Abstract
Viral infections are among the most complex medical problems and have been a major threat to the economy and global health. Several epidemics and pandemics have occurred due to viruses, which has led to a significant increase in mortality and morbidity rates. Natural products have always been an inspiration and source for new drug development because of their various uses. Among all-natural sources, plant sources are the most dominant for the discovery of new therapeutic agents due to their chemical and structural diversity. Despite the traditional use and potential source for drug development, natural products have gained little attention from large pharmaceutical industries. Several plant extracts and isolated compounds have been extensively studied and explored for antiviral properties against different strains of viruses. In this review, we have compiled antiviral plant extracts and natural products isolated from plants reported since 2015.
Keywords: natural products, extracts, mechanism of action, antiviral activity, drug development
1. Introduction
Viruses are small infectious particles ranging from 20 to 300 nm in size and containing nucleic acids, proteins, and lipids [1]. The viruses are simple in their structure, but their interactions with the host are very complex. Viruses have always been a major threat to the economy and global health because of their epidemics and pandemics nature, and they are prone to mutation and resistance to therapy as well [2,3]. There are several examples of viruses that are known to have caused either an epidemic or pandemic in the last twenty years. These include avian influenza A (H5N1) in 1997, paramyxovirus (Nipah virus) in 1999, coronavirus (CoV), known as SARS-CoV in 2002, swine H1N1 influenza A virus in 2009, Middle East Respiratory Syndrome virus (MERS-CoV) in 2012, Ebola outbreak in 2014, and COVID-19, known as SARS-CoV-2, which is seen today (declared as a pandemic by WHO on March 2020). Millions of people have died because of these viruses [4]. As of September 2021, COVID-19 has affected more than 219 million people with 4.55 million deaths worldwide, and the number continues to rise [5]. Currently, there are few preferred antiviral drugs available such as Acyclovir used to treat herpes simplex virus or amantadine used to treat influenza type A, to name a few; unfortunately, of these, none are effective against all types of viruses [6]. Therefore, it is imperative to discover new antiviral drugs. Several FDA-approved drugs (oseltamivir, ritonavir, remdesivir, ribavirin, favipiravir, chloroquine, hydroxychloroquine) are currently being considered for the treatment of COVID-19 (the so-called “drug repurposing” approach) to expedite the process of drug development as well as reduce time and cost [7,8]. Researchers are considering currently available resources (from the synthetic and natural world) for the development of new drugs by molecular modifications of known antiviral scaffolds.
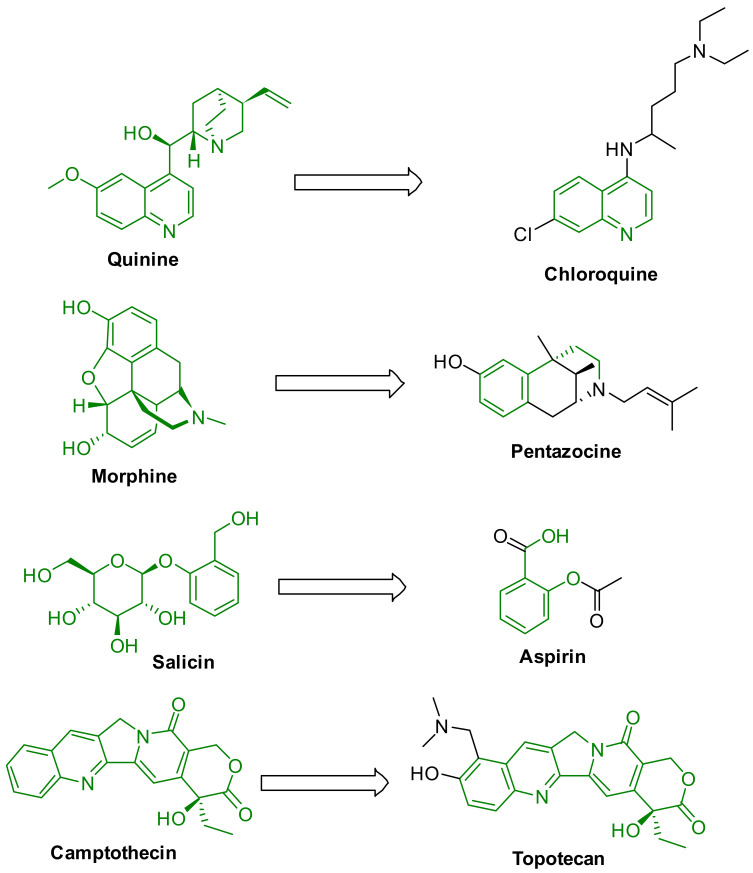
Exploring natural products could be an effective strategy to develop new potent antiviral drugs. The world has a long history of using natural products for medical purposes. Among all-natural sources, plant sources are the most dominant for the discovery of new therapeutic agents because of their chemical and structural diversity. Many natural products were identified as potential drugs such as morphine, quinine, paclitaxel, penicillin, digitoxin, lovastatin, berberine, and doxorubicin. In addition, nature in one guise or another has continued to influence the design of small drug-like molecules. Many natural products are used as scaffolds for developing new synthetic drugs such as chloroquine, atorvastatin, captopril, aspirin, and pentazocine (Figure 1) [9]. However, less than 15% of the natural sources have been explored so far, leaving many opportunities in natural product chemistry research. Many review articles have been published on natural products with their diverse uses including antiviral properties [6,10,11,12,13,14,15,16], although few have covered the breadth needed, and an update in the development of drug discovery from the natural sources would provide researchers an effective beginning toward such efforts. We believe that plant-based natural products could play a vital role in developing potential antiviral drug candidates. Recently, several review articles reported on the antiviral properties of plant extracts and isolated compounds [12,17,18]. The previous review articles were focused either on a class of phytochemicals, plant extracts against specific viral strains, or targets [17,19,20,21]. This review aims to provide an update of plant extracts and isolated compounds (secondary metabolites) with structures that show antiviral properties (we have included the EC50 or IC50 values) since 2015. In addition, we have determined the drug-like properties of the most active isolated antiviral compounds to understand the possible durability as medicinal agents. We believe this review will help the researcher in the design and development of potential antiviral drug candidates.
Figure 1.
Natural products-inspired synthetic drugs.
2. Antiviral Activity from Plant Extracts and Secondary Metabolites
Extraction is the initial and most crucial step in the investigation of medicinally important plants. It is crucial to extract the desired chemical components from the plant materials by following the appropriate extraction process for further isolation and characterization, which is an especially challenging task for researchers. Essential precautions must be taken to not lose the activity or the desired component during the process of extraction. Various traditional and modern methods are used to prepare the plant extract from different parts of the plants such as Soxhlet extraction, reflux extraction, sonification, decoction, maceration, pressurized-liquid extraction, solid-phase extraction, microwave-assisted extraction, hydro distillation, and enzyme-assisted extraction [22,23]. Spectroscopic techniques including X-ray studies play an important role in structure determination and confirmation [24].
Extracts are mixtures of secondary metabolites. Most often, the activity of the extracts is not due to a single constituent; instead, the activity may be because of the synergetic effect of two or more active constituents. Diverse classes of compounds are found in plants and their extracts; however, most of the bioactive compounds come from four major classes: alkaloids, glycosides, polyphenols, and terpenes. Several extracts were collected from different parts of various plants and were reported for their antiviral properties against a wide range of strains.
In this section, we have discussed the natural compounds, which were isolated, characterized, and evaluated for their antiviral properties. Their different chemical structures, wide therapeutic use, and the urgent need for new drugs has led to the rising attention of natural products as a source for drug development [2].
2.1. Influenza Virus
Influenza is a respiratory virus that affects the nose, throat, and respiratory system [25]. The influenza virus enters the body and attacks healthy cells, typically, epithelial cells. Once the virus enters the healthy cell, it replicates and spreads to infect other healthy cells [25]. The key to stopping the infection from spreading is to not allow the virus to enter the cell and replicate. According to the CDC, 35.5 million people were infected by influenza viruses, and 34,200 people died from the virus during the 2018–2019 influenza season [25]. There are several different classes of influenza: class A, B, C, and D influenza. Classes A, B, and C can infect humans, whereas class D infects cows [26]. Class A influenza is the most common type of influenza and has several subtypes based on its hemagglutinin and neuraminidase surface proteins. The hemagglutinin refers to the HA portion of the subtype, and the neuraminidase refers to the NA portion of the subtype [26]. After each letter, there are corresponding numbers that relate to the different strains. There are 18 different types of HA and 11 different types of NA [26]. A common subtype of influenza is known as A(H1N1), which is the subtype responsible for the 2009 swine flu pandemic. Later, the A(H3N2) variant of A(H1N1) predominated in human infections as a result of inclusion of A(H1N1) variant genetic information in the 2010–2011 seasonal flu vaccine [27]. With influenza rapidly evolving, it is important to be constantly researching and finding other ways to treat this virus [28]. Natural products are one way to find and develop new drugs and tend to be safer and less expensive. Below, we include studies that examine plant extracts’ potency and explore their mechanisms of action.
Brazil, a country known for having the largest biodiversity, has had several lectins isolated and reported for their antiviral properties. Recently, Gondim et al. screened the lectins from the Northeastern Brazilian flora, Canavalia brasiliensis (ConBr), Canavalia maritima (ConM), Dioclea lasiocarpa (DLasiL), and Dioclea sclerocarpa (DSclerL) against 18 different viruses. DSclerL and DLasiL exhibited EC50 values of 9 nM for HIV-1 and 46 nM for the respiratory syncytial virus (RSV). DLasiL also showed inhibitory property against feline coronavirus at an EC50 of 5 nM, and DSclerL, ConBr, and ConM revealed significantly low EC50 against influenza A virus strain H3N2 (0.4 nM) and influenza B virus (6 nM) [29].
Wang et al. isolated fractions from the twigs and leaves of Laggera pterodonta to evaluate its antiviral properties. Of the fractions, they identified their fraction 14 (Fr 14) as the most active against H3N3 with an IC50 of 43.5 μg/mL. It also showed activity against two different H1N1 strains. Through time addition assays, they observed that Fr 14 acts on the early stages of viral replication. Mechanistically, it inhibited the p38/MAPK pathway and further inhibited the COX-2 and NF-kB pathway. Despite these findings, further studies must be done to determine a more detailed mechanism [30].
Yu et al. extracted dried plant leaves from the Mosla scabra plant. The extracted compound showed antiviral activity against the influenza A virus (IAV). Data showed that the inhibitory rate of Mosla scabra on the lung index of IAV-infected mice treated with the total flavonoids extracted from Mosla scabra (MF) (40 mg/kg) was 10.02%; that of IAV-infected mice treated with MF (120 mg/kg) was 33.54%; and that of IAV-infected mice treated with MF (360 mg/kg) was 52.44%. MF was shown to increase the expression of INF-α in the blood and decrease the expression of pro-inflammatory cytokines. This finding suggests that it may not activate the NF-κB and apoptosis pathway. While the mechanism of Mosla scabra against IAV is still elusive, these findings suggest a possible avenue for future studies [31].
In addition, there are several plant extracts that were reported for the antiviral properties against various strains of influenza virus, which are listed in Table 1. These extracts’ mechanism of actions were not elucidated and warrant further research.
Table 1.
Active natural extracts against influenza strains.
| S. No. | Plant Name | Plant Extract | Virus | Activity | Ref. |
|---|---|---|---|---|---|
| 1 | Allium sativum | Methanolic extract of roots | H1N1 | EC50: 5 mg/mL | [32] |
| 2 | Plumbago indica | Ethanolic extract of roots | H1N1 | EC50: 1 mg/mL | [32] |
| 3 | Arachis hypogaea L. | Peanut skin extracted with hexane | H1N1 | IC50: 1.0–1.5 µg/mL | [33] |
| 4 | Caesalpinia decapetala | Aqueous ethanolic extract of leaves | H1N1 | EC50: 5.7 µg/mL | [34] |
| 5 | Carpesium abrotanoides L. | Dried herbal ethanolic extraction | H1N1 | IC50: 15.9 µM | [35] |
| H3N2 | IC50: 11.6 µM | ||||
| 6 | Cayratia pedata | DMSO extract of leaves | H1N1 | IC50: 65.99 µg/mL | [36] |
| 7 | Cayratia pedata | DMSO extract of stem bark | H1N1 | IC50: 20.50 µg/mL | [36] |
| 8 | Diotacanthus albiflorus | DMSO extract of leaves | H1N1 | IC50: 60.09 µg/mL | [36] |
| 9 | Diotacanthus albiflorus | DMSO extract of stem bark | H1N1 | IC50: 33.98 µg/mL | [36] |
| 10 | Embelia Ribes | Fruits extracted with ethyl acetate | H1N1 | IC50: 0.2 µM | [37] |
| 11 | Hippophae rhamnoides L. | Methanolic extracts of leaves | H1N1 | IC50: 7.2l µg/mL | [38] |
| 12 | Hippophae rhamnoides L. | Ethyl acetate extracts of leaves | H1N1 | IC50: 10.3l µg/mL | [38] |
| 13 | Murraya paniculata L. | Petroleum ether extraction of plant leaves | H5N1 | IC50: 0.15 µg/mL | [39] |
| 14 | Piper longum | Methanolic and chloroform extract from seeds | H1N1 | IC50: 33.43–46.24 µg/mL | [40] |
| 15 | Piper nigrum | Methanolic and chloroform extract from seeds | H1N1 | IC50: 17.47 µg/mL | [40] |
| 16 | Polygonum chinense Linn | Methanolic extract of dried and ground whole plant | H1N1 | EC50: 38.4–55.5 µg/mL | [41] |
| 17 | Poncirus trifoliata | Seeds extracted with ethanol | H1N1 | EC50: 2.51 µg/mL | [42] |
| 18 | Psoralae Semen | Aqueous extract of unknown part | H1N1 | Inhibitory (%): 30 | [43] |
| 19 | Radix isatidis | Hot methanol and ethanol extraction | H1N1 | IC50: 3.34 mg/mL | [44] |
| 20 | Ruta graveolens L. | Petroleum ether extraction of plant leaves | H5N1 | IC50: 7.8 µg/mL | [39] |
| 21 | Strychnos minor | DMSO extract of leaves | H1N1 | IC50: 46.69 µg/mL | [36] |
| 22 | Strychnos minor | DMSO extract of stem bark | H1N1 | IC50: 22.43 µg/mL | [36] |
| 23 | Strychnos nux-vomica | DMSO extract of leaves | H1N1 | IC50: 33.36 µg/mL | [36] |
| 24 | Strychnos nux-vomica | DMSO extract of stem bark | H1N1 | IC50: 23.60 µg/mL | [36] |
Reference drugs: Ribavirin (EC50: 20.5–49.9 µM); Osehamivir (IC50: 0.015–0.025 µM); Oseltamivir (IC50: 3.71–6.44 µM).
Of the extracts that are obtained from plant-based material, natural compounds can be isolated, characterized, and explored for their mechanism of actions as anti-influenza agents, such as the following studies.
Inoue et al. investigated the antiviral effects of the extract from the stems and roots of Salacia reticulata on H1N1. The authors observed there is an 80% decrease in the incidence of coughing after oral administration of 0.6 mg/day. The major phytochemical constituents in Salacia reticulata are salacinol, kotalanol, and catechin [45].
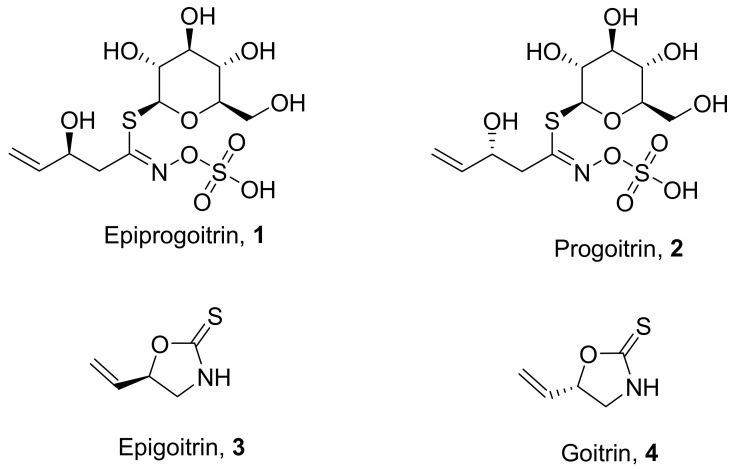
Ma et al. isolated four natural products from the sun-dried roots (Isatidis Radix) of the plant Isatis indigotica (Figure 2). The isolated compounds showed potential antiviral properties against influenza virus A (H1N1) in the order of progoitrin (2) > goitrin (4) > epigoitrin (3) > epiprogoitrin (1). These compounds did not show promising in vitro antiviral activity. However, in vivo studies show activity at a concentration of 5 mg/mL. Hemagglutination (HA) and neuraminidase (NA) inhibition assays were performed to understand the antiviral mechanism, but interestingly, these compounds did not show any inhibition effect, even at higher concentrations [46].
Figure 2.
Isolated natural products from Isatis indigotica.
Wang et al. isolated and characterized an active fraction from Laggera pterodonta. The isolated compound illic acid (5) (Figure 3) from the active fraction showed potential antiviral properties against influenza virus A (H1N1 and H3N2) and avian influenza virus (H6N2 and H9N2). In vitro studies show that the active fraction is effective against influenza strains A/PR/8/34 (H1N1), A/Guangzhou/GIRD07/09 (H1N1), and A/Aichi/2/68 (H3N2) with IC50 values of 79.4, 43.4, and 75 μg/mL, respectively. However, they are not as effective as the standard Oseltamivir (0.05 µg/mL). Time of addition, bio-plex, and Western blotting assays were performed to understand the antiviral mechanism. The results suggest that the active fraction inhibits the early stage of the virus replication. Western blotting assay results show that the fraction inhibits the p38/MAPK, NF-κB, and COX-2. Lastly, it was shown to increase the expression of cytokines and chemokines [30].
Figure 3.
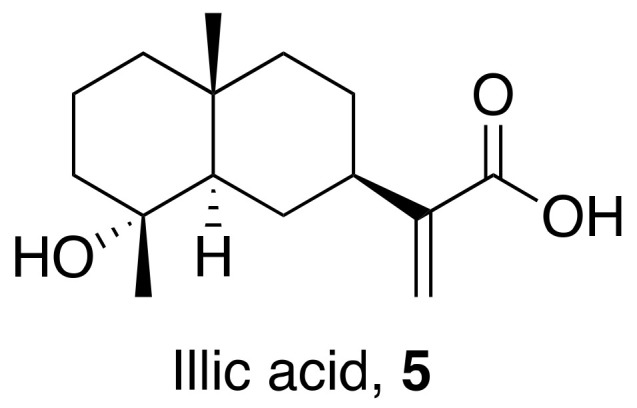
Isolated compound from Laggera pterodonta.
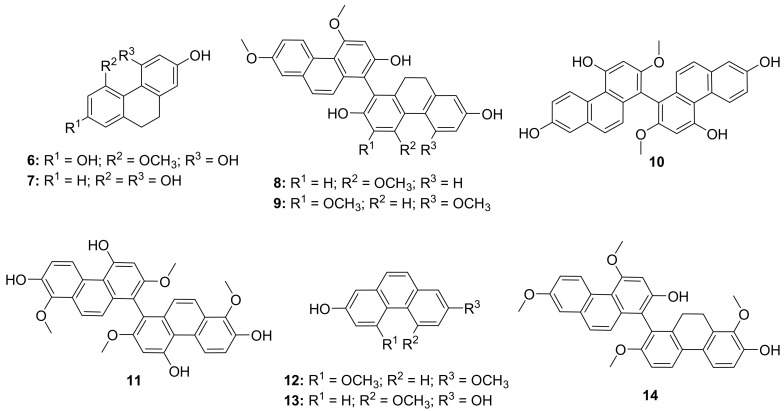
Shi et al. isolated twelve phenanthrene natural products from Bletilla striata (Figure 4). The isolated compounds showed potential antiviral properties against influenza virus A (H3N2) in the order of compound 9 > 6 > 11 = 10 = 7 = 13 > 12 > 14 > 8 and percent inhibition values of 17.2, 20.7, 34.5, 48.8, 75.9, and 79.3% respectively. These compounds did not show promising results as pretreatment. However, in vivo studies show that compounds 8, 9, 10, 11, 12, and 14 have strong inhibition in both simultaneous treatment (IC50 from 14.6 ± 2.4 to 43.3 ± 5.3 μM) and post-treatment (18.4 ± 3.1 to 42.3 ± 3.9 μM) assays. Hemagglutination (HA) and neuraminidase (NA) inhibition assays were performed to understand the antiviral mechanism. Compounds 6, 9, 10, 11, 12, and 13 showed strong inhibition on NA; however, no compound was able to inhibit hemagglutination. Oseltamivir was used as a reference drug for this study (100% inhibition). Lastly, the presence of compounds 8, 9, 10, 11, 12, and 14 led to a reduction in transcription of viral matrix protein mRNA [47].
Figure 4.
Isolated natural products natural products from Bletilla striata.
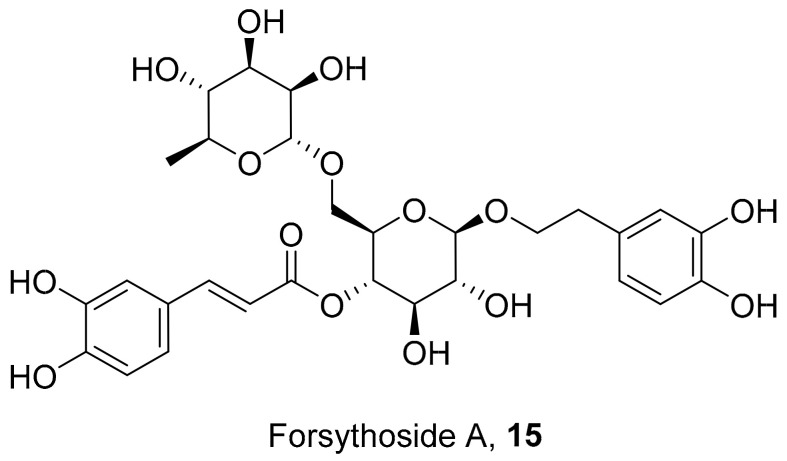
Law et al. isolated a compound from seven medicinal herbs to determine the antiviral activity against influenza (H1N1) viruses (Figure 5). Forsythoside A (15) was isolated as the active compound from the fruit of Forsythia suspensa and was found to be active against the various influenza subtypes. The treatment of compound 15 led to a slower and abnormal release mechanism of the virus via electron microscopy. Western blotting assay results showed that it reduced M1 protein expression. This may be contributing to the inhibitory effects seen on viral replication. However, the mechanism in which compound 15 leads to the reduced expression still needs further investigation [48].
Figure 5.
Isolated a compound from Forsythia suspensa.
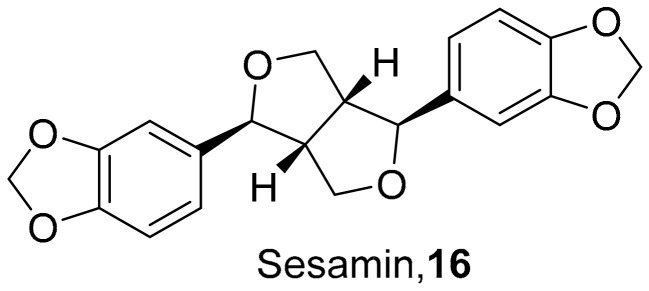
Fanhchaksai et al. used combinatorial screening along with computational methods to determine the effects of sesamin (16) (Figure 6) on target proteins against influenza H1N1. The computational data from 16 showed promising results that sesamin could be used as an alternative antiviral H1N1 compound. Compound 16 reduced the neuraminidase activity of influenza H1N1. Western blotting revealed that sesamin decreased the expression of pro-inflammatory cytokines via the MAPK pathway at concentrations of 5 μg/mL. However, 16 does exhibit some unwanted side effects, making this sesamin a starting point for future studies to optimize it as a lead [49].
Figure 6.
Structure of sesamin (16).
In addition to the above-mentioned compounds, many other compounds isolated from various plants are listed in Table 2 along with their IC50/EC50 values against influenza strains.
Table 2.
Isolated natural compounds against influenza strains.
| S. No. | Plant Name (Part) |
Compound | Virus | Activity | Ref. |
|---|---|---|---|---|---|
| 1 |
Forsythia suspensa (Fruits) |
|
H1N1 | IC50: 19.9 µM | [50] |
| 2 |
Forsythia suspensa (Fruits) |
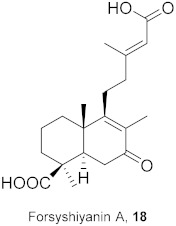
|
H1N1 | IC50: 18.4 µM | [50] |
| 3 |
Forsythia suspensa (Fruits) |
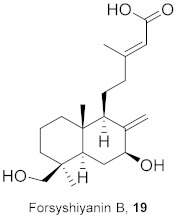
|
H1N1 | IC50: 26.2 µM | [50] |
| 4 |
Forsythia suspensa (Fruits) |
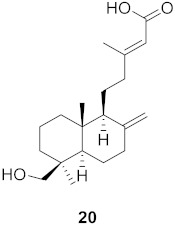
|
H1N1 | IC50: 25.7 µM | [50] |
| 5 |
Forsythia suspensa (Fruits) |
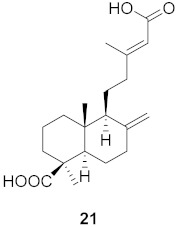
|
H1N1 | IC50: 24.1 µM | [50] |
| 6 |
Forsythia suspensa (Fruits) |
|
H1N1 | IC50: 24.9 µM | [50] |
| 7 |
Forsythia suspensa (Fruits) |
|
H1N1 | IC50: 23.5 µM | [50] |
| 8 |
Forsythia suspensa (Fruits) |
|
H1N1 | IC50: 18.6 µM | [50] |
| 9 |
Basilicum polystachyon (Whole plant) |
|
H1N1 | IC50: 4.1 μM | [51] |
| H3N3 | IC50: 18 μM | ||||
| 10 |
Curcuma aeruginosa (Rhizomes) |
|
H1N1 | IC50: 30.4 μg/mL | [52] |
| 11 | Sonneratia paracaseolaris |
|
H1N1 | IC50: 28.4 µg/mL | [53] |
| 12 |
Salvia plebeian R. Br (Roots) |
|
H1N1 | IC50: 16.65–19.83 µM | [54] |
| 13 |
Ilex asprella (Roots) |
|
H1N1 | EC50: 4.1 µM | [55] |
| 14 | Ilex asprella(Roots) |
|
H1N1 | EC50: 1.7 µM | [55] |
| 15 | Abies beshanzuensis (Bark) |
|
H3N2 | IC50: 30.8 µg/mL | [56] |
| 16 | Abies beshanzuensis (Bark) |
|
H3N2 | IC50: 30.9 µg/mL | [56] |
| 17 |
Cleistocalyx operculatus (Leaves) |
|
H1N1 | IC50: 5.07 µM | [57] |
| H9N2 | IC50: 9.34 µM | ||||
| 18 |
Cleistocalyx operculatus (Leaves) |
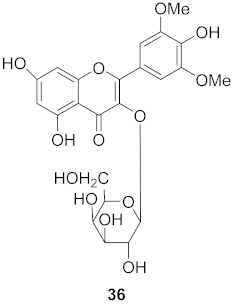
|
H1N1 | IC50: 5.07 µM | [57] |
| H9N2 | IC50: 9.34 µM | ||||
| 19 |
Elaeocarpus tonkinesis (Leaves and Twigs) |
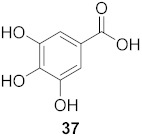
|
H1N1 | EC50: 8.1 µg/mL | [58] |
| 20 |
Elaeocarpus tonkinesis (Leaves and Twigs) |
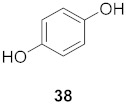
|
H1N1 | EC50: 19.7 µg/mL | [58] |
Reference drugs: Ribavirin (IC50: 24.6 µg/mL, EC50: 52.2–56.9 µM); Oseltamivir (IC50: 0.10 µM, EC50: 0.01–2.36 µM).
2.2. Human Immunodeficiency Virus (HIV)
Human Immunodeficiency Virus type 1 (HIV-1) is a retrovirus that attacks healthy immune cells, thus affecting and eventually destroying the human immune system. More than 33 million deaths have been reported globally since its first major outbreak. However, the outbreaks have been reduced by up to 40% from the initial rates because of prevention strategies and treatment plans [59]. Antiretroviral therapy (ART) has shown success in preventing viral replication and improving the lives of those living with HIV-1 but is not useful in eliminating the virus within the body. As a result of this, many ART drugs are used in combination to treat the infection. This allows for a decrease in the viral load, which may lower the levels of the virus to nearly undetectable amounts [60]. Drug resistance has been documented in all six of the antiretroviral drug classes and as such often requires resistance testing before beginning the therapies. This often requires the use of multiple drugs in combination with ART to have a significant effect on the viral load. The molecular mechanism involved in the reverse transcription process of retroviruses such as HIV-1 makes it likely for errors to occur. Often, these result in mutations and subsequently cause an increased genetic diversity in the HIV-1 virus, ultimately allowing for potential drug resistance [60].
Current HIV-1 treatments have undergone prominent advances over the past three decades since the major outbreak of the virus but are far from being perfect at HIV-1 treatment or prevention. No current treatment can effectively cure the viral infection despite being able to reduce the viral load. The current medications often have side effects and require lifetime administration of the drugs, further burdening the patients. Cessation of medication use can result in a spike in the viral load, increasing the chance of mutations and a need for alterations to patient treatments. Natural products have been researched for suppressing HIV [59]. In its early history, ART drug research did focus on several natural compounds including Calanolides for NNRTI activity, Kuwanon-L from the black mulberry tree Morus nigra for anti-reverse transcriptase and anti-integrase activity, Bowman–Birk inhibitor from soybeans used as a protease inhibitor, and many others [61]. As a result of the versatility and the vast number of compounds synthesized from plants, ART small-molecule drug candidates could likely be derived from natural products, allowing for a basic understanding of the pharmacokinetic properties in the anti-HIV-1 activity. Table 3 shows a list of plant species extracts with inhibition data, followed by a recent study that investigates the mechanism actions of an isolated compound that shows potent anti- HIV activity.
Table 3.
Extracts from plants that are active against HIV.
| S. No. | Plant name | Plant Extract | Virus | Activity | Ref. |
|---|---|---|---|---|---|
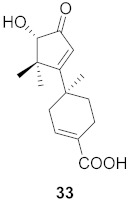
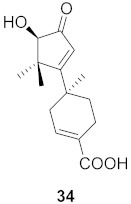
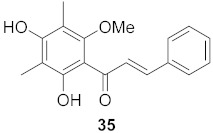
| 1 | Artemisia campestris | Aqueous ethanolic extract of the whole plant | HIV-1 | IC50: 14.62 μg/mL | [62] |
| 2 | Cassia Siberiana | Chloroform methanolic extract of roots | HIV-1 | IC50: 84.8 μg/mL | [63] |
| 3 | Croton megalobotrys | Chloroform methanolic extract of bark | HIV-1 | IC50: 0.05 μg/mL | [63] |
| 4 | Daphne gnidium L. | Ethyl acetate extraction of branches | HIV-1 | EC50: 0.08 μg/mL | [64] |
| 5 | Eclipta alba | Leaves extracted with chloroform | HIV-1 | IC50: 250 μg/mL | [65] |
| 6 | Euphorbia kansui | Methanolic extract of roots | HIV-1 | EC50: 110 ng/mL | [66] |
| 7 | Terminalia chebula | Methanolic/aqueous extract of fruit | HIV-1 | IC50: ≤5 μg/mL | [67] |
| 8 | Vitex doniana | Chloroform methanolic extract of roots | HIV-1 | IC50: 25 μg/mL | [63] |
Reference drugs: Efavirenz (EC50: 0.0007–0.002 μM); Zidovudine (EC50: 0.005 μg/mL, 0.02 μM).
Chen et al. isolated a novel phorbol ester, hop-8 (39) (Figure 7), from the dried leaves and twigs of Ostodes katharinae that shows potent antiviral activity against wild-type HIV-1 and HIV-2 and drug-resistant strains in peripheral blood mononuclear cells (PBMCs). The cytotoxicity assay of Hop-8 shows EC50 values ranging from 0.396 to 6.915 μM, which are better than the standard, Prostratin. To determine the mode of action for hop-8, Western blotting and cell transfection techniques were used. One of Hop-8′s mechanisms to resist infection by HIV verified in this study involves stimulating A3G expression, which prevents Vif-mediated degradation. This discovery may be considered as a potent strategy for therapeutic development in the future [68]. Other isolated natural compounds have been found to show significant anti-HIV properties (Table 4) and may also be considered to serve as therapeutic agents.
Figure 7.
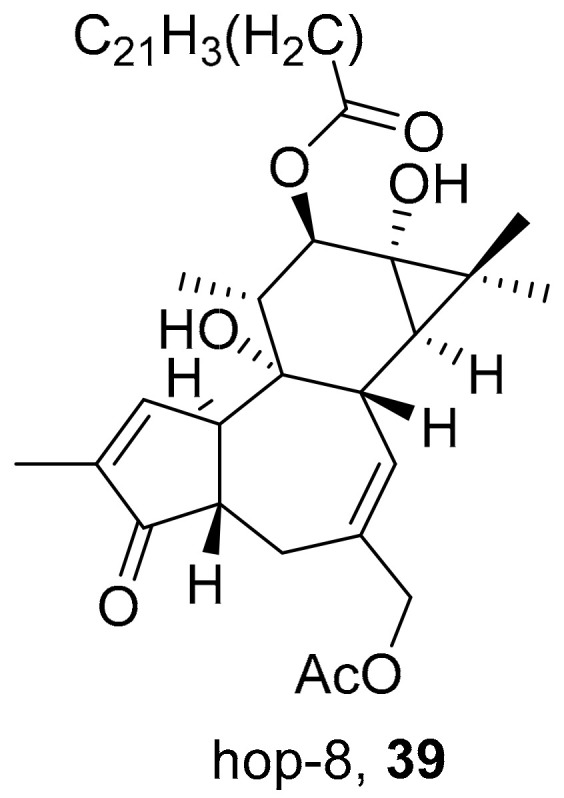
Isolated compound from Ostodes katharinae.
Table 4.
Compounds isolated from plants against HIV.
| S. No. | Plant Name (Part) |
Compound | Virus | Activity | Ref. |
|---|---|---|---|---|---|
| 1 |
Clausena anisum-olens (Leaves and twigs) |
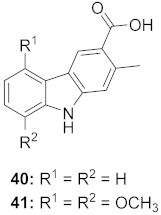
|
HIV |
40: EC50: 2.4 μg/mL 41: EC50: 3.7 μg/mL |
[69] |
| 2 |
Manilkara zapota (Fruit) |
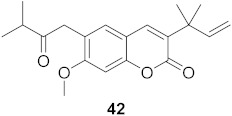
|
HIV | EC50: 8.69 μM | [70] |
| 3 |
Manilkara zapota (Fruit) |
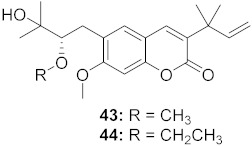
|
HIV |
43: EC50: 0.33 μM 44: EC50: 0.42 μM |
[70] |
| 4 |
Manilkara zapota (Fruit) |
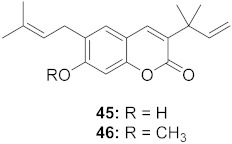
|
HIV |
45: EC50: 2.28 μM 46: EC50: 3.49 μM |
[70] |
| 5 |
Manilkara zapota (Fruit) |
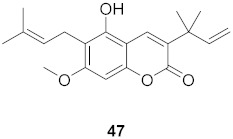
|
HIV | EC50: 4.26 μM | [70] |
| 6 |
Manilkara zapota (Fruit) |
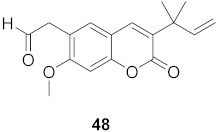
|
HIV | EC50: 0.97 μM | [70] |
| 7 |
Manilkara zapota (Fruit) |
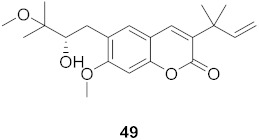
|
HIV | EC50: 5.26 μM | [70] |
| 8 |
Manilkara zapota (Fruit) |
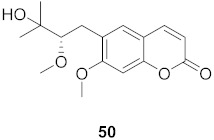
|
HIV | EC50: 6.73 μM | [70] |
| 9 |
Manilkara zapota (Fruit) |
|
HIV | EC50: 0.12 μM | [70] |
| 10 |
Euphorbia semiperfoliata (Whole plant) |
|
HIV-1 |
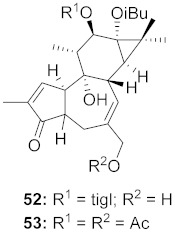
52: EC50: 0.013 μM 53: EC50: 0.054 μM |
[71] |
| 11 | Salvia miltiorrhiza Bunge |
|
HIV-1 |
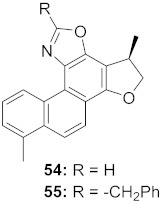
54: IC50: 0.03 μM 55: IC50: 1.2 μM |
[72] |
| 12 |
Marcetia taxifolia (Aerial parts) |
|
HIV-1 |
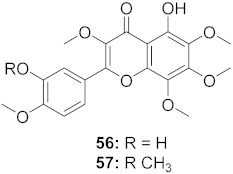
56: IC50: 4.1 μM 57: IC50: 0.4 μM |
[73] |
| 13 |
Justicia gendarussa (Stem and bark) |
|
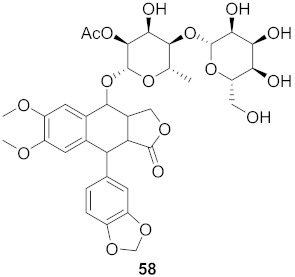
HIV-1 | IC50: 15–21 nM | [74] |
| 14 |
Justicia gendarussa (Root and stem) |
|
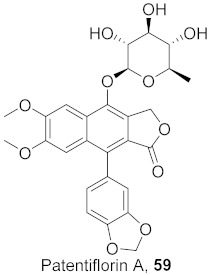
HIV- 1 | IC50: 26.9 nM | [75] |
| 15 |
Rheum palmatum L. and Rheum officinale Baill (Roots) |
|
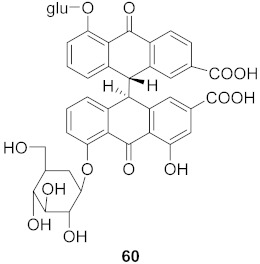
HIV | IC50: 1.9 μM | [76] |
| 16 |
Rheum palmatum L. and Rheum officinale Baill (Roots) |
|
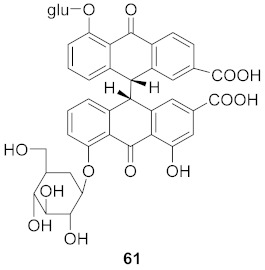
HIV | IC50: 2.1 μM | [76] |
| 17 |
Flueggea virosa (Roots) |
|
HIV |
62: EC50: 19.2 μM 63: EC50: 20.5 μM 64: EC50: 40.1 μM |
[77] |
| 18 |
Flueggea virosa (Roots) |
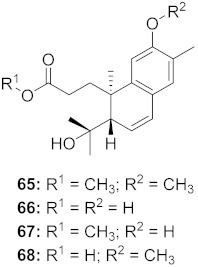
|
HIV |
65: EC50: 51.8 μM 66: EC50: >100 μM 67: EC50: 87.8 μM 68: EC50: 7.1 μM |
[77] |
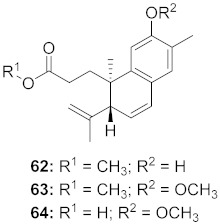
| 19 |
Flueggea virosa (Roots) |
|
HIV | EC50: 58.0 μM | [77] |
| 20 |
Flueggea virosa (Roots) |
|
HIV | EC50: >100 μM | [77] |
| 21 |
Flueggea virosa (Roots) |
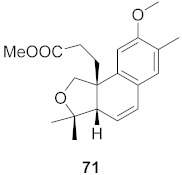
|
HIV | EC50: 53.9 μM | [77] |
| 22 |
Flueggea virosa (Roots) |
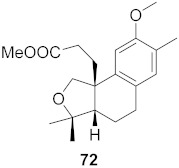
|
HIV | EC50: 48.6 μM | [77] |
| 23 |
Flueggea virosa (Roots) |
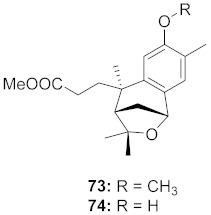
|
HIV |
73: EC50: 40.6 μM 74: EC50: >100 μM |
[77] |
| 24 |
Flueggea virosa (Roots) |
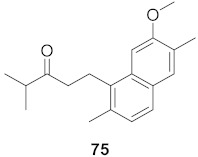
|
HIV | EC50: 69.4 μM | [77] |
| 25 |
Chloranthus japonicus (Roots) |
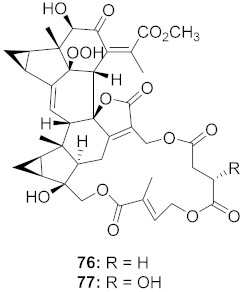
|
HIV-1 |
76: EC50: 3.08 μM 77: EC50: 3.29 μM |
[78] |
| 26 | Chloranthus japonicus (Roots) |
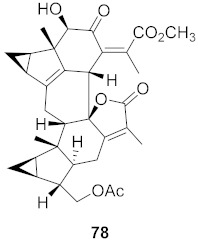
|
HIV-1 | EC50: 5.41 μM | [78] |
| 27 | Kaempferia pulchra (Rhizomes) |
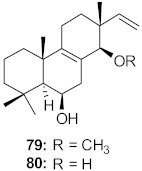
|
HIV-1 | IC50: 1.56–6.25 μM | [79] |
| 28 | Kaempferia pulchra (Rhizomes) |
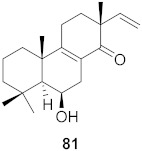
|
HIV-1 | IC50: 1.56–6.25 μM | [79] |
| 29 | Kaempferia pulchra (Rhizomes) |
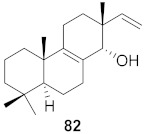
|
HIV-1 | IC50: 1.56–6.25 μM | [79] |
| 30 | Kaempferia pulchra (Rhizomes) |
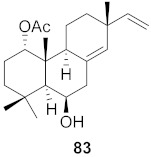
|
HIV-1 | IC50: 1.56–6.25 μM | [79] |
| 31 | Kaempferia pulchra (Rhizomes) |
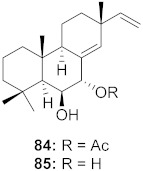
|
HIV-1 | IC50: 1.56–6.25 μM | [79] |
| 32 |
Stillingia lineata (Bark) |
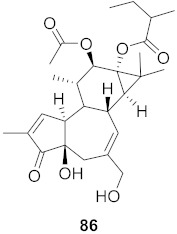
|
HIV-1 | EC50: 0.271 μM | [80] |
| HIV-2 | EC50: 0.107 μM | ||||
| 33 |
Stillingia lineata (Bark) |
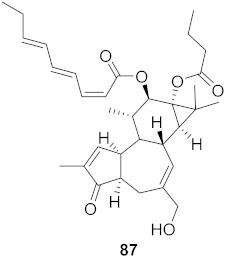
|
HIV-1 | EC50: 0.233 μM | [80] |
| HIV-2 | EC50: 0.174 μM | ||||
| 34 |
St35illingia lineata (Bark) |
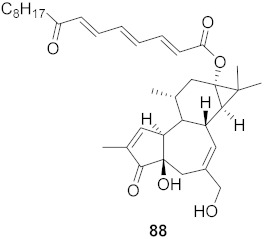
|
HIV-1 | EC50: 0.043 μM | [80] |
| HIV-2 | EC50: 0.018 μM | ||||
| 35 |
Moquiniastrm floribundum (Leaves) |
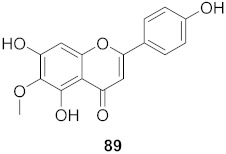
|
HIV-1 | IC50: 0.345 mM | [81] |
| 36 |
Moquiniastrm floribundum (Leaves) |
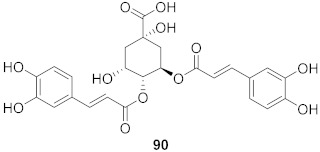
|
HIV-1 | IC50: 0.240 mM | [81] |
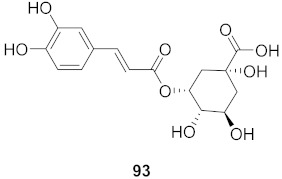
| 37 |
Moquiniastrm floribundum (Leaves) |
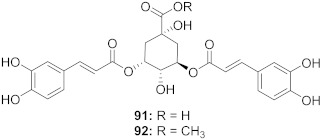
|
HIV-1 |
91: IC50: 0.315 mM 92: IC50: 0.250 mM |
[81] |
| 38 |
Moquiniastrm floribundum (Leaves) |
|
HIV-1 | IC50: 0.374 mM | [81] |
| 39 |
Moquiniastrm floribundum (Leaves) |
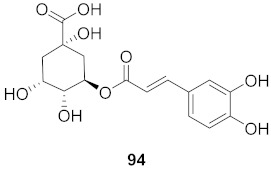
|
HIV-1 | IC50: 0.489 mM | [81] |
Reference drugs: Zidovudine (EC50: 0.005 μg/mL, 0.02 μM); Prostratin (EC50: 0.226 μM); Efavirenz (IC50: 0.0007–0.002 μM); Nevaripine (IC50: 0.1–0.5 μM); Honokiol (IC50: 45.9 μM); Myricetin (0.2 mM); Foscarnet (0.001 mM).
2.3. Arthropod-Borne Flaviviruses
Dengue virus (DENV), West Nile virus (WNV), and Chikungunya virus (CHIKV) are examples of mosquito-borne RNA viruses apart of the Flaviviridae family that cause flu-like symptoms transmitted by the Ae albopictus mosquito. Of these viruses, DENV is the most prevalent mosquito-borne virus affecting up to 400 million people each year globally according to the CDC [82]. There is an available vaccine; however, the vaccine may lead to a higher risk of developing severe DENV symptoms for individuals not previously infected with the virus and is only effective in the age group 9–45 years old [83]. DENV has four different serotypes (DENV 1–4). Currently, there are no anti-DENV therapeutics that have been approved by the FDA; however, there are several in the clinical trial phase. The most targeted proteins when developing these anti-DENV drugs include but are not limited to the envelope protein, methyltransferases, and genes important for coding nonstructural (NS) proteins, such as RNA polymerases, protease, and helicase, to name a few [80]. NS2B, NS3, and NS5 are examples of NS protein targets that may be important in developing these therapeutics [84]. Given the lack of availability of therapeutics, natural extracts and compounds may provide a helpful avenue to discover lead compounds or natural treatments. For example, Angelina et al. found that the ethanol extract from the leaves Cassia Alata effectively inhibited DENV serotype 2 infection in every step of the virus’s replication cycle [85].
WNV is the leading cause of mosquito-transmitted disease in the continental United States [86]. There are no vaccines for prevention or therapeutics against WNV available. Therefore, there is an urgent need for research for the development of therapeutics against this virus. Potential targets include but are not limited to the envelope protein, NS proteins 3 and 5 [87]. Some natural product extracts are active against WNV, and once the active compounds are structurally elucidated, they may serve potent anti-WNV therapeutics [88].
CHIKV viral infection is mainly seen in countries in the Eastern hemisphere such as Africa, Asia, Europe, and other tropical and subtropical islands [89]. Currently, there are no vaccines or therapeutics available against CHIKV. Similar to DENV, there are current potential targets that include but are not limited to NS proteins 1 and 2, CHIKV capsid, and proteins important to fusion. However, there are many proteins involved in the CHIKV replication cycle that have no structural information. There has been extensive screening for activating anti-CHIKV natural products; while most only show moderate activity, they may serve as lead compounds for developing helpful therapeutics [90]. There is an obvious need for discovery in preventative and curing treatments for these and other viruses, which is a need that may be satisfied through natural sources. Extract isolates in the below studies show inhibitory effects that were further studied to decipher the mechanism in which the extracts manifested its inhibitory effects.
Panya et al. extracted the bioactive peptides of 33 Thai medicinal plants via digestion to evaluate the antiviral activity against DENV. Of these plants, Thunbergia laurifolia Lindl and Acacia catechu peptide extracts showed potent antiviral activity against DENV. Foci-forming unit assay results showed that both peptide extracts have inhibitory potential with an IC50 value of 0.18 and 1.54 μg/mL for Acacia catechu and Thunbergia laurifolia Lindl, respectively. Acacia catechu peptide extract was further studied to determine the active peptide sequence and mechanism of action. The identified peptide inhibitors in the extract of Acacia catechu were also found to be effective against all four DENV serotypes. The results of the time of addition assay suggest that the Acacia catechu peptide extract inhibits DENV2 in the early stages of infection; however, the mechanism in which the peptides inhibit the early step of infection is still unknown [91].
Leite et al. sought to find the anti-DENV-2 activity of extracts from the leaves of Cissampelos sympodialish. Using MTT assays and infection of Huh-7 cells with DENV-2, researchers found that the leaf hydroalcoholic extract (AFL) showed significant inhibitory effects at 10 μg/mL. AFL did not decrease the expression of DENV but rather the expression of cytokines important in its infections. Then, 72 h after infection with the virus showed there was a decrease in the production of migration inhibitory factor (MIF) and TNF-α, both of which are important in proliferating the effects of the DENV infection. Further isolation and elucidation of two AFL alkaloids were found to have no activity, therefore eliminating them as the components responsible for AFL’s activity. Further research must be done to isolate the active components of this fraction to give a clear mechanism of action for AFL [92]. A list of plant species with IC50/EC50 data is summarized in Table 5.
Table 5.
Plant extracts with antiviral activity against arthropod-borne flaviviruses.
| S. No. | Plant Name | Plant Extract | Virus | Activity | Ref. |
|---|---|---|---|---|---|
| 1 | Andrographis paniculata | Pure andrographolide in DMSO | DENV2 | EC50: 21.304 µM | [93] |
| 2 | Dryopteris crassirhizoma | Aqueous extract of whole plant | DENV | IC50: 130 µg/mL | [94] |
| 3 | Euphorbia amygdaloides semiperfoliata | Ethyl acetate extract of whole plant | CHIKV | EC50: <0.8 µg/mL | [95] |
| 4 | Euphorbia characias | Ethyl acetate extract of stems | CHIKV | EC50: 2.9 µg/mL | [95] |
| 5 | Euphorbia hyberna insularis | Ethyl acetate extract of aerial parts | CHIKV | EC50: 1.0 µg/mL | [95] |
| 6 | Euphorbia pithyusa | Ethyl acetate extract of leaves | CHIKV | EC50: <0.8 µg/mL | [95] |
| 7 | Euphorbia pithyusa | Ethyl acetate extract of stems | CHIKV | EC50: <0.8 µg/mL | [95] |
| 8 | Euphorbia pithyusa | Methanolic and ethyl acetate extract of roots. | CHIKV | EC50: <0.8 µg/mL | [95] |
| 9 | Euphorbia segetalis pinea | Ethyl acetate extract of roots | CHIKV | EC50: 1.8 µg/mL | [95] |
| 10 | Euphorbia segetalis pinea | Ethyl acetate extract of arial parts | CHIKV | EC50: 3.7 µg/mL | [95] |
| 11 | Euphorbia segetalis pinea | Ethyl acetate extract of stems | CHIKV | EC50: 3.5 µg/mL | [95] |
| 12 | Euphorbia spinosa | Ethyl acetate extract of roots | CHIKV | EC50: <0.8 µg/mL | [95] |
| 13 | Euphorbia spinosa | Methanolic extract of roots | CHIKV | EC50: 2.3 µg/mL | [95] |
| 14 | Euphorbia spinosa | Ethyl acetate extract of stems | CHIKV | EC50: 3.4 µg/mL | [95] |
| 15 | Justicia adhatoda | Aqueous extract of leaf | DENV | IC50: 60 µg/mL | [96] |
| 16 | Morus alba | Aqueous extract of the whole plant | DENV | IC50: 221 µg/mL | [94] |
| 17 | Psidium guajoava | Aqueous extract of leaf | DENV | IC50: 60 µg/mL | [96] |
| 18 | Syzygium campanulatum | Ethyl acetate extract of leaves | DENV2 | Inhibitory (%): 64.77 | [97] |
| 19 | Syzygium grande | Ethyl acetate extract of leaves | DENV2 | Inhibition (%): 61.46 | [97] |
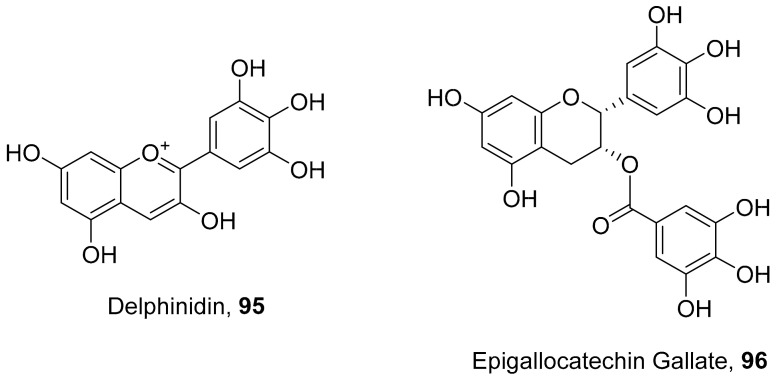
Vazquez-Calvo et al. used many polyphenols found in green tea and wine to analyze their effects against the West Nile virus (WNV), Zika virus (ZIKV), and Dengue virus (DENV). Cell viability assays, Quantitative-PCR, and LysoSensor assays were done to determine the effect of these polyphenols. Of the different polyphenols tested, delphinidin (95) and epigallocatechin gallate (96) (Figure 8) showed the most inhibition against WNV at 10 μM. To determine the action on the viral particle, the two polyphenols were added at different times of infection. Compounds 95 and 96 were found to affect the early stages of infection by a suggested virucidal effect. The LysoSensor assay supports 95 and 96 effects via virucidal effect rather than a pH-dependent fusion. The investigation supports that the mechanism of action is due to a direct effect on the viral particle rather than a pH-dependent mechanism. Compounds 95 and 96 also show inhibitory activity against ZIKV and DENV [98].
Figure 8.
Structure of polyphenolic compounds delphinidin (95) and epigallocatechin gallate (96).
Many secondary metabolites have been reported to have antiviral properties against arthropod-borne flaviviruses. In Table 6, we have mentioned the compounds that exhibited viral inhibition with inhibitory activity with IC50 or EC50 dose.
Table 6.
Compounds isolated from plants that are active against arthropod-borne flaviviruses.
| S. No. | Plant Name (Part) |
Compound | Virus | Activity | Ref |
|---|---|---|---|---|---|
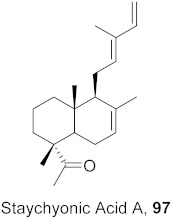
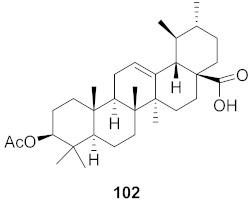
| 1 | Basilicum poly-stachyon |
|
DENV | IC50: 1.4 µM | [99] |
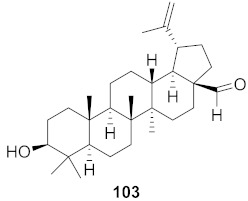
| 2 |
Basilicum polystachyon (Whole plant) |
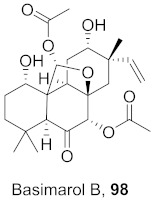
|
WNV | IC50: 100 μM | [51] |
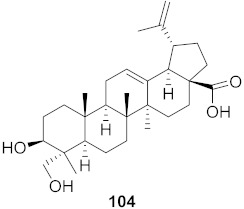
| 3 | Mammea americana (Seeds) |
|
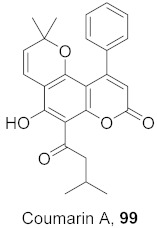
DENV2 | EC50: 9.6 µg/mL | [100] |
| CHIKV | EC50: 10.7 µg/mL | ||||
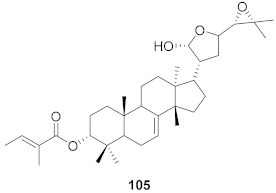
| 4 | Mammea americana (Seeds) |
|
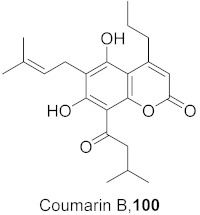
DENV2 | EC50: 2.6 µg/mL | [100] |
| CHIKV | EC50: 0.5 µg/mL | ||||
| 5 |
Diospyros Ebenaceae (Bark) |
|
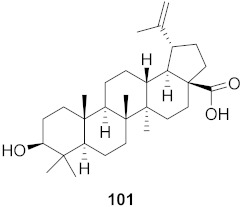
DENV | IC50: 6.6 µM | [101] |
| 6 |
Diospyros Ebenaceae (Bark) |
|
DENV | IC50: 7.0 µM | [101] |
| 7 |
Diospyros Ebenaceae (Bark) |
|
DENV | IC50: 6.1 µM | [101] |
| 8 |
Diospyros Ebenaceae (Bark) |
|
DENV | IC50: 5.3 µM | [101] |
| 9 |
Melia azedarach (Fruits) |
|
DENV2 | EC50: 3.0 µM | [102] |
| 10 |
Melia azedarach (Fruits) |
|
DENV2 | EC50: 12 µM | [102] |
| 11 |
Stillingia lineata (Bark) |
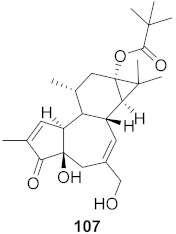
|
CHIKV | EC50: 1.2 μM | [80] |
| 12 |
Stillingia lineata (Stem bark) |
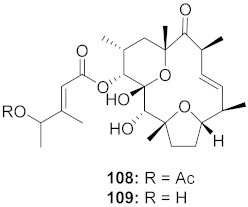
|
CHIKV |
108: EC50: 7 μM 109: EC50: 34 μM |
[103] |
| 13 |
Stillingia lineata (Bark) |
|
CHIKV | EC50: 3.3 μM | [80] |
| 14 |
Stillingia lineata (Bark) |
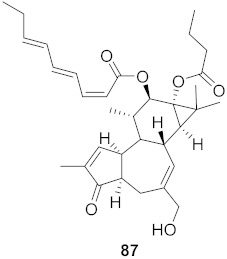
|
CHIKV | EC50: 1.4 μM | [80] |
| SINV | EC50: 5.0 μM | ||||
| 15 |
Stillingia lineata (Bark) |
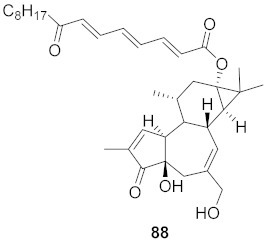
|
CHIKV | EC50: 2.2 μM | [80] |
| SINV | EC50: 11 μM | ||||
| 16 |
Euphorbia semiperfoliata (Whole plant) |
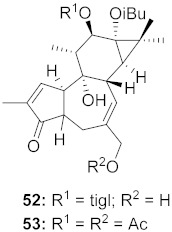
|
CHIKV |
52: EC50: 1.0 μM 53: EC50: 0.44 μM |
[71] |
2.4. Herpes Simplex Virus
Herpes simplex virus (HSV), commonly known as herpes, is typically found in two different strands: herpes simplex virus type 1 (HSV-1) and herpes simplex virus type 2 (HSV-2). HSV-1 is a highly contagious infection typically transmitted by oral contact but can also be contracted via oral–genital contact [104,105]. HSV-1, based on 2016 statistics, affected approximately 3.7 billion globally in individuals under 50 years old [105]. Typically, individuals infected with this strain are asymptomatic, so much so that infected individuals are unaware that they are carriers. Often, infected patients present with painful ulceration or blisters on or around their mouth [105].
HSV-2 is considered a sexually transmitted infection, and it is almost exclusively transmitted through genital–genital contact [100]. In 2016, HSV-2 affected approximately 419 million people globally within the age group of 15–49 [101]. Individuals with this strain also experience similar ulceration and blisters by their genitals. Newly infected patients may also experience mild cold symptoms, including fever, body aches, and swollen lymph nodes. Transmission is highest in HSV-2 patients when sores are present; however, they can still pass the virus when they are asymptomatic [105]. HSV-2 has been shown to increase the likelihood of contracting HIV by weakening the skin and mucous membrane responsible for protection [106].
According to the CDC, there is currently no cure for HSV [107], but many medications are used to lessen the severity of an outbreak and the frequency at which the virus enters its lytic cycle. Acyclovir, famciclovir, and valacyclovir are examples of medications that can help patients control the symptoms of an outbreak [105]. In 1988, Dr. Gertrude Elion obtained the Nobel Prize for the discovery of acyclovir, which is one of the first-line drugs currently used for the treatment of HSV infections. Studies also suggest HSV reactivation in the brain can lead to Alzheimer’s disease (AD) [108]. Despite their efficiency in symptomatic patients, they do not cure symptoms or entirely prevent HSV transmission. Further research must be done to lessen symptoms for those infected and develop a vaccine to prevent the transmission.
Tannins are an important class of compounds isolated from plants and possess various biological properties, including antiviral properties. Vilhelmova-Ilieva et al. study the antiviral properties of ellagitannins against HSV over the last decade [109]. They also investigated the effect of ellagitannins on acyclovir (ACV)-resistant herpes. The ellagitannin(s)–ACV combination applied against ACV-resistant HSV-1 produced a much stronger synergistic effect compared to the effect observed against ACV-resistant HSV-2 [110].
Bisignano et al. examined the anti-HSV-1 activity of the methanolic extract of Prunus dulcis, specifically from the almond skins. Using plaque-forming assays, Western blotting, and other techniques, they were able to demonstrate the extract’s activity in blocking the replication of HSV-1 particles. It was also found that the extract was able to prevent the absorption of the virus in Vero cells. This effect was observed after 1 h post-infection when cells were treated with 0.4 mg/mL of the extract. Further studies must be done to determine the mechanism by which this inhibitory effect takes place [111]. Table 7 summarizes other plant species’ extracts that show inhibitory effects against HSV.
Table 7.
Extracts from plants active against HSV.
| S. No. | Plant Name | Plant Extract | Virus | Activity | Ref. |
|---|---|---|---|---|---|
| 1 | Arctium lappa L. | Ethanolic extract of fruits | HSV-1 | IC50: 400 μg/mL | [112] |
| 2 | Epimedium koreanum | Herbal aqueous extraction | HSV | EC50: 0.62 μg/mL | [113] |
| 3 | Eucalyptus sideroxylon Cunn. ex Woolls | Methanolic extract of leaves | HSV-2 | IC50: 199.34 μg/mL | [114] |
| 4 | Juncus Compressus | Whole plant extracted with methanol | HSV-2 | IC50: 12.4 μM | [115] |
| 5 | Punica granatum L. | Aqueous extract of pomegranate rind | HSV | EC50: 0.02 μg/mL | [116] |
| 6 | Ribes multiflorum | Methanol and aqueous extraction of leaves and fruit | HSV-1 | EC50: 9710 μg/mL | [117] |
| 7 | Ribes uva-crispa | Methanol and aqueous extraction of leaves and fruit | HSV-1 | EC50: 9710 μg/mL | [117] |
| 8 | Rosmarinus officinalis | Aqueous extract of the whole plant | HSV-1 | EC50: 67.34 μg/mL | [118] |
| 9 | Syzygium jambos | Ethanolic extract of leaves | HSV-1 | IC50: 50.00 μg/mL | [119] |
| 10 | Terminalia chebula Retz | Ethanolic extract of fruit | HSV-2 | IC50: 0.01 μg/mL | [120] |
Reference drug: Acyclovir (EC50: 0.8–2.1 μg/mL, 0.41 μM; IC50: 0.1 μg/mL).
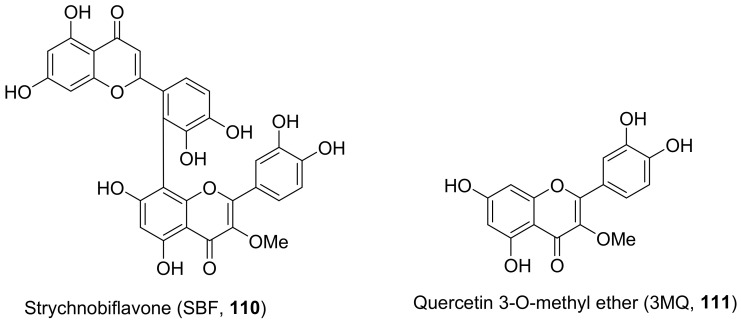
The mechanisms of action of standardized ethyl acetate extract from the stem bark of Strychnos pseudoquina (SEAE) and isolated compound strychnobiflavone (SBF, 110) were shown to affect the early stages of viral infection accompanied by reduced HSV-1 protein expression. Both flavonoids (Figure 9) elicited a concentration-dependent inhibition of monocyte chemoattractant protein-1(MCP-1), whereas (3MQ, 111) reduced the chemokine release more significantly than SBF. Conversely, both compounds stimulated the production of the cytokines TNF-a and IL-1 in LPS-stimulated cells. It can be concluded that SEAE and SBF interfered with various steps of the HSV replication cycle, mainly adsorption, post-adsorption, and penetration, as well as with band c viral proteins expression. Incidentally, the direct inactivation of viral particles was observed. The results are significant as they suggest that the compounds present anti-HSV and anti-inflammatory activities [121].
Figure 9.
Isolated compounds from Strychnos pseudoquina.
In Table 8, we have mentioned the compounds that exhibited inhibitory activity on viral inhibition against HSV with the IC50 or EC50 dose.
Table 8.
Compounds isolated from plants against HSV.
| S. No. | Plant Name (Part) |
Compound | Virus | Activity | Ref. |
|---|---|---|---|---|---|
| 1 | Houttuynia cordata |
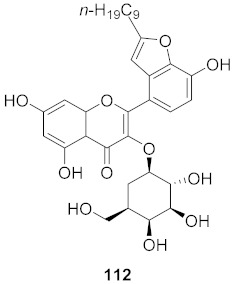
|
HSV | IC50: 23.5 μM | [122] |
| 2 |
Boswellia serrata (Oleo-gum-resin) |
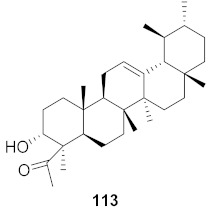
|
HSV-1 | EC50: 5.2 μg/mL | [123] |
| 3 |
Angelica archangelica L. (Fruits) |
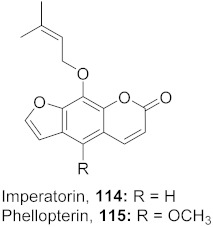
|
HSV-1 |
114: IC50: 15.62 μg/mL 115: IC50: 3.90 μg/mL |
[124] |
| 4 |
Rhododendron capitatum (Aerial parts) |
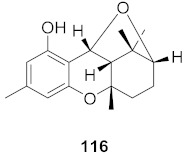
|
HSV-1 | IC50: 4.2 μM | [125] |
| 5 | Cnidium monnieri (Fruit) |
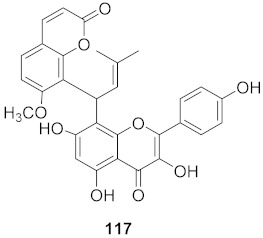
|
HSV-1 | IC50: 1.23 μM | [126] |
| 6 | Kalanchoe daigremontiana (Leaves) |
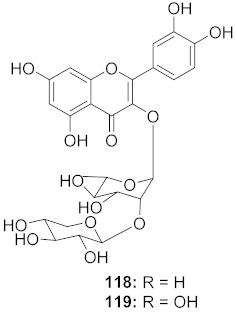
|
HSV-1 | EC50: 0.97 μg/mL | [127] |
| HSV-2 | EC50: 0.72 μg/mL | ||||
| 7 | Morus alba L. |
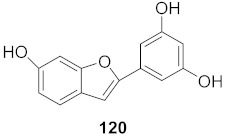
|
HSV-1 | IC50: 2.2 ± 0.1 μg/mL | [128] |
| HSV-2 | IC50: 2.5 ± 0.3 μg/mL | ||||
| 8 | Morus alba L. |
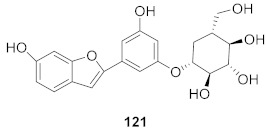
|
HSV-1 | IC50: 5.0 μg/mL | [128] |
| HSV-2 | IC50: 3.2 μg/mL | ||||
| 9 | Morus alba L. |
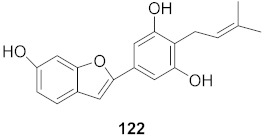
|
HSV-1 | IC50: 8.4 μg/mL | [128] |
| HSV-2 | IC50: 8.2 ± 0.4 μg/mL | ||||
| 10 | Morus alba L. |
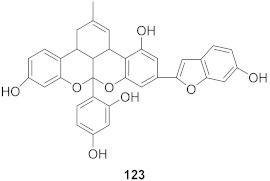
|
HSV-1 | IC50: 5.2 μg/mL | [128] |
| HSV-2 | IC50: 3.7 μg/mL | ||||
| 11 | Morus alba L. |
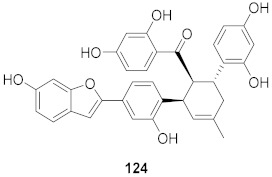
|
HSV-1 | IC50: 12.5 μg/mL | [128] |
| HSV-2 | IC50: 12.5 μg/mL | ||||
| 12 | Morus alba L. |
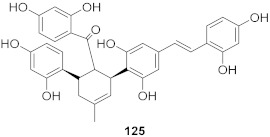
|
HSV-1 | IC50: 6.3 μg/mL | [128] |
| HSV-2 | IC50: 25.0 μg/mL | ||||
| 13 | Camellia sinensis (Leaves) |
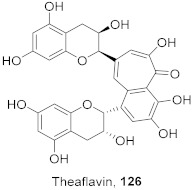
|
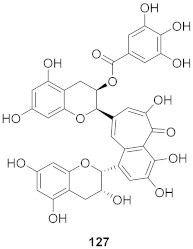
HSV-1 | EC50: 50 μM | [129] |
| 14 | Camellia sinensis (Leaves) |
|
HSV-1 | EC50: 25 μM | [129] |
| 15 | Camellia sinensis (Leaves) |
|
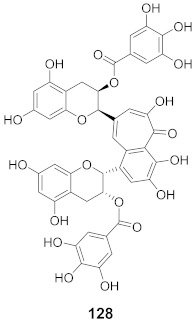
HSV-1 | EC50: 20 μM | [129] |
| 16 | Kalanchoe pinnata (Root) |
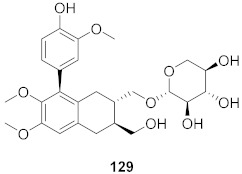
|
HSV-1 | EC50: 0.97 μg/mL | [130] |
| HSV-2 | EC50: 0.72 μg/mL | ||||
| 17 | Kalanchoe pinnata (Root) |
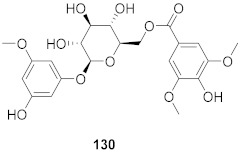
|
HSV-1 | EC50: 0.97 μg/mL | [130] |
| HSV-2 | EC50: 0.72 μg/mL |
Reference drug: Acyclovir (EC50: 0.8–2.1 μg/mL, 0.41 μM; IC50: 0.1 μg/mL).
2.5. Hepatitis Virus
Hepatitis is a viral infection of the liver that causes inflammation, leading to either short-term or long-term damage to the organ’s structure and function and ultimately to the individual. The most prevalent hepatitis strains in the world are Hepatitis A (HAV), Hepatitis B (HBV), and Hepatitis C virus (HCV) [131]. While all three classes of the virus are similar in their responses to the body, they differ in symptoms and treatments.
Of the three, Hepatitis A is considered to be the most contagious. The virus is easily ingested through contaminated food or proximity of infected individuals. However, some are unaware of their infection status unless they are tested, as the symptoms are minor and common to other illnesses. Although Hepatitis A is easily spread, the virus’s short-term effects, minor symptoms, and easy prevention through vaccinations categorize it as the least dangerous [132].
Hepatitis B is also vaccine-preventable; however, HBV is spread internally through bodily fluids from sexual intercourse or injections with contaminated needles. Symptoms of infection may or may not show, and effects can be short-term or long-term. Tenofovir and entecavir are examples of antivirals used to suppress viral replication and other complications associated with an active outbreak. Unfortunately, there are no cures for this viral infection [133].
Similar to Hepatitis B, HCV individuals can be asymptomatic. HCV also has a slight potential to have short-term effects but mainly leads to life-threatening issues [133]. Many people infected with HBV and HCV do not realize they have the virus and spread it mainly through blood from injections or even sexually through open wounds, making them more dangerous than HAV.
Hepatitis is a global issue, and over 320 million people worldwide are affected with just HBV and HCV alone. These statistics do not include consideration of those living unknowingly with the infection [128]. Not only are individuals living with these illnesses, but hepatitis contributes to a large portion of liver cancer cases, leading to close to 2 million deaths per year just from liver cancer [133]. While current therapies and preventions exist against hepatitis, they lead to undesirable and painful side effects and sometimes ineffective treatments. Therefore, it is essential to continue studying hepatitis viruses to develop and improve vaccinations of this disease to make preventative therapies more effective. Traditionally, plant sources are dependable resources for antivirals for hepatitis infections, as we do not have better treatment plans. Several plant extracts were reported and summarized in Table 9 for their antiviral properties against hepatitis A, B, and C.
Table 9.
Extracts from plants against hepatitis virus.
| S. No. | Plant Name | Plant Extract | Virus | Activity | Ref. |
|---|---|---|---|---|---|
| 1 | Abutilon figarianum | Ethanolic then dichloromethane extract of whole plant | HBV | IC50: 99.76 μg/mL | [134] |
| 2 | Acacia oerfota | Total ethanolic extract of whole plant | HBV | IC50: 101.46 μg/mL | [134] |
| 3 | Alectryon serratus | Ethanolic extract of leaves | HCV | IC50: 9.8 μg/mL | [135] |
| 4 | Alectryon serratus | Chloroform methanolic extract of leaves | HCV | IC50: 1.2 μg/mL | [135] |
| 5 | Alectryon serratus | Chloroform methanolic and water extract of leaves | HCV | IC50: 0.43 μg/mL | [135] |
| 6 | Boerhavia diffusa | Methanolic extract of whole plant | HCV | IC50: 12.5–25 μM | [136] |
| 7 | Capparis decidua | Ethanolic then aqueous extract of the whole plant | HBV | IC50: 66.82 μg/mL | [134] |
| 8 | Coccinea grandis | Total ethanolic extract of whole plant | HBV | IC50: 31.57 μg/mL | [134] |
| 9 | Corallocarpus epigeus | Total ethanolic extract of whole plant | HBV | IC50: 71.9 μg/mL | [134] |
| 10 | Curcuma domestica | Dried powder of rhizomes was extracted with ethanol | HCV | IC50:1.68 μg/mL | [137] |
| 11 | Curcuma heyneana | Dried powder of rhizomes was extracted with ethanol | HCV | IC50: 5.49 μg/mL | [137] |
| 12 | Curcuma xanthorrhiza | Dried powder of rhizomes was extracted with ethanol | HCV | IC50:4.93 μg/mL | [137] |
| 13 | Fumaria parviflora | Ethanolic then hexane extract of the whole plant | HBV | IC50: 35.44 μg/mL | [134] |
| 14 | Glycine max | A fermented extract of defatted soybean meal | HAV | IC50: 27 μg/mL | [138] |
| 15 | Glycine max | A fermented extract of defatted soybean meal with Aspergillus fumigatus F-993 | HAV | IC50: 8.60 μg/mL | [138] |
| 16 | Glycine max | A fermented extract of defatted soybean meal with A. awamori FB-113 | HAV | IC50: 16.88 μg/mL | [138] |
| 17 | Guiera senegalensis | Ethanolic then dichloromethane extract of the whole plant | HBV | IC50: 10.65 μg/mL | [134] |
| 18 | Indigofera caerulea | Methanolic extract of whole plant | HBV | IC50: 73.21 μg/mL | [134] |
| 19 | Juncus maritimus Lam. | Methanolic extract of rhizomes | HCV | Inhibition (%): >50 | [139] |
| 20 | Lentinula edodes | Hot water extraction of mycelia | HCV | IC50: 5 μg/mL | [140] |
| 21 | Limonium sinense | Aqueous extract from underground part of plant | HCV | EC50: 9.71 μg/mL CC50: 343.47 μg/mL |
[141] |
| 22 | Phyllanthus reticulates Poir. | Aqueous and ethanol extracts | HBV | EC50: 0.56μg/mL | [142] |
| 23 | Pinus pinaster | Pine extract from bark | HCV | IC50: 5.78 μg/mL EC50: 4.33 μg/mL |
[143] |
| 24 | Pulicaria crispa | Ethyl acetate extract of whole plant | HBV | IC50: 14.45 μg/mL | [134] |
| 25 | Taraxacum officinale | Methanolic extract of leaves | HCV | Inhibition (%): >65% | [144] |
| 26 | Valeriana wallichii | Methanolic extract of roots | HCV | CC50: 252.2 μg/mL | [145] |
Effective plant extracts against hepatitis infection were further investigated to identify the active component/molecules responsible for the antiviral properties. The list of isolated compounds was summarized in Table 10 with their IC50, EC50, and/or CC50 values.
Table 10.
Compounds isolated from plants with antiviral activity against hepatitis virus.
| S. No. | Plant Name (Part) |
Compound | Virus | Activity | Ref. |
|---|---|---|---|---|---|
| 1 |
Phyllantus acidus (Stem) |
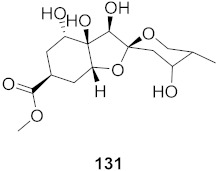
|
HBV | IC50: 11.2 μM | [146] |
| 2 |
Phyllantus acidus (Stem) |
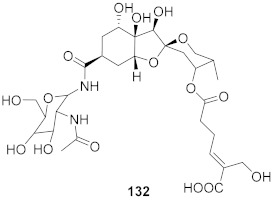
|
HBV | IC50: 57.1 μM | [146] |
| 3 |
Vitis vinifera (Root) |
|
HCV | EC50: 0.006 μM | [147] |
| 4 |
Vitis vinifera (Root) |
|
HCV | EC50: 2.37 μM | [147] |
| 5 |
Wikstroemia chamaedaphne (Buds) |
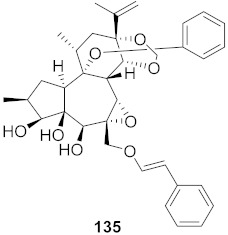
|
HBV | IC50: 46.5 μg/mL | [148] |
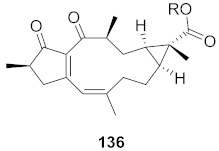
| 6 |
Wikstroemia chamaedaphne (Buds) |
|
HBV | IC50: 88.3 μg/mL | [148] |
| 7 | Multiple Fumaria and Corydalis species from Turkey |
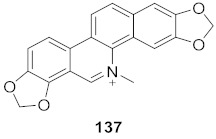
|
HBV | IC50: 15 mg | [149] |
| 8 | Multiple Fumaria and Corydalis species from Turkey |
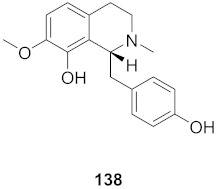
|
HBV | IC50: 23 mg | [149] |
| 9 |
Candida albicans (Root) |
|
HCV | IC50: 0.57 μM/L | [150] |
| 10 |
Cyanara Cardunculus L.var. sylvestris (Lam.) Fiori (Leaves) |
|
HCV | EC50: 0.4–1.4 μM | [151] |
| 11 |
Cyanara Cardunculus L.var. sylvestris (Lam.) Fiori (Leaves) |
|
HCV | EC50: 2.7–14.0 μM | [151] |
| 12 | Green Tea |
|
HBV | CC50: 247.28 μM | [152] |
| 13 | Caulis trachelospermi |
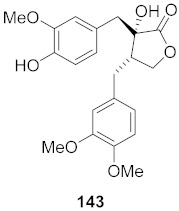
|
HCV | IC50: 0.325 μg/mL | [101] |
| 14 | Selaginella moellendorffii |
|
HBV | IC50: 0.026 μg/mL | [153] |
| 15 | Phyllanthus urinaria |
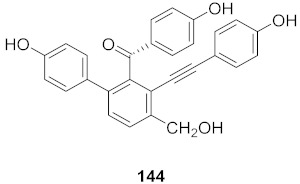
|
HCV | EC50: 2.48 μM | [154] |
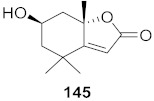
| 16 |
Viola diffusa Ging (Whole plant) |
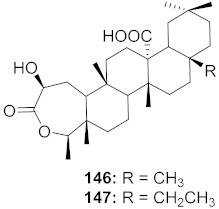
|
HBV |
146: IC50: 26.2 μM 147: IC50: 33.7 μM |
[155] |
| 17 |
Viola diffusa Ging (Whole plant) |
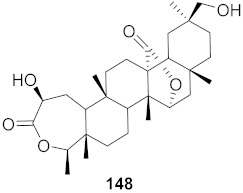
|
HBV | IC50: 104.0 μM | [155] |
| 18 |
Viola diffusa Ging (Whole plant) |
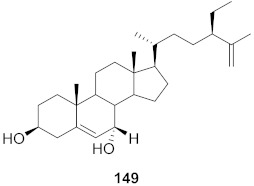
|
HBV | IC50: 62.0 μM | [155] |
| 19 |
Viola diffusa Ging (Whole plant) |
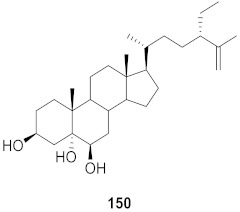
|
HBV | IC50: 32.7 μM | [155] |
| 20 |
Viola diffusa Ging (Whole plant) |
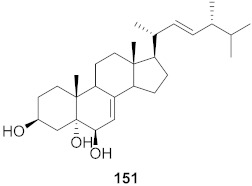
|
HBV | IC50: 112.8 μM | [155] |
| 21 |
Perovskia atriplicifolia (Whole plant) |
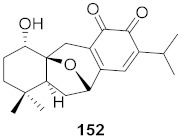
|
HBV | IC50: 1.03 μM | [156] |
| 22 |
Perovskia atriplicifolia (Whole plant) |
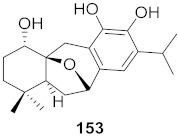
|
HBV | IC50: 0.59 μM | [156] |
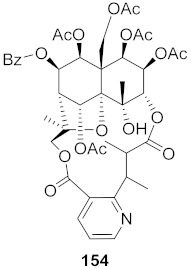
| 23 |
Maytrenus ilicifolia (Root bark) |
|
HCV | EC50: 2.3 μM | [157] |
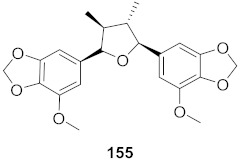
| 24 |
Peperomia blanda (Aerial parts) |
|
HCV | EC50: 4.0 μM | [157] |
| 25 |
Peperomia blanda (Aerial parts) |
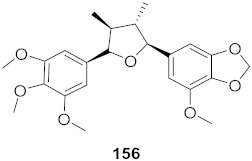
|
HCV | EC50: 8.2 μM | [157] |
| 26 |
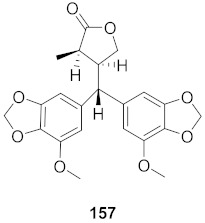
Peperomia blanda (Aerial parts) |
|
HCV | EC50: 38.9 μM | [157] |
| 27 |
Illicium jiadifengpi (Fruits) |
|
HBV | Inhibitory (%): 28.85 | [158] |
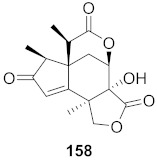
| 28 |
Illicium jiadifengpi (Fruits) |
|
HBV | Inhibitory (%): 37.93 | [158] |
| 29 |
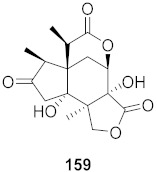
Chloranthus japonicus (Roots) |
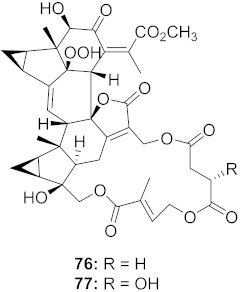
|
HCV |
76: EC50: 3.07 μM 77: EC50: 9.34 μM |
[78] |
| 30 |
Chloranthus japonicus (Roots) |
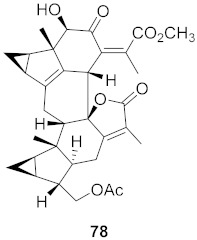
|
HCV | EC50: 1.62 μM | [78] |
| 31 |
Aloe vera (Leaves) |
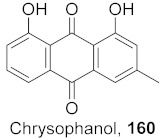
|
HBV | Inhibitory (%): 62 | [159] |
| 32 |
Aloe vera (Leaves) |
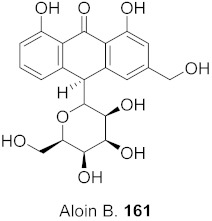
|
HBV | Inhibitory (%): 61 | [159] |
| 33 |
Aloe vera (Leaves) |
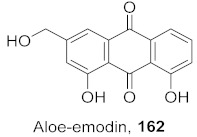
|
HBV | Inhibitory (%): 83 | [159] |
Reference drug: Lamivudine (IC50: 23.50 mM); Plumbagin (IC50: 0.57 µM).
In addition to the above discussed natural products against viruses that affect diverse demographics, there are many other natural products reported against viruses such as HCoV, PRRSV, MNV-1, CV-B, HR3V, RSV, etc., that target more specific demographics. The variation in antiviral activity reflects that plant sources are the treasure of lead compounds for the development of antiviral agents.
Cheng et al. evaluated the antiviral activity of extracts from leaves and twigs of Houttuynia cordata against MNV-1. There were three extracts obtained: the aqueous extract (HWE), the purified polysaccharide from the aqueous extract (HP), and the ethanolic extract (HEE). The plaque assay results showed that HWE had the most potent antiviral activity with the highest selectivity index of 16.14 with HP having a lesser effect and HEE having the lowest antiviral activity. Previous literature identified that the aqueous extract exhibited great antiviral activity and therefore, HP was further studied to determine its mechanism. Structural analysis suggests that HP may be a pectin-like acidic polysaccharide with a 1,4-linked Galp core. Using time- and dosage-dependent studies, HP was found to reduce the residual infectivity after 10 min of incubation. The mechanism was further studied, and HP was found to be responsible for deforming and inflating viral particles per the results of decimal reduction time and transmission electron microscopic studies. Therefore, HP’s antiviral mode of action inhibits viral penetration in target cells [160].
Different parts of the plant Nuphar lutea L., also known as yellow water lily, are used to treat various diseases such as inflammation and pathogen-related diseases. Winer et al. reported the effect of methanolic extract of Nuphar lutea leaves on the measles virus (MV). The antiviral property against MV was quantified by using qRT-PCR and the IC50 value was determined (0.3 μg/mL). The authors also claim the inhibitory activity of the methanolic extract against Respiratory Syncytial Virus (RSV) [161].
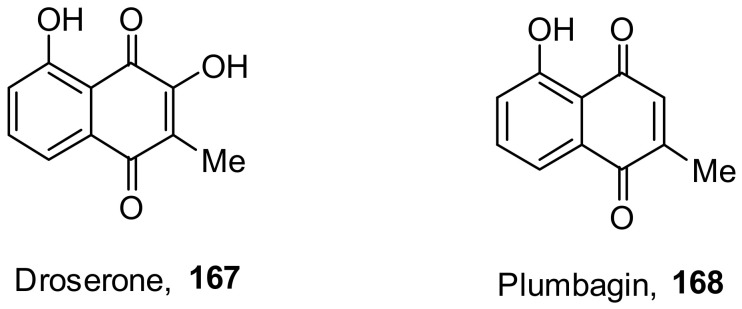
Lieberherr et al. isolated two natural compounds, droserone (167) and plumbagin (168), from Triphyophyllum peltatum (Figure 10). Other structurally similar naphthoquinones were synthesized to determine their antiviral potential against Measle Virus (MV). Infection inhibition and cell viability assays were performed on the compounds. The results showed that droserone had inhibitory activity against MV. Further analysis using the addition of droserone at a different time of the MV cycle found that it must be present during the early stages of infection to inhibit MV. To verify if droserone was acting on the virus or the cells, the cells were preincubated with the compound and then washed, and the absence of droserone showed no significant inhibition. The results suggest that the inhibition of MV is due to interaction with the viral particle. The results from the plaque assay showed that droserone may have interactions with receptor recognition and/or membrane fusion induction processes [162].
Figure 10.
Isolated compounds from Triphyophyllum peltatum.
Porcine reproductive and respiratory syndrome virus (PRRSV) is endemic in most pig-producing countries. The infections because of this virus affect enormous economic losses to the swine industry. Arjin et al. reported the strong inhibition (IC50: 625–1250 μg/mL) of PRRSV replication by the ethanolic extract of the whole plant of Caesalpinia sappan and Tiliacora triandra [163].
Thabti et al. investigated the water and water–alcohol plant extracts of leaves and stem bark of three different species of mulberry—Morus alba var. alba, Morus alba var. rosa, and Morus rubra. The authors observed that the leaves’ water–alcohol extracts exhibited maximum antiviral activity on human coronavirus (67–100% inhibition), while stem bark and leaves’ water and water–alcohol extracts were the most effective on picornaviruses (3–15% inhibition) [164].
Human rotavirus (HRoV) is known as the leading cause of severe gastroenteritis in infants and children under the age of five years. Unfortunately, there is no specific antiviral drug for this virus. Civra et al. reported that the methanolic extract of Rindera lanata (Boraginaceae) showed the most favorable selectivity index with EC50: 25.5 μg/mL. The authors also confirm that the methanolic extract was inactive or barely active against other RNA viruses, namely human rhinovirus and respiratory syncytial virus (RSV) [165].
Coxsackievirus B (CV-B) is a small nonenveloped single-stranded and common enterovirus that produces central nervous system disease as well as various systemic inflammatory diseases. Snene et al. determined the antiviral property of ethyl acetate and methanolic maceration extraction of aerial parts of the plant Daucus virgatus (Poir.) Maire by the plaque reduction assay. The authors claim that ethyl acetate and methanol extracts exhibited significant inhibitory effects against CV-B4 virus with IC50 values of 98.16 and 60.08 μg/mL, respectively. The cytotoxicity study of the crude extracts on the HEp-2cell line indicates moderate toxicity [166].
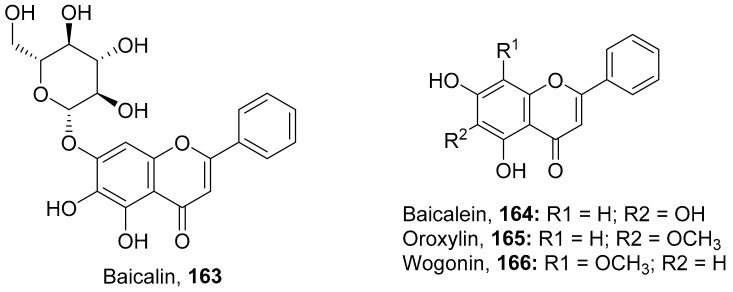
It was hypothesized that the compounds baicalin (163), baicalein (164), oroxylin A (165), and wogonin (166) (Figure 11) of Scutellaria baicalensis extracts (SBE) were capable of interacting with one another to play an effective role against coxsackievirus group B type 3 (CVB3) via various signaling pathways, although this needs further investigation. The data suggest that there was an inhibitory effect on CVB3 viral-induced myocarditis accompanied by a downregulation of the AKT and p38 expressions in viral-infected primary myocardial cells and a viral myocarditis animal model. The results demonstrated that SBE has anti-CVB3 properties both in vitro and in vivo, which are capable of repairing tissue injury and prolong survival in mice with viral myocarditis. However, the exact compounds and the molecular mechanisms by which SBE mediates these antiviral effects against CVB3 remain to be elucidated. From the study, it is heavily indicated that SBE possesses potent antiviral activity with a significant effect on the survival and pathological changes in CVB3-induced myocarditis [167].
Figure 11.
Isolated compounds from Scutellaria baicalensis.
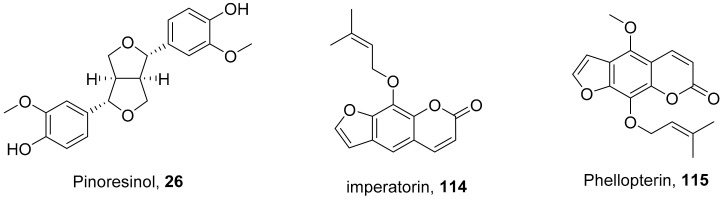
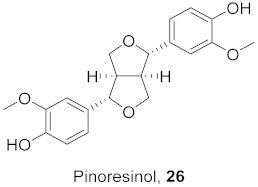
The compound (pinoresinol, 26) isolated from Curcuma aeruginosa, as well as compounds (imperatorin, 114; phellopterin, 115) isolated from Angelica archangelica (Figure 12), also shows antiviral properties against CV-B at IC50 values of 7.1, 15.6, and 3.9 μg/mL [52,124].
Figure 12.
Isolated compounds from Curcuma aeruginosa and Angelica archangelica.
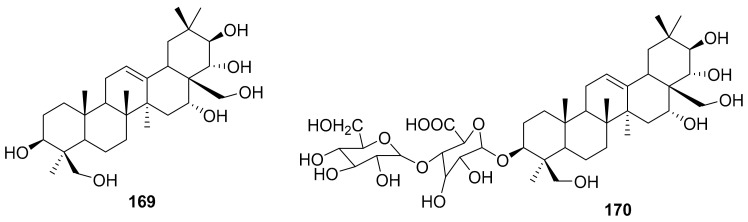
Kim et al. isolated ten oleanane-type triterpenoids from the seeds of Aesculus turbinata (Figure 13). The isolated compounds 169 and 170 showed potential antiviral properties against porcine epidemic diarrhea virus (PEDV). Western blotting showed that compounds 169 and 170 showed significant inhibition of nucleocapsid protein synthesis and inhibition of RNA expression of nucleocapsid and spike when treated in Vero cells at a concentration of 40 μM. Compound 169 was found to show inhibition of the RNA expression in a dose-dependent manner. Compound 169 was the more potent of the isolates; thus, further docking modeling of ARS-CoV 3CLpro (PDB ID code 3V3M) was performed. The docking study resulted in a proposed mechanism of action for 169 as a 3C-Chymotrypsin-Like protease (3CL protease) inhibitor [168].
Figure 13.
Isolated compounds from of Aesculus turbinata.
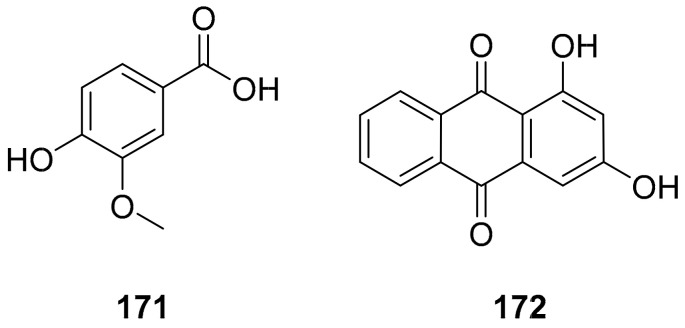
Sun et al. used the aqueous extract from the aerial parts of Rubia cordifolia (RCAP) to explore its antiviral activity against the human rotavirus. Antiviral assays, qPCR, and other techniques were utilized to determine the potency of this extract. At concentrations of 15.63 mg/mL and above, the rotavirus becomes undetectable and accelerates rotavirus-induced apoptosis. The researcher also isolated compounds 171 and 172 (Figure 14) from RCAP; while no formal tests were done, previous research suggests that they may exhibit similar anti-rotavirus activity [169].
Figure 14.
Isolated compounds from Rubia cordifolia.
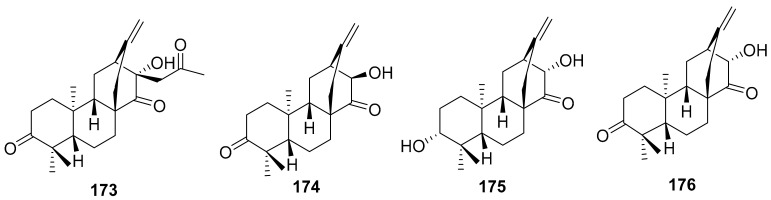
Human rhinovirus (HRV) is one of the most important causative etiological agents of the common cold. Even though upper respiratory infection because of HRV is mild and self-limiting, there are many reports that HRV infection leads to severe medical complications, including asthma aggravation. Wang et al. extracted unusual ent-atisane type diterpenoids with 2-oxopropyl skeleton (173–176) from the roots of Euphorbia ebracteolate (Figure 15) showing antiviral properties against human rhinovirus 3 (HRV3) with an IC50 value range from 25 to 90 μM [170].
Figure 15.
Isolated compounds from Euphorbia ebracteolate.
Respiratory syncytial virus (RSV), an enveloped negative-sense RNA virus, is the most common cause of acute lower respiratory infections in infants and children. Every year, RSV causes millions of hospitalizations and thousands of deaths. Currently, very few drugs are available for the treatment of RSV, and new drug development against RSV is urgently needed. Plant sources are an attractive source for the identification of lead compounds or drug candidates for RSV. Isolated compounds from different plants that show potential antiviral properties against RSV are listed in Table 11.
Table 11.
Compounds isolated from plants with antiviral activity against RSV.
| S. No. | Plant Name (Part) |
Compound | Virus | IC50/EC50 | Ref. |
|---|---|---|---|---|---|
| 1 |
Lilium speciosum var gloriosoides Barker (Bulbs) |
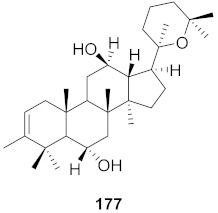
|
RSV | IC50: 2.9 μg/mL | [171] |
| 2 |
Lilium speciosum var gloriosoides Barker (Bulbs) |
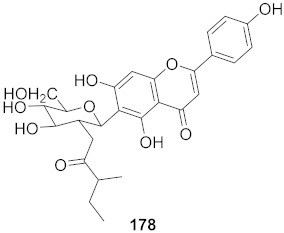
|
RSV | IC50: 2.1 μg/mL | [172] |
| 3 | Erycibe obtusifolia |
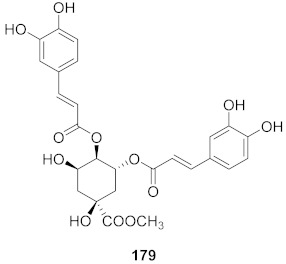
|
RSV A2 | EC50: 0.52 μg/mL | [173] |
| RSV Long | EC50: 0.59 μg/mL |
Reference drug: Ribavirin (EC50: 2.42–2.63 μg/mL).
Ebola virus (EBOV) is a negative-stranded RNA virus, which recently caused the outbreak in West Africa. The outbreak caused more than 28,000 cases and about 11,000 deaths. This epidemic reveals the need of an effective drug candidate for EBOV due to its mutations counteracting innate immune system responses. The EBOV VP35 protein is essential for viral inhibition of IFN production, and the protein is considered as an effective viral target. Petrillo et al. investigated the ethanolic extract of Asphodelus microcarpus for EBOV. The studies indicate that the ethanolic extract significantly reverted the EBOV VP35 inhibition of the vRNA-induced IFN response at concentrations of 3–0.1 μg/mL [174].
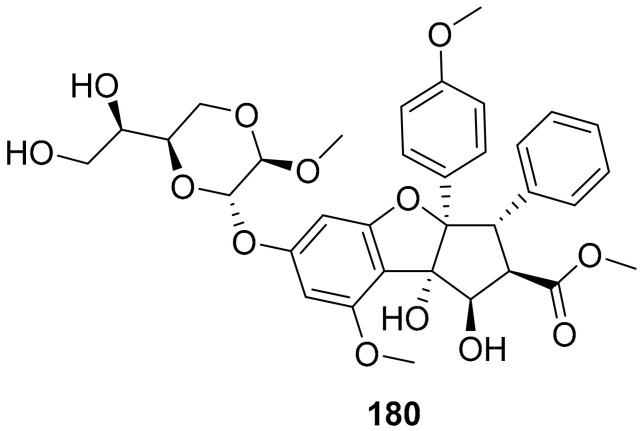
Biedenkopf et al. identified silvestrol (180) (Figure 16) as a potential inhibitor (IC50: 10 nM) of EBOV replication. The authors isolated silvestrol (180) from the plant Aglaia foveolate. Effective silvestrol concentrations were non-toxic in the tested cell systems. Silvestrol could be considered as a potential drug candidate or lead molecule for EBOV [175].
Figure 16.
Structure of silvestrol (180).
In our search for plant extracts and isolated compounds for the antiviral properties against various viruses, we observed that polar extracts (aqueous or methanolic/ethanolic extracts) and polar secondary metabolites of the plant materials showed the most potency. We observed diverse classes of compounds isolated from different parts of various plants. We believe it is difficult to categorize based on chemical diversity. However, the predominant class of secondary metabolites reported for potential antiviral properties are polyphenolic compounds, glycosides, terpenoids, anthraquinones, and coumarins. In addition, most of the reported compounds show antiviral properties are oxygen-rich molecules, including antioxidants.
Many antioxidant molecules slow or stop viral virus replication and show antiviral properties [176]. The cellular injury due to viral infections caused by the over generation of free radicals has been linked to over 200 clinical disorders [177,178]. The overproduction of free radicals that lead to the development of oxidative stress is associated with pathogenic factors in a variety of viral infections [179].
In addition to the biological potential of the molecules, having balanced pharmacokinetic (ADME—Absorption, Distribution, Metabolism, and Excretion) properties of drug-like molecules is one the most difficult and challenging parts of the drug development process. We used a computational software STARDROP to determine the properties such as lipophilicity (logP), human intestine absorption (HIA), blood–brain barrier ability (BBB), hERG inhibition potential (hERG pIC50), rotatable bonds, hydrogen bond donor (HBD), hydrogen bond acceptor (HBA), and molecular weight (MW) [180]. The properties of the most effective natural compounds (included in this manuscript) isolated from different plants are shown in Table 12.
Table 12.
Drug-likeness properties of selected potential natural products.
| Entry | Compound | logP | HIA | BBB | hERG pIC50 | Rotatable Bonds | HBD | HBA | MW |
|---|---|---|---|---|---|---|---|---|---|
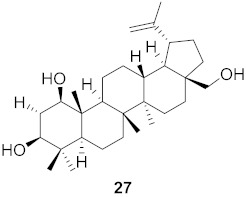
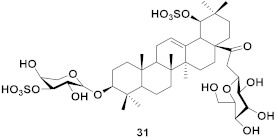
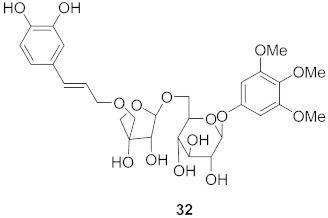
| 1 | 25 | 1.991 | + | - | 3.836 | 8 | 2 | 9 | 494.6 |
| 2 | 31 | 1.129 | - | - | 4.580 | 10 | 8 | 18 | 925.1 |
| 3 | 32 | −0.989 | - | - | 4.561 | 13 | 7 | 15 | 626.6 |
| 4 | 43 | 3.258 | + | - | 5.332 | 7 | 1 | 5 | 360.4 |
| 5 | 44 | 3.635 | + | - | 5.456 | 8 | 1 | 5 | 374.5 |
| 6 | 48 | 2.752 | + | + | 4.855 | 5 | 0 | 4 | 286.3 |
| 7 | 51 | 2.307 | + | + | 4.953 | 4 | 0 | 4 | 260.3 |
| 8 | 54 | 5.480 | + | + | 6.054 | 0 | 0 | 3 | 289.3 |
| 9 | 55 | 7.230 | + | + | 6.668 | 2 | 0 | 3 | 379.5 |
| 10 | 56 | 2.076 | - | - | 4.810 | 6 | 2 | 9 | 404.4 |
| 11 | 57 | 2.270 | - | - | 4.872 | 7 | 1 | 9 | 418.4 |
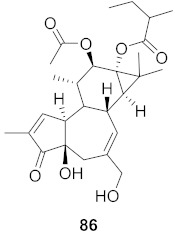
| 12 | 86 | 3.411 | + | - | 4.463 | 7 | 2 | 7 | 474.6 |
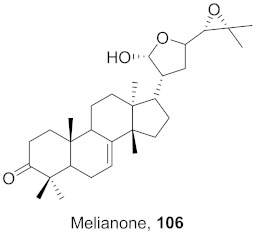
| 13 | 88 | 4.945 | + | - | 5.576 | 14 | 2 | 6 | 578.8 |
| 14 | 97 | 5.158 | + | + | 5.625 | 4 | 0 | 1 | 300.5 |
| 15 | 100 | 4.480 | + | - | 5.680 | 7 | 2 | 5 | 372.5 |
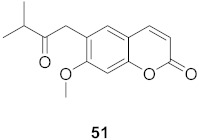
| 16 | 105 | 5.770 | + | - | 5.498 | 5 | 1 | 5 | 554.8 |
| 17 | 107 | 3.564 | + | - | 4.708 | 4 | 2 | 5 | 416.6 |
| 18 | 117 | 4.049 | + | - | 5.981 | 5 | 4 | 9 | 528.5 |
| 19 | 118 | −0.320 | - | - | 4.146 | 5 | 8 | 14 | 564.5 |
| 20 | 119 | −0.504 | - | - | 3.960 | 5 | 9 | 15 | 580.5 |
| 21 | 129 | 0.348 | - | - | 4.587 | 8 | 5 | 10 | 506.5 |
| 22 | 130 | −0.473 | - | - | 4.216 | 9 | 5 | 12 | 482.4 |
| 23 | 133 | 3.561 | + | - | 5.617 | 4 | 5 | 6 | 454.5 |
| 24 | 139 | 2.345 | + | + | 3.865 | 0 | 1 | 3 | 188.2 |
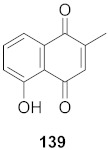
| 25 | 140 | 1.284 | + | - | 3.963 | 4 | 2 | 6 | 346.4 |
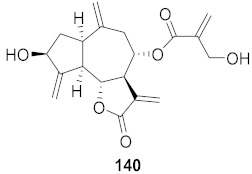
| 26 | 143 | 2.531 | + | - | 4.917 | 7 | 2 | 7 | 388.4 |
| 27 | 144 | 3.605 | + | - | 5.396 | 6 | 4 | 5 | 436.5 |
| 28 | 153 | 3.472 | + | - | 5.208 | 1 | 3 | 4 | 332.4 |
| 29 | 179 | 1.414 | - | - | 4.310 | 10 | 6 | 12 | 530.5 |
| 30 | 180 | 0.821 | + | - | 4.432 | 11 | 4 | 13 | 654.7 |
| 31 | Ribavirin | −1.85 | 3.325 | 3 | 4 | 9 | 244.2 | ||
| 32 | Oseltamivir | 1.767 | + | - | 3.737 | 9 | 2 | 6 | 312.4 |
| 33 | Efavirenz | 4.013 | + | + | 4.992 | 3 | 1 | 3 | 315.7 |
| 34 | Zidovudine | −0.018 | + | - | 4.089 | 3 | 2 | 9 | 267.2 |
| 35 | Prostratin | 1.971 | + | - | 4.365 | 3 | 3 | 6 | 390.5 |
| 36 | Nevaripine | 1.828 | + | - | 4.890 | 1 | 0 | 5 | 300.7 |
| 37 | Honokiol | 4.362 | + | + | 5.358 | 5 | 2 | 2 | 266.3 |
| 38 | Myricetin | 1.303 | - | - | 4.274 | 1 | 6 | 8 | 318.2 |
| 39 | Foscarnet | −1.535 | + | - | 2.667 | 1 | 3 | 5 | 126.0 |
| 40 | Acyclovir | −1.649 | - | - | 4.302 | 4 | 3 | 8 | 225.2 |
| 41 | Lamivudine | −1.036 | + | - | 3.955 | 2 | 2 | 6 | 229.3 |
| 42 | Plumbagin | 2.345 | + | + | 3.865 | 0 | 1 | 3 | 188.2 |
logP: lipophilicity; HIA: human intestine absorption (+ = can absorb through intestine, - = cannot absorb through intestine); BBB: blood–brain barrier (+ = can cross BBB, - = cannot cross BBB); hERG pIC50: hERG (human ether-a-go-go-related gene) activity (pIC50); HBD: hydrogen bond donor; HBA: hydrogen bond acceptor; MW: molecular weight.
Among the potent compounds, most of them follow the “Rule of Five” with some violations. The hERG pIC50 values are also important to consider, since these values indicate possible cardiac toxicity (especially compounds with >5 hERG pIC50 value). We believe this compiled information, including the drug-like properties, will enrich the process of developing new potential antiviral drug candidates.
3. Methodology
The references considered for this review article were retrieved from PubMed, SciFinder, Springer, ScienceDirect, ACS, Google Scholar, and Wiley databases from 2015 to 2020, and the search keywords used antiviral combined with natural products and further filter by the plant(s). Both plant extracts and isolated compounds along with their IC50 and/or EC50 values were reported in this review. We also used the terms viruses, plants, H1N1, HIV, HSV, phytochemical, etc. to identify missing relevant articles to include in the review. The search strategy identified 1319 publications, and 102 references were excluded for duplication. We have also searched current clinical trials on natural products from plant source as potential therapy for viral infection using www.clinicaltrials.gov (accessed on 6 October 2021). Currently, there are 52 studies being conducted; however, most of the studies are preventive treatments (using heparin and vitamin C).
4. Conclusions
As viruses become more prevalent around the world, it is important to continue to look for new and improved antiviral drugs. Based on the extensive research efforts from 2015, there are a plethora of plant resources that show potential antiviral properties against various strains of the epidemic and pandemic-causing viruses. This review shows antiviral activity against the pandemic and epidemic-causing viruses: avian influenza A (H5N1 and H1N1), Ebola virus, and SARS-CoV-2. These viruses and others are responsible for the death of millions, warranting an expanded research effort into avenues that are not normally taken, such as natural resources. We have found that most polar components of the plants show antiviral properties. The secondary metabolites reported for antiviral properties are in the class of coumarins, polyphenolics, glycosides, and terpenoids. Modes of actions of the isolated compounds from plant sources may provide insight for the design of novel derivatives that show potent antiviral activity. Overall, this extensive review may serve as inspiration for the development of novel drug candidates that take advantage of the unique and diverse chemical structures of isolated compounds and extracts from plant sources, including those against drug-resistant and vaccine immunity escaping viral strains.
Acknowledgments
We thank the Department of Chemistry & Physics, Augusta University and CSM Student Research Funding at Augusta University for financial support.
Abbreviations
| 229E | Human coronavirus |
| AV | Adenovirus |
| B/Lee/40 | Influenza B/Lee/40 |
| CHIKV | Chikungunya virus |
| CV-B | Coxsackievirus B |
| CV | Coxsackieviruses |
| CyHV-3 | Cyprinid herpesvirus 3 |
| DENV | Dengue virus |
| EBV | Epstein–Barr virus |
| EHV-1 | Equid herpesvirus 1 |
| EV | Ebola virus |
| FV | Flavivirus |
| H1N1 | Influenza virus |
| H3N2 | Influenza A/Victoria virus |
| H5N1 | Avian influenza virus |
| H9N2 | Novel Reassortant avian influenza A virus |
| HAV | Hepatitis A Virus |
| HBV | Hepatitis B Virus |
| HCoV 229E | Human coronavirus |
| HCV | Hepatitis C virus |
| HIV-1 | Human immunodeficiency virus 1 |
| HIV-2 | Human immunodeficiency virus 2 |
| HIV | Human immunodeficiency virus |
| HR3V | Human rhinovirus 3 virus |
| HRoV | Human rotavirus |
| HSV | Herpes simplex viruses |
| HRV | Human rhino virus |
| HSV-1 | Herpes simplex viruses 1 |
| HSV-2 | Herpes simplex viruses 2 |
| HuNoVs | Human noroviruses |
| MV | Measles virus |
| MNV-1 | Murine Norovirus-1 |
| NDV | Newcastle disease virus |
| POV | Poliovirus |
| PRRSV | Porcine reproductive and respiratory syndrome virus |
| PRV | Pseudorabies Virus |
| PV | Pestivirus |
| PV1 | Picornavirus |
| RSV | Respiratory syncytial virus |
| SARS-CoV-2 | COVID19 |
| SFV | Semliki forest virus |
| SINV | Sindbis virus |
| SuHV-1 | Suid herpesvirus 1 |
| TMV | Tobacco mosaic virus |
| VSV | Vesicular stomatitis virus |
| VV | Vaccinia virus |
| WNV | West Nile virus |
| WSSV | White spot syndrome virus |
| YFV | Yellow fever virus |
| ZIKV | Zika virus |
Author Contributions
Conceptualization, S.S.P.; methodology, E.T., L.E.S., B.A.D., A.M.P., I.E. and S.S.P.; software, S.S.P.; validation, E.T., L.E.S. and S.S.P.; investigation, E.T., L.E.S., B.A.D., A.M.P., I.E. and S.S.P.; resources, S.S.P.; writing—original draft preparation, E.T., L.E.S., B.A.D., A.M.P., I.E. and S.S.P.; writing—review and editing, E.T., L.E.S., B.A.D., A.M.P., I.E. and S.S.P.; supervision, S.S.P.; project administration, S.S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lorizate M., Krausslich H.-G. Role of lipids in virus replication. Cold Spring Harb. Perspect. Biol. 2011;3:a004820. doi: 10.1101/cshperspect.a004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattio L.M., Catinella G., Pinto A., Dallavalle S. Natural and nature-inspired stilbenoids as antiviral agents. Eur. J. Med. Chem. 2020;202:112541. doi: 10.1016/j.ejmech.2020.112541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aleksandr I., Eva Z., Suvi K., Marten S., Hilde L., Mona T., Laura K., Henrik P., Mira L., Hannimari K.K., et al. Novel ac-tivities of safe-in-human broad-spectrum antiviral agents. Antivir. Res. 2018;154:174–182. doi: 10.1016/j.antiviral.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO WHO Publishes List of Top Emerging Diseases Likely to Cause Major Epidemics. [(accessed on 15 December 2020)]. Available online: https://www.who.int/medicines/ebola-treatment/WHO-list-oftop-emerging-diseases/en/
- 5.Guangxiang L., Shou-Jiang G. Global health concerns by emerging viral infections. J Med. Virol. 2020;92:399–400. doi: 10.1002/jmv.25683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M., Lei Y. Antiviral Effects of Plant-Derived Essential Oils and Their Compounds: An Updated Review. Molecules. 2020;25:2627. doi: 10.3390/molecules25112627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Divyabharathi M., Ashish W., Praveen T.K. Drug Repurposing in Antiviral Research: A Current Scenario. J. Young Pharm. 2019;11:117–121. [Google Scholar]
- 8.Daisuke A., Hideki N. Pathogenic Viruses Commonly Present in the Oral Cavity and Relevant Antiviral Compounds De-rived from Natural Products. Medicines. 2018;5:120. doi: 10.3390/medicines5040120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark A.M. Natural Products as a Resource for New Drugs. Pharm. Res. 1996;13:1133–1141. doi: 10.1023/A:1016091631721. [DOI] [PubMed] [Google Scholar]
- 10.Lin L.-T., Hsu W.-C., Lin C.-C. Antiviral Natural Products and Herbal Medicines. J. Tradit. Complement. Med. 2014;4:24–35. doi: 10.4103/2225-4110.124335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Sayed K.A. Natural Products as Antiviral Agents. Stereoselective Synth. (Part K) 2000;24:473–572. doi: 10.1016/s1572-5995(00)80051-4. [DOI] [Google Scholar]
- 12.Goh V.S.L., Mok C.-K., Chu J.J.H. Antiviral Natural Products for Arbovirus Infections. Molecules. 2020;25:2796. doi: 10.3390/molecules25122796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chojnacka K., Witek-Krowiak A., Skrzypczak D., Mikula K., Młynarz P. Phytochemicals containing biologically active polyphenols as an effective agent against Covid-19-inducing coronavirus. J. Funct. Foods. 2020;73:104146. doi: 10.1016/j.jff.2020.104146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riccio G., Ruocco N., Mutalipassi M., Constantini M., Zupo V., Coppola D., de Pascale D., Lauritano C. Ten-Year Re-search Update Review: Antiviral Activities from Marine Organisms. Biomolecules. 2020;10:2627. doi: 10.3390/biom10071007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teixeira R.R., Pereira W.L., da Silveira Oliveira A.F.C., da Silva A.M., de Oliveira A.S., da Silva M.L., da Silva C.C., de Paula S.O. Natural products as source of potential dengue antivirals. Molecules. 2014;19:8151–8176. doi: 10.3390/molecules19068151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2014. J. Nat. Prod. 2014;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 17.Mohan S., Taha M.M.E., Makeen H.A., Alhazmi H.A., Al Bratty M., Sultana S., Ahsan W., Najmi A., Khalid A. Bioactive natural antivirals: An updated review of the available plants and isolated molecules. Molecules. 2020;25:4878. doi: 10.3390/molecules25214878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar A., Singh A.K., Tripathi G. Phytochemicals as Potential Curative Agents against Viral Infection: A Review. Curr. Org. Chem. 2020;24:2356–2366. doi: 10.2174/1385272824999200910093524. [DOI] [Google Scholar]
- 19.Remali J., Aizat W.M. A Review on Plant Bioactive Compounds and Their Modes of Action against Coronavirus Infection. Front. Pharmacol. 2021;11:589044. doi: 10.3389/fphar.2020.589044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ben-Shabat S., Yarmolinsky L., Porat D., Dahan A. Antiviral effect of phytochemicals from medicinal plants: Applications and drug delivery strategies. Drug Deliv. Transl. Res. 2020;10:354–367. doi: 10.1007/s13346-019-00691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Attah A.F., Fagbemi A.A., Olubiyi O., Dada-Adegbola H., Oluwadotun A., Elujoba A., Babalola C.P. Therapeutic Potentials of Antiviral Plants Used in Traditional African Medicine With COVID-19 in Focus: A Nigerian Perspective. Front. Pharmacol. 2021;11:589044. doi: 10.3389/fphar.2021.596855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sasidharan S., Chen Y., Saravanan D., Sundram K.M., Latha L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. AJTCAM. 2011;8:1–10. doi: 10.4314/ajtcam.v8i1.60483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q.-W., Lin L.-G., Ye W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018;13:1–26. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kellogg J.J., Paine M.F., McCune J.S., Oberlies N.H., Cech N.B. Selection and characterization of botanical natural products for research studies: A NaPDI center recommended approach. Nat. Prod. Rep. 2019;36:1196–1221. doi: 10.1039/C8NP00065D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CDC Key Facts about Influenza (Flu) [(accessed on 15 December 2020)]; Available online: https://www.cdc.gov/flu/about/keyfacts.htm.
- 26.CDC Influenza (Flu) Types of Iinfluenza Viruses, Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD) [(accessed on 20 September 2020)]; Available online: https://www.cdc.gov/flu/about/viruses/types.htm.
- 27.CDC Influenza (Flu) 2009 H1N1 Pandemic Timeline. [(accessed on 15 December 2020)]; Available online: https://www.cdc.gov/flu/pandemic-resources/2009-pandemic-timeline.html.
- 28.Nabel G.J., Fauci A.S. Induction of unnatural immunity: Prospects for a broadly protective universal influenza vaccine. Nat. Med. 2010;16:1389–1391. doi: 10.1038/nm1210-1389. [DOI] [PubMed] [Google Scholar]
- 29.Gondim A.C.S., da Silva S.R., Mathys L., Noppen S., Liekens S., Sampaio A.H., Nagano C.S., Rocha C.R.C., Nascimento K.S., Cavada B.S., et al. Potent antiviral activity of carbohydrate-specific algal and leguminous lectins from the Brazilian biodiversity. MedChemComm. 2019;10:390–398. doi: 10.1039/C8MD00508G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y.T., Zhou B., Lu J., Chen Q.L., Ti H., Huang W.Y., Li J., Yang Z.F., Jiang Z., Wang X.H. Inhibition of influenza virus via a sesquiterpene fraction isolated from Laggera pterodonta by targeting the NF-κB and p38 pathways. BMC Complement. Altern. Med. 2017;17:25. doi: 10.1186/s12906-016-1528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu C.-H., Yu W.-Y., Fang J., Zhang H.-H., Ma Y., Yu B., Wu F., Wu X.-N. Mosla scabra flavonoids ameliorate the influenza A virus-induced lung injury and water transport abnormality via the inhibition of PRR and AQP signaling pathways in mice. J. Ethnopharmacol. 2016;179:146–155. doi: 10.1016/j.jep.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 32.Chavan R.D., Shinde P., Girkar K., Madage R., Chowdhary A. Assessment of Anti-Influenza Activity and Hemagglutina-tion Inhibition of Plumbago indica and Allium sativum Extracts. Pharmacogn. Res. 2016;8:105–111. doi: 10.4103/0974-8490.172562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makau J.N., Watanabe K., Mohammed M.M., Nishida N. Antiviral Activity of Peanut (Arachis hypogaea L.) Skin Extract against Human Influenza Viruses. J. Med. Food. 2018;21:777–784. doi: 10.1089/jmf.2017.4121. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L., Chen J., Ke C., Zhang H., Zhang S., Tang W., Liu C., Liu G., Chen S., Hu A., et al. Ethanol extract of Caesal-pinia decapetala inhibits influenza virus infection in vitro and in vivo. Viruses. 2020;12:557. doi: 10.3390/v12050557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J.-F., He W.-J., Zhang X.-X., Zhao B.-Q., Liu Y.-H., Zhou X.-J. Dicarabrol, a new dimeric sesquiterpene from Carpesium abrotanoides L. Bioorg. Med. Chem. Lett. 2015;25:4082–4084. doi: 10.1016/j.bmcl.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 36.Enkhtaivan G., John K.M., Ayyanar M., Sekar T., Jin K.-J., Kim D.H. Anti-influenza (H1N1) potential of leaf and stem bark extracts of selected medicinal plants of South India. Saudi J. Biol. Sci. 2015;22:532–538. doi: 10.1016/j.sjbs.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hossan S., Fatima A., Rahmatullah M., Khoo T.J., Nissapatorn V., Galochkina A.V., Slita A.V., Shtro A.A., Nikolaeva Y., Zarubaev V.V., et al. Antiviral activity of Embelia ribes Burm. f. against influenza virus in vitro. Arch. Virol. 2018;163:2121–2131. doi: 10.1007/s00705-018-3842-6. [DOI] [PubMed] [Google Scholar]
- 38.Enkhtaivan G., John K.M., Pandurangan M., Hur J.H., Leutou A.S., Kim D.H. Extreme effects of Seabuckthorn extracts on influenza viruses and human cancer cells and correlation between flavonol glycosides and biological activities of extracts. Saudi J. Biol. Sci. 2017;24:1646–1656. doi: 10.1016/j.sjbs.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker D.H.A., Ibrahim E.A., Kandeil A., El Baz F.K. Sterols Bioactivity of Ruta graveolens L. and Murraya paniculata L. Int. J. Pharm. Pharm. Sci. 2017;9:103–108. doi: 10.22159/ijpps.2017v9i2.15790. [DOI] [Google Scholar]
- 40.Priya N.C., Kumari P.S. Antiviral activities and cytotoxicity assay of seed extracts of Piper longum and Piper nigrum on human cell lines. Int. J. Pharm. Sci. Rev. Res. 2017;44:197–202. [Google Scholar]
- 41.Tran T.T., Kim M., Jang Y., Lee H.W., Nguyen H.T., Nguyen T.N., Park H.W., Le Dang Q., Kim J.-C. Characterization and mechanisms of anti-influenza virus metabolites isolated from the Vietnamese medicinal plant Polygonum chinense. BMC Complement. Altern. Med. 2017;17:162. doi: 10.1186/s12906-017-1675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heo Y., Cho Y., Ju K.S., Cho H., Park K.H., Choi H., Yoon J.K., Moon C., Kim Y.B. Antiviral activity of Poncirus trifo-liata seed extract against oseltamivir-resistant influenza virus. J. Microbiol. 2018;56:586–592. doi: 10.1007/s12275-018-8222-0. [DOI] [PubMed] [Google Scholar]
- 43.Choi J.-G., Jin Y.-H., Kim J.-H., Oh T.W., Yim N.-H., Cho W.-K., Ma J.Y. In vitro Anti-viral Activity of Psoraleae Semen Water Extract against Influenza A Viruses. Front. Pharmacol. 2016;7:460. doi: 10.3389/fphar.2016.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Z.-F., Hu P., Zhong S., Chen Y., Li L., Li X.-Z., Li C., Li J., Wang Y.-T. Extraction and Separation of Polysaccharides from Radix isatidis and their Effects towards Hemagglutinin Protein of Influenza Virus. Asian J. Chem. 2015;27:1615–1620. doi: 10.14233/ajchem.2015.17394. [DOI] [Google Scholar]
- 45.Romero-Perez G., Egashira M., Harada Y., Tsuruta T., Oda Y., Ueda F., Tsukahara T., Tsukamota Y., Inoue R. Orally ad-ministered Salacia reticulata extract reduces h1n1 influenza clinical symptoms in Murine lung Tissues Putatively Due to enhanced natural Killer cell activity. Front. Immunol. 2016;7:115–125. doi: 10.3389/fimmu.2016.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nie L.-X., Wu Y.-L., Dai Z., Ma S.-C. Antiviral activity of Isatidis Radix derived glucosinolate isomers and their breakdown products against influenza A in vitro/ovo and mechanism of action. J. Ethnopharmacol. 2020;251:112550. doi: 10.1016/j.jep.2020.112550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi Y., Zhang B., Lu Y., Qian C., Feng Y., Fang L., Ding Z., Cheng D. Antiviral activity of phenanthrenes from the me-dicinal plant bletilla striata against influenza a virus. BMC Complement. Altern. Med. 2017;17:273. doi: 10.1186/s12906-017-1780-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Law A.H.-Y., Yang C.L.-H., Lau A.S.-Y., Chan G.C.-F. Antiviral effect of forsythoside A from Forsythia suspensa (Thunb.) Vahl fruit against influenza A virus through reduction of viral M1 protein. J. Ethnopharmacol. 2017;209:236–247. doi: 10.1016/j.jep.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 49.Fanhchaksai K., Kodchakorn K., Pothacharoen P., Kongtawalert P. Effect of sesamin against cytokine production from influenza typeA H1N1-induced peripheral blood mononuclear cells:computational and experimental studies. In Vitro Cell. Dev. Biol. Anim. 2016;52:107–119. doi: 10.1007/s11626-015-9950-7. [DOI] [PubMed] [Google Scholar]
- 50.Zhao L., Xiang K.-L., Liu R.-X., Xie Z.-P., Zhang S.-M., Dai S.-J. Anti-inflammatory and anti-viral labdane diterpenoids from the fruits of Forsythia suspensa. Bioorg. Chem. 2020;96:103651. doi: 10.1016/j.bioorg.2020.103651. [DOI] [PubMed] [Google Scholar]
- 51.Tan Y.P., Xue Y., Savchenko A.I., Houston S.D., Modhiran N., McMillan C.L.D., Boyle G.M., Bernhardt P.V., Young P.R., Watterson D., et al. Basimarols A, B, and C, Highly Oxygenated Pimarane Diterpenoids from Basilicum polystachyon. J. Nat. Prod. 2019;82:2828–2834. doi: 10.1021/acs.jnatprod.9b00522. [DOI] [PubMed] [Google Scholar]
- 52.Azman N.S.N., Hossan S., Nissapatorn V., Uthaipibull C., Prommana P., Jin K.T., Rahmatullah M., Mahboob T., Raju C.S., Jindal H.M., et al. Anti-infective activities of 11 plants species used in traditional medicine in Malaysia. Exp. Parasitol. 2018;194:67–78. doi: 10.1016/j.exppara.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 53.Gong K.-K., Li P.-L., Qiao D., Zhang X.-W., Chu M.-J., Qin G.-F., Tang X.-L., Li G.-Q. Cytotoxic and antiviral triterpenoids from the mangrove plant Sonneratia paracaseolaris. Molecules. 2017;22:1319. doi: 10.3390/molecules22081319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bang S., Ha T.K.Q., Lee C., Li W., Oh W.-K., Shim S.H. Antiviral activities of compounds from aerial parts of Salvia plebeia R. Br. J. Ethnopharmacol. 2016;192:398–405. doi: 10.1016/j.jep.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 55.Peng M.-H., Dai W.-P., Liu S.-J., Yu L.-W., Wu Y.-N., Liu R., Chen X.-L., Lai X.-P., Li X., Zhao Z.-X., et al. Bioactive glycosides from the roots ofIlex asprella. Pharm. Biol. 2016;54:2127–2134. doi: 10.3109/13880209.2016.1146779. [DOI] [PubMed] [Google Scholar]
- 56.Hu C.-L., Xiong J., Li J.-Y., Gao L.-X., Wang W.-X., Cheng K.-J., Yang G.-X., Li J., Hu J.-F. ChemInform Abstract: Rare Sesquiterpenoids from the Shed Trunk Barks of the Critically Endangered Plant Abies beshanzuensis and Their Bioactivities. Eur. J. Org. Chem. 2016;47:1832–1835. doi: 10.1002/ejoc.201600165. [DOI] [Google Scholar]
- 57.Ha T.K.Q., Dao T.T., Nguyen N.H., Kim J., Kim E., Cho T.O., Oh W.K. Antiviral phenolics from the leaves of Cleistocalyx operculatus. Fitoterapia. 2016;110:135–141. doi: 10.1016/j.fitote.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 58.Dao N.T., Jang Y., Kim M., Nguyen H.H., Pham D.Q., Le Dang Q., Van Nguyen M., Yun B.-S., Pham Q.M., Kim J.-C., et al. Chemical Constituents and Anti-influenza Viral Activity of the Leaves of Vietnamese Plant Elaeocarpus tonkinensis. Rec. Nat. Prod. 2018;13:71–80. doi: 10.25135/rnp.76.18.04.266. [DOI] [Google Scholar]
- 59.UNAIDS UNAIDS Fact Sheet. [(accessed on 15 December 2020)]. Available online: https://www.unaids.org/en/resources/fact-sheet.
- 60.Arts E.J., Hazuda D.J. HIV-1 Antiretroviral Drug Therapy. Cold Spring Harb. Perspect. Med. 2012;2:a007161. doi: 10.1101/cshperspect.a007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cary D.C., Peterlin B.M. Natural Products and HIV/AIDS. AIDS Res. Hum. Retrovir. 2018;34:31–38. doi: 10.1089/aid.2017.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ticona L.A., Bermejo P., Guerra J.A., Abad M.J., Beltran M., Lazaro R.M., Alcami J., Bedoya L.M. Ethanolic extract of Artemisia campestris subsp. glutinosa (Besser) Batt. inhibits HIV-1 replication in vitro through the activity of terpenes and flavonoids on viral entry and NF-κB pathway. J. Ethnopharmacol. 2020;263:113163. doi: 10.1016/j.jep.2020.113163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tietjen I., Gatonye T., Ngwenya B.N., Namushe A., Simonambanga S., Muzila M., Mwimanzi P., Xiao J., Fedida D., Brumme Z., et al. Croton megalobotrys Müll Arg. and Vitex doniana (Sweet): Traditional medicinal plants in a three-step treatment regimen that inhibit in vitro replication of HIV-1. J. Ethnopharmacol. 2016;191:331–340. doi: 10.1016/j.jep.2016.06.040. [DOI] [PubMed] [Google Scholar]
- 64.Sanna G., Farci P., Busonera B., Murgia G., La Colla P., Giliberti G. Antiviral properties from plants of the Mediterranean flora. Nat. Prod. Res. 2015;29:2065–2070. doi: 10.1080/14786419.2014.1003187. [DOI] [PubMed] [Google Scholar]
- 65.Gurrapu S., Mamidala E. In vitro HIV-1 reverse transcriptase inhibition by alkaloids isolated from leaves of eclipta alba. Int. Res. J. Pharm. 2018;9:66–70. doi: 10.7897/2230-8407.09110. [DOI] [Google Scholar]
- 66.Liu O., Li W., Huang L., Asada Y., Morris-Natschke S.L., Chen C., Lee K., Koike K. Identification, structural modification, and dichotomous effects on human immunodeficiency virus type 1 (HIV-1) replication of ingenane esters from Euphorbia kansui. Eur. J. Med. Chem. 2018;156:618–627. doi: 10.1016/j.ejmech.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar N., Paul K.S. Phytochemistry and medicinal value of Harad (Terminalia chebula Retz.) the ‘king of medicinal plants. Pharma Chem. 2018;10:186–195. [Google Scholar]
- 68.Chen H., Zhang R., Luo R.-H., Yang L.-M., Wang R.-R., Hao X.-J., Zheng Y.-T. Anti-HIV Activities and Mechanism of 12-O-Tricosanoylphorbol-20-acetate, a Novel Phorbol Ester from Ostodes katharinae. Molecules. 2017;22:1498. doi: 10.3390/molecules22091498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang J.-H., Wang X.-Y., Zhou Y.-P., Lu R., Chen C.-H., Zhang M.-H., Cheng Y.-Y., Morris-Natschke S.L., Lee K.-H., Wang Y.-S. Carbazole Alkaloids from Clausena anisum-olens: Isolation, Characterization, and Anti-HIV Evaluation. Molecules. 2020;25:99. doi: 10.3390/molecules25010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Y.-P., Yan G., Guo J.-M., Liu Y.-Y., Li Y.-J., Zhao Y.-Y., Qiang L., Fu Y.-H. Prenylated Coumarins from the Fruits of Manilkara zapota with Potential Anti-inflammatory Effects and Anti-HIV Activities. J. Agric. Food Chem. 2019;67:11942–11947. doi: 10.1021/acs.jafc.9b04326. [DOI] [PubMed] [Google Scholar]
- 71.Nothias L.-F., Boutet-Mercey S., Cachet X., De La Torre E., Laboureur L., Gallard J.-F., Retailleau P., Brunelle A., Dorrestein P.C., Costa J., et al. Environmentally Friendly Procedure Based on Supercritical Fluid Chromatography and Tandem Mass Spectrometry Molecular Networking for the Discovery of Potent Antiviral Compounds from Euphorbia semiperfoliata. J. Nat. Prod. 2017;80:2620–2629. doi: 10.1021/acs.jnatprod.7b00113. [DOI] [PubMed] [Google Scholar]
- 72.Zhang D., Guo J., Zhang M., Liu X., Ba M., Tao X., Yu L., Guo Y., Dai J. Oxazole-containing diterpenoids from cell cultures of Salvia miltiorrhiza and their anti-HIV-1 activities. J. Nat. Prod. 2017;80:3241–3246. doi: 10.1021/acs.jnatprod.7b00659. [DOI] [PubMed] [Google Scholar]
- 73.Ortega J.T., Serrano M.L., Suárez A.I., Baptista J., Pujol F.H., Rangel H.R. Methoxyflavones from Marcetia taxifolia as HIV-1 Reverse Transcriptase Inhibitors. Nat. Prod. Commun. 2017;12:1677–1680. doi: 10.1177/1934578X1701201104. [DOI] [Google Scholar]
- 74.Zhang H.-J., Rumschlag-Booms E., Guan Y.-F., Liu K.-L., Wang D.-Y., Li W.-F., Nguyen V.H., Cuong N.M., Soejarto D.D., Fong H.H.S., et al. Anti-HIV diphyllin glycosides from Justicia gendarussa. Phytochemistry. 2017;136:94–100. doi: 10.1016/j.phytochem.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 75.Zhang H.-J., Rumschlag-Booms E., Guan Y.-F., Wang D.-Y., Liu K.-L., Li W.-F., Nguyen V.H., Cuong N.M., Soejarto D.D., Fong H.H.S., et al. Potent Inhibitor of Drug-Resistant HIV-1 Strains Identified from the Medicinal Plant Justicia gendarussa. J. Nat. Prod. 2017;80:1798–1807. doi: 10.1021/acs.jnatprod.7b00004. [DOI] [PubMed] [Google Scholar]
- 76.Esposito F., Carli I., DEL Vecchio C., Xu L., Corona A., Grandi N., Piano D., Maccioni E., Distinto S., Parolin C., et al. Sennoside A, derived from the traditional chinese medicine plant Rheum L., is a new dual HIV-1 inhibitor effective on HIV-1 replication. Phytomedicine. 2016;23:1383–1391. doi: 10.1016/j.phymed.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 77.Chao C.-H., Cheng J.-C., Shen D.-Y., Huang H.-C., Wu Y.-C., Wu T.-S. 13-Methyl-3,4-seco-ent-podocarpanes, rare C18-diterpenoids from the roots of Flueggea virosa. RSC Adv. 2016;6:34708–34714. doi: 10.1039/C6RA00843G. [DOI] [Google Scholar]
- 78.Yan H., Ba M.-Y., Li X.-H., Guo J.-M., Qin X.-J., He L., Zhang Z.-Q., Guo Y., Liu H.-Y. Lindenane sesquiterpenoid dimers from Chloranthus japonicus inhibit HIV-1 and HCV replication. Fitoterapia. 2016;115:64–68. doi: 10.1016/j.fitote.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 79.Win N.N., Ito T., Matsui T., Aimaiti S., Kodama T., Ngwe H., Okamota Y., Tanaka M., Asakawa Y., Abe I., et al. Isopi-marane diterpenoids from Kaempferia pulchrarhizomes collected in Myanmar and their Vpr inhibitory activity. Bioorg. Med. Chem. Lett. 2016;26:1789–1793. doi: 10.1016/j.bmcl.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 80.Olivon F., Palenzuela H., Girard-Valenciennes E., Neyts J., Pannecouque C., Roussi F., Grondin I., Leyssen P., Litaudon M. Antiviral Activity of Flexibilane and Tigliane Diterpenoids from Stillingia lineata. J. Nat. Prod. 2015;78:1119–1128. doi: 10.1021/acs.jnatprod.5b00116. [DOI] [PubMed] [Google Scholar]
- 81.Tamayose C.I., Torres P.B., Roque N., Ferreira M.J.P. HIV-1 reverse transcriptase inhibitory activity of flavones and chlorogenic acid derivatives from Moquiniastrum floribundum (Asteraceae) S. Afr. J. Bot. 2019;123:142–146. doi: 10.1016/j.sajb.2019.02.005. [DOI] [Google Scholar]
- 82.CDC About Dengue: What You Need to Know. [(accessed on 15 December 2020)]; Available online: https://www.cdc.gov/dengue/about/index.html.
- 83.CDC Dengue Vaccine. [(accessed on 15 December 2020)]; Available online: https://www.cdc.gov/dengue/prevention/dengue-vaccine.html.
- 84.Dighe S.N., Ekwudu O., Dua K., Chellappan D.K., Katavic P.L., Collet T.A. Recent update on anti-dengue drug discovery. Eur. J. Med. Chem. 2019;176:431–455. doi: 10.1016/j.ejmech.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 85.Angelina M., Hanafi M., Suyatna F.D., Dewi B.E. Drug of Action Cassia Alata Leaves Extract as Antiviral to Dengue Virus Serotype-2 In vitro. Pharmacogn. J. 2020;12:864. doi: 10.5530/pj.2020.12.124. [DOI] [Google Scholar]
- 86.CDC West Nile Virus. [(accessed on 15 December 2020)]; Available online: https://www.cdc.gov/westnile/index.html.
- 87.Acharya D., Bai F. An Overview of Current Approaches toward the Treatment and Prevention of West Nile Virus Infection. Methods Mol. Biol. 2016;1435:249–291. doi: 10.1007/978-1-4939-3670-0_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abubakr M., Mandal S.C., Banerjee S. Natural Compounds against Flaviviral Infections. Nat. Prod. Commun. 2013;8:1487–1492. doi: 10.1177/1934578X1300801039. [DOI] [PubMed] [Google Scholar]
- 89.CDC Chikungunya Virus. [(accessed on 15 December 2020)]; Available online: https://www.cdc.gov/chikungunya/index.html.
- 90.Pérez-Pérez M.-J., Delang L., Ng L.F.P., Priego E.-M. Chikungunya virus drug discovery: Still a long way to go? Expert Opin. Drug Discov. 2019;14:855–866. doi: 10.1080/17460441.2019.1629413. [DOI] [PubMed] [Google Scholar]
- 91.Panya A., Yongpitakwattana P., Budchart P., Sawasdee N., Krobthong S., Paemanee A., Roytrakul S., Rattanabunyong S., Choowongkomon K., Yenchitsomanus P.-T. Novel bioactive peptides demonstrating anti-dengue virus activity isolated from the Asian medicinal plant Acacia Catechu. Chem. Biol. Drug Des. 2019;93:100–109. doi: 10.1111/cbdd.13400. [DOI] [PubMed] [Google Scholar]
- 92.Leite F.C., Mello C.S., Fialho L.G., Marinho C.F., Lima A.L.A., Filho J.M.B., Kubelka C.F., Piuvezam M.R. Cissampelos sympodialish as anti-viral effect inhibiting denguenon-structural viral protein-1 and pro-inflammatory mediators. Rev. Bras. Farmacogn. 2016;26:502–506. doi: 10.1016/j.bjp.2016.03.013. [DOI] [Google Scholar]
- 93.Panraksa P., Ramphan S., Khongwichit S., Smith D.R. Activity of andrographolide against dengue virus. Antivir. Res. 2017;139:69–78. doi: 10.1016/j.antiviral.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 94.Maryam M., Te K.K., Wong F.C., Chai T.T., Low G.K.K., Gan S.C., Chee H.Y. Antiviral activity of traditional Chinese medicinal plants Dryopteris crassirhizoma and Morus alba against dengue virus. J. Integr. Agric. 2020;19:1085–1096. doi: 10.1016/S2095-3119(19)62820-0. [DOI] [Google Scholar]
- 95.Nothias-Scaglia L.F., Dumontet V., Neyts J., Roussi F., Costa J., Leyssen P., Litaudon M., Paolini J. LC-MS2-Based derep-lication of Euphorbia extracts with anti-Chikungunya virus activity. Fitoterapia. 2015;105:202–209. doi: 10.1016/j.fitote.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 96.Wilson D.K., Shyamala G., Paulpandi M., Narayanasamy A., Siram K., Karuppaiah A., Sankar V. Development and Characterization of Phytoniosome Nano Vesicle Loaded with Aqueous Leaf Extracts of Justicia adhatoda and Psidium guajoava Against Dengue Virus (DEN-2) J. Clust. Sci. 2021;32:297–304. doi: 10.1007/s10876-020-01788-6. [DOI] [Google Scholar]
- 97.Zakaria I.I., Salin N.H., Amanah A., Othman S., Khairuddin F., Khawory M.H., Wahab R.A., Rahaman M.R.A., Chern P.P., Johari N.A., et al. Potential anti-viral compounds from Malaysian Plant Natural Product Repository and Data-base (MyNature50000) for DENV2. Biotechnol. Biotechnol. Equip. 2019;33:379–389. doi: 10.1080/13102818.2019.1578184. [DOI] [Google Scholar]
- 98.Vázquez-Calvo Á., de Oya N.J., Martin-Acebes M.A., Garcia-Moruno E., Saiz J.-C. Antiviral Properties of the Natural Polyphenols Delphinidin and Epigallocatechin Gallate against the Flaviviruses West Nile Virus, Zika Virus, and Dengue Virus. Front. Microbiol. 2017;8:1314. doi: 10.3389/fmicb.2017.01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yuen P.T., Sevan D.H., Naphak M., Andrei I.S., Glen M.B., Paul R.Y., Daniel W., Craig M.W. Stachyonic Acid: A Dengue Virus Inhibitor from Basilicum polystachyon. Chem. Eur. J. 2019;25:5664–5667. doi: 10.1002/chem.201900591. [DOI] [PubMed] [Google Scholar]
- 100.Gómez-Calderónet C., Mesa-Castro C., Robledo S., Gómez S., Bolivar-Avila S., Diaz-Caastillo F., Martinez-Gutierrez M. Antiviral effect of compounds derived from the seeds of Mammea americana and Tabernaemontana cymose on Dengue and Chickugunya virus infections. BMC Complement. Altern. Med. 2017;17:57. doi: 10.1186/s12906-017-1562-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Qian X.-J., Jin Y.-S., Chen H.-S., Xu Q.-Q., Ren H., Zhu S.-Y., Tang H.-L., Wang Y., Zhao P., Qi Z.-T., et al. Trachelogenin, a novel inhibitor of hepatitis C virus entry through CD81. J. Gen. Virol. 2016;97:1134–1144. doi: 10.1099/jgv.0.000432. [DOI] [PubMed] [Google Scholar]
- 102.Sanna G., Madeddu S., Giliberti G., Nikoletta G.N., Cottiglia F., Caboni P., De Logu A., Agus E. Limonoids from Melia azedarach Fruits as Inhibitors of Flaviviruses and Mycobacterium tuberculosis. PLoS ONE. 2015;10:e0141272. doi: 10.1371/journal.pone.0141272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Techer S., Girard-Valenciennes E., Retailleau P., Neyts J., Gueritte F., Leyssen P., Litaudon M., Smadja J., Grondin I. Tonantzitlolones from Stillingia lineata ssp. lineata as potential inhibitors of chikungunya virus. Phytochem. Lett. 2015;12:313–319. doi: 10.1016/j.phytol.2015.04.023. [DOI] [Google Scholar]
- 104.Arduino P.G., Porter S.R. Herpes Simplex Virus Type 1 infection: Overview on relevant clinico-pathological features. J. Oral Pathol. Med. 2007;37:107–121. doi: 10.1111/j.1600-0714.2007.00586.x. [DOI] [PubMed] [Google Scholar]
- 105.WHO Herpes Simplex Virus. [(accessed on 15 December 2020)]. Available online: https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus.
- 106.CDC Genital herpes-CDC Fact Sheet (Detailed) [(accessed on 6 October 2021)]; Available online: https://www.cdc.gov/std/herpes/stdfact-herpes-detailed.htm.
- 107.CDC Genital Herpes Treatment and Care. [(accessed on 6 October 2021)]; Available online: https://www.cdc.gov/std/herpes/treatment.htm.
- 108.Itzhaki R.F., Wozniak M.A. Herpes simplex virus type 1, apolipoprotein E, and cholesterol: A dangerous liaison in Alzheimer’s disease and other disorders. Prog. Lipid Res. 2006;45:73–90. doi: 10.1016/j.plipres.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 109.Vilhelmova-Ilieva N., Jacquet R., Deffieux D., Pouységu L., Sylla T., Chassaing S., Nikolova I., Quideau S., Galabov A.S. Anti-Herpes Simplex Virus Type 1 Activity of Specially Selected Groups of Tannins. Drug Res. 2019;69:374-373. doi: 10.1055/a-0640-2557. [DOI] [PubMed] [Google Scholar]
- 110.Vilhelmova-Ilieva N., Jacquet R., Quideau S., Galabov A.S. Ellagitannins as synergists of ACV on the replication of ACV-resistant strains of HSV 1 and 2. Antivir. Res. 2014;110:104–114. doi: 10.1016/j.antiviral.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 111.Bisignano C., Mandalari G., Smeriglio A., Trombetta D., Pizzo M.M., Pennisi R., Sciortino M.T. Almond Skin Extracts Abrogate HSV-1 Replication by Blocking Virus Binding to the Cell. Viruses. 2017;9:178. doi: 10.3390/v9070178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dias M.M., Zuza O., Riani L.R., Faria-Pinto P., Pinto P.L.S., Silva M.P., de Moraes J., Ataíde A.C.Z., Silva F.D.O., Cecílio A.B., et al. In vitro schistosomicidal and antiviral activities of Arctium lappa L. (Asteraceae) against Schistosoma mansoni and Herpes simplex virus-1. Biomed. Pharmacother. 2017;94:489–498. doi: 10.1016/j.biopha.2017.07.116. [DOI] [PubMed] [Google Scholar]
- 113.Cho W.-K., Weeratunga P., Lee B.-H., Park J.-S., Kim C.-J., Ma J.Y., Lee J.-S. Epimedium koreanum Nakai Displays Broad Spectrum of Antiviral Activity in Vitro and in Vivo by Inducing Cellular Antiviral State. Viruses. 2015;7:352–377. doi: 10.3390/v7010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Okba M.M., Gedaily R.A.E., Ashour R.M. UPLC-PDA-ESI-qTOF-MS profiling and potent anti-HSV-II activity of Eucalyptus sideroxylon leaves. J. Chromatogr. B. 2017;1068–1069:335–342. doi: 10.1016/j.jchromb.2017.10.065. [DOI] [PubMed] [Google Scholar]
- 115.Bús C., Kúsz N., Jakab G., Tahaei S.A.S., Zupkó I., Endrész V., Bogdanov A., Burián K., Csupor-Löffler B., Hohmann J., et al. Phenanthrenes from Juncus Compressus Jacq. with Promising Antiproliferative and Anti-HSV-2 Activities. Molecules. 2018;23:2085. doi: 10.3390/molecules23082085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Houston D.M.J., Bugert J.J., Denyer S.P., Heard C.M. Potentiated virucidal activity of pomegranate rind extract (PRE) and punicalagin against Herpes simplex virus (HSV) when co-administered with zinc (II) ions, and antiviral activity of PRE against HSV and aciclovir-resistant HSV. PLoS ONE. 2017;12:e0179291. doi: 10.1371/journal.pone.0179291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Duman R., Hasan H., Dogan H.H., Dinc M., Tuncer P. Cytotoxic and antiviral activity of Ribes Uva Crispa Linn. and Ribes Multflorum kit. Ex romer and schultes extracts. Int. J. Pharm. Sci. Res. 2018;9:1779–1787. [Google Scholar]
- 118.Al-Megrin W.A., Alsadhan N.A., Metwally D.M., Al-Talhi R.A., El-Khadragy M.F., Abdel-Hafez L.J.M. Potential antiviral agents of Rosmarinus officinalis extract against herpes viruses 1 and 2. Biosci. Rep. 2020;40 doi: 10.1042/BSR20200992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Twilley D., Langhansová L., Palaniswamy D., Lall N. Evaluation of traditionally used medicinal plants for anticancer, antioxidant, anti-inflammatory and anti-viral (HPV-1) activity. S. Afr. J. Bot. 2017;112:494–500. doi: 10.1016/j.sajb.2017.05.021. [DOI] [Google Scholar]
- 120.Kesharwani A., Polachira S.K., Nair R., Agarwal A., Mishra N.N., Gupta S.K. Anti-HSV-2 activity of Terminalia chebula Retz extract and its constituents, chebulagic and chebulinic acids. BMC Complement. Altern. Med. 2017;17:110. doi: 10.1186/s12906-017-1620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Boff L., Silva I.T., Argenta D.F., Farias L.M., Alvarenga L.F., Pádua R.M., Braga F.C., Leite J.P.V., Kratz J.M., Simões C.M.O. Strychnos pseudoquina A. St. Hil.: A Brazilian medicinal plant with promising in vitro antiherpes activity. J. Appl. Microbiol. 2016;121:1519–1529. doi: 10.1111/jam.13279. [DOI] [PubMed] [Google Scholar]
- 122.Kerl T., Berger F., Schmalz H.-G. Total Synthesis of the Antiviral Natural Product Houttuynoid B. Chem. Eur. J. 2016;22:2935–2938. doi: 10.1002/chem.201505118. [DOI] [PubMed] [Google Scholar]
- 123.Goswami D., Mahapatra A.D., Banerjee S., Kar A., Ojha D., Mukherjee P.K., Chattopadhyay D. Boswellia serrata oleo-gun-resin and β-boswellic acid inhibits HSV-1 infection in vitro through modulation of NF-κB and p38 MAP kinase signaling. Phytomedicine. 2018;51:94–103. doi: 10.1016/j.phymed.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 124.Rajtar B., Skalicka-Woźniak K., Świątek Ł., Stec A., Boguszewska A., Polz-Dacewicz M. Antiviral effect of compounds derived from Angelica archangelica L. on Herpes simplex virus-1 and Coxsackievirus B3 infections. Food Chem. Toxicol. 2017;109:1026–1031. doi: 10.1016/j.fct.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 125.Liao H.-B., Huang G.-H., Yu M.-H., Lei C., Hou A.-J. Five Pairs of Meroterpenoid Enantiomers from Rhododendron capi-tatum. J. Org. Chem. 2017;82:1632–1637. doi: 10.1021/acs.joc.6b02800. [DOI] [PubMed] [Google Scholar]
- 126.Su F., Zhao Z., Ma S., Wang R., Li Y., Liu Y., Li Y., Li L., Qu J., Yu S. Cnidimonins A–C, Three Types of Hybrid Dimer from Cnidium monnieri: Structural Elucidation and Semisynthesis. Org. Lett. 2017;19:4920–4923. doi: 10.1021/acs.orglett.7b02290. [DOI] [PubMed] [Google Scholar]
- 127.Ürményi F.G.G., Saraiva G.D.N., Casanova L.M., Matos A.D.S., Camargo L.M.D.M., Romanos M.T.V., Costa S.S. Anti-HSV-1 and HSV-2 Flavonoids and a New Kaempferol Triglycoside from the Medicinal Plant Kalanchoe daigremontiana. Chem. Biodivers. 2016;13:1707–1714. doi: 10.1002/cbdv.201600127. [DOI] [PubMed] [Google Scholar]
- 128.Ma F., Shen W., Zhang X., Li M., Wang Y., Zou Y., Li Y., Wang H. Anti-HSV Activity of Kuwanon X from Mulberry Leaves with Genes Expression Inhibitory and HSV-1 Induced NF-κB Deactivated Properties. Biol. Pharm. Bull. 2016;39:1667–1674. doi: 10.1248/bpb.b16-00401. [DOI] [PubMed] [Google Scholar]
- 129.de Oliveira A., Prince D., Lo C.Y., Lee L.H., Chu T.C. Antiviral activity of theaflavin digallate against herpes simplex virus type 1. Antivir. Res. 2015;118:56–67. doi: 10.1016/j.antiviral.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cryer M., Lane K., Greer M., Cates R., Burt S., Andrus M., Zou J., Rogers P., Hansen M.D.H., Burgado J., et al. Isolation and identification of compounds from Kalanchoe pinnata having human alphaherpesvirus and vaccinia virus antiviral activity. Pharm. Biol. 2017;55:1586–1591. doi: 10.1080/13880209.2017.1310907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.CDC Global Viral Hapatitus: Millions of People are Affected. [(accessed on 15 December 2020)]; Available online: https://www.cdc.gov/hepatitis/global/index.htm.
- 132.WHO . WHO Guidelines on Hepatitis B and C Testing. World Health Organization; Geneva, Switzerland: 2017. pp. 1–32. [PubMed] [Google Scholar]
- 133.CDC Viral Hepatitis. [(accessed on 15 December 2020)]; Available online: https://www.cdc.gov/hepatitis/abc/index.htm.
- 134.Arbab A.H., Parvez M.K., Al-Dosari M.S., Al-Rehaily A.J. In vitro evaluation of novel antiviral activities of 60 medicinal plants extracts against hepatitis B virus. Exp. Ther. Med. 2017;14:626–634. doi: 10.3892/etm.2017.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tumewu L., Apryani E., Santi M.R., Wahyuni T.S., Permanasari A.A., Adianti M., Aoki C., Widyawaruyanti A., Hafid A.F., Lusida M.I., et al. AntiHepatitis C Virus Activity of Alectryon serratus Leaves Extract. Procedia Chem. 2016;18:169–173. doi: 10.1016/j.proche.2016.01.026. [DOI] [Google Scholar]
- 136.Bose M., Kamra M., Mullick R., Bhattacharya S., Das S., Karande A.A. A plant-derived dehydrorotenoid: A new inhibitor of hepatitis C virus entry. FEBS Lett. 2017;591:1305–1317. doi: 10.1002/1873-3468.12629. [DOI] [PubMed] [Google Scholar]
- 137.Wahyuni T.S., Permatasari A.A., Widiandani T., Fuad A., Widyawaruyanti A., Aoki-Utsubo C., Hotta H. Antiviral Activities of Curcuma Genus against Hepatitis C Virus. Nat. Prod. Commun. 2018;13:1579. doi: 10.1177/1934578X1801301204. [DOI] [Google Scholar]
- 138.Ghanem K.Z., Mahran M.Z., Ramadan M.M., Ghanem H.Z., Fadel M., Mahmoud M.H. A comparative study on flavour components and therapeutic properties of unfermented and fermented defatted soybean meal extract. Sci. Rep. 2020;10:5998. doi: 10.1038/s41598-020-62907-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sahli R., Rivière C., Neut C., Bero J., Sahuc M.-E., Smaoui A., Beaufay C., Roumy V., Hennebelle T., Rouillé Y., et al. An ecological approach to discover new bioactive extracts and products: The case of extremophile plants. J. Pharm. Pharmacol. 2017;69:1041–1055. doi: 10.1111/jphp.12728. [DOI] [PubMed] [Google Scholar]
- 140.Matsuhisa K., Yamane S., Okamoto T., Watari A., Kondoh M., Matsuura Y., Yagi K. Anti-HCV effect of Lentinula edodes mycelia solid culture extracts and low-molecular-weight lignin. Biochem. Biophys. Res. Commun. 2015;462:52–57. doi: 10.1016/j.bbrc.2015.04.104. [DOI] [PubMed] [Google Scholar]
- 141.Hsu W.-C., Chang S.-P., Lin L.-C., Li C.-L., Richardson C.D., Lin C.-C., Lin L.-T. Limonium sinense and gallic acid suppress hepatitis C virus infection by blocking early viral entry. Antivir. Res. 2015;118:139–147. doi: 10.1016/j.antiviral.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 142.Aarthi C., Babu P.B.R. Studies On in vitro Culture and Secondary Metabolite Production of Phyllanthus reticulates Poir. J. Pharm. Sci. Res. 2017;9:307–313. [Google Scholar]
- 143.Ezzikouri S., Nishimura T., Kohara M., Benjelloun S., Kino Y., Inoue K., Matsumori A., Tsukiyama-Kohara K. Inhibitory effects of Pycnogenol® on hepatitis C virus replication. Antivir. Res. 2015;113:93–102. doi: 10.1016/j.antiviral.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 144.Rehman S., Ijaz B., Fatima N., Muhammad S.A., Riazuddin S. Therapeutic potential of Taraxacum officinale against HCVNS5B polymerase: In-vitro and In silico study. Biomed. Pharmacother. 2016;83:881–891. doi: 10.1016/j.biopha.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 145.Ganta K.K., Mandal A., Debnath S., Hazra B., Chaubey B. Anti-HCV Activity from Semi-purified Methanolic Root Extracts ofValeriana wallichii. Phytother. Res. 2017;31:433–440. doi: 10.1002/ptr.5765. [DOI] [PubMed] [Google Scholar]
- 146.Gu C., Yin A.P., Yuan H.Y., Yang K., Luo J., Zhan Y.J., Yang C.R., Zuo D.M., Li H.Z., Xu M. New anti-HBV nor-bisabolane sesquiterpenes from Phyllantus acidus. Fitoterapia. 2019;137:104151. doi: 10.1016/j.fitote.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 147.Lee S., Mailar K., Kim M.I., Park M., Kim J., Min D.-H., Heo T.-H., Bae S.K., Choi W., Lee C. Plant-Derived Purification, Chemical Synthesis, and In Vitro/In Vivo Evaluation of a Resveratrol Dimer, Viniferin, as an HCV Replication Inhibitor. Viruses. 2019;11:890. doi: 10.3390/v11100890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li S.-F., Jiao Y.-Y., Zhang Z.-Q., Chao J.-B., Jia J., Shi X.-L., Zhang L.-W. Diterpenes from buds of Wikstroemia chamaedaphne showing anti-hepatitis B virus activities. Phytochemistry. 2018;151:17–25. doi: 10.1016/j.phytochem.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 149.Subaiea G.M., Aljofan M., Devadasu V.R., Alshammari T.M. Acute toxicity testing of newly discovered potential antihep-atitis B virus agents of plant origin. Asian J. Pharm. Clin. Res. 2017;10:210–213. [Google Scholar]
- 150.Hassan S.T.S., Berchová-Bímová K., Petráš J. Plumbagin, a Plant-Derived Compound, Exhibits Antifungal Combinatory Effect with Amphotericin B against Candida albicans Clinical Isolates and Anti-hepatitis C Virus Activity. Phytother. Res. 2016;30:1487–1492. doi: 10.1002/ptr.5650. [DOI] [PubMed] [Google Scholar]
- 151.Elsebai M.F., Koutsoudakis G., Saludes V., Pérez-Vilaró G., Turpeinen A., Matilla S., Pirttilä A.M., Fontaine-Vive F., Me-hiri M., Meyerhans A., et al. Pan-genotypic Hepatitis C Virus Inhibition by Natural Products Derived from the Wild Egyptian Artichoke. J. Virol. 2016;90:1918–1930. doi: 10.1128/JVI.02030-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xu J., Gu W., Li C., Li X., Xing G., Li Y., Song Y., Zheng W. Epigallocatechin gallate inhibits hepatitis B virus via farnesoid X receptor alpha. J. Nat. Med. 2016;70:584–591. doi: 10.1007/s11418-016-0980-6. [DOI] [PubMed] [Google Scholar]
- 153.Zhu B., Wang T.B., Hou L.J., Lv H.X., Liu A.M., Zeng P., Li A.H. A New Selaginellin from Selaginella moellendorffii In-hibits Hepatitis B Virus Gene Expression and Replication. Chem. Nat. Compd. 2016;52:624–629. doi: 10.1007/s10600-016-1725-1. [DOI] [Google Scholar]
- 154.Chung C.-Y., Liu C.-H., Burnouf T., Wang G.-H., Chang S.-P., Jassey A., Tai C.-J., Tai C.-J., Huang C.-J., Richardson C.D., et al. Activity-based and fraction-guided analysis of Phyllanthus urinaria identifies loliolide as a potent inhibitor of hepatitis C virus entry. Antivir. Res. 2016;130:58–68. doi: 10.1016/j.antiviral.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 155.Dai J.-J., Tao H.-M., Min Q.-X., Zhu Q.-H. Anti-hepatitis B virus activities of friedelolactones from Viola diffusa Ging. Phytomedicine. 2015;22:724–729. doi: 10.1016/j.phymed.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 156.Jiang Z.-Y., Yu Y.-J., Huang C.-G., Huang X.-Z., Hu Q.-F., Yang G.-Y., Wang H.-B., Zhang X.-Y., Li G.-P. Icetexane Diterpenoids from Perovskia atriplicifolia. Planta Med. 2015;81:241–246. doi: 10.1055/s-0034-1396151. [DOI] [PubMed] [Google Scholar]
- 157.Jardim A.C.G., Igloi Z., Shimizu J.F., Santos V.A.F.F.M., Felippe L.G., Mazzeu B.F., Amako Y., Furlan M., Harris M., Rahal P. Natural compounds isolated from Brazilian plants are potent inhibitors of hepatitis C virus replication in vitro. Antivir. Res. 2014;115:39–47. doi: 10.1016/j.antiviral.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu J.-F., Wang L., Wang Y.-F., Song X., Yang L.-J., Zhang Y.-B. Sesquiterpenes from the fruits of Illicium jiadifengpi and their anti-hepatitis B virus activities. Fitoterapia. 2015;104:41–44. doi: 10.1016/j.fitote.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 159.Parvez M.K., Al-Dosari M.S., Alam P., Rehman M., Alajmi M.F., Alqahtani A.S. The anti-hepatitis B virus therapeutic potential of anthraquinones derived from Aloe vera. Phytother. Res. 2019;33:2960–2970. doi: 10.1002/ptr.6471. [DOI] [PubMed] [Google Scholar]
- 160.Cheng D., Sun L., Zou S., Chen J., Mao H., Zhang Y., Liao N., Zhang R. Antiviral Effects of Houttuynia cordata Polysaccharide Extract on Murine Norovirus-1 (MNV-1)—A Human Norovirus Surrogate. Molecules. 2019;24:1835. doi: 10.3390/molecules24091835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Winer H., Ozer J., Shemer Y., Reichenstein I., Eilam-Frenkel B., Benharroch D., Golan-Goldhirsh A., Gopas J. Nuphar lu-tea extracts exhibit anti-viral activity against the measles virus. Molecules. 2020;25:1657. doi: 10.3390/molecules25071657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lieberherr C., Zhang G., Grafen A., Singethan K., Kendl S., Vogt V., Maier J., Bringmann G., Schneider-Schaulies J. The Plant-Derived Naphthoquinone Droserone Inhibits In Vitro Measles Virus Infection. Planta Med. 2016;83:232–238. doi: 10.1055/s-0042-111825. [DOI] [PubMed] [Google Scholar]
- 163.Arjin C., Pringproa K., Hongsibsong S., Ruksiriwanich W., Seel-Audom M., Mekchay S., Sringarm K. In vitro screening antiviral activity of Thai medicinal plants against porcine reproductive and respiratory syndrome virus. BMC Vet. Res. 2020;16:102. doi: 10.1186/s12917-020-02320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Thabti I., Albert Q., Philippot S., Dupire F., Westerhuis B., Fontanay S., Risler A., Kassab T., Elfalleh W., Aferchichi A., et al. Advances on Antiviral Activity of Morus spp. Plant Extracts: Human Coronavirus and Virus-Related Respiratory Tract Infections in the Spotlight. Molecules. 2020;25:1876. doi: 10.3390/molecules25081876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Civra A., Francese R., Sinato D., Donalisio M., Cagno V., Rubiolo P., Ceylan R., Uysal A., Zengin G., Lembo D. In vitro screening for antiviral activity of Turkish plants revealing methanolic extract of Rindera lanata var. lanata active against human rotavirus. BMC Complement. Altern. Med. 2017;17:74. doi: 10.1186/s12906-017-1560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Snene A., El Mokni R., Jmii H., Jlassi I., Jaïdane H., Falconieri D., Piras A., Dhaouadi H., Porcedda S., Hammami S. In vitro antimicrobial, antioxidant and antiviral activities of the essential oil and various extracts of wild (Daucus virgatus (Poir.) Maire) from Tunisia. Ind. Crop. Prod. 2017;109:109–115. doi: 10.1016/j.indcrop.2017.08.015. [DOI] [Google Scholar]
- 167.Fu Q., Gao L., Fu X., Meng Q., Lu Z. Scutellaria baicalensis Inhibits Coxsackievirus B3-Induced Myocarditis Via AKT and p38 Pathways. J. Microbiol. Biotechnol. 2019;29:1230–1239. doi: 10.4014/jmb.1904.04050. [DOI] [PubMed] [Google Scholar]
- 168.Kim J.W., Ha T.-K.-Q., Cho H.M., Kim E., Shim S.H., Yang J.-L., Oh W.K. Antiviral escin derivatives from the seeds of Aesculus turbinata Blume (Japanese horse chestnut) Bioorg. Med. Chem. Lett. 2017;27:3019–3025. doi: 10.1016/j.bmcl.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sun Y., Gong X., Tan J.Y., Kang L., Li D., Vikash , Yang J., Du G. In vitro Antiviral Activity of Rubia cordifolia Aerial Part Extract against Rotavirus. Front. Pharmacol. 2016;7:308–318. doi: 10.3389/fphar.2016.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang B., Wei Y., Zhao X., Tian X., Ning J., Zhang B., Deng S., Li D., Ma X., Wang C. Unusual ent-atisane type diterpenoids with 2-oxopropyl skeleton from the roots of Euphorbia ebracteolate and their antiviral activity against human rhinovirus 3 and enterovirus 71. Bioorg. Med. 2018;81:234–240. doi: 10.1016/j.bioorg.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 171.Chen W., Zhang H., Wang J., Hu X. A New Triterpenoid from the Bulbs of EW Lilium speciosum var. gloriosoides. Chem. Nat. Compd. 2019;55:289–291. doi: 10.1007/s10600-019-02669-9. [DOI] [Google Scholar]
- 172.Chen W., Zhang H., Wang J., Hu X. Flavonoid Glycosides from Bulbs of Lilium speciosum var. gloriosoides and their Po-tential Antiviral Activity against RSV. Chem. Nat. Compd. 2019;55:461–464. doi: 10.1007/s10600-019-02714-7. [DOI] [Google Scholar]
- 173.Tang W., Li M., Liu Y., Liang N., Yang Z., Zhao Y., Wu S., Lu S., Li Y., Liu F. Small molecule inhibits respiratory syncytial virus entry and infection by blocking the interaction of the viral fusion protein with the cell membrane. FASEB J. 2019;33:4287–4299. doi: 10.1096/fj.201800579R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Di Petrillo A., Fais A., Pintus F., Santos-Buelga C., González-Paramás A.M., Piras V., Orrù G., Mameli A., Tramontano E., Frau A. Broad-range potential of Asphodelus microcarpus leaves extract for drug development. BMC Microbiol. 2017;17:159. doi: 10.1186/s12866-017-1068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Biedenkopf N., Lange-Gruenweller K., Schulte F.W., Weisser A., Mueller C., Becker D., Becker S., Hartmann R.K., Gruenweller A. The natural compound silvestrol is a potent inhibitor of Ebola virus replication. Antivir. Res. 2017;137:76–81. doi: 10.1016/j.antiviral.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 176.Schreck R., Rieber P., Baeuerle P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kumar S., Misra U.K., Kalita J., Khanna V.K., Khan M.Y. Imbalance in oxidant/antioxidant system in different brain regions of rat after the infection of Japanese encephalitis virus. Neurochem. Int. 2009;55:648–654. doi: 10.1016/j.neuint.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 178.Gacche R., Khsirsagar M., Kamble S., Bandgar B., Dhole N., Shisode K., Chaudhari A. Antioxidant and Anti-inflammatory Related Activities of Selected Synthetic Chalcones: Structure-Activity Relationship Studies Using Computational Tools. Chem. Pharm. Bull. 2008;56:897–901. doi: 10.1248/cpb.56.897. [DOI] [PubMed] [Google Scholar]
- 179.Gullberg R.C., Steel J.J., Moon S.L., Soltani E., Geiss B.J. Oxidative stress influences positive strand RNA virus genome synthesis and capping. Virology. 2015;475:219–229. doi: 10.1016/j.virol.2014.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.StarDrop A Complete Platform for Small Molecule Design, Optimisation and Data Analysis. [(accessed on 6 October 2021)]. Available online: https://www.optibrium.com/stardrop/