Abstract
Soil flooding severely impairs agricultural crop production. Plants can cope with flooding conditions by embracing an orchestrated set of morphological adaptations and physiological adjustments that are regulated by the elaborated hormonal signaling network. The most prominent of these hormones is ethylene, which has been firmly established as a critical signal in flooding tolerance. ABA (abscisic acid) is also known as a “stress hormone” that modulates various responses to abiotic stresses; however, its role in flooding tolerance remains much less established. Here, we discuss the progress made in the elucidation of morphological adaptations regulated by ABA and its crosstalk with other phytohormones under flooding conditions in model plants and agriculturally important crops.
Keywords: abscisic acid, phytohormones, morphological adaptations, flooding stress
1. Introduction
Flooding is a major environmental constraint affecting crop production systems. While it is particularly acute in Asia [1], this is a world-wide problem that will come with a significant economic cost [2]. In light of climate change and global warming, it is anticipated that the future threats of flooding are likely to increase, affecting more regions [3]. Thus, the importance of dealing with impacts of flooding on the agricultural sector has emerged as one of the critical issues for food security [4]. The term flooding encompasses both waterlogging and submergence conditions. Waterlogging refers to the conditions in which the root-zones are saturated with excessive water, whereas submergence is defined as a situation in which the aboveground parts of plants are partially or completely covered by water [5]. The adversity of flooding stress is largely due to the dramatically restricted availability of oxygen for submerged plant tissues since gases’ diffusion is many-fold slower in water than in air [6]. Reduced O2 diffusion in saturated soils suppresses aerobic respiration and subsequently causes an energy crisis and accumulation of toxic substances [7]. Waterlogging of the rhizosphere or partial flooding of the aboveground parts of a plant lead to a gradual hypoxia (deficiency of oxygen), and long-term complete submergence brings about anoxia (the total absence of oxygen [8]).
Plants have developed several strategies to cope with the adverse consequences of submergence conditions. One strategy is known as the quiescence strategy where plants do not elongate shoots under flooding conditions, to minimize energy and carbohydrate consumption, but continue to regrow after stress. This strategy has been efficiently implemented by wetland species, which could survive relatively deep and transient floods (e.g., Rumex acetosa) [9,10,11,12,13]. Another strategy (known as an escape strategy) is utilized by plants such as deepwater rice and the wetland dicot Rumex palustris. Here, plants rapidly extend their petioles or stems to allow leaves to reach the water surface to aerate the remainder of the plant [10,14,15,16,17]. The escape strategy is effective under prolonged, but relatively shallow, flooding events [17,18].
The key regulator of these flooding-induced acclimations is ethylene (ET), which, owing to its gaseous nature, rapidly accumulates in flooded tissues [6,14,19]. In addition, a permeability barrier for ET in the roots prevents ET losses and leads to the accumulation of high levels of ET in root tissues [20]. ET is an early and reliable signal for different complementary pathways required for morphological and anatomical acclimations under flooding conditions, like aerenchyma formation, adventitious roots (ARs) formation, and shoot growth of plants in response to flooding stress. The rapid accumulation of ET may work as a priming factor for these adaptive responses of plants to flooding [8].
A wide range of studies found that interactions of ET with other phytohormones, and specifically, ABA, were also important for adaptations to flooding stress. ABA is known as a “stress hormone” that accumulates under different abiotic stresses and mediates cross-adaptation in plants [21]. Under drought, salinity, or cold stress, ABA accumulation causes stomatal closure to conserve water in leaves [22,23,24]. However, compared with ET or GA (gibberellin), the role of ABA in flooding tolerance remains much less established. The aim of this work was to fill this gap in the knowledge and review the progress made in the elucidation of morphological adaptations regulated by ABA and its crosstalk with other phytohormones under flooding conditions.
2. Changes in ABA Content and ABA-Regulated Responses during Submergence
The changes of ABA content during flooding stress are summarized in Table 1, and they are species-dependent. In an early study, ABA was considered as a potent antagonist of GA action in rice internodes through influencing GA responsiveness, evidenced by both applied ethylene and submergence, which reduced endogenous ABA levels in internodes significantly within 3 h [25]. The submergence- and ethylene-mediated decline of ABA levels in deepwater and lowland rice has also been demonstrated by researchers [26,27,28].
Table 1.
Changes of ABA concentration affected by flooding stress in different plant species.
| Stress | Species | Tissue | Response | Reference |
|---|---|---|---|---|
| flooding | Solanum dulcamara | stems, AR primordia | decreased | [11,33] |
| flooding | Rumex palustris | petioles | sharply decreased | [36] |
| submergence | Lolium perenne | leaf | decreased | [5] |
| submergence | Oryza sativa, deepwater | internodes | decreased | [25] |
| submergence | Oryza sativa, lowland | shoot | decreased | [28] |
| submergence | Scirpus micronatus | shoots | decreased | [34] |
| flooding | citrus | root | decrease | [37] |
| submergence | Rumex palustris | petioles | decrease significantly | [11] |
| submergence | Nasturtium officinale | petioles, stem | decrease | [38] |
| waterlogging | Carrizo citrange | roots | decreased, back to control | [35] |
| flooding | citrus | leaf | sharp increase | [37] |
| flooding | Pilsum sativum L. | shoot | increase | [39] |
| flooding | Pisum sativum L. | root | early increase | [39] |
| flooding | Nicotiana tabacum L. | leaf | increase | [40] |
| waterlogging | Vigna radiata L. | leaf | increase | [41] |
| anoxia | Lactuca sativa L. | roots | unchanged | [42] |
| flooding | Glycine max | seedlings | unchanged | [43] |
The accumulated ET mimics a submerged environment and reduces ABA concentration via activating the ABA breakdown to inactive ABA catabolite phaseic acid in rice [29]. In addition, under completely submerged conditions, the accumulated ET in Rumex palustris causes a massive depletion of ABA levels, and the decline in the endogenous ABA concentration stimulates the expression of gibberellin 3-oxidase (GA3OX1), which catalyzes the conversion to bioactive gibberellin GA1 [11,12].
However, ET does not significantly alter the expression of genes involved in ABA metabolism, as is evident from the analysis of several Arabidopsis ET receptor mutants [30]. In another study, the Ethylene Response Factor VII (ERF-VII) transcription factors RELATED TO APETALA2.12 (RAP2.12), RAP2.2, and RAP2.3 were all induced by exogenous ABA treatment in Arabidopsis. In addition, the enhanced ABA sensitivity of the RAP2 genes overexpressors might be due to the activation of abscisic acid insensitive 5 (ABI5) [31]. Moreover, hypoxia disturbs ABA metabolism and increases ABA sensitivity in embryos of dormant barley grains [32]. Similarly, two perennial ryegrass accessions exhibited a dramatic decrease in ABA content, which resulted from enhanced ABA catabolism via the upregulation of ABA8Ox1, although the expression of zeaxanthin epoxidase and 9-cis-epoxycarotenoid dioxygenase 1 (NCED1) was upregulated under submergence stress [5]. The decrease of the ABA content in plants during flooding stress was also reported in Solanum dulcamara, Scirpus mucronatus, and Carrizo citrange [33,34,35].
However, in other species, such as Nasturtium officinale (watercress), ABA breakdown is not likely regulated by ET, since ACC (l-aminocyclopropane-l-carboxylic acid) application did not alter NoNCED3 or NoCYP707A1/A2 gene expression, and submergence did not induce GA biosynthesis genes in this species [38]. Taken together, this suggests that although ABA depletion is a common feature in hypoxic plant tissues, it might be independently regulated in a species-specific manner rather than necessarily being under ET control.
On the contrary, elevated levels of ABA content in response to flooding have also been reported in the leaves of alfalfa, pea, and tobacco, as well as in the roots of Gerbera jamesonii [40,44,45,46]. Meanwhile, the endogenous ABA concentration remained unchanged in response to flooding in soybean (Glycine max) and lettuce (Lactuca sativa) [42,43].
The above controversy in results poses a question as to the real relationship among ABA, ET, and submergence-induced reactions. It could be suggested that plants grow quickly to escape submergence stress by the fast drop in the endogenous ABA level, while hypoxia-susceptible plants usually respond to oxygen shortage by a drastic increase of the ABA concentration [8].
Another routine to study the function of phytohormones is to measure the response of plants after the exogenous phytohormone application of mutants (see Table 2 for selected examples).
Table 2.
The effect of ABA on flooding stress in different plant species.
| Species | Chemicals | Concentrations (uM) | Effect | Treatment | Reference |
|---|---|---|---|---|---|
| Lactuca sative L. | ABA | 1, 3, 10, 30, 100, 300 | increase survivability | anoxia 24 h | [42] |
| Glycine max | ABA | 5, 10, 50 | increase the survival | flooding | [43] |
| Oryza sativa | ABA | 0.1 uM, 24 h | improving seeds resistance | submergence | [53] |
| Arabidopsis | ABA | 10, 50, 100 | increase tolerance | anoxia | [54] |
| Zea mays. L. | ABA | 100 uM, 24 h | increase tolerance | anoxia | [55,56] |
| Nasturtium officinale | ABA | 0.5, 1, 5 | inhibits shoot elongation | submergence | [38] |
| Rumex palustris | ABA | - | inhibits petiole elongation | submergence | [57] |
| Oryza sativa | ABA | 1 | inhibits petiole elongation | submergence | [25] |
| Oryza sativa | fluridone (biosynthesis inhibitor) | - | induced AR emergence | submergence | [58,59,60] |
The interaction between flooding and other soil factors, such fertilizers and pollutants, needs to be accounted for, as ABA levels in plants are subjected to orchestrated actions exerted by these factors. For example, the concentration of ABA in the soil solution was highest in acid soils and in soils with reduced moisture, while it was lowest in moist, neutral, and moderately alkaline soils [47]. Pollutants, like heavy metals and other toxic substances, result in a shift in the biological activity of the soil [48,49]. ABA content is also much higher in plants grown under conditions of soil salinity [50] and changes in plant ontogeny [51].
The climate also exerts a major impact on ABA production; thus, plant responses to flooding in a dry/warm climate will be strikingly different from those under humid or cold conditions. For example, ABA content is significantly higher in cold-grown plants (compared with optimal temperature conditions) [52]. This difference in the basal ABA levels will impact patterns of ABA signaling and, hence, plant adaptation to hypoxia.
3. Stomatal Closure and Root-Shoot Response
Stress-induced ABA-mediated stomata closure has been observed in plants exposed to hyperosmotic conditions, such as drought or salinity [22,23,24]. Surprisingly, plants also close stomata in response to waterlogging. This process delays leaf chlorosis and senescence, although the underlying mechanisms are poorly understood [61].
ABA is known to act in root-to-shoot signaling in the drought response; it is therefore reasonable to suggest that ABA may act as a mobile signal for root-to-shoot communication during waterlogging stress as well [62]. However, a transcriptome study performed on Arabidopsis revealed an upregulation of ABA biosynthesis genes in leaves and a downregulation in roots under flooding conditions [63], which was reflected in the actual ABA levels in shoots and roots. Interestingly, NCED3 was slightly induced in the ET insensitive mutant ein2-5, indicating that ET alters ABA biosynthesis and signaling in systemic responses [63]. The ABA concentration was also decreased in the xylem sap in flooded tomato roots [64]. It was suggested that ABA may accumulate in leaves of flooded plants because of reduced translocation of photoassimilates out of leaves, and roots do not act as the source of ABA because most roots collapse rapidly and die within the first few days of flooding [45]. This model was further supported by experiments with wilty mutants of peas and tomatoes, showing that ABA accumulation in the leaves of flooded plants is determined by the shoot rather than the root genotype [45]. This notion is rather similar to the modern view of ABA-mediated signaling in response to salinity and drought [22,23,24] that also questions the root origin of ABA signals.
The nature of the mobile signal that mediates stomatal closure remains elusive, and no solid evidence could support the transport of phytohormones from the root to the shoot prior to flooding-induced stomatal closure [65,66,67]. In waterlogged citrus leaves, JA (jasmonic acid) levels rapidly but transiently increased preceding the progressive accumulation of ABA. In citrus roots, as indole-3-acetic acid increased, JA and ABA levels decreased rapidly and significantly in all genotypes under flooded conditions [37]. Similarly, the ET precursor ACC, JA, or metabolite fluxes might contribute to systemic flooding stress adaptation.
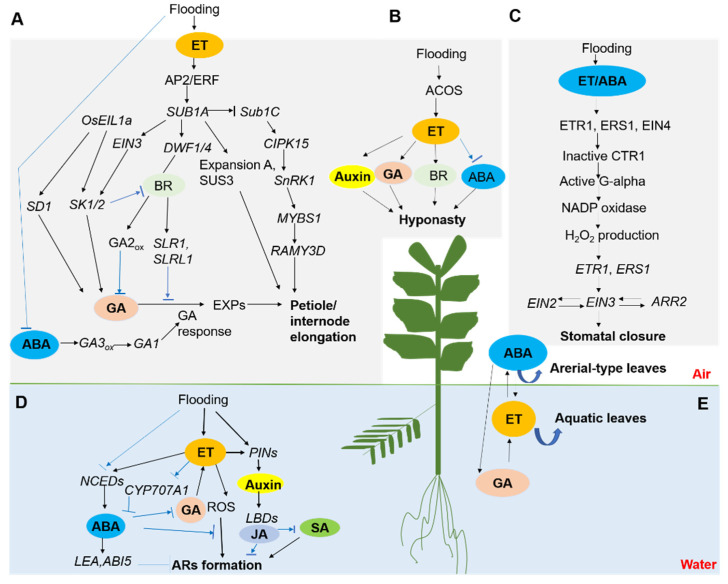
The morphological adaptations regulated by ET, GA, and ABA are summarized in Figure 1.
Figure 1.
A schematic model of plants’ morphological adaptations during flooding stress (adapted from [17,33,68,69,70,71,72,73,74]). To cope with flooding stress, plants undergo multifaceted anatomical, metabolic, and morphologic alterations. The morphological adaptations include shoot elongation (A), hyponasty (B), stomatal closure (C), adventitious roots formation (D), and heterophylly induction (E). The model is collective and integrates findings reported in one or more species. The interactions and hierarchy of signaling components can vary depending on species. (A): Processes, hormones, and genes involved in submergence-induced shoot elongation (blue arrows indicate an inhibitory effect). Submergence causes accumulation of ethylene, and subsequently, it induces reduction of ABA biosynthesis and promotes the ABA catabolism, which leads to a lower endogenous ABA concentration in rice. This stimulates GA signaling and ultimately enhances petiole elongation. BR is also involved in this process. (B): Increased ethylene biosynthesis in waterlogged Arabidopsis is linked to transcript accumulation of ACO5. GA, BR, auxin, and ABA positively regulate the hyponasty, while the accumulation of ABA was inhibited by ethylene. (C): A proposed model for the hormone-mediated stomatal closure under waterlogging stress. Ethylene and/or ABA directly or indirectly enhance H2O2 production under waterlogging conditions. The binding of ethylene to ETR1, ERS1, and EIN4 induced the inactivation of CTR1, resulting in the activation of G alpha, which promotes H2O2 production via NADPH oxidases. ETR1 and ERS1 translocate the signals of H2O2 to EIN2, EIN3, and ARR2, which are essential for stomatal closure functioning. (D): Adventitious root biogenesis and growth regulation in plants. Ethylene, which accumulates in submerged tissues, promotes adventitious root growth. GA enhances ethylene-induced root growth, while ABA acts as a root growth inhibitor. ROS act downstream of ethylene to mediate the root growth response. Auxin also promotes adventitious root biogenesis. (E): In aquatic plants, ABA could initiate and maintain the development of aerial-type leaves, and this process is dependent on the cross-talk with ethylene- and GA-signaling pathways, which initiate and maintain the formation of submerse leaves. Abbreviations—AP2/ERF: apelata2/ethylene response factor; CYP707A1: encode abscisic acid 8′-hydroxylases; ROS: reactive oxygen species; PINs: pin-formed protein; LBDs: lateral organ boundaries domain; ARs: adventitious root; DWF1/4: dwarf1/4; BR: brassinosteroids; SD1: semidwarf1; SK1/2: snorkel 1/2; EIL1a: ethylene insensitive like 1a; SLR1: Slender Rice-1; SUS3: sucrose synthetase 3; SnRK1: SNF1-related kinase 1; CIPK15: calcineurin B-like–interacting protein kinase gene; ACO: 1-aminocyclopropane-1-carboxylic acid oxidase, ACC oxidase; ACS: ACC synthase; AMY: amylase; EXP: expansins; SUB1: submergence1; NCED: 9-cis-epoxycarotenoid dioxygenase; GA3ox: gibberellin 3-oxidase; ARR2: 2-component response regulator; CTR1: constitutive triple response 1; EIN: ethylene insensitive; ERS1: Ethylene response sensor 1; ETR1: Ethylene receptor 1; G alpha: G protein alpha subunit; ARR2: 2-component response regulator.
4. Petioles Elongation and Hyponastic Growth
For plants using the escape strategy, the accumulation of ET causes a reduction of ABA content, which subsequently enhances the concentration of bioactive GA and plants’ sensitivity to GA [30,75]. GA is the ultimate hormone that stimulates both cell division in the intercalary meristem and internodal elongation. Under hypoxic conditions, the exogenous application of GA upregulates the activity of ACC synthases (OsACS5) in both lowland and deep-water rice seedlings, whereas the application of ABA has an adverse effect on the activity of OsACS5 [76]. The external application of ABA to deep-water rice and Rumex palustris restricts submergence-induced petiole elongation, indicating that ABA may function as a negative regulator of enhanced petiole growth [11,76]. The adverse effect of ABA on petiole growth could be reversed by GA, suggesting that submergence-induced growth is manipulated through the balance between ABA and GA [30]. Similarly, the rapid petiole growth of lotuses might also result from an altered balance between growth-inhibiting ABA and growth-promoting GA hormones [77], summarized in Figure 1.
The proposed sequence of events mediated by ET, GA, and ABA is that a low oxygen concentration leads to ET accumulation, which causes a decrease in ABA levels and an increase in GA1 concentration as well as GA sensitivity, and internodal elongation was promoted ultimately [78,79]. Different endogenous ABA contents determine different petioles elongation rates of two accessions of the wetland plant Rumex palustris under submerged conditions [80]. In addition, an elongating rice variety exhibited a stronger reduction in ABA concentration than a non-elongating variety [14]. ABA could inhibit H+ pumps in guard cells, and the reduced ABA content might stimulate acidification of the apoplast [18]. In addition, auxin can facilitate the activation of plasma membrane H+-ATPases, which leads to apoplast acidification and expansin activation [18,81,82]. Secondly, ABA might work as an antagonist of GA and suppress root growth for survival during flooding. A reduction in ABA content might lead to an increased GA signal strength, which enhances starch breakdown in the shoot to generate sugars to fuel proton pump activity and provide building blocks for cell wall synthesis (Figure 1). Together with cell wall acidification, the higher expression level of expansins promoted by GA can enhance cell wall extensibility, which is beneficial for elongation. GA biosynthesis inhibitor PAC or GA3 application did not significantly influence the elongation of watercress stem or petioles, which indicates that the role that GA played in watercress shoot growth underwater might be different from other plants [38].
IAA also plays a role in plants’ shoot elongation under flooding stress. However, neither removal of the lamina (putative auxin source) nor addition of N-1-naphthylphthalamic acid (NPA), which inhibit the polar auxin transport, had any effect on the submergence-induced elongation of R. palustris petioles over a two-day treatment [83]. In the same work, the application of a polar IAA transport inhibitor on Ranunculus scleratus prevented petiole extension, and ET was found to induce polar auxin transport [83]. Interestingly, a role of auxin in R. palustris has also been identified. Inhibiting polar auxin transport, chemically or by lamina removal, transiently reduced the underwater elongation response [84]. Furthermore, a rapid accumulation of auxin in the ab- and adaxial fragments of the elongating petioles was observed [75]. In addition, internode elongation in peas requires an interaction between auxin and GA [85]. Decapitation (removal of the auxin source) results in a downregulation of GAβ-hydroxylase, responsible for bioactive GA1 synthesis, and a reduction of the GA1 concentration in pea internodes, and this down-regulation can be completely rescued by the application of IAA [85]. This is indicative of internode elongation; a certain amount of endogenous IAA biosynthesis is required by GA1.
Hyponastic growth, together with petiole elongation, enables plants to reach the air and restore gaseous exchange [75,86,87]. The orientation of plant petioles and leaf blades changes from horizontal to almost vertical during complete submergence, and this phenomenon is known as a hyponastic growth [88]. During early submergence, a sharp decline of ABA, mediated by ET, is a prerequisite to avoid the inhibitory effect of ABA on hyponastic growth. ET-induced hyponastic leaf and petiole movement is dependent on GA [89]. In R. palustris, the lateral redistribution of auxin to the outer cell layers in the petiole is promoted by ET, and it likely leads to differential growth, given the expansion of specific cells [75]. ET was unable to induce differential cell expansion and leaf hyponasty in the Arabidopsis ROTUNDIFOLIA3 mutant, defective in the P450 cytochrome involved in brassinosteroids (BRs) biosynthesis. Chemical perturbation of BR biosynthesis also generates similar results, predicating the involvement of BRs in ET-regulated hyponasty [90] (Figure 1).
5. Heterophylly Initiation
Heterophylly is defined as abrupt changes in leaf morphology in a single plant in response to ambient environmental cues [91]. Leaves of constitutively submerged plants exhibit a distinct morphology with a narrow shape, lack of stomata, and reduced vessel development compared to terrestrial leaves. ET seems to be a key regulator of heterophyte formation in aquatic plants [92]. Exogenous ET application promotes the formation of submerged leaves, and the endogenous levels of ET are elevated in submerged plants compared to terrestrial plants [92,93]. Anatomical and developmental studies revealed that alterations in cell division patterns, promoted by ET, resulted in changes in leaf form. The modified cell division patterns were attributed to the overactivation of genetic networks composed of the ET signaling transducer ET INSENSITIVE3 and abaxial genes that repress genes underlying xylem and stomatal development, while the higher levels of ABA produced in terrestrial leaves played a positive role [94,95,96].
Submerged leaves produced higher levels of ET but lower levels of ABA compared with terrestrial leaves [96]. The exogenous application of ABA to submerged plants resulted in terrestrial-type leaves’ formation under submerged conditions [93,97]. Moreover, ET treatment reduces endogenous ABA levels, indicating that ET regulates heterophylly by suppressing ABA and regulating cell division and elongation [93] (Figure 1).
GA concentrations in leaf primordia change in response to circumambient environmental cues, and the application of exogenous GA alters leaf complexity in different plant species. Furthermore, GA reduces leaf complexity by inducing leaf primordia differentiation and disabling the formation of marginal serrations and leaflets by suppressing transient organogenetic activity in the leaf margins [98]. The expression levels of the KNOTTED1-like homeobox (KNOX1) gene, a negative regulator of GA biosynthesis, were altered in response to submergence, and consequently, the accumulation of GA was changed in the leaf primordia. Variations in the expression of KNOX1 appear to underlie differences in leaf shape [99,100]. Unsurprisingly, tGA has an adverse effect on heterophylly in aquatic plants [101]. Besides this, the effects of GA can either be enhanced by ET or inhibited by ABA.
Auxin polarization is crucial for leaf primordia initiation and for the outgrowth of leaf lamina during leaf development [102,103]. Auxins are also involved in vascular patterning in leaves, which affects leaf morphology [68]. Therefore, auxins could play a role in heterophylly as downstream targets of other upstream phytohormones.
In R. palustris, leaves acclimate by thinner epidermal cell walls and cuticles and by lying chloroplasts closer to the epidermis, which helps CO2 enter mesophyll cells through diffusion rather than stomata; an increase in the specific leaf’s area was also observed [104,105].
6. Formation of Adventitious Roots (AR) and Aerenchyma
The formation of ARs is a typical adaptive response to flooding stress in many plants [60,87,106,107]. Submergence-tolerant species develop larger adventitious root systems than intolerant species, and these newly emerged adventitious roots often contain more aerenchyma [108]. ET triggers ARs growth, and other phytohormones are also involved in this process. ET can promote cell division in rice AR primordia [109]. Besides this, ET promotes adventitious root formation, either by increasing plant sensitivity to auxin or by stimulating the formation of root primordia in flooded plants [106].
In tomatoes, flooding induces ET synthesis and stimulates auxin accumulation and transport in the stem, which triggers additional ET synthesis and thus further stimulates a flux of auxin towards the flooded parts of the plant [19,60,110]. Although the endogenous auxin concentration remained unchanged in Rumex plants during waterlogging-induced adventitious rooting, continuous basipetal (shoot to the rooting zone) transport of auxin increased auxin sensitivity, which is required for adventitious root formation [58,59,106]. In addition, auxin activates plasma membrane H+-ATPases, leading to apoplast acidification [81] and the expression of expansin genes [82]. For example, the emergence of tomatoes’ adventitious roots is favored by cell wall loosening through the regulation of apoplastic pH or the upregulation of the expansin gene LeEXP1, which promotes cell wall disassembly and cell enlargement [111].
ABA might play negative roles in ARs’ formation during flooding stress. During partial submergence, the ABA content in AR primordia of S. dulcamara prominently decreased due to a downregulation of ABA biosynthesis and an up-regulation of ABA degradation [11,112]. The exogenous application of ABA negatively affects submergence-induced AR formation, whereas the removal of ABA using an ABA biosynthesis inhibitor induced AR emergence [58,59,60,112].
Furthermore, the role of JA in AR outgrowth seems to be ambiguous, and it depends on the plant species. Two JA-deficient Arabidopsis mutants produced more adventitious roots compared to the wild type, indicating that JAs function negatively in AR formation [113]. In contrast, Lischweski et al. found that JA acted positively on AR formation in petunias [114]. Cytokinin and JA are antagonists of auxin-induced adventitious root formation, but little information is available on how these regulators mediate adventitious root formation under submergence stress [114]. Transgenic Arabidopsis carrying the IPT (isopentenyl transferase, a key enzyme of cytokinin biosynthesis) gene exposed to waterlogging accumulated greater and faster quantities of cytokinins than WT plants. Cytokinin accumulation was accompanied by better chlorophyll retention and increased biomass and carbohydrate content relative to WT plants. IPT plants also showed an improved recovery ability [115]. The protective effect of CK was also reported in wheat. Transgenic wheat (Triticum aestivum L.) plants carrying the ipt gene were more tolerant to flooding than wildtype plants, with a higher yield and less growth inhibition during flooding [116]. Cytokinins might play a role due to their nature of activity (cell division promotion and cell expansion, but also senescence delay) not only in the roots, but in the above ground plant organs as well, probably even in the escape strategy.
Under waterlogging conditions, the content of SA was significantly increased in waterlogging-tolerant soybean cultivars, which may further stimulate ARs and enhance gas exchange and finally show tolerance to waterlogging stress [117]. A proper concentration of SA significantly increases the adventitious root formation in soybeans, and it increases SA-triggered PCD responses and peroxidation, and therefore, aerenchyma cells develop in the root, which improve oxygen supply. Secondly, accumulated SA may stimulate adventitious root primordium formation [118] (Figure 1).
ET is implicated in the development of aerenchyma, which is the intercellular space that facilitates gaseous exchange and maintains the physical strength of tissues. The development of secondary aerenchyma in a flooded soybean was inhibited in response to the exogenous application of ABA, indicating that aerenchyma formation requires a reduction in ABA content [119,120,121,122,123]. ABA promotes suberin deposition in cell walls to regulate abiotic stress responses. For soybean aerenchyma cells’ development, a root cell must be unsuberized; therefore, the biosynthesis of suberin must be suppressed by the down-regulation of ABA. In waterlogging-tolerant plants, aerenchyma cells were well-developed, with a significant decrease in whole plant ABA contents compared to sensitive and control plants [124,125,126].
Besides aerenchyma, hypertrophied lenticels are formed to facilitate the connection between underwater hypoxic tissues and the atmosphere [123,127]. In Glycine max, hypertrophied lenticels were the swollen tissues at the stem base, which was attribute to radial cell division and expansion [123]. Hypertrophied lenticels are associated with auxin and ET production and are observed in many gymnosperms and angiosperms during flooding [123,128].
7. Unanswered Questions and an Outlook
Most studies have paid close attention to the detrimental effects of a low-oxygen environment on plant metabolism, whereas the molecular responses and signaling events occurring in the reoxygenation stage have been largely ignored. However, when floodwater subsides, high levels of ROS (reactive oxygen species)-related lipid peroxidation are observed in plants during reoxygenation [129]. Although excessive water exists in the soil, some plants show symptoms of water deficiency after reoxygenation, as evidenced by wilted leaves and the induced expression of dehydration-responsive genes. Thus, the post-flooding period is associated with oxidative and drought-induced damages, and a true resilience to flooding should include the ability to tolerate both post-flooding and flooding phases [129,130,131,132]. Enhanced expression of ET biosynthetic enzymes was found in Arabidopsis during reoxygenation, and the ET-insensitive mutants ein2-5 and ein3eil1 exhibited impaired genes expression associated with ABA biosynthesis, dehydration, and enhanced sensitivity to post-anoxic stress [132]. The role of ABA in regulating post-submergence responses, its interaction with ROS, and its control of plant ionic homeostasis during post-submergence recovery are some interesting aspects for future research.
In Arabidopsis, the transcript levels of JA biosynthesis genes increased, and jasmonates accumulated rapidly during reoxygenation, and the JA-inducible accumulation of antioxidants may relieve oxidative damage caused by reoxygenation and improve plant survival after submergence [132,133,134]. Although ABA has always been regarded as a negative regulator of the development of morphological adaptations to soil flooding in different plant species, recent evidence suggests a positive role of ABA in the modulation of plant responses to hypoxia and the ability to recover during the post-hypoxic period [73]. In rice, the flooding tolerance-associated SUB1A gene confers tolerance to drought and oxidative stress during reoxygenation through increased ROS scavenging and enhanced ABA responsiveness [129].
Targeted protein proteolysis via the N-end rule pathway is evolutionarily conserved, and it is a key mechanism involved in the low-oxygen response in plants, and ERFVII transcription factors are substrates for the N-end rule pathway, acting as oxygen sensors [135,136]. In recent years, NO has arisen as an important modulator of low oxygen conditions [137]. Under hypoxic conditions, NO depletion is regulated by ET through the RAP2.3 ERFVII transcription factor in Arabidopsis [138], which, in turn, promotes the stabilization of ERFVII transcription factors [139]. NO has been shown to act downstream on ABA signaling in the regulation of seed germination and stomatal conductance (at least partially [140]). Moreover, it has been shown that NO might antagonize ABA signaling through posttranslational Tyr nitration of the PYR/PYL/RCAR ABA receptors that significantly reduce their PP2CA inhibitory activity in the presence of ABA [141]. Gibbs et al. [142] reported that NO and oxygen in Arabidopsis seeds promote the degradation of ERFVII, leading to the downregulation of ABI5 and germination. Conversely, recent evidence supports the involvement of ABA, at least partially, in the induction of NO biosynthesis genes’ expression in hypoxic conditions [143], suggesting a complex crosstalk interaction between NO, ABA, and ET. Thus, it is plausible that that ET can interact with NO (nitric oxide)–oxygen sensing pathways in concert with ABA and modulate plant physiological and metabolic responses to low oxygen environments. Specific details of this interaction warrant a separate investigation.
Author Contributions
L.X. conceived and designed this paper. Y.Z., L.X. and S.S. wrote the original draft. S.S., Y.Z., W.Z. and S.F.A.-E. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by funding from the National Natural Science Foundation of China (31901438).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bubeck P., Otto A., Weichselgartner J. Societal impacts of flood hazards. Nat. Hazards. 2017 doi: 10.1093/acrefore/9780199389407.013.281. [DOI] [Google Scholar]
- 2.Razzaq A., Wani S.H., Saleem F., Yu M., Zhou M., Shabala S. Rewilding crops for climate resilience: Economic analysis and de novo domestication strategies. J. Exp. Bot. 2021:erab276. doi: 10.1093/jxb/erab276. [DOI] [PubMed] [Google Scholar]
- 3.Monre . Climate Change and Sea Level Rise Scenarios for Vietnam. Natural Resources-Environment and Mapping Publishing House; Ha Noi, Vietnam: 2011. [Google Scholar]
- 4.Chakraborty K., Guru A., Jena P., Ray S., Guhey A., Chattopadhyay K., Sarkar R.K. Rice with SUB1 QTL possesses greater initial leaf gas film thickness leading to delayed perception of submergence stress. Ann. Bot. 2020;127:251–265. doi: 10.1093/aob/mcaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X., Li M., Jannasch A.H., Jiang Y. Submergence stress alters fructan and hormone metabolism and gene expression in perennial ryegrass with contrasting growth habits. Environ. Exp. Bot. 2020;179:104–202. doi: 10.1016/j.envexpbot.2020.104202. [DOI] [Google Scholar]
- 6.Armstrong W. Aeration in higher plants. Adv. Bot. Res. 1980;7:255–332. doi: 10.1016/s0065-2296(08)60089-0. [DOI] [Google Scholar]
- 7.Voesenek L.A.C.J., Bailey-Serres J. Flood adaptive traits and processes: An overview. New Phytol. 2015;206:57–73. doi: 10.1111/nph.13209. [DOI] [PubMed] [Google Scholar]
- 8.Yemelyanov V.V., Shishova M.F. Phytohormones and Abiotic Stress Tolerance in Plants. Springer; Berlin/Heidelberg, Germany: 2012. The Role of Phytohormones in the Control of Plant Adaptation to Oxygen Depletion. [DOI] [Google Scholar]
- 9.Fukao T., Bailey-Serres J. Plant responses to hypoxia—Is survival a aalancing act? Trends Plant Sci. 2014;9:449–456. doi: 10.1016/j.tplants.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Colmer T.D., Voesenek L.A.C.J. Flooding tolerance: Suits of plant traits in variable environments. Funct. Plant Biol. 2009;36:665–681. doi: 10.1071/FP09144. [DOI] [PubMed] [Google Scholar]
- 11.Parlanti S., Kudahettige N.P., Lombardi L., Mensuali-Sodi A., Alpi A., Perata P. Distinct mechanisms for aerenchyma formation in leaf sheaths of rice genotypes displaying a quiescence or escape strategy for flooding tolerance. Ann. Bot. 2011;107:1335–1343. doi: 10.1093/aob/mcr086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Veen H., Mustroph A., Barding G.A., Van Vergeer E.M., Welschen-Evertman R.A.M., Pedersen O., Visser E.J.W., Larive C.K., Pierik R., Bailey-Serres J., et al. Two Rumex species from contrasting hydrological niches regulate flooding tolerance through distinct mechanisms. Plant Cell. 2013;25:4691–4707. doi: 10.1105/tpc.113.119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasidharan R., Mustroph A., Boonman A., Akman M., Ammerlaan A.M.H., Breit T., Schranz M.E., Voesenek L.A.C.J., Van Tienderen P.H. Root transcript profiling of two Rorippa species reveals gene clusters associated with extreme submergence tolerance. Plant Physiol. 2013;163:1277–1292. doi: 10.1104/pp.113.222588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hattori Y., Nagai K., Furukawa S., Song X.J., Kawano R., Sakakibara H., Wu J., Matsumoto T., Yoshimura A., Kitano H. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature. 2009;460:1026–1030. doi: 10.1038/nature08258. [DOI] [PubMed] [Google Scholar]
- 15.Nagai K., Hattori Y., Ashikari M. Stunt or elongate? Two opposite strategies by which rice adapts to floods. J. Plant Res. 2010;123:303–309. doi: 10.1007/s10265-010-0332-7. [DOI] [PubMed] [Google Scholar]
- 16.Keisuke N., Yuma K., Takuya K., Tomonori N., Takeshi K., Angeles-Shim R.B., Hideshi Y., Atsushi Y., Motoyuki A. QTL analysis of internode elongation in response to gibberellin in deepwater rice. AoB. Plants. 2014;6:plu028. doi: 10.1093/aobpla/plu028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey-Serres J., Voesenek L.A.C.J. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008;59:313–339. doi: 10.1146/annurev.arplant.59.032607.092752. [DOI] [PubMed] [Google Scholar]
- 18.Voesenek L.A.C.J., Bailey-Serres J. Flooding tolerance: O2 sensing and survival strategies. Curr. Opin. Plant Biol. 2013;16:647–653. doi: 10.1016/j.pbi.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Grichko V.P., Glick B.R. Flooding tolerance of transgenic tomato plants expressing the bacterial enzyme ACC deaminase controlled by the 35s, rold or prb-1b promoter. Plant Physiol. Biochem. 2001;39:19–25. doi: 10.1016/S0981-9428(00)01217-1. [DOI] [Google Scholar]
- 20.Armstrong W., Cousins D., Armstrong J., Turner D.W., Beckett P.M. Distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: A microelectrode and modelling study with phragmites australis. Ann. Bot. 2000;86:687–703. doi: 10.1006/anbo.2000.1236. [DOI] [Google Scholar]
- 21.Bharath P., Gahir S., Raghavendra A.S. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021;12:615114. doi: 10.3389/fpls.2021.615114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim C.W., Baek W., Jung J., Kim J.H., Lee S.C. Function of ABA in stomatal defense against biotic and drought stresses. Int. J. Mol. Sci. 2015;16:15251–15270. doi: 10.3390/ijms160715251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y., Gao J., Im Kim J., Chen K., Bressan R.A., Zhu J.K. Control of plant water use by ABA induction of senescence and dormancy: An overlooked lesson from evolution. Plant Cell Physiol. 2017;58:1319–1327. doi: 10.1093/pcp/pcx086. [DOI] [PubMed] [Google Scholar]
- 24.Niu M., Xie J., Chen C., Cao H., Sun J., Kong Q., Shabala S., Shabal L., Huang Y., Bie Z. An early ABA induced stomatal closure, Na+ sequestration in leaf vein and K+ retention in mesophyll confer salt tissue tolerance in Cucurbita species. J. Exp. Bot. 2018;69:4945–4960. doi: 10.1093/jxb/ery251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann-Benning S., Kende H. On the role of abscisic acid and gibberellin in the regulation of growth in rice. Plant Physiol. 1992;99:1156–1161. doi: 10.1104/pp.99.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azuma T., Hirano T., Deki Y., Uchida N., Yamaguchi T. Involvement of the decrease in levels of abscisic acid in the internodal elongation of submerged floating rice. J. Plant Physiol. 1995;146:323–328. doi: 10.1016/S0176-1617(11)82062-6. [DOI] [Google Scholar]
- 27.Fukao T., Xu K., Ronald P.C., Bailey-Serres J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell. 2006;18:2021–2034. doi: 10.1105/tpc.106.043000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ram P.C., Singh B.B., Singh A.K., Ram P., Singh P.N., Singh H.P., Boamfa I., Harren F., Santosa E., Jackson M.B. Submergence tolerance in rainfed lowland rice: Physiological basis and prospects for cultivar improvement through marker-aided breeding. Field Crops Res. 2002;76:131–152. doi: 10.1016/S0378-4290(02)00035-7. [DOI] [Google Scholar]
- 29.Hiroaki S., Masanori O., Kentaro M., Tetsuo K., Shoko S., Yusuke J., Masaru F., Taku A., Hirokazu T., Miho A. Ethylene promotes submergence-induced expression of OsABA8ox1, a gene that encodes ABA 8-hydroxylase in rice. Plant Cell Physiol. 2007;48:287–298. doi: 10.1093/pcp/pcm003. [DOI] [PubMed] [Google Scholar]
- 30.Bakshi A., Piy S., Fernandez J.C., Chervin C., Hewezi T., Binder B.M. Eethylene receptors signal via a noncanonical pathway to regulate abscisic acid responses. Plant Physiol. 2018;176:910–929. doi: 10.1104/pp.17.01321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papdi C., Pérez-Salamó I., Joseph M.P., Giuntoli B., Szabados L. The low oxygen, oxidative and osmotic stress responses synergistically act through the ethylene response factor vii genes RAP2.12, RAP 2.2 and RAP 2.3. Plant J. 2015;82:772–784. doi: 10.1111/tpj.12848. [DOI] [PubMed] [Google Scholar]
- 32.Benech-Arnold R.L., Gualano N., Leymarie J., Come D., Corbineau F. Hypoxia interferes with ABA metabolism and increases ABA sensitivity in embryos of dormant barley grains. J. Exp. Bot. 2006;57:1423–1430. doi: 10.1093/jxb/erj122. [DOI] [PubMed] [Google Scholar]
- 33.Yang X., Jansen M.J., Zhang Q., Sergeeva L., Ligterink W., Mariani C., Rieu I., Visser E.J.W. A disturbed auxin signaling affects adventitious root outgrowth in solanum dulcamara under complete submergence. J. Plant Physiol. 2018;224–225:11–18. doi: 10.1016/j.jplph.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Lee T.-M., Shieh Y.-J., Chou C.-H. Abscisic acid inhibits shoot elongation of Scirpus mucronatus. Physiol. Plant. 1996;97:1. doi: 10.1111/j.1399-3054.1996.tb00470.x. [DOI] [Google Scholar]
- 35.Arbona V., Zandalinas S.I., Manzi M., Gonzalez-Guzman M., Rodriguez P.L., Gómez-Cadenas A. Depletion of abscisic acid levels in roots of flooded carrizo citrange (Poncirus trifoliata L. raf. × Ctrus sinensis L. osb.) plants is a stress-specific response associated to the differential expression of pyr/pyl/rcar receptors. Plant Mol Biol. 2017;93:623–640. doi: 10.1007/s11103-017-0587-7. [DOI] [PubMed] [Google Scholar]
- 36.Benschop J.J., Jackson M.B., Gühl K., Vreeburg R., Voesenek L. Contrasting interactions between ethylene and abscisic acid in rumex species differing in submergence tolerance. Plant J. 2010;44:756–768. doi: 10.1111/j.1365-313X.2005.02563.x. [DOI] [PubMed] [Google Scholar]
- 37.Arbona V., Cadenas A.G. Hormonal modulation of citrus responses to flooding. Aust. Econ. Pap. 2008;16:194–210. doi: 10.1007/s00344-008-9051-x. [DOI] [Google Scholar]
- 38.Müller J.T., Veen H.V., Bartylla M.M., Akman M., Pedersen O., Sun P., Schuurink R.C., Takeuchi J., Todoroki Y., Weig A.R. Keeping the shoot above water—Submergence triggers antithetical growth responses in stems and petioles of watercress (Nasturtium officinale) New Phytol. 2019;229:140–155. doi: 10.1111/nph.16350. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J., Zhang X. Can early wilting of old leaves account for much of the ABA accumulation in flooded pea plants? J. Exp Bot. 1994;45:1335–1342. doi: 10.1093/jxb/45.9.1335. [DOI] [Google Scholar]
- 40.Hurng W.P., Lur H.S., Liao C.K., Kao C.H. Role of abscisic acid, ethylene and polyamines in flooding-promoted senescence of tobacco leaves. J. Plant Physiol. 1994;143:102–105. doi: 10.1016/S0176-1617(11)82104-8. [DOI] [Google Scholar]
- 41.Ahmed S., Nawata E., Sakuratani T. Changes of endogenous ABA and ACC, and their correlations to photosynthesis and water relations in mungbean (Vigna radiata L. Wilczak cv. KPS1) during waterlogging. Environ. Exp. Bot. 2006;57:278–284. doi: 10.1016/j.envexpbot.2005.06.006. [DOI] [Google Scholar]
- 42.Noguchi H.K. Abscisic acid and hypoxic induction of anoxia tolerance in roots of lettuce seedlings. J. Exp. Bot. 2000;352:1939–1944. doi: 10.1093/jexbot/51.352.1939. [DOI] [PubMed] [Google Scholar]
- 43.Komatsu S., Han C., Nanjo Y., Altaf-Un-Nahar M., Yang P. Label-free quantitative proteomic analysis of abscisic acid effect in early-stage soybean under flooding. J. Proteome Res. 2013;12:4769–4784. doi: 10.1021/pr4001898. [DOI] [PubMed] [Google Scholar]
- 44.Castonguay Y., Nadeau P., Simard R.R. Effects of flooding on carbohydrate and aba levels in roots and shoots of alfalfa. Plant Cell Environ. 2010;16:695–702. doi: 10.1111/j.1365-3040.1993.tb00488.x. [DOI] [Google Scholar]
- 45.Jackson M.B., Young S.F., Hall K.C. Are roots a source of abscisic acid for the shoots of flooded pea plants? J. Exp. Bot. 1988;39:1631–1637. doi: 10.1093/jxb/39.12.1631. [DOI] [Google Scholar]
- 46.Olivella C., Biel C., Vendrell M., Save R. Hormonal and physiological responses of Gerbera jamesonii to flooding stress. Hortic. Sci. 2000;35:222–225. doi: 10.21273/HORTSCI.35.2.222. [DOI] [Google Scholar]
- 47.Hartung W., Sauter A., Turner N.C., Fillery I., Heilmeier H. Abscisic acid in soils: What is its function and which factors and mechanisms influence its concentration? Plant Soil. 1996;184:105–110. doi: 10.1007/BF00029279. [DOI] [Google Scholar]
- 48.Bungau S., Behl T., Aleya L., Bourgeade P., Aloui-Sossé B., Purza A.L., Abid A., Samuel A.D. Expatiating the impact of anthropogenic aspects and climatic factors on long-term soil monitoring and management. Environ. Sci. Pollut. Res. Int. 2021;28:30528–30550. doi: 10.1007/s11356-021-14127-7. [DOI] [PubMed] [Google Scholar]
- 49.Samuel A.D., Bungau S.G., Tit D.M., Frunzulica C.E.M., Badea G.E. Effects of long term application of organic and mineral fertilizers on soil enzymes. Rev. Chim. 2018;69:2608–2612. doi: 10.37358/RC.18.10.6590. [DOI] [Google Scholar]
- 50.Wang Y., Mopper S., Hasenstein K.H. Effects of salinity on endogenous ABA, IAA, JA, and SA in Iris hexagona. J. Chem. Ecol. 2001;27:327–342. doi: 10.1023/A:1005632506230. [DOI] [PubMed] [Google Scholar]
- 51.Gitea M.A., Gitea D., Tit D.M., Purza L., Samuel A.D., Bungău S., Badea G.E., Aleya L. Orchard management under the effects of climate change: Implications for apple, plum, and almond growing. Environ. Sci. Pollut. Res. Int. 2019;26:9908–9915. doi: 10.1007/s11356-019-04214-1. [DOI] [PubMed] [Google Scholar]
- 52.Liu H., Fang Y., Junyan W.U., Chen Q., Sun W., Liu Z., Fang Y., Chao M.I., Yuanyuan P.U., Zhao Y. Response of endogenous ABA and GA to cold resistance of Brassica rapa L. and Brassica napus L. Chin. J. Eco-Agric. 2016;24:1529–1538. doi: 10.13930/j.cnki.cjea.160421. [DOI] [Google Scholar]
- 53.Chen S.Y., Zou H.W. ABA improving rice seeds resistance to waterlogging stress during germinating period. J. Anhui Agric. Sci. 2013;41:593–594. doi: 10.13989/j.cnki.0517-6611.2013.02.129. [DOI] [Google Scholar]
- 54.Ellis M.H., Dennis E.S., Peacock W.J. Arabidopsis roots and shoots have different mechanisms for hypoxic stress tolerance. Plant Physiol. 1999;119:57–64. doi: 10.1104/pp.119.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hwang S.Y., VanToai T.T. Abscisic acid induced anaerobiosis tolerance in corn. Plant Physiol. 1991;97:593–597. doi: 10.1104/pp.97.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vantoai T.T. Field Performance of Abscisic Acid-Induced Flood-Tolerant Corn. Crop Sci. 1993;33:344–346. doi: 10.2135/cropsci1993.0011183X003300020028x. [DOI] [Google Scholar]
- 57.Voesenek L.A.C.J., Jackson M.B., Toebes A.H.W., Vriezen W.H., Colmer T.D. De-submergence-induced ET production in Rumex palustris: Regulation and ecophysiological significance. Plant J. 2003;33:341–352. doi: 10.1046/j.1365-313X.2003.01632.x. [DOI] [PubMed] [Google Scholar]
- 58.Steffens B., Sauter M. Epidermal cell death in rice (Oryza sativa L.) is regulated by ethylene, gibberellins and abscisic acid. Plant Physiol. 2005;139:713–721. doi: 10.1104/pp.105.064469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steffens B., Wang J., Sauter M. Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta. 2006;223:604–612. doi: 10.1007/s00425-005-0111-1. [DOI] [PubMed] [Google Scholar]
- 60.Vidoz M.L., Loreti E., Mensuali A., Alpi A., Perata P. Hormonal interplay during adventitious root formation in flooded tomato plants. Plant J. 2010;63:551–562. doi: 10.1111/j.1365-313X.2010.04262.x. [DOI] [PubMed] [Google Scholar]
- 61.Liu P., Sun F., Gao R., Dong H. RAP2.6L Overexpression delays waterlogging induced premature senescence by increasing stomatal closure more than antioxidant enzyme activity. Plant Mol. Biol. 2012;79:609–622. doi: 10.1007/s11103-012-9936-8. [DOI] [PubMed] [Google Scholar]
- 62.Joshi S.A., Valon C., Leung J.A. Brand new start: Abscisic acid perception and transduction in the guard cell. Sci. Signal. 2011;4:re4. doi: 10.1126/scisignal.2002164. [DOI] [PubMed] [Google Scholar]
- 63.Hsu F.C., Chou M.Y., Peng H.P., Chou S.J., Shih M.C. Insights into hypoxic systemic responses based on analyses of transcriptional regulation in Arabidopsis. PLoS ONE. 2011;6:e28888. doi: 10.1371/journal.pone.0028888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Janowiak F., Else M.A., Jackson M.B. Leaf-area-specific delivery rates of indole acetic acid and abscisic acid in the transpiration stream of flooded tomato plants in relation to stomatal closure. Zeszyty Problemowe Postępów Nauk Rolniczych. 2010;545:151–159. [Google Scholar]
- 65.Else M.A., Coupland D., Dutton L., Jackson M.B. Decreased root hydraulic conductivity reduces leaf water potential, initiates stomatal closure and slows leaf expansion in flooded plants of castor oil (Ricinus communis) despite diminished delivery of ABA from the roots to shoots in xylem sap. Physiol. Plant. 2001;111:46–54. doi: 10.1034/j.1399-3054.2001.1110107.x. [DOI] [Google Scholar]
- 66.Jackson M.B. Long-distance signalling from roots to shoots assessed: The flooding story. J. Exp. Bot. 2002;53:175–181. doi: 10.1093/jexbot/53.367.175. [DOI] [PubMed] [Google Scholar]
- 67.Else M.A., Taylor J.M., Atkinson C.J. Anti-transpirant activity in xylem sap from flooded tomato (Lycopersicon esculentum Mill.) plants is not due to pH-mediated redistributions of root- or shoot-sourced ABA. J. Exp. Bot. 2006;57:3349–3357. doi: 10.1093/jxb/erl099. [DOI] [PubMed] [Google Scholar]
- 68.Sasidharan R., Voesenek L.A.C.J. Ethylene-Mediated Acclimations to Flooding Stress. Plant Physiol. 2015;169:3–12. doi: 10.1104/pp.15.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bailey-Serres J., Fukao T., Gibbs D.J., Holdsworth M.J., van Dongen J.T.V. Making sense of low oxygen sensing. Trends Plant Sci. 2012;17:129–138. doi: 10.1016/j.tplants.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 70.Dongen J.T.V., Licausi F. Low-oxygen stress in plants. Plant Cell Monogr. 2014;21 doi: 10.1007/978-3-7091-1254-0. [DOI] [Google Scholar]
- 71.Bashar K., Tareq M., Amin M., Honi U., Tahjib-Ul-Arif M., Sadat M., Hossen Q. Phytohormone-mediated stomatal response, escape and quiescence strategies in plants under flooding stress. Agronomy. 2019;9:43. doi: 10.3390/agronomy9020043. [DOI] [Google Scholar]
- 72.Wanke D. The ABA-mediated switch between submersed and emersed life-styles in aquatic macrophytes. J. Plant Res. 2011;124:467–475. doi: 10.1007/s10265-011-0434-x. [DOI] [PubMed] [Google Scholar]
- 73.Guzmán G.M., Cadenas G.A., Arbona V. Abscisic acid as an emerging modulator of the responses of plants to low oxygen conditions. Front. Plant Sci. 2021;12:661–789. doi: 10.3389/fpls.2021.661789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Ollas C., Guzmán G.M., Pitarch Z., Matus J.T., Candela H., Rambla J.L., Granell A., Cadenas G.A., Arbona V. Identification of ABA-Mediated Genetic and Metabolic Responses to Soil Flooding in Tomato (Solanum lycopersicum L. Mill) Front. Plant Sci. 2021;12:613059. doi: 10.3389/fpls.2021.613059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cox M.C.H., Benschop J.J., Vreeburg R.A.M., Wagemaker C.A.M., Moritz T., Peeters A.J.M., Voesenek L.A.C.J. The roles of ethylene, auxin, abscisic acid, and gibberellin in the hyponastic growth of submerged Rumex palustris petioles. Plant Physiol. 2004;136:2948–2960. doi: 10.1104/pp.104.049197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van der Straeten D., Zhou Z., Prinsen E., Van Onckelen H., Van Montagu M.C. A comparative molecular-physiological study of submergence response in lowland and deepwater rice. Plant Physiol. 2001;125:955–968. doi: 10.1104/pp.125.2.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jin Q., Wang Y., Li X., Wu S., Wang Y., Luo J., Mattson N., Xu Y. Interactions between ethylene, gibberellin and abscisic acid in regulating submergence induced petiole elongation in Nelumbo nucifera. Aquat. Bot. 2016;137:9–15. doi: 10.1016/j.aquabot.2016.11.002. [DOI] [Google Scholar]
- 78.Kende H. Deepwater Rice: A Model Plant to Study Stem Elongation. Plant Physiol. 1998;118:1105–1110. doi: 10.1104/pp.118.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Raskin I., Kende H. Regulation of growth in stem sections of deep-water rice. Planta. 1984;160:66–72. doi: 10.1007/BF00392467. [DOI] [PubMed] [Google Scholar]
- 80.Chen X., Pierik R., Peeters A.J.M., Poorter H., Visser E.J.W., Huber H., Kroon H.D., Voesenek L.A.C.J. Endogenous abscisic acid as a key switch for natural variation in flooding-induced shoot elongation. Plant Physiol. 2010;154:969–977. doi: 10.1104/pp.110.162792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Becker D., Hedrich R. Channeling auxin action: Modulation of ion transport by indole-3-acetic acid. Plant Mol. Biol. 2002;49:349–356. doi: 10.1023/A:1015211231864. [DOI] [PubMed] [Google Scholar]
- 82.Cosgrove D.J. Loosening of plant cell walls by expansins. Nature. 2000;407:321–326. doi: 10.1038/35030000. [DOI] [PubMed] [Google Scholar]
- 83.Rijnders J.G.H.M., Yang Y.Y., Kamiya Y., Takahashi N., Barendse G.W.M., Blom C.W.P.M., Voesenek L.A.C.J. Ethylene enhances gibberellin levels and petiole sensitivity in flooding-tolerant Rumex palustris but not in flooding-intolerant R. acetosa. Planta. 1997;203:20–25. doi: 10.1007/s004250050160. [DOI] [Google Scholar]
- 84.Cox M.C.H., Peeters A.J.M., Voesenek L.A.C.J. The stimulating effects of ethylene and auxin on petiole elongation and on hyponastic curvature are independent processes in submerged Rumex palustris. Plant Cell Environ. 2010;29:282–290. doi: 10.1111/j.1365-3040.2005.01420.x. [DOI] [PubMed] [Google Scholar]
- 85.Ross J.J., O’Neill D.P., Smith J.J., Kerckhoffs L.H.J., Elliott R.C. Evidence that auxin promotes gibberellin A1 biosynthesis in pea. Plant J. 2000;21:547–552. doi: 10.1046/j.1365-313x.2000.00702.x. [DOI] [PubMed] [Google Scholar]
- 86.Rauf M., Arif M., Fisahn J., Xue G.P., Balazadeh S., Mueller-Roeber B. NAC transcription factor speedy hyponastic growth regulates flooding induced leaf movement in Arabidopsis. Plant Cell. 2013;25:4941–4955. doi: 10.1105/tpc.113.117861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Phukan U.J., Mishra S., Timbre K., Luqman S., Shukla R.K. Mentha arvensis exhibits better adaptive characters in contrast to Mentha piperita when subjugated to sustained waterlogging stress. Protoplasma. 2014;251:603–614. doi: 10.1007/s00709-013-0561-4. [DOI] [PubMed] [Google Scholar]
- 88.Voesenek L.A.C.J., Blom C.W.P.M. Growth responses of Rumex species in relation to submergence and ethylene. Plant Cell Environ. 2010;12:433–439. doi: 10.1111/j.1365-3040.1989.tb01959.x. [DOI] [Google Scholar]
- 89.Pierik R., Cuppens M.L.C., Voesenek L.A.C.J., Visser E.J.W. Interactions between ethylene and gibberellins in phytochrome mediated shade avoidance responses in tobacco. Plant Physiol. 2004;136:28–36. doi: 10.1104/pp.104.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Polko J.K., Pierik R., van Zanten M., Tarkowská D., Strnad M., Voesenek L.A.C.J., Peeters A.J.M. Ethylene promotes hyponastic growth through interaction with ROTUNDIFOLIA3/CYP90C1 in Arabidopsis. J. Exp. Bot. 2013;64:613–624. doi: 10.1093/jxb/ers356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zotz G., Wilhelm K., Becker A. Heteroblasty-A Review. Bot. Rev. 2011;77:109–151. doi: 10.1007/s12229-010-9062-8. [DOI] [Google Scholar]
- 92.Kuwabara A., Ikegami K., Koshiba T., Nagata T. Effects of ethylene and abscisic acid upon heterophylly in Ludwigia arcuata (Onagraceae) Planta. 2003;217:880–887. doi: 10.1007/s00425-003-1062-z. [DOI] [PubMed] [Google Scholar]
- 93.Kuwabara A., Tsukaya H., Nagata T. Identification of factors that cause heterophylly in Ludwigia arcuate walt. (Onagraceae) Plant Biol. 2000;3:98–105. doi: 10.1055/s-2001-11748. [DOI] [Google Scholar]
- 94.Kuwabara A., Nagata T. Cellular basis of developmental plasticity observed in heterophyllous leaf formation of Ludwigia arcuata (Onagraceae) Planta. 2006;224:761–770. doi: 10.1007/s00425-006-0258-4. [DOI] [PubMed] [Google Scholar]
- 95.Ling B.L., Wang H.J., Wang J.S., Irina Z.L., Abrams S.R. Abscisic acid regulation of heterophylly in Marsilea quadrifolia L.: Effects of R-(−) and S-(+) isomers. J. Exp. Bot. 2005;56:2935–2948. doi: 10.1093/jxb/eri290. [DOI] [PubMed] [Google Scholar]
- 96.Juhyun K., Youngsun J., Jinseul K., Myeongjune J., Yoon P.J., Gyun L.H., Soo C.D., Eunju L., Ilha L., Hao Y. A molecular basis behind heterophylly in an amphibious plant, Ranunculus trichophyllus. PLoS Genet. 2018;14:e1007208. doi: 10.1371/journal.pgen.1007208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walbot V. Abscisic acid induces pink pigmentation in maize aleurone tissue in the absence of bronze-2. Maydica. 1994;1:19–28. [Google Scholar]
- 98.Yanai O., Shani E., Russ D., Ori N. Gibberellin partly mediates LANCEOLATE activity in tomato. Plant J. 2011;68:571–582. doi: 10.1111/j.1365-313X.2011.04716.x. [DOI] [PubMed] [Google Scholar]
- 99.Farquharson K.L. Examining the molecular basis of heterophylly in NorthAmerican Lake cress. Plant Cell. 2014;26:45–67. doi: 10.1105/tpc.114.135434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nakayama H., Nakayama N., Seiki S., Kojima M., Sakakibara H., Sinha N., Kimura S. Regulation of the KNOX-GA gene module induces heterophyllic alteration in North American Lake Cress. Plant Cell. 2014;26:4733–4748. doi: 10.1105/tpc.114.130229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Deschamp P.A., Cooke T.J. Leaf dimorphism in the aquatic angiosperm Callitriche heterophylla. Am. J. Bot. 1985;72:1377–1387. doi: 10.1002/j.1537-2197.1985.tb08394.x. [DOI] [Google Scholar]
- 102.Townsley B.T., Sinha N.R. A new development: Evolving concepts in leaf ontogeny. Annu. Rev. Plant Biol. 2012;63:535–562. doi: 10.1146/annurev-arplant-042811-105524. [DOI] [PubMed] [Google Scholar]
- 103.Byrne M.E. Making leaves. Curr. Opin. Plant Biol. 2011;15:24–30. doi: 10.1016/j.pbi.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 104.Mommer L., Pons T.L., Visser E.J. Photosynthetic consequences of phenotypic plasticity in response to submergence: Rumex palustris as a case study. J. Exp. Bot. 2006;57:283–290. doi: 10.1093/jxb/erj015. [DOI] [PubMed] [Google Scholar]
- 105.Mommer L., Pons T.L., Wolters-Arts M., Venema J.K., Visser E.J.W. Submergence induced morphological, anatomical, and biochemical responses in a terrestrial species affects gas diffusion resistance and photosynthetic performance. Plant Physiol. 2005;139:497–508. doi: 10.1104/pp.105.064725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Visser E.J.W., Cohen J.D., Barendse G.W.M., Blom C.W.P.M., Voesenek L.A. An ethylene-mediated increase in sensitivity to auxin induces adventitious root formation in flooded Rumex palustris Sm. Plant Physiol. 1996;112:1687–1692. doi: 10.1104/pp.112.4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jackson W.T. The role of adventitious roots in recovery of shoots following flooding of the original root systems. Am. J. Bot. 1955;42:816–819. doi: 10.1002/j.1537-2197.1955.tb10428.x. [DOI] [Google Scholar]
- 108.Yamauchi T., Abe F., Kawaguchi K., Oyanagi A., Nakazono M. Adventitious roots of wheat seedlings that emerge in oxygen-deficient conditions have increased root diameters with highly developed lysigenous aerenchyma. Plant Signal Behav. 2014;9:e28506-1. doi: 10.4161/psb.28506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lorbiecke R., Sauter M. Adventitious root growth and cell-cycle induction in deepwater rice. Plant Physiol. 1999;119:21–29. doi: 10.1104/pp.119.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ecker J.R. The ethylene signal transduction pathway in plants. Science. 1995;268:667–675. doi: 10.1126/science.7732375. [DOI] [PubMed] [Google Scholar]
- 111.Xue Z.H., Kou X.H., Luo Y.B., Zhu B.Z., Xu W.T. Effect of ethylene on polygalacturonase, lipoxygenase and expansin in ripening of tomato fruits. Trans. Tianjin Univ. 2009;15:173–177. doi: 10.1007/s12209-009-0031-4. [DOI] [Google Scholar]
- 112.Dawood T., Yang X., Visser E.J., Te Beek T.A., Kensche P.R., Cristescu S.M., Rieu I. A co-opted hormonal cascade activates dormant adventitious root primordia upon flooding in Solanum dulcamara. Plant Physiol. 2016;170:2351–2364. doi: 10.1104/pp.15.00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gutierrez L., Mongelard G., Floková K., Pacurar D.I., Novák O., Staswick P., Kowalczyk M., Pacurar M., Demailly H., Geiss G. Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell. 2012;24:2515–2527. doi: 10.1105/tpc.112.099119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lischweski S., Muchow A., Guthörl D., Hause B. Jasmonates act positively in adventitious root formation in petunia cuttings. BMC Plant Biol. 2015;15:229. doi: 10.1186/s12870-015-0615-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Le Nguyen Huynh N.L., VanToai T., Streeter J., Banowetz G. Regulation of flooding tolerance of SAG12:ipt Arabidopsis plants by cytokinin. J. Exp. Bot. 2005;56:1397–1407. doi: 10.1093/jxb/eri141. [DOI] [PubMed] [Google Scholar]
- 116.Shtratnikova V.Y., Kudryakova N.V., Kudoyarova G.R., Korobova A.V., Akhiyarova G.R., Danilova M.N., Kusnetsov V.V., Kulaeva O.N. Effect of the ipt gene expression on wheat tolerance to root flooding. Russ. J. Plant Physiol. 2011;58:799–807. doi: 10.1134/s1021443711050244. [DOI] [Google Scholar]
- 117.Yoon-Ha K., Sun-Joo H., Muhammad W., Khan A.L., Joon-Hee L., Jeong-Dong L., Nguyen H.T., In-Jung L. Comparative analysis of endogenous hormones level in two soybean (Glycine max L.) lines differing in waterlogging tolerance. Front. Plant Sci. 2015;6:714. doi: 10.3389/fpls.2015.00714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhou W., Chen F., Meng Y., Chandrasekaran U., Shu K. Plant waterlogging/flooding stress responses: From seed germination to maturation. Plant Physiol. Biochem. 2020;148:228–236. doi: 10.1016/j.plaphy.2020.01.020. [DOI] [PubMed] [Google Scholar]
- 119.Dengler N.G. Anisophylly and dorsiventral shoot symmetry. Int. J. Plant Sci. 1999;160:S67–S80. doi: 10.1086/314218. [DOI] [PubMed] [Google Scholar]
- 120.Thomas A.L., Guerreiro S.M.C., Sodek L. Aerenchyma formation and recovery from hypoxia of the flooded root system of nodulated soybean. Ann. Bot. 2005;96:1191–1198. doi: 10.1093/aob/mci272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shimamura S., Mochizuki T., Nada Y., Fukuyama M. Secondary aerenchyma formation and its relation to nitrogen fixation in root nodules of soybean plants (Glycine max) grown under flooded conditions. Plant Prod. Sci. 2002;5:294–300. doi: 10.1626/pps.5.294. [DOI] [Google Scholar]
- 122.Shimamura S., Mochizuki T., Nada Y., Fukuyama M. Formation andfunction of secondary aerenchyma in hypocotyl, roots, and nodules of soybean (Glycine max) under flooded conditions. Plant Soil. 2003;251:351–359. doi: 10.1023/A:1023036720537. [DOI] [Google Scholar]
- 123.Shimamura S., Yamamoto R., Nakamura T., Shimada S., Komatsu S. Stem hypertrophic lenticels and secondary aerenchyma enable oxygen transport to roots of soybean in flooded soil. Ann. Bot. 2010;106:277–284. doi: 10.1093/aob/mcq123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shimamura S., Yoshioka T., Yamamoto R., Hiraga S., Nakamura T., Shimada S., Komatsu S. Role of abscisic acid in flood-induced secondary aerenchyma formation in soybean (Glycine max) hypocotyls. Plant Prod. Sci. 2014;17:131–137. doi: 10.1626/pps.17.131. [DOI] [Google Scholar]
- 125.Larré C.F., Fernando J.A., Marini P., Bacarin M.A., Peters J.A. Growth and chlorophyll a fluorescence in Erythrina crista-galli L. plants under flooding conditions. Acta Physiol. Plant. 2013;35:1463–1471. doi: 10.1007/s11738-012-1187-4. [DOI] [Google Scholar]
- 126.Larson K.D., Schaffer B., Davies F.S. Floodwater oxygen content, ethylene production and lenticel hypertrophy in flooded mango (Mangifera indica L) trees. J. Exp. Bot. 1993;44:665–671. doi: 10.1093/jxb/44.3.665. [DOI] [Google Scholar]
- 127.Syed N.H., Prince S.J., Mutava R.N., Patil G., Song L., Chen W., Babu V., Joshi T., Khan S., Nguyen H.T. Core clock, SUB1, and ABAR genes mediate flooding and drought responses via alternative splicing in soybean. J. Exp. Bot. 2015;66:7129. doi: 10.1093/jxb/erv407. [DOI] [PubMed] [Google Scholar]
- 128.Covington M.F., Harmer S.L. The circadian clock regulates auxin signaling and responses in Arabidopsis. PloS Biol. 2007;5:1773–1784. doi: 10.1371/journal.pbio.0050222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fukao T., Yeung E., Bailey-Serres J. The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice. Plant Cell. 2011;23:412–427. doi: 10.1105/tpc.110.080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Setter T.L., Bhekasut P., Greenway H. Desiccation of leaves after desubmergence is one cause for intolerance to complete submergence of the rice cultivar IR42. Funct. Plant Biol. 2010;37:1096–1104. doi: 10.1071/FP10025. [DOI] [Google Scholar]
- 131.Tamang B.G., Magliozzi J.O., Maroof M.A.S., Fukao T. Physiological and transcriptomic characterization of submergence and reoxygenation responses in soybean seedlings. Plant Cell Environ. 2014;37:2350–2365. doi: 10.1111/pce.12277. [DOI] [PubMed] [Google Scholar]
- 132.Tsai K.J., Chou S.J., Shih M.C. ET plays an essential role in the recovery of Arabidopsis during post-anaerobiosis reoxygenation. Plant Cell Environ. 2014;37:2391–2405. doi: 10.1111/pce.12292. [DOI] [PubMed] [Google Scholar]
- 133.Yuan L.B., Dai Y.S., Xie L.J., Yu L.J., Zhou Y., Lai Y.X., Yang Y.C., Xu L., Fang Q., Chen S.X. Jasmonate regulates plant responses to postsubmergence reoxygenation through transcriptional activation of antioxidant synthesis. Plant Physiol. 2017;173:1864–1880. doi: 10.1104/pp.16.01803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tsai K.J., Lin C.Y., Ting C.Y., Shih M.C. ET-regulated glutamate dehydrogenase fine-tunes metabolism during anoxia-reoxygenation. Plant Physiol. 2016;172:1548–1562. doi: 10.1104/pp.16.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gibbs D.J., Lee S.C., Isa N.M., Gramuglia S., Fukao T., Bassel G.W., Correia C.S., Corbineau F., Theodoulou F.L., Bailey-Serres J. Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature. 2011;479:415–418. doi: 10.1038/nature10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Licausi F., Kosmacz M., Weits D.A., Giuntoli B., Giorgi F.M., Voesenek L.A.C.J., Perata P., van Dongen J.T.V. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature. 2011;479:419–422. doi: 10.1038/nature10536. [DOI] [PubMed] [Google Scholar]
- 137.Prakash V., Singh V.P., Tripathi D.K., Sharma S., Corpas F.J. Crosstalk between nitric oxide (NO) and abscisic acid (ABA) signalling molecules in higher plants. Environ. Exp. Bot. 2018;161:41–49. doi: 10.1016/j.envexpbot.2018.10.033. [DOI] [Google Scholar]
- 138.León J., Costa-Broseta Á., Castillo M.C., Spoel S. RAP2.3 negatively regulates nitric oxide biosynthesis and related responses through a rheostat-like mechanism in Arabidopsis. J. Exp. Bot. 2020;71:3157–3171. doi: 10.1093/jxb/eraa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hartman S., Liu Z., Van Veen H., Vicente J., Reinen E., Martopawiro S., Zhang H., Dongen N.V., Bosman F., Bassel G.W. Ethylene-mediated nitric oxide depletion pre-adapts plants to hypoxia stress. Nat. Commun. 2019;10:4020. doi: 10.1038/s41467-019-12045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kolbert Z., Barroso J.B., Brouquisse R., Corpas F.J., Hancock J.T. A forty year journey: The generation and roles of NO in plants. Nitric Oxide Biol. Chem. 2019;93:53–70. doi: 10.1016/j.niox.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 141.Castillo M., Lozano-Juste J., Gonzalez-Guzmán M., Rodriguez L., Rodriguez P.L., León J. Inactivation of PYR/PYL/RCAR ABA receptors by tyrosine nitration may enable rapid inhibition of ABA signaling by nitric oxide in plants. Sci. Signal. 2015;8:ra89. doi: 10.1126/scisignal.aaa7981. [DOI] [PubMed] [Google Scholar]
- 142.Gibbs D.J., Md Isa N., Movahedi M., Lozano-Juste J., Mendiondo G.M., Berckhan S., Rosa N.M., Conde J.V., Correia C.S.P., Pearce S. Nitric oxide sensing in plants is mediated by proteolytic control of group VII ERF transcription factors. Mol. Cell. 2014;53:369–379. doi: 10.1016/j.molcel.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang H., Gannon L., Jones P.D., Rundle C.A., Hassall K.L., Gibbs D.J., Holdsworth M.J., Theodoulou F.L. Genetic interactions between ABA signalling and the Arg/N-end rule pathway during Arabidopsis seedling establishment. Sci. Rep. 2018;8:15192. doi: 10.1038/s41598-018-33630-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.