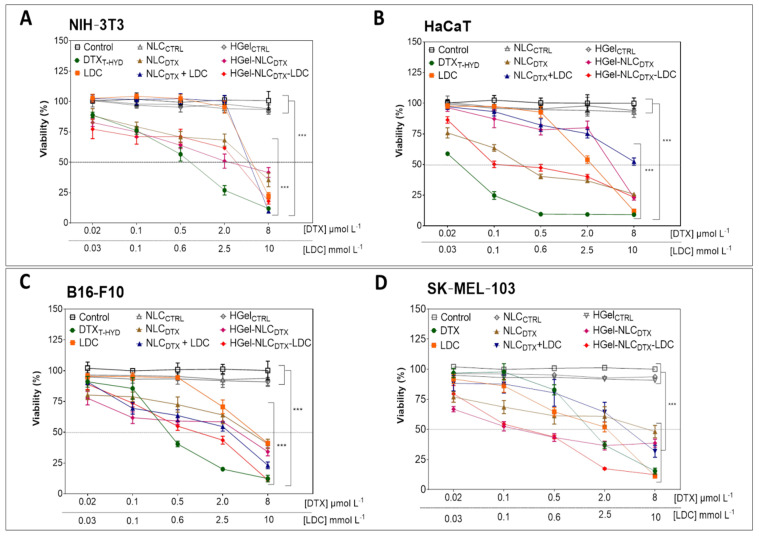
Figure 2.
Viability of NIH-3T3 (A), HaCaT (B), B16-F10 (C) and SK-MEL-103 (D) cells after 24 h of treatment with DTXT-HYD, LDC, NLCDTX, NLCDTX + LDC, HGel-NLCDTX or HGel-NLCDTX-LDC at 0.02, 0.1, 0.5, 2 and 8 µmol L−1 (equivalent DTX concentrations) and 0.03, 0.1, 0.6, 2.5 and 10 mmol L−1(equivalent LD concentrations, evaluated by MTT assay. In the statistical analyses (by two-Way ANOVA plus Bonferroni post-hoc) each treatment was compared to untreated (control) cells. In NIH-3T3 and B16-F10 cells, the DTXT-HYD, NLCDTX, NLCDTX + LDC, HGel-NLCDTX and HGel-NLCDTX-LDC formulations showed a significant reduction (*** p < 0.001) in cell viability at the concentrations of 0.02 µmol L−1 docetaxel and above. The same pattern of cytotoxicity was observed in SK-MEL-103 cells, except for the DTX formulation for which a significant reduction in cell viability (*** p < 0.001) was only observed from the concentration of 0.5 µmol L−1 docetaxel on. For HaCaT cells the DTX, NLCDTX and HGel-NLCDTX-LDC formulations promoted significant reduction (*** p < 0.001) in cell viability beginning at 0.02 µmol L−1 docetaxel, while the NLCDTX + LDC formulations and HGel-NLCDTX significantly reduced (*** p < 0.001) cell viability from the concentration of 0.1 µmol L−1 docetaxel and higher. Data expressed as mean ± SD (n = 3).