Abstract
Resistance to therapy is a frequently observed phenomenon in the treatment of cancer, and as with other cancer therapeutics, therapies based on oncolytic viruses also face the challenges of resistance, such as humoral and cellular antiviral responses, and tumor-associated interferon-mediated resistance. In order to identify additional mechanisms of resistance that may contribute to therapeutic failure, we developed a systematic search strategy for studies published in PubMed. We analyzed 6143 articles on oncolytic virotherapy and found that approximately 8% of these articles use resistance terms in the abstract and/or title. Of these 439 articles, 87 were original research. Most of the findings reported pertain to resistance mediated by tumor-cell-dependent interferon signaling. Yet, mechanisms such as epigenetic modifications, hypoxia-mediated inhibition, APOBEC-mediated resistance, virus entry barriers, and spatiotemporal restriction to viral spread, although not frequently assessed, were demonstrated to play a major role in resistance. Similarly, our results suggest that the stromal compartment consisting of, but not limited to, myeloid cells, fibroblasts, and epithelial cells requires more study in relation to therapy resistance using oncolytic viruses. Thus, our findings emphasize the need to assess the stromal compartment and to identify novel mechanisms that play an important role in conferring resistance to oncolytic virotherapy.
Keywords: oncolytic virotherapy, therapeutic resistance, stromal cells, cancer cells, resistance mechanisms
1. Introduction
Therapeutic resistance has been studied as a mechanism that allows the target to escape and evolve against the treatment, for example in the context of antibiotic resistance by pathogenic bacteria and resistance by tumors towards radiotherapy, chemotherapy, or immunotherapy. In the early phase of research assessing cancer resistance, most of the resistance mechanisms were attributed to be innate to tumors [1]. However, recent findings have revealed that therapeutic resistance may also arise via evolutionary mechanisms. A detailed understanding of such mechanisms is therefore required to improve therapeutic outcomes.
Recently, oncolytic virotherapy has been considered a promising biotherapy, where native or genetically engineered viruses are utilized to infect and kill cancer cells [2,3]. Moreover, it has been demonstrated that such oncolytic viruses, in addition to tumor killing, can also promote local and systemic antitumor immune responses and thus contribute to improved therapeutic outcomes [4]. Nevertheless, oncolytic virotherapy is not equally effective in all patients and cancer types, thus suggesting the possibility of therapeutic resistance. Although cellular and humoral antiviral immune responses have been well documented in the context of infectious virology, there have been fewer studies that explore resistance mechanisms in terms of cancer and virotherapy. To this end, we conducted a systematic review with the aim to provide a comprehensive overview of the resistance mechanisms associated with cancer and stromal cells and viruses employed for oncolytic virotherapy. We considered that resistance would not only be a result of an antiviral immune response, but also be a response mediated by tumor cells and stromal cells present in the tumor microenvironment. Therefore, through this systematic review, we hope to shed light on some of the overlooked players and mechanisms of resistance that undermine the therapeutic efficacy of oncolytic viruses.
2. Materials and Methods
Protocol: We used the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocol (PRISMA-P) statement as a guide to formulate the research question and process articles for analysis.
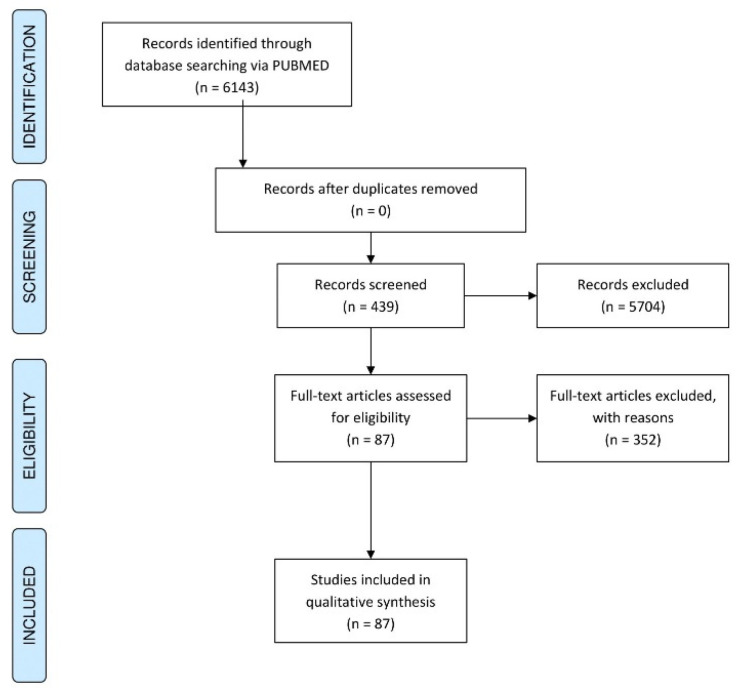
Eligibility criteria: We included all studies published to date that had the terms “oncolytic” and “resistance” in their abstract and/or title. The search strategy was thus defined as “resistance”[tiab] AND “oncolytic”[tiab] for exploring articles on PubMed. Only original research, in the form of full-text English publications, was included for analysis. Abstracts, letters, reviews, and commentaries were excluded (indicated in Figure 1).
Figure 1.
PRISMA flow diagram indicating the steps of article screening for a systematic analysis.
Search strategy and article screening: A systematic search strategy was developed for PubMed. Titles that met our eligibility criteria were identified and processed for study selection. Title and abstract screenings were performed. Subsequently, a detailed analysis of the selected 87 articles was performed. Any conflicts were resolved through discussion. All data and results are provided in Supplementary Table S1 and are intended to serve as a resource and inspiration for future studies in the field of oncolytic virotherapy. Figures were made using the Orange Data Mining software [5] and the RawGraphs platform [6]. The graphical abstract was made using Biorender.
3. Results
A systematic search on PubMed performed on articles retrieved on 31 January 2021 found that 6143 articles contained “oncolytic”-related terms in the abstract and/or title, while only 439 of these articles (7.2%) contained both the terms “resistance” and “oncolytic” in the abstract or title.
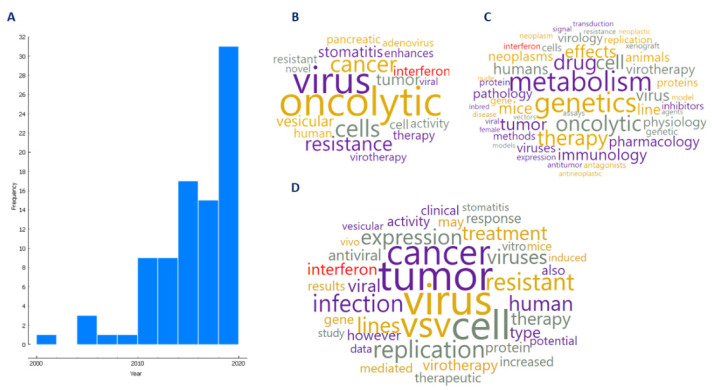
A detailed screening of the 439 articles revealed 87 original research articles (1.5%) that studied resistance to virotherapy in the context of cancer. Regarding these 87 articles, Figure 2A shows that from the year 2000 to 2020, there has been an increase in the number of original research articles pertaining to resistance in oncolytic virotherapy. The most frequent terms used in the article title (Figure 2B) MeSH heading terms (Figure 2C) and terms from the abstract (Figure 2D) are an indication of the diversity of the concepts that relate to this subject. Of note, “interferon” (marked in red in Figure 2B–D) was found to be one of the most frequently retrieved terms from titles, abstracts, and MeSH headings, thus indicating that it might be a well-studied resistance mechanism in the literature.
Figure 2.
Literature review in the context of resistance to oncolytic viruses. (A) Number of original research articles (a total of 87) that studied resistance to virotherapy in the context of cancer from 2000 to 2020. (B) Frequently used terms in article titles, (C) MESH heading terms, and (D) abstracts.
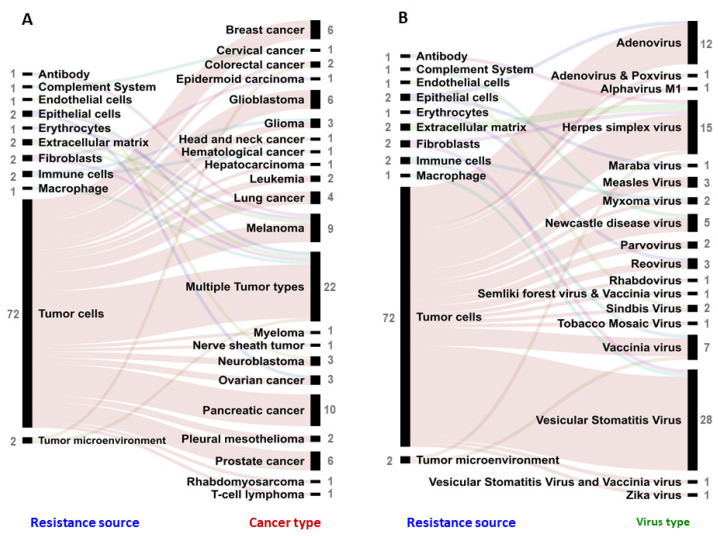
These 87 articles were published in more than 40 journals by various authors. Seventeen viral vector types (natural or genetically modified) were studied in relation to resistance developed in twenty-two cancer types represented by hundreds of cell lines and preclinical animal models. This analysis also indicated an increasing interest in understanding the role of therapeutic resistance towards oncolytic viruses since the last decade (since 2010). Resistance mechanisms could be classified based on molecular features along with cellular mechanisms and cell-type-specific mechanisms (indicated in Figure 3A,B). Through this approach, we found that there has been a selective focus on assessing interferon (IFN)-mediated resistance by tumor cells (twenty-seven studies), with only two studies reporting on the role of fibroblast-mediated resistance [7,8] and three other studies assessing the role of epithelial cells and endothelial cells as physical barriers in regulating the spatiotemporal spread of the virus in tumors [9,10,11] (Figure 3B). Interestingly, two studies assessed the role of tumor-associated myeloid cells (TAMs) in conferring resistance to oncolytic virotherapy; however, only one of them elaborated on macrophage-mediated interferon signaling as a mechanism of resistance [12].
Figure 3.
Resistance mechanisms undermining the efficacy of oncolytic viruses. (A) Types of resistance mechanisms and responsible molecular factors studied in reference to oncolytic viruses. The reference number of articles corresponding to the respective resistance mechanisms is provided in brackets. (B) Frequency of studies focusing on a particular resistance source and corresponding mechanism. Each link indicates an article. The number of articles studying a particular resistance source and resistance mechanism is indicated next to the label in grey.
Resistance to virotherapy has been studied in different cancer types. Pancreatic cancer (10 studies), melanoma (9 studies), prostate cancer (6 studies), breast cancer, and glioblastoma have been the most studied individual cancer types in the context of resistance, where tumor-cell-mediated resistance mechanisms are frequently explored (72 studies) (Figure 4A). Yet, resistance mechanism studies also often include cancer cell lines belonging to different cancer types (Figure 4A) in a single study (22 studies), thus enabling high-throughput screening of various pathways associated with resistance [8,10,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31].
Figure 4.
Resistance to oncolytic virotherapy exhibited by different sources or cell types present in the tumor microenvironment. Resistance source assessed in research articles in the context of (A) different cancers and (B) different virotherapy platforms. Each link indicates an article. The number of articles studying a particular resistance source, cancer type, and virus type is indicated next to the label in grey.
A wide range of virus types have been described to exhibit decreased therapeutic efficacy due to resistance mechanisms (Figure 4B). The most frequently described viral vectors in this context were vesicular stomatitis virus (VSV, 29 studies), which is an enveloped RNA virus, herpes simplex virus (HSV, 15 studies,) an enveloped DNA virus, and adenovirus (AdV, 12 studies), a nonenveloped DNA virus (Figure 4B). Interestingly, more research with VSV, HSV, and AdV has led researchers to uncover a wide range of resistance mechanisms that stem from tumor cell heterogeneity (10 studies), epigenetic modifications (5 studies), hypoxia (1 study), blocking viral entry (3 study), and association of novel cell survival genes (25 studies) in the process of either preventing productive infection and/or subsequent oncolysis (Figure 4B).
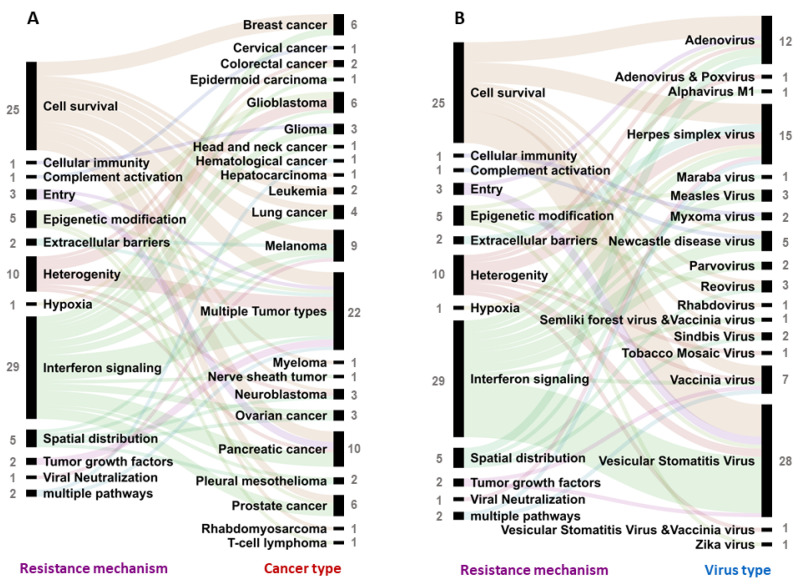
A common mechanism explored in various cancer types is the activation of interferon-mediated antiviral signals (29 studies) and the activation of cell-survival molecular mechanisms that protect tumor cells against viral infection and infection-induced oncolysis (25 studies) (Figure 5A). It is moreover important to note that resistance mechanisms associated with tumor growth factors (2 studies), physical barriers (2 studies), and preventing viral spread in tumor tissue (5 studies) and mechanisms that involve systemic responses of a multidimensional nature are frequently associated with the tumor microenvironment and not a particular cell type (Figure 5A and Figure 3B). Once more, interferon-mediated resistance and cell survival mechanisms have been studied more often across a wide range of virus types (Figure 5B). It is important to note here that other resistance mechanisms may or may not play a role in reducing the efficacy of different oncolytic viruses; however, the absence of such a study prevents one from reaching such a conclusion.
Figure 5.
Assessing resistance towards oncolytic viruses in various cancers. Resistance to oncolytic virotherapy exhibited by different mechanisms studied in various articles in the context of (A) different cancer types and (B) towards different virotherapy platforms. Each link indicates an article. The number of articles studying a particular cancer type, virus type, and resistance mechanism is indicated next to the label in grey.
4. Discussion
This systematic analysis was based on 87 articles retrieved from published literature describing current practices in assessing resistance to oncolytic virotherapy. The aim of many of these articles was to explore which cellular pathways are associated with resistance and, if possible, to regulate these pathways and overcome resistance for better therapeutic outcomes. Of note, the most frequently studied resistance mechanisms, irrespective of virotherapy type, were based on cellular signaling post-virus infection, often focusing on interferon responses (29 studies) mediated by tumor (27 studies) and stromal cells (2 studies). Tumor-cell-mediated resistance mechanisms were explored more (72 studies) in comparison to stromal-cell-based mechanisms (8 studies), where pancreatic cancer (10 studies), melanoma (9 studies), prostate cancer (6 studies), breast cancer (6 studies), and glioblastoma (6 studies) were the cancer types most explored. The present results indicate the complexity of resistance phenomena and the challenge in targeting such mechanisms while designing virotherapy. Thus, for a comprehensive understanding of these mechanisms retrieved from the systematic analysis, we discuss in detail below how molecular processes regulated by various cell types present in the tumor microenvironment can impact the efficacy of virotherapy. Accordingly, we classify the resistance mechanisms studied in these articles as: tumor-cell-mediated responses, stromal cell responses, antiviral immune responses, and systemic responses.
Tumor cell-mediated responses: Tumor cells are the primary targets of oncolytic virotherapy. Therefore, resistance developed by, or innate in, tumor cells against viral activity dictates the clinical outcome of the therapy. This might explain why most of the studies (72 studies) retrieved from the systematic analysis had a focus on tumor-cell-mediated resistance. Resistance to viral infection and replication by tumor cells can be achieved at four distinct stages of (1) virus binding and entry, (2) viral transcription–translation, (3) cell survival, and (4) cell signaling. Here, it is important to note that a decrease in the oncolytic potential of a virus could be a result of resistance mechanisms that directly prevent virus replication and the translation of viral proteins or be due to virus inactivation in the extracellular space and disposal due to systemic processes. To assess various mechanisms associated with resistance to viral gene therapy, a study by Song et al. analyzed and identified changes in tumor gene expression post-virus infection. They found that these differentially expressed genes were involved in regulating the above-mentioned four stages by altering cell morphology, the expression of cell surface proteins affecting virus binding and entry, and altering cell development, movement, growth, and proliferation and cell-to-cell signaling and interaction [16].
Virus binding and entry: Virus entry depends on multiple factors, including the presence of host cell receptors for virus binding and the presence of neutralizing antibodies that block uptake, but also the presence of proteases in the extracellular matrix, which can either promote or prevent virus binding. Few studies (<5 studies) retrieved from the systematic analysis, were designed to study virus entry as a mechanism of resistance of oncolytic virotherapy.
For pleural mesothelioma, downregulation of heparanase enzymes in the tumor microenvironment has been described to result in the formation of a dense extracellular matrix, preventing deep tumor infiltration of viruses and attachment to target cells [32]. Pancreatic ductal adenocarcinoma cell lines have been described to have a low expression of the low-density lipoprotein receptor, the receptor for vesicular stomatitis virus, thus conferring a resistant phenotype to the tumor cells [33]. Moreover, epidermal growth factor receptor-targeted HSV has shown to be sensitivity to key neutralizing antibodies that pre-dominantly target glycoprotein D as a viral attachment protein [34].
Additionally, protein kinase B (AKT) activity and virus-induced activation of the pathway regulated by the mammalian target of rapamycin (AKT/mTOR axis) is linked to resistance in various cancers. Choi et al. described how the endogenous activity of AKT and related mutations promoted virus entry of Orthopoxvirus in triple-negative breast cancer (cells) [35]. Tong and colleagues showed that the AKT/mTOR axis can be regulated by blocking phosphoinositide 3-kinase (PI3K) activation, thus sensitizing multiple cancer cell lines in vitro for viral infection [36]. Rapamycin was observed to reverse mTOR-mediated tumor cell resistance against herpes simplex virotherapy, indicating that molecular mechanisms lay at the center of this phenotypic resistance to therapy [14].
Lucas et al. observed that modification of the capsid protein hexon of adenovirus increased the transduction and cytotoxicity of pancreatic tumors [37]. These findings indicate a close association among cell survival mechanisms that regulate virus binding and entry to tumor cells, and thus, these can be exploited to improve therapeutic efficacy.
Viral transcription–translation: After successful entry in the host cell, the next challenge of the virus is productive transcription and translation of its genome, the production of viral progeny, and oncolysis. Therefore, the regulation of host transcription and translational machinery is another crucial step. However, tumor cells can regulate molecular processes and shut down virus transcription via epigenetic modification of either the host genome or the viral genome and by the expression of antiviral proteins such as the apolipoprotein B mRNA-editing enzyme (APOBEC).
For example, Shulak and colleagues demonstrated the role of tumor cell epigenetic modifications in the promotion of resistance against virotherapy. They observed that treatment with histone deacetylase inhibitors promoted VSV-mediated tumor cell death in a nuclear factor kappa-B (NF-kB) autophagy-dependent manner [38]. This study was in accordance with an earlier observation made by Bieler et al., showing that adenovirus oncolytic activity was promoted against glioblastoma cell lines [39]. Another confirmation came when it was observed that NF-κB inhibition could reverse the resistance phenotype, promoting the oncolytic potential of virotherapy [26]. Alternatively, epigenetic modifications in viruses can play a role as they can aid in circumventing the resistance developed by tumor cells. For example, it was observed that Zika virus with a higher frequency of genomic CpG dinucleotides exhibited a better oncolytic activity in glioblastoma cell lines [40].
Huff et al. demonstrated that APOBEC3 upregulation in an IFN-β dependent manner by tumor cells is an innate mechanism of resistance to RNA viral infection and worsens treatment outcome [41,42]. This suggests that APOBEC enzymes, via their mRNA editing nature, can have an important role in dictating the outcome of any nucleic-acid-based therapeutic or viral therapy. Similar mechanisms that are sensitive to nonself RNA molecules were observed against alphaviruses. Here, the activation of double-stranded-RNA-sensitive protein kinases in tumor cells led to restricted translation and increased sensitivity to interferon responses [43]. This limited viral replication in tumor cells exhibits a decreased interferon response.
Cell survival: Several survival proteins and their respective pathways play an important role in protecting tumor cells against viral infections and virus-induced cell death. Most of the studies (25 studies) retrieved from the systematic analysis found various molecular factors that play an important role in resistance towards oncolytic virotherapy by regulating cell survival. In addition to epigenetic modifications, promoting cell survival, regulation of autophagy, and apoptosis in tumor cells were found to promote resistance to virotherapy. For example, a study by Lv and colleagues demonstrated that vaccinia virus expressing IL-24 resulted in caspase-dependent apoptosis and decreased expression of the signal transducer and activator of transcription-3 (STAT3). In vivo, the modified virus also promoted apoptosis in lung cancer that was otherwise resistant to virus-induced cell death [44]. A similar study by Li et al. showed an increased oncolytic activity of virotherapy where tumor cell apoptosis was promoted by Beclin-1 activity expressed by adenoviruses [45]. A study by Colunga et al. indicated that loss of the autophagy protein sequestosome-1 (p62/SQSTM1) via calpain-dependent mechanisms can promote oncolytic activity in melanoma [46]. A role of autophagy in regulating oncolysis was supported by the observation that pharmacological regulation of autophagy improved Newcastle disease virus-mediated oncolysis in drug-resistant lung cancer cell lines [47]. Furthermore, several studies demonstrated that master regulators such as tumor protein p53 (TP53) and its activation abrogate viral infectivity and replication in tumors; however, this is limited to nonmalignant cells [48] and tumor cells that do not harbor TP53 mutations [48,49]. Various other cell survival regulators such as the inhibitor of nuclear factor kappa-B kinase subunit-beta (IKK) [50], inositol polyphosphate 5-phosphatase (Inpp5e) [51], mitogen-activated protein kinase (MAPK) [52], multidrug-resistance protein-1 (MDR1) [22], mitogen-activated protein kinase kinase (MEK) [53], second mitochondria-derived activator of caspases (SMAC) [54,55], Sirtuin 1 (SIRT1) [56], and N-myc proto-oncogene protein (MYCN) [57] have also been found associated with resistance to viral infection and replication in tumor cells.
Cell signaling: Intercellular communication between tumor cells has been well studied in the context of tumor progression and resistance to various immunotherapies, where tumor cells have been found to communicate and influence neighboring tumor cells via secreting cytokines, growth factors, metabolites, and extracellular vesicles to promote a protumoral environment [58]. Soluble signals released by infected cells can protect surrounding cells from viral infection, e.g., interferon responses [28,29,59,60,61,62,63,64,65,66,67]. Secretion of interferons by tumor cells infected with oncolytic viruses and the subsequent activation of the interferon-receptor (IFNR)-based Janus-kinase (JAK-STAT) pathway in surrounding tumor cells has been demonstrated; thus, also in this context, this pathway is an important mode of antiviral resistance [68]. Activation of interferon signaling has been observed to induce the expression of various antiviral proteins such as interferon-induced transmembrane protein 1 (IFITM1), interferon-induced protein with tetratricopeptide repeats (IFIT3), IFN-α inducible gene 6 (IFI6), 2′-5′-oligoadenylate synthetase 2 (OAS2), adenosine deaminases acting on RNA (ADAR), interferon-β (IFNβ), and tripartite motif containing 25 (TRIM25), which play a role in attenuating viral replication and the oncolysis of tumor cells [69]. These studies are supported by the observation that inhibitors of interferon signaling such as ruxolitinib can enhance the oncolytic potential of virotherapy in both in vitro and in vivo models [15,20,70,71].
Alternatively, the expression of the estrogen receptor on palbociclib-resistant breast cancer cells has been described to sensitize them towards adenoviral replication and oncolysis due to their low antiviral IFN response and increased cyclin-dependent kinase-2 activation [72].
Stromal cell responses: Although stromal cells make up an essential part of the tumor microenvironment, only eight studies explored them in relation to resistance against oncolytic virotherapy. For example, cancer-associated fibroblasts are often abundantly present in solid tumors. Unlike tumor cells, these cells do not have a compromised interferon signaling pathway and are not as permissive to viral infection [2]. Therefore, antiviral resistance demonstrated by these cells may affect the therapeutic efficiency of oncolytic virotherapy. Furthermore, Arwert et al. demonstrated that cancer-associated fibroblasts, via stimulator of interferon genes (STING) and interferon regulatory transcription factor 3 (IRF3), can influence neighboring cells and enable sensing of genomic stress in cancer cells to prevent infection [7]. Alternatively, in a secretome analysis study, fibroblasts were found to secrete protumoral growth factors during virotherapy that impeded viral replication in cancer cells [8]. Similarly, tumor-associated macrophages have been reported to induce a protective antiviral state via secretion of IFN-β in ovarian and breast tumors, rendering them resistant to oncolytic virotherapy [12]. This suggests that the regulatory role of such stromal cells requires more frequent and detailed observation in the context of preventing effective virotherapy.
Antiviral immune responses: Antiviral immune responses are generated during oncolytic virotherapy. For example, oncolytic virotherapy has been found to induce local immune responses and convert an immunologically “cold” tumor into a “hot”, immune-cell-infiltrated, tumor via the release of inflammatory signals post-viral oncolysis [73]. Here, interferon-based signaling is a major component of such inflammatory signals and plays an important role in the transformation of “cold-to-hot” tumors [73]. Although studies have demonstrated that blocking interferon responses improves viral infectivity in tumor cells, this may also limit the induction of interferon-dependent antitumor immune responses [33,68]. For example, Melero et al. demonstrated that intratumoral injections of Semliki forest virus (WT or replicon particles) depends on virus-induced interferon responses that enhance CD8+ T-cell-mediated antitumor activity, while furthermore, increased interferon signaling leads to a better survival [74]. In noncancer settings as well, interferon responses have been found to influence T-cell maturation and clonal development, where suppressing interferon signaling post-viral infection impedes antigen-specific T-cell development and activity [75]. Apart from interferon-based cellular immunity to viral infection, adaptive humoral and cellular responses mediated via lymphocytes also interfere with viral infection in the tumor.
Antibody responses can neutralize therapeutic viruses posing a challenge for multiple-dose regimens. Studies suggest that the use of a higher virus dosage for booster injections could overcome this limitation [4]. Alternatively, Biswas et al., using Newcastle disease virus, showed that complement-mediated opsonization was evaded when regulators of complement activating factors were incorporated in the viral envelope [76]. Zemp et al. reported that resistance to oncolytic myxoma virus in a glioma models involves a multifaceted cellular immune response that can be reversed with cyclophosphamide-mediated lymphoablation [77]. Similarly, cytolysis of virus-infected tumor cells by T-cells or NK cells has been observed to prevent the multiplication of viruses in the tumor microenvironment, decreasing the infection of surrounding tumor cells [78]. Tumor-associated macrophages may also prevent the infection of tumor cells by engulfing a large proportion of virus particles.
Systemic responses: Certain mechanisms that impede virotherapy occur at complex levels and exist due to the multidimensional interaction of various cells present in the microenvironment. For example, epithelial cells have been observed to act as a physical barrier and regulate the spatiotemporal spread of viruses in the tumor and periphery to target and lyse cancer cells [9,10]. Furthermore, angiogenesis and the vascular leakiness at local tumor sites have been proven to be a regulatory factor in viral spread and infection [11,79]. Limited viral replication in hypoxic zones of tumors is also responsible for resistance against virotherapy and requires engineering of a hypoxia-inducible expression system to restore oncolytic activity [80]. Alternatively, the presence of a dense extracellular matrix seems a limiting factor for spreading of viruses in tumors as well [32] and indicates the possibility of the inhibition of viral replication at a stage beyond viral entry [19,81]. Other than physical constraints, soluble signals such as prostaglandin [24] and vascular endothelial growth factor (VEGF) [11] have been observed to promote the accumulation of granulocytic myeloid suppressor cells correlating with resistance in the tumor microenvironment. Finally, healthy erythrocytes were recently discovered to play a role in decreasing viral spread in the body [82], indicating that certain mechanisms could be overlooked due to the choice of the model or by limiting our focus on disease pathology and not on the healthy state.
Thus, to successfully infect cancer cells, viruses need to cross a multitude of obstacles that arise at a systemic level. Challenges in the design of novel viral-vector-based therapeutics are therefore related to inefficient bio-distribution, suboptimal infection due to tumor hypoxia, overcoming vascular barriers, escaping antiviral immune responses, and intracellular antiviral molecular mechanisms. Moreover, it should be noted that such mechanisms when considered together might confer a certain degree of resistance to viral infection and subsequent oncolysis. However, due to the limited number of studies, the importance of the contribution of the different resistance mechanisms remains difficult to estimate.
Alternatively, multiple mechanisms in the tumor microenvironment have also been observed to promote virotherapy, which can be exploited to improve therapeutic efficacy. For example, an anti-inflammatory crosstalk between tumor cells and tumor-associated fibroblasts mediated by transforming growth factor-β (TGF-β) and fibroblast growth factor 2 (FGF2), respectively, has been found to sensitize both tumor cells and fibroblasts for viral infection [83]. Moreover, reovirus internalized by tumor-associated dendritic cells has been reported to promote virotherapy, providing protection against antibody-mediated neutralization [84,85]. Similarly, myeloid-derived suppressor cells have been found to act as a vehicle for tumor-specific delivery and targeting of oncolytic viruses [86]. Apart from viral delivery and protection, the role of myeloid cells has been studied extensively in the context of tumor antigen presentation [87] and antitumor immune responses post-virotherapy [88]. Therefore, exploiting mechanisms that allow us to sensitize tumor cells for viral infection and utilize stromal cells for viral delivery and tumor-associated myeloid cells for antitumor immunity could be some of the primary objectives for future research in the field of cancer virotherapy.
5. Conclusions
Through a systematic analysis of published literature, we identified key resistance mechanisms and cell types that are involved in undermining the therapeutic efficacy of oncolytic viruses. Importantly, our analysis demonstrated that resistance mechanisms can be classified as tumor-cell-mediated responses, stromal cell responses, antiviral immune responses, and systemic responses, which are involved in conferring resistance to various virus types in different cancers. Our results indicate the need to further explore known mechanisms such as interferon-mediated resistance and find novel factors such as epigenetic modifications, tumor heterogeneity, hypoxia, secreted factors, etc., that are responsible for promoting resistance to virotherapy. Of note, the analysis revealed the need to extensively assess the role of stromal cells such as fibroblasts, epithelial cells, endothelial cells, and tumor-associated myeloid cells, as to how they protect cancer cells from viral infection and oncolysis.
An extensive knowledge of such resistance mechanisms will aid researchers and clinicians in the rational design of virotherapy and for the implementation of appropriate therapeutic strategies. Research to date has shown the importance of acknowledging cancer as an evolving disease and the need to consider strategic therapeutics to combat resistance. Cell-based vector delivery to escape neutralizing antibodies, synthetic transgene expression circuits for efficient viral replication, suppression of antiviral interferon signaling, and directed evolution of viral vectors are a few of the existing techniques that can allow us to engineer and screen for viral vectors that can overcome their previous limitations. Thus, through our analysis and results, we encourage studies that explore resistance towards oncolytic viruses and hope to inspire the rational design of oncolytic viruses that can overcome therapeutic resistance.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/vaccines9101166/s1, Supplementary Information File S1 (.pdf), and Table S1: List of articles included in the systematic analysis, with the information of the first authors’ name, PUBMED identity number, DOI number, year of publication, cancer type, viral vector platforms, resistance mechanisms, and source of resistance to virotherapy. Table S1 is attached as a .xlsx file.
Author Contributions
All authors made substantial contributions to the manuscript. Conceptualization and design, D.K.B. and T.D.; supervision and funding acquisition, T.D. and R.C.; collection and assembly of data, D.K.B.; data analysis and interpretation, D.K.B., T.D. and R.C.; manuscript writing and editing, D.K.B., R.C. and T.D. All authors have read and agreed to the published version of the manuscript.
Funding
The authors’ work was supported by CNPq (Grant Award 305700/2017-0 to R.C.), CAPES (Finance Code 001, to D.B.), and an ATTP-GSMS scholarship (Abel Tasman Talent Program to D.K.B.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Attached as Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aktipis C.A., Kwan V.S.Y., Johnson K.A., Neuberg S.L., Maley C.C. Overlooking Evolution: A Systematic Analysis of Cancer Relapse and Therapeutic Resistance Research. PLoS ONE. 2011;6:e26100. doi: 10.1371/journal.pone.0026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaufman H.L., Kohlhapp F.J., Zloza A. Oncolytic Viruses: A New Class of Immunotherapy Drugs. Nat. Rev. Drug. Discov. 2015;14:642–662. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Twumasi-Boateng K., Pettigrew J.L., Kwok Y.Y.E., Bell J.C., Nelson B.H. Oncolytic Viruses as Engineering Platforms for Combination Immunotherapy. Nat. Rev. Cancer. 2018;18:419–432. doi: 10.1038/s41568-018-0009-4. [DOI] [PubMed] [Google Scholar]
- 4.Buijs P.R.A., Verhagen J.H.E., van Eijck C.H.J., van den Hoogen B.G. Oncolytic Viruses: From Bench to Bedside with a Focus on Safety. Hum. Vaccin Immunother. 2015;11:1573–1584. doi: 10.1080/21645515.2015.1037058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demšar J., Curk T., Erjavec A., Gorup C., Hocevar T., Milutinovic M., Možina M., Polajnar M., Toplak M., Staric A., et al. Orange: Data Mining Toolbox in Python. J. Mach. Learn. Res. 2013;14:2349–2353. [Google Scholar]
- 6.Mauri M., Elli T., Caviglia G., Uboldi G., Azzi M. RAWGraphs: A Visualisation Platform to Create Open Outputs; Proceedings of the 12th Biannual Conference on Italian SIGCHI Chapter; Cagliari, Italy. 18 September 2017; Cagliari, Italy: ACM; 2017. pp. 1–5. [Google Scholar]
- 7.Arwert E.N., Milford E.L., Rullan A., Derzsi S., Hooper S., Kato T., Mansfield D., Melcher A., Harrington K.J., Sahai E. STING and IRF3 in Stromal Fibroblasts Enable Sensing of Genomic Stress in Cancer Cells to Undermine Oncolytic Viral Therapy. Nat. Cell Biol. 2020;22:758–766. doi: 10.1038/s41556-020-0527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Asten S.D., Raaben M., Nota B., Spaapen R.M. Secretome Screening Reveals Fibroblast Growth Factors as Novel Inhibitors of Viral Replication. J. Virol. 2018;92:e00260-18. doi: 10.1128/JVI.00260-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong J.G., Valdes Y.R., Sivapragasam M., Barrett J.W., Bell J.C., Stojdl D., DiMattia G.E., Shepherd T.G. Spatial and Temporal Epithelial Ovarian Cancer Cell Heterogeneity Impacts Maraba Virus Oncolytic Potential. BMC Cancer. 2017;17:594. doi: 10.1186/s12885-017-3600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yumul R., Richter M., Lu Z.-Z., Saydaminova K., Wang H., Wang C.-H.K., Carter D., Lieber A. Epithelial Junction Opener Improves Oncolytic Adenovirus Therapy in Mouse Tumor Models. Hum. Gene Ther. 2016;27:325–337. doi: 10.1089/hum.2016.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng J.-C., Granot T., DiGiacomo V., Levin B., Meruelo D. Enhanced Specific Delivery and Targeting of Oncolytic Sindbis Viral Vectors by Modulating Vascular Leakiness in Tumor. Cancer Gene Ther. 2010;17:244–255. doi: 10.1038/cgt.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y.-P., Suksanpaisan L., Steele M.B., Russell S.J., Peng K.-W. Induction of Antiviral Genes by the Tumor Microenvironment Confers Resistance to Virotherapy. Sci. Rep. 2013;3:2375. doi: 10.1038/srep02375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janelle V., Brassard F., Lapierre P., Lamarre A., Poliquin L. Mutations in the Glycoprotein of Vesicular Stomatitis Virus Affect Cytopathogenicity: Potential for Oncolytic Virotherapy. J. Virol. 2011;85:6513–6520. doi: 10.1128/JVI.02484-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu X., Tao L., Rivera A., Zhang X. Rapamycin Enhances the Activity of Oncolytic Herpes Simplex Virus against Tumor Cells That Are Resistant to Virus Replication. Int. J. Cancer. 2011;129:1503–1510. doi: 10.1002/ijc.25808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paglino J.C., van den Pol A.N. Vesicular Stomatitis Virus Has Extensive Oncolytic Activity against Human Sarcomas: Rare Resistance Is Overcome by Blocking Interferon Pathways. J. Virol. 2011;85:9346–9358. doi: 10.1128/JVI.00723-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song T.-J., Haddad D., Adusumilli P., Kim T., Stiles B., Hezel M., Socci N.D., Gönen M., Fong Y. Molecular Network Pathways and Functional Analysis of Tumor Signatures Associated with Development of Resistance to Viral Gene Therapy. Cancer Gene Ther. 2012;19:38–48. doi: 10.1038/cgt.2011.64. [DOI] [PubMed] [Google Scholar]
- 17.Noll M., Berchtold S., Lampe J., Malek N.P., Bitzer M., Lauer U.M. Primary Resistance Phenomena to Oncolytic Measles Vaccine Viruses. Int. J. Oncol. 2013;43:103–112. doi: 10.3892/ijo.2013.1914. [DOI] [PubMed] [Google Scholar]
- 18.Le Bœuf F., Batenchuk C., Vähä-Koskela M., Breton S., Roy D., Lemay C., Cox J., Abdelbary H., Falls T., Waghray G., et al. Model-Based Rational Design of an Oncolytic Virus with Improved Therapeutic Potential. Nat. Commun. 2013;4:1974. doi: 10.1038/ncomms2974. [DOI] [PubMed] [Google Scholar]
- 19.Voros A., Kormos B., Valyi-Nagy T., Valyi-Nagy K. Increased Resistance of Breast, Prostate, and Embryonic Carcinoma Cells against Herpes Simplex Virus in Three-Dimensional Cultures. ISRN Oncol. 2013;2013:104913. doi: 10.1155/2013/104913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paglino J.C., Andres W., van den Pol A.N. Autonomous Parvoviruses Neither Stimulate nor Are Inhibited by the Type I Interferon Response in Human Normal or Cancer Cells. J. Virol. 2014;88:4932–4942. doi: 10.1128/JVI.03508-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cronin M., Le Boeuf F., Murphy C., Roy D.G., Falls T., Bell J.C., Tangney M. Bacterial-Mediated Knockdown of Tumor Resistance to an Oncolytic Virus Enhances Therapy. Mol. Ther. 2014;22:1188–1197. doi: 10.1038/mt.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su B.-H., Shieh G.-S., Tseng Y.-L., Shiau A.-L., Wu C.-L. Etoposide Enhances Antitumor Efficacy of MDR1-Driven Oncolytic Adenovirus through Autoupregulation of the MDR1 Promoter Activity. Oncotarget. 2015;6:38308–38326. doi: 10.18632/oncotarget.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vähä-Koskela M., Tähtinen S., Grönberg-Vähä-Koskela S., Taipale K., Saha D., Merisalo-Soikkeli M., Ahonen M., Rouvinen-Lagerström N., Hirvinen M., Veckman V., et al. Overcoming Tumor Resistance by Heterologous Adeno-Poxvirus Combination Therapy. Mol. Ther. Oncolytics. 2015;1:14006. doi: 10.1038/mto.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou W., Sampath P., Rojas J.J., Thorne S.H. Oncolytic Virus-Mediated Targeting of PGE2 in the Tumor Alters the Immune Status and Sensitizes Established and Resistant Tumors to Immunotherapy. Cancer Cell. 2016;30:108–119. doi: 10.1016/j.ccell.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleinlützum D., Hanauer J.D.S., Muik A., Hanschmann K.-M., Kays S.-K., Ayala-Breton C., Peng K.-W., Mühlebach M.D., Abel T., Buchholz C.J. Enhancing the Oncolytic Activity of CD133-Targeted Measles Virus: Receptor Extension or Chimerism with Vesicular Stomatitis Virus Are Most Effective. Front. Oncol. 2017;7:127. doi: 10.3389/fonc.2017.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selman M., Ou P., Rousso C., Bergeron A., Krishnan R., Pikor L., Chen A., Keller B.A., Ilkow C., Bell J.C., et al. Dimethyl Fumarate Potentiates Oncolytic Virotherapy through NF-ΚB Inhibition. Sci. Transl. Med. 2018;10:eaao1613. doi: 10.1126/scitranslmed.aao1613. [DOI] [PubMed] [Google Scholar]
- 27.Zamarin D., Ricca J.M., Sadekova S., Oseledchyk A., Yu Y., Blumenschein W.M., Wong J., Gigoux M., Merghoub T., Wolchok J.D. PD-L1 in Tumor Microenvironment Mediates Resistance to Oncolytic Immunotherapy. J. Clin. Investig. 2018;128:1413–1428. doi: 10.1172/JCI98047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurokawa C., Iankov I.D., Anderson S.K., Aderca I., Leontovich A.A., Maurer M.J., Oberg A.L., Schroeder M.A., Giannini C., Greiner S.M., et al. Constitutive Interferon Pathway Activation in Tumors as an Efficacy Determinant Following Oncolytic Virotherapy. J. Nat. Cancer Inst. 2018;110:1123–1132. doi: 10.1093/jnci/djy033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao X., Liang J., Huang C., Li K., Xing F., Zhu W., Lin Z., Xu W., Wu G., Zhang J., et al. DNA-PK Inhibition Synergizes with Oncolytic Virus M1 by Inhibiting Antiviral Response and Potentiating DNA Damage. Nat. Commun. 2018;9:4342. doi: 10.1038/s41467-018-06771-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Nakatake M., Kurosaki H., Kuwano N., Horita K., Ito M., Kono H., Okamura T., Hasegawa K., Yasutomi Y., Nakamura T. Partial Deletion of Glycoprotein B5R Enhances Vaccinia Virus Neutralization Escape While Preserving Oncolytic Function. Mol. Ther. Oncolytics. 2019;14:159–171. doi: 10.1016/j.omto.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berchtold S., Beil J., Raff C., Smirnow I., Schell M., D’Alvise J., Gross S., Lauer U.M. Assessing and Overcoming Resistance Phenomena against a Genetically Modified Vaccinia Virus in Selected Cancer Cell Lines. Int. J. Mol. Sci. 2020;21:7618. doi: 10.3390/ijms21207618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe Y., Kojima T., Kagawa S., Uno F., Hashimoto Y., Kyo S., Mizuguchi H., Tanaka N., Kawamura H., Ichimaru D., et al. A Novel Translational Approach for Human Malignant Pleural Mesothelioma: Heparanase-Assisted Dual Virotherapy. Oncogene. 2010;29:1145–1154. doi: 10.1038/onc.2009.415. [DOI] [PubMed] [Google Scholar]
- 33.Felt S.A., Droby G.N., Grdzelishvili V.Z. Ruxolitinib and Polycation Combination Treatment Overcomes Multiple Mechanisms of Resistance of Pancreatic Cancer Cells to Oncolytic Vesicular Stomatitis Virus. J. Virol. 2017;91:e00461-17. doi: 10.1128/JVI.00461-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuzmen C., Cairns T.M., Atanasiu D., Lou H., Saw W.T., Hall B.L., Cohen J.B., Cohen G.H., Glorioso J.C. Point Mutations in Retargeted GD Eliminate the Sensitivity of EGFR/EGFRvIII-Targeted HSV to Key Neutralizing Antibodies. Mol. Ther. Methods Clin. Dev. 2020;16:145–154. doi: 10.1016/j.omtm.2019.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi A.H., O’Leary M.P., Lu J., Kim S.-I., Fong Y., Chen N.G. Endogenous Akt Activity Promotes Virus Entry and Predicts Efficacy of Novel Chimeric Orthopoxvirus in Triple-Negative Breast Cancer. Mol. Ther. Oncolytics. 2018;9:22–29. doi: 10.1016/j.omto.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tong Y., Zhu W., Huang X., You L., Han X., Yang C., Qian W. PI3K Inhibitor LY294002 Inhibits Activation of the Akt/MTOR Pathway Induced by an Oncolytic Adenovirus Expressing TRAIL and Sensitizes Multiple Myeloma Cells to the Oncolytic Virus. Oncol. Rep. 2014;31:1581–1588. doi: 10.3892/or.2014.3020. [DOI] [PubMed] [Google Scholar]
- 37.Lucas T., Benihoud K., Vigant F., Schmidt C.Q., Wortmann A., Bachem M.G., Simmet T., Kochanek S. Hexon Modification to Improve the Activity of Oncolytic Adenovirus Vectors against Neoplastic and Stromal Cells in Pancreatic Cancer. PLoS ONE. 2015;10:e0117254. doi: 10.1371/journal.pone.0117254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shulak L., Beljanski V., Chiang C., Dutta S.M., Van Grevenynghe J., Belgnaoui S.M., Nguyên T.L.-A., Di Lenardo T., Semmes O.J., Lin R., et al. Histone Deacetylase Inhibitors Potentiate Vesicular Stomatitis Virus Oncolysis in Prostate Cancer Cells by Modulating NF-ΚB-Dependent Autophagy. J. Virol. 2014;88:2927–2940. doi: 10.1128/JVI.03406-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bieler A., Mantwill K., Dravits T., Bernshausen A., Glockzin G., Köhler-Vargas N., Lage H., Gansbacher B., Holm P.S. Novel Three-Pronged Strategy to Enhance Cancer Cell Killing in Glioblastoma Cell Lines: Histone Deacetylase Inhibitor, Chemotherapy, and Oncolytic Adenovirus Dl520. Hum. Gene Ther. 2006;17:55–70. doi: 10.1089/hum.2006.17.55. [DOI] [PubMed] [Google Scholar]
- 40.Trus I., Berube N., Jiang P., Rak J., Gerdts V., Karniychuk U. Zika Virus with Increased CpG Dinucleotide Frequencies Shows Oncolytic Activity in Glioblastoma Stem Cells. Viruses. 2020;12:579. doi: 10.3390/v12050579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huff A.L., Wongthida P., Kottke T., Thompson J.M., Driscoll C.B., Schuelke M., Shim K.G., Harris R.S., Molan A., Pulido J.S., et al. APOBEC3 Mediates Resistance to Oncolytic Viral Therapy. Mol. Ther. Oncolytics. 2018;11:1–13. doi: 10.1016/j.omto.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evgin L., Huff A.L., Kottke T., Thompson J., Molan A.M., Driscoll C.B., Schuelke M., Shim K.G., Wongthida P., Ilett E.J., et al. Suboptimal T-Cell Therapy Drives a Tumor Cell Mutator Phenotype That Promotes Escape from First-Line Treatment. Cancer Immunol. Res. 2019;7:828–840. doi: 10.1158/2326-6066.CIR-18-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toribio R., Díaz-López I., Berlanga J.J., Molina-Jiménez F., Majano P., Ventoso I. Naturally Occurring and Engineered Alphaviruses Sensitive to Double-Stranded-RNA-Activated Protein Kinase Show Restricted Translation in Mammalian Cells, Increased Sensitivity to Interferon, and Marked Oncotropism. J. Virol. 2020;94:e01630-19. doi: 10.1128/JVI.01630-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lv C., Su Q., Liang Y., Hu J., Yuan S. Oncolytic Vaccine Virus Harbouring the IL-24 Gene Suppresses the Growth of Lung Cancer by Inducing Apoptosis. Biochem. Biophys. Res. Commun. 2016;476:21–28. doi: 10.1016/j.bbrc.2016.05.088. [DOI] [PubMed] [Google Scholar]
- 45.Li L., You L.-S., Mao L.-P., Jin S.-H., Chen X.-H., Qian W.-B. Combing Oncolytic Adenovirus Expressing Beclin-1 with Chemotherapy Agent Doxorubicin Synergistically Enhances Cytotoxicity in Human CML Cells in Vitro. Acta Pharm. Sin. 2018;39:251–260. doi: 10.1038/aps.2017.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colunga A., Bollino D., Schech A., Aurelian L. Calpain-Dependent Clearance of the Autophagy Protein P62/SQSTM1 Is a Contributor to ΔPK Oncolytic Activity in Melanoma. Gene Ther. 2014;21:371–378. doi: 10.1038/gt.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang K., Li Y., Zhu Q., Xu J., Wang Y., Deng W., Liu Q., Zhang G., Meng S. Pharmacological Modulation of Autophagy Enhances Newcastle Disease Virus-Mediated Oncolysis in Drug-Resistant Lung Cancer Cells. BMC Cancer. 2014;14:551. doi: 10.1186/1471-2407-14-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hastie E., Cataldi M., Steuerwald N., Grdzelishvili V.Z. An Unexpected Inhibition of Antiviral Signaling by Virus-Encoded Tumor Suppressor P53 in Pancreatic Cancer Cells. Virology. 2015;483:126–140. doi: 10.1016/j.virol.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sunamura M., Hamada H., Motoi F., Oonuma M., Abe H., Saitoh Y., Hoshida T., Ottomo S., Omura N., Matsuno S. Oncolytic Virotherapy as a Novel Strategy for Pancreatic Cancer. Pancreas. 2004;28:326–329. doi: 10.1097/00006676-200404000-00022. [DOI] [PubMed] [Google Scholar]
- 50.Cataldi M., Shah N.R., Felt S.A., Grdzelishvili V.Z. Breaking Resistance of Pancreatic Cancer Cells to an Attenuated Vesicular Stomatitis Virus through a Novel Activity of IKK Inhibitor TPCA-1. Virology. 2015;485:340–354. doi: 10.1016/j.virol.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoang H.-D., Graber T.E., Jia J.-J., Vaidya N., Gilchrist V.H., Xiang X., Li W., Cowan K.N., Gkogkas C.G., Jaramillo M., et al. Induction of an Alternative MRNA 5’ Leader Enhances Translation of the Ciliopathy Gene Inpp5e and Resistance to Oncolytic Virus Infection. Cell Rep. 2019;29:4010–4023.e5. doi: 10.1016/j.celrep.2019.11.072. [DOI] [PubMed] [Google Scholar]
- 52.Gholami S., Chen C.-H., Gao S., Lou E., Fujisawa S., Carson J., Nnoli J.E., Chou T.-C., Bromberg J., Fong Y. Role of MAPK in Oncolytic Herpes Viral Therapy in Triple-Negative Breast Cancer. Cancer Gene Ther. 2014;21:283–289. doi: 10.1038/cgt.2014.28. [DOI] [PubMed] [Google Scholar]
- 53.Bommareddy P.K., Aspromonte S., Zloza A., Rabkin S.D., Kaufman H.L. MEK Inhibition Enhances Oncolytic Virus Immunotherapy through Increased Tumor Cell Killing and T Cell Activation. Sci. Transl. Med. 2018;10:417. doi: 10.1126/scitranslmed.aau0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li W., Turaga R.C., Li X., Sharma M., Enadi Z., Dunham Tompkins S.N., Hardy K.C., Mishra F., Tsao J., Liu Z.-R., et al. Overexpression of Smac by an Armed Vesicular Stomatitis Virus Overcomes Tumor Resistance. Mol. Ther. Oncolytics. 2019;14:188–195. doi: 10.1016/j.omto.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dobson C.C., Naing T., Beug S.T., Faye M.D., Chabot J., St-Jean M., Walker D.E., LaCasse E.C., Stojdl D.F., Korneluk R.G., et al. Oncolytic Virus Synergizes with Smac Mimetic Compounds to Induce Rhabdomyosarcoma Cell Death in a Syngeneic Murine Model. Oncotarget. 2017;8:3495–3508. doi: 10.18632/oncotarget.13849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muscolini M., Castiello L., Palermo E., Zevini A., Ferrari M., Olagnier D., Hiscott J. SIRT1 Modulates the Sensitivity of Prostate Cancer Cells to Vesicular Stomatitis Virus Oncolysis. J. Virol. 2019;93:e00626-19. doi: 10.1128/JVI.00626-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y., Zhuo B., Yin Y., Han T., Li S., Li Z., Wang J. Anti-Cancer Effect of Oncolytic Adenovirus-Armed ShRNA Targeting MYCN Gene on Doxorubicin-Resistant Neuroblastoma Cells. Biochem. Biophys. Res. Commun. 2017;491:134–139. doi: 10.1016/j.bbrc.2017.07.062. [DOI] [PubMed] [Google Scholar]
- 58.Li I., Nabet B.Y. Exosomes in the Tumor Microenvironment as Mediators of Cancer Therapy Resistance. Mol. Cancer. 2019;18:32. doi: 10.1186/s12943-019-0975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Urbiola C., Santer F.R., Petersson M., van der Pluijm G., Horninger W., Erlmann P., Wollmann G., Kimpel J., Culig Z., von Laer D. Oncolytic Activity of the Rhabdovirus VSV-GP against Prostate Cancer. Int. J. Cancer. 2018;143:1786–1796. doi: 10.1002/ijc.31556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allagui F., Achard C., Panterne C., Combredet C., Labarrière N., Dréno B., Elgaaied A.B., Pouliquen D., Tangy F., Fonteneau J.-F., et al. Modulation of the Type I Interferon Response Defines the Sensitivity of Human Melanoma Cells to Oncolytic Measles Virus. Curr. Gene Ther. 2017;16:419–428. doi: 10.2174/1566523217666170102110502. [DOI] [PubMed] [Google Scholar]
- 61.Tarasova I.A., Tereshkova A.V., Lobas A.A., Solovyeva E.M., Sidorenko A.S., Gorshkov V., Kjeldsen F., Bubis J.A., Ivanov M.V., Ilina I.Y., et al. Comparative Proteomics as a Tool for Identifying Specific Alterations within Interferon Response Pathways in Human Glioblastoma Multiforme Cells. Oncotarget. 2018;9:1785–1802. doi: 10.18632/oncotarget.22751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaowinn S., Cho I.-R., Moon J., Jun S.W., Kim C.S., Kang H.Y., Kim M., Koh S.S., Chung Y.-H. Pancreatic Adenocarcinoma Upregulated Factor (PAUF) Confers Resistance to Pancreatic Cancer Cells against Oncolytic Parvovirus H-1 Infection through IFNA Receptor-Mediated Signaling. Biochem. Biophys. Res. Commun. 2015;459:313–318. doi: 10.1016/j.bbrc.2015.02.107. [DOI] [PubMed] [Google Scholar]
- 63.Westcott M.M., Liu J., Rajani K., D’Agostino R.J., Lyles D.S., Porosnicu M. Interferon Beta and Interferon Alpha 2a Differentially Protect Head and Neck Cancer Cells from Vesicular Stomatitis Virus-Induced Oncolysis. J. Virol. 2015;89:7944–7954. doi: 10.1128/JVI.00757-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vähä-Koskela M.J.V., Le Boeuf F., Lemay C., De Silva N., Diallo J.-S., Cox J., Becker M., Choi Y., Ananth A., Sellers C., et al. Resistance to Two Heterologous Neurotropic Oncolytic Viruses, Semliki Forest Virus and Vaccinia Virus, in Experimental Glioma. J. Virol. 2013;87:2363–2366. doi: 10.1128/JVI.01609-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moerdyk-Schauwecker M., Shah N.R., Murphy A.M., Hastie E., Mukherjee P., Grdzelishvili V.Z. Resistance of Pancreatic Cancer Cells to Oncolytic Vesicular Stomatitis Virus: Role of Type I Interferon Signaling. Virology. 2013;436:221–234. doi: 10.1016/j.virol.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liikanen I., Monsurrò V., Ahtiainen L., Raki M., Hakkarainen T., Diaconu I., Escutenaire S., Hemminki O., Dias J.D., Cerullo V., et al. Induction of Interferon Pathways Mediates in Vivo Resistance to Oncolytic Adenovirus. Mol. Ther. 2011;19:1858–1866. doi: 10.1038/mt.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saloura V., Wang L.-C.S., Fridlender Z.G., Sun J., Cheng G., Kapoor V., Sterman D.H., Harty R.N., Okumura A., Barber G.N., et al. Evaluation of an Attenuated Vesicular Stomatitis Virus Vector Expressing Interferon-Beta for Use in Malignant Pleural Mesothelioma: Heterogeneity in Interferon Responsiveness Defines Potential Efficacy. Hum. Gene Ther. 2010;21:51–64. doi: 10.1089/hum.2009.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patel M.R., Dash A., Jacobson B.A., Ji Y., Baumann D., Ismail K., Kratzke R.A. JAK/STAT Inhibition with Ruxolitinib Enhances Oncolytic Virotherapy in Non-Small Cell Lung Cancer Models. Cancer Gene Ther. 2019;26:411–418. doi: 10.1038/s41417-018-0074-6. [DOI] [PubMed] [Google Scholar]
- 69.Udayakumar T.S., Betancourt D.M., Ahmad A., Tao W., Totiger T.M., Patel M., Marples B., Barber G., Pollack A. Radiation Attenuates Prostate Tumor Antiviral Responses to Vesicular Stomatitis Virus Containing IFNβ, Resulting in Pronounced Antitumor Systemic Immune Responses. Mol. Cancer Res. 2020;18:1232–1243. doi: 10.1158/1541-7786.MCR-19-0836. [DOI] [PubMed] [Google Scholar]
- 70.Dold C., Rodriguez Urbiola C., Wollmann G., Egerer L., Muik A., Bellmann L., Fiegl H., Marth C., Kimpel J., von Laer D. Application of Interferon Modulators to Overcome Partial Resistance of Human Ovarian Cancers to VSV-GP Oncolytic Viral Therapy. Mol. Ther. Oncolytics. 2016;3:16021. doi: 10.1038/mto.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diallo J.-S., Le Boeuf F., Lai F., Cox J., Vaha-Koskela M., Abdelbary H., MacTavish H., Waite K., Falls T., Wang J., et al. A High-Throughput Pharmacoviral Approach Identifies Novel Oncolytic Virus Sensitizers. Mol. Ther. 2010;18:1123–1129. doi: 10.1038/mt.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lypova N., Lanceta L., Gibson A., Vega S., Garza-Morales R., McMasters K.M., Chesney J., Gomez-Gutierrez J.G., Imbert-Fernandez Y. Targeting Palbociclib-Resistant Estrogen Receptor-Positive Breast Cancer Cells via Oncolytic Virotherapy. Cancers. 2019;11:684. doi: 10.3390/cancers11050684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sivanandam V., LaRocca C.J., Chen N.G., Fong Y., Warner S.G. Oncolytic Viruses and Immune Checkpoint Inhibition: The Best of Both Worlds. Mol. Ther. Oncolytics. 2019;13:93–106. doi: 10.1016/j.omto.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Melero I., Quetglas J.I., Reboredo M., Dubrot J., Rodriguez-Madoz J.R., Mancheño U., Casales E., Riezu-Boj J.I., Ruiz-Guillen M., Ochoa M.C., et al. Strict Requirement for Vector-Induced Type I Interferon in Efficacious Antitumor Responses to Virally Encoded IL12. Cancer Res. 2015;75:497–507. doi: 10.1158/0008-5472.CAN-13-3356. [DOI] [PubMed] [Google Scholar]
- 75.Pinto A.K., Daffis S., Brien J.D., Gainey M.D., Yokoyama W.M., Sheehan K.C.F., Murphy K.M., Schreiber R.D., Diamond M.S. A Temporal Role of Type I Interferon Signaling in CD8+ T Cell Maturation during Acute West Nile Virus Infection. PLoS Pathog. 2011;7:e1002407. doi: 10.1371/journal.ppat.1002407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Biswas M., Johnson J.B., Kumar S.R.P., Parks G.D., Elankumarana S. Incorporation of Host Complement Regulatory Proteins into Newcastle Disease Virus Enhances Complement Evasion. J. Virol. 2012;86:12708–12716. doi: 10.1128/JVI.00886-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zemp F.J., McKenzie B.A., Lun X., Reilly K.M., McFadden G., Yong V.W., Forsyth P.A. Cellular Factors Promoting Resistance to Effective Treatment of Glioma with Oncolytic Myxoma Virus. Cancer Res. 2014;74:7260–7273. doi: 10.1158/0008-5472.CAN-14-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carey B.L., Ahmed M., Puckett S., Lyles D.S. Early Steps of the Virus Replication Cycle Are Inhibited in Prostate Cancer Cells Resistant to Oncolytic Vesicular Stomatitis Virus. J. Virol. 2008;82:12104–12115. doi: 10.1128/JVI.01508-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gil M., Seshadri M., Komorowski M.P., Abrams S.I., Kozbor D. Targeting CXCL12/CXCR4 Signaling with Oncolytic Virotherapy Disrupts Tumor Vasculature and Inhibits Breast Cancer Metastases. Proc. Natl. Acad. Sci. USA. 2013;110:E1291–E1300. doi: 10.1073/pnas.1220580110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reinblatt M., Pin R.H., Federoff H.J., Fong Y. Utilizing Tumor Hypoxia to Enhance Oncolytic Viral Therapy in Colorectal Metastases. Ann. Surg. 2004;239:892–899. doi: 10.1097/01.sla.0000128308.36393.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Valyi-Nagy K., Dosa S., Kovacs S.K., Bacsa S., Voros A., Shukla D., Folberg R., Valyi-Nagy T. Identification of Virus Resistant Tumor Cell Subpopulations in Three-Dimensional Uveal Melanoma Cultures. Cancer Gene Ther. 2010;17:223–234. doi: 10.1038/cgt.2009.73. [DOI] [PubMed] [Google Scholar]
- 82.Sun C.W., Willmon C., Wu L.-C., Knopick P., Thoerner J., Vile R., Townes T.M., Terman D.S. Sickle Cells Abolish Melanoma Tumorigenesis in Hemoglobin SS Knockin Mice and Augment the Tumoricidal Effect of Oncolytic Virus In Vivo. Front. Oncol. 2016;6:166. doi: 10.3389/fonc.2016.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ilkow C.S., Marguerie M., Batenchuk C., Mayer J., Ben Neriah D., Cousineau S., Falls T., Jennings V.A., Boileau M., Bellamy D., et al. Reciprocal Cellular Cross-Talk within the Tumor Microenvironment Promotes Oncolytic Virus Activity. Nat. Med. 2015;21:530–536. doi: 10.1038/nm.3848. [DOI] [PubMed] [Google Scholar]
- 84.Ilett E.J., Bárcena M., Errington-Mais F., Griffin S., Harrington K.J., Pandha H.S., Coffey M., Selby P.J., Limpens R.W.A.L., Mommaas M., et al. Internalization of Oncolytic Reovirus by Human Dendritic Cell Carriers Protects the Virus from Neutralization. Clin. Cancer Res. 2011;17:2767–2776. doi: 10.1158/1078-0432.CCR-10-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jennings V.A., Ilett E.J., Scott K.J., West E.J., Vile R., Pandha H., Harrington K., Young A., Hall G.D., Coffey M., et al. Lymphokine-activated Killer and Dendritic Cell Carriage Enhances Oncolytic Reovirus Therapy for Ovarian Cancer by Overcoming Antibody Neutralization in Ascites. Int. J. Cancer. 2014;134:1091–1101. doi: 10.1002/ijc.28450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eisenstein S., Coakley B.A., Briley-Saebo K., Ma G., Chen H., Meseck M., Ward S., Divino C., Woo S., Chen S.-H., et al. Myeloid-Derived Suppressor Cells as a Vehicle for Tumor-Specific Oncolytic Viral Therapy. Cancer Res. 2013;73:5003–5015. doi: 10.1158/0008-5472.CAN-12-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guillerme J.-B., Boisgerault N., Roulois D., Ménager J., Combredet C., Tangy F., Fonteneau J.-F., Gregoire M. Measles Virus Vaccine–Infected Tumor Cells Induce Tumor Antigen Cross-Presentation by Human Plasmacytoid Dendritic Cells. Clin. Cancer Res. 2013;19:1147–1158. doi: 10.1158/1078-0432.CCR-12-2733. [DOI] [PubMed] [Google Scholar]
- 88.Kilinc M.O., Ehrig K., Pessian M., Minev B.R., Szalay A.A. Colonization of Xenograft Tumors by Oncolytic Vaccinia Virus (VACV) Results in Enhanced Tumor Killing Due to the Involvement of Myeloid Cells. J. Transl. Med. 2016;14:340. doi: 10.1186/s12967-016-1096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Attached as Supplementary Materials.