Abstract
The ratio of proapoptotic versus antiapoptotic Bcl-2 members is a critical determinant that plays a significant role in altering susceptibility to apoptosis. Therefore, a reduction of antiapoptotic protein levels in response to proximal signal transduction events may switch on the apoptotic pathway. In endothelial cells, tumor necrosis factor alpha (TNF-α) induces dephosphorylation and subsequent ubiquitin-dependent degradation of the antiapoptotic protein Bcl-2. Here, we investigate the role of different putative phosphorylation sites to facilitate Bcl-2 degradation. Mutation of the consensus protein kinase B/Akt site or of potential protein kinase C or cyclic AMP-dependent protein kinase sites does not affect Bcl-2 stability. In contrast, inactivation of the three consensus mitogen-activated protein (MAP) kinase sites leads to a Bcl-2 protein that is ubiquitinated and subsequently degraded by the 26S proteasome. Inactivation of these sites within Bcl-2 revealed that dephosphorylation of Ser87 appears to play a major role. A Ser-to-Ala substitution at this position results in 50% degradation, whereas replacement of Thr74 with Ala leads to 25% degradation, as assessed by pulse-chase studies. We further demonstrated that incubation with TNF-α induces dephosphorylation of Ser87 of Bcl-2 in intact cells. Furthermore, MAP kinase triggers phosphorylation of Bcl-2, whereas a reduction in Bcl-2 phosphorylation was observed in the presence of MAP kinase-specific phosphatases or the MAP kinase-specific inhibitor PD98059. Moreover, we show that oxidative stress mediates TNF-α-stimulated proteolytic degradation of Bcl-2 by reducing MAP kinase activity. Taken together, these results demonstrate a direct protective role for Bcl-2 phosphorylation by MAP kinase against apoptotic challenges to endothelial cells and other cells.
Programmed cell death is critical for the successful development of multiple tissues and the maintenance of normal tissue homeostasis. The signaling pathways involved in apoptosis have been intensively studied (reviewed in references 2, 3, 19, 21, 23, and 24). Key regulatory proteins in apoptotic events are the Bcl-2 family of proteins, which can either promote cell survival (Bcl-2, Bcl-XL, A1, Mcl-1, and Bcl-W) or promote cell death (Bax, Bak, Bcl-XS, and Bok) (1). The relative amounts or equilibrium between these pro- and antiapoptotic proteins influences the susceptibility of cells to apoptosis.
Bcl-2 is the prototype member of a protein family that functions to suppress apoptosis in a variety of cell systems. Bcl-2 is localized in the endoplasmatic reticulum, nuclear envelope, and outer mitochondrial membrane, whereby its NH2-terminal part is facing the cytosol. Several mechanisms have been proposed to explain the antiapoptotic function of Bcl-2. Bcl-2 might act as a regulator of Ca2+ homeostasis (4) or as an antioxidant (34). Bcl-2 forms heterodimers with the proapoptotic protein Bax and might thereby neutralize its death effector properties (46). In addition, Bcl-2 prevents the release of potent mitochondrial activators of the cytosolic death effector proteases, the caspase protease family, which mediates the intracellular proteolysis that is characteristic of apoptosis (60). The association of Bcl-2 with the mitochondrial apoptosis-activating factor Apaf1 and the blockade of cytochrome c release may prevent the activation of the two death proteases, caspases 9 and 3 (39, 61). Bcl-2 also acts by modulating the collapse of the mitochondrial transmembrane potential that occurs during apoptosis (60). Whereas any one or a combination of these potential functions of Bcl-2 may operate to suppress apoptosis, the mechanism by which Bcl-2 may be regulated to preserve mitochondrial integrity has not been identified.
The regulation of Bcl-2 on the transcriptional level seems to be a critical factor in the development of cancer, as has been demonstrated by enhanced expression of Bcl-2 protein in cancer tissues (36). In contrast, Bcl-2 protein levels have been shown to be reduced in endotoxic shock in vivo (26), in endothelial cells exposed to lipopolysaccharide (27), in neuronal cells treated with β-amyloid (47), and in neurons following cerebral ischemia (37), suggesting a potential role of Bcl-2 turnover under inflammatory conditions. Moreover, a potential role for Bcl-2 degradation is supported by our recent finding that, in human umbilical vein endothelial cells (HUVEC), tumor necrosis factor alpha (TNF-α) induces dephosphorylation and subsequent ubiquitin-dependent degradation of Bcl-2 leading to apoptosis (16).
The ubiquitin-proteolytic pathway is a major system for selective protein degradation in eukaryotic cells. One of the first steps in this process includes selective modification of ɛ-NH2 groups of lysine residues in the corresponding protein by ubiquitination, which targets the protein for ubiquitin-dependent degradation by the proteasome complex. Although the mechanisms that underlie this multicatalytic process are very well characterized (reviewed in references 13, 14, 29, and 30), the signals that target proteins for ubiquitination and, therefore, determine their stability, are often unclear. In some cases, different patterns of phosphorylation, a partially conserved sequence motif, or specific structural features (as shown for mitotic cyclins, other cell cycle regulators, or transcription factors) are required (reviewed in reference 29).
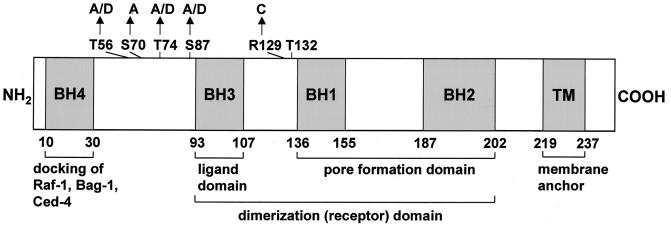
The objectives of this study were to get further insights into the signal transduction stimulating Bcl-2 degradation. Bcl-2 contains several consensus protein kinase sites, such as a protein kinase B/Akt site (RXRXXS/T) at position 132, a protein kinase Cα (PKC) or cyclic AMP (cAMP)-dependent protein kinase (PKA) site (RXS/T) at an evolutionarily conserved serine site (Ser70), and several mitogen-activated protein (MAP) kinase sites for extracellular signal-regulated kinases 1 and 2 (ERK1/2) (PXXS/TP) at positions 56, 74, and 87. The role of these different putative phosphorylation sites for facilitating selective Bcl-2 degradation was examined. We demonstrate here that dephosphorylation of the MAP kinase site Ser87 within Bcl-2 appears to play a major role as a signal for its ubiquitin-dependent degradation. Furthermore, we show that oxidative stress mediates TNF-α-stimulated proteolytic degradation of Bcl-2 by inactivation of the MAP kinases ERK1/2. Thus, Bcl-2 represents an attractive target for posttranslational modification in response to proapoptotic stimuli in endothelial cells and other cells.
MATERIALS AND METHODS
Cell culture.
HUVEC were purchased from Cell Systems/Clonetics, Solingen, Germany, and cultured in endothelial basal medium supplemented with hydrocortisone (1 μg/ml), bovine brain extract (3 μg/ml), gentamicin (50 μg/ml), amphotericin B (50 μg/ml), epidermal growth factor (10 μg/ml), and 10% fetal calf serum (FCS) until the third passage. COS-7 and HeLa cells were cultured in Dulbecco's modified Eagle's medium with 10% FCS and 2 mM l-glutamine.
Plasmid constructs.
Human Bcl-2 and wild-type MAP kinase phosphatase 3 (MKP-3) were amplified and subcloned into pcDNA3.1(−)MycHis or pcDNA3.1(−) (Invitrogen, Groningen, The Netherlands) under the control of the cytomegalovirus immediate-early gene as described recently (16). The MAP kinase phosphatase MKP-4 was amplified by PCR with oligonucleotides containing EcoRV and BamHI restriction sites and cloned into the respective sites of pcDNA3.1(−)MycHis under the transcriptional control of the cytomegalovirus promoter. Desired mutant Bcl-2 constructs and a dominant negative form of MKP-3 (Cys293 to Ser) were obtained by site-directed mutagenesis (Stratagene, Heidelberg, Germany). Sequences were determined by an ABI automated sequencer.
Transient-transfection system.
Transient transfections of HUVEC and COS-7 cells were performed as described previously (16). HeLa cells were transfected with plasmids by the calcium phosphate method (53). The transfection of oligonucleotides into HUVEC or HeLa cells was carried out by using the Lipofectamine procedure (GIBCO BRL, Karlsruhe, Germany). Briefly, 1.5 μg of phosphorothiolated sense (5′-GGGAAGGATGGCGCACGCTG-3′) or antisense (5′-CAGCGTGCGCCATCCTTCCC-3′) oligonucleotides (Roth, Karlsruhe, Germany) corresponding to the Bcl-2 sequence (49) or phosphorothiolated sense (5′-CCTGAGTTCCACTGAGTTCC-3′) or antisense (5′-GGAACTCAGTGGAACTCAGG-3′) oligonucleotides corresponding to a highly conserved sequence within the MAP kinase-specific phosphatases (31) was incubated in 100 μl of RPMI medium in the presence of 5 μl of Lipofectamine (GIBCO BRL) for 30 min at room temperature. HUVEC or HeLa cells (5 × 105 cells in 6-cm2 wells) were washed with RPMI medium and incubated with 2 ml of RPMI medium before adding the Lipofectamine-oligonucleotide mixture. After further incubation for 5 h, 3 ml of complete RPMI medium was added and the cells were again incubated for 18 h before the determination of apoptosis.
Western blot analysis and immunoprecipitation.
Cells were lysed as described (16). Western blots were performed with either anti-Bcl-2 antibody (Boehringer Mannheim, Mannheim, Germany) or anti-myc antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.). For the detection of phosphorylated forms of Bcl-2, immunoprecipitation was carried out with either an anti-phosphoserine-specific antibody (clone 4A9; Alexis Corporation, Grünberg, Germany) or an anti-myc antibody (Santa Cruz Biotechnology). Immunoprecipitates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and Western blot analysis was carried out with anti-myc antibody or an anti-phosphoserine-specific antibody.
To identify ubiquitinated forms of Bcl-2, HeLa cells transiently transfected with various myc-tagged Bcl-2 constructs were incubated with the proteasome inhibitor lactacystin (10 μM) for 2 h (7). Cells were lysed, and protein concentrations were determined by the Bradford method (6). Equal amounts of protein were subjected to immunoprecipitation with anti-myc antibody. The immunoprecipitate was resolved via SDS–10% PAGE and transferred onto polyvinylidene difluoride (PVDF) membrane, and the conjugates were detected with anti-ubiquitin antibody (Sigma-Aldrich Chemie GmbH, Deisenhofen, Germany) and the enhanced chemiluminescence method (Amersham, Braunschweig, Germany). As a control for expression of myc-tagged Bcl-2 protein, Western blot analysis was performed with an anti-myc antibody (Santa Cruz Biotechnology).
For the assay of TNF-α-induced degradation, cells were incubated for the time periods indicated with 100 ng of TNF-α per ml, and cell extracts were obtained by lysis of cells in 10 mM Tris-HCl (pH 8), 1% Triton X-100, and 0.32 M sucrose on ice for 20 min. Then, homogenates were centrifuged and the resulting supernatant was used for Western blotting. Proteins (30 μg/lane) were loaded onto SDS–12.5% PAGE gels and probed with anti-Bcl-2 antibody (Boehringer Mannheim) or anti-myc antibody (Santa Cruz Biotechnology), and enhanced chemiluminescence was performed according to the instructions of the manufacturer (Amersham).
Stability of proteins in vivo.
HeLa cells were starved in Dulbecco's modified Eagle's medium without methionine and cysteine for 1 h and then were metabolically labeled with l-[35S]methionine and l-[35S]cysteine for 2 h. HeLa cells were then chased in nonradioactive medium for the time periods indicated. Cells were lysed (10 mM Tris-HCl, pH 8; 1% Triton X-100; 0.32 M sucrose) at 4°C for 20 min. Samples containing equal amounts of protein were immunoprecipitated with an anti-myc antibody. Immunocomplexes were collected with immobilized Protein A/G-plus Sepharose (Amersham) and resolved on SDS–12.5% PAGE. The gel was dried, and proteins were visualized by a PhosphorImager (Molecular Dynamics).
In vitro kinase assay.
For the detection of in vitro phosphorylation of Bcl-2 by MAP kinase or Akt kinase, respectively, HeLa cells were transiently transfected with various myc-tagged Bcl-2 constructs and overexpressed proteins were immunoprecipitated with anti-myc antibody. Isolated immunoprecipitated myc-tagged Bcl-2 proteins were incubated at 30°C in a kinase reaction mixture containing 25 mM Tris (pH 7.5), 5 mM β-glycerolphosphate, 0.1 mM Na3VO4, 2 mM dithiothreitol, 10 mM MgCl2, 15 μM ATP, and 5 μCi of [γ-32P]ATP with activated MAP kinase ERK2 (New England Biolabs, Schwabach, Germany) for 30 min. Active Akt was obtained by immunoprecipitation of myc-tagged, constitutive active Akt (Thr308Asp and Ser473Asp) from transiently transfected COS-7 cells as described recently (17). Immunoprecipitated myc-tagged Akt kinase was combined with immunoprecipitated myc-tagged Bcl-2, and in vitro kinase assays were carried out as described above. The reaction was terminated by the addition of SDS loading dye, and samples were subjected to SDS–12.5% PAGE and analyzed by a PhosphorImager.
Cell death analysis.
DNA fragmentation was demonstrated and quantified by morphological analysis of apoptotic nuclei after fluorescence staining with 4′,6′-diamidino-2-phenylindole (DAPI) as described previously (16). The data were confirmed by an enzyme-linked immunosorbent assay specific for histone-associated DNA fragments as recently described (18). To determine the influence of the various Bcl-2 constructs on apoptosis, HUVEC were transiently cotransfected with β-galactosidase reporter and test plasmids. Cells were fixed in 4% formaldehyde, and transfected cells were identified by β-galactosidase staining for 12 h. The percentage of morphologically altered cells typical for apoptotic cell death was determined by phase-contrast microscopy and then represented as a function of the total number of blue cells under each condition. The number of dead versus viable cells was counted by two independent investigators in a total number of 600 cells.
Northern blot.
Total RNA was prepared as described recently (5) and 10 μg of total RNA was loaded on 0.8% formamide agarose gels. RNA was blotted on nylon membranes, and the blots were hybridized with a [α-32P]dCTP-labeled DNA probe specific for human MKP-3. After incubation for 24 h, blots were washed (0.1% SDS, 0.2× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]) and exposed to X-ray films.
Statistics.
Data are expressed as the mean ± standard error of the mean (SEM) from at least three independent experiments. Statistical analysis was performed with analysis of variance followed by a modified least significant difference test (SPSS-Software).
RESULTS
Bcl-2 stability is mainly regulated by phosphorylation of its MAP kinase sites Thr74 and Ser87.
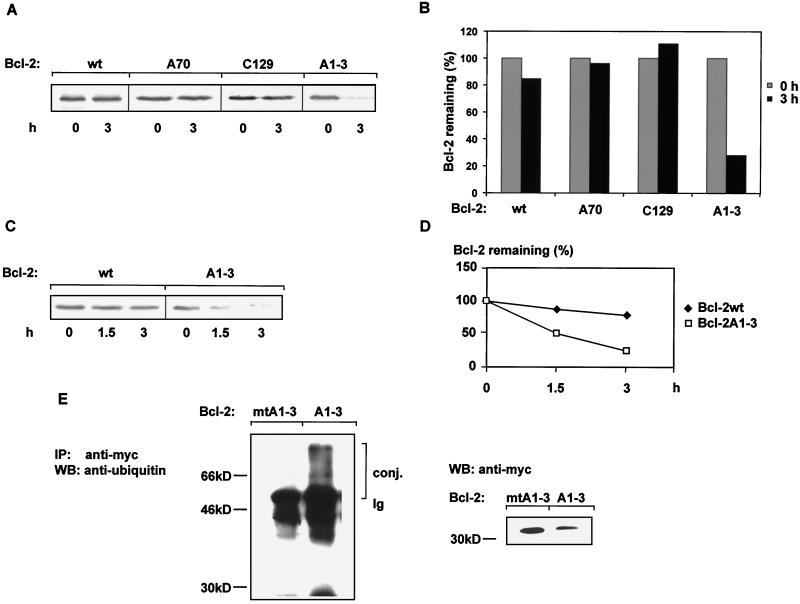
We previously showed that incubation with TNF-α stimulates ubiquitin-dependent Bcl-2 proteolysis involving dephosphorylation of Bcl-2 as a prerequisite (16). Since Bcl-2 contains several consensus protein kinase sites, we investigated the involvement of these putative phosphorylation sites in the regulation of Bcl-2 protein stability. The consensus Akt site at position 132 was mutated by an Arg-to-Cys substitution at position 129 (Bcl-2C129). The potential PKC or PKA site at position 70 was changed from Ser to Ala (Bcl-2A70) (Fig. 1). To determine the influence of these mutations on Bcl-2 stability, pulse-chase analyses of the corresponding constructs were carried out. Neither inactivation of the potential Akt site nor inactivation of the PKC or PKA site affected Bcl-2 stability (Fig. 2A and B). No significant difference in Bcl-2 protein levels could be observed after a 3-h chase following metabolic labeling of HeLa cells (Fig. 2A to D). In contrast, simultaneous mutation of all MAP kinase sites at positions 56, 74, and 87 (Bcl-2A1-3) to the amino acid Ala resulted in a Bcl-2 protein that was rapidly degraded by the 26S proteasome (Fig. 2A, 2B, and 3B; P < 0.001). While the half-life of wild-type Bcl-2 was found to be more than 6 h, inactivation of MAP kinase sites (Bcl-2A1-3) resulted in a half-life of less than 1.5 h (Fig. 2C and D).
FIG. 1.
Schematic diagram of Bcl-2 (1). Different homologue domains (BH) are indicated by hatched boxes. Putative phosphorylation sites investigated in this study are indicated. MAP kinase sites, T56, T74, and S87; PKC or PKA sites, S70; Akt kinase site, T132. Arrows indicate substitutions. A, alanine; C, cysteine; D, aspartic acid; S, serine; T, threonine; TM, transmembrane domain.
FIG. 2.
Stability of various Bcl-2 constructs with different inactivated phosphorylation sites. (A) Bcl-2 stability is affected neither by the evolutionarily conserved PKA or PKC site Ser70 nor by the Akt site Thr132. Bcl-2 proteins metabolically labeled with 35S were chased (3 h) as described in Materials and Methods. Labeled Bcl-2 was immunoprecipitated from aliquots containing equal amounts of proteins. Bcl-2wt, wild-type Bcl-2; Bcl-2A70, Ser-to-Ala substitution at position 70; Bcl-2C129, destruction of the Akt consensus site by an exchange of Arg with Cys; Bcl-2A1-3, MAP kinase consensus sites Thr56, Thr74, and Ser87 were changed to Ala. A representative autoradiogram of three independent experiments is shown. (B) Quantitative (PhosphorImaging) analysis of the data depicted in panel A is shown. Quantities are relative to the amount of protein at time zero. (C) Representative time course degradation of wild-type Bcl-2 (wt) and of a Bcl-2 construct in which all MAP kinase consensus sites were changed to Ala (Bcl-2A1-3) (n = 3). (D) Quantitative analysis of the data depicted in panel C. Quantities are relative to the amount of protein at time zero. (E) Detection of ubiquitin-Bcl-2 conjugates in HeLa cells. HeLa cells were transiently transfected with expression vector containing either a lysine-free Bcl-2A1-3 construct (Bcl-2mtA1-A3) or Bcl-2A1-3. Following 42 h of transfection, cells were incubated for an additional 2 h with the proteasome inhibitor lactacystin. Equal amounts of protein, as determined by the Bradford method (6), were subjected to immunoprecipitation with anti-myc antibody, and ubiquitin conjugates were identified by Western blot (WB) analysis and anti-ubiquitin antibody. Expression of Bcl-2 protein is detected by Western blot analysis with anti-myc antibody. conj., conjugates; Ig, the heavy chain of the immunoglobulin molecule.
FIG. 3.
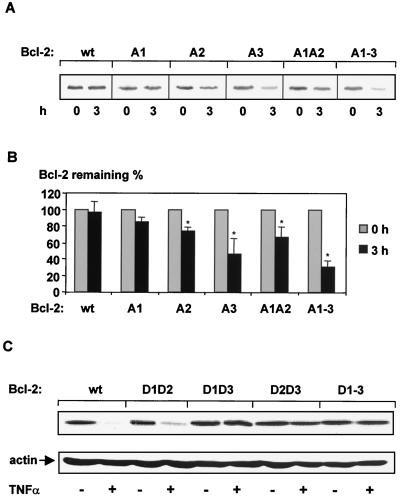
In vivo degradation of Bcl-2 mutated proteins in which the MAP kinase sites were progressively substituted with Ala. (A) HeLa cells transiently transfected with Bcl-2 cDNA were pulse labeled (2 h) with [35S]methionine and chased (3 h) as described in Materials and Methods. Bcl-2A1, Thr56 was replaced with Ala; Bcl-2A2, Thr74 was changed to Ala; Bcl-2A3, Ser87 was mutated to Ala; Bcl-2A1A2, combined substitution of Thr56 and Thr74 with Ala; Bcl-2A1-A3, Thr56, Thr74, and Ser87 were replaced with Ala. A representative autoradiogram is shown (n = 3). (B) Quantitative analysis of three independent experiments described for panel A after a chase period of 3 h. Quantities are relative to the amount of protein at time zero. Data are mean ± SEM (error bars) (∗, significantly different from amount of wild-type Bcl-2 protein after a 3-h chase [P < 0.05], n = 3). (C) Sensitivity of various Bcl-2 constructs containing phosphate-mimetic amino acids at relevant MAP kinase sites to TNF-α. HUVEC transiently transfected with Bcl-2 in pcDNA3.1 were incubated with TNF-α (100 ng/ml) for 6 h. Bcl-2D1D2, Thr56 and Thr74 were replaced with Asp; Bcl-2D1D3, Thr56 and Ser87 were replaced with Asp; Bcl-2D2D3, Thr74 and Ser87 were changed to Asp; Bcl-2D1-D3, Thr56, Thr74, and Ser87 were mutated to Asp. Western blot analysis was carried out with anti-myc antibody. Following stripping of the PVDF membrane, equal loading of the samples was demonstrated by Western blot analysis with antiactin antibody.
To demonstrate the intermediary of ubiquitin conjugates in the degradation of Bcl-2A1-3, HeLa cells transiently transfected with Bcl-2A1-3 were incubated with the proteasome inhibitor lactacystin. As shown in Fig. 2E, highly ubiquitinated forms of Bcl-2A1-3 could be observed. Similar analyses with HeLa cells transiently transfected with a Bcl-2A1-3 construct that lacks all ubiquitin acceptor amino acids (lysine-free Bcl-2A1-3 or Bcl-2mtA1-3) revealed no ubiquitin conjugates, clearly demonstrating the specificity of the ubiquitin conjugates of the Bcl-2A1-3 construct (Fig. 2E). To demonstrate equal loading and expression of the various Bcl-2 constructs, Western blot analysis was carried out with an anti-myc antibody. The lower intensity of the nonubiquitinated Bcl-2A1-3 band is due to its increased ubiquitinated forms (Fig. 2E, right panel). These data indicate that ubiquitin conjugates are formed during the degradation of the Bcl-2A1-3 protein.
To explore the role of single MAP kinase sites for Bcl-2 stability, Thr56, Thr74, and Ser87 were individually mutated to alanine (Fig. 1). Replacement of Thr56 with Ala (Bcl-2A1) resulted in only slight degradation (∼12%) of Bcl-2, whereas substitution of Thr74 by Ala (Bcl-2A2) was accompanied by an approximately 25% reduction of protein levels (Fig. 3A and B). The combined mutation of Thr56 and Thr74 to Ala (Bcl-2A1A2) led to a Bcl-2 construct that is degraded by ∼35%, implicating a cumulative effect of both MAP kinase sites. However, the Ser87-to-Ala mutation (Bcl-2A3) resulted in 50% degradation (Fig. 3A and B). In order to ensure that phosphorylation of the MAP kinase sites in Bcl-2 is sufficient to inhibit TNF-α-induced degradation, Thr56, Thr74, and Ser87 were replaced with phosphate-mimetic aspartic acid residues, which mimic continuous phosphorylation of the protein (45). Mutation of the single site Thr56 or Thr74 did not significantly affect TNF-α-induced degradation, whereas the Ser87Asp Bcl-2 mutant profoundly reduced TNF-α-triggered Bcl-2 degradation in vivo and in vitro (data not shown). Furthermore, the combination Thr56Asp-Thr74Asp (Bcl-2D1D2) mutation slightly reduced TNF-α-stimulated proteolysis, whereas both Thr56Asp-Ser87Asp (Bcl-2D1D3) and Thr74Asp-Ser87Asp (Bcl-2D2D3) mutants, as well as a triple Thr56Asp-Thr74Asp-Ser87Asp Bcl-2 construct (Bcl-2D1-3), were resistant towards TNF-α-stimulated degradation in endothelial cells (Fig. 1 and 3C). These data indicate that dephosphorylation of Ser87 appears to play a major role as a signal for Bcl-2 degradation.
MAP kinase site phosphate-mimetic Bcl-2 constructs inhibit TNF-α-induced apoptosis.
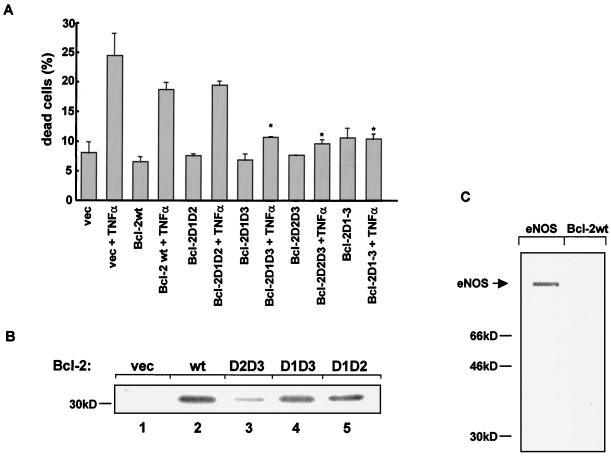
We previously showed that a reduction of Bcl-2 protein levels preceded the induction of apoptosis (16). To establish that TNF-α-induced dephosphorylation of Bcl-2 at its MAP kinase sites signals degradation of Bcl-2 and, therefore, stimulates apoptosis, Bcl-2 constructs carrying phosphate-mimetic amino acid substitutions at the corresponding MAP kinase sites were transfected into HUVEC. TNF-α-induced apoptosis was examined by detecting the morphological change of β-galactosidase-stained nuclei. According to the results illustrated above, resistance to TNF-α-induced apoptosis paralleled the inhibition of degradation of various Bcl-2 mutants, in which different MAP kinase sites were mutated. TNF-α-induced apoptosis was significantly reduced in cells expressing Bcl-2 constructs with the relevant phospho-mimetic amino acid substitutions (Fig. 4A). A Bcl-2 construct with substitutions of Asp for Thr56 and Thr74 (Bcl-2D1D2) showed significantly less resistance to TNF-α-induced apoptosis compared to Bcl-2 constructs with substitutions of Asp for both Thr56 and Ser87 (Bcl-2D1D3) or Thr74 and Ser87 (Bcl-2D2D3) (Fig. 4A).
FIG. 4.
Influence of phosphate-mimetic Bcl-2 mutant proteins on apoptosis and in vitro kinase assays of various Bcl-2 proteins. (A) HUVEC were transiently cotransfected with a vector carrying either wild-type Bcl-2 or various phosphate-mimetic Bcl-2 constructs and a lacZ reporter. Apoptosis was induced by incubation with TNF-α (100 ng/ml) for 18 h. Transfected cells were identified by β-galactosidase staining as described under Materials and Methods. Data are mean + SEM (error bars) (∗, significantly different from Bcl-2wt + TNF-α [P < 0.05]; n = 4). vec, empty vector. (B) Phosphorylation of various Bcl-2 constructs by active MAP kinase. Wild-type Bcl-2 or mutant Bcl-2 forms lacking two of the three putative MAP kinase acceptor amino acids were expressed in HeLa cells and myc-tagged Bcl-2 was immunoprecipitated with anti-myc antibody. Isolated immunocomplexes were incubated with active MAP kinase as described under Materials and Methods and resolved by SDS-PAGE. Lane 1, empty vector (vec); lane 2, wild type (wt); lane 3, Bcl-2D2D3 (Thr74 and Ser87 were changed to Asp); lane 4, Bcl-2D1D3 (Thr56 and Ser87 are with Asp); lane 5, Bcl-2D1D2 (Thr56 and Thr74 were replaced by Asp). (C) In vitro phosphorylation of Bcl-2wt and endothelial NO synthase (eNOS) by constitutive active kinase Akt. The kinase assay was carried out as described in Materials and Methods. Experiments were repeated three times, with identical results.
The MAP kinase sites Thr74 and Ser87 within Bcl-2 are dominantly phosphorylated by activated MAP kinase in vitro.
To determine if all three MAP kinase sites within Bcl-2 are potential targets of activated MAP kinase, in vitro kinase assays were performed. Bcl-2 constructs containing only one intact MAP kinase site, either Thr56 (Bcl-2D2D3), Thr74 (Bcl-2D1D3), or Ser87 (Bcl-2D1D2), were transiently expressed in HeLa cells and isolated by immunoprecipitation with an anti-myc antibody. These immunocomplexes were added as substrates to a MAP kinase assay. In agreement with the data described above, the phosphorylation efficiency of the single MAP kinase sites correlated with their functional role in regulating Bcl-2 stability. Activated MAP kinase dominantly phosphorylated Bcl-2 proteins containing the intact MAP kinase site Thr74 (Bcl-2D1D3) or Ser87 (Bcl-2D1D2), whereas the MAP kinase site Thr56 (Bcl-2D2D3) was only slightly phosphorylated (Fig. 5, lanes 3 to 5). Bcl-2wt served as a control for MAP kinase phosphorylation activity (Fig. 5, lane 2). A Bcl-2 mutant in which all MAP kinase sites were inactivated could not be phosphorylated by MAP kinase in vitro (16) (data not shown).
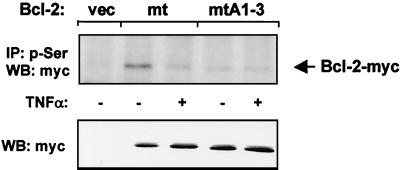
FIG. 5.
Effect of TNF-α on Ser87 phosphorylation of Bcl-2. HUVEC were transfected with empty vector (vec), lysine-free myc-tagged Bcl-2 (Bcl-2mt [Lys17Arg, Lys22Arg, Lys218Arg, and Lys239Arg]), or lysine-free Bcl-2A1-3 (Bcl-2mtA1-3 [Thr56Ala, Thr74Ala, Ser87Ala, Lys17Arg, Lys22Arg, Lys218Arg, and Lys239Arg]) and incubated with or without TNF-α (100 ng/ml) for 6 h. Serine-phosphorylated Bcl-2 was immunoprecipitated (IP) with antiphosphoserine antibody. Bcl-2 protein levels were detected by Western blot (WB) analysis with anti-myc antibodies. Western blot of cell lysates with antibody against myc (lower panel) served as a control for expression. A representative blot of three independent experiments is shown.
PKA and PKC have been shown to act as kinases for Bcl-2 phosphorylation (33, 52, 56). To assess if Bcl-2 could also be phosphorylated by Akt in vitro, COS-7 cells were transiently transfected with a constitutive active, myc-tagged Akt construct. Akt was immunoprecipitated with an anti-myc antibody, and isolated immunocomplexes were used in a kinase assay. Phosphorylation by Akt was observed for the known Akt targets histone 2B or endothelial NO synthase (Fig. 4C) (17). In contrast, Bcl-2 was not phosphorylated by Akt (Fig. 4C). Thus, Akt does not appear to be a principal Bcl-2 kinase.
Incubation with TNF-α induces dephosphorylation of Ser87 within Bcl-2.
Our results suggested that dephosphorylation of Ser87 plays a predominant role as a signal for ubiquitin-dependent degradation of Bcl-2. To demonstrate directly that TNF-α indeed triggers dephosphorylation of Ser87, we transiently expressed myc-tagged Bcl-2 constructs in HUVEC. Cells were lysed following treatment with or without TNF-α. To determine Ser87 phosphorylation, immunoprecipitation was carried out with an anti-phosphoserine-specific antibody, and Western blot analysis was performed with an anti-myc antibody. Bcl-2 degradation in consequence to dephosphorylation was prevented by using degradation-resistant, lysine-free Bcl-2 constructs. As shown in Fig. 5, TNF-α stimulation completely reduced Ser87 phosphorylation of Bcl-2. The same experiment was performed with a lysine-free Bcl-2 construct, in which all MAP kinase sites were inactivated to prevent phosphorylation at these sites. No serine phosphorylation of this mutated Bcl-2 protein was determined, which demonstrates the specificity for Ser87 phosphorylation (Bcl-2mtA1-3) (Fig. 5). Taken together, these data provide compelling evidence that Ser87 phosphorylation is directly affected by TNF-α.
The MAP kinase ERK2 induces phosphorylation of Bcl-2 in vivo.
To show that activated ERK phosphorylates Bcl-2 in vivo, we transiently cotransfected HeLa cells with ERK2 and a degradation-resistant myc-tagged form of Bcl-2 (Bcl-2mt). myc-tagged Bcl-2 was immunoprecipitated with an anti-myc antibody, and Western blot analysis was performed with an anti-phosphoserine antibody. An increased amount of phosphorylated Bcl-2 protein was observed in the presence of activated ERK2, whereas less phosphorylated Bcl-2 was detectable in the absence of ERK2 (Fig. 6A). In addition, the MAP kinase-specific inhibitor PD98059 significantly reduced the amount of serine-phosphorylated Bcl-2 compared with untreated cells (Fig. 6B). Thus, Bcl-2 is phosphorylated by ERK2 in vivo.
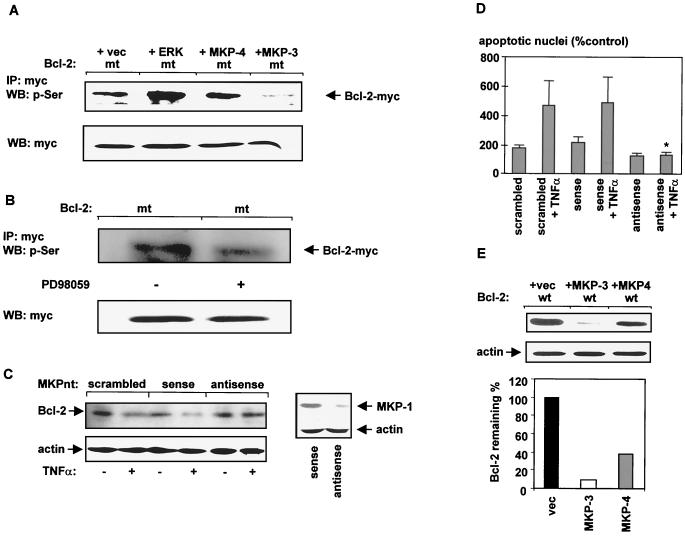
FIG. 6.
ERK2 induces Bcl-2 phosphorylation whereas ERK-specific phosphatases induce Bcl-2 dephosphorylation and its subsequent degradation. (A) Plasmids encoding a myc-tagged lysine-free Bcl-2 protein (Bcl-2mt) were cotransfected with either MKP-3, MKP-4, ERK in pcDNA3.1, or empty vector (vec) in HeLa cells. After 42 h of transfection, ERK activity was stimulated by starving cells in FCS-free medium for 2 h and a subsequent addition of FCS for 1 h. Immunoprecipitation (IP) was performed with anti-myc antibody. Immunocomplexes were resolved by SDS-PAGE as described under Materials and Methods. Western blot (WB) analysis was carried out with antiphosphoserine antibody. Western blot analysis of protein homogenates with anti-myc antibody served as a control for Bcl-2 expression. (B) Effect of PD98059 on serine phosphorylation. Lysine-free myc-tagged Bcl-2 protein (Bcl-2mt) was transfected into HeLa cells and 30 h after transfection, cells were incubated with PD98059 (15 μM) for 18 h. Proteins were immunoprecipitated with anti-myc antibody, and the presence of phosphorylated Bcl-2 was determined with antiphosphoserine antibody. (C) Effect of antisense MKP oligonucleotides on TNF-α-induced degradation of Bcl-2. HUVEC were transfected with either sense, antisense, or scrambled MKP oligonucleotides (MKPnt) by the Lipofectamine method as described in Materials and Methods and incubated for 6 h with or without TNF-α (100 ng/ml). Suppression of MKP-1 after antisense oligonucleotide treatment is shown in the right panel via Western blot analysis with anti-MKP-1 antibody. Stripping of the PVDF membrane followed by reprobing with antiactin demonstrated equal loading of the samples. (D) TNF-α-induced apoptosis in HUVEC is completely inhibited in the presence of antisense MKP oligonucleotides. Lipofectamine-treated cells served as controls. (∗, significantly different from sense + TNF-α [P < 0.05]; n = 3; mean ± SEM [error bars] are shown; apoptosis in cells transfected with Lipofectamine was about 10%). (E) Effect of MKP-3 and MKP-4 on Bcl-2 stability. HeLa cells were cotransfected with Bcl-2wt and either empty vector (vec), MKP-3, or MKP-4. Forty-two hours after transfection, cells were lysed and proteins were separated by SDS–12.5% PAGE. Western blot analysis was performed with anti-myc antibody. Reprobe of the PVDF membrane with antiactin demonstrated equal loading of the samples (middle panel). The lower panel shows a quantitative analysis of the data depicted in the upper panel. Quantities are relative to the amount of Bcl-2 protein cotransfected with empty vector (Bcl-2 + vec). Each experiment was performed three times, with identical results.
ERK-specific phosphatases induce Bcl-2 dephosphorylation and its subsequent degradation.
MAP kinase phosphorylation is a reversible process, indicating that protein phosphatases play a crucial role in controlling cellular activities. An emerging class of dual-specificity phosphatases has been shown to regulate directly and specifically MAP kinase family members. The dual-specificity phosphatase family that mainly dephosphorylates MAP kinases at both the Tyr and Thr residues necessary for enzymatic activity comprises MAP kinase phosphatase 1 (MKP-1), MKP-2, MKP-3, and MKP-4 (12, 43, 44). Transfection of HUVEC with MKP antisense oligonucleotides directed against a conserved domain within the MKP family completely inhibited TNF-α-induced degradation of Bcl-2 in vivo, whereas neither sense nor scrambled oligonucleotides affected the degradation process (Fig. 6C). Furthermore, pretreatment with MKP antisense oligonucleotides completely prevented ERK dephosphorylation after 6 h of TNF-α stimulation, whereas MKP sense oligonucleotides did not affect ERK activity (data not shown). This inhibition of ERK dephosphorylation was associated with the complete inhibition of TNF-α-induced apoptosis of HUVEC (P < 0.05; Fig. 6D) indicating a pivotal role for the MAP kinase pathway to modulate TNF-α-mediated apoptotic processes.
MKP-1 and MKP-2 are strictly localized to the nucleus (25, 38), whereas MKP-3 is exclusively spotted in the cytosol (58). MKP-4 is located in the cytosol and in the nucleus (44). Since Bcl-2 is primarily located at the outer mitochondrial membrane, whereby its NH2-terminal part is facing the cytosol, the reversible phosphorylation process of Bcl-2 should mainly take place in the cytosol. Therefore, we investigated the direct influence of MKP-3 and MKP-4 on Bcl-2 phosphorylation and degradation. Cotransfection of MKP-3 with a lysine-free, degradation-resistant form of Bcl-2 (Bcl-2mt) in HeLa cells resulted in a significantly reduced amount of phosphorylated Bcl-2, as demonstrated by Western blot analysis with an anti-phosphoserine antibody following immunoprecipitation with anti-myc antibody (Fig. 6A). Cotransfection with MKP-4 revealed no significant reduction of phosphorylated Bcl-2 (Fig. 6A).
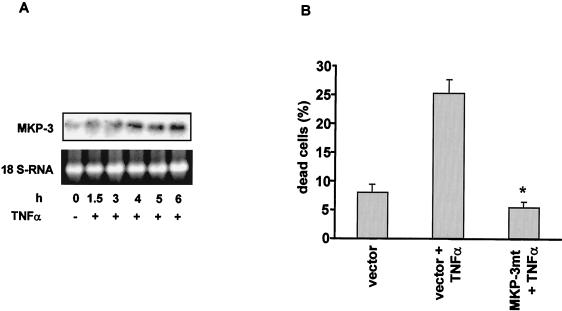
The presence of both phosphatases affected Bcl-2 stability (Fig. 6E). However, in the presence of MKP-3, Bcl-2 degradation was at least fourfold higher than that in the presence of MKP-4 (Fig. 6E). Moreover, a crucial role of MKP-3 for Bcl-2 degradation was further indicated by the finding that TNF-α stimulated the time-dependent transcription of MKP-3 mRNA, as assessed by Northern blot analysis (Fig. 7A). Finally, a dominant negative MKP-3 mutant in which Cys293 was changed to Ser (8) significantly inhibited TNF-α-induced Bcl-2 degradation (not shown) and apoptosis in HUVEC (Fig. 7B). Taken together, these data demonstrate a pivotal role for MKP-3, not only for the TNF-α-triggered degradation process of Bcl-2 but also for TNF-α-mediated apoptosis induction.
FIG. 7.
Effect of TNF-α on MKP-3 expression. (A) Northern blot analysis of MKP-3 mRNA after stimulation of HUVEC with TNF-α. Equal loading of the samples is demonstrated by determining 18S RNA concentration. (B) TNF-α-induced apoptosis in HUVEC is completely inhibited by overexpression of a dominant negative MKP-3 (MKP-3mt) mutant protein. Data are mean ± SEM (error bars) (∗, significantly different from vector + TNF-α [P < 0.05], n = 3). MKP-3mt, Cys293 was mutated to Ser.
Effect of antioxidants on TNF-α-stimulated Bcl-2 degradation.
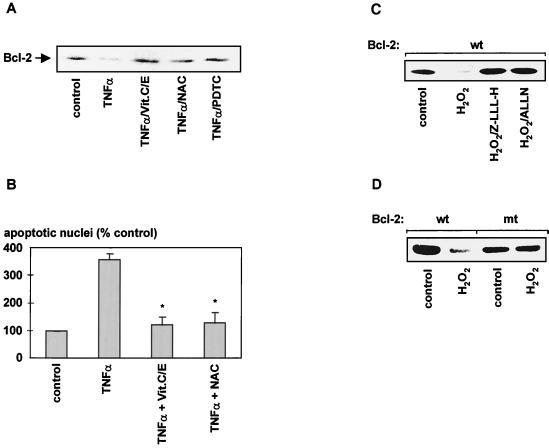
The role of reactive oxygen species (ROS) in TNF-α-stimulated cell death has been previously reported (22, 55, 58). Studies using mitochondrial inhibitors and respiration-deficient cells have shown that TNF-α cytotoxicity is primarily mediated by ROS generated at the ubiquinone site of the mitochondrial electron transport (55). Inhibition of the electron flow to ubiquinone by respiratory chain inhibitors prevented formation of superoxide anions and strongly reduced TNF-α-induced cell death (55). To investigate whether oxygen radicals are involved in the TNF-α-induced degradation of Bcl-2, we studied the effects of a series of antioxidants on the Bcl-2 degradation process. Figure 8A demonstrates that coincubation with antioxidants, such as N-acetylcysteine (NAC), pyrrolidinedithiocarbamate, or a combination of vitamins E and C, significantly inhibited stimulus-dependent degradation of Bcl-2 in intact cells. To further demonstrate the physiological relevance of ROS-mediated Bcl-2 degradation, the effects of antioxidants on TNF-α-stimulated apoptosis in HUVEC were studied. As illustrated in Fig. 8B, antioxidants profoundly inhibited TNF-α-induced apoptosis.
FIG. 8.
Effect of antioxidants on TNF-α-stimulated Bcl-2 degradation and apoptosis in HUVEC. (A) Inhibition of TNF-α-stimulated degradation of Bcl-2 in vivo. HUVEC were incubated for 12 h in the presence of 100 ng of TNF-α/ml and vitamins C and E (Vit.C/E; 10 μM concentrations of each), NAC (200 μM), or pyrrolidinedithiocarbamate (PDTC; 10 μM). Bcl-2 protein levels were determined by Western blotting. (B) HUVEC were incubated with TNF-α (100 ng/ml) in the presence or absence of NAC (200 μM) or vitamins C and E (10 μM) for 18 h, and apoptosis was assessed by morphological analysis of DAPI-stained nuclei (∗, significantly different from TNF-α [P < 0.05]; apoptosis in nontransfected cells was about 1.0%). (C) Effect of ROS on Bcl-2 stability in vivo. HUVEC were incubated with H2O2 (200 μM) for 12 h in the presence or absence of the proteasome inhibitors Z-LLL-H (20 μM) or ALLN (0.5 μg/ml). (D) HUVEC transiently transfected with either a wild-type or a degradation-resistant Bcl-2 construct (Bcl-2mt) were incubated with H2O2 (200 μM) for 12 h. A representative Western blot against myc-tagged Bcl-2 is shown. Each experiment was repeated three times, with identical results.
Having demonstrated that the generation of ROS is involved in TNF-α-stimulated degradation of Bcl-2 and subsequent apoptosis, we next examined the effect of H2O2 on Bcl-2 stability in vivo. Incubation with H2O2 for 6 to 12 h drastically reduced Bcl-2 levels in HUVEC (Fig. 8C) and HeLa cells (not shown). Proteasome inhibitors, such as Z-LLL-H or ALLN, completely inhibited ROS-triggered degradation of Bcl-2 (Fig. 8C), demonstrating the involvement of the ubiquitin-proteasome pathway in this proteolytic process. Furthermore, the stability of a lysine-free, degradation-resistant Bcl-2 construct transiently transfected into HUVEC was not affected by H2O2 (Fig. 8D). Thus, the degradation of Bcl-2 by ROS also requires activation of the ubiquitin-dependent proteasome complex.
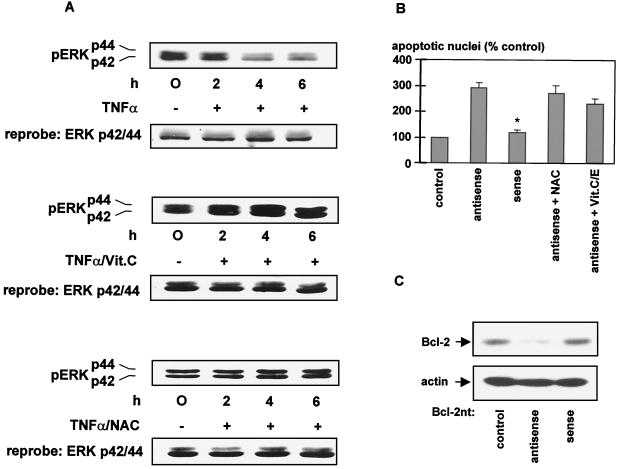
Since previous studies demonstrated that prolonged incubation with TNF-α induced dephosphorylation and deactivation of ERK1/2 (16), we speculated whether ROS might influence the activity of these kinases. We first examined the long-term effect of H2O2 on ERK1/2 activation. Transient activation of ERK1/2 could be observed within the first 30 min of H2O2 stimulation in HUVEC and HeLa cells. However, after incubation of HUVEC with H2O2 for more than 1 h, phosphorylated active ERK was significantly reduced (data not shown). Moreover, TNF-α-induced dephosphorylation of ERK1/2 could be completely prevented by addition of antioxidants, such as vitamin C or NAC (Fig. 9A), indicating an important role of ROS in TNF-α-triggered ERK1/2 deactivation.
FIG. 9.
(A) Antioxidants inhibit TNF-α-triggered ERK1/2 deactivation. HUVEC were incubated with TNF-α (100 ng/ml) in the presence of either 100 μM vitamin C (Vit.C) or 200 μM NAC. Cells were lysed, and Western blot analysis was carried out with anti-phospho-ERK1/2 antibody to determine activated, phosphorylated ERK1/2. Following stripping of the PVDF membrane, equal loading of the samples was demonstrated by Western blot analysis with anti-ERK antibody. Experiments were performed three times, with identical results. (B) Effect of antioxidants on apoptosis induced by antisense oligonucleotides against Bcl-2. HeLa cells were transfected with antisense or sense oligonucleotides directed against Bcl-2 for 18 h, and apoptosis was determined by morphological analysis of DAPI-stained nuclei (∗, significantly different from antisense oligonucleotides [P < 0.05]). Experiments were performed three times, with identical results. (C) Effect of antisense Bcl-2 oligonucleotides on Bcl-2 expression. HUVEC were transfected with either sense or antisense Bcl-2 oligonucleotides (Bcl-2nt) by the Lipofectamine method described in Materials and Methods. Suppression of Bcl-2 after antisense oligonucleotide treatment is shown via Western blot analysis with anti-Bcl-2 antibody. Stripping of the PVDF membrane followed by reprobing with antiactin demonstrates equal loading of the samples.
To further localize the effect of antioxidants within the signal transduction of Bcl-2 degradation, Bcl-2 was down-regulated by specific antisense oligonucleotides (50). Reduced Bcl-2 levels by transfection of HeLa cells with Bcl-2 antisense oligonucleotides were associated with significant apoptosis induction, whereas sense oligonucleotides affected neither Bcl-2 protein levels nor apoptosis (Fig. 9B and C). Importantly, addition of antioxidants was not capable of preventing apoptosis induced by Bcl-2 antisense-oligonucleotide treatment (Fig. 9B). Thus, antioxidants interfere with the signal transduction upstream of Bcl-2 degradation, as apoptosis induced by antisense Bcl-2 down-regulation does not appear to be dependent on ROS generation.
DISCUSSION
Under basal conditions, the antiapoptotic protein Bcl-2 is a long-lived protein. Proapoptotic stimuli, such as TNF-α or H2O2, induce its degradation via the ubiquitin-proteasome pathway both in vivo and in vitro. We have previously demonstrated that the protein appears to be specifically stabilized by phosphorylation processes (16). To further dissect the mechanisms involved in Bcl-2 degradation, the present study aimed to identify the crucial phosphorylation site(s) within Bcl-2 and to determine its (or their) specificity for Bcl-2 stability. The results of this study reveal the following novel findings: destruction of the three putative MAP kinase sites at positions 56, 74, and 87 results in ubiquitination and subsequent degradation of the protein. Progressive inactivation of these MAP kinase sites revealed that Bcl-2 stability is mainly regulated by phosphorylation at Thr74 and Ser87, with Ser87 phosphorylation playing a predominant role. TNF-α or the MAP kinase-specific inhibitor PD98059 diminishes Ser87 phosphorylation of Bcl-2 in vivo, while activated ERK2 induces phosphorylation of Bcl-2 in vivo and in vitro. In addition, phosphorylation of Bcl-2 was demonstrated in 32P-labeled HUVEC transiently transfected with wild-type Bcl-2 (data not shown). In particular, the 60-amino-acid loop domain of Bcl-2 has been suggested to play a significant role for Bcl-2 phosphorylation (9, 20). However, the functional consequences of this phosphorylation event are discussed controversially (9, 20). The present data indicate that signaling through the MAP kinase pathway by phosphorylation of Thr74 and Ser87 located within or in close proximity to the loop domain plays a critical role in the maintenance of Bcl-2 stability and, as a consequence, apoptosis susceptibility. Overexpression of degradation-resistant Bcl-2 proteins such as lysine-free or phospho-mimetic Bcl-2 proteins significantly inhibits TNF-α-induced apoptosis in HUVEC (16) (Fig. 4A), supporting the important physiological role of Bcl-2 protein stability for cell survival. Appropriate subcellular targeting of phospho-mimetic and lysine-free Bcl-2 proteins with inactivated MAP kinase sites was confirmed by immunolocalization experiments (data not shown). These data suggest that mutations of MAP kinase sites within Bcl-2 mainly affect its phosphorylation and, thus, Bcl-2 stability, whereas potential other effects, such as alteration of protein conformation or of subcellular localization, appear to be less important.
It has been proposed that caspases are capable of directly cleaving Bcl-2. This caspase-mediated cleavage was suggested to be, at least in part, responsible for the inability of Bcl-2 to block interleukin-3 withdrawal- or Fas-induced apoptosis in Jurkat or Ba/F3 cells (11). However, Johnson and Boise (35) recently demonstrated that Bcl-2 is incapable of inhibiting TNF-α-induced cell death in the murine pro-B cell line FL5.12 and cleavage-defective Bcl-2 proteins do not block TNF-α-induced apoptosis. In accordance with this report, we have previously shown that Bcl-2 mutants which are resistant to caspase-mediated cleavage (Asp32Ala and Asp34Ala) are avidly degraded by the proteasome complex following TNF-α stimulation (16). Therefore, caspase-mediated Bcl-2 cleavage does not appear to be important for TNF-α-induced apoptosis in endothelial cells.
MAP kinases ERK1/2 are at the center of many signal transduction pathways in eukaryotic cells (for review, see reference 50). These cascades are composed of a trio of sequentially acting protein kinases that transmit an extracellular physiological signal to the targets that orchestrate the appropriate cellular response. ERK1/2 phosphorylate a number of substrates participating in cell cycle regulation, including transcription factors (42). In these cases, ERK1/2 translocate to the nucleus upon activation and activate immediate-early gene transcription. Those periodically occurring, temporal events are essential for the maintenance of normal cell growth. In contrast, involvement of the ERK MAP kinase pathway in Bcl-2 stability appears to be a single, irreversible incident, as Bcl-2 dephosphorylation induces its degradation and then subsequent cell death. This implicates that long-term down-regulation of MAP kinases causes cytosolic signaling events, which lead to irreversible effects. There are other examples for cytosolic substrates of MAP kinases. They can phosphorylate and, thus, activate cytoplasmic phospholipase A2 in vitro (40) or the gap junction protein connexin 43 (59). However, the biological significance of these events has yet to be determined.
Protein phosphorylation is a reversible process involving protein phosphatases as key regulators in cellular activities. MAP kinase members are directly regulated by dual-specific phosphatases. MKP-1, MKP-2, MKP-3, and MKP-4 have been previously implicated in the in vivo inactivation of ERK1/2 (8, 12, 25, 36, 39, 40). As demonstrated in the present study, Bcl-2 degradation was either inhibited by overall suppression of MKP expression or triggered by overexpression of MKP-3 or MKP-4, respectively. Compared with MKP-4, MKP-3 induced a more pronounced Bcl-2 dephosphorylation at Ser87. This correlates with the minor effect of MKP-4 on Bcl-2 protein stability compared with the stronger effect of MKP-3 on Bcl-2 degradation. Therefore, we postulate that MKP-3 is the major phosphatase involved in TNF-α-induced Bcl-2 degradation by dephosphorylation of ERK. This conclusion is also supported by the finding that TNF-α induces MKP-3 expression. Moreover, we have previously shown that overexpression of MKP-3 induces apoptosis in HUVEC (16), whereas overexpression of a dominant negative form of MKP-3 dramatically inhibits TNF-α-induced Bcl-2 degradation and TNF-α-induced apoptosis in HUVEC. It is of note that MKP-1 is strictly localized to the nucleus, whereas MKP-4 can be found in the cytoplasm and in the nucleus. MKP-3 appears exceptional insofar that it is exclusively cytosolic and, in contrast to other dual specificity phosphatases, highly selective towards ERK inactivation (8). In this respect, MKP-3 appears to play a superior role in the TNF-α-induced signaling cascade of Bcl-2 degradation. Such compartmentalized regulation of ERK may be of fundamental importance in molecular processes underlying apoptosis induction.
Alternatively, regulation of Bcl-2 stability might be directly affected by yet-unknown phosphatases, which could dephosphorylate Bcl-2 on Thr74 and Ser87. While we do not know the nature of the protein phosphatase responsible for direct Bcl-2 dephosphorylation, several phosphatases, such as the protein phosphatase 2A (PP2A) or the Ca2+ calmodulin serine/threonine phosphatase PP2B/calcineurin, have been implicated to interact directly with Bcl-2 in murine factor-dependent myeloid cells (15), baby hamster kidney cells, and Jurkat cells and in the SU-DHL-4B-cell lymphoma cell line (56). However, there is no evidence that the binding of either PP2A or PP2B is involved in the regulation of Bcl-2 degradation. An intact Ser70 site within Bcl-2 is required for the reversible association of PP2A with Bcl-2 (15). In our studies, neither an intact nor an inactive Ser70 site influenced Bcl-2 stability and, furthermore, the potent PP2A and PP2B inhibitor okadaic acid did not influence TNF-α-induced Bcl-2 degradation (data not shown). The interaction between PP2B and Bcl-2 apparently facilitates T-cell survival by preventing the nuclear localization of the transcription factor NF-AT (56). Therefore, PP2A and PP2B may have different functional consequences indicating the potential versatility of Bcl-2 in interacting with and regulating other components involved in apoptosis. In addition, these intrinsic differences might be due to the different cell lines used for the studies mentioned above.
The results of the present study further demonstrate that degradation of Bcl-2 by the ubiquitin-dependent proteasome complex is regulated by oxidative stress. Scavenging of ROS by antioxidants prevents TNF-α-induced Bcl-2 degradation in HUVEC, suggesting the involvement of ROS for TNF-α-stimulated Bcl-2 proteolysis. In addition, the treatment of HUVEC with exogenous H2O2 also stimulated Bcl-2 degradation. However, the intracellular target(s) of the ROS is not yet clear. A direct interaction resulting in activation of the ubiquitin-dependent proteasome complex seems rather unlikely, since the proteasome-dependent degradation of IκBα induced by TNF-α (51) was not ameliorated by antioxidants (data not shown). This demonstrates clearly the specificity of the contribution of ROS in TNF-α-stimulated degradation of Bcl-2. Otherwise, several kinases and phosphatases have been shown to be redox regulated (22, 32). This is supported by our findings that the stimulation of HUVEC and HeLa cells with H2O2 leads, after a short-term initial activation, to a time-dependent ERK inactivation after 1 h. Moreover, dephosphorylation and, therefore, inactivation of ERK1/2 was completely inhibited in the presence of various antioxidants, indicating a central role of ROS in TNF-α-triggered ERK1/2 deactivation. Finally, stimulation of apoptosis by antisense oligonucleotides against Bcl-2 was not affected by antioxidants. These data clearly indicate that antioxidants interfere with the apoptosis signal transduction leading to Bcl-2 degradation. However, further studies are required to investigate in detail the ROS-modulated ERK1/2 deactivation.
The involvement of ROS in TNF-α-stimulated cell death has previously been reported (22, 55, 58) and is supported by the present data, which demonstrate that antioxidants abrogate endothelial cell apoptosis induced by TNF-α. The proteolysis of Bcl-2 seems to play an important role in apoptosis signal transduction, since proteasome complex inhibitors or overexpression of degradation-resistant Bcl-2 mutants lacking the ubiquitin-acceptor amino acids prevent TNF-α-triggered cell death (16).
Inhibition of apoptosis correlates with the prevention of the proteasome complex-mediated proteolytic degradation of Bcl-2, which has been shown to be activated by ROS. The degradation of Bcl-2 might directly induce apoptosis or render the endothelial cells more susceptible for proapoptotic stimuli. Therefore, the inhibition of endothelial cell apoptosis and Bcl-2 degradation by antioxidants might importantly contribute to the functional integrity of the endothelial monolayer and thereby inhibit damage to the vessel wall, which is a key event for the initiation of atherosclerotic lesion development.
Taken together, our study suggests an important, regulatory role of Bcl-2 stability for cell survival. In response to death signals, posttranslational modifications like dephosphorylation result in the inactivation of the antiapoptotic function of Bcl-2 by diminishing the Bcl-2 protein level via ubiquitin-proteolytic degradation. In light of the ambivalent reports about the role of Bcl-2 phosphorylation as both an apoptosis promoting and an apoptosis inhibitory process (10, 15, 28, 33, 52, 57) or in cell cycle progression (41, 48, 54), our data demonstrate a defined function of Bcl-2 phosphorylation and dephosphorylation in determining its stability. Activation of the Bcl-2 degradation process might therefore alter the balance between pro- and antiapoptotic proteins within a cell and, moreover, may initiate the apoptotic machinery. Thus, phosphorylation of Bcl-2 at Thr74 and Ser87 might be of substantial importance for the maintenance of cell survival.
ACKNOWLEDGMENTS
This work was supported by a grant for young research scientists from the Faculty of Medicine, University of Frankfurt, and by the Sonderforschungsbereich SFB-553(C2).
We thank Alexandra Bittner, Christine Goebel, and Susanne Ficcus for expert technical assistance.
REFERENCES
- 1.Adams J M, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1325. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Arch R H, Gedrich R W, Thompson C B. Tumor necrosis factor receptor-associated factors (TRAFs)—a family of adapter proteins that regulates life and death. Genes Dev. 1998;12:2821–2830. doi: 10.1101/gad.12.18.2821. [DOI] [PubMed] [Google Scholar]
- 3.Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 4.Baffy G, Miyashita T, Williamson J R, Reed J C. Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hemapoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production. J Biol Chem. 1993;268:6511–6519. [PubMed] [Google Scholar]
- 5.Batt D B, Carmichael G G, Liu Z. An improved rapid method of isolating RNA from cultured cells. Methods Mol Biol. 1998;86:15–17. doi: 10.1385/0-89603-494-1:15. [DOI] [PubMed] [Google Scholar]
- 6.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Breitschopf K, Bengal E, Ziv T, Admon A, Ciechanover A. A novel site for ubiquitination: the N-terminal residue, and not internal lysines of MyoD, is essential for conjugation and degradation of the protein. EMBO J. 1998;20:5964–5973. doi: 10.1093/emboj/17.20.5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camps M, Nichols A, Gillieron C, Antonsson B, Muda M, Chabert C, Boschert U, Arkinstall S. Catalytic activation of the phosphatase MKP-3 by ERK2-activated protein kinase. Science. 1998;280:1262–1265. doi: 10.1126/science.280.5367.1262. [DOI] [PubMed] [Google Scholar]
- 9.Chang B S, Minn A J, Muchmore S W, Fesik S W, Thompson C B. Identification of a novel regulatory domain in Bcl-2-xL and Bcl-2. EMBO J. 1997;16:968–977. doi: 10.1093/emboj/16.5.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C-Y, Faller D V. Phosphorylation of Bcl-2 protein and association with p21Ras in Ras-induced apoptosis. J Biol Chem. 1996;271:2376–2379. doi: 10.1074/jbc.271.5.2376. [DOI] [PubMed] [Google Scholar]
- 11.Cheng E H-Y, Kirsch D G, Clem R J, Ravi R, Kastan M B, Bedi A, Ueno K, Hardwick J M. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 12.Chu Y, Solski P A, Khosravi-Far R, Der C J, Kelly K. The mitogen-activated protein kinase phosphatases PAC1, MKP-1, and MKP-2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation. J Biol Chem. 1996;271:6497–6501. doi: 10.1074/jbc.271.11.6497. [DOI] [PubMed] [Google Scholar]
- 13.Ciechanover A, Schwartz A L. The ubiquitin-proteasome pathway: the complexity and myriad functions of protein death. Proc Natl Acad Sci USA. 1998;95:2727–2730. doi: 10.1073/pnas.95.6.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coux O, Tanaka K, Goldberg A L. Structure and function of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 15.Deng X, Ito T, Carr B, Mumby M, May W S., Jr Reversible phosphorylation of Bcl2 following interleukin 3 or byrostatin 1 is mediated by direct interaction with protein phosphatase 2A. J Biol Chem. 1998;273:34157–34163. doi: 10.1074/jbc.273.51.34157. [DOI] [PubMed] [Google Scholar]
- 16.Dimmeler S, Breitschopf K, Haendeler J, Zeiher A M. Dephosphorylation targets Bcl-2 for ubiquitin-dependent degradation: a link between the apoptosome and the proteasome pathway. J Exp Med. 1999;189:1815–1822. doi: 10.1084/jem.189.11.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher A M. A novel Ca2+-independent pathway for NOS activation in endothelial cells via Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 18.Dimmeler S, Haendeler J, Nehls M, Zeiher A M. Suppression of apoptosis by nitric oxide via inhibition of interleukin-1beta-converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like protease. J Exp Med. 1997;185:601–607. doi: 10.1084/jem.185.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evan G, Littlewood T. A matter of life and death. Science. 1998;281:1317–1321. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- 20.Fang G, Chang B S, Kim C N, Perkins C, Thompson C B, Bhalla K N. “Loop” domain is necessary for taxol-induced mobility shift and phosphorylation of Bcl-2 as well as for inhibiting taxol-induced cytosolic accumulation of cytochrome c and apoptosis. Cancer Res. 1998;58:3202–3208. [PubMed] [Google Scholar]
- 21.Gaiewski T F, Thompson C B. Apoptosis meets signal transduction: elimination of a BAD influence. Cell. 1996;87:589–592. doi: 10.1016/s0092-8674(00)81377-x. [DOI] [PubMed] [Google Scholar]
- 22.Gotoh Y, Cooper J A. Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-α signal transduction. J Biol Chem. 1998;273:17477–17482. doi: 10.1074/jbc.273.28.17477. [DOI] [PubMed] [Google Scholar]
- 23.Green D R. Apoptotic pathways: the roads to ruin. Cell. 1998;94:695–698. doi: 10.1016/s0092-8674(00)81728-6. [DOI] [PubMed] [Google Scholar]
- 24.Green D R, Reed J C. Mitochondria and apoptosis. Science. 1998;281:1309–1316. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 25.Guan K L, Butch E. Isolation and characterization of a novel dual specific phosphatase, HVH2, which selectively dephosphorylates the mitogen-activated protein kinase. J Biol Chem. 1995;270:7197–7203. doi: 10.1074/jbc.270.13.7197. [DOI] [PubMed] [Google Scholar]
- 26.Haendeler J, Messmer U K, Brune B, Neugebauer E, Dimmeler S. Endotoxic shock leads to apoptosis in vivo and reduces Bcl-2. Shock. 1996;6:405–409. doi: 10.1097/00024382-199612000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Haendeler J, Zeiher A M, Dimmeler S. Vitamin C and E prevent lipopolysaccharide-induced apoptosis in human endothelial cells by modulation of Bcl-2 and Bax. Eur J Pharmacol. 1996;317:407–411. doi: 10.1016/s0014-2999(96)00759-5. [DOI] [PubMed] [Google Scholar]
- 28.Haldar S, Jena N, Croce C M. Inactivation of Bcl-2 by phosphorylation. Proc Natl Acad Sci USA. 1995;92:4507–4511. doi: 10.1073/pnas.92.10.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 30.Hochstrasser M. Ubiquitin-dependent protein degradation. Annu Rev Genet. 1996;30:405–439. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]
- 31.Horiuchi M, Hayashida W, Kambe T, Yamada T, Dzau V J. Angiotensin type 2 receptor dephosphorylates Bcl-2 by activating mitogen-activated protein kinase phosphatase-1 and induces apoptosis. J Biol Chem. 1997;272:19022–19026. doi: 10.1074/jbc.272.30.19022. [DOI] [PubMed] [Google Scholar]
- 32.Huot J, Houle F, Marceau F, Landry J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ Res. 1997;80:383–392. doi: 10.1161/01.res.80.3.383. [DOI] [PubMed] [Google Scholar]
- 33.Ito T, Deng X, Carr B, May W S. Bcl-2 phosphorylation required for anti-apoptosis function. J Biol Chem. 1997;272:11671–11673. doi: 10.1074/jbc.272.18.11671. [DOI] [PubMed] [Google Scholar]
- 34.Jacobson M D, Raff M C. Programmed cell death and Bcl-2 protection in very low oxygen. Nature. 1995;374:814–816. doi: 10.1038/374814a0. [DOI] [PubMed] [Google Scholar]
- 35.Johnson B W, Boise L H. Bcl-2 and caspase inhibition cooperate to inhibit tumor necrosis factor-α-induced cell death in a Bcl-2 cleavage-independent fashion. J Biol Chem. 1999;274:18552–18558. doi: 10.1074/jbc.274.26.18552. [DOI] [PubMed] [Google Scholar]
- 36.Krajewski S, Bodrug S, Gascoyne R, Berean K, Krajewska M, Reed J C. Immunohistochemical analysis of Mcl-1 and Bcl-2 proteins in normal and neoplastic lymph nodes. Am J Pathol. 1994;145:515–525. [PMC free article] [PubMed] [Google Scholar]
- 37.Krajewski S, Mai J K, Krajewska M, Sikorska M, Mossakowski M J, Reed J C. Upregulation of bax protein levels in neurons following cerebral ischemia. J Neurosci. 1995;15:6364–6376. doi: 10.1523/JNEUROSCI.15-10-06364.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwak S P, Dixon J E. Multiple dual specificity protein tyrosine phosphatases are expressed and regulated differentially in liver cell lines. J Biol Chem. 1995;270:1156–1160. doi: 10.1074/jbc.270.3.1156. [DOI] [PubMed] [Google Scholar]
- 39.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 40.Lin L L, Wartmann M, Lin A Y, Knopf J L, Seth A, Davis R J. CPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 41.Ling Y-H, Tornos C, Perez-Soler R. Phosphorylation of Bcl-2 is a marker of M phase events and not a determinant of apoptosis. J Biol Chem. 1998;273:18984–18991. doi: 10.1074/jbc.273.30.18984. [DOI] [PubMed] [Google Scholar]
- 42.Marshall C J. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev. 1994;4:82–89. doi: 10.1016/0959-437x(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 43.Muda M, Boschert U, Dickinson R, Martinou J-C, Martinou I, Camps M, Schlegel W, Arkinstall S. MKP-3, a novel cytosolic protein-tyrosine phosphatase that exemplifies a new mitogen-activated protein kinase phosphatase. J Biol Chem. 1996;271:4319–4326. doi: 10.1074/jbc.271.8.4319. [DOI] [PubMed] [Google Scholar]
- 44.Muda M, Boschert U, Smith A, Antonsson B, Gillieron C, Chabert C, Camps M, Martinou I, Ashworth A, Arkinstall S. Molecular cloning and functional characterization of a novel mitogen-activated protein kinase phosphatase, MKP-4. J Biol Chem. 1997;272:5141–5151. doi: 10.1074/jbc.272.8.5141. [DOI] [PubMed] [Google Scholar]
- 45.Musti A M, Treier M, Bohmann D. Reduced ubiquitin-dependent degradation of c-Jun after phosphorylation by MAP kinases. Science. 1997;275:400–402. doi: 10.1126/science.275.5298.400. [DOI] [PubMed] [Google Scholar]
- 46.Oltvai Z N, Milliman C L, Korsmeyer S J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 47.Paradis E, Douillard H, Koutroumanis M, Goodyer C, LeBlanc A. Amyloid beta peptide of Alzheimer's disease downregulates Bcl-2 and upregulates Bax expression in human neurons. J Neurosci. 1996;16:7533–7539. doi: 10.1523/JNEUROSCI.16-23-07533.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poommipanit P B, Chen B, Oltvai Z N. Interleukin-3 induces the phosphorylation of a distinct fraction of Bcl-2. J Biol Chem. 1999;274:1033–1039. doi: 10.1074/jbc.274.2.1033. [DOI] [PubMed] [Google Scholar]
- 49.Reed J C, Stein C, Subasinghe C, Haldar S, Croce C M, Yum S, Cohen J. Antisense-mediated inhibition of Bcl2 protooncogene expression and leukemic cell growth and survival: comparisons of phosphodiester and phosphorothioate oligodeoxynucleotides. Cancer Res. 1990;50:6565–6570. [PubMed] [Google Scholar]
- 50.Robinson M J, Cobb M H. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 51.Roff M, Thompson J, Rodriguez M S, Jacque J M, Baleux F, Arenzana-Seisdedos F, Hay R T. Role of iκBα ubiquitination in signal-induced activation of NFκB in vivo. J Biol Chem. 1996;271:7844–7850. doi: 10.1074/jbc.271.13.7844. [DOI] [PubMed] [Google Scholar]
- 52.Ruvolo P P, Deng X, Carr B K, May W S. A functional role for mitochondrial protein kinase Cα in Bcl2 phosphorylation and suppression of apoptosis. J Biol Chem. 1998;273:25436–25442. doi: 10.1074/jbc.273.39.25436. [DOI] [PubMed] [Google Scholar]
- 53.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 54.Scatena C D, Stewart Z A, Mays D, Tang L J, Keefer C J, Leach S D, Pietenpol J A. Mitotic phosphorylation of Bcl-2 during normal cell cycle progression and taxol-induced growth arrest. J Biol Chem. 1998;273:30777–30784. doi: 10.1074/jbc.273.46.30777. [DOI] [PubMed] [Google Scholar]
- 55.Schulze-Osthoff K, Krammer P H, Dröge W. Divergent signalling via APO-1/Fas and the TNF receptor, two homologous molecules involved in physiological cell death. EMBO J. 1994;13:4587–4596. doi: 10.1002/j.1460-2075.1994.tb06780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shibasaki F, Kondo E, Akagi T, McKeon F. Suppression of signalling through transcription factor NF-AT by interactions between calcineurin and Bcl-2. Nature. 1997;386:728–731. doi: 10.1038/386728a0. [DOI] [PubMed] [Google Scholar]
- 57.Shrivastava R K, Srivastava A R, Korsmeyer S J, Nesterova M, Cho-Chung Y S, Longo D L. Involvement of microtubules in the regulation of Bcl2 phosphorylation and apoptosis through cyclic AMP-dependent protein kinase. Mol Cell Biol. 1998;18:3509–3517. doi: 10.1128/mcb.18.6.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sidon-de Fraisse C, Rincheval V, Risler Y, Mignotte B, Vayssiere J L. TNFα activates at least two apoptotic signaling cascades. Oncogene. 1998;17:1639–1651. doi: 10.1038/sj.onc.1202094. [DOI] [PubMed] [Google Scholar]
- 59.Warn-Cramer B J, Lampe P D, Kurata W E, Kanemitsu M Y, Loo L W, Eckhart W, Lau A F. Characterization of the mitogen-activated protein kinase phosphorylation sites on the connexin-43 gap junction protein. J Biol Chem. 1996;271:3779–3789. doi: 10.1074/jbc.271.7.3779. [DOI] [PubMed] [Google Scholar]
- 60.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T I, Jones D P, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 61.Zou H, Wenzel W J, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]