Abstract
Standard repetitive extragenic palindromic (REP)-PCR, enterobacterial repetitive intergenic consensus-PCR, and Salmonella enteritidis repetitive element-PCR methods for bacterial strain typing were performed with DNA extracted by boiling members of each of the currently recognized species of human viridans group streptococci. Each of the methods was reproducible. The unique isolates (n = 72) from 15 species of viridans group streptococci were readily distinguishable, with no two isolates showing greater than 90% per cent similarity. The majority of strains exhibited much less than 90% similarity. Isolates identical by REP-PCR were also identical by the other two methods. These PCR-based typing methods, although they do not permit determination of the species of the isolates, are simple to perform and are suitable for clinical and ecological investigations of viridans group streptococci.
The viridans group, or oral, streptococci are a heterogeneous group of bacteria primarily isolated from the oral cavity and the gastro- and urogenital tracts (40). These bacteria and, in particular, members of the anginosus group (Streptococcus anginosus, Streptococcus intermedius, and Streptococcus constellatus) and the mitis group (Streptococcus mitis, Streptococcus oralis, Streptococcus sanguinis [Streptococcus sanguis], Streptococcus parasanguinis [Streptococcus parasanguis], Streptococcus gordonii, Streptococcus cristatus [Streptococcus crista], Streptococcus infantis, and Streptococcus peroris) may be associated with extraoral diseases including deep-seated abscesses in the liver and brain, infective endocarditis, and septicemia (2, 4–7, 9, 15, 16, 19, 29, 41, 42), while members of the mutans group (Streptococcus mutans and Streptococcus sobrinus) are associated with dental caries (24). The typing of strains of individual species of viridans group streptococci is not often reported, and consequently, the range of techniques used to type members of these species has been limited. Ribotyping has been used to demonstrate the oral origin of viridans group streptococci isolated from patients with infective endocarditis (29), while restriction fragment length polymorphism analysis, ribotyping, and arbitrary primed PCR (AP-PCR) have been used to study the ecology and person-to-person transmission of S. mutans (20, 21, 23, 32). The genotypic variation of S. mitis biovar 1 has also been studied by ribotyping (10, 13). However, the non-PCR methods are time-consuming and technically demanding, and especially with regard to restriction fragment length polymorphism analyses in particular the resulting DNA fragment patterns can be very difficult to interpret given the large number of bands present in the patterns that are produced. Rudney et al. (30) used pulsed-field gel electrophoresis (PFGE) to examine a small number of strains from a limited number of species of viridans group streptococci and found that PFGE was unable to distinguish between species but also reported that PFGE revealed great diversity between strains. PFGE is also laborious and time-consuming, and the method is also not readily applicable to the examination of large numbers of strains.
PCR-based typing methods have apparently not been used extensively to examine viridans group streptococci. However, such approaches are widely used to type clinically important bacterial species, and the basic equipment and techniques for PCRs are available in most microbiology laboratories. The demonstration of useful PCR methods for the typing of viridans group streptococci would be an important step toward a better understanding of the ecology and epidemiology of these bacteria. We have therefore determined the usefulness of selected PCR-typing methods that have previously been used to type mainly gram-negative bacteria for their ability to amplify DNAs isolated from representatives of each of the currently recognized species of viridans group streptococci. Repetitive extragenic palandromic (REP)-PCR (39), enterobacterial repetitive intergenic consensus (ERIC)-PCR (39), Salmonella enteritidis repetitive element (SERE)-PCR (28), and BOX-PCR (37) methods have been tested; and the ability of each PCR-based typing method to differentiate between independent strains of these species was investigated.
MATERIALS AND METHODS
Bacteria.
The following strains, which represent all of the currently recognized species of viridans group streptococci isolated from humans, were included in this study: S. oralis NCTC 11427T, GPD1, H362, and PC1467; S. mitis NCTC 10712, PP53, K208, NCTC 12261T, and HV51; S. gordonii NCTC 7868, NCTC 7865T, M5, and GPF1; S. sanguinis AC59, P695, KPE2, NP506, SK96, and NCTC 7863T; S. parasanguinis FW 213, SS895, MGH 143, 85-81, SS-897, and ATCC 151912T; S. cristatus CC5A, AK1, and ATCC 19642T; S. constellatus AM699, NCTC 10714, NCTC 11063, NCDO 2226T, and NCTC 5389; S. intermedius NMH 2, UNS 35, 415-87, and NCDO 2227T; S. anginosus NCTC 10713T, NMH 10, PC4890, NCTC 11062, KR 687, and NCTC 8037; S. mutans SE11, 161, KPSK2, B48, 4177, and NCTC 10449T; S. sobrinus ATCC 33478T, OMZ 65, 279, TH62, and B13; S. vestibularis LV71, NCTC 12166T, JW3, and OP81; S. salivarius A385, NCTC 8606, H50, KPS1, T267, and NCTC 8618T; S. peroris JCM 10158T, 105, and 091; and S. infantis JCM 10157T, 0103, 092, 0101, and 0134. NCTC, NCDO, ATCC, and JCM are abbreviations for National Type Culture Collection, National Collection of Dairy Organisms, American Type Culture Collection, and Japanese Collection of Microorganisms, respectively. Strains marked with a superscript T are type strains. In this collection of strains we have included the two most recently described species, S. peroris and S. infantis (17), and in this communication have used the new nomenclature for certain species within the mitis group (35). Each of these strains is apparently epidemiologically unlinked, being isolated from different individuals. These bacteria have been investigated extensively in previous studies, and the identity of each has been established by DNA-DNA homology or by comparison of each with the reported biochemical reactions of these species (3, 17). The bacteria were stored frozen at −80°C in brain heart infusion (Oxoid) supplemented with glycerol (30% [vol/vol]), routinely subcultured on Columbia agar (Oxoid Ltd.) supplemented with 5% (vol/vol) horse blood, and incubated anaerobically for 24 to 48 h before the colonies were harvested for DNA extraction.
Extraction of DNA.
A 5-μl bacteriological loopful of cells was removed from the surface of the agar plates, and the bacteria were evenly suspended, by vigorous vortexing for 20 s, in 300 μl of sterile water (MilliQ) in 1.5-ml microcentrifuge tubes. The DNA was extracted by cell lysis, which was achieved by immersing the tubes in boiling water for 10 min. The cell debris was pelleted by centrifugation (Microfuge; MSE; 13,000 rpm for 10 s), and the supernatant containing the DNA was carefully removed from the underlying cell debris and transferred to a fresh microcentrifuge tube. Bisbenzimide (Hoescht 33258; Sigma) was used to estimate the concentration of DNA in these extracts, and examination of various extracts from the same and different strains indicated that the DNA concentration was approximately 50 to 60 ng/μl. The DNA extracts were stored at 4°C for no longer than 14 days, until they were used in the PCR assays.
REP-PCR.
The primers REP1R-Dt (5′-III NCG NCG NCA TCN GCC-3′) and REP2-Dt (5′-NCG NCT TAT CNG GCC TAC-3′) used in this study were previously described by Versalovic et al. (39). The reaction mixture contained each of the four deoxynucleoside triphosphates (dNTPs; Advanced Biotechnologies, Surrey, England) at a concentration of 125 μM, 150 ng of each primer, 3.75 mM MgCl2, 2.5 U of Taq polymerase (Advanced Biotechnologies), 20 mM (NH4)2SO4, 75 mM Tris-HCl (pH 9.0), 0.01% (wt/vol) Tween 80, and 12 μl of DNA solution made up to a final volume of 25 μl with sterile water. PCR amplification was performed in an automated thermocycler (Techne Thermocycler) with an initial denaturation (7 min at 95°C), followed by 32 cycles of denaturation (30 s at 94°C), annealing (1 min at 40°C), and extension (8 min at 65°C), with a final extension (16 min at 65°C).
ERIC-PCR.
ERIC-PCR primer pairs ERIC 1R (5′-ATG TAA GCT CCT GGG GAT TCA C-3′) and ERIC 2 (5′-AAG TAA GTG ACT GGG GTG AGC G-3′) were as described by Versalovic et al. (39). The ERIC-PCR mixture consisted of 50 mM KCl, 10 mM Tris-HCl (pH 9.0), 0.1% (vol/vol) Triton X-100, 1.8 mM MgCl2, each of the four dNTPs at a concentration of 125 μM, 100 pmol of each primer, 2.5 U of Taq polymerase, and 4 μl of DNA solution made up to a final volume of 20 μl with sterile water. ERIC-PCR amplification conditions were an initial denaturation cycle (5 min at 95°C), 35 cycles each consisting of denaturation (45 s at 92°C), annealing (1 min at 52°C), and extension (10 min at 70°C), with a final extension (20 min at 70°C).
SERE-PCR.
The SERE-PCR primer (5′-GTG AGT ATA TTA GCA TCC GCA-3′) used for amplification was as described by Rajashekara et al. (28). The reaction mixture contained 5 mM MgCl2, 10 mM (NH4)2SO4, 37.5 mM Tris-HCl (pH 9.0), 0.005% (wt/vol) Tween 80, each of the dNTPs at a concentration of 125 μM, 2.5 U of Taq polymerase, 50 pmol of primer, and 12 μl of DNA solution made up to a final volume of 30 μl with sterile water. The amplification of DNA was initiated by denaturation (95°C for 5 min), followed by 35 cycles each consisting of denaturation (1 min at 94°C), annealing (1 min at 50°C), and extension (2 min at 72°C), with a final extension (15 min at 72°C).
BOX repeat PCR.
The BOXA primer (5′-ATA CTC TTC GAA AAT CTC TTC AAA C-3′) described by van Belkum et al. (37) was used; and the reaction mixture contained each of the four dNTPs at a concentration of 125 μM, 50 pmol of primer, 3.75 mM MgCl2, 2.5 U of Taq polymerase, 20 mM (NH4)2SO4, 75 mM Tris-HCl (pH 9.0), 0.01% (wt/vol) Tween 80, and 12 μl of DNA solution made up to a final volume of 25 μl with sterile water. Amplification began with an initial denaturation (4 min at 94°C), followed by 40 cycles consisting of denaturation (1 min at 94°C), annealing (2 min at 45°C), and extension (2 min at 74°C), with a final extension (5 min at 74°C).
Reproducibilities of PCR methods.
To test the reproducibilities of the PCR amplifications, DNA was extracted from four independent strains of S. oralis on three separate occasions, and the PCR methods were performed with each DNA extract in duplicate.
Comparison of REP-, SERE-, and ERIC-PCRs.
To determine the interrelationship between the different PCR-based typing methods, 10 S. oralis strains were used. These strains were selected such that two strains with identical REP-PCR patterns were selected from each of 5 different individuals. Each of these strains was then examined by both ERIC-PCR and SERE-PCR.
Visualization of amplicons.
The amplification products of REP-PCR and ERIC-PCR (15 μl) were analyzed with 2% Metaphor agarose (Flowgen, Staffordshire, England) containing 0.5 μg of ethidium bromide per ml and were separated electrophoretically on gels (20 by 25 cm) at 140 V for 3 h in TBE (Tris-borate-EDTA) buffer. SERE-PCR products (15 μl) were resolved on 0.8% agarose (Sigma) containing 0.5 μg of ethidium bromide per ml by electrophoretic separation at 140 V for 3 h. To all samples 3 μl of tracking dye (0.25% bromophenol blue, 0.25% xylene cyanol FF, 30% glycerol) was added, and a molecular size marker (pGEM DNA Markers; Promega) was included on all gels, in three separate lanes, to facilitate comparison of tracks between gels. BOX PCR products were resolved on 1.5% agarose gels run at 140 V for 3 h in 0.5× TBE buffer. The gels were examined on a transilluminator and were photographed with Polaroid type 665 positive/negative film (Sigma).
Computer-assisted analysis of DNA patterns.
All of the DNA patterns were analyzed with the Windows version of GelCompar (version 4.0; Applied Maths, Kortrijk, Belgium). The individual bands in each of the patterns produced by the different PCR methods were analyzed by applying the Dice coefficient to the peaks. For clustering, the unweighted pair group method with arithmetic means (UPGMA) was used, and a band position tolerance of 1.5% was used for comparison of the DNA patterns. The analysis of the patterns was undertaken in accordance with the instructions of the manufacturer.
RESULTS AND DISCUSSION
Three different PCR methods, REP-PCR, ERIC-PCR, and SERE-PCR, gave complex PCR products with each of the strains, representing all 15 currently recognized species of viridans group streptococci. The three PCR-based typing methods were each found to be highly reproducible, in that the patterns produced in replicate PCRs of each of four independent isolates were indistinguishable (>98% similarity; data not shown).
None of the S. oralis strains examined with the BOXA primer yielded any PCR products, so these primers were not tested further with any of the other species. The BOXA primer is based on the sequence of a repetitive element identified in Streptococcus pneumoniae (37). It was expected that at least S. oralis might produce amplicons with this primer since these species are phylogenetically closely related, and it follows that less closely related species would be even less likely to produce amplicons. The present findings agree with those of van Belkum et al. (37), who reported that only a small number of strains of S. mutans were amplified with the BOX primer, while none of any other species of streptococci produced amplicons. It is interesting, however, that Azoarcus spp., Xanthomonas spp., and Pseudomonas spp., all gram-negative bacteria, produced amplicons with the BOXA primer and permitted differentiation between isolates (14, 25).
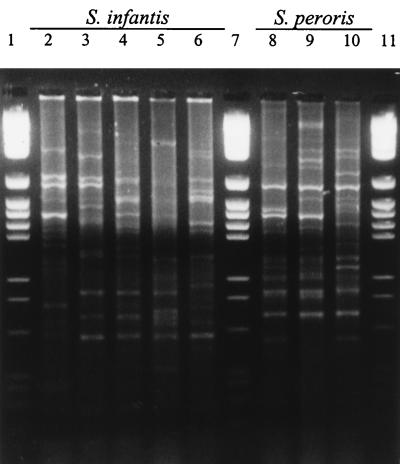
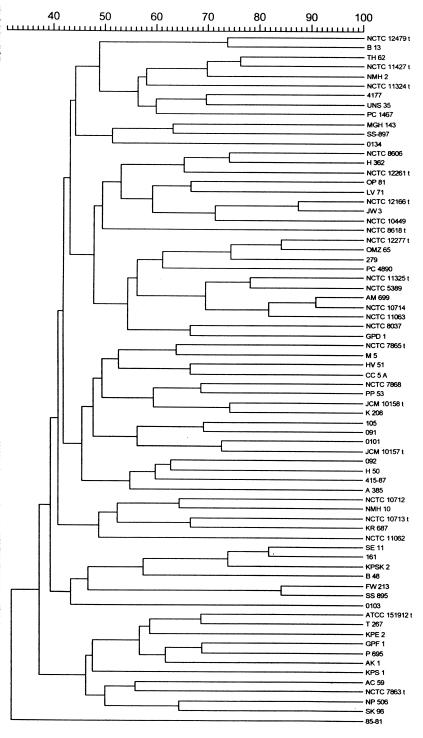
Representative reaction products for the REP-PCRs are shown in Fig. 1. The sizes of the PCR products for the strains varied widely, and many discrete amplicons were produced; the number of amplicons was dependent upon the strain examined. The REP-PCR data were subjected to cluster analysis, and the dendrogram is shown in Fig. 2. From this it can be seen that all strains were distinguishable from each other and that no two strains exhibited >90% similarity. Although the REP primers were designed to amplify sequences originally identified in gram-negative bacteria, it was apparent from the first application of these primers that they also amplified sequences in gram-positive bacteria, including streptococci. REP-PCR primers have been used successfully to type and differentiate among many gram-negative bacterial genera and species, sometimes in combination with other PCR-based typing methods (1, 18). There have also been several reports that REP-PCR may be useful for the typing of gram-positive genera including S. pneumoniae (26, 38), vancomycin-resistant Enterococcus faecium (8), and methicillin-resistant Staphylococcus aureus (22). These primers have not previously been reported to have been used to type oral streptococci, and the apparent ease with which amplicons have been obtained indicates that the REP sequences may be distributed widely among these species, as they are among gram-negative enteric bacteria (43).
FIG. 1.
Representative REP-PCR patterns of viridans group streptococci. Lanes 2 to 6, S. infantis 0103, 092, 0101, JCM 10157T, and 0134, respectively; lanes 8 to 10, S. peroris 105, 091, and JCM 10158T, respectively; lanes 1, 7, and 11, molecular size markers.
FIG. 2.
Dendrogam showing relatedness between REP-PCR band patterns of viridans group streptococci. Bands were analyzed by applying the Dice coefficient, and the matrix was clustered by the UPGMA method.
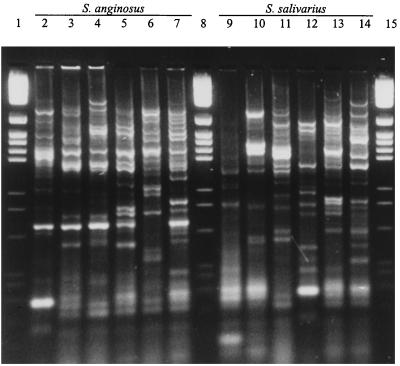
Each of the viridans group streptococci produced a distinct pattern in the ERIC-PCRs; examples of representative patterns are shown in Fig. 3. The ERIC-PCR patterns contained multiple bands over a wide range of sizes, and when these were subjected to cluster analysis it was found that no two strains were >88% similar (data not shown). These ERIC primers have been used extensively in molecular typing studies of a wide range of gram-negative bacteria, and recently, they have been used to study clonal diversity among Mycobacterium tuberculosis strains and were found to be more discriminatory than other established molecular typing schemes (34). They do not appear to have been used extensively in the study of streptococci, although Versalovic et al. (39) reported on the amplification of DNA extracted from a limited number of gram-positive bacteria.
FIG. 3.
Representative ERIC-PCR patterns of viridans group streptococci. Lanes 2 to 7, S. anginosus NCTC 10713T, NMH 10, PC4890, NCTC 11062, KR 687, and NCTC 8037, respectively; lanes 9 to 14, S. salivarius A385, NCTC 8606, H50, KPS1, T267, and NCTC 8618T, respectively; lanes 1, 8, and 15, molecular size markers.
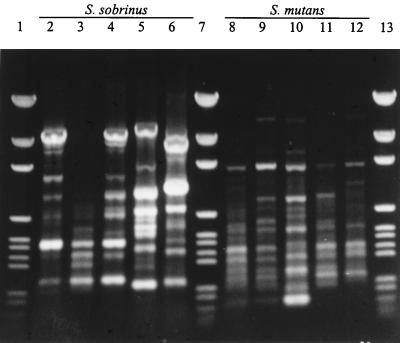
By the SERE-PCRs each strain was again found to be distinct, and representative patterns are shown in Fig. 4. Examination of the banding patterns indicated that in excess of 15 discrete bands were often produced per strain, and cluster analysis of the data showed that each strain was distinct, with no two strains exhibiting greater than 90% similarity (data not shown). The SERE-PCR typing method has not previously been reported for streptococci, apparently having been used previously to type only S. enteritidis strains (28). The results indicate that the SERE sequences may be widely dispersed among streptococci.
FIG. 4.
Representative SERE-PCR patterns of viridans group streptococci. Lanes 2 to 6, S. sobrinus ATCC 33478T, OMZ 65, 279, TH62, and B13, respectively; lanes 8 to 12, S. mutans SE11, 161, KPSK2, B48, and NCTC 10449T, respectively; lanes 1, 7, and 13, molecular size markers.
The ease with which amplicons could be generated in these PCRs suggests that the repetitive sequences are present in viridans group streptococci. However, Sander et al. (33) have indicated that these PCRs may simply be instances in which the primers act as random or arbitrary primers as in randomly amplified polymorphic DNA (RAPD) analysis (or AP-PCR assays). In our experience, with streptococcal DNA prepared as described here, RAPD analyses-PCRs are usually unsuccessful and certainly irreproducible with random-sequence octanucleotides (data not shown). Increasing the length of the primer in RAPD analysis-PCR assays decreases the discriminatory power of the assay, yet here the primers were considerably longer than octanucleotides yet were extremely discriminatory (36). However, Gillings and Holley (12) have reported that ERIC-PCR produced complex patterns in various bacteria, bacteriophages, invertebrates, fungi, plants, and vertebrates at low stringency (52°C), while in reactions performed at high stringency (66°C) most bands failed to be amplified. Those bands that were amplified, it was claimed, were those from the enterobacterial target sequences, and they concluded that ERIC-PCR does not necessarily amplify bands directly from genuine ERIC sequences. It is therefore clear that although the patterns were reproducible and were able to discriminate between strains, the real basis of this discrimination, and consequently, the sequences that acted as targets for the primer or primer pairs, cannot be stated with certainty.
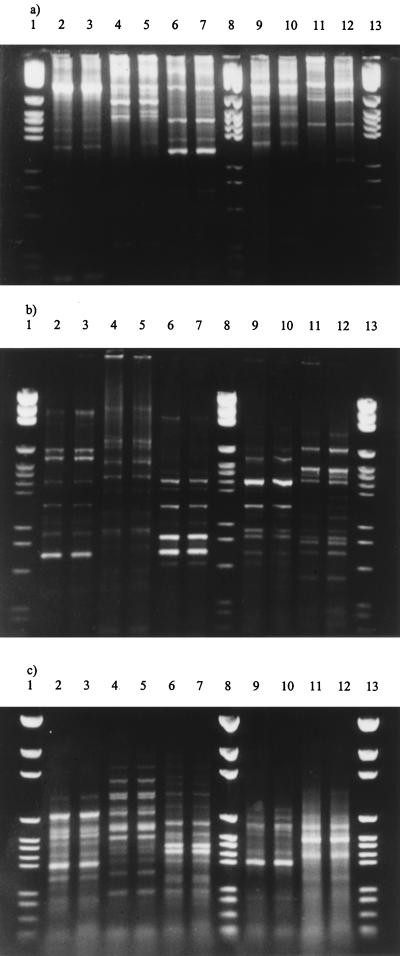
A comparison of the three PCR typing methods was examined by performing each PCR with 5 pairs of independent S. oralis strains. It was found that the patterns produced with each pair of strains were identical by each PCR method (Fig. 5). Thus, strains which were identical by one method were identical by both of the other methods. However, the pattern for each pair of strains was different by each typing method.
FIG. 5.
Comparison of patterns produced by REP-PCR (a), ERIC-PCR (b), and (c) SERE-PCR with duplicate strains of S. oralis isolated from five different individual strains. Isolates from the same individual are in adjacent lanes (from left to right), and in each gel the same isolate occupies the same lane. Lanes 1, 8, and 13, molecular size markers.
Other PCR-based methods may be useful in identifying isolates to the species level. Garnier et al. (11) described PCR primers based on internal fragments of genes encoding for d-alanine–d-alanine ligases for the identification of clinically relevant viridans group streptococcal species, while an identification protocol based upon amplification and sequencing of fragments of the superoxide dismutase gene for the identification of many species of viridans group streptococci has been described (27). Recently, Rudney and Larson (31) have developed AP-PCR protocols which may be useful in identifying members of the mitis group of viridans group streptococci. However, examination of the distribution of the 15 species of viridans group streptococci within each of the dendrograms indicates that none of the three PCR-based typing methods examined in the study described in this report permitted the identification of viridans group streptococci to the species level (data not shown).
In conclusion, the ERIC-, SERE-, and REP-PCR approaches produced amplicons with each of these independent strains of viridans group streptococci, and the pattern of the bands was unique for each strain. Furthermore, there was no evidence that the band patterns, or even an individual band, could be used for the identification of these streptococcal strains to the species level. These techniques are robust and reproducible, can be performed with DNA that has undergone minimal preparation, and makes use of routine laboratory equipment. These methods would seem to be suitable for studying the epidemiology and relatedness of individual isolates of viridans group streptococci.
REFERENCES
- 1.Appuhamy S, Parton R, Coote J G, Gibbs H A. Genomic fingerprinting of Haemophilus somnus by a combination of PCR methods. J Clin Microbiol. 1997;35:288–291. doi: 10.1128/jcm.35.1.288-291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beighton D, Carr A D, Oppenheim B A. Identification of viridans streptococci associated with bacteraemia in neutropenic cancer patients. J Med Microbiol. 1994;40:202–204. doi: 10.1099/00222615-40-3-202. [DOI] [PubMed] [Google Scholar]
- 3.Beighton D, Hardie J M, Whiley R A. A scheme for the identification of viridans streptococci. J Med Microbiol. 1991;35:367–372. doi: 10.1099/00222615-35-6-367. [DOI] [PubMed] [Google Scholar]
- 4.Bochud P Y, Calandra T, Francioli P. Bacteremia due to viridans streptococci in neutropenic patients: a review. Am J Med. 1994;97:256–264. doi: 10.1016/0002-9343(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 5.Bouvet A, Durand A, Devine C, Etienne J, Leport C . the Groupe d’Enquêsue l’Endocardite en France 1990–1991. In vitro susceptibility to antibiotics of 200 strains of streptococci and enterococci isolated during infective endocarditis. In: Totalian A, editor. Pathogenic streptococci: present and future. St. Petersburg, Russia: Lancer Publications; 1994. pp. 72–73. [Google Scholar]
- 6.Carratala J, Alcaide F, Fernandez-Sevilla A, Corbella X, Linares J, Gudiol F. Bacteremia due to viridans streptococci that are highly resistant to penicillin: increase among neutropenic patients with cancer. Clin Infect Dis. 1995;20:1169–1173. doi: 10.1093/clinids/20.5.1169. [DOI] [PubMed] [Google Scholar]
- 7.Douglas C W I, Heath J, Hampton K K, Preston F E. Identity of viridans streptococci isolated from cases of infective endocarditis. J Med Microbiol. 1993;39:179–182. doi: 10.1099/00222615-39-3-179. [DOI] [PubMed] [Google Scholar]
- 8.Dunne M W, Jr, Wang W. Clonal dissemination and colony morphotype variation of vancomycin-resistant Enterococcus faecium isolates in metropolitan Detroit, Michigan. J Clin Microbiol. 1997;35:388–392. doi: 10.1128/jcm.35.2.388-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elting L S, Bodey G P, Keefe B H. Septicemia and shock syndrome due to viridans streptococci: a case-control study of predisposing factors. Clin Infect Dis. 1992;14:1201–1207. doi: 10.1093/clinids/14.6.1201. [DOI] [PubMed] [Google Scholar]
- 10.Fitzsimmons S, Evans M, Pearce C, Sheridan M J, Wientzen R, Bowden G, Cole M F. Clonal diversity of Streptococcus mitis biovar 1 isolates from the oral cavity of human neonates. Clin Diagn Lab Immunol. 1996;3:517–522. doi: 10.1128/cdli.3.5.517-522.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garnier F, Gerbaud G, Courvalin P, Galimand M. Identification of clinically relevant viridans group streptococci to the species level by PCR. J Clin Microbiol. 1997;35:2337–2341. doi: 10.1128/jcm.35.9.2337-2341.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillings M, Holley M. Repetitive element PCR fingerprinting (rep-PCR) using enterobacterial repetitive intergenic consensus (ERIC) primers is not necessarily directed at ERIC elements. Lett Appl Microbiol. 1997;25:17–21. doi: 10.1046/j.1472-765x.1997.00162.x. [DOI] [PubMed] [Google Scholar]
- 13.Hohwy J, Kilian M. Clonal diversity of the Streptococcus mitis biovar 1 population in the human oral cavity and pharynx. Oral Microbiol Immunol. 1995;10:19–25. doi: 10.1111/j.1399-302x.1995.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 14.Hurek T, Wagner B, Reinhold-Hurek B. Identification of N2-fixing plant- and fungus-associated Azoarcus species by PCR-based genomic fingerprints. Appl Environ Microbiol. 1997;63:4331–4339. doi: 10.1128/aem.63.11.4331-4339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs J A, Pietersen H G, Stobberingh E E, Soeters P B. Streptococcus anginosus, Streptococcus constellatus and Streptococcus intermedius. Clinical relevance, hemolytic and serological characteristics. Clin Microbiol Infect Dis. 1995;104:547–553. doi: 10.1093/ajcp/104.5.547. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs J A, Schouten H C, Stobberingh E E, Soeters P B. Viridans streptococci isolated from the bloodstream. Relevance of species identification. Diagn Microbiol Infect Dis. 1995;22:267–273. doi: 10.1016/0732-8893(95)00137-y. [DOI] [PubMed] [Google Scholar]
- 17.Kawamura Y, Hou X-G, Todome Y, Sultana F, Hirose K, Shu S-E, Ezaki T, Ohkuni H. Streptococcus peroris sp. nov. and Streptococcus infantis sp. nov.: new members of the Streptococcus mitis group, isolated from human clinical specimens. Int J Syst Bacteriol. 1998;48:921–927. doi: 10.1099/00207713-48-3-921. [DOI] [PubMed] [Google Scholar]
- 18.Kerouanton A, Brisabois A, Grout J, Picard B. Molecular epidemiological tools for Salmonella dublin typing. FEMS Immunol Med Microbiol. 1996;14:25–29. doi: 10.1111/j.1574-695X.1996.tb00263.x. [DOI] [PubMed] [Google Scholar]
- 19.Kitada K, Nagata K, Yakushiji T, Eifuku H, Inoue M. Serological and biological characteristics of ’Streptococcus milleri’ isolates from systemic purulent infections. J Med Microbiol. 1992;36:143–148. doi: 10.1099/00222615-36-3-143. [DOI] [PubMed] [Google Scholar]
- 20.Kozai K, Wang D S, Sandham H J, Phillips H I. Changes in strains of mutans streptococci induced by treatment with chlorhexidine varnish. J Dent Res. 1991;70:1252–1257. doi: 10.1177/00220345910700090401. [DOI] [PubMed] [Google Scholar]
- 21.Kreulen C M, de-Soet H J, Hogeveen R, Veerkamp J S. Streptococcus mutans in children using nursing bottles. J Dent Child. 1997;64:107–111. [PubMed] [Google Scholar]
- 22.Lessing M P, Jordens J Z, Bowler I C. Molecular epidemiology of a multiple strain outbreak of methicillin-resistant Staphylococcus aureus amongst patients and staff. J Hosp Infect. 1995;31:253–260. doi: 10.1016/0195-6701(95)90204-x. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Caufield P W. The fidelity of initial acquisition of mutans streptococci by infants from their mothers. J Dent Res. 1995;74:681–685. doi: 10.1177/00220345950740020901. [DOI] [PubMed] [Google Scholar]
- 24.Loesche W J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louws F J, Fulbright D W, Stephens C T, de Bruijn F J. Specific genomic fingerprints of phytopathogenic Xanthomonas and Pseudomonas pathovars and strains generated with repetitive sequences and PCR. Appl Environ Microbiol. 1994;60:2286–2295. doi: 10.1128/aem.60.7.2286-2295.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGee L, Klugman K P, Friedland D, Lee H J. Spread of Spanish multi-resistant serotype 23F clone of Streptococcus pneumoniae to Seoul, Korea. Microb Drug Resist. 1997;3:253–257. doi: 10.1089/mdr.1997.3.253. [DOI] [PubMed] [Google Scholar]
- 27.Poyart C, Quesne G, Coulon S, Berche P, Trieu-cuot P. Identification of streptococci to species level by sequencing the gene encoding the manganese-dependent superoxide dismutase. J Clin Microbiol. 1998;36:41–47. doi: 10.1128/jcm.36.1.41-47.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajashekara G, Koeuth T, Nevile S, Back A, Nagaraja K V, Lupski J R, Kapur V. SERE, a widely dispersed bacterial repetitive DNA element. J Med Microbiol. 1998;47:489–498. doi: 10.1099/00222615-47-6-489. [DOI] [PubMed] [Google Scholar]
- 29.Richard P, Amador Del Valle G, Moreau P, Milpied N, Felice M P, Daeschler T, Harousseau J L, Richet H. Viridans streptococcal bacteraemia in patients with neutropenia. Lancet. 1995;345:1607–1609. doi: 10.1016/s0140-6736(95)90117-5. [DOI] [PubMed] [Google Scholar]
- 30.Rudney J D, Neuvar E K, Soberay A H. Endonuclease-fragment polymorphisms of oral viridans streptococci, compared by conventional and field-inversion gel electrophoresis. J Dent Res. 1992;71:1182–1188. doi: 10.1177/00220345920710051001. [DOI] [PubMed] [Google Scholar]
- 31.Rudney J D, Larson C J. Identification of oral mitis group streptococci by arbitrary primed polymerase chain reaction. Oral Microbiol Immunol. 1999;14:33–42. doi: 10.1034/j.1399-302x.1999.140104.x. [DOI] [PubMed] [Google Scholar]
- 32.Saarela M, Hannula J, Matto J, Asikainen S, Alaluusua S. Typing of mutans streptococci by arbitrarily primed polymerase chain reaction. Arch Oral Biol. 1996;41:821–826. doi: 10.1016/s0003-9969(96)00049-0. [DOI] [PubMed] [Google Scholar]
- 33.Sander A, Ruess M, Bereswill S, Schuppler M, Steinbrueckner B. Comparison of different DNA fingerprint techniques for molecular typing of Bartonella hensalae isolates. J Clin Microbiol. 1998;36:2973–2981. doi: 10.1128/jcm.36.10.2973-2981.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sechi L A, Zanetti S, Dupre I, Delogu G, Fadda G. Enterobacterial repetitive intergenic consensus sequences as molecular targets for typing of Mycobacterium tuberculosis strains. J Clin Microbiol. 1998;36:128–132. doi: 10.1128/jcm.36.1.128-132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trüper H G, De Clari L. Taxonomic note: necessary correction of specific epithets formed as substantives (nouns) “in apposition.”. Int J Syst Bacteriol. 1997;47:908–909. [Google Scholar]
- 36.van Belkum A. DNA fingerprinting of medically important microorganisms by use of PCR. Clin Microbiol Rev. 1994;7:174–184. doi: 10.1128/cmr.7.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Belkum A, Sluijuter M, de Groot R, Verbrugh H, Hermans P W. Novel BOX repeat PCR assay for high-resolution typing of Streptococcus pneumoniae strains. J Clin Microbiol. 1996;34:1176–1179. doi: 10.1128/jcm.34.5.1176-1179.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Versalovic J, Kapur V, Mason E O, Jr, Shah U, Koeuth T, Lupski J R, Musser J M. Penicillin-resistant Streptococcus pneumoniae strains recovered in Houston: identification and molecular characterization of multiple clones. J Infect Dis. 1993;167:850–856. doi: 10.1093/infdis/167.4.850. [DOI] [PubMed] [Google Scholar]
- 39.Versalovic J, Koeuth T, Lupski J R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whiley R A, Beighton D. Current classification of the oral streptococci. Oral Microbiol Immunol. 1998;13:195–216. doi: 10.1111/j.1399-302x.1998.tb00698.x. [DOI] [PubMed] [Google Scholar]
- 41.Whiley R A, Beighton D, Winstanely T G, Fraser H Y, Hardie J M. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (the Streptococcus milleri group): association with different body sites and clinical infections. J Clin Microbiol. 1992;30:243–244. doi: 10.1128/jcm.30.1.243-244.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whiley R A, Fraser H, Hardie J M, Beighton D. Phenotypic differentiation of Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus strains within the “Streptococcus milleri group.”. J Clin Microbiol. 1990;28:1497–1501. doi: 10.1128/jcm.28.7.1497-1501.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woods C R, Versalovic J, Koeuth T, Lupski J R. Whole-cell repetitive element sequence-based polymerase chain reaction allows rapid assessment of clonal relationships of bacterial isolates. J Clin Microbiol. 1993;31:1927–1931. doi: 10.1128/jcm.31.7.1927-1931.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]