece J., Olsho L.E.W., Caban-Martinez A.J., Fowlkes A.L., Lutrick K., et al. Prevention and Attenuation of COVID-19 by BNT162b2 and mRNA-1273 Vaccines. medRxiv. 2021 doi: 10.1101/2021.06.01.21257987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chung H., He S., Nasreen S., Sundaram M.E., Buchan S.A., Wilson S.E., Chen B., Calzavara A., Fell D.B., Austin P.C., et al. Effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe COVID-19 outcomes in Ontario, Canada. medRxiv. 2021 doi: 10.1101/2021.05.24.21257744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.NIH A Study to Evaluate Efficacy, Safety, and Immunogenicity of mRNA-1273 Vaccine in Adults Aged 18 Years and Older to Prevent COVID-19. [(accessed on 10 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04470427?term=vaccine&cond=covid-19&draw=5.
- 199.Hall V.J., Foulkes S., Saei A., Andrews N., Oguti B., Charlett A., Wellington E., Stowe J., Gillson N., Atti A., et al. Effectiveness of BNT162b2 mRNA Vaccine Against Infection and COVID-19 Vaccine Coverage in Healthcare Workers in England, Multicentre Prospective Cohort Study (the SIREN Study) SSRN. 2021 doi: 10.2139/ssrn.3790399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pawlowski C., Lenehan P., Puranik A., Agarwal V., Venkatakrishnan A.J., Niesen M.J.M., O’Horo J.C., Badley A.D., Halamka J., Soundararajan V. FDA-authorized COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. medRxiv. 2021 doi: 10.1101/2021.02.15.21251623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., Hernán M.A., Lipsitch M., Reis B., Balicer R.D. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Björk J., Inghammar M., Moghaddassi M., Rasmussen M., Malmqvist U., Kahn F. Effectiveness of the BNT162b2 vaccine in preventing COVID-19 in the working age population—First results from a cohort study in Southern Sweden. medRxiv. 2021 doi: 10.1101/2021.04.20.21254636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Public Health England Annex A: Report to JCVI on estimated efficacy of a single dose of Pfizer BioNTech (BNT162b2 mRNA) vaccine and of a single dose of ChAdOx1 vaccine (AZD1222) [(accessed on 22 December 2020)]; Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/949505/annex-a-phe-report-to-jcvi-on-estimated-efficacy-of-single-vaccine-dose.pdf.
- 204.Chodick G., Tene L., Patalon T., Gazit S., Ben Tov A., Cohen D., Muhsen K. Assessment of Effectiveness of 1 Dose of BNT162b2 Vaccine for SARS-CoV-2 Infection 13 to 24 Days After Immunization. JAMA Netw. Open. 2021;4:e2115985. doi: 10.1001/jamanetworkopen.2021.15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Moustsen-Helms I.R., Emborg H.-D., Nielsen J., Nielsen K.F., Krause T.G., Mølbak K., Møller K.L., Berthelsen A.-S.N., Valentiner-Branth P. Vaccine effectiveness after 1st and 2nd dose of the BNT162b2 mRNA Covid-19 Vaccine in long-term care facility residents and healthcare workers—A Danish cohort study. medRxiv. 2021 doi: 10.1101/2021.03.08.21252200. [DOI] [Google Scholar]
- 206.Chodick G., Tene L., Patalon T., Gazit S., Tov A.B., Cohen D., Muhsen K. The effectiveness of the first dose of BNT162b2 vaccine in reducing SARS-CoV-2 infection 13-24 days after immunization: Real-world evidence. medRxiv. doi: 10.1001/jamanetworkopen.2021.15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.CARPHA Carpha Situation Report NO. 145. [(accessed on 1 April 2021)]. Available online: https://carpha.org/Portals/0/Documents/COVID%20Situation%20Reports/Situation%20Report%20145%20-%20April%201,%202021.pdf.
- 208.Creech C.B., Walker S.C., Samuels R.J. SARS-CoV-2 Vaccines. JAMA. 2021;325:1318–1320. doi: 10.1001/jama.2021.3199. [DOI] [PubMed] [Google Scholar]
- 209.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.NIH A Study to Evaluate the Safety and Immunogenicity of Vaccine CVnCoV in Healthy Adults in Germany for COVID-19. [(accessed on 18 June 2021)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04674189?id=NCT04639466+OR+NCT04655625+OR+NCT04662697+OR+NCT04683224+OR+NCT04668339+OR+NCT04674189+OR+NCT04665258+OR+NCT04646590+OR+NCT04642638+OR+NCT04656613+OR+NCT04648800+OR+NCT04649515+OR+NCT04677660+OR+NCT04668625+OR+NCT04649021+OR+NCT04649151+OR+NCT04659486+OR+NCT04664075&draw=2&rank=3&load=cart.
- 211.CureVac CureVac’s mRNA-based vaccine candidate against COVID-19. [(accessed on 16 June 2021)]. Available online: https://www.curevac.com/en/covid-19/
- 212.Rauch S., Roth N., Schwendt K., Fotin-Mleczek M., Mueller S.O., Petsch B. mRNA-based SARS-CoV-2 vaccine candidate CVnCoV induces high levels of virus-neutralising antibodies and mediates protection in rodents. NPJ Vaccines. 2021;6:57. doi: 10.1038/s41541-021-00311-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.NIH A Phase III Clinical Study of a SARS-CoV-2 Messenger Ribonucleic Acid (mRNA) Vaccine Candidate Against COVID-19 in Population Aged 18 Years and Above. [(accessed on 17 May 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04847102.
- 214.Zhang N.-N., Li X.-F., Deng Y.-Q., Zhao H., Huang Y.-J., Yang G., Huang W.-J., Gao P., Zhou C., Zhang R.-R., et al. A Thermostable mRNA Vaccine against COVID-19. Cell. 2020;182:1271–1283. doi: 10.1016/j.cell.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.NIH A Study to Evaluate the Immunogenicity and Safety of mRNA-1273.211 Vaccine for COVID-19 Variants. [(accessed on 15 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04927065.
- 216.Wu K., Choi A., Koch M., Ma L., Hill A., Nunna N., Huang W., Oestreicher J., Colpitts T., Bennett H., et al. Preliminary Analysis of Safety and Immunogenicity of a SARS-CoV-2 Variant Vaccine Booster. medRxiv. 2021 doi: 10.1101/2021.05.05.21256716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.NIH Dose-Confirmation Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of mRNA-1273 COVID-19 Vaccine in Adults Aged 18 Years and Older. [(accessed on 5 May 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04405076.
- 218.NIH Safety and Immunogenicity Study of a SARS-CoV-2 (COVID-19) Variant Vaccine (mRNA-1273.351) in Naïve and Previously Vaccinated Adults. [(accessed on 2 July 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04785144.
- 219.NIH A Trial Evaluating the Safety and Effects of an RNA Vaccine ARCT-021 in Healthy Adults. [(accessed on 29 March 2021)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04668339?term=vaccination&cond=covid&draw=1.
- 220.NIH Open Label Extension Study to Assess the Safety and Long-Term Immunogenicity of ARCT-021. [(accessed on 28 January 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04728347.
- 221.Holdings A.T. Arcturus—A Clinical-Stage mRNA Therapeutics and Vaccines Company. [(accessed on 10 June 2021)];Biopharma Deal. 2021 Available online: https://www.nature.com/articles/d43747-021-00073-3. [Google Scholar]
- 222.NIH Study of mRNA Vaccine Formulation against COVID-19 in Healthy Adults 18 Years of Age and Older (VAW00001) [(accessed on 29 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04798027?term=Sanofi&cond=COVID-19&draw=2&rank=2.
- 223.Kalnin K.V., Plitnik T., Kishko M., Zhang J., Zhang D., Beauvais A., Anosova N.G., Tibbitts T., DiNapoli J., Ulinski G., et al. Immunogenicity and efficacy of mRNA COVID-19 vaccine MRT5500 in preclinical animal models. NPJ Vaccines. 2021;6:61. doi: 10.1038/s41541-021-00324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sanofi Sanofi and Translate Bio Initiate Phase 1/2 Clinical Trial of mRNA COVID-19 Vaccine Candidate. [(accessed on 12 March 2021)]. Available online: https://www.sanofi.com/en/media-room/press-releases/2021/2021-03-12-07-00-00-2191846#.
- 225.NIH Study of DS-5670a (COVID-19 Vaccine) in Japanese Healthy Adults and Elderly Subjects. [(accessed on 8 July 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04821674.
- 226.Yan Z.P., Yang M., Lai C.L. COVID-19 Vaccines: A Review of the Safety and Efficacy of Current Clinical Trials. Pharmaceuticals. 2021;14:406. doi: 10.3390/ph14050406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.NIH Safety and Immunogenicity of EXG-5003. [(accessed on 30 April 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04863131.
- 228.ISRCTN Registry Clinical Trial to Assess the Safety of a Coronavirus Vaccine in Healthy Men and Women. 2020. [(accessed on 7 September 2021)]. Available online: https://www.isrctn.com/ISRCTN17072692.
- 229.Imperial College London COVAC1: How the Trial Works. [(accessed on 7 June 2021)]. Available online: https://www.imperial.ac.uk/covid-19-vaccine-trial/trial-info/
- 230.Wise N. The Latest Development in ChulaCov19 Vaccine. [(accessed on 30 March 2021)]. Available online: https://www.newswise.com/coronavirus/the-latest-development-in-chulacov19-vaccine.
- 231.NIH ChulaCov19 mRNA Vaccine in Healthy Adults. [(accessed on 6 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04566276.
- 232.NIH PTX-COVID19-B, an mRNA Humoral Vaccine, is Intended for Prevention of COVID-19 in a General Population. This Study is Designed to Evaluate Safety, Tolerability, and Immunogenicity of PTX-COVID19-B Vaccine in Healthy Seronegative Adults Aged 18–64. [(accessed on 30 June 2021)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04765436?id=NCT04747821+OR+NCT04756830+OR+NCT04733807+OR+NCT04728828+OR+NCT04760704+OR+NCT04765436&draw=2&rank=1&load=cart.
- 233.Susha Cheriyedath M.S. SARS-CoV-2 mRNA Vaccine PTX-COVID19-B Safe and Highly Immunogenic in Preclinical Study. [(accessed on 18 May 2021)]. Available online: https://www.news-medical.net/news/20210518/SARS-CoV-2-mRNA-vaccine-PTX-COVID19-B-safe-and-highly-immunogenic-in-preclinical-study.aspx.
- 234.NIH A Study of the Safety of and Immune Response to Varying Doses of a Vaccine Against COVID-19 in Healthy Adults. [(accessed on 21 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04758962.
- 235.NIH Phase 1 Study to Assess Safety, Reactogenicity and Immunogenicity of the HDT-301 Vaccine Against COVID-19. [(accessed on 19 April 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04844268.
- 236.NIH A Study to Evaluate Safety, Reactogenicity, and Immunogenicity of mRNA-1283 and mRNA-1273 Vaccines in Healthy Adults Between 18 Years and 55 Years of Age to Prevent COVID-19. [(accessed on 10 May 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04813796.
- 237.Moderna, Inc First Participants Dosed in Phase 1 Study Evaluating mRNA-1283, Moderna’s Next Generation COVID-19 Vaccine. [(accessed on 15 March 2021)]. Available online: https://investors.modernatx.com/news-releases/news-release-details/first-participants-dosed-phase-1-study-evaluating-mrna-1283.
- 238.ChiCTR Randomized, Double-Blind, Placebo-Controlled Phase I Clinical Trial to Evaluate the Safety and Immunogenicity of mRNACOVID-19 Vaccine in Healthy Susceptible Populations Aged 18 Years and Older People. [(accessed on 7 September 2021)]. Available online: http://www.chictr.org.cn/showprojen.aspx?proj=126046.
- 239.BioWorld Stemirna Raises Nearly $200M to Advance mRNA COVID-19 Vaccine. [(accessed on 4 June 2021)]. Available online: https://www.bioworld.com/articles/507855-stemirna-raises-nearly-200m-to-advance-mrna-covid-19-vaccine?v=preview.
- 240.NIH Safety and Immunogenicity of LNP-nCOV saRNA-02 Vaccine against SARS-CoV-2, the Causative Agent of COVID-19 (COVAC-Uganda) [(accessed on 22 June 2021)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04934111?term=vaccine&type=Intr&cond=Covid19&draw=2.
- 241.NIH A Study to Evaluate the Efficacy, Immune Response, and Safety of a COVID-19 Vaccine in Adults ≥18 Years With a Pediatric Expansion in Adolescents (12–17 Years) at Risk for SARS-CoV-2. [(accessed on 6 May 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04611802.
- 242.Shinde V., Bhikha S., Hoosain Z., Archary M., Bhorat Q., Fairlie L., Lalloo U., Masilela M.S.L., Moodley D., Hanley S., et al. Efficacy of NVX-CoV2373 Covid-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021;384:1899–1909. doi: 10.1056/NEJMoa2103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Novavax, Inc. Novavax COVID-19 Vaccine Demonstrates 89.3% Efficacy in UK Phase 3 Trial. 2021. [(accessed on 7 September 2021)]. Available online: https://ir.novavax.com/news-releases/news-release-details/novavax-covid-19-vaccine-demonstrates-893-efficacy-uk-phase-3.
- 244.Yang S., Li Y., Dai L., Wang J., He P., Li C., Fang X., Wang C., Zhao X., Huang E., et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: Two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect. Dis. 2021;21:1107–1119. doi: 10.1016/S1473-3099(21)00127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.NIH A Phase III Clinical Trial to Determine the Safety and Efficacy of ZF2001 for Prevention of COVID-19. [(accessed on 7 May 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04646590.
- 246.NIH Study of Monovalent and Bivalent Recombinant Protein Vaccines Against COVID-19 in Adults 18 Years of Age and Older (VAT00008) [(accessed on 11 June 2021)];2021 Available online: https://clinicaltrials.gov/ct2/show/study/NCT04904549.
- 247.Sanofi Sanofi and GSK Initiate Global Phase 3 Clinical Efficacy Study of COVID-19 Vaccine Candidate. [(accessed on 7 September 2021)]. Available online: https://www.sanofi.com/en/media-room/press-releases/2021/2021-05-27-07-30-00-2236989.
- 248.ZAWYA Cuba Encouraged by Early Efficacy Results of Homegrown COVID-19 Vaccine. [(accessed on 20 June 2021)]. Available online: https://www.zawya.com/mena/en/economy/story/Cuba_encouraged_by_early_efficacy_results_of_homegrown_COVID19_vaccine-TR20210620nL2N2O2003X1/
- 249.RPCEC SOBERANA 02-FaseIII. [(accessed on 7 September 2021)]. Available online: https://rpcec.sld.cu/en/trials/RPCEC00000354-En.
- 250.Chang-Monteagudo A., Ochoa-Azze R., Climent-Ruiz Y., Macías-Abraham C., Rodríguez-Noda L., Valenzuela-Silva C., Sánchez-Ramírez B., Perez-Nicado R., González-Mugica R., Hernández-García T., et al. A single dose of SARS-CoV-2 FINLAY-FR-1A dimeric-RBD recombinant vaccine enhances neutralization response in COVID-19 convalescents, with excellent safety profile. A preliminary report of an open-label phase 1 clinical trial. medRxiv. 2021 doi: 10.1101/2021.02.22.21252091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.NIH A Global Phase III Clinical Trial of Recombinant COVID- 19 Vaccine (Sf9 Cells) [(accessed on 30 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04887207.
- 252.NIH Study of the Tolerability, Safety, Immunogenicity and Preventive Efficacy of the EpiVacCorona Vaccine for the Prevention of COVID-19. [(accessed on 3 March 2021)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04780035?term=vaccine&cond=Covid19&draw=2.
- 253.News D. Two More Russian Vaccines: What We Do and Don’t Know. [(accessed on 3 March 2021)]. Available online: https://www.dw.com/en/two-more-russian-vaccines-what-we-do-and-dont-know/a-56811025.
- 254.Mahase E. Covid-19: Russian vaccine efficacy is 91.6%, show phase III trial results. BMJ. 2021;372:n309. doi: 10.1136/bmj.n309. [DOI] [PubMed] [Google Scholar]
- 255.RPCEC ABDALA Clinical Study—Phase III. [(accessed on 8 September 2021)]. Available online: https://rpcec.sld.cu/trials/RPCEC00000359-En.
- 256.NIH Study to Evaluate the Safety, Immunogenicity, and Efficacy of Nanocovax Vaccine Against COVID-19. [(accessed on 8 September 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04922788.
- 257.NIH A Controlled Phase 2/3 Study of Adjuvanted Recombinant SARS-CoV-2 Trimeric S-protein Vaccine (SCB-2019) for the Prevention of COVID-19 (SCB-2019) [(accessed on 11 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04672395.
- 258.Liang J.G., Su D., Song T.-Z., Zeng Y., Huang W., Wu J., Xu R., Luo P., Yang X., Zhang X., et al. S-Trimer, a COVID-19 subunit vaccine candidate, induces protective immunity in nonhuman primates. Nat. Commun. 2021;12:1346. doi: 10.1038/s41467-021-21634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Richmond P., Hatchuel L., Dong M., Ma B., Hu B., Smolenov I., Li P., Liang P., Han H.H., Liang J., et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: A phase 1, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397:682–694. doi: 10.1016/S0140-6736(21)00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.NIH A Study to Evaluate the Safety, Immunogenicity, and Efficacy of UB-612 COVID-19 Vaccine. [(accessed on 24 December 2020)]; Available online: https://www.clinicaltrials.gov/ct2/show/study/NCT04683224?id=NCT04639466+OR+NCT04655625+OR+NCT04662697+OR+NCT04683224+OR+NCT04668339+OR+NCT04674189+OR+NCT04665258+OR+NCT04646590+OR+NCT04642638+OR+NCT04656613+OR+NCT04648800+OR+NCT04649515+OR+NCT04677660+OR+NCT04668625+OR+NCT04649021+OR+NCT04649151+OR+NCT04659486+OR+NCT04664075&draw=2&rank=1&load=cart.
- 261.RPCEC SOBERANA 01. [(accessed on 3 July 2021)]. Available online: https://rpcec.sld.cu/en/trials/RPCEC00000332-En.
- 262.IRCT A phase II, Randomized, Two-armed, Double-Blind, Placebo Controlled Trial to Evaluate Efficacy and Safety of an Adjuvanted Recombinant SARS-CoV-2 Spike (S) Protein Subunit Vaccine (SpikoGen®) Produced by CinnaGen Co. (Two doses of 25 µg with Dosing Interval of 21 days) 2021. [(accessed on 3 July 2021)]. Available online: https://www.irct.ir/trial/56287.
- 263.NIH A Study to Evaluate MVC-COV1901 Vaccine Against COVID-19 in Adult (COVID-19) [(accessed on 30 March 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04695652.
- 264.NIH A Study to Evaluate MVC-COV1901 Vaccine Against COVID-19 in Elderly Adults. [(accessed on 15 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04822025.
- 265.Hsieh S.-M., Liu W.-D., Huang Y.-S., Lin Y.-J., Hsieh E.-F., Lian W.-C., Chen C., Janssen R., Shih S.-R., Huang C.-G., et al. Safety and immunogenicity of a Recombinant Stabilized Prefusion SARS-CoV-2 Spike Protein Vaccine (MVC COV1901) Adjuvanted with CpG 1018 and Aluminum Hydroxide in healthy adults: A Phase 1, dose-escalation study. EClinicalMedicine. 2021;38:100989. doi: 10.1016/j.eclinm.2021.100989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.IRCT Phase II, Safety and Immunogenicity of RAZI SARS-CoV-2 Recombinant Spike Protein Vaccine (RAZI Cov Pars) in Adults Aged 18–70 Years; a Randomised, Double Blind, parallel 2 Arms Clinical Trial. 2021. [(accessed on 8 September 2021)]. Available online: https://en.irct.ir/trial/55238.
- 267.ChiCTR A Randomized, Double-Blind, Placebo-Controlled Phase II Clinical Trial to Evaluate the Immunogenicity and Safety of Recombinant SARS-CoV-2 Fusion Protein Vaccine (V-01) in Healthy Subjects. 2021. [(accessed on 9 July 2021)]. Available online: http://www.chictr.org.cn/showproj.aspx?proj=124702.
- 268.RPCEC MAMBISA Study. 2021. [(accessed on 9 July 2021)]. Available online: https://rpcec.sld.cu/en/trials/RPCEC00000345-En.
- 269.EurekAlert BAT Progresses COVID-19 Candidate Vaccine into Phase I Human Clinical Trials. [(accessed on 1 July 2021)]. Available online: https://www.eurekalert.org/pub_releases/2020-12/raba-bpc121520.php.
- 270.NIH KBP-201 COVID-19 Vaccine Trial in Healthy Volunteers. [(accessed on 10 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04473690.
- 271.Biological E’s Novel Covid-19 Vaccine of SARS-CoV-2 for Protection against Covid-19 Disease. [(accessed on 10 November 2020)]. Available online: http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=48329.
- 272.JRCT A Phase 1/2, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study of S-268019 in Japanese Adult Participants. [(accessed on 9 December 2020)]. Available online: https://jrct.niph.go.jp/en-latest-detail/jRCT2051200092.
- 273.NIH Anti-COVID19 AKS-452—ACT Study (ACT) [(accessed on 23 April 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04681092?term=NCT04681092&draw=2&rank=1.
- 274.NIH A Clinical Trial of COVAC-2 in Healthy Adults. [(accessed on 1 April 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04702178.
- 275.NIH Safety and Immunogenicity Study of SARS-CoV-2 Nanoparticle Vaccine (GBP510) Adjuvanted with Aluminum Hydroxide (COVID-19) [(accessed on 8 February 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04742738.
- 276.NIH Reactogenicity, Safety and Immunogenicity of QazCoVac-P COVID-19 Vaccine. [(accessed on 18 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04930003?term=vaccine&recrs=adf&cond=COVID-19&phase=0123&sort=nwst&draw=2.
- 277.NIH Safety, Tolerance and Immunogenicity of EuCorVac-19 for the Prevention of COVID-19 in Healthy Adults. [(accessed on 26 April 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04783311.
- 278.NIH A Clinical Trial to Evaluate the Recombinant SARS-CoV-2 Vaccine (CHO Cell) for COVID-19. [(accessed on 7 May 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04869592.
- 279.NIH A Study on the Safety, Tolerability and Immune Response of SARS-CoV-2 Sclamp (COVID-19) Vaccine in Healthy Adults. [(accessed on 23 April 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04495933.
- 280.Watterson D., Wijesundara D.K., Modhiran N., Mordant F.L., Li Z., Avumegah M.S., McMillan C.L., Lackenby J., Guilfoyle K., van Amerongen G., et al. Preclinical development of a molecular clamp-stabilised subunit vaccine for severe acute respiratory syndrome coronavirus 2. Clin. Transl. Immunol. 2021;10:e1269. doi: 10.1002/cti2.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Chappell K.J., Mordant F.L., Li Z., Wijesundara D.K., Ellenberg P., Lackenby J.A., Cheung S.T.M., Modhiran N., Avumegah M.S., Henderson C.L., et al. Safety and immunogenicity of an MF59-adjuvanted spike glycoprotein-clamp vaccine for SARS-CoV-2: A randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 2021;21:1383–1394. doi: 10.1016/S1473-3099(21)00200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.NIH Safety and Immunogenicity Trial of Multi-peptide Vaccination to Prevent COVID-19 Infection in Adults (pVAC) [(accessed on 1 December 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04546841?term=vaccine&cond=covid-19&draw=2.
- 283.NIH A Study to Evaluate the Safety and Immunogenicity of COVID-19 (AdimrSC-2f) Vaccine. [(accessed on 13 January 2021)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT04522089.
- 284.NIH Safety and Immunogenicity of a SARS-CoV-2 Vaccine (NBP2001) in Healthy Adults (COVID-19) [(accessed on 18 February 2021)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04760743.
- 285.NIH Safety, Reactogenicity and Immunogenicity Study of ReCOV. [(accessed on 26 March 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04818801.
- 286.NIH SARS-COV-2-Spike-Ferritin-Nanoparticle (SpFN) Vaccine With ALFQ Adjuvant for Prevention of COVID-19 in Healthy Adults. [(accessed on 20 April 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04784767.
- 287.Joyce M.G., King H.A.D., Naouar I.E., Ahmed A., Peachman K.K., Cincotta C.M., Subra C., Chen R.E., Thomas P.V., Chen W.-H., et al. Efficacy of a Broadly Neutralizing SARS-CoV-2 Ferritin Nanoparticle Vaccine in Nonhuman Primates. bioRxiv. 2021 doi: 10.1101/2021.03.24.436523. [DOI] [Google Scholar]
- 288.Carmen J.M., Shrivastava S., Lu Z., Anderson A., Morrison E.B., Sankhala R.S., Chen W.-H., Chang W.C., Bolton J.S., Matyas G.R., et al. A spike-ferritin nanoparticle vaccine induces robust innate immune activity and drives polyfunctional SARS-CoV-2-specific T cells. bioRxiv. 2021 doi: 10.1101/2021.04.28.441763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.NIH To Evaluate the Safety, and Immunogenicity of Vaccine Candidate against COVID-19, in Healthy Adults (COVEPIT 3) [(accessed on 9 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04885361.
- 290.NIH First-in-Human Study Of Orally Administered CoV2-OGEN1 in Healthy Subjects. [(accessed on 7 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04893512.
- 291.NIH Study of a Recombinant Coronavirus-Like Particle COVID-19 Vaccine in Adults. [(accessed on 24 May 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04636697.
- 292.Ward B.J., Gobeil P., Séguin A., Atkins J., Boulay I., Charbonneau P.-Y., Couture M., D’Aoust M.-A., Dhaliwall J., Finkle C., et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat. Med. 2021;27:1071–1078. doi: 10.1038/s41591-021-01370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.ACT A Phase 1/2 Randomized, Placebo-Controlled, Multi-Centre Study to Evaluate the Safety and Immunogenicity of COVID-19 Vaccine in Healthy Adults. [(accessed on 4 July 2021)];2020 Available online: https://www.australianclinicaltrials.gov.au/anzctr/trial/ACTRN12620000817943.
- 294.NIH Safety, Tolerability, and Immunogenicity of the COVID-19 Vaccine Candidate (VBI-2902a) [(accessed on 21 April 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04636697https://clinicaltrials.gov/ct2/show/NCT04773665.
- 295.NIH Study of a Severe Acute Respiratory Syndrome CoV-2 (SARS-CoV-2) Virus-Like Particle (VLP) Vaccine in Healthy Adults (COVID-19) [(accessed on 8 April 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04818281?cond=NCT04818281&draw=2.
- 296.NIH Safety and Tolerability of COVID-19 Vaccine (ABNCoV2) (COUGH-1) [(accessed on 9 April 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04839146.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in the review were retrieved from the World Health Organization (WHO) vaccine tracker and landscape website (https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines) (accessed on 15 June 2021) and/or other publicly available resources as detailed through in-text citation and the references section of the review. Figures were created with BioRender.com.