Abstract
An outbreak of mupirocin-resistant (MuR) staphylococci was investigated in two wards of a large hospital in Warsaw, Poland. Fifty-three MuR isolates of Staphylococcus aureus, S. epidermidis, S. haemolyticus, S. xylosus, and S. capitis were identified over a 17-month survey which was carried out after introduction of the drug for the treatment of skin infections. The isolates were collected from patients with infections, environmental samples, and carriers; they constituted 19.5% of all staphylococcal isolates identified in the two wards during that time. Almost all the MuR isolates were also resistant to methicillin (methicillin-resistant S. aureus and methicillin-resistant coagulase-negative staphylococci). Seven of the outbreak isolates expressed a low-level-resistance phenotype (MuL), whereas the remaining majority of isolates were found to be highly resistant to mupirocin (MuH). The mupA gene, responsible for the MuH phenotype, has been assigned to three different polymorphic loci among the strains in the collection analyzed. The predominant polymorph, polymorph I (characterized by a mupA-containing EcoRI DNA fragment of about 16 kb), was located on a specific plasmid which was widely distributed among the entire staphylococcal population. All MuR S. aureus isolates were found to represent a single epidemic strain, which was clonally disseminated in both wards. The S. epidermidis population was much more diverse; however, at least four clusters of closely related isolates were identified, which suggested that some strains of this species were also clonally spread in the hospital environment. Six isolates of S. epidermidis were demonstrated to express the MuL and MuH resistance mechanisms simultaneously, and this is the first identification of such dual MuR phenotype-bearing strains. The outbreak was attributed to a high level and inappropriate use of mupirocin, and as a result the dermatological formulation of the drug has been removed from the hospital formulary.
Methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant coagulase-negative staphylococci (MRCoNS) belong to the most important pathogens that cause nosocomial infections worldwide. Their incidence varies between different countries, hospitals, and hospital wards; and in a given hospital they may constitute from 1 to 80% of all staphylococcal isolates (14, 39). Many other resistance genes have readily been acquired by MRSA and MRCoNS, which, alarmingly, limits the choice of therapeutic options for the treatment of infections caused by these microorganisms.
One of the few antibiotics which is still effective against MRSA is mupirocin. It was introduced in 1985 in the United Kingdom and has successfully been used to treat various staphylococcal skin infections or to eradicate intranasal MRSA carriage. The unique bactericidal action of mupirocin is dependent on its ability to inhibit the isoleucyl-tRNA synthetase (15). The first mupirocin-resistant (MuR) strains appeared shortly after introduction of this drug. At first, the low-level-resistance phenotype (MuL; MICs, ≤256 μg/ml) was reported (16, 30), and this was followed soon after by the emergence of the high-level-resistance phenotype (MuH; MICs, ≥512 μg/ml) (26). In almost all cases reported to date, MuR staphylococci have also been found to be resistant to methicillin.
The MuL phenotype probably results from mutations in the staphylococcal native ileS gene, which encodes the isoleucyl-tRNA synthetase, whereas MuH has been attributed to the presence of the additional plasmid-borne gene, mupA, which codes for the isoleucyl-tRNA synthetase, which has no affinity to mupirocin (11). The mupA gene has been cloned (8) and sequenced (13). Sequence analysis has revealed that it shows a low degree of homology with the staphylococcal ileS gene. This has suggested that the gene responsible for the MuH phenotype has originated in other bacterial species.
The first survey, which encompassed four medical centers in Great Britain and which was reported on in 1990 (2), has revealed that only 0.3% of all S. aureus isolates and 3% of CoNS isolates were MuR at that time, and most of these were MuL. A questionnaire survey performed by the Central Public Health Laboratory in London, United Kingdom, in 1994 has indicated, however, that MuR staphylococci were present in 100 of 136 participating centers and that MuH strains were present in 35 of 136 participating centers all over Great Britain (21). A recent European multicenter study comprising 19 hospitals in which isolates collected in 1997 were analyzed has revealed mupirocin resistance in 3.9% (1.6% MuH) of all S. aureus isolates and in 12.8% (6.6% MuH) of CoNS isolates (29). Several outbreaks caused by MuH MRSA but also MuH MSSA have already been reported (4, 17, 18, 26, 34).
In the study presented here, we examined a group of staphylococcal isolates collected in the University Hospital in Warsaw, Poland, over a period of 17 months starting in February 1993. The collection included the first MuR staphylococci reported in Poland (32). The aim of the project was to study the prevalence of MuR strains in this hospital, determine their resistance phenotypes, and reveal the mechanisms of spread of the mupA gene among various species of staphylococci by the use of molecular methods.
(Part of this work was presented during the 8th European Congress of Clinical Microbiology and Infectious Diseases, Lausanne, Switzerland, May 1997, and the 9th European Congress of Clinical Microbiology and Infectious Diseases, Berlin, Germany, March 1999.)
MATERIALS AND METHODS
Clinical isolates.
A total of 272 Staphylococcus isolates were collected between February 1993 and June 1994 at the neonatal and pediatric wards of the University Hospital in Warsaw, which is a large teaching hospital with 720 beds and 15 wards. The collection included all the staphylococcal isolates recovered from the infection sites of patients (26%), isolates recovered from environmental samples (17%) taken over the 17-month period, and all staphylococcal isolates obtained in two rounds of carriage testing of personnel (nasal and throat swabs) both at the beginning and at the end of June 1994 (57%). Tests of environmental samples and for carriage were performed only in the neonatal ward. The hospital microbiology laboratory performed the collection and initial identification of bacterial isolates. All the isolates were subsequently subjected to screening for mupirocin resistance; and 53 MuR isolates of S. aureus, S. epidermidis, S. haemolyticus, S. capitis, and S. xylosus were selected. Fourteen MuR isolates (26%) were collected from sites of infection; they were recovered from conjunctiva, blood, and skin. Nineteen MuR isolates (36%) were detected in environmental samples, and 20 isolates (38%) were collected from carriers. S. aureus was identified by coagulase production (bound and free), and CoNS were identified to the species level by the ID32 STAPH ATB test (bioMérieux, Charbonnieres-les-Bains, France).
Antimicrobial susceptibility testing.
Susceptibility to a panel of 17 antimicrobial agents was performed by the disk diffusion method according to National Committee for Clinical Laboratory Standards (NCCLS) guidelines (24). Disks containing the following antimicrobial agents were used: amikacin, chloramphenicol, ciprofloxacin, clindamycin, erythromycin, fusidic acid (10 μg), gentamicin, kanamycin, lincomycin (2 μg), mupirocin (5 μg), neomycin (10 μg), penicillin, rifampin, streptomycin, tetracycline, trimethoprim-sulfamethoxazole, and vancomycin (Oxoid, Basingstoke, United Kingdom). The NCCLS criteria were applied for interpretation of the results (24). For fusidic acid, neomycin, and lincomycin, French guidelines were adopted (3). The results of mupirocin susceptibility testing were interpreted as described by Finlay et al. (10). The levels of mupirocin resistance were analyzed by evaluation of MICs by the agar dilution method as described by NCCLS (24) over a concentration range from 0.125 to 8,192 μg/ml. The mupirocin powder was supplied by SmithKline Beecham (London, United Kingdom). Isolates for which mupirocin MICs were higher than 4 μg/ml but not in excess of 256 μg/ml were regarded as MuL, and isolates for which mupirocin MICs were higher than 256 μg/ml were regarded as MuH (1). Resistance to methicillin was identified by the agar screening method as described by NCCLS (25) and on tryptic soy agar (bioMérieux) containing 25 mg of methicillin (SmithKline Beecham) per liter (6). Strains were classified as heterogeneous if growth of countable colonies was observed on plates with methicillin after 40 h of incubation at 37°C and as homogeneously resistant when confluent or semiconfluent growth was observed after 20 h of incubation, as described previously (20, 23, 33–35). Methicillin-susceptible S. aureus ATCC 25923 and methicillin-resistant S. aureus MR-3 (33) were used as standard strains for susceptibility testing.
Detection of mupA gene.
The mupA gene was detected by specific PCR with the use of MI and MII primers complementary to regions that encompass NcoI restriction sites located within the coding region of the mupA gene. The MI (5′-CTT ACC AGT TGA ATT) and MII (5′-TGG AGC ACT ATC CGA) oligonucleotides were modified versions of the original primers used by Gilbart et al. (11). PCRs were run in a GeneAmp 2400 apparatus (Perkin-Elmer, Norwalk, Conn.) under the conditions reported previously (11). mupA detection was later confirmed by hybridization studies (see restriction fragment length polymorphism [RFLP] analysis of the mupA locus).
RAPD analysis.
Total DNA from staphylococcal spheroplasts was purified with the Genomic DNA Prep Plus kit (A&A Biotechnology, Gdańsk, Poland). In order to obtain spheroplasts, bacterial cells were digested with lysostaphin (AMBI Inc., Tarrytown, N.Y.) at 37°C. Randomly amplified polymorphic DNA (RAPD) analysis was performed with RAPD-7 (37) and ERIC-1 (38) primers by using a GeneAmp 2400 cycler (Perkin-Elmer) under the conditions reported previously (12).
Macrorestriction analysis of genomic DNA (PFGE).
The genomic DNAs of the staphylococcal isolates were prepared in agarose discs by a previously described methodology (5) and were digested with the SmaI restriction enzyme (MBI Fermentas, Vilnius, Lithuania). The resulting DNA fragments were separated by pulsed-field gel electrophoresis (PFGE) with a CHEF DRII system (Bio-Rad, Hercules, Calif.) under conditions reported previously (5).
Restriction endonuclease analysis of plasmid DNA (REAP).
Plasmid DNA was purified from staphylococcal spheroplasts by the modified alkaline lysis method (28). Plasmid DNA was digested with HindIII (MBI Fermentas), and the digested plasmid DNA was run in 1% agarose gels.
RFLP analysis of mupA locus.
A molecular probe representing the mupA gene-coding sequence was obtained by PCR with MI and MII primers and plasmid pMZ2, in which the mupA gene is cloned (7) as a template. The PCR product was gel purified and labelled with the ECL Random Prime Labeling System (Amersham, Little Chalfont, United Kingdom) as recommended by the manufacturer. Plasmid DNA was digested with EcoRI, HindIII, and Bsu15I (an isoschizomer of ClaI) (all the enzymes were purchased from MBI Fermentas), and the digested plasmid DNA was run in 0.8% agarose gels. Transfer of DNA onto a positively charged nylon membrane (Boehringer Mannheim, Mannheim, Germany) was performed with the use of a vacuum blotter (Vacuus Transfere; Kucharczyk TE, Warsaw, Poland). Hybridization and detection were performed with the ECL system (Amersham) according to the manufacturer’s recommendations.
Plasmid curing.
Curing of plasmids from MuH isolates was performed by culturing the cells overnight at 43°C, and subsequent screening for loss of the MuH phenotype was done by replica plating as described earlier (36). The mupirocin susceptibility of the resulting strains was tested by the disk diffusion method with 5- and 200-μg mupirocin disks (9) and by evaluation of MICs. The plasmid contents of these strains were determined and characterized by REAP. The loss of the mupA gene was confirmed by hybridization. RAPD and PFGE analyses were used to compare the chromosomal background of the cured strains with the parental ones.
RESULTS
Species identification and antimicrobial susceptibility testing.
The results of the species identification and antimicrobial susceptibility testing analyses are presented in Tables 1 and 2. Fifty-three staphylococcal isolates were identified as MuR with 5-μg mupirocin disks, and these constituted 19.5% of all staphylococci recovered in the two wards of the hospital over the 17-month period between February 1993 and June 1994. The first MuR isolate (S. epidermidis BNM 2) was recovered from an environmental sample in August 1993, and the remaining MuR staphylococci were recovered over the whole collection interval (Fig. 1). Only 7 MuR isolates were identified during the 11 months from February to December 1993, while 46 isolates were collected within the 6 months between January and June 1994. MuR isolates were observed only in the neonatal ward until December 1993, when first isolates from the pediatric ward were recovered. Nineteen of the MuR isolates were identified as S. aureus (11.3% of all S. aureus isolates collected); of the remainder, classified as CoNS, 31 isolates were identified as S. epidermidis and 3 others were identified as single isolates each of S. haemolyticus, S. xylosus, and S. capitis. Mupirocin resistance was found in 32.7% of all CoNS isolates collected. The mupirocin MIC assessment has revealed that both mupirocin resistance phenotypes (MuL and MuH) were present among the MuR isolates. The MuL phenotype was manifested by seven S. epidermidis isolates (mupirocin MICs, 32 to 128 μg/ml), while all remaining MuR isolates were determined to have the MuH phenotype (MICs, ≥1,024 μg/ml). The first MuR isolate (S. epidermidis BNM 2) was identified as MuL, and isolates of this phenotype substantially contributed to the total number of MuR isolations only in 1993 (Fig. 1). The first MuH isolate (S. xylosus BNM 4) was identified in October 1993; in 1994 the MuH isolates became absolutely predominant among all the MuR strains. All but two MuR strains (single isolates of S. xylosus and S. capitis) were identified as being methicillin resistant; the S. aureus as well as the CoNS isolates expressed the heterogeneous phenotype of resistance to this drug. Detailed susceptibility testing was carried out with all the MuR isolates for epidemiological purposes (Tables 1 and 2). All S. aureus isolates were resistant to penicillin due to β-lactamase production, 14 isolates (74%) were resistant to tetracycline, and 10 isolates (53%) were resistant to kanamycin. A single S. aureus isolate expressed resistance to rifampin. Twenty-three S. epidermidis isolates (74%) were resistant to tetracycline; 18 isolates (58%) were resistant to erythromycin. Eleven (36%) and eight (26%) isolates were resistant to kanamycin and gentamicin, respectively. Sporadic isolates were resistant to trimethoprim-sulfamethoxazole (four isolates), streptomycin (three isolates), and amikacin (two isolates). The methicillin-susceptible S. capitis strain was resistant only to penicillin and mupirocin, and the S. xylosus isolate was sensitive to all antibiotics tested except mupirocin.
TABLE 1.
Typing, susceptibility, and mupA analysis results for S. aureus isolates
| Isolate no. | Isolation date (day.mo.yr) | Source of isolationa | Mupirocin MIC (μg/ml) | REAP pattern | PFGE patternb | RAPD pattern | mupA locus polymorph | mupA PCR result | Resistancec |
|---|---|---|---|---|---|---|---|---|---|
| BNM 12 | 16.12.1993 | i | 8,192 | A1 | a6 | A/A | I | + | KP |
| BNM 46 | 7.6.1994 | c | 4,096 | A1 | a5 | A/A | I | + | KP |
| BNM 47 | 7.6.1994 | c | 4,096 | A1 | a5 | A/A | I | + | KP |
| BNM 55 | 23.6.1994 | c | 4,096 | A1 | a5 | A/A | I | + | KP |
| BNM 26 | 28.2.1994 | c | 4,096 | A2 | a4 | A/A | I | + | K |
| BNM 15 | 4.1.1994 | e | 5,120 | A3 | a4 | A/A | I | + | KPT |
| BNM 28 | 12.4.1994 | e | 7,168 | A3 | a1 | A/A | I | + | PT |
| BNM 30 | 12.4.1994 | e | 5,120 | A3 | a1 | A/A | I | + | PT |
| BNM 31 | 12.4.1994 | e | 4,096 | A3 | a1 | A/A | I | + | KPT |
| BNM 32 | 25.4.1994 | i | 4,096 | A3 | a1 | A/A | I | + | KPT |
| BNM 34 | 25.4.1994 | i | 4,096 | A3 | a1 | A/A | I | + | PT |
| BNM 38 | 4.5.1994 | e | 4,096 | A3 | a1 | A/A | I | + | PT |
| BNM 50 | 9.6.1994 | c | 4,096 | A3 | a2 | A/A | I | + | PT |
| BNM 53 | 22.6.1994 | c | 4,096 | A3 | a1 | A/A | I | + | KPT |
| BNM 66 | 21.4.1994 | i | 4,096 | A3 | a4 | A/A | I | + | PRT |
| BNM 69 | 13.6.1994 | i | 4,096 | A3 | a1 | A/A | I | + | KPT |
| BNM 70 | 13.6.1994 | i | 4,096 | A3 | a1 | A/A | I | + | KPT |
| BNM 49 | 9.6.1994 | c | 4,096 | A3 | a6 | A/A | I | + | PT |
| BNM 63 | 22.6.1994 | c | 4,096 | B1 | a3 | A/A | I | + | PT |
Isolation sources: i, site of infection; e, environment; c, carriers.
Results of PFGE typing of S. aureus isolates have already been included in the multicenter analysis of MRSA clones present in Poland (19).
Explanation of resistance patterns: K, kanamycin resistance; P, penicillin resistance; R, rifampin resistance; S, streptomycin resistance; T, tetracycline resistance.
TABLE 2.
Typing, susceptibility, and mupA analysis results of CoNS isolates
| Isolate no. | Isolation date (day.mo.yr) | Source of isolationa | Mupirocin MIC (μg/ml) | REAP pattern | PFGE pattern | RAPD pattern | mupA PCR result | mupA locus polymorph | Resistanceb |
|---|---|---|---|---|---|---|---|---|---|
| S. epidermidis | |||||||||
| BNM 8 | 23.11.1993 | e | 32 | C1 | b | C/C | − | − | PT |
| BNM 51 | 9.6.1994 | c | 64 | C1 | b | C/C | − | − | EP |
| BNM 2 | 4.8.1993 | e | 64 | C2 | b | C/C | − | − | EPT |
| BNM 9 | 23.11.1993 | e | 128 | C2 | b | C/C | − | − | EPT |
| BNM 19 | 12.1.1994 | i | 64 | C2 | b | C/C | − | − | EPT |
| BNM 20 | 2.2.1994 | i | 32 | C2 | b | C/C | − | − | EPT |
| BNM 21 | 31.1.1994 | e | 32 | C2 | b | C/C | − | − | EPT |
| BNM 16 | 4.1.1994 | e | 5,120 | C3 | b | C/C | + | I | EKPT |
| BNM 27 | 1.4.1994 | i | 5,120 | C3 | b | C/C | + | I | EPT |
| BNM 37 | 4.5.1994 | e | 4,096 | C4 | b | C/C | + | I | EPT |
| BNM 44 | 7.6.1994 | c | 4,096 | D1 | b | C/C | + | II | PT |
| BNM 54 | 24.6.1994 | c | 4,096 | D1 | b | C/C | + | II | EPT |
| BNM 23 | 28.2.1994 | e | 1,024 | D2 | b | C/C | + | II | EGPSX |
| BNM 61 | 21.6.1994 | c | 8,192 | E | d | G/C | + | I | GKPST |
| BNM 18 | 4.1.1994 | e | 4,096 | F | h | B/B | + | I | EPT |
| BNM 22 | 28.02.94 | e | 2,048 | F | h | B/B | + | I | EPT |
| BNM 29 | 12.04.94 | e | 2,048 | G1 | h | B/B | + | I | EPT |
| BNM 35 | 24.04.94 | i | 4,096 | G1 | h | B/B | + | I | EPT |
| BNM 11 | 16.12.93 | i | 4,096 | G2 | h | B/B | + | I | EPT |
| BNM 56 | 21.06.94 | c | 4,096 | H1 | h | B/B | + | I | EPT |
| BNM 52 | 20.06.94 | c | 4,096 | H2 | h | B/C | + | I | EP |
| BNM 42 | 6.06.94 | e | 2,048 | I | f1 | F/C | + | II | AGKPT |
| BNM 59 | 20.06.94 | c | 4,096 | I | f1 | F/C | + | II | GKPST |
| BNM 43 | 6.06.94 | e | 4,096 | J | f2 | F/C | + | II | GKPTX |
| BNM 48 | 8.06.94 | c | 4,096 | J | f1 | F/C | + | II | GKPTX |
| BNM 57 | 20.06.94 | c | 4,096 | K | f3 | F/E | + | I | GKPT |
| BNM 5 | 16.11.93 | i | 4,096 | B2 | c | H/B | + | I | KP |
| BNM 65 | 24.06.94 | c | 4,096 | B2 | c | H/B | + | I | KP |
| BNM 67 | 30.05.94 | e | 4,096 | B2 | c | H/B | + | I | KP |
| BNM 36 | 6.05.94 | i | 4,096 | L | g | D/C | + | I | KPT |
| BNM 40 | 4.05.94 | e | 4,096 | M | e | E/D | + | I | AGKP |
| S. xylosus BNM 4 | 20.10.93 | c | 4,096 | N | j | I/F | − | III | |
| S. haemolyticus BNM 33 | 26.04.94 | i | 8,192 | O | i | G/J | + | I | EGKPS |
| S. capitis BNM 64 | 22.06.94 | c | 2,048 | P | k | C/H | + | I | P |
Isolation sources: i, site of infection; e, environment; c, carriers.
Explanation of resistance patterns: A, amikacin resistance; E, erythromycin resistance; G, gentamicin resistance; K, kanamycin resistance; P, penicillin resistance; S, streptomycin resistance; T, tetracycline resistance; X, trimethoprim-sulfamethoxazole resistance.
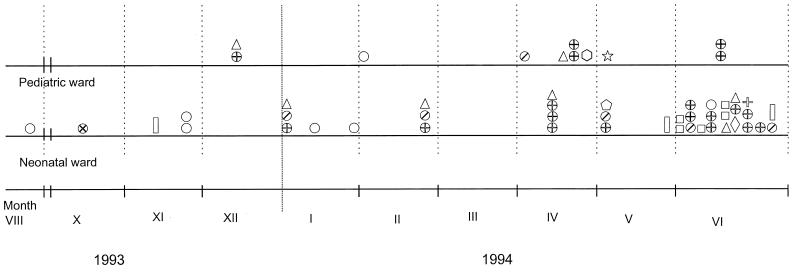
FIG. 1.
Timing of MuR staphylococci isolations. Symbols
correspond to the different PFGE types of the isolates, as follows:
⊕, type a (S. aureus); ○, type b (S.
epidermidis MuL);
 , type b
(S. epidermidis MuH); ▯, type c
(S. epidermidis); ◊, type d (S. epidermidis);
, type b
(S. epidermidis MuH); ▯, type c
(S. epidermidis); ◊, type d (S. epidermidis);
 , type e
(S. epidermidis); □, type f (S. epidermidis);
⋆, type g (S. epidermidis); ▵, type h (S.
epidermidis);
, type e
(S. epidermidis); □, type f (S. epidermidis);
⋆, type g (S. epidermidis); ▵, type h (S.
epidermidis);
 , type i (S.
haemolyticus); ⊗, type j (S. xylosus);
, type k
(S. capitis). Symbols located directly over each other
indicate isolates recovered on the same day.
, type i (S.
haemolyticus); ⊗, type j (S. xylosus);
, type k
(S. capitis). Symbols located directly over each other
indicate isolates recovered on the same day.
PFGE typing.
All 53 MuR staphylococcal isolates were subjected to PFGE with the SmaI restriction enzyme. Results of the analysis are shown in Tables 1 and 2. Nineteen S. aureus isolates produced six banding patterns. The degree of similarity of two of the least-similar patterns was characterized by a Dice coefficient value of 0.82. According to the interpretative criteria proposed by Tenover et al. (31), all the patterns represented six subtypes of a single type and were designated a1 to a6. The most prevalent subtype was a1, in which nine different isolates were grouped. Among 31 S. epidermidis isolates, nine different PFGE patterns were revealed, and these constituted seven distinct types. The types that had more than 1 isolate were type b, with 13 isolates (all the MuL isolates and 6 MuH isolates); type h, with 7 isolates; type f, with 5 isolates (subtypes f1, f2, and f3), and type c, with 3 isolates. The results of PFGE typing of S. aureus isolates have already been included in the description of a multicenter analysis of MRSA clones present in Poland (19).
RAPD typing.
All MuR isolates were typed by RAPD analysis with the use of RAPD-7 (37) and ERIC-1 (38) primers. Results of the analysis are listed in Tables 1 and 2. The RAPD analysis results were consistent with the PFGE data. All 19 isolates of S. aureus had identical RAPD patterns with both primers, denoted as combined pattern A/A (PFGE type a). Typing of the S. epidermidis isolates revealed nine different combined RAPD types. The C/C type was represented by 13 isolates, including all the MuL isolates and 6 MuH isolates (of PFGE type b). Six other isolates were of the B/B type (PFGE type h), four isolates were of the F/C type (PFGE type f), three isolates were of the H/B type (PFGE type c), and single isolates were of other unique types.
REAP.
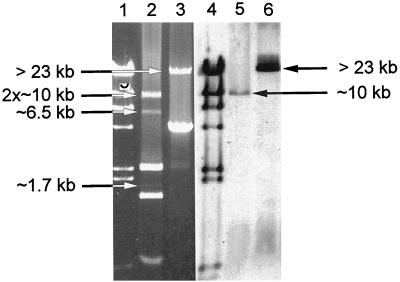
The REAP patterns that were revealed (Tables 1 and 2) were found to be very complex due to the variable compositions of multiple plasmids of various sizes and sequences in the cells of the isolates studied. Almost all isolates contained more than two plasmid molecules that were repeatedly purified with different efficiencies. REAP has shown that the plasmid DNAs of 18 of 19 S. aureus isolates produced very similar complex restriction patterns, patterns A1 to A3. REAP types A1 and A2 differed slightly from each other by the restriction patterns of their high-molecular-mass plasmids, and A3 was different from A2 only by the presence of a single additional small molecule, which has correlated with tetracycline resistance (data not shown). A single S. aureus isolate, isolate BNM 63, was specified by a unique, relatively simple REAP pattern, pattern B1, which was produced by one large plasmid (about 28 kb) and one small plasmid (about 4.7 kb). The ca. 28-kb plasmid was digested with HindIII into a double band of ca. 10 kb and bands of ca. 6.5 and 1.7 kb, whereas bands of about 2.5, 1.5, and 0.7 kb were obtained from the much more abundant smaller plasmid (Fig. 2; for details, see Discussion). Plasmid DNAs purified from S. epidermidis isolates revealed a much higher degree of variability. Nineteen different REAP patterns have been distinguished among 31 isolates that differed according to the bands obtained from either large or small plasmids, or both. The largest group of S. epidermidis isolates with similar REAP patterns was formed by 13 isolates of the PFGE type b, with REAP patterns C1 to C5, D1, and D2. These patterns, which were formed by multiple plasmids, shared with each other several DNA bands, and the differences between some of these were due to the presence or absence of a single molecule (data not shown; for details, see Discussion).
FIG. 2.
Identification of the pMupA I plasmid. The plasmid DNA digestion patterns for isolate BNM 63 is obtained with HindIII (lane 1) and Bsu15I (ClaI) (lane 2) and the results of hybridization of the digested DNA with the mupA probe (HindIII, lane 5; Bsu15I [ClaI], lane 6) are shown. Lanes 1 and 4, bacteriophage λ digested by HindIII DNA molecular size marker (Kucharczyk TE, Warsaw, Poland). DNA bands specific for the pMupA I plasmid are indicated by arrows.
mupA gene detection and RFLP analysis of mupA locus.
The mupA gene was detected by specific PCR in all but one of the isolates that expressed the MuH phenotype; the exception was the single isolate of S. xylosus (isolate BNM 4). No amplification was obtained for any of the S. epidermidis isolates with the MuL phenotype. Plasmid DNA extracted from all MuR isolates and digested with HindIII, EcoRI, and Bsu15I (ClaI) was hybridized with the mupA probe. Different sizes of hybridizing plasmid fragments distinguished three different mupA gene polymorphs. The mupA polymorph I was characterized by the EcoRI-hybridizing fragment of ca. 16 kb, the HindIII-hybridizing fragment of ca. 10 kb, and the Bsu15I (ClaI)-hybridizing fragment larger than 23 kb. Polymorph II was specified by hybridizing fragments of the following sizes: EcoRI, ca. 4 kb; HindIII, ca. 8 kb; and Bsu15I (ClaI), two fragments of ca. 2 and 8.5 kb. Polymorph III was defined by an EcoRI-hybridizing fragment of ca. 8 kb, an HindIII-hybridizing fragment of ca. 5.5 kb, and a Bsu15I (ClaI)-hybridizing fragment ca. 8 kb (data not shown). mupA polymorph I was the most prevalent polymorph among the collection of isolates analyzed (Tables 1 and 2). It was present in all S. aureus isolates, 17 S. epidermidis isolates (of all PFGE types), and single strains each of S. haemolyticus and S. capitis. Polymorph II characterized eight S. epidermidis isolates (PFGE types b and f). The single S. xylosus strain, for which no product by PCR with MI and MII primers was obtained, had the unique mupA polymorph, polymorph III.
Detection of dual MuR phenotype-bearing strains.
The representative MuH isolates from the PFGE type b cluster, BNM 27 (REAP type C3, mupA polymorph I) and BNM 44 (REAP type D1, mupA polymorph II), were subjected to the plasmid curing experiment. From 2 to 4% of cells cultured overnight at 43°C were found to have lost the MuH phenotype on replica plates containing 512 μg of mupirocin per ml. Single selected descendants of strains of each isolate were subsequently shown to express the MuL phenotype by both the disk diffusion method and the MIC evaluation (MICs, 32 μg/ml). They were confirmed to produce the same PFGE and RAPD patterns as the default clinical strains (data not shown). Plasmids were purified from the descendant strains and were digested with HindIII and EcoRI, and the restriction patterns were compared with the HindIII and EcoRI restriction patterns of plasmids obtained from the parental isolates. In all cases the plasmid DNA patterns of the descendants differed from those of the original strains by the lack of at least one visible DNA band. No hybridization with the mupA probe was observed with plasmid DNAs purified from descendant strains (data not shown).
DISCUSSION
The objective of the present study was to investigate the mechanisms of spread of mupirocin resistance in a population of staphylococcal strains from a large hospital that overused the dermatological formulation of mupirocin. The prevalence of mupirocin resistance among staphylococci isolated over the complete collection period was high (19.5%) compared with the overall level of resistance reported in surveys to date (26, 29). These data indicated that an outbreak of MuR staphylococci has occurred in the hospital, and this was of special concern as no mupirocin resistance among staphylococci had previously been reported in Poland. Identification of the MuR isolates has revealed that this group included S. aureus as well as several species of CoNS. Outbreaks of MuR S. aureus infection accompanied by the isolation of MuR CoNS have already been described (17, 26). The high proportion of MuR isolates among the CoNS isolates (32.7%) versus that (11.3%) among the S. aureus isolates indicates that CoNS constitute a significant reservoir of MuR strains. The resistance profiles of the isolates have shown that the overwhelming majority of these were resistant to methicillin. This is also typical for outbreaks caused by MuR staphylococci and can be explained by the fact that mupirocin is preferentially used for treatment of nosocomial infections caused by methicillin-resistant microorganisms, and so the selective pressure of mupirocin use is focused mainly on such strains.
Two widely used typing approaches, PFGE and RAPD analysis, supplemented by other methods (REAP, mupA polymorphism studies, susceptibility testing) were used to characterize the outbreak isolates in detail. Results have shown that the entire group of S. aureus (Table 1) isolates was of the MuH phenotype and produced DNA patterns which were either identical (the RAPD type A/A) or closely related (subtypes of the PFGE type a). They were also characterized by very similar REAP patterns (patterns A1 to A3 for all isolates except isolate BNM 63), the same mupA polymorph (polymorph I), and similar antimicrobial susceptibility profiles. A different situation was observed among the CoNS (Table 2). The analysis was mainly focused on the S. epidermidis population, which was represented by 7 MuL isolates and 24 MuH isolates. PFGE and RAPD analyses have defined a number of genetically diverse clusters of related S. epidermidis isolates. Some of the clusters (MuH PFGE types c, f, and h) grouped isolates which were also similar to each other in terms of their REAP, mupA polymorphism, or antimicrobial susceptibility patterns, although some degree of diversity in these aspects was observed within each group. The most interesting cluster was the S. epidermidis cluster that contained the largest number (n = 13) isolates which were indistinguishable both by PFGE (type b) and by RAPD analysis (pattern C/C). These isolates were differentiated by their MuR phenotypes (MuL and MuH), mupA polymorph types for the MuH isolates (I and II), and REAP patterns. These differences correlated with each other and illustrated a process of diversification of the originally single epidemic strain that involved various genetic events (discussed in detail below). When all the typing results are considered together, it may be postulated that at least one S. aureus strain and four different S. epidermidis MuR strains were clonally spread at the same time in the hospital.
Mupirocin resistance may be a result of either point mutations in the chromosomal ileS gene (MuL) or acquisition of the plasmid-located mupA gene (MuH) (8). Recently, staphylococcal strains in which the mupA gene has a chromosomal location have been isolated (27) and have been found to have a low level of mupirocin resistance. This effect has been attributed to lower levels of expression of the mupA gene in the context of chromosomal sequences. REAP, mupA detection, and analysis of mupA locus polymorphism were applied to investigate the mechanisms of mupirocin resistance and the possible routes of their spread in the hospital (Tables 1 and 2). As mentioned above, only seven clonally related S. epidermidis isolates were found to be of the MuL phenotype (MICs, 32 to 128 μg/ml), whereas all the other isolates were characterized by the MuH phenotype (MICs, 1,024 to 8,192 μg/ml). The MuL isolates did not contain the mupA gene, as demonstrated by both PCR and hybridization experiments. PCR with mupA-specific primers has detected the mupA gene in the DNAs of all but one of the MuH isolates. The only exception was the single S. xylosus isolate, which was nevertheless shown to possess the mupA gene by hybridization with the mupA-specific probe. The lack of DNA amplification with MI and MII primers in this case has suggested that this particular strain had a unique mupA gene allele, which has correlated with the RFLP data (discussed below).
The mupA locus hybridization studies have provided several important data. mupA-hybridizing fragments were assigned to specific DNA bands in each of the plasmid restriction patterns identified among the MuH isolates. This has suggested that in all strains analyzed the mupA gene was located on a plasmid, and this result is consistent with other data (40) on the correlation of the MuH phenotype with such a location of the gene. All restriction digestions of plasmid DNA performed in the study have distinguished three different polymorphs of the mupA locus. Polymorph I (characterized by mupA-containing EcoRI DNA fragment of about 16 kb) was found to dominate the others. It was found in four species (S. aureus, S. epidermidis, S. haemolyticus, and S. capitis) and in different types or subtypes of particular species (e.g., S. epidermidis strains of all PFGE types) (Tables 1 and 2). This has suggested that a plasmid or a plasmid DNA fragment (a transposon) containing mupA polymorph I must have been exchanged between various staphylococcal strains that also belonged to different species. Polymorph II (located on EcoRI fragment of ca. 4 kb) was restricted to S. epidermidis; however, it was also found in isolates of different PFGE types (b and f). The single S. xylosus strain was specified by polymorph III (present on the EcoRI fragment of about 8 kb), and this strain, as was mentioned above, was the only MuH isolate for which no mupA gene was detected by PCR with MI and MII primers. These data may suggest that the MuH phenotype in this isolate was determined by a unique mupA allele with a mismatch(es) within the priming site(s) for the PCR primers. Different mupA locus polymorphs have been reported by British and American investigators. The polymorphs characterized by the EcoRI-hybridizing band of about 4 kb have been detected several times in isolates from Great Britain and the United States (2, 21, 22, 27, 40); however, it remains to be elucidated whether they were identical to the polymorph II analyzed in our study.
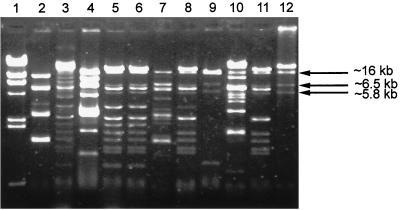
The mupA locus polymorphism analyzed in the context of REAP results has brought some interesting insights into understanding of the mechanism of spread of the mupA gene in the staphylococcal population studied. mupA polymorph I was found in a predominant number of both S. aureus and CoNS isolates, which were characterized by different REAP patterns resulting from the variable compositions of multiple plasmid molecules. Hybridization of the mupA probe to the single DNA band of more than 23 kb following Bsu15I (ClaI) digestion (the unique ClaI site is located within the coding part of the mupA gene [13]) has suggested that the polymorph I mupA allele could have been carried by a separate plasmid shared by different isolates. Of the mupA polymorph I-containing isolates, S. aureus BNM 63 presented the least complex REAP pattern (pattern B1), and this allowed us to identify the putative mupA-bearing plasmid, pMupA I. Pattern B1 was specified by two plasmids, of which the one of about 28 kb was cut by HindIII into a double band of ca. 10 kb and bands of ca. 6.5 and 1.7 kb (Fig. 2). The Bsu15I (ClaI) restriction enzyme has linearized this plasmid and produced a band of more than 23 kb (Fig. 2), and EcoRI has digested it into fragments of about 16, 6.5, and 5.5 kb (data not shown). Hybridization with the mupA probe with the ca. 10-kb band obtained after HindIII digestion (Fig. 2), >23-kb band obtained after Bsu15I (ClaI)-digestion (Fig. 2), and 16-kb band obtained after EcoRI digestion (data not shown) has confirmed that this molecule is pMupA I. Detailed analysis of the plasmid DNA restriction patterns that characterized other mupA polymorph I containing isolates has revealed the pMupA I-specific bands in all of these isolates; however, in many isolates some of the bands obtained by HindIII or EcoRI restriction digestion could be masked by bands for other plasmids (Fig. 3). These data have suggested that the polymorph I mupA gene has spread in both S. aureus and CoNS populations by means of dissemination of a specific plasmid. All mupA polymorph II gene-containing isolates were characterized by complex REAP patterns determined by multiple plasmid molecules. However, some bands were common to all the patterns obtained following each restriction digestion, and this has suggested that polymorph II may also have been located in a discrete plasmid that was spread to all isolates of that group.
FIG. 3.
EcoRI restriction patterns of plasmid DNA extracted from selected MuR isolates belonging to several different REAP types and carrying different mupA polymorphs. Patterns characteristic only for the mupA polymorph I containing isolates shared the three bands identified as pMupA I specific. In several cases, some of these bands could be masked by other plasmids. Lane 2, BNM 4 (S. xylosus, REAP type N, mupA polymorph III); lane 3, BNM 11 (S. epidermidis, type G, polymorph I); lane 4, BNM 15 (S. aureus, type A, polymorph I); lane 5, BNM 18 (S. epidermidis, type F, polymorph I); lane 6, BNM 22 (S. epidermidis, type F, polymorph I); lane 7, BNM 23 (S. epidermidis, type D, polymorph II); lane 8, BNM 29 (S. epidermidis, type G, polymorph I); lane 9, BNM 33 (S. haemolyticus, type O, polymorph I); lane 10, BNM 40 (S. epidermidis, type M, polymorph I); lane 11, BNM 52 (S. epidermidis, type H, polymorph I); lane 12, BNM 65 (S. epidermidis, type B, polymorph I). The DNA bands produced by the pMupA I plasmid are indicated by arrows. Lane 1, phage λ DNA digested by HindIII molecular size marker (Kucharczyk TE).
The cluster of 13 closely related S. epidermidis isolates characterized by PFGE pattern b and RAPD combined type C/C (Table 2) was especially interesting in terms of the spread of mupirocin resistance in the hospital. As mentioned above, this group was internally diversified by the phenotype of mupirocin resistance, and both MuL and MuH phenotypes were represented. Among the six MuH isolates, three (BNM 16, BNM 27, and BNM 37) had mupA polymorph I and three others (BNM 23, BNM 44, and BNM 54) had polymorph II. Especially interesting were comparisons of the plasmid restriction patterns for particular isolates. REAP patterns C4 and C3, characteristic of the MuH mupA polymorph I isolates, differed from patterns C1 and C2 of some of the MuL isolates, respectively, only by the presence of the pMupA I-specific bands (data not shown). REAP pattern D1 of some of the MuH mupA polymorph II isolates among other bands contained all the bands typical of REAP pattern C5 of a MuL isolate. Obvious similarities were also found between the C1, C2, and C5 patterns of plasmids from the MuL isolates (data not shown) and indicated that one pattern could evolve from the other by the acquisition or loss of a single plasmid molecule. All these observations have suggested that the PFGE type b S. epidermidis population was originally of the MuL phenotype, has diversified in time in terms of the plasmid content, and has subsequently produced the MuH subpopulation. This subpopulation has probably emerged due to several independent events of introduction of mupA-carrying plasmids into the MuL genetic background. The results of the plasmid curing experiment carried out with representative REAP type C3 and D1 isolates support this hypothesis. For the descendant strains that were obtained, the MuR phenotype was found to have changed from MuH to MuL, and this has correlated with the loss from the REAP patterns of characteristic DNA bands, including those that hybridize with the mupA probe. The evidence has indicated that two different mupirocin resistance mechanisms were active simultaneously in the MuH S. epidermidis isolates of PFGE type b. Although the existence of such strains has been anticipated by Cookson (1), this is the first report of such a dual mechanism of mupirocin resistance in strains of staphylococci.
Clinical analysis of the outbreak is difficult, as testing of samples from the environmental and carriers was performed only for the neonatal ward and not for the pediatric ward; therefore, it is not possible to establish the exact time points of important epidemiological events. The first MuR isolate (BNM 2) was detected about 8 months after introduction of the drug into clinical practice (Fig. 1). It was identified in the environmental sample from the neonatal ward as an S. epidermidis strain of the MuL phenotype. However, the possibility that the MuH phenotype was selected first but remained undetected at the beginning of the study cannot be excluded. The frequency distribution of isolation of MuR strains has suggested that the outbreak rapidly developed at the end of 1993 and the beginning of 1994. This is supported by the fact that the first-identified infections caused by MuR staphylococci occurred in both wards in November and December 1993 and by the fact that the frequency of isolation of MuR strains from the environment of the neonatal ward increased at that time. This outbreak was mainly due to strains with the MuH phenotype, as they constituted 86.8% of all MuR isolates and 91.7% of all MuR isolates identified from December 1993 to the end of the study. Different epidemiological phenomena contributed to the outbreak. The presence of three mupA gene polymorphs, located in different plasmid molecules, may suggest that more than one selection event for the gene has occurred in the hospital over the outbreak period. At least one of the plasmids that carried the mupA gene (pMupA I) has undergone a very efficient dissemination process among the strains and has also crossed the species barriers. On the other hand, several strains, one S. aureus strain and at least four S. epidermidis strains, have spread clonally in the hospital environment. Identification of the same strains in both the neonatal and the pediatric wards has revealed that they must have been transmitted between the wards, which are located next each to other on the same floor of the hospital building. All aspects of the spread of MuR staphylococci in the hospital were facilitated by the high rate of carriage by personnel, as demonstrated by the tests performed in June 1994. The MuR isolates recovered from carriers at this time accounted for about half of the whole collection and comprised several strains that belonged to a number of staphylococcal species. Movement of the personnel as well as transfer of patients with incubators between the two wards has been a routine practice in the hospital. The spread of MuR staphylococci in the hospital was promoted by over- and misuse of the dermatological formulation of mupirocin in the two wards. Following the outbreak, mupirocin has been removed from the hospital formulary as a drug for the treatment of skin infections.
ACKNOWLEDGMENTS
We thank Keith Dyke, who kindly supplied the plasmid (plasmid pMZ2) used for preparation of the mupA probe and Neil Woodford for helpful discussions concerning the PCR primer design.
This work was partly supported by KBN (Polish Scientific Research Committee) grant 4 P05E 026 16. The collection of strains was financed by grant 6 P204 011 04 from KBN.
REFERENCES
- 1.Cookson B D. The emergence of mupirocin resistance: a challenge to infection control and antibiotic prescribing practice. J Antimimicrob Chemother. 1998;41:11–18. doi: 10.1093/jac/41.1.11. [DOI] [PubMed] [Google Scholar]
- 2.Cookson B D, Lacey R W, Noble W C, Reeves D S, Wise R, Redhead R J. Mupirocin-resistant Staphylococcus aureus. Lancet. 1990;335:1095–1096. doi: 10.1016/0140-6736(90)92667-7. [DOI] [PubMed] [Google Scholar]
- 3.Courvalin P, Soussy C J. Report of the comité de l’antibiogramme de la SocietéFancaise de Microbiologie. Clin Microbiol Infect. 1996;2:S11–S25. [Google Scholar]
- 4.Dawson S J, Finn L F, McCulloch J E, Kilvington S, Lewis D A. Mupirocin-resistant MRSA. J Hosp Infect. 1994;28:75–78. doi: 10.1016/0195-6701(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 5.de Lencastre H, Couto I, Santos I, Melo-Cristino J, Torres-Pereira A, Tomasz A. Methicillin-resistant Staphylococcus aureusdisease in a Portuguese hospital: characterization of clonal types by a combination of DNA typing methods. Eur J Clin Microbiol Infect Dis. 1994;13:64–73. doi: 10.1007/BF02026129. [DOI] [PubMed] [Google Scholar]
- 6.de Lencastre H, Figueiredo A M S, Urban C, Rahal J, Tomasz A. Multiple mechanisms of methicillin resistance and improved methods for detection in clinical isolates of Staphylococcus aureus. Antimicrob Agents Chemother. 1991;35:632–639. doi: 10.1128/aac.35.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyke, K. G. H. Personal communication.
- 8.Dyke K G H, Curnock S P, Golding M, Noble W C. Cloning of the gene conferring resistance to mupirocin in Staphylococcus aureus. FEMS Microbiol Lett. 1991;77:195–198. doi: 10.1016/0378-1097(91)90550-t. [DOI] [PubMed] [Google Scholar]
- 9.Eltringham I. Mupirocin resistance and methicillin-resistant Staphylococcus aureus(MRSA) J Hosp Infect. 1997;35:1–8. doi: 10.1016/s0195-6701(97)90162-6. [DOI] [PubMed] [Google Scholar]
- 10.Finlay J E, Miller L A, Poupard J A. Interpretive criteria for testing susceptibility of staphylococci to mupirocin. Antimicrob Agents Chemother. 1997;41:1137–1139. doi: 10.1128/aac.41.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbart J, Perry C R, Slocombe B. High-level mupirocin resistance in Staphylococcus aureus: evidence for two distinct isoleucyl-tRNA synthetases. Antimicrob Agents Chemother. 1993;37:32–38. doi: 10.1128/aac.37.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gniadkowski M, Schneider I, Jungwirth R, Hryniewicz W, Bauernfeind A. Ceftazidime-resistant Enterobacteriaceaeisolates from three Polish hospitals: identification of three novel TEM- and SHV-5-type extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1998;42:514–520. doi: 10.1128/aac.42.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodgson J E, Curnock S P, Dyke K G H, Morris R, Sylvester D R, Gross M S. Molecular characterization of the gene encoding high-level mupirocin resistance in Staphylococcus aureusJ2870. Antimicrob Agents Chemother. 1994;38:1205–1208. doi: 10.1128/aac.38.5.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hryniewicz W, Trzciński K. The current situation and perspectives of standardisation in susceptibility testing in Poland. Antiinfect Drugs Chemother. 1994;13:35–40. [Google Scholar]
- 15.Hughes J, Mellows G. Interaction of pseudomonic acid A with Escherichia coliB isoleucyl-tRNA synthetase. Biochem J. 1980;191:209–219. doi: 10.1042/bj1910209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huskisson S C, Wainscott G. A comparative trial of mupirocin and chlortetracycline in general practice and a children’s casualty department. In: Wilkinson D S, Price J D, editors. Mupirocin—a novel topical antibiotic. London, United Kingdom: Royal Society of Medicine; 1985. pp. 109–112. [Google Scholar]
- 17.Irish D, Eltringham I, Teall A, Pickett H, Farrelly H, Reith S, Woodford N, Cookson B. Control of an outbreak of an epidemic methicillin and mupirocin resistant Staphylococcus aureus. J Hosp Infect. 1998;39:19–26. doi: 10.1016/s0195-6701(98)90239-0. [DOI] [PubMed] [Google Scholar]
- 18.Layton M C, Patterson J E. Mupirocin resistance among consecutive isolates of oxacillin-resistant and borderline oxacillin-resistant Staphylococcus aureusat a university hospital. Antimicrob Agents Chemother. 1994;38:1664–1667. doi: 10.1128/aac.38.7.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leski T, Oliveira D, Trzcinski K, Santos Sanches I, Aires de Sousa M, Hryniewicz W, de Lencastre H. Clonal distribution of methicillin-resistant Staphylococcus aureusin Poland. J Clin Microbiol. 1998;36:3532–3539. doi: 10.1128/jcm.36.12.3532-3539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Łęski T A, Gniadkowski M, Trzcinski K, Hryniewicz W. Comparison of genetic characteristics of MRSA strains present in the Warsaw hospital in 1992 and 1996. Zentbl Bakteriol Parasitenko Infektionskr Hyg Abt I Orig. 1998;287:363–373. doi: 10.1016/s0934-8840(98)80172-2. [DOI] [PubMed] [Google Scholar]
- 21.Marples R R, Speller D C E, Cookson B D. Prevalence of mupirocin resistance in Staphylococcus aureus. J Hosp Infect. 1995;29:153–161. doi: 10.1016/0195-6701(95)90197-3. [DOI] [PubMed] [Google Scholar]
- 22.Morton T M, Johnston J L, Patterson J, Archer G L. Characterization of a conjugative staphylococcal mupirocin resistance plasmid. Antimicrob Agents Chemother. 1995;39:1272–1280. doi: 10.1128/aac.39.6.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murchan S, Trzciński K, Skoczynska A, van Leeuven W, van Belkum A, Pietuszko S, Gadomski T, Hryniewicz W. Spread of old and new clones of epidemic methicillin-resistant Staphylococcus aureusin Poland. Clin Microbiol Infect. 1998;4:481–490. [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards. Document M2-A5. Vol. 13 1993. , no. 24. National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. Document M7-A3. Vol. 13 1993. , no. 24. National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 26.Rahman M, Noble W C, Cookson B. Mupirocin-resistant Staphylococcus aureus. Lancet. 1987;ii:387. doi: 10.1016/0140-6736(90)92667-7. [DOI] [PubMed] [Google Scholar]
- 27.Ramsey M A, Bradley S F, Kauffman C A, Morton T M. Identification of chromosomal location of mupAgene, encoding low-level mupirocin resistance in staphylococcal isolates. Antimicrob Agents Chemother. 1996;40:2820–2823. doi: 10.1128/aac.40.12.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Schmitz F J, Lindenlauf E, Hofmann B, Fluit A C, Verhoef J, Heinz H P, Jones M E. The prevalence of low- and high-level mupirocin resistance in staphylococci from 19 European hospitals. J Antimicrob Chemother. 1998;42:489–495. doi: 10.1093/jac/42.4.489. [DOI] [PubMed] [Google Scholar]
- 30.Simpson N B, Fitzsimons C P, MacKenzie J, Gemmell C G. Bactroban ointment in flaring atopic dermatitis. In: Dobson R L, Leyden J J, Noble W C, Price J D, editors. Bactroban (mupirocin). Proceedings of an international symposium. Amsterdam, The Netherlands: Excerpta Medica; 1985. pp. 171–174. [Google Scholar]
- 31.Tenover F C, Arbeit R D, Goering V R, Mickelsen P A, Murray B E, Pershing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trzciński K, Dulny G, Tyski S, Zaręba T, Hryniewicz W. Program and abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Staphylococcus aureus resistant to mupirocin and methicillin in neonatal unit of Warsaw postgraduate teaching hospital, abstr. C-34; p. 45. [Google Scholar]
- 33.Trzciński K, Hryniewicz W, Claus H, Witte W. Characterization of two different clusters of clonally related methicillin-resistant Staphylococcus aureusstrains by conventional and molecular typing. J Hosp Infect. 1994;28:113–126. doi: 10.1016/0195-6701(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 34.Trzciński K, Hryniewicz W, Kluytmans J, van Leeuven W, Sijmons M, Dulny G, Verbrugh H, van Belkum A. Simultaneous persistence of methicillin-resistant and methicillin susceptible clones of Staphylococcus aureusin a neonatal ward of a Warsaw Hospital. J Hosp Infect. 1997;36:291–303. doi: 10.1016/s0195-6701(97)90056-6. [DOI] [PubMed] [Google Scholar]
- 35.Trzciński K, van Leeuven W, van Belkum A, Grzesiowski P, Kluytmans J, Sijmons M, Verbrugh H, Witte W, Hryniewicz W. Two clones of methicillin-resistant Staphylococcus aureusin Poland. Clin Microbiol Infect. 1997;3:198–207. doi: 10.1111/j.1469-0691.1997.tb00598.x. [DOI] [PubMed] [Google Scholar]
- 36.Udo E E, Pearman J W, Grubb W B. Emergence of high-level mupirocin resistance in methicillin-resistant Staphylococcus aureusin western Australia. J Hosp Infect. 1994;26:157–165. doi: 10.1016/0195-6701(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 37.van Belkum A, Kluytmans J, van Leeuven W, Quint W, Peters E, Fluit A, Vandenbroucke-Grauls C, van den Brule A, Koeleman H. Multicenter evaluation of arbitrarily primed PCR for typing of Staphylococcus aureusstrains. J Clin Microbiol. 1995;33:1537–1547. doi: 10.1128/jcm.33.6.1537-1547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Versalovic J, Koeuth T, Lupski J R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1995;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voss A, Milatovic D, Wallrauh-Shwarz C, Rosdahl V T, Braveny I. Methicillin resistant Staphylococcus aureusin Europe. Eur J Clin Microbiol Infect Dis. 1994;13:50–55. doi: 10.1007/BF02026127. [DOI] [PubMed] [Google Scholar]
- 40.Woodford N, Watson A P, Patel S, Waghorn D J, Cookson B D. Heterogeneous location of the novel mupA gene which confers high-level mupirocin resistance in Staphylococcus aureus. J Med Microbiol. 1998;47:829–835. doi: 10.1099/00222615-47-9-829. [DOI] [PubMed] [Google Scholar]